Abstract
Aim: To evaluate the efficacy and safety of third-line chemotherapy (CTx) for patients with unresectable or recurrent gastric cancer (GC) refractory to S-1 with or without platinum and taxanes. Patients and Methods: We retrospectively analyzed clinicopathological and survival data of 26 patients who underwent third-line CTx. Results: Irinotecan therapy (odds ratio=0.12, 95% confidence interval=0.02-0.38; p<0.01) and ≥2 cycles of third-line CTx (odds ratio=0.01, 95% confidence intervaI=0.01-0.11; p<0.01) were independent predictors of longer progression-free survival in multivariate Cox regression analysis. In 18 patients (69%) receiving irinotecan, the overall response rate was 11%, and the disease control rate was 44%. Median progression-free and overall survival were 3.5 and 11.3 months, respectively. Ten patients (56%) had grade 3-4 toxicities, which were managed. Conclusion: Irinotecan therapy may become optimal and tolerated in the third-line setting to prolong progression-free survival by increasing the number of treatment cycles.
Keywords: Gastric cancer, irinotecan, third-line chemotherapy, progression-free survival
Gastric cancer (GC) is the third leading cause of cancer death worldwide, and it remains the second leading cause of cancer death in Japan (1). Generally, patients with unresectable or recurrent GC have poor prognosis and are treated with chemotherapy (CTx). In Japan, the SPIRITS trial, a phase-III study, established S-1 plus cisplatin as a standard first-line CTx regimen for unresectable or recurrent GC (2). Several phase-III studies showed a survival benefit with irinotecan or docetaxel as second-line CTx compared with best supportive care (3-5). In Japan, paclitaxel is commonly used as second-line CTx for unresectable or recurrent GC, and has provided overall response rates (ORR) of 16-24%, median overall survival (OS) of 5-6 months, and modest toxicity in several phase-II studies (6-8). Moreover, nanoparticle albumin-bound (nab)-paclitaxel is also reported to be an option for second-line CTx, with an ORR of 24-28% and median OS of 9-10 months, comparable to the efficacy of paclitaxel (9,10).
In the phase-III WJOG4007 trial that compared weekly paclitaxel with irinotecan as second-line CTx for patients with advanced GC refractory to fluoropyrimidines plus platinum, OS did not differ between the two groups (11). Furthermore, in the recent phase-III RAINBOW trial, ramucirumab plus paclitaxel significantly improved OS compared with placebo plus paclitaxel in patients with advanced GC refractory to fluoropyrimidines plus platinum (12). This regimen has been regarded as the new standard second-line CTx for these patients.
Third-line CTx of advanced GC is expected to be developed in the near future. Therefore, it is important to evaluate the survival benefit of third-line CTx. After the WJOG4007 (11) and the RAINBOW (12) trials, taxanes are used as second-line CTx, and irinotecan more often as third-line CTx in Japanese clinical practice. Moreover, each of four types of CTx, fluoropyrimidines, platinum, taxanes and irinotecan, were independently associated with longer OS in patients with advanced GC (13). However, there have been few reports on the efficacy and safety of irinotecan in the third-line setting for patients with advanced GC refractory to the other three types of CTx (14,15). In this study, we retrospectively evaluated the efficacy and safety of irinotecan, compared with other agents, for patients with unresectable or recurrent GC refractory or those intolerant to S-1 with or without platinum and subsequent taxane therapy.
Patients and Methods
Patients. We retrospectively reviewed a database of 26 patients who had unresectable or recurrent GC refractory or were intolerant to S-1 with or without platinum and taxanes who underwent third-line CTx with irinotecan or other agents at Saitama Medical Center of Saitama Medical University from September 2012 to January 2017. We evaluated the efficacy and safety of irinotecan monotherapy and the outcomes compared with other agents in these 26 patients. This retrospective study was approved by the local Ethics Committee of Saitama Medical Center of Saitama Medical University (no. 613-III).
Tumor classification and histopathological grading were performed according to the Union for International Cancer Control pTNM staging guidelines, seventh edition (16). Terminology defined by the Japanese Gastric Cancer Association was used to avoid unnecessary confusion (17). All patients had at least one proven lesion with any noncurative factor such as peritoneal (P1), hepatic (H1) and distant metastasis (M1). Additionally, eligible patients were required to have an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0-2.
Chemotherapy schedule. Patients received intravenous infusion of irinotecan (100 mg/m2) during a 90-minute period weekly for 3 weeks followed by 1 week’s rest (18). Treatment was discontinued at onset of disease progression, development of severe toxic effects, or the patient’s request. Dose reduction or treatment delays were dependent on the toxicity or each physician’s discretion in any course. Tumor response was objectively assessed after each treatment course according to the Response Evaluation Criteria in Solid Tumors (19). Adverse events were evaluated by the Common Terminology Criteria for Adverse Events, version 4.0 (20).
Follow-up schedule. Disease progression and development of new lesions were evaluated as needed by computed tomography. Carcinoembryonic antigen and cancer antigen 19-9 as tumor markers were measured every 4 weeks during treatment. Responses were evaluated every 8 weeks or earlier in patients with evidence of treatment failure. Physical examinations and laboratory tests were performed every week or 2 weeks during treatment.
Statistical analysis. Continuous variables are expressed as medians and ranges. Grouping of categorical and continuous variables was carried out using standard thresholds. Cox proportional hazard regression analysis was used to identify significant independent factors for progression-free survival (PFS). Factors with differences at p<0.05 according to univariate analysis were assessed by multivariate analysis. In the univariate and multivariate analyses, odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. All statistical analyses were performed with JMP 5.0 software (SAS Institute, Cary, NC, USA). Values of p<0.05 were considered significant.
Results
Patient characteristics. Characteristics of the 26 patients who underwent third-line CT are presented in Table I. These patients included 19 men and seven women with a median age of 65 years (range=33-75 years). Of the 26 patients, 16 (62%) underwent gastrectomy. The median serum albumin level was 3.7 (range=2.2-4.4) g/dl on initial induction of third-line CTx. The median time from first- to third-line CTx and of second-line CTx was 15 (range=6.1-64) and 2.6 (range=0.7-25) months, respectively.
Table I. Characteristics of 26 patients with unresectable or recurrent gastric cancer receiving third-line chemotherapy.
Nab: Nanoparticle albumin-bound; CTx: chemotherapy. *Overlapping cases.
Efficacy. The median follow-up time was 145 days in censored cases, as of the cutoff date of March 31, 2017. In 26 patients treated with third-line CTx, the median PFS was 2.3 months, and the median OS was 5.4 months. We selected the following 17 variables for univariate analysis with regard to PFS: Age, gender, PS, location, histological type, primary lesion, P1, H1, M1, number of noncurative factors, irinotecan as third-line CTx, cycles of CTx, relative dose intensity, toxicity grade, serum albumin, time from first- to third-line CTx and time of second-line CTx. In univariate analysis, good PS (0, 1; p=0.01), no peritoneal metastasis (p=0.01), therapy with irinotecan (p<0.01), ≥2 cycles of CTx (p<0.01) and high serum albumin (≥3.7 g/dl; p=0.34) were significantly associated with longer PFS. In multivariate Cox regression analysis, irinotecan (OR=0.12, 95% CI=0.02-0.38; p<0.01) and ≥2 cycles of CT (OR=0.01; 95% CI=0.01-0.11; p<0.01) were independent predictors of longer PFS (Table II).
Table II. Univariate and multivariate predictors of progression-free survival in 26 patients with unresectable or recurrent gastric cancer receiving third-line chemotherapy.
CI: Confidence interval; CTx: chemotherapy; OR: odds rate.
Eighteen patients receiving irinotecan were assessable for response (Table III). One patient (6%) had complete response (CR), one (6%) had partial response, six (33%) had stable disease, and 10 (56%) had progressive disease. The ORR was 11%, and the disease control rate was 44%. The median number of cycles was three (range=1-17) and the RDI administered per patient was 69% (range=24-100%). Median PFS was 3.5 months, and median OS was 11.3 months. After discontinuing irinotecan, 10 patients (56%) received subsequent CTx.
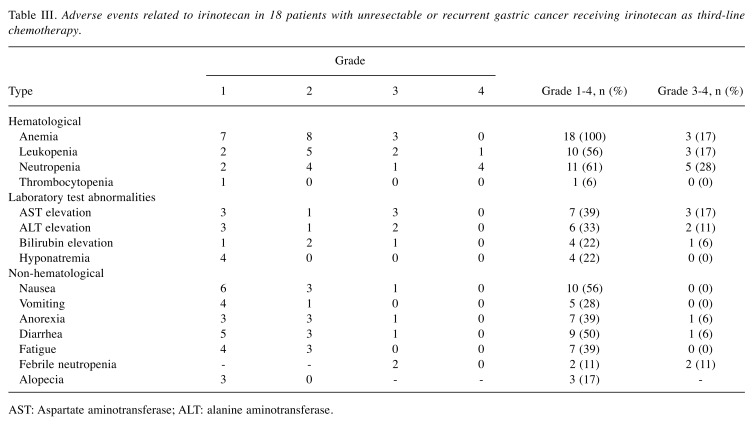
Table III. Adverse events related to irinotecan in 18 patients with unresectable or recurrent gastric cancer receiving irinotecan as third-line chemotherapy.
AST: Aspartate aminotransferase; ALT: alanine aminotransferase.
Adverse effects. The adverse events associated with irinotecan are shown in Table III. Ten patients (56%) had grade 3 or 4 toxicities and these were managed well. The most frequent hematological grade 3 or 4 toxicities were neutropenia (28%), leukopenia (17%) and anemia (17%). Grade 3 or 4 non-hematological toxicities were nausea (6%), anorexia (6%), diarrhea (6%) and febrile neutropenia (11%). Two patients (11%) died of disease progression within 30 days of the last administration of irinotecan. No treatment-related death occurred.
Discussion
Despite the lack of evidence of survival benefit of third-line CTx, two retrospective studies have been reported about the outcome of irinotecan as third-line CTx for patients with unresectable or recurrent GC refractory to fluoropyrimidines, platinum and taxanes. In results, irinotecan produced an ORR of 3-18%, median PFS of 2.2-2.3 months and median OS of 4.0-6.0 months for advanced GC in the third-line setting (14,15). Our data indicate that irinotecan as third-line CTx gave an ORR of 11%, a median PFS of 3.5 months and OS of 11.3 months, comparable to those two studies. Additionally, in the phase-III WJOG4007 trial, irinotecan as second-line CTx produced a 14% ORR, a median PFS of 2.3 months and OS of 8.4 months (11). Based on these findings, irinotecan may be a clinically effective drug for third-line therapy for selective patients with unresectable or recurrent GC. However, the prolonged OS in this study might be attributed to the higher proportion of patients (10; 56%) who underwent four or more lines of CTx, compared with the proportion of 21-40% in the two former studies.
Some studies reported on favorable predictors affecting survival following third-line CTx (4,21). A good PS, high serum albumin, low histological grade, an increased number of noncurative factors, a long time from first- to third-line CTx and a long time of second-line CTx were independent predictors for better survival in multivariate analysis. In this study, irinotecan and increasing number of treatment cycles of third-line CTx were independent predictors of longer PFS in multivariate analysis. However, two patients (11%) died of disease progression within 30 days of the final dosage of irinotecan, although no treatment-related death occurred. Thus, the careful selection of patients who are suitable for irinotecan as third-line setting seems to be required, while irinotecan therapy may be regarded as an available option in third-line setting to prolong PFS by increasing the number of treatment cycles.
In this study, the most frequent grade 3 or 4 toxicities caused by irinotecan were neutropenia (28%), leukopenia (17%) and anemia (17%), with 11% developing febrile neutropenia. The most common grade 3 or 4 non-hematological toxicities were nausea (6%), anorexia (6%) and diarrhea (6%). These incidences of grade 3 or 4 toxicities were not very different from those in previous studies of irinotecan as second-line or third-line CTx (3,4,11,14,15), indicating the safety of irinotecan in the third-line setting. However, 16 patients (89%) needed dose reduction or delays of irinotecan, with lower RDI of 69% in this study compared with that of 77-78% in two previous studies (14,15). By these findings, it is suggested that the appropriate dose or delays of irinotecan should be based on the patient’s condition.
Conclusion
In conclusion, irinotecan therapy may become optimal and tolerated in the third-line setting to prevent disease progression for selective patients with unresectable or recurrent GC refractory to fluoropyrimidines with or without platinum and taxanes, by increasing the number of treatment cycles, even if requiring dose reductions or delays. Although the current retrospective study was performed at a single center on a limited patient population, and was therefore subject to selection bias, our findings should stimulate further inquiry into how to manage in these patients treated with irinotecan as third-line CTx.
Conflicts of Interest
Minoru Fukuchi and the other co-authors have no conflicts of interest in regard to this study.
Authors’ Contributions
MF, KK and TI collected clinical information and performed statistical analysis. MF, YK, KI, EM and HI designed the study protocol and wrote the article.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H, Takeuchi M. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): A phase III trial. Lancet Oncol. 2008;9:215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 3.Thuss-Patience PC, Kretzschmar A, Bichev D, Deist T, Hinke A, Breithaupt K, Dogan Y, Gebauer B, Schumacher G, Reichardt PThuss-Patience PC, Kretzschmar A, Bichev D. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer-A randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO) Eur J Cancer. 2011;47:2306–2314. doi: 10.1016/j.ejca.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Kang JH, Lee SI, Lim DH, Park KW, Oh SY, Kwon HC, Hwang IG, Lee SC, Nam E, Shin DB, Lee J, Park JO, Park YS, Lim HY. Salvage chemotherapy for pretreated gastric cancer: A randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol. 2012;30:1513–1518. doi: 10.1200/JCO.2011.39.4585. [DOI] [PubMed] [Google Scholar]
- 5.Ford HE, Marshall A, Bridgewater JA, Janowitz T, Coxon FY, Wadsley J, Mansoor W, Fyfe D, Madhusudan S, Middleton GW, Swinson D, Falk S, Chau I, Cunningham D, Kareclas P, Cook N, Blazeby JM, Dunn JA. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): An open-label, phase 3 randomised controlled trial. Lancet Oncol. 2014;15:78–86. doi: 10.1016/S1470-2045(13)70549-7. [DOI] [PubMed] [Google Scholar]
- 6.Hironaka S, Zenda S, Boku N, Fukutomi A, Yoshino T, Onozawa Y. Weekly paclitaxel as second-line chemotherapy for advanced or recurrent gastric cancer. Gastric Cancer. 2006;9:14–18. doi: 10.1007/s10120-005-0351-6. [DOI] [PubMed] [Google Scholar]
- 7.Kodera Y, Ito S, Mochizuki Y, Fujitake S, Koshikawa K, Kanyama Y, Matsui T, Kojima H, Takase T, Ohashi N, Fujiwara M, Sakamoto J, Akimasa N. A phase II study of weekly paclitaxel as second-line chemotherapy for advanced gastric Cancer (CCOG0302 study) Anticancer Res. 2007;27:2667–2671. [PubMed] [Google Scholar]
- 8.Matsuda G, Kunisaki C, Makino H, Fukahori M, Kimura J, Sato T, Oshima T, Nagano Y, Fuii S, Takagawa R, Kosaka T, Ono HA, Akiyama H, Ichikawa Y. Phase II study of weekly paclitaxel as a second-line treatment for S-1-refractory advanced gastric cancer. Anticancer Res. 2009;29:2863–2867. [PubMed] [Google Scholar]
- 9.Sasaki Y, Nishina T, Yasui H, Goto M, Muro K, Tsuji A, Koizumi W, Toh Y, Hara T, Miyata Y. Phase II trial of nanoparticle albumin-bound paclitaxel as second-line chemotherapy for unresectable or recurrent gastric cancer. Cancer Sci. 2014;105:812–817. doi: 10.1111/cas.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukuchi M, Mochiki E, Ishiguro T, Ogura T, Sobajima J, Kumagai Y, Ishibashi K, Ishida H. Efficacy of nab-paclitaxel as second-line chemotherapy for unresectable or recurrent gastric cancer. Anticancer Res. 2016;36:6699–6703. doi: 10.21873/anticanres.11281. [DOI] [PubMed] [Google Scholar]
- 11.Hironaka S, Ueda S, Yasui H, Nishina T, Tsuda M, Tsumura T, Sugimoto N, Shimodaira H, Tokunaga S, Moriwaki T, Esaki T, Nagase M, Fujitani K, Yamaguchi K, Ura T, Hamamoto Y, Morita S, Okamoto I, Boku N, Hyodo I. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol. 2013;31:4438–4444. doi: 10.1200/JCO.2012.48.5805. [DOI] [PubMed] [Google Scholar]
- 12.Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, Cunningham D, Rougier P, Komatsu Y, Ajani J, Emig M, Carlesi R, Ferry D, Chandrawansa K, Schwartz JD, Ohtsu A. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–1235. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 13.Shitara K, Matsuo K, Mizota A, Kondo C, Nomura M, Takahari D, Yokota T, Ura T, Ito S, Sawaki A, Tajika M, Kawai H, Muro K. Association of fluoropyrimidines, platinum agents, taxanes, and irinotecan in any line of chemotherapy with survival in patients with advanced gastric cancer. Gastric Cancer. 2011;14:155–160. doi: 10.1007/s10120-011-0019-3. [DOI] [PubMed] [Google Scholar]
- 14.Kawakami T, Machida N, Yasui H, Kawahira M, Kawai S, Kito Y, Yoshida Y, Hamauchi S, Tsushima T, Todaka A, Yokota T, Yamazaki K, Fukutomi A, Onozawa Y. Efficacy and safety of irinotecan monotherapy as third-line treatment for advanced gastric cancer. Cancer Chemother Pharmacol. 2016;78:809–814. doi: 10.1007/s00280-016-3138-z. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura T, Iwasa S, Nagashima K, Okita N, Takashima A, Honma Y, Kato K, Hamaguchi T, Yamada Y, Shimada Y, Boku N. Irinotecan monotherapy as third-line treatment for advanced gastric cancer refractory to fluoropyrimidines, platinum, and taxanes. Gastric Cancer. 2017;20:655–662. doi: 10.1007/s10120-016-0670-9. [DOI] [PubMed] [Google Scholar]
- 16.Sobin LH, Gospodarowicz MK, Wittekind CH. Oxford: Wiley-Blackwell. 2009. TNM Classification of Malignant Tumours. Seven Edition. [Google Scholar]
- 17.Japanese Gastric Cancer Association Japanese classification of gastric carcinoma: Third English edition. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 18.Futatsuki K, Wakui A, Nakao I, Sakata Y, Kambe M, Shimada Y, Yoshino M, Taguchi T, Ogawa N. Late phase II study of irinotecan hydrochloride (CPT-11) in advanced gastric cancer. CPT-11 Gastrointestinal Cancer Study Group. Gan To Kagaku Ryoho. 1994;21:1033–1038. [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Cancer Therapy Evaluation Program: Common Terminology Criteria for Adverse Events v4.03. 2009. Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40.
- 21.Shim HJ, Yun JY, Hwang JE, Bae WK, Cho SH, Chung IJ. Prognostic factor analysis of third-line chemotherapy in patients with advanced gastric cancer. Gastric Cancer. 2011;14:249–256. doi: 10.1007/s10120-011-0032-6. [DOI] [PubMed] [Google Scholar]