Abstract
This chapter includes diseases of animals in the order Rodentia, in which there are over 2000 species representing 40% of all mammals. This incredibly diverse order includes members inhabiting every continent, either naturally or in human-made environments. While rodents have been the cause or implicated in disease transmission that has lead to human pandemics, such as the Black Death, and the decimation of certain animal species, like island-dwelling birds; genetically modified rodents have contributed significantly to the advancement of biomedical research and human health. There are more than 50 species of endangered rats, mice, voles, squirrels, and marmots. The recent extinction of the Bramble Cay melomys represents the first human-induced rodent extinction linked to climate change. Rodents are the reservoir host of several human and domestic pathogens of concern listed by OIE. Herein, we highlight those diseases of rodents that lead to clinically important gross and microscopic lesions.
Keywords: Capybara, guinea pig, mouse, mole-rat, pathology, porcupine, rat, rodent, squirrel, zoonosis
Introduction
The order Rodentia includes 29 families, 468 genera, and over 2052 species. Two separate systems exist to classify rodents (Carleton and Musser, 2005). The first classification scheme by J.F. Brandt (1855) is based on morphology of the jaw and skull together with masticatory muscle position and has three suborders: Sciuromorpha (squirrel-like); Myomorpha (mouse-like); and Hystricomorpha (porcupine-like). The second separates rodents into two suborders, Sciurognathi and Hysticognathi, based on the position of incisors and the angle of the jaw (Carleton and Musser, 2005). Molecular studies yield seven clades containing three phylogenetic lineages: squirrel-related (Sciuriodea and Gliridae); mouse-related (Anomaluromorpha, Castoridae, Geomyoidae, and Myodonta); and Ctenohystrica. With diverse ecologic strategies, rodents are found naturally in a variety of habitats globally except Antarctica. The natural history and biology of members of this extensive order exhibit substantial variation. Nevertheless, similarities exist among rodents including dentition, anatomy, and disease susceptibility.
Unique features
The unifying characteristic of all rodents is their dentition, which includes two pairs of continuously growing (elondont) incisors. Brachydonts have elondont incisors with brachydont molars; in Hystricomorphs, all of the teeth are elondont. Dentition is important when considering the diet and husbandry needs of these groups. Brachydonts, which include mice, rats, hamsters, and gerbils, need high calorie diets with low amounts of fiber, whereas the hystricomorphs (porcupine-like; guinea pigs, chinchillas, agoutis) are strictly herbivores and require ample abrasive roughage. Dental formulae vary but in general, rodents have four incisors, no canines, 1–2 premolars, and numerous (up to 12) molars.
As most rodents are omnivorous, their digestive system typically consists of a simple stomach (monogastric) and a variably adapted intestinal tract depending on the degree of hind gut fermentation (Stevens and Hume, 1998). Most practice coprophagy or cecotrophy to maintain normal microflora and absorb microbially derived amino acids and vitamins (e.g., B and K). As such, herbivorous, omnivorous, insectivorous, and carnivorous rodents have different gastric and large intestinal anatomy. Some rodents lack gall bladders (e.g., rats and pocket gophers). Rats and hamsters are unable to vomit due to a muscular sphincter at the esophageal-gastric junction. Members of Caviidae lack the enzyme l-gulonolactone oxidase, making vitamin C (ascorbic acid) a necessary dietary requirement for this group (see scurvy in section, Nutritional Diseases).
Rodents are typically small (as small as 10 g) and rotund with short legs, though the largest rodents are almost 70 kg (e.g., capybaras). Rodents are nocturnal and though some species hibernate (e.g., woodchucks) but most of the species do not. There are numerous specializations among rodents including cheek pouches (food transport), extremity and claw development (e.g., burrowing, jumping, climbing, flying), sensory organ variations (e.g., vibrissae and large ears or eyes), special integument and pelage (e.g., porcupine quills and chinchilla fur), and tail adaptations (e.g., prehensile, fat storage). Rodents have a strong sense of smell and a well-developed vomeronasal organ, which is important for reproduction and social behavior through the sensing of pheromones. Since rodents lack sweat glands, they are susceptible to overheating.
Reproductive anatomy varies though most female rodents ovulate spontaneously and are polyestrous. Like rabbits, chinchillas have two cervices that communicate with individual uterine horns. Vaginas are imperforate prior to sexual maturing and during nonbreeding and/or postpartum intervals. Placentation is discoid and hemochorial. Mammary gland anatomy varies among species. The suborders Scuirognatha and Stricognatha have altricial and precocial young, respectively. Some male rodents have testes within a scrotum, though the testes of porcupines, agoutis, chinchillas, cavies, and capybaras are located within the inguinal canal, which remains open in most male rodents. Male rodents also have well developed accessory sex glands and an os penis. Prairie dogs have perianal sacs similar to canids and felids, which are important for sexual communication. Male beavers have persistent paramesonephric ducts that resemble a uterus and are referred to as masculine uterus (Doboszynska and Zurowski, 1981, Meier et al., 1998).
South American rodents including guinea pigs and related species have unique pulmonary anatomy as do burrow-dwelling, subterranean mole-rats that live in low oxygen tension environments, for example, large and dilated, primitive bronchi and bronchioles (Ilgun et al., 2014, Widmer et al., 1997). Lung morphology of 40 different rodent species representing 11 families is well described (Wallau et al., 2000).
Common microscopic findings in rodents that may be misinterpreted as lesions include: multinucleated, karyomegalic, and cytomegalic hepatocytes are common in several rodent species and can increase with age (Fig. 20.1 ); hepatocellular intranuclear cytoplasmic invaginations (pseudoinclusions) (Fig. 20.1); eosinophilic cytoplasmic spherical inclusions in renal tubular epithelial cells and hepatocytes seen predominantly male mice, rats, and hamsters; splenic extramedullary hematopoiesis, which is very common in healthy rodents of all ages (Fig. 20.2 ); hemosiderin, lipofuscin, ceroid, and melanin (in dark or black coated animals) are commonly detected in various tissues, such as spleen, liver, kidney, and adrenal glands; cardiac muscle in the tunica of pulmonary veins in the lung is a normal finding in mice; male rodents may have refluxed seminal coagula in the urinary bladder and urethra that is thought to occur peri mortem; and adrenal X-zone vacuolation in female mice. Furthermore, there are evident sexual dimorphisms of some organs and glands, such as the cuboidal parietal epithelium of the glomeruli in male rodents.
Figure 20.1.
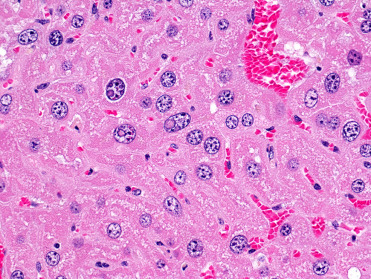
Normal liver in an aged mouse.
Common findings inlcude anisocytosis, anisokaryosis, and eosinophilic intranuclear pseudoinclusions of invaginated cytoplasm.
Figure 20.2.
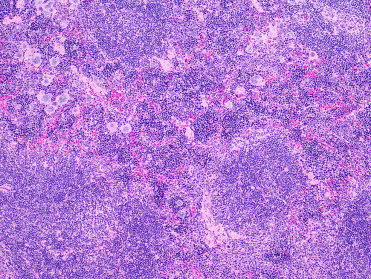
Splenic extramedullary hematopoiesis in an aged mouse.
Within the red pulp are hematopoietic elements including erythroid and myeloid lineages. Note the large multinucleated megakaryocytes, which are readily visible at this magnification.
Hematological reference ranges have been established for some common domestic, zoo-housed, and ubiquitous free-ranging species. In general, the lifespan of rodent erythrocytes is shorter than other mammals, thus, higher levels of circulating reticulocytes (1%–6%), seen as polychromasia and anisocytosis, is considered normal. Howell-Jolly bodies may also be present. In all age groups, lymphocytes are more numerous than neutrophils. In fact, lymphocytes are the predominant white blood cells in guinea pigs (45%–80%). In addition, guinea pigs and capybaras have Kurloff cells (Fig. 20.3 ), which are lymphocytes containing a single, large intracytoplasmic mucopolysaccharide inclusion body, known as a Kurloff body (Jara et al., 2005). Though the actual function of these cells is unknown, they are presumed to be natural killer cells. The number of Kurloff cells in females fluctuate with the reproductive cycle stage, thus they are considered to be estrogen-dependent. Pregnant females may have 2%–5% Kurloff cells in circulation while males typically have fewer. Guinea pigs have heterophils similar to rabbits. Rats, mice, and hamsters commonly have neutrophils with ring-form nuclei. Chinchillas and porcupines have well-defined and segmented neutrophil nuclei. Band neutrophils may be a normal finding in some species including New World porcupines, coypu, and agoutis.
Figure 20.3.
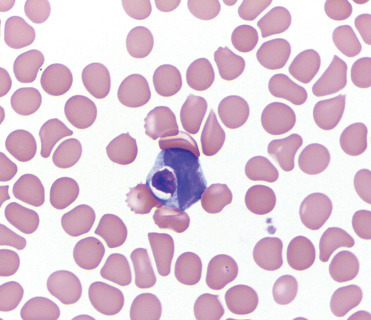
Normal Kurloff cell in a blood smear from a guinea pig.
A Kurloff body is a mucopolysaccharide inclusion in the cytoplasm of lymphocytes. They are found in lymphocytes of guinea pigs and capybaras. In this image, it causes mild peripheralization of the nucleus.
(Photo Courtesy of L. Black, University of Florida)
Serum biochemical parameters have been compiled for some rodent species (Kurtz et al., 2017, Yarto-Jaramillo, 2015). Of note is that serum levels of alanine aminotransferase (ALT) are not sensitive biomarkers of liver health in guinea pigs and some other rodents as they normally have low hepatic levels of this enzyme. In contrast, gamma glutamyl transferase is present in the liver of all rodents and can be evaluated to determine the hepatic injury or disease. Hamsters and other desert species may have high levels of serum calcium. Rodents like mice and rats, and specifically those evolved in arid regions, have a profound ability to concentrate their urine, thus it is often turbid and contain calcium crystals.
The following list of diseases of rodents is not exhaustive but instead highlights the more common findings in species of which there is published literature or knowledge. A plethora of information exists regarding the anatomy, physiology, and diseases of rodents used as animal models in biomedical research (i.e., laboratory animals) and is a valuable resource when examining lesser-studied rodent species using a comparative approach (Barthold et al., 2016, Greaves, 2011, Suckow et al., 2012, Treuting and Dintzis, 2017). As many rodents are important prey species, diseased or dead rodents are exceedingly difficult to detect in nature using the passive surveillance techniques of traditional wildlife disease monitoring. Thus, less is known about the diseases and pathology of free-ranging rodents as compared to larger and charismatic mammals.
Non-infectious diseases
Nutritional
One of the most well described nutritional diseases in rodents is hypovitaminosis C or scurvy. Guinea pigs and a number of other species including capybaras and humans, lack the enzyme l-gulonolactone oxidase which is required for l-ascorbic acid (vitamin C) production therefore, these species require dietary vitamin C (Cueto et al., 2000). Vitamin C is necessary for the formation of collagen via hydroxylation of proline/lysine for cross linking of fibrillar collagen. Hypovitaminosis C results in decreased collagen fibril cross linking, leading to the increased fragility of blood vessels, cartilage, and osteoid. In affected individuals, gross and histologic lesions are characteristic and include capillary hemorrhage and lack of bone deposition and remodeling as a result of poorly formed and weak collagen. Other gross lesions include evidence of anemia (e.g., pallor, reactive bone marrow), periarticular hemorrhage, and swollen joints, specifically at the costochondral junctions (scorbutic lattice). Characteristic oral lesions include gingival swelling, erythema, hemorrhage, erosion, ulceration, and tooth loss. Histological examination is necessary to confirm and define bone lesions, which include: dilation of metaphyseal blood vessels, irregular columnization and hypertrophy of physeal cartilage, calcification of metaphyseal cartilage with reduced or absent osteoid, microfractures with surrounding and subperiosteal hemorrhage, medullary fibrosis, and decreased osteoclastic activity and remodeling. Tooth loss may result from a combination of abnormal odontoblasts, dentin resorption, pulp fibrosis, and a lack of/abnormal alveolar bone remodeling.
Like lagomorphs, some hindgut-fermenting rodents are susceptible to gut stasis and related diseases if they are not able to obtain enough fiber from the diet. Obesity can be common in rodent species if fed a high caloric diet and can predispose to diabetes mellitus, cardiac disease, pregnancy toxemia, and hepatic lipidosis in females.
Metabolic
Dystrophic mineralization occurs in guinea pigs, hamsters, and free-ranging rats (Rothenburger et al., 2015a). Mineralization most often affects the myocardium but can also occur in other tissues, such as the diaphragm, tongue, liver, kidney, lungs, cornea, and aorta (see section Congenital/Genetic). Though the pathogenesis is not fully elucidated, mitochondria appear to be preferentially mineralized based on ultrastructural studies. Gross lesions may include cardiomegaly with chalky to gritty white material and streaking within the myocardium of the atria and ventricles. Histologically, affected cardiac myocytes are degenerative and/or necrotic with rupture of the sarcolemma. Damaged myocytes will have variable and progressive mineralization until the entire myofiber is densely mineralized. Variably mature fibrosis surrounds individual and clusters of dead and mineralized myocytes. Satellite cells are frequently hypertrophic.
Pregnancy toxemia occurs in overweight, pregnant, and lactating female guinea pigs and hamsters. There are two forms in guinea pigs, a fasting type that results from decreased carbohydrate intake and subsequent mobilization of fat stores and a circulatory type that occurs when the gravid uterus compresses the aorta and causes reduced blood flow to abdominal organs. Characteristic gross and histologic lesions include hepatic lipidosis and fat deposition in renal tubular epithelial cells. Similarly, hepatic lipidosis and associated metabolic effects occasionally affect overweight nongravid sows and boars.
Urolithiasis is common in guinea pigs, rats, and mice due to high urine pH and calcium concentrations. Obstructive urolithiasis has also been reported in captive male agoutis resulting in bilateral hydronephrosis, urinary bladder rupture, and peritonitis (Batista et al., 2010).
A number of other endocrine diseases have rarely been documented in various rodent species. They have typically been associated with hormone-producing neoplasms (adrenal, islet, thyroid, parathyroid, and pituitary tumors).
Toxic
Fluorosis is well studied in rats as they are used as models in toxicology studies. Guinea pigs and rabbits are also susceptible and fluorosis has occurred in free-ranging cotton rats in sites contaminated by petrochemical waste (Rafferty et al., 2000). Pathognomonic lesions occur in teeth and bones. Periosteal hyperostosis occurs first along the medial surface of the metatarsals, then progresses to affect the mandible, metacarpals, and ribs though all bones can be affected in severe, chronic disease. Bones are brittle and chalky-white. Pathological fractures are common but joints are typically spared. Although characteristic, enamel hypoplasia only occurs when fluorosis is present during tooth development. Tooth lesions include dull, dry, and chalky hypomineralized or hypoplastic enamel with pitting, grooves, and discoloration (due to oxidation); excessive attrition affects the incisors first. Irregular wear, malocclusion, and fractures may be present. Histologically, ameloblasts are small while osteoblasts are vacuolated and disorganized. There is abundant globular dentin and hypomineralization of the outer enamel layer. A differential for enamel hypoplasia in young Syrian hamsters is Hamster Parvovirus (HaPV); concurrent testicular hypoplasia, and cerebral malacia is present with this viral infection.
Cholecalciferol (Vitamin D) is used as a rodenticide. The deliberate poisoning of free-ranging rats and mice results in inadvertent toxicity of nontarget animals including other rodents, such as squirrels or carnivorous predators that consume affected carcasses. Multisystemic mineralization occurs prior to death in affected individuals. Unlike other rodents, naked mole-rats, beavers, and woodchucks do not require dietary vitamin D and toxicity can develop if they are fed rodent chow or diets supplemented with vitamin D. Lesions consistent with calcinosis cutis and circumscripta can develop in naked mole-rats fed such diets. Soft tissue calcification is not limited to pressure points and can be found throughout the body in severe cases. Resolution occurs with correction of the diet (Delaney et al., 2013).
Anticoagulant rodenticides including warfarin and its derivatives are frequently used to control free-ranging mice and rat populations. These compounds antagonize vitamin K and thus inhibit production of vitamin K-dependent clotting factors (I, II, VII, IX, X). Gross lesions include multisystemic hemorrhage, particularly subcutaneous and periarticular hematomas. “Bait,” some of which are brightly colored (e.g., teal-green), may be present within the intestines. Inadvertent consumption by nontarget wildlife may have fatal consequences.
Hindgut fermenting rodents are susceptible to similar antibiotic-related toxicities as rabbits (see Chapter 19). Nephrotoxicosis can be the result of exposure to numerous agents and has been extensively described in laboratory animals. For example, Swainsonine toxicity occurs following the ingestion of plants from the genus Swainsona and results in acquired alpha-mannosidosis in rats. Histologically, renal tubular epithelial cells are severely and diffusely vacuolated.
Congenital/Genetic
Dystrophic mineralization, also known as dystrophic cardiac calcinosis is considered as a genetic disease in inbred mouse strains, particularly in aged individuals (Eaton et al., 1978). Cardiac mineralization is detected grossly as pinpoint foci to larger irregular crusts composed of white, gritty material within and overlying the epicardium. Histologic lesions vary depending on severity and chronicity with degeneration and necrosis of individual myocytes to extensive regions of myocyte loss and replacement by deeply basophilic granular material. In severe cases, mineralization extends into the myocardium resulting in scar formation and can also be seen in the kidneys and lungs. The prevalence of this disease in wild mice from which these strains originated is unknown.
Diabetes mellitus (DM) in Chinese hamsters is a heritable, autosomal recessive disease (Green et al., 1963). Symptomology and lesions are similar to those found in humans and include hydropic degeneration and degranulation of pancreatic islet β-cells with secondary vascular lesions, such as arteriosclerosis and glomerulosclerosis. In addition, affected hamsters are predisposed to pancreatic adenocarcinomas. Serum levels of α-2 globulins are a useful indicator of risk for diabetes mellitus development in these populations.
Polycystic kidney disease has been diagnosed in captive populations of Brazilian agoutis (Müller et al., 2009). Most affected individuals have no clinical signs and are potentially related genetically. Grossly, affected agoutis have a range of bilateral renal changes including atrophy, roughened granular cortical surfaces, and multiple cysts. Histologically, there is cystic dilatation of renal tubules and Bowman’s capsules with mesangial and capsular thickening accompanied by mononuclear interstitial nephritis and fibrosis.
Age-Related/Degenerative
Many degenerative and age-related diseases are described in rodents used in toxicology studies (Barthold et al., 2016, Pettan-Brewer and Treuting, 2011). A significant disease confounding these studies is chronic progressive nephropathy, which is best described in rats though mice, naked mole-rats, and Australian rodents, particularly the stick-nest rat, are also affected (Delaney et al., 2016a). The exact pathogenesis is unknown; however genetic factors may play a role. Grossly, kidneys are bilaterally pale and either enlarged or shrunken with numerous pinpoint to large, 1 mm cortical microcysts creating a nodular surface. Histologically, renal tubules are markedly ectatic and tortuous and many contain brightly eosinophilic fluid (proteinosis) (Fig. 20.4 ). Tubules have a range of degeneration, necrosis, and regeneration with occasional tubular hyperplasia and adenoma formation (predominantly in rats). Glomeruli are variably affected by membranous and/or proliferative changes with thickened basement membranes, sclerosis, and obsolescence. Synechiae, dilated Bowman’s capsules, and hypertrophied parietal epithelium are common. The interstitium is expanded by lymphoplasmacytic infiltrates with pigment-laden macrophages, mild hemorrhage, and edema. There is often substantial interstitial fibrosis and in severe cases, infarction. Diagnosis is based on histologic evaluation though in some species, clinical pathology may be preferentially used to determine renal function.
Figure 20.4.
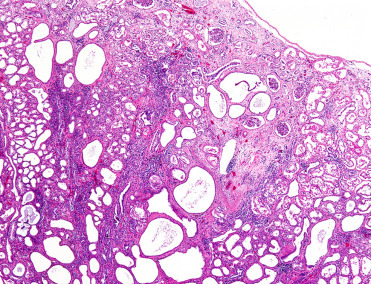
Chronic progressive nephropathy in a naked mole-rat.
Renal tubules are variably ectatic, atrophied, degenerate, necrotic, and replaced by collagenous fibrous tissue. Within the interstitium are accumulations of mononuclear cells (primarily lymphocytes and fewer macrophages). There is also sclerosis of glomeruli and periglomerular fibrosis.
Cardiomyopathy is common in aged mice, rats and hamsters; it is particularly common in Syrian hamsters and is reported in woodchucks (Chanut et al., 2013, Rothenburger et al., 2015a; Roth and King, 1986). Lesion severity varies though it appears to increase with age and is more common in males. Though considered strain and thus possibly genetically related in laboratory rats, free-ranging rats develop similar disease (Rothenburger et al., 2015a). Grossly, there may be cardiomegaly but macroscopic changes are not always apparent. Histologically, myocardial lesions consist of multifocal and perivascular lymphoplasmacytic infiltrates accompanied by fibrosis, myocyte degeneration, and drop out (Fig. 20.5 A,B). Trichrome, Movat pentachrome, or other stains that highlight the fibrosis are useful to evaluate severity and chronicity. Syrian hamsters develop cardiomyopathy associated spontaneous atrial thrombosis and subsequent coagulopathy that is often fatal (Fig. 20.6 ). Females appear to be affected earlier in the life than males.
Figure 20.5.
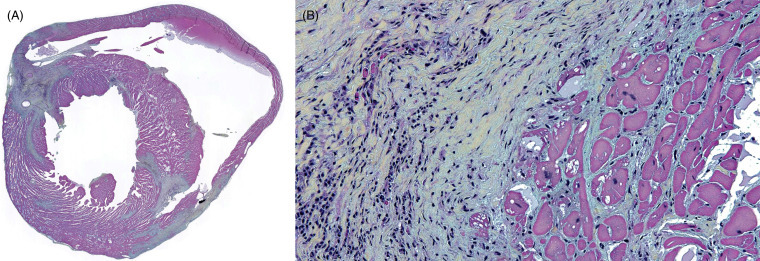
Cardiomyopathy in a free-ranging black rat.
(A) There is multifocal disruption and replacement of the myocardium by fibrosis (blue-green staining), Movat pentachrome. (B) Cardiomyocyte degeneration is characterized by cell swelling, anisocytosis, and anisokaryosis and loss of cross striations. Cells are separated and surrounded by increased amounts of fibrosis (blue-green staining) and lymphoplasmacytic inflammation, Movat pentachrome.
Figure 20.6.
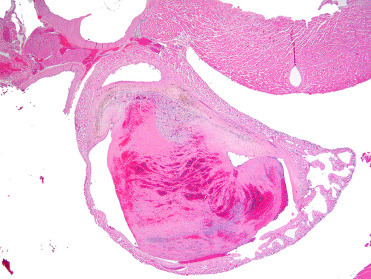
Atrial thrombosis in a Syrian hamster.
The atrium is expanded by a large luminal thrombus that is regionally adherent to the endocardium. The thrombus is composed of laminated layers of fibrin, leukocytes, and erythrocytes, and intermixed hemosiderin-laden macrophages.
(Photo Courtesy of J. Landolfi, University of Illinois Zoological Pathology Program)
Like other species, rodents are susceptible to skeletal degeneration and related diseases. Guinea pigs, specifically the Duncan-Hartley breed and Syrian hamsters, frequently develop degenerative joint disease. Gross lesions include thickened joint capsules, variable osteophyte formation, roughened cartilage surfaces, eburnation, joint effusion, and synovial proliferation. Depending on which joints are affected and chronicity, crepitus may be present when joints are manipulated. Microscopic examination of joints reveals degeneration, necrosis, and ulceration of the articular cartilage with proliferation and fibrosis of the synovium and joint capsule, periarticular and periosteal bone formation, and remodeling. There may also be variable inflammation and hemorrhage within joint spaces.
Amyloidosis is typically seen in aged animals. Amyloid is an extracellular accumulation of insoluble protein. Excessive amyloid deposits can lead to tissue atrophy and dysfunction. Amyloidosis is most common in myomorphs (mice, rats, hamsters, gerbils) and appears in the kidneys, spleen, adrenals, and liver as well as the gastrointestinal lamina propria (mouse) (Fig. 20.7 ). There is a higher prevalence in females. Of note is the eosinophilic substance within the nasal planum of aged mice that was once thought to be amyloid, through histochemical and ultrastructural studies, has instead been shown to consist of collagen and complex carbohydrates produced by the nasal gland epithelial cells (Doi et al., 2007).
Figure 20.7.
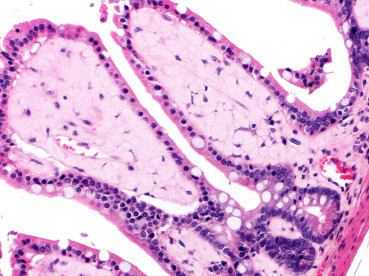
Intestinal amyloidosis in an aged mouse.
The lamina propria is markedly expanded by homogenous, pale eosinophilic extracellular fibrillar material consistent with amyloid. There are few lymphocytes embedded within the amyloid.
(Photo courtesy of C. Pettan-Brewer and P. Treuting, University of Washington)
Hemosiderosis is the accumulation of iron within various tissues, most commonly the liver. It is described in numerous species including primates, birds, rhinoceros, hyraces, and rodents. In one zoological institution, hemosiderosis was present in approximately 65% of adult naked mole rats (Delaney et al., 2013). Grossly, livers appear bronze. Iron accumulation appears histologically as zonal to diffuse hepatocellular accumulation of brown granular to globular pigment. Pigment is most intense in the periportal hepatocytes. The pigment stains positively with Prussian blue and is not refractile with polarized light.
Aged mice can develop intrapulpal denticles, which are dysplastic tooth-like growths arising in the pulp of incisors. These may result in abnormal wear and or breakage of the incisors contributing to periodontitis and decreased ability to effectively gnaw (Pettan-Brewer and Treuting, 2011).
Trauma
Despite their protective quills, porcupines are particularly susceptible to cutaneous trauma and tears due to an exquisitely fragile integument. Secondary infections resulting in pyoderma and persistent ulceration may occur.
Inflammatory, Non-infectious
Stress dermatosis (or dermatitis) is a condition of several South American rodents including, the agouti, acouchis, and capybaras. This syndrome is seen in captive and free-ranging individuals in densely populated groups. It presents as alopecic skin lesions and lacerations along the lumbosacral spine. Stress is thought to increase skin fragility and overcrowding with intraspecific aggression contributes to lesion development. Sun exposure, pruritus, and self-mutilation result in large patches of alopecia with erythema and excoriated skin with loss of the long hairs along the dorsum and tail. Microscopic lesions correlate to severity and chronicity and include variable epidermal degeneration, necrosis, erosion/ulceration, and associated dermatitis with hemorrhage and edema. Diagnosis is based on clinical history, demonstrated absence of a parasitic etiology in skin scrapings and histopathology.
Miscellaneous
Malocclusion is common especially in hysticomorphs, though it is also seen in brachydonts. It is a significant disease in captivity and may be related to genetics, nutrition and diet, and husbandry practices (Losco, 1995).
Ovarian cysts are common in rodents. Cystic rete ovarii are prevalent in female guinea pigs (Veiga-Parga et al., 2016) and can be distinguished from other ovarian cysts as they arise from the rete and compress adjacent ovarian tissue (Fig. 20.8 ). Other ovarian cystic structures reported in female rodents include: bursal cysts, epithelial cysts, follicular cysts, luteal cysts, and paraovarian cysts (Dixon et al., 2014).
Figure 20.8.
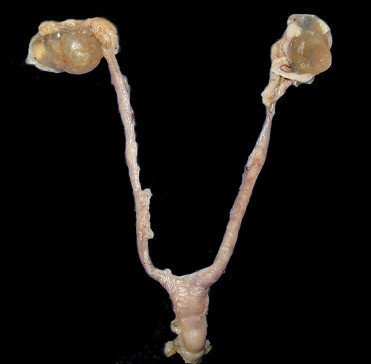
Cystic rete ovarii in a guinea pig.
The ovaries are bilaterally enlarged by multiple, intraovarian rete cysts.
(Photo Courtesy of J. Landolfi, University of Illinois Zoological Pathology Program)
The hemochorial placentation in rodents predisposes them to trophoblast emboli. This incidental lesion is best described in chinchillas and is not associated with clinical ramifications or gross lesions. Histologically, intravascular trophoblasts are present in the lung, uterus, spleen, liver, and other sites, such as the adrenal glands with no associated inflammation. Trophoblasts are large (>100 μm) with abundant amphophilic granular cytoplasm and large nuclei with prominent nucleoli.
Neoplastic
Many neoplasms of inbred mice and rats appear to be strain related. Nevertheless, certain neoplasms occur more frequently among free-ranging and domesticated outbred rodents than in other mammalian orders. Several of them are described below. A list of other common neoplasms in rodent species is listed in the Supplemental Materials (Table e1).
Table e1.
Rodent Neoplasia
| System | Species Affected | Neoplasia Type | References |
|---|---|---|---|
| Cardiovascular | |||
| Rats | Endocardial schwannoma | Berridge (2016) | |
| Guinea pig | Myocardial rhabdomyoma | Barthold (2016) | |
| Endocrine | |||
| Rats | Adrenocortical adenoma | Strandberg (1983) | |
| Rats | C cell carcinoma | Boorman (1996) | |
| Rats | Pituitary adenoma | Benitz (1965), Barthold (2016) | |
| Glands | |||
| Mice, Rats | Mammary adenocarcinoma | Rudman (2012); Krinke (1994), Krinke (1996) | |
| Mice, Rats | Mammary fibroadenoma | Rudman (2012); Krinke (1994), Krinke (1996) | |
| Gray squirrel | Mammary (mixed malignant) | Williams (1989) | |
| Mice, Rats | Zymbal gland adenoma/carcinoma | Rudman (2012); Krinke (1994), Krinke (1996) | |
| Mice, Rats | Preputial/Clitoral adenoma/carcinoma | Rudman (2012), Barthold (2016), Krinke (1994), Krinke (1996) | |
| Female reproductive | |||
| Mice, Rats | Ovarian adenocarcinoma | Dixon (2014), Krinke (1994), Krinke (1996) | |
| Mice | Teratoma | Dixon (2014), Barthold (2016) | |
| Mice, Rats | Uterine decidual reaction/deciduoma | Dixon (2014) | |
| Woodchuck, mice, rats | Uterine leiomyoma | Foley (1993), Dixon (2014) | |
| Mice, Rats | Uterine leiomyosarcoma | Dixon (2014) | |
| Male reproductive | |||
| Woodchuck | Adenoma of the rete testis | Foley (1993) | |
| Rat, woodchuck | Interstitial cells tumors | Foley (1993), Barthold (2016), Creasy (2012) | |
| Woodchuck | Sertoli cell tumors | Foley (1993) | |
| Woodchuck | Seminoma | Foley (1993) | |
| Woodchuck, mice | Teratoma | Foley (1993), Barthold (2016), Creasy (2012) | |
| Hepatobilliary | |||
| Rat | Foci of cellular alteration | Thoolen (2010) | |
| Woodchuck, rat, mice | Hepatocellular adenoma | Roth (1991), Thoolen (2010), Krinke (1994), Krinke (1996) | |
| Woodchuck, rat, mice | Hepatocellular carcinoma | Roth (1985), Thoolen (2010), Krinke (1994), Krinke (1996) | |
| Hemolymphatic | |||
| Mice, Rats | Histiocytic sarcoma | Krinke (1994), Krinke (1996), Kogan (2002), Barthold (2016) | |
| Guinea pig, rat | Lymphocytic leukemia | Yarto-Jaramillo (2011), Barthold (2016) | |
| Guinea pig, hamster, mice, woodchuck, rat | Lymphoma | Nagy (2002), Simmons (2001), Yarto-Jaramillo (2011), Kogan (2002), Barthold (2016) | |
| Woodchuck, mice, rats | Myeloproliferative disease | Roth (1991), Kogan (2002), Barthold (2016) | |
| Gastrointestinal | |||
| Black-tailed prairie dogs | Odontoma | Phalen (2000) | |
| Tree squirrels | Elodontoma | Boy (2006) | |
| Red squirrels | Gastric spindle cell tumor | Simpson (2013) | |
| Muskrat, free-ranging rats | Gastric squamous papillomas | Cosgrove (1968), Rothenburger (2014) | |
| Woodchuck | Oral leiomyosarcoma | Kang (2005) | |
| Integument | |||
| Red backed vole | Adnexal carcinoma | Williams (1989) | |
| Gerbils, guinea pigs | Aural polyps | Benitz (1965) | |
| Gerbils | Ear canal fibropapilloma | Benitz (1965) | |
| Gerbils | Cholesteatomas | Benitz (1965) | |
| Gray, red, and fox squirrels | Fibroma | Bangari (2009) | |
| Beechey ground squirrel, rats, mice | Harderian gland carcinoma | Ranck (2008), Barthold (2016), Krinke (1994), Krinke (1996) | |
| Ground squirrel | Integumentary gland adenocarcinoma | Carminato (2012) | |
| Damaraland mole rat | Melanomas | Sura (2011) | |
| Yellow cheeked vole | Sebacious gland adenoma | Williams (1989) | |
| Capybara, tundra vole, arctic ground squirrel,woodchuck | Squamous cell carcinoma | Hamanno (2014), Takahisa (2014), Anderson (1990) | |
| Beaver, insular vole, porcupine | Squamous papilloma | Williams (1989) | |
| Hamsters, red squirrels | Trichoepithelioma | Foster (2002), Simpson (2013) | |
| Guinea pig | Trichofolliculoma | Barthold (2016) | |
| Musculoskeletal, Adipose, Connective tissue | |||
| Damaraland mole rat | Fibrosarcoma | Sura (2011) | |
| Rat | Hibernomas | Brunner (2009) | |
| Woodchuck | Lipoma | Anderson (1990) | |
| Liposarcoma | |||
| Mice, Rats | Mesencyhmal tumors | Greaves (2013) | |
| Rat, woodchuck | Mesothelioma | Barthold (2016), Creasy (2012), Kang (2004) | |
| Beaver | Rhabdomyoma | Williams (1989) | |
| Red backed vole | Rhabdomyosarcoma | Williams (1989) | |
| Tundra vole | Osteoma | Williams (1989) | |
| Respiratory | |||
| Guinea pig | Alveologenic carcinoma | Yarto-Jaramillo (2015) | |
| Guinea pig | Bronchogenic carcinoma | Yarto-Jaramillo (2015) | |
| Guinea pig | Papillary adenoma | Yarto-Jaramillo (2015) | |
| Gray squirrels | Pulmonary adenomatosis | Kirschstein (1958) | |
| Red squirrels, mice | Pulmonary carcinoma | Simpson (2013), Barthold (2016), Renne (2009) | |
| Mice, Rats | Pulomnary adenoma | Barthold (2016), Renne (2009) | |
| Urinary | |||
| Red and Gray squirrels, mice, rats | Renal adenoma | Simpson (2013), Williams (1989), Frazier (2012) | |
| Mice, Rats | Renal carcinoma | Frazier (2012) | |
Anderson, W. I., Scott, D. W., Hornbuckle, W. E., King, J. M., Tennant, B. C., 1990. Spontaneous neoplastic and hyperplastic skin lesions of the woodchuck. Vet. Derm. 1, 177–180.
Bangari, D. S., Miller, M. A., Stevenson, G. W., Thacker, H. L. Sharma, A., Mittal, S. K., 2009. Cutaneous and systemic poxviral disease in red (Tamiasciurus hudsonicus) and Gray (Sciurus carolinensis) squirrels. Vet. Pathol. 46, 667–672.
Barthold, S. W., Griffey, S. M., and Barthold, D. H., 2016. Pathology of Laboratory Rodents and Rabbits, fourth ed. John Wiley & Sons, Ames.
Benitz, K. F., Kramer, A. W., 1965. Spontaneous tumors in the Mongolian gerbil. Lab. Anim. Care. 15, 281–294.
Berridge, B. R., Mowat, V., Nagai, H., Nyska, A., Okazaki, Y., Clements, P. J., Rinke, M., Snyder, P. W., Boyle, M. C., Wells, M., 2016. Non-proliverative and proliferative lesions of the cardiovascular system of the rat and mouse. Toxicol. Pathol. 29, 1–47.
Boorman, G.A., DeLellis, R.A., Elwell, M.R. 1996. Endocrine System. In: Monographs on Pathology of Laboratory Animals. first ed. Jones, T.C., Capen, C.C., Mohr, U. (eds), Springer Berlin Heidelberg, pp. 262–274.
Boy, S. C. and Steenkamp, G., 2006. Odontoma-like tumours of squirrel elodont incisors – elodontomas. J. Comp. Path. 135, 56–61.
Brunner, R. H., Novilla, M. N, Picut, C. A., Kirkpatrick, J. B., O’Neill, T. P., Scully, K. L., Lawrence, W. B., Goodman, D. G., Saladino, B. H., Peters, D. G., Parker, G.A., 2009. Spontaneous hibernomas in Sprague-Dawley rats. Toxicol. Pathol. 37, 547–552.
Carminato, A., Nassuato, C., Vascellari, M., Bozzato, E., Mutinelli, F., 2012. Adenocarcinoma of the dorsal glands in 2 European ground squirrels (Spermophilus citellus). Comp. Med. 62, 279–281.
Cosgrove, G. E., Lushbaugh, W. B., Humason, G., Anderson, M. G., 1968. Synhimantus (Nematoda) associated with gastric squamous tumors in muskrats. Bull. Wild. Dis. Assoc. 4, 54–57.
Creasy, D., Bube, A., deRijk, E., Kandori, H., Kuwahara, M., Masson, R., Nolte, T., Reams, R., Regan, K., Rehm, S., Rogerson, P., Whitney, K., 2012. Proliferative and nonproliferative lesions of the rat and mouse male reproductive system. Toxicol. Pathol. 40, 40–121.
Dixon, D., Alison, R., Bach, U., Colman, K., Foley, G.L., Harleman, J.H., Haworth, R., Herbert, R., Heuser, A., Long, G., Mirsky, M., Regan,K., Van Esch, E., Westwood, F.R., Vidal, J., Yoshida, M., 2014. Nonproliferative and proliferative lesions of the rat and mouse female reproductive system. J. Toxicol. Path. 27(3–4 Suppl.), 1S–107S.
Foley, G. L., Anderson, W. I., Schlager, D. H., Hornbuckle, W. E., Baldwin, B. H., Tennant, B. C., 1993. Neoplastic and nonneoplastic lesions of the reproductive tract of the woodchuck (Marmota monax). J. Zoo Wild. Med. 24, 475–481.
Foster, A. P., Brown, P. J., Jandrig, B., Grosch, A., Voronkoca, T., Scherneck, S., Ulrich, R., 2002. Polyomavirus infection in hamsters and trichoepitheliomas/cutaneous adnexal tumors. Vet. Rec. 151, 13–17.
Frazier, K. S., Seely, J. C., Hard, G. C., Betton, G., Burnett, R. Nakatsuji, S., Nishikawa, A., Durchfeld-Meyer, B., Bube, A., 2012. Proliferative and nonproliferative lesions of the rat and mouse urinary system. Toxicol. Pathol. 40, 14–86.
Greaves, P., Chouinard, L., Ernst, H., Mecklenburg, L., Pruimboombrees, I. M., Rinke, M., Rittinghausen, S., Thibault, S., Vonerichsen, J., Yoshida, T., 2013. Proliferative and non-proliferative lesions of the rat and mouse soft tissue, skeletal muscle and mesothelium. Toxicol. Pathol. 26, 1–26.
Hamanno, T., Terasawa, F., Tachikawa, Y., Murai, A., Mori, T., El-Dakhly, K., Sakai, H., Yanai, T., 2014. Squamous cell carcinoma in a capybara (Hydrochoerus hydrochaeris). J. Vet. Med. Sci. 76, 1301–1304.
Kang, B. C., Lee, Y. S. Lee, S. K., 2004. Malignant pleural mesothelioma in a woodchuck (Marmota monax). J. Vet. Med. Sci. 66, 1617–1619.
Kang, B. C., Jang, D. D., Lees, S. K., 2005. Oral leiomyosarcoma in a woodchuck (Marmota monax). J. Vet. Med Sci. 67, 353–355.
Krinke, A. L., Schaetti, P. R., Krinke, G. J., 1994. Changes in the major ocular glands. In: Mohr, U., Dungworth, D. L., Capen, C. C., Carlton, W. W., Sundberg, J. P., Ward, J. M. (Eds).Pathobiology of the Aging Rat, Vol. 1. International Life Sciences Institute Press, Washington, DC, pp. 109–119.
Krinke, G. J, Schaetti, P. R., Krinke, A., 1996. Nonneoplastic and neoplastic changes in the Harderian and lacrimal glands. In: Mohr, U., Dungworth, D. L., Capen, C. C., Carlton, W. W., Sundberg, J. P., Ward, J. M. (Eds). Pathobiology of the Aging Mouse, Vol. 2. International Life Sciences Institute Press, Washington, DC, pp. 139–152.
Kirschstein, R. L., Rabson, A. S., Kilham, L., 1958. Pulmonary lesions produced by fibromas viruses in squirrels and rabbits. Cancer Res. 18, 1340–1344.
Kogan, S. C., Ward, J. M., Anver, M. R., Berman, J. J., Brayton, C., Cardiff, R. D., Carter, J. S., de Coronado, S., Downing, J. R., Fredrickson, T. N., Haines, D. C., Harris, A. W., Harris, N. L., Hiai, H., Jaffe, E. S., MacLennan, I. C., Pandolfi, P. P., Pattengale, P. K., Perkins, A. S., Simpson, R. M., Tuttle, M. S., Wong, J. F., Morse, H. C., 2002.
Bethesda proposal for classification of nonlymphoind hematopoietic neoplasms in mice. Blood. 100, 238–245.
Nagy, T., McDonough, S. P., Erb, H. N., Smith, C. A., Baldwin, B. H. Tennant, B. C., 2002. Lymphosarcoma in the laboratory woodchuck (Marmota monax). Comp. Med. 52, 152–159.
Phalen, D. N., Antinoff, N., Fricke, M. E., 2000. Obstructive respiratory disease in prairie dogs with odontomas. Vet. Clin. North Am. Exot. Anim. Pract. 3, 513–517.
Renne, R., Brix, A., Harkema, J., Herbert, R., Kittel, B., Lewis, D., March, T., Nagano, K., Pino, M., Rittinghausen, S., Rosenbruch, M., Tellier, P., Wohrmann, T., 2009. Proliferative and nonproliferative lesions of the rat and mouse respiratory tract. Toxicol. Pathol. 37(7 Suppl.), 5S–73S.
Ranck, R.S., Cullen, J.M., Wagie, K.S., Marion, P.L. 2008. Harderian gland neoplasms in captive, wild-caught Beechey ground squirrels (Spermophilus beecheyi). Vet. Pathol. 45, 388–392.
Roth, L., King, J. M., Hornbuckle, W. E., Harvey, J., Tennant, B. C., 1985. Chronic hepatitis and hepatocellular carcinoma associated with persistent woodchuck hepatitis virus infection. Vet. Pathol. 22, 338–343.
Roth, L., King, J. M., Tennant, B. C., 1991. Hepatic lesions in woodchucks (Marmota monax) serongative for woodchuck hepatitis virus. J. Wildl. Dis. 2, 281–287.
Rothenburger, J. L., Himsworth, C. G., Lejune, M., Treuting, P. M., Leighton, F. A., 2014. Lesions associated with Eucoleus sp. in the non-glandular stomach of wild urban rats (Rattus norvegicus). Int. J. Parasitol. Parasites Wildl. 3, 95–101.
Rudmann, D., Cardiff, R., Chouinard, L., Goodman, D., Kuttler, K., Marxfeld, H., Molinolo, A., Treumann, S., Yoshizawa, K., 2012. Proliferative and nonproliferative lesions of the rat and mouse mammary, Zymbal’s preputial and clitoral glands. Toxicol. Pathol. 40, 7–39.
Simmons, J. H., Riley, L. K., Franklin, C. L., Besch-Williford, C. L., 2001. Hamster polyomavirus infection in a pet Syrian hamster (Mesocricetus auratus). Vet. Pathol. 38, 441–446.
Simpson, V. R., Hargreaves, J., Butler, H. M., Davison, N. J., Everest, D. J., 2013. Causes of mortality and pathological lesions observed post-mortem in red squirrels (Sciurus vulgaris) in Great Britain. BMC Vet. Res. 9, 229.
Strandberg, J. 1996. Endocrine System. Endocrine System. In: Monographs on Pathology of Laboratory Animals. first ed. Jones, T.C., Capen, C.C., Mohr, U. (eds), Springer Berlin Heidelberg, pp. 440–448.
Sura, R., French, R.A., Goldman, B. D., Schwartz, D. R., 2011. Neoplasia and granulomas surrounding microchip transponders in Damaraland mole rats (Cryptomys damarensis). Vet. Pathol. 48, 896–902.
Takahisha, H., Terasawa, F., Tachikawa, Y., Murai, A., Mori, T., El-Dakhly, K., Sakai, H., Yanai, T., 2014. Squamous cell carcinoma in a capybara (Hydrochoerus hydrochaeris). J. Vet. Med. Sci. 76, 1301–1304.
Thoolen, B., ten Kate, F. J. W., van Diest, P. J., Malarkey, D. E., Elmore, S. A., Maronpot, R. R., 2012. Comparative histomorphological review of rat and human hepatocellular proliferative lesions. Toxicol. Pathol. 25, 189–199.
Williams, E. S., Thorne, E. T., 1989. Spontaneous tumors of free-ranging terrestrial mammals of North America. In: Kaiser, H.E. (Ed) Comparative Aspects of Tumor Development. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp. 214–225.
Yarto-Jaramillo, E., 2015. Rodentia. In: Miller R. E., Folwer, M. E. (Eds.), Fowler’s Zoo and Wild Animal Medicine, eighth ed. Elsevier Saunders, St. Louis, pp. 384–422.
Naked mole-rats are a popular zoo species and are heralded as animal models for cancer and aging due to their extreme longevity and purported cancer resistance. Rare cases of neoplasia have been reported in naked mole-rats and in the related Damaraland mole-rat, which is also long-lived and appears to be relatively resistant to cancer and age-related diseases (Delaney et al., 2016b, Sura et al., 2011, Taylor et al., 2016,). Other subterranean rodents used as animal models for carcinogenesis research include some members of Spalacidae (mole-rats, blind mole-rats, zokors, and bamboo rats). There are currently no reported spontaneous tumors in this group; however, subcutaneous tumors (fibromas and fibrosarcomas) may be induced with application of carcinogenic compounds.
Odontoma, or more aptly named odontogenic dysplasia, is described in several rodent species (rats, mice, guinea pig, prairie dogs, degus, chinchilla, chipmunk, squirrels; Boy et al., 2006). Most odontomas are not true neoplasms and instead represent dysplastic changes resulting from chronic inflammation or trauma and associated regional tissue response. As there is no demonstrated metastatic potential or criteria for malignancy, these lesions are increasingly identified as hamartomas versus neoplasms (and also referred to as pseudo-odontomas). Nevertheless, these toothy masses are regionally destructive and almost exclusively affect the incisors. Grossly, odontogenic dysplasia presents as nodular swellings around the maxillary or mandibular incisors. Microscopically, these lesions are composed of disorganized and dysplastic proliferations of odontogenic epithelium, enamel matrix, and mineralized enamel, dentin, pulp, and cementum. In rodents, odontomas have been described as complex because they contain fully differentiated tooth elements but do not form the tooth-like structures grossly present in compound odontomas seen in other species (e.g., horse). Histologically, rodent odontomas are composed of well-differentiated odontogenic epithelium among an enamel organ with dental pulp mesenchyme and a variably mineralized enamel matrix.
Infectious diseases
Several important infectious and zoonotic diseases of rodents described are later with a focus on those that cause significant lesions in rodent species. Additional pathogens, including zoonotic pathogens that occur in rodent species are listed in the Supplemental Materials (Table e2). Several references exist pertaining to rodent reservoirs of emerging and reemerging zoonotic pathogens (Han et al., 2015, Meerburg et al., 2009).
Table e2.
Summary of Zoonotic Pathogens and Associated Diseases of Rodents*
| Etiology | Disease Name | Known Host | Disease in Host (if any) | Other Species infected | Disease in nonhost species | Disease in Humans | Transmission Route | References | |
|---|---|---|---|---|---|---|---|---|---|
| Bacteria | |||||||||
| Bartonella spp. | Bartonellosis | Many rodent species | None | Dogs, cats | None, endocarditis, myocarditis | Lymphadenopathy, neuroretinitis, endocarditis, myocarditis, acute febrile illness, anemia and chronic fatigue | Fleas, ticks, lice, flies, contact with infected blood | Gutiérrez (2015) | |
| Francisella tularensis | Tularemia | Beavers, muskrat, rabbits | NA | Mice, squirrels, hares, many mammal suseptible | Sudden death, necrotizing hepatitis and splenitis | Febrile illness, lymphadenitis, pneumonia, sepsis | Direct contact with infected animals, ticks, flies, contaminated water | Wobeser (2007), Wobeser (2009), Mörner (1992) | |
| Leptospira spp. | Leptospirosis | Rats, many rodent species | None, interstitial nephritis | Guinea pigs, hamsters | Sudden death, vasculitis, hemorrhage | Fever, icterus, pulmonar hemorrhage | Contact with urine-contaminated water | Adler (2010) | |
| Listeria monocytogenes | Listeriosis | Rats, mice, many rodent species | None | Cattle, sheep, rabbits, hares | Abortion, septicemia, encephalitis | Abortion, septicemia, encephalitis | Fecal-oral | Low (1997) | |
| Rickettsia akari | Rickettsialpox | House mice and rats | None | Other rodents, dogs | NA | Febrile illness, lymphadenopathy, papulovesicular rash | Biting mites | Meerburg (2009) | |
| Rickettsia typhi | Murine/Endemic typhus | Rats | None | House mice, cats, dogs, opossums, skunks | NA | Febrile illness, rash | Fleas, other ectoparasites | Meerburg (2009) | |
| Streptobacillus moniliformis and S. minus | Rat bite fever | Norway and black rats | Infected bite wounds, abscesses | NA | NA | Rash, arthritis, variably severe febrile illness, death | Rodent bites (also in urine and blood) | Meerburg (2009) | |
| Yersinia entercolitica | Yersiniosis (syn. Pseudotuberculosis) | Many rodent species | Necrotizing hepatitis, splenitis, entercolitis | Mice, voles, beavers, muskrats, agoutis, hares | Necrotizing hepatitis, splenitis, entercolitis | Entercolitis, fever | Fecal-oral via contaminated food and water | Mair (1973), Gasper (2009) | |
| Yersinia pestis | Plague | Voles, deer mice, prairie dogs, rats, squirrrels | Bubonic, pneumonic, septicemic | Cats | Bubonic, pneumonic, septicemic | Bubonic, pneumonic, septicemic | Fleas, contact with infected animals, inhalation | Gage (2005), Wobeser (2009) | |
| Yersinia pseudotuberculosis | Yersiniosis (syn. Pseudotuberculosis) | Many rodent species | Necrotizing hepatitis, splenitis, entercolitis | Mice, voles, beavers, muskrats, agoutis, hares | Necrotizing hepatitis, splenitis, entercolitis | Entercolitis, fever | Fecal-oral via contaminated food and water | Mair (1973), Gasper (2009) | |
| Viruses | |||||||||
| Orthopoxviridae | Pox (Mousepox, Cowpox) | Mice and other rodents | Ectromelia (mice): acute mortality, chronic (crusting dermatosis of face, legs and tail; conjuctivitis, organ and lymph node necrosis | Many rodent species susceptible | Cutaneous lesions | Cutaneous lesions | Direct contact | Meerburg (2009) | |
| Orthopoxviridae | Monkeypox | Monkeys, Gambian giant rat, squirrels | Cutaneous lesions | Prairie dogs | Necrotizing bronchopneumonia, conjunctivitis, and tongue ulcers | Fever, vascular rash | Direct contact | Meerburg (2009) | |
| Arenaviridae | Hemorrhagic fever | Many rodent species (coevolution with host), Mice, hamsters, guinea pigs and muskrats | None, may include conjunctivitis, ascites, splenomegaly, and hepatic lipidosis with multicentric vasculitis and perivascular lymphocytic infiltrates | None | None | Fever, malaise, headache, vomiting, diarrhea, mucosal hemorrhage | Contact with excretions or contaminated materials, inhalation | Meerburg (2009) | |
| Arenaviridae | Lassa fever | Multimammate rat | None | None | None | Fever, malaise, headache, vomiting, diarrhea, mucosal hemorrhage | Contact with excretions or contaminated materials, inhalation | Meerburg (2009) | |
| Arenaviridae | Lymphocytic choriomeningitis | House mice | None | None | None | Meningitis, encephalitis, fetal defects (intrauterine infection) | Contact with excretions or contaminated materials, inhalation | Meerburg (2009) | |
| Bunyaviridae | Hantavirus cardiopulmonary syndrome | Deer mice, cotton rats, rice rats, whitefooted mice | None | None | None | Pneumonia, pulmonary edema, death | Contact with excretions or contaminated materials, inhalation, bites (rare) | Meerburg (2009) | |
| Bunyaviridae | Hemorrhagic fever with renal syndrome | Norway and black rats, field mice, voles | None | None | None | Fever, multisystemic vascular leakage, renal failure | Contact with excretions or contaminated materials, inhalation, bites (rare) | Meerburg (2009) | |
| Flaviviridae | Omsk hemorrhagic fever | Muskrats (Siberia) | Fatal encephalitis (muskrat) | Watervoles, Norway rats, mice, some bird species | None | Fever, hemorrhage without encephalitis | Direct contact with infected muskrats, Ticks (Dermacentor & Ixodes) | Meerburg (2009) | |
Adler, B. and la Peña Moctezuma, de,A. 2010. Leptospira and leptospirosis. Vet. Microbiol. 140, 287–296.
Gage, K. L., Kosoy, M. Y., 2005. Natural history of Plague: perspectives from more than a century of research. Ann. Rev. Entomol. 50, 505–528.
Gasper, P. W., Watson, R. P. Plague and yersiniosis. In: Williams, E. S., Barker, I. K. (Eds.), Infectious Diseases of Wild Mammals, third ed. Ames, Iowa, pp. 313–330.
Gutiérrez, R., Krasnov, B., Morick, D., Gottlieb, Y., Khokhlova, I. S., Harrus, S., 2015. Bartonella infection in rodents and their flea ectoparasites: an overview. Vector Borne Zoonotic Dis. 15, 27–39.
Han, B. A., Schmidt, J. P., Bowden, S. E., Drake, J. M., 2015. Rodent reservoirs of future zoonotic diseases. Proc. Natl. Acad. Sci. USA. 112, 7039–7044.
Low, J. C., Donachie, W., 1997. A review of Listeria monocytogenes and listeriosis. Vet. J. 153, 9–29.
Mair, N.S., 1973. Yersiniosis in wildlife and its public health implications. J. Wildl. Dis. 9, 64–71.
Meerburg, B. G., Singleton, G. R., Kijlstra, A., 2009. Rodent-borne diseases and their risks for public health. Critical Rev. Microbiol. 35, 221–270.
Mörner, T., 1992. The ecology of tularaemia. Rev. Sci. Tech. 11, 1123–1130.
Wobeser, G., Ngeleka, M., Appleyard, G., Bryden, L., Mulvey, M. R., 2007. Tularemia in deer mice (Peromyscus maniculatus) during a population irruption in Saskatchewan, Canada. J. Wildl. Dis. 43, 23–31.
Wobeser, G., Campbell, G. D., Dallaire, A., McBurney, S., 2009. Tularemia, plague, yersiniosis, and Tyzzer’s disease in wild rodents and lagomorphs in Canada: a review. Can. Vet. J. 50, 1251–1256
This list includes the zoonotic pathogens carried by rodents that cause disease in wild animals and those deemed most relevent to wildlife and zoo animal specialists; therefore it is not an exhaustive list. Readers are directed to Meerburg (2009) and Han (2015) for more information.
DNA Viruses
Poxviral diseases in rodents are caused by members of Orthopoxviridae, which includes Mousepox and Cowpox (OIE listed reportable zoonotic diseases). Several species of rodents serve as the reservoir for Cowpox virus, which results in cutaneous lesions in humans. In laboratory mice, Mousepox, more commonly known as ectromelia, is an important disease. This infection can be acutely fatal with minimal lesions. In the chronic form, gross lesions include crusting lesions of face, legs, and tail, conjunctivitis, an enlarged, swollen and friable liver and spleen, and in cases of recovery, splenic fibrosis. Necrosis of the kidney, thymus, and lymph nodes may also be grossly evident. The small intestine, urinary bladder, and vagina can have mucosal erosions and hemorrhage. Histologically, skin and mucosal lesions include epithelial ballooning degeneration, hyperplasia, and erosions with characteristic intraepithelial, intracytoplasmic inclusion bodies. Within the parenchymal organs, splenic and hepatocellular necrosis is typically coagulative and multifocal to coalescing. Diagnosis is based on characteristic lesions with viral inclusions. Electron microscopy, PCR, and virus isolation are useful for confirmation of the viral strain.
In 2003, an outbreak of monkeypox (Orthopoxviridae), a zoonotic virus, occurred in prairie dogs sold as companion animals in the United States (Gaurner et al., 2004). Fatal infections in infected prairie dogs were characterized by necrotizing bronchopneumonia, conjunctivitis, and glossal ulcers. Viruses and viral antigens were detected in multiple tissues and cell types via immunohistochemical staining, PCR, and electron microscopy. Infected humans developed fever and vascular rashes. Forty seven confirmed and probable cases of monkeypox were reported from six states. The prairie dogs contracted the virus following exposure to infected African rodents. Other rodents that also developed disease and died included: rope squirrels, tree squirrels, Gambian giant rats, brush-tailed porcupine, dormice, and striped mice.
Fibromas and fibromatosis of gray squirrels are potentially fatal conditions caused by squirrel fibroma virus (Leporipoxviridae). Disease is mainly reported in the eastern North America (Terrell et al., 2002) but also occurs in red squirrels and has been reported in a fox squirrel (Wilcoxen et al., 2015). Transmission is likely through biting arthropods (fleas, ticks, and mosquitoes). Gross and histologic lesions are similar to Shope fibromas in rabbits (both are Leporipoxviruses). Multifocal to coalescing tan, firm nodules may cover all parts of the body and begin as alopecic nodules with progression to plaque-like to pedunculated masses (Fig. 20.9 A). Systemic disease manifests as renomegaly and multiple pulmonary nodules (Kirschstein et al., 1958). Histologically, the skin nodules exhibit marked epidermal hyperplasia with ballooning degeneration of keratinocytes, spongiosis, and intracytoplasmic eosinophilic viral inclusions (Fig. 20.9B,C). Pulmonary nodules correlate histologically to adenomatous hyperplasia with similar viral inclusions. Other microscopic lesions may include atypical mesencyhmal proliferation in the liver and seminal vesicles, and renal tubular epithelial hyperplasia with viral inclusions. Diagnosis is based on characteristic lesions and detection of virus by PCR or isolation.
Figure 20.9.
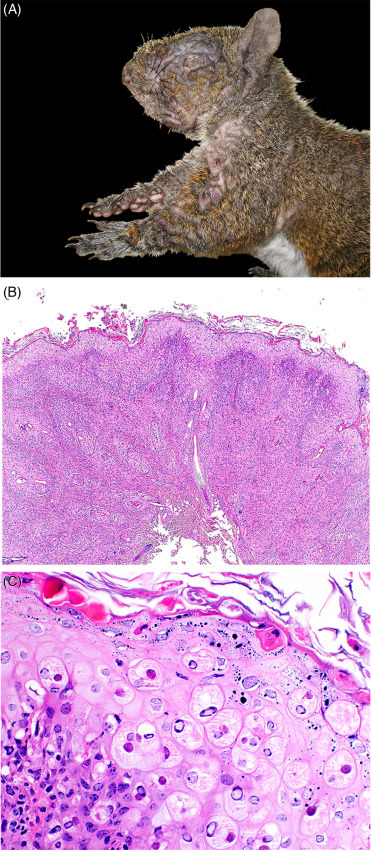
Fibromatosis in a free-ranging gray squirrel.
(A) Multifocal, variably sized dermal plaque-like fibromas (coalescing areas of dermal thickening and alopecia) are present throughout the skin (B) The epidermis is severely thickened and there is dermal inflammation. (C) Numerous epithelial cells contain intracytoplasmic eosinophilic inclusions and are undergoing ballooning keratinocyte degeneration. Lymphocytic inflammation is present in the underlying dermis.
(Part A: Photo Courtesy of J. Landolfi, University of Illinois Zoological Pathology Program; Part C: Courtesy of D. Campbell, Canadian Wildlife Health Cooperative Ontario-Nunavut)
Parapoxvirus is an important cause of disease in red squirrels in the United Kingdom and Ireland where it is a suspected factor in severe population declines (Bangari et al., 2009; Simpson et al., 2013, Tompkins et al., 2002). The causative agent is referred to as Red squirrel parapoxvirus. Grey squirrels are the likely maintenance hosts since a large percentage of healthy gray squirrels are positive for the virus and their range overlaps with that of red squirrels. Lesions in red squirrels include exudative dermatitis with crusting that is similar to other poxviral diseases. Viral inclusions can be detected histologically within the cytoplasm of infected cells. Electron microscopy and PCR are used to detect the virus.
Woodchuck hepatitis virus (WHV; Hepadnaviridae) is a disease in captive and free-ranging animals (Roth et al., 1985). Although not zoonotic, this disease is a valuable animal model for viral hepatitis and hepatocarcinogenicity associated with hepatitis B in humans. It affects both sexes and is typically found in animals greater than 4 years of age. Activated T cells (CD8 +) cause cellular and DNA damage leading to persistent hepatitis. Simultaneously, viral DNA integrates into the host genome at loci containing oncogenes, creating the potential for carcinogenic transformation. Hepatocellular carcinomas are reported in 100% of experimentally infected woodchucks. Grossly, most carcinomas are solitary and involve one hepatic lobe. Histologically, neoplastic masses have differing growth patterns: trabecular, adenoid, or solid with and without cystic spaces, hemorrhage, and necrosis. An interesting feature of these tumors is the presence of atypical, bizarre, and giant neoplastic cells. Adenomas may be present and are considered a precursor to carcinomas. Mitotic index and cellular morphology are used to classify tumors as adenomas versus carcinomas. The remaining hepatic parenchyma often contains acute and/or chronic hepatitis with neutrophilic to mononuclear inflammation, variable hepatocellular degeneration, necrosis, fibrosis, and biliary changes. Electron microscopic identification of the virus is definitive. Beechey ground squirrels have a similar virus with variable hepatic lesions (Cullen and Marion, 1996).
Hamster polyomavirus (HaPyV), a papovavirus, causes cutaneous trichoepitheliomas in Syrian hamsters (Foster et al., 2002). The virus is transmitted via urine and is highly contagious, with reported 50% morbidity in affected breeding colonies. Early lesions consist of wart-like growths and alopecic nodules of the face and perineum with progression to severe, multifocal to coalescing nodules throughout the body. Histologically, these characteristic skin tumors contain multiple islands of basilar to variably differentiated follicular epithelium and abrupt central keratinization with faded anuclear keratinocytes (ghost cells). Rudimentary hairs may be present and follicular rupture may be associated with inflammation.
Other polyomaviruses-associated neoplasms include other cutaneous tumors, lymphomas and salivary gland tumors. In virally induced lymphomas, affected tissues are effaced by diffuse sheets of neoplastic lymphocytes among scant stroma. Viral inclusions are not present. In a case of polyomavirus-induced, multicentric lymphoma in an 8-week old Syrian hamster, the virus was detected in tumor cells, renal tubular epithelial cells, and enterocytes via in situ PCR indicating some degree of tissue tropism (Simmons et al., 2001). Diagnosis is based on clinical history, gross and histologic lesions, and detection of the virus via PCR and/or electron microscopy.
RNA Viruses
Mouse hepatitis caused by MHV (Coronaviridae) is enzootic in some colonies of inbred laboratory mice (Barthold et al., 2016). Mouse hepatitis may also occur in nonlaboratory strains of mice in zoo settings. MHV causes polytropic and enterotropic disease syndromes, both of which occur following highly contagious viral infection by the oronasal route. Grossly, there is hepatomegaly, splenomegaly, lymphadenomegaly, and lymphadenopathy with jaundice and ascites. There may be gross evidence of intestinal (ileal and cecal) disease with thickened and necrotic mucosa. Within the liver, spleen, and lymph nodes, there are multiple coalescing and random white necrotic foci. Histologically, polytropic disease includes necrotizing hepatitis with syncytial cells within necrotic foci, and demyelination in CNS in immunodeficient mice. The enterotropic disease lesions are age-dependent and may include villous attenuation and syncytial cell formation with eosinophilic intracytoplasmic inclusion bodies in enterocytes, mesenteric lymph nodes, and endothelial cells; granulomatous serositis may also be present. Diagnosis is based on histologic features and immunohistochemical demonstration of viral antigens within lesions. Virus isolation, PCR, and serology are used to confirm infection and exposure, respectively.
Sendai virus infection (parainfluenza-1; Paramyxoviridae) is common in wild and pet rodents and is actively excluded from laboratory populations of mice, rats, and gerbils (Easterbrook et al., 2008). Disease manifestations occur quickly following the aerosol inhalation, direct contact, or in utero exposure. The virus targets respiratory epithelium and type II pneumocytes, which may impair ciliary activity and predispose affected individuals to secondary Mycoplasma pulmonis and other bacterial infections. Gross lesions include dark purple discoloration, atelectasis, and consolidation of the cranioventral lung lobes and diffuse pulmonary edema. Splenomegaly and lymphadenomegaly are common and if infection occurs in a gravid female, fetal death may occur. Histologically, lesions include necrotizing rhinitis, laryngotracheitis, bronchitis, and bronchointerstitial pneumonia. In immunocompromised animals (e.g., athymic and SCID mice), intracytoplasmic and intranuclear inclusion bodies may be present. Diagnosis is based on supportive clinical history, histologic findings, and PCR detection of the virus.
Sialodacryoadenitis is a disease specific to rats and is caused by sialodacryoadenitis virus (SDAV; Coronaviridae). The virus may be enzootic in some research colonies and in pet and free-ranging rats (Easterbrook et al., 2008). Virus is shed in nasal secretions and/or saliva. SDAV infects and replicates in the respiratory tract and spreads to salivary and lacrimal glands resulting in secondary lesions of the nasopharynx, respiratory tract, and eyes. Grossly, the parotid and submandibular salivary glands, Harderian glands, exorbital glands, and cervical lymph nodes are enlarged, pale, and edematous. The Harderian glands may exhibit brown pigmentation, which correlates to accumulations of porphyrin within acini. Histologically, there is acute necrotizing adenitis. In chronic cases, there is fibrosis and extensive squamous metaplasia of glandular tissue. There may be rhinitis, tracheitis, bronchitis, and bronchiolitis in severe and chronic cases. Histologic lesions are characteristic and supportive of the diagnosis.
Bacteria
Within this section are several zoonotic and notable nonzoonotic bacteria that infect rodents. Several others that are notable but not associated with disease in their rodent host are listed in the Supplemental Materials (Table e2).
Zoonotic Bacteria
Plague is an OIE-listed and WHO-reportable zoonotic disease that is important to consider when working with rodents. Fleas spread the causative agent, Yersinia pestis, between hosts. Cats and other rodent-eating predators can also contract infection through ingestion and rarely via inhalation. Historically, plague has been implicated in the deaths of millions of humans, most notably in 14th century Europe during the Black Death. There are two main host types: enzootic reservoirs (voles and deer mice) and epizootic amplifiers (prairie dogs, rats, and squirrels). With close to 100% mortality, plague outbreaks in prairie dog colonies are a devastating and significant threat not only this species but to endangered black-footed ferrets, who are susceptible to disease because prairie dogs are their primary food source (Cully et al., 2010). There are three disease forms: bubonic (lymphadenomegaly with abscessation); pneumonic (necrotizing pneumonia with fibrinous pleuritis); and septicemic (multifocal necrosis of liver, lung, spleen, kidney, eye, brain). Gross lesions include multifocal pulmonary hemorrhage (Fig. 20.10 ), hemorrhagic lymph nodes, erythema of axillary and inguinal skin, splenomegaly and localized hemorrhage and necrosis in the dermis and subutis (suspected site of flea bite). Histologic lesions of bubonic disease include necrosuppurative lymphadenitis; in the pneumonic form, necrotizing pneumonia; and in the septicemic form, multifocal abscessation. All lesions contain Gram and Giemsa positive coccobacilli with characteristic bipolar morphology resembling a safety pin. Bacterial culture must be completed at a certified, biosecure laboratory; identification using immunohistochemistry or immunofluorescence is a beneficial diagnostic aid.
Figure 20.10.
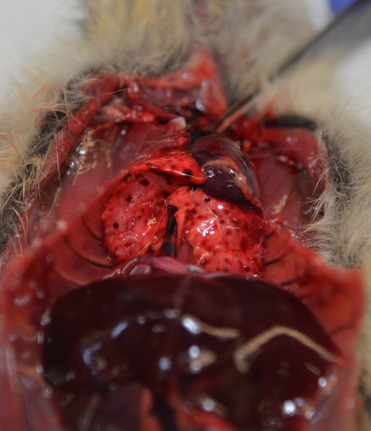
Plague in a prairie dog.
Multifocal pulmonary hemorrhage is consistent with Yersinia pestis infection.
(Photo Courtesy of K. Fox, Colorado Parks and Wildlife)
Yersiniosis (Pseudotuberculosis) is caused by Y. pseudotuberculosis and Y. entercolitica. Rodents may serve as carriers of this zoonotic bacterial agent, though some species, including mice, voles, beavers, and muskrats may succumb to acutely fatal disease or develop more characteristic lesions and symptoms. Gross and histologic lesions include multifocal necrotizing hepatitis and splenitis and/or fibrinonecrotizing and ulcerative enterocolitis (Fig. 20.11 ). Agoutis appear to be uniquely susceptible in zoo-settings (K. Terio, personal communication).
Figure 20.11.
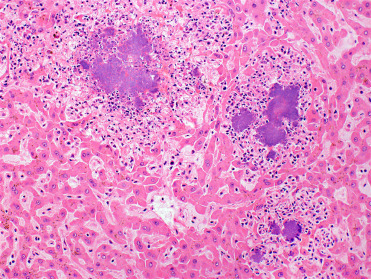
Yersiniosis in the liver of a free-ranging North American beaver.
Bacterial infection causes multifocal, random, necrosis throughout the hepatic parenchyma. Large, central colonies of the causative agent, Yersinia pseudotuberculosis are surrounded by mild neutrophilic inflammation and necrotic cell debris.
(Photo Courtesy of D. Campbell, Canadian Wildlife Health Cooperative Ontario-Nunavut)
Tularemia caused by Francisella tularensis is an important zoonotic and OIE listed reportable disease that is maintained by wild rodents and lagomorphs. Disease occurs in many rodents including mice, beavers, and muskrats, while voles appear to be subclinical carriers and serve as a reservoir (Nelson et al., 2014, Rossow et al., 2014). Lesions of affected species are similar to those described in rabbits (see Chapter 19). Miliary to multifocal and coalescing hepatic necrosis with variable inflammation (depending on chronicity) characterize the disease (Fig. 20.12 ). Unlike yersiniosis and similar to listeriosis, bacteria are not readily detected but can be better visualized with special stains.
Figure 20.12.
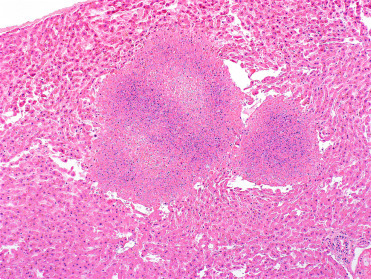
Tularemia in the liver of a free-ranging North American beaver.
Large, multifocal random areas of coagulative necrosis are present throughout the hepatic parenchyma. Note the relative lack of inflammation. Unlike Yersiniosis, bacterial colonies are not readily detected with routine hematoxylin and eosin staining.
(Photo Courtesy of D. Campbell, Canadian Wildlife Health Cooperative Ontario-Nunavut)
Listeriosis is a common zoonotic bacterium in rodent species. It can cause variable, yet similar lesions as for rabbits (see Chapter 19). The systemic form is most common. During an outbreak in bushy-tailed jirds, death occurred without clinical signs (Tappe et al., 1984).
Salmonellosis is reported in guinea pigs, rats, and mice, though any rodent species can be infected and develop disease. Salmonella enterica Enteritidis or Typhimurium are the most common zoonotic serovars. Guinea pigs become septicemic and often die acutely. Other rodents may develop gastrointestinal disease that includes diarrhea and abdominal pain prior to systemic spread. In hamsters, salmonellosis must be considered and ruled out in cases of “wet tail,” which is typically associated with infection by Lawsonia intracellularis. Gross lesions of salmonellosis include cyanosis of mucous membranes and polyserositis with splenomegaly. Hepatic, splenic, and lymph node necrosis are typical, as are multifocal necrosis and button ulcers in the colon with segmental intestinal infarction. Histologic lesions correlate to gross findings and include small foci of hepatocellular necrosis containing necrotic cell debris and variable numbers of macrophages, lymphocytes, and fewer neutrophils (paratyphoid nodules) and Kupffer cell hyperplasia; granulomatous hepatitis, splenitis, and lymphadenitis; and fibrinonecrotic ileotyphlocolitis. Intestinal infarction can be attributed to vasculitis and thrombosis of the mesenteric or mesocolic vessels. Culture of the causative agent and histologic lesions confirm the diagnosis.
Mycobacteriosis is a zoonotic disease that is rarely reported in rodents, though some species may serve as reservoirs. There are reports of mycobacteriosis (Mycobacterium microti) in British voles and wood mice (Cavanagh et al., 2002), hamsters, and Korean and Richardson’s ground squirrels in Spain.
Chlamydophila caviae and less frequently C. psittaci cause disease in guinea pigs, primarily juveniles. Conjunctivitis, bronchitis, and pneumonia may occur and result in debilitation and death. Histologically, there is heterophilic keratoconjunctivitis and uveitis. Pneumonia has a cranioventral pattern and is heterophilic. Some animals may be asymptomatic. Diagnosis is achieved through cytology of ocular discharge and/or PCR of affected tissues. Immunohistochemistry of tissue sections is also available.
Nonzoonotic Bacteria
Bordetella bronchiseptica causes bordetellosis (epizootic pneumonia), a common respiratory disease in guinea pigs though is also reported in free-ranging squirrel populations (Simpson et al., 2013). Similar disease can occur in other rodents, particularly in the context of immunosuppression or coinfection. Grossly, there is cranioventral pneumonia with multifocal to coalescing, discrete, reddish-gray consolidated regions affecting multiple lobes, and pleuritis. Mucopurulent exudate may be present in the nares, nasal passages, trachea, and tympanic bullae. There is often crusting conjunctivitis. Females may have metritis and pyosalpinx. Histologically, lung lesions consist of necrotizing and heterophilic bronchopneumonia with obliteration of the airways. Diagnosis is confirmed with bacterial culture or PCR. Other predisposing pathogens (e.g., adenovirus, Mycoplasma spp.) must be ruled out.
Ciliated associated respiratory (CAR) bacillus is an unclassified bacterium that colonizes the ciliated epithelium of the respiratory tract of rats, mice, rabbits, and a variety of other species. This agent acts synergistically with other respiratory pathogens including Mycoplasma pulmonis, Sendai virus, coronavirus, Streptococcus pneumoniae, Corynebacterium kutscheri, Bordetella bronchiseptica, Klebsiella pneumoniae, or Pasteurella spp. Lesions include suppurative to mucopurulent bronchopneumonia, perivascular and bronchiolar lymphoid hyperplasia, rhinitis and lymphoplasmacytic tracheitis in free-ranging, pet and laboratory rats (Baker, 1998, Rothenburger et al., 2015b). Coinfection of CAR bacillus and mycoplasmas has been reported in rats and spinifex hopping mice with pneumonia (Mackie et al., 2001).
Murine respiratory mycoplasmosis is caused by Mycoplasma pulmonis (Baker, 1998). Gross lesions include catarrhal exudate in the upper respiratory tract with cranioventral bronchopneumonia and mucopurulent exudate in airways with atelectasis. Histologic lesions include peribronchial lymphoid cuffing with BALT hyperplasia; neutrophilic bronchopneumonia; type II pneumocyte hyperplasia of alveolar epithelium; and emphysema; bronchiectasis is a characteristic finding. Histology alone cannot differentiate M. pulmonis and CAR bacillus respiratory infections (Rothenburger et al., 2015b). Other lesions associated with M. pulmonis include endometritis, salpingitis, and perioophoritis. Diagnosis is based on histologic features, culture or PCR detection, and ELISA.
Pasteurellosis caused by P. multocida affects young and immunocompromised rodents, most frequently following direct transmission from the dam. Strain virulence and species determine lesions, which in general, are similar to those reported in other species, including rabbits (see Chapter 19). Prairie dogs develop upper respiratory disease and pneumonia, while conjunctivitis is more common in rats and mice. Hamsters have variable susceptibility.
Cervical lymphadenitis, a disease classically described in guinea pigs, is caused by Streptococcus equi subsp. zooepidemicus. Infection is associated with coarse foods that cause traumatic lesions of the oral mucosa; bite wounds may serve as another route of entry. Grossly, the cervical and submandibular lymph nodes are markedly enlarged and contain thick purulent yellow-white to red-gray exudate. Lymph nodes in severely affected and chronic cases may rupture. Histologically, affected lymph nodes are effaced by heterophilic lymphadenitis with central necrosis and peripheral fibrosis. Thoracic lesions include fibrinous pleural adhesions, fibrinosuppurative bronchopneumonia, pleuritis, and pericarditis. Chains of Gram-positive cocci are present within lesions. Diagnosis is based on gross and histologic lesions and culture of causative agent.
Tyzzer’s Disease, caused by Clostridium piliforme, is a potentially fatal disease of many rodent species. It has been reported in captive gerbils, hamsters, and spinifex hopping mice (Stannard et al., 2017). Infection results in a triad of gross lesions including icterus and hepatomegaly with military gray foci, congestion, and edema of the intestine, and lymphadenomegaly with edema and hemorrhage. Sudden death without clinical disease or lesions is possible in peracute cases. Histologically, multifocal random to coalescing hepatic necrosis is surrounded by hemorrhage, heterophils, and macrophages (Fig. 20.13 A). At the necrotic margins, hepatocytes may accumulate characteristic criss-crossed bundles of faintly staining bacilli that are best visualized with silver stains (Fig. 20.13B).
Figure 20.13.
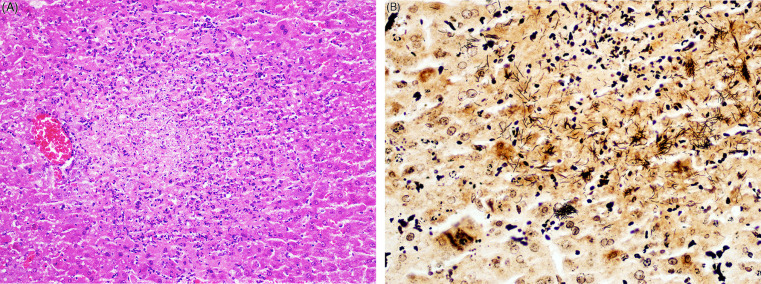
Tyzzer’s disease (Clostridium piliforme) in the liver of a gerbil.
(A) There is multifocal to coalescing necrosis of hepatic parenchyma with low numbers of faintly basophilic bacilli. (B) Multiple individual and clusters of filamentous Clostridium piliforme stain brown-black. Steiner’s silver stain.
(Photo Courtesy of J. Landolfi, University of Illinois Zoological Pathology Program)
Proliferative ileitis (wet tail) due to Lawsonia intracellularis is most commonly reported in hamsters. Similar to this infection in other species, there is segmental thickening of ileum with serosal nodules. Histologically, there is marked crypt hyperplasia and herniation and villous elongation, hyperplasia, and fusion with variable necrosis and hemorrhage. Silver stains and PCR are useful to confirm infection.
Fungi
A diverse array of Pneumocystis spp. are carried without clinical signs by a variety of wild rodents; these organisms are thought to be highly host-specific. Pneumocystis spp. are found within alveoli and alveolar macrophages and appear as small, eosinophilic round organisms with a small central body. In severe cases, organisms within alveolar spaces and alveolar macrophages can obliterate alveoli; in some cases they can be associated with interstitial pneumonia (Fig. 20.14 A,B). This fungus can cause significant opportunistic infections in immunocomromised individuals.
Figure 20.14.
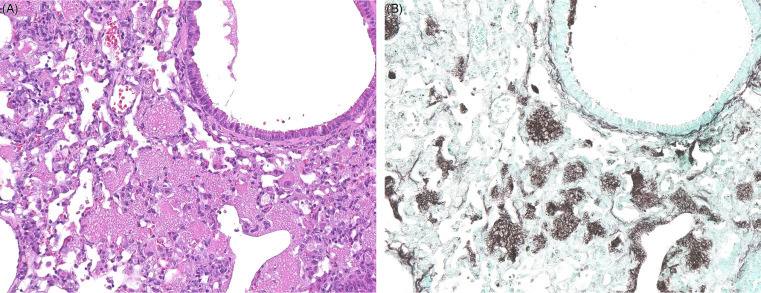
Pneumocystisspp. in the lungs of an immunocompromised mouse.
(A) Alveoli are filled with lightly eosinophilic material that appears foamy to honeycomb and is associated with chronic pneumonitis. (B) Silver stains are useful to highlight intralesional and intracellular pneumocystis cysts. GMS
Cryptococcosis (Cryptococcus neoformans) can be a significant pathogen in some rodent species. For example, approximately 20% of slender-tailed cloud rats at one zoological institution died with or as a result of cryptococcal pneumonia over a 15-year period (Berliner et al., 2007). These large, arboreal rodents were likely infected by contaminated substrates and may be a highly susceptible species, similar to some species of nonhuman primates and felids. Lesions included none or mild to severe, chronic granulomatous pneumonia with or without necrosis and regional lymphadenitis. Disseminated disease can produce subcutaneous nodules (cryptococcoma) and meningoencephalitis. Microscopically, characteristic fungal yeasts with narrow-based budding are present in cytologic preparations and histologic sections. GMS staining can enhance detection of fungal yeasts, which are 5–20 μm diameter with a distinct, thick (5–10 μm), nonstaining mucopolysaccharide capsule, the latter of which is best visualized using the PAS reaction and mucin stains. Culture can confirm infection (Fig. 20.15 )
Figure 20.15.
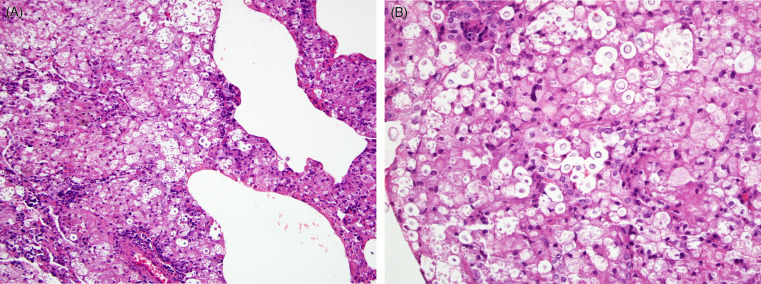
Cryptococcosis in the lungs of a slender-tailed cloud rat.
(A) The interstitium is moderately to markedly expanded and distorted by myriad fungal yeasts and associated histiocytic to granulomatous inflammation. Aggregates of lymphoplasmacytic inflammation can be seen but are typically infrequent. (B) Yeast vary in size, have narrow-based budding, and are surrounded by a thick mucopolysaccharide capsule that does not stain with hematoxylin and eosin and appears as a clear halo.
(Photos Courtesy of D. McAloose, Wildlife Conservation Society)
Adiaspiromycosis is a systemic fungal disease reported in several captive and free-ranging rodent species. It is caused by the saphrophytic fungi Chrysosporium parum and C. crescens. Once inhaled, these fungi cause pulmonary granulomas with fungal spherules and conidia. Additional diagnostic tests may be useful, including immunodiffusion and complement fixation. These fungi are zoonotic.
Other systemic mycoses of rodents include infections caused by the endemic (and zoonotic) fungi Blastomcyes dermatitides and Histoplasma capsulatum. Reported cases include juvenile mice and chinchillas. Immunity to fungal pathogens appears limited in mice.
Dermatophytosis is a cutaneous fungal disease that can be found in any rodent species and is similar to that of other mammalian species. Causative agents include Trichophyton mentagrophytes, Microsporum canis, M. gypseum, and Epidermophyton. Of note, these fungi are zoonotic and can cause skin lesions in other animals. Additionally, Sporothrix schneckii associated cutaneous disease is reported in several rodent species and can be zoonotic. Dermatitis lesions vary from suppurative and necrotizing to granulomatous and fibrosing depending on chronicity. PAS-positive yeast are present within macrophages. Characteristic organisms on cytologic preparations of skin scrapes prepared with potassium hydroxide support the diagnosis.
Metazoa
Capillaria hepatica (synonymn Calodium hepaticum) affects a broad host range worldwide, including rodents and humans. It is a ubiquitous parasite of globally invasive Norway and black rat populations (Rothenburger et al., 2014). Gross lesions consist of multifocal to coalescing, tortuous, white tracks in the liver parenchyma (Fig. 20.16 A). Histologically, varying combinations of lymphoplasmocytic and granulomatous inflammatory infiltrates, fibrosis, and bioperculate eggs and occasionally adult parasites replace hepatic parenchyma (Fig. 20.16B). Cross sections of viable or mineralized capillarid nematodes may be present. Eggs are only released from the liver following carcass decomposition or ingestion by a predator/cannibalistic conspecific. Therefore, diagnosis is based on the presence of the nematode and/or eggs in the liver and not fecal parasitology.
Figure 20.16.
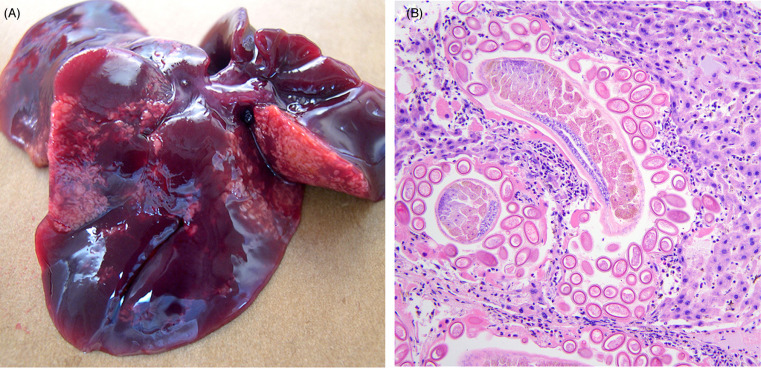
Capillaria hepatica in the liver of a free-ranging Norway rat.
(A) Multiple liver lobes contain locally extensive tortuous, white nematode tracks. (B) Adult nematodes and eggs replace the liver parenchyma. The eggs are characteristically bioperculate. Inflammation is lymphoplasmacytic to granulomatous and may be associated with fibrosis in chronic infections.
Many species of free-ranging rodents are susceptible to fatal visceral larval migrans associated with the raccoon nematode Baylisascaris procyonis (Fig. 20.17 ). Significant clinical disease is often related to parasite migration in the brain, where they may be accompanied by low numbers of eosinophils, gliosis, spheroids, and rarefaction or necrosis of the neuropil.
Figure 20.17.
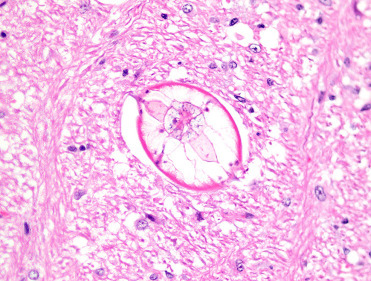
Visceral larval migrans due to Baylisascaris procyonis in the brain of a free-ranging groundhog.
The larval nematode parasites contain prominent lateral alae and lateral cords, a thick cuticle, and coelomyarian musculature.
(Photo Courtesy of J. Landolfi, Of Mice and Microflora Considerations for Genetically Engineered Mice. P. M. Treuting, C. B. Clifford, R. S. Sellers, C. F. Brayton. First Published December 14, 2011)
Ectoparasites
Rodents, particularly squirrels are prone to Notoedric mange (Anholt et al., 2014, Cornish et al., 2001; Stephenson et al., 2013). Mites burrow into the stratum corneum inducing marked hypekeratosis, which results in patchy alopecia, and crusting and thickening of the skin (lichenification), which can appear yellow to gray. Gross lesions are predominantly found on the ears, nose, tail, external genitalia, inguinal and perianal regions, and feet though they can cover the entire body in severe cases. Peripheral lymphadenopathy may also be seen. Skin scrapings and fecal floats are beneficial to best examine mite features and presence of eggs, respectively. Histologically, there is marked epidermial hyperplasia with parakeratotic hyperkeratosis forming caps over tunnels containing mites with superficial, perivascular eosinophilic dermatitis (Fig. 20.18 A,B). Secondary bacterial infections are common. Additional notable parasites of rodents are listed in the Supplemental Materials (Table e3).
Figure 20.18.
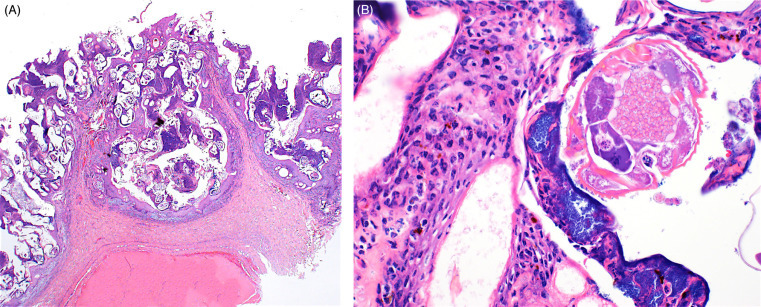
Notoedric mange in a free-ranging grey squirrel.
(A) Severe hyperkeratosis and epidermal hyperplasia contain multiple large mite tunnels. (B) Cross section of a Notoedres centrifera mite adjacent to colonies of coccoid bacteria, parakeratotic hyperkeratosis, and neutrophilic cellular debris.
(Photos Courtesy of D. Campbell, Canadian Wildlife Health Cooperative Ontario-Nunavut)
Table e3.
Summary of Parasites and Associated Diseases of Rodents
| Etiology | Location in Host | Associated Disease (if any) | Species Affected | References | |
|---|---|---|---|---|---|
| Metazoan | |||||
| Baylisascaris procylonis | Muliple systems | Granulomatous and eosinophilic inflammation from larval migrans | All | Bauer (2013) | |
| Capillaria hepatica (syn. Calodium hepaticum) | Liver | Multifocal granulomatous hepatitis with fibrosis | All | Rothenburger (2014a), Simpson (2013) | |
| Cysticercus fasciolaris | Liver | Parasitic cysts | Mice and rats | Morrisey (1996) | |
| Cysticercus mustelae | Liver | Parasitic cysts | Squirrels | Langham (1990) | |
| Cysticercus serialis (syn. Muticeps serialis) | Subcutaneous tissues, abdomen, liver, spleen, thorax | Parasitic cysts | Chinchilla | Morrisey (1996) | |
| Echinococcus cruzi (syn. oligarthrus) | Subcutaneous tissues, abdomen, liver, spleen, thorax | Parasitic cysts | Agouti | Rausch (1984), Zimmerman (2009) | |
| Echinococcus granulosus | Liver | Parasitic cysts | Ground squirrels (Spermophilus dauricus/alashanicus) | Yang (2009) | |
| Echinococcus multilocularis | Liver, other organs | Parasitic cysts | All | Janovsky (2002), Campbell-Palmer (2015), Miller (2016) | |
| Echinococcus vogeli | Liver, other organs | Parasitic cysts | Agoutis, spiny rats | Gardner (1988) | |
| Eucoleus sp. | Esophagus, stomach | Hyperplasia, hyperkeratosis, inflammation | Rats | Rothenburger (2014b) | |
| Gongylonema neoplasticum | Stomach | Hyperplasia, hyperkeratosis, inflammation | Rats | Hitchcock (1952) | |
| Hymenolepis sp. | Intestines | Diarrhea, enteritis | Chincilla, mice, rats, hamsters | Morrisey (1996) | |
| Obeliscoides cuniculi | Stomach | None | Woodchuck | Measures (1983) | |
| Synhimantus sp. | Stomach | Squamous papillomas | Muskrats | Cosgrove (1967) | |
| Syphacia sp. | Cecum | None | Mice, rats, hamsters | Morrisey (1996) | |
| Taenia crassiceps cysticercosis | Subcutaneous tissues, abdomen, liver, spleen, thorax | Parasitic cysts | Woodchuck, squirrel | Klein (2011) | |
| Trichosomoides crassicauda | Urinary bladder, renal pelvis | Mild inflammation, predisposes to calculi formation | Rats | Morrisey (1996) | |
| Protozoan | |||||
| Babesia sp. | Blood | None | Agouti, porcupine, spiny rat | de Thoisy (2000) | |
| Besnoitia sp. | Bone, inner ear, scleral conjunctiva, subcutaneous tissues, mesentery, large intestines, pancreas | Range from one to acute, fatal infections | Kangaroo rat, hamesters, mice, rats | Ernst (1968) | |
| Cryptosporidium sp. | Intestine | Diarrhea, enteritis | Chinchilla, guinea pig, hamster, rat, mice | Morrisey (1996) | |
| Eimeria sp. | Intestine | Diarrhea, enteritis | Guinea pig, rat, mice | Morrisey (1996) | |
| Encephalitozoon cuniculi | Brain, kidney, liver, heart, lung, spleen | Multisystemic inflammation & necrosis | All | Hinney (2016) | |
| Frenkelia | Brain | None, lymphoid hyperplasia, lymphocytic meningoencephalitis | All | Geisel (1979), Kennedy (1986) | |
| Giardia sp. | Intestine | Diarrhea, enteritis | Hamster, mice, rat | Morrisey (1996) | |
| Hepatozoon sp. | Mutliple organs, blood | None | Squirrel, porcupine | Simpson (2013), de Thoisy (2000) | |
| Klossiella sp. | Kidney | Interstitial nephritis and fibrosis | Guinea pigs, mice | Pearce (1961), Morrisey (1996) | |
| Leishmania lainsoni | Skin | None | Agouti | Silveira (1991) | |
| Pneumocystis carinii | Lungs | Interstitial pneumonia, perivascular lymphoid cuffs | All | Henderson (2012) | |
| Syphacia spp. | Cecum and colon | None to rectal prolapse, intussusception, enteritis | Mice and rats | Baker (1998) | |
| Spironucleus muris | Intestine | None to fatal enteritis | Mice and rats | Baker (1998) | |
| Toxoplasma gondii | Brain, liver, spleen, lung, skeletal muscle, heart | None to multisystemic inflammation & necrosis | all | Bangari (2007), Forzan (2004), Simpson (2013) | |
| Trypanosomatidae | Blood | None to thrombocytosis, ascites, immunodepression, lymphoid & erythroid hyperplasia | agouti, porcupine, spiny rat, Norway rats, mice | Albright (1991), de Thoisy (2000) | |
| Ectoparasite | |||||
| Chirodiscoides caviae | Skin | None | Guinea pigs | Morrisey (1996) | |
| Demodex | Skin | Alopecia, dermatitis, pruritis | Gerbil, hamster | Morrisey (1996) | |
| Gliricola porcelli | Skin | Alopecia, crusting dermatitis, pruritis | Guinea pigs | Morrisey (1996) | |
| Gyropus ovalis | Skin | Alopecia, crusting dermatitis, pruritis | Guinea pigs | Morrisey (1996) | |
| Myobia musculi | Skin | Alopecia, pruritis, self-mutilation | Mice | Baker (1998) | |
| Myocoptes musculinis | Skin | Alopecia, pruritis, self-mutilation | Mice | Morrisey (1996) | |
| Notoedres sp. | Skin | Alopecia, crusting dermatitis | Hamster, grey squirrel, rats | Anholt (2014), Cornish (2001), Morrisey (1996), Stephenson (2013) | |
| Ornithonyssus bacoti | Skin | Anemia | Rats | Morrisey (1996) | |
| Polyplax serrata | Skin | Anemia, pruritis, dermatitis | Mice | Morrisey (1996) | |
| Polyplax spinulosa | Skin | Anemia, pruritis | Rats | Morrisey (1996) | |
| Psoregates simplex | Skin | Follicular nodules | Mice | Morrisey (1996) | |
| Radfordia affinis | Skin | Alopecia, pruritis, self-mutilation | Mice | Baker (1998) | |
| Radfordia ensifera | Skin | Anemia, pruritis, dermatitis | Rats | Morrisey (1996) | |
| Trixacarus caviae | Skin | Alopecia, crusting dermatitis | Guinea pigs | Morrisey (1996) | |
Anholt, H., Himsworth, C., Rothenburger, J., Patrick, D.M. 2014. Ear mange mites (Notoedres muris) in black and Norway rats (Rattus rattus and Rattus norvegicus) from inner-city Vancouver, Canada. J. Wild. Dis. 50, 104–108.
Anholt, H., Himsworth, C., Rothenburger, J., Patrick, D.M. 2014. Ear mange mites (Notoedres muris) in black and Norway rats (Rattus rattus and Rattus norvegicus) from inner-city Vancouver, Canada. J. Wild. Dis. 50, 104–108.
Baker, D.G. 1998. Nathural pathogens of laboratory mice, rats, and rabbits and their effects on research. Clin. Microbiol Rev. 11, 231–266.
Bangari, D. S., Mouser, P., Miller, M. A., Stevenson, G. W., Vemulapalli, R., Thacker, H. L., 2007. Toxoplasmosis in a woodchuck (Marmota monax) and two American red squirrels (Tamiasciurus hudsonicus). J. Vet. Diagn. Invest. 19, 705–709.
Bauer, C., 2013. Baylisascariosis—Infections of animals and humans with ‘unusual’roundworms. Vet. Parasitol, 193, 404–412.
Campbell-Palmer, R., Del Pozo, J., Gottstein, B., Girling, S., Cracknell, J., Schwab, G., Rosell, F., Pizzi, R., 2015. Echinococcus multilocularis detection in live Eurasian beavers (Castor fiber) using a combination of laparoscopy and abdominal ultrasound under field conditions. PloS One 10, e0130842.
Cornish, T.E., Linders, M.J., Little, S.E., Vander Haegen, W.M. 2001. Notoedric mange in western gray squirrels from Washington. J. Wild. Dis. 37, 630–633.
Cosgrove, G. E., Lushbaugh, W. B., Humason, G., Anderson, M. G., 1968. Synhimantus (Nematoda) associated with gastric squamous tumors in muskrats. Bull. Wild. Dis. Assoc. 4, 54–57.
Ernst, J. V., Chobotar, B., Oaks, E. C., Hammond, D. M., 1968. Besnoitia jellisoni (Sporozoa: Toxoplasmea) in rodents from Utah and California. J. Parasitol. 54, 545–549.
de Thoisy, B., Michel, J. C., Vogel, I., Vié, J. C., 2000. A survey of hemoparasite infections in free-ranging mammals and reptiles in French Guiana. J. Parasitol. 86, 1035–1040.
Forzan, M. J., Frasca, S., 2004. Systemic toxoplasmosis in a five month old beaver, (Castor Canadensis). J. Zoo Wild. Med. 35, 113–115.
Gardner, S. L., Rausch, R. L., Camacho, O. C. J., 1988. Echinococcus vogeli Rausch and Bernstein, 1972, from the paca, Cuniculus paca l.(Rodentia: Dasyproctidae), in the Departamento de Santa Cruz, Bolivia. J. Parasitol. 74, 399–402.
Geisel, O., Kaiser, E., Vogel, O., Krampitz, H. E., Rommel, M., 1979. Pathomorphologic findings in short-tailed voles (Microtus agrestis) experimentally-infected with Frenkelia microti. J. Wildl. Dis. 15, 267–270.
Henderson, K. S., Dole, V., Parker, N. J., Momtsios, P., Banu, L., Brouillette, R., Simon, M. A., Albers, T. M., Pritchett-Corning, K. R., Clifford, C. B., Shek, W. R., 2012. Pneumocystis carinii causes a distinctive interstitial pneumonia in immunocompetent laboratory rats that had been attributed to “rat respiratory virus”. Vet. Path. 49, 440–452.
Hinney, B., Sak, B., Joachim, A., Kváč, M., 2016. More than a rabbit’s tale–Encephalitozoon spp. in wild mammals and birds. Int. J. Parasitol. Parasites Wildl., 5, 76–87.
Hitchcock, C. R., Bell, E. T., 1952. Studies on the nematode parasite, Gongylonema neoplasticum (Spiroptera neoplasticum) and avitaminosis A in the forestomach of rats: comparison with Fibiger’s results. J. Natl. Cancer Inst. 12, 1345–1388.
Janovsky, M., Bacciarini, L., Sager, H., Grone, A., Gottstein, B. 2002. Echinococcus multilocularis in a European beaver from Switzerland. J. Wild. Dis. 8, 618–620.
Kennedy, M. J., Frelier, P. F., 1986. Frenkelia sp. from the brain of a porcupine (Erethizon dorsatum) from Alberta, Canada. J. Wildl. Dis. 22, 112–114.
Klein, P. N., Driscoll, C. P., 2011. The role of wildlife rehabilitation as sentinels for One Health issues at the wildlife and public health interface: reports of Taenia crassiceps Cysticercosis in woodchucks (Marmota monax) and squirrels (Sciurus carolinensis) in Maryland and Virginia. WCV Call of the Wild Conference.
Langham, R. F., Rausch, R. L., Williams, J. F., 1990. Cysticerci of Taenia mustelae in the fox squirrel. J. Wild. Dis. 26, 295–296.
Measures, L. N., Anderson, R. C., 1983. Characteristics of natural infections of the stomach worm, Obeliscoides cuniculi (Graybill), in lagomorphs and woodchucks in Canada. J. Wildl. Dis. 19, 219–224.
Miller, A. L., Olsson, G. E., Walburg, M. R., Sollanberg, S., Skarin, M., Ley, C., Wahlstrom, H., Hoguln, J., 2016. First identification of Echinococcus multilocularis in rodent intermediate hosts in Sweden. Int. J. Parasitol. 5, 56–63.
Morrisey, J.K. 1996. Parasites of ferrets, rabbits, and rodents. J. Exotic Pet. Med. 5, 106–114.
Pearce, L. 1961. Klossiella infection of the guinea pig. J. Exp. Med. 23, 431–441.
Rausch, R. L., D’Alessandro, A., Ohbayashi, M., 1984. The taxonomic status of Echinococcus cruzi Brumpt and Joyeux, 1924 (Cestoda: Taeniidae) from an agouti (Rodentia: Dasyproctidae) in Brazil. J. Parasitol. 70, 295–302.
Rothenburger, J. L., Himsworth, C. G., Chang, V., Lejune, M., Leighton, F. A., 2014a. Capillaria hepatica in wild Norway Rats (Rattus norvegicus) from Vancouver, Canada. J. Wildl. Dis. 50, 628–633.
Rothenburger, J. L., Himsworth, C. G., Lejune, M., Treuting, P. M., Leighton, F. A., 2014b. Lesions associated with Eucoleus sp. in the non-glandular stomach of wild urban rats (Rattus norvegicus). Int. J. Parasitol. 3, 95–101.
Silveira, F. T., Lainson, R., Shaw, J. J., Braga, R. R., Ishikawa, E. E. A., Souza, A. A. A., 1991. Cutaneous leishmaniasis in the Amazon: isolation of Leishmania (V.) lainsoni rodent Agouti paca (Rodentia: Dasyproctidae) in the state of Para, Brazil. Rev. Inst. Med. Trop. S. Paolo. 33, 18–22.
Simpson, V. R., Hargreaves, J., Butler, H. M., Davison, N. J., Everest, D. J., 2013. Causes of mortality and pathological lesions observed post-mortem in red squirrels (Sciurus vulgaris) in Great Britain. BMC Vet. Res. 9, 229.
Stephenson, N., Swift, P., Villepique, J. T., Clifford, D. L., Nyaoke, A., De la Mora, A., Moore, J., Foley, J., 2013. Pathologic findings in Western gray squirrels (Sciurus griseus) from a notoedric mange epidemic in San Bernardino Moutnains, California. J. Parasitol. 2, 266–270.
Yang, Y. R., Liu, T., Bai, X., Boufana, B., Craig, P.S., Nakao, M., Ito, A., Zhang, J. Z., Giraudoux, P., McManus, D. P., 2009. Natural infection of the ground squirrel (Spermophilus spp.) with Echinococcus granulosus in China. PLoS Negl. Trop. Dis. 3, e518.
Zimmerman, D. M., Douglass, M., Reavill, D. R., Greiner, E. C., 2009. Echinococcus oligarthrus cystic hydatidosis in Brazilian agouti (Dasyprocta leporine). J. Zoo Wild. Med. 40, 551–558.
E-Slides
-
20.e1
Chronic progressive nephropathy (CPN), naked mole-rat, kidney. In the kidney, there is marked ectasia of tubules and Bowman’s spaces forming numerous microcysts seen at low magnification. Some ectatic tubules contain luminal proteinaceous material and there is multifocal tubular degeneration, necrosis, and regeneration. Interstitium has areas of mixed inflammation, fibrosis and edema. Glomeruli have segmental to global membranous change and multifocal sclerosis. (see Fig. 20.4). Hepatic hemosiderosis is seen in sections of the liver. eSlide: VM05054
-
20.e2
Chronic progressive nephropathy (CPN), naked mole-rat, kidney. In the kidney, there is marked ectasia of tubules and Bowman’s spaces forming numerous microcysts seen at low magnification. Some ectatic tubules contain luminal proteinaceous material and there is multifocal tubular degeneration, necrosis, and regeneration. Interstitium has areas of mixed inflammation, fibrosis and edema. Glomeruli have segmental to global membranous change and multifocal sclerosis. (see Fig. 20.4). Hepatic hemosiderosis is seen in sections of the liver. eSlide: VM05053
-
20.e3
Chronic progressive nephropathy (CPN), naked mole-rat, kidney. There is mild to moderate ectasia of tubules and Bowman’s spaces forming numerous microcysts seen at low magnification. Some ectatic tubules contain luminal proteinaceous material and there is multifocal tubular degeneration, necrosis, and regeneration. Interstitium has areas of mixed inflammation, fibrosis and edema. Glomeruli have segmental to global membranous change and multifocal sclerosis. (see Fig. 20.4). eSlide: VM05057
-
20.e4
Hepatic hemosiderosis, naked mole-rat, liver. Prussian blue staining highlights bright blue granular intracytoplasmic (iron) pigments throughout the liver. The kidney has changes consistent with chronic progressive nephropathy. eSlide: VM05058
-
20.e5
Yersiniosis, beaver, liver and diaphragm. Yersiniosis in the liver and diaphragm of a free-ranging North American beaver. Bacterial infection causes multifocal random,necrosis throughout the hepatic parenchyma. Large, central colonies of the causative agent, Yersinia pseudotuberculosis are surrounded by mild neutrophilic inflammation. (see Fig. 20.11). eSlide: VM04954
-
20.e6
Tularemia, beaver, liver and spleen. Tularemia in the liver and spleen of a free-ranging North American beaver. Large, multifocal random areas of coagulative necrosis are present throughout the hepatic and splenic parenchyma. Note the relative lack of inflammation. Unlike Yersiniosis, bacterial colonies are not readily detected with routine hematoxylin and eosin staining. (see Fig. 20.12). eSlide: VM04952
-
20.e7
Pneumocystis murina pneumonia, mouse, lung. Multifocally, alveoli are partially to completely filled with large macrophages containing small round yeasts.(see Fig. 20.14). eSlide: VM05178
-
20.e8
Pneumocystis murina pneumonia, mouse, lung, GMS. Gomori methenamine silver (GMS) staining highlights the intrahistiocytic yeasts within the alveoli. (see Fig. 20.14). eSlide: VM05275
-
20.e9
Pulmonary cryptococcosis, slender-tailed cloud rat, lung. The interstitium and air spaces are moderately to markedly expanded and distorted by myriad fungal yeasts that are associated with histiocytic to granulomatous inflammation. Aggregates of lymphoplasmacytic inflammation are present but infrequent. (see Fig. 20.15). eSlide: VM05089
-
20.e10
Pulmonary cryptococcosis, slender-tailed cloud rat, lung. GMS. Intralesional yeasts are hhighlighed gray/blue with silver staining. GMS (see Fig. 20.15). eSlide: VM05096
-
20.e11
Pulmonary cryptococcosis, slender-tailed cloud rat, lung. Mucicarmine. Mucicarmine staining highlights the thick mucopolysaccharide capsule of the crytpococcal yeast and stains it bright pink. Mucicarmine (see Fig. 20.15). eSlide: VM05097
-
20.e12
Capillaria hepatica (Calodium hepaticum), Norway rat, liver. Capillaria hepatica in the liver of a free-ranging Norway rat. Adult nematodes and eggs invade and efface the liver parenchyma. There are examples of viable and degenerative adults. The eggs are characteristically bioperculate with a thick shell. Inflammation is lymphoplasmacytic to granulomatous and is associated with fibrosis indicating chronic infection. In some areas, there is multifocal mineralization adjacent to eggs. (see Fig. 20.16). eSlide: VM04956
-
20.e13
Baylisacaris visceral larval migrans, groundhog, brain. Multifocally, multiple sections of larval nematodes with prominent lateral alae and lateral cords, a thick cuticle, and coelomyarian musculature, features consistent with larval ascarids, are present in the brain. Inflammation, including eosinophils, gliosis, and spheroids and rarefaction of the neuropil due to parasite migration, are multifocal and occasionally associated with intralesional nematodes. (see Fig. 20.17). eSlide: VM04961
References
- Anholt H., Himsworth C., Rothenburger J., Patrick D.M. Ear mange mites (Notoedres muris) in black and Norway rats (Rattus rattus and Rattus norvegicus) from inner-city Vancouver. Can. J. Wildl. Dis. 2014;50:104–108. doi: 10.7589/2013-02-046. [DOI] [PubMed] [Google Scholar]
- Baker D.G. Nathural pathogens of laboratory mice, rats, and rabbits and their effects on research. Clin. Microbiol. Rev. 1998;11:231–266. doi: 10.1128/cmr.11.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangari D.S., Stevenson G.W., Thacker H.L., Sharma A., Mittal S.K. Cutaneous and systemic poxviral disease in red (Tamiasciurus hudsonicus) and gray (Sciurus carolinensis) squirrels. Vet Pathol. 2009;46:667–672. doi: 10.1354/vp.08-VP-0305-B-BC. [DOI] [PubMed] [Google Scholar]
- Barthold S.W., Griffey S.M., Percy D.H. fourth ed. John Wiley & Sons; Ames: 2016. Pathology of Laboratory Rodents and Rabbits. [Google Scholar]
- Batista J.S., Olinda R.G., Silva T., Rodrigues C., Oliveira A.F., Queiroz S., Morais S., Oliveira M.F. Diseases of agouti (Dasyprocta agouti) rained in captivity diagnosed by pathological examination. Pesq. Vet. Bras. 2010;30:497–502. [Google Scholar]
- Berliner A.L., Calle P.P., James S.B., McAloose D., Moore R.P., Raphael B.L. Cryptococcus neoformans pneumonia in slender tailed cloud rats (Phloeomys pallidus): a review of seven cases. Proceedings of the Annual Meeting of American Association of Zoo Veterinarians; Scottsdale, AZ; 2007. pp. 205–206. [Google Scholar]
- Boy S.C., Steenkamp G. Odontoma-like tumors of squirrel elodont incisors - elondontomas. J. Comp. Pathol. 2006;135:56–61. doi: 10.1016/j.jcpa.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Carleton, M.D., Musser, G.G., 2005. Order Rodentia. In: Wilson, D.E., Reeder, D.M. (Eds.), Mammal Species of the World, A., Taxonomic, Geographic Reference, Johns Hopkins University, Press, Baltimore, pp. 745–752.
- Cavanagh R., Begon M., Bennett M., Ergon T., Graham I.M., de Haas P.E., Hart C.A., Koedam M., Kremer K., Lambin X., Roholl P. Mycobacterium microti infection (vole tuberculosis) in wild rodent populations. J. Clin. Microbiol. 2002;40:3281–3285. doi: 10.1128/JCM.40.9.3281-3285.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanut F., Kimbrough C., Hailey R., Berridge B., Hughes-Earle A., Davies R., Roland K., Stokes A., Casartelli A., York M., Jordan H. Spontaneous cardiomyopathy in young Sprague-Dawley rats: evaluation of biological and environmental variability. Toxicol. Path. 2013;41:1126–1136. doi: 10.1177/0192623313478692. [DOI] [PubMed] [Google Scholar]
- Cornish T.E., Linders M.J., Little S.E., Vander Haegen W.M. Notoedric mange in western gray squirrels from Washington. J. Wildl. Dis. 2001;37:630–633. doi: 10.7589/0090-3558-37.3.630. [DOI] [PubMed] [Google Scholar]
- Cueto G.R., Allekotte R., Kravetz F.O. Scurvy in capybaras bred in captivity Argentine. J. Wildl. Dis. 2000;36:97–101. doi: 10.7589/0090-3558-36.1.97. [DOI] [PubMed] [Google Scholar]
- Cullen J.M., Marion P.L. Non-neoplastic liver disease associated with chronic ground squirrel hepatitis virus infection. Hepatology. 1996;23:1324–1329. doi: 10.1002/hep.510230605. [DOI] [PubMed] [Google Scholar]
- Cully J.F., Johnson T.L., Collinge S.K., Ray C. Disease limits populations: plague and black-tailed prairie dogs. Vector-borne Zoonotic Dis. 2010;10:7–15. doi: 10.1089/vbz.2009.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney M.A., Nagy L., Kinsel M.K., Treuting P.M. Spontaneous histologic lesions of the adult naked mole-rat (Heterocephalus glaber): a retrospective survey of lesions in a zoo population. Vet. Pathol. 2013;50:607–621. doi: 10.1177/0300985812471543. [DOI] [PubMed] [Google Scholar]
- Delaney M.A., Kinsel M.K., Treuting P.M. Renal pathology in a non-traditional aging model: the naked mole-rat (Heterocephalus glaber) Vet. Pathol. 2016;53:493–503. doi: 10.1177/0300985815612557. [DOI] [PubMed] [Google Scholar]
- Delaney M.A., Ward J.M., Walsh T.F., Chinnadurai S.K., Kerns K., Kinsel M.J., Treuting P.M. Initial case reports of cancer in naked mole-rats (Heterocephalus glaber) Vet. Pathol. 2016;53:691–696. doi: 10.1177/0300985816630796. [DOI] [PubMed] [Google Scholar]
- Dixon D., Alison R., Bach U., Colman K., Foley G.L., Harleman J.H., Haworth R., Herbert R., Heuser A., Long G., Mirsky M., Regan K., Van Esch E., Westwood F.R., Vidal J., Yoshida M. Nonproliferative and proliferative lesions of the rat and mouse female reproductive system. J. Toxicol. Path. 2014;27(3–4 Suppl.):1S–107S. doi: 10.1293/tox.27.1S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doboszynska T., Zurowski W. Anatomical studies of the male genital organs of the European beaver. Acta Theriologica. 1981;26:331–340. [Google Scholar]
- Doi T., Kotani Y., Kokoshima H., Kanno T., Wako Y., Tsuchitani M. Eosinophilic substanceis “Not amyloid” in the mouse nasal septum. Vet. Pathol. 2007;44:796–802. doi: 10.1354/vp.44-6-796. [DOI] [PubMed] [Google Scholar]
- Easterbrook J.D., Kaplan J.E., Glass G.E., Watson J., Klein S.L. A survey of rodent-borne pathogens carried by wild-caught Norway rats: a potential threat to laboratory rodent colonies. Lab. Anim. 2008;42:92–98. doi: 10.1258/la.2007.06015e. [DOI] [PubMed] [Google Scholar]
- Eaton G.J., Custer R.P., Johnson F.N., Stabenow K.T. Dystrophic cardiac calcinosis in mice. Am. J. Pathol. 1978;90:173–186. [PMC free article] [PubMed] [Google Scholar]
- Foster A.P., Brown P.J., Jandrig B., Grosch A., Voronkoca T., Scherneck S., Ulrich R. Polyomavirus infection in hamsters and trichoepitheliomas/cutaneous adnexal tumors. Vet. Rec. 2002;151:13–17. doi: 10.1136/vr.151.1.13. [DOI] [PubMed] [Google Scholar]
- Veterinary Monkeypox Virus Working Group. Gaurner J., Johnson B.J., Paddock C.D., Shieh W.-J., Goldsmith C.S., Reynolds M.G., Damon I.K., Regnery R.L., Zaki S.R. Monkeypox transmission and pathogenesis in prairie dogs. Emerg. Infect. Dis. 2004;10:426–431. doi: 10.3201/eid1003.030878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves P. fourth ed. Elsevier Academic Press; San Diego: 2011. Histopathology of Preclinical Toxicity Studies. pp. 263–324. [Google Scholar]
- Green M.N., Yerganian G., Gagnon H.J. Prediction of spontaneous hereditary diabetes mellitus in Chinese hamsters by means of elevated alpha-2 serum levels. Nature. 1963;197:396. doi: 10.1038/197396a0. [DOI] [PubMed] [Google Scholar]
- Han B.A., Schmidt J.P., Bowden S.E., Drake J.M. Rodent reservoirs of future zoonotic diseases. Proc. Natl. Acad. Sci. USA. 2015;112:7039–7044. doi: 10.1073/pnas.1501598112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilgun R., Yoldas A., Kuru N., Ozkan Z.E. Macroscopic anatomy of the lower respiratory system in mole rats (Spalax leucodon) Anat. Histol. Embryol. 2014;43:474–481. doi: 10.1111/ahe.12098. [DOI] [PubMed] [Google Scholar]
- Jara L.F., Sanchez J.M., Alvarado H., Nassar-Montoya F. Kurloff cells in peripheral blood and organs of wild capybaras. J. Wildl. Dis. 2005;41:431–434. doi: 10.7589/0090-3558-41.2.431. [DOI] [PubMed] [Google Scholar]
- Kirschstein R.L., Rabson A.S., Kilham L. Pulmonary lesions produced by fibromas viruses in squirrels and rabbits. Cancer Res. 1958;18:1340–1344. [PubMed] [Google Scholar]
- Kurtz D.M., Prescott J.S., Travlos G.S. third ed. CRC Press; Boca Raton: 2017. The Clinical Chemistry of Laboratory Animals. [Google Scholar]
- Losco P.E. Dental dysplasia in rats and mice. Toxicol. Pathol. 1995;23:677–688. doi: 10.1177/019262339502300605. [DOI] [PubMed] [Google Scholar]
- Mackie J.T., Booth R., Caton W., Stevenson R. Concurrent infection with cilia-associated respiratory bacillus and mycoplasmas in spinifex hopping-mice (Notomys alexis) with pneumonia. Aust. Vet. J. 2001;79:502–504. doi: 10.1111/j.1751-0813.2001.tb13026.x. [DOI] [PubMed] [Google Scholar]
- Meerburg B.G., Singleton G.R., Kijlstra A. Rodent-borne diseases and their risks for public health. Crit. Rev. Microbiol. 2009;35:221–270. doi: 10.1080/10408410902989837. [DOI] [PubMed] [Google Scholar]
- Meier C., Partlow G.D., Fisher K.R.S., Rennie B. Persistent paramesonpehric ducts (masculine uterus) in the male North American beaver (Castor candadensis) Can. J. Zool. 1998;76:1188–1193. [Google Scholar]
- Müller D.W.H., Szentiks C.A., Wibbelt G. Polycystic kidney disease in adult Brazilian agoutis (Dasyprocta leporina) Vet. Pathol. 2009;46:656–661. doi: 10.1354/vp.08-VP-0107-W-FL. [DOI] [PubMed] [Google Scholar]
- Nelson D.D., Haldorson G.J., Stanton J.B., Noh S.M., Bradway D.S., Mansfield K.G., Baszler T.V. Francisella tularensis infection without lesions in gray tree squirrels (Sciurus griseus): a diagnostic challenge. J. Vet. Diag. Invest. 2014;26:312–315. doi: 10.1177/1040638713520541. [DOI] [PubMed] [Google Scholar]
- Pettan-Brewer C., Treuting P.M. Practical pathology of aging mice. Pathobiol. Aging Age Relat. Dis. 2011;1 doi: 10.3402/pba.v1i0.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafferty D.P., Lochmiller R.L., Kim S., Qualls C.W., Schroder J., Basta N., McBee K. Fluorosis risks to resident hispid cotton rats on land-treatment facilities for petrochemical wastes. J. Wildl. Dis. 2000;36:636–645. doi: 10.7589/0090-3558-36.4.636. [DOI] [PubMed] [Google Scholar]
- Rossow H., Sissonen S., Koskela K.A., Kinnunen P.M., Hemmila H., Niemimaa J., Huitu O., Kuusi M., Vapalahti O., Henttonen H., Nikkari S. Detection of Francisella tularensis in voles in Finland. Vector Borne Zoonotic Dis. 2014;14:193–198. doi: 10.1089/vbz.2012.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth L., King J.M. Congestive cardiomyopathy in the woodchuck, Marmota monax. J. Wildl. Dis. 1986;22:533–537. doi: 10.7589/0090-3558-22.4.533. [DOI] [PubMed] [Google Scholar]
- Roth L., King J.M., Hornbuckle W.E., Harvey J., Tennant B.C. Chronic hepatitis and hepatocellular carcinoma associated with persistent woodchuck hepatitis virus infection. Vet. Pathol. 1985;22:338–343. doi: 10.1177/030098588502200407. [DOI] [PubMed] [Google Scholar]
- Rothenburger J.L., Himsworth C.G., Chang V., LeJeune M., Leighton F.A. Capillaria hepatica in wild Norway rats (Rattus norvegicus) from Vancouver, Canada. J. Wildl. Dis. 2014;50:628–633. doi: 10.7589/2013-09-256. [DOI] [PubMed] [Google Scholar]
- Rothenburger J.L., Himsworth C.G., Treuting P.M., Leighton F.A. Survey of cardiovascular pathology in wild urban Rattus norvegicus and Rattus rattus. Vet. Pathol. 2015;52:201–208. doi: 10.1177/0300985814528220. [DOI] [PubMed] [Google Scholar]
- Rothenburger J.L., Himsworth C.G., Clifford C.B., Ellis J., Treuting P.M., Leighton F.A. Respiratory pathology and pathogens in wild urban rats (Rattus norvegicus and Rattus rattus) Vet. Pathol. 2015;52:1210–1219. doi: 10.1177/0300985815593123. [DOI] [PubMed] [Google Scholar]
- Simmons J.H., Riley L.K., Franklin C.L., Besch-Williford C.L. Hamster polyomavirus infection in a pet Syrian hamster (Mesocricetus auratus) Vet. Pathol. 2001;38:441–446. doi: 10.1354/vp.38-4-441. [DOI] [PubMed] [Google Scholar]
- Simpson V.R., Hargreaves J., Butler H.M., Davison N.J., Everest D.J. Causes of mortality and pathological lesions observed post-mortem in red squirrels (Sciurus vulgaris) in Great Britain. BMC Vet. Res. 2013;9:229. doi: 10.1186/1746-6148-9-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stannard H.J., Tulk M.L., Old J.M. Dead mouse hopping: Tyzzer’s disease in spinifex hopping-mice (Notomys alexis) Vet. Microbiol. 2017;201:201–207. doi: 10.1016/j.vetmic.2017.01.018. [DOI] [PubMed] [Google Scholar]
- Stephenson N., Swift P., Villepique J.T., Clifford D.L., Nyaoke A., De la Mora A., Moore J., Foley J. Pathologic findings in Western gray squirrels (Sciurus griseus) from a notoedric mange epidemic in San Bernardino Moutnains, California. J. Parasitol. 2013;2:266–270. doi: 10.1016/j.ijppaw.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C.E., Hume J.D. Contributions of microbes in vertebrate gastrointestinal tract to production and conservation of nutrients. Physiol. Rev. 1998;78:393–427. doi: 10.1152/physrev.1998.78.2.393. [DOI] [PubMed] [Google Scholar]
- Suckow M.A., Stevens K.A., Wilson R.P. Academic Press; London: 2012. The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents. [Google Scholar]
- Sura R., French R.A., Goldman B.D., Schwartz D.R. Neoplasia and granulomas surrounding microchip transponders in Damaraland mole rats (Cryptomys damarensis) Vet. Pathol. 2011;48:896–902. doi: 10.1177/0300985810377184. [DOI] [PubMed] [Google Scholar]
- Taylor K.R., Milone N.A., Rodriguez C.E. Four cases of spontaneous neoplasia in the naked mole-rat (Heterocephalus glaber), a putative cancer-resistant species. J. Gerentol. A Biol. Sci. Med. Sci. 2016;72:38–43. doi: 10.1093/gerona/glw047. [DOI] [PubMed] [Google Scholar]
- Terrell S.P., Forrester D.J., Mederer H., Regan T.W. An epizootic of fibromatosis in gray squirrels (Sciurus carolinensis) in Florida. J. Wildl. Dis. 2002;38:303–312. doi: 10.7589/0090-3558-38.2.305. [DOI] [PubMed] [Google Scholar]
- Tompkins D.M., Sainsbury A.W., Nettleton P., Buxton D., Gurnell J. Parapoxvirus causes a deleterious disease in red squirrels associated with UK population declines. Proc. R Soc. Lond. Biol. Sci. 2002;269:529–533. doi: 10.1098/rspb.2001.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappe J.P., Chandler F.W., Westrom W.K., Liu S.K., Dolensek E.P. Listeriosis in seven bushy-tailed jirds. JAVMA. 1984;185:1367–1370. [PubMed] [Google Scholar]
- Treuting P.M., Dintzis S.M. second ed. Academic Press; London: 2017. Comparative Anatomy and Histology: A Mouse and Human Atlas (Expert Consult) [Google Scholar]
- Veiga-Parga T., LaPerle K.M., Newman S.J. Spontaneous reproductive pathology in female guinea pigs. J. Vet. Diagn. Invest. 2016;28:656–661. doi: 10.1177/1040638716665429. [DOI] [PubMed] [Google Scholar]
- Wallau B.R., Schmitz A., Perry S.F. Lung morphology in rodents (Mammalia Rodentia) and its implication for systematics. J. Morphol. 2000;246:228–248. doi: 10.1002/1097-4687(200012)246:3<228::AID-JMOR6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Widmer H.R., Hoppeler H., Nevo E., Taylor C.R., Weibel E.R. Working underground: respiratory adaptations in the blind mole rat. Proc. Natl. Acad. Sci. 1997;94:2062–2067. doi: 10.1073/pnas.94.5.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcoxen T.E., Seitz J., Nuzzo J.T. Fibroma virus infection in an Eastern fox squirrel (Sciurus niger) from Sangamon County Illinois. Trans. Illinois State Acad. Sci. 2015;108:27–28. [Google Scholar]
- Yarto-Jaramillo E. Rodentia. In: Miller R.E., Folwer M.E., editors. Fowler’s Zoo and Wild Animal Medicine. eighth ed. Elsevier Saunders; St. Louis: 2015. pp. 384–422. [Google Scholar]


