Abstract
Objective
To investigate the prognostic value of change in liver stiffness following surgery, in patients with hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC).
Methods
Patients with HBV-related HCC were included. Preoperative (baseline) liver stiffness and postoperative dynamic change in liver stiffness was evaluated.
Results
Out of 158 patients in total, postoperative liver stiffness was increased in 98 patients and decreased in 60 patients compared with baseline values. Kaplan-Meier analysis revealed that patients with elevated liver stiffness had significantly worse overall survival outcomes than those with decreased liver stiffness. Similar trends were observed for diseases-free survival and recurrence outcomes. Multivariate analyses showed that Child–Turcotte–Pugh score (hazard ratio [HR] 1.209) and liver stiffness changes (HR 1.891) were independent factors associated with overall survival. Liver stiffness changes (HR 1.521) and α-fetoprotein level (HR 1.210) were found to be independent factors for disease-free survival in patients with HCC.
Conclusion
Increased postoperative liver stiffness may be an independent risk factor of HCC prognosis. Patients with increased liver stiffness following surgery should undergo additional examinations during follow-up.
Keywords: Liver stiffness, chronic hepatitis B, hepatocellular carcinoma, FibroScan, prognosis
Introduction
Due to the prevalence of hepatitis B virus (HBV) and alcohol consumption, hepatocellular carcinoma (HCC) has become one of the most common cancers, with an increasing incidence worldwide, particularly in China.1,2 Ablation and liver transplantation are effective in treating HCC, however, surgical resection remains the most important therapy, and is conducted in most patients with early stage HCC.3–5 Despite the effectiveness of these treatments, recurrence and metastasis are still major problems for long-term survival in patients with HCC.6–8
Chronic HBV infection is well-known to be associated with the development of cirrhosis and HCC, resulting in hepatic disease-related deaths.9–11 The incidence of HCC is reported to be 0.5% in patients infected with HBV, and ranges between 2.5% and 6% in patients with cirrhosis.6,12–14 In Asia, over 60% of patients with HCC have an aetiology of chronic HBV infection.15–17 Liver stiffness, measured by transient elastography, has proven to be an effective non-invasive procedure for evaluating liver fibrosis and assessing portal vein hypertension.18–21 Liver stiffness can be measured using a FibroScan™ device (Echosens, Paris, France) that detects the propagation speed of an elastic sheer wave triggered by a transducer, which is then converted to a measurement of liver stiffness.22 High levels of liver stiffness have been reported to be associated with the risk of HCC occurrence.23,24
To the best of the authors’ knowledge, there is no published study to date that evaluates the prognostic effectiveness of liver stiffness measurements in patients with HCC, and thus, the prognostic value of liver stiffness in patients with HCC remains a clinical issue that requires exploration. The aim of the present study was to assess the prognostic value of change in liver stiffness from preoperative values, within one year following hepatic resection in patients with HBV-related HCC.
Patients and methods
Study population
This retrospective study included consecutive patients who were diagnosed with HCC and who had received liver surgery at Heze Municipal Hospital (Shandong, China) between February 2011 and May 2015. Anti-virus treatment and postoperative clinical examinations were conducted at Yantai Infectious Disease Hospital and Yantai Yuhuangding Hospital (both in Yantai, Shandong, China). Liver stiffness measurements, using Fibroscan™ (Echosens), were conducted at Jining No.1 People’s Hospital (Jining, Shandong, China). Inclusion criteria were: (1) Histologically confirmed HCC with HBV infection; (2) Child–Turcotte–Pugh score ≤9; (3) Single lesion located in the liver; and (4) Valid clinical characteristics and laboratory outcomes. Exclusion criteria were: (1) Coinfection with hepatitis C virus and/or human immunodeficiency virus; (2) Alcoholic hepatic diseases; (3) Schistosomiasis; and (4) Invalid clinical characteristics and laboratory outcomes.
This study was conducted under compliance with the Declaration of Helsinki and was approved by the Human Ethics Committees of Yantai Infectious Disease Hospital, Yantai Yuhuangding hospital and Jining No.1 People’s Hospital (Approval No. YIDH-201703-K3). All patients provided written informed consent prior to enrolment into the study.
Transient elastography
All patients underwent liver stiffness measurements by transient elastography on at least two occasions: one prior to surgery (baseline) and the other within 1 year following liver resection surgery. Transient elastography was conducted by Fibroscan™ (Echosens) as previously described.25 Results were expressed as kPa and the median value of 10 successful measurements in each patient was used as the liver stiffness value.
Diagnosis and anti-virus treatment
Fibrosis and HCC were diagnosed by histopathology of tissue specimens acquired during liver surgery. The maximum tumour diameter was calculated with images obtained during preoperative contrast-enhanced computed tomography (CT) using a GE LightSpeed VCT system (GE Healthcare, Tokyo, Japan). HBV infection was diagnosed with a positive serum viral marker and/or elevated serum HBV-DNA level (>1000 copies/ml during two consecutive detections). Briefly, serum HBV DNA was detected using a Daan test (Daan Gene Co., Ltd., Guangdong, China) according to the manufacturer’s instructions. DNA was extracted from 100 μl of serum and detected by real-time polymerase chain reaction (PCR) with HBV-DNA specific fluorescence probing, using a Roche LightCycler 480 system (Roche Diagnostics Ltd., Rotkreuz, Switzerland). HCC recurrence during follow-up was screened by contrast-enhanced CT and ultrasonography (GE Volusion™ E10 ultrasound system; GE Healthcare, Shanghai, China). Blood laboratory parameters were obtained for each patient, and Child–Turcotte–Pugh score classification was applied for consideration of prognosis, as previously described.25,26 All patients received anti-HBV regimens (entecavir) for at least 6 months prior to surgery and after surgery, as previously described.27,28
Study outcomes
The primary outcome of this study was change in liver stiffness following hepatic resection, from preoperative (baseline) value, as a prognostic marker in patients with HBV-related HCC. Secondary outcomes included independent factors associated with prognosis following hepatic resection among patients with HBV-related HCC.
Statistical analyses
Data are presented as n (%) prevalence, median (range) or mean ± SD. Student’s t-test and Wilcoxon signed–rank test was used to analyse continuous variables with or without normal distribution, respectively. Categorical variables were analysed using χ2-test and Fisher’s exact test. Survival was assessed using Kaplan-Meier estimator and Log-rank test, as appropriate. Multivariate analysis was performed using Cox proportional hazards model. All statistical analyses were conducted using SPSS software, version 16.0 (SPSS Inc., Chicago, IL, USA) and a P value < 0.05 was regarded as statistically significant.
Results
Baseline patient characteristics
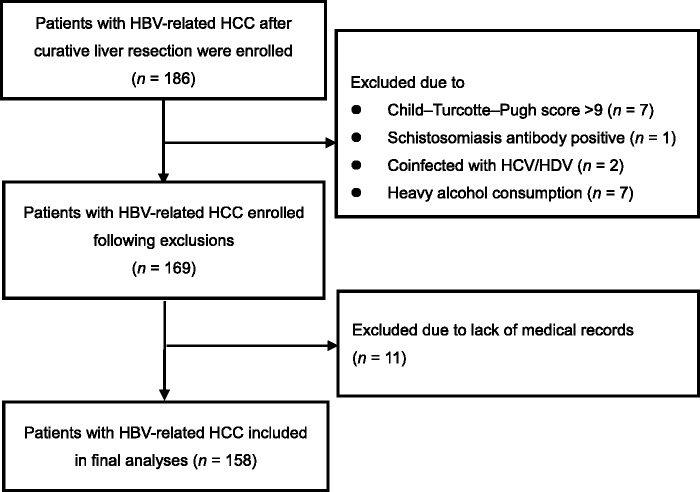
Out of 186 patients initially enrolled, a total of 158 patients were included in the final analyses (Figure 1; Table 1). Male patients were predominant (n = 123 [77.85%]), and median age was 50 years (range, 22–74 years). More than half of the patients were confirmed by pathology to have fibrosis (67.72%). Most patients were Child–Turcotte–Pugh class A (n = 147 [93.04%]), and all patients received anti-virus therapy prior to hepatic resection. The median serum HBV DNA level was 4.1 log copies/ml. A total of 102 patients (64.56%) had serum HBV DNA level <3 log copies/ml (the lower limit of detection) prior to surgery, and 56 patients (35.44%) were serum HBV DNA positive. The median maximum tumour diameter was 4.1 (range, 3.0–5.3) cm, and median liver stiffness was 6.7 (range, 4.3–12.5) kPa (Table 1).
Figure 1.
Flow chart showing selection of the study population. HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HDV, hepatitis D virus.
Table 1.
Baseline characteristics of patients with hepatitis B virus-related hepatocellular carcinoma.
| Variable | Study population, n = 158 |
|---|---|
| Sex, male | 123 (77.85) |
| Age, years | 50 (22–74) |
| Fibrosis | 107 (67.72) |
| Cirrhosis | 81 (51.27) |
| CTP class | |
| A | 147 (93.04) |
| B | 11 (6.96) |
| C | 0 (0) |
| Tumour maximum size, cm | 3.6 (1–8) |
| HBV DNA, log copies/ml | 4.1 (3.0–5.3) |
| HBeAg positive | 96 (60.76) |
| AFP, ng/ml | 347.82 (1.19–1210) |
| TBIL, mmol/l | 6.87 (2.60–24.70) |
| ALT, IU/l | 24 (11–46) |
| AST, IU/l | 21 (17–58) |
| ALP, IU/l | 69 (45–203) |
| ALB, g/l | 4.2 (2.8–6.1) |
| Platelet count, × 109/l | 193.67 (89–267) |
| Prothrombin time, s | 12.7 (11.0–15.6) |
| INR | 0.97 (0.78–1.54) |
| Liver stiffness, kPa | 6.7 (4.3–12.5) |
Data presented as n (%) prevalence or median (range).
AFP, α-fetoprotein; ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTP, Child–Turcotte–Pugh score; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; INR, international normalized ratio; TBIL, total bilirubin.
Demographic and clinical characteristics in patients with increased or decreased postoperative liver stiffness
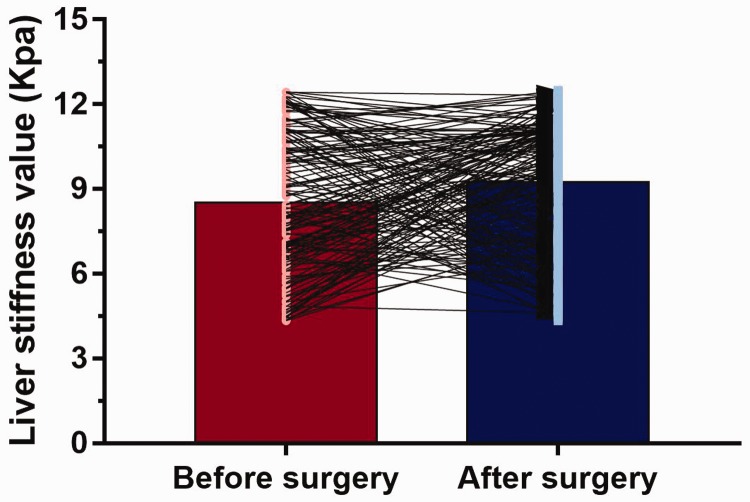
All patients received liver stiffness assessments on at least two occasions. Mean baseline liver stiffness was 8.53 ±2.41 Kpa and was 9.26 ± 2.29 Kpa after surgery. Compared with baseline values prior to surgery, 98 patients (62.03%) experienced increased liver stiffness (mean change, 3.8 ± 1.3 kPa) and 60 patients (37.97%) had decreased liver stiffness (mean change, 2.4 ± 1.1 kPa) within 12 months following surgery (dynamic changes are shown in Figure 2). In patients categorised according to postoperative increase or decrease in liver stiffness, there was a statistically significant between-group difference in platelet count only (P = 0.04; Table 2).
Figure 2.
Dynamic changes in liver stiffness values from baseline (before surgery) to within 1 year after surgery in 158 patients with hepatitis B virus-related hepatocellular carcinoma. Mean ± SD liver stiffness was 8.53 ± 2.41 Kpa at baseline and 9.26 ± 2.29 Kpa post-surgery; 98 patients (62.03%) experienced increased liver stiffness (mean change, 3.8 ± 1.3 kPa) and 60 patients (37.97%) had decreased liver stiffness (mean change, 2.4 ± 1.1 kPa) following surgery.
Table 2.
Comparison of baseline variables in 158 patients with increased or decreased liver stiffness following surgery.
| Variable | Study subgroup |
Statistical significance | |
|---|---|---|---|
| Increased LS | Decreased LS | ||
| Patients | 98 (62.03) | 60 (37.97) | |
| Sex, male | 71 (72.45) | 52 (86.67) | NS |
| Age, years | 48 (27–73) | 50 (22–74) | NS |
| Fibrosis | 68 (69.39) | 39 (65.00) | NS |
| CTP class | NS | ||
| A | 89 (90.82) | 58 (96.67) | |
| B | 9 (9.18) | 2 (3.33) | |
| C | 0 | 0 | |
| Tumour maximum size, cm | 4.7 (2.4–8) | 3.3 (1–8) | NS |
| HBV DNA, log copies/ml | 3.7 (3.0–4.7) | 3.9 (3.0–5.3) | NS |
| HBeAg positive | 61 (62.24) | 31 (58.33) | NS |
| AFP, ng/ml | 379.42 (2.56–1210) | 319.87 (1.19–1210) | NS |
| TBIL, mmol/l | 4.78 (2.60–24.70) | 7.41 (3.90–21.60) | NS |
| ALT, IU/l | 21 (11–38) | 27 (19–46) | NS |
| AST, IU/l | 28 (17–46) | 18 (17–58) | NS |
| ALP, IU/l | 71 (47–203) | 58 (45–102) | NS |
| ALB, g/l | 4.7 (2.8–5.7) | 3.9 (2.8–6.1) | NS |
| Platelet count, ×109/l | 189.77 (103–267) | 213.52 (89–241) | P = 0.04 |
| Prothrombin time, s | 13.2 (11.0–14.7) | 12.6 (11.0–15.6) | NS |
| Differentiation | NS | ||
| Well–moderate | 48 (49.0) | 31 (51.7) | |
| Poor–undifferentiated | 50 (51.0) | 29 (48.3) | |
| Vascular invasion | NS | ||
| Yes | 22 (22.4) | 16 (26.7) | |
| No | 76 (77.6) | 44 (73.3) | |
| INR | 1.01 (0.87–1.54) | 0.88 (0.78–1.21) | NS |
Data presented as n (%) prevalence or median (range).
AFP, α-fetoprotein; ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTP, Child–Turcotte–Pugh score; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; INR, international normalized ratio; LS, liver stiffness; TBIL, total bilirubin.
NS, no statistically significant between-group difference (P > 0.05).
Prognostic performance of liver stiffness changes in patients with HCC
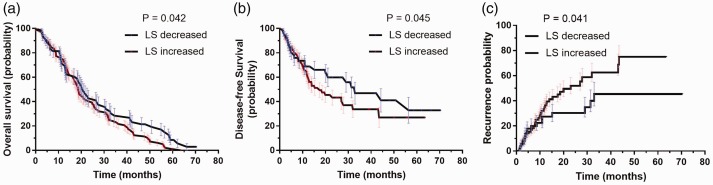
Patient outcomes were analysed using Kaplan–Meier estimator in patients categorised according to postoperative increase or decrease in liver stiffness, in order to investigate the potential difference in prognosis between the two groups. Patients with increased postoperative liver stiffness were revealed to have significantly worse overall survival outcomes than those with decreased postoperative liver stiffness (P = 0.042). Similar trends were observed for diseases-free survival and recurrence outcomes, which showed that patients with increase postoperative liver stiffness had significantly worse outcomes than those with decreased liver stiffness (P = 0.045 for disease-free survival and P = 0.041 for recurrence outcomes; Figure 3).
Figure 3.
Kaplan-Meier estimator curves in 158 patients with hepatitis B virus-related hepatocellular carcinoma, categorised according to increased or decreased postoperative liver stiffness (LS), showing: (a) significantly worse overall survival outcomes in patients with increased LS versus those with decreased LS (P = 0.042); (b) significantly worse diseases-free survival in patients with increased LS versus those with decreased LS (P = 0.045); and (c) significantly worse recurrence outcomes in patients with increased LS versus those with decreased LS (P = 0.041).
Univariate and multivariate analyses of prognostic variables in patients with HCC
The potential correlations between clinical parameters and overall survival, disease-free survival and recurrence were analysed using Cox proportional hazards model in patients with HCC. Univariate analyses showed that age, Child–Turcotte–Pugh score and postoperative change in liver stiffness were all prognostic variables for overall survival. Multivariate analysis revealed that only Child–Turcotte–Pugh score (hazard ratio [HR] 1.209, P = 0.039) and liver stiffness changes (HR 1.891, P = 0.042) were independent prognostic variables that were associated with overall survival (Table 3).
Table 3.
Univariate and multivariate analyses of prognostic variables for overall survival in patients with hepatitis B virus-related hepatocellular carcinoma.
| Variable | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | Statistical significance | HR | 95% CI | Statistical significance | |
| Age | 0.935 | 0.165, 0.974 | P = 0.004 | |||
| Sex | 0.805 | 0.236, 2.110 | NS | |||
| CTP score | 1.235 | 1.156, 2.502 | P = 0.011 | 1.209 | 1.010, 1.742 | P = 0.039 |
| HBV DNA | 0.905 | 0.818, 1.290 | NS | |||
| HBeAg status | 1.606 | 0.270, 1.962 | NS | |||
| AFP level | 1.604 | 0.516, 2.021 | NS | |||
| Platelet count | 1.363 | 0.296, 2.366 | NS | |||
| Total bilirubin | 2.129 | 0.724, 2.363 | NS | |||
| LS changes | 2.112 | 1.839, 2.326 | P = 0.003 | 1.891 | 1.441, 2.620 | P = 0.042 |
AFP, α-fetoprotein; CTP, Child–Turcotte–Pugh score; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; LS, liver stiffness; HR, hazard ratio; CI, confidence interval.
NS, no statistically significant correlation (P > 0.05; Cox proportional hazards).
To evaluate whether postoperative change in liver stiffness was an independent risk factor for disease-free survival and recurrence outcomes, both univariate and multivariate analyses were conducted (Table 4 and Table 5). Multivariate analyses showed that α-fetoprotein (AFP) level (HR 1.210, P = 0.029) and change in liver stiffness (HR 1.521, P = 0.040) were independent prognostic variables for HCC disease-free survival (Table 4) while serum HBV DNA viral load (HR 1.011, P = 0.040), AFP level (HR 1.929, P = 0.035) and change in liver stiffness (HR 1.052, P = 0.032) were independent prognostic variables for HCC recurrence (Table 5).
Table 4.
Univariate and multivariate analyses of prognostic variables for disease-free survival in patients with hepatitis B virus-related hepatocellular carcinoma.
| Variable | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | Statistical significance | HR | 95% CI | Statistical significance | |
| Age | 1.001 | 0.991, 1.012 | NS | |||
| Sex | 0.972 | 0.670, 1.594 | NS | |||
| CTP score | 0.662 | 0.221, 0.976 | P = 0.037 | |||
| HBV DNA | 0.495 | 0.330, 1.109 | NS | |||
| HBeAg status | 1.230 | 1.065, 1.692 | P = 0.021 | |||
| AFP level | 1.291 | 1.061, 2.182 | P = 0.015 | 1.210 | 1.115, 1.692 | P = 0.029 |
| Platelet count | 1.211 | 1.067, 1.877 | P = 0.027 | |||
| Total bilirubin | 1.159 | 0.698, 1.912 | NS | |||
| LS changes | 1.530 | 1.051, 2.248 | P = 0.035 | 1.521 | 1.074, 2.081 | P = 0.040 |
AFP, α-fetoprotein; CTP, Child–Turcotte–Pugh score; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; LS, liver stiffness; HR, hazard ratio; CI, confidence interval.
NS, no statistically significant correlation (P > 0.05; Cox proportional hazards).
Table 5.
Univariate and multivariate analyses of prognostic variables for recurrence in patients with hepatitis B virus-related hepatocellular carcinoma.
| Variable | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | Statistical significance | HR | 95% CI | Statistical significance | |
| Age | 0.172 | 0.129, 1.284 | NS | |||
| Sex | 1.201 | 0.882, 1.729 | NS | |||
| CTP score | 1.209 | 1.019, 1.812 | P = 0.025 | |||
| HBV DNA | 1.029 | 1.008, 1.892 | P = 0.037 | 1.011 | 1.002, 1.928 | P = 0.040 |
| HBeAg status | 0.662 | 0.197, 1.236 | NS | |||
| AFP level | 1.861 | 1.081, 2.162 | P = 0.029 | 1.929 | 1.028, 2.788 | P = 0.035 |
| Platelet count | 1.191 | 0.772, 1.962 | NS | |||
| Total bilirubin | 1.099 | 0.294, 1.621 | NS | |||
| LS changes | 1.012 | 1.008, 1.425 | P = 0.012 | 1.052 | 1.005, 1.752 | P = 0.032 |
AFP, α-fetoprotein; CTP, Child–Turcotte–Pugh score; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; LS, liver stiffness; HR, hazard ratio; CI, confidence interval.
NS, no statistically significant correlation (P > 0.05; Cox proportional hazards).
Discussion
The present study demonstrated that increased postoperative liver stiffness may be a potential risk factor for poor HCC outcomes in patients with HBV-related HCC. The study showed that patients with decreased postoperative liver stiffness values had better survival outcomes than patients with elevated liver stiffness values. In addition, Child–Turcotte–Pugh score and liver stiffness changes were found to be independent variables associated with overall survival. AFP level and liver stiffness changes were found to be independent variables associated with HCC disease-free survival while serum HBV DNA viral load, AFP level and liver stiffness changes were independent variables associated with HCC recurrence. As such, these factors may have prognostic value in patients with HBV-related HCC.
Although liver histology is useful regarding diagnosis of hepatic diseases, such as cirrhosis, the invasive nature of the procedure limits its wider use in clinical practice.29,30 FibroScan™ (Echosens) has been routinely applied for liver diseases detection worldwide, and allows clinicians to quantitatively evaluate the status of the liver.31 As a non-invasive procedure, FibroScan™ is also suitable for patients following liver resections. Previous advances have indicated that elevated liver stiffness is associated with HCC, but the mechanisms for liver regeneration after surgery are complicated in patients with underlying HBV infection.32 Postoperative screening with transient elastography provides a reproducible method of quantification to assess the alteration of liver regeneration, as well as potential impact on the prognosis of patients with HCC.33 The present study found a correlation between dynamic changes of liver stiffness and prognosis in patients with HBV-related HCC, which may be utilized as a dynamic approach to monitor HCC or evaluate the recovery of patients.
Several markers have been identified as effective in evaluating prognosis in patients with HCC.34–36 For example, AFP has been widely reported as a prognostic marker in patients with HCC,37–39 and is a serum HCC marker that is used to diagnosis and monitor HCC progression. However, AFP levels are not only elevated in HCC. Other diseases, such as those involving tumours of the female reproductive system, may also manifest with elevated AFP levels.40,41 Although high postoperative AFP levels are associated with poorer HCC prognosis, there is a certain proportion of patients with HCC who are AFP negative.42 For those AFP-negative patients with HCC, AFP is not suitable as a prognostic marker. According to the present study, patients with HCC with abnormal or normal AFP levels may instead be effectively stratified using liver stiffness measured by transient elastography.
The present study results may be limited by several factors. The relatively small sample size may limit the generalizability of the results, and the retrospective study design may have biased the results. In the present study, the antiviral treatment was determined by clinical experts at Yantai Infectious Diseases Hospital, liver stiffness was measured at Jining No.1 People’s Hospital, and HCC diagnosis and surgery were performed at Heze Municipal Hospital. In addition, patients included in the present study received various different treatments following surgery, for example, some received no further treatment following radical resection, while some were treated with sorafenib, and these factors were not included in the present analyses. All of these additional variables may have affected the present results, and whether or not postoperative treatment may further improve the prognosis of patients with HCC remains unclear. Data were collected and analysed at a single centre. Larger multicentre prospective studies are required to validate the present results and further investigate the value of liver stiffness as a prognostic marker in patients with HCC.
In conclusion, the present study demonstrated an association between dynamic change in liver stiffness and prognosis in patients with HCC. Liver stiffness measurement with transient elastography may allow the evaluation of outcomes in patients with HCC, and thus, may be used to guide routine follow-up for these patients. In particular, patients with HCC with increased liver stiffness following surgery should undergo more frequent follow-up examinations, even if the most recent exam following liver resection indicates no cause for concern.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs
Youde Liu https://orcid.org/0000-0001-5313-7367
Yinghua Zhang https://orcid.org/0000-0002-0191-3296
References
- 1.European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018; 69: 182–236. [DOI] [PubMed] [Google Scholar]
- 2.Bertuccio P, Turati F, Carioli G, et al. Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol 2017; 67: 302–309. [DOI] [PubMed] [Google Scholar]
- 3.Chaudhari VA, Khobragade K, Bhandare M, et al. Management of fibrolamellar hepatocellular carcinoma. Chin Clin Oncol 2018; 7: 51. [DOI] [PubMed] [Google Scholar]
- 4.Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology 2019; 156: 477–491.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitin T, Degnin C, Chen Y, et al. Radiotherapy for hepatocellular carcinoma in Russia: a survey-based analysis of current practice and the impact of an educational workshop on clinical expertise. J Cancer Educ 2020; 35: 105–111. [DOI] [PubMed] [Google Scholar]
- 6.Yu MW, Chang HC, Chen PJ, et al. Increased risk for hepatitis B-related liver cirrhosis in relatives of patients with hepatocellular carcinoma in northern Taiwan. Int J Epidemiol 2002; 31: 1008–1015. [DOI] [PubMed] [Google Scholar]
- 7.Cai SH, Lu SX, Liu LL, et al. Increased expression of hepatocyte nuclear factor 4 alpha transcribed by promoter 2 indicates a poor prognosis in hepatocellular carcinoma. Therap Adv Gastroenterol 2017; 10: 761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai SH, Lv FF, Zhang YH, et al. Dynamic comparison between Daan real-time PCR and Cobas TaqMan for quantification of HBV DNA levels in patients with CHB. BMC Infect Dis 2014; 14: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai S, Cao J, Yu T, et al. Effectiveness of entecavir or telbivudine therapy in patients with chronic hepatitis B virus infection pre-treated with interferon compared with de novo therapy with entecavir and telbivudine. Medicine (Baltimore) 2017; 96: e7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ou H, Cai S, Liu Y, et al. A noninvasive diagnostic model to assess nonalcoholic hepatic steatosis in patients with chronic hepatitis B. Therap Adv Gastroenterol 2017; 10: 207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu X, Cai S, Li Z, et al. Potential effects of telbivudine and entecavir on renal function: a systematic review and meta-analysis. Virol J 2016; 13: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu CM, Liaw YF. Hepatitis B virus-related cirrhosis: natural history and treatment. Semin Liver Dis 2006; 26: 142–152. [DOI] [PubMed] [Google Scholar]
- 13.Chen YC, Chu CM, Yeh CT, et al. Natural course following the onset of cirrhosis in patients with chronic hepatitis B: a long-term follow-up study. Hepatol Int 2007; 1: 267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai S, Li Z, Yu T, et al. Serum hepatitis B core antibody levels predict HBeAg seroconversion in chronic hepatitis B patients with high viral load treated with nucleos(t)ide analogs. Infect Drug Resist 2018; 11: 469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang FS, Fan JG, Zhang Z, et al. The global burden of liver disease: the major impact of China. Hepatology 2014; 60: 2099–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai S, Ou Z, Liu D, et al. Risk factors associated with liver steatosis and fibrosis in chronic hepatitis B patient with component of metabolic syndrome. United European Gastroenterol J 2018; 6: 558–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemoine M, Shimakawa Y, Njie R, et al. Food intake increases liver stiffness measurements and hampers reliable values in patients with chronic hepatitis B and healthy controls: the PROLIFICA experience in The Gambia. Aliment Pharmacol Ther 2014; 39: 188–196. [DOI] [PubMed] [Google Scholar]
- 19.Arena U, Vizzutti F, Corti G, et al. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology 2008; 47: 380–384. [DOI] [PubMed] [Google Scholar]
- 20.Xue X, Cai S. Comment on “Assessment of liver stiffness in pediatric Fontan patients using transient elastography”. Can J Gastroenterol Hepatol 2016; 2016: 9343960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng J, Cai S, Liu J, et al. Dynamic changes in liver stiffness measured by transient elastography predict clinical outcomes among patients with chronic hepatitis B. J Ultrasound Med 2017; 36: 261–268. [DOI] [PubMed] [Google Scholar]
- 22.Adler M, Larocca L, Trovato FM, et al. Evaluating the risk of hepatocellular carcinoma in patients with prominently elevated liver stiffness measurements by FibroScan: a multicentre study. HPB (Oxford) 2016; 18: 678–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foucher J, Chanteloup E, Vergniol J, et al. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut 2006; 55: 403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masuzaki R, Tateishi R, Yoshida H, et al. Prospective risk assessment for hepatocellular carcinoma development in patients with chronic hepatitis C by transient elastography. Hepatology 2009; 49: 1954–1961. [DOI] [PubMed] [Google Scholar]
- 25.Wu D, Chen E, Liang T, et al. Predicting the risk of postoperative liver failure and overall survival using liver and spleen stiffness measurements in patients with hepatocellular carcinoma. Medicine (Baltimore) 2017; 96: e7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973; 60: 646–649. [DOI] [PubMed] [Google Scholar]
- 27.Wang ZY, Tao QF, Wang ZH, et al. Antiviral therapy improves post-operative survival outcomes in patients with HBV-related hepatocellular carcinoma of less than 3cm - A retrospective cohort study. Am J Surg Epub ahead of print 13 June 2019. DOI: 10.1016/j.amjsurg.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 28.Huang G, Lau WY, Wang ZG, et al. Antiviral therapy improves postoperative survival in patients with hepatocellular carcinoma: a randomized controlled trial. Ann Surg 2015; 261: 56–66. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Tsao G, Friedman S, Iredale J, et al. Now there are many (stages) where before there was one: in search of a pathophysiological classification of cirrhosis. Hepatology 2010; 51: 1445–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hytiroglou P, Snover DC, Alves V, et al. Beyond “cirrhosis”: a proposal from the International Liver Pathology Study Group. Am J Clin Pathol 2012; 137: 5–9. [DOI] [PubMed] [Google Scholar]
- 31.Feier D, Lupsor Platon M, Stefanescu H, et al. Transient elastography for the detection of hepatocellular carcinoma in viral C liver cirrhosis. Is there something else than increased liver stiffness? J Gastrointestin Liver Dis 2013; 22: 283–289. [PubMed] [Google Scholar]
- 32.Wong GL, Chan HL, Wong CK, et al. Liver stiffness-based optimization of hepatocellular carcinoma risk score in patients with chronic hepatitis B. J Hepatol 2014; 60: 339–345. [DOI] [PubMed] [Google Scholar]
- 33.Jung KS, Kim SU, Choi GH, et al. Prediction of recurrence after curative resection of hepatocellular carcinoma using liver stiffness measurement (FibroScan®). Ann Surg Oncol 2012; 19: 4278–4286. [DOI] [PubMed] [Google Scholar]
- 34.Li D, Satomura S. Biomarkers for hepatocellular carcinoma (HCC): an update. Adv Exp Med Biol 2015; 867: 179–193. [DOI] [PubMed] [Google Scholar]
- 35.Cai S, Yu T, Jiang Y, et al. Comparison of entecavir monotherapy and de novo lamivudine and adefovir combination therapy in HBeAg-positive chronic hepatitis B with high viral load: 48-week result. Clin Exp Med 2016; 16: 429–436. [DOI] [PubMed] [Google Scholar]
- 36.Xiao YB, Cai SH, Liu LL, et al. Decreased expression of peroxisome proliferator-activated receptor alpha indicates unfavorable outcomes in hepatocellular carcinoma. Cancer Manag Res 2018; 10: 1781–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Komorowski AL, Hsu CC, Julka KD, et al. AFP role in predicting recurrence of hepatocellular carcinoma after living donor liver transplantation in HCV patients. Neoplasma 2018; 65: 455–460. [DOI] [PubMed] [Google Scholar]
- 38.Lim TS, Kim DY, Han KH, et al. Combined use of AFP, PIVKA-II, and AFP-L3 as tumor markers enhances diagnostic accuracy for hepatocellular carcinoma in cirrhotic patients. Scand J Gastroenterol 2016; 51: 344–353. [DOI] [PubMed] [Google Scholar]
- 39.Notarpaolo A, Layese R, Magistri P, et al. Validation of the AFP model as a predictor of HCC recurrence in patients with viral hepatitis-related cirrhosis who had received a liver transplant for HCC. J Hepatol 2017; 66: 552–559. [DOI] [PubMed] [Google Scholar]
- 40.Isonishi S, Ogura A, Kiyokawa T, et al. Alpha-fetoprotein (AFP)-producing ovarian tumor in an elderly woman. Int J Clin Oncol 2009; 14: 70–73. [DOI] [PubMed] [Google Scholar]
- 41.Lu JB, Cai SH, Pan YH, et al. Altered epidermal fatty acid-binding protein expression in hepatocellular carcinoma predicts unfavorable outcomes. Cancer Manag Res 2018; 10: 6275–6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shu H, Li W, Shang S, et al. Diagnosis of AFP-negative early-stage hepatocellular carcinoma using Fuc-PON1. Discov Med 2017; 23: 163–168. [PubMed] [Google Scholar]