Abstract
Acute hypoxia and chronic hypoxia induce pulmonary vasoconstriction. While hypoxic pulmonary vasoconstriction is a physiological response if parts of the lung are affected, global exposure to hypoxic conditions may lead to clinical conditions like high-altitude pulmonary hypertension. Nitric oxide is the major vasodilator released from the vascular endothelium. Nitric oxide-dependent vasodilation is impaired in hypoxic conditions. Inhibition of nitric oxide synthesis is the most rapid and easily reversible molecular mechanism to regulate nitric oxide-dependent vascular function in response to physiological and pathophysiological stimuli. Asymmetric dimethylarginine is an endogenous, competitive inhibitor of nitric oxide synthase and a risk marker for major cardiovascular events and mortality. Elevated asymmetric dimethylarginine has been observed in animal models of hypoxia as well as in human cohorts under chronic and chronic intermittent hypoxia at high altitude. In lowlanders, asymmetric dimethylarginine is high in patients with pulmonary hypertension. We have recently shown that high asymmetric dimethylarginine at sea level is a predictor for high-altitude pulmonary hypertension. Asymmetric dimethylarginine is a highly regulated molecule, both by its biosynthesis and metabolism. Methylation of L-arginine by protein arginine methyltransferases was shown to be increased in hypoxia. Furthermore, the metabolism of asymmetric dimethylarginine by dimethylarginine dimethylaminohydrolases (DDAH1 and DDAH2) is decreased in animal models of hypoxia. Whether these changes are caused by transcriptional or posttranslational modifications remains to be elucidated. Current data suggest a major role of asymmetric dimethylarginine in regulating pulmonary arterial nitric oxide production in hypoxia. Further studies are needed to decipher the molecular mechanisms regulating asymmetric dimethylarginine in hypoxia and to understand their clinical significance.
Keywords: hypoxic pulmonary vasoconstriction, endothelium-dependent vasodilation, nitric oxide, dimethylarginine dimethylaminohydrolase (DDAH)
Both acute hypoxia and chronic hypoxia (CH) induce pulmonary vasoconstriction. While regional hypoxic pulmonary vasoconstriction (HPV) is a physiological response if parts of the bronchial tree are obstructed, global exposure to hypoxic conditions may lead to severe clinical conditions like high-altitude pulmonary hypertension (HAPH). Being the major endothelium-derived vasodilator, nitric oxide (NO) counterbalances the impact of vasoconstrictor stimuli like endothelin-1, angiotensin II, and others.1,2 NO formation is enhanced by hypoxia in the systemic vasculature, where hypoxia causes compensatory vasodilation and enhanced blood flow. 3 The role of NO in modulating HPV has remained much less clear. For example, inhibition of NO formation in porcine pulmonary arterioles resulted in enhanced hypoxic vasoconstriction, 4 but acute exposure to high-altitude-associated hypobaric hypoxia was paralleled by increased generation of NO in healthy mountaineers. 5 Thus, it appears that NO release in the pulmonary circulation modulates the pulmonary vasoconstrictor response to hypoxia, but it does not completely prevent it. Asymmetric dimethylarginine (ADMA) is the major endogenous inhibitor of NO synthase (NOS); it has been shown to be increased in animal models of hypoxia and in human studies. This review aims to summarize our current understanding of the role of the endothelial L-arginine–ADMA–NO pathway in the responses of the systemic and pulmonary circulation to hypoxia, in HAPH, and in pulmonary arterial hypertension (PAH) in general.
Biology of the dimethylarginines
Dimethylarginines are formed during the methylation of L-arginine residues within specific proteins, a process that is catalyzed by a family of enzymes named protein arginine methyltransferases (PRMTs). The complex process of arginine methylation is a mechanism of posttranslational modification of proteins which affects protein function and has been extensively been reviewed elsewhere. 6 When methylated proteins are cleaved during physiological protein turnover, monomethyl-L-arginine (NMMA) as well as ADMA and symmetric dimethylarginine (SDMA) are released. ADMA and NMMA are competitive inhibitors of NO synthesis, while SDMA does not directly inhibit NOS activity (for an extensive review, cf., Böger 7 ). ADMA has been shown to be associated with numerous cardiovascular and metabolic diseases, cardiovascular risk factors, and it has been characterized as a prospective marker of major adverse cardiovascular events and mortality. 8 Furthermore, ADMA and SDMA interfere with the cellular uptake of L-arginine by the y + carrier for basic amino acids, 9 thereby potentially reducing the bioavailability of L-arginine as substrate for NOS activity.
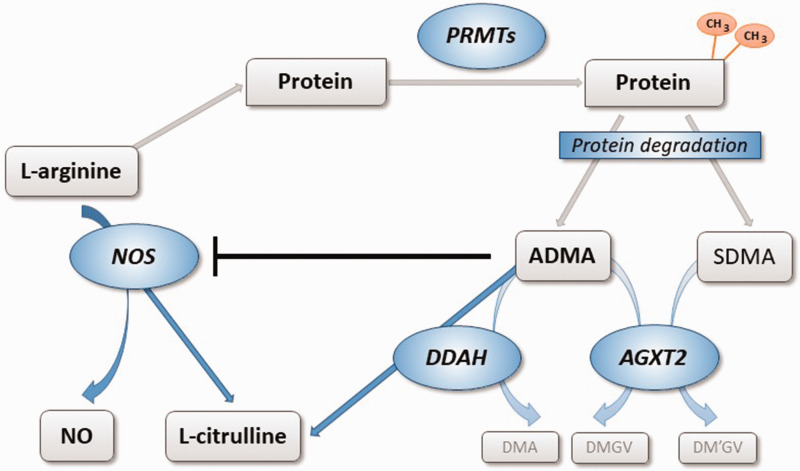
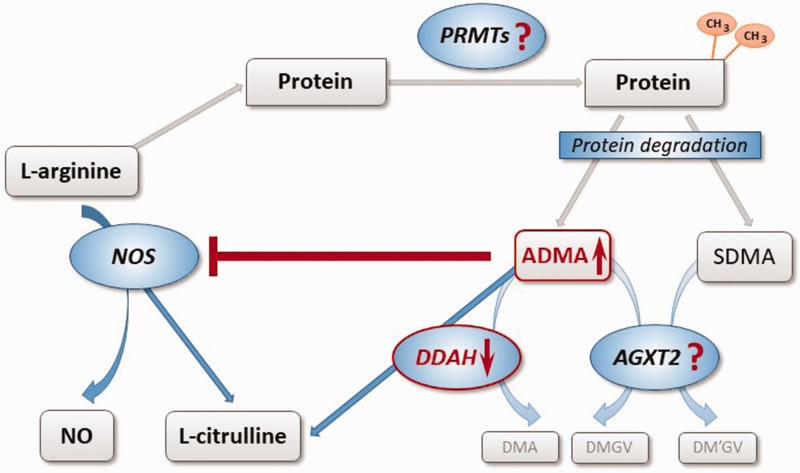
ADMA, but not SDMA, is a substrate for dimethylarginine dimethylaminohydrolase (DDAH), an enzyme that occurs in at least two isoforms with distinct tissue distribution and regulation. 10 DDAH degrades ADMA to L-citrulline and dimethylamine. An alternative metabolic pathway for both dimethylarginines has recently been identified: Alanine glyoxylate aminotransferase-2 (AGXT2) can utilize both, ADMA and SDMA, as alternative substrates, degrading them to dimethylguanidinovaleric acid.11,12 Fig. 1 depicts the complex pathways involved in the regulation of endogenous methylarginines.
Fig. 1.
Biosynthesis and metabolism of ADMA and SDMA. L-arginine residues within specific proteins are subject to methylation by PRMTs. Degradation of di-methylated proteins during physiological protein turnover results in the release of ADMA and SDMA. ADMA is a competitive inhibitor of NOS, while SDMA does not directly impair NOS activity. ADMA, but not SDMA, is enzymatically degraded by DDAH into L-citrulline and DMA. DDAH exists in two distinct isoforms with different regulation and tissue distribution. Both dimethylarginines may be cleaved by an alternative pathway through the activity of AGXT2, resulting in the formation of symmetric or asymmetric dimethylguanidinovaleric acid (DMGV and DM'GV). AGXT2: alanine glyoxylate aminotransferase 2; ADMA: asymmetric dimethylarginine; PRMT: protein arginine N-methyltransferase; NO: nitric oxide; NOS: NO synthase; SDMA: symmetric dimethylarginine; DDAH: dimethylarginine dimethylaminohydrolase; DMA: dimethylamine.
ADMA has been shown to be a risk marker of major cardiovascular events and mortality in numerous studies including populations with a broad range of cardiovascular risk. 13
ADMA is increased in hypoxia and in PAH: Observational human studies
Multiple observational studies in human cohorts that included a broad range of different populations as well as patients with chronic lung diseases reported elevated ADMA concentrations. Table 1 gives an overview of published observational studies of ADMA in human cohorts.
Table 1.
Observational studies of ADMA in chronic hypoxia and pulmonary arterial hypertension in humans.
| Clinical condition | Study design | Observation | Reference |
|---|---|---|---|
| High altitude | |||
| CIH | 72 healthy Chilean lowlanders exposed to CIH during three months; 16 Andean highlander natives | ADMA ↑ by x% in CIH; no change in SDMA in CIH; highest ADMA in highland natives | Lüneburg et al. 14 |
| CIH | 100 healthy Chilean lowlanders exposed to CIH during six months; echocardiography at six months | ADMA ↑ by x% in CIH; SDMA ↓ by x% in CIH | Siques et al. 15 |
| CIH | 120 Chilean mining workers after exposure to CIH for a mean 14 ± 0.5 years | ADMA, but not SDMA, ↑ as compared to reference levels; higher ADMA in workers with HAPH (mPAP > 30 mm Hg) than in those without | Brito et al. 16 |
| HAPE | 200 HAPE patients, 200 HAPE-free altitude sojourners, and 450 healthy highlanders | ADMA significantly ↑ in HAPE-patients and in highlanders than in HAPE-free sojourners | Ali et al. 17 |
| Acute hypobaric hypoxia (hypobaric chamber) | 12 healthy humans during a 24 h stay in a hypobaric chamber | N = 5 developed AMS, high mPAP, and decreased ADMA; N = 4 had mild AMS, mildly elevated mPAP, and elevated ADMA | Tannheimer et al. 18 |
| Lung diseases | |||
| OSAS | 40 OSAS patients, 20 healthy controls | ADMA ↑ in OSAS vs. controls | In et al. 19 |
| COPD | COPD patients with or without PAH (sPAP > 35 mm Hg), healthy controls | ADMA ↑ in COPD with PAH vs. both other groups | Telo et al. 20 |
| PAH | |||
| IPAH | Patients with IPAH, healthy controls | ADMA ↑ in IPAH | Kielstein et al. 21 |
| PAH in systemic sclerosis | 66 European patients with systemic sclerosis (24 with PAH, 42 without PAH), 30 age-matched healthy controls | ADMA ↑ in systemic sclerosis with PAH, not in systemic sclerosis without PAH | Dimitroulas et al. 22 |
| PAH in connective tissue disease | 88 Chinese patients with connective tissue diseases (43 with PAH, 45 without PAH), and 40 healthy controls | ADMA ↑ in connective tissue diseases with PAH, not in connective tissue diseases without PAH | Liu et al. 23 |
| HIV-associated PAH | 214 HIV patients, of whom 85 underwent right heart catheterization for suspected PAH | ADMA ↑ in HIV patients with PAH than in those without; mPAP 14.2% higher per each 0.1 µmol/l increase in ADMA | Parikh et al. 24 |
| CTEPH | 135 CTEPH patients, 40 healthy controls | ADMA ↑ in CTEPH patients than in controls | Skoro-Sajer et al. 25 |
AMS: acute mountain sickness; CIH: chronic-intermittent hypobaric hypoxia; COPD: chronic obstructive lung disease; CTEPH: chronic thromboembolic pulmonary hypertension; HAPE: high-altitude pulmonary edema; HAPH: high-altitude pulmonary hypertension; HIV: human immunodeficiency virus; IPAH: idiopathic PAH; mPAP: mean pulmonary arterial pressure; OSAS: obstructive sleep apnea syndrome; PAH: pulmonary arterial hypertension; sPAP: systolic pulmonary arterial pressure; ADMA: asymmetric dimethylarginine; SDMA: symmetric dimethylarginine.
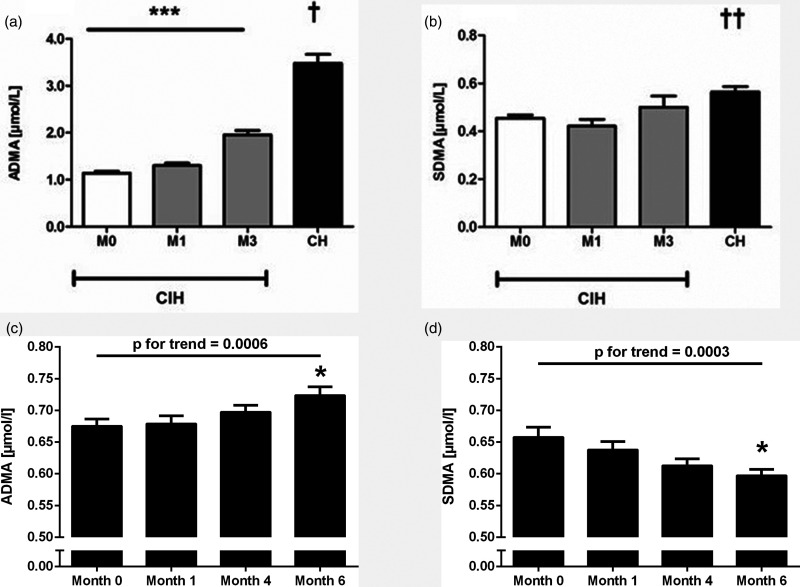
Acute or chronic exposure to high-altitude results in hypobaric hypoxia, a condition enabling us to dissect the effects of hypoxia in its purest manner if primarily healthy human subjects are being studied. Our group longitudinally followed a group of 72 primarily healthy male army draftees during three months of military services at high altitude in the Andean mountains (five days at 3550 m, followed by two days at sea level per week). 26 We compared these individuals, who were exposed to chronic-intermittent hypobaric hypoxia (CIH), with a group of 16 male Andean natives who had lived at an altitude above 3500 m for most of their lives (CH). In this study, we were the first to report a continuous increase of ADMA but not of SDMA in CIH; Andean natives had significantly higher ADMA levels than the CIH cohort after three months of exposure (Fig. 2a and b). We recently validated these results in another cohort of 100 primarily healthy male Chilean subjects who had never been exposed to altitude before and were followed for up to six months of CIH exposure. 15 In this cohort, ADMA continued to increase throughout the six months' observational period, while SDMA levels significantly dropped during the same time period (Fig. 2c and d). In another study, we measured ADMA and SDMA in a cohort of 120 Chilean mining workers who had been exposed to CIH for a mean of 14 ± 0.5 years. 16 As compared to healthy reference populations, ADMA was elevated in this group, while SDMA was within published reference ranges.
Fig. 2.
Time courses of ADMA and SDMA during long-term exposure to CIH in humans. (a and b) The plasma concentrations of ADMA, but not SDMA, increase continuously in a cohort of 72 healthy young males during three months of CIH residents native to the Andean plateau have significantly more elevated plasma levels of both, ADMA and SDMA. Figure reproduced with permission from Lüneburg et al. 26 . ***p<0.001 for trend during 3 months of follow-up; *p<0.001 for differences between CH and M0, M1, and M3; **p<0.01 for differences between CH and M0, M1. In a prospective cohort of 100 healthy male individuals who were exposed to CIH during six months, there was a continuous, significant increase in ADMA (c) and a significant decrease in SDMA plasma concentrations (d). Figure reproduced with permission from Siques et al. 15 ADMA: asymmetric dimethylarginine; SDMA: symmetric dimethylarginine; CIH: chronic-intermittent hypobaric hypoxia; CH: chronic hypoxia. *p<0.01 vs. Month 0.
Both chronic and chronic intermittent hypoxia exposure may eventually result in PAH and right ventricular hypertrophy. 16 The cut-off level of mean pulmonary arterial pressure (mPAP) to diagnose PAH in lowlanders is 25 mm Hg. For individuals exposed to high altitude, this threshold has been set to 30 mm Hg to account for the fact that mild to moderate increases in mPAP occur even in healthy individuals. 27 In our recent study in Chilean mining workers, individuals who had been diagnosed with HAPH (mPAP >30 mm Hg) had significantly higher ADMA concentrations (1.01 ± 0.15 µmol/l) than those without HAPH (0.81 ± 0.18 µmol/l; p < 0.001). 16 Although it cannot be excluded that possible confounders contributed to this difference, this remarkable result may help to identify people at risk at an early time point. Further clinical validation is urgently required for this purpose.
Another condition that may arise from high-altitude exposure is high-altitude pulmonary edema (HAPE)—a condition associated with leakage of the pulmonary endothelium and fluid accumulation in the lung interstitium. Although it is still unclear whether a change in endothelial NO formation is involved in the development of HAPE, NO clearly is a mediator that affects endothelial integrity.28–30 Ali et al. studied a group of 400 primarily healthy sojourners at high altitude (3500 m), of whom 200 had developed HAPE and 200 had remained healthy, and compared them with high-altitude residents of Tibeto-Burman ethnicity. 17 They found elevated levels of the vasoconstrictor mediator, endothelin-1, and increased activity of the renin-angiotensin-aldosterone system, but decreased levels of NO, in altitude sojourners as compared to altitude residents. These changes were significantly more prominent in HAPE patients than in HAPE-free individuals; HAPE patients also had a significantly elevated mean ADMA concentration and lower levels of superoxide dismutase as compared to both, HAPE-free sojourners and altitude residents. These data, however, are difficult to interpret because another study yielded opposite results: Tannheimer et al. studied 12 healthy human volunteers who stayed at hypobaric hypoxia in a hypobaric chamber for 24 h. 18 Five of the investigated individuals (42%) developed acute mountain sickness (AMS, Lake Louise score (LLS) > 5), had a massive increase in mPAP above 40 mm Hg, and showed a decrease in plasma ADMA concentration (−36.2%), while four individuals with mild AMS (LLS 0–3) and less increase in mPAP (<40 mm Hg) had a significant increase in ADMA (+36.3 %); the remaining three study participants had values in-between. On the other hand, we observed a significant positive association of ADMA with acclimatization status to high altitude, 26 where poor acclimatization was defined as the presence of AMS plus arterial oxygen saturation below 89%). Thus, the association of ADMA with AMS and HAPE remains to be further elucidated.
Besides exposure to high altitude, chronic or chronic intermittent hypoxia may also occur in lung diseases like sleep apnea syndrome and the advanced stages of chronic obstructive lung diseases. A group of Turkish investigators compared 40 patients with obstructive sleep apnea syndrome (OSAS) to 20 healthy controls. 19 They observed higher ADMA but lower L-arginine serum concentrations in OSAS patients than in healthy controls. These differences were independent of the presence of traditional cardiovascular risk factors, as the subgroup of OSAS patients without risk factors showed similar differences to controls. In another study, Telo et al. 20 compared patients with chronic-obstructive lung disease (COPD) who had developed PAH (defined as systolic PAP (sPAP) > 35mm Hg) to COPD patients without elevated sPAP and to healthy controls. They found a significantly elevated mean ADMA concentration in COPD patients with PAH as compared to both other groups. In this study, ADMA was negatively correlated with arterial oxygen saturation and positively correlated with sPAP.
In adult patients with congenital heart disease, plasma ADMA levels were significantly higher in patients with PAH than in those without PAH. 31
Another series of studies analyzed ADMA concentration in various subtypes of PAH according to the current Nice classification of the World Health Organization. 32 Kielstein et al. reported significantly higher ADMA plasma concentrations in patients with idiopathic PAH than in healthy controls (0.53 ± 0.15 µmol/l; controls, 0.36 ± 0.05 µmol/l; p < 0.001). 21 ADMA is also elevated in PAH associated with connective tissue disease: Dimitroulas et al. studied 66 patients with systemic sclerosis who had developed PAH (N = 24) or not (N = 42) and 30 age-matched healthy controls. 22 ADMA was significantly elevated in patients with PAH as compared to patients without PAH. In another study, 88 Chinese patients with connective tissue diseases, among whom 43 had developed PAH, were compared with 40 healthy controls. 23 ADMA concentration was significantly elevated in patients with connective tissue disease and PAH but not in those without PAH. PAH may also result from human immunodeficiency virus (HIV) infection. Parikh et al. 24 studied ADMA concentration in 214 HIV-infected individuals, among whom 85 underwent right heart catheterization for suspected PAH. ADMA was significantly associated with the presence of PAH; mPAP was 14.2% higher per each increase in ADMA by 0.1 µmol/l.
Finally, in chronic thromboembolic pulmonary hypertension (CTEPH), ADMA was demonstrated to be significantly elevated as compared to healthy controls: 25 mean plasma ADMA concentration was 0.62 [0.51–0.73] vs. 0.51 [0.45–0.6] µmol/l in 135 CTEPH patients vs. 40 healthy controls (p < 0.0002). CTEPH patients who underwent surgery had lower ADMA concentration at baseline than inoperable patients, suggesting a correlation of ADMA with the extent and severity of CTEPH.
In summary, these observational studies suggest that PAH, irrespectively of its pathogenesis, is associated with elevated circulating ADMA concentration, as is any regimen of CH or chronic-intermittent hypoxia. Derangements in the biosynthesis and/or metabolism od ADMA are therefore likely to be involved in the pathophysiology of PAH, even if the observational studies cited above do not prove any causal relationship.
ADMA as a prognostic and predictive biomarker of PAH
Several clinical studies analyzed the prospective association of ADMA with physical performance and health status in individuals exposed to hypoxia or in patients with PAH: Dimitroulas et al. reported elevated ADMA concentrations in patients with systemic sclerosis who had developed PAH as compared to patients without PAH. 22 Within the group of patients with PAH, ADMA showed a significant inverse correlation with the results of the 6-min' walk test as a measure of physical performance. In another study, ADMA concentration showed a significant inverse correlation with mixed-venous oxygen saturation and a weak but significant positive correlation with right atrial pressure in 57 patients with idiopathic PAH. 21 In the latter study, survival of patients with an ADMA concentration above the median (0.51 µmol/l) was significantly reduced as compared to patients with ADMA below the median.
Skoro-Sajer et al. calculated an ADMA concentration of 0.64 µmol/l to be the optimal cut-off value to predict mortality with a sensitivity of 81.1% and a specificity of 79.3% in patients with chronic thromboembolic PAH. 25 They also showed that ADMA plasma concentrations above that level predicted increased mortality in patients who underwent surgical thrombectomy as well as in patients who were inoperable.
In a clinical study of patients with COPD who had developed PAH or not, a significant negative correlation was determined between serum ADMA levels and arterial oxygen saturation, and a significant positive correlation was found between ADMA and systolic pulmonary artery pressure. 20 Another group of investigators reported that ADMA plasma concentration was positively correlated with the severity of bronchial obstruction in COPD patients. 33 These investigators confirmed that there was a trend toward higher pulmonary arterial pressure with higher ADMA concentrations.
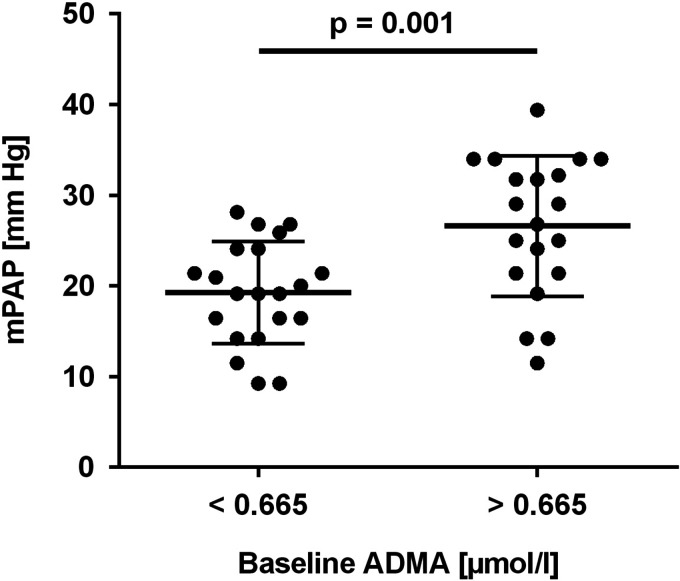
While all of these studies addressed the prognostic association of ADMA with disease outcome and/or physical performance in patients with established disease, we recently reported the first data on the predictive value of ADMA for PAH in primarily healthy individuals: 15 Out of a group of 100 young healthy male Chileans who were for the first time exposed to chronic-intermittent hypoxia at high altitude for a period of six months, we had echocardiographic estimations of mPAP available for 43 individuals. Nine of these subjects had developed HAPH after six months of CIH (i.e., they had mPAP levels >30 mm Hg). Baseline ADMA concentration, which was measured in a blood sample taken at sea level before the first exposure to high altitude, was a highly significant predictor for the development of HAPH, with an optimal cut-off level of 0.665 µmol/l (sensitivity, 100%, specificity, 63.6%). Individuals with ADMA concentration below this cut-off had significantly lower mPAP than those with ADMA above the cut-off level (Fig. 3). Table 2 summarizes the results of prospective studies of ADMA.
Fig. 3.
Baseline ADMA concentration at sea level predicts the elevation of pulmonary arterial pressure during chronic intermittent hypobaric hypoxia. In a prospective study, baseline ADMA concentration at sea level was significantly associated with mPAP after six months of exposure to chronic intermittent hypobaric hypoxia. A cut-off ADMA level of 0.665 µmol/l was optimal to predict the development of high-altitude pulmonary arterial hypertension (i.e., mPAP >30 mm Hg). Figure reproduced with permission from Siques et al. 15 ADMA: asymmetric dimethylarginine; mPAP: mean pulmonary arterial pressure.
Table 2.
Prospective clinical studies of ADMA in chronic hypoxia and pulmonary arterial hypertension in humans.
| Clinical condition | Study design | Prospective observation | Reference |
|---|---|---|---|
| IPAH | 57 patients with IPAH, follow-up for up to 40 months | Survival significantly reduced in patients with ADMA > median (i.e., 0.51 µmol/l) | Kielstein et al. 21 |
| CTEPH | 135 CTEPH patients | ADMA cut-off 0.64 µmol/l differentiates high vs. low mortality (sensitivity, 81.1%, specificity, 79.3%) | Skoro-Sajer et al. 25 |
| PAH in systemic sclerosis | 66 patients with systemic sclerosis (24 with PAH, 42 without PAH) | ADMA inversely associated with performance in the 6-min walk test | Dimitroulas et al. 22 |
| COPD | COPD patients with or without PAH (sPAP >35 mm Hg) | ADMA correlated positively with sPAP and inversely with arterial oxygen saturation | Telo et al. 20 |
| COPD | 42 COPD patients | ADMA positively correlated with severity of bronchial obstruction; trend toward higher PAP with higher ADMA | Parmaksiz et al. 33 |
| CIH | 100 healthy Chilean lowlanders exposed to CIH during six months; 43 participants with echocardiography at six months | Baseline ADMA at sea level predicts HAPH after six months of CIH; ADMA cut-off 0.665 µmol/l (sensitivity, 100%, specificity, 63.6%) | Siques et al. 15 |
CIH: chronic-intermittent hypobaric hypoxia; COPD: chronic obstructive lung disease; HAPH: high-altitude pulmonary hypertension; PAH: pulmonary arterial hypertension; sPAP: systolic pulmonary arterial pressure; IPAH: idiopathic PAH; ADMA: asymmetric dimethylarginine; CTEPH: chronic thromboembolic pulmonary hypertension.
The latter study suggests that not only is there an increase of ADMA in hypoxia, but the pre-existence of high ADMA concentration predisposes to maladaptation of the pulmonary circulation to hypoxia. Therefore, we believe that it might be worthwhile studying single-nucleotide polymorphisms (SNPs) in genes involved in the regulation of the L-arginine–ADMA–NO pathway, such as eNOS, PRMTs, DDAHs, and/or AGXT2, to better understand the genetic basis of genetic susceptibility for maladaptation to CH. Accordingly, evidence is given in a study by Trittmann et al. showing an association of DDAH1 SNP rs480414 with a lower risk of PAH in children with bronchopulmonary dysplasia. 34
Mechanisms of regulation of the L-arginine–ADMA–NO pathway in hypoxia: Evidence from animal studies
Experimental studies in various animal models and focusing on the different enzymes in this complex pathway have been performed to unravel the potential mechanisms of ADMA regulation and its pathophysiological consequences.
Bulau et al. determined whether enzymes contributing to ADMA biosynthesis and metabolism are present in the lung. 35 They showed expression of PRMT1-6 but not PRMT7 in the lung of which PRMT2 was almost exclusively found in the lung. This corresponded to high levels of protein-bound ADMA in lung tissue homogenates, compared to liver, kidneys, and heart tissues. The lungs also contained DDAH1 and DDAH2 proteins and DDAH enzymatic activity, suggesting that the complete pathway of ADMA biosynthesis and metabolism is present in the lungs.
Yildirim et al. investigated changes in PRMT expression during exposure to three weeks of normobaric hypoxia (10% oxygen) in adult BALB/c mice. 36 This study showed that among all investigated PRMT isoforms (PRMT1-6), only expression of PRMT2, which was barely detectable in normoxia, increased at the mRNA and protein levels under hypoxic conditions, accompanied by an increase in ADMA concentration. These results suggest that hypoxia might influence ADMA metabolism in the lung by upregulating L-arginine methylation by PRMT2.
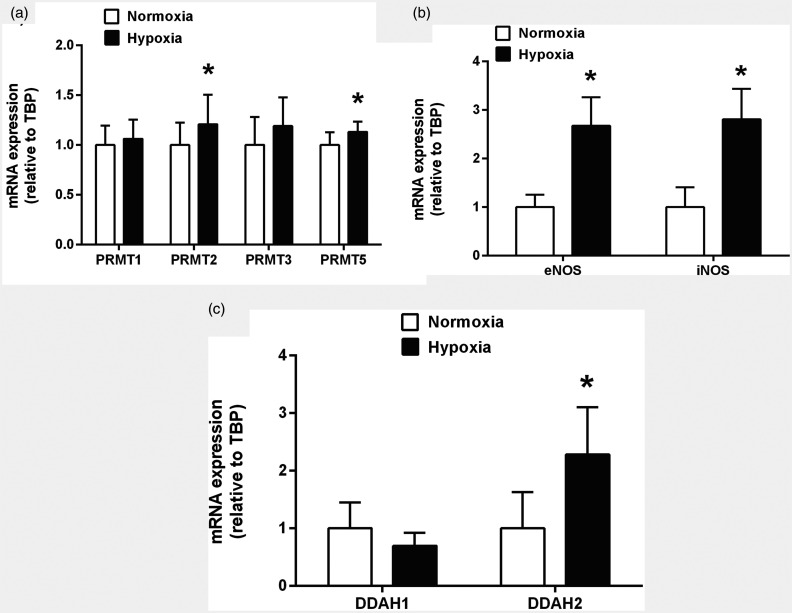
We recently analyzed mRNA expression of PRMT1, PRMT2, PRMT3, and PRMT5 in C57/bl6 mice subjected to 21 days of normobaric hypoxia (10% oxygen) vs. normoxic controls by quantitative real-time polymerase chain reaction. We found small but significant increases in PRMT2 and PRMT5 mRNA expression after three weeks of CH, while PRMT1 and PRMT3 remained unchanged (own, unpublished data; Fig. 4a). All PRMTs analyzed by us showed relatively small absolute changes in mRNA expression, raising the question whether upregulation of PRMTs is the major molecular mechanism behind the increase in ADMA during hypoxia.
Fig. 4.
Expression of genes involved in the regulation of ADMA concentration during chronic hypoxia in mice. Lung tissue was collected and homogenized after 21 days of exposure to hypoxia (10% O2), total RNA was extracted, and mRNA expression was determined by real-time quantitative polymerase chain reaction. There were moderate changes in PRMT expression, with a slight, but significant upregulation of PRMT2 and PRMT5 mRNAs (a). NOS isoenzymes II and III (iNOS and eNOS) were consistently and strongly upregulated (b), and DDAH2 was also significantly upregulated, while DDAH1 showed no differential expression in chronic hypoxia (c). PRMT: protein arginine methyltransferase; NOS: nitric oxide synthase; DDAH: dimethylarginine dimethylaminohydrolase; TBP: TATA-box binding protein. *p<0.05 vs. normoxia.
Several animal studies investigated the metabolism of ADMA by the DDAH enzymes in hypoxia. The reported findings are controversial. Millatt et al. 37 reported a 37% decrease in DDAH1 protein expression in the lungs of adult male rats exposed to one week of hypoxia (10% oxygen) as compared to rats kept under normoxic conditions. This was accompanied by 37% decrease in lung DDAH activity and a 2.3-fold elevation of ADMA concentration in lung tissue. Despite an enhanced eNOS protein expression in hypoxic lungs, NO metabolite levels in lung homogenates were reduced by 22% in this study. These results suggest that eNOS expression was upregulated in hypoxia, but its catalytic activity is impaired by increased ADMA secondary to its impaired degradation by DDAH1.
More recently, Sasaki et al. investigated changes in the L-arginine–ADMA–NO pathway in monocrotaline-induced PAH in rats. 38 They observed significantly reduced eNOS protein expression and NOS activity and increased arginase activity in PAH. In addition, they found a 41% decrease in DDAH activity which was mainly caused by a nearly complete loss of DDAH1 protein expression, while DDAH2 protein levels remained unchanged. This study therefore suggests that NO production in pulmonary hypertension is impaired by reduced eNOS expression, enhanced competition for L-arginine as a substrate by arginase, and accumulation of endogenous NOS inhibitors caused by downregulation of DDAH1.
Incubation of pulmonary arterial endothelial cells in hypoxic conditions resulted in downregulation of DDAH1 and DDAH2 mRNA and protein expression, DDAH enzymatic activity, and increased ADMA levels. 39 In this study, mRNA 21 was suggested to mediate the hypoxia-induced downregulation of DDAH1.
By contrast, DDAH2 was the main isoform changed by hypoxia in four other studies: Arrigoni et al. 40 studied the effects of three days of hypoxia on newborn piglets. They reported an about 90% reduction of DDAH2 protein but not mRNA, which was paralleled by an about 70% reduction in DDAH activity and no change in DDAH1 protein or mRNA.
A marked increase in plasma ADMA and SDMA levels was observed in chronic pulmonary hypertensive rats and patients with idiopathic PAH by Pullamsetti et al. 41 In this study, expression of DDAH2 was reduced at mRNA and protein levels with no significant changes in DDAH1 expression.
Hypoxia-reoxygenation caused an increase in DDAH2 protein expression in murine peritoneal macrophages in vitro, which was associated with 24% lower ADMA concentration and enhanced NO production by these cells. 42 DDAH2-deficient murine monocytes demonstrated no increase in NO production after hypoxia-reoxygenation challenge. However, the results of this study are not comparable to the other studies cited here, as measurements performed after several hours of reoxygenation constitute an experimental model different from acute hypoxia or CH as such, and DDAH2 is the only DDAH isoform found in immune cells.
Our group recently reported the effects of 30 days of exposure to either chronic or CIH on the L-arginine–ADMA–NO pathway in adult Wistar rats. 14 Similar to the findings of Millatt et al., 37 we found a strong increase in eNOS expression but no change in NOS catalytic activity. In our study, this was accompanied by decreased arginase and DDAH activities and elevated concentrations of ADMA in lung tissue homogenates. These effects were observed in both, chronic and chronic-intermittent hypoxia, but they were stronger in CH. These results are in line with our observations in humans studied under the conditions of chronic or chronic-intermittent hypoxia (see above; Lüneburg et al. 26 ).
While our recent data from C57/bl6 mice exposed to normobaric hypoxia for 21 days confirmed significant upregulation of eNOS and iNOS mRNA levels in the lung (own, unpublished data; Fig. 4b), we observed upregulation of DDAH2 mRNA, while DDAH1 mRNA levels were not significantly changed (own, unpublished data; Fig. 4c).
Overexpression of DDAH1 in mice resulted in reduced ADMA concentration and increased NO metabolites in isolated lung perfusates during hypoxic ventilation. 43 This was paralleled by reduced pulmonary arterial pressure in response to chronic, but not to acute hypoxia.
Taken together, available data on the effects of hypoxia on PRMT and DDAH isoforms suggest complex mechanisms of regulation, which appear to be highly variable between animal strains and species, experimental models, and age of the investigated animals.
Species differences were also observed by Mizuno et al. 44 in comparing components of the L-arginine–ADMA–NO pathway between yak and cow. Yaks, a species with a genetically determined high level of adaptation to life at high altitude, displayed higher expression levels of eNOS, DDAH1, and DDAH2 protein, significantly higher DDAH activity, and significantly lower mean ADMA plasma concentration. These results may explain previously reported differences in the pulmonary endothelial response to acetylcholine between yak and domestic cow, suggesting that pulmonary endothelial function and structure critically determine adaptation to life at high altitude. 45
In another study, Lopez et al. 46 studied expression and activity of components of the L-arginine–ADMA–NO pathway between newborn llamas and sheep at high and low altitude. Newborn sheep had significantly elevated ADMA plasma concentration and decreased DDAH2 mRNA expression in lung tissue as compared to sheep born in lowland. By comparison, llamas had extremely low ADMA concentrations and likewise low DDAH2 expression irrespectively of the altitude where they were born. Unfortunately, this study did not report expression levels for DDAH1. While downregulation of DDAH2 expression in sheep born at high altitude may explain high ADMA levels in these animals, the extremely low expression levels of DDAH2 in llamas cannot explain why this species had more than 10 times lower ADMA levels.
Conclusions and future perspectives
By contrast to the vasodilator response to hypoxia in the systemic circulation, the pulmonary vascular bed shows vasoconstriction in hypoxia. There is plenty of evidence that inhibition of endothelial NO production by endogenous methylarginines, among which ADMA is the most abundant, contributes to this regulation. The concentration of ADMA is highly regulated by multiple enzymatic pathways. Recent data from observational and prospective clinical studies suggest that not only is there upregulation of ADMA in hypoxia, but individuals with high ADMA at baseline have an exacerbated response of pulmonary arterial pressure to chronic hypoxic exposure. Fig. 5 summarizes our current understanding of the enzymatic pathways affecting ADMA that have been shown to be altered in hypoxia.
Fig. 5.
Effects of chronic hypoxia on enzymatic pathways involved in the biosynthesis and metabolism of asymmetric and symmetric dimethylarginine. There are consistent data showing upregulation of ADMA concentrations, both in plasma and tissues, while SDMA levels are either unchanged or decreased in chronic hypoxia. The majority of studies suggest downregulation of DDAH expression and/or activity, with some controversy whether DDAH1 or DDAH2 is more affected. There is uncertainty about the effects of chronic hypoxia on the expression and activities of PRMTs and AGXT2 (marked by “?” in the chart), which is due to sparsity of available published data. AGXT2: alanine glyoxylate aminotransferase 2; ADMA: asymmetric dimethylarginine; PRMT: protein arginine N-methyltransferase; NO: nitric oxide; NOS: NO synthase; SDMA: symmetric dimethylarginine; DDAH: dimethylarginine dimethylaminohydrolase; DMA: dimethylamine; DMGV and DM'GV: symmetric or asymmetric dimethylguanidinovaleric acid.
Genetic selection during hundreds of years at high altitude may have led to changes in expression and activity of enzymes involved in the L-arginine–ADMA–NO pathway, which may have contributed to adaptation to life at high altitude in these animal species. Whether similar findings can be made in human highland populations and how the genes involved have been altered during evolution remains to be elucidated in future studies. Such studies may also shed light on SNPs and/or epigenetic modifications in the relevant genes which convey sensitivity toward HAPH and/or AMS.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work by the authors was supported by the German Federal Ministry of Education and Research under grant no. 01DN17046 (DECIPHER), by the Georg & Jürgen Rickertsen Foundation, and the Werner Otto Foundation.
ORCID iDs
Juliane Hannemann https://orcid.org/0000-0003-3499-4827
Rainer Böger https://orcid.org/0000-0003-3770-5357
References
- 1.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Medicine 1993; 329: 2002–2012. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka M, Abe K, Oka M, et al. Inhibition of nitric oxide synthase unmasks vigorous vasoconstriction in established pulmonary arterial hypertension. Physiol Rep 2017; 5: e13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Justice JM, Tanner MA, Myers PR. Endothelial cell regulation of nitric oxide production during hypoxia in coronary microvessels and epicardial arteries. J Cell Physiol 2000; 182: 359–365. [DOI] [PubMed] [Google Scholar]
- 4.Archer SL, Tolins JP, Raij L, et al. Hypoxic pulmonary vasoconstriction is enhanced by inhibition of the synthesis of an endothelium derived relaxing factor. Biochem Biophys Res Commun 1989; 164: 1198–1205. [DOI] [PubMed] [Google Scholar]
- 5.Janocha AJ, Koch CD, Tiso M, et al. Nitric oxide during altitude acclimatization. N Engl J Med 2011; 365: 1942–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fulton MD, Brown T, Zheng YG. The biological axis of protein arginine methylation and asymmetric dimethylarginine. Int J Mol Sci 2019; 20: 3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Böger RH. Asymmetric dimethylarginine (ADMA): a novel risk marker in cardiovascular medicine and beyond. Ann Med 2006; 38: 126–136. [DOI] [PubMed] [Google Scholar]
- 8.Böger RH, Sullivan LM, Schwedhelm E, et al. Plasma asymmetric dimethylarginine and incidence of cardiovascular disease and death in the community. Circulation 2009; 119: 1592–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Closs EI, Basha FZ, Habermeier A, et al. Interference of L-arginine analogues with L-arginine transport mediated by the y + carrier hCAT-2B. Nitric Oxide Biol Chem 1997; 1: 65–73. [DOI] [PubMed] [Google Scholar]
- 10.Leiper JM, Santa Maria J, Chubb A, et al. Identification of two human dimethylarginine dimethylaminohydrolases with distinct tissue distributions and homology with microbial arginine deiminases. Biochem J 1999; 343: 209–214. [PMC free article] [PubMed] [Google Scholar]
- 11.Lüneburg N, Lieb W, Zeller T, et al. Genome-wide association study of L-arginine and dimethylarginines reveals novel metabolic pathway for symmetric dimethylarginine. Circ Cardiovasc Genet 2014; 7: 864–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodionov RN, Murry DJ, Vaulman SF, et al. Human alanine-glyoxylate aminotransferase 2 lowers asymmetric dimethylarginine and protects from inhibition of nitric oxide production. J Biol Chem 2010; 285: 5385–5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Böger RH, Maas R, Schulze F, et al. Asymmetric dimethylarginine (ADMA) as a prospective marker of cardiovascular disease and mortality – an update on patient populations with a wide range of cardiovascular risk. Pharmacolog Res 2009; 60: 481–487. [DOI] [PubMed] [Google Scholar]
- 14.Lüneburg N, Siques P, Brito J, et al. Long-term chronic intermittent hypobaric hypoxia in rats causes an imbalance in the asymmetric dimethylarginine/nitric oxide pathway and ROS activity: a possible synergistic mechanism for altitude pulmonary hypertension?. Pulm Med 2016; 2016: 6578578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siques P, Brito J, Schwedhelm E, et al. Asymmetric dimethylarginine at sea level is a predictive marker of hypoxic pulmonary arterial hypertension at high altitude. Front Physiol 2019; 10: 651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brito J, Siques P, Lopez R, et al. Long-term intermittent work at high altitude: right heart functional and morphological status and associated cardiometabolic factors. Front Physiol 2018; 9: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali Z, Mishra A, Kumar R, et al. Interactions among vascular-tone modulators contribute to high altitude pulmonary edema and augmented vasoreactivity in highlanders. PLoS One 2012; 7: e44049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tannheimer M, Hornung K, Gasche M, et al. Decrease of asymmetric dimethylarginine predicts acute mountain sickness. J Travel Med 2012; 19: 338–343. [DOI] [PubMed] [Google Scholar]
- 19.In E, Özdemir C, Kaman D, et al. Heat shock proteins, L-arginine, and asymmetric dimethylarginine levels in patients with obstructive sleep apnea syndrome. Arch Bronconeumol 2015; 51: 544–550. [DOI] [PubMed] [Google Scholar]
- 20.Telo S, Kirkil G, Kuluöztürk M, et al. Can ADMA play a role in determining pulmonary hypertension related to chronic obstructive pulmonary disease?. Clin Respir J 2018; 12: 1433–1438. [DOI] [PubMed] [Google Scholar]
- 21.Kielstein JT, Bode-Böger SM, Hesse G, et al. Asymmetrical dimethylarginine in idiopathic pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol 2005; 25: 1414–1418. [DOI] [PubMed] [Google Scholar]
- 22.Dimitroulas T, Giannakoulas G, Sfetsios T, et al. Asymmetrical dimethylarginine in systemic sclerosis-related pulmonary arterial hypertension. Rheumatology 2008; 47: 1682–1685. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Fu Q, Jiang L, et al. Clinical value of asymmetrical dimethylarginine detection in patients with connective tissue disease-associated pulmonary arterial hypertension. Cardiol Res Pract 2019; 2019: 3741909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parikh RV, Scherzer R, Nitta EM, et al. Increased levels of asymmetric dimethylarginine are associated with pulmonary arterial hypertension in HIV infection. AIDS 2014; 28: 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skoro-Sajer N, Mittermayer F, Panzenboeck A, et al. Asymmetric dimethylarginine is increased in chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med 2007; 176: 1154–1160. [DOI] [PubMed] [Google Scholar]
- 26.Lüneburg N, Siques P, Brito J, et al. Long-term intermittent exposure to high altitude elevates asymmetric dimethylarginine in first exposed young adults. High Alt Med Biol 2017; 18: 226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leon-Velarde F, Maggiorini M, Reeves JT, et al. Consensus statement on chronic and subacute high altitude diseases. High Alt Med Biol 2005; 6: 147–157. [DOI] [PubMed] [Google Scholar]
- 28.Duran WN, Beuve AV, Sanchez FA. Nitric oxide, S-nitrosation, and endothelial permeability. IUBMB life 2013; 65: 819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kubes P. Nitric oxide affects microvascular permeability in the intact and inflamed vasculature. Microcirculation 1995; 2: 235–244. [DOI] [PubMed] [Google Scholar]
- 30.Swenson ER, Bartsch P. High-altitude pulmonary edema. Compr Physiol 2012; 2: 2753–2773. [DOI] [PubMed] [Google Scholar]
- 31.Fang ZF, Huang YY, Tang L, et al. Asymmetric dimethyl-L-arginine is a biomarker for disease stage and follow-up of pulmonary hypertension associated with congenital heart disease. Pediatr Cardiol 2015; 36: 1062–1069. [DOI] [PubMed] [Google Scholar]
- 32.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D34–D41. [DOI] [PubMed] [Google Scholar]
- 33.Parmaksiz ET, Inal A, Salepci B, et al. Relationship of asymmetric dimethylarginine levels with disease severity and pulmonary hypertension in chronic obstructive pulmonary disease. Lung India 2018; 35: 199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trittmann JK, Gastier-Foster JM, Zmuda EJ, et al. A single nucleotide polymorphism in the dimethylarginine dimethylaminohydrolase gene is associated with lower risk of pulmonary hypertension in bronchopulmonary dysplasia. Acta Paediatr 2016; 105: e170–e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bulau P, Zakrzewicz D, Kitowska K, et al. Analysis of methylarginine metabolism in the cardiovascular system identifies the lung as a major source of ADMA. Am J Physiol Lung Cell Mol Physiol 2007; 292: L18–L24. [DOI] [PubMed] [Google Scholar]
- 36.Yildirim AO, Bulau P, Zakrzewicz D, et al. Increased protein arginine methylation in chronic hypoxia: role of protein arginine methyltransferases. Am J Respir Cell Mol Biol 2006; 35: 436–443. [DOI] [PubMed] [Google Scholar]
- 37.Millatt LJ, Whitley GS, Li D, et al. Evidence for dysregulation of dimethylarginine dimethylaminohydrolase I in chronic hypoxia-induced pulmonary hypertension. Circulation 2003; 108: 1493–1498. [DOI] [PubMed] [Google Scholar]
- 38.Sasaki A, Doi S, Mizutani S, et al. Roles of accumulated endogenous nitric oxide synthase inhibitors, enhanced arginase activity, and attenuated nitric oxide synthase activity in endothelial cells for pulmonary hypertension in rats. Am J Physiol Lung Cell Mol Physiol 2007; 292: L1480–L1487. [DOI] [PubMed] [Google Scholar]
- 39.Iannone L, Zhao L, Dubois O, et al. miR-21/DDAH1 pathway regulates pulmonary vascular responses to hypoxia. Biochem J 2014; 462: 103–112. [DOI] [PubMed] [Google Scholar]
- 40.Arrigoni FI, Vallance P, Haworth SG, et al. Metabolism of asymmetric dimethylarginines is regulated in the lung developmentally and with pulmonary hypertension induced by hypobaric hypoxia. Circulation 2003; 107: 1195–1201. [DOI] [PubMed] [Google Scholar]
- 41.Pullamsetti S, Kiss L, Ghofrani HA, et al. Increased levels and reduced catabolism of asymmetric and symmetric dimethylarginines in pulmonary hypertension. FASEB J 2005; 19: 1175–1177. [DOI] [PubMed] [Google Scholar]
- 42.Lambden S, Martin D, Vanezis K, et al. Hypoxia causes increased monocyte nitric oxide synthesis which is mediated by changes in dimethylarginine dimethylaminohydrolase 2 expression in animal and human models of normobaric hypoxia. Nitric Oxide Biol Chem 2016; 58: 59–66. [DOI] [PubMed] [Google Scholar]
- 43.Bakr A, Pak O, Taye A, et al. Effects of dimethylarginine dimethylaminohydrolase-1 overexpression on the response of the pulmonary vasculature to hypoxia. Am J Respir Cell Mol Biol 2013; 49: 491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mizuno S, Ishizaki T, Toga H, et al. Endogenous asymmetric dimethylarginine pathway in high altitude adapted yaks. BioMed Res Int 2015; 2015: 196904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Durmowicz AG, Hofmeister S, Kadyraliev TK, et al. Functional and structural adaptation of the yak pulmonary circulation to residence at high altitude. J Appl Physiol 1993; 74: 2276–2285. [DOI] [PubMed] [Google Scholar]
- 46.Lopez V, Moraga FA, Llanos AJ, et al. Plasmatic concentrations of ADMA and homocystein in llama (Lama glama) and regulation of arginase type II: an animal resistent to the development of pulmonary hypertension induced by hypoxia. Front Physiol 2018; 9: 606. [DOI] [PMC free article] [PubMed] [Google Scholar]