Abstract
Background:
Due to their history of renal disease and exposure to immunosuppression, kidney transplant recipients with a failing graft may be at higher risk of adverse outcomes compared to nontransplant controls. Understanding the burden of disease in transplant recipients may inform treatment decisions of people whose native kidneys are failing and may be eligible for a transplant.
Objective:
To compare mortality and morbidity in kidney transplant recipients with a failing graft to matched nontransplant controls.
Design:
Retrospective cohort study.
Setting:
Alberta, Canada.
Patients:
Kidney transplant recipients with a failing graft were identified as having at least 2 estimated glomerular filtration rate (eGFR) measurements between 15-30 mL/min/1.73 m2 (90-365 days apart). We also identified nontransplant controls with a similar degree of kidney dysfunction.
Measurements:
Mortality and hospitalization.
Methods:
We propensity-score matched 520 kidney transplant recipients with a failing graft to 520 nontransplant controls.
Results:
The median age of the matched cohort was 57 years and 40% were women. Compared to matched nontransplant controls, recipients with a failing graft had a higher hazard of death (hazard ratio, 1.54; 95% confidence interval [CI], 1.28-1.85; p < .001) and a higher rate of all-cause hospitalization (rate ratio, 1.67; 95% CI, 1.42-1.97; p < .001). Kidney transplant recipients also had a higher rate of several cause-specific hospitalizations including genitourinary, cardiovascular, and infectious causes.
Limitations:
Observational design with the risk of residual confounding.
Conclusions:
A failing kidney transplant is associated with an increased burden of mortality and morbidity beyond chronic kidney disease. This information may assist the discussion of prognosis in kidney transplant recipients with a failing graft and the design of strategies to minimize risks.
Keywords: Alberta, CKD (chronic kidney disease), estimated glomerular filtration rate, kidney transplantation, mortality
Abrégé
Contexte:
En raison de leurs antécédents de néphropathie et de leur exposition aux immunosuppresseurs, les receveurs d’une greffe rénale dont le greffon est défaillant pourraient être plus susceptibles de souffrir de pathologies associées que les patients non transplantés (contrôles). Comprendre le fardeau de la maladie pour les receveurs d’une greffe pourrait orienter les décisions de traitement pour les patients dont les reins natifs sont défaillants et qui sont admissibles à une greffe.
Objectif:
Comparer la mortalité et les comorbidités de receveurs d’une greffe rénale dont le greffon est défaillant à celles de patients non greffés (contrôles).
Type d’étude:
Étude de cohorte rétrospective.
Cadre:
Alberta, Canada.
Sujets:
Le statut de receveur avec greffon défaillant a été établi par au moins deux mesures de DFGe se situant entre 15 et 30 ml/min/1.73 m2 (de 90 à 365 jours d’intervalle). Des patients non greffés présentant un dysfonctionnement rénal similaire ont servi de contrôles.
Mesures:
Mortalité et nombre d’hospitalisations.
Méthodologie:
Nous avons jumelé 520 receveurs avec greffon défaillant à 520 patients non greffés sur la base du score de propension.
Résultats:
L’âge médian des sujets était de 57 ans et 40 % étaient des femmes. Les patients avec un greffon défaillant ont présenté un risque de mortalité (rapport de risque : 1.54; IC 95 % : 1.28-1.85; p < .001) et un taux d’hospitalization toutes causes confondues (rapport des taux : 1.67; IC 95%, 1.42-1.97; p < .001) plus élevés que les patients non greffés. Ils étaient également hospitalisés plus fréquemment, notamment pour des problèmes génito-urinaires ou cardiovasculaires, ou pour des infections.
Limites:
La nature observationnelle de l’étude pourrait comporter des facteurs de confusion résiduels.
Conclusion:
Une transplantation rénale défaillante a été associée à un plus grand risque de morbidité et de mortalité que l’insuffisance rénale chronique. Cette information pourrait orienter les discussions concernant le pronostic des receveurs d’un rein dont le greffon est défaillant et guider l’élaboration de stratégies pour minimiser les risques.
What was known before
Kidney transplant recipients with a failing graft are at a high risk of mortality and morbidity. Information to assist the discussion of prognosis and management of kidney transplant recipients with a failing graft is insufficient and largely indirect.
What this adds
Compared to matched nontransplant controls, recipients with a failing graft have a higher hazard of death (hazard ratio, 1.54; 95% confidence interval [CI], 1.28-1.85; p < .001) and a higher rate of all-cause hospitalization (rate ratio, 1.67; 95% CI, 1.42-1.97; p <.001). For the first time, this study provides measures of disease burden that can be used to inform kidney transplant recipients with a failing graft about their prognosis, discuss treatment options for people whose native kidneys are failing or have kidney failure and are eligible for a kidney transplant, and review transplant policies and strategies to minimize risks.
Introduction
Advances in immunosuppression regimens have improved short-term graft survival without significant changes to long-term graft survival.1,2 Most kidney transplants will fail at some point during the lifetime of the recipient, and 1 in 5 transplant recipients of a deceased-donor kidney will experience graft failure within 5 years of their transplant.2 During the period of graft dysfunction, the recipient may be at increasing risk of adverse events.
Information to assist the discussion of prognosis and management of kidney transplant recipients with a failing graft is insufficient and largely indirect. Available evidence is based on studies including people who survived to kidney failure and were treated with dialysis. In a systematic review, we found that the risk of mortality for kidney transplant recipients who received dialysis was highest in the first year after returning to dialysis (annual mortality: 12% in year 1 vs 5%-6% in years 2-4),3 suggesting a high risk of mortality during the transition from predialysis to postdialysis care. Unfortunately, there was insufficient information on cardiovascular and infection-related events in this population. We also found that outcomes of kidney transplant recipients with a failing graft not yet treated with dialysis were understudied. Understanding the disease burden of transplant recipients with a failing graft may help discussion about their prognosis and treatment decisions. In addition, this information may help discuss treatment options for people whose native kidneys are failing or have kidney failure and are eligible for a kidney transplant, and review transplant policies and strategies to minimize risks in transplant recipients.
In this study, we assessed clinically important outcomes in kidney transplant recipients with a failing graft. We examined the rate of death and hospitalizations in kidney transplant recipients with a failing graft and compared these outcomes to matched nontransplant controls with a similar degree of chronic kidney disease. We hypothesized that kidney transplant recipients with a failing graft may have an increased rate of complications compared to the nontransplant population.
Materials and Methods
Design and Setting
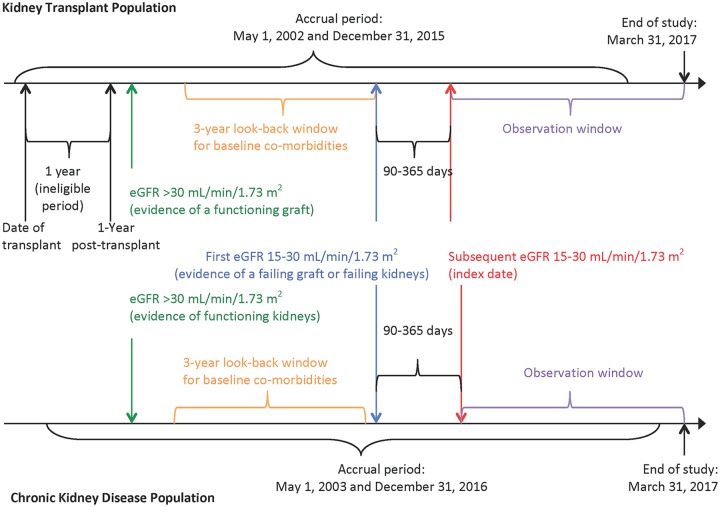
We conducted a retrospective population-based cohort study using linked healthcare databases within the Alberta Kidney Disease Network, which incorporates data from Alberta Health, the provincial health ministry.4 Over 99% of Alberta residents are registered with Alberta Health and have universal access to hospital care and physician services. We followed guidelines for the reporting of observational studies (Supplemental Table S1) and a protocol approved by the research ethics boards at the University of Alberta and the University of Calgary, with a waiver of patient consent. A schematic of the study design is presented as Figure 1.
Figure 1.
Study design.
Note. eGFR = estimated glomerular filtration rate.
Data Sources
We ascertained baseline characteristics, covariate information, and outcome data from database records (Supplemental Table S2). The Alberta Health database contains information on demographic data, vital statistics, and diagnostic and procedural information for inpatient and outpatient physician services. We identified kidney transplant recipients from the Northern and Southern Alberta Renal Program databases, which provide care to all patients treated with chronic dialysis or kidney transplantation in the province. We linked these data sources to a provincial laboratory repository via unique, encoded, patient identifiers.5,6 We used validated coding algorithms applied to physician claims and hospitalization data7,8 to define comorbidities at baseline based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and International Statistical Classification of Diseases, Tenth Revision (ICD-10). We identified comorbidities using one or more diagnostic or procedural codes in the 3 years prior to the index date or validated algorithms to capture diagnoses, such as hypertension and diabetes mellitus (Supplemental Table S2).9,10
Populations
Kidney transplant recipient population
We considered all prevalent kidney transplant recipients between May 1, 2002 and December 31, 2015 in Alberta. We excluded pediatric recipients (<18 years old), recipients with a previous organ transplant, and recipients who had received a simultaneous multiorgan transplant (eg, kidney-pancreas).
We used laboratory data to identify kidney transplant recipients who achieved a functioning graft posttransplant and then experienced a loss of graft function during the study period (Supplemental Figure S1). We calculated the estimated glomerular filtration rate (eGFR) using the Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) equation.11 Although data on race were not available, misclassification of eGFR was expected to be minimal since ~3% of the Alberta population are black.12 We included recipients who survived at least 1 year with a functioning graft defined as at least one eGFR >30 mL/min/1.73 m2 measurement after the first transplant year. We considered only eGFR measurements beyond the first year posttransplant to ensure stability of renal function and immunosuppression regimen.13-15 We excluded recipients who had graft failure (death or return to dialysis) within 1 year posttransplant or whose baseline eGFR was <30 mL/min/1.73 m2 throughout the entire follow-up period. From this initial cohort, we identified recipients who developed a loss of graft function based on at least 2 outpatient eGFR measurements between 15 and 30 mL/min/1.73 m2 that were obtained between 90 and 365 days apart. We excluded recipients who had an eGFR <15 mL/min/1.73 m2 or who received dialysis in between these 2 measurements. To maintain independence between matched samples, we also excluded transplant recipients who were captured in the control sample. We used the second of the 2 eGFR measurements as the index date for follow-up.
Chronic kidney disease population
We identified members of the general population in Alberta with a similar degree of chronic kidney disease between May 1, 2003 and December 31, 2016, to coincide with the eligible laboratory dates of the recipients (Supplemental Figure S2). As in the transplant recipient population, we identified adults (≥18 years old) with at least 2 outpatient eGFR measurements between 15 and 30 mL/min/1.73 m2 that were obtained between 90 and 365 days apart to ensure chronicity. The second of these 2 measurements was used as the index date for follow-up. We excluded patients who had evidence of a previous transplant or were on maintenance dialysis prior to their index date.
Matching
We used propensity score methods to match kidney transplant recipients with a failing graft to nontransplant controls with chronic kidney disease in a 1:1 ratio. We estimated the propensity score as the conditional probability of receiving a transplant using a logistic regression model in which we regressed transplant status on the following baseline covariates: age (and its squared term), sex, socioeconomic status (quintile of neighborhood income), location of residence (urban vs rural), distance from home to transplant center, year of cohort entry, index eGFR, index albuminuria, and baseline comorbidities. We modeled age, distance from home to transplant center, and index eGFR as continuous variables. To enhance group comparability, we specified exact matching for categories of index year (<2005, 2005-2010, and >2010), age (<65 and ≥65), sex (men and women), nonmetastatic cancer (present and absent), and albuminuria. Index albuminuria was defined by albumin-to-creatinine ratio (ACR), protein-to-creatinine ratio (PCR), or urine dipstick based on outpatient random spot urine measurements. Measurements were categorized as none/mild (A1: dipstick negative/trace, PCR <15 mg/mmol, ACR <30 mg/g), moderate (A2: dipstick 1+, PCR 15-50 mg/mmol, ACR 30-300 mg/g), or severe (A3/A4: dipstick ≥2+, PCR >50 mg/mmol, ACR >300 mg/g).16 ACR was the primary measure of albuminuria, and if unavailable, was supplemented with PCR. When both ACR and PCR were unavailable, urine dipstick was used. All outpatient ACR or PCR measurements or urine dipsticks in the 90 days before the index eGFR were used to establish baseline albuminuria. For those with multiple albuminuria measurements, we used the median value rounded down to the nearest category.
For matching, demographic data were complete except for socioeconomic status, location of residence, and index albuminuria (≤5% missing in the kidney transplant recipient cohort). Those with missing socioeconomic data were reclassified in the third (middle) quintile of neighborhood income and those with missing location of residence data were reclassified as urban. Due to its potential to indicate a lower level of quality of care, we treated missing index albuminuria as a separate category such that the resulting variable was categorical with 4 levels (ie, none/mild, moderate, severe/nephrotic, or missing). We matched transplant recipients to nontransplant controls on the logit of the propensity score using a caliper width equal to 0.2 of the standard deviation of the logit of the propensity score within categories defined by exact matching variables.17 We matched without replacement using a greedy nearest neighbor algorithm in random order. We compared differences in baseline characteristics between transplant recipients and nontransplant chronic kidney disease patients using graphical methods and standardized differences. A standardized difference less than 10% was considered to be indicative of a negligible difference between groups.18 We used the MatchIt package (version 3.0.2) in R (version 3.4.4) for matching.19,20
Outcomes
We followed participants from their index date until the first of death, emigration from the province, or end of study (March 31, 2017). The primary outcome was time to all-cause mortality. Secondary outcomes included all-cause hospitalization and hospitalization (most responsible diagnosis) for genitourinary (ICD-10: N00-N99), cardiovascular (ICD-10: I00-I99), infectious (ICD-10: A00-A99, B00-B99), cancer (ICD-10: C00-C97, D00-D48), endocrine (ICD-10: E00-E90), respiratory (ICD-10: J00-J99), and gastrointestinal (ICD-10: K00-K93) causes. In these analyses, we considered hospitalization counts (ie, each participant could have multiple hospitalizations).
Statistical Analysis
Analysis approach
We summarized mortality data using the Kaplan-Meier method. We estimated the hazard ratio (HR) for mortality and corresponding 95% confidence interval (CI) using Cox proportional hazards regression. We assessed model validity and goodness of fit by means of formal tests of significance and graphical methods based on residuals. We used negative binomial regression to compare rates of hospital admissions, by including in each count model an offset term representing the log of the time at risk. Individuals were considered not at risk while hospitalized for the outcome of interest. We accounted for the matched nature of the sample using robust variance estimation.21 We used STATA, version 14 (www.STATA.com) and R, version 1.1.442 (R-project.org) for all analyses.
Power considerations
Based on data from southern Alberta, we hypothesized that over the study period, approximately 500 transplant recipients would have met the eligibility criteria. Assuming a mortality rate of 8% per year in the nontransplant control group5 and an exponential distribution of the hazard function, we estimated that this study would have more than an 80% probability of detecting a between-group difference in mortality as low as 2% per year (eg, 8% vs 10%; HR exposed vs unexposed = 1.25) at the 1% significance level with a 2-sided test.
Results
Baseline Characteristics
There were 2723 prevalent kidney transplant recipients in Alberta, Canada between May 1, 2002 and December 31, 2015. Of these, 562 (21%) met the study inclusion criteria (Supplemental Figure S1) and were matched 1:1 with nontransplant controls with a similar degree of chronic kidney dysfunction (Supplemental Figure S2). The matching algorithm excluded 42 recipients (7%). Thus, the final cohort consisted of 520 kidney transplant recipients and 520 nontransplant controls. As expected, the 2 populations were substantially different prior to matching, particularly in terms of age and comorbidities such as heart failure, dementia, and cancer (Supplemental Table S3). After matching, the 2 groups were comparable with all measured standardized differences <10% (Table 1).
Table 1.
Baseline Characteristics of Kidney Transplant Recipients With a Failing Graft and Matched Nontransplant Patients With Chronic Kidney Disease at the Time of Cohort Entry.
| Characteristic | Transplant recipients (n = 520) | Nontransplant controls (n = 520) | Standardized differencea |
|---|---|---|---|
| Age, years | 56.6 (45.4-65.4) | 56.7 (46.3-65.6) | 2.2 |
| >65 years | 134 (26) | 134 (26) | 0 |
| Women | 208 (40) | 208 (40) | 0 |
| Socioeconomic statusb | |||
| Lowest quintile | 130 (25) | 115 (22) | 6.8 |
| Second quintile | 132 (25) | 133 (26) | 0.4 |
| Middle quintile | 106 (20) | 113 (22) | 3.3 |
| Fourth quintile | 68 (13) | 76 (15) | 4.5 |
| Highest quintile | 84 (16) | 83 (16) | 0.5 |
| Urban residencec | 450 (87) | 451 (87) | 0.6 |
| Distance to transplant center, kmd | 25.9 (13.5-164.4) | 23.4 (13.0-139.5) | 5.4 |
| <50 km | 329 (63) | 340 (65) | 4.4 |
| 50.1-150 km | 54 (10) | 61 (12) | 4.3 |
| 150.1-300 km | 67 (13) | 53 (10) | 8.4 |
| >300 km | 70 (13) | 66 (13) | 2.3 |
| Northern Alberta recipient | 363 (63) | N/A | N/A |
| Year of transplant | |||
| 1994-2000 | 178 (34) | N/A | N/A |
| 2001-2007 | 202 (39) | N/A | N/A |
| 2008-2015 | 58 (11) | N/A | N/A |
| Missing | 82 (16) | N/A | N/A |
| Transplant to index date, years | 7.0 (3.6-10.8) | N/A | N/A |
| Index date | |||
| 2002-2006 | 162 (31) | 155 (30) | 2.9 |
| 2007-2011 | 187 (36) | 196 (38) | 3.6 |
| 2012-2017 | 171 (33) | 169 (32) | 0.8 |
| Index eGFR, mL/min/1.73 m2 | 26.7 (24.1-28.4) | 27.0 (24.0-28.6) | 2.7 |
| 26-30 | 353 (68) | 362 (70) | 3.7 |
| 21-25 | 121 (23) | 114 (22) | 3.2 |
| 15-20 | 46 (9) | 44 (8) | 1.4 |
| Index albuminuria | |||
| None/mild | 152 (29) | 152 (29) | 0 |
| Moderate | 132 (25) | 132 (25) | 0 |
| Severe | 210 (40) | 210 (40) | 0 |
| No measurement | 26 (5) | 26 (5) | 0 |
| Comorbiditiese | |||
| Hypertension | 343 (66) | 354 (68) | 4.5 |
| Diabetes mellitus | 194 (37) | 198 (38) | 1.6 |
| Myocardial infarction | 31 (6) | 34 (7) | 2.4 |
| Percutaneous coronary intervention/coronary artery bypass graft | 21 (4) | 18 (3) | 3.0 |
| Heart failure | 63 (12) | 56 (11) | 4.2 |
| Atrial fibrillation | 35 (7) | 32 (6) | 2.4 |
| Stroke/transient ischemic attack | 38 (7) | 40 (8) | 1.5 |
| Peripheral vascular disease | 41 (8) | 45 (9) | 2.8 |
| Chronic pulmonary disease | 69 (13) | 60 (12) | 5.3 |
| Peptic ulcer disease | 15 (3) | 17 (3) | 2.2 |
| Liver disease | 17 (3) | 12 (2) | 5.8 |
| Dementia | 8 (2) | 10 (2) | 2.9 |
| Lymphoma | 10 (2) | 9 (2) | 1.4 |
| Cancer (nonmetastatic) | 34 (7) | 34 (7) | 0 |
| Cancer (metastatic) | 3 (1) | 3 (1) | 0 |
| Human immunodeficiency virus/acquired immunodeficiency syndrome | 2 (0) | 1 (0) | 3.6 |
Note. Data are presented as number (%) or as median (interquartile range). The time of cohort entry is the date of the second of 2 eligible estimated glomerular filtration rate measurements. N/A, not applicable.
Standardized differences provide a measure of the difference between groups divided by the pooled standard deviation; >10% is interpreted as a meaningful difference between the groups.
Income was categorized according to fifths of average neighborhood income (1 = low, 5 = high).
Urban indicates a population >10 000 or a population >1000 with population density >400/km2.
Values >500 km were imputed as 500 km.
Assessed by the presence of diagnostic or procedural codes in the 3 years prior to the index date, based on validated algorithms, where applicable (Supplemental Table S2).
The median age of the matched cohort was 57 years and 40% were women. The median time from transplant to the index date (ie, time to moderate-severe graft dysfunction) for the matched recipients was 7 years (interquartile range [IQR] 4-11) with an index eGFR of 27 mL/min/1.73 m2 (IQR, 24-28). Of these recipients, 66% of patients had a history of hypertension and 37% of patients had diabetes mellitus.
Mortality
After a median follow-up of 5 years (range, 0.01-14.2 years; 5824 patient-years at risk), 348 participants died. There were 206 (40%) deaths in the kidney transplant recipient group and 142 (27%) deaths in the matched nontransplant group (73 vs 47 deaths per 1000 person-years; Table 2). The median survival time was 4.9 years among the transplant recipients and 5.4 years among the nontransplant controls. The hazard for death was constant over follow-up time. Figure 2 shows the survival probabilities over time. The hazard of death was 54% higher in kidney transplant recipients compared to matched nontransplant controls (HR, 1.54; 95% CI, 1.28-1.85; p < .001).
Table 2.
Mortality and Morbidity in Kidney Transplant Recipients (n = 520) and Matched Nontransplant Patients (n = 520).
| Number of events | Events per 1000 person-years (95% CI) | Hazard or rate ratio (95% CI)a | P-value | |
|---|---|---|---|---|
| All-cause mortality | ||||
| Transplant recipients | 206 | 73 (64-84) | 1.54 (1.28-1.85) | <.001 |
| Nontransplant controls | 142 | 47 (40-56) | ||
| All-cause hospitalizations | ||||
| Transplant recipients | 2508 | 1202 (1051-1354) | 1.67 (1.42-1.97) | <.001 |
| Nontransplant controls | 1770 | 720 (631-810) | ||
| Genitourinary hospitalizations | ||||
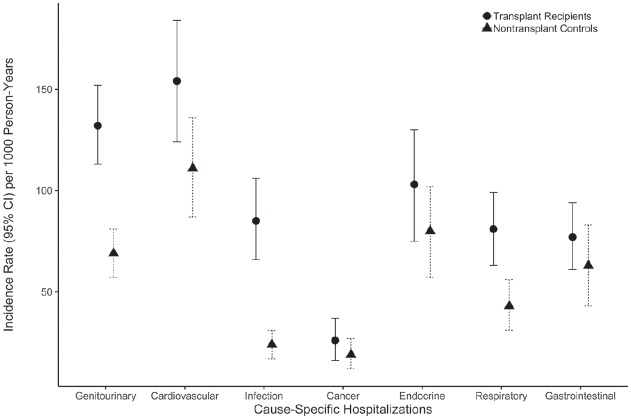
| Transplant recipients | 353 | 132 (113-152) | 1.91 (1.51-2.41) | <.001 |
| Nontransplant controls | 208 | 69 (57-81) | ||
| Cardiovascular hospitalizations | ||||
| Transplant recipients | 355 | 154 (124-184) | 1.38 (1.04-1.84) | .024 |
| Nontransplant controls | 276 | 111 (87-136) | ||
| Infection hospitalizations | ||||
| Transplant recipients | 212 | 85 (66-106) | 3.52 (2.47-5.01) | <.001 |
| Nontransplant controls | 71 | 24 (17-31) | ||
| Cancer hospitalizations | ||||
| Transplant recipients | 62 | 26 (16-37) | 1.37 (0.78-2.39) | .27 |
| Nontransplant controls | 53 | 19 (12-27) | ||
| Endocrine hospitalizations | ||||
| Transplant recipients | 244 | 103 (75-130) | 1.29 (0.88-1.89) | .19 |
| Nontransplant controls | 215 | 80 (57-102) | ||
| Respiratory hospitalizations | ||||
| Transplant recipients | 203 | 81 (63-99) | 1.87 (1.30-2.69) | .001 |
| Nontransplant controls | 111 | 43 (31-56) | ||
| Gastrointestinal hospitalizations | ||||
| Transplant recipients | 207 | 77 (61-94) | 1.22 (0.84-1.77) | .29 |
| Nontransplant controls | 187 | 63 (43-83) | ||
| Other hospitalizations | ||||
| Transplant recipients | 872 | 354 (307-400) | 1.46 (1.21-1.77) | <.001 |
| Nontransplant controls | 649 | 241 (207-277) | ||
Cox proportional hazard regression models were used to calculate the hazard ratio with 95% CI for death and negative binomial regression was used to compare hospitalization rate ratios with 95% CI. CI = confidence interval.
Figure 2.
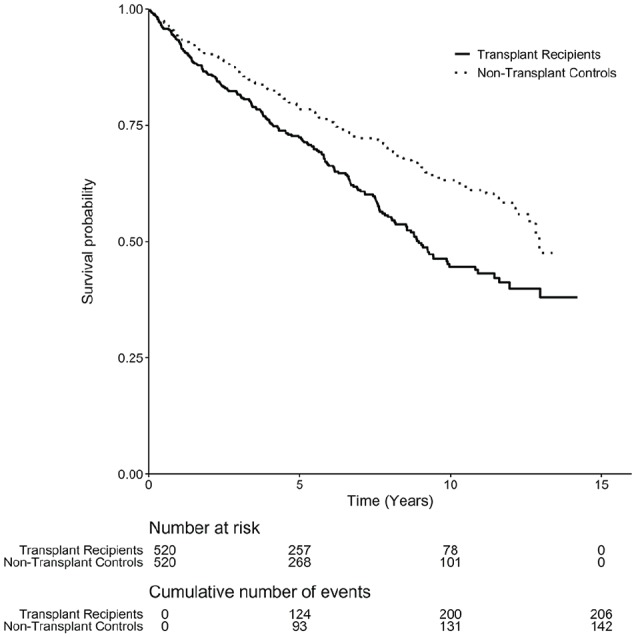
Kaplan-Meier estimated survival probabilities stratified by transplant status.
Morbidity
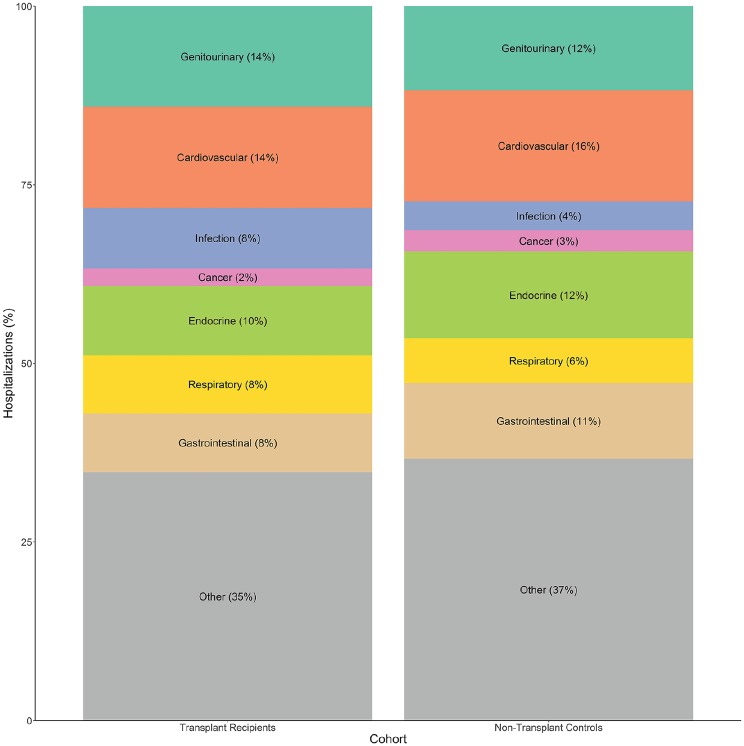
The rate of all-cause hospitalization was 67% higher in kidney transplant recipients compared to matched nontransplant controls (rate ratio [RR], 1.67; 95% CI, 1.42-1.97; p < .001). Kidney transplant recipients also had significantly higher rates of hospitalization for several causes (Table 2), including genitourinary, cardiovascular, infectious, and respiratory disease causes (Figures 3 and 4). There were no statistically significant differences between the transplant recipients and nontransplant controls with respect to the rate of hospitalization for cancer, endocrine, and gastrointestinal causes.
Figure 3.
Cause-specific hospital admission rates (most responsible diagnosis, bars indicate 95% CI) per 1000 person-years by transplant status.
Note. CI = confidence interval.
Figure 4.
Proportion of cause-specific hospitalizations (most responsible diagnosis) by transplant status.
Discussion
In this study of 520 kidney transplant recipients with a failing graft, we found that both mortality and morbidity were higher compared to matched nontransplant controls with a similar degree of chronic kidney disease. With the exception of cancer, endocrine, and gastrointestinal causes, kidney transplant recipients with a failing graft experienced a significantly higher rate of hospitalizations for various causes, especially infections. Our findings suggest that mortality and morbidity is higher amongst patients with a kidney transplant compared to moderate-severe nondialysis-dependent chronic kidney disease. These results are likely to be of even great significance in the future given that transplant centers are increasingly accepting recipient candidates who are older and have more comorbidities.1,22 As a result, the population of kidney transplant recipients with a failing graft will be older and more medically complex than ever and may require specialized care to mitigate the risk of mortality and morbidity.
To our knowledge, this is one of the largest studies comparing the outcomes of kidney transplant recipients with a failing graft to nontransplant controls with a similar degree of chronic kidney disease. Previous studies have shown that in the chronic kidney disease population and kidney transplant recipient population, decreased kidney function is associated with an increased risk of all-cause mortality.5,6,23 For kidney transplant recipients, poor graft function at 1 year is associated with long-term posttransplant death and graft loss.6,13 In our study, we report on the risk of death for recipients who initially had good graft function after their first transplant year, and subsequently developed poor graft function in follow-up. We observed a 54% higher mortality in transplant recipients compared to nontransplant controls. Thus, our results extend upon previous findings by showing that there is a mortality risk for recipients who have moderate-severe graft dysfunction, not just for those who experience complete graft failure.3,24
In addition to all-cause mortality, Meier-Kriesche et al reported on 58 900 kidney transplant recipients between 1988 and 1998 and found that recipients with higher serum creatinine values at 1-year posttransplant had progressively increased risk of cardiovascular deaths and infectious deaths, but not malignancy-related deaths.25 While we did not assess cause-specific death in our study due to potential measurement error, we did find a similar pattern in our morbidity outcome, as recipients with a failing graft had a higher risk of hospitalization due to cardiovascular events and infection, but not cancer.
Hospitalizations are associated with morbidity and significant costs to the healthcare system. A 2013 study of 6079 Canadian kidney transplant recipients from 2001 to 2008 reported that the risk of all-cause hospitalization was 6 times higher than the nontransplant general population.26 Compared to the general population, recipients had a higher risk of hospitalizations due to genitourinary disease (standardized hospitalization ratios [SHR] 18), circulatory disease (SHR 4), infectious disease (SHR 31), and cancer (SHR 3). In this study, recipients with a failing graft had an increased rate of all-cause hospitalization and various cause-specific hospitalizations when compared to nontransplant controls with chronic kidney disease. This may be due to the prolonged history of chronic kidney disease for recipients as well as the continued exposure to immunosuppression. Although the rate of hospitalization due to cancer was higher in recipients with a failing graft compared to nontransplant controls with chronic kidney disease, it did not reach statistical significance (Table 2). Despite being at higher risk of skin cancers and viral-related cancers, such as posttransplant lymphoproliferative disorder, transplant recipients may be at lower risk of developing other types of cancers, such as prostate and breast cancer, due to the rigorous screening and evaluation process for transplant candidacy.27 We found similar associations with endocrine and gastrointestinal causes.
Our study has a number of strengths including the identification and follow-up of more than 500 kidney transplant recipients with a failing graft based on multiple serum creatinine and eGFR measurements in a large Canadian province. We also compared our outcomes to nontransplant controls with a similar degree of chronic kidney disease to assess the excess risk among transplant recipients with severe nondialysis-dependent chronic kidney disease. Our rich data sources allowed us to apply eligibility criteria that maximized the identification of stable patients with sustained chronic deterioration of kidney function and link this information to important clinical outcomes using validated algorithms. There are limitations worth noting. First, given its observational nature, our study remains at risk of bias due to residual confounding. While we used recommended methods to minimize the impact of measured confounding, only a randomized design would allow inference about causation. Second, we lacked data on certain characteristics such as smoking, blood pressure control, and body mass index, transplant-related factors, such as cause of kidney failure, as well as medical history variables such as medication use and kidney biopsy results or pathology. However, we were able to identify and control for several other important confounders associated with mortality and morbidity. We used broad codes to characterize cause-specific hospitalizations, which may lead to misclassification; however, we would not expect this to be significantly different between the recipient and CKD groups. Finally, our study population had universal access to specialist care, and thus our findings may not be generalizable to health systems without universal access to care.
In summary, among 520 kidney transplant recipients with a failing graft, the rate of mortality and all-cause hospitalization was higher than nontransplant controls with a similar degree of chronic kidney disease. This information can be used to assist the discussion of prognosis in kidney transplant recipients with a failing graft. These data can also be used to assist decision making about treatment options for transplant failure and the design of clinical strategies to minimize the risk and management of complications in this patient population.
Supplemental Material
Supplemental material, RTx_Failing_-_Appendix_-_20190528 for Mortality and Morbidity in Kidney Transplant Recipients With a Failing Graft: A Matched Cohort Study by Ngan N. Lam, Devon J. Boyne, Robert R. Quinn, Peter C. Austin, Brenda R. Hemmelgarn, Patricia Campbell, Gregory A. Knoll, Lee Anne Tibbles, Serdar Yilmaz, Hude Quan and Pietro Ravani in Canadian Journal of Kidney Health and Disease
Acknowledgments
This study is based in part on data provided by Alberta Health and Alberta Health Services. The interpretation and conclusions contained herein are those of the researchers and do not necessarily represent the views of the Government of Alberta or Alberta Health Services. Neither the Government of Alberta nor, Alberta Health or Alberta Health Services express any opinion in relation to this study. We are not able to make our data set available to other researchers due to our contractual arrangements with the provincial health ministry (Alberta Health), who is the data custodian. Researchers may make requests to obtain a similar data set at https://albertainnovates.ca/programs/strategy-for-patient-oriented-research/.
Footnotes
List of Abbreviations: ACR, albumin-to-creatinine ratio; CDK-EPI, Chronic Kidney Disease-Epidemiology Collaboration; CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; ICD-10, International Statistical Classification of Diseases, Tenth Revision; IQR, interquartile range; PCR, protein-to-creatinine ratio; RR, rate ratio; SHR, standardized hospitalization ratios.
Ethics Approval and Consent to Participate: This study was approved by the institutional review board at the University of Alberta and the University of Calgary.
Consent for Publication: All authors consent to the publication of this study.
Availability of Data and Materials: The data analyzed during this study are available from the corresponding author on reasonable request.
Author Contributions: N.N.L., R.R.Q., and P.R. participated in research design. D.J.B. and P.R. participated in data analysis. N.N.L. drafted and revised the manuscript. All authors were involved in data interpretation and final approval of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article. This study was supported by a Canadian Institutes of Health Research (CIHR) Operative grant (grant no. FRN 142314). D.J.B. is supported by the Barrie I Strafford Doctoral Scholarship for Interdisciplinary Studies on Aging. Dr. Austin is supported by a Mid-Career Investigator Award from the Heart and Stroke Foundation.
ORCID iDs: Ngan N. Lam  https://orcid.org/0000-0002-0129-7091
https://orcid.org/0000-0002-0129-7091
Pietro Ravani  https://orcid.org/0000-0001-6973-8570
https://orcid.org/0000-0001-6973-8570
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Knoll G. Trends in kidney transplantation over the past decade. Drugs. 2008;68(suppl 1):3-10. [DOI] [PubMed] [Google Scholar]
- 2. Canadian Organ Replacement Register. Annual report: Treatment of end-stage organ failure in Canada, 2006-2015. https://www.cihi.ca/en/corr-annual-statistics-2017. Published August 30, 2017. Accessed February 10, 2020.
- 3. Kabani R, Quinn RR, Palmer S, et al. Risk of death following kidney allograft failure: a systematic review and meta-analysis of cohort studies. Nephrol Dial Transplant. 2014;29(9):1778-1786. [DOI] [PubMed] [Google Scholar]
- 4. Hemmelgarn BR, Clement F, Manns BJ, et al. Overview of the Alberta Kidney Disease Network. BMC Nephrol. 2009;10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303:423-429. [DOI] [PubMed] [Google Scholar]
- 6. Lam NN, Tonelli M, Lentine KL, et al. Albuminuria and posttransplant chronic kidney disease stage predict transplant outcomes. Kidney Int. 2017;92(2):470-478. [DOI] [PubMed] [Google Scholar]
- 7. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. [DOI] [PubMed] [Google Scholar]
- 8. Tonelli M, Wiebe N, Fortin M, et al. Methods for identifying 30 chronic conditions: application to administrative data. BMC Med Inform Decis Mak. 2015;15:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hux JE, Ivis F, Flintoft V, Bica A. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care. 2002;25(3):512-516. [DOI] [PubMed] [Google Scholar]
- 10. Quan H, Khan N, Hemmelgarn BR, et al. Validation of a case definition to define hypertension using administrative data. Hypertension. 2009;54(6):1423-1428. [DOI] [PubMed] [Google Scholar]
- 11. Shaffi K, Uhlig K, Perrone RD, et al. Performance of creatinine-based GFR estimating equations in solid-organ transplant recipients. Am J Kidney Dis. 2014;63(6):1007-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Statistics Canada. 2016 Census of Population. https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/details/page.cfm?Lang=E&Geo1=PR&Code1=48&Geo2=PR&Code2=01&Data=Count&SearchText=alberta&SearchType=Begins&SearchPR=01&B1=All&TABID=1. Published November 29, 2017. Accessed February 10, 2020.
- 13. Hariharan S, McBride MA, Cherikh WS, Tolleris CB, Bresnahan BA, Johnson CP. Post-transplant renal function in the first year predicts long-term kidney transplant survival. Kidney Int. 2002;62(1):311-318. [DOI] [PubMed] [Google Scholar]
- 14. Lenihan CR, O’Kelly P, Mohan P, et al. MDRD-estimated GFR at one year post-renal transplant is a predictor of long-term graft function. Ren Fail. 2008;30:345-352. [DOI] [PubMed] [Google Scholar]
- 15. Schnitzler MA, Johnston K, Axelrod D, Gheorghian A, Lentine KL. Associations of renal function at 1-year after kidney transplantation with subsequent return to dialysis, mortality, and healthcare costs. Transplantation. 2011;91(12):1347-1356. [DOI] [PubMed] [Google Scholar]
- 16. Andrassy KM. Comments on ‘KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease’. Kidney Int. 2013;84(3):622-623. [DOI] [PubMed] [Google Scholar]
- 17. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Comm Stat Simulat Comput. 2009;38:1228–1234. [Google Scholar]
- 19. Ho DE, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42:1-28. [Google Scholar]
- 20. R Foundation for Statistical Computing. R: a language and environment for statistical computing; 2017. https://www.r-project.org/.
- 21. Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med. 2013;32(16):2837-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lam NN, Kim SJ, Knoll GA, et al. The risk of cardiovascular disease is not increasing over time despite aging and higher comorbidity burden of kidney transplant recipients. Transplantation. 2017;101:588-596. [DOI] [PubMed] [Google Scholar]
- 23. Coresh J, Turin TC, Matsushita K, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311(24):2518-2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rao PS, Schaubel DE, Jia X, Li S, Port FK, Saran R. Survival on dialysis post–kidney transplant failure: results from the Scientific Registry of Transplant Recipients. Am J Kidney Dis. 2007;49(2):294-300. [DOI] [PubMed] [Google Scholar]
- 25. Meier-Kriesche HU, Baliga R, Kaplan B. Decreased renal function is a strong risk factor for cardiovascular death after renal transplantation. Transplantation. 2003;75(8):1291-1295. [DOI] [PubMed] [Google Scholar]
- 26. Jiang Y, Villeneuve PJ, Schaubel D, Mao Y, Rao P, Morrison H. Long-term follow-up of kidney transplant recipients: comparison of hospitalization rates to the general population. Transplant Res. 2013;2(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stengel B. Chronic kidney disease and cancer: a troubling connection. J Nephrol. 2010;23(3):253-262. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, RTx_Failing_-_Appendix_-_20190528 for Mortality and Morbidity in Kidney Transplant Recipients With a Failing Graft: A Matched Cohort Study by Ngan N. Lam, Devon J. Boyne, Robert R. Quinn, Peter C. Austin, Brenda R. Hemmelgarn, Patricia Campbell, Gregory A. Knoll, Lee Anne Tibbles, Serdar Yilmaz, Hude Quan and Pietro Ravani in Canadian Journal of Kidney Health and Disease