Approach to the Vomiting Cat
Vomiting can be defined as the ejection of part or all of the contents of the stomach and/or upper intestine through the mouth, usually in a series of involuntary spasmodic movements. The disturbances in gastrointestinal (GI) motility are coordinated with respiratory and abdominal muscle contractions and mediated by the central nervous system (CNS).
Vomiting begins with retching, a series of brief negative intrathoracic pressure pulses that coincide with positive abdominal contractions. These pressure changes occur as a result of repeated herniations of the abdominal esophagus and cardiac portion of the stomach into the esophagus. During retching, food freely moves back and forth in the esophagus, which is now dilated because of the ingesta. Ultimately, the diaphragm rapidly moves cranially, resulting in positive intrathoracic pressure that leads to expulsion of these contents.12 Vomiting is such an active process that it seems to involve the whole cat, and so it is little wonder that it concerns owners so much.
Since vomiting is mediated by the CNS with input and influence from just about anywhere in the body, it is important to summarize this physiology so it can be appreciated when managing clinical cases. Vomiting results from stimulation of the “vomiting center,” which is located in the brainstem; there are four main pathways that stimulate the vomiting center,12 and these are summarized below and in Figure 23-1 .
-
1Peripheral sensory receptors
-
aIntraabdominal
-
iFrom stomach, intestines, pancreas, liver, peritoneum, kidneys, bladder
-
iiVia visceral afferent fibers in sympathetic and vagal nerves
-
i
-
bHeart and large vessels
-
iVia vagus nerve
-
i
-
cPharynx
-
iVia glossopharyngeal nerve
-
i
-
a
-
2Bloodborne substances can stimulate the chemoreceptor trigger zone (CTZ). The CTZ lacks a blood-brain barrier so that substances diffuse to it freely.
-
aUremia
-
bElectrolyte imbalances
-
cBacterial toxins
-
dDrugs (e.g., antibiotics, nonsteroidal antiinflammatories, chemotherapeutics)
-
a
-
3Vestibular input
-
aInflammatory processes
-
bMotion sickness
-
iVia acoustic nerve
-
i
-
a
-
4Higher CNS centers
-
aPsychogenic
-
iFear, stress, excitement by catecholamine release
-
i
-
bInflammatory CNS lesions
-
a
FIGURE 23-1.
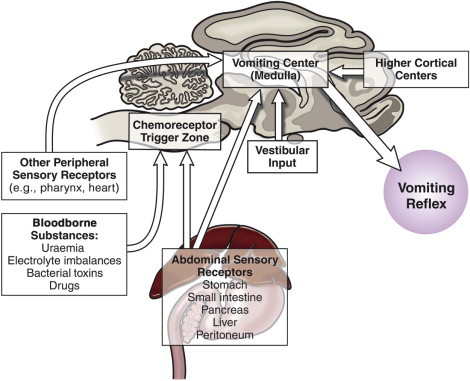
Summary of the main pathways that stimulate the vomiting center.
These complex pathways highlight the need to consider the whole cat and not just the cat's gastrointestinal tract when assessing a cat presenting for vomiting. The approach to managing a cat with vomiting must follow logical steps. When the underlying cause is gastrointestinal disease, a precise diagnosis can only be reached after obtaining biopsy samples. A summary of diagnostic steps and possible underlying causes is shown in Figure 23-2 .
FIGURE 23-2.
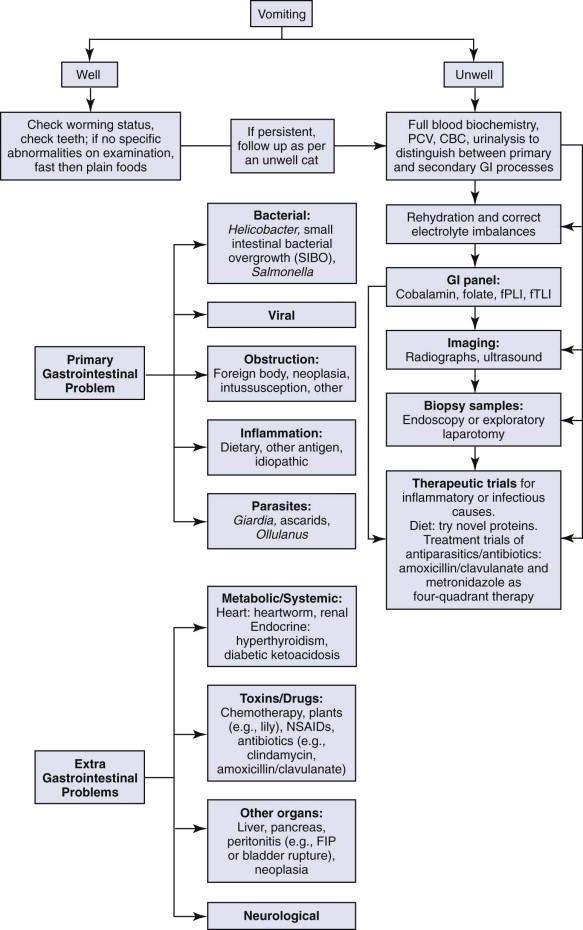
Summary of diagnostic steps and possible underlying causes of vomiting.
The diagnostic steps are
-
1
Signalment and clinical history
-
2
Physical examination
-
3
Blood and urine testing
-
4
Imaging (radiography, ultrasonography)
-
5
Biopsy samples
-
6
Treat and manage underlying problem
The decision to proceed to steps 4 and 5 is based on the assumption that the prior steps have narrowed down the underlying cause as gastrointestinal, pancreatic, or hepatic in origin.
Step 1: Signalment and Clinical History
The important aspects of the clinical history are given in Table 23-1 . Signalment is important, because younger cats are more likely to have ingested foreign bodies (though not all older cats have grown out of this habit). Some extragastrointestinal problems, such as hyperthyroidism and renal disease are more likely to occur in older cats.
TABLE 23-1.
Clinical History for Vomiting Cats
| Question | Interpretation |
|---|---|
| Signalment |
|
| Diet |
|
| Environment |
|
| Duration and frequency |
|
| Relationship to eating |
|
| Describe vomiting process |
|
| Appearance of vomitus |
|
| Deworming history |
|
| Previous illnesses |
|
| Current medications |
|
| Behavioral changes |
|
| Co-existing systemic signs |
|
Adapted from Hall JE: Clinical approach to chronic vomiting. In August JR, editor: Consultations in feline internal medicine, ed 3, Philadelphia, 1997, Saunders, p 63.
Most texts and references instruct clinicians to distinguish between vomiting and regurgitation, with the latter noted as being quite passive.3, 11, 12 In practice, it can be hard to make this distinction, because it is the author's experience that cats with esophageal disease can have quite forceful, spasmodic movements when ejecting ingesta by regurgitation—although it is also possible for regurgitation to be a passive process. Given that the physiology of vomiting, as described above, results in ingesta being forced to and then evacuated from the esophagus, it is hardly surprising that it can resemble regurgitation. Fortunately, regurgitation and esophageal disease do vary from vomiting in other ways! Vomiting is usually preceded by the cat licking its lips, salivating, or making attempts to swallow. Regurgitated ingesta is often in a tubelike structure and if undigested can be covered with frothy saliva. Partially digested food suggests vomitus, and the presence of bile or digested blood confirms this.
It is important to determine if the cat vomits regularly. Many owners have seen their cats vomit on a regular basis with no evidence of the cat being unwell, and this is noted frequently in the veterinary literature.3, 12 Hairballs can cause gastric irritation, and it may be that eating quickly also stimulates the peripheral sensory receptors that contribute to vomiting. If a cat does vomit regularly, it is important to assess if the cat is presenting for a change in the vomiting pattern (e.g., frequency or timing in relation to eating) and if the cat is unwell in any way, such as anorexia or weight loss.
The pattern of vomiting is important in all cases, because cats presenting with acute gastritis usually have a sudden onset of frequent vomiting compared with those with chronic disease processes that may vomit every few days. The timing in relation to eating can be helpful, because the stomach should empty by 6 to 8 hours after a meal; so, vomiting longer than 8 hours after a meal can suggest motility or retention disorders. The description of the vomitus can be helpful. If bile is present, the pylorus is not obstructed; the presence of blood (digested or fresh) indicates ulceration. Hair in the vomitus can indicate hairball gastritis, and the possibility of trichobezoar obstruction should be considered.
Access to foreign bodies or toxins is an important aspect of the clinical history. Has the cat been seen playing with an insect, mouse, or other prey? Are there any medications unaccounted for (e.g., a dropped aspirin tablet)? Are lilies present in the house?
Step 2: Physical Examination
Vomiting is the major sign of gastric disease, but given the number of potential organ systems that can be involved, a thorough physical examination should be undertaken. Because linear foreign bodies are a common cause of vomiting, all cats presenting for anorexia or vomiting should have the underside of the tongue evaluated for the presence of string caught there. Applying gentle pressure with a thumb in the intermandibular space to elevate the tongue is an effective way to visualize lesions or foreign bodies in the sublingual area (see Figure 3-8).
A thorough examination may reveal specific signs, such as a palpable thyroid nodule and tachycardia in the case of hyperthyroidism or palpably small kidneys with chronic kidney disease. The author has found that some cats with dental disease can gorge their food, resulting in vomiting; so, paying attention to the state of the teeth and gums is important. Of course, some cats have multiple problems, and correction of dental disease may not resolve vomiting if there is another process. In the examination, it is also important to note consequences of both the underlying process and the vomiting itself; these include the demeanor of the cat, hydration status, and abdominal pain.
The physical examination findings, together with the clinical history, help determine the next appropriate steps. Well cats that are not continually vomiting and are appropriately hydrated, with no other specific signs, may be treated as outpatients by fasting them for 24 hours, then returning to food with a bland diet, such as plain cooked chicken or commercial, low-residue prescription diets designed for this purpose. Follow-up is important to ensure signs do not progress. Cats with nonspecific signs may require supportive care with subcutaneous or intravenous fluids and perhaps analgesia (with opioids). If clinical signs do not resolve, the pursuit of a specific diagnosis should be attempted. The practitioner must ask the following important questions:
-
•
Are ancillary tests appropriate?
-
•
Is supportive care necessary?
-
•
Are any medications required?
Step 3: Blood and Urine Testing
Routine Tests
Routine serum/plasma biochemistries, hematology, urinalysis, and total thyroxine (T4) (for older cats) testing is not only important to distinguish primary from secondary gastrointestinal disease but to look for consequences of vomiting that may need to be addressed, such as hydration status and electrolyte abnormalities. Careful interpretations should be made. Severe azotemia, even with hyperphosphatemia, can occur as a result of primary gastrointestinal disease, and the distinction from renal disease usually requires an assessment of urine specific gravity.
Blood Tests for Gastrointestinal Disease
Cobalamin, folate, feline trypsin-like immunoreactivity (fTLI), and feline pancreatic lipase immunoreactivity (fPLI) tests are useful markers of intestinal and pancreatic disease,7, 8, 9, 10 but it is important to note that they mostly do not give a precise diagnosis. More detail about the utility of these tests is noted below in the section Approach to the Cat with Diarrhea.
Step 4: Imaging: Ultrasonography and Radiology
Radiography is most useful for identifying foreign bodies or signs of intestinal obstruction from other causes. The major findings are noted below in the section Intestinal Obstruction. Contrast radiography can aid the diagnosis for both discrete and linear foreign bodies but should be used with caution, because intestinal perforation may be present. Nonionic iodinated agents that are typically used for myelography (such as iopamidol or iohexol) should be used, since barium irritates the peritoneum and oral iodine compounds are hypertonic. Hypertonic compounds may draw fluid into the stomach and intestines after oral administration, with the potential of creating further fluid and electrolyte imbalances in an already compromised patient.6
Ultrasonography is a useful diagnostic adjunct and helps to detect and characterize localized thickening of the stomach or intestinal wall, lymphadenopathy, radiolucent foreign bodies, and changes in the size and echogenicity of the pancreas, liver, kidneys, or spleen. Abdominal effusions can be assessed and sampled. Ultrasound-guided fine-needle aspiration can be used to sample masses, bile, or peritoneal fluid. It should be recognized that in most cases of gastrointestinal disease, imaging will not give a definitive diagnosis and biopsy will be required, usually using either endoscopy or laparotomy. Ultrasonography can be a considered as a means to “survey the field,” assessing
-
•The nature of the underlying disease, such as
-
•Thickened intestines with or without discrete layers
-
•Lymph node involvement
-
•Other organ involvement
-
•
-
•The location of disease, for example,
-
•Diffuse or focal
-
•Proximal duodenum (reachable by endoscope) versus distal ileum
-
•
These factors may be used to assess the appropriateness of endoscopy versus laparotomy to obtain diagnostic samples.
Step 5: Intestinal (and other Organ) Biopsies
Histologic evaluation of affected tissue is usually needed for diagnosis of most chronic gastrointestinal diseases. Intestinal biopsy samples can be obtained by the use of endoscopy, laparotomy, or laparoscopy, each of which has advantages and disadvantages. Laparotomy allows gross examination of and access to the entire intestinal tract as well as other abdominal organs. Laparotomy is the most invasive alternative, but with careful anesthesia and analgesia, many cats recover uneventfully. One survey assessed that 83% of cats undergoing exploratory laparotomy survived the hospitalization, and although complications occurred in 26% of cats, these were more likely to be associated with the underlying disease process and not surgery or anesthesia.4
Laparoscopy is not readily available in all veterinary clinics. This alternative is less invasive and allows exploration of the abdomen but not as thoroughly as with laparotomy. Organs are usually exteriorized for biopsy. There is the possibility of anesthetic complications associated with insufflating the abdomen.
Endoscopy is the least invasive procedure and is the only alternative that allows examination of the intestinal lumen. This option limits the parts of the gastrointestinal tract that can be biopsied; it does not allow examination or sampling of any other part of the gastrointestinal tract and does not enable full-thickness biopsy samples. One study found that, of cats investigated for gastrointestinal disease, 9 of 33 cats (27%) had no pathology recognized proximal to the jejunum (i.e., the effective length of diagnostic endoscopes would have precluded diagnosis), and other organs were affected in 9 of 10 cats with inflammatory bowel diseases and 7 of 8 cats with intestinal small cell lymphoma.1 Careful case selection for endoscopy from survey ultrasonography can reduce the number of missed diagnoses from endoscopy, but the possibility still remains.
The quality of endoscopically obtained biopsy samples varies greatly with the skill of the endoscopist. It has been stated that “it is exceedingly easy to take inadequate tissue samples with a flexible endoscope.”5 In an assessment of endoscopically obtained biopsy samples, two laboratories were compared, one that received samples from any practitioner and the other that received samples ONLY from practitioners trained to take, mount, and submit endoscopy samples. All slides were reviewed by three pathologists who found that, of samples from the first laboratory, 15% of the slides were considered inadequate for diagnosis, 71% were considered questionable, and only 14% were adequate. By comparison, in the second laboratory (with samples from experienced practitioners) 0% of slides were inadequate, 21% were questionable, and 79% were considered adequate for diagnosis.13 In the case of distinguishing between lymphocytic intestinal infiltrates (commonly known as inflammatory bowel disease) and lymphocytic neoplasia (small cell lymphoma), endoscopically obtained samples can give an incorrect diagnosis.2 Many of these problems can be minimized with experienced operators and careful case selection from prior ultrasonography.
References
- 1.Baral RM. Australian College of Veterinary Scientists; Gold Coast, Queensland, Australia: 2006. Laparotomy for gastro-intestinal biopsies, Science Week Conference Proceedings (Small Animal Medicine chapter) p 70. [Google Scholar]
- 2.Evans SE, Bonczynski JJ, Broussard JD. Comparison of endoscopic and full-thickness biopsy specimens for diagnosis of inflammatory bowel disease and alimentary tract lymphoma in cats. J Am Vet Med Assoc. 2006;229:1447. doi: 10.2460/javma.229.9.1447. [DOI] [PubMed] [Google Scholar]
- 3.Hall J. Clinical Approach to chronic vomiting. In: August J, editor. Consultations in feline internal medicine. ed 3. Saunders; Philadelphia: 1997. p. 61. [Google Scholar]
- 4.Lester S, Welsh E, Pratschke K. Complications of exploratory coeliotomy in 70 cats. J Small Anim Pract. 2004;45:351. doi: 10.1111/j.1748-5827.2004.tb00247.x. [DOI] [PubMed] [Google Scholar]
- 5.Mansell J, Willard MD. Biopsy of the gastrointestinal tract. Vet Clin North Am Small Anim Pract. 2003;33:1099. doi: 10.1016/s0195-5616(03)00056-1. [DOI] [PubMed] [Google Scholar]
- 6.Shaiken L. Radiographic appearance of linear foreign bodies in cats. Vet Med. 1999;94:417. [Google Scholar]
- 7.Simpson KW, Fyfe J, Cornetta A. Subnormal concentrations of serum cobalamin (vitamin B12) in cats with gastrointestinal disease. J Vet Intern Med. 2001;15:26. doi: 10.1892/0891-6640(2001)015<0026:scoscv>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Steiner JM, Williams DA. Serum feline trypsin-like immunoreactivity in cats with exocrine pancreatic insufficiency. J Vet Intern Med. 2000;14:627. doi: 10.1892/0891-6640(2000)014<0627:sftiic>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Steiner JM, Wilson BG, Williams DA. Development and analytical validation of a radioimmunoassay for the measurement of feline pancreatic lipase immunoreactivity in serum. Can J Vet Res. 2004;68:309. [PMC free article] [PubMed] [Google Scholar]
- 10.Suchodolski JS, Steiner JM. Laboratory assessment of gastrointestinal function. Clin Tech Small Anim Pract. 2003;18:203. doi: 10.1016/S1096-2867(03)00075-6. [DOI] [PubMed] [Google Scholar]
- 11.Tams TR. A diagnostic approach to vomiting in dogs and cats. Vet Med. 1992;87:785. [Google Scholar]
- 12.Twedt DC. Diseases of the stomach. In: Sherding RG, editor. The cat: clinical diseases and management. ed 2. Churchill Livingstone; New York: 1994. p. 1181. [Google Scholar]
- 13.Willard MD, Lovering SL, Cohen ND. Quality of tissue specimens obtained endoscopically from the duodenum of dogs and cats. J Am Vet Med Assoc. 2001;219:474. doi: 10.2460/javma.2001.219.474. [DOI] [PubMed] [Google Scholar]
Therapeutics for Vomiting and Diarrhea
Therapeutic strategies used in the treatment of feline gastrointestinal diseases include nonspecific supportive therapies and targeted therapies based on the primary underlying disease process identified. The most effective therapies for treating feline vomiting and diarrhea are those directed at treating the primary underlying disease process. However, symptomatic supportive care is often necessary prior to arriving at a definitive diagnosis at the onset of targeted therapy or during periods of clinical relapse.
Nonspecific Supportive Therapies for Vomiting
Antiemetics and Prokinetics
Antiemetics and prokinetics are used to control or prevent vomiting through specific receptor interactions mediated either centrally or peripherally, making some more effective in cats than others. The five most commonly used antiemetics all control vomiting by different mechanisms and include mirtazapine, metoclopramide, dolasetron/ondansetron, maropitant, and the phenothiazines (TABLE 23-2, TABLE 23-3 ). Metoclopramide functions both as an antiemetic and prokinetic in cats, while cisapride functions solely as a prokinetic.
TABLE 23-2.
Mechanism of Action and Adverse Effects of the Common Antiemetic and Prokinetic Drugs Used to Treat Vomiting in Cats
| Drug | Mechanism of Action | Adverse Effects |
|---|---|---|
| Metoclopramide (antiemetic and prokinetic) | D2 antagonism 5-HT3 antagonism 5-HT4 agonist |
Extrapyramidal signs |
| Dolasetron (antiemetic) Ondansetron (antiemetic) |
5-HT3 antagonism | Prolongation QT interval Arrhythmias |
| Maropitant (antiemetic) | NK-1 antagonist | |
| Phenothiazines (antiemetic) Prochlorperazine Chlorpromazine |
D2 antagonism H1, H2 antagonism Cholinergic antagonism alpha1, alpha2 antagonism |
Extrapyramidal signs Sedation Decreases seizure threshold Hypotension |
| Cisapride (prokinetic) | 5-HT4 agonist | Prolongation QT interval Arrhythmias |
| Mirtazapine (appetite stimulant and antiemetic) | 5-HT2 , 5-HT3 antagonism H1 antagonism |
Behavior changes Tremors, muscle twitching Hyperactivity |
TABLE 23-3.
Dosage Recommendations, Contraindications, Potential Drug Interactions, and Clinical Indications for Dosage Adjustments for the Common Antiemetic and Prokinetic Drugs Used to Treat Vomiting in Cats
| Drug | Dosage (Cats) | C: Contraindications DI: Drug Interactions DA: Dosage Adjustments |
|---|---|---|
| Metoclopramide | 0.2-0.4 mg/kg SC, PO q8h 1-2 mg/kg/day CRI |
C: GI obstruction DI: Phenothiazines: extrapyramidal signs DA: Azotemia |
| Dolasetron Ondansetron |
0.5-1.0 mg/kg IV, SC, PO q12-24h 0.22-0.5 mg/kg IV, PO q8-12h |
DI: Cisapride: prolonged QT interval and arrhythmias |
| Maropitant | 1 mg/kg IV, SC, PO q24h (up to 5 days) | |
| Phenothiazines prochlorperazine chlorpromazine | 0.2-0.4 mg/kg SC q8h | C: Dehydration; hypotension; seizure hx DI: Metoclopramide: extrapyramidal signs |
| Cisapride | 1.5 mg/kg PO q12h | C: GI obstruction DI: Dolasetron: prolonged QT interval and arrhythmias; azole antifungals: inhibition CYP3A isoenzyme |
| Mirtazapine | 1.88 mg/cat PO q48h | DI: Concurrent administration with other MAO inhibitors (i.e., selegiline, amitraz, tramadol, amitriptyline, clomipramine) and/or SSRIs (i.e., fluoxetine) contraindicated DA: Kidney or liver dysfunction |
CRI, Constant rate infusion; hx, history.
Mirtazapine
Mirtazapine, a piperazinoazepine, antagonizes the presynaptic alpha2-adrenergic receptor, increasing noradrenergic and serotonergic neurotransmission; the primary mechanism targeted for its use is as an antidepressant in humans. Mirtazapine is also a potent antagonist of the postsynaptic serotonergic receptors (5-HT2 and 5-HT3) and histamine H1 receptors. Because of its antiserotonergic and antihistaminic effects, mirtazapine is used as an entiemetic and appetite stimulant in cats.
Anorexia is a common clinical problem in ill cats, and in some anorexic or partially anorexic cats the use of an appetite stimulant as adjunctive therapy to nutritional support (i.e. feeding tubes) may be of clinical benefit. Prior to the development of mirtazapine, cyproheptadine was used as an appetite stimulant in cats, with variable clinical results.
Recently, the pharmacokinetics and pharmacodynamics of mirtazapine have been reported in cats. In a group of healthy cats, mirtazapine was found to be an effective appetite stimulant, with a shorter half-life than that reported in humans. The recommended oral dose is 1.88 mg/cat every 48 hours.55a In humans, age and kidney and liver dysfunction affect mirtazapine metabolism (hepatic CYP 450 enzymes) and clearance (excreted in urine and feces), suggesting that dose adjustment may be necessary.69a Side effects reported in cats treated with mirtazapine include behavior changes (vocalization and interaction), tremors, muscle twitching, and hyperactivity.9a, 55a
Metoclopramide
Metoclopramide is both an antiemetic and prokinetic drug that acts peripherally on the gastrointestinal tract and centrally within the central nervous system (CNS). At low doses metoclopramide inhibits dopaminergic (D2) transmission, and at higher doses it inhibits serotonergic 5-HT3 receptors in the chemoreceptor trigger zone (CRTZ).15, 23 Metoclopramide also acts peripherally as a prokinetic at the level of the gastrointestinal smooth muscle of the stomach and duodenum, triggering gastric emptying and duodenal contractions. Multiple mechanisms mediate metoclopramide's prokinetic activity, including augmentation of acetylcholine release and increased smooth muscle sensitivity to cholinergic neurotransmission, which may in part be because of antagonism of dopamine, but more recently, serotonergic 5HT4 receptor activation has been suggested.23, 56 Metoclopramide has been reported to increase the lower esophageal sphincter tone in humans,20 although in cats metoclopramide's affect on the lower esophageal sphincter is reported to be weak.32
Adverse central nervous system, extrapyramidal signs occur secondary to dopamine (D2) antagonism, including excitement and behavior changes. Extrapyramidal signs are most often seen at the higher doses needed to block 5-HT3 receptors. Because of metoclopramide's prokinetic properties, an intestinal obstruction should be ruled out prior to its use.
Dopamine is a less important neurotransmitter in the chemoreceptor trigger zone of cats than alpha2-adrenergic and 5-HT3-serotonergic receptors, suggesting that D2-dopaminergic antagonist may be a less effective antiemetic in cats. Clinically metoclopramide commonly controls vomiting in cats, although this clinical response may be secondary to 5-HT3 antagonism and/or its prokinetic effects.32, 44
Extrapolated from the short elimination half-life of metoclopramide in dogs (90 minutes), frequent intermittent dosing or delivery by a constant rate infusion (CRI) is necessary. Empirical dosing in cats is 0.2 to 0.4 mg/kg subcutaneously or orally every 8 hours or 1 to 2 mg/kg/day as a CRI. Approximately 25% of metoclopramide is excreted in the urine, thus dose reduction is recommended in cats with underlying renal azotemia.42
Dolasetron and Ondansetron
Dolasetron and ondansetron are selective serotonin antagonists that inhibit central and peripheral 5-HT3 receptors. Their main antiemetic effect is through antagonism of the peripheral 5-HT3 receptors in the gastrointestinal tract. In cats 5-HT3 antagonism of the CRZT is also likely important in the antiemetic effect of dolasetron and ondansetron. Dolasetron and ondansetron were originally used for vomiting secondary to chemotherapy because of their superior clinical efficacy.
The clinical use of dolasetron and ondansetron in cats has not been associated with reported side effects, and experimental studies report minimal toxicity in animals at doses 30 times the antiemetic dose.15 Side effects reported in humans include headaches, elevated liver enzymes, rare hypersensitivity reactions, prolongation of the QT interval, and arrhythmias.14, 24
Dolasetron is commonly used for parenteral administration and ondansetron for oral administration, dictated primarily based on the tablet sizes available and cost. Recommended dosing of dolasetron is 0.5 to 1 mg/kg intravenously every 24 hours and ondansetron 0.5 mg/kg orally every 12 hours.
Maropitant
Maropitant is a neurokinin-1 (NK-1) receptor antagonist, blocking the binding of substance P to the NK-1 receptors located in the emetic center, CRTZ, and the enteric plexus.55 In cats maropitant has been reported to be efficacious in treating xylazine-induced vomiting and motion sickness.31 Recommended dosing in cats is 1 mg/kg intravenously, subcutaneously or orally every 24 hours for up to 5 days.31 Maropitant is reported to be well tolerated in cats.
Phenothiazines
Prochlorperazine and chlorpromazine are considered broad-spectrum antiemetics by antagonism of D2-dopaminergic, histaminergic (H1 and H2), and cholinergic (muscarinic) receptors within the CRTZ and, at high doses, the alpha-adrenergic receptors (alpha1 and alpha2) within the vomiting center. In cats alpha2-receptors play a key role in emesis (recall xylazine is the emetic of choice in cats), suggesting cats may be more sensitive to the antiemetic effects of the phenothiazines.
Prochlorperazine and chlorpromazine produce an antiemetic effect at relatively low doses, thus avoiding profound sedation; although, because of antagonism of the alpha-receptors, vasodilation and hypotension can be clinically significant side effects. Phenothiazines have the potential to lower the seizure threshold; their use is not recommended in patients with a known seizure history. Other CNS-associated side effects linked to D2 antagonism occur at higher doses and produce extrapyramidal signs, including rigidity, tremors, weakness, and restlessness. Antagonism of the histaminergic receptors carries the risk of sedation.
Because of the need for frequent dosing (0.2 to 0.4 mg/kg subcutaneously every 8 hours) and the risk of hypotension and sedation, the clinical use of phenothiazine antiemetics is limited to hospitalized patients with refractory vomiting and should be avoided in patients who are dehydrated or hypotensive.
Cisapride
Cisapride is a serotonergic 5-HT4 agonist that increases propulsive gastrointestinal motility from the lower esophageal sphincter to the colon. Cisapride binds serotonergic 5-HT4 receptors in the myenteric plexus, increasing the release of acetylcholine in gastrointestinal smooth muscle. In dogs cisapride has greater prokinetic activity in the stomach relative to metoclopramide.29 Cisapride has no direct antiemetic effect, although it is indicated in a vomiting cat with colonic dysmotility secondary to megacolon. Colonic distention can trigger the vomiting reflex in cats. Cisapride induces colonic smooth muscle contractions in cats with megacolon that is dependent on the influx of extracellular calcium and is only partially cholinergic dependent.30 Other potential indications include refractory generalized ileus or gastroesophageal reflux. Dosage recommendations based on the pharmacokinetics in healthy cats is 1.5 mg/kg orally every 12 hours.41 Prior to the use of cisapride, an intestinal obstruction should be ruled out because of its strong prokinetic effects.
Side effects reported in humans are cramping and diarrhea. Potentially life-threatening side effects include QT prolongation and ventricular arrhythmias, the primary concern in humans that led to cisapride's removal from the market in the United States.47 In cats QT prolongation associated with cisapride administration requires 20 times the therapeutic dose.37 Because of the risk of prolongation of the QT interval and ventricular arrhythmias, the concurrent use of cisapride and dolasetron is not recommended.14 Other potential drug interactions associated with cisapride include concurrent therapy with azole antifungals (ketoconazole and itraconazole), because of their inhibition of hepatic CYP3A isoenzyme system and the inhibition of cisapride metabolism.47
Dietary Modification
Diet trials are commonly used in cats with idiopathic gastrointestinal signs or in cats with suspected or known food hypersensitivities. Dietary strategies used to control vomiting in cats focus on either a highly digestible diet or an elimination (novel protein/carbohydrate or hydrolyzed protein) diet.72 The empirical use of elimination diets in cats is reported to be relatively successful, with approximately 50% of cats with idiopathic gastrointestinal signs responsive to a novel protein/carbohydrate diets within 2 to 3 days.28 Interestingly, traditional diet trials are recommended for a minimum of 8 to 12 weeks, but in this group of diet-responsive cats with chronic gastrointestinal disease, clinical improvement was reported within days.28 Thus if a cat is going to be diet responsive, clinical improvement to a diet trial should be noted relatively early.
Highly Digestible Diets
Highly digestible diets enable more effective absorption and assimilation of nutrients in the face of a compromised digestive tract. These diets contain highly digestible proteins and carbohydrates, moderate to low fat, soluble fiber but low concentrations of insoluble fiber, and are supplemented with omega-3 fatty acids.
Novel Protein/Carbohydrate or Elimination Diets
These diets are recommended when food allergy or intolerance is suspected. These diets contain a single highly digestible novel carbohydrate source and novel protein source. Alternatively, diets formulated with hydrolyzed proteins can be used as an alternative to novel protein/carbohydrate diets.
Targeted Therapies with Specific Indications for Vomiting
Gastrointestinal Ulcers
See TABLE 23-4, TABLE 23-5 for information on gastrointestinal ulcers.
TABLE 23-4.
Mechanism of Action and the Adverse Effects of the Common Drugs Used to Treat Gastric Ulcers in Cats
| Drug | Mechanism of Action | Adverse Effects |
|---|---|---|
| Famotidine (increases gastric pH) | H2 antagonism | |
| Ranitidine (increases gastric pH) (prokinetic) |
H2 antagonism Anticholinesterase |
Hypotension (IV) |
| Omeprazole (increased gastric pH) | H+/K+ ATPase inhibitor | |
| Sucralfate (gastric ulcer healing) | Prevents H+ back diffusion, inactivates pepsin, absorbs bile acids, and increases gastric mucosal prostaglandin synthesis | |
TABLE 23-5.
Dosage Recommendations, Contraindications, Potential Drug Interactions, and Clinical Indications for Dosage Adjustments for the Common Drugs Used to Treat Gastric Ulcers in Cats
| Drug | Dosage (Cats) | C: Contraindications DI: Drug Interactions DA: Dosage Adjustments |
|---|---|---|
| Famotidine | 0.5 mg/kg IV, SC, PO q12-24h | DA: azotemia |
| Ranitidine | 2.5 mg/kg IV q12h 3.5 mg/kg PO q12h |
DA: azotemia |
| Omeprazole | 0.5-1 mg/kg PO q24h | DI: inhibition CYP2C: diazepam Do not crush enteric coated tablets |
| Sucralfate | 250 mg PO q12h | DI: decreases oral absorption of fluoroquinolones, tetracyclines, and digoxin |
Famotidine
Famotidine has no direct antiemetic effect but is a competitive inhibitor of the histamine (H2) receptors associated with the gastric parietal cells. The H2-receptor is the dominant receptor involved in gastric acid secretion. H2-receptor antagonism is reported to result in a 70% to 90% reduction in acid production.13 Famotidine is more effective at suppressing gastric acid secretion relative to ranitidine. Famotidine is well tolerated, although, with chronic therapy, there is the potential for hypoacidity and gastric bacterial overgrowth. In humans dose reduction is recommended in association with renal dysfunction.21 Famotidine is not an inhibitor of the hepatic microsomal cytochrome P-450 enzyme system, therefore significant drug interactions are not anticipated.
Hyperacidity alone is not considered a common cause for vomiting in cats, but famotidine is effective in treating vomiting in cats associated with gastric ulcers or gastritis. Recommended dosage in cats is 0.5 mg/kg every 12 to 24 hours.
Ranitidine
Ranitidine is also a competitive inhibitor of the H2 receptor associated with gastric parietal cells. In addition, ranitidine increases lower esophageal sphincter tone and functions as a prokinetic agent (increasing gastric emptying and stimulating intestinal motility, including colonic motility), because of its anticholinesterase activity.40, 54 Significant drug interactions associated with hepatic microsomal cytochrome P-450 enzyme system inhibition are not a clinical concern with ranitidine.46 An adverse effect to be aware of in cats treated with ranitidine is transient hypotension associated with ranitidine administered as an IV bolus.19 In humans dose reduction is recommended in patients with renal azotemia.39
Ranitidine is effective in decreasing gastric acid in cats.22 Ranitidine would be a logical choice in a cat with gastrointestinal ulceration and/or atony. The reported dosage recommendation for ranitidine in cats is 3.5 mg/kg orally every 12 hours or 2.5 mg/kg intravenous every 12 hours.19
Omeprazole
Omeprazole is a proton pump inhibitor that targets the H+/K+ ATPase pump on the luminal surface of partial cells. Omeprazole is effective at suppressing parietal cell acid secretion, and its effects persist for ≈24 hours after drug withdrawal because of drug accumulation in the parietal cell (by ion trapping). Indications for omeprazole therapy are for the treatment and prevention of nonsteroidal antiinflammatory drug (NSAID)–induced ulcers.9 Omeprazole is enteric coated to prevent its degradation by gastric acid; therefore oral formulations should not be crushed. Based on human studies, omeprazole is a hepatic microsomal cytochrome P-450 enzyme inhibitor with known drug interactions with diazepam.2 The extent of clinically significant drug interactions in cats has yet to be studied.
Omeprazole is reported to be effective in reducing gastric acid secretion in cats.22 The recommended empirical dosage in cats is 0.5 to 1 mg/kg orally once daily. Long-term use in humans33 and dogs11 is associated with gastric polyps and parietal cell hyperplasia, respectively, but the effect of long-term use in cats is currently unknown.
Sucralfate
Sucralfate is a disaccharide complexed with aluminum that dissociates to sucrose octasulfate and aluminum hydroxide upon exposure to gastric acid. The sucrose octasulfate spontaneously polymerizes, producing a viscous material capable of binding ulcerative lesions in the gastric mucosa. Once bound to the exposed mucosa, it prevents back diffusion of H+, inactivates pepsin, absorbs bile acids, and increases mucosal prostaglandin synthesis, collectively supporting ulcer healing.
Sucralfate is not systemically absorbed but does prevent the absorption of drugs capable of chelating with aluminum, including fluoroquinolones, tetracyclines, and digoxin. If sucralfate is indicated in a cat being treated concurrently with fluoroquinolones, tetracyclines, or digoxin, the recommendation is to administer the other drug 2 hours prior to the administration of sucralfate to optimize drug absorption.
Clinical indications for the use of sucralfate in cats are for the treatment of gastric ulcers and esophagitis.36 Dosage recommendation in cats is 250 mg orally every 12 hours. Sucralfate can be crushed, suspended in water, and administered as slurry.
Nonspecific Supportive Therapies for Diarrhea
Dietary Modification
Diet trials are used in some cats with diarrhea if the underlying cause is from known or suspected food hypersensitivities. Dietary management includes either a highly digestible diet, an elimination (novel protein/carbohydrate or hydrolyzed protein) diet (see above for both), or a diet high in fiber.72
High-Fiber Diets
High-fiber diets contain a mixture of both soluble and insoluble fiber that can be beneficial in patients with signs of large bowel diarrhea. Insoluble fiber, such as cellulose, functions to increase the bulk of the stool, bind fluid, and regulate intestinal motility. Soluble fiber, including fruit and vegetable pectins and beet pulp, functions as a source of butyric acid that can be used by the colonic mucosa and decreases proinflammatory cytokines.69, 72
Vitamin Supplementation
Cobalamin
Cobalamin (vitamin B12) is an essential vitamin needed by a number of different enzymes, including key enzymes involved in methionine metabolism and the conversion of methylfolate to tetrahydrofolate needed for DNA synthesis. Cobalamin and folate are intimately linked, and hypocobalaminemia can lead to a functional deficiency of folate.57 Ingested cobalamin requires intrinsic factor binding for enterocyte absorption at the level of the ileum.
Hypocobalaminemia is commonly associated with distal small intestine diseases in cats, including inflammatory bowel disease. In addition, low cobalamin has a negative impact on enterocyte function; therefore in many cats with intestinal disease and hypocobalaminemia, cobalamin supplementation is necessary for resolution of clinical signs.60, 64 Quantification of serum cobalamin levels is recommended in cats with clinical signs of small bowel diarrhea, ones suspected to have an infiltrative disease of the small intestine (inflammatory bowel disease or gastrointestinal lymphoma), or ones with pancreatic dysfunction. When hypocobalaminemia is identified, supplementation is recommended (250 µg/cat every 7 days) while the underlying cause of cat's malabsorption is being investigated and at initiation of targeted therapy.
Probiotics and Prebiotics
Probiotics
Probiotics are ingested live microorganisms intended to benefit the host, specifically to support the microflora environment of the gastrointestinal tract as well as to provide an overall benefit to the body's immune function by immunomodulation.8, 18, 51 Probiotics chemically modify ingesta and intestinal mucus, as well as affect immune cells, enterocytes, and goblet cells within the intestinal mucosa through direct receptor interactions and indirectly through the action of cytokines.
The microorganisms commonly used are nonpathogenic bacteria and yeast that have a vital role in gastrointestinal health, including Lactobacillus spp., Enterococcus faecium, Bifidobacterium spp., and Saccharomyces spp. For example, lactobacilli synthesize B vitamins, digestive enzymes, and folate coenzymes.63 Clinical indications for the use of probiotics are diverse, including primary gastrointestinal disease, chronic renal disease, and pancreatitis.71
The rational use of probiotics in the treatment of gastrointestinal diseases include their ability to modulate gastrointestinal flora, minimize colonization by pathogenic bacteria, and decrease the likelihood of bacterial translocation.17 In healthy cats, Lactobacillus acidophilus is reported to reduce fecal Clostridium counts.45 When Lactobacillus acidophilus was used adjunctively with antimicrobial therapy, fecal shedding of Campylobacter was reduced in cats with Campylobacter-induced diarrhea relative to cats treated with antimicrobials alone.3 Specifically, in cats with gastrointestinal disease, available research supports the probiotic Enterococcus faecium as clinically beneficial in resolving diarrhea in kittens.16 Relative to the control group, the kittens treated with probiotics had increased fecal Bifidobacteria and blood IgA concentrations and decreased fecal counts of Clostridium perfringens.
Prebiotics
Prebiotics are dietary supplements used to select for the more beneficial enteric flora, support gastrointestinal function, and prevent the overgrowth of pathogenic bacteria, including Salmonella, Escherichia coli, Clostridium, or Campylobacter. For a food additive to be considered a prebiotic, it must be nondigestible by the gastrointestinal tract (resistant to gastric acidity, gastrointestinal hydrolysis and absorption), yet fermentable by gastrointestinal microflora to short-chain fatty acids to stimulate the growth of “good” intestinal bacterial.72
Prebiotics include nondigestible oligosaccharides—commonly, oligofructose, fructo-oligosaccharides, mannanoligosaccharides, inulin, chicory, and lactosucrose.72 Reports on the use of prebiotics in cats are limited to their use in healthy cats; healthy cats fed fructo-oligosaccharides were reported to have a trend toward an increase in fecal concentrations of Lactobacilli and a decrease in concentration of C. perfringens and E. coli relative to the controls.65 To date no reports are available on the use of prebiotics in cats with gastrointestinal disease.
Probiotics and prebiotics potentially have a supportive role in the treatment of gastrointestinal disease in cats. The important clinical consideration in the use of probiotics as an adjunctive therapy is to ensure the use of live nonpathogenic microorganisms that have been documented to colonize the intestinal tract of cats. Gastrointestinal flora co-evolve with their host. Gastrointestinal microorganism colonization varies among species and within each individual animal. The distribution of fecal microflora for a given individual is considered unique but stable over time.68
Targeted Therapies with Specific Indications for Diarrhea
Antimicrobials and Antiparasitics
Antimicrobial and antiparasitic therapies for the treatment of feline diarrhea are indicated based on the specific diagnosis of infectious diarrhea, bacterial enteritis, or as adjunctive therapy for inflammatory bowel disease. Infectious pathogens more commonly associated with feline diarrhea include bacterial enteropathies (Clostridium, Campylobacter), protozoal enteropathies (Tritrichomonas foetus, Giardia spp.), and helminthic enteropathies associated with ascarids, hookworms, whipworms, and tapeworms. Only the more common anthelminthic, antimicrobial, and antiprotozoal therapies are discussed below (TABLE 23-6, TABLE 23-7 ). More information about antimicrobials and antiparasitics is found under specific infections in the discussions of Infectious Enteritis and Gastrointestinal Parasites.
TABLE 23-6.
Mechanism of Action and Adverse Effects of the Common Antimicrobials and Antiparasitics Used to Treat Specific Causes of Diarrhea in Cats
| Drug | Mechanism of Action | Adverse Effects |
|---|---|---|
| Fenbendazole (anthelmintic) | Binds microtubule beta-tubulin subunits preventing polymerization |
|
| Pyrantel pamoate (anthelmintic) | Targets nicotinic acetylcholine receptors of parasites: depolarization and spastic paralysis | |
| Metronidazole (antimicrobial) | Anaerobic environment: converted to unstable intermediates that disrupt bacterial DNA synthesis |
|
| Ronidazole (antimicrobial) | Anaerobic environment: converted to unstable intermediates that disrupt bacterial DNA synthesis |
|
TABLE 23-7.
Dosage Recommendations and Spectrum of Activity of the Common Antimicrobial and Antiparasitic Drugs Used to Treat Specific Causes of Diarrhea in Cats
| Drug | Dosage (Cats) | Spectrum |
|---|---|---|
| Fenbendazole | 50 mg/kg PO every 24h × 5 days | Ascarids, hookworms, whipworms, Taenia pisiformis |
| Pyrantel pamoate | 5 mg/kg PO once, repeat in 3 weeks | Ascarids, hookworms, Physaloptera |
| Metronidazole Metronidazole benzoate |
10-15 mg/kg/day 20 mg/kg/day |
Anaerobes, Giardia spp. |
| Ronidazole | 30 mg/kg PO q24h | T. foetus |
Fenbendazole
Fenbendazole is an anthelmintic used to treat common helminth infections, including ascarids, hookworms, whipworms, and a single species of tapeworm, Taenia pisiformis. Giardia spp. are also considered susceptible to fenbendazole. Fenbendazole binds beta-tubulin subunits of microtubules, interfering with their polymerization. Side effects include vomiting and diarrhea, although both are considered rare. Fenbendazole is not approved for use in cats in North America but is commonly used clinically, and an empirical dosage of 50 mg/kg orally every 24 hours for 5 consecutive days is recommended.
Pyrantel Pamoate
Pyrantel pamoate is a nicotinic anthelmintic used primarily for the treatment of ascarids, but its spectrum of activity also includes hookworms and the stomach worm, Physaloptera spp. Pyrantel is toxic to susceptible parasites through its selective action on their nicotinic acetylcholine receptors, resulting in depolarization and spastic paralysis. Pyrantel is not approved for use in cats but is considered safe in cats and is commonly used clinically. The dosage recommendation in cats is 5 mg/kg orally once, repeat in 3 weeks, and finally repeated in 3 months.
Metronidazole
Metronidazole is a nitroimidazole antibiotic with an anaerobic antibacterial spectrum with antiprotozoal activity against Giardia spp. In an anaerobic environment, metronidazole is converted to unstable intermediates (nitroso free radicals) that disrupt bacterial DNA synthesis. Immunomodulatory properties capable of inhibiting cell-mediated immunity have been described for metronidazole, although its immunomodulatory properties are reported at dosages well beyond what is recommended for clinical use,62 raising questions about the clinical use of metronidazole as an adjunctive therapy for treating inflammatory bowel disease.34, 43
Resistance to metronidazole is considered rare.43 The most common adverse reaction is gastrointestinal upset, including inappetence, anorexia, nausea, and vomiting. Profuse salivation can occur in cats after oral administration of metronidazole base (formulation used in standard tablets), which has lead to the use of metronidazole benzoate (a compounded formulation not approved by the Food and Drug Administration) in some cats because of its better oral palatability.61 At high doses (>200 mg/kg/day) benzoic acid is reported to be neurotoxic in cats, but with appropriate clinical dosing of metronidazole benzoate benzoic acid toxicity is unlikely.6 Dose-related metronidazole toxicity in cats results in cerebellovestibular ataxia secondary to gamma-aminobutyric acid (GABA) inhibition at dosages greater than or equal to 58 mg/kg/day12, 52; clinical signs include nystagmus, head tilt, ataxia, seizures, and obtundation.
In cats with inflammatory bowel disease, the dosage recommendation for the metronidazole base is 10 to 15 mg/kg/day. Metronidazole benzoate contains approximately 60% metronidazole base by weight, translating to an empirical dosage of 20 mg/kg/day of metronidazole benzoate (equivalent to 12.4 mg/kg/day of metronidazole base).61 Little is known about the safety of chronic metronidazole use in cats, but oral metronidazole has been reported to disrupt DNA within feline peripheral mononuclear cells following 7 days of therapy.61 This metronidazole-induced genotoxicity is reversible and is no longer detected 6 days after antibiotic therapy is discontinued.
Ronidazole
Ronidazole is a nitroimidazole antibiotic (similar to metronidazole) and available as a powder-on-feed antibiotic. Ronidazole is not approved for use in cats but has been used off-label to effectively treat tritrichomoniasis in naturally and experimentally infected cats (30 mg/kg orally every 12 hours for 14 days).25 T. foetus reduces nitroimidazoles to their nitroso free radicals. Ronidazole has been reported to have better in vitro and 10-fold higher in vivo activity against T. foetus relative to metronidazole.25, 35, 49 Ronidazole resistance is beginning to be reported in T. foetus isolates from cats with diarrhea.26
Side effects include hepatoxicity and neurotoxicity. Neurotoxicity is associated with high doses and has been reported in cats.59 The use of ronidazole is recommended only for confirmed cases of T. foetus, and dosing should not exceed 30 mg/kg once daily in cats, especially in cats at risk for neurotoxicity. Ronidazole is not registered for human or veterinary use in the United States; therefore its use in cats requires owner informed consent and client education of the potential human hazards.
Immunosuppressive Therapies
Immunosuppressive therapies are considered the standard of care for cats with gastrointestinal biopsies consistent with inflammatory bowel disease (lymphoplasmacytic or eosinophilic inflammation). The common immunosuppressive therapies used in cats with inflammatory bowel disease include glucocorticoids, cyclosporine, and chlorambucil (TABLE 23-8, TABLE 23-9 ). More information on the treatment of inflammatory bowel disease is found elsewhere in this chapter.
TABLE 23-8.
Mechanism of Action and Adverse Effects of the Common Immunosuppressive Drugs Used to Treat Inflammatory Bowel Disease in Cats
| Drug | Mechanism of Action | Adverse Effects |
|---|---|---|
| Glucocorticoids | Immunomodulation: decreasing leukocyte phagocytosis, chemotaxis, and antigen expression |
|
| Cyclosporine | Attenuates T-lymphocyte activation and proliferation by inhibition of interleukin-2 |
|
| Chlorambucil | Alkylates and cross links DNA Lymphocyte cytotoxicity |
|
TABLE 23-9.
Dosage Recommendations and Drug Interactions for the Common Immunosuppressive Drugs Used to Treat Inflammatory Bowel Disease in Cats
| Drug | Types | Dosage (Cats) | Drug Interactions |
|---|---|---|---|
| Glucocorticoids |
Prednisone/prednisolone | NSAIDs: gastrointestinal ulceration |
|
| Antiinflammatory | 0.5-1 mg/kg/day | ||
| Immunosuppressive | 2-4 mg/kg/day | ||
| Dexamethasone | Prednisone dose divided by 7 | ||
| Budesonide | 0.5-1 mg/cat/day | ||
| Cyclosporine | Cyclosporine modified (microemulsion) | 4 mg/kg PO q12-24h | Ketoconazole: CYP3A inhibition |
| Chlorambucil | — | <4-kg cat: 2 mg/cat q72h >4-kg cat: 2 mg/cat q48h |
— |
NSAIDs, Nonsteroidal antiinflammatory drugs.
Glucocorticoids
Glucocorticoids are considered first-line therapy in the treatment of cats with inflammatory bowel disease. Glucocorticoids bind their intracellular glucocorticoid receptors, modifying the expression of genes with glucocorticoid response elements. Immunomodulation is achieved through inhibition of cytokine release and response, including decreasing leukocyte phagocytosis, chemotaxis, and antigen expression. The more common side effects in cats include gastrointestinal ulceration, opportunistic infections (e.g., urinary tract infections), pancreatitis, and diabetes mellitus. Cats are less susceptible to iatrogenic hyperadrenocorticism than dogs.
Initial therapy is usually with oral prednisone or prednisolone. Prednisone is a prodrug that is metabolized to its active form prednisolone. Cats are reported to be less efficient in the conversion of prednisone to prednisolone27; therefore prednisolone may be preferred in cats, especially in cats refractory to prednisone therapy.
Alternative forms of glucocorticoids can be considered in specific patient populations. In patients with severe malabsorption, injectable dexamethasone may provide improved bioavailability and clinical response. Also dexamethasone maybe preferred in patients with a history of heart failure, fluid retention, or hypertension because of its lack of mineralocorticoid activity relative to prednisone/prednisolone. Dexamethasone's potency is 4 to 10 times that of prednisolone; therefore a dose reduction is necessary when prescribing dexamethasone (the dexamethasone dose is one seventh that of prednisolone).4, 10 Budesonide is an oral, locally active, high-potency glucocorticoid that is formulated to be released in the distal gastrointestinal tract (based on the pH differential between the proximal and distal small intestine), where it is absorbed and is locally immunomodulating at the level of the enterocyte. The amount of systemically absorbed budesonide is minimized, because 80% to 90% of the budesonide absorbed from the gastrointestinal tract undergoes first-pass metabolism in the liver. Some systemic absorption does occur, as evidenced by a blunted adrenocorticotropic hormone (ACTH) stimulation test in dogs treated with budesonide at 3 mg/m2 for 30 days.66, 70 The use of budesonide in cats remains anecdotal, with a suggestive empirical dose of 0.5 to 1 mg/cat/day.
Initial glucocorticoid therapy for cats with inflammatory bowel disease consists of antiinflammatory (0.5 to 1 mg/kg/day) to immunosuppressive (2 to 4 mg/kg/day) dosages, with dosages based on the potency of prednisone/prednisolone. The goal of therapy is to achieve clinical remission and slowly taper the dose of glucocorticoids to the lowest dose that will control the cat's clinical signs.67 Some cats may be completely weaned off therapy, while others require long-term low-dose therapy. The tapering of therapy should be slow, with a 25% to 50% dose reduction every 3 to 4 weeks.
Cyclosporine
Cyclosporine is considered a second-tier immunosuppressive drug used to treat inflammatory bowel disease in cats. Use of cyclosporine in the treatment of diarrhea associated with inflammatory bowel disease in cats is extrapolated from its use in dogs to treat glucocorticoid refractory inflammatory bowel diarrhea.1 Cyclosporine suppresses T-lymphocyte–mediated inflammation in the gastrointestinal tract secondary to suppression of inflammatory cytokines. Specifically, cyclosporine attenuates T-lymphocyte activation and proliferation through the inhibition of interleukin-2 (IL-2) production. Side effects of cyclosporine in cats include dose-dependent inappetence and vomiting, which may occur at the onset of therapy and are generally responsive to dose reduction. Other less common side effects reported in cats are opportunistic infections, including toxoplasmosis5 and hepatoxicity.
The microemulsion formulation of cyclosporine has higher oral bioavailability and less variable pharmacokinetics.58 A suggested initial dosage of cyclosporine is 4 mg/kg every 12 or 24 hours. Serum cyclosporine levels can be used to monitor for excessive trough plasma concentration (>400 ng/mL) as determined using a high-performance liquid chromotography (HPLC) analytical method.53
Chlorambucil
Chlorambucil is a slow-acting nitrogen mustard that alkylates and effectively cross links DNA, leading to altered protein production. The immunosuppressive effects of chlorambucil are the result of its cytotoxic effect on lymphocytes, similar to other nitrogen mustards. Bone marrow suppression is considered mild to moderate and is rapidly reversible. Neurotoxicity and myoclonus has been reported in a cat accidently overdosed with chlorambucil.7
Chlorambucil is used as a second-tier drug in cats to treat immune-mediated disorders, in part because of ease of administration and its low risk of myelosuppression. For the treatment of inflammatory bowel disease, the recommended dosing in cats is 2 mg/cat every 48 hours in cats greater than 4 kg and 2 mg/cat every 72 hours in cats less than 4 kg.50 Chlorambucil is commonly used in combination with glucocorticoids in the treatment of immune-mediated diseases, including inflammatory bowel disease,48, 50 and as a chemotherapeutic agent in the treatment of gastrointestinal small cell lymphoma in cats.38
References
- 1.Allenspach K, Rufenacht S, Sauter S. Pharmacokinetics and clinical efficacy of cyclosporine treatment of dogs with steroid-refractory inflammatory bowel disease. J Vet Intern Med. 2006;20:239. doi: 10.1892/0891-6640(2006)20[239:paceoc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 2.Andersson T. Omeprazole drug interaction studies. Clin Pharmacokinet. 1991;21:195. doi: 10.2165/00003088-199121030-00004. [DOI] [PubMed] [Google Scholar]
- 3.Baillon ML, Butterwick RF. The efficacy of a probiotic strain, Lactobacillus acidophilus DSM, in the recovery of cats from clinical Campylobacter infection [abstract] J Vet Intern Med. 2003;17:416. [Google Scholar]
- 4.Ballard PL, Carter JP, Graham BS. A radioreceptor assay for evaluation of the plasma glucocorticoid activity of natural and synthetic steroids in man. J Clin Endocrinol Metab. 1975;41:290. doi: 10.1210/jcem-41-2-290. [DOI] [PubMed] [Google Scholar]
- 5.Barrs VR, Martin P, Beatty JA. Antemortem diagnosis and treatment of toxoplasmosis in two cats on cyclosporin therapy. Aust Vet J. 2006;84:30. doi: 10.1111/j.1751-0813.2006.tb13119.x. [DOI] [PubMed] [Google Scholar]
- 6.Bedford PG, Clarke EG. Experimental benzoic acid poisoning in the cat. Vet Rec. 1972;90:53. doi: 10.1136/vr.90.3.53. [DOI] [PubMed] [Google Scholar]
- 7.Benitah N, de Lorimier LP, Gaspar M. Chlorambucil-induced myoclonus in a cat with lymphoma. J Am Anim Hosp Assoc. 2003;39:283. doi: 10.5326/0390283. [DOI] [PubMed] [Google Scholar]
- 8.Benyacoub J, Czarnecki-Maulden GL, Cavadini C. Supplementation of food with Enterococcus faecium (SF68) stimulates immune functions in young dogs. J Nutr. 2003;133:1158. doi: 10.1093/jn/133.4.1158. [DOI] [PubMed] [Google Scholar]
- 9.Bersenas AM, Mathews KA, Allen DG. Effects of ranitidine, famotidine, pantoprazole, and omeprazole on intragastric pH in dogs. Am J Vet Res. 2005;66:425. doi: 10.2460/ajvr.2005.66.425. [DOI] [PubMed] [Google Scholar]
- Cahil C. Mirtazapine as an antiemetic. Vet Forum. 2006;23:34. [Google Scholar]
- 10.Cantrill HL, Waltman SR, Palmberg PF. In vitro determination of relative corticosteroid potency. J Clin Endocrinol Metab. 1975;40:1073. doi: 10.1210/jcem-40-6-1073. [DOI] [PubMed] [Google Scholar]
- 11.Carlsson E. A review of the effects of long-term acid inhibition in animals. Scand J Gastroenterol Suppl. 1989;166:19. doi: 10.3109/00365528909091238. [DOI] [PubMed] [Google Scholar]
- 12.Caylor KB, Cassimatis MK. Metronidazole neurotoxicosis in two cats. J Am Anim Hosp Assoc. 2001;37:258. doi: 10.5326/15473317-37-3-258. [DOI] [PubMed] [Google Scholar]
- 13.Coruzzi G, Bertaccini G, Noci MT. Inhibitory effect of famotidine on cat gastric secretion. Agents Actions. 1986;19:188. doi: 10.1007/BF01966205. [DOI] [PubMed] [Google Scholar]
- 14.Cubeddu LX. Iatrogenic QT abnormalities and fatal arrhythmias: mechanisms and clinical significance, Curr. Cardiol Rev. 2009;5:166. doi: 10.2174/157340309788970397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunningham RS. 5-HT3-receptor antagonists: a review of pharmacology and clinical efficacy. Oncol Nurs Forum. 1997;24:33. [PubMed] [Google Scholar]
- 16.Czarnecki-Maulden G, Cavadini C, Lawler D. Incidence of naturally occurring diarrhea in kittens fed Enterococcus faecium SF68. Supplement to Compend Contin Edu Vet. 2007;29:37. [Google Scholar]
- 17.Damaskos D, Kolios G. Probiotics and prebiotics in inflammatory bowel disease: microflora “on the scope”. Br J Clin Pharmacol. 2008;65:453. doi: 10.1111/j.1365-2125.2008.03096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dotan I, Rachmilewitz D. Probiotics in inflammatory bowel disease: possible mechanisms of action. Curr Opin Gastroenterol. 2005;21:426. [PubMed] [Google Scholar]
- 19.Duran S, Jernigan A, Ravis W. Proceedings of 9th Annual ACVIM Forum. 1991. Pharmacokinetics of oral and intravenous ranitidine in cats [abstract] p. 902. [Google Scholar]
- 20.Durazo FA, Valenzuela JE. Effect of single and repeated doses of metoclopramide on the mechanisms of gastroesophageal reflux. Am J Gastroenterol. 1993;88:1657. [PubMed] [Google Scholar]
- 21.Echizen H, Ishizaki T. Clinical pharmacokinetics of famotidine. Clin Pharmacokinet. 1991;21:178. doi: 10.2165/00003088-199121030-00003. [DOI] [PubMed] [Google Scholar]
- 22.Fandriks L, Jonson C. Effects of acute administration of omeprazole or ranitidine on basal and vagally stimulated gastric acid secretion and alkalinization of the duodenum in anaesthetized cats. Acta Physiol Scand. 1990;138:181. doi: 10.1111/j.1748-1716.1990.tb08831.x. [DOI] [PubMed] [Google Scholar]
- 23.Freeman AJ, Cunningham KT, Tyers MB. Selectivity of 5-HT3 receptor antagonists and anti-emetic mechanisms of action. Anticancer Drugs. 1992;3:79. [PubMed] [Google Scholar]
- 24.Goodin S, Cunningham R. 5-HT(3)-receptor antagonists for the treatment of nausea and vomiting: a reappraisal of their side-effect profile. Oncologist. 2002;7:424. doi: 10.1634/theoncologist.7-5-424. [DOI] [PubMed] [Google Scholar]
- 25.Gookin JL, Copple CN, Papich MG. Efficacy of ronidazole for treatment of feline Tritrichomonas foetus infection. J Vet Intern Med. 2006;20:536. doi: 10.1892/0891-6640(2006)20[536:eorfto]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 26.Gookin JL, Stauffer SH, Dybas D. Documentation of in vivo and in vitro aerobic resistance of feline Tritrichomonas foetus isolates to ronidazole. J Vet Intern Med. 2010;24:1003. doi: 10.1111/j.1939-1676.2010.0534.x. [DOI] [PubMed] [Google Scholar]
- 27.Graham-Mize CA, Rosser EJ., Jr Comparison of microbial isolates and susceptibility patterns from the external ear canal of dogs with otitis externa. J Am Anim Hosp Assoc. 2004;40:102. doi: 10.5326/0400102. [DOI] [PubMed] [Google Scholar]
- 28.Guilford WG, Jones BR, Markwell PJ. Food sensitivity in cats with chronic idiopathic gastrointestinal problems. J Vet Intern Med. 2001;15:7. doi: 10.1892/0891-6640(2001)015<0007:fsicwc>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 29.Gullikson GW, Loeffler RF, Virina MA. Relationship of serotonin-3 receptor antagonist activity to gastric emptying and motor-stimulating actions of prokinetic drugs in dogs. J Pharmacol Exp Ther. 1991;258:103. [PubMed] [Google Scholar]
- 30.Hasler AH, Washabau RJ. Cisapride stimulates contraction of idiopathic megacolonic smooth muscle in cats. J Vet Intern Med. 1997;11:313. doi: 10.1111/j.1939-1676.1997.tb00472.x. [DOI] [PubMed] [Google Scholar]
- 31.Hickman MA, Cox SR, Mahabir S. Safety, pharmacokinetics and use of the novel NK-1 receptor antagonist maropitant (Cerenia) for the prevention of emesis and motion sickness in cats. J Vet Pharmacol Ther. 2008;31:220. doi: 10.1111/j.1365-2885.2008.00952.x. [DOI] [PubMed] [Google Scholar]
- 32.Hillemeier C, McCallum R, Oertel R. Effect of bethanechol and metoclopramide on upper gastrointestinal motility in the kitten. J Pediatr Gastroenterol Nutr. 1986;5:134. doi: 10.1097/00005176-198601000-00025. [DOI] [PubMed] [Google Scholar]
- 33.Jalving M, Koornstra JJ, Wesseling J. Increased risk of fundic gland polyps during long-term proton pump inhibitor therapy. Aliment Pharmacol Ther. 2006;24:1341. doi: 10.1111/j.1365-2036.2006.03127.x. [DOI] [PubMed] [Google Scholar]
- 34.Jergens A. Feline idiopathic inflammatory bowel disease. Compend Contin Educ Prac Vet. 1992;14:509. [Google Scholar]
- 35.Kather EJ, Marks SL, Kass PH. Determination of the in vitro susceptibility of feline Tritrichomonas foetus to 5 antimicrobial agents. J Vet Intern Med. 2007;21:966. doi: 10.1892/0891-6640(2007)21[966:dotivs]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 36.Katz PO, Ginsberg GG, Hoyle PE. Relationship between intragastric acid control and healing status in the treatment of moderate to severe erosive oesophagitis. Aliment Pharmacol Ther. 2007;25:617. doi: 10.1111/j.1365-2036.2006.03235.x. [DOI] [PubMed] [Google Scholar]
- 37.Kii Y, Nakatsuji K, Nose I. Effects of 5-HT(4) receptor agonists, cisapride and mosapride citrate on electrocardiogram in anaesthetized rats and guinea-pigs and conscious cats. Pharmacol Toxicol. 2001;89:96. doi: 10.1034/j.1600-0773.2001.d01-142.x. [DOI] [PubMed] [Google Scholar]
- 38.Kiselow MA, Rassnick KM, McDonough SP. Outcome of cats with low-grade lymphocytic lymphoma: 41 cases (1995-2005) J Am Vet Med Assoc. 2008;232:405. doi: 10.2460/javma.232.3.405. [DOI] [PubMed] [Google Scholar]
- 39.Koch KM, Liu M, Davi IM. Pharmacokinetics and pharmacodynamics of ranitidine in renal impairment. Eur J Clin Pharmacol. 1997;52:229. doi: 10.1007/s002280050279. [DOI] [PubMed] [Google Scholar]
- 40.Kounenis G, Koutsoviti-Papadopoulou M, Elezoglou A. Comparative study of the H2-receptor antagonists cimetidine, ranitidine, famotidine and nizatidine on the rabbit stomach fundus and sigmoid colon. J Pharmacobiodyn. 1992;15:561. doi: 10.1248/bpb1978.15.561. [DOI] [PubMed] [Google Scholar]
- 41.LeGrange SN, Boothe DM, Herndon S. Pharmacokinetics and suggested oral dosing regimen of cisapride: a study in healthy cats. J Am Anim Hosp Assoc. 1997;33:517. doi: 10.5326/15473317-33-6-517. [DOI] [PubMed] [Google Scholar]
- 42.Lehmann CR, Heironimus JD, Collins CB. Metoclopramide kinetics in patients with impaired renal function and clearance by hemodialysis. Clin Pharmacol Ther. 1985;37:284. doi: 10.1038/clpt.1985.41. [DOI] [PubMed] [Google Scholar]
- 43.Lofmark S, Edlund C, Nord CE. Metronidazole is still the drug of choice for treatment of anaerobic infections. Clin Infect Dis. 2010;50(Suppl 1):S16. doi: 10.1086/647939. [DOI] [PubMed] [Google Scholar]
- 44.Mangel AW, Stavorski JR, Pendleton RG. Effects of bethanechol, metoclopramide, and domperidone on antral contractions in cats and dogs. Digestion. 1983;28:205. doi: 10.1159/000198989. [DOI] [PubMed] [Google Scholar]
- 45.Marshall-Jones ZV, Baillon ML, Croft JM. Effects of Lactobacillus acidophilus DSM13241 as a probiotic in healthy adult cats. Am J Vet Res. 2006;67:1005. doi: 10.2460/ajvr.67.6.1005. [DOI] [PubMed] [Google Scholar]
- 46.Martinez C, Albet C, Agundez JA. Comparative in vitro and in vivo inhibition of cytochrome P450 CYP1A2, CYP2D6, and CYP3A by H2-receptor antagonists. Clin Pharmacol Ther. 1999;65:369. doi: 10.1016/S0009-9236(99)70129-3. [DOI] [PubMed] [Google Scholar]
- 47.Michalets EL, Williams CR. Drug interactions with cisapride: clinical implications. Clin Pharmacokinet. 2000;39:49. doi: 10.2165/00003088-200039010-00004. [DOI] [PubMed] [Google Scholar]
- 48.Miller E. The use of cytotoxic agents in the treatment of immune-mediated diseases of dogs and cats. Semin Vet Med Surg (Small Anim) 1997;12:157. doi: 10.1016/s1096-2867(97)80027-8. [DOI] [PubMed] [Google Scholar]
- 49.Miwa GT, Wang R, Alvaro R. The metabolic activation of ronidazole [(1-methyl-5-nitroimidazole-2-yl)-methyl carbamate] to reactive metabolites by mammalian, cecal bacterial and T. foetus enzymes. Biochem Pharmacol. 1986;35:33. doi: 10.1016/0006-2952(86)90551-4. [DOI] [PubMed] [Google Scholar]
- 50.Moore L. Proceedings 22nd Am Coll Vet Intern Med Forum. 2004. Beyond corticosteroids for therapy of inflammatory bowel disease in dogs and cats [abstract] p. 611. [Google Scholar]
- 51.Nomoto K. Prevention of infections by probiotics. J Biosci Bioeng. 2005;100:583. doi: 10.1263/jbb.100.583. [DOI] [PubMed] [Google Scholar]
- 52.Olson EJ, Morales SC, McVey AS. Putative metronidazole neurotoxicosis in a cat. Vet Pathol. 2005;42:665. doi: 10.1354/vp.42-5-665. [DOI] [PubMed] [Google Scholar]
- 53.Papich MG. Proceedings of 14th Annual Members Meeting of the American Academy of Veterinary Dermatology and American College of Veterinary Dermatology. 1998. Immunnosuppressive drug therapy; p. 41. [Google Scholar]
- 54.Petroianu GA, Arafat K, Schmitt A. Weak inhibitors protect cholinesterases from strong inhibitors (paraoxon): in vitro effect of ranitidine. J Appl Toxicol. 2005;25:60. doi: 10.1002/jat.1036. [DOI] [PubMed] [Google Scholar]
- 55.Prommer E. Aprepitant (EMEND): the role of substance P in nausea and vomiting. J Pain Palliat Care Pharmacother. 2005;19:31. [PubMed] [Google Scholar]
- Quimby JM, Gustafson DL, Samber BJ et al: Studies on the pharmacokinetics and pharmacodynamics of mirtazapine in healthy young cats, J Vet Pharmacol Ther (in press). [DOI] [PubMed]
- 56.Rao AS, Camilleri M. Review article: metoclopramide and tardive dyskinesia. Aliment Pharmacol Ther. 2010;31:11. doi: 10.1111/j.1365-2036.2009.04189.x. [DOI] [PubMed] [Google Scholar]
- 57.Reed N, Gunn-Moore D, Simpson K. Cobalamin, folate and inorganic phosphate abnormalities in ill cats. J Feline Med Surg. 2007;9:278. doi: 10.1016/j.jfms.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robson D. Review of the pharmacokinetics, interactions and adverse reactions of cyclosporine in people, dogs and cats. Vet Rec. 2003;152:739. doi: 10.1136/vr.152.24.739. [DOI] [PubMed] [Google Scholar]
- 59.Rosado TW, Specht A, Marks SL. Neurotoxicosis in 4 cats receiving ronidazole. J Vet Intern Med. 2007;21:328. doi: 10.1111/j.1939-1676.2007.tb02968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ruaux CG, Steiner JM, Williams DA. Early biochemical and clinical responses to cobalamin supplementation in cats with signs of gastrointestinal disease and severe hypocobalaminemia. J Vet Intern Med. 2005;19:155. doi: 10.1892/0891-6640(2005)19<155:ebacrt>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 61.Sekis I, Ramstead K, Rishniw M. Single-dose pharmacokinetics and genotoxicity of metronidazole in cats. J Feline Med Surg. 2009;11:60. doi: 10.1016/j.jfms.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sen P, Chakravarty AK, Kohli J. Effects of some imidazoles on cellular immune responses—an experimental study. Indian J Exp Biol. 1991;29:867. [PubMed] [Google Scholar]
- 63.Shahani KM, Ayebo AD. Role of dietary lactobacilli in gastrointestinal microecology. Am J Clin Nutr. 1980;33:2448. doi: 10.1093/ajcn/33.11.2448. [DOI] [PubMed] [Google Scholar]
- 64.Simpson KW, Fyfe J, Cornetta A. Subnormal concentrations of serum cobalamin (vitamin B12) in cats with gastrointestinal disease. J Vet Intern Med. 2001;15:26. doi: 10.1892/0891-6640(2001)015<0026:scoscv>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 65.Sparkes AH, Papasouliotis K, Sunvold G. Effect of dietary supplementation with fructo-oligosaccharides on fecal flora of healthy cats. Am J Vet Res. 1998;59:436. [PubMed] [Google Scholar]
- 66.Stroup ST, Behrend EN, Kemppainen RJ. Effects of oral administration of controlled-ileal-release budesonide and assessment of pituitary-adrenocortical axis suppression in clinically normal dogs. Am J Vet Res. 2006;67:1173. doi: 10.2460/ajvr.67.7.1173. [DOI] [PubMed] [Google Scholar]
- 67.Tams TR. Feline inflammatory bowel disease. Vet Clin North Am Small Anim Pract. 1993;23:569. doi: 10.1016/s0195-5616(93)50306-6. [DOI] [PubMed] [Google Scholar]
- 68.Tannock GW. New perceptions of the gut microbiota: implications for future research. Gastroenterol Clin North Am. 2005;34:361. doi: 10.1016/j.gtc.2005.05.006. vii. [DOI] [PubMed] [Google Scholar]
- 69.Tedelind S, Westberg F, Kjerrulf M. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease. World J Gastroenterol. 2007;13:2826. doi: 10.3748/wjg.v13.i20.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmer CJ, Sitsen JM, Delbressine LP. Clinical pharmacokinetics of mirtazapine. Clin Pharmacokinet. 2000;38:461. doi: 10.2165/00003088-200038060-00001. [DOI] [PubMed] [Google Scholar]
- 70.Tumulty JW, Broussard JD, Steiner JM. Clinical effects of short-term oral budesonide on the hypothalamic-pituitary-adrenal axis in dogs with inflammatory bowel disease. J Am Anim Hosp Assoc. 2004;40:120. doi: 10.5326/0400120. [DOI] [PubMed] [Google Scholar]
- 71.Wynn SG. Probiotics in veterinary practice. J Am Vet Med Assoc. 2009;234:606. doi: 10.2460/javma.234.5.606. [DOI] [PubMed] [Google Scholar]
- 72.Zoran DL. Nutritional management of feline gastrointestinal diseases. Top Companion Anim Med. 2008;23:200. doi: 10.1053/j.tcam.2008.08.003. [DOI] [PubMed] [Google Scholar]
Diseases of the Esophagus
Esophageal disease is uncommon in the cat when compared with dogs, but it is also likely that problems such as esophagitis and esophageal strictures are often overlooked. Awareness about feline esophageal diseases is low, the clinical signs are often not specific, and imaging beyond survey radiographs may be required for diagnosis.
The esophagus is composed of four layers (from inner to outer): mucosa, submucosa, muscularis, and adventitia (there is no serosal layer). In the dog, the muscle layer is entirely composed of skeletal muscle, but in cats, the distal third of the esophagus is composed of smooth muscle. The upper esophageal sphincter prevents reflux of esophageal contents into the pharynx and minimizes aerophagia. The lower esophageal sphincter prevents gastroesophageal reflux and relaxes during swallowing to allow food and fluid to enter the stomach.
Clinical Presentation
Clinical signs of esophageal disease include drooling, dysphagia, pain on swallowing (odynophagia), and, most classically, regurgitation. Weight loss may occur secondary to inadequate food intake when disease is severe or chronic. Other clinical signs, such as anorexia, cough, dyspnea, and fever, may occur if complications such as aspiration pneumonia or esophageal perforation occur.
Regurgitation is passive expulsion of food or fluid from the esophagus. The food is undigested and often accompanied by mucus and saliva. Mucosal erosions may produce frank blood in the regurgitated material. Regurgitation must be differentiated from vomiting (Table 23-10 ). Vomiting is typically preceded by salivation, retching, and abdominal contractions. The vomitus consists of partially digested food from the stomach and/or intestines and may be mixed with bile-stained fluid. Some cats will have both vomiting and regurgitation. Expectoration may also be confused with vomiting or regurgitation. Expectoration is associated with coughing, but cats that cough excessively may also stimulate vomition so that a careful history is needed to characterize the clinical signs correctly. Coughing may also occur in cats that have aspirated as a result of regurgitation.
TABLE 23-10.
How to Differentiate Vomiting from Regurgitation
| Sign | Regurgitation | Vomiting |
|---|---|---|
| Prodromal nausea (salivation, licking lips, anxiety) | No | Usually |
| Retching (dry heaves) | No | Usually |
| Material produced: | ||
| Food | Sometimes | Sometimes |
| Bile | No | Sometimes |
| Blood | Sometimes undigested | Sometimes (undigested or digested) |
| Volume produced | Variable | Variable |
| Timing relative to eating | Variable | Variable |
| Distention of cervical esophagus | Sometimes | No |
Adapted from Willard MD: Clinical manifestations of gastrointestinal disorders. In Nelson RW, Couto CG, editors: Small animal internal medicine, St Louis, 2009, Mosby Elsevier, Table 28-1, p 354.
Drooling, dysphagia, and odynophagia are most commonly seen with conditions of the oropharynx and/or proximal esophagus. Odynophagia is most commonly associated with esophagitis and foreign bodies. Dysphagia and regurgitation together most commonly indicate oral or pharyngeal dysfunction; if regurgitation is not accompanied by dysphagia, esophageal dysfunction is likely.55 Regurgitation in cats with esophageal disease is caused by obstruction or muscular dysfunction. Causes of obstruction include vascular ring anomaly, foreign object, stricture, and neoplasia. Causes of muscular dysfunction include congenital disease, esophagitis, myopathies, neuropathies, and dysautonomia.
Regurgitation may occur immediately after eating if the lesion is in the proximal esophagus. However, a dilated esophagus provides a reservoir for food and fluid so that regurgitation may not be associated in time with eating.
Young cats with signs of esophageal disease should be suspected of congenital defects, such as vascular ring anomaly, or a foreign body. Adult cats with esophageal disease may have a recent history of general anesthesia, administration of certain oral medications, or ingestion of irritant chemicals. Acute onset of clinical signs may suggest a foreign body, while chronic, slowly worsening signs may indicate a stricture or tumor.
Diagnostic Approach
All cats suspected of esophageal disease should have a minimum database as part of the diagnostic plan (complete blood cell count, serum chemistries, urinalysis, and other tests as indicated by age or concurrent diseases, such as serum total T4 and blood pressure measurement). An important part of diagnosis is observation of the cat while eating food, to localize the location of the dysfunction. If the cat is unwilling to eat while in the veterinary clinic, the owner can make a video of the cat eating at home for the clinician to view.
The general diagnostic approach to regurgitation in cats is found in Figure 23-3 . Plain and contrast radiography and endoscopy are important diagnostic tools for esophageal disease. Fluoroscopy is valuable for the diagnosis of motility disorders, but availability is limited to universities and referral centers because of the cost of equipment. Ultrasonography is limited to evaluation of the cervical esophagus and a small segment of abdominal esophagus between the cardia of the stomach and the diaphragm.
FIGURE 23-3.
Diagnostic approach to regurgitation.
(Adapted from Willard MD: Clinical manifestations of gastrointestinal disorders. In Nelson RW, Couto CG, editors: Small animal internal medicine, St Louis, 2009, Mosby Elsevier, Figure 28-1, p 354.)
The entire esophagus should be evaluated with cervical and thoracic radiographs. Thoracic radiographs may also show evidence of complications such as aspiration pneumonia or esophageal perforation. The normal esophagus is not visualized on plain radiographs, but may be seen if food or fluid are retained or a foreign body or mass is present. Radiographic contrast agents useful for esophagrams in cats include liquid or paste barium. A water-soluble iodinated contrast agent (e.g., iohexol, Gastrografin) is preferred if there is any risk the esophagus is perforated, because these agents are less irritating and more rapidly reabsorbed. Esophagrams are most useful for diagnosis of luminal obstructions, extraluminal compression, mucosal irregularities, and possibly alterations in motility.
Dilute liquid barium can be administered with a syringe or it may be mixed with canned food, especially if a motility disorder or stricture is suspected. Multiple lateral radiographs are taken rapidly, starting within 10 seconds of swallowing the contrast agent. Contrast is rapidly cleared from the normal esophagus by peristalsis. If the contrast in the esophagus terminates abruptly, an obstruction is likely. If the contrast is retained throughout the esophagus, muscular dysfunction is suspected. Some conditions, such as esophagitis, are difficult to diagnose radiographically, because contrast agents may or may not adhere to ulcerated mucosa.
Flexible endoscopy is a noninvasive diagnostic tool for esophageal disorders and is often used if plain and contrast radiographs have failed to establish a diagnosis. It is most sensitive for diagnosis of masses, ulcers, perforations, and obstructions. In addition, it is often possible to retrieve foreign bodies using endoscopy as well as to assist with dilatation of strictures or placement of gastrostomy feeding tubes if required. Biopsy of the esophageal mucosa is more difficult than biopsy of gastric or intestinal mucosa and is not commonly performed with the exception of mass lesions.
Specific Diseases
Esophagitis and Esophageal Strictures
Esophagitis may result from various causes of inflammation, such as contact irritation from foreign bodies (including trichobezoars lodged in the esophagus), chemical irritants or caustic medications, gastroesophageal reflux, persistent vomiting, hiatal hernia, or general anesthesia. Inflammation disrupts the esophageal mucosa and exposes the submucosa. An important part of the treatment plan is identification and treatment of the underlying cause.
Clinical signs include dysphagia, regurgitation, salivation, and repeated swallowing, although signs may be absent in cats with mild esophagitis. Cats with odynophagia may repeatedly extend the head and neck while swallowing. If the esophagitis or underlying disease is severe, weight loss and dehydration may occur secondary to anorexia.
If the submucosa and muscularis are damaged, strictures may form as a result of the production of fibrous connective tissue and compromise the esophageal lumen.54 Neoplasia is an important cause of esophageal stricture in humans, but not in cats. Most cases have single strictures, but multiple strictures are possible. In two studies, the mean stricture diameter was reported as 5 mm.26, 32 Most strictures are less than 1 cm in length. Clinical signs associated with strictures appear 5 to 14 days after the esophageal injury and may be present for weeks before definitive treatment is pursued. Regurgitation typically occurs immediately after eating, although if the stricture is long standing, a pouch may form cranial to the lesion where food accumulates.
Survey radiographs may be normal in cats with esophagitis and strictures, but are useful to rule out other causes for the clinical signs, such as a foreign body, or to detect related problems, such as aspiration pneumonia. In some patients, dilation of the esophagus with fluid or air may be seen.45 A contrast esophagram may disclose irregularities of the mucosa in cats with severe esophagitis. Segmental dilation may occur with severe inflammation. Strictures may be diagnosed with an esophagram (Figure 23-4 ); however, in some cases, it may be difficult to differentiate a stricture from intramural thickening (e.g., because of neoplasia).
FIGURE 23-4.
Lateral barium contrast esophagram of a 4-month-old domestic shorthair (DSH) cat with an esophageal stricture associated with administration of doxycycline tablets.
(Courtesy Dr. Emma Thom.)
Endoscopy is useful for diagnosis of esophagitis; findings include mucosal erythema, hemorrhage, and erosions or ulcerations. If gastroesophageal reflux is present, the lesions will be most severe in the distal esophagus, and the lower esophageal sphincter may be dilated. Endoscopy is often used for definitive diagnosis of esophageal stricture as well as to visualize the lesion during treatment by bougienage or balloon catheter dilation. Strictures appear as a ring of white fibrous tissue that narrows the esophageal lumen. If endoscopy is performed after a barium esophagram, 24 hours should be allowed to elapse between the procedures or the barium will obscure visualization with the endoscope.19
General anesthesia is an important cause of esophagitis (sometimes leading to stricture formation) in cats, probably because gastroesophageal reflux appears to occur commonly in anesthetized cats.* For example, in a series of seven cats with benign esophageal stricture, recent anesthesia for ovariohysterectomy was the suspected cause in five cases.1 Clinical signs appeared up to 21 days after anesthesia.
Many preanesthetic drugs and induction agents reduce lower esophageal sphincter pressure.27, 28 Other predisposing factors may be intraabdominal surgery and a head-down position on the surgery table. Reflux fluid with a pH less than 4 is likely to cause esophageal mucosal damage, as is prolonged contact time. Esophageal defense mechanisms include clearance of the reflux fluid by peristalsis and neutralization of the acidic pH by the bicarbonate present in saliva.
In a study of 40 kittens less than 15 weeks of age, risk of gastroesophageal reflux during anesthesia was evaluated with use of a laryngeal airway mask versus endotracheal intubation.47 Gastroesophageal reflux was observed in 50% of kittens with use of the laryngeal airway mask but more importantly in 22% of kittens with endotracheal intubation. The reflux episodes occurred shortly after anesthesia induction. In a study of 50 cats anesthetized with thiopentone or propofol, gastroesophageal reflux occurred in 14%.16 Reflux also occurred shortly after anesthesia was induced and lasted for a mean of 23 minutes. It is unknown why esophageal strictures form only in a small number of cats that experience gastroesophageal reflux during anesthesia.
Gastroesophageal reflux disease (GERD) is a commonly reported cause of esophagitis in humans, but it is rarely reported in cats when not associated with general anesthesia.24, 33 The true incidence is unknown, and diagnosis may be hampered by scant knowledge about the clinical presentation and diagnosis. Clinical signs and diagnostic procedures are as for other causes of esophagitis. In one case series of three cats, diagnosis of GERD was based on clinical signs, contrast radiography, and endoscopic findings.24 Biopsy and histopathology of abnormal esophageal tissue was performed in two cases. The authors noted that the esophageal mucosa may appear grossly normal, but submucosal inflammation may be found on histopathologic examination of biopsies.
A consequence of chronic severe GERD in humans is the development of metaplastic columnar epithelium (Barrett esophagus) that replaces the normal squamous epithelium. One case series reported on Barrett-like esophagus in three cats.23 Two cases were associated with hiatal hernia and one with cardial incompetence.
Drug-induced esophageal damage and stricture formation is well known in humans and cats (see Figure 4-4). In humans over 70 drugs have been implicated, and most are antibacterials or NSAIDs.30 Implicated drugs in the cat include tetracycline, doxycycline, and clindamycin in tablet or capsule form administered without a food or water bolus.4, 17, 32, 36, 37 Clinical signs (dysphagia, regurgitation, salivation, anorexia) appear 3 to 16 days after drug treatment is started. Strictures commonly form in the midcervical esophagus or over the heart base in the thoracic esophagus. Doxycycline hyclate is most commonly associated with esophageal strictures in cats, and the principle reason for its irritating properties is an acidic pH. The monohydrate salt of doxycycline is less irritating and is marketed as tablets and a palatable paste licensed for use in dogs and cats in some countries.48 In humans esophageal ulceration after doxycycline therapy is more common than stricture formation. Although the development of strictures in cats would appear to be uncommon, it seems possible the incidence of esophagitis is underestimated, because the clinical signs (e.g., odynophagia, chest pain) may go unrecognized.
Esophageal transit studies of normal cats have shown that the passage time of dry-swallowed tablets and capsules is often prolonged (longer than 30 seconds).20, 53 Complete entrapment (retention for more than 4 minutes) in the midcervical region occurs commonly. However, a small bolus of food or water is sufficient to ensure immediate passage of the medication into the stomach.20, 53 The risk of esophageal retention can also be lessened by coating a tablet or capsule with butter or a gel dietary supplement (Nutri-Cal; Vétoquinol, Fort Worth, Tex.).21 One study determined that tablets or capsules administered using a one-step pill gun with flavored liquid (FlavoRx Pill Glide; FLAVORx, Columbia, Md.) or a pill delivery treat (Greenies Pill Pockets; Nutro Products, Franklin, Tenn.) ensured an average transit time of 60 seconds or less.6
Delayed esophageal transit of medications allows tablets and capsules to disintegrate within the esophagus, exposing the mucosa to irritating chemicals. Cats may be at risk of delayed esophageal transit, because they do not typically drink water with medication, and they do not have an upright posture. In addition, medications are often given to sick or dehydrated patients that may be at greater risk of esophageal retention of medication. All oral medication given to cats in tablet or capsule form should be followed with food or a liquid.
Mild esophagitis will resolve on its own, especially if an underlying cause can be removed or treated. Frequent meals of canned food should be provided. Cats with moderate to severe esophagitis will require medical therapy, and those with difficulty eating or weight loss may also require gastrostomy tube feeding. Esophagostomy or pharyngostomy feeding tubes should be avoided in these patients. Treatment is provided to control inflammation and promote healing while reducing gastric acid secretion and increasing lower esophageal sphincter tone. The length of medical treatment will vary from about one week to several weeks, depending on the underlying cause and severity of disease. Medications indicated for esophagitis include prokinetics, H2-receptor antagonists, proton pump inhibitors, and sucralfate (Table 23-11 ).
TABLE 23-11.
Drugs Used in the Treatment of Esophagitis and Esophageal Strictures
| Drug | Dose | Mechanism |
|---|---|---|
| Cisapride | 1.5 mg/kg, q12h, PO | Prokinetic; increases lower gastroesophageal sphincter pressure, promotes gastric emptying |
| Famotidine | 0.5-1.0 mg/kg, q12-24h, PO or IV | H2-receptor antagonist; reduces gastric acid secretion |
| Metoclopramide | 0.2-0.4 mg/kg, q6h, SC or PO | Prokinetic; increases lower gastroesophageal sphincter pressure, promotes gastric emptying |
| Omeprazole | 0.5-1.0 mg/kg, q24h, PO | Proton pump inhibitor; reduces gastric acid secretion |
| Ranitidine | 2.5 mg/kg, q12h, IV or 3.5 mg/kg, q12h, PO | H2-receptor antagonist; reduces gastric acid secretion |
| Sucralfate | 0.25 g/cat, q6-8h, PO | Adheres to and protects damaged mucosa |
Drug doses from Trepanier L: Acute vomiting in cats: rational treatment selection, J Feline Med Surg 12:225, 2010.
Prokinetic drugs enhance gastric emptying and increase lower esophageal sphincter tone. Metoclopramide also has antiemetic effects, which may be beneficial in patients with chronic vomiting. It can be administered by the subcutaneous (SC) route, an advantage in a vomiting or regurgitating patient. Cisapride may be more effective at enhancing both gastric emptying and lower esophageal sphincter tone, but it must be obtained from a compounding pharmacy in most countries and can only be given orally.
H2-receptor antagonists are competitive inhibitors that block parietal H2 receptors and decrease the amount of gastric acid produced. Proton pump inhibitors are noncompetitive inhibitors that act on the H+/K+ ATPase enzyme system at the secretory surface of gastric parietal cells. They are considered superior for decreasing gastric acid secretion and are therefore the first choice, despite their greater cost.45 A drawback of proton pump inhibitors is that they must be administered orally. Sucralfate may be beneficial for reflux esophagitis, because it binds to mucosal erosions in an acid environment and provides a protective barrier. It is given as oral slurry, ideally separate from meals or other medications.
Antibiotics are not commonly recommended unless aspiration pneumonia is present or the eroded mucosa is at risk of bacterial infection in a patient with severe disease or a compromised immune system. Corticosteroids are often recommended for cats with esophagitis to reduce esophageal inflammation and impair the formation of fibrous connective tissue. However, the benefit of corticosteroids in cats with esophagitis has not been investigated and administration must be weighed against potential adverse effects, especially in patients with aspiration pneumonia.
Treatment of esophageal stricture typically requires dilation with either bougienage or a balloon catheter; both are used with endoscopic visualization under general anesthesia. Appropriate analgesia should be provided, because dilating the stricture is painful. It does not appear that placement of a gastrostomy feeding tube is specifically required to recover from dilation procedures, although a tube may be placed in some anorexic cats to ensure nutritional intake and administer oral medications.
A bougie is a long, narrow, oblong, mechanical dilator available in various sizes (typically 9- to 12-mm sizes are used in cats) that is gently passed through the stricture, usually over a guide wire. Established criteria for selection of bougie diameter and dilation end points are not available. In one study, the initial bougie chosen was approximately the same size as the estimated diameter of the stricture, or no more than 2 mm larger.8 Once the first bougie is passed, subsequent bougies of increasing diameter are employed. Two to four bougies of increasing size may be passed in a single session, with the goal of dilating the stricture without causing esophageal tear or perforation. Determining when dilation should be stopped is a matter of clinical judgment. The procedure may be repeated as needed to maintain improvement; the total number of procedures required is variable. In one retrospective case series of eight cats treated with bougienage, the median number of procedures was 4.5, and a good outcome was achieved in 75% of the cases.8 In some cases, the endoscope tip itself has been used for bougienage when bougies or balloon catheters were not available.
Balloon catheter dilation has become a popular method in recent years.26, 32, 38 Although some clinicians feel this is a safer procedure than bougienage, there is no data in the literature to support this assumption. The catheter can be placed through the endoscope biopsy channel, alongside the endoscope, or with the aid of a preplaced guide wire. As for bougienage, established criteria for selection of balloon diameter and dilation end points are not available, and the clinician's best judgment must be used. Various balloon sizes are available; in one study, the size was selected so that the inflated diameter was 4 mm larger than the stricture diameter.32 The balloon is passed into the stricture with endoscopic guidance. It is then inflated to a predetermined pressure for 1 to 2 minutes to stretch the stricture, usually with saline, but contrast agents may also be used if fluoroscopy is used. As for bougienage, some cases may require more than one dilation procedure (typically two to four). Cuffed endotracheal tubes are not appropriate substitutes for balloon catheters.
Regardless of the method used, after the dilation procedure, the endoscope should be used to look for other strictures and should be passed into the stomach to look for potential causes, such as causes of chronic vomiting. After treatment, medical management to decrease ongoing gastroesophageal reflux, resolve inflammation, and prevent further stricture formation should be instituted (as described previously). Most cats are able to eat the day following the dilation procedure. Corticosteroid treatment after dilation is controversial, and no controlled studies in animals are available. Antibiotics are not routinely recommended.
The prognosis for cats undergoing esophageal dilation is generally good based on the ability to eat canned food with minimal episodes of regurgitation. However, published studies show 10% to 30% of cats died or were euthanized despite multiple episodes of dilation, and up to 30% could only be fed liquid diets.1, 8, 32, 38 Even among cats with good outcomes, a return to a dry kibble diet may not be possible.
The dilation technique employed may be dictated by the clinician's experience, the equipment available, and the cost. Potential complications of both methods include esophageal tear or perforation, hemorrhage, infection, and aspiration. Esophageal tears or perforations may lead to pneumothorax or pneumomediastinum. Repeated stricture formation is also possible, leaving only less desirable treatment options, such as long-term percutaneous gastrostomy tube feeding or surgery.
Esophageal surgery is generally avoided whenever possible, because it is difficult and invasive (requiring a thoracotomy), with risk of serious complications, such as failure of anastomosis, necrosis, and stricture formation. Closure of incisions in the esophagus is difficult, because there is no serosa, and the muscles are oriented longitudinally. Indications for esophageal surgery include repair of perforations, treatment of strictures that fail to respond to dilation, and tumor resection.
Stent placement has recently been described in cats with esophageal strictures with variable results. A 1-year-old cat presented with a 4-week history of dysphagia and regurgitation caused by a single cervical esophageal stricture after treatment with oral clindamycin.18 Guided balloon dilation was performed 6 times over a period of 3 weeks, but stricture formation always recurred. A self-expanding metal stent was placed using endoscopy and fluoroscopy after another dilation procedure. The cat did well eating a canned diet from an elevated position for 10 months, but by 12 months, the cat was no longer able to eat even liquid food and was euthanized. On necropsy, the stent had migrated and was obstructed by swallowed hair.
In another case, a biodegradable self-expanding stent was used to successfully treat an 11-year-old cat that presented with a stricture in the cervical esophagus after anesthesia for dentistry.3 Balloon dilation was performed twice, but regurgitation recurred 5 days after the last procedure. The stricture was dilated a third time with a balloon catheter, and a tubular self-expanding polydioxanone stent was placed with fluoroscopic guidance. The life span of the stent was estimated to be 10 to 12 weeks, sufficient time to allow healing of the esophagus.
Foreign Bodies
Foreign bodies are less commonly found in the esophagus of the cat than in other gastrointestinal locations. Reported foreign bodies include string, needles, fish hooks, and bones. Trichobezoars may cause obstruction when they become lodged in the esophagus during vomiting (Figure 23-5 ). Recurrent esophageal trichobezoars have been infrequently reported in the literature.12, 51 It is not known if an esophageal motility disorder is the underlying cause for recurrent obstructions. In one case, an esophageal diverticulum developed in association with recurrent trichobezoars.12 Treatment for recurrent trichobezoars includes prokinetic drug therapy (e.g., cisapride), moderate to high-fiber diets, and shaving of long-haired cats.
FIGURE 23-5.
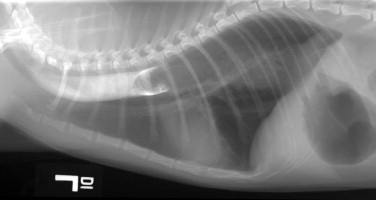
Lateral esophagram of a cat with a trichobezoar esophageal foreign body. Trichobezoars may cause obstruction when they become lodged in the esophagus during vomiting.
(Courtesy Dr. John Graham.)
Common areas for foreign bodies to lodge include the thoracic inlet, the heart base, and the esophageal hiatus in the diaphragm.5 Obstruction of the esophageal lumen may be complete or partial. Clinical signs include acute onset of gagging, salivation, repeated swallowing, dysphagia, and regurgitation. However, chronic esophageal foreign bodies have been reported in cats with dysphagia, intermittent regurgitation, and weight loss over a period of weeks or months.2
Cough, mucopurulent nasal discharge, and fever may be found if aspiration has occurred. Trauma to the esophagus may cause esophagitis and even esophageal stricture. Perforation of the esophagus by the foreign body may lead to pneumothorax, pneumomediastinum, or pyothorax with signs of depression, anorexia, fever, and dyspnea. If the perforation occurs in the cervical esophagus, swelling, cellulitis, and drainage of serous or purulent material may be noted.
Many foreign bodies are readily diagnosed with survey radiographs, especially if they are radiopaque. Other radiographic findings include an esophagus dilated with fluid or air. Radiolucent objects may be detected with an esophagram. Care must be taken when performing esophagrams on cats that may have an obstruction, because aspiration is a concern. If abnormalities that could be consistent with an esophageal perforation (e.g., periesophageal gas or fluid, pleural effusion) are detected on survey radiographs, an aqueous iodine contrast solution should be used.
Removal of esophageal foreign bodies should be performed as soon as possible to minimize esophageal trauma and pressure necrosis. Endoscopy can be used to confirm the diagnosis and often to remove the object. Both rigid and flexible endoscopes may be used along with accessories such as various forceps and Foley catheters. Care should be taken to remove the object as atraumatically as possible, especially if the object is sharp or pointed. If the object is in the caudal esophagus and it cannot be grasped and removed, an attempt should be made to gently push it into the stomach, where it can be retrieved using laparotomy and gastrotomy. If esophageal perforation has occurred, esophagotomy is recommended and is described elsewhere.5, 14
Removal of fish hooks may require a combination of surgery and endoscopy.5, 39 A surgical approach to the esophagus is made, but the esophagus is not incised; rather, the portion of the hook protruding through the esophagus is cut and removed, and the endoscope is used to retrieve the remainder.
Following uncomplicated foreign body removal, the esophagus should be carefully inspected for lesions and bleeding before the endoscope is withdrawn. Food and water should be withheld for 24 to 48 hours. Supportive care includes fluid therapy and analgesia; a gastrostomy feeding tube may be required in selected cases for nutritional support. Broad-spectrum antibiotics are administered to control bacterial infection and therapy for esophagitis should be instituted as described previously. Careful follow-up should include evaluation for stricture formation.
If an esophageal perforation has occurred, conservative management may be sufficient if the defect is small. A broad-spectrum antibiotic should be administered along with other supportive care, such as fluid therapy and analgesia. Feeding through a gastrostomy tube for several days is recommended as well as close monitoring for complications such as pleuritis. Large perforations require thoracotomy for surgical repair.
Megaesophagus
Megaesophagus is a diffuse hypomotility disorder that may be classified as congenital versus acquired or idiopathic versus secondary to other diseases. It is uncommon in cats compared with dogs. At least two dog breeds have been identified with heritable congenital megaesophagus. A heritable form of megaesophagus has been suggested for cats, particularly for Siamese cats, although no detailed studies have been performed.13, 29 It is often frustrating to determine the underlying cause of acquired megaesophagus. Megaesophagus may be a manifestation of neuromuscular diseases, such as dysautonomia or myasthenia gravis (see Chapter 27). Megaesophagus may also develop secondary to esophagitis from chronic vomiting or GERD.24, 43
Other uncommon causes of megaesophagus are found in the literature. One case report describes a young cat with megaesophagus secondary to a large nasopharyngeal polyp that extended into the cervical esophagus.10 Megaesophagus resolved once the polyp was removed. In another report, a young cat with diaphragmatic hernia was diagnosed with megaesophagus and gastric dilation.31 Megaesophagus resolved with medical treatment and surgical correction of the diaphragmatic defect.
Clinical signs are typically those of esophageal dysfunction; regurgitation is the most consistently found sign. Regurgitation may not be closely related in time to eating if the esophagus is markedly distended and holds food. Cats with long-standing disease may suffer from weight loss or secondary rhinitis. The appetite is typically normal or increased. Additional signs may occur if systemic neuromuscular disease is present. Aspiration pneumonia may cause fever, dyspnea, and cough. Two case reports describe cats with idiopathic megaesophagus and chronic vomiting associated with intermittent gastroesophageal intussusception.35, 50 Survey and contrast radiographs may identify a dilated esophagus (Figure 23-6 ), but contrast fluoroscopy is the diagnostic tool of choice when available, because it allows for assessment of peristalsis. Care must be taken with contrast studies because of the risk of aspiration.
FIGURE 23-6.
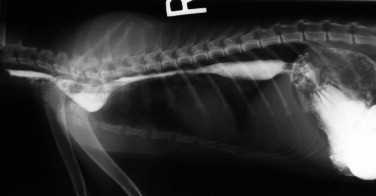
Lateral esophagram of a 5-month-old kitten with megaesophagus.
(Courtesy Dr. Emilia Monachino.)
Treatment of megaesophagus is largely symptomatic and supportive unless an underlying disorder can be identified and treated. Frequent small meals are offered with the cat feeding in an upright position. The upright position should be maintained for at least 10 minutes after eating to allow for gravity-assisted passage of food into the stomach. This is best accomplished by having the owner hold the cat over their shoulder so that the esophagus is in a vertical position.44 Different types of diets should be offered to determine which is best for the individual patient; calorically dense diets may be beneficial for patients with weight loss. Prokinetic drugs, such as cisapride, stimulate smooth muscle, but since most of the esophagus is skeletal muscle, the efficacy of such drugs is questionable for treatment of megaesophagus. Prokinetic drugs also increase lower esophageal sphincter tone and may increase esophageal transit time, neither of which is desirable in patients with megaesophagus.
Vascular Ring Anomaly
Vascular ring anomalies are congenital malformations of the great vessels that entrap the thoracic esophagus and cause obstruction. The most commonly reported anomaly is persistent right aortic arch. The esophagus is entrapped by the aorta on the right, the ligamentum arteriosum and the pulmonary trunk on the left, and the heart base ventrally. Other vascular anomalies are rarely described in cats, such as a double aortic arch described in a Siamese cat.56
Onset of clinical signs occurs around the time of weaning to solid food so that most affected cats are presented at less than 6 months of age. The most common clinical sign is regurgitation, and most patients are underweight. A distended cervical esophagus may be palpated, and secondary aspiration pneumonia may occur.
A history of regurgitation since weaning is very suggestive of a vascular ring anomaly, but other causes of regurgitation must be ruled out. Survey radiographs show a dilated esophagus cranial to the heart, while the caudal esophagus is usually normal. The bulge of the aortic arch normally seen on a ventrodorsal radiographic view is absent. An esophagram is used to confirm the location of the obstruction and the severity of disease.
Definitive treatment is surgical repair of the vascular defect (i.e., ligation and transection of the ligamentosum arteriosum). Some patients will require nutritional support through gastrostomy tube feeding and treatment for aspiration pneumonia before surgery. Early diagnosis and surgical intervention brings the best prognosis for return of normal esophageal function. Some affected cats are left with residual esophageal hypomotility, which is managed as for idiopathic megaesophagus.
Neoplasia
Esophageal neoplasia is rare in the cat as in the dog. Although parasitic granulomas caused by Spirocerca lupi are associated with esophageal neoplasia in dogs, this parasite does not infect cats. Both primary and metastatic esophageal tumors can occur in the cat. Squamous cell carcinoma is the most common primary esophageal tumor in cats and is often found in the caudal two thirds of the esophagus.7, 22, 25, 46 Affected cats are middle aged or older. Clinical signs are typically those associated with esophageal obstruction, such as regurgitation, dysphagia, odynophagia, and salivation. Patients with advanced disease may suffer anorexia, depression, and weight loss. On physical examination, an esophageal mass may or may not be palpable.
Survey and contrast radiographs reveal esophageal dilation, a soft tissue mass, or periesophageal lesions that displace the esophagus. Computed tomography is useful to identify periesophageal or intraluminal masses. Definitive diagnosis is made with endoscopy and biopsy. Mucosal biopsies are difficult to obtain, because the esophageal mucosa is tough; exfoliative cytology may also be helpful. Treatment is rarely undertaken, because disease is often advanced at the time of diagnosis, and many patients have complications such as aspiration pneumonia. Palliation may be attempted with chemotherapy or radiation, although data on efficacy is unavailable. In general, squamous cell carcinomas in other anatomic locations respond poorly to treatment. Surgical resection may be attempted if anastomosis can be accomplished without excessive tension.
Hiatal Hernia
Disorders of the hiatus are rare in cats. Hiatal hernia is protrusion of the distal esophagus and stomach through the esophageal hiatus of the diaphragm into the thoracic cavity; the protrusion may be intermittent (“sliding”) or persistent. Other organs are occasionally involved, such as the omentum.40 This is distinct from a gastroesophageal intussusception where the stomach is prolapsed into the lumen of the distal esophagus.35, 49 Both congenital and traumatic hiatal hernias have been described in cats.9, 23, 41, 42, 52 Congenital hernias appear to be more common than acquired hernias, and affected cats typically present with clinical signs before 1 year of age. It is suspected that increased inspiratory effort associated with upper airway obstruction, such as a nasopharyngeal polyp, may also lead to development of hiatal hernia.23
Hiatal herniation reduces lower esophageal sphincter pressure. Clinical signs associated with hiatal hernia, such as intermittent vomiting and regurgitation, may be because of reflux esophagitis, hypomotility, or obstruction. Large hernias and secondary aspiration pneumonia may be associated with respiratory distress. Survey radiographs may reveal a gas-filled soft tissue density in the caudal dorsal mediastinum. An esophagram will show the gastroesophageal junction and gastric rugae cranial to the diaphragm (Figure 23-7 ). Both fluoroscopy and endoscopy may be useful for diagnosis but are not typically necessary.
FIGURE 23-7.
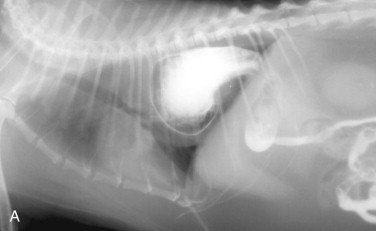
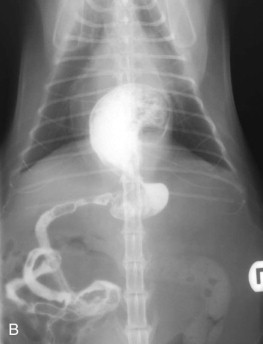
Lateral (A) and ventrodorsal (B) esophagrams of a cat with a hiatal hernia showing protrusion of the distal esophagus and stomach through the esophageal hiatus of the diaphragm into the thoracic cavity.
(Courtesy Dr. John Graham.)
The prognosis for cats with hiatal hernia is considered to be good. A trial of medical management (as for reflux esophagitis) for 1 month has been recommended before surgery.34 Surgery is the treatment of choice for large defects, especially in young cats with congenital disease or cats that have failed medical management. Various reconstructive surgical techniques have been described.14
References
- 1.Adamama-Moraitou KK, Rallis TS, Prassinos NN. Benign esophageal stricture in the dog and cat: a retrospective study of 20 cases. Can J Vet Res. 2002;66:55. [PMC free article] [PubMed] [Google Scholar]
- 2.Augusto M, Kraijer M, Pratschke KM. Chronic oesophageal foreign body in a cat. J Feline Med Surg. 2005;7:237. doi: 10.1016/j.jfms.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battersby I, Doyle R. Use of a biodegradable self-expanding stent in the management of a benign oesophageal stricture in a cat. J Small Anim Pract. 2009;51:49. doi: 10.1111/j.1748-5827.2009.00868.x. [DOI] [PubMed] [Google Scholar]
- 4.Beatty JA, Swift N, Foster DJ. Suspected clindamycin-associated oesophageal injury in cats: five cases. J Feline Med Surg. 2006;8:412. doi: 10.1016/j.jfms.2006.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bebchuk TN. Feline gastrointestinal foreign bodies. Vet Clin North Am Small Anim Pract. 2002;32:861. doi: 10.1016/s0195-5616(02)00030-x. [DOI] [PubMed] [Google Scholar]
- 6.Bennett AD, MacPhail CM, Gibbons DS. A comparative study evaluating the esophageal transit time of eight healthy cats when pilled with the FlavoRx pill glide versus pill delivery treats. J Feline Med Surg. 2010;12:286. doi: 10.1016/j.jfms.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berube D, Scott-Moncrieff JC, Rohleder J. Primary esophageal squamous cell carcinoma in a cat. J Am Anim Hosp Assoc. 2009;45:291. doi: 10.5326/0450291. [DOI] [PubMed] [Google Scholar]
- 8.Bissett SA, Davis J, Subler K. Risk factors and outcome of bougienage for treatment of benign esophageal strictures in dogs and cats: 28 cases (1995-2004) J Am Vet Med Assoc. 2009;235:844. doi: 10.2460/javma.235.7.844. [DOI] [PubMed] [Google Scholar]
- 9.Brinkley CH. Hiatus hernia in a cat. Vet Rec. 1990;127:46. [PubMed] [Google Scholar]
- 10.Byron JK, Shadwick SR, Bennett AR. Megaesophagus in a 6-month-old cat secondary to a nasopharyngeal polyp. J Feline Med Surg. 2010;12:322. doi: 10.1016/j.jfms.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cottrell BD. Post anaesthetic oesophageal stricture in the cat. Vet Rec. 1986;118:645. doi: 10.1136/vr.118.23.645. [DOI] [PubMed] [Google Scholar]
- 12.Durocher L, Johnson SE, Green E. Esophageal diverticulum associated with a trichobezoar in a cat. J Am Anim Hosp Assoc. 2009;45:142. doi: 10.5326/0450142. [DOI] [PubMed] [Google Scholar]
- 13.Forbes DC, Leishman DE. Megaesophagus in a cat. Can Vet J. 1985;26:354. [PMC free article] [PubMed] [Google Scholar]
- 14.Fossum T, Hedlund C. Surgery of the digestive system. In: Fossum TW, editor. Small animal surgery. ed 3. Mosby Elsevier; St Louis: 2007. p. 339. [Google Scholar]
- 15.Galatos AD, Rallis T, Raptopoulos D. Post anaesthetic oesophageal stricture formation in three cats. J Small Anim Pract. 1994;35:638. [Google Scholar]
- 16.Galatos AD, Savas I, Prassinos NN. Gastro-oesophageal reflux during thiopentone or propofol anaesthesia in the cat. J Vet Med A Physiol Pathol Clin Med. 2001;48:287. doi: 10.1046/j.1439-0442.2001.00357.x. [DOI] [PubMed] [Google Scholar]
- 17.German AJ, Cannon MJ, Dye C. Oesophageal strictures in cats associated with doxycycline therapy. J Feline Med Surg. 2005;7:33. doi: 10.1016/j.jfms.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glanemann B, Hildebrandt N, Schneider MA. Recurrent single oesophageal stricture treated with a self-expanding stent in a cat. J Feline Med Surg. 2008;10:505. doi: 10.1016/j.jfms.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glazer A, Walters P. Esophagitis and esophageal strictures. Comp Contin Edu Pract Vet. 2008;30:281. [PubMed] [Google Scholar]
- 20.Graham J, Lipman A, Newell S. Esophageal transit of capsules in clinically normal cats. Am J Vet Res. 2000;61:655. doi: 10.2460/ajvr.2000.61.655. [DOI] [PubMed] [Google Scholar]
- 21.Griffin B, Beard DM, Klopfenstein KA. Use of butter to facilitate the passage of tablets through the esophagus in cats [abstract] J Vet Intern Med. 2003;17:445. [Google Scholar]
- 22.Gualtieri M, Monzeglio MG, Di Giancamillo M. Oesophageal squamous cell carcinoma in two cats. J Small Anim Pract. 1999;40:79. doi: 10.1111/j.1748-5827.1999.tb03041.x. [DOI] [PubMed] [Google Scholar]
- 23.Gualtieri M, Olivero D. Reflux esophagitis in three cats associated with metaplastic columnar esophageal epithelium. J Am Anim Hosp Assoc. 2006;42:65. doi: 10.5326/0420065. [DOI] [PubMed] [Google Scholar]
- 24.Han E, Broussard J, Baer K. Feline esophagitis secondary to gastroesophageal reflux disease: clinical signs and radiographic, endoscopic, and histopathologic findings. J Am Anim Hosp Assoc. 2003;39:161. doi: 10.5326/0390161. [DOI] [PubMed] [Google Scholar]
- 25.Happe RP, van der Gaag I, Wolvekamp WT. Esophageal squamous cell carcinoma in two cats. Tijdschr Diergeneeskd. 1978;103:1080. [PubMed] [Google Scholar]
- 26.Harai BH, Johnson SE, Sherding RG. Endoscopically guided balloon dilatation of benign esophageal strictures in 6 cats and 7 dogs. J Vet Intern Med. 1995;9:332. doi: 10.1111/j.1939-1676.1995.tb01093.x. [DOI] [PubMed] [Google Scholar]
- 27.Hashim MA, Waterman AE. Effects of thiopentone, propofol, alphaxalone-alphadolone, ketamine and xylazine-ketamine on lower oesophageal sphincter pressure and barrier pressure in cats. Vet Rec. 1991;129:137. doi: 10.1136/vr.129.7.137. [DOI] [PubMed] [Google Scholar]
- 28.Hashim MA, Waterman AE. Effects of acepromazine, pethidine and atropine premedication on lower oesophageal sphincter pressure and barrier pressure in anaesthetised cats. Vet Rec. 1993;133:158. doi: 10.1136/vr.133.7.158. [DOI] [PubMed] [Google Scholar]
- 29.Hoenig M, Mahaffey MB, Parnell PG. Megaesophagus in two cats. J Am Vet Med Assoc. 1990;196:763. [PubMed] [Google Scholar]
- 30.Jaspersen D. Drug-induced oesophageal disorders: pathogenesis, incidence, prevention and management. Drug Saf. 2000;22:237. doi: 10.2165/00002018-200022030-00007. [DOI] [PubMed] [Google Scholar]
- 31.Joseph R, Kuzi S, Lavy E. Transient megaoesophagus and oesophagitis following diaphragmatic rupture repair in a cat. J Feline Med Surg. 2008;10:284. doi: 10.1016/j.jfms.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leib MS, Dinnel H, Ward DL. Endoscopic balloon dilation of benign esophageal strictures in dogs and cats. J Vet Intern Med. 2001;15:547. doi: 10.1892/0891-6640(2001)015<0547:ebdobe>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 33.Lobetti R, Leisewitz A. Gastroesophageal reflux in two cats. Feline Pract. 1996;24:5. [Google Scholar]
- 34.Lorinson D, Bright RM. Long-term outcome of medical and surgical treatment of hiatal hernias in dogs and cats: 27 cases (1978-1996) J Am Vet Med Assoc. 1998;213:381. [PubMed] [Google Scholar]
- 35.Martinez NI, Cook W, Troy GC. Intermittent gastroesophageal intussusception in a cat with idiopathic megaesophagus. J Am Anim Hosp Assoc. 2001;37:234. doi: 10.5326/15473317-37-3-234. [DOI] [PubMed] [Google Scholar]
- 36.McGrotty Y, Knottenbelt C. Oesophageal stricture in a cat due to oral administration of tetracycline. J Small Anim Pract. 2002;43:221. doi: 10.1111/j.1748-5827.2002.tb00062.x. [DOI] [PubMed] [Google Scholar]
- 37.Melendez L, Twedt D, Wright M. Suspected doxycycline-induced esophagitis with esophageal stricture formation in three cats. Feline Pract. 2000;28:10. [Google Scholar]
- 38.Melendez LD, Twedt DC, Weyrauch EA. Conservative therapy using balloon dilation for intramural, inflammatory esophageal strictures in dogs and cats: a retrospective study of 23 cases (1987-1997) Eur J Comp Gastroenterol. 1998;3:31. [Google Scholar]
- 39.Michels G, Jones B, Huss B. Endoscopic and surgical retrieval of fishhooks from the stomach and esophagus in dogs and cats: 75 cases (1977-1993) J Am Vet Med Assoc. 1995;207:1194. [PubMed] [Google Scholar]
- 40.Mitsuoka K, Tanaka R, Nagashima Y. Omental herniation through the esophageal hiatus in a cat. J Vet Med Sci. 2002;64:1157. doi: 10.1292/jvms.64.1157. [DOI] [PubMed] [Google Scholar]
- 41.Owen MC, Morris PJ, Bateman RS. Concurrent gastro-oesophageal intussusception, trichobezoar and hiatal hernia in a cat. N Z Vet J. 2005;53:371. doi: 10.1080/00480169.2005.36579. [DOI] [PubMed] [Google Scholar]
- 42.Papazoglou L, Patsikas M, Rallis T. Hiatal hernia with esophageal stricture in a cat. Feline Pract. 2000;28:10. [Google Scholar]
- 43.Pearson H, Darke PG, Gibbs C. Reflux oesophagitis and stricture formation after anaesthesia: a review of seven cases in dogs and cats. J Small Anim Pract. 1978;19:507. doi: 10.1111/j.1748-5827.1978.tb05532.x. [DOI] [PubMed] [Google Scholar]
- 44.Ridgway MD, Graves TK. Megaesophagus. Clin Brief. 2010;8:43. [Google Scholar]
- 45.Sellon RK, Willard MD. Esophagitis and esophageal strictures. Vet Clin North Am Sm Anim Pract. 2003;33:945. doi: 10.1016/s0195-5616(03)00075-5. [DOI] [PubMed] [Google Scholar]
- 46.Shinozuka J, Nakayama H, Suzuki M. Esophageal adenosquamous carcinoma in a cat. J Vet Med Sci. 2001;63:91. doi: 10.1292/jvms.63.91. [DOI] [PubMed] [Google Scholar]
- 47.Sideri AI, Galatos AD, Kazakos GM. Gastro-oesophageal reflux during anaesthesia in the kitten: comparison between use of a laryngeal mask airway or an endotracheal tube. Vet Anaesth Analg. 2009;36:547. doi: 10.1111/j.1467-2995.2009.00499.x. [DOI] [PubMed] [Google Scholar]
- 48.Trumble C. Oesophageal stricture in cats associated with use of the hyclate (hydrochloride) salt of doxycycline [letter] J Feline Med Surg. 2005;7:241. doi: 10.1016/j.jfms.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Van Camp S, Love N, Kumaresan S. Gastroesophageal intussusception in a cat. Vet Radiol Ultrasound. 1998;39:190. doi: 10.1111/j.1740-8261.1998.tb00338.x. [DOI] [PubMed] [Google Scholar]
- 50.Van Geffen C, Saunders JH, Vandevelde B. Idiopathic megaoesophagus and intermittent gastro-oesophageal intussusception in a cat. J Small Anim Pract. 2006;47:471. doi: 10.1111/j.1748-5827.2006.00024.x. [DOI] [PubMed] [Google Scholar]
- 51.Van Stee EW, Ward CL, Duffy ML. Recurrent esophageal hairballs in a cat (a case report) Vet Med. 1980;75:1873. [PubMed] [Google Scholar]
- 52.Waldron DR, Moon M, Leib MS. Oesophageal hiatal hernia in two cats. J Small Anim Pract. 1990;31:259. [Google Scholar]
- 53.Westfall D, Twedt D, Steyn P. Evaluation of esophageal transit of tablets and capsules in 30 cats. J Vet Intern Med. 2001;15:467. doi: 10.1892/0891-6640(2001)015<0467:eoetot>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 54.Weyrauch E, Willard M. Esophagitis and benign esophageal strictures. Comp Contin Edu Pract Vet. 1998;20:203. [Google Scholar]
- 55.Willard M. Clinical manifestations of gastrointestinal disorders. In: Nelson RW, Couto CG, editors. Small animal internal medicine. ed 4. Mosby Elsevier; St Louis: 2009. p. 351. [Google Scholar]
- 56.Yarim M, Gultiken ME, Ozturk S. Double aortic arch in a Siamese cat. Vet Pathol. 1999;36:340. doi: 10.1354/vp.36-4-340. [DOI] [PubMed] [Google Scholar]
Diseases of the Stomach
The stomach is a frequent site for gastrointestinal problems in cats, and the most common gastric problems are described in this chapter. Some conditions such as gastric dilatation-volvulus are often reported in dogs but rarely reported in cats. In one report of three feline cases, all were associated with diaphragmatic hernia.15 Gastric parasites, the diagnostic approach to the vomiting cat, and therapeutics for vomiting are covered elsewhere in this chapter.
The anatomy of the feline stomach is similar to that of other mammals having a simple glandular stomach. Most of the stomach is situated on the left side of the abdominal cavity. It has five regions, starting from the lower esophageal sphincter: cardia, fundus, body, antrum, and pylorus (Figure 23-8 ). The pylorus of the cat is unique compared with other species in that it is narrow and has high resistance in order to maintain a tight seal (Figure 23-9 ). The normal stomach has a characteristic appearance when viewed using endoscopy (Figure 23-10 ) or ultrasonography (Figure 23-11 ).
FIGURE 23-8.
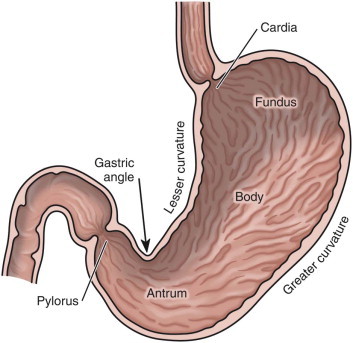
The five regions of the feline stomach.
(From Twedt DC: Diseases of the stomach. In Sherding RG, editor: The cat: diseases and clinical management, ed 2, Philadelphia, 1994, Saunders, Figure 38-1, p 1182.)
FIGURE 23-9.
Endoscopic view of the normal feline pylorus. The pylorus is readily visible during endoscopic examination, and may be open or closed.
(Courtesy Prof. Danièlle Gunn-Moore.)
FIGURE 23-10.
Endoscopic appearance of normal gastric folds in the cat. Prominent rugal folds are visible in the greater curvature of the stomach.
(Courtesy Prof. Danièlle Gunn-Moore.)
FIGURE 23-11.
Ultrasonographic appearance of the normal feline stomach showing the characteristic rosette or wagon wheel appearance.
(Courtesy Dr. John Graham.)
The gastric emptying time of normal cats is shorter than that of other mammals. In one study, the gastric emptying half-time for solid food in normal cats was 1.4 to 3.6 hours.53 This implies prolonged fasting (longer than 8 hours) in preparation for anesthesia and surgery is unnecessary.
Clinical Presentation
The main clinical sign of gastric disease is vomiting, but it is important to note that vomiting is also associated with many nongastric problems, including concurrent intestinal disease, such as enteritis or colitis. Vomiting patients therefore require a thorough physical examination and diagnostic plan to determine the cause. Vomiting must be distinguished from regurgitation, which is primarily associated with esophageal disease (see Table 23-10). Vomitus often contains food, hair, refluxed bile, or blood. Fresh blood may appear as large or small clots. Older blood clots have a brown “coffee ground” appearance. Gastric bleeding may also cause melena. Other clinical signs may be associated with gastric disease, such as anorexia, weight loss, pain, lethargy, bloating, and nausea.
Specific Diseases
Gastritis
Gastritis may be acute or chronic in nature, and this distinction may be useful in assessing the potential cause. For example, cats with acute gastritis may be suspected of foreign body or plant ingestion, drug or toxin exposure (see Chapter 31), or dietary indiscretion. Cats with chronic gastritis may be suspected of parasitism, Helicobacter spp. infection, or dietary intolerance or hypersensitivity (see Chapter 17). Chronic lymphocytic plasmacytic gastritis of unknown etiology is also a common cause of chronic vomiting. Whenever possible, a specific underlying cause should be sought and treated.
Acute Uncomplicated Gastritis
Patients with sudden onset of vomiting may have an obvious cause in the history (e.g., dietary indiscretion), but in many cases, the cause is not apparent. Abdominal radiographs should be taken if foreign body ingestion is possible, especially in a young cat. If the patient is systemically well, further diagnostic testing may be postponed pending response to therapy. Treatment for uncomplicated acute gastritis is symptomatic and supportive. Clinical signs are expected to resolve in 24 to 48 hours; if signs persist, re-evaluation and further investigation is warranted. Subcutaneous fluid therapy using an isotonic balanced electrolyte solution may be used to correct mild fluid deficits (<5%). Oral intake of fluids and food should be discontinued for up to 24 hours. A highly digestible diet, either commercial or homemade, is introduced with a gradual transition back to the normal diet over the next several days.
Antiemetic therapy may be indicated for acute uncomplicated gastritis if the vomiting is frequent or the cat has signs of nausea (see Table 23-3). Protectants, such as kaolin and pectin, are difficult to administer to cats and are without proven efficacy. Bismuth subsalicylate is controversial; it is considered contraindicated by some experts, because of the cat's sensitivity to salicylates,39 yet is commonly used in clinical practice.
Foreign Body Ingestion
Cats ingest foreign bodies less commonly than dogs. In one study of 208 cases of gastrointestinal foreign body ingestion, only 12% were in cats.22 Foreign body ingestion is most likely to be seen in young cats and may involve a wide variety of objects, including linear objects (e.g., dental floss, thread with or without a needle, tinsel, string). The owner may or may not be aware of the ingestion. Ingestion of multiple foreign bodies may be seen in cats with pica (Figure 23-12 ). In one case report, a young domestic shorthair cat required gastrotomy for removal of 32 copper pennies.43 Some patients require multiple surgeries, because of repeated foreign body ingestion.22 In such cases, a behavioral diagnosis should be sought and treatment instituted (see Chapter 13).
FIGURE 23-12.
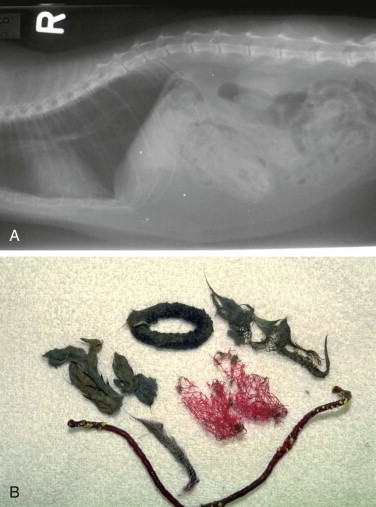
A, Abdominal radiograph of a 4-year-old female Siamese cat presented for vomiting pieces of hair elastics. B, Variety of objects removed by gastrotomy from the same cat; this was the second surgery for this patient for ingesting multiple foreign objects.
Trichobezoars (large masses of hair) also represent a type of foreign object. Both long- and shorthaired cats may be affected. Hair is normally ingested during grooming and is eliminated in vomitus and feces. Cats lack the strong peristaltic contractions (“housekeeper” contractions) that clear the stomach of undigested contents normally found in other species. This may explain why cats seem to be susceptible to gastric trichobezoars. Gastric motility dysfunction is suspected to cause repeated gastric trichobezoars in some cats. Intestinal3, 22 and esophageal14, 59 obstruction with trichobezoars has also been documented. Traditional treatments for cats with recurrent trichobezoars include regular grooming, shaving the hair coat of long-haired cats, flea control, treatment of underlying dermatologic disorders, and administration of semisolid petroleum laxatives. More recently, commercial diets have been formulated for control of trichobezoars. Cats with recurrent trichobezoars causing illness and suspected motility disorders may benefit from treatment with prokinetic drugs such as cisapride.
Clinical signs of gastric foreign bodies are variable but typically involve intermittent or persistent vomiting because of gastric outflow obstruction, distention, and mucosal irritation. Gastric obstruction may be partial or total. Patients with complete obstruction will present with more dramatic signs, including anorexia and depression. The base of the tongue should always be examined, because linear foreign bodies are sometimes anchored either in this location, or they may be lodged in the pylorus, causing intestinal plication. Gastric foreign bodies may also be asymptomatic and found incidentally.5 Physical examination may be unremarkable or may reveal dehydration or abdominal pain. If the stomach is markedly distended, the foreign body may be palpable in some patients.
Survey radiographs are always indicated when foreign body ingestion is suspected. Radiopaque foreign bodies may be readily diagnosed, although some, along with radiolucent objects, will require a contrast study for diagnosis (Figure 23-13 ). Barium is commonly used as a contrast agent, although if gastric perforation is suspected, an aqueous iodinated agent is preferred. Ultrasonography is also useful for detection of gastrointestinal foreign bodies.56
FIGURE 23-13.
Abdominal radiograph of a cat with a large gastric trichobezoar.
(Courtesy Dr. John Graham.)
Removal of some foreign bodies can be attempted endoscopically, particularly if the object does not have sharp edges and is not too large. Successful removal of fish hooks, particularly single-barb hooks, using endoscopy has been described.35 Otherwise, foreign objects are best removed using gastrotomy through a ventral midline laparotomy. A radiograph should always be taken just before surgery to ensure the object has not moved further down the gastrointestinal tract.
Postoperative management after gastrotomy includes maintenance of hydration and electrolyte balance. Hypokalemia is common with anorexia and vomiting and should be treated by supplementation of IV fluids with 20 to 40 mEq/L potassium chloride (not to exceed 0.5 mEq/kg/hour). Refractory vomiting should be treated with an antiemetic. A highly digestible diet can be introduced the day after surgery. In general, the prognosis for recovery is good. In one study, 88% of cats with gastrointestinal foreign bodies survived to discharge.22 Those cats that did not survive had linear foreign bodies of long-standing duration with subsequent peritonitis.
Helicobacter Gastritis
Helicobacter are spiral or curved gram-negative bacteria that inhabit the glands, parietal cells, and mucus of the gastric antrum and fundus. Helicobacter contain large amounts of urease, which alters the pH in the vicinity of the bacteria and allows for colonization of the acidic environment of the stomach. In the early 1980s, the discovery of the association of Helicobacter pylori with gastric disease (gastritis, peptic ulcers, and neoplasia) in humans revolutionized treatment of those diseases. Since then, Helicobacter spp. have been associated with gastric disease in various veterinary species, including cats and dogs. Several Helicobacter spp. (e.g., H. heilmannii, H. bizzozeronii, H. felis) have been identified in cats, some of which have the potential to infect humans, although transmission is thought to be rare.19, 48 The prevalence of Helicobacter infection in cats varies geographically and may be very high (>40%) in some locations.1, 27, 37, 47, 58
The importance of Helicobacter as a cause of gastric disease is cats is unclear; the bacteria may be found in the stomach of both clinically normal cats and cats with gastritis. The prevalence of Helicobacter infection is not higher in cats with gastritis compared with normal cats.61 Determination of the role of Helicobacter is also hampered by the paucity of controlled clinical trials that evaluate eradication of gastritis and clinical signs in infected cats.
An immune response to infection characterized by gastric lymphoid hyperplasia is common, although the local immune response in cats is generally less severe than the response in humans infected with H. pylori. To date gastrointestinal ulcers have not been associated with Helicobacter infection in cats. Recent studies have suggested a possible association between Helicobacter infection and gastric lymphoma in cats, although more research is needed to confirm the association and understand the pathogenesis.7, 32 Helicobacter spp. may be commensal in most cats, and perhaps loss of tolerance explains the development of gastritis in some individuals.49 Another possibility is that the inflammatory response is normally well managed and disease may result when there is an abnormality of the immunoregulatory system.21
The most commonly used methods for diagnosis of Helicobacter infection in cats are based on gastric specimens obtained during endoscopy (or laparotomy): exfoliative cytology, histopathologic examination of biopsy specimens, and rapid urease testing of biopsy specimens.28 However, it is important to note that even when Helicobacter organisms are identified, the infection may not be the cause of the patient's clinical signs, and other causes of vomiting should always be evaluated.
Exfoliative cytology is the least expensive and most easily performed diagnostic test. In one study, it was also the most sensitive diagnostic method when compared with urease testing and histologic examination.20 Brush cytology samples gathered during endoscopy are air-dried on microscope slides and stained with Wright's stain. The slide is examined at 100× magnification under oil immersion. Spiral bacteria are readily seen if present. At least 10 oil-immersion fields on two slides should be examined before determining a specimen is negative for Helicobacter-like organisms.28
Since Helicobacter produce abundant urease, a rapid urease test (e.g., CLOtest, Ballard Medical Products, Draper, Utah) may be used for diagnosis.38 The kit consists of an agar gel impregnated with urea and a pH indicator. A gastric biopsy sample is applied to the gel, and if urease is present, ammonia will form and change the pH (and thus the color) of the gel. The gel may change color rapidly (within 30 minutes), but 24 hours must elapse before the test can be considered negative.28 The more rapidly the color changes, the higher the bacterial load. Both false-positive and false-negative results are possible with rapid urease testing for various reasons, giving the test a sensitivity of 70% to 90%.28, 37
Histopathologic examination of gastric biopsy samples using hemotoxylin and eosin (H&E) or silver stains is highly sensitive and specific in human studies for detection of Helicobacter-like organisms. The organisms are not equally distributed; so, examination of biopsy specimens from multiple sites will increase sensitivity. The bacteria may be seen in mucus on the surface epithelium as well as in the gastric pits, glandular lumen, and parietal cells. Organisms may also be seen submucosally within gastric lymphoid follicles.46 Histopathologic examination of biopsy samples also allows for assessment of other abnormalities. Mild to severe lymphocytic-plasmacytic or lymphocytic gastritis may be present.
In humans combination therapy with antibiotics and antisecretory drugs is recommended to reduce the risk of gastric ulcers and cancer from H. pylori infection. Treatment is highly successful at eradicating both clinical signs and histologic changes in the gastric mucosa. Since Helicobacter infection is common in cats, yet no clear pathogenic role has been established, it is difficult to know when treatment should be attempted. One expert recommends treating only patients with clinical signs of gastritis that have biopsy-confirmed Helicobacter infection with a treatment regimen of amoxicillin (20 mg/kg, every 12 hours, PO), clarithromycin (7.5 mg/kg, every 12 hours, PO) and metronidazole (10 mg/kg, every 12 hours, PO) for 14 days.49 A common dilemma would be determining the treatment of choice for patients with lymphoplasmacytic inflammation of the stomach and small intestine and confirmed Helicobacter infection. Are such patients best treated for inflammatory bowel disease, Helicobacter infection, or both? Currently, guidelines for determining the best treatment approach are lacking.
Also, few studies on the efficacy of combination therapy have been conducted in cats. Long-term eradication of infection may be difficult, and histopathologic resolution of gastritis may not be possible, which raises the question of whether Helicobacter is the true underlying cause.38, 41 In one study, two cats with clinical gastritis and Helicobacter infection were treated with oral metronidazole, amoxicillin, and bismuth subsalicylate for 3 weeks and were also fed a commercial elimination diet.25 Posttreatment gastric biopsies were obtained a mean of 7 weeks after the cessation of treatment. Resolution of clinical signs occurred rapidly, and clearance of Helicobacter spp. was achieved at that time point, but gastric inflammation persisted in post-treatment biopsies. In another study, 13 cats with asymptomatic Helicobacter infection were treated with oral omeprazole, amoxicillin, metronidazole, and clarithromycin for 14 days.26 Treatment failed to eradicate infection in 4 of the cats based on molecular analysis of post-treatment gastric biopsies. It is unclear if treatment failure is because of recrudescence or reinfection.
The reader is referred to excellent reviews of Helicobacter in cats for more information.27, 38, 48
Chronic Gastritis
Chronic gastritis is common in cats with chronic intermittent vomiting. Ollulanus tricuspis is a worm that infects the stomach of cats, causing chronic gastritis, and it is difficult to diagnose (see below, Gastrointestinal Parasites). The worm is occasionally found on histologic examination of gastric biopsy samples.9 It is reasonable to treat empirically (fenbendazole 10 mg/kg, once daily, PO × 2 days) for this parasite when the cause of gastritis is not apparent.49
The frequency of vomiting in cats with chronic gastritis is highly variable, ranging from once or twice per week (and not necessarily every week) to more than once daily. Most patients are otherwise well, although other clinical signs (inappetence, anorexia, depression, or weight loss) are possible depending on disease severity. Results of routine laboratory testing are typically normal but may show neutrophilic leukocytosis, eosinophilia, or hypoproteinemia. Survey and contrast radiographs are often normal.
The most common finding on histopathologic examination of biopsy samples is lymphocytic plasmacytic (LP) gastritis (Figure 23-14 ). Some patients will also have concurrent evidence of LP inflammation in the small intestine, pancreas, and/or liver. Such patients will be treated for their concurrent problem; treatment of inflammatory bowel disease, pancreatitis, and cholangiohepatitis is covered elsewhere in this chapter.
FIGURE 23-14.
Histopathologic image (40×) of a stomach biopsy from a 10-year-old cat with a history of chronic vomiting. Small lymphocytes are increased in number in the lamina propria; cellular debris is evident within some gastric glands.
(Courtesy Dr. Sally Lester.)
Some cats with chronic LP gastritis respond to treatment for dietary intolerance or hypersensitivity with a limited antigen diet (see Chapter 17). Patients with moderate to severe LP gastritis may be best treated with a limited antigen diet and immunosuppressive therapy (prednisolone 1 to 2 mg/kg/day, PO tapering to every other day at the lowest dose that controls clinical signs). Patients that fail this initial treatment approach may require additional immunosuppressive therapy, such as chlorambucil (see Table 23-9).
Occasionally, cats with chronic gastritis are diagnosed with eosinophilic inflammation on histopathologic examination of biopsy specimens. Treatment is similar to that for LP gastritis, although such patients should be evaluated for evidence of hypereosinophilic syndrome and eosinophilic enteritis. Eosinophilic fibrosing gastritis was suspected to be caused by toxoplasmosis in one case report.33
Gastric Ulceration
Gastric or gastroduodenal ulcerations are uncommon in the cat compared with the dog and may be caused by a variety of disorders, both gastric and nongastric.29 Classical clinical signs include vomiting, hematemesis, and melena. However, in one review of eight cats, hematemesis and melena were present in less than one third of cases.29 Depending on the underlying cause and severity of disease, abdominal pain, anorexia, lethargy, pale mucous membranes, and drooling may also be seen. Cats with neoplastic disease may have prolonged clinical signs and are more likely to present with anorexia and weight loss.29 Cats with perforated ulcers may or may not present with signs of shock. Diagnosis may be problematic because the clinical signs and physical examination findings are often not specific, even in cats with perforated ulcerations.8
The causes of gastric ulceration in cats are not well characterized. In dogs the most common cause is the administration of ulcerogenic drugs, particularly NSAIDs, either alone or in combination with corticosteroids. Several cases of NSAID-induced gastroduodenal ulceration or perforation have been reported in cats.8, 34, 45 Additional cases may be reported in the future, because long-term administration of these drugs is gaining in popularity for treatment of chronic diseases such as osteoarthritis. NSAIDs cause direct mucosal damage and interfere with prostaglandin synthesis. Although inhibition of the COX-1 enzyme is thought to be the cause of adverse effects, such as gastric ulceration, even COX-2–selective drugs have been associated with adverse effects, and safety in sick cats is not well evaluated. Recently, guidelines for the long-term use of NSAIDs in cats were published by the International Society of Feline Medicine and the American Association of Feline Practitioners.51 The recommendations include administering NSAIDs either with or shortly after food, withholding therapy if inappetence or anorexia develops, determining dose based on lean body weight, and titrating to the lowest effective dose.
Neoplastic causes of gastric ulceration include systemic mastocytosis, mast cell tumor, lymphosarcoma, adenocarcinoma, and gastrinoma (Zollinger-Ellison syndrome). Cats with chronic renal disease may suffer mucosal damage from uremic toxins and increased gastric acid production secondary to hypergastrinemia (because of decreased renal metabolism of gastrin).18 Hepatic disease is a cause of gastric ulceration in dogs but is uncommonly reported in cats.23 Recent anesthesia and surgery have been implicated as a cause of gastric ulceration and perforation, perhaps through hypovolemia, hypoperfusion, or stress.8, 29 Other non-neoplastic causes reported for gastric or gastroduodenal ulceration in cats include parasites (e.g., Ollulanus tricuspis, Toxocara cati, Aonchotheca putorii, Gnathostoma spp.), bacterial infections, toxins, inflammatory bowel disease, and foreign bodies. One case report describes a cat with severe gastric ulceration caused by intoxication with Dieffenbachia leaves.36 In some case reports, the cause for the gastric ulcerations could not be determined.
A minimum database should be collected for cats suspected of gastric ulceration, to identify underlying diseases. Anemia, usually regenerative, may be present. Other findings will be dependent on the presence of underlying diseases; for example, azotemia and isosthenuria may indicate renal disease. Electrolyte and acid–base abnormalities may be because of chronic vomiting and anorexia.
Survey and contrast radiographs and ultrasonography are primarily useful to rule out other causes for the clinical signs, such as foreign bodies. Cats with perforated ulcers may have evidence of pneumoperitoneum (sometimes severe) on plain radiographs or ultrasonographs, and this is an indication for surgical exploration.6, 8, 24, 31, 34 Evidence of peritonitis on imaging studies should be followed with peritoneal fluid analysis. A definitive diagnosis may be made using endoscopy, which allows direct visualization of lesions and collection of biopsy samples. However, some cats with gastric ulceration present in poor condition, which may preclude the use of endoscopy because of anesthetic risk and risk of ulcer perforation.29 The location of ulcers is typically pyloroantral or fundic in cats with non-neoplastic disease.8, 29 Areas of erosion may appear pale or hemorrhagic; the mucosa is often friable and bleeds easily. Fresh or clotted blood may be seen in the stomach lumen. In some cases, mucosal ulceration must be distinguished from ulcerated tumors. NSAID-induced ulcers are typically found in the antrum and do not have marked mucosal thickening; ulcerated tumors frequently have thickened edges and surrounding mucosa.49 Biopsy samples should be taken at the periphery of the ulcer to avoid perforation.
Treatment should be directed at any underlying disorder. Treatment for NSAID toxicity is described in Chapter 31. General supportive measures include fluid therapy and electrolyte replacement; blood transfusion may also be required (see Chapter 25). Gastric acid production can be decreased with the use of H2-receptor blockers or proton pump inhibitors, and sucralfate is used as a mucosal protectant (see Table 23-5). Sucralfate may inhibit absorption of other oral medications and should be given 2 hours apart from other drugs. If vomiting is severe or persistent, antiemetic therapy is warranted (see Table 23-3). Analgesia should be provided for painful patients; a good choice is the opioid buprenorphine (see Table 6-1). Broad-spectrum antibiotic therapy is indicated for patients with significant mucosal barrier dysfunction, perforation, leukopenia and/or neutrophilia, fever, and melena.
Surgical intervention is warranted for patients with life-threatening hemorrhage, failure to respond to medical management, or evidence of perforation.29 The entire abdominal cavity and gastrointestinal tract should be thoroughly explored to locate extragastrointestinal lesions, non-perforated ulcers, and multiple ulcers. In one case series, nonperforated ulcers were detected at laparotomy by association with adhesions or a gastric mass.29 Surgical management includes débridement and suturing of the ulcer site as well as collection of biopsy samples for histopathologic examination. The prognosis for recovery was excellent in two studies, particularly for cats with non-neoplastic causes of gastric or gastroduodenal ulceration.8, 29 In one study of seven cats with perforated gastric or duodenal ulcers, the survival rate was low (14%).23
Gastric Motility Disorders and Delayed Gastric Emptying
Disorders of gastric motility are better characterized in dogs than in cats. The most common clinical sign is vomiting of undigested food 8 hours or more after a meal. If outflow obstruction is present, vomiting may be projectile. There may also be a history of recurrent trichobezoars. Various disorders are associated with impaired gastric motility, such as chronic gastritis, drug therapy (e.g., anticholinergic and narcotic drugs), dysautonomia, gastric neoplasia, metabolic disorders (e.g., hypokalemia), and temporary postsurgical gastroparesis. In some cases of chronic motility dysfunction, no cause can be identified. Outflow obstruction may be caused by neoplasia, foreign bodies, and extragastric masses. Pyloric stenosis is infrequently documented in young cats, often Siamese cats.4, 40, 55
Since the range of underlying disorders is diverse, the diagnostic approach should allow for detection of both gastric and nongastric disorders. A minimum database (CBC, serum chemistries, urinalysis, feline leukemia virus [FeLV] and feline immunodeficiency virus [FIV] serology) is used to establish overall health status. Radiographs are used to confirm presence of food in the stomach for longer than 8 hours. Ultrasonography may detect gastric lesions, such as masses. Endoscopy is used to identify outflow obstruction as well as other lesions, such as ulcers, and evidence of gastritis.
Assessment of gastric emptying using nuclear scintigraphy is the most accurate method but is limited to referral centers. Gastric emptying times for liquids, canned food, and dry diets have been established using nuclear scintigraphy.11, 16, 17 However, emptying times are variable, depending on the amount and type of diet fed as well as the amount of water ingested. Even the shape of kibble affects emptying time.2 Radiographic contrast series are widely used, but gastric emptying times are variable for barium in either liquid form or mixed with canned food. Contrast radiography using liquid barium (8 to 10 mL/kg) is performed in a fasted patient. Radiographs are taken immediately after administration of the barium and again at 15 and 30 minutes, in some cases, also at 1 and 3 hours. Liquid barium is expected to enter the duodenum no more than 30 minutes after administration, and the stomach should be completely empty of barium within 3 hours. The clinician should be aware that some cats with gastric motility disorders will have a normal gastric emptying time with liquid barium. Barium can also be mixed with canned food and fed as a meal; retention of barium-containing food in the stomach for more than 8 to 12 hours is abnormal.
Gastric emptying time may also be established with the use of barium impregnated polyspheres (BIPS; Med I.D. Systems, Grand Rapids, Mich.) and radiography. Gastric emptying times for BIPS have been established in healthy fasted and fed cats as well as in sedated cats,10, 52 but the values do not correlate well with scintigraphic studies.17 A mixture of small (1.5 mm) and large (5 mm) spheres are administered with food, and two to four radiographs are taken over the next 24 hours. The small spheres are intended to mimic liquid transit time and the large spheres solid transit time. However, studies assessing the clinical relevance of this method are lacking. One review concluded that BIPS are probably sufficiently sensitive to detect grossly delayed gastric emptying.60
Treatment of gastric emptying disorders is directed at identifiable causes. Treatment for gastric ulcers, chronic gastritis, and foreign bodies is described elsewhere in this chapter. Pyloric stenosis is managed surgically. If no outflow obstruction exists, treatment with prokinetic agents, such as metoclopramide or cisapride, may be beneficial (see Table 23-3).
Gastric Neoplasia
Gastric tumors account for less than 1% of malignancies in dogs and cats.30 Benign gastric tumors are even less common than gastric malignancies. Gastric smooth muscle hamartoma has been reported in one 11-year-old cat.50 Although adenocarcinoma is the most common gastric cancer of the dog, lymphoma is the most common gastric cancer in the cat. Feline gastrointestinal lymphoma occurs as two major types: small cell (lymphocytic) and the more aggressive large cell (lymphoblastic) form. Small cell lymphomas are more frequently enteric.57 In one study of 12 cats with gastric lymphoma, diffuse large B-lymphocyte tumors of immunoblastic nuclear type predominated.42 Gastric lymphoma is not associated with FeLV, and the role of Helicobacter in the development of gastric lymphoma in cats requires investigation.7 Adenocarcinoma,12, 13, 54 plasmacytoma,62 and gastric carcinoid44 have also been described. The Siamese cat may be predisposed to adenocarcinoma.12, 54 As would be expected, most cats with gastric neoplasia are older cats.
As for most gastric diseases, vomiting is the most common clinical sign of neoplasia. The vomitus may contain blood and melena may be present. Other clinical signs include anorexia, weight loss, bloating, and depression. Perforation of the tumor may occur, leading to pneumoperitoneum or septic peritonitis. Clinical signs present gradually and are often present for weeks to months. Physical examination findings are nonspecific, although occasionally a gastric mass or gastric thickening may be palpated if the stomach is markedly enlarged.
Results of routine diagnostic testing are generally nonspecific; anemia may be associated with ulceration. Survey or contrast radiography may reveal a mass (Figure 23-15, A ); other findings include delayed gastric emptying, impaired motility, and mucosal ulceration. Ultrasonography is also useful for diagnosis and can be used to guide needle aspirates of masses (Figure 23-15, B). Endoscopy allows for visualization of lesions as well as the ability to obtain partial thickness biopsy samples. Problems with interpretation of endoscopic biopsy samples include detection of necrosis, inflammation, and ulceration rather than the primary lesion. In dogs some neoplastic lesions are submucosal, making it very difficult to obtain diagnostic samples by endoscopy. Therefore several biopsies should be taken and masses should be biopsied multiple times in the same place to sample deeper tissues. The center of ulcerated lesions should not be biopsied. Surgical biopsies are more reliable for diagnosis.
FIGURE 23-15.
Radiographic (A) and ultrasonographic (B) images of a cat with a gastric mass that was determined to be lymphoma on biopsy.
(Courtesy Dr. John Graham.)
Surgical resection is the most common treatment for gastric neoplasia other than lymphoma (Figure 23-16 ). The prognosis for most patients is poor, typically because of debilitation, concurrent diseases, and recurrent or metastatic disease.30 The success of chemotherapy for lymphoma depends on cell type, with small cell tumors carrying a better prognosis than large cell tumors.
FIGURE 23-16.
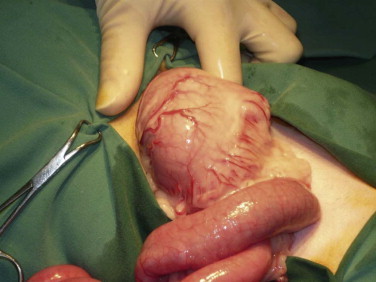
Surgical appearance of a gastric mass caused by small cell lymphoma.
(Courtesy Dr. Randolph Baral.)
References
- 1.Araujo IC, Mota SB, de Aquino MHC. Helicobacter species detection and histopathological changes in stray cats from Niterói, Brazil. J Feline Med Surg. 2010;12:509. doi: 10.1016/j.jfms.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armbrust LJ, Hoskinson JJ, Lora-Michiels M. Gastric emptying in cats using foods varying in fiber content and kibble shapes. Vet Radiol Ultrasound. 2003;44:339. doi: 10.1111/j.1740-8261.2003.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 3.Barrs VR, Beatty JA, Tisdall PL. Intestinal obstruction by trichobezoars in five cats. J Feline Med Surg. 1999;1:199. doi: 10.1053/jfms.1999.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumberger A. [Pyloric dysfunction as a cause of chronic vomiting in the cat] Schweiz Arch Tierheilkd. 1977;119:415. [PubMed] [Google Scholar]
- 5.Bebchuk TN. Feline gastrointestinal foreign bodies. Vet Clin North Am Small Anim Pract. 2002;32:861. doi: 10.1016/s0195-5616(02)00030-x. [DOI] [PubMed] [Google Scholar]
- 6.Boysen SR, Tidwell AS, Penninck DG. Ultrasonographic findings in dogs and cats with gastrointestinal perforation. Vet Radiol Ultrasound. 2003;44:556. doi: 10.1111/j.1740-8261.2003.tb00507.x. [DOI] [PubMed] [Google Scholar]
- 7.Bridgeford EC, Marini RP, Feng Y. Gastric Helicobacter species as a cause of feline gastric lymphoma: a viable hypothesis. Vet Immunol Immunopathol. 2008;123:106. doi: 10.1016/j.vetimm.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cariou MPL, Halfacree ZJ, Lee KCL. Successful surgical management of spontaneous gastric perforations in three cats. J Feline Med Surg. 2010;12:36. doi: 10.1016/j.jfms.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cecchi R, Wills SJ, Dean R. Demonstration of Ollulanus tricuspis in the stomach of domestic cats by biopsy. J Comp Pathol. 2006;134:374. doi: 10.1016/j.jcpa.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Chandler M, Guilford G, Lawoko C. Radiopaque markers to evaluate gastric emptying and small intestinal transit time in healthy cats. J Vet Intern Med. 1997;11:361. doi: 10.1111/j.1939-1676.1997.tb00481.x. [DOI] [PubMed] [Google Scholar]
- 11.Costello M, Papasouliotis K, Barr FJ. Determination of solid- and liquid-phase gastric emptying half times in cats by use of nuclear scintigraphy. Am J Vet Res. 1999;60:1222. [PubMed] [Google Scholar]
- 12.Cribb AE. Feline gastrointestinal adenocarcinoma: a review and retrospective study. Can Vet J. 1988;29:709. [PMC free article] [PubMed] [Google Scholar]
- 13.Dennis MM, Bennett N, Ehrhart EJ. Gastric adenocarcinoma and chronic gastritis in two related Persian cats. Vet Pathol. 2006;43:358. doi: 10.1354/vp.43-3-358. [DOI] [PubMed] [Google Scholar]
- 14.Durocher L, Johnson SE, Green E. Esophageal diverticulum associated with a trichobezoar in a cat. J Am Anim Hosp Assoc. 2009;45:142. doi: 10.5326/0450142. [DOI] [PubMed] [Google Scholar]
- 15.Formaggini L, Schmidt K, De Lorenzi D. Gastric dilatation-volvulus associated with diaphragmatic hernia in three cats: clinical presentation, surgical treatment and presumptive aetiology. J Feline Med Surg. 2008;10:198. doi: 10.1016/j.jfms.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goggin JM, Hoskinson JJ, Butine MD. Scintigraphic assessment of gastric emptying of canned and dry diets in healthy cats. Am J Vet Res. 1998;59:388. [PubMed] [Google Scholar]
- 17.Goggin JM, Hoskinson JJ, Kirk CA. Comparison of gastric emptying times in healthy cats simultaneously evaluated with radiopaque markers and nuclear scintigraphy. Vet Radiol Ultrasound. 1999;40:89. doi: 10.1111/j.1740-8261.1999.tb01845.x. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein R, Marks S, Kass P. Gastrin concentrations in plasma of cats with chronic renal failure. J Am Vet Med Assoc. 1998;213:826. [PubMed] [Google Scholar]
- 19.Haesebrouck F, Pasmans F, Flahou B. Gastric helicobacters in domestic animals and nonhuman primates and their significance for human health. Clin Microbiol Rev. 2009;22:202. doi: 10.1128/CMR.00041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Happonen I, Saari S, Castren L. Comparison of diagnostic methods for detecting gastric Helicobacter-like organisms in dogs and cats. J Comp Pathol. 1996;115:117. doi: 10.1016/s0021-9975(96)80034-x. [DOI] [PubMed] [Google Scholar]
- 21.Harbour S, Sutton P. Immunogenicity and pathogenicity of Helicobacter infections of veterinary animals. Vet Immunol Immunopathol. 2008;122:191. doi: 10.1016/j.vetimm.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Hayes G. Gastrointestinal foreign bodies in dogs and cats: a retrospective study of 208 cases. J Small Anim Pract. 2009;50:576. doi: 10.1111/j.1748-5827.2009.00783.x. [DOI] [PubMed] [Google Scholar]
- 23.Hinton L, McLoughlin M, Johnson S. Spontaneous gastroduodenal perforation in 16 dogs and seven cats (1982-1999) J Am Anim Hosp Assoc. 2002;38:176. doi: 10.5326/0380176. [DOI] [PubMed] [Google Scholar]
- 24.Itoh T, Nibe K, Naganobu K. Tension pneumoperitoneum because of gastric perforation in a cat. J Vet Med Sci. 2005;67:617. doi: 10.1292/jvms.67.617. [DOI] [PubMed] [Google Scholar]
- 25.Jergens AE, Pressel M, Crandell J. Fluorescence in situ hybridization confirms clearance of visible Helicobacter spp. associated with gastritis in dogs and cats. J Vet Intern Med. 2009;23:16. doi: 10.1111/j.1939-1676.2008.0211.x. [DOI] [PubMed] [Google Scholar]
- 26.Khoshnegah J, Jamshidi S, Mohammadi M, Sasani F. The efficacy and safety of long-term Helicobacter species quadruple therapy in asymptomatic cats with naturally acquired infection. J Feline Med Surg. 2011;13:88. doi: 10.1016/j.jfms.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lecoindre P, Chevallier M, Peyrol S. Gastric heliocbacters in cats. J Feline Med Surg. 2000;2:19. doi: 10.1053/jfms.2000.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leib M, Duncan R. Diagnosing gastric Helicobacter infections in dogs and cats. Comp Contin Edu Pract Vet. 2005;27:221. [Google Scholar]
- 29.Liptak J, Hunt G, Barrs V. Gastroduodenal ulceration in cats: eight cases and a review of the literature. J Feline Med Surg. 2002;4:27. doi: 10.1053/jfms.2001.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liptak JM, Withrow SJ. Cancer of the gastrointestinal tract. In: Withrow SJ, Vail DM, editors. Withrow & MacEwen's small animal clinical oncology. ed 4. Saunders Elsevier; St Louis: 2007. p. 455. [Google Scholar]
- 31.Lykken JD, Brisson BA, Etue SM. Pneumoperitoneum secondary to a perforated gastric ulcer in a cat. J Am Vet Med Assoc. 2003;222:1713. doi: 10.2460/javma.2003.222.1713. [DOI] [PubMed] [Google Scholar]
- 32.Marini RP, Fox JG, White H. Helicobacter spp. influences the development of primary gastric lymphoma in cats: a viable hypothesis. Gut. 2001;49:A52. [Google Scholar]
- 33.McConnell JF, Sparkes AH, Blunden AS. Eosinophilic fibrosing gastritis and toxoplasmosis in a cat. J Feline Med Surg. 2007;9:82. doi: 10.1016/j.jfms.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mellanby RJ, Baines EA, Herrtage ME. Spontaneous pneumoperitoneum in two cats. J Small Anim Pract. 2002;43:543. doi: 10.1111/j.1748-5827.2002.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 35.Michels G, Jones B, Huss B. Endoscopic and surgical retrieval of fishhooks from the stomach and esophagus in dogs and cats: 75 cases (1977-1993) J Am Vet Med Assoc. 1995;207:1194. [PubMed] [Google Scholar]
- 36.Muller N, Glaus T, Gardelle O. [Extensive stomach ulcers because of Dieffenbachia intoxication in a cat] Tierarztl Prax Ausg K Klientiere Heimtiere. 1998;26:404. [PubMed] [Google Scholar]
- 37.Neiger R, Dieterich C, Burnens A. Detection and prevalence of Helicobacter infection in pet cats. J Clin Microbiol. 1998;36:634. doi: 10.1128/jcm.36.3.634-637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neiger R, Simpson K. Helicobacter infection in dogs and cats: facts and fiction. J Vet Intern Med. 2000;14:125. doi: 10.1892/0891-6640(2000)014<0125:iidacf>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 39.Papich MG, Davis CA, Davis LE. Absorption of salicylate from an antidiarrheal preparation in dogs and cats. J Am Anim Hosp Assoc. 1987;23:221. [Google Scholar]
- 40.Pearson H, Gaskell CJ, Gibbs C. Pyloric and oesophageal dysfunction in the cat. J Small Anim Pract. 1974;15:487. doi: 10.1111/j.1748-5827.1974.tb06527.x. [DOI] [PubMed] [Google Scholar]
- 41.Perkins SE, Yan LL, Shen Z. Use of PCR and culture to detect Helicobacter pylori in naturally infected cats following triple antimicrobial therapy. Antimicrob Agents Chemother. 1996;40:1486. doi: 10.1128/aac.40.6.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pohlman LM, Higginbotham ML, Welles EG. Immunophenotypic and histologic classification of 50 cases of feline gastrointestinal lymphoma. Vet Pathol. 2009;46:259. doi: 10.1354/vp.46-2-259. [DOI] [PubMed] [Google Scholar]
- 43.Poortinga E. Copper penny ingestion in a cat. Can Vet J. 1995;36:634. [PMC free article] [PubMed] [Google Scholar]
- 44.Rossmeisl JH, Jr, Forrester SD, Robertson JL. Chronic vomiting associated with a gastric carcinoid in a cat. J Am Anim Hosp Assoc. 2002;38:61. doi: 10.5326/0380061. [DOI] [PubMed] [Google Scholar]
- 45.Runk A, Kyles A, Downs M. Duodenal perforation in a cat following the administration of nonsteroidal anti-inflammatory medication. J Am Anim Hosp Assoc. 1998;35:52. doi: 10.5326/15473317-35-1-52. [DOI] [PubMed] [Google Scholar]
- 46.Serna JH, Genta RM, Lichtenberger LM. Invasive Helicobacter-like organisms in feline gastric mucosa. Helicobacter. 1997;2:40. doi: 10.1111/j.1523-5378.1997.tb00056.x. [DOI] [PubMed] [Google Scholar]
- 47.Shojaee Tabrizi A, Jamshidi S, Oghalaei A. Identification of Helicobacter spp. in oral secretions vs. gastric mucosa of stray cats. Vet Microbiol. 2010;140:142. doi: 10.1016/j.vetmic.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 48.Simpson K, Neiger R, DeNovo R. The relationship of Helicobacter spp. infection to gastric disease in dogs and cats. J Vet Intern Med. 2000;14:228. [PubMed] [Google Scholar]
- 49.Simpson KW. Diseases of the stomach. In: Ettinger SJ, Feldman EC, editors. Textbook of veterinary internal medicine. ed 6. Saunders Elsevier; St Louis: 2005. p. 1310. [Google Scholar]
- 50.Smith TJ, Baltzer WI, Ruaux CG. Gastric smooth muscle hamartoma in a cat. J Feline Med Surg. 2010;12:334. doi: 10.1016/j.jfms.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sparkes AH, Heiene R, Lascelles BDX. ISFM and AAFP consensus guidelines: Long-term use of NSAIDs in cats. J Feline Med Surg. 2010;12:521. doi: 10.1016/j.jfms.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sparkes AH, Papasouliotis K, Barr FJ. Reference ranges for gastrointestinal transit of barium-impregnated polyethylene spheres in healthy cats. J Small Anim Pract. 1997;38:340. doi: 10.1111/j.1748-5827.1997.tb03481.x. [DOI] [PubMed] [Google Scholar]
- 53.Steyn PF, Twedt D, Toombs W. The scintigraphic evaluation of solid phase gastric emptying in normal cats. Vet Radiol Ultrasound. 1995;36:327. doi: 10.1111/j.1740-8261.1997.tb00874.x. [DOI] [PubMed] [Google Scholar]
- 54.Turk MA, Gallina AM, Russell TS. Nonhematopoietic gastrointestinal neoplasia in cats: a retrospective study of 44 cases. Vet Pathol. 1981;18:614. doi: 10.1177/030098588101800506. [DOI] [PubMed] [Google Scholar]
- 55.Twaddle AA. Congenital pyloric stenosis in two kittens corrected by pyloroplasty. N Z Vet J. 1971;19:26. doi: 10.1080/00480169.1971.33924. [DOI] [PubMed] [Google Scholar]
- 56.Tyrrell D, Beck C. Survey of the use of radiography vs. ultrasonography in the investigation of gastrointestinal foreign bodies in small animals. Vet Radiol Ultrasound. 2006;47:404. doi: 10.1111/j.1740-8261.2006.00160.x. [DOI] [PubMed] [Google Scholar]
- 57.Valli VE, Jacobs RM, Norris A. The histologic classification of 602 cases of feline lymphoproliferative disease using the National Cancer Institute working formulation. J Vet Diagn Invest. 2000;12:295. doi: 10.1177/104063870001200401. [DOI] [PubMed] [Google Scholar]
- 58.Van den Bulck K, Decostere A, Baele M. Identification of non-Helicobacter pylori spiral organisms in gastric samples from humans, dogs, and cats. J Clin Microbiol. 2005;43:2256. doi: 10.1128/JCM.43.5.2256-2260.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Stee EW, Ward CL, Duffy ML. Recurrent esophageal hairballs in a cat (a case report) Vet Med. 1980;75:1873. [PubMed] [Google Scholar]
- 60.Wyse CA, McLellan J, Dickie AM. A review of methods for assessment of the rate of gastric emptying in the dog and cat: 1898-2002. J Vet Intern Med. 2003;17:609. doi: 10.1111/j.1939-1676.2003.tb02491.x. [DOI] [PubMed] [Google Scholar]
- 61.Yamasaki K, Suematsu H, Takahashi T. Comparison of gastric lesions in dogs and cats with and without gastric spiral organisms. J Am Vet Med Assoc. 1998;212:529. [PubMed] [Google Scholar]
- 62.Zikes CD, Spielman B, Shapiro W. Gastric extramedullary plasmacytoma in a cat. J Vet Intern Med. 1998;12:381. doi: 10.1111/j.1939-1676.1998.tb02138.x. [DOI] [PubMed] [Google Scholar]
Approach to the Cat with Diarrhea
Overview
Diarrhea can be defined as increased volume and/or increased frequency of defecation of stools with increased water content. Approaches to diarrhea, as for any clinical sign, need to take into account the individual animal. For example, neoplasia is much less likely to occur in a kitten than in a geriatric cat. In many cases, the precise diagnosis of gastrointestinal disease cannot be reached without biopsy samples. The decision to obtain biopsy samples should follow a logical pathway that is appropriate to the cat's condition. These are summarized in Figure 23-17 . For example, many cases of acute diarrhea in a well cat can resolve with limited or no intervention, and so do not require a precise diagnosis.
FIGURE 23-17.
Diagnostic approach to the cat with diarrhea.
The diagnostic steps are
-
1
Signalment and clinical history
-
2
Physical examination
-
3
Fecal assessment
-
4
Blood and urine testing
-
5
Imaging (radiography, ultrasonography)
-
6
Biopsy samples
These steps do not include treatment/diet trials or other empiric therapies that are appropriate in many cases. Steps 3 and 4 are often undertaken at the same time, and there is no definite order for these steps. They are divided here for reasons of clarity. In a younger cat, where infectious causes are more likely, thorough fecal testing is more important; in an older cat, extra-gastrointestinal diseases, such as hyperthyroidism, are more likely; so, blood and urine testing is more important, but fecal assessment should not be neglected.
The decision to proceed to Step 4 (and each subsequent step) should take into account several considerations. The main considerations in assessing and managing a cat with diarrhea are
-
•
Is there an acute onset or a chronic time course?
-
•
Are there any dietary changes or indiscretion?
-
•
Is the cat well or unwell?
-
•
Is there primary or secondary gastrointestinal disease?
-
•
Is there small or large bowel diarrhea?
Step 1: Signalment and Clinical History
The components of the clinical history for cats with diarrhea are detailed in Table 23-12 . After establishing the cat's age, breed, vaccination, and deworming history, it is important to establish the duration and nature of the diarrhea. Chronic diarrhea is usually defined as greater than 3 weeks in duration and mostly warrants at least some degree of a diagnostic workup, whereas acute diarrhea is often self-limiting in a well cat.
TABLE 23-12.
Clinical History for Cats with Diarrhea
| Signalment | Age? Young cats are prone to dietary, infectious, and parasitic causes of diarrhea; older cats are more likely to have inflammatory, metabolic, and neoplastic causes of diarrhea. |
| Vaccination status | Appropriate panleukopenia, FeLV vaccination? |
| Diet | Detailed dietary history as well as recent dietary changes. Adverse reactions to food are common causes of diarrhea. Diarrhea that ceases when an animal is not fed suggests osmotic diarrhea. |
| Environment | Presence of various plants, chemicals, and foreign objects? Health status of cats in the household? Outdoor cats are more likely to develop parasitic, toxic, and infectious disorders. |
| Travel history | Infectious potential or enzootic area (fungal or parasitic diarrhea)? |
| Current medications | Drug reaction or toxicity? Drug therapies that can cause diarrhea should be noted (e.g., antibiotics, anti-inflammatory agents, cardiac glycosides). |
| Past medical and surgical problems | Organ system affected? Recurrence? Response to previous treatment? |
| Onset and duration of diarrhea | Acute versus chronic? Acute diarrheas are abrupt in onset and of short duration, and generally they are self-limiting. Chronic diarrheas persist usually longer than 3 weeks and fail to respond to symptomatic therapy. |
| Appearance of diarrhea | Quantity and quality of the stool (color, consistency, character, presence of blood or mucus)? Loose to watery feces that contain fat droplets, undigested food, melena, and variable colors suggests small intestinal disease. The volume is always increased with small intestinal disease. Loose to semisolid feces containing excess mucus and fresh blood (hematochezia) indicates large intestinal disease. The volume may be normal to slightly decreased with large intestinal disease. |
| Description of defecation process | Tenesmus (straining) and dyschezia (painful defecation)? These are hallmarks of large intestinal disease (e.g., inflammatory or obstructive lesions of the colon, rectum, or anus). |
| Frequency of defecation | Frequency is normal to slightly increased with small bowel disease, but greatly increased with large bowel disease. |
| Associated physical signs | Vomiting, anorexia, weight loss, and dyschezia may help localize the disorder to a specific part of the gastrointestinal tract. Clinical signs relating to problems in other organs or body systems should be noted and may suggest a more generalized disease. Vomiting may occur as a consequence of small intestinal inflammation in some cats with diarrhea. Weight loss may result from decreased caloric intake (anorexia), decreased nutrient assimilation (maldigestion/malabsorption), or excessive caloric loss (protein-losing enteropathy or nephropathy). Weight loss is observed uncommonly with large bowel disease. |
FeLV, Feline leukemia virus.
From Hall JE: Clinical approach to chronic diarrhea In August JR, editor: Consultations in feline internal medicine, ed 4, Philadelphia, 2001, Saunders, p 130.
A description of the feces helps determine whether the diarrhea is small or large bowel in origin (Table 23-13 ); this will affect how any investigations might proceed. Important questions to ask concern frequency of defecation (and how this compares with the normal state), tenesmus (straining usually indicates large bowel diarrhea, since an irritated colon leads to urgency), volume of feces (smaller volumes are typical of large bowel diarrhea; larger volumes are more typical of small bowel), how formed the stool is (from soft stool to cow-pat consistency to liquid tea; usually more watery stool relates to small intestinal disease), color (darker indicates digested blood), and presence of any mucus or blood (presence relates to large bowel).
TABLE 23-13.
Distinctions Between Small and Large Bowel Diarrhea
| Observation | Small Bowel Diarrhea | Large Bowel Diarrhea |
|---|---|---|
| Frequency of defecation | Normal to slightly increased | Very increased |
| Fecal output | Large volumes | Small volumes frequently |
| Urgency or tenesmus | Absent | Present |
| Dyschezia | Absent | Present with rectal disease |
| Mucus in feces | Absent | Present |
| Exudate in feces | Absent | Present sometimes |
| Hematochezia (red blood) | Absent | Present sometimes |
| Melena (digested blood) | Present sometimes | Absent |
| Steatorrhea | Present sometimes | Absent |
| Flatulence and borborygmus | Present sometimes | Absent |
| Weight loss | Present sometimes | Rare |
| Vomiting | Present sometimes | Rare |
From Sherding RG: Diseases of the intestines. In Sherding RG, editor: The cat: diseases and clinical management, ed 2, New York, 1994, Churchill Livingstone, p 1215.
Most household toxins, such as plants, cause signs additional to diarrhea such vomiting or neurological signs,11 but it is important to ascertain if the cat has had access to anything unusual. Likewise, it is important to find out if the cat has had any possible exposure to dietary indiscretions; this can include if the cat has been seen with or is known to hunt prey including insects. Cockroaches carry pathogenic bacteria12, 18 and other prey such as birds and rats can carry Salmonella; salmonellosis in cats has been dubbed songbird fever.6
Simple causes of self-limiting diarrhea include dietary change (either a new flavor or a new style of food, such as dry food for the first time); so, the owner must also be quizzed if anything new has been offered, either new cat food or treats (such as greasy fish or chicken).
Although the physical examination will usually determine how unwell a cat is, the owner's impressions are also important, because cats can hide signs from strangers, particularly in a practice setting. Lethargy and inappetence are important signs, as ill cats typically do not eat well.
Step 2: Physical Examination
The cat's general demeanor can be an indicator of how unwell a cat is and therefore dictate the extent of diagnostic testing required. This can be noted by assessing how interested the cat is in its surroundings or any behavior changes from previous visits, such as if a normally difficult-to-handle cat is placid. Body weight should be assessed and, if possible, compared with that of previous visits (even those noted on a clinical record from another veterinarian). The body condition score (BCS) should also be assessed and can be very important when there is no prior weight information.
Dehydration is usually a sign that a cat needs more involved management. Abdominal palpation should be performed to assess pain (where?), any masses (foreign bodies, lymph nodes, or even focally thickened intestines, such as with neoplasia), or turgid intestines. Fever often indicates infection but can also reflect neoplasia or other inflammatory changes. A thorough examination of all body systems should always be performed, no matter what a cat presents for. In the case of diarrhea, extragastrointestinal signs can be of vital importance, such as a palpable thyroid and tachycardia suggesting hyperthyroidism. After the clinical history has been taken and the physical examination performed, the veterinarian must make the important decisions of whether any interventions are required and whether the patient should be managed as an inpatient or outpatient. The veterinarian should be asking
-
•
Are ancillary tests appropriate?
-
•
Is supportive care necessary?
-
•
Are any medications required?
In many cases, the answers to these questions are obvious. For example, a cat may seem well but has had access to lilies (the author has seen diarrhea as a primary presenting sign for this!) or has a palpable abdominal mass. Substantial weight loss is an indicator that further investigations are warranted sooner rather than later. If the decision is made for empiric management and outpatient care, it is vital to follow up either by scheduling a recheck visit or calling the client, because simple acute problems can turn into complicated chronic problems.
The Well Cat with Acute Onset Diarrhea
If the diarrhea has been present for less than a week and the cat has no weight loss, dehydration, fever, or palpable abdominal abnormalities, it is appropriate to manage the cat as an outpatient. Even in the absence of fecal testing, it is appropriate to deworm the cat (see the section Gastrointestinal Parasites). The cat should be fasted for 24 hours (12 hours, if less than 4 months old) and then fed a bland diet (such as plain, cooked, skinless chicken, or low-residue prescription diets designed for cats with gastrointestinal problems). It is appropriate to maintain the cat on the low-residue diet for at least 7 to 10 days and then slowly reintroduce the regular diet.
Step 3: Fecal Assessment +/− Culture
Fecal assessment is mostly used to assess infectious agents, such as parasite-associated diarrhea, but the importance of assessing feces, even when parasitic or bacterial infections are not suspected, should not be underestimated. Gross examination of feces can determine if melena or fresh blood or mucus are present to help distinguish large from small bowel disease when the owner's observations may be misleading. Occult fecal blood can be an indicator of gastrointestinal inflammation in cases of subtle disease, and undigested starches and fats can indicate maldigestion or malabsorption.8
For assessment of feces for parasites, the fecal sample should ideally be fresh (<1 hour old). Refrigeration (for no longer than one week) can preserve ova, oocysts, and cysts but not protozoal trophozoites. Feces should be assessed by
-
1Direct wet preparation
-
aTo assess for trophozoites
-
a
-
2Stained fecal smear
-
aCan aid in the visualization of internal structures of some protozoa
-
a
-
3Fecal flotation (preferably with centrifugation)
-
aTo find cysts, oocysts, and ova
-
a
These techniques are described in Box 23-1 . Specific fecal analyses can be performed to assess for
- 1
-
2
Giardia: SNAP Giardia Test Kit (IDEXX Laboratories, Westbrook, Me.)10
-
3
Cryptosporidium enzyme-linked immunosorbent assay (ELISA) (but care should be taken, since different ELISAs have varying sensitivity and specificity)9
BOX 23-1. Methods of Fecal Analysis.
Direct Wet Preparation
Used to evaluate the smear for the presence of trophozoites, such as Giardia spp. and Tritrichomonas foetus.
-
1
Place peppercorn size amount of feces on a warm slide and mix with a drop of 0.9% saline (smear must not be too thick, because trophozoites will be easily missed).
-
2
Apply coverslip.
-
3
Evaluate systematically for motile organisms using the 10× magnification.
-
4
Confirmation at 40× magnification.
Stained Fecal Smear
Adding iodine to a wet mount through the edge of the coverslip can aid in the visualization of internal structures of some protozoa. The direct wet preparation must be examined without any stain for motility first, because staining the preparation kills the organism. Methylene blue is useful for identifying trophozoites, particularly those of Entamoeba histolytica. This method has little to no diagnostic value for the diagnosis of bacterial-associated diarrhea.
Fecal Flotation
Used to find cysts, oocysts, and ova in feces. Standing (gravitational) flotation methods are easier and quicker but have much poorer sensitivity than centrifugation methods.2 Solutions used in centrifugation flotation methods include zinc sulfate and Sheather sugar.
Procedure for Single Centrifugal Flotation3
-
1
Weigh out 2 to 5 g of feces.
-
2
Mix feces with approximately 10 mL of flotation solution.
-
3
Pour mixture through a tea strainer into a beaker or fecal cup.
-
4
Pour strained solution into a 15-mL centrifuge tube.
-
5
Fill tube with flotation solution so that a slight positive meniscus forms, being sure not to overfill the tube.
-
6
Place a coverslip on the tube, and put the tube in the centrifuge.
-
7
Make sure the centrifuge is balanced.
-
8
Centrifuge at 1200 rpm (280× g) for 5 minutes.
-
9
Remove the tube and let stand 10 minutes.
-
10
Remove the coverslip, and place it on a glass slide. Systematically examine the entire area under the coverslip at 100× magnification (i.e., 10× objective). You may wish to use the 40× objective lens to confirm your diagnosis and make measurements; however, with practice, most parasites can be identified using the 10× objective (100× magnification).
Citations are from the references in the section Approach to the Cat with Diarrhea.
Adapted from Marks SL: The scoop on poop—maximizing the diagnostic yield of the fecal examination. WSAVA Conference Proceedings 2007; Dryden MW, Payne PA, Smith V: Accurate diagnosis of Giardia spp. and proper fecal examination procedures, Vet Ther 7:4, 2006.
Fecal culture should be undertaken with the understanding that bacteria will be cultured; so, interpretation is based on the relevance of the positive culture result. Factors affecting interpretation include whether the growth is a heavy and pure growth of a known pathogen, such as Salmonella, Campylobacter, Yersinia, or Clostridium difficile. Further information about the relevance of culture and PCR results is contained below in the section Infectious Enteritis.
Step 4: Blood and Urine Testing
Routine Tests
Investigations begin by assessing if the diarrhea is the result of primary gastrointestinal disease or secondary to another process, by performing routine serum/plasma biochemistries, hematology, urinalysis, and total T4 (for older cats). In most cases of secondary gastrointestinal disease, diarrhea is not usually the primary presenting complaint, but since the approach to investigations and management diverge so much, this is an important step to take. Biochemistry and urine tests may also show the consequences of diarrhea, such as dehydration and electrolyte abnormalities.
Hematology can be normal in some cats, with changes expected, and so should not be used to rule out any condition. It can be useful, for example, if there is a left shift neutrophilia, indicating acute infection, or eosinophilia, reflecting parasitism. Monocytosis can suggest chronic disease that was not suggested by the clinical history.
The Unwell Cat with Acute Onset Diarrhea
In the case of acute onset diarrhea, the cat may be unwell as a consequence of the diarrhea (e.g., from dehydration) and not because of the cause of the diarrhea. If rehydration is required (with intravenous or subcutaneous fluids, depending on severity of illness), then it is important that biochemistry tests are performed before fluid administration so that any diagnostic clues are not lost by alteration of the profile from the fluid therapy. Fever and neutrophilia may indicate the need for antibiotic therapy. If infection is suspected, fecal sampling (see Step 4) should occur before starting antibiotics. If a cat is unwell from dehydration, then further testing may not be warranted. The clinician should be alert that linear foreign bodies can result in diarrhea (see the section Intestinal Obstruction).
The Well Cat with Chronic Diarrhea but No Weight Loss
Diarrhea of chronic duration (greater than 3 weeks) does require a more thorough investigation at the outset. However, if clinically well, the cat can be managed as an outpatient in the first instance, at least while waiting for results of diagnostic testing. A diet trial with a novel protein is appropriate for a well cat with stable weight. As with any patient managed as an outpatient, follow-up is vital and, in this scenario, includes scheduling revisits.
Blood Tests for Gastrointestinal Disease
Cobalamin, folate, feline trypsin-like immunoreactivity (fTLI), and feline pancreatic lipase immunoreactivity (fPLI) are useful markers of intestinal and pancreatic disease,14, 15, 16, 17 but it is important to note that they typically do not give a precise diagnosis.
Cobalamin and folate are water-soluble vitamins and are readily found in commercial cat foods so that dietary insufficiency is rare, and decreased levels are almost always because of GI disease. These vitamins are taken up by specific receptors in different areas of the small intestine. Chronic inflammatory gastrointestinal disease may damage the receptors and lead to decreased serum concentrations of one or both vitamins, provided the disease process is severe and long standing enough to deplete body stores. Serum cobalamin and folate concentrations may also be decreased in cats with exocrine pancreatic insufficiency (EPI).
Trypsin-like immunoreactivity is a pancreas-specific marker, and assessment of serum TLI is used for diagnosis of EPI and pancreatitis in the cat, although the sensitivity of the assay for pancreatitis is low. PLI is a marker for pancreatic inflammation and is more sensitive than TLI for the diagnosis of pancreatitis. Since inflammation of the small intestine may be seen concurrently with pancreatitis, serum TLI and PLI are useful adjunctive tests in the diagnosis of diarrhea.
TLI, PLI, and cobalamin are stable in serum at room temperature for several days, but folate is unstable so that samples for cobalamin/folate analysis should be frozen (Table 23-14 ). Samples submitted for folate concentration should not be hemolyzed, because red blood cells contain high levels of folate. In addition, folate is light-sensitive, and samples should be wrapped to exclude light. Severe lipemia may interfere with common assays for TLI and PLI.
TABLE 23-14.
Sample Handling Factors for Tests Used to Assess Gastrointestinal Function
| Parameter | Cobalamin | Folate | fTLI | fPLI |
|---|---|---|---|---|
| Stable at room temperature? | Yes | No | Yes | Yes |
| Hemolysis interferes? | No | Yes | No | No |
| Lipemia interferes? | No | No | Yes | Yes |
| Fasting required? | No | No | Yes (12-18 h) | Yes (12-18 h) |
| Species-specific? | No | No | Yes | Yes |
fPLI, Feline pancreatic lipase immunoreactivity; fTLI, feline trypsin-like immunoreactivity.
The main utility of these tests are
-
1
To indicate that further investigation of gastrointestinal disease is warranted.
When a cat presents for weight loss with no overt signs of GI disease, decreased cobalamin or folate can indicate that further investigations with imaging and, ultimately, biopsy sampling are warranted. Many clients are more willing to proceed with invasive diagnostics when a specific marker of the disease in the organ involved has been recognized. Caution should be exercised, because either cobalamin or folate may not be reduced with GI disease. In one study of small cell lymphoma, only 78% of cats were hypocobalaminemic,7 meaning that if this was the only instigating factor to investigate, nearly one fourth of cats would not have been investigated further. Also, cobalamin may be reduced in non-alimentary illness.1
-
2
To detect hypocobalaminemia that may indicate the need for supplementation for clinical improvement.13
-
3
To recognize pancreatic pathology when fPLI is increased.17 It is important to note that an elevated value gives no indication of the nature of the pancreatic pathology.
-
4
To make a diagnosis of EPI when the fTLI is low.15 It should be noted that EPI can result from other pathology that may require further investigations.
Step 5: Imaging
Radiology alone is seldom useful in cats with diarrhea. Plicated intestines can indicate linear foreign bodies and masses may be recognized. In contrast, ultrasonography is a very useful modality to assess intestinal wall thickening and to distinguish the five layers of the intestinal wall. Abdominal masses can be assessed to determine if they are focal, mural intestinal thickening, or lymph nodes (or of other origin). All intraabdominal organs should be assessed to determine if there is multiorgan disease (even if not indicated by biochemistry results). Despite its utility, it must be recognized that a diagnosis cannot be reached by ultrasonography alone. Fine-needle aspiration (or Tru-Cut biopsy) of discrete masses is required, and if no masses are present, biopsy samples are required, using either endoscopy, laparotomy, or laparoscopy.
Ultrasonography can be considered as a means to “survey the field,” assessing
-
•The nature of the underlying disease, such as
-
•Thickened intestines with or without discrete layers
-
•Lymph node involvement
-
•Other organ involvement
-
•
-
•Location of disease, for example,
-
•Diffuse or focal
-
•Proximal duodenum (reachable by endoscope) versus distal ileum
-
•
These factors may be used to assess the appropriateness of endoscopy versus laparotomy to obtain diagnostic samples.
Step 6: Intestinal (and other Organ) Biopsy
This diagnostic step coincides with the section Approach to the Vomiting Cat and is covered in that section.
References
- 1.Barron P, Mackie J, Evans N. Serum cobalamin concentrations in healthy cats and cats with non-alimentary tract illness in Australia. Aust Vet J. 2009;87:280. doi: 10.1111/j.1751-0813.2009.00449.x. [DOI] [PubMed] [Google Scholar]
- 2.Dryden MW, Payne PA, Ridley RK. Comparison of common fecal flotation techniques for the recovery of parasite eggs and oocysts. Vet Ther. 2005;6:15. [PubMed] [Google Scholar]
- 3.Dryden MW, Payne PA, Smith V. Accurate diagnosis of Giardia spp and proper fecal examination procedures. Vet Ther. 2006;7:4. [PubMed] [Google Scholar]
- 4.Gookin JL, Birkenheuer AJ, Breitschwerdt EB. Single-tube nested PCR for detection of Tritrichomonas foetus in feline feces. J Clin Microbiol. 2002;40:4126. doi: 10.1128/JCM.40.11.4126-4130.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gookin JL, Foster DM, Poore MF. Use of a commercially available culture system for diagnosis of Tritrichomonas foetus infection in cats. J Am Vet Med Assoc. 2003;222:1376. doi: 10.2460/javma.2003.222.1376. [DOI] [PubMed] [Google Scholar]
- 6.Greene CE. Salmonellosis. In: Greene CE, editor. Infectious diseases of the dog and cat. ed 3. Saunders Elsevier; St Louis: 2006. p. 355. [Google Scholar]
- 7.Kiselow MA, Rassnick KM, McDonough SP. Outcome of cats with low-grade lymphocytic lymphoma: 41 cases (1995-2005) J Am Vet Med Assoc. 2008;232:405. doi: 10.2460/javma.232.3.405. [DOI] [PubMed] [Google Scholar]
- 8.Lassen ED. Laboratory investigation of digestion and intestinal absorption. In: Thrall MA, editor. Veterinary hematology and clinical chemistry. ed 1. Lippincott Williams & Wilkins; Baltimore, Maryland: 2004. p. 387. [Google Scholar]
- 9.Marks SL, Hanson TE, Melli AC. Comparison of direct immunofluorescence, modified acid-fast staining, and enzyme immunoassay techniques for detection of Cryptosporidium spp in naturally exposed kittens. J Am Vet Med Assoc. 2004;225:1549. doi: 10.2460/javma.2004.225.1549. [DOI] [PubMed] [Google Scholar]
- 10.Mekaru SR, Marks SL, Felley AJ. Comparison of direct immunofluorescence, immunoassays, and fecal flotation for detection of Cryptosporidium spp. and Giardia spp. in naturally exposed cats in 4 Northern California animal shelters. J Vet Intern Med. 2007;21:959. doi: 10.1892/0891-6640(2007)21[959:codiia]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 11.Milewski LM, Khan SA. An overview of potentially life-threatening poisonous plants in dogs and cats. J Vet Emerg Crit Care. 2006;16:25. [Google Scholar]
- 12.Miller P, Peters B. Overview of the public health implications of cockroaches and their management. NSW Public Health Bulletin. 2004;15:208. doi: 10.1071/nb04046. [DOI] [PubMed] [Google Scholar]
- 13.Ruaux CG, Steiner JM, Williams DA. Early biochemical and clinical responses to cobalamin supplementation in cats with signs of gastrointestinal disease and severe hypocobalaminemia. J Vet Intern Med. 2005;19:155. doi: 10.1892/0891-6640(2005)19<155:ebacrt>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 14.Simpson KW, Fyfe J, Cornetta A. Subnormal concentrations of serum cobalamin (vitamin B12) in cats with gastrointestinal disease. J Vet Intern Med. 2001;15:26. doi: 10.1892/0891-6640(2001)015<0026:scoscv>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Steiner JM, Williams DA. Serum feline trypsin-like immunoreactivity in cats with exocrine pancreatic insufficiency. J Vet Intern Med. 2000;14:627. doi: 10.1892/0891-6640(2000)014<0627:sftiic>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Steiner JM, Wilson BG, Williams DA. Development and analytical validation of a radioimmunoassay for the measurement of feline pancreatic lipase immunoreactivity in serum. Can J Vet Res. 2004;68:309. [PMC free article] [PubMed] [Google Scholar]
- 17.Suchodolski JS, Steiner JM. Laboratory assessment of gastrointestinal function. Clin Tech Small Anim Pract. 2003;18:203. doi: 10.1016/S1096-2867(03)00075-6. [DOI] [PubMed] [Google Scholar]
- 18.Zarchi AAK, Vatani H. A survey on species and prevalence rate of bacterial agents isolated from cockroaches in three hospitals. Vector Borne Zoonotic Dis. 2009;9:197. doi: 10.1089/vbz.2007.0230. [DOI] [PubMed] [Google Scholar]
Diseases of the Intestines
Inflammatory Bowel Disease
Inflammatory bowel disease (IBD) refers to intestinal inflammatory infiltrates of the small or large intestine (or both) of unknown etiology. The term IBD should strictly be applied to mean idiopathic IBD, thus excluding inflammatory enteritis because of food sensitivities, although common usage has led to IBD referring to intestinal inflammatory infiltrates of both known and unknown causes. IBD is not a diagnostic end point but a description of a series of intestinal diseases that have similar histopathology. Recent efforts by the World Small Animal Veterinary Association (WSAVA) Gastrointestinal Standardization Group have led to both diagnostic and classification guidelines35, 168, 169 that encompass chronicity, nonresponse to symptomatic treatment, no specific cause found, as well as histologic confirmation of non-neoplastic intestinal inflammatory changes.
Clinical Presentation
There are no obvious breed or gender predispositions, and although cats of any age can be affected, inflammatory intestinal diseases are more likely to occur in middle-aged to older cats (5 to 10 years of age or older) than in younger cats. Presenting clinical signs include vomiting, diarrhea, and weight loss with increased or decreased appetite. These signs can occur in isolation or together. Weight loss without vomiting or diarrhea deserves special mention because not only have several studies38, 58 shown this to be the most common presenting sign for IBD, but many veterinarians do not consider primary intestinal disease without the presence of vomiting or diarrhea. Weight loss despite normal to increased caloric intake can represent poor absorption of food because of small intestinal disease, although it can also represent maldigestion associated with exocrine pancreatic insufficiency or increased metabolism associated with hyperthyroidism, or even lack of energy utilization associated with diabetes mellitus. Conversely, appetite may be reduced, most likely because of nausea. If the large bowel is affected, signs are typically discomfort when defecating, resulting in frequent small volumes of diarrhea, often with mucus and blood; if the large bowel alone is affected, there may be no weight loss.
Physical examination findings are often nonspecific, but the most consistent findings for small intestinal disease are weight loss (or being underweight in a cat not seen previously) and palpably “thickened” intestines. Noting a cat as underweight can be subjective, and prior recorded weights (even from previous veterinarians if the patient is new to the practice) are usually helpful. Body condition scoring (using a 5-point or 9-point scale) for every cat seen is helpful in recognizing those that are underweight. Weight loss often occurs with loss of muscle mass in cats, and muscle mass can be assessed over the ribs and pelvis as well as scapulae and nuchal crest. Thickened intestines are also a subjective finding; it is the author's opinion that thickened intestines are actually intestines with increased turgidity, since differences between normal intestines and those with inflammatory infiltrates can be as little as 0.5 mm.
Perhaps more important during the history taking and physical examination are those signs that can point to extragastrointestinal disease. When confronted with a cat showing weight loss or vomiting or diarrhea (or a combination of signs), the clinician should start with trying to distinguish the signs as being either primary gastrointestinal or secondary signs. Examples of clues pointing to extragastrointestinal diseases include tachycardia and palpable thyroid nodule, indicating hyperthyroidism, or polydipsia/polyuria, which has a variety of causes but is not typical of primary intestinal disease.
Pathophysiology
Inflammatory bowel disease has traditionally been considered an immune-mediated disease. The local immune system of the intestinal mucosa no doubt plays an important role, but recent work has also shown the importance of the normal bacterial population in perpetuating and, perhaps, even initiating pathology. It is known for certain that IBDs are an expression of an overanxious immune response, with a recent study104 indicating increases in inflammatory (IL-6), type-1 immunity (IL-12 p40), and immunomodulatory (transforming growth factor [TGF]-beta, IL-10) cytokines. Other researchers have found an association with bacterial counts (Enterobacteriaceae, E. coli, and Clostridium spp.) and abnormalities in mucosal architecture, indicating that mucosal bacteria are involved in the etiopathogenesis.65
We can summarize these theories by saying that IBDs are likely to be a consequence of hypersensitivity reactions to antigens from the intestinal lumen (e.g., bacterial, parasitic, or dietary antigens). This hypersensitivity may occur because of failed immunoregulation (suppressive function) of the gut-associated lymphoid tissue (GALT). It is known that granulomatous colitis in Boxer dogs is associated with infection,143 and pathogens may well be found in at least some cases of IBD in cats that cause the immune response and subsequent inflammatory infiltrate of the lamina propria typically seen. Although not described specifically in cats, chronic intestinal inflammatory change can impair motility.
Diagnosis
Investigations should follow the guidelines presented elsewhere in this chapter (see Approach to the Vomiting Cat and Approach to the Cat with Diarrhea), even if the cat is not showing signs of vomiting or diarrhea. These investigations culminate in obtaining biopsy samples, either endoscopically or with full-thickness samples taken during laparotomy (Figure 23-18 ) or laparoscopy.
FIGURE 23-18.
Appearance of the small intestine of a cat with inflammatory bowel disease (IBD) at surgery. Note the turgidity of intestine that remains in arched position. This is an extreme case, and in many cases the intestine can look grossly normal.
There are no typical laboratory findings in IBD, and many cats may have entirely normal results from routine biochemical and hematologic investigations. Moderate liver enzyme elevations may be seen5, 38, 58, 66 even in the absence of recognizable hepatic pathology, and this may reflect subclinical secondary hepatic disease, secondary cholestasis, or showering of the liver with inflammatory cells from the small intestine through the portal circulation.66 Other changes can reflect consequences of the intestinal disease, such as azotemia or hemoconcentration reflecting dehydration,58 or hypokalemia reflecting inappetance.38 The chronic inflammation may be reflected by neutrophilia, monocytosis,5, 38, 58, 66 or hyperglobulinaemia.38 Hypocobalaminemia can reflect ileal inflammation, and low serum folate can reflect proximal small intestinal inflammation.149
Typical ultrasonographic findings consistent with IBD are focal or diffuse intestinal wall thickening (Figure 23-19 ); normal wall thickness is less than or equal to 2.8 mm for the duodenum and less than or equal to 3.2 mm for the ileum,50 and large mesenteric lymph nodes with hypoechoic changes may be seen. One study found that ultrasonographic findings correlated with histologic grade of IBD.5 There is no clear distinction between ultrasonographic changes from IBD and those from small cell lymphoma. One recent paper has suggested that ultrasonographic thickening of the muscularis layer is more likely in cats with intestinal small cell lymphoma than those with IBD, but this change was also seen in 12% of cats with normal small intestine.177
FIGURE 23-19.
Ultrasonographic image of a cat with inflammatory bowel disease (IBD); the duodenal wall measurements at the two locations numbered D1 and D2 are 3.8 and 3.2 mm, respectively, compared with normal measurements of less than or equal to 2.8 mm. Note that most of the thickening is of the mucosal layer. The layers (from center) are lumen (white), mucosa (black), submucosa (white), muscularis (black), serosa (white).
Inflammatory bowel diseases require histologic findings obtained from biopsy samples for diagnosis, but diagnosis should not be made solely on these findings. The WSAVA International Gastrointestinal Standardization Group has proposed “An All Encompassing Definition of Inflammatory Bowel Disease” that comprises clinical criteria, imaging criteria, as well as pathophysiologic criteria.169
Clinical Criteria
The clinical criteria for the diagnosis of IBD include
-
1
Chronic duration (>3 weeks) of gastrointestinal signs, including vomiting, diarrhea, and weight loss
-
2
Nonresponse to symptomatic therapy alone (such as parasiticides, antibiotics, and dietary trials)
-
3
No specific cause after thorough investigation
-
4
Histologic confirmation of non-neoplastic intestinal inflammatory changes
These distinctions are not always straightforward to make for either the clinician or the pathologist reading the histology! The most common inflammatory infiltrates30, 66, 74, 80, 171 are
-
1
Lymphocytic/plasmacytic (70% to 100% of cases)
-
2
Eosinophilic
-
3
Neutrophilic
-
4
Mixed inflammatory
-
5
Granulomatous
Clinicians often consider the assessment of histologic samples to be out of their hands; however, it is important to work with the pathologist by providing good quality samples and a good clinical history, as well as having an open dialogue if the findings are not within expectations.
For example, with lymphocytic/plasmacytic infiltrations, the pathologist has the difficult task of distinguishing diseased from normal tissue in a site that is laden with lymphocytes in the healthy state. Once deciding the tissue has pathology, the pathologist's next task is distinguishing inflammatory infiltrate from neoplastic infiltrate with normal, mature lymphocytes (as seen in small cell lymphoma). Inflammatory change also results in changes to normal tissue architecture, with thickened villi, edema, or erosion of the epithelium being typical changes. Clinicians should expect morphologic descriptions as well as assessments of degree and type of inflammation.
These difficulties are further compounded with the recognition that histologic grading of mild, moderate, or severe does not necessarily correlate with severity of clinical signs. This means that a cat with severe clinical signs of weight loss and vomiting or diarrhea may have only mild histologic changes (and vice versa).
Other Organs Concurrently Affected
Concurrent inflammation of the pancreas and liver with intestinal inflammation was first described in the mid-1990s,171 and despite constant reference to this phenomenon at conferences and veterinary websites, there has been little description since then, though one study found 70% of IBD cases had liver inflammation and 30% had pancreatic inflammation.6 The term “triaditis” has frequently been used, but the author prefers to spell this “tri-iditis” to distinguish it from inflammation of the hepatic portal triads. There has been no assessment of prognosis when the pancreas and/or liver are involved, but the author has found no difference in prognosis.
Dietary Therapy
Many cases diagnosed with intestinal inflammatory infiltrates have these changes because of dietary sensitivity. In one study, 29% of cats with histologic gastrointestinal changes improved with dietary elimination therapy alone. Interestingly, improvement was noted within 4 days compared with the longer duration of 8 weeks often recommended for improvement of dermatologic manifestations of food sensitivities. This careful study made note of the cat's prior diets and likely dietary causes of sensitivities.55 Another study found dietary therapy to be unsuccessful in 52 of 60 cats but no specifics of diets tried are noted.58 As with any therapeutic trial, follow-up visits are vitally important. Many cats with small intestinal disease may show initial improvement simply because of the diet having lower residue, since there is decreased substrate for intestinal bacteria to digest and lower osmotic potential. The corollary of this is that failure of one novel protein diet does not mean that all novel protein diets will fail.
When food sensitivities are responsible for gastrointestinal clinical signs in cats, the responsible food ingredient is usually a dietary staple. Commonly incriminated ingredients are beef, fish, wheat, and corn gluten.55 A careful dietary history is therefore important. Large bowel inflammation typically improves with higher-fiber diets,38, 58 and attempting a trial with such a diet is certainly appropriate.
Drug Therapy
Immune suppressive therapy is the mainstay of IBD treatment, and glucocorticoids, such as prednisolone, are most commonly used. Sulfasalazine use for large bowel signs has not been critically evaluated but seems safe and effective.
In cats with substantial weight loss or severe clinical signs, such as chronic diarrhea, the author prefers to start with corticosteroid therapy, even if dietary causes have not yet been ruled out. The diet should also be changed to one containing a novel protein, and, if and when clinical signs resolve, an attempt is made to wean the cat from corticosteroid therapy, hopefully to the point of being discontinued. A diet challenge can then be used to confirm the diagnosis of food sensitivity.
There are no universal guidelines for doses of corticosteroids. The author prefers the use of orally administered prednisolone to reduce the chance of side effects and will choose the starting dose based on the severity of disease. The starting dose is usually 2 mg/kg, once daily, PO (10 mg/cat/day for most cats) starting 10 days after biopsies have been obtained to allow time for the mucosa to heal. If there is an improvement noted after a recheck at 2 weeks, the higher dose is maintained for a further 2 to 4 weeks, at which point, many cats are back to their normal weight and are not exhibiting clinical signs. If this is the case, the corticosteroid dose can be weaned down to 1 mg/kg, PO (often 5 mg/cat/day) for several months, with continued rechecks scheduled to assess weight, clinical signs, and diet. The goal is to wean down to the lowest effective dose.
If hypocobalaminemia is present, cobalamin supplementation may be required.144 Cobalamin is administered parenterally at 250 µg/cat subcutaneously weekly for 6 weeks, then every second week for 6 weeks, then monthly. Owners can be shown how to inject their cats (as practitioners routinely do with diabetics).
Some cats seem resistant to conventional therapy. If this is the case, the diagnostic findings should be re-assessed to ensure no steps were missed or findings disregarded; the cat should be reexamined to look for emergence of other signs; and the pathologist who reads the histology should be contacted to recheck the findings. Some cases of apparently resistant IBD are actually food sensitive, but it can be difficult to find the incriminating diet source, and commercial diets are not always effective. If underlying infectious causes have been entirely ruled out and the practitioner is certain of the diagnosis of idiopathic disease, immune suppressive therapy can be increased by either increasing the dose of prednisolone or using other agents, such as chlorambucil, typically at 2 mg/cat, PO, every second day. It has been suggested that cats with eosinophilic inflammation may be more likely to be refractory to standard therapy. Side effects of immunosuppressive therapy are rare but include inducing diabetes mellitus, immune suppression, delayed healing, and gastrointestinal ulceration.
Reported doses of sulfasalazine to manage large bowel IBD are 10 to 20 mg/kg, PO, once daily for 7 to 10 days.174 Because this drug is usually only available as 500 mg tablets, one eighth of a tablet, providing a dose of 62.5 mg, is usually appropriate for most cats. In some countries, it is possible to have a compounding pharmacist formulate the drug into more convenient tablet sizes or as an oral suspension. Cats are generally regarded as susceptible to salicylates, and possible side effects include vomiting or diarrhea, or anemia. The exact pharmacodynamics of this drug are not known; so, caution for extended use should be exercised and the drug withdrawn if any possible adverse signs are noted, but there are anecdotal reports of extended use of this drug without adverse consequences.
Intestinal Neoplasia
A survey of the online Veterinary Cancer Registry (http://www.vetcancerregistry.com) identified 6% of all submitted feline tumors to be intestinal tumors. Approximately 74% of reported feline small intestinal tumors were lymphomas. Adenocarcinomas accounted for 17%, and other tumor types reported included mast cell tumors and leiomyosarcomas.135
“Lymphoma in veterinary medicine: no longer a one-word diagnosis” was the title of an editorial in a recent issue of the Veterinary Clinical Pathology journal,95 and this is nowhere truer than in the feline gastrointestinal tract! A recent study classified 50 cases of feline gastrointestinal lymphoma both histologically and immunophenotypically, and it found eight different categories according to the Revised European and American Lymphoma/World Health Organization (REAL/WHO) classification system and six categories according to the National Cancer Institute Working Formulation (NCI WF) system.116
For most veterinarians in practice, the most important distinction is the histologic grade, because low-grade (lymphocytic or small cell) lymphoma has a much better prognosis (and requires different treatment) compared with high-grade (often lymphoblastic) or intermediate-grade lymphoma. For the purposes of simplicity and practicality, only small cell lymphoma and high-grade lymphoma will be addressed here. The prognosis and treatment for intermediate-grade intestinal lymphoma should be considered as for high-grade lymphoma.
Intestinal Small Cell (Low-Grade) Lymphoma
Small cell lymphoma was first described in human pathology in 1966.122 Earlier, small lymphocytes were considered end-stage cells without the ability to divide. In cats small cell lymphoma is most commonly associated with the gastrointestinal tract or skin.163
Small cell neoplasia can be a confusing concept, since our traditional ideas of malignant neoplasia focus on rapidly dividing cells. The confusion is compounded by various terms used in the literature, such as lymphocytic lymphoma, low-grade lymphoma, well-differentiated lymphoma, or diffuse lymphoma; another term, epitheliotropic malignant lymphoma predominantly applies to small cell lymphoma, and other papers fail to distinguish these lymphomas from lymphoblastic lymphosarcoma (the traditional, aggressive form). “Small cell lymphoma” seems to be most widely used term, though the author prefers “lymphocytic lymphosarcoma,” since it is more descriptive.
Intestinal small cell lymphoma can be considered as a severe lymphocytic intestinal infiltrate, the most common form of which is commonly called IBD. Not only is lymphocytic IBD hard to distinguish histologically from lymphocytic lymphosarcoma, but the approaches and treatments are similar. Several reports have suggested a relationship between the two conditions in that inflammatory infiltrates may become neoplastic over time.27, 43, 90
Prevalence
The true prevalence of intestinal small cell lymphoma is unknown, but several recent studies have indicated similar rates to inflammatory bowel diseases, with Kleinschmidt et al noting 10 small cell lymphoma cats compared with 14 with intestinal lymphocytic infiltrates,74 Evans et al reporting 10 cases compared with 12 with IBDs40, and Baral et al diagnosing 8 cases compared with 10 with IBDs.6 Traditionally, 90% of feline lymphosarcoma is regarded as intermediate or high grade,163 but this may not be the case within the gastrointestinal tract. Fondacaro et al found 75% of gastrointestinal lymphoma to be lymphocytic43; a more recent paper found approximately equal numbers of high-grade and low-grade gastrointestinal lymphoma.84
Patient Signalment and Risk Factors
Older cats are more at risk of small cell lymphoma, with mean or median ages reported from 9 to 13 years. Younger cats with the disease have, however, been recognized.40, 43, 73, 84 No breed or gender predispositions have been definitively recognized. Two larger studies have suggested a skew to males with 28 males compared with 22 females in one report,43 and 24 males compared with 17 females in the other73; most other studies looking at gender and breed did not clearly distinguish between lymphoblastic and lymphocytic neoplasia.
Clinical Signs
Clinically, it is impossible to distinguish cats with IBDs from cats with small cell lymphoma. This is hardly surprising when even histologic distinction can be difficult! Therefore cats will present with weight loss or vomiting or diarrhea at a similar frequency to those with IBD. Weight loss has been recognized as a presenting sign in 82% to 100% of cases, diarrhea in 25% to 60% of cases, and vomiting in 25% to 73% of cases, with various combinations of these signs also possible. Other variable signs are lethargy and inappetence or, conversely, polyphagia.40, 43, 73, 84 These findings can be summarized by stating that cats with gastrointestinal small cell lymphoma can present with any combination of signs relating to the gastrointestinal tract.
Location and Other Organ Involvement
Intestinal small cell lymphoma is typically a diffuse disease, and therefore multiple areas of the alimentary tract are usually affected. In studies where different locations of the small intestine were assessed, the jejunum was most commonly affected (100%), with the ileum frequently affected (93% to 100%), and duodenal pathology slightly less prevalent (83% to 90%).40, 84 Although the numbers of cats assessed in these studies are small, the important fact that the duodenum is not always affected needs to be recognized, which has important implications for how biopsy samples are obtained, because lesions beyond the duodenum are likely to be beyond the reach of an endoscope. Further difficulties in precise diagnosis may arise, since non-neoplastic lymphocytic infiltrates (e.g., IBD) are often found in other locations along the intestinal tract.27, 40, 84 The stomach is also affected in 14% to 40% of small cell lymphoma cases.40, 43, 84 Although not fully assessed, involvement of the colon appears rare.84
Local lymph node involvement is common, being noted in up to 59% of cases.84 This percentage may be even higher, because many studies assessed lymph node cytology from ultrasound-guided fine-needle aspirates, which may miss spread to the lymph node, because the population of neoplastic lymphocytic cells is indistinguishable from the normal population of lymph node cells. Histology is required to assess changes in lymph node architecture.
Liver involvement is not uncommon but not thoroughly assessed. One study noted liver lymphocytic neoplasia in 8 of 38 cats with small intestinal lymphocytic neoplasia,73 another found 5 of 15 affected cats in which the liver was biopsied,84 another noted 2 of 4 cats had liver involvement,27 and a further study detected neoplasia “in the lymph nodes, liver, or both” in all 10 cats with intestinal small cell lymphoma.40
The pancreas may also be involved.73, 84 This may be akin to the noted association of lymphocytic inflammation of intestine, pancreas, and liver171 that has been dubbed tri-iditis.
Ultrasound findings may not suggest extragastrointestinal involvement. In the case of liver pathology, ultrasonography may show no changes in as many as 75% to 80% of cases.40, 84 Focal nodular changes and hepatomegaly have been recognized as ultrasonographic signs of hepatic small cell lymphoma.7
Pathophysiology
Both lymphocytic IBD and lymphocytic neoplasia are often recognized simultaneously in the same cat,40, 84 and numerous authors have suggested that lymphocytic IBD may be a precursor to intestinal lymphoid neoplasia.90, 125 If this is the case, then antigenic factors, such as bacterial population changes or food sensitivities, could be considered primary initiating factors for small cell lymphoma since they are potential underlying etiologies of IBDs.79 However, neoplasia also requires genetic mutations to occur (often affecting regulation of cell death and cell survival), and these may be initiated by the inciting antigenic factors or the ongoing inflammatory changes.154 As opposed to other feline lymphoid neoplasia, no association has been made with FeLV infection.27, 43, 73, 125
Intestinal lymphocytic lymphosarcoma begins in the superficial mucosa and progresses to involve the entire mucosa and submucosa; then advancing in a perivascular pattern into the tunica muscularis, eventually infiltrating all four intestinal tunics.43 Lymph node and other organ (such as liver or pancreas) involvement likely represent metastasis through lymphatics and perhaps hematogenously. More distant metastasis is not reported.
Diagnosis
Serum or plasma biochemistry and hematologic findings are typically nonspecific. However, this testing is important as part of the diagnostic workup to rule out extra-GI disease, such as hyperthyroidism or diabetes mellitus. Common biochemistry findings are mild to moderate increase of liver enzymes, such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), and/or alkaline phosphatase (ALP).27, 40, 43, 84 As with IBDs, these liver enzyme changes may or may not represent overt hepatic disease.67 Albumin may be reduced43 but is normal in most cases27, 43, 84; azotemia may be present and may be of prerenal origin or represent concurrent renal disease. In one study, 25 of 32 cats were hypocobalaminemic; 1 of 27 cats had low folate, but 10 of 27 had elevated folate; and 12 of 16 cats had increased fTLI.73
Hematologically, a mature neutrophilia with or without monocytosis is sometimes present, representing the inflammatory response; lymphopenia may be present as a stress response. Anemia may be present and may occur as a result of chronic slow GI blood loss, and in some cases, ulceration, or it may be because of chronic disease; hemoconcentration is also possible, reflecting dehydration.27, 40, 43, 84
Palpable or ultrasonographically visible thickened intestines (30% to 41% of cases)27, 40, 43, 84 or mesenteric lymph nodes (20% to 50% of cases)27, 40, 43, 84 are no more or less likely to be present in comparison with IBDs. There are no defined ultrasound guidelines for cats with intestinal small cell lymphoma, because most prior papers do not distinguish between small cell and lymphoblastic neoplasia.54, 113 A more recent paper found 9 of 15 cats undergoing ultrasound examination had diffuse small intestinal wall thickening, with a mean of 4.3 mm (range, 3.4 to 5.0 mm; median, 4.5 mm), and focal mural thickening of 20 mm was noted in one cat.84 In many cases, against expectations, intestinal wall layering was preserved. These findings also mean that 5 of 15 cats had ultrasonographically normal intestinal wall thickness (≤2.8 mm for the duodenum and ≤3.2 mm for the ileum).50 If affected, jejunal lymph nodes may appear as hypoechoic and enlarged; in the same study, 12 of 15 cats had lymph node changes with a mean diameter of 15.9 mm (range, 6.5 to 30 mm; median, 10 mm)84 compared with the normal diameter of less than or equal to 5.0 mm.132 None of these findings can definitively distinguish small cell lymphoma from IBDs; although one recent paper has suggested that ultrasonographic thickening of the muscularis layer is more likely in cats with intestinal small cell lymphoma (Figure 23-20 ) than those with IBD, this change was also seen in 12% of cats with a normal small intestine. However, thickening of the muscularis layer together with lymphadenopathy was recognized in 26% of those cats with small cell lymphoma compared with 4% of those with IBD and 2% of cats with no small intestinal pathology.177
FIGURE 23-20.
Ultrasonographic findings of small cell lymphoma; duodenal wall measurements at the two locations numbered D1 and D2 are 2.9 mm and 3.4 mm, respectively. Note that the prominence of the muscularis propria layer in the second measurement compared with the ultrasonographic appearance of inflammatory bowel disease (IBD) in Figure 23-19, in concordance with findings of Zwingenberger et al.177
Biopsy samples and histopathology are required for definitive diagnosis. An example of jejunal and mesenteric lymph node appearance at laparotomy is shown in Figure 23-21 . As noted for IBDs, it is important to work with the pathologist by providing good quality samples and a thorough clinical history, as well as having an open dialogue if the findings are not within expectations.
FIGURE 23-21.
The appearance of intestinal small cell lymphoma at laparotomy. Note the erythematous and generally thickened intestines and prominent mesenteric lymph nodes.
It is difficult to distinguish between lymphocytic inflammation and small cell lymphocytic neoplasia in any location; some histopathologic features that might help in differentiating the ends of the spectrum may include
-
1
Demonstration of small lymphocyte domination (sometimes referred to as monotonous or monomorphous population) in small cell lymphoma, compared with morphologically mixed cell populations in IBD27, 40
-
2
Infiltration into deeper layers (submucosa and muscle wall) in lymphocytic neoplasia27, 40, 43
-
3
No mucosal congestion, edema, or fibrosis in lymphocytic neoplasia,43 compared with IBD
-
4
Epitheliotropism, or homing of neoplastic T lymphocytes to the mucosal epithelium in lymphocytic neoplasia27
These features can be seen in Figure 23-22 . Each of these criteria may be useful but are unlikely to be definitive. Further studies that may not be routinely available but which may be helpful are
-
1
Immunophenotyping; most reports have found purely T lymphocytes in most cases of intestinal small cell lymphoma27, 73, 84, 116 (Figure 23-23 ).
-
2
Clonality; the detection of a clonal population of cells, as recently described for intestinal lymphocytic lymphosarcoma,101 would be closest to providing the basis for definitive diagnosis.
FIGURE 23-22.
Photomicrograph of small intestinal mucosa in a cat with intestinal small cell lymphoma. There is marked cellular infiltrate of the lamina propria, extending through the lamina muscularis into the submucosa. Villous morphology is distorted by the infiltrate.
FIGURE 23-23.
Photomicrograph of small intestinal mucosa in a cat with intestinal small cell lymphoma. Immunohistochemical labeling with a pan–T-lymphocyte marker reveals positive labeling of the infiltrating cells, consistent with a T-lymphocyte origin. CD3-positive cells can be seen in the mucosal epithelium but no Pautrier abscesses are evident.
Drug Therapy
Effective treatment of feline intestinal small cell lymphoma was brought to light by Fondacaro et al43 and has therefore become known as the Fondacaro protocol. This consists of a combination of prednisolone and chlorambucil given orally by the client at home (Table 23-15 ). The rationale is that a slow alkylating agent, such as chlorambucil, is more appropriate to use for the slowly dividing, well-differentiated lymphocytes that cause disease. This can be contrasted to the aggressive chemotherapeutic agents required for the rapidly proliferating cells in lymphoblastic neoplasia that is typically associated with lymphosarcoma.
TABLE 23-15.
Comparison of Small Cell Lymphoma Protocols, All Drugs Dosed Orally
| Protocol | Chlorambucil | Prednisolone | Median Survival (Months) |
|---|---|---|---|
| Pulsed | 15 mg/m2/24h for 4 days every 3 weeks43 |
10 mg/cat/24h43 or 3 mg/kg/24h84 |
17 (range 0.33-50)43 15 (range 0.5-77)84 |
| Continuous | 2 mg/cat/48h73 or 1 mg/cat/24h |
10 mg/cat/24h73 or 5 mg/cat/12-24h73 |
25 (range 1.5-67)73 |
Reference citations are from the section Diseases of the Intestines.
Reported response rates to this protocol are excellent, with 59% to 76% of cats achieving complete clinical remission, reported median survival times ranging from 20 to 30 months for those cats responding to therapy, and reports of individual cats surviving as long as 76 months.43, 73, 84 The original reported protocol comprised prednisolone (10 mg/cat, PO or 2 mg/kg, PO) given daily with chlorambucil pulsed by administration of 15 mg/m2 for 4 days every 3 weeks. A more recent study73 dosed prednisolone similarly, but chlorambucil was given as continuous therapy of 2 mg/cat, PO every second or third day.
Similar protocols are used in humans with both low-grade (i.e., lymphocytic) lymphosarcoma and chronic lymphocytic leukemia.117, 151 Some studies with humans have indicated that continuous therapy with chlorambucil results in prolonged survival,64 although meta-analyses have not been able to determine optimum dosing and scheduling of administration of chlorambucil or other alkylating agents in these conditions in humans.20, 72
Although we do not have enough data to critically compare pulsed therapy to continuous dosing, the study assessing continuous dosing appeared to have a lower number of cats completely responding, although those cats that did respond had a longer median survival73 than those in the studies assessing pulsed chlorambucil dosing.43, 84 The differences may also relate to the definitions used for complete response. The chlorambucil dose of 2 mg/cat, PO every second day (or third day) is often chosen because of the ready availability of 2 mg coated tablets, the breaking of which can expose the owner to these cytotoxic medications. Chlorambucil can be compounded into smaller doses, thus allowing daily dosing of 1 mg capsules. The author has used this dose to apparent good effect, but there has been no critical assessment.
It is unknown whether involvement of lymph nodes or other organs, such as the liver, affects prognosis. The only study of substantial size to include extra-GI locations found anatomic location was not prognostic for response or survival time.73 In another study, of the five cats with liver involvement, two cats did not survive more than 5 months, yet the other three lived longer than  years, with two surviving longer than
years, with two surviving longer than  years.84 A study of hepatic small cell lymphoma suggests the density of neoplastic lymphocytes may influence survival, and density may relate to the stage of the disease when diagnosis occurs.7
years.84 A study of hepatic small cell lymphoma suggests the density of neoplastic lymphocytes may influence survival, and density may relate to the stage of the disease when diagnosis occurs.7
Adverse effects of chlorambucil are rare, but gastrointestinal signs, myelosuppression, and myoclonus have all been reported. Gastrointestinal signs, such as vomiting, diarrhea, or inappetence, can be difficult to distinguish from continuation of the gastrointestinal disease diagnosed. These signs are usually self limiting. Myelosuppression is also possible with thrombocytopenia reported.57, 84 Monoclonus has been reported on one occasion.17 It is ideal to check hematologic parameters every 2 months for cats receiving chlorambucil. Continuous therapy using lower doses of chlorambucil may be less likely to lead to these adverse effects.
High doses of corticosteroids can induce diabetes mellitus, and thus blood glucose should be checked regularly. If diabetes occurs, the author has found that budesonide (1 mg budesonide is generally considered to be equivalent of 5 mg prednisolone) can be substituted for prednisolone, since it has reputed lower systemic effects (though no assessments of this drug's effectiveness in cats have been made). An alternative is for the cat to be weaned off corticosteroids, with chlorambucil continued as monotherapy (as is often the case with humans). Iatrogenic diabetes mellitus usually needs to be managed with insulin therapy, at least initially (see Chapter 24).
Intestinal High-Grade (Lymphoblastic) Lymphoma
High-grade lymphoma or lymphosarcoma is the traditional style of aggressive, rapidly dividing lymphoid neoplasia that carries a much poorer prognosis than small cell lymphoma.
Prevalence
Most early studies do not distinguish grade of neoplasia; so, the prevalence of low-grade and high-grade alimentary lymphoma are difficult to assess. Several recent studies found a similar prevalence of each,84, 116 but the seminal paper describing small cell lymphoma found only 17 cases of lymphoblastic lymphoma compared with 50 cases of small cell lymphoma.43 This ratio of approximately one high-grade GI lymphoma case for every three low-grade cases more closely approximates the rate found in the author's practice.
Patient Signalment and Risk Factors
The reported median ages of affected cats range from 10 to 12 years, but cats as young as 1 year old have been diagnosed. Most papers note that males are overrepresented, and Siamese cats may also be overrepresented although most affected cats are domestic shorthairs.43, 49, 90, 116, 176 Precise signalment is difficult to determine from the literature, because many papers assess all anatomic locations of lymphoma without necessarily breaking down epidemiologic data for each anatomic site. Also, there are few comparisons to a reference population.
Pathophysiology
The association of lymphoma with FeLV infection is well established and documented139 and is covered in Chapter 28; FIV has also been shown to be lymphomagenic.12, 139 Since the control of FeLV through vaccination began in the 1980s, nonretroviral-associated lymphoid neoplasia has become more common, and the rates of intestinal lymphoma have, in fact, increased since FeLV infection rates have decreased.86 The underlying causes for this increase are not known. The association with inflammation from IBDs was noted for small cell lymphomas, and perhaps there is a spectrum from lymphocytic IBD to small cell lymphoma to high-grade lymphoma. That some cats are more likely to have inflammatory changes become neoplastic is suggested by a paper noting higher lymphoma rates in cats with vaccine-associated sarcomas (a neoplastic condition where the role of chronic inflammation is well noted).89
Whether the underlying cause is retroviral or chronic inflammation or anything else, the pathogenesis of high-grade intestinal lymphoma, as with small cell lymphoma and other neoplasia, depends on chromosomal changes that affect regulation of cell growth and death, resulting in malignant transformation and clonal expansion of immature lymphocytes.152
Metastasis can occur in one third to two thirds of cases,90, 92 with involvement of mesenteric lymph nodes most commonly noted, but spread to liver, spleen, kidneys, and thorax is also possible.90
A recent survey of gastrointestinal lymphoma found that most cases (37 of 50) involved the small intestine (including 3 that also involved the stomach and 4 that also involved the large intestine), and 4 of 50 cases involved the large intestine only.116
Clinical Signs
Cats with high-grade alimentary lymphoma often present similarly to those with other gastrointestinal diseases. Typical clinical signs are weight loss, anorexia, lethargy, vomiting, diarrhea, or a combination of these signs. Repeated studies have found cats with no vomiting or diarrhea; in one study, 13 of 28 cats had only anorexia or weight loss on presentation.90
Cats with large bowel pathology usually present, as with other causes of colitis, with increased urgency, and small, frequent amounts of diarrhea, often with blood or mucus. Cats with large bowel neoplasia of any form can present for constipation caused by intestinal obstruction.
Palpation of an abdominal mass has been recognized in 59% to 85% of cases,43, 90 but the corollary of this is that 15% to 41% of cases did not have a palpable mass. It is also important to note that up to 50% of cats with intestinal small cell lymphoma, and a number with IBDs, have palpable mesenteric lymph nodes; so, a palpable abdominal mass is not a specific indication of high-grade neoplasia. Many cats have palpably thickened bowel loops.124
Diagnosis
Hematology and plasma or serum biochemistry findings are also nonspecific. Increased liver enzymes may or may not indicate liver involvement. Anemia may be recognized and can be non-regenerative, reflecting chronic disease or slow blood loss, or regenerative if there is more substantial blood loss associated with mucosal ulceration. Hypoalbuminemia can be because of blood loss or intestinal protein loss. Hypercalcemia of malignancy is a possibility but not commonly reported. Despite nonspecific signs, laboratory testing is important to rule out extra-GI diseases and help manage consequences of enteric disease, as with small cell lymphoma and IBDs.
Ultrasonography commonly shows a focal intestinal thickening (of 5 to 25 mm) with partial or complete loss of distinction of intestinal layering as shown in Figure 23-24 . The area of lymphomatous infiltration is hypoechoic, because it contains a uniform cell population without much reactionary fibrous tissue. Mesenteric lymphadenomegaly is common (Figure 23-25 ), as are changes in other organs, such as kidney, liver, or pancreas. Ascites may also be seen.54, 113 Although ultrasonographic distinctions predominate, there is considerable overlap between ultrasonographic findings with small cell lymphoma and high-grade lymphoma. The clinician must not lose sight of the fact that microscopic distinctions are required to diagnose either condition.
FIGURE 23-24.
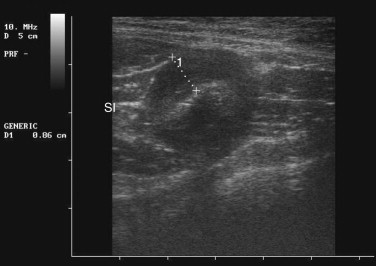
Ultrasonographic image of intestinal lymphoblastic lymphoma; the intestinal wall measurement is 8.6 mm. Note the total loss of layering.
FIGURE 23-25.
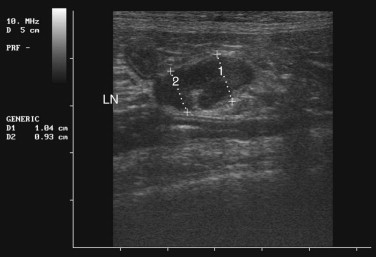
Ultrasonographic image of lymph node of a cat with intestinal lymphoblastic lymphoma, measurements are 10.4 mm and 9.3 mm (normal lymph nodes are <5 mm).
Cytologic diagnosis of high-grade lymphoma from fine-needle aspirates (FNA) is much more likely than with small cell lymphoma. This is because there is usually a focal lesion, and the neoplastic cells are a monomorphic population of large, immature cells (i.e., that are not normally seen in tissue). Sometimes, mixed lymphoid populations (of immature lymphoblasts and mature lymphocytes) are seen if a germinal lymphoid follicle is aspirated, and precise diagnosis may be difficult if there are a large number of lymphoblasts.160 FNA samples are best obtained with ultrasound guidance. The cytologic sample quality is greatly improved by not aspirating when the needle is visualized in the mass but merely “pecked” into the mass so that the needle is merely acting to finely “core” the mass. On removing the syringe and needle, the hub of the needle is removed before drawing air into the syringe, the hub is replaced, and the sample within the needle is expressed onto a slide.
Usually, the decision to diagnose by cytology from FNA is based on the ultrasonographic appearance of a mass. Since there is substantial crossover of ultrasonographic appearance of intestinal masses, laparotomy for excision is often performed with the affected bowel submitted for histology. Except when intestinal obstruction has resulted, there is no therapeutic benefit of excising a gastrointestinal lymphoma (which requires excision and anastomosis), but there is minimal room for doubt when a histologic diagnosis is achieved.
Chemotherapy
The response to therapy for high-grade intestinal lymphoma is significantly worse than that for small cell lymphoma.43, 124 Further, response to therapy for high-grade intestinal lymphoma appears to be worse than for lymphoma in other anatomic locations.142 Precise remission rates and survival times are difficult to quantify, because many studies assess lymphoma from multiple locations and do not necessarily differentiate response of gastrointestinal lymphoma or report the grade of lymphoma. With remission rates reported from 18% to 80%43, 92, 176 and a median survival time of up to 41 weeks (range, 4 to 120 weeks),176 it can be said with some certainty that some cats respond to therapy for reasonable durations. Multiple authors have noted that the best prognostic indicator is response to an initial treatment cycle,92, 100, 176 which should prompt clinicians to encourage owners to start therapy and decide whether to continue based on the cat's response.
There are several published chemotherapeutic protocols,43, 92, 100, 142, 176 but all follow the same principles of using medications to target specific phases of the cell division cycle (such as L-asparaginase and vincristine) with other medications that interrupt multiple phases of the cell cycle (cyclophosphamide and doxorubicin). Targeting the cancer cell in different ways enables more cells to be killed, reduces the toxicity of the individual drug used, and reduces the likelihood of resistance to a specific drug. Several authors have noted increased success with the addition of L-asparaginase and doxorubicin to protocols.92, 162, 176
Chemotherapy for lymphoma is covered in more detail in Chapter 28, Oncology.
Adenocarcinoma
Although noted as the next most common intestinal neoplasia, after the various forms of lymphoma, adenocarcinoma is seen relatively infrequently in practice. Most cats are more than 10 years old,32, 76, 126 males may be overrepresented, and several studies have recognized a distinct overrepresentation of Siamese cats.32, 76
Three distinct forms have been described140:
-
1
Infiltrative: characterized by a thickened, annular, stenotic lesion (Figure 23-26 ) that ultimately results in intestinal obstruction
-
2
Ulcerative: characterized by a deep, indurated mucosal ulcer with raised edges
-
3
Proliferative: characterized by a lobulated, expanding intestinal mass
FIGURE 23-26.
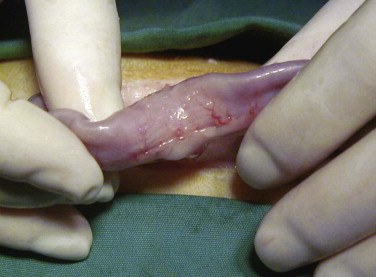
Appearance of the infiltrative form of intestinal adenocarcinoma at surgery; the thickened, annular, stenotic lesion can be seen.
Cats typically present with nonspecific signs of gastrointestinal disease but can present with obstructive signs. Cats with large bowel neoplasia can present for tenesmus or hematochezia and even constipation, if the lesion is obstructive (or partially obstructive). On physical examination, an abdominal mass is palpable in approximately 50% of cases, but other findings are usually nonspecific. Anemia can be found if mucosal ulceration has occurred, but there are no distinctive laboratory findings.
Lesions can occur anywhere along the intestinal tract. One study of 100 cases found 40% of feline intestinal adenocarcinoma lesions were present at the ileum or ileocolic junction.32 Twenty-five to 50 percent of cases have metastasis at the time of the diagnosis, and this is a poor prognostic indicator.32, 76, 145
Radiology may show a mass lesion or intestinal obstruction, and ultrasonography can localize lesions to an intestinal origin. The ultrasonographic appearance of the proliferative, circumferential, outwardly expansile form is better described than the annular, constricting-band form with minimal outward enlargement. In these cases, sonographically, a solitary segmental intestinal mural mass is present and characterized by circumferential bowel wall thickening with transmural loss of normal sonographic wall layers. The thickening can vary in echogenicity but may be hypoechoic and may be symmetric or asymmetric. There is no definitive distinction, however, from lymphosarcoma, mast cell tumor, smooth muscle origin tumors, or even segmental benign inflammatory bowel disease.126
Surgical resection is the treatment of choice. There seem to be two distinct groups in terms of survival time postresection:
-
1
Short-duration survival (euthanasia or death within 2 weeks of surgery)
-
2
Long-duration survival (mean survival time of 15 months,76 with a number of cats surviving greater than 2 years)76, 109
Because clean margins improve prognosis, for large bowel adenocarcinoma, subtotal colectomy may be required for complete excision.145 Because of the potential for success after resection, it is recommended to excise unidentified masses at the time of surgery.145
Other Intestinal Neoplasia
Other forms of intestinal neoplasia are recognized infrequently, and include intestinal mast cell tumors, adenomatous polyps, eosinophilic sclerosing fibroplasia, gastrointestinal stromal tumors (leiomyosarcoma), and hemangiosarcoma.
Intestinal Mast Cell Tumors
Mast cell tumors (MCT) are often cited as the third most common form of feline gastrointestinal tumor,85 but the intestines are a far less common site than cutaneous, splenic, or hepatic mast cell neoplasia.85, 124 Masses are usually segmental nodular thickenings that occur in older cats.140 The masses are indistinguishable ultrasonographically from other tumors, such as lymphoblastic lymphosarcoma.126 A recent series of 50 cases described a variant of feline intestinal mast cell tumor, dubbed sclerosing mast cell tumor, for which neoplastic cells form a trabecular pattern with dense stromal collagen. Additionally, eosinophilic infiltrates were moderate to marked in most cases. These cases can be confused histologically with eosinophilic enteritis, gastrointestinal stromal tumor, or fibrosarcoma.56 Surgical resection is recommended, but lesions are commonly infiltrative or metastasize widely, and there are few reports of successful treatment. Lomustine (dosed 50 to 60 mg/m2, PO every 4 to 6 weeks) has recently been assessed as adjunctive chemotherapy for mast cell neoplasia in various locations,123 and results look promising, but only two cats assessed had gastrointestinal mast cell neoplasia. Lomustine was used unsuccessfully in one cat with sclerosing MCT; another cat with sclerosing MCT received eight treatments of vinblastine and had a survival time of greater than 4 years.56
Adenomatous Polyps
Adenomatous polyps have been reported in the duodenum87 and ileum106 and can result in intussusception.133 Cats of Asian ancestry, predominantly Siamese, are greatly overrepresented, and most reported cases have been males.87 Cats usually present for vomiting or hematemesis that, surprisingly, can be very acute in onset; complete intestinal obstruction may result.87, 106, 133 Resection is curative, with survival times of more than 4 years reported.87
Gastrointestinal Eosinophilic Sclerosing Fibroplasia
Eosinophilic sclerosing fibroplasia has recently been described in a series of 25 cases and is not strictly neoplasia.29 The ulcerating mass lesions that can occur anywhere from the stomach to the colon are often grossly and histologically mistaken for neoplasia. There appears to be no breed predisposition or age predisposition (with ages ranging from 14 weeks to 16 years), but 18 of 25 cases (72%) were castrated males cats compared with 7 of 25 (28%) female spayed cats. Eighty-four percent of cats presented for vomiting, 68% presented for weight loss, and 7 of 12 (58%) cats had peripheral eosinophilia. All cases had a palpable abdominal mass. The pyloric sphincter was the most common site, and lesions in this location were mostly considered unresectable. Fourteen of 25 cats (56%) had bacterial colonies within microabscesses and necrotic foci within the lesion. The bacteria recognized were predominantly gram-negative rods, but antibiotics did not seem to be clinically effective. The bacteria are suspected to initiate the lesions, having been embedded after foreign body penetration. There are no specific treatment recommendations, but excision, where possible, would be prudent; corticosteroids appear to be helpful adjunctive therapy. Survival times are difficult to estimate since many cats were euthanized because neoplasia was suspected and follow-up times were short (up to 6 months) for the remaining cats.29
Gastrointestinal Stromal Tumors (Leiomyosarcoma)
There are very few reports of intestinal leiomyosarcomas in cats,8, 159 which have been reclassified as gastrointestinal stromal tumors.99 These tumors may be more likely to arise from the ileocecocolic junction. Resection, if possible, is usually recommended, with survival times of 3 to 4 months reported before recurrence. The author owns a cat with this tumor where resection was not possible, and the cat appears healthy 24 months past diagnosis (Figure 23-27 ).
FIGURE 23-27.
Ultrasonographic image (A) and photomicrograph (B) of a cat with gastrointestinal stromal tumor (leiomyosarcoma). The ultrasonographic image (A) demonstrates that notable thickening of both mucosal and muscularis layers. The histology (B) shows spindle cell proliferation extending between the smooth muscle bundles of the tunic muscularis.
Hemangiosarcoma
Cats with intestinal hemangiosarcoma often present with anemia, and the disease appears to be highly metastatic.33 The intestines appear grossly thickened by dark red tissue.138 The small and large intestines seem to be affected with similar frequency. Removal of macroscopic disease is recommended, but often the full extent of the severity is only recognized at surgery.33 The prognosis is poor.
Infectious Enteritis
Approach to Diagnosis
Suspicions of infectious causes of diarrhea should be aroused in younger cats, cats from shelters, or cats with immune suppression. When considering infectious causes of diarrhea, clinicians should assess whether the diarrhea is large bowel or small bowel in origin and correlate this with specific pathogens that are likely to cause clinical signs as shown in Table 23-16 . To increase the diagnostic yield of fecal examination for parasitic causes of diarrhea, wet smears and appropriate fecal flotations should be performed on fresh fecal samples (<1 hour old). It is appropriate to administer broad-spectrum anthelminthics, even if fecal tests are negative.
TABLE 23-16.
Common Intestinal Pathogens Differentiated by Most Likely Location
| Small Intestine | Both | Large Intestine |
|---|---|---|
| Coronavirus | Campylobacter spp. | Parvovirus (panleukopenia) |
| Toxocara cati | Escherichia coli | Trichuris vulpis |
| Toxascaris leonina | Salmonella | Tritrichomonas foetus |
| Ancylostoma tubaeforme | Campylobacter spp. | |
| Giardia spp. | Clostridium spp. | |
| Cryptosporidium parvum | Yersinia enterocolitica | |
Bacterial and viral causes of diarrhea should be considered when the cat is systemically unwell with fever. Fecal culture should be performed in these circumstances, but the limitations of this testing need to be recognized in that many intestinal bacteria can be found in healthy animals.70 Further antibiotic administration can result in increase of other bacteria.69
Fungal causes of diarrhea are usually recognized from histology of biopsy samples.
It remains to be seen whether the recent ready availability of PCR panels looking at a number of infectious causes of diarrhea will be beneficial for recognizing pathogens that had previously been misdiagnosed or a hindrance for readily recognizing commensal organisms not necessarily causative of the clinical signs being investigated.
The most common viral, bacterial, and mycotic causes of diarrhea in cats are described below. Parasitic gastrointestinal diseases are covered later in this chapter.
Viral Enteritis
Viral causes of diarrhea are not usually specifically diagnosed, since, with the exception of the canine fecal ELISA for parvovirus, routine definitive tests are not available.
Panleukopenia/Parvovirus
Clinical signs of panleukopenia (feline parvovirus infection) are more likely to occur in kittens, with the highest morbidity and mortality occurring between 3 and 5 months of age. Subclinical cases in older (susceptible) cats probably go unrecognized. The organism is very stable in most environments, and infections mostly occur from environmental contact.
Peracute cases can result in death within 12 hours, with little or no warning signs. Acute cases often have fever, depression, and anorexia, with signs beginning approximately 3 to 4 days before presentation. Vomiting is usually bile tinged and unrelated to eating. Diarrhea does not always occur, and when it does, it is usually later in the course of the illness. Leukopenia is not pathognomonic, because this can also occur with acute bacterial infection (e.g., salmonellosis can present identically).53
Commercially available ELISA tests for canine parvovirus antigen in feces can detect feline parvovirus; however, shedding may have ceased by the time clinical signs occur, and vaccination can result in positive test results for up to 2 weeks.111
Aggressive fluid therapy, usually at twice maintenance rates, is usually required. Broad-spectrum antibiotic coverage is used to prevent or treat secondary bacterial infection from viral injury of intestinal mucosa. Parenteral antibiotics are preferred to prevent the possibility of further gastrointestinal irritation. The author recommends calculating IV doses and introducing appropriate amounts of antibiotics to the fluids bag to create a constant rate infusion (CRI); cefazolin can be used in this way at 100 mg/kg/24 hours, and beta-lactam CRIs are commonly used in human medicine.127 Aminoglycosides or fluoroquinolones can be used concurrently at routine doses if fever persists after 24 hours or the cat is moribund on presentation, but care must be used with these agents. Aminoglycosides are potentially nephrotoxic, and fluoroquinolones have been reported to result in cartilage damage in growing animals, although this has not been demonstrated clinically in cats.134 Fluoroquinolone retinal toxicity has been seen in all animals. Cats that survive the first week usually recover, and prior infection imparts lifelong immunity.28 Vaccinations are highly effective for disease prevention.
Coronavirus
Feline enteric coronavirus (FECV) mostly causes mild, self-limiting diarrhea and must be distinguished from feline infectious peritonitis coronavirus (FIPCV), which is essentially always fatal and for which diarrhea is not a typical sign (but is possible). The most widely accepted current theory of FIP pathogenesis involves initial infection with FECV and then mutation to FIPCV in small numbers of susceptible individuals.112, 165
Routine serologic testing for FECV in cats with diarrhea would neither prove correlation with the clinical signs nor affect how the disease is managed and so is not recommended. Cats with FECV diarrhea should be managed with symptomatic therapy of fasting, then reintroducing a bland diet and supportive care with fluid therapy if necessary.
Other Viral Enteridities
Other viruses, such as astrovirus, reovirus, rotavirus, and torovirus-like agent, have been recognized to cause diarrhea in cats, but their roles as pathogens are unclear. They are not routinely recognized in practice, since electron microscopy of fecal samples is necessary for diagnosis and is not routinely performed. Management is supportive care with appropriate fasting, then reintroduction of bland diets and fluid and electrolyte replacement if necessary.51
Bacterial Enteritis
Successful identification of a known bacterial pathogen from a fecal sample does not necessarily mean that the agent found is the cause of disease in the cat. Although a number of bacterial pathogens have been demonstrated to cause diseases when specific pathogen-free (SPF) cats are experimentally infected,42 these same organisms can be found in healthy cats.93 The differences between healthy and diarrheic cats that have bacteria found in their feces may relate to virulence factors of the organism, or host factors (local or systemic immunity) of the cat. There is no definitive answer for this quandary. The author's opinion is that
-
•
If a diarrheic cat is systemically unwell and has a fever, then feces should be cultured.
-
•
If an organism is isolated that is known to cause signs consistent with those the cat is showing, the cat should be treated appropriately.
Campylobacter
Campylobacter diarrhea is usually caused by C. jejuni. Clinical signs of infection are poorly documented, but most cats are asymptomatic. Younger cats are more likely to have clinical signs and hemorrhagic, mucoid diarrhea has been reported. Diagnosis can be from culture of feces or swabs, and the organism is quite hardy; so, it usually survives transport to the laboratory.28 In individual cases, the organism has not been cultured after antibiotic treatment,45, 46 but it is not definitively proven that antibiotic therapy affects the natural course of the disease. Antibiotics that can be used are amoxicillin-clavulanate (15 mg/kg, every 12 hours, PO) or fluoroquinolones, such as enrofloxacin (5 mg/kg, once daily, PO) for durations of 14 to 21 days.44 Macrolides, such as erythromycin (10 to 15 mg/kg, every 8 hours, PO), are regarded as the drug of choice for humans but can cause gastrointestinal side effects.93
Clostridium
Clostridium difficile has been recognized in up to 5% of diarrheic cats.93 Clinical signs are typically acute onset watery diarrhea and anorexia. Diagnosis has been made with detection of toxin A or toxin B in fecal samples using ELISA. Although these tests have not yet been validated for cats, they may prove to be a useful aid to diagnosis94 and are available for testing of equine feces at some commercial laboratories. Nontoxigenic strains exist; so, positive culture alone does not ensure diagnosis. Metronidazole (10 mg/kg, every 12 hours, PO) for approximately 7 days is the treatment of choice.93
Clostridium perfringens typically results in large bowel diarrhea with tenesmus, mucus, and hematochezia, but small bowel signs can also be seen.42 PCR testing for enterotoxin A is commercially available and may prove to be a useful adjunct in diagnosis. Antibiotics that can be used include metronidazole (10 mg/kg, every 12 hours, PO), tylosin (10 to 20 mg/kg, twice daily, PO), or amoxicillin-clavulanate (22 mg/kg, every 12 hours, PO) for 7 days.94
Escherichia coli
Escherichia coli is a ubiquitous organism within the feline intestinal tract, and it would be unusual not to successfully culture E. coli from the feces of both healthy and unwell cats. When E. coli is associated with clinical signs of gastrointestinal disease, it is mostly as an opportunistic pathogen, with overgrowth resulting from changed environmental conditions, such as inflammation from other pathology or another pathogen. There are also specific strains of E. coli that are true pathogens because of virulence factors not present in commensal E. coli; these include enteropathogenic E. coli and enterotoxigenic E. coli, which both induce a watery diarrhea, and enterohemorrhagic E. coli, which produces a diarrheal syndrome with copious bloody discharge and no fever.77 PCR testing is commercially available to identify pathogenic strains of E. coli 16, 148; although not offered at routine veterinary laboratories, this testing is available to veterinarians, and laboratories offering these services can readily be found online. Diagnosis should also document histologic lesions corresponding to the strain of E. coli identified.77 There is emerging resistance to E. coli worldwide in all species of animals, including humans. This includes the typical therapies for gram-negative bacteria of beta-lactam–enhanced penicillins and fluoroquinolones.167 A major risk factor is prior antibiotic usage, because commensal organisms are exposed to antibiotics. PCR testing does not enable antibiotic sensitivity testing, and fecal culture may not be able to distinguish pathogenic from nonpathogenic strains; so, sensitivities may not be an accurate reflection of the pathogenic organism. PCR testing for genes that impart resistance to E. coli have recently been described but are not yet commercially available.34 In some circumstances, supportive care with fluid and electrolyte replacement may be all that is required while the cat's immune system combats the infection. Empiric therapy could include beta-lactam–enhanced penicillins (such as amoxicillin-clavulanate at 20 mg/kg, every 12 hours, PO), fluoroquinolones (such as enrofloxacin at 5 mg/kg, once daily, PO), or cefovecin (8 mg/kg, every 2 weeks, SC), but the clinician must be aware of possible drug resistance.
Salmonella
Salmonella typhimurium infection is possible from ingestion of infected prey, infected food sources, or from a contaminated environment, including the veterinary hospital. The resulting clinical signs depend on the number of infecting organisms, the immune status of the cat, and the presence of concurrent diseases. Infection rates in cats (and humans) have been correlated with seasonal bird migrations,153 and the illness has been dubbed songbird fever,52 but there is no distinction between this and other Salmonella infections. Clinical signs usually begin 3 to 5 days after exposure, starting with fever (often >40° C [104° F]), malaise and anorexia, and progressing to diarrhea, vomiting, and abdominal pain. Hematology can show leukopenia with a left shift and nonregenerative anemia, and biochemistry results are usually nonspecific. Diagnosis is based on isolation of the organism by culture or identification with PCR, but care should be taken to correlate pathogen identification with clinical signs since, as with most GI pathogens, the organism can be isolated from healthy animals.147 As with E. coli, antibiotic resistance is widespread,120 with one United Kingdom survey finding the multiple drug-resistant strain DT104 to be the most frequent bacteriophage type identified.115 Treatment should be reserved only for those cats showing systemic signs, because routine antibiotic use in treating salmonellosis induces drug-resistant strains and prolongs the convalescent excretion period.52 Antibiotic choice should be based solely on sensitivity findings, since resistances are so widespread and unpredictable.98 This means that if the organism has been identified by PCR, then culture of feces must also be undertaken. The duration of treatment must be long enough to eliminate fecal excretion of the organism, prevent the chance of relapse, and reduce the chance of resistance developing; up to 28 days has been advocated.4, 164 These cautions are particularly important because of the zoonotic potential of salmonellosis.
Other Bacterial Enteridities
Other bacterial causes of diarrhea have been reported in cats, such as Yersinia enterocolitica,48 Yersinia pseudotuberculosis,63 Clostridium piliforme (Tyzzer's disease),71 and Anaerobiospirillum sp.36 Specific diagnosis of these (and other bacterial infections) may be found in the course of investigation. Management follows the principles of supportive care and appropriate antibiosis based on sensitivity testing.
Small Intestinal Bacterial Overgrowth
Small intestinal bacterial overgrowth (SIBO) has not been specifically described in cats. The criteria defined for dogs is a fasting bacterial count in duodenal juice of greater than 105 organisms/mL11 and is often recognized with other chronic gastrointestinal diseases.130 Healthy cats appear to have at least this number of upper intestinal bacterial with a range of 105 to 108/mL recognized.68 Bacterial overgrowth could potentially occur with ileus or intestinal inflammation of any underlying cause. Foul-smelling small bowel diarrhea with no specific pathogen recognized may be an indicator of this condition, as could an increase in bacterial metabolites, such as folate. If suspected, it is appropriate to manage with broad-spectrum antibiotics, such as metronidazole (10 to 15 mg/kg, every 12 hours, PO) or amoxicillin (10 mg/kg, every 12 hours, PO) for an extended duration such as 21 to 28 days. Alterations in intestinal flora have been recognized after such treatment69; however, any advice for this “condition” is entirely empirical. All efforts should be directed at identifying a precise underlying cause.
Mycotic Enteritis
Mycotic and other infectious agents are only rarely recognized as intestinal pathogens in cats. Diagnosis is made by histologic and microbial analysis of samples obtained at biopsy. Possible agents include Histoplasma capsulatum,24 Aspergillus spp., Candida albicans, 140 and Pythium insidiosum. 119
Intestinal Obstruction
Intestinal obstructions arise most commonly as a result of neoplasia in older cats and foreign body ingestion predominantly in younger cats.10, 41, 60 Less common causes include intussusception83 and granulomatous inflammation (e.g., from FIP)59; tapeworm infection, with greater than 30 worms acting as a linear foreign body, has also been reported.173 Other listed causes are volvulus, intestinal torsion, incarceration of bowel in a hernia, adhesions or stricture, intramural abscess or hematoma, and congenital malformations.140
Intestinal Foreign Bodies
Patient Signalment and Risk Factors
Linear foreign bodies have traditionally been considered more common than discrete foreign bodies in cats,10, 14, 41 but a study from a primary care facility indicated only 33% of foreign body cases were because of linear foreign bodies.60 The larger case load of linear foreign bodies at referral institutions noted in earlier studies may indicate the abilities of primary care practitioners to recognize and effectively deal with discrete foreign body obstructions.
Most studies have found that cats with intestinal foreign bodies are generally younger (mean, 1.0 to 2.7 years), with a notable exception being obstruction from trichobezoars where three of five cats in one study were 10 years or older; the greatest risk factor appears to be length of hair coat.9 No specific breed predispositions have been described but Siamese and Siamese-related cats have been noted to have oral fixations13 and so may be expected to be overrepresented with intestinal foreign bodies.
Clinical Signs
Clinical signs will vary depending on the type of foreign body (linear or discrete), the position of obstruction, and the time since obstruction. Most cats present for anorexia or vomiting. Partial obstruction can result in diarrhea (which can be bloody). Foreign body obstruction is typically considered an acute condition, with duration of obstruction because of a linear foreign body, measured from the onset of clinical signs to diagnosis, reported to range from 1 to 10 days.10, 41, 60 However, one paper demonstrated chronic, intermittent, gastrointestinal disease from a linear foreign body of a 1-month duration175 demonstrating that partial obstruction can result in a chronic course.
Physical examination may or may not reveal abdominal pain, palpable abdominal mass (or plication), dehydration, or fever. All cats presenting for anorexia or vomiting should have the underside of the tongue evaluated for the presence of a linear foreign body. Applying gentle pressure with a thumb in the intermandibular space to elevate the tongue is an effective way to visualize lesions or foreign bodies in the sublingual area (see Figure 3-8).
Pathophysiology
Life-threatening consequences can result from the interactions of local and systemic factors that arise from intestinal obstruction. Locally, damage to the mucosa from traction and pressure of the foreign object can cause hemorrhage, ischemia, and necrosis. Systemically, hypovolemia, toxemia, and acid–base and electrolyte imbalances can ensue.
Complete intestinal obstruction by discrete masses results in gas and fluid distention of the lumen proximal to the obstruction. Most gas accumulation is a result of swallowed air, which is predominantly nitrogen that cannot be absorbed by the intestinal mucosa. Gas also arises from bacterial fermentation. Fluid accumulates as a result of increased secretions (saliva, bile, and secretions of gastric, pancreatic, and small intestinal origin) and retention of fluid already ingested, and it can be augmented by local hemorrhage.39 Since most intestinal obstructions in cats do not reach the midjejunum,60 reabsorption of fluids that normally occurs at the jejunum and ileum is impaired.39
Linear foreign bodies, such as string, dental floss, or elastic toys, require proximal anchoring, usually under the tongue or in the pylorus (for example, by part of a toy attached to elastic). Peristalsis moves the free end of the “string” through the intestinal tract, resulting in pleats of intestines around the foreign body. As the foreign body is forced against the intestinal mucosa, the mucosa becomes edematous, and even partial penetration affects mucosal integrity, allowing systemic entry of bacteria.
Intraluminal bacterial populations increase for both discrete and linear foreign bodies as a result of stasis. Mucosal permeability can be affected by prolonged luminal distention, allowing entry of bacteria and toxins systemically or into the peritoneal cavity. Direct entry of bacteria to the peritoneal cavity, causing septic peritonitis, can result from perforation of the intestinal wall from linear foreign bodies or sharp discrete foreign bodies, such as toothpicks or plastic toys.
Diagnosis
Definitive diagnosis requires identification of the foreign body retrieved at surgery or in some cases, by endoscopy. This may be aided greatly prior to surgery by diagnostic imaging. However, imaging findings, particularly in the case of partial obstructions, may be subtle enough that obstruction of no identifiable cause is recognized or no overt signs are apparent. Laboratory findings are not helpful in the precise diagnosis but are important to assess fluid and electrolyte balances that must be corrected.
Cats rarely help practitioners by ingesting radiopaque objects, but on the rare occasions that they do, these can be observed easily on plain radiographs. Nonopaque foreign bodies depend on dilatation of the intestine from gas and fluid accumulation proximal to the obstruction for radiographic recognition (Figure 23-28 ). One study has suggested that if the jejunal diameter is greater than 2.5 times the length of the cranial end plate of the second lumbar vertebra, then intestinal obstruction is the most likely abnormality. Care must be taken that the jejunal and not duodenal diameter is measured and that the radiographs must be positioned strictly lateral, because an oblique view can alter the measurement of the lumbar vertebra.1 However, dilatation of an obstructed intestine may not occur if the obstruction is partial or intermittent, or if vomiting results in less fluid present. Since most foreign body obstructions in cats are proximal, identifiable dilatation may not be recognized for this reason.78 Linear foreign bodies present further challenges for radiographic recognition; the following typical radiographic signs129, 137 may or may not be present:
-
•
Accordion-like pleating or gathering (plication) of the jejunum
-
•
Most of the small intestine localized in midventral region of the abdominal cavity instead of being dispersed uniformly throughout the peritoneal cavity on the lateral view (Figure 23-29 )
-
•
Gathering of small intestine to the right side of the midline in the ventrodorsal view (Figure 23-30 )
-
•
Altered gas pattern with luminal gas collecting in small bubbles instead of normal curved tubular columns. This can be subtle when there is only minimal involvement of the intestine but overt when involving the entire small intestine. Comma-shaped gas patterns are more likely to occur with linear foreign bodies.1
FIGURE 23-28.
Radiography of discrete foreign body. Note that the dilatation of the intestine from gas and fluid accumulation proximal to the foreign body appears caudal to the obstruction. The foreign body was a piece of leather, and the obstruction was in the duodenum. The gross appearance is shown in Figure 23-33.
FIGURE 23-29.
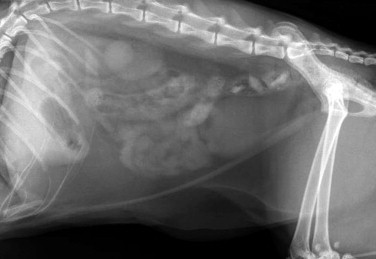
Radiographic appearance of a linear foreign body (lateral view). Note that most of the small intestine is localized in midventral region of the abdominal cavity instead of being dispersed uniformly throughout the peritoneal cavity.
FIGURE 23-30.
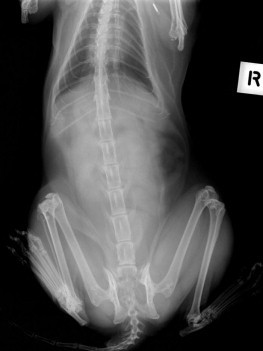
Radiographic appearance of a linear foreign body (ventrodorsal view). Note the gathering of most of the small intestine to the right side of the midline.
Contrast radiography can aid diagnosis for both discrete and linear foreign bodies but should be used with caution because intestinal perforation may be present. Nonionic iodinated agents that are typically used for myelography (such as iopamidol or iohexol) should be used, since barium is irritating to the peritoneum and oral iodine compounds are hypertonic. Hypertonic compounds may draw fluid into the stomach and intestines after oral administration, with the potential of creating further fluid and electrolyte imbalances in an already compromised patient.137
Ultrasonography is a very useful diagnostic tool, particularly for discrete foreign bodies, where, in most cases, there is overt distention of the small intestines with intraluminal fluid apparent (Figure 23-31 ). This modality has not been extensively assessed as an adjunct to diagnosis of foreign body intestinal obstruction in cats specifically, although there are several papers assessing dogs and small numbers of cats that agree with its utility.114, 156, 161 Linear foreign bodies are more difficult to assess ultrasonographically, but plicated bowel can be recognized, sometimes with the foreign body seen as a hyperechoic line centrally.156
FIGURE 23-31.
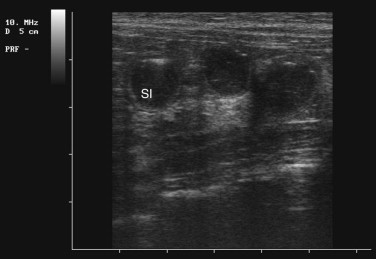
Ultrasonographic image of a grossly distended intestinal lumen because of intestinal obstruction with a discrete foreign body.
Conservative Management
Successful treatment of foreign body obstruction requires evacuation or removal of the foreign body as well as correction of any bacteremia or endotoxemia, acid–base or fluid imbalances. Discrete foreign body obstruction requires surgery or endoscopy to remove the object. In some specific circumstances, linear foreign body obstruction may be managed conservatively by cutting the anchor point below the tongue and allowing the cat to pass the foreign body by peristalsis. However, the decision to manage a cat conservatively must be done with the cat hospitalized, with fluid therapy and antibiotic coverage and a clear recognition on behalf of the practitioner and the owner that surgery may subsequently be required.
Cutting a sublingual linear foreign body may be achieved in a conscious cat by applying pressure with the thumb of one hand in the intermandibular space to elevate the tongue and gently grasping it using gauze with the other hand while a second person cuts the line with a suture cutter. There is a chance of a small nick on the sublingual surface. If the cat will not tolerate the procedure, sedation is appropriate. When cutting the line, the nature of the linear foreign body should be assessed (i.e., is it more or less likely to cut mucosa). In one study,10 19 cats with linear foreign bodies were managed conservatively with 10 cats subsequently requiring surgery. The authors of that paper created guidelines that will be adapted here.
Conservative management should be attempted if the cat
-
•
Is presented acutely (within 2 days) after known ingestion of a linear foreign body
-
•
Has a sublingually fixed linear foreign body that can be cut
-
•
Has no overt signs of peritonitis
Surgical intervention is mandatory if
-
•
Clinical signs (e.g., vomiting or anorexia) persist or deterioration occurs with conservative management
-
•
The cat has overt signs of peritonitis
-
•
The linear foreign body is fixed at the pylorus
Some authors disagree with attempting conservative management, since a perforated intestine from a linear foreign body reportedly carries a 50% mortality rate,41, 88 and early surgical intervention is never an incorrect decision. This should be balanced with the observation that cats can carry a linear intestinal foreign body, such as an elastic cord for a 1-month duration without intestinal perforation.175 However, fishing line, for example, would not be so forgiving!
Surgical Management
Surgery to remove an intestinal foreign body (FIGURE 23-32, FIGURE 23-33 ) should be considered an exploratory laparotomy. That is, the aim of the surgery is not only to remove the foreign body but to assess the entire intestinal tract and abdomen for other foreign bodies or pathology.
FIGURE 23-32.
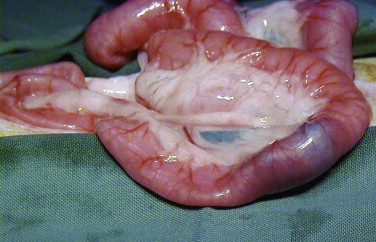
Appearance of a discrete foreign body at laparotomy. Note intestines are distended distal to obstruction (top and right of picture) but not proximally (to the left and bottom of picture).
FIGURE 23-33.
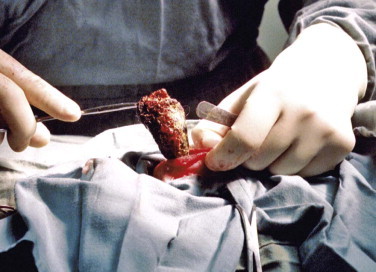
Removal of a discrete foreign body (a piece of leather) at laparotomy. Enterotomy to remove discrete foreign bodies should always be distal to the obstruction, because the intestine is likely to be compromised proximal to as well as overlying the obstruction. This is the same cat as in the radiology image in Figure 23-28.
Enterotomy to remove discrete foreign bodies should always be distal to the obstruction, because the intestine is likely to be compromised proximal to as well as overlying the obstruction, thus delaying healing and creating the potential for surgical dehiscence. Linear foreign bodies require multiple enterotomy incisions, since pulling the object out through a single incision could create iatrogenic intestinal perforation. The anchor point (either sublingual or pylorus by gastrotomy) must be released in the first instance. Enterotomy incisions are closed with 5/0 synthetic, monofilament, absorbable suture material, such as polydioxyanone (PDS) or equivalent, in either a simple interrupted or simple continuous pattern.14, 88
A technique has been described for removal of linear foreign bodies by making a single enterotomy incision proximally and passing a red rubber catheter over the linear foreign body aborally, milking the foreign body within the catheter through the colon for retrieval from the cat's anus by an assistant.2 This technique is not always effective, because it can be hampered if the foreign body is knotted or does not run smoothly through the red rubber catheter.102
If the affected bowel segment demonstrates evidence of necrosis or perforation on the mesenteric border of the intestine, resection and anastomosis should be performed. Necrosis is indicated by dark discoloration, thin intestinal wall, poor arterial pulsation, poor capillary bleeding, or lack of peristalsis. End-to-end anastomosis can be accomplished using a simple interrupted appositional pattern or a modified simple continuous appositional pattern with the same type of suture material used for enterotomy closure.14, 88
Mass Obstruction
Intraabdominal masses causing intestinal obstruction are often presumed to be neoplastic but can also be of infectious origin. Resection, where possible, is always recommended, because resection of neoplasia (if no metastasis) can offer a good prognosis,76, 87, 109, 145 and infectious causes may be managed with adjunctive therapy after definitive diagnosis.
Neoplasia
Intestinal obstruction in older cats is more likely to be secondary to neoplasia. Any neoplasia can cause obstruction, but adenocarcinoma32, 76 and adenomatous polyps88, 106 are reported to cause obstruction more often than other types of neoplasia. Please refer to the sections on intestinal neoplasia earlier in this chapter for more details.
Granulomatous Inflammation
Granulomatous inflammation causing a single focal intestinal lesion can lead to obstruction in the same way that neoplastic change can. Feline infectious peritonitis (FIP) can present as focal lesions,59 often in the colon or ileocecocolic junction. In the case of FIP, the focal lesion is usually an indicator of multisystemic disease; so, resection does not help prognosis.
The fungus-like organism, Pythium insidiosum has also been reported to cause granulomatous lesions, resulting in intestinal obstruction119 from large extraluminal masses that are approximately fist sized. Resection with adjunctive itraconazole (10 mg/kg) for 2 months after surgery was a successful treatment.
Intussusception
Intussusception refers to invagination or prolapse of one portion of the intestine into the part of the tract that either precedes or follows it. There is a bimodal age distribution with intussusceptions in older cats, most likely associated with neoplasia (or IBD in some cases)25; underlying causes for younger cats are ill defined and may be idiopathic in many cases,15, 25 but associations with parasitism and, in one case, a linear foreign body, have been made. Siamese and Burmese cats seem to be overrepresented. The most common locations are the ileocolic region and the jejunum.15, 25, 83, 110
Affected cats present with nonspecific signs of gastrointestinal disease, such as anorexia and lethargy. Vomiting is not necessarily a presenting sign; diarrhea may occur. Abdominal palpation reveals a mass in most cases. Plain and contrast radiography only show evidence of obstruction and usually do not help define that the bowel has intussuscepted.15, 25, 83, 110
Ultrasonography is very useful for diagnosis, because a distinctive pattern of alternating hypoechoic and hyperechoic concentric rings (Figure 23-34 ) is present in transverse sections.25, 110 Sometimes, the target lesion seen can be hard to distinguish from the pathology of other intraabdominal masses, such as lymph nodes, and in these cases, the size of the lesions can help, because the width will always be greater than 11 mm with an intussusception (because the sum of at least four intestinal wall widths cannot be less) and is often greater than16 mm.110
FIGURE 23-34.
Ultrasonographic image of ileocolic intussusceptions. Note concentric circles alternating increased and decreased echogenicity within grossly distended intestines.
(Courtesy Dr. Karon Hoffmann, University of Sydney.)
Surgical correction is always required, and manual reduction is typically not possible because there is usually significant venous infarction, edema, and congestion (Figure 23-35 ) as well as adhesions from fibrin and effusions from the affected bowel.15, 25 If the intussusception does not reduce manually, resection of the affected bowel is required, with anastomosis of the healthy tissue. There appears to be no benefit to enteroplication, which can result in significant ileus. There is no benefit to performing resection-anastomosis if the intussusception does reduce manually.15, 25
FIGURE 23-35.
Gross appearance of ileal intussusception at laparotomy.
The prognosis depends on the underlying disease process and the chronicity of the intussusception, and therefore how debilitated the cat is at presentation, However, prognosis is mostly good, with survival reported in up to 80% of cases, though recurrence can occur in some cats with idiopathic disease, often at different locations of the intestinal tract.15
Constipation and Megacolon
Constipation is defined as infrequent or difficult defecation associated with retention of feces within the colon and rectum. Prolonged constipation results in harder and drier feces that become impacted, and this is known as obstipation. 140 Chronic, recurrent constipation and obstipation can result in megacolon, which refers to persistent increased bowel diameter that is not responsive to therapy. Megacolon is not a specific disease entity; it may be considered the most advanced stage in the spectrum of chronic constipation.18
In most cases, constipation can be managed quite simply if the underlying cause is determined and dealt with. A comprehensive list of causes of constipation is noted in Table 23-17 , but the underlying causes can usually be attributed to
-
1Reticence to defecate, because of
-
aColorectal or anal pain
-
bDifficulty squatting
-
a
-
2Inability to defecate, because of
-
aFecal factors (including dehydration and fecal bulk)
-
bColon factors (poor peristalsis)
-
a
-
3Physical obstruction (less common), such as
-
aMass (discrete foreign bodies do not obstruct distally in cats)
-
bTrauma resulting in pelvic canal narrowing
-
a
TABLE 23-17.
Classifications and Causes of Constipation
| Category | Cause |
|---|---|
| Dietary factors |
|
| Environmental/psychological factors |
|
| Difficulty posturing for defecation |
|
| Painful anorectal disorders |
|
| Anorectal obstruction |
|
| Neuromuscular dysfunction |
|
| Fluid and electrolyte abnormalities |
|
| Drug-related effects |
|
From Sherding R: Diseases of the intestines. In Sherding R, editor: The cat: diseases and clinical management, ed 2, New York, 1994, Churchill Livingstone, p 1211.
Of course, multiple factors can interact. For example, an older cat may have renal disease and so will be dehydrated to some degree and have arthritic hips and so be reticent to squat.
Clinical Signs
The presenting signs of constipation are usually evident to owners and include straining in the litter box and producing hard dry feces, if at all. Sometimes, however, owners can misinterpret signs. Cats can strain because of lower urinary tract problems, and, if no urine is produced, some owners assume the problem is because of constipation. Some constipated cats can intermittently have diarrhea because of direct colonic irritation from hard dry feces and so may present for diarrhea and not constipation. Cats can also present for less specific signs, such as anorexia, lethargy, weight loss, and even vomiting.140, 170 Vomiting can occur because of colonic receptors stimulating vagal afferent endings, which, in turn, can stimulate the chemoreceptor trigger zone.61 Sometimes owners are concerned that their cat is defecating less, but the cat has just changed its diet to a much lower–residue diet and so is producing less feces. A full dietary history is an important aspect of the initial assessment.
Physical examination should confirm presence of feces in the colon and assess the degree of impaction. The presence of feces can usually be confirmed by abdominal palpation. In constipated cats, the colon is often palpated as a long firm tube extending cranially; sometimes, feces can be palpated to and around the colic flexure. Alternatively, the feces may be palpated as large, discrete fecal concretions (that can sometimes be hard to distinguish from intraabdominal masses such as lymph nodes). If there is any doubt of the presence or degree of fecal impaction, survey abdominal radiographs should be taken. A lateral view taken in a conscious cat should be adequate to confirm the diagnosis.
The physical examination should also assess for contributing causes, including musculoskeletal conditions. Any recent trauma should be taken into account. The hips and lumbosacral region should be assessed for pain. The degree of flexion and extension of the hips should be gently assessed. The lumbosacral spine can be assessed by running two fingers on either side of the spinous processes. The cat will flinch in painful areas. Any arthritic change is magnified in an underweight cat, since there may be less muscle mass and the joints may bear a heavier load. Any suspicions of underlying musculoskeletal abnormalities can be confirmed with radiographs.
Neurologic assessment should also be performed. Subtle changes just affecting colonic innervation will not be apparent on physical examination alone. However, an assessment of proprioception, placing reflexes, and gait should at least be performed to assess for lumbosacral spinal cord disease. Anorectal abnormalities or lesions should be evaluated. Impacted or infected anal sacs can lead to reticence to defecate; and therefore anal sacs should be assessed and expressed. Because this is painful for most cats, the cat should be held by an experienced assistant. The author prefers expressing one anal sac at a time with well-lubricated gloves and the index finger within the rectum and the thumb positioned externally. A rectal exam can be performed with a well-lubricated (gloved!) middle finger, feeling over the pelvic rim for masses as well as assessing if the colon closes over (squeezes) the finger. If the colon feels open around the finger, this can be an indicator of impaired colonic innervation but does not imply that this is a permanent change. If there are impacted feces continuing to the anus, rectal examination is not possible until this has been cleared. If a cat finds anal gland expression or rectal examination too painful to tolerate (based on the clinician's judgment), these procedures should be done under sedation.
Hydration and electrolyte status are also important factors in the constipated cat. Chronic renal disease is defined by azotemia (in conjunction with inadequately concentrated urine), which means the cat must be dehydrated to some degree. Plasma or serum biochemistry and urinalysis can be used to diagnose renal disease, assess degree of renal disease, or recognize prerenal dehydration. Electrolyte changes including hypokalemia and hypocalcemia may also contribute to reduced colonic smooth muscle function.140 In young to middle-aged cats of apparent good health and hydration, blood and urine assessments are usually not required at an initial presentation for constipation.
Management
In all cases, the same principles of management apply:
-
1
Ensure removal of obstructing feces
-
2
Ensure colonic motility and smooth passage of feces
-
3
Reduce fecal bulk
-
4
Ensure adequate hydration
-
5
Manage underlying problems
Management of the First Episode in a Well Cat with Minimal Obstructing Feces
The first step is to ensure obstructing feces are removed. In simple cases, the cat will evacuate feces after use of a glycerin or sorbitol pediatric rectal suppository. Another option is administration of a microenema, such as Microlax (McNeil Consumer Healthcare, Fort Washington, Pa.), which contains 5 mL of sodium lauryl sulfoacetate. These products act to lubricate the colon wall and therefore facilitate the passage of feces. The author prefers to use one or two of these within the consult room to observe the cat defecating (the cat must be provided with a litter tray!). The outside tube should be lubricated with the suppository contents before carefully inserting and then expressing the rest of the contents. There are also stimulant laxatives (containing bisacodyl) and emollient laxatives (containing sodium docusate) that have reportedly been used.140
If a rectal suppository vial cannot easily be inserted because hardened fecal content obstructs its entry, a more substantive enema will be required (sometimes requiring sedation or anesthesia), and this is covered in the next section on management. Some cats present for difficulty defecating and pass hard, dry stools but do not have fecal impaction at the time of examination.
After the obstructing feces have been removed, steps must be taken to ensure colonic motility and smooth passing of feces. Medical management of constipation traditionally involves laxatives and prokinetic agents. These may not be required in straightforward cases. As long as there is no obstructive lesion, cisapride at 2.5 mg/cat, every 12 hours, PO82 is very safe and can be instituted with a view to reducing the dose to once daily after 10 to 14 days and discontinuing if signs remain abated. Doses of up to 7.5 mg/cat, every 8 hours, PO have been reported. Cisapride is only available from compounding agencies in most countries. An osmotic or lubricant laxative (Table 23-18 ) may be used concurrently at reduced doses as necessary.
TABLE 23-18.
Medical Management of Constipation
| Classification | Examples | Comment |
|---|---|---|
| Lubricant laxatives |
Petrolatum | Over-the-counter pet products, very safe |
| Mineral oil | AVOID, risk of inhalation lipid pneumonia | |
| Hyperosmotic laxatives | Lactulose Polyethylene glycol (e.g., MiraLAX, Schering-Plough Healthcare Products, Kennilworth, NJ) |
Starting dose 2 mL/cat q12h Starting dose  tsp q12h tsp q12hNo controlled data |
| Emollient laxatives | Docusate (e.g., Colace, Purdue Products, Stamford, Conn.) | 50 mg/cat PO q12h |
| Bulk laxatives | High fiber diets Psyllium (Metamucil, Procter & Gamble, Cincinnati, Ohio) |
Increase colonic motility but also increase bulk of feces |
| Stimulant laxatives | Bisacodyl (Dulcolax, Boehringer Ingelheim, Ridgefield, Conn.) | 5 mg/cat PO q24h |
| Prokinetic agents | Cisapride | 2.5-5.0 mg/cat q12-24h Must be compounded |
Reducing the fecal bulk produced is an important part of long-term management. Traditional dietary recommendations are to increase the amount of fiber.18, 26, 140, 170 Increased dietary fiber results in production of short-chain fatty acids, which have been demonstrated to stimulate feline colonic smooth muscle contractions.128 However, dietary fiber is also classified as a bulk laxative and so, by definition, will increase fecal bulk. In humans dietary fiber has been considered a mainstay of therapy for constipation, but a recent review concluded that many patients with more severe constipation have worsening symptoms when increasing dietary fiber intake.103 Because megacolon is believed to be the end result of chronic dilatation,140, 170 it is the author's firm belief that initial dietary efforts should be directed to reducing fecal bulk and thus introducing a low-residue diet. Reduced dry matter intake reduces stool volume,31 and the author has found that recurrence rates of constipation reduce greatly when cats are transitioned to entirely wet food diets. Wet food diets also help ensure adequate water intake and therefore help maintain hydration. However, increased dietary fiber is beneficial for some cats, and trial and error may be required to determine whether a high-fiber or low-residue diet will be of benefit to each individual cat. In one report, 15 cats with recurrent constipation refractory to traditional medical and dietary management were successfully treated with a psyllium-enriched dry extruded diet.48a After 1 month on the diet, 14 cats had no clinical signs of constipation. The remaining cat was clinically normal after 2 months on the diet. Improvement was noted in 10 of 15 cats after only 7 days of dietary therapy.
Measures should be taken to ensure adequate hydration. Maintaining adequate hydration is particularly relevant for cats with chronic kidney disease that have impaired ability to conserve water. Changing to wet food diets helps increase water intake. Some cats with chronic kidney disease may need additional fluid support, such as subcutaneous fluids administered by the owner at home on a regular basis.
Underlying problems may be minor and simple to manage, such as an anal gland abscess, or more involved, such as reduced pelvic outflow, as a result of prior trauma. Arthritis is a common underlying factor in many older cats and may be managed with prudent use of nonsteroidal agents (see Chapter 26).
Management of Cats with Repeat Episodes and Obstipation
In cases of obstipation, the cat is more likely to be debilitated to some degree; so, laboratory investigations to assess plasma or serum biochemistry parameters as well as hematology and urinalysis are ideal. Any hydration deficit or electrolyte abnormalities should be corrected before the anesthesia that is often required to remove the obstructing feces. Rectal suppositories and microenemas are usually ineffective in obstipated cats. Enemas are often required to remove impacted feces in such circumstances. The enema solution must be warmed and introduced slowly to avoid vomiting. The typical volume required is 5 to 10 mL/kg (so, up to approximately 50 mL/cat). The enema solution can be an isotonic electrolyte solution or tap water, and mild soap can be added (but any soap used must not contain hexachlorophene, which is neurotoxic if absorbed); mineral oil can be used (5 to 10 mL/cat) as a lubricant or docusate as an emollient (5 to 10 mL/cat), but the two agents must not be used together since docusate promotes mucosal absorption. Sodium phosphate–containing enemas must not be used, because they can induce severe hypernatremia, hyperphosphatemia, and hypocalcemia in cats.
Often, the enema solution alone is insufficient to reduce the fecal mass, and manual manipulation of the feces by abdominal palpation is required. Sometimes the feces must be broken down by a gloved finger per-rectum while the colon is massaged manually through the abdominal wall with the other hand, but great care must be taken with this maneuver, because the devitalized colon can be perforated more easily.140, 170 Enemas as described are painful for the cat, and opioid analgesia is recommended at the time of anesthesia. Opioids can reduce peristalsis in humans,96 but having evacuated the bowel, the pain relief is more important than this transient effect.
An alternative to enemas is administration of an oral polyethylene glycol (PEG 3350) solution (e.g., CoLyte, GoLytely). A nasoesophageal tube is placed and the solution is given as a slow trickle (6 to 10 mL/kg/hour) over 4 to 18 hours. Defecation usually results in 6 to 12 hours. In a retrospective study of 9 cats, median time to defecation was 8 hours and the median total dose of PEG 3350 was 80 mL/kg.26a No adverse effects were noted.
A cat that has been obstipated needs supportive therapy when discharged. There are no controlled comparisons of the various therapies noted in Table 23-21; the author prefers cisapride 2.5 mg, every 12 hours to every 8 hours, PO82 (first thing in the morning, when the owner returns from work, when the owner goes to bed), and lactulose syrup 2 mL/cat, every 12 hours, PO. A cat that has been so severely obstipated that an enema under anesthesia is required can be expected to continue these medications lifelong.
TABLE 23-21.
Physical Characteristics of Tritrichomonas foetus versus Giardia duodenalis
| Characteristic | Tritrichomonas foetus | Giardia duodenalis |
|---|---|---|
| Size | 15 µm × 5 µm | 15 µm × 8 µm |
| Motility | Erratic, forward motion | Falling leaf, rolling motion |
| Structures | Undulating membrane | Ventral disk, median bodies |
| Flagellae | 3 anterior, 1 posterior | 8 |
| Nuclei | 1 | 2 |
To reduce fecal bulk and decrease the opportunity for recurrence, low-residue canned foods (or sachets) are preferred for cats that have become obstipated. Some cats may benefit from high-fiber diets. As with simple initial episodes, canned food helps maintain adequate hydration, and at home subcutaneous fluids may be used additionally in cats with chronic kidney disease. With repeat episodes or severe obstipation, investigations for an underlying cause should be thorough and include evaluation for colonic mass obstructions. A review of published cases indicated that 96% of cases of megacolon are accounted for by idiopathic megacolon (62%), pelvic canal stenosis (23%), nerve injury (6%), or Manx sacral spinal cord deformity (5%).170 Although most cases are idiopathic, an attempt should be made to identify and treat any specific underlying causes.
Megacolon
Megacolon is not specifically defined in cats. It has been described as “generalized colonic dysfunction manifesting as severe colonic dilation and fecal impaction,” or a “severely and irreversibly dilated and hypomotile” colon140 and “a subjective evaluation of the diameter of the colon, usually based on radiographic assessment.”18 There are specific radiographic guidelines for humans with megacolon, in that a colonic diameter of more than 6.5 cm at the level of the pelvic brim is considered diagnostic.118
Radiographically, in the lateral view, the normal colon should be approximately the same diameter as the length of the body of the second lumbar vertebra.81 In cats, however, “there are no published guidelines for determining megacolon, so, diagnosis of abnormal colonic dilatation is subjective.”75 However, one author has suggested that “as a rule of thumb, the diameter of the colon should be less than the length of the body of the seventh lumbar vertebra (L7).”105 This author continues, “Enlargement of the diameter of the colon beyond 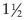 times the length of the body of L7 is indicative of chronic large bowel dysfunction and an explanation must be sought.”105 A recent paper found that 15 of 20 cats with no gastrointestinal disease had a colon diameter greater than the length of L7; however, no assessment of constipated cats was made.1 In practice, many cats with megacolon have a colonic diameter far exceeding this guideline (Figure 23-36
). One study of 11 cats with megacolon found the mean diameter of the colon was 2.7 times greater than the length of the seventh lumbar vertebra (median, 2.4; range, 1.8 to 3.3),107 but in general, objective descriptions of this condition are lacking in the veterinary literature.
times the length of the body of L7 is indicative of chronic large bowel dysfunction and an explanation must be sought.”105 A recent paper found that 15 of 20 cats with no gastrointestinal disease had a colon diameter greater than the length of L7; however, no assessment of constipated cats was made.1 In practice, many cats with megacolon have a colonic diameter far exceeding this guideline (Figure 23-36
). One study of 11 cats with megacolon found the mean diameter of the colon was 2.7 times greater than the length of the seventh lumbar vertebra (median, 2.4; range, 1.8 to 3.3),107 but in general, objective descriptions of this condition are lacking in the veterinary literature.
FIGURE 23-36.
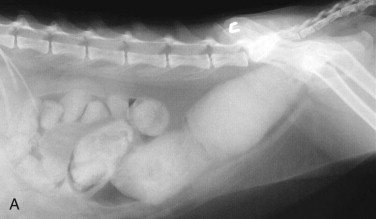
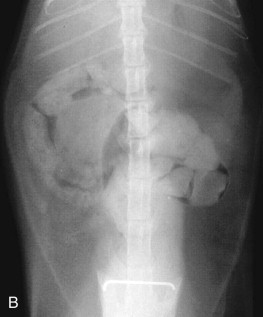
Radiographic appearance of an obstipated cat with megacolon, lateral (A) and ventrodorsal (B) views.
The definition of megacolon in cats should include functional as well as radiographic guidelines. In the absence of broadly recognized radiographic recommendations, the author proposes that the O’Brien rule-of-thumb guidelines105 (as noted above) be introduced until a more comprehensive study can establish other radiographic diagnostic criteria (or confirm these). The author therefore proposes to define megacolon as dilatation of the colon, to more than 1.5 times the length of the seventh lumbar vertebra, which is refractory to medical and dietary management. Practitioners can expect the radiographic assessment of colonic dilatation to exceed this guideline in cats with megacolon and, conversely, there are likely to be cats having colonic distention greater than this amount that will respond to medical and dietary management and can therefore not be defined as having megacolon.
Management of Megacolon
By the definition used above, megacolon is refractory to medical and dietary therapy; so, to be defined as having megacolon, a cat may have had several episodes of obstipation managed by enema as well as dietary trials (with both low-residue and high-fiber diets) and medical therapy with cisapride and an osmotic or emollient laxative; yet the cat will still obstruct with feces. In these circumstances, the only possible therapy is subtotal colectomy.
Subtotal colectomy refers to surgical excision of 95% to 98% of the colon, whether it is grossly diseased or not with preservation of the ileocolic junction (ICJ). This approach has resulted in a more favorable clinical response than when the ICJ is also excised.22, 170 When preserving the ICJ, it has been noted that, in some rare cases, it can be difficult to join the proximal segment of colon to the distal piece of descending colon because of the tethering effect of the ileocecocolic blood vessels. In these cases, sacrificing these vessels and removing the ICJ (i.e., total colectomy) is recommended to facilitate approximation of the ileum to the distal colonic segment.21 A recently described technique using a biofragmentable anastomosis ring, compared with sutured anastomoses, showed no discernible effect on prognosis.131 Prognosis following subtotal colectomy is generally good. A review of multiple papers, totaling over 100 cats that had undergone subtotal colectomy, found the most commonly reported perioperative complication was diarrhea or loose stools immediately after surgery. In the majority of individuals, stool consistency improves without further treatment so that within 1 to 6 weeks of the surgery soft, formed stools are developed. Diarrhea can persist in a small number of cases. In the longer term, in some cats, constipation can eventually return, but this can usually be managed by dietary and medical therapies.172
Anorectal Diseases
Pathology of the rectum or anus is relatively rare in cats and therefore poorly described in the veterinary literature. Consequently, published information is often not referenced, suggesting it expresses the authors’ opinions. Readers are directed to surgical texts for details and approaches about surgical corrections.
Anal Sac Diseases
The anal sacs are paired cutaneous evaginations situated between the internal and external sphincter muscles. These sacs store secretions from alveolar and sebaceous glands that reside within the sacs. Each anal gland has an associated duct that opens to the skin surface just lateral to the anus.136, 155 Normal anal gland secretions have only very recently been described47 and vary markedly; the color can be white, brown, orange, yellow, tan or gray, and consistency can range from watery to thick and creamy, with two thirds of cats having solid portions within the secretion. On microscopic examination, epithelial cells are commonly seen, with most cats having some neutrophils present. Bacteria are commonly recognized as are, on some occasions, yeasts. Bacteria seen in this study were mainly gram-positive cocci (63%) or gram-negative cocci (30%). Gram-negative or gram-positive rods were also seen but were rarely the dominant bacterial population.47 With such a wide range of normal secretions, it is difficult to diagnose any pathology from the nature of the secretion alone. However, blood is infrequently recognized, and neutrophils are typically present in only small numbers in normal secretions.
Anal sac diseases described in cats include impaction, inflammation (sacculitis), infection, abscessation, and neoplasia (essentially the same as in dogs).140, 155
Anal Sac Impaction/Inflammation/Infection/Abscessation
It has been contended in dogs that sacculitis and abscessation are an extension of impaction. It is not known in dogs or cats what the predisposing causes are, but suggested underlying reasons are loose stools (that are less effective at expressing the sac during defecation), local swelling or edema occluding the duct, and obesity.155 The author's observations have also indicated that constipation can result in anal sac impaction because of less frequent expulsion of the sac contents; the resultant pain of the anal sac impaction can lead to further constipation, thus establishing a cycle. The retention of secretions may predispose to sacculitis, but impacted anal sacs do not always result in inflammation. Abscessation is a likely sequel to sacculitis.155
Cats usually present for licking, scratching, or biting at the perineal area and can present for scooting (or dragging their anus) as dogs do. Other presenting signs can be inability to sit or settle, a lump seen by the owner, or a generally unwell state.
Expression is the only management required for impacted (and not infected) anal sacs. The author prefers expressing one anal sac at a time with well-lubricated gloves and the index finger within the rectum and the thumb externally. This is painful for most cats; so, the cat should be held by an experienced assistant, and it is sometimes not possible without some degree of sedation. With frequent episodes, underlying causes should be investigated. Sometimes, trial-and-error diet change to manipulate the nature of the feces to either more (high-fiber diets) or less (low-residue diets) bulk help reduce the frequency of episodes. Obesity should be managed by reduced caloric intake, but dietary management for this should also take into account the nature of the feces.
Overt infection may be recognized by pus secretion from the anal sacs, which will have a high numbers of neutrophils. This can be managed by broad-spectrum antibiotics, such as amoxicillin/clavulanate or cephalosporins. A single treatment with a nonsteroidal antiinflammatory drug, such as meloxicam, can be given in animals with appropriate hydration and without other illness.
Anal sac abscesses often present already open and draining. Many heal well by secondary intention with antibiotic treatment until they are closed over; so rechecks are required before the completion of an antibiotic course. Large abscesses may require surgical drainage with the insertion of a Penrose drain and management as for a cat fight abscess. It must be remembered that wounds in this area are easily re-infected by fecal contamination. Recurrent impaction, sacculitis, or infection may require anal sacculectomy (as in dogs). This procedure should be delayed until infection is cleared. The procedure is similar to that performed in dogs.
Neoplasia
Reports of anal sac/gland neoplasia were confined to sporadic case reports,97, 108 until a large case series was recently published.141 In this study, 64 cases of anal gland carcinoma were recognized at a private diagnostic laboratory during a 12-year period, with submissions from 62 practices. This indicates that, for most practices, this condition will be seen, at most, once every 12 years. Affected cats ranged in age from 6 to 17 years (median and mean, 12 years); female (mostly spayed) cats were overrepresented (61% of cases), and Siamese cats may have been over-represented (7.8% of cases). The number of Siamese cats with anal sac neoplasia was 3 times greater than the number of Siamese cats in the laboratory reference population. Affected cats presented for dyschezia, recurrent constipation, change in the nature or volume of feces, and/or perineal swelling or ulceration, sometimes with purulent or hemorrhagic discharge. Most tumors were originally interpreted as and initially managed as anal sac abscess. Presumptive metastasis in liver, lung, or abdominal lymph nodes was recognized by physical examination or radiography in six cats; one cat was hypercalcemic. Excision appeared to be curative (with a 3- to 4-year follow-up period) in 3 of 29 cats undergoing surgery for resection or debulking (others had only incisional biopsy performed). For the remaining 36 cats with known postsurgical outcome, median survival was only 3 months, with a 19% 1-year survival rate (with none of these cats surviving to 2 years).141
Anal Diseases
Atresia Ani
Atresia ani is a developmental defect of the anal opening or terminal rectum (see Figure 41-4). Kittens usually present within days or weeks of birth with abdominal distention, discomfort, tenesmus, restlessness, vomiting, and/or loss of appetite. There are several anatomic variations22:
-
•
Type I: A membrane over the anal opening remains, with the rectum ending as a blind pouch just cranial to the closed anus.
-
•
Type II: The anus is closed as in type I, but the rectal pouch is located somewhat cranial to the membrane overlying the anus.
-
•
Type III: The rectum ends as a blind pouch cranially within the pelvic canal (rectal atresia), whereas the terminal rectum and anus are normal.
-
•
Type IV: Occurs in females and atresia ani exists with a persistent communication between the rectum and the vagina (rectovaginal fistula). This fistula can occur with a normal anal opening as well.
Most reported cases have been type IV,23, 146, 150, 158, 166 and this has also been recognized with concurrent sacrococcygeal agenesis.3 Surgical correction has been described for type II157 and type IV19, 91 atresia ani in cats. The reader should consult these references for surgical advice; possible complications include megacolon after prolonged obstruction, postsurgical anal stricture, and fecal incontinence because of sphincter dysfunction.
Fecaliths
Foreign bodies in cats rarely obstruct the gastrointestinal tract distal to the jejunum60; however, large fecal balls resulting from constipation can, additional to constipation or obstipation, cause distention of the anus. This distention can result in inflammation of the anal sphincter with loss of tone (Figure 23-37, A ), which, in the author's experience, is temporary with correction of the underlying cause of constipation. It can take some weeks for the dilated anus to return to normal (Figure 23-37, B). A low-residue wet diet is recommended to reduce fecal bulk during the healing period.
FIGURE 23-37.
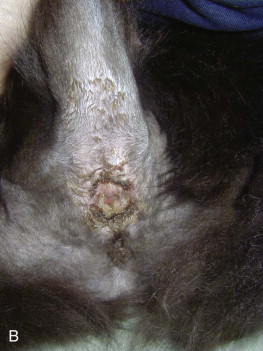
A, Dilated anus with loss of anal tone after removal of a fecalith. B, This is the same cat as in A 2 weeks later. Note the reduction in anal dilation. The cat's anal tone returned to entire normalcy in the following week. The only management used was feeding of a low-residue diet.
Rectal Prolapse
Rectal prolapse occurs as a result of a disease process that causes chronic straining, such as
-
1
Intestinal conditions that result in diarrhea and tenesmus
-
2
Conditions that result in constipation or other intestinal obstruction
-
3
Lower urinary tract diseases
-
4
Dystocia
Prolapses are usually classified in three ways.62
-
1
First degree: prolapse of only mucous membrane
-
2
Second degree: prolapse of full rectal wall thickness
-
3
Third degree: prolapse is sufficient to bring mesorectum outside the anus
The prolapsed rectum is obvious but must be differentiated from ileocolic intussusceptions, which have been described with neoplasia.37 This distinction can be made by inserting a thermometer through the anus alongside the prolapsed mass. Insertion will not be possible for an intussusception but will be for an anorectal prolapse.
The prolapsed tissue must be assessed for viability, and management must include determining and managing the underlying cause as well as management of the prolapse. In simple cases where the mucosa is viable, the prolapse can be reduced with lubrication and gentle pressure. A temporary purse-string suture may be required to prevent recurrence.
Perineal Dermatitis
Perineal dermatitis is often confused with gastrointestinal or urogenital disease, because there are often copious sebaceous secretions that can mimic fecal or urinary secretions. Perineal dermatitis can result from flea or other allergies but also fecal or urine scalding associated with diarrhea or urinary incontinence, respectively. Skin fold dermatitis can also occur in obese cats (Figure 23-38 ). Episioplasty has been described to correct this,121 but the author has found that stringent dieting can result in improvement while managing the skin fold dermatitis with antibiotics, such as cephalosporins, and regular cleansing.
FIGURE 23-38.
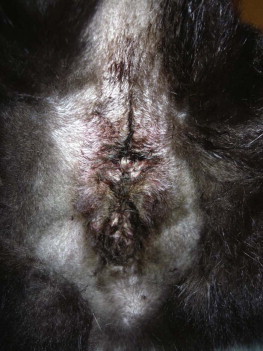
Perineal/skin fold dermatitis associated with obesity. Antibiotics were needed to manage the pyoderma while the cat was on a diet during a 12-week period. The condition resolved with weight loss, and no further management was required.
References
- 1.Adams WM, Sisterman LA, Klauer JM. Association of intestinal disorders in cats with findings of abdominal radiography. J Am Vet Med Assoc. 2010;236:880. doi: 10.2460/javma.236.8.880. [DOI] [PubMed] [Google Scholar]
- 2.Anderson S, Lippincott C, Gill P. Single enterotomy removal of gastrointestinal linear foreign bodies. J An Anim Hosp Assoc. 1992;28:487. [Google Scholar]
- 3.Araújo FPD, Araújo BM, Kemper B. Sacrococcygeal agenesis association and anal atresia in mixed breed cats. Ciencia Rural. 2009;39:1893. [Google Scholar]
- 4.Asperilla MO, Smego RA, Scott LK. Quinolone antibiotics in the treatment of Salmonella infections. Rev Infect Dis. 1990;12:873. doi: 10.1093/clinids/12.5.873. [DOI] [PubMed] [Google Scholar]
- 5.Baez J, Hendrick M, Walker L. Radiographic, ultrasonographic, and endoscopic findings in cats with inflammatory bowel disease of the stomach and small intestine: 33 cases (1990-1997) J Am Vet Med Assoc. 1999;215:349. [PubMed] [Google Scholar]
- 6.Baral RM. Australian College of Veterinary Scientists; Gold Coast, Queensland, Australia: 2006. Laparotomy for gastro-intestinal biopsies, Science Week Conference Proceedings (Small Animal Medicine chapter) p 70. [Google Scholar]
- 7.Baral RM, Krockenberger MB, Foster DJ et al: Hepatic small cell lymphosarcoma in four cats, J Feline Med Surg, in press.
- 8.Barrand KR, Scudamore CL. Intestinal leiomyosarcoma in a cat. J Small Anim Pract. 1999;40:216. doi: 10.1111/j.1748-5827.1999.tb03063.x. [DOI] [PubMed] [Google Scholar]
- 9.Barrs VR, Beatty JA, Tisdall PL. Intestinal obstruction by trichobezoars in five cats. J Feline Med Surg. 1999;1:199. doi: 10.1053/jfms.1999.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basher AW, Fowler JD. Conservative versus surgical management of gastrointestinal linear foreign bodies in the cat. Vet Surg. 1987;16:135. doi: 10.1111/j.1532-950x.1987.tb00925.x. [DOI] [PubMed] [Google Scholar]
- 11.Batt RM, Needham JR, Carter MW. Bacterial overgrowth associated with a naturally occurring enteropathy in the German Shepherd dog. Res Vet Sci. 1983;35:42. [PubMed] [Google Scholar]
- 12.Beatty J, Terry A, MacDonald J. Feline immunodeficiency virus integration in B-cell lymphoma identifies a candidate tumor suppressor gene on human chromosome 15q15. Cancer Res. 2002;62:7175. [PubMed] [Google Scholar]
- 13.Beaver BV. Disorders of behavior. In: Sherding RG, editor. The cat: diseases and clinical management. ed 2. Churchill Livingstone; New York: 1994. p. 191. [Google Scholar]
- 14.Bebchuk TN. Feline gastrointestinal foreign bodies. Vet Clin North Am Small Anim Pract. 2002;32:861. doi: 10.1016/s0195-5616(02)00030-x. [DOI] [PubMed] [Google Scholar]
- 15.Bellenger CR, Beck JA. Intussusception in 12 cats. J Small Anim Pract. 1994;35:295. [Google Scholar]
- 16.Bellin T, Pulz M, Matussek A. Rapid detection of enterohemorrhagic Escherichia coli by real-time PCR with fluorescent hybridization probes. J Clin Microbiol. 2001;39:370. doi: 10.1128/JCM.39.1.370-374.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benitah N, de Lorimier L-P, Gaspar M. Chlorambucil-induced myoclonus in a cat with lymphoma. J Am Anim Hosp Assoc. 2003;39:283. doi: 10.5326/0390283. [DOI] [PubMed] [Google Scholar]
- 18.Bertoy RW. Megacolon in the cat. Vet Clin North Am Small Anim Pract. 2002;32:901. doi: 10.1016/s0195-5616(02)00020-7. [DOI] [PubMed] [Google Scholar]
- 19.Bornet JP. Recto-vaginal fistula and anal imperforation in a cat: surgical treatment. Bull Acad Vet Fr. 1990;63:53. [Google Scholar]
- 20.Brandt L, Kimby E, Nygren P. A systematic overview of chemotherapy effects in indolent non-Hodgkin's lymphoma. Acta Oncol. 2001;40:213. doi: 10.1080/02841860151116286. [DOI] [PubMed] [Google Scholar]
- 21.Bright RM. Proc Am Assoc Feline Pract Fall Meeting. 1997. GI surgery. Atlanta, Ga. [Google Scholar]
- 22.Bright RM, Bauer MS. Surgery of the digestive system. In: Sherding RG, editor. The cat: diseases and clinical management. ed 2. Churchill Livingstone; New York, NY: 1994. p. 1353. [Google Scholar]
- 23.Broek AHM, Else RW, Hunter MS. Atresia ani and urethrorectal fistula in a kitten. J Small Anim Pract. 1988;29:91. [Google Scholar]
- 24.Brömel C, Sykes JE. Histoplasmosis in dogs and cats. Clin Tech Small Anim Pract. 2005;20:227. doi: 10.1053/j.ctsap.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Burkitt JM, Drobatz KJ, Saunders HM. Signalment, history, and outcome of cats with gastrointestinal tract intussusception: 20 cases (1986-2000) J Am Vet Med Assoc. 2009;234:771. doi: 10.2460/javma.234.6.771. [DOI] [PubMed] [Google Scholar]
- 26.Byers C, Leasure C, Sanders NA. Feline idiopathic megacolon. Comp Contin Edu Pract Vet. 2006;28:658. [Google Scholar]
- Carr AP, Gaunt MC. Constipation resolution with administration of polyethylene glycol solution in cats (abstract) J Vet Intern Med. 2010;24:753. [Google Scholar]
- 27.Carreras JK, Goldschmidt M, Lamb M. Feline epitheliotropic intestinal malignant lymphoma: 10 Cases (1997-2000) J Vet Intern Med. 2003;17:326. doi: 10.1111/j.1939-1676.2003.tb02456.x. [DOI] [PubMed] [Google Scholar]
- 28.Cook AK. Feline infectious diarrhea. Top Companion Anim Med. 2008;23:169. doi: 10.1053/j.tcam.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Craig LE, Hardam EE, Hertzke DM. Feline gastrointestinal eosinophilic sclerosing fibroplasia. Vet Pathol. 2009;46:63. doi: 10.1354/vp.46-1-63. [DOI] [PubMed] [Google Scholar]
- 30.Crandell J, Jergens A, Morrison J. Development of a clinical scoring index for disease activity in feline inflammatory bowel disease. J Vet Intern Med. 2006;20:788. [Google Scholar]
- 31.Crane SW, Griffin RW, Messent PR. Introduction to commercial pet Ffoods. In: Hand MS, Thatcher CD, Remillard RL, editors. Small animal clinical nutrition. ed 4. Mark Morris Institute; Topeka, Kans: 2000. p. 111. [Google Scholar]
- 32.Cribb A. Feline gastrointestinal adenocarcinoma: A review and retrospective study. Can Vet J. 1988;29:709. [PMC free article] [PubMed] [Google Scholar]
- 33.Culp WTN, Drobatz KJ, Glassman MM. Feline visceral hemangiosarcoma. J Vet Intern Med. 2008;22:148. doi: 10.1111/j.1939-1676.2008.0022.x. [DOI] [PubMed] [Google Scholar]
- 34.Dallenne C, Da Costa A, Decre D. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae. J Antimicrob Chemother. 2010;65:490. doi: 10.1093/jac/dkp498. [DOI] [PubMed] [Google Scholar]
- 35.Day MJ, Bilzer T, Mansell J. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: a report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J Comp Pathol. 2008;138(Suppl 1):S1. doi: 10.1016/j.jcpa.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 36.De Cock HEV, Marks SL, Stacy BA. Ileocolitis associated with Anaerobiospirillum in cats. J Clin Microbiol. 2004;42:2752. doi: 10.1128/JCM.42.6.2752-2758.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demetriou J, Welsh E. Rectal prolapse of an ileocaecal neoplasm associated with intussusception in a cat. J Feline Med Surg. 1999;1:253. doi: 10.1053/jfms.1999.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dennis JS, Kruger JM, Mullaney TP. Lymphocytic/plasmacytic colitis in cats: 14 cases (1985-1990) J Am Vet Med Assoc. 1993;202:313. [PubMed] [Google Scholar]
- 39.Ellison G. Intestinal obstruction. In: Bojrab M, editor. Disease mechanisms in small animal surgery. ed 2. Lea & Febiger; Philadelphia: 1993. p. 252. [Google Scholar]
- 40.Evans SE, Bonczynski JJ, Broussard JD. Comparison of endoscopic and full-thickness biopsy specimens for diagnosis of inflammatory bowel disease and alimentary tract lymphoma in cats. J Am Vet Med Assoc. 2006;229:1447. doi: 10.2460/javma.229.9.1447. [DOI] [PubMed] [Google Scholar]
- 41.Felts J, Fox P, Burk R. Thread and sewing needles as gastrointestinal foreign bodies in the cat: a review of 64 cases. J Am Vet Med Assoc. 1984;184:56. [PubMed] [Google Scholar]
- 42.Foley J, Hirsh DC, Pedersen NC. An outbreak of Clostridium perfringens enteritis in a cattery of Bengal cats and experimental transmission to specific pathogen free cats. Feline Pract. 1996;24:31. [Google Scholar]
- 43.Fondacaro JV, Richter KP, Carpenter JL. Feline gastrointestinal lymphoma: 67 cases (1988-1996) Eur J Comp Gastroenterol. 1999;4:5. [Google Scholar]
- 44.Fox JG. Campylobacter infections. In: Greene CE, editor. Infectious diseases of the dog and cat. ed 3. Saunders Elsevier; St Louis: 2006. p. 339. [Google Scholar]
- 45.Fox JG, Ackerman JA, Newcomer CE. The prevalence of Campylobacter jejuni in random-source cats used in biomedical research [correspondence] J Infect Dis. 1985;151:743. doi: 10.1093/infdis/151.4.743. [DOI] [PubMed] [Google Scholar]
- 46.Fox JG, Claps M, Beaucage CM. Chronic diarrhea associated with Campylobacter jejuni infection in a cat. J Am Vet Med Assoc. 1986;189:455. [PubMed] [Google Scholar]
- 47.Frankel JL, Scott DW, Erb HN. Gross and cytological characteristics of normal feline anal-sac secretions. J Feline Med Surg. 2008;10:319. doi: 10.1016/j.jfms.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fredriksson-Ahomaa M, Korte T, Korkeala H. Transmission of Yersinia enterocolitica 4/O:3 to pets via contaminated pork. Lett Appl Microbiol. 2001;32:375. doi: 10.1046/j.1472-765x.2001.00922.x. [DOI] [PubMed] [Google Scholar]
- Freiche V, Deswarte G, Soulard Y. A psyllium-enriched dry extruded diet improves recurrent feline constipation (abstract) J Vet Intern Med. 2010;24:1547. [Google Scholar]
- 49.Gabor LJ, Malik R, Canfield PJ. Clinical and anatomical features of lymphosarcoma in 118 cats. Aust Vet J. 1998;76:725. doi: 10.1111/j.1751-0813.1998.tb12300.x. [DOI] [PubMed] [Google Scholar]
- 50.Goggin JM, Biller DS, Debey BM. Ultrasonographic measurement of gastrointestinal wall thickness and the ultrasonographic appearance of the ileocolic region in healthy cats. J Am Anim Hosp Assoc. 2000;36:224. doi: 10.5326/15473317-36-3-224. [DOI] [PubMed] [Google Scholar]
- 51.Greene CE. Feline enteric viral infections. In: Greene CE, editor. Infectious diseases of the dog and cat. ed 3. Saunders Elsevier; St Louis: 2006. p. 103. [Google Scholar]
- 52.Greene CE. Salmonellosis. In: Greene CE, editor. Infectious diseases of the dog and cat. ed 3. Saunders Elsevier; St Louis: 2006. p. 355. [Google Scholar]
- 53.Greene CE, Addie DD. Feline parvovirus infections. In: Greene CE, editor. Infectious diseases of the dog and cat. ed 3. Elsevier; St Louis: 2006. p. 78. [Google Scholar]
- 54.Grooters AM, Biller DS, Ward H. Ultrasonographic appearance of feline alimentary lymphoma. Vet Radiol Ultrasound. 1994;35:468. [Google Scholar]
- 55.Guilford WG, Jones BR, Markwell PJ. Food sensitivity in cats with chronic idiopathic gastrointestinal problems. J Vet Intern Med. 2001;15:7. doi: 10.1892/0891-6640(2001)015<0007:fsicwc>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 56.Halsey CH, Powers BE, Kamstock DA. Feline intestinal sclerosing mast cell tumour: 50 cases (1997-2008) Vet Comp Oncol. 2010;8:72. doi: 10.1111/j.1476-5829.2009.00206.x. [DOI] [PubMed] [Google Scholar]
- 57.Handagma PJ, Feldman BF. Drug-induced thrombocytopenia. Vet Res Commun. 1986;10:1. doi: 10.1007/BF02213961. [DOI] [PubMed] [Google Scholar]
- 58.Hart J, Shaker E, Patnaik A. Lymphocytic-plasmacytic enterocolitis in cats: 60 cases (1988-1990) J Am Anim Hosp Assoc. 1994;30:505. [Google Scholar]
- 59.Harvey CJ, Lopez JW, Hendrick MJ. An uncommon intestinal manifestation of feline infectious peritonitis: 26 cases (1986-1993) J Am Vet Med Assoc. 1996;209:1117. [PubMed] [Google Scholar]
- 60.Hayes G. Gastrointestinal foreign bodies in dogs and cats: a retrospective study of 208 cases. J Small Anim Pract. 2009;50:576. doi: 10.1111/j.1748-5827.2009.00783.x. [DOI] [PubMed] [Google Scholar]
- 61.Hicks GA, Coldwell JR, Schindler M. Excitation of rat colonic afferent fibres by 5-HT3 receptors. J Physiol. 2002;544:861. doi: 10.1113/jphysiol.2002.025452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holt P. Anal and perianal surgery in dogs and cats. In Pract. 1985;7:82. doi: 10.1136/inpract.7.3.82. [DOI] [PubMed] [Google Scholar]
- 63.Iannibelli F, Caruso A, Castelluccio A. Yersinia pseudotuberculosis in a persian cat. Vet Record. 1991;129:103. doi: 10.1136/vr.129.5.103. [DOI] [PubMed] [Google Scholar]
- 64.Jaksic B, Brugiatelli M. High dose continuous chlorambucil vs intermittent chlorambucil plus prednisone for treatment of B-CLL–IGCI CLL-01 trial. Nouv Rev Fr Hematol. 1988;30:437. [PubMed] [Google Scholar]
- 65.Janeczko S, Atwater D, Bogel E. The relationship of mucosal bacteria to duodenal histopathology, cytokine mRNA, and clinical disease activity in cats with inflammatory bowel disease. Vet Microbiol. 2008;128:178. doi: 10.1016/j.vetmic.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 66.Jergens A, Moore F, Haynes J. Idiopathic inflammatory bowel disease in dogs and cats: 84 cases (1987-1990) J Am Vet Med Assoc. 1992;201:1603. [PubMed] [Google Scholar]
- 67.Jergens AE, Moore FM, Haynes JS. Idiopathic inflammatory bowel disease in dogs and cats: 84 cases (1987-1990) J Am Vet Med Assoc. 1992;201:1603. [PubMed] [Google Scholar]
- 68.Johnson K, Lamport A, Batt RM. An unexpected bacterial flora in the proximal small intestine of normal cats. Vet Record. 1993;132:362. doi: 10.1136/vr.132.14.362. [DOI] [PubMed] [Google Scholar]
- 69.Johnston KL, Lamport AI, Ballèvre OP. Effects of oral administration of metronidazole on small intestinal bacteria and nutrients of cats. Am J Vet Res. 2000;61:1106. doi: 10.2460/ajvr.2000.61.1106. [DOI] [PubMed] [Google Scholar]
- 70.Johnston KL, Swift NC, Forster-van Hijfte MF. Comparison of the bacterial flora of the duodenum in healthy cats and cats with signs of gastrointestinal tract disease. J Am Vet Med Assoc. 2001;218:48. doi: 10.2460/javma.2001.218.48. [DOI] [PubMed] [Google Scholar]
- 71.Jones BR, Greene CE. Tyzzer's disease. In: Greene CE, editor. Infectious diseases of the dog and cat. ed 3. Saunders Elsevier; St Louis: 2006. p. 362. [Google Scholar]
- 72.Kimby E, Brandt L, Nygren P. A systematic overview of chemotherapy effects in B-cell chronic lymphocytic leukaemia. Acta Oncol. 2001;40:224. doi: 10.1080/02841860151116303. [DOI] [PubMed] [Google Scholar]
- 73.Kiselow MA, Rassnick KM, McDonough SP. Outcome of cats with low-grade lymphocytic lymphoma: 41 cases (1995-2005) J Am Vet Med Assoc. 2008;232:405. doi: 10.2460/javma.232.3.405. [DOI] [PubMed] [Google Scholar]
- 74.Kleinschmidt S, Harder J, Nolte I. Chronic inflammatory and non-inflammatory diseases of the gastrointestinal tract in cats: diagnostic advantages of full-thickness intestinal and extraintestinal biopsies. J Feline Med Surg. 2010;12:97. doi: 10.1016/j.jfms.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Konde LJ, Pugh CR. Radiology and sonography of the digestive system. In: Tams TR, editor. Handbook of small animal gastroenterology. Saunders; Philadelphia: 1996. p. 75. [Google Scholar]
- 76.Kosovsky J, Matthiesen D, Patnaik A. Small intestinal adenocarcinoma in cats: 32 cases (1978-1985) J Am Vet Med Assoc. 1988;192:233. [PubMed] [Google Scholar]
- 77.Kruth SA. Gram-negative bacterial infections. In: Greene CE, editor. Infectious diseases of the dog and cat. ed 3. Elsevier; St Louis: 2006. p. 320. [Google Scholar]
- 78.Lamb CR, Hansson K. Radiological identification of nonopaque intestinal foreign bodies. Vet Radiol Ultrasound. 1994;35:87. [Google Scholar]
- 79.Lecoindre P. Chronic inflammatory bowel diseases, etiopathogeny, diagnosis. Bull Acad Vét France. 2006;159:333. [Google Scholar]
- 80.Lecoindre P, Chevallier M. Contribution to the study of feline inflammatory bowel disease: 51 cases (1991-1994) Rev Méd Vét. 1997;11:893. [Google Scholar]
- 81.Lee R, Leowijuk C. Normal parameters in abdominal radiology of the dog and cat. J Small Anim Pract. 1982;23:251. [Google Scholar]
- 82.LeGrange S, Boothe D, Herndon S. Pharmacokinetics and suggested oral dosing regimen of cisapride: a study in healthy cats. J Am Anim Hosp Assoc. 1997;33:517. doi: 10.5326/15473317-33-6-517. [DOI] [PubMed] [Google Scholar]
- 83.Levitt L, Bauer MS. Intussusception in dogs and cats: a review of thirty-six cases. Can Vet J. 1992;33:660. [PMC free article] [PubMed] [Google Scholar]
- 84.Lingard AE, Briscoe K, Beatty JA. Low-grade alimentary lymphoma: clinicopathological findings and response to treatment in 17 cases. J Feline Med Surg. 2009;11:692. doi: 10.1016/j.jfms.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Litster AL, Sorenmo KU. Characterisation of the signalment, clinical and survival characteristics of 41 cats with mast cell neoplasia. J Feline Med Surg. 2006;8:177. doi: 10.1016/j.jfms.2005.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Louwerens M, London CA, Pedersen NC. Feline lymphoma in the post-feline leukemia virus era. J Vet Intern Med. 2005;19:329. doi: 10.1892/0891-6640(2005)19[329:flitpl]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 87.MacDonald J, Mullen H, Moroff S. Adenomatous polyps of the duodenum in cats: 18 cases (1985-1990) J Am Vet Med Assoc. 1993;202:647. [PubMed] [Google Scholar]
- 88.MacPhail C. Gastrointestinal obstruction. Clin Tech Small Anim Pract. 2002;17:178. doi: 10.1053/svms.2002.36606. [DOI] [PubMed] [Google Scholar]
- 89.Madewell BR, Gieger TL, Pesavento PA. Vaccine site-associated sarcoma and malignant lymphoma in cats: a report of six cases (1997-2002) J Am Anim Hosp Assoc. 2004;40:47. doi: 10.5326/0400047. [DOI] [PubMed] [Google Scholar]
- 90.Mahony OM, Moore AS, Cotter SM. Alimentary lymphoma in cats: 28 cases (1988-1993) J Am Vet Med Assoc. 1995;207:1593. [PubMed] [Google Scholar]
- 91.Makkena S, Suryawanshi RV, Rambabu K. Management of atresia ani and recto-vaginal fistula in a Persian cat. Indian Vet J. 2008;85:985. [Google Scholar]
- 92.Malik R, Gabor LJ, Foster SF. Therapy for Australian cats with lymphosarcoma. Aust Vet J. 2001;79:808. doi: 10.1111/j.1751-0813.2001.tb10923.x. [DOI] [PubMed] [Google Scholar]
- 93.Marks SL. Nev, 2008. Critical appraisal of infectious diarrhea in cats. Proceedings 80th Western Veterinary Conference, Las Vegas. [Google Scholar]
- 94.Marks SL, Kather EJ. Clostridium perfringens- and Clostridium difficile-associated diarrhea. In: Greene CE, editor. Infectious diseases of the dog and cat. ed 3. Saunders Elsevier; St Louis: 2006. p. 363. [Google Scholar]
- 95.McManus P. Lymphoma in veterinary medicine: no longer a one-word diagnosis. Vet Clin Pathol. 2008;37:360. doi: 10.1111/j.1939-165X.2008.00095.x. [DOI] [PubMed] [Google Scholar]
- 96.Mehendale SR, Yuan CS. Opioid-induced gastrointestinal dysfunction. Dig Dis. 2006;24:105. doi: 10.1159/000090314. [DOI] [PubMed] [Google Scholar]
- 97.Mellanby RJ, Foale R, Friend E. Anal sac adenocarcinoma in a Siamese cat. J Feline Med Surg. 2002;4:205. doi: 10.1053/jfms.2002.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Michael GB, Butaye P, Cloeckaert A. Genes and mutations conferring antimicrobial resistance in Salmonella: an update. Microbes Infect. 2006;8:1898. doi: 10.1016/j.micinf.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 99.Miettinen M, Lasota J. Gastrointestinal stromal tumors—definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Archiv. 2001;438:1. doi: 10.1007/s004280000338. [DOI] [PubMed] [Google Scholar]
- 100.Mooney SC, Hayes AA, MacEwen EG. Treatment and prognostic factors in lymphoma in cats: 103 cases (1977-1981) J Am Vet Med Assoc. 1989;194:696. [PubMed] [Google Scholar]
- 101.Moore PF, Woo JC, Vernau W. Characterization of feline T cell receptor gamma (TCRG) variable region genes for the molecular diagnosis of feline intestinal T cell lymphoma. Vet Immunol Immunopathol. 2005;106:167. doi: 10.1016/j.vetimm.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 102.Muir P, Rosin E. Failure of the single enterotomy technique to remove a linear intestinal foreign body from a cat. Vet Rec. 1995;136:75. doi: 10.1136/vr.136.3.75. [DOI] [PubMed] [Google Scholar]
- 103.Müller-Lissner SA, Kamm MA, Scarpignato C. Myths and misconceptions about chronic constipation. Am J Gastroenterol. 2005;100:232. doi: 10.1111/j.1572-0241.2005.40885.x. [DOI] [PubMed] [Google Scholar]
- 104.Nguyen Van N, Taglinger K, Helps CR. Measurement of cytokine mRNA expression in intestinal biopsies of cats with inflammatory enteropathy using quantitative real-time RT-PCR. Vet Immunol Immunopathol. 2006;113:404. doi: 10.1016/j.vetimm.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 105.O’Brien T. Large intestine. In: O’Brien TR, editor. Radiographic diagnosis of abdominal disorders in the dog and cat. Saunders; Philadelphia: 1978. p. 352. [Google Scholar]
- 106.Orr CM, Gruffydd-Jones TJ, Kelly DF. Ileal polyps in Siamese cats. J Small Anim Pract. 1980;21:669. doi: 10.1111/j.1748-5827.1980.tb05959.x. [DOI] [PubMed] [Google Scholar]
- 107.Özak A, Beşaltı Ö, Gökçe P. Megacolon in cats: 11 cases (1995-2001) Veteriner Cerrahi Dergisi. 2001;7:28. [Google Scholar]
- 108.Parry NMA. Anal sac gland carcinoma in a cat. Vet Pathol. 2006;43:1008. doi: 10.1354/vp.43-6-1008. [DOI] [PubMed] [Google Scholar]
- 109.Patnaik A, Johnson G, Greene R. Surgical resection of intestinal adenocarcinoma in a cat, with survival of 28 months. J Am Vet Med Assoc. 1981;178:479. [PubMed] [Google Scholar]
- 110.Patsikas MN, Papazoglou LG, Papaioannou NG. Ultrasonographic findings of intestinal intussusception in seven cats. J Feline Med Surg. 2003;5:335. doi: 10.1016/S1098-612X(03)00066-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Patterson EV, Reese MJ, Tucker SJ. Effect of vaccination on parvovirus antigen testing in kittens. J Am Vet Med Assoc. 2007;230:359. doi: 10.2460/javma.230.3.359. [DOI] [PubMed] [Google Scholar]
- 112.Pedersen NC, Allen CE, Lyons LA. Pathogenesis of feline enteric coronavirus infection. J Feline Med Surg. 2008;10:529. doi: 10.1016/j.jfms.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Penninck DG, Moore AS, Tidwell AS. Ultrasonography of alimentary lymphosarcoma in the cat. Vet Radiol Ultrasound. 1994;35:299. [Google Scholar]
- 114.Penninck DG, Nyland TG, Kerr LY. Ultrasonographic evaluation of gastrointestinal diseases in small animals. Vet Radiol Ultrasound. 1990;31:134. [Google Scholar]
- 115.Philbey AW, Brown FM, Mather HA. Salmonellosis in cats in the United Kingdom: 1955 to 2007. Vet Rec. 2009;164:120. doi: 10.1136/vr.164.4.120. [DOI] [PubMed] [Google Scholar]
- 116.Pohlman LM, Higginbotham ML, Welles EG. Immunophenotypic and histologic classification of 50 cases of feline gastrointestinal lymphoma. Vet Pathol. 2009;46:259. doi: 10.1354/vp.46-2-259. [DOI] [PubMed] [Google Scholar]
- 117.Portlock CS, Fischer DS, Cadman E. High-dose pulse chlorambucil in advanced, low-grade non-Hodgkin's lymphoma. Cancer Treat Rep. 1987;71:1029. [PubMed] [Google Scholar]
- 118.Preston D, Lennard-Jones J, Thomas B. Towards a radiologic definition of idiopathic megacolon. Abdom Imaging. 1985;10:167. doi: 10.1007/BF01893094. [DOI] [PubMed] [Google Scholar]
- 119.Rakich PM, Grooters AM, Tang KN. Gastrointestinal pythiosis in two cats. J Vet Diagn Invest. 2005;17:262. doi: 10.1177/104063870501700310. [DOI] [PubMed] [Google Scholar]
- 120.Randall LP, Cooles SW, Osborn MK. Antibiotic resistance genes, integrons and multiple antibiotic resistance in thirty-five serotypes of Salmonella enterica isolated from humans and animals in the UK. J Antimicrob Chemother. 2004;53:208. doi: 10.1093/jac/dkh070. [DOI] [PubMed] [Google Scholar]
- 121.Ranen E, Zur G. Perivulvar dermatitis in a cat treated by episioplasty. J Small Anim Pract. 2005;46:582. doi: 10.1111/j.1748-5827.2005.tb00290.x. [DOI] [PubMed] [Google Scholar]
- 122.Rappaport H. Tumors of the hematopoietic system. Atlas of tumor pathology. Ann Intern Med. 1967;67:686. [Google Scholar]
- 123.Rassnick KM, Williams LE, Kristal O. Lomustine for treatment of mast cell tumors in cats: 38 cases (1999-2005) J Am Vet Med Assoc. 2008;232:1200. doi: 10.2460/javma.232.8.1200. [DOI] [PubMed] [Google Scholar]
- 124.Richter K. Feline gastrointestinal lymphoma. In: Bonagura J, Twedt D, editors. Kirk's current veterinary therapy XIV. Saunders Elsevier; St Louis: 2009. [Google Scholar]
- 125.Richter KP. Feline gastrointestinal lymphoma. Vet Clin North Am Small Anim Pract. 2003;33:1083. doi: 10.1016/s0195-5616(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 126.Rivers BJ, Walter PA, Feeney DA. Ultrasonographic features of intestinal adenocarcinoma in five cats. Vet Radiol Ultrasound. 1997;38:300. doi: 10.1111/j.1740-8261.1997.tb00859.x. [DOI] [PubMed] [Google Scholar]
- 127.Roberts JA, Webb S, Paterson D. A systematic review on clinical benefits of continuous administration of beta-lactam antibiotics. Crit Care Med. 2009;37:2071. doi: 10.1097/CCM.0b013e3181a0054d. [DOI] [PubMed] [Google Scholar]
- 128.Rondeau M, Meltzer K, Michel K. Short-chain fatty acids stimulate feline colonic smooth muscle contraction. J Feline Med Surg. 2003;5:167. doi: 10.1016/S1098-612X(03)00002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Root CR, Lord PF. Linear radiolucent gastrointestinal foreign bodies in cats and dogs: their radiographic appearance. Vet Radiol Ultrasound. 1971;12:45. [Google Scholar]
- 130.Rutgers HC, Batt RM, Kelly DF. Lymphocytic-plasmacytic enteritis associated with bacterial overgrowth in a dog. J Am Vet Med Assoc. 1988;192:1739. [PubMed] [Google Scholar]
- 131.Ryan S, Seim H, 3rd, Macphail C. Comparison of biofragmentable anastomosis ring and sutured anastomoses for subtotal colectomy in cats with idiopathic megacolon. Vet Surg. 2006;35:740. doi: 10.1111/j.1532-950X.2006.00218.x. [DOI] [PubMed] [Google Scholar]
- 132.Schreurs E, Vermote K, Barberet V. Ultrasonographic anatomy of abdominal lymph nodes in the normal cat. Vet Radiol Ultrasound. 2008;49:68. doi: 10.1111/j.1740-8261.2007.00320.x. [DOI] [PubMed] [Google Scholar]
- 133.Schwandt CS. Low-grade or benign intestinal tumours contribute to intussusception: a report on one feline and two canine cases. J Small Anim Pract. 2008;49:651. doi: 10.1111/j.1748-5827.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- 134.Seguin MA, Papich MG, Sigle KJ. Pharmacokinetics of enrofloxacin in neonatal kittens. Am J Vet Res. 2004;65:350. doi: 10.2460/ajvr.2004.65.350. [DOI] [PubMed] [Google Scholar]
- 135.Selting KA. Intestinal tumors. In: Withrow SJ, Vail DM, editors. Withrow and MacEwen's small animal clinical oncology. ed 4. Saunders Elsevier; St Louis: 2007. p. 491. [Google Scholar]
- 136.Shabadash SA, Zelikina TI. Unknown hepatoid glands of certain cats and deer. Biol Bull Russ Acad Sci. 2003;30:383. [PubMed] [Google Scholar]
- 137.Shaiken L. Radiographic appearance of linear foreign bodies in cats. Vet Med. 1999;94:417. [Google Scholar]
- 138.Sharpe A, Cannon MJ, Lucke VM. Intestinal haemangiosarcoma in the cat: clinical and pathological features of four cases. J Small Anim Pract. 2000;41:411. doi: 10.1111/j.1748-5827.2000.tb03235.x. [DOI] [PubMed] [Google Scholar]
- 139.Shelton GH, Grant CK, Cotter SM. Feline immunodeficiency virus and feline leukemia virus infections and their relationships to lymphoid malignancies in cats: a retrospective study (1968-1988) J Acquir Immune Defic Syndr. 1990;3:623. [PubMed] [Google Scholar]
- 140.Sherding R. Diseases of the intestines. In: Sherding R, editor. The cat: diseases and clinical management. ed 2. Churchill Livingstone; New York: 1994. p. 1211. [Google Scholar]
- 141.Shoieb AM, Hanshaw DM. Anal sac gland carcinoma in 64 cats in the United Kingdom (1995-2007) Vet Pathol. 2009;46:677. doi: 10.1354/vp.08-VP-0257-S-FL. [DOI] [PubMed] [Google Scholar]
- 142.Simon D, Eberle N, Laacke-Singer L. Combination chemotherapy in feline lymphoma: treatment outcome, tolerability, and duration in 23 cats. J Vet Intern Med. 2008;22:394. doi: 10.1111/j.1939-1676.2008.0057.x. [DOI] [PubMed] [Google Scholar]
- 143.Simpson KW, Dogan B, Rishniw M. Adherent and invasive Escherichia coli is associated with granulomatous colitis in Boxer dogs. Infect Immun. 2006;74:4778. doi: 10.1128/IAI.00067-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Simpson KW, Fyfe J, Cornetta A. subnormal concentrations of serum cobalamin (vitamin B12) in cats with gastrointestinal disease. J Vet Intern Med. 2001;15:26. doi: 10.1892/0891-6640(2001)015<0026:scoscv>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 145.Slawienski M, Mauldin G, Mauldin G. Malignant colonic neoplasia in cats: 46 cases (1990-1996) J Am Vet Med Assoc. 1997;211:878. [PubMed] [Google Scholar]
- 146.Souza HJM, Corgozinho KB, Rosário JMP. Rectovaginal fistula and atresia ani in a kitten: case report. Clin Vet (Milano) 2000;5:26. [Google Scholar]
- 147.Spain CV, Scarlett JM, Wade SE. Prevalence of enteric zoonotic agents in cats less than 1 year old in Central New York State. J Vet Intern Med. 2001;15:33. doi: 10.1892/0891-6640(2001)015<0033:poezai>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 148.Stacy-Phipps S, Mecca JJ, Weiss JB. Multiplex PCR assay and simple preparation method for stool specimens detect enterotoxigenic Escherichia coli DNA during course of infection. J Clin Microbiol. 1995;33:1054. doi: 10.1128/jcm.33.5.1054-1059.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Suchodolski JS, Steiner JM. Laboratory assessment of gastrointestinal function. Clin Tech Small Anim Pract. 2003;18:203. doi: 10.1016/S1096-2867(03)00075-6. [DOI] [PubMed] [Google Scholar]
- 150.Suess RP, Jr, Martin RA, Moon ML. Rectovaginal fistula with atresia ani in three kittens. Cornell Vet. 1992;82:141. [PubMed] [Google Scholar]
- 151.Summerfield GP, Taylor PR, Mounter PJ. High-dose chlorambucil for the treatment of chronic lymphocytic leukaemia and low-grade non-Hodgkin's lymphoma. Br J Haematol. 2002;116:781. doi: 10.1046/j.0007-1048.2002.03362.x. [DOI] [PubMed] [Google Scholar]
- 152.Sweetenham J. Lymphoblastic lymphoma in adults. Curr Hematol Malig Rep. 2006;1:241. doi: 10.1007/s11899-006-0005-8. [DOI] [PubMed] [Google Scholar]
- 153.Tauni MA, Österlund A. Outbreak of Salmonella typhimurium in cats and humans associated with infection in wild birds. J Small Anim Pract. 2000;41:339. doi: 10.1111/j.1748-5827.2000.tb03214.x. [DOI] [PubMed] [Google Scholar]
- 154.Thieblemont C, Nasser V, Felman P. Small lymphocytic lymphoma, marginal zone B-cell lymphoma, and mantle cell lymphoma exhibit distinct gene-expression profiles allowing molecular diagnosis. Blood. 2004;103:2727. doi: 10.1182/blood-2003-06-2160. [DOI] [PubMed] [Google Scholar]
- 155.Thompson MS. Diseases of the anal sacs. In: Bonagura J, editor. Current veterinary therapy XIII small animal practice. Saunders; Philadelphia: 2000. p. 591. [Google Scholar]
- 156.Tidwell AS, Penninck DG. Ultrasonography of gastrointestinal foreign bodies. Vet Radiol Ultrasound. 1992;33:160. [Google Scholar]
- 157.Tsioli V, Papazoglou LG, Anagnostou T. Use of a temporary incontinent end-on colostomy in a cat for the management of rectocutaneous fistulas associated with atresia ani. J Feline Med Surg. 2009;11:1011. doi: 10.1016/j.jfms.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tudury EA, Lorenzoni OD. Colostomy in persian female cat with atresia ani and rectovaginal fistula. Rev Centr Cienc Rurai. 1989;19:155. [Google Scholar]
- 159.Turk M, Gallina A, Russell T. Nonhematopoietic gastrointestinal neoplasia in cats: a retrospective study of 44 cases. Vet Pathol. 1981;18:614. doi: 10.1177/030098588101800506. [DOI] [PubMed] [Google Scholar]
- 160.Twomey L, Alleman AR. Cytodiagnosis of feline lymphoma. Comp Contin Educ Pract Vet. 2005;27:17. [Google Scholar]
- 161.Tyrrell D, Beck C. Survey of the use of radiography vs. ultrasonography in the investigation of gastrointestinal foreign bodies in small animals. Vet Radiol Ultrasound. 2006;47:404. doi: 10.1111/j.1740-8261.2006.00160.x. [DOI] [PubMed] [Google Scholar]
- 162.Vail DM, Moore AS, Ogilvie GK. Feline lymphoma (145 cases): proliferation indices, cluster of differentiation 3 immunoreactivity, and their association with prognosis in 90 cats. J Vet Intern Med. 1998;12:349. doi: 10.1111/j.1939-1676.1998.tb02134.x. [DOI] [PubMed] [Google Scholar]
- 163.Valli V, Jacobs R, Norris A. The histologic classification of 602 cases of feline lymphoproliferative disease using the National Cancer Institute working formulation. J Vet Diagn Invest. 2000;12:295. doi: 10.1177/104063870001200401. [DOI] [PubMed] [Google Scholar]
- 164.van Duijkeren E, Houwers DJ. A critical assessment of antimicrobial treatment in uncomplicated Salmonella enteritis. Vet Microbiol. 2000;73:61. doi: 10.1016/s0378-1135(00)00156-5. [DOI] [PubMed] [Google Scholar]
- 165.Vennema H, Poland A, Foley J. Feline infectious peritonitis viruses arise by mutation from endemic feline enteric coronaviruses. Virology. 1998;243:150. doi: 10.1006/viro.1998.9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Waknitz D, Greer DH. Urethrorectal fistula in a cat. Vet Med Small Anim Clin. 1983;78:1551. [Google Scholar]
- 167.Warren AL, Townsend KM, King T. Multi-drug resistant Escherichia coli with extended-spectrum β-lactamase activity and fluoroquinolone resistance isolated from clinical infections in dogs. Aust Vet J. 2001;79:621. doi: 10.1111/j.1751-0813.2001.tb10783.x. [DOI] [PubMed] [Google Scholar]
- 168.Washabau RJ. Report from: WSAVA Gastrointestinal Standardization Group. 2005. http://www.wsava.org/GIStandards1.htm Available at. Accessed January 17, 2010.
- 169.Washabau RJ, Day MJ, Willard MD. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J Vet Intern Med. 2010;24:10. doi: 10.1111/j.1939-1676.2009.0443.x. [DOI] [PubMed] [Google Scholar]
- 170.Washabau RJ, Hasler AH. Constipation, obstipation, and megacolon. In: August JR, editor. Consultations in feline internal medicine. ed 3. Saunders; Philadelphia: 1997. p. 104. [Google Scholar]
- 171.Weiss DJ, Gagne JM, Armstrong PJ. Relationship between inflammatory hepatic disease and inflammatory bowel disease, pancreatitis, and nephritis in cats. J Am Vet Med Assoc. 1996;209:1114. [PubMed] [Google Scholar]
- 172.White R. Surgical management of constipation. J Feline Med Surg. 2002;4:129. doi: 10.1053/jfms.2002.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wilcox RS, Bowman DD, Barr SC. Intestinal obstruction caused by Taenia taeniaeformis infection in a cat. J Am Anim Hosp Assoc. 2009;45:93. doi: 10.5326/0450093. [DOI] [PubMed] [Google Scholar]
- 174.Willard MD. Feline inflammatory bowel disease: a review. J Feline Med Surg. 1999;1:155. doi: 10.1016/S1098-612X(99)90204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Willis SE, Farrow CS. Partial gastrointestinal obstruction for one month because of a linear foreign body in a cat. Can Vet J. 1991;32:689. [PMC free article] [PubMed] [Google Scholar]
- 176.Zwahlen CH, Lucroy MD, Kraegel SA. Results of chemotherapy for cats with alimentary malignant lymphoma: 21 cases (1993-1997) J Am Vet Med Assoc. 1998;213:1144. [PubMed] [Google Scholar]
- 177.Zwingenberger AL, Marks SL, Baker TW. Ultrasonographic evaluation of the muscularis propria in cats with diffuse small intestinal lymphoma or inflammatory bowel disease. J Vet Intern Med. 2010;24:289. doi: 10.1111/j.1939-1676.2009.0457.x. [DOI] [PubMed] [Google Scholar]
Gastrointestinal Parasites
A common theme when discussing the prevalence of most gastrointestinal parasites in cats is that they occur more commonly in younger cats and in cats housed in crowded conditions, such as catteries and shelters. It is likely an increased chance for transmission exists in these populations.48 The reported prevalence for each parasite varies greatly with the population studied, the geographic location of the population, and the sensitivity of the diagnostic test used to study that population.48
The presence or absence of diarrhea is not a reliable predictor of whether a particular cat is infected with or shedding a parasite.42 In fact, most cats with diarrhea do not harbor enteric protozoa.48 On the other hand, most cats with diarrhea because of enteric pathogens will shed those organisms, often intermittently.
It is important to remember that infection with most gastrointestinal parasites may not cause clinical signs. Therefore detection of a pathogenic parasite in a cat with diarrhea does not necessarily prove causation.48 A search should always be undertaken to identify other causes of diarrhea prior to convicting a cat of having diarrhea because of a particular parasite. In addition, co-infections or the presence of other noninfectious causes of diarrhea can result in more severe diarrhea that is often refractory to treatment for the parasite. Treatment will be more rewarding if all potential causes of diarrhea are identified in the patient.
Enteric parasites with zoonotic potential occur commonly enough that cats, particularly those with diarrhea and who are owned by immunocompromised persons, should be evaluated for those pathogens.26, 27 The following is a discussion of the most common enteric parasites found in cats. For more on parasite prevention and control, see Chapter 8, and for more on zoonotic enteric parasites, see Chapter 34.
Nematodes
Ollulanus tricuspis
Ollulanus tricuspis is an almost microscopic nematode worm infecting the stomach of domestic and wild cats.5 The worm measures less than 1 mm long.3
Life Cycle
The larvae of O. tricuspis develop and hatch within the uterus of the female worm. They develop to maturity in the stomach of the cat where it is capable of re-infecting the host.3 The worm is transmitted to other cats that ingest the vomitus of an infected cat.41
Clinical Signs and Pathophysiology
Clinical signs shown by infected cats include vomiting, anorexia, and weight loss.2, 5 Histologic findings in infected cats include lymphocytic-plasmacytic gastritis, lymphoid hyperplasia, and mucosal fibrosis. Gross lesions may be absent, or the cat may develop nodular gastritis.41 One report suggested the parasite may have been a contributing factor in the carcinogenesis of a gastric adenocarcinoma in an infected cat.10
Diagnosis and Treatment
The diagnosis of infection with O. tricuspis is difficult, because ova are not shed in the feces; rather, the vomitus must be examined for worms or larvae. The worms may also appear in gastric mucosal biopsy samples.41 A report of 131 cats undergoing endoscopic examinations found the parasite in gastric biopsy samples from 4 cats.5
Fenbendazole may be effective in treating infections with O. tricuspis.41 Preparations with febantel may also be expected to successfully treat these infections.
Prevention and Zoonotic Potential
Transmission can be prevented by appropriately treating infected cats. Other cats should not be allowed to ingest infected vomit. This parasite is of no zoonotic concern.
Physaloptera
Another parasite rarely inhabiting the stomach in cats is in the genus Physaloptera. Larger than Ollulanus tricuspis, this blood-sucking worm infects cats that have ingested intermediate hosts, such as cockroaches, crickets, or flour beetles.11 Preying on transport hosts, such as mice that have eaten an intermediate host, is another way cats become infected with this parasite. Clinical signs of infection with Physaloptera spp. include vomiting, anorexia, and melena. A diagnosis of Physaloptera infection can be made after identifying the ova in the patient's feces or adult worms in the vomitus. Occasionally, the worms may be seen during gastroscopy. The adult worms must be differentiated from ascarids.11 Infection can be treated with ivermectin, pyrantel pamoate, or fenbendazole.3 Because there is no migratory phase of the life cycle, the treatment does not need to be repeated.11
Strongyloides
Three species of Strongyloides infect cats. Strongyloides felis infects cats in India and tropical Australia,1, 43 S. tumefaciens is a rare parasite of cats in the southeastern United States,3 and S. planiceps is found in cats in Malaya and Japan.1 Strongyloides stercoralis, found in dogs and humans, produces experimental infections in cats, but natural infection with this species has not been observed.1 Feline infection with Strongyloides spp. is considered by most to be rare. However, one report from Australia identified S. felis in 169 of 504 necropsied cats.43
Life Cycle
Infection with Strongyloides spp. occurs after ingestion of infective larvae. Infection can also take place after the larvae penetrate the skin of the cat.11 Ingested larvae penetrate the intestinal wall and migrate through the diaphragm into the lungs. After cutaneous penetration, the larvae enter the venous circulation and enter the lungs. After further development in the lungs, the parasite migrates up the trachea and is swallowed. Adult S. felis and S. planiceps burrow into the wall of the small intestine, while adult S. tumefaciens lives in the colonic mucosa. Ova may be shed in the feces or hatch in the intestinal tract. Autoinfection occurs if larvae become infective and penetrate the intestinal wall before being shed. Ova and larvae that are shed develop into free-living adult worms.11 The prepatent period is between 7 and 10 days.1, 11
Clinical Signs and Diagnosis
Signs of a Strongyloides spp. infection are usually absent.1, 40 Lung migration may cause cough or respiratory distress. The presence of the parasite in the intestinal tract may result in diarrhea and weight loss.11 Strongyloides tumefaciens is associated with the formation of small, worm-filled nodules in the colon.40
Identification of Strongyloides spp. larvae using the Baermann fecal concentration technique is required to diagnose most infections. Unless the infection is heavy, examination of a fresh fecal smear is insensitive for identification of these larvae.1 The nodules formed by S. tumefaciens infection can be visualized during colonoscopy. Histopathology of the biopsied nodules should reveal many adult worms.40
Treatment and Prevention
Infection with Strongyloides spp. can be treated with fenbendazole,11 pyrantel pamoate,40 thiabendazole,1, 11 or ivermectin.3 To evaluate efficacy, repeat a fecal examination 2 to 3 days after the treatment ends. Because of the presence of free-living adult worms in the environment and the ability of larvae to cause infection by penetrating intact skin, prevention is difficult. Keeping cats indoors in warm, humid climates may be an owner's only means of preventing infection with Strongyloides spp. parasites.
Whipworms
Infections with Trichuris vulpis rarely occur in cats and are considered to be clinically unimportant.3, 14
Roundworms
The two species of roundworms commonly infecting cats are Toxocara cati (Figure 23-39 ) and Toxascaris leonina (Figure 23-40 ). The latter also has the ability to infect dogs.14
FIGURE 23-39.
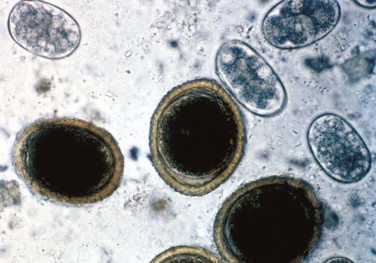
Fecal flotation showing the large, brown, thick-walled ova of Toxocara cati. The other ova are of Ancylostoma caninum (magnification 400×).
(From Marks SL, Willard MD: Diarrhea in kittens. In August JR, editor: Consultations in feline internal medicine, ed 5, St Louis, 2006, Saunders Elsevier, p 138.)
FIGURE 23-40.
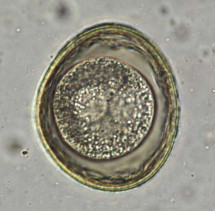
Ovum from Toxascaris leonina (magnification 400×).
(From Bowman DD: Helminths. In Bowman DD, editor: Georgis’ parasitology for veterinarians, ed 9, St Louis, 2009, Saunders Elsevier, p 313.)
Life Cycle
Cats are infected with T. cati in several ways. Most commonly, infection is by ingestion of contaminated food, water, or infected paratenic hosts such as rodents. Transuterine transmission has not been reported.14 Transmammary infection occurs, but only if the queen is acutely infected late in pregnancy. Chronically infected queens do not pass T. cati ova in their milk.14
After ingestion, T. cati larvae migrate through the small intestinal wall, into the liver, and then to the lungs where they are coughed up and swallowed. These larvae then infect the small intestine. Some of the migrating larvae become encysted in the cat's muscle tissue. Larvae from ova ingested through the milk tend not to undergo migration and mature directly in the small intestine.14 The prepatent period is approximately 8 weeks.
Infection with T. leonina occurs after ingestion of infective ova or an infected paratenic host. Unlike T. cati, very few T. leonina larvae migrate through the cat's tissues. Most develop in the wall of the small intestine. The prepatent period is 7 to 10 weeks. Toxascaris leonina ova can become infective within 8 days of being passed in the feces when the ambient temperature is 27° C but normally require 3 to 4 weeks.14
Clinical Signs
Clinical illness because of roundworm infection is uncommon. Illness, when it does happen, most often occurs in kittens26 Signs may be mild and can include vomiting,14 diarrhea, weight loss, poor growth, and a “pot belly.”26 A heavy infection with T. cati can result in catarrhal enteritis. Severe infections can lead to intestinal obstruction and, possibly, perforation.26 Much less dramatic changes arise after infection with T. leonina, although enteritis may occur.14
Diagnosis and Treatment
Roundworms are frequently diagnosed with a fecal floatation. The centrifugal floatation technique is more sensitive than the simple fecal floatation technique many hospitals use.14 Occasionally, adult worms will be passed with the feces.
The goals of treating roundworms include disease prevention in an individual cat or kitten, prevention of environmental contamination by cats defecating outside, and the prevention of zoonotic infections. Many effective and safe anthelmintics are available (Table 23-19 ). Benzimidazoles, such as fenbendazole, act on the parasite's microtubular structure, leading to disintegration of the worm's intestines, muscular layer, and hypodermis.14 Pyrantel in the pamoate formulation is poorly absorbed and causes paralytic parasite death. Macrocyclic lactones, such as milbemycin, also lead to paralytic parasite death. These compounds act on the parasite's gamma-aminobutyric acid (GABA)– and glutamate-controlled ion channels. These channels are lacking in tapeworms, accounting for the lack of efficacy against these parasites.14 Lastly, emodepside (a cyclic octadepsipeptide) has been combined with praziquantel in the product Profender (Bayer Animal Health). This topical parasiticide has been shown to be both safe and effective.14
TABLE 23-19.
Anthelmintic Drugs
| Drug | Trade Name | Dosage | Route and Duration | Susceptible Parasites |
|---|---|---|---|---|
| Emodepside | *Profender (Bayer Animal Health) | 3 mg/kg | Topical, once |
|
| Epsiprantel | Cestex (Pfizer) | 2.75 mg/kg | PO, once |
|
| Febantel | †Drontal Plus (Bayer Animal Health) | 15 mg/kg | PO |
|
| Fenbendazole | Panacur (Intervet) | 50 mg/kg q24h | PO, 3-5 days |
|
| Flubendazole | Flubenol (Janssen) | 22 mg/kg q24h | PO, 2-3 days |
|
| Ivermectin | Various | 200 µg/kg | PO, once |
|
| Milbemycin | Interceptor, Milbemax* (Novartis) | 2 mg/kg | PO, once |
|
| Piperazine | Pipa-Tabs (Vet-A-Mix) | 110 mg/kg | PO, repeat in 3 weeks |
|
| Praziquantel | Droncit (Bayer) | 20 to 25 mg/kg | PO, SC, once |
|
| Praziquantel | Droncit (Bayer) | 5 mg/kg | PO, SC, once daily for 3 to 5 days |
|
| Pyrantel pamoate | Nemex (Pfizer), Strongid (Pfizer) | 5-20 mg/kg | PO, repeat in 3 weeks |
|
| Pyrantel plus praziquantel | Drontal (Bayer) | 1 tablet/4 kg | PO, once |
|
| Selamectin | Revolution, Stronghold (Pfizer) | 6 mg/kg | Topical, once |
|
Combined with praziquantel.
Combined with praziquantel and pyrantel.
These drugs appear to be so safe that overdosing is almost impossible.14 Kittens can be dewormed starting at two weeks of age and again at 4, 6, 8, 12, and 16 weeks.26 Older kittens and adults can be dewormed every month to 4 months.14 Because of the safety of these drugs, the possibility of false-negative tests and, more importantly, the zoonotic potential of these infections, perhaps all kittens should be dewormed, not just those testing positive.
Prevention
Roundworm ova are very hardy and can remain infective for years.14 They survive sewage treatment and composting, and there is no practical means of decreasing the ova population once the environment is contaminated. Thus it is best to attempt to prevent contamination in the first place. When practical, keeping cats indoors allows appropriate control of potentially contaminated fecal material. If the pet cat is allowed outdoors, attempts at preventing hunting may reduce the possibility of infection. Keep children's play areas, such as sand boxes, inaccessible to cats when children are not at play. Feeding only well-cooked food can prevent infection by contaminated food. Finally, empirical, preventative deworming for cats that go outdoors should be performed 3 to 4 times yearly. Any less frequently does not lead to an appreciable decrease in the prevalence of the parasite.7
Zoonotic Potential
Roundworms easily infect humans who ingest the ova, particularly children. Visceral larval migrans occurs after infection with Toxocara canis in humans. Infection can lead to the formation of nodules in the brain, liver, lungs, and kidneys. Ocular larval migrans results in granulomatous retinitis that is often misdiagnosed as retinoblastoma in older children.3 This can lead to unnecessary enucleation. Toxocara cati appears, however, to be less important than T. canis as an infection in humans.3
Hookworms
The species of hookworms that infect cats are Ancylostoma tubaeforme and Ancylostoma braziliense (see Figure 23-39). They are reported to be an uncommon infection in cats.26, 34 Ancylostoma braziliense can also infect dogs.
Life Cycle
Hookworm infections occur after ingesting food or water contaminated with hookworm larvae or eating infected paratenic hosts. The larvae can survive for months in the tissues of paratenic hosts.14 Infection also occurs after larval migration through the skin. In either case, the worm matures in the small intestine.14 Unlike dogs, transmammary infection has not been reported in cats.3, 14
The prepatent period is between 19 and 28 days, depending on the route of infection. The time to patency after transcutaneous infection is longer than for direct colonization. Infective L3 larva develop 2 to 7 days after the ova are passed.3
Clinical Signs
Developing larvae attach to the mucosa of the small intestine where they ingest copious amounts of blood. Because the worms can remove a significant volume of blood from kittens, weakness from iron-deficiency anemia34 or blood-loss anemia may be noted.14 Melena and diarrhea may also be recognized. Signs are uncommon in adult cats.
Diagnosis and Treatment
Identification and treatment of hookworm infections are similar to that for roundworm infections (see Table 23-19).
Prevention
Hookworm larvae are not as hardy as roundworm eggs. Soil contamination may be a temporary problem in areas that experience a hard frost.3 Hookworm larvae will not develop in temperatures less then 15° C or greater than 37° C. Frequent, appropriate disposal of feces, cleaning surfaces with a 1% bleach solution, and deterring hunting may prevent infections.
Zoonotic Potential
Migration through the skin of persons coming into contact with the larvae of A. braziliense is the most common cause of cutaneous larval migrans, particularly in the southeastern United States.3 This is an erythematous, pruritic skin eruption often found on the soles of the feet of infected children.
Cestodes
The tapeworms most commonly found in cats are Dipylidium caninum and Taenia taeniaeformis. Diphyllobothrium latum, Spirometra spp., and Echinococcus multilocularis occasionally infect cats. The latter is important, because it can lead to alveolar echinococcosis in humans.8 Spirometra tapeworms are found in North America (S. mansonoides) and far-East Asia (S. mansoni and erinacei), while D. latum prefers temperate climates.11
Life Cycle
The life cycle of tapeworms is indirect. This means a cat must ingest the tissues of an infected intermediate host. For D. caninum, this is the flea Ctenocephalides felis.3 The intermediate hosts for T. taeniaeformis are small mammals such as rodents. Both D. latum and Spirometra spp. require two intermediate hosts for complete development. The first host for both is an aquatic copepod. The second intermediate host for D. latum is freshwater fish, while Spirometra tapeworms use tadpoles, snakes, mammals,11 and birds as second intermediate hosts.3 Cats become infected with these tapeworms only after ingesting the second intermediate host. After ingestion, the parasite attaches to the wall of the small intestine and begins to produce segments. These tapeworms rarely migrate through host tissues, but when they do, the infection can be life threatening. Patent infections become apparent in 17 days for D. caninum 8, 34 to 80 days for T. taeniaeformis,3 5 to 6 weeks for D. latum, and 10 days for Spirometra spp.3 Segment shedding may last for years unless the infection is treated. Taenia taeniaeformis eggs are immediately infective and survive for variable periods of time depending on the environment. The eggs prefer low temperatures and high humidity and may live up to a year in these conditions.8 Both D. latum and Spirometra spp. pass ova in the feces, not segments.11
Clinical Signs
Tapeworm infections are well tolerated by the cat. Usually there are no signs of infection other than finding segments on the feces or attached to perianal hair. Because both D. latum and Spirometra tapeworms absorb vitamin B12 across the cuticle, megaloblastic anemia is possible, but unlikely.11
Diagnosis and Treatment
Tapeworm infections are diagnosed by identifying the typical appearance of the segments8 or the egg packets within the segments.26 The segments of T. taeniaeformis are flat, while those of D. caninum have been described as appearing like a grain of rice. The segments should be handled carefully, because they are friable and rupture may result in exposure of the handler.8 The operculated ova of D. latum and Spirometra spp. must be differentiated from trematode ova.
Even though tapeworm infections are well tolerated, cats should be treated for reasons of owner discomfort and public health concerns (see Table 23-19). These infections are easily treated, because drug treatment is highly effective. Re-infection must be controlled using preventative measures, especially flea control to prevent re-infection with D. caninum. Praziquantel and epsiprantel are safe and effective. Fenbendazole is effective against T. taeniaeformis, but not D. caninum.
Prevention
Without controlling exposure to intermediate hosts, tapeworm infections are difficult to eliminate. Flea control is imperative in eradicating infections with D. caninum. Controlling predation helps prevent ingestion of T. taeniaeformis–infected rodents.
Zoonotic Potential
Infection with D. caninum occurs in young children who are most likely to eat fleas. Infection results in only minimal signs of illness.8 The larval stage of T. taeniaeformis is of little zoonotic importance.3 Although cats are uncommonly infected with Echinococcus multilocularis, potentially life-threatening alveolar damage occurs in North American humans infected with this tapeworm.8 Plerocercoids of Spirometra spp. can penetrate the mucous membranes or open skin wounds of humans and migrate around the subcutaneous connective tissue, forming nodules, a condition called sparganosis.3 Megaloblastic anemia, as a result of vitamin B12 deficiency, may occur in humans infected with D. latum or Spirometra spp. tapeworms.11
Trematodes
Alaria
Alaria marcianae flukes reside in the intestinal tract of cats and the mammary glands of lactating queens. Miracidia hatch underwater from ova shed in the feces and penetrate the skin of a snail. After further development, cercariae penetrate the skin of leopard frog tadpoles and are able to survive the metamorphosis to the adult frog.3 If the tadpole is eaten by a snake, bird, or mammal, the parasite enters the host's tissues but does not undergo further development. After a male or nonlactating female cat ingests the infected intermediate host, the parasite penetrates the wall of the small intestine, passes through the diaphragm, and enters the lungs for further development. Finally, the parasite is coughed up and swallowed to complete maturation and reproduce in the small intestine.
If, however, an infected host is ingested by a lactating queen, the parasite migrates through the tissues to the mammary glands, rather than the lungs.3 Once shed in the milk, the parasites develop into mature adults in the kittens. Some of the mesocercariae remain in the mammary glands to infect future litters. Clinical signs associated with worms in the small intestine are uncommon.11 Migration through the lungs often goes unnoticed, but the cat may cough or experience hemoptysis.3 Diagnosis involves demonstration of fluke ova in the feces. Although therapy may be unnecessary,11 praziquantel or epsiprantel are effective in eliminating the intestinal population of the fluke.
Platynosomum
Platynosomum spp. are flukes living in the gall bladder, bile ducts,46 and pancreatic ducts.3 These flukes are most prevalent in the southeast United States and Caribbean islands3 and require two intermediate hosts. The first host is a snail, while the second intermediate host is a lizard, toad, gecko, or skink.46 Cats become infected with this fluke after ingesting an infected second intermediate host. The prepatent period for the fluke is 8 weeks.46 Most infections are subclinical. If clinical signs do occur, they may include weight loss, vomiting, diarrhea, icterus, hepatomegaly, or abdominal distention. Diagnosis involves identification of ova shed in the feces using a fecal sedimentation method46 or by finding adult flukes in the gall bladder or bile ducts during abdominal surgery. Treatment involves administering praziquantel (20 mg/kg, q24h, PO for 3 to 5 days) and/or surgical removal of the flukes.3
Protozoa
Coccidia
Two species of coccidians are the most common to infect cats, Isospora felis and Isospora rivolta (Figure 23-41 ). The genus Isospora may be renamed Cystoisospora. These are species-specific obligate intracellular parasites.4, 13 They are able to survive in the environment for months.13
FIGURE 23-41.
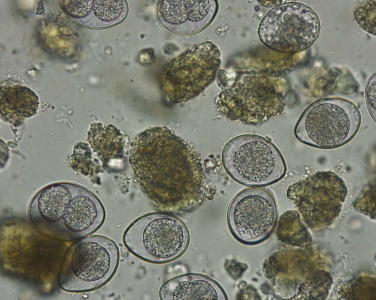
Zinc sulfate fecal flotation showing Isospora spp. oocysts from a kitten with diarrhea (magnification 1000×).
(From Marks SL, Willard MD: Diarrhea in kittens. In August JR, editor: Consultations in feline internal medicine, ed 5, St Louis, 2006, Saunders Elsevier, p 138.)
Life Cycle
A detailed description of the coccidial life cycle can be found elsewhere.4, 13 Simply put, direct transmission is by ingesting oocyst-contaminated food or water or by grooming contaminated body parts. Indirect transmission occurs after ingesting a mechanical vector or the infected tissues of paratenic hosts.39 After ingestion by a cat, the oocyst excysts in the small intestine and enters the enterocyte where further development occurs.26
The parasite may also migrate through the intestinal wall to form cysts in mesenteric lymph nodes. These cysts may serve as a source for reinfection.4, 13 The prepatent period is 4 to 11 days26 and the shed oocyst becomes infective after several days of exposure to warmth and moisture.13
Clinical Signs
Infection with Isospora spp. is usually subclinical.26 Signs, if they occur, range from mild, transient watery diarrhea to severe mucohemorrhagic diarrhea with vomiting and resultant dehydration and weight loss.4, 13 Signs are most commonly recognized in severely infected neonatal kittens,26 particularly those with concurrent illness, and arise because of small intestinal congestion, mucosal erosion, or villus atrophy.39 Signs may also be noted in immunosuppressed adult cats.26
Diagnosis and Treatment
Isospora species are readily found in fecal floatation or wet-mount examinations. Shedding can be intermittent, but most cats with diarrhea caused by coccidial infection shed large numbers of oocsyts.39
Fortunately, in most cats, the diarrhea from Isospora spp. infection is self-limiting.26 In fact, if a kitten is persistently shedding oocysts despite appropriate treatment13 or the parasite is identified in an adult cat with chronic diarrhea, attempts should be made to identify co-infections or other diseases that may cause diarrhea.39
Anticoccidial drugs are either coccidiostatic or coccidiocidal (Table 23-20 ). Coccidiostatic drugs are the most commonly used drugs for individual pet cats. Trimethoprim-augmented sulfadiazine (Tribrissen; Intervet/Schering-Plough Animal Health, Summit, NJ) or another sulfa-containing antibiotic, sulfadimethoxine (Albon; Pfizer Animal Health, Madison, NJ), can be used. Supportive care for severely affected kittens, such as parenteral rehydration, should be used as needed.
TABLE 23-20.
Antiprotozoal Drugs
| Drug | Trade Name | Dosage | Route, Duration | Susceptible Parasites |
|---|---|---|---|---|
| Azithromycin | Zithromax (Pfizer) | 10 mg/kg q24h | PO, 10 days minimum; 28 days for T. gondii | Cryptosporidium spp., Toxoplasma gondii |
| Clindamycin | Antirobe (Pfizer) | 25 mg/kg q12h | PO, 14 to 21 days | Toxoplasma gondii shedding |
| Clindamycin | Antirobe (Pfizer) | 10 mg/kg q12h | PO, 28 days | Toxoplasma gondii |
| Febantel | †Drontal Plus (Bayer) | 56.5 mg/kg q24h | PO, 5 days | Giardia spp. |
| Fenbendazole | Pancur (Intervet) | 50 mg/kg q24h | PO, 5 days | Giardia spp. |
| Metronidazole | Flagyl (Pharmacia) | 25 mg/kg q12h | PO, 7 days | Giardia spp. |
| Nitazoxanide | Alinia (Romark Laboratories) | 25 mg/kg q12h | PO, 5-28 days | Giardia, Cryptosporidium |
| Paromomycin | Humatin (Parke-Davis) | 125-165 mg/kg q12h | PO, 5 days | Giardia, Cryptosporidium |
| Ponazuril | *Marquis (Bayer) | 20-50 mg/kg q24h | PO, 1-2 days | Isospora spp., Toxoplasma gondii shedding |
| Ronidazole | None | 30 mg/kg q24h | PO, 14 days | Tritrichomonas foetus |
| Sulfadimethoxine | Albon (Pfizer Animal Health) | 50 mg/kg once, then 25 mg/kg q24h | PO, 14-21 days | Isospora spp. |
| Trimethoprim-Sulfa | Tribrissen (Schering-Plough) | 30 mg/kg q12h | PO, 14 days | Isospora spp. |
| Trimethoprim-Sulfa | Tribrissen (Schering-Plough) | 15 mg/kg q12h | PO, 28 days | Toxoplasma gondii |
Dilute 1 gram of the paste in 3 mL of water to yield 37.5 mg of ponazuril per mL of solution.39
Combined with praziquantel and pyrantel.
Coccidiocidal drugs are often reserved for use in densely populated situations such as catteries or shelters.26 However, many veterinarians are now using them as a first-line defense against Isospora spp. infection.13 Ponazuril (Marquis Oral Paste; Bayer Animal Health, Shawnee Mission, Kan.), formulated for horses, is effective and can be safely administered to cats. For more on the use of ponazuril in cats, see Chapter 46. A related drug, diclazuril, is also available and may be administered once at 25 mg/kg PO.13 While not available in North America, toltrazuril (Baycox, Bayer Animal Health) may be administered once at 30 mg/kg PO or 15 mg/kg PO once daily for 3 days.32a A second course of therapy 10 days later may be required to completely eliminate the oocysts.
Prevention
Sanitation is very important, because the oocyst requires several days to become infective. Frequent removal of feces, preferably daily, is recommended to prevent re-infection and transmission to other cats.39 Controlling a cat's ability to hunt reduces the chance of ingesting an Isospora-infected rodent. Control of mechanical vectors, such as cockroaches and flies, is also useful.13 Since a cat can become infected after grooming an infected cat's perineum, consideration should be given to treating all cats in contact with the patient.39
In addition, catteries and shelters should ensure all food is well cooked, litter boxes are cleaned daily, and surfaces are well cleaned with steam13 or 10% ammonia.39 Where recurrent Isospora spp. infections are a problem, prophylactic treatment of all 2- to 3-week-old kittens with ponazuril should be considered.13 Despite all well-intentioned efforts at hygiene and treatment, Isospora spp. infection can still be transmitted to other cats.13
Zoonotic Potential
Because these are species-specific parasites, transmission of I. felis and I. rivolta from cats to humans does not occur.
Giardia
The flagellated protozoal parasite, Giardia duodenalis, has seven microscopically indistinguishable genotypes or assemblages.26 Assemblages A and B infect humans, while assemblage F is harbored by cats. Cats will occasionally harbor assemblages A and B.39
Life Cycle
Infection with G. duodenalis occurs after ingesting cyst-contaminated feces, by grooming an infected cat or from contaminated fomites.37 Re-infection may occur by self-grooming. Only a small number of cysts need be ingested to establish an infection. In humans as few as 10 cysts are required to cause infection.37
After ingestion of infective cysts, trophozoites begin to excyst in the stomach.37 This process is completed in the proximal duodenum.26 The trophozoites adhere to enterocytes along the length of the small intestine using the ventral suction disk. Intermittent shedding of immediately infective cysts begins 5 to 16 days after infection.28 Proteins released during encystment of the trophozoites are detected by the fecal antigen tests.37 Cysts may adhere to the perianal region, facilitating re-infection by self-grooming.28 Occasionally, trophozoites are found in examinations of fresh, watery feces. These do not survive for long and are not infective.4
Pathogenesis
The mechanisms of disease induced by G. duodenalis are still unclear. After the trophozoite attaches to the brush border of the enterocyte, the tight junction between cells is disrupted, increasing intestinal permeability.37 The brush border becomes attenuated, further exacerbating malabsorption of water, electrolytes, and other nutrients.39 The alteration in intercellular adhesion results in T-lymphocyte activation and mucosal cell injury.37 Infection also promotes mucosal cell apoptosis (preprogrammed cell death).28 In addition, small intestinal bacterial overgrowth may accompany G. duodenalis infections, resulting in more severe clinical signs.39
Clinical Signs
Fortunately, most cats infected with G. duodenalis show no clinical signs.28, 39 The most common sign is acute, transient, small bowel diarrhea28 without systemic illness, such as fever or vomiting.39 Less commonly, a cat might have profuse, watery malodorous diarrhea37 with mucus.39 Also possible, but uncommon, is weight loss26, 28 or abdominal pain.37 The severity of clinical signs exhibited in an individual cat depends on the age and general health of the cat.37 Cats co-infected with Cryptosporidium felis or Tritrichomonas foetus may have more severe diarrhea that is more difficult to control,39 as will the presence of bacterial overgrowth.
Diagnosis
The diagnosis of G. duodenalis requires demonstration of trophozoites or cysts in a fecal examination, or detection of encystment proteins or giardial DNA in a fecal sample. A reliable diagnosis may be difficult to obtain for several reasons. Cysts are small, easily missed, and must be differentiated from plant debris or yeast.37 Trophozoites are short lived outside the body and can only be found in very fresh, watery feces or, better yet, in diarrheic feces collected directly from the cat's rectum.37 Shedding of cysts is usually intermittent, and the intensity of shedding varies greatly.28, 37 Because of these difficulties, the absence of the organism in a fecal sample does not eliminate it as the cause of diarrhea. It is often necessary to test multiple fecal samples, using at least two different techniques in order to find the organism.37, 39
The easiest test to perform is a fecal smear or wet mount examination to identify trophozoites or cysts (FIGURE 23-42, FIGURE 23-43 ). The sample examined should be very fresh, warm, diarrheic feces.39 One drop of feces is placed on a slide along with a drop of 0.9% saline or Lugol iodine.28 Trophozoites are identified by their characteristic structure (Table 23-21 ). The motile trophozoites have a motion described as appearing like the back and forth rolling motion of a falling leaf. Since Lugol iodine stain kills the trophozoite, there will be no motion to detect.28 This test is not very sensitive; however, with trained examiners, the test has a high specificity.
FIGURE 23-42.
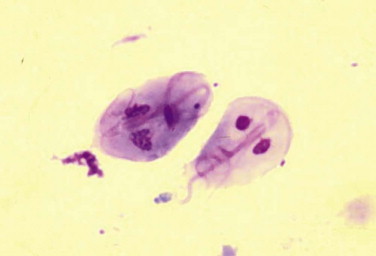
Giemsa-stained fecal smear showing two trophozoites of Giardia duodenalis. The trophozoite on the right, viewed from the top, displays the characteristic pear-shaped face appearance with the bilateral symmetry, two nuclei, posterior median bodies, and fibrils running the length of the parasite.
(From Marks SL, Willard MD: Diarrhea in kittens. In August JR, editor: Consultations in feline internal medicine, ed 5, St Louis, 2006, Saunders Elsevier, p 136.)
FIGURE 23-43.
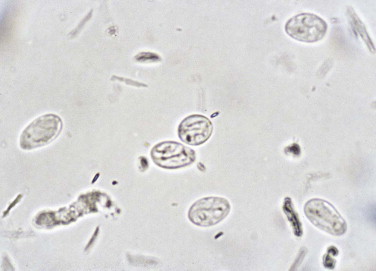
Zinc sulfate fecal flotation showing Giardia duodenalis cysts (magnification 400×).
(From Marks SL, Willard MD: Diarrhea in kittens. In August JR, editor: Consultations in feline internal medicine, ed 5, St Louis, 2006, Saunders Elsevier, Figure 15-6, p 137.)
Increased sensitivity can be gained by performing a centrifugal flotation using zinc sulfate. The sample should be warm, fresh feces or feces refrigerated for no more than 2 days.28 The processed sample is examined for the same structures as the wet mount. The sensitivity of examining one sample is 70%28 and increases as more samples are examined. The sensitivity of looking at three samples is 95%26, 28; therefore the test is not considered negative until three specimens have been found free of the organism.39
A fecal antigen test that identifies the encystment protein is available. The SNAP Giardia antigen test (IDEXX Laboratories) uses fresh or frozen feces, or feces refrigerated for less than 7 days.34 Since the antigen is continuously shed, this test avoids the problem of intermittent shedding of the whole organism.28 The sensitivity of the test is 85%, with a specificity of 100%.35 By combining the antigen test with a zinc sulfate fecal centrifugal flotation, the sensitivity improves to 97.8%.11 It is unknown how long the antigen remains in the feces after treatment. Thus a zinc sulfate centrifugal flotation examination should be used to evaluate therapeutic efficacy.28, 39 The use of this test in cats without diarrhea is controversial, because these cats are unlikely to shed cysts. The zoonotic significance of a positive antigen test in a cat not shedding cysts is unknown and may cause confusion.39
Polymerase chain reaction detection of Giardia DNA is available, but the test has not been standardized across all diagnostic laboratories. One needs to ensure the laboratory performing the test has validated it for assemblage F. The test may also be used to identify cats harboring the zoonotic assemblages A and B. The sensitivity of this test is unknown.39
Treatment
Two commonly available drugs are used most frequently to treat infections with G. duodenalis (see Table 23-20). Fenbendazole may be effective and can be used in pregnant queens28 and in cats co-infected with roundworms, hookworms, and Taenia spp. tapeworms.39 However, in one small study, only four of eight cats infected with both G. duodenalis and Cryptosporidium felis stopped shedding Giardia permanently after receiving fenbendazole.29 Febantel, in the combination product Drontal Plus (Bayer Animal Health), is converted to fenbendazole. When six experimentally infected cats received 56.5 mg/kg of febantel q24h PO for 5 days, four of them stopped shedding G. duodenalis cysts.39
Metronidazole has been the traditional drug used to treat G. duodenalis in pets.28 The drug is also useful for treating concurrent small intestinal bacterial overgrowth and clostridial infections.39 The administration of metronidazole may eliminate shedding in 67% of cats.26 Neurologic side effects may occur at the dose recommended for treatment of Giardia (see above, Therapeutics for Vomiting and Diarrhea). The use of a Giardia vaccine was ineffective in clearing infection by itself.44
The combination of fenbendazole and metronidazole has been suggested as the initial treatment of choice for G. duodenalis infections.39 Although controlled studies are lacking, they may work synergistically by acting on two different targets within the parasite.28 Febantel would be expected to have the same synergism with metronidazole.
Drug therapy may not be necessary in cats without diarrhea that are infected with G. duodenalis, 37 because it is uncommon for a cat to carry the assemblages required to infect humans. The veterinarian may be obligated to treat a healthy cat if the owner wants to treat, the owner is immunocompromised, or the goal is eradication of an infection from a multicat home or prevention of parasite transmission to Giardia-naïve cats is attempted.28
What may appear to be treatment failure is more likely to be re-infection. In addition to drug therapy, steps should be taken to prevent re-infection. All cats with diarrhea positive for G. duodenalis should be treated along with their housemates.37 Sanitation is imperative in the fight against re-infection and transmission of G. duodenalis. Dispose of old litter pans and scoops and use disposable litter boxes during treatment. When the infection is eliminated, not just controlled, new litter boxes and scoops may be purchased. Bathe all cats during treatment to remove cysts from the hair coat. Since Giardia spp. cysts are susceptible to desiccation,28 blow-dry all cats using a warm air blower, paying particular attention to the perineal area. Disinfect bowls, housing, and other utensils with bleach.28
In addition to antiprotozoal drugs and sanitation, supportive care may become necessary. Probiotics and a highly digestible, bland diet may be offered to cats with small bowel diarrhea, while a high-fiber diet may be useful for those few cats with large bowel diarrhea.39 Where required, hydration and electrolyte imbalances must be corrected and antiemetics used to control vomiting.
Therapy can be evaluated by retesting feces with a zinc sulfate centrifugal flotation examination 1 to 3 days after the end of treatment and again 3 weeks later. A positive test immediately posttreatment is most likely because of therapeutic failure. If the cat is negative immediately after treatment ends, but is positive 3 weeks later, re-infection is likely.28 Since the fecal antigen test may remain positive long after the infection is eradicated, this test is inappropriate for evaluating therapy.28, 39
Re-treatment of fecal flotation-positive, recovered cats may be handled in a manner similar to the positive healthy cat mentioned above.28 Cats with diarrhea that continue to shed cysts may be re-treated for G. duodenalis infection along with dietary modification and empirical treatment for other common intestinal parasites. However, serious consideration should be given to investigation into other potential causes of diarrhea.28
Prevention
The Giardia vaccine has been found to be ineffective in preventing infection37 and production has been discontinued.39 This means prevention of Giardia infection involves avoiding exposure, stress and re-infections. Providing a clean environment, feeding only processed foods, and controlling potential transport hosts will help reduce the chances of exposure. Isolation of cats with diarrhea may be important, too.39 Municipal sanitation control is difficult as the cyst survives for weeks in cool, moist environments.28 Cysts are also able to survive water treatment and can pass through attempts at water filtration.37
Zoonotic Potential
Giardiasis is associated with debilitating diarrhea in some humans, particularly those who are immunocompromised.35 However, cats do not commonly carry the assemblages needed to infect humans. Transmission of G. duodenalis from cats to humans is rare and unproven.28 Still, it seems prudent to consider the owner's health when contemplating management of giardial infections in cats. To avoid human health risks, cats with diarrhea that test positive for G. duodenalis should be treated with the goal of controlling the diarrhea.39 Since no treatment for G. duodenalis is completely effective or 100% safe, treatment of positive cats without diarrhea should only begin after a discussion of the benefits and risks of the treatment with the owner.39
Tritrichomonas foetus
Tritrichomonas foetus is best known for causing bovine reproductive infections. It is an obligate anaerobic parasite26 that also colonizes the lower intestinal tract of cats. There are enough differences between the two isolates that the feline isolate does not cause disease in heifers and vice versa.39 The parasite depends on the host's normal intestinal flora and secretions for obtaining nutrition.7 A report from the United States of purebred cats tested at an international cat show found T. foetus in 36 of the 117 cats tested, a prevalence of 31%.23 This parasite seems to have a higher prevalence in purebred cats than nonpurebred cats. A study of pet cats visiting veterinary hospitals across the United States reported 12 of 32 purebred cats were positive for T. foetus, while only 5 of 141 nonpurebred cats were positive. In this same study, 12 of the 17 positive tests were from purebred cats.45 A study from the United Kingdom of diarrheic fecal samples sent to a veterinary diagnostic laboratory reported similar results. Purebred cats represented 14 of the 16 cats testing positive for T. foetus. The U.K. study also found the Siamese and Bengal breeds each represented 6 of 14 positive cats; only two other breeds tested positive.25
Transmission
Like most other protozoal parasites, T. foetus is transmitted by ingestion of the parasite, in this case, the trophozoite. Unlike most of the other parasites, T. foetus does not form cysts and only survives up to 3 days outside the body in moist feces.47 A cat becomes infected through the use of a shared litter box with an infected cat. After walking into the box, the parasite is transferred from the infected feces of one cat to the paws of the other. Infection then occurs through ingestion of the trophozoites during grooming.47 After infection, T. foetus colonizes the distal ileum and colon,15 followed by shedding of infective trophozoites 2 to 7 days later.19
Clinical Signs
There are several mechanisms by which T. foetus causes diarrhea. These include alteration of the cat's normal bacterial flora population, increases in local inflammatory cytokine concentrations, production of enzymes, and direct mucosal injury. The resulting injury leads to plasmacytic-lymphocytic49 and neutrophilic colitis.37 Although most infections involve only the mucosa of the colon, one study reported two of seven cats with diarrhea and T. foetus infections as having trophozoites in deeper layers of the colonic wall.49 Co-infection with Cryptosporidium felis 17 or Giardia duodenalis 39 can be associated with increased numbers of T. foetus trophozoites and increased severity of diarrhea.
Signs of infection are most frequent in kittens and young cats, although infections without clinical signs can occur.39 Adult cats, however, may also show signs of T. foetus infection. The most common sign is a foul-smelling large bowel diarrhea with increased frequency of defecation,39 mucus, blood,15 and flatulence.37 The consistency of the diarrhea may wax and wane, but the presence of diarrhea does not.47 Cats with diarrhea are otherwise in good health and maintain their body condition.15, 39 Severe diarrhea can result in anal swelling and fecal incontinence.39 Diarrhea may respond to the use of antibiotics because of changes in the cat's intestinal microbial flora. However, it always returns at the cessation of therapy.39, 47 Many cats experience a spontaneous resolution of the diarrhea within 2 years of diagnosis.15, 38
Since T. foetus causes reproductive infections in heifers and bulls, there is speculation the parasite also infects the reproductive tract in cats. Tritrichomonas foetus was found in the uterus of a queen with pyometra.9 However, in a study of 60 breeding male and female cats from 33 catteries, no cytologic or molecular evidence of T. foetus was found in the reproductive tract. The authors reported colonic infection with T. foetus in 15 of the 60 cats representing 22 of the 33 catteries.24
Diagnosis
Detection of the trophozoites in a sample of feces is the most expedient means of diagnosing an infection with T. foetus (Figure 23-44 ). An index of suspicion is required, because the clinical presentation of T. foetus infection is often mistaken for infection with Giardia duodenalis. If a cat is not responding to treatment for that parasite, consider T. foetus as a cause of the diarrhea.
FIGURE 23-44.
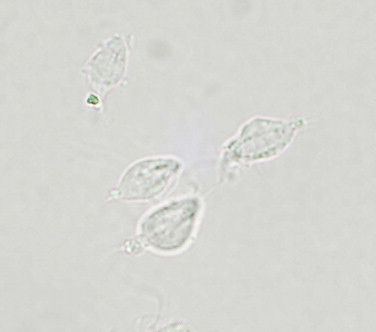
Tritrichomonas foetus trophozoites from culture. Notice the undulating membrane on the trophozoite on the right.
(From Scorza AV, Lappin MR: Gastrointestinal protozoal infections. In August JR, editor: Consultations in feline internal medicine, ed 6, St Louis, 2010, Saunders Elsevier, p 207.)
The sample required for the diagnosis of T. foetus is a fresh, nonrefrigerated sample of watery feces. Refrigeration kills the trophozoites, and they are not found in normal feces.34 The sample may be freshly passed diarrhea, feces collected using a wire loop passed into the colon, or collected by a colonic flush using a red rubber catheter and 10 mL of saline.47
A wet mount or smear examination of the feces should be performed on all cats with diarrhea. Examination of multiple samples may be required to find the T. foetus trophozoites with this technique because it is insensitive.39 The trophozoites must be differentiated from Giardia duodenalis based on structural differences and motility patterns (see Table 23-21).
The trophozoites of T. foetus can be cultured using the InPouch TF system (Biomed Diagnostics). This test is more sensitive than the fecal wet mount examination and detects 1000 trophozoites per sample.15 The number of parasites shed by a cat with diarrhea is high enough to be routinely detected with this method.18 The test should be performed in-house, because the parasite is unlikely to survive the trip to the laboratory.47 The test pouch is inoculated with 50 µg of freshly collected feces, about the size of a peppercorn.18 Any more than this increases the chances of bacterial overgrowth.15 The pouch is incubated at 25° C and examined under the microscope for motile trophozoites every other day for 12 days. The pouch should be tapped gently to dislodge the parasites, which tend to collect along the seams.15 The test is considered negative if parasites are not found after 12 days. One benefit of this system is that it does not support growth of Giardia duodenalis or Pentatrichomonas hominis.18
If a fecal wet mount examination and culture are both negative and infection with T. foetus is still under consideration, a PCR test can be performed. This test detects DNA from live or dead trophozoites, but is more expensive than other diagnostic methods.47 This test is more sensitive than the other two methods and can detect 10 parasites per sample.16 The sample size is 200 mg of feces not contaminated by litter preserved in 3 to 5 mL of rubbing alcohol shipped at room temperature.15 Trophozoites of T. foetus are sometimes found in colonic biopsy samples adhered to the surface or in the lumen of crypts.15
Treatment
The most effective drug for the treatment of T. foetus in cats is ronidazole.17 The drug has a bitter taste and should be compounded into capsules. Veterinary staff and owners should use gloves when handling ronidazole.15 If a confirmed relapse occurs, another course of treatment may eliminate the parasite.39 Diarrhea may take several weeks to resolve after elimination of the parasite, because significant colitis is often present.47 Effectiveness of treatment can be evaluated by performing fecal PCR tests 2 and 20 weeks after the end of treatment.15 Apparent treatment failures may occur because of re-infection, co-infection with Giardia duodenalis or Cryptosporidium felis, or the presence of another concurrent diarrhea-causing disorder. A more worrisome cause for treatment failure is a recent report of parasite resistance to ronidazole in two cats.22 Fortunately, diarrhea ultimately resolved in both cats despite the continued presence of the parasite. If the cat retests negative and the diarrhea is not improving after 2 weeks, consider the possibility that another disease may exist.
Nonspecific treatment for diarrhea is unhelpful37 and may prolong the duration of diarrhea.15 Diarrhea may respond to antibiotics as they alter the intestinal flora population; however, once treatment is stopped, the diarrhea will return.47
An important and potentially serious adverse effect of ronidazole administration in cats is a reversible neurotoxicity. Onset of signs often begins within 1 week of the onset of therapy and may last between 1 and 4 weeks after cessation of therapy.38 These signs can include depression, ataxia, seizures,47 behavioral changes, weakness, hyperesthesia, and trembling.38 Neurotoxicosis usually requires only supportive care along with discontinuation of the drug. The neurologically affected cat should be retested for the parasite, because it may have been eliminated.38 Because of the potential for neurotoxicity, the use of ronidazole should be restricted to cats with confirmed infections with T. foetus.47
Prevention and Zoonotic Potential
Crowded conditions should be avoided, because transmission of T. foetus trophozoites is more efficient in these settings.39 Cats testing positive should be isolated from other cats during treatment.37 Providing a clean environment will help prevent transmission of trophozoites.
Although there is a report of an infection in one immunocompromised person, transmission of T. foetus trophozoites from cats to healthy humans has not been reported.39 Still, prudence dictates handling feces infected with T. foetus trophozoites carefully.
Cryptosporidium felis
Recent genetic evaluations have shown that most feline infections with Cryptosporidium spp. are with C. felis; not, as previously thought, with C. parvum.36 Cryptosporidium parvum seems to be limited to farm animals.4 Cryptosporidium felis is an obligate intracellular parasite infecting the small intestine.4
Life Cycle
Infective oocysts are ingested from contaminated feces during self-grooming of contaminated body parts and from contaminated food and water.32, 39 After infection, the parasite attaches to the brush border of the enterocyte. The prepatent period is 3 to 6 days,39 and the oocysts are infective as soon as they are shed, making this a very contagious disease.20 Like most intestinal parasites, shedding is often intermittent.
Clinical Signs
The pathogenic effects of C. felis infections are not well understood. Direct cytotoxicity and inflammation causes villus atrophy and decreased surface area for absorption of water, electrolytes, and other nutrients.20, 32 Apoptosis (preprogrammed cell death) of the mucosal cells may be accelerated, adding to the malabsorption.20
Most infections with C. felis are subclinical.39 Signs, if present, range from a mild, self-limiting small bowel diarrhea33 to chronic intermittent small bowel diarrhea.32 Severe diarrhea with weight loss and anorexia may also occur.32, 33 Clinically apparent infections are most common in kittens, adult cats with concurrent gastrointestinal diseases, and cats co-infected with Giardia duodenalis or Tritrichomonas foetus.39 Cats with co-infections may experience more severe clinical signs.32
Diagnosis
A fecal flotation, which should be performed on all cats with diarrhea, may reveal C. felis if there are large numbers of oocysts (Figure 23-45 ). The fecal floatation test, however, is often negative39 because of intermittent shedding. The parasite is small and floats in a higher plane than helminth ova; the high-power lens and appropriate adjustment of the microscope stage is required to find the parasite.32 The small size of the oocyst makes identification difficult, particularly if the examiner is not specifically looking for them.34
FIGURE 23-45.
Fecal smear from a cat with diarrhea showing a single oocyst of Cryptosporidium felis colored with a modified Ziehl-Neelsen stain (magnification 1000×).
(From Marks SL, Willard MD: Diarrhea in kittens. In August JR, editor: Consultations in feline internal medicine, ed 5, St Louis, 2006, Saunders Elsevier, p 135.)
A modified Ziehl-Neelsen stain of a thin fecal smear may help in the identification of the oocysts.39 This technique works well in humans with large numbers of oocysts.33 Once signs resolve or the oocyst numbers decline, a single examination of a stained smear becomes insensitive. When only one sample is available, testing for C. felis antigen is a good choice.34 The ProSpecT Microplate Assay (Alexon Biomedical, Sunnyvale, Calif.) is more sensitive and specific for the diagnosis of C. felis than is the examination of a stained smear.6 Immunofluorescent antibody testing is available from some laboratories.
Fecal C. felis DNA can be detected using PCR testing. This test is available at many veterinary diagnostic laboratories; however, at present, there is no test standardization among laboratories.39 The clinical and zoonotic significance of a positive PCR test combined with an oocyst negative test is unknown.39 Therefore a positive PCR test in a cat without diarrhea presents a confusing situation for the attending veterinarian with regard to recommendations for the owner.
Treatment
Unfortunately, there are no completely effective and safe treatment protocols available for C. felis.32, 39 A concerted attempt to find other causes of diarrhea should take place prior to convicting a cat of having diarrhea solely from C. felis infection. Most reports on therapy for C. felis are uncontrolled and anecdotal. A number of drugs have been discussed. Azithromycin for at least 10 days appears safe but produces variable results.39 Paromomycin, an oral aminoglycoside, may be effective. However, one study reported acute renal failure in 4 of 32 cats receiving the drug. Deafness also occurred in three of those four cats.21 Nitazoxanide is a drug approved for treating humans with diarrhea caused by Cryptosporidium spp. infections. The administration of nitazoxanide to cats at 25 mg/kg q12h PO for at least 5 days39 up to 28 days32 may be effective. However, nitazoxanide is a gastrointestinal irritant and commonly results in vomiting and foul-smelling diarrhea.
Co-infections with Giardia duodenalis and/or Tritrichomonas foetus are more difficult to control. If diarrhea from C. felis infection improves but does not resolve at the end of therapy, the duration of treatment may be prolonged.39 Additional diagnostic testing should also be performed to ensure the only cause of the diarrhea is infection with C. felis.
Prevention
Environmental control of C. felis is difficult, because it is extremely hardy. It is resistant to chlorination and most disinfectants.32 Oocysts remain viable at temperatures above freezing up to 65° C.4 The parasite is difficult to filter and survives treatment at municipal water treatment facilities.20 Steam-cleaned housing and utensils may be beneficial in controlling parasite numbers, and they are susceptible to 5% ammonia solutions; however, the required contact time is 18 hours.39
Zoonotic Potential
Cryptosporidium spp. are relatively species specific, and there are no reports of waterborne outbreaks of human cryptosporidiosis associated with C. felis.32 Cryptosporidiosis can cause life-threatening diarrhea in HIV-positive persons.20 Fortunately, humans are rarely infected with C. felis.39 In fact, the zoonotic species most commonly found in humans (often veterinary students), is C. parvum found in young heifers.4 Regardless of a person's health, feces from a cat with diarrhea should be handled carefully. If a cat infected with Cryptosporidium spp. is owned by an immunocompromised person, a PCR test may be useful in determining the species of the parasite and its zoonotic risk.
Toxoplasma gondii
Like other coccidians, Toxoplasma gondii is an obligate intracellular parasite.12 Domestic cats and other felids are the only animals that shed oocysts. Any warm-blooded animal, including humans, can be infected with this parasite.
Life Cycle
Toxoplasma gondii can be transmitted by ingestion of infective oocysts in fecally contaminated food or water after ingestion of tissue cysts through carnivorism, or by transplacental or trans-mammary transmission of the parasite. The parasite enters into one of two cycles, depending on the host species. The enteroepithelial cycle only occurs in cats and results in shedding of oocysts after sexual reproduction of the parasite. After a cat ingests an infective oocyst or a tissue cyst, the parasite enters the mucosal cells of the small intestine, where it may undergo development and sexual reproduction, after which oocysts are shed.12 The prepatent period after ingesting an infective oocyst is 19 to 48 days, while shedding after ingesting tissue cysts starts in 3 to 10 days.4 Fecal shedding, which occurs only after initial infection, lasts for 2 to 3 weeks4, 31 and the oocysts become infective 1 to 5 days after they are shed.12
The extraintestinal cycle occurs in any animal, including cats. After ingestion, the parasite penetrates the cells of the small intestine and rapidly replicates in the enterocytes and associated lymph nodes into tachyzoites. After hematogenous and lymphatic spread, tachyzoites infect cells in all tissues of the body.4 Tissues most commonly infected include the brain, liver, pancreas, and lungs.30 If a pregnant queen becomes infected, tachyzoites cause placentitis, after which they infect the fetus.13 In 3 weeks, the host's immune response slows parasite replication, and the resultant bradyzoites form tissue cysts30 in the brain, striated muscle, and liver, and they remain viable for the life of the animal.4 Immunosuppressive drugs or disease may dull the suppression of parasite division by the host immune system and allow the slowly dividing bradyzoites in tissue cysts to begin rapid division, thereby reactivating the infection with tachyzoites.30
Pathogenesis
None of the forms of T. gondii produces a toxin. Rapid replication of tachyzoites within a cell leads to rupture of the cell and necrosis of the tissue in which they are located.12 The most commonly injured tissues are the brain, lungs, liver, and pancreas. Prenatal infection leads to more severe illness, because the immature immune system is unable to slow down replication by tachyzoites, allowing continued damage to tissues. Prenatal infection is more likely to result in ocular infections, and neonatal death is usually caused by pulmonary or hepatic infection.30 Type II and IV hypersensitivities may be involved in the pathogenesis of chronic disease from bradyzoites in tissue cysts.30
Clinical Signs
Kittens infected perinatally can be stillborn or die shortly after birth. They may also suffer from hepatomegaly and ascites, central nervous system signs resulting from encephalitis, respiratory distress, or uveitis.12, 13
Clinical signs of infection in healthy adult cats are uncommon (Box 23-2 ).31 Diarrhea from enteroepithelial development of the parasite is rare.39 Cats that develop clinical disease often have an episodic course with vague signs30 that depend on the body system affected. Onset of illness may be acute or chronic, and the most commonly affected organs include the brain, lungs, liver, heart, pancreas, and the eyes.13 Signs are the result of spread of tachyzoites after initial infection or after reactivation of tissue cysts. Cats suffering from uveitis may develop lens luxation and glaucoma.
BOX 23-2. Clinical Findings in Feline Toxoplasmosis.
Fever
Anorexia, lethargy
Weight loss
Muscle pain, hyperesthesia
- Respiratory tract disease
- Conjunctivitis
- Rhinitis
- Coughing
- Respiratory distress, tachypnea
- Diffuse harsh lung sounds
Vomiting, diarrhea
Abdominal discomfort
Icterus
Abdominal effusion
Arthritis, joint pain, shifting lameness
Cardiac arrhythmias, sudden death
Splenomegaly
Lymphadenomegaly
Pyogranulomatous dermatitis
- Neurologic signs
- Ataxia
- Circling
- Behavioral changes
- Twitching
- Tremors
Ocular signs
- Retinochoroiditis, retinal hemorrhages
- Optic neuritis
- Optic nerve atrophy
- Anisocoria
- Blindness
- Anterior uveitis, aqueous flare, hyphema, velvety iris
- Glaucoma
- Lens luxation
- Retinal detachment
- Neonatal disease (after transplacental transmission)
- Stillbirth
- Fading kittens
- Organ dysfunction
Liver: hepatomegaly, icterus, ascites
Lung: respiratory distress
Central nervous system: excessive sleep, crying
Adapted from Dubey JP, Lappin MR: Toxoplasmosis and neosporosis. In Greene CE, editor: Infectious diseases of the dog and cat, ed 3, St Louis, 2006, Saunders Elsevier, p 759.
Diagnosis
The best way to identify a cat shedding T. gondii oocysts is to demonstrate them with a centrifugal fecal flotation technique using Sheather sugar solution. The oocysts are about a quarter of the size of Isospora felis oocysts (Figure 23-46 ).12 Oocysts of T. gondii are morphologically indistinguishable from Hammondia or Besnoitia spp. oocysts.13 Detection of fecal T. gondii DNA using a PCR test can be used to definitively differentiate T. gondii oocysts from similar coccidians.31 It is probably best, however, to assume suspicious oocysts are those of T. gondii until proven otherwise.
FIGURE 23-46.
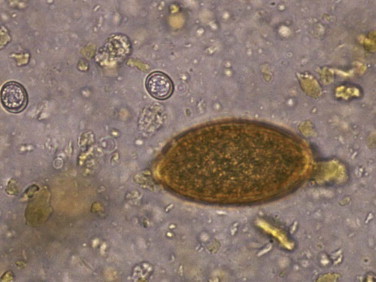
An unstained fecal sample from a naturally infected cat showing oocysts of Toxoplasma gondii compared with a Capillaria spp. ovum (magnification 400×).
(From Dubey JP, Lappin MR: Toxoplasmosis and neopsporosis. In Greene CE, editor: Infectious diseases of the dog and cat, ed 3, St Louis, 2006, Saunders Elsevier, p 756.)
Proving infection with T. gondii is responsible for a cat's systemic illness is also difficult. Finding tachyzoites in cytology samples is uncommon. They are most likely to be identified from body cavity effusions.13 The most common method of identifying an infected cat is by detecting T. gondii–associated immunoglobulins using immunofluorescent antibody or ELISA techniques. Since cats are infected for life, a seropositive cat has been infected at some point in its life. However, use of serology alone is insufficient to diagnose an active T. gondii infection.
Serum immunoglobulin M (IgM) is produced within 1 to 2 weeks after infection, but increased IgM titers may persist for months to years. Serum immunoglobulin G (IgG) begins to rise later; in some cats, IgG may not be detectable for 4 to 6 weeks.12 By the time IgG is detectable, shedding will have ceased. Maternally acquired IgG persists in kittens for 8 to 12 weeks.13 A rising IgG titer is associated with an active infection, but the degree of increase is not associated with the severity of the clinical signs. If a cat becomes seronegative, it is more likely the titer has fallen below the sensitivity of the test rather than the parasite has been eliminated from the body.31 Because of the vague nature of the clinical signs, many cats are presented later in the course of the disease. By this time, they may have switched from IgM to IgG production or passed the time of maximal IgG production. Thus a negative IgM titer or a lack of rising IgG titer does not rule out T. gondii infection.30 Also, reactivation of tissue cysts is rarely associated with rising IgG titers.31
Ultimately, the diagnosis of an active systemic T. gondii infection requires demonstration of an IgM titer greater than 1 : 64 or a fourfold increase in IgG titers over a 2- to 3-week period along with signs consistent with toxoplasmosis, the exclusion of other disorders that may cause the clinical signs, and response to appropriate anti–T. gondii treatment.31 Although serum IgM titers may be increased in otherwise healthy cats, increased IgM titers in cerebrospinal fluid or aqueous humor only occurs in cats with active CNS or ocular infections.30
Treatment
The goals of treating a cat infected with T. gondii are to reduce shedding of oocysts and to control the clinical signs in sick cats. Shedding can be reduced by using ponazuril,13 toltrazuril, or high doses of clindamycin.
The drug options for treating a sick cat include clindamycin, trimethoprim-augmented sulfadiazine, or azithromycin for at least 4 weeks (see Table 23-20). Recurrences are more common if the cat is treated for less than 4 weeks.13, 30 The antifolate drug pyrimethamine may be more effective than trimethoprim, but megaloblastic anemia develops in many cats. Supplementation with folinic acid (5 mg/cat, once daily, PO) or brewer's yeast (100 mg/kg, once daily, PO) may prevent or reverse the anemia.12 No drug clears all of the tissue cysts; so, cats remain infected for life. If uveitis is also present, use appropriate topical, oral, or parenteral corticosteroids. For a cat with proven T. gondii–associated uveitis alone, a topical ocular glucocorticosteroid is the only required treatment; no antibiotics are necessary unless the uveitis is persistent or recurrent.31
Clinical signs such as malaise, fever, and muscle pain should begin to resolve in 2 to 3 days.30 If there is no response within 7 days, switch to or add another drug.31 If there is still no response, search for another condition that may cause the observed clinical signs. However, ocular and CNS signs resolve more slowly and thoracic radiographic changes may take weeks to resolve.30 Some CNS changes may never completely resolve. Cats co-infected with feline immunodeficiency virus (FIV) do not respond to anti–T. gondii treatment as well as FIV-negative cats respond.12
Prevention
Feeding cats commercially processed cat food and avoiding undercooked or raw meat can prevent exposure to T. gondii. Controlling hunting reduces access to paratenic hosts with infective tissue cysts. Access to mechanical carriers of T. gondii, such as earthworms or cockroaches, should be minimized.
Zoonotic Potential
Human infection with T. gondii is common, more so in warm, humid climates where the prevalence of T. gondii seropositive persons approaches 100%. The number of persons seropositive for T. gondii is estimated to be around 500,000,000 worldwide.12 Infective oocysts are hardy and may remain viable in the environment for up to 18 months.12 Human infection most often occurs after eating raw or undercooked meat infected with tissue cysts or by transplacental infection.31 Seropositive cats are finished shedding and are unlikely to resume shedding even if the infection becomes reactivated.31 Cats found to be shedding oocysts should be quarantined at a veterinary hospital until shedding ends. Oocysts of T. gondii have not been found on the hair coat13; so, transmission of toxoplasmosis does not occur after touching a cat.31
Pregnant women infected with T. gondii for the first time, or chronically infected women who are also HIV positive, can transmit the parasite to their unborn child. Transplacental infection can result in stillbirths, CNS, or ocular disease.30 More severe fetal disease may occur if the infection happens in the first half of the woman's pregnancy.12 Toxoplasma gondii infection of immunocompetent humans usually results in a self-limiting fever and malaise.30 Steps useful in preventing transmission of T. gondii to humans can be found in Box 23-3 .
BOX 23-3. Guidelines for Cat Owners to Avoid Acquiring Toxoplasmosis.
-
•
Wash hands after handling cats, especially if you are pregnant or immunocompromised.
-
•
Remove fecal material from the home environment daily, since shed oocysts require a minimum of 24 hours to become infective.
-
•
Do not have immunocompromised persons clean the litter box. If they must clean the litter box, they should wear gloves and wash hands thoroughly when finished.
-
•
Use litter box liners, and periodically wash the litter box with scalding water and detergent.
-
•
Wear gloves when gardening, and wash hands thoroughly when finished.
-
•
Cover children's sandboxes when not in use to avoid fecal contamination by outdoor cats.
-
•
Only feed cats cooked or commercially processed food.
-
•
Control potential transport hosts, such as flies and cockroaches, that may bring the organism into the home.
-
•
Filter or boil water from sources in the environment.
-
•
Cook meat for human consumption to 80° C for 15 minutes minimum (because of uneven heating, microwave cooking does not kill all T. gondii 12).
-
•
Freeze meat at −12° C for 24 hours.12
-
•
Wear gloves when handling meat, and wash hands thoroughly with soap and water when finished.
Citation 12: Dubey JP, Lappin MR: Toxoplasmosis and neopsporosis. In Greene CE, editor: Infectious diseases of the dog and cat, ed 3, St Louis, 2006, Saunders Elsevier, p 754.
Adapted from Box 274-1 in Lappin MR: Toxoplasmosis. In Bonagura JD, Twedt DC, editors: Kirk's current veterinary therapy XIV, St Louis, 2009, Saunders Elsevier, p 1257.
References
- 1.Bowman D, Hendrix C, Lindsay D. Feline clinical parasitology. ed 1. Iowa State University Press; Ames, Iowa: 2002. Strongyloides species; p. 235. [Google Scholar]
- 2.Bowman DD. Diagnostic parasitology. In: Bowman DD, editor. Georgis’ parasitology for veterinarians. ed 9. Saunders Elsevier; St Louis: 2009. p. 295. [Google Scholar]
- 3.Bowman DD. Helminths. In: Bowman DD, editor. Georgis’ parasitology for veterinarians. ed 9. Saunders Elsevier; St Louis: 2009. p. 115. [Google Scholar]
- 4.Bowman DD. Protozoans. In: Bowman DD, editor. Georgis’ parasitology for veterinarians. ed 9. Saunders Elsevier; St Louis: 2009. p. 84. [Google Scholar]
- 5.Cecchi R, Wills SJ, Dean R. Demonstration of Ollulanus tricuspis in the stomach of domestic cats by biopsy. J Comp Pathol. 2006;134:374. doi: 10.1016/j.jcpa.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Cirak VY, Bauer C. Comparison of conventional coproscopical methods and commercial coproantigen ELISA kits for the detection of Giardia and Cryptosporidium infections in dogs and cats. Berl Munch Tierarztl Wochenschr. 2004;117:410. [PubMed] [Google Scholar]
- 7.Coati N, Hellmann K, Mencke N. Recent investigation on the prevalence of gastrointestinal nematodes in cats from France and Germany. Parasitol Res. 2003;90(Suppl 3):S146. doi: 10.1007/s00436-003-0921-7. [DOI] [PubMed] [Google Scholar]
- 8.Conboy G. Cestodes of dogs and cats in North America. Vet Clin North Am Small Anim Pract. 2009;39:1075. doi: 10.1016/j.cvsm.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Dahlgren SS, Gjerde B, Pettersen HY. First record of natural Tritrichomonas foetus infection of the feline uterus. J Small Anim Pract. 2007;48:654. doi: 10.1111/j.1748-5827.2007.00405.x. [DOI] [PubMed] [Google Scholar]
- 10.Dennis MM, Bennett N, Ehrhart EJ. Gastric adenocarcinoma and chronic gastritis in two related Persian cats. Vet Pathol. 2006;43:358. doi: 10.1354/vp.43-3-358. [DOI] [PubMed] [Google Scholar]
- 11.Dimski DS. Helminth and noncoccidial protozoan parasites of the gastrointestinal tract. In: Sherding RG, editor. The cat: diseases and clinical management. ed 2. Saunders; Philadelphia: 1994. p. 585. [Google Scholar]
- 12.Dubey JP, Lappin MR. Toxoplasmosis and neosporosis. In: Greene CE, editor. Infectious diseases of the dog and cat. ed 3. Saunders Elsevier; St Louis: 2006. p. 754. [Google Scholar]
- 13.Dubey JP, Lindsay DS, Lappin MR. Toxoplasmosis and other intestinal coccidial infections in cats and dogs. Vet Clin North Am Small Anim Pract. 2009;39:1009. doi: 10.1016/j.cvsm.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Epe C. Intestinal nematodes: biology and control. Vet Clin North Am Small Anim Pract. 2009;39:1091. doi: 10.1016/j.cvsm.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Gookin JL. Tritrichomonas. In: Bonagura JD, Twedt DC, editors. Kirk's current veterinary therapy XIV. Saunders Elsevier; St Louis: 2009. p. 509. [Google Scholar]
- 16.Gookin JL, Birkenheuer AJ, Breitschwerdt EB. Single-tube nested PCR for detection of Tritrichomonas foetus in feline feces. J Clin Microbiol. 2002;40:4126. doi: 10.1128/JCM.40.11.4126-4130.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gookin JL, Copple CN, Papich MG. Efficacy of ronidazole for treatment of feline Tritrichomonas foetus infection. J Vet Intern Med. 2006;20:536. doi: 10.1892/0891-6640(2006)20[536:eorfto]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 18.Gookin JL, Foster DM, Poore MF. Use of a commercially available culture system for diagnosis of Tritrichomonas foetus infection in cats. J Am Vet Med Assoc. 2003;222:1376. doi: 10.2460/javma.2003.222.1376. [DOI] [PubMed] [Google Scholar]
- 19.Gookin JL, Levy MG, Law JM. Experimental infection of cats with Tritrichomonas foetus. Am J Vet Res. 2001;62:1690. doi: 10.2460/ajvr.2001.62.1690. [DOI] [PubMed] [Google Scholar]
- 20.Gookin JL, Nordone SK, Argenzio RA. Host responses to Cryptosporidium infection. J Vet Intern Med. 2002;16:12. doi: 10.1892/0891-6640(2002)016<0012:hrtci>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 21.Gookin JL, Riviere JE, Gilger BC. Acute renal failure in four cats treated with paromomycin. J Am Vet Med Assoc. 1999;215:1821. [PubMed] [Google Scholar]
- 22.Gookin JL, Stauffer SH, Dybas D. Documentation of in vivo and in vitro aerobic resistance of feline Tritrichomonas foetus isolates to ronidazole. J Vet Intern Med. 2010;24:1003. doi: 10.1111/j.1939-1676.2010.0534.x. [DOI] [PubMed] [Google Scholar]
- 23.Gookin JL, Stebbins ME, Hunt E. Prevalence of and risk factors for feline Tritrichomonas foetus and Giardia infection. J Clin Microbiol. 2004;42:2707. doi: 10.1128/JCM.42.6.2707-2710.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gray SG, Hunter SA, Stone MR. Assessment of reproductive tract disease in cats at risk for Tritrichomonas foetus infection. Am J Vet Res. 2010;71:76. doi: 10.2460/ajvr.71.1.76. [DOI] [PubMed] [Google Scholar]
- 25.Gunn-Moore DA, McCann TM, Reed N. Prevalence of Tritrichomonas foetus infection in cats with diarrhoea in the UK. J Feline Med Surg. 2007;9:214. doi: 10.1016/j.jfms.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall EJ, German AJ. Diseases of the small intestine. In: Ettinger SJ, Feldman EC, editors. Textbook of veterinary internal medicine. ed 7. Saunders Elsevier; St Louis: 2010. p. 1526. [Google Scholar]
- 27.Hill SL, Cheney JM, Taton-Allen GF. Prevalence of enteric zoonotic organisms in cats. J Am Vet Med Assoc. 2000;216:687. doi: 10.2460/javma.2000.216.687. [DOI] [PubMed] [Google Scholar]
- 28.Janeczko S, Griffin B. Giardia infection in cats. Compend Contin Educ Vet. 2010;32:E1. [PubMed] [Google Scholar]
- 29.Keith CL, Radecki SV, Lappin MR. Evaluation of fenbendazole for treatment of Giardia infection in cats concurrently infected with Cryptosporidium parvum. Am J Vet Res. 2003;64:1027. doi: 10.2460/ajvr.2003.64.1027. [DOI] [PubMed] [Google Scholar]
- 30.Lappin MR. Toxoplasmosis. In: Bonagura JD, Twedt DC, editors. Kirk's current veterinary therapy XIV. Saunders Elsevier; St Louis: 2009. p. 1254. [Google Scholar]
- 31.Lappin MR. Update on the diagnosis and management of Toxoplasma gondii infection in cats. Top Companion Anim Med. 2010;25:136. doi: 10.1053/j.tcam.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Lindsay DS, Zajac AM. Cryptosporidium infections in cats and dogs. Compend Contin Educ Vet. 2004;26 [Google Scholar]
- Lloyd S, Smith J. Activity of toltrazuril and diclazuril against Isospora species in kittens and puppies. Vet Rec. 2001;148:509. doi: 10.1136/vr.148.16.509. [DOI] [PubMed] [Google Scholar]
- 33.Marks SL, Hanson TE, Melli AC. Comparison of direct immunofluorescence, modified acid-fast staining, and enzyme immunoassay techniques for detection of Cryptosporidium spp in naturally exposed kittens. J Am Vet Med Assoc. 2004;225:1549. doi: 10.2460/javma.2004.225.1549. [DOI] [PubMed] [Google Scholar]
- 34.Marks SL, Willard MD. Diarrhea in kittens. In: August JR, editor. Consultations in feline internal medicine. ed 5. Saunders Elsevier; St Louis: 2006. p. 133. [Google Scholar]
- 35.Mekaru SR, Marks SL, Felley AJ. Comparison of direct immunofluorescence, immunoassays, and fecal flotation for detection of Cryptosporidium spp. and Giardia spp. in naturally exposed cats in 4 Northern California animal shelters. J Vet Intern Med. 2007;21:959. doi: 10.1892/0891-6640(2007)21[959:codiia]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 36.Palmer CS, Traub RJ, Robertson ID. Determining the zoonotic significance of Giardia and Cryptosporidium in Australian dogs and cats. Vet Parasitol. 2008;154:142. doi: 10.1016/j.vetpar.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 37.Payne PA, Artzer M. The biology and control of Giardia spp and Tritrichomonas foetus. Vet Clin North Am Small Anim Pract. 2009;39:993. doi: 10.1016/j.cvsm.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 38.Rosado TW, Specht A, Marks SL. Neurotoxicosis in 4 cats receiving ronidazole. J Vet Intern Med. 2007;21:328. doi: 10.1111/j.1939-1676.2007.tb02968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scorza AV, Lappin MR. Gastrointestinal protozoal infections. In: August JR, editor. Consultations in feline internal medicine. ed 6. Saunders Elsevier; St Louis: 2010. p. 201. [Google Scholar]
- 40.Sherding RG, Johnson S. Diseases of the intestines. In: Birchard S, Sherding RG, editors. Saunders manual of small animal practice. ed 3. Saunders Elsevier; St Louis: 2006. p. 702. [Google Scholar]
- 41.Simpson KW. Diseases of the stomach. In: Ettinger SJ, Feldman EC, editors. Textbook of veterinary medicine. ed 7. Saunders Elsevier; St Louis: 2010. p. 1504. [Google Scholar]
- 42.Spain CV, Scarlett JM, Wade SE. Prevalence of enteric zoonotic agents in cats less than 1 year old in central New York State. J Vet Intern Med. 2001;15:33. doi: 10.1892/0891-6640(2001)015<0033:poezai>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 43.Speare R, Tinsley DJ. Survey of cats for Strongyloides felis. Aust Vet J. 1987;64:191. doi: 10.1111/j.1751-0813.1987.tb09682.x. [DOI] [PubMed] [Google Scholar]
- 44.Stein JE, Radecki SV, Lappin MR. Efficacy of Giardia vaccination in the treatment of giardiasis in cats. J Am Vet Med Assoc. 2003;222:1548. doi: 10.2460/javma.2003.222.1548. [DOI] [PubMed] [Google Scholar]
- 45.Stockdale HD, Givens MD, Dykstra CC. Tritrichomonas foetus infections in surveyed pet cats. Vet Parasitol. 2009;160:13. doi: 10.1016/j.vetpar.2008.10.091. [DOI] [PubMed] [Google Scholar]
- 46.Tams TR. Hepatobiliary parasites. In: Sherding RG, editor. The cat: diseases and clinical management. ed 2. Saunders; Philadelphia: 1994. p. 607. [Google Scholar]
- 47.Tolbert MK, Gookin J. Tritrichomonas foetus: a new agent of feline diarrhea. Compend Contin Educ Vet. 2009;31:374. [PubMed] [Google Scholar]
- 48.Tzannes S, Batchelor DJ, Graham PA. Prevalence of Cryptosporidium, Giardia and Isospora species infections in pet cats with clinical signs of gastrointestinal disease. J Feline Med Surg. 2008;10:1. doi: 10.1016/j.jfms.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yaeger MJ, Gookin JL. Histologic features associated with Tritrichomonas foetus-induced colitis in domestic cats. Vet Pathol. 2005;42:797. doi: 10.1354/vp.42-6-797. [DOI] [PubMed] [Google Scholar]
Diseases of the Exocrine Pancreas
Pancreatitis
Pancreatitis refers to inflammation of the pancreas only, with no implication of the underlying cause or pathology. For example, acute necrotizing pancreatitis (ANP) with pancreatic auto-digestion, requiring predominantly supportive care by maintaining fluid and electrolyte balances and pain relief, must not be confused with chronic pancreatitis (CP) caused by lymphocytic infiltration, and commonly associated with lymphocytic inflammatory bowel disease (IBD), and often requires corticosteroids to manage. These two conditions (and others) can only be definitively distinguished histologically. In many cases, the clinical signs of cats with acute pancreatitis will resolve with supportive care before a precise diagnosis is reached and will thus remain undiagnosed.
There are no formal classifications for feline pancreatitis, but most authors78, 89, 90 use the terms
-
•Acute pancreatitis
-
•Acute necrotizing pancreatitis, characterized by severe peri-pancreatic fat necrosis
-
•Acute suppurative pancreatitis, characterized by neutrophilic infiltration
-
•
-
•
Chronic pancreatitis, characterized by lymphocytic infiltration
Prevalence
The exact prevalence of feline pancreatitis is unknown. Necropsy studies from the 1970s to 1990s reported prevalence of feline pancreatitis ranging from 0.45% to 2.4%.21, 67 A more recent study17 found 67% of 115 cats had evidence of pancreatitis. However, this included pancreatic pathology in 45% of apparently healthy cats, which suggests that mild pathology is unlikely to cause clinical signs. These studies all show lymphocytic pancreatitis to be significantly more prevalent than acute pancreatitis. This may underestimate the true prevalence of acute pancreatitis, since it is understood that no permanent histopathologic changes are present after resolution of acute pancreatitis.89 It is also possible that studies assessing pathology in necropsy cases do not reflect clinical practice.
Patient Signalment and Risk Factors
There are no specific age, breed, or sex predispositions. Although one study reported Siamese cats to be at increased risk of acute pancreatitis,33 subsequent studies have recognized the majority of cases are domestic shorthair cats, suggesting no specific breed predispositions.22, 29, 60, 71 Most studies have indicated older cats (8 to 10 years of age) are more likely to be affected,22, 29, 60, 71 but these studies most likely underrepresent cats with less severe clinical disease for which definitive diagnosis may not be reached and which may be younger. No association has been made with a high-fat diet or obesity.
Etiology and Disease Associations
In most cases of both acute and chronic pancreatitis, no specific cause is found, and the disease is primarily considered to be idiopathic.22, 90 There are, however, some specific underlying causes that are sporadically recognized. These include infections with herpesvirus,75 calicivirus,37, 49 feline infectious peritonitis (FIP),44 liver fluke58 and pancreatic fluke,26, 77 and toxoplasmosis.20 However, a recent paper found no association between serum feline pancreatic lipase immunoreactivity (fPLI) concentrations and Toxoplasma gondii serology.8 Pancreatitis has also been recognized subsequent to trauma81 and organophosphate poisoning.33
The association of pancreatitis with inflammatory bowel disease and cholangitis is frequently mentioned (triaditis) but poorly described in the literature.80 One study found 30% of IBD cases to have histologic evidence of pancreatic involvement,6 and another found fPLI concentrations were elevated in 70% of cases with histologically confirmed IBD.3 It is the author's experience that many cases of pancreatitis recognized with IBD have no specific clinical signs attributable to pancreatitis and should therefore be diagnosed and treated as intestinal disease.
Diabetes mellitus is a recognized co-morbidity of pancreatitis in cats. A recent study found fPLI concentrations were significantly higher in 29 diabetic cats compared with 23 non-diabetics. No association could be made between fPLI concentrations and the degree of diabetic control.23
One study found 5 of 13 cats (38%) histologically diagnosed with hepatic lipidosis were also histologically diagnosed with acute pancreatitis. It is not known if pancreatitis is a cause, consequence, or coincident disease of hepatic lipidosis. For example, anorexia associated with acute pancreatitis could predispose to fatty infiltration of the liver. However, the high rate of concurrent disease has important implications for ensuring cats with pancreatitis receive adequate caloric intake.1
Ongoing or recurrent pancreatitis may lead to pancreatic cysts10 or exocrine pancreatic insufficiency,74 which are both covered later in this chapter.
Pathophysiology
Although pancreatitis has been experimentally induced in cats,18, 41, 56 the pathophysiology of spontaneous pancreatitis remains unknown. Acute pancreatitis is initiated by an increase in secretion of pancreatic enzymes that leads to inappropriate cellular activation of trypsin and subsequently other digestive zymogens. These activated digestive enzymes lead to local effects including inflammation, hemorrhage, acinar cell necrosis, and peripancreatic fat necrosis.43, 78, 86 Chronic pancreatitis may result from any of several underlying processes: ongoing, low-grade acute pancreatitis episodes may instigate chronicity; chronic pancreatitis, with a predominance of lymphocytic inflammation has been induced experimentally within 5 weeks by narrowing the main pancreatic duct to approximately 25% of its normal diameter18; and the association with IBD80 may suggest an immune-mediated cause.
Clinical Signs
The clinical signs of pancreatitis in cats are nonspecific. A review of eight prior series totaling 159 cases of acute pancreatitis in cats found anorexia (87% of cases) and lethargy (81%) to be the most common historical findings.78 Vomiting was recognized in 46% of cases, diarrhea in 12%, and weight loss in 47%. Physical examination findings were similarly nonspecific with dehydration (54%) being the major finding; fever was recognized in only 25% of cases and abdominal pain in 19%. It is important to note that vomiting and abdominal pain, key features of pancreatitis in dogs, are not consistently recognized in cats. Similar, nonspecific findings indistinguishable from IBD are recognized in cats with chronic pancreatitis.3, 6
Diagnosis
Because the presenting signs and physical examination findings are nonspecific, the diagnosis of pancreatitis can be challenging, requiring not only clinical suspicion but a combination of diagnostic modalities. For the most part, hematology and plasma biochemistry findings are unremarkable, although a combination of findings may increase clinical suspicion. For example, moderate elevations in liver enzymes, bilirubin, and glucose are present in approximately 50% of cases and hypocalcemia in approximately two of three of cases; hypocalcemia infers a poorer prognosis. Hypoalbuminemia is seen in approximately one of three of cases and has important implications for fluid therapy.78 Amylase and lipase elevations are not reflective of pancreatitis in cats.47 Feline trypsin-like immunoreactivity (fTLI) is the diagnostic test of choice of exocrine pancreatic insufficiency, but elevations in pancreatitis are not seen consistently enough to warrant use of this test for this purpose.29, 47, 71
The biggest recent advance in feline pancreatic diagnostics has been the characterization of feline pancreatic lipase,69 leading to the development of a radioimmunoassay for the measurement of feline pancreatic lipase immunoreactivity (fPLI).70 It must be remembered, however, that an increase in fPLI only tells the clinician that pancreatic pathology is present, but not the cause of pathology, which may be, for example, neutrophilic or lymphocytic pancreatitis or neoplasia, and it may or may not involve the intestines or liver. fPLI should therefore be used as a screening test, with elevated results not suggesting a diagnostic end point. Further, the high interassay variability of this test70 would suggest that mild cases may be missed as shown in one study24 and that the test may not be appropriate for serial monitoring. fPLI is currently available as “Spec fPL” from commercial laboratories and has a sensitivity of 79% and a specificity of 82% when 5.4 µg/L is used as the diagnostic cut off25 compared with 3.5 µg/L, which is the listed reference range high point.
In an acutely unwell cat (less than 2 days) with only mild to moderate signs of disease, further diagnostics may not be warranted, and many cats will improve with supportive therapy of balancing fluid and electrolytes, pain relief, and antinausea/vomiting therapy.
Cats with chronic duration of signs and acutely unwell cats that do not improve with supportive therapy warrant further diagnostics. The underlying disease process cannot be assumed from an elevated fPLI; in one study of 63 cases, acute necrotizing pancreatitis could not be distinguished from chronic nonsuppurative pancreatitis by signalment, duration of signs, or clinical findings.22
The major utility of diagnostic imaging is to rule out other differential diagnoses, such as an intestinal foreign body, and perhaps confirm that the pancreas is affected. Radiography is non-specific for diagnosis of pancreatitis, but findings may include decreased abdominal detail (sometimes associated with ascites), soft tissue density in the right cranial quadrant of the abdomen, hepatomegaly, or gas-filled intestines22, 60, 64 (see Figure 23-47 ). Additionally, thoracic radiographs may show pleural effusion. One study found 5 of 20 cats with pancreatic necrosis had such a change60; the mechanisms resulting in pleural effusion are not precisely defined. Ultrasonography has high specificity (>85%) but low sensitivity (<35%) for recognizing pancreatitis in cats,22, 29, 60, 71 with findings dependent on operator skills, quality of equipment, and severity of lesions. Typical findings are hypoechogenicity of the pancreas, which may be enlarged or irregular; hyperechogenicity of the peripancreatic fat; the possible presence of abdominal effusion; and abnormal findings with other organs, such as liver or intestine, may add to the clinical picture22, 60, 64, 71 (Figure 23-48 ). One study indicated that contrast-enhanced Doppler ultrasonography can provide further diagnostic insights.54 A recent study suggested that endosonography may be useful in cases where transabdominal ultrasonography is difficult, for example, because of obesity, hyperechoic mesentery, or excessive intestinal gas.61
FIGURE 23-47.
Abdominal radiograph (lateral view) of a cat with acute pancreatitis. The findings are essentially unremarkable; there is increased gas in the stomach and minor loss of serosal detail cranially.
(Courtesy Small Animal Specialist Hospital, North Ryde, Sydney, Australia.)
FIGURE 23-48.
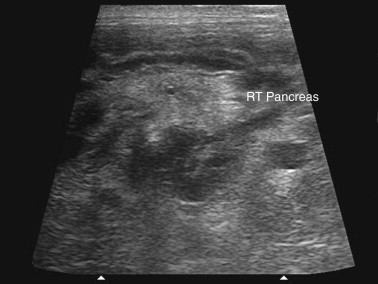
Ultrasonography of the right limb of the pancreas of a cat with acute pancreatitis. The pancreas is outlined by the duodenum (above) and the presence of abdominal effusion (to the sides and below); the hyperechoic areas (for example, to the right of the image, near the text) are most likely peripancreatic fat.
(Courtesy Small Animal Specialist Hospital, North Ryde, Sydney, Australia.)
For more than 20 years, computed tomography (CT) has been a commonly used modality to confirm pancreatitis in humans,55 but this reliability has not been demonstrated in cats, where sensitivity may be as low as 20%.24, 29
Definitive diagnosis of pancreatitis, including differentiation of the inflammatory process, can only be made by cytologic assessment of pancreatic tissue. In most cases, ultrasound-guided fine-needle aspiration (FNA) of the pancreas is technically difficult because of the small dimension of the feline pancreas; there appears to be no assessment of feline pancreatic FNA findings in the literature. Gross inspection of the pancreas and samples for histologic assessment can be obtained during laparotomy22, 64 (see FIGURE 23-49, FIGURE 23-50 ) or laparoscopy.16, 79 Because pancreatitis often occurs concurrently with pathology of other organs,22 thorough evaluation of the abdomen by ultrasonography or gross inspection is recommended, as are multiple biopsies of, for example, intestines, liver, and mesenteric lymph nodes, where appropriate. Clinicians may be reluctant to biopsy the pancreas because of perceived risks of deleterious effects. Studies of pancreatic biopsy in healthy cats dispel the concern that the pancreas is unforgiving to mild manipulation and biopsy16,42a and the author's clinical experience is consistent with these findings.
FIGURE 23-49.
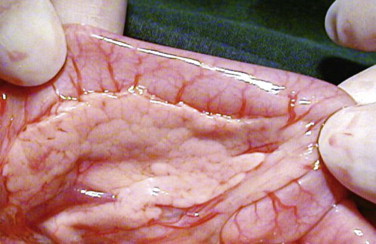
Gross appearance of pancreas at laparotomy; this was histologically diagnosed as chronic pancreatitis (i.e., lymphocytic infiltration was recognized).
FIGURE 23-50.
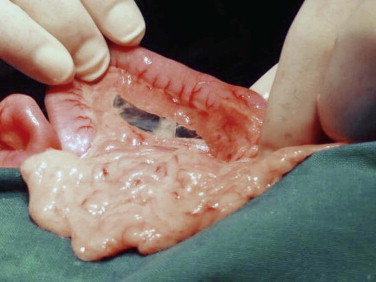
Gross appearance of pancreas at laparotomy; this pancreas was found to be histologically normal. It does look smaller than is typically seen; pancreatic atrophy can look similar to this, grossly.
Therapy
Supportive care comprising correction of fluid/electrolyte imbalances, pain management, and nutritional support are the mainstay of therapy for cats with pancreatitis.78, 86, 89 Specific underlying causes, when diagnosed, should be managed, as should concurrent diseases. Follow-up evaluation is determined on a case-by-case basis; reduction or resolution of clinical signs is the main criterion for success of therapy. Serial fPLI values may be monitored when initial results are extremely high but are of limited value for mild increases because of assay variability.
Fluid Therapy
Dehydration, acid–base and electrolyte abnormalities should be corrected during the first 12 to 24 hours. Hypocalcemia, if present, should be treated with a calcium gluconate infusion of 50 to 150 mg/kg during 12 to 24 hours, with continued assessment of plasma calcium concentrations.
Plasma transfusions can be considered in cats with hypoalbuminemia.78, 86, 90
Pain Management
Although abdominal pain is not commonly described in cats with pancreatitis, it is likely to be present in most cases and may contribute to anorexia. Historical concern about exacerbation of pancreatitis with opioids is no longer accepted, and this class of drugs is considered appropriate. Meperidine (1 to 2 mg/kg SC or IM) every 1 to 2 hours, butorphanol (0.2 to 0.4 mg/kg SC) every 6 hours, or sustained-release buprenorphine (120 µg/kg SC) every 72 hours are alternatives.67, 78, 86 The author uses one dose of methadone (0.1 to 0.2 mg/kg SC, IM, or IV) initially and places a fentanyl patch for longer-term pain management.
Nutritional Support
The traditional recommendation for management of pancreatitis across all species has been nil per os for several days. This recommendation is appropriate for cats with severe vomiting, but there is no evidence to support this approach in cats that are not vomiting and that are eating normally. Further, nutritional support is vital for those cats with concurrent hepatic lipidosis. If the cat is not eating voluntarily, nutritional support by tube feeding is often warranted.67, 78, 86 A recent paper found nasogastric tube feeding of cats with pancreatitis was tolerated well and resulted in few clinically significant complications.42 Other reported nutritional strategies for cats with pancreatitis incorporate partial parenteral nutrition (PPN; 8.5% amino acids, 20% lipids), or total parenteral nutrition (TPN; 6% amino acids, 20% lipids, 50% dextrose), or both instead of enteral feeding.14, 39, 53 Cats do not seem to benefit from feeding of specially formulated low-fat diets; commercially available, veterinary liquefied diets appear to be well tolerated despite their high-fat contents.86
Drug Therapy
Other therapy may be appropriate in individual cases.
Antiemetics
All cats with pancreatitis that are vomiting should be treated with antiemetics. Examples of drugs that can be used are 5-HT3 antagonists, such as dolasetron (0.5 to 1.0 mg/kg IV or PO, once to twice daily); ondansetron (0.1 to 0.2 mg/kg IV every 6 to 12 hours); and maropitant, an NK1-inhibitor (0.5 to 1.0 mg/kg SC once daily). These drugs are covered in detail earlier in this chapter under Therapeutics for Vomiting and Diarrhea. Dopaminergic antagonists, such as metoclopramide, are less effective antiemetic agents in cats than the other choices mentioned.78, 89
Antibiotics
In most cases, pancreatitis begins as a sterile process, and antibiotic therapy is controversial. Pancreatic necrosis and inflammation may predispose to bacterial colonization of the pancreas as demonstrated in experimental models.82, 84 This has not been demonstrated in spontaneous disease, and no comparison of outcomes has been made of cats with pancreatitis treated with or without antibiotics. Cefotaxime (20 to 80 mg/kg IV, IM) has been used to prevent bacterial colonization in experimental models.83 Other broad-spectrum cephalosporins or ampicillin may act similarly. Antibiotic considerations are possibly more important for acute pancreatitis than for treatment of chronic disease.
Corticosteroids
Cats with demonstrated lymphocytic pancreatitis, with or without concurrent IBD or lymphocytic cholangitis, should be treated with corticosteroids (e.g., prednisolone, 1 to 2 mg/kg once to twice daily) with tapering to the lowest effective dose. There is no justification for use of corticosteroids in cats with acute necrotizing or acute suppurative pancreatitis, or cats for which the cause of pancreatitis has not been diagnosed histologically. Use of corticosteroids in cats with pancreatic disease creates a risk of iatrogenic diabetes mellitus.
Surgical Intervention
Surgical intervention is warranted to relieve any bile duct obstruction that may result or for the débridement of pancreatic abscesses or necrotic tissue; in many cases, cats will survive multiple years after such corrective surgery.65
Pancreatic Cysts, Pseudocysts, and Bladders
Pancreatic cysts, pseudocysts, and bladders have been described sporadically in cats.* Pancreatic cysts are lined by a single layer of cuboidal epithelium and do not communicate with the pancreatic duct; pseudocysts are enclosed by a wall of fibrous tissue, lacking the epithelial lining characteristic of true cysts and can form secondary to pancreatic inflammation; cystic dilations of the pancreatic duct are referred to as pancreatic bladder. True pancreatic cysts have been described in three cats9, 10, 15; a congenital pancreatic cyst with associated inflammation was described as an incidental finding in an adult cat15; multiple pancreatic cysts were described in a cat with concurrent polycystic disease in the kidney and liver9; and a another cat had multiple recurrent pancreatic cysts with concurrent mild pancreatic inflammation and atrophy associated a with rapid clinical course resulting in diabetes mellitus.10
Cysts, pseudocysts, and bladders may be identified ultrasonographically or by CT. They may be benign, but the associated pancreatic inflammation and other sequelae, such as diabetes mellitus, may need to be managed. Pancreatic bladders may result in biliary obstruction, and surgical correction may be required.
Pancreatic Nodular Hyperplasia
Pancreatic nodular hyperplasia is recognized quite frequently as an incidental finding in older cats or at necropsy.45, 67 Disseminated small nodules can be found throughout the exocrine portion of the pancreas (FIGURE 23-51, FIGURE 23-52 ). These lesions can be differentiated from pancreatic adenomas by the absence of a capsule in cases of nodular hyperplasia.45, 67 Nodular hyperplasia does not lead to functional changes and so does not cause any clinical signs unless the bile duct is obstructed.38 Choledochenterostomy or cholenterostomy are feasible options in this circumstance13 with survival much longer than reported when these procedures are performed in cats with neoplastic disease.
FIGURE 23-51.
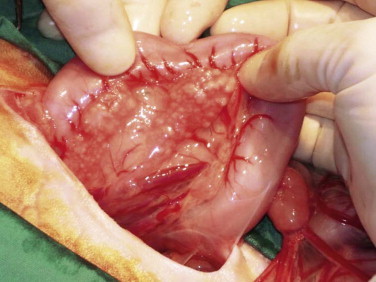
Gross appearance of pancreatic nodular hyperplasia at laparotomy; this was an incidental finding.
FIGURE 23-52.
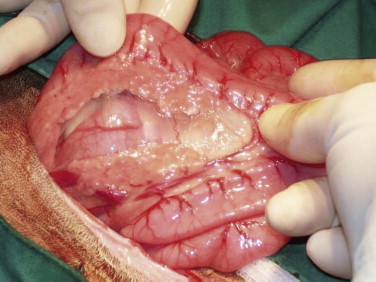
Gross appearance of pancreatic nodular hyperplasia at laparotomy; this was an incidental finding. Note the changes are more subtle than those in Figure 23-51.
Pancreatic Neoplasia
Neoplasia of the exocrine pancreas is rare in cats. Its frequency was assessed in the 1970s when one study estimated 12.6 cases per 100,000 patients per year at risk,52 and another found pancreatic tumors in 5 of 800 feline necropsies.45 A more recent study recognized, from 15,764 feline admissions over a 20-year study period, only two cats with pancreatic adenomas (0.013% of admissions) and eight with pancreatic adenocarcinomas (0.05% of admissions).62
Adenomas appear as small, solitary or multifocal nodules and are not typically associated with adjacent pancreatic inflammation. They do not cause clinical signs, unless large, when any clinical signs result from the physical size and are usually an incidental finding.45, 86
Few generalities can be made about the presentation for pancreatic adenocarcinoma. The age range is large (4 to 20 years), there is no sex predisposition, and no clear breed predispositions are present.62, 86 Only cytology or histopathology can distinguish pancreatitis from pancreatic carcinoma in cats antemortem, yet it is important to differentiate the two conditions, because, in contrast to adenomas, pancreatic adenocarcinoma is associated with a grave prognosis.
The presence of lesions consistent with metastases on radiography or ultrasonography may suggest malignancy, but one study could not distinguish neoplasia from pancreatic nodular hyperplasia ultrasonographically based on the appearance of the pancreas alone32 (FIGURE 23-53, FIGURE 23-54 ).
FIGURE 23-53.
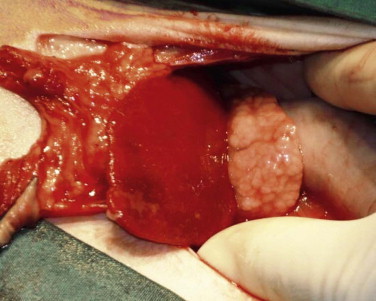
Gross appearance of pancreatic adenocarcinoma at laparotomy. Note that the appearance of the pancreas is very similar to that of pancreatic nodular hyperplasia in Figure 23-51. Additionally, ascites can be seen between the surgeon's thumb and the pancreas, and the mesentery is very inflamed.
FIGURE 23-54.
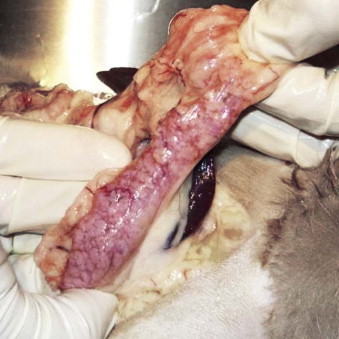
Gross appearance of pancreatic adenocarcinoma at necropsy. Note that the appearance of the pancreas is very similar to that of pancreatic nodular hyperplasia in Figure 23-51.
Pancreatic adenocarcinomas in cats can result in a paraneoplastic dermatologic condition consisting of nonpruritic, symmetric alopecia affecting the face, ventral body, and medial aspect of the limbs of cats. The skin is usually glistening but not fragile, and there can be crusty lesions on the footpads.* The pathogenesis of this dermatologic disease is unknown. In one case, surgical excision of the pancreatic carcinoma resulted in resolution of dermatologic disease, indicating that the process is reversible (although signs recurred as the tumor re-emerged).73
Diabetes mellitus is a recognized complication of pancreatic adenocarcinoma. The mechanism is unknown and may simply be secondary to compression or invasion of islet cells by the tumor. In some cats, diabetes is recognized ahead of pancreatic neoplasia.31, 40, 62
Obstructive jaundice has also been described with pancreatic adenocarcinoma.13
Most cases of pancreatic adenocarcinoma in cats have metastasized by the time of diagnosis, and most reported cases die or are euthanized within 7 days of diagnosis.62 Surgical excision is a potential option if neoplasia is confined to one limb of the pancreas, but recurrence is possible even if there is no evidence of metastasis and excision seems complete at the time of surgery.73
Exocrine Pancreatic Insufficiency
Exocrine pancreatic insufficiency (EPI) is a condition caused by insufficient synthesis and secretion of pancreatic digestive enzymes from the exocrine portion of the pancreas.66 In humans it has been reported that 90% of pancreatic acinar cells must be lost before clinical signs of EPI are seen.19
Prevalence and Patient Signalment
EPI is considered rare in cats but is perhaps being recognized more frequently because of increased awareness. There are less than fifty cases described in the veterinary literature* with one of these papers describing only 16 cases from five institutions, with prevalence described as 0.01% to 0.1% of cats seen over a 15-year period.74 In contrast to this, the Gastrointestinal Laboratory at Texas A&M University recognized 1342 samples with serum fTLI concentrations at or less than 8.0 µg/L, which is diagnostic for EPI, out of 84,523 submissions,66 which equates to 1.6% of cats with known or suspected gastrointestinal disease.
All studies indicate a wide age range of cats can be affected, from kittens less than 6 months of age to cats more than 15 years old, with a median age of approximately 7 years. There is no apparent breed predisposition.66, 68, 74 One paper recognized 10 of 16 (62.5%) cats to be male,74 and another recognized 15 of 20 (75%) male cats,68 suggesting a possible sex predisposition.
Etiology and Disease Associations
Chronic pancreatitis is believed to be the most common cause of EPI in cats,66, 85 although most reported cases have not had histologic confirmation of this. Pancreatic acinar atrophy (PAA) is recognized as the most common cause of EPI in dogs, and has been definitively described in two feline cases74 and mentioned as a cause for three other cases.85 Other potential causes of EPI include disruption of pancreatic enzyme flow at the duodenal papilla following duodenal resection72 and pancreatic fluke infection (Eurytrema procyonis), 2, 26 and amyloid deposition and neoplasia are other possible causes of pancreatic cell damage that have not definitively been described in cats.66 Congenital pancreatic hypoplasia or aplasia has not definitively been reported in cats, but reports of EPI in cats as young as 3 months of age63, 74 suggest this possibility.
Since chronic pancreatitis is a common cause of EPI and chronic pancreatitis has a strong association with IBD, many cats may have concurrent lymphocytic pancreatitis and enteritis.66, 74, 85 Therefore cats failing to respond to therapy for EPI may require further diagnostics and management of an underlying condition. Further, destruction of functional exocrine pancreatic tissue can also affect pancreatic endocrine tissue, resulting in concurrent diabetes mellitus.35
Clinical Signs
Several studies have indicated that all cats with EPI will have weight loss when diagnosed, unless a kitten, in which case ill-thrift is recognized.68, 74 Diarrhea is not necessarily present, being described in 50% to 75% of cats; the nature of feces can vary from voluminous, malodorous stools that can be discolored (yellow or pale), sometimes with steatorrhea, to normal feces in other cats. Increased frequency of defecation and the presence of mucus in the feces of some cats can lead to the diarrhea being characterized as large bowel. Only about 20% to 30% of cats are polyphagic, some described as having a ravenous appetite; conversely, some cats present with anorexia. Vomiting has also been described. Since cats with EPI often have concurrent disorders, such as IBD, the clinical signs recognized may reflect the concurrent disease and not necessarily EPI alone. Physical examination findings are similarly nonspecific, with thin/emaciated body condition being the most common finding. Hematologic findings are non-specific, but a mild nonregenerative, normocytic, normochromic anemia may be recognized as well as lymphopenia or neutrophilia. Plasma biochemistry results may show a mild to moderate increase in alanine aminotransferase (ALT) and a mild increase in alkaline phosphatase in some cats. Mild to moderate hyperglycemia may be seen, as may mild hypoglycemia or normoglycemia.66, 68, 74 Hypocobalaminemia is recognized in nearly all cats with EPI.66, 68, 74, 85, 87 This may be because of insufficient production of intrinsic factor, a cobalamin-binding protein only produced by the pancreas in cats and necessary for ileal absorption of cobalamin27; it may also be because of failure of pancreatic enzymes to liberate cobalamin from binding by R protein in the duodenum or small intestinal bacterial overgrowth (SIBO), not yet specifically described in cats.74 Folate concentrations may be reduced (because of concurrent intestinal malabsorption),68 normal,68, 74 or increased,74 which may relate to reduced pancreatic bicarbonate secretion, secondary to severe hypocobalaminemia,59 or associated with SIBO.7
None of these presenting complaints, physical examination findings, or routine testing results are specific to EPI. Therefore EPI requires a degree of clinical suspicion and/or thorough diagnostics to ensure the diagnosis is not missed.
Diagnosis
A low level of serum fTLI is diagnostic for EPI.66, 68, 74 Samples can be sent to the Gastrointestinal Laboratory at Texas A&M University from anywhere worldwide (with instructions about sample handling requirements on their website: http://vetmed.tamu.edu/gilab/). The reference range for serum fTLI is 12 to 82 µg/L, with concentrations at or less than 8.0 µg/L diagnostic for EPI.
Since the clinical signs and routine laboratory findings are nonspecific for EPI, it is ideal to test serum for fTLI in any cat with weight loss or ill-thrift. The Texas A & M gastrointestinal panel also includes testing for levels of cobalamin, folate, and fPLI, ensuring concurrent hypocobalaminemia will not be missed and potentially providing indications of other gastrointestinal disease.
Conversely, although a low level of serum fTLI confirms a diagnosis of EPI, it is not necessarily a diagnostic end point, since EPI is so often recognized concurrently with other gastrointestinal disease. Failure to respond to therapy should prompt the clinician to consider and investigate further for concurrent processes.
Management
Most cats with EPI can be successfully managed with dietary supplementation of pancreatic enzymes. Commercial products (e.g., Viokase [Axcan Pharma, Birmingham, Ala.], Pancrezyme [Virbac, Fort Worth, Tex.], and Creon [Abbott Laboratories, Abbott Park, Ill.]) are available, and powder is considered more effective than tablets or capsules (some capsules can be opened and the contents sprinkled onto food, like powder). The required dose can vary quite substantially from cat to cat. It is appropriate to start with one teaspoon of powder with food twice daily, and adjustments can be made depending on the response; most cats accept the powder readily if it is mixed thoroughly through canned food, but other flavors (e.g., fish oil or brine from canned tuna) can be used to disguise the taste if necessary. Raw pancreas (e.g., from beef or pork) may also be used, with 30 to 60 g twice daily an appropriate starting dose.66
Since most cats with EPI are hypocobalaminemic, supplementation by subcutaneous injection is required (oral supplementation is not effective since cobalamin deficiency leads to cobalamin malabsorption). An appropriate dose for most cats is 250 µg, and it is usually given weekly for 6 weeks, then every second week for a further six doses; it is appropriate to continue dosing every month beyond that. Owners can be taught to inject their cats at home (as owners of diabetic animals are taught to do with insulin).66 Because some cats may have SIBO, antibiotics such as metronidazole (15 to 25 mg/kg PO every 12 hours for 14 days) may be warranted. An elevation of folate may arouse suspicion of SIBO, but it is appropriate to try antibiotics in a cat failing to respond to enzyme and cobalamin supplementation.
Concurrent diseases, such as lymphocytic, chronic pancreatitis, or IBD may need to be managed with corticosteroids, or diabetes mellitus with insulin. No studies have assessed specific dietary requirements in cats with EPI.
Most cats respond to appropriate treatment, with a return to normal weight and normal feces. With ongoing therapy, cats can lead normal lives for a full life span.
References
- 1.Akol KG, Washabau RJ, Saunders HM. Acute pancreatitis in cats with hepatic lipidosis. J Vet Intern Med. 1993;7:205. doi: 10.1111/j.1939-1676.1993.tb01008.x. [DOI] [PubMed] [Google Scholar]
- 2.Anderson W, Georgi M, Car B. Pancreatic atrophy and fibrosis associated with Eurytrema procyonis in a domestic cat. Vet Rec. 1987;120:235. doi: 10.1136/vr.120.10.235. [DOI] [PubMed] [Google Scholar]
- 3.Bailey S, Benigni L, Eastwood J. Comparisons between cats with normal and increased fPLI concentrations in cats diagnosed with inflammatory bowel disease. J Small Anim Pract. 2010;51:484. doi: 10.1111/j.1748-5827.2010.00973.x. [DOI] [PubMed] [Google Scholar]
- 4.Bailiff NL, Norris CR, Seguin B. Pancreatolithiasis and pancreatic pseudobladder associated with pancreatitis in a cat. J Am Anim Hosp Assoc. 2004;40:69. doi: 10.5326/0400069. [DOI] [PubMed] [Google Scholar]
- 5.Banner BF, Alroy J, Kipnis RM. Acinar cell carcinoma of the pancreas in a cat. Vet Pathol. 1979;16:543. doi: 10.1177/030098587901600506. [DOI] [PubMed] [Google Scholar]
- 6.Baral RM. Australian College of Veterinary Scientists; Gold Coast, Queensland, Australia: 2006. Laparotomy for gastro-intestinal biopsies, Science Week Conference Proceedings (Small Animal Medicine chapter) p 70. [Google Scholar]
- 7.Batt RM, Rutgers HC, Sancak AA. Enteric bacteria: friend or foe? J Small Anim Pract. 1996;37:261. doi: 10.1111/j.1748-5827.1996.tb02376.x. [DOI] [PubMed] [Google Scholar]
- 8.Bayliss DB, Steiner JM, Sucholdolski JS. Serum feline pancreatic lipase immunoreactivity concentration and seroprevalences of antibodies against Toxoplasma gondii and Bartonella species in client-owned cats. J Feline Med Surg. 2009;11:663. doi: 10.1016/j.jfms.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosje JT, van den Ingh TS, van der Linde-Sipman JS. Polycystic kidney and liver disease in cats. Vet Q. 1998;20:136. doi: 10.1080/01652176.1998.9694858. [DOI] [PubMed] [Google Scholar]
- 10.Branter EM, Viviano KR. Multiple recurrent pancreatic cysts with associated pancreatic inflammation and atrophy in a cat. J Feline Med Surg. 2010;12:822. doi: 10.1016/j.jfms.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooks DG, Campbell KL, Dennis JS. Pancreatic paraneoplastic alopecia in three cats. J Am Anim Hosp Assoc. 1994;30:557. [Google Scholar]
- 12.Browning T. Exocrine pancreatic insufficiency in a cat. Aust Vet J. 1998;76:104. doi: 10.1111/j.1751-0813.1998.tb14537.x. [DOI] [PubMed] [Google Scholar]
- 13.Buote NJ, Mitchell SL, Penninck D. Cholecystoenterostomy for treatment of extrahepatic biliary tract obstruction in cats: 22 cases (1994-2003) J Am Vet Med Assoc. 2006;228:1376. doi: 10.2460/javma.228.9.1376. [DOI] [PubMed] [Google Scholar]
- 14.Chan DL, Freeman LM, Labato MA. Retrospective evaluation of partial parenteral nutrition in dogs and cats. J Vet Intern Med. 2002;16:440. doi: 10.1892/0891-6640(2002)016<0440:reoppn>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Coleman MG, Robson MC, Harvey C. Pancreatic cyst in a cat. N Z Vet J. 2005;53:157. doi: 10.1080/00480169.2005.36495. [DOI] [PubMed] [Google Scholar]
- 16.Cosford KL, Shmon CL, Myers SL. Prospective evaluation of laparoscopic pancreatic biopsies in 11 healthy cats. J Vet Intern Med. 2010;24:104. doi: 10.1111/j.1939-1676.2009.0420.x. [DOI] [PubMed] [Google Scholar]
- 17.De Cock HEV, Forman MA, Farver TB. Prevalence and histopathologic characteristics of pancreatitis in cats. Vet Pathol. 2007;44:39. doi: 10.1354/vp.44-1-39. [DOI] [PubMed] [Google Scholar]
- 18.De Giorgio R, Sternini C, Widdison AL. Differential effects of experimentally induced chronic pancreatitis on neuropeptide immunoreactivities in the feline pancreas. Pancreas. 1993;8:700. doi: 10.1097/00006676-199311000-00006. [DOI] [PubMed] [Google Scholar]
- 19.DiMagno EP, Go VLW, Summerskill WHJ. Relations between pancreatic enzyme outputs and malabsorption in severe pancreatic insufficiency. N Engl J Med. 1973;288:813. doi: 10.1056/NEJM197304192881603. [DOI] [PubMed] [Google Scholar]
- 20.Dubey JP, Carpenter JL. Histologically confirmed clinical toxoplasmosis in cats: 100 cases (1952-1990) J Am Vet Med Assoc. 1993;203:1556. [PubMed] [Google Scholar]
- 21.Duffell SJ. Some aspects of pancreatic disease in the cat. J Small Anim Pract. 1975;16:365. doi: 10.1111/j.1748-5827.1975.tb05757.x. [DOI] [PubMed] [Google Scholar]
- 22.Ferreri JA, Hardam E, Kimmel SE. Clinical differentiation of acute necrotizing from chronic nonsuppurative pancreatitis in cats: 63 cases (1996-2001) J Am Vet Med Assoc. 2003;223:469. doi: 10.2460/javma.2003.223.469. [DOI] [PubMed] [Google Scholar]
- 23.Forcada Y, German AJ, Noble PJ. Determination of serum fPLI concentrations in cats with diabetes mellitus. J Feline Med Surg. 2008;10:480. doi: 10.1016/j.jfms.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forman MA, Marks SL, De Cock HE. Evaluation of serum feline pancreatic lipase immunoreactivity and helical computed tomography versus conventional testing for the diagnosis of feline pancreatitis. J Vet Intern Med. 2004;18:807. doi: 10.1892/0891-6640(2004)18<807:eosfpl>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 25.Forman MA, Shiroma J, Armstrong PJ. Evaluation of feline pancreas-specific lipase (Spec fPLTM) for the diagnosis of feline pancreatitis [abstract] J Vet Intern Med. 2009;23:733. [Google Scholar]
- 26.Fox JN, Mosley JG, Vogler GA. Pancreatic function in domestic cats with pancreatic fluke infection. J Am Vet Med Assoc. 1981;178:58. [PubMed] [Google Scholar]
- 27.Fyfe JC. Feline intrinsic factor (IF) is pancreatic in origin and mediates ileal cobalamin (CBL) absorption [abstract] J Vet Intern Med. 1993;7:133. [Google Scholar]
- 28.Garvey MS, Zawie DA: Feline pancreatic disease. Vet Clin North Am Small Anim Pract. 1984;14:1231. doi: 10.1016/s0195-5616(84)50155-7. [DOI] [PubMed] [Google Scholar]
- 29.Gerhardt A, Steiner JM, Williams DA. Comparison of the sensitivity of different diagnostic tests for pancreatitis in cats. J Vet Intern Med. 2001;15:329. [PubMed] [Google Scholar]
- 30.Godfrey DR. A case of feline paraneoplastic alopecia with secondary Malassezia-associated dermatitis. J Small Anim Pract. 1998;39:394. doi: 10.1111/j.1748-5827.1998.tb03739.x. [DOI] [PubMed] [Google Scholar]
- 31.Goossens MMC, Nelson RW, Feldman EC. Response to insulin treatment and survival in 104 cats with diabetes mellitus (1985-1995) J Vet Intern Med. 1998;12:1. doi: 10.1111/j.1939-1676.1998.tb00489.x. [DOI] [PubMed] [Google Scholar]
- 32.Hecht S, Penninck DG, Keating JH. Imaging findings in pancreatic neoplasia and nodular hyperplasia in 19 cats. Vet Radiol Ultrasound. 2007;48:45. doi: 10.1111/j.1740-8261.2007.00203.x. [DOI] [PubMed] [Google Scholar]
- 33.Hill RC, Van Winkle TJ. Acute necrotizing pancreatitis and acute suppurative pancreatitis in the cat. J Vet Intern Med. 1993;7:25. doi: 10.1111/j.1939-1676.1993.tb03165.x. [DOI] [PubMed] [Google Scholar]
- 34.Hines B, Salisbury S, Jakovljevic S. Pancreatic pseudocyst associated with chronic-active necrotizing pancreatitis in a cat. J Am Anim Hosp Assoc. 1996;32:147. doi: 10.5326/15473317-32-2-147. [DOI] [PubMed] [Google Scholar]
- 35.Holzworth J, Coffin DL. Pancreatic insufficiency and diabetes mellitus in a cat. Cornell Vet. 1953;43:502. [PubMed] [Google Scholar]
- 36.Hoskins JD, Turk JR, Turk MA. Feline pancreatic insufficiency. Vet Med Small Anim Clin. 1982;77:1745. [Google Scholar]
- 37.Hurley KF, Pesavento PA, Pedersen NC. An outbreak of virulent systemic feline calicivirus disease. J Am Vet Med Assoc. 2004;224:241. doi: 10.2460/javma.2004.224.241. [DOI] [PubMed] [Google Scholar]
- 38.Kelly DF, Baggott DG, Gaskell CJ. Jaundice in the cat associated with inflammation of the biliary tract and pancreas. J Small Anim Pract. 1975;16:163. doi: 10.1111/j.1748-5827.1975.tb05730.x. [DOI] [PubMed] [Google Scholar]
- 39.Kerry H. Placement of jejunal feeding tubes for post-gastric feeding. Clin Tech Small Anim Pract. 2004;19:32. doi: 10.1053/S1096-2867(03)00082-3. [DOI] [PubMed] [Google Scholar]
- 40.Kipperman BS, Nelson RW, Griffey SM. Diabetes mellitus and exocrine pancreatic neoplasia in two cats with hyperadrenocorticism. J Am Anim Hosp Assoc. 1992;28:415. [Google Scholar]
- 41.Kitchell BE, Strombeck DR, Cullen J. Clinical and pathologic changes in experimentally induced acute pancreatitis in cats. Am J Vet Res. 1986;47:1170. [PubMed] [Google Scholar]
- 42.Klaus JA, Rudloff E, Kirby R. Nasogastric tube feeding in cats with suspected acute pancreatitis: 55 cases (2001-2006) J Vet Emer Crit Car. 2009;19:337. doi: 10.1111/j.1476-4431.2009.00438.x. [DOI] [PubMed] [Google Scholar]
- Lutz TA, Rand JS, Watt P. Pancreatic biopsy in normal cats. Aust Vet J. 1994;17:223. doi: 10.1111/j.1751-0813.1994.tb03410.x. [DOI] [PubMed] [Google Scholar]
- 43.Mansfield CS, Jones BR. Review of feline pancreatitis part one: the normal feline pancreas, the pathophysiology, classification, prevalence and aetiologies of pancreatitis. J Feline Med Surg. 2001;3:117. doi: 10.1053/jfms.2001.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montali RJ, Strandberg JD. Extraperitoneal lesions in feline infectious peritonitis. Vet Pathol. 1972;9:109. doi: 10.1177/030098587200900204. [DOI] [PubMed] [Google Scholar]
- 45.Owens JM, Drazner FH, Gilbertson SR. Pancreatic disease in the cat. J Am Anim Hosp Assoc. 1975;11:83. [Google Scholar]
- 46.Packer RA, Cohn LA, Wohlstadter DR. D-Lactic acidosis secondary to exocrine pancreatic insufficiency in a cat. J Vet Intern Med. 2005;19:106. doi: 10.1892/0891-6640(2005)19<106:dastep>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 47.Parent C, Washabau RJ, Williams DA. Serum trypsin-like immunoreactivity, amylase, lipase in the diagnosis of feline acute pancreatitis [abstract] J Vet Intern Med. 1995;9:194. [Google Scholar]
- 48.Pascal-Tenorio A, Olivry T, Gross TL. Paraneoplastic alopecia associated with internal malignancies in the cat. Vet Derm. 1997;8:47. doi: 10.1111/j.1365-3164.1997.tb00263.x. [DOI] [PubMed] [Google Scholar]
- 49.Pedersen NC, Elliott JB, Glasgow A. An isolated epizootic of hemorrhagic-like fever in cats caused by a novel and highly virulent strain of feline calicivirus. Vet Microbiol. 2000;73:281. doi: 10.1016/S0378-1135(00)00183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perrins N, Gaudiano F, Bond R. Carriage of Malassezia spp. yeasts in cats with diabetes mellitus, hyperthyroidism and neoplasia. Med Mycol. 2007;45:541. doi: 10.1080/13693780701435333. [DOI] [PubMed] [Google Scholar]
- 51.Perry LA, Williams DA, Pidgeon GL. Exocrine pancreatic insufficiency with associated coagulopathy in a cat. J Am Anim Hosp Assoc. 1991;27:109. [Google Scholar]
- 52.Priester WA. Data from eleven United States and Canadian colleges of veterinary medicine on pancreatic carcinoma in domestic animals. Cancer Res. 1974;34:1372. [PubMed] [Google Scholar]
- 53.Pyle SC, Marks SL, Kass PH. Evaluation of complications and prognostic factors associated with administration of total parenteral nutrition in cats: 75 cases (1994-2001) J Am Vet Med Assoc. 2004;225:242. doi: 10.2460/javma.2004.225.242. [DOI] [PubMed] [Google Scholar]
- 54.Rademacher N, Ohlerth S, Scharf G. Contrast-enhanced power and color doppler ultrasonography of the pancreas in healthy and diseased cats. J Vet Intern Med. 2008;22:1310. doi: 10.1111/j.1939-1676.2008.0187.x. [DOI] [PubMed] [Google Scholar]
- 55.Ranson J, Shamamian P. Diagnostic standards for acute pancreatitis. World J Surg. 1997;21:136. doi: 10.1007/s002689900205. [DOI] [PubMed] [Google Scholar]
- 56.Reber PU, Lewis MP, Patel AG. Ethanol-mediated neutrophil extravasation in feline pancreas. Dig Dis Sci. 1998;43:2610. doi: 10.1023/a:1026634807335. [DOI] [PubMed] [Google Scholar]
- 57.Root MV, Johnson KH, Allen WT. Diabetes mellitus associated with pancreatic endocrine insufficiency in a kitten. J Small Anim Pract. 1995;36:416. doi: 10.1111/j.1748-5827.1995.tb02972.x. [DOI] [PubMed] [Google Scholar]
- 58.Rothenbacher H, Lindquist WD. Liver cirrhosis and pancreatitis in a cat infected with Amphimerus Pseudofelineus. J Am Vet Med Assoc. 1963;143:1099. [PubMed] [Google Scholar]
- 59.Ruaux CG, Steiner JM, Williams DA. Early biochemical and clinical responses to cobalamin supplementation in cats with signs of gastrointestinal disease and severe hypocobalaminemia. J Vet Intern Med. 2005;19:155. doi: 10.1892/0891-6640(2005)19<155:ebacrt>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 60.Saunders HM, VanWinkle TJ, Drobatz K. Ultrasonographic findings in cats with clinical, gross pathologic, and histologic evidence of acute pancreatic necrosis: 20 cases (1994-2001) J Am Vet Med Assoc. 2002;221:1724. doi: 10.2460/javma.2002.221.1724. [DOI] [PubMed] [Google Scholar]
- 61.Schweighauser A, Gaschen F, Steiner J. Evaluation of endosonography as a new diagnostic tool for feline pancreatitis. J Feline Med Surg. 2009;11:492. doi: 10.1016/j.jfms.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seaman RL. Exocrine pancreatic neoplasia in the cat: a case series. J Am Anim Hosp Assoc. 2004;40:238. doi: 10.5326/0400238. [DOI] [PubMed] [Google Scholar]
- 63.Sheridan V. Pancreatic deficiency in the cat [letter] Vet Rec. 1975;96:229. doi: 10.1136/vr.96.10.229-c. [DOI] [PubMed] [Google Scholar]
- 64.Simpson KW, Shiroma JT, Biller DS. Ante mortem diagnosis of pancreatitis in four cats. J Small Anim Pract. 1994;35:93. [Google Scholar]
- 65.Son TT, Thompson L, Serrano S. Retrospective study: surgical intervention in the management of severe acute pancreatitis in cats: 8 cases (2003-2007) J Vet Emer Crit Car. 2010;20:426. doi: 10.1111/j.1476-4431.2010.00554.x. [DOI] [PubMed] [Google Scholar]
- 66.Steiner JM. Exocrine pancreatic insufficiency. In: August JR, editor. Consultations in feline internal medicine. ed 6. Saunders Elsevier; St Louis: 2010. p. 225. [Google Scholar]
- 67.Steiner JM, Williams DA. Feline exocrine pancreatic disorders. Vet Clin North Am Small Anim Pract. 1999;29:551. [PubMed] [Google Scholar]
- 68.Steiner JM, Williams DA. Serum feline trypsin-like immunoreactivity in cats with exocrine pancreatic insufficiency. J Vet Intern Med. 2000;14:627. doi: 10.1892/0891-6640(2000)014<0627:sftiic>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 69.Steiner JM, Wilson BG, Williams DA. Purification and partial characterization of feline classical pancreatic lipase. Comp Biochem Physiol B Biochem Mol Biol. 2003;134:151. doi: 10.1016/s1096-4959(02)00222-1. [DOI] [PubMed] [Google Scholar]
- 70.Steiner JM, Wilson BG, Williams DA. Development and analytical validation of a radioimmunoassay for the measurement of feline pancreatic lipase immunoreactivity in serum. Can J Vet Res. 2004;68:309. [PMC free article] [PubMed] [Google Scholar]
- 71.Swift NC, Marks SL, MacLachlan NJ. Evaluation of serum feline trypsin-like immunoreactivity for the diagnosis of pancreatitis in cats. J Am Vet Med Assoc. 2000;217:37. doi: 10.2460/javma.2000.217.37. [DOI] [PubMed] [Google Scholar]
- 72.Tangner CH, Turrel JM, Hobson HP. Complications associated with proximal duodenal resection and cholecystoduodenostomy in two cats. Vet Surg. 1982;11:60. [Google Scholar]
- 73.Tasker S, Griffon DJ, Nuttall TJ. Resolution of paraneoplastic alopecia following surgical removal of a pancreatic carcinoma in a cat. J Small Anim Pract. 1999;40:16. doi: 10.1111/j.1748-5827.1999.tb03248.x. [DOI] [PubMed] [Google Scholar]
- 74.Thompson KA, Parnell NK, Hohenhaus AE. Feline exocrine pancreatic insufficiency: 16 cases (1992-2007) J Feline Med Surg. 2009;11:935. doi: 10.1016/j.jfms.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van Pelt CS, Crandell RA. Pancreatitis associated with a feline herpesvirus infection. Compan Anim Pract. 1987;1:7. [Google Scholar]
- 76.VanEnkevort BA, O’Brien RT, Young KM : Pancreatic pseudocysts in 4 dogs and 2 cats: ultrasonographic and clinicopathologic findings. J Vet Intern Med. 1999;13:309. [PubMed] [Google Scholar]
- 77.Vyhnal KK, Barr SC, Hornbuckle WE. Eurytrema procyonis and pancreatitis in a cat. J Feline Med Surg. 2008;10:384. doi: 10.1016/j.jfms.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Washabau RJ. Acute necrotizing pancreatitis. In: August JR, editor. Consultations in feline internal medicine. ed 5. Saunders Elsevier; St Louis: 2006. p. 109. [Google Scholar]
- 79.Webb CB, Trott C. Laparoscopic diagnosis of pancreatic disease in dogs and cats. J Vet Intern Med. 2008;22:1263. doi: 10.1111/j.1939-1676.2008.0176.x. [DOI] [PubMed] [Google Scholar]
- 80.Weiss DJ, Gagne JM, Armstrong PJ. Relationship between inflammatory hepatic disease and inflammatory bowel disease, pancreatitis, and nephritis in cats. J Am Vet Med Assoc. 1996;209:1114. [PubMed] [Google Scholar]
- 81.Westermarck E, Saario E. Traumatic pancreatic injury in a cat: a case history. Acta Vet Scand. 1989;30:359. doi: 10.1186/BF03548044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Widdison AL, Alvarez C, Chang Y-B. Sources of pancreatic pathogens in acute pancreatitis in cats. Pancreas. 1994;9:536. doi: 10.1097/00006676-199407000-00019. [DOI] [PubMed] [Google Scholar]
- 83.Widdison AL, Karanjia ND, Reber HA. Antimicrobial treatment of pancreatic infection in cats. Br J Surg. 1994;81:886. doi: 10.1002/bjs.1800810631. [DOI] [PubMed] [Google Scholar]
- 84.Widdison AL, Karanjia ND, Reber HA. Routes of spread of pathogens into the pancreas in a feline model of acute pancreatitis. Gut. 1994;35:1306. doi: 10.1136/gut.35.9.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Williams DA. Feline exocrine pancreatic insufficiency. In: Bonagura JD, editor. Kirk's current veterinary therapy XII. Saunders; St Louis: 1995. p. 732. [Google Scholar]
- 86.Williams DA. Feline exocrine pancreatic disease. In: Bonagura JD, Twedt DC, editors. Kirk's current veterinary therapy XIV. Saunders Elsevier; St Louis: 2009. p. 538. [Google Scholar]
- 87.Williams DA, Reed SD, Perry L. Fecal proteolytic activity in clinically normal cats and in a cat with exocrine pancreatic insufficiency. J Am Vet Med Assoc. 1990;197:210. [PubMed] [Google Scholar]
- 88.Wolff A. An unusual unidentified abdominal mass in a cat. Vet Med Small Anim Clin. 1979;74:162. [PubMed] [Google Scholar]
- 89.Xenoulis PG, Steiner JM. Current concepts in feline pancreatitis. Top Companion Anim Med. 2008;23:185. doi: 10.1053/j.tcam.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 90.Zoran DL. Pancreatitis in cats: diagnosis and management of a challenging disease. J Am Anim Hosp Assoc. 2006;42:1. doi: 10.5326/0420001. [DOI] [PubMed] [Google Scholar]
Diseases of the Liver
The feline liver is a large, complex organ involved in a variety of essential metabolic, functional, and detoxification processes that can be affected, individually or collectively, by disease or dysfunction. Cats have a unique set of liver diseases that occur more commonly in this species compared with the typical diseases that occur in dogs, and these include hepatic lipidosis, feline cholangitis syndrome, and infectious hepatopathies (e.g., FIP, flukes, histoplasmosis, toxoplasmosis).2, 15, 34, 58, 61 Nevertheless, these conditions often present with characteristic clinical, laboratory, and histopathologic changes that are necessary for proper diagnosis and management. The goal of this section is to review the interpretation of clinical and laboratory changes that occur in these feline liver diseases, provide an approach for separating the more common diseases by their clinical footprint, and then discuss therapy of each liver disease based on our current level of understanding of hepatoprotectants, antioxidants, and drugs used for specific therapeutic purposes.
Clinical Signs
The clinical signs of liver disease in cats are often vague and nonspecific; however, recognition of certain clinical and laboratory abnormalities and their association with liver disease can greatly aid the diagnostic process. The most common early clinical signs observed in cats with liver disease are anorexia, lethargy, and weight loss, which are signs present in many (if not most!) feline diseases.2, 15 Because these early indicators of disease do not point specifically toward liver disease, a delay in diagnosis will occur unless the clinician carefully considers all possibilities and performs other tests to further evaluate the situation. For example, feline hepatic lipidosis is the most common form of liver disease in cats in the United States, United Kingdom, Japan, and Western Europe, occurring with a prevalence of nearly 16% in one study.2 However, the most common, and often only, clinical sign associated with onset of this condition is anorexia; the signs of serious hepatic disease (especially jaundice and vomiting) do not occur until later (days or weeks) in the course of the disease.2, 21 Recognition that anorexia in a cat, even for a few days, is a risk factor for development of hepatic lipidosis is essential, and this risk is increased in obese cats.11, 21 Further, the clinical signs of liver failure develop much more slowly; many cats with hepatic lipidosis present alert and responsive until much later in the course of the disease, thus delaying onset of appropriate therapy. A similar clinical situation exists for the second most common form of liver disease in cats, feline cholangitis syndrome.15, 28, 58 This complex of diseases in the cat can be associated with signs ranging from anorexia and lethargy to vomiting and jaundice, and these signs can vary in severity and prevalence. The key point is that except for development of jaundice, there is no constellation of clinical signs that are classic clinical indicators of liver disease in cats.15, 28 As with many feline diseases, the subtle clinical signs of anorexia, lethargy, or inactivity are often the only signs of illness and should be further investigated.
Routine Laboratory Tests
There are few changes that occur in the complete blood count that are specific indicators of primary liver disease in cats. The most common finding is the presence of poikilocytes, which are red blood cells with an irregular shape, speculated to be caused by changes in membrane lipids as a result of liver dysfunction.14 Other abnormalities may occur, such as anemia of chronic disease or neutrophilia, but these findings are nonspecific and occur with variable frequency. Perhaps the most important reason for obtaining a hemogram is in icteric cats, because this test is essential to help rule out hemolysis as the cause of the hyperbilirubinemia.
The serum chemistry profile can be very helpful, but there are several critical points in interpretation of these values that are important to review. The hepatic transaminases (alanine aminotransferase [ALT] and aspartate aminotransferase [AST]) are leakage enzymes but do not discriminate among hepatobiliary disorders, nor do they provide an indicator of severity or disease origin. Thus although increases in ALT may be noted in cats with liver disease, they are also present in a variety of other systemic infectious, inflammatory, neoplastic, and endocrine diseases, including hyperthyroidism, feline heartworm disease, FIP, and neoplasia.* Alternatively, the cholestatic membrane–associated enzymes alkaline phosphatase (ALP) and gamma glutamyltransferase (GGT) are especially useful for recognizing disorders involving biliary or pancreatic ductal components. Unlike the dog, these enzymes will only increase modestly in cats, even in severe disease, and there is no glucocorticosteroid or drug induction of the enzymes to influence interpretation.14, 37 Thus increases in ALP in the adult cat represent a release of enzyme from the hepatobiliary tree and should be considered clinically important. Both ALP and GGT are produced in other tissues than the liver, with the highest GGT activity present in the kidney and pancreas; however, sources other than the liver do not contribute to the activity of these enzymes in health. Recent studies of the effects on these enzymes in cats with pancreatitis, cholangitis, extrahepatic bile duct obstruction (EHBDO), and hepatic lipidosis reveal some important characteristics in interpreting increases in these enzymes.14 First, both ALP and GGT are increased in cats with pancreatitis, cholangitis, or EHBDO, because inflammation in the biliary tree also affects the pancreatic ducts (and vice versa, Figure 23-55 ), and if the fold increases in these enzymes are similar, the diagnosis is likely one of the three.15 Conversely, in cats with hepatic lipidosis (without concurrent inflammatory disease of the biliary or pancreatic duct system), large increases in ALP are observed, but GGT will remain normal or only slightly increased. Thus if the increase in ALP is 5 to 10 times, while GGT is not increased or is only increased 1 to 2 times, then the likely diagnosis is hepatic lipidosis.14, 15, 16
FIGURE 23-55.
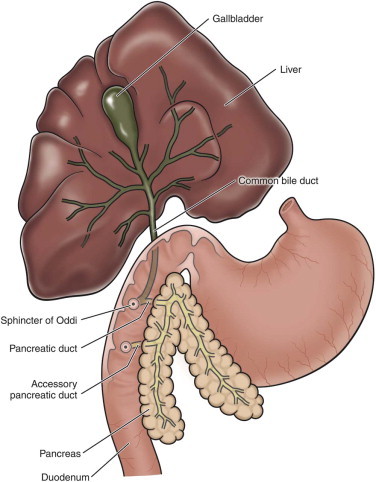
Diagram of feline anatomy of the biliary tree, pancreatic ducts, and connection to the duodenum.
(Available athttp://media.gradvet.com/show10MinuteTopUp.php?type=&Entity=10MinuteTopUps&ID=31.)
Other than enzymes on the biochemistry panel, which are of limited value for assessing liver function, there are several key tests that can be used to help assess liver function cats with elevated liver enzymes. These five tests found on most routine biochemistry panels are helpful functional indicators: cholesterol, bilirubin, glucose, albumin, and urea nitrogen (BUN). However, none are immune to outside influences on their interpretation, including bilirubin and cholesterol, which are the most liver specific. In cats with severe liver disease or failure, bilirubin levels tend to be quite elevated, while BUN, albumin, cholesterol, and glucose concentrations tend to be significantly decreased, reflecting inability to metabolize urea (lack of arginine), inability to produce albumin or cholesterol, and abnormal metabolization of glucose. However, these changes represent severe loss of liver function and thus are not sensitive indicators of liver function because the changes occur quite late in the course of the disease. Nevertheless, in cats with elevated liver enzymes and clinical signs of liver disease, these values should be carefully assessed. Because GI disease and protein-losing nephropathies can also cause loss of albumin and affect cholesterol, it is essential to evaluate the cat for these problems when interpreting these results. Finally, bilirubin metabolism is a critical function of the liver, but interpretation of hyperbilirubinemia requires a careful consideration of bilirubin disposition. Hyperbilirubinemia develps because of one of three possible causes: (1) excessive hemolysis of red blood cells (RBC) (also known as prehepatic icterus)—high bilirubin in the blood stream occurs because of an overload of the mononuclear/phagocyte system with heme pigments from RBC destruction, (2) hepatic parenchymal disease or insufficiency (also known as hepatic icterus)—resulting in lack of normal bilirubin metabolism in hepatocytes and regurgitation of the pigments into the blood stream when they are not taken up into cells and excreted in bile, and (3) disease of gall bladder, biliary tract, or pancreatic duct (also known as posthepatic icterus)—resulting in obstruction of the bile ducts or loss of bile into the abdomen (duct or gall bladder rupture and bile peritonitis).41 The bottom line is that in any cat with hyperbilirubinemia, an assessment of the packed cell volume and RBC morphology should be completed to determine whether icterus is caused by hemolysis. Once hemolysis is ruled out, then assessment of primary parenchymal disease versus disease of the biliary tree is completed by evaluating the clinical presentation, laboratory values, and imaging of the biliary tree and abdomen for possible evidence of biliary or pancreatic disease.
A urinalysis is also an important part of the minimum database, and it is no different in a sick cat with suspected liver disease. In cats the presence of hyperbilirubinuria is abnormal at any urine concentration, because they do not conjugate bilirubin in their renal tubules.14 However, like bilirubinemia, presence of bilirubin in the urine can occur because of any of the three possible causes of hyperbilirubinemia: prehepatic, hepatic, and posthepatic; thus further evaluation is necessary once bilirubin is detected. Ammonium biurate crystalluria suggests the presence of hyperammonemia, which in the cat is either because of a congenital portosystemic shunt (less common in cats than in dogs) or because of severe, end-stage liver disease resulting in portal hypertension, which is typically caused by cirrhosis or advanced polycystic liver disease.6, 41
Liver Function Tests
The most common feline liver diseases are hepatic lipidosis and feline cholangitis syndrome, which are two diseases that often result in development of clinical or biochemical icterus. Thus because hyperbilirubinemia is a more sensitive indicator of liver function than bile acids or other liver function tests, the need for further testing is moot. However, there will be circumstances when further assessment of liver function is indicated, and for this, serum bile acids, blood ammonia levels, and urine bile acids may be needed. There are several situations where liver function testing may be indicated, but the most common indications for additional testing would be a cat with persistently elevated liver enzymes of unknown origin, a cat that develops urethral obstruction because of urate stones (suggestive of portosystemic shunting) or a cat with possible polycystic liver disease.5 One of the oldest tests of liver function, because of its association with development of hepatoencephalopathy, is measurement of blood ammonia levels.38 However, although this test is the only practical way to diagnose hepatoencephalopathy in dogs, the test has a number of limitations, including differences in ammonia levels between arterial and venous (lower) samples and significant sample handling issues (ammonia is labile and results are affected by improper sample handling or lack of immediate measurement) that make its use difficult in practice.43 In cats hyperammonemia is even less common than in dogs likely because of their high-functioning urea cycle pathways14; the assays have not been validated for feline blood in most laboratories, and as such, the test is not recommended as the sole indicator of hepatic failure. In nonicteric cats with severe liver disease or in young cats suspected of having a portosystemic shunt, serum bile acids are the more reliable indicator of hepatic insufficiency.18
The measurement of serum bile acid concentrations, preprandially and postprandially, is the most reliable, readily available, and sensitive test of hepatic function in nonicteric cats.5, 14 That being said, although increases in bile acids are accurate indicators of hepatic insufficiency, the levels cannot be used to assess severity of disease or the type of dysfunction. Further, bile acid assays are most effective when paired samples (preprandial- and postprandial) are compared, because single, fasting, or random bile acid samples can result in a false-negative (normal) result. However, cats will often not eat in the hospital or when they are sick, and this prevents collection of a postprandial sample. However, this does not invalidate the results, because if the result of the single bile acid sample is abnormal, it does reliably indicate liver dysfunction.
An alternative to using serum for testing bile acids in cats is urine bile acid analysis. Healthy cats excrete a small percentage of conjugated bile acids in the urine14; however, in cats with liver disorders that cause increased serum bile acids (and especially cholestatic liver diseases) a significant increase in urine bile acid excretion occurs. When urine bile acids (UBA) were collected 4 to 8 hours after a meal and measured (normalizing the value with urine creatinine: UBA/UCr) and compared with serum bile acids in a study of 54 cats with hepatic disease, 17 cats with nonhepatic disease, and 8 normal cats, the results were highly correlated.47 The utility of the urine bile acid test is that it does not require a paired sample (postprandial test), and it is not as affected as the serum test is by hemolysis or lipemia of the blood sample. Normal cats will have an UBA/UCr of less than 4.4 µmol/mg, while values greater than 4.4 are considered evidence of significant hepatic dysfunction.47
It is well known that the liver plays a central role in coagulation homeostasis and is the single site of synthesis of many coagulation proteins, anticoagulant proteins, and fibrinolytic factors. Vitamin K is one of the most common factors found to be inactive or deficient in cats with liver dysfunction, and it is essential for normal functioning of factors II, VII, IX, and X; protein C and S; and thrombin. Insufficient or inactive vitamin K can occur for a variety of reasons, including dietary restriction (e.g., anorexia or diet deficiency), disruption of the enteric microflora that synthesize vitamin K (e.g., chronic antibiotic therapy), diseases causing fat malabsorption (e.g., IBD, exocrine pancreatic insufficiency), ingestion of vitamin K antagonists, or liver dysfunction.20 For example, in cats with hepatic lipidosis, approximately 25% will have an increased prothrombin time (PT), 35% will have an increased partial thromboplastin time (PTT), but 60% of cats will have increased PIVKA (proteins induced by vitamin K antagonists or absence).20 Nevertheless, although PIVKA is a very sensitive test for abnormalities of vitamin K function, most cats with liver disease that have a normal PT/PTT, but abnormal PIVKA do not represent clinical evidence of bleeding. In any case, abnormalities in the clotting cascade related to vitamin K deficiency in cats with liver disease are common, whether or not they show evidence of active bleeding. And because the balance of the coagulation system in a cat with liver disease can be disrupted by a procedure that initiates small amounts of bleeding (e.g., a biopsy), all cats with liver disease should be given vitamin K as a precautionary measure before and after invasive procedures, even if the clotting times (PT and PTT) are normal. This may be especially important in cats with hepatic lipidosis, because their vitamin K clotting status is likely to be even more affected by the concurrent anorexia and disruption of enteric microflora.14 The dose of vitamin K1 (phytonadione, aquaMEPHYTON [Merck, West Point, Pa.]) used prophylactically is 2.5 mg SC, IM, or PO q12h for 3 to 5 days, then weekly until recovered.
Cholestasis and Icterus
See Box 23-4 for a summary of the causes of icterus. Cholestasis is the reduction of bile flow, which can occur at any point along the biliary tree; bile production occurs in hepatocytes, and flow is connected to the distal concentrating components (gallbladder and common bile duct) by the bile ductules. Thus cholestasis can occur inside the liver's biliary tree (intrahepatic cholestasis) or outside the liver in the gallbladder and common bile duct (extrahepatic cholestasis). Intrahepatic cholestasis most often occurs in diseases involving hepatocellular damage, leakage, or swelling, such as infections (e.g., bacterial cholangiohepatitis, toxoplasmosis, FIP, or other diseases causing inflammation), infiltrative diseases (e.g., lymphoma), metabolic diseases (e.g., hepatic lipidosis), or diseases causing disruption of architecture (e.g., cirrhosis or severe polycystic disease).41 Intrahepatic cholestasis occurs in zone 1 of the liver lobules (periportal zone); at the level of hepatocytes, canaliculi or bile ductules; and is damaging to cells because of the emulsifying properties of lipid on membrane lipids. However, because the liver has a large reserve capacity, clinical icterus (e.g., jaundice) only occurs in the most severe cases when the liver is affected diffusely. Thus severe or persistent intrahepatic cholestasis can serve to perpetuate the inflammation and cell damage if it is not corrected.
BOX 23-4. Summary of the Causes of Icterus.
Icterus is the result of cholestasis, and the underlying cause can be either hemolysis or hepatobiliary disease, for which further clinical examination will be needed to determine if RBC destruction or liver disease is occurring. In most hepatobiliary diseases of cats, cholestasis is occurring, but there may be no clinically apparent icterus because the degree of hyperbilirubinemia must be at least 2 to 3 times greater than the normal values to exceed the capacity of the liver to process the excess bilirubin. In cats with hyperbilirubinemia not caused by hemolysis, whether it is clinical or subclinical, there is no need for further evaluation of liver function (e.g., bile acid assays), because bilirubin is a more sensitive indicator of liver function than bile acids. The degree of hyperbilirubinemia does not suggest differentiation of intrahepatic versus extrahepatic cholestasis; however, the presence of acholic feces (white feces) is diagnostic for extrahepatic bile duct obstruction (EHBDO), because lack of stercobilinogen (the brown/black pigment in feces) is only found in cats with complete obstruction of the bile duct. Finally, the presence of intrahepatic cholestasis and clinical icterus in a cat indicates a diffuse hepatobiliary disease, such as cholangitis or hepatic lipidosis, as focal liver disease, even if severe, will not cause clinical hyperbilirubinemia because of the tremendous reserve capacity of the liver for bilirubin uptake.
Extrahepatic cholestasis or extrahepatic bile duct obstruction (EHBDO) is less common than intrahepatic cholestasis and is most commonly associated with obstruction of the common bile duct. Since gallstones are uncommon in cats, the most common causes are neoplasia (primarily of the pancreas, but cholangiocarcinomas can occur) or chronic pancreatitis, which can occur concurrently with cholangitis in cats, resulting in both intrahepatic and extrahepatic cholestasis in some cats.23, 41 The bile ducts are affected in cats with chronic pancreatitis, because the feline biliary system and pancreatic duct system merge at the level of the pancreas to form a single duct that empties into the duodenum. Thus in cats with either pancreatitis or biliary disease, recent evidence has shown that the inflammation affects both organs.54, 59 Further, in chronic pancreatitis, either persistent inflammation or development of fibrosis can result in dilation or obstruction of the common bile duct.33 In cats with chronic EHBDO, the common bile duct will become widely dilated and tortuous, a finding easily seen on abdominal ultrasonography but a problem not easily managed (FIGURE 23-56, FIGURE 23-57 ). Interestingly, the gallbladder is often not enlarged, and may in fact be small in cats with this condition, because the remaining fluid in the gallbladder is white bile (highly concentrated mucinous bile from which the pigment has been resorbed).41 In addition, variable filling of the gall bladder is a normal phenomenon; thus gallbladder size is not an indicator of EHBDO.
FIGURE 23-56.
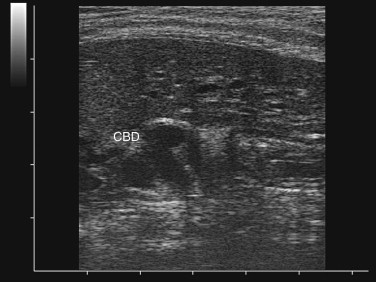
Ultrasonographic appearance of distended tortuous common bile duct (labeled CBD) in a cat with infectious cholangitis.
(Courtesy Dr. Randolph Baral.)
FIGURE 23-57.
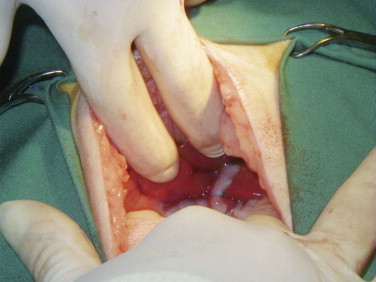
Appearance of distended bile duct at laparotomy of cat with infectious cholangitis (same cat as in Figure 23-56).
(Courtesy Dr. Randolph Baral.)
Portal Hypertension and Hepatic Encephalopathy, Ascites, and Acquired Portosystemic Shunting
Portal hypertension is an abnormally high venous pressure in the portal system and is typically caused by increased resistance to portal blood flow. There are potentially three regional causes of portal hypertension: prehepatic (disease in the portal vein itself), hepatic (intrahepatic diseases causing compression or decreased flow), and posthepatic (diseases of the caudal vena cava, right heart or pulmonary vasculature). The most common cause of portal hypertension in the cat is cirrhosis or portal venous thrombosis, because portal vein hypoplasia (formerly known as microvascular dysplasia) is known to occur only in the dog, and the other causes of portal hypertension (Budd-Chiari syndrome, heartworm caval syndrome, pulmonary hypertension) are rare and more likely to occur in the dog.41, 44 In any case, the clinically recognizable effects of portal hypertension are development of ascites (unusual in the cat), acquired portosystemic shunting (reported in cats), and development of hepatic encephalopathy (less common in cats than in dogs, because of their profound ability to handle protein wastes).5, 36, 41 Most cats and dogs that develop hepatic encephalopathy (HE) secondarily to portal hypertension do so because of reduced liver function (because of portosystemic vascular shunting [PSS] or cirrhosis and the acquired shunting that develops). Cats can develop another form of chronic HE because of hepatic lipidosis, but this is believed to be because of the combination of liver failure and prolonged fasting, resulting in arginine deficiency and impaired ammonia detoxification.2
Portosystemic vascular anomalies, also called portosystemic shunts or portovenous shunts (PSS), although less common than in dogs, also occur in cats. These vascular anomalies can be either congenital or acquired, single or multiple in number, and occur as extrahepatic vascular shunts or within the liver itself (intrahepatic shunts).5 The shunting of blood around the liver is the cause of hepatic atrophy and reduced hepatic function that results in an accumulation of toxins, particularly ammonia that leads to the development of hepatoencephalopathy. The two most common veins that serve as the connection point for the shunting portal venous blood are the caudal vena cava and the azygous.5 In cats a single, extrahepatic, portocaval shunt is the most commonly reported form, and occurs in 75% of cats with PSS.5 As in dogs, specific breeds of cats may have PSS more commonly, and these include domestic shorthair cats, Burmese, Siamese, Persian, and Himalayan breeds.5 In contrast to dogs, males may be more predisposed to PSS than females, but the clinical signs relate to the three body systems most affected: the central nervous system, GI tract, and urinary tract. The most common presenting complaints in cats are weight loss or poor/stunted growth, and dull, bizarre or lethargic behavior, especially after eating. Signs of GI disease common in dogs, such as vomiting, diarrhea, or inappetence, are less common in cats, but in one report, 75% of cats with PSS drooled.5 Finally, cats with PSS often present with signs of lower urinary tract disease (e.g., hematuria, stranguria, or even obstruction) because of the development of urate uroliths (which are radiolucent, thus difficult to detect).5
Because the most common signs of HE are apathy, listlessness, and decreased mental alertness, they are often not recognized specifically as indicative of brain dysfunction but as part of the constellation of signs of the liver disease. However, with progression of the disease, other signs will develop, including ataxia, salivation, stupor, or coma. The best and only practical diagnostic test for HE is plasma measurement of ammonia levels.43 However, as previously noted, the test has many technical issues that make its clinical utility in the practice setting difficult at best, and there are few laboratories that have validated ammonia measurement in the cat.
Miscellaneous Diseases: Hepatobiliary Neoplasia and Amyloidosis
Cancer of the liver can occur as a primary disease (Table 23-22 ) or as a result of metastasis of neoplastic disease occurring elsewhere and, most typically, the abdominal cavity. The most common neoplastic infiltration of the liver that is not a primary liver tumor is lymphoma (FIGURE 23-58, FIGURE 23-59 ), followed by visceral mastocytosis.4 As with many other types of cancer, hepatobiliary neoplasia is most common in middle-aged to older cats, and it is relatively rare, with a reported incidence of 1.5% to 2.3%.4 Benign tumors, such as biliary cystadenoma (Figure 23-60 ), carry a good prognosis if they are amenable to surgical resection. The incidence of metastatic neoplasia (including lymphoma and mast cell tumors) has not been reported. The clinical presentation is typically nonspecific (the most common signs are vomiting, lethargy, and anorexia), and there are no laboratory changes that are suggestive of hepatic neoplasia. Thus the diagnosis must be made by identification of structural abnormalities by hepatobiliary imaging and subsequent examination of the tissue either by FNA or biopsy techniques.
TABLE 23-22.
Hepatobiliary Neoplasia
| Tumor Type | Incidence/Species | Comments |
|---|---|---|
| Hepatocyte Tumors: | ||
|
Cats > dogs Most common primary tumor in dogs |
Diagnosis by biopsy (FNA cannot differentiate normal from adenoma) Surgical removal is curative |
| Tumors of Bile Duct Epithelium: | ||
|
Adenoma most common bile duct tumor in cats and frequent cause of cholestasis (rare in dogs) Carcinoma more common in dogs |
Carcinomas (both species) are highly metastatic (lymph nodes and lung) and 80% have metastasized at the time of diagnosis in cats |
| Neuroendocrine Tumors: | ||
|
Unusual in cats but carcinoid tumors reported occasionally | Can be intrahepatic or extrahepatic; if solitary can be excised, but no therapy if diffuse (more common in dogs) |
| Stromal Cell Tumors: | ||
|
All are rare, but reported occasionally in cats (<13% of all hepatic tumors in dogs) |
All behave aggressively and likely represent metastatic disease |
| Other Common Tumors: | ||
|
Most common round cell tumor in liver of cats (can be multicentric, alimentary, or hepatosplenic), followed by mast cell tumor (typically metastasis from spleen) Histiocytic tumors are more common in dogs |
Both low-grade lymphocytic (small cell) and high-grade (large cell, blastic) lymphoma occur; the prognosis varies with the tumor type Prognosis with splenectomy and lomustine therapy is very good |
FNA, Fine-needle aspiration.
Data from Balkman C: Hepatobiliary neoplasia in dogs and cats, Vet Clin North Am Small Anim Pract 39:617, 2009.
FIGURE 23-58.
Ultrasonographic image of nodular lesion associated with hepatic small cell lymphoma. The nodule appears as a target lesion, being hypoechoic peripherally, yet hyperechoic centrally. Hypoechogenicity typically occurs with inflammatory cell infiltration, and dense hyperechogenicity as seen here is often associated with fibrosis.
(Courtesy Dr. Randolph Baral.)
FIGURE 23-59.
Gross appearance of liver from a cat diagnosed with hepatic small cell lymphoma. Note the mottled patchiness.
(Courtesy Dr. Randolph Baral.)
FIGURE 23-60.
Gross appearance of a biliary cystadenoma (bile duct adenoma). These tumors account for more than 50% of all feline hepatobiliary tumors and may reach a large size by the time of diagnosis.
(Courtesy Dr. Susan Little.)
Historically, amyloidosis has been recognized as primarily a renal disease, especially in Abyssinian cats. More recently, cases of hepatic amyloidosis without renal involvement have been diagnosed in Siamese and related breeds, as well as in nonpedigreed cats.4a, 10a, 30a The majority of cases have been described in Australia, the United Kingdom, and Europe. Amyloid A is deposited in the liver, probably in response to chronic inflammation in another organ. In the Siamese breed, a genetic component may contribute.48a The amyloid A protein occurring in the Siamese breed differs from that known in the Abyssinian breed.48a
The most common clinical signs are related to spontaneous rupture of the enlarged and friable liver. Affected cats may present with lethargy, anorexia, pale mucous membranes, and a heart murmur secondary to anemia. Clinical signs of liver disease are usually absent. Hepatomegaly and hypotension may also be found. Results of routine laboratory testing (mild to marked increases in ALT and globulins while ALP and GGT are typically normal) and ultrasonographic examination (hepatomegaly, generalized increase in hepatic parenchymal echogenicity)4a of the liver may be supportive, but definitive diagnosis relies on histopathologic examination of a liver biopsy. FNA of the liver is not helpful because amyloid is rarely detected with this method. Hemostasis should be evaluated carefully before any biopsy procedure is planned. The most important differential diagnoses are FIP, hepatic lipidosis, and hepatic lymphoma. Scintigraphic imaging using I-123 serum amyloid P component has potential as a noninvasive test.39a There is no specific treatment for amyloidosis in cats, so therapy is primarily supportive care (antioxidants, vitamin K, blood transfusion). Attention should be paid to identification and control of any underlying chronic inflammatory disease. Unfortunately, the long-term prognosis is poor as most affected cats die of intra-abdominal bleeding.
Hepatobiliary Imaging
Survey abdominal radiography is the simplest and most readily available imaging modality to assess structures in the abdominal cavity. Radiographs are most useful to assess liver size, will reveal large hepatic masses, and provide evidence of radiopaque masses or other abnormalities in the abdomen. However, the preferred imaging modality used to assess hepatic structures in cats with suspected liver disease is abdominal ultrasonography (AUS). The reasons why ultrasonography is a more useful tool for assessment of the liver in cats are numerous, but because feline liver diseases are primarily diffuse, infiltrative, or metabolic diseases that also affect the biliary tree, ultrasonography is the only imaging tool that will give reliable diagnostic information. This widely available diagnostic tool can be helpful in determining liver size and parenchymal echogenicity, in identifying mass lesions, evaluating the biliary tree and gallbladder, quantifying flow (Doppler techniques), and identifying vascular anomalies.29 As with all diagnostic modalities, the skill and experience of the operator is vital to accurate procurement and interpretation of the images. Further, it is important to remember that although ultrasonographic images are extremely useful in the clinical evaluation of a cat with possible liver disease, the images themselves do not represent a histologic diagnosis.
For the most common liver diseases of cats (hepatic lipidosis, feline cholangitis syndrome, and neoplasia/lymphoma), AUS examination provides a useful means of obtaining clinical clues and tissue to support or refute the differentials. For example, in cats with hepatic lipidosis, the liver is quite enlarged and typically diffusely hyperechoic, while in cholangitis or other inflammatory diseases, the liver is more often diffusely hypochoic.29 However, these sonographic findings are very nonspecific and can easily lead to errors in diagnosis if the tissue is not subsequently sampled for confirmation.24, 35 Thus one of the most important utilities of the AUS is the ability to obtain liver tissue (either by aspiration or guided-needle biopsy) and for aspiration of the gallbladder to obtain bile for culture.24, 49 These techniques alone have made the AUS an extremely important diagnostic tool in the evaluation of liver disease in cats.
Liver Histopathology (Aspirates and Biopsies)
The diagnosis of most liver diseases requires a histopathologic sample of liver tissue, and this is particularly true in the most common feline liver diseases, which tend to be diffuse diseases affecting the entire liver. Cats with one of these diffuse diseases can be sampled randomly using any one of these commonly employed techniques: ultrasound-guided fine-needle aspirates (FNA), ultrasound-guided needle biopsy, laparoscopic biopsies, or biopsies obtained surgically. Some types of neoplasia (particularly round cell tumors) and vacuolar hepatopathies (hepatic lipidosis) can often be diagnosed by cytology using FNA techniques. However, differentiation of liver cell tumors (adenomas and carcinomas) and inflammatory diseases of the liver cannot be diagnosed without a larger sample of tissue and histopathologic examination.30, 50 Further, even in cats with classic hepatic lipidosis changes, concurrent diseases such as cholangitis or lymphoma can be missed if only FNA techniques are employed.60 Thus it is essential to consider that in many liver diseases the lesions, although typically diffuse, may also have focal components; for example, inflammation may be throughout the liver, but fibrosis will be present only in focal areas. Thus the results of FNA or Tru-Cut needle biopsies should always be considered in the light of the clinical, laboratory, and ultrasonographic evidence.
Prior to scheduling a cat for a biopsy, the risk-to-benefit ratio of performing a liver biopsy should always be considered. This is primarily because heavy sedation or anesthesia will be essential in most cats undergoing a liver FNA, and for all cats undergoing a liver biopsy (needle or otherwise). In addition to anesthesia risks, the use of automatic spring-loaded biopsy guns to obtain ultrasound-guided biopsies of liver tissue is contraindicated in cats, because they may cause a lethal shock reaction.40 A similar reaction may be seen with penetration of the larger bile ducts or gallbladder with a large-bore biopsy needle, because these tissues have a significant autonomic innervation in the cat that may result in bradycardia and shock following the procedure.40, 48, 54 It is particularly important to recognize this as a risk in cats with EHDBO or dilated bile ducts, and this risk factor reiterates the need for ultrasound examination of the liver prior to making biopsy decisions. Nonetheless, owners should be informed of these potential risks, in addition to the risk of bleeding from biopsy sites in any cat undergoing liver sampling.7, 48
Biopsy Techniques
Liver biopsies, whether they are obtained by needle, laparoscopy, or surgical means, should be taken from a location that represents the primary liver pathology, handled appropriately to ensure accurate interpretation of the sample, and the histopathologic description should be interpreted according to the guidelines set by the WSAVA Standards for Clinical and Histologic Diagnosis of Canine and Feline Liver Disease.42, 61 Guidelines for obtaining and handling surgical biopsies of the liver are reviewed elsewhere27 and will not be further discussed. Because needle aspirates/biopsies, Tru-Cut–type biopsies, and laparoscopic biopsies are commonly used to obtain liver tissue in cats, the benefits and limitations of each of these techniques will be discussed. As a general rule, the more tissue that can be obtained, the better the pathologist's interpretation of the tissue abnormalities will be. For example, most pathologists believe that at least six portal areas are necessary to make a diagnosis of inflammation liver disease in cats.42 This will require either a 16- or 18-gauge needle size or larger piece of tissue than is obtained with smaller needles or an aspirate. The amount of tissues required to view at least six portal areas is approximately 15 mg, and 5 mg will be required for culture of the tissue.42 If other analyses of the tissues are considered (e.g., metal analysis), approximately 20 to 40 mg of liver is needed.42 A typical laparoscopic cup biopsy forceps will provide 45 mg of liver tissue, a 14-g Tru-Cut–type biopsy needle provides 15 to 20 mg, and an 18-g needle biopsy provides only 3 to 5 mg of liver tissue.42 Thus, depending on the clinical circumstances and considered differentials, the best approach for obtaining the needed tissue must be considered prior to planning the procedure.
Fine-needle aspiration to obtain liver tissue for cytologic examination is commonly performed in cats with liver disease for good reason. The procedure is inexpensive, easy to do, is relatively low risk, and often requires only sedation to complete.57 Further, samples obtained by this method can be diagnostic for hepatic lipidosis, hepatic lymphoma or other round cell tumors, and in areas where appropriate, definitive diagnosis of certain infectious diseases (e.g., histoplasmosis).57 However, even with these relatively straightforward diseases, FNA of liver tissue has significant limitations, the most important of which is the failure to accurately identify the primary disease. For example, although it is easy to make a diagnosis of hepatic lipidosis using this technique, a paper recently showed four cats that were incorrectly diagnosed with hepatic lipidosis instead of lymphoma because the FNA samples were obtained from areas that did not have lymphoma infiltration.60 In another study, reviewing the agreement between FNA cytologic samples of liver and the histopathologic diagnosis, only 51% of the cases had overall agreement.50 Thus although cytology of FNA samples of liver tissue in cats with diffuse hepatic disease remains a useful first step, it is important for the clinician to carefully interpret the results and discuss the potential limitations of this technique with owners.
There are several needle biopsy techniques available for sampling liver tissue, but not all are suitable or safe for use in cats. The Menghini technique is one such approach that is not suitable for use in cats, because it is a blind procedure using a large-bore needle that cannot be used with ultrasound guidance.42 The second option among the needle biopsy techniques that is not recommended for cats is the biopsy gun device. Tru-Cut biopsy guns are operated by a triggering device that can result in the induction of a lethal vagotonic shock reaction in the cat immediately following the procedure.40 For most ultrasound-guided liver biopsy procedures, either the manual or, preferably, the semiautomatic Tru-Cut device is recommended for use in obtaining needle biopsies from cats. As a general rule, the Tru-Cut device will advance into the liver to a depth of 2 cm; so, it is essential to carefully note the amount of liver tissue available during the ultrasound assessment before advancing the needle for tissue collection. Properly obtained Tru-Cut needle biopsies are a valuable technique for obtaining a representative sample of liver tissue61; however, because of the risk for bleeding or liver fracture with any movement, it is essential that cats be anesthetized for this procedure.
Laparoscopy is an intermediate step between needle biopsy and surgical laparotomy for obtaining liver tissue for histopathology in cats.48, 54 This technique is becoming more widely used as more specialists are trained for this procedure that allows visualization of tissues to be biopsied without opening the entire abdomen. Although this technique does require general anesthesia, the limited degree of invasiveness, the large biopsy sample size, and rapid patient recovery make laparoscopy a valuable tool for obtaining liver tissue,48 and it can be used to obtain biopsies from the spleen, pancreas, kidneys, lymph nodes, or to aspirate the gallbladder. For a detailed discussion of laparoscopic techniques and equipment, the interested reader is referred to several recent reviews on the subject.48, 54 To maximize the histopathologic accuracy, biopsies taken at laparoscopy or surgically should be taken from both normal-appearing and abnormal areas in the liver. Further, if there is a need to obtain samples from the deeper tissues, the laparoscope can be used to direct a Tru-Cut needle biopsy to the best location for sampling. One of the major advantages of the laparoscopic technique is that it allows the operator to observe the biopsy sites for excessive bleeding, which is unusual, but if observed can be staunched by using pressure on the site, gelatin coagulation material placement, or electrocautery. With experienced operators, the complication rate for laparoscopy is very low (less than 2%), and most complications were because of anesthesia, bleeding, or air embolism.48 Finally, although not necessary to have direct visualization to obtain an aspirate of gallbladder bile, laparoscopy allows easy sampling of bile for culture, which is important in all cats with suspected inflammatory liver disease or hepatobiliary disease.
Therapy of Liver Disease
Once a diagnosis of liver disease is made in the cat, specific therapy for the cause (if available) should be instituted; however, for many feline liver diseases, no specific therapy is available, and thus hepatoprotective therapy is used concurrently to aid in the recovery of the liver from the insult. In this section, therapy of two of the most common diseases of the feline liver will be considered, with a special emphasis on nutritional aspects of treatment, nutraceutical therapy, and the unique needs of cats.
Idiopathic Hepatic Lipidosis
The most common liver disease of cats is idiopathic hepatic lipidosis (FIGURE 23-61, FIGURE 23-62 ), a disease that results in liver failure because of a combination of factors including hepatic lipid accumulation, insulin resistance, fasting, and protein (especially arginine) deficiency.2, 8, 9, 11 Thus, unlike many diseases of the liver, the primary focus of therapy and the essential component for recovery is nutritional support. As in any patient with serious liver disease, initial therapy is always aimed at correction of any fluid or electrolyte abnormalities that may exist, because these may be profound if the cat has been vomiting. In addition, normalization of electrolytes is particularly important in cats that have been anorexic for an especially long time (1 to 2 weeks), because refeeding syndrome may be triggered with the initiation of feeding, resulting in sudden drops in potassium, phosphate, and magnesium.1 Although this phenomenon is less common and usually less profound in cats fed enterally versus those started on intravenous nutrition, it can be a significant source of morbidity if electrolyte replacement and monitoring are not carefully attended.
FIGURE 23-61.
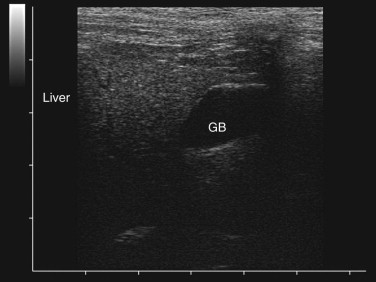
Ultrasonographic appearance of liver with hepatic lipidosis. The echogenicity of the parenchyma is uniformly increased, which is more apparent when compared with other ultrasonographic images presented in this chapter. Additionally, the gall bladder is distended. Hepatic lipidosis in this cat was secondary to anorexia associated with primary intestinal disease.
(Courtesy Dr. Randolph Baral.)
FIGURE 23-62.
Gross appearance of liver from a cat with hepatic lipidosis. Note the pale tan and exaggerated reticular pattern. In most cases, the edges appear more rounded than is evident here. Hepatic lipidosis in this cat was secondary to anorexia associated with primary intestinal disease (same cat as in Figure 23-61).
(Courtesy Dr. Randolph Baral.)
Once the cat is hemodynamically stable, the next step in treatment planning in cats with hepatic lipidosis is re-introduction of nutrition, which must include placement of a feeding tube (Box 23-5 ). However, because many of these cats are extremely ill and are not good candidates for anesthesia, placement of a nasoesophageal (NE) tube to allow initiation of enteral feeding is often the most appropriate step for the first few days. Force feeding is to be strongly discouraged in these sick cats for several reasons:
-
•
It is highly stressful and will further increase the stress response and insulin resistance phenomena that are perpetuating the hepatic lipidosis.
-
•
It can be dangerous to the cat (aspiration) or operator (scratches/biting).
-
•
It is rarely able to meet the necessary nutritional goals set for the patient.
-
•
It may induce food aversion, a phenomenon unique to cats, but creating a profound aversion to the chosen food that can be lifelong.12
BOX 23-5. Considerations for Feeding Tubes in Patients with Liver Disease.
When administering food through a feeding tube, there are several important points:
-
1
The food should be room temperature (not too hot or cold).
-
2
The tube should be flushed with water following feeding, to remove any particles of food or medication that may cause the tube to clog.
-
3
If the cat is volume sensitive, it is important to carefully calculate how much water is used for flushing the tube, because a significant volume of fluid can be infused, creating a potential fluid overload. If the cat is fluid sensitive, the total amount of fluids (amount in the food, amount added to food if blenderized, and amount of flush) must be determined, and the amount of fluid used in flushes or food preparation may have to be reduced.
Although NE tubes are excellent choices for short-term feeding of cats unwilling to eat, there are several disadvantages to their long-term use, including the nasal irritation that occurs, the relative ease with which cats can (and will) remove them, and the need to use liquid enteral diets.62 Thus once the cat is deemed stable enough for general anesthesia, a long-term feeding tube solution is needed, and this typically is either an esophageal (E) tube (Figure 23-63 ) or percutaneous endoscopic gastrostomy (PEG) tube.22, 62 Both feeding options are generally well tolerated methods for providing long-term feeding, but E tubes have the advantage of being placed without the need for any specialized equipment, and if complications occur, they are generally easily addressed, because the most common complications are infection at the tube site or premature removal of the tube by the cat. Placement of a PEG tube, although relatively easy to learn to place, requires having the appropriate endoscopic equipment, and if complications occur as a result of infection or tube removal, more significant morbidity can result. Because there is no advantage to placement of PEG tubes in cats versus E tubes, placement of E tubes is advocated as the best approach for most practice situations. Interested readers are referred to several recent reviews on tube placement for specific details on each method and to Chapter 18.22, 62
FIGURE 23-63.
Esophageal feeding tubes are ideal for enteral nutrition in the cat, because they are easily placed and are associated with few serious complications.
Diet selection is the next step in treatment planning for cats with hepatic lipidosis. In contrast to the belief that animals in liver failure need lower quantities of protein to reduce the workload on the liver, cats with hepatic lipidosis actually need protein to recover. In fact, the work of Biourge and coworkers showed that protein was the essential nutrient in reducing hepatic lipid accumulation, was essential to eliminate the negative nitrogen balance, and also appeared to minimize muscle catabolism.9 Further, diets high in protein can improve insulin sensitivity and assist weight loss in recovery from obesity.8, 11 Conversely, although carbohydrates are a readily available energy source, they are often associated with gastrointestinal distress (diarrhea, abdominal cramping) and hyperglycemia (secondary to the insulin resistance in place as a result of obesity and hepatic lipidosis).2 Thus diets selected for cats with hepatic lipidosis should ideally be high in protein (>40% metabolizable energy [ME]) and have lower amounts of carbohydrates (<20% ME), with the remaining calories coming from fat. The diets that best fit this profile are the diets formulated for diabetic cats; however, kitten food, many adult cat foods, and some of the enteral recovery diets have this high protein/low carbohydrate profile. Many of the intestinal diets are not higher protein and are higher in carbohydrates, and so would not be the ideal choice. The key to using any of the foods that are not designed for use in a feeding tube is to blenderize them (and if necessary, strain the food) so that the food will easily go through the 14- or 16-g feeding tube without clogging it. Enteral diets designed for use in feeding tubes are the easiest to use, and are an acceptable choice in most situations. Finally, because many cat stomachs are volume sensitive with initiation of feeding, it is very important to start conservatively with small-volume feeding on a more frequent schedule. With prolonged fasting, the stomach volume of a cat with hepatic lipidosis may be reduced dramatically, preventing normal expansion and limiting intake to as little as 10% of normal. Thus to avoid vomiting when feeding, the starting volume may have to be as small as 10 to 15 mL every 2 to 3 hours. A good rule of thumb is to start with estimation of resting energy requirement (RER) (40 to 50 kcal/kg is a good estimate of RER), and then attempt to meet 25% of RER the first day. If no problems are encountered, increase the amount to 50% of RER the second day, and so on, but during this period, keep the frequency as high as possible (feed four to six meals per day) so that the volume remains relatively small at each meal. Once full RER has been achieved with multiple meals per day, the frequency of feeding can be gradually reduced to three to four meals per day. Most cats will eventually tolerate three meals per day well, and some can tolerate two meals per day, but this is quite variable and should not be attempted during the first weeks of feeding. In general, most cats with hepatic lipidosis will require tube feeding for a minimum of 3 to 6 weeks before they will show interest in food and begin eating again on their own. The tube should be retained until the cat has been eating on his or her own for at least 1 week or longer and can be maintained for a longer duration if it is being used to administer medications, because cats can eat normally with the E tube in place.
The other therapeutic considerations for cats diagnosed with hepatic lipidosis are directed toward dealing with the complications of the disease and reducing the oxidative stress on the liver with hepatoprotective therapy (Table 23-23 ). In cats that are vomiting, antiemetic therapy is beneficial, because it is imperative that the cat continues to receive some food, and vomiting will complicate this. Metoclopramide is often used in cats because of its ready availability and low cost, but it is a very weak antiemetic in cats and thus may not be the best choice. In most cats, the novel NK-1 receptor antagonist maropitant has been a safe and effective choice.32 The most commonly used antiemetics in the author's feline practice are maropitant (1 mg/kg IV, SC or through the E tube q24h), ondansetron (0.22 mg/kg IV q8-12h), or dolasetron (0.5 mg/kg IV, SC q24h). In addition to control of vomiting, all cats with hepatic lipidosis should be given vitamin K1 (2.5 mg/cat PO, SC) daily for a week, then weekly until the cat has recovered, and vitamin B12 (cobalamin) (250 µg/cat SC) weekly for 6 weeks, then monthly until blood values are normal.46 Other vitamins may become deficient, such as some of the B vitamins and vitamin E; however, feeding is likely to rapidly replenish these deficiencies if they exist. This is also likely true of amino acid deficiencies, but supplementation of L-carnitine (250 mg/day PO) may be beneficial by improving fatty acid oxidation.10 Finally, hepatoprotectant and antioxidant therapy with S-adenosylmethionine (SAMe) (20 mg/kg PO q24h) has been advocated to increase glutathione and may be beneficial in cats with hepatic lipidosis.13, 19, 56 It is important to note that if SAMe is given through the tube (and thus the tablets must be crushed), the dose must be increased by approximately 50% to allow for the loss of absorption from loss of the enteric coating.
TABLE 23-23.
Medications and Supplements Used in the Treatment of Hepatic Lipidosis
| Medication | Dose |
|---|---|
| Antiemetics: | |
| Maropitant | 1 mg/kg, IV/SC/PO, q24h |
| Ondansetron | 0.22-0.50 mg/kg, IV/PO, q8-12h |
| Dolasetron | 0.5-1.0 mg/kg, IV/SC/PO, q12-24h |
| Vitamin K1 | 2.5 mg/cat/day for 1 week, then weekly until recovered, PO, SC |
| Vitamin B12 | 250 µg/cat weekly for 6 weeks, then monthly until blood values are normal, SC |
| L-carnitine | 250 mg/cat/day, PO |
| SAMe | 20 mg/kg, PO, q24h |
SAMe, S-adenosylmethionine.
Because drug metabolism is often impaired in cats with hepatic lipidosis, appetite stimulants, such as mirtazapine, cyproheptadine, and clonazepam, should not be used in cats because dosing and side effects can be unpredictable. Benzodiazepine agonist drugs (e.g., diazepam) should be completely avoided in cats with possible lipidosis-induced hepatoencephalopathy, because they will exacerbate the signs and may cause fulminant liver failure.2, 14 Fortunately, most cats with idiopathic hepatic lipidosis that receive immediate and aggressive therapy and feeding for their disease will recover completely. Cats that develop hepatic lipidosis secondary to other serious diseases (e.g., lymphoma) have a much lower chance of complete recovery and often die of their disease or its complications.
Feline Cholangitis Syndrome
The most common inflammatory liver disease in the cat is a complex syndrome with multiple subgroups of disease previously termed cholangitis/cholangiohepatitis complex (CCH) but currently recognized under the terminology feline cholangitis syndrome.61 This disease is quite variable in both its presentation and severity, and it may occur as a primary process or secondary to/concurrent with other diseases (e.g., pancreatitis, IBD). Because the primary starting point of the inflammatory disease in cats is the bile ducts (cholangitis), with inflammation extending to the hepatic parenchyma (cholangiohepatitis) only with time and severity, the term cholangitis syndrome has become the preferred terminology. The disease syndrome has been further classified by the WSAVA Liver Diseases Group into one of three primary types: neutrophilic or suppurative, chronic lymphoplasmacytic (FIGURE 23-64, FIGURE 23-65 ), and lymphocytic (non-suppurative).61 Each of the forms appears to behave quite differently clinically as well as in their progression and outcome. In general, cats with the suppurative form of CCH typically have an acute onset of illness, which often includes fever, anorexia, and vomiting, and they may become icteric quite quickly (Figure 23-66 ).28, 52 The nonsuppurative form of CCH (lymphocytic form) tends to be a more chronic condition, with affected cats showing nonspecific signs of illness that may include partial anorexia and lethargy, but the signs may wax and wane or are non-progressive.28, 52 Because of the feline pancreatic and bile duct anatomy, it is common for cats with CCH to have pancreatitis and vice versa, and in some cases, cats will also have concurrent IBD; the constellation of the three conditions occurring together is called triaditis.59 This combination is increasingly recognized in cats, and recent reports suggest from 50% to 85% of cats with one syndrome have all three diseases.25, 51, 54, 59 At this time, the etiology of each of these syndromes and the pathogenesis is not well understood; however, the enteric microflora are presumed to play an important role in the suppurative form, and immune mechanisms are presumed to be the cause of the chronic inflammation found in the chronic nonsuppurative forms. However, whether or not these syndromes are related, a continuum of disease or completely different diseases remains undetermined.
FIGURE 23-64.
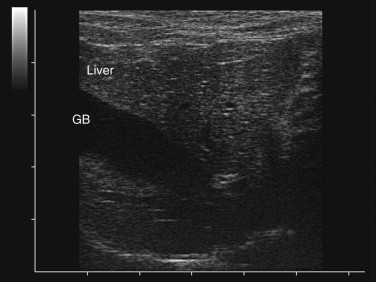
Ultrasonographic appearance of liver with lymphocytic/plasmacytic inflammation. Note the varying echogenicity throughout the hepatic parenchyma; areas of hypoechogenicity likely reflect inflammatory cell infiltration. The gall bladder is distended; its shape is distorted by pressure from the transducer.
(Courtesy Dr. Randolph Baral.)
FIGURE 23-65.
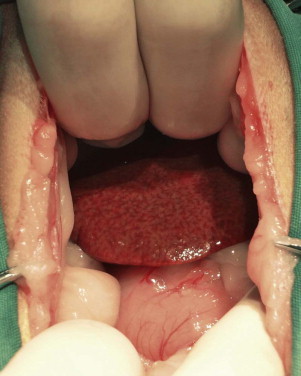
Gross appearance of liver from a cat diagnosed with lymphocytic/plasmacytic inflammation. Note the liver appears thickened with rounded edges and has a reticular pattern.
(Courtesy Dr. Randolph Baral.)
FIGURE 23-66.
Icterus is the result of cholestasis and will only be apparent clinically when bilirubin levels are 2 to 3 times normal values.
Once a definitive diagnosis is obtained by histopathology of the liver tissue and culture of bile, treatment can be tailored to needs of the cat. Cats with the more aggressive suppurative form of cholangitis often require intravenous fluid therapy, antibiotic therapy (based on results of culture whenever possible), and supportive therapy (antiemetics, vitamin K1, hepatoprotectants such as SAMe [20 mg/kg PO q24h] and ursodeoxycholic acid [10 mg/kg PO q24h]), and if pancreatitis is concurrent, pain control with opioid pain relievers (e.g., buprenorphine 0.05 to 0.1 mg/kg PO, SQ q8-12h).52 If culture is not possible, combination therapy with enrofloxacin (4 mg/kg PO q24h) and metronidazole (5 mg/kg PO q12h) is reasonable. In cats with chronic lymphoplasmacytic forms of cholangitis, management must be tailored to the individual situation and often requires therapy with either immunosuppressive doses of prednisolone (2 to 4 mg/kg PO q24h) or chlorambucil (4 mg/m2 PO q2d), along with the hepatoprotectants and cholerectics, and concurrent treatment of other diseases (pancreatitis or IBD) that may be occurring.52 The lymphocytic or lymphoplasmacytic forms of cholangitis may wax and wane in intensity over time, and may require long-term continuous or intermittent therapy to control the disease. There is no specific diet that is recommended for cats with inflammatory liver disease, but protein restriction should not be initiated unless the cat has clear evidence of severe hepatoencephalopathy. The diet should be selected based on other conditions (such as IBD), for which the diet may be more critical in the management. Monitoring of serum chemistry values (especially glucose), clotting times, cobalamin levels, and PLI/TLI concentrations are recommended every few months, as well as careful monitoring of the CBC for all cats on chlorambucil. In all cats with chronic inflammatory liver disease, prior to initiation of immunosuppressive therapy, a careful assessment of the cat for other possible causes of inflammation should be completed (Box 23-6 ).
BOX 23-6. Infectious Diseases Associated with Hepatopathy or Inflammation in the Feline Liver.
Bacterial agents: hepatic abscess, ascending infection/bacterial translocation, leptospirosis, Clostridium spp., Helicobacter spp., bartonellosis
Viral agents: feline infectious peritonitis, feline herpesvirus, virulent calicivirus
Protozoal agents: toxoplasmosis, visceral leishmaniasis
Parasitic agents: flukes (Platynosomum), visceral migrans (Toxocara), heartworm (Dirofilaria)
Fungal agents: histoplasmosis, paecilomycosis
From Kearns S: Infectious hepatopathies in dogs and cats, Top Comp Anim Med 24:189, 2009.
Hepatoencephalopathy/Portosystemic Shunting
As in dogs, if a cat with PSS can have surgical closure of the shunting vessel (ligation, placement of an ameroid constrictor, intravenous coiling), the long-term prognosis for function and quality of life is generally very good.5 However, even if surgical correction is anticipated, and especially if surgical correction is impossible or not completely successful, medical management of HE is indicated. See Table 23-24 for the basic therapeutic approach to medical management of cats with HE resulting from PSS.
TABLE 23-24.
Medical Management of Hepatoencephalopathy in Cats
| Specific Problem to Address | Therapeutic Options |
|---|---|
| Decreasing bacterial production of ammonia |
|
| Coagulopathy (postoperative or if symptomatic) |
|
| Gastrointestinal ulcers and gastritis |
|
| Seizures |
|
| Nutritional support |
|
| Hepatoprotectants |
|
SAMe, S-adenosylmethionine.
From Berent AC, Tobias KM: Portosystemic vascular anomalies, Vet Clin North Am Small Anim Pract 39:513, 2009.
Nutraceutical Therapy
Because hepatocytes, by their position in the body between the GI tract and rest of the body, as well as their critical role in metabolism and detoxification, are uniquely susceptible to oxidative injury and reactive intermediates of metabolism, they must be able to protect themselves. The natural defenses of the liver include superoxide dismutase and glutathione, free-radical scavengers such as vitamin E and ascorbate, and other prosurvival signaling pathways that are controlled by hormones and growth factors.56 However, in injury or overwhelming infection or inflammation, the natural defenses of the liver can be overwhelmed, and then it is essential for medicines and nutraceutical therapy to be included in the treatment plan to help reduce inflammation and fibrosis, protect against oxidant injury, and enhance bile flow. The cytoprotective agents most commonly used in liver diseases to assist in these processes (Table 23-25 ) are:
-
•
S-Adenosylmethionine (SAMe)—a precursor in the synthesis of glutathione and an important methyl donor to DNA and proteins, is an important antioxidant and stabilizes membrane functions
-
•
N-acetylcysteine—a precursor to glutathione and antioxidant, also improves tissues oxygen delivery
-
•
Ursodeoxycholic acid (tertiary bile acid)—used to replace hepatotoxic, hydrophobic bile acids and increase bile flow
-
•
Silymarin (milk thistle)—a free radical scavenger and anti-inflammatory/antifibrotic agent
-
•
Vitamin E—an antioxidant and antiinflammatory vitamin*
TABLE 23-25.
Dosages and Indications of Commonly Used Hepatoprotective Agents in Feline Liver Disease
| Agent | Indications | Dosage | Veterinary Products |
|---|---|---|---|
| S-Adenosylmethionine (SAMe) | Inflammatory hepatopathies, hepatic lipidosis, cholestatic hepatopathies, acetaminophen toxicity | 20 mg/kg/day PO Breaking/crushing enteric coated tablets reduces bioavailability |
Denosyl-SD4 or Denomarin (Nutramax Labs, Edgewood, Md.) Zentonil (Vetoquinol, Buena, New Jersey) |
| N-Acetylcysteine (NAC) | Acute liver failure, acetaminophen toxicity | 140 mg IV once, then 70 mg/kg IV q6h or 100 mg/kg/24h in CRI | No veterinary products, but powder is widely available and is used in a 10% solution with saline |
| Silymarin (Milk thistle has four isomers: isosilybin, silydianin, silychristin, and silybin; this is the active component of silymarin) | Toxic hepatopathies, metabolic hepatopathies (hepatic lipidosis), and inflammatory hepatopathies | 20-50 mg/kg/day PO divided q8h | Marin or Denomarin (Nutramax Labs) |
| Ursodeoxycholic acid (UDCA) | Cholestatic and inflammatory hepatopathies, and metabolic hepatopathies | 10-15 mg/kg/day PO | No veterinary products |
| Vitamin E | Cholestatic and inflammatory hepatopathies | 10-15 IU/kg/day PO | No veterinary products |
CRI, Constant rate infusion; IU, international units.
From Webster CRL, Cooper J: Therapeutic use of cytoprotective agents in canine and feline hepatobiliary disease, Vet Clin North Am Small Anim Pract 39:631, 2009.
Although few clinical trials of these nutraceuticals have been performed in feline liver disease, a few studies have recently appeared showing that SAMe, ursodeoxycholic acid, silymarin, and N-acetylcysteine all are hepatoprotective, have few adverse side effects, and may be beneficial in many types of liver disease in cats.3, 26, 39, 53, 55
Summary
Feline liver disease is a common problem that requires careful consideration of the presenting complaint, clinicopathologic findings, imaging results, and, if available, histopathologic interpretation to be able to provide an accurate diagnostic and therapeutic plan. A variety of insults can be responsible for liver dysfunction or failure, but hepatic lipidosis and feline cholangitis syndrome remain the most common reasons for cats to present with icterus or liver failure. Therapy must be tailored to the individual, but nutritional support is critical in the management of hepatic lipidosis, and appropriate supportive therapy with hepatoprotectants may be crucial to treatment success.
References
- 1.Armitage-Chan E, O’ Toole T, Chan DL. Management of prolonged food deprivation, hypothermia, and refeeding syndrome in a cat. J Vet Emerg Crit Care. 2006;16:S34. [Google Scholar]
- 2.Armstrong PJ, Blanchard G. Hepatic lipidosis in cats. Vet Clin North Am Small Anim Pract. 2009;39:599. doi: 10.1016/j.cvsm.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Avizeh R, Najafzadeh H, Razijalali M. Evaluation of prophylactic and therapeutic effects of silymarin and N-acetylcysteine in acetaminophen-induced hepatotoxicity in cats. J Vet Pharmacol Therap. 2009;33:95. doi: 10.1111/j.1365-2885.2009.01100.x. [DOI] [PubMed] [Google Scholar]
- 4.Balkman C. Hepatobiliary neoplasia in dogs and cats. Vet Clin North Am Small Anim Pract. 2009;39:617. doi: 10.1016/j.cvsm.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Beatty JA, Barrs VR, Martin PA. Spontaneous hepatic rupture in six cats with systemic amyloidosis. J Small Anim Pract. 2002;43:355. doi: 10.1111/j.1748-5827.2002.tb00086.x. [DOI] [PubMed] [Google Scholar]
- 5.Berent AC, Tobias KM. Portosystemic vascular anomalies. Vet Clin North Am Small Anim Pract. 2009;39:513. doi: 10.1016/j.cvsm.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Bertolini G. Acquired portal collateral circulation in dogs and cats. Vet Radiol. 2010;51:25. doi: 10.1111/j.1740-8261.2009.01616.x. [DOI] [PubMed] [Google Scholar]
- 7.Bigge LA, Brown DJ, Pennick DG. Correlation between coagulation profile findings and bleeding complications after ultrasound guided biopsy: 434 cases (1993-1996) J Am Anim Hosp Assoc. 2001;37:228. doi: 10.5326/15473317-37-3-228. [DOI] [PubMed] [Google Scholar]
- 8.Biourge V, Nelson RW, Feldman EC. Effect of weight gain and subsequent weight loss on glucose tolerance and insulin response in healthy cats. J Vet Int Med. 1997;11:86. doi: 10.1111/j.1939-1676.1997.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 9.Biourge V, Massat B, Groff JM. Effects of protein, lipid or carbohydrate supplementation on hepatic lipid accumulation during rapid weight loss in obese cats. Am J Vet Res. 1994;55:1406. [PubMed] [Google Scholar]
- 10.Blanchard G, Paragon BM, Mullat F. Dietary L-carnitine supplementation in obese cats alters carnitine metabolism and decreases ketosis during fasting and induced hepatic lipidosis. J Nutr. 2002;132:204. doi: 10.1093/jn/132.2.204. [DOI] [PubMed] [Google Scholar]
- Blunden A, Smith A. Generalized amyloidosis and acute liver haemorrhage in four cats. J Small Anim Pract. 1992;33:566. [Google Scholar]
- 11.Brown B, Mauldin GF, Armstrong PF. Metabolic and hormonal alterations in cats with hepatic lipidosis. J Vet Int Med. 2000;14:20. doi: 10.1892/0891-6640(2000)014<0020:mahaic>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Brunetto MA, Gomes MOS, Andre MR. Effects of nutritional support on hospital outcomes in dogs and cats. J Vet Emerg Crit Care. 2010;20:224. doi: 10.1111/j.1476-4431.2009.00507.x. [DOI] [PubMed] [Google Scholar]
- 13.Center SA. Metabolic, antioxidant, nutraceutical, probiotic, and herbal therapy relating to the management of hepatobiliary disorders. Vet Clin North Am Small Anim Pract. 2004;34:67. doi: 10.1016/j.cvsm.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Center SA. Current considerations for evaluating liver function in the cat. In: August JR, editor. Consultations in feline internal medicine. ed 5. Elsevier; Philadelphia: 2006. p. 89. [Google Scholar]
- 15.Center SA. Diseases of the gallbladder and biliary tree. Vet Clin North Am Small Anim Pract. 2009;39:543. doi: 10.1016/j.cvsm.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Center SA, Baldwin BH, Dillingham S. Diagnostic value of serum gamma glutamyl transferase and alkaline phosphatase in hepatobiliary disease in the cat: 1975-1990. J Am Vet Med Assoc. 1986;188:507. [PubMed] [Google Scholar]
- 17.Center SA, Crawford MA, Guida L. Retrospective study of 77 cats with severe hepatic lipidosis: 1975-1990. J Vet Int Med. 1993;7:349. doi: 10.1111/j.1939-1676.1993.tb01030.x. [DOI] [PubMed] [Google Scholar]
- 18.Center SA, Erb HN, Joseph SA. Measurement of serum bile acids concentrations for diagnosis of hepatobiliary disease in cats. J Am Vet Med Assoc. 1995;207:1048. [PubMed] [Google Scholar]
- 19.Center SA, Randolph JF, Warner KL. The effects of s-adenosylmethionine on clinical pathology and redox potential in the red blood cell, liver and bile of normal cats. J Vet Int Med. 2005;19:303. doi: 10.1892/0891-6640(2005)19[303:teosoc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 20.Center SA, Warner D, Corbett J. Proteins invoked by vitamin K absence in clotting times in clinically ill cats. J Vet Int Med. 2000;14:292. doi: 10.1892/0891-6640(2000)014<0292:pibvka>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 21.Center SA, Warner KL, Erb HN. Liver glutathione concentrations in dogs and cats with naturally occurring liver disease. Am J Vet Res. 2003;63:1187. doi: 10.2460/ajvr.2002.63.1187. [DOI] [PubMed] [Google Scholar]
- 22.Chan DL. Critical care nutrition. In: August JR, editor. Consultations in feline internal medicine. ed 6. Elsevier; Philadelphia: 2010. p. 116. [Google Scholar]
- 23.Fahie MA, Martin RA. Extrahepatic biliary tract obstruction, a retrospective study of 45 cases (1983-1993) J Am Anim Hosp Assoc. 1995;31:478. doi: 10.5326/15473317-31-6-478. [DOI] [PubMed] [Google Scholar]
- 24.Feeney DA, Anderson KL, Ziegler LE. Statistical relevance of ultrasound criteria in the assessment of different liver diseases in dogs and cats. Am J Vet Res. 2008;69:212. doi: 10.2460/ajvr.69.2.212. [DOI] [PubMed] [Google Scholar]
- 25.Ferrari J, Hardam E, Van Winkle TJ. Clinical differentiation of acute and chronic feline pancreatitis. J Am Vet Med Assoc. 2003;223:469. doi: 10.2460/javma.2003.223.469. [DOI] [PubMed] [Google Scholar]
- 26.Flora K, Hahn M, Rosen H. Milk thistle (Silybum marianum) for the therapy of liver disease. Am J Gastroenterol. 1998;93:139. doi: 10.1111/j.1572-0241.1998.00139.x. [DOI] [PubMed] [Google Scholar]
- 27.Fossum TW, Hedlund CS. Surgery of the liver. In: Fossum TW, editor. Small animal surgery. Mosby; St Louis: 1997. p. 367. [Google Scholar]
- 28.Gagne JM, Armstrong PF, Weiss DJ. Clinical features of inflammatory liver disease in cats: 41 cases (1983-1993) J Am Vet Med Assoc. 1999;214:513. [PubMed] [Google Scholar]
- 29.Gaschen L. Update on hepatobiliary imaging. Vet Clin North Am Small Anim Pract. 2009;39:439. doi: 10.1016/j.cvsm.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Giordano A, Paltrinieri S, Bertazzolo W. Sensitivity of tru-cut and fine needle aspirate biopsies of liver and kidney for the diagnosis of feline infectious peritonitis. Vet Clin Path. 2004;34:368. doi: 10.1111/j.1939-165X.2005.tb00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey D, Day M. Generalised amyloidosis in two Siamese cats: spontaneous liver haemorrhage and chronic renal failure. J Small Anim Pract. 1998;39:442. doi: 10.1111/j.1748-5827.1998.tb03753.x. [DOI] [PubMed] [Google Scholar]
- 31.Haney DR, Christiansen JS, Toll J. Severe cholestatic liver disease secondary to liver fluke (Platynosomum concinnum) infection in three cats. J Am Anim Hosp Assoc. 2006;42:234. doi: 10.5326/0420234. [DOI] [PubMed] [Google Scholar]
- 32.Hickman MA, Cox SR, Mahabir S. Safety, pharmacokinetics and use of the novel NK-1 antagonist maropitant (Cerenia) for the prevention of emesis and motion sickness in cats. J Vet Pharmacol Ther. 2008;31:220. doi: 10.1111/j.1365-2885.2008.00952.x. [DOI] [PubMed] [Google Scholar]
- 33.Karanjia ND, Singh SM, Widdison AL. Pancreatic ductal and interstitial pressures in cats with chronic pancreatitis. Gastroenterol. 1992;37:268. doi: 10.1007/BF01308182. [DOI] [PubMed] [Google Scholar]
- 34.Kearns S. Infectious hepatopathies in dogs and cats. Top Comp Anim Med. 2009;24:189. doi: 10.1053/j.tcam.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis KM, O’Brien RT. Abdominal ultrasonographic findings associated with feline infectious peritonitis: a retrospective review of 16 cases. J Am Anim Hosp Assoc. 2010;46:152. doi: 10.5326/0460152. [DOI] [PubMed] [Google Scholar]
- 36.Lipscomb VL, Jones HJ, Brockman DJ. Complications and long-term outcomes of the ligation of congenital portosystemic shunts in 49 cats. Vet Rec. 2007;160:465. doi: 10.1136/vr.160.14.465. [DOI] [PubMed] [Google Scholar]
- 37.Lowe AD, Campbell KL, Barger A. Clinical, clinicopathological, and histological changes observed in 14 cats treated with glucocorticoids. Vet Rec. 2009;162:777. doi: 10.1136/vr.162.24.777. [DOI] [PubMed] [Google Scholar]
- 38.Maddison JE. Hepatic encephalopathy. Current concepts of the pathogenesis. J Vet Int Med. 1992;6:341. doi: 10.1111/j.1939-1676.1992.tb00367.x. [DOI] [PubMed] [Google Scholar]
- 39.Nicolson BT, Center SA, Randolph JF. Effects of oral ursodeoxycholic acids in healthy cats on clinicopathological parameters, serum bile acids and light microscopic and ultrastructural features of the liver. Res Vet Sci. 1996;61:258. doi: 10.1016/s0034-5288(96)90074-0. [DOI] [PubMed] [Google Scholar]
- Piirsalu K, McLean R, Zuber R. Role of I-123 serum amyloid protein in the detection of familial amyloidosis in Oriental cats. J Small Anim Pract. 1994;35:581. [Google Scholar]
- 40.Proot SJM, Rothuizen J. High complication rate of an automatic tru-cut biopsy gun device for liver biopsy in cats. J Vet Int Med. 2006;20:1327. doi: 10.1892/0891-6640(2006)20[1327:hcroaa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 41.Rothuizen J. Important clinical syndromes associated with liver disease. Vet Clin North Am Small Anim Pract. 2009;39:419. doi: 10.1016/j.cvsm.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Rothuizen J, Twedt D. Liver biopsy techniques. Vet Clin North Am Small Anim Pract. 2009;39:469. doi: 10.1016/j.cvsm.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 43.Rothuizen J, van den Ingh TS. Arterial and venous ammonia concentrations in the diagnosis of canine hepatoencephalopathy. Res Vet Sci. 1982;33:17. [PubMed] [Google Scholar]
- 44.Rogers CL, O’Toole TE, Keating JH. Portal vein thrombosis in cats: 6 cases (2001-2006) J Vet Int Med. 2008;22:282. doi: 10.1111/j.1939-1676.2008.0048.x. [DOI] [PubMed] [Google Scholar]
- 45.Sergeeff JS, Armstrong PJ, Bunch SE. Hepatic abscesses in cats: 14 cases (1985-2002) J Vet Int Med. 2004;18:295. doi: 10.1892/0891-6640(2004)18<295:haicc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 46.Simpson KW, Fyfe I, Cornetta A. Subnormal concentrations of serum cobalamin (vitamin B12) in cats with gastrointestinal disease. J Vet Int Med. 2001;15:26. doi: 10.1892/0891-6640(2001)015<0026:scoscv>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 47.Trainor D, Center SA, Randolph JF. Urine sulfated and non-sulfated bile acids as a diagnostic test for liver disease in cats. J Vet Int Med. 2003;17:145. doi: 10.1111/j.1939-1676.2003.tb02425.x. [DOI] [PubMed] [Google Scholar]
- 48.Twedt DC. Laparoscopy of the liver and pancreas. In: Tams TR, editor. Small animal endoscopy. ed 2. Mosby; St Louis: 1999. p. 44. [Google Scholar]
- van der Linde-Sipman J, Niewold T, Tooten P. Generalized AA-amyloidosis in Siamese and Oriental cats. Vet Immunol Immunopathol. 1997;56:1. doi: 10.1016/s0165-2427(96)05717-0. [DOI] [PubMed] [Google Scholar]
- 49.Wagner KA, Hartman FA, Trepanier LA. Bacterial culture results from liver, gallbladder, or bile in 248 dogs and cats evaluated for hepatobiliary disease: 1998-2003. J Vet Int Med. 2007;21:417. doi: 10.1892/0891-6640(2007)21[417:bcrflg]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 50.Wang KY, Panciera DL, Al Rukivati RK. Accuracy of ultrasound guided fine needle aspirate of the liver and cytologic finding in dogs and cats: 97 cases (1990-2000) J Am Vet Med Assoc. 2004;224:75. doi: 10.2460/javma.2004.224.75. [DOI] [PubMed] [Google Scholar]
- 51.Washabau RJ. Acute necrotizing pancreatitis. In: August JR, editor. Consultations in feline internal medicine. ed 5. Elsevier; St Louis: 2006. p. 109. [Google Scholar]
- 52.Webb C. Feline cholangitis syndrome. In: Cote EC, editor. Veterinary clinical advisor. ed 2. Elsevier; Philadelphia: 2010. p. 196. [Google Scholar]
- 53.Webb CB, McCord KW, Twedt DC. Oxidative stress and neutrophil function following oral supplementation of a silibinin-phophatidylcholine complex in cats. J Vet Int Med. 2008;22:808A. doi: 10.2460/ajvr.70.1.57. [DOI] [PubMed] [Google Scholar]
- 54.Webb CB, Trott C. Laparoscopic diagnosis of pancreatic disease in dogs and cats. J Vet Intern Med. 2008;22:1263. doi: 10.1111/j.1939-1676.2008.0176.x. [DOI] [PubMed] [Google Scholar]
- 55.Webb CB, Twedt DC, Fettman MJ. S-adenosylmethionine in a feline acetominophen model of oxidative injury. J Feline Med Surg. 2003;38:246. doi: 10.1016/S1098-612X(02)00017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Webster CRL, Cooper J. Therapeutic use of cytoprotective agents in canine and feline hepatobiliary disease. Vet Clin North Am Small Anim Pract. 2009;39:631. doi: 10.1016/j.cvsm.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 57.Weiss DJ, Moritz A. Liver cytology. Vet Clin North Am Small Anim Pract. 2002;32:1267. doi: 10.1016/s0195-5616(02)00047-5. [DOI] [PubMed] [Google Scholar]
- 58.Weiss DJ, Armstrong PJ, Gagne MJ. Inflammatory liver disease. Sem Vet Med Surg. 1997;12:22. doi: 10.1016/s1096-2867(97)80040-0. [DOI] [PubMed] [Google Scholar]
- 59.Weiss DJ, Gagne JM, Armstrong PJ. Relationship between feline inflammatory liver disease and inflammatory bowel disease, pancreatitis, and nephritis. J Am Vet Med Assoc. 1996;209:1114. [PubMed] [Google Scholar]
- 60.Willard MD. Fine needle aspiration cytology suggests hepatic lipidosis in 4 cats with infiltrative hepatic disease. J Feline Med Surg. 1999;1:215. doi: 10.1053/jfms.1999.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.WSAVA Standards for clinical and histological diagnosis of canine and feline liver diseases. Churchill Livingston; Edinburgh: 2006. WSAVA Liver Standardization Group. [Google Scholar]
- 62.Zoran DL. Nutrition for anorectic, critically ill or injured cats. In: August JR, editor. Consultations in feline internal medicine. ed 5. Elsevier; Philadelphia: 2006. p. 145. [Google Scholar]
Approach to the Cat with Ascites and Diseases Affecting the Peritoneal Cavity
The peritoneum is the serous membrane lining the abdominal cavity, as well as covering the organs of the abdomen. It comprises a single layer of squamous mesothelial cells resting on a deeper layer of loose connective tissue. The layer of peritoneum that lines the inner surface of the abdomen is called parietal peritoneum; the abdominal organs are lined by visceral peritoneum. The total surface area of the peritoneum is one to one-and-a-half times that of the total cutaneous area of the body.5, 30
The peritoneal cavity contains a small amount of fluid (less than 1 mL/kg body weight) that reduces friction between the abdominal organs as they slide over each other. The fluid is a pure transudate and contains solutes in the same concentration as serum (Box 23-7 ). This fluid is absorbed from the abdominal cavity predominantly through lymphatic vessels lying beneath the mesothelial basement membrane on the surface of the diaphragm. Lymphatic drainage occurs predominantly to the sternal lymph nodes.5, 30 Ascites is the abnormal effusion and accumulation of fluid in the abdominal cavity.
BOX 23-7. Characteristics of Normal Peritoneal Fluid.
-
•
Clear, slightly yellow
-
•
Specific gravity <1.016
-
•
Protein: 20 g/L (mainly albumin)
-
•Total white cell count: 2000 to 2500/mL
-
•50% macrophages
-
•Some eosinophils, mast cells
-
•Few neutrophils
-
•
-
•
No fibrinogen (does not clot on standing)
-
•
Fibronectin (a bacterial opsonizing protein)
From Bray J: Diagnosis and management of peritonitis in small animals, In Practice 18:403, 1996.
Pathophysiology of Ascites
Fluid exchange across the capillary bed is determined by Starling forces, that is, the balance between hydrostatic pressure, which causes transudation of fluid out of blood vessels, and the colloid osmotic pressure, which acts to retain fluid within blood vessels. The amount of peritoneal fluid is therefore determined by the balance of these, as well as vascular permeability, with excess fluid drained by the lymphatic system. Accumulation of fluid within a body cavity results when the rate of filtration of fluid into a space is greater than the rate of fluid resorption from that space. Effusion accumulation is therefore correlated to increased capillary hydrostatic pressure, widening of the oncotic pressure gradient, increased endothelial permeability, increased interstitial hydrostatic pressure, or loss of effective lymphatic drainage or a combination of these factors.10, 23, 32
Peritonitis of any cause results in vascular dilation, increased capillary permeability, and the migration of inflammatory cells into the peritoneum in response to immunomodulatory mediators. The inflamed peritoneum becomes a freely diffusible membrane, allowing a massive outpouring of fluid and plasma proteins from the circulation.5, 30
Clinical Evaluation of Ascites
Ascites is not commonly seen in practice; one study recognized ascites in only three cats out of 1000 admissions to an American veterinary teaching hospital,34 but the prevalence may be greater in primary care practice. In that study, dilated cardiomyopathy (DCM) was the most common disease associated with peritoneal effusion; however, DCM was diagnosed in most of these cats before 1987, when taurine deficiency was found to be a primary cause of this form of cardiomyopathy in cats. Neoplasia was the most common cause after 1987.34 Feline infectious peritonitis (FIP) was by far the most common cause of feline ascites diagnosed over a 10-year period at the Feline Centre at the University of Bristol, comprising 50% of all cats with recognized ascites.32
Presentation and Clinical Signs
Cats with ascites usually present with nonspecific clinical signs, such as anorexia or lethargy. The owners may present the cat because they recognize abdominal enlargement (Figure 23-67 ), but in many cases, owners perceive this as weight gain. Clinicians should be aware that sudden weight gain in a chronically underweight cat may be because of fluid accumulation (which can be intrathoracic fluid if ascites is not present), particularly if muscle mass seems reduced. Ascitic cats presenting subsequent to trauma may have intraabdominal hemorrhage or urinary tract rupture. Fever in a young ascitic cat will often suggest FIP, and cats with FIP may or may not be jaundiced. Presence of jugular distention or even a jugular pulse can suggest right-sided heart failure.
FIGURE 23-67.
Abdominal distention because of ascites in an elderly cat. This cat had reduced muscle mass, despite weight gain because of the abdominal fluid.
A palpable fluid thrill can help to distinguish ascites from other causes of abdominal enlargement, such as organomegaly, abdominal masses, bladder distention, abdominal wall weakness, obesity or, occasionally, accumulations of gas within the abdominal cavity27 (Table 23-26 ). Recognizing a fluid thrill involves gently tapping one side of the abdominal wall with the fingers of one hand while feeling for a sensation of fluid movement with the fingers of the other hand positioned on the opposite side of the abdomen.
TABLE 23-26.
Causes of Abdominal Distention
| Weak abdominal wall | Hyperadrenocorticism |
| Organomegaly |
Hepatomegaly/renomegaly/splenomegaly |
| Mesenteric lymphadenopathy | |
| Gastric distention/bladder distention/advanced obstipation | |
| Pregnancy/pyometra | |
| Neoplasia | |
| Obesity | |
| Gas accumulation (pneumoperitoneum) |
Traumatic penetration of abdominal wall |
| Rupture of the stomach or bowel | |
| Gas-forming bacterial infection | |
| Extension from pneumomediastinum or pneumothorax | |
| Fluid Accumulation (Ascites) | |
| Transudates | |
| Pure transudate* |
Hypoproteinemia: glomerular disease, intestinal malabsorption or protein loss, severe chronic liver disease (Neoplasia) |
| (Obstruction of lymphatic drainage/lymphangiectasia) | |
| Modified transudate |
Congestive heart failure |
| Hepatic disease: cirrhosis, neoplasia | |
| Neoplasia: obstruction of blood vessels and/or lymphatics | |
| Portal hypertension/obstruction of posterior vena cava | |
| Exudates | |
| Nonseptic exudates |
Feline infectious peritonitis |
| Hepatitis (particularly lymphocytic cholangitis) | |
| Bile or urine peritonitis | |
| Pancreatic peritonitis | |
| Diaphragmatic or pericardial hernia | |
| Steatitis | |
| Neoplasia | |
| Septic exudates |
Extension of infection from elsewhere |
| Intestinal perforation/bowel rupture | |
| Ruptured pyometra | |
| Penetrating wound | |
| Migrating foreign body | |
| Hematogenous spread | |
| Caused by a Ruptured Vessel | |
| Hemorrhagic effusion |
Organ or major blood vessel rupture; associated with trauma or secondary to ruptured neoplasm |
| Perforation of stomach or intestine | |
| Bleeding disorders | |
| Splenic or gastric torsion | |
| Thrombosis | |
| Chyle | Ruptured lymphatic drainage |
| Obstruction of lymphatic drainage/lymphangiectasia | |
| Neoplasia | |
| Congestive heart failure | |
| Steatitis | |
| Caused by a Ruptured Viscus | |
| Urine | Ruptured urinary tract. Since urine is irritant it usually results in a secondary nonseptic exudate. |
| Bile | Ruptured biliary tract. Since bile is irritant it usually results in a secondary nonseptic exudate. |
When present for any length of time, a pure transudate will become modified. This is particularly true of transudates that develop slowly, such as those associated with congestive heart failure or portal hypertension. Modified transudates are therefore more common than pure transudates.
Adapted from Tasker S, Gunn-Moore D: Differential diagnosis of ascites in cats, In Practice 22:472, 2000.
Diagnostic Approaches
Hematology, Biochemistry, and Urinalysis
Routine laboratory findings are usually nonspecific but may provide clues to the underlying cause of ascites. For example, neutrophilia may point towards septic peritonitis but can also occur with FIP; most cats with hemoperitoneum are anemic at presentation8; uroperitoneum often results in azotemia and electrolyte abnormalities; hypoglycemia may reflect sepsis with septic peritonitis, and a recent study recognized 83% of cases of septic peritonitis had ionized hypocalcemia17; elevated liver enzymes may be associated with inflammatory, infectious or neoplastic hepatopathies including FIP; elevation of serum globulins occurs in many cats with FIP but can also be associated with neoplasia or septic peritonitis; and a finding of hypoalbuminemia (which can cause a pure transudate) should prompt for an assessment of urine protein : creatinine ratio to assess if there is renal protein loss.
Imaging
Imaging may be required to confirm the presence of fluid as well as to aid in diagnosis of the underlying cause. Radiographic findings can vary greatly depending on the amount of abdominal fluid present and the underlying etiology. Loss of normal detail or presence of a “ground glass” appearance to the abdominal cavity is suggestive of the presence of fluid (FIGURE 23-68, FIGURE 23-69 ). Very young, thin or dehydrated cats may also have a loss of detail that can mimic the presence of fluid. Ultrasonography of the abdomen (FIGURE 23-70, FIGURE 23-71 ) can allow the detection of even very small volumes of fluid. It also enables evaluation of the size and structure of intraabdominal organs, such as the liver and spleen, which can help determine the underlying cause of ascites.
FIGURE 23-68.
Radiographic appearance (right lateral view) of the same cat as in Figure 23-67. Note that, additional to the abdominal distention, the loss of serosal detail creates difficulty to discern abdominal organs.
FIGURE 23-69.
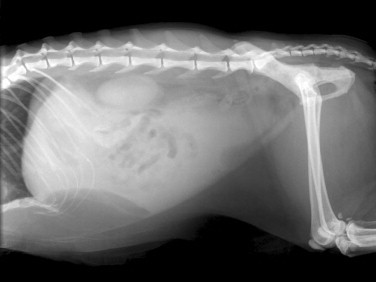
Radiographic appearance (right lateral view) of the same cat as in Figure 23-67 after drainage of a substantial amount of fluid. The abdominal distention is reduced but the remaining fluid still somewhat obscures the midabdominal organs.
FIGURE 23-70.
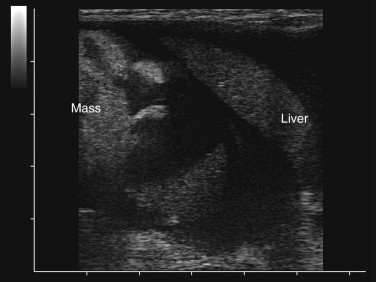
Ultrasonographic appearance of ascites. Note that organs are highlighted by the dark background of the hypoechoic fluid. This is particularly emphasized with the low cellularity of this particular effusion.
FIGURE 23-71.
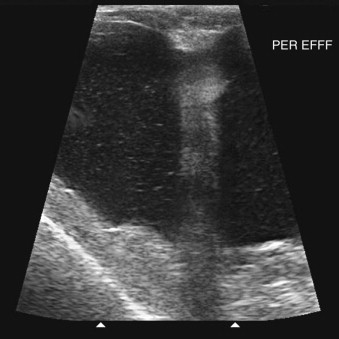
Ultrasonographic appearance of ascites, note the echogenic debris through the fluid that reflects the cellular nature of this chylous effusion.
(Courtesy Small Animal Specialist Hospital, North Ryde, Sydney, Australia.)
Abdominocentesis
Abdominocentesis confirms the presence of abdominal fluid (in cases of low-volume effusion) and assessment of the fluid is required to diagnose the underlying cause of ascites. Most cats tolerate abdominocentesis without sedation and the cat can be held in a standing position or in lateral recumbency (whichever is more comfortable for the cat and familiar to the clinician). The abdomen is clipped and aseptically prepared. A 20- to 22-gauge butterfly needle may be used with a 5- to 10-mL syringe. In cases with low-volume effusion, ultrasonography can help to guide fine needle aspiration from small pockets of abdominal fluid. Diagnostic peritoneal lavage can be used if ultrasound-guided aspiration is unsuccessful. For this procedure, 10 to 20 mL/kg of warmed, sterile fluid is infused into the abdomen over 2 to 5 minutes after aseptic preparation of the site. The cat is gently rolled from side to side or allowed to stand; gentle massage of the abdomen also helps distribute the fluid. The fluid is allowed to dwell for a minimum of 2 to 5 minutes before aseptic preparation is repeated before paracentesis. No attempt is made to remove all the fluid. It must be remembered that, since the recovered fluid has been diluted by this procedure, cell counts and biochemical analyses will be affected.33
If a large volume effusion causes discomfort because of abdominal distention, a three-way stopcock may be used so large volumes can be drained from one puncture (Figure 23-72 ). However, removal of large volumes of ascitic fluid can be detrimental, because it may prevent the subsequent reabsorption of valuable protein and/or red blood cells; the resulting reduction in intraabdominal pressure may encourage further accumulation of fluid; and rapid removal of large volumes can lead to fluid shifts causing cardiovascular collapse.32 Fluid can be collected into ethylenediaminetetraacetic acid (EDTA) tubes (for total nucleated cell count, packed cell volume, total protein, and cytology), serum tubes (for biochemistry, such as albumin, bilirubin, creatinine, potassium, triglyceride, glucose, lactate, and lipase), sterile tubes for culture, and/or other tubes for effusion-specific tests such as PCR. Samples should be prioritized according to the volume of fluid available and to the suspected underlying disease process.10
FIGURE 23-72.
Drainage of large-volume abdominal effusion using a butterfly catheter, three-way stopcock, and 10-mL syringe.
Fluid Analysis and Classification
Initial assessment of fluid retrieved is made on the basis of color and protein concentration, and much information can be gleaned from this simple assessment, even before cell numbers and types are assessed. Although this brief, initial assessment is useful to refine the differential diagnoses, a thorough assessment based on underlying etiology and pathophysiology is required for definitive diagnosis and therefore appropriate management (Table 23-27 ). Ascitic fluid, classified according to its pathophysiologic cause, can be divided into transudates, modified transudates, exudates (septic or nonseptic), or effusions (chylous or hemorrhagic).10, 23
TABLE 23-27.
Characteristics of Peritoneal Effusion Fluid
| Pure Transudate | Modified Transudate | Exudate | Chyle | Hemorrhage | |
|---|---|---|---|---|---|
| Appearance of fluid | Usually clear and colorless or occasionally amber-colored | Yellow or blood tinged, can be turbid | Turbid fluid | Milky or pinkish opaque | Red (blood) |
| Total protein (g/L) | <25 | >25 | >25, usually >30 | 25-60 | 35-75 |
| Specific gravity | <1.015 | 1.015-1.025 | >1.025 | Not applicable | Not applicable |
| Nucleated cells (×109/L) | <1 | 1-7 | >5 | 0.25-20 | 1-20, depending on peripheral count |
| Predominant cell types | Macrophages Mesothelial cells |
Macrophages Mesothelial cells Lymphocytes Erythrocytes Neutrophils (nondegenerate) +/−Neoplastic cells |
Neutrophils (non-degenerate, or degenerate, if bacterial) Macrophages Erythrocytes +/− Neoplastic cells |
Small lymphocytes Neutrophils Macrophages |
Erythrocytes Neutrophils Macrophages Mesothelial cells Neoplastic cells |
Adapted from Tasker S, Gunn-Moore D: Differential diagnosis of ascites in cats, In Practice 22:472, 2000.
Transudates
Transudates are a consequence of altered fluid dynamics. Protein-poor transudates (commonly referred to as pure transudates) form predominantly as a result of severe hypoalbuminemia, which causes a lowered colloid osmotic pressure. Since there is no change in endothelial or mesothelial permeability, as fluid accumulates, there is no concurrent cell leakage; so, there is a decrease in the cell count through a dilutional effect. Consequently, transudative effusions are typically clear and colorless.10, 23, 32 Other pathologic causes of protein-poor transudates include cirrhosis, lymphatic obstruction, and noncirrhotic portal hypertension (presinusoidal and sinusoidal). Since hypoalbuminemia is the most common cause of transudates, serum albumin concentrations must be measured to guide further diagnostics. If the serum albumin concentration is normal (or only minimally decreased), then radiographs, abdominal ultrasonography, and/or echocardiography are indicated to assess cardiac function and for urinary bladder rupture.10 One review of feline ascitic cases found 24% of effusions were protein-poor transudates, of which 82% were the result of hepatic failure or primary renal disease.34
Modified Transudates
Modified transudates can result from increased hydrostatic pressure within the postsinusoidal vessels of the liver secondary to right-sided congestive heart failure (e.g., tricuspid insufficiency) or potentially from mass lesions (such as neoplastic masses) obstructing blood flow from the hepatic vein or caudal vena cava into the right side of the heart. The increase in hydrostatic pressure within the vessels of the liver causes a protein-rich fluid to leach out of the liver into the abdominal cavity. Since cell membrane permeability does not change, cells do not accumulate in the effusion.10 Modified transudates can also result from increased vascular permeability in the early stages of an inflammatory process, in which case cellularity will be increased. Modified transudates were described as the most common type of ascitic effusion identified in cats in one study, with most being resulting from neoplasia and congestive cardiac failure; however, this study partially included cases prior to 1987, when right-sided heart failure associated with dilated cardiomyopathy (DCM) was prevalent.34 The recognition of the role of taurine deficiency in this condition and the subsequent addition of this amino acid to feline diets now means that right-sided heart failure is only rarely encountered as a cause of ascites in cats.
Exudates
Exudates are a consequence of altered mesothelial and/or endothelial permeability. This permeability results from a cytokine-mediated inflammatory response of any underlying cause (e.g., infectious, neoplastic, immune mediated). Exudates have high protein and moderate to high cell concentrations and are classified as nonseptic or septic.
Exudates are often primarily composed of neutrophils. Nondegenerate neutrophils (and the absence of organisms) points to a nonseptic exudate (mostly FIP but also neoplasia). FIP is the most common cause of exudative effusion in cats and was the most common cause of feline ascites diagnosed over a 10-year period at the Feline Centre at the University of Bristol.32 The presence of neoplastic cells rules in neoplasia, but the absence of such cells does not rule out this diagnosis since many cases of neoplastic ascites are not associated with exfoliated neoplastic cells. Other causes of nonseptic exudates include pancreatitis, lymphocytic cholangitis, and viscus rupture, such as the gall bladder or urinary bladder.
Degenerate neutrophils typify septic exudates (i.e., septic peritonitis), and their presence should instigate investigation for causes of infection (mostly leakage of gastrointestinal contents).34
Chylous Effusions
Chylous effusions appear as milky or pink opaque fluid, and small mature lymphocytes initially predominate in cell counts. After drainage, more macrophages and nondegenerate neutrophils may be found. Chyle is typically classified as an exudate, but its physical characteristics can be consistent with a modified transudate (protein content between 25 and 40 g/L); biochemical analysis of triglyceride and cholesterol levels in the fluid are required to confirm the diagnosis. Pseudochylous effusions resemble true chyle both in appearance and cytology but do not contain fat. Similar conditions result in both chylous and pseudochylous effusions. Chylous abdominal effusions are rarely reported in the cat and only accounted for 7% of cases of ascites in one study.34 The described causes of chylous ascites in cats are predominantly neoplastic. In a series of nine cats, chylous ascites was associated with nonresectable abdominal neoplasia in four cases (i.e., hemangiosarcoma and paraganglioma), with intestinal and mesenteric lymphoma in two cases and lymphangiosarcoma of the abdominal wall in another.13 One described case in a 10-year-old cat was thought to be because of FIP.28 Figure 23-71 shows an ultrasonographic image of a cat with chylous abdominal effusion associated with pancreatitis. Other potential causes include right-sided congestive cardiac failure, steatitis (inflammation of fat), biliary cirrhosis, and lymphangiectasia.
Hemorrhagic Effusions
Hemoperitoneum in companion animals is categorized as traumatic or spontaneous. Traumatic hemoperitoneum is further divided into blunt causes of trauma (i.e., motor vehicle accidents and high-rise falls) and penetrating trauma (i.e., gunshot wounds and bite wounds).8, 21
Inadvertent splenic aspiration, venipuncture, or acute severe hemorrhage should be suspected if the cytology is consistent with peripheral blood including platelets but without erythrophagocytosis or if the blood clots readily.
When there is no history of trauma, coagulopathy or spontaneous rupture of a vascular neoplasm should be considered. In one study of 16 feline cases of spontaneous hemoperitoneum, 12 cases (75%) were associated with hepatic pathology such as neoplasia, necrosis, and amyloidosis.21 In another study of 65 cases of spontaneous hemoperitoneum, 46% (30 of 65) of cats had abdominal neoplasia, and 54% (35 of 65) had non-neoplastic conditions. Cats with neoplasia were significantly older and had significantly lower packed cell volumes (PCVs) than cats with non-neoplastic disease. Hemangiosarcoma was the most often diagnosed neoplasm (18 of 30, 60%), and the spleen was the most common location for neoplasia (11 of 30, 37%). Coagulopathies (8 of 35, 23%) and hepatic necrosis (8 of 35, 23%) were the most common causes of non-neoplastic hemoperitoneum.8 Other non-neoplastic causes of hemoperitoneum include ruptured bladder, hepatic rupture secondary to hepatic amyloidosis, gastric/duodenal ulcer, hepatic hematoma, hepatitis, perinephric pseudocyst, feline infectious peritonitis–induced liver rupture, and feline infectious peritonitis–induced nephritis.8, 21
The prognosis of cats with spontaneous hemoperitoneum is poor. In two studies, only approximately 12% of cases survived to be discharged from hospital.8, 21 Median survival time for cats that were discharged in one of those studies was 54 days (range, 5 to 1825 days).8
Specific Causes of Ascites
Feline Infectious Peritonitis
Feline infectious peritonitis (FIP) comprised 50% of cats with recognized ascites over a 10-year period at the Feline Centre at the University of Bristol,32 and, as a rule of thumb, when ascites is recognized in a younger cat, FIP should be considered the major rule-out. The abdominal effusion found with FIP is typically straw to golden yellow (although the color can be very variable, for example, chyle may be present), may contain fibrin clots, and has a high protein concentration. The total protein content is greater than 35 g/L and often greater than 45 g/L, with globulins comprising 50% or more.31 One study described an effusion with total protein greater than 80 g/L as 90% specific, 55% sensitive, and having a 0.78 positive predictive value to diagnose FIP.31 The Rivalta test evaluates the fluid's globulin content, and was found to be very sensitive but only 80% specific; this test is performed by adding one drop of acetic acid (98%) to 5 mL of distilled water. This fluid is mixed thoroughly, and then one drop of effusion is gently placed on the surface of the mixture. If the drop stays at the top of the fluid or slowly floats to the bottom, the test is considered to be positive. This test can give inaccurate results if inappropriate technique is used or if there is a significant temperature difference between the fluid sample and the acetic acid solution. A positive Rivalta test can result from lymphosarcoma, septic, or FIP effusions; these can be distinguished by cytology and culture. Immunofluorescence staining of coronavirus antigen in macrophages had a positive predictive value of 1.00 but a negative predictive value of 0.57.15 The potential clinical presentations, diagnosis, and management of FIP are covered in detail in Chapter 33.
Neoplasia
One study found neoplasia to be the most common cause of ascites in cats,34 and neoplasia should be considered the major rule-out in older cats with ascites. The effusion from cats with ascites resulting from neoplasia may be a modified transudate, resulting from compression of hepatic veins or the caudal vena cava, or metastases to the peritoneum; hemorrhage from neoplasia can cause hemoperitoneum; chylous effusions may result from reduced lymphatic drainage or rupture of lymphatic vessels; and raised vascular permeability caused by neoplastic infiltration can result in an exudative effusion. Carcinomas, mesotheliomas, and discrete (round) cell neoplasms (e.g., lymphoma, mast cell tumors, malignant histiocytosis) exfoliate cells into effusions more readily than sarcomas, and of these, lymphosarcoma is the most common malignancy of cats. Cytology of ascitic fluid reveals neoplastic cells in less than a quarter of cases; so the absence of such cells does not rule out a diagnosis of neoplasia. In these circumstances, the diagnosis may be achieved by ultrasound-guided fine-needle aspiration of affected organs, or even biopsy samples obtained at laparotomy. The specific approaches will depend on the specific neoplasia diagnosed.
Septic Peritonitis
Exudates caused by septic inflammation usually result from bacterial contamination of the peritoneal cavity secondary to gastrointestinal tract leakage or penetrating wounds associated with trauma. Gastrointestinal tract leakage may occur as a result of ulceration associated with neoplasia or inflammatory disease or as a result of penetration of a sharp object ingested (such as a toothpick), it can also occur subsequent to prior abdominal surgery.7, 9, 17, 24 Primary septic peritonitis in which no apparent cause can be identified has also been described in cats.26
Septic exudates are usually yellow to tan in color, with yellow particulate matter and are foul-smelling. Microscopically, the fluid is characterized by the presence of degenerate neutrophils and bacteria. Bacteria are often seen intracellularly within neutrophils. The condition is associated with high morbidity and mortality rates, with survival rates reported between 32% and 80%.7, 9, 17, 24, 26 The history and clinical signs are often vague and nonspecific but can include abdominal pain, vomiting, lethargy/depression, and anorexia. Abdominal pain is an inconsistent finding, being recognized in only 62% of cats in one study7 and 43% in another.24 Some cats may have an inappropriately low heart rate.7, 26 Hematologic and serum biochemistry findings are also inconsistent; neutrophilia with a left shift may be present, as may neutropenia or a normal neutrophil count. Similarly, cats may be hypoglycemic, hyperglycemic, or normoglycemic, and they may be hypoalbuminemic.7, 24, 26 One study recognized ionized hypocalcemia in 89% of cats with septic peritonitis at the time of diagnosis,17 and another suggested hyperlactatemia, when present, may be associated with a poorer prognosis.24 Radiographic findings are usually typical of ascites of any cause, but presence of pneumoperitoneum in a cat that has not undergone recent surgery may suggest the presence of gas-forming bacteria or rupture of an abdominal viscus and warrants immediate surgical intervention. Ultrasonography does not directly aid the diagnosis of septic peritonitis.7 Exploratory laparotomy to determine and correct an underlying problem, such as full-thickness gastrointestinal perforation (often requiring partial resection) is required, as is copious abdominal lavage with sterile, warmed fluids (Figure 23-73 ). There are no statistically significant survival differences between postsurgical primary closure, open peritoneal drainage, or closed suction drainage postsurgical lavage; however, a trend toward a higher survival rate has been seen in cats treated with primary closure.24, 26 Treatment also involves antibiotics, initially parenterally, based on culture and sensitivity findings. Consistent with intestinal contents, most bacteria recognized are gram-negative aerobes, such as E. coli or Enterobacter spp., but mixed infections are usually found.7, 24 Anaerobes seem more common in cats with primary septic peritonitis,26 which perhaps suggests these cases may result from healed over-bite wounds into the abdomen. Amoxicillin/clavulanate would be an appropriate empirical choice of antibiotics while awaiting sensitivity results. There are no definitive guidelines for duration of antibiotic treatment; the author uses extended treatment courses of 4 to 6 weeks. Supportive care with intravenous fluids to maintain fluid and electrolyte balances is also required perioperatively.
FIGURE 23-73.
Fulminant peritonitis associated with gastrointestinal perforation. In this case, the effusion volume was low but the high degree of serosal inflammation is evident.
Bile Peritonitis
Bile peritonitis is infrequently reported in cats but has been recognized in association with gunshot20 or motor vehicle2 trauma, with biliary obstruction from gall stones2, 22 and subsequent to percutaneous ultrasound-guided cholecystocentesis in a cat with infectious cholangitis.4 Concurrent bacterial infection was recognized in each case; this increases severity of inflammation and worsens the prognosis, although full recovery was achieved in most reported cases.2, 4, 20 Bile peritonitis has the potential to result in small-volume effusions; so, if abdominocentesis does not yield a sample of effusion but bile peritonitis is high on the differential list, then diagnostic peritoneal lavage is appropriate. Since repair of or removal of the gall bladder and abdominal lavage are required, exploratory laparotomy is an appropriate means to diagnose this condition. Management should be considered as for septic peritonitis of other causes.
Uroabdomen
Trauma, including blunt abdominal trauma, urethral catheterization, and bladder expression, is the most common cause of uroperitoneum in cats.1 It is also recognized as a complication of ureteral surgery.18 The bladder is the most frequent site of urine leakage after blunt abdominal trauma, whereas the urethra is most commonly injured following catheterization. Cats with ruptured bladders may still have a palpable bladder and the ability to urinate. Common historical complaints are anuria (53.8%) and vomiting (50%). Azotemia is a common finding, and hyperkalemia is seen in around 50% of cases. Drainage of urine from the peritoneal cavity seems to improve patient stabilization. Morbidity and mortality depended largely on the severity of associated injuries.1 Regardless of the site of injury or the cause of uroabdomen, the first goal of treatment is patient stabilization. Isotonic replacement fluids are used for initial resuscitation. Treatment of hypovolemic shock, if present, is the first order of fluid therapy. After fluid resuscitation, drainage of urine from the abdomen should be established. Continuous passive drainage of the urine is necessary for stabilization and allows effective diuresis to occur. Indwelling catheterization of the urinary bladder is recommended to keep the bladder decompressed and reduce urine flow into the abdominal cavity in patients with bladder and proximal urethral injury. If the urethra is traumatized and a catheter cannot be placed, prepubic tube cystostomy may be necessary to achieve temporary urinary diversion. The decision to treat the uroabdomen patient surgically or conservatively should be based on the location and severity of the underlying injury, the condition of the patient at presentation, and the patient's response to initial stabilization.1, 12
Right-Sided Congestive Heart Failure
Congestive heart failure has become an uncommon cause of ascites in cats since the late 1980s/early 1990s, from which time dilated cardiomyopathy has been largely eradicated.32, 34 Ascites does still result from right-sided congestive heart failure in conditions such as tricuspid insufficiency,6 arrhythmogenic right ventricular cardiomyopathy,16 myocardial fibrofatty infiltration,14 or restrictive cardiomyopathy.29, 34 Concurrent pleural effusion or pulmonary edema is often, but not necessarily, present with cardiac induced ascites. A heart murmur is not necessarily noted. Noting a jugular pulse or thrill is helpful diagnostically, if present. The ascitic fluid is typically a modified transudate, but a chylous effusion is also possible. Cardiac diseases are covered in Chapter 20.
Hepatopathies
In some cases, hepatic lipidosis has been reported to cause ascites, particularly in association with pancreatitis. These cats are often hypoalbuminemic, with the possibility of intravenous fluid therapy contributing to the ascites by raising hydrostatic pressure.11 Other liver diseases which can result in ascites include lymphocytic cholangitis,19, 25 neutrophilic cholangitis, cirrhosis,13 necrosis, neoplasia, and suppurative cholangiohepatitis.34 Portosystemic shunts in cats rarely result in ascites, compared with dogs.3 Hypoalbuminemia and hepatic failure result in transudates; portal hypertension and cirrhosis cause higher protein ascites because of raised capillary hydrostatic pressure causing leakage of high protein lymph. Hepatopathies are covered in detail elsewhere in this chapter.
References
- 1.Aumann M, Worth L, Drobatz K. Uroperitoneum in cats: 26 cases (1986-1995) J Am Anim Hosp Assoc. 1998;34:315. doi: 10.5326/15473317-34-4-315. [DOI] [PubMed] [Google Scholar]
- 2.Bacon NJ, White RAS. Extrahepatic biliary tract surgery in the cat: a case series and review. J Small Anim Pract. 2003;44:231. doi: 10.1111/j.1748-5827.2003.tb00149.x. [DOI] [PubMed] [Google Scholar]
- 3.Blaxter AC, Holt PE, Pearson GR. Congenital portosystemic shunts in the cat: a report of nine cases. J Small Anim Pract. 1988;29:631. [Google Scholar]
- 4.Brain PH, Barrs VR, Martin P. Feline cholecystitis and acute neutrophilic cholangitis: clinical findings, bacterial isolates and response to treatment in six cases. J Feline Med Surg. 2006;8:91. doi: 10.1016/j.jfms.2005.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bray J. Diagnosis and management of peritonitis in small animals. In Practice. 1996;18:403. [Google Scholar]
- 6.Closa J, Font A. Traumatic tricuspid insufficiency in a kitten. J Am Anim Hosp Assoc. 1999;35:21. doi: 10.5326/15473317-35-1-21. [DOI] [PubMed] [Google Scholar]
- 7.Costello MF, Drobatz KJ, Aronson LR. Underlying cause, pathophysiologic abnormalities, and response to treatment in cats with septic peritonitis: 51 cases (1990-2001) J Am Vet Med Assoc. 2004;225:897. doi: 10.2460/javma.2004.225.897. [DOI] [PubMed] [Google Scholar]
- 8.Culp WTN, Weisse C, Kellogg ME. Spontaneous hemoperitoneum in cats: 65 cases (1994-2006) J Am Vet Med Assoc. 2010;236:978. doi: 10.2460/javma.236.9.978. [DOI] [PubMed] [Google Scholar]
- 9.Culp WTN, Zeldis TE, Reese MS. Primary bacterial peritonitis in dogs and cats: 24 cases (1990-2006) J Am Vet Med Assoc. 2009;234:906. doi: 10.2460/javma.234.7.906. [DOI] [PubMed] [Google Scholar]
- 10.Dempsey SM, Ewing PJ. A review of the pathophysiology, classification, and analysis of canine and feline cavitary effusions. J Am Anim Hosp Assoc. 2011;47:1. doi: 10.5326/JAAHA-MS-5558. [DOI] [PubMed] [Google Scholar]
- 11.Dimski DS. Feline hepatic lipidosis. Clin Tech Small Anim Pract. 1997;12:28. doi: 10.1016/s1096-2867(97)80041-2. [DOI] [PubMed] [Google Scholar]
- 12.Gannon KM, Moses L. Uroabdomen in dogs and cats. Compend Contin Educ Vet. 2002;24:604. [Google Scholar]
- 13.Gores BR, Berg J, Carpenter JL. Chylous ascites in cats: nine cases (1978-1993) J Am Vet Med Assoc. 1994;205:1161. [PubMed] [Google Scholar]
- 14.Harjuhahto TAI, Leinonen MR, Simola OTM. Congestive heart failure and atrial fibrillation in a cat with myocardial fibro-fatty infiltration. J Feline Med Surg. 2011;13:109. doi: 10.1016/j.jfms.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartmann K, Binder C, Hirschberger J. Comparison of different tests to diagnose feline infectious peritonitis. J Vet Int Med. 2003;17:781. doi: 10.1111/j.1939-1676.2003.tb02515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harvey AM, Battersby IA, Faena M. Arrhythmogenic right ventricular cardiomyopathy in two cats. J Small Anim Pract. 2005;46:151. doi: 10.1111/j.1748-5827.2005.tb00306.x. [DOI] [PubMed] [Google Scholar]
- 17.Kellett-Gregory LM, Mittleman Boller E, Brown DC. Retrospective study: ionized calcium concentrations in cats with septic peritonitis: 55 cases (1990-2008) J Vet Emerg Crit Care. 2010;20:398. doi: 10.1111/j.1476-4431.2010.00562.x. [DOI] [PubMed] [Google Scholar]
- 18.Kyles AE, Hardie EM, Wooden BG. Management and outcome of cats with ureteral calculi: 153 cases (1984-2002) J Am Vet Med Assoc. 2005;226:937. doi: 10.2460/javma.2005.226.937. [DOI] [PubMed] [Google Scholar]
- 19.Lucke VM, Davies JD. Progressive lymphocytic cholangitis in the cat. J Small Anim Pract. 1984;25:249. [Google Scholar]
- 20.Ludwig LL, McLoughlin MA, Graves TK. Surgical treatment of bile peritonitis in 24 dogs and 2 cats: a retrospective study (1987-1994) Vet Surg. 1997;26:90. doi: 10.1111/j.1532-950x.1997.tb01470.x. [DOI] [PubMed] [Google Scholar]
- 21.Mandell DC, Drobatz K. Feline hemoperitoneum 16 cases (1986-1993) J Vet Emerg Crit Care. 1995;5:93. [Google Scholar]
- 22.Moores AL, Gregory SP. Duplex gall bladder associated with choledocholithiasis, cholecystitis, gall bladder rupture and septic peritonitis in a cat. J Small Anim Pract. 2007;48:404. doi: 10.1111/j.1748-5827.2006.00268.x. [DOI] [PubMed] [Google Scholar]
- 23.O’Brien PJ, Lumsden JH. The cytologic examination of body cavity fluids. Semin Vet Med Surg (Small Anim) 1988;3:140. [PubMed] [Google Scholar]
- 24.Parsons KJ, Owen LJ, Lee K. A retrospective study of surgically treated cases of septic peritonitis in the cat (2000-2007) J Small Anim Pract. 2009;50:518. doi: 10.1111/j.1748-5827.2009.00790.x. [DOI] [PubMed] [Google Scholar]
- 25.Prasse KW, Mahaffey EA, DeNovo R. Chronic lymphocytic cholangitis in three cats. Vet Path. 1982;19:99. doi: 10.1177/030098588201900201. [DOI] [PubMed] [Google Scholar]
- 26.Ruthrauff CM, Smith J, Glerum L. Primary bacterial septic peritonitis in cats: 13 cases. J Am Anim Hosp Assoc. 2009;45:268. doi: 10.5326/0450268. [DOI] [PubMed] [Google Scholar]
- 27.Saunders WB, Tobias KM. Pneumoperitoneum in dogs and cats: 39 cases (1983-2002) J Am Vet Med Assoc. 2003;223:462. doi: 10.2460/javma.2003.223.462. [DOI] [PubMed] [Google Scholar]
- 28.Savary KC, Sellon RK, Law JM. Chylous abdominal effusion in a cat with feline infectious peritonitis. J Am Anim Hosp Assoc. 2001;37:35. doi: 10.5326/15473317-37-1-35. [DOI] [PubMed] [Google Scholar]
- 29.Saxon B, Hendrick M, Waddle JR. Restrictive cardiomyopathy in a cat with hypereosinophilic syndrome. Can Vet J. 1991;32:367. [PMC free article] [PubMed] [Google Scholar]
- 30.Seim HB. Management of peritonitis. In: Bonagura JD, Kirk RW, editors. Kirk's current veterinary therapy XII: small animal practice. Saunders; Philadelphia: 1995. p. 764. [Google Scholar]
- 31.Sparkes A, Gruffydd-Jones T, Harbour D. Feline infectious peritonitis: a review of clinicopathological changes in 65 cases, and a critical assessment of their diagnostic value. Vet Rec. 1991;129:209. doi: 10.1136/vr.129.10.209. [DOI] [PubMed] [Google Scholar]
- 32.Tasker S, Gunn-Moore D. Differential diagnosis of ascites in cats. In Practice. 2000;22:472. [Google Scholar]
- 33.Walters JM. Abdominal paracentesis and diagnostic peritoneal lavage. Clin Tech Small Anim Pract. 2003;18:32. doi: 10.1016/1096-2867(03)90023-5. [DOI] [PubMed] [Google Scholar]
- 34.Wright KN, Gompf RE, DeNovo RC., Jr Peritoneal effusion in cats: 65 cases (1981-1997) J Am Vet Med Assoc. 1999;214:375. [PubMed] [Google Scholar]


