ABSTRACT
Given the dramatic impact of the COVID-19 pandemic, it is imperative to divulge all the available technologies with the potential to fight against this virus. Plant biotechnology offers potential solutions to this pandemic through the development of low-cost vaccines and antibodies useful for therapy, prophylaxis, and diagnosis. The technology to produce plant-made biopharmaceuticals is already established; two examples of these are: a therapeutic enzyme that has entered the market and the influenza vaccines that are currently under clinical trials with encouraging results. Thus far, some companies have started developing anti-COVID-19 antibodies and vaccines. In particular, plant-made antibodies might be timely produced and approved for human use in the short term, while the development of vaccines will take longer time (clinical evaluations could be concluded by the end of 2021); nonetheless, the candidates obtained will be valuable tools for future outbreaks. The key aspects that will define the exploitation of this technology in the fight against COVID-19 are discussed.
KEYWORDS: SARS-CoV-2 virus, plantibody, biomanufacturing, COVID-19 vaccine, mucosal immunization
1. Introduction
The COVID-19 pandemic, caused by SARS-CoV-2 virus, is having a dramatic impact on human lifestyle and on economic, social, and global relations. At the end of March 2020 (three months after its emergence), it has caused >800,000 cases with >40,000 deaths. When compared to the H1N1 pandemic, which has a mortality rate of about 0.02%, COVID-19 has higher mortality rate (~3%), as well as higher transmissibility (it spread to >200 countries, territories, or areas within a three-month period). This situation demands accelerating the race to produce and validate new vaccines, antibodies, and drugs. A special concern that will be dimensioned in the coming months is the impact of this pandemic in developing and low-income countries. Under the context of this rapidly evolving situation, several efforts have been announced around the world involving pharma companies and public institutions to accelerate transmission blockade and develop effective treatments and vaccines. Given the urgency to develop vaccines, the International Coalition of Medicines Regulatory Authorities has published a compilation of the first global regulatory workshop on COVID-19 vaccine development; in which the key points comprise the pre-clinical data requirements that include the risk of disease enhancement by the test vaccine [1]. The latter aspect deserves special attention given the precedents of candidate vaccines against SARS, in which such phenomenon has been observed. This situation highlights the need for rational vaccine design avoiding epitopes that trigger those undesired immune responses [2]. The models developed to evaluate SARS vaccine candidates will be a valuable starting point for performing preclinical evaluations; comprising models based on mice, ferrets, and monkeys [3].
In the case of the antibody-mediated therapy (passive immunization), an interesting fact is the cross-reactivity of anti-SARS-CoV-1 virus with SARS-CoV-2 virus, suggesting that these already developed biologicals could enter the arena to fight COVID-19 [4]. Therefore, the availability of monoclonal antibodies against SARS-CoV-1 virus is a valuable arsenal to study their potential for the treatment of COVID-19.
2. Molecular farming: a mature technology
Molecular farming is a biotechnology application that involves the use of plant species as hosts for the production of highly valuable recombinant proteins, which include antibodies, vaccines, hormones, and enzymes [5]. While the initial efforts in the plant-based manufacturing arena, reported three decades ago, suffered of low protein yields and a precarious processing, the plant genetic engineering approaches have been refined and sophisticated expression platforms are currently available, which allow obtaining protein yields up to 5 g of protein per kg of plant leaves [6]. For instance, under cGMP processes, N. benthamiana plants can be grown and subjected to transient transformation, which triggers efficient expression of the target protein [6]. Within few days, plant leaf biomass is harvested and processed to purify the target biopharmaceuticals. Injectable formulations are obtained in this type of platforms, which can be easily scaled up [7,8]. Other platforms are based on plant biomass propagation using bioreactors with suspensions of cell cultures (e.g. carrot or rice cells) [9,10].
Moreover, the refinement of production processes has positively impacted the quality and fulfillment of the requirements of regulatory agencies, e.g. compliance of the ICH guidelines [11,12]. In this sense the work done by some researchers has achieved the implementation of processes prone to scale-up according to the principles of the current Good Manufacturing Practices (cGMP) for pharmaceuticals indicated by the US FDA.
Today, plant-made biopharmaceuticals have become a reality. At least one product has entered the market, namely taliglucerase alfa, a carrot-made enzyme obtained in bioreactors that is prescribed as replacement therapy for Gaucher´s disease [10]. Other products are close to be approved, for instance, clinical trials are ongoing to evaluate influenza vaccines produced by Medicago Inc [13]. Moreover, Zmapp (a plant-made monoclonal antibody cocktail) was placed in the spotlight during the Ebola outbreak in 2014 since it was administered to two patients on a compassionate basis to treat a few patients [14]. Although other antibodies have shown better performance, this plant-made antibody cocktail has revealed the potential of the technology in the fight against emerging diseases [15]. Today glycoengineering approaches are available to yield specific antibody glycoforms, which will allow optimizing the functional activity and safety of the target antibodies [16]. Moreover, functional single chain antibodies are also produced in plant systems; providing simpler molecules for viral neutralization [17]. Plant-made antigens and antibodies can be also convenient tools for diagnosis; providing low-cost proteins with preserved antigenic determinants and specificity [18,19].
3. Expert opinion
Upon the extraordinary and unprecedented public health crisis caused by COVID-19, a solid involvement and collaboration across governments, societies, and businesses is urgently required. Under this context and especially considering that the pandemic is in the initial stage in developing and low-income countries, governments and academic institutions should turn attention to low-cost technologies for the production of biopharmaceuticals against COVID-19. Given that the processes for the production of plant-made vaccines, therapeutic enzymes, and antibodies are already established, a rapid progress in the generation of candidates is anticipated, which might comprise, immediately, the preexisting anti-SARS-CoV-1 virus antibodies that could cross-react with SARS-CoV-2 virus and vaccines based on VLPs assembled with the S protein (currently considered the primary target for virus neutralization) Figure 1.
Figure 1.
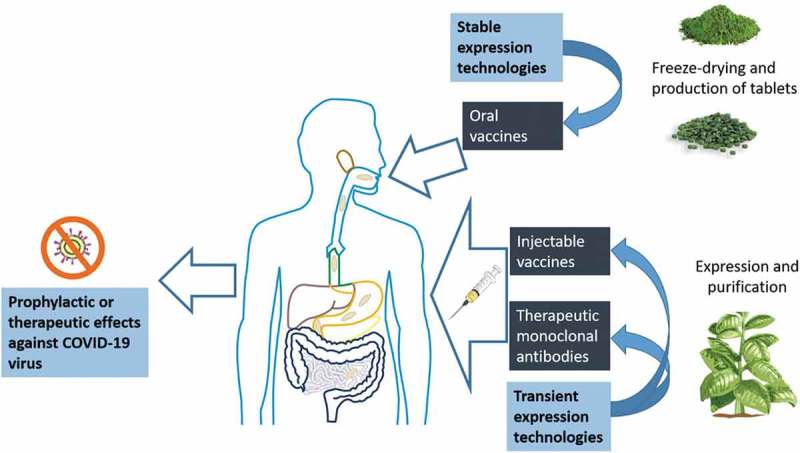
Developmental paths for the production of plant-made antibodies or vaccines against SARS-CoV-2 virus. Transient transformation approaches allow high protein yields in the transformed plants, which are processed to purify the target biopharmaceutical and obtain injectable vaccines or monoclonal antibodies. Stable genetic transformation technologies applied in edible plant species can render oral vaccine formulations (e.g. capsules or tables containing freeze-dried leaves), which can be applied as boosting agents following a parenteral priming.
At least two companies have announced the beginning of the race for the development of plant-made vaccines and antibodies against SARS-CoV-2 virus. After 21 days of having access to the SARS-CoV-2 virus S protein sequence, the production of VLPs in a transient expression system has been announced by the company Medicago Inc. (www.medicago.com/en/pipeline/), therefore a rapid progress is expected for this development. This company has estimated to hold a production capacity of 10 million doses a month of VLPs-based vaccines, which is an attractive production capacity. This number of doses could allow, for instance, immunizing 10 million US adults who are at severe risk of critical illness or death if infected with SARS-CoV-2 virus. In addition, developing epitope-based vaccines is advised to diminish the possibility of disease enhancement. iBio Inc. also has a VLP vaccine in its pipeline (www.ibioinc.com/pipeline). Other companies with the potential to enter this race include Nomad (www.nomadbioscience.com/), Ventria (ventria.com/), Greenovation Biopharmaceuticals (www.greenovation.com/), protalix (ww.protalix.com), and Kentucky Bioprocessing (www.kentuckybioprocessing.com). The academic institutions will have with no doubt a key role in this field. In fact the Infectious Disease Research Centre at Laval University has joint efforts with Medicago to develop therapeutic antibodies. Several Universities and Institutes from several countries including the US, Germany, UK, South Africa, South Corea, Mexico, and Thailand are working in the molecular farming field and investing efforts in the COVID-19 topic. Establishing partnerships with Institutes specialized in infectious diseases will lead to a synergy.
The development of transgenic plants (stably transformed) takes a long time (>3 months); therefore this approach is unpractical to respond to pandemics. Nonetheless, transient transformation approaches offer rapid methods (expression within a week) for the efficient expression of the target antigen or antibody; rendering injectable formulations. Despite this, in my expert opinion, the development of stably transformed plant species (especially those yielding edible biomass) is still necessary to generate models of oral vaccines, which could find their niche at the post-pandemic stage. It is highly probable that the SARS-CoV-2 virus will become a seasonal pathogen, which will generate the need for having constant immunization programs. Therefore, having already developed oral vaccine production platforms will be useful to cope with such situation given that this approach is the most practical to perform large vaccination campaigns at low cost and under easy-to-implement logistics; moreover, they could also be used as boosting agents that might complement the parenteral priming performed with pure antigens. Under this scheme, optimal immune profiles (in terms of not only protecting the systemic compartment but the mucosal compartments) could be induced [20]. In fact, mucosal immunization is considered of superior potential to protect the mucosal compartments through the induction of secretory IgA response that could neutralize the virus.
Sadly the projections on the impact of this pandemic in developing countries can be devastating and this is just in the initial scenarios. Therefore, it is urgent to implement low-cost production platforms for biologicals to fight COVID-19 in those scenarios. What will be the source of low-cost biologicals? Governments and the current companies working on the molecular farming field, e.g. Medicago and iBio, should form alliances to benefit the most unprotected populations. In this regard, plant-based manufacturing should be contemplated as one alternative.
Although the most straightforward approach to produce anti-SARS-CoV-2 virus biopharmaceuticals will rely on conventional platforms (i.e. CHO cells and yeast), plant-made biopharmaceuticals can be incorporated into the fight against the SARS-CoV-2 virus. This will only occur if a timely development is accomplished through a stretch cooperation between companies, government agencies, and public institutions. In the case of vaccines, the developmental pipeline will necessarily require extensive safety and efficacy evaluations, which will take at least a year according to the estimations from Medicago Inc. (www.medicago.com/en/pipeline/). However, the experience gained in those evaluations will be critical to fight against COVID-19 if it becomes a seasonal pathogen and in the case of emergence of new Coronavirus strains. In fact the FDA has established an Advanced Technologies Team to stimulate discussion, divulgation, and feedback among FDA staff and between them and prospective innovators/developers of advanced manufacturing technologies. This action is a hope to get more expedite processes for the approval of biopharmaceuticals produced under innovative platforms; especially for those against emerging pathogens [21].
Since plant-made biopharmaceuticals have not been adopted systematically around the world thus far, regulatory agencies must increase their knowledge in this technology and adapt their requirements. The approval of a plant-made vaccine will perhaps be achieved for the case of COVID-19. Will regulatory agencies display flexibility to validate and approve anti- SARS-CoV-2 virus plant-made biopharmaceuticals (e.g. by adapting/simplifying the regulatory requirements for this specific technology)? Will regulatory agencies accelerate the evaluation process of plant-made biopharmaceuticals against SARS-CoV-2 virus? Will the existing clinical trials of plant-made vaccines against influenza (with encouraging findings) and the enzyme already approved make the approval pathway smoother? Will the developing and low-income countries ultimately benefit from the plant-based technologies in the fight against COVID-19? The coming month will be critical to answer these key questions.
The main features of plant-based platforms that include low cost, scalability, and safety should be divulged to promote their adoption by governments in association with spin-off companies or private small companies seeking to crystalize products to benefit the global health; especially in developing and low-income countries that have limited resources to cope with pandemics.
Funding Statement
This paper is not funded.
Declaration of interest
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Global regulatory workshop on COVID-19 Vaccine development. [Cited 2020 March25 http://www.icmra.info/drupal/sites/default/files/2020-03/First%20regulatory%20COVID-19%20workshop%20-%20meeting%20report_March%202020.pdf
- 2.Wang Q, Zhang L, Kuwahara K, et al, Immunodominant SARS coronavirus epitopes in humans elicited both enhancing and neutralizing effects on infection in non-human primates. ACS Infect Dis. 2(5): 361–376. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Highlights the need for rational vaccine design.
- 3.Gretebeck LM, Subbarao K.. Animal models for SARS and MERS coronaviruses. Curr Opin Virol. 2015;13:123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian X, Li C, Huang A, et al, Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg Microbes Infect. 9(1): 382–385. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Suggest the use of anti-SARS-CoV-1 virus antibodies against SARS-CoV-2 virus.
- 5.Fischer R, Buyel JF. Molecular farming - The slope of enlightenment. Biotechnol Adv. 2020;40:107519. [DOI] [PubMed] [Google Scholar]; •• A key review describing the evolution of molecular farming.
- 6.Gleba Y, Klimyuk V, Marillonnet S. Magnifection–a new platform for expressing recombinant vaccines in plants. Vaccine. 2005;23(17–18):2042–2048. [DOI] [PubMed] [Google Scholar]
- 7.Klimyuk V, Pogue G, Herz S, et al. Production of recombinant antigens and antibodies in Nicotiana benthamiana using ‘magnifection’ technology: GMP-compliant facilities for small- and large-scale manufacturing. Curr Top Microbiol Immunol. 2014;375:127–154. [DOI] [PubMed] [Google Scholar]; • Describes a prominent technology for transient expression in plants.
- 8.Marsian J, Fox H, Bahar MW, et al. Plant-made polio type 3 stabilized VLPs-a candidate synthetic polio vaccine. Nat Commun. 2017;8(1):245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi YS, Moon JH, Kim TG, et al. Potent In Vitro and In Vivo activity of plantibody specific for porphyromonas gingivalis FimA. Clin Vaccine Immunol. 2016;23(4):346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mor TS. Molecular pharming’s foot in the FDA’s door: protalix’s trailblazing story. Biotechnol Lett. 2015;37(11):2147–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Transmits the path for the approval of a plant-made enzyme.
- 11.Buyel JF, Fischer R. Predictive models for transient protein expression in tobacco (Nicotiana tabacum L.) can optimize process time, yield, and downstream costs. Biotechnol Bioeng. 2012;109:2575–2588. [DOI] [PubMed] [Google Scholar]
- 12.Buyel JF, Woo JA, Cramer SM, et al. The use of quantitative structure–activity relationship models to develop optimized processes for the removal of tobacco host cell proteins during biopharmaceutical production. J Chromatog A. 2013;1322:18–28. [DOI] [PubMed] [Google Scholar]
- 13.Pillet S, Couillard J, Trépanier S, et al, Immunogenicity and safety of a quadrivalent plant-derived virus like particle influenza vaccine candidate-two randomized phase II clinical trials in 18 to 49 and ≥50 years old adults. PLoS One. 14(6): e0216533 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Exemplifies the potential of plant-made vaccines.
- 14.Tully CM, Lambe T, Gilbert SC, et al. Emergency Ebola response: a new approach to the rapid design and development of vaccines against emerging diseases. Lancet Infect Dis. 2015;15:356–359. [DOI] [PubMed] [Google Scholar]
- 15.New antibodies best ZMapp in Ebola trial. Nat Biotechnol. 2019;37:1105 10.1038/s41587-019-0284-y [DOI] [PubMed] [Google Scholar]
- 16.Stelter S, Paul MJ, Teh AY, et al. Engineering the interactions between a plant-produced HIV antibody and human Fc receptors. Plant Biotechnol J. 2020;18(2):402–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phoolcharoen W, Banyard AC, Prehaud C, et al. In vitro and in vivo evaluation of a single chain antibody fragment generated in planta with potent rabies neutralisation activity. Vaccine. 2019;37(33):4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LÉC M, Silva BB, Dutra RF, et al. Transient expression of dengue virus NS1 antigen in Nicotiana benthamiana for use as a diagnostic antigen. Front Plant Sci. 2020;10:1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rybicki EP. Plant molecular farming of virus-like nanoparticles as vaccines and reagents. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12(2):e1587. [DOI] [PubMed] [Google Scholar]; •• Shows the potential of plant-made VLPs.
- 20.Kardani K, Bolhassani A, Shahbazi S. Prime-boost vaccine strategy against viral infections: mechanisms and benefits. Vaccine. 2016;34(4):413–423. [DOI] [PubMed] [Google Scholar]
- 21.CBER Advanced Technologies Program. [Cited 2020 March25]. https://www.fda.gov/vaccines-blood-biologics/industry-biologics/cber-advanced-technologies-program
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Global regulatory workshop on COVID-19 Vaccine development. [Cited 2020 March25 http://www.icmra.info/drupal/sites/default/files/2020-03/First%20regulatory%20COVID-19%20workshop%20-%20meeting%20report_March%202020.pdf
- CBER Advanced Technologies Program. [Cited 2020 March25]. https://www.fda.gov/vaccines-blood-biologics/industry-biologics/cber-advanced-technologies-program


