Abstract
Background/Aim: To evaluate the association between programmed cell death ligand 1 (PD-L1) expression on both tumor cells (TC) and inflammatory cells (IC), tumor infiltrating lymphocytes (TILs), CD3+ and CD8+ lymphocytes and other clinicopathological parameters in primary infiltrative breast cancer (IBC) of young women, a population shown to have a worse prognosis. Materials and Methods: A retrospective study was performed collecting data from patients younger than 40 years old. Forty-five young women with IBC were included. Whole tissue sections were used to evaluate all parameters. Results: Twenty percent (20%) of cases showed PD-L1 expression by tumor cells (PDL1TC) and 44.4% showed PD-L1 expression by immune cells (PDL1IC). Furthermore, 28.88% revealed high stromal TILs. PDL1TC and PDL1IC expression were significantly associated with tumor diameter and expression of estrogen (ER) and progesterone (PR) receptors and Ki67. PDL1TC expression was also associated with grade. High TILs were associated with tumor diameter, ER and Ki67 expression. PDL1TC, PDL1IC expression and TILs were associated with the density of CD3+ and CD8+ lymphocytes. Conclusion: Our results are similar to those of other age groups, as reported in the literature.
Keywords: Breast cancer, young women, PD-L1, TILs
Breast cancer is the most common malignancy diagnosed among women of all ages and the second malignancy to cause death, after lung cancer (1). Younger women with breast cancer have a higher risk of recurrence and death, compared to older women (2-6). Additionally, this patient group shares considerations that must be taken into account when treated, like fertility preservation, pregnancy, sexual life and external appearance (7-11).
Over the past decade great improvement in diagnosis, prognosis and treatment has been achieved, complementing the already established targeted-therapy against estrogen (ER) and progesterone receptors (PR), as well as human epidermal growth factor receptor 2 (HER2). The molecular subtyping of breast cancer, compared to the morphological features, have opened new horizons to the understanding of the complex pathogenetic pathways leading to this common neoplasm (12). From the available studies it appears that for primary infiltrative breast cancer (IBC) there are not considerable differences regarding the biological profile in various ages, excluding the genetically predisposed cases (13).
The development and progression of IBC is due to a complex system of multiple factors including those of the microenvironment such as stromal and immune cells (14). The latter knowledge in combination with perceiving the cancer – immunity cycle, has led numerous studies to focus on the tumor microenvironment of the host in several solid cancers, including IBC (15,16). Over the decades, it has been proven that the presence of high tumor infiltrating lymphocytes (TILs) in solid tumors, such as lung, ovarian cancer, colorectal cancer, renal cell carcinoma, prostate cancer and head and neck cancers, as well as in breast cancer, is associated with better prognosis (17-25).
Most recently, researchers have been focusing on programmed cell death 1 and programmed cell death ligand 1 (PD1/PD-L1) axis, one of the most common mechanisms of tumor cell escape in cancer (26). The programmed cell PD-1 is a 55-kDa transmembrane protein located on the membrane surface of CD4+ T cells, NK T cells, B lymphocytes and dendritic cells. The most studied ligand of PD-1 is programmed cell death ligand 1 (PD-L1) or B7H1 or CD274 as otherwise known (27). PD-L1 is located on the membrane of various cell types such as hematopoietic cells e.g. B and T lymphocytes, dendritic cells, macrophages and mast cells, but also on non-hematopoietic cells such as endothelial, epithelial and muscle cells (28). Its levels in normal tissues are extremely low, whereas it is found overexpressed on neoplastic cells (29). PD-1 is commonly expressed on T regulatory cells found in TILs of several solid cancers. Its interaction with PD-L1 located on the surface of neoplastic cells leads to decreased cytokine expression, suppression of further activation of T lymphocytes gets and elimination of immune response (30,31).
The aim of the present study was to examine the expression of PD-L1 on both tumor cells (TC) and immune cells (IC), quantify TILs, CD3+ and CD8+ in IBC and investigate the association between these parameters, well as with other clinicopathologic parameters in the population of young women ≤40-years-old.
Materials and Methods
Study group. We retrospectively searched the electronic data-base of the Pathology Department of the University Hospital of Ioannina, Ioannina, Greece. All patients were young women (≤40 years old) with IBC treated surgically at the same Institution between 2011 and 2016. Forty-five cases of breast cancer were found, all of which were of the nonspecific type (NST); several parameters were studied including tumor size, histological grade, estrogen receptor (ER), progesterone receptor (PR), HER2 and Ki67 expression. Due to the limited number of cases of triple-negative cancer (TNC), further analysis of the IBC into molecular subtypes was not performed. Similarly, grade 1 IBC were excluded from the statistical analysis due to their limited number.
Quantification of TILs. Histopathological analysis of the lymphocytic infiltrate was performed according to the guidelines for clinical and research practice (32). Briefly, using the hematoxylin/eosin stained tissue sections, the percentage of stromal mononuclear cells, lymphocytes and plasma cells (polymorphonuclear leukocytes were excluded) were quantified within the tumor border. The evaluation did not include hot spots, TILs outside the tumor border, TILs in tumor zones with crush artifacts, necrosis, regressive hyalinization or areas of previous biopsy site. The quantification was as detailed as possible, dividing TILs in three groups (≤10%, 11-60%, and >60%). Ultimately, they were divided into two groups, low TILs (<60%) and high TILs (>60%). Further analysis was performed, identifying the composition of the TILs, using CD3 and CD8 antibodies. Each antibody was counted in five randomly selected high power fields at 400x magnification, and the counts were averaged (33). Positive CD3 or CD8 TILs up to 25 cells were considered as low (+1-25 cells), whereas medium density was the count of 26 up to 50 cells (++26-50 cells) and high the count of 51 cells or more (+++≥51 cells). As with TILs quantification, CD3 and CD8 were divided in two groups, low (1-25 cells) and high (>26 cells) for statistical purposes.
PD-L1 immunohistochemistry. PD-L1 immunohistochemistry assessment was performed on whole-tissue sections using the rabbit monoclonal antibody, clone E1L3N (Cell Signaling Technologies, Danvers, MA, USA). Both tumor cells (TC) and immune cells (IC) were evaluated. Human placenta tissue was used as positive control in parallel with the sections. TC staining was defined as partial or complete membranous staining, while IC staining was defined as cytoplasmic or membranous staining in lymphocytes or macrophages, using the number of IC within the stroma of the tumor. Positivity was defined as ≥1% in both TC and IC (34).
Statistical analysis. Statistical analysis was performed using V.22.0 Statistical Package for the Social Sciences (SPSS). The Fisher’s exact test was used to examine associations between categorical variables and the Mann Whitney U-test to examine differences in groups of quantitative measurements. The significance level was set at <0.05 in all cases.
Results
The mean age of the patients was 34.71 years. The studied parameters included tumor size, histologic grade, estrogen receptor (ER), progesterone receptor (PR), HER2 and Ki67 expression and are shown in Table I.
Table I. Clinicopathological characteristics of young women with breast cancer.
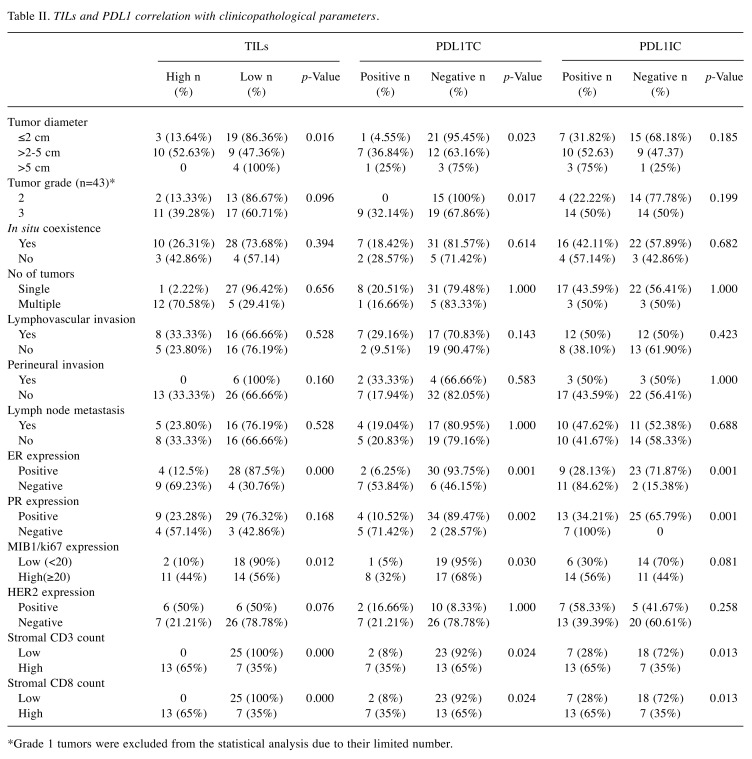
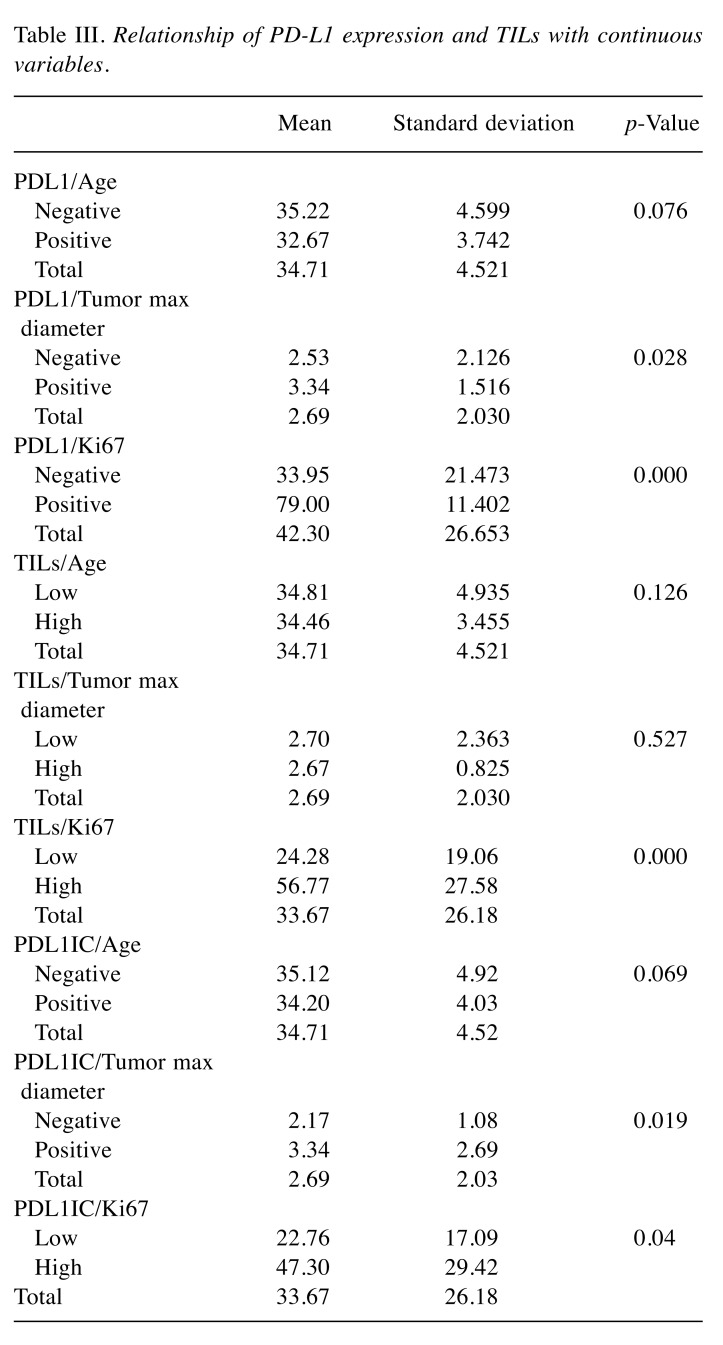
Association of PDL1TC expression with clinicopathologic characteristics. Of all forty-five cases of breast cancer collected for this study, 20% were found to have PDL1TC expression. Correlation of PDL1TC with clinicopathologic parameters showed significant association with tumor diameter, breast cancer grade, ER, PR and Ki67 expression (Table II). Specifically, PDL1TC expression revealed a significant association with carcinomas measuring more than 2 cm (36.84%, p=0.023), grade 3 IBC (32.14%, p=0.017%), ER-negative status (53.84%, p=0.001), PR-negative status (71.42%, p=0,002) and high Ki76(32%, p=0.030) expression. PD-L1 expression assessed with continuous variables like the patients’ age, tumor diameter as well as Ki67 showed significant association with the latter two (p=0.028, p=0.000) (Table III). No significant association was shown between PD-L1 expression and other clinicopathologic studied parameters.
Table II. TILs and PDL1 correlation with clinicopathological parameters.
*Grade 1 tumors were excluded from the statistical analysis due to their limited number
Table III. Relationship of PD-L1 expression and TILs with continuous variables.
Association of PDL1IC expression with clinicopathological characteristics. Inflammatory expression of PD-L1 was observed in 44.44% (20/45) of IBC. PD-L1 positivity in TILs showed significant association with ER, PR expression, large tumor diameter and Ki67 expression. More detailed, PDL1IC expression is most likely to be found in ER- and PR-negative cases (84.62%, p=0.001, 100%, p=0.001, irrespectively) (Table II). Furthermore, PDL1IC expression is most commonly found in large tumor diameters and high Ki67 (p=0.019, p=0.04), as was shown estimating the latter two parameters as continuous variables (Table III). No other significant association was shown between PDL1IC expression and other clinicopathological parameters studied.
Association of stromal TILs with clinicopathological characteristics. Of all forty-five cases collected for this study, thirteen (28.88%) revealed high stromal TILs. The presence of high stromal TILs was significantly increased in IBC measuring more than 2 cm (52.63%, p=0.016) and in ER-negative cases (69.23%, p=0,000) and cases with high Ki67 (44%, p=0.012). Furthermore, associating stromal TILs as a continuous variable with the patients’ age, tumor diameter and Ki67, revealed that high stromal TILs are more likely to be seen in cases of IBC with a high Ki67 (p=0.000) (Table III). No significant association was found between stromal TILs and other clinicopathologic parameters.
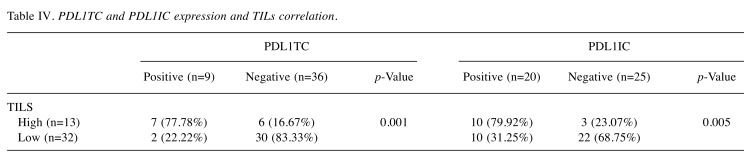
Association of PDL1TC and PDL1IC expression, stromal TILs, CD3 and CD8 lymphocytes. Both PDL1TC and PDL1IC expression in IBC cases revealed a significant association with high stromal TILs (77.78%, p=0.001, 79.92%, p=0.005, respectively) (Table IV). All PDL1TC expression (35%, p=0.024), PDL1IC (65%, p=0.013), and high TILs (100%, p=0.000) showed a significant association with the presence of CD3 lymphocytes. Similarly, PDL1TC expression is significantly associated with the presence of high CD8 infiltrate (35%, p=0.024), as is PDL1IC expression (65%, p=0.013) and high stromal TILs (100%, p=0.000) (Table II).
Table IV. PDL1TC and PDL1IC expression and TILs correlation.
Discussion
Breast cancer in premenopausal women is being increasingly detected (8,9). This subgroup of women draws particular attention, because compared to older women, young age itself is a negative prognostic factor, associated with a higher risk of relapse and death (2-6). Moreover, additional concerning issues regarding the quality of life of this population are being encountered such as fertility preservation, pregnancy, sexual life and external appearance (8,11). It is therefore important, to enrich our knowledge in the tumor microenvironment, a hallmark of cancer in this particular group (14). Although various studies exist examining PD-L1 expression and TILs in breast cancer, this is one of the few studies to focus on this specific age subgroup.
The results of this study indicate that high stromal TILs are only found in a minority of cases, which is consistent with other reports studying TILs in IBC in women of all ages (26,35). PD-L1 on the contrary, has a very wide spectrum of expression on TC and IC in IBC cases, as is reported in the literature, with a positivity range approximately from 20% to almost 60% and 2% to 90%, respectively (36-41). Of all forty-five cases of IBC included in our study, we observed nine (20%) cases of PDL1TC expression and twenty cases of PDL1IC expression (44.44%), both of which apply in the range. A possible explanation for having only a minority of cases expressing PDL1TC and high stromal TILs, is the fact that high stromal TILs and PD-L1 expression are most commonly encountered in TNC, which are less frequent (37). Thus, a possible explanation for having only a minimum positivity of PDL1TC in this study is the limited number of TNC in our cohort. Furthermore, we have shown that PDL1TC positive cases are more seldom found than PDL1IC positive cases, which is consistent with other studies (42).
Associations between PDL1TC and clinicopathological parameters revealed a positive correlation between this expression and grade 3 IBC, ER and PR negativity, as well as large tumor diameter and higher Ki67 expression. No statistically significant associations were found between PDL1TC and other clinicopathologic parameters. These findings are consistent with most studies found in the literature (43-46). The association between PDL1TC and HER2 status remains controversial, though (41,47). In our study we did not find any correlation between PDL1TC expression and HER2 positive IBC which is consistent with a meta-analysis performed from Huang et al. (41) in which 47 studies were included, conducted between the years 2006-2018. A possible explanation for this heterogeneity between PDL1TC expression and HER2 status in the various studies could be the lack of standardized methodology of measurement of PD-L1 (41).
There are not many studies focusing on the expression of PD-L1 on TILs, but the results regarding the relationship between the latter with various clinicopathologic parameters are consistent (39,48,49). Similarly, the results of our study indicated that patients with PDL1IC IBC are most likely to have ER or PR negative carcinomas, large tumor diameter and high Ki67, all of which are established indicators of poor prognosis.
High stromal TILs, PLD1TC and PDL1IC expression were found to be associated with each other, which validates previous reports (35,37,39,50). Furthermore, we indicated that high stromal TILs are more commonly answered in ER negative cases and high Ki67 expression, which shows no deviation from existing reports (51,52). We already know that high stromal TILs are more commonly answered in HER2+ IBC, but this conclusion could not have been conducted in our study, possibly due to the limited number of HER2+ cases (52).
Further analysis of stromal TILs revealed a statistically significant association between high stromal TILs, PDL1TC and PDL1IC with the presence of high CD3+ and CD8+T lymphocytes. TILs are composed by several B and T types of lymphocytes, each of them with a different prognostic value (53).There are various types of T lymphocytes and it is worth mentioning that most CD8+ T lymphocytes are also CD3+ positive (54,55). Denkert et al. (26) indicate that TILs, regardless of their composition, are linked to an improved prognosis, but there are also many studies focusing on the prognostic value of the various types of cells composing TILs. For instance, Seo et al. (56) showed that the increased number of CD8+ T cells is linked to a better clinical outcome and CD3+ lymphocytes are associated with a better prognosis in IBC while Teschendorff et al. (57) indicated that Th2 cells, a CD4+ subpopulation, are linked to mediating the antitumor response, thus having a negative prognostic value. Mori et al. (58) conjectured that the subtypes of TILs should be considered in future studies, because their variations lead to a heterogeneity of conclusions conducted regarding the RFS and OS. Nevertheless, despite having an heterogeneity of opinions between various studies, it is safe to say that our results indicate that young women with high stromal TILs and/or PDL1TC and/or PDL1IC expression are more likely to have a high CD8+ lymphocyte and a high CD3+ infiltrate. Both findings are linked to a better clinical outcome as proven from the studies mentioned above.
Many studies have been conducted in order to evaluate the prognostic value of PDL1TC, PDL1IC and TILs in IBC, and even after many meta-analyses some results remain conflicting (41,59,60). Kim et al. (60), conducted an meta-analysis which included 7,877 cases and was led to the conclusion that PDL1TC expression is associated with poorer DFS. No association was found between PDL1 expression and OS, to the contrast with the meta-analysis conducted by Huang et al. which found that PDL1TC expression is linked to a poorer DFS but also OS. In the present study PDL1TC expression was proven to be linked to several unfavorable prognostic factors, which could indicate a poor prognosis. Although the PD-L1 expression on TILs has not yet been thoroughly examined in the literature, it seems from the existing studies that it is of great prognostic value (61). From these studies PDL1IC expression has shown to have opposite prognostic value compared to PDL1TC expression, since most cases are related to higher DFS, RFS as well as OS (41,62). Zhao et al. (61) studied the prognostic value of PDL1IC expression on several solid tumors, and indicated that it is a positive prognostic factor, especially in IBC. Briefly, a possible explanation for this result, is the fact that PDL1TC expression is mostly driven intercellularly through the tumor-intrinsic mechanism, whereas the PDL1IC expression is driven via adaptive mechanisms, thus related to high stromal TILs which are an anti-tumor answer as a result of an activated PD1/PDL1 pathway (63). The diversity of prognosis between these two factors indicates the importance of the evaluation of PD-L1 expression on both TC and IC in carcinomas. High stromal TILs per se are linked to an improved patient survival and numerous studies focusing on treating such patients with neoadjuvant systemic therapy have shown that the presence of high stromal TILs is in favor of complete pathological response, thus excellent prognosis. Similarly results were found in patients with high CD3+ infiltrate (37,39).
Undeniable, the discovery of TILs as well as the PD1/PD-L1 axis has opened new horizons in understanding the response of the host immune system and the way it associates with tumor progression. Thus, established targeted therapy against ER and PR, as well as human epidermal growth factor receptor 2 (HER2), are already getting enriched with the introduction of immunotherapy, showing very promising results (64). Our opinion is that as in other age groups, stromal TILs and PDL1TC and PDL1IC could be used as prognostic factors and immunotherapy will complement the existent targeted therapies.
Our study has certain limitations especially because of its retrospective nature and its small number of cases studied, as well as the absence of prognostic information. However, it is conducted in a particular patient group where scarce data actually exist. Breast cancer in younger women was rarely identified in the past but now shows an increased number of diagnoses and unfavorable prognosis (8,9). This indicates that although major progress has been achieved in prognosis, diagnosis and treatment in elderly women, this does not yet apply in young women. Several studies have been conducted focusing on whether young breast cancer could have special characteristic or not (13,65). This is the first study to compare clinicopathologic parameters with PD-L1 expression and stromal TILs focusing on this specific age group.
In conclusion, the results of our study show that the PD-L1, CD3 and CD8 expression status in younger women are similar to other age groups as reported in the literature. Thus, the latter parameters should be evaluated and reported in order to be used in cases of young women with IBC and complement existing targeted therapies with immunotherapy, aiming for a better outcome.
Conflicts of Interest
All Authors declare that they have no conflicts of interest.
Authors’ Contributions
Zoi Evangelou and Anna Batistatou have designed the study. Zoi Evangelou, Alexandra Papoudou-Bai, Georgia Karpathiou, Helen Kourea, Sevasti Kamina and Anna Goussia have contributed to the data. Haralambos Harissis has contributed to the acquisition and examination of the original material. Dimitrios Peschos has contributed to the analysis of data. All Authors have read and approved the manuscript.
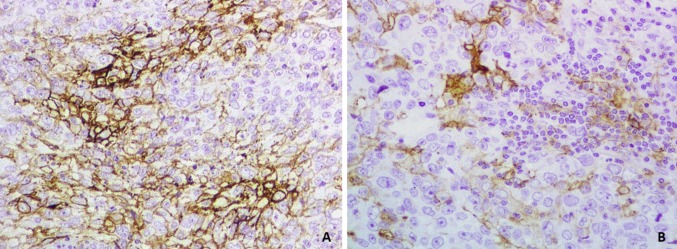
Figure 1. (A) PD-L1 expression in tumor cells (×20). (B) PD-L1 expression in TILs and tumor cells (×40).
References
- 1.Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends-an update. Cancer Epidemiol Biomarkers Prev. 2016;25:16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 2.Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, Wang Y, Marcom PK, Marks JR, Febbo PG, Nevins JR, Potti A, Blackwell KL. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330. doi: 10.1200/JCO.2007.14.2471. [DOI] [PubMed] [Google Scholar]
- 3.Bartelink H, Horiot J-C, Poortmans PM, Struikmans H, Van den Bogaert W, Fourquet A, Jager JJ, Hoogenraad WJ, Oei SB, Wárlám-Rodenhuis CC, Pierart M, Collette L. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881-10882 trial. J Clin Oncol. 2007;25:3259–3265. doi: 10.1200/JCO.2007.11.4991. [DOI] [PubMed] [Google Scholar]
- 4.Anders CK, Johnson R, Litton J, Phillips M, Bleyer A. Breast cancer before age 40 years. Semin Oncol. 2009;36:237–249. doi: 10.1053/j.seminoncol.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fredholm H, Eaker S, Frisell J, Holmberg L, Fredriksson I, Lindman H. Breast cancer in young women: poor survival despite intensive treatment. PLoS One. 2009;4:e7695. doi: 10.1371/journal.pone.0007695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bharat A, Aft RL, Gao F, Margenthaler JA. Patient and tumor characteristics associated with increased mortality in young women (≤40 years) with breast cancer. J Surg Oncol. 2009;100:248–251. doi: 10.1002/jso.21268. [DOI] [PubMed] [Google Scholar]
- 7.Maltaris T, Beckmann MW, Dittrich R. Review. Fertility preservation for young female cancer patients. In Vivo. 2009;23:123–130. [PubMed] [Google Scholar]
- 8.Cardoso F, Loibl S, Pagani O, Graziottin A, Panizza P, Martincich L, Gentilini O, Peccatori F, Fourquet A, Delaloge S, Marotti L, Penault-Llorca F, Kotti-Kitromilidou AM, Rodger A, Harbeck N. The European Society of Breast Cancer Specialists recommendations for the management of young women with breast cancer. Eur J Cancer. 2012;48:3355–3377. doi: 10.1016/j.ejca.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Brinton LA, Sherman ME, Carreon JD, Anderson WF. Recent trends in breast cancer among younger women in the United States. JNCI J Natl Cancer Inst. 2008;100:1643–1648. doi: 10.1093/jnci/djn344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ribnikar D, Ribeiro JM, Pinto D, Sousa B, Pinto AC, Gomes E, Moser EC, Cardoso MJ, Cardoso F. Breast cancer under age 40: a different approach. Curr Treat Options Oncol. 2015;16:16. doi: 10.1007/s11864-015-0334-8. [DOI] [PubMed] [Google Scholar]
- 11.Paluch-Shimon S, Pagani O, Partridge AH, Bar-Meir E, Fallowfield L, Fenlon D, Friedman E, Gelmon K, Gentilini O, Geraghty J, Harbeck N, Higgins S, Loibl S, Moser E, Peccatori F, Raanani H, Kaufman B, Cardoso F. Second international consensus guidelines for breast cancer in young women (BCY2) The Breast. 2016;26:87–99. doi: 10.1016/j.breast.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Reis-Filho JS, Pusztai L. Gene expression profiling in breast cancer: classification, prognostication, and prediction. Lancet. 2011;378:1812–1823. doi: 10.1016/S0140-6736(11)61539-0. [DOI] [PubMed] [Google Scholar]
- 13.Fu J, Wu L, Fu W, Tan Y, Xu T, Hong Z, Wang F, Li S. How young is too young in breast cancer? Young breast cancer is not a unique biological subtype. Clin Breast Cancer. 2018;18:e25–e39. doi: 10.1016/j.clbc.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Ma W, Gilligan BM, Yuan J, Li T. Current status and perspectives in translational biomarker research for PD-1/PD-L1 immune checkpoint blockade therapy. J Hematol Oncol. 2016;9:47. doi: 10.1186/s13045-016-0277-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asano Y, Kashiwagi S, Goto W, Takada K, Takahashi K, Hatano T, Takashima T, Tomita S, Motomura H, Ohsawa M, Hirakawa K, Ohira M. Prediction of treatment response to neoadjuvant chemotherapy in breast cancer by subtype using tumor-infiltrating lymphocytes. Anticancer Res. 2018;38:2311–2321. doi: 10.21873/anticanres.12476. [DOI] [PubMed] [Google Scholar]
- 17.Papaioannou E, Sakellakis M, Melachrinou M, Tzoracoleftherakis E, Kalofonos H, Kourea E. A standardized evaluation method for FOXP3+ Tregs and CD8+ T-cells in breast carcinoma: Association with breast carcinoma subtypes, stage and prognosis. Anticancer Res. 2019;39:1217–1232. doi: 10.21873/anticanres.13232. [DOI] [PubMed] [Google Scholar]
- 18.Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc P-H, Trajanoski Z, Fridman W-H, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 20.Bromwich EJ, McArdle PA, Canna K, McMillan DC, McNicol A-M, Brown M, Aitchison M. The relationship between T-lymphocyte infiltration, stage, tumour grade and survival in patients undergoing curative surgery for renal cell cancer. Br J Cancer. 2003;89:1906–1908. doi: 10.1038/sj.bjc.6601400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McArdle PA, Canna K, McMillan DC, McNicol A-M, Campbell R, Underwood MA. The relationship between T-lymphocyte subset infiltration and survival in patients with prostate cancer. Br J Cancer. 2004;91:541–543. doi: 10.1038/sj.bjc.6601943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karpathiou G, Casteillo F, Giroult J-B, Forest F, Fournel P, Monaya A, Froudarakis M, Dumollard JM, Prades JM, Peoc’h M. Prognostic impact of immune microenvironment in laryngeal and pharyngeal squamous cell carcinoma: Immune cell subtypes, immuno-suppressive pathways and clinicopathologic characteristics. Oncotarget. 2017;8 doi: 10.18632/oncotarget.14242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ, Wolff AC, Wood WC, Davidson NE, Sledge GW, Sparano JA, Badve SS. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32:2959–2966. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, Rouas G, Francis P, Crown JPA, Hitre E, de Azambuja E, Quinaux E, Di Leo A, Michiels S, Piccart MJ, Sotiriou C. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31:860–867. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 25.Lee M-C, Buitrago DH, Kadota K, Ujiie H, Woo K, Sima CS, Travis WD, Jones DR, Adusumilli PS. The tumor immune microenvironment in octogenarians with stage I non-small cell lung cancer. Oncoimmunology. 2014;3:e967142. doi: 10.4161/21624011.2014.967142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C, von Törne C, Weichert W, Engels K, Solbach C, Schrader I, Dietel M, von Minckwitz G. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–113. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 27.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 28.Woo S-R, Turnis ME, Goldberg M V., Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, Tangsombatvisit S, Grosso JF, Netto G, Smeltzer MP, Chaux A, Utz PJ, Workman CJ, Pardoll DM, Korman AJ, Drake CG, Vignali DAA. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate t-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brahmer JR, Tykodi SS, Chow LQM, Hwu W-J, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gajewski TF, Schreiber H, Fu Y-X. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Azuma T, Yao S, Zhu G, Flies AS, Flies SJ, Chen L. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111:3635–3643. doi: 10.1182/blood-2007-11-123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F, Perez EA, Thompson EA, Symmans WF, Richardson AL, Brock J, Criscitiello C, Bailey H, Ignatiadis M, Floris G, Sparano J, Kos Z, Nielsen T, Rimm DL, Allison KH, Reis-Filho JS, Loibl S, Sotiriou C, Viale G, Badve S, Adams S, Willard-Gallo K, Loi S. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo X, Fan Y, Lang R, Gu F, Chen L, Cui L, Pringle GA, Zhang X, Fu L. Tumor infiltrating lymphocytes differ in invasive micropapillary carcinoma and medullary carcinoma of breast. Mod Pathol. 2008;21:1101–1107. doi: 10.1038/modpathol.2008.72. [DOI] [PubMed] [Google Scholar]
- 34.Downes MR, Slodkowska E, Katabi N, Jungbluth AA, Xu B. Inter- and intraobserver agreement of programmed death ligand 1 scoring in head and neck squamous cell carcinoma, urothelial carcinoma and breast carcinoma. Histopathology. 2019;76:189–190. doi: 10.1111/his.13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee AH, Happerfield LC, Millis RR, Bobrow LG. Inflammatory infiltrate in invasive lobular and ductal carcinoma of the breast. Br J Cancer. 1996;74:796–801. doi: 10.1038/bjc.1996.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schalper KA, Velcheti V, Carvajal D, Wimberly H, Brown J, Pusztai L, Rimm DL. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res. 2014;20:2773–2782. doi: 10.1158/1078-0432.CCR-13-2702. [DOI] [PubMed] [Google Scholar]
- 37.Sabatier R, Finetti P, Mamessier E, Adelaide J, Chaffanet M, Ali HR, Viens P, Caldas C, Birnbaum D, Bertucci F. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget. 2015;6 doi: 10.18632/oncotarget.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghebeh H, Tulbah A, Mohammed S, ElKum N, Amer SM Bin, Al-Tweigeri T, Dermime S. Expression of B7-H1 in breast cancer patients is strongly associated with high proliferative Ki-67-expressing tumor cells. Int J Cancer. 2007;121:751–758. doi: 10.1002/ijc.22703. [DOI] [PubMed] [Google Scholar]
- 39.Ghebeh H, Mohammed S, Al-Omair A, Qattant A, Lehe C, Al-Qudaihi G, Elkum N, Alshabanah M, Bin Amer S, Tulbah A, Ajarim D, Al-Tweigeri T, Dermime S. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: Correlation with important high-risk prognostic factors. Neoplasia. 2006;8:190–198. doi: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muenst S, Schaerli AR, Gao F, Däster S, Trella E, Droeser RA, Muraro MG, Zajac P, Zanetti R, Gillanders WE, Weber WP, Soysal SD. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014;146:15–24. doi: 10.1007/s10549-014-2988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang W, Ran R, Shao B, Li H. Prognostic and clinicopathological value of PD-L1 expression in primary breast cancer: a meta-analysis. Breast Cancer Res Treat. 2019 doi: 10.1007/s10549-019-05371-0. [DOI] [PubMed] [Google Scholar]
- 42.Dill EA, Gru AA, Atkins KA, Friedman LA, Moore ME, Bullock TN, Cross JV, Dillon PM, Mills AM. PD-L1 Expression and intratumoral heterogeneity across breast cancer subtypes and stages. Am J Surg Pathol. 2017;41:334–342. doi: 10.1097/PAS.0000000000000780. [DOI] [PubMed] [Google Scholar]
- 43.Bae SB, Cho HD, Oh M-H, Lee J-H, Jang S-H, Hong SA, Cho J, Kim SY, Han SW, Lee JE, Kim HJ, Lee HJ. Expression of programmed death receptor ligand 1 with high tumor-infiltrating lymphocytes is associated with better prognosis in breast cancer. J Breast Cancer. 2016;19:242. doi: 10.4048/jbc.2016.19.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buisseret L, Garaud S, de Wind A, Van den Eynden G, Boisson A, Solinas C, Gu-Trantien C, Naveaux C, Lodewyckx J-N, Duvillier H, Craciun L, Veys I, Larsimont D, Piccart-Gebhart M, Stagg J, Sotiriou C, Willard-Gallo K. Tumor-infiltrating lymphocyte composition, organization and PD-1/PD-L1 expression are linked in breast cancer. Oncoimmunology. 2017;6:e1257452. doi: 10.1080/2162402X.2016.1257452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qin T, Zeng Y, Qin G, Xu F, Lu J, Fang W, Xue C, Zhan J, Zhang X, Zheng Q, Peng R, Yuan Z, Zhang L, Wang S. High PD-L1 expression was associated with poor prognosis in 870 Chinese patients with breast cancer. Oncotarget. 2015;6 doi: 10.18632/oncotarget.15532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Z-Q, Milne K, Derocher H, Webb JR, Nelson BH, Watson PH. PD-L1 and intratumoral immune response in breast cancer. Oncotarget. 2017;8 doi: 10.18632/oncotarget.18305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li S, Chen L, Jiang J. Role of programmed cell death ligand-1 expression on prognostic and overall survival of breast cancer. Medicine (Baltimore) 2019;98:e15201. doi: 10.1097/MD.0000000000015201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim A, Lee SJ, Kim YK, Park WY, Park DY, Kim JY, Lee CH, Gong G, Huh GY, Choi KU. Programmed death-ligand 1 (PD-L1) expression in tumour cell and tumour infiltrating lymphocytes of HER2-positive breast cancer and its prognostic value. Sci Rep. 2017;7:11671. doi: 10.1038/s41598-017-11905-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Botti G, Collina F, Scognamiglio G, Rao F, Peluso V, De Cecio R, Piezzo M, Landi G, De Laurentiis M, Cantile M, Di Bonito M. Programmed death ligand 1 (PD-L1) tumor expression is associated with a better prognosis and diabetic disease in triple negative breast cancer patients. Int J Mol Sci. 2017;18:459. doi: 10.3390/ijms18020459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cimino-Mathews A, Thompson E, Taube JM, Ye X, Lu Y, Meeker A, Xu H, Sharma R, Lecksell K, Cornish TC, Cuka N, Argani P, Emens LA. PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum Pathol. 2016;47:52–63. doi: 10.1016/j.humpath.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Polónia A, Pinto R, Cameselle-Teijeiro JF, Schmitt FC, Paredes J. Prognostic value of stromal tumour infiltrating lymphocytes and programmed cell death-ligand 1 expression in breast cancer. J Clin Pathol. 2017;70:860–867. doi: 10.1136/jclinpath-2016-203990. [DOI] [PubMed] [Google Scholar]
- 52.Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer. 2016;4:59. doi: 10.1186/s40425-016-0165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gooden MJM, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105:93–103. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G, Tamada K, Chen L. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- 56.Seo S-K, Seo H-M, Jeong H-Y, Choi I-W, Park Y-M, Yagita H, Chen L, Choi I-H. Co-inhibitory role of T-cell-associated B7-H1 and B7-DC in the T-cell immune response. Immunol Lett. 2006;102:222–228. doi: 10.1016/j.imlet.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 57.Teschendorff AE, Gomez S, Arenas A, El-Ashry D, Schmidt M, Gehrmann M, Caldas C. Improved prognostic classification of breast cancer defined by antagonistic activation patterns of immune response pathway modules. BMC Cancer. 2010;10:604. doi: 10.1186/1471-2407-10-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mori H, Kubo M, Yamaguchi R, Nishimura R, Osako T, Arima N, Okumura Y, Okido M, Yamada M, Kai M, Kishimoto J, Oda Y, Nakamura M. The combination of PD-L1 expression and decreased tumor-infiltrating lymphocytes is associated with a poor prognosis in triple-negative breast cancer. Oncotarget. 2017;8 doi: 10.18632/oncotarget.14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uhercik M, Sanders A, Owen S, Davies E, Sharma A, Jiang W, Mokbel K. Clinical significance of PD1 and PDL1 in human breast cancer. Anticancer Res. 2017;37:4249–4254. doi: 10.21873/anticanres.11817. [DOI] [PubMed] [Google Scholar]
- 60.Kim HM, Lee J, Koo JS. Clinicopathological and prognostic significance of programmed death ligand-1 expression in breast cancer: a meta-analysis. BMC Cancer. 2017;17:690. doi: 10.1186/s12885-017-3670-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao T, Li C, Wu Y, Li B, Zhang B. Prognostic value of PD-L1 expression in tumor infiltrating immune cells in cancers: A meta-analysis. PLoS One. 2017;12:e0176822. doi: 10.1371/journal.pone.0176822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li X, Wetherilt CS, Krishnamurti U, Yang J, Ma Y, Styblo TM, Meisel JL, Peng L, Siddiqui MT, Cohen C, Aneja R. Stromal PD-L1 expression is associated with better disease-free survival in triple-negative breast cancer. Am J Clin Pathol. 2016;146:496–502. doi: 10.1093/ajcp/aqw134. [DOI] [PubMed] [Google Scholar]
- 63.Kim HR, Ha S-J, Hong MH, Heo SJ, Koh YW, Choi EC, Kim EK, Pyo KH, Jung I, Seo D, Choi J, Cho BC, Yoon SO. PD-L1 expression on immune cells, but not on tumor cells, is a favorable prognostic factor for head and neck cancer patients. Sci Rep. 2016;6:36956. doi: 10.1038/srep36956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Katz H, Alsharedi M. Immunotherapy in triple-negative breast cancer. Med Oncol. 2018;35:13. doi: 10.1007/s12032-017-1071-6. [DOI] [PubMed] [Google Scholar]
- 65.Fredholm H, Magnusson K, Lindström LS, Tobin NP, Lindman H, Bergh J, Holmberg L, Pontén F, Frisell J, Fredriksson I. Breast cancer in young women and prognosis: How important are proliferation markers. Eur J Cancer. 2017;84:278–289. doi: 10.1016/j.ejca.2017.07.044. [DOI] [PubMed] [Google Scholar]