Abstract
Background/Aim: Neutrophil-to-lymphocyte ratio (NLR) has been reported to be associated with poor prognosis for radioactive iodine ablation refractory differentiated thyroid cancer (RR-DTC). Little is known whether NLR can be a tumor marker for RR-DTC patients treated with lenvatinib. Patients and Methods: We retrospectively analyzed RR-DTC patients treated with lenvatinib. NLR was calculated at 4 points before and during lenvatinib treatment. Results: The median NLR value increased at the start of lenvatinib treatment, compared to 6 months prior to initiation of lenvatinib treatment. The median overall survival was significantly longer in patients with the lower NLR (< 3) at the start of lenvatinib treatment. The median NLR values decreased when the patients achieved best tumor response, and increased again upon disease progression. Conclusion: NLR values vary before and during lenvatinib treatment, suggesting that this ratio can reflect disease activity of RR-DTC. NLR can supportively be used as a tumor marker of RR-DTC and an indicator for starting lenvatinib treatment.
Keywords: Thyroid cancer, lenvatinib, neutrophil-to-lymphocyte ratio, prognostic factors
According to a recent survey by the National Cancer Center Japan, it is estimated that 19,800 new patients will be newly diagnosed and 1,800 patients will die from thyroid cancer in Japan in 2019 (1). The treatment for metastatic or recurrent thyroid cancer depends on its histological subtype. Radioactive iodine ablation (RAI) therapy is effective for patients with differentiated thyroid cancer (DTC) including papillary thyroid cancer (PTC) and follicular thyroid cancer (FTC), though up to 15% of DTC patients will develop RAI refractory differentiated thyroid cancer (RR-DTC) (2).
Lenvatinib is an oral multi-kinase inhibitor targeting vascular endothelial growth factor receptor (VEGFR) 1−3, fibroblast growth factor receptor (FGFR) 1-4, ret proto- oncogene (RET), stem cell factor receptor (KIT), and platelet-derived growth factor receptor alpha (PDGFRα) (3-5). In the SELECT (study of E7080“Lenvatinib”in differentiated cancer of the thyroid) trial, lenvatinib demonstrated prolonged progression-free survival (PFS) compared to placebo [18.3 versus 3.6 months, hazard ratio (HR) 0.21, p< 0.001] for patients with RR-DTC (6). Lenvatinib also showed preferable efficacy and safety for patients with MTC [objective response rate (ORR) 36%] and ATC (ORR 24%) in the phase II trial (7).
The neutrophil-to-lymphocyte ratio (NLR) is defined as the number of absolute neutrophil counts divided by the number of absolute lymphocyte counts, based on complete blood cell counts. A systematic review and meta-analysis has shown that NLR reveals the balance of the immune system, while being associated with survival in solid tumors (8). Neutrophils have been reported to promote tumor progression, while lymphocytes are associated with the elimination of cancer cells (9). Anti-tumor immunity is mainly regulated by CD4 and CD8 T lymphocytes, and various cytokines and chemokines mediate the activities of these immune cells. NLR has been reported to reflect the profile of these cytokines and chemokines (10). Therefore, NLR may reflect the anti-tumor immunity status and the associated prognosis in cancer patients.
An elevated NLR was associated with a higher risk of recurrence and poor prognosis in PTC in a previous study (11). Higher NLR was correlated with extrathyroidal invasive, bilateral, multifocal, and lymph node-positive tumors in PTC (12). NLR was significantly elevated in ATC patients compared to PTC patients, and can therefore be used as a marker to discriminate between ATC and DTC (13). In the Japanese phase II trial of lenvatinib, NLR was higher in ATC and MTC patients than in DTC patients, and a higher pre-treatment NLR may predispose to a lower PFS following lenvatinib treatment (7). However, few studies have been reported on the changes of NLR during lenvatinib treatment in RR-DTC patients. We therefore performed a sequential analysis of NLR before and during lenvatinib treatment for RR-DTC patients, and evaluated the outcomes of the treatment.
Patients and Methods
We performed a retrospective analysis of patients with recurrent or metastatic RR-DTC, who were treated with lenvatinib from January 2012 to September 2018 at our hospital's Department of Medical Oncology. For all patients, the treatment consisted of 24 mg of oral lenvatinib once-daily. Treatment was continued until disease progression, unacceptable toxicity despite the appropriate dose reduction and interruption, or the patient's refusal of treatment.
This study was approved by the institutional review board of the Cancer Institute Hospital of JFCR (No. 2017-1052). This study was conducted in accordance with the Helsinki Declaration of 1964 and later versions. In light of the retrospective nature of this study, the requirement for patients’ informed consent was waived by our hospital's institutional review board.
Treatment response was evaluated by computed tomography (CT) scans according to Response Evaluation Criteria in Solid Tumors (RECIST) criteria (ver. 1.1). PFS was defined as the time between the initiation of treatment and disease progression or death by any cause. We defined overall survival (OS) as the time between the initiation of treatment and death by any cause.
NLR was defined as the number of absolute neutrophil counts divided by the number of absolute lymphocyte counts, based on complete blood cell counts. The values of NLR were evaluated at four time points: 6 months before starting lenvatinib, at the start of lenvatinib treatment, at the time the patient achieved best tumor response, and at the time of disease progression. Considering previous reports, we set the cutoff value of NLR at 3 (7).
We used EZR (R ver. 2.13.0) to perform the statistical analyses (14). The PFS and OS were estimated by the Kaplan-Meier method and compared using a log-rank test. The survival results are expressed as the median value with a 95% confidence interval (CI). Wilcoxon signed-rank test was applied to analyze continuous data. Fisher's exact test was applied to compare categorical variables. Statistical significance was defined as p< 0.05.
Results
Patients. Between January 2012 and September 2018, 33 RR-DTC patients were treated with lenvatinib at our hospital. Patient characteristics are shown in Table I. The median age was 68 years (range=22-83 years), and 14 (42%) patients were male. The histological subtypes were PTC in 29 (88%) and FTC in 4 (12%) patients. All patients had measureable lesions and received surgery for the primary tumor. Six (18%) patients had previously received sorafenib. Three patients were enrolled in the phase III trial for lenvatinib. Six (18%) of the patients were anti-thyroglobulin antibody (TgAb)-positive.
Table I. Patient characteristics (n=33).
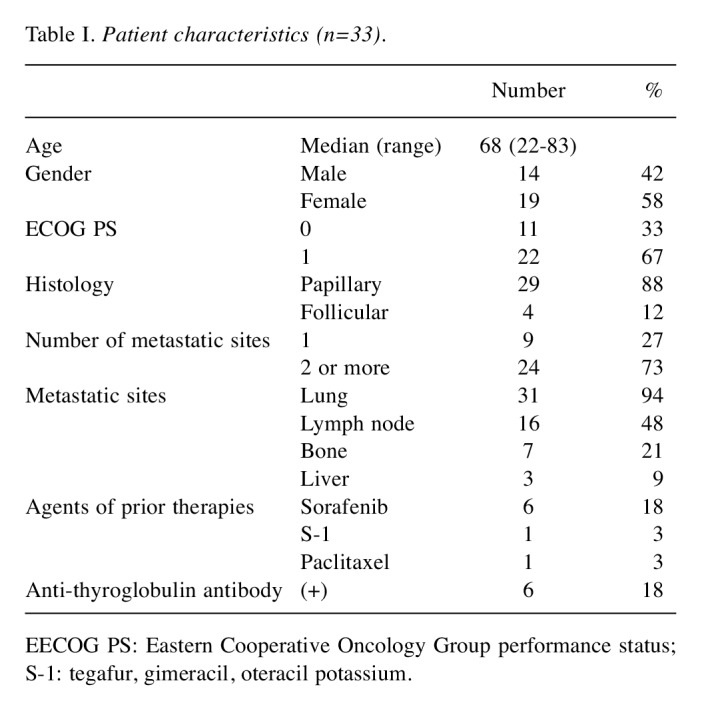
EECOG PS: Eastern Cooperative Oncology Group performance status; S-1: tegafur, gimeracil, oteracil potassium.
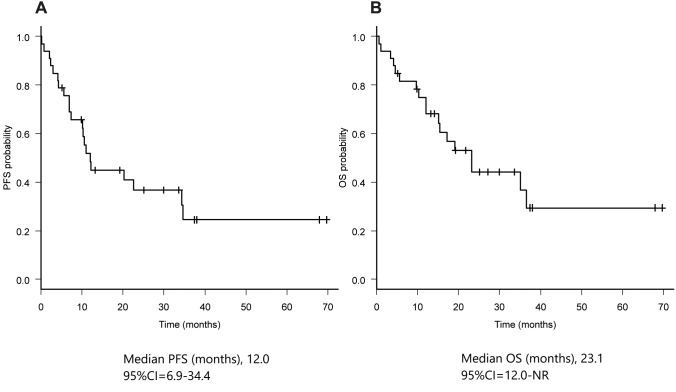
Efficacy of lenvatinib for all patients. As of February 14th 2019, the median follow-up time was 15.4 months (range=0.6-69.0 months). The median PFS was 12.0 months (95%CI=6.9-34.4) and the median overall survival (OS) was 23.1 months (95%CI=12.0-NR) (Figure 1). Objective response rate (ORR) was 60.6% (95%CI=42.1-77.1).
Figure 1. Kaplan-Meier curves of progression-free survival (A) and overall survival (B).
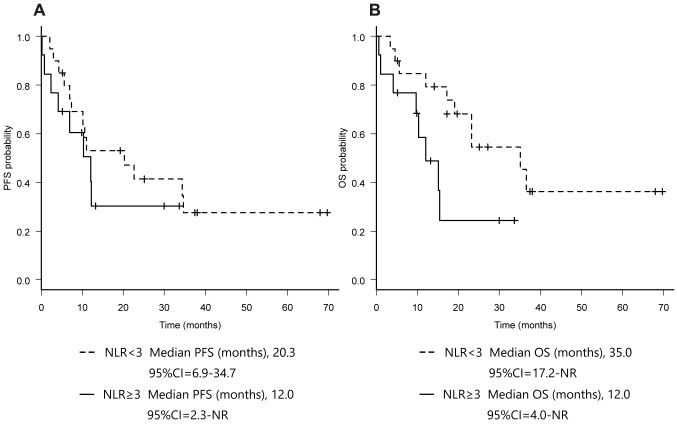
NLR values and treatment outcomes of lenvatinib for RR-DTC patients. We compared the treatment outcomes of lenvatinib for RR-DTC patients, according to the NLR values at the start of lenvatinib treatment. ORR was 69.6% (95%CI=47.1-86.8) in the lower NLR (NLR< 3) group and 40.0% (95%CI=12.2-73.8) in the higher NLR (NLR≥3) group (p=0.28). Disease control rate (DCR) was 95.7% (95%CI=78.1-99.9) in the lower NLR group and 60.0% (95%CI=26.2-87.8) in the higher NLR group (p=0.07) (Table II). The median PFS was 20.3 months (95%CI=6.9-34.7) in the lower NLR group and 12.0 months (95%CI=2.3-NR) in the higher NLR group (p=0.35). The median OS was significantly longer in the lower NLR group [35.0 months (95%CI=17.2-NR)] than in the higher NLR group [11.9 months (95%CI=4.0-NR)] (p< 0.05) (Figure 2).
Table II. Efficacy of lenvatinib according to neutrophil to lymphocyte ratio (NLR) value.
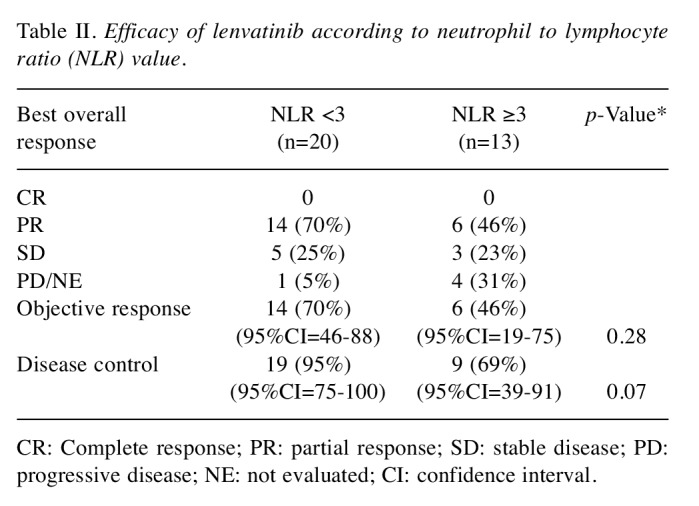
CR: Complete response; PR: partial response; SD: stable disease; PD: progressive disease; NE: not evaluated; CI: confidence interval.
Figure 2. Kaplan-Meier curves of progression-free survival (A) and overall survival (B) according to NLR value.
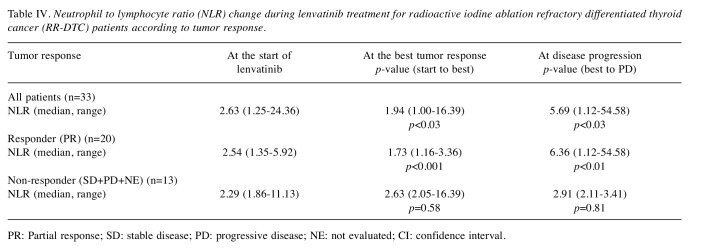
Changes of NLR values before and during lenvatinib treatment. We next evaluated the changes in NLR values before and during lenvatinib treatment for RR-DTC patients. The median NLR value significantly increased at the start of lenvatinib treatment [2.63 (range=1.35-24.35)] (p< 0.01) compared to that at 6 months (median 5.8 months, range=3.5-9.7 months) before starting lenvatinib [2.17 (range=1.13-11.80)] (Table III). For patients who achieved partial response (N=20), the median NLR significantly decreased from 2.54 (range=1.35-5.92) to 1.73 (range=1.16-3.36) at the time patients achieved best tumor response (p< 0.001). However, the median NLR values increased again up to 6.36 (range=1.12-54.58) upon disease progression (p< 0.01). This trend was not observed in patients who did not respond to lenvatinib (N=13) (Table IV).
Table III. Change of neutrophil to lymphocyte ratio (NLR) for radioactive iodine ablation refractory differentiated thyroid cancer (RRDTC) patients before treatment.
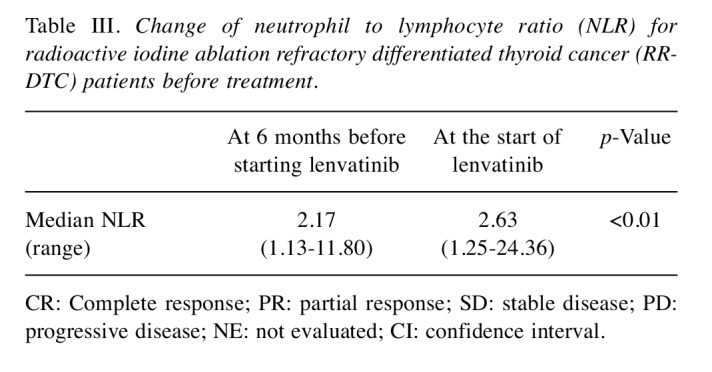
CR: Complete response; PR: partial response; SD: stable disease; PD: progressive disease; NE: not evaluated; CI: confidence interval.
Table IV. Neutrophil to lymphocyte ratio (NLR) change during lenvatinib treatment for radioactive iodine ablation refractory differentiated thyroid cancer (RR-DTC) patients according to tumor response.
PR: Partial response; SD: stable disease; PD: progressive disease; NE: not evaluated; CI: confidence interval.
Discussion
To the best of our knowledge, no other study has evaluated the NLR values sequentially before and during lenvatinib treatment in RR-DTC patients.
Between 6 months before starting lenvatinib and at the start of lenvatinib treatment, the NLR values significantly increased in patients with RR-DTC. During these 6 months, disease progression was observed and it became necessary to start lenvatinib in all of the patients. In the previous large surgical cohort of thyroid cancer patients, the median NLR value was 1.57, which was lower than the value observed in our study at the start of lenvatinib treatment (13). This may be explained by the differences in patient characteristics. In the surgical cohort, few patients had recurrent or metastatic disease, whereas all of the patients had recurrent or metastatic disease in our study. During lenvatinib treatment, the NLR values significantly decreased in RR-DTC patients who responded to lenvatinib, whereas NLR did not decrease in patients who did not respond to lenvatinib. The patients with lower baseline NLR values demonstrated better OS. The PFS, ORR, and DCR were also slightly better in patients with lower baseline NLR. In the phase II trial of lenvatinib, a similar non-significant trend was observed in PFS (baseline NLR ≥3 vs. < 3, HR=1.67, 95%CI=0.78-3.55, p=0.18), as shown by the multivariate analysis (7). Consequently, NLR values correlate with tumor activity in RR-DTC patients, and can supportively be used as a prognostic and predictive marker for RR-DTC patients treated with lenvatinib.
Although RR-DTC is originally an indolent malignancy with slow progression, it sometimes becomes an aggressive and life-threatening disease in metastatic or recurrent patients. Therefore, it is an important and difficult question when to start systemic therapy including lenvatinib. The phase III trials of multi-kinase inhibitors for RR-DTC, enrolled patients who had confirmed disease progression by the RECIST criteria within 12-14 months (6,15). This can be one of the indicators for deciding to start lenvatinib treatment. Thyroglobulin levels have also been studied as a tumor marker for DTC (16). In the SELECT trial, baseline thyroglobulin levels were associated with poorer PFS in RR-DTC (17). It has been reported that a shorter thyroglobulin doubling time (< 1 year) in patients is associated with a poor prognosis (18). Therefore, in clinical practice, a shorter thyroglobulin doubling time (< 1 year) is also one of the indicators to start lenvatinib treatment. However, these indicators are not always available. It is often difficult to measure diffuse pulmonary metastases or bone metastases using the RECIST criteria. The measurement of thyroglobulin is affected by anti-thyroglobulin antibodies (TgAb) (19). It has been reported that 17-29% of DTC patients have anti-thyroglobulin antibodies, which makes it difficult to use thyroglobulin as a universal tumor marker for RR-DTC (20,21). In fact, 18% of the patients were TgAb-positive in our study. Our results suggested that NLR can be supportively used as an indicator to start lenvatinib treatment, in combination with tumor size and thyroglobulin doubling time.
Our study has several limitations. First, this was a retrospective analysis of a small number of patients at a single institute. Second, the value of NLR is easily affected not only by tumor progression but also by infection, corticosteroids, radiotherapy, or other physiological stresses. Though we used a cutoff value of 3 for NLR according to the previous report, the appropriate cutoff value is still controversial. We are now planning a prospective cohort study to resolve these limitations.
In conclusion, our study suggested that the NLR values may reflect tumor activity in RR-DTC patients before and during lenvatinib treatment. NLR can supportively be used as a tumor marker and an indicator for starting lenvatinib treatment.
Conflicts of Interest
Takahashi S received grants and personal fees from Eisai, Novartis, Taiho, MSD, Chugai, Daiichi-Sankyo, Bayer and AstraZeneca. Fukuda N and Tomomatsu J received personal fees from Eisai. The Authors declare no other conflicts of interest associated with this manuscript.
Authors’ Contributions
Fukuda N wrote the manuscript; Wang X, Omoto A, Urasaki T, Sato Y, Nakano K, Nishizawa M, Yunokawa M, Ono M, Tomomatsu J, and Takahashi S contributed to the critical revision of the manuscript.
References
- 1.N.C.C.I. Japan, Projected Cancer Statistics. 2018. Available at: http://ganjoho.jp/en/public/statistics/short_pred.html.
- 2.Pacini F, Ito Y, Luster M, Pitoia F, Robinson B, Wirth L. Radioactive iodine-refractory differentiated thyroid cancer: unmet needs and future directions. Expert Rev Endocrinol Metab. 2012;7(5):541–554. doi: 10.1586/eem.12.36. [DOI] [PubMed] [Google Scholar]
- 3.Matsui J, Funahashi Y, Uenaka T, Watanabe T, Tsuruoka A, Asada M. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res. 2008;14(17):5459–5465. doi: 10.1158/1078-0432.CCR-07-5270. [DOI] [PubMed] [Google Scholar]
- 4.Matsui J, Yamamoto Y, Funahashi Y, Tsuruoka A, Watanabe T, Wakabayashi T, Uenaka T, Asada M. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008;122(3):664–671. doi: 10.1002/ijc.23131. [DOI] [PubMed] [Google Scholar]
- 5.Okamoto K, Kodama K, Takase K, Sugi NH, Yamamoto Y, Iwata M, Tsuruoka A. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett. 2013;340(1):97–103. doi: 10.1016/j.canlet.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff AO, Gianoukakis AG, Kiyota N, Taylor MH, Kim SB, Krzyzanowska MK, Dutcus CE, de las Heras B, Zhu J, Sherman SI. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372(7):621–630. doi: 10.1056/NEJMoa1406470. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi S, Kiyota N, Yamazaki T, Chayahara N, Nakano K, Inagaki L, Toda K, Enokida T, Minami H, Imamura Y, Fukuda N, Sasaki T, Suzuki T, Ikezawa H, Dutcus CE, Tahara M. A Phase II study of the safety and efficacy of lenvatinib in patients with advanced thyroid cancer. Future Oncol. 2019;15(7):717–726. doi: 10.2217/fon-2018-0557. [DOI] [PubMed] [Google Scholar]
- 8.Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B, Tannock IF, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411(6835):380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 10.Chen ZY, Raghav K, Lieu CH, Jiang ZQ, Eng C, Vauthey JN, Chang GJ, Qiao W, Morris J, Hong D, Hoff P, Tran H, Menter DG, Heymach J, Overman M, Kopetz S. Cytokine profile and prognostic significance of high neutrophil-lymphocyte ratio in colorectal cancer. Br J Cancer. 2015;112(6):1088–1097. doi: 10.1038/bjc.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JY, Park T, Jeong SH, Jeong CY, Ju YT, Lee YJ, Hong SC, Ha WS, Choi SK, Jung EJ. Prognostic importance of baseline neutrophil to lymphocyte ratio in patients with advanced papillary thyroid carcinomas. Endocrine. 2014;46(3):526–531. doi: 10.1007/s12020-013-0089-6. [DOI] [PubMed] [Google Scholar]
- 12.Manatakis DK, Tseleni-Balafouta S, Balalis D, Soulou VN, Korkolis DP, Sakorafas GH, Plataniotis G, Gontikakis E. Association of baseline neutrophil-to-lymphocyte ratio with clinicopathological characteristics of papillary thyroid carcinoma. Int J Endocrinol. 2017;2017:8471235. doi: 10.1155/2017/8471235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho JS, Park MH, Ryu YJ, Yoon JH. The neutrophil to lymphocyte ratio can discriminate anaplastic thyroid cancer against poorly or well differentiated cancer. Ann Surg Treat Res. 2015;88(4):187–192. doi: 10.4174/astr.2015.88.4.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanda Y. Investigation of the freely-available easy-to-use software "EZR" (Easy R) for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R, Shong YK, Sherman SI, Smit JW, Chung J, Kappeler C, Peña C, Molnár I, Schlumberger MJ. DECISION investigators. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384(9940):319–328. doi: 10.1016/S0140-6736(14)60421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malandrino P, Latina A, Marescalco S, Spadaro A, Regalbuto C, Fulco RA, Scollo C, Vigneri R, Pellegriti G. Risk-adapted management of differentiated thyroid cancer assessed by a sensitive measurement of basal serum thyroglobulin. J Clin Endocrinol Metab. 2011;96(6):1703–1709. doi: 10.1210/jc.2010-2695. [DOI] [PubMed] [Google Scholar]
- 17.Tahara M, Schlumberger M, Elisei R, Habra MA, Kiyota N, Paschke R, Dutcus CE, Hihara T, McGrath S, Matijevic M, Kadowaki T, Funahashi Y, Sherman SI. Exploratory analysis of biomarkers associated with clinical outcomes from the study of lenvatinib in differentiated cancer of the thyroid. Eur J Cancer. 2017;75:213–221. doi: 10.1016/j.ejca.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Miyauchi A, Kudo T, Miya A, Kobayashi K, Ito Y, Takamura Y, Higashiyama T, Fukushima M, Kihara M, Inoue H, Tomoda C, Yabuta T, Masuoka H. Prognostic Impact of serum thyroglobulin doubling-time under thyrotropin suppression in patients with papillary thyroid carcinoma who underwent total thyroidectomy. Thyroid. 2011;21(7):707–716. doi: 10.1089/thy.2010.0355. [DOI] [PubMed] [Google Scholar]
- 19.Rosário PW, Maia FF, Fagundes TA, Vasconcelos FP, Cardoso LD, Purisch S. Antithyroglobulin antibodies in patients with differentiated thyroid carcinoma: methods of detection, interference with serum thyroglobulin measurement and clinical significance. Arq Bras Endocrinol Metabol. 2004;48(4):487–492. doi: 10.1590/s0004-27302004000400008. [DOI] [PubMed] [Google Scholar]
- 20.Kumar A, Shah DH, Shrihari U, Dandekar SR, Vijayan U, Sharma SM. Significance of antithyroglobulin autoantibodies in differentiated thyroid carcinoma. Thyroid. 1994;4(2):199–202. doi: 10.1089/thy.1994.4.199. [DOI] [PubMed] [Google Scholar]
- 21.Görges R, Maniecki M, Jentzen W, Sheu SN, Mann K, Bockisch A, Janssen OE. Development and clinical impact of thyroglobulin antibodies in patients with differentiated thyroid carcinoma during the first 3 years after thyroidectomy. Eur J Endocrinol. 2005;153(1):49–55. doi: 10.1530/eje.1.01940. [DOI] [PubMed] [Google Scholar]