MID3: Mission Impossible, or Model‐Informed, Drug Discovery and Development? At the 2019 American Society for Clinical Pharmacology and Therapeutics (ASCPT) annual meeting, point‐counterpoint discussions were held on key challenges that limit, and future directions that enhance the adoption of model‐informed drug discovery and development (MID3) across the drug discovery, development, regulatory, and utilization continuum. We envision that the opportunities discussed and lessons learned from having contrasting perspectives on issues that lack consensus may aid our discipline in more effectively implementing MID3 principles.
Background
The evolution of the science and application of quantitative approaches over the past 50 years in drug discovery, development, regulatory approval, and clinical utilization (DDRU) is incontrovertible. Numerous publications have extensively documented case studies demonstrating the impact of modeling and simulation (M&S) in decision making in industry, regulatory, and practice settings.1, 2, 3, 4, 5, 6, 7 At the same time, there appears to be consensus within the community that the discipline needs to continue to evolve from one‐off case studies to a paradigm of systematic, best practices‐driven approaches to improving decision making across the DDRU continuum.
Over the past two decades, significant efforts have been made to appropriately frame the scope and promise of the quantitative discipline of pharmacometrics. This is apparent in the evolution of the terms used to describe the discipline, from M&S, to model‐based drug development and model‐based drug discovery, to the current usage: MID3. Marshall and colleagues have described MID3 as a “quantitative framework for prediction and extrapolation centered on knowledge and inference generated from integrated models of compound, mechanism, and disease level data aimed at improving the quality, efficiency, and cost effectiveness of decision making.”1 It should be noted that in the context of regulatory decision making, reference is often made to MIDD, which excludes the discovery term in MID3.
There has been tangible progress towards the goal of MID3 as “business as usual.” Best practices have been developed for MID3 with the objective of improving implementation, standardization, and acceptance to various stakeholders. Reviews of the literature and standard practice across organizations have generally affirmed the documented standards, acknowledged the modest improvements in organizational awareness, and set expectations for future wider use and impact, and have also highlighted areas for further improvement.8 There have been significant developments in the regulatory domain, with model‐informed drug development (MIDD) being formally noted in the Prescription Drug User Fee Act VI, highlighting model‐informed decisions made in the areas of extrapolation and dose optimization, inference about efficacy, clinical trial design, and informing policy.9 Additionally, the US Food and Drug Administration (FDA) has implemented a new Model‐Informed Drug Development Paired Meeting Pilot Program. Their early experience has been encouraging and may ultimately help achieve transformative applications of MIDD approaches in drug development programs as a matter of routine.10, 11
While there is a general appreciation of the positive impact of MID3 on the quality and efficiency of decision making and its potential to have a significant impact on the well‐documented research and development (R&D) productivity challenges3, 4, 5, 6, MID3 in practice can be isolated and inconsistently applied across the community, with its full potential yet unrealized. We posit that this may be due to a number of factors ranging from unresolved scientific and technical issues, lack of standardized processes, operational and organizational barriers, and educational and knowledge gaps both within the scientific and clinical research community.12, 13, 14 Moreover, there is continued lack of clarity of the return on investment among decision makers, the novelty and ever‐increasing complexity of treatment modalities, and need for more precisely addressing the needs of patient populations.
To tackle a few of these foundational challenges, an interactive discussion using a point‐counterpoint format was held at the ASCPT 2019 annual meeting. The format was chosen to encourage critical thinking based on opposing views from a panel of experts, who occasionally took positions that were extreme and not necessarily aligned with their own personal opinions. The diversity of perspectives was felt to be particularly helpful as the selected topics lack consensus within the community and may have multiple plausible/correct answers depending on the context (Table 1). The debate themes were chosen to be of broad value to students, early career members, and experienced scientists within and outside the clinical pharmacology and drug development community at ASCPT, and regardless of the organization and setting in which they reside. The themes ranged from those that baselined the state of the art by defining success now and into the future (theme 1), behaviors and approaches to delivering useful models for the right questions and decisions (themes 2 and 3), looking into the future in terms of underserved areas of great potential (theme 4), disruptive innovations (theme 5), and educational and organizational opportunities to prepare and position the discipline for success (themes 6 and 7, respectively). To encourage interactivity, the audience was polled prior to the session, and after each debate and in real time, to assess level of agreement between the panelists and audience, and to what extent the debates changed audiences’ perceptions. The presentation slides from the session can be found in the Supplementary Material.
Table 1.
Overview of MID3 themes, points, and counterpoints discussed at the 2019 ASCPT Annual Meeting
| Theme | Point (speaker abbreviations)a | Counterpoint (speaker abbreviations)a |
|---|---|---|
| 1. State of the art | MID3 has been a smashing success (J.G.) | No. MID3 has fallen short of expectations (O.D.P.) |
| 2. Primary limitations to success | Talk more, model less (S.T.) | Talk less, model more (O.D.P.) |
| 3. All models are wrong, but some are useful | Wrong models are dangerous (S.T.) | Wrong models are useful (P.H.v.d.G.) |
| 4. Transforming clinical trial design decision making | All clinical trials should be informed by simulations (M.R.G.) | Simulations are unnecessary and time consuming in most cases (D.O.) |
| 5. Disruptive innovations necessary for the future | Industrialize current models & methodologies (J.G.) | Future lies in machine learning and systems models (M.R.G.) |
| 6. The ideal MID3 scientist for the future | Best pharmacometricians have training in mathematics and statistics (D.A.) | Best pharmacometricians have training in medicine and pharmacology (P.H.v.d.G.) |
| 7. Organizational opportunities in R&D | Pharmacometricians have a strategic role and hence need to be part of the core development team (D.O.) | Pharmacometricians provide technical solutions but are not part of drug development teams (D.A.) |
MID3, model‐informed drug discovery and development; R&D, research and development.
Views expressed were to facilitate debate and did not always reflect personal opinion.
Theme 1: State of the Art
Point: MID3 has been a smashing success (J.G.)
The assessment of whether MID3 has been a success can be made based on the following four considerations (Figure 1). First, the evidence is clear that pharmacometricians are highly sought after to help inform important decisions that span across the DDRU continuum. The fact that pharmaceutical companies, regulatory authorities, and other domains have recognized and created dedicated departments to formalize input from quantitative disciplines is a primary indicator of the power and influence that scientists in the field of pharmacometrics have gained over the past 40‐plus years. Second, because of the demonstrated ability to integrate data to generate knowledge, pharmacometricians today have clearly defined career paths and leadership opportunities as well as organizational infrastructure that enable them to climb the career ladder, take up increasingly complex challenges, and expand the spheres of influence. Third, perhaps the most important metric of success of MID3 progression is the level of impact that scientists in the discipline have had on advancing public health. There are numerous, well‐documented innovations in the areas of dose optimization, alternative approaches to evidence generation, novel clinical trial designs, and drug approvals that would not have been possible without applying pharmacometric concepts to problem solving. Finally, from an economic standpoint, there has been a sustained investment in the area over the past 20‐plus years and the current demand for pharmacometric scientists continues to outstrip supply.
Figure 1.
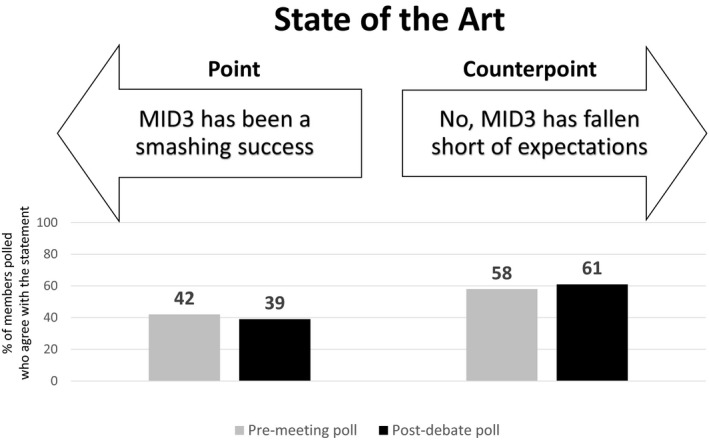
Premeeting and postdebate polling results for theme 1: State of the art.
It is important to contextualize the speed and depth of these achievements and recognize that the field is still in its nascent stages. This is evident when compared with related disciplines such as statistics, where for instance, the concept of confidence intervals was introduced in 1937 and it was not until 1962 that the regulatory amendment to expectations around the efficacy of medicines was introduced. Therefore, the progression and translation of techniques and methodologies into the critical path in drug development can take decades. MID3 has been a smashing success when viewed from this context. Moreover, the future holds tremendous promise as new developments and opportunities emerge in the areas of physiologically‐based pharmacokinetics/pharmacodynamics, quantitative systems pharmacology, real‐world evidence, decision‐support systems, and patient care.
Counterpoint: No. MID3 has fallen short of expectations (O.D.P.)
The problem with MID3 as a tool supporting clinical and regulatory decision making is that the beauty is in the eye of the beholder. The points made in favor of it represent a self‐assessment that is biased with what may be close to the heart. However, when looking at the facts from an external perspective, it is readily apparent that critical decisions continue to be made using P values and rarely embrace the principles founded in the learn‐and‐confirm paradigm, which is the foundation of MID3.
The fact that M&S is undervalued is reflected in its conspicuous absence from the pyramid of evidence‐based medicine that includes not just randomized controlled trials and systematic reviews but also observational studies, uncontrolled cohort/case studies, and animal research.15 Furthermore, MID3 is essentially disconnected from the promised land of artificial intelligence (big data and personalized medicine). A literature search has highlighted that even the zebrafish model is advertised as a predictive tool for personalized medicine, whereas the term MID3 is barely mentioned, despite the fact that data integration and knowledge generation can play a central role in the development of dosing algorithms. From a regulatory perspective, it should be noted that the 2018 review on new therapy approvals by the FDA’s Center of Drug Evaluation and Research does not mention the role of M&S, pharmacometrics, or MID3 as an enabler of the successful programs, raising questions regarding whether it is a core element of the modern toolkit for innovation.
This is not to say that MID3 has had no impact, as the volume of ad hoc case studies clearly demonstrate. Unfortunately, one could argue that drug development decision making would not be very different if pharmacometrics did not exist as a discipline. Therefore, for MID3 to emerge as a core driver of innovation, the discipline needs to rechannel its resources to a renewed focus on knowledge generation and management and less on developing productivity tools (e.g., standardizing its outputs). In contrast to what has been observed with Big Data, MID3 has fallen short of expectations. One needs a similar momentum to ensure MID3 becomes mainstream, elevating the drug developing and decision‐making process.
Theme 2: Primary Limitations to Success
Point: Talk more, model less (S.T.)
Pharmacometricians enjoy the operational aspects and the challenge of model development—modeling is fun! The first inclination is to immediately start model building upon receipt of data (Figure 2). An analysis plan may exist, but such plans generally contain template text with vague objectives rather than focusing on the development, clinical, and/or regulatory question that M&S will support. However, even if the question is clear and the modeler begins with the end in mind, it is easy to be distracted by interesting trends in the data, or the obsession with getting a perfect fit.
Figure 2.
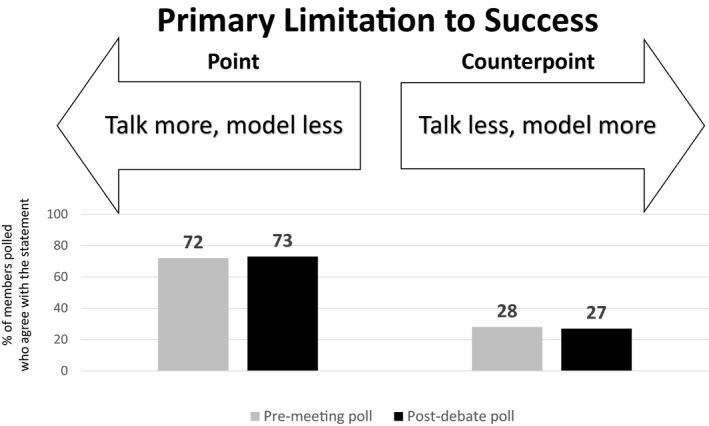
Premeeting and postdebate polling results for theme 2: Primary limitations to success.
Pharmacometricians often add unnecessary complexity to models to “get the line through the points.” It’s uncomfortable to accept that a model does not have a perfect fit. For example, if a model is effectively estimating the exposure metric (area under the curve (AUC)) for subsequent pharmacokinetics/pharmacodynamics modeling, one may consider the model complete. But we are often compelled to try complex absorption models to better characterize Cmax and improve the fit. Indeed, the diagnostics look better, but this adds time and complexity and reduces understanding and credibility. Thus, while the model “fits,” it is of limited or no value if it does not answer the question for which it was developed or is not available before the deadline.
To ensure that M&S adds value (and to avoid doing more than what is required), pharmacometricians must communicate with their project teams before any data analysis starts to understand the key strategic development questions, clinical context, available data, assumptions, and decision criteria. A M&S plan should be developed accordingly and shared with (and agreed upon by) the team. It is also imperative to regularly check in with the team during model development as well to ensure that the model being developed remains consistent with their needs and to course correct as needed.
Lastly, pharmacometricians must become better scientific communicators whose objective should be to impact and influence quantitative decisions, rather than impress (or more likely confuse) teams with technical progress. Modelers often relay the features and processes associated with model development evaluation (e.g., goodness of fit, parameter tables), using technical “lingo” that is incomprehensible to other stakeholders. Unfortunately, this usually obliterates any impact that the model may have. For pharmacometricians to be successful, they must understand WHY M&S is being used, WHAT questions should be answered, WHO will use the results, and HOW they will be used. Thus, the primary limitation to success of MID3 is communication: We need to talk more and model less!
Counterpoint: Talk less, model more (O.D.P.)
While the importance of communication is fully acknowledged, it is equally critical, as noted by Alexander Pope in his poem "An Essay on Criticism" composed in 1711, “a little learning is a dang’rous thing,” to understand that communication without subject mastery and elucidation of the strengths and weaknesses can be misleading at best and dangerous at worst. In fact, politicians are a great example of such a setting. One needs therefore to eliminate the root causes or the primary limitations for the successful implementation of MID3 by the ad hoc nature of MID3. Instead, models should become an integral part of the evidence synthesis framework, in a similar continuum to what is currently done for systematic reviews. One should integrate knowledge, building up on prior evidence, and by doing so summarize and scrutinize the predictive performance, strengths, and limitations of the models.
The real problem pharmacometricians face is not poor communication, it is the deception of perception within the community that performs and presents models without full understanding of context, assumptions, and limitations. Evidence synthesis is more than fitting lines through data points. In this regard, the MID3 community is a long way away from the successes of prognostic, predictive, and diagnostic models, which are based on extensive and continuous data collection and exhaustive characterization of model performance. Because currently models are not continuously verified and improved upon, the ability to build trust by demonstrating reliability over time is absent. It therefore stands to reason that pharmacometric models are not seen by decision makers and stakeholders as predictive instruments with the appropriate performance attributes around specificity, sensitivity, and predictive performance. This has the consequence of eroding trust and credibility.
The need of the hour is to recognize that the core competency of MID3 is professional excellence. MID3 thus needs to become a process at the enterprise level, with a well‐defined development path for scientists in the field, as to ensure the acquisition of core competencies across different knowledge domains. 13 The competencies required to address existing limitations are lacking and cannot be compensated for by communicating better or more.
Theme 3: All models are wrong, but some are useful
Point: Wrong models are dangerous (S.T.)
Pharmacometric models range from the oversimplified compartment models to complex quantitative systems pharmacology models where it is impossible to estimate every parameter with precision (Figure 3). Are these “wrong” models dangerous, though? It depends! If the only purpose of a model was to be descriptive, a cubic spline would be sufficient; however, one can’t extrapolate from a model whose sole purpose is to “connect the dots.” Empirical models have a useful place in MID3 but must be used with caution; if a model optimized only based on goodness‐of‐fit is used to extrapolate and make subsequent drug development or regulatory decisions, danger can arise. Some illustrative examples are highlighted below.
Figure 3.
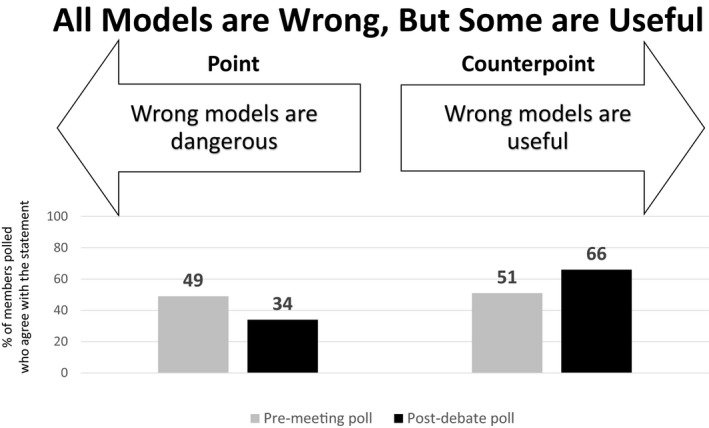
Premeeting and postdebate polling results for theme 3: All models are wrong, but some are useful.
Indirect response models with two different mechanisms represent similar though not identically shaped response‐time curves, and could, in theory, both be successfully fit to data at hand.16 If a naïve modeler chooses a model based on goodness‐of‐fit rather than the underlying mechanism, they may select the wrong one whose parameters will not have physiological/pharmacological meaning, and extrapolation to make decisions on study design or dosing may have future negative consequences.
There is an ongoing debate in the pharmacometrics community around fixing versus estimating allometric scaling exponents; one against the latter is that if the population being modeled has a relatively narrow weight range, an estimated exponent will likely not be representative of a wider patient population. If the model is used to extrapolate to another population (e.g., adults to children), the subsequent estimate of clearance in the new population may be off by several fold, propagating the error to the calculation of AUC, and subsequent estimates of dose in the new population.
In population pharmacokinetics, spurious covariates that are statistically significant but not clinically relevant may be added to the model if modelers let the data rather than the clinical question drive the selection. A dose modification for an irrelevant subgroup ultimately may lead to an FDA‐mandated label change! Similarly, rejecting a known important/influential covariate based on lack of statistical significance may lead to a decision based on incomplete information.
Lastly, pharmacometrics is a very heterogeneous field. Modelers with excellent technical skills but limited understanding of pharmacology or physiology may not be aware that they are using the wrong model or getting parameter values that are impossible or not physiologically reasonable, because they are relying on goodness of fit to indicate that the model is appropriate. In addition, they may make incorrect modifications that violate the biology to decrease computational complexity, which improves the fit but invalidates model utility.
A wrong model may lead to incorrect dose predictions or incomplete understanding of the mechanism of action, which may cause real harm to patients. Decisions based on the faulty model may bring an ineffective or unsafe drug forward, or suggest study designs that will fail, making patients wait that much longer to get a drug that they need. Therefore, wrong models can indeed be dangerous!
Counterpoint: Wrong models are useful (P.H.v.d.G.)
Probably the most‐cited quotation in pharmacometrics is Box’s statement that “all models are wrong, but some are useful.” Arguably, this has done the discipline more harm than good and has undermined the credibility and scientific status of its practitioners, since it implies that M&S is intrinsically flawed and detached from reality in the laboratory and clinic. Nonmodelers typically interpret this defensive statement in the following ways, both of which erode the confidence they have in the pharmacometrician:
The model is too simple and therefore does not represent reality
The model is too complex and therefore is based on too many assumptions
However, there seems to be no good reason to treat pharmacometrics (and M&S in general) different from any other scientific discipline and instead inculcate Karl Popper’s universal principles and consider models as hypotheses which are testable and falsifiable.17 Viewed in this way, a wrong model becomes a rejected hypothesis which may be very impactful in the learning phase of drug development. In particular, if the model is mechanistic, it tells us that our current understanding of biology, pathophysiology, and disease is incorrect. Based on this, the model can be modified and/or expanded and becomes the next hypothesis which can be tested in the next experiment/trial. In this extended version of Sheiner’s learn and confirm paradigm, the difference between having a model or not becomes the difference between having an explicit, quantifiable, and testable hypothesis or not. It is difficult to see how anyone could not be in favor of the former. Thus, we propose that the pharmacometrics mantra changes to “all models are hypotheses” and that the field embraces the concept that “wrong models can be very useful.”
Theme 4: Transforming Clinical Trial Design Decision Making
Point: All clinical trials should be informed by simulations (M.R.G.)
The first point to consider is that the decision‐making process in pharmaceutical R&D is broken (Figure 4). Relative to other science‐based industries, it can be argued that the rigor with which decisions are made in drug development pales in comparison to the scientific excellence with which data are gathered from clinical trials and other data sources. Decisions are often too dependent on individual intuition and power structures, often limited to a statistical assessment at the protocol level without any integration of totality of evidence, leading to a lack of objective quantitative assessments in many cases. The most urgent need for our industry is to evolve toward the formal decision processes used in other science‐based industries, driving all decisions with quantitative analyses.
Figure 4.
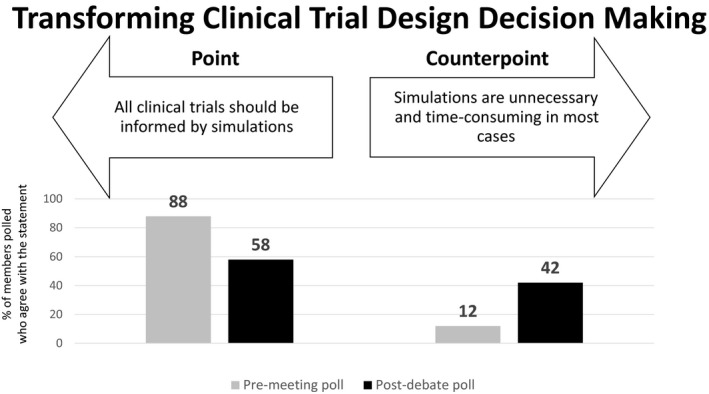
Premeeting and postdebate polling results for theme 4: Transforming clinical trial design decision making.
This requires a fundamental change to the drug development decision‐making process, which is currently an empirical and opaque exercise that involves subjective intuition based on clinical trial results and several factors external to the trial observations, with uncertain assumptions about the translation of these results. The proposed approach would align with those used by other science‐based industries by making assumptions explicit and transparent, considering the clinical trial data as a single element of the decision, augmented by prior knowledge, and utilizing model‐based projections of clinically meaningful outcomes to quantitatively characterize all inputs to that decision. In other words, this is a model‐based projection of decision criteria informed by a clinical trial, where the clinical trial data is input into the decision but not the ultimate decision criterion itself. When viewed in this context, it becomes clear that every clinical trial becomes an opportunity to provide a valuable data point to the decision process. Therefore, by extension, all decisions, not just trials, would need to be informed by simulations with the mechanics in place to deliver decision guidance at the time of the top line report of the trial. This has been accomplished in other industries and can be achieved in drug development with a change in mindset (proactive and long‐term oriented vs. reactive and short term) and a shift in our area of focus from late‐stage to early‐stage drug development. This change in emphasis is aimed at conditions where the uncertainty in the decision space and, thereby, the probability of inaccurate decision making is largest. In doing so, the totality of all available evidence can be integrated and utilized in an effective and transparent manner to inform decisions and ultimately improve R&D productivity.
Counterpoint: Simulations are unnecessary and time consuming in most cases (D.O.)
While M&S is routinely deployed to understand exposure‐response, support dose selection, and extrapolate to special populations, its application in supporting trial designs has been less systematic. We can all agree that gaining efficiency in drug development is critical to improving the probably of success and reducing the cost of development. There are many approaches other than M&S to enhance the efficiency of the study design, including implementation of futility analysis, supporting design with Bayesian approach, and/or using an adaptive design. Implementing a full M&S approach requires adequate resources, planning, and relevant data and models that can be used to predict study outcome. It should be considered a unique tool, ideal in certain situations, but should not be considered essential for every program. For certain established therapeutic areas, some aspects of the study design such as duration of treatment may be set by regulatory expectations, while other programs which have the potential to be transformational require companies to react rapidly and initiate the next clinical study as soon as possible to provide breakthrough drugs to patients. M&S still lack enough automation, standardization, and efficiency to become a routine deliverable to support ALL clinical studies rather than be deployed when it can have greatest impact.
Theme 5: Disruptive Innovations Necessary for Future
Point: Industrialize current models and methodologies (J.G.)
The need to prioritize investment toward industrializing mainstream pharmacometric approaches cannot be overemphasized, particularly as the majority of the audience considered MID3 not to be a smashing success (Figure 5). An analogy can be drawn to the evolution of the automobile industry. The first commercially used internal combustion engine was invented in 1859, and the modern version of it in 1876. However, horse carriages continued to be popular for several decades, and it was not until 1913 when the Ford motor company incorporated its first moving assembly line, triggering large‐scale production and industrialization, which led to the success of the technology. Pharmacometrics is facing a similar situation today as it not a sustaining technology but a disruptive innovation. This is still a relatively new market, for which many may not even be aware of the need and certainly may not realize its full potential.
Figure 5.
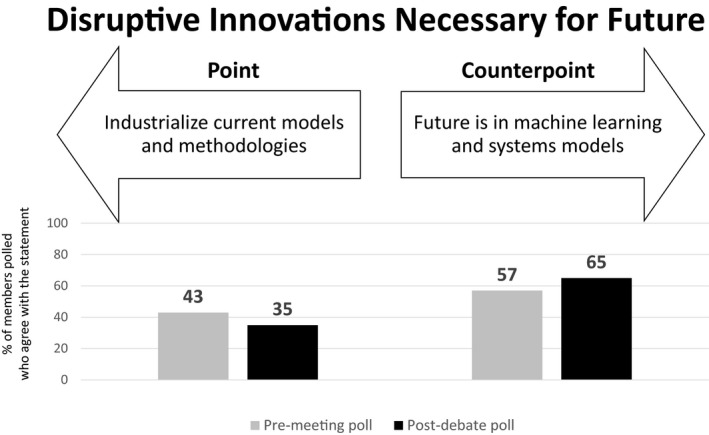
Premeeting and postdebate polling results for theme 5: Disruptive innovations necessary for the future.
The discipline has brought tools together from mathematics, statistics, pharmacology, physical chemistry, and medicine domains to solve new and complex problems to optimize therapy, and such disruptive innovations can only become mainstream if they are more accessible. A useful example here is the Kaplan‐Meier analysis of survival data, which is based on relatively complex statistical principles, and yet is used widely by many scientists and clinicians, including those not trained in statistics, due to accessibility and interpretability. Similarly, there are widely‐used pharmacometric methodologies (e.g., population PK modeling of sparse data, application of sigmoidal drug effect models) that do not need greater sophistication but unfortunately consume disproportionate time and resources due to lack of standardization. Therefore, the success of the disciplines depends on standardizing routine pharmacometric applications and making them predictable. Here, the emphasis around standardization is beyond documentation of the methodology and processes, which most organizations already do, but the consistent application of models that have stood the test of time (e.g., sigmoidal drug effect models) and which enable the majority of drug development decisions (e.g., phase 3 dose selection) today. It is proposed that this will not only enhance trust and credibility among stakeholders but will create the much‐needed but scarcely available space to embrace new tools and ultimately allow the discipline to expand its influence into newer areas.
Counterpoint: Future is in machine learning and systems models (M.R.G.)
Do we need to focus more on developing sophisticated tools and methods? Yes, we do. In fact, the analogy of the internal combustion engine illustrates this need as it will likely be obsolete within the next 10 years. Automation of methods without innovation leads to stagnation as this allows us to not to think about the problem. More important, the impact of MID3 today is limited to a small set of problems. Pharmacometrics as a tool has been marginalized to a small part of the M&S sandbox, limited to addressing traditional questions such as dose selection and dose adjustments for labeling. Thus, when resources are geared toward automation of the status quo, we limit future innovation and the scope of influence on drug development decision making.
Advancements in quantitative sciences create a much larger M&S playground. We would limit ourselves if we don’t expand beyond the typical pharmacometrics sandbox. Our discipline should embrace new methods, such as systems biology/pharmacology, artificial intelligence, machine learning, computational genomics, big data, Bayesian networks, etc. Although we need to be beware of unfounded enthusiasm (hype), a thoughtful application of these new methods has the potential to broaden the scope of influence and increase the opportunity to interact with other disciplines. For example, these tools and methods could serve as bridges to other disciplines, as the quantitative underpinning will help address new questions in in areas such as translational medicine, health economics, digital health, portfolio strategies, and real‐world evidence. Innovating at the intersection of multiple disciplines has historically led to novel solutions in this discipline, and continuing to emphasize that over automation of current methods is more likely to have a positive impact on drug development and patients' lives.
Theme 6: Ideal MID3 scientist for the future
Point: Best pharmacometricians have training in mathematics and statistics (D.A.)
We have seen the FDA’s MIDD pilot program, but perhaps missed the FDA’s original pilot program in Statistical Inference? Oh, that’s right, there wasn’t one. In fact, after Neyman‐Pearson methods of statistical inference were introduced in 1933, they were rapidly adopted as a means of interpreting data (Figure 6). Without a shadow of doubt, it is statistical thinking that has done more to influence drug development from the foundations of the FDA, through to the requirements for demonstration of efficacy in 1962 (Kefauver‐Harris Drug Amendments). That does not mean that models are not of interest—inference falls into two camps: Interpolation (which is really the realm of statistics) and extrapolation outside the “data comfort zone” (pharmacometrics). Technology now allows the simple application of models by the uninformed hobbyist. Want to fit population‐PK models—aka "nonlinear mixed‐effects statistical models" (some of the MOST sophisticated statistics)—no problem, there is a software that can do that for you; even better, one does not even have to see an equation!
Figure 6.
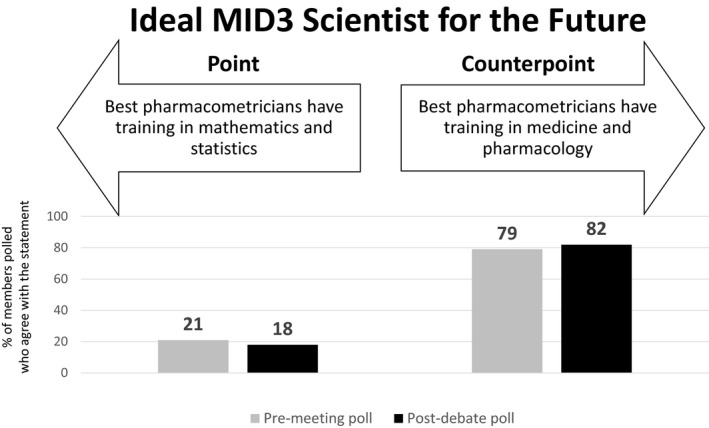
Premeeting and postdebate polling results for theme 6: The ideal MID3 scientist for the future.
Pharmacometricians must blend the application of models—with all their assumptions and flaws, with a thorough grounding in the methods of statistical inference necessary for decision making. Without the former, there will be no means of extrapolation. Without the latter there can be no acceptance. Pharmacometricians, by proper training in mathematics and statistics, should know the limitations of models—the pharmacometrician knows that the data is described by a poly‐exponential function (the model), nothing more, but does not have to ascribe physiological importance to the parameters of the model. The nonexpert believes that there is an underlying (and often misplaced) confidence in the physiological basis for the model, parameterized by numbers that have profound meaning. That blind faith falls down with the volume of distribution of chloroquine, for example, which is not a real volume (it is > 100 L/kg). The pharmacometrician accepts that the scaling is nonphysiological but a useful parameter to describe the data. The nonexpert might wonder what a 7‐ton human looks like. Thus, without training in both mathematics and statistics, there will be no appreciation by the practitioner of the limitations, or otherwise, of the (very sophisticated) methodology their driverless software is capable of.
Next time you have a medical procedure, would you prefer the physician to be an enthusiastic anatomist with a liking for human biology and a sharp knife, or a trained surgeon? When you board a flight, would you like the pilot to be a keen private pilot interested in aeronautical engineering? Or would you prefer skilled professionals with many years of certified training and a thorough understanding of the challenges, implications, and what to do when things go wrong? If one believes that modeling is relevant, then surely it is too important to be left to enthusiastic amateurs.
Without a thorough training in statistics and mathematics, the work of pharmacometricians will be viewed either as irrelevant, or worse, a distraction from the important decision‐making P value–based business of developing new medicines. With that background and training, pharmacometrics will become a collaboration that starts where existing statistics finishes and extends knowledge to make the very best decisions.
Counterpoint: Best pharmacometricians have training in medicine and pharmacology (P.H.v.d.G.)
If Lewis Sheiner had been a statistician (and had not trained as an MD), would pharmacometrics have developed into the distinct discipline as we currently know it or have evolved as a branch within statistics? We will never know the answer of course, but it seems reasonable to think that the latter would have been a likely outcome (in fact, the Chief Statistical Advisor of this journal recently stated that “…a pharmacometrician is a special type of statistician”).18 Continuing the thought experiment, it can then be argued that this would probably not have made much difference to what pharmacometrics does and looks like in the confirm phase of later‐stage drug development. However, it would have been very unlikely that MID3 had developed as we now know it and M&S applied in earlier stages of R&D might still be in its infancy.
If we now turn this analysis upside down and apply it to what training pharmacometricians of the future need, it really becomes a question of where and how the discipline wants to impact MID3. An ideal outcome would of course be everywhere, however with a limited talent, training and resource pool this strategy might dilute the impact and result in a “Jack of all trades, master of none” outcome. Given the choice, it might be tempting to be drawn to the later stages of drug development, where budgets are larger, visibility is higher and M&S is already well accepted. However, design and analysis of late‐stage trials is not commonly seen as a major bottleneck in pharma R&D; the real challenge is the lack of translation of early research to successful therapeutics, illustrated by the fact that the majority of phase 2, proof‐of‐concept studies have a negative outcome. Statistics plays an important but minor role in this, since the uncertainty is mainly related to the (lack of) understanding of the biology and disease.
Thus, the emphasis of MID3 approaches to make an impact here should be on extrapolation to reduce the uncertainty associated with the novel biology, disease, and patient population, and there is no scientific basis to make the case that sophisticated statistics can make much difference at this stage. In order to be considered a valuable and equal partner and make an impact at this stage of R&D, pharmacometricians need to have a deep understanding of biology and disease and be able to translate this into models that can guide novel ideas and approaches toward becoming therapeutics. The logical consequence of this argument is that for MID3 to reach its full potential, the next generation of pharmacometricians should be trained as quantitative biomedical scientists (which of course does include a solid grounding in statistics as well). Statisticians should be more than capable (perhaps more so than pharmacometricians) to cover the confirm part of MID3 and arguably, given the bounded nature of the questions that need to be addressed by models in this territory, the need for human, hands‐on modelers may diminish over time through application of artificial intelligence, machine learning methods, and technology.
Theme 7: Organizational Opportunities in R&D
Point: Pharmacometricians have a strategic role and hence need to be part of the core development team (D.O.)
Pharmacometric approaches have been steadily expanding to support drug development with renewed interest coming recently from both external (e.g., implementation of FDA’s MIDD pilot program, initiation of discussion for International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines) and internal (e.g., wider acceptance of M&S within pharmaceutical companies) influences (Figure 7). This demand has justified the hiring and retaining of experienced pharmacometricians to provide that expertise. Some of the best impact of pharmacometric applications include using M&S to avoid conducting a study or extrapolate efficacy/safety data to special populations. Identification of drug development questions to be answered using a M&S approach and thus influence not only the design of a study but the strategy of development of a compound requires the input and expertise of a person with the appropriate skill set. Not only that, but as pharmacometricians participate in discussion and have the opportunity to debate strategy with clinicians, statisticians, clinical pharmacologists, regulatory leads, and other member of the team, they deepen their understanding of the therapeutic area and can provide more insightful comments and suggestions and in return have the opportunity establish the credibility needed to influence decisions.
Figure 7.
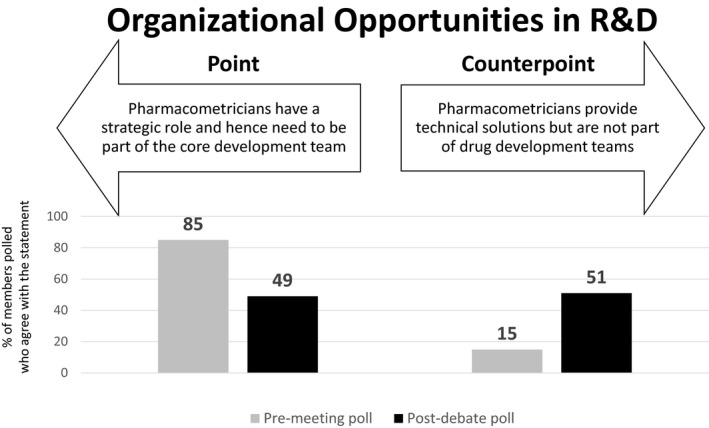
Premeeting and postdebate polling results for theme 7: Organizational opportunities in R&D.
Many opportunities for implementation of a novel approach are lost as the new methods are proposed too late in the process or after a more traditional path has been selected. Similarly, not all companies have expertise sitting in committees when the development decisions are made. Having the expert part of the development team ensures that the right person will be present at the right time and right place to achieve the best decision.
Counterpoint: Pharmacometricians provide technical solutions but are not part of drug development teams (D.A.)
Pharmacometrics is a perishable skill—if you do not use that skill frequently then you will lose it. In some industries, if you have not kept certified every 28 days, then you lose currency. Sitting on project teams, fending meeting after meeting will sap time and energy that could be put to better use. Of course, problems that can be addressed by pharmacometric approaches may be identified at such team meetings, but they are seldom the place to solve them. Being a core member will not solve those problems, having good communication with clinical pharmacologists and statisticians will. In truth, everyone wants to be in the Colosseum when the lions come out. When the project leader goes into the big decision‐making boards to present the latest shiny data, more often than not it is a spectator sport for most of the team. It is the clear communication of the pharmacometric results and implications that matter. If those results are incapable of being explained by the (layman) project leader, why do we think they are likely to be accepted?
Pharmacometrics is essentially a portfolio‐level activity, identifying common problems across projects. Rising above the trees to see the forest and lay of the land is necessary. The questions it seeks to answer are common to multiple projects and even diseases. Sitting down in the middle of the wood means it can only help put out local fires.
In this 50th anniversary year of the first moon landing, it is clearer than ever how important team effort is to major collaborative projects like developing a drug. Not everyone needs to be in the lunar module—where conditions are cramped and issues immediate. Pharmacometrics should have its rightful seat at mission control among the other key disciplines. Without the knowledge, analysis, guidance, and support these disciplines provide, there will be no new drug and no moon landing, but not everyone needs to be first man on the moon.
Discussion and Concluding Remarks
The interactive, point‐counterpoint format was designed with the intent of highlighting contrasting perspectives on a range of topics regarding the future of MID3 that were thought to be of value to the ASCPT community. As summarized above, the debates led to a range of insights around the key challenges that limit, and future directions that can enhance, the adoption of MID3.
The first observation is that if one drew a parallel with the development of pharmaceutical statistics in pharmaceutical drug development, the essential ingredients (power, people, impact on public health, economics) are there for MID3 to grow for years to come and reach a similar level of success and be considered an integral component of the modern toolkit for innovation. This will be facilitated both by harmonizing and industrializing processes and approaches, continuing to improve predictive capabilities, and innovating at the intersection of emerging areas to inform a broader array of decisions. Recently, an ICH draft reflection paper has been initiated that calls for a strategic approach to harmonizing the use of MIDD in regulatory submissions across geographies. While such an approach will entail a multiyear commitment, it is envisioned to lead to wider acceptance and utilization of MIDD.
The debate on the primary limitations to the success of MID3 highlighted the need to intensify the focus on qualifying and validating the predictive performance of our models by adopting a systematic and continuous assessment approach versus the ad hoc, one‐off approach that is widespread in the community. This is especially important as the scope of influence expands beyond the traditional paradigm (e.g., dose selection) to areas such as driving go/no‐go decisions, generating pivotal evidence for regulatory decisions, personalized/precision therapeutics, etc. At the same time, the importance of communication and training in nontechnical presentation and engagement with stakeholders was overwhelmingly acknowledged, emphasizing the underappreciated aspects of problem framing, continuous engagement with stakeholders, and achieving a common understanding of strengths and limitations of solutions. While the significant dangers of making decisions based on wrong models was well articulated and widely recognized, the discussions called for a change in mindset to treat all models as hypothesis that can be tested and falsified, and to embrace the notion that wrong models, especially when mechanistically designed, are almost always useful. This is very relevant in the discovery phase of R&D, where the uncertainty is large.
Discussions regarding the use of simulations to optimize clinical trial designs appeared to reach common ground supporting a selective rather than a ubiquitous application of pharmacometric simulations, considering the limited resources and the fact that core elements of efficacy/safety trials are generally well established and accepted by different stakeholders. The debate, however, spotlighted the opportunity to fundamentally change the decision‐making process itself using model‐based projections of decision criteria at the time of topline clinical trial results, generated by integrating all available evidence as well as external factors in a transparent manner. The types of simulations necessary for such a paradigm were envisioned to be multifaceted and multifunctional rather than purely pharmacologically based. Looking into the medium to long‐term future, the debate around disruptive innovations was seen as a false dichotomy in that the limited resources necessitated the standardization of routine pharmacometric applications and to create space for continued expansion into newer technologies and disciplines. Conversely, it was recognized that for MID3 to be successful, continued innovations at the intersection of emerging disciplines was considered imperative, as has been the hallmark of pharmacometrics for decades.
Spirited exchanges around organizational opportunities to best position MID3 for success and educational backgrounds of future MID3 scientists gave some interesting insights. While the panelists agreed on distinguishing roles within the pharmacometric disciplines, there is a need for modelers to keep their advanced skills fresh, up to speed, and expanding. At the same time, in order to ensure right utilization to the right question, the need for a strategic and therapeutic area specialist mindset that can operate on the decision‐making level was also considered paramount. From an educational standpoint, scientists in the drug discovery and early development domain may need a greater focus in pharmacology, biology, and medicine, while colleagues contributing to late‐stage development may benefit more from a stronger mathematical and statistical training focus. Ultimately, the community may need to accept that these roles require different background/role descriptions and move to more specialized roles to improve efficiency and impact for the discipline. A second educational dimension would be to ensure that scientists have the skills necessary to help standardized current methodologies and apply them appropriately, while also focusing on novel areas for further impact.
One limitation of this session was the fact that the discussions and voting were largely among members of the clinical pharmacology and pharmacometrics community and without the participation of more diverse stakeholder groups, including statisticians, therapeutic area experts, clinicians, development leaders, and others. The insights gained should be viewed with that caveat; nevertheless, the lessons learned may facilitate discussions within organizations involving relevant stakeholders, including engaging executive leadership to drive home the point that MIDD should be seen as a key component of the tool kit to enhance R&D efficiency and productivity.
The core capability of pharmacometrics as a discipline remains the integration of data through models to generate knowledge. The debates highlighted areas of further emphasis, including the need to enhance the robustness and predictive performance of models used for decision making, modify educational curricula to sharpen core competencies including communication skills and therapeutic area knowledge, standardize time‐tested models in order to expand the field to embrace newer domains, and incorporate organization changes to maximize impact on decision making, with the overall intent to widen the scope of influence and help realize the full potential of MID3.
Funding
No funding was received for this work.
Conflict of Interest Statement
All authors are employees of their stated companies and have nothing to disclose beyond their affiliation. As Editor‐in‐Chief for Clinical Pharmacology & Therapeutics, Piet H. van der Graaf was not involved in the peer review and editorial decision of this manuscript.
Supporting information
Supplemental Material . MID3 Point/Counterpoint PowerPoint.
Acknowledgments
The authors gratefully acknowledge the ASCPT staff for their excellent support to create this session and providing for the logistics. Also, Aline Barth is acknowledged for her assistance during the session.
References
- 1. Marshall, S.F. et al Good practices in model‐informed drug discovery and development: practice, application, and documentation. CPT Pharmacometrics Syst. Pharmacol. 5, 93–122 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Dijkman, S.C. , Wicha, S.G. , Danhof, M. & Della Pasqua, O.E. Individualized dosing algorithms and therapeutic monitoring for antiepileptic drugs. Clin. Pharmacol. Ther. 103, 663–673 (2018). [DOI] [PubMed] [Google Scholar]
- 3. Milligan, P.A. et al Model‐based drug development: a rational approach to efficiently accelerate drug development. Clin. Pharmacol. Ther. 93, 502–514 (2013). [DOI] [PubMed] [Google Scholar]
- 4. Visser, S.A.G. , De Alwis, D.P. , Kerbusch, T., Stone, J.A. & Allerheiligen, S.R.B. Implementation of quantitative and systems pharmacology in large pharma. CPT Pharmacometrics Syst. Pharmacol. 3, e142 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nayak, S. et al Getting innovative therapies faster to patients at the right dose: impact of quantitative pharmacology towards first registration and expanding therapeutic use. Clin. Pharmacol. Ther. 103, 378–383 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Allerheiligen, S.R.B. Impact of modeling and simulation: myth or fact? Clin. Pharmacol. Ther. 96, 413–415 (2014). [DOI] [PubMed] [Google Scholar]
- 7. Lalonde, R.L. et al Model‐based drug development. Clin. Pharmacol. Ther. 82, 21–32 (2007). [DOI] [PubMed] [Google Scholar]
- 8. Marshall, S. et al Model‐informed drug discovery and development: current industry good practice and regulatory expectations and future perspectives. CPT Pharmacometrics Syst. Pharmacol. 8, 87–96 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Madabushi, R. et al The US Food and Drug Administration's model‐informed drug development paired meeting pilot program: early experience and impact. Clin. Pharmacol. Ther. 106, 74–78 (2019). [DOI] [PubMed] [Google Scholar]
- 10. Madabushi, R. , Wang, Y. & Zineh, I. A holistic and integrative approach for advancing model informed drug development. CPT Pharmacometrics Syst. Pharmacol. 8, 9–11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang, Y. , Zhu, H. , Madabushi, R. , Liu, Q. , Huang, S.‐M. & Zineh, I. Model‐informed drug development: current US regulatory practice and future considerations. Clin. Pharmacol. Ther. 105, 899–911 (2019). [DOI] [PubMed] [Google Scholar]
- 12. Grasela, T.H. & Slusser, R. The paradox of scientific excellence and the search for productivity in pharmaceutical research and development. Clin. Pharmacol. Ther. 95, 521–527 (2014). [DOI] [PubMed] [Google Scholar]
- 13. Vlasakakis, G. et al White paper: landscape on technical and conceptual requirements and competence framework in drug/disease modeling and simulation. CPT Pharmacometrics Syst. Pharmacol. 2, e40 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harnisch, L. , Shepard, T. , Pons, G. & Della Pasqua, O. Modeling and simulation as a tool to bridge efficacy and safety data in special populations. CPT Pharmacometrics Syst. Pharmacol. 2, e28 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Murad, M.H. , Asi, N. , Alsawas, M. & Alahdab, F. New evidence pyramid. Evid. Based Med. 21, 125–127 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sharma, A. & Jusko, W.J. Characterization of four basic models of indirect pharmacodynamic responses. J. Pharmacokinet. Biopharm. 24, 611–635 (1996). [DOI] [PubMed] [Google Scholar]
- 17. Popper, K. Conjectures and Refutations (Routledge and Kegan Paul, Abingdon, 1963). [Google Scholar]
- 18. Smith, B.P. Where will statistical sciences for clinical pharmacology be in 2030? Clin. Pharmacol. Ther. 107, 17–21 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material . MID3 Point/Counterpoint PowerPoint.


