Changes in Body Temperature
Assessment of body temperature is an essential part of every physical examination. As with all mammalian species, horses normally maintain their core body temperatures within a narrow range despite extremes in environmental conditions.1, 2 The core temperatures may vary by approximately 1°C (2°F) between individuals. In adult horses the normal body temperature ranges from 37.2° to 38.3°C (99.0°–101.0°F), whereas in neonatal foals the temperature tends to be slightly higher, ranging from 37.8° to 38.9°C (100.0°–102.0°F). Diurnal variation of up to 1°C (2°F) may occur, with the low point typically occurring in the morning and the peak in the late afternoon.
Mechanisms Controlling Body Temperature
The set-point, which is the crucial temperature that the body attempts to maintain, is regulated primarily via neuronal control operating through temperature centers in the hypothalamus.3, 4, 5 Both peripheral and central thermoreceptors sense changes in ambient and core body temperatures and activate feedback mechanisms that bring the temperature back to the set-point. Specifically, peripheral thermoreceptors, which are generally most sensitive to low temperatures, are located in the skin and around certain great veins, as well as in some deep tissues, such as the spinal cord and abdominal viscera. Central thermoreceptors include large numbers of heat-sensitive neurons and lower numbers of cold-sensitive neurons in the preoptic area of the anterior hypothalamus (POA). In response to changes in temperature, the peripheral and central thermoreceptors transmit signals into the posterior hypothalamic area, subsequently activating autonomic and behavioral effector responses to regulate body temperature. These responses affect the balance between heat loss and heat production.
There are multiple mechanisms for cooling in response to elevations in body temperature, which include both means of increasing heat loss and decreasing heat production.3, 4, 5 One means of increasing heat loss is by transferring heat from the body core to the surface by increasing blood flow to the skin. Changes in core body temperature and environmental temperature cause the sympathetic nervous system to regulate the degree of vasoconstriction and thus the amount of blood flow, with increases in temperature resulting in cutaneous vasodilation and increased skin blood flow. Heat is lost from body surfaces to the surroundings by several physical mechanisms, including radiation, conduction, and convection. Evaporation is also an important mechanism of heat loss in horses.5 To some extent, the amount of evaporative heat loss is controlled by the rate of sweating. However, even when the animal is not sweating, water evaporates insensibly from the skin and lungs, resulting in continual heat loss. In horses, evaporative heat loss, primarily through increased sweating but also through increased respiration, becomes more important as the ambient temperature rises and during exercise.5, 6 In addition to increased heat loss when the body temperature rises, the horse also decreases temperature further by inhibiting means of heat production, such as shivering, and by behavioral responses, such as seeking shade and wind currents and wading into water.6, 7, 8
Mechanisms that increase body temperature are triggered when the body temperature is too low.2 Heat is conserved by stimulation of the posterior hypothalamic sympathetic centers, leading to cutaneous vasoconstriction and piloerection. Heat production also increases and may occur through increased muscle activity ranging from inapparent contractions to generalized shivering. Shivering may increase heat production by 4 to 5 times baseline. The primary motor center for shivering is in the posterior hypothalamus, which normally is stimulated by cold signals from the peripheral receptors and to some extent central receptors in the POA. Digestion of food also contributes to total body heat. Sympathetic stimulation may increase the rate of cellular metabolism, increasing heat production by chemical thermogenesis. Cooling also increases the production of thyrotropin-releasing hormone, ultimately increasing thyroid hormones and cellular metabolism and further contributing to chemical thermogenesis. In addition to these physiologic adaptations, behavioral responses to conserve heat also occur, such as adopting a huddled stance, aggregating in groups, and seeking shelter.9, 10, 11 It has been demonstrated that horses voluntarily select shelter, especially under wet, windy conditions and that shelter selection is affected by breed, body condition score, and hair coat weight.9
Increased Body Temperature: Hyperthermia and Fever
Elevation of the body temperature above normal is one of the most common clinical problems encountered, and although classically associated with infection, a variety of disorders may cause increased body temperature.3, 4, 5 Veterinarians should distinguish between conditions of hyperthermia, in which the temperature set-point is unaltered, and true fever, in which the set-point actually increases.
Hyperthermia
Mechanisms of Hyperthermia
The body temperature may become elevated without an increase in the set-point when there is a loss of equilibrium in the heat balance equation.4 Increased heat production or absorption of heat beyond the ability of the body to dissipate heat may occur. In some conditions impaired heat loss also may occur.
Conditions Associated with Hyperthermia
Hyperthermic conditions include problems such as exercise-related hyperthermia, heat stroke, anhidrosis, malignant hyperthermia, central nervous system disorders, and reactions to certain toxins or drugs (Box 7.1 ). In general, these conditions do not respond to treatment with antipyretic drugs.
BOX 7.1. Causes of Changes in Body Temperature.
Hyperthermia
Exercise-related hyperthermia
Heat stroke
Anhidrosis
Malignant hyperthermia
Central nervous system disorders
Toxins or drugs
Macrolide-induced hyperthermia
Fever
Infection
Neoplasia
Immune-mediated disease
- Other
- Toxic hepatopathy
- Inflammatory bowel disease
- Other
Hypothermia
Accidental Hypothermia
Exposure to harsh environmental conditions
Surgical procedures/anesthesia
Pathologic Hypothermia
Sepsis/inflammation (maladaptive response)
Intracranial disease
Hypothyroidism (neonates)
Exercise-Related Hyperthermia
During sustained or high-intensity exercise, increased heat production is associated with muscular activity.5, 6, 12, 13 The heat produced may exceed the ability of the body to lose heat, resulting in an increased core body temperature. Typically, the temperature returns to normal with rest as heat loss mechanisms remain activated. There is some evidence that ageing compromises the ability of horses to thermoregulate during exercise.14 Elevated temperature also may occur with the intense muscle activity associated with generalized seizures.
Heat Stroke
Heat stroke occurs when the body temperature rises above a critical temperature, leading to multisystemic problems. In horses signs of heat stroke may develop when the body temperature is above 41.5°C (107°F), which most often occurs in association with exercise in environmentally stressful conditions.13, 15 Although horses can acclimatize to various weather conditions to some extent, the efficiency of evaporative heat loss may be compromised significantly in hot, humid weather.6, 12, 13, 15 Susceptibility to heat stroke may increase if sweating leads to dehydration and electrolyte imbalances. Once the body temperature reaches the critical point, the homeostatic mechanisms of thermoregulation fail, resulting in peripheral vasoconstriction, decreased cardiac output, and decreased blood pressure. Affected horses are lethargic with weak, flaccid muscles. Prostration, circulatory shock, disseminated intravascular coagulation, multiple organ failure, and death may occur.
Anhidrosis
Anhidrosis is an inappropriate response to prolonged climatic stress characterized by a partial or total loss of the ability to sweat.16, 17, 18 This condition occurs primarily in horses living in hot, humid environments and has been documented in about 2% to 6% of horses living in Florida.16, 19 The impaired sweating results in diminished heat loss, frequently resulting in hyperthermia. Other clinical signs include tachypnea, poor performance, and poor hair coat.
Malignant Hyperthermia
Malignant hyperthermia (MH) encompasses a group of inherited skeletal muscle disorders in which calcium metabolism is altered. Although the condition is most common in human beings and pigs, it has been reported in several species, including dogs and horses.20, 21, 22, 23 MH is characterized by a hypermetabolic state of muscle that generally is induced by halogenated inhalation anesthetics, depolarizing skeletal muscle relaxants, and occasionally local anesthetics or stressors such as heat or vigorous exercise. Clinical signs include a rapid increase in core body temperature, skeletal muscle rigidity, and tachycardia. Affected animals may develop significant acidosis and muscle necrosis, and the condition can be fatal. MH is most often associated with a defect in the ryanodine receptor.20, 23 In Quarter Horses and American Paint Horses, MH is inherited as an autosomal dominant trait that is linked to a single point mutation in the skeletal muscle ryanodine receptor 1 (RyR1) gene at nucleotide C7360G.23, 24 This mutation can be identified by genetic testing. Horses with polysaccharide storage myopathy tend to have a more severe clinical phenotype if they are concurrently affected with the MH mutation.24
Central Nervous System
Alterations in body temperature have been documented with a variety of conditions affecting areas of the hypothalamus involved in thermoregulation, with hyperthermia being more common than hypothermia.1, 3, 4, 25 Central hyperthermia has been associated with conditions such as hemorrhage, traumatic brain injury, neoplasms or abscesses, infectious/inflammatory changes, and degenerative disorders. It is usually characterized by a lack of diurnal variation, absence of sweating, resistance to antipyretic drugs, and excessive response to external cooling. In some cases, damage to the hypothalamus results in a disruption in the hypothalamic set-point temperature, resulting in neurogenic fever rather than true hyperthermia.25
Toxins or Drugs
Hyperthermia has occasionally been associated with some toxins or drugs.26, 27, 28 Exposure to compounds that uncouple oxidative phosphorylation, such as the wood preservative pentachlorophenol, could potentially cause a significant rise in body temperature.27 Foals treated with macrolide antibiotics are at risk of developing hyperthermia.28, 29 Erythromycin causes a drug-induced anhidrosis that is the likely cause of the hyperthermia.29 Because the sweat response of treated foals is impaired, these foals are at particular risk of hyperthermia when exposed to hot, humid environments and direct sunlight.
Fever
Mechanisms of Fever
In true fever the set-point for the desired core body temperature increases and then is maintained by the same mechanisms that maintain the normal body temperature. Fever is a part of the acute phase response to infection or inflammation. The pathogenesis of fever is complex, and multiple integrative signaling pathways come into play3, 4, 30, 31 (Fig. 7.1 ). Currently it is thought that both neuronal and humoral mechanisms are involved in the induction of fever. Neuronal mechanisms in part involve stimulation of vagal afferents, whereas humoral mechanisms involve several proinflammatory cytokines that act as endogenous pyrogens.32, 33 Prostaglandin E2 (PGE2), which may be produced peripherally or centrally, is regarded as the principal downstream mediator of fever, acting on thermosensitive hypothalamic neurons to influence the febrile response.34, 35 Other mediators, such as orexin, may also play a role in thermoregulation.36, 37
Fig. 7.1.

Approach to changes in body temperature.
The substance most often used in experimental studies of the febrile response is bacterial lipopolysaccharide (LPS).38, 39, 40 Like many microbial products acting as exogenous pyrogens, LPS initiates fever by activation of the innate immune system, specifically via the complement cascade and Toll-like receptors (TLRs). The response is generally biphasic, with early and late phases. In the early phase of fever, LPS taken up by the Kupffer cells of the liver activates the complement cascade, with C5a rapidly stimulating the Kupffer cells to produce PGE2. This peripherally produced PGE2 may then act primarily via two pathways to affect thermoregulation. First, this PGE2 may be transported by the bloodstream to the ventromedial POA where it acts on thermoregulatory neurons to increase the set-point. Second, it may interact with receptors on hepatic vagal afferents, leading to activation of pathways projecting to the medulla oblongata and then to the POA via the ventral noradrenergic bundle. Consequently, norepinephrine is secreted, which then stimulates α1-adrenoreceptors on thermoregulatory neurons, evoking a rise in core temperature. In the late phase of fever, there is further production of PGE2 following both direct induction of its synthesizing enzymes via TLR signaling and the production of endogenous pyrogens resulting in the central production of PGE2.
Endogenous pyrogen was initially assumed to be a single molecule produced by leukocytes, thus the name leukocytic or granulocytic pyrogen. Currently, at least 11 cytokines have been shown to be intrinsically pyrogenic.30, 32, 33 These cytokines are produced predominantly by monocytes and macrophages. Among the most potent of the endogenous pyrogens are interleukin-1 (IL-1) α and β and tumor necrosis factor–alpha (TNF-α). Others include TNF-β, IL-6, and interferon-α. The precise means by which these cytokines influence thermoregulation are complex and remain to be fully elucidated. It appears that endogenous pyrogens have both peripheral and central mechanisms of action that include stimulation of afferent neuronal pathways, induction of PGE2 synthesis in endothelial cells in both the periphery and brain, and direct action in the brain via neuronal cytokine receptors.
The organum vasculosum laminae terminalis (OVLT) is a rich vascular network associated with neurons of the POA that plays an important role in the generation of fever.33, 35, 41, 42 This region is part of the circumventricular organs, which are unique structures of the brain with extensive vasculature and a minimal blood-brain barrier. As such, endothelial cells lining this region may allow direct movement of exogenous or endogenous pyrogens and peripherally produced PGE2 into the brain. Also, exogenous and endogenous pyrogens may interact with specific receptors on endothelial cells of the OVLT to further produce PGE2. Ablation of the OVLT prevents fever after a peripheral injection of endogenous pyrogens but has no effect when endogenous pyrogens are injected directly into the brain tissue.42
The production of both peripheral and central PGE2 is key in the pathogenesis of fever. PGE2 is basically synthesized in three steps involving phospholipase A2, cyclooxygenase, and terminal PGE synthetase. These enzymes are upregulated in fever.34 The cyclooxygenase 2 (COX-2) pathway is clearly important in the pathogenesis of fever because COX inhibitors, and specifically COX-2 inhibitors, effectively reduce the febrile response but have no effect on the normal body temperature.43, 44 At the same time that there is upregulation of proteins involved in prostaglandin production, there is transcriptional downregulation of proteins involved in PGE2 inactivation, such as 15-hydroxy-prostaglandin dehydrogenase. Once produced, PGE2 interacts with E type prostaglandin receptors (EP receptors), of which there are currently four identified subtypes with differential expression in various areas of the hypothalamus and brainstem.45 It appears that the EP3 receptor in the median preoptic nucleus within the POA of the hypothalamus is particularly important in the febrile response. The interaction of prostaglandins with their receptors initiates neuronal signaling by producing a cascade of changes in cyclic nucleotides, calcium, and monoamines leading to a higher set-point in the hypothalamic thermoregulatory center. For example, interaction with the EP3 receptor mediates decreases in intracellular cyclic adenosine monophosphate (cAMP), which results in decreased firing of warm-sensitive neurons, increased firing of cold-sensitive neurons, and ultimately fever.
Feedback mechanisms exist to prevent an excessive rise in body temperature. A number of antipyretic substances, sometimes referred to as either endogenous or autogenous cryogens, may be liberated systemically or within the brain during fever.31, 46, 47, 48, 49, 50 Some of the antipyretic effects occur via an inhibitory influence on the formation or action or endogenous pyrogens or via effects on neuronal thermoregulatory circuits that are activated during fever. The interactions between pyrogens and cryogens are complex, and some cytokines function as either depending on the circumstance. Some endogenous antipyretic substances include glucocorticoids, neuropeptides/hormones (arginine vasopressin, adrenocorticotropic hormone, α-melanocyte-stimulating hormone MSH, γ-MSH), cytokines (TNF-α, IL-10), lipocortin, nitric oxide, and epoxyeicosanoids.31, 47, 49 One of the major antipyretics is IL-10. Following induction by pyrogenic cytokines, IL-10 inhibits further production of IL-1 and TNF. Also, α-MSH has significant antipyretic effects, being more effective in controlling fever than acetaminophen when administered to human beings.49 Nitric oxide also has an antipyretic role, mediated by cyclic guanosine monophosphate in the POA.50 Even PGE2, whose role is primarily in the induction of fever, may produce hypothermic responses when interacting with the EP4 receptor.45
The cytokines that act as endogenous pyrogens are generally proinflammatory, with a variety of biologic effects in addition to fever.1, 4, 30, 40, 40, 51 Thus fever is usually accompanied by additional hematologic, immunologic, and metabolic changes referred to as the acute phase response. Among these effects is the synthesis of acute phase proteins by hepatocytes, including fibrinogen, C-reactive protein, haptoglobin, serum amyloid A, and others. Also, hypoferremia, hypozincemia, and hypercupremia are cytokine mediated, as is the activation of lymphocytes, which in turn produce additional cytokines.
Prostaglandins induced by endogenous pyrogens stimulate the muscle catabolism associated with fever and induce collagenase synthesis from synovial cells, contributing to the muscle and joint pain often seen with fever. Local tissue responses to IL-1β and TNF-α may stimulate afferent neural impulses that lead to behavioral responses associated with fever, such as lethargy and anorexia. As expected, treatment with COX inhibitors can diminish many of the signs of fever.
Fever is a host defense mechanism that has been preserved within the animal kingdom. Although much attention is focused on the adverse effects of fever, including patient discomfort, there is evidence that fever can be beneficial.52, 53, 54, 55, 56, 57, 58 Some studies have demonstrated an association between a rise in body temperature and a decrease in mortality and morbidity during infection. Although the elevation in temperature may directly impair organism growth in some cases, the beneficial effects are thought to be primarily associated with enhanced host defenses.40, 52, 53, 54, 55, 56, 57 Fever has beneficial effects on multiple aspects of both the innate and adaptive immune response. As part of the acute phase response associated with fever, the concentration of iron, which is required by many bacteria for multiplication, decreases.59, 60, 61 The beneficial effects of fever, however, are not universal, and many of the positive effects of fever are reversed when temperature becomes extremely high.43, 62 In rabbits experimentally infected with Pasteurella multocida, the survival rate increased in association with elevations in body temperature up to 2.25°C (4.5°F) above normal but decreased with elevations above this level.63 The increased catabolism, variable anorexia, and increased metabolic rate can lead to muscle wasting and weakness when fever is prolonged. Although seizures induced by fever are uncommon in horses, they can be seen in neonates when the temperature is above 42°C (108°F).2 In debilitated animals, prolonged fever has been associated with cardiovascular failure.
Conditions Associated with Fever
Fever is part of the physiologic response to infection and inflammation (see Box 7.1). It is considered a cardinal sign of infection and has been associated with infections caused by essentially all types of organisms. Although there is considerable variation, viral infections are often associated with high fevers. In addition to infection, fever can be a prominent component of many inflammatory, neoplastic, and immunologic conditions. In intensive care units in human hospitals, the incidence of fever ranges from 23% to 70% and is related to an infectious process in approximately half of the cases.64, 65
Fever of Unknown Origin
Fever of unknown origin in human medicine is defined specifically as a fever that occurs on several occasions over at least 3 weeks in which the diagnosis remains unclear after an initial diagnostic workup. In veterinary medicine, the term fever of unknown origin is often used more loosely, referring to any prolonged, unexplained fever. In many cases, the cause of a fever of unknown origin is a common disease with an unusual presentation. In a review of 63 cases of fever of unknown origin in the horse, the specific criteria used to define fever of unknown origin included (1) illness of at least 3 weeks’ duration associated with nonspecific signs, (2) body temperature of at least 38.6°C (101.5°F) on several occasions, and (3) no clear diagnosis after an initial complete blood count and serum biochemical profile.66 The most common cause was found to be infection, which was responsible for 43% of the cases. Other causes included neoplasms in 22% of cases; immune-mediated diseases in 6.5%; and miscellaneous diseases such as toxic hepatopathy, parasitism, and others in 19%. In 9.5% of cases no diagnosis was made. Specifically, the most common conditions identified were abdominal infections (peritonitis/abdominal abscessation) and lymphosarcoma, each accounting for approximately 16% of cases. Pneumonia, pleuropneumonia, or both were found in 11% of cases. Three horses (approximately 5%) were found to have bacterial endocarditis, and in each case a murmur was not identified initially but developed within several weeks of the onset of illness. Thus the diagnosis of fever of unknown origin requires a systematic approach with emphasis on the evaluation of infectious disease.
Diagnostic Approach to Elevations in Body Temperature
Increased body temperature is a common clinical sign with diverse causes. Fortunately, in many cases the cause may be readily apparent on the basis of the signalment, history, and physical examination. In other cases, additional diagnostic testing may be required (see Fig. 7.1).
Hyperthermia
Many causes of hyperthermia, such as exercise-related hyperthermia and malignant hyperthermia, can be distinguished from fever based largely on the signalment and history of the patient. Older horses may be more susceptible to exercise-related hyperthermia.14 Treatment with macrolide antibiotics is significant due to the potential for macrolides to cause hyperthermia.28, 29 A sweat test, such as the quantitative intradermal terbutaline sweat test, can be useful in confirming anhidrosis.18, 67 Genetic testing is available for detection of the RyR1 mutation in cases of suspect malignant hyperthermia.23, 24
Fever
Multiple conditions may result in fever. Infectious diseases remain the most common cause, although other inflammatory conditions such as neoplasia and immune-mediated disease may also cause fever. Often localizing clinical signs such as nasal discharge or diarrhea aid in determining the specific etiology. If the underlying condition responsible for the fever is not readily apparent, additional diagnostic tests are warranted.
Documentation of Fever
It may be useful to have the body temperature taken twice daily over a period of time to document fever and identify any pattern. Fevers can be categorized as intermittent, remittent, biphasic, or sustained, although some inconsistencies in the precise definitions of these patterns exist. In general, intermittent fevers are characterized by recurring paroxysms of elevated temperature followed by periods of normal temperature, such as those fevers that demonstrate diurnal variation. In most cases of intermittent fever the temperature tends to peak in the late afternoon or evening. Intermittent fevers most often are associated with infectious causes, particularly viral infections, although they may be seen with a variety of other conditions. Remittent fevers are those in which diurnal variation is exaggerated without a return to normal body temperature or those with a cyclic pattern in which the temperature elevation lasts for several days, such as may be seen with equine infectious anemia virus. Biphasic fevers, in which an initial rise in body temperature precedes a period of normal temperature and then a second rise, are characteristic of certain diseases such as equine neorickettsiosis (Potomac horse fever). Sustained fevers are those in which the elevation of temperature is consistent.
Signalment and History
The signalment and history should be considered when investigating fever. Some causes of fever may be more common in certain age groups. For example, although not a consistent finding, fever is often seen in neonatal foals in association with septicemia, omphalophlebitis, or septic arthritis. Rhodococcus equi is predominantly seen in foals 1 to 6 months of age and is often associated with fever. Young horses, especially those that have been recently exposed to new horses, may be particularly at risk for respiratory tract infections. Any exposure to Streptococcus equi subspecies equi (strangles) may be significant because of the association of this organism with internal abscessation. Geographic location and travel history may be relevant, as some diseases, such as equine neorickettsiosis, piroplasmosis, and coccidioidomycosis, are more common in certain geographic locations.
Physical Examination
A thorough physical examination, including auscultation of the thorax with a rebreathing bag and rectal palpation, is indicated in the evaluation of fever. Repeating the physical examination may yield new information. A neurologic examination can also be useful as disorders of the central nervous system may cause aberrations in temperature through pyrogenic cytokines or in some cases through direct effects on thermoregulatory centers.
Ancillary Diagnostic Aids
Ancillary diagnostic tests are commonly used in the diagnosis of fever, particularly in cases of fever of unknown origin. Localizing signs may help direct selection of the most appropriate tests but are not always present.
Clinical Pathology/Laboratory Testing
A database, including complete blood count (CBC), fibrinogen, biochemical profile with bile acids, and urinalysis, should be obtained. Hemoparasites may occasionally be seen on the blood smear, but the apparent absence of organisms does not rule out a parasitemia that is below detectable limits. Abnormalities consistent with chronic infection or inflammation, including anemia, hyperfibrinogenemia, hyperglobulinemia, and thrombocytosis, are common but nonspecific findings. Although inflammation is the most common cause of hyperglobulinemia, further assessment by serum protein electrophoresis and specific immunoglobulin quantitation may be indicated in some cases. A monoclonal gammopathy is characteristic of plasma cell myeloma but may also be seen with other tumors of the reticuloendothelial system and occasionally other conditions. Immunodeficiencies, which in some cases are associated with low lymphocyte counts or low immunoglobulin concentrations, can predispose affected horses to chronic infections. In cases of hypoalbuminemia, gastrointestinal or renal loss, third space loss, and decreased production associated with significant hepatic disease should be investigated. The presence of hypercalcemia can be helpful in the diagnosis of disease, as it is most often linked with either renal disease or certain neoplasms in horses. Bone marrow aspiration may be useful, particularly in those horses with persistent abnormalities in circulating cell populations.
Infections of the respiratory tract and abdomen frequently are associated with fever in the horse, and therefore these systems should be thoroughly evaluated. In many cases this includes cytologic evaluation and culture and/or polymerase chain reaction (PCR) of samples from the respiratory tract and abdomen. The upper airway is often sampled by nasal swabs, nasal washes, or guttural pouch lavage, and the lower airway can be sampled by bronchoalveolar lavage or transtracheal aspiration. Thoracocentesis may be considered because abnormalities of pleural fluid are occasionally present even without increases in the volume of fluid. Similarly, abnormalities in peritoneal fluid can be found without increases in fluid volume, and abdominocentesis should be considered as part of the diagnostic plan. It should be remembered that neoplastic cells often do not exfoliate into fluid; therefore neoplasia cannot be ruled out based on cytologic evaluation of fluid.
Although only occasionally associated with fever, the presence of gastrointestinal parasites is so common that feces from horses with fever of unknown origin should be examined for parasite ova. In cases of suspected gastrointestinal protein loss, diarrhea, or melena, one should consider diagnostic procedures such as fecal culture and analysis for clostridial toxins, culture and PCR for Salmonella, rectal mucosal biopsy, or absorption tests.
Blood cultures are generally most useful in neonates but can yield valuable information in adult horses with fever as well. Ideally, three to five samples should be collected at least 45 minutes apart when the horse is not on a regimen of antibiotic therapy. Sampling just before and during a temperature rise is most likely to yield a positive culture.
Serologic evaluation can be useful in the assessment of fever, and specific tests can be prioritized based on the patient and the geographic area. Due to variability in the clinical presentation and regulatory concerns, equine infectious anemia should be considered as a differential diagnosis for horses with fever of unknown origin, and testing should be performed. A serologic test for detection of antibodies to the M protein of Streptococcus equi ssp. equi has been developed and may be a useful aid in the diagnosis of metastatic abscessation associated with the strangles organism.68 Serologic tests for several other infectious diseases are also available, including tests for Corynebacterium pseudotuberculosis, equine piroplasmosis, brucellosis, and coccidioidomycosis, among others.
Immune-mediated disorders such as immune-mediated hemolytic anemia, immune-mediated thrombocytopenia, systemic lupus erythematosis, vasculitides, and rheumatoid arthritis have been implicated as causes of fever of unknown origin but more commonly in human beings and small animals than in horses. Appropriate diagnostic tests, such as the Coombs’ test, skin biopsy, and antinuclear antibody testing, may be useful in some patients.
Endoscopy
Endoscopy is commonly used for evaluation of the respiratory tract, especially the upper respiratory tract including the guttural pouches. Pleuroscopy allows direct visual examination of the pleural space and may facilitate biopsy of any masses. Also, endoscopy can be useful in evaluation of the esophagus, stomach, and urinary tract.
Diagnostic Imaging
Ultrasound is a practical, noninvasive means of assessing the thorax and parts of the abdomen. It can help identify abnormalities that need further evaluation, such as consolidated lung, abdominal masses, or pathologic liver and kidney conditions. It may also help identify fluid for collection. Echocardiography can aid in the diagnosis of bacterial endocarditis.
Radiographs of the thorax are helpful in the evaluation of pulmonary disease. Although the practicality and utility of abdominal radiographs are limited, especially in adult horses, they may be of value in individual cases, especially in neonates. Nuclear imaging using labeled white blood cells may identify a site of infection or inflammation.
Other
Exploratory laparoscopy or laparotomy is indicated when abdominal involvement is suspected or the animal is becoming progressively debilitated. In horses for which a specific diagnosis has not been made, therapeutic trials with antimicrobials may help, and in cases of suspected immune-mediated disease, corticosteroids may help.
Decreased Body Temperature: Hypothermia
Mechanisms of Hypothermia
Hypothermia occurs when the core body temperature drops below accepted normal values.4 Clinically, hypothermia can be characterized as either accidental or pathologic. In accidental hypothermia the body’s ability to produce heat is overwhelmed, often in association with harsh environmental conditions. There is a spontaneous decrease in the core body temperature independent of actual disruption to the thermoregulatory system.
Pathologic causes of hypothermia should be considered when no clear reason for accidental hypothermia is evident. Pathologic hypothermia occurs in association with disorders that decrease metabolic activity or directly affect the thermoregulatory center, such as endocrine disorders, sepsis, and intracranial disease. When hypothermia is seen with systemic inflammation, it is often considered a maladaptive thermoregulatory response. The mechanisms involved in producing hypothermia are not fully understood, but several cytokines (including TNF-α, interleukins, and interferon-gamma [IFN-γ]) are involved.69 Some of these same cytokines may also act as pyrogens under other conditions.
The ability to generate heat through shivering is impaired or lost once the body temperature becomes too low. The animal experiences a decrease in the metabolic rate of most tissues. Heart rate, cardiac output, glomerular filtration, and blood pressure may decrease.
Conditions Associated with Hypothermia
Accidental Hypothermia
Accidental hypothermia is most often associated with cold or cold, damp, windy environments (see Box 7.1). Occasionally extreme situations occur, such as an animal falling through ice. Mild accidental hypothermia sometimes occurs with surgical procedures. Accidental hypothermia is particularly common in neonates and geriatric or debilitated adult horses. Although central thermoregulation through the hypothalamus is normal in neonates, foals have a large ratio of surface area to body weight, enhancing heat loss.2 Sick or debilitated animals often have decreases in activity and nutritional intake and alterations in circulation that can contribute to hypothermia. Severe hypothermia may result in significant metabolic changes and death.
Pathologic Hypothermia
Pathologic hypothermia can be associated with sepsis, neurologic disorders, and endocrine disorders (see Box 7.1). Hypothermia has been observed with septicemia and shock, especially in neonates, in which 24% of septic foals were found to have a decreased body temperature.2
Hypothyroidism is an uncommon clinical problem in horses; however, impaired thermoregulation has been seen in foals with congenital hypothyroidism.70, 71 Hypothermia may be more common in donkeys than horses. In one study, histologic lesions of the thyroid gland were identified in four of five hypothermic donkeys.72
Approach to Hypothermia
Accidental Hypothermia
Accidental hypothermia can generally be identified from the signalment and history (see Fig. 7.1). It is important to assess environmental conditions to determine whether environmental stress, especially cold, wet, and windy conditions, is contributing to the hypothermia. Neonatal, geriatric, and debilitated patients are at high risk.
Pathologic Hypothermia
Signalment and History
The signalment and history should be considered when assessing cases of potential pathologic hypothermia (see Fig. 7.1). It is important to consider environmental conditions, as in some cases both accidental and pathologic causes may contribute to the hypothermia, such as in septic neonates born in cold, damp environments.
Physical Examination
A thorough physical examination should be performed in horses with hypothermia. Patients should be assessed for potential underlying conditions such as sepsis or inflammation and neurologic or endocrine disorders. A neurologic examination may be useful.
Ancillary Diagnostic Tests
A CBC, serum biochemical profile, fibrinogen, and urinalysis can be useful in determining the underlying cause of hypothermia. Blood cultures are indicated in cases of potential sepsis. Hypothyroidism is uncommon, but if suspected, diagnostic testing may include a thyroid-stimulating hormone or thyrotropin-releasing hormone (TRH) response test. Ultrasound and aspiration or biopsy of the thyroid may also be useful, especially in foals with suspected congenital or nutritional hypothyroidism.
Changes in Body Weight
Changes in body weight and condition are common clinical problems in horses. Both weight loss and obesity have the potential to adversely affect the health of the horse. Weight loss can range from mild physiologic weight loss, such as that seen with increased exercise, to dramatic, life-threatening weight loss.73, 74 Severe malnutrition, regardless of the underlying cause, can result in starvation and the accompanying signs of marked weakness and wasting, known as inanition. Ultimately starvation can lead to organ failure and death. Obesity appears to be increasing in prevalence in the horse population and has been linked to serious health concerns, particularly laminitis.75, 76, 77, 78 Some horses that are prone to obesity (“easy keepers”) have an endocrinologic disorder known as equine metabolic syndrome (EMS).79, 80, 81
The most accurate means of assessing body weight and monitoring changes in weight is to use a scale, but this has practical limitations. Thus a variety of weight tapes and equations based on morphometric measurements have been developed to estimate body weight.82, 83 To account for differences in body type between breeds, some breed-specific equations are available.82 The ideal body weight depends on the type and use of the horse, and equations have been developed to calculate the ideal body weight for horses of certain breeds. Several online tools are available to aid in the calculation of actual and ideal body weight.
Weight alone does not necessarily reflect the body condition of the horse. Because muscle weighs more than fat, athletic horses may be heavier than nonathletic horses of similar size. To better evaluate body condition, a number of scoring systems have been developed to estimate the extent of adiposity.83, 84, 85, 86, 87 One commonly used system to determine a body condition score (BCS) is the Henneke system, which uses a scale of 1 to 9, with 1 representing an extremely emaciated horse, 9 representing an extremely fat one, and 5 being close to ideal86, 87 (Table 7.1 ). A BCS system that ranges from 0 to 5 is also occasionally used, with 0 being emaciated, 3 good, and 5 very fat. In addition to body condition scoring, a number of morphometric measurements can be used to help estimate adiposity, some of which may be particularly useful in evaluating localized adiposity.83, 88, 89 The girth-to-height ratio correlates well with the BCS and is a suitable morphometric measure for assessment of overall adiposity.83 The cresty neck score, which ranges from 0 to 5, can be used to evaluate adiposity in the neck, with a score of 3 or greater being considered a cresty neck (Table 7.2 ). Some additional measurements used to assess neck adiposity include crest height, neck circumference, and neck circumference–to–height ratio. It has been demonstrated in ponies that measures of generalized and localized obesity, including a BCS of 7 or greater, a cresty neck score of 4 or greater, and a neck circumference–to–height ratio greater than 0.71, are useful in the prediction of laminitis.88 A more quantitative means of measuring adiposity is by ultrasound.90 One method is to take a measurement 5 cm lateral from the midline at the midpoint of the pelvic bone and the percent body fat is calculated by the following equation: % body fat = 2.47 + 5.47 (rump fat in cm). Most lean horses are approximately 8% to 14% body fat, whereas overconditioned horses are 16% to 30%. Ultrasound can also be used to assess retroperitioneal fat. A topline score can be used to evaluate epaxial musculature. Body condition scoring is also valuable in evaluating thin horses and is often used to assess horses in animal welfare cases.
Table 7.1.
Horse Body Condition Scores on the Henneke Scale86
| Score | Description |
|---|---|
|
Extremely emaciated; no fatty tissue; vertebrae, ribs, tail head, and bones of withers, shoulder, and neck are visible |
|
Emaciated; slight tissue cover over bones; vertebrae, ribs, tail head, and bones of withers, shoulder, and neck are visible |
|
Slight fat cover over body; individual vertebrae and ribs no longer visibly discernible; withers, shoulders, and neck do not appear overly thin |
|
Ridge of spine and outline of ribs are visible; tail head may or may not be visible depending on the breed; withers, shoulders, and neck do not appear overly thin |
|
Spine and ribs cannot be seen, but ribs can be felt; tail head is spongy; withers, shoulders, and neck are rounded and smooth |
|
Slight crease down spine; ribs and tail head feel spongy; fat deposits along withers and neck and behind shoulders |
|
Crease down spine; ribs have fat filling between them; tail head spongy; fat deposits along withers and neck and behind shoulders |
|
Apparent crease down spine; ribs difficult to feel; soft fat surrounding tail head; fat deposits along withers, behind shoulders, and on inner thighs; neck is large |
|
Obvious crease down spine; patchy fat on ribs; bulging fat on tail head, withers, behind shoulders, and on neck; fat fills in flank and on inner thighs |
Table 7.2.
Cresty Neck Score Used in the Assessment of Regional Adiposity83
| Score | Description |
|---|---|
| 0 | No palpable crest |
| 1 | No visual appearance of a crest but slight filling felt with palpation |
| 2 | Noticeable appearance of a crest Fat deposited fairly evenly from poll to withers Crest easily cupped in one hand and bent from side to side |
| 3 | Crest enlarged and thickened Fat deposited most heavily in middle of neck giving a mounded appearance Crest fills cupped hand and begins losing side-to-side flexibility |
| 4 | Crest grossly enlarged and thickened May have wrinkles perpendicular to the topline Crest can no longer be cupped in one hand or easily bent from side to side |
| 5 | Crest is so large it falls to one side |
Mechanisms Controlling Body Weight
The stability of body weight and body composition is related to the balance of energy intake and energy expenditure.73 Dietary carbohydrates, fats, and proteins provide energy to support the body’s metabolic needs. When the energy intake exceeds the expenditure, the excess energy is stored, primarily as fat, resulting in an increase in body weight. When the energy intake is insufficient to meet metabolic needs, energy stores are used and there is a loss of body weight. Both weight loss and obesity can be serious clinical problems.
Weight Gain/Obesity
Obesity is being increasingly recognized in the equine population. In the 1990s, a study by the National Animal Health Monitoring System of the U.S. Department of Agriculture (USDA) estimated that approximately 5% of the horse population was obese. Several more recent studies in the United States and Europe have found that 45% to 50% of horses are overconditioned, with 10% to 35% being obese.75, 76, 77, 78, 91, 92, 93 Breed has been shown to be strongly associated with the risk of obesity. In many cases, owners do not recognize their horse as obese. Horses that are not ridden or used for pleasure riding rather than competition appear to be at increased risk of obesity.75, 76 In a study of outdoor-living horses in the United Kingdom, seasonal variation in BCS was documented, with the prevalence of obesity increasing from 27% at the end of winter to 35% at the end of summer.76 In this study, providing supplementary feed was not a strong predictor of obesity, supporting the belief that grass consumption is an important factor influencing obesity in outdoor-living horses.
Obesity has been linked to adverse health effects in many species. In horses, the increased risk of laminitis associated with obesity is a major concern.81, 94 Other potential problems seen with obesity include thermoregulatory inefficiency, exercise intolerance, altered reproductive performance, hyperlipemia syndrome, and the development of lipomas in mesenteric adipose.
Mechanisms of Weight Gain
Weight gain occurs when energy intake exceeds energy expenditure. Although this is a fairly straightforward concept, the actual etiology of obesity can be complex, involving interactions between genetics, hormones, and management. In many cases, management factors including diets high in sugar and starch coupled with a lack of sufficient exercise contribute to obesity. Even when horses are ridden regularly as pleasure horses, the relatively low-intensity exercise provided often does not use significant energy.75, 76
Some horses are particularly susceptible to obesity. At one time, it was proposed that this was due to hypothyroidism, but evidence did not support this. In 2002 EMS was introduced as a metabolic and endocrine disorder contributing to obesity.79 EMS is a complex problem that shares some characteristics with metabolic syndrome in people.79, 80, 81, 94, 95 The major phenotypic features of EMS include obesity and/or regional adiposity, insulin resistance, and a predisposition for laminitis.
The pathophysiologic mechanisms involved in both obesity in general and EMS are complex, involving interactions between multiple factors including genetics. It appears that some individuals may inherit traits that facilitate survival in harsh environments, making them especially thrifty. EMS can be recognized in any breed of horse, but an increased susceptibility has been recognized in certain breeds, including several pony breeds, Morgan Horses, Paso Finos, Arabians, Saddlebreds, Spanish Mustangs, and Warmblood breeds.81 Studies are ongoing to help better define the role of genetics in EMS.
Insulin dysregulation is a key factor in the diverse physiologic processes that contribute to EMS.79, 80, 81, 96, 97 Insulin dysregulation refers collectively to abnormalities of insulin metabolism including excessive insulin responses to sugars, fasting hyperinsulinemia, and insulin resistance (IR). The relationship between IR and obesity might be described as a vicious circle, as each may promote the other. In one study that demonstrated differences in the insulin response to glucose between ponies and horses, all animals were in moderate body condition, suggesting that the breed-related differences in insulin dynamics were independent of obesity.98 In addition to genetics, EMS, and obesity itself, some factors that can potentially cause IR include physiologic responses to stress or pregnancy, systemic inflammation, and pituitary pars intermedia dysfunction (PPID).
There is increasing awareness of the role fat itself plays in the development of metabolic disease and inflammation.99, 100, 101 Previously, adipose tissue was considered simply an energy storage depot with a fixed number of adipocytes. Any increase in adipose mass was thought to occur due to increased fat storage within existing cells resulting in hypertrophy. However, it is now recognized that adipose tissue is a complex endocrine organ that contains a large pool of stem cells and preadipocytes that can be recruited once existing adipocytes reach a critical level of hypertrophy. Thus in obesity there is generally both hypertrophy and proliferation of adipocytes. The adipocytes are biologically active and influence multiple physiologic processes including energy metabolism, cardiovascular function, reproductive function, inflammation, and immunity. The cells secrete a wide variety of cell-signaling proteins, or cytokines, known as adipokines, that function in both an autocrine/paracrine and endocrine fashion.100 Due to local influences, adipocytes from differing anatomic locations may vary in their specific pattern of expression of these adipokines. Currently at least 100 substances are thought to be secreted by adipocytes. Some important adipose-derived hormones include leptin, adiponectin, resistin, visfatin, and angiotensinogen. Adipokines that act as proinflammatory cytokines and inflammatory mediators include TNF-α, IL-1α and IL-1β, IL-6, and IL-8, as well as monocyte chemotactic protein–1 and plasminogen activator inhibitor. The altered expression of adipokines in obesity can influence a number of metabolic processes and contribute to EMS.101, 102, 103, 104, 105, 106, 107 Adipokines may inhibit insulin signal transduction pathways contributing to insulin resistance. Leptin, which is involved in satiety and regulation of obesity, is elevated in horses with IR, suggesting leptin resistance. Adiponectin, an insulin-sensitizing hormone, can be decreased in obese horses. In humans and rodents, obesity is linked with an upregulation of proinflammatory cytokines secreted by adipocytes. This results in a chronic inflammatory state and contributes to insulin dysregulation. Studies in horses, however, have yielded variable results regarding the expression of inflammatory mediators in obese horses, and further investigation is needed to understand their role in EMS.95, 104, 105, 106
Several other factors may influence the development of obesity in addition to insulin dysregulation and the action of adipokines. The mechanisms of central control of body weight remain somewhat obscure, and it has been proposed that there is a set-point for body weight regulated through a region of the hypothalamus.108 Myostatin, a myokine and negative regulator of skeletal muscle mass, has been implicated in obesity in other species, and limited data in the horse suggest a possible role for myostatin and its receptor in equine obesity.109 Recently, the central role of the intestinal microbiota in the progression and prevention of metabolic dysfunction has become apparent, and the role of the gut microbiota in obesity and insulin dysregulation is under investigation in multiple species, including horses.110, 111, 112
An increased risk of laminitis is an important feature of EMS.79, 80, 81, 113 The mechanisms involved in the relationship of obesity and insulin dysregulation to laminitis are not fully understood, and this remains an active area of research. The pathogenesis is likely multifactorial, and some proposed mechanisms include impaired glucose uptake by epidermal laminar cells, altered function of epidermal cells, endothelial cell dysfunction within the vasculature of the foot, digital vasoconstriction, and activation of metalloproteinase by glucose deprivation or reactive oxygen species.
Conditions Associated with Weight Gain
An increase in body weight occurs when the energy intake is greater than the energy expenditure. Being overweight should be distinguished from the normal physiologic weight gain associated with pregnancy and from pathologic conditions that cause abdominal distention, such as ascites or peritonitis (Box 7.2 ).
BOX 7.2. Causes of Weight Gain/Obesity.
Physiologic Weight Gain
Growth
Pregnancy
Management
Overfeeding/excessive grass consumption
Lack of exercise
Endocrinologic/Metabolic Disorders
Equine metabolic syndrome
Pituitary pars intermedia dysfunction
Often causes weight loss
Overconditioning and obesity are often related to management problems associated with supplemental feed or excessive grass consumption, often combined with a lack of significant exercise. Horses with EMS are particularly susceptible to weight gain, and EMS is an important cause of obesity and regional adiposity. PPID can have variable effects on body weight. Whereas many horses with PPID may experience weight loss and muscle wasting, others may exhibit obesity or regional adiposity, especially if they have concurrent IR.
Diagnostic Approach to Weight Gain
Signalment and History
Any breed may develop EMS, but some appear to be at increased risk, including pony breeds, Morgan Horses, Paso Finos, Arabians, Saddlebreds, Spanish Mustangs, and Warmblood breeds.81 The condition has also been commonly recognized in American Miniature Horses, draft breeds, and Norwegian Fjords, as well as donkeys and mules. It appears to be less common in Thoroughbreds and Standardbreds. The prevalence of PPID increases with age.
A thorough history is important. Particular attention should be given to the feeding practices as well as the amount and type of exercise that the horse receives. Previous episodes of laminitis may point to an increased likelihood of EMS or PPID. A history of abnormal shedding or polyuria/polydipsia increases the suspicion of PPID. The breeding history should be established. In some cases, the breeding history may be unknown, especially if the horse has changed ownership.
Physical Examination
A complete physical examination should be performed in cases of weight gain. This may help to rule out medical conditions that may cause abdominal distention such as ascites, bloat, uroperitoneum, hemoabdomen, or peritonitis. Palpation per rectum may be indicated to rule out pregnancy, especially in horses that have an unknown breeding history.
The body weight and BCS should be assessed, and the horse should be evaluated for regional adiposity.82, 83, 84, 85, 86, 87 Several morphometric measurements can be useful in the evaluation of body weight and condition. It is important to palpate carefully over the shoulder, elbow, ribs, withers, sternum, loin, and tailhead. The girth:height ratio can be useful in the assessment of overall adiposity.83 A cresty neck score, as well as the neck crest height and neck circumference–to–height ratio, are useful in the assessment of neck adiposity.83 In many cases, careful evaluation for lameness, increased digital pulses, and sensitivity to hoof testers is warranted due to the association of obesity with laminitis.
Ancillary Diagnostic Tests
Routine blood work is often unremarkable in cases of weight gain. As normal values may vary among laboratories, it is ideal to use reference ranges specific for the laboratory being used, especially when evaluating IR and PPID. Also, any significant ongoing pain or stress should be taken into account as these conditions can affect glucose and insulin dynamics, as well as adrenocorticotropic hormone (ACTH) and cortisol. Horses with both suspected EMS and PPID should be screened for IR. Current tests for evaluating IR focus primarily on measurements of glucose and insulin.80, 81 It is generally recommended to limit feed before sampling to one flake of grass hay low in nonstructural carbohydrates per 500 kg of body weight given no later than 10 pm the night before. As IR is often well compensated, baseline concentrations of glucose and insulin may be normal or in many cases the glucose is normal whereas the insulin is elevated. Occasionally horses will develop diminished glycemic control and become hyperglycemic (greater than 150 mg/dL) consistent with type 2 diabetes mellitus. There are several mathematical calculations such as the glucose:insulin ratio and reciprocal inverse square of insulin (RISQI) that are occasionally used to help assess insulin sensitivity.114 Because resting concentrations of both glucose and insulin can be normal in IR, dynamic testing is often indicated. Practical dynamic tests include the oral sugar test (OST) and the combined intravenous glucose-insulin test (CGIT). Other diagnostic tests that may be useful in assessing IR include measurement of triglyceride and leptin concentrations, both of which may be elevated in IR.
Evaluation for PPID should be considered in horses with weight gain or regional adiposity, as well as in those with loss of body condition.115, 116 There are several options for diagnostic testing. Currently, frequently recommended tests include measurement of baseline ACTH, a low-dose dexamethasone suppression test, and a TRH stimulation test with measurement of ACTH.
Weight Loss
Mechanisms of Weight Loss
Weight loss occurs when the energy intake is exceeded by the expenditure. Thus there are basically two general mechanisms of weight loss, decreased energy intake and increased energy demand. In many horses with weight loss, both of these mechanisms come into play. Muscle loss often occurs with weight loss but can also occur as a result of neurogenic or disuse muscle atrophy, in which case the muscle loss may be independent of generalized weight loss.
Causes of Decreased Energy Intake
-
1.
Limited access to feed or poor quality feed: The most direct cause of protein-calorie malnutrition and weight loss is an inadequate volume or quality of feed to meet the dietary requirements of the animal. This can result from actual underfeeding or from other factors such as excessive competition between horses or lameness preventing the horse from accessing the feed easily.117 Also, the nutritional value of feed, particularly forage, can vary widely, and poor quality feed can be a significant factor in malnutrition.
-
2.
Dysphagia: Dysphagia, difficulty with prehension, mastication, or swallowing, can occur in horses for many reasons with resultant decreased feed intake and weight loss. One common cause of difficulty eating, especially in older horses, is abnormal dentition.
-
3.
Malabsorption/malassimilation: Malabsorption is the inadequate assimilation of dietary substances due to defects in digestion, absorption, or transport.118 Both macronutrients (carbohydrates, fats, and proteins) and micronutrients (vitamins and minerals) can be affected. Normal absorption is a complex process involving multiple organs, enzymes, and hormones, as well as transport and secretory mechanisms. Impairment of any of the steps in this process can result in malabsorption. Rapid gastrointestinal transit time can also contribute to malabsorption. In horses, malabsorption is often associated with conditions that damage the intestinal wall, such as inflammatory bowel diseases, infiltrative neoplasms, parasitism, and certain gastrointestinal infections.119 In foals, both primary and secondary lactase deficiency have been identified as causes of malabsorption.120 Depending on the underlying cause of the malabsorption, weight loss may occur in the face of normal dietary intake.
-
4.
Anorexia: Anorexia, which is a loss of appetite, frequently contributes to weight loss, especially when prolonged. Anorexia may be either partial or complete, and occasionally the term hyporexia is used to describe partial anorexia. A wide variety of diseases are associated with anorexia, and in many cases, the decreased desire for feed is combined with increased energy expenditure, resulting in significant weight loss. The pathophysiologic mechanisms involved in anorexia are not fully understood, but it appears that there are disturbances of hypothalamic pathways controlling energy homeostasis and that multiple mediators including hormones, neuropeptides, and cytokines such as IL-1 and TNF-α are involved.121, 122
Causes of Increased Energy Demand
In general, causes of increased energy demand can be categorized as physiologic or pathologic.
-
1.
Physiologic causes of increased energy demand: Physiologic weight loss may be seen with conditions requiring increased energy, such as late pregnancy, early lactation, and intense exercise. Environmental stress, especially decreased ambient temperature, can also have a significant impact on caloric requirements.123, 124, 125 In adult horses, the lower critical temperature, which is the temperature below which the horse must increase metabolic heat production to maintain normal body temperature, ranges from 5°C (4°F) to −15°C (5°F) depending on the horse’s adaptation to the environment. The digestible energy requirements are estimated to increase by 2.5% for every degree Celsius below the lower critical temperature. Energy requirements are further increased by wet, windy conditions. It is estimated that in cold temperatures when the hair coat is wet the maintenance digestible energy requirement may increase by as much as 50%.
-
2.
Pathologic causes of increased energy demand: Many disease processes will result in weight loss, not only because anorexia is common, but because there is frequently a concomitant increase in energy expenditure.126 Fever itself appears to increase energy needs. In human patients, the resting energy requirement is estimated to increase by approximately 14% for each degree C increase in body temperature. However, the increase in energy requirement associated with disease is not consistent. For example, it was determined that the resting energy requirement of critically ill neonatal foals (40–50 kcal/kg body weight per day) was less than that of control foals (60–80 kcal/kg body weight per day).127 The regulation of energy requirements in disease is complex and remains poorly understood.
-
3.
Cachexia is a specific multifactorial syndrome associated with underlying illness that is defined by the loss of skeletal muscle with or without the loss of fat mass.128, 129, 130 Cachexia is characterized by a negative protein and energy balance driven by a variable combination of reduced feed intake and abnormal metabolism, and it cannot be fully reversed by nutritional support. The pathophysiology of this syndrome involves multiple mechanisms.130, 131, 132, 133 It is in part mediated by cytokines, including TNF-α, IL-6, IL-1β, IFN-γ, and proteolysis inducing factor. TNF-α was originally designated as cachectin due to its catabolic effects. In human patients, muscle catabolism has been associated with TNF-α in a variety of conditions such as cancer, congestive heart failure, and chronic obstructive pulmonary disease. Based on studies in cultured muscle cells, it appears the increased muscle catabolism associated with TNF-α is mediated by reactive oxygen species and nuclear factor-κβ, which upregulate ubiquitin/proteasome activity.134 There are several additional mechanisms of cytokine mediated cachexia, including activation of the hypothalamic melanocortin system and upregulation of the cytokine-activated transcription factor STAT3.128, 129, 132, 133, 134 The endocrine system also has a role in cachexia. There is often a reduction in circulating anabolic hormones associated with disease. In cancer patients, tumor-derived parathyroid hormone–related protein (PTHrP) appears to play a role in cachexia.131 In chronic disease states such as congestive heart failure and chronic kidney disease, it appears that the renin-angiotensin system has a major role in producing cachexia.133 Angiotensin II concentrations are often elevated in chronic disease, and treatment with angiotensin converting enzyme inhibitors can improve weight loss.
-
4.
Several additional mechanisms may contribute to increased energy requirements during systemic disease.126, 128, 129, 130 The stress associated with disease may alter metabolism due to increases in sympathetic and hormonal activity. Chronic pain may also result in elevated systemic catecholamine and cortisol concentrations, resulting in a catabolic state. In recurrent airway obstruction, the work of breathing can increase energy requirements. An increase in nutrient demand can be seen in association with protein loss in conditions such as peritonitis, pleuritis, colitis, inflammatory bowel disease, and burns. Also, although thyroid disease is uncommon in horses, weight loss has been recognized in association with hyperthyroidism and the accompanying increase in basal metabolic rate.135, 136
-
5.
Aging in multiple species has been associated with unintentional weight loss and sarcopenia, which is a loss in muscle mass, quality, and strength associated specifically with aging. The mechanisms behind this structural and functional decline in skeletal muscle are complex.137 In horses, aging alone has been associated with a loss of weight and muscles.138, 139 Horses affected with PPID may be particularly susceptible to muscle loss due to atrophy of type 2A and 2B muscle fibers and loss of type 2B fibers.115, 140 Proposed mechanisms of steroid-related myopathy in horses with PPID include myocyte apoptosis, negative regulation of nuclear factor kappa B (NF-κΒ) activation and function, and oxidative stress.
-
6.
It is not uncommon for weight loss to involve multiple mechanisms at the same time. Not only may decreased energy intake and increased energy demand be present concurrently, but there may be multiple mechanisms contributing to the decreased intake and increased demand. For example, in recurrent airway obstruction there may be increased demands from both the work of breathing and the presence of inflammatory cytokines. In parasitism, there is competition for nutrients within the gastrointestinal tract, as well as damage to the gastrointestinal wall resulting in inflammation and malabsorption of both macronutrients and micronutrients.
Conditions Associated with Weight Loss
A wide variety of problems can be associated with weight loss (Box 7.3 ). An inadequate amount or quality of feed for the age and use of the horse is an important cause that should not be overlooked. In some cases, adequate feed is provided, but the horse is unable to eat a sufficient amount due to factors such as competition with other horses or the presence of a gait deficit that limit’s the horse’s ability to access feed readily. Also, actual dysphagia can contribute to weight loss if prolonged. Some of the more common problems causing dysphagia include dental disorders, oral or pharyngeal foreign bodies, esophageal disorders, masseter myopathy, and several neurologic conditions such as neuropathy associated with guttural pouch disease, nigropallidal encephalomalacia, equine protozoal myelitis, and botulism.
BOX 7.3. Mechanisms and Selected Differential Diagnoses for Decreased Body Weight.
| Mechanism | Differential Diagnoses |
|---|---|
| Lack of access to appropriate food |
|
| Lack of ingestion of available nutrients |
|
| Abnormal digestion, absorption, or metabolism of nutrients |
|
| Inadequate delivery of nutrients to peripheral tissues |
|
| Increased rate of protein and energy use or loss |
|
| Primary muscle wasting disorders |
|
Almost any disease process, including parasitism, infectious diseases, neoplasms, immunologic disorders, toxicities, chronic organ dysfunction, and endocrinopathies, can result in weight loss. In some instances the underlying cause of weight loss is readily apparent, such as in cases of pleuropneumonia or diarrhea. Weight loss is common with many gastrointestinal diseases, particularly protein losing enteropathies. In a retrospective study of 40 horses presented for weight loss despite a good appetite, a definitive diagnosis was established in 24 of 40 cases (60%), and 16 of 40 cases (40%) were idiopathic.141 The most common diagnoses were inflammatory bowel disease (13 of 40, 32.5%) and intestinal lymphosarcoma (4 of 40, 10%). Hypoalbuminemia was identified in 58% of the total cases, and the severity of hypoalbuminemia was related with nonsurvival. A review of inflammatory bowel diseases of the horse identified cases of granulomatous enteritis, multisystemic eosinophilic epitheliotropic disease, lymphocytic-plasmacytic enterocolitis, and idiopathic eosinophilic enterocolitis.142 Some other potential gastrointestinal causes of weight loss and hypoproteinemia in horses include parasitism, right dorsal colitis, and proliferative enteropathy due to Lawsonia intracellularis.
Other important causes of weight loss include internal abscessation, neoplasia, and chronic organ dysfunction.141, 143, 144, 145, 146, 147, 148, 149 Internal abscesses can be difficult to diagnose. Although a multitude of organisms may be involved, Streptococcus equi subspecies equi (strangles) and Corynebacterium pseudotuberculosis (pigeon fever) are particularly important. Weight loss is a common clinical finding in horses with either chronic hepatic or renal disease, and weight loss has also been recognized with heart failure. In some cases, recurrent airway obstruction can also be associated with significant weight loss. Several neoplastic conditions can cause weight loss, and in horses, lymphosarcoma is particularly common.
Endocrine disorders have also been associated with changes in body weight. In horses, PPID is a common disorder that can be associated with either abnormal fat deposition or weight loss and muscle wasting.115, 140 Although uncommon, hyperthyroidism has been documented in horses, with weight loss being a major feature.135, 136, 150, 151 Rarely, diabetes mellitus has been identified in horses.152
Weight loss can be a component of a variety of other conditions. Persistent infection with equine infectious anemia virus can result in weight loss, although many cases are subclinical.153 Several toxins have been associated with weight loss through a variety of mechanisms. For example, yellow star thistle toxicity causes significant dysphagia, and pyrollizidine alkaloids and alsike clover cause hepatic disease. Some other potential toxic causes of weight loss include selenium, lead, wild jasmine (Cestrum diurnum), and hairy vetch (Vicia villosa), among others. Chronic pain may also result in weight loss.
Muscle atrophy may occur either as a component of generalized weight loss, as in starvation and cachexia, or as a separate entity. Muscle loss is often prominent in association with aging and PPID. Some direct causes of muscle atrophy include disuse and neurogenic muscle atrophy. Progressive muscle wasting and weight loss are prominent features of equine motor neuron disease, which is a neurodegenerative disorder linked to vitamin E deficiency.154 Other signs include weakness, muscle fasciculations, shifting weight, and increased recumbency. Also, rapid muscle atrophy affecting primarily the epaxial and gluteal muscles has been associated with immune-mediated myositis, particularly in Quarter Horses.155
Diagnostic Approach to Weight Loss
Signalment and History
The signalment and history should be considered when evaluating a horse with weight loss. Some conditions, such as certain dental disorders and PPID, are more common in older horses. Lawsonia intracellularis primarily affects weanling-age foals.
An accurate diet history is particularly important. The type, amount, and quality of feed should be documented, and it should be determined whether additional micronutrients or supplements are being fed. It is important to ensure that assessment of the amount is accurate by weight, because many owners feed by volume. The age, use, and condition of the horse should be considered when evaluating the feeding program because these may affect nutritional requirements. The feed should be evaluated for the presence of mold or toxic plants. The potential availability of vitamin E in the diet should be considered. Feed analysis can often be beneficial in assessing feed quality. The particulars of the feeding management should be determined, such as the feeding schedule, the types of feeders, potential access to sand, and the water source. It should be established if there are other horses on the property and if so whether there is competition among horses for feed and if the weight loss affects more than one individual. Also, the appetite of the horse should be determined.
General historical information is also important when evaluating weight loss. This includes the history of deworming, dental care, and any use of medications, such as nonsteroidal antiinflammatory drugs. Any history of abnormal shedding, as well as any history of previous disease, such as strangles, pigeon fever, or laminitis, can be relevant.
Physical Examination
A complete general physical examination should be performed. The BCS and, if possible, the body weight should be determined. Observing the animal while eating can be important to establish whether it can prehend, masticate, and swallow normally. Neonatal foals should be observed suckling and evaluated for milk coming from the nostrils. The mare’s udder and the milk should be examined. The clinical examination should include a thorough oral examination, rebreathing examination, and rectal palpation. A neurologic examination may also yield useful information. The presence of concurrent clinical signs, such as fever, diarrhea, cough, nasal discharge, a heart murmur, polyuria/polydipsia, icterus, and abnormal shedding or hypertrichosis, can point more quickly to a specific cause of weight loss and help direct additional testing.
Ancillary Diagnostic Tests
A number of ancillary tests can be useful in the diagnosis of weight loss, and tests should be prioritized based on the signalment, history, and physical examination findings.
Clinical Pathology/Laboratory Testing
A CBC, fibrinogen, and serum chemistry can be useful and is generally part of the minimum database. The white blood cell count and fibrinogen may be elevated with active infection. Several parameters may support a diagnosis of chronic inflammation including anemia, hyperfibrinogenemia, hyperglobulinemia, and thrombocytosis. These changes may support internal abscessation. Hypoproteinemia, and particularly hypoalbuminemia, is seen with protein losing conditions, such as gastrointestinal or renal disease and third space loss, or with decreased protein production, which can be seen with significant liver disease. A common cause of hypoalbuminemia in horses with weight loss is protein losing enteropathy. The serum chemistry may support a diagnosis of renal or hepatic disease, and a urinalysis can help in the evaluation of renal disease. The presence of hypercalcemia may indicate possible renal disease, hyperparathyroidism, or neoplasia. However, it should be remembered that hypercalcemia of malignancy is an inconsistent finding in horses with neoplasia.
Parasitism can contribute to weight loss even if it is not the primary problem. Assessment of parasite status should include fecal flotation with a fecal egg count; a Baermann analysis and tapeworm enzyme-linked immunosorbent assay (ELISA) should be considered. Feces should also be assessed for the consistency and fiber length, as well as the presence of sand.
Sampling of the airway via transtracheal aspirate or bronchoalveolar lavage can be useful in evaluating pulmonary disease. Culture and cytology of pleural or peritoneal fluid can also be useful; however, it should be remembered that many neoplastic conditions do not exfoliate significant numbers of cells, making diagnosis of neoplasia often challenging. Aspiration or biopsy of appropriate tissues may be indicated when possible.
Additional diagnostic testing, such as serum or whole blood trace mineral analysis, determination of vitamin E concentrations, thyroid testing, and testing for PPID, may be indicated for some horses with weight loss, determination of vitamin E concentrations, thyroid testing, and testing for PPID. Baseline concentrations of thyroid hormones often do not accurately reflect thyroid status due to the number of confounding variables, and additional testing is generally recommended.150, 151, 156, 157, 158
Endoscopy
Endoscopic evaluation may aid in the evaluation of pharyngeal function and guttural pouch disease. It can also be helpful in assessing the esophagus and examining the stomach for equine gastric ulcer syndrome or gastric squamous cell carcinoma.
Diagnostic Imaging
Imaging of the thorax by ultrasound and radiographs can be useful in the evaluation of thoracic disease. Ultrasound can be used to assess the abdomen and can help to assess fluid volume and character, intestinal wall thickness, and abdominal masses. Abdominal radiographs may be useful in foals, but in adult horses they are often impractical, and the diagnostic utility is generally limited to the identification of enteroliths or excessive sand accumulation. Echocardiography may be indicated especially in cases where a murmur or arrhythmia is identified on physical examination.
Other
Oral absorption tests using either D(+)-xylose or glucose to identify malabsorption can be useful in the evaluation of unexplained weight loss.142, 159, 160 Although xylose is less affected by metabolic factors, glucose absorption is a valid, practical means of assessing malabsorption. Intestinal biopsy is often necessary to identify the specific cause of the malabsorption.
Cough
Cough is a sudden, forceful expulsion of air through the glottis that generates a characteristic sound. Although cough is a normal reflex mechanism that is important in protection of the airways, it is also a common sign of disease in multiple species. Cough promotes ciliary activity and generates the high air flows necessary to shear mucus from the airway walls, thus acting to remove secretions and foreign particles from the respiratory tract and prevent pulmonary aspiration. Specific data related to the importance of an intact cough mechanism in horses are limited, but in human patients an inefficient cough reflex has been associated with atelectasis and recurrent pneumonia.161, 162, 163 Although cough is an important protective mechanism, excessive cough is a common sign of disease and can have deleterious effects. In human medicine, cough has been estimated to account for up to 40% of outpatient practice.164, 165, 166 Cough is also a common clinical problem in horses. Even when not associated with significant systemic disease, cough can be performance limiting in the equine athlete.
Mechanisms of Cough
Cough is a highly coordinated reflex action that can be modulated by voluntary input. All mammalian species that have been studied either cough or display a similar respiratory reflex.167 Despite the prevalence and clinical importance of cough, the precise mechanisms involved are not fully understood. Much of the information reported regarding the mechanism of cough in horses has been extrapolated from other species. Some species-related differences, however, are known to exist.167 Age and gender are also known to influence cough. The cough reflex can be poorly developed in neonates and can be compromised in association with aging.168, 169 These age-related differences may contribute to an increased susceptibility to aspiration and respiratory infections in these age groups. Interestingly, in humans an increased sensitivity of the cough reflex has been recognized in females following puberty, and women are more likely to develop chronic cough.170, 171, 172
Cough Cycle
There are three phases of the cough cycle: inspiratory, compressive, and expiratory.173, 174, 175 These phases are generally followed by a period of relaxation. In the inspiratory phase, inhalation essentially generates the volume necessary for an effective cough. A variable amount of air is inhaled and serves to lengthen the expiratory muscles, optimizing the length-tension relationship. This expansion of lung volume allows for an increase in the subsequent velocity of expiratory airflow due to greater force of contraction from the lengthened muscles and greater elastic recoil pressure.
The compressive phase of cough consists of a very brief (200 msec) closure of the glottis to maintain lung volume as isometric contraction of the expiratory muscles occurs. Muscles of the chest wall, diaphragm, and abdominal wall contract against the closed glottis, resulting in a rapid rise in intrathoracic pressure.
The expiratory phase starts with opening of the glottis as the expiratory muscles continue to contract, resulting in the release of air. There is a brief period of supramaximal expiratory flow, sometimes referred to as the cough spike, which is then followed by lower expiratory flows. Due to relative changes in pleural and airway pressures there is dynamic compression of the larger airways during expiration, which helps to maximize the velocity of airflow by decreasing the airway diameter. The high-velocity turbulent airflow results in airway vibration, which is largely responsible for the sound recognized as cough. Importantly, the high flows generated in the expiratory phase dislodge mucus and facilitate the removal of mucus and debris from the tracheobronchial tree. At the end of cough, expiratory muscles relax, intrapleural pressure decreases and transient bronchodilation may occur.
The specific pattern of cough can vary and is sometimes broadly categorized into two types.173 Laryngeal cough, which functions primarily to protect the airway from aspiration, is triggered by mechanical stimulation of laryngeal receptors and is a true reflex, sometimes referred to as the expiratory reflex. The inspiratory phase is minimal, and expiration occurs rapidly. Tracheobronchial cough is stimulated primarily by receptors distal to the larynx and has a more prominent inspiratory phase in order to generate the greater airflows necessary to remove secretions and debris from the tracheobronchial tree. It may occur either as a reflex or by voluntary control.
Neurophysiology of Cough
Coughing is a complex visceral reflex that can be both voluntarily and involuntarily regulated.176, 177, 178, 179 The neurologic control of cough is still under investigation, and an improved understanding has therapeutic implications for the control of cough. Basically, cough can be compartmentalized into three components: (1) stimulation of sensory afferent nerves, (2) integration of the information in the brainstem and higher brain centers, and (3) activation of efferent pathways to the muscles involved in the generation of cough176, 177, 178, 179 (Fig. 7.2 ).
Fig. 7.2.
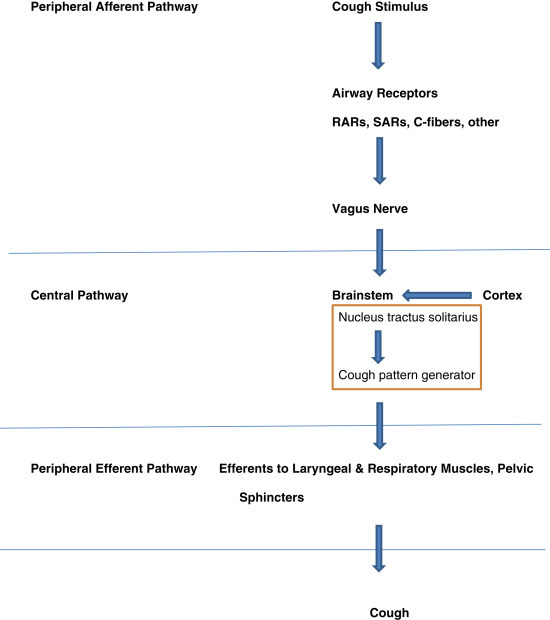
Simplified schematic of the neurophysiology of cough.
Afferent Pathways Regulating Cough
Cough is initiated by the activation of sensory vagal afferent nerves that primarily innervate the respiratory tract, although they may be found in additional sites such as the ear in some species.178, 179, 180, 181, 182 Though all vagal in origin, this population of afferent nerves is not homogeneous, and several subtypes have been identified. Some of the differences between subtypes include their distribution within the respiratory tract as well as the presence of myelination, the receptor type and sensitivity to various stimuli, and the location of the cell bodies, which can be within either the nodose and/or jugular ganglia. In general, these nerves respond to chemical and/or mechanical stimuli, with different thresholds for the various stimuli. There is no single classification scheme, but the subtypes are often functionally classified broadly as being either primarily sensitive to mechanical stimuli (mechanosensors) or to chemical stimuli (chemosensors, sometimes referred to as nociceptors). Although both types can be located throughout the airways, mechanosensors tend to predominate in the larynx and large airways. Among the most studied subtypes of sensory afferents are the rapid-adapting receptors (rapidly adapting stretch receptors), slow-adapting receptors (slowly adapting stretch receptors), and C-fibers. Several other receptors, including a possible specific cough receptor, have been described. Overall, the relative contribution of the various subtypes in the generation of cough is still uncertain and may be species dependent. These nerves often contribute to other functions within the respiratory tract, such as enhancing mucus secretion, initiating bronchoconstriction, and influencing the rate and depth of respiration.
Rapid-Adapting Receptors and Slow-Adapting Receptors
Rapid-adapting receptors (RARs) and slow-adapting receptors (SARs) are myelinated nerves that originate primarily in the nodose ganglion.178, 179, 180, 181 RARs are present in both extrapulmonary and intrapulmonary airways, whereas SARs are predominantly within the intrapulmonary airways and lung parenchyma and are sparse in extrapulmonary airways. Although there are some differences in the exact stimuli they respond to, they are both predominantly low threshold mechanosensors that may respond to a variety of stimuli such as changes in lung volume and changes in airway diameter due to smooth muscle constriction, airway wall edema, or the presence of mucus.
C-Fibers
The majority of vagal airway afferent nerves are unmyelinated C-fibers that originate primarily in the jugular ganglion.178, 179, 180, 181 They innervate the epithelial layer throughout the extrapulmonary and intrapulmonary respiratory tract. A type of nociceptor, C-fibers are predominantly chemosensors that respond to a wide variety of stimuli including both high and low temperatures, nonisotonicity, low pH, irritants, and inflammatory mediators. They can have multiple receptors, including neuronal transient receptor potential (TRP) channels such as ankyrin 1 (TRPA1) and vanilloid 1 (TRPV1).183, 184 Most C-fibers are known to react with capsaicin, and it appears that this occurs primarily through the TRPV1 receptor. Also, the TRP receptors may be important in disease states as they can be activated by reactive oxygen species generated during inflammation, and TRP channel antagonists are being investigated as potential antitussive agents.185, 186, 187 PGE2, which is released in association with airway inflammation, acts directly on pulmonary C-fibers to increase their sensitivity and lower the cough threshold.188
The initiation of cough is a complex activity with multiple interactions between the afferent nerve subtypes.178, 179, 180, 181, 189 For example, activated C-fibers release neuropeptides including substance P, neurokinin A, and calcitonin gene–related peptide. These neuropeptides can cause bronchospasm, edema formation, and mucus secretion, which in turn can lead to activation of the RARs. Without the activity of the RARs and SARs, C-fibers are limited in their ability to evoke cough.
Bronchoconstriction and cough are closely interrelated but can also have distinct pathways.190, 191 Some, but not all, stimuli for cough will also cause reflex bronchoconstriction, which generally has a slower onset and longer duration than cough. Bronchoconstriction itself can be a stimulus for cough and, up to a point, can improve the efficiency of cough by decreasing airway diameter and thus increasing airflow velocity. Some therapies affect either bronchoconstriction or cough, whereas others affect both. For example, some bronchodilator drugs can also decrease cough by desensitizing airway receptors that initiate cough.
Central Regulation of Cough
The mechanisms controlling the central regulation of cough are complex, involving both the brainstem and higher brain centers.177, 179, 192, 193, 194 The afferent sensory nerve input arising from the respiratory tract is centrally integrated primarily within the nucleus tractus solitarius (nTS) of the medulla oblongata in the brainstem. Neurons within the nTS communicate with other brainstem neurons as part of a multifunctional, dynamic neural network that functions in both generation of the normal breathing pattern and cough. This neural network has been referred to as the cough pattern generator as well as the respiratory pattern generator or central controller. When cough occurs, there is reshaping of the discharge patterns of respiratory neurons within this neural network resulting in discharges being transmitted to the motor neurons involved in cough. Projections between the brainstem and cortical centers add complexity to the regulation of both breathing and cough. Due to cortical modulation, some cough can be voluntarily initiated and suppressed. Among the receptors involved in the generation of cough in the brainstem are N-methyl-d-aspartate (NMDA) receptors, and in several species antagonists of NMDA-type glutamate receptors such as dextromethorphan have been effective antitussives.195, 196
Activation of Efferent Pathways
Signals from the brainstem and cerebral cortex are transmitted via a number of efferent nerves to the muscles involved in cough.173, 178 Important efferent nerves include the vagus, phrenic, intercostal, and lumbar nerves. In addition to the vagus, several other cranial nerves play a role, including the trigeminal, facial, hypoglossal, and accessory nerves. The efferent nerves involved in cough supply the muscles of the larynx and tracheobronchial tree, the diaphragm, and the intercostal, abdominal, and pelvic muscles, as well as secretory glands of the respiratory tract. The coordinated activity of these muscles results in cough.
Cough Efficiency/Adverse Effects
The effectiveness of cough is dependent on the ability to generate high flow velocities and the interaction of the flowing air with airway secretions.173 In general, cough is more effective in clearing large airways as opposed to smaller peripheral airways. Cough efficiency is affected by the integrity of the neurophysiologic pathway of cough as well as physical aspects, such as respiratory muscle strength and mucus quality. Normal mucus acts to improve airway clearance and protect receptors from irritants, and changes in the amount or tenacity of mucus can both mechanically stimulate cough receptors and cause flow limitation.173, 197, 198 Cough is often less effective as a clearance mechanism when airway disease is present.
Not only does cough lose its defensive function is some disease conditions, but it may even have deleterious effects.173, 199 Lung expansion during the inspiratory phase may allow for the spread of infectious agents and particulate matter into the smaller airways. Paroxysmal or persistent coughing can cause fatigue and may decrease feed intake, particularly in foals. Chronic cough can result in bronchial muscle hypertrophy. Cough can also have significant cardiovascular effects due to changes in abdominal and intrathoracic pressures.199, 200 During the expiratory phase of cough there is an initial rise in systemic arterial blood pressure and a concurrent rise in cerebral venous and cerebrospinal fluid pressures. This is followed by a period of hypotension, which can reduce the effective perfusion pressure of the brain, especially when there is high cerebral venous pressure. The resulting cerebral hypoperfusion and anoxia have been associated with cough-induced syncope in dogs as well as people.201, 202 Some other potential adverse effects of cough that have been reported in small animals and people include pneumothorax, pneumomediastinum, and lung lobe torsion, as well as rib and vertebral fractures.199, 202
Conditions Associated with Cough
The potential causes of cough are diverse, as the cough receptors can respond to a wide variety of mechanical and irritant stimuli.173, 174, 175, 176, 177, 178, 179, 180, 181, 183, 203 Some general stimuli of cough include inhaled particles or irritants, inflammatory mediators, bronchoconstriction, excessive mucus, exposure to cold or hot air, sloughing of airway epithelial cells, and pulmonary edema. Intramural or extramural tension on the airways, such as that seen in association with masses or decreased pulmonary compliance, can result in cough. The sensitivity of cough receptors is influenced by a number of factors, such as genetics, age, and the presence of disease. Most often, the cough reflex becomes hyperresponsive once respiratory disease is established due to both increased exposure of the cough receptors due to a loss of the integrity of the epithelial lining and increased sensitivity of the receptors in response to inflammation. It is important to remember, however, that significant respiratory disease can be present without cough.
The specific causes of cough have been categorized in a number of ways, such as the duration of signs (acute or chronic), the presence or absence of fever, whether the cough is productive or not (wet or dry), and whether it originates from the upper or lower airway. Categorizing cough can be useful in establishing a list of differentials for cough, although there can be considerable overlap between the categories. The presence or absence of fever may be particularly useful when considering the causes of cough (Box 7.4 ).
BOX 7.4. Causes of Cough with Fever.
| Common Causes | Major Diagnostic Test(s) |
|---|---|
|
|
|
|
| Less Common Causes | Major Diagnostic Test(s) |
|---|---|
|
|
|
|
|
|
|
|
|
Causes of Cough Associated with Fever
Cough in conjunction with fever is most often associated with infection of the respiratory tract, although some noninfectious conditions such as neoplasia can also occasionally present with fever. Infection of the respiratory tract may be primary or may be secondary to an underlying condition such as inflammation of the airways. Viral infections of the respiratory tract are a common cause of cough in horses and are particularly important as they can cause outbreaks of respiratory disease.203, 204 Affected horses often present with an acute onset of a dry cough in conjunction with fever, lethargy, and anorexia. Some important equine respiratory viruses include equine influenzavirus, equine herpesviruses (EHVs), equine rhinitis A and B, and equine viral arteritis. With regard to the equine herpesviruses, EHV-4 and EHV-1 are the major pathogens associated with acute respiratory disease in the domestic horse population.204, 205 Respiratory viruses frequently cause exposure and sensitization of cough receptors, along with injury to epithelial cells and decreased mucociliary clearance, which can predispose the affected horse to secondary bacterial infection. In addition to viruses, both bacterial and fungal agents can cause infection of the respiratory tract, and are often associated with a periodic wet cough due to increased mucus production and neutrophil accumulation. Bacterial infection is particularly common in the lower respiratory tract and can cause bronchitis, pneumonia, or pleuropneumonia.206, 207 A variety of organisms have been isolated and polymicrobial infections may occur. Some common pathogens include Streptococcus equi subspecies zooepidemicus, Pasteurella, Actinobacillus spp., Escherichia coli, and Klebsiella pneumoniae. Rhodococcus equi is an important cause of pneumonia in foals. Anaerobic organisms, such as Bacteroides fragilis and Peptostreptococcus anaerobius, may also infect the lower airways of horses. Mycobacterium and Mycoplasma spp. are occasionally isolated from horses with respiratory tract disease.206, 208 Fungal respiratory disease in horses is uncommon and is most often recognized in the paranasal sinuses, guttural pouches, and lungs.209, 210 Some primary fungal pathogens that have been identified in association with respiratory disease in the horse include Coccidioides immitis, Cryptococcus neoformans, Histoplasma capsulatum, Blastomyces dermatitidis, and Conidiobolus coronatus. Opportunistic fungi, such as Aspergillus spp., Candida spp., and Pneumocystis carinii, can also cause respiratory tract disease. Following recovery from infectious respiratory tract disease of any cause, horses will often have a persistent wet or dry cough as the cough receptors remain hypersensitive.
Interstitial pneumonia has been reported in horses of all ages in association with both acute and chronic respiratory disease.211, 212 Horses with interstitial pneumonia frequently have marked tachypnea with increased respiratory effort, and cough and fever can be present as well. Some specific types of interstitial pneumonias include equine multinodular pulmonary fibrosis and syndromes of acute lung injury and acute respiratory distress. Although interstitial pneumonia has been linked to a variety of causes, in many cases an underlying cause is not identified. Equine multinodular pulmonary fibrosis has been linked to EHV-5 infection.212
Other conditions may occasionally present with cough in association with fever. Thoracic neoplasia is uncommon in horses and presents with variable clinical signs that may include cough with or without fever.213 Smoke inhalation may cause a significant inflammatory response and edema resulting in tachypnea, cough, and fever.214 Fever may occasionally be present with parasitic or cardiogenic cough.
Causes of Cough Without Fever
Among the most common causes of cough without fever in horses is a spectrum of chronic inflammatory airway disease that is now referred to as equine asthma syndrome.215, 216, 217, 218 Included in this syndrome are inflammatory airway disease (IAD, mild to moderate equine asthma) and recurrent airway obstruction (RAO, severe equine asthma). Summer pasture-associated RAO (SPARAO, summer pasture-associated obstructive pulmonary disease, SPAOPD) is a subset of RAO in which signs occur after exposure to pasture in warm summer months. RAO primarily affects horses over 7 years of age. The prevalence of RAO is approximately 10% to 20%, and a familial predisposition has been identified.219, 220, 221, 222 Affected horses typically have frequent coughing, increased respiratory effort at rest, and exercise intolerance. With IAD, horses may be affected at any age, and the clinical signs are more subtle. Both RAO and IAD are characterized by increased mucus in the airways. With regard to the specific cytologic abnormalities in the bronchoalveolar lavage fluid (BALF), RAO is typically characterized by pronounced neutrophilia (greater than 25%), whereas in IAD the abnormalities tend to be milder and more variable. Other conditions that can resemble equine asthma clinically include silicosis, lungworm infection, and idiopathic eosinophilic pneumonia.223, 224, 225, 226
It is not uncommon for horses to cough occasionally, especially at the beginning of exercise. These coughs are sometimes referred to as arena cough, warm-up cough, or nuisance cough depending on the exact circumstances. This type of cough may reflect cough’s function as part of the normal defense mechanisms of the respiratory tract, as it is not uncommon for clinically normal horses to have some mucus accumulated behind the larynx waiting to be expelled. Also, horses breathe more deeply during exercise, and the environment in some riding arenas can be dusty. Although this type of cough is generally relatively innocuous, it may indicate poor air quality and may also be a sign of early disease. Thus all horses with cough should be monitored closely.
Parasitic pneumonitis is another cause of cough that is generally afebrile, although occasionally fever may be seen. In foals, the larvae of Parascaris equorum migrate through the lungs and can occasionally cause a significant inflammatory response resulting in cough and nasal discharge.203 Horses infected with the lungworm Dictyocaulus arnfeldi can have chronic cough and mucoid nasal discharge.224, 225 Affected horses often have a history of contact with donkeys or mules. In donkeys and mules, infection is generally asymptomatic but is patent, providing a source of eggs in the feces. In horses and ponies, infection often results in an inflammatory response and cough and is typically not patent, with larval development arrested in the lungs.
Exercise-induced pulmonary hemorrhage (EIPH) is defined by the presence of hemorrhage in the airways of a horse after exercise.227, 228 It is common in Thoroughbred and Standardbred racehorses and in other horses performing strenuous exercise, such as barrel racing horses. The clinical abnormalities seen with EIPH are not consistent but may include epistaxis, increased respiratory rate, poor performance, and coughing.
Congestive heart failure, although not common in horses, can result in cough.229, 230 In particular, in left-sided heart failure, blood is not effectively pumped from the left side of the heart, leading to increased blood in the pulmonary circulation and increased pulmonary vascular pressure. Vascular congestion and pulmonary edema follow, and cough receptors are stimulated. In a study of 14 horses with congestive heart failure, all horses presented with a heart murmur and tachycardia.229 Other common clinical signs included cough, crackles, tachypnea, ventral edema, and either jugular distention or pulsation. The underlying causes for heart failure included congenital defects, traumatic vascular rupture, pericarditis, pulmonary hypertension secondary to RAO, and valvular dysplasia. Fever is not generally associated with cardiogenic cough but may be present in some horses, especially those with pericarditis or endocarditis.
A number of disorders of the upper airway can result in cough. Pharyngitis, also referred to as pharyngeal or follicular lymphoid hyperplasia, is a relatively common disorder that is recognized primarily in young horses.203, 231, 232 It is most likely the result of the lymphoid response to a variety of irritant or infectious stimuli. Although many cases are subclinical, coughing, nasal discharge, and mild submandibular lymphadenopathy may be present. Dynamic or static compression of the upper airway such as may be seen with dorsal displacement of the soft palate, fourth branchial arch defect (rostral displacement of the palatopharyngeal arch, laryngeal dysplasia), aryepiglottic fold entrapment, subepiglottic cyst, arytenoid chondritis/chondrosis, and laryngeal hemiplegia, may also be associated with cough, which is often dry in nature.203 Although clinical signs vary, these disorders are often characterized by abnormal breath sounds such as stridor or stertor at rest or during exercise as well as normal lung sounds and absent or minimal mucopurulent discharge. Soft palate problems, including soft palate paresis, dorsal displacement of the soft palate and cleft palate, and other causes of dysphagia, can result in cough that is often associated with eating. In some cases, disorders such as sinusitis and guttural pouch mycosis or empyema will result in cough due to the presence of exudate in the upper airways.
Tracheal abnormalities may also result in cough. These include tracheal stenosis, collapse, and partial obstruction such as may be seen with a foreign body or neoplasia. Tracheal collapse is typically associated with an inspiratory honking sound. It is most often diagnosed in American Miniature Horses but may be seen in other horses as well and has been reported in association with pneumonia.233, 234, 235
Diagnostic Approach to Cough
Cough is a common clinical problem that can be associated with a number of diverse disorders. The diagnosis of the specific cause of cough is based on consideration of the signalment, history, and clinical signs, as well as the use of appropriate diagnostic aids.
Signalment and History
The signalment can help to prioritize the differential diagnoses in a horse presented for cough. Pneumonia caused by Rhodococcus equi is most common in foals 1 to 6 months of age.207 Although viral respiratory diseases can be seen in any age horse, they are particularly common in weanling and yearling horses.204 Pharyngitis is also most common in young horses.231, 232 Horses with RAO are typically over 7 years of age, and a familial predisposition has been identified.215, 216, 217, 218 The risk of developing RAO is threefold when one parent is affected and almost fivefold when both parents are affected.221, 222
Several components of the history are important in the assessment of a horse with cough. Routine management factors such as vaccination, deworming, stabling, and bedding type, as well as the feed type, feed quality, and feeding practices, are of particular importance. Risk factors for infectious respiratory disease include exposure to new horses, either at an event or through new arrivals to the farm, and stress, such as recent transport, surgery, weaning, or strenuous activity. A history of other clinical signs such as fever, lethargy, and anorexia, as well as the involvement of other horses, may increase the suspicion of infectious respiratory disease. The risk of lungworm infection is increased by exposure to donkeys or mules.224, 225 EIPH is associated with strenuous exercise.227 The geographic area may raise the suspicion of some conditions such as silicosis, which has been reported primarily in California, and certain mycotic infections, such as Coccidioides immitis, which is found primarily in the southwestern United States.209, 210, 223 Any history of previous disease, as well as a history of signs such as stridor or exercise intolerance, can also be important.
The historical aspects of the cough itself can be helpful in the evaluation of horses with cough. These include whether the cough is acute or chronic, any seasonal pattern, the frequency of the cough, and any association with feeding or exercise. It can be helpful to establish if the cough is nonproductive or productive, although this can be difficult to discern.
Physical Examination
A complete physical examination, including a detailed examination of the respiratory system, should be performed in horses presented for evaluation of cough. The character of any nasal discharge should be noted. The presence of a fever increases the likelihood of infectious respiratory tract disease, although noninfectious conditions may occasionally be associated with fever. It should be remembered that fever can be intermittent and can be suppressed by the administration of nonsteroidal antiinflammatory drugs. Careful auscultation of the thorax should be performed. The presence of a heart murmur and tachycardia may suggest congestive heart failure, especially if there is jugular distention or pulsation.229 If the horse can tolerate it, a rebreathing examination should be performed, especially if there are no obvious abnormal sounds during quiet breathing. The rebreathing examination, which is performed by loosely placing a plastic bag over the nostrils, will cause the horse to increase tidal volume, accentuating breath sounds. Cough may be induced, especially if there is exudate in the airways. The presence of crackles and/or wheezes on auscultation suggests pulmonary parenchymal disease. With pulmonary consolidation, atelectasis, or pleural fluid, breath sounds are typically decreased or absent ventrally, although occasionally sounds may be accentuated due to referral of sounds from aerated lung. Percussion can be used to identify areas of dullness. In horses with significant RAO, percussion can be used to identify a shift of the caudal lung border.236
Ancillary Diagnostic Tests
Clinical Pathology
A CBC and fibrinogen are frequently indicated in the assessment of horses with cough, and in some cases a serum chemistry may also be useful. Acute viral infection can be associated with leukopenia characterized primarily by lymphopenia.204 A transient anemia may be seen as well. Neutrophilia and hyperfibrinogenemia are seen with many inflammatory conditions, especially bacterial or fungal pneumonia.206 Inflammatory conditions may also be associated with anemia, hyperglobulinemia, and thrombocytosis, especially if chronic. Hematologic values are usually unremarkable in horses with uncomplicated equine asthma.216 Although not consistent, peripheral eosinophilia may be seen with idiopathic eosinophilic pneumonia, parasitic pneumonitis, and occasionally eosinophilic IAD.216, 224, 225, 226 Hypercalcemia can be seen in some horses with malignancy.
An arterial blood gas evaluates air exchange and helps to monitor the severity of respiratory disease. In some horses with chronic or recurrent infectious disease, measurement of immunoglobulin concentrations may help identify an underlying immunodeficiency. There has been a growing interest in the use of biomarkers to evaluate disease states. Although findings are often nonspecific, serum amyloid A and fibrinogen are two biomarkers commonly used to evaluate inflammatory disease.237 In racehorses with IAD, increased concentrations of surfactant protein D (SP-D) have been described, and in horses with RAO, soluble CD14 concentrations are increased compared with controls.238, 239
Airway Cytology
Several techniques have been described for sampling the respiratory tract of horses.240, 241, 242, 243, 244, 245, 246 The primary means of sampling the lower respiratory tract include bronchoalveolar lavage and tracheal wash. Tracheal wash is often used for the diagnosis of infectious pneumonia. Analysis of BALF is recommended for the diagnosis of equine asthma.215, 216 RAO is characterized by a moderate to severe neutrophilia (greater than 25%), whereas in IAD the changes are more varied and may include a mild to moderate increase in neutrophils (greater than 10%), eosinophils (greater than 5%), and/or mast cells (greater than 5%). BALF fluid cytology is also useful in the diagnosis of EIPH, which is characterized by the presence of erythrocytes and hemosiderophages.
Assessment of Infection
Currently a nasal or nasopharyngeal swab is the preferred sample for diagnosis of most viral respiratory infections.204 A Dacron or rayon swab is preferred compared with cotton. Detection of genetic material by PCR is a common means of virus identification, although virus isolation can be performed as well. There are also several means of viral antigen detection. For example, direct immunofluorescence can be used to demonstrate EHV antigens and several enzyme-linked immunosorbent assays are available for the detection of influenza-virus nucleoprotein.
Appropriate samples for the identification of bacterial or fungal agents in respiratory tract disease depend on the suspected site of infection and organism. Common techniques for sampling the upper airways include nasal or nasopharyngeal swab, nasal wash, guttural pouch wash, and sinus trephination. Transtracheal aspiration is commonly used for sampling the lower airway in cases of suspected pneumonia. Other techniques include endoscopic tracheal aspiration or brushing and sterilely performed BAL. Pleural fluid can be sampled by thoracocentesis. Cytology and Gram stain of the samples can support the presence of infection. Culture and PCR are used for identification of specific pathogens. Many laboratories now offer diagnostic panels for respiratory disease that test for multiple common pathogens.
Serologic testing is available to detect the host response to several infectious agents. Depending on the organism, however, it may be difficult to distinguish active infection from exposure or vaccination. Serology can be useful in the diagnosis of several fungal infections, such as cryptococcosis, coccidiomycosis, blastomycosis, and conidiobolomycosis.209, 210
The Baermann fecal flotation technique can be used in horses with suspected lungworm infection. However, as infections in horses are often not patent, a negative Baermann does not rule out the presence of lungworms.224, 225 Routine fecal flotation can be used to detect Parascaris equorum in foals with suspected parasitic pneumonitis, but in some cases the infection may not yet be patent.
Endoscopy
Endoscopic examination is particularly valuable in evaluation of the upper airways and trachea. Because sedation can alter airway function, endoscopy is best performed without sedation if possible. Endoscopy can identify exudate, anatomic or functional abnormalities, mass lesions, and airway obstruction. On occasion, lungworm larvae may be seen in the trachea. Pleuroscopy can be used to evaluate the pleural space.
An endoscopic mucus scoring system has been developed to quantify mucus in the trachea (0—no visible mucus, 1—single to multiple small blobs of mucus, 2—larger but nonconfluent blobs, 3—confluent or stream forming mucus, 4—pool forming mucus, 5—profuse amounts of mucus).247 Normal horses tend to be either grade 0 or 1, whereas those with equine asthma are 2 to 5.216, 248 Studies have shown associations between the amount of mucus present in the airways with coughing and poor performance.232, 249, 250, 251
Diagnostic Imaging
Radiographs and ultrasound are useful in the evaluation of horses with either suspected upper or lower airway cough.246 Radiographs of the upper airway can demonstrate fluid accumulation, soft tissue masses, and abnormalities of pharyngeal and laryngeal structures. Ultrasound can be used to evaluate laryngeal problems such as left laryngeal hemiplegia, laryngeal dysplasia, arytenoid chondritis, and congenital malformations. Thoracic ultrasonography is a practical means of assessing the thorax and can provide diagnostic information about the pleural cavity, lung, and mediastinum. The presence and character of pleural fluid can be determined, and abnormalities such as consolidation and abscessation in the periphery of the lung can be identified. In addition, the caudal lung borders can be determined. If congestive heart failure is suspected, the heart can be evaluated by echocardiography. Thoracic radiography can identify abnormalities of the pleural space, pulmonary parenchyma, mediastinum, and diaphragm. In the lungs, pulmonary consolidation, abscesses, peribronchial disease, and interstitial disease may be evident.
Other Diagnostic Aids
Several additional diagnostic tests can be helpful in the evaluation of equine asthma, especially pulmonary function testing.215, 216, 217, 218, 249, 252 Other tests that may be useful include a hay challenge to induce disease, histamine provocation tests to assess airway reactivity, and bronchodilator response testing, typically with atropine, N-butylscopolammonium bromide, or albuterol. Although allergen testing has limitations in horses, it may be useful in some cases to identify specific triggers. Endobronchial biopsy specimens have also been used in the evaluation of horses with asthma.253
Percutaneous lung biopsy, although not commonly performed, can be used to evaluate respiratory disease.254 Both histologic and microbiologic evaluation of tissue may be useful. Lung biopsy may be particularly helpful in the diagnosis of pulmonary neoplasia, equine multinodular pulmonary fibrosis, and silicosis.
Respiratory Distress
Respiratory distress is defined as labored breathing and is characterized by an inappropriate degree of effort to breathe based on rate, rhythm, and subjective evaluation of respiratory effort.255 Dyspnea refers to the sensation of arduous, uncomfortable, or difficult breathing that occurs when the demand for ventilation exceeds the patient’s ability to respond.256 Because dyspnea describes a subjective feeling, it is technically a symptom rather than a clinical sign and thus not strictly applicable in veterinary medicine although the term is often used. The clinical signs of respiratory distress vary with the severity and origin of impaired gas exchange. Clinical signs commonly observed in horses with respiratory distress include flared nostrils, exercise intolerance, inactivity, exaggerated abdominal effort, abnormal respiratory noise (stertor or stridor), anxious expression, extended head and neck, and synchronous pumping of the anus with the respiratory cycle.255, 257, 217 In severe cases, cyanosis may be seen. Horses with chronic respiratory distress may develop a heave line resulting from hypertrophy of the cutaneous trunci and abdominal muscles, which assist during forced expiration.257, 217 Weight loss may be seen in association with decreased feed intake and a high energy demand for respiration. In fact, it has been shown that in horses with recurrent airway obstruction (RAO), which is part of the syndrome of equine asthma, the work of breathing has the potential to approximate the energy expenditure of a horse trotting for 12 hours a day.258, 216
Mechanisms of Respiratory Distress
Problems with both ventilation, which is the process of moving air into and out of the lungs, and respiration, which is the process of gas exchange, can impair the efficient exchange of oxygen and carbon dioxide leading to respiratory distress. Common causes of respiratory distress include primary pulmonary disease, airway obstruction, or impairment of the muscles and supporting structures necessary for ventilation.255, 256, 259 In some horses, respiratory distress can occur in the absence of impaired gas exchange in response to pain, metabolic acidosis, or high environmental temperature. Familiarity with the mechanics of breathing and control of ventilation in healthy and diseased lungs facilitates the diagnosis and treatment of respiratory distress.217, 257
Control of Ventilation
The partial pressure of oxygen (Pao 2) and the partial pressure of carbon dioxide (Paco 2) in arterial blood are maintained within a narrow range through rigid control of gas exchange.256 The control of ventilation is complex but essentially involves three components: (1) stimulation of sensory afferents via chemoreceptors and mechanoreceptors, (2) integration of the information in the brainstem, and (3) efferent signals to the muscles of respiration256, 260, 261, 262 (Fig. 7.3 ). Within the brainstem, ventilation is controlled by a complex neuronal network known as the central controller or respiratory pattern generator, which is the same neuronal network involved in the cough reflex. In response to afferent signals, the central controller alters the rate and depth of respiration via efferent signals to the muscles of respiration. The central controller therefore adjusts alveolar ventilation to the metabolic rate of the individual. Although this process is involuntary, higher brain centers can influence ventilation, and there can be voluntary, conscious control of ventilation.
Fig. 7.3.

Simplified schematic outlining the control of ventilation. CFRs, C-fiber receptors; RARs, rapid-adapting receptors; SARs, slow-adapting receptors.
Neuromuscular Physiology of Ventilation
Sensory Afferents
Afferent input into the central respiratory centers in the brainstem arises primarily from three groups of neural receptors: (1) peripheral arterial chemoreceptors, (2) central chemoreceptors in the brainstem, and (3) chemoreceptors and mechanoreceptors in the respiratory tract260, 261, 262, 263, 264 (see Fig. 7.3). In addition, mechanoreceptors in the diaphragm and thoracic wall have some influence on the level and timing of ventilatory activity in response to changes in length, tension, or movement. All of these receptors collectively provide information to the central controller, resulting in the modification of ventilation.
Central and Peripheral Arterial Chemoreceptors
The major chemoreceptors include the central chemoreceptors in the ventral medulla of the brainstem and the peripheral chemoreceptors in the arterial vasculature.256 These chemoreceptors identify changes in metabolism and oxygenation, providing feedback to the central controller and thus influencing ventilation.
The central chemoreceptors monitor alterations in the pH of intracerebral interstitial fluid and cerebrospinal fluid. As the blood-brain barrier is impermeable to bicarbonate and hydrogen ions but is freely permeable to carbon dioxide, acidification of the intracerebral interstitial fluid and thus stimulation of the central chemoreceptors occur predominantly in response to hypercapnia and respiratory acidosis. The severity of acidosis in the intracerebral interstitial fluid caused by hypercapnia is amplified by two features of the central nervous system: (1) hypercapnia produces cerebral vasodilation, increasing the delivery of CO2 to the central nervous system, and (2) cerebrospinal fluid has poor buffering capacity because of low total protein concentrations.256
Peripheral chemoreceptors include the carotid bodies, situated at the bifurcation of the common carotid artery, and the aortic bodies, which are located near the aortic arch.256 Carotid bodies are generally considered the primary peripheral chemoreceptor, but the aortic bodies can also influence ventilation. These receptors relay information to the central controller regarding arterial gas tensions via the glossopharyngeal and vagus nerves. Their responsiveness to alterations in Paco 2 is less consequential than the central chemoreceptors. They also can have a slight response to metabolic acidosis. The peripheral chemoreceptors are highly sensitive to changes in the partial pressure of oxygen, however, and are solely responsible for the hypoxic ventilatory drive. The peripheral chemoreceptors demonstrate a nonlinear response to low arterial oxygen tension. They are insensitive to alterations in Pao 2 above 100 mm Hg, exhibit moderate response to arterial O2 tensions between 50 and 100 mm Hg, and demonstrate a dramatic increase in responsiveness when the partial pressure of oxygen falls below 50 mm Hg in the arterial circulation.256 The respiratory pattern elicited by hypoxia differs from that stimulated by hypercapnia.265, 266 Hypoxia evokes an increase in respiratory frequency, whereas hypercapnia triggers an elevation in tidal volume. In addition, hypoxia stimulates recruitment of the inspiratory muscles, whereas hypercapnia potentiates the activity of inspiratory and expiratory muscles.
The sensitivity of peripheral chemoreceptors should be considered in the treatment of patients with complex acid-base and blood-gas abnormalities. A patient suffering simultaneously from impaired gas exchange caused by pulmonary disease and metabolic acidosis resulting from shock can manifest respiratory distress in response to hypoxemia, hypercapnia, and acidosis. Oxygen supplementation likely will improve the patient’s arterial oxygen tension; however, such treatment may abolish the hypoxic ventilatory drive and consequently slow the ventilatory rate. This decreased ventilation could exacerbate respiratory acidosis and result in decompensation of the patient.259 Although treatment of metabolic acidosis may be indicated, it can also contribute to respiratory acidosis by ultimately increasing CO2. Thus to prevent life-threatening acidemia, treatment of respiratory acidosis, often by assisted ventilation, may be indicated depending on the specifics of the case.
Respiratory Tract Receptors
Receptors located in the upper and lower respiratory tract respond to mechanical and chemical stimuli and relay afferent information to the central controller of respiration via the vagus nerve.255, 256, 263, 264 Vagal blockade abolishes tachypnea in horses with pulmonary disease; therefore these receptors are likely to play an important role in development of respiratory distress associated with primary pulmonary disease.267, 268, 269
As discussed earlier, some of the major respiratory tract receptors influencing ventilation are the slow-adapting receptors (SARs, slowly adapting stretch receptors), rapid-adapting receptors (RARs, rapidly adapting stretch receptors), and C-fiber receptors (CFRs).261, 263, 264 These receptors also function in mucus secretion, bronchoconstriction, and the generation of cough.180 The SARs, also known as pulmonary stretch receptors, are located primarily within smooth muscle fibers in the walls of the trachea and bronchi and are sparse in extrapulmonary airways.256, 259, 263 The SARs are primarily mechanosensors and are stimulated in part by pulmonary inflation, inhibiting further inflation of the lung (Hering-Breuer reflex). Conversely, at end expiration these receptors stimulate inspiratory activity. These receptors are considered to be partially responsible for controlling the depth and rate of respiration.
The RARs are found in and under the epithelium of the respiratory tract from the nasopharynx to the bronchi, and their pattern of response varies with their location.256, 264 Although they are highly sensitive to mechanical stimuli, those in the bronchi are more chemosensitive and are sometimes referred to as irritant receptors. The wide variety of stimuli they may respond to include exogenous agents such as smoke, irritant gases, and dust, as well as endogenously produced inflammatory mediators, including histamine and prostaglandins. RARs are not likely to function in regulation of breathing in a normal resting horse.259 Stimulation of these receptors by noxious stimuli triggers bronchoconstriction, cough, tachypnea, mucus production, and release of inflammatory mediators.255, 256, 180 In horses with RAO (severe equine asthma), the production of histamine, prostaglandins, and other inflammatory mediators increases, and the resulting stimulation of RARs by these inflammatory mediators may be in part responsible for the bronchoconstriction, mucus production, and tachypnea observed in horses with this disorder.217, 270, 271, 272 In addition to their role as chemoreceptors, the RARs or irritant receptors also function as mechanoreceptors throughout the airways.263, 264 An abrupt change in end-expiratory lung volume, such as occurs with pneumothorax or pleural effusion, can produce tachypnea in response to stimulation of these receptors. Increased negative pressure (upper airway obstruction) within the airway stimulates the mechanoreceptors of the larynx and produces prolongation of inspiratory time and activation of upper airway dilator muscles.273, 274
Unmyelinated C-fibers innervate receptors found in the epithelial layer throughout the intrapulmonary and extrapulmonary respiratory tract.256, 263 C-fiber receptors are predominantly chemosensors, although they may also respond to hyperinflation of the lung. These receptors respond to pulmonary edema, congestion, and inflammatory mediators, and stimulation activates tachypnea. In addition, C-fiber receptors may stimulate the release of pulmonary neuropeptides, which produce bronchoconstriction, vasodilation, protein extravasation, and cytokine production. C-fiber receptors located within the alveolar walls in juxtaposition to pulmonary capillaries are sometimes referred to as juxtacapillary receptors. Information from other species suggests that these receptors can respond to increased interstitial fluid volume and may be involved in the sensation of difficult breathing.256
Central Control of Ventilation
Brainstem Control
The mechanisms involved in the central control of ventilation are still not completely understood. The central controller of the brainstem integrates signals from the sensory afferent neurons and then initiates phasic activity of the diaphragmatic, intercostal, and abdominal respiratory muscles.256 Although it is a complex neuronal network, the central controller consists of two basic regions with several subregions: (1) the medullary respiratory center in the reticular formation, which includes the ventral and dorsal respiratory groups, and (2) the pontine respiratory center, which includes the pneumotaxic and apneustic centers. The medullary respiratory center controls the rhythmic pattern of respiration. The dorsal respiratory group helps to coordinate inspiratory activity by assimilating afferent information from the glossopharyngeal and vagus nerves and transmitting efferent signals to the muscles of inspiration and neurons in the ventral respiratory group. The ventral respiratory group consists of inspiratory and expiratory motor neurons. One subregion, the pre-Botzinger complex, appears to have a major role in generating the basic breathing rhythm.260, 261, 262, 275 Closely associated neurons in the retrotrapezoid nucleus and parafacial respiratory group are important in regulating expiration.275, 276 The ventral respiratory group also helps to regulate ventilation during exercise. In the pontine respiratory center, the apneustic center provides stimulatory input to inspiratory motor neurons, activating and prolonging inhalation.260, 261, 262 Damage to this region, which can result from problems such as trauma or neonatal encephalopathy, results in prolonged inspiratory gasps interrupted by transient expiratory efforts.259 The pneumotaxic center, also in the pons, inhibits the inspiratory centers and helps to regulate the volume and rate of respiration. The pneumotaxic center is not required to maintain a normal respiratory rhythm; instead, this center functions to fine-tune the respiratory rhythm, receiving afferent input from the vagus nerve regarding Pao 2, Paco 2, and pulmonary inflation.
Input of Higher Brain Centers
Ventilation is primarily under involuntary control via the brainstem, but higher brain centers can also play a role.260, 261, 262 Conditions that influence ventilation via higher brain centers include the emotional state, via input from the limbic system, and temperature, via input from the hypothalamus, as well as conscious control. Conscious or voluntary control of ventilation, which is centered in the cerebral cortex, can be overridden by the chemoreceptor reflex.
Effectors of Ventilation
The muscles required for ventilation include the diaphragm, the external and internal intercostal muscles, and the abdominal muscles.256 Horses have a somewhat unique biphasic inspiratory and expiratory airflow pattern in which there is both a passive and an active phase to inspiration and expiration.277 The single most important muscle required for the inspiratory phase of the respiratory cycle is the diaphragm.278 The relaxation volume is the static equilibrium volume of the relaxed respiratory system at which the net elastic recoil pressure is zero. In horses, the first part of inhalation following exhalation is passive until the relaxation volume is reached, at which point the diaphragm and external intercostal muscles complete the inspiratory phase. Contraction of the diaphragm forces the abdominal contents back, increasing the length of the thoracic cavity, and pulls the ribs abaxially, increasing the width of the abdominal cavity. In addition, the external intercostal muscles participate in inspiration by pulling the ribs abaxially to increase the width of the thoracic cavity. The net effect is an increase in the size of the thoracic cavity, producing subatmospheric intrathoracic pressure that drives inspiration and pulmonary inflation. In most species, expiration at rest is a passive process and relies on elastic recoil of the lung to create positive intrathoracic pressure.256 This is the case during the first portion of expiration in horses, which relies on elastic recoil to the point of relaxation volume, when the tendency for pulmonary collapse equals the tendency for expansion by the thoracic wall. Horses then further decrease lung volume by active compression of the chest wall through contraction of the internal intercostal muscles and muscles of the abdominal wall.278 The passive phase of inhalation follows. Dysfunction of the diaphragm and intercostal muscles due to either mechanical (abdominal distention, trauma to the thoracic wall) or neuromuscular (botulism, phrenic nerve damage, nutritional myodegeneration) problems prevents expansion of the thoracic wall and can produce hypoventilation, hypoxemia, and respiratory distress.255, 257 Horses with torsion of the large colon can develop significant abdominal distention and respiratory distress, and respiratory failure caused by impaired diaphragmatic function can play an important role in the pathophysiology and mortality associated with this intestinal accident.
Control of Airway Diameter
The diameter of the conducting airways, which is primarily controlled by the autonomic nervous system, is an important determinant of the degree of pulmonary resistance and work of breathing.256 The control of airway diameter by the autonomic nervous system involves both sympathetic and parasympathetic innervation with activation of adrenergic and muscarinic receptors, respectively.256, 279, 280, 281 The receptors are generally widely expressed in the lung, although the pattern of specific receptor expression can vary among species. The control of normal airway function and airway diameter is complex, requiring interactions or “crosstalk” between receptors. Vagal-mediated parasympathetic stimulation is important in the regulation of airway smooth muscle tone, and stimulation can cause bronchoconstriction as well as mucus secretion and bronchial vasodilation. Specifically, bronchoconstriction can occur due to the action of acetylcholine from parasympathetic fibers on M3 muscarinic receptors located on airway smooth muscle. This mechanism of bronchoconstriction has been associated with allergic airway disease in several species. The administration of anticholinergics such as atropine or N-butylscopolammonium bromide can result in rapid relief of bronchoconstriction in some horses with RAO, demonstrating the important role of parasympathetic bronchoconstriction in the pathogenesis of this disorder.257, 259, 282, 283 With regard to sympathetic effects, both α- and β-adrenergic receptors are found in the lung.28, 29, 30 However, direct sympathetic innervation of airway smooth muscle is minimal to none, and thus stimulation of the adrenergic receptors is primarily via circulating catecholamines.279, 280, 281 β2-Adrenergic receptors are abundant throughout the lung on multiple cell types, including bronchial smooth muscle, bronchial epithelial cells, and several immune cells, and thus activation can have multiple effects. The stimulation of β2-receptors on airway smooth muscle can cause smooth muscle relaxation and bronchodilation. Airways must be constricted for β2-receptor stimulation or the anticholinergic action of atropine to increase airway diameter.284, 285 β2-Receptors appear to be present in normal or increased numbers on asthmatic airway smooth muscle but appear to be hyporesponsive, thus allowing bronchoconstriction.281, 286 β1-Adrenergic receptors are less abundant than β2-receptors in the airways. The effects of both β1- and α1-adrenergic receptors in the regulation of airway diameter in horses appear to be minimal.285, 286, 287
Nonadrenergic-noncholinergic (NANC) innervation, including both excitatory and inhibitory components, also contributes to large airway diameter.288, 289 Stimulation of excitatory NANC nerves initiates a process of neurogenic inflammation that involves bronchoconstriction, mucus secretion, increased vascular permeability, vasodilation, and cough. Inhibitory NANC nerves appear to be important neural pathways for bronchodilation, and activation results in the relaxation of smooth muscles of the trachea and bronchi. Dysfunction of the NANC system may play a role in asthma in both people and horses, although the precise role remains to be elucidated. In RAO-affected horses with clinical signs of airway obstruction, inhibitory NANC function is absent.290 Failure of the inhibitory NANC system may result from the inflammatory response during acute RAO or may be an inherent autonomic dysfunction of the conducting airways of RAO-affected horses.
Role of Hypoxemia and Hypercapnia in Respiratory Distress
Respiratory distress most often originates from inadequate pulmonary gas exchange to meet the metabolic demands of the individual, resulting in hypoxemia and hypercapnia. Hypoxemia results from one or more of five basic pathophysiologic mechanisms: hypoventilation, ventilation-perfusion mismatch, right-to-left shunting of blood, diffusion impairment, and reduced inspired oxygen concentration.256 The degree of associated hypercapnia and the response to oxygen supplementation vary depending on the mechanism of impaired gas exchange, and determination of these two parameters is useful in defining the pathophysiologic process predominantly responsible for the development of hypoxemia.256
Hypoventilation
Alveolar hypoventilation is defined as insufficient ventilation leading to hypercapnia.256 The elevation in Paco 2 is inversely proportional to the reduction in alveolar ventilation; halving alveolar ventilation doubles Paco 2.256 The reduction in arterial oxygen tension is almost directly proportional to the increase in CO2. For instance, if Paco 2 increases from 40 to 80 mm Hg, the Pao 2 decreases from 100 to 60 mm Hg. Therefore hypoxemia resulting from hypoventilation is rarely life threatening. In addition, oxygen supplementation easily abolishes hypoxemia caused by pure hypoventilation. Acidosis caused by hypercapnia is the most clinically significant feature of hypoventilation and may be life threatening.256 Metabolic alkalosis or central nervous system depression (e.g., from head trauma, encephalitis, narcotic drugs) can produce hypoventilation; however, horses with these disorders may not demonstrate clinical signs of respiratory distress. Disorders that can cause alveolar hypoventilation that are often associated with clinical signs of respiratory distress include dysfunction of the respiratory muscles from mechanical (abdominal distention, trauma to the thoracic wall) or neuromuscular (botulism, phrenic nerve damage, nutritional myodegeneration) conditions, restrictive pulmonary disease (silicosis, pulmonary fibrosis, pneumothorax, pleural effusion), and upper airway obstruction.255, 257, 259
Ventilation-Perfusion Mismatch
Ventilation-perfusion (V-Q) mismatch is the most common cause of hypoxemia and is characterized by unequal distribution of alveolar ventilation and blood flow.256, 259 Either a low or high V-Q ratio can result in hypoxemia. Pulmonary regions that are overperfused in relation to ventilation (low V-Q ratio) contribute disproportionate amounts of blood with low arterial oxygen content to the systemic circulation.256 Respiratory diseases characterized by low V-Q ratios include RAO, pulmonary atelectasis, and consolidation.259 If ventilation exceeds perfusion (high V-Q ratio), the ventilated pulmonary units are inefficient for CO2 elimination and O2 uptake. Ventilation of poorly or nonperfused units is wasted ventilation, termed alveolar dead space. 256 Conditions associated with high V-Q ratios include pulmonary thromboembolism and shock (low pulmonary artery pressure). Patients with V-Q mismatch often have a normal arterial Pco 2. The ventilatory drive to maintain normal Paco 2 is powerful, and as the CO2 dissociation curve is basically a straight line (direct relationship), increased ventilation efficiently decreases Paco 2 at high and low V-Q ratios. However, also due to the nearly flat shape of the O2 dissociation curve, increasing ventilation is inefficient for proportionally increasing the arterial Po 2. Only pulmonary units with moderate to low V-Q ratios benefit from increased ventilation. Therefore the increased ventilatory effort to maintain normal Paco 2 is wasted and unnecessarily increases the work of breathing. Oxygen supplementation tends to increase Paco 2 in patients with a V-Q mismatch. Also, elevation in arterial O2 is delayed compared with hypoventilation and in some cases may be incomplete.256 Compensatory mechanisms are present to minimize unequal distribution of ventilation and perfusion in diseased lungs to prevent the development of hypoxemia until the pulmonary pathologic condition is severe.256 Reflex pulmonary arterial constriction (hypoxic vasoconstriction) prevents perfusion of unventilated alveolar units and attempts to redirect blood flow to alveoli that are ventilated adequately. Airway hypocapnia causes bronchoconstriction of airways that conduct to unperfused alveolar units, redirecting air flow to better perfused alveoli.
Shunt
Shunt is defined as blood that is not exposed to ventilated areas of the lung and is added to the arteries of the systemic circulation.256 Shunting can occur as an extreme form of V-Q mismatch or with direct addition of unoxygenated blood to the arterial system. Physiologic shunting is defined as perfusion of nonventilated or collapsed regions of the lung and occurs with pulmonary consolidation, atelectasis, and edema. Certain congenital heart diseases, such as tetralogy of Fallot and some cardiac septal defects, are examples of direct right-to-left shunts wherein unoxygenated blood from the right side of the heart is added to oxygenated blood from the left side of the heart. Right-to-left shunting may also contribute to hypoxemia in some cases of persistent pulmonary hypertension. In these conditions hypoxemia cannot be abolished by increasing the oxygen content of inspired air. The shunted blood is never exposed to the higher concentration of inspired oxygen in the alveolus, and the addition of a small amount of shunted blood with its low O2 content greatly reduces the Po 2 of arterial blood. Compared with breathing room air, the decrement in Po 2 is much greater at Po 2 levels associated with the inhalation of O2-enriched air because the O2 dissociation curve is so flat at high Po 2 levels. Only hypoxemia caused by right-to-left shunting behaves in this manner when the patient is permitted to inspire high percentages of oxygen (70%–100%). Shunts do not usually cause hypercapnia.256 Chemoreceptors detect excess arterial CO2, and ventilation increases to reduce the content of CO2 in unshunted blood until arterial Pco 2 reaches the normal range. In some cases of shunt the arterial Pco 2 is below normal because of hyperventilation stimulated by the hypoxemic ventilatory drive.
Diffusion Impairment
Gas exchange between the alveolus and the capillary occurs by passive diffusion, which is driven by the property of molecules to move randomly from an area of high concentration to one of low concentration.256 Factors that determine the rate of gas exchange include the concentration gradient between the alveolus and capillary blood, solubility of the gas, surface area available for diffusion, and width of the air-blood barrier. Diseases characterized by pure diffusion impairment are rare in veterinary medicine.259 However, some degree of diffusion impairment can occur with disorders such as pulmonary fibrosis, interstitial pneumonia, silicosis, or pulmonary edema and is most often associated with increased width of the barrier or decreased surface area available for gas exchange. Although the major component of hypoxemia in these conditions is a V-Q mismatch, diffusion impairment can contribute to the severity of hypoxemia. Supplemental oxygen therapy is effective in treating hypoxemia caused by diffusion impairment because it creates a more favorable concentration gradient and increases the driving pressure of oxygen to move from the alveolus into the blood. Transport of CO2 is less affected by diseases of diffusion impairment because of its greater solubility compared with O2.256
Reduction of Inspired Oxygen
Hypoxemia resulting from decreased inspired oxygen content is uncommon and occurs only under special circumstances. High altitude and iatrogenic ventilation with a low oxygen concentration are the most common circumstances in which hypoxemia is attributed to reduction of inspired oxygen content.256
Most pulmonary diseases in horses incorporate more than one of these pathophysiologic mechanisms for the development of hypoxemia. Horses with pleuropneumonia, for example, may develop hypoxemia caused by hypoventilation (extrapulmonary restriction by pleural effusion), V-Q mismatch (accumulation of exudate and edema within alveoli and conducting airways), and diffusion impairment (exudate and edema within the interstitial spaces).
Role of Obstructive Disease in Respiratory Distress
Airway obstruction can limit air flow and contribute to respiratory distress. The location (intrathoracic or extrathoracic) and nature (fixed or dynamic) of airway obstruction determine whether impedance to air flow occurs during inspiration, expiration, or both.257 The phase of the respiratory cycle that is affected by air flow obstruction is prolonged and may be associated with a respiratory noise (stertor, stridor, or wheeze).256, 257, 291
The horse is an obligate nasal breather and can breathe efficiently only through the nares.259 Therefore upper airway obstruction within the nasal passages cannot be bypassed by mouth breathing. In addition, approximately 80% of the total airway resistance to air flow is located in the upper airway.291 A 50% decrease in the radius of an airway increases its resistance by 16 times (Poiseuille’s law).256 Therefore small changes in the upper airway diameter dramatically affect the overall resistance to air flow and work of breathing for the horse. Extrathoracic airway pressures are subatmospheric during inspiration; therefore poorly supported structures in the upper airway narrow or collapse during inspiration (dynamic collapse). There are several causes of upper airway obstruction in horses, the most common being laryngeal hemiplegia.
Of the total airway resistance 20% is attributable to the small airways.291 Although the radius of individual bronchioles is small, many of them exist and the sum or collective radius is large, with the result that their overall contribution to pulmonary resistance is low.256 Because the resistance of the bronchioles is low, advanced disease must be present for routine measurements of airway resistance to detect an abnormality, and obstruction of these airways must be extensive before a horse would suffer from respiratory distress. During pulmonary inflation intrathoracic pressures are subatmospheric. Small airways are pulled open by negative intrathoracic pressure and stretched parenchymal attachments at high lung volumes. Thus resistance to air flow in small airways is low during the inspiratory phase of respiration.256 During exhalation intrathoracic pressure is positive and the diameter of small airways is decreased, and bronchioles may even close at low lung volumes. Therefore resistance to air flow in small airways is greatest during the expiratory phase. In horses with RAO the airway diameter is reduced by inflammatory exudate, edema, and bronchoconstriction.257, 259, 290 As lung volume decreases during expiration, the narrowed bronchioles are compressed shut (dynamic airway collapse) and trap air distal to the site of closure.259 This is an example of severe flow limitation, which may lead ultimately to the development of emphysema. Flow limitation forces horses with RAO to breathe at higher lung volumes and maintain a higher functional residual capacity to reduce or prevent dynamic airway collapse. Affected horses attempt to reduce the end-expiratory lung volume by recruiting abdominal muscles to increase the intrathoracic pressures during expiration. However, the greater the end-expiratory pressure, the greater is the likelihood of small airway compression and collapse. Hypertrophy of the cutaneous trunci and expiratory abdominal muscles, especially the external abdominal oblique, produces the characteristic “heave line” associated with RAO.259 Because dynamic airway narrowing and collapse occur during exhalation, wheezes are typically loudest at end expiration in horses with RAO.257, 259
Role of Restrictive Disease in Respiratory Distress
Restrictive disease is less common than obstructive pulmonary disease in horses.259 By definition, restrictive disease inhibits pulmonary expansion and leads to inspiratory respiratory distress.256 The vital capacity and compliance (pulmonary or chest wall) decrease, expiratory flow rates and elastic recoil increase, and airway resistance is normal. The characteristic respiratory pattern in horses with restrictive pulmonary disease is rapid, shallow respiration at low lung volumes.259 This strategy takes advantage of high pulmonary compliance at low lung volumes and decreases the work of breathing. This respiratory pattern has the disadvantage of increased ventilation of anatomic dead space.256 Restrictive diseases may be classified as intrapulmonary (pulmonary fibrosis, silicosis, and interstitial pneumonia) and extrapulmonary (pleural effusion, pneumothorax, mediastinal mass, botulism, and nutritional myodegeneration).255, 259 Hypoxemia observed in horses with intrapulmonary restrictive disease is largely attributed to V-Q mismatch and diffusion impairment, although hypoventilation may also have a role. Stimulation of juxtacapillary receptors may contribute to respiratory distress observed in these patients.256 The pathophysiologic mechanism for hypoxemia in horses with extrapulmonary restriction is hypoventilation.259 In horses with pleural effusion and pneumothorax, respiratory distress is likely to be exacerbated by thoracic pain.
Nonpulmonary Respiratory Distress
Respiratory distress does not always originate from dysfunction of the pulmonary system and its supporting structures. Nonpulmonary respiratory distress can occur because of inadequate oxygen-carrying capacity of the blood, pain, hyperthermia, or compensation for metabolic acidosis.255, 256, 257
Impaired oxygen-carrying capacity of the blood may occur because of anemia (blood loss, hemolytic, or aplastic) or dysfunction of red blood cells (methemoglobinemia, carbon monoxide toxicity). In these cases the arterial Po 2 tension (quantity of dissolved oxygen) is normal; however, the total oxygen content of the blood is reduced greatly.256 Tachypnea and respiratory distress occur in response to impaired oxygen delivery and tissue hypoxia.
The respiratory system can compensate for metabolic acidosis by increasing ventilation to lower Paco 2 and attenuate acidemia.256 The ventilatory drive increases in response to stimulation by peripheral chemoreceptors by circulating hydrogen ions. Hypocarbic compensation for mild to moderate metabolic acidosis is effective in returning blood pH to normal until renal compensatory mechanisms can be established.256
Pain and anxiety are physiologic causes of tachypnea and hyperpnea. Horses with musculoskeletal pain are unlikely to demonstrate significant respiratory distress; however, rhabdomyolysis and laminitis are painful musculoskeletal conditions that may produce tachypnea.257 Marked respiratory distress is observed frequently in horses with abdominal pain; however, the respiratory distress is not caused solely by pain and is exacerbated by abdominal distention, shock, acidosis, and endotoxemia.
Elevations in body temperature caused by fever or hyperthermia associated with exercise, heat stroke, anhidrosis, or macrolide-induced hyperthermia can produce respiratory distress in horses. Hyperpnea is an effective means of heat dissipation in human beings, dogs, and ruminants.257 Unfortunately, in horses increased ventilation is an inefficient mechanism for heat dissipation.257, 259
Conditions Associated with Respiratory Distress
A number of conditions, both respiratory and nonrespiratory in origin, can cause some degree of respiratory distress in horses (Box 7.5 ). Among the more common respiratory causes are infectious conditions. In particular, bacterial infections of the respiratory tract such as bacterial pneumonia, aspiration pneumonia, pleuropneumonia, pleuritis, and pulmonary abscessation can result in respiratory distress.206, 207 Strangles can result in respiratory distress in association with airway obstruction from lymph node abscessation and less frequently due to pneumonia. Viral respiratory tract infections, which are common in horses, may sometimes result in respiratory distress.204 Fungal pneumonia can result in significant respiratory distress in some patients but is uncommon in horses.209, 210 Respiratory distress is often a prominent feature of syndromes of interstitial pneumonia, including acute lung injury, acute respiratory distress, and equine multinodular pulmonary fibrosis.211, 212, 292, 293 In neonatal foals, immature lungs and lack of surfactant can contribute to respiratory distress.294 Idiopathic or transient tachypnea has been reported in neonatal foals, especially in Clydesdales, Thoroughbreds, and Arabians.294 RAO is a common condition in horses that has the potential to cause significant respiratory distress, both in association with acute exacerbations of the condition and with chronic disease.215, 216, 217, 218, 290 Some other respiratory problems that can be associated with respiratory distress include exercise-induced pulmonary hemorrhage, eosinophilic pulmonary disease, silicosis, smoke inhalation, and neoplasia.213, 214, 223, 226, 227, 295, 296, 297
BOX 7.5. Causes of Respiratory Distress.
Respiratory Causes
- Respiratory tract infection
- Bacterial pneumonia, pleuropneumonia (including aspiration, pulmonary abscesses)
- Strangles
- Viral respiratory tract infection
- Fungal respiratory tract infection
- Interstitial pneumonia
- Equine multinodular pulmonary fibrosis
- Syndromes of acute lung injury, acute respiratory distress
Recurrent airway obstruction (severe asthma)
- Upper airway obstruction
- Left laryngeal hemiplegia
- Arytenoid chondritis
- Fourth branchial arch defect
- Subepiglottic cyst
- Dorsal displacement of the soft palate
- Aryepiglottic fold entrapment
- Intraluminal masses, abscessation (strangles, other)
- Laryngeal and pharyngeal dysfunction associated with HYPP
- Choanal atresia
- Nasopharyngeal cicatrix syndrome
Exercise-induced pulmonary hemorrhage
Pneumothorax
Diaphragmatic hernia
- Tracheal abnormalities
- Tracheal collapse, stenosis
- Foreign body
- Intraluminal mass
- Cardiac disease
- Pulmonary edema
- Left-to-right shunting
- Other
- Silicosis
- Smoke inhalation
- Neoplasia
- Eosinophilic pulmonary disease
- Crotolaria equorum—pyrrolizidine alkaloid toxicity
- Additional causes in foals
- Inadequate surfactant, pulmonary immaturity
- Idiopathic tachypnea (transient)
Nonrespiratory Causes
Systemic inflammatory response syndrome
Pain
Elevated body temperature
Fever
- Hyperthermia
- Exercise-related hyperthermia
- Heat stroke
- Anhidrosis
- CNS disorders
- Macrolide-induced hyperthermia
Anemia
Monensin
Snake envenomation
Neuroborreliosis
Upper airway obstruction is another important cause of respiratory distress in horses, especially when oxygen demand is increased by exercise.255, 257, 259 The most common cause of non–fixed upper airway obstruction in horses is laryngeal hemiplegia, which produces inspiratory stridor during exercise.257, 298, 299, 300 Intraluminal masses and arytenoid chondritis cause fixed upper airway obstruction and produce inspiratory and expiratory respiratory distress.257 Several other upper airway abnormalities have been documented, such as fourth branchial arch defect, subepiglottic cyst, aryepiglottic fold entrapment, and dorsal displacement of the soft palate.257, 301 Laryngeal and pharyngeal dysfunction has been reported in horses that are homozygous for hyperkalemic periodic paralysis.302, 303 Infrequently, laryngeal paralysis has been associated with hepatic dysfunction.304 A wide variety of other conditions have been associated with respiratory distress such as pneumothorax, diaphragmatic hernia, choanal atresia, nasopharyngeal cicatrix syndrome, tracheal collapse, and others.233, 234, 235, 305, 306 Although heart disease is relatively uncommon in horses, it may result in respiratory distress via pulmonary edema or right-to-left shunting.229, 230 Respiratory distress is a prominent sign of a rare form of pyrollizidine alkaloid toxicity associated with Crotolaria equorum. 307
Several nonrespiratory conditions can also cause respiratory distress. Respiratory distress is often a feature of the systemic inflammatory response syndrome.255, 257 Pain, fever, or hyperthermia may lead to respiratory distress. Tachypnea and elevation in body temperature are the most prominent clinical signs in horses with anhidrosis.16 Other nonrespiratory causes of respiratory distress include disorders that cause anemia, such as blood loss, autoimmune hemolytic activity, neonatal isoerythrolysis, and red maple toxicosis. In addition to red maple, other toxins that can affect respiration include monensin and snake envenomization. Episodic respiratory distress was seen in 5 of 16 horses diagnosed with neuroborreliosis.308
Approach to Respiratory Distress
Signalment and History
Both breed and age have been linked with specific causes of respiratory distress. For example, American Miniature Horses are at increased risk of tracheal collapse.233 Idiopathic laryngeal hemiplegia is more common in large, long-necked horses such as draft horses, Thoroughbreds, and Warmbloods.298, 299 In Quarter Horses and related breeds, some foals affected with hyperkalemic periodic paralysis will show signs of respiratory stertor usually within the first week of life as a result of laryngeal dysfunction, especially if homozygous.302, 303 Inadequate lung development, surfactant deficiency, persistent pulmonary hypertension, and meconium aspiration are problems unique to neonatal foals.294 Congenital problems such as choanal atresia and cardiac defects should also be considered in neonates. Rhodococcal pneumonia is primarily a disease of foals 1 to 6 months of age.207 Horses affected with RAO are generally over 7 years of age, and a familial predisposition has been identified.215, 216
Some important historical considerations include environmental conditions such as heat and humidity and relocation to high altitude. A history of recent transport may raise the index of suspicion for pleuropneumonia. Useful information can also be obtained from specifics related to the respiratory distress such as the speed of onset, progression of signs, history of previous episodes, and association with exercise or specific locations/housing. Any history of recent trauma or potential exposure to toxins should be determined. A history of any other problems such as cough, nasal discharge, dysphagia, anorexia, or lethargy can also be important.
In neonatal foals, the circumstances of gestation and parturition can help to establish the likelihood of problems such as immaturity, rib fractures, meconium aspiration, and sepsis.
Physical Examination
A thorough physical examination is essential to determine the origin of respiratory distress, identify concurrent disease, and direct further diagnostic testing. Prolonged inspiration is consistent with restrictive or extrathoracic, nonfixed, obstructive disease, whereas horses with intrathoracic airway obstruction exhibit expiratory difficulty.256, 257 Respiratory distress associated with inspiration and expiration may indicate an extrathoracic fixed obstruction. Stertor is a low-pitched, “snoring” respiratory noise caused by partial obstruction above the larynx and is inspiratory. In comparison, stridor is a more musical sound that is generated by obstruction of the larynx and less frequently the trachea or bronchi. Stridor can be heard during inspiration and/or expiration but is most often audible during inspiration.257
Thoracic auscultation identifies abnormal respiratory sounds (crackles and wheezes) or regions of decreased breath sounds caused by pleural effusion, pneumothorax, or pulmonary consolidation.259, 308 Percussion of the thoracic wall generates a resonant and hollow sound when performed over regions of normal lung. Pleural effusion and pulmonary consolidation sound dull and flat during thoracic percussion, whereas pneumothorax produces a hyperresonant sound.246, 259
Normal air flow occurs in laminar flow; therefore normal horses at rest do not generate easily audible sounds.259 If the horse is not in significant distress, auscultation with a rebreathing bag can help enhance sounds by increasing tidal volume. Respiratory sounds are generated from vibration in tissue and sudden changes in pressure of gas moving within the airway lumen. Airway narrowing and exudate generate audible sounds by creating disturbances in laminar flow, turbulence, and sudden changes in pressure of moving gas.256 Crackles are intermittent or explosive sounds, generated by bubbling of air through secretions or by equilibration of airway pressures after sudden opening of collapsed small airways. The generation of crackles requires an air-fluid interface, and these abnormal lung sounds occur in horses with pneumonia, interstitial fibrosis, RAO, pulmonary edema, and atelectasis.246, 259 Wheezes are continuous, musical sounds that originate from oscillation of small airway walls before complete closing (expiratory wheeze) or opening (inspiratory wheeze).256 Expiratory wheezes are the hallmark of obstructive pulmonary disease.256
Horses with nonpulmonary respiratory distress demonstrate increased rate and/or depth of respiration without producing abnormal respiratory noise. Stertor and stridor are absent, and auscultation of the thorax is normal.
Ancillary Diagnostic Tests
Clinical Pathology
Blood Work
A CBC and fibrinogen can help to evaluate hemoconcentration, anemia, and the presence of inflammation. Serum amyloid A can also be used in the assessment of inflammation.
Arterial blood gas analysis provides a quantitative evaluation of pulmonary function, alveolar ventilation, and acid-base status and may identify the origin of respiratory distress (hypercapnia, hypoxemia, or acidemia).256 The clinician may determine the pathophysiologic mechanism of hypoxemia by examining the Paco 2 level and by investigating the response of Pao 2 to supplemental oxygen therapy. In addition, serial blood gas monitoring can determine response to bronchodilator, parasympathomimetic, or antiinflammatory therapy.
Airway Cytology/Assessment of Infection
Appropriate samples for cytologic evaluation and culture can be obtained from the respiratory tract depending on the primary differential diagnoses.240, 241, 246 The degree of respiratory distress should be considered, as some diagnostic procedures can be stressful in horses with significant respiratory distress, especially foals. Currently, for most viral respiratory tract infections, the most common means of diagnosis is detection of viral genetic material by PCR from a nasal or nasopharyngeal swab. For strangles, PCR and/or culture of a nasal or nasopharyngeal swab, nasal wash, guttural pouch wash, or abscess can be diagnostic. Several techniques have been described for sampling the lower airways of horses including transtracheal aspiration, bronchoalveolar lavage, and various transendoscopic procedures. Thoracocentesis can be useful in cases of suspected pleuropneumonia and in some cases of suspected neoplasia. For the diagnosis of RAO, cytologic analysis of bronchoalveolar lavage fluid is recommended and is typically characterized by a significant increase in neutrophils (greater than 25%). Bronchoalveolar lavage can also be helpful in the evaluation of suspected EIPH.
Endoscopy
An endoscopic examination of the upper airway is indicated in horses with stertor or stridor and suspected upper airway obstruction.246, 259 Endoscopy can be used to evaluate the trachea and can help to identify tracheal collapse, foreign bodies, mucus, or hemorrhage. Horses with extreme respiratory distress may resent endoscopic examination, and forced examination may precipitate a respiratory crisis.
Diagnostic Imaging
The findings during thoracic auscultation and percussion are valuable in determining whether ultrasonography rather than radiography is indicated.246, 259 Pulmonary consolidation, abscessation, fibrosis, interstitial pneumonia, peribronchial infiltration, and mediastinal masses are typically imaged best via thoracic radiography. Thoracic ultrasonography is superior to radiography in detecting and characterizing pleural fluid and peripheral pulmonary abscessation and consolidation in horses.309 Air reflects the ultrasound beam; therefore ultrasonography does not image deep pulmonary lesions if the overlying lung is aerated.
Other Diagnostic Aids
The administration of atropine or N-butylscopolammonium bromide in horses with RAO may provide rapid relief of respiratory distress if the major component of airway obstruction is reversible bronchoconstriction.282 The bronchodilatory properties of N-butylscopolammonium bromide appear to be similar to those of atropine, although of shorter duration and associated with fewer systemic side effects.283 Horses that respond to an atropine or N-butylscopolammonium bromide challenge likely will respond favorably to bronchodilator therapy. Incomplete response to these bronchodilators in horses with RAO indicates that exudate or fibrosis is contributing to airway obstruction, and limited response to bronchodilator therapy is anticipated.257, 259 Other tests that may be of value in the assessment of equine asthma include a hay challenge, histamine provocation test, and pulmonary function testing.
Both endobronchial and percutaneous lung biopsies may be used in the diagnosis of respiratory disease but are not commonly performed.253, 254 In horses with suspected anhidrosis, a terbutaline sweat test can be used to evaluate the patient’s ability to sweat.67
Edema
Edema is defined as the excessive and abnormal accumulation of fluid in the interstitium, which is the intercellular connective tissue that lies between the cellular elements of tissues.310, 311 Interstitial fluid accumulates as a result of imbalances between the rates at which fluid enters and exits the interstitium. Factors that either increase the rate of fluid flux from the capillary or impair reabsorption and lymph drainage to the extent that normal compensatory mechanisms are overwhelmed result in the accumulation of fluid in the interstitial space and the development of edema.
Edema is often classified according to the anatomic location. The most frequently recognized type of edema in horses is peripheral edema, which is often seen in the limbs or along the ventrum. Most peripheral edema is “pitting,” which means that an indentation persists after pressure is applied to an area and released—for example, using the tip of a finger to indent the fluid. Occasionally nonpitting peripheral edema will be recognized, which in horses is most often associated with lymphedema, which is fluid retained in the interstitium as a result of impaired lymphatic drainage. Organ-specific edema, such as cerebral, pulmonary, and corneal edema, also occurs and is often clinically important. Generalized edema occurs when edema is present both peripherally and in multiple organs.
General Physiology of Fluid Movement
The volume of interstitial fluid and lymph fluid in the normal horse is 8% to 10% of body mass,310 or 36 to 45 L in a 450-kg horse. Interstitial fluid consists largely of water, protein, and electrolytes. Compared with plasma, interstitial fluid has a slightly lower concentration of cationic electrolytes, a slightly higher concentration of chloride, and a much lower concentration of protein (1.2 vs. 0.2 mOsm/L of water).311 The overall amount and function of the proteins within the interstitial space are not inconsequential. A constant circulation of plasma proteins occurs between the vascular and interstitial spaces, with about half of the protein circulating every 24 hours in human beings. More than half of the plasma protein content of the body is contained within the interstitial space at any one time. Plasma proteins within the interstitial space are important in the transport of water-insoluble substances from the vascular space and in resistance to infection.312
The extracellular tissue of the interstitium, except in the case of bone, consists of a three-dimensional collagen fiber network embedded in a proteoglycan gel matrix.313 Normally most of the interstitial water is contained within the proteoglycan interstitial gel, with a small proportion existing as free water. However, in edematous states, the proportion of fluid as free water within the interstitium increases.311
The source of interstitial fluid is the intravascular space. The volume of interstitial fluid is determined by the functional relationships of three major anatomic structures: the capillary, the interstitial space, and the lymphatics.314 Functionally, the volume of fluid that accumulates in the interstitium is determined by the rate of ingress of fluid from the vascular space, the compliance of the interstitium, and the rate at which fluid is evacuated from the interstitium.
Traditionally the fundamental principle guiding microvascular filtration and transcapillary fluid shifts has been defined by the Starling equation, first introduced in 1896. In this model, fluid movement is passive and dependent on pressure gradients across the endothelium, which are determined by hydrostatic and colloid osmotic pressures.311, 315 However, it is now recognized that the regulation of fluid flux is not simply governed by transcapillary hydrostatic and oncotic pressure differences but is much more complex. The role of non-Starling mechanisms of barrier regulation and fluid movement are an area of active investigation. Important factors that mediate processes central to the development of edema include the endothelial glycocalyx layer, the endothelial basement membrane, and selective water channels known as aquaporins.312, 316, 317, 318, 319
The Starling Equation
The net rate of ingress of fluid from capillaries into the interstitium is determined by a number of factors acting across the capillary membrane, the effects of which are related by Starling’s equation:
in which J equals the volume flow across the capillary wall; Kf equals the filtration coefficient of the capillary wall (volume flow per unit time per 100 g of tissue per unit pressure); P c equals capillary hydrostatic pressure; P t equals interstitial fluid hydrostatic pressure; σ equals the osmotic reflection coefficient; πp equals the colloid osmotic (oncotic) pressure of the plasma; and πt equals the colloid osmotic (oncotic) pressure of the interstitial fluid.315 Although all these factors act in concert to determine the rate of net fluid efflux from the capillary, considering them individually is conceptually easier.
Filtration (Kf) and Reflection (σ) Coefficients
Together the filtration and reflection coefficients describe the properties of the capillary membrane that determine the ease with which water, protein, and other plasma constituents move from the vascular space to the interstitium. The filtration coefficient, which is the product of the hydraulic permeability and surface area of the capillary, is a measure of the ease with which water crosses the capillary membrane. The reflection coefficient is a mathematical expression of the capillary permeability to a particular substance and ranges from 0, indicating the substance crosses the membrane as easily as water, to 1, indicating that the substance does not cross the membrane. The reflection coefficient is dependent on both the substance in question and the tissue bed.311, 320, 321 For example, in lung endothelium, the reflection coefficients for urea, glucose and albumin are approximately 0.3, 0.5, and 0.7. Reflection coefficients for albumin vary among tissues, being 0.1 in the hepatic sinusoids, 0.9 in muscle, and 0.99 in brain.
The filtration and reflection coefficients together partially determine the rate of fluid flux across the capillary wall and the composition of the fluid. For a given hydrostatic and oncotic pressure difference, tissues with higher filtration coefficients (whether because of a larger capillary surface area or more porous capillaries) will have a greater fluid flux. Conversely, under the same circumstance, increases in the reflection coefficient will reduce solute movement across the capillary wall, affecting the osmotic pressure gradient and reducing fluid flux. The differential permeability of the capillary membrane to water and protein has important consequences in the maintenance of the oncotic pressure difference between plasma and interstitial fluid. Multiple factors influence the movement of fluid and solutes across the endothelium, including the concentration of the solutes on either side of the membrane, solute charge and interaction with other solutes, and capillary pore configuration.322
Hydrostatic and Colloid Osmotic Pressures (Starling’s Forces)
Transcapillary fluid flow results from an imbalance between the hydraulic forces favoring movement of water from the capillary into the interstitium and the forces favoring movement of water in the reverse direction. The forces contributing to fluid movement out of the capillary are the intracapillary hydrostatic pressure and the interstitial colloid osmotic pressure, whereas those forces favoring movement of fluid from the interstitium to the capillary are the interstitial hydrostatic pressure (if it is positive) and the plasma colloid osmotic pressure323 (Fig. 7.4 ).
Fig. 7.4.
Basic Starling’s forces—mean forces influencing fluid movement into or out of the capillary.
From Guyton AC, Hall JE: The microcirculation and the lymphatic system: capillary fluid exchange, interstitial fluid and lymph flow. The body fluid compartments: extracellular and intracellular fluids; interstitial fluid and edema. In Guyton AC, Hall JE, editors: Textbook of medical physiology, ed 11, Philadelphia: Elsevier/Saunders; 2006.
The principal force favoring fluid efflux from the capillary is the hydrostatic pressure within the capillary. Capillary hydrostatic pressure varies among different tissues and decreases along the length of the capillary. Hydrostatic pressure within a capillary is determined by the arterial and venous pressures and by the precapillary and postcapillary resistances.324 Specifically, capillary pressure (Pc) is determined by the ratio of the postcapillary resistance (Ra) to the precapillary resistance (Rv), and by the arterial (Pa) and venous (Pv) pressures:
Thus although an increase in either arterial or venous pressure will increase capillary pressure, a small increase in venous pressure has a much greater effect than does an increase in arterial pressure. For this reason the hydrostatic pressure is greater in capillaries below the heart (e.g., legs) than in those above the heart (e.g., head).
The colloid osmotic pressure of the plasma is the principal force minimizing fluid efflux from the capillary. The colloid osmotic pressure is generated both because the plasma and interstitial fluid are separated by a semipermeable membrane, the endothelium, and because they vary slightly, but significantly, in composition. As noted previously, the interstitial fluid has a lower protein concentration than does plasma but has an essentially identical electrolyte concentration. The difference in protein concentration across the semipermeable endothelium generates an osmotic force that tends to draw water from the interstitium into the plasma.
In addition to the capillary hydrostatic pressure, the colloid osmotic pressure and negative hydrostatic pressure of the interstitial fluid favor fluid movement out of the capillary. Fluid flux across the capillary results from the summation of these forces (see Fig. 7.4). These figures should be recognized as representing the forces at the midpoint of an idealized capillary; the forces are dynamic, changing between tissues and even along the length of the capillary. In fact, a large net flux of fluid from the capillary occurs at its arteriolar end, where capillary hydrostatic forces are greatest and the oncotic gradient is least, whereas a net flux of fluid into the capillary occurs toward its venous end, where capillary hydrostatic forces are least and the oncotic pressure gradient favoring reabsorption is greatest following dilution of protein in the interstitium (Fig. 7.5 ).
Fig. 7.5.

Schematic of normal fluid movement in a capillary bed. Filtration occurs at the arterial end where capillary hydrostatic pressure is highest, and absorption occurs at the venous end where capillary hydrostatic pressure is lower, allowing plasma colloid oncotic pressure to predominate. Excess fluid is removed by the lymphatics.
The small imbalance in filtration forces results in a net efflux of fluid from the capillary into the interstitial tissue. Normally this fluid does not accumulate in the interstitium, as it is removed by the lymphatics.
Other Factors Influencing Fluid and Solute Movement
Vascular Endothelium
The endothelium forms a dynamic barrier between the blood and the tissue. Although endothelial cells can have distinct morphologies and functions depending on the tissue and even the specific vessel, in general one function is to restrict the extravasation of larger molecules and cells from the vasculature to the interstitium.325 The endothelial glycocalyx, which is a layer of macromolecules at the luminal surface of the vascular endothelium, plays an important role in fluid homeostasis and solute exchange.317, 325, 326 The composition of the endothelial glycocalyx is dynamic, but the major constituents are hyaluronic acid and the negatively charged heparin sulfate proteoglycans. Changes in the endothelial glycocalyx appear to play a key role in endothelial dysfunction and the formation of edema. Also, endothelial permeability is in part regulated by the dynamic opening and closing of cell-cell adherens junctions that are composed primarily of vascular endothelial-cadherin.318, 327, 328
Aquaporins
Aquaporins are a diverse family of membrane proteins that are expressed predominantly in tissues in which edema and fluid imbalances are of major concern.319, 329 Water movement across cell membranes is driven by osmotic and hydrostatic forces, but the speed of this process can be influenced by the presence of specific aquaporin channels. These channels are primarily water channels, although some are also permeable to small solutes. Aquaporin-4 water channels play a central role in brain water regulation in neurologic disorders.330, 331, 332 The pharmacologic modulation of the expression and activity of various aquaporins potentially could provide novel treatments for a variety of disorders, including brain edema.
Lymphatics
The lymphatics drain the interstitium of fluid and substances, notably proteins, that are not absorbed by the capillaries.311 The lymphatics represent the only means by which interstitial protein is returned to the circulation. Interstitial fluid (and, with it, protein) moves down a pressure gradient into lymphatic capillaries through clefts between the lymphatic endothelial cells. Lymphatic endothelial cells are supported, and the lymphatic capillaries maintained patent, by anchoring filaments that attach the endothelial cells to surrounding connective tissue. Lymphatic fluid progresses centripetally through progressively larger vessels before draining into the great veins of the chest. Lymphatic valves prevent the retrograde flow of fluid from the lymphatics. Lymph is propelled by factors extrinsic to the lymphatics, including muscle activity, active and passive motion, posture, respiration, and blood vessel pulsation. Exercise increases lymph flow, at least in part because of the increase in tissue pressure that is associated with muscle contraction, although passive motion also increases lymph flow. In human beings, standing results in significant diminution or cessation of lymph flow from the lower extremities, which can result in the accumulation of interstitial fluid and edema. In addition to the extrinsic factors affecting lymph flow, coordinated contractions of lymphatic vessels contribute substantially to the centripetal flow of lymph.313
Mechanisms of Edema Formation
Simply stated, accumulation of excessive fluid in the interstitial spaces—edema—results from an imbalance of the rates of fluid filtration from the capillaries and drainage by the lymphatics. The exact pathophysiologic mechanisms involved in edema formation are complex and involve some tissue-specific mechanisms. Basic mechanisms of edema include alterations in Starling’s forces, changes in vascular permeability, and impaired lymph drainage.
Alterations in Starling’s Forces
Perturbations of one or more of the forces that affect filtration across the capillary alter the rate at which fluid enters the interstitium.311, 312 Increases in capillary hydrostatic pressure, decreases in plasma oncotic pressure, and increases in interstitial oncotic pressure all favor increased fluid filtration. Conversely, increased interstitial hydrostatic pressure and decreased interstitial oncotic pressure act to inhibit fluid filtration.
There are several fundamental mechanisms by which excessive interstitial fluid can accumulate (Box 7.6 ). Increases in capillary hydrostatic pressure, which occur with venous obstruction or arteriolar dilation, such as that associated with inflammation, increase net fluid efflux. The edema that occurs with congestive heart failure likely has an increase in capillary hydrostatic pressure as one of its causes, although the mechanism is complex.313 Posture also affects capillary hydrostatic pressure; capillaries below the level of the heart have higher hydrostatic pressures than do capillaries above the level of the heart.
BOX 7.6. Mean Forces (mm Hg) Influencing Fluid Movement into or out of the Capillary.
| Type of Pressure | Mean Force |
|---|---|
| Hydrostatic Pressures | |
| Mean capillary pressure | 17.0 |
| Interstitial pressure | –5.3 |
| Total hydrostatic pressure favoring filtration | 22.3 |
| Colloid Oncotic Pressures | |
| Plasma oncotic pressure | 28.0 |
| Interstitial oncotic pressure | 6.0 |
| Total oncotic pressure opposing filtration | 22.0 |
| Total pressure favoring filtration | 0.3 |
Data from Guyton AC: Textbook of medical physiology, ed 11, Philadelphia, Saunders, 2005.
A decrease in the oncotic gradient across the capillary endothelium, which occurs with a decreased plasma oncotic pressure or an increased interstitial oncotic pressure, results in an increase in efflux of fluid from the capillary. A decrease in plasma oncotic pressure decreases the oncotic gradient that favors movement of fluid into the capillary. Consequently, the capillary hydrostatic pressure, which favors filtration, predominates and fluid accumulates in the interstitium. Plasma oncotic pressure decreases when plasma protein concentration declines. Albumin is the plasma protein that exerts the preponderance of the oncotic force and edema often is associated with hypoalbuminemia.311, 321
Changes in Vascular Permeability
An increase in the permeability of the capillary membrane can increase fluid and protein transport into the interstitium and decrease the ability of the membrane to maintain a difference in oncotic pressure between the plasma and the interstitium.314 Thus changes in vascular permeability can affect fluid flux both directly and via changes in Starling’s forces.
The regulation of vascular permeability is complex with multiple pathophysiologic triggers contributing to increased permeability.325, 326, 327 The microvasculature is sensitive to damage via several mechanisms including mechanical forces, ischemia-reperfusion injury, sepsis, and inflammation. Vasodilation and increased blood flow alone can increase vascular permeability. Numerous molecular regulators affect vascular permeability, including hormones, inflammatory cytokines, and other plasma constituents. Vascular endothelial growth factor is a potent angiogenic factor that can affect regulation of the vascular barrier.325, 333, 334 Also, a number of inflammatory cytokines contribute to vasculitis. Among these are histamine and bradykinin, both of which can ultimately increase the generation of nitric oxide.325
The precise means by which vascular permeability is increased after stress to the endothelium are complicated and still under investigation. Inflammatory mediators and hormones can affect the integrity and function of the endothelial glycocalyx layer, and the changes in this layer frequently result in increased permeability and edema.317, 325, 326 The organization and function of vascular endothelial-cadherin and other proteins at the adherens junctions can also be affected.318, 325, 327, 328 In addition, changes in the transcriptional, translational, and posttranslational regulation of transporters and ion and water channels, including aquaporins, occur.325, 329, 330, 331, 332 The complexity of the regulation of vascular permeability and other factors contributing to edema can in part explain some of the limitations of colloid therapy. Improved understanding of the mechanisms controlling vascular permeability might lead to the formulation of pharmacologic strategies to control the barrier function of the endothelium.
Impaired Lymph Drainage
Lymphatic obstruction prevents the removal of interstitial fluid and protein.311 Filtration of fluid and passage of small amounts of protein into the interstitial space continues in the presence of lymphatic obstruction. The interstitial fluid is reabsorbed by the capillaries, but the protein is not. Consequently, the protein content of the interstitial fluid gradually increases, with a resultant increase in interstitial oncotic pressure that favors filtration of fluid. The increased interstitial oncotic pressure causes fluid to accumulate in the interstitium, thus exacerbating the edema.
Compensatory Mechanisms Limiting Edema Formation
Alterations in the magnitude of one or more of Starling’s forces can be offset by compensatory changes in lymph flow and other of Starling’s forces.311 In concert Starling’s forces and lymph flow act as “edema safety factors” to prevent the excess accumulation of interstitial fluid and development of frank edema. For example, lymph flow increases with the increased filtration associated with increased capillary hydrostatic pressure. Thus a larger volume of fluid enters and is removed from the interstitial space. The interstitial protein concentration decreases as increased fluid flow washes protein out of the interstitial space. Reduced interstitial space protein concentration minimizes oncotic forces drawing fluid from the capillary to the interstitial space.315
Conditions Associated with Edema
Edema is not in itself a disease; rather, it is a sign of a disease process. Therefore the identification of conditions leading to the accumulation of edema is based on an understanding of the pathogenesis of edema. The most common form of edema recognized in horses is peripheral edema, and multiple causes have been identified (Table 7.3 ). Some specific causes of peripheral edema are discussed later.
Table 7.3.
Common Causes of Peripheral or Ventral Edema in Horses
| Congestive Heart Failure |
| Valvular disease Myocarditis Monensin toxicosis |
| Vasculitis |
| Equine viral arteritis Equine ehrlichiosis Purpura hemorrhagica Equine infectious anemia |
| Venous Obstruction and Congestion |
| Catheter-related thrombophlebitis Disseminated intravascular coagulation Tight bandages Tumors Immobility |
| Cellulitis |
| Staphylococcal Clostridial Counterirritant application |
| Lymphatic Obstruction |
| Ulcerative lymphangitis Lymphadenitis (Streptococcus equi, Corynebacterium pseudotuberculosis) Lymphosarcoma Tumors |
| Hypoalbuminemia |
| Parasitism Pleural and peritoneal effusions Protein loss (gastrointestinal, renal, or wounds) Inadequate production (starvation) Hemodilution (subsequent to hemorrhage) |
| Shock |
| Hemorrhagic Endotoxic |
| Pleuritis |
| Late-term pregnancy Prepubic tendon rupture Starvation (inadequate intake; malabsorption) |
A nonpainful edema of the limbs is often seen in horses in association with immobility. This condition, commonly referred to as “stocking up,” is apparently associated with venous congestion and decreased lymph flow. It can affect all four limbs but is most common in the hindlimbs.
Hypoproteinemia, particularly hypoalbuminemia, is an important cause of edema.311 In horses, the most common cause of hypoalbuminemia is protein loss, particularly through the gastrointestinal tract. In addition to acute colitis, some specific gastrointestinal conditions that are frequently associated with protein loss include right dorsal colitis, proliferative enteropathy due to Lawsonia intracellularis, and inflammatory bowel disease.142, 335, 336 Other potential causes of protein loss include renal disease, especially chronic renal failure, amyloidosis, extensive skin wounds (burns), and third space loss as is seen in some cases of pleuritis or peritonitis. Occasionally hypoalbuminemia can be seen in association with decreased albumin production due to liver disease.337 Severe burn injuries can induce generalized edema independent of protein loss, likely secondary to endothelial injury.214, 338
Vasculitis is another important cause of edema in horses.339, 340, 341, 342, 343 Some infectious diseases in which vasculitis is often prominent include equine viral arteritis, equine infectious anemia virus, African horse sickness, and equine granulocytic anaplasmosis (Anaplasma phagocytophilum). Purpura hemorrhagica is an immune-mediated vasculitis that is often associated with marked edema.343 Many, but not all, horses affected with purpura hemorrhagica have been exposed to or infected with Streptococcus equi subspecies equi.
Several other causes of edema have been recognized in horses. Congestive heart failure is relatively uncommon in horses, but when it does occur ventral edema is among the most common clinical signs along with a heart murmur accompanied by either jugular distention or pulsation, tachycardia, and tachypnea.229 Lymphangitis is an inflammation of the lymphatic vessels that often results in impaired lymphatic drainage and swelling. The condition can occur in any limb but is more common in the hindlimbs. It is usually caused by a bacterial infection, although culture is frequently unrewarding. Some cases of Corynebacterium pseudotuberculosis infection manifest as an ulcerative lymphangitis.344 Chronic progressive lymphedema is a condition recognized in some draft horse breeds including Clydesdales, Shires, and Belgians.345 The condition is thought to be caused by altered elastin metabolism leading to impaired lymphatic function. Affected horses have chronic lymphedema with swelling of the distal limbs and development of fibrosis. Massive envenomation by bees (bee stings) causes generalized edema in horses.346 Snake envenomation can also cause edema.347
Diagnostic Approach to the Patient with Edema
The diagnostic approach to the patient with edema is dependent on an understanding of the mechanisms of edema formation and a knowledge of the diseases likely to be involved. The diagnostic approach to an animal with edema should not be any different from that for any other sign of disease. A clinical examination, including history and physical examination, permits the development of a list of potential diagnoses and dictates the appropriate subsequent steps in confirming the diagnosis. The reader is referred to those sections of the text that deal with specific diseases for a more in-depth description of the conditions and their diagnosis.
Signalment and History
The signalment can be important when considering specific conditions associated with edema. For example, Lawsonia intracellularis is primarily a disease of weanling-age horses.335 Chronic progressive lymphedema is particularly common in certain draft breeds, including Shires, Clydesdales, and Belgians.229
When taking the history of a horse that has edema, the veterinarian should focus on acquiring facts that have the greatest diagnostic use in differentiating among those diseases that have edema as a sign. The veterinarian should consider the following aspects: housing, season, and geographic region; vaccine and parasiticide administration; exposure to other horses and diseases present within the herd; the duration of the edema and its distribution; and the presence of any other clinical signs. The veterinarian should investigate the remainder of the history depending on the responses to initial questions.
Physical Examination
The physical examination should begin with a visual evaluation of the attitude and physical condition of the horse. The temperature, pulse rate, and respiratory rate should be recorded. Although the physical examination should be complete, particular attention should be paid to those body systems that the preliminary examination indicates might be involved in the disease process. The physical examination reveals the distribution and severity of edema. Edema that is localized to one extremity or is not bilaterally symmetric is more likely to be caused by local factors (e.g., lymphangitis, venous obstruction) than by systemic disease. Conversely, edema that involves several areas of the body and has a symmetric distribution is likely to be associated with systemic disease, such as the ventral edema of congestive heart failure. It is important to note that distribution of edema associated with hypoproteinemia can vary and can be widespread or limited to the ventrum, limbs, or submandibular area.
Ancillary Diagnostic Tests
The clinician will have developed an ordered list of potential diagnoses after the initial clinical examination, and this list will help direct the most appropriate ancillary diagnostic tests. Confirmation or elimination of the potential diagnoses may depend on the subsequent diagnostic procedures, including the response to therapy. A CBC and serum biochemical profile are often useful as part of a minimum database and will establish whether hypoproteinemia is present. Sections of this text deal with the specific disease processes and appropriate diagnostic procedures.
Collapse in Horses: Syncope, Seizures, and Sleep Disorders
Collapse is characterized by a loss of postural tone with or without progression to recumbency and with or without loss of consciousness. Collapse in horses is uncommon, but it has serious implications for both horse welfare and human safety. In horses, it appears that collapse most often occurs at rest, but episodes can occur during exercise.348
Collapse can be categorized as syncopal or nonsyncopal. Syncopal collapse, which is sometimes referred to as fainting, is a self-limiting loss of consciousness and postural tone resulting from cerebral hypoperfusion.349, 350 Nonsyncopal collapse includes a number of disorders that can be further subdivided into those that are associated with some degree of loss of consciousness, including seizures, sleep disorders, and hypoglycemia, and those with no loss of consciousness, including musculoskeletal and metabolic problems, such as hyperkalemic periodic paralysis.
Mechanisms of Collapse
Syncope
Syncope is defined as a temporary interruption of cerebral perfusion resulting in a sudden and transient loss of consciousness and postural tone.349, 350 The brain has a high metabolic demand and is highly sensitive to changes in perfusion. Normally cerebral blood flow is tightly regulated by a complex system of cerebral autoregulation involving metabolic, myogenic, and neurologic components. This system maintains cerebral blood flow despite changes in blood pressure, and syncope can occur when this system fails. As syncope is uncommon in horses, much of the information regarding syncope is extrapolated from other species.
The causes of syncope can be broadly classified as cardiovascular, noncardiovascular, and unknown.350, 351 Cardiovascular syncope is due to reduced cardiac output and has been associated with structural heart disease, arrhythmias, and, in people, coronary heart disease. Causes of noncardiovascular syncope include primarily neurologic and metabolic disorders. One cause of noncardiogenic syncope in people is orthostatic or postural hypotension, which is defined as a decrease of 20 mm Hg in systolic blood pressure or 10 mm Hg in diastolic blood pressure within 3 minutes of changing position from sitting or supine to standing.350, 351, 352 It results from an inadequate physiologic response to postural changes and has been associated with an impairment of peripheral vasoconstriction or reduction of intravascular volume. Specific causes of syncope related to orthostatic hypotension include dehydration or blood loss, as well as a variety of neurologic, cardiovascular, and endocrine disorders. Another type of noncardiogenic syncope is neurally mediated syncope, which is associated with disturbances of reflex cardiovascular control.350, 351, 353, 354 Some cases of neurally mediated syncope are associated with pain, stresss, or specific triggers. Although the pathogenesis of this phenomenon is not fully understood, it is characterized by peripheral vasodilation and/or transient bradycardia that is associated with inappropriate triggering of neural reflexes. In some but not all cases, neurally mediated syncope is associated with concurrent cardiac disease. The most common form of neurally mediated syncope is neurocardiogenic or vasovagal syncope, in which there is an increase in vagal parasympathetic tone and a decrease in sympathetic tone that results in bradycardia and hypotension associated with peripheral vasodilation.353, 354 In two cases of presumptive neurocardiogenic syncope in horses, no bradycardia was noted before collapse, suggesting a purely vasodepressor response in those cases.348 Some other forms of neurally mediated syncope that have been recognized in people include carotid sinus syndrome, postmicturition syncope, and swallow syncope.
Syncope is common in people, with approximately one third of the population experiencing syncope at least once during their lifetime.350, 351 In humans, presyncopal signs such as weakness, blurred vision, and nausea frequently precede the collapse. Recovery is spontaneous, and following the episode people can experience persistent drowsiness, dizziness, headache, or nausea but typically not confusion. In horses, signs such as stumbling, head elevation, or anxiousness can precede collapse, but in most cases there are little or no premonitory warning signs.355 The collapse is associated with a transient comatose state followed by recovery. Horses may struggle during recovery but are normal thereafter unless they sustain significant trauma during the episode. A syndrome of convulsive syncope that is difficult to differentiate from seizure activity has been described in people, but it is uncertain if this occurs in horses.356, 357
Seizures
Seizures are the manifestation of the excessive and/or hypersynchronous discharge of neurons in the cerebral cortex that leads to voluntary alterations of motor activity, consciousness, and autonomic or sensory functions.349, 355, 358, 359, 360 Normal neuronal function reflects a balance between excitatory and inhibitory activity. When intracranial neurons experience either excessive excitation or loss of inhibition (disinhibition) the resulting excessive depolarization can result in seizure activity. The seizure threshold reflects the level of neuronal inhibition that must be exceeded for uncontrolled discharge of a population of neurons to occur.
Normally there are sporadic low-frequency spontaneous depolarizations within the brain that are contained by a process known as surround inhibition. However, in some cases, groups of cells will depolarize with recurrent high-frequency bursts of action potentials that can manifest as focal seizure activity. Depolarizations of sufficient magnitude can overcome the surrounding inhibitory zone and be conducted to a more widespread area via normal anatomic connections between neurons, thus propagating seizure activity and potentially involving the entire cortex. Although occasionally depolarizations in the brainstem and thalamus can result in seizures, these depolarizations are projected to the cortex. The mechanisms of seizure termination are not fully understood, but factors that may be involved include metabolic exhaustion of neurons and the activity of extracortical inhibitory centers within the cerebellum. Other regions of the brain, such as the caudate and reticular formation, may also affect seizure activity.
Multiple factors may trigger the uncontrolled synchronous neuronal activity involved in the generation of seizures, reflecting the complexity of the neuronal environment.358, 359, 360, 361 Among the factors influencing this environment are the neuronal lipoprotein cell membrane with its associated ion channels and enzymes. Also, neuronal function is influenced by the ionic environment, including concentrations of sodium, chloride, calcium, and potassium. A variety of neurotransmitters are present, including both excitatory neurotransmitters such as glutamate, aspartate, and acetylcholine, and also inhibitory neurotransmitters such as γ-aminobutyric acid, glycine, taurine, and norepinephrine. Several hormones and neurosteroids can influence epileptogenesis, having variable effects depending on the substance.361 Any alterations in the complex neuronal environment can potentiate seizures. For example, hypoglycemia can result in a loss of energy substrate for the Na+-K+ ATPase pump, causing cells to move toward lower positivity and allowing excessive excitation. In hepatoencephalopathy, there may be a decrease in the function of inhibitory neurotransmitters, resulting in a lack of inhibition and unregulated depolarization. Multiple factors contribute to the development of seizures associated with traumatic brain injury, infection, or neoplasia including neuronal loss, altered cellular metabolism and blood flow, and neuroinflammation, among others.358, 359, 360, 361, 362
Seizures can present with a variety of forms and severities, and the classification and terminology can be confusing. In humans, the Commission on Classification and Terminology of the International League Against Epilepsy has developed a comprehensive scheme for the classification of seizures to more accurately describe seizures and develop a systematic approach to their diagnosis and treatment.363 Efforts have been made to similarly classify seizures in horses.364, 365 The term epilepsy is defined as two or more seizures that occur in more than 24 hours regardless of the cause. Seizures can be described as either focal or generalized, both of which have been described in horses. Focal seizures, which have previously been referred to as partial, represent a focal area of abnormal discharge within the brain and result in localized motor signs or sensations. Focal seizures can be further categorized as simple, if alertness and normal mentation are maintained, and complex, if impairment of consciousness is present. Generalized seizures involve the entire cerebral cortex and typically result in generalized bilateral motor activity and loss of consciousness. Generalized seizures can be primary, meaning they are generalized from the outset, or secondary, meaning they progress from a focal seizure. Seizures can also be classified by the cause. Reactive seizures are those in which the seizure is a result of a temporary systemic disease with normal brain function such as seizures seen in association with acute hemorrhage or hypoglycemia. Other causes of seizures have been grouped as follows: (1) symptomatic, in which there is a documented structural brain disorder such as brain damage from trauma or hypoxia, congenital anomalies, neoplasia, and infection, including meningitis, encephalitis, and abscessation; (2) cryptogenic, in which symptomatic causes are suspected but cannot be documented; and (3) idiopathic, in which there are no underlying structural or metabolic abnormalities and there is a suspected genetic predisposition.
The clinical presentation of seizures in horses is similar to that seen in other species.355, 364 In a study of 104 horses over 3 weeks of age with seizures, precipitating factors were identified in approximately 11% of cases and included xylazine, noise, trimming the mane, touching the neck, and estrus.364 Prodromal or preictal signs are not consistently recognized but can be seen in some horses with either focal or generalized seizures and include anxious behavior, isolation from other horses, standing in a corner, swishing of the tail, and changes in personality. Focal seizures may involve the face or body and some of the more common signs include ear or lip twitching, tongue prolapse, opening and closing of jaw, compulsive kicking, head turning to one side, and tremors.364 In some cases, impaired consciousness and behavior changes have also been reported. Generalized seizures are typically characterized by tonic-clonic motor activity. Eye globe deviation and nystagmus are common. Vocalization and autonomic disturbances such as mydriasis, urination, defecation, and profuse sweating have also been reported. Affected horses may have generalized rigidity or a loss of muscle tone. Postictal signs have also been reported in horses. In the study of 104 horses with seizures, 31% of horses affected by complex focal or generalized seizures had postictal signs typically lasting from minutes to hours.364 These signs included ataxia, disorientation, lethargy, agitation, blindness, hypersensitivity, and shallow breathing.
Sleep Disorders
Sleep is defined as unconsciousness from which one can be aroused by sensory or other stimuli.349, 355 Sleep has been investigated in domestic horses, and four stages of vigilance have been identified: wakefulness, drowsiness, slow wave sleep (SWS), and rapid eye movement (REM) sleep, also known as paradoxic or desynchronized sleep.366, 367, 368, 369 REM sleep appears to be an important regenerative part of the sleep cycle. Adult horses typically require 3 to 5 hours per day of sleep with approximately 0.5 hour per day of that being REM sleep. The majority of REM sleep in horses appears to occur during recumbency, although occasionally REM sleep has been observed in standing horses that are not habituated to their environment.368
One cause of excessive drowsiness and possible collapse in horses is recumbent sleep deprivation. The level of tolerance for sleep deprivation among individual horses appears to vary widely, with some horses being able to tolerate weeks of being unable to lie down to sleep whereas others will show signs of sleep deprivation after just several days.355, 368, 370
Narcolepsy is a sleep disorder that is primarily characterized by excessive drowsiness and sleep attacks with or without cataplexy, which is a sudden loss of muscle tone and somatic areflexia.355, 371, 372 In people, disturbed nocturnal sleep, hypnagogic hallucinations, and sleep paralysis can also be seen in association with narcolepsy. The sleep cycle is disrupted, and people with narcolepsy frequently enter REM sleep at the onset of sleep rather than later in the sleep cycle. Both sporadic and familial forms of narcolepsy have been identified in humans, dogs, and horses.370, 371, 372, 373, 374, 375, 376, 377, 378, 379 The pathophysiology of narcolepsy remains unclear and may vary among species and individuals. It appears to be a complex biochemical disorder affecting several regions of the brain (brainstem, hypothalamus, limbic system, and possibly the striatum and cortex) with dysfunction of dopaminergic, cholinergic, and noradrenergic neurotransmitters.372, 381 Defective hypocretin signaling has been described in both familial and sporadic narcolepsy in some species.371, 372, 373, 382 Hypocretin 1 and 2, also known as orexins, are neuropeptides that appear to be specifically expressed in certain hypothalamic neurons. They appear to have an important role in the regulation of sleep and arousal states, as well as appetite. In some dog breeds, a familial form of narcolepsy has been identified in association with a mutation in hypocretin-2 receptor gene.374 In these dogs, hypocretin concentration in the cerebrospinal fluid (CSF) is normal. In humans, narcolepsy is only rarely associated with mutations in the hypocretin receptor gene. In both humans and dogs, the sporadic form of narcolepsy has been associated with decreased hypocretin concentration in the CSF. Many cases of narcolepsy in people are linked to the human leukocyte antigen, and an autoimmune component may play a role in the development of disease. It has been postulated that there is an immune-mediated destruction of hypothalamic neurons producing hypocretin, resulting in a progressive decline in the concentration of hypocretin. The role of hypocretin in equine narcolepsy is currently uncertain. The concentration of hypocretin in the CSF of an Icelandic foal with narcolepsy without cataplexy was similar to controls, although it was speculated that concentrations could decrease over time.377 Occasionally secondary narcolepsy may develop after disease or organic insult to the brain, such as following traumatic brain injury.383, 384
Most cases of narcolepsy in horses have been diagnosed in foals, but sporadic cases have been seen in adults.355, 374, 375, 376, 377, 378, 379, 380 Given the difficulties in diagnosing narcolepsy, some of these cases may represent recumbent sleep deprivation or idiopathic hypersomnia, which is another disorder of the central nervous system characterized by excessive sleepiness. Better characterization of sleep disorders in horses is needed.
Sleep disorders in horses are often characterized by drowsiness, with gradual lowering of head and buckling, which most often occurs in the forward direction. In some cases there is complete collapse. In cases of narcolepsy with cataplexy there will be limb atonia and areflexia. Occasionally swaying and stumbling when walking have been described in narcoleptic horses. In cases of narcolepsy in other species, cataplexy can sometimes be induced by play or feeding, and similar triggers have been described occasionally in equine cases of narcolepsy. Many normal newborn foals can be induced into a sleeplike cataplectic state by firm, whole-body restraint or squeezing.355
Conditions Causing Collapse
Several conditions have been reported in association with collapse in horses, but information is somewhat limited (Box 7.7 ). It can be challenging to make a definitive diagnosis for the cause of collapse. In a retrospective study of 25 horses with episodic collapse, a final diagnosis was established in 11 cases and a presumptive diagnosis was made in 8, with 6 being undiagnosed.348 Overall, the most common cause of collapse in this series of cases was syncope, which accounted for 63% of the cases in which a definitive or presumptive diagnosis was made. In the 11 horses with a specific final diagnosis, causes included cardiac disease (5 of 11), generalized seizures (2 of 11), hypoglycemia secondary to neoplasia (2 of 11), and sleep disorders (2 of 11). In the 8 horses with a presumptive diagnosis, causes included neurocardiogenic syncope (5 of 8), syncope associated with severe EIPH (2 of 8), and seizures (1 of 8).
BOX 7.7. Causes of Collapse.
Syncope
- Cardiovascular
- Arrhythmia
- 3° atrioventricular (AV) block
- Atrial tachycardia with 2° AV block
- Atrial fibrillation
- Other
- Right-sided heart failure
- Ruptured chordae tendinae
- Myocardial infarction, myocardial fibrosis
- Endocarditis
- Pericarditis
- Noncardiovascular
- Neurocardiogenic (vasovagal) syncope
- Polycythemia
- Airway obstruction
Undetermined
Seizures
- Reactive (no structural brain lesion)
- Hepatoencephalopathy
- Hemorrhage
- Electrolyte abnormalities
- Hypoglycemia
- Symptomatic
- Traumatic brain injury (skull fracture, cerebral hemorrhage)
- Cerebral edema
- Neonatal encephalopathy
- Neoplasia
- Cholesterol granuloma (can be incidental)
- Vasculitis
- Viral, bacterial, or verminous meningoencephalitis
- Abscessation
- Equine protozoal myeloencephalitis
- Intracranial vascular events
- Leukoencephalomalacia
- Congenital anomalies
- Ear ticks
- Cryptogenic
- Lesion suspected but cannot be identified
- Idiopathic
- Juvenile idiopathic epilepsy of Arabian foals
Sleep Disorders
Recumbent sleep deprivation
Narcolepsy
Other
Hyperkalemic periodic paralysis
Hypoglycemia
Hyperthermia
Undetermined
Syncope
Syncope in horses has been associated with cardiovascular, noncardiovascular, and unexplained causes, with cardiovascular and presumed neurocardiogenic syncope being the most common. Specific cardiovascular problems that have been reported in association with syncope in horses include cardiac arrhythmias, including third-degree atrioventricular (AV) block, atrial tachycardia with advanced second-degree AV block, and atrial fibrillation.348, 355, 385, 386 Right-sided heart failure, ruptured chordae tendineae, myocardial infarction, myocardial fibrosis, aortic endocarditis, and pericarditis have also been associated with syncope.355, 385, 386 In some cases, a definitive cardiac disease can be hard to confirm. Depending on the underlying problem some horses with cardiovascular syncope may have other signs of cardiac failure, but this is not consistent. Presumptive neurocardiogenic syncope has been diagnosed in horses in which there are no abnormal findings on clinical, neurologic, or cardiac examinations.348, 355 In general, collapse was reported to occur without warning at rest, and horses were described to sink abruptly or to raise their heads and fall backward to the ground. Confirmation of neurocardiogenic syncope would require ECG and blood pressure monitoring. Polycythemia and upper airway obstruction have also been reported to cause syncopal episodes.387, 388
Seizures
A number of disorders have been associated with seizures in horses. The clinical presentation of the seizure appears to be independent of the underlying cause, reflecting the area and extent of the cerebral cortex involved.364 In the study evaluating 104 horses with seizures, 2 horses were found to have reactive seizures not associated with abnormal brain function. These horses were diagnosed with liver failure and severe acute systemic hemorrhage. The remaining 102 horses were felt to have underlying brain disease, although a specific diagnosis was not always possible. Of these, 28% had a single seizure episode and 70% had two or more seizures and were diagnosed with epilepsy. Of those horses with a single seizure episode, an underlying structural brain lesion was confirmed or suspected in 16 of 29 horses, with acute head trauma being the most common (6 of 16). In those horses with epilepsy, the condition was categorized as symptomatic in 35.6% of cases and cryptogenic in 54.8%, and 2 foals were diagnosed with idiopathic epilepsy. Overall there was no breed, age, or gender association in horses with epilepsy, although juvenile idiopathic epilepsy is seen in Arabian foals. Specific causes of symptomatic seizures that were identified included skull fracture, cerebral hemorrhage, cerebral edema, neoplasia, cholesterol granuloma, vasculitis, meningoencephalitis, abscessation, intracranial vascular events, leukoencephalomalacia, congenital abnormalities, and equine protozoal myeloencephalitis (EPM). The presence of ear ticks has rarely been associated with seizures.355 Meningoencephalitis due to infection with the nematode Halicephalobus gingivalis has been associated with multiple neurologic deficits, including seizures.389 Neonatal foals have a lower seizure threshold than adults, and seizures have been reported in association with sepsis, neonatal encephalopathy, and electrolyte abnormalities, including hyponatremia.355, 390, 391, 392 Hypoglycemia may cause seizures but is more often associated with weakness and lethargy. Hyperbilirubinemic encephalopathy (kernicterus) may rarely occur in foals.
Sleep Disorders
Sleep deprivation can be induced by preventing horses from being able to lie down, such as when horses are kept on a tie-line to prevent them from exacerbating a musculoskeletal injury by getting up and down.355, 370 Other conditions in which horses do not lie down to sleep include musculoskeletal, abdominal, thoracic, or neurologic problems that either create pain or mechanical difficulties on attempt at recumbency or rising to stand. Environmental insecurity, including such things as herd dynamics, stall size, and excessive noise, may prevent horses from feeling comfortable enough to lie down. It has also been proposed that monotony, such as being in cross-ties for an extended time, may cause horses to exhibit drowsiness, with the head lowering to the point of near collapse as the horse begins to transition from SWS to REM sleep.
Familial narcolepsy has been identified in American Miniature Horses, Shetland Ponies, and Lipizzaners.355, 375, 376, 378 At this time the precise genetics have not been determined. Sporadic narcolepsy, generally without cataplexy, has been identified in several breeds including Warmbloods, Icelandic Horses, and Thoroughbreds.355, 378
Other
A number of other disorders can occasionally result in collapse. Hyperkalemic periodic paralysis can cause weakness, muscle fasciculations, and collapse without alterations in consciousness.302 Other electrolyte abnormalities in addition to hyperkalemia may result in collapse including hypocalcemia, hyponatremia, and hypokalemia.355 Hypoglycemia can be associated with weakness and collapse, and in some cases seizures may occur. Some other disorders that can be result in collapse include trauma to the motor pathways, botulism, and hyperthermia.355
Diagnostic Approach to Collapse
The diagnosis of the cause of collapse can be challenging, and in many cases a definitive cause is not determined. Often the clinician is unable to witness the episode, and although accounts by the owner can be helpful, they can also be inaccurate and may omit key information if the owner is unaware of the significance. A final diagnosis was found to be more likely in horses that experienced multiple episodes of collapse than in horses that were observed to collapse only once.348 This may be because they may be more likely to have ongoing signs of disease and also because the sensitivity of diagnostic procedures may be improved by carrying them out either during or immediately after a collapsing episode. For example, some cardiac arrhythmias are paroxysmal and may not be present between episodes.
Signalment and History
There are some age, breed, and sex associations with some causes of collapse. In general, neonatal foals are more susceptible to seizures than adult horses and may seize in response to a number of metabolic and systemic diseases.355, 390, 391, 392 Juvenile idiopathic epilepsy is a disease of Egyptian Arabian foals with an age of onset ranging from 2 days to 6 months.355, 393 Familial forms of narcolepsy have been identified in American Miniature Horses, Shetland Ponies, and Lipizzaners.355, 375, 376, 378 Hyperkalemic periodic paralysis should be considered as a possible cause of collapse in Quarter Horses and related breeds.302 In the study of 104 horses with seizures, seizure type was significantly associated with gender, with females being more prone to generalized seizures and one mare demonstrating generalized seizures during estrus.364 Estrogen and progesterone concentrations are related to alterations in seizure threshold in women and female dogs, and a similar risk factor may be present in horses.361, 394
A detailed history should be obtained, including a performance history and the occurrence of any other problems, such as EIPH or lameness. With regard to the episodes of collapse, the onset and duration as well as any specific triggers and the features of the episode should be determined. Possible reasons for recumbent sleep deprivation should be investigated, including underlying medical problems and management changes such as moving the horse to a new environment with different horses, excessive noise, or limited space.
Physical Examination
A thorough physical examination should be performed. In some cases, additional clinical signs may be present depending on the underlying disease. For example, fever and lethargy may be present in horses with viral or bacterial encephalitis and icterus, and weight loss may be seen in horses with hepatoencephalopathy. Horses with sleep disorders may have abrasions on the fetlocks from repeatedly buckling forward. Particular attention should be focused on the cardiovascular and neurologic systems. In some cases of potential cardiac disease, evaluating the horse after exercise may be useful.
Video surveillance may be needed to allow observation of the actual episode of collapse. Although differentiation of the conditions can be difficult, clinical features can vary among syncope, seizures, and sleep disorders. Generalized seizures generally differ from syncope in that they involve tonic-clonic convulsive activity and may be associated with opisthotonos or nystagmus, as well as urination and defecation. Seizures are also often followed by a postictal phase that is not seen in syncope or narcolepsy. In horses with sleep disorders, the collapse is often preceded by a gradual lowering of the head, and the horse most often falls forward. Eye movement may be seen in association with REM sleep during a sleep attack, but this appears to be uncommon in horses.
Ancillary Diagnostic Aids
Clinical Pathology
A CBC, fibrinogen, and serum chemistry can be helpful in identifying metabolic or systemic abnormalities, such as sepsis, liver disease, or electrolyte abnormalities. In some horses, such as those with hyperkalemic periodic paralysis (HYPP), the abnormality may only be present during the episode. Measurement of cardiac troponin can be useful to identify cardiac disease.395, 396 Analysis of CSF and testing for EPM can provide useful information.
Electrodiagnostic Testing
An electrocardiogram (ECG) is valuable in evaluating the cardiac rhythm in horses with episodes of collapse. As some arrhythmias are intermittent, continuous ECG monitoring may be necessary to identify the arrhythmia. This is most often done with a 24-hour Holter monitor. An insertable cardiac monitor, also known as insertable or implantable loop recorder, has been used in horses to record an ECG during the collapse episodes and can improve the diagnostic yield.348 Blood pressure monitoring during an episode could be useful but has not been done in horses.
An electroencephalogram (EEG) assesses electrical brain activity and can be helpful in the identification of seizures. However, due to the paroxysmal nature of epileptiform activity, a normal EEG does not rule out the presence of seizures, especially if performed during the interictal period. In Arabian foals with juvenile idiopathic epilepsy, epileptiform activity was found on EEG in 9 of 13 foals.393 Continuous EEG monitoring improves the chance of detecting abnormal brain activity. A device for ambulatory EEG has been used in horses for long-duration EEG recording and has been shown to identify abnormal brain acitivity.397, 398 EEG can also evaluate electrical activity in the sleep cycle and may be useful in the assessment of narcolepsy.
Diagnostic Imaging
Echocardiography can be useful in the evaluation of cardiac disease. It can also be used to evaluate the liver in horses with suspected hepatic disease and hepatoencephalopathy.
Radiographs, computed tomography (CT), and magnetic resonance imaging (MRI) can all be useful in the diagnostic evaluation of horses with collapse, especially with suspected seizure activity.348, 364, 399, 400 Skull radiographs and CT are particularly useful in horses with skull trauma.400 CT can be used to identify other lesions as well but has some limitations in identifying inflammatory disease and small or diffuse parenchymal lesions. MRI has been shown to be a valuable diagnostic tool, especially in the evaluation of symptomatic epilepsy.364, 399 There has been a good correlation between MRI findings and intraoperative or postmortem results. It is important to remember, however, that it is not uncommon for seizures to occur in the absence of a structural brain lesion.
Additional Testing
Challenge testing has been used in the assessment of possible narcolepsy in horses, but results have not been consistent.374, 375, 376, 377 Physostigmine may induce narcoleptic episodes, and atropine may eliminate signs. In humans and dogs, hypocretin concentration in CSF can be used in the evaluation of narcolepsy, but at this time the value of hypocretin testing in horses is uncertain.377, 382
Dysphagia
Dysphagia is defined as difficulty swallowing. Normal swallowing, or deglutition, occurs as a complex sequence of events with three phases: oral, pharyngeal, and esophageal.401 In equine medicine, dysphagia is often used in the broad sense to include any difficulty in the prehension and uptake of feed, as well as in actual swallowing. The clinical signs of dysphagia vary depending on the cause but frequently include dropping feed or packing feed between the cheek teeth and buccal mucosa, ptyalism, extension of the neck, gagging, coughing, and the presence of feed-tinged nasal discharge. Dysphagia can be a significant problem for the patient due to the risk of aspiration and the potential compromise in nutritional and hydration status.
Mechanisms of Dysphagia
Normal eating and drinking are complex neuromuscular activities that are initiated with the uptake of material into the oral cavity.401, 402, 403 The process of swallowing allows the transport of this material from the oral cavity to the stomach while protecting the airway. The initial stage of swallowing is the oral phase, which encompasses the formation and positioning of a feed bolus, which is defined as feed that is of suitable size and consistency to be swallowed. Mastication and moistening by saliva lead to formation of the bolus and also initiate digestion by mechanical breakdown of the feed and addition of digestive enzymes from saliva. The bolus is moved to the base of the tongue, where coordinated movements of the tongue and pharynx initiate the pharyngeal phase of swallowing. The oropharynx relaxes and the soft palate elevates to seal the palatopharyngeal arch and nasopharynx. As the bolus enters the oropharynx, the hyoid apparatus moves rostrodorsally, drawing the larynx and common pharynx forward while at the same time the epiglottis tips caudally to prevent the bolus from entering the larynx. The bolus is moved through the pharynx by sequential contraction of the pharyngeal constrictor muscles along with some driving force from the tongue. The lower portion of the inferior pharyngeal constrictor muscle, known as the cricopharyngeus, is the major muscle in the upper esophageal sphincter. At the end of the pharyngeal stage of the swallow, this muscle relaxes, allowing the bolus to enter the esophagus, and then contracts to prevent oroesophageal reflux and aerophagia. As the bolus enters the esophagus, the esophagus, including the lower esophageal sphincter, relaxes and the bolus is propelled toward the stomach by primary peristaltic waves. Gravity can assist in moving a liquid bolus toward the stomach. After the bolus enters the stomach, the lower esophageal sphincter contracts to prevent gastroesophageal reflux. Any minor reflux that does occur is typically cleared by secondary peristaltic waves, which are waves that occur in the thoracic esophagus without an associated pharyngeal contraction. Active antiperistalsis does not normally occur in horses, and thus horses generally do not actively vomit. All of the activity involved in swallowing occurs relatively rapidly. In herbivores, breathing can continue uninterrupted during swallowing.
Several anatomic structures and at least 30 muscles, including both striated and smooth muscles, are involved in the process of eating and drinking.401, 402 Among the muscles used are the masseter, temporalis, and medial and lateral pterygoid muscles, often referred to as the muscles of mastication. Also muscles within the lips, oral cavity, pharynx, larynx, and esophagus are involved. Sophisticated integration among these muscles and the peripheral and central nervous systems is required for normal swallowing to occur.
Each phase of swallowing is under complex neurologic control, with both voluntary (somatic) and involuntary (autonomic) input into the process.401, 402, 403 Cranial nerves (CNs) that play a major role in prehension and swallowing include the trigeminal (CN V), facial (CN VII), glossopharyngeal (CN IX), vagus (CN X), spinal accessory (CN XI), and hypoglossal (CN XII). The activity of these six CNs is mediated centrally via the medulla oblongata of the brainstem, where a network of sensory and motor nuclei and interneurons form the swallowing center. In addition to the brainstem, the cortical and subcortical regions of the brain play an integral part in mediating the swallow, especially the oral phase.
Both sensory and motor function play a role in the uptake of feed, as well as throughout swallowing.403 With regard to the uptake of feed, sensory input from the olfactory and optic nerves is important in providing smell and sight while the trigeminal nerve provides sensation to the rostral oral mucosa and lips. Once feed is in the oral cavity and oropharynx, peripheral receptors stimulate the trigeminal, facial, and glossopharyngeal nerves providing sensory input to the brainstem, which leads to activation of the swallowing reflex. Patterned discharges of excitation and inhibition are sent from neurons in the swallowing center to motor nuclei of the cranial nerves, resulting in swallowing. Swallowing can also be initiated by stimulation of cortical neurons, and often cortical and peripheral inputs act together in eliciting swallowing. Once swallowing is initiated, continuing sensory input modulates motor activity.
A variety of problems, either congenital or acquired, can disrupt swallowing.404, 405, 406 Problems causing dysphagia may be grouped by the anatomic location as oral, pharyngeal, or esophageal. Also, general mechanisms of dysphagia may be classified as either morphologic or functional. Morphologic causes include pain and inflammatory conditions, as well as mechanical problems, such as anatomic abnormalities and obstruction. Functional causes include a variety of neurologic, neuromuscular, and muscular disorders that can interfere with the highly regulated activity of swallowing. Functional disorders may affect any phase in the process of eating and drinking, but most often affect the oral and pharyngeal phases of swallowing. Some disorders, such as guttural pouch empyema, have the potential to cause both physical obstruction and neurologic impairment.
Conditions Associated with Dysphagia
Morphologic Causes
Several morphologic causes of dysphagia have been identified in horses (Box 7.8 ). Anatomic abnormalities that have been recognized in association with dysphagia include a cleft palate, wry nose, branchial remnant cysts, and subepiglottic cysts.405, 406, 407, 408 Pain and inflammation from a number of underlying causes often contribute to dysphagia. Dental problems can cause both pain and mechanical problems with mastication.409, 410 Some painful dental conditions include ulcerations from points or hooks, periodontitis, infundibular caries, fractured teeth, and equine odontoclastic tooth resorption and hypercementosis syndrome (EOTRH). Problems such as retained deciduous premolars, as well as a wave, step, or shear mouth, and occasionally severe malocclusions may make it mechanically difficult to chew effectively. Oral ulceration causing discomfort may be seen with plant awns, vesicular stomatitis virus, cantharidin toxicosis, and exposure to caustic substances or some medications, such as phenylbutazone or enrofloxacin.404, 405, 406 Dysphagia has been reported in a foal with equine herpesvirus 2–associated oral and esophageal ulceration.411 Other painful conditions include epiglottitis, foreign bodies, temporomandibular joint disease, mandibular or maxillary fractures, and other traumatic injuries, such as hyoid bone injury or tongue lacerations.405, 406, 412, 413 Although uncommon, septic sialoadenitis involving either the parotid or mandibular salivary gland can be a significant cause of pain, inappetance, and dysphagia.414
BOX 7.8. Morphologic Causes of Dysphagia.
- Anatomic abnormalities
- Cleft palate
- Wry nose, severe malocclusions
- Branchial remnant cysts
- Subepiglottic cysts
- Dental conditions
- Points, hooks
- Retained deciduous premolars
- Wave, step, or shear mouth
- Periodontitis
- Fractured teeth
- Equine odontoclastic tooth resorption and hypercementosis
- Other
Temporomandibular joint disease
- Oral ulceration
- Dental problems
- Vesicular stomatitis virus
- Plant awns
- Caustic substances, medications
- Cantharidin toxicosis
- Trauma
- Fractures of skull, mandible, or maxilla
- Tongue lacerations
Foreign bodies
- Neoplasia
- Oral, esophageal—squamous cell carcinoma, dental
Epiglottitis
- Retropharyngeal masses causing compression
- Abscesses
- Guttural pouch enlargement
- Empyema, mycosis
- Tympany
- Hemorrhage associated with rupture of the longus capitus
- Neoplasia
- Septic sialoadenitis
- Esophageal disorders
- Obstruction (choke)
- Esophagitis
- Perforation, fistula
- Stricture
- Diverticula, megaesophagus
- Congenital abnormalities—cysts
- Neoplasia
Snakebite
The most common obstructive problem causing dysphagia is esophageal obstruction, or choke.404, 405, 406, 415 This most often results from a feed impaction, although esophageal foreign bodies may occur as well. In addition to obstruction, other esophageal conditions that can result in dysphagia include esophagitis, esophageal rupture, fistula, strictures, diverticula, megaesophagus, and congenital disorders, such as esophageal cysts.415, 416 Esophageal tumors are uncommon, but primary esophageal squamous cell carcinoma has been reported.415, 417, 418, 419 Both squamous cell carcinoma and leiomyosarcoma have been seen to extend from the stomach into the esophagus. Idiopathic muscular hypertrophy of the distal esophagus has been recognized but in many cases is of no clinical significance.420
External compression of the pharynx or esophagus may also result in obstruction. Pharyngeal collapse may be seen with enlargement of the retropharyngeal lymph nodes, most often due to abscessation associated with Streptococcus equi subspecies equi, as well as with severe guttural pouch disease, including empyema, mycosis, or tympany, and occasionally neoplasia.405, 406, 421, 422, 423, 424 Pharyngeal collapse is often associated with dyspnea, as well as dysphagia. Oral neoplasms of either dental or nondental origin are relatively uncommon but can cause pain or act as a space-occupying mass resulting in dysphagia.405, 406, 425, 426 A number of dental tumors, as well as osteogenic tumors of nondental origin, such as osteomas, ossifying fibromas, and osteochondromas, have been described. Squamous cell carcinoma is the most common soft tissue neoplasm reported in the oral cavity, but others have been identified, including melanoma and lymphosarcoma. Adenocarcinoma of the tongue and epiglottis has been reported as a cause of severe dysphagia in a horse.427 Snakebite may cause severe edema of the head and dysphagia.347, 428
Functional Causes
Guttural pouch diseases affecting the cranial nerves within the pouch are one of the more common functional problems causing dysphagia404, 405, 406, 424, 429, 430, 431 (Box 7.9 ). The dorsolateral wall of the medial compartment of the guttural pouch contains several nerves, including the glossopharyngeal (IX); branches of the vagus (X), spinal accessory (XI), and hypoglossal nerves (XII); and the cranial cervical ganglion. In addition, a small portion of the facial nerve (VII) is located in the dorsal wall of the lateral compartment. Thus guttural pouch disease can result in pharyngeal dysfunction, dysphagia, and occasionally other neurologic signs such as laryngeal paralysis, Horner’s syndrome, and facial paralysis.404, 405, 424, 429, 430, 431, 406 Important diseases of the guttural pouch causing dysphagia include guttural pouch empyema and guttural pouch mycosis. Skull trauma resulting in rupture of the longus capitus muscle at the site of attachment to the basisphenoid bone may result in significant hemorrhage into the pouch, potentially affecting the cranial nerves.429 Occasionally flushing of the guttural pouch and surgical correction of guttural pouch tympany results in damage to the cranial nerves coursing through the pouch with consequent neurologic dysfunction.405, 406, 431 Although temporohyoid osteoarthropathy most often results in deficits of the facial (CN VII) and vestibulocochlear (CN VIII) nerves, occasionally deficits of the glossopharyngeal (CN IX) and vagus (X) nerves occur, resulting in dysphagia.432, 433 Lead toxicity may result in a peripheral neuropathy with dysphagia.434 Some horses with polyneuritis equi, an inflammatory condition affecting primarily the cauda equina, will have involvement of the cranial nerves and present with cranial nerve signs including dysphagia, occasionally even before the development of signs related to cauda equina dysfunction.435, 436
BOX 7.9. Functional Causes of Dysphagia.
Peripheral Nerve Disease
- Guttural pouch disease affecting the cranial nerves
- Empyema, mycosis
- Tympany
- Hemorrhage associated with rupture of the longus capitus
- Neoplasia
Temporohyoid osteoarthropathy
Lead poisoning
Polyneuritis equi
Central Neurologic Disease
- Viral encephalitis/encephalomyelitis
- Rabies
- Eastern, western, and Venezuelan encephalitis (EEE, WEE, VEE)
- West Nile virus (WNV)
- Equine herpes myeloencephalopathy
Equine protozoal myeloencephalitis
- Toxicity
- Nigropallidal encephalomalacia
- Leukoencephalomalacia
Trauma
Tetanus
Migrating parasites
- Equine grass sickness
- Affects central, peripheral, and enteric nervous systems
Pharyngeal dysfunction in neonatal foals
Neuromuscular Disease
Botulism
Tetanus
- Organophosphate toxicity
- Primarily acute cholinergic signs, dysphagia rare
Tick paralysis
Muscle Disease
Nutritional myodegeneration (white muscle disease)
Hyperkalemic periodic paralysis
Central neurologic diseases and neuromuscular or muscle disorders that can result in dysphagia often have other concurrent signs in addition to problems eating. Some important central neurologic diseases that can be associated with dysphagia include viral encephalitis (rabies, eastern and western encephalitis, West Nile virus, equine herpesvirus), equine protozoal myeloencephalitis, toxic neuropathies (leukoencephalomalacia and nigropallidal encephalomalacia), tetanus, cerebral trauma, and rarely migrating parasites.404, 405, 406, 437, 438, 439, 440, 441 Dysphagia is a relatively common finding in horses affected with rabies.404 Horses with nigropallidal encephalomalacia have lesions of the basal ganglia and can swallow but are unable to prehend feed as they lack coordination of the lips and tongue. Tetanus toxin acts at several sites within the nervous system, including the inhibitory interneurons in the central nervous system and the neuromuscular junction, causing muscle paralysis.442 It typically results in increased tonus of the masticatory muscles, known as trismus, and dysphagia. The mouth may be difficult to open, thus the term lockjaw. Some cases of marked hypocalcemia may result in tetany and dysphagia. A syndrome of dysphagia related to presumed pharyngeal dysfunction has been reported in neonatal foals.443 Although the pathogenesis is not fully understood, some affected foals were premature and/or diagnosed with neonatal encephalopathy. Other central neurologic problems that may potentially cause dysphagia include bacterial or fungal meningitis and intracranial masses.
Neuromuscular problems that can result in dysphagia include organophosphate toxicity, tick paralysis, botulism, and to some extent tetanus, which also has a central component.404, 405, 406, 442, 444, 445 One of the early signs of botulism can be that the horse eats slowly. Equine grass sickness (EGS), also referred to as equine dysautonomia, is a largely fatal neurodegenerative disease affecting the peripheral, central, and enteric nervous systems of grazing equids.446, 447, 448 The gastrointestinal tract is the most severely affected body system, with the predominant clinical signs being colic, weight loss, and dysphagia. Although the disease primarily occurs within Great Britain, cases have been identified in regions of mainland Europe and rarely in other locations including the United States. A possible link to Clostridium botulinum types C and D has been proposed, but the cause of EGS remains unclear.446
The primary muscle disease resulting in dysphagia is nutritional myodegeneration (nutritional muscular dystrophy, white muscle disease) associated with selenium deficiency.449, 450, 451 Although reported more frequently in foals, nutritional myodegeneration can affect horses of any age. Although multiple muscles can be affected, myodegeneration may be limited to the masseter muscle and/or tongue in some cases, making dysphagia the predominant sign. Foals affected with HYPP may have dysphagia, particularly when they are homozygous.302
Diagnostic Approach to Dysphagia
The evaluation of dysphagia focuses on determining whether morphologic and/or functional abnormalities are present. The initial assessment should include consideration of the signalment and history and a thorough physical examination, including observation of the horse eating. Additional tests such as a neurologic examination and ancillary diagnostic aids are often indicated.
Signalment and History
Some age and breed associations have been associated with dysphagia. Anatomic abnormalities such as a cleft palate or wry nose are generally apparent at an early age. Some dental disorders can be age related.409, 410 The deciduous premolars are lost between 2 and 5 years of age and may be retained as caps. Older horses are more are more likely to have a severe wave mouth, missing teeth, or EOTRH and can be at increased risk of esophageal obstruction. Dysphagia associated with pharyngeal dysfunction may be seen in neonatal foals, sometimes in association with prematurity or neonatal encephalopathy.443 In foals with Quarter Horse breeding, HYPP can be a cause of dysphagia.302 Friesian horses have an increased prevalence of esophageal disorders, specifically megaesophagus.452
A thorough general history is important in the evaluation of dysphagia. The history may help to differentiate dysphagia from a decrease in appetite. The diet, dental history, and any recent disease and treatment should be determined. A history of dropping feed during mastication, or quidding, may suggest dental disease. The potential for exposure to toxins such as lead, organophosphates, or yellow star thistle and Russian knapweed should be assessed. The vaccination status of the horse should be established, especially with regard to tetanus, West Nile virus, eastern equine encephalitis virus (EEE), western equine encephalitis virus (WEE), equine herpesvirus, and rabies. It should be determined whether other animals on the farm are having problems as certain conditions, such as strangles and botulism, may affect more than one individual. Trauma is often associated with a history of an acute onset of dysphagia. In some cases there is a known history of a traumatic event such as a fall or kick, but in other cases the cause of the trauma in unknown. Occasionally trauma may occur during the administration of medication or passage of a nasogastric tube. In addition, a history of flushing the guttural pouch can be of significance as the procedure may rarely cause inflammation and tissue damage.
Physical Examination
Biosecurity procedures should be followed during the initial assessment of horses with dysphagia as rabies is a potential differential. Ideally, veterinarians and assistants should have an adequate rabies antibody titer.
A complete physical examination should be performed. The presence of a fever suggests an infectious or inflammatory process. Observing the horse while eating, and if possible drinking, can be valuable. Some horses can continue to drink despite difficulty swallowing feed whereas others cannot. Offering feed may help distinguish dysphagia from anorexia. Depending on the underlying cause, many dysphagic horses are hungry and will attempt to eat. Some horses, such as those with retropharyngeal abscessation due to strangles, may have both dysphagia and a decreased appetite. Observation of eating can also help determine the phase of eating affected, which can help in prioritizing differentials. In cases where prehension is primarily affected, nigropallidal encephalomalacia should be considered in areas where yellow star thistle or Russian knapweed is present. Dropping feed while chewing is consistent with abnormal mastication, which is often associated with dental disease. The regurgitation of feed through the nares while eating is generally associated with pharyngeal or esophageal disorders rather than oral cavity problems. Those horses with feed in the nares are at increased risk of aspiration of feed material, and coughing or increased respiratory rate and effort may be seen. Careful auscultation of the thorax, including a rebreathing evaluation, should be performed.
A thorough oral examination using a mouth speculum is usually indicated. The teeth should be examined for the presence of problems such as retained deciduous caps, sharp points or hooks, wave mouth or step mouth, missing teeth, diastema, periodontitis, infundibular caries, dental fractures, and EOTRH.409, 410 The oral cavity and tongue should be examined for the presence of ulcers or other abnormalities, such as lacerations, foreign bodies, and masses. Foreign bodies may become wedged between the molars or under the tongue, and wire foreign bodies or awns may become embedded in the tissue. The submandibular and throat latch area should be examined for evidence of lymphadenopathy or guttural pouch enlargement. Occasionally an esophageal obstruction can be palpated in the neck. An esophageal perforation should be considered if there is heat or swelling in the neck. If ptyalism is observed without dysphagia, ingestion of clover that is contaminated with Rhizoctonia leguminicola, a fungus that produces the mycotoxin slaframine, should be considered.453 Excess salivation stops once the horse is no longer exposed to the toxin.
Nasogastric Intubation
A complete esophageal obstruction can be ruled out if a nasogastric tube can be passed into the stomach. Common sites for esophageal obstruction to occur are the proximal esophagus just distal to the larynx, at the thoracic inlet, and at the base of the heart.
Neurologic Examination
A complete neurologic examination is often indicated in the evaluation of dysphagia, especially when morphologic causes have been ruled out. The neurologic examination helps establish a neuroanatomic localization. In general, horses with dysphagia due to peripheral neurologic disease may exhibit only dysphagia, whereas those with central neurologic or neuromuscular disease tend to have additional deficits. For example, the presence of generalized weakness in association with dysphagia often suggests a neuromuscular problem, such as botulism or organophosphate toxicity.404, 405, 406, 444, 445 Equine motor neuron disease results in generalized weakness and muscle atrophy; however, affected horses do not exhibit cranial nerve deficits and are not dysphagic.454 Tetanus causes spastic paralysis and can present with a variety of signs, including dysphagia, hyperesthesia, and prolapse of third eyelid, in addition to a stiff gait, which may progress to recumbency.442 Spinal ataxia associated with dysphagia suggests a diffuse or multifocal disease affecting the spinal cord and brainstem, such as equine protozoal myelitis, rabies, equine herpes myeloencephalopathy, or a migrating parasite.437, 438, 439, 440 Viral encephalitis should be considered in dysphagic horses with mentation changes, especially if the horse is febrile. Horses with polyneuritis equi occasionally have cranial nerve involvement in addition to the classic signs of cauda equina involvement, which include slowly progressive paralysis of the tail, rectum, anus, and bladder.435, 436 Affected horses may also have ataxia and weakness of the hindlimbs.
Ancillary Diagnostic Aids
Clinical Pathology/Laboratory Testing
A CBC, fibrinogen, and serum chemistry may be helpful in documenting an inflammatory process. In cases of suspected nutritional myodegeneration, measurement of selenium and glutathione peroxidase can help in evaluating the selenium status of the patient.
Further diagnostic tests are often indicated in cases of suspected neurologic disease. These may include an evaluation of CSF for cytologic and biochemical abnormalities, as well as specific testing for antibodies to Sarcocystis neurona or Neospora hughsei. 440 In cases of suspected West Nile virus infection, the immunoglobulin M (IgM) capture ELISA can be used. Cranial nerve involvement is infrequent with equine herpesvirus myeloencephalopathy, but testing by PCR or virus isolation on nasal swabs and/or blood should be considered if appropriate.438, 439 Botulism can be a difficult disease to diagnose, and diagnosis is often based on the history and clinical signs along with the exclusion of other diseases.444, 445 Several tests are available for definitive diagnosis, including testing for toxin by mouse bioassay or PCR in feed, manure, gastrointestinal contents, serum, or wound exudate. The antemortem diagnosis of polyneuritis equi can be difficult, but a biopsy of skeletal muscle innervated by nerves arising from the cauda equina may demonstrate extensive cellular infiltrates in intramuscular nerve fibers.435, 436
Endoscopy
Endoscopic examination allows visualization of the nasal passageways, nasopharynx, pharynx, larynx, and guttural pouches. Pharyngeal and laryngeal function can be assessed, although the use of sedation should be taken into account as it may affect function. Endoscopic examination of the guttural pouch can identify the presence of empyema or guttural pouch mycosis, as well as other less common conditions such as trauma-related hemorrhage and neoplasia. The stylohyoid bone should be carefully evaluated. In temporohyoid osteoarthropathy there is often osseous proliferation primarily affecting the proximal portion of the stylohyoid.432, 433 Depending on the length of the endoscope, the esophagus may also be evaluated for evidence of esophagitis, obstruction, strictures, diverticula, perforations, or other abnormalities.
Diagnostic Imaging
Diagnostic imaging can provide useful information in the evaluation of dysphagia. Skull radiographs, CT, or MRI can help in the diagnosis of fractures, dental disease, temporohyoid osteoarthropathy, temporomandibular joint disease, guttural pouch disease, and retropharyngeal masses.405, 406, 409, 410, 412, 421 Radiopaque foreign bodies, such as a wire in the oral cavity, pharynx, or esophagus, can be identified on radiographs.413 In cases of esophageal perforation, extraluminal radiolucencies consistent with subcutaneous air may be visualized. Contrast radiography with the use of barium sulfate can be useful in the diagnosis of certain esophageal disorders such as strictures, diverticula, or megaesophagus.415 Due to the risk of aspiration in horses with dysphagia, radiographs and ultrasound of the thorax are often indicated, especially in horses with abnormal thoracic auscultation, nasal discharge, cough, increased respiratory rate, or increased respiratory effort as evidenced by increased abdominal effort or nasal flare. Ultrasound may also be useful in the detection of foreign bodies and in evaluation of swelling in the throatlatch region.
Colic
Colic is defined as the manifestation of abdominal pain. In horses colic is a serious medical and economic problem worldwide. In the United States the annual incidence of equine colic has been reported to be anywhere between 3.5% and 26%.455, 456, 457, 458 A study performed by the National Animal Health Monitoring System in 1998 assessed the annual incidence of colic to be 4.2% with an estimated total cost of approximately $115 million.455 In this study only 1.4% of colic episodes resulted in surgery, but the overall fatality rate for all colic was 11%. When evaluating general causes of mortality in horses over 6 months of age, including euthanasia, colic was found to be responsible for 22.2% of equine deaths in 1998 and 15.2% in 2005.459 This makes colic among the most common causes of death in horses along with old age (24.8% and 30.4% of deaths) and injury (12.7% and 16.0% of deaths). Even in less serious cases, colic can be associated with a significant loss of use. In a study of working horses in Egypt, the prevalence of colic was found to be 54.6%.460 These numbers illustrate the enormous impact that colic has on the equine population.
Horses express clinical signs of colic in a variety of ways.461, 462 Often the first sign recognized by owners is inappetence, but other early signs may include general restlessness and extended periods of lying down. More overt signs, such as kicking or biting at the abdomen, pawing, and stretching out as if to urinate, may also be observed. Bruxism is occasionally seen. As the level of discomfort increases, horses may repeatedly get up and down and try to roll, sometimes violently.461 In some cases, the horse may acutely have much pain. The level of stoicism varies widely among individual horses, and the severity of signs does not always correlate to the severity of the lesion.
Mechanisms of Colic
The precise pathophysiologic mechanisms involved in cases of colic can vary because of the myriad causes for colic in the horse. Most cases of colic, sometimes referred to as “true” colics, are gastrointestinal in origin. However, disorders of other body systems, such as cholelithiasis and uterine torsion, may also manifest as colic. True colic can be classified on the basis of small intestinal versus large intestinal disorders, physical versus functional disorders, obstructive versus nonobstructive lesions, and strangulating versus nonstrangulating lesions. In all of these colic classifications, the simplest basic etiologies for damage to the gastrointestinal tract are inflammation and ischemia.462, 463, 464 Gastrointestinal distention, ileus, mesenteric tension, and endotoxemia also play a role in the development of disease in many cases.
Ischemia of the intestine can result from a strangulating lesion or even from a simple obstruction. Strangulating lesions cause acute direct occlusion of vessels leading to rapid tissue hypoxia, ischemia, and ultimately necrosis. With obstructions significant pressure within the distended intestine may lead to venous collapse, which over time may result in ischemia as the intestinal vasculature becomes increasingly compromised.462, 463, 464 The mucosa is the layer most sensitive to hypoxia because of its high metabolic activity. Damage to the mucosa can be assessed on a scale of Grade I through Grade V, with Grade V being the most severe.462 The villous tips are especially sensitive to ischemia, and damage typically begins there. Crypt cells are affected later as the ischemia becomes more complete and longer in duration. In the equine colon complete ischemia leads to necrosis and detachment of surface epithelial cells and most likely to capillary thrombosis and occlusion.462 Smooth muscle is less sensitive to hypoxia, and therefore it is destruction of the mucosa that is the main factor leading to the pathologic changes associated with colic.
Prolonged intestinal distention proximal to an obstructive lesion can eventually lead to tissue ischemia. Distention also results in edema and additional secretion of fluid into the lumen of the gut as venous collapse occurs.462, 463, 464 As veins are occluded, hydrostatic pressure within the capillaries increases, causing increased filtration. Lymphatic drainage is also often impaired, and the resultant excess fluid becomes edema and secreted intestinal fluid. Distention is one of the main causes of pain associated with colic, as stretch pain receptors are triggered within the wall of the intestine.462
Inflammation plays a major role in almost every type of colic and often occurs secondary to ischemia. The inflammatory response is generally meant to protect the intestine against long-term damage. The pathophysiology of inflammation is complex and beyond the scope of this section, but the basic process in the gastrointestinal tract is similar to that of other body systems. Almost all cells in the intestine can play a role in the development of inflammation by either cytokine production or cell activation. Some specific cell types involved in the initiation of intestinal inflammation include mucosal cells, endothelial cells, fibroblasts, neutrophils, macrophages, neurons, eosinophils, and mast cells.463 Cytokines, growth factors, and adhesion molecules produced by these cells are important in the initiation of inflammation. Some important cytokines include IL-1, TNF-α, platelet activating factor, complement, interferon, and histamine.463
Endothelial cells are stimulated by ischemia or macrophage cytokines to attract neutrophils, which subsequently migrate to the affected tissue facilitated by adhesion molecules. Vascular permeability is also increased, resulting in the formation of edema and facilitating the migration of inflammatory cells. Neurons, fibroblasts, and muscle cells detect and release other cytokines, leading to the activation of many effector cell types.463, 464
Eventually the intestinal barrier is compromised to the extent that endotoxin begins to leak into systemic circulation. Endotoxemia (discussed in detail in Chapter 12) is most common with colitis and to a lesser extent with strangulating lesions. The response to endotoxin is complex, involving a massive release of cytokines and mediators that results in systemic inflammation and fever. Classical endotoxemia has more recently been described as systemic inflammatory response syndrome (SIRS), because it likely involves more than just endotoxin as an initiating factor.463 The result is hemodynamic responses that include both vasodilation and vasoconstriction, platelet aggregation, and eventual development of a hypercoagulable state and consumptive coagulopathy.462, 463
Finally, ileus should be addressed as an important component of the pathophysiology of colic. Ischemic bowel has decreased motility, although bowel more proximal to the lesion tends to have an increase in motility until it becomes so distended that it can no longer contract normally. Postoperative ileus appears to involve both dopaminergic and adrenergic stimulation. It has also been suggested that prostaglandins E1 and E2, as well as nitric oxide, play a role in disrupting intestinal motility patterns.462
Owners often question why horses have colic, and several studies have attempted to identify risk factors for colic with varying results.465, 466, 467, 468, 469, 470, 471, 472, 473 Some factors that may increase the risk of colic in horses include stall confinement, feeding of excessive concentrate, infrequent large meals, recent feed changes, decreased water consumption or lack of access to water even during turnout, feeding of Coastal Bermuda grass hay, and off-the-ground feeding. Other risk factors include inadequate deworming or dentistry, exposure to sand, transport, changes in activity, previous colic episodes, and the use of nonsteroidal antiinflammatory drugs (NSAIDs). Colic has also been linked to stereotypic behaviors such as crib-biting, which has been shown in some studies to be associated with an increased risk of both colic in general and epiploic foramen entrapment.470, 474, 475, 476 It is important to remember that colic can occur regardless of management practices.
Conditions Associated with Colic
Colic encompasses a wide variety of conditions, both gastrointestinal and nongastrointestinal in origin (Table 7.4 ). The actual incidence of specific disorders causing colic in the general equine population is not known, partially because a definitive diagnosis is not always established. In general, most colics are gastrointestinal in origin, with simple gas or spasmodic colics being most common.456, 477, 478, 479 Other common causes include large colon impaction and large colon displacement. In one study of 604 horses that were referred for evaluation of colic, 327 cases were medical (54.1%) and 277 cases were surgical (45.9%).479 Of the medical cases, the most common problems were large colon impaction (39.6%) and spasmodic colic (20.8%). Of the surgical cases, the most common problems were large colon displacement (24.5%), followed by large colon torsion (14.3%) and strangulating lipoma (13.5%).479
Table 7.4.
Gastrointestinal Causes of Colic
| Gas and spasmodic | |
| Impactions Feed Sand (primarily pelvic flexure, large colon) Other intraluminal obstructions |
Pelvic flexure Large colon Cecum Small colon Ileal Gastric Meconium impaction (foals) Enteroliths Fecaliths Foreign body |
| Large colon displacements | Right dorsal displacement Left dorsal displacement (nephrosplenic entrapment) Other |
| Large colon torsion and volvulus Small intestinal volvulus |
|
| Strangulating lipoma | |
| Entrapment of small intestine | Epiploic foramen Mesenteric rent |
| Intussusception | |
| Ulceration | Gastric Right dorsal colitis |
| Enteritis/colitis Inflammatory bowel disease |
Duodenitis/proximal jejunitis (anterior enteritis) Colitis |
| Parasites | Ascarid impactions Tapeworms Strongylus vulgaris Cyathostomes |
| Herniation | Inguinal Umbilical Diaphragmatic |
| Peritonitis Abdominal abscessation Hemoperitoneum |
|
| Toxins | Cantharidin Monensin Other |
| Other | Ileus Overo lethal white syndrome Congenital anomalies Neoplasia |
Disorders of several body systems other than the gastrointestinal tract can present with signs of colic.480, 481, 482, 483, 484 Causes of colic involving the reproductive tract include uterine torsion or tears in mares and testicular torsion in stallions. Occasionally ovarian activity may cause transient colic in mares. Some other potential nongastrointestinal causes of colic include cholelithiasis, liver lobe torsion, urolithiasis, and pheochromocytoma.
Diagnostic Approach to Colic
Colic can be caused by a number of different conditions. Several other clinical problems may be confused with colic, such as pleuritis, exertional rhabdomyolysis, laminitis, hyperkalemic periodic paralysis, renal disease, and even neurologic abnormalities.485 When a horse presents with colic, it is important to determine that the horse is exhibiting signs of abdominal pain and then arrive at a specific diagnosis if possible. In some cases the evaluation of a horse with colic may be straightforward, but other cases may be more challenging. Fortunately, although a specific diagnosis may not be made, many cases of colic either resolve spontaneously or with minimal intervention. However, in some horses, the condition can be life threatening, and a delay in surgical exploration can result in increased mortality. Thus the initial goal in the assessment of a horse with colic is often to determine whether the case is an uncomplicated one, such as a gas or spasmodic colic, rather than one requiring either extensive medical management or surgical exploration. Signalment, history, physical examination, clinicopathologic data, imaging findings, and endoscopy may all contribute to the evaluation of a patient with colic.485, 486 A number of studies have focused on identifying means to aid in the early classification of colic cases as medical or surgical and to determine a prognosis. Most of these studies have assessed a number of physical examination and laboratory variables.486, 487, 488, 489, 490, 491, 492
Signalment and History
The signalment of the horse can provide information that may increase the degree of suspicion for a specific cause of colic. Weanling-age foals are more prone to ascarid impactions, whereas younger foals are at higher risk for small intestinal volvulus or intussusception.485, 486, 493, 494 Older horses have a far greater likelihood of developing strangulating lipomas.462, 477
Colic may affect horses of any breed, but some breed predispositions have been recognized. An increased incidence of colic has been recognized in Arabians and Thoroughbreds.455, 457, 485, 495 Arabians are specifically predisposed to ileal impaction, small colon impaction, and enterolith formation. Miniature horses, especially when young, are prone to small colon impactions and fecaliths.462, 485 Standardbred, Tennessee Walking Horse, and Warmblood stallions have a higher risk of developing inguinal hernias than stallions of other breeds, which is felt to be related to increased size of the inguinal rings.462, 485 Overo lethal white syndrome is a fatal, recessive genetic condition that causes ileocolonic aganglionosis in neonatal foals.496 Affected foals are white or predominantly white with small dark markings and generally develop signs of colic shortly after birth. The defective gene is found predominantly in American Paint Horses but has also been found in horses of other breeds with overo coloring, such as American Miniature Horses and half-Arabians.
Gender is also important to consider in cases of colic. In addition to inguinal hernias, testicular torsion can be a cause of colic in stallions.462 Postpartum mares are predisposed to torsion of the large colon, whereas colic in a late-gestation mare may be associated with normal parturition or dystocia.462, 485 Pregnant mares exhibit signs of colic with uterine torsion.480, 485 Nonpregnant mares can have mild transient signs of colic associated with ovarian activity.
The history of the patient can provide vital clues as to the severity and possible etiology of the colic.485, 486 With regard to the current episode, it is important to determine the duration and severity of pain, recent defecation and character of feces, appetite, previous treatment, and response to treatment. Previous history of colic, including colic surgery, history of other surgeries, recent management changes, geographic area, breeding history, and pregnancy status, are all important components in the medical history of a colicky horse. Additionally, specific details as to feed, access to sand, water source, deworming, dentistry, history of medications (e.g., NSAIDs, antibiotics), activity level, and stereotypic behavior such as crib-biting/windsucking may also provide valuable diagnostic information.
Physical Examination
A thorough physical examination is essential to the evaluation of colic. It is important to assess the degree of pain, and in some cases the horse may be so uncomfortable initially that sedation will be necessary for safe completion of the examination. If possible, heart rate, respiratory rate, and auscultation of gut sounds should be evaluated before sedation because these can change significantly in response to sedation.497, 498 Because rectal temperature can also be significantly decreased after rectal palpation, it is advisable to obtain the body temperature before palpation.461 A finding of pyrexia may increase suspicion of colitis, enteritis, peritonitis, or intraabdominal abscessation in the patient, although previous administration of an NSAID may mask this clinical finding.462, 485, 486 Tachycardia can be an indicator of pain, hypovolemia, tachyarrhythmia, or endotoxemia, and rates of greater than 80 beats per minute generally indicate serious disease.462, 485, 486 During auscultation the patient should be evaluated for the presence of any cardiac abnormalities that would put the horse at increased risk under sedation. Respiratory rate may be increased in horses with colic as a response to some combination of pain, fever, and/or metabolic acidosis. Mucous membranes and capillary refill time (CRT) give a rough assessment of cardiovascular status and peripheral perfusion. Normal mucous membranes should be pink and moist with a CRT of less than 2 seconds. Tacky mucous membranes are generally observed with dehydration of at least 5% to 7%.485, 486 Prolonged CRT and either grayish or dark red mucous membranes indicate impaired cardiovascular status and poor perfusion. With poor perfusion, there may also be cool extremities and reduced jugular fill. Significant changes in mucous membrane color, often with a dark “toxic” line adjacent to the teeth, may accompany endotoxemia, which is common in gastrointestinal disease, particularly in horses with colitis, proximal enteritis, and strangulating lesions. Icterus is relatively common in horses that have been off feed for more than 48 hours due to equine fasting hyperbilirubinemia. However, icterus may also be seen with hepatobiliary disease or hemolysis. Other important considerations during the physical examination include assessment of hydration status, attitude, abdominal distention, and the presence of injuries that indicate self-trauma, which often reflects the degree of pain. As previously mentioned, in stallions careful palpation of the inguinal region must be performed to identify an inguinal hernia or testicular torsion. Severe abdominal distention may markedly increase the respiratory effort and eventually lead to respiratory distress. Auscultation of abdominal quadrants (left dorsal, left ventral, right dorsal, right ventral) can give an estimation of gastrointestinal motility.485, 486 Assessing the abdomen for pings can help to localize gas. Auscultation of the most ventral part of the abdomen should also be performed to assess for the presence of sand.499 Although not always present, a characteristic “waves on the beach” sound can sometimes be heard, indicating the presence of sand in the colon. If feces can be collected from the horse, it can be useful to evaluate for the presence of sand. A simple way to do this is to mix a few fecal balls with warm water in a rectal sleeve and hang the sleeve with the fingers down. After about 5 minutes any sand in the feces should sediment into the ends of the glove fingers.485
Additional diagnostics for horses with colic include rectal palpation, nasogastric intubation, and, in many cases, abdominocentesis. Adequate restraint is important for these procedures because not only is the veterinarian at increased risk of injury with an uncooperative horse, but the horse also is at increased risk of rectal tears, significant epistaxis, and abdominocentesis complications such as enterocentesis or contamination of the collection site. The most appropriate means of restraint is dependent on the individual patient and may include sedation and/or the application of a nose twitch. When selecting the method of restraint the safety of the examiner and the horse must be considered, while at the same time it is important to not further compromise an unstable patient or dramatically affect assessment of the horse’s level of pain. Use of xylazine hydrochloride intravenously at 0.2 to 0.5 mg/kg (typically 100–250 mg for a 500-kg horse) is often adequate, but butorphanol tartrate at 0.01 to 0.08 mg/kg intravenously can be added when necessary.461 Use of detomidine is usually discouraged in the initial management of colic because of its long duration of action and heightened ability to mask more significant pain that may affect determination of the horse’s treatment.465, 466 However, if indicated, detomidine can be administered at 0.01 to 0.04 mg/kg intravenously or intramuscularly.461 It is most commonly used when a longer duration of sedation is required to keep a horse quiet during transport to a referral center.
Nasogastric intubation should be performed as a part of a complete colic examination. If a horse is exhibiting significant pain or has a heart rate greater than 60 beats per minute on initial evaluation, nasogastric intubation should be performed before other diagnostic procedures because it may have both diagnostic and therapeutic value. If a horse has significant gastric distention, gastric decompression may help prevent gastric rupture and decrease pain, allowing for a more thorough physical examination. The nasogastric tube can be left in place if a significant amount of net reflux is obtained to allow for repeated decompression. If no reflux is obtained and a simple colic is suspected, oral fluids with or without electrolytes or magnesium sulfate (Epsom salt) can be administered via the tube. Although mineral oil has long been considered a part of standard colic treatment, its use is controversial. Mineral oil may be valuable as a diagnostic tool, providing an estimate of gastrointestinal transit time. In a normal horse mineral oil can be observed in the feces and on the perineum and tail in 12 to 24 hours.500 The absence of mineral oil after this period may indicate delayed gastrointestinal transit. However, mineral oil can sometimes pass around an impaction that is present, thereby falsely suggesting adequate gastrointestinal transit when in fact digesta is not moving normally.500 In addition, if a horse ultimately requires abdominal surgery, the presence of mineral oil can complicate an enterotomy.501
Palpation per rectum can be important in the assessment of colic. Even with the use of appropriate sedation, some horses remain resistant to rectal examination, placing them at increased risk for rectal tears. To potentially improve the quality and safety of palpation per rectum, intravenous administration of N-butylscopolammonium can help to decrease rectal pressure and peristalsis.502 Application of lidocaine within the rectal lumen has also been used to facilitate palpation. Although rectal palpation can provide valuable diagnostic information, the presence or absence of a particular lesion cannot always be definitively determined. For instance, although a pelvic flexure or cecal impaction may be palpable, if no impaction is palpated, it cannot be ruled out. Additionally, gas distention might be palpable in gas colic, but it may also reflect a more serious problem, such as a displacement or torsion. Rectal palpation therefore can provide evidence to support a diagnosis but is often not diagnostic in itself. Nephrosplenic entrapment may be suspected if the spleen is displaced medially or the gut is palpated between the spleen and kidney.485, 486 Another useful finding is the identification of distended small intestine. Although small intestinal distention may occasionally occur secondary to a large intestinal problem such as a displacement, it is most often an indication of small intestinal disease. Although rectal palpation cannot differentiate a surgical small intestinal problem such as a strangulating lesion from a medical problem such as proximal enteritis, small intestinal disease is almost always an indication for referral.485, 486, 503 Therefore palpable loops of distended small intestine are a significant finding on rectal examination. Rectal palpation can also be useful for identifying masses in the abdomen, which may be a cause of chronic colic.
Ancillary Diagnostic Tests
Clinical Pathology
A packed cell volume (PCV) and total protein should ideally be evaluated to assist with assessment of hydration status and possible protein loss. These values can be affected by stress, which can increase the PCV, anemia, protein loss, or hyperproteinemia, confounding the effects of dehydration.462, 504 Therefore these values must be evaluated in the context of the history and physical examination of the patient, as well as their relationship to each other. In general, the greatest negative indicator is a significantly increased PCV (i.e., greater than 65%) coupled with a significantly low plasma protein concentration (i.e., less than 4 g/dL), especially if serial values are trending toward these extremes.462, 485, 486, 504
Although further blood work usually is not performed on a standard simple colic, a CBC and serum chemistry can provide valuable information if a colic goes beyond simple treatment and requires more advanced care.504, 505 In acute colic CBC values will typically be normal or reflect stress, but in more chronic conditions nonspecific signs of inflammation such as leukocytosis and hyperfibrinogenemia can develop, as well as a possible normochromic normocytic anemia of inflammation and chronic disease. If significant colon wall compromise occurs, leukopenia with neutropenia and a left shift with toxic changes can occur as a result of endotoxemia.461, 485, 504 Thrombocytopenia may also be present with endotoxemia.
Electrolyte abnormalities, especially hypokalemia and hypocalcemia, are relatively common abnormalities observed on a chemistry panel in horses with colic.505 Anorexia, dehydration, diarrhea, and excessive nasogastric reflux can all contribute to derangement of electrolytes. In addition to calcium and potassium, sodium, chloride, magnesium, and bicarbonate can be lost with diarrhea or significant gastric reflux.472 Leakage from damaged cells may result in elevated serum phosphate. Metabolic acidosis caused by elevation of lactate, loss of bicarbonate, or both may also be observed. Hyperglycemia is relatively common in horses with acute gastrointestinal disease in association with dysregulation of glucose homeostasis. Significant elevations in glucose have been associated with a worse prognosis for hospital discharge.506 Occasionally, hepatic enzyme activities are increased, especially γ-glutamyltransferase (GGT). This most commonly occurs with right dorsal displacement of the large colon or proximal enteritis.507, 508 In a study of horses with large colon displacements, 49% of horses with right dorsal displacements had concentrations of GGT above the reference range compared with 1% of horses with left dorsal displacements.508 Dehydration often results in elevations in creatinine and serum urea nitrogen, and these parameters should be monitored, especially when NSAIDs or aminoglycoside antibiotics are used. Elevations in muscle enzymes are common in horses with colic. In a study of horses undergoing celiotomy for acute gastrointestinal pain, elevations in creatine kinase (CK) and aspartate aminotransferase (AST) activity were significantly associated with the presence of lesions resulting in intestinal ischemia.509
Abdominocentesis can be performed both in the field and in a referral setting. Most often the procedure is performed with either an 18-g, 1.5-inch needle or a teat cannula for the collection of fluid. The typical location in which the procedure is performed is to the right of midline (to avoid splenocentesis) on the most ventral point of the abdomen at least several inches caudal to the xiphoid.462, 485 However, occasionally other locations, such as on the ventral midline, will be used. Also, ultrasound of the abdomen can be used to identify pockets of fluid, but it should be remembered that fluid can often still be obtained even when a significant volume of fluid is not apparent on ultrasound. If severe gas distention or small intestinal distention is present, or if a sand enterocolitis is suspected, it may be advisable to forego abdominocentesis to avoid puncture of a distended compromised viscus or a heavy sand-filled viscus that is sitting flush with the ventral body wall. Although a practitioner may not have access to a full fluid analysis and cytology, a basic assessment of abdominal fluid can be made by visual evaluation for color and clarity, and total protein can be evaluated with a refractometer. Normal fluid should be odorless, nonturbid, and a clear to pale yellow color.462, 510, 511 Increased turbidity can indicate increased protein, increased total nucleated cell count, or both. Total protein should be less than 2.5 g/dL. A normal cell count is typically less than 5000 cells/μL, although it may be normal up to 10,000/μL.458, 475 Serosanguineous fluid usually reflects a strangulating obstruction or significant compromise to the bowel wall. With a ruptured gastrointestinal structure, the fluid is generally dark and smells of ingesta. These results should be confirmed, however, because an accidental enterocentesis will appear the same.462 If the spleen is accidentally punctured, the fluid will look like frank blood and will usually have a PCV equal to or greater than the peripheral PCV.511
Measurement of lactate concentrations in both the circulation and peritoneal fluid is useful in the assessment of colic.512, 513, 514, 515, 516 Several point-of-care analyzers are available, making measurement of lactate practical.516, 517 In general, in horses with colic, an elevation in lactate concentration in either the circulation or peritoneal fluid suggests intestinal hypoperfusion and correlates with more severe disease. Elevations in peritoneal fluid lactate, especially if higher than the simultaneously measured blood lactate, as well as increases in peritoneal fluid lactate over time, are predictors of intestinal ischemia secondary to a strangulating obstruction.512, 515, 516 There is some evidence that pony and miniature breeds presenting for gastrointestinal disease have higher blood lactate concentrations compared with large-breed horses, suggesting that different cutoff values should be used when determining the necessity of surgery and the prognosis in these breeds.518
Diagnostic Imaging
Ultrasound examination of the abdomen is often useful in the evaluation of horses with colic.519, 520, 521, 522, 523, 524, 525 Adequate transabdominal ultrasound examination is most easily performed with a 2.5- to 5-MHz curved linear transducer; however, an examination can also be performed using a transrectal probe.519, 520, 525 A protocol for fast localized abdominal sonography of horses admitted for colic (FLASH) has been developed.524 This protocol allows for rapid evaluation of specific abdominal regions of particular importance in the assessment of colic. On the left side of the horse the regions evaluated include the ventral abdomen, gastric window, splenorenal window, and left middle third of the abdomen, and on the right side the regions evaluated include the duodenal window, right middle third of the abdomen, and cranial ventral thorax.
Ultrasonography is particularly valuable in the evaluation of small intestinal distention and motility.519, 520, 522, 524 Small intestine can most reliably be found in the inguinal region or on the ventral abdomen, and it should normally be collapsed or have a very small diameter with frequent peristalsis. Small intestine can be evaluated for dilation, motility, and thickening of the intestinal wall. The presence of dilated turgid small intestinal loops is highly sensitive and specific (80% and 96.15%) for small intestinal obstruction, although enteritis cannot entirely be ruled out. An intussusception may be identified as a target-shaped lesion on cross-sectional view.519, 520, 526 Ultrasound can also be used to identify a left dorsal displacement of the large colon, also referred to as a nephrosplenic entrapment.519, 520, 527 With this condition, the spleen cannot be visualized against the body wall, or the kidney cannot be visualized on the left side because of the interference of large colon. Visualization of colonic mesenteric vasculature on the right side of the abdomen can be a predictor of right dorsal displacement of the large colon, a 180° large colon volvulus, or both, although this change is not present in all cases.528 Ultrasonographic assessment of peritoneal fluid volume and character may be useful in assessing the patient and in identifying pockets of representative fluid for abdominocentesis.519, 520 The thickness of intestinal wall should be evaluated at numerous locations, especially the right dorsal colon, which may be extraordinarily thickened with right dorsal colitis.519, 520 Investigation for abdominal masses also is often undertaken using ultrasound, coupled with rectal palpation.
A study comparing the findings of rectal examination and ultrasonographic findings in horses with colic demonstrated that ultrasonography was a more sensitive diagnostic technique than rectal examination in horses with small intestinal obstruction, left dorsal displacement of the large colon (nephrosplenic entrapment), and large colon torsion in the long axis, but was less sensitive than rectal examination in horses with impactions and most large colon displacements.521 It was concluded that although ultrasonography is advisable in evaluation of the equine acute abdomen, it should not replace traditional rectal palpation.
Radiographs of the abdomen are of limited usefulness but may be diagnostic for two problems associated with colic in the adult horse: sand accumulation and enterolithiasis.529, 530 Sand is usually visualized best in the ventral large colon. It is important to keep in mind that some sand can be visualized in many normal horses, so the presence of sand in and of itself has no diagnostic relevance. Radiographs may, however, provide some information as to the volume of sand present.504 Enteroliths represent the other main lesion identifiable by radiographs, although, depending on the location, they may be difficult to visualize. In a study of 141 horses evaluated for enterolithiasis, abdominal radiography had a sensitivity of 76.9% and a specificity of 94.4%.530 The abdomen can be more thoroughly assessed via radiography in a neonate as opposed to an adult horse, although results still cannot be considered conclusively diagnostic. Radiographs of a foal’s abdomen can identify gas distention of the stomach, small intestine, or large colon, which are nonspecific but generally pathologic findings.494, 531 Radiographs can aid in the diagnosis of meconium impaction. A radiographic pattern known as pneumatosis intestinalis, characterized by linear radiolucencies within the bowel wall, is typical for necrotizing enterocolitis.494 Excessive peritoneal fluid and pneumoperitoneum can also be identified.
Endoscopy/Other
Gastroscopy is important in cases of colic where equine gastric ulcer syndrome is suspected. Ideally the squamous and glandular regions of the stomach, as well as the pyloric antrum, should be examined.
Evaluation for parasites may be indicated for some patients. Ideally both fecal flotation and a fecal egg count should be performed. Because standard flotation and fecal egg count methods are unreliable in the diagnosis of tapeworm infestation, however, a tapeworm ELISA, which detects antibody to tapeworms, should be considered.
An underlying cause is not found in some horses with chronic colic after routine diagnostic evaluation. In these cases, an exploratory laparotomy or laparoscopy may be indicated.
Referral Indications
Referral to a secondary care center is indicated when there is a strong likelihood that the horse will require surgery or more extensive medical care is necessary. The most common basis for referral is severe or persistent pain with a lack of response to analgesics.485, 486, 503 If a horse demonstrates intractable pain or requires repeated doses of analgesics to remain comfortable, referral is warranted. Referral is also indicated when a horse has not resolved the signs of colic in 24 to 48 hours after onset, or if a 1.1-mg/kg dose of flunixin meglumine does not maintain comfort for at least 8 to 12 hours.503 A persistently elevated heart rate can be a good indicator of pain or endotoxemia and necessitates referral. Other indications of endotoxemia or impaired cardiovascular status include weak pulses, abnormal mucous membrane color (pale, hyperemic, injected, purple, or cyanotic), and delayed capillary refill time. Rectal palpation of a potentially surgical lesion or small intestinal distention, an abnormal abdominocentesis, net nasogastric reflux of greater than 2 to 3 L, or significant depression are all findings that would also support referral.485, 486, 503 In addition, significant dehydration may require extensive treatment with intravenous or oral fluids or both because dehydration has the potential to exacerbate even apparently simple colic problems and affects the patient’s systemic health. Dehydration can result in worsening of an impaction that is already present or contribute to development of an impaction when coupled with ileus and an inciting primary colic problem such as colonic displacement.
Diarrhea
Diarrhea, defined as an increase in the fluidity, frequency, or volume of feces, is a commonly encountered clinical problem in the horse. It is often the result of a primary gastrointestinal disease but may also occur as a secondary response to another disease process, such as sepsis, endotoxemia, or hepatic disease. In some cases, diarrhea may rapidly develop into a life-threatening condition.
The function of the equine gastrointestinal tract is complex and involves maintenance of normal fluid balance and digestion and absorption.532, 533, 534 As a result of dietary intake and endogenous secretions, a large volume of fluid normally enters the gastrointestinal tract, most of which is reabsorbed. In the adult horse absorption occurs predominantly in the large bowel, where a volume of water approximately equal to the total extracellular fluid volume of the animal, or about 100 L, is recovered during the course of the day. Because the large colon is the primary site of water resorption, most significant diarrheal disease in the adult horse involves the colon. In young foals, however, small intestinal disorders such as rotaviral infection also may result in diarrhea.535
A second critical function of the large bowel is that of microbial digestion of carbohydrates and, to some extent, protein or nonprotein nitrogen.532, 533, 534 Microbial fermentation of carbohydrates in the cecum and colon results primarily in the production of volatile fatty acids (VFAs), which are absorbed readily, providing up to 75% of the energy requirement of the horse. The intestinal microbiome is a complex polymicrobial ecosystem that appears to play an important role in the maintenance of health.534, 536, 537 Therefore maintaining a stable environment for the microbial population is important. In general, efficient function of the large bowel requires mechanisms that limit the rate of digesta passage, provide optimal conditions for microbial digestion, and allow for efficient transport of solutes and water.
The characteristics of normal equine feces and the frequency of defecation can vary somewhat with diet, age, and sex.538 On average, adult horses defecate approximately 8 to 10 times daily. The frequency of defecation tends to be slightly higher in stallions and foals. Generally, equine feces are tan, brown, or greenish, and, although approximately 75% water, they are well formed. An average size adult horse on a typical diet of grass hay and grain produces about 20 to 28 g of feces per kilogram of body weight per day, or about 11 to 13 kg of feces per day.538 In cases of diarrhea the amount of feces may increase up to tenfold, with some horses producing more than 200 g/kg/day, which can equate to more than 90 L of diarrhea. As a result, diarrhea can cause significant losses of electrolytes and water. Especially in cases of acute diarrhea, these losses may result in severe dehydration, electrolyte imbalances, and acid-base abnormalities. However, horses with chronic diarrhea seldom develop these abnormalities as they are often able to compensate for increased fecal losses. Protein loss may be seen with both acute and chronic diarrhea.
Mechanisms of Diarrhea
Several basic mechanisms of diarrhea have been described, and in many diarrheal diseases more than one mechanism is involved. Inflammation within the bowel often plays a central role in the pathogenesis of diarrhea and may contribute to diarrhea via multiple mechanisms. Basic mechanisms of diarrhea include the following:
-
1.
Malabsorption: Malabsorption results from a decrease in the functional absorptive surface area of the gastrointestinal tract. In the small intestine, villus atrophy, such as that seen with rotaviral enteritis, equine proliferative enteropathy (Lawsonia intracellularis), and infiltrative bowel disease, can result in malabsorption because of the loss of functional epithelium and maldigestion caused by decreased production of digestive enzymes.142, 535, 539, 540, 541, 542, 543, 544, 545, 546, 547, 548, 549, 550, 551, 552, 553, 554, 555, 556, 557, 558, 559, 560, 561, 562, 563, 564, 565, 566, 567, 568, 569, 570, 571, 572, 573, 142,535,539-577 In the colon, a number of insults can result in inflammation and disruption of absorptive cells and tight junctions, leading to decreased absorptive capacity and decreased ability to retain absorbed fluid (i.e., increased loss). Several inflammatory mediators, such as TNF-α, histamines, and prostaglandins, can contribute to the colonic inflammation. These mediators are produced primarily by inflammatory cells in the lamina propria and inhibit absorption through a variety of mechanisms.542, 543, 544, 545, 546
-
2.
Increased secretion: The increased secretion of solutes and water by the inflamed colon can contribute significantly to the development of diarrhea. Although the precise mechanisms of secretion in the equine colon are not understood fully, active secretion and passive fluid loss occur.542, 543, 544, 545, 546, 547, 548, 549 Control of active secretion is complex, involving two primary pathways: first, the activation of adenyl cyclase, resulting in an increase of intracellular cyclic adenosine monophosphate concentrations, and second, the activation of calcium channels, leading to increased intracellular calcium concentrations.547, 548 Cyclic adenosine monophosphate and calcium stimulate specific secretory activities, primarily through chloride channels. In some cases of diarrhea, bacterial enterotoxins such as those produced by certain strains of Escherichia coli and Salmonella spp. stimulate adenyl cyclase activity, thus increasing active secretion. This is true hypersecretory diarrhea. Also, a number of inflammatory mediators produced by the inflamed colon, particularly prostaglandin E2, increase intracellular concentrations of cyclic adenosine monophosphate and to some extent calcium, thereby increasing active secretion by mucosal cells.547, 548, 549 Inflammation also enhances passive fluid loss through a number of factors, such as changes in hydrostatic pressure in the colonic capillaries, mucosal damage, and loss of tight junctions. In horses with severe mucosal injury, the loss of protein can decrease vascular oncotic pressure and further potentiate fluid exchange across the endothelium.
-
3.
Decreased transit time (abnormal motility): Sufficient retention time and thorough mixing are required for digestion and absorption of nutrients and fluid to occur, and thus decreased intestinal transit time can contribute to diarrhea.532, 533, 534, 550 Primary motility disorders causing diarrhea are not well recognized, although diarrhea associated with stress or excitement may represent this phenomenon. However, it appears that secondary motility disorders are common in response to a variety of gastrointestinal problems, although the exact role that these changes in motility play in the pathogenesis of diarrhea is not entirely clear. Both inflammatory and infectious conditions may influence motility in addition to potentially altering absorption and secretion. Absorption of endotoxin and the release of inflammatory mediators, including prostaglandins, disrupts normal motility patterns.551 In general, these changes result in hypermotility and decreased intestinal transit time, although hypomotility has been recognized as well. Some progressive motility must be present for diarrhea to occur. In some cases of acute colitis, a period of ileus may occur without diarrhea. This is usually transient and diarrhea develops, although occasionally ileus may persist. With diarrheal diseases, the elimination of gastrointestinal contents is part of the normal host defense mechanism, and thus treatments specifically aimed at decreasing motility are not indicated in most cases.
-
4.
Osmotic overload: Any increase in osmotically active particles within the intestinal lumen can result in diarrhea. The increase can be associated with the administration or ingestion of osmotically active substances such as magnesium sulfate. The increase also may be associated with overloading of the intestine with carbohydrates or occasionally lipids beyond the amount that can be digested and absorbed. Therefore sudden dietary changes that result in significant shifts in gut flora and changes in fermentation or gastrointestinal diseases that result in malabsorption or maldigestion also may result in an osmotic diarrhea. In foals the loss of villous epithelial cells in the small intestine associated with disorders such as rotavirus infection and clostridiosis may lead not only to malabsorption but also to maldigestion caused by the decreased production of lactase and secondary lactose intolerance.535, 539, 552 Although uncommon, primary lactose intolerance has also been reported in foals.553 Lactose intolerance allows excess lactose to enter the large intestine, increasing the osmotic load.
-
5.
Increased hydraulic pressure from the blood to the lumen: The pressure change may result in increased fluid movement to the lumen and decreased net fluid absorption. This mechanism of diarrhea is more common in chronic conditions, such as congestive heart failure or inflammatory bowel disease. The condition may result from decreased oncotic pressure associated with hypoproteinemia, increased capillary hydrostatic pressure (as in heart failure), or decreased lymphatic drainage associated with inflammation of lymphatics and lymph nodes.
The normal equine fecal microbiota is very complex. In horses with diarrhea, the bacterial diversity of the gut microbiota may be lower than in horses with normal feces.537 This microbial imbalance, referred to as microbiome dysbiosis, may be both a result and a cause of intestinal disease and diarrhea. Characterization of the microbiome and its role in health and disease is a very active area of research.
Understanding the mechanisms of diarrhea can be helpful in directing therapy. It is important to remember that most disorders that cause diarrhea, whether infectious or noninfectious, do so through inflammatory mechanisms resulting in multiple functional alterations.
Conditions Associated with Diarrhea
Diarrhea is a common, and sometimes fatal, clinical problem of adult horses and foals. A number of specific causes for acute and chronic diarrhea have been identified (Table 7.5, Table 7.6, Table 7.7 ). In some cases, rapid dietary changes or stress can result in diarrhea that is generally mild and transient. Also, although sand ingestion is frequently associated with impactions, it may also result in diarrhea due to mechanical irritation to the gastrointestinal tract.
Table 7.5.
Differential Diagnoses for Acute Diarrhea in Adult Horses
| Common Causes | Major Diagnostic Test(s) |
|---|---|
| Salmonellosis | Fecal culture or polymerase chain reaction (PCR), culture of rectal mucosal biopsy |
| Potomac horse fever (Neorickettsia risticii) | PCR (feces, peripheral blood), paired serologic tests |
| Clostridiosis (Clostridium difficile, C. perfringens) | Fecal culture, toxin analysis |
| Antibiotic-associated diarrhea | History |
| Nonsteroidal antiinflammatory toxicity (primarily right dorsal colitis) | History and supportive clinicopathologic findings, ultrasonography, exploratory surgery with biopsy |
| Undiagnosed | Other conditions ruled out |
| Less Common | |
|---|---|
| Cantharidin toxicity Parasitism (strongylosis, cyathostomiasis, other) Aeromonas, Campylobacter spp. Sand Carbohydrate overload Arsenic toxicity, other toxicities Thromboembolic disease Anaphylaxis |
Table 7.6.
Differential Diagnoses for Chronic Diarrhea in Adult Horses
| Cause of Diarrhea | Major Diagnostic Test(s) |
|---|---|
| Chronic salmonellosis | Fecal culture or polymerase chain reaction, culture |
| Sand | Fecal sedimentation |
| Parasitism (strongylosis, cyathostomiasis) | Fecal egg count, empirical deworming |
| Nonsteroidal antiinflammatory toxicity (primarily right dorsal colitis) | History and supportive clinicopathologic findings, ultrasonography, exploratory surgery with biopsy |
| Inflammatory or infiltrative disorders Inflammatory bowel disease (granulomatous, lymphocytic-plasmacytic, or eosinophilic enterocolitis) Mucosal lymphosarcoma Amyloidosis |
Histopathologic examination, absorption tests (supportive but nonspecific) |
| Dietary: abnormal fermentation | History |
| Neoplasms: lymphosarcoma, squamous cell carcinoma | Histopathologic examination |
| Peritonitis, abdominal abscessation | Peritoneal fluid analysis, ultrasound, exploratory surgery |
| Nongastrointestinal causes (chronic liver disease, congestive heart failure, renal disease) | Physical examination, clinicopathologic findings |
Table 7.7.
Differential Diagnoses for Diarrhea in Foals
| Cause of Diarrhea | Major Diagnostic Test(s) |
|---|---|
| Salmonellosis | Fecal culture or PCR |
| Clostridiosis (Clostridium difficile,C.perfringens) | Fecal culture, toxin analysis |
| Endotoxemia, gram-negative septicemia | Blood culture, physical examination, complete blood count, sepsis score |
| Antibiotic-associated diarrhea | History |
| Foal heat diarrhea | History, physical examination |
| Viral: rotavirus; rarely coronavirus or adenovirus | Electron microscopy, enzyme immunoassay |
| Protozoan: cryptosporidiosis | Fecal analysis |
| Secondary lactose intolerance therapy | Oral lactose tolerance test, response to |
| Rhodococcusequi | Culture, PCR |
| Lawsoniaintracellulare | Fecal PCR, serologic testing |
| Gastric ulcer disease syndrome | Gastric endoscopy |
| Strongyloideswesteri | Fecal egg count |
| Sand | Fecal sedimentation |
PCR, Polymerase chain reaction.
Several infectious agents are associated with diarrhea in horses. Salmonella and Clostridium species are among the most common causes of infectious diarrhea in horses of any age.554, 555 Salmonella is of particular importance because under certain circumstances it can be highly contagious, leading to significant outbreaks of disease, especially in hospitalized patients.556 Clostridium difficile and Clostridium perfringens are the most common clostridia associated with colitis in horses, although occasionally other species may be involved.552, 557, 558, 559, 560, 561 In certain geographic areas equine neorickettsiosis (Potomac horse fever) caused by Neorickettsia risticii is also common.562 Lawsonia intracellularis can cause equine proliferative enteropathy resulting in diarrhea, colic, edema, and weight loss.335, 335,601-565 Lawsonia intracellularis can potentially affect horses of any age, but it is most common in weanling-age foals. Although primarily a respiratory pathogen, Rhodococcus equi also can cause diarrhea, particularly in foals 2 to 4 months of age.566 Escherichia coli is an uncommon cause of diarrhea in foals, unlike in calves and piglets. However, enterotoxigenic strains, characterized by the presence of virulence factors, have been identified in foals.567 Other, less common bacterial agents potentially associated with diarrhea include Campylobacter spp. and Aeromonas spp.568, 569
Both equine rotavirus and coronavirus can be important enteropathogens. Rotaviral infection is a common cause of diarrhea in foals and is most often recognized in foals from 1 to 4 weeks of age.535, 539, 570, 571 Equine coronavirus, also sometimes referred to as equine enteric coronavirus, has been recognized in both foals and adult horses.571, 572, 573, 574, 575 Since 2011 several outbreaks of equine coronavirus have been identified in adult horses, causing it to be named as an emerging pathogen. The most common signs include anorexia, lethargy, and fever; diarrhea and colic are less common.
The signs associated with internal parasites can vary depending on the type and severity of infestation, but diarrhea may occur. Often it is a chronic diarrhea, but acute diarrhea may be seen. Encysted cyathosomin larvae are ubiquitous in grazing horses, but large numbers of encysted larvae place the horse at risk for developing larval cyathostominosis.576, 577 This condition occurs when there is a mass emergence of the larvae, resulting in acute typhlocolitis. The inflammatory responses trigger diarrhea, especially in individuals with high parasite burdens. The case fatality rate may be as high as 50%. The majority of infections with Strongyloides westeri are subclinical, but occasionally diarrhea can occur in foals with high worm burdens.578, 579 A 2014 study in Thoroughbred foals in central Kentucky found a mean prevalence of S. westeri infection of 30%, which was greater than previous reports from the area.579 Cryptosporidium spp. are coccidian parasites that have been linked to diarrhea in foals and occasionally adult horses.580, 581, 582
Medications may be involved in the pathogenesis of diarrhea, with the two classes of drugs most commonly incriminated being antibiotics and NSAIDs. Potentially any systemic antibiotic may cause diarrhea, but the risk appears to be higher with certain antibiotics such as trimethoprim-potentiated sulfonamides and erythromycin.28, 583, 584 Although the mechanisms of antibiotic-associated diarrhea may be multifactorial, an important factor is that the use of systemic antimicrobials leads to changes in the intestinal microbiota.585, 586 The use of NSAIDs has been linked to adverse gastrointestinal effects including gastric ulcers, right dorsal colitis, and inhibition of mucosal barrier healing.336, 587, 588, 589, 626 Phenylbutazone causes changes in right dorsal colon arterial blood flow and changes in VFA production.589
Diarrhea is a component of the clinical syndrome associated with several toxins. Cantharidin (blister beetle toxin) can cause severe colitis in horses.590 Some plants associated with diarrhea include alfalfa dodder (Cuscuta campestris), acorns, and oleander.591, 592, 593 Other toxins that may result in diarrhea include lead, selenium, and arsenic.594, 595
Cellular infiltrative disorders such as inflammatory bowel disease and neoplasia can cause diarrhea, which is generally chronic in nature. Alimentary lymphosarcoma is the most common intestinal neoplasia of horses, and it can cause significant cellular infiltration of the intestine and associated lymph nodes.596 It has been recognized across a broad age range, including relatively young horses. Several inflammatory bowel diseases have been recognized in horses, including granulomatous enteritis, lymphocytic-plasmacytic enterocolitis, eosinophilic enterocolitis, and multisystemic eosinophilic epitheliotropic disease.142
Some additional causes of diarrhea occur in neonatal foals. Foal heat diarrhea is a normal physiologic diarrhea that occurs typically between 5 and 15 days of age.597 Foals are otherwise healthy and the diarrhea is generally mild and self-limiting. With respect to infectious agents, coinfections appear to be common in neonatal foals.571 Secondary lactose intolerance has been documented in foals with gastrointestinal infections, and primary lactose intolerance has been reported as well.539, 552, 553 Diarrhea has been seen in neonatal foals in association with hypoxic-ischemic gastrointestinal damage, sepsis, and rarely acute pancreatitis.598
Diagnostic Approach to Diarrhea
A comprehensive evaluation may help in establishing a diagnosis and developing a treatment plan in cases of diarrhea (Box 7.10 ). However, even in severe cases a definitive diagnosis often is not made, making the problem particularly frustrating.554, 555, 568, 571, 599
BOX 7.10. Outline of Diagnostic Approach to Diarrhea.
I. Signalment, History, and Physical Examination
II. Clinical Pathology
-
1.Minimum database: complete blood count, fibrinogen, and serum chemistry profile
-
a.Assess hydration, acid-base status, electrolyte abnormalities, and protein status
-
b.Assess renal and hepatic function
-
c.Assess endotoxemia
-
a.
-
2.
Serum protein electrophoresis and immunoglobulin quantitation
-
3.
Serologic testing: Neorickettsia risticii and Lawsonia intracellulare
-
4.
Peritoneal fluid analysis
III. Evaluation of Feces
-
1.
Gross appearance: severity, hemorrhage, odor, and presence of sand
-
2.
Direct smear: evaluation of protozoan populations and presence of leukocytes and epithelial cells
-
3.
Parasite evaluation: including evaluation for Cryptosporidium parvum, especially in foals
-
4.Evaluation of bacterial pathogens
-
a.Gram stain and spore stain
-
b.Aerobic and anaerobic culture (culture of multiple samples or rectal mucosal biopsy for Salmonella spp.)
-
c.Clostridial toxin analysis
-
d.Polymerase chain reaction: Salmonella spp., N.risticii, and L.intracellulare
-
a.
-
5.
Foals: evaluation of viral pathogens, primarily rotavirus (electron microscopy and enzyme immunoassay)
IV. Diagnostic Imaging: Radiography and Ultrasonography
V. ENDOSCOPIC EXAMINATION: STOMACH, RECTUM, AND DESCENDING COLON
VI. ABSORPTION TESTS (GLUCOSE OR XYLOSE ABSORPTION): PRIMARILY FOR CHRONIC PROTEIN-LOSING ENTEROPATHY
VII. HISTOPATHOLOGIC EXAMINATION
VIII. TOXIN EVALUATION: CANTHARIDIN IN URINE OR GASTROINTESTINAL CONTENTS, ARSENIC IN LIVER, OR OTHER
IX. RESPONSE TO THERAPY
Signalment and History
The veterinarian should consider the signalment and history carefully when evaluating a patient with diarrhea. Age is particularly important because several disorders, such as foal heat diarrhea, rotaviral diarrhea, and equine proliferative enteropathy, are age related. The genetic background also may be significant because diarrhea has been associated with certain heritable immunodeficiencies, and granulomatous bowel disease has been identified in three sibling horses.600, 601, 602 Establishing whether the diarrhea is acute or chronic is important. Other historical questions of particular relevance include the type and source of feed, as well as any dietary changes; deworming program; involvement of single versus multiple animals; exposure to sand; and the use of medications, especially antibiotics and NSAIDs. Other concurrent diseases, stress, possible exposure to toxins, weight loss, water consumption, and salt availability also may be significant. The information obtained helps the veterinarian prioritize differential diagnoses and direct further testing.
Physical Examination
The clinician should perform a complete physical examination. Attention should be given to good biosecurity measures, especially as some causes of equine diarrhea are potentially zoonotic in addition to being transmissible among horses. The body condition of the horse and the presence of any edema should be noted. The presence of fever, dehydration, or signs of endotoxemia may help in assessing the severity of the disease and differentiating the cause because some causes of diarrhea are not typically associated with systemic signs of illness. The presence of oral ulcers and synchronous diaphragmatic flutter increase the likelihood of cantharidin toxicity especially in horses being fed alfalfa.590 In horses presented for diarrhea with a concurrent arrhythmia, oleander toxicity should be considered if exposure is possible.593 Careful evaluation of the abdomen should be performed. Visible abdominal distention is often an indication of large intestinal distention, which may occur in association with acute colitis. However, distention also may be visible with extreme dilation of multiple loops of small intestine. Careful auscultation of the abdomen can be useful in assessing motility. Although the frequency of borborygmi in horses is variable, horses with normal motility generally have 1 to 3 borborygmi in a 60-second time period. Auscultation behind the xiphoid process may help identify the presence of sand or gravel if particles can be heard grinding together during contractions of the colon.499 Percussion of the abdomen while ausculting may help to identify high-pitched resonant sounds associated with gas-distended bowel. Particularly in foals, transabdominal palpation and ballottement may be useful to identify increased abdominal fluid or large masses near the body wall. Transrectal palpation can be helpful in assessing the size of intestinal segments, consistency of contents, and wall thickness, as well as in identifying masses, enlarged lymph nodes, or mesenteric arteritis.
Ancillary Diagnostic Tests
Clinical Pathology
Routine analysis of blood work typically does not identify a specific cause of diarrhea, although occasionally N. risticii can be found in circulating monocytes. However, blood work is often important in directing appropriate supportive care and may help to establish whether diarrhea is caused by another condition, such as hepatic or renal disease. Some important parameters to evaluate include the presence of leukopenia, particularly neutropenia with a left shift, and toxic changes in the white blood cells. These abnormalities suggest a systemic inflammatory response, often associated with endotoxemia. Thrombocytopenia and coagulopathies may also be present. The clinician also should evaluate the concentration of protein, as well as the albumin-to-globulin ratio. Both acute and chronic diarrhea can cause significant protein loss, resulting in hypoproteinemia and particularly hypoalbuminemia. Hyperglobulinemia may indicate a chronic inflammatory condition. Disturbances in acid-base balance, especially metabolic acidosis, and electrolyte abnormalities frequently occur in horses with acute diarrhea but are uncommon with chronic diarrhea. Although hypocalcemia is relatively common in horses with colitis, marked hypocalcemia may be suggestive of possible cantharidin toxicity.63 Because of the dehydration frequently seen with acute diarrhea, prerenal azotemia is common and is important to recognize because some therapies, especially NSAIDs, may worsen the condition. In a study of 122 horses with acute diarrhea, horses with azotemia and clinicopathologic findings consistent with hemoconcentration and hypoproteinemia were less likely to survive.599
The diagnostic and prognostic value of serum protein electrophoresis has been evaluated in horses with chronic diarrhea.603 Significantly higher levels of β1-globulin were found in horses with larval cyathostominosis than in other horses, and such values in conjunction with a decreased albumin were helpful in diagnosing intestinal parasitism. However, a normal β1-globulin concentration was not a reliable indicator of the absence of the disease. Significantly lower albumin concentrations and significantly higher α2-globulin concentrations were found in horses that did not survive, suggesting that these parameters are nonspecific indicators of the severity of inflammatory changes within the intestinal wall. Parasitic infections, particularly strongylosis, also may be associated with elevated serum concentrations of the immunoglobulin G (IgG) isotype IgG(T).604
Because immunodeficiencies are infrequently associated with diarrhea,600, 601 further evaluation of immune status may be indicated in some horses. This evaluation may include specific immunoglobulin quantitation, evaluation of specific lymphocyte subsets, or functional assays. The clinician should consider genetic testing for severe combined immunodeficiency in sick foals of Arabian breeding.
Analysis of peritoneal fluid may be useful in some horses with diarrhea. Abnormalities in the peritoneal fluid may reflect the severity of inflammation and in some cases may help to establish a specific diagnosis. Increases in protein and sometimes nucleated cell count may be seen in association with ulcerative colitis. In horses with bacterial peritonitis, the veterinarian may find organisms on cytologic examination or culture. Occasionally, the veterinarian may identify neoplastic cells in the peritoneal fluid, although their absence does not rule out the presence of neoplasia.
Evaluation of Feces
Evaluation of the feces may yield important information in horses with diarrhea. Even the gross appearance of the feces can be helpful. For example, profuse, watery diarrhea is not generally consistent with a diagnosis of right dorsal colitis, which is most often associated with soft, poorly formed feces. Frank blood in the feces suggests bleeding into the distal colon from mucosal damage. Hemorrhagic, foul-smelling feces often are seen in association with clostridial diarrhea. The clinician also can assess the feces for the presence of occult blood, which indicates bleeding from any source. Although excess sand in the feces is readily apparent in some cases, other cases require mixing the feces in a rectal sleeve with water and allowing the sand to settle. Acorn husks may be present in the feces of horses with acorn toxicity. Less commonly used tests include evaluation of fecal osmolality and electrolyte concentrations (sodium and potassium). If the concentration of sodium plus potassium is much less than the osmolality, the result indicates the presence of osmotically active nonelectrolytes, confirming an osmotic diarrhea.
Microscopic examination of the feces for evidence of parasitism and evaluation of viable protozoal populations also may be useful. A direct smear of fresh feces allows for observation of the motility of ciliates and can be used as a screen for the presence of ova and oocysts. Ideally evaluation of parasitism should include fecal flotation and a quantitative method that allows for estimation of the number of eggs per gram of feces, such as McMaster’s or Stolley’s. Cryptosporidial oocysts are typically difficult to identify on routine fecal flotation, but detection can be improved by acid-fast or acridine orange staining as well as immunofluorescent staining or flow cytometry.579, 580, 581, 582 It is important to remember that fecal examination for parasites sometimes can be misleading, giving false-negative results.
Fecal samples also can be examined microscopically for leukocytes and epithelial cells. In general, the cellularity increases with the severity of diarrhea. Fecal leukocytes and epithelial cells are increased in salmonellosis but are not specific for this disorder.605 The presence of more than 10 leukocytes per high-power field has been associated with salmonellosis.
Evaluation of the feces for infectious agents is essential in the diagnostic evaluation of horses with diarrhea. Gram stain and spore stain of fecal smears can help to identify and quantitate the bacterial populations present, particularly clostridial species. However, although large numbers of gram-positive rods or spores have been identified in horses with clostridial enterocolitis, the results of direct staining may be misleading.557, 558, 559, 560, 561 Clostridium perfringens has been cultured from 59% of samples in which no gram-positive rods were visible. Some clostridial strains also are likely parts of the normal microflora.561, 606 Large numbers of yeast in the feces should alert the clinician to the possibility of candidiasis, especially in compromised neonatal foals.
Fecal culture is used commonly to establish a diagnosis in horses with bacterial diarrhea. When culturing feces, especially if an outside laboratory is used, the clinician must consider proper sample handling, particularly for anaerobic clostridia.607 Salmonella spp. are one of the most significant bacterial pathogens in equine feces.556 Although the number of Salmonella organisms isolated from the feces of horses with clinical salmonellosis is generally greater than from horses with asymptomatic infections, the volume of feces in horses with profuse diarrhea may decrease the likelihood of positive culture. Culture of multiple fecal samples, typically five, is recommended to increase the sensitivity.608 Culture of a rectal mucosal biopsy or rectal scraping is an alternative to fecal cultures and may increase sensitivity because Salmonella spp. are intracellular organisms. Identifying clostridial species requires anaerobic culture. However, evaluating the presence of toxin in cases of suspected clostridial diarrhea also is critical because Clostridium spp., particularly C. perfringens type A, may be present in normal equine feces.557, 558, 559, 560, 561, 606 Depending on the clostridial species and the laboratory, toxin can be assessed by detecting preformed toxin in the feces, toxin being produced by the isolate in culture, or the toxin gene in the isolate.
An increasing number of polymerase chain reaction (PCR) assays are available for detecting causative agents of equine diarrhea. In comparing a PCR with microbial culture for detection of salmonellae in equine feces and environmental samples, the PCR method was found to be more sensitive and more rapid and required submission of fewer samples.609, 610 For diagnosis of equine neorickettsiosis (Potomac horse fever), PCR is currently recommended for detection of Neorickettsia risticii in feces and peripheral blood.562, 611, 612 Fecal PCR analysis is also useful in documenting Clostridium, Lawsonia intracellularis, and Cryptosporidium spp., as well as both equine rotavirus and equine coronavirus.335, 539, 563, 572, 613, 614 Both rotavirus and coronavirus can also be identified in the feces by electron microscopy.539, 572, 615 A number of commercial assays are also available for the detection of rotavirus antigen.539, 616
Serologic methods, evaluating the presence of antibodies, have been used in the diagnosis of Neorickettsia risticii and Lawsonia intracellularis. 562-564,612,614 In the case of N. risticii, the serologic tests are of limited value in the diagnosis of disease.562 For L. intracellularis, it is recommended to use both PCR and serologic diagnostic testing as these assays have high analytic specificity but variable sensitivity depending on the circumstances.563, 614
Coinfections have been documented in both adult horses and foals with diarrhea. In a study of neonatal foals, coinfections were significantly associated with the risk of gastrointestinal disease.571 The use of diagnostic panels rather than individual tests in combination with quantitative toxin gene analysis may be helpful in the diagnosis of coinfections.
Diagnostic Imaging
Diagnostic imaging, although particularly useful in foals, also can be valuable in adult horses. In foals radiographs can detect gas distention in the lumen of the gastrointestinal tract, and the gas pattern may help to differentiate ileus from mechanical obstruction. Occasionally, gas may be seen within the bowel wall in severe cases of clostridial necrotizing enterocolitis. In adult horses abdominal radiography is limited somewhat by having the proper facilities and equipment to perform the procedure safely. However, radiographs can be effective in identifying radiodense material, such as enteroliths and sand. Ultrasonography can be used in horses of all ages to evaluate the amount and character of the peritoneal fluid, masses, small intestinal motility, intestinal distention, and wall thickness. In horses with right dorsal colitis the diagnosis has been supported by ultrasonographic evidence of an increase in mural thickness of the right dorsal colon.617 The right dorsal colon may have a prominent hypoechoic layer most likely associated with submucosal edema and inflammatory infiltrates. Although isotope-labeled white blood cell scintigraphic scans also may help identify colonic ulcerations, the availability and sensitivity of the procedure are limited.618 In cases of L. intracellularis, ultrasonography may reveal thickened small intestinal loops and possibly excess free abdominal fluid.335, 563
Endoscopy
Endoscopic examination of the stomach and proximal duodenum may reveal the presence of neoplasms or ulceration. Diarrhea and inappetence can be seen in symptomatic foals with ulceration of the squamous gastric mucosa. Endoscopy also can be used for inspection of the mucosa of the rectum and descending colon, allowing for evaluation of mural masses or mucosal inflammation.
Additional Diagnostic Tests
Absorption tests are used primarily in horses with chronic diarrhea or weight loss to evaluate the small intestinal absorptive capacity. Oral glucose and oral xylose absorption tests have been used.160, 619 Although the plasma concentration of glucose may reflect glucose metabolism as well as absorption from the gastrointestinal tract, the assay is reliable in the diagnosis of significant malabsorptive conditions. Xylose is influenced less by the metabolic status of the horse, but the compound is more expensive than glucose, and the assay is not available in many laboratories. Results of both assays are nonspecific, but abnormal results support malabsorption and may indicate the necessity of biopsy.
Diagnosing neoplasms and chronic inflammatory or infiltrative disorders often requires histopathologic examination. A rectal mucosal biopsy is easy to collect and can be cultured, but the area that can be reached for biopsy is limited. Laparoscopy allows for visualization of the abdomen and certain biopsies. The veterinarian can obtain a full-thickness intestinal biopsy during exploratory celiotomy.
The diagnosis of cantharidin toxicity can be made in suspect cases by the identification of blister beetles in the feed source and detection of cantharidin in urine or gastrointestinal contents.590 If a heavy metal or trace mineral toxicity is suspected, lead and selenium can be measured in the blood and liver or arsenic can be measured in the blood, urine, liver, or kidney as appropriate.594, 595 Oleandrin is detectable in urine and gastrointestinal contents.593
Evaluation of Response to Therapy
Evaluating the response to empirical therapy may be helpful in some horses with undiagnosed diarrhea, especially in chronic cases. Dietary changes may decrease diarrhea in some cases, and often a diet of grass hay alone is recommended. In cases in which right dorsal colitis is suspected but cannot be confirmed, using pelleted feed and the addition of psyllium mucilloid and corn oil to the diet may be beneficial. Psyllium mucilloid also has been used for treatment of horses in which sand was suspected as contributing to the diarrhea. Any medications that the horse has been receiving, especially NSAIDs or antibiotics, should be discontinued in case they are contributing to the diarrhea.
Fecal microbiota transplant (FMT), also known as transfaunation, can be used in an attempt to restore normal flora.620 Although not a new concept, there has been renewed interest in FMT due in part to increased awareness of the importance of the microbiota and the success of FMT in the management of Clostridium difficile infection in humans. Currently, data regarding FMT in horses are limited. Some guidelines for the use of FMT in horses, including case selection and FMT procedure, have been proposed.620 Several commercial prebiotics and probiotics are available, and their efficacy is under investigation.621 The yeast Saccharomyces boulardii may decrease the severity and duration of clinical signs in horses with acute enterocolitis.622
A course of corticosteroids can be tried in cases of chronic diarrhea in which infectious causes have been ruled out. Treatment with a larvicidal anthelmintic may be beneficial in some cases and sometimes is used with corticosteroids. Some horses with chronic diarrhea have responded to iodochlorhydroxyquin (10 g/450 kg/day for 2 weeks). This drug sometimes has been used concurrently with trimethoprim-sulfa. Occasionally, transfusion with plasma seems to suppress diarrhea in young horses.
Polyuria and Polydipsia
Polyuria and polydipsia are infrequently reported clinical problems in the horse that can be a source of inconvenience to owners, may indicate a major abnormality in the mechanisms controlling water balance, and can be associated with significant disease. As water intake and urine output are closely linked, polydipsia and polyuria generally occur together. Establishing whether the primary problem is polyuria or polydipsia can be helpful in developing a management strategy and providing an accurate prognosis.
Normal fluid intake and urine production in horses are influenced by a number of factors, including age, diet, physiologic demands such as exercise or lactation, environmental temperature and humidity, and gastrointestinal water absorption.623, 624, 625, 626, 627, 628, 629, 630 In adult horses, water requirements range from 25 to 70 mL/kg per day. These requirements are proportional to metabolic body size rather than body mass, with larger horses requiring less water per kilogram than smaller horses or ponies. Because fat is low in water content compared with lean body tissue, horses with significant body fat require less water than lean horses. Fluid requirements are higher in neonatal foals than adult horses due to their high total body water.
Water is obtained from three sources: drinking water, water in feed, and metabolic water from the catabolism of fats, carbohydrates, and protein. Water is lost in urine and feces as well as through evaporation across the skin and respiratory tract. In adult horses, normal water intake through drinking is approximately 50 to 60 mL/kg per day (20–30 L/day for an average adult horse) and normal urine production is approximately 15 to 30 mL/kg per day (5–15 L/day for an average adult horse).623, 624, 625, 626, 627 Diet is a major influence on drinking and urine output. In neonatal foals on a milk diet, fluid intake is approximately 250 mL/kg per day to meet their caloric needs.631, 632, 633 As a result, the kidneys actively excrete water and the urine output approaches 148 mL/kg per day, which is almost 5 times that of adults. In adult horses, water intake correlates with dry matter intake.628, 629 Depending on weather conditions, water consumption may be minimal in horses on good quality pasture due to the high moisture content of grass. Water requirements are higher for horses consuming hay compared with pasture or grain-based rations and vary with the type of hay. Horses on legume hay have significantly higher urine production than those on grass hay. Voluntary water intake is also affected by ambient temperature, increasing when temperatures are high and water losses in sweat are increased623, 625, 634, 635 (Table 7.8 ). Water consumption may increase significantly in heavily exercised horses such as endurance horses to replace the additional fluid lost in sweat.634, 635 Horses with diarrhea may also have significantly increased water consumption.
Table 7.8.
Causes of Polyuria and Polydipsia
| Solute Diuresis |
|
| Water Diuresis |
|
| Iatrogenic |
|
PPID, Pituitary pars intermedia dysfunction.
Not reported in horses.
Modified from Fenner WR: Quick reference to veterinary medicine, ed 3, Philadelphia, Wiley-Blackwell, 2001.
Polyuria and polydipsia may be clearly evident in some affected horses but in others may be more subtle. The conditions are sometimes hard for owners to recognize, particularly in horses housed at pasture and especially if they are with other horses. Some owners may misinterpret frequent urination, or pollakiuria, as polyuria. Objective documentation of polyuria and polydipsia requires measurement of water consumption and 12- to 24-hour urine production. In adult horses, polydipsia has been defined as water intake greater than 100 mL/kg per day and polyuria as urine output greater than 50 mL/kg per day. Consideration of factors that can influence water consumption and urine production, such as diet, climatic conditions, and level of exercise, should be taken into consideration when documenting polyuria and polydipsia.
Mechanisms of Polyuria and Polydipsia
Maintenance of Normal Water Balance
The maintenance of adequate body water that has a relatively constant concentration of electrolytes and other solutes is essential for normal cellular function. Under normal conditions, plasma osmolality remains relatively constant, varying only by approximately 2%.636 Body water is controlled by two general mechanisms: (1) fluid intake, which is regulated by factors that influence thirst, and (2) renal excretion of water, which is regulated by factors that affect glomerular filtration and tubular reabsorption. In normal horses, glomerular filtration exceeds 1000 L/day, but 99% is reabsorbed in the renal tubules and collecting ducts. Normal urine is 3 to 4 times more concentrated than plasma with an osmolality of 900 to 1200 mOsm/kg and a specific gravity of 1.025 to 1.050. The ability to concentrate urine is dependent primarily on three factors: (1) the production of arginine vasopressin (AVP), also known as antidiuretic hormone (ADH); (2) the presence of a sufficient number of nephrons that are sensitive to AVP; and (3) the presence of a hyperosmotic renal medullary interstitium.636, 637
Role of Arginine Vasopressin
Arginine vasopressin is an important peptide hormone that is highly conserved among species.636, 637, 638, 639 Initially, AVP was recognized as having two functions, antidiuresis and vasoconstriction. It is now known to have diverse effects, some of which include modulation of pituitary functions, as well as immune responses and behavior. A small peptide of nine amino acids, AVP is synthesized primarily in the supraoptic and paraventricular nuclei of the hypothalamus, with smaller quantities produced by various tissues outside the hypothalamus. It is produced as a precursor, prepro-vasopressin, which is packaged into neurosecretory granules and transported axonally via the pituitary stalk (infundibulum) to the posterior pituitary (neurohypophysis). During transport, prepro-vasopressin is processed into the active hormone, which is stored for secretion in the posterior pituitary.
AVP is primarily released in response to signals from osmoreceptors located in the anterior hypothalamus near the supraoptic nuclei and in the anteroventral region of the third ventricle.636, 637, 638, 639 These osmoreceptors rapidly respond to small increases in the osmolarity of the extracellular fluid, which is primarily determined by the sodium concentration. Once stimulated, osmoreceptors send signals to nerve cells in the supraoptic nuclei, which relay the signals down the pituitary stalk to the posterior pituitary stimulating the release of AVP from secretory granules in the nerve endings. Although the exact threshold for AVP release in the horse is unknown, in healthy ponies, an increase of approximately 8 mOsm/kg in plasma osmolality (about 3%) following water deprivation was associated with an increase in plasma AVP concentration from 1.53 ± 0.36 pg/mL to 4.32 ± 1.12 pg/mL.640 When the extracellular fluid becomes hypo-osmolar, less AVP is released, causing less water to be reabsorbed. Although changes in osmolarity are the major stimulus for AVP release, the stimulation of arterial and cardiopulmonary baroreceptors by a reduction in circulating volume and hypotension can also result in AVP release.636, 638 Other stimuli for AVP release that have been reported in some species include stress, pain, nausea, hypoglycemia, and certain drugs such as morphine.
There are three different receptors for AVP, V1a, V1b, and V2, with V2 being the receptor found on the epithelial cells of the distal convoluted tubule and collecting duct of the kidney.638 Extrarenal V2 receptors are present in the endothelium. Within the kidney, one mechanism of action of AVP is to influence aquaporins, which are integral membrane proteins that can form pores in the cell membrane that act primarily as water channels. Binding of AVP to the renal V2 receptor increases aquaporin-2 synthesis and leads to aquaporin-2 water channel insertion into the apical membrane. This aquaporin channel then allows water to move down the osmotic gradient and out of the nephron, increasing water reabsorption by the kidney. AVP also acts to increase urea permeability in the terminal inner medullary collecting duct by regulating urea transporters, increasing the concentration of urea in the medullary interstitium.641 Also, the V2 receptors are active in the loop of Henle, where activation stimulates the reabsorption of sodium.
Role of the Renal Hyperosmotic Medullary Interstitium
The osmolarity of the interstitial fluid in the medulla of the kidney is approximately 1200 to 1400 mOsm/L compared with about 300 mOsm/L for interstitial fluid in other locations. This high osmolarity of the renal medullary interstitium surrounding the collecting ducts provides the osmotic gradient necessary for water reabsorption to occur when AVP concentrations are high. The primary mechanism maintaining the hyperosmolar interstitium is countercurrent multiplication, which depends on the structure and function of the loop of Henle636 (Fig. 7.6 ). Essentially, the ongoing active reabsorption of ions from the thick ascending loop of Henle combined with the continued flow of new ions into the loop of Henle ultimately multiplies the medullary solute concentration. Due to the presence of aquaporin channels, the descending loop of Henle is permeable to water, but it is relatively impermeable to solute. Thus as tubular fluid enters the descending loop and moves toward the inner medulla, it gradually becomes more hypertonic as water moves out into the interstitium. In the thin ascending loop of Henle, ions begin to move into the interstitium by passive diffusion. Once fluid enters the thick ascending loop of Henle, sodium, potassium, and chloride are actively reabsorbed via the apical Na+/K+/2Cl− cotransporter. This active transport of ions, combined with the impermeability of this section to water, results in an increased osmolarity in the renal medullary interstitium. This creates an osmotic pressure gradient, drawing water from the descending limb and increasing the osmolarity of the tubular fluid. This process is repeated as fluid continues to flow through the tubules, with the net effect of continuing to add sodium to the medullary interstitium until the osmolarity reaches 1200 to 1400 mOsm/L. The collecting ducts also contribute to the hyperosmolar interstitium through the active transport of ions and the facilitated diffusion of urea into the interstitium. The relatively low blood flow in the renal medulla and the unique characteristics of the vasa recta help to maintain the hyperosmolar interstitium.
Fig. 7.6.
Simplified schematic of the countercurrent multiplier in the loop of Henle. ECF, Extracellular fluid, concentrations in mOsm/L.
Countercurrent multiplication and maintenance of the hyperosmolar interstitium play important roles in the concentration of urine. When luminal fluid enters the thick ascending loop of Henle, approximately 80% of the glomerular filtrate has been reabsorbed. The active resorption of NaCl in the thick ascending loop of Henle, which is impermeable to water, causes the fluid entering the distal tubule in the renal cortex to be hypotonic. Water is then reabsorbed from the distal tubules and cortical portions of the collecting ducts under the action of AVP. Reabsorbed water is transported rapidly out of the interstitium by the extensive cortical capillary network, and interstitial hypertonicity is preserved.
Thirst
Adequate fluid intake, which is regulated by thirst, contributes to the maintenance of water balance along with the renal excretion of water.630, 634, 636, 642 The thirst center in the hypothalamus consists of osmoreceptors in the anteroventral wall of the third ventricle and anterolateral preoptic nucleus that are in close proximity to those that regulate AVP secretion. A major stimulus for thirst is increased osmolarity of the extracellular fluid, which causes intracellular dehydration in the thirst centers, stimulating the sensation of thirst. In human beings the threshold for stimulation of thirst is approximately 2 to 5 mOsm/kg greater than that for stimulation of AVP release. Also, decreases in extracellular fluid volume and arterial pressure can stimulate thirst via mechanisms that are distinct from osmolarity. These mechanisms include neural input from cardiopulmonary and systemic baroreceptors and the release of angiotensin II, which can both stimulate thirst and decrease renal fluid excretion. Peripherally, mechanoreceptors in the oropharynx can sense dryness of the mouth, stimulating thirst. Following experimental water deprivation, ponies drank when their plasma osmolalities increased by 3%, when plasma Na concentrations increased by approximately 5%, and after induction of a plasma volume deficit of 6%.8
General Mechanisms of Polyuria and Polydipsia
General mechanisms of polyuria and polydipsia include water or solute diuresis, and in some cases both mechanisms may contribute.625, 630, 634, 636 Water diuresis is caused by either decreased water absorption in the collecting tubules or excessive voluntary water intake. In water diuresis, either polyuria or polydipsia may be primary. Solute diuresis is caused by excessive renal excretion of a nonreabsorbed solute such as glucose or sodium, resulting in primary polyuria.
Polydipsia as the Primary Problem
Excessive water intake results in water diuresis.636 By expanding the extracellular fluid volume and decreasing plasma osmolarity, excessive fluid intake causes a suppression of AVP secretion. This then makes the collecting ducts less permeable to water, increasing urine output.
Polyuria as the Primary Problem
Polyuria can result from renal disease or several systemic disorders. Although polyuria is commonly associated with renal failure, the precise mechanisms are not fully understood and are most likely multiple.643 With primary renal insufficiency or failure, there is a decrease in functional nephrons. This can result in solute diuresis, as increasing amounts of glomerular filtrate are presented to the remaining tubules, often exceeding their reasborptive capacity. The resultant increase in urinary solute is accompanied by an increase in water loss. Also, the increased tubular flow rate in the remaining tubules may simply allow less time for the reabsorption of water. In addition, diuresis may result from decreased hypertonicity of the medullary interstitium associated with decreased transport of sodium and chloride out of the loop of Henle when there is renal compromise. Another factor that may contribute to polyuria in renal failure is acquired nephrogenic diabetes insipidus due to an inability of the diseased collecting ducts to respond to AVP.
The syndrome of diabetes insipidus, which is characterized by the excretion of large volumes of dilute urine associated with abnormalities related to AVP, has several fundamental causes.644, 645 In primary polydipsia, AVP secretion is suppressed by excessive fluid intake. Also, in human patients, gestational diabetes insipidus can occur due to degradation of AVP by a placental enzyme. Central or neurogenic diabetes insipidus occurs when there is inadequate production or secretion of AVP. This form of diabetes, or inflammatory disease involves the hypothalamus or posterior pituitary. In nephrogenic diabetes insipidus there is insensitivity of the renal collecting duct epithelial cells to AVP, and the condition may be primary or acquired. Primary nephrogenic diabetes insipidus implies isolated dysfunction of the response to AVP not associated with actual structural or metabolic problems of the kidney. In people this occurs as a familial disorder with an X-linked autosomal semirecessive mode of inheritance, and there is some evidence this may occur as a familial problem in horses as well. Acquired nephrogenic diabetes insipidus can be associated with renal disease or altered sensitivity of nephrons to AVP due to endotoxemia or cortisol excess. Also, in some species, hypercalcemia, potassium depletion, and the administration of certain drugs such as gentamicin have been reported to cause insensitivity of the collecting duct receptors to AVP.
Renal medullary washout is a syndrome in which the relative hypertonicity of the renal medulla is reduced, typically from prolonged diuresis of any cause.636, 643 The increased tubular flow rates during diuresis may result in an inability to resorb sufficient sodium and urea from the tubular lumen. The resulting decrease in medullary hypertonicity leads to impairment of renal concentrating ability and polyuria with secondary polydipsia. Enhanced medullary blood flow may deplete medullary solute further.
Polyuria as a result of solute diuresis can occur in association with excessive salt consumption and hyperglycemia in addition to renal failure.643, 646, 647, 648, 649 When blood glucose concentrations increase above the renal threshold of approximately 150 to 200 mg/dL in the horse, the resultant glucosuria results in increased urine flow. Solute diuresis may also occur following obstructive urinary tract disease, but this is not commonly reported in horses.
Conditions Associated with Polyuria and Polydipsia
There are several potential specific causes of polyuria and polydipsia in horses.650, 651 The most common causes include renal failure, PPID, and primary psychogenic polydipsia. Other less common causes include excessive salt consumption, central and nephrogenic diabetes insipidus, and diabetes mellitus. Polyuria and polydipsia may also occur secondary to sepsis and endotoxemia or may be iatrogenic following the use of α2-agonists, corticosteroids, or diuretics.650, 651, 652
Polydipsia
The most common reason for excessive water intake in horses is psychogenic water drinking. The extent of polydipsia and therefore polyuria seen with psychogenic water drinking can be substantial. Psychogenic water drinking can be a stable vice associated with confinement and boredom.642, 653 The behavior has also been associated with changes in environmental conditions, stabling, and diet. It has been suggested that primary polydipsia is particularly common in horses residing in the southern United States when ambient temperature and humidity are high.
Excessive salt consumption may also result in increased thirst, although a significant amount of salt needs to be consumed to stimulate thirst in horses.648 In human patients, polydipsia has been reported in association with conditions affecting the osmoreceptors involved in the regulation of thirst, such as neurologic disease affecting the hypothalamic thirst center.654, 655 This could potentially occur in horses. For example, the enlarged pituitary gland in horses with PPID could compress the hypothalamus.
The ability to concentrate urine is retained in cases of primary polydipsia. However, occasionally the long-standing diuresis can result in medullary washout and a decrease in concentrating ability.
Polyuria
Important causes of primary polyuria in horses include renal failure and pituitary pars intermedia dysfunction.650, 651 Diabetes insipidus and diabetes mellitus are less common causes.
Renal Failure
In the acute stage of renal failure there is frequently a period of oliguria or anuria, which is then followed by polyuria.643, 646 The repair of tubules following an acute insult may take several weeks, and in some cases the repair may not be complete, resulting in chronic renal failure and persistently diminished concentrating ability.643, 647 Some potential causes of renal failure in horses include failure to recover from ischemic renal damage, pigmenturia, chronic infection, urinary obstruction, and exposure to nephrotoxins such as nonsteroidal antiinflammory drugs, gentamicin, and polymixin.646, 647, 656, 657 Other possible causes include immune-mediated disease, glomerulonephritis, congenital abnormalities, renal helminthiasis, and amyloidosis. In some horses, the inciting cause of chronic renal failure may be hard to identify.
Pituitary Pars Intermedia Dysfunction
Polyuria and polydipsia are recognized in approximately 30% of horses with pituitary pars intermedia dysfunction (PPID), although numbers vary among studies.658, 659, 660 Other clinical signs that are commonly associated with PPID include hypertrichosis, laminitis, muscle atrophy, abnormal fat deposition, and recurrent infections. The pathogenesis of polyuria and polydipsia in PPID is not completely understood and most likely involves multiple factors. As some horses with PPID are hyperglycemic, osmotic diuresis associated with glucosuria may play a role. Although it has not been documented, the enlarged pars intermedia may impinge on the posterior pituitary and/or hypothalamus, potentially causing secondary neurogenic diabetes insipidus by interfering either with the production or release of AVP or possibly with osmoreceptors involved in thirst. Also, an increase in plasma cortisol associated with PPID may result in antagonism of the action of AVP at the renal tubule.
Diabetes Insipidus
Neurogenic diabetes insipidus appears to be rare in the horse, with two cases reported in the literature.661, 662 In both cases, the horses were unable to concentrate urine in response to water deprivation but responded to the administration of exogenous vasopressin with a decrease in urine volume and increase in urine concentration. In one case the condition was felt to be idiopathic, and in the other it was felt to be acquired secondary to encephalitis.
Primary nephrogenic diabetes insipidus also appears to be rare in horses, having been reported in three colts and a 14-year-old gelding.663, 664, 665 Two of the colts were sibling Thoroughbreds, suggesting that an inherited form of nephrogenic diabetes insipidus similar to what is seen in people may occur in horses.664 In addition to signs of polyuria and polydipsia, two of the three colts were underweight. Following water deprivation, affected colts could not increase urine concentration although plasma AVP concentrations increased. In addition, affected horses had minimal response to the administration of exogenous vasopressin, confirming resistance of the collecting ducts to the antidiuretic action of vasopressin.
Nephrogenic diabetes insidipus has been recognized in horses with some frequency as a secondary problem in which there is an acquired decrease in renal responsiveness to AVP.645 This acquired nephrogenic diabetes insipidus has been associated with a variety of conditions, including renal disease. It has also been described following drug therapy, as well as with a variety of endocrine, metabolic, infectious, neoplastic, and postobstructive disorders.
Diabetes Mellitus
Diabetes mellitus is characterized by chronic hyperglycemia. In type 1 or insulin-dependent diabetes mellitus, there is a lack of insulin, whereas in type 2 or non–insulin-dependent diabetes mellitus, insulin is adequate but tissue sensitivity to insulin is impaired. Although it is a relatively common cause of hyperglycemia and glucosuria in other species, type 1 diabetes mellitus has been infrequently diagnosed in the horse.152, 666, 667, 668, 669, 670, 671 Type 2 diabetes mellitus has been recognized in horses in association with several conditions, especially PPID and equine metabolic syndrome.80, 672, 673, 674, 675, 676 Diabetes mellitus has also been reported in a horse with bilateral granulosa cell tumors.672
Other
Medullary washout may result from chronic diuresis of any cause and may be a more common complication of primary diseases and their therapy in horses than has been reported to date. Polyuria and polydipsia have occasionally been reported in association with sepsis and endotoxemia in multiple species, including horses. The mechanisms are unclear but may involve endotoxin-induced production of PGE2, which can cause renal vasodilation and inhibit the actions of AVP. Although infrequently reported in horses, polyuria and polydipsia have been recognized in association with severe liver disease. Some conditions that have been associated with polyuria and polydipsia in other species include primary polycythemia, hypercalcemia, and potassium depletion.
Diagnostic Approach to Polyuria and Polydipsia
Signalment and History
The signalment and history can be valuable in the assessment of polyuria and polydipsia. Congenital problems such as renal anomalies are generally recognized in young animals, whereas PPID increases in prevalence with age. Primary nephrogenic diabetes insipidus has been described in two related Thoroughbred colts. A detailed history should be obtained with particular emphasis on the duration of signs, any management changes, diet including any supplements, and any current or previous medication use. In some horses, iatrogenic causes of polyuria and polydipsia can be ruled out by assessment of the history and return to normal urine output and water intake after discontinuing medications, intravenous fluids, or excess dietary salt.
Physical Examination
A complete physical examination should be performed with careful attention to hydration status. Palpation per rectum can be useful in evaluation of the urinary tract. Horses with psychogenic polydipsia are generally normal on physical examination. Chronic renal insufficiency is often associated with a poor body condition, and in some cases, appetite may be decreased. The clinical signs of PPID can be quite variable depending on the individual and the stage of disease, but commonly recognized signs include hypertrichosis, muscle atrophy, abnormal fat deposition, chronic infections, and laminitis.
Measurement of Water Intake and Urine Volume
The measurement of 24-hour water intake and urine production can be helpful in confirming the problem, especially in those cases in which polyuria and polydipsia are less obvious.677, 678, 679, 680 It can also establish the severity, which can help to prioritize differentials. In general, the degree of polydipsia and polyuria tends to be more pronounced with psychogenic polydipsia and diabetes insipidus than with renal failure or PPID. Several means of urine collection have been devised. Generally close observation and cross-tying are required to ensure the device stays in place and measurements are accurate. In general water intake over 100 mL/kg per day and urine output over 50 mL/kg per day are consistent with polydipsia and polyuria in adult horses. Factors that can influence water intake and urine production, such as diet and environmental conditions, should be taken into consideration. When measuring water consumption, behavioral tendencies to spill or play in the water should be taken into account.
Ancillary Diagnostic Aids
Clinical Pathology
A hemogram, serum biochemistries, and urinalysis should be assessed in horses presented for polyuria and polydipsia. Urine specific gravity (USG) is decreased in horses with polyuria and polydipsia. The extent of the decrease in USG, along with the presence or absence of other abnormalities on laboratory tests, can help prioritize differential diagnoses and may direct additional testing. Horses with psychogenic polydipsia tend to present with hyposthenuria (USG less than 1.008 and osmolality less than 260 mOsm/kg) without other laboratory abnormalities. As hyposthenuria indicates that renal diluting ability is intact, horses with renal failure or complete medullary washout are unlikely to have hyposthenuria, although it may occasionally be present in horses recovering from acute renal failure. Chronic renal failure is suggested by the presence of isothenuria (USG 1.008–1.014 and osmolality 260–300 mOsm/kg) with concurrent azotemia.681, 682, 683 Other laboratory abnormalities that may be seen with chronic renal failure include mild anemia, hypoalbuminemia, hyponatremia, hypochloridemia, hyperkalemia, hypercalcemia, and hypophosphatemia. Some additional laboratory tests that may be used to assess renal function include fractional excretion of electrolytes, urinary protein–to–urinary creatinine ratio, creatinine clearance, and measurement of urinary enzymes. Measurement of glomerular filtration rate is a sensitive indicator of renal dysfunction but is infrequently performed primarily for practical reasons. Measurement of symmetric dimethylarginine, a methylated form of arginine that is eliminated primarily by renal excretion, is useful in the evaluation of renal function in small animals and is under investigation in horses.684
There are no consistent laboratory abnormalities in horses with PPID, although neutrophilia, lymphopenia, hyperglycemia, and glucosuria may be present.658, 659, 660 Additional endocrine testing is required to confirm the presence of PPID. Some recommended tests include measurement of baseline ACTH concentration, measurement of ACTH concentration following the administration of thyrotropin-releasing hormone, or a low-dose dexamethasone suppression test. Hyperglycemia and glucosuria may also be seen with equine metabolic syndrome, underlying systemic disease and diabetes mellitus.
Water Deprivation Testing
Water deprivation evaluates the ability to conserve water and is most often used to differentiate psychogenic polydipsia from diabetes insipidus.624, 685, 686 It is most clearly indicated in horses that are hyposthenuric rather than isossthenuric. Water deprivation testing is contraindicated in horses that are dehydrated or azotemic. In normal animals, the increase in plasma osmolality that occurs with water deprivation results in the release of AVP, which acts on the renal tubules and collecting ducts to conserve water, increasing USG. Before initiating the test, baseline values for USG, serum urea nitrogen, creatinine, and body weight should be obtained and the bladder emptied by catheterization. After water is removed, USG and body weight should be monitored every 6 to 12 hours. Serum urea nitrogen and creatinine may be monitored as well. In response to 24 to 72 hours of water deprivation, normal horses will increase their USG over 1.045 with an osmolality over 1500 mOsm/kg. For practical purposes, if USG increases above 1.025 within 24 hours or by the time that 5% of body weight is lost, concentrating ability is adequate and the test can be discontinued. The test should be terminated if there is a loss of more than 5% of the body weight or if clinical signs of dehydration or azotemia develop. Typically, horses with psychogenic polydipsia can concentrate their urine in response to water deprivation whereas those with diabetes insipidus cannot. Occasionally horses with psychogenic polydipsia will have incomplete concentrating ability due to medullary washout and loss of the medullary interstitial osmotic gradient. These horses may respond to a modified water deprivation test where water intake is gradually restricted for several days allowing for restoration of the medullary interstitial osmotic gradient.687 Horses with either central or nephrogenic diabetes insipidus cannot effectively concentrate their urine in response to water deprivation, and to distinguish these conditions additional testing such as measurement of endogenous AVP concentrations and evaluation of the response to exogenous AVP is indicated.
Measurement of Arginine Vasopressin and Response to Exogenous Arginine Vasopressin or Analogs
The plasma concentration of AVP can be measured during water deprivation testing or following the administration of 7.5% sodium chloride solution to increase plasma osmolality. In normal ponies, AVP concentrations increased from baseline values of 1.53 ± 0.36 pg/mL to 4.32 ± 1.12 pg/mL after 24 hours of water deprivation.13 Plasma concentrations of AVP will increase in horses with psychogenic polydipsia or nephrogenic diabetes insipidus, but any increase will be minimal with central diabetes insipidus. In nephrogenic diabetes insipidus, the elevation in AVP is not accompanied by an increase in USG.
The ability of renal tubules to respond to AVP can be assessed by a challenge test.688 USG is monitored following the administration of either exogenous synthetic vasopressin or desmopressin acetate, a synthetic vasopressin analog. USG is expected to increase over 1.020, and failure to do so is consistent with a diagnosis of nephrogenic diabetes insipidus if medullary washout is not present.
Diagnostic Imaging
Transabdominal and transrectal ultrasound examination can be useful in evaluation of the urinary tract. Although not consistent, changes in the size and/or architecture of the kidneys may be present in both acute and chronic renal failure. Ultrasound can also be helpful in the diagnosis of urolithiasis.
Poor Performance
Poor performance is the inability of an individual to perform at a level that can be reasonably expected based on the individual’s physical characteristics, level of training, and/or previous performance. Any decrease in performance may be critical to the equine athlete. In some cases, poor performance may be easily documented, such as when the problem is severe or when there is a drop in performance that can be objectively measured (e.g., in a discipline such as racing). However, in other cases, poor performance may be subtle and difficult to document.
Mechanisms Affecting Performance
Numerous factors influence performance, including genetics, training, desire, body composition, and overall health. Peak athletic performance requires optimal function of all body systems, particularly those involved in locomotion and oxygen transport.
Genetic Factors
Genetic factors play a role in several aspects of performance. In multiple species, genetics are known to influence athletic potential. As the knowledge of equine genomics expands, understanding of the heritability of specific traits associated with elite athletic performance will increase.689, 690, 691 For example, variation at the myostatin gene locus has been found to be associated with a horse’s best racing distance.690 In addition to influencing inherent athletic ability, genetic traits can potentially impair performance either by direct effects on the individual’s health or by increasing the likelihood of the individual developing secondary problems. For example, heritable muscle disorders can directly affect performance, and poor structural conformation can increase the risk of injury.3, 4 Similarly, it has been suggested that Warmblood horses that must work in top gear to achieve the speeds required for upper levels of 3-day eventing are more prone to injury.692
Locomotion
Movement is a key component of athletic performance, and therefore musculoskeletal, neurologic, and neuromuscular disorders can all have a negative impact on performance.691, 692, 693, 694, 695 The mechanisms by which they affect performance are multiple and include pain, mechanical restrictions, incoordination, and weakness. Pain can make the horse reluctant to exercise and can also limit the amount of training so that the horse is unable to achieve the level of fitness required for optimal performance. Any gait deficit can potentially make efficient movement mechanically difficult. Also, lameness at one site can contribute to the development of secondary problems such as back pain or chronic myopathy that can further impair performance. Neurologic disease can cause ataxia and weakness, adversely affecting performance.
Physical activity requires muscles to generate and sustain the power for movement. Horses are generally considered to be a highly athletic species, and there are several adaptations in equine skeletal muscle that contribute to this athletic ability, including an increased muscle mass relative to body weight, high locomotor efficiency based on muscle-tendon architecture, high intrinsic shortening velocities, and efficient muscle energetics.696, 697 In horses, muscle tissue comprises about 45% of body weight in most equine breeds and up to 55% of body weight in Thoroughbreds, compared with 30% to 40% in many other species. Horses that have the ability to run fast tend to have a large overall skeletal muscle mass, longer muscle fascicles, a high percentage of fast-twitch muscle fibers, and a low percentage of body fat.90 Any change in muscle mass, architecture, or function can have a profound impact on performance. If muscle fatigues prematurely or is painful, such as with rhabdomyolysis, the horse may not be able to generate the necessary power for efficient movement.
Oxygen Uptake and Transport
Any process that impairs the uptake or transport of oxygen can limit the ability to produce the energy required for optimal performance. Thus diseases of both the upper and lower respiratory tract, and less frequently the cardiovascular system, are important causes of poor performance. A number of upper airway obstructive disorders have been recognized that restrict the movement of air into and/or out of the lung.301, 739, 301, 698, 699, 700, 701, 702 Disease of the lower respiratory tract may increase the work of breathing and impede normal gas exchange in the lung. For example, inflammatory airway disease (IAD) has been shown to alter pulmonary mechanics through changes in lung compliance, greater viscous lung resistance, and rate of dynamic work of breathing.703 Due to the increase in ventilatory load, the energy required for breathing is significantly higher in affected horses, which may limit their performance. Also, a higher neutrophil percentage in the bronchoalveolar lavage fluid of horses with poor racing performance has been correlated with significantly lower relative gas flows during inspiration and expiration.704 Although the effects of exercise-induced pulmonary hemorrhage (EIPH) on performance are not entirely clear, in a study of 132 horses with a history of poor performance, exercise-induced hypoxemia was seen in horses with IAD and EIPH.705 Gas exchange was most significantly impaired in those horses with concurrent EIPH and upper airway obstruction. Once oxygen is taken up into the circulation from the lung, it must be delivered to the tissue, especially working muscle, for optimal performance. Thus cardiovascular disease can impair performance by decreasing the effectiveness of oxygen delivery to the tissues.
Other
Almost any systemic disease or source of pain has the potential to make the horse reluctant to work. For example, poor performance has been reported in association with equine gastric ulcer syndrome and possibly dental disease.700, 706, 707 Also, in horses with anhidrosis, their decreased ability to maintain normal body temperature can limit performance in warm environmental conditions.708
Conditions Associated with Poor Performance
It is not possible to list all potential causes of poor performance as so many factors influence performance (Box 7.11 ). Certainly almost any systemic disease, as well as desire and training, can affect performance. When evaluating horses presented specifically for the complaint of poor performance, the conditions most often identified have been abnormalities of the respiratory and musculoskeletal systems.695, 699, 700, 709, 710 It is not uncommon for multiple problems to occur concurrently. In a study of 275 racehorses with a history of poor racing performance, 84% were found to have more than one abnormality.699 Similarly, in a study of 27 endurance horses with poor performance, 66.7% had multiple concomitant disorders.710
BOX 7.11. Causes of Poor Performance.
Systemic Disease
Body Condition
Overconditioned
Underconditioned
Training
Inadequate
Overtraining
Musculoskeletal
Lameness
Back pain
Neck pain
- Muscle disorders
- Sporadic exertional rhabdomyolysis
- Subclinical exertional rhabdomyolysis
- Polysaccharide storage myopathy
- Recurrent exertional rhabdomyolysis
- Vitamin E myopathy
- Muscle injury/strain
- Mitochondrial myopathy
- Myofibrillar myopathy
Neurologic Disease
Respiratory Tract Disease
- Upper airway obstruction
- Dorsal displacement of the soft palate
- Recurrent laryngeal neuropathy
- Axial deviation of the aryepiglottic folds
- Dynamic pharyngeal collapse
- Epiglottic entrapment
- Subepiglottic cyst
- Rostral displacement of the palatopharyngeal arch
- Redundant alar folds
- Lower airway disease
- Inflammatory airway disease; recurrent airway obstruction
- Exercise-induced pulmonary hemorrhage
Cardiovascular Disease
Cardiac arrhythmia
Myocardial dysfunction
Murmurs (often not clinically significant)
Other
Dental disorders
Equine gastric ulcer syndrome
Anhidrosis
Saddle fit; horse-saddle-rider interaction
Disease in early neonatal period
Locomotor Disorders
A surprising number of horses presented for poor performance are found to be lame even when lameness has not been reported by the owner or trainer.692, 695, 699, 700, 709, 710 Although some horses may compete successfully with low-grade lameness, any degree of lameness has the potential to impair performance. A wide variety of musculoskeletal conditions have been identified in association with poor performance.692, 695, 699, 700, 709, 710, 711, 712 In addition to those horses with forelimb or hindlimb lameness, horses with other sites of musculoskeletal pain, such as neck, back, or sacroiliac joint pain, may also have poor performance.693, 694, 712 Neurologic deficits also have the potential to adversely affect performance.
Several muscle disorders have been linked to poor performance, including both sporadic and recurrent rhabdomyolysis (tying-up).698, 700, 713, 714 In many cases, affected horses do not exhibit the classic clinical signs of rhabdomyolysis but have increased muscle enzymes in response to exercise. In one study, 53 of 348 horses examined for poor performance had this form of subclinical myopathy identified only by increased creatine kinase concentrations after exercise, and 10 of 348 had clinical exertional rhabdomyolysis.700
Polysaccharide storage myopathy (PSSM) is an inherited glycogen storage disorder that is an important cause of muscle disease in a number of breeds, including Quarter Horses and related breeds, many draft and Warmblood breeds, and others.714 Clinical signs vary depending on the individual and breed, and the clinical presentation of affected horses ranges from being asymptomatic to exhibiting muscle atrophy and progressive weakness, muscle soreness, and gait abnormalities, or acute rhabdomyolysis. In Quarter Horses and related breeds it appears there is a second mutation, malignant hyperthermia, that potentially worsens the signs of PSSM in affected horses.24
Several other muscle disorders have been identified in horses. Recurrent exertional rhabdomyolysis (RER) is a heritable abnormality of intramuscular calcium regulation seen primarily in Thoroughbreds and to a lesser extent in Standardbreds and possibly Arabians.715 A skeletal muscle mitochondrial myopathy has been described in an Arabian in association with impaired oxidative energy metabolism; muscle stiffness and exercise intolerance due to a suspected myofibrillar myopathy have also been reported in Arabian horses.716, 717 Vitamin E deficiency has been associated with several clinical problems, one of which is a myopathy.718 Horses affected with myopathy related to vitamin E deficiency may present with signs of muscle atrophy, weakness, toe dragging, and muscle fasciculations, as well as poor performance.
Muscle strain or soreness can also be a cause of decreased performance.698 Equine activities can often be athletically challenging and may require either high intensity or prolonged effort. Some disciplines require a combination of activities and may require sudden moves or maintaining a precise body position. In some cases, the horse is not adequately conditioned, making the risk of muscle injury greater. Any muscle group may be affected, and clinical signs vary depending on the muscle involved and the severity of the injury.
Respiratory Disorders
Respiratory disease has been the most common cause of poor performance in several studies. Among the most common respiratory problems associated with poor performance in the equine athlete is dynamic airway obstruction, affecting both racing and nonracing performance horses.699, 700, 702, 709, 300, 719 In the study of 275 racehorses evaluated for poor performance, 110 (40%) were found to have dynamic airway obstruction.699 Similarly, in the study of 348 racehorses and show horses with poor performance, 148 (42.6%) had dynamic airway obstruction.700 Of these 148 affected horses, 39 were found to have multiple airway abnormalities, and an additional 22 were found to have a concurrent cardiac arrhythmia. The most common conditions causing airway obstruction include dorsal displacement of the soft palate and idiopathic left laryngeal hemiplegia with arytenoid collapse, also referred to as recurrent laryngeal neuropathy. Other conditions diagnosed have included axial deviation of the aryepiglottic folds, dynamic pharyngeal collapse, epiglottic entrapment, subepiglottic cyst, fourth branchial arch defect (rostral displacement of the palatopharyngeal arch, laryngeal dysplasia), and redundant alar folds. Horses of Quarter Horse breeding with hyperkalemic periodic paralysis (HYPP) may suffer dynamic collapse of the airway when exercising.720
Disease of the lower respiratory tract is also a common cause of decreased performance.227, 251, 755, 710, 721, 722 In the study of 27 endurance horses with poor performance, respiratory tract abnormalities, specifically tracheal fluid neutrophilia and IAD, were the most commonly identified problems.710 IAD was identified in 70% of national hunt horses presented for poor athletic performance.721 Excess mucus accumulation in the airway has been associated with poor willingness to perform in sport horses.251, 721 In addition to IAD, other subtypes of equine asthma, including recurrent airway obstruction (RAO) and summer-pasture–associated obstructive pulmonary disease, can limit athletic performance. The effects of EIPH on performance have been difficult to measure because there are so many confounding factors, but current evidence suggests that moderate to severe EIPH adversely affects athletic capacity.227, 722 Clinical bacterial or viral respiratory tract infection significantly affects performance, but the role of subclinical infections is less clear. Studies in Standardbred trotters have not found an association between subclinical viral infection and poor performance.723, 724
Cardiovascular Disorders
Cardiovascular disease can also cause poor performance, although it is less common than either musculoskeletal or respiratory disease.699, 700, 709, 710 The prevalence and clinical relevance of cardiac arrhythmias have varied between studies.699, 700, 709, 710, 725, 726, 727 In the study of 348 horses evaluated for poor performance, a clinically significant cardiac arrhythmia was the sole abnormality found in 33 horses, and an arrhythmia in conjunction with dynamic airway obstruction was found in 22 horses.700 The most frequent arrhythmias observed were atrial and ventricular premature depolarizations. However, in the study of 275 racehorses, arrhythmias were noted in just two horses.699 Isolated premature depolarizations were common in a study of 88 Thoroughbreds with poor performance, with 55 horses having at least one premature ventricular or supraventricular premature depolarization.727 The clinical significance of these depolarizations was uncertain. Ventricular tachycardia and paroxysmal atrial fibrillation have also been identified in horses with poor performance. Changes in the T wave, once thought to be related to poor performance, and second-degree atrioventricular block have been found to have no effect on exercise capacity.728
Many horses have murmurs that are of little clinical significance.699, 700, 709, 710, 729, 730 In the study of 348 horses with poor performance, 102 were found to have murmurs, the most common being mitral regurgitation.700 In all cases the murmur was determined to be clinically unimportant. Similarly, in the study of endurance horses, neither the prevalence nor the grade of valvular regurgitation was significantly different between well-performing horses and those with a decreased performance level.710 Although many murmurs appear to be clinically unimportant, in some individual cases a murmur may be associated with more significant cardiac disease and poor performance.
Echocardiography has been used to evaluate cardiac function in cases of poor performance. In the study of 348 horses, decreased fractional shortening indicating left ventricular myocardial dysfunction after exercise was found in 19 horses, only 8 of which had echocardiographic changes at rest.700 Six of the 19 horses had what was felt to be a clinically significant ventricular arrhythmia.
Other
A wide variety of other disorders have been linked to poor performance. Body condition plays a role in performance, and horses that are both overconditioned and underconditioned may have suboptimal performance.90, 697, 731, 732 A study in endurance horses demonstrated that body condition score at the start of the ride had a significant effect on performance, with horses with lower scores being less likely to complete the ride.732 Some other problems potentially associated with poor performance include dental disease, gastric ulcers, anhidrosis, and both undertraining and overtraining.706, 707, 708, 733 Poor saddle fit may cause focal areas of increased pressure resulting in an unwillingness to work. The dynamic interaction between the horse, saddle, and rider may not be optimal.734 Some foals that were sick within the first 18 hours of birth may have decreased performance as an adult.735
Diagnostic Approach to Poor Performance
Determining the cause of poor performance in those horses without overt clinical disease often is challenging.695, 698, 699, 700, 701 In the study of 348 cases of poor performance, a definitive diagnosis was established in 73.5% of cases after in-depth examination, which included the use of a high-speed treadmill.700 Multiple factors can contribute to the difficulty in establishing a definitive diagnosis. First, it can even be hard to determine whether the horse has actual poor performance or if it is simply not performing up to expectations that are unrealistic for the individual’s inherent ability or current level of training. Medical conditions can certainly influence the willingness to work, but there may also be a behavioral component that can be difficult to assess. The abnormalities that are sufficient to impair performance can be quite subtle and may be evident only during exercise. Multiple problems may be present concurrently, which can make it hard to determine the actual clinical significance of any given problem.
Equine athletes presented for poor performance should undergo a comprehensive evaluation, the basic components of which include a history, detailed physical examination, and laboratory screening. The clinician should emphasize examination of the respiratory, musculoskeletal, and cardiovascular systems because these systems most often are linked to performance problems. In many cases standardized exercise testing, generally on a high-speed treadmill, is critical in identifying the problem. Endoscopic examination of the upper airways during exercise has proven particularly useful. It may also be helpful to evaluate the horse while it’s being ridden.
Signalment and History
The signalment may be helpful in the assessment of some cases of poor performance. Horses that present at a young age may be more likely to have a congenital or heritable condition or may have limited genetic potential. Diseases with a heritable basis are more often seen in certain breeds. PSSM is found in a wide variety of breeds but is common in Quarter Horses and related breeds as well as some draft horse and Warmblood breeds, whereas RER is primarily seen in Thoroughbreds and to a lesser extent Standardbreds and possibly Arabians.713, 714, 715 RER is more common in females and in horses with a nervous temperament. Recurrent laryngeal neuropathy appears to be more common in large, long-necked horses especially of Thoroughbred or draft horse breeding.736
Obtaining a complete history is a fundamental part of evaluating poor performance. The clinician should establish the use of the horse, the time in training, and the specifics of the training program. Determining whether the horse has never performed as expected or has experienced a decline in the level of performance is crucial. If the horse has never performed as expected, the veterinarian should consider a lack of ability, congenital abnormalities, and training problems. A change in performance, either sudden or insidious, often is associated with an acquired problem. The clinician should characterize specifically the decline in performance, including the intensity of exercise at which signs are observed and whether performance is abnormal from the onset of exercise or declines during an exercise bout. In those cases in which performance drops off during exercise, the clinician should determine whether the decline is acute or gradual and whether any other signs, such as stridor, are associated with it.
Other elements of the history with particular relevance to athletic performance include any previous respiratory disease, respiratory noise, cough, or respiratory distress associated with exercise. Any change in gait also may be significant. Establishing the housing environment, feeding practices, changes in appetite or body condition, the type of tack used, and whether sweating is appropriate is important. The clinician should determine the response to any medications that have been used, such as phenylbutazone or furosemide. The information obtained in the history may help direct the investigation.
Physical Examination
The clinician should perform a complete physical examination in all cases. The body condition score should be determined. In-depth assessment of the gait, as well as the respiratory and cardiovascular systems, is generally indicated.
Respiratory Examination
The examination should include evaluation of air flow from the nares and percussion of the sinuses, as well as assessment of any cough or nasal discharge. Careful palpation of the larynx may reveal an increase in prominence of the muscular process of the left arytenoid cartilage resulting from a loss of mass of the left dorsal cricoarytenoid muscle associated with idiopathic hemiplegia. The clinician can use the laryngeal adductor response test, or slap test, to evaluate adduction of the arytenoid cartilages by slapping the withers during expiration and evaluating movement of the contralateral arytenoid by endoscopy or palpation. It is important to note that many of the horses with airway obstruction did not have a history of abnormal respiratory noise and did not have abnormalities at rest.300, 699, 700, 702, 709, 719 At the same time, not all abnormalities observed at rest caused obstruction. These findings emphasize the importance of videoendoscopy during exercise as a component of a performance evaluation.
Thorough auscultation of the trachea and lungs should be performed. Having the horse rebreathe from a plastic bag placed over the nostrils increases the respiratory rate and tidal volume, thus accentuating sounds. In addition to auscultation, the clinician should note the character and pattern of respiration, including the presence of any abdominal component, and the recovery time. Percussion of the thorax may be useful in establishing the lung border and any dull or hyperresonant areas, as well as in detecting pleural pain.
Cardiovascular Examination
Any decrease in cardiac output potentially can limit performance, making thorough evaluation of the cardiovascular system essential. On basic physical examination the clinician should evaluate the mucous membrane color, capillary refill time, and arterial and venous peripheral pulses, although finding abnormalities in these parameters in horses presented for decreased performance is uncommon. The clinician should perform careful auscultation of the heart on both sides of the thorax to evaluate the cardiac rhythm and murmurs. Electrocardiography can be used to further evaluate the cardiac rhythm and ideally should be performed before, during, and after exercise using radiotelemetry.
Musculoskeletal and Neurologic Examination
A lameness examination should be performed in all horses presented for evaluation of poor performance both because lameness is a common cause of poor performance and because it is important to establish that the horse is sound enough to safely perform an exercise test if indicated. In some horses presented for poor performance, the gait asymmetry may be subtle and only discernible at high speed, making diagnosis by traditional methods difficult. In these cases use of a sensor-based motion analysis system, gait analysis on the treadmill, and advanced diagnostic techniques such as nuclear scintigraphy, thermography, and CT or MRI may be useful. In some cases it can be helpful to perform a ridden evaluation. Careful palpation of the muscles should be performed to identify any heat, swelling, or reactivity. In many cases a neurologic examination is indicated to identify any neurologic deficits that could contribute to poor performance.
Ancillary Diagnostic Aids
Clinical Pathology/Selected Laboratory Tests
Hematologic testing and a biochemical profile are indicated, although in most horses presented for poor performance without obvious clinical abnormalities, routine evaluation of a single sample is within normal limits. Because exercise can induce some changes in laboratory parameters, such as an increase in the packed cell volume and neutrophil count, considering the time of sample collection relative to exercise is important.737, 738, 739 Potentially significant findings include changes consistent with chronic inflammation, such as anemia, hyperglobulinemia, and possibly hyperfibrinogenemia or thrombocytosis. Subclinical infections may have only slight alterations in the leukocyte count and differential. Viral infections, especially in the early stages, may be associated with a leukopenia and neutropenia. A decrease in the neutrophil-to-lymphocyte ratio has been associated with overtraining, although this is not a reliable correlation.739 Changes in the hormonal response to exercise have been reported with overtraining, primarily a decrease in cortisol.740
Horses at rest normally maintain a significant proportion of red blood cells and hemoglobin in the splenic reserve.737, 738, 741 Although total body hemoglobin increases in response to training and may correlate with performance, this cannot be determined from a resting sample. Special techniques must be used to document total red cell mass or hemoglobin.741,792 Anemia can decrease the oxygen-carrying capacity during exercise, resulting in suboptimal performance.
Signs of organ dysfunction in horses presented for poor performance are not common findings but are occasionally present. Much attention has been paid to the importance of electrolytes and exercise; however, abnormalities seldom are found. In general, circulating electrolyte concentrations are regulated tightly and may not reflect closely the total body electrolyte status.628 However, a concentration of potassium consistently below 3 mEq/L may suggest a potassium deficit. Performing renal fractional excretion of electrolytes can help in detecting chronic electrolyte deficiencies.
Myopathy can lead to decreased performance, and muscle enzymes may be elevated, although many cases of myopathy are subclinical and require evaluation of muscle enzymes after exercise.700, 713 Creatine kinase is measured before exercise and ideally 4 to 6 hours after an exercise bout consisting of 15 to 30 minutes at the trot. In normal horses this light exercise rarely causes more than a threefold increase in creatine kinase. An increase of fivefold or more indicates exertional rhabdomyolysis. Genetic testing, muscle biopsies, and measurement of vitamin E and selenium can also be helpful in defining myopathies.
Myocardial disease may contribute to left ventricular dysfunction and arrhythmias. Elevations in myocardial fractions of creatine kinase, lactate dehydrogenase, and troponin support myocardial disease but are not present in all cases.
Analysis of fluid from the lower airways is important in the diagnosis of lower airway disease. Although exact sampling techniques can vary, in most cases cytologic evaluation of bronchoalveolar lavage fluid is used to aid in the diagnosis of EIPH and forms of equine asthma such as IAD and RAO.
Endoscopy
Endoscopic examination of the upper airway is an important part of the evaluation of poor performance. As many of the abnormalities associated with poor performance are related to dynamic airway obstruction, either treadmill or overground videoendoscopy is generally indicated regardless of the history and physical examination findings.300, 719, 743, 744, 745 Endoscopy also can be useful in identifying respiratory problems other than dynamic airway collapse. For example, the clinician can identify narrowing of the ventral nasal meatus associated with sinusitis, nasal masses, and pharyngitis. In some cases evidence of inflammation and retropharyngeal lymphadenopathy on endoscopic examination of the guttural pouches has been associated with dorsal displacement of the soft palate, which may result from neuropathy of the pharyngeal branch of the vagus nerve.746
Tracheal injury and secretions in the lower respiratory tract can be visualized if the endoscope is sufficiently long. Airway secretions may be sampled via the endoscope or via a bronchoalveolar lavage catheter. Endoscopy can also be useful in diagnosing equine gastric ulcer syndrome.
Diagnostic Imaging
Diagnostic imaging can be useful in the evaluation of horses with poor performance. Radiographs and ultrasound are routinely used in the evaluation of musculoskeletal problems. In some cases, additional imaging techniques may be indicated such as nuclear scintigraphy, thermography, CT, or MRI.
Radiographs and ultrasound can also be helpful in evaluation of the respiratory tract. Thoracic ultrasound and radiographs are often used in the evaluation of lower respiratory tract disease but may be normal in conditions commonly associated with poor performance such as IAD. Radiographs of the upper airways can allow for the evaluation of soft tissue masses or fluid accumulations. In addition, abnormalities of the pharyngeal and laryngeal structures such as thickening of the soft palate or hypoplasia of the epiglottis may be seen. Upper airway ultrasonography can also be helpful, particularly in the assessment of recurrent laryngeal neuropathy.747 The denervated laryngeal muscles are hyperechoic and more homogeneous than normal muscle. Other conditions that can be diagnosed by ultrasound include arytenoid chondritis, laryngeal dysplasia, and congenital malformations of the larynx. CT and MRI have also been used to evaluate the upper airway.
Echocardiography can be used in addition to electrocardiography for further assessment of the heart.698, 748 In assessing cardiac function, it is ideal to measure indices such as percentage fractional shortening and wall motion indices, both before and after exercise. It should be taken into account that there is considerable normal variation in postexercise indices.749
Exercise Testing
Exercise testing provides a mechanism for evaluating a range of body systems under standard exercise conditions.695, 698, 750, 751 In particular, measurements of cardiorespiratory and metabolic function taken during an exercise test provide information about the capacity and efficiency of key body systems involved in energy production. From a clinical standpoint exercise testing is generally most useful in assessing the significance of abnormalities found on a physical examination; however, testing may also help to establish the reason for reduced athletic capacity in horses that have no abnormalities on basic examinations. Exercise testing can be done in the field, which mimics the condition in which the horse actually performs. However, most testing is currently done on a treadmill, which provides more consistent conditions and an opportunity to perform a greater range of measurements. The specific protocol used for exercise testing may vary somewhat.695, 698, 750, 751, 752 Occasionally a high-speed test is performed in which the horse is accelerated rapidly to maximal speed and run to fatigue. However, the most common type of test is an incremental test in which the speed increases every 1 to 2 minutes until the horse reaches fatigue, allowing for the generation of data during submaximal and maximal exercise. In most cases the test is performed with the treadmill at a slope of 10%. This slope is not so steep as to be completely unrepresentative of normal exercise, yet it ensures that maximum-intensity exercise can be performed without reaching speeds that may be too fast for horse safety. Some parameters that can be assessed in an exercise test include heart rate, blood lactate level, arterial blood gases, total red cell volume, stride length, and oxygen uptake. Various spirometers, which are masks for measuring pulmonary ventilation, can be used to measure parameters such as air flow rates, tidal volume, and the durations of phases of the respiratory cycle.698 As previously discussed, treadmill videoendoscopy is often valuable.
Heart Rate during Exercise
Evaluation of the heart rate during exercise provides an indirect index of cardiovascular capacity and function. Several heart rate monitors are available.753 Radiotelemetry also can be used to evaluate the heart rate and rhythm, particularly at the end of exercise. Because the stroke volume does not change greatly with increasing exercise speed, the heart rate provides a guide to changes in cardiac output. In general, a linear increase in heart rate occurs with increasing exercise speed up to the point at which the maximal heart rate is reached.752, 754, 755, 756 The maximal heart rate (HRmax) is identified when no further increase in heart rate occurs despite an increase in exercise speed. The HRmax does not change with training state, although the speed at which it is reached increases with increasing fitness.
One reference point for comparison of cardiovascular capacity is the treadmill speed at a heart rate of 200 beats per minute (V200). At a heart rate of 200 beats per minute, most horses are close to the point of onset of blood lactate accumulation. The V200 can be calculated by linear regression analysis or plotted using measurements taken at three to four submaximal exercise speeds, without the horse reaching maximal exercise. The clinician should take care when using the V200 to assess exercise capacity because at a heart rate of 200 beats per minute, horses may be exercising at different proportions of their HRmax and therefore their maximal oxygen uptake (VO2max). In general, however, horses with the highest cardiovascular and metabolic capacities have the highest V200 values; that is, the better horses reach a heart rate of 200 beats per minute at higher speeds than those with a lower exercise capacity. The V200 increases with training and can be useful for monitoring changes in fitness. The better quality Thoroughbreds have a V200 of 8 to 9 m/sec in an exercise test with the treadmill set at a 10% slope. Values less than 7 m/sec are abnormal and if found in a fit horse indicate decreased cardiac capacity.
Another measurement of cardiovascular capacity is the treadmill speed at which the horse reaches HRmax, known as VHRmax. This value correlates with VO2max and exercise capacity but requires the horse to exercise up to maximal speeds so that a plateau in heart rate can be identified.
Heart rate measurements are helpful in determining the actual significance of cardiac abnormalities such as murmurs and arrhythmias. In horses with functional cardiac disease, the reduced stroke volume necessitates higher heart rates to maintain adequate cardiac output. It should be taken into account that a high heart rate does not always indicate a cardiac problem. For example, studies in Standardbred racehorses have suggested that horses with musculoskeletal problems have an increased V200 and that monitoring the V200 may help to identify subclinical lameness.
Blood or Plasma Lactate Measurement
Exercising muscles produce lactate to some extent during all intensities of exercise, but production increases exponentially with the intensity of exercise.756, 757, 758 As exercise becomes more intense, the aerobic energy contribution becomes insufficient to meet total energy requirements, and increased anaerobic metabolism results in increased lactate production. Lactate diffuses from muscle to blood, and therefore blood or plasma concentrations of lactate reflect muscle lactate. Some evidence suggests that whole blood concentrations most accurately measure lactate accumulation because red blood cells actively take up lactate.758, 759, 760, 761
The rate of increase of lactate in the blood may be used as an indirect indicator of cardiovascular and metabolic capacity. Horses with the highest aerobic capacities because of a high maximal cardiac output tend to have lower lactate values at submaximal exercise intensities than those with lower aerobic capacities. Lactate values can be used to compare horses or to evaluate training in the same horse. The treadmill speed at which a plasma lactate of 4 mmol/L (VLA4) is reached is one measure of lactate production, and a high value reflects good aerobic capacity. The VLA4 has been used to monitor changes in fitness. In fit Thoroughbred horses 3 years of age and older, values for VLA4 range from 8.0 to 9.5 m/sec. Horses that are not fit or that have respiratory disease have lower values. Another useful reference is the blood or plasma lactate at conclusion of the 10-m/sec exercise step of the incremental test, and highly fit, athletic horses usually have values below 5 mmol/L. High-quality sprint horses, which perform largely under anaerobic conditions and have a high anaerobic capacity, may have high peak lactate values.
Oxygen Uptake
The measurement of oxygen uptake (VO2) is critical to assessing athletic performance.754, 755 The VO2max has been used as a key indicator of exercise capacity in human athletes since the 1950s. As the VO2 increases linearly with increasing treadmill speed, VO2max can be identified when VO2 reaches a plateau despite an increase in speed. The Thoroughbred horse has VO2max values that are higher than those of many other mammalian species when expressed on a mass-specific basis. The major factor responsible for the high VO2max in athletic horses is their high oxygen-carrying capacity, which arises from a high maximum stroke volume and to some extent a large arteriovenous oxygen content difference. The VO2max is a good index of changes in fitness and a measurement of exercise capacity in performance horses.
Maximum Oxygen Pulse
The oxygen pulse is defined as the VO2/heart rate and is expressed as mL/kg/beat. This value provides an indication of the maximum stroke volume, and in high-quality horses values range from 0.66 to 0.76 mL/kg/beat. Those horses with cardiac problems resulting in low cardiac outputs and individuals with low VO2max values usually have values in the range of 0.5 to 0.56 mL/kg/beat. The maximum oxygen pulse also has been shown to correlate with treadmill total run time.
Arterial Blood Gas Analysis during Exercise
Arterial blood gas analysis during exercise may be indicated, especially in horses in which respiratory disorders are the suspected cause of poor performance. For an accurate blood gas analysis, the clinician should take into account the temperature of the blood because it may reach 42°C during maximal exercise. At exercise intensities above 65% VO2max, athletic horses become hypoxemic, although the extent varies among individuals.762, 763, 764, 765 Horses with low VO2max values do not necessarily have a significant decrease in arterial oxygen tension.
Additional Pulmonary Function Evaluation
Assessment of pulmonary ventilation can be facilitated by measurements of respiratory resistance, air flow rates, tidal volume, and the durations of phases of the respiratory cycle. Tests can be performed during and after exercise. This testing generally requires specialized equipment such as spirometers and ergospirometers, which may become more widely available for use.
Hematocrit and Total Red Cell Volume during Exercise
The total volume of red cells is a major determinant of oxygen-carrying capacity, and therefore measurement of red cell volume can give some index of exercise capacity. A postexercise packed cell volume test is not a reliable indicator of total red cell volume primarily because of plasma volume variations, but it does provide a rough estimate of total circulating red cells.
The clinician can make an accurate determination of red cell volume by techniques that use dye dilution after mobilization of the splenic erythrocyte pool to measure the plasma volume. Although total red cell volume increases with training, some evidence indicates that Standardbred racehorses with overtraining syndrome may develop an abnormal red cell hypervolemia that contributes to poor performance.792
Peak Running Speed and Total Run Time
The peak treadmill running speed and the total run time may indicate exercise capacity. In some studies of human athletes, the peak treadmill running speed during an exercise test was shown to be a predictor of performance. Athletic Thoroughbred racehorses can complete 60 seconds at 13 m/sec during an incremental exercise test at a 10% slope.
Stride Length
Athletic horses are thought to have better stride characteristics.701, 766 Some studies have shown a correlation between maximum stride length and the treadmill run time. An accelerometric device has been used to provide quantitative information about locomotor variables that may be useful in evaluating performance.766
It is important to remember that although exercise testing can be useful in the evaluation of poor performance, a diagnosis usually cannot be made based on a single measurement. An integrative approach taking into account the history, clinical findings, and all ancillary diagnostic testing is generally required.
Footnotes
The editors and authors acknowledge and appreciate the contributions of Bonnie R. Rush, Kenneth W. Hinchcliff, and Siddra A. Hinesas as previous contributors to this chapter. Some of their original work has been incorporated into this edition.
References
- 1.Klein B.G. Thermoregulation. In: Klein B.G., editor. Cunningham’s textbook of veterinary physiology. 5th ed. Elsevier; St. Louis: 2012. [Google Scholar]
- 2.Koterba A.M. Physical examination, neonatal infection. In: Koterba A.M., Drummond W.H., Kosch P.C., editors. Equine clinical neonatology. Lea and Febiger; Philadelphia: 1990. [Google Scholar]
- 3.Dinarello C.A. Thermoregulation and the pathogenesis of fever. Infect Dis Clin North Am. 1996;10:433. doi: 10.1016/s0891-5520(05)70306-8. [DOI] [PubMed] [Google Scholar]
- 4.Guyton A.C., Hall J.E. Body temperature, temperature regulation, and fever. In: Guyton A.C., Hall J.E., editors. Textbook of medical physiology. 11th ed. Elsevier/Saunders; Philadelphia: 2006. [Google Scholar]
- 5.Guthrie A.J., Lund R.J. Thermoregulation: base mechanisms and hyperthermia. Vet Clin North Am Equine Pract. 1998;14:45. doi: 10.1016/s0749-0739(17)30211-0. [DOI] [PubMed] [Google Scholar]
- 6.Marlin D.J., Schroter R.C., White S.L. Recovery from transport and acclimatisation of competition horses in a hot humid environment. Equine Vet J. 2001;33:371. doi: 10.2746/042516401776249507. [DOI] [PubMed] [Google Scholar]
- 7.Holcomb K.E., Tucker C.B., Stull C.L. Preference of domestic horses for shade in a hot, sunny environment. J Anim Sci. 2014;92:1708. doi: 10.2527/jas.2013-7386. [DOI] [PubMed] [Google Scholar]
- 8.Holcomb K.E., Stull C.L. Effect of time and weather on preference, frequency and duration of shade use by horses. J Anim Sci. 2016;94:1653. doi: 10.2527/jas.2015-0160. [DOI] [PubMed] [Google Scholar]
- 9.Jorgensen G.H., Aanensen L., Mejdell C.M. Preference for shelter and additional heat in horses exposed to Nordic winter conditions. Equine Vet J. 2015 doi: 10.1111/evj.12522. [DOI] [PubMed] [Google Scholar]
- 10.Stachurska A., Robovsky J., Bocian K. Changes of coat cover in primitive horses living on a reserve. J Anim Sci. 2015;93:1411. doi: 10.2527/jas.2014-8668. [DOI] [PubMed] [Google Scholar]
- 11.Brinkmann L., Gerken M., Hambly C. Saving energy during hard times: energetic adaptations of Shetland pony mares. Exp Biol. 2014;217(Pt 24):4320. doi: 10.1242/jeb.111815. [DOI] [PubMed] [Google Scholar]
- 12.Geor R.J., McCutcheon L.J., Ecker G.L. Heat storage in horses during submaximal exercise before and after humid heat acclimation. J Appl Physiol. 2000;89:2283. doi: 10.1152/jappl.2000.89.6.2283. [DOI] [PubMed] [Google Scholar]
- 13.Geor R.J., McCutcheon L.J. Thermoregulatory adaptations associated with training and heat acclimation. Vet Clin North Am Equine Pract. 1998;14:97. doi: 10.1016/s0749-0739(17)30214-6. [DOI] [PubMed] [Google Scholar]
- 14.McKeever K.H., Eaton T.L., Geiser S. Age related decreases in thermoregulation and cardiovascular function in horses. Equine Vet J Suppl. 2010;38:220. doi: 10.1111/j.2042-3306.2010.00259.x. [DOI] [PubMed] [Google Scholar]
- 15.Lindinger M.I. Exercise in the heat: thermoregulatory limitations to performance in humans and horses. Can J Appl Physiol. 1999;24:152. doi: 10.1139/h99-013. [DOI] [PubMed] [Google Scholar]
- 16.Mayhew I.G., Ferguson H.O. Clinical, clinicopathological, and epidemiological features of anhidrosis in central Florida Thoroughbred horses. J Vet Intern Med. 1987;1:136. doi: 10.1111/j.1939-1676.1987.tb02001.x. [DOI] [PubMed] [Google Scholar]
- 17.McEwan Jenkinson D., Elder H.Y. Bovell DL. Equine sweating and anhidrosis part 1—equine sweating. Vet Dermatol. 2006;17:361. doi: 10.1111/j.1365-3164.2006.00545.x. [DOI] [PubMed] [Google Scholar]
- 18.McEwan Jenkinson D., Elder H.Y., Bovell D.L. Vet Dermatol. 2007;18:2. doi: 10.1111/j.1365-3164.2007.00571.x. Equine sweating and anhidrosis part 2: anhidrosis. [DOI] [PubMed] [Google Scholar]
- 19.Johnson E.B., MacKay R.J., Hernandez J.A. An epidemiologic study of anhidrosis in horses in Florida. J Am Vet Med Assoc. 2010;236:1091. doi: 10.2460/javma.236.10.1091. [DOI] [PubMed] [Google Scholar]
- 20.Fujii J., Otsu K., Zorzato F. Identification of a mutation in porcine ryanodine receptor associated with malignant hyperthermia. Science. 1991;253:448. doi: 10.1126/science.1862346. [DOI] [PubMed] [Google Scholar]
- 21.Manley S.V., Kelly A.B., Hodgson D. Malignant hyperthermia-like reactions in three anesthetized horses. J Am Vet Med Assoc. 1983;183:85. [PubMed] [Google Scholar]
- 22.Smyth G.B. Spinal cord decompression and stabilization of a comminuted axis fracture complicated by intraoperative malignant hyperthermia-like reaction in a filly. Aust Equine Vet. 1992;10:133. [Google Scholar]
- 23.Aleman M., Nieto J.E., Magdesian K.G. Malignant hyperthermia associated with ryanodine receptor 1 (C7360G) mutation in Quarter Horses. J Vet Intern Med. 2009;23:329. doi: 10.1111/j.1939-1676.2009.0274.x. [DOI] [PubMed] [Google Scholar]
- 24.McCue M.E., Valberg S.J., Jackson M. Polysaccharide storage myopathy phenotype in quarter horse-related breeds is modified by the presence of an RYR1 mutation. Neuromuscul Disord. 2009;19:37. doi: 10.1016/j.nmd.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Agrawal A., Timothy J., Thapa A. Neurogenic fever. Singapore Med. 2007;48:492. [PubMed] [Google Scholar]
- 26.Cuddy M.L. The effects of drugs on thermoregulation. AACN Clin Issues. 2004;15:238. doi: 10.1097/00044067-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Exon J.H. A review of chlorinated phenols. Vet Hum Toxicol. 1984;26:508. [PubMed] [Google Scholar]
- 28.Stratton-Phelps M., Wilson W.D., Gardner I.A. Risk of adverse effects in pneumonic foals treated with erythromycin versus other antibiotics: 143 cases (1986–1996) J Am Vet Med Assoc. 2000;68(217) doi: 10.2460/javma.2000.217.68. [DOI] [PubMed] [Google Scholar]
- 29.Stieler A.L., Sanchez L.C., Mallicote M.F. Macrolide-induced hyperthermia in foals: role of impaired sweat responses. Equine Vet J. 2015 doi: 10.1111/evj.12481. [DOI] [PubMed] [Google Scholar]
- 30.Dinarello C.A. Infection, fever, and exogenous and endogenous pyrogens: some concepts have changed. J Endotoxin Res. 2004;10:201. doi: 10.1179/096805104225006129. [DOI] [PubMed] [Google Scholar]
- 31.Kozak W., Kluger M.J., Tesfaigzi J. Molecular mechanisms of fever and endogenous antipyresis. Ann N Y Acad Sci. 2000;917:121. doi: 10.1111/j.1749-6632.2000.tb05376.x. [DOI] [PubMed] [Google Scholar]
- 32.Dinarello C.A. Cytokines as endogenous pyrogens. J Infect Dis. 1999;179(suppl 2):S294. doi: 10.1086/513856. [DOI] [PubMed] [Google Scholar]
- 33.Luheshi G.N. Cytokines and fever: mechanisms and sites of action. Ann N Y Acad Sci. 1998;856:83. doi: 10.1111/j.1749-6632.1998.tb08316.x. [DOI] [PubMed] [Google Scholar]
- 34.Ivanov A.I., Romanovsky A.A. Prostaglandin E2 as a mediator of fever: synthesis and catabolism, Front Biosci. 1977;9:2004. doi: 10.2741/1383. [DOI] [PubMed] [Google Scholar]
- 35.Simm B., Ott D., Pollatzek E. Effects of prostaglandin E2 on cells cultured from the rat organum vasculosum laminae terminalis and median preoptic nucleus. Neuroscience. 2016;28:313. doi: 10.1016/j.neuroscience.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 36.Rusyniak D.E., Zaretsky D.V., Zaretskaia The role of Orexin-1 receptors in physiologic responses evoked by microinjection of PgE2 or muscimol into the medial preoptic area. Neurosci Lett. 2011;498:162. doi: 10.1016/j.neulet.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kozak W., Fraifeld V. Non-prostaglandin eicosanoids in fever and anapyrexia. Front Biosci. 2004;9:3339. doi: 10.2741/1486. [DOI] [PubMed] [Google Scholar]
- 38.Roth J., Blatteis C.M. Mechanisms of fever production and lysis: lessons learned from experimental LPS fever. Compr Physiol. 2014;4:1563. doi: 10.1002/cphy.c130033. [DOI] [PubMed] [Google Scholar]
- 39.Blatteis C.M., Li S., Feleder C. Cytokines, PGE2 and endotoxic fever: a re-assessment. Prostglandins Other Lipid Mediat. 2005;76:1. doi: 10.1016/j.prostaglandins.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Evans S.S., Repasky E.A., Fisher D.T. Fever and the thermal regulation of immunity: the immune system feels the heat. Nature Reviews Immunol. 2015;15:335. doi: 10.1038/nri3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Still J.T. Evidence for the involvement of the organum vasculosum laminae terminalis in the febrile response of rabbits and rats. J Physiol. 1985;368:501. doi: 10.1113/jphysiol.1985.sp015872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blatteis C.M., Bealer S.L., Hunter W.S. Suppression of fever after lesions of the anteroventral third ventricle in guinea pigs. Brain Res Bull. 1983;11:519. doi: 10.1016/0361-9230(83)90124-7. [DOI] [PubMed] [Google Scholar]
- 43.Plaisacne K.I., Mackowiak P.A. Antipyretic therapy: physiologic rationale, diagnostic implications and clinical consequences. Arch Intern Med. 2000;160:449. doi: 10.1001/archinte.160.4.449. [DOI] [PubMed] [Google Scholar]
- 44.Aronoff D.M., Neilson E.G. Antipyretics: mechanisms of action and clinical use in fever suppression. Am J Med. 2001;111:304. doi: 10.1016/s0002-9343(01)00834-8. [DOI] [PubMed] [Google Scholar]
- 45.Lazarus M. The differential role of prostaglandin E2 receptors EP3 and EP4 in regulation of fever. Mol Nutr Food Res. 2006;50:451. doi: 10.1002/mnfr.200500207. [DOI] [PubMed] [Google Scholar]
- 46.Tatro J.B. Endogenous antipyretics. Clin Infect Dis. 2000;5(suppl):S190. doi: 10.1086/317519. [DOI] [PubMed] [Google Scholar]
- 47.Roth J. Endogenous antipyretics. Clinica Chimica Acta. 2006;371:13. doi: 10.1016/j.cca.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 48.Catania A., Lipton J.M. Peptide modulation of fever and inflammation within the brain. Ann N Y Acad Sci. 1998;856:62. doi: 10.1111/j.1749-6632.1998.tb08313.x. [DOI] [PubMed] [Google Scholar]
- 49.Lipton J.M., Catania A. Anti-inflammatory actions of the neuroimmunomodulator alpha-MSH. Immunol Today. 1997;18:140. doi: 10.1016/s0167-5699(97)01009-8. [DOI] [PubMed] [Google Scholar]
- 50.Steiner A.A., Antunes-Rodrigues J., McCann S.M. Antipyretic role of the NP-cGMP pathway in the anteroventral preoptic region of the rat brain. Am J Physiol Regul Integr Comp Physiol. 2002;282:R584. doi: 10.1152/ajpregu.00391.2001. [DOI] [PubMed] [Google Scholar]
- 51.Morimota A., Sakata Y., Watanabe N. Charactistics of fever and acute-phase response induced in rabbits by IL-1 and TNF. Am J Physiol. 1989;256:R35. doi: 10.1152/ajpregu.1989.256.1.R35. [DOI] [PubMed] [Google Scholar]
- 52.Soszynski D. The pathogenesis and adaptive value of fever. Postepy Hig Med Dosw. 2003;57:531. [PubMed] [Google Scholar]
- 53.Repasky E.A., Evans S.S., Dewhirst M.W. Temperature matters! And why it should matter to tumor immunologists. Cancer Immunol Res. 2013;1:210. doi: 10.1158/2326-6066.CIR-13-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee C.T., Zhong L., Mace T.A. Elevation in body temperature to fever range enhances and prolongs subsequent responsiveness of macrophages to endotoxin challenge. PLoS One. 2012;7:e30077. doi: 10.1371/journal.pone.0030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang Q., Cross A.S., Singh I.S. Febrile core temperature is essential for optimal host defense in bacterial peritonitis. Infect Immun. 2000;68:1265. doi: 10.1128/iai.68.3.1265-1270.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weinstein M.P., Iannini P.B., Stratton C.W., Eickhoff T.C. Spontaneous bacterial peritonitis: a review of 28 cases with emphasis on improved survival and factors influencing prognosis. Am J Med. 1978;64:592. doi: 10.1016/0002-9343(78)90578-8. [DOI] [PubMed] [Google Scholar]
- 57.Banet M. Fever and survival in the rat: the effect of enhancing fever. Pflugers Arch. 1979;381:35. doi: 10.1007/BF00582329. [DOI] [PubMed] [Google Scholar]
- 58.Launey Y., Nesseler N., Malledant Clinical review: fever in septic ICU patients—friend or foe? Critical Care. 2011;15:222. doi: 10.1186/cc10097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grieger T.A., Kluger M.J. Fever and survival: the role of serum iron. J Physiol. 1978;279:187. doi: 10.1113/jphysiol.1978.sp012339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kluger M.J., Rothenburg B.A. Fever and reduced iron: their interaction as a host defense response to bacterial infection. Science. 1979;203:374. doi: 10.1126/science.760197. [DOI] [PubMed] [Google Scholar]
- 61.Ballantyne G.H. Rapid drop in serum iron concentrations as a host defense mechanism: a review of experimental and clinical evidence. Am Surg. 1984;50:405. [PubMed] [Google Scholar]
- 62.Mackowiak P.A., Plaisacne K.I. Benefits and risks of antipyretic therapy. Ann N Y Acad Sci. 1998;856:214. doi: 10.1111/j.1749-6632.1998.tb08328.x. [DOI] [PubMed] [Google Scholar]
- 63.Kluger M.J., Vaughn L.K. Fever and survival in rabbits infected with Pasteurella multocida. J Physiol. 1978;282:243. doi: 10.1113/jphysiol.1978.sp012460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laupland K.B. Fever in the critically ill medical patient. Crit Care Med. 2009;37:S273. doi: 10.1097/CCM.0b013e3181aa6117. [DOI] [PubMed] [Google Scholar]
- 65.Circiumara B., Baldock G., Cohen J. A prospective study of fever in the intensive care unit. Intensive Care Med. 1999;25:668. doi: 10.1007/s001340050928. [DOI] [PubMed] [Google Scholar]
- 66.Mair T.S., Taylor F.G., Pinsent P.J. Fever of unknown origin in the horse: a review of 63 cases. Equine Vet J. 1989;21:260. doi: 10.1111/j.2042-3306.1989.tb02163.x. [DOI] [PubMed] [Google Scholar]
- 67.MacKay R.J. Quantitative intradermal terbutaline sweat test in horses. Equine Vet J. 2008;40:518. doi: 10.2746/042516408X322409. [DOI] [PubMed] [Google Scholar]
- 68.Sheoran A.S., Sponseller B.T., Holmes N. Serum and mucosal antibody isotype responses to M-like protein (SeM) of Streptococcus equi in convalescent and vaccinated horses. Vet Immunol Immunopathol. 1997;59:239. doi: 10.1016/s0165-2427(97)00074-3. [DOI] [PubMed] [Google Scholar]
- 69.Leon L.R. Hypothermia in systemic inflammation: role of cytokines. Front Biosci. 1877;9:2004. doi: 10.2741/1381. [DOI] [PubMed] [Google Scholar]
- 70.Irvine C.H. Hypothyroidism in the foal. Equine Vet J. 1984;16:302. doi: 10.1111/j.2042-3306.1984.tb01932.x. [DOI] [PubMed] [Google Scholar]
- 71.Murray M.J. Hypothyroidism and respiratory insufficiency in a neonatal foal. J Am Vet Med Assoc. 1990;197:1635. [PubMed] [Google Scholar]
- 72.Stephen J.O., Baptiste K.E., Townsend H.G. Clinical and pathologic findings in donkeys with hypothermia: 10 cases (1988–1998) J Am Vet Med Assoc. 2000;216(725) doi: 10.2460/javma.2000.216.725. [DOI] [PubMed] [Google Scholar]
- 73.Guyton A.C., Hall J.E. Dietary balances; regulation of feeding; obesity and starvation; vitamins and minerals. In: Guyton A.C., Hall J.E., editors. Textbook of medical physiology. 11th ed. Elsevier/Saunders; Philadelphia: 2006. [Google Scholar]
- 74.Kronfeld D.S. Starvation and malnutrition of horses: recognition and treatment. J Equine Vet Sci. 1993;13:298. [Google Scholar]
- 75.Robin C.A., Ireland J.L., Wylie C.E. Prevalence of and risk factors for equine obesity in Great Britain based on owner-reported body condition scores. Equine Vet J. 2015;47:196. doi: 10.1111/evj.12275. [DOI] [PubMed] [Google Scholar]
- 76.Giles S.L., Rands S.A., Nicol C.J. Obesity prevalence and associated risk factors in outdoor living domestic horses and ponies. PeerJ. 2014;2:e299. doi: 10.7717/peerj.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ireland J.L., Wylie C.E., Collins S.N. Preventive health care and owner-reported disease prevalence of horses and ponies in Great Britain. Res Vet Sci. 2013;95:418. doi: 10.1016/j.rvsc.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 78.Thatcher C.D., Pleasant R.S., Geor R.J. Prevalence of overconditioning in mature horses in southwest Virginia during the summer. J Vet Intern Med. 2012;26:1413. doi: 10.1111/j.1939-1676.2012.00995.x. [DOI] [PubMed] [Google Scholar]
- 79.Johnson P.J. The equine metabolic syndrome peripheral Cushing’s syndrome. Vet Clin North Am Equine Pract. 2002;18:271. doi: 10.1016/s0749-0739(02)00006-8. [DOI] [PubMed] [Google Scholar]
- 80.Johnson P.J., Wiedmeyer C.E., LaCarrubba A. Diabetes, insulin resistance and metabolic syndrome in horses. J Diabetes Sci Technol. 2012;6:534. doi: 10.1177/193229681200600307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Frank N., Geor R.J., Bailey S.R. Equine metabolic syndrome. J Vet Intern Med. 2010;24:467. doi: 10.1111/j.1939-1676.2010.0503.x. [DOI] [PubMed] [Google Scholar]
- 82.Martinson K.L., Coleman R.C., Rendahl A.K. Estimation of body weight and development of a body weight score for adult equids using morphometric measurements. J Anim Sci. 2014;92:2230. doi: 10.2527/jas.2013-6689. [DOI] [PubMed] [Google Scholar]
- 83.Carter R.A., Geor R.J., Burton Staniar W. Apparent adiposity assessed by standardized scoring systems and morphometric measurements in horses and ponies. Vet J. 2009;179:204. doi: 10.1016/j.tvjl.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 84.Hallebeek J.M. The body condition score in horses. Tijdschr Diergeneeskd. 2014;139:30. [PubMed] [Google Scholar]
- 85.Dugdale A.H., Grove-White D., Curtis G.C. Body condition scoring as a predictor of body fat in horses and ponies. Vet J. 2012;194 doi: 10.1016/j.tvjl.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 86.Henneke D.R., Potter G.D., Kreider J.L. Relationship between condition score, physical measurements and body fat percentage in mares. Equine Vet J. 1983;15:371. doi: 10.1111/j.2042-3306.1983.tb01826.x. [DOI] [PubMed] [Google Scholar]
- 87.Mottet R., Onan G., Hiney K. Revisiting the Henneke body condition scoring system: 25 years later. J Equine Vet Sci. 1983;29:417. [Google Scholar]
- 88.Carter R.A., Treiber K.H., Geor R.J. Prediction of incipient pasture-associated laminitis from hyperinsulinaemia, hyperleptinaemia and generalized and localized obesity in a cohort of ponies. Equine Vet J. 2009;41:171. doi: 10.2746/042516408x342975. [DOI] [PubMed] [Google Scholar]
- 89.Giles S.L., Nicol C.J., Rands S.A. Assessing the seasonal prevalence and risk factors for nuchal crest adiposity in domestic horses and ponies using the cresty neck score. BMC Vet Res. 2015;11:13. doi: 10.1186/s12917-015-0327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kearns C.F., McKeever K.H., Kumagai K. Fat-free mass is related to one-mile race performance in elite Standardbred horses. Vet J. 2002;163:260. doi: 10.1053/tvjl.2001.0656. [DOI] [PubMed] [Google Scholar]
- 91.Wyse CA, McNie KA, Tannahill VJ, et al: Prevalence of obesity in riding horses in Scotland. 162:590, 2008. [DOI] [PubMed]
- 92.Thatcher C.D., Pleasant R.S., Geor R.J. Prevalence of obesity in mature horses: an equine body condition study. J Anim Physiol Anim Nutr. 2008;92:222. [Google Scholar]
- 93.Stephenson H.M., Green M.J., Freeman S.L. Prevalence of obesity in a population of horses in the UK. Vet Rec. 2011;168:131. doi: 10.1136/vr.c6281. [DOI] [PubMed] [Google Scholar]
- 94.Johnson P.J., Wiedmeyer C.E., Messer N.T. Medical implications of obesity in horses—lessons for human obesity. J Diabetes Sci Technol. 2009;3:163. doi: 10.1177/193229680900300119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ertelt A., Barton A.K., Schmitz R.R. Metabolic syndrome: is equine disease comparable to what we know in humans? Endocr Connect. 2014;3:R81. doi: 10.1530/EC-14-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.de Graaf-Roelfsema E. Glucose homeostasis and the enteroinsular axis in the horse: a possible role in equine metabolic syndrome. Vet J. 2014;199:11. doi: 10.1016/j.tvjl.2013.09.064. [DOI] [PubMed] [Google Scholar]
- 97.Frank N., Tadros E.M. Insulin dysregulation. Equine Vet J. 2014;46:103. doi: 10.1111/evj.12169. [DOI] [PubMed] [Google Scholar]
- 98.Bamford N.J., Potter S.J., Harris P.A. Breed differences in insulin sensitivity and insulinemic responses to oral glucose in horses and ponies of moderate body condition score. Domest Anim Endocrinol. 2014;47:101. doi: 10.1016/j.domaniend.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 99.Fischer-Posovszky P., Wabitsch M., Hochberg Z. Endocrinology of adipose tissue—an update. Horm Metab Res. 2007;39:314. doi: 10.1055/s-2007-976539. [DOI] [PubMed] [Google Scholar]
- 100.Radin M.J., Sharkey L.C., Holycross B.J. Adipokines: a review of biological and analytical principles and an update in dogs, cats, and horses. Vet Clin Pathol. 2009;38:136. doi: 10.1111/j.1939-165X.2009.00133.x. [DOI] [PubMed] [Google Scholar]
- 101.Bamford N.J., Potter S.J., Harris P.A. Effect of increased adiposity on insulin sensitivity and adipokine concentrations in horses and ponies fed a high fat diet, with or without a once daily high glycaemic meal. Equine Vet J. 2015 doi: 10.1111/evj.12434. doi: 10:111/evj.12434. [DOI] [PubMed] [Google Scholar]
- 102.Kearns C.F., McKeever K.H., Roegner V. Adiponectin and leptin are related to fat mass in horses. Vet J. 2006;172:460. doi: 10.1016/j.tvjl.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 103.Pleasant R.S., Suagee J.K., Thatcher C.D. Adiposity, plasma insulin, leptin, lipids, and oxidative stress in mature light breed horses. J Vet Intern Med. 2013;27:576. doi: 10.1111/jvim.12056. [DOI] [PubMed] [Google Scholar]
- 104.Suagee J.K., Corl B.A., Crisman M.V. Relationships between body condition score and plasma inflammatory cytokines, insulin, and lipids in a mixed population of light-breed horses. J Vet Intern Med. 2013;27:157. doi: 10.1111/jvim.12021. [DOI] [PubMed] [Google Scholar]
- 105.Suagee J.K., Corl B.A., Geor R.J. A potential role for pro-inflammatory cytokines in the development of insulin resistance in horses. Animals (Basel) 2012;2:243. doi: 10.3390/ani2020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Holbrook T.C., Tipton T., McFarlane D. Neutrophil and cytokine dysregulation in hyperinsulinemic obese horses. Vet Immunol Immunopathol. 2012;145:283. doi: 10.1016/j.vetimm.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 107.Tadros E.M., Frank N., Donnell R.L. Effects of equine metabolic syndrome on inflammatory responses of horses to intravenous lipopolysaccharide infusion. Am J Vet Res. 2013;74:1010. doi: 10.2460/ajvr.74.7.1010. [DOI] [PubMed] [Google Scholar]
- 108.Farr O.M., Li C.S., Mantzoros C.S. Central nervous system regulation of eating: insight from human brain imaging. Metabolism. 2016;65:699. doi: 10.1016/j.metabol.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Morrison P.K., Bing C., Harris P.A. Preliminary investigation into a potential role for myostatin and its receptor (ActRIIB) in lean and obese horses and ponies. PLos One. 2014;9:e112621. doi: 10.1371/journal.pone.0112621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Raoult D. Microbiota, obesity and malnutrition. Microb Pathog. 2016 doi: 10.1016/j.micpath.2016.02.001. doi: 10.1016. [DOI] [PubMed] [Google Scholar]
- 111.Shepherd M.L., Ponder M.A., Burk A.O. Fibre digestibility, abundance of faecal bacteria and plasma acetate concentrations in overweight adult mares. J Nutr Sci. 2014;3:e10. doi: 10.1017/jns.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Patterson E., Ryan P.M., Cryan J.F. Gut microbiota, obesity and diabetes. Postgrad Med J. 2015 doi: 10.1136/postgradmedj-2015-133285. [DOI] [PubMed] [Google Scholar]
- 113.Treiber K.H., Kronfeld D.S., Hess T.M. Evaluation of genetic and metabolic predispositions and nutritional risk factors for pasture-associated laminitis in ponies. J Am Vet Med Assoc. 2006;228:1538. doi: 10.2460/javma.228.10.1538. [DOI] [PubMed] [Google Scholar]
- 114.Treiber K.H., Kronfeld D.S., Hess T.M. Use of proxies and reference quintiles obtained from minimal model analysis for determination of insulin sensitivity and pancreatic beta-cell responsiveness in horses. Am J Vet Res. 2005;66:2114. doi: 10.2460/ajvr.2005.66.2114. [DOI] [PubMed] [Google Scholar]
- 115.Schott H.C. Pituitary pars intermedia dysfunction: challenges of diagnosis and treatment. Proc Am Assoc Equine Pract. 2006;52:60. [Google Scholar]
- 116.Beech J., Boston R., Lindborg S. Comparison of cortisol and ACTH responses after administration of thyrotropin releasing hormone in normal horses and those with pituitary pars intermedia dysfunction. J Vet Intern Med. 2011;25:1431. doi: 10.1111/j.1939-1676.2011.00810.x. [DOI] [PubMed] [Google Scholar]
- 117.Giles S.L., Nicol C.J., Harris P.A. Dominance rank is associated with body condition in outdoor-living domestic horses (Equus caballus) Appl Anim Behav Sci. 2015;166:71. doi: 10.1016/j.applanim.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kiela P.R., Ghishan F.K. Physiology of intestinal absorption and secretion. Best Pract Res Clin Gastroenterol. 2016;30:145. doi: 10.1016/j.bpg.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Roberts M.C. Malabsorption syndromes in the horse. Compend Cont Educ Pract Vet. 1985;7:S637. [Google Scholar]
- 120.Sloet van Oldruitenborgh-Oosterbaan MM Lactose intolerance in foals. Equine Vet Educ. 2008;20:252. [Google Scholar]
- 121.Laviano A., Inui A., Marks D.L. Neural control of the anorexia-cachexia syndrome. Am J Physiol Endocrinol Metab. 2008;295:E1000–E1008. doi: 10.1152/ajpendo.90252.2008. [DOI] [PubMed] [Google Scholar]
- 122.Ramos E.J., Suzuki S., Marks D. Cancer anorexia-cachexia syndrome: cytokines and neuropeptides. Curr Opin Clin Nutr Metab Care. 2004;7:427. doi: 10.1097/01.mco.0000134363.53782.cb. [DOI] [PubMed] [Google Scholar]
- 123.Cymbaluk N.E., Christison G.I. Environmental effects on thermoregulation and nutrition of horses. Vet Clin North Am Equine Pract. 1990;6:355. doi: 10.1016/s0749-0739(17)30546-1. [DOI] [PubMed] [Google Scholar]
- 124.Morgan K. Thermoneutral zone and critical temperatures of horses. J Thermal Biol. 1998;23:59. [Google Scholar]
- 125.Ott E.A. Influence of temperature stress on the energy and protein metabolism and requirements of the working horse. Livestock Proc Sci. 2005;92:123. [Google Scholar]
- 126.Plank L.D., Hill G.L. Energy balance in critical illness. Proc Nutr Soc. 2003;62:545. doi: 10.1079/pns2003259. [DOI] [PubMed] [Google Scholar]
- 127.Jose-Cunilleras E., Viu J., Corradini I. Energy expenditure of critically ill neonatal foals. Equine Vet J Suppl. 2012;41:48. doi: 10.1111/j.2042-3306.2011.00500.x. [DOI] [PubMed] [Google Scholar]
- 128.Evans W.J., Morley J.E., Argiles J. Cachexia: a new definition. Clin Nutr. 2008;27:793. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 129.Fearon K., Strasser F., Anker S.D. Definition and classification of cancer cachexia. The Lancet. 2011;12:489. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 130.Anker S.D., Morleoy J.E. Cachexia: a nutritional syndrome? J Cachexia Sarcopenia Muscle. 2015;6:269. doi: 10.1002/jcsm.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kir S., Komaba H., Garcia A.P. PTH/PTHrP receptor mediates cachexia in models of kidney failure and cancer. Cell Metab. 2015 doi: 10.1016/jcmet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Burfeind K.G., Michaelis K.A., Marks D.L. The central role of hypothalamic inflammation in the acute illness response and cachexia. Semin Cell Dev Biol. 2015 doi: 10.1016/j.semcdb.2015.10.038. doi: 10:1016/jsemcbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yoshida T., Delafontaine P. Mechanisms of cachexia in chronic disease states. Am J Med Sci. 2015;350:250. doi: 10.1097/MAJ.0000000000000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Reid M.B., Yi-Ping L. Tumor necrosis factor-α and muscle wasting: a cellular perspective. Respir Rec. 2001;2:269. doi: 10.1186/rr67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ramirez S., McClure J.J., Moore R.M. Hyperthyroidism associated with a thyroid adenocarcinoma in a 21-year-old gelding. J Vet Intern Med. 1998;12:475. doi: 10.1111/j.1939-1676.1998.tb02153.x. [DOI] [PubMed] [Google Scholar]
- 136.Frank N., Sojka J., Messer N.T. Equine thyroid dysfunction. Vet Clin North Am Equine Pract. 2002;18:305. doi: 10.1016/s0749-0739(02)00007-x. [DOI] [PubMed] [Google Scholar]
- 137.Rygiel K.A., Piccard M., Turnbull D.M. The ageing neuromuscular system and sarcopenia—a mitochondrial perspective. J Physiol. 2016 doi: 10.1113/JP271212. doi: 10.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lehnhard K.A., McKeever K.H., Kearns C.F. Myosin heavy chain profiles and body composition are different in old versus young Standardbred mares. Vet J. 2004;167:59. doi: 10.1016/s1090-0233(03)00045-5. [DOI] [PubMed] [Google Scholar]
- 139.Kim J., Hinchcliff K.W., Yamaguchi M. Age-related changes in metabolic properties of equine skeletal muscle associated with muscle plasticity. Vet J. 2005;169:397. doi: 10.1016/j.tvjl.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 140.Aleman M., Watson J.K., Williams D.C. Myopathy in horses with pituitary pars intermedia dysfunction (Cushing’s disease) Neuromusc Disord. 2006;16:737. doi: 10.1016/j.nmd.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 141.Metcalfe L.V.A., More S.J., Duggan V. A retrospective study of horses investigated for weight loss despite a good appetite (2002–2011) Equine Vet J. 2013;45(340) doi: 10.1111/j.2042-3306.2012.00624.x. [DOI] [PubMed] [Google Scholar]
- 142.Schumacher J., Edwards J.F., Cohen N.D. Chronic inflammatory bowel diseases of the horse. J Vet Intern Med. 2000;14:258. doi: 10.1892/0891-6640(2000)014<0258:ciibdo>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 143.Traub J.L., Bayly W.M., Reed S.M. Intra-abdominal neoplasia as a cause of chronic weight loss in the horse. Compend Cont Educ Pract Vet. 1983;5:S526. [Google Scholar]
- 144.Rumbaugh G.E., Smith B.P., Carlson G.P. Internal abdominal abscesses in the horse: a study of 25 cases. J Am Vet Med Assoc. 1978;172:304. [PubMed] [Google Scholar]
- 145.Foreman J.H., Weidner J.P., Parry B.A. Pleural effusion secondary to thoracic metastatic mammary adenocarcinoma in a mare. J Am Vet Med Assoc. 1990;197:1193. [PubMed] [Google Scholar]
- 146.McGorum B.C., Murphy D., Love S. Clinicopathological features of equine primary hepatic disease: a review of 50 cases. Vet Rec. 1999;145:134. doi: 10.1136/vr.145.5.134. [DOI] [PubMed] [Google Scholar]
- 147.Moore B.R., Abood A.S., Hinchcliff K.W. Hyperlipemia in 9 miniature horses and miniature donkeys. J Vet Intern Med. 1994;8:376. doi: 10.1111/j.1939-1676.1994.tb03253.x. [DOI] [PubMed] [Google Scholar]
- 148.West H.J. Clinical and pathological studies in horses with hepatic disease. Equine Vet J. 1996;28:146. doi: 10.1111/j.2042-3306.1996.tb01607.x. [DOI] [PubMed] [Google Scholar]
- 149.McLeland S. Diseases of the equine urinary system. Vet Clin North Am Equine Pract. 2015;31:377. doi: 10.1016/j.cveq.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 150.Tan R.H., Davies S.E., Crisman M.V. Propylthiouracil for treatment of hyperthyroidism in a horse. J Vet Intern Med. 2008;22:1253. doi: 10.1111/j.1939-1676.2008.0169.x. [DOI] [PubMed] [Google Scholar]
- 151.Alberts M.K., McCann J.P., Woods P.R. Hemithyroidectomy in a horse with confirmed hyperthyroidism. J Am Vet Med Assoc. 2000;217:1051. doi: 10.2460/javma.2000.217.1051. [DOI] [PubMed] [Google Scholar]
- 152.Johnson P.J., Scotty N.C., Wiedmeyer C. Diabetes mellitus in a domesticated Spanish Mustang. J Am Vet Med Assoc. 2005;226:584. doi: 10.2460/javma.2005.226.584. [DOI] [PubMed] [Google Scholar]
- 153.Clabough D.L. Equine infectious anemia: the clinical signs, transmission, and diagnostic procedures. Vet Med. 1990;85:1007. [Google Scholar]
- 154.Divers T.J., Mohammed H.O., Hintz H.F. Equine motor neuron disease: a review of clinical and experimental studies. Clin Techniques Equine Pract. 2006;5:24. [Google Scholar]
- 155.Lewis S.S., Valberg S.J., Nielsen I.L. Suspected immune-mediated myositis in horses. J Vet Intern Med. 2007;21:495. doi: 10.1892/0891-6640(2007)21[495:simih]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 156.Meredith T.B., Dobrinski I. Thyroid function and pregnancy status in broodmares. J Am Vet Med Assoc. 2004;224:892. doi: 10.2460/javma.2004.224.892. [DOI] [PubMed] [Google Scholar]
- 157.Breuhaus B.A. Thyroid-stimulating hormone in adult euthyroid and hypothyroid horses. J Vet Intern Med. 2002;16:109. doi: 10.1892/0891-6640(2002)016<0109:tshiae>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 158.Morris D.D., Garcia M. Thyroid-stimulating hormone response test in healthy horses, and effect of phenylbutazone on equine thyroid hormones. Am J Vet Res. 1983;44:503. [PubMed] [Google Scholar]
- 159.Jacobs K.A., Bolton J.R. Effect of diet on the oral D-xylose absorption test in the horse. Am J Vet Res. 1856;43:1982. [PubMed] [Google Scholar]
- 160.Mair T.S., Hillyer M.H., Taylor F.G. Small intestinal malabsorption in the horse: an assessment of the specificity of the oral glucose tolerance test. Equine Vet J. 1991;23:334. doi: 10.1111/j.2042-3306.1991.tb03735.x. [DOI] [PubMed] [Google Scholar]
- 161.Perrin C., Unterborn J.N., Ambrosio C.D., Hill N.S. Pulmonary complications of chronic neuromuscular diseases and their management. Muscle Nerve. 2004;29:5. doi: 10.1002/mus.10487. [DOI] [PubMed] [Google Scholar]
- 162.Schramm C.M. Current concepts of respiratory complications of neuromuscular disease in children. Curr Opin Pediatr. 2000;12:203. doi: 10.1097/00008480-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 163.Niimi A., Matsumoto H., Ueda T. Impaired cough reflex in patients with recurrent pneumonia. Thorax. 2003;58:152. doi: 10.1136/thorax.58.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Irwin R.S., Curley F.J., French C.L. Chronic cough. The spectrum and frequency of causes, key components of the diagnostic evaluation, and outcome of specific therapy. Am Rev Respir Dis. 1990;141:640. doi: 10.1164/ajrccm/141.3.640. [DOI] [PubMed] [Google Scholar]
- 165.Irwin R.S., Baumann M.H., Bolser D.C. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:1S. doi: 10.1378/chest.129.1_suppl.1S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schappert S.M., Burt C.W. Ambulatory care visits to physicians’ offices, hospital outpatient departments, and emergency departments: United States, 2001–02. Vital Health Stat. 2006;13:1. [PubMed] [Google Scholar]
- 167.Canning B.J. The cough reflex in animals: relevance to human cough research. Lung. 2008;186:S23. doi: 10.1007/s00408-007-9054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chang A.B., Widdicombe J.G. Cough throughout life: children, adults and the senile. Pulm Pharmacol Ther. 2007;20:371. doi: 10.1016/j.pupt.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 169.Chang A.B. Pediatric cough: children are not miniature adults. Lung. 2010;1:S33. doi: 10.1007/s00408-009-9166-2. [DOI] [PubMed] [Google Scholar]
- 170.Fujjimura M., Sakamoto S., Kamio Y. Female gender as a determinant of cough threshold to inhaled capsaicin. Eur Resp J. 1996;9:1624. doi: 10.1183/09031936.96.09081624. [DOI] [PubMed] [Google Scholar]
- 171.Kavlcikova-Bogdanova N., Buday T., Plevkova J., Song W.J. Chronic cough as a female gender issue. Adv Exp Med Biol. 2016;905:69. doi: 10.1007/5584_2015_182. [DOI] [PubMed] [Google Scholar]
- 172.Morice A.H. Chronic cough hypersensitivity syndrome. Cough. 2013;9:14. doi: 10.1186/1745-9974-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chang A.B. The physiology of cough. Paed Resp Rev. 2006;7:2. doi: 10.1016/j.prrv.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 174.McCool F.D. Global physiology and pathophysiology of cough. Chest. 2006;129:48S. doi: 10.1378/chest.129.1_suppl.48S. [DOI] [PubMed] [Google Scholar]
- 175.Robinson N.E. Pathophysiology of coughing. Proceedings of the thirty-second convention of the American Association of Equine Practitioners. Nashville. 1986;291 [Google Scholar]
- 176.Canning B.J., Chang A.B., Bolser D.C. Anatomy and neurophysiology of cough: CHEST guideline and expert panel report. Chest. 2014;146:1633. doi: 10.1378/chest.14-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Canning B.J. Encoding of the cough reflex. Pulm Pharmacol Ther. 2007;20:396. doi: 10.1016/j.pupt.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Polverino M., Polverino F., Fasolino Anatomy and neuro-pathophysiology of the cough reflex arc. Multidisciplinary Resp Med. 2012;7:5. doi: 10.1186/2049-6958-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Canning B.J. Anatomy and neurophysiology of the cough reflex: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:33S. doi: 10.1378/chest.129.1_suppl.33S. [DOI] [PubMed] [Google Scholar]
- 180.Canning B.J., Mori N., Mazzone S.B. Vagal afferent nerves regulating the cough reflex. Respir Physiol Neurobiol. 2006;152:223. doi: 10.1016/j.resp.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 181.Mazzone S.B. An overview of the sensory receptors regulating cough. Cough. 2005;1:2. doi: 10.1186/1745-9974-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bloustine S., Langston L., Miller T. Ear-cough (Arnold’s) reflex. Ann Otol Rhinol Laryngol. 1976;85:496. doi: 10.1177/000348947608500315. [DOI] [PubMed] [Google Scholar]
- 183.Zholos A.V. TRP channels in respiratory pathophysiology: the role of oxidative, chemical irritant and temperature stimuli. Curr Neuropharmacol. 2015;13:279. doi: 10.2174/1570159X13666150331223118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Geppetti P., Patacchini R., Nassini R. Cough: the emerging role of the TRPA1 channel. Lung. 2010;1:S63. doi: 10.1007/s00408-009-9201-3. [DOI] [PubMed] [Google Scholar]
- 185.Taylor-Clark T.E. Role of reactive oxygen species and TRP channels in the cough reflex. Cell Calcium. 2016 doi: 10.1016/j.ceca.2016.03.007. doi.org/10.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bonvini S.J., Birrell M.A., Smith J.A., Belvisi M.G. Targeting TRP channels for chronic cough: from bench to bedside. Naunyn Schmiedebergs Arch Pharmacol. 2015;388:401. doi: 10.1007/s00210-014-1082-1. [DOI] [PubMed] [Google Scholar]
- 187.Grace M.S., Dubuis E., Birrell M.A., Belvisi M.G. TRP channel antagonists as potential antitussives. Lung. 2012;190:11. doi: 10.1007/s00408-011-9322-3. [DOI] [PubMed] [Google Scholar]
- 188.Lee L.Y., Kwong K., Lin Y.S. Hypersensitivity of bronchopulmonary C-fibers induced by airway mucosal inflammation: cellular mechanisms. Pulm Pharmacol Ther. 2002;15:199. doi: 10.1006/pupt.2002.0338. [DOI] [PubMed] [Google Scholar]
- 189.Canning B.J. Interactions between vagal afferent nerve subtypes mediating cough. Pulm Pharmacol Ther. 2002;15:187. doi: 10.1006/pupt.2002.0363. [DOI] [PubMed] [Google Scholar]
- 190.Karlsson J.A., Sant’Ambrogio G., Widdicombe J. Afferent neuronal pathways in cough and reflex bronchoconstriction. J Appl Physiol. 1988;65:1007. doi: 10.1152/jappl.1988.65.3.1007. [DOI] [PubMed] [Google Scholar]
- 191.Pounsford J. Cough and bronchoconstriction. Bull Eur Physiopathol Respir. 1987;23(S10):37s. [PubMed] [Google Scholar]
- 192.Pantaleo T., Bongianni F., Mutolo D. Central nervous mechanisms of cough. Pulm Pharmacol Ther. 2002;15:227. doi: 10.1006/pupt.2002.0358. [DOI] [PubMed] [Google Scholar]
- 193.Haji A., Kimura S., Ohi Y. A model of the central regulatory system for cough reflex. Biol Pharm Bull. 2013;36:501. doi: 10.1248/bpb.b13-00052. [DOI] [PubMed] [Google Scholar]
- 194.Shannon R., Baekey D.M., Morris K.F. Production of reflex cough by brainstem respiratory networks. Pulm Pharmacol Ther. 2004;17:369. doi: 10.1016/j.pupt.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 195.Canning B.J. Central regulation of the cough reflex: therapeutic implications. Pulm Pharmacol Ther. 2009;22:75. doi: 10.1016/j.pupt.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Allain H., Bentue-Ferrer D., Daval G. Mechanisms of chronic cough pathophysiology. Rev Mal Respir. 2004;21:763. doi: 10.1016/s0761-8425(04)71417-5. [DOI] [PubMed] [Google Scholar]
- 197.Foster W.M. Mucociliary transport and cough in humans. Pulm Pharmacol Ther. 2002;15:277. doi: 10.1006/pupt.2002.0351. [DOI] [PubMed] [Google Scholar]
- 198.Rubin B.K. Secretion properties, clearance and therapy in airway disease. Transl Respir Med. 2014;2:6. doi: 10.1186/2213-0802-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Irwin R.S. Complications of cough: Evidence-based clinical practice guidelines. Chest. 2006;129:55S. doi: 10.1378/chest.129.1_suppl.54S. [DOI] [PubMed] [Google Scholar]
- 200.Johansson A.M., Gardner S.Y., Atkins C.E. Cardiovascular effects of acute pulmonary obstruction in horses with recurrent airway obstruction. J Vet Intern Med. 2007;21:302. doi: 10.1892/0891-6640(2007)21[302:ceoapo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 201.Dicpinigaitis P.V., Lim L., Farmakidis C. Cough syncope. Respir Med. 2014;108:244. doi: 10.1016/j.rmed.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 202.Nelson R.W., Couto C.G., editors. Essentials of small animal internal medicine. 2nd ed. Mosby; St. Louis: 1992. [Google Scholar]
- 203.Clayton H., Murphy J. The coughing horse. In. Practice. 1980;2:25. [Google Scholar]
- 204.Gilkerson J.R., Bailey K.E., Diaz-Mendez A., Hartley C.A. Update on viral diseases of the equine respiratory tract. Vet Clin North Am Equine Pract. 2015;31:91. doi: 10.1016/j.cveq.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 205.Ma G., Azab W., Osterrieder N. Equine herpes viruses type 1 (EHV-1) and 4 (EHV-4)—masters of co-evolution and a constant threat to equids and beyond. Vet Microbiol. 2013;167:123. doi: 10.1016/j.vetmic.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 206.Reuss S.M., Giguere S. Update on bacterial pneumonia and pleuropneumonia in the adult horse. Vet Clin North Am Equine Pract. 2015;31:105. doi: 10.1016/j.cveq.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 207.Reuss S.M., Cohen N.D. Update on bacterial pneumonia in the foal and weanling. Vet Clin North Am Equine Pract. 2015;31:121. doi: 10.1016/j.cveq.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 208.Ogilvie T.H., Rosendal S., Blackwell T.E. Mycoplasma felis as a cause of pleuritis in horses. J Am Vet Med Assoc. 1983;192:1374. [PubMed] [Google Scholar]
- 209.Stewart A.J., Cuming R.S. Update on fungal respiratory disease in horses. Vet Clin North Am Equine Pract. 2015;31:43. doi: 10.1016/j.cveq.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 210.Cafarchia C., Figueredo L.A., Otranto D. Fungal diseases of horses. Vet Microbiol. 2013;167:215. doi: 10.1016/j.vetmic.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 211.Wilkins P.A., Lascola K.M. Update on interstitial pneumonia. Vet Clin North Am Equine Pract. 2015;31:137. doi: 10.1016/j.cveq.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 212.Williams K.J., Maes R., Del Piero F. Equine multinodular pulmonary fibrosis: a newly recognized herpesvirus-associated fibrotic lung disease. Vet Pathol. 2007;44:849. doi: 10.1354/vp.44-6-849. [DOI] [PubMed] [Google Scholar]
- 213.Scarrett W.K., Crisman M.V. Neoplasia of the respiratory tract. Vet Clin North Am Equine Pract. 1998;14:451. doi: 10.1016/s0749-0739(17)30180-3. [DOI] [PubMed] [Google Scholar]
- 214.Marsh P.S. Fire and smoke inhalation injury in horses. Vet Clin North Am Equine Pract. 2007;23:19. doi: 10.1016/j.cveq.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 215.Couetil L.L., Hoffman A.M., Hodgson J. Inflammatory airway disease of horses. J Am Vet Intern Med. 2007;21:356. doi: 10.1892/0891-6640(2007)21[356:iadoh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 216.Couetil L.L., Cardwell J.M., Gerber V. Inflammatory airway disease of horses—revised consensus statement. J Vet Intern Med. 2016;30:503. doi: 10.1111/jvim.13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Mazan M.R. Update on noninfectious inflammatory diseases of the lower airway. Vet Clin North Am Equine Pract. 2015;31:159. doi: 10.1016/j.cveq.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 218.Pirie R.S. Recurrent airway obstruction: a review. Equine Vet J. 2014;46:276. doi: 10.1111/evj.12204. [DOI] [PubMed] [Google Scholar]
- 219.Ireland J.L., Christley R.M., McGowan C.M. Prevalence of and risk factors for recurrent airway obstruction in geriatric horses and ponies. Equine Vet J. 2015;47:25. [Google Scholar]
- 220.Hotchkiss J.W., Reid S.W., Christley R.M. A survey of horse owners in Great Britain regarding horses in their care. Part 2: risk factors for recurrent airway obstruction. Equine Vet J. 2007;39:301. doi: 10.2746/042516407x180129. [DOI] [PubMed] [Google Scholar]
- 221.Gerber V., Swinburne J.E., Blott S.E. Genetics of recurrent airway obstruction (RAO) Dtsch Tierarztl Wochenschr. 2008;115:271. [PubMed] [Google Scholar]
- 222.Gerber V., Tessier C., Marti E. Genetics of upper and lower airway diseases in the horse. Equine Vet J. 2015;47:390. doi: 10.1111/evj.12289. [DOI] [PubMed] [Google Scholar]
- 223.Schwartz L.W., Knight H.D., Whittig L.D. Silicate pneumoconiosis and pulmonary fibrosis in horses from the Monterey-Carmel peninsula. Chest. 1981;80:82. doi: 10.1378/chest.80.1_supplement.82s. [DOI] [PubMed] [Google Scholar]
- 224.Lyons E.T., Tolliver S.C., Drudge J.H. Lungworms (Dictyocaulus arnfeldi): prevalence in live equids in Kentucky. Am J Vet Res. 1985;46:921. [PubMed] [Google Scholar]
- 225.Goetz T.E. Dictyocaulus arnfeldi as a possible cause of chronic cough in 14 horses. Equine Pract. 1984;6:33. [Google Scholar]
- 226.Bell S.A., Drew C.P., Wilson W.D., Pusterla N. Idiopathic chronic eosinophilic pneumonia in 7 horses. J Vet Intern Med. 2008;22:648. doi: 10.1111/j.1939-1676.2008.0100.x. [DOI] [PubMed] [Google Scholar]
- 227.Hinchcliff K.W., Couetil L.L., Knight P.K. Exercise induced pulmonary hemorrhage in horses: American College of Veterinary Internal Medicine consensus statement. J Am Vet Intern Med. 2015;29:743. doi: 10.1111/jvim.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Leguillette R., Steinmann M., Bond S.L., Stanton B. Tracheobronchoscopic assessment of exercise-induced pulmonary hemorrhage and airway inflammation in barrel racing horses. J Vet Intern Med. doi:10.1111/jvim. 2016;13959 doi: 10.1111/jvim.13959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Davis J.L., Gardner S.Y., Schwabenton B. Congestive heart failure in horses: 14 cases (1984–2001) J Am Vet Med Assoc. 2002;220(1512) doi: 10.2460/javma.2002.220.1512. [DOI] [PubMed] [Google Scholar]
- 230.Leroux A.A., Detilleux J., Sandersen C.F. Prevalence and risk factors for cardiac diseases in a hospital-based population of 3,434 horses (1994–2011) J Vet Intern Med. 2013;27(1563) doi: 10.1111/jvim.12197. [DOI] [PubMed] [Google Scholar]
- 231.Auer D.E., Wilson R.G., Groenendyk S. Pharyngeal lymphoid hyperplasia in Thoroughbred racehorses in training. Aust Vet J. 1985;62:124. doi: 10.1111/j.1751-0813.1985.tb07259.x. [DOI] [PubMed] [Google Scholar]
- 232.Christley R.M., Hodgson D.R., Rose R.J. Coughing in Thoroughbred racehorses: risk factors and tracheal endoscopic and cytological findings. Vet Rec. 2001;148:99. doi: 10.1136/vr.148.4.99. [DOI] [PubMed] [Google Scholar]
- 233.Aleman M, Nieto JE, Benak J, et al: Tracheal collapse in American Miniature Horses: 13 cases (1985–2007). 2008;233:1302. [DOI] [PubMed]
- 234.Tetens J., Hubert J.D., Eddy A.L. Dynamic tracheal collapse as a cause of exercise intolerance in a Thoroughbred. J Am Vet Med Assoc. 2000;216:722. doi: 10.2460/javma.2000.216.722. [DOI] [PubMed] [Google Scholar]
- 235.Fenger C.K., Kohn C.W. Tracheal obstruction from tracheal collapse associated with pneumonia in a horse. J Am Vet Med Assoc. 1992;200:1698. [PubMed] [Google Scholar]
- 236.Bakos Z., Voros K., Kellokoski H., Reiczigel J. Comparison of the caudal lung borders determined by percussion and ultrasonography in horses with recurrent airway obstruction. Acta Vet Hung. 2003;51:249. doi: 10.1556/AVet.51.2003.3.1. [DOI] [PubMed] [Google Scholar]
- 237.Kusano K., Hobo S., Ode H., Ishikawa Y. Tracheal endoscopic and cytologic findings and blood examination results in Thoroughbred racehorses suspected to have lower respiratory tract disease. J Equine Sci. 2008;19:97. doi: 10.1294/jes.19.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Richard E.A., Pitel P.H., Christmann U. Serum concentration of surfactant protein D in horses with lower airway inflammation. Equine Vet J. 2012;44:277. doi: 10.1111/j.2042-3306.2011.00421.x. [DOI] [PubMed] [Google Scholar]
- 239.Wagner B., Ainsworth D.M., Freer H. Analysis of soluble CD14 and its use as a biomarker in neonatal foals with septicemia and horses with recurrent airway obstruction. Vet Immunol Immunopathol. 2013;155:124. doi: 10.1016/j.vetimm.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 240.Hoffman A.M., Viel L. Techniques for sampling the respiratory tract of horses. Vet Clin North Am Equine Pract. 1997;13:463. doi: 10.1016/s0749-0739(17)30224-9. [DOI] [PubMed] [Google Scholar]
- 241.Hoffman A.M. Bronchoalveolar lavage: sampling technique and guidelines for cytologic preparation and interpretation. Vet Clin North Am Equine Pract. 2008;24:423. doi: 10.1016/j.cveq.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 242.Wysocka B., Klusinski W. Cytological evaluation of tracheal aspirate and broncho-alveolar lavage fluid in comparison to endoscopic assessment of lower airways in horses with recurrent airways obstruction or inflammatory airway disease. Pol J Vet Sci. 2015;18:587. doi: 10.1515/pjvs-2015-0076. [DOI] [PubMed] [Google Scholar]
- 243.Sweeney C.R., Sweeney R.W., Benson C.E. Comparison of bacteria isolated from specimens obtained by use of endoscopic guarded tracheal swabbing and percutaneous tracheal aspiration in horses. J Am Vet Med Assoc. 1989;195:1225. [PubMed] [Google Scholar]
- 244.Moore B.R., Dradowka S., Robertson J.T. Cytologic evaluation of bronchoalveolar lavage fluid obtained from Standardbred racehorses with inflammatory airway disease. Am J Vet Res. 1995;56:562. [PubMed] [Google Scholar]
- 245.Rossier Y., Sweeney C.R., Ziemer E.L. Bronchoalveolar lavage fluid cytologic findings in horses with pneumonia or pleuropneumonia. J Am Vet Med Assoc. 1991;198:1001. [PubMed] [Google Scholar]
- 246.Hewson J., Arroyo L.G. Respiratory disease: diagnostic approaches in the horse. Vet Clin North Am Equine Pract. 2015;31:307. doi: 10.1016/j.cveq.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 247.Gerber V., Straub R., Marti E. Endoscopic scoring of mucus quantity and quality: observer and horse variance and relationship to inflammation, mucus viscoelasticity and volume. Equine Vet J. 2004;36:576. doi: 10.2746/0425164044864525. [DOI] [PubMed] [Google Scholar]
- 248.Koblinger K., Nicol J., McDonald K. Endoscopic assessment of airway inflammation in horses. J Vet Intern Med. 2011;25:1118. doi: 10.1111/j.1939-1676.2011.00788.x. [DOI] [PubMed] [Google Scholar]
- 249.Rettmer H., Hoffman A.M., Lanz S. Owner-reported coughing and nasal discharge are associated with clinical findings, arterial oxygen tension, mucus score and bronchoprovocation in horses with recurrent airway obstruction in a field setting. Equine Vet J. 2015;47:291. doi: 10.1111/evj.12286. [DOI] [PubMed] [Google Scholar]
- 250.Holcombe S.J., Robinson N.E., Derksen F.J. Effect of tracheal mucus and tracheal cytology on racing performance in Thoroughbred racehorses. Equine Vet J. 2006;38:300. doi: 10.2746/042516406777749191. [DOI] [PubMed] [Google Scholar]
- 251.Widmer A., Doherr M.G., Tessier C. Association of increased tracheal mucus accumulation with poor willingness to perform in show-jumpers and dressage horses. Vet J. 2009;182:430. doi: 10.1016/j.tvjl.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 252.Bedenice D., Mazan M.R., Hoffman A.M. Association between cough and cytology of bronchoalveolar lavage fluid and pulmonary function in horses diagnosed with inflammatory airway disease. J Vet Intern Med. 2008;22:1022. doi: 10.1111/j.1939-1676.2008.0109.x. [DOI] [PubMed] [Google Scholar]
- 253.Bullone M., Helie P., Joubert P., Lavoie J.P. Development of a semiquantitative histological score for the diagnosis of heaves using endobronchial biopsy specimens of horses. J Vet Intern Med. 2016;14556 doi: 10.111/jvim. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Venner M., Schmidbauer S., Drommer W., Deegen E. Percutaneous lung biopsy in the horse: comparsion of two instruments and repeated biopsy in horses with induced acute interstitial pneumopathy. J Vet Intern Med. 2006;20:968. doi: 10.1892/0891-6640(2006)20[968:plbith]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 255.Ainsworth D.M., Davidow E. Respiratory distress in large animals. Proc Forum ACVIM. 1994;12:589. [Google Scholar]
- 256.West J.B. 9th ed. Lippincott, Williams & Wilkins; Baltimore: 2012. Respiratory physiology: the essentials. [Google Scholar]
- 257.Wilson W.D., Lakritz J. Alterations in respiratory function. In: Smith B.P., editor. Large animal internal medicine. 5th ed. Elsevier Mosby; St. Louis: 2015. [Google Scholar]
- 258.Mazan M.R., Deveney E.F., DeWitt S. Energetic cost of breathing, body composition, and pulmonary function in horses with recurrent airway obstruction. J Appl Physiol. 2004;97:91. doi: 10.1152/japplphysiol.00629.2003. [DOI] [PubMed] [Google Scholar]
- 259.Beech J., editor. Equine respiratory disorders. Lea & Febiger; Philadelphia: 1991. [Google Scholar]
- 260.Garcia A.J., Zanella S., Koch H. Chapter 3—networks within networks: the neuronal control of breathing. Prog Brain Res. 2011;188:31. doi: 10.1016/B978-0-444-53825-3.00008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Dempsey J.A., Smith C.A. Pathophysiology of human ventilatory control. Eur Respir J. 2014;44:495. doi: 10.1183/09031936.00048514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.McCrimmon D.R., Ramirez J.M., Alford S., Zuperku E.J. Unraveling the mechanism for respiratory rhythm generation. Bioessays. 2000;22:6. doi: 10.1002/(SICI)1521-1878(200001)22:1<6::AID-BIES3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 263.Widdicombe J. Reflexes from the lungs and airways: historical perspective. J Appl Physiol. 2006;102:268. doi: 10.1152/japplphysiol.00155.2006. [DOI] [PubMed] [Google Scholar]
- 264.Widdicombe J. Functional morphology and physiology of pulmonary rapidly reacting receptors (RARs) Anat Rec A Discov Mol Cell Evol Biol. 2003;270:2. doi: 10.1002/ar.a.10003. [DOI] [PubMed] [Google Scholar]
- 265.Ainsworth D.M., Ducharme N.G., Hackett R.P. Regulation of equine respiratory muscles during acute hypoxia and hypercapnia. Am Rev Respir Dis. 1993;147:A700. [Google Scholar]
- 266.Muir W.W., Moore C.A., Hamlin R.L. Ventilatory alterations in normal horses in response to changes in inspired oxygen and carbon dioxide. Am J Vet Res. 1975;36:155. [PubMed] [Google Scholar]
- 267.Derksen F.J., Robinson N.E., Slocombe R.F. Ovalbumin induced allergic lung disease in the pony: role of vagal mechanisms. J Appl Physiol. 1982;53:719. doi: 10.1152/jappl.1982.53.3.719. [DOI] [PubMed] [Google Scholar]
- 268.Derksen F., Robinson N., Slocombe R. 3-Methylindole-induced pulmonary toxicosis in ponies. Am J Vet Res. 1982;43:603. [PubMed] [Google Scholar]
- 269.Derksen F.J., Robinson N.E., Stick J.A. Technique for reversible vagal blockade in the standing conscious pony. Am J Vet Res. 1981;42:523. [PubMed] [Google Scholar]
- 270.McGorum B.C. Quantification of histamine in plasma and pulmonary fluids from horses with chronic obstructive pulmonary disease, before and after “natural” (hay and straw) challenges. Vet Immunol Immunopathol. 1993;36:223. doi: 10.1016/0165-2427(93)90021-u. [DOI] [PubMed] [Google Scholar]
- 271.Watson E., Sweeney C., Steensma K. Arachidonate metabolites in bronchoalveolar lavage fluid from horses with and without COPD. Equine Vet J. 1992;24:379. doi: 10.1111/j.2042-3306.1992.tb02859.x. [DOI] [PubMed] [Google Scholar]
- 272.Grunig G., Hermann M., Winder C. Procoagulant activity in respiratory tract secretions from horses with chronic pulmonary disease. Am J Vet Res. 1988;49:705. [PubMed] [Google Scholar]
- 273.Sant’Ambrogio G., Mathew O.P., Fisher J.T. Laryngeal receptors responding to transmural pressure, airflow, and local muscle activity. J Appl Physiol. 1983;65:317. doi: 10.1016/0034-5687(83)90075-0. [DOI] [PubMed] [Google Scholar]
- 274.Sant’Ambrogio G., Mathew O.P. Laryngeal receptors and their reflex responses. Clin Chest Med. 1986;7:211. [PubMed] [Google Scholar]
- 275.Feldman J.L. Chapter 14—Looking forward to breathing. Prog Brain Res. 2011;188:213. doi: 10.1016/B978-0-444-53825-3.00019-X. [DOI] [PubMed] [Google Scholar]
- 276.Silva J.N., Tanabe F.M., Moreira T.S., Takura A.C. Neuroanatomical and physiological evidence that the retrotrapezoid nucleus/parafacial region regulates expiration in adult rats. Respir Physiol Neurobiol. 2016;227:9. doi: 10.1016/j.resp.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 277.Koterba A.M., Kosch P.C., Beech J. The breathing strategy of the adult horse (Equus caballus) at rest. J Appl Physiol. 1988;64:337. doi: 10.1152/jappl.1988.64.1.337. [DOI] [PubMed] [Google Scholar]
- 278.Lessa T.B., de Abreu D.K., Bertasoli B.M., Ambrosio C.E. Diaphragm: a vital respiratory muscle in mammals. Ann Anat. 2016;205:122. doi: 10.1016/j.aanat.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 279.Nijkampf F.P. β-adrenergic receptors in the lung: an introduction. Life Sci. 1993;52:2073. doi: 10.1016/0024-3205(93)90722-f. [DOI] [PubMed] [Google Scholar]
- 280.Proskocil B.J., Fryer A.D. Beta2-agonist and anticholinergic drugs in the treatment of lung disease. Proc Am Thorac Soc. 2005;3:395. doi: 10.1513/pats.200504-038SR. [DOI] [PubMed] [Google Scholar]
- 281.Bai T.R. Beta 2 adrenergic receptors in asthma: a current perspective. Lung. 1992;170:125. doi: 10.1007/BF00174316. [DOI] [PubMed] [Google Scholar]
- 282.Couetil L., Hammer J., Miscovis Feutz M. Effects of N-butylscopolammonium bromide on lung function in horses with recurrent airway obstruction. J Vet Intern Med. 2012;26:1433. doi: 10.1111/j.1939-1676.2012.00992.x. [DOI] [PubMed] [Google Scholar]
- 283.De Lagarde M., Rodrigues N., Chevigny M. N-butylscopolammonium bromide causes fewer side effects than atropine when assessing bronchoconstriction reversibility in horses with heaves. Eq Vet J. 2014;46:474. doi: 10.1111/evj.12229. [DOI] [PubMed] [Google Scholar]
- 284.Derksen F.J., Scott J.S., Slocombe R.F. Effect of clenbuterol on histamine-induced airway obstruction in ponies. Am J Vet Res. 1987;48:423. [PubMed] [Google Scholar]
- 285.Scott J., Broadstone R., Derksen F. Beta-adrenergic blockade in ponies with recurrent obstructive pulmonary disease. J Appl Physiol. 1988;64:2324. doi: 10.1152/jappl.1988.64.6.2324. [DOI] [PubMed] [Google Scholar]
- 286.Scott J.S., Berney C.E., Derksen F.J., Robinson N.E. Beta-adrenergic receptor activity in ponies with recurrent obstructive pulmonary disease. Am J Vet Res. 1991;52:1416. [PubMed] [Google Scholar]
- 287.Scott J.S., Garon H.E., Broadstone R.V. Alpha 1 adrenergic induced airway obstruction in ponies with recurrent pulmonary disease. J Appl Physiol. 1988;65:686. doi: 10.1152/jappl.1988.65.2.687. [DOI] [PubMed] [Google Scholar]
- 288.van der Veldenr V.H., Hulsmann A.R. Autonomic innervation of human airways: structure, function, and pathophysiology in asthma. Neuroimmunomodulation. 1999;6:145. doi: 10.1159/000026376. [DOI] [PubMed] [Google Scholar]
- 289.Andersson R.G., Grundstrom N. Innervation of airway smooth muscle. Efferent mechanisms. Pharmacol Ther. 1987;32:107. doi: 10.1016/0163-7258(87)90055-6. [DOI] [PubMed] [Google Scholar]
- 290.Robinson N.E., Derksen F.J., Olszewski M.A. The pathogenesis of chronic obstructive pulmonary disease of horses. Br Vet J. 1996;152:283. doi: 10.1016/s0007-1935(96)80101-1. [DOI] [PubMed] [Google Scholar]
- 291.Macklem P.T., Mead J. Resistance of central and peripheral airways measured by retrograde catheter. J Appl Physiol. 1967;22:395. doi: 10.1152/jappl.1967.22.3.395. [DOI] [PubMed] [Google Scholar]
- 292.Buergelt C.D., Hines S.A., Cantor G. A retrospective study of proliferative interstitial lung disease of horses in Florida. Vet Pathol. 1986;23:750. doi: 10.1177/030098588602300614. [DOI] [PubMed] [Google Scholar]
- 293.Lakritz J., Wilson W.D., Berry C.R. Bronchointerstitial pneumonia and respiratory distress in young horses: clinical, clinicopathologic, radiographic, and pathological findings in 23 cases. J Vet Intern Med. 1984–1989;7(277):1993. doi: 10.1111/j.1939-1676.1993.tb01020.x. [DOI] [PubMed] [Google Scholar]
- 294.Wilkins P.A. Lower respiratory problems of the neonate. Vet Clin North Am Equine Pract. 2003;19:19. doi: 10.1016/s0749-0739(02)00064-0. [DOI] [PubMed] [Google Scholar]
- 295.Berry C.R., O’Brien T.R., Madigan J.E. Thoracic radiographic features of silicosis in 19 horses. J Vet Intern Med. 1991;5:248. doi: 10.1111/j.1939-1676.1991.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 296.Marques F.J., Hehenberger E., Dickinson R. Respiratory distress due to retropharyngeal and neck swelling in a horse with mediastinal lymphosarcoma. Compend Contin Educ Vet. 2012;34:E5. [PubMed] [Google Scholar]
- 297.Singh K., Holbrook T.C., Gilliam L.L. Severe pulmonary disease due to multisystemic eosinophilic epitheliotropic disease in a horse. Vet Pathol. 2006;43:189. doi: 10.1354/vp.43-2-189. [DOI] [PubMed] [Google Scholar]
- 298.Brakenhoff J.E., Holcombe S.J., Hauptman J.G. The prevalence of laryngeal disease in a large population of competition draft horses. Vet Surg. 2006;35:579. doi: 10.1111/j.1532-950X.2006.00192.x. [DOI] [PubMed] [Google Scholar]
- 299.Dixon P.M., McGorum B.C., Railton D.I. Laryngeal paralysis: a study of 375 cases in a mixed-breed population of horses. Equine Vet J. 2001;33:452. doi: 10.2746/042516401776254790. [DOI] [PubMed] [Google Scholar]
- 300.Davidson E.J., Martin B.B., Boston R.C. Exercising upper respiratory videoendoscopic evaluation of 100 nonracing performance horses with abnormal respiratory noise and/or poor performance. Equine Vet J. 2011;43:3. doi: 10.1111/j.2042-3306.2010.00132.x. [DOI] [PubMed] [Google Scholar]
- 301.Franklin S.H., Naylor J.R., Lane J.G. Effect of dorsal displacement of the soft palate on ventilation and airflow during high-intensity exercise. Equine Vet J Suppl. 2002;34:379. doi: 10.1111/j.2042-3306.2002.tb05452.x. [DOI] [PubMed] [Google Scholar]
- 302.Naylor J.M., Nickel D.D., Trimino G. Hyperkalemic periodic paralysis in homozygous and heterozygous horses: a co-dominant genetic condition. Equine Vet J. 1999;31:153. doi: 10.1111/j.2042-3306.1999.tb03809.x. [DOI] [PubMed] [Google Scholar]
- 303.Carr E.A., Spier S.J., Kortz G.D., Hoffman E.P. Laryngeal and pharyngeal dysfunction in horses homozygous for hyperkalemic periodic paralysis. J Am Vet Med Assoc. 1996;209:798. [PubMed] [Google Scholar]
- 304.Hughes K.J., McGorum B.C., Love S., Dixon P.M. Bilateral laryngeal paralysis associated with hepatic dysfunction and hepatic encephalopathy in six ponies and four horses. Vet Rec. 2009;164:142. doi: 10.1136/vr.164.5.142. [DOI] [PubMed] [Google Scholar]
- 305.Norman T.E., Chaffin M.K., Bisset W.T., Thompson J.A. Association of clinical signs with endoscopic findings in horses with nasopharyngeal cicatrix syndrome: 118 cases (2003–2008) J Am Vet Med Assoc. 2012;240(734) doi: 10.2460/javma.240.6.734. [DOI] [PubMed] [Google Scholar]
- 306.James F.M., Parente E.J., Palmer J.E. Management of bilateral choanal atresia in a foal. J Am Vet Med Assoc. 2006;229:1784. doi: 10.2460/javma.229.11.1784. [DOI] [PubMed] [Google Scholar]
- 307.Botha C.J., Lewis A., du Plessis E.C. Crotalariosis equorum (“jaagsiekte”) in horses in southern Mozambique, a rare form of pyrrolizidine alkaloid poisoning. J Vet Diagn Invest. 2012;24:1099. doi: 10.1177/1040638712460673. [DOI] [PubMed] [Google Scholar]
- 308.Johnstone L.K., Engiles J.B., Aceto H. Retrospective evaluation of horses diagnosed with neuroborreliosis on postmortem examination: 16 cases (2004–2015) J Vet Intern Med. 2016;30(1350) doi: 10.1111/jvim.14369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Reimer J.M. Diagnostic ultrasonography of the equine thorax. Compend Cont Educ Pract Vet. 1990;12:1321. [Google Scholar]
- 310.Carlson G.P. Blood chemistry, body fluids, and hematology. In: Gillespie J.R., Robinson N.E., editors. Equine exercise physiology. 2nd ed. ICEEP Publications; Davis, CA: 1987. [Google Scholar]
- 311.Guyton A.C., Hall J.E. The microcirculation and the lymphatic system: capillary fluid exchange, interstitial fluid and lymph flow. The body fluid compartments: extracellular and intracellular fluids; interstitial fluid and edema. In: Guyton A.C., Hall J.E., editors. Textbook of medical physiology. 11th ed. Elsevier/Saunders; Philadelphia: 2006. [Google Scholar]
- 312.Renkin E.M. Some consequences of capillary permeability to macromolecules: Starling’s hypothesis revisited. Am J Physiol. 1986;250:H706. doi: 10.1152/ajpheart.1986.250.5.H706. [DOI] [PubMed] [Google Scholar]
- 313.Staub N.C., Taylor A.E., editors. Edema. Raven Press; New York: 1984. [Google Scholar]
- 314.Demling R.H. Effect of plasma and interstitial protein content on tissue edema formation. Curr Stud Hematol Blood Transfus. 1986;53:36. doi: 10.1159/000413163. [DOI] [PubMed] [Google Scholar]
- 315.Taylor A.E. Capillary fluid filtration: Starling forces and lymph flow. Circ Res. 1981;49:557. doi: 10.1161/01.res.49.3.557. [DOI] [PubMed] [Google Scholar]
- 316.Levick J.R., Michel C.C. Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res. 2010;87:198. doi: 10.1093/cvr/cvq062. [DOI] [PubMed] [Google Scholar]
- 317.Collins S.R., Blank R.S., Deatherage L.S. The endothelial glycocalyx: emerging concepts in pulmonary edema and acute lung injury. Anesth Analg. 2013;117:664. doi: 10.1213/ANE.0b013e3182975b85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Dejana E., Orsenigo F., Lampugnani M.G. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008;121:2115. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- 319.Frigeri A., Nicchia G.P., Svelto M. Aquaporins as targets for drug discovery. Curr Pharm Des. 2007;13:2421. doi: 10.2174/138161207781368738. [DOI] [PubMed] [Google Scholar]
- 320.Renkin E.M., Michel C.C., editors. Handbook of physiology. Oxford University Press; New York: 1984. [Google Scholar]
- 321.Raj J.U., Anderson J. Regional differences in interstitial fluid albumin concentration in edematous lamb lungs. J Appl Physiol. 1992;72:699. doi: 10.1152/jappl.1992.72.2.699. [DOI] [PubMed] [Google Scholar]
- 322.Costanzo L., editor. Physiology. 3rd ed. Saunders; St. Louis: 2006. [Google Scholar]
- 323.Michel C.C. Microvascular permeability, venous stasis and oedema. Inter Angiol. 1984;8:9–13. [PubMed] [Google Scholar]
- 324.Green J.F. Lea & Febiger; Philadelphia: 1987. Fundamental cardiovascular and pulmonary physiology. [Google Scholar]
- 325.Claesson-Welsh L. Vascular permeability—the essentials. Ups J Med Sci. 2015;120:135. doi: 10.3109/03009734.2015.1064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Golden M.H. Nutritional and other types of oedema, albumin, complex carbohydrates and the interstitium—a response to Malcom Coulthard’s hypothesis: oedema in kwashiorkor is caused by hypoalbuminemia. Paediatr Int Child Health. 2015;35:90. doi: 10.1179/2046905515Y.0000000010. [DOI] [PubMed] [Google Scholar]
- 327.Trani M., Dejana E. New insights in the control of vascular permeability: vascular endothelial-cadherin and other players. Curr Opin Hematol. 2015;22:267. doi: 10.1097/MOH.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 328.Vestweber D. VE-cadherin: the major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler Thromb Vasc Biol. 2008;28:223. doi: 10.1161/ATVBAHA.107.158014. [DOI] [PubMed] [Google Scholar]
- 329.Verkman A.S. Mammalian aquaporins: diverse physiological roles and potential clinical significance. Expert Rev Mol Med. 2008;10:e13. doi: 10.1017/S1462399408000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Stokum J.A., Kurland D.B., Gerzanich V. Mechanisms of astrocyte-mediated cerebral edema. Neurochem Res. 2015;40:317. doi: 10.1007/s11064-014-1374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Stokum J.A., Gerzanich V., Simard J.M. Molecular pathophysiology of cerebral edema. J Cereb Blood Flow Metab. 2016;36:513. doi: 10.1177/0271678X15617172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Hsu Y., Tran M., Linninger A.A. Dynamic regulation of aquaporin-4 water channels in neurological disorders. Croat Med J. 2015;56:401. doi: 10.3325/cmj.2015.56.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Sun Z., Li X., Massena S. VEGFR2 induces c-Src signaling and vascular permeability in vivo via the adaptor protein TSAd. J Exp Med. 2012;209:1363. doi: 10.1084/jem.20111343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Eliceiri B.P., Paul R., Schwartzberg P.L. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4:915. doi: 10.1016/s1097-2765(00)80221-x. [DOI] [PubMed] [Google Scholar]
- 335.Page A.E., Slovis N.M., Horohov D.W. Lawsonia intracellularis and equine proliferative enteropathy. Vet Clin North Am Equine Pract. 2014;30:641. doi: 10.1016/j.cveq.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 336.Galvin N., Dillon H., McGovern F. Right dorsal colitis in the horse: minireview and reports on three cases in Ireland. Ir Vet J. 2004;57:467. doi: 10.1186/2046-0481-57-8-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Parraga M.E., Carlson G.P., Thurmon M. Serum protein concentrations in horses with severe liver disease: a retrospective study and review of the literature. J Vet Intern Med. 1995;9:154. doi: 10.1111/j.1939-1676.1995.tb03289.x. [DOI] [PubMed] [Google Scholar]
- 338.Demling R.H. The burn edema process: current concepts. J Burn Care Rehabil. 2005;26:207. [PubMed] [Google Scholar]
- 339.Morris D.D. Cutaneous vasculitis in horses: 19 cases (1978–1985) J Am Vet Med Assoc. 1987;191(460) [PubMed] [Google Scholar]
- 340.White S.D., Affolter V.K., Dewey J. Cutaneous vasculitis in equines: a retrospective study of 72 cases. Vet Dermatol. 2009;20:600. doi: 10.1111/j.1365-3164.2009.00827.x. [DOI] [PubMed] [Google Scholar]
- 341.Balasuriya U.B. Equine viral arteritis. Vet Clin North Am Equine Pract. 2014;30:543. doi: 10.1016/j.cveq.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 342.Cook R.F., Leroux C., Issel C.J. Equine infectious anemia and equine infectious anemia virus in 2013: a review. Vet Microbiol. 2013;29:167. doi: 10.1016/j.vetmic.2013.09.031. [DOI] [PubMed] [Google Scholar]
- 343.Pusterla N., Watson J.L., Affolter V.K. Purpura haemorrhagica in 53 horses. Vet Rec. 2003;153:118. doi: 10.1136/vr.153.4.118. [DOI] [PubMed] [Google Scholar]
- 344.Kilcoyne I., Spier S.J., Carter C.N. Frequency of Corynebacterium pseudotuberculosis infection in horses across the United States during a 10-year period. J Am Vet Med Assoc. 2014;245:309. doi: 10.2460/javma.245.3.309. [DOI] [PubMed] [Google Scholar]
- 345.Affolter V.K. Chronic progressive lymphedema in draft horses. Vet Clin North Am Equine Pract. 2013;29:589. doi: 10.1016/j.cveq.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 346.Lewis N., Racklyeft D.J. Mass envenomation of a mare and foal by bees. Aust Vet J. 2014;92:141. doi: 10.1111/avj.12177. [DOI] [PubMed] [Google Scholar]
- 347.Fielding C.L., Pusterla N., Magdesian K.G. Rattlesnake envenomation in horses: 58 cases (1992–2009) J Am Vet Med Assoc. 2011;238(631) doi: 10.2460/javma.238.5.631. [DOI] [PubMed] [Google Scholar]
- 348.Lyle C.H., Turley G., Blissitt K.J. Retrospective evaluation of episodic collapse in the horse in a referred population: 25 cases (1995–2009) J Vet Intern Med. 2010;24(1498) doi: 10.1111/j.1939-1676.2010.0610.x. [DOI] [PubMed] [Google Scholar]
- 349.Guyton A.C., Hall J.E. 11th ed. Elsevier; Philadelphia: 2006. Textbook of medical physiology. [Google Scholar]
- 350.Gauer R.L. Evaluation of syncope. Am Fam Physician. 2011;84:640. [PubMed] [Google Scholar]
- 351.Hilz M.J., Marthol H., Neundorfer B. Syncope—a systematic overview of classification, pathogenesis, diagnosis and management. Fortschr Neurol Psychiatr. 2002;70:95. doi: 10.1055/s-2002-19923. [DOI] [PubMed] [Google Scholar]
- 352.Lanier J.B., Mote M.B., Clay E.C. Evaluation and management of orthostatic hypotension. Am Fam Physician. 2011;84:527. [PubMed] [Google Scholar]
- 353.Benditt D.G. Neurally mediated syncopal syndromes: pathophysiological concepts and clinical evaluation. Pacing Clin Electrophysiol. 1997;20:572. doi: 10.1111/j.1540-8159.1997.tb06211.x. [DOI] [PubMed] [Google Scholar]
- 354.Gatzoulis K.A., Toutouzas P.K. Neurocardiogenic syncope: aetiology and management. Drugs. 2001;61:1415. doi: 10.2165/00003495-200161100-00005. [DOI] [PubMed] [Google Scholar]
- 355.Mayhew J. 2nd ed. Wiley Blackwell; Sussex, UK: 2009. Large animal neurology. [Google Scholar]
- 356.Sabu J., Regeti K., Mallappallil M. Convulsive syncope induced by ventricular arrhythmia masquerading as epileptic seizures: case report and review of the literature. J Clin Med Res. 2016;8:610. doi: 10.14740/jocmr2583w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Kanjwal K., Karabin B., Kanjwal Y. Differentiation of convulsive syncope from epilepsy with an implantable loop recorder. Int J Med Sci. 2009;6:296. doi: 10.7150/ijms.6.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Jefferys J.G.R. Advances in understanding basic mechanisms of epilepsy and seizures. Seizure. 2010;19:638. doi: 10.1016/j.seizure.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 359.McNamara J.O. Cellular and molecular basis of epilepsy. J Neurosci. 1994;14:3413. doi: 10.1523/JNEUROSCI.14-06-03413.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Staley K. Molecular mechanisms of epilepsy. Nature. 2015;18:367. doi: 10.1038/nn.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Reddy D.S. Role of hormones and neurosteroids in epileptogenesis. Front Cell Neurosci. 2013;7:115. doi: 10.3389/fncel.2013.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Lucke-Wold B.P., Nguyen L., Turner R.C. Traumatic brain injury and epilepsy: underlying mechanisms leading to seizure. Seizure. 2015;33:13. doi: 10.1016/j.seizure.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 363.Engel J. A proposal diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on classification and terminology, ILAE Commission Report. Epilepsia. 2001;42:796. doi: 10.1046/j.1528-1157.2001.10401.x. [DOI] [PubMed] [Google Scholar]
- 364.Lacombe V.A., Mayes M., Mosseri S. Distribution and predictive factors of seizure types in 104 cases. Equine Vet J. 2013;46:441. doi: 10.1111/evj.12149. [DOI] [PubMed] [Google Scholar]
- 365.Lacombe V.A., Mayes M., Mosseri S. Epilepsy in horses: aetiological classification and predictive factors. Equine Vet J. 2012;44:646. doi: 10.1111/j.2042-3306.2011.00527.x. [DOI] [PubMed] [Google Scholar]
- 366.Zepelin H. Mammalian sleep. In: Kryger M.H., Roth T., Dement W.C., editors. Principles and practice of sleep medicine. 3rd ed. WB Saunders Co; Philadelphia: 2000. [Google Scholar]
- 367.Ruckebusch Y. The relevance of drowsiness in the circadian cycle of farm animals. Anim Behav. 1972;20:637. doi: 10.1016/s0003-3472(72)80136-2. [DOI] [PubMed] [Google Scholar]
- 368.Williams D.C., Aleman M., Holliday T.A. Qualitative and quantitative characteristics of the electroencephalogram in normal horses during spontaneous drowsiness and sleep. J Vet Intern Med. 2008;22:630. doi: 10.1111/j.1939-1676.2008.0096.x. [DOI] [PubMed] [Google Scholar]
- 369.Hendricks J.C., Morrison A.R. Normal and abnormal sleep in mammals. J Am Vet Med Assoc. 1981;178:121. [PubMed] [Google Scholar]
- 370.Bertone J.J. Excessive drowsiness secondary to recumbent sleep deprivation in two horses. Vet Clin North Am Equine Pract. 2006;22:157. doi: 10.1016/j.cveq.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 371.Mignot E.J., Dement W.C. Narcolepsy in animals and man. Equine Vet. 1993;25:476. doi: 10.1111/j.2042-3306.1993.tb02996.x. [DOI] [PubMed] [Google Scholar]
- 372.Scammell T.E. Narcolepsy. N Engl J Med. 2015;373:2654. doi: 10.1056/NEJMra1500587. [DOI] [PubMed] [Google Scholar]
- 373.Ripley B., Fujiki N., Okura M. Hypocretin levels in sporadic and familial cases of canine narcolepsy. Neurobiol Dis. 2001;8:525. doi: 10.1006/nbdi.2001.0389. [DOI] [PubMed] [Google Scholar]
- 374.Lin L., Faraco J., Li R. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 375.Lunn D.P., Cuddon P.A., Shaftoe S. Familial occurrence of narcolepsy in miniature horses. Equine Vet J. 1993;25:483. doi: 10.1111/j.2042-3306.1993.tb02998.x. [DOI] [PubMed] [Google Scholar]
- 376.Ludvikova E., Nishino S., Sakai N. Familial narcolepsy in the Lipizzaner horse: a report of three fillies born to the same sire. Vet Q. 2012;32:99. doi: 10.1080/01652176.2012.714089. [DOI] [PubMed] [Google Scholar]
- 377.Bathen-Nothen A., Heider C., Fernandez A.J. Hypocretin measurement in an Icelandic foal with narcolepsy. J Vet Intern Med. 2009;23:1299. doi: 10.1111/j.1939-1676.2009.0400.x. [DOI] [PubMed] [Google Scholar]
- 378.van Nieuwstadt R.A., van der Want C.J., Binkhorst G.J. Narcolepsy in horses. Tijdschr Diergeneeskd. 1993;118:765. [PubMed] [Google Scholar]
- 379.Dreifuss F.E., Flynn D.V. Narcolepsy in a horse. J Am Vet Med Assoc. 1984;184:131. [PubMed] [Google Scholar]
- 380.Sweeney C.R., Hendricks J.C., Beech J. Narcolepsy in a horse. J Am Vet Med Assoc. 1983;183:126. [PubMed] [Google Scholar]
- 381.Faull K.F., Guillemainault C., Berger P.A. Cerebrospinal fluid monoamine metabolites in narcolepsy and hypersomnia. Ann Neurol. 1983;13:258. doi: 10.1002/ana.410130306. [DOI] [PubMed] [Google Scholar]
- 382.Mignot E., Lammers G.J., Ripley B. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59:1553. doi: 10.1001/archneur.59.10.1553. [DOI] [PubMed] [Google Scholar]
- 383.Viola-Saltzman M., Watson M.D. Traumatic brain injury and sleep disorders. Neurol Clin. 2012;30:1299. doi: 10.1016/j.ncl.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Baumann C.R., Bassetti C.L., Scammell T.E. Loss of hypocretin (orexin) neurons with traumatic brain injury. Ann Neurol. 2010;66:555. doi: 10.1002/ana.21836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Jesty S.A., Reef V.B. Evaluation of the horse with acute cardiac crisis. Clin Tech Equine Pract. 2006;5:93. [Google Scholar]
- 386.Reef V.B., Clark E.S., Oliver J.A. Implantation of a permanent transvenous pacing catheter in a horse with a complete heart block and syncope. J Am Vet Med Assoc. 1986;189:449. [PubMed] [Google Scholar]
- 387.Hay W.P., Baskett A., Abdy M.J. Complete upper airway obstruction and syncope caused by a subepiglottic cyst in a horse. Equine Vet J. 1997;29:75. doi: 10.1111/j.2042-3306.1997.tb01642.x. [DOI] [PubMed] [Google Scholar]
- 388.Steiger R., Feige K. Case report: polycythemia in a horse. Schweiz Arch Tierheilkd. 1995;137:306. [PubMed] [Google Scholar]
- 389.Bryant U.K., Lyons E.T., Bain F.T. Halicephalobus gingivalis-associated meningoencephalitis in a Thoroughbred foal. J Vet Diagn Invest. 2006;18:612. doi: 10.1177/104063870601800618. [DOI] [PubMed] [Google Scholar]
- 390.Hardefeldt L.Y. Hyponatremic encephalopathy in azotaemic neonatal foals: four cases. Aust Vet J. 2014;92:488. doi: 10.1111/avj.12265. [DOI] [PubMed] [Google Scholar]
- 391.Viu J., Monreal L., Jose-Cunilleras E. Clinical findings in 10 foals with bacterial meningoencephalitis. Equine Vet J Suppl. 2012;41:100. doi: 10.1111/j.2042-3306.2011.00508.x. [DOI] [PubMed] [Google Scholar]
- 392.Wong D., Wilkins P.A., Bain F.T. Neonatal encephalopathy in foals. Compend Contin Educ Vet. 2011;33:E5. [PubMed] [Google Scholar]
- 393.Aleman M., Gray L.C., Williams D.C. Juvenile idiopathic epilepsy in Egyptian Arabian foals: 22 cases (1985–2005) J Vet Intern Med. 2006;20(1443) doi: 10.1892/0891-6640(2006)20[1443:jieiea]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 394.Berendnt M., Gredal H., Pedersen L.G. A cross-sectional study in Danish Labrador Retrievers: prevalence and selected risk factors. J Vet Intern Med. 2002;16:262. doi: 10.1892/0891-6640(2002)016<0262:acsoei>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 395.Cornelisse C.J., Schott H.C., Olivier N.B. Concentration of cardiac troponin I in horse with a ruptured aortic regurgitation jet lesion and ventricular tachycardia. J Am Vet Med Assoc. 2000;217:231. doi: 10.2460/javma.2000.217.231. [DOI] [PubMed] [Google Scholar]
- 396.Nath L.C., Anderson G.A., Hinchcliff K.W. Serum cardiac troponin I concentrations in horses with cardiac disease. Aust Vet J. 2012;90:351. doi: 10.1111/j.1751-0813.2012.00970.x. [DOI] [PubMed] [Google Scholar]
- 397.Wijnberg I.D., van der Ree M., van Someren P. The applicability of ambulatory electroencephalography (AEEG) in healthy horses and horses with abnormal behavior or clinical signs of epilepsy. Vet Q. 2013;33:121. doi: 10.1080/01652176.2013.842075. [DOI] [PubMed] [Google Scholar]
- 398.van der Ree M, Wijnberg I. A review on epilepsy in the horse and the potential of ambulatory EEG as a diagnostic tool, 332:159, 2012. [DOI] [PubMed]
- 399.Manso-Diaz G., Dyson S.J., Dennis R. Magnetic resonance imaging characteristics of equine head disorders: 84 cases (2000–2013) Vet Radiol Ultrasound. 2015;56(176) doi: 10.1111/vru.12210. [DOI] [PubMed] [Google Scholar]
- 400.Sogaro-Robinson C., Lacombe V.A., Reed S.M. Factors predictive of abnormal results for computed tomography of the head in horses affected by neurologic disorders. J Am Vet Med Assoc. 2009;235:176. doi: 10.2460/javma.235.2.176. [DOI] [PubMed] [Google Scholar]
- 401.Goyal R.K., Mashimo H. Physiology of oral, pharyngeal, and esophageal motility. GI Motility online. 2006 doi: 10.1038/gimo1. [DOI] [Google Scholar]
- 402.Shaw S., Martino R. The normal swallow: muscular and neurophysiological control. Otolaryngol Clin N Am. 2013;46:937. doi: 10.1016/j.otc.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 403.Steele C.M., Miller A.J. Sensory input pathways and mechanisms in swallowing: a review. Dysphagia. 2010;25:323. doi: 10.1007/s00455-010-9301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Aleman M. Dysphagia of neurogenic origin. In: Robinson N.E., Sprayberry K.A., editors. Current therapy in equine medicine 6. Saunders/Elsevier; St. Louis: 2009. [Google Scholar]
- 405.Baum K.H., Modransky P.D., Halpern N.E. Dysphagia in horses: the differential diagnosis, part I. Compend Cont Educ Pract Vet. 1988;10:1301. [Google Scholar]
- 406.Baum G.H., Halpern N.E., Banish L.D. Dysphagia in horses: the differential diagnosis, part II. Compend Cont Educ Pract Vet. 1988;10:1405. [Google Scholar]
- 407.Stick J.A., Boles C. Subepiglottic cyst in three foals. J Am Vet Med Assoc. 1980;177:62. [PubMed] [Google Scholar]
- 408.Nolen-Walston R.D., Parente E.J., Madigan J.E. Branchial remnant cysts of mature and juvenile horses. Equine Vet J. 2009;41:918. doi: 10.2746/042516409x452161. [DOI] [PubMed] [Google Scholar]
- 409.Dixon P.M., Dacre I. A review of equine dental disorders. Vet J. 2005;169:165. doi: 10.1016/j.tvjl.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 410.Staszyk C., Bienert A., Kreutzer R. Equine odontoclastic tooth resorption and hypercementosis. Vet J. 2008;178:372. doi: 10.1016/j.tvjl.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 411.Vengust M., Baird J.D., van Dreumel T. Equid herpesvirus 2-associated oral and esophageal ulceration in a foal. J Vet Diag Invest. 2008;20:811. doi: 10.1177/104063870802000618. [DOI] [PubMed] [Google Scholar]
- 412.Baker G.J. Equine temporomandibular joints (TMJ): morphology, function, and clinical disease. Proc Am Assoc Equine Pract. 2002;48:442. [Google Scholar]
- 413.Pusterla N., Latson K.M., Wilson W.D. Metallic foreign bodies in the tongues of 16 horses. Vet Rec. 2006;159:485. doi: 10.1136/vr.159.15.485. [DOI] [PubMed] [Google Scholar]
- 414.Kilcoyne I., Watson J.L., Spier S.J. Septic sialoadenitis in equids: a retrospective study of 18 cases (1998–2010) Equine Vet J. 2010;47(54) doi: 10.1111/evj.12228. [DOI] [PubMed] [Google Scholar]
- 415.Bezdekova B. Esophageal disorders in horses—a review of the literature. Pferdeheilkunde. 2012;28:187. [Google Scholar]
- 416.Broekamn L.E., Kuiper D. Megaesophagus in the horse. A short review of the literature and 18 own cases. Vet Q. 2002;24:199. doi: 10.1080/01652176.2002.9695136. [DOI] [PubMed] [Google Scholar]
- 417.Booth T.M., Marmion W.J., Cullimore A.M. Esophageal obstruction in an aged pony associated with squamous cell carcinoma. Equine Vet Educ. 2008;20:627. [Google Scholar]
- 418.Brazil T.J. Recurrent esophageal obstruction caused by gastro-esophageal squamous cell carcinoma: a diagnostic challenge? Equine Vet Educ. 2008;20:633. [Google Scholar]
- 419.Green S., Green E.M., Aronson E. Squamous cell carcinoma: an unusual case of esophageal choke in a horse. Mod Vet Pract. 1986;65:870. [Google Scholar]
- 420.Benders N.A., Velduis Kroez E.J., van der Kolk J.H. Idiopathic muscular hypertrophy of the esophagus in the horse: a retrospective study of 31 cases. Equine Vet J. 2004;36:46. doi: 10.2746/0425164044864697. [DOI] [PubMed] [Google Scholar]
- 421.Todhunter R.J., Brown C.M., Stickle R. Retropharyngeal infections in five horses. J Vet Med Assoc. 1985;187:600. [PubMed] [Google Scholar]
- 422.Sweeney C.R., Timoney J.F., Newton J.R. Streptococcus equi infections in horses: guidelines for treatment, control and prevention of strangles. J Vet Intern Med. 2005;19:123. [PubMed] [Google Scholar]
- 423.McCue P.M., Freeman D.E., Donawick W.J. Guttural pouch tympany: 15 cases (1977–1986) J Am Vet Med Assoc. 1989;12(1761) [PubMed] [Google Scholar]
- 424.Greet T.R.C. Outcome of treatment in 35 cases of guttural pouch mycosis. Equine Vet J. 1987;19:483. doi: 10.1111/j.2042-3306.1987.tb02649.x. [DOI] [PubMed] [Google Scholar]
- 425.Tremaine WH: Oral cavity neoplasia, ivis.org. AAEP Focus Meeting, Indianapolis, 2006.
- 426.Casey M. A new understanding of the oral and dental pathology of the equine cheek teeth. Vet Clin North Am Equine Pract. 2013;29:301. doi: 10.1016/j.cveq.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 427.Laus F., Rossi G., Paggi E. Adenocarcinoma involving the tongue and epiglottis in a horse. J Vet Med Sci. 2014;76:467. doi: 10.1292/jvms.13-0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Anlen K.G. Effects of bites by the European adder (Vipera berus) in seven Swedish horses. Vet Rec. 2008;162:652. doi: 10.1136/vr.162.20.652. [DOI] [PubMed] [Google Scholar]
- 429.Sweeny C.R., Freeman D.E., Sweeny R.W. Hemorrhage into the guttural pouch (auditory tube diverticulum) associated with rupture of the longus capitis muscle in three horses. J Am Vet Med Assoc. 1993;202:1129. [PubMed] [Google Scholar]
- 430.Dobesova O., Schwarz B., Velde K. Guttural pouch mycosis in horses: a retrospective study of 28 cases. Vet Rec. 2012;171:561. doi: 10.1136/vr.100700. [DOI] [PubMed] [Google Scholar]
- 431.Bell C. Pharyngeal neuromuscular dysfunction associated with bilateral guttural pouch tympany in a foal. Can Vet J. 2007;48:192. [PMC free article] [PubMed] [Google Scholar]
- 432.Koch C., Witte T. Temporohyoid osteoarthropathy in the horse. Equine Vet Ed. 2014;26:121. [Google Scholar]
- 433.Walker A.M., Sellon D.C., Cornelisse C.J. Temporohyoid osteoarthropathy in 33 horses. J Vet Intern Med. 2002;16:697. doi: 10.1892/0891-6640(2002)016<0697:toih>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 434.Burrows G.E., Borchard R.E. Experimental lead toxicosis in ponies: comparison of the effects of smelter effluent-contaminated hay and lead acetate. Am J Vet Res. 1982;43:2129. [PubMed] [Google Scholar]
- 435.Yvorchuk-St. Jean K. Neuritis of the cauda equina. Vet Clin North Am Equine Pract. 1987;3:421–426. doi: 10.1016/s0749-0739(17)30684-3. [DOI] [PubMed] [Google Scholar]
- 436.Hahn C.N. Polyneuritis equi: the role of T-lymphocytes and importance of differential clinical signs. Equine Vet J. 2008;40:100. doi: 10.2746/042516408X276924. [DOI] [PubMed] [Google Scholar]
- 437.Long M.T. West Nile virus and equine encephalitis viruses: new perspectives. Vet Clin North Am Equine Pract. 2014;30:523. doi: 10.1016/j.cveq.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 438.Pusterla N., Hussey G.S. Equine herpesvirus 1 myeloencephalopathy. Vet Clin North Am Equine Pract. 2014;30:489. doi: 10.1016/j.cveq.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 439.Kohn C.W., Fenner W.R. Equine herpes myeloencephalopathy. Vet Clin North Am Equine Pract. 1987;3:405. doi: 10.1016/s0749-0739(17)30683-1. [DOI] [PubMed] [Google Scholar]
- 440.Howe D.K., MacKay R.J., Reed S.M. Equine protozoal myeloencephalitis. Vet Clin North Am Equine Pract. 2014;30:659. doi: 10.1016/j.cveq.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 441.Uhlinger C. Clinical and epidemiologic features of an epizootic of equine leukoencephalomalacia. J Am Vet Med Assoc. 1991;198:126. [PubMed] [Google Scholar]
- 442.van Galen G., Delguste C., Sandersen C. Tetanus in the equine species: a retrospective study of 31 cases. Tijdschr Diergeneeskd. 2008;133:512. [PubMed] [Google Scholar]
- 443.Holcombe S.J., Hurcombe S.D., Barr B.S. Dysphagia associated with presumed pharyngeal dysfunction in 16 neonatal foals. Equine Vet J Suppl. 2012;41:105. doi: 10.1111/j.2042-3306.2011.00451.x. [DOI] [PubMed] [Google Scholar]
- 444.Swerczek T.W. Toxicoinfectious botulism in foals and adult horses. J Am Vet Med Assoc. 1980;176:217. [PubMed] [Google Scholar]
- 445.Wilkins P.A., Palmer J.E. Botulism in foals less than 6 months of age: 30 cases (1989–2002) J Vet Intern Med. 2003;17(702) doi: 10.1111/j.1939-1676.2003.tb02503.x. [DOI] [PubMed] [Google Scholar]
- 446.Doxey D.L., Milne E.M., Gilmour J.S. Clinical and biochemical features of grass sickness (equine dysautonomia) Equine Vet J. 1991;23:360. doi: 10.1111/j.2042-3306.1991.tb03738.x. [DOI] [PubMed] [Google Scholar]
- 447.Wylei C.E., Proudman C.J. Equine grass sickness: epidemiology, diagnosis, and global distribution. Vet Clin North Am Equine Pract. 2009;25:381. doi: 10.1016/j.cveq.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 448.McGorum B.C., Scholes S., Milne E.M. Equine grass sickness, but not botulism, causes autonomic and enteric neurodegeneration and increases soluble N-ethylmaleimide-sensitive factor attachment receptor protein expression within neuronal perikarya. Equine Vet J. 2016 doi: 10.1111/evj.13543. [DOI] [PubMed] [Google Scholar]
- 449.Moore R.M., Kohn C.W. Nutritional muscular dystrophy in foals. Compend Cont Educ Pract Vet. 1991;13:476. [Google Scholar]
- 450.Step D.L., Divers T.J., Cooper B. Severe masseter myonecrosis in a horse. J Am Vet Med Assoc. 1991;198:117. [PubMed] [Google Scholar]
- 451.Pearson E.G., Snyder S.P., Saulez M.N. Masseter myodegeneration as a cause of trismus or dysphagia in adult horses. Vet Rec. 2005;156:642. doi: 10.1136/vr.156.20.642. [DOI] [PubMed] [Google Scholar]
- 452.Komine M., Langohr I.M., Kiupel M. Megaesophagus in Friesian horses associated with muscular hypertrophy of the caudal esophagus. Vet Pathol. 2014;51:979. doi: 10.1177/0300985813511126. [DOI] [PubMed] [Google Scholar]
- 453.Sockett D.C., Baker J.C., Stowe C.M. Slaframine (Rhizoctonia leguminicola) intoxication in horses. J Am Vet Med Assoc. 1982;181:606. [PubMed] [Google Scholar]
- 454.Divers T.J., Mohammed H.O., Cummings J.R. Equine lower motor neuron disease: findings in 28 horses and proposal of a pathophysiological mechanism. Equine Vet J. 1994;26:409. doi: 10.1111/j.2042-3306.1994.tb04411.x. [DOI] [PubMed] [Google Scholar]
- 455.USDA-APHIS: Incidence of colic in U.S. horses. Available at http://www.aphis.usda.gov/vs/ceah/ncahs/nahms/equine/equine98/colic.PDF. Accessed Mar 4, 2009.
- 456.Tinker M.K., White N.A., Lessard P. Prospective study of equine colic incidence and mortality. Equine Vet J. 1997;29:448. doi: 10.1111/j.2042-3306.1997.tb03157.x. [DOI] [PubMed] [Google Scholar]
- 457.Traub-Dargatz J.L., Kopral C.A., Seitzinger A.H. Estimate of the national incidence of and operation-level risk factors for colic among horses in the United States, spring 1998 to spring 1999. J Am Vet Med Assoc. 2001;219:67. doi: 10.2460/javma.2001.219.67. [DOI] [PubMed] [Google Scholar]
- 458.Uhlinger C. Investigations into the incidence of field colic. Equine Vet J. 1992;13:11. [Google Scholar]
- 459.USDA-APHIS: Trends in equine mortality, 1998–2005. Available at http://www.aphis.usda.gov/animal_health/nahms/equine/download/equine05/Equine05_is_Mortality.PDF
- 460.Salem S.E., Scantlebury C.E., Ezzat E. Colic in a working horse population in Egypt: prevalence and risk factors. Equine Vet J. 2016 doi: 10.1111/wvj.12573. [DOI] [PubMed] [Google Scholar]
- 461.Orsini J.A., Divers T.J., editors. Equine emergencies: treatment and procedures. Saunders; St. Louis: 2008. [Google Scholar]
- 462.Mair T., Divers T., Ducharme N., editors. Manual of equine gastroenterology. Saunders; St. Louis: 2002. [Google Scholar]
- 463.White N.A. II: Intestinal response to injury. Proc Ann Conv AAEP. 2006;52:115. [Google Scholar]
- 464.Snyder J.R. The pathophysiology of intestinal damage: effects of luminal distention and ischemia. Vet Clin North Am Equine Pract. 1989;5:247. doi: 10.1016/s0749-0739(17)30587-4. [DOI] [PubMed] [Google Scholar]
- 465.Kaya G., Sommerfeld-Stur I., Iben C. Risk factors of colic in horses in Austria. J Anim Physiol Anim Nutr (Berl) 2009;93:339. doi: 10.1111/j.1439-0396.2008.00874.x. [DOI] [PubMed] [Google Scholar]
- 466.Cohen N.D., Gibbs P.G., Woods A.M. Dietary and other management factors associated with colic in horses. J Am Vet Med Assoc. 1999;215:53. [PubMed] [Google Scholar]
- 467.Tinker M.K., White N.A., Lessard P. Prospective study of equine colic risk factors. Equine Vet J. 1997;29:454. doi: 10.1111/j.2042-3306.1997.tb03158.x. [DOI] [PubMed] [Google Scholar]
- 468.Hudson J.M., Cohen N.D., Gibbs P.G. Feeding practices associated with colic in horses. J Am Vet Med Assoc. 2001;219:1419. doi: 10.2460/javma.2001.219.1419. [DOI] [PubMed] [Google Scholar]
- 469.Archer D.C., Proudman C.J. Epidemiological clues to preventing colic. Vet J. 2006;172:29. doi: 10.1016/j.tvjl.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 470.Scantlebury C.E., Archer D.C., Proudman C.J. Management and horse-level risk factors for recurrent colic in the UK general equine practice population. Equine Vet J. 2015;47:202. doi: 10.1111/evj.12276. [DOI] [PubMed] [Google Scholar]
- 471.Suthers J.M., Pinchbeck G.L., Proudman C.J. Risk factors for large colon volvulus in the UK. Equine Vet J. 2013;45:558. doi: 10.1111/evj.12039. [DOI] [PubMed] [Google Scholar]
- 472.Scantlebury C.E., Archer D.C., Proudman C.J. Recurrent colic in the horse: incidence and risk factors for recurrence in the general practice population. Equine Vet J Suppl. 2011;39:81. doi: 10.1111/j.2042-3306.2011.00383.x. [DOI] [PubMed] [Google Scholar]
- 473.White N.A. II: Causes and risks for colic. Proc Ann Conv AAEP. 2006;52:115. [Google Scholar]
- 474.Malamed R., Berger J., Bain M.J. Retrospective evaluation of crib-biting and windsucking behaviours and owner-perceived behavioural traits as risk factors for colic in horses. Equine Vet J. 2010;42:686. doi: 10.1111/j.2042-3306.2010.00096.x. [DOI] [PubMed] [Google Scholar]
- 475.Escalona E.E., Okell C.N., Archer D.C. Prevalence of and risk factors for colic in horses that display crib-biting behavior. BMC Vet Res. 2014;1:S3. doi: 10.1186/1746-6148-10-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Archer D.C., Pinchbeck G.K., French N.P. Risk factors for epiploic foramen entrapment colic: an international study. Equine Vet J. 2008;40:224. doi: 10.2746/042516408X266079. [DOI] [PubMed] [Google Scholar]
- 477.Proudman C.J. A two year, prospective study of equine colic in general practice. Equine Vet J. 1992;24:90. doi: 10.1111/j.2042-3306.1992.tb02789.x. [DOI] [PubMed] [Google Scholar]
- 478.Voigt A., Saulex M.N., Donnellan C.M. Causes of gastrointestinal colic at an equine referral hospital in South Africa (1998–2007) J S Afr Vet Assoc. 2009;80(92) doi: 10.4102/jsava.v80i3.201. [DOI] [PubMed] [Google Scholar]
- 479.Abutarbush S.M., Carmalt J.L., Shoemaker J.L. Causes of gastrointestinal colic in horses in western Canada: 604 cases (1992–2002) Can Vet J. 2005;46(800) [PMC free article] [PubMed] [Google Scholar]
- 480.Yorke E.H., Caldwell F.J., Johnson A.K. Uterine torsion in mares. Compend Contin Educ Vet. 2012;34:E2. [PubMed] [Google Scholar]
- 481.Duesterdieck-Zellmer K.F. Equine urolithiasis. Vet Clin North Am Equine Pract. 2007;23:613. doi: 10.1016/j.cveq.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 482.Johnston J.K., Divers T.J., Reef V.B. Cholelithiasis in horses: ten cases (1982–1986) J Am Vet Med Assoc. 1989;194(405) [PubMed] [Google Scholar]
- 483.Tennent-Brown B.S., Mudge M.C., Hardy J. Liver lobe torsion in six horses. J Am Vet Med Assoc. 2012;241:615. doi: 10.2460/javma.241.5.615. [DOI] [PubMed] [Google Scholar]
- 484.Luethy D., Habecker P., Murphy B Clinical and pathological features of pheochromocytoma in the horse: a multi-center retrospective study of 37 cases (2007–2014) J Vet Intern Med. 2016;30(309) doi: 10.1111/jvim.13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Moore B.R., Moore R.M. Examination of the equine patient with gastrointestinal emergency. Vet Clin North Am Equine Pract. 1994;10:549. doi: 10.1016/s0749-0739(17)30346-2. [DOI] [PubMed] [Google Scholar]
- 486.Cook V.L., Hassel D.M. Evaluation of the colic in horses: decision for referral. Vet Clin North Am Equine Pract. 2014;30:383. doi: 10.1016/j.cveq.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 487.Furr M.O., Lessard P., White N.A., 2nd Development of a colic severity score for predicting the outcome of equine colic. Vet Surg. 1995;24:97. doi: 10.1111/j.1532-950x.1995.tb01302.x. [DOI] [PubMed] [Google Scholar]
- 488.Ebert R. Prognostic parameters in equine colic. Tiearztl Prax. 1994;22:256. [PubMed] [Google Scholar]
- 489.Orsini J.A., Elser A.H., Galligan D.T. Prognostic index for acute abdominal crisis (colic) in horses. Am J Vet Res. 1969;49:1988. [PubMed] [Google Scholar]
- 490.Parry B.W., Anderson G.A., Gay C.C. Prognosis in equine colic: a study of individual variables used in case assessment. Equine Vet J. 1983;15:337. doi: 10.1111/j.2042-3306.1983.tb01818.x. [DOI] [PubMed] [Google Scholar]
- 491.Parry B.W., Anderson G.A., Gay C.C. Prognosis in equine colic: a comparative study of variables used to assess individual cases. Equine Vet J. 1983;15:211. doi: 10.1111/j.2042-3306.1983.tb01768.x. [DOI] [PubMed] [Google Scholar]
- 492.Parry B.W., Gay C.C., Anderson G.A. Assessment of the necessity for surgical intervention in cases of equine colic: a retrospective study. Equine Vet J. 1983;15:216. doi: 10.1111/j.2042-3306.1983.tb01770.x. [DOI] [PubMed] [Google Scholar]
- 493.Cribb N.C., Cote N.M., Boure L.P. Acute small intestinal obstruction associated with Parascaris equorum infection in young horses: 25 cases (1985–2004) N Z Vet J. 2006;54(338) doi: 10.1080/00480169.2006.36721. [DOI] [PubMed] [Google Scholar]
- 494.Chaffin M.K., Cohen N.D. Diagnostic assessment of foals with colic. Proc Ann Conv AAEP. 1999;45:235. [Google Scholar]
- 495.Cohen N.D., Matejka P.L., Honnas C.M. Case-control study of the association between various management factors and the development of colic in horses. Texas Equine Colic Study Group. J Vet Med Assoc. 1995;206:667. [PubMed] [Google Scholar]
- 496.Santschi E.M., Purdy A.K., Valberg S.J. Endothelin receptor B polymorphism associated with lethal white foal syndrome in horses. Mamm Genome. 1998;9:306. doi: 10.1007/s003359900754. [DOI] [PubMed] [Google Scholar]
- 497.Wagner A.E., Muir W.W., 3rd, Hinchcliff K.W. Cardiovascular effects of xylazine and detomidine in horses. Am J Vet Res. 1991;52:651. [PubMed] [Google Scholar]
- 498.Daunt D.A., Steffey E.P. Alpha-2 adrenergic agonists as analgesics in horses. Vet Clin North Am Equine Pract. 2002;18:39. doi: 10.1016/s0749-0739(02)00004-4. [DOI] [PubMed] [Google Scholar]
- 499.Ragle C.A., Meagher D.M., Schrader J.L. Abdominal auscultation in the detection of experimentally induced gastrointestinal sand accumulation. J Vet Intern Med. 1989;3:12. doi: 10.1111/j.1939-1676.1989.tb00322.x. [DOI] [PubMed] [Google Scholar]
- 500.Tillotson K., Traub-Dargatz J.L. Gastrointestinal protectants and cathartics. Vet Clin North Am Equine Pract. 2003;19:599. doi: 10.1016/j.cveq.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 501.White N.A., editor. The equine acute abdomen. Lea & Febiger; Malvern: 1990. [Google Scholar]
- 502.Luo T., Bertone J.J., Greene H.M. A comparison of N-butylscopolammonium and lidocaine for control of rectal pressure in horses. Vet Ther. 2006;7:243. [PubMed] [Google Scholar]
- 503.White N.A. Equine colic: how to make the decision for surgery. In: AAEP Focus Proceedings. Philadelphia; 2005
- 504.Parry B.W. Use of clinical pathology in evaluation of horses with colic. Vet Clin North Am Equine Pract. 1987;3:529. doi: 10.1016/s0749-0739(17)30663-6. [DOI] [PubMed] [Google Scholar]
- 505.Navarro M., Monreal L., Segura D. A comparison of traditional and quantitative analysis of acid-base and electrolyte imbalances in horses with gastrointestinal disorders. J Vet Intern Med. 2005;19:871. doi: 10.1892/0891-6640(2005)19[871:acotaq]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 506.Hassel D.M., Hill A.E., Rorabeck R.A. Association between hyperglycemia and survival in 228 horses with acute gastrointestinal disease. J Vet Intern Med. 2009;23:1261. doi: 10.1111/j.1939-1676.2009.0395.x. [DOI] [PubMed] [Google Scholar]
- 507.Davis J.L., Blikslager A.T., Catto K. A retrospective analysis of hepatic injury in horses with proximal enteritis (1984–2002) J Vet Intern Med. 2003;17(896) doi: 10.1111/j.1939-1676.2003.tb02530.x. [DOI] [PubMed] [Google Scholar]
- 508.Gardner R.B., Nydam D.V., Mohammed H.O. Serum gamma glutamyl transferase activity in horses with right or left dorsal displacements of the large colon. J Vet Intern Med. 2005;19:761. doi: 10.1892/0891-6640(2005)19[761:sggtai]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 509.Krueger C.R., Ruple-Czerniak A., Hackett E.S. Evaluation of plasma muscle enzyme activity as an indicator of lesion characteristics and prognosis in horses undergoing celiotomy for acute gastrointestinal pain. BMC Vet Res. 2014;1:S7. doi: 10.1186/1746-6148-10-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Fischer A.T., Jr. Advances in diagnostic techniques for horses with colic. Vet Clin North Am Equine Pract. 1997;13:203. doi: 10.1016/s0749-0739(17)30237-7. [DOI] [PubMed] [Google Scholar]
- 511.Cowell R.L., Tyler R.D., editors. Diagnostic cytology and hematology of the horse. 2nd ed. Mosby; St. Louis: 2007. [Google Scholar]
- 512.Peloso J.G., Cohen N.D. Use of serial measurements of peritoneal fluid lactate concentration to identify strangulating intestinal lesions in referred horse with signs of colic. J Am Vet Med Assoc. 2012;240:1208. doi: 10.2460/javma.240.10.1208. [DOI] [PubMed] [Google Scholar]
- 513.Radcliffe R.M., Divers T.J., Fletcher D.J. Evaluation of L-lactate and cardiac troponin I in horses undergoing emergency abdominal surgery. J Vet Emerg Crit Care (San Antonio) 2012;22:313. doi: 10.1111/j.1476-4431.2012.00744.x. [DOI] [PubMed] [Google Scholar]
- 514.Yamout S.Z., Nieto J.E., Beldomenico P.M. Peritoneal and plasma D-lactate concentrations in horses with colic. Vet Surg. 2011;40:817. doi: 10.1111/j.1532-950X.2011.00859.x. [DOI] [PubMed] [Google Scholar]
- 515.Latson K.M., Nieto J.E., Beldomenico P.M. Evaluation of peritoneal fluid lactate as a marker of intestinal ischemia in equine colic. Equine Vet J. 2005;37:342. doi: 10.2746/0425164054529319. [DOI] [PubMed] [Google Scholar]
- 516.Tennent-Brown B.S. Interpreting lactate measurement in critically ill horses: diagnosis, treatment, and prognosis. Compend Contin Educ Vet. 2012;34:E2. [PubMed] [Google Scholar]
- 517.Nieto J.E., Dechant J.E., le Jeune S.S. Evaluation of 3 handheld portable analyzers for measurement of L-lactate concentrations in blood and peritoneal fluid of horses with colic. Vet Surg. 2015;44:366. doi: 10.1111/j.1532-950X.2014.12231.x. [DOI] [PubMed] [Google Scholar]
- 518.Dunkel B., Kapff J.E., Naylor R.J. Blood lactate concentrations in ponies and Miniature Horses with gastrointestinal disease. Equine Vet J. 2013;45:666. doi: 10.1111/evj.12043. [DOI] [PubMed] [Google Scholar]
- 519.Freeman S.L. Diagnostic ultrasonography of the mature equine abdomen. Equine Vet Educ. 2003;15:319. [Google Scholar]
- 520.le Jeune S Whitcomb MB: Ultrasound of the equine acute abdomen. Vet Clin North Am Equine Pract. 2014;30:353. doi: 10.1016/j.cveq.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 521.Scharner D., Bankert J., Brehm W. Comparison of the findings of rectal examination and ultrasonographic findings in horses with colic. Tieraztl Prax Ausg G Grosstiere Nutztiere. 2015;43:278. doi: 10.15653/TPG-150234. [DOI] [PubMed] [Google Scholar]
- 522.Cavalleri J.M., Biernert-Zeit A., Feige K. Examination of horses with acute colic—clinical pathology and diagnostic imaging. Tierarztl Prax Ausg G Grosstiere Nutztiere. 2013;41:124. [PubMed] [Google Scholar]
- 523.Beccati F., Pepe M., Gialletti R. Is there a statistical correlation between ultrasonographic findings and definitive diagnosis in horses with acute abdominal pain? Equine Vet J Suppl. 2011;39:98. doi: 10.1111/j.2042-3306.2011.00428.x. [DOI] [PubMed] [Google Scholar]
- 524.Busoni V., De Busscher V., Lopez D. Evaluation of a protocol for fast localized abdominal sonography of horses (FLASH) admitted for colic. Vet J. 2011;188:77. doi: 10.1016/j.tvjl.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 525.Bradecamp E.A. How to image the adult equine abdomen and thorax in ambulatory practice using a 5-mHz rectal probe. Proc Ann Conv AAEP. 2007;53:537. [Google Scholar]
- 526.Bernard W.V., Reef V.B., Reimer J.M. Ultrasonographic diagnosis of small-intestinal intussusception in three foals. J Am Vet Med Assoc. 1989;194:395. [PubMed] [Google Scholar]
- 527.Santschi E.M., Slone D.E., Frank W.M. Use of ultrasound in horses for diagnosis of left dorsal displacement of the large colon and monitoring its nonsurgical correction. Vet Surg. 1993;22:281. doi: 10.1111/j.1532-950x.1993.tb00398.x. [DOI] [PubMed] [Google Scholar]
- 528.Ness S.L., Bain F.T., Zantingh A.J. Ultrasonographic visualization of colonic mesenteric vasculature as an indicator of large colon right dorsal displacement or 180° volvulus (or both) in horses. Can Vet J. 2012;53:378. [PMC free article] [PubMed] [Google Scholar]
- 529.Keppie N.J., Rosensein D.S., Holcombe S.J. Objective radiographic assessment of abdominal sand accumulation in horses. Vet Radiol Ultrasound. 2008;49:122. doi: 10.1111/j.1740-8261.2008.00337.x. [DOI] [PubMed] [Google Scholar]
- 530.Yarbrough T.B., Langer D.L., Snyder J.L. Abdominal radiography for diagnosis of enterolithiasis in horses: 141 cases (1990–1992) J Am Vet Med Assoc. 1994;205(592) [PubMed] [Google Scholar]
- 531.Gerhards H., Klein H.J., Offeney F. Radiographic diagnosis of abdominal diseases in foals and ponys. II. Pathologic findings in 60 cases. Tierarztl Prax. 1990;18:383. [PubMed] [Google Scholar]
- 532.Argenzio R.A., Lowe J.E., Pickard D.W. Digesta passage and water exchange in the equine large intestine. Am J Physiol. 1974;226:1035. doi: 10.1152/ajplegacy.1974.226.5.1035. [DOI] [PubMed] [Google Scholar]
- 533.Argenzio R.A., Stevens C.E. Cyclic changes in ionic composition of digesta in the equine intestinal tract. Am J Physiol. 1975;228:1224. doi: 10.1152/ajplegacy.1975.228.4.1224. [DOI] [PubMed] [Google Scholar]
- 534.Argenzio R.A. Functions of the large intestine and their interrelationship with disease. Cornell Vet. 1975;65:303. [PubMed] [Google Scholar]
- 535.Conner M.E., Darlington R.W. Rotavirus infection in foals. Am J Vet Res. 1980;41:1699. [PubMed] [Google Scholar]
- 536.Costa M.C., Stampfli H.R., Allen-Vercoe E. Development of the faecal microbiota in foals. Equine Vet J. 2015 doi: 10.1111/evj.12532. doi: 10.1111. [DOI] [PubMed] [Google Scholar]
- 537.Rodriguez C., Taminiau B., Brevers B. Faecal microbiota characterization of horses using rdna barcoded pyrosequencing, and carriage rate of Clostridium difficile at hospital admission. BMC Microbiol. 2015;15:181. doi: 10.1186/s12866-015-0514-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Holland J.L., Kronfeld D.S., Sklan D. Calculation of fecal kinetics in horses fed hay or hay and concentrate. J Anim Sci. 1934;76:1998. doi: 10.2527/1998.7671937x. [DOI] [PubMed] [Google Scholar]
- 539.Bailey KE, Gilkerson J.R., Browning G.F. Equine rotaviruses—current understanding and continuing challenges. Vet Microbiol. 2013;167:135. doi: 10.1016/j.vetmic.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Vannucci F.A., Gebhart C.J. Recent advances in understanding the pathogenesis of Lawsonia intracellularis infections. Vet Pathol. 2014;51:465. doi: 10.1177/0300985813520249. [DOI] [PubMed] [Google Scholar]
- 541.Wong D.M., Alcott C.J., Sponseller B.A. Impaired intestinal absorption of glucose in 4 foals with Lawsonis intracellularis infection. J Vet Intern Med. 2009;23:940. doi: 10.1111/j.1939-1676.2009.0334.x. [DOI] [PubMed] [Google Scholar]
- 542.O’Louglin E.V., Scott R.B., Gall D.G. Pathophysiology of infectious diarrhea: changes in intestinal structure and function. J Pediatr Gastroenterol Nutr. 1991;12:5. doi: 10.1097/00005176-199101000-00004. [DOI] [PubMed] [Google Scholar]
- 543.Rachmilewitz D. Prostaglandins and diarrhea. Dig Dis Sci. 1980;25:897. doi: 10.1007/BF01308037. [DOI] [PubMed] [Google Scholar]
- 544.Hardcastle J., Hardcastle P.T. Involvement of prostaglandin in histamine-induced fluid and electrolyte secretion by rat colon. J Pharm Pharmacol. 1988;40:106. doi: 10.1111/j.2042-7158.1988.tb05191.x. [DOI] [PubMed] [Google Scholar]
- 545.Wang Y.Z., Su H.C., Cooke Histamine augments colonic secretion in guinea pig distal colon. Am J Physiol. 1990;258:G432. doi: 10.1152/ajpgi.1990.258.3.G432. [DOI] [PubMed] [Google Scholar]
- 546.Clarke L.L., Argenzio R.A. NaCl transport across equine proximal colon and the effect of endogenous prostaglandins. Am J Physiol. 1990;259:G62. doi: 10.1152/ajpgi.1990.259.1.G62. [DOI] [PubMed] [Google Scholar]
- 547.Halm D.R., Rechkemmer G.R., Schoumache R.A. Apical membrane chloride channels in a colonic cell line activated by secretory agonists. Am J Physiol. 1988;254:C505. doi: 10.1152/ajpcell.1988.254.4.C505. [DOI] [PubMed] [Google Scholar]
- 548.Cliff W.H., Frizzell R.A. Separate Cl− conductances activated by cAMP and Ca2 in Cl(−)-secreting epithelial cells. Proc Natl Acad Sci USA. 1990;87:4956. doi: 10.1073/pnas.87.13.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Ling B.N., Kokko K.E., Eaton D.C. Prostaglandin E2 activates clusters of apical Cl− channels in principal cells via a cyclic adenosine monophosphate-dependent pathway. J Clin Invest. 1994;93:829. doi: 10.1172/JCI117037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Guirl M.J., Hogenauer C., Santa Ana C.A. Rapid intestinal transit as a primary cause of severe chronic diarrhea in patients with amyloidosis. Am J Gastroenterol. 2003;98:2219. doi: 10.1111/j.1572-0241.2003.07695.x. [DOI] [PubMed] [Google Scholar]
- 551.King J.N., Gerring E.L. The action of low dose endotoxin on equine bowel motility. Equine Vet J. 1991;23:11. doi: 10.1111/j.2042-3306.1991.tb02705.x. [DOI] [PubMed] [Google Scholar]
- 552.Weese J.S., Parsons D.A., Staempfli H.R. Association of Clostridium difficile with enterocolitis and lactose intolerance in a foal. J Am Vet Med Assoc. 1999;214:229. [PubMed] [Google Scholar]
- 553.Roberts V.L.H., Knottenbelt D.C., Williams A. Suspected primary lactose intolerance in neonatal foals. Equine Vet Educ. 2008;20:249. [Google Scholar]
- 554.Larsen J. Acute colitis in adult horses. A review with emphasis on aetiology and pathogenesis. Vet Q. 1997;19:72. doi: 10.1080/01652176.1997.9694745. [DOI] [PubMed] [Google Scholar]
- 555.Stewart M.C., Hodgson J.L., Kim H. Acute febrile diarrhoea in horses: 86 cases (1986–1991) Aust Vet J. 1995;72(41) doi: 10.1111/j.1751-0813.1995.tb15327.x. [DOI] [PubMed] [Google Scholar]
- 556.Smith B.P. Salmonella infection in horses. Compend Cont Educ Pract Vet. 1981;3:S4. [Google Scholar]
- 557.Jones R.L. Clostridial enterocolitis. Vet Clin North Am Eq Pract16. 2000;471 doi: 10.1016/s0749-0739(17)30090-1. [DOI] [PubMed] [Google Scholar]
- 558.Diab S.S., Songer G., Uzal F.A. Clostridium difficile infection in horses: a review. Vet Microbiol. 2013;167:42. doi: 10.1016/j.vetmic.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 559.East L.M., Savage C.J., Traub-Dargatz J.L. Enterocolitis associated with Clostridium perfringens infection in neonatal foals: 54 cases (1988–1997) J Am Vet Med Assoc. 1998;212(1751) [PubMed] [Google Scholar]
- 560.Magdesian K.G., Hirsh D.C., Jang S.S. Characterization of Clostridium difficile isolates from foals with diarrhea: 28 cases (1993–1997) J Am Vet Med Assoc. 2002;220(67) doi: 10.2460/javma.2002.220.67. [DOI] [PubMed] [Google Scholar]
- 561.Tillotson K., Traub-Dargatz J.L., Dickinson C.E. Population-based study of fecal shedding of Clostridium perfringens in broodmares and foals. J Am Vet Med Assoc. 2002;220:342. doi: 10.2460/javma.2002.220.342. [DOI] [PubMed] [Google Scholar]
- 562.Berlin F.R., Reising A., Slovis N.M. Clinical and clinicopathological findings associated with survival in 44 horses with equine neorickettsiosis (Potomac horse fever) J Vet Intern Med. 2013;27:1528. doi: 10.1111/jvim.12209. [DOI] [PubMed] [Google Scholar]
- 563.Pusterla N., Gebhart C.J. Equine proliferative enteropathy—a review of recent developments. Equine Vet J. 2013;45:403. doi: 10.1111/evj.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Platell J.L., Gibson J.S. Proliferative enteropathy in foals: disease, diagnostics and transmission. Vet J. 2013;195:135. doi: 10.1016/j.tvjl.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 565.Lavoie J.P., Drolet R., Parsons D. Equine proliferative enteropathy: a cause of weight loss, colic, diarrhoea and hypoproteinemia in foals on three breeding farms in Canada. Equine Vet J. 2000;32:418. doi: 10.2746/042516400777591110. [DOI] [PubMed] [Google Scholar]
- 566.Cimprich R.E., Rooney J.R. Corynebacterium equi enteritis in foals. Vet Pathol. 1977;14:95. doi: 10.1177/030098587701400201. [DOI] [PubMed] [Google Scholar]
- 567.Holland R.E., Sriranganathan N., Dupont L. Isolation of enterotoxigenic Escherichia coli from a foal with diarrhea. J Am Vet Med Assoc. 1989;194:389. [PubMed] [Google Scholar]
- 568.Hurcombe S.D., Fox J.G., Kohn C.W. Isolation of Campylobacter fetus subspecies fetus in a 2-year-old quarter horse with chronic diarrhea of an undetermined etiology. J Vet Diagn Invest. 2009;21:266. doi: 10.1177/104063870902100218. [DOI] [PubMed] [Google Scholar]
- 569.Hathcock T.L., Schumacher J., Wright J.C. The prevalence of Aeromonas species in feces of horses with diarrhea. J Vet Intern Med. 1999;13:357. doi: 10.1111/j.1939-1676.1999.tb02194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Browning G.F., Chalmers R.M., Snodgrass D.R. The prevalence of enteric pathogens in diarrhoeic Thoroughbred foals in Britain and Ireland. Equine Vet J. 1991;23:397. doi: 10.1111/j.2042-3306.1991.tb03751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Slovis N.M., Elam J., Estrada M. Infectious agents associated with diarrhoea in neonatal foals in central Kentucky: a comprehensive molecular study. Equine Vet J. 2014;46:311. doi: 10.1111/evj.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Pusterla N, Mapes S, Wademan C, et al. Emerging outbreaks associated with equine coronavirus in adult horses. Vet Microbiol 2013;162:228. [DOI] [PMC free article] [PubMed]
- 573.Fielding C.L., Higgins J.K., Higgins J.C. Disease associated with equine coronavirus infection and high case fatality rate. J Vet Intern Med. 2015;29:307. doi: 10.1111/jvim.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Oue Y., Morita Y., Kondo T. Epidemic of equine coronavirus at Obihiro Racecourse, Hokkaido, Japan in 2012. J Vet Med Sci. 2013;75:1261. doi: 10.1292/jvms.13-0056. [DOI] [PubMed] [Google Scholar]
- 575.Guy J.S., Breslin J.J., Breuhaus B. Characterization of a coronavirus isolated from a diarrheic foal. J Clin Microbiol. 2000;38:4523. doi: 10.1128/jcm.38.12.4523-4526.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Corning S. Equine cyathostomins: a review of biology, clinical significance and therapy. Parasit Vectors Suppl. 2009;2:S1. doi: 10.1186/1756-3305-2-S2-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Nielsen M.K., Loynachan A.T., Jacobsen S. Local and systemic inflammatory and immunologic reactions to cyathostomin larvicidal therapy in horses. Vet Immunol and Immunopathol. 2015;168:203. doi: 10.1016/j.vetimm.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 578.Netherwood T., Wood J.L., Townsend H.G. Foal diarrhoea between 1991 and 1994 in the United Kingdom associated with Clostridium perfringens, rotavirus, Strongyloides westeri and Cryptosporidium spp. Epidemiol Infect. 1996;117:375. doi: 10.1017/s0950268800001564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Lyons E.T., Tolliver S.C. Prevalence of patent Strongyloides westeri infections in Thoroughbred foals in 2014. Parasitol Res. 2014;113:4163. doi: 10.1007/s00436-014-4088-1. [DOI] [PubMed] [Google Scholar]
- 580.Galuppi R., Piva S., Castagnetti C. Cryptosporidium parvum: from foal to veterinary students. Vet Parasitol. 2016;219:53. doi: 10.1016/j.vetpar.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 581.Liu A., Zhang J., Zhao The first report of Cryptosporidium andersoni in horses with diarrhea and multilocus subtype analysis. Parasit Vectors. 2015;8:483. doi: 10.1186/s13071-015-1102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Cole D.J., Cohen N.D., Snowden K. Prevalence of and risk factors for fecal shedding of Cryptosporidium parvum oocysts in horses. J Am Vet Med Assoc. 1998;213:1296. [PubMed] [Google Scholar]
- 583.Wilson D.A., MacFadden K.E., Green E.M. Case control and historical cohort study of diarrhea associated with administration of trimethoprim-potentiated sulphonamides to horses and ponies. J Vet Intern Med. 1996;10:258. doi: 10.1111/j.1939-1676.1996.tb02059.x. [DOI] [PubMed] [Google Scholar]
- 584.Baverud V., Franklin A., Gunnarson A. Clostridium difficile associated with acute colitis in mares when their foals are treated with erythromycin and rifampin for Rhodococcus equi pneumonia. Equine Vet J. 1998;30:482. doi: 10.1111/j.2042-3306.1998.tb04523.x. [DOI] [PubMed] [Google Scholar]
- 585.Costa M.C., Stampfli H.R., Arroyo L.G. Changes in the equine fecal microbiota associated with the use of systemic antimicrobial drugs. BMC Vet Res. 2015;11:19. doi: 10.1186/s12917-015-0335-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Harlow B.E., Lawrence L.M., Flythe M.D. Diarrhea-associated pathogens, lactobacilli and cellulolytic bacteria in equine feces: responses to antibiotic challenge. Vet Microbiol. 2013;166:225. doi: 10.1016/j.vetmic.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 587.Karcher L.F., Dill S.G., Anderson W.I. Right dorsal colitis. J Vet Intern Med. 1990;4:247. doi: 10.1111/j.1939-1676.1990.tb03117.x. [DOI] [PubMed] [Google Scholar]
- 588.Marshall J.F., Blikslager A.T. The effect of nonsteroidal anti-inflammatory drugs on the equine intestine. Equine Vet J Suppl. 2011;39:140. doi: 10.1111/j.2042-3306.2011.00398.x. [DOI] [PubMed] [Google Scholar]
- 589.McConnico R.S., Morgan T.W., Williams C.C. Pathophysiologic effects of phenylbutazone on the right dorsal colon in horses. Am J Vet Res. 2008;69:1496. doi: 10.2460/ajvr.69.11.1496. [DOI] [PubMed] [Google Scholar]
- 590.Schmitz D.G. Cantharidin toxicosis in horses. J Vet Intern Med. 1989;3:208. doi: 10.1111/j.1939-1676.1989.tb00859.x. [DOI] [PubMed] [Google Scholar]
- 591.Abutarbush S.M. Alfalfa dodder (Cuscuta campestris) toxicity in horses: clinical, haematological and serum biochemical findings. Vet Rec. 2013;173:95. doi: 10.1136/vr.101635. [DOI] [PubMed] [Google Scholar]
- 592.Smith S., Naylor R.J., Knowles E.J. Suspected acorn toxicity in nine horses. Equine Vet J. 2015;47:568. doi: 10.1111/evj.12306. [DOI] [PubMed] [Google Scholar]
- 593.Renier A.C., Kass P.H., Magdesian K.G. Oleander toxicosis in equids: 30 cases (1995–2010) J Am Vet Med Assoc. 2013;242(540) doi: 10.2460/javma.242.4.540. [DOI] [PubMed] [Google Scholar]
- 594.Pace L.W., Turnquist S.E., Casteel S.W. Acute arsenic toxicosis in five horses. Vet Pathol. 1997;34:160. doi: 10.1177/030098589703400211. [DOI] [PubMed] [Google Scholar]
- 595.Casteel S.W. Metal toxicosis in horses. Vet Clin North Am Equine Pract. 2001;17:517. doi: 10.1016/s0749-0739(17)30049-4. [DOI] [PubMed] [Google Scholar]
- 596.Taylor S.D., Pusterla N., Vaugan B. Intestinal neoplasia in horses. J Vet Intern Med. 2006;20:1429. doi: 10.1892/0891-6640(2006)20[1429:inih]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 597.Gianini M., Sutter O., Burger D. Gastrointestinal and endocrine function during “foal heat diarrhea” in healthy foals. J Reprod Fertil Suppl. 2000;56:717. [PubMed] [Google Scholar]
- 598.Olivett T.L., Divers T.J., Cushing T. Acute pancreatitis in two 5-day-old Appaloosa foals. Equine Vet J Suppl. 2012;41:96. doi: 10.1111/j.2042-3306.2011.00435.x. [DOI] [PubMed] [Google Scholar]
- 599.Cohen N.D., Woods A.M. Characteristics and risk factors for failure of horses with acute diarrhea to survive: 122 cases (1990–1996) J Am Vet Med Assoc. 1999;214(382) [PubMed] [Google Scholar]
- 600.Mair T.S., Taylor F.G., Harbour D.A., Pearson G.R. Concurrent cryptosporidium and coronavirus infections in an Arabian foal with combined immunodeficiency syndrome. Vet Rec. 1990;126:127. [PubMed] [Google Scholar]
- 601.Richards A.F., Kelly D.F., Knottenbelt D.C. Anaemia, diarrhoea and opportunistic infections in Fell ponies. Equine Vet J. 2000;32:386. doi: 10.2746/042516400777591174. [DOI] [PubMed] [Google Scholar]
- 602.Sweeney R.W., Sweeney C.R., Saik J. Chronic granulomatous bowel disease in three sibling horses. J Am Vet Med Assoc. 1986;188:1192. [PubMed] [Google Scholar]
- 603.Mair T.S., Cripps P.J., Ricketts S.W. Diagnostic and prognostic value of serum protein electrophoresis in horses with chronic diarrhoea. Equine Vet J. 1993;25:324. doi: 10.1111/j.2042-3306.1993.tb02973.x. [DOI] [PubMed] [Google Scholar]
- 604.Patton S., Mock R.E., Drudge J.H. Increase of immunoglobulin T concentrations in ponies as a response to experimental infection with the nematode Strongylus vulgaris. Am J Vet Res. 1978;39:19. [PubMed] [Google Scholar]
- 605.Morris D.D., Whitlock R.H., Palmer J.E. Fecal leukocytes and epithelial cells in horses with diarrhea. Cornell Vet. 1983;73:265. [PubMed] [Google Scholar]
- 606.Schoster A., Arroyo L.G., Staempfli H.R. Presence and molecular characterization of Clostridium difficile and Clostridium perfringens in intestinal compartments of healthy horses. BMC Vet Res. 2012;8:94. doi: 10.1186/1746-6148-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Weese J.S., Staemplfi H.R., Prescott J.F. Test selections and interpretation in the diagnosis of Clostridium difficile–associated colitis. Proc Ann AAEP Conv. 1999;45:502. [Google Scholar]
- 608.Hyatt D.R., Weese J.S. Salmonella culture: sampling procedures and laboratory techniques. Vet Clin North Am Equine Pract. 2004;20:577. doi: 10.1016/j.cveq.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 609.Cohen N.D., Neibergs H.L., Wallis D.E. Genus-specific detection of salmonellae in equine feces by use of the polymerase chain reaction. Am J Vet Res. 1994;55:1049. [PubMed] [Google Scholar]
- 610.Cohen N.D., Martin L.J., Simpson R.B. Comparison of polymerase chain reaction and microbiological culture for detection of salmonellae in equine feces and environmental samples. Am J Vet Res. 1996;57:780. [PubMed] [Google Scholar]
- 611.Barlough J.E., Rikihisa Y., Madigan J.E. Nested polymerase chain reaction for detection of Ehrlichia risticii genomic DNA in infected horses. Vet Parasitol. 1997;68:367. doi: 10.1016/s0304-4017(96)01083-7. [DOI] [PubMed] [Google Scholar]
- 612.Mott F., Rikihisa T., Zhang Y. Comparison of PCR and culture to the indirect fluorescent-antibody test for diagnosis of Potomac horse fever. J Clin Microbiol. 1997;35:2215. doi: 10.1128/jcm.35.9.2215-2219.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Magdesian K.G., Leutenegger C.M. Real-time PCR and typing of Clostridium difficile isolates colonizing mare foal pairs. Vet J. 2011;190:119. doi: 10.1016/j.tvjl.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 614.Page A.E., Slovis N.M., Gebhart C.J. Serial use of serologic assays and fecal PCR assays to aid in identification of subclinical Lawsonia intracellularis infection for targeted treatment of Thoroughbred foals and weanlings. J Am Vet Med Assoc. 2011;238:1482. doi: 10.2460/javma.238.11.1482. [DOI] [PubMed] [Google Scholar]
- 615.Ellis G.R., Daniels E. Comparison of direct electron microscopy and enzyme immunoassay for the detection of rotaviruses in calves, lambs, piglets and foals. Aust Vet J. 1988;65:133. doi: 10.1111/j.1751-0813.1988.tb14439.x. [DOI] [PubMed] [Google Scholar]
- 616.Mino S., Kern A., Barrandeguy M. Comparison of two commercial kits and an in-house ELISA for the detection of equine rotavirus in foal feces. J Virol Methods. 2015;222:1. doi: 10.1016/j.jviromet.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 617.Jones S.L., Davis J., Rowlingson K. Ultrasonographic findings in horses with right dorsal colitis: five cases (2000–2001) J Am Vet Med Assoc. 2003;222(1248) doi: 10.2460/javma.2003.222.1248. [DOI] [PubMed] [Google Scholar]
- 618.East L.M., Trumble T.N., Steyn P.F. The application of technetium-99m hexamethylpropyleneamine oxime (99mTc-HMPAO) labeled white blood cells for the diagnosis of right dorsal ulcerative colitis in two horses. Vet Radiol Ultrasound. 2000;41:360. doi: 10.1111/j.1740-8261.2000.tb02088.x. [DOI] [PubMed] [Google Scholar]
- 619.Roberts M.C., Norman P. A re-evaluation of the D (+) xylose absorption test in the horse. Equine Vet J. 1979;11:239. doi: 10.1111/j.2042-3306.1979.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 620.Mullen K.R., Yasuda K., Divers T.J. Equine faecal microbiota transplant: current knowledge, proposed guidelines and future directions. Equine Vet Educ. 2016 doi: 10.1111/eve. 12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Schoster A., Weese J.S., Guardabassi L. Probiotic use in horses—what is the evidence for their clinical efficacy? J Vet Intern Med. 2014;28:1640. doi: 10.1111/jvim.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622.Desrochers A.M., Dolente B.A., Roy M.F. Efficacy of Saccharomyces boulardii for treatment of horses with acute enterocolitis. J Am Vet Med Assoc. 2005;227:954. doi: 10.2460/javma.2005.227.954. [DOI] [PubMed] [Google Scholar]
- 623.Groenendyk S., English P.B., Abetz I. External balance of water and electrolytes in the horse. Equine Vet J. 1988;20:189. doi: 10.1111/j.2042-3306.1988.tb01497.x. [DOI] [PubMed] [Google Scholar]
- 624.Rumbaugh G.E., Carlson G.P., Harrold D. Urinary production in the healthy horse and in horses deprived of feed and water. Am J Vet Res. 1982;43:735. [PubMed] [Google Scholar]
- 625.Tasker J.B. Fluid and electrolyte studies in the horses. III. Intake and output of water, sodium and potassium in normal horses. Cornell Vet. 1967;57:649. [Google Scholar]
- 626.Morris D.D., Divers T.J., Whitlock R.H. Renal clearance and fractional excretion of electrolytes over a 24-hour period in horses. Am J Vet Res. 1984;45:2431. [PubMed] [Google Scholar]
- 627.Kohn C.W., Strasser S.L. 24-hour renal clearance and excretion of endogenous substances in the mare. Am J Vet Res. 1986;47:1332. [PubMed] [Google Scholar]
- 628.Rose R.J. Electrolytes: clinical application. Vet Clin North Am Equine Pract. 1990;6:281. doi: 10.1016/s0749-0739(17)30542-4. [DOI] [PubMed] [Google Scholar]
- 629.Cymbaluk N.F. Water balance of horses fed various diets. Equine Pract. 1989;11:19. [Google Scholar]
- 630.Sufit E., Houpt K.A., Sweeting M. Physiological stimuli of thirst and drinking patterns in ponies. Equine Vet J. 1985;17:12. doi: 10.1111/j.2042-3306.1985.tb02028.x. [DOI] [PubMed] [Google Scholar]
- 631.Brewer B.D., Clement S.F., Lotz W.S. Renal clearance, urinary excretion of endogenous substances and urinary diagnostic indices in healthy neonatal foals. J Vet Intern Med. 1991;5:28. doi: 10.1111/j.1939-1676.1991.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 632.Edwards D.J., Brownlow M.A., Hutchins D.R. Indices of renal function; values in eight normal foals from birth to 56 days. Aust Vet J. 1990;67:251. doi: 10.1111/j.1751-0813.1990.tb07779.x. [DOI] [PubMed] [Google Scholar]
- 633.Martin R.G., McMeniman N.P., Dowsett K.F. Milk and water intakes of foals sucking grazing mares. Equine Vet J. 1992;24:295. doi: 10.1111/j.2042-3306.1992.tb02839.x. [DOI] [PubMed] [Google Scholar]
- 634.Rose B.D. McGraw-Hill Information Services; New York: 1989. Clinical physiology of acid-base and electrolyte disorders. [Google Scholar]
- 635.McCutcheon L.J., Geor R.J. Sweat fluid and ion losses in horses during training and competition in cool vs. hot ambient conditions: implications for ion supplementation. Equine Vet J. 1996;22(S):54. doi: 10.1111/j.2042-3306.1996.tb05032.x. [DOI] [PubMed] [Google Scholar]
- 636.Guyton A.C., Hall J. Textbook of medical physiology. Philadelphia; Elsevier Saunders: 2006. Regulation of extracellular fluid osmolarity and sodium concentration. [Google Scholar]
- 637.Jamison R.L., Maffly R.H. The urinary concentrating mechanism. N Engl J Med. 1976;295:1059. doi: 10.1056/NEJM197611042951908. [DOI] [PubMed] [Google Scholar]
- 638.Rotondo F., Butz H., Syro L.V. Arginine vasopressin (AVP): a review of its historical perspectives, current research and multifunctional role in the hypothalmo-hypophysial system. Pituitary. 2016;19:345. doi: 10.1007/s11102-015-0703-0. [DOI] [PubMed] [Google Scholar]
- 639.Boone M., Deen P.M. Physiology and pathophysiology of the vasopressin-regulated renal water reabsorption. Pflugers Arch. 2008;456:1005. doi: 10.1007/s00424-008-0498-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Houpt K.A., Thornton S.N., Allen W.R. Vasopressin in dehydrated and rehydrated ponies. Physiol Behav. 1989;45:659. doi: 10.1016/0031-9384(89)90087-5. [DOI] [PubMed] [Google Scholar]
- 641.Sands J.M., Blount M.A., Klein J.D. Regulation of renal urea transport by vasopressin. Trans Am Clin Climatol Assoc. 2011;122:82. [PMC free article] [PubMed] [Google Scholar]
- 642.Houpt K.A. Thirst in horses: the physiological and psychological causes. Equine Pract. 1987;9:28. [Google Scholar]
- 643.Guyton A.C., Hall J. Textbook of Medical Physiology. Philadelphia; Elsevier Saunders: 2006. Kidney diseases and diuretics. [Google Scholar]
- 644.Robertson G.L. Diabetes insipidus: differential diagnosis and management. Best Pract Red Clin Endocrinol Metab. 2016;30:205. doi: 10.1016/j.beem.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 645.Schott H.C. Water homeostasis and diabetes insipidus in horses. Vet Clin North Am Equine Pract. 2011;27:175. doi: 10.1016/j.cveq.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 646.Geor R.J. Acute renal failure in horses. Vet Clin North Am Equine Pract. 2007;23:577. doi: 10.1016/j.cveq.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 647.Schott H.C. Chronic renal failure in horses. Vet Clin North Am Equine Pract. 2007;23:593. doi: 10.1016/j.cveq.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 648.Buntain B.J., Coffman J.R. Polyuria and polydipsia in a horse induced by psychogenic salt consumption. Equine Vet J. 1981;13:266. doi: 10.1111/j.2042-3306.1981.tb03517.x. [DOI] [PubMed] [Google Scholar]
- 649.Taylor F.G.R., Hillyer M.H. The differential diagnosis of hyperglycaemia in horses. Equine Vet Educ. 1992;4:135. [Google Scholar]
- 650.Knottenbelt D.C. Polyuria-polydipsia in the horse. Equine Vet Educ. 2000;12:179. [Google Scholar]
- 651.McKenzie E.C. Polyuria and polydipsia in horses. Vet Clin North Am Equine Pract. 2007;23:641. doi: 10.1016/j.cveq.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 652.Rye S.H., Kim B.S., Lee C.W. Glucocorticoid-induced laminitis with hepatopathy in a Thoroughbred filly. J Vet Sci. 2004;5:271. [PubMed] [Google Scholar]
- 653.Browning A.P. Polydipsia and polyuria in two horses caused by psychogenic polydipsia. Equine Vet Educ. 2000;12:175. [Google Scholar]
- 654.Andersson B., Rundgren M. Thirst and its disorders. Ann Rev Med. 1982;33:231. doi: 10.1146/annurev.me.33.020182.001311. [DOI] [PubMed] [Google Scholar]
- 655.Robertson G.L. Abnormalities of thirst regulation. Kidney International. 1984;25:460. doi: 10.1038/ki.1984.39. [DOI] [PubMed] [Google Scholar]
- 656.Sturgeon B.P., Bassett H. Polydipsia in a foal with renal helminthiasis. Vet Rec. 2000;147:23. doi: 10.1136/vr.147.1.23. [DOI] [PubMed] [Google Scholar]
- 657.Wooldridge A.A., Seahorn T.L., Williams J. Chronic renal failure associated with nephrolithiasis, ureterolithiasis, and renal dysplasia in a 2-year-old Quarter Horse gelding. Vet Radiol Ultrasound. 1999;40:361. doi: 10.1111/j.1740-8261.1999.tb02126.x. [DOI] [PubMed] [Google Scholar]
- 658.McGowan T.W., Pinchbeck G.P., McGowan C.M. Prevalence, risk factors and clinical signs predictive for equine pituitary pars intermedia dysfunction in aged horses. Equine Vet J. 2013;45:74. doi: 10.1111/j.2042-3306.2012.00578.x. [DOI] [PubMed] [Google Scholar]
- 659.McFarlane D. Equine pituitary pars intermedia dysfunction. Vet Clin North Am Equine Pract. 2011;27:93. doi: 10.1016/j.cveq.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 660.Schott H.C. Pituitary pars intermedia dysfunction: equine Cushing’s disease. Vet Clin North Am Equine Pract. 2002;18:237. doi: 10.1016/s0749-0739(02)00018-4. [DOI] [PubMed] [Google Scholar]
- 661.Breukink H.J., Van Wegen P., Schotman A.J.H. Idiopathic diabetes insipidus in a Welsh pony. Equine Vet J. 1983;15:284. doi: 10.1111/j.2042-3306.1983.tb01796.x. [DOI] [PubMed] [Google Scholar]
- 662.Filar J., Ziolo T., Szalecki J. Diabetes insipidus in the course of encephalitis in the horse. Med Weter. 1971;27:205. [Google Scholar]
- 663.Morgan R.A., Malalana F., McGowan C.M. Nephrogenic diabetes insipidus in a 14-year-old gelding. N Z Vet J. 2012;60:254. doi: 10.1080/00480169.2012.669723. [DOI] [PubMed] [Google Scholar]
- 664.Schott H.C., Bayly W.M., Reed S.M. Nephrogenic diabetes insipidus in sibling colts. J Vet Intern Med. 1993;7:68. doi: 10.1111/j.1939-1676.1993.tb03172.x. [DOI] [PubMed] [Google Scholar]
- 665.Brashier M. Polydipsia and polyuria in a weanling colt caused by nephrogenic diabetes insipidus. Vet Clin North Am Equine Pract. 2006;22:219. doi: 10.1016/j.cveq.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 666.Corke M.J. Diabetes mellitus: the tip of the iceberg. Equine Vet J. 1986;18:87. doi: 10.1111/j.2042-3306.1986.tb03549.x. [DOI] [PubMed] [Google Scholar]
- 667.Menzies-Gow N. Diabetes in the horse: a condition of increasing clinical awareness for differential diagnosis and interpretation of tests. Equine Vet J. 2009;41:841. doi: 10.2746/042516409x471412. [DOI] [PubMed] [Google Scholar]
- 668.Collobert C., Gillet J.P., Sorel P., Minnelo J. Chronic pancreatitis associated with diabetes mellitus in a Standardbred racehorse: a case report. J Eq Vet Sci. 1990;10:58. [Google Scholar]
- 669.Giri J.K., Magdesian K.G., Gaffney P.M. Insulin-dependent diabetes mellitus associated with presumed autoimmune polyendocrine syndrome in a mare. Can Vet J. 2011;52:506. [PMC free article] [PubMed] [Google Scholar]
- 670.Navas de Solis C. Foreman JH: Transient diabetes mellitus in a neonatal Thoroughbred foal. J Vet Emerg Critical Care. 2010;20:611. doi: 10.1111/j.1476-4431.2010.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671.Jeffrey J.R. Diabetes mellitus secondary to chronic pancreatitis in a pony. J Am Vet Med Assoc. 1968;153:1168. [PubMed] [Google Scholar]
- 672.McCoy D.J. Diabetes mellitus associated with bilateral granulosa cell tumors in a mare. J Am Vet Med Assoc. 1986;188:733. [PubMed] [Google Scholar]
- 673.Ruoff W.W., Baker D.C., Morgan S.J. Type II diabetes mellitus in a horse. Equine Vet J. 1986;18:143. doi: 10.1111/j.2042-3306.1986.tb03571.x. [DOI] [PubMed] [Google Scholar]
- 674.Baker J.R., Ritchie H.E. Diabetes mellitus in the horse: a case report and review of the literature. Equine Vet J. 1974;6:7. doi: 10.1111/j.2042-3306.1974.tb03919.x. [DOI] [PubMed] [Google Scholar]
- 675.Durham A.E., Hughes K.J., Cottle H.J. Type 2 diabetes mellitus with pancreatic beta cell dysfunction in 3 horses confirmed with minimal model analysis. Equine Vet J. 2009;41:924. doi: 10.2746/042516409x452152. [DOI] [PubMed] [Google Scholar]
- 676.Muylle E., van den Hende C. Non–insulin dependent diabetes mellitus in a horse. Equine Vet J. 1986;18:145. doi: 10.1111/j.2042-3306.1986.tb03572.x. [DOI] [PubMed] [Google Scholar]
- 677.Sneddon J.C., Colyn P. A practical system for measuring water intake in stabled horses. Equine Vet Sci. 1991;11:141. [Google Scholar]
- 678.Harris P. Collection of urine. Equine Vet J. 1988;20:86. doi: 10.1111/j.2042-3306.1988.tb01465.x. [DOI] [PubMed] [Google Scholar]
- 679.van den Berg I.S. Modified apparatus for collection of free-flow urine from mares. J S Afr Vet Assoc. 1996;67:214. [PubMed] [Google Scholar]
- 680.Tasker J.B. Fluid and electrolyte studies in the horse. II. An apparatus for the collection of total daily urine and feces from horses. Cornell Vet. 1966;56:77. [PubMed] [Google Scholar]
- 681.Kohn C.W., Chew D.J. Laboratory diagnosis and characterization of renal disease in horses. Vet Clin North Am Equine Pract. 1987;3:585. doi: 10.1016/s0749-0739(17)30666-1. [DOI] [PubMed] [Google Scholar]
- 682.Bickhardt K., Deegen E., Espelage W. Kidney function tests in horses—methods and reference values in healthy animals. Dtsch Tierarztl Wochenschr. 1996;103:117. [PubMed] [Google Scholar]
- 683.Satoh H., Abe S., Kato M. Optimum conditions for serum clearance of iodixanol, applicable to the estimation of glomerular filtration rate in horses. Vet Res Commun. 2011;35:463. doi: 10.1007/s11259-011-9485-7. [DOI] [PubMed] [Google Scholar]
- 684.Nabity M.B., Lees G.E., Boggess M.M. Symmetric dimethylarginine assay validation, stability and evaluation as a marker for the early detection of chronic kidney disease in dogs. J Vet Intern Med. 2015;29:1036. doi: 10.1111/jvim.12835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 685.Brobst D.F., Bayly W.M. Responses of horses to a water deprivation test. J Equine Vet Sci. 1982;2:51. [Google Scholar]
- 686.Genetzky R.M., Loparco F.V., Ledet A.E. Clinical pathologic alterations in horses during a water deprivation test. Am J Vet Res. 1987;48:1007. [PubMed] [Google Scholar]
- 687.Brown C.M. Lea & Febiger; Philadelphia: 1989. Problems in equine medicine. [Google Scholar]
- 688.Zimmer E.L. Equine medicine and surgery. American Veterinary Publications; Goleta, CA: 1991. Water deprivation test and vasopressin challenge. [Google Scholar]
- 689.Velie B.D., Hamilton N.A., Wade C.M. Heritability of racing performance in the Australian Thoroughbred racing population. Anim Genet. 2015;46:23. doi: 10.1111/age.12234. [DOI] [PubMed] [Google Scholar]
- 690.Hill E.W., McGivney B.A., Gu J. A genome-wide SNP-association study confirms a sequence variant (g.66493737C>T) in the equine myostatin (MSTN) gene as the most powerful predictor of optimum racing distance for Thoroughbred racehorses. BMC Genomics. 2010;11:552. doi: 10.1186/1471-2164-11-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 691.Jonsson L., Egenvall A., Roepstorff L. Associations of health status and conformation with longevity and lifetime competition performance in young Swedish Warmblood riding horses. J Am Vet Med Assoc. 2014;244:1449. doi: 10.2460/javma.244.12.1449. [DOI] [PubMed] [Google Scholar]
- 692.Dyson S. Lameness and poor performance in the sports horse: dressage, show jumping and horse trials (eventing) AAEP Proceedings. 2000;46:308. [Google Scholar]
- 693.Dyson S.J. Lesions of the equine neck resulting in lameness or poor performance. Vet Clin North Am Equine Pract. 2011;27:417. doi: 10.1016/j.cveq.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 694.Girodroux M., Dyson S., Murray R. Osteoarthritis of the thoracolumbar synovial intervertebral articulations: clinical and radiographic features in 77 horses with poor performance and back pain. Equine Vet J. 2009;41:130. doi: 10.2746/042516408x345099. [DOI] [PubMed] [Google Scholar]
- 695.Rose R.J. Poor performance: a clinical and physiological perspective. Proceedings of the 19th American College of Veterinary Internal Medicine Forum. 2001;224 [Google Scholar]
- 696.Rivero J.L., Hill E.W. Skeletal muscle adaptations and muscle genomics of performance horses. Vet J. 2016;209:5. doi: 10.1016/j.tvjl.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 697.Kearns C.F., McKeever K.H., Abe T. Overview of horse body composition and muscle architecture: implications for performance. Vet J. 2002;164:224. doi: 10.1053/tvjl.2001.0702. [DOI] [PubMed] [Google Scholar]
- 698.Evans D.L. Physiology of equine performance and associated tests of function. Equine Vet J. 2007;39:373. doi: 10.2746/042516407x206418. [DOI] [PubMed] [Google Scholar]
- 699.Morris E.A., Seeherman H.J. Clinical evaluation of poor performance in the racehorse: the results of 275 evaluations. Equine Vet J. 1991;23:169. doi: 10.1111/j.2042-3306.1991.tb02749.x. [DOI] [PubMed] [Google Scholar]
- 700.Martin B.B., Reef V.B., Parente E.J. Causes of poor performance of horses during training, racing or showing: 348 cases (1992–1996) J Am Vet Med Assoc. 2000;216(554) doi: 10.2460/javma.2000.216.554. [DOI] [PubMed] [Google Scholar]
- 701.Seeherman H.J., Morris E., O’Callaghan M.W. The use of sports medicine techniques in evaluating the problem equine athlete. Vet Clin North Am Equine Pract. 1991;7:259. doi: 10.1016/s0749-0739(17)30565-5. [DOI] [PubMed] [Google Scholar]
- 702.Brown J.A., Hinchcliff K.W., Jackson M.A. Prevalence of pharyngeal and laryngeal abnormalities in Thoroughbreds racing in Australia, and their association with performance. Equine Vet J. 2005;37:397. doi: 10.2746/042516405774480021. [DOI] [PubMed] [Google Scholar]
- 703.Pirrone F., Albertini M., Clement M.G. Respiratory mechanics in Standardbred horses with sub-clinical inflammatory airway disease and poor athletic performance. Vet J. 2007;173:144. doi: 10.1016/j.tvjl.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 704.Evans D.L., Kiddell L., Smith C.L. Pulmonary function measurements immediately after exercise are correlated with neutrophil percentage in tracheal aspirates in horses with poor racing performance. Res Vet Sci. 2011;90:510. doi: 10.1016/j.rvsc.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 705.Sanchez A., Couetil L.L., Ward M.P. Effect of airway disease on blood gas exchange in racehorses. J Vet Intern Med. 2005;19:87. doi: 10.1892/0891-6640(2005)19<87:eoadob>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 706.Tamzali Y., Marguet C., Priymenko N. Prevalence of gastric ulcer syndrome in high-level endurance horses. Equine Vet J. 2011;43:141. doi: 10.1111/j.2042-3306.2010.00129.x. [DOI] [PubMed] [Google Scholar]
- 707.Leahy E.R., Burk A.O., Greene E.A. Nutrition-associated problems facing elite level three-day eventing horses. Equine Vet J Suppl. 2010;38:370. doi: 10.1111/j.2042-3306.2010.00233.x. [DOI] [PubMed] [Google Scholar]
- 708.Hubert J.D., Beadle R.E., Norwood G. Equine anhidrosis. Vet Clin North Am Equine Pract. 2002;18:355. doi: 10.1016/s0749-0739(02)00016-0. [DOI] [PubMed] [Google Scholar]
- 709.Richard E.A., Fortier G.D., Pitel P.H. Sub-clinical diseases affecting performance in Standardbred trotters: diagnostic methods and predictive parameters. Vet J. 2010;184:282. doi: 10.1016/j.tvjl.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 710.Fraipont A., Van Erck E., Ramery E. Subclinical diseases underlying poor performance in endurance horses: diagnostic methods and predictive tests. Vet Rec. 2011;169:154. doi: 10.1136/vr.d4142. [DOI] [PubMed] [Google Scholar]
- 711.Dabareiner R.M., Cohen N.D., Carter G.K. Lameness and poor performance in horses used for team roping: 118 cases (2000–2003) J Am Vet Med Assoc. 2005;226(1694) doi: 10.2460/javma.2005.226.1694. [DOI] [PubMed] [Google Scholar]
- 712.Gorgas D., Luder P., Lang J. Scintigraphic and radiographic appearance of the sacroiliac region in horses with gait abnormalities or poor performance. Vet Radiol Ultrasound. 2009;50:208. doi: 10.1111/j.1740-8261.2009.01519.x. [DOI] [PubMed] [Google Scholar]
- 713.Aleman M. A review of equine muscle disorders. Neuromuscul Disord. 2008;18:277. doi: 10.1016/j.nmd.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 714.McCue M.E., Valberg S.J., Lucio M. Glycogen synthase (GYS1) mutation in diverse breeds with polysaccharide storage myopathy. J Vet Intern Med. 2008;22:1228. doi: 10.1111/j.1939-1676.2008.0167.x. [DOI] [PubMed] [Google Scholar]
- 715.Fritz K.L., McCue M.E., Valberg S.J. Genetic mapping of recurrent exertional rhabdomyolysis in a population of North American Thoroughbreds. Anim Genet. 2012;43:730. doi: 10.1111/j.1365-2052.2012.02351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 716.Valberg S.J., Carlson G.P., Cardinet G.H. Skeletal muscle myopathy as a cause of exercise intolerance in a horse. Muscle Nerve. 1994;17:305. doi: 10.1002/mus.880170308. [DOI] [PubMed] [Google Scholar]
- 717.Valberg S.J., McKenzie E.C., Eyrich L.V. Suspected myofibrillar myopathy in Arabian horses with a history of exertional rhabdomyolysis. Equine Vet J. 2015 doi: 10.1111/evj.12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 718.Bedford H.E., Valberg S.J., Firshman A.M. Histopathologic findings in the sacrocaudalis dorsalis medialis muscle of horses with vitamin E–responsive muscle atrophy and weakness. J Am Vet Med Assoc. 2013;242:1127. doi: 10.2460/javma.242.8.1127. [DOI] [PubMed] [Google Scholar]
- 719.Tan R.H., Dowling B.A., Dart A.J. High-speed treadmill videoendoscopic examination of the upper respiratory tract in the horse: the results of 291 clinical cases. Vet J. 2005;170:243. doi: 10.1016/j.tvjl.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 720.Maxson-Sage A., Parente E.J., Beech J. Effect of high-intensity exercise on arterial blood gas tensions and upper airway and cardiac function in clinically normal Quarter Horses and horses heterozygous and homozygous for hyperkalemic periodic paralysis. Am J Vet Res. 1998;59:615. [PubMed] [Google Scholar]
- 721.Allen K.J., Tremaine W.H., Franklin S.H. Prevalence of inflammatory airway disease in national hunt horses referred for investigation of poor athletic performance. Equine Vet J Suppl. 2006;36:529. doi: 10.1111/j.2042-3306.2006.tb05599.x. [DOI] [PubMed] [Google Scholar]
- 722.Salz R.O., Ahern B.J., Boston R. Association of tracheal mucus or blood and airway neutrophilia with racing performance in Thoroughbred horses in an Australian racing yard. Aust Vet J. 2016;94:96. doi: 10.1111/avj.12422. [DOI] [PubMed] [Google Scholar]
- 723.Back H., Penell J., Pringle J. A longitudinal study of poor performance and subclinical respiratory viral activity in Standardbred trotters. Vet Rec Open. 2015;2:e000107. doi: 10.1136/vetreco-2014-000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 724.Back H., Ullman K., Treiberg Berndtsson L. Viral load of equine herpesviruses 2 and 5 in nasal swabs of actively racing Standardbred trotters: temporal relationship of shedding to clinical findings and poor performance. Vet Microbiol. 2015;179:142. doi: 10.1016/j.vetmic.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 725.Barbesgaard L., Buhl R., Meldgaard C. Prevalence of exercise-associated arrhythmias in normal performing dressage horses. Equine Vet J Suppl. 2010;38:202. doi: 10.1111/j.2042-3306.2010.00223.x. [DOI] [PubMed] [Google Scholar]
- 726.Buhl R., Meldgaard C., Barbesgaard L. Cardiac arrhythmias in clinically healthy showjumping horses. Equine Vet J Suppl. 2010;38:196. doi: 10.1111/j.2042-3306.2010.00185.x. [DOI] [PubMed] [Google Scholar]
- 727.Jose-Cunilleras E., Young L.E., Newton J.R. Cardiac arrhythymias during and after treadmill exercise in poorly performing Thoroughbred racehorses. Equine Vet J Suppl. 2006;36:163. doi: 10.1111/j.2042-3306.2006.tb05534.x. [DOI] [PubMed] [Google Scholar]
- 728.King C.M., Evans D.L., Rose R.J. Significance for exercise capacity of some electrocardiographic findings in racehorses. Aust Vet J. 1994;71:200. doi: 10.1111/j.1751-0813.1994.tb03401.x. [DOI] [PubMed] [Google Scholar]
- 729.Kriz N.G., Hodgson D.R., Rose R.J. Prevalence and clinical importance of heart murmurs in racehorses. J Am Vet Med Assoc. 2000;216:1441. doi: 10.2460/javma.2000.216.1441. [DOI] [PubMed] [Google Scholar]
- 730.Zucca E., Ferrucci F., Stancari G. The prevalence of cardiac murmurs among Standardbred racehorses presented with poor performance. J Vet Med Sci. 2010;72:781. doi: 10.1292/jvms.09-0217. [DOI] [PubMed] [Google Scholar]
- 731.Leleu C., Cotrel C. Body composition in young Standardbreds in training: relationships to body condition score, physiologic and locomotor variables during exercise. Equine Vet J Suppl. 2006;36:98. doi: 10.1111/j.2042-3306.2006.tb05521.x. [DOI] [PubMed] [Google Scholar]
- 732.Garlinghouse S.E., Burrill M.J. Relationship of body condition score to completion rate during 160 km endurance races. Equine Vet J Suppl. 1999;30:591. doi: 10.1111/j.2042-3306.1999.tb05290.x. [DOI] [PubMed] [Google Scholar]
- 733.McGowan C.M., Golland L.C., Evans D.L. Effects of prolonged training, overtraining and detraining on skeletal muscle metabolites and enzymes. Equine Vet J Suppl. 2002;34:257. doi: 10.1111/j.2042-3306.2002.tb05429.x. [DOI] [PubMed] [Google Scholar]
- 734.Greve L., Dyson S. The horse-saddle-rider interaction. Vet J. 2013;195:275. doi: 10.1016/j.tvjl.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 735.Hemberg E., Kindahl H., Lundeheim N. Relationships between early foal health, future performance and their dams reproductive health. Reprod Domest Anim. 2010;45:817. doi: 10.1111/j.1439-0531.2009.01360.x. [DOI] [PubMed] [Google Scholar]
- 736.Boyko A.R., Brooks S.A., Behan-Braman A. Genomic association establishes correlation between growth and laryngeal neuropathy in Thoroughbreds. BMC Genomics. 2014;15:259. doi: 10.1186/1471-2164-15-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 737.Rose R.J., Allen J.R., Hodgson D.R. Response to submaximal treadmill exercise and training in the horse: changes in haematology, arterial blood gas and acid base measurements, plasma biochemical values and heart rate. Vet Rec. 1983;113:612. [PubMed] [Google Scholar]
- 738.Rose R.J., Allen J.R. Hematologic responses to exercise and training. Vet Clin North Am Equine Pract. 1985;1:461. doi: 10.1016/s0749-0739(17)30745-9. [DOI] [PubMed] [Google Scholar]
- 739.Tyler-McGowan C.M., Golland L.S., Evans D.L. Haematological and biochemical responses to training and overtraining. Equine Exerc Physiol Suppl. 1999;30:621. doi: 10.1111/j.2042-3306.1999.tb05297.x. [DOI] [PubMed] [Google Scholar]
- 740.Hamlin M.J., Shearman J.P., Hopkins W.G. Changes in physiologic parameters in overtrained Standardbred racehorses. Equine Vet J. 2002;34:383. doi: 10.2746/042516402776249146. [DOI] [PubMed] [Google Scholar]
- 741.McKeever K.H., Hinchcliff K.W., Reed S.M. Role of decreased plasma volume in hematocrit alterations during incremental treadmill exercise in horses. Am J Physiol. 1993;265:R404. doi: 10.1152/ajpregu.1993.265.2.R404. [DOI] [PubMed] [Google Scholar]
- 742.Persson S.G.B., Osterberg I. Racing performance in red blood cell hypervolaemic Standardbred trotters. Equine Vet J Suppl. 1999;30:617. doi: 10.1111/j.2042-3306.1999.tb05296.x. [DOI] [PubMed] [Google Scholar]
- 743.Dart A.J., Dowling B.A., Hodgson D.R. Evaluation of high-speed treadmill videoscopy for diagnosis of upper respiratory tract dysfunction in horses. Aust Vet J. 2001;79:109. doi: 10.1111/j.1751-0813.2001.tb10713.x. [DOI] [PubMed] [Google Scholar]
- 744.Christley R.M., Hodgson D.R., Evans D.L. Cardiorespiratory responses to exercise in horses with different grades of idiopathic laryngeal hemiplegia. Equine Vet J. 1997;29:6. doi: 10.1111/j.2042-3306.1997.tb01629.x. [DOI] [PubMed] [Google Scholar]
- 745.King C.M., Evans D.L., Rose R.J. Cardiorespiratory and metabolic responses to exercise in horses with various abnormalities of the upper respiratory tract. Equine Vet J. 1994;71:200. doi: 10.1111/j.2042-3306.1994.tb04373.x. [DOI] [PubMed] [Google Scholar]
- 746.Holcombe S.J., Derksen F.J., Stick J.A. Pathophysiology of dorsal displacement of the soft palate in horses. Equine Vet J Suppl. 1999;30:45. doi: 10.1111/j.2042-3306.1999.tb05186.x. [DOI] [PubMed] [Google Scholar]
- 747.Chalmers H.J., Cheetham J., Yeager A.E. Ultrasonography of the equine larynx. Vet Radiol Ultrasound. 2006;47:476. doi: 10.1111/j.1740-8261.2006.00170.x. [DOI] [PubMed] [Google Scholar]
- 748.Meyer C., Gerber R., Guthrie A.J. The use of the standard exercise test to establish the clinical significance of mild echocardiographic changes in a Thoroughbred poor performer. J S Afr Vet Assoc. 2004;75:100. doi: 10.4102/jsava.v75i2.461. [DOI] [PubMed] [Google Scholar]
- 749.Durando M.M., Reef V.B., Birks E.K. Right ventricular pressure dynamics during exercise: relationship to stress echocardiography. Equine Vet J Suppl. 2002;34:472. doi: 10.1111/j.2042-3306.2002.tb05468.x. [DOI] [PubMed] [Google Scholar]
- 750.Seeherman H.J., Morris E.A. Methodology and repeatability of a standardized treadmill exercise test for clinical evaluation of fitness in horses. Equine Vet J Suppl. 1990;9:20. doi: 10.1111/j.2042-3306.1990.tb04729.x. [DOI] [PubMed] [Google Scholar]
- 751.Seeherman H.J. Treadmill exercise testing: treadmill installation and training protocols used for clinical evaluations of equine athletes. Vet Clin North Am Equine Pract. 1991;7:259. doi: 10.1016/s0749-0739(17)30500-x. [DOI] [PubMed] [Google Scholar]
- 752.Leleu C., Cotrel C., Courouce-Malblanc A. Relationships between physiological variables and race performance in French Standardbred trotters. Vet Rec. 2005;156:339. doi: 10.1136/vr.156.11.339. [DOI] [PubMed] [Google Scholar]
- 753.Evans D.L., Rose R.J. Method of investigation of the accuracy of four digital-display heart rate meters suitable for use in the exercising horse. Equine Vet J. 1986;18:129. doi: 10.1111/j.2042-3306.1986.tb03567.x. [DOI] [PubMed] [Google Scholar]
- 754.Evans D.L., Rose R.J. Cardiovascular and respiratory responses in Thoroughbred horses during treadmill exercise. J Exp Biol. 1988;134:397. doi: 10.1242/jeb.134.1.397. [DOI] [PubMed] [Google Scholar]
- 755.Evans D.L., Rose R.J. Determination and repeatability of maximum oxygen uptake and other cardiorespiratory measurements in the exercising horse. Equine Vet J. 1988;20:94. doi: 10.1111/j.2042-3306.1988.tb01467.x. [DOI] [PubMed] [Google Scholar]
- 756.Rose R.J., Hendrickson D.K., Knight P.K. Clinical exercise testing in the normal Thoroughbred racehorse. Aust Vet J. 1990;67:345. doi: 10.1111/j.1751-0813.1990.tb07394.x. [DOI] [PubMed] [Google Scholar]
- 757.Evans D.L., Harris R.C., Snow D.H. Correlation of racing performance with blood lactate and heart rate after exercise in Thoroughbred horses. Equine Vet J. 1993;25:441. doi: 10.1111/j.2042-3306.1993.tb02987.x. [DOI] [PubMed] [Google Scholar]
- 758.Rasanen, Lampinen K.F. Poso AR. Responses of blood and plasma lactate and plasma purine concentrations to maximal exercise and their relation to performance in Standardbred trotters. Am J Vet Res. 1995;56:1651. [PubMed] [Google Scholar]
- 759.Vaihkonen L.K. Hyyppa, Poso AR. Factors affecting accumulation of lactate in red blood cells. Equine Vet J Suppl. 1999;30:443. doi: 10.1111/j.2042-3306.1999.tb05263.x. [DOI] [PubMed] [Google Scholar]
- 760.Rainger J.E., Evans D.L., Hodgson D.R. Distribution of lactate in plasma and erythrocytes during and after exercise in horses. Br Vet J. 1995;151:299. doi: 10.1016/s0007-1935(95)80180-4. [DOI] [PubMed] [Google Scholar]
- 761.Poso A.R., Lampinen K.J., Rasanen L.A. Distribution of lactate between red blood cells and plasma after exercise. Equine Vet J Suppl. 1995;18:231. [Google Scholar]
- 762.Bayly W.M., Shultz D.A., Hodgson D.R. Ventilatory responses of the horse to exercise: effect of gas collection systems. J Appl Physiol. 1987;63:1210. doi: 10.1152/jappl.1987.63.3.1210. [DOI] [PubMed] [Google Scholar]
- 763.Christley R.M., Evans D.L., Hodgson D.R. Blood gas changes during incremental and sprint exercise. Equine Vet J Suppl. 1999;30:24. doi: 10.1111/j.2042-3306.1999.tb05182.x. [DOI] [PubMed] [Google Scholar]
- 764.Bayly W.M., Hodgson D.R., Schulz D.A. Exercise-induced hypercapnia in the horse. J Appl Physiol. 1989;67:958. doi: 10.1152/jappl.1989.67.5.1958. [DOI] [PubMed] [Google Scholar]
- 765.Christley R.M., Hodgson D.R., Evans D.L. Effects of training on the development of exercise-induced arterial hypoxemia in horses. Am J Vet Res. 1997;58:653. [PubMed] [Google Scholar]
- 766.Barrey E., Evans S.E., Evans D.L. Locomotion evaluation for racing in Thoroughbreds. Equine Vet J Suppl. 2001;33:99. doi: 10.1111/j.2042-3306.2001.tb05369.x. [DOI] [PubMed] [Google Scholar]


