Abstract
Wastewater treatment is the means by which water that has been used and/or contaminated by humans or nature is restored to a desirable quality. Treatment may consist of chemical, biological, or physical processes or a combination thereof. Water may be treated to any level of quality desired; however, as its purity increases, so does the cost of attaining that purity. The required quality of water is dictated by its intended use, for example, aquatic life, drinking water, or irrigation. The purpose of this chapter is to describe wastewater treatment technologies predominantly in use today. Ultimately, the technology selected as appropriate for one application may not be the optimal for another. Selection will be based on site-specific factors, such as resources available, climate, land availability, economics, etc.
Keywords: Ecotoxicology, Environmental toxicology, Environmental and human health risk assessment, Soil and groundwater pollution, Transport and fate of chemicals in the environment, Waste reclamation, Resource management, Wastewater treatment, Water pollution control, Water resources management
Wastewater treatment is the means by which water that has been used and/or contaminated by humans or nature is restored to a desirable quality. Treatment may consist of chemical, biological, or physical processes or a combination thereof. Water may be treated to any level of quality desired; however, as its purity increases, so does the cost of attaining that purity. The required quality of water is dictated by its intended use, for example, aquatic life, drinking water, or irrigation. The purpose of this chapter is to describe wastewater treatment technologies predominantly in use today. Ultimately, the technology selected as appropriate for one application may not be the optimal for another. Selection will be based on site-specific factors, such as resources available, climate, land availability, economics, etc.
Wastewater Management Goals
The overall goal of wastewater management is the sustainable development of natural resources, including protection of public health and the environment. Sustainable development may be defined as the meeting of the needs of society today without compromise of those for future generations. In the context of water quality, sustainable development may be considered as the management of water resources such that current and future uses of this resource are not impaired. Consequently, objectives for water use, treatment, reclamation, and reuse should be consistent with this goal.
Water Quality Management
To understand the need for a particular degree of wastewater treatment, the concept of water quality management must be introduced. Water must be used and treated in such a way that deleterious effects are minimized, both for the environment and for the next user. From an engineering perspective, the intended use of a water should be determined, water quality requirements for that use delineated, and then treatment or other management techniques based on those requirements accomplished.
The dictionary defines pollution as something that makes the water physically impure, foul or filthy, dirty, stained, tainted, or defiled. Actually, the addition of anything to a water that adversely changes its existing quality constitutes pollution, including heat and sediment. Pollution may be caused by both humans and nature, and the effects may be similar.
The philosophy of water pollution control has changed considerably since the passage of the Public Health Service Act of 1912, which was primarily for the investigation of the spread of waterborne disease. Today, water quality standards are usually limited by aquatic life considerations. For example, the primary drinking water standard for zinc is 5 mg l− 1, while concentrations of zinc as low as 0.001 mg l− 1 may be toxic to aquatic life. The implications of this difference are obvious as the cost to protect aquatic life may be much greater than that for satisfying water quality standards for drinking water. It should be noted that the cost of removal of a contaminant increases considerably when the percentage of removal increases. From the technical standpoint, water can be made as pure as desired; however, from the practical perspective, the cost of achieving a given purity must be considered.
The 1987 Clean Water Act required that wastewater effluents potentially containing toxic contaminants undergo bioassay (whole effluent toxicity) testing to determine if such discharge may result in adverse effects on receiving water biota. Several aquatic species are employed and evaluated for acute and chronic affects. If it is determined that aquatic toxicity is a concern, then evaluation to reduce the toxicity must be conducted. This includes a Toxic Reduction Evaluation (TRE) and if necessary a Toxic Identification Evaluation (TIE) to identify and eliminate the toxicity causing agent (s).
Because of the increasingly stringent limits for selected pollutants due to potential toxicity and/or other deleterious effects on critical ecosystems and public health, the focus on wastewater treatment has shifted to one of wastewater management. This includes prevention instead of treatment/remediation and promotion of ‘clean technologies.’ Emphasis is now given by industry to waste minimization and product life cycle analysis to reduce raw materials, energy and environmental releases, thereby conserving natural resources, reducing risks/liability, and at the same time providing significant cost savings.
Receiving surface waters (lake, river, ocean, or estuary) have an innate ability to accept some contaminants without adverse environmental impact. This so-called self-purification capacity is denoted as the waste assimilative capacity. It is defined as the amount of contaminant that may be discharged into a receiving water, under defined low-flow (and in some cases high flow) conditions which will not result in deleterious effects. This concept often dictates the wastewater treatment requirements imposed on a wastewater discharger. However, where sufficient assimilative capacity does exist, regulations or standards may dictate at least a minimal degree of treatment obtainable by technology-based standards and are based on what can be achieved technologically rather than what is needed environmentally.
Where assimilative capacity is not sufficient to accommodate wastewater effluents and maintain stream standards, compliance to water-quality-based standards is required. Based on the designated use of the stream and results from ecotoxicological evaluations, total maximum daily loads (TMDLs) are determined by state environmental agencies. Considering contributions from non-point-source (diffuse) pollution, background conditions, and a factor of safety, a determination for each discharger is made as to acceptable point source effluent loadings. Accordingly, discharge permit restrictions are then imposed and enforced. These actions typically require a high degree of treatment, thereby shifting focus to resource management, waste reduction, and water reuse options.
Classification of Pollutants
It is convenient to classify pollutants into four categories as follows: chemical, physical, physiological, and biological. A brief discussion of these is in order as is their method of removal. It should be noted that, depending on the intended use of the water, every receiving water will have a limit as to how much of each of these kinds of wastes can be discharged into the water without adverse effects.
Chemical pollutants can be broadly categorized into inorganic and organic pollutants, where organic materials may be defined as those compounds containing organic carbon. The major problem with organic materials is their conversion to carbon dioxide and water as follows:
In as much as aquatic life requires a certain level of dissolved oxygen to live and propagate, it is obvious that if sufficient organic material is placed into the water, oxygen levels may be reduced to inimical concentrations. It is interesting to note that the process described is the same as that taking place in aerobic (presence of free oxygen) wastewater treatment processes. The common measure of oxygen depleting substances is the biochemical oxygen demand (BOD).
A prime consideration of inorganic chemicals is their toxicity. For example, changes in pH may occur from the discharge of soluble salts, and toxicity may occur directly from heavy metals. It should be noted, however, that organics may cause toxicity (e.g., pesticides), and inorganics may cause oxygen depletion (e.g., sulfurous acid). Also, not all materials containing carbon may cause an oxygen demand (e.g., bicarbonate). Some compounds containing organic carbon may be very difficult to biodegrade or non-biodegradable. Aquatic toxicity must be evaluated where appropriate and action taken to eliminate or reduce toxicity when necessary.
Other organic materials include compounds of a toxic nature, such as pesticides, and taste- and odor-producing compounds, including phenols and oils with their tendency to form surface films. The USEPA has defined a list of 126 toxic organic and inorganic chemicals that appear as specific limitations in discharge permits. These are identified as priority pollutants and can be found on the USEPA website www.epa.gov. Volatile organic chemicals (VOCs) such as benzene and toluene may result in public health problems and must be controlled under Clean Air Act legislation. The refractory organics are of particular concern because of the potential long-term cumulative effects of these materials in drinking water and the food chain. The Safe Drinking Water Act (SDWA) also requires the USEPA to list unregulated contaminants that are known or anticipated to occur in public water systems and may require regulation in the future. This list is called the Contaminant Candidate List (CCL) and is updated every five years and can be found on the USEPA website. The EPA uses the CCL to prioritize research efforts in order to make informed regulatory decisions about specific chemicals. The agency determines whether or not to regulate at least five chemicals on the CCL with each publication cycle. Newer organic pollutants, such as pharmaceuticals and personal care products (PPCPs) and endocrine disrupting chemicals (EDCs), have received increasing attention over the last decade. This is because of their wide distribution in surface waters, groundwater, and wastewater effluents, and their effects on the development of a variety of aquatic species, the development of antibiotic resistant pathogens, and possible effects on human health. Regulations for a number of these newer pollutants are currently being evaluated by the EPA. Some are currently banned in the United States and the European Union.
Physical pollutants include color, turbidity, temperature, suspended solids, foam, and radioactivity. Although color is not necessarily harmful, it may be aesthetically unacceptable for drinking water and some industrial uses. Color is often caused by organic colloids, thus making its removal expensive. Color may be the result of natural decay of vegetative organics such as fluvic and humic acids. These can react with free chlorine to form Trihalomethanes (THMs) which are of public health concern in drinking waters. Temperature is an important factor in biological activity and significantly affects chemical, biological, and physical reactions. It may also act synergistically with toxic materials, for example, heavy metal toxicity increases with increasing temperature. Turbidity is caused by colloidal material and/or suspended solids, and its removal requires coagulation and filtration. Suspended solids, which may cause turbidity, can result from wastewater discharges or from natural processes, such as erosion. This may inhibit photosynthesis by reducing light penetration, decrease benthic organism activity by covering the water bottom with sediment, and interfere with fish activity by clogging their gills. Solids may be organic or inorganic. Dissolved solids tend to increase in concentration with reuse. In most cases these are difficult and expensive to remove. Foam resulting from surface active agents may cause aesthetic problems, but developments by the detergent industry have minimized these effects. Surface active agents also may cause a reduction in the rate of oxygen gas transfer into the water. Radioactivity may be the result of fallout, natural sources, or waste discharges and can be incorporated into sludges or biological life or dissolved in the water. Because of the unique effects of radioactive substances, they must be controlled at the source.
Physiological effects of pollution are primarily the result of taste and odor. Although taste and odor problems may be minor in effect, public reaction many times results in magnification of problems and concomitant adverse publicity of the water purveyor. Taste and odor are particularly objectionable when present in drinking water or process water for food where palatability is important. It is significant to note that phenol is detectable at concentrations of 0.001 mg l− 1 and is pervasive in the wastewater discharges of the petrochemical industry. The ability of taste and odor-producing materials to taint fish is also important.
The biological category can also be divided into two subdivisions, public health considerations from waterborne diseases and eutrophication and/or biological growths resulting from nutrient additions.
The causative agents for waterborne disease include virus, protozoa, bacteria, and the helminths,. Contemporary epidemic problems include hepatitis (virally borne), giardiasis, and cryptosporidium (protozoan origin). Probably the most serious waterborne disease is cholera (bacterial origin), which caused major epidemics with high fatalities and morbidities in the 1800s in the United States and continues to be a problem in developing countries. Examples of some water borne diseases are presented in Table I .
Table I.
Examples of waterborne infectious diseases
| Bacteria | Campylobacter jejuni. |
| Enteropathogenic Escheria coli(gastroenteritis) | |
| Vibrio cholerae (cholera) | |
| Leptospira spp. (leptospirosis) | |
| Salmonella typhi, S. paratyphi (enteric fever) | |
| Shigella spp. (bacillary dysentery) | |
| Yersinia enterocolitica (gastroenteritis) Legionella pneumophillia (acute respiratory illness) | |
| Viruses | Rotavirus (gastroenteritis, upper respiratory infection) |
| Norovirus (gastroenteritis) | |
| Hepatitis A (infectious hepatitis) | |
| Hepatitis E (infectious hepatitis) | |
| Enteroviruses (including poliovirus) | |
| Protozoa | Entamoeba histolytica (amoebic dysentery) |
| Cryptosporidia spp. (cryptosporidiosis) | |
| Giardia lambia (giardiasis) | |
| Helminths | Dracunculiasis (guinea-worm disease) |
| Schistosoma spp. (schistosomiasis; Bilharzia) |
The second category, which may be called secondary biological pollution, is the deterioration of water quality resulting from the addition of phosphorus and/or nitrogen to receiving waters that may be from wastewater discharges or from pollution with no single point source (diffuse pollution). When excessive biological growths and associated water quality problems occur in lakes and estuaries, the phenomenon is known as eutrophication. Eutrophication is a natural geological process that may be accelerated by human cultural activities. Cyanobacteria (blue-green algae) can flourish in eutrophic waters. Some of these organisms are capable of producing toxins that have adverse human health effects, including liver, gastrointestinal, and nervous system effects. In many parts of the world including the U.S. water supply systems have been compromised due to these toxins. This impact is expected to worsen due to the effects of climate change.
It should be noted that nutrient removal (i.e., phosphorus and nitrogen removal) has been a major impetus for advanced wastewater treatment processes for discharges being made into affected water bodies.
Pollutants may also be classified as conventional, toxic, or nonconventional. Conventional contaminants are typical of domestic sewage and include BOD, suspended solids, pH, coliform, and oil and grease. Toxic pollutants include priority pollutants and other constituents resulting in aquatic toxicity and/or affecting public health. These may include behavioral or physiological abnormalities, cancer or genetic mutations upon exposure. Nonconventional pollutants are defined by USEPA as those that are neither conventional nor toxic. These include chemical oxygen demand (COD), total organic carbon (TOC), and nutrients (phosphorous and nitrogen) among others. The type and degree of treatment will depend on the classification of contaminants and that of the receiving waters.
Classification of Wastewater Treatment Methods
Wastewater treatment processes are categorized as source treatment, pretreatment, primary treatment, secondary treatment, and tertiary or advanced wastewater treatment.
Source treatment is used to remove toxics and/or other undesirable contaminants to prevent intermingling with other waste streams. This approach offers opportunities for reuse of these constituents such as metals, etc. Conventional treatment includes pre- and primary treatment followed by secondary-treatment processes. When necessary tertiary treatment processes are included in the treatment sequence to remove specific constituents to very low residue levels. Pretreatment is employed to render the raw wastewater compatible and/or amenable for subsequent treatment processes. Consideration is given to those constituents that pass through, interfere with, or accumulate in the sludge or are otherwise incompatible with following treatment processes. Equalization, spill retention, neutralization for pH adjustment, nutrient addition, toxics or inhibitory substance removal, oil and grease removal, and solids removal by flotation, sedimentation, or filtration are typical pretreatment processes. Primary treatment is a subset of pretreatment methods and involves physical separation by screening, grit removal, and sedimentation.
Depending on the amount of organics contained in the solid material, primary treatment may remove a significant portion of the oxygen-demanding substances (BOD). A well-designed and operated primary plant may remove as much as 35–40% of the BOD and as much as 60–65% of the settleable solids for municipal wastewaters.
Secondary treatment adds a biological process after primary treatment, which is commonly either activated sludge or trickling filtration for municipal wastewaters. Typically activated sludge or a modification of suspended growth treatment systems is used for industrial wastewater to achieve a high-quality effluent. These biochemical processes are typically aerobic and are the same as previously described as occurring in a river where organics are oxidized to carbon dioxide and water.
A well-operated and designed secondary treatment plant can be expected to remove 85–95% of both BOD and suspended solids. Under existing regulations in the United States, all discharges must be subjected to at least secondary treatment.
Conventional treatment is described by Best Conventional Treatment Technology (BCT) and is designed to remove conventional pollutants such as BOD, TSS, etc. common to municipal wastewaters. However, BCT does not effectively remove many constituents of present-day concern, especially those of industrial wastewater origin. These include many VOCs, toxics, non-biodegradable organics, persistent organic pollutants (POPs), nutrients and emerging contaminants such as EDCs and PPCPs. Hence, additional treatment technology is required.
Tertiary treatment may be defined as treatment in addition to primary and secondary processes. It may include precipitation, filtration, coagulation and flocculation, air stripping, ion exchange, adsorption, membrane processes, nitrification, and/or denitrification, and other processes. Those processes may be integrated into the secondary treatment plant or added onto the secondary effluent. Tertiary or advanced wastewater treatment can attain virtually any removal efficiency desired. However, as previously noted, as the percentage of contaminant removal increases, so does the cost of attaining it. These technologies are often referred to as Best Available Technology Economically Achievable (BTEA) and are applied to meet stream standards or to comply with TMDL requirements.
Tertiary treatment is employed to remove toxics, persistent organics, nonconventional pollutants, nutrients, etc. and is typically considered as BATEA. Tertiary treatment systems following secondary treatment, however, may not be efficient at industrial facilities because of the need for treatment of a large volume of flow with low contaminant concentrations. Many tertiary processes also may not be pollutant-specific. Generally, the most cost-effective approach is to address the problem at the source where flows are low and specific pollutants are present at high concentrations. Applicable technologies for source treatment will depend on the constituents in the process wastewater targeted for removal. For example, if VOCs and ammonia are to be removed, then air or steam stripping should be evaluated. If heavy metals are of concern, then oxidation/reduction, precipitation, filtration, ion exchange, and membrane processes may be investigated. Organic chemicals may require chemical oxidation, wet air oxidation, anaerobic treatment, granular activated carbon (GAC), polymeric resins, or reverse osmosis for effective removal.
Special mention should be made of the solid material removed in any of these processes since residuals disposal is a major problem in wastewater treatment. Sludges are particularly troublesome because of their high water content, concomitant large volumes, and concentration of heavy metals, viruses, protozoa, and other constituents capable of causing environmental and public health harm. Costs of treatment may be one-half or more of that of the aqueous waste stream. Many of the processes mentioned in the preceding paragraphs produce solids. Solids from primary and secondary clarifiers must be effectively managed. Residual solids usually undergo a series of treatment steps involving thickening, dewatering, and final disposition or reuse. Organic sludge may also require stabilization prior to final disposal. Stabilization may be achieved by digestion [aerobic or anaerobic (no oxygen present)], lime stabilization, or by other means. Sludge thickening is typically accomplished by gravity, flotation, or centrifugation methods. Centrifuges, belt filters, and filter presses are dewatering options. The selection of unit processes and their sequence will depend primarily on the characteristics and volume of the sludge and on the final disposition or reuse option selected.
Ultimate disposal choices include incineration, landfill, land disposal (lagooning or application to land for reuse) or other reuse alternatives. Since this material is a resource, it is important that opportunities for reuse be fully considered. It is the ultimate disposal method selected that may significantly influence the unit operations selected for treatment.
Wastewater Characterization
It is mandatory that the composition and characteristics of a wastewater be known and understood in order to design and operate a wastewater treatment plant. For example, the BOD is a measure of the organic material present and its removal depends on its form. This may be suspended, colloidal, dissolved, molecular type, etc. Solids characteristics will determine the sludge handling and transport facilities. pH may indicate the metals speciation, toxicity, the and/or the need for neutralization. Oxygen content and oxidation reduction potential (ORP) may demonstrate the need for odor control if a lack of dissolved oxygen is present as well as chemical speciation. Grease and oil may cause operational problems and may require special removal facilities.
Wastes originating from industry may be toxic or inhibitory to biological processes, and thus their discharge to a municipal treatment plant must be regulated. Pollutants may include heavy metals, VOCs, priority pollutants, grease and oil, etc. Pretreatment ordinances are used by municipalities to control discharges into municipal sewerage systems.
A major consideration in characterizing wastewaters is the flow since it may vary considerably and contain different contaminants in different concentrations at different times. For example, one would expect to find low flows occurring at night and peaks occurring during periods of maximum water use for domestic wastewaters. Industrial waste flows are generally more random and must be analyzed statistically based on probability analysis. Sampling of wastewaters is often done in accordance with flow so that the mass of constituents can be determined on a weighted average basis (composite sampling).
Whereas the characteristics of municipal sewage are relatively constant, industrial waste characteristics and parameters of concern can change significantly. Important industrial waste parameters that are of significance to a given industry are defined by a Standard Industrial Classification (SIC) grouping. Wastewater characterization and effluent discharge requirements under the National Pollution Discharge Elimination System (NPDES) permit system are based on SIC classifications. The strength and volume of industrial wastewaters are usually defined in terms of units of production (i.e., gal bbl− 1 beer and lb BOD bbl− 1 beer for a brewery) and variation in characteristics and flow by statistical distribution. Industrial waste of organic nature can be correlated to municipal waste loadings by the use of population equivalents. Typically 0.17 lb BOD per capita per day is generated for domestic wastewaters. Assuming an industry generates 17 000 lb BOD per day, then this would be equivalent to a population of 100 000 people. Because of the inherent variability in industrial waste characteristics, treatability studies are often necessary to determine design parameters and potential pretreatment requirements.
Objectives of Wastewater Treatment
The processes introduced will be examined in the following for their role in treating the various components constituting a wastewater. The prime objectives are threefold: (1) separate the solid from the liquid fraction and concentrate the solids collected from the carrier water; (2) remove and/or render innocuous materials that will cause adverse effects when the effluent and resultant residuals are subjected to ultimate disposition; and (3) maximize reuse potential of treated wastewater and residuals. It is instructive to note that only 0.1% of domestic sewage is solids, the remainder being the carrier water.
In general, less than 50% of the waste material in domestic sewage remains in suspension, thus allowing separation by straining, skimming, or settling. These residuals must be destroyed or rendered removable. This is accomplished by biological, physical, or chemical means.
During chemical treatment the coagulating chemicals combine with the finely divided and colloidal (non-settleable) material to form settleable flocs. In aerobic biological treatment, living organisms metabolize finely divided colloidal and dissolved biodegradable substances and convert them into carbon dioxide and water and settleable films, slimes, or flocs, primarily consisting of cell material.
Physical treatment may involve adsorption processes; where, for example, contaminants containing a high affinity for activated carbon may be removed.
Wastewater Treatment Processes
Wastewater treatment plant design is based on the selection and sequencing of various unit operations. A schematic illustrating integration of processes capable of treating a variety of wastewaters is shown in Figure 1 . Selection of a combination of processes depends on the characteristics of the wastewaters; the required effluent quality (including potential future restrictions); costs; and, availability of land. As previously indicated, treatment methods can be classified as pretreatment/primary treatment; secondary treatment; tertiary treatment; sludge treatment/stabilization; and, ultimate disposition or reuse treatment technologies for residuals.
Figure 1.
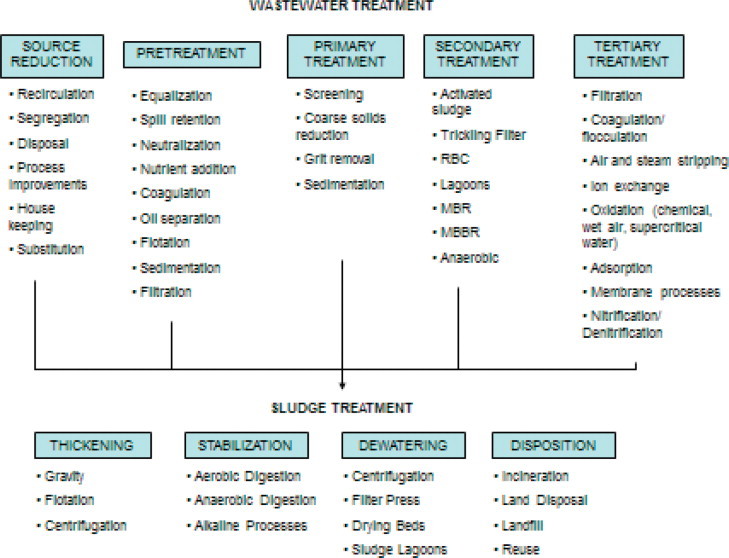
Typical wastewater treatment processes.
Pretreatment/Primary Treatment
Pretreatment methods are used to render the wastewater compatible with and/or amenable to subsequent treatment processes. Typical processes include equalization, neutralization, and oil and grease separation. Primary treatment involves physical separation for municipal wastewaters and usually consists of screening or comminution, followed by grit removal, and then sedimentation prior to secondary treatment.
Screening/Comminution
Screening is used to remove large, objectionable, solid matter that is removed periodically to prevent flow obstruction and head loss. The removed materials are putrescible and are usually buried or incinerated. A comminutor acting like a large ‘garbage grinder’ may be used to homogenize the solids as an alternative to screening. To protect the machinery the comminutor generally follows grit removal. The homogenized solids contribute to the organic load to the treatment facility, and hence screening is often the method of choice.
Grit Removal
Grit is composed of small coarse particles of sand, gravel, or other minute mineral material. Grit is removed to prevent damage to mechanical equipment and to maintain tank volume capacities. Grit may be removed in an aerated chamber where the amount of air is just sufficient to keep the organic matter in suspension and allow the heavier inorganic material to settle. Grit may also be removed by controlling the velocity of flow through a chamber such that the gritty material will settle and the organics remain suspended. Grit removed is typically washed and land disposed.
Oil and Grease Removal
Excessive oil, grease, and finely divided suspended solids should be removed prior to discharge into the primary sedimentation tank or aeration basin. This can be accomplished by employing processes including gravity separation with skimming and/or flotation. An API separator or parallel plate/corrugated plate oil separator may be employed effectively to separate free oil by gravity allowing the lighter-than-water oil globules to rise to the tank surface to be skimmed off. The API gravity separator is designed to remove oil globules 0.015 cm or greater and can achieve an effluent oil of less than 50 mg l− 1. The corrugated plate separator (CPS) with a narrow separation space can remove oil globules 0.01 cm or greater to as low as 10 mg l− 1; however, it is more affected by hydraulic variation. Flotation can be accomplished by introducing air under pressure into recycled effluent water and then allowing the pressurized air–water mixture to escape at atmospheric pressure in the flotation unit as minute air bubbles. Oil globules, sludge flocs, and suspended solids are floated by these bubbles. The air–oil and/or air–solids mixture rises to the surface where it is skimmed off. Chemical coagulants are generally added to help break oil emulsions and promote flocculation and enhance bubble attachment and flotation. Flotation usually follows API separation. A schematic diagram of a dissolved air flotation unit is shown in Figure 2 . Where VOCs are of concern, API separators and flotation units are often covered and the space purged with methane or nitrogen to minimize explosion hazard.
Figure 2.
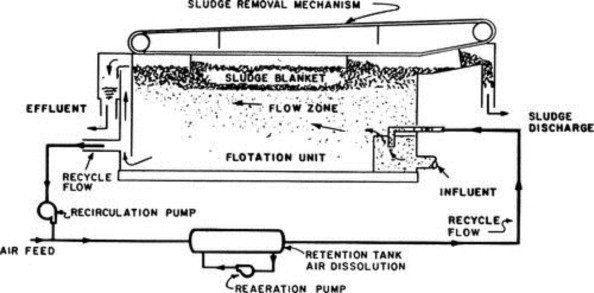
Dissolved air flotation unit.
(Courtesy of Komline–Sanderson Company)
Equalization and Neutralization
It is sometimes necessary, especially for industrial wastewaters, to install a basin to neutralize large fluctuations in pH and/or concentrations of contaminants in the incoming flow to the treatment plant. Control of fluctuations in flow with time may be required and necessitates a holding tank to equalize the flow variability. The use of equalization will assist in maintaining a relatively uniform flow and/or concentration through the plant and to the receiving water. Equalization is perhaps the most important unit operation in the treatment train for industrial wastewaters because of the desire to approach quasi steady-state conditions for design equation assumptions to hold true. Neutralization usually follows equalization so that acidic and alkaline streams can be partially neutralized in the equalization basin. Acidic wastewaters can be neutralized with lime, caustic, or limestone. Alkaline wastewaters can be neutralized with H2SO4 or HCl or by using flue gas (CO2). Usually a two-step process is required for pH control because of the logarithmic nature of pH. A pH of 6.5–8.5 is generally required prior to biological treatment.
Primary Sedimentation
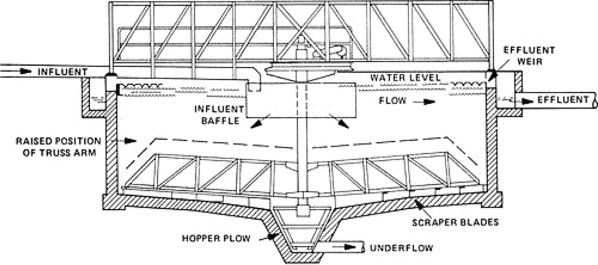
Settleable solids are removed by introducing the wastewater, after pretreatment, into a large rectangular or circular tank where solids settle by gravity. The supernatant overflows weirs and proceeds to secondary treatment for aerobic conversion to CO2 and water by biological oxidation. Primary clarification also acts as a barrier for oil and grease to prevent operational problems during subsequent treatment. Since solids will be collected at the bottom of the settling tank, provision must be made for their removal. This is usually accomplished by utilizing a continuous belt device as shown in Figure 3 . The solids (sludge) are then pumped from the sludge hopper to a sludge digester or other sludge treatment unit process. Note that the secondary sedimentation tank follows biological treatment. As shown in Figure 3, it differs from the primary tank because the provision for scum removal will generally not be required. Additionally, in place of a belt conveyor, a vacuum draw-off is often employed to remove solids quickly for activated sludge applications. It should be noted that chemicals are sometimes added prior to settling to enhance solids removal. The efficiency of solids removal for both primary and secondary clarification is a function of the overflow rate (gal ft− 2 day) which is essentially the velocity of the liquid exiting the tank. The settling velocity of the solids must be greater than the overflow rate if removal is to be effective. Liquid retention time must also be sufficient for efficient solids separation. It should be noted that for treatment of soluble industrial waste the primary settling tank is usually replaced with an equalization basin.
Figure 3.
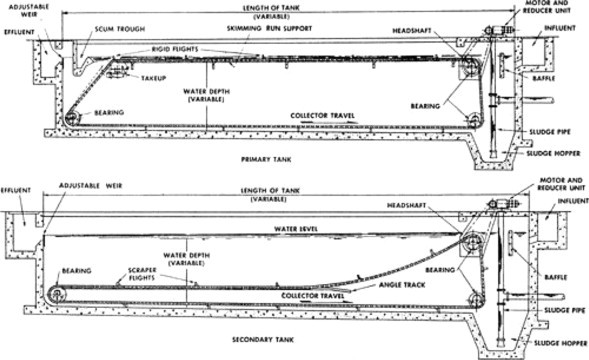
Gravity settling tank
Secondary Treatment
Secondary treatment utilizes some form of biological treatment following pretreatment/primary treatment. Since much of the organic material in a wastewater may be colloidal or dissolved, the processes described thus far will be ineffective in its treatment. It has previously been shown that organic matter will be oxidized to carbon dioxide and water in the presence of microorganisms, oxygen, and nutrients. Thus, the conditions required for wastewater treatment are an adequate number of acclimated microorganisms that can metabolize the organic material, an oxygen supply, nutrients, a means of intimate contact between the microorganisms and the food (wastewater), and a method of containment. The process is identical to that occurring in an aerobic waterway, except that in the wastewater treatment plant, the process is optimized by reduced time requirements and a continuous supply of oxygen to maintain an aerobic state.
There are numerous ways to design a biological wastewater treatment process; however, the two most commonly used are the activated sludge process and the trickling filtration process. In the activated sludge process, the fine, suspended, and colloidal and soluble organic materials are brought into intimate contact with a biologically active sludge maintained in suspension in the tank by introducing air that not only serves to maintain turbulence and maximum contact, but supplies the microorganisms with the oxygen required for their metabolism. The activated sludge performs the work of adsorbing, assimilating, and flocculating the waste material.
The trickling filtration process is identical to the activated sludge process in principle. However, instead of the water flowing through the suspended sludge containing the microorganisms, the waste material flows over a suitable surface to which the microorganisms adhere. When wastewater flows over the fixed film surface, growth of bacteria and other microorganisms occurs. These microorganisms create a slimy, gelatinous film that transfers the matter held in suspension, both colloidal and in solution, to the microorganisms, which remove food (substrate) needed for their growth and transfer back to the liquid the end products of decomposition, including (in the aerobic process) nitrates, carbon dioxide, and sulfates. As is the case with the activated sludge process, the microorganisms working in the trickling filtration process need a continuous food supply, adequate oxygen, a suitable support, and appropriate nutrients.
The processes taking place in any aerobic biological treatment plant can best be described schematically as shown in Figure 4 , where cell material is produced by synthesis (cell growth) and end products are generated by endogenous respiration (cell death), depending on the existing ratio of food to microorganisms (F M− 1 ratio) in the process. Thus, part of the energy may be utilized in reproducing new bacterial cells and the rest of the energy used in converting waste products to carbon dioxide and water. If the food supply becomes meager, the bacteria will eat themselves and bacterial cells will be converted into end products. This process, when the food-to-microorganism ratio is low is called endogenous respiration. It can also be seen from Figure. 4 that part of the waste material will be non-biodegradable. This is the residue of the waste material that will not be removed, either as cell material or as non-biodegradable residues produced during metabolism termed soluble microbial products (SMPs). The endogenous respiration process is analogous to a starving human who uses his own tissue to provide energy to sustain life processes. Note that SMPs are also generated during synthesis.
Figure 4.
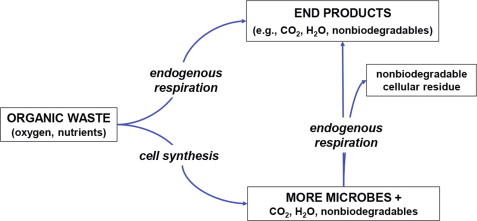
Biological stabilization of organic waste materials.
Figure 5 schematically demonstrates a complete-mix biological wastewater treatment process, where Q is the flow volume, S is the concentration of organics (substrate), V is the volume of the tank, M is the mass of microorganisms in the tank, s is the concentration of organics in the tank, and R is the fraction of return flow. The mass of microorganisms per day wasted divided into the inventory of microbes in the reactor is termed the sludge age or mean cell residence time (MCRT). This is used for system control to maintain a desired physiology of the biomass present. The required time for stabilization for industrial wastewaters, which are often soluble, is usually longer than that required for municipal wastewaters and will vary depending on the complexity of the waste. Retention time required may be hours to days and must be determined by treatability studies. Temperature effects are also significant depending on the nature of the wastewater, temperature extremes and temperature variation.
Figure 5.
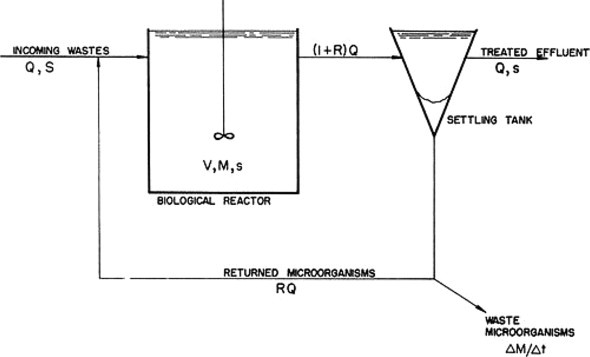
Complete mix biological reactor.
Activated Sludge Process
The activated sludge process consists of maintaining an active biological floc in a tank supplied with oxygen so that maximum contact is made between the incoming wastewater and the microorganisms in the floc. In the conventional process, a rectangular tank is usually used and the wastewater is introduced into a concentration of microorganisms maintained in the tank. Typically, air is introduced, either in the form of bubbles through diffusers or by turbulent agitation of the liquid by an impeller. In some cases pure oxygen is used in place of air. The microbe concentration is maintained in the tank by returning a certain portion of the sludge that passes through the tank and is settled out in a secondary sedimentation basin. The activated sludge process produces new cell material by synthesis which will become part of the activated sludge mass. Part of the settled material therefore, must be disposed of, and a portion must be introduced into the incoming raw wastewater in order to have an active population of microorganisms that will feed on the organic compounds.
As might be expected, the design of an activated sludge process will depend on the ratio of the food, or waste, to the microorganisms, or activated sludge. For domestic sewage characterized by a high suspended solids and colloidal content, most organic materials are adsorbed by the sludge floc in 15–45 min, although most conventional plants are designed with at least 30–90 min of contact time for adequate adsorption by the sludge floc. Retention times may be 24 h or longer for extended aeration package plants. With the activated sludge process, it is possible to obtain removals of BOD on the order of 95% or greater with a higher quality effluent than with most other biological oxidation processes. Many modifications of the activated sludge process exist, including a high-rate process where the food to-microorganism ratio is high, thus producing more sludge, typically used for pretreatment; a step-aeration process, where the influent is added at intervals along the aeration tank; tapered aeration, where the introduction of air is varied along the length of the tank, the higher concentration being at the influent point of the tank; and the contact stabilization process, where a portion of the sludge is aerated separately, thus adding flexibility to the process. Most conventional activated sludge basins treating municipal wastewaters are designed for plug flow to minimize hydraulic retention time and optimize settling properties of the floc. In a true plug flow unit all particles entering the reactor stay for an equal amount of time. This of course is not possible in practice. However, plug flow can be approached by dividing the aeration tank into a series of reactors. With complete-mix reactors the incoming wastewater is completely mixed with the reactor contents upon entry. Because of its ability to absorb shock loadings and reduce toxic/inhibitory constituents by dilution to below threshold levels it is often used for treating industrial wastewaters. With the increasing need for nutrient removal staged reactors are often used with combinations of anaerobic, anoxic and aerobic cells incorporating internal recycle for both phosphorus and nitrogen biological removal.
A modification of the activated sludge process is called extended aeration, where long detention times are given to the aeration operation. It is usually designed for complete-mix conditions. This means that a high solids content or a low food-to-microorganism ratio and long sludge age will exist. Thus, endogenous respiration of the sludge will occur and the sludge will ‘burn itself up.’ The process is sometimes called complete oxidation, although there will always be some biological residue, inorganics, and solids that will necessarily emanate from the system. Because of the long sludge age, nitrogen is converted to nitrate within the reactor, thereby reducing the oxygen demand of the effluent on the receiving water. The process of extended aeration is frequently used in small installations such as schools, subdivisions, and motels. Many industrial plants employ extended aeration to enhance priority pollutant removal, reduce toxicity, and PPCPs and EDCs due to the longer mean cell residence time, MCRT (sludge age). Priority pollutants can be reduced to μg l− 1 levels and BOD soluble to<10 mg l− 1. Figure 6 shows some of the modifications of the activated sludge process commonly employed.
Figure 6.
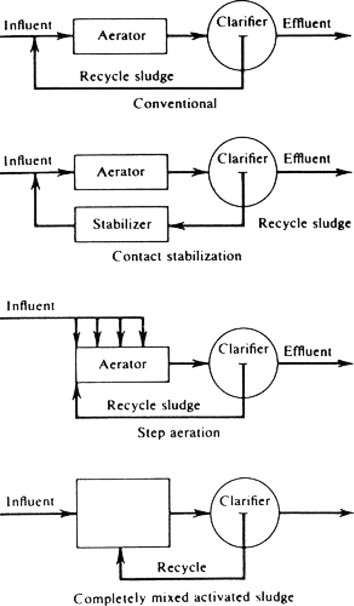
Activated sludge process modifications
The complete mix activated sludge system is generally employed for industrial waste treatment since it maximizes dampening of fluctuations of influent wastewater quality, including toxicity. However, the process for readily degradable wastes may tend to promote the growth of filamentous microbes, which do not settle well in the secondary clarifier. For these cases a selector may be employed preceding the aeration basin. In a selector, degradable organics are removed by the floc due to biosorption and therefore are not available as a food source for the filaments. A contact time of approximately 15 min is generally used.
Another modification of the activated sludge process popular for both municipal and industrial wastewaters is the sequencing batch reactor (SBR). This is a combination of complete mix and plug flow operated on an intermittent basis,that is, aeration, sedimentation, and decanting of effluent all in the same basin. This system has been shown to provide a good settling floc and an effluent of high quality low in nutrients without the need for an external clarifier. The system offers low cost and high flexibility. The SBR utilizes two or more basins operating in parallel such that when one is filling the other is emptying.
Lagoons and Oxidation Ponds
Stabilization ponds are probably one of the oldest wastewater treatment processes in existence and are still used in many locations in the United States today. They can be used alone or in combination with other wastewater treatment processes. Design and utilization depend on many factors, including weather, land availability, purpose, and location. Detention time varies from 7 to 180 days, depending on the type of pond and climate conditions.
Ponds can be classified into four categories: facultative, aerated, aerobic, and anaerobic, the most common being the facultative pond.
Facultative ponds are usually 6–8 ft in depth and operate aerobically in the upper layers and anaerobically in the lower depths. Oxygen in the upper portion is supplied by photosynthesis and surface reaeration. The organic loading is based on climate and on surface area which must be sufficiently low to maintain dissolved oxygen produced by algae. The algae present in the effluent may create problems however. The detention time of a facultative pond will vary from 30 to 180 days or more depending on climate conditions and nature of the wastewater. Effluent BOD values range from 20 to 60 mg l− 1 and effluent suspended solids from 30 to 150 mg l− 1 in warmer climates due to algae content. Multiple ponds in series are recommended for greater operational flexibility and to prevent short-circuiting.
Aerated lagoons are supplied with oxygen by mechanical or diffused aeration and are usually 6–20 ft deep with detention times varying from 3 to 12 days. Hence, large land areas are required, although less than for facultative ponds. The aerated lagoon can be used for pretreatment or may be designed in series to achieve a higher quality effluent. In a two or three basin system, the first basin is fully mixed, thereby maintaining all solids in suspension. This maximizes the organic removal rate. A second basin operates at a lower power level, thereby permitting solids to deposit on the bottom. The solids undergo anaerobic degradation and stabilization. A third basin is frequently employed for further removal of suspended solids and enhanced clarification. Aerated lagoons are employed for the treatment of nontoxic or nonhazardous wastewaters such as food processing and pulp and paper.
Aerobic or maturation ponds are about 18–36 in. deep and maintain oxygen throughout their depth. The oxygen is supplied by photosynthesis and surface reaeration, which is sometimes aided by mixing. These high-rate ponds are limited to warm, sunny climates and have a detention time of 3–5 days. In addition, unless algae removal is practiced, the effluent will contain a high percentage of suspended solids. These ponds will usually produce an effluent of high microbial quality.
Anaerobic ponds have such heavy loading that no aerobic zone exists. They are usually used for strong industrial or agricultural wastes as pretreatment. They are 8–15 ft deep and have detention times of 20–50 days. A major problem is with odor produced from the anaerobic process. Addition of sodium nitrate may be used to reduce or eliminate this problem.
Trickling Filtration
As previously mentioned, the trickling filtration process is similar to the activated sludge process, except that the microorganisms working to stabilize the organic waste material are attached to a fixed bed rather than being suspended. Following pretreatment and sedimentation the wastewater is distributed from rotary nozzles over the bed, which usually consists of coarse, rough, hard material that gives support to the biological film. The organics in the wastewater are oxidized after assimilation by the bacteria. Periodically, the film will become so thick that it can no longer be supported on the medium and will slough off and be discharged into the effluent from the filter. This sludge will then go to the secondary settling tank and be removed with that from the primary settling tank. The trickling filtration process is said to be advantageous because it can provide good performance with a minimum of skilled operator attention and use less energy. However, the process is highly temperature dependent and will not always perform in accord with the requirements of present-day regulatory agencies. Other variables affecting performance include the organic and hydraulic loading rates and the biodegradability of the wastewater. While effluent quality is less than that from the activated sludge process, quality can be enhanced by following the trickling filter with a biological contactor. The contactor is usually designed for a retention time of 15 min and a solids content similar to that maintained in the activated sludge process. For the treatment of industrial wastewaters, a trickling filter is considered a pretreatment process usually designed to remove approximately 50% of the BOD. Note that recirculation of the effluent into the influent is commonly practiced to reduce influent organic concentration and maintain a ‘wetted’ filter growth. Both carbonaceous and nitrogenous oxygen demand can be reduced using trickling filters.
Figure 7 is a schematic diagram of a typical ‘old style’ rock filled trickling filter installation. Oxygen is supplied because of the ‘open-air’ nature of the filter, and the wastewater is applied to the filter medium via the distributor, which rotates because of the momentum induced by the carrier water. The medium shown is stone, although today filters utilize synthetic media such as PVC, which allows greater depths of filtration and a larger specific surface for microbial growth. Multi-stage filters can be used if needed to comply with more stringent effluent standards. Filters may also be covered and off gases treated to reduce or eliminate odor problems.
Figure 7.
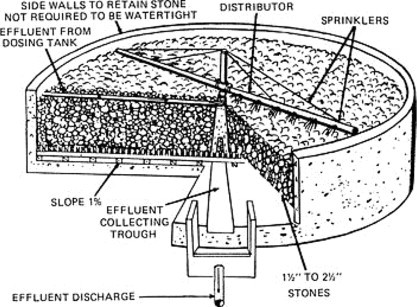
Schematic diagram of a rock filled trickling filter.
Membrane Bioreactors (MBRs) and Moving Bed Bioreactors (MBBRs)
Membrane Bioreactors (MBRs) consist of a biological reactor with suspended biomass and solids removal by ultra- and microfiltration membranes. These can be used for municipal or industrial wastewaters. The MBRs allow for a much higher biomass concentration to be maintained, thereby allowing smaller bioreactors to be used and saving space. Separation of solids by membrane filtration eliminates the need for secondary sedimentation, and small pore size prevents the discharge of most pathogens. The MBRs can be operated with a longer solids retention time, allowing for more complete oxidation of organics and the maintenance of a population of slow-growing bacteria capable of nutrient, EDC and PPCPs removals and reduced biosolids generation. The disadvantage of MBRs is their high capital costs, periodic membrane replacement, high energy costs and need to control membrane fouling. They offer a competitive alternative when nutrient removal is required. Energy costs have recently been significantly reduced and comparable with advanced wastewater treatment systems.
Moving Bed Bioreactor (MBBR) technology is an advanced secondary wastewater treatment process that incorporates attached growth media within a suspended growth reactor in order to increase the amount of biomass in the treatment basin. It has a smaller footprint compared to conventional suspended growth systems. Existing aerated treatment process can be easily retrofitted to an MBBR process by adding the plastic media and effluent screens. Because of longer sludge retention times, enhanced nitrification and priority pollutant removal is achieved.
Anaerobic Treatment
Anaerobic treatment processes involve the biological breakdown of organic waste to methane and carbon dioxide gases in the absence of oxygen. Because of lower cost compared to aerobic processes, the beneficial use of methane production, low sludge production, and low energy and nutrient requirements, anaerobic processes have become more attractive alternatives for the stabilization of certain wastes, in particular high-strength industrial wastes (e.g., food industries) and biological sludge. Now over 850 anaerobic treatment systems are in operation globally with about 75% of these treating wastewaters from the food or related industries. Today, more than 60 anaerobic treatment systems are being employed in the chemical and petro-chemical industries. Examples of anaerobic processes are anaerobic filter reactor, anaerobic contact process, fluidized-bed reactor, upflow anaerobic sludge blanket, ADI-BVF process, and expanded granular sludge bed process. These processes are discussed in detail in other texts including Eckenfelder et al., Metcalf & Eddy, etc.
Septic Tanks
A discussion of biological treatment would not be complete without mentioning the septic tank, inasmuch as a large segment of the population is still served by this primitive device, as shown in Figure 8 . From previous discussion, it can be seen that the process is anaerobic, will accumulate solids, and unless periodically pumped out (every 2 to 3 years) will discharge objectionable material. The discharge from such a tank usually flows into a designed drain field and ultimately into the groundwater and/or surface water. A well-designed drainage system into a suitable soil is mandatory since the septic tank itself acts only as a settler and its discharge remains high in soluble organic and microbial contaminants. Proper hydrogeologic conditions are therefore critical in effective drainage field treatment.
Figure 8.
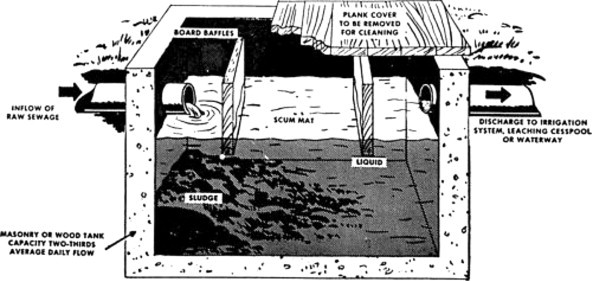
Septic tank.
Residuals (Sludge) Treatment
One of the most pervasive problems in water pollution control is the disposal of the solids produced in the various separation processes. If chemicals are added, the sludge produced may be different from a biological sludge and will increase the sludge volume produced. Thus, the nature of the solids produced may vary considerably and therefore necessitate different treatment scenarios prior to ultimate disposition.
The choices for the ultimate disposition of sludge are few and consist of some type of land application, incineration, or reuse. Incineration leaves a residue that must be disposed of, and concern exists regarding air-quality impacts. Thus, the ultimate residing place of the solids produced in wastewater treatment is into/onto the land or some other reuse alternative.
The objectives of the processes used to treat sludge prior to its final disposition are to reduce the volume, destroy pathogens, remove water, improve efficiency of subsequent processes, control putrescibility, and stabilize organics. Because the final product must be transported to its ultimate disposal or reuse site, attainment of these objectives will reduce transportation costs and minimize adverse environmental/public health effects. As indicated by Figure 9 , the sequence of unit processes for residuals management typically includes thickening, stabilization, dewatering, and ultimate disposal or reuse.
Figure 9.
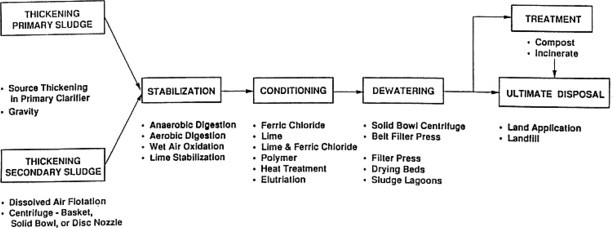
Sequence of unit processes for residuals management.
Thickening
The primary objective of sludge thickening is to concentrate the solids, thus reducing the volume of sludge. Thickeners may increase the solids concentration by a factor of 2–5 and produce a clarified liquid effluent. Thickening is accomplished by either gravity or dissolved-air flotation. A gravity thickener is shown in Figure 10 . The operation of a dissolved-air flotation unit is similar to that described for pretreatment. Decanted liquid is recirculated to the treatment plant influent.
Figure 10.
Gravity thickener.
(Courtesy of Link-Belt, FMC Co.)
Sludge Stabilization
Organic sludge must be stabilized before reuse or ultimate disposal except in the case of incineration. The most common method to stabilize residual solids is by aerobic or anaerobic digestion. Aerobic digestion stabilizes excess biological solids by the oxidation of cellular organic matter through endogenous metabolism. Conventional aerobic digestion employs aeration of primary and secondary clarifier underflow in one or more completely mixed aeration basins. Diffused air or surface mechanical aerators are used to provide mixing and oxygen requirements. An aeration time of 10–20 days is usually provided based on temperature. Volatile solids reduction are in the range of 30–50%, with greater than 44% being most desirable. The digested sludge can be disposed of without causing odor or other nuisance conditions. The oxygen required for aerobic digestion can be estimated as 1.4 lb of oxygen consumed for each pound of volatile suspended solids destroyed. Nitrogen and phosphorus will be released by the oxidation process and nitrification will usually occur. As the sludge age is increased in the bioreactor, more of the degradable biomass is oxidized and less will be oxidized in the aerobic digester. The digester can be used as a source of biological seed if toxicity or inhibition is observed in the secondary treatment unit.
Anaerobic fermentation or sludge digestion decomposes organic matter in the absence of molecular oxygen. In contrast to the aerobic stabilization process, anaerobic digestion produces methane and carbon dioxide from the organic material as follows:
The process actually has two reactions occurring: one where complex organics are hydrolyzed and fermented into simple organic acids, and a second in which the organic acids are converted to methane and carbon dioxide. Anaerobic digesters are usually operated at a temperature near 95 F, which means that heat must be supplied. Depending on the process used, detention times may vary from 10 to 60 days, and the gas composition of a well-operated digester is about 70% methane and 30% carbon dioxide. The methane gas may be used to heat the digester or to run machinery in the plant; however, any hydrogen sulfide present must be removed because of its corrosive nature. Thermophilic digestion may be employed. It occurs at temperatures between 120 and 135 F. At the higher temperatures reaction rates are greater and thus the required retention time is reduced compared to mesophilic digestion. Other reported advantages include: improved dewaterability and increased pathogen destruction. Disadvantages are high energy requirements, poorer quality supernatant, odors and less process stability.
A two-stage anaerobic digestion operation is shown schematically in Figure 11 . Note that if the gas is not utilized, it must be flared. In addition, the supernatant shown is high in BOD and solids and must be directed into the plant influent for treatment.
Figure 11.
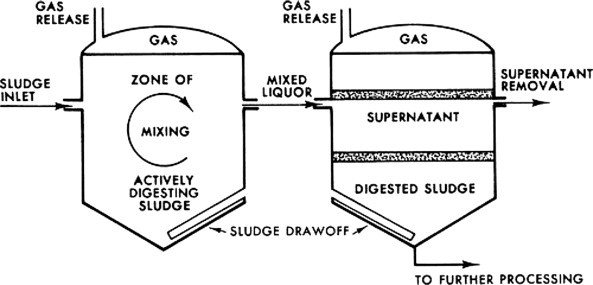
Two-stage anaerobic digester
Advantages of the process include no oxygen required, a reduction in sludge volume, methane production, inactivation of pathogens, and the production of a good soil conditioner. The major disadvantages are a high capital cost and the need for careful operation.
Sludge Dewatering
Several methods of sludge dewatering are available, depending on the size of the plant, the kind of sludge, and site-specific considerations.
A commonly employed unit process for dewatering thickened municipal and industrial sludge is the belt filter press. As shown in Figure 12 , chemically conditioned sludge is fed through two filter belts and is squeezed by force to drive water through these belts. Chemical polymers are required to promote flocculation and enhance dewatering. A final solids concentration range of from 20 to 35% can be expected depending on the characteristics of the sludge and the method of operation.
Figure 12.
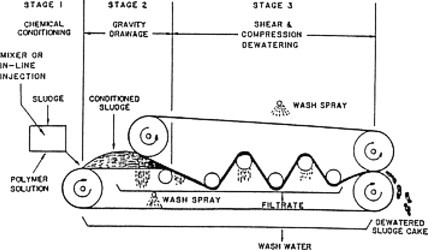
Belt filter press
The pressure filter, as shown in Figure 13 (a) and Figure 13 (b), is another alternative. The sludge is fed into the press and pressure is applied so that the filtrate passes through the cloth with the solids being retained and forming a cake on the cloth. The medium is usually precoated. The filtrate is collected in the drainage ports provided at the bottom of each press chamber. The pressure operation ceases when the filtrate flow is near zero, at which time the press is opened and the cakes released. Most municipal sludge can be dewatered to produce a cake solids of 40–50% with 225-lb in− 2 filters. Conditioning chemicals such as lime, ferric chloride, or polymers are often used to enhance dewatering and minimize cloth plugging. Fly ash and coal fines have also been employed to reduce cake compressibility, thereby enhancing dewatering but adding to the sludge volume.
Figure 13.
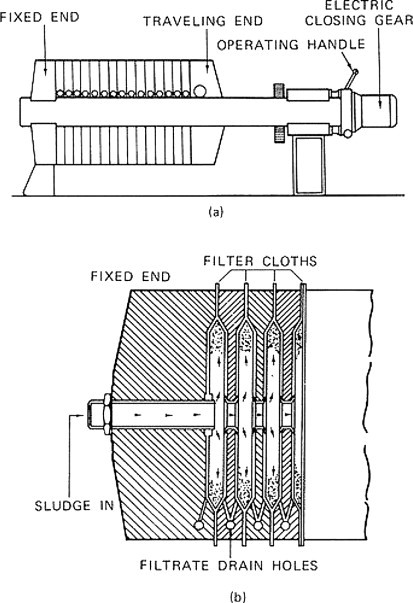
(a) Side view of a filter press; (b) filter press cutaway view.
Several types of centrifugal dewatering are also used, where the centrifuge uses centrifugal force to enhance the sludge particle sedimentation rate and concentrate the solids. Flocculants are added with the sludge to increase removal of fines. Solid-bowl centrifuges are typically employed for dewatering to 20–35% solids. Both centrate and cake solids are continuously discharged from the machine. The centrate must be returned to the plant influent for treatment.
Sludge drying beds are often used for small sludge volumes, which drain and dry rapidly. Application is usually restricted to the more arid climates. A schematic is presented in Figure 14 . The filtrate is returned to the treatment plant. About 10–30% solids can be expected depending on specific application. With good weather, well-digested sludge may achieve as high as 45% solids concentration in 6 weeks. Covered beds have also been employed in wetter climates.
Figure 14.
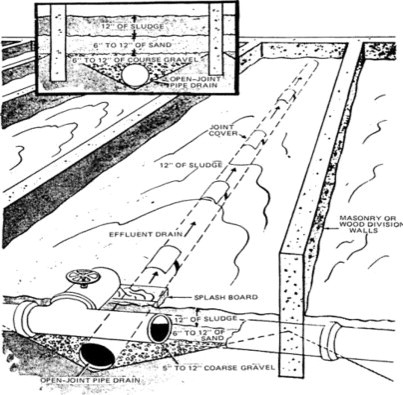
Sand drying beds
Other methods of sludge treatment include composting, thermal treatment, incineration, advanced alkaline stabilization, and wet oxidation. Of course, combustion methods may lead to air pollution problems and have residual ash to be disposed. It should be noted that other less utilized methods exist, and treatment may be used prior to the methods described for stabilization and/or conditioning.
Ultimate Disposition of Solids
The quality/ultimate disposition of sludge (termed biosolids for municipal wastewater residuals) is currently governed by the USEPA through 40 CFR Part 503 of the 1987 Clean Water Act Amendments. The EPA promotes practices that provide for the beneficial use of municipal sewage sludge biosolids, while maintaining or improving environmental quality and protecting public health. These practices typically include land application of biosolids as a soil amendment or fertilizer supplement and various procedures that derive energy from biosolids or convert them to useful products.
Part 503 standards provide for a wide range of end-use possibilities for biosolids that depend on sludge characteristics and treatment methods. Processes defined as Processes to Significantly Reduce Pathogens (PSRP) generate a ‘Class B’ sludge that may be used under restricted conditions. Those biosolids processed by Processes to Further Reduce Pathogens (PFRP) are termed a ‘Class A’ sludge and can be used unrestrictedly. The term ‘Exceptional Quality’ is often used to describe a biosolids product which meets Class A requirements. The end product must be stable (i.e., no odors, no vector attraction), noninfectious, and of sufficiently low metals content so as not to translocate and bioaccumulate to undesirable levels. Some treatment technologies capable of producing a Class A sludge include composting, heat drying, auto-thermal thermophilic aerobic digestion, pasteurization and gamma radiation.
The constraints on the use of sludge for growing edible crops are primarily associated with the presence of heavy metals, the nitrogen content, and the possibility of the presence of phytotoxic materials in the sludge. Particular care must be given if the solids contain contributions from industry.
Some metals of concern include arsenic, mercury, lead, zinc, copper, and nickel, although the limiting metal is usually cadmium. Cadmium is of particular concern because of its potential translocation from the soil to the fruit and the ability of certain crops, such as chard, to accumulate significant quantities in the edible portion of the plant. Other metals such as arsenic may also be accumulated in fruit and grain to levels exceeding FDA and other regulatory limits.
Nitrogenous material is also of concern and may be a limiting factor in sludge application rates. Because soil biochemical reactions are mostly aerobic, nitrification will occur, and if the nitrogen is not used by the crops, it will be converted to nitrate. Nitrate in groundwater can cause methemoglobinemia in infants.
The helminths may pose a pathogen threat because they concentrate in the sludge and possess the ability to survive extremely adverse environments. For example, Ascaris ova have been found to survive for as long as 7 years. Other pathogens may cause restrictions on crops grown in sludge application sites.
Groundwater is of primary concern in areas where sludge is applied and potential adverse effects must be considered. The travel of various contaminants through soil depends on many factors, including the soil composition, the contaminant, its concentration and speciation, and the groundwater hydrology.
As previously indicated, when biosolids are effectively treated and recycled they can be used as a significant resource. Reduced disposal volume conserves land disposal facility capacity. Other benefits include improved soil fertility and tilth, reduced need for fertilizers, better growth and quality of crops, and decreased energy consumption. According to the USEPA Biosolids Fact Sheet for Land Application of Biosolids in 2000, nationally about 60% of biosolids were land applied. In California it was 70% and in Oregon 95%. Stabilized biosolids have also been employed as intermediate and final landfill cover; to recover lands devastated by mining; and, smelting activities, and for wetlands reclamation.
Advanced Wastewater (Tertiary) Treatment
Thus far, the conventional treatment of wastewaters has been described (BCT), and one may conclude that organic waste materials (BOD), suspended solids, and bacteria can be reduced with relatively high efficiency, as shown in Table II . The treatment plant flow scheme may be arranged as shown in Figure. 15(a) (activated sludge) or Figure 15(b) (trickling filtration). Considering the concepts previously discussed, one can trace the flow and removal of the three parameters of primary concern (BOD, suspended solids, and the bacterium Escherichia coli) by conventional treatment.
Table II.
Efficiencies of treatment processes for domestic sewage
| Treatment | BOD (%) | Suspended solids (%) | Escherichia coli (%) |
|---|---|---|---|
| Plain sedimentation | 25–40 | 40–70 | 25–75 |
| Chemical precipitation | 50–85 | 70–90 | 40–80 |
| High-rate trickling filtration preceded and followed by plain sedimentation | 65–95 | 65–92 | 80–95 |
| Low-rate trickling filtration preceded and followed by plain sedimentation | 80–95 | 70–92 | 90–95 |
| High-rate activated sludge preceded and followed by plain sedimentation | 65–95 | 65–95 | 80–95 |
| Conventional activated sludge preceded and followed by plain sedimentation | 75–95 | 85–95 | 90–98 |
| Chlorination of biologically treated sewage | – | – | 98–99 |
Figure 15.
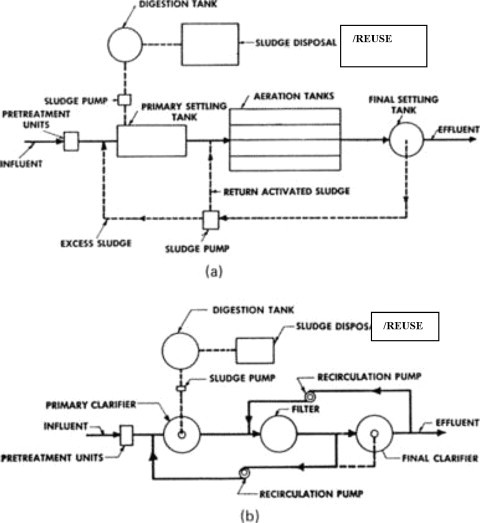
Flow schemes for conventional municipal wastewater treatment
Other contaminants may not be significantly reduced using secondary treatment and therefore must be subjected to other removal techniques or tertiary treatment (BATEA). These contaminants include toxic organics, VOCs, phosphorus, nitrogen, heavy metals, refractory organics, and difficult-to-remove pathogens such as Giardia lamblia. Some processes investigated and applied with success for removals include coagulation and flocculation, filtration, ion exchange, nitrification, denitrification, membrane processes, air stripping, adsorption, and chemical oxidation. These will be discussed briefly in the following sections.
Suspended Solids Removal
Total Suspended solids (TSS) removal by tertiary treatment implies the removal of those materials that have been carried over from a secondary clarification process. Pretreatment is required prior to physical chemical treatment processes. Influent suspended solids concentration must be less than about 100 mg l− 1 or backwashing requirements become excessive for sand filtration. Finely dispensed suspended solids may require the addition of coagulant prior to filtration. Several means for removal of suspended solids have been proposed and tested. These include the use of diatomaceous earth filtration, pressure filtration, chemical clarification, sand filtration with conventional and multimedia units, ultrafiltration, and the moving-bed filter. With the exception of the chemical clarification processes, these methods all involve removal by physical straining of the finely divided solids.
Diatomaceous earth filtration is a form of mechanical separation that uses diatomaceous earth, a powdered filter aid, that is built up on a supporting medium. As filtration proceeds, the solid material that will not pass through the diatomaceous earth is retained and eventually builds up a pressure that will no longer allow filtration. At that point, the unit is backwashed and made ready for another cycle. It should be noted that the diatomaceous earth filtration process was developed during World War II to remove the cysts that cause amoebic dysentery and is presently quite common in swimming pool filtration.
Chemical clarification consists of four phases: coagulation, flocculation, sedimentation, and filtration. Coagulation, essentially the addition of chemicals that are rapidly mixed with the water, usually involves the use of polymers and/or the oxides of aluminum, iron, or calcium. Once the coagulation step (charge neutralization) is accomplished, the flocculation process then allows aggregation, neutralization, and adsorption of the floc particles. This process is followed by the sedimentation process, where the flocs that have been previously formed are allowed to settle. Although most of the flocculated material will be removed in the sedimentation tank, it is usually required to further remove the floc particles that do not settle by the use of a filtration process that is conducted in beds of porous media such as sand.
The usual means of filtration has been through sand beds containing graded sand placed on a supporting medium containing an underdrain to collect the filtered effluent. As wastewater containing solids is passed through this type of filter, the solids will accumulate and eventually clog up the openings causing high head loss and/or poor effluent quality. Thus, some provision must be made to remove the collected material. The procedure usually followed is to backwash the sand, that is, to reverse the flow, usually with air scour, so that the sand is suspended and the lighter material is washed away. It should be noted that subsequent to a backwashing operation, the sand will settle according to size and thus become stratified, with the smaller particles at the top. Since these particles will filter out most of the solid material, it may be concluded that the entire depth of a sand bed is not utilized in a filtration process. To alleviate this problem, a multimedia filter is employed, involving different filtration materials, each having a different specific gravity. Materials commonly used are sand, coal, and garnet, as shown in Figure. 16 . This type of filter extends the length of the filtration runs and demonstrates high efficiency. Filter loadings are usually designed from 2 to 6 gpm/ft2 with 4 gpm/ft2 being the most typical.
Figure 16.
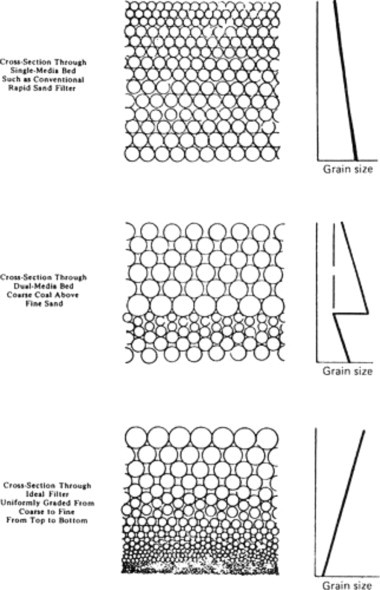
Stratification of different types of filters. (a) The activated sludge process; (b) the trickling filtration process.
The moving-bed filter is a technique that is essentially a form of countercurrent extraction, that is, feeding the sand countercurrent to the filtering water. As the filter surface becomes clogged, the filter medium is moved forward and a new surface is exposed. It is said that, theoretically, 1% of the filter is being backwashed 100% of the time as compared to a conventional backwashing operation where 100% of the filter is backwashed 1% of the time.
Ultrafiltration or nanofiltration uses pressure to drive a liquid through a membrane permeable to some consituents including fine particles, viruses, bacteria and protozoans. This process is similar to reverse osmosis, but differs with respect to the size of the particles that are separated. Ultrafiltration will generally not retain a particle whose weight is 500–1000 times the molecular weight, and thus it fails to separate inorganic salts. The pressures used in ultrafiltration are on the order of 50 lb/in2 as contrasted to pressures exceeding 500 lb/in2 in reverse osmosis. The process is essentially one of pressure filtration, using semipermeable membranes that act as molecular screens and separate colloidal and molecular materials that are dissolved or suspended in the liquid phase.
Organic Removal
As previously indicated, wastewaters may contain measurable amounts of refractory soluble organics, toxics, VOCs and other contaminants not removed by conventional processes. These problem organics may be removed at the source (usually recommended), within the biological treatment step, and/or in the tertiary treatment stage following conventional treatment. The composition of refractory organics is not always precisely known. Although they are not easily biologically oxidizable, they may exercise a BOD in a receiving water over time. In addition, these materials may cause taste and odor problems in a water supply, and more important, the potential toxicological effects on the aquatic environment and on humans have not been precisely delineated. Toxics are of concern and must be removed or reduced to below threshold concentrations prior to biological treatment. VOCs may result in noncompliance with air regulations and result in worker and public health concerns.
Volatile organics (benzene, toluene, etc.) should be removed by pretreatment prior to biological treatment. Air stripping or steam stripping can be used depending on the characteristics of the volatile organics. Air stripping is accomplished in packed or tray towers with countercurrent air–liquid flow. The off gas must be treated through vapor-phase carbon, biofilters, combustion or other methods. Steam stripping introduces steam in a packed tower, causing volatiles to be removed in the vapor phase. Biofilters can also be employed to treat off-gases from the stripper and/or aeration basin. This low-cost method employs a porous medium that promotes sorption into a liquid film on the medium surface followed by microbial stabilization. Powdered activated carbon (PAC) has been used in the aeration basin to adsorb VOCs and toxic compounds.
Chemical oxidation is typically employed to treat contaminants that may be non-biodegradable (refractory), toxic or inhibitory to microbial growth, and odor causing. Oxidizing agents used include ozone, O3; hydrogen peroxide, H2 O 2; permanganate, MnO− 4; various chlorine compounds; or even oxygen. These reactions frequently require catalysts to be cost effective. Such catalysts include UV light for ozone and hydrogen peroxide. Ferrous iron (FeSO4 or Fenton's reagent) at pH of about 3.5 is frequently employed for use with H2O2. Metal oxides such as titanium dioxide have also been employed for O3 catalysis. Ozone and UV plus various catalysts may be combined for specific waste in advanced oxidation processes. It is often not cost effective to carry the chemical oxidation to completion. Partial oxidation to compounds may be sufficient to render specific compounds such as many priority pollutants less toxic and more amenable to subsequent and less expensive biological treatment.
Adsorption may also be used to remove toxics, refractory organics, some heavy metals, and VOCs. Activated carbon possesses the property of attracting many contaminants to adhere to its surface via adsorption. Activated carbon is very porous with a very high surface-area-to-unit-weight ratio and thus possesses a high adsorption capacity for the hydrophobic soluble organics in wastewater. It can be contacted with wastewater either as a very fine powder or as granules. Since the powdered form is not conducive to column operation, granular activated carbon is used most frequently in advanced wastewater treatment plants. As the wastewater passes through the bed and the constituents from the wastewater are adsorbed onto the carbon, the sites available for adsorption will be exhausted and the bed must be replaced. The replacement can either be by new activated carbon material or by thermal regeneration of the exhausted bed. The sorption capacity of regenerated carbon however may be reduced as compared to the virgin material. In large plants, regeneration by high temperatures is usually practiced. Powdered activated carbon (PAC) can be added to the activated sludge process for enhanced performance (the PACT process). This has been shown effective for removing many priority pollutants and refractory organics. It can also remove color and enhance nitrification.
Inorganic Removal
Many of the inorganic constituents of a water will be at a higher than desirable concentration in a secondary treatment plant effluent. Although in most cases these excessive concentrations will not be harmful, continuous reuse of the water will cause a rapid buildup to prohibitive levels. Thus, a means of removing inorganics via a tertiary treatment process may be necessary. Pretreatment may also be needed to remove inorganics such as heavy metals, which may cause toxicity/inhibition to the biological treatment process. In many cases source treatment allows for reuse.
Ion exchange is a process that has been used for many years in the removal of hardness from water and of specific ions such as certain metals. The process takes advantage of the ability of certain natural and synthetic materials to exchange an ion contained in the material for another contained in the water passing through it. For example, if water containing calcium ions is passed through an ion-exchange medium that preferred calcium ions instead of the sodium ions that were already attached to it, the calcium ions will adhere to the exchange medium and the sodium ions would pass out into the effluent. The higher valence, higher molecular weight constituents replace the lesser ones. As with adsorption on carbon, if a solution containing an ion to be removed is continuously applied to the ion-exchange medium, the medium will eventually become exhausted. It may then be subjected to a process called regeneration, where the exchange medium is converted back to its original state by passing a concentrated solution of the original replaced ion through it. There are many modifications and combinations of ion-exchange units that have been used in both water and wastewater treatment technology. It should be noted that it is possible to recover material from an ion-exchange unit that might be economically advantageous. The use of ion-exchange media probably most familiar to the layman is the device that can be placed on the faucet in the home to obtain soft water for steam irons and other uses.
The electrodialysis process is used for demineralizing wastewaters. The principle of the process is shown in Figure. 17 . When voltage is applied across a cell containing mineralized water, the negatively charged ions (anions) will migrate to the positive electrode and the positively charged ions (cations) will migrate to the negative electrode. As shown in the diagram, alternate placement of anion and cation permeable membranes causes alternate compartments to become more concentrated in salts while others become more dilute. It should be noted that both suspended solids and dissolved organics should be eliminated from the feed water to the electrodialysis unit because of the possibilities of plugging and fouling. Like ion exchange, this process can be used for pretreatment of heavy metals. Note that for both ion exchange regeneration and electrodialysis concentrate wastes are produced which require proper treatment and management.
Figure 17.
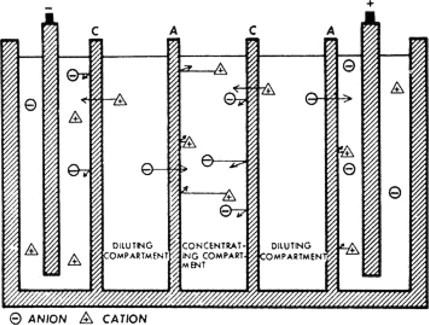
Electrodialysis unit. A, anion-permeable membrane; C, cation-permeable membrane.
Theoretically, the process of reverse osmosis (RO) is capable of removing greater than 90% of certain inorganic ions, organic material, and colloids. The principle involved is shown in Figure. 18 , which demonstrates various osmotic systems leading to the reverse osmosis process, and in Figure. 19 , which shows the construction of a spiral-wound reverse osmotic module. In operation, the water containing the dissolved materials is placed in contact with the membrane at a pressure in excess of the osmotic pressure of the solution. Under these conditions, the water will permeate the membrane and concentrate the dissolved materials. Problems with the reverse osmosis process have primarily been with the membranes because they are subject to fouling. Increasing effluent quality standards and the growing need for reuse of water resources has resulted in increased research, development, and implementation of RO technologies. As with ion exchange and electrodialysis processes a concentrated waste is generated which must be properly managed.
Figure 18.
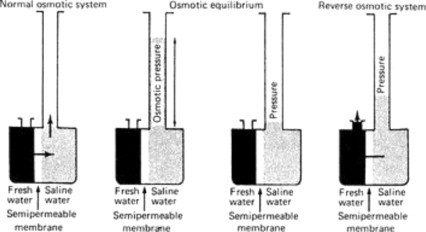
Principle of reverse osmosis: pressure on an osmotic system leads to reverse osmosis
Figure 19.
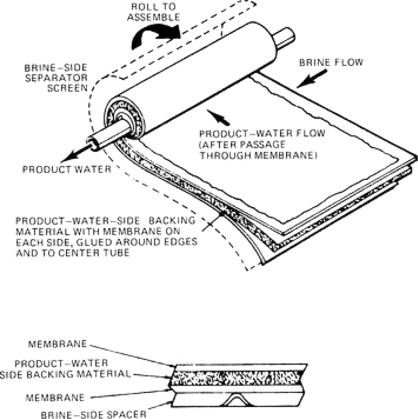
A spiral-wound reverse osmosis module.
Nitrogen Removal
As previously mentioned, the presence of the various forms of nitrogen in water can lead to several problems, including eutrophication, oxygen demand in a receiving water, toxicity (ammonia at high pH), and methemoglobinemia in infants. The removal of nitrogen is quickly becoming an integral part of a tertiary or modified secondary treatment facility. Successful removal of ammonia nitrogen has been accomplished by raising the pH to about 10 and removing the converted ammonia by aeration. The pH is usually raised by the use of lime and a packed tower is used for the gas-stripping operation. Nitrogen can also be removed by selective ion exchange, where ammonium ion is removed by using clinoptilolite, a naturally occurring medium.
The biological process used for nitrogen removal is attained by modifying the activated sludge process to allow for the oxidation of organic nitrogen to nitrate and then subsequently denitrifying the nitrate by placing it under anoxic conditions where bacteria convert the nitrate to nitrogen gas. Both of these processes have been used successfully. However, the bacteria involved are highly sensitive to temperature and to toxic organics. Appropriate consideration must therefore be given accordingly. The conversion of ammonia nitrogen to nitrate is called nitrification, and nitrate to nitrogen gas conversion is called denitrification. The microorganisms involved for nitrification are the autotrophic genera Nitrosomonas and Nitrobacter. The growth rates of these bacteria are very slow, and hence sludge age must be sufficiently long to prevent their washout. The microbes responsible for denitrification are heterotrophic, and hence organic carbon must be available for their metabolism. The biological processes involved are shown in Figure. 20 . The same reactions occur in surface waters and ground waters. The Bardenpho process and other modifications stage anoxic and aerobic sequencing basins with significant internal recycle. Nitrification occurs in the aerobic reactor and denitrification in the anoxic basin.
Figure 20.
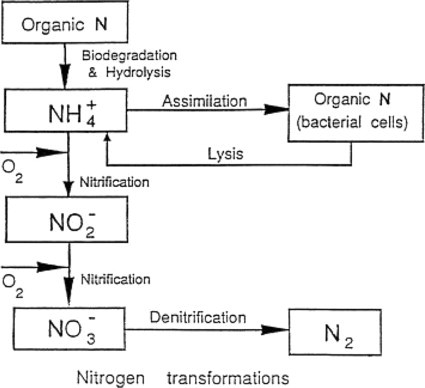
Nitrogen transformations under aerobic conditions.
Phosphorus Removal
Because of its ability to stimulate biological growth, phosphorus in wastewater effluents is limited to very low levels whenever the receiving water discharges into a lake or impoundment. The major source of phosphorus in domestic wastewater is detergents. This has been reduced in the United States and provinces in Canada where phosphates have been controlled in detergents.
Phosphorus removal can be accomplished by chemical precipitation and by a modification of biological oxidation referred to as ‘luxury uptake.’ This involves an anaerobic step in the presence of volatile fatty acids (mainly acetic) in which P is released into solution and acetate absorbed by specialized microbes called Acinetobacter. An aerobic step follows in which P is rapidly taken up by the microbes. Phosphorous levels less than 1 mg l− 1 and often less than 0.1 mg l− 1 can be achieved by this method for municipal wastewaters. Activated sludge has also been shown to remove significant phosphorus, especially when aluminum or iron salts are added at the end of the aeration tank or before the clarifier.
MBRs have successfully been used to enhance both nitrogen and phosphorus removals. An initial anaerobic basin followed by anoxic and aerobic zones using membrane separation of mixed liquor suspended solids has been shown effective in reducing these nutrients to very low levels.
Chemical removal of phosphorus can also be accomplished with aluminum, iron, or lime. The methodology for its addition varies depending on the particular plant. Lime precipitation of phosphorus is accomplished at pH levels above 10.5. Alum can be added prior to filtration. Note that the precipitation of phosphorus will increase sludge production. Phosphorous resources are becoming limiting, hence reclamation is becoming more important in regards to sustainability goals.
Water Reuse
It can be concluded from the previous discussion that wastewater can be treated to any quality desired and, therefore, reused, provided cost is not a controlling factor. The degree of treatment should be based on the quality requirements of the intended use of the water. Thus, if the wastewater can be used in a beneficial manner without causing adverse environmental or health effects, it should be considered as a valuable resource and used accordingly.
Drivers for water reuse vary from region to region and the nature of the application. Many years ago, there was no compelling business reason for water reuse. Non stringent regulatory requirements for the treatment and discharge of wastewater to the environment and abundance of good quality water supplies provided little incentive to water users to do anything but wastewater. Nowadays, water reuse is driven by more than a moral obligation to preserve this valuable resource. It has become good business practice by many industries and municipalities and the only option to sustain life in small to large communities in many parts of the world. Drivers such as water scarcity and droughts, lack of good quality water supplies, the cost of treating wastewater to very high standards due to more stringent regulations, permitting requirements, public relations, energy conservation, etc. have become strong motivators for industry and communities to look at wastewater as a resource not a waste.
The force field diagram shown in Figure 21 illustrates the various drivers and motivators for water reuse.
Figure 21.
Water Reuse Motivatorsa
a Taken from Industrial Wastewater Management- A Systems Approach, American Institute of Chemical Engineers July 2003, 2–6.
Perhaps one of the most compelling drivers for water conservation and reuse is the scarcity of water supplies and the uneven distribution of water resources across the globe. The ever increase in population growth will undoubtedly place extreme pressures on the world conventional water supplies (fresh surface water and groundwater) and energy resources required to tap into unconventional supplies (brackish and sea water). In areas such as the Middle East, water scarcity is an everyday concern. In the Gulf Cooperation Council (GCC) countries, there are virtually no rivers, little precipitation, and limited renewable fresh groundwater supplies. Dependence on desalination is high and is extremely energy intensive and expensive. Water reuse in these countries is an everyday practice. GCC countries lead many others in the world in the reuse of their treated sewage effluent (TSE) for landscape irrigation.
Introduction
Using wastewaters in a beneficial manner is not a new concept. In fact, water reuse has been practiced for many years. Because of the many discharges into our waterways, unless one lives in the backwaters of a mountain stream, the drinking water supply contains some wastewater. Even the Romans recognized that some waters were not suitable for drinking and had separate water for drinking and another supply for other purposes. Today, this practice is called a dual water system and is used in several locations in the United States and globally as well.
Probably the most widespread reuse of wastewaters has been for agricultural purposes, i.e., applying wastewater discharges after varying degrees of treatment, or in some cases, no treatment, for irrigation of crops. Subsequent to the advent of sewerage collection systems, numerous disposal sites existed throughout the world by the early 1900s. These were called sewage farms and were primarily used for disposal, although some of the wastewater was used for crop production and other beneficial uses. One of the more notable of these sewage farms was at Melbourne, Australia, which was started in 1893 and was still operating in the 1980s.
As water shortages became acute and the cost of wastewater treatment increased, many locations started investigating the potential for reusing wastewater for nonpotable purposes, thus alleviating some of the demand for potable water and giving an alternative to additional wastewater treatment. For example, in 1929, the city of Pomona, California, started a project using reclaimed wastewater for landscape and garden irrigation; in 1932, Golden Gate Park in San Francisco started using water from an on-site activated sludge plant for landscape irrigation and for small lakes in the park.
Probably the first introduction of treated wastewater into a municipal groundwater source was at Whittier Narrows in California in 1962, and direct injection of reclaimed wastewater into a groundwater aquifer was started in Orange County, California, in 1976. Although the primary purpose of the Orange County injection is to prevent saltwater intrusion, a high percentage of the reclaimed wastewater enters an aquifer used as a drinking-water source.
The use of dual distribution systems should also be mentioned inasmuch as, where feasible, a dual system significantly reduces potable water demand by using one system for potable water and the other for irrigation and other uses not requiring potable water. Notable of these systems is one installed in 1960 in Colorado Springs, Colorado, which supplies reclaimed water in a separate system for landscape irrigation. St. Petersburg, Florida, installed a similar system in 1977 where reclaimed water is supplied via a 260-mile dual system for irrigation of public parks, landscape irrigation, golf courses, and cooling-tower makeup water. It is interesting to note that although the initial impetus for the system was pollution abatement, the greatest benefit has been in water conservation by significantly reducing potable water demand.
The first direct potable water reuse project in the world was at Windhoek, Namibia. Here highly treated wastewater is introduced directly into the potable water reservoir/ distribution system. During drought periods, the reclaimed water is as much as 30% of the supply. This project was started in 1960 and was expanded in 2000.
As the world's population increases and the number of people living in urban centers grows, the need for water is becoming increasingly acute, particularly in semiarid and arid areas and when droughts occur. In many locations, especially under drought conditions, the demand for water exceeds the supply. Thus, there is no doubt that reclaimed water will play a major role in the water supply of the future. Many reuse programs in the arid and semiarid United States have been instigated by a need for water; however, many reuse projects have also been instigated in order to avoid costly compliance with requirements for wastewater discharges into surface waters. These requirements usually involve nitrogen and phosphorus removal and concomitant advanced wastewater treatment. It should be noted that these nutrients may actually be of benefit in agricultural water reuse and in wetlands enhancement.
It is significant to note that the major uses of water do not require potable water quality. Industry, for instance, uses significantly large volumes of water for cooling, processing, steam production, and other general uses. The majority of that water evaporates (evaporative cooling) or leaves the site with the product. However, a good portion remains and ends up as process water requiring disposal. Industry, depending on geographic location, also has to address storm water runoff and discharge, particularly when it becomes contaminated with raw materials, product, by products, or other industrial operations.
Industry is driven more and more towards water conservation, recycling, and reuse as public, media, and regulatory scrutiny regarding how industry sources, treats, and manages water continues to increase. Companies who treat water and wastewater as a commodity do so at great risk. Others who value water and wastewater as a precious resource have a greater chance of sustaining their operations and improving their viability in the market place.
From the perspective of industrial water users, water reuse can be approached from different angles. One such approach is to conserve water and reuse effluent within the fence of their operation. Traditionally, the industry has made strides in the design and operation of processes to minimize water use and optimize reuse through practices such as segregation of clean and contaminated streams, cascading water reuse (where the quality of the effluent discharged from one process is good enough to be used in another before treatment or disposal is required), and reuse of their wastewater effluent for cooling operations, fire fighting, and wash water requirements.
A second approach is for industry to look outside the fence of their operation for water conservation and reuse opportunities. An example would be to address water management throughout their entire supply chain from production, transportation, logistics, reprocessing of intermediate products by others, to final product use. Another outside the fence approach, which is gaining favor in countries such as Saudi Arabia and is driving the establishment of an entire infrastructure to support it, is the reuse of municipal effluent for industrial applications such as industrial cooling, mining, district cooling, etc. The success of such an approach greatly depends on realizing the true value of treating water without considering government subsidies as is the case in some countries.
Wastewater Contaminants Inimical to Reuse
Municipal and industrial wastewaters can contain anything that is contributed to the sewer as attributed by day to day human activities and the specific industrial manufacturing processes. Therefore, constraints on the use of the wastewater may be imposed by its chemical, biological, and physical characteristics. Consideration of potential adverse effects of these many parameters on public health and/or the environment is mandatory. The presence of some contaminants may preclude use of the water for some purposes if their removal is not economical. An economic analysis of the cost of treating and delivering the wastewater as compared to the benefits of its beneficial use must be made. This may include costs of advanced wastewater treatment that could be negated by avoiding discharge into a water-quality-limited waterbody. Parameters of concern are as follows.
Inorganic Chemicals
The list of inorganic chemicals that could be present in a wastewater is all inclusive. Many inorganic ions are naturally found in water such as calcium, sodium, magnesium, potassium, bicarbonates, chlorides, sulfates, and nitrates. Wastewaters may contain these ions and many others which may be contributed by a myriad of sources. The presence of some ions, such as boron, may totally preclude the use of a wastewater for irrigation because of its toxicity to plants. Also, the potential bioaccumulation and toxicity of the heavy metals to plants, humans and animals must be considered along with other potentially harmful materials. Selected drinking and irrigation standards are shown in Table III . It should be noted that because of pretreatment standards imposed on industrial waste discharges to municipal sewers in the U.S., the presence of many of these materials is being minimized. As previously noted, advanced wastewater treatment can remove these ions to acceptable levels.
Table III.
Selected drinking and irrigation water standards (mg l− 1)a
| Constituent | Drinking water | For fine-textured soils | For any soil | For livestock |
|---|---|---|---|---|
| Aluminum | 20 | 5 | ||
| Arsenic | 0.01 | 2 | 0.1 | 0.2 |
| Barium | 1.0 | |||
| Boron | 0.75 | 2 | 5.0 | |
| Cadmium | 0.01 | 0.05 | 0.01 | 0.05 |
| Chromium | 0.05 | 1.0 | 0.1 | 1.0 |
| Copper | 1.0 | 5 | 0.2 | 0.5 |
| Iron | 0.3 | 20 | 5 | |
| Lead | 0.05 | 10 | 5 | 0.1 |
| Manganese | 0.05 | 10 | 0.02 | |
| Mercury | 0.002 | 0.01 | ||
| Nickel | 2.0 | 0.2 | ||
| Selenium | 0.01 | 0.02 | 0.02 | 0.05 |
| Zinc | 5 | 10 | 2 | 25 |
Organic Chemicals
Listing the organic chemicals that may be in municipal and industrial wastewater is difficult if not impossible, primarily because of the numerous species existing, some of which may be formed by interactions with each other. Many organics are relatively new and the long-term health effects are unknown, as are potential synergistic effects (e.g., endocrine disrupters and pharmaceuticals and personal care products). Although drinking-water standards exist for some organics, compliance does not guarantee that the water is safe for potable use. Furthermore, the traditional measures of organics, such as BOD, COD, and TOC, reveal little concerning the toxicity and health effects of the organics measured. It should be noted that organics such as pesticides, insecticides, and herbicides may be found in any drinking-water source where runoff contributes to the waterbody. Numerous organics have been detected in water and wastewater at very low levels; however, many remain unidentified. Even with advances in analytical techniques, technology, and epidemiological studies, it cannot be concluded that many of these trace organics found in wastewater do not pose a potential health hazard.
Microbiological Considerations
Protection from waterborne disease is paramount, both in drinking-water supplies and in recreational waters. Pathogenic microorganisms found in wastewater may include bacteria, viruses, protozoans, algae, and helminths. The major waterborne pathogenic microorganisms that have been found in the United States are tabulated in Table IV
Table IV.
Major waterborne pathogens and related disease
| Pathogen | Disease |
|---|---|
| Bacteria | |
| Shigella (spp.) | Shigellosis (bacillary dysentery) |
| Salmonella typhi | Typhoid fever |
| Salmonella (1700 serotypes spp.) | Salmonellosis |
| Vibro cholerae | Cholera |
| Escherichia coli (enteropathogenic) | Gastroenteritis and septicemia, hemolytic uremic svndrome (HUS) |
| Yersinia enterocolitica | Yersiniosis |
| Leptospira (spp.) | Leptospirosis |
| Campylobacter jejune | Gastroenteritis, reactive arthritis |
| Protozoa | |
| Entamoeba histolytica | Amebiasis (amebic dysentery) |
| Giardia lamblia | Giardiasis (gastroenteritis) |
| Cryptosporidium | Cryptosporidiosis, diarrhea, fever |
| Microsporidia | Diarrhea |
| Helminths | |
| Ascaris lumbricoides | Ascariasis (roundworm infection) |
| Ancylostoma (spp) | Ancylostomiasis (hookworm infection) |
| Necator americanus | Necatoriasis (roundworm infection) |
| Ancylostoma (spp.) | Cutaneous larva migrams (hookworm infection) |
| Strongloides stercoralis | Strongyloidiasis (threadworm infection) |
| Trichuris trichiura | Trichuriasis (whipworm infection) |
| Taenia (spp.) | Taeniasis (tapeworm infection) |
| Enterobius vermicularis | Enterobiasis (pinwork infection) |
| Echinococcus granulosus (spp.) | Hydatidosis (tapeworm infection) |
| Viruses | |
| Enteroviruses (polio, echo, coxsackie, new enteroviruses, serotype 68–71) | Gastroenteritis, heart anomolies, meningitis, others |
| Hepatitis A and E virus | infectious hepatitis |
| Adenovirus | Respiratory disease eye infectons, gastroenteritis (serotype 40 and 41) |
| Rotavirus | Gastroenteritis |
| Parvovirus | Gastroenteritis |
| Noroviruses | Diarrhea, vomiting, fever |
| Astrovirus | Gastroenteritis |
| Calicivirus | Gastroenteritis |
| Coronavirus | Gastroenteritis |
Adapted from National Research Council, 1996; Sagik et. al., 1978; and Hurst et. al., 1989
The presence of these microorganisms, which clearly exist in untreated wastewater, are of obvious public health concern, and every effort must be made to minimize the possibility of human contact when using reclaimed wastewater for any purpose. As previously discussed, conventional wastewater treatment can reduce the levels of these pathogens to acceptable concentrations, although advanced wastewater treatment may be required for indirect potable reuse. Bacteria are most susceptible to conventional treatment when adequate disinfection is included. Viruses and protozoans are more difficult to remove and may require additional treatment, such as ultrafiltration and reverse osmosis. The protozoans, such as giardia and cryptosporidium, are much more resistant and may survive even high concentrations of chlorine.
Serious outbreaks of giardiasis and cryptosporidiosis have occurred in cities with excellent water-treatment facilities and are of major concern in the water industry. Therefore, every effort must be made to minimize human contact with reclaimed water that may contain any of these pathogens. Of particular concern is the possibility of pathogens being carried in aerosols emitted by spray irrigation inasmuch as aerosols in the 2–5 mm size are primarily removed in the respiratory tract. Notably, there are no documented reports of disease outbreaks attributed to spray irrigation with disinfected reclaimed wastewater. The potential transmission of Legionnaires' disease (Legionella) by drift from cooling towers should also be noted.
Survival of pathogens depends on several factors, including temperature, soil organic content, humidity, solar radiation, and foliage. Typical survival times are shown in Table V .
Table V.
Typical Pathogen survival times at 20–30 °C. (in days) d
| Pathogen | Survival Time (days) |
||
|---|---|---|---|
| Fresh Water & Sewage | Crops | Soil | |
| Virusesa | |||
| Enterovirusesb | < 120 but usually < 50 | < 60 but usually < 15 | < 100 but usually < 20 |
| Bacteria | |||
| Fecal conforms a,c | < 60 but usually < 30 | < 30 but usually < 15 | < 70 but usually < 20 |
| Salmonella spp.a | < 60 but usually < 30 | < 30 but usually < 15 | < 70 but usually < 20 |
| Shigella spp.a | < 30 but usually < 10 | < 10 but usually < 5 | --- |
| Vibrio choleraed | < 30 but usually < 10 | < 5 but usually < 2 | < 20 but usually < 10 |
| Protozoa | |||
| Entamoeba histolytica cysts | < 30 but usually < 15 | < 10 but usually < 2 | < 20 but usually < 10 |
| Helminths | |||
| Ascaris lumbricoides eggs | Many months | < 60 but usually < 30 | Many months |
In seawater, viral survival is less, and bacterial survival is very much less than in fresh water.
Includes polio, echo-, and Coxsackie viruses.
V. cholerae survival in aqueous environments is uncertain.
Taken from USEPA Guidelines for Water Reuse, 2004 (http://nepis.epa.gov/Adobe/PDF/30006MKD.pdf).
Adapted from Feacham et. al., 1983
Hence the use of reclaimed wastewater may pose some risks with respect to potential exposure to microorganisms. However, with good design and operation, proper monitoring, and appropriate precautions maintained, the risk from any reclaimed water application is minimal. According to the Water Environment Federation, water reuse has not been implicated as the cause in any infectious disease outbreak.
Other Water Quality Parameters of Concern
The presence of excessive solids can cause problems in the distribution system by settling out in the system and by clogging the nozzles in spray irrigation. They can also cause problems when reclaimed water is applied to soils by clogging the soils and reducing the infiltration rate. Also, some solids adsorb heavy metals and solids can also shield microorganisms from disinfection.
Excessive salinity may have a profound effect on both plants and soils. It is usually measured by electroconductivity, which is related to total dissolved solids (TDS). As salts infiltrate into the ground, they tend to concentrate in the root zone because of evapotranspiration (evaporation from soil and surface water and transpiration) by plants. With an increase in salinity, plants expend energy in adjusting for osmotic effects to obtain water from the soil. Thus, less energy is available for plant growth and a reduction in productivity occurs. Adequate drainage is mandatory for long-term use of reclaimed water for irrigation, and it may be necessary to leach more water through the soil than the plants require in order to maintain a proper salt balance.
Sodium is of particular concern in irrigation water because of its ability to cause changes in soil structure, infiltration, and permeability rates. A high percentage of sodium in a soil with some clays causes conditions unfavorable for water movement and plant growth. The hazard from sodium can be estimated by its ratio to calcium and magnesium, and methods are available to determine its effect on soils and plants.
Nutrients important to plants include nitrogen, phosphorus, potassium, zinc, boron, and sulfur, all of which may be present in wastewater. The most beneficial nutrient in reclaimed water is nitrogen, which may replace an equal amount of nitrogen in fertilizer during the growing period. However, nitrogen in excessive amounts may be detrimental to crops and monitoring may be required. It should also be noted that under aerobic conditions, nitrogen in the wastewater existing as organic or ammonia nitrogen will ultimately be converted to nitrate, of concern because of its relationship to methemoglobinemia.
Applications of Water Reuse
As previously indicated, water is becoming a scarce resource and the use of reclaimed water for many purposes is receiving increasing attention. Possible uses of reclaimed water and potential restraints are shown in Table VI .
Table VI.
| Waste water reuse categories | Potential constraints |
|---|---|
| 1. Agricultural irrigation Crop irrigation Commercial nurseries |
|
| 2. Landscape irrigation Parks Schoolyards Freeway medians Golf courses Cemeteries Greenbelts Residential |
|
| 3. Industrial recycling and reuse Cooling Boiler feed Process water Heavy construction |
|
| 4. Groundwater recharge Groundwater replenishment Salt water intrusion control Subsidence control |
|
| 5. Recreational/environmental uses Lakes and ponds Marsh enhancement Streamflow augmentation Fisheries Snowmaking |
|
| 6. Nonpotable urban uses Fire protection Air conditioning Toilet flushing |
|
| 7. Potable reuse |
|
Taken from Waste water Engineering, Metcalf and Eddy, McGraw-Hill, 2003.
Arranged in descending order of projected volume of use.
Thus a wide spectrum of applications for reclaimed water exists, and each use has special concerns and requirements for treating the wastewater that helps to ameliorate these concerns. More cities, in particular those in arid, semiarid, and water-short areas, will increase their interest in water reuse. It is unlikely that direct potable reuse will be common in the near future; however, as is the case in Namibia, where no other source is available, pipe-to-pipe use is possible with no apparent adverse effects. One only has to consider the many wastewater discharges into the Mississippi River and the use of that waterbody as a drinking water source for New Orleans to conclude that indirect potable reuse is viable. Advanced wastewater treatment can produce almost any water quality desired and has become more reliable and effective through experience and innovation.
With regards to planned indirect potable reuse, the National Resources Council stated in 1998 that any community considering potable reuse should do so only after a careful, thorough, site-specific assessment that includes contaminant monitoring, health and safety testing, and system reliability evaluation.
It is instructive to examine several successful water-reuse projects to illustrate the viability and utility of wastewater reuse and its potential in helping to solve critical water shortages.
St. Petersburg Dual Water System
Dual water systems have the capability of minimizing the use of potable water inasmuch as non-potable water is used is used for purposes not requiring potable water. This reduces the demand for potable water and its concomitant cost of treatment. The use of dual water systems is much more attractive when developing new systems, when the cost of constructing another distribution system can be minimized, as was the case in St. Petersburg. The St. Petersburg system, which has been in operation since 1977, uses 20 MGD of reclaimed water via 260 miles of distribution piping. The reclaimed water is used for parks, golf courses, industrial processes, commercial uses, and residences. The St. Petersburg dual water system approach has allowed the city to meet the demand from a 10% population increase without increasing supplies. When excess reclaimed water exists, forty million gallons of treated wastewater can be stored onsite; after that the reclaimed water is pumped into a saltwater aquifer some 900 ft below the ground surface.
The Orange County, California, Groundwater Replenishment System
At Orange County, CA groundwater discharge with direct injection of reclaimed wastewater has been practiced since 1976. The original plant treated 15 MGD of activated sludge effluent using, reverse osmosis to treat part of the effluent to satisfy a 500 mg l− 1 TDS maximum requirement because of limitations imposed. The water was then injected into four aquifers to form a seawater intrusion barrier. Before injection, the water was blended 2:1 with well water from a deep non-contaminated aquifer. The water could then flow to the ocean or to augment the potable water supply, or in both directions. The water produced from the treatment plant had no measurable viruses, non-detectable coliform organisms, and an average turbidity of 0.22 TU. Continuing monitoring showed the treatment process removed potential contaminants to non-detect levels for drinking water. Epidemiological studies showed no measurable adverse health effects in the populations ingesting the reclaimed water. It must be recognized that the reclaimed water was diluted with non-contaminated water and had time in the aquifer for natural purification. This facility was recently demolished to make room for a new Groundwater Replenishment (GWR) system. The system opened in 2008 at a cost of $480 million to build and $29 million a year to operate. This enhancement was necessary due to increased population growth, salt water intrusion due to over pumping from the ground water aquifer, the on going chronic drought, and the increased cost of importing water from Northern California. The treatment train consist of filter screens followed by microfiltration. Reverse osmosis is next and the effluent is disinfected using UV light with hydrogen peroxide, About 70 MGD of water is produced. Approximately half filters through the soil into deep aquifers. The rest is injected into the Orange County seawater barrier to prevent ocean seawater intrusion.
The Tahoe-Truckee Sanitation District
This advanced 9.6 MGD wastewater treatment plant, which is located near Lake Tahoe, California, is probably the longest-operating sophisticated plant in the world. This treatment process consist of screening, grit removal, sedimentation, activated sludge, biological phosphorous removal, chemical treatment, filtration, ion exchange ammonia removal and chlorination for disinfection. A unique system of ammonia removal is employed. A combination of ion exchange using clinoptilolite (a natural ion-exchange medium) in conjunction with an indoor air stripping operation results in the recovery of ammonium sulfate, a fertilizer. The effluent has better water quality than many municipal drinking waters. In spite of the high degree of treatment, the effluent is injected into the groundwater system via a leach field where it eventually finds its way into the Truckee River, which is the major source of water for Reno, Nevada.
Regulations for Water Reuse
There are presently no existing federal standards for water reclamation and reuse. However, several states have adopted applicable regulations, and as the need for water becomes more critical, more stringent state regulations will undoubtedly be promulgated. Arizona, California, Colorado, Florida, Hawaii, Nevada, Oregon, and Texas have adopted regulations that encourage water reuse. Some states have based their regulations on providing an alternative to surface water discharge. In states with no regulations, reuse may be permitted on a case-by-case basis. It should also be noted that many foreign countries have pertinent regulations and the World Health Organization has published guidelines for water reuse.
The prime objective of existing regulations is to protect public health and the major consideration has been for irrigation; however, little attention has been given to the potential adverse effects of some constituents of wastewater on plants and soil. As previously mentioned, these effects can be profound and should be considered. The regulations address the following categories of reuse with appropriate restrictions and requirements (taken from Guidelines for Water Reuse, USEPA, 2004)
-
1.
Unrestricted urban reuse. Irrigation in areas in which public access is not restricted, such as parks, playgrounds, school yards and residences, toilet flushing, fire fighting, air conditioning, construction, ornamental fountains, and aesthetic impoundments.
-
2.
Restricted urban reuse. Irrigation of areas in which public access can be controlled, such as golf courses, cemeteries, and highway medians.
-
3.
Agricultural reuse on food crops. Irrigation of food crops that are intended for direct human consumption, often further classified as to whether the food crop is to be processed or consumed raw.
-
4.
Agricultural reuse on nonfood crops. Irrigation of fodder, fiber and seed crops, pastureland, commercial nurseries, and sod farms.
-
5.
Unrestricted recreational use. An impoundment of water in which no limitations are imposed on body-contact water-recreation activities.
-
6.
Restricted recreational reuse. An impoundment of reclaimed water in which recreation is limited to fishing, boating, and other noncontact recreational activities.
-
7.
Environmental reuse. Reclaimed water used to create artificial wetlands, to enhance natural wetlands, and to sustain stream flows.
-
8.
Industrial reuse. Reclaimed water used in industrial facilities primarily for cooling system makeup water, boiler feed water, process water, and general washdown.
Requirements may include water quality and treatment, monitoring, system reliability, storage for system or excess reclaimed water, application rates, groundwater monitoring, and setback or buffer zones. The most restrictive regulations are for indirect potable use via groundwater recharge by surface spreading or direct injection and augmentation of surface supplies. An important consideration in indirect potable reuse is public perception. Several projects have been rejected because of public and/or political pressure, health concerns being the major reasons for rejection. These categories of reuse and pertinent restrictions have been further refined and expanded by USEPA Guidelines for Water Reuse, 2012.
Finally, the question of water rights must be investigated. While states east of the Mississippi use the Riparian Rights Doctrine, states west of the Mississippi use the Doctrine of Prior Appropriation. In Western water-short areas, water rights may play a major role in any water resource project.
Footnotes
Change History: March 2015. AJ Englande Jr., P Krenkel, and J Shamas made minor grammar and substantial changes throughout the article including new sections on new Membrane Bioreactors (MBRs) and Moving Bed Bioreactors (MBRs), and Anaerobic Treatment. Other additions were new Figures 1 and 21, updated figures 4, 15a and 15b, a new table 1 and updated Tables 3, 4, 5 and 6. Finally there was an updated Bibliography and a new website citations section.
Further Reading
- Byers W., Lindgren G., Noling C., Peters D. 2nd edn. American Institute of Chemical Engineers; New York: 2003. Industrial Water Management A Systems Approach. [Google Scholar]
- Culp G.L., Culp R.L. Van Nostrand Reinhold; Princeton, NJ: 1974. New Concepts in Water Purification. [Google Scholar]
- Droste R.L. John Wiley & Sons, Inc.; New York: 1997. Theory and Practice of Water and Wastewater Treatment. [Google Scholar]
- Eckenfelder W.W., Ford D.L., Englande A.J. 4th edn. McGraw-Hill; New York: 2009. Industrial Water Quality. [Google Scholar]
- Holdsworth R. Avebury Technical; U.K.: 1991. New Health Considerations in Water Treatment. [Google Scholar]
- Krenkel P.A., Novotny V. Academic Press; New York: 1980. Waste Quality Management. [Google Scholar]
- Metcalf W.J., Eddy . 3rd ed. McGraw-Hill; New York: 2003. Wastewater Engineering Treatment & Reuse. [Google Scholar]
- Metcalf W.J., Eddy A.E.C.O.M. McGraw-Hill; New York: 2007. Water Reuse Issues, Technologies and Applications. [Google Scholar]
- National Academy of Science and National Academy of Engineering . U.S. Government Printing Office; Washington, D.C.: 1972. Water Quality Criteria. [Google Scholar]
- State of California . Department of Health Services; Berkeley, California: 1999. Wastewater Reclamation Criteria. California Administrative Code, Title 22, Division 4. [Google Scholar]
- USEPA . USEPA; Washington, D.C.: 2000. Biosolids Technology Fact Sheet, EPA 832-F-00-064. [Google Scholar]
- USEPA . USEPA; Washington, D.C.: 2015. 40 CFR Part 503 Standards for the Use or Disposal of Sewage Sludge. [Google Scholar]
- USEPA . USEPA; Washington, D.C.: 2004. Guidelines for Water Reuse EPA/625/R-04/108. [Google Scholar]
- USEPA . USEPA; Washington, D.C.: 2012. Guidelines for Water Reuse EPA/625/R-04/108. [Google Scholar]
- Water Pollution Control Federation . 2nd edn. Water Pollution Control Federation; Washington, D.C.: 1989. Water Reuse, Manual of Practice SM-3. [Google Scholar]
- Weber W.J., Jr. Wiley (Interscience); New York: 1972. Physicochemical Processes for Water Quality Control. [Google Scholar]
Relevant Websites
- (http://nepis.epa.gov/Adobe/PDF/30006MKD.pdf)–USEPA Guidelines for Water Reuse, 2004.
- http://water.epa.gov/lawsregs/rulesregs/sdwa/regulations.cfm.
Glossary
- Aerobic
Living or occurring only in the presence of oxygen.
- Anaerobic
Living or occurring only in the absence of free oxygen.
- Ascaris ova
Eggs of a nematode worm, which is an intestinal parasite.
- Benthic
Organisms and material on the bottom of a waterbody.
- Biochemical oxygen demand (BOD)
Measure of oxygen uptake by microorganisms when stabilizing biodegradable organic material.
- Chemical oxidation
Stabilization of a substance by an oxidizing chemical, such as chlorine.
- Chemical oxygen demand (COD)
Measure of oxygen equivalent of organic matter susceptible to oxidation by a strong chemical oxidant (e.g., potassium dichromate).
- Colloidal
Suspension of finely divided particles in a water that will pass through a filter paper and will not settle out of solution. In water, colloids usually possess a negative charge and thus will migrate to a positively charged electrode.
- Effluent
Discharge, after treatment, from a wastewater treatment plant or process.
- Eutrophication
Natural aging of a lake where it changes from a pristine state to a swamp or land. The geologic process takes millions of years, but human cultural activities hasten the process by the addition of nutrients and erosion.
- Facultative
Ability of microorganisms to live under aerobic or anaerobic conditions.
- Flocculation
Rapid to slow mixing that causes agglomeration of particles for more efficient sedimentation.
- Giardiasis
Enteric disease transmitted by a protozoan, Giardia lamblia, which can cause severe effects in humans and is carried in water.
- Helminth
Worm, especially a parasitic intestinal nematode or trematode.
- Influent
Flow into a process or body of water.
- Methemoglobinemia
Cyanosis in infants that may be caused by excessive concentration of nitrates in the water.
- Supernatant
Clear liquid overlying material deposited by settling, precipitation, or centrifugation.
- Synergistic
Action of two or more substances that amplifies the toxicity of either or both. Antagonistic is its antithesis.


