Lymphadenopathy is defined as an enlargement or a change in the character of a lymph node. Pathologic lymphadenopathy is usually a symptom of infectious, noninfectious conditions, or, in rare cases, malignant disease. Lymphadenopathy, especially cervical lymphadenopathy, is quite common in childhood, with a reported prevalence of 28% to 55% in otherwise normal infants and children.1 , 2 In addition, children have palpable nodes in most of the superficial lymphatic basins, including cervical, axillary, and inguinal regions that are nonpathologic; there is progressive increase in lymphoid mass from birth until early adolescence. This lymphoid tissue then normally diminishes throughout puberty.3
Many lymph nodes are palpable in children, and generally, cervical nodes less than 2 cm, axillary nodes less than 1 cm, and inguinal nodes less than 1.5 cm are considered physiologic in young children. Palpable epitrochlear and supraclavicular nodes should, however, be viewed with suspicion and trigger investigations.
The primary goal of a consulting surgeon is to determine the need for a tissue diagnosis. A key consideration is to resolve the family's fears of malignancy in an efficient and cost-effective manner. This chapter focuses primarily on lymphadenopathy in the cervical region. Some comments are made about other regions.
Anatomy
The regional lymph node groups of the head and neck are shown in Figure 57-1 . The precise borders for these groups have been classified by the American Head and Neck Society and are shown in Figure 57-2 .4 Drainage to lymphatic basins usually follows predictable, anatomic routes, with the nomenclature reflecting the site of the lymph nodes. The face and oropharynx drain predominantly to the preauricular, submandibular, and submental nodes; the posterior scalp drains to the occipital nodal group; and the mouth, tongue, tonsils, oropharynx, and nasopharynx drain to superficial and deep chains of the anterior cervical nodes. Significant lymphatic collateralization exists.
Figure 57-1.
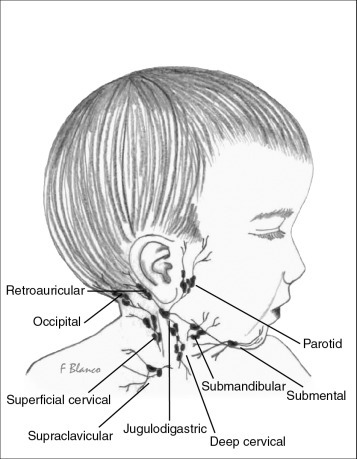
Regional lymph node groups of the head and neck.
Figure 57-2.
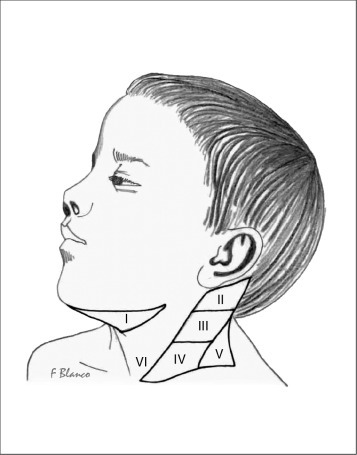
Lymphatic node levels of the neck. Level I: submental and submandibular; level II: superior jugular; level III: middle jugular; level IV: inferior jugular; level V: supraclavicular or posterior; and level VI: central or anterior.
Differential Diagnosis
Most lymphadenopathy is benign in nature and is generally associated with a short duration of symptoms. Table 57-1 shows a list of differential diagnoses. Generalized lymphadenopathy is defined as enlargement of more than two noncontiguous lymph node groups.
Table 57-1.
Differential Diagnosis of Lymphadenopathy in Children
| Generalized lymphadenopathy: infectious |
Viral: CMV, HIV, rubella, varicella, measles, EBV, herpes, hepatitis Bacterial: typhoid, tuberculosis, mycobacterial, syphilis, LGV, leptospirosis, brucellosis Protozoal: for instance, toxoplasmosis, leishmaniasis Fungal: for instance, coccidioidomycosis, Cryptococcus, histoplasmosis Other: syphilis, Lyme disease |
| Generalized lymphadenopathy: malignant |
Lymphoma, leukemia, neuroblastoma, thyroid tumor, metastasis (e.g., osteosarcoma, glioblastoma) |
| Generalized lymphadenopathy: others |
Autoimmune disorders: for instance, JRA, SLE, drug reactions, CGD, lymphohistiocytosis, LCH, dermatomyositis Storage disorders: for instance, Gaucher disease, Niemann-Pick disease Miscellaneous: Addison disease, Castleman disease, Churg-Strauss syndrome, Kawasaki disease, Kikuchi disease, lipid storage disease, sarcoidosis |
| Localized lymphadenopathy: infectious |
Staphylococcus aureus, group A Streptococcus (e.g., pharyngitis), anaerobes (periodontal disease), acute bacterial lymphadenitis, cat-scratch disease, tularemia, bubonic plague, diphtheria, chancroid, viral URI, mononucleosis, tuberculosis/atypical mycobacterium |
| Localized lymphadenopathy: malignant |
Lymphoma, leukemia, neuroblastoma, rhabdomyosarcoma, parotid tumor, nasopharyngeal tumor, solid tumor metastasis |
| Localized lymphadenopathy: site specific |
Cervical: Kawasaki disease Occipital: tinea capitis, pediculosis capitis Preauricular: cat-scratch disease, chronic eye infections Supraclavicular: histoplasmosis, coccidioidomycosis Mediastinal: sarcoidosis, cystic fibrosis, histoplasmosis, Axillary: local infection, brucellosis, immunization reactions, JRA Inguinal: syphilis, LGV, diaper rash |
| Localized cervical masses: non-nodal masses |
Mumps, thyroglossal duct, branchial cleft cyst, sternocleidomastoid tumor, cervical ribs, lymphatic malformation, hemangiomas, laryngocele, dermoid cyst |
CGD, chronic granulomatous disease; CMV, cytomegalovirus; EBV, Epstein-Barr virus; HIV, human immunodeficiency virus; JRA, juvenile rheumatoid arthritis; LCH, Langerhans cell histiocytosis; LGV, lymphogranuloma venereum; SLE: systemic lupus erythematosus; URI, upper respiratory infection.
Malignancy
Malignancy accounts for 11% to 24% of the diagnoses, depending on the nature of the group reporting their result. The higher rates are reported in series from oncology practices.5 , 6 Malignant processes are more common in the age group of 2 to 12 years old and very rare in the age group of less than 2 years old. Malignancy as a cause is also more common in children with chronic generalized lymphadenopathy, nodes greater than 3 cm in diameter, and nodes in the supraclavicular region. Associated symptoms of night sweats, weight loss, and hepatosplenomegaly also increase the chance of malignancy. Finally abnormal laboratory and radiologic evaluation are associated with increased malignancy rates.7 Soldes and colleagues reviewed predictors of malignancy in children with peripheral lymphadenopathy and determined that increasing node size, increasing number of sites of adenopathy, and age were associated with an increasing risk of malignancy (P < 0.05).8 In addition, supraclavicular adenopathy, an abnormal chest radiograph, and fixed nodes were all significantly associated with malignancy.
The most common malignancies as a cause of lymphadenopathy are Hodgkin and non-Hodgkin lymphomas, leukemia, and metastatic disease.
Evaluation
A careful history, physical examination, appropriate laboratory evaluation, and targeted imaging will usually help in deciding the need for tissue sampling. Persistent or progressive new-onset lymphadenopathy of greater than 4 to 6 weeks duration usually triggers a workup by the referring pediatrician. Indeed, most children with acute lymphadenopathy are rarely ever evaluated by pediatric surgeons. Most will improve with antibiotic therapy initiated by their pediatrician or the lymphadenopathy will resolve spontaneously when related to viral illnesses.
Once a child is referred to a surgeon, important historical questions include duration, progression, location, and associated symptoms, such as pain, fever, weight loss, and night sweats. Additional clinical information includes recent illnesses, especially upper respiratory tract symptoms, infections, trauma, bites, and dental problems. Drug use and sexual activity are important questions, especially in adolescents. Recent immunizations, especially bacillus Calmette-Guérin (BCG) should be evaluated. Social history, including recent travel, animal exposure, and exposure to tuberculosis and tropical diseases should be sought.
Once a general physical examination is completed, including a search for organomegaly, specific evaluation of the enlarged lymph nodes and other nodal basins should be performed. The skin and subcutaneous tissue that is drained by the affected lymph nodes should be evaluated; the characteristics of the lymph node should be noted. Normal nodes are usually soft, mobile, small, and nontender. Lymphadenopathy secondary to infections is also usually soft and can be mobile. However, on occasion, bacterial invasion of lymph nodes can result in erythema, tenderness, and fluctuance. With time, infected nodes can become adherent and have no inflammatory signs. Firm, fixed, nontender rubbery nodes can indicate a neoplastic process in older children.8
A thorough history and physical examination usually help separate local from generalized processes and help guide further evaluation, including laboratory and radiologic evaluation.
Investigation
Laboratory Studies
Most patients have had a laboratory evaluation prior to referral to surgery. These tests usually include a complete blood count (CBC) with manual differential, sedimentation rate, and C-reactive protein. However, these are not always helpful in determining the specific etiology of the disease process. Pancytopenia can be seen in leukemia; lymphocytosis is seen with mononucleosis, cytomegalovirus (CMV), and toxoplasmosis.
Based on the history and physical examination, more specific tests for Epstein-Barr virus (EBV), CMV, toxoplasmosis, brucellosis, histoplasmosis, syphilis, bartonellosis, and coccidioidomycosis should be considered. Tests for human immunodeficiency virus (HIV) should also be considered, based on the history as well as the tuberculin skin test.
Serum lactate dehydrogenase should be assayed when suspecting leukemia or lymphoma as a byproduct of high cell turnover.
Radiologic Evaluation
Diagnostic imaging can be used to determine the characteristic of the lymphadenopathy, identify potential sources of infection, identify mediastinal and abdominal masses, and to help differentiate enlarged lymph nodes from other pathology. Chest radiographs, ultrasonography with Doppler, and computer tomography have all been used in the evaluation of adenopathy.
In children with long-term lymphadenopathy, a two-view chest radiograph is helpful to rule out mediastinal masses that may compress the airway with or without significant symptoms. A chest radiograph should be performed prior to any operative intervention, including biopsies done under general anesthesia. Patients with large mediastinal masses compressing the airway should not undergo general anesthesia, because this could result in airway collapse (see Chapter 38).9
Ultrasonography (US) is helpful when the nodes are difficult to palpate and to help differentiate nodes from other structures, such as thyroglossal duct cysts and dermoid cysts in the neck, and undescended testis and inguinal hernias in the groin, US may also be helpful in determining the characteristics of the node. Fluctuance and abscess formation will help guide therapies such as needle aspiration or incision and drainage.
Attempts have been made to use ultrasonography and Doppler characteristics to differentiate neoplastic from non-neoplastic etiologies. Reactive lymphadenopathy is associated with central necrosis, central hyperechogenicity, long to short–axis ratio (>2.0), hilar vascularity, and low pulsatility index.10, 11, 12, 13, 14 However, these modalities are not sensitive or specific enough to primarily rule out neoplastic processes. The decision to delay biopsy diagnosis should not be dependent on US/Doppler findings.
Computed tomography (CT) is useful in patients with mediastinal masses and suspected intra-abdominal malignancies. Airway compromise may be best evaluated by chest CT. Interventional radiologists sometimes use CT scans to help guide biopsies from mediastinal masses.
Diagnostic Procedures
The decision to proceed with obtaining tissue from the involved lymph node is made in conjunction with the referring physician and after appropriate physical, laboratory, and radiologic evaluation as required. Often, the child has been observed for several weeks prior to referral to a surgeon. Small, soft, mobile nodes should not undergo biopsy, because these are most likely benign unless they are in the supraclavicular region. Tissue diagnosis is helpful when lymph nodes persist or enlarge after adequate antibiotic therapy, when they are associated with signs or symptoms of malignancy, and, finally, if the diagnosis is questioned.
Most authors recommend waiting at least 4 to 6 weeks before obtaining tissue samples. Earlier biopsy should be considered for nodes in the supraclavicular or epitrochlear region, nodes greater than 3 cm in diameter, and for children with a history of malignancy, weight loss, night sweats, fever, or hepatosplenomegaly. Similarly, physical characteristics of the lymph node may also indicate earlier biopsy.15 , 16
Fine-Needle Aspiration
Fine-needle aspiration (FNA) has been used extensively in adults, with practical advantages, including its simplicity, speed in the outpatient setting without sedation, as well as its cost effectiveness. In addition, the sensitivity and specificity reaches more than 90%.
The use of FNA in children has increased, especially in countries where tuberculosis is prevalent.17, 18, 19, 20 Aspirates should be sent for Gram stain, acid-fast stain, and cultures for aerobic/anaerobic bacteria, mycobacteria, and fungi.
However, the use of FNA in children has not become universal, because the aspirate usually provides a small sample, which limits the ability to perform flow cytometry, chromosomal analysis, and electron microscopy. Most pediatric hematologists and pathologists prefer excisional biopsy, because it allows the assessment of nodal architecture and permits the use of special stains. In addition, some children will not permit FNA without some sedation, which negates a primary benefit of FNA. Aspirates may also have a higher rate of false-negative rates in the diagnosis of Hodgkin disease, a common malignant condition in children. Finally, the risk of seeding the needle site tract with malignant cells, although small, is a legitimate concern of physicians and parents alike.21
Excisional Biopsy
Excisional biopsy provides enough tissue to perform flow cytometry, chromosomal analysis, electron microscopy, and the use of special stains. Indications for an excisional biopsy include
-
1.
Lymph nodes that are hard/matted
-
2.
Lymph nodes fixed to surrounding tissue
-
3.
Progressively enlarging nodes without response to antibiotic therapy
-
4.
Presence of abnormally enlarged nodes after 4 to 6 weeks
-
5.
Supraclavicular, epitrochlear lymph nodes
-
6.
Hepatosplenomegaly
-
7.
Mediastinal or hilar masses
-
8.
Laboratory anomalies, especially anemia, leukocytosis, leucopenia, and thrombocytopenia
-
9.
Symptoms such as fever, weight loss, and night sweats
-
10.
Suspicion of atypical mycobacterial adenitis
-
11.
Diagnostic dilemma
Most excisional biopsies are done under general anesthesia or sedation and, very rarely, under local anesthesia. The biopsy should be coordinated with pathology so that the lymph node can be sent as a fresh specimen. The nodes should not be fixed in formalin. As discussed earlier, a chest radiograph should be obtained to rule out a mediastinal mass that may compromise the airway prior to exposing children to general anesthesia or sedation.
In a recent review, Oguz et al. reviewed their experience with 457 children (2 months to 19 years old) with lymphadenopathy who were referred to their oncology group; 346 (75.7%) had benign processes, and 111 (24.3%) had malignant disease. Of these, 134 patients underwent excisional biopsy for indications highlighted previously. Table 57-2 highlights the findings on excisional biopsy and compares them to findings by other authors.7
Table 57-2.
Excisional Biopsy Results
| Excisional Biopsy Results | Oguz et al, 20067 (n = 134) | Moore et al, 20035 (n = 1332) | Yaris et al, 200621a (n = 38) |
|---|---|---|---|
| Malignant | 79.8% | 11.8% | 50% |
| Hodgkin lymphoma | 40.2% | 6% | |
| Non-Hodgkin lymphoma | 29.1% | 2.1% | |
| Nasopharyngeal cancer | 3.7 % | ||
| Thyroid cancer | 2.2 % | ||
| Miscellaneous | 4.2 % | 3.9% | |
| Benign | 20.1% | 88.2% | 50% |
| Chronic lymphadenitis | 5.9 % | 11.3% | |
| Hyperplasia | 5.9 % | 47.8% | 25% |
| Tuberculosis | 2.9% | 25% | 15.7% |
| Reactive | 2.2% | ||
| Miscellaneous | 3.2% | 4.1% |
As can be seen, the pathologic diagnosis varied depending on the reporting group and the associated referral pattern, with higher malignant rates documented by oncology groups7 , 21a and higher infectious rates reported by authors in developing countries.5
Management of Adenopathy
Darville and colleagues have suggested a helpful algorithm for the management of cervical lymphadenopathy (Fig. 57-3 ).22 This algorithm is a useful tool to help surgeons determine their role in the management of enlarged lymph nodes. As suggested elsewhere in this chapter, most of the medical evaluation and management has usually been performed by the referring physician; however, it is the surgeon's responsibility to review each case prior to intervention.
Figure 57-3.
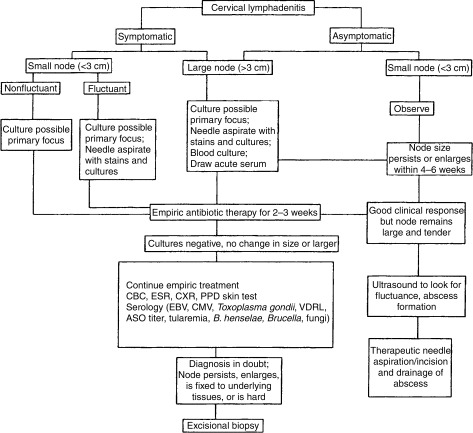
Evaluation and treatment algorithm. ASO, antistreptolysin titer; CBC, complete blood count; CMV, cytomegalovirus; CXR, chest radiograph; EBV, Epstein-Barr virus; ESR, erythrocyte sedimentation rate; PPD, purified protein derivative; VDRL, Venereal Disease Research Laboratory.
(Reprinted with permission from Elsevier.22)
Surgical Management
Surgical management is usually limited to diagnostic FNA, excisional biopsy, incision and drainage, and total excision. Further details are provided under the specific conditions discussed later.
Acute Lymphadenitis
The most common cause of self-limiting, acute, inflammatory lymph node is a viral infection.23 Acute bilateral cervical adenopathy is most often caused by a viral respiratory tract infection (rhinovirus, parainfluenza virus, influenza virus, respiratory syncytial virus, coronavirus, adenovirus, reovirus) and is usually hyperplastic in nature.24 Viral-associated adenopathy does not suppurate and usually resolves spontaneously.
Unilateral lymphadenitis is usually caused by streptococcal or staphylococcal infection in 40% to 80% of the cases.25 These are usually large (>2 cm), solitary, and tender in the preschool child.26 The submandibular, upper cervical, submental, occipital, and lower cervical nodes are affected in decreasing order of frequency.27 Suppurative adenitis is associated with group A streptococcal or penicillin-resistant staphylococci. Staphylococcus infection leading to lymphadenitis seems to occur more commonly in infants.28 Other less frequent causal organisms include Hemophilus influenzae type B, group B streptococci, and anaerobic bacteria. Community-acquired methicillin-resistant Staphylococcus aureus (MRSA) is now more commonly being isolated from superficial abscesses and suppurative lymphadenitis in children. Clindamycin is an appropriate agent to use under these circumstances.25 , 29
Suppurative lymphadenitis presents with local inflammatory signs, including unilateral tender adenopathy involving the submandibular or deep cervical nodes draining the oropharynx. Erythema, fever, malaise, and signs of systemic illness may occur. The primary infection in the head and neck regions should be looked for with careful attention to the oropharynx and middle ear. Appropriate treatment should be started, usually an empirical 5- to 10-day course of an oral β-lactamase–resistant antibiotic. Intravenous antibiotics should be started if systemic signs are present or in very young infants. A response should be observable within 72 hours, and failure of therapy usually necessitates additional diagnostic testing. This is usually fine-needle aspiration or ultrasonography.
Aspirate culture by FNA can guide further organism-specific antibiotic treatment, including clindamycin if MRSA is encountered. If no fluid is aspirated, sterile saline can be injected and then aspirated to obtain material for culture.26 In addition, repeated aspiration together with antibiotics is an effective treatment for fluctuant lymphadenitis.30 As stated previously, however, FNA may require sedation or anesthesia in young children.
Ultrasonography may help to differentiate between solid and cystic masses and can identify fluid that may require operative drainage. Incision and drainage is a more definitive surgical approach to suppurative fluctuant lymphadenitis. Gauze packing has been used to prevent early skin closure and achieve hemostasis; however, the use of minimal incisions, with vessel loops functioning as drains, has been gaining wider acceptance recently.31
Persistent Lymphadenitis
Persistent lymphadenitis that does not resolve despite 2 to 4 weeks of appropriate therapy warrants additional diagnostic workup. Some common causes of persistent lymphadenitis are discussed in this section.
Atypical Mycobacterial Adenitis
The genus Mycobacterium is characterized on light microscopy to be bacilli distinguished by their dense lipid capsules. The lipid capsules resist decolorization by acid alcohol after staining and thus are termed acid-fast bacilli. In the United States, 70% to 95% of mycobacterial lymphadenitis cases are caused by atypical mycobacteria (nontuberculous strains). The most common agents include M. avium-intracellulare, M. scrofulaceum, M. fortuitum, and M. chelonei.32 In contrast to tuberculous adenitis, atypical (or nontuberculous) mycobacterial adenitis is generally considered a local infectious process, without systemic involvement in immunocompetent hosts. Disseminated disease is more commonly observed in patients with underlying acquired or congenital immunodeficiency states. Atypical mycobacterial adenitis is not contagious, and the portal of entry in otherwise healthy children is the oropharynx.33
Atypical mycobacterial adenitis usually occurs in young children between 1 and 5 years of age. The common clinical presentation is focal, unilateral involvement of the jugulodigastric, preauricular, or submandibular nodal group. There is rapid onset of nodal enlargement, and the skin gradually develops a pink or red hue; with time, the overlying skin becomes thin.26 In contrast to acute suppurative lymphadenitis, there is no response to first-line antibiotics, and the clinical course is described as indolent, with the involved nodal group being minimally tender, firm, and rubbery to palpation, well circumscribed, and sometimes adherent to underlying structures. Although remarkably nontender, these lesions develop a draining sinus tract in 10% of patients.34 , 35 Signs of systemic illness or inflammation are usually minimal or nonexistent. Chest radiographs are usually normal.
Differentiating atypical mycobacterial and mycobacterial tuberculous cervical lymphadenitis can occasionally be challenging, based purely on epidemiologic and clinical features. Age (<5 years), race (white), place of residence (rural), bilaterality (rare) all point toward atypical mycobacterial infection. Purified protein derivative (PPD) skin testing in children with atypical mycobacterial lymphadenitis can result in an intermediate reaction because of cross reactivity, usually less than 15 mm. Blood interferon-gamma release assay is emerging as the discriminating test of choice; it was originally described for pulmonary disease but is now being used for nodal disease as well.36 Other criteria that point toward a diagnosis of tuberculous lymphadenitis are (1) a positive PPD, (2) abnormal chest radiograph, and (3) contact with a person with infectious tuberculosis. Spyridis and colleagues have shown that fulfilling two of three criteria results in diagnosis of tuberculous lymphadenitis with a 92% sensitivity.37
Unlike tuberculous adenitis, atypical mycobacterial adenitis generally does not respond to chemotherapy. The treatment of choice is complete surgical excision with primary wound closure. In a literature review of the surgical treatment of atypical mycobacterial cervicofacial adenitis in children, excision, incision and drainage, curettage, and needle aspiration were compared across 16 studies. The cure rates were 92%, 10%, 86%, and 41%, respectively.38 Incision and drainage should be avoided, because it often results in a chronically draining sinus. There have been reports of adequate medical treatment of atypical mycobacterial lymphadenitis; however, in a recent multicenter randomized trial comparing surgical excision and antibiotics, surgical excision was superior, with a 96% cure rate compared with 66% with antibiotic therapy.39 Multidrug antibiotic therapy, usually including clarithromycin and rifabutin, may be used as an adjunct for unresectable or recurrent disease.40 Surgical treatment should include elliptic excision of the overlying skin when it is thinned out, debridement of subcutaneous granulation tissue, and complete excision of the involved node(s) with closure of the overlying skin; formal lymph node dissection is not required. Curettage is recommended only if surgical excision is not possible because of unacceptable cosmetic outcomes or risk of injuries to adjacent nerves. A nerve stimulator may be helpful for lesions at the angle of the mandible to avoid injury to branches of the facial nerve.
Mycobacterial Adenitis
In developed countries, tuberculous adenitis or scrofula is almost exclusively caused by M. tuberculosis. Before control of bovine tuberculosis, the predominant cause of tuberculous adenitis was M. bovis. Occasional cases of M. bovis are observed from underdeveloped regions in which consumption of contaminated raw meat occurs. Patients proven to have human tuberculous adenitis often report previous exposure to a known carrier of tuberculosis, but most patients do not have active disease on a chest radiograph.37 Differentiation between tuberculosis and atypical mycobacterial adenitis has been highlighted previously. Tuberculous adenitis is considered to be a local manifestation of a systemic disease and not an initial primary focus of tuberculous infection.41
Clinically, children with tuberculous adenitis are usually older and present with nonsuppurative lymphadenitis, which may be bilateral.42 A retrospective review of 24 immunocompetent children with tuberculous lymphadenitis showed that no patient had bilateral disease, and the submandibular (29%) and the anterior cervical (71%) sites were the only areas of involvement.37 However, posterior triangle nodal involvement does occur.
The diagnosis of tuberculous adenitis can be made on the criteria established by Spyridis and colleagues37 and positive acid-fast bacteria on stain or culture of nodal tissue. Diagnostic confirmation may be aided by FNA with aspirate culture and cytologic examination.43 Rapid diagnosis of tuberculous adenitis by DNA amplification of nodal material using polymerase chain reaction (PCR) has been reported.44 Blood testing and PPD are also used. A negative tuberculin PPD test essentially excludes the diagnosis of tuberculous adenitis. If a diagnostic dilemma persists, surgical excisional biopsy is warranted. Incisional biopsy or incision and drainage should be avoided to prevent development of chronic, draining sinus tracts.23 , 45 Fistula and cheloid formation can be seen in up to 100% of patients who undergo incision and drainage of tuberculous infected lymph nodes.37
Tuberculous adenitis generally responds to medical management that consists of multiple-agent chemotherapy. The World Health Organization recommends directly observed short-course therapy, including isoniazid, rifampin, ethambutol, and pyrazinamide for the first 2 months, followed by isoniazid and rifampin for an additional 4 months.46 Nodal regression usually occurs within 3 months. Although antituberculous chemotherapy remains essential, the role of complete surgical excision of involved nodes is more controversial.47 Complete excision of involved nodes is prudent when biopsy is required for diagnosis, when a chronically draining sinus tract evolves during medical treatment, or when optimal medical management fails.
Cat-Scratch Disease
Cat-scratch disease is a common cause of lymphadenitis in children, with an estimated incidence in the United States of 9.3 per 100,000 ambulatory pediatric and adult patients per year.48 The highest age-specific incidence is among children younger than 10 years of age.2 Current microbiologic and PCR-directed DNA analysis demonstrates that the pleomorphic, gram-negative bacillus Bartonella henselae (formerly Rochalimaea) is the causative organism of cat-scratch disease.49 Most cases can be directly related to contact with a cat, and the usual site of inoculation is an extremity. Subsequent adenitis occurs at regional lymphatic drainage basins (inguinal, axillary, epitrochlear nodes) 5 days to 2 months later.50 Similarly, cervical lymphadenopathy is observed with scratches in the head and neck region. Although the primary manifestation of Bartonella henselae infection is lymphadenopathy, some series report up to 25% of cases resulting in severe systemic illnesses.51
Initial infection occurs at the portal of entry in the skin, such as a scratch or bite. Papule formation may be observed at the site of inoculation in 3 to 5 days, with development of subacute lymphadenopathy at regional nodal drainage beginning within 1 to 2 weeks. Early systemic symptoms of fever, malaise, myalgia, and anorexia are commonly reported.
Although most cases involve the lymph nodes of limbs, approximately 25% of cases involve the cervical nodes.50 Diagnosis is based on a history of exposure to cats, presence at a site of inoculation, and regional lymphadenopathy. Identification of Bartonella henselae from involved lymph nodes using Warthin-Starry silver impregnation stain has traditionally been used for diagnosis, but the stain has been found to be unreliable and lacking specificity. PCR for Bartonella henselae using paraffin sections from lymph nodes or other tissue is more reliable and specific.52 To confirm diagnosis without obtaining tissue, many centers use serologic testing, which has been available for several years; it has a low sensitivity but is highly specific.53
Lymphadenitis associated with cat-scratch disease is usually benign, self-limiting, and resolves within 6 to 8 weeks without specific treatment.54 Antibiotic treatment has thus been controversial, although azithromycin has been associated with rapid resolution of the adenitis.55 Suppuration is unusual; however, if it occurs, needle aspiration may provide symptomatic relief. Excisional biopsy is generally unnecessary but may be warranted if a draining sinus tract develops or if the diagnosis is uncertain and the potential for malignancy cannot be excluded.
Miscellaneous Lesions
Various other infectious and inflammatory conditions can produce lymphadenopathy in infants and children. Most patients with these disorders do not require surgical management or, in particular, excisional biopsy of the lesions. A systematic approach to evaluation of these patients, as outlined previously, generally leads to the correct diagnosis. Surgical management of these lesions should be directed to patients who present diagnostic dilemmas and have nodal disease in suspicious areas, or have persistent adenopathy despite adequate medical therapy.
Infectious lymphadenopathy
Lymphadenopathy caused by infectious agents include toxoplasmosis (caused by Toxoplasma gondii), tularemia (caused by Francisella tularensis), and mononucleosis (caused by Epstein-Barr virus). Infection with Actinomyces israelii in the head and neck may lead to cervicofacial actinomycosis that is characterized by a woody indurated cervical mass and development of chronic, draining fistulas. Direct involvement of the lymph nodes is uncommon, but the induration can make the clinical differentiation difficult.56 Infection with HIV can produce general lymphadenopathy in infants and children.57
Inflammatory disorders
Inflammatory disorders include Kawasaki disease, Kikuchi disease, Castleman disease, and Rosai-Dorfman disease.
Kawasaki disease, or mucocutaneous lymph node syndrome, is a febrile disorder of childhood that is characterized in part by the abrupt onset of erythematous changes in the oropharyngeal mucosa, acute vasculitis, and extensive nonsuppurative, nontender cervical adenopathy.58 Diagnosis is made on clinical grounds, and the resolution of the nodal disease occurs relatively quickly in the course of the disease.
Kikuchi disease, or histiocytic necrotizing lymphadenitis, may present as cervical lymphadenopathy that resolves spontaneously. It typically presents in the older child with bilateral, painful cervical nodes. There are associated fevers, night sweats, splenomegaly, leucopenia with atypical lymphocytosis, and elevated erythrocyte sedimentation rate (ESR). This disease can be clinically confused with malignant disease, and the patients often appropriately undergo excisional biopsy for definitive diagnosis.59
Castleman disease, also called angiofollicular or giant lymph node hyperplasia may also occasionally present as a solitary, enlarged cervical lymph node. The enlarged node appears hypervascular on US/Doppler or CT scan. Surgical excision is curative in the localized form.60 The multicentric form of the disease, often accompanied by visceral involvement, is considered a type of lymphoproliferative disorder and requires systemic therapy.
Rosai-Dorfman disease, or sinus histiocytosis with massive lymphadenopathy, is a rare disorder affecting predominantly African-American children in the first decade of life. Disease progresses from unilateral cervical adenopathy to massive bilateral cervical involvement and extension to other nodal groups or extra nodal sites. The disorder is benign but has a slow rate of resolution spanning 6 to 9 months. Excisional biopsy may aid diagnosis.61
Malignant disorders
Although lymphoma is the most common malignant disorder manifested by cervical adenopathy, neuroblastoma and thyroid carcinoma are other childhood cancers that can present as enlarged cervical lymph nodes.
Lymphomas are one of the more common malignant conditions in children. They may present as primary neck adenopathy that does not resolve with antibiotics or is enlarging. Patients with congenital or acquired immunodeficiency states, including HIV infection, are at greater risk for developing malignant lymphoproliferative conditions. Excisional biopsy is often used to help diagnose lymphomas.
In neuroblastoma, adenopathy is usually bilateral. These patients often have stage 4 disease, and if the primary is not evident on examination and radiologic evaluation, excisional biopsy is performed for initial diagnosis of neuroblastoma.
Metastatic thyroid carcinoma may present with unilateral cervical lymph node enlargement that should not be mistaken for ectopic thyroid gland. If a thorough neck examination does not reveal a thyroid nodule, and a history of neck irradiation or other high-risk factors is obtained, thyroid ultrasonography should be performed as part of the evaluation of neck lymphadenopathy.
Summary
Most adenopathy in children is nonpathologic and spontaneously resolves. Pathologic lymphadenopathy has a large differential diagnosis, with viral lymphadenitis being the most common. Surgical consultation is often obtained when the lymph nodes do not spontaneously resolve, if there is concern for malignancy, or if there is a diagnostic dilemma. Most of the investigation is usually performed prior to surgical consult, but the surgeon must be aware of an adequate workup prior to intervention. The surgeon's role is usually limited to excisional biopsy, incision and drainage, and, rarely, aspiration in children, depending on the pathology suspected. FNA for diagnosis has a more limited role in children but may be useful in selected cases.
The complete reference list is available online atwww.expertconsult.com.
References
- 1.Herzog L.W. Prevalence of lymphadenopathy of the head and neck in infants and children. Clin Pediatr (Phila) 1983;22:485–487. doi: 10.1177/000992288302200703. [DOI] [PubMed] [Google Scholar]
- 2.Larsson L.O., Bentzon M.W., Berg Kelly K., et al. Palpable lymph nodes of the neck in Swedish schoolchildren. Acta Paediatr. 1994;83:1091–1094. doi: 10.1111/j.1651-2227.1994.tb12992.x. [DOI] [PubMed] [Google Scholar]
- 3.Nield L.S., Kamat D. Lymphadenopathy in children: When and how to evaluate. Clin Pediatr (Phila) 2004;43:25–33. doi: 10.1177/000992280404300104. [DOI] [PubMed] [Google Scholar]
- 4.Robbins K.T., Clayman G., Levine P.A., et al. Neck dissection classification update: Revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology-Head and Neck Surgery. Arch Otolaryngol Head Neck Surg. 2002;128:751–758. doi: 10.1001/archotol.128.7.751. [DOI] [PubMed] [Google Scholar]
- 5.Moore S.W., Schneider J.W., Schaaf H.S. Diagnostic aspects of cervical lymphadenopathy in children in the developing world: A study of 1,877 surgical specimens. Pediatr Surg Int. 2003;19:240–244. doi: 10.1007/s00383-002-0771-x. [DOI] [PubMed] [Google Scholar]
- 6.Lake A.M., Oski F.A. Peripheral lymphadenopathy in childhood. Ten-year experience with excisional biopsy. Am J Dis Child. 1978;132:357–359. doi: 10.1001/archpedi.1978.02120290029003. [DOI] [PubMed] [Google Scholar]
- 7.Oguz A., Karadeniz C., Temel E.A., et al. Evaluation of peripheral lymphadenopathy in children. Pediatr Hematol Oncol. 2006;23:549–561. doi: 10.1080/08880010600856907. [DOI] [PubMed] [Google Scholar]
- 8.Soldes O.S., Younger J.G., Hirschl R.B. Predictors of malignancy in childhood peripheral lymphadenopathy. J Pediatr Surg. 1999;34:1447–1452. doi: 10.1016/s0022-3468(99)90101-x. [DOI] [PubMed] [Google Scholar]
- 9.Shamberger R.C., Holzman R.S., Griscom N.T., et al. CT quantitation of tracheal cross-sectional area as a guide to the surgical and anesthetic management of children with anterior mediastinal masses. J Pediatr Surg. 1991;26:138–142. doi: 10.1016/0022-3468(91)90894-y. [DOI] [PubMed] [Google Scholar]
- 10.Ahuja A., Ying M. An overview of neck node sonography. Invest Radiol. 2002;37:333–342. doi: 10.1097/00004424-200206000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Ahuja A., Ying M., King A., Yuen H.Y. Lymph node hilus: Gray scale and power Doppler sonography of cervical nodes. J Ultrasound Med. 2001;20:987–992. doi: 10.7863/jum.2001.20.9.987. quiz 994. [DOI] [PubMed] [Google Scholar]
- 12.Asai S., Miyachi H., Oshima S., et al. A scoring system for ultrasonographic differentiation between cervical malignant lymphoma and benign lymphadenitis. Rinsho Byori. 2001;49:613–619. [PubMed] [Google Scholar]
- 13.Papakonstantinou O., Bakantaki A., Paspalaki P., et al. High-resolution and color Doppler ultrasonography of cervical lymphadenopathy in children. Acta Radiol. 2001;42:470–476. doi: 10.1080/028418501127347197. [DOI] [PubMed] [Google Scholar]
- 14.Ying M., Ahuja A., Brook F. Accuracy of sonographic vascular features in differentiating different causes of cervical lymphadenopathy. Ultrasound Med Biol. 2004;30:441–447. doi: 10.1016/j.ultrasmedbio.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Kubba H. A child with cervical lymphadenopathy. Clin Otolaryngol. 2006;31:433–434. doi: 10.1111/j.1749-4486.2006.01308.x. [DOI] [PubMed] [Google Scholar]
- 16.Chesney P.J. Cervical adenopathy. Pediatr Rev. 1994;15:276–284. doi: 10.1542/pir.15-7-276. quiz 285. [DOI] [PubMed] [Google Scholar]
- 17.Wright C.A., Hesseling A.C., Bamford C., et al. Fine-needle aspiration biopsy: A first-line diagnostic procedure in paediatric tuberculosis suspects with peripheral lymphadenopathy? Int J Tuberc Lung Dis. 2009;13:1373–1379. [PubMed] [Google Scholar]
- 18.Ponder T.B., Smith D., Ramzy Lymphadenopathy in children and adolescents: Role of fine-needle aspiration in management. Cancer Detect Prev. 2000;24:228–233. [PubMed] [Google Scholar]
- 19.van de Schoot L., Aronson D.C., Behrendt H., Bras J. The role of fine-needle aspiration cytology in children with persistent or suspicious lymphadenopathy. J Pediatr Surg. 2001;36:7–11. doi: 10.1053/jpsu.2001.19991. [DOI] [PubMed] [Google Scholar]
- 20.Khan R.A., Wahab S., Chana R.S., et al. Children with significant cervical lymphadenopathy: Clinicopathological analysis and role of fine-needle aspiration in Indian setup. J Pediatr (Rio J) 2008;84:449–454. doi: 10.2223/JPED.1840. [DOI] [PubMed] [Google Scholar]
- 21.Chhieng D.C., Cangiarella J.F., Symmans W.F., Cohen J.M. Fine-needle aspiration cytology of Hodgkin disease: A study of 89 cases with emphasis on false-negative cases. Cancer. 2001;93:52–59. doi: 10.1002/1097-0142(20010225)93:1<52::aid-cncr9007>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Yaris N., Cakir M., Sozen E., Cobanoglu U. Analysis of children with peripheral lymphadenopathy. Clin Pediatr (Phila) 2006;45:544–549. doi: 10.1177/0009922806290609. [DOI] [PubMed] [Google Scholar]
- 22.Darville T., Jacobs R.F. In: Pediatric Infectious Diseases: Principles and Practice. 2nd ed. Jenson H.B., Baltimore R.S., editors. Stamford; CT, Saunders: 2002. Lymphadenopathy, lymphadenitis and lymphangitis; pp. 610–629. [Google Scholar]
- 23.Bodenstein L., Altman R.P. Cervical lymphadenitis in infants and children. Semin Pediatr Surg. 1994;3:134–141. [PubMed] [Google Scholar]
- 24.Leung A.K., Robson W.L. Childhood cervical lymphadenopathy. J Pediatr Health Care. 2004;18:3–7. doi: 10.1016/j.pedhc.2003.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnigan R.H., Pereira K.D., Poole M.D. Community-acquired methicillin-resistant Staphylococcus aureus in children and adolescents: Changing trends. Arch Otolaryngol Head Neck Surg. 2003;129:1049–1052. doi: 10.1001/archotol.129.10.1049. [DOI] [PubMed] [Google Scholar]
- 26.Gosche J.R., Vick L. Acute, subacute, and chronic cervical lymphadenitis in children. Semin Pediatr Surg. 2006;15:99–106. doi: 10.1053/j.sempedsurg.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly C.S., Kelly R.E., Jr Lymphadenopathy in children. Pediatr Clin North Am. 1998;45:875–888. doi: 10.1016/s0031-3955(05)70051-1. [DOI] [PubMed] [Google Scholar]
- 28.Hieber J.P., Davis A.T. Staphylococcal cervical adenitis in young infants. Pediatrics. 1976;57:424–426. [PubMed] [Google Scholar]
- 29.Martinez-Aguilar G., Hammerman W.A., Mason E.O., Jr, Kaplan S.L. Clindamycin treatment of invasive infections caused by community-acquired, methicillin-resistant and methicillin-susceptible Staphylococcus aureus in children. Pediatr Infect Dis J. 2003;22:593–598. doi: 10.1097/01.inf.0000073163.37519.ee. [DOI] [PubMed] [Google Scholar]
- 30.Brodsky L., Belles W., Brody A., et al. Needle aspiration of neck abscesses in children. Clin Pediatr (Phila) 1992;31:71–76. doi: 10.1177/000992289203100202. [DOI] [PubMed] [Google Scholar]
- 31.Tsoraides S.S., Pearl R.H., Stanfill A.B., et al. Incision and loop drainage: A minimally invasive technique for subcutaneous abscess management in children. J Pediatr Surg. 2010;45:606–609. doi: 10.1016/j.jpedsurg.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 32.Albright J.T., Pransky S.M. Nontuberculous mycobacterial infections of the head and neck. Pediatr Clin North Am. 2003;50:503–514. doi: 10.1016/s0031-3955(03)00024-5. [DOI] [PubMed] [Google Scholar]
- 33.Altman R.P., Margileth A.M. Cervical lymphadenopathy from atypical mycobacteria: Diagnosis and surgical treatment. J Pediatr Surg. 1975;10:419–422. doi: 10.1016/0022-3468(75)90106-2. [DOI] [PubMed] [Google Scholar]
- 34.Thorell E.A., Chesney P.J. In: Principles and Practice of Pediatric Infectious Diseases. 3rd ed. Long S.S., Pickering L.K., Prober C.G., editors. Elsevier; Philadelphia: 2008. Cervical lymphadenitis and neck infections; pp. 143–155. [Google Scholar]
- 35.Mair I.W., Elverland H.H. Cervical mycobacterial infection. J Laryngol Otol. 1975;89:933–939. doi: 10.1017/s0022215100081214. [DOI] [PubMed] [Google Scholar]
- 36.Kobashi Y., Obase Y., Fukuda M., et al. Clinical reevaluation of the QuantiFERON TB-2G test as a diagnostic method for differentiating active tuberculosis from nontuberculous mycobacteriosis. Clin Infect Dis. 2006;43:1540–1546. doi: 10.1086/509327. [DOI] [PubMed] [Google Scholar]
- 37.Spyridis P., Maltezou H.C., Hantzakos A., et al. Mycobacterial cervical lymphadenitis in children: Clinical and laboratory factors of importance for differential diagnosis. Scand J Infect Dis. 2001;33:362–366. doi: 10.1080/003655401750174002. [DOI] [PubMed] [Google Scholar]
- 38.Flint D., Mahadevan M., Barber C., et al. Cervical lymphadenitis due to non-tuberculous mycobacteria: Surgical treatment and review. Int J Pediatr Otorhinolaryngol. 2000;53:187–194. doi: 10.1016/s0165-5876(00)82006-6. [DOI] [PubMed] [Google Scholar]
- 39.Lindeboom J.A., Kuijper E.J., Bruijnesteijn van Coppenraet E.S., et al. Surgical excision versus antibiotic treatment for nontuberculous mycobacterial cervicofacial lymphadenitis in children: A multicenter, randomized, controlled trial. Clin Infect Dis. 2007;44:1057–1064. doi: 10.1086/512675. [DOI] [PubMed] [Google Scholar]
- 40.Berger C., Pfyffer G.E., Nadal D. Treatment of nontuberculous mycobacterial lymphadenitis with clarithromycin plus rifabutin. J Pediatr. 1996;128:383–386. doi: 10.1016/s0022-3476(96)70288-3. [DOI] [PubMed] [Google Scholar]
- 41.Cantrell R.W., Jensen J.H., Reid D. Diagnosis and management of tuberculous cervical adenitis. Arch Otolaryngol. 1975;101:53–57. doi: 10.1001/archotol.1975.00780300057016. [DOI] [PubMed] [Google Scholar]
- 42.Lai K.K., Stottmeier K.D., Sherman I.H., McCabe W.R. Mycobacterial cervical lymphadenopathy. Relation of etiologic agents to age. JAMA. 1984;251:1286–1288. doi: 10.1001/jama.251.10.1286. [DOI] [PubMed] [Google Scholar]
- 43.Lau S.K., Wei W.I., Kwan S., Yew W.W. Combined use of fine-needle aspiration cytologic examination and tuberculin skin test in the diagnosis of cervical tuberculous lymphadenitis. A prospective study. Arch Otolaryngol Head Neck Surg. 1991;117:87–90. doi: 10.1001/archotol.1991.01870130093023. [DOI] [PubMed] [Google Scholar]
- 44.Narita M., Shibata M., Togashi T., Kobayashi H. Polymerase chain reaction for detection of Mycobacterium tuberculosis. Acta Paediatr. 1992;81:141–144. doi: 10.1111/j.1651-2227.1992.tb12190.x. [DOI] [PubMed] [Google Scholar]
- 45.Siu K.F., Ng A., Wong J. Tuberculous lymphadenopathy: A review of results of surgical treatment. Aust N Z J Surg. 1983;53:253–257. [PubMed] [Google Scholar]
- 46.World Health Organization . 4th ed. 2010. Treatment of Tuberculosis: Guidelines.http://whqlibdoc.who.int/publications/2010/9789241547833_eng.pdf (WHO/HTM/TB/2009.420) Available at. Accessed September 5, 2011. [Google Scholar]
- 47.Castro D.J., Hoover L., Zuckerbraun L. Cervical mycobacterial lymphadenitis. Medical vs surgical management. Arch Otolaryngol. 1985;111:816–819. doi: 10.1001/archotol.1985.00800140060011. [DOI] [PubMed] [Google Scholar]
- 48.Jackson L.A., Perkins B.A., Wenger J.D. Cat scratch disease in the United States: An analysis of three national databases. Am J Public Health. 1993;83:1707–1711. doi: 10.2105/ajph.83.12.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bergmans A.M., Groothedde J.W., Schellekens J.F., et al. Etiology of cat scratch disease: Comparison of polymerase chain reaction detection of Bartonella (formerly Rochalimaea) and Afipia felis DNA with serology and skin tests. J Infect Dis. 1995;171:916–923. doi: 10.1093/infdis/171.4.916. [DOI] [PubMed] [Google Scholar]
- 50.Carithers H.A. Cat-scratch disease. An overview based on a study of 1,200 patients. Am J Dis Child. 1985;139:1124–1133. doi: 10.1001/archpedi.1985.02140130062031. [DOI] [PubMed] [Google Scholar]
- 51.Centers for Disease Control Cat-scratch disease in children—Texas, September 2000-August 2001. MMWR Morb Mortal Wkly Rep. 2002;51:212–214. [PubMed] [Google Scholar]
- 52.Margolis B., Kuzu I., Herrmann M., et al. Rapid polymerase chain reaction-based confirmation of cat scratch disease and Bartonella henselae infection. Arch Pathol Lab Med. 2003;127:706–710. doi: 10.5858/2003-127-706-RPCRCO. [DOI] [PubMed] [Google Scholar]
- 53.Vermeulen M.J., Herremans M., Verbakel H., et al. Serological testing for Bartonella henselae infections in The Netherlands: Clinical evaluation of immunofluorescence assay and ELISA. Clin Microbiol Infect. 2007;13:627–634. doi: 10.1111/j.1469-0691.2007.01700.x. [DOI] [PubMed] [Google Scholar]
- 54.Margileth A.M. Antibiotic therapy for cat-scratch disease: Clinical study of therapeutic outcome in 268 patients and a review of the literature. Pediatr Infect Dis J. 1992;11:474–478. [PubMed] [Google Scholar]
- 55.Bass J.W., Freitas B.C., Freitas A.D., et al. Prospective randomized double blind placebo-controlled evaluation of azithromycin for treatment of cat-scratch disease. Pediatr Infect Dis J. 1998;17:447–452. doi: 10.1097/00006454-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 56.Burden P. Actinomycosis. J Infect. 1989;19:95–99. doi: 10.1016/s0163-4453(89)91739-8. [DOI] [PubMed] [Google Scholar]
- 57.Falloon J., Eddy J., Wiener L., Pizzo P.A. Human immunodeficiency virus infection in children. J Pediatr. 1989;114:1–30. doi: 10.1016/s0022-3476(89)80596-7. [DOI] [PubMed] [Google Scholar]
- 58.Wood L.E., Tulloh R.M. Kawasaki disease in children. Heart. 2009;95:787–792. doi: 10.1136/hrt.2008.143669. [DOI] [PubMed] [Google Scholar]
- 59.Payne J.H., Evans M., Gerrard M.P. Kikuchi-Fujimoto disease: A rare but important cause of lymphadenopathy. Acta Paediatr. 2003;92:261–264. doi: 10.1111/j.1651-2227.2003.tb00539.x. [DOI] [PubMed] [Google Scholar]
- 60.Parez N., Bader-Meunier B., Roy C.C., Dommergues J.P. Paediatric Castleman disease: Report of seven cases and review of the literature. Eur J Pediatr. 1999;158:631–637. doi: 10.1007/s004310051166. [DOI] [PubMed] [Google Scholar]
- 61.Gupta P., Babyn P. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): A clinicoradiological profile of three cases including two with skeletal disease. Pediatr Radiol. 2008;38:721–728. doi: 10.1007/s00247-007-0701-0. quiz 821–822. [DOI] [PubMed] [Google Scholar]


