Abstract
The last century has witnessed several assaults from RNA viruses, resulting in millions of death throughout the world. The 21st century appears no longer an exception, with the trend continued with escalated fear of SARS coronavirus in 2002 and further concern of influenza H5N1 in 2003. A novel influenza virus created the first pandemic of the 21st century, the pandemic flu in 2009 preceded with the emergence of another deadly virus, MERS-CoV in 2012. A novel coronavirus “SARS-CoV-2” (and the disease COVID-19) emerged suddenly, causing a rapid outbreak with a moderate case fatality rate. This virus is continuing to cause health care providers grave concern due to the lack of any existing immunity in the human population, indicating their novelty and lack of previous exposure. The big question is whether this novel virus will be establishing itself in an endemic form or will it eventually die out? Endemic viruses during circulation may acquire mutations to infect naïve, as well as individual with pre-existing immunity. Continuous monitoring is strongly advisable, not only to the newly infected individuals, but also to those recovered individuals who were infected by SARS-CoV-2 as re-infection may lead to the selection of escape mutants and subsequent dissemination to the population.
Keywords: Coronavirus, SARS, COVID-19, SARS-CoV-2, pre existing immunity
Introduction
Towards the end of the first decade of the 21st century, during December 2019, numerous pneumonia incidences of unidentified cause appeared in Wuhan, Hubei, China, with clinical presentations greatly resembling Flu and viral pneumonia. After virus isolation and analysis of viral genome sequence from infected patient’s samples, a novel coronavirus named as severe acute respiratory syndrome-related coronavirus 2 or SARS-CoV-2 (initially designated as novel coronavirus or nCoV-2019) was identified from an unknown source. SARS-CoV-2 is the causative agent of respiratory disease which is recently named as Coronavirus disease 2019 (COVID-19) by the World Health Organisation (WHO). Human-to-human transmission of SARS-CoV-2 is a major concern for the health care workers and a preliminary R0 (Reproductive number: as the number of new infection one infected person generates on average throughout its infectious period) measure of 1.4–2.5 was reported by the WHO (https://www.who.int/health-topics/coronavirus). Since its appearance within a months’ time enormous number of new cases are piling up and the actual R0 value may be quite higher than previously calculated (Wu et al. 2020). Epidemiological data suggest that approximately (as of February 29 2020) 79,251 people infected with this virus in mainland China and multiple cases also reported from other parts of the world (https://who.sprinklr.com;https://www.medpagetoday.com/infectiousdisease/publichealth/84698). So far 60 other countries reported SARS-CoV-2 infection; although most of the cases are migrants of China, indicating a single introduction of this virus. Among the other countries, South Korea (3,150 cases), Italy (889 cases), Japan (234 cases), Iran (388 cases), Singapore (96 cases), HongKong (94 cases) and USA (64 cases) reported significant number of cases and the situation is getting complicated with time. Current findings indicate that various subtypes of coronaviruses are in circulation within the bat population including other species such as birds, cats, dogs, pigs, mice, horses, whales before they acquire the ability to cross the species barriers to cause human infection. It is still unanswered that how SARS-CoV-2 has caused an outbreak in Wuhan. The initial wave of infection found associated with the seafood market of Wuhan, strengthening the hypothesis that close contact between the live or dead animal with an individual may be responsible for the initiation of the outbreak. In the recent past there are examples of zoonotic transmission of bird flu which directly jumped from avian species to human, but no transmission between the humans were established which kept the disease under control. However, the ability to spread among human to human upon close contact makes SARS-CoV-2 as a very important contagious agent to study and monitor whose devastation has already been established in Wuhan, Hubei, China.
COVID-19 is one of the most alarming diseases in the globe at this moment. The number of patients infected with SARS-CoV-2 is increasing in almost steady rate, although in some days less number of cases was reported. Total number of cases reached to 20,000 in first 12 days, 40,000 in next 7 days and more than 80,000 in just 33 days. Infections are causing varied clinical manifestation from mild symptoms to severe respiratory attacks, although there is possibility of asymptomatic infection. It has spread in many other countries beyond China, therefore proper handling and management of the disease is critically important to prevent a pandemic.
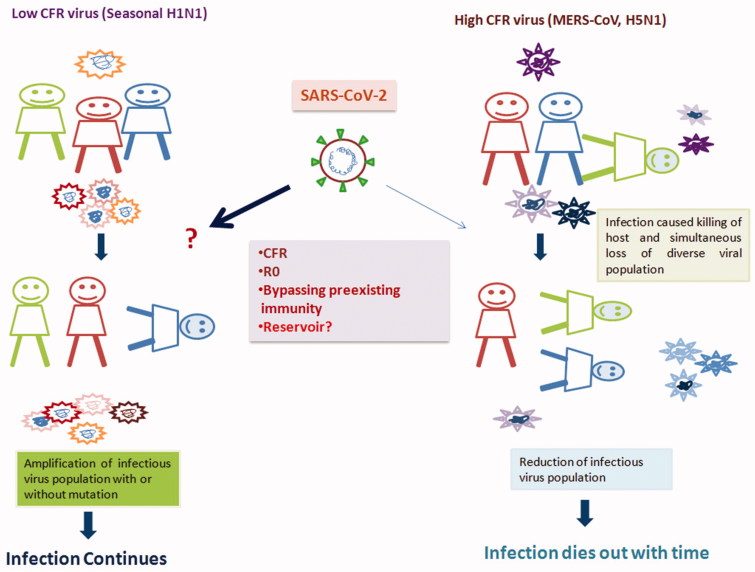
History has taught several lessons regarding the endemic nature of emerging viral infections. Virus faces major challenges from pre-existing immunity to remain in the population. Other important factors such as case fatality rate (CFR), R0 and the reservoir hosts are crucial in influencing viral endemicity. By analysing CFR of different RNA viruses and their longevity in circulation throughout the globe, it was found that viruses with higher CFR (CFR >5%) die out after few passages of infections sooner or later, whereas viruses with low CFR remain endemic with a seasonal outbreak like common flu caused by influenza viruses (Figure 1). Although it is very difficult to calculate actual CFR until the outbreak ends, an initial estimate suggests that CFR for SARS-CoV-2 is 2.58%, much closer to seasonal flu than the other coronaviruses (SARS-CoV and MERs-CoV) of the recent past (i.e. 10% and 35%, respectively, https://www.who.int/emergencies/mers-cov/en/) indicating the ability of the SARS-CoV-2 to remain in circulation with low CFR throughout the world. As per the recent data, COVID-19 has already claimed 2,924 lives, mainly in China although few deaths reported from other parts of the world. However, as of February 29 2020, 39,556 infected individuals already recovered from SARS-CoV2 infection, who will eventually acquire immunity to the virus. (https://www.medpagetoday.com/infectiousdisease/publichealth/84698). The Covid-19 recovered population increases day by day, with herd immunity of the population. A virus may re-infect a person with pre-existing immunity, either in its present form (which is very unlikely), or with acquired mutations to persist in the circulation. SARS-CoV-2 recovered populations may enable the selection of mutant viruses, and their spread in the community.
Figure 1.
Relationship between high and low CFR virus infection and outbreak outcome. When viruses with low CFR infect an individual, gradually, it induces the host immune system. The induced immunity may enable the spread of mutant viruses in the population, whereas viruses with high CFR causes robust infection and rapid immune responses leading to death. Imprisonment of the different viral species within the dead host will lead to a reduction in the diverse or mutant virus population in the circulation result the outbreak to its conclusion.
In this review, we will discuss the critical factors influencing the continued viral appearance and periodical infection. We will also discuss the novelty of this virus and its potential threat to cause periodic infection. We have collected data from different studies and also analysed the genome sequence to define the evolutionary relationship of this virus. Moreover, we have predicted the reason for the severity of this disease with literature support.
Coronavirus and its genome
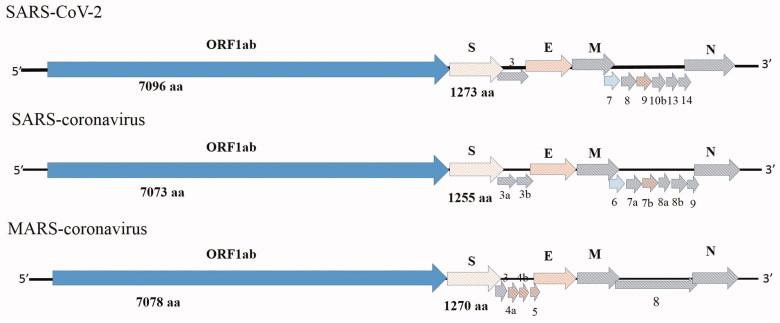
Coronaviruses are enveloped and belongs to the family Coronaviridae which is further subdivided into four genera: alpha, beta, gamma and delta coronaviruses (Woo et al. 2007; Lefkowitz et al, 2018). They possess a long positive-sense single-stranded RNA genome ranging between 26.4 kb to 31.7 kb which is largest for any RNA virus. Big RNA genome of coronavirus provides additional flexible power in host adaptation and genome modification. The genome organisation is overall same in all coronaviruses (Figure 2). Two short untranslated regions (UTR) are present at the 5′ and 3′ end of the genome and the coding genes are present in between the UTR. The arrangement of the coding gene is; 5′-replicase ORF1ab, spike (S), envelope (E), membrane (M), nucleocapsid (N)−3′. However, variable numbers of additional ORFs are present in between spike and nucleocapsid genes in different strains of coronaviruses. The transcription regulatory motif (TRS) is present at the 3′ end of the genome, which plays an important role in RNA replication and recombination (Lai et al. 1985).
Figure 2.
Schematic presentation of coronavirus genome orientation. SARS-coronavirus (SARS-CoV), MERS-coronavirus (MERS-CoV) and novel coronavirus (SARS-CoV-2).
The complete genome size of the SARS-CoV-2 is approximately 29 kb (29825 nt-29903nt).The ORF1ab gene is the largest gene segment of the coronavirus and it constitutes two ORF i.e. ORF1a and ORF1b. The position of the ORF1ab gene in SARS-CoV-2 (251-21541 nt) slightly changes to starting codon position as compared to SARS-CoV (265-21486 nt) and MERS-CoV (279-21514). Generally, a slippery sequence (UUUAAAC) followed by a putative pseudoknot structure sequence is present in between the ORFs. The replicase ORF1ab is cleaved by papain-like protease (PLpro) and 3 C-like protease (3CLpro). The ORF1ab gene of the coronavirus genome encodes15–16 non-structural proteins (nsp) at the consensus cleavage site. The nsp 12 and nsp 13 encode the RNA-dependent RNA polymerase and helicase protein respectively. The newly emerging SARS-CoV-2 possesses a single point mutation at a slippery sequence. A haemagglutinin esterase (HE) gene is present downstream to ORF1ab and upstream to the S gene which is absent in coronaviruses infecting humans.
Three surface glycoproteins are found in all classes of coronaviruses i.e. spike (S), envelope (E) and membrane (M). The S proteins are type I membrane glycoproteins responsible for the formation of “spikes”, present on the surface of coronaviruses. The S proteins are cleaved into the receptor binding S1 domain, and cell membrane fusion S2 domains. The SARS-CoV-2 S2 domain represents higher identity with bat derived coronavirus strains than the S1 domain. Therefore, the receptor-binding domain of SARS-CoV-2 is more similar to that of SARS-CoV 2002-03 and it has found that it uses angiotensin-converting enzyme 2 (ACE2) as cellular receptor (Lu et al. 2020). However, some key amino acid substitution (at positions 439, 501, 493, 485 and 486) were observed in the SARS-CoV-2 receptor-binding domain those were thought to be important in SARS-CoV (Lu et al. 2020). The E and M genes are conserved among all the coronaviruses. These two genes encode two small transmembrane proteins associated with the envelope of all coronaviruses.
The coronavirus nucleocapsid (N) protein is a virion structural protein. The N protein interacts with the viral genomic RNA and helps in packaging of RNA genome into virus particles by recognising a specific sequence. The N protein-dependent assembly of the viral RNA packaging signal is already established in SARS-CoV. Intracellular co-localization of N with replicase components is required for RNA synthesis. Variable numbers of small ORFs are present between the various conserved genes downstream to the N gene. The 3a protein of SARS-CoV modulates virus release by forming a transmembrane homotetramer complex within channel protein. Other than the virus release, the 3a protein helps in SARS-CoV-induced cell death, Golgi fragmentation, and the accumulation of intracellular vesicles. Like the SARS-CoV and MERS-CoV the SARS-CoV-2 virus possesses several other small ORFs (ORF9, ORF13, ORF14, ORF10) at the downstream to N gene (Marra et al. 2003). The function of the N gene and small ORFs of SARS-CoV-2 is not yet known.
Phylogenetic analysis
Before the emergence of SARS-CoV in 2002, construction of phylogenetic tree for coronaviruses based on the Pol or N gene was a standard practice. Using this method, initially, SARS-CoV was proposed as a member of gammacoronavirus (Marra et al. 2003; Rota et al. 2003). However, further analysis of the amino-terminal domain of the spike protein of the SARS-CoV revealed that 19 out of the 20 cysteine residues was spatially conserved within the betacoronavirus group (Rota et al. 2003). On the other hand, only five residues were found conserved within the alphacoronavirus and gammacoronavirus group (Rota et al. 2003). Furthermore, the subsequent whole genome-based phylogenetic analysis concluded that SARS-CoV is a member of the betacoronavirus lineage. Phylogenetic analysis based on RdRP gene of SARS-CoV-2 revealed that it belongs to the genus betacoronavirus (Eickmann 2003).
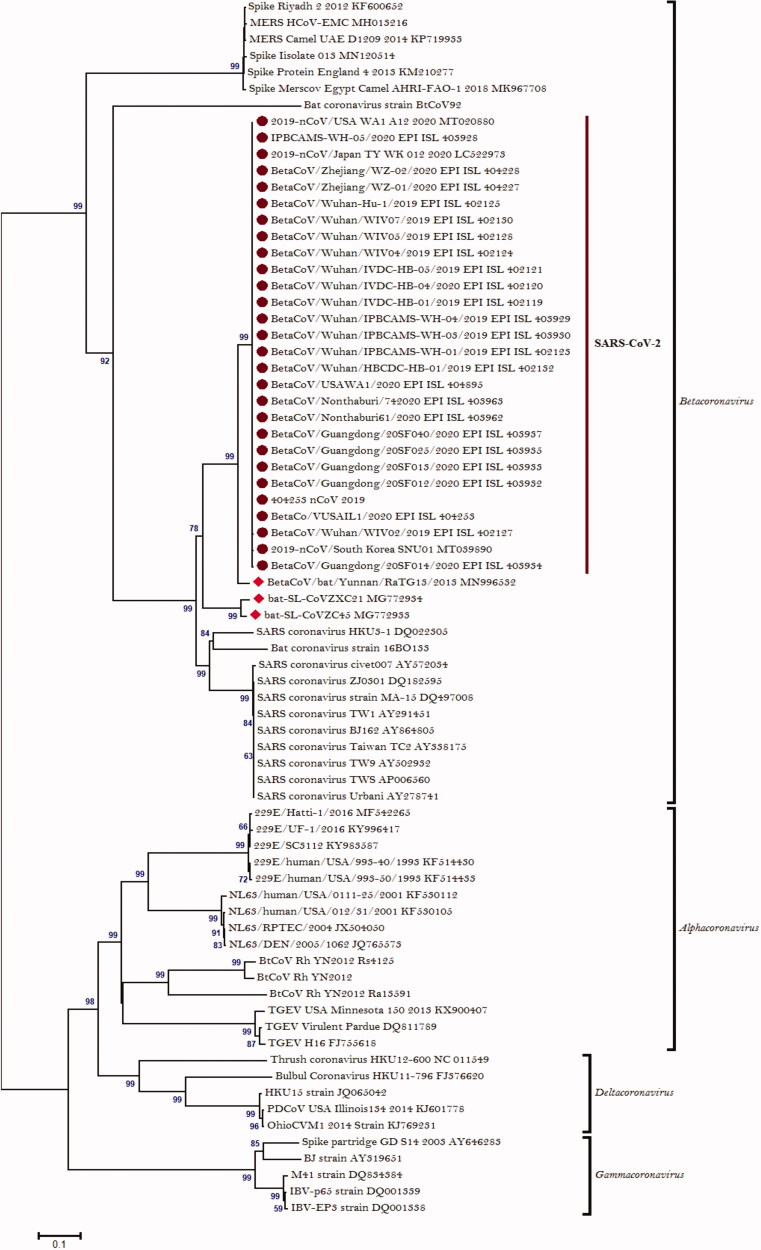
We have constructed a phylogenetic tree of the SARS-CoV-2 based on available sequences in GenBank and GISAID data bank (Figure 3). Phylogenetic analysis based on the spike gene of the SARS-CoV-2 represents that it belongs to the genus betacoronaviruses. From the analysis of the sequences published since the emergence of SARS-CoV-2, it is evident that this virus is stably spreading without any noticeable mutation as they are clustered in a single group. Analysis from the phylogenetic tree represents that all the SARS-CoV-2 strains are distinctly separated from previously reported coronavirus endemic strains; SARS-CoV and MERS-CoV. Hence, the SARS-CoV-2 is termed as “novel” coronavirus. Analysis performed in our study also revealed that this virus is very similar to the bat derived coronavirus strain. The SARS-CoV-2 and bat derived Yunnan/RaTG13/2013 coronavirus strain derived from common ancestor, although they differs in in the size of the spike genes (spike gene size in RaTG13:3809 bp; SARS-CoV-2:3822bp). Since SARS-CoV and MERS-CoV also derived from the bat, bat originated human infecting coronavirus strain is not a novel event. As shown in earlier studies that SARS-CoV and MERS-CoV used civets and camel, respectively, as an intermediate host to gain infection potentiality towards human (Guan et al. 2003; Alagaili et al. 2014). It may be predicted that in case of SARS-CoV-2 transmission there may be one or more intermediate host present in between bats and human.
Figure 3.
Phylogenetic analysis of coronaviruses. Phylogenetic tree constructed based on the spike gene using MEGA 6 software with the neighbor-joining method and 1000 bootstrap values. Novel coronavirus, SARS-CoV-2 symbolises as a solid circle and bat-derived coronaviruses symbolises as a solid hexagonal shape.
Immunopathogenesis of SARS-CoV-2 infection
Previous cases of human coronavirus infection commenced by SARS-CoV and MERS-CoV was accountable for severe pneumonia leading to high mortality. The mechanistic explanation of their high morbidity and mortality is inadequately explained. Rapid virus replication reaching to high titres and associated enhanced inflammation is believed to be a cause of disease severity. Initial data from China shows that the SARS-CoV-2 produced observable symptoms, primarily ARDS (Acute Respiratory Distress Syndrome) in elderly populations similar to human cases of H7N9 infection in China (Table 1). An earlier study with H7N9 infection also found the clinical exhibition of disease with high fever and rapidly progressive pneumonia that did not respond to antibiotics (Chen et al. 2013). A recent study on SARS-CoV-2 infection showed that all the 41 patients had pneumonia and manifestations of a critical respiratory ailment similar to severe acute respiratory syndrome (SARS) coronavirus and was associated with ICU admission and high mortality (Huang et al. 2020).
Table 1.
The Comparison between Corona virus infection and Influenza Infection.
| Virus | Origin | Receptor | Lineage | Genome | Clinical manifestation | Morbidity and mortality | Incubation period | CFR (Approx.) | R0 | Susceptible population | Antiviral and treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| pdmH1N1 2009 |
Mexico, U.S | SA α − 2, 6 | Influenza A | Segmented negative sense RNA | Upper respiratory tract infection, Secondary bacterial pneumonia | 89.3 million cases & 18,436 deaths* | 4–6 days | <1% | 1.2 − 1.6 | Children & young adults without underlying diseases | Neuramindase inhibitor (oseltamivir and zanamivir) |
| H5N1 | Hong Kong, Europe, Africa, Southeast Asia | SA α − 2, 3 | Influenza A | Segmented negative sense RNA | Viral pneumonia, ARDS, Extra pulmonary symptoms: renal failure, multiple organ failure, CNS involvement, etc | 861 cases & 455 deaths globally** | 2–5 days | 53% | 0.05–0.98 | People in close contact with live poultry or contaminated environment | Oseltamivir and zanamivir |
| H7N9 | China, Taiwan | SA α − 2, 3 and SA α − 2,6 | Influenza A | Segmented negative sense RNA | Viral replication in upper & lower airways, virulent in the lower | 1567cases & 615 deaths*** | 1–10 days | 39% | 0.03-0.4 | Elderly male with substantial historical poultry exposure | Oseltamivir and zanamivir |
| SARS-CoV | Fu Shan city, China | ACE2 | Beta-Corona virus | Linear positive sense RNA | Fever, pneumonia Shortness of breath, Coughing | 8000 and 774 | 3 − 10 days | 10% | 3 | had direct, close contact with someone who’s infected with SARS | Rivaverin, Cortico sterioids and supportive |
| MERS-CoV | Qater | DPP4 | Beta-Corona virus | Linear positive sense RNA | Fever, Shortness of breath rapidly progressive pneumonitis, respiratory failure, septic shock and multi-organ failure resulting in death | 2 494 and 858 | 2–15 days | 35% | 1< | people close contact with camels and infected human, 50 years, male predominance | Supportive, Corticosteroids |
| SARS-CoV-2 (nCoV-2019) | Wuhan, Hubei province, China | ACE2 and Unknown? | Beta-Corona virus | Linear positive sense RNA | Fever, Coughing, Shortness of breath, Pneumonia, ARDS | Infected 85,406, Died 2,924 and Recovered 39,561**** | 2–14 days | 2.50% | 2.68 | had direct, close contact with someone who’s infected with SARS-CoV-2 | Supportive, Corticosteroids |
*https://www.cdc.gov/flu/pandemic-resources/2009-h1n1-pandemic.html. **https://www.who.int/influenza/human_animal_interface/H5N1_cumulative_table_archives/en/. ***https://www.who.int/csr/don/05-september-2018-ah7n9-china/en/. ****As of February 29 2020. The significance of shading is to differentiate two different families of viruses (Orthomyxoviridae and Coronaviridae).
It is known that coronaviruses such as human SARS-CoV and, bat SARS-like CoV SL-CoVZXC21 utilizes Angiotensin-converting enzyme 2 (ACE2), as their receptor and recent reports suggest that SARS-CoV-2 also uses the identical receptor for entry into the host cell (Zhou et al. 2020). ACE2 is an essential enzyme in the renin-angiotensin system (RAS) that plays a significant role in regulating blood pressure and maintaining electrolyte and fluid homeostasis. ACE2 also protects an individual against severe acute lung damage that can be triggered by sepsis, acid aspiration, severe acute respiratory syndrome (SARS) and the lethal avian influenza A H5N1 and H7N9 virus infection (Kuba et al. 2005; Yang et al. 2014; Zou et al. 2014). Moreover, recent studies have shown that ACE2 negatively regulates inflammatory responses during bacterial or viral infections (Yang et al. 2014; Sodhi et al. 2019). SARS-CoV-2 infection utilizes ACE2 for viral attachment and subsequent entry into cytosol, therefore rapid replication of SARS-CoV-2 may reduce the surface expression of ACE2 in the lung tissue which may cause further intensification of the inflammation and severity of the disease pathology (Glowacka et al. 2010) (Figure 4). A recent study have found that smoking is associated with higher expression of ACE2 when compared with the non-smokers, which may be one of the reasons of some population for the vulnerability towards novel SARS-CoV-2 infection (Liu et al. 2017; Cai 2020).
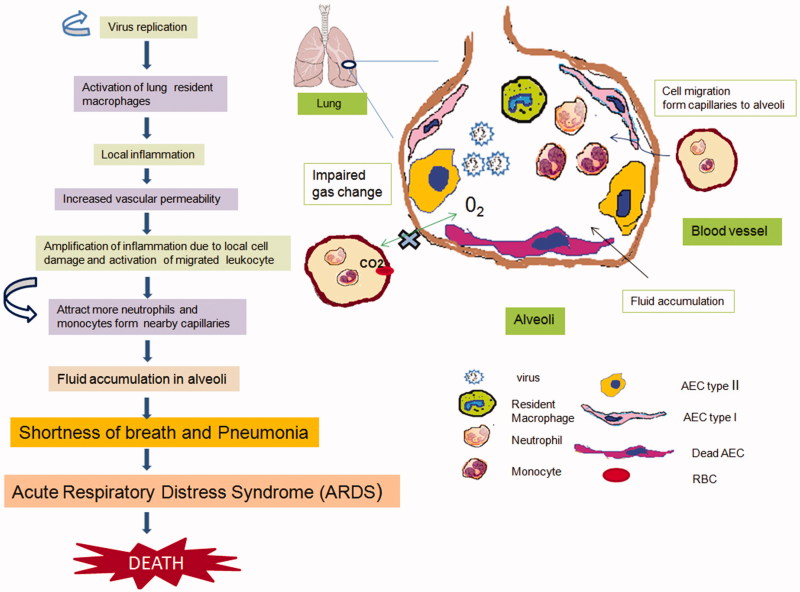
Figure 4.
Immunopathogenesis of Coronavirus infection. Robust viral replication in the lung causes activation of alveolar macrophages and epithelial cell damages which, results in the induction of inflammatory cytokines and release upon activation through innate immune receptors. Cyclic amplification of inflammatory responses lead to pneumonia and hypoxia as depicted in figure. Alveolar Epithelial Cells (AEC); Red Blood Cells (RBCs).
The pathophysiology of disease outcome depends on the extent of inflammatory responses within the host. In absence of any specific antiviral drugs, the primary therapeutic strategy to treat SARS-CoV-2 infection should be managing the inflammatory responses with anti-inflammatory drugs such as corticosteroids. Previous studies on SARS patients have found a positive outcome when treated with corticosteroids (Chen et al. 2006). WHO recommendations for managing this novel coronavirus (SARS-CoV-2) infection discourage the application of corticosteroids. The explanation for not using corticosteroids could be delayed viral clearance from the respiratory tract or blood with other corticosteroid-induced complications in the treated patients previously infected with SARS and MERS (Lee et al. 2004; Arabi et al. 2018; Russel et al. 2020). During the pandemic in 2009 of influenza A infection, a prospective cohort study showed reduced mortality in patients with (H1N1) pdm09 viral pneumonia when treated with mild doses of corticosteroid (Li et al. 2017). Therefore these disparities in findings suggest that randomized controlled trials must be carried out to conclude the beneficial effect of corticosteroid medication in coronavirus-induced inflammation associated difficulties. However, doctors from the Chinese Thoracic Society have recommended the application of corticosteroids to critically ill patients with SARS-CoV-2 pneumonia with a low to moderate dose (≤0.5–1 mg/kg per day methylprednisolone or equivalent) for short term (<7 days) (Zhao et al. 2020).
Factors influencing endemicity of novel corona virus
There are several attributes of any infection caused by an organism for persistence presence in circulation that must fulfil some basics criteria. SARS-CoV-2 is no longer an exception if it able to cause endemic disease. The criteria for endiminicity are as follows.
Virus must have low CFR, has the ability to cause mild infection and asymptomatic moderate incubation period within its host.
Virus must be contagious (R0 > 2)
Virus must have steady mutation rate to avoid pre-existing immunity.
Virus must have reservoir other than its primary host or it must have latent phase in its primary host.
Current findings suggest that SARS-CoV-2 has very lower CFR when compared with SARS-CoV, MERS-CoV or influenza A H5N1 infection in human and the virus spread by a German individual and a Chinese woman before the onset of the symptoms, or disease allow us to believe fulfilling of the first criteria for periodic infection (Rothe et al. 2020). Although later it was found that they have mild symptoms like fever. WHO predicted the R0 measure for SARS-CoV-2 remain 1.4–2.5 although recent estimates suggest R0 of 2.2 indicating their contagious potential (Li et al. 2020).
Role of host immunity in viral selection
Viruses face potent challenges from the host pre-existing immunity before they establish periodical infection. The role of pre-existing immunity broadly studied for influenza virus infection (Biswas et al 2020). Host immunity also allows the selection of mutant viruses to cause a new round of infection within the host with pre-existing immunity towards the earlier strains. If the SARS-CoV-2 can withstand the new mutations and remains flexible to infect its host towards disease progression, it will persist in circulation. The primary wave of SARS-CoV-2 infection is under progress at present. The majority of the exposed population will acquire some immunity eventually and the real hurdles for the viruses to defeat or bypass existing immunity will reflect with the acquired mutations within the virus in future. To defeat host immunity, viruses must mutate without losing their infectivity. So far there are no published data regarding infection ability of SARS-CoV-2 and their mutation potential under robust immunological selection pressure within the host. Previous data from SARS-CoV and MERS-CoV infected individuals indicated that antibody response tends to be short-lived but remain longer depending on the severity of the infection. T cell responses often target highly conserved internal proteins and are long-lived (Channappanavar et al. 2014; Alshukairi et al. 2016). SARS-CoV-specific memory T cells but not memory B cells or antibody could be detected six years after infection in SARS survivors (Tang et al 2011). In conclusion, these studies present some ideas regarding selection pressure from the host immunity, i.e. milder coronavirus infection may produce a short-lived humoral immune response. As of February 29 2020, SARS-CoV-2 infected 85,406 people and the infection turns into fatality for more than 2,924 cases. However, as per the available data, 39,561 individual got recovered after infection. Percent recovery from this population indicates that ∼ 93% of the population already recovered from SARS-CoV2 infection. This percentage was calculated as below within infected population: (total no recovered/total no of recovered + total no of death). It can be predicted that at least 93% of the total population, which may likely to go higher with the end of this outbreak, will develop immunity towards this novel virus. So far there is no vaccine available, although in future vaccinated individual will also acquire some immunity. The possibility of the future outbreak by SARS-CoV-2 will depend on the longevity of host immunity and bypassing of existing or pre-existing immunity by the mutant virus (Figure 5).
Figure 5.
Viral selection during an outbreak. Host induces immune response when infected by the virus, which can protect the individual from the same virus but not from the mutated one. Reservoir host allows the virus to replicate and mutate, leading to an expansion in viral diversity with altered antigenicity within the viral pool. The antigenically mutated virus can be selected when it infects a host with pre-existing immunity towards the earlier strain. This will allow the selection of mutated viruses within a host and dissemination if the mutant virus remains contagious.
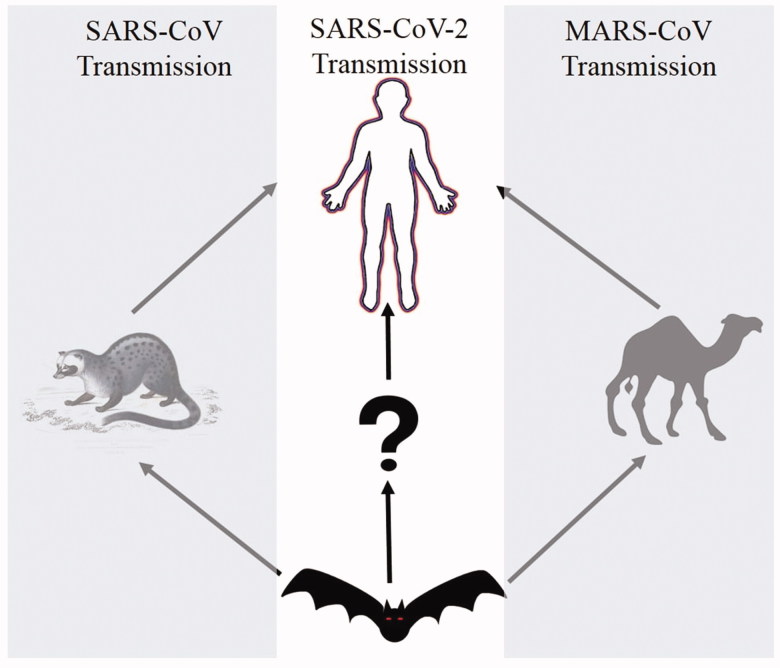
Reservoir for SARS-CoV-2
The transmission dynamics of infectious diseases critically depend on reservoir hosts, which can sustain the pathogen with a moderate or asymptomatic infection in the population, even in the presence of resistance from primary hosts. Coronaviruses are found widely within bat, pig, cow and several other species. Previous reports also suggest that this virus is also presents in birds. Molecular phylogenetic analysis performed in this study showed that neighbouring virus to SARS-CoV-2 was from bats with 96% similarity suggesting novelty of SARS-CoV-2. We don’t know how this virus mutated and its time frame. However, recent estimates with Bedford’s analysis predicted that SARS-CoV-2 and RaTG13 derived from a common ancestor sometimes between 25 and 65 years ago (https://www.sciencemag.org/news/2020/01/mining-coronavirus-genomes-clues-outbreak-s-origins). These observations are indicative of the existence of intermediate host or reservoir before the SARS-CoV-2 gained entry into the human population (Figure 4). It is utmost important to find out its intermediate host to control the ongoing and future outbreak.
Based on the gene bank data, apart from the lung and oropharyngeal fluid this virus also isolated from faces and blood. This observation represents that SARS-CoV-2 transmitted through droplet and human to human, might be through the orofecal route but immune-pathogenesis of this novel virus is probably different from the previous endemic strain of coronaviruses. The faecal oral route is very common in birds for transmission of avian viruses. Migratory birds play a very important role in the dissemination of avian influenza viruses from different parts of the globe during seasonal migrations (Bailey et al. 2018). Coronaviruses such as human SARS-CoV and bat SARS-like CoV SL-CoVZXC21 uses angiotensin-converting enzyme 2 (ACE2) as their receptor and recent reports suggest that SARS-CoV-2 also uses the same receptor for entry into the host cell. We have compared the ACE2 gene from avian species and the data suggest conservation of amino acids of ACE2 protein within most of the birds (data not shown). We examined the interacting residues of the receptor-binding domain (RBD) of the spike protein of SARS-CoV-2 with critical residues of ACE2 from different species (from human, poultry; Gallus gallus, Mallard; Anasplatyrhynchos, swan goose; Ansercygnoidesdomesticus, shelduck; Tadornacana) and we found limited amino acids conservation in comparison to human counterpart (Table 2). Moreover, there is no experimental proof for betacoronavirus (like SARS-CoV and SARS-CoV-2) infection in the birds; although a recent study has shown the significant presence of alpha and gammacoronaviruses in ducks, shorebirds, and herons in Australia (Chamings et al. 2018). These observations allow us to believe that the birds are less prone to betacoronavirus infection or the least chance of bird infection from betacoronaviruses. Significantly, the bird poses alpha and gammacoronaviruses that are incompetent to infect a human. Hence, the risks of spread of the SARS-CoV-2 through bird to human are very unlikely at its present form. Qinghai Lake in China is one of the biggest breeding sites for different migratory bird species. There may be a possibility that this novel virus may acquire the ability to infect other animals including the migratory birds. Therefore, it is extremely important to find out the reservoir of this novel coronavirus within the other animals including the migratory birds, if there are any (Figure 6).
Table 2.
Key interacting residues between RBD of spike protein of different corona viruses and host receptor ACE2 of human, bat, mouse civet and different avian species (modified from Dong et al. 2020).
| 41 | 42 | 45 | 79 | 82 | 83 | 90 | 325 | 329 | 353 | Amino Acid (aa) position relative to human ACE2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Y | E | L | N | R | F | D | E | T | K | Gallas ACE2 |
| Y | E | L | N | N | F | D | E | K | K | Anas ACE2 |
| Y | E | L | N | N | F | D | E | K | K | Tadorna ACE2 |
| Y | E | L | N | S | F | D | E | K | K | Anser ACE2 |
| Y | Q | V | L | T | Y | D | Q | E | K | Civet ACE2 |
| Y | Q | L | T | S | F | T | Q | A | H | Mouse ACE2 |
| Y | Q | L | I | N | F | N | P | T | H | Rat ACE2 |
| H | E | L | L | T | Y | N | P | N | K | Bat ACE2 |
| Y | Q | L | L | M | Y | N | Q | E | K | Human ACE2 |
| Y484 | Y436 | Y484 | L472 | L472 | Y475 | T402 | R426 | R426 | Y491 | Human SARS |
| Y485 | Y437 | Y485 | F473 | F473 | Y476 | T403 | R427 | R427 | Y492 | BatSARS-likeCoV-WIV1 |
| Q498 | Y449 | Q498 | F486 | F486 | Y489 | T415 | N439 | N439 | Y455 | SARS-CoV-2 |
| N474 | G439 | N474 | E462 | E462 | V465 | T410 | A434 | A434 | Y481 | Bat SARS-like CoV SL-CoVZXC21 |
| N473 | G435 | N471 | G459 | G459 | V462 | T406 | A430 | A430 | Y481 | Bat SARSr-CoV HKU3-1 |
The table is shaded to make its representation more attractive.
Figure 6.
Transmission pattern of SARS-coronavirus (SARS-CoV), MERS-coronavirus (MERS-CoV and novel coronavirus, SARS-CoV-2.
Conclusions
One of the most worrying things about any outbreak is the spreading of viruses from mild or asymptomatic individuals, which is probably happening in case of SARS-CoV-2 infection. If this trend continues then the virus may establish itself in the population and quarantine will be very difficult to stop the virus spread. Phylogenetic analysis based on the spike gene nucleotide sequence performed in this study has shown that all the SARS-CoV-2 sequences are forming a single cluster without any branching indicating SARS-CoV-2 conservation throughout this ongoing outbreak. Earlier studies have shown that spike protein was a target of a robust humoral immune response in case of SARS or MERS viral infection. These findings advocate that virus that caused the initial outbreak of SARS-CoV-2 is still in circulation without any major changes. Very little or absence of pre-existing immunity in the community or selection pressure within the population probably allowed SARS-CoV-2 to remain in circulation in its original form. Moreover, SARS-CoV-2 has very limited CFR as opposed to SARS-CoV and MERS-CoV without killing its host (Table 1), will able to produce new virus particles alongside some mutations. Till now most of the deaths have occurred in the elderly population due to novel coronavirus infection whereas, children and young adults exhibit relatively milder disease. Therefore, SARS-CoV-2 infection will enable the host to develop humoral and cell-mediated immunity and simultaneously the immune selected viruses may acquire mutations with higher infection potential or altered antigenicity. This will likely empower them to evade pre-existing immunity (towards their predecessor counterpart) in the host during the subsequent waves of infection (as depicted in Figure 1). Current estimates based on the available information, suggest that more than ∼93% individual have recovered from this novel coronavirus infections. The virus may adapt itself to produce infection within the recovered population in future to sustain its presence in the environment. “The ability of the virus to cause infection in the host with pre-existing immunity” will be the most important criteria to establish itself in the circulation. The recovered population may keep under monitor to find if any reinfection leads to the creation of any mutant strain due to the selection pressure. Therefore, we must prepare ourselves for mutant strains of this novel virus to avoid future devastation.
Since there is no antiviral and vaccine to fight against SARS CoV-2, a vaccine or therapeutics is a primary need at this moment. Currently, several companies have started preparing vaccines for this novel virus although efficacy will depend on the selection of antigen and the virus’s ability to bypass the vaccine-induced immunity. We must look at the conservation among different epitopes in immune selected strains while choosing the right vaccine candidates. Structurally spike protein carries RBD like Haemagglutinin (HA) of the Influenza virus with a similar function. We must look at the amino acid conservation in different domain of the spike protein. Mutation in the immune dominance region of the vaccine candidate will make them ineffective for future outbreaks. Recently, physicians from China are urging recovered patients to donate plasma which may be useful to protect COVID-19, something like a 100 years old practice during 1918 flu. In case of pandemic 1918 influenza infection, three waves of infection killed more than 50 million worldwide in two years, where the second wave was the most devastating. The first wave of the 1918 flu pandemic flu had resembled common flu epidemics; people most at risk were the weak and elderly, while younger, healthier people recovered easily apparently resembling COVID-19 in its present form. Most of the studies have found an analogy between COVID-19 and Influenza disease pathophysiology. Time will tell us whether SARS-CoV-2 and associated COVID-19 will be established as the new flu of 21st century.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Alagaili AN, Briese T, Mishra N, Kapoor V, Sameroff SC, de Wit E, Munster VJ, Hensley LE, Zalmout IS, Kapoor A, et al. . 2014. Middle east respiratory syndrome coronavirus infection in dromedary camels in Saudi Arabia. MBio. 5(4):e01482–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshukairi AN, Khalid I, Ahmed WA, Dada AM, Bayumi DT, Malic LS, Althawadi S, Ignacio K, Alsalmi HS, Al-Abdely HM, et al. . 2016. Antibody response and disease severity in healthcare worker MERS survivors. Emerg Infect Dis. 22(6):1113–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabi YM, Mandourah Y, Al-Hameed F, Sindi AA, Almekhlafi GA, Hussein MA, Jose J, Pinto R, Al-Omari A, Kharaba A, et al. . 2018. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med. 197(6):757–767. [DOI] [PubMed] [Google Scholar]
- Bailey ES, Fieldhouse JK, Choi JY, Gray GC. 2018. A Mini Review of the Zoonotic Threat Potential of Influenza Viruses, Coronaviruses, Adenoviruses, and Enteroviruses. Front. Public Heal. 6:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas A, Chakrabarti AK, Dutta S. 2020. Current. challenges: from the path of “original antigenic sin” towards the development of universal flu vaccines: flu vaccine efficacy encounters significant hurdles from pre-existing immunity of the host suggesting assessment of host immunity before vaccination. Int. Rev. Immunol. 39:21–36. [DOI] [PubMed] [Google Scholar]
- Cai G. 2020. Tobacco-Use Disparity in Gene Expression of ACE2, the Receptor of 2019-nCov. Preprints. DOI: 10.20944/preprints202002.0051.v1 [DOI] [Google Scholar]
- Chamings A, Nelson TM, Vibin J, Wille M, Klaassen M, Alexandersen S. 2018. Detection and characterisation of coronaviruses in migratory and non-migratory Australian wild birds. Sci. Rep. 8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R, Zhao J, Perlman S. 2014. T cell-mediated immune response to respiratory coronaviruses. Immunol. Res. 59:118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Liang W, Yang S, Wu N, Gao H, Sheng J, Yao H, Wo J, Fang Q, Cui D, et al. . 2013. Human infections with the emerging avian influenza A H7N9 virus from wet market poultry: clinical analysis and characterisation of viral genome. Lancet. 381(9881):1916–1925.,. (13)60903-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RC, Tang XP, Tan SY, Liang BL, Wan ZY, Fang JQ, Zhong N. 2006. Treatment of severe acute respiratory syndrome with glucosteroids: the guangzhou experience. Chest. 129(6):1441–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong N, Yang X, Ye L, Chen K, Chan EW-C, Yang M, Chen S. 2020. Genomic and protein structure modelling analysis depicts the origin and infectivity of 2019-nCoV, a new coronavirus which caused a pneumonia outbreak in Wuhan, China. bioRxiv 2020.01.20.913368.
- Eickmann M. 2003. Phylogeny of the SARS Coronavirus. Science. 302(5650):1504b–11505. [DOI] [PubMed] [Google Scholar]
- Glowacka I, Bertram S, Herzog P, Pfefferle S, Steffen I, Muench MO, Simmons G, Hofmann H, Kuri T, Weber F, et al. . 2010. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J. Virol. 84(2):1198–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Zheng BJ, He YQ, Liu XL, Zhuang ZX, Cheung CL, Luo SW, Li PH, Zhang LJ, Guan YJ, et al. . 2003. Isolation and characterization of viruses related to the SARS coronavirus from animals in Southern China. Science (80-.). 302(5643):276–278. [DOI] [PubMed] [Google Scholar]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. . 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 395(10223):497–506. (20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, et al. . 2005. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 11(8):875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai MM, Baric RS, Makino S, Keck JG, Egbert J, Leibowitz JL, Stohlman SA. 1985. Recombination between nonsegmented RNA genomes of murine coronaviruses. J. Virol. 56(2):449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N, Allen Chan KC, Hui DS, Ng EKO, Wu A, Chiu RWK, Wong VWS, Chan PKS, Wong KT, Wong E, et al. . 2004. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J. Clin. Virol. 31(4):304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz EJ, Dempsey DM, Hendrickson RC, Orton RJ, Siddell SG, Smith DB. 2018. Virus taxonomy: the database of the International Committee on Taxonomy of Viruses (ICTV). Nucleic Acids Res. 46(D1):D708–D717. 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, et al. . 2020. Early transmission dynamics in wuhan, china, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 382(13):1199–1207. DOI: 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Zhang M, Yang L, Li Y, Wang L, Huang Z, Wang L, Chen Z, Zhou M. 2017. Prevalence and patterns of tobacco smoking among Chinese adult men and women: findings of the 2010 national smoking survey. J Epidemiol Community Health . 71(2):154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Yang SG, Gu L, Zhang Y, Yan XX, Liang ZA, Zhang W, Jia HY, Chen W, Liu M, Yu KJ, 2017. Effect of low-to-moderate-dose corticosteroids on mortality of hospitalized adolescents and adults with influenza A(H1N1)pdm09 viral pneumonia. Influenza Other Respi Viruses. 11(4):345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, et al. . 2020. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 6736:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra MA, Jones SJ, Astell CR, Holt RA, Brooks-Wilson A, Butterfield YSN, Khattra J, Asano JK, Barber SA, Chan SY, et al. . 2003. The genome sequence of the SARS-associated coronavirus. Science. 300(5624):1399–1404. [DOI] [PubMed] [Google Scholar]
- Rota P,A, Oberste MS, Monroe SS, Nix WA, Campagnoli R, Icenogle JP, Peñaranda S, Bankamp B, Maher K, Chen M-H, et al. . 2003. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 300(5624):1394–1399. [DOI] [PubMed] [Google Scholar]
- Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, Zimmer T, Thiel V, Janke C, Guggemos W, et al. . 2020. Transmission of 2019-nCoV infection from an asymptomatic contact in germany. N. Engl. J. Med. 382:970–971. DOI: 10.1056/NEJMc2001468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell CD, Millar JE, Baillie JK. 2020. Clinical evidence does not support corticosteroid treatment for SARS-CoV-2 lung injury. Lancet. 395:473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi CP, Nguyen J, Yamaguchi Y, Werts AD, Lu P, Ladd MR, Fulton WB, Kovler ML, Wang S, Prindle T, et al. . 2019. A dynamic variation of pulmonary ACE2 is required to modulate neutrophilic inflammation in response to pseudomonas aeruginosa lung infection in mice. J. Immunol. 203(11):3000–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F, Quan Y, Xin Z-T, Wrammert J, Ma M-J, Lv H, Wang T-B, Yang H, Richardus JH, Liu W, et al. . 2011. Lack of peripheral memory B cell responses in recovered patients with severe acute respiratory syndrome: a six-year follow-up study. J. Immunol. 186(12):7264–7268. [DOI] [PubMed] [Google Scholar]
- Woo PCY, Wang M, Lau SKP, Xu H, Poon RWS, Guo R, Wong BHL, Gao K, Tsoi H-W, Huang Y, et al. . 2007. Comparative analysis of twelve genomes of three novel group 2c and group 2d coronaviruses reveals unique group and subgroup features. J. Virol. 81(4):1574–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JT, Leung K, Leung GM. 2020. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 395(10225):689–697. DOI: 10.1016/S0140-6736(20)30260-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Gu H, Zhao Z, Wang W, Cao B, Lai C, Yang X, Zhang LY, Duan Y, Zhang S, et al. . 2014. Angiotensin-converting enzyme 2 (ACE2) mediates influenza H7N9 virus-induced acute lung injury. Sci Rep. 4:7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JP, Hu Y, Du RH, Chen ZS, Jin Y, Zhou M, Zhang J, Qu JM, Cao B. 2020. [Expert consensus on the use of corticosteroid in patients with 2019-nCoV pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi. 43(0):E007. [DOI] [PubMed] [Google Scholar]
- Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L, et al. . 2020. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 4579(7798):270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z, Yan Y, Shu Y, Gao R, Sun Y, Li X, Ju X, Liang Z, Liu Q, Zhao Y, et al. . 2014. Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nat Commun. 5:3594. [DOI] [PMC free article] [PubMed] [Google Scholar]