Abstract
Extracellular vesicles (EVs) have been described as novel bio-markers and bio-activators in vascular dysfunction in hypertension. However, the mechanism(s) by which EVs affect vascular function is unknown. To examine the effects of EVs on endothelial-dependent vasodilation [acetylcholine (ACh)], we isolated circulating EVs from platelet-poor plasma using a low centrifugation speed (17,000 g), and mesenteric resistance arteries from 12-wk old normotensive Wistar-Kyoto rats (WKY) and spontaneously hypertensive rats (SHR). Arteries were cannulated on a pressure myograph and EVs were added to the vessel lumen and circulating bath. We found that circulating EVs from normotensive WKY reduced vasodilation of normotensive WKY arteries, but had no effect on hypertensive SHR arteries. In contrast, EVs from hypertensive SHR failed to reduce vasodilation of arteries from both WKY and SHR. The restraining effect on vasodilation by EVs from normotensive WKY may be mediated by inhibition of eNOS, as addition of L-NAME did not provide any additive effect. Moreover, circulating EVs from normotensive 6 week old SHR, an age where SHR have not yet developed hypertension, had similar restraining effect on vasodilation. In addition, delipidation of EVs did not alter the restraining effect of EVs from WKY, but did restore the restraining effect of EVs from SHR. Finally, EVs from normotensive humans also restrained vasodilation of normotensive mouse arteries, an effect not observed in EVs from hypertensive humans. Taken together, our data supports a vasoactive role of EVs that is altered in hypertension.
Keywords: Extracellular vesicles, eNOS, acetylcholine, vascular reactivity, vasodilation, hypertension
Introduction
Hypertension is a key risk factor for cardiovascular disease, a leading cause of death worldwide1. Although treatments for hypertension can reduce the risk for cardiovascular events, existing therapies are only partially effective. Hypertension is commonly associated with endothelial dysfunction, which can contribute to or cause further exacerbation of this disease1–3. Thus, development of minimally- or non-invasive diagnostic tools is necessary to evaluate the stage of disease and establish early indicators of hypertension, such as the beginning stages of endothelial dysfunction. Circulating extracellular vesicles (EVs) may be such tools, as they recently have been reported to be associated with numerous cardiovascular diseases, including hypertension2, 4.
EVs are submicron vesicles that belong to a heterogeneous population of small membrane fragments secreted from various cell types upon many stimuli, although some have been found to be several microns in size5. Smaller EVs, or “exosomes”, are <100nm in size and released from multivesicular bodies, whereas larger EVs, or microvesicles, majority ranging from 100nm to 1000nm, are thought to be the product of exocytic budding6. Initially thought of as “cell trash”, EVs have emerged as novel bio-markers and bio-activators in health and disease, particularly in cardiovascular disease as they carry protein, lipids and nucleic acids that can regulate cardiovascular function7, 8. Therefore, it is important to understand the physiological roles of these circulating EVs and how they change, both compositionally and functionally, during hypertension.
There is accumulating evidence indicating that circulating EVs likely have functional effects within the vasculature, including atherosclerosis, ischemic injury, and hypertension9. Studies have suggested that circulating EVs may be increased in individuals with cardiovascular risk factors, and, therefore, may contribute to endothelial dysfunction in hypertension and serve as biomarkers for development of hypertension9, 10. Most patients with essential HTN have higher levels of circulating EVs which correlate with the degree of HTN and vascular dysfunction11–13. Other groups have analyzed EV function and shown that EVs reduce endothelial-dependent vasodilation, potentially via a nitric oxide (NO)-dependent mechanism14–17. In particular, Brodsky et al. and Burger et al. showed that endothelial derived EVs from cell culture impaired acetylcholine induced vasorelaxation of aortic and second order mesenteric rings, respectively, in a concentration-dependent manner17, 18. Horn et al. analyzed EV cargo from human plasma and showed that EVs can carry functional endothelial nitric oxide synthase (eNOS)8. However, previously published studies provided only a limited characterization of EVs. In addition, the vessels used in these published studies were predominantly conduit or feed arteries14–17. Thus, it is not known how circulating EVs may affect resistance artery function during the basal state and after induction of hypertension.
In this study, we examined the role of an enriched EV preparation using low centrifugation of platelet-poor plasma (17,000 g centrifugation speed) from normotensive Wistar-Kyoto rats (WKY) and spontaneous hypertensive rats (SHR) on acetylcholine (ACh)-mediated vasodilation of resistance mesenteric arteries. We next determined the contribution of nitric oxide synthase (NOS) in the vasoactive effect of EVs, and whether their effect depends on an intact membrane. Furthermore, we compared the effect of circulating EVs isolated from normotensive and hypertensive human subjects (20,000 g centrifugation speed) on normotensive mouse resistance mesenteric arteries.
Methods
The data supporting the findings of this study are available from the corresponding author on reasonable request.
Expanded methods and materials details are available in the online-only supplement (please see http://hyper.ahajournals.org).
Animals
Five week old male Wistar-Kyoto rats (WKY) and spontaneously hypertensive rats (SHR) from Envigo were employed. C57BL/6J wild-type (WT) mice purchased from Taconic were used between 10–20 weeks of age. Blood and vessels were collected from the same rats when possible to minimize the number of animals used.
EV isolation
Blood was removed from rats (WKY and SHR) from a carotid artery central access. Under anesthesia (Inactin, Sigma), an incision was made along the right side of the trachea exposing the right carotid artery. A PE-50 tube was inserted into the right carotid artery and after ensuring blood flow, ligated into place with 4–0 silk ligature19. Blood was collected into 0. 218 M, 0.1 μm filtered citrate buffer and processed within 30 minutes of collection. Platelet poor plasma was obtained by centrifugation with a bench top centrifuge (Centrifuge 5810R Eppendorf) at 3,250 g (max speed) at room temperature for 10 minutes in a swing bucket rotor (A-4–62, Eppendorf). An EV pellet was obtained from platelet poor plasma by a second centrifugation spin (accuSpin Micro 17; Fisher Scientific) at 17,000 g (P17) for 30 min. For western blot analysis and obtaining a positive EV fraction, the supernatant 17,000 g was further centrifuged at 116,000 g (P116) for 75 min at 4°C in polycarbonate tubes in a Beckman Coulter XL-90 ultracentrifuge and a type 90 Ti Rotor (Beckman coulter) (k factor at maximum speed 25). The EV pellet was then suspended either in 100 μL of phosphate buffer saline (PBS) buffer or 100 μL electrophoresis solubilization buffer (ESB). For all vasoreactivity experiments, samples were resuspended in 120 μl of Krebs-HEPES buffer containing (in mM) NaCl 118.4, KCl 4.7, MgSO4 1.2, NaHCO3 4, KH2PO4 1.2, CaCl2 2, Hepes 10, glucose 6 (pH 7.40). From 12 week old rats we obtained about 7–8 mL of whole blood and 5 mL from 6 week old rats. For pressure myography experiments, EVs were isolated from 1 mL of blood from each animal.
Human EV isolation
Under IRB-HSR Study protocol #17192, blood samples were collected from normotensive and hypertensive (persistently elevated blood pressure above 140 mm Hg systolic) human volunteers. Citrated blood was also collected from hypertensive humans with informed consent and with evaluation and approval from the University of Virginia Institutional Review Board. An EV pellet was generated from platelet poor plasma as it was done with the rat blood samples using a low centrifugation speed (20,000 g). Of note, EVs from rats were isolated using a slightly lower speed of 17,000 g. As plasma samples from rats were more dilute and less viscous, speed for isolation of human EVs was slightly increased to 20,000 g to achieve a similar pull down of EVs. However this is a minimal change compared to high speed ultracentrifugation (116,000 g) and did not affect the biological function of the preparation as demonstrated in the result section. We obtained approximately 7.5 mL of blood from each patient. Three patients were healthy controls (2 female, one male, average age 35) and three patients were hypertensive (2 female, one male, average age 50).
EV morphology
Cryo-transmission electron microscopy was performed to study the morphology of EVs20–22.
EV size detection
Nanoparticle Tracking Analysis (NTA) was carried out using the ZetaView PMX 110 multiple parameter particle tracking analyzer (Particle Metrix, Meerbusch, Germany) configured with a 488 nm laser with a long wave-pass cut-off filter (500 nm) and a sensitive CMOS camera 640 × 480 pixels in size mode using ZetaView software version 8.02.28 provided from the manufacturer23.
Rat kidney microsomes enrichment (Mi)
A standard rough microsomal fraction was obtained by centrifugation of kidney homogenates obtained from 12 weeks old WKY for western blot analysis24.
EV and non-EV protein analysis by western blotting
Protein assessment of isolated EVs and Mi (kidney microsomes) was performed25. Proteins were separated by hand cast SDS-PAGE gradient gels (Resolving gel T= 5–20% (w/v); C=2.6%) and transferred to nitrocellulose membranes. Membranes were incubated with primary antibodies against TSG 101 or calreticulin. Acquisition of the fluorescent signal was performed by Odyssey infrared imaging system (LI-COR Biosciences).
EV protein analysis by flow cytometry
EV protein content was detected with imaging flow cytometry according to our recent publications26, 27. The following antibodies were added to EV samples: CD105-fluorescein isothiocyanate (FITC), CD31-phycoerythrin (PE), Annexin V (AV)- Alexa Fluor®568, CD45- Alexa Fluor®405 (AF405), and CD42d-Allophycocyanin (APC). Imaging flow cytometry [Amnis ImageStreamX Mark II (ISX)] and analysis software [ISX (IDEAS, Amnis Corporation; Application Version 6.2.64.0)] was used to detect and determine the source and count of the EVs as described previously by our group26, 27.
EV delipidation
EVs pellets were resuspended in 100 μL of 0.1 μm filtered PBS and delipidated by chloroform methanol and resuspended in Krebs-HEPES buffer28.
Blood Pressure Measurements
Mean arterial pressure (MAP) on rats 7 weeks and older was measured with telemetry probes [Data Sciences International, (DSI)], as previously described19. Due to the smaller size of 6 week old rats, MAP at 6 weeks of age was measured via direct carotid artery cannulation using a Digi-Med Blood Pressure Analyzer (Micro-Med, Inc)19.
Pressure Myography
Freshly isolated 3rd or 4th order rat or mouse mesenteric arteries (< 220 μm) were mounted in a pressure arteriograph [Danish MyoTechnology (DMT)] and equilibrated for 30 min at 80 mmHg and 37°C, as previously described29. If treated with L-nitro-arginine methyl ester (L-NAME, 100 μM), L-NAME was added 10 minutes into the equilibration step. The lumen and bath solutions were then replaced with EV containing Krebs-HEPES solution while control vessels were perfused with Krebs-HEPES. 30 μL of the isolated EV solution (or liposomes) were placed into 3 mL buffer for the lumen solution and 70 μL of EVs were placed into the 10 mL circulating bath. The vessels were then equilibrated for an additional 10 min followed by pre-constriction with 10 μM phenylephrine (PE). To complete a full ACh dose response curve for each vessel, increasing concentrations of ACh were then added into the bath to examine the endothelial-dependent vasodilation of these vessels. Endothelial cell health was confirmed using NS309 and smooth muscle cell health was confirmed by PE. Maximal passive diameter of the vessels was then obtained as previously described29. Internal diameter was measured at each step using the DMT MyoVIEW software. Data were replotted in multiple figures (such as control WKY arteries) because comparisons were done across all groups as all treatment groups underwent the same experimental design. The EC50 of the ACh dilation and raw lumen diameters for resting diameter (after EV treatment), maximal constriction, maximum dilation for each treatment group were calculated in the online-only supplemental Table S1 and Table S2 (please see http://hyper.ahajournals.org).
Statistics
Data were analyzed using Prism 8 software (GraphPad Software Inc.) and displayed in mean ± SEM. Two-tailed Student’s t-test and one-way ANOVA followed by a Tukey’s multiple comparisons post-hoc test, were used as appropriate. For pressure myography acetylcholine curve data, a mixed-effects model (REML) for repeated measures followed by Sidak’s multiple comparisons test was used and comparisons were made at each dose of ACh between groups. p < 0.05 was considered to be statistically significant.
Study Approval
All animal procedures were approved by the University of Virginia School of Medicine Animal Care and Use Committee.
Results
EVs isolated from normotensive and hypertensive rats exhibited different phenotype populations based on cell surface markers
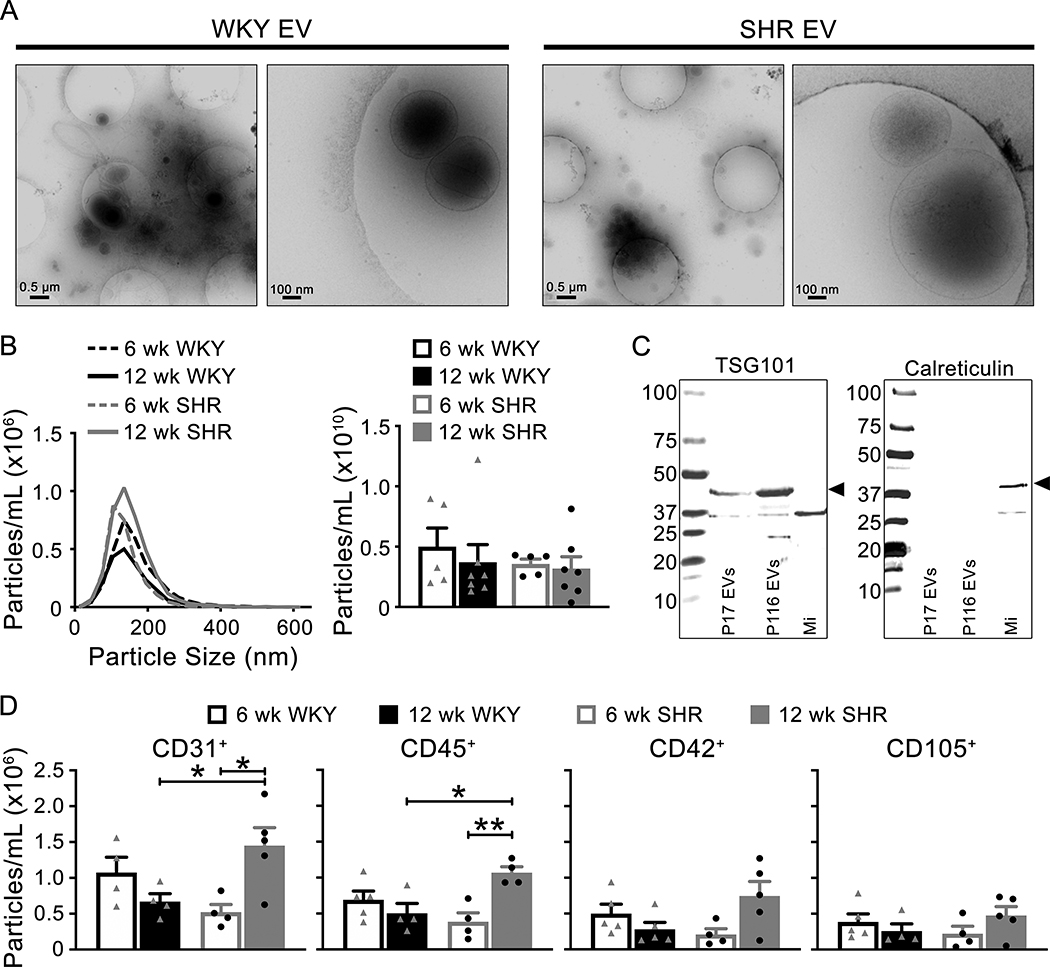
Morphology, size, concentration and protein cargo of circulating EVs were analyzed using the low centrifugation speed pellet. Initially, cryo-electron microscopy was used to describe morphology and demonstrated lipid bi-layer vesicles of <100nm to 1000nm from WKY and SHR isolated enriched EV pellets (Figure 1A). Size and concentration of EVs were measured with NTA (Figure 1B). EVs isolated from 6 and 12 week old WKY and SHR plasma had a similar size distribution and concentrations (Table 1), suggesting that age and blood pressure status did not affect size or concentration of circulating EVs. The presence of EV specific-protein, TSG101, and lack of calreticulin, which is specifically absent in EVs, were confirmed in the isolated EV pellets (Figure 1C). To determine the cell origin of EVs from plasma, targeted phenotyping was performed with imaging flow cytometry for markers of endothelial (CD105+), platelet (CD42d+), leukocyte (CD45+) and platelet cell adhesion molecule (PECAM, CD31+) origin. Leukocyte (CD45+) and PECAM (CD31+) derived EVs were significantly higher in hypertensive SHR (12 weeks old) compared to normotensive SHR (6 weeks old) (Figure 1D and Supplemental Figure S1, please see http://hyper.ahajournals.org). No significant difference was observed in the other surface markers, CD105 and CD42d.
Figure 1: Characterization of EV morphology, size, concentration and protein cargo.
(A) Cryo-electron microscopy images show EVs of different sizes (<100nm to 1000nm) from 12 week old WKY and SHR (low magnification (left panel) and high magnification (right panel) images). (B) EV size distribution and concentration were not different between age and genotypes of animals using NTA (WKY: triangle symbols; SHR: circle symbols). (C) Western blots of known EV protein (TSG101) and non-EV protein (Calreticulin) was performed on EV pellets (P17 and P116 are EV containing samples, while a crude microsome membrane preparation (Mi) was used as a positive control for calreticulin). (D) Summarized targeted phenotyping data of EVs from WKY (triangle symbols) and SHR (circle symbols) using the following markers: CD31, CD45, CD42, and CD105. Hypertension resulted in a significant increase in CD31 and CD45 positive EVs. Data are presented as mean ± sem. One-way ANOVA: * p < 0.05, ** p < 0.01.
Table 1:
Concentration and size of EVs from 6 and 12 week WKY and SHR.
| Animal | Age (weeks) | Individual Animal | Particles/mL | Particle Size (nm) | ||
|---|---|---|---|---|---|---|
| Median | Mode | Mean | ||||
| WKY | 6 | 1 | 9.81×1009 ± 9.81×1008 | 135.6±5.7 | 145.3±12.6 | 149.9±59.4 |
| 2 | 1.31×1010 ± 1.46×1009 | 150.6±7.3 | 151.8±13.2 | 171.1±70.3 | ||
| 3 | 8.05×1009 ± 1.82×1009 | 146.2±7.4 | 149.6±15.9 | 163.8±63.8 | ||
| 4 | 3.59×1010 ± 4.11×1009 | 133.3±5.4 | 138.9±12.3 | 148.3±60.6 | ||
| 5 | 3.40×1010 ± 3.88×1009 | 142.7±5.1 | 149.1±12.8 | 155.5±54.6 | ||
| Average | 2.00×1010 ± 1.38×1010 | 141.7 ± 7.2 | 146.9 ± 5.1 | 157.7 ± 9.6 | ||
| WKY | 12 | 1 | 5.23×1009 ± 6.55×1008 | 145.7±10.6 | 148.6±16.3 | 159.4±56.4 |
| 2 | 7.59×1009 ± 8.50×1008 | 145.5±9.4 | 153.6±15.9 | 157.6±54.4 | ||
| 3 | 1.06×1010 ± 1.78×1009 | 136.2±10.0 | 144.6±15.1 | 151.3±60.9 | ||
| 4 | 4.89×1010 ± 7.47×1009 | 133.9±5.3 | 141.7±9.9 | 147.0±59.4 | ||
| 5 | 1.48×1010 ± 1.70×1009 | 152.3±8.7 | 157.0±20.7 | 157.5±57.3 | ||
| 6 | 1.04×1010 ± 3.75×1009 | 138.6±9.5 | 145.0±17.5 | 153.7±56.3 | ||
| 7 | 6.49×1009 ± 6.69×1008 | 141.8±12.4 | 147.9±20.5 | 157.5±57.3 | ||
| Average | 1.49×1010 ± 1.53×1010 | 142.0 ± 6.4 | 145.6 ± 4.2 | 154.9 ± 4.4 | ||
| SHR | 6 | 1 | 1.07×1010 ± 1.08×1009 | 143.7±6.7 | 152.4±11.4 | 155.1±57.9 |
| 2 | 1.01×1010 ± 1.82×1009 | 134.3±33.9 | 143.3±37.3 | 156.3±59.5 | ||
| 3 | 1.67×1010 ± 2.35×1009 | 136.7±6.0 | 147.1±14.1 | 148.2±55.7 | ||
| 4 | 1.72×1010 ± 2.32×1009 | 137.3±3.6 | 143.0±14.0 | 150.4±55.5 | ||
| 5 | 1.68×1010 ± 1.62×1009 | 138.6±7.9 | 142.4±15.4 | 151.4±50.2 | ||
| Average | 1.43×1010 ± 3.57×1009 | 138.1 ± 3.5 | 148.3 ± 5.4 | 152.3 ± 3.4 | ||
| SHR | 12 | 1 | 1.24×1010 ± 6.33×1008 | 135.9±4.8 | 144.7±11.2 | 148.9±58.0 |
| 2 | 1.49×1009 ± 2.25×1008 | 175.0±13.7 | 195.6±52.4 | 199.3±92.8 | ||
| 3 | 6.79×1009 ± 4.70×1008 | 136.4±5.9 | 136.6±12.4 | 151.4±58.8 | ||
| 4 | 1.15×1010 ± 1.12×1009 | 141.1±5.4 | 145.3±12.6 | 155.8±59.7 | ||
| 5 | 3.25×1010 ± 2.78×1009 | 144.9±8.0 | 151.6±14.0 | 158.1±56.5 | ||
| 6 | 5.34×1009 ± 4.07×1008 | 137.7±7.3 | 143.9±16.6 | 152.4±55.3 | ||
| 7 | 1.91×1010 ± 3.23×1009 | 133.0±6.3 | 138.7±12.5 | 146.2±57.0 | ||
| Average | 1.27×1010 ± 1.04×1010 | 143.4 ± 14.5 | 150.9 ± 20.3 | 158.9 ± 18.3 | ||
Data are mean ± s.d. One-way ANOVA.
SHR resistance arteries exhibit similar vasodilation to acetylcholine compared to normotensive WKY resistance arteries
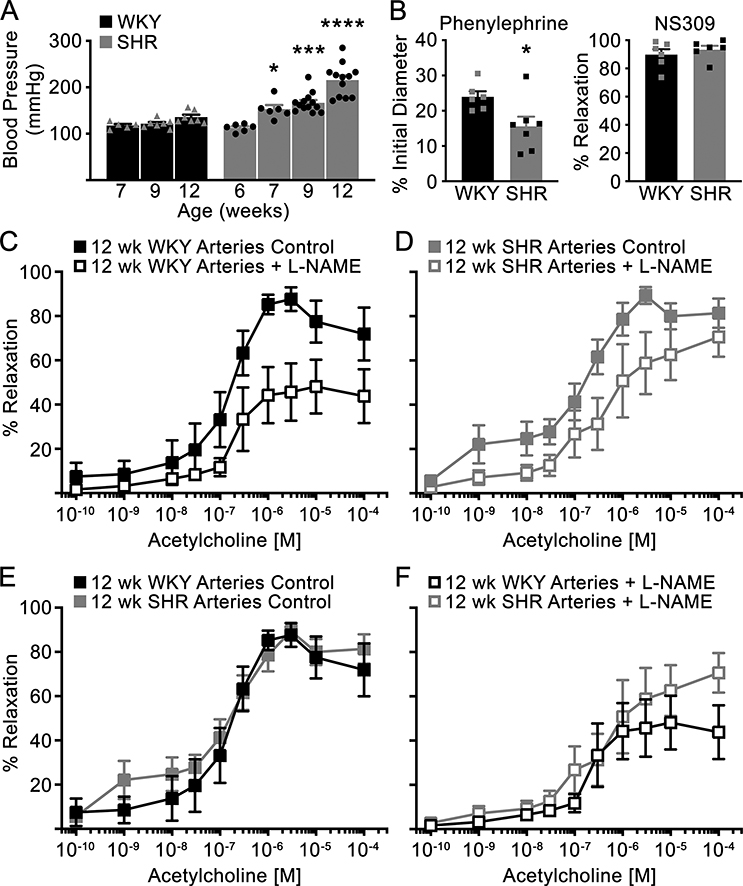
We confirmed that SHR are hypertensive compared to control WKY beginning at 7 weeks of age (Figure 2A). Next, we assessed endothelial-dependent vasodilation (ACh) of isolated 3rd and 4th order mesenteric arteries from 12 week old WKY and SHR. We confirmed functional smooth muscle and endothelial cells using phenylephrine and NS309, an alpha adrenergic agonist and SKCa/IKCa agonist, respectively (Figure 2B). Both WKY and SHR arteries were able to constrict and dilate, however, SHR arteries displayed hyperconstriction in response to PE. Following pre-constriction with PE, ACh was added in increasing doses and resulted in similar vasodilation response curve of mesenteric arteries from both WKY and SHR. L-NAME, an inhibitor of endothelial nitric oxide synthase (eNOS), slightly reduced vasodilation in WKY arteries (Figure 2C) and to a lesser extent SHR arteries (Figure 2D). The maximal vasodilation of WKY arteries treated with L-NAME was significantly reduced compared to control WKY arteries (p=0.05, Supplemental Table S1, please see http://hyper.ahajournals.org). Vasodilation of 12 week old SHR arteries, either untreated control or L-NAME treated, was not different compared to age-matched WKY arteries (Figures 2E, F).
Figure 2: WKY and SHR mesenteric arteries similarly vasodilate to ACh, although SHR arteries are hyperconstrictive to phenylephrine.
(A) MAP was measured over time in WKY (triangle symbols) and SHR (circle symbols) via direct carotid cannulation (6 weeks of age) and radiotelemetry (>7 weeks of age). (B) Isolated mesenteric arteries from WKY and SHR were constricted with phenylephrine and dilated with NS309. (C-F) Arteries pre-constricted with phenylephrine were exposed to increasing concentrations of ACh in the present or absence of 100 μM L-NAME (12 wk WKY Arteries Control and 12 wk SHR Arteries + L-NAME: n=6; 12 wk WKY Arteries + L-NAME: n=5; and 12 wk SHR Arteries Control: n=7). Data presented as mean ± sem. One-way ANOVA (vs baseline MAP recording, WKY 7 wk or SHR 6 wk was used as baseline for comparisons) (A), Student’s t-test (B), and Mixed-effects model for repeated measures with Sidak’s multiple comparisons test (C-F) were used; * p < 0.05, *** p < 0.001, **** p < 0.0001.
EVs isolated from normotensive rats, but not hypertensive rats, reduced vasodilation in healthy resistance arteries
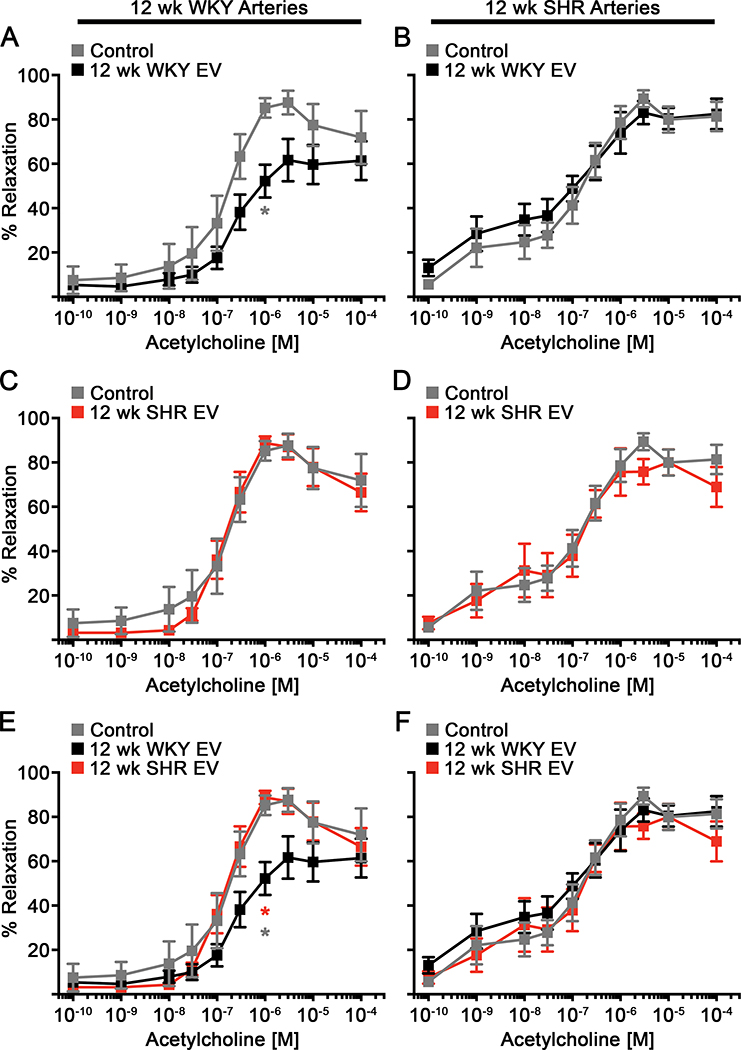
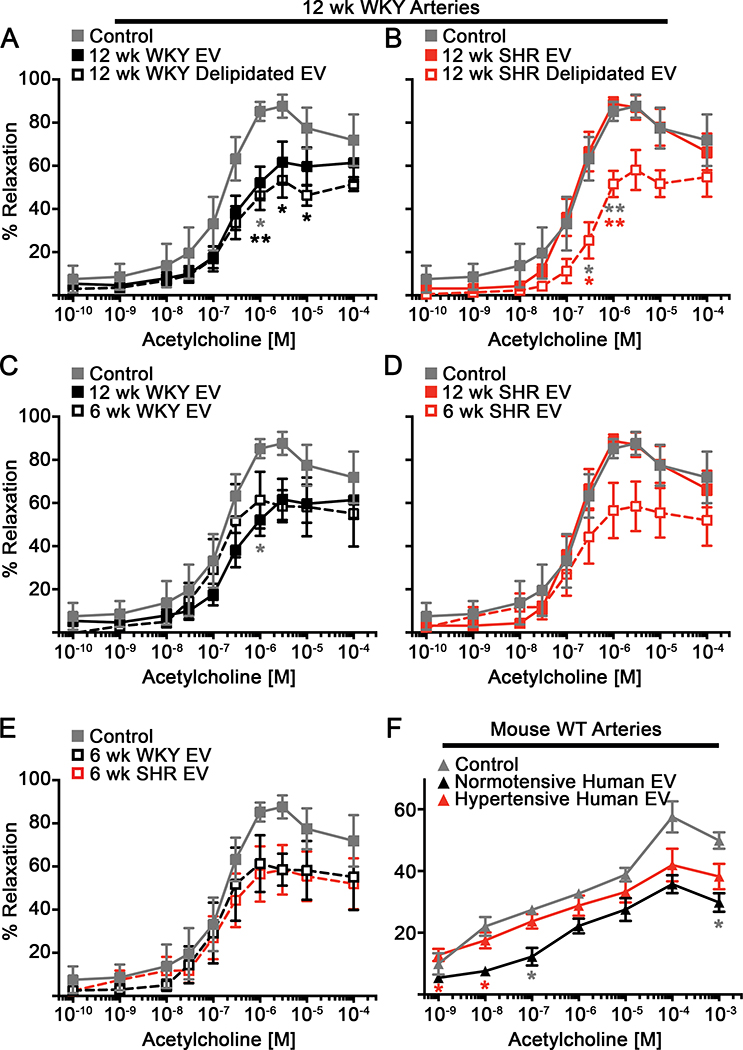
To determine the acute effects of EVs on endothelial-dependent vasodilation, we first treated WKY and SHR arteries with the enriched EV preparation from 12 week old WKY or SHR blood for 10 min and then performed ACh dose response curves. For simplicity we will call the enriched EV pellet from WKY “WKY EVs” and from SHR “SHR EVs”. WKY arteries treated with WKY EVs displayed significantly reduced vasodilation and maximal percent relaxation (Figure 3A and Supplemental Table S1, please see http://hyper.ahajournals.org). In contrast, ACh-mediated vasodilation was not altered by WKY EV treatment in SHR mesenteric arteries (Figure 3B). SHR EVs failed to alter vasodilation in both WKY and SHR arteries (Figures 3C, D). Treatment with WKY EVs significantly reduced vasodilation compared to SHR EVs only in normotensive WKY arteries, but not in hypertensive SHR arteries (Figures 3E, F). Based on previous data suggesting EVs may regulate vascular function30, we pretreated WKY arteries with L-NAME, a NOS inhibitor, followed by treatment with WKY or SHR EVs. Interestingly, pre-treatment with L-NAME followed by WKY EV treatment did not result in any additional reduction in vasodilation of WKY arteries compared to WKY EVs alone or L-NAME alone (Figures 4A, C, E). Vasodilation of WKY vessels pretreated with L-NAME and SHR EVs was similarly reduced compared to WKY vessels treated with L-NAME alone, but not significantly reduced compared to control WKY vessels or vessels treated with SHR EVs alone (Figure 4B, D, F). These data suggest that reduced vasodilation mediated by the enriched WKY EV preparation is due to reduced NO signaling.
Figure 3: Extracellular vesicles from WKY blood, but not SHR blood, reduces vasodilation of arteries from WKY, an effect lost in SHR arteries.
WKY (A, C, E) and SHR (B, D, F) mesenteric arteries were exposed to EVs isolated from WKY or SHR EVs and treated with increasing doses of ACh (12 wk WKY Arteries: Control (n=6), 12 wk WKY EV (n=5); 12 wk SHR EV (n=4); 12 wk SHR Arteries: Control (n=7); 12 wk WKY EV (n=5); 12 wk SHR EV (n=6)). Data presented as mean ± sem. Mixed-effects model for repeated measures with Sidak’s multiple comparisons test (A,E: 12 wk WKY EV vs. Control (grey *); 12 wk WKY EV vs. 12 wk SHR EV (red *)): * p < 0.05, ** p < 0.01.
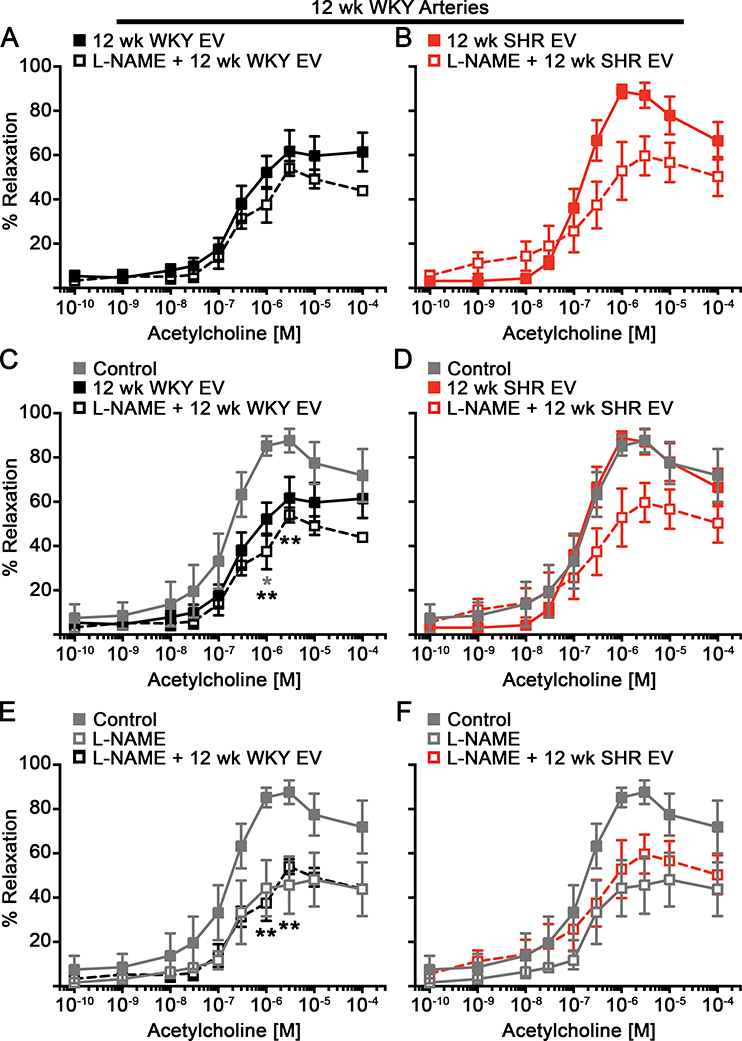
Figure 4: Inhibition of NOS does not further reduce the vasodilation of WKY arteries treated with WKY extracellular vesicles.
ACh-mediated dilation was performed on WKY mesenteric arteries were exposed to WKY (A, C, E) or SHR EVs (B, D, F) with or without L-NAME (Control: n=6; L-NAME, 12 wk WKY EV, and L-NAME + 12 wk SHR EV: n=5; 12 wk SHR EV and L-NAME + 12 wk WKY EV: n=4). Data presented as mean ± sem. Mixed-effects model for repeated measures with Sidak’s multiple comparisons test (C: Control vs 12 wk WKY EV (grey *); Control vs. L-NAME + 12 wk WKY EV (black *); E: Control vs. L-NAME + 12 wk WKY EV (black *)): * p < 0.05, ** p < 0.01.
Removal of the EV lipid membrane of both WKY and SHR EVs reduced vasodilation of resistance arteries
To evaluate if intact EVs are necessary for the EV-mediated regulation of vasodilation, we removed the lipid membrane of the EVs following isolation from the blood and treated WKY arteries with these delipidated EVs. Delipidation of WKY EVs resulted in significantly reduced vasodilation compared to untreated control arteries, similar to that of arteries treated with intact WKY EVs (Figure 5A and Supplemental Table S1, please see http://hyper.ahajournals.org). Interestingly, SHR delipidated EVs also significantly reduced ACh-mediated vasodilation compared to both untreated and SHR intact EVs treated WKY arteries (Figure 5B, and Supplemental Table S1, please see http://hyper.ahajournals.org). These data suggest that a vasoactive factor within the EV cargo is sufficient to impair vasodilation and that an intact lipid membrane of SHR EVs inhibits its effect. To confirm that the presence of lipids alone do not impact vasodilation, we treated WKY arteries with liposomes and found no significant reduction in vasodilation compared to untreated control arteries (Supplemental Figure S2A, B, C; please see http://hyper.ahajournals.org).
Figure 5: Delipidated SHR EVs or EVs from normotensive young SHR retain the ability to reduce vasodilation of WKY arteries.
ACh curves were performed on WKY arteries were exposed to WKY (A) or SHR (B) EVs that have been delipidated (12 wk WKY Delipidated EV and 12 wk SHR Delipidated EV: n=4). Alternatively, arteries were treated with EVs isolated from 6 week old WKY or SHR, prior to development of hypertension in SHR animals, and exposed to increasing concentrations of ACh (C-E) (6 wk WKY EV and 6 wk SHR EV: n=4). (F) ACh-mediated dilation was evaluated in control mouse WT mesenteric arteries (n=3 vessels) and arteries treated with EVs isolated from normotensive (3 patients; n=3 vessels) or hypertensive human patients (3 patients; n=5 vessels). Data presented as mean ± sem. Mixed-effects model for repeated measures with Sidak’s multiple comparisons test (A,C: Control vs 12 wk WKY EV (grey *); Control vs. 12 wk WKY Delipidated EV (black *). B: Control vs 12 wk SHR Delipidated EV (grey *); 12 wk SHR EV vs 12 wk SHR Delipidated EV (red *). F: Control vs Normotensive Human EV (grey *); Normotensive Human EV vs Hypertensive Human EV (red *)): * p < 0.05, ** p < 0.01, *** p < 0.001.
SHR EVs isolated prior to the development of hypertension reduced vasodilation of resistance arteries
We next asked whether blood pressure status alters EV function. To do this, we collected blood from 6 week old SHR, an age prior to the development of hypertension, or 6 week old WKY and analyzed vasodilation of 12 week old WKY arteries. WKY EVs from 6 and 12 week WKY similarly reduced vasodilation (Figure 5C). EVs isolated from 6 week old SHR retained their ability to reduced ACh-mediate vasodilation (Figure 5D), similar to that of 6 and 12 week old WKY EVs (Figure 5E). To evaluate if there is a functional difference in an enriched EV preparation from normotensive and hypertensive humans, we isolated mesenteric resistance arteries from wildtype (WT) C57BL/6 mice and treated them with the EVs isolated from blood samples from normotensive or hypertensive human subjects from the low centrifugation speed pellet (20,000 g). ACh-mediated vasodilation was significantly reduced when mouse arteries were treated with EVs from normotensive subjects, but not from hypertensive subjects (Figure 5F). These data suggest that hypertension alters circulating EVs and, therefore, their impact on endothelial-mediated vasodilation.
Discussion
EVs have been identified as potential novel bio-markers and bio-activators in the development of hypertension, in particular they have been found to affect vascular tone in patients with endothelial dysfunction8, 31. As they carry proteins, lipids and nucleic acids, EVs represent novel messengers of signaling molecules in the circulation to nearby or remote sites in the body32. Here we demonstrate that normotensive 12 week old WKY EVs from the low centrifugation speed pellet regulate vascular tone by restraining the vasodilatory response to ACh in 12 week old WKY resistance arteries without altering the EC50 to ACh. This effect is lost in SHR arteries, where ACh-mediated vasodilation of SHR arteries is not changed following treatment with 12 week old WKY EVs. Interestingly, EVs isolated from 12 week old SHR fail to reduce ACh-mediated vasodilation in WKY and SHR resistance arteries. These data suggest that at 12 weeks of age, the functions of both resistance arteries and circulating EVs are altered in SHR.
Isolation of SHR EVs prior to the development of hypertension (6 weeks old) results in a similar reduction in vasodilation as WKY EVs isolated at either 6 weeks or 12 weeks of age (when animals are normotensive); indicating that alteration of EV function occurs during or after the development of hypertension in SHR animals. It is also of clinical significance that EVs from hypertensive human patients exerted a similar effect on normotensive mouse resistance arteries. This lack of effect on vasodilation by EVs isolated from hypertensive subjects may be a failed attempt to compensate for the increased peripheral resistance. During hypertension, adaptive processes may alter the properties of EVs, rendering them more vasodilatory (or less anti-vasodilatory) as a compensatory response to counteract the increased vasoconstricted state.
A focus of future studies will be to determine how EVs or their cargo/content are altered with the development of or during hypertension. In vitro experiments have shown that different stimuli lead to biogenesis of different types of EVs, e.g. a hypoxic stimulus produces a different EV phenotype in regards to size and content33. Also of significance, some of the EV cargo can be found inside the EVs but are also carried within the lipid bilayer facilitating different mechanisms of paracrine/endocrine communication6, 34. Ligand-receptor-interaction, fusion with the recipient cell, or endocytosis are some possible ways of interaction of EVs with their recipient cells34. Our data suggests that a vasoactive factor within the EV cargo could be responsible for the reduced ACh-mediated vasodilation.
Delipidation of the enriched EV preparation both retains WKY EV function and recovers the reduced vasodilation with SHR EVs. EVs can contain a number of potential factors, such as mRNAs, miRNAs, or proteins35. As we are examining the effects of these EVs within a relatively acute period, alteration of transcription or translation is not a likely mechanism. The delipidation experiments suggest that the important vasoactive factor is positioned on the outer side of the EVs or found within the enriched EV cargo. EVs from SHR induced an anti-vasodilatory function after delipidation, indicating that the factor responsible for regulating endothelial-mediated vasodilation is likely localized within the EVs and was released with removal of the lipid bilayer. Thus, the intact membrane of EVs from 12 week old SHR may be altered, thereby prohibiting access of the EV-derived factor to the endothelium. However, changes to the EV structure, be it the lipid bilayer structure or the mechanism by which the cargo is released/delivered, following development of hypertension remains unknown25, 34. These novel findings suggest the importance of future studies to examine the changes to the lipid bilayer or cargo release/delivery mechanisms following development of hypertension.
With regards to potential vasoactive factors within EVs, earlier studies have advocated a role for NOS and nitric oxide (NO) signaling via EVs where, during disease, the expression of eNOS is reduced and flow-mediated dilation is impaired15–17, 36. However, a large portion of these data are correlative or utilized conduit arteries, where NO signaling is the major regulator of vasodilation. We show that in resistance arteries from normotensive rats, ACh-mediated vasodilation is partially reduced by inhibition of endothelial NOS (eNOS) via L-NAME. This inhibition of vasodilation is not as profound in SHR arteries, suggesting an alteration in the vasodilatory signals within hypertensive arteries. When WKY arteries were treated with L-NAME and WKY EVs, there appeared to be no additive effect, suggesting that EVs from healthy normotensive WKY may inhibit eNOS or NO-mediated signaling similar to the eNOS inhibitory effects of LNAME. While speculative, it is possible that EVs provide a break to vasodilation in normotension to maintain blood pressure in an optimal range for tissue perfusion.
An anti-vasodilatory effect of EVs has been demonstrated in other studies, however, the origin of the EVs differed between studies and may be important in understanding the role of EVs in vascular tone regulation. For example, T-cell derived EVs have been shown to reduce vasodilation, potentially through reduction in endothelial-mediated NO production30, 37, 38, whereas platelet derived EVs have resulted in a thromboxane A2-dependent vasoconstriction39. Endothelial derived EVs have been shown to limit vasodilation in both mouse and human arteries in an in vitro system. In particular, Densmore et al showed that EVs isolated from cultured endothelial cells (HUVECS) impaired ACh mediated vasodilation in both healthy C57BL/6 facialis arteries and human colon microvessels30. Interestingly, treatment of cultured endothelial cells with endothelial-derived EVs resulted in reduced NO production, likely due to a reduction in Ser1179 phosphorylation of eNOS30. These data are congruent with our data demonstrating a lack of additive effect by L-NAME, suggesting a potential role for changes in NO-mediated vasodilatory signaling following treatment of EVs from normotensive animals. As the EVs may alter the functional reactivity of smooth muscle cells and/or endothelial cells, examination of eNOS activity in endothelial cells and smooth muscle cell sensitivity to NO within normotensive and hypertensive arteries following exposure to EVs will need to be done in future experiments to determine the direct role of EVs in regulating eNOS/NO-mediated vasodilation30.
Our EV preparation included a mix of EVs deriving from all circulating cells in the blood and the endothelium. However targeted phenotyping with imaging flow cytometry indicates that leukocyte derived EVs (CD45+) and PECAM positive (CD31+) EVs were significantly higher in hypertensive SHR (12 weeks old) compared to normotensive SHR at 6 weeks. These populations of EVs might be the potential dominant EV subtype(s) responsible for the differential vasodilatory effect of EVs from SHR and WKY. CD45 is found on many cells of lymphoid and myeloid cell lines, indicating that the immune system may play a role. Isolation of adequate subpopulations of circulating EVs and increasing the purity of EV preparations to limit contamination of soluble proteins, will enable further understanding of which EVs derived from normotensive animals regulate endothelial-dependent vasodilation and identify those EVs and their content that are affected by hypertension. Although we had limited material, our EV preparation is enriched for EVs as demonstrated by cryo-electron microscopy, the presence of known EV specific-protein TSG101, and the lack of calreticulin, a protein not associated with EVs. It is also of clinical significance that EVs from hypertensive human patients exerted a similar effect on normotensive mouse resistance arteries. Further experiments will need to delineate if EVs from normotensive and hypertensive animals have dose-dependent effects, and whether they have endothelial or vascular smooth muscle-specific effects using endothelial denuded vessels.
Supplementary Material
Perspectives.
We demonstrate that an enriched EV preparation from normotensive humans and rats limit vasodilation in response to endothelial-dependent vasodilatory signals, potentially through an eNOS-mediated mechanism. These data strongly support a paracrine/endocrine role for circulating EVs to regulate vascular tone and, thereby, maintain organ perfusion by limiting vasodilatory signaling in resistance arteries. Interestingly, this function is lost in EVs isolated from subjects with hypertension, likely due to changes of their lipid bilayer composition and cargo. Endothelial dysfunction is extensively studied in the hypertension literature; however, we find that circulating EVs from enriched preparations also become dysfunctional due to increased blood pressure. Therefore, while circulating EVs are valuable biomarkers for hypertension, they may also contribute to the development of hypertension and ultimately end-organ failure through an inability to regulate vascular tone.
Novelty and Significance.
What is new?
Using extracellular vesicles (EVs) isolated from normotensive and hypertensive rats, we show that normotensive, but not hypertensive, EVs reduce endothelial-dependent vasodilation, possibly through a NOS-dependent mechanism.
Delipidation of EVs isolated from hypertensive rats reduce endothelial-dependent vasodilation suggesting a change in the EV structural composition or mechanism of cargo release/delivery following development of hypertension.
EVs isolated from normotensive, but not hypertensive, patients, demonstrate similar reduction in endothelial-mediated vasodilation compared to rat EVs.
What is Relevant?
These data suggest that the vasoactive function of EVs is altered during hypertension
Summary
EVs regulate endothelial-mediated vascular tone in resistance arteries, an effect that is lost with hypertension, and may provide a source of novel biomarkers for identifying the development of hypertension.
Acknowledgements
The authors would like to thank Jennie Ma, PhD, Professor of Biostatistics at University of Virginia for her guidance on our statistical analyses, Joanne Lannigan, MS Director of the Flow Cytometry Core Facility at University of Virginia for her assistance and guidance on phenotyping our EVs, Abigail Antoine for her assistance in figure design, and Sasha Klibanov, PhD for preparing the liposome preparations
Sources of Funding
This work was supported by K99HL143165 (MEG), R01 DK113632 and R01 DK094907 (THL), R01HL088554 (BEI), K23HL126101 (UE), and NCI P30 CA044579-28 (Flow Cytometry Center Grant).
Footnotes
Conflicts of Interest/Disclosures: None
References
- 1.Khaddaj MR, Mathew JC, Kendrick DJ and Braun AP. The vascular endothelium: A regulator of arterial tone and interface for the immune system. Crit Rev Clin Lab Sci. 2017;54:458–470. [DOI] [PubMed] [Google Scholar]
- 2.Deanfield JE, Halcox JP and Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–95. [DOI] [PubMed] [Google Scholar]
- 3.Dharmashankar K and Widlansky ME. Vascular endothelial function and hypertension: insights and directions. Curr Hypertens Rep. 2010;12:448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickhout A and Koenen RR. Extracellular Vesicles as Biomarkers in Cardiovascular Disease; Chances and Risks. Front Cardiovasc Med. 2018;5:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Vizio D, Kim J, Hager MH, Morello M, Yang W, Lafargue CJ, True LD, Rubin MA, Adam RM, Beroukhim R, Demichelis F and Freeman MR. Oncosome formation in prostate cancer: association with a region of frequent chromosomal deletion in metastatic disease. Cancer Res. 2009;69:5601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Niel G, D’Angelo G and Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–228. [DOI] [PubMed] [Google Scholar]
- 7.Hafiane A and Daskalopoulou SS. Extracellular vesicles characteristics and emerging roles in atherosclerotic cardiovascular disease. Metabolism. 2018;85:213–222. [DOI] [PubMed] [Google Scholar]
- 8.Horn P, Cortese-Krott MM, Amabile N, Hundsdorfer C, Kroncke KD, Kelm M and Heiss C. Circulating microparticles carry a functional endothelial nitric oxide synthase that is decreased in patients with endothelial dysfunction. J Am Heart Assoc. 2012;2:e003764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savoia C, Sada L, Zezza L, Pucci L, Lauri FM, Befani A, Alonzo A and Volpe M. Vascular inflammation and endothelial dysfunction in experimental hypertension. Int J Hypertens. 2011;2011:281240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berezin A, Zulli A, Kerrigan S, Petrovic D and Kruzliak P. Predictive role of circulating endothelial-derived microparticles in cardiovascular diseases. Clin Biochem. 2015;48:562–8. [DOI] [PubMed] [Google Scholar]
- 11.Preston RA, Jy W, Jimenez JJ, Mauro LM, Horstman LL, Valle M, Aime G and Ahn YS. Effects of severe hypertension on endothelial and platelet microparticles. Hypertension. 2003;41:211–7. [DOI] [PubMed] [Google Scholar]
- 12.Wang JM, Su C, Wang Y, Huang YJ, Yang Z, Chen L, Wu F, Xu SY and Tao J. Elevated circulating endothelial microparticles and brachial-ankle pulse wave velocity in well-controlled hypertensive patients. J Hum Hypertens. 2009;23:307–15. [DOI] [PubMed] [Google Scholar]
- 13.Sansone R, Baaken M, Horn P, Schuler D, Westenfeld R, Amabile N, Kelm M and Heiss C. Endothelial microparticles and vascular parameters in subjects with and without arterial hypertension and coronary artery disease. Data Brief. 2018;19:495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu L, Hu XX, Lin ZB, Chang FJ, Ou ZJ, Wang ZP and Ou JS. Circulating microparticles from patients with valvular heart disease and cardiac surgery inhibit endothelium-dependent vasodilation. J Thorac Cardiovasc Surg. 2015;150:666–72. [DOI] [PubMed] [Google Scholar]
- 15.Lovren F and Verma S. Evolving role of microparticles in the pathophysiology of endothelial dysfunction. Clinical chemistry. 2013;59:1166–74. [DOI] [PubMed] [Google Scholar]
- 16.Freed JK, Durand MJ, Hoffmann BR, Densmore JC, Greene AS and Gutterman DD. Mitochondria-regulated formation of endothelium-derived extracellular vesicles shifts the mediator of flow-induced vasodilation. Am J Physiol Heart Circ Physiol. 2017;312:H1096–h1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brodsky SV, Zhang F, Nasjletti A and Goligorsky MS. Endothelium-derived microparticles impair endothelial function in vitro. Am J Physiol Heart Circ Physiol. 2004;286:H1910–5. [DOI] [PubMed] [Google Scholar]
- 18.Burger D, Turner M, Munkonda MN and Touyz RM. Endothelial Microparticle-Derived Reactive Oxygen Species: Role in Endothelial Signaling and Vascular Function. Oxid Med Cell Longev. 2016;2016:5047954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kemp BA, Howell NL, Keller SR, Gildea JJ, Padia SH and Carey RM. AT2 Receptor Activation Prevents Sodium Retention and Reduces Blood Pressure in Angiotensin II-Dependent Hypertension. Circ Res. 2016;119:532–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeager M, Berriman JA, Baker TS and Bellamy AR. Three-dimensional structure of the rotavirus haemagglutinin VP4 by cryo-electron microscopy and difference map analysis. EMBO J. 1994;13:1011–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DiMaio F, Yu X, Rensen E, Krupovic M, Prangishvili D and Egelman EH. Virology. A virus that infects a hyperthermophile encapsidates A-form DNA. Science. 2015;348:914–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banizs AB, Huang T, Dryden K, Berr SS, Stone JR, Nakamoto RK, Shi W and He J. In vitro evaluation of endothelial exosomes as carriers for small interfering ribonucleic acid delivery. Int J Nanomedicine. 2014;9:4223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehdiani A, Maier A, Pinto A, Barth M, Akhyari P and Lichtenberg A. An innovative method for exosome quantification and size measurement. J Vis Exp. 2015:50974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graham JM. Preparation of crude subcellular fractions by differential centrifugation. ScientificWorldJournal. 2002;2:1638–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thery C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erdbrugger U, Rudy CK, Etter ME, Dryden KA, Yeager M, Klibanov AL and Lannigan J. Imaging flow cytometry elucidates limitations of microparticle analysis by conventional flow cytometry. Cytometry A. 2014;85:756–70. [DOI] [PubMed] [Google Scholar]
- 27.Lannigan J and Erdbruegger U. Imaging flow cytometry for the characterization of extracellular vesicles. Methods. 2017;112:55–67. [DOI] [PubMed] [Google Scholar]
- 28.Wessel D and Flugge UI. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984;138:141–3. [DOI] [PubMed] [Google Scholar]
- 29.Good ME, Eucker SA, Li J, Bacon HM, Lang SM, Butcher JT, Johnson TJ, Gaykema RP, Patel MK, Zuo Z and Isakson BE. Endothelial cell Pannexin1 modulates severity of ischemic stroke by regulating cerebral inflammation and myogenic tone. JCI Insight. 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Densmore JC, Signorino PR, Ou J, Hatoum OA, Rowe JJ, Shi Y, Kaul S, Jones DW, Sabina RE, Pritchard KA Jr., Guice KS and Oldham KT. Endothelium-derived microparticles induce endothelial dysfunction and acute lung injury. Shock. 2006;26:464–71. [DOI] [PubMed] [Google Scholar]
- 31.Agouni A, Ducluzeau PH, Benameur T, Faure S, Sladkova M, Duluc L, Leftheriotis G, Pechanova O, Delibegovic M, Martinez MC and Andriantsitohaina R. Microparticles from patients with metabolic syndrome induce vascular hypo-reactivity via Fas/Fas-ligand pathway in mice. PLoS One. 2011;6:e27809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khaddaj Mallat R, Mathew John C, Kendrick DJ and Braun AP. The vascular endothelium: A regulator of arterial tone and interface for the immune system. Crit Rev Clin Lab Sci. 2017;54:458–470. [DOI] [PubMed] [Google Scholar]
- 33.Dignat-George F and Boulanger CM. The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol. 2011;31:27–33. [DOI] [PubMed] [Google Scholar]
- 34.Mulcahy LA, Pink RC and Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawson C, Vicencio JM, Yellon DM and Davidson SM. Microvesicles and exosomes: new players in metabolic and cardiovascular disease. J Endocrinol. 2016;228:R57–71. [DOI] [PubMed] [Google Scholar]
- 36.Amabile N, Guerin AP, Leroyer A, Mallat Z, Nguyen C, Boddaert J, London GM, Tedgui A and Boulanger CM. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol. 2005;16:3381–8. [DOI] [PubMed] [Google Scholar]
- 37.Mostefai HA, Agouni A, Carusio N, Mastronardi ML, Heymes C, Henrion D, Andriantsitohaina R and Martinez MC. Phosphatidylinositol 3-kinase and xanthine oxidase regulate nitric oxide and reactive oxygen species productions by apoptotic lymphocyte microparticles in endothelial cells. J Immunol. 2008;180:5028–35. [DOI] [PubMed] [Google Scholar]
- 38.Martin S, Tesse A, Hugel B, Martinez MC, Morel O, Freyssinet JM and Andriantsitohaina R. Shed membrane particles from T lymphocytes impair endothelial function and regulate endothelial protein expression. Circulation. 2004;109:1653–9. [DOI] [PubMed] [Google Scholar]
- 39.Pfister SL. Role of platelet microparticles in the production of thromboxane by rabbit pulmonary artery. Hypertension. 2004;43:428–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.