Abstract
Background/Aim: There are two strategies for the interpretation of tumor markers (TM) in fluid effusions: i) high cut-off and ii) fluid/serum ratio (F/S) and low cut-off. The objective of this study is to compare these two strategies and to determine whether diagnostic accuracy improves by the identification of possible false positives using Adenosine deaminase (ADA), C reactive protein (CRP) and % of polymorphonuclear cells (%PN). Patients and Methods: We studied 157 ascitic fluids, 74 of which were malignant. ADA, CRP and %PN were determined in ascitic fluid, and Carcinoembryonic antigen (CEA), Cancer antigen 72-4 (CA72-4), Cancer antigen CA19-9 and Cancer antigen 15-3 (CA15-3) in both fluid and serum. Results: The strategy of high cut-off showed 59.5% sensitivity at 100% specificity. The F/S strategy showed 75.7% sensitivity at 95.2% specificity. Subclassifying cases with ADA, CRP and %PN negative showed 67.5% sensitivity at 100% specificity for high cut-off and for the F/S strategy was 81.7% sensitivity at 98.7% specificity. Conclusion: The strategy of F/S with negative ADA, CRP and %PN allow the best interpretation for TM in the ascitic fluid.
Keywords: Diagnosis, cancer, tumour markers, malignant ascites, differential diagnosis
Ascites is the abnormal accumulation of fluid in the peritoneal cavity. It is mostly seen in patients with liver disease, pancreatic disease, tuberculous peritonitis, congestive heart failure, kidney disease, acquired immune deficiency syndrome and cancer (1). Cytology is the gold standard for confirming the presence of malignant cells in ascitic fluid, but its sensitivity only ranges between 50 and 70% (2). The main cause of this low sensitivity is the fact that a primary tumor may infiltrate the peritoneum but may not shed cells, with a negative result in the cytological analysis. In these cases, other invasive procedures, such as laparoscopy may be needed to confirm the presence of malignant cells.
The potential of tumor markers (TM) for improving the diagnosis of malignant pleural and peritoneal effusions has been mentioned by several authors but there are major discrepancies between the reports regarding their specificity and sensitivity, and also in terms of the cut-off values used (3-5): for Carcinoembryonic antigen (CEA) the range is 2-50 ng/ml and for Cancer antigen 19-9 (CA19-9) it is 14.5-200 ku/l (6-10). Their sensitivity ranges between 24% and 77% and their specificity from 82% to 100% (6-10). Establishing cut-off points is particularly difficult, because, among other reasons, different immunoassay kits obtain different concentrations in the same samples (11).
Another cause of the differences between studies is found in the types of benign diseases recorded. Tuberculosis and peritonitis may have high TM concentrations in peritoneal fluid, due to the inflammation of the mesothelial cells or nearby tissues (12,13). Adenosine deaminase (ADA), C-reactive protein (CRP) and granulocyte count are all used in the differential diagnosis of peritoneal inflammations, such as tuberculous effusions (14) and peritonitis (15-17).
Paramalignant effusions are another source of discrepancy. Although patients with these effusions have cancer, no neoplastic cells are present in the mesothelium; however, TM concentrations in serum may be high. So, tumour markers may be found in effusion fluids alongside other macromolecules, such as albumin, and they may be present in high concentrations in the serum of patients in the absence of neoplasia (18).
Taken together, to achieve high specificity using only TM values in effusions, high cut-off points have normally been used. However, in a previous study our group reported a strategy based on two criteria: i) low cut-off points and ii) the fluid/serum (F/S) ratio (19). Analyzing pleural, peritoneal and pericardial effusions and using a combination of CEA, cancer antigen 15-3 (CA15-3) and (CA19-9), we obtained a sensitivity of 76.2% and a specificity of 97%. Similarly, subclassifying these effusions according to their ADA, CRP and % of polymorphonuclear cells (%PN) value, we obtained a sensitivity of 80% and a specificity of 100% in patients with negative ADA, CRP and %PN using the F/S ratio. In a recent study using this strategy we found similar sensitivities for CEA, CA15-3, CA19-9 and Cancer antigen 72-4 (CA72-4) in pleural effusion (20). This strategy is less accurate for tumor markers secreted by mesothelial cells, such as cancer antigen 125 (CA125) and cytokeratin 19 fragments (CYFRA 21-1) that are best interpreted with a single determination in fluid effusion (21,22).
In the present study, we aimed to compare and assess the diagnostic accuracy of these two strategies: i) a cut-off point for each TM in fluid effusion to obtain maximum specificity, and ii) the F/S in ascitic fluid in order to validate previous results. We also sought to determine whether the classification in groups according to ADA, CRP and %PN might help improve diagnostic accuracy.
Patients and Methods
From January 2008 to December 2017, ascitic fluid and serum samples were collected from consecutive patients of all medical specialties at our center presenting a first episode of ascites. Patients with previously diagnosed liver cirrhosis were excluded. Diagnostic procedures were performed by ratters who were blind to the study data.
The reference method used was pathological confirmation of cancer in serous effusions or a definitive diagnosis within three months off the determination of TM. Malignant ascites was defined as the presence of neoplastic cells detected by cytology, biopsy or autopsy. Paramalignant effusions were defined as effusions in which none of the methods described above detected neoplastic cells in patients diagnosed with cancer.
The following tests were performed in fluid and/or serum in order to identify benign effusions: protein (biuret method, albumin (bromochresol purple), lactate dehydrogenase (LDH) (lactate to pyruvate), rheumatoid factor in LX-20 autoanalyzer (Beckman Coulter, Madrid, Spain) N-terminal probrain natriuretic peptide (Nt-ProBNP) in Cobas 601 analyzer (Roche Diagnostics, Barcelona, Spain), microbiological cultures, and (if required) antinuclear antibodies immunofluorescence in Hep 2 cells (Innova, Barcelona, Spain), anti-cyclic citrullinated peptide Unicap 250 (Thermofisher, Barcelona, Spain), thyrotropin, in DxI 800 analyzer (Beckman Couter), and serological tests for viruses (Vidas Biomerieux, Madrid, Spain), bacteria and fungi, among others.
Effusion fluid and serum samples were obtained and analyzed on the same day. CEA, CA15-3, CA 72-4 and CA19-9 were assessed using an electrochemiluminescence method on a Cobas 601 analyzer (Roche Diagnostics). The mean analytical variations expressed as the between-assay coefficient of variation were: 5% for CEA at a concentration of 5 μg/l, 3% for CA15-3 at a concentration of 32 ku/l, 4.2% for CA72-4 at a concentration of 8.3 ku/l and 4.5% for CA19-9 at a concentration of 29 ku/l, There were no changes in the two techniques used throughout the study period. In the first, using a single measurement in ascitic fluid, we determined the cut-off for each TM at a specificity of 100% with the ROC curve. For the second approach, simultaneous measurements were made in fluid and serum, while effusions were deemed malignant when CEA, CA15-3, CA72-4 or CA19-9 in fluids were above the upper reference limit (URL) and the F/S ratio was above 1.2. TM in serum were measured only in patients with higher TM values in the effusion fluid compared to the URL in the serum (5 mg/l for CEA; 30 ku/l for CA15-3; 6.9 ku/l for CA72-4, and 37 ku/l for CA19-9).
The criteria used to indicate a possible false positive effusion (i.e., peritonitis, inflammation, or a tuberculous effusion) were %PN>90, CRP>50 mg/l or ADA >45 u/l (19,20). The use of biomarkers ADA, CRP and %PN identified two groups of effusions: i) group A, with effusions containing all biomarkers under the cut-off point, and ii) group B, with effusions containing at least one positive biomarker. ADA (EC3.5.4.4) (ITC Diagnostics, Barcelona, Spain) and CRP (Tina-quant CRP latex, Roche Diagnostics) were assessed using an LX-20 autoanalyzer (Beckman Coulter). Leukocyte count was determined using a Neubauer chamber and May-Grünwald-Giemsa stain. The analytical variation expressed as the between-assay coefficient of variation was 7.4% for ADA and 2.3% for CRP at concentrations of 10.3 u/l and 76.6 mg/l respectively. The Papanicolaou stain in Leica Autostainer XL (Leica, Barcelona, Spain) was used for cytology staining. This study was approved by the Ethical committee of the Unió Catalana d’Hospitals (n° 12/05).
Statistical analysis. ROC analysis was used to establish cut-off points for each TM at a specificity of 100%. Sensitivity, specificity, negative predictive values (NPV), positive predictive values (PPV), likelihood ratio negative (LHR-) and likelihood ratio positive (LHR+), were all calculated for each TM and for all TMs combined. All statistical analyses were conducted using IBM® SPSS® Statistics for Windows v.20 (IBM Corporation, Armonk, NY, USA) and Stata® v.10 (StataCorp LP, College Station, TX, USA).
Results
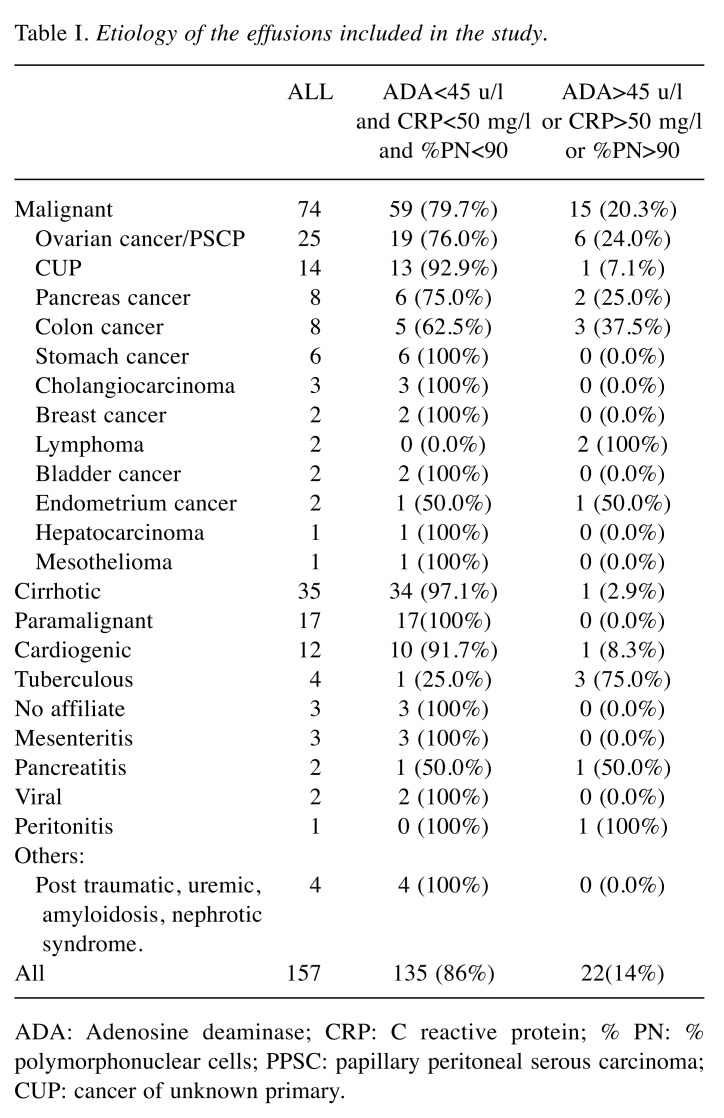
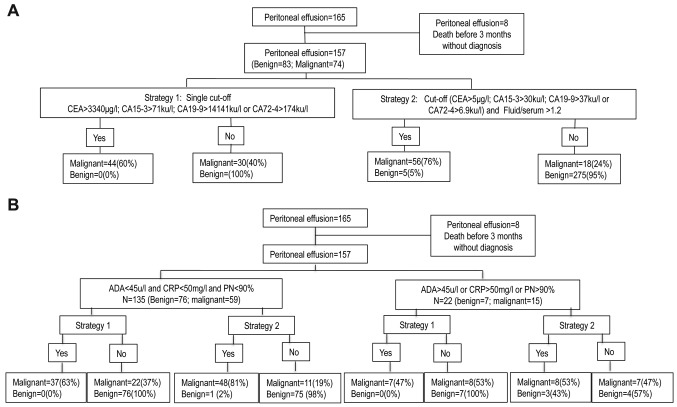
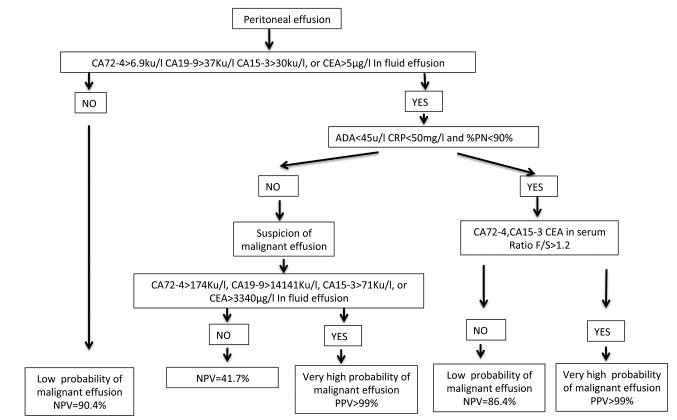
One hundred and fifty-seven consecutive peritoneal effusions were included, from 84 women and 73 men with age ranging from 35 to 96 years (mean=67.7; SD=13.4). Of the effusions evaluated, 83 (52.9%) had a benign etiology and 74 (47.1%) were malignant (Table I). The staging of tumors involved in malignant ascites were: i) 18 stage IIIC ovarian cancers, ii) 7 stage IV ovarian cancers, iii) 48 stage IV peritoneal mesotheliomas and iv) one stage IIIA. The effusions were assigned to one of two groups: i) group A, with ADA<45 u/l, CRP<50 mg/l and %PN<90%, and ii) group B, with at least one of the following: ADA >45 u/l, CRP>50 mg/l and %PN>90% (Table I). Figure 1 shows the flow charts for this study.
Table I. Etiology of the effusions included in the study.
ADA: Adenosine deaminase; CRP: C reactive protein; % PN: % polymorphonuclear cells; PPSC: papillary peritoneal serous carcinoma; CUP: cancer of unknown primary.
Figure 1. Flow chart of study. A. Flow chart for all group. B. Flow chart subclassifying according to adenosine deaminase (ADA), C reactive protein (CRP) and % polymorphonuclear cells (%PN). CA15-3: cancer antigen 15-3; CA19-9: cancer antigen 19-9; CA72-4: cancer antigen 72-4. CEA: carcinoembryonic antigen; F/S: fluid serum ratio.
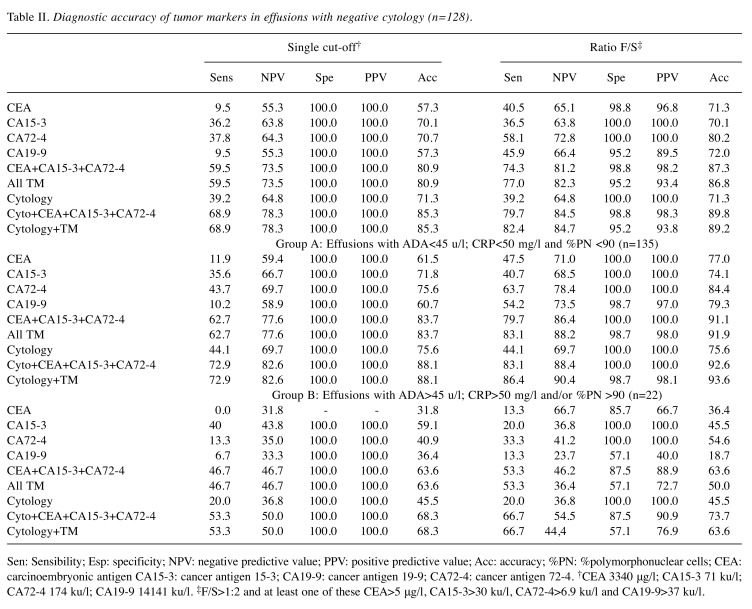
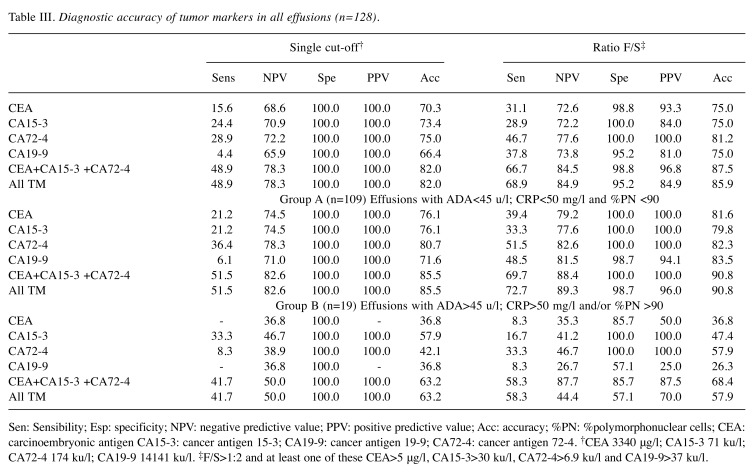
The cut-offs with a specificity of 100% obtained using the ROC curve method, with a single measurement in peritoneal effusions were: 71 ku/l, 3340 μg/l, 14141 ku/l and 174 ku/l for CA15-3, CEA, CA19-9 and CA72-4, respectively. Sensitivity and negative predictive value (NPV) for effusions with at least one TM above the cut-off were: 59.5% and 75.3% (Table II), respectively, and in patients with negative cytology (Table III), the sensitivity was 48.9% and NPV 73.8%. Considering only group A patients, the sensitivity was 62.7% for all patients and 51.5% in patients with negative cytology.
Table II. Diagnostic accuracy of tumor markers in all effusions (157).
Sen: Sensibility; Esp: specificity; NPV: negative predictive value; PPV: positive predictive value; Acc: accuracy; %PN: %polymorphonuclear cells; CEA: carcinoembryonic antigen CA15-3: cancer antigen 15-3; CA19-9: cancer antigen 19-9; CA72-4: cancer antigen 72-4. †CEA 3340 μg/l; CA15-3 71 ku/l; CA72-4 174 ku/l; CA19-9 14141 ku/l. ‡F/S>1:2 and at least one of these CEA>5 μg/l, CA15-3>30 ku/l, CA72-4>6.9 ku/l and CA19-9>37 ku/l.
Table III. Diagnostic accuracy of tumor markers in effusion with negative cytology (n=128).
Sen: Sensibility; Esp: specificity; NPV: negative predictive value; PPV: positive predictive value; Acc: accuracy; %PN: %polymorphonuclear cells; CEA: carcinoembryonic antigen CA15-3: cancer antigen 15-3; CA19-9: cancer antigen 19-9; CA72-4: cancer antigen 72-4. †CEA 3340 μg/l; CA15-3 71 ku/l; CA72-4 174 ku/l; CA19-9 14141 ku/l. ‡F/S>1:2 and at least one of these CEA>5 μg/l, CA15-3>30 ku/l, CA72-4>6.9 ku/l and CA19-9>37 ku/l.
The alternative approach (i.e., at least one TM above the URL in peritoneal effusion and an F/S ratio>1.2) showed results for sensitivity, specificity, NPV and PPV of 75.7%, 95,2%, 81.4% and 93.3%, respectively for the whole group, and 68.9%, 95.2%, 84.9% and 84.9%, respectively, in patients with negative cytology (Tables II and III). In group A, the specificity reached 98.7% and the sensitivity was 81.4% for all patients and 72.7% in patients with negative cytology (Tables II and III). In group A only CA19-9 showed a false positive result. The sensitivities of CEA, CA15-3 and CA72-4 were similar to the figures obtained when using four TMs, but the specificity was 100% at a sensitivity of 79.7% for all patients and at a sensitivity of 69.7% in the negative cytology group.
Figure 2 shows the algorism for the interpretation tumor markers in ascitic fluids. In patients with positive ADA, CRP or %PN the negative predictive value of the TM was very low, due to the high prevalence (68%) of neoplastic ascites in this group. Additionally, in the group with negative ADA, CRP and %PN the prevalence was 43.7%.
Figure 2. Algorithm for the interpretation of tumor markers in peritoneal fluid. ADA: Adenosine deaminase; CA15-3: cancer antigen 15-3; CA19- 9: cancer antigen 19-9; CA72-4: cancer antigen 72-4. CEA: carcinoembryonic antigen; CRP: C reactive protein; F/S: fluid serum ratio; NPV: negative predictive value; PPV: positive predictive value.
Discussion
This study assessed two strategies for evaluating tumor markers in ascites. Our results are in agreement with previous studies that have used the strategy of the F/S ratio (19-21), showing better sensitivity values (75.7%) compared to the strategy of a single measurement in fluid with a high cut-off point. For the whole group of patients analyzed, the specificity obtained was 95.2%. In group A, sensitivity rose to 81.4% and specificity to 98.7%. The rates did not reach the level of 100% specificity obtained in previous studies (19-20) due to a false positive in CA19-9.
For all effusions in group A the combination of CEA, CA15-3 and CA72-4 showed the maximum specificity (100%) with a sensitivity of 79.7% and in patients with negative cytology from group A the sensitivity of three TMs was 69.7% at a specificity of 100%. On the other hand, the tumor marker that appeared most frequently as a false positive was CA19-9 in group B (3 out of 7 of patients with benign disease). With regard to the data obtained with the cut-off strategy, the values required for CA19-9 and CEA with the single determination were higher compared to the figures reported by other authors (6,9,23). This was due to the values of one paramalignant effusion, which showed concentrations in serum of 3631 mg/l, 1161 ku/l and 89896 ku/l for CEA, CA72-4 and CA19-9 respectively and concentrations in peritoneal fluid lower compared to that of the serum (3230 ng/ml, 166 ku/l and 12884 ku/l for CEA, CA72-4 and CA19-9 respectively).
It should be noted that most false positives correspond only to CA19-9 (5% in the patient group as a whole, and 2% in group A). Three out of seven patients with benign diseases in group B were false positives. One of them, a patient with pancreatitis, has a CA19-9 concentration in fluid of 6370 ku/l. In a previous study (19) we found a similar value, 7700 ku/l, in a patient with peritonitis of biliary origin. Indeed, many organs of the abdominal cavity are rich in CA19-9, and inflammation or necrosis may release this marker in large concentrations in the peritoneal fluid. These data suggest that false positives to this marker may be obtained even if high cut-off values are used.
Using the cut-off values obtained by our group in pleural effusions (20), which are similar to those described by Lui et al. (9) in peritoneal fluid (CEA:60 μg/l, CA19-9:209 ku/l, CA72-4:21 ku/l and CA15-3:80 ku/l), we obtained sensitivities of 73% for the whole group and 66.7% in patients with negative cytology, but with lower specificities (94% and 94.7%); therefore, these results were not better compared to those obtained with the ratio strategy.
There was a notable difference between pleural and ascitic effusions in group B, the prevalence of malignant effusions in pleural (20,23) described previously was lower than we found in the present study for peritoneal effusions, since ascitic effusions were more likely to be malignant. Higher concentrations of CRP have been described in peritonitis (15,16) but also in malignant ascites (25,26). Some ovarian, pancreatic and colonic cancer may have an important inflammatory component. This group may also include lymphomas, which can release ADA in a similar way to pleural effusions (18). In fact, in that study all lymphomas were included in this group. Nevertheless, classifying the effusions according to ADA, CRP and %PN allowed us to use the best strategy for obtaining higher sensitivity, specificity and diagnostic accuracy.
In this study, more than 50% of patients with malignant ascites have negative cytology. In these, as yet, undiagnosed patients, more evidence is required to guide the choice of more invasive procedures, such as laparoscopy in order to establish the diagnosis. In this regard, the study of biomarkers in fluid and serum allows identification of 70% of patients with malignant ascites and negative cytology and has a positive predictive value greater than 99% in 24 hours, allowing faster assignment to invasive procedures and thus improving efficiency. In summary, patients with a previous diagnosis of neoplasia and the appearance of ascites with negative cytology and a positive F/S ratio for tumor markers can be considered to present disease progression. Similarly, the presence of ascites and positive tumor markers but negative cytology in newly diagnosed localized tumors may indicate peritoneal metastasis. These and the other practical considerations raised by our data should be addressed in case by case team discussions.
The main limitation of this study is the fact that it was conducted at a single center. Multicenter studies are now needed to validate clinical data and to establish whether they may also be applicable to other measurement systems.
We can conclude that to obtain the maximum diagnostic yield from the measurement of CA72-4, CA15-3 and CEA in peritoneal effusions, we should determination markers in fluid and serum with a low cut-off point simultaneously in patients who are unlikely to present increases in TM due to benign disease (i.e., with ADA, CRP and % PN below the discriminant values).
Conflicts of Interest
The Authors declare that they have no conflicts of interests regarding this study.
Authors’ Contributions
Conception and design was done by JT, RM. Analysis and interpretation was done by JT, JM, AA, MS, CF, JF, CG-F. Histological examination was performed by FS. EE-V, RP, JA, SC, EC, MD, JT-U, CB, FV, PS, DR, MB, and JO acquired the data. All authors were involved with critical revisions, as well as read and approved the final manuscript.
Acknowledgements
The Authors also thank Michael Maudsley for help with the translation and edition of the manuscript.
References
- 1.Oey RC, van Buuren HR, de Man RA. The diagnostic work-up in patients with ascites: current guidelines and future prospects. Neth J Med. 2016;74(8):330–335. [PubMed] [Google Scholar]
- 2.Motherby H, Nadjari B, Friegel P, Kohaus J, Ramp U, Böcking A. Diagnostic accuracy of effusion cytology. Diagn Cytopathol. 1999;20:350–357. doi: 10.1002/(sici)1097-0339(199906)20:6<350::aid-dc5>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 3.Shi HZ, Liang QL, Jiang J, Qin XJ, Yang HB. Diagnostic value of carcinoembryonic antigen in malignant pleural effusion: a meta-analysis. Respirology. 2008;13(4):518–527. doi: 10.1111/j.1440-1843.2008.01291.x. [DOI] [PubMed] [Google Scholar]
- 4.Liang QL, Shi HZ, Qin XJ, Liang XD, Jiang J, Yang HB. Diagnostic accuracy of tumour markers for malignant pleural effusion: a meta-analysis. Thorax. 2008;63(1):35–41. doi: 10.1136/thx.2007.077958. [DOI] [PubMed] [Google Scholar]
- 5.Ahadi M, Tehranian S, Memar B, Vossoughinia H, Salari M, Eskandari E, Farzanehfar M, Sadeghi R. Diagnostic value of carcinoembryonic antigen in malignancy-related ascites: systematic review and meta-analysis. Acta Gastroenterol Belg. 2014;77(4):418–424. [PubMed] [Google Scholar]
- 6.Zhu FL, Ling AS, Wei Q, Ma J, Lu G. Tumor markers in serum and ascites in the diagnosis of benign and malignant ascites. Asian Pac J Cancer Prev. 2015;16(2):719–722. doi: 10.7314/apjcp.2015.16.2.719. [DOI] [PubMed] [Google Scholar]
- 7.Gulyás M, Kaposi AD, Elek G, Szollár LG, Hjerpe A. Value of carcinoembryonic antigen (CEA) and cholesterol assays of ascitic fluid in cases of inconclusive cytology. J Clin Pathol. 2015;54(11):831–835. doi: 10.1136/jcp.54.11.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaleta EJ, Tolan NV, Ness KA, O'Kane D, Algeciras-Schimnich A. CEA, AFP and CA 19-9 analysis in peritoneal fluid to differentiate causes of ascites formation. Clin Biochem. 2013;46(9):814–818. doi: 10.1016/j.clinbiochem.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Liu F, Kong X, Dou Q, Ye J, Xu D, Shang H, Xu K, Song Y. Evaluation of tumor markers for the differential diagnosis of benign and malignant ascites. Ann Hepatol. 2014;13(3):357–363. [PubMed] [Google Scholar]
- 10.Han N, Sun X, Qin C, Bakari Hassan K, Wu Z, Zhang Y, Lan X. Value of (18)F-FDG PET/CT combined with tumor markers in the evaluation of ascites. AJR Am J Roentgenol. 2018;210(5):1155–1163. doi: 10.2214/AJR.17.18733. [DOI] [PubMed] [Google Scholar]
- 11.Slev PR, Rawlins ML, Roberts WL. Performance characteristics of seven automated CA 15-3 assays. Am J Clin Pathol. 2006;125(5):752–757. doi: 10.1309/G6X6-PR75-26FA-KV0E. [DOI] [PubMed] [Google Scholar]
- 12.Mansour M, Linden ER, Colby S, Posner G, Marsh F Jr. Elevation of carcinoembryonic antigen and CA-125 in a patient with multivisceral tuberculosis. J Natl Med Assoc. 1997;89(2):142–143. [PMC free article] [PubMed] [Google Scholar]
- 13.Wu SS, Lin OS, Chen YY, Hwang KL, Soon MS, Keeffe EB. Ascitic fluid carcinoembryonic antigen and alkaline phosphatase levels for the differentiation of primary from secondary bacterial peritonitis with intestinal perforation. J Hepatol. 2001;34(2):215–221. doi: 10.1016/s0168-8278(00)00039-8. [DOI] [PubMed] [Google Scholar]
- 14.Tao L, Ning HJ, Nie HM, Guo XY, Qin SY, Jiang HX. Diagnostic value of adenosine deaminase in ascites for tuberculosis ascites: a meta-analysis. Diagn Microbiol Infect Dis. 2014;79(1):102–107. doi: 10.1016/j.diagmicrobio.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Yildirim B, Sari R, Isci N. Patients with spontaneous bacterial peritonitis, and malignant and cirrhotic ascites. J Natl Med Assoc. 2005;97(2):276–280. [PMC free article] [PubMed] [Google Scholar]
- 16.Mousa N, Besheer T, Abdel-Razik A, Hamed M, Deiab AG, Sheta T, Eldars W. Can combined blood neutrophil to lymphocyte ratio and C-reactive protein be used for diagnosis of spontaneous bacterial peritonitis. Br J Biomed Sci. 2018;75(2):71–75. doi: 10.1080/09674845.2017.1396706. [DOI] [PubMed] [Google Scholar]
- 17.Lutz P, Goeser F, Kaczmarek DJ, Schlabe S, Nischalke HD, Nattermann J, Hoerauf A, Strassburg CP, Spengler U. Relative ascites polymorphonuclear cell count indicates bacterascites and risk of spontaneous bacterial peritonitis. Dig Dis Sci. 2017;62(9):2558–2568. doi: 10.1007/s10620-017-4637-4. [DOI] [PubMed] [Google Scholar]
- 18.Trapé J, Filella X, Alsina-Donadeu M, Juan-Pereira L, Bosch-Ferrer Á, Rigo-Bonnin R. Oncology Section of the Catalan Association of Clinical Laboratory Science. Increased plasma concentrations of tumour markers in the absence of neoplasia. Clin Chem Lab Med. 2011;49(10):1605–1620. doi: 10.1515/CCLM.2011.694. [DOI] [PubMed] [Google Scholar]
- 19.Trapé J, Molina R, Sant F, Montesinos J, Arnau A, Franquesa J, Blavia R, Martín E, Marquilles E, Perich D, Pérez C, Roca JM, Doménech M, López J, Badal JM. Diagnostic accuracy of tumour markers in serous effusions: a validation study. Tumour Biol. 33(5):1661–1668. doi: 10.1007/s13277-012-0422-3. [DOI] [PubMed] [Google Scholar]
- 20.Trapé J, Sant F, Franquesa J, Montesinos J, Arnau A, Sala M, Bernadich O, Martín E, Perich D, Pérez C, Lopez J, Ros S, Esteve E, Pérez R, Aligué J, Gurt G, Catot S, Domenech M, Bosch J, Badal JM, Bonet M, Molina R, Ordeig J. Evaluation of two strategies for the interpretation of tumour markers in pleural effusions. Respir Res. 2017;18(1):103. doi: 10.1186/s12931-017-0582-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trapé J, Molina R, Sant F. Clinical evaluation of the simultaneous determination of tumor markers in fluid and serum and their ratio in the differential diagnosis of serous effusions. Tumour Biol. 2004;25(5-6):276–281. doi: 10.1159/000081392. [DOI] [PubMed] [Google Scholar]
- 22.Trapé J, Gurt G, Franquesa J, Montesinos J, Arnau A, Sala M, Sant F, Casado E, Ordeig JM, Bergos C, Vida F, Sort P, Isava Á, González M, Molina R. Diagnostic accuracy of tumor markers CYFRA21-1 and CA125 in the differential diagnosis of ascites. Anticancer Res. 2015;35(10):5655–5660. [PubMed] [Google Scholar]
- 23.Trapé J, Sant F, Montesinos J, Arnau A, Sala M, Bernadich O, Martín E, Perich D, Lopez J, Ros S, Esteve-Valverde E, Pérez R, González-Fernández C, Aligue J, Catot S, Domenech M, Ruiz D, Bonet M, Molina R, Ordeig J. Diagnostic accuracy of CYFRA21-1 in the differential diagnosis of pleural effusions. Anticancer Res. 2019;39(9):5071–5076. doi: 10.21873/anticanres.13700. [DOI] [PubMed] [Google Scholar]
- 24.Chen SJ, Wang SS, Lu CW, Chao Y, Lee FY, Lee SD, Wu SL, Cherng KL, Lo KJ. Clinical value of tumour markers and serum-ascites albumin gradient in the diagnosis of malignancy-related ascites. J Gastroenterol Hepatol. 1994;9(4):396–400. doi: 10.1111/j.1440-1746.1994.tb01262.x. [DOI] [PubMed] [Google Scholar]
- 25.Yuksel I, Karaahmet F, Coskun Y, Kılıncalp S, Hamamci M, Akinci H, Ustun Y, Simsek Z, Erarslan E, Coban S. Significance of serum and ascitic fluid C-reactive protein in differential diagnosis of benign and malignant ascites. Dig Dis Sci. 2014;59(10):2588–2593. doi: 10.1007/s10620-014-3205-4. [DOI] [PubMed] [Google Scholar]
- 26.Abdel-Razik A, Mousa N, Elalfy H, Sheta TF, Awad M, Abdelsalam M, Elhelaly R, Elzehery R, Gouda NS, Eldars W. A novel combination of C-reactive protein and vascular endothelial growth factor in differential diagnosis of ascites. J Gastrointest Cancer. 2017;48(1):50–57. doi: 10.1007/s12029-016-9873-x. [DOI] [PubMed] [Google Scholar]