
Typically there is little fluid present in the peritoneal, pleural, and pericardial cavities, and thus they are considered potential spaces. Detailed physiologic descriptions of serous body cavity homeostasis are available (Bouvy and Bjorling, 1991, Dempsey and Ewing, 2011, Forrester et al., 1988). These serous body cavities are lined by specialized cells, termed mesothelial cells. Accumulation of fluid in these potential spaces results from an imbalance in fluid production and removal. Clinical signs of the presence of increased amounts of fluid include abdominal distension, abdominal pain, dyspnea with an obstructive breathing pattern, muffled heart sounds, and cardiac arrhythmias. Collection and evaluation of fluid from these sites may be therapeutic as well as diagnostic for the presence of inflammatory, hemorrhagic, neoplastic, or lymphatic conditions. Further diagnostic tests may be indicated as per the cytologic characteristics. Removal and examination of fluid is a relatively low-risk procedure, particularly for the diagnostic yield that can be produced.
Collection Techniques
Abdominal Fluid
Place the patient in left lateral recumbency and restrain. Clip and surgically prepare an area (e.g., 10 × 10 inches square) with the umbilicus in the center. The urinary bladder should be emptied before performing paracentesis. Infiltrate a small area with a local anesthetic, if desired. Use a 20- to 22-gauge needle or over-the-needle catheter to penetrate the abdomen. Attempt to obtain fluid in four quadrants, allowing the fluid to flow freely by gravity and capillary action. If needed, gentle suction with a 3- or 6-mL syringe can be employed. For a complete description of the technique, the reader is referred elsewhere (Walters, 2003). Allow the animal to rest quietly while fluid is being removed. Moving the animal or allowing the patient to move while the needle is in the abdomen can result in laceration or puncture of organs. Some investigators prefer to have the patient standing for fluid removal; however, it is more likely that the omentum will occlude the needle in this position.
If an adequate sample is not obtained using the traditional four-quadrant abdominocentesis, a diagnostic peritoneal lavage (DPL) may be used. This technique is identical to that described above; however, a catheter is employed over a needle. After removing the stylet, 10 to 20 mL/kg of warmed isotonic fluids are introduced via an IV fluid set. The animal is then gently rolled from side to side, walked briefly, and massaged to distribute the fluid throughout the abdomen. The sample is then obtained using the four-quadrant approach. Detailed descriptions of this technique can be found elsewhere (Walters, 2003). Using the DPL technique doubles the accuracy of a straight needle abdominocentesis (Crowe, 1984). A significant drawback of using this technique is the undeterminable effect of dilution on the total nucleated cell count and total protein.
Pleural Fluid
For removal of fluid from the thorax, the patient should be in standing or in ventral /sternal recumbency. Clip the hair and surgically prepare the thoracic wall from the fifth to eleventh intercostal space. Infiltrate a small area at the seventh to eighth intercostal space at the level of the costochondral junction with local anesthetic. It is best to attach extension tubing to the hub of the needle or over-the-needle catheter and a three-way stopcock for removal of pleural fluid. Insert the needle or catheter into the chest wall at the surgically prepared site, taking care to avoid the intercostal vessels located just caudal to each rib. For a complete description of this technique, the reader is referred elsewhere (Tseng and Waddell, 2000).
Pericardial Fluid
For removal of fluid from the pericardial sac, sedate the patient if necessary. Surgically prepare an area over the lower to mid fifth to seventh intercostal space bilaterally. Place the patient in left lateral or sternal recumbency. Attach ECG leads to monitor for dysrhythmias during the procedure. Infiltrate an area at the costochondral junction, or approximately where the lower and mid-thorax meet, with local anesthetic. Use a 16- to 18-gauge over-the-needle catheter with a three-way valve to which a 30- or 60-mL syringe is attached. Always maintain negative pressure on the syringe as the chest wall is punctured. Carefully advance the needle into the fourth intercostal space through a nick incision in the direction of the heart. Advance the needle until resistance is met (from the pericardium). A release will be felt as the needle enters the pericardial sac, and a flash of blood is often seen. Thread the tubing or catheter so that it is securely within the pericardial sac. For a complete description of this technique, the reader is referred elsewhere (Gidlewski and Petrie, 2005, Shaw and Rush, 2007).
Sample Handling
Note the color and character of the fluid initially upon removal (Fig. 6-1 ). If the fluid is clear initially and then turns red, iatrogenic blood contamination is likely. Conversely, if a sample is red throughout collection, a hemorrhagic fluid should be suspected. The fluid should be collected into both a lavender-top tube (EDTA anticoagulant) for evaluation of nucleated cells and red blood cells. A portion of the sample should also be placed in a red-top tube (or any sterile tube without additives) for biochemical assays such as potassium, creatinine, lactate, and glucose. Finally, a portion of the fluid should be placed in a sterile tube for aerobic, anaerobic, mycoplasma, and fungal cultures. Samples intended for culture should not be refrigerated and should be processed within 24 hours of obtaining. Tubes that contain EDTA should not be used for bacterial culture because EDTA is bacteriostatic (Songer and Post, 2005). For cytologic examination, make both a direct nonconcentrated smear by a squash or blood film spread technique and smears from the pellet of a centrifuged sample using either a blood film spread or squash technique. Samples can be spun for 3 to 5 minutes at 450 g (approximately 1500 to 2000 rpm) to obtain the pellet. From the resulting supernatant, a total protein should be measured by refractometry. The remaining pellet is reconstituted using an equal amount of remaining liquid by flicking the tube with a finger or using a stir stick. A small amount of unspun fluid may additionally be placed in a cytocentrifuge if available, and a cytospin preparation be produced for improved visibility of cytoplasmic features. Methanolic Romanowsky stains such as Wright stain or an aqueous Romanowsky stain can be applied to a few slides for immediate in-house evaluation. The remaining unstained smears and an EDTA and serum tube filled with fluid may be submitted to the laboratory for diagnostic confirmation. Maher et al (2010) showed that after 24 and 48 hours of storage, there is a significant decrease in the total nucleated cell count, a decrease in the number of neutrophils and neoplastic cells, and an increase in the number of unrecognizable cells. In addition, bacteria that were seen in fresh samples were no longer found at 24 and 48 hours (Maher et al., 2010). Whenever possible, the tubes should be sent overnight on ice to prevent in vitro changes along with freshly prepared slides. This will allow the clinical pathologist evaluating the sample to compare the cellularity and appearance of the cells in the sample submitted with those in the tube at the time of collection.
Figure 6-1.

Effusion color and character.
Gross appearance of various effusions. From left to right these are: (a) clear and colorless—transudate; (b) yellow and slightly turbid—modified transudate; (c) red and slightly turbid (likely hemolyzed red blood cells)—hemorrhage; (d) orange and turbid—likely inflammatory fluid with blood; (e) sedimented fluid—note thick pellet of cells on the bottom of the tube; (f) red and turbid—bloody as a result of either hemorrhage or iatrogenic blood contamination; (g) brown and slightly turbid—possible bile or red blood cell breakdown.
Laboratory Evaluation
Protein Quantitation
Protein quantitation is typically done via refractometry; however, some institutions will determine protein via spectrophotometry or automated analysis. Both methods offer accurate readings in a wide range of protein concentrations (Braun et al., 2001, George, 2001, George and O’Neill, 2001). It has been shown with canine effusions that refractometry underestimates the protein content when the concentration is less than 2.0 g/dL, and that spectrophotometry using the biuret method is more accurate when there is high protein content (Braun et al., 2001). Others have found that refractometry can be used accurately down to 1.0 g/dL (George and O’Neill, 2001). A similar finding of underestimating the protein content in feline effusions with refractometry may also occur (Papasouliotis et al., 2002). In that same study, a dry chemistry analyzer produced increased globulin concentration and therefore lower albumin:globulin (A:G) ratios when compared with a reference wet analyzer using the same biuret and bromocresol green methodologies. This finding is particularly important because decreased A:G ratios support a diagnosis of feline infectious peritonitis (Hartmann et al., 2003, Shelly et al., 1988). A recent study (Hetzel et al., 2012) showed acceptable results for total protein were produced using a VetScan table top analyzer, whereas the VetTest and SpotChem analyzers were determined to be unacceptable for evaluating total protein in canine effusions.
For cloudy or turbid samples and bloody samples, the fluid should be centrifuged and the protein measured on the supernatant. Turbidity may interfere with evaluation of protein by either refractometry or spectrophotometry. The protein content is used with the nucleated cell count to classify the effusion and help formulate a list of possible etiologies (Fig. 6-2 ).
KEY POINT The measurement of specific gravity using a standard refractometer has not been validated for use with body cavity fluids, only urine. Therefore, the use of specific gravity values in low protein fluids should be regarded with caution (George, 2001).
Figure 6-2.
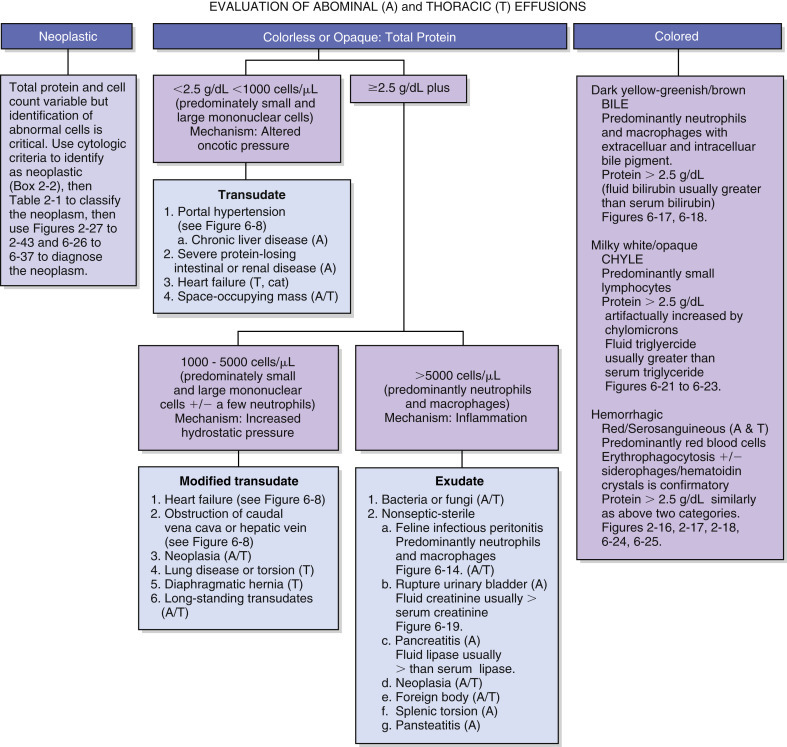
Algorithm for effusion classification.
Abdominal and thoracic effusions are easily classified by color and content. Pathophysiology of the near colorless effusions depends on the etiology. Mild increases of cells and/or protein related to increased hydrostatic pressure (modified transudate) often result from chronic transudation of fluid or passage across a membrane. Exudation of significant numbers of neutrophils and/or macrophages from injured lymphatic and blood vessels (exudate) results from infectious or noninfectious etiologies. Colored effusions are distinctive forms of exudation.
(Modified from Meyer DJ, Harvey JW: Veterinary laboratory medicine—interpretation and diagnosis, ed 3, Elsevier, St Louis, 2004.)
Red Blood Cell and Total Nucleated Cell Count
Although an initial impression of the cellularity and amount of blood can usually be made by visual inspection of the sample, knowledge of the actual cell counts for erythrocytes and nucleated cells is important for further classification of the type of fluid. With this information, one can begin to narrow down the list of possible causes for the abnormal fluid accumulation. For samples being submitted to a reference laboratory, placing some of the sample in a lavender-top tube (EDTA) and some in a red-top tube is recommended. The lavender-top tube contains anticoagulant, which prevents the sample from clotting if there is a high protein content. Red blood cell mass can be roughly estimated by spinning a microhematocrit tube of fluid and measuring the packed cell volume (PCV). This is mainly applicable to fluids that are red and semi-translucent to opaque.
The nucleated cell counts will be determined either with a hemocytometer or an automated cell-counting instrument. Although traditional, the hemacytometer method for determining the total nucleated cell count (TNCC) is slow, laborious, and inherently inaccurate. Similarly, estimation of leukocyte cell counts can be performed for overall evaluation from well-made direct smears. Each microscope differs in magnification, but often the estimate can be accomplished by counting nucleated cells from an average field in the monolayer and multiplying the count by the square of the lens objective. For example, using the 40× objective, the average presence of 6 cells/field × 1600 (402) = 9600/μL. The Sysmex XT-2000iV provides a rapid, precise, and accurate TNCC (Pinta da Cunha, 2009). If the amount of fibrinogen in the fluid sample is high, then the sample in the red-top tube is likely to clot, producing erroneous results.
KEY POINT Do not use gel-containing serum separator tubes for submission of fluid to a reference laboratory. Cells may bind to the gel in these tubes and result in an artifactual low cell count.
Nucleated Cell Differential
Standard procedures for performing a differential of the nucleated cells vary among laboratories. Some laboratories do no differential, some perform a three-part differential of 100 cells (large mononuclear cells, small mononuclear cells, and neutrophils), and yet others will provide a 100-cell differential of all cell types observed. Automated differentials have been evaluated and shown to only have modest concordance with human observations and are likely best used as a screening tool (Pinta da Cunha et al., 2009, Bauer, 2012). The differential provides a relative picture of the types and numbers of cells and aids in establishing a list of potential causes for the fluid accumulation. A differential is not a substitute for a cytologic evaluation because it includes the noncellular components of the smear. The cytologic evaluation is performed in an attempt to determine a specific diagnosis.
Normal Cytology and Hyperplasia
Normally, only a very small amount of fluid is found in the peritoneal, pleural, and pericardial spaces (i.e., potential spaces); thus, cytologic evaluation is generally not performed unless an increased amount of fluid accumulates. The physiology and pathophysiology of effusions is well described elsewhere (Dempsey and Ewing, 2011, O’Brien and Lumsden, 1988, Shaw and Rush, 2007). Normal fluid is clear and colorless (Fig. 6-1A). Several types of cells may be found in body cavity effusions, and their relative proportions vary depending on the cause of the fluid accumulation. Cells expected to be in normal fluid include mesothelial, mononuclear phagocytes, lymphocytes, and rare neutrophils. Mesothelium will easily become hyperplastic or reactive when increased body cavity fluid or inflammation is present.
Mesothelial Cells
In most cases the cytologist will find reactive mesothelial cells in body cavity fluids. These are considered as large mononuclear cells for the purpose of the three-part cell differential. Mesothelial cells may be seen as individualized cells (Fig. 6-3 ) or in variably sized clusters. They have a moderate amount of homogeneous medium-blue cytoplasm and occasionally cytoplasmic blebs (Fig. 6-4A&B ). Hyperplastic mesothelial cells are large (12 to 30 μm) with homogeneous deep-blue cytoplasm and may display a pink to red “fringed” cytoplasmic border (Fig. 6-4A&B). This feature helps identify these cells as mesothelial cells, rather than macrophages or other large mononuclear cells. These cells may contain one or more nuclei of equal size (Fig. 6-4B). Nucleoli may be visible and occasional mitotic figures may be evident.
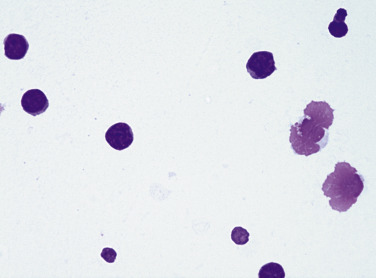
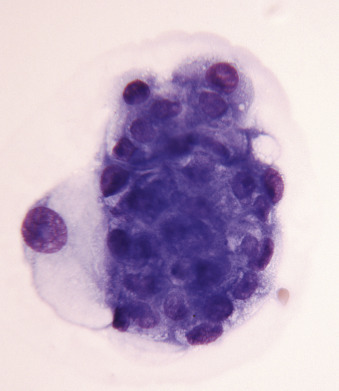
Figure 6-3.
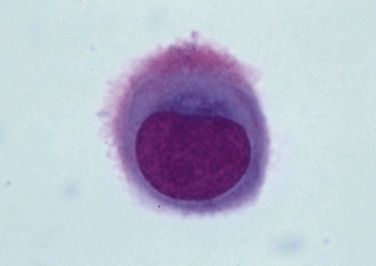
Normal mesothelial cell.
Exfoliated cell in an effusion with its characteristic pink fringe along the cytoplasmic border. (Wright-Giemsa; HP oil.)
(From Meyer DJ, Franks PT: Classification and cytologic examination, Compend Contin Educ Pract Vet 9:123-29, 1987.)
Figure 6-4.
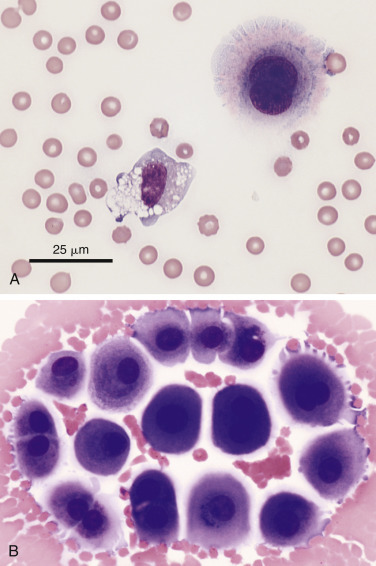
Reactive mesothelial cell.
A, Exfoliated binucleate mesothelial cell (upper right) and mildly vacuolated and basophilic macrophage in an effusion. The mesothelial cell has a characteristic pink fringe along the cytoplasmic border. (Modified Wright; HP oil.) B, A loose group of variably reactive mesothelial cells at the feathered edge of a smear made from an effusion. These cells may contain one or more nuclei. Note the presence of the “fringe” (glycocalyx) on the mesothelial cells as well as prominent cytoplasmic blebbing of a few cells at the periphery. Several cells contain paranuclear dark granules, the significance of which is unknown. (Modified Wright; HP oil.)
Macrophages
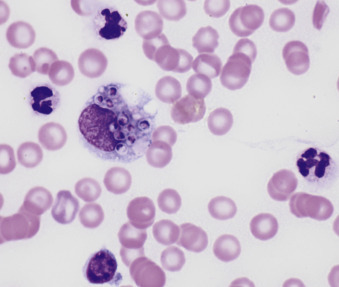
Macrophages are large mononuclear cells with abundant pale-gray to light-blue cytoplasm and a round to kidney bean–shaped nucleus (Figs. 6-4A and 6-5 ). The chromatin may be fine, and small, round nucleoli may be visible. Macrophages often contain vacuoles or previously phagocytized cells and/or debris if there is inflammation or if the fluid has been present for a long time (Fig. 6-6 ). Macrophages are considered as large mononuclear cells for the purpose of the three-part cell differential.
Figure 6-5.
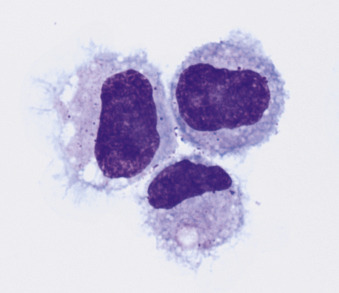
Macrophages.
Three unremarkable to mildly basophilic and vacuolated macrophages from an effusion are present. (Modified Wright; HP oil.)
Figure 6-6.
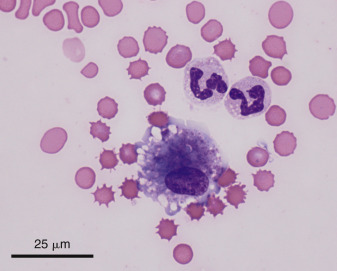
Macrophage. Neutrophils.
Shown are a moderately vacuolated and basophilic macrophage and two nondegenerate neutrophils. (Modified Wright; HP oil.)
Lymphoid Cells
Small and medium lymphocytes found in effusions appear similar to those found in peripheral blood and solid tissues. These nucleated cells often have a thin rim of lightly basophilic cytoplasm and a round nucleus. Lymphocytes are considered small mononuclear cells for the purpose of the three-part cell differential. In normal fluids they are present in higher proportions in cats than in dogs. The nucleus nearly fills the cell, producing a uniformly high nuclear-to-cytoplasmic (N:C) ratio. The chromatin is finely stippled to evenly clumped; nucleoli are typically not visible (Fig. 6-7A&B ).
Figure 6-7.
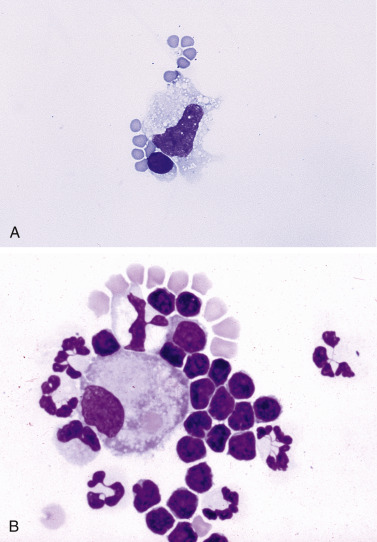
A, Normal fluid/transudate.
Note the macrophage and small lymphocyte with several erythrocytes. Normal fluid and transudates contain very low nucleated cell counts (less than 1000/μL) and low protein content (less than 2.5 g/dL). (Methanolic Romanowsky; HP oil.) B, Modified transudate. Pleural. Cat. The effusion cells have been concentrated in this animal with cardiomyopathy and display mild inflammation. Note a large macrophage, many small lymphocytes, and nondegenerate neutrophils. This fluid had a protein of 2.5 g/dL and an increased nucleated cell count of 4000 cells/μL. The macrophage has phagocytized a red blood cell. (Methanolic Romanowsky; HP oil.)
Neutrophils
Neutrophils appear similar to those found in peripheral blood. They are medium-sized cells with pale to clear cytoplasm and a segmented nucleus. Neutrophils should be absent or present in very low numbers in normal fluid, but they will be found in increased numbers with chronic fluid accumulation or with inflammation (Figure 6-6, Figure 6-7).
General Classification of Effusions
Effusions are usually classified as transudate, modified transudate, and exudate, related to the protein concentration, nucleated cell count, and cell types present (Fig. 6-2).
Transudate
Fluids are classified as transudates when they have low protein content and a low total nucleated cell count (protein less than 2.5 g/dL and cells less than 1000/μL). These fluids increase in volume in response to physiologic mechanisms, such as increased hydrostatic vascular pressure or decreased colloidal osmotic pressure, which cause the normal homeostatic mechanisms of fluid production and resorption to be overwhelmed, as described by Starling principles (Dempsey and Ewing, 2011, O’Brien and Lumsden, 1988, Stewart, 2000). Some causes for transudate accumulation include severe hypoalbuminemia, presinusoidal portal hypertension (Buob, 2011, James et al., 2008), hepatic insufficiency, portosystemic shunt, portal vein thrombosis, acute uroabdomen, and early myocardial insufficiency (Fig. 6-8 ). The cells commonly found in transudates are similar to those in normal fluid, which are mostly mononuclear cells consisting of macrophages, small lymphocytes, and nonreactive mesothelial cells (Fig. 6-7A). Nondegenerate neutrophils may comprise a small proportion of the population.
Figure 6-8.
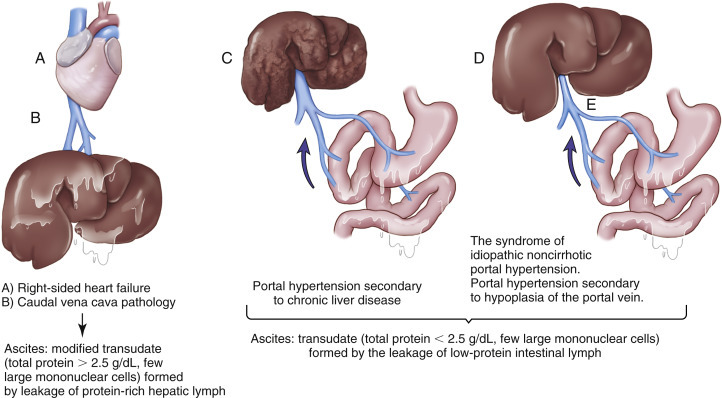
Pathophysiology of ascites formation.
(Modified from Meyer DJ, Harvey JW: Veterinary laboratory medicine—interpretation and diagnosis, ed 3, Elsevier, St Louis, 2004.)
Modified Transudate
A fluid is classified as a modified transudate when a transudate changes its physical features. The accumulation of a transudate in a body cavity causes increased pressure, which is irritating to the mesothelial cells lining the space. They respond by proliferating and sloughing into the effusion. With time, the sloughed mesothelial cells die and in so doing release chemoattractants that draw small numbers of phagocytes into the effusion to remove cellular debris. The result is a mild increase in both total protein (greater than 2.5 g/dL) and nucleated cell count (less than 5000/μL). Thus, modified transudates are generally transudates that have been present long enough to elicit a mild inflammatory reaction (Fig. 6-7B). They are most often associated with cardiovascular disease (Fig. 6-8) or neoplastic conditions; however, posthepatic, postsinusoidal, and sinusoidal portal hypertension usually presents with a modified transudate abdominal effusion (Buob, 2011).
In cases of extended duration, modified transudates can have a cloudy or milky appearance. Such fluids strongly resemble chyle and, in fact, have in the past been called “pseudochylous effusions” (a term no longer used). The gross appearance of these fluids is the result of high lipid content (due to higher cholesterol content than serum) but is in no way related to a true chylous effusion, because there are no triglycerides or chylomicrons present (Fossum et al., 1986b, Hillerdal, 1997, Meadows and MacWilliams, 1994). The phagocytes attracted to remove cellular debris from transudates are rich in enzymes that digest protein but are virtually devoid of enzymes that will break down complex lipids. Consequently, while most of the constituents of dying cells are removed by phagocytosis, lipid content of the cells simply accumulates in the effusion. Effusions formed in this manner are easily distinguished cytologically from true chylous effusions (Fossum et al., 1986b).
The principle cellular constituent of the modified transudate is the reactive mesothelial cell (Fig. 6-4B). Because of the ability of mesothelial cells to respond to irritation by proliferation, the presence of increased numbers of mesothelial cell clusters and rafts is a common finding in reactivity (Fig. 6-4B). Mitoses are increased, and occasional multinucleated reactive mesothelial cells are seen. Reactive mesothelial cells in clusters are capable of imbibing lipid from the effusion fluid, and when they do, they take on the characteristics of secretory cells. In this form they must be differentiated from metastatic adenocarcinoma or mesothelioma. This may be done by critically evaluating the cell populations for criteria of malignancy. However, differentiation between neoplasia and mesothelial hyperplasia can be occasionally challenging to the less experienced, and a second opinion is recommended.
As modified transudates mature, the proportion of inflammatory cells they contain will increase. In most cases the principal inflammatory cell is the nondegenerate neutrophil, but neutrophils rarely account for more than 30% of the total cell population. Over time, modified transudates gradually become cytologically indistinguishable from nonspecific exudates. One method used to distinguish transudates or modified transudates from exudates is measurement of C-reactive protein (CRP) concentration in canine effusions (Parra et al., 2006). A CRP level of 4 μg/mL could be used to reliably differentiate transudates from exudates, whereas a level of 11 μg/mL could be used to differentiate modified transudates from exudates. Zoia et al (2009) advocate using a combination of variables, including lactate dehydrogenase and protein ratios, in feline pleural effusions and the serum to distinguish transudate from exudate by applying a set of criteria adopted from human medicine. This method of differentiation has not gained widespread acceptance.
Exudate
Exudates are the result of either increased vascular permeability secondary to inflammation or vessel injury/leakage (hemorrhagic effusion, chylous effusion). An exudative fluid usually contains both increased protein and an increased nucleated cell count. The total protein concentration is usually greater than 3.0 g/dL, along with cell counts greater than 5000/μL. Infectious causes for exudates include bacteria (Figure 6-9, Figure 6-10 ), fungi, viruses, protozoa such as Toxoplasma (Toomey et al., 1995) (Fig. 6-11 ), Neospora caninum (Holmberg, 2006) (Fig. 6-12 ), or helminthes such as Mesocestoides sp. (Caruso et al., 2003). Noninfectious causes involve organ inflammation such as pancreatitis, steatitis, and inflammatory neoplasia, and irritants such as bile and urine. Cytologic evaluation is useful to determine an underlying cause in cases of exudative effusions.
Figure 6-9.
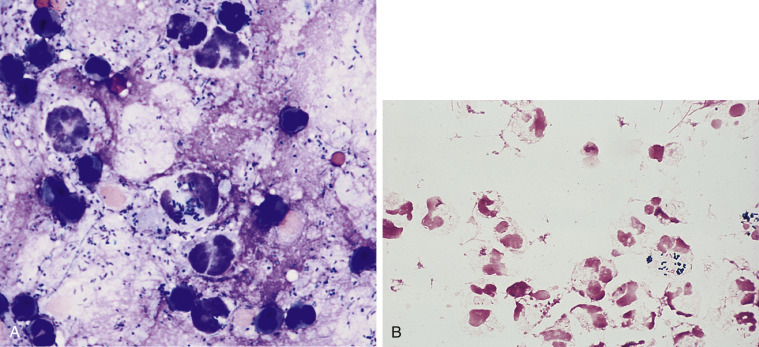
A, Exudate. Septic peritonitis. Dog.
Numerous degenerate neutrophils are noted with a large number of pleomorphic bacteria seen, both in the background and within neutrophils. This concentrated smear was made from a dog with a ruptured pancreatic abscess. (Modified Wright; HP oil.) B, Septic exudate. Pleural. Cat. Degenerate neutrophils are bloated, with foamy or vacuolated cytoplasm and also swollen lytic nuclei. Note presence of small gram-positive, pleomorphic bacterial rods. Aerobic and anaerobic cultures are recommended in cases of pyothorax. This sample contains many nucleated cells (greater than 100,000 cells/μL) and greater than 3.0 g/dL protein. (Gram; HP oil.)
Figure 6-10.
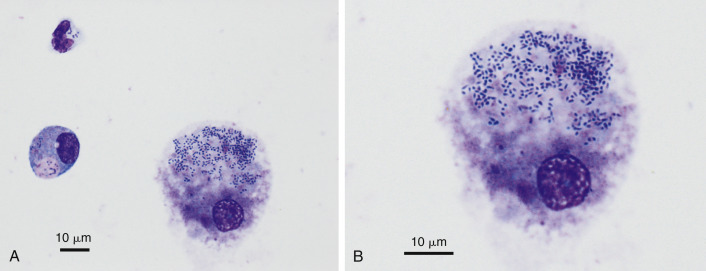
Septic exudate. Rhodococcosis. Pyothorax. Cat. Same case A-B.
A, One neutrophil and two macrophages contains a uniform population of bacteria. (Wright-Giemsa; HP oil.) B, Higher magnification of the largest macrophage in A. Note the pleomorphic coccobacillus shape of the bacteria. (Wright-Giemsa; HP oil.) Diagnosis of Rhodococcus equi confirmed by culture.
(Material courtesy of Eric Morissette et al., University of Florida; Case 7 of 2007 ASVCP case review session.)
Figure 6-11.
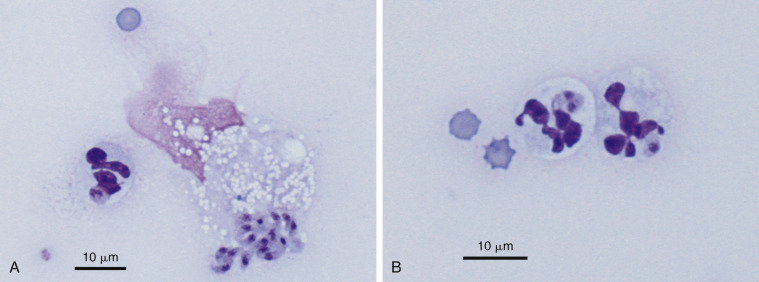
Septic exudate. Toxoplasmosis. Pleural. Cat. Same case A-B.
A, Note the extracellular bunch of banana-shaped tachyzoites. (Wright-Giemsa; HP oil.) B, Neutrophils with intracellular tachyzoites. (Wright-Giemsa; HP oil.) Diagnosis supported by histopathologic examination of the lung.
(Material courtesy of Deborah Davis et al., IDEXX Laboratories; Case 1 of 2005 ASVCP case review session.)
Figure 6-12.
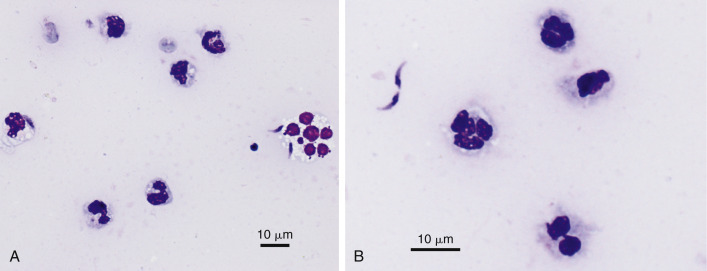
Septic exudate. Neosporosis. Abdominal. Dog. Same case A-B.
A, Two extracellular crescent-shaped tachyzoites measuring approximately 7 microns in length along with many markedly degenerate neutrophils. (Wright-Giemsa; HP oil.) B, Zoom magnification of degenerate neutrophils with two elongated extracellular tachyzoites containing a prominent focal nucleus. (Wright-Giemsa; HP oil.) Diagnosis confirmed by serum titer and PCR analysis.
(Material courtesy of Tara Holmberg et al., University of California; Case 1 of 2005 ASVCP case review session.)
Inflammatory effusions are classified according to the standard rules for inflammation as neutrophilic, mixed, or macrophagic. The terms granulomatous and pyogranulomatous are not generally applied to effusions because granuloma is a solid structure and thus not applicable to a fluid environment. In neutrophilic reactions, neutrophils (either nondegenerate or degenerate) comprise more than 70% of the inflammatory cells seen. Mixed reactions are characterized by a mixture of neutrophils and macrophages. In histiocytic inflammation, macrophages are the prevalent cell seen.
Most inflammatory effusions are cytologically nonspecific in terms of an etiologic diagnosis. However, as with inflammatory responses elsewhere, cytomorphology provides significant clues as to the underlying cause. Neutrophilic inflammatory effusions indicate severe active irritation (Fig. 6-13 ). If neutrophils are degenerate, an effort should be made to identify bacterial organisms within phagocytes (primarily neutrophils). This is generally easiest at the feathered edge of the smear. If organisms are not seen, the fluid should still be cultured. Mixed and macrophagic inflammatory effusions reflect less severe irritation and are found with resolving acute effusions or in association with less irritating etiologic agents than bacteria (e.g., fungal organisms or foreign bodies). Chemical evaluation of effusion fluid is also useful in recognizing sepsis.
Figure 6-13.
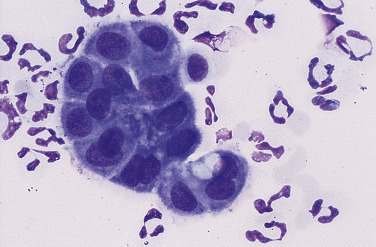
Nonseptic exudate. Peritoneal. Dog.
Concentrated fluid from an animal with pancreatitis showing one cluster of reactive mesothelial cells and neutrophils. The protein content in this sample was 3.0 g/dL with a cell count of 8000 cells/μL. Most of the cells are neutrophils, suggesting an underlying inflammatory condition. Further testing (e.g., fluid and serum lipase or ultrasonography) is required to determine the specific cause for the fluid accumulation. (Romanowsky; HP oil.)
A study involving peritoneal fluid in dogs and cats evaluated the difference between blood and effusion fluid glucose concentrations. This study found that a difference of greater than 20 mg/dL provided a rapid and reliable means to differentiate septic and nonseptic effusion fluid (Bonczynski et al., 2003). In the same study, lactate was also measured in blood and effusions from dogs. A difference of less than 2.0 mmol/L was 100% sensitive and 100% specific for the diagnosis of septic peritonitis. A later study (Levin, 2004) found a peritoneal effusion lactate of greater than 2.5 mmol/L to be diagnostic for a septic process in dogs but not in cats.
Specific Types of Effusions
While most inflammatory effusions are cytologically nonspecific, some etiologies cause reactions with characteristic diagnostic features. These effusions are discussed below.
Feline Infectious Peritonitis
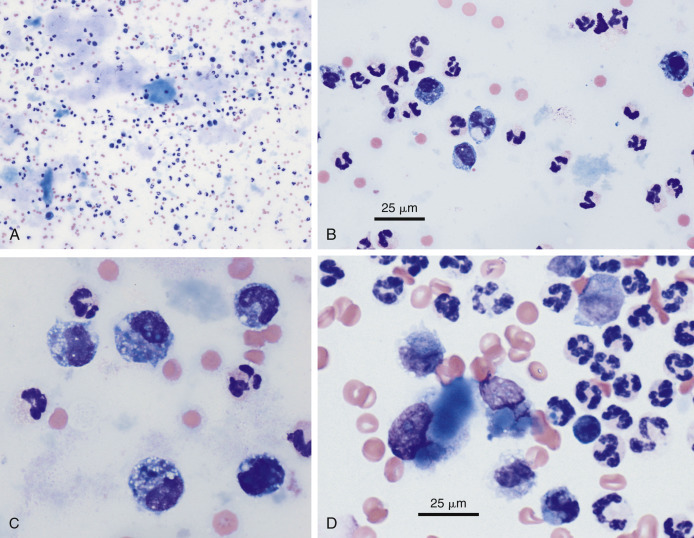
Feline infectious peritonitis (FIP) is unique among the causes of inflammatory effusion in that the fluid that accumulates is usually high in protein, yet it has a relatively low cellularity (Fischer et al., 2012, McReynolds and Macy, 1997, Norris et al., 2005). FIP has been noted to cause 18% of pleural (Davies and Forrester, 1996), 9.8% of pericardial (Davidson et al., 2008), and 5% of abdominal (Wright, 1999) effusions. Classification as an inflammatory effusion is based primarily on the presence of high total protein (often greater than 4.5 g/dL), which is a reflection of a similar elevation in serum protein, as well as the product of vasculitis and increased vascular permeability. Cytologically these fluids are usually relatively low in cell number (1000 to 30,000/μL) and inconsistent with regard to the cell types present. In a majority of cases the predominant cell is the nondegenerate neutrophil (Fig. 6-14A ). However, activated macrophages are commonly observed (Fig. 6-14B) (Norris et al., 2005, Paltrinieri et al., 1999). In rare cases the lymphocyte is prevalent and the effusion can even appear chyle-like (Savary et al., 2001). Regardless of the predominant cell type, slides in all cases have a bluish-gray to purple granular background that results from the high protein content (Fig. 6-14A&B), and clumps and strands of fibrin may be observed.
Figure 6-14.
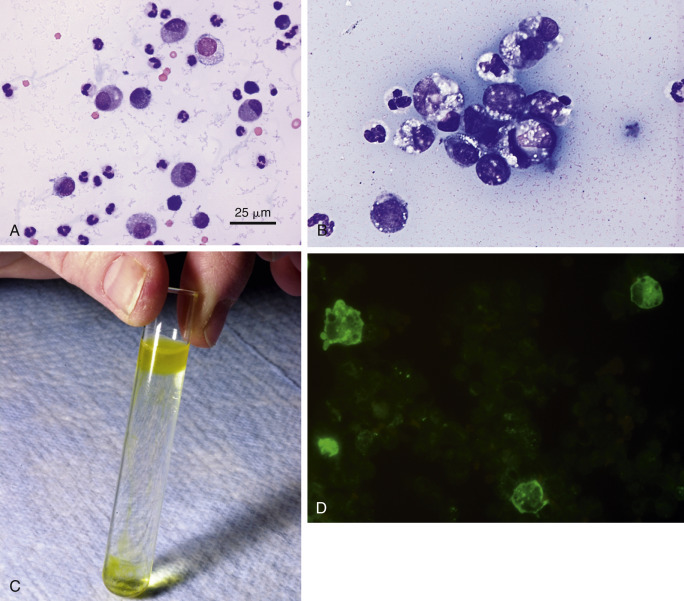
Abdominal effusion. FIP. Cat.
A, This concentrated smear of the exudate contains moderately basophilic macrophages and nondegenerate neutrophils. The background contains basophilic, coarsely granular protein as well as basophilic protein crescents and strands of fibrin. (Modified Wright; HP oil.) B, This exudate mostly contains foamy, vacuolated macrophages with lower numbers of mildly degenerate neutrophils and intermediate to large lymphoid cells. Lymphocytes may be intermediate in size and appear reactive in some cases of FIP. Also note the granular precipitated protein throughout the background. (Methanolic Romanowsky; HP oil.) C, Rivalta test. Positive test results are indicated by a layer of gel on top of the acetic acid solution. Fluid is from a cat with PCR-confirmed FIP. The cat was moderately icteric. Note the yellow streaks of gel in the middle of the tube from partially floating material. D, FCoV immunofluorescence test. A specific cytologic test for FIP involves immunofluorescence of intracellular feline coronavirus (FCoV) within the effusion. Shown are three infected and intact macrophages (green). (FCoV immunofluorescence; HP oil.)
(C, Photo by Sam Royer, Purdue University. D, Courtesy of Jacqueline Norris, University of Sydney, Australia.)
Electrophoresis of either the effusion fluid or the serum reveals a polyclonal gammopathy. The total protein, A:G ratio, and percent globulin in effusions have all been evaluated as a tool for diagnosing FIP from effusions. Paltrinieri et al (1999) found that a total protein greater than 3.5 alone had a sensitivity of 87.1% and a specificity of 60%. Shelly et al (1988) found that gamma globulin values of greater than or equal to 32% had a 100% positive predictive value (PPV). This same study found an A:G greater than 0.81 had a negative predictive value (NPV) of 100%. Sparkes et al (1994) found a 94% PPV and 100% NPV for FIP when the total protein of an effusion was greater than 3.5 mg/L at the same time globulins made up greater than 50% of the protein.
An additional test that can help rule out FIP is the Rivalta test. To perform this relatively simple test, place one drop of 98% acetic acid into 5-mL distilled water and mix well in a clear reagent tube. Slowly layer one drop of the effusion fluid to the surface of the acetic acid solution. A positive test requires that the drop of effusion retain its form on the surface or slowly sink to the bottom as a droplet or jellyfish-like shape (Fig. 6-14C). This test indicates a high protein content as well as fibrin and inflammatory mediators. A recent study reported no major differences using acetic acid, distilled white vinegar, and wine vinegar (Fischer et al., 2013). This same study showed that refrigerated storage for up to 21 days did not affect results. This test has a recently reported PPV of 58.4% to 88.4% and NPV of 66.7% to 93.4% (Fischer et al., 2012) in contrast to PPV of 86% and NPV of 97% in an earlier study (Hartmann et al., 2003). The sensitivity and specificity of the Rivalta test are reported to be 91.3% and 65.5%, respectively (Fischer et al., 2012).
A more specific cytologic test for FIP involves immunofluorescence of intracellular feline coronavirus (FCoV) within effusion macrophages. A positive result (Fig. 6-14D) is diagnostic for FIP. Unfortunately, a negative result does eliminate FIP diagnostically because the NPV of 57% is poor (Hartmann et al., 2003, Parodi et al., 1993). Using direct immunofluorescence to detect FCoV antigens within effusion macrophages, there was a sensitivity of 100% and a specificity of 71.4% (Litster et al., 2013)—results similar to those previously reported (Paltrinieri et al., 1999).
Nocardial/Actinomycotic Effusions
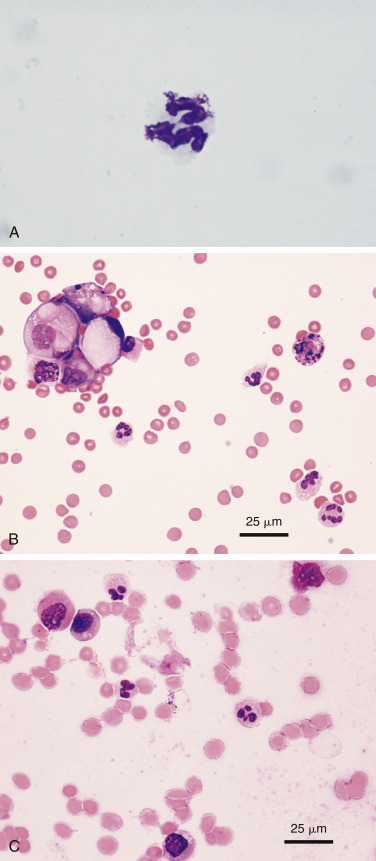
Complex bacteria such as Nocardia asteroides and Actinomyces sp. are important causes of both peritoneal and pleural effusions in dogs and cats. Grossly, these effusions are turbid and yellow to blood-tinged “tomato soup.” Even when collected in EDTA they typically contain visible particulates or granules (the so-called “sulfur granules”) (Fig. 6-15A ; Songer and Post, 2005).
Figure 6-15.
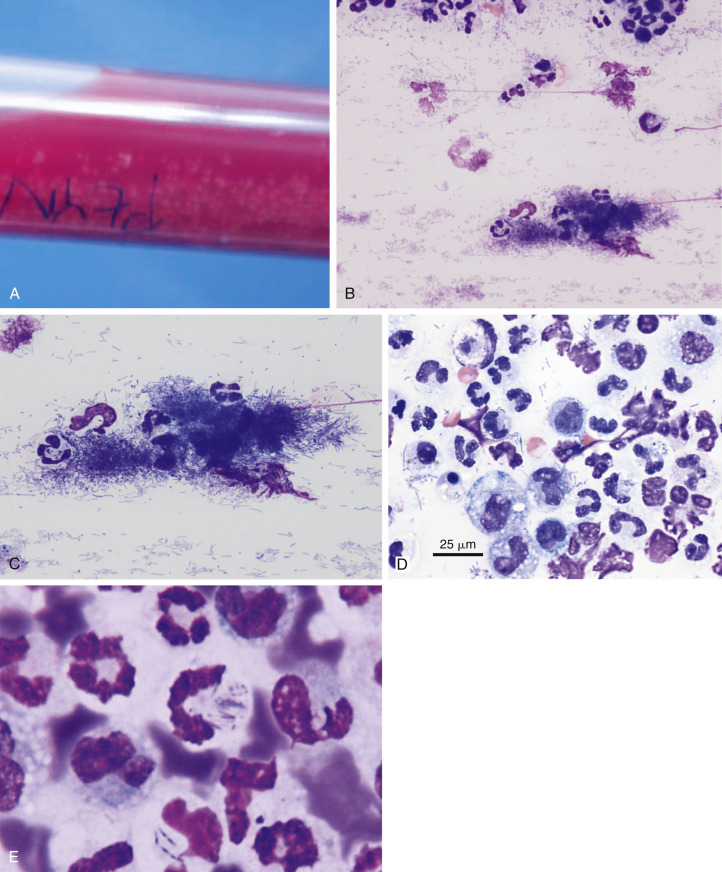
Septic exudate. Actinomycosis. Pleural. Same cat case A and E and same dog case B-D.
A, Gross fluid appearance showing presence of blood and numerous light yellow particles (i.e., sulfur granules). B, Direct smear from a pleural effusion demonstrates the cytologic appearance of a particle along with many lysed cells and nuclear streaks. These granules often are dragged to the feathered edge, as was the case with this smear. (Modified Wright; IP.) C, Higher magnification of the same particle in A. (Modified Wright; HP oil.) D, The fluid has marked suppurative inflammation with many degenerate neutrophils and several foamy macrophages. Short and long filamentous organisms and lysed cells are seen throughout the background. In areas of the smear lacking bacteria (not shown), the neutrophils were only mildly degenerate. (Modified Wright; HP oil.) E, Slender often beaded filamentous bacteria are present within two degenerate neutrophils. (Aqueous Romanowsky; HP oil.)
(A and E, Images courtesy of Janina Łukaszewska, Wrocław, Poland.)
On the basis of physical parameters, these effusions are typical exudates, with high total protein and markedly high cellularity. Because of the high cellularity, direct smears are generally adequate for cytologic examination. If particles are observed in the fluid, it is important to make squash preparations of these particles in addition to making smears of the fluid alone.
Microscopically, nocardial and actinomycotic infections are characterized by neutrophilic to mixed inflammation, probably dependent on the duration of the disease. In the more chronic reactions, there is generally a significant reactive mesothelial cell component to the response. A striking feature of the inflammatory response is the morphology of neutrophils. Whereas most cases of septic pleuritis and peritonitis are signaled by the presence of predominantly degenerating neutrophils, in nocardial and actinomycotic effusions the majority of the neutrophils away from the organisms are nondegenerate or show signs of aging (hypersegmentation) or apoptosis (karyorrhexis and/or pyknosis). Degenerating neutrophils are only seen immediately in the vicinity of the bacterial organisms because these agents, in contrast to most other bacteria, produce only weak local toxins. The net effect of this phenomenon is that smears in these cases may be easily misinterpreted as noninfectious, particularly if the organisms are not widespread. Because the particles seen grossly often are composed of bacterial colonies, it is important that squash preparations of these particles be examined to ensure that the diagnosis is not missed (Fig. 6-15B&C). In addition, the feathered edge of blood film smears should also be carefully examined because small colonies may be dragged to the edge.
Microscopic morphology of the organisms is quite characteristic. Colonies are composed of delicate, filamentous, often beaded organisms, and are often found at the feathered edge of smears (Fig. 6-15D&E). The most significant diagnostic feature of these organisms is that these filaments are branched. Using standard hematologic stains, Nocardia organisms cannot be differentiated from Actinomyces. However, Nocardia is gram positive and variably acid-fast, whereas Actinomyces is gram positive but not acid-fast (Songer and Post, 2005). Dense mats of organisms and sulfur granule formation (microscopic or macroscopic) are more commonly seen with Actinomyces infections, rather than Nocardia (Sykes, 2012).
Cytologic diagnosis should be confirmed by bacterial culture of the effusion and/or sulfur granules. Because these species have special culture requirements, it is important that the bacteriology laboratory be aware of the provisional diagnosis at the time of sample submission.
Systemic Histoplasmosis
Systemic histoplasmosis, caused by Histoplasma capsulatum, is an occasional cause of peritoneal effusions, and a rare cause of pleural effusions in dogs and cats living in endemic areas. Because the fungus is ubiquitous in the area, serology cannot be relied upon for diagnosis. In many cases cytologic identification of the organism is essential.
Effusions due to histoplasmosis present in a variety of ways. On the basis of physical characteristics, the fluid has been reported as a pure transudate (Stickle and Hribernik, 1978), a modified transudate (Dillon et al., 1982, VanSteenhouse and DeNovo, 1986), and an exudate (Kowalewich et al., 1993). Cytologically, it has inflammatory characteristics with a mixture of neutrophils and macrophages. Histoplasmosis is considered to be disseminated when observed in effusions and can be found in the lungs, liver, spleen, bone marrow, rectal wall, and peripheral blood.
Demonstration of the organism is best done within macrophages (Fig. 6-16 ) at the feathered edge of sediment or direct smears. Histoplasma organisms measure approximately 2 to 4 μm in diameter, appear round to slightly ovoid in shape, and have a single basophilic nucleus surrounded by a thick, colorless cell wall (pseudocapsule). In fluids it is common to see individual organisms free in the background. Two other organisms have similar cytomorphology. One organism is Sporothrix. However, it differs in size (3 to 5 μm in diameter) and shape (elongated to cigar shaped), and the infection is usually restricted to the skin (see Fig. 3-24); disseminated disease has been described in cats and dogs. The other organism is Leishmania. However, it is distinguished by the presence of an internal kinetoplast, which gives it the appearance of having two nuclei (see Fig. 3-25).
Figure 6-16.
Histoplasmosis. Dog.
A macrophage containing numerous oval 2 to 3 μm yeast organisms of Histoplasma capsulatum is seen at left center. There are numerous red cells in the background of the smear. (Modified Wright; HP oil.)
If there is uncertainty in the cytologic diagnosis, a urine antigen test is available. This test detects a glycoprotein antigen released from viable Histoplasma yeast and excreted in urine (Kauffman, 2007, Wheat, 2003). A similar test has been evaluated for the detection of Blastomyces spp. infections in dogs (Spector et al., 2008). The test crossreacts with antigens from Histoplasma spp. and may prove to be a useful tool in detecting disease as well as monitoring resolution/recurrence.
Bilious Effusion
Rupture of the gall bladder or common bile duct may occur in any species secondary to direct trauma or disease of the biliary tree. Etiologies such as gastric dilatation and volvulus, cholelithiasis, gunshot wounds, and iatrogenic secondary to fine needle aspiration or surgery have all been reported. In addition, it is an infrequent accompaniment to diaphragmatic hernia from any cause in the dog and cat. When the results of direct trauma are mainly in the biliary system, leakage of bile is virtually always restricted to the peritoneal cavity, with a resulting peritonitis. When associated with diaphragmatic hernia, leakage of bile occurs when liver is trapped in the diaphragmatic rent and there is rupture or necrosis of the gall bladder or common bile duct. In this circumstance, both peritonitis and pleuritis can result. Bile is a very irritating substance; its presence quickly elicits an inflammatory response. Grossly, the fluid may be initially brown (Fig. 6-1G) to yellow to greenish; however, as the response becomes more and more cellular, this discoloration may become masked. Large volumes of fluid can usually be obtained. Based on physical characteristics, these effusions are usually exudates (Ludwig et al., 1997, Owens et al., 2003).
Cytologically, the striking feature of bilious effusion is the presence of bile in the smear. Frequently, bile is seen as yellow to green to blue-black granular material scattered in the slide background (Fig. 6-17A-C ) and in the cytoplasm of neutrophils, reactive mesothelial cells, and macrophages. In reactions of greater duration, bile granules may have all been converted to rhomboidal to amorphous golden crystals of bile pigment. When such crystals are found in the cytoplasm of effusion phagocytes in the absence of evidence of prior hemorrhage (e.g., erythrophagocytosis), the possibility of bilious effusion should be strongly considered. In addition to the typical appearance of bilious effusions, acellular, amorphous, fibrillar blue-grey mucinous material (Fig. 6-18A-D ) has been associated with biliary tree rupture, particularly of the common bile duct in dogs (Owens et al., 2003). Accumulations of this extracellular material are the predominant cytologic finding in contrast to the more typical green or yellow bile granules. It is suspected that this bile-free material is produced by biliary and gallbladder epithelium as a consequence to extrahepatic biliary obstruction with regurgitation of normal bile into hepatic lymph and venous blood. This material has been termed “white bile” (Owens et al., 2003).
Figure 6-17.
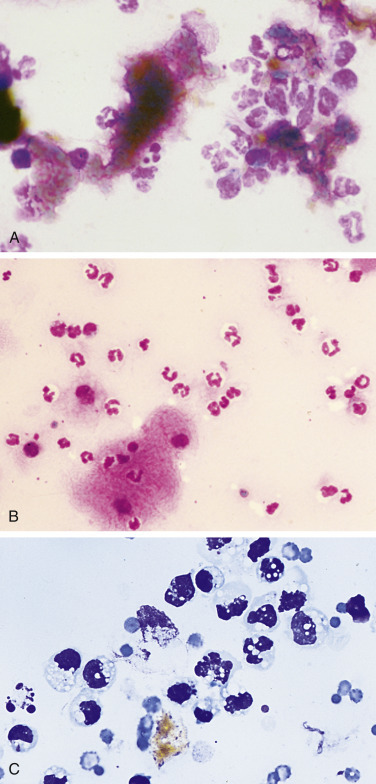
Bilious effusion. Peritoneal. Dog. Same case A-B.
A, Degenerate neutrophils are surrounded by dark yellow to black amorphous bile material that is free in the background. (Wright-Giemsa; HP oil.) B, Large numbers of mostly nondegenerate neutrophils accompany the presence of amorphous material. The basophilic bile material is coated by stain precipitate producing a pink, granular appearance. This greenish, flocculent fluid had a protein level of 3.0 g/dL and an estimated nucleated cell count of greater than 60,000/μL. (Wright-Giemsa; HP oil.) C, Note extracellular gold-brown crystalline material, vacuolated neutrophils, and macrophages. Some of the neutrophils contain pyknotic nuclei and others contain karyolytic nuclei. (Romanowsky; HP oil.)
(A and B, Courtesy of Rose Raskin, University of Florida.)
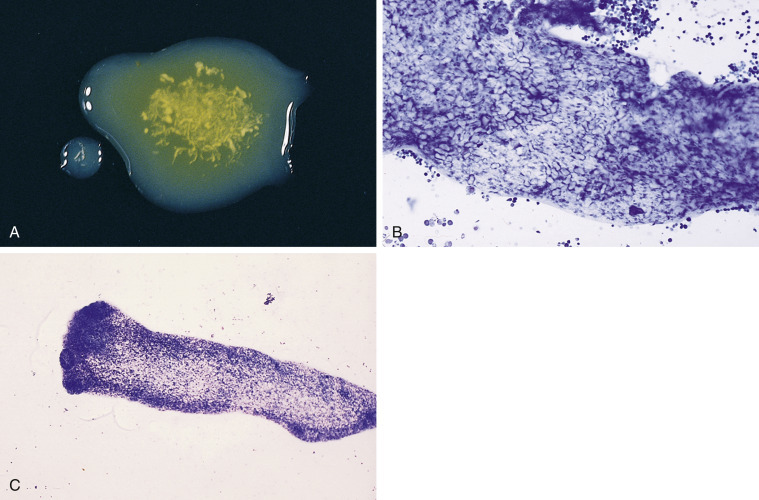
Figure 6-18.
Bile peritonitis. Dog. Same case A-C.
A, This is a concentrated smear of fluid from a dog with a ruptured gall bladder. Note the numerous neutrophils and lakes of blue-grey amorphous mucinous material that are present throughout the background. (Modified Wright; IP.) B, Note the suppurative inflammation with variably degenerate neutrophils as well as basophilic and foamy macrophages. Amorphous blue-gray material, likely mucin, is seen in the background. (Modified Wright; HP oil.) C, Note the suppurative inflammation and foamy macrophages that contain blue-grey to dark blue granular material. The background contains mucinous and finely granular protein. (Modified Wright; HP oil.) D, In abdominal fluid from another case, neutrophils appear mostly nondegenerate. Intracellular mucus material is dense, hyalinized, and medium blue. (Modified Wright; HP oil)
(D, Courtesy of Rose Raskin, Purdue University.)
The inflammatory response to bile is generally composed of nondegenerate neutrophils—84 to 98% of TNCC (Ludwig et al., 1997) (Fig. 6-18B-D). Varying numbers of macrophages and reactive mesothelial cells can be admixed depending on duration of contact with the bile irritant. Fluid bilirubin concentrations are several times higher than serum concentrations, a finding that is 100% diagnostic (Ludwig et al., 1997, Owens et al., 2003). There is a poor survival rate when the bilious effusion is also septic (Ludwig et al., 1997).
Eosinophilic Effusion
Effusions with more than 10% eosinophils are termed eosinophilic effusions regardless of the protein content or cell count. This condition is uncommonly seen in veterinary medicine. With large numbers of eosinophils, the fluid grossly may have a green tint. The presence of eosinophils does not provide a specific diagnosis, and the cause is often unknown in these cases. Neoplasia such as lymphoma or mast cell disease (e.g., visceral mast cell tumor, systemic mastocytosis) involved half of the cases in one study (Fossum et al., 1993). Several case reports exist noting these specific neoplasms as the etiology of the eosinophilic effusion (Barrs et al., 2002, Bounous et al., 2000, Cowgill and Neel, 2003, Harris et al., 2013, Peaston and Griffey, 1994, Tomiyasu et al., 2010). Heartworm disease, interstitial pneumonia, disseminated eosinophilic granulomatosis, peritoneal cestodiasis (Patten et al., 2013), and sarcocystosis (Allison et al., 2006) are other possibilities in dogs. A case of lung worms in a cat with an eosinophilic effusion has been reported (Miller et al., 1984). Dacron implants have also been implicated as an iatrogenic etiology in eosinophilic effusions (Macintire et al., 1995).
Uroperitoneum
Uroperitoneum in cats and dogs is frequently seen secondary to a ruptured bladder, although compromise of any of the urinary tract that lies within the peritoneal cavity can produce uroperitoneum. The most common cause of a ruptured bladder is trauma (Aumann et al., 1998, Burrows and Bovee, 1974). Sources of trauma include blunt abdominal trauma (e.g., vehicular), aggressive catheterization or aggressive palpation/expression. Urine in the peritoneal space results in chemical irritation. The protein content and total nucleated cell count may be variable as a result of dilution from the urine. Early in the condition, a mononuclear cell population may predominate, suggestive of a modified transudate. Later the neutrophil is usually the predominant cell type as effusion becomes an exudate (Fig. 6-2). Bacteria may or may not be present. Neutrophils exposed to the irritant material may show karyorrhexis, pyknosis, or karyolysis with ragged nuclear borders (Fig. 6-19A-C ). In some cases urinary crystals are found on cytologic examination, which supports the diagnosis of uroperitoneum. In cats the creatinine and potassium concentrations in the effusion were found to be higher than serum concentrations (generally a ratio of 2:1) (Aumann et al., 1998). A higher effusion creatinine concentration vs. serum creatinine concentration tends to persist longer than a higher effusion urea nitrogen (BUN) concentration vs. serum BUN concentration because creatinine equilibrates more slowly than urea nitrogen. One study found that 85% of dogs with uroperitoneum had an abdominal fluid:serum creatinine ratio greater than 2:1 (all of these dogs had an effusion creatinine concentration that was four times the serum concentration), and 100% had an abdominal fluid:serum potassium greater than 1.4:1 (Schmiedt et al., 2001). In addition, serum Na:K also tends to be decreased in cases of uroabdomen (Aumann et al., 1998, Burrows and Bovee, 1974). Cases of urothorax have been described in a dog and a cat (Klainbart et al., 2011, Störk et al., 2003).
Figure 6-19.
Uroperitoneum. Dog.
A, The fluid contained a high number of neutrophils, many of which appeared similar to this “ragged” cell. Urine acts as a chemical irritant, causing karyolytic changes to cells. (Wright-Giemsa; HP oil.) B, Cytospin preparation of abdominal fluid with a cluster of reactive mesothelial cells and one karyorrhectic and several karyolytic neutrophils. (Modified Wright; HP oil.) C, Sedimented fluid from same case as B. Several vacuolated macrophages along with mildly karyolytic neutrophils against a background of erythrocytes. Elevated fluid creatinine confirmed the suspicion of uroperitoneum.
(Courtesy of Rose Raskin, A, University of Florida; B-C, Purdue University.)
Parasitic Ascites (Abdominal Cestodiasis)
In a small number of dogs with ascites, often from western North America, the etiology is aberrant cestodiasis associated with Mesocestoides infection (Caruso et al., 2003, Crosbie et al., 1998, Patten et al., 2013, Stern et al., 1987). This disease has been termed canine peritoneal larval cestodiasis, or CPLC (Patten et al., 2013). Pleural involvement has also been reported (Toplu et al., 2004). Rare reports of infection involve cats (Eleni et al., 2007, Jabbar et al., 2012, Venco et al., 2005). Peritoneal aspirates from anorexic, ascitic dogs have the gross appearance of tapioca pudding or cream of wheat (Fig. 6-20A ). Motile cestodes can be seen in fluid with the unaided eye. Microscopically, the fluid is usually a suppurative exudate (Caruso et al., 2003); however, eosinophilic inflammation has also been described (Patten et al., 2013). Microscopic examination may also show acephalic metacestodes or acoelomic tissue with calcareous corpuscles (Fig. 6-20B), which may be seen in nonspecific cestode infections. Less often seen microscopically are metacestodes with visible tetrathyridia, a unique larval form having four suckers that represents the asexual reproductive form of Mesocestoides spp. infection (Fig. 6-20CC). Cestode ova are not usually found in the feces (Crosbie et al., 1998). Molecular testing is necessary for identification of the different Mesocestoides species (Crosbie et al., 2000). Cases of this parasitic disease have been reported in Italy, Germany, and Japan (Bonfanti et al., 2004, Kashiide et al., 2014, Wirtherle et al., 2007). See Appendix for more images.
Figure 6-20.
Cestodiasis. Peritoneal. Dog. Same case A and C.
A, Gross view of the ascitic fluid revealed a tapioca pudding appearance. Motility of these granules may be observed with the unaided eye. B, Acoelomic metacestode tissue with amorphous degenerate debris and calcareous corpuscles. Inflammatory cells are also present surrounding the structure. (Aqueous Romanowsky; LP.) C, Tetrathyridia larval stage. Note the oval structures at the left end that represent suckers and identify the parasite as Mesocestoides spp. (Aqueous Romanowsky; LP.)
(A and C, Courtesy of Jocelyn Johnsrude, IDEXX, West Sacramento, CA.)
Chylous Effusions
Chyle is a mixture of lymph and chylomicrons. Triglyceride-rich chylomicrons are derived from dietary lipids processed in the intestine and transported via lymphatics. Historically, chylous effusions were thought to be primarily a result of thoracic duct rupture. It is now known that there are a variety of causes for chylous effusions and that rupture of the thoracic duct is uncommon. Causes for chylous effusions in the thoracic cavity include cardiovascular disease, neoplasia (e.g., lymphoma, thymoma, and lymphangiosarcoma), heartworm disease, diaphragmatic hernia (Kerpsack et al., 1994), lung torsion, mediastinal fungal granulomas, chronic coughing, vomiting, primary lymphedema, iatrogenic, or idiopathic (Forrester et al., 1991, Fossum et al., 1986a, Fossum et al., 1991, Fossum, 1993, Mclane and Buote, 2011, Meakin et al., 2013, Neath et al., 2000, Schuller et al., 2011, Singh and Brisson, 2010, Small et al., 2008, Waddle and Giger, 1990). In one study (Fossum et al., 1986a), Afghan Hounds appeared to have a higher incidence of chylothorax than other breeds of dogs. This may be due to Afghans being overrepresented in cases of lung lobe torsion (Neath et al., 2000). Purebred cats were overrepresented in a 1991 retrospective study by Fossum et al. Chylous pleural effusions are more prevalent in cats (30.5%) than dogs (18.9%) (Davies and Forrester, 1996, Mellanby et al., 2002); however, the prevalence of chylothorax in the Mellanby study may be underestimated as mediastinal effusions were also included in that study. Chylomediastinum has not been described in animals.
Chyloperitoneum is less common than chylothorax with a prevalence of 6.7% in a retrospective study (Wright et al., 1999). Causes for chyloperitoneum include cardiovascular disease, FIP, neoplasia, steatitis, biliary cirrhosis, lymphatic rupture or leakage, postoperative accumulation following ligation of the thoracic duct, congenital lymphatic abnormalities, and other causes (Fossum et al., 1992, Gores et al., 1994, Nelson, 2001, Savary et al., 2001).
Grossly, chylous effusions have a milk-like, white to pink-white appearance, depending on the quantity of dietary fat content and the presence or absence of hemorrhage (Fig. 6-21A&B ). Chylous effusions may be clear or serosanguineous depending on the diet. Chylous effusions have a triglyceride concentration that is greater than the serum triglycerides concentration; often the effusion:serum ratio is greater than 3:1 (Fossum et al., 1986b, Meadows and MacWilliams, 1994). In addition, a cholesterol-to-triglyceride (C:T) ratio of less than one was proposed to be characteristic of a chylous effusion (Fossum et al., 1986b). However, another study found it to be less reliable (Waddle and Giger, 1990). Based on lipoprotein electrophoretic studies, pleural chylous effusions can be better identified by fluid triglyceride concentrations greater than 100 mg/dL and nonchylous effusions by concentrations less than 100 mg/dL (Waddle and Giger, 1990).
Figure 6-21.

Chylous effusion. Pleural. Cat.
A, A pink tint is found in this chylous effusion, indicating some degree of hemorrhage is present. The fluid had 13,000/μL nucleated cell count, 267 mg/dL triglycerides, and 169 mg/dL cholesterol. B, Turbid “milky” fluid in most cases, due to the presence of chyle. Measurement of high fluid triglyceride levels confirms the diagnosis. The cell count of this sample is less than 10,000 cells/μL and the protein is 4.0 g/dL.
(A, Courtesy of Rose Raskin, University of Florida.)
Cell counts and protein concentrations are typically increased vs. pure transudate parameters (Fig. 6-2). Therefore, chylous effusions generally fit into modified transudate or, more frequently, exudate categories depending on the degree of chronicity (Fossum et al., 1986a, Fossum et al., 1986b, Fossum et al., 1991). Notably, the total protein measured by refractometry can be very high due to artifactual interference by the lipid in the solution (George, 2001).
Initially, the chylous effusion is characterized by predominantly small lymphocytes (Figure 6-22, Figure 6-23 ) (Fossum et al., 1991). Chyle is an irritant and over time, neutrophils and/or macrophages become prominent, although small lymphocytes with lesser numbers of reactive lymphocytes are readily observed as are reactive mesothelial cells. “Smeared” nuclear material from ruptured lymphocytes may also be admixed (Fossum et al., 1986a, Fossum et al., 1986b, Schuller et al., 2011, Small et al., 2008). In addition, the inflammatory cells may contain phagocytized lipid, which appear as multiple discrete, colorless vacuoles within the cytoplasm (Fig. 6-23B).
Figure 6-22.
Chylous effusion. Peritoneum. Dog.
This direct smear is characteristic of the entire smear. It is composed of essentially all small lymphocytes. Numerous lysed cells are present, as the lipid in the solution acts as a detergent, making the cells particularly fragile. (Modified Wright; HP oil.)
Figure 6-23.
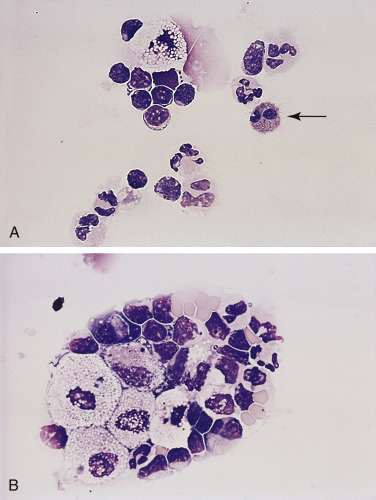
Chylous effusion. Pleural. Cat. A-B same case as in Figure 6-21B.
A, Note small lymphocytes, neutrophils, one eosinophil (arrow), and one large macrophage. Initially chylous effusions contain predominantly small lymphocytes and macrophages. As the duration of fluid presence increases, neutrophils and eosinophils will increase in number. (Romanowsky; HP oil.) B, Concentrated preparation. Note the punctate lipid vacuoles within macrophages along with small lymphocytes, neutrophils, and low number of red blood cells. (Romanowsky; HP oil.)
Hemorrhagic Effusions
Hemorrhagic effusions can occur in any of the major body cavities, including the pericardial sac. These effusions are serosanguineous to red depending on the duration of the exudate and the extent of the hemorrhage. Physical evaluation reveals a protein concentration slightly less than that of peripheral blood. PCV of effusions is often the same or slightly less than that of peripheral blood (Culp et al., 2010, Mandell and Drobatz, 1995, Mongil et al., 1995).
Hemoperitoneum is generally divided into traumatic and nontraumatic (spontaneous), with the former being subdivided into blunt trauma (e.g., motor vehicle accident) and penetrating (e.g., gunshot wound). Malignant neoplasia was the final diagnosis in 68.3% to 80% of canine cases of acute nontraumatic hemoperitoneum (Aronsohn et al., 2009, Pintar et al., 2003). Of them, 63.3% to 88% were determined to be hemangiosarcoma. Other causes of canine hemoperitoneum include hematomas, torsion (liver and splenic), and coagulopathies such as rodenticide intoxication (Aronsohn et al., 2009, Beal et al., 2008, Pintar et al., 2003). Similar studies in cats implicate malignant neoplasia as the etiology in feline hemoperitoneum in 44% to 46% of cases (Culp et al., 2010, Mandell and Drobatz, 1995). Hemangiosarcoma comprised 28% and 60% of these tumors. Other etiologies include coagulopathies, hepatic necrosis, ruptured urinary bladder, hepatic torsion, hepatic rupture secondary to amyloidosis, and FIP-associated lesions of the liver and kidney (Culp et al., 2010, Mandell and Drobatz, 1995, Swann and Brown, 2001).
Hemothorax is associated with a variety of etiologies (Mellanby et al., 2002). In the dog these include rodenticide intoxication (DuVall et al., 1989), neoplasia (Slensky et al., 2003), secondary to parasitic infestation (Chikweto et al., 2012, Sasanelli et al., 2008), and iatrogenic (Cohn et al., 2003). Feline hemothorax has been associated with rodenticide intoxication (DuVall et al., 1989) and fat embolism (Sierra et al., 2007).
Cytology can differentiate true hemorrhagic effusions from sample contamination at the time of collection. Hemorrhagic effusions contain predominantly red blood cells with relatively few numbers of leukocytes compared with that in peripheral blood. Noteworthy is the microscopic observation of macrophages containing phagocytized red cells (erythrophagocytosis) and/or hemosiderin, which confirms the effusion as hemorrhagic (Figure 6-24, Figure 6-25 ). These cells are best observed at the feathered edge of sediment smears. Hemorrhagic effusions do not contain platelets; if observed, an indication that the effusion was contaminated by peripheral blood during collection. A positive Prussian blue stain confirms the iron-rich pigment as hemosiderin (Fig. 6-25B).
Figure 6-24.
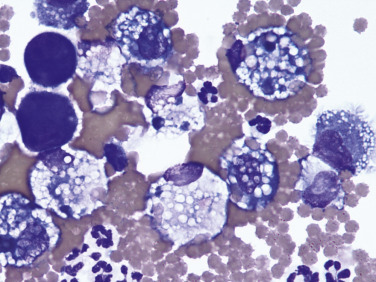
Hemorrhagic effusion. Pleura. Dog.
This direct smear shows numerous enlarged and vacuolated macrophages, a few nondegenerate neutrophils, and two deeply basophilic reactive mesothelial cells (left). Many macrophages contain varying amounts of hemosiderin and erythrophagia is occurring in several cells. (Modified Wright; HP oil.)
Figure 6-25.
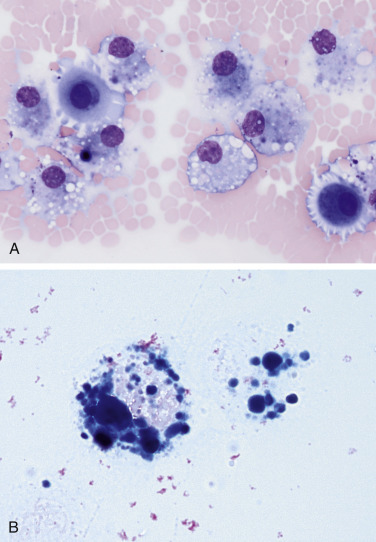
Hemorrhagic effusion. Cat. Same case A-B.
A, Several moderately foamy macrophages contain variable amounts of blue-grey, finely granular pigment, presumed to be hemosiderin. Also noted are two mildly basophilic and granular mesothelial cells, likely reactive. (Modified Wright; HP oil.) B, Two hemosiderophages are noted filled with Prussian blue positive material, confirming it as hemosiderin. (Prussian blue; HP oil.)
Neoplastic Effusions
Neoplasia is a common cause of abdominal and thoracic effusions in dogs and cats (Fig. 6-2). Gross appearance and cell count and total protein concentration parameters are generally not helpful in classifying neoplastic effusions. The salient cytologic characteristic is the microscopic observation of neoplastic cells.
In dogs and cats, the common causes of neoplastic effusions are lymphoma (pleural), and adenocarcinoma or carcinoma (pleural and peritoneal), with sarcomas and mesothelioma less common causations.
Diagnostic cells may not be evident in an effusion, so if a mass is present, fluid analysis along with fine-needle aspiration cytology of the mass increases the likelihood of identifying neoplasia. In one study, detection of malignant tumors in abdominal and thoracic fluids had a sensitivity of only 64% for dogs and 61% for cats; however, the specificity was high at 99% for dogs and 100% for cats (Hirschberger et al., 1999). One study found that telomerase activity had only 50% sensitivity and 83% specificity; the test was not recommended as a standalone diagnostic tool (Spangler et al., 2000). Cytology had a diagnostic accuracy of 94% and 99% in diagnosing ovarian carcinoma in effusions, as determined using two different cytopathologists (Bertazzolo et al., 2012).
Lymphoma
Effusions associated with lymphoma are generally highly cellular and contain an immature population of discrete round cells that are morphologically consistent with lymphocytes (Fig. 6-26A-C ). The neoplastic cells have high N:C ratios; scant to moderate amounts of cytoplasm; and often scattered, small, colorless cytoplasmic vacuoles. Occasionally granular lymphocytes are the predominant neoplastic cell (Fig. 6-27 ). Moderate numbers of mitoses are observed. Variable number of red blood cells, reactive mesothelial cells, and inflammatory cells may be admixed.
Figure 6-26.
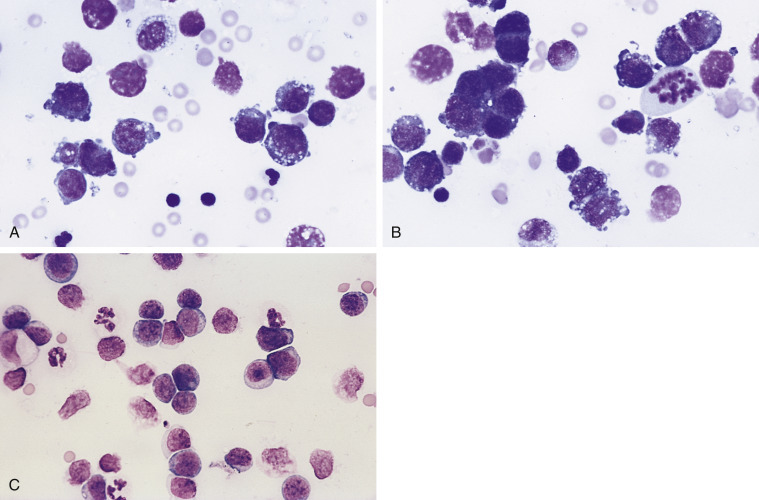
Neoplastic effusion. Lymphoma. Pleural. A, Dog.
Note the individual large round cells with high nuclear-to-cytoplasmic ratio and mildly vacuolated cytoplasm. Also evident are two small lymphocytes and one neutrophil. Light-purple, round structures are free nuclei from lysed cells. These cells cannot be evaluated. The cellularity of this sample is 15,000 cells/μL with a protein of 3.4 g/dL. (Romanowsky.) B, Dog. Note individual lymphoblasts, one mitotic figure, two intermediate-size lymphocytes, and one small lymphocyte. A few lysed cells and red blood cells are also present. (Romanowsky.) C, Cat. Note the monomorphic population of large lymphoblasts, few neutrophils, and few lysed cells. The lymphoblasts are larger than neutrophils and contain a small rim of cytoplasm with a large, round nucleus. The nuclear chromatin is fine and nucleoli are visible in many of the cells. The nucleated cell count of this fluid is 14,000 cells/μL with increased protein (4.0 g/dL). (Romanowsky; × 200.)
Figure 6-27.
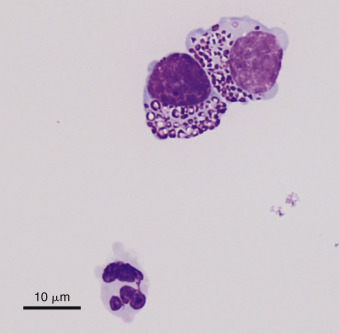
Neoplastic effusion. Granular cell lymphoma. Pleural. Cat.
This fluid was light yellow, hazy with a protein of 4.2 g/dL and WBC of 5600/μL. In addition, 63% of nucleated cells were granulated; two are shown. Granules varied from fine to coarse (as shown) and were frequently eccentrically placed to one side of the cell. Nondegenerate neutrophils (one shown), small lymphocytes, and occasional phagocytes were also present. (Wright-Giemsa; HP oil.)
(Courtesy of Rose Raskin, University of Florida.)
Although chyle can be associated with lymphoma (Fossum et al., 1986b, Fossum et al., 1991, Gores et al., 1994), the prominent malignant lymphocytes are readily observed (Fig. 6-26A&B). Rare cases of lymphoma have cells that are morphologically similar to normal small lymphocytes. These cases are problematic, and cytology can only suggest the possibility of lymphoma. In these cases additional diagnostic testing is required (Ridge and Swinney, 2004).
Evaluation of the types of lymphoid cells found within the fluid by immunocytochemistry or flow cytometry may provide useful information. A monotypic population of medium and/or large lymphocytes is more likely associated with neoplasia (Figs. 6-26C, Figure 6-28, Figure 6-29 ) than with a reactive process. Immunocytochemistry with antibodies against the molecules CD3 (T-cell) and CD79a or CD20 (B-cell) is helpful in these cases for minimal phenotyping. Additional information is found in Chapter 17.
Figure 6-28.
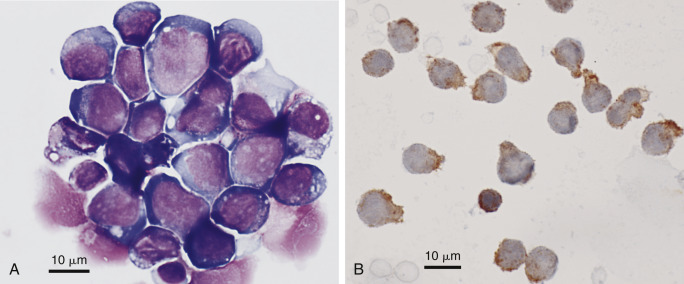
Neoplastic effusion. Pleural. T-cell lymphoma. Dog. Same case A-B.
A, Cytocentrifugated specimen demonstrating a variably sized round cell population having scant to moderate amounts of basophilic cytoplasm, fine to moderately coarse chromatin with occasional prominent nucleoli, and variable N:C. (Modified Wright; HP oil.) B, Sediment smear displays positive cell surface staining in all lymphoid cells for the CD3 antigen, which supports a clonal population of T-lymphocytes. Note the small, likely normal, lymphocyte below center. Red cells are barely visible beneath the size bar. Rare small normal lymphocyte reacted with CD79a antibody (not shown). (CD3 antibody; HP oil.)
(A and B, Courtesy of Rose Raskin, Purdue University.)
Figure 6-29.
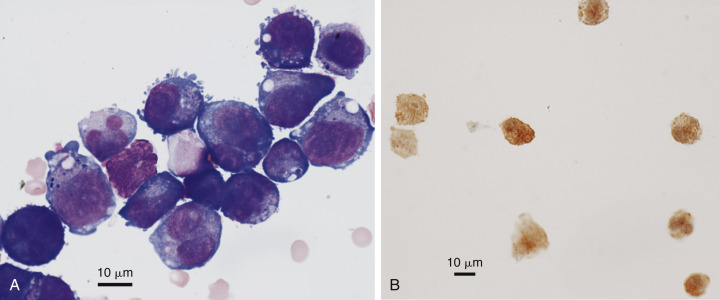
Neoplastic effusion. Pleural. B-cell lymphoma. Cat. Same case A-B.
A, Cytocentrifuged specimen demonstrating marked pleomorphism of round cells. Some cells have multiple nuclei and irregularly shaped nuclei. Nucleoli are usually large and multiple. There is surface blebbing on several cells, which may be mesothelial in origin. (Modified Wright; HP oil.) B, All large round cells are positively stained for the CD79a antigen in this sediment smear of pleural fluid supporting a B-cell neoplasm. CD3 antigen was absent on all cells (not shown). (CD79a antibody; HP oil.)
(A and B, Courtesy of Rose Raskin, Purdue University.)
Carcinoma and Adenocarcinoma
Effusions associated with carcinomas and adenocarcinomas may be the result of either a primary or secondary neoplasia. In the thorax the predominant neoplasm is pulmonary adenocarcinoma. In order for neoplastic cells from this tumor to be present in pleural effusions, the neoplasm has to invade either into pulmonary vessels and lymphatics or directly through the pleural surface of the lung and into the pleural cavity.
Pleural effusions associated with carcinomas are generally secondary to metastatic disease. Metastatic mammary carcinoma in females and prostatic carcinoma and transitional cell carcinoma in males are the more common neoplasms.
In the peritoneal cavity the predominant causes of effusions associated with carcinomas are those that spread by implantation on the peritoneal surface. These include cholangiocarcinoma, pancreatic adenocarcinoma, ovarian adenocarcinoma, and mammary carcinoma in females and prostatic carcinoma in males.
Cytologically, these tumors are morphologically similar and the organ of origin cannot be determined (Clinkenbeard, 1992). Effusions associated with carcinomas are characterized by the presence of rafts and acinar arrangements (adenocarcinoma) of round to polygonal cells with variable amounts of often extremely basophilic cytoplasm. Cytoplasmic basophilia may be so intense as to obscure nuclear detail. Inflammation may or may not be present, but reactive mesothelial cells are often present (Figs. 6-30A&B and 6-31A&B ).
Figure 6-30.
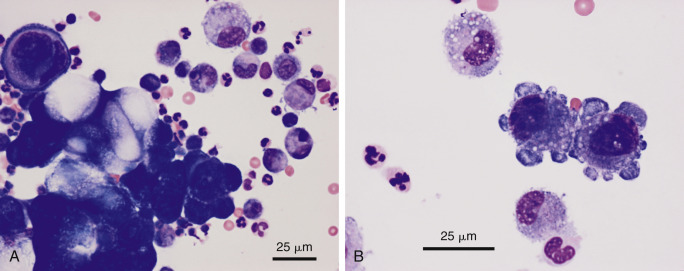
Neoplastic effusion. Adenocarcinoma. Pleural. Dog. Same case A-B.
A, Note the cohesive cluster of neoplastic epithelial cells. The large, distorting vacuoles suggest a secretory nature to the tissue of origin. Prominent nuclear molding is seen in the upper left side of the image. The background contains a mixture of inflammatory cells. (Modified Wright; HP oil.) B, Note the two neoplastic cells that exhibit marked pleomorphism of their nucleoli. Compare the neoplastic cells to the nearby macrophages. (Modified Wright; HP oil.)
Figure 6-31.
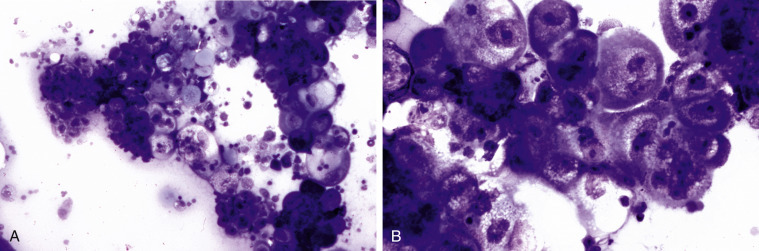
Neoplastic effusion. Adenocarcinoma. Pleural. Cat. Same case A-B.
A, Note the presence of clusters and sheets of large cells. This fluid is highly cellular (23,000 cells/μL) and has an increased protein content (3.6 g/dL). Inflammatory cells are often found in effusions associated with neoplasia. (Romanowsky; IP.) B, Note that the cells are large with abundant basophilic, lightly vacuolated cytoplasm. The nuclei are round with granular coarse chromatin and a large prominent nucleolus. Neutrophils can be seen within the cytoplasm of some of the neoplastic cells. (Romanowsky; HP oil.)
Carcinoma cells can resemble reactive mesothelial cells and be a challenge to differentiate. Generally, a diagnosis of neoplasia can be made if the cell population in question fulfills five strong nuclear criteria. Areas where nuclear detail can be seen must be found (Figs. 6-30B and 6-31B). When there is still cytologic ambivalence, a second opinion is recommended.
Adenocarcinomas are formed from secretory tissue, which often contains cytoplasmic vacuoles; this differentiates adenocarcinoma from other types of carcinoma. In some cells the amount of secretory product is sufficient to displace the nucleus peripherally, forming a balloon or signet ring cell (Fig. 6-32A&B ).
Figure 6-32.
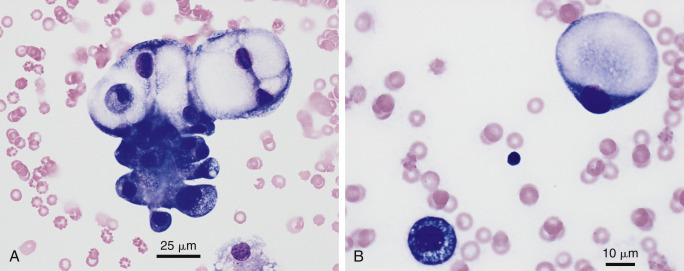
Neoplastic effusion. Signet ring formation. A, Pleural. Adenocarcinoma. Dog.
Marked anisocytosis and anisokaryosis are present within the same cell cluster. Abundant secretory product appears as clear material expanding the cytoplasm. (Modified Wright; HP oil.) B, Pleural. Mediastinal neurocarcinoma. Dog. Signet ring formation appears as the clear abundant cytoplasm expands, pushing the nucleus off to one side of the cell. Small lymphocyte in the center for size comparison. Diagnosis supported by co-expression of cytokeratin and chromogranin A. (Wright-Giemsa; HP oil.)
(A, Courtesy of Rose Raskin, Purdue University. B, Material courtesy of Mary Leissinger, Louisiana State University; Case 9 of 2013 ASVCP case review session.)
Mesothelioma
Mesothelioma is an uncommon tumor that arises from the mesothelial lining of the serous body cavities. One report involving five dogs suggested that mesotheliomas can arise in dogs secondary to chronic idiopathic pericardial hemorrhage (Machida et al., 2004). Cytologically, mesothelioma is extremely challenging to differentiate from carcinoma or marked reactive mesothelial hyperplasia. Mesothelioma cells are generally round to slightly polygonal in shape and are arranged primarily in clusters; however, spindle shapes have also been observed. This variation in cytomorphology likely arises from the multiple histologic subtypes, including a granular cell morphology, deciduoid (Morini et al., 2006), epithelioid (Leisewitz and Nesbit, 1992), cystic, sclerosing (Geninet et al., 2003), and even a lipid-rich form (Avakian et al., 2008). Nuclei are hyperchromic and located centrally. Often nuclei of adjacent cells within a cluster appear to press against each other resulting in deformation of nuclei (nuclear molding). Because of the difficulty of differentiating mesothelioma from reactive mesothelial hyperplasia, caution must be used when evaluating suspect populations for criteria of malignancy. As with effusions associated with carcinomas, at least five strong nuclear criteria of malignancy must be seen before a presumptive diagnosis is made. When there is still cytologic ambivalence, a second opinion is recommended, especially related to the variable cytologic morphology of mesothelioma (Figure 6-33, Figure 6-34, Figure 6-35 ).
Figure 6-33.
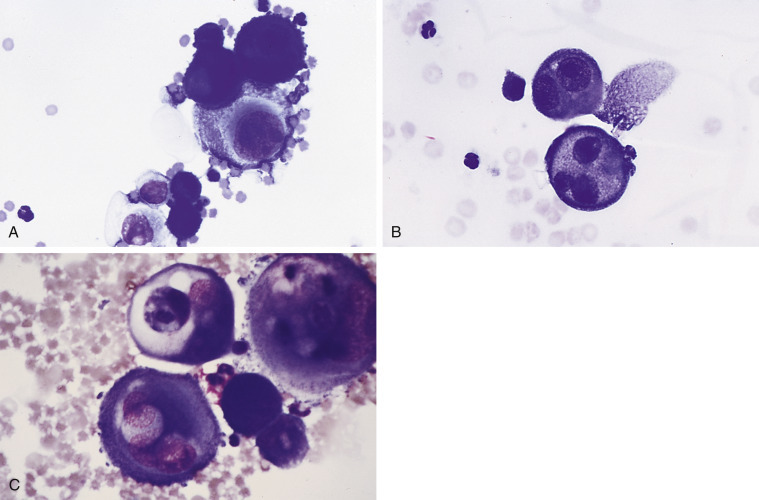
Neoplastic effusion. Mesothelioma. Same case A-C. A, Pleural. Dog.
The neoplastic cells are present as individual cells and in small clusters. The nucleoli are variably shaped and very prominent. (Romanowsky; HP oil.) B, These cells contain a variable amount of cytoplasm with one or more nuclei. The nuclei may be of odd number and variable size. (Romanowsky; HP oil.) C, The nuclear chromatin is coarsely granular and irregularly clumped with large prominent nucleoli. Many cells retain the fringed glycocalyx border. Also present are low numbers of small lymphocytes, neutrophils, and red blood cells. (Romanowsky; HP oil.)
Figure 6-34.
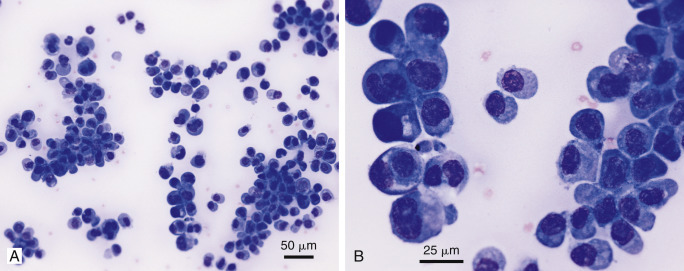
Neoplastic effusion. Mesothelioma. Pleural. Cat.
Same case A and B. A, Large cell clusters with papillary formation comprise the majority of the nucleated cell population. (Modified-Wright; IP.) B, Malignant features include anisokaryosis, multinucleation, high and variable nuclear to cytoplasmic ratio, coarse chromatin, and prominent nucleoli. (Modified-Wright; HP oil.) Diagnosis confirmed by immunochemical expression of cytokeratin, vimentin, and calretin.
(Material courtesy of Cheryl Swenson et al., Michigan State University; Case 3 of 2005 ASVCP case review session.)
Figure 6-35.
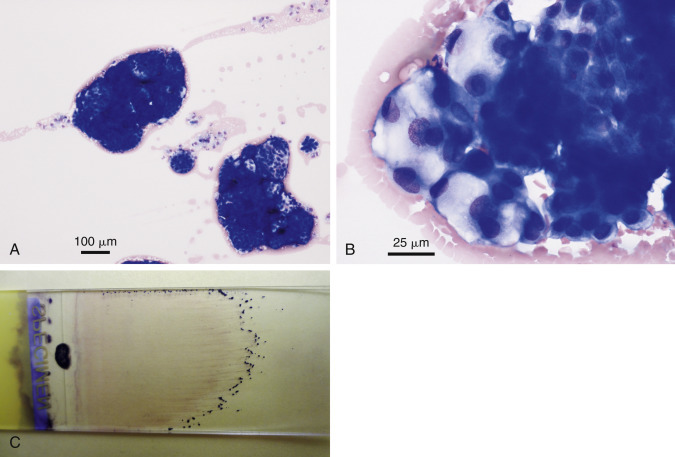
Neoplastic effusion. Mesothelioma. Abdominal. Dog.
Same case A-C. A, One small cluster of reactive mesothelium is shown in comparison with two large neoplastic cell clusters. (Wright-Giemsa; LP.) B, Cell clusters are variable in size and cytoplasmic vacuolation. Morphology is indistinguishable from that of secretory adenocarcinoma. (Wright-Giemsa; HP oil.) C, Large cell clusters are grossly visible at the feathered edge of a direct smear of this abdominal fluid.
(Material courtesy of Sarah Hammond et al., Virginia Tech University; Case 6 of 2012 ASVCP case review session.)
Once the diagnosis of malignancy has been established, cytology cannot further differentiate mesothelioma from carcinoma. A presumptive diagnosis of carcinoma is logical based on relative frequency of their occurrence. One tool to attempt to diagnose mesothelioma is the dual expression of cytokeratin and vimentin immunomarkers (Avakian et al., 2008, Bacci et al., 2006, Geninet et al., 2003, Gumber et al., 2011, Morini et al., 2006) (Fig. 6-36A&B ). A case of mesothelioma has been recognized by the use of the immunohistochemical marker calretinin in a horse (Stoica et al., 2004). Although calretinin has been used to diagnose mesothelioma in humans and one horse, this immunomarker has consistently been shown to be negative in dogs and cats (Bacci et al., 2006, Geninet et al., 2003, Morini et al., 2006).
Figure 6-36.
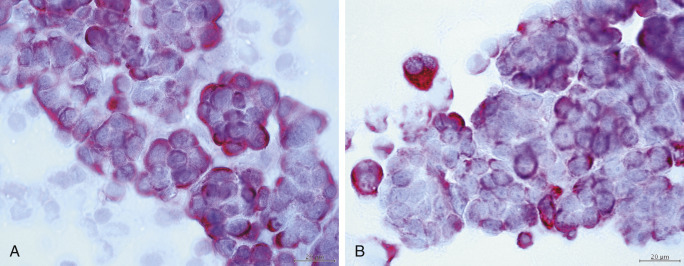
Neoplastic effusion. Mesothelioma. Dog.
Same case A and B. A, Immunocytochemistry from histologically confirmed case of mesothelioma. Many cell clusters react positively. (Pan-cytokeratin antibody; HP oil.) B, Immunocytochemistry demonstrates strong reaction within the cytoplasm of several multinucleated cells. (Vimentin antibody; HP oil.) Diagnosis of mesothelioma supported by concurrent reactivity of both vimentin and cytokeratin.
(Material courtesy of Kansas State University.)
Other Neoplasms
Several other tumors have been reported to be associated with effusions. These include a visceral mast cell tumor associated with a peritoneal effusion in a dog, malignant melanoma associated with a pleural effusion in a cat, and myxosarcoma associated with pleural effusions (de Souza et al., 2001, Morges and Zaks, 2011, Riegel et al., 2008, CC1 et al., 2012). Myxosarcoma has extensive background of dense, mucinous matrix, which often induces windrowing of the cells within the sample. The mediastinal area can produce neoplasms involving the thymus, thyroid, and neuroendocrine organs (Figs. 6-32B and 6-37 ).
Figure 6-37.
Neoplastic effusion. Pleural. Mediastinal neurocarcinoma. Dog.
Same case as Figure 32B. Cluster of cohesive cells with variable vacuolation and indistinct cytoplasmic cell borders. Diagnosis supported by co-expression of cytokeratin and chromogranin A. (Wright-Giemsa; HP oil.)
(Material courtesy of Mary Leissinger, Louisiana State University; Case 9 of 2013 ASVCP case review session.)
Pericardial Effusions
Pericardial effusions may also be classified as transudates, modified transudates, or exudates, and can be further subdivided into canine and feline effusions.
Canine
In many cases pericardial effusions are hemorrhagic (Fig. 6-38A-C ). The incidence of pericardial effusions associated with neoplasia in the dog varies between 38% and 71% (Berg and Wingfield, 1984, Kerstetter et al., 1997, MacDonald et al., 2009, Sisson et al., 1984). The incidence of pericarditis without an identifiable cause (idiopathic) varies between 19% and 67% (Berg and Wingfield, 1984, Sisson et al., 1984, Kerstetter et al., 1997, Atencia et al., 2013). Other infrequent reported causes include bacterial and fungal infections, cardiac insufficiency, uremia, trauma, foreign body, coagulopathy, hernia, and left atrial rupture (Aronson and Gregory, 1995, Berg and Wingfield, 1984, Bouvy and Bjorling, 1991, Peterson et al., 2003, Petrus and Henik, 1999, Shubitz et al., 2001).
Figure 6-38.
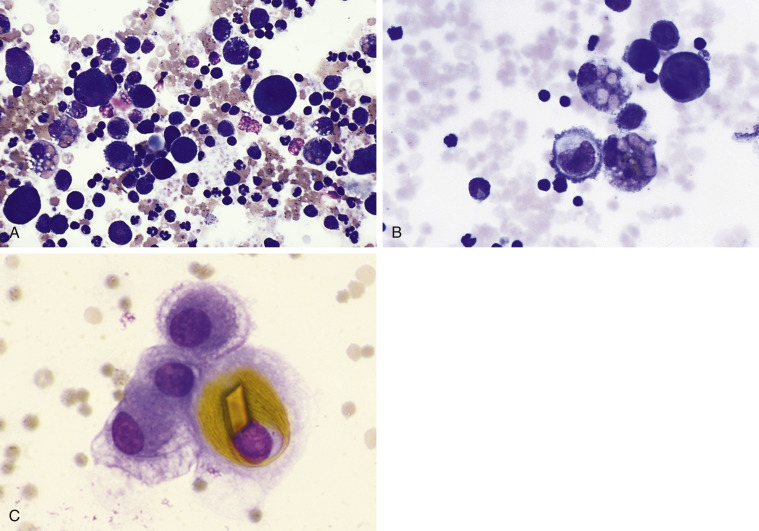
A, Hemorrhagic effusion. Pericardial. Dog.
Buffy-coat preparation. Note the variety of cells, including neutrophils, lymphocytes, erythrophages, and reactive mesothelial cells. This fluid was red and turbid with 5,000,000 red blood cells/μL, 7000 nucleated cells/μL, and protein of 4.0 g/dL. (Romanowsky; HP oil.) B, Hemorrhagic effusion. Pericardial. Dog. Observe the erythrophagia, reactive mesothelial cells, macrophage, and small lymphocytes with many red blood cells. Also note that one macrophage contains brown hemosiderin pigment. One mesothelial cell contains two nuclei. Cytologic evaluation of pericardial fluid is important to rule out infection and some types of neoplasia. Often additional testing is required to determine the cause of hemorrhagic pericardial effusion. (Romanowsky; HP oil.) C, Four mesothelial cells are present, one of which contains a golden rhomboid hematoidin crystal within the cytoplasm. (Wright-Giemsa; HP oil.)
(C, Courtesy of Rose Raskin, University of Florida.)
Cytologic evaluation of pericardial effusion is most valuable in diagnosing infection and inflammation (Fig. 6-39A&B ). The pericardial effusion is often associated with moderate to marked mesothelial hyperplasia, which mimics carcinoma cells. The mesothelial cells are very large with dark basophilic cytoplasm and are often binucleated with prominent nucleoli (Fig. 6-4B). Mitotic figures that are occasionally bizarre may mimic carcinoma. Consequently, cytology often cannot reliably differentiate mesothelial cell hyperplasia from neoplasia. Unlike hemangiosarcoma, which may cause pericardial hemorrhage and exfoliate large individualized neoplastic endothelial cells, other neoplasms of mesenchymal origin typically do not shed neoplastic cells into effusions. To underscore the difficulty in diagnosis, 74% of 19 neoplastic effusions were not detected with cytology and 13% of 31 nonneoplastic effusions were falsely reported as neoplastic. In another study that evaluated 47 pericardial effusions, cytology identified the cause in only six (12.8%); five were infectious and one was lymphoma (MacDonald et al., 2009). Interestingly, when PCV of the fluid was less than 10%, a cytologic diagnosis was made in 20.3% vs. only 7.7% when PCV was greater than 10% (Cagle et al., 2014).
Figure 6-39.
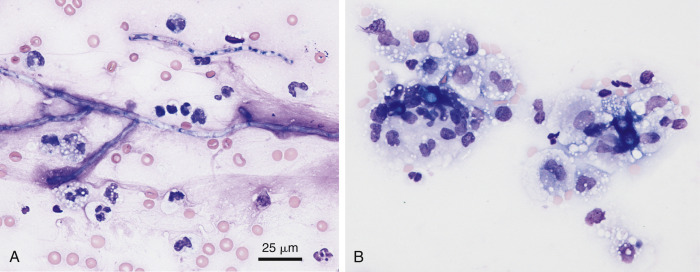
Mixed cell fungal exudate. Pericardial. Dog.
A, Aspergillosis. Degenerative neutrophils and macrophages surround septate, branching hyphae of Aspergillus sp. as confirmed by fungal culture. (Modified Wright; HP oil.) B, Blastomycosis. Within the feathered edge of a direct smear, several dark blue fungal thick-walled yeast of Blastomyces dermatitides are surrounded by many vacuolated macrophages and small numbers of degenerate neutrophils. (Wright-Giemsa; IP.)
(A and B, Courtesy of Rose Raskin, Purdue University.)
Serum cardiac troponin I (cTnI) and cardiac troponin T (cTnT) were evaluated in 37 dogs with pericardial effusions. Higher serum cTnI concentrations (mean 2.77 ng/dL; range 0.09 to 47.18 ng/dL) were more frequently associated with effusions related to hemangiosarcoma than with idiopathic pericardial effusions (mean 0.05 ng/dL; range 0.03 to 0.09 ng/dL). Serum cTnT was not diagnostically useful (Shaw et al., 2004). In another study a plasma cTnI concentration greater than 0.25 ng/mL had a sensitivity of 81% and specificity of 100% in identifying pericardial effusions associated with cardiac hemangiosarcoma (Chun et al., 2010).
Further testing is often required (e.g., ultrasonography, coagulation testing, pericardiectomy) to determine the underlying cause of the pericardial effusion. Measurement of pericardial fluid pH was found to be diagnostically helpful when measured by precise instrumentation (Edwards, 1996). A pH greater than 7.3 was more likely associated with noninflammatory disease, usually neoplasia. Using a urine dipstick to measure pH, a pericardial fluid pH greater than or equal to 7.0 was associated with neoplasia in 93% of the cases, whereas a value less than 7.0 was more often associated with nonneoplastic disease. Other studies using the urine dipstick to measure the pH of pericardial effusions generally found that there was too much overlap for it to be diagnostically reliable (Fine et al., 2003, de Laforcade et al., 2005).
Feline
Although pericardial disease is rare in cats (2.3%), FIP or congestive heart failure was associated with a pericardial effusion 88% of the time (Rush et al., 1990). A broader study (Hall et al., 2007) involving 146 samples found that 75.4% of cases of pericardial effusion had an assumed cause of congestive heart failure. Neoplasia accounted for 5.4% of cases, most of which were lymphoma. FIP was the assumed cause in one case (0.7%). Rush et al (1990) found neoplasia associated with 18% of cases, whereas Hall et al (2007) found neoplasia in 5.5% of cases. Lymphoma made up approximately half of the cases in both studies. Amati et al (2014) found six of seven cases of pericardial lymphoma being T-cell.
Miscellaneous Effusion Findings
MacGregor et al (2004) reported the presence of elongate to rhomboid-shaped cholesterol crystals in pericardial effusion from a dog. This was in contrast to a more typical plate appearance as from a case of canine peritoneal fluid (Fig. 6-40A&B ). Another uncommon finding was ciliated columnar cells arising from an oviductal hamartoma as described in the abdominal fluid from a dog (Fry et al., 2003). Barium crystals may be found in effusions postradiographic imaging following tissue injury (Fig. 6-41 ).
Figure 6-40.
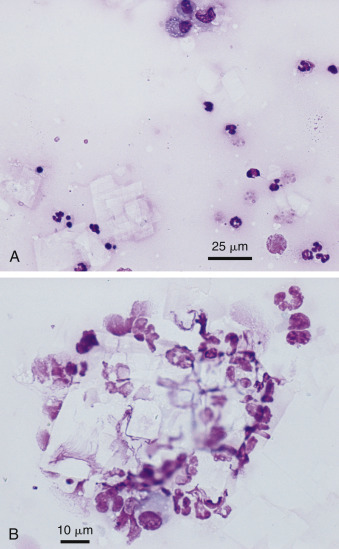
Cholesterol crystals. Abdominal. Dog.
Same case A-B. A, Rectangular crystals appear against the lightly eosinophilic background along with mononuclear phagocytes and degenerate neutrophils. (Romanowsky; HP oil.) B, Numerous degenerate neutrophils surround colorless rectangular crystals. (Romanowsky; HP oil.) Necropsy revealed sclerosing encapsulating peritonitis.
(Material courtesy of Tara Arndt, University of California/Ontario Veterinary College; Case 1 of 2010 ASVCP case review session.)
Figure 6-41.
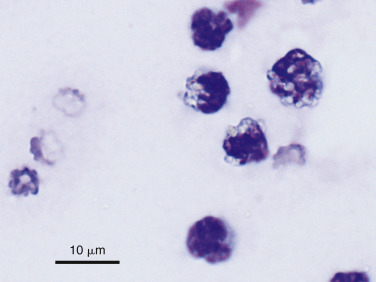
Barium crystals. Abdominal. Dog.
Degenerate neutrophils contain light yellow to colorless crystals. This may follow intestinal rupture after barium contrast imaging. (Romanowsky; HP oil.)
(Material courtesy of Colorado State University; Case 5 of 2000 ASVCP case review session.)
Ancillary Tests
In some instances, other laboratory testing of effusions may contribute useful information for differential diagnosis. A summary of previously described tests is shown in Table 6-1 .
Table 6-1.
Biochemical and Electrophoretic Tests Used to Evaluate Effusions
| Test | Use/Expected Result | Effusion |
|---|---|---|
| Creatinine/potassium | Fluid values are higher than those of serum creatinine and/or potassium | Uroperitoneum |
| Triglycerides | Fluids often contain triglycerides that exceed 100 mg/dL | Chylous |
| Cholesterol | Fluid level that is higher than that of serum cholesterol | Nonchylous |
| Bilirubin | Fluid level that is higher than serum bilirubin | Bile peritonitis/pleuritis |
| Lipase/amylase | Fluid values are higher than those of serum lipase and/or amylase | Pancreatitis |
| Protein electrophoresis | A:G ratio < 0.8 on the fluid is very suggestive for FIP | FIP infection |
| Lipoprotein electrophoresis | Presence of chylomicrons in fluid when triglyceride levels are equivocal | Chylous |
| pH | pH <7.0 may suggest benign or nonneoplastic conditions | Pericardial |
| pH, pCO2, glucose, lactate | Fluids with pH < 7.2, pCO2 > 55 mm Hg, glucose < 50 mg/dL, or lactate > 5.5 mmol/L are likely to have bacterial infection | Septic |
| Serum-effusion glucose | A difference of serum-effusion glucose of >20 mg/dL indicates bacterial infection | Septic peritonitis |
| Serum-effusion lactate | A difference in serum-effusion lactate of <−2.0 mmol/L (in dogs) indicates bacterial infection | Septic peritonitis |
References
- Allison R., Williams P., Lansdowne J. Fatal hepatic sarcocystosis in a puppy with eosinophilia and eosinophilic peritoneal effusion. Vet Clin Pathol. 2006;35(3):353–357. doi: 10.1111/j.1939-165x.2006.tb00148.x. [DOI] [PubMed] [Google Scholar]
- Amati M., Venco L., Roccabianca P. Pericardial lymphoma in seven cats. J Feline Med Surg. 2014;16:507–512. doi: 10.1177/1098612X13506199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronsohn M.G., Dubiel B., Roberts B. Prognosis for acute nontraumatic hemoperitoneum in the dog: a retrospective analysis of 60 cases (2003-2006) J Am Anim Hosp Assoc. 2009;45(2):72–77. doi: 10.5326/0450072. [DOI] [PubMed] [Google Scholar]
- Aronson L.R., Gregory C.R. Infectious pericardial effusion in five dogs. Vet Surg. 1995;24(5):402–407. doi: 10.1111/j.1532-950x.1995.tb01351.x. [DOI] [PubMed] [Google Scholar]
- Atencia S., Doyle R.S., Whitley N.T. Thoracoscopic pericardial window for management of pericardial effusion in 15 dogs. J Small Anim Pract. 2013;54(11):564–569. doi: 10.1111/jsap.12138. [DOI] [PubMed] [Google Scholar]
- Aumann M., Worth L.T., Drobatz K.J. Uroperitoneum in cats: 26 cases (1986-1995) J Am Anim Hosp Assoc. 1998;34:315–324. doi: 10.5326/15473317-34-4-315. [DOI] [PubMed] [Google Scholar]
- Avakian A., Alroy J., Rozanski E. Lipid-rich pleural mesothelioma in a dog. J Vet Diagn Invest. 2008;20(5):665–667. doi: 10.1177/104063870802000525. [DOI] [PubMed] [Google Scholar]
- Bacci B., Morandi F., De Meo M. Ten cases of feline mesothelioma: an immunohistochemical and ultrastructural study. J Comp Pathol. 2006;134(4):347–354. doi: 10.1016/j.jcpa.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Barrs V.R., Beatty J.A., McCandlish I.A. Hypereosinophilic paraneoplastic syndrome in a cat with intestinal T cell lymphosarcoma. J Small Anim Pract. 2002;43:401–405. doi: 10.1111/j.1748-5827.2002.tb00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer N. Flow cytometric analysis of effusions in dogs and cats with the automated haematology analyser ADVIA 120. Vet Record. 2012;156(21):674–678. doi: 10.1136/vr.156.21.674. [DOI] [PubMed] [Google Scholar]
- Beal M.W., Doherty A.M., Curcio K. Peliosis hepatis and hemoperitoneum in a dog with diphacinone intoxication. J Vet Emerg Crit Care. 2008;18:388–392. [Google Scholar]
- Berg R.J., Wingfield W. Pericardial effusion in the dog: a review of 42 cases. J Am Anim Hosp Assoc. 1984;30(5):721–730. [Google Scholar]
- Bertazzolo W., Bonfanti U., Mazzotti S. Cytologic features and diagnostic accuracy of analysis of effusions for detections of ovarian carcinoma in dogs. Vet Clin Pathol. 2012;41(1):127–132. doi: 10.1111/j.1939-165X.2011.00385.x. [DOI] [PubMed] [Google Scholar]
- Bonczynski J.J., Ludwig L.L., Barton L.J. Comparison of peritoneal fluid and peripheral blood pH, bicarbonate, glucose, and lactate concentrations as a diagnostic tool for septic peritonitis in dogs and cats. Vet Surg. 2003;32:161. doi: 10.1053/jvet.2003.50005. [DOI] [PubMed] [Google Scholar]
- Bonfanti U., Bertazzolo W., Pagliaro L. Clinical, cytological and molecular evidence of Mesocestoides sp. infection in a dog from Italy. J Vet Med A. 2004;51:435–438. doi: 10.1111/j.1439-0442.2004.00664.x. [DOI] [PubMed] [Google Scholar]
- Bounous D.I., Bienzle D., Miller-Liebl D. Pleural effusion in a dog. Vet Clin Pathol. 2000;29:55–58. doi: 10.1111/j.1939-165x.2000.tb00399.x. [DOI] [PubMed] [Google Scholar]
- Bouvy B.M., Bjorling D.E. Pericardial effusion in dogs and cats. Part I. Normal pericardium and causes and pathophysiology of pericardial effusion. Compend Contin Educ Pract Vet. 1991;13:417–424. [Google Scholar]
- Braun J.P., Guelfi J.F., Pagès J.P. Comparison of four methods for determination of total protein concentrations in pleural and peritoneal fluid from dogs. Am J Vet Res. 2001;62:294. doi: 10.2460/ajvr.2001.62.294. [DOI] [PubMed] [Google Scholar]
- Buob S. Portal hypertension: pathophysiology, diagnosis and treatment. J Vet Intern Med. 2011;25(2):169–186. doi: 10.1111/j.1939-1676.2011.00691.x. [DOI] [PubMed] [Google Scholar]
- Burrows C.F., Bovee K.C. Metabolic changes due to experimentally induced rupture of the canine urinary bladder. Am J Vet Res. 1974;35(8):1083–1088. [PubMed] [Google Scholar]
- Cagle L.A., Epstein S.E., Owens S.D. Diagnostic yield of cytologic analysis of pericardial effusion in dogs. J Vet Intern Med. 2014;28(1):66–71. doi: 10.1111/jvim.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso K.J., James M.P., Fisher D. Cytologic diagnosis of peritoneal cestodiasis in dogs caused by. Mesocestoides sp., Vet Clin Pathol. 2003;32(1):50–60. doi: 10.1111/j.1939-165x.2003.tb00314.x. [DOI] [PubMed] [Google Scholar]
- Chikweto A., Bhaiyat M.I., Tiwari K.P. Spirocercosis in owned and stray dogs in Grenada. Vet Parasitol. 2012;190(3-4):613–616. doi: 10.1016/j.vetpar.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Chun R., Kellihan H.B., Henik R.A. Comparison of plasma cardiac troponin I concentrations among dogs with cardiac hemangiosarcoma, noncardiac hemangiosarcoma, other neoplasms, and pericardial effusion of nonhemangiosarcoma origin. J Am Vet Med Assoc. 2010;237(7):806–811. doi: 10.2460/javma.237.7.806. [DOI] [PubMed] [Google Scholar]
- Clinkenbeard K.D. Diagnostic cytology: carcinomas in pleural effusions. Compend Contin Educ Pract Vet. 1992;14(2):187–194. [Google Scholar]
- Cohn L.A., Stoll M.R., Branson K.R. Fatal hemothorax following management of an esophageal foreign body. J Am Anim Hosp Assoc. 2003;39(3):251–256. doi: 10.5326/0390251. [DOI] [PubMed] [Google Scholar]
- Cowgill E., Neel J. Pleural fluid from a dog with marked eosinophilia. Vet Clin Pathol. 2003;32(3):147–149. doi: 10.1111/j.1939-165x.2003.tb00329.x. [DOI] [PubMed] [Google Scholar]
- Crosbie P.R., Boyce W.M., Platzer E.G. Diagnostic procedures and treatment of eleven dogs with peritoneal infections caused by Mesocestoides spp. J Am Vet Med Assoc. 1998;213:1578–1583. [PubMed] [Google Scholar]
- Edwards N.J. The diagnostic value of pericardial fluid pH determination. J Am Anim Hosp Assoc. 1996;32:63–67. doi: 10.5326/15473317-32-1-63. [DOI] [PubMed] [Google Scholar]
- Crosbie P.R., Platzer E.G., Nadler S.A. Molecular systematics of Mesocestoides spp (Cestoda: Mesocestoididae) from domestic dogs (Canis familiaris) and coyotes (Canis latrans) J Parasitol. 2000;82:350–357. doi: 10.1645/0022-3395(2000)086[0350:MSOMSC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Crowe DT: Diagnostic abdominal paracentesis techniques clinical evaluation in 129 dogs and cats. J Am Anim Hosp Assoc. 1984;20(2):223–230. [Google Scholar]
- Culp W.T., Weisse C., Kellogg M.E. Spontaneous hemoperitoneum in cats: 65 cases (1994-2006) J Am Vet Med Assoc. 2010;236(9):978–982. doi: 10.2460/javma.236.9.978. [DOI] [PubMed] [Google Scholar]
- Davidson B.J., Paling A.C., Lahmers S.L. Disease association and clinical assessment of feline pericardial effusion. J Am Anim Hosp Assoc. 2008;44(1):5–9. doi: 10.5326/0440005. [DOI] [PubMed] [Google Scholar]
- Davies C., Forrester S.D. Pleural effusion in cats: 82 cases (1987 to 1995) J Small Anim Pract. 1996;37(5):217–224. doi: 10.1111/j.1748-5827.1996.tb01772.x. [DOI] [PubMed] [Google Scholar]
- de Laforcade A.M., Freeman L.M., Rozanski E.A. Biochemical analysis of pericardial fluid and whole blood in dogs with pericardial effusion. J Vet Intern Med. 2005;19(6):833–836. doi: 10.1892/0891-6640(2005)19[833:baopfa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- de Souza M.L., Torres L.F., Rocha N.S. Peritoneal effusion in a dog secondary to visceral mast cell tumor. A case report. Acta cytologica. 2001;45(1):89–92. doi: 10.1159/000327194. [DOI] [PubMed] [Google Scholar]
- Dempsey S.M., Ewing P.J. A review of the pathophysiology, classification, and analysis of canine and feline cavitary effusions. J Am Anim Hosp Assoc. 2011;47(1):1–11. doi: 10.5326/JAAHA-MS-5558. [DOI] [PubMed] [Google Scholar]
- Dillon A.R., Teer P.A., Powers R.D. Canine abdominal histoplasmosis: a report of four cases. J Am Anim Hosp Assoc. 1982;18(3):498–502. [Google Scholar]
- DuVall M.D., Murphy M.J., Ray A.C. Case studies on second-generation anticoagulant rodenticide toxicities in nontarget species. J Vet Diagn Invest. 1989;1(1):66–68. doi: 10.1177/104063878900100118. [DOI] [PubMed] [Google Scholar]
- Eleni C., Scaramozzino P., Busi M. Proliferative peritoneal and pleural cestodiasis in a cat caused by metacestodes of Mesocestoides sp. Anatomohistopathological findings and genetic identification. Parasite. 2007;14(1):71–76. doi: 10.1051/parasite/2007141071. [DOI] [PubMed] [Google Scholar]
- Fine D.M., Tobias A.H., Jacob K.A. Use of pericardial fluid pH to distinguish between idiopathic and neoplastic effusions. J Vet Intern Med. 2003;17(4):525–529. doi: 10.1111/j.1939-1676.2003.tb02473.x. [DOI] [PubMed] [Google Scholar]
- Fischer Y., Sauter-Louis C., Hartmann K. Diagnostic accuracy of the Rivalta test for feline infectious peritonitis. Vet Clin Pathol. 2012;41(4):558–567. doi: 10.1111/j.1939-165X.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer Y., Weber K., Sauter-Louis C. The Rivalta’s test as a diagnostic variable in feline effusions – evaluation of optimum reaction and storage conditions. Tierarztliche Praxis Kleintiere. 2013;41(5):297–303. [PubMed] [Google Scholar]
- Forrester S.D., Fossum T.W., Rogers K.S. Diagnosis and treatment of chylothorax associated with lymphoblastic lymphosarcoma in four cats. J Am Vet Med Assoc. 1991;198:291–294. [PubMed] [Google Scholar]
- Forrester S.D., Troy G.C., Fossum T.W. Pleural effusions: pathophysiology and diagnostic considerations. Compend Contin Educ Pract Vet. 1988;10:121–136. [Google Scholar]
- Fossum T.W. Feline chylothorax. Compend Contin Educ Pract Vet. 1993;15:549–567. [Google Scholar]
- Fossum T.W., Birchard S.J., Jacobs R.M. Chylothorax in 34 dogs. J Am Vet Med Assoc. 1986;188:1315–1318. [PubMed] [Google Scholar]
- Fossum T.W., Forrester S.D., Swenson C.L. Chylothorax in cats: 37 cases (1969-1989) J Am Vet Med Assoc. 1991;198(4):672–678. [PubMed] [Google Scholar]
- Fossum T.W., Hay W.H., Boothe H.W. Chylous ascites in three dogs. J Am Vet Med Assoc. 1992;200:70–76. [PubMed] [Google Scholar]
- Fossum T.W., Jacobs R.M., Birchard S.J. Evaluation of cholesterol and triglyceride concentrations in differentiating chylous and nonchylous pleural effusions in dogs and cats. J Am Vet Med Assoc. 1986;188:49–51. [PubMed] [Google Scholar]
- Fry M.M., DeCock H.E.V., Greeley M.A. Abdominal fluid from a dog. Vet Clin Pathol. 2003;32:77–80. doi: 10.1111/j.1939-165x.2003.tb00318.x. [DOI] [PubMed] [Google Scholar]
- Fossum T.W., Wellman M., Relford R.L. Eosinophilic pleural or peritoneal effusions in dogs and cats: 14 cases (1986-1992) J Am Vet Med Assoc. 1993;202:1873–1876. [PubMed] [Google Scholar]
- Geninet C., Bernex F., Rakotovao F. Sclerosing peritoneal mesothelioma in a dog - a case report. J Vet Med A Physiol Pathol Clin Med. 2003;50(8):402–405. doi: 10.1046/j.0931-184x.2003.00566.x. [DOI] [PubMed] [Google Scholar]
- George J.W. The usefulness and limitations of hand-held refractometers in veterinary laboratory medicine: an historical and technical review. Vet Clin Pathol. 2001;30(4):201–210. doi: 10.1111/j.1939-165x.2001.tb00432.x. [DOI] [PubMed] [Google Scholar]
- George J.W., O’Neill S.L. Comparison of refractometer and biuret methods for total protein measurement in body cavity fluids. Vet Clin Pathol. 2001;30(1):16–18. doi: 10.1111/j.1939-165x.2001.tb00250.x. [DOI] [PubMed] [Google Scholar]
- Gidlewski J., Petrie J.-P. Therapeutic pericardiocentesis in the dog and cat. Clin Tech Small Anim Pract. 2005;20(3):151–155. doi: 10.1053/j.ctsap.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Gores B.R., Berg J., Carpenter J.L. Chylous ascites in cats: Nine cases (1978-1993) J Am Vet Med Assoc. 1994;205:1161–1164. [PubMed] [Google Scholar]
- Gumber S., Fowlkes N., Cho D.Y. Disseminated sclerosing peritoneal mesothelioma in a dog. J Vet Diagn Invest. 2011;23:1046–1050. doi: 10.1177/1040638711416625. [DOI] [PubMed] [Google Scholar]
- Hall D.J., Shofer F., Meier C.K. Pericardial effusion in cats: a retrospective study of clinical findings and outcome in 146 cats. J Vet Intern Med. 2007;21(5):1002–1007. doi: 10.1892/0891-6640(2007)21[1002:peicar]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Harris B.J., Constantino-Casas F., Archer J. Loeffler’s endocarditis and bicavity eosinophilic effusions in a dog with visceral mast cell tumour and hypereosinophilia. J Comp Pathol. 2013;149(4):429–433. doi: 10.1016/j.jcpa.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Hartmann K., Binder C., Hirschberger J. Comparison of different tests to diagnose feline infectious peritonitis. J Vet Intern Med. 2003;17:781–790. doi: 10.1111/j.1939-1676.2003.tb02515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetzel N., Papasouliotis K., Dodkin S. Biochemical assessment of canine body cavity effusions using three bench-top analysers. J Small Anim Pract. 2012:53459–53464. doi: 10.1111/j.1748-5827.2012.01240.x. [DOI] [PubMed] [Google Scholar]
- Hillerdal G. Chylothorax and pseudochylothorax. Euro Resp J. 1997;10(5):1157–1162. doi: 10.1183/09031936.97.10051157. [DOI] [PubMed] [Google Scholar]
- Hirschberger J., DeNicola D.B., Hermanns W. Sensitivity and specificity of cytologic evaluation in the diagnosis of neoplasia in body fluids from dogs and cats. Vet Clin Pathol. 1999;28:142–146. doi: 10.1111/j.1939-165x.1999.tb01065.x. [DOI] [PubMed] [Google Scholar]
- Holmberg T.A., Vernau W., Melli A.C. Neospora caninum associated with septic peritonitis in an adult dog. Vet Clin Pathol. 2006;35(2):235–238. doi: 10.1111/j.1939-165x.2006.tb00121.x. [DOI] [PubMed] [Google Scholar]
- Jabbar A., Papini R., Ferrini N. Use of a molecular approach for the definitive diagnosis of proliferative larval mesocestoidiasis in a cat. Infect Genet Evol. 2012;12(7):1377–1380. doi: 10.1016/j.meegid.2012.04.014. [DOI] [PubMed] [Google Scholar]
- James F.E., Knowles G.W., Mansfield C.S. Ascites due to pre-sinusoidal portal hypertension in dogs: a retrospective analysis of 17 cases. Aust Vet J. 2008;86(2):180–186. doi: 10.1111/j.1751-0813.2008.00284.x. [DOI] [PubMed] [Google Scholar]
- Kashiide T., Matsumoto J., Yamaya Y. Case report: first confirmed case of canine peritoneal larval cestodiasis caused by Mesocestoides vogae (syn. M. corti) in Japan. Vet Parasitol. 2014;201:154–157. doi: 10.1016/j.vetpar.2014.01.021. [DOI] [PubMed] [Google Scholar]
- Kauffman C.A. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev. 2007;20(1):115–132. doi: 10.1128/CMR.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerpsack S.J., McLouglin M.A., Graves T.K. Chylothorax associated with lung lobe torsion and a peritoneopericardial diaphragmatic hernia in a cat. J Am Anim Hosp Assoc. 1994;30(4):351–354. [Google Scholar]
- Kerstetter K.K., Krahwinkel D.J., Millis D.L. Pericardiectomy in dogs: 22 cases (1978-1994) J Am Vet Med Assoc. 1997;211:736–740. [PubMed] [Google Scholar]
- Klainbart S., Merchav R., Ohad D.G. Traumatic urothorax in a dog: a case report. J Small Anim Pract. 2011;52(10):544–546. doi: 10.1111/j.1748-5827.2011.01107.x. [DOI] [PubMed] [Google Scholar]
- Kowalewich N., Hawkins E.C., Skowronek A.J. Identification of Histoplasma capsulatum organisms in the pleural and peritoneal effusions of a dog. J Am Vet Med Assoc. 1993;202(3):423–426. [PubMed] [Google Scholar]
- Leisewitz A.L., Nesbit J.W. Malignant mesothelioma in a seven-week-old puppy. J S Afr Vet Assoc. 1992;63(2):70–73. [PubMed] [Google Scholar]
- Levin G.M., Bonczynski J.C., Ludwig L.L. Lactate as a diagnostic test for septic peritoneal effusions in dogs and cats. J Am Anim Hosp Assoc. 2004;40(5):364–371. doi: 10.5326/0400364. [DOI] [PubMed] [Google Scholar]
- Litster A.L., Pogranichniy R., Lin T.L. Diagnostic utility of a direct immunofluorescence test to detect feline coronavirus antigen in macrophages in effusive feline infectious peritonitis. Vet J. 2013;198(2):362–366. doi: 10.1016/j.tvjl.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig L.L., McLoughlin M.A., Graves T.K. Surgical treatment of bile peritonitis in 24 dogs and 2 cats: A retrospective study (1987–1994) Vet Surg. 1997;26(2):90–98. doi: 10.1111/j.1532-950x.1997.tb01470.x. [DOI] [PubMed] [Google Scholar]
- MacDonald K.A., Cagney O., Magne M.L. Echocardiographic and clinicopathologic characterization of pericardial effusion in dogs: 107 cases (1985–2006) J Am Vet Med Assoc. 2009;235(12):1456–1461. doi: 10.2460/javma.235.12.1456. [DOI] [PubMed] [Google Scholar]
- MacGregor J.M., Rozanski E.A., McCarthy R.J. Cholesterol-based pericardial effusion and aortic thromboembolism in a 9-year-old mixed-breed dog with hypothyroidism. J Vet Intern Med. 2004;18:354–358. doi: 10.1892/0891-6640(2004)18<354:cpeaat>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Machida N., Tanaka R., Takemura N. Development of pericardial mesothelioma in golden retrievers with a long-term history of idiopathic haemorrhagic pericardial effusion. J Comp Pathol. 2004;131(2-3):166–175. doi: 10.1016/j.jcpa.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Macintire D.K., Henderson R.H., Banfield C. Budd-Chiari syndrome in a kitten caused by membranous obstruction of the caudal vena cava. J Am Anim Hosp Assoc. 1995;31(6):484–491. doi: 10.5326/15473317-31-6-484. [DOI] [PubMed] [Google Scholar]
- Maher I., Tennant K.V., Papasouliotis K. Effect of storage time on automated cell count and cytological interpretation of body cavity effusions. Vet Rec. 2010;167(14):519–522. doi: 10.1136/vr.c4606. [DOI] [PubMed] [Google Scholar]
- Mandell D.C., Drobatz K. Feline hemoperitoneum 16 cases (1986-1993) J Vet Emerg Crit Care. 1995;5(2):93–97. [Google Scholar]
- Mclane M.J., Buote N.J. Lung lobe torsion associated with chylothorax in a cat. J Feline Med Surg. 2011;13(2):135–138. doi: 10.1016/j.jfms.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds C., Macy D. Feline infectious peritonitis. Part I: etiology and diagnosis. Compend Contin Educ Pract Vet. 1997;19(9):1007–1016. [Google Scholar]
- Meadows R.L., MacWilliams P.S. Chylous effusions revisited. Vet Clin Pathol. 1994;23:54–62. doi: 10.1111/j.1939-165x.1994.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Meakin L.B., Salonen L.K., Baines S.J. Prevalence, outcome and risk factors for postoperative pyothorax in 232 dogs undergoing thoracic surgery. J Small Anim Pract. 2013;54(6):313–317. doi: 10.1111/jsap.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellanby R.J., Villiers E., Herrtage M.E. Canine pleural and mediastinal effusions: a retrospective study of 81 cases. J Small Anim Pract. 2002;43:447–451. doi: 10.1111/j.1748-5827.2002.tb00011.x. [DOI] [PubMed] [Google Scholar]
- Meyer D.J., Franks P.T. Effusion: classification and cytologic examination. Compend Contin Educ Pract Vet. 1987;9:123–129. [Google Scholar]
- Miller B.H., Roudebush P., Ward H.G. Pleural effusion as a sequel to aelurostrongylosis in a cat. J Am Vet Med Assoc. 1984;185(5):556–557. [PubMed] [Google Scholar]
- Mongil C.M., Drobatz K., Dendricks J.C. Traumatic hemoperitoneum in 28 cases: A retrospective review. J Am Anim Hosp Assoc. 1995;31(3):217–222. doi: 10.5326/15473317-31-3-217. [DOI] [PubMed] [Google Scholar]
- Morges M.A., Zaks K. Malignant melanoma in pleural effusion in a 14-year-old cat. J Feline Med Surg. 2011;13(7):532–535. doi: 10.1016/j.jfms.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morini M., Bettini G., Morandi F. Deciduoid peritoneal mesothelioma in a dog. Vet Pathol. 2006;43(2):198–201. doi: 10.1354/vp.43-2-198. [DOI] [PubMed] [Google Scholar]
- Neath P.J., Brockman D.J., King L.G. Lung lobe torsion in dogs: 22 cases (1981–1999) J Am Vet Med Assoc. 2000;217(7):1041–1044. doi: 10.2460/javma.2000.217.1041. [DOI] [PubMed] [Google Scholar]
- Nelson K.L. Chyloabdomen in a mature cat. Can Vet J. 2001;42(5):381–383. [PMC free article] [PubMed] [Google Scholar]
- O’Brien P.J., Lumsden J.H. The cytologic examination of body cavity fluids. Semin Vet Med Surg (Small Anim) 1988;3(2):140–156. [PubMed] [Google Scholar]
- Norris J.M., Bosward K.L., White J.D. Clinicopathological findings associated with feline infectious peritonitis in Sydney, Australia: 42 cases (1990-2002) Aust Vet J. 2005;83(11):668–673. doi: 10.1111/j.1751-0813.2005.tb13044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens S.D., Gossett R., McElhaney M.R. Three cases of canine bile peritonitis with mucinous material in abdominal fluid as the prominent cytologic finding. Vet Clin Pathol. 2003;32:114–120. doi: 10.1111/j.1939-165x.2003.tb00324.x. [DOI] [PubMed] [Google Scholar]
- Paltrinieri S., Parodi M.C., Cammarata G. In vivo diagnosis of feline infectious peritonitis by comparison of protein content, cytology, and direct immunofluorescence test on peritoneal and pleural effusions. J Vet Diagn Invest. 1999;11(4):358–361. doi: 10.1177/104063879901100411. [DOI] [PubMed] [Google Scholar]
- Papasouliotis K., Murphy K., Dodkin S. Use of the Vettest 8008 and refractometry for determination of total protein, albumin, and globulin concentrations in feline effusions. Vet Clin Pathol. 2002;31:162–166. doi: 10.1111/j.1939-165x.2002.tb00297.x. [DOI] [PubMed] [Google Scholar]
- Parodi M.C., Cammarata G., Paltrinieri S. Using direct immunofluorescence to detect coronaviruses in peritoneal and pleural effusions. J Small Anim Pract. 1993;34(12):609–613. [Google Scholar]
- Parra M.D., Papasouliotis K., Ceron J.J. Concentrations of C-reactive protein in effusions in dogs. Vet Rec. 2006;158:753–757. doi: 10.1136/vr.158.22.753. [DOI] [PubMed] [Google Scholar]
- Patten P.K., Rich L.J., Zaks K. Cestode infection in 2 dogs: cytologic findings in liver and mesenteric lymph node. Vet Clin Pathol. 2013;42(1):103–105. doi: 10.1111/vcp.12016. [DOI] [PubMed] [Google Scholar]
- Peaston A.E., Griffey S.M. Visceral mast cell tumour with eosinophilia and eosinophilic peritoneal and pleural effusions in a cat. Aust Vet J. 1994;71(7):215–217. doi: 10.1111/j.1751-0813.1994.tb03405.x. [DOI] [PubMed] [Google Scholar]
- Peterson P.B., Miller M.W., Hansen E.K. Septic pericarditis, aortic endarteritis, and osteomyelitis in a dog. J Am Anim Hosp Assoc. 2003;39(6):528–532. doi: 10.5326/0390528. [DOI] [PubMed] [Google Scholar]
- Petrus D.J., Henik R.A. Pericardial effusion and cardiac tamponade secondary to brodifacoum toxicosis in a dog. J Am Vet Med Assoc. 1999;215:647–648. [PubMed] [Google Scholar]
- Pinta da Cunha N., Giordano A., Caniatti M. Analytical validation of the Sysmex XT-2000iV for cell counts in canine and feline effusions and concordance with cytologic diagnosis. Vet Clin Pathol. 2009;38(2):230–241. doi: 10.1111/j.1939-165X.2008.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintar J., Breitschwerdt E.B., Hardie E.M. Acute nontraumatic hemoabdomen in the dog: a retrospective analysis of 39 cases (1987-2001) J Am Anim Hosp Assoc. 2003;39(6):518–522. doi: 10.5326/0390518. [DOI] [PubMed] [Google Scholar]
- Ridge L., Swinney G. Angiotrophic intravascular lymphosarcoma presenting as bi-cavity effusion in a dog. Aust Vet J. 2004;82(10):616–618. doi: 10.1111/j.1751-0813.2004.tb12604.x. [DOI] [PubMed] [Google Scholar]
- Riegel C.M., Stockham S.L., Patton K.M. What is your diagnosis? Muculent pleural effusion from a dog. Vet Clin Pathol. 2008;37(3):353–356. doi: 10.1111/j.1939-165X.2008.00043.x. [DOI] [PubMed] [Google Scholar]
- Rush J.E., Keene B.W., Fox P.R. Pericardial disease in the cat: a retrospective evaluation of 66 cases. J Am Anim Hosp Assoc. 1990;26(1):39–46. [Google Scholar]
- Sasanelli M., Paradies P., Otranto D. Haemothorax associated with Angiostrongylus vasorum infection in a dog. J Small Anim Pract. 2008;49(8):417–420. doi: 10.1111/j.1748-5827.2008.00551.x. [DOI] [PubMed] [Google Scholar]
- Savary K.C.M., Sellon R.K., Law J.H. Chylous abdominal effusion in a cat with feline infectious peritonitis. J Am Anim Hosp Assoc. 2001;37(1):35–40. doi: 10.5326/15473317-37-1-35. [DOI] [PubMed] [Google Scholar]
- Schmiedt C., Tobias K.M., Otto C.M. Evaluation of abdominal fluid:peripheral blood creatinine and potassium ratios for diagnosis of uroperitoneum in dogs. J Vet Emerg Crit Care. 2001;11(4):275–280. [Google Scholar]
- Schuller S., Garreres A.L., Remy I. Idiopathic chylothorax and lymphedema in 2 whippet littermates. Can Vet J. 2011;52(11):1243–1245. [PMC free article] [PubMed] [Google Scholar]
- Shaw S.P., Rozanski E.A., Rush J.E. Cardiac troponins I and T in dogs with pericardial effusion. J Vet Intern Med. 2004;18(3):322–324. doi: 10.1892/0891-6640(2004)18<322:ctiati>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Shaw S.P., Rush J.E. Canine pericardial effusion: diagnosis, treatment, and prognosis. Compend Contin Educ Pract Vet. 2007;29(7):405–411. [PubMed] [Google Scholar]
- Shelly S.M., Scarlett-Kranz J., Blue J.T. Protein electrophoresis on effusions from cats as a diagnostic test for feline infectious peritonitis. J Am Anim Hosp Assoc. 1988;24:495–500. [Google Scholar]
- Shubitz L.F., Matz M.E., Noon T.H. Constrictive pericarditis secondary to Coccidioides immitis infection in a dog. J Am Vet Med Assoc. 2001;218(4):537–540. doi: 10.2460/javma.2001.218.537. [DOI] [PubMed] [Google Scholar]
- Sierra E., Rodríguez F., Herráez P. Post-traumatic fat embolism causing haemothorax in a cat. Vet Rec. 2007;161(5):170–172. doi: 10.1136/vr.161.5.170. [DOI] [PubMed] [Google Scholar]
- Singh A., Brisson B.A. Chylothorax associated with thrombosis of the cranial vena cava. Can Vet J. 2010;51(8):847–852. [PMC free article] [PubMed] [Google Scholar]
- Sisson D., Thomas W.P., Ruehl W.W. Diagnostic value of pericardial fluid analysis in the dog. J Am Vet Med Assoc. 1984;184:51–55. [PubMed] [Google Scholar]
- Slensky K.A., Volk S.W., Schwarz T. Acute severe hemorrhage secondary to arterial invasion in a dog with thyroid carcinoma. J Am Vet Med Assoc. 2003;223(5):649–653. doi: 10.2460/javma.2003.223.649. [DOI] [PubMed] [Google Scholar]
- Small M.T., Atkins C.E., Gordon S.G. Use of a nitinol gooseneck snare catheter for removal of adult Dirofilaria immitis in two cats. J Am Vet Med Assoc. 2008;233(9):1441–1445. doi: 10.2460/javma.233.9.1441. [DOI] [PubMed] [Google Scholar]
- Sommerey CC1., Borgeat K.A., Hetzel U. Intrathoracic myxosarcoma in a dog. J Comp Pathol. 2012;147(2-3):199–203. doi: 10.1016/j.jcpa.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Songer J.G., Post K.W. Saunders; St. Louis: 2005. Veterinary microbiology: bacterial and fungal agents of animal disease. pp 10-12, 55-59, 83–86. [Google Scholar]
- Spangler E.A., Rogers K.S., Thomas J.S. Telomerase enzyme activity as a diagnostic tool to distinguish effusions of malignant and benign origin. J Vet Intern Med. 2000;14(2):146–150. doi: 10.1892/0891-6640(2000)014<0146:teaaad>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Sparkes A.H., Gruffydd-Jones T.J., Harbour D.A. An appraisal of the value of laboratory tests in the diagnosis of feline infectious peritonitis. J Am Anim Hosp Assoc. 1994;30(4):345–350. [Google Scholar]
- Spector D., Legendre A.M., Wheat J. Antigen and antibody testing for the diagnosis of blastomycosis in dogs. J Vet Intern Med. 2008;22(4):839–843. doi: 10.1111/j.1939-1676.2008.0107.x. [DOI] [PubMed] [Google Scholar]
- Stern A., Walder E.J., Zontine W.J. Canine Mesocestoides infections. Compend Contin Educ Pract Vet. 1987;9:223–231. [Google Scholar]
- Stewart R.H. Editorial: the case for measuring plasma colloid osmotic pressure. J Vet Intern Med. 2000;14(5):473–474. [PubMed] [Google Scholar]
- Stickle J.E., Hribernik T.N. Clinicopathologic observations in disseminated histoplasmosis in dogs. J Am Anim Hosp Assoc. 1978;14(1):105–110. [Google Scholar]
- Stoica G., Cohen N., Mendes O. Use of immunohistochemical marker calretinin in the diagnosis of a diffuse malignant metastatic mesothelioma in an equine. J Vet Diagn Invest. 2004;16(3):240–243. doi: 10.1177/104063870401600313. [DOI] [PubMed] [Google Scholar]
- Störk C.K., Hamaide A.J., Schwedes C. Hemiurothorax following diaphragmatic hernia and kidney prolapse in a cat. J Feline Med Surg. 2003;5(2):91–96. doi: 10.1016/S1098-612X(02)00097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann H.M., Brown D.C. Hepatic lobe torsion in 3 dogs and a cat. Vet Surg. 2001;30(5):482–486. doi: 10.1053/jvet.2001.25877. [DOI] [PubMed] [Google Scholar]
- Sykes J.E. Actinomycosis and nocardiosis. In: Greene C.E., editor. Infectious diseases of the dog and cat. ed 4. Elsevier Saunders; St. Louis: 2012. pp. 484–520. [Google Scholar]
- Tomiyasu H., Fujino Y., Ugai J. Eosinophilia and eosinophilic infiltration into splenic B-cell high-grade lymphoma in a dog. J Vet Med Sci. 2010;72(10):1367–1370. doi: 10.1292/jvms.09-0544. [DOI] [PubMed] [Google Scholar]
- Toomey J.M., Carlisle-Nowak M.M., Barr S.C. Concurrent toxoplasmosis and feline infectious peritonitis in a cat. J Am Anim Hosp Assoc. 1995;31:425–428. doi: 10.5326/15473317-31-5-425. [DOI] [PubMed] [Google Scholar]
- Toplu N., Yildiz K., Tunay R. Massive cystic tetrathyridiosis in a dog. J Small Anim Pract. 2004;45(8):410–412. doi: 10.1111/j.1748-5827.2004.tb00257.x. [DOI] [PubMed] [Google Scholar]
- Tseng L.W., Waddell L.S. Approach to the patient in respiratory distress. Clin Tech Small Anim Pract. 2000;15(2):53–62. doi: 10.1053/svms.2000.6805. [DOI] [PubMed] [Google Scholar]
- VanSteenhouse J.L., DeNovo R.C. Atypical Histoplasma capsulatum infection in a dog. J Am Vet Med Assoc. 1986;188(5):527–528. [PubMed] [Google Scholar]
- Venco L., Kramer L., Pagliaro L. Ultrasonographic features of peritoneal cestodiasis caused by Mesocestoides sp. in a dog and in a cat. Vet Radiol Ultrasound. 2005;46(5):417–422. doi: 10.1111/j.1740-8261.2005.00076.x. [DOI] [PubMed] [Google Scholar]
- Waddle J.R., Giger U. Lipoprotein electrophoresis differentiation of chylous and nonchylous pleural effusions in dogs and cats and its correlation with pleural effusion triglyceride concentration. Vet Clin Pathol. 1990;19:80–85. doi: 10.1111/j.1939-165x.1990.tb00548.x. [DOI] [PubMed] [Google Scholar]
- Walters J.M. Abdominal paracentesis and diagnostic peritoneal lavage. Clin Tech Small Anim Pract. 2003;18(1):32–38. doi: 10.1016/1096-2867(03)90023-5. [DOI] [PubMed] [Google Scholar]
- Wheat L.J. Current diagnosis of histoplasmosis. Trends Microbiol. 2003;11(10):488–494. doi: 10.1016/j.tim.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Wirtherle N., Wiemann A., Ottenjann M. First case of canine peritoneal larval cestodosis caused by Mesocestoides lineatus in Germany. Parasitol International. 2007;56:317–320. doi: 10.1016/j.parint.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Wright K.N., Gompf R.E., DeNovo R.C., Jr. Peritoneal effusion in cats: 65 cases (1981-1997) J Am Vet Med Assoc. 1999;214(3):375–381. [PubMed] [Google Scholar]
- Zoia A., Slater L.A., Heller J. A new approach to pleural effusion in cats: markers for distinguishing from exudates. J Feline Med Surg. 2009;11(10):847–855. doi: 10.1016/j.jfms.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


