Abstract
Background
Gallbladder cancer (GBC) is the most common tumor of the biliary tract. The incidence of GBC shows a large geographic variability, being particularly frequent in Native American populations. In Chile, GBC represents the second cause of cancer-related death among women. We describe here the establishment of three novel cell lines derived from the ascitic fluid of a Chilean GBC patient, who presented 46% European, 36% Mapuche, 12% Aymara and 6% African ancestry.
Results
After immunocytochemical staining of the primary cell culture, we isolated and comprehensively characterized three independent clones (PUC-GBC1, PUC-GBC2 and PUC-GBC3) by short tandem repeat DNA profiling and RNA sequencing as well as karyotype, doubling time, chemosensitivity, in vitro migration capability and in vivo tumorigenicity assay. Primary culture cells showed high expression of CK7, CK19, CA 19-9, MUC1 and MUC16, and negative expression of mesothelial markers. The three isolated clones displayed an epithelial phenotype and an abnormal structure and number of chromosomes. RNA sequencing confirmed the increased expression of cytokeratin and mucin genes, and also of TP53 and ERBB2 with some differences among the three cells lines, and revealed a novel exonic mutation in NF1. The PUC-GBC3 clone was the most aggressive according to histopathological features and the tumorigenic capacity in NSG mice.
Conclusions
The first cell lines established from a Chilean GBC patient represent a new model for studying GBC in patients of Native American descent.
Keywords: Gallbladder cancer, Cancer cell lines, Ascites, Native American ancestry, Gene expression profile
Background
Gallbladder cancer (GBC; ICD-10 diagnosis code C23) accounts for 80–95% of biliary tract cancers and is the sixth most common gastrointestinal cancer worldwide [1, 2]. This aggressive disease is relative rare in most high-income countries, but GBC affects at least 219.420 persons every year worldwide. The geographic areas with the highest mortality rates include Chile, Bolivia, Korea, Nepal, Bangladesh, Japan, Peru, Czech Republic and Slovakia [3]. In Chile, GBC is one of the most common neoplasms and represents the second cause of cancer-related death in women, the mortality rate in 2015 was 10.2 deaths per 100 000 women [4]. The regional distribution of GBC in Chile is correlated with the prevalence of its main risk factor, gallstone disease, together with the regional proportions of Mapuche Native American ancestry and other socioeconomic factors [5, 6].
One of the major characteristics of GBC is its late diagnosis and ineffective treatment [7]. The lack of specific symptoms in early stages and effective diagnostic biomarkers results in most patients being diagnosed with locally advanced or metastatic disease [8, 9]. Unfortunately, the 5-year survival rate is less than 10% for these patients [10].
To improve the clinical prognosis of GBC patients, more efficient methods for early diagnosis and more effective therapeutic approaches must be developed. Research on GBC is challenging not only because it is a rare tumor worldwide, but also due to the difficulty of accessing human tissue samples and the lack of experimental models. Obtaining fresh frozen tissue samples is extremely demanding because most of the early lesions are thin, flat, incidental and masked with gross acute inflammatory changes, and chemotherapy and palliative care is frequently offered to patients with advanced tumor after diagnosis confirmation by fine needle aspiration biopsy [11]. The prophylactic removal of gallbladders with stones hampers the study of the natural history of GBC, and the non-existence of animal models mimicking the progression of pre-neoplastic lesions to invasive cancer translates into the present basic and clinical know-how relying on other types of gastrointestinal cancer.
A large proportion of the current knowledge on GBC biology comes from studies performed using GBC cell lines, which have played a critical role in identifying therapeutic targets and facilitating the rapid screening of new anticancer compounds. Most GBC cell lines derive from primary tumors and metastatic sites and have been isolated from Japanese [12–16], Korean [17] and Chinese [18, 19] patients. Cell lines from GBC patients with Native American ancestry are not available yet.
To our knowledge, this is the first report on the development and characterization of cell lines derived from a GBC patient of Native American descent. We describe the isolation of three cell lines from the ascites of a single patient, which conserved the genomic features and malignant behavior of the primary GBC tumor. Our finding suggest that the three established cell lines reflect the heterogeneity of GBC tumors and provide a useful model to investigate the mechanisms underlying late stage GBC progression and metastasis, gain new insight into drug resistance mechanisms and test new therapeutic strategies.
Results
Establishment of GBC cell lines from one primary cell culture
We successfully established a primary culture of tumor cells derived from the ascitic fluid of a 60-year-old man diagnosed with metastatic GBC, who showed the following percentages of genetic ancestry: 46% European, 36% Mapuche Native American, 12% Aymara Native American and 6% African. Moderate to strong expression (2+ to 3+) of the epithelial markers CK7 (cytokeratin 7) and CK19 (cytokeratin 19) was observed in 100% of the ascites-derived cells, mesothelin was not expressed. Almost 90% of the cells showed high expression levels (3+) of the tumor carbohydrate antigen 19-9 (CA 19-9) and moderate expression (2+) of the mucins MUC1 (mucin 1) and MUC16 (mucin 16) (Fig. 1). Taken together, all these markers indicate an epithelial origin of the primary culture.
Fig. 1.
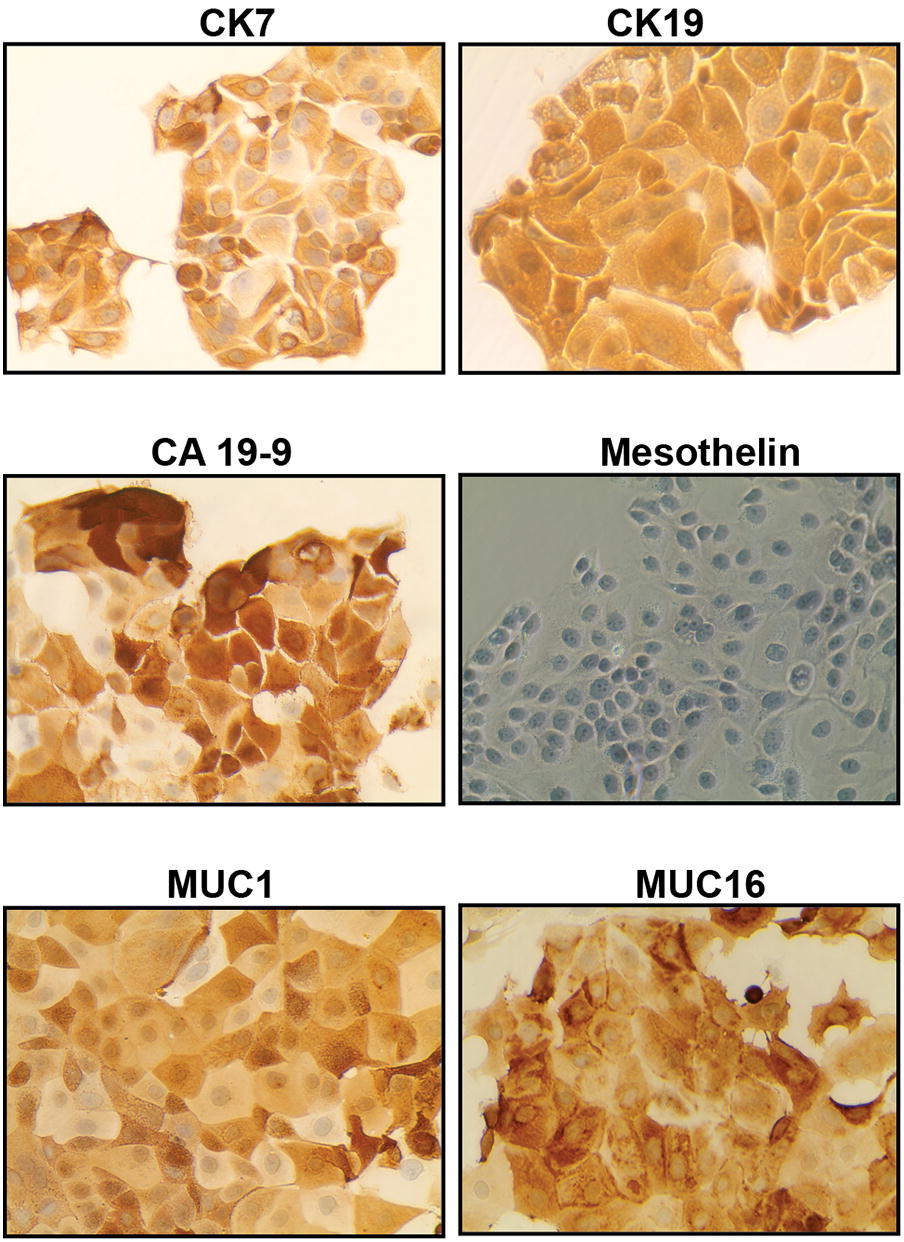
Ascites-derived primary culture has an epithelial phenotype. Representative micrographs showing the expression of characteristic epithelial, mesenchymal and tumor markers in the ascites-derived primary culture. Cells showed strong positive staining for CK7, CK19, CA 19-9, MUC1 and MUC16, whereas mesothelin was absent. All pictures were taken at ×40 magnification
The primary culture consisted of epithelial cells with different morphologies, which grew as heterogeneous cell populations. In order to generate monoclonal cell lines, we used a limiting dilution procedure to isolate and spread individual cells. We established three cell lines as separate cultures, namely PUC-GBC1, PUC-GBC2 and PUC-GBC3. In consistency with the primary culture, the newly established cell lines showed high expression levels of the epithelial markers CK7 and CK19 (3+, 2+) and no mesothelin expression (see Additional file 1). As depicted in Fig. 2, the three cell lines showed positive staining for CA 19-9, MUC1 and MUC16, with moderate to strong (2+ to 3+) intensity in more than 80% of tumor cells, with the exception of CA 19-9, which was only expressed in 20% of PUC-GBC2 cells. We also evaluated the protein expression of the tumor suppressor p53 since its stabilization/activation has been associated with mutations in the TP53 (Tumor Protein P53) gene and poor prognosis of GBC [20–25]. All three cell lines were p53 positive, with a strong brown nuclear staining in 100% of the tumor cells.
Fig. 2.
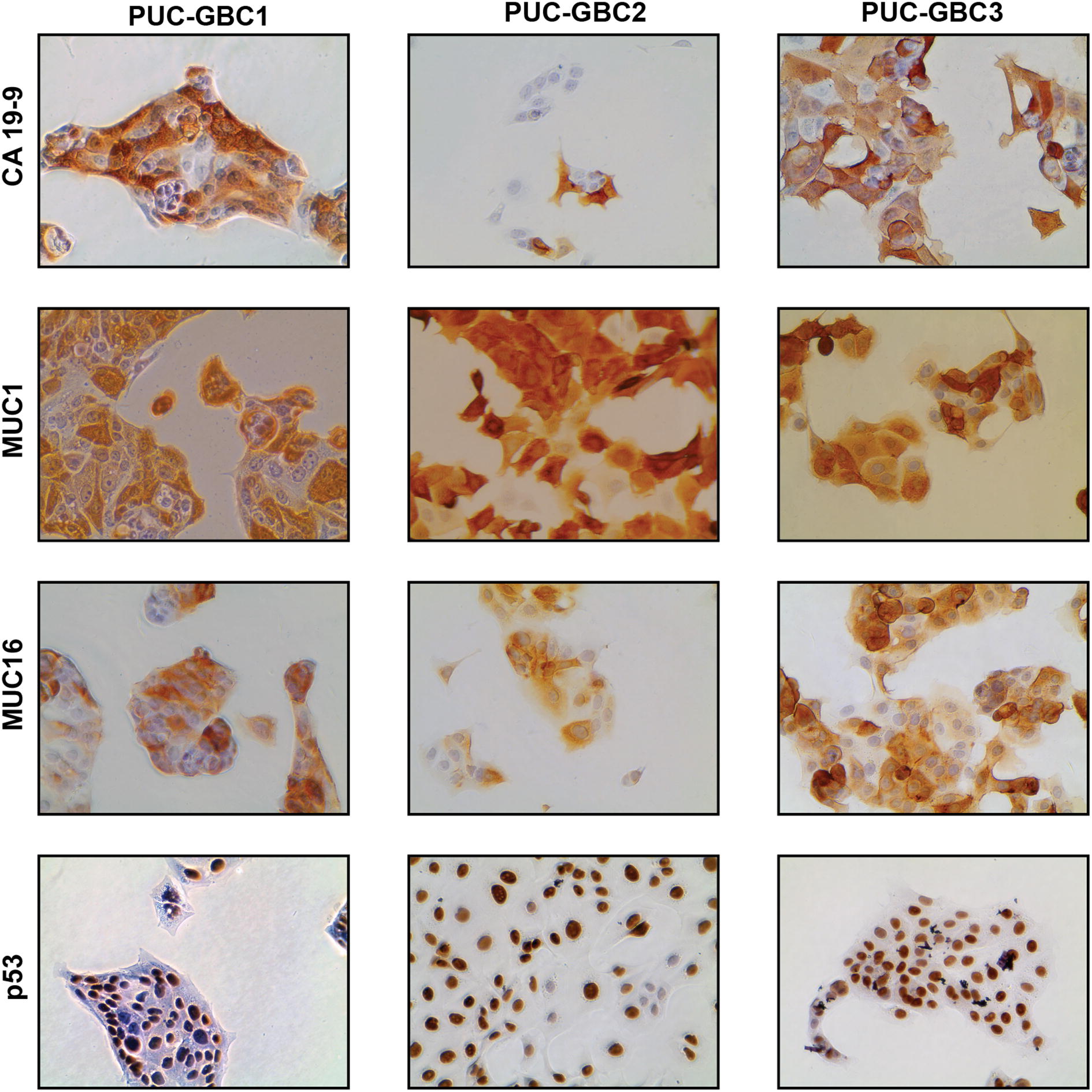
All three cell lines retain the epithelial phenotype of the primary culture. Representative micrographs of immunocytochemical staining for CA 19-9, MUC1, MUC16 and p53 in the three clones isolated from the ascites-derived primary culture. All pictures were taken at ×40 magnification
Short tandem repeat (STR) profiling, chromosome analyses and RNA sequencing
The STR DNA profile analysis revealed very minor discrepancies among the three cell lines: only PUC-GBC3 lost one allele at D7S820 (Table 1).
Table 1.
Short tandem repeat (STR) DNA profiling of ascites-derived gallbladder cancer cell lines
| STR marker | Allelle(s) | ||
|---|---|---|---|
| PUC-GBC1 | PUC-GBC2 | PUC-GBC3 | |
| AMEL | X Y | X Y | X Y |
| CSF1PO | 10 11 | 10 11 | 10 11 |
| D13S317 | 11 12 | 11 12 | 11 12 |
| D16S539 | 10 12 | 10 12 | 10 12 |
| D21S11 | 33 2 | 33 2 | 33 2 |
| D5S818 | 7 11 | 7 11 | 7 11 |
| D7S820 | 7 10 | 7 10 | 10 |
| TH01 | 7 | 7 | 7 |
| TPOX | 8 | 8 | 8 |
| vWA | 16 | 16 | 16 |
The modal chromosome number was in the hyperdiploid range (> 50) for the three cell lines. The karyotype for PUC-GBC1 was: XY,-X, +3, +4, +5, +5, −7, +10, +11, +12, +12, +12, +13, add(13)(q33), +14, +15, +16, +16, −17,+ 19, −21, −21, −22, −22. For PUC-GBC2: XY, +1, +2, +3, +4, +5, +9, +10, +10, +13, +14, +14, add(q31), +19, −20, −22 [10]. And for PUC-GBC3: XY, +2, +4, + 4, der(5), −6, −8, −9, −11, i(12)(q10), add(13)(q34), +14, +15 ps+, +16, −18, −19, −20, −21, −21, −21, −22 (Fig. 3).
Fig. 3.
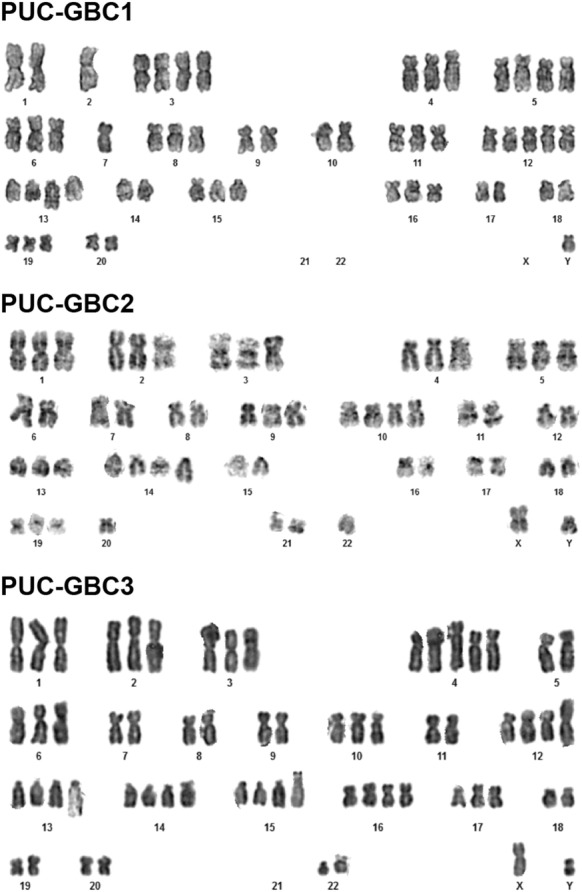
Karyotype analysis of cancer cell lines reveals chromosomal heterogeneity
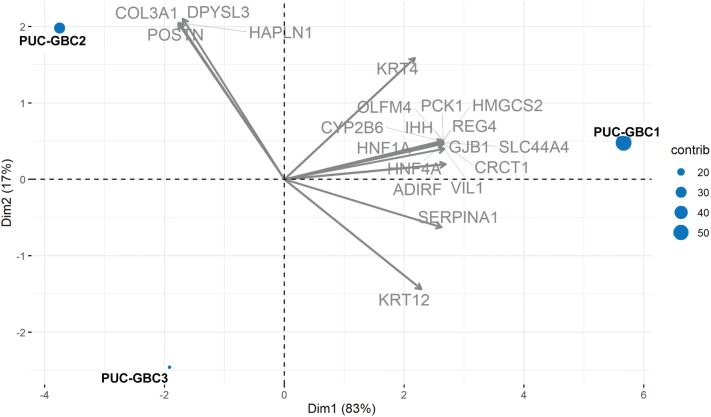
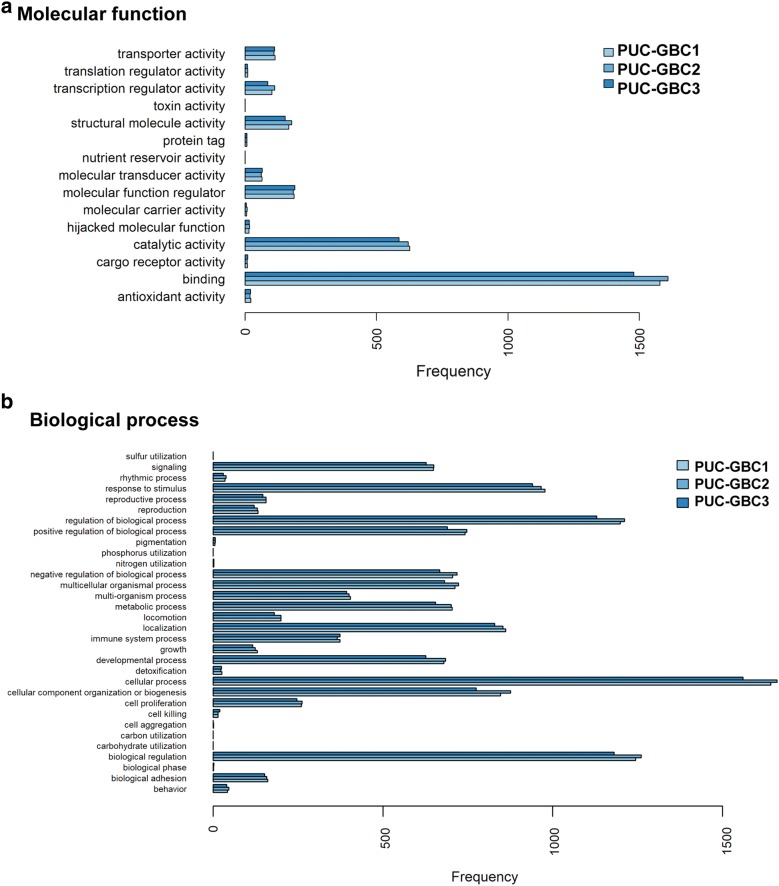
The analysis of RNA sequencing data confirmed the increased expression of cytokeratin (KRT7 and KRT19) and mucin (MUC1 and MUC16) genes, as well as TP53 with some differences in expression among the cell lines. For example, TP53 was weakly expressed in PUC-GBC3. The principal component analysis (PCA) of the three cell lines and the 20 genes with the largest variability in gene expression (highest coefficient of variation) revealed that the expression profiles of PUC-GBC2 and PUC-GBC3 were more similar to each other than to PUC-GBC1 (Fig. 4). Based on the genes with the largest expression variability, PUC-GBC2 showed an enrichment of genes coding for proteins that form the extracellular matrix, such as COL3A1, POSTN and HAPLN1. On the other hand, genes involved in the regulation of cancer stem cell properties (HNF1A, OLFM4, PCK1 and REG4) and cell metabolism (CYP2B6 and SLC44A4) were overrepresented in PUC-GBC1. Additional file 2 provides the expression values for the three newly established GBC cell lines as normalized transcripts per million. We also performed a gene ontology (GO) enrichment analysis, processing genes in terms of their associated molecular function and biological process. The results showed a similar enrichment of GO terms among the three cell lines (Fig. 5). Binding was the largest sub-category in the molecular function category, followed by catalytic activity, while the major biological processes were cellular process, biological regulation, and regulation of biological process.
Fig. 4.
Principal component analysis (PCA) and biplot of the three novel cell lines and the 20 genes with the largest variability in gene expression. Points represent the cell lines, the distance among the points indicates the similarity among the gene expression profiles and arrows show the differentially expressed genes. The proportion of variance explained by each principal component is shown in parentheses
Fig. 5.
Distribution of level 2 gene ontology (GO) terms including biological process and molecular function among all annotated genes
According to the MSK-IMPACT database [26], mutations in at least 10% of primary GBC tumors are expected in ten genes: TP53, ATM, SMAD4, ARID1A, CTNNB1, NF1, NOTCH3, PTPRD, KEAP1 and ARID1B. Among these ten candidate genes, RNA sequencing revealed a novel non-synonymous mutation in NF1 shared by the three GBC cell lines (chr17:31232174, exon25:c.C3299T). According to SIFT (Sorting Intolerance from Tolerance) and Polyphen-2, this mutation has a damaging amino acid impact (p.S1100L). Additional file 3 lists the number of identified exonic mutations and the annotated genetic variants in the three GBC cell lines. Synonymous variants were more common (n = 5923) than non-synonymous (n = 4368) and frameshift (n = 208), with multiple overlapping variants between the different cell lines (Additional file 4). Considering that these cell lines derived from a metastatic site, genomic alterations were expected in multiple genes, many of them involved in relevant oncogenic signaling pathways. These included non-synonymous single-nucleotide variations (nsSNV) in CDKN1A (rs1801270), MDM4 (rs4252716), PIK3CA (rs2230461), ERBB2 (rs1058808) and ERBB3 (rs55699040), a frameshift deletion in CDKN2A (NA), among others (see Additional file 3).
In vitro characterization
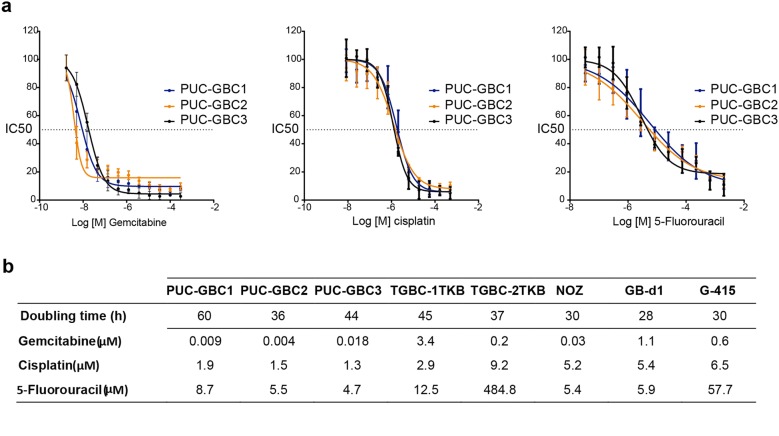
Growth curves were examined for the three cell lines. The population doubling time was 60 h for PUC-GBC1, 36 h for PUC-GBC2 and 44 h for PUC-GBC3. Representative dose–response curves to gemcitabine, cisplatin and fluorouracil (5-FU), along with IC50 values are shown in Fig. 6a. Comparisons with calculated IC50 from five commercially available GBC cell lines are shown in Fig. 6b. Our three newly established cell lines showed higher sensitivity to gemcitabine and cisplatin than the commercially available ones. Sensitivity to 5-FU was similar to that shown by GB-d1 and NOZ.
Fig. 6.
Growth characteristics and chemosensitivity analysis. a Dose–response curves of the ascites-derived gallbladder cancer cell lines treated with chemotherapeutic agents. Cells were incubated for 72 h with each drug as single agent before cell viability was assessed via MTS assay. Data are representative of three independent experiments with three technical replicates (mean ± SD) b Mean doubling times (hours) and half maximal inhibitory concentration (IC50) values of cytotoxic drugs of ascites-derived clones and commercial established GBC cell lines
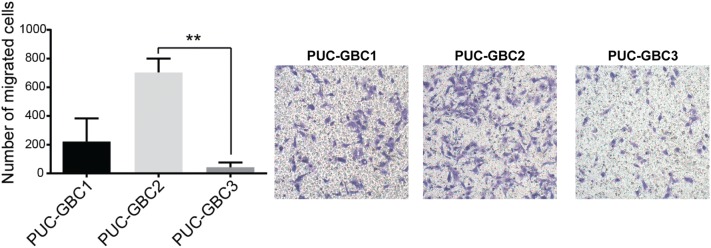
In vitro migration assays showed that the relative migration rate after 24 h was highest for PUC-GBC2 and lowest for PUC-GBC3 (P = 0.0073) (Fig. 7).
Fig. 7.
GBC cell lines have differential migratory capacities. Cells were seeded in the upper side of a Transwell® membrane and, after 6 h, migrated cells were stained with crystal violet and counted. PUC-GBC2 had the strongest migration ability compared to PUC-GBC1 and PUC-GBC3. Results are expressed as mean ± SD of 3 independent experiments (**P = 0.0073 by Kruskal–Wallis with Dunn’s post-test)
Tumorigenic potential
In vivo tumorigenicity assays revealed palpable tumors in all mice within 15 days. As shown in Fig. 8, PUC-GBC3 exhibited the highest growth kinetics, followed by PUC-GBC1, whereas PUC-GBC2 had the lowest tumor-growing potential comparing to PUC-GBC3 (P = 0.0002) and PUC-GBC1 (P = 0.031) at day 38.
Fig. 8.
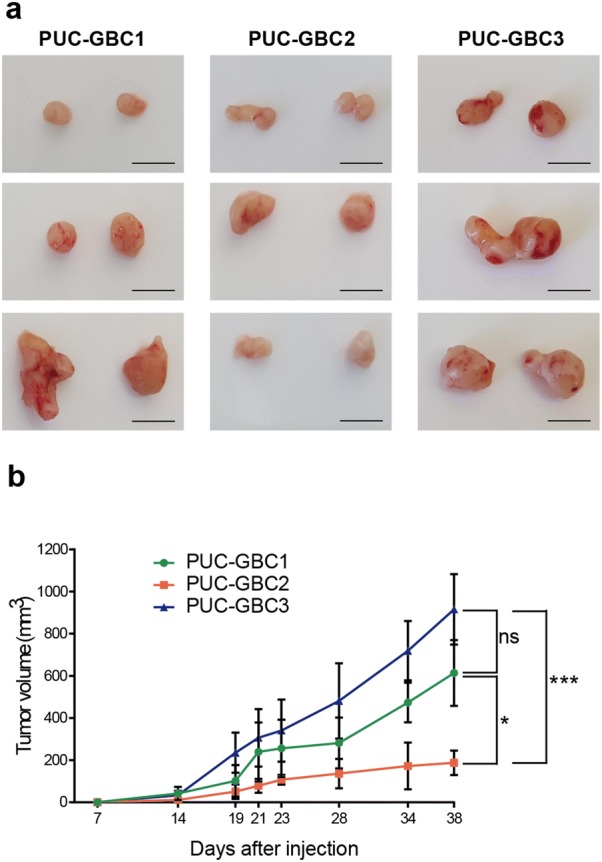
Tumorigenicity of ascites-derived gallbladder cancer cell lines. a Subcutaneous tumors induced by transplantation of the tumor cells (3 × 106 cells) in NSG™ mice (scale bar, 1 cm). b Tumor growth curves of the subcutaneous mouse xenografts. Results are expressed as mean ± SD (n = 3 mice per group, *P = 0.031 and ***P = 0.0002 by Kruskal–Wallis with Dunn’s post-test at day 38)
The hematoxylin- and eosin-stained tissue sections of the mice tumor xenografts showed histological features of adenocarcinoma, with high mitotic index, anisokaryosis and anisocytosis. Particularly, PUC-GBC1 and PUC-GBC2 xenografts were histologically similar and characterized by the presence of tubular structures constituted by cells with enlarged, hyperchromatic nuclei, frequent mitotic activity, abundant apoptotic bodies, and murine stroma dominating the tumor mass. In contrast, PUC-GBC3 tumors had a more aggressive appearance, with a more solid growth and less gland formation; also, isolated signet ring cells and scarce infiltration of the host stroma were noted (Fig. 9).
Fig. 9.
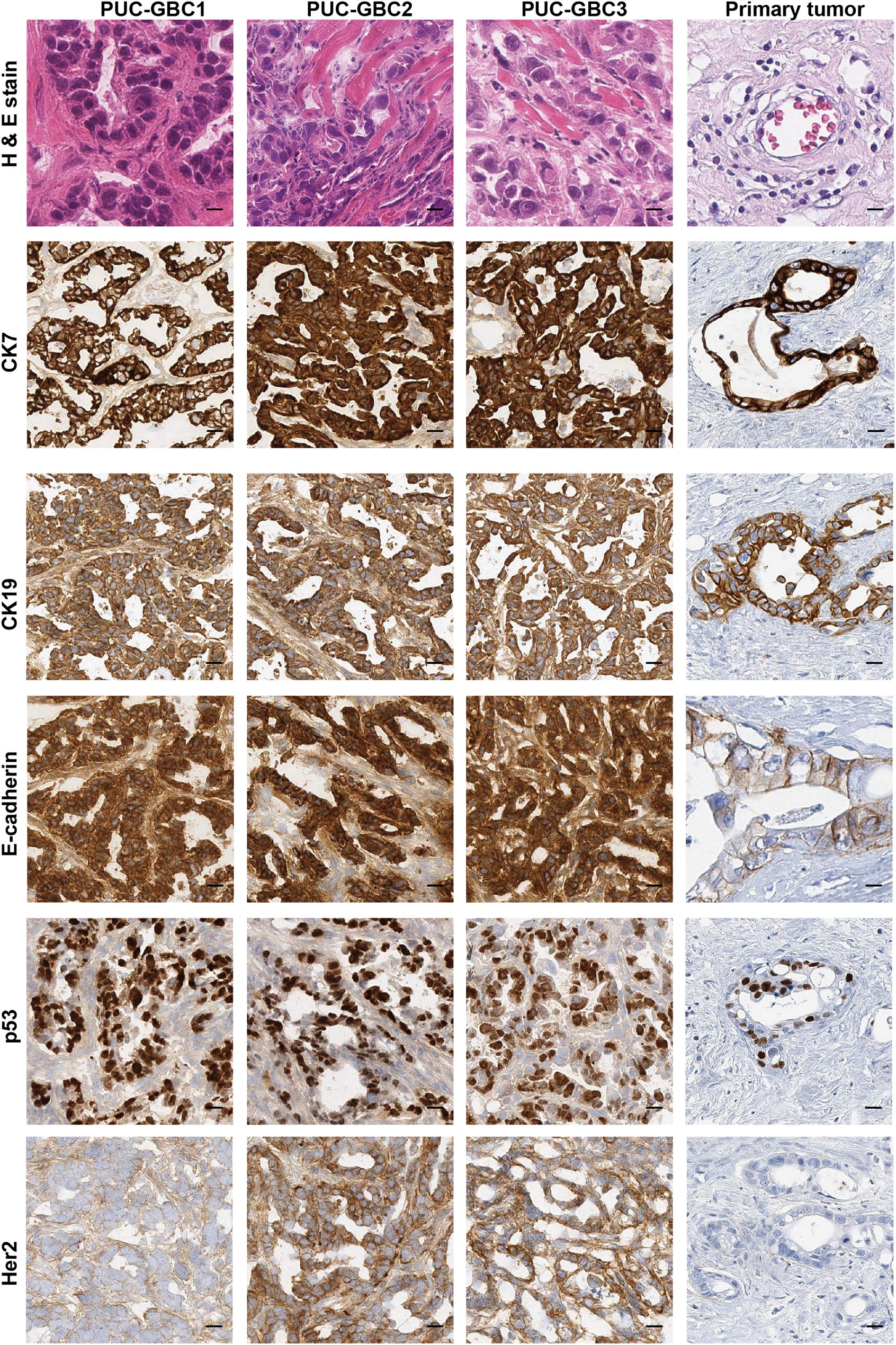
Xenograft tumors display high similarities with the original primary tumor. Xenografts showed histological features of adenocarcinoma, with tumor infiltration of muscle tissue. Expression of all markers was observed in the tumor cells of xenografts, except for HER2, which was present with different intensity only in cells derived from peritoneal metastasis. Scale bar, 20 µm; magnification, ×20
Immunohistochemistry (IHC) showed a strong positivity of the epithelial markers CK7, CK19 and E-cadherin (3+, 100% of the cells), which were also expressed in the primary tumor (Fig. 9 and Additional file 2 with RNA sequencing expression results). Nuclear positive p53 expression was observed in more than 70% of the cells in the xenografts in agreement with the expression observed in the primary tumor (Fig. 9). We also decided to evaluate the expression of human epidermal growth factor receptor 2 (HER2) because there is a subset of HER2-positive patients who might be candidates for an anti-HER2 therapy [27, 28]. We did not observe HER2 expression in the tissue section of the primary tumor or in the xenograft model of PUC-GBC1. However, PUC-GBC2 and PUC-GBC3 showed moderate and high HER2 intensity, and RNA sequencing revealed an increased ERBB2 expression for all GBC cell lines, and particularly PUC-GBC1 and PUC-GBC2 (Additional file 2).
Discussion
We report in this study on the phenotypic, genomic and functional characteristics of three new GBC cell lines derived from the metastatic ascites of a Chilean male patient; these were named PUC-GBC1, PUC-GBC2 and PUC-GBC3. Mapuche Native American ancestry has been strongly associated with the risk of GBC, and we found that the percentage of Mapuche Native American ancestry of the male donor of the primary tumor cells was 36%. For comparison, the first (third) quartiles of Mapuche ancestry reported in a recent study that included 1805 Chileans were 28% (43%) [6], confirming the representativeness of the established GBC cell lines for the Chilean population. The three new cell lines displayed an epithelial phenotype and showed abnormality in structure and number of chromosomes, with a tendency to triploidy. The doubling time varied from 36 to 60 h and was very similar to that observed for commercially available Asian cell lines. Evaluation of chemosensitivity indicated that our ascites-derived lines are more chemosensitive to gemcitabine and cisplatin than Asian GBC cell lines, which could be due to differences in molecular patterns. Furthermore, PUC-GBC1, PUC-GBC2 and PUC-GBC3 were tumorigenic when injected subcutaneously in NSG™ mice. Interestingly, the tumor volume of the PUC-GBC2 xenografts was the lowest despite PUC-GBC2 cells having the shortest doubling time and a higher migration capacity than PUC-GBC1 and PUC-GBC3 cells. In vivo, PUC-GBC3 cells showed a more aggressive behavior in agreement with the histopathological characteristics of the derived xenografts. The analysis of RNA sequencing data revealed differentially expressed genes that could be responsible for the phenotypic differences observed between the three cell lines. For instance, PUC-GBC2 showed elevated expression of COL3A1, which encodes an extracellular matrix (ECM) protein (collagen α‑1(III)) associated with tumor progression [29, 30] and has been reported as a marker of poor prognosis [31–33]. At the functional level, recent reports have demonstrated that COL3A1 promotes cell proliferation and migration [34, 35]. Similarly, high expression of POSTN, also overrepresented in PUC-GBC2, might explain the higher migratory ability of these cells. POSTN encodes for periostin, a multifunctional ECM protein that contributes to the remodeling of the tumor microenvironment during tumor progression [36]. In CCA and hepatoblastoma, overexpressed periostin was found to induce cell migration and epithelial-to-mesenchymal transition (EMT) features [37, 38]. On the other hand, PUC-GBC1 cells exhibited a slower growth rate and an enhanced in vivo tumorigenic capability. The transcriptome profile showed a high expression of genes related with cancer stem cell properties, such as HNF1A, a transcription factor recently reported to be enriched in pancreatic cancer stem cells [39] and a key player in the transcriptional reprogramming of colorectal cancer cells that promote liver metastasis [40]. RNA-Seq also confirmed the increased expression of cytokeratin and mucin genes in all three cell lines, showed an elevated TP53 and ERBB2 expression for PUC-GBC1 and PUC-GBC2, and revealed a novel exonic mutation in NF1.
We confirmed the epithelial origin of the primary culture and the derived cell lines by evaluation of the immunocytochemical expression of CK7 and CK19 (Additional file 1 and Fig. 9). Keratins are a complex subclass of the intermediate filaments, made up of more than 20 different polypeptides. Among them, CK19 belongs to the type I group of cytokeratins and is specifically expressed in the periderm, whereas CK7 is a type II cytokeratin specifically expressed in the simple epithelia lining the cavities of the internal organs and in the gland ducts and blood vessels [41]. Both CKs have shown normal expression in gallbladder epithelium [42] and are considered markers of biliary tract tumors [43].
We also evaluated the expression of CA 19-9, which is a glycolipid synthesized by the pancreatic, biliary, gastric, colonic cells, as well as the endometrial and salivary epithelia [43]. We found elevated CA 19-9 levels in almost all the cells of the primary culture and of the clones PUC-GBC1 and PUC-GBC3. The intensity of CA 19-9 expression was also high in PUC-GBC2, but only in 20% of cells (Fig. 2). Recently, Barnett et al. reported that differential expression of CA 19-9 and sTRA (Sialyl-TRA), another carbohydrate antigen, made it possible to identify morphologically distinct subsets of pancreatic cancer cells. Subpopulations expressing both markers were part of well differentiated and mucin-secreting pancreatic adenocarcinomas, whereas those expressing just one were often poorly differentiated and vacuolated and never mucin-secreting. In addition, the authors observed cases displaying sometimes more than one subpopulation in the same tumor [44]. A heterogeneous distribution of CA 19-9 expression in GBC could explain our findings, especially considering that the clones were isolated from malignant ascites, characterized by containing a heterogeneous group of tumor cells. However, more research is needed to determine if cells with different patterns of CA 19-9 expression co-exist in gallbladder tumors.
Other tumor markers strongly expressed by the primary culture and individual clones were the mucins MUC1 and MUC16, particularly MUC1 according to RNA sequencing results (Additional file 2). MUC1 and MUC16 are transmembrane glycoproteins, located in 1q22 and 19p13 locus respectively, which contribute to forming physiological barriers and transmitting growth and survival signals to the cells [45]. High MUC1 immunoreactivity has been associated with vascular invasion and the malignant progression of many tumors [46–51], including GBC [52, 53]. Studies have reported that MUC1 expression is higher in GBC than in normal and inflammatory gallbladder tissue [54–57] and more frequently and extensively expressed in poorly differentiated adenocarcinoma [58]. The upregulation of MUC1 in cancer is a product of the aberrant O-linked glycosylation that lead to an increased sialylation of this protein [59, 60]. Interestingly, the expression rate of stromal localization of sialylated MUC1 at the deepest invading sites of pT2 gallbladder carcinoma seems to be associated with a more frequent postsurgical peritoneal dissemination and lower survival rate of these patients [54]. Similar findings have reported that sialylated MUC1 mucin plays an important role in the progression of prostate cancer [61] and may be involved in the metastatic potential of pancreatic ductal adenocarcinoma [62].
On the other hand, the upregulation of MUC16 in primary tumor and ascites-derived clones could be associated with a biologically aggressive phenotype (Figs. 1, 2). Indeed, MUC16 (cancer antigen 125, CA 125) is used in clinics to diagnose and predict prognosis in ovarian cancer patients, but it is also upregulated in a large percentage of digestive tract adenocarcinomas and has been proposed as a prognostic marker for gastrointestinal cancers [63]. In a recent study, positive immunohistochemical staining of MUC16 (immunoreactivity in more than 10% of the tumor cells) was observed in more than 75% of extrahepatic pancreatobiliary tumors (n = 234 cases), including 37 gallbladder cancers [64]. Chaube et al. proposed MUC16 as a potential diagnostic marker because serum levels are increased in patients with GBC and benign disease can be differentiated from malignant disease with a sensitivity of 64% and a specificity of 90% [65].
These newly established cell lines, in particular PUC-GBC1 and PUC-GBC2, showed overexpression of p53, which was also observed in the primary tumor and xenografts (Fig. 9). In GBC, most studies have reported a frequency over 50% of p53 overexpression [20, 23, 66–68]. In addition, there is a progressive increase in p53 positivity from dysplastic lesions to carcinoma in situ and invasive carcinoma [23, 66, 69], and a high correlation between protein overexpression and the presence of TP53 mutations [20, 21]. The cell lines described here conserved the p53 overexpression observed in the primary tumor, although our analysis did not show any TP53 mutation. It has been generally accepted that mutations in TP53 may result in the synthesis of a functionally defective p53 protein that exhibits an extended half-life, which tends to accumulate in the cell nucleus and can be detected by immunohistochemistry [70, 71]. However, studies in other tumors have described immunohistochemical overexpression of p53 in absence of gene mutations due to the deregulation of upstream factors of p53 pathway, which lead to protein stabilization and accumulation in the nucleus [72, 73]. Based on our data, it is highly probable that the p53 signaling pathway, along with other relevant oncogenic pathways, is dysfunctional in all three cell lines. For instance, genomic analysis revealed a nsSNV in TP53 (dbSNP identifier: rs121912651) in all cell lines, which has not been reported as an oncogenic mutation but likely possesses a pathogenic role according to the ClinVar database (https://www.ncbi.nlm.nih.gov/clinvar/). Additionally, we found a nsSNV in MDM4 and a frameshift deletion in CDKN2A, both genes encoding proteins related with regulation of p53 (Additional file 3). However, further studies are needed in order to determine the level of p53 activity in these novel cell lines, as well as the functional and clinical significance of those genetic variants in gallbladder cancer.
We also assessed the expression of HER2 in primary tumors and xenografts. Overexpression of this protein has been reported in 14–48% of GBC cases [27, 28, 74–78], and its amplification and mutation has been observed in 5–20% and 10% of the cases, respectively [27, 75–77, 79]. The newly established GBC cell lines did not present any ERBB2 mutation. HER2 upregulation has been associated with poor prognosis in GBC [77, 78], and experimental tumor models have suggested that HER2 signaling is involved in gallbladder carcinogenesis [80, 81]. In this study, immunohistochemistry for HER2 was negative for the primary tumor and PUC-GBC1 xenografts but was 2+ for PUC-GBC2 and 3+ for PUC-GBC3 (Fig. 9). ERBB2 was particularly highly expressed in PUC-GBC1 and PUC-GBC2, and showed a somewhat lower expression in PUC-GBC3. This is not an unexpected finding considering that intratumor heterogeneity is a common event in cancer, and genetic heterogeneity of HER2 has been reported for other tumors [82–88]. For instance, focal or patchy positivity of HER2 is a pattern encountered in primary gastric tumors, which is consequence of the intratumor heterogeneity and could explain discordances observed in HER2 status between the primary tumor and metastatic sites [82, 85]. Recently, Toshiba et al. investigated the status of HER2 in a large cohort of patients with GBC and observed intratumor heterogeneity in 51% (20/39) of the cases with IHC scores of 2+ and 3+. Among them, around 80% showed amplification of HER2/neu gene as determined by fluorescent in situ hybridization (FISH) [27]. Based on these observations, it would be relevant to perform the immunohistochemical test for HER2 in primary and metastatic sites if clinical trials with anti-HER2 therapies are considered for patients with this disease. In this regard, it would be even more advisable to implement the technology of liquid biopsies and take advantage of their clinical value for the identification of heterogeneous subclonal populations of tumor cells.
Conclusions
In summary, we established and characterized three new GBC cell lines derived from a patient with metastatic disease, who represents well the Chilean population with a 36% percentage of Mapuche Native American ancestry. The new cell lines share some biological characteristics, but also show genomic and phenotypic differences that reflect intratumor heterogeneity. The established GBC cell lines provide a new experimental model for future research, including the study of cellular and molecular mechanisms involved in metastasis, the identification of new tumor-associated markers, and the evaluation of responses to new therapeutic agents.
Materials and methods
Cell lines and growth conditions
A panel of five cell lines of Asian origin was used for comparison of doubling times and drug sensitivity. GB-d1 [14] was provided by Dr. Anirban Maitra (Department of Pathology, Division of Pathology and Laboratory Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX, USA); NOZ was obtained from the Health Science Research Resources Bank (Osaka, Japan; No JCRB1033); and G-415, TGBC-1TKB and TGBC-2TKB were purchased from RIKEN BioResource Center (Ibaraki, Japan; No RCB2640, RCB1129 and RCB1130). G-415 and GB-d1 were grown in RPMI 1640 medium (Thermo Scientific HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS), 10 units/mL penicillin and 10 mg/mL streptomycin (1% penicillin/streptomycin, Thermo Scientific HyClone). NOZ, TGBC-1TKB and TGBC-2TKB were grown in Dulbecco’s Modified Eagle Medium (DMEM high glucose; Corning, New York, NY, USA) supplemented with 5% FBS and 1% penicillin/streptomycin.
Isolation and establishment of human gallbladder cancer cell lines
The primary cell culture was established from the ascites of a 60-year-old male patient with GBC. Histological examination classified this tumor as moderately differentiated tubular adenocarcinoma with a T3NxM1 stage. There was evidence of vascular and lymphatic system invasion and peritoneal carcinomatosis. Ascitic fluid (50 mL) was obtained from the patient, delivered to the laboratory and centrifuged at 1000 rpm for 10 min, and the pellet was rinsed twice with sterile phosphate-buffered saline (PBS; Corning, New York, NY, USA) containing antibiotics (1% penicillin–streptomycin solution, P/S). The supernatant was removed and saved as ascitic fluid supplement at -20 °C, and the remaining cells were resuspended in DMEM/F12 medium (HyClone, GE Healthcare Bio-Sciences, Pittsburgh, PA, USA) supplemented with 5% FBS, 50% of ascitic fluid (HyClone, GE Healthcare Bio-Sciences, Pittsburgh, PA, USA) and 1% P/S, seeded into 6-well culture plates, and incubated at 37 °C in a humidified atmosphere of 5% CO2 in the air. The growth medium was replaced every 2–3 days, and the plate was regularly checked for epithelial cells and fibroblast outgrowth. The ascitic fluid supplement was maintained in the culture and reduced 10 times every week until the cells reached confluency. After the first subculture, the cells were grown in complete culture medium alone (without added ascitic fluid) and submitted to immunocytochemistry analysis to confirm the epithelial origin and evaluate the expression of tumor markers. This analysis was repeated at passage 17. Primary culture cells were subcultured until they reached more than 20 passages. Then, three individual clones were obtained by serial dilution of the primary cell culture in a 96-well plate. Once established, all cell lines were cultured in DMEM supplemented with 5% FBS and 1% P/S. All cultures were tested for mycoplasma contamination with a commercial PCR kit (EZ-PCR Mycoplasma Test Kit, Biological Industries, Cromwell, CT, USA).
Immunostaining procedures
Primary antibodies and working dilutions used for immunostaining analysis were mouse monoclonal anti-cytokeratin 7 (CK7; Cat. No M7018; Clone OV-TL 12/30) and mouse monoclonal anti-p53 (Cat. No IS616: Clone DO-7) from Dako (Agilent Technologies, Santa Clara, CA, USA); mouse monoclonal anti-mesothelin (Cat. No ACI 3175; Clone MSLN-15C11) from Biocare Medical, LLC (Pacheco, CA, USA); mouse monoclonal anti-CA 15-3 (MUC1; Cat. No MU323-UC; Clone BGX323A) from Biogenex (Fremont, NE, USA); mouse monoclonal anti-cytokeratin 19 (CK19; Cat. No 760-4281; Clone A53-B/A2.26), mouse monoclonal anti-CA 19-9 (Cat. No 760-2609; Clone 121SLE), mouse monoclonal anti-CA 125 (MUC16; Cat. No 760-2610; Clone OC125), rabbit monoclonal anti-calretinin (Cat. No 790-4467; Clone SP65), mouse monoclonal anti-vimentin (Cat. No 790-2917; Clone V9), mouse monoclonal anti-E-cadherin (Cat. No 790-4497; Clone 36) and rabbit monoclonal anti-HER2 (Cat. No 790-2991; Clone 4B5) from Roche Tissue Diagnostics (Oro Valley, AZ, USA).
The epithelial and tumor origin of the primary culture from the ascites and the derived cell lines was evaluated by immunocytochemistry. Cells were seeded at 2 × 105 per well in 24-well plates and allowed to attach for 24 h. Following fixation in 4% paraformaldehyde for 15 min, PBS wash and permeabilization with 0.1% Triton X-100, cells were incubated with primary antibodies at 1:100 dilution for 1 h. Then, cells were washed four times with 0.2% BSA (bovine serum albumin) in PBS and incubated for 30 min with the SuperPicture™ Polymer Detection Kit (Thermo Fisher Scientific Inc., Waltham, MA, USA). The bound antibody was visualized with a red chromogen (ImmPACT™ NovaRED™ Substrate; Vector Laboratories Inc., Burlingame, CA, USA). Antibody for HER2/neu was immunostained on the Benchmark XT automated stainer (Ventana Medical Systems, Tucson, AZ, USA) according to the manufacturer’s instructions. Images were acquired using the EVOS XL Core Imaging System (Thermo Fisher Scientific Inc., Waltham, MA, USA).
The primary tumor and xenografts were analyzed immunohistochemically on formalin-fixed paraffin-embedded (FFPE) sections (2 μm thickness) using the automated immunostainer BenchMark ULTRA and the ultraVIEW universal DAB detection kit (Ventana Medical Systems Inc., Roche Group, Tucson, AZ, USA). Digital images were captured using the Aperio AT2 Digital Pathology Scanner (Leica Biosystems, Nussloch, Germany). The expression was evaluated by the pathologist (J.C.R.) considering the intensity of the staining (1 + , weak; 2 + , moderate; 3 + , strong) and the percentage of labeled cells. Immunohistochemical scoring of HER-2 expression was based on the system proposed for gastric and gastroesophageal junction cancer [89].
Short tandem repeat (STR) DNA profiling
The genetic profiling of the cell lines derived from the primary cell culture was established using the polymorphic STR loci detection service offered by the Department of Molecular Medicine at Aarhus University Hospital (IdentiCell service for human cell line authentication). DNA of each cell line was purified using the PureLink® Genomic DNA mini kit (Thermo Fisher Scientific Inc., Waltham, MA, USA).
Chromosome analysis
The cytogenetic karyotyping analysis was performed using a standard air-dried method. Briefly, cells in an exponential growth phase were treated with a final concentration of 0.1 µg/mL colcemid (Colcemid® Solution, Irvine Scientific, Santa Ana, CA, USA) for 45 min at 37 °C. Then, cells were harvested to arrest metaphases and treated with the hypotonic solution (0.075 M KCl) for 10 min at 37 °C. After two changes in the fixative (3:1, methanol: glacial acetic acid), the cell suspension was incubated overnight at -20 °C. The next day, the fixative was changed another three times and two drops of the cell suspension were dropped from a distance of about 50 cm onto clean dry slides tilted at an angle of about 45°, allowing the cells to roll across the slide. After air drying, one slide was stained with Giemsa (3:1 ratio of Gurr Buffer and Giemsa Stain) and analyzed under a light microscope at 10× and 100× magnification. If the metaphase cells were abundant and well spread, the remaining slides were used for chromosome analysis using trypsin G-banding. To determine ploidy, chromosomes were counted from a minimum of 50 metaphase spreads using GenASIs Karyotyping (Applied Spectral Imaging Inc. Carlsbad, CA, USA).
Cell growth characteristics
The doubling time was calculated by seeding the cells at a density of 20 000 cells/well in 12-well plates and counting them by Trypan blue dye exclusion in a Neubauer camera every 24 h for 15 days (the medium was replaced every 3 days). The doubling times were determined from the growth curves using the data generated from 3 independent experiments, each with three technical replicates.
Chemosensitivity assay
The cytotoxic drugs used for in vitro chemosensitivity assays were gemcitabine (Sigma-Aldrich, St. Louis, MO, USA), cisplatin (Calbiochem, Merck group, Darmstadt, Germany) and Fluorouracil (Laboratorios Kampar, Santiago, Chile). These drugs are the main chemotherapy agents used to treat GBC. Cells were seeded into 96-well plates at a density of 5 × 103 cells/well in 100 μL cell culture medium. After an overnight attachment period, cells were treated with either gemcitabine (starting at 300 µM), cisplatin (starting at 350 µM) or fluorouracil (starting at 2 mM) with a serial dilution of 1/3. Following 72 h of drug incubation, cell viability was assessed by incubating the cells for 2 h at 37 °C with a MTS-PMS colorimetric solution (Promega Corp., Madison, WI, USA), and absorbance of each well was read at a wavelength of 490 nm using a multiwell plate reader (Epoch Microplate Spectrophotometer, BioTek Instruments Inc., Winooski, VT, USA). The half maximal inhibitory concentration was calculated from the dose response curves (IC50: dose of drug required to reduce final cell number or optical density in MTS assay to 50% of control). Three independent experiments were performed, each with three technical replicates.
Cell migration assay
Migration assays were performed using Transwell® 24-well plates containing polycarbonate filters with an 8-micron pore size (BD Biosciences, Bedford, MA, USA). Complete medium was placed in the lower chamber to act as a chemoattractant, and 5 × 104 cells were seeded with serum-free medium into the upper chamber. After 24 h, cells were fixed in methanol for 15 min and stained with 0.05% crystal violet in 25% methanol/PBS for 15 min. Cells on top of the membrane were removed using a cotton swab, and filters were washed with PBS. Cells on the underside of filters were viewed and counted under a microscope in 10 randomly selected fields. Three independent experiments were performed, each with three technical replicates.
Tumorigenicity assay
The in vivo tumorigenicity assay was performed using 7 to 8-week-old male NOD scid gamma (NSG™) mice obtained from The Jackson Laboratory (Bar Harbor, ME, USA). The animals (n = 9) were randomized into three groups, anesthetized with isoflurane (3% for induction of anesthesia and 1.5% for maintenance) and 3 x 106 cells suspended in 150 μL PBS/matrigel (Matrigel™ Basement Membrane Matrix, BD Biosciences, Bedford, MA, USA) were injected bilaterally and subcutaneously on the back of the anesthetized mice. Animals were examined every week for the development of tumors. Tumor volumes were estimated twice a week from caliper measurements (volume = 0.52 × (width)2 × length). When tumors had grown to 1.5–2.0 cm3 after 4 weeks, mice were euthanized by carbon dioxide (CO2) and the tumor xenografts were removed, weighted and photographed. Then, tumors were fixed in 10% neutral buffered formalin, and processed for routine histopathological examination.
Variant and gene expression analysis from RNA sequencing, and estimation of genetic ancestry proportions
Total RNA was extracted and sequenced on an Illumina Hi-Seq 2000. The quality of the sequencing reads was assessed using FastQC (0.11.2) (http://www.bioinformatics.bbsrc.ac.uk/projects/fastqc). PRINSEQ (0.20.3) [90] and cutadapt (1.9) [91] were used to filter the reads based on quality parameters and to remove adapters. Variant calling was performed following the GATK guidelines for RNA sequencing data, including a two-pass alignment with STAR (2.5.2b) [92], towards the GRCh38 version of the human genome, removal of PCR duplicates with picard tools (2.1.0) (http://broadinstitute.github.io/picard/) and variant calling with GATK 3.5 [93]. Thresholds for the detection of mutations were read depth > 20, allelic depth > 10, mapping quality > 40 and Fisher’s strand < 60. Variants were annotated with Annovar [94]. Gene expression values were calculated using feature Counts from the subread package (1.6.4) [95] and normalized as transcripts per million. More than 30 M reads were generated per each cell line sample. Additional file 5 provides the sequencing statistics for the three newly established GBC cell lines. Gene ontology analyses were conducted with the R package goProfiles.
The identified variants with an allele frequency > 5% according to the 1000 Genome Project (more than 5000 variants in total) were used to infer the genetic ancestry proportions of the donor of the primary GBC tumor cells [96]. Surrogates of African and European ancestry were 87 Yorubans in Ibadan, Nigeria, and 80 Utah residents with Northern and Western European ancestry from the 1000 Genome Project. Nine Mapuche and nine Aymara individuals were selected to represent the two largest indigenous peoples in Chile based on the three following criteria: four grandparental Mapuche or Aymara surnames, estimated Native American proportion of at least 74% for Mapuche and at least 99% for Aymara reference individuals, and mitochondrial DNA haplogroups consistent with Mapuche (haplogroup C or D) or Aymara (haplogroup B) descent [6]. The ADMIXTURE software was used for supervised estimation of the African, European, Mapuche Native American and Aymara Native American ancestry components relying on the above-mentioned identified common variants, reference individuals and genotype data from a recent study that included 1805 Chileans [6, 97].
Statistical analysis
Statistical analyses were performed using R programming environment in Rstudio© version 1.0 (Rstudio, Inc., Boston, MA). A Kruskal–Wallis test was used to compare multiple groups, followed by Dunn’s post hoc multiple comparisons test. Probability values smaller than 0.05 were considered statistically significant.
Supplementary information
Additional file 1. Representative micrographs of immunocytochemical staining for CK7, CK19, Mesothelin, Vimentin and Calretinin in the three clones isolated from the ascites-derived primary culture (magnification, 40×).
Additional file 2. Gene expression of normalized transcripts per million in Chilean GBC cell lines.
Additional file 3. Exonic mutations in Chilean GBC cell lines.
Additional file 4. Venn diagrams depicting unique and shared (overlapping circles) variants in the three cell lines. (a) non-synonymous; (b) synonymous; and (c) frameshift. The green circle depicts the variants identified in PUC-GBC1, the orange circle depicts the variants identified in PUC-GBC2, and the blue circle depicts the variants identified in PUC-GBC3.
Additional file 5. Sequencing statistics.
Acknowledgements
Not applicable.
Abbreviations
- GBC
Gallbladder cancer
- CK7
Cytokeratin 7
- CK19
Cytokeratin 19
- CA 19-9
Carbohydrate antigen 19-9
- MUC1
Mucin 1
- MUC16
Mucin 16
- TP53
Tumor Protein P53
- STR
Short tandem repeat
- GO
Gene ontology
- nsSNV
Nonsynonymous single-nucleotide variation
- SIFT
Sorting intolerance from tolerance
- 5-FU
Fluorouracil
- HER2
Human epidermal growth factor receptor 2
- ERBB2
Erb-B2 Receptor Tyrosine Kinase 2
- IHC
Immunohistochemistry
- CKs
Cytokeratins
- sTRA
Sialyl-TRA
- CA 125
Cancer antigen 125
- FISH
Fluorescent in situ hybridization
- DMEM
Dulbecco’s Modified Eagle Medium
- FBS
Fetal bovine serum
- PBS
Phosphate-buffered saline
- P/S
Penicillin-streptomycin solution
- BSA
Bovine serum albumin
- FFPE
Formalin-fixed paraffin-embedded
- NSG
NOD scid gamma
Authors’ contributions
PG, CB and LR contributed equally to this work. PG, CB, LR, JAE and JCR conceived and designed the experiments. PG, CB, LR, FA and CL-A performed the in vitro experiments and characterized the primary culture and derived clones. JC-I, MS and VPM performed the in vivo experiments. JL-B, MD-L and FB performed the gene expression analysis from RNA sequencing and the estimation of genetic ancestry proportions. FA, ZP and ML provided help with the karyotyping. SK provided help with the primary culture establishment. DR assisted with tissue processing and imaging. PG, CB, LR, JAE, HW, PL, JL-B and JCR analyzed the data and drafted the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by grants from the Fondo Nacional de Desarrollo Científico y Tecnológico, FONDECYT No. 1170893 (JCR), 1171463 (CB), 1201734 (PL), 11180987 (HW) and 1160800 (SK); Dirección de Investigación Medicina UC-Pontificia Universidad Católica de Chile, Project No 16-240 (JCR); and Instituto Milenio IMII P09/016-F (JCR). Lorena Rosa is a student of the Ph.D Program “Doctorado en Ciencias mención Biología Celular y Molecular Aplicada”, Universidad de La Frontera and was supported by CONICYT (#21140027). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
All data generated and/or analyzed in this study are included in this published article and its additional files. Additionally, they are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The present study was approved by the Scientific Ethics Committee of the Faculty of Medicine, Pontificia Universidad Católica de Chile, and was conducted in accordance with the tenets of the 1964 Helsinki declaration and its subsequent amendments. The clinical sample used to develop and establish the primary culture was obtained after the patient signed the written informed consent form. Mouse husbandry and animal experiments were conducted in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. For injections of cancer cells, mice were anesthetized under 3% isoflurane for induction of anesthesia and 1.5% for maintenance. At the end of the assay, mice were euthanized using carbon dioxide (CO2) overdose. The protocol was approved by the Committee on Animal Experimentation, Pontificia Universidad Católica de Chile (Protocol No CBB-224/2014).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Patricia García, Carolina Bizama and Lorena Rosa contributed equally to this work
Supplementary information
Supplementary information accompanies this paper at 10.1186/s40659-020-00282-7.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99–109. doi: 10.2147/clep.s37357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 4.Series y Gráficos de Mortalidad. Ministerio de Salud. http://www.deis.cl/series-y-graficos-de-mortalidad/. Accessed 05 Feb 2019.
- 5.Villanueva L. Cancer of the gallbladder-Chilean statistics. Ecancer Med Sci. 2016;10:704. doi: 10.3332/ecancer.2016.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lorenzo Bermejo J, Boekstegers F, Gonzalez Silos R, Marcelain K, Baez Benavides P, Barahona Ponce C, et al. Subtypes of Native American ancestry and leading causes of death: Mapuche ancestry-specific associations with gallbladder cancer risk in Chile. PLoS Genet. 2017;13(5):e1006756. doi: 10.1371/journal.pgen.1006756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andren-Sandberg A. Diagnosis and management of gallbladder cancer. N Am J Med Sci. 2012;4(7):293–299. doi: 10.4103/1947-2714.98586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazcano-Ponce EC, Miquel JF, Munoz N, Herrero R, Ferrecio C, Wistuba II, et al. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin. 2001;51(6):349–364. doi: 10.3322/canjclin.51.6.349. [DOI] [PubMed] [Google Scholar]
- 9.Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer. 2006;118(7):1591–1602. doi: 10.1002/ijc.21683. [DOI] [PubMed] [Google Scholar]
- 10.Compton CC, Byrd DR, Garcia-Aguilar J, Kurtzman SH, Olawaiye A, Washington MK, editors. AJCC Cancer Staging Atlas. 2. New York: Springer-Verlag; 2012. [Google Scholar]
- 11.Goldin RD, Roa JC. Gallbladder cancer: a morphological and molecular update. Histopathology. 2009;55(2):218–229. doi: 10.1111/j.1365-2559.2008.03192.x. [DOI] [PubMed] [Google Scholar]
- 12.Homma S, Hasumura S, Nagamori S, Kameda H. Establishment and characterization of a human gall bladder carcinoma cell line NOZ. Hum Cell. 1988;1(1):95–97. [PubMed] [Google Scholar]
- 13.Koyama S, Yoshioka T, Mizushima A, Kawakita I, Yamagata S, Fukutomi H, et al. Establishment of a cell line (G-415) from a human gallbladder carcinoma. Gan. 1980;71(4):574–575. [PubMed] [Google Scholar]
- 14.Shimura H, Date K, Matsumoto K, Nakamura T, Tanaka M. Induction of invasive growth in a gallbladder cancer cell line by hepatocyte growth factor in vitro. Jpn J Cancer Res. 1995;86(7):662–669. doi: 10.1111/j.1349-7006.1995.tb02450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghosh M, Koike N, Yanagimoto G, Tsunoda S, Kaul S, Hirano T, et al. Establishment and characterization of unique human gallbladder cancer cell lines. Int J Oncol. 2004;24(5):1189–1196. [PubMed] [Google Scholar]
- 16.Yamada N, Chung Y, Ohtani H, Ikeda T, Onoda N, Sawada T, et al. Establishment and characterization of a new human gallbladder carcinoma cell line (OCUG-1) producing TA-4. Int J Oncol. 1997;10(6):1251–1255. doi: 10.3892/ijo.10.6.1251. [DOI] [PubMed] [Google Scholar]
- 17.Ku JL, Yoon KA, Kim IJ, Kim WH, Jang JY, Suh KS, et al. Establishment and characterisation of six human biliary tract cancer cell lines. Br J Cancer. 2002;87(2):187–193. doi: 10.1038/sj.bjc.6600440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang JH, Li LF, Yu Y, Li B, Jin HJ, Shen DH, et al. Establishment and characterization of a cell line, EH-GB2, derived from hepatic metastasis of gallbladder cancer. Oncol Rep. 2012;27(3):775–782. doi: 10.3892/or.2011.1570. [DOI] [PubMed] [Google Scholar]
- 19.Liu ZY, Xu GL, Tao HH, Yang YQ, Fan YZ. Establishment and characterization of a novel highly aggressive gallbladder cancer cell line, TJ-GBC2. Cancer Cell Int. 2017;17:20. doi: 10.1186/s12935-017-0388-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Itoi T, Watanabe H, Yoshida M, Ajioka Y, Nishikura K, Saito T. Correlation of p53 protein expression with gene mutation in gall-bladder carcinomas. Pathol Int. 1997;47(8):525–530. doi: 10.1111/j.1440-1827.1997.tb04535.x. [DOI] [PubMed] [Google Scholar]
- 21.Roa I, Melo A, Roa J, Araya J, Villaseca M, de Aretxabala X. P53 gene mutation in gallbladder cancer. Rev Med Chil. 2000;128(3):251–258. [PubMed] [Google Scholar]
- 22.Quan ZW, Wu K, Wang J, Shi W, Zhang Z, Merrell RC. Association of p53, p16, and vascular endothelial growth factor protein expressions with the prognosis and metastasis of gallbladder cancer. J Am Coll Surg. 2001;193(4):380–383. doi: 10.1016/S1072-7515(01)01012-2. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh M, Sakhuja P, Singh S, Agarwal AK. p53 and beta-catenin expression in gallbladder tissues and correlation with tumor progression in gallbladder cancer. Saudi J Gastroenterol. 2013;19(1):34–39. doi: 10.4103/1319-3767.105922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim K, Kim DH, Chae SW, Shin JH, Kim HJ, Do SI, et al. Expression of cell cycle-related proteins, p16, p53 and p63 as important prognostic markers in gallbladder adenocarcinoma. Pathol Oncol Res. 2014;20(2):409–415. doi: 10.1007/s12253-013-9710-5. [DOI] [PubMed] [Google Scholar]
- 25.Singh A, Mishra PK, Saluja SS, Talikoti MA, Kirtani P, Najmi AK. Prognostic significance of HER-2 and p53 expression in gallbladder carcinoma in North Indian patients. Oncology. 2016;91(6):354–360. doi: 10.1159/000450999. [DOI] [PubMed] [Google Scholar]
- 26.Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23(6):703–713. doi: 10.1038/nm.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida H, Shimada K, Kosuge T, Hiraoka N. A significant subgroup of resectable gallbladder cancer patients has an HER2 positive status. Virchows Arch. 2016;468(4):431–439. doi: 10.1007/s00428-015-1898-1. [DOI] [PubMed] [Google Scholar]
- 28.Halder S, Kundu S, Chakraborty J, Chakrabarti S. Significance of HER2 and Ki-67 in preneoplastic lesions and carcinoma of gallbladder. J Gastrointest Cancer. 2018 doi: 10.1007/s12029-018-0162-8. [DOI] [PubMed] [Google Scholar]
- 29.Tapper J, Kettunen E, El-Rifai W, Seppala M, Andersson LC, Knuutila S. Changes in gene expression during progression of ovarian carcinoma. Cancer Genet Cytogenet. 2001;128(1):1–6. doi: 10.1016/s0165-4608(01)00386-7. [DOI] [PubMed] [Google Scholar]
- 30.Yue H, Wang J, Chen R, Hou X, Li J, Lu X. Gene signature characteristic of elevated stromal infiltration and activation is associated with increased risk of hematogenous and lymphatic metastasis in serous ovarian cancer. BMC Cancer. 2019;19(1):1266. doi: 10.1186/s12885-019-6470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan L, Shu B, Chen L, Qian K, Wang Y, Qian G, et al. Overexpression of COL3A1 confers a poor prognosis in human bladder cancer identified by co-expression analysis. Oncotarget. 2017;8(41):70508–70520. doi: 10.18632/oncotarget.19733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi S, Tian B. Identification of biomarkers associated with progression and prognosis in bladder cancer via co-expression analysis. Cancer Biomark. 2019;24(2):183–193. doi: 10.3233/cbm-181940. [DOI] [PubMed] [Google Scholar]
- 33.Engqvist H, Parris TZ, Kovacs A, Nemes S, Werner Ronnerman E, De Lara S, et al. Immunohistochemical validation of COL3A1, GPR158 and PITHD1 as prognostic biomarkers in early-stage ovarian carcinomas. BMC Cancer. 2019;19(1):928. doi: 10.1186/s12885-019-6084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao YF, Zhu T, Chen J, Liu L, Ouyang R. Knockdown of collagen alpha-1(III) inhibits glioma cell proliferation and migration and is regulated by miR128-3p. Oncol Lett. 2018;16(2):1917–1923. doi: 10.3892/ol.2018.8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carpino G, Overi D, Melandro F, Grimaldi A, Cardinale V, Di Matteo S, et al. Matrisome analysis of intrahepatic cholangiocarcinoma unveils a peculiar cancer-associated extracellular matrix structure. Clin Proteomics. 2019;16:37. doi: 10.1186/s12014-019-9257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma H, Wang J, Zhao X, Wu T, Huang Z, Chen D, et al. Periostin promotes colorectal tumorigenesis through integrin-FAK-Src Pathway-mediated YAP/TAZ activation. Cell Rep. 2020;30(3):793–806. doi: 10.1016/j.celrep.2019.12.075. [DOI] [PubMed] [Google Scholar]
- 37.Chen L, Tian X, Gong W, Sun B, Li G, Liu D, et al. Periostin mediates epithelial-mesenchymal transition through the MAPK/ERK pathway in hepatoblastoma. Cancer Biol Med. 2019;16(1):89–100. doi: 10.20892/j.issn.2095-3941.2018.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sonongbua J, Siritungyong S, Thongchot S, Kamolhan T, Utispan K, Thuwajit P, et al. Periostin induces epithelialtomesenchymal transition via the integrin alpha5beta1/TWIST2 axis in cholangiocarcinoma. Oncol Rep. 2020;43(4):1147–1158. doi: 10.3892/or.2020.7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abel EV, Goto M, Magnuson B, Abraham S, Ramanathan N, Hotaling E, et al. HNF1A is a novel oncogene that regulates human pancreatic cancer stem cell properties. Elife. 2018 doi: 10.7554/elife.33947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teng S, Li YE, Yang M, Qi R, Huang Y, Wang Q, et al. Tissue-specific transcription reprogramming promotes liver metastasis of colorectal cancer. Cell Res. 2020;30(1):34–49. doi: 10.1038/s41422-019-0259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chougule P, Sumitran-Holgersson S. Cytokeratins of the Liver and Intestine Epithelial Cells During Development and Disease. In: Hamilton G, editor. Cytokeratins tools in oncology. London: IntechOpen; 2012. pp. 15–32. [Google Scholar]
- 42.Uhlen M, Bjorling E, Agaton C, Szigyarto CA, Amini B, Andersen E, et al. A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol Cell Proteomics. 2005;4(12):1920–1932. doi: 10.1074/mcp.m500279-mcp200. [DOI] [PubMed] [Google Scholar]
- 43.Malaguarnera G, Giordano M, Paladina I, Rando A, Uccello M, Basile F, et al. Markers of bile duct tumors. World J Gastrointest Oncol. 2011;3(4):49–59. doi: 10.4251/wjgo.v3.i4.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barnett D, Liu Y, Partyka K, Huang Y, Tang H, Hostetter G, et al. The CA19-9 and Sialyl-TRA antigens define separate subpopulations of pancreatic cancer cells. Sci Rep. 2017;7(1):4020. doi: 10.1038/s41598-017-04164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGuckin MA. Introduction to mucins in cancer. In: Taylor-Papadimitriou J, Burchell JM, editors. Mucins and Cancer. London: Future Medicine Ltd; 2013. pp. 6–18. [Google Scholar]
- 46.Xu F, Liu F, Zhao H, An G, Feng G. Prognostic significance of mucin antigen MUC1 in various human epithelial cancers: a meta-analysis. Medicine. 2015;94(50):e2286. doi: 10.1097/md.0000000000002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang XT, Kong FB, Mai W, Li L, Pang LM. MUC1 immunohistochemical expression as a prognostic factor in gastric cancer: meta-analysis. Dis Markers. 2016;2016:9421571. doi: 10.1155/2016/9421571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin X, Gu Y, Kapoor A, Wei F, Aziz T, Ojo D, et al. Overexpression of MUC1 and genomic alterations in its network associate with prostate cancer progression. Neoplasia. 2017;19(11):857–867. doi: 10.1016/j.neo.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang X, Sun Q, Chen C, Zhang Y, Kang X, Zhang JY, et al. MUC1 overexpression predicts worse survival in patients with non-small cell lung cancer: evidence from an updated meta-analysis. Oncotarget. 2017;8(52):90315–90326. doi: 10.18632/oncotarget.19861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ozcan HEA, Anuk T, Ozden O. Expression profile and cellular localizations of mucin proteins, CK7, and cytoplasmic p27 in Barrett’s esophagus and esophageal adenocarcinoma. Adv Med Sci. 2018;63(2):296–300. doi: 10.1016/j.advms.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 51.Saltos A, Khalil F, Smith M, Li J, Schell M, Antonia SJ, et al. Clinical associations of mucin 1 in human lung cancer and precancerous lesions. Oncotarget. 2018;9(86):35666–35675. doi: 10.18632/oncotarget.26278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toba T, Kijima H, Hakamada K, Igarashi Y. Histological phenotype is correlated with the wall-invasion pattern of gallbladder adenocarcinoma. Biomed Res. 2014;35(5):295–302. doi: 10.2220/biomedres.35.295. [DOI] [PubMed] [Google Scholar]
- 53.Hiraki T, Yamada S, Higashi M, Hatanaka K, Yokoyama S, Kitazono I, et al. Immunohistochemical expression of mucin antigens in gallbladder adenocarcinoma: MUC1-positive and MUC2-negative expression Is associated with vessel invasion and shortened survival. Histol Histopathol. 2017;32(6):585–596. doi: 10.14670/hh-11-824. [DOI] [PubMed] [Google Scholar]
- 54.Kawamoto T, Shoda J, Irimura T, Miyahara N, Furukawa M, Ueda T, et al. Expression of MUC1 mucins in the subserosal layer correlates with postsurgical prognosis of pathological tumor stage 2 carcinoma of the gallbladder. Clin Cancer Res. 2001;7(5):1333–1342. [PubMed] [Google Scholar]
- 55.Chang HJ, Kim SW, Lee BL, Hong EK, Kim WH. Phenotypic alterations of mucins and cytokeratins during gallbladder carcinogenesis. Pathol Int. 2004;54(8):576–584. doi: 10.1111/j.1440-1827.2004.01666.x. [DOI] [PubMed] [Google Scholar]
- 56.Ghosh M, Kamma H, Kawamoto T, Koike N, Miwa M, Kapoor VK, et al. MUC 1 core protein as a marker of gallbladder malignancy. Eur J Surg Oncol. 2005;31(8):891–896. doi: 10.1016/j.ejso.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 57.Xiong L, Yang Z, Yang L, Liu J, Miao X. Expressive levels of MUC1 and MUC5AC and their clinicopathologic significances in the benign and malignant lesions of gallbladder. J Surg Oncol. 2012;105(1):97–103. doi: 10.1002/jso.22055. [DOI] [PubMed] [Google Scholar]
- 58.Yamato T, Sasaki M, Watanabe Y, Nakanuma Y. Expression of MUC1 and MUC2 mucin core proteins and their messenger RNA in gall bladder carcinoma: an immunohistochemical and in situ hybridization study. J Pathol. 1999;188(1):30–37. doi: 10.1002/(sici)1096-9896(199905)188:1<30::aid-path291>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 59.Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15(9):540–555. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- 60.Beatson R, Tajadura-Ortega V, Achkova D, Picco G, Tsourouktsoglou TD, Klausing S, et al. The mucin MUC1 modulates the tumor immunological microenvironment through engagement of the lectin Siglec-9. Nat Immunol. 2016;17(11):1273–1281. doi: 10.1038/ni.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arai T, Fujita K, Fujime M, Irimura T. Expression of sialylated MUC1 in prostate cancer: relationship to clinical stage and prognosis. Int J Urol. 2005;12(7):654–661. doi: 10.1111/j.1442-2042.2005.01112.x. [DOI] [PubMed] [Google Scholar]
- 62.Masaki Y, Oka M, Ogura Y, Ueno T, Nishihara K, Tangoku A, et al. Sialylated MUC1 mucin expression in normal pancreas, benign pancreatic lesions, and pancreatic ductal adenocarcinoma. Hepatogastroenterology. 1999;46(28):2240–2245. [PubMed] [Google Scholar]
- 63.Streppel MM, Vincent A, Mukherjee R, Campbell NR, Chen SH, Konstantopoulos K, et al. Mucin 16 (cancer antigen 125) expression in human tissues and cell lines and correlation with clinical outcome in adenocarcinomas of the pancreas, esophagus, stomach, and colon. Hum Pathol. 2012;43(10):1755–1763. doi: 10.1016/j.humpath.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fernandez Moro C, Fernandez-Woodbridge A, Alistair D’souza M, Zhang Q, Bozoky B, Kandaswamy SV, et al. Immunohistochemical typing of adenocarcinomas of the pancreatobiliary system improves diagnosis and prognostic stratification. PLoS ONE. 2016;11(11):e0166067. doi: 10.1371/journal.pone.0166067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chaube A, Tewari M, Singh U, Shukla HS. CA 125: a potential tumor marker for gallbladder cancer. J Surg Oncol. 2006;93(8):665–669. doi: 10.1002/jso.20534. [DOI] [PubMed] [Google Scholar]
- 66.Roa I, Villaseca M, Araya J, Roa J, de Aretxabala X, Melo A, et al. p53 tumour suppressor gene protein expression in early and advanced gallbladder carcinoma. Histopathology. 1997;31(3):226–230. doi: 10.1046/j.1365-2559.1997.2420850.x. [DOI] [PubMed] [Google Scholar]
- 67.Wang SN, Chung SC, Tsai KB, Chai CY, Chang WT, Kuo KK, et al. Aberrant p53 expression and the development of gallbladder carcinoma and adenoma. Kaohsiung J Med Sci. 2006;22(2):53–59. doi: 10.1016/s1607-551x(09)70221-9. [DOI] [PubMed] [Google Scholar]
- 68.Hidalgo Grau LA, Badia JM, Salvador CA, Monso TS, Canaleta JF, Nogues JM, et al. Gallbladder carcinoma: the role of p53 protein overexpression and Ki-67 antigen expression as prognostic markers. HPB. 2004;6(3):174–180. doi: 10.1080/13651820410025110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wistuba II, Gazdar AF, Roa I, Albores-Saavedra J. p53 protein overexpression in gallbladder carcinoma and its precursor lesions: an immunohistochemical study. Hum Pathol. 1996;27(4):360–365. doi: 10.1016/S0046-8177(96)90109-4. [DOI] [PubMed] [Google Scholar]
- 70.Yemelyanova A, Vang R, Kshirsagar M, Lu D, Marks MA, Shih IM, et al. Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: an immunohistochemical and nucleotide sequencing analysis. Mod Pathol. 2011;24:1248. doi: 10.1038/modpathol.2011.85. [DOI] [PubMed] [Google Scholar]
- 71.Murnyak B, Hortobagyi T. Immunohistochemical correlates of TP53 somatic mutations in cancer. Oncotarget. 2016;7(40):64910–64920. doi: 10.18632/oncotarget.11912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koga T, Hashimoto S, Sugio K, Yoshino I, Nakagawa K, Yonemitsu Y, et al. Heterogeneous distribution of P53 immunoreactivity in human lung adenocarcinoma correlates with MDM2 protein expression, rather than with P53 gene mutation. Int J Cancer. 2001;95(4):232–239. doi: 10.1002/1097-0215(20010720)95:4<232::aid-ijc1040>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 73.Wang YC, Lin RK, Tan YH, Chen JT, Chen CY, Wang YC. Wild-type p53 overexpression and its correlation with MDM2 and p14ARF alterations: an alternative pathway to non-small-cell lung cancer. J Clin Oncol. 2005;23(1):154–164. doi: 10.1200/jco.2005.03.139. [DOI] [PubMed] [Google Scholar]
- 74.Kalekou H, Miliaras D. Immunohistochemical study of microvessel density, CD44 (standard form), p53 protein and c-erbB2 in gallbladder carcinoma. J Gastroenterol Hepatol. 2004;19(7):812–818. doi: 10.1111/j.1440-1746.2004.03357.x. [DOI] [PubMed] [Google Scholar]
- 75.Nakazawa K, Dobashi Y, Suzuki S, Fujii H, Takeda Y, Ooi A. Amplification and overexpression of c-erbB-2, epidermal growth factor receptor, and c-met in biliary tract cancers. J Pathol. 2005;206(3):356–365. doi: 10.1002/path.1779. [DOI] [PubMed] [Google Scholar]
- 76.Kawamoto T, Krishnamurthy S, Tarco E, Trivedi S, Wistuba II, Li D, et al. HER receptor family: novel candidate for targeted therapy for gallbladder and extrahepatic bile duct cancer. Gastrointest Cancer Res. 2007;1(6):221–227. [PMC free article] [PubMed] [Google Scholar]
- 77.Harder J, Waiz O, Otto F, Geissler M, Olschewski M, Weinhold B, et al. EGFR and HER2 expression in advanced biliary tract cancer. World J Gastroenterol. 2009;15(36):4511–4517. doi: 10.3748/wjg.15.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roa I, de Toro G, Schalper K, de Aretxabala X, Churi C, Javle M. Overexpression of the HER2/neu gene: a new therapeutic possibility for patients with advanced gallbladder cancer. Gastrointest Cancer Res. 2014;7(2):42–48. [PMC free article] [PubMed] [Google Scholar]
- 79.Li M, Zhang Z, Li X, Ye J, Wu X, Tan Z, et al. Whole-exome and targeted gene sequencing of gallbladder carcinoma identifies recurrent mutations in the ErbB pathway. Nat Genet. 2014;46(8):872–876. doi: 10.1038/ng.3030. [DOI] [PubMed] [Google Scholar]
- 80.Kiguchi K, Carbajal S, Chan K, Beltran L, Ruffino L, Shen J, et al. Constitutive expression of ErbB-2 in gallbladder epithelium results in development of adenocarcinoma. Cancer Res. 2001;61(19):6971–6976. [PubMed] [Google Scholar]
- 81.Wu Q, Kiguchi K, Kawamoto T, Ajiki T, Traag J, Carbajal S, et al. Therapeutic effect of rapamycin on gallbladder cancer in a transgenic mouse model. Cancer Res. 2007;67(8):3794–3800. doi: 10.1158/0008-5472.can-06-3214. [DOI] [PubMed] [Google Scholar]
- 82.Kim MA, Lee HJ, Yang HK, Bang YJ, Kim WH. Heterogeneous amplification of ERBB2 in primary lesions is responsible for the discordant ERBB2 status of primary and metastatic lesions in gastric carcinoma. Histopathology. 2011;59(5):822–831. doi: 10.1111/j.1365-2559.2011.04012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seol H, Lee HJ, Choi Y, Lee HE, Kim YJ, Kim JH, et al. Intratumoral heterogeneity of HER2 gene amplification in breast cancer: its clinicopathological significance. Mod Pathol. 2012;25(7):938–948. doi: 10.1038/modpathol.2012.36. [DOI] [PubMed] [Google Scholar]
- 84.Yoon HH, Shi Q, Sukov WR, Lewis MA, Sattler CA, Wiktor AE, et al. Adverse prognostic impact of intratumor heterogeneous HER2 gene amplification in patients with esophageal adenocarcinoma. J Clin Oncol. 2012;30(32):3932–3938. doi: 10.1200/jco.2012.43.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cho EY, Park K, Do I, Cho J, Kim J, Lee J, et al. Heterogeneity of ERBB2 in gastric carcinomas: a study of tissue microarray and matched primary and metastatic carcinomas. Mod Pathol. 2013;26(5):677–684. doi: 10.1038/modpathol.2012.205. [DOI] [PubMed] [Google Scholar]
- 86.Kurozumi S, Padilla M, Kurosumi M, Matsumoto H, Inoue K, Horiguchi J, et al. HER2 intratumoral heterogeneity analyses by concurrent HER2 gene and protein assessment for the prognosis of HER2 negative invasive breast cancer patients. Breast Cancer Res Treat. 2016;158(1):99–111. doi: 10.1007/s10549-016-3856-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hou Y, Nitta H, Wei L, Banks PM, Portier B, Parwani AV, et al. HER2 intratumoral heterogeneity is independently associated with incomplete response to anti-HER2 neoadjuvant chemotherapy in HER2-positive breast carcinoma. Breast Cancer Res Treat. 2017;166(2):447–457. doi: 10.1007/s10549-017-4453-8. [DOI] [PubMed] [Google Scholar]
- 88.Rye IH, Trinh A, Saetersdal AB, Nebdal D, Lingjaerde OC, Almendro V, et al. Intratumor heterogeneity defines treatment-resistant HER2+ breast tumors. Mol Oncol. 2018;12(11):1838–1855. doi: 10.1002/1878-0261.12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ruschoff J, Dietel M, Baretton G, Arbogast S, Walch A, Monges G, et al. HER2 diagnostics in gastric cancer-guideline validation and development of standardized immunohistochemical testing. Virchows Arch. 2010;457(3):299–307. doi: 10.1007/s00428-010-0952-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schmieder R, Edwards R. Quality control and preprocessing of metagenomic datasets. Bioinformatics. 2011;27(6):863–864. doi: 10.1093/bioinformatics/btr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. %J EMBnet journal. 2011;17(1):3. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 92.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38(16):e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30(7):923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 96.Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature. 2015;526(7571):68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19(9):1655–1664. doi: 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Representative micrographs of immunocytochemical staining for CK7, CK19, Mesothelin, Vimentin and Calretinin in the three clones isolated from the ascites-derived primary culture (magnification, 40×).
Additional file 2. Gene expression of normalized transcripts per million in Chilean GBC cell lines.
Additional file 3. Exonic mutations in Chilean GBC cell lines.
Additional file 4. Venn diagrams depicting unique and shared (overlapping circles) variants in the three cell lines. (a) non-synonymous; (b) synonymous; and (c) frameshift. The green circle depicts the variants identified in PUC-GBC1, the orange circle depicts the variants identified in PUC-GBC2, and the blue circle depicts the variants identified in PUC-GBC3.
Additional file 5. Sequencing statistics.
Data Availability Statement
All data generated and/or analyzed in this study are included in this published article and its additional files. Additionally, they are available from the corresponding author on reasonable request.