Key Readings Index
Structure and Function, 724
Dysfunction/Responses to Injury, 730
Portals of Entry/Pathways of Spread, 744
Defense Mechanisms/Barrier Systems, 744
Disorders of Domestic Animals, 744
Disorders of Horses, 758
Disorders of Ruminants (Cattle, Sheep, and Goats), 758
Disorders of Dogs, 759
Disorders of Cats, 759
Structure and Function, 761
Dysfunction/Responses to Injury, 763
Portals of Entry/Pathways of Spread, 764
Defense Mechanisms/Barrier Systems, 764
Structure, 764
Function, 766
Dysfunction/Responses to Injury, 771
Portals of Entry/Pathways of Spread, 772
Defense Mechanisms/Barrier Systems, 772
Structure, 772
Function, 775
Dysfunction/Responses to Injury, 775
Portals of Entry/Pathways of Spread, 777
Defense Mechanisms/Barrier Systems, 777
Structure and Function, 777
Structure and Function, 777
Dysfunction/Responses to Injury, 778
Portals of Entry/Pathways of Spread, 778
Defense Mechanisms/Barrier Systems, 778
Disorders of the Lymphoid/Lymphatic System of Domestic Animals
Thymus, 778
Spleen, 780
Lymph Nodes, 789
Mucosa-Associated Lymphoid Tissue, 796
Disorders of the Lymphoid/Lymphatic System by Species
Disorders of Horses, 796
Disorders of Ruminants (Cattle, Sheep, and Goats), 797
Disorders of Pigs, 798
Disorders of Dogs, 799
Disorders of Cats, 803
E-Glossary 13-1 Glossary of Abbreviations and Terms
AA amyloidosis—Serum amyloid A amyloidosis
ACT—Activated clotting time
ADP—Adenosine diphosphate
AL amyloidosis—Amyloid–light chain amyloidosis
ALL—Acute lymphoblastic leukemia
AML—Acute myeloid leukemia
AP3—Adaptor protein complex
ATALT—Auditory tube–associated lymphoid tissue
ATP—Adenosine triphosphate
BALT—Bronchus-associated lymphoid tissue
BCR-ABL—Breakpoint cluster region-Abelson
BLAD—Bovine leukocyte adhesion deficiency
BLL—Burkitt-like lymphoma
BLP—B lymphocyte progenitor
BLV—Bovine leukemia virus
BNP—Bovine neonatal pancytopenia
BVD—Bovine viral diarrhea
BVDV—Bovine viral diarrhea virus
CalDAG-GEFI—Calcium diacylglycerol guanine nucleotide exchange factor I
CALT—Conjunctiva-associated lymphoid tissue
CBC—Complete blood count
C-bilirubin—Conjugated bilirubin
CD—Cluster of differentiation
CH—Cutaneous histiocytosis
CHS—Chédiak-Higashi syndrome
CLAD—Canine leukocyte adhesion deficiency
CLL—Chronic lymphocytic leukemia
CLP—Common lymphoid progenitor
CML—Chronic myeloid leukemia
CMP—Common myeloid progenitor
CPV-2—Canine parvovirus type 2
CTCL—Cutaneous T lymphocyte lymphoma
DC—Dendritic cell
DIC—Disseminated intravascular coagulation
DLBCL—Diffuse large B cell lymphoma
DNA—Deoxyribonucleic acid
DNA-PKcs—DNA-dependent protein kinase catalytic subunit
2,3-DPG—2,3-diphosphoglycerate
EATCL—Enteropathy-associated T cell lymphoma
EBL—Enzootic bovine leukosis
EBV—Epstein-Barr virus
EHV-1—Equine herpes virus 1
EHV-5—Equine herpes virus 5
EIAV—Equine infectious anemia virus
EMH—Extramedullary hematopoiesis
EMP—Extramedullary plasmacytoma
EP—Erythroid progenitor
Epo—Erythropoietin
FAD—Flavin adenine dinucleotide
FAE—Follicle-associated epithelium
FcaGHV1—Felis catus gammaherpesvirus 1
Fe3+—Ferric iron
FeLV—Feline leukemia virus
FIV—Feline immunodeficiency virus
FL—Follicular lymphoma
FPV—Feline parvovirus
GALT—Gut-associated lymphoid tissue
GMP—Granulocyte-macrophage progenitor
GP—Glycoprotein
GP—Granulocyte progenitor
G6PD—Glucose-6-phosphate dehydrogenase
Gr.—Greek
GSH—Reduced glutathione
GT—Glanzmann thrombasthenia
H&E—Hematoxylin and eosin
HEV—High endothelial venule
Hgb—Hemoglobin
Hpt—Haptoglobin
Hpx—Hemopexin
HS—Histiocytic sarcoma
HSC—Hematopoietic stem cell
IBD—Inflammatory bowel disease
iDC—Interstitial dendritic cell
Ig—Immunoglobulin
IgA—Immunoglobulin A
IgG—Immunoglobulin G
IgM—Immunoglobulin M
IL—Interleukin
IMHA—Immune-mediated hemolytic anemia
IMTP—Immune-mediated thrombocytopenia
INF—Interferon
IRF4—Interferon regulatory factor 4
LAD—Leukocyte adhesion deficiency
LALT—Larynx-associated lymphoid tissue
LBL—Lymphoblastic lymphoma
LC—Langerhans cell
LGL—Large granular lymphocyte
LYST—Lysosomal trafficking regulator
MAC—Membrane attack complex
MALT—Mucosa-associated lymphoid tissue
MAP—Mycobacterium avium ssp. paratuberculosis
M cell—Microfold cell
MCF—Malignant catarrhal fever
MCH—Mean cell hemoglobin
MCHC—Mean cell hemoglobin concentration
MCL—Mantle cell lymphoma
MCP—Mast cell progenitor
MCT—Mast cell tumor
MCV—Mean cell volume
MDS—Myelodysplastic syndrome
MEP—Megakaryocyte-erythroid progenitor
MetHgb—Methemoglobin
MHC—Major histocompatibility complex
miRNA—MicroRNA
MKP—Megakaryocyte progenitor
MM—Multiple myeloma
MP—Macrophage progenitor
MPV—Mean platelet volume
MUM1—Melanoma-associated antigen (mutated) 1
MZL—Marginal zone lymphoma
NADH—Reduced nicotinamide adenine dinucleotide
NADPH—Reduced nicotinamide adenine dinucleotide phosphate
NALT—Nasal-associated lymphoid tissue
NCI—National Cancer Institute
NI—Neonatal isoerythrolysis
NK cell—Natural killer cell
NKP—Natural killer cell progenitor
nRBC—Nucleated red blood cell
PALS—Periarteriolar lymphoid sheath
PAMS—Periarteriolar macrophage sheath
PARR—Polymerase chain reaction for antigen receptor rearrangement
PCR—Polymerase chain reaction
PCV2—Porcine circovirus type 2
PCVAD—Porcine circovirus–associated disease
PFK—Phosphofructokinase
PHA—Pelger-Huët anomaly
PK—Pyruvate kinase
PL—Persistent lymphocytosis
PMWS—Postweaning multisystemic wasting syndrome
PPP—Pentose phosphate pathway
PRCA—Pure red cell aplasia
PRDC—Porcine respiratory disease complex
PrPSc—Scrapie prion protein
PRRS—Porcine reproductive and respiratory syndrome
PT—Prothrombin time
PTCL—Peripheral T cell lymphoma
PTT—Partial thromboplastin time
RBC—Red blood cell
REAL—Revised European-American Classification of Lymphoid Neoplasms
rhEpo—Recombinant human erythropoietin
SCID—Severe combined immunodeficiency disease
SFHN—Splenic fibrohistiocytic nodule
SH—Systemic histiocytosis
SPF—Specific pathogen–free
TCRLBCL—T cell–rich large B cell lymphoma
TGF-β—Transforming growth factor-β
TLP—T lymphocyte progenitor
TNF—Tumor necrosis factor
TNKP—T lymphocyte–natural killer cell progenitor
Tpo—Thrombopoietin
TZL—T zone lymphoma
U-bilirubin—Unconjugated bilirubin
vWD—von Willebrand disease
vWF—von Willebrand factor
WHO—World Health Organization
Bone Marrow and Blood Cells2
Structure and Function
Hematopoiesis, from haima (Gr., blood) and poiein (Gr., to make), is the production of blood cells, including erythrocytes, leukocytes, and platelets. Also known as hemopoiesis, hematopoiesis first occurs in the blood islands of the yolk sac and then transitions to the liver and spleen during gestation. After birth the primary hematopoietic site is the central cavities of bone, termed bone marrow (Fig. 13-1 ). Hematopoiesis occurring elsewhere is called extramedullary hematopoiesis (EMH), which is most common in the spleen.
Figure 13-1.

Structure of Bone Marrow.
(Courtesy Dr. K.M. Boes, College of Veterinary Medicine, Virginia Polytechnic Institute and State University; and Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
The bone marrow is supported by an anastomosing network of trabecular bone that radiates centrally from the compact bone of the cortex. Trabecular bone is covered by periosteum, consisting of an inner osteogenic layer of endosteal cells, osteoblasts, and osteoclasts, and an outer fibrous layer that anchors the stromal scaffolding of the marrow spaces.
Within the marrow spaces, a network of stromal cells and extracellular matrix provides metabolic and structural support to hematopoietic cells. These stromal cells consist of adipocytes and specialized fibroblasts, called reticular cells. The latter provides structural support by producing a fine network of a type of collagen, called reticulin, and by extending long cytoplasmic processes around other cells and structures. Both reticulin and cytoplasmic processes are not normally visible with light microscopy but are visible with silver reticulin stains (e.g., Gordon and Sweet's and sometimes with periodic acid–Schiff).
Bone marrow is highly vascularized but does not have lymphatic drainage. Marrow of long bones receives part of its blood supply from the nutrient artery, which enters the bone via the nutrient canal at midshaft. The remaining arterial supply enters the marrow through an anastomosing array of vessels that arise from the periosteal arteries and penetrate the cortical bone. Vessels from the nutrient and periosteal arteries converge and form an interweaving network of venous sinusoids that permeates the marrow. These sinusoids not only deliver nutrients and remove cellular waste but also act as the entry point for hematopoietic cells into blood circulation. Sinusoidal endothelial cells function as a barrier and regulate traffic of chemicals and particles between the intravascular and extravascular spaces. Venous drainage parallels that of the nutrient artery and its extensions.
Hematopoiesis occurs in the interstitium between the venous sinusoids in the so-called hematopoietic spaces. There is a complex functional interplay among hematopoietic cells with the supporting connective tissue cells, extracellular matrix, and soluble factors, which form the hematopoietic microenvironment. Behavior of hematopoietic cells is influenced by direct cell-to-cell and cell-matrix interactions and by soluble mediators, such as cytokines and hormones that interact with cells and with matrix proteins. Cells localize to specific niches within the hematopoietic microenvironment via adhesion molecules, such as integrins, immunoglobulins, lectins, and other receptors, which recognize ligands on other cells or matrix components. Cells also express receptors for soluble molecules such as chemokines (chemoattractant cytokines) and hormones that influence cell trafficking and metabolism.
Other components of the marrow include myelinated and nonmyelinated nerves, as well as low numbers of resident macrophages, lymphocytes, and plasma cells. Of note, the macrophages play an important role in iron storage and erythrocyte maturation.
The following basic concepts provide a framework for understanding the mechanisms of injury and diseases presented later in the chapter.
-
•
Hematopoietic tissue is highly proliferative. Billions of cells per kilogram of body weight are produced each day.
-
•
Pluripotent hematopoietic stem cells are a self-renewing population, giving rise to cells with committed differentiation programs, and are common ancestors of all blood cells. The process of hematopoietic differentiation is shown in Fig. 13-2 .
-
•
Hematopoietic cells undergo sequential divisions as they develop, so there are progressively higher numbers of cells as they mature. Cells also continue to mature after they have stopped dividing. Conceptually, it is helpful to consider cells in the bone marrow as belonging to mitotic and postmitotic compartments. Examples of developing hematopoietic cells are shown in Fig. 13-3 .
-
•
Mature cells released into the blood circulation have different normal life spans, varying from hours (neutrophils), to days (platelets), to months (erythrocytes), and to years (some lymphocytes).
-
•
The hematopoietic system is under exquisite local and systemic control and responds rapidly and predictably to various stimuli.
-
•
Production and turnover of blood cells are balanced so that numbers are maintained within normal ranges (steady-state kinetics) in healthy individuals.
-
•
Normally the bone marrow releases mostly mature cell types (and very low numbers of cells that are almost fully mature) into the circulation. In response to certain physiologic or pathologic stimuli, however, the bone marrow releases immature cells that are further back in the supply “pipeline.”
Figure 13-2.

Classic and Spatial Model of Hematopoietic Cell Differentiation, Canine Blood Smears, and Bone Marrow Aspirate.
The bone marrow consists of (1) hematopoietic stem cells, pluripotent cells capable of self-renewal; (2) progenitor cells that evolve into more differentiated cells with each cell division; (3) precursor cells that can be identified by light microscopy (not shown, see Fig. 13-3); and (4) mature hematopoietic cells awaiting release into the blood vasculature. The earliest lineage commitment is to either the common myeloid progenitor (CMP), which produces platelets, erythrocytes, and nonlymphoid leukocytes, or the common lymphoid progenitor (CLP), which differentiates into various lymphocytes and plasma cells. The cell origin of mast cells is unclear, but they may originate from a stem cell or a myeloid progenitor. Megakaryocytes remain in the bone marrow and release cytoplasmic fragments, or platelets, into blood sinusoids. T lymphocyte progenitor (TLP) cells travel from the bone marrow to the thymus during normal T lymphocyte maturation. During homeostasis, platelets and erythrocytes remain in circulation, but the leukocytes leave blood vessels to enter the tissues, where they actively participate in immune responses. In particular, monocytes and B lymphocytes undergo morphologic and immunologic changes to form macrophages and plasma cells, respectively. Macrophages, granulocytes, and mast cells migrate unidirectionally into tissues, but lymphoid cells can recirculate between the blood, tissues, and lymphatic vessels. BLP, B lymphocyte progenitor; EP, erythroid progenitor; GMP, granulocyte-macrophage progenitor; GP, granulocyte progenitor; MCP, mast cell progenitor; MKP, megakaryocyte progenitor; MEP, megakaryocyte-erythroid progenitor; MP, macrophage progenitor; NK cell, natural killer cell; NKP, natural killer cell progenitor; TLP, T lymphocyte progenitor; TNKP, T lymphocyte–natural killer cell progenitor.
(Courtesy Dr. K.M. Boes, College of Veterinary Medicine, Virginia Polytechnic Institute and State University; and Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
Figure 13-3.

Hematopoietic Cell Morphology, Feline (Erythroid and Granulocyte Lineages) and Canine (Monocyte Lineage) Blood Smears and Bone Marrow Aspirates.
As erythroid cells mature from a rubriblast to a mature erythrocyte, their nuclei become smaller and more condensed. The nucleus is eventually extruded to form a polychromatophil. Erythroid cells also become less basophilic and more eosinophilic as more hemoglobin is produced and as RNA-rich organelles are lost during maturation. (Hemoglobin stains eosinophilic, and RNA stains basophilic with routine Romanowsky's stains.) As granulocytes (e.g., neutrophils, eosinophils, and basophils) mature from a myeloblast to their mature forms, their nuclei become dense and segmented. Granulocytes acquire their secondary or specific granules during the myelocyte stage and can be morphologically differentiated starting at this stage. Neutrophils have neutral-staining secondary granules, eosinophil secondary granules have an affinity for acidic or eosin dyes, and basophil secondary granules have an affinity for basic dyes. Monoblasts differentiate into promonocytes with ruffled nuclear boarders and then into monocytes. (Courtesy Dr. K.M. Boes, College of Veterinary Medicine, Virginia Polytechnic Institute and State University; and Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
The composition of the marrow changes with age. The general pattern is that hematopoietic tissue (red marrow) regresses and is replaced with nonhematopoietic tissue, mainly fat (yellow marrow). Thus in newborns and very young animals the bone marrow consists largely of hematopoietically active tissue, with relatively little fat, whereas in geriatric individuals the marrow consists largely of fat. In adults, hematopoiesis occurs primarily in the pelvis, sternum, ribs, vertebrae, and the proximal ends of humeri and femora. Even within these areas of active hematopoiesis, fat may constitute a significant proportion of the marrow volume.
Hematopoiesis
Immature hematopoietic cells can be divided into three stages: stem cells, progenitor cells, and precursor cells. Hematopoietic stem cells (HSCs) have the capacity to self-renew, differentiate into mature cells, and repopulate the bone marrow after it is obliterated. Progenitor cells and precursor cells cannot self-renew; with each cell division, they evolve into more differentiated cells. Later-stage precursors cannot divide. Stem cells and progenitor cells require immunochemical stains for identification, but precursor cells can be identified by their characteristic morphologic features (see Fig. 13-3).
Control of hematopoiesis is complex, with many redundancies, feedback mechanisms, and pathways that overlap with other physiologic and pathologic processes. Many cytokines influence cells of different lineages and stages of differentiation. Primary growth factors for primitive cells are interleukin (IL) 3, produced by T lymphocytes, and stem cell factor, produced by monocytes, macrophages, fibroblasts, endothelial cells, and lymphocytes. Interleukin 7 is an early lymphoid growth factor. Lineage-specific growth factors are discussed in their corresponding sections.
Erythropoiesis.
Erythropoiesis—from erythros (Gr., red)—refers to the production of red blood cells, or erythrocytes, whose primary function is gas exchange; oxygen is delivered from the lungs to the tissues, and carbon dioxide is transported from the tissues to the lungs. During maturation, erythroid precursors synthesize a large quantity of a metalloprotein, called hemoglobin, to facilitate gas transportation. Erythrocytes have secondary functions, such as blood acid-base buffering.
The dominant regulator of erythropoiesis is a glycoprotein aptly named erythropoietin (Epo). Other direct or indirect stimulators of erythropoiesis include interleukins (e.g., IL-3, IL-4, and IL-9), colony-stimulating factors (e.g., granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor), and hormones (e.g., growth hormone, insulin-like growth factor, testosterone, and thyroid hormone). Epo is synthesized primarily in the kidney and exerts its effects by promoting proliferation and inhibiting apoptosis of developing erythroid cells. The stimulus for increased Epo production is hypoxia.
Within the bone marrow, erythroid precursors surround a central macrophage in specialized niches, termed erythroblastic islands (Fig. 13-4 ). The central macrophage, also known as a nurse cell, anchors the precursors within the island niche, regulates erythroid proliferation and differentiation, transfers iron to the erythroid progenitors for hemoglobin synthesis, and phagocytizes extruded metarubricyte nuclei. Although erythroblastic islands occur throughout the marrow, those with more differentiated erythroid cells neighbor sinusoids, whereas nonadjacent islands contain mostly undifferentiated precursors.
Figure 13-4.
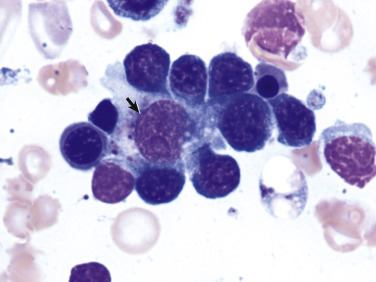
Erythroblastic Island, Canine Splenic Aspirate.
Erythroid precursors surround and adhere to a central macrophage, or nurse cell (arrow), which regulates the erythroid cell's maturation and iron acquisition.
(Courtesy Dr. K.M. Boes, College of Veterinary Medicine, Virginia Polytechnic Institute and State University.)
Iron is essential to hemoglobin synthesis and function. It is acquired through the diet and is transported to the bone marrow via the iron transport protein, transferrin. Central macrophages either store iron as ferritin or hemosiderin, or transfer the iron to erythroid precursors for hemoglobin synthesis. Hemosiderin is identifiable in routinely stained marrow preparations as an intracellular brown pigment. However, Perls's Prussian blue stain is more sensitive and specific for iron detection.
The earliest erythroid precursor identifiable by routine light microscopy is the rubriblast, which undergoes maturational division to produce 8 to 32 progeny cells. Late-stage erythroid precursors, known as metarubricytes, extrude their nuclei and become reticulocytes, and subsequently mature erythrocytes. The normal transit time from rubriblast to mature erythrocyte is approximately 1 week.
Reticulocytes start maturing in the bone marrow but finish their maturation in the blood circulation and spleen. Horses are an exception in that they do not release reticulocytes into circulation, even in situations of increased demand. Unlike mature erythrocytes, which lack organelles, reticulocytes still contain ribosomes and mitochondria, mainly to support completion of hemoglobin synthesis. These remaining organelles impart a bluish-purple cast (polychromasia) to reticulocytes on routine blood smear examination. The resultant cells are termed polychromatophils. Because older reticulocytes do not exhibit polychromasia, more sensitive laboratory techniques must be used for accurate reticulocyte quantification. When a blood sample is incubated with new methylene blue stain, the reticulocytes' ribosomal RNA precipitates to form irregular, dark aggregates (Fig. 13-5 ). Cats also have a more mature form of reticulocyte, termed punctate reticulocyte, which is stippled when stained with new methylene blue. Punctate reticulocytes indicate prior, not active, regeneration and do not appear polychromatophilic on routine blood smear evaluation.
Figure 13-5.
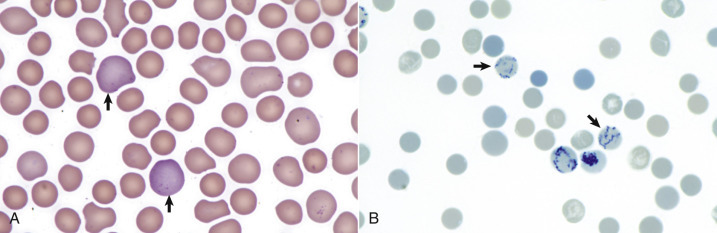
Reticulocytosis, Canine Blood Smears.
A, Reticulocytes (arrows) appear polychromatophilic with routine staining. Wright's stain. B, Reticulocytes. Precipitated aggregates of RNA are stained blue (arrows) with new methylene blue.
(Courtesy Dr. M.M. Fry, College of Veterinary Medicine, University of Tennessee.)
In most mammals, mature erythrocytes have a biconcave disk shape, called a discocyte. Microscopically, these cells are round and eosinophilic with a central area of pallor. However, the central concavity may not be microscopically apparent in species other than the dog. Camelids normally have oval erythrocytes, termed ovalocytes or elliptocytes, which facilitate better gas exchange at high altitudes. The erythrocytes of some animals are prone to in vitro shape change, including those of cervids, pigs, and some goat breeds (e.g., Angora).
Erythrocyte size during health depends on the species, breed, and age of the animal. In dogs, some breeds have relatively smaller (e.g., Akitas and Shibas) or larger (e.g., some poodles) erythrocytes. Akitas and Shibas also have a high concentration of potassium, unlike erythrocytes in other dogs. Juvenile animals may have larger erythrocytes because of the persistence of fetal erythrocytes, which is followed by a period of relatively smaller cells before reaching adult reference intervals.
Mature mammalian erythrocytes lack nuclei and organelles and are thus incapable of transcription, translation, and oxidative metabolism. However, they do require energy for various functions, including maintenance of shape and deformability, active transport, and prevention of oxidative damage. Red blood cells generate this energy entirely through glycolysis (also known as the Embden-Meyerhof pathway). Except in pigs, glucose enters erythrocytes from the plasma through an insulin-independent, integral membrane glucose transporter.
Within circulation the erythrocyte mean life span varies between species and is related to body weight and metabolic rate: approximately 150 days in horses and cattle, 100 days in dogs, and 70 days in cats. When erythrocytes reach the end of their life span, they are destroyed in a process termed hemolysis. Hemolysis may occur within blood vessels (intravascular hemolysis) or by sinusoidal macrophages (extravascular hemolysis). During intravascular hemolysis, erythrocytes release their contents, mostly hemoglobin, directly into blood. However, during extravascular hemolysis, macrophages phagocytize entire erythrocytes, leaving little or no hemoglobin in the blood. Normal turnover of erythrocytes occurs mainly by extravascular hemolysis within the spleen, and to a lesser extent in other organs such as the liver and bone marrow. The exact controls are not clear, but factors that likely play a role in physiologic hemolysis include the following:
-
•
Exposure of membrane components normally sequestered on the inner leaflet of the erythrocyte membrane, particularly phosphatidylserine.
-
•
Decreased erythrocyte deformability.
-
•
Binding of immunoglobulin G (IgG) and/or complement to erythrocyte membranes. Complement binding may be secondary clustering of the membrane anion exchange protein, band 3.
-
•
Oxidative damage to erythrocytes.
Macrophages degrade erythrocytes into reusable components, such as iron and amino acids, and the waste product bilirubin. Bilirubin is then exported into circulation, where it is transported to the liver by albumin. The liver conjugates and subsequently excretes bilirubin into bile for elimination from the body.
Intravascular hemolysis normally occurs at only extremely low levels. Hemoglobin is a tetramer that, when released from the erythrocyte into the blood, splits into dimers that bind to a plasma protein called haptoglobin. The hemoglobin-haptoglobin complex is taken up by hepatocytes and macrophages. This is the major pathway for handling free hemoglobin. However, free hemoglobin may also oxidize to form methemoglobin, which dissociates to form metheme and globin. Metheme binds to a plasma protein called hemopexin, which is taken up by hepatocytes and macrophages in a similar manner to hemoglobin-haptoglobin complexes. Free heme in the reduced form binds to albumin, from which it is taken up in the liver and converted into bilirubin.
The concentration of circulating erythrocytes typically decreases postnatally and remains below normal adult levels during the period of rapid body growth. The age at which erythrocyte numbers begin to increase and the age at which adult levels are reached vary among species. In dogs, adult values are usually reached between 4 and 6 months of age; in horses, this occurs at approximately 1 year of age.
Granulopoiesis and Monocytopoiesis (Myelopoiesis).
Granulopoiesis is the production of neutrophils, eosinophils, and basophils, whereas monocyte production is termed monocytopoiesis. Granulocytic and monocytic cells are sometimes collectively referenced as myeloid cells. However, the term myeloid and the prefix myelo- can be confusing because they have other meanings; they may reference the bone marrow, all nonlymphoid hemic cells (erythrocytes, leukocytes, and megakaryocytes), only granulocytes, or the spinal cord.
The main purpose of granulocytes and monocytes is to migrate to sites of tissue inflammation and function in host defense (see Chapters 3 and 5 ). Briefly, these cells have key immunologic functions, including phagocytosis and microbicidal activity (neutrophils and monocyte-derived macrophages), parasiticidal activity and participation in allergic reactions (eosinophils and basophils), antigen processing and presentation, and cytokine production (macrophages). Neutrophils are the predominant leukocyte type in blood of most domestic species.
Primary stimulators of granulopoiesis and monocytopoiesis are granulocyte-macrophage colony-stimulating factor and IL-1, IL-3, and IL-6 (granulocytes and monocytes), granulocyte colony-stimulating factor (granulocytes), and macrophage colony-stimulating factor (monocytes). In general, these cytokines are produced by various inflammatory cells, with or without contribution from stromal cells.
The earliest granulocytic precursor identifiable by routine light microscopy is the myeloblast, which undergoes maturational division over 5 days to produce 16 to 32 progeny cells (see Fig. 13-3). These granulocytic precursors are conceptually divided into those stages that can divide, including myeloblasts, promyelocytes, and myelocytes (proliferation pool), and those that cannot, including metamyelocytes, and band and segmented forms (maturation pool). Within the neutrophil maturation pool is a subpool, termed the storage pool, which consists of a reserve of fully mature neutrophils. The size of the storage pool varies by species; it is large in the dog, but small in ruminants. In homeostasis mostly mature segmented granulocytes are released from the marrow into the blood.
The first monocytic precursor identifiable by morphologic features is the monoblast, which develops into promonocytes and subsequently monocytes (see Fig. 13-3). Unlike granulocytes, monocytes do not have a marrow storage pool; they immediately enter venous sinusoids upon maturation. After migrating into the tissues, monocytes undergo morphologic and immunophenotypic maturation into macrophages.
Within blood vessels there are two pools of leukocytes: the circulating pool and the marginating pool. Circulating cells are free flowing in blood, whereas marginating cells are temporarily adhered to endothelial cells by selectins. In most healthy mammals there are typically equal numbers of neutrophils in the circulating and marginal pools. However, there are threefold more marginal neutrophils relative to circulating neutrophils in cats. Only the circulating leukocyte pool is sampled during phlebotomy. The concentration of myeloid cells in blood depends on the rate of production and release from the bone marrow, the proportions of cells in the circulating and marginating pools, and the rate of migration from the vasculature into tissues.
The fate of neutrophils after they leave the bloodstream in normal conditions (i.e., not in the context of inflammation) is poorly understood. They migrate into the gastrointestinal and respiratory tracts, liver, and spleen and may be lost through mucosal surfaces or undergo apoptosis and be phagocytized by macrophages.
Lymphopoiesis.
Lymphopoiesis—from lympha (Latin, water)—refers to the production of new lymphocytes, including B lymphocytes, T lymphocytes, and natural killer (NK) cells. B lymphocytes primarily produce immunoglobulins, also known as antibodies, and are key effectors of humoral immunity. They are distinguished by the presence of an immunoglobulin receptor complex, termed the B lymphocyte receptor. Plasma cells are terminally differentiated B lymphocytes that produce abundant immunoglobulin. T lymphocytes, effectors of cell-mediated immunity, possess T lymphocyte receptors that bind antigens prepared by antigen-presenting cells. A component of innate immunity, NK cells kill a variety of infected and tumor cells in the absence of prior exposure or priming. Main growth factors for B lymphocytes, T lymphocytes, and NK cells are IL-4, IL-2, and IL-15, respectively.
Lymphocytes are derived from HSCs within the bone marrow. B lymphocyte development occurs in two phases, first in an antigen-independent phase in the bone marrow and ileal Peyer's patches (the site of B lymphocyte development in ruminants), then in an antigen-dependent phase in peripheral lymphoid tissues (such as spleen, lymph nodes, and mucosa-associated lymphoid tissue [MALT]). T lymphocyte progenitors migrate from the bone marrow to the thymus, where they undergo differentiation, selection, and maturation processes before migrating to the peripheral lymphoid tissue as effector cells.
Unlike granulocytes, which circulate only in blood vessels and migrate unidirectionally into target tissues, lymphocytes travel in both blood and lymphatic vessels and continually circulate between blood, tissues, and lymphatic vessels. Also in contrast to nonlymphoid hematopoietic cells, blood lymphocyte concentrations in adult animals are primarily dependent upon extramedullary lymphocyte production and kinetics, and not lymphopoiesis by the marrow.
In healthy nonruminant mammals, lymphocytes are the second most numerous blood leukocyte. According to conventional wisdom, cattle normally have higher numbers of lymphocytes than neutrophils in circulation. However, recent studies suggest that is no longer the case, most likely due to changes in genetics and husbandry. In most species the majority of lymphocytes in blood circulation are T lymphocytes. The concentration of blood lymphocytes decreases with age.
Thrombopoiesis.
Thrombopoiesis—from thrombos (Gr., clot)—refers to the production of platelets, which are small (2 to 4 µm), round to ovoid, anucleate cells within blood vessels. Platelets have a central role in primary hemostasis but also participate in secondary hemostasis (coagulation) and inflammatory pathways (see Chapters 2 and 3).
Thrombopoietin (Tpo) is the primary regulator of thrombopoiesis. The liver and renal tubular epithelial cells constantly produce Tpo, which is then cleared and destroyed by platelets and their precursors. Therefore plasma Tpo concentration is inversely proportional to platelet and platelet precursor mass. If the platelet mass is decreased, less Tpo is cleared, and there is subsequently more free plasma Tpo to stimulate thrombopoiesis.
The earliest morphologically identifiable platelet precursor is the megakaryoblast, which undergoes nuclear reduplications without cell division, termed endomitosis, to form a megakaryocyte with 8 to 64 nuclei. As the name suggests, megakaryocytes are very large cells, much larger than any other hematopoietic cell (Fig. 13-6 ; also see Fig. 13-1). Megakaryocytes neighbor venous sinusoids, extend their cytoplasmic processes into vascular lumens, and shed membrane-bound cytoplasmic fragments (platelets) into blood circulation. Orderly platelet shedding is partially facilitated by β1-tubulin microtubules within megakaryocytes.
Figure 13-6.
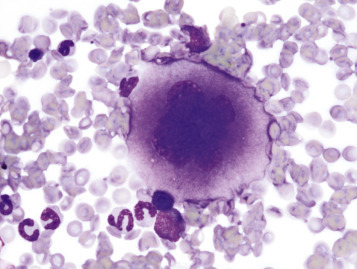
Megakaryocyte, Canine Bone Marrow Aspirate.
Note the cell's very large size, lobulated nucleus, and abundant granular cytoplasm. Wright's stain.
(Courtesy Dr. M.M. Fry, College of Veterinary Medicine, University of Tennessee.)
Platelets circulate in a quiescent form and become activated by binding platelet agonists, including thrombin, adenosine diphosphate (ADP), and thromboxane. Platelet activation causes shape change, granule release, and relocation of procoagulant phospholipids and glycoproteins (GPs) to the outer cell membrane. Specific procoagulant actions include release of calcium, von Willebrand factor (vWF), factor V, and fibrinogen, as well as providing phosphatidylserine-rich binding sites for the extrinsic tenase (factors III, VII, and X), intrinsic tenase (factors IX, VIII, and X), and prothrombinase (factors X, V, and II) coagulation complexes. Platelet GP surface receptors include those for binding vWF (GPIb-IX-V), collagen (GPVI), and fibrinogen (GPIIb-IIIa), which facilitate platelet aggregation and adherence to subendothelial collagen. Expansion of surface area and release of granule contents is aided by a network of membrane invaginations known as the open canalicular system. This system is not present in horses, cattle, and camelids.
Methods for Examination of the Bone Marrow
Gross and Microscopic Examination
Bone marrow is not routinely sampled during postmortem examinations. However, indications for bone marrow evaluation include suspected leukemia, metastatic neoplasia within bone marrow, or infectious myelitis, as well as cytopenia(s) or hematopoietic dysplasia of unknown cause. Multimodal evaluation is ideal, including a recent (<24 hours) complete blood count with bone marrow cytologic and histopathologic examination. However, antemortem blood analyses are not always available, and interpretation of hematopoietic cytomorphologic examination results becomes difficult to impossible shortly after death.
Postmortem bone marrow should be collected as soon as possible after death or euthanasia, preferably within 30 minutes. Samples may be collected from the proximal femur, rib, sternum or vertebrae. When collecting from the femur, the femoral neck is removed with a bone saw, or a fragment of the shaft is removed with bone-cutting shears. Cytologic samples are first collected using the paintbrush technique: gently sample the red marrow with a clean, dry, natural-bristle brush, and then carefully brush the material onto a clean glass microscope slide in two to four parallel wavy lines. The brush should be cleaned and dried before its use on a different animal. The slide is then air dried, stored away from formalin fumes, and then stained with a routine (Romanowsky) stain. For histologic evaluation the entire femoral head or femoral shaft or rib fragment with exposed red marrow is submersed in 10% neutral buffered formalin. For cosmetic necropsies, samples may be obtained by antemortem techniques, such as needle biopsies for cytologic examination and core biopsies for histopathologic examination.
Complete Blood Count
The complete blood count (CBC) is the cornerstone for diagnosis of hematologic disturbances and is often part of a minimum database in sick patients. The CBC includes numeric data indicating the concentration of different cell types, as well as other estimations of red blood cell mass (hemoglobin concentration, packed-cell volume, and hematocrit), red blood cell volume (mean cell volume), and red blood cell hemoglobin content (mean cell hemoglobin and mean cell hemoglobin concentration). Cell morphologic features and the presence or absence of hemic parasites are assessed upon microscopic review of a blood smear and are also included in a CBC report. (note: Some parasites may infect blood cells, such as Hepatozoon organisms within circulating neutrophils or monocytes or Bartonella organisms within erythrocytes, but mainly cause disease in other body systems and are therefore not discussed in this chapter.) Learning to evaluate blood smears is a valuable skill for any practicing veterinarian. The CBC also may include the plasma protein concentration, as measured with a refractometer. It is important to remember that changes in hydration status and in the distribution of body fluids between the vascular and extravascular compartments affect the concentration of both cells and proteins in the blood.
Additional Tests
Other tests that may help with evaluation of the hematopoietic system include cell or tissue biopsies, the direct antiglobulin test, flow cytometry, immunophenotyping, and polymerase chain reaction (PCR). Aspiration cytology and/or histopathology of organs other than the bone marrow can be pursued to assess for the presence of EMH, increased destruction of erythrocytes, neoplasia, or infection. The Coombs test, or direct antiglobulin test, detects excessive antibody or complement bound to red blood cells' surfaces and is the standard assay for immune-mediated hemolytic anemia. Flow cytometry and immunofluorescent antibody tests may also be used to detect autoantibody bound to erythrocytes or other hematopoietic cells. Immunophenotyping and PCR are further discussed in the section on Hematopoietic Neoplasia.
Hemostasis Testing
Structural or functional abnormalities of blood vessels, platelets, or coagulation factors may result in a tendency toward hypocoagulability (bleeding), hypercoagulability (inappropriate thrombosis), or both. In veterinary medicine there has been a great deal of work on specific mechanisms of hypocoagulability, whereas mechanisms of hypercoagulability are less fully characterized. Disorders of primary hemostasis typically result in “small bleeds” (e.g., petechiation, mild ecchymosis, bleeding from mucous membranes, bleeding immediately after venipuncture), whereas disorders of secondary hemostasis typically result in “big bleeds” (e.g., hemorrhage into body cavities/joints, marked ecchymosis, large hematomas, delayed bleeding after venipuncture). This chapter concentrates on primary disorders of hemostasis and also covers disseminated intravascular coagulation, which is the secondary condition. However, it is important to note that coagulation disorders can also result from other underlying disease processes. For example, advanced liver disease can lead to abnormal hemostasis through decreased or defective synthesis of coagulation factors or impaired clearance of fibrinolytic products that inhibit coagulation reactions and platelet function. Vascular disorders may also result in a bleeding tendency because of abnormalities of endothelial function or collagen-platelet interactions. Specific diseases involving abnormal structure or function of hematopoietic or hemostatic elements are discussed later in this chapter.
The CBC provides basic information about platelets, including numeric values for platelet concentration and mean platelet volume (MPV), subjective assessment of platelet morphologic features (size, shape, and granularity), and a rough estimation of platelet numbers based on examination of a blood smear. Some laboratories measure reticulated platelets (platelets recently released from the bone marrow), although this test is mostly used in the research setting at present. Increased MPV and increased numbers of reticulated platelets tend to indicate increased thrombopoiesis. Bone marrow examination is indicated with any unexplained cytopenia, including thrombocytopenia, to evaluate production. Tests to evaluate the components of the hemostatic process are described and listed in E-Appendix 13-1.
Dysfunction/Responses to Injury
Bone Marrow
Mechanisms of bone marrow disease are summarized in Box 13-1 . Hematopoietic cells' response to injury is dependent upon whether the insult is on the marrow or within extramarrow tissues. In general, marrow-directed injury or disturbances result in production of abnormal hematopoietic cells (dysplasia), fewer hematopoietic cells (hypoplasia), or a failure of hematopoietic cell development (aplasia). Dysplasia, hypoplasia, and aplasia may be specific for one cell line, such as pure red cell aplasia, or affect multiple lineages, as seen with aplastic anemia. Accordingly, decreased blood concentrations of the involved cell types are expected with hypoplasia or aplasia. Erythroid, myeloid, and megakaryocytic hypoplasia or aplasia causes nonregenerative anemia, neutropenia, and thrombocytopenia, respectively. Bicytopenia is used to describe decreased blood concentrations of two cell lines, whereas pancytopenia indicates decreased blood concentrations of all three cell types. Bicytopenia or pancytopenia may indicate generalized marrow disease, such as occurs with aplastic anemia or marrow malignancies (leukemia), necrosis, fibrosis (myelofibrosis), or inflammation (myelitis). Replacement of hematopoietic tissue within the bone marrow by abnormal tissue, including neoplastic cells, fibrosis, or inflammatory cells, is termed myelophthisis.
Box 13-1. Mechanisms of Disease in Bone Marrow and Blood Cells.
Bone Marrow
Hypoplasia
Hyperplasia
Dysplasia
Aplasia
Neoplasia
Myelophthisis (fibrosis, metastatic neoplasia)
Necrosis
Inflammation
Blood Cells
Increased destruction
Hemorrhage (especially erythrocytes)
Consumption (platelets)
Neoplasia
Altered distribution
Abnormal function
Insults to extramarrow tissues and cells tend to cause increased production of the involved cell types (hyperplasia) with or without dysplasia. Loss of erythrocytes from blood vessels (hemorrhage), or premature destruction of erythrocytes (hemolysis) causes erythroid hyperplasia. Tissue inflammation may cause neutrophilic, eosinophilic, basophilic, and/or monocytic hyperplasia, depending on the type of inflammation. Megakaryocytic hyperplasia may occur with increased platelet use during hemorrhage or disseminated intravascular coagulation (DIC) or with immune-mediated platelet destruction. Exceptions to these generalizations, such as anemia of chronic disease, iron deficiency anemia, and anemia of renal failure, are discussed in more detail later.
Endothelial cell response to injury specifically within the marrow is poorly characterized, but it is likely similar to that of endothelial cells elsewhere, playing active roles in coagulation and inflammation (see Chapters 2 and 3). However, one potential sign of marrow sinusoidal injury is the presence of circulating nucleated erythrocytes in the absence of erythrocyte regeneration, termed inappropriate metarubricytosis. It is proposed that injured marrow endothelial cells allow premature passage of metarubricytes into blood circulation during times of stress. However, a conflicting theory proposes that marrow stress causes decreased metarubricyte attachment to central macrophages, and subsequent release into circulation. Specific causes of marrow injury–induced metarubricytosis include sepsis, hyperthermia, malignancies, hypoxia, and certain drugs and toxins. Inappropriate metarubricytosis may also occur with erythroid dysplasia and splenic disorders.
In addition to a suspected role in inappropriate metarubricytosis, marrow macrophages are integral to altered iron metabolism, including anemia of chronic disease and hemosiderosis. Anemia of chronic disease is a mild to moderate nonregenerative anemia observed in animals with a variety of inflammatory and metabolic disorders. This anemia is discussed in more detail later, but briefly, it is primarily a result of iron sequestration within macrophages. Hemosiderosis is the excessive accumulation of iron in tissues, typically macrophages. Accumulation of iron in parenchymal organs, leading to organ toxicity, is termed hemochromatosis. In animals, iron overload due to blood transfusions or chronic hemolytic anemias may cause marrow hemosiderosis and hemochromatosis.
Myelitis can take different forms. Granulomatous myelitis occurs with systemic fungal infections (e.g., histoplasmosis) or mycobacteriosis. Acute or neutrophilic myelitis may occur with lower-order bacterial infections or those with an immune-mediated component. Dogs and cats with nonregenerative immune-mediated hemolytic anemia (IMHA) often have myelitis, in addition to myelofibrosis and necrosis. The inflammation is evident as fibrin deposition, edema, and multifocal neutrophilic infiltrates; immune-mediated cytopenias may also concurrently occur with bone marrow lymphocytic and/or plasma cell hyperplasia.
Bone marrow necrosis is the necrosis of medullary hematopoietic cells, stromal cells, and stroma in large areas of bone marrow. Potential causes include leukemias, extramarrow malignancies, infection (bovine viral diarrhea virus [BVDV], Ehrlichia canis, and feline leukemia virus [FeLV]), sepsis, drugs or toxins (carprofen, chemotherapeutic agents, estrogen, metronidazole, mitotane, and phenobarbital), and irradiation. Direct hematopoietic or stromal cytotoxicity and altered marrow microvasculature (disseminated intravascular coagulation) are proposed pathogeneses. Extensive marrow necrosis results in decreased hematopoiesis and subsequent blood cytopenias, including anemia, neutropenia, and thrombocytopenia. If the animal survives the initial insult, the marrow may recover and resume normal hematopoiesis, or it may undergo scar formation, termed myelofibrosis.
Secondary myelofibrosis is the enhanced deposition of collagen within the marrow by nonneoplastic fibroblasts and reticular cells. Disease pathogenesis is unclear, but there are two leading theories. First, it may represent scar formation after marrow necrosis, as previously presented. And second, high concentrations of growth factors present during times of marrow injury or activation may stimulate fibroblast proliferation. In particular, stimulated megakaryocytes and macrophages produce fibrogenic cytokines, including platelet-derived growth factor, transforming growth factor-β, and epidermal growth factor. Early in disease there is reticulin deposition without reduction of hematopoietic elements. However, fibrous collagen replaces hematopoietic cells with disease progression. Histologic identification of reticulin and collagen fibers can be aided with reticulin silver and Masson's trichrome stains, respectively. In animals, secondary myelofibrosis occurs most commonly with leukemias, extramarrow malignancies, and chronic hemolytic anemias, but many cases are idiopathic. Experimental whole-body gamma irradiation, dietary strontium-90 exposure, and certain drugs and toxins can also induce myelofibrosis.
The responses of marrow adipocytes to systemic and localized disease are under current investigation, especially in relation to energy metabolism, inflammation, and bone trauma. During times of severe energy imbalance, such as cachexia, the marrow may undergo serous atrophy of fat, also known as gelatinous marrow transformation (E-Fig. 13-1). The pathogenesis of this phenomenon is unknown, but it is characterized by adipocyte atrophy, hematopoietic cell hypoplasia with subsequent cytopenias, and replacement of the marrow with extracellular hyaluronic acid–rich mucopolysaccharides. Positive Alcian blue staining identifies the extracellular material as mucin.
E-Figure 13-1.

Serous Atrophy of Fat (Gelatinous Transformation), Bone Marrow, Calf.
Serous atrophy of fat is caused by starvation and/or cachexia and is characterized by replacement of normal fat and hematopoietic elements by a gelatinous extracellular matrix.
(Courtesy Department of Pathobiology, College of Veterinary Medicine, University of Tennessee).
Marrow adipocytes secrete adipose-derived hormones, termed adipokines, including leptin and adiponectin. In general, leptin is proinflammatory, prothrombotic, and mitogenic for various cell types, including lymphocytes, hematopoietic progenitors, and leukemic cells. Conversely, adiponectin has antiinflammatory and growth inhibitory properties. During times of inflammation and infection, leptin production is increased.
In response to marrow trauma, such as orthopedic surgery, fat may enter the vasculature, embolize to various tissues, and cause tissue ischemia. The severity of tissue injury caused by fat embolism is dependent upon the quantity of fat entering circulation and the tissue's susceptibility to ischemia (see Chapter 2).
Blood Cells
Responses of circulating blood cells to injury include decreased survival (destruction, consumption, or loss), altered distribution, and altered structure or function (see Box 13-1). These responses are not mutually exclusive—for example, altered erythrocyte structure may lead to decreased survival. Often, but not always, these responses result in decreased concentrations of blood cells in circulation.
Abnormal Concentrations of Blood Cells.
The concentration of blood cells may be decreased, termed cytopenia (from kytos [Gr., hollow vessel] and penia [Gr., poverty]) or increased, designated cytosis (from osis [Gr., condition]). A specific blood cell type is denoted as being decreased by using the suffix -penia (Table 13-1 ). A decreased concentration of erythrocytes is the exception and is termed anemia (from a [Gr., without] and haima [Gr., blood]). Decreased concentrations of blood basophils are not recognized in domestic animals because the lower reference interval is typically zero. An increased blood cell type is denoted with the suffix -osis or -philia (see Table 13-1). Postmortem quantification of blood cell concentrations is not possible due to perimortem coagulation. However, a complete blood count (CBC) with microscopic blood smear evaluation is the foundation for antemortem assessment of blood cells.
Table 13-1.
Terminology for Increases or Decreases in Hematopoietic Cells in Blood
| Cell type | Decreased | Increased |
|---|---|---|
| Erythrocytes | Anemia | Erythrocytosis |
| Reticulocytes | Reticulopenia | Reticulocytosis |
| Leukocytes | Leukopenia | Leukocytosis |
| Neutrophils | Neutropenia | Neutrophilia |
| Lymphocytes | Lymphopenia | Lymphocytosis |
| Monocytes | Monocytopenia | Monocytosis |
| Eosinophils | Eosinopenia | Eosinophilia |
| Basophils | Basopenia | Basophilia |
| Platelets | Thrombocytopenia | Thrombocytosis |
Anemia.
Anemia causes clinical signs referable to decreased red hemoglobin pigment (e.g., pale mucous membranes), decreased oxygen-carrying capacity (e.g., depression, lethargy, weakness, and exercise tolerance), and decreased blood viscosity (e.g., heart murmur). Recumbency, seizures, syncope, or coma may occur with severe anemia. Anemia is confirmed by identifying a decreased hemoglobin concentration or reduced erythrocyte mass, as measured by the packed-cell volume, hematocrit, or red blood cell concentration.
The three general causes of anemia are blood loss (hemorrhage), red blood cell destruction or lysis (hemolysis), and decreased red blood cell production (erythroid hypoplasia). Classifying anemia as regenerative or nonregenerative is clinically useful because it provides information about the mechanism of disease; regenerative anemia indicates hemorrhage or hemolysis, whereas erythroid hypoplasia or aplasia causes nonregenerative anemia (Table 13-2 ).
Table 13-2.
Causes of Regenerative and Nonregenerative Anemia
| Regenerative Anemia | Nonregenerative Anemia |
|---|---|
|
|
|
|
The hallmark of regenerative anemias, except in horses, is reticulocytosis (i.e., increased numbers of circulating reticulocytes [immature erythrocytes]), which is evident as polychromasia on a routinely stained blood smear (see Fig. 13-5). Reticulocytosis indicates increased bone marrow erythropoiesis (Fig. 13-7 ) and release of erythrocytes before they are fully mature. Reticulocytosis is an appropriate marrow response to anemia and is often seen with hemorrhage or hemolysis. On a CBC a strong regenerative response may produce an increased mean cell volume (MCV) and decreased mean cell hemoglobin concentration (MCHC) because reticulocytes are larger and have a lower hemoglobin concentration than mature erythrocytes. Horses are an exception to this classification scheme because they do not release reticulocytes into circulation, even with erythroid hyperplasia. Horses with a regenerative response may have an increased MCV and red cell distribution width (an index of variation in cell size). But definitive determination of regeneration in a horse requires demonstration of erythroid hyperplasia via bone marrow examination or an increasing red cell mass over sequential CBCs.
Figure 13-7.
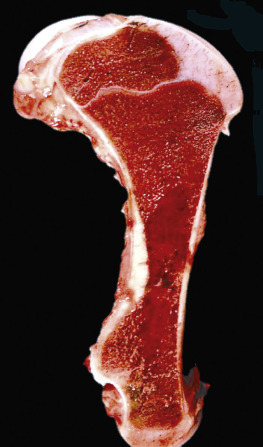
Hemopoietically Active Bone Marrow, Femur, Calf.
Note that the bone marrow has a uniform consistency and is red to dark red. These responses are characteristic of hemopoietically active bone marrow.
(Courtesy Dr. Ramos, Autonomous University of Barcelona; and Noah's Arkive, College of Veterinary Medicine, The University of Georgia.)
In addition to reticulocytosis there may be increased numbers of nucleated red blood cells (nRBCs) in circulation with erythrocyte regeneration, termed appropriate metarubricytosis. When nRBCs are present as part of a regenerative response, they should be in low numbers relative to the numbers of reticulocytes. However, the presence of circulating nRBCs is not in itself definitive evidence of regeneration and may signify dyserythropoiesis (e.g., lead poisoning or bone marrow disease) or splenic dysfunction. These processes should be suspected when nRBCs are increased without reticulocytosis, or their numbers are high relative to the degree of reticulocytosis, termed inappropriate metarubricytosis.
In ruminants, reticulocytosis is often accompanied by basophilic stippling (Fig. 13-8 ). However, like metarubricytosis, basophilic stippling without reticulocytosis is concerning for lead poisoning or other causes of dyserythropoiesis.
Figure 13-8.

Basophilic Stippling and Polychromasia, Bovine Blood Smear.
Erythrocytes from this cow with regenerative anemia include several cells with basophilic stippling (arrow) and two polychromatophilic cells (reticulocytes) (arrowheads). Wright's stain. (Courtesy Dr. M.M. Fry, College of Veterinary Medicine, University of Tennessee.)
Recall that the stimulus for increased erythropoiesis is increased secretion of Epo in response to tissue hypoxia. Although the action of Epo on erythropoiesis is rapid, evidence of a regenerative response is not immediately apparent in a blood sample. One of the main effects of Epo is to expand the pool of early-stage erythroid precursors, and it takes time for these cells to differentiate to the point where they are released into circulation. In a case of acute hemorrhage or hemolysis, for example, it typically takes 3 to 4 days until reticulocytosis is evident on the CBC and several more days until the regenerative response peaks. The term preregenerative anemia is sometimes used to describe anemia with a regenerative response that is impending but not yet apparent on the CBC. Confirming a regenerative response in such cases requires either evidence of erythroid hyperplasia in the bone marrow or emergence of a reticulocytosis on subsequent days.
Hemorrhage results in escape of erythrocytes and other blood components, such as protein, from the vasculature. As a result, a decreased plasma or serum protein concentration, termed hypoproteinemia, may be evident on a CBC or chemistry panel. If the hemorrhage is into the gastrointestinal lumen, some of the protein may be resorbed and converted to urea, resulting in an increased urea nitrogen concentration relative to creatinine in plasma. Hemorrhage within the urinary tract may cause red urine with erythrocytes observed in the urine sediment. Causes of hemorrhage include trauma, abnormal hemostasis, certain parasitisms, ulceration, and neoplasia.
Hemorrhage may be acute or chronic, or internal or external. During acute hemorrhage, there are ample iron stores within the body for hemoglobin synthesis and erythrocyte regeneration. However, with chronic external hemorrhage, continued loss of iron may deplete the body's iron stores. As iron stores diminish, so does erythrocyte regeneration, eventually leading to iron deficiency anemia. Iron deficiency anemia is either poorly regenerative or nonregenerative and is discussed in more detail later in the chapter. Iron deficiency anemia does not occur with chronic internal hemorrhage, such as into the peritoneal cavity, because iron is not lost from the body and can be reused for erythropoiesis.
In hemolytic anemia, erythrocytes are destroyed at an increased rate. Whether the mechanism is intravascular or extravascular, or a combination, depends on the specific disease process (specific diseases are discussed later in this chapter). Some clinical indicators of hemolytic anemia and their pathogeneses are summarized in Fig. 13-9 and are further described in the following discussion.
Figure 13-9.
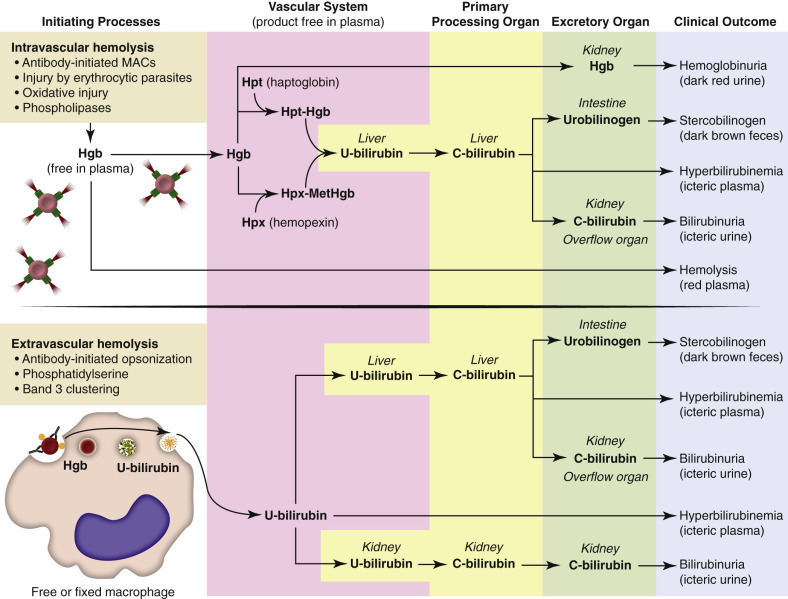
Mechanisms of Color Abnormalities of Plasma, Urine, and Feces during Hemolysis.
Intravascular hemolysis: Several initiating processes can cause intravascular hemolysis; formation of the complement membrane attack complex is pictured. With intravascular hemolysis, free hemoglobin is release directly into the plasma, where it is scavenged by haptoglobin and hemopexin. When haptoglobin and hemopexin are saturated, the cell-free hemoglobin causes red discoloration of the plasma (hemolysis) and is excreted in the urine (hemoglobinuria; dark red urine). The liver clears haptoglobin-hemoglobin and hemopexin-methemoglobin complexes from plasma and converts hemoglobin to unconjugated bilirubin and then conjugated bilirubin. Conjugated bilirubin is normally excreted in the bile and then converted to urobilinogen (yellow) and subsequently stercobilinogen (dark brown). However, excessive bilirubin will spill over into the plasma, resulting in hyperbilirubinemia, icteric plasma (if severe enough), and urinary excretion of bilirubin (bilirubinuria; icteric urine). Extravascular hemolysis: During extravascular hemolysis, erythrocytes are phagocytized by macrophages, which digest erythrocytes, and convert hemoglobin to unconjugated bilirubin. Excessive bilirubin in plasma causes hyperbilirubinemia with or without icteric plasma. Unconjugated bilirubin is processed and excreted by the liver (as previously described) and in dogs, the kidney. C-bilirubin, Conjugated bilirubin; Hgb, hemoglobin; Hpt, haptoglobin; Hpx, hemopexin; MACs, membrane attack complexes; MetHgb, methemoglobin; U-bilirubin, unconjugated bilirubin.
(Courtesy Dr. K.M. Boes, College of Veterinary Medicine, Virginia Polytechnic Institute and State University; and Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
A classic sequela of hemolytic anemias in general is hyperbilirubinemia, which is an increase in the plasma bilirubin concentration. Bilirubin is a yellow pigment, which explains why hyperbilirubinemia, if severe enough, causes icterus—the grossly visible yellowing of fluid or tissues (Fig. 13-10 ). Icterus, also known as jaundice, is usually detectable when the plasma bilirubin concentration exceeds 2 mg/dL. However, it is important to note that hyperbilirubinemia and icterus are not pathognomonic for hemolysis and may also occur with conditions of impaired bile flow (cholestasis), such as hepatopathy or cholangiopathy.
Figure 13-10.
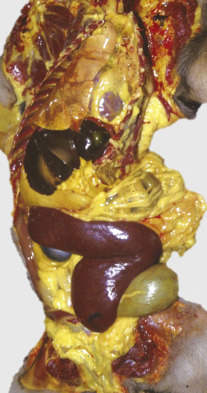
Icterus, Immune-Mediated Hemolytic Anemia, Subcutaneous Fat, Splenomegaly, Spleen, Dog.
The marked yellow discoloration of tissues, most strikingly visible in the subcutaneous fat, is from high concentrations of serum bilirubin produced as a result of the hemolytic anemia.
(Courtesy Dr. J.A. Ramos-Vara, College of Veterinary Medicine, Michigan State University; and Noah's Arkive, College of Veterinary Medicine, The University of Georgia.)
In addition to icterus, hemolytic anemia often results in splenomegaly (Fig. 13-11 ), which is secondary to extravascular hemolysis and macrophagic hyperplasia within the spleen, as well as splenic EMH. Splenomegaly may also occur in other conditions, as discussed elsewhere in this chapter.
Figure 13-11.
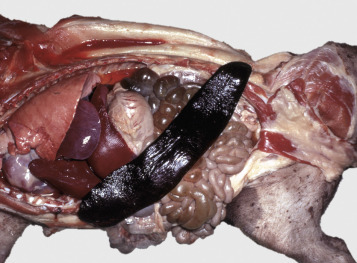
Splenomegaly, Fatal Hemolytic Anemia, Mycoplasma suis, Pig.
The spleen is extremely enlarged, meaty, and congested.
(Courtesy College of Veterinary Medicine, University of Illinois.)
Intravascular hemolysis is grossly evident as pink-tinged plasma or serum, termed hemolysis or hemoglobinemia. Hemolysis is not apparent until the concentration of extracellular hemoglobin is greater than approximately 50 mg/dL. Cell-free hemoglobin is scavenged by haptoglobin until haptoglobin becomes saturated with hemoglobin at a concentration of approximately 150 mg/dL. When haptoglobin is saturated, any remaining free hemoglobin has a low enough molecular weight to pass through the renal glomerular filter into the urine. This imparts a pink or red discoloration to the urine, called hemoglobinuria. Thus extracellular hemoglobin can cause gross discoloration of the plasma, where it is bound to haptoglobin, before becoming grossly visible in urine. The half-life of haptoglobin is markedly decreased when bound to hemoglobin, so when large amounts of haptoglobin-hemoglobin complex are formed, the concentration of haptoglobin in the blood decreases and hemoglobin can pass through the glomerulus at even lower concentrations. Hemoglobinuria is a contributing factor in the renal tubular necrosis (hemoglobinuric nephrosis) that often occurs in cases of acute intravascular hemolysis (see Chapter 11). A similar lesion occurs in the kidneys of individuals with marked muscle damage and resulting myoglobinuria (see Chapters 11 and 15).
Hemoglobinuria cannot be distinguished grossly from hematuria (erythrocytes in the urine) or myoglobinuria (myoglobin in the urine), and all three processes cause a positive reaction for “blood protein” on urine test strips. Comparing the colors of the plasma and the urine may be informative. In contrast to hemoglobin, myoglobin causes gross discoloration of the urine before the plasma is discolored. This is because myoglobin is a low-molecular-weight monomer, freely filtered by the glomerulus, and does not bind plasma proteins to a significant degree. Hematuria can be distinguished from hemoglobinuria on the basis of microscopic examination of urine sediment (i.e., erythrocytes are present in cases of hematuria).
In addition to red plasma and urine, hemoglobinemia may also be identified by increased MCH or MCHC values on a CBC. This is because the hemoglobin concentration is measured by lysing all erythrocytes in the sample and then measuring the total hemoglobin via spectrophotometry. By this method, hemoglobin that originated within or outside of erythrocytes is measured together. However, calculations for MCH and MCHC, which include results for the hemoglobin and red blood cell concentrations, assume that all of the hemoglobin originated within erythrocytes. In the case of hemoglobinemia, the excess extracellular hemoglobin may cause an artifactual increase in the calculated MCH and MCHC. It is important to remember that similar artifactual increases may also occur with lipemia.
Once hemolytic anemia has been identified, the specific cause for hemolysis should be investigated based on signalment, clinical history, and microscopic blood smear evaluation. The most common causes of hemolytic anemia in domestic animals are immune-mediated, infectious, oxidative, and mechanical fragmentation (i.e., microangiopathic) disorders (Table 13-3 ).
Table 13-3.
Four Common Causes of Hemolytic Anemia, and Their Main Hematologic Characteristics
| Immune-Mediated | Infectious | Oxidative | Mechanical Fragmentation |
|---|---|---|---|
|
|
|
|
Spherocytosis and autoagglutination are hallmarks of immune-mediated hemolytic anemia, either primary (also known as idiopathic) or secondary to infectious disease, drugs/toxins, or neoplasms. Spherocytes form when macrophages (mainly in the spleen) phagocytize part of an erythrocyte plasma membrane bound with autoantibody (Fig. 13-12 ). The remaining portion of the erythrocyte assumes a spherical shape, thus preserving maximal volume. This change in shape results in decreased deformability of the cells. Erythrocytes must be extremely pliable to traverse the splenic red pulp and sinusoidal walls; spherocytes therefore tend to be retained in the spleen in close association with macrophages with risk for further injury and eventual destruction. In the dog, spherocytes appear smaller than normal and have uniform staining (Fig. 13-13, A ), in contrast to normal erythrocytes, which have a region of central pallor imparted by their biconcave shape. This difference in staining between spherocytes and normal erythrocytes is not consistently discernible in many other domestic animals (including horses, cattle, and cats), whose erythrocytes differ from those of the dog in that they are smaller and have less pronounced biconcavity and therefore less pronounced central pallor. Autoagglutination occurs because of cross-linking of antibodies bound to erythrocytes (see Fig 13-12). Autoagglutination is evident macroscopically as blood with a grainy consistency (see Fig. 13-13, B), and microscopically as clusters of erythrocytes (see Fig. 13-13, C). Autoagglutination may also result in a falsely increased MCV and decreased red blood cell concentration when clustered cells are mistakenly counted as single cells by automated hematology analyzers. When autoagglutination is present, the packed-cell volume is the most reliable measurement of red blood cell mass.
Figure 13-12.
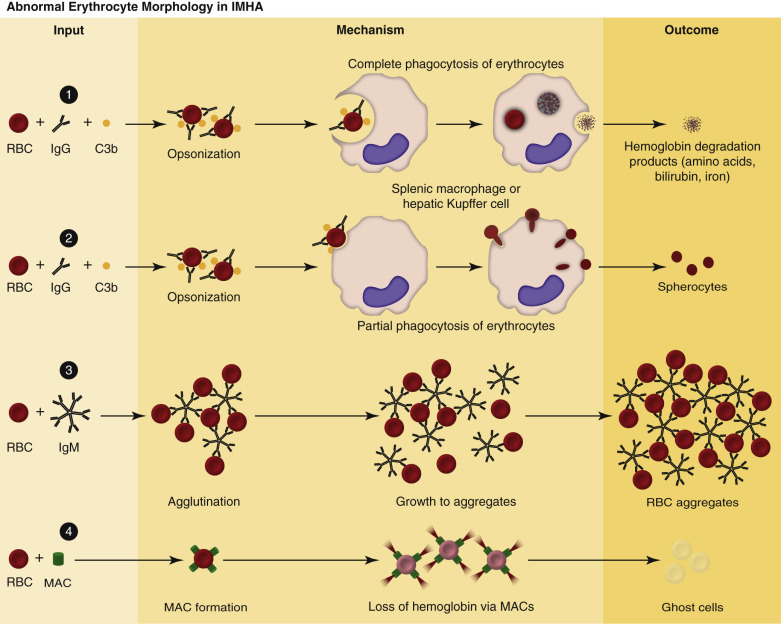
Pathogenesis of Abnormal Erythrocyte Morphologic Changes in Immune-Mediated Hemolytic Anemia.
1, Red blood cell (RBC) degradation. Antierythrocyte antibodies bind RBC surface antigens, resulting in RBC opsonization by immunoglobulins (mainly immunoglobulin G [IgG]) and complement (primarily C3b). Immunoglobulin- or C3b-bound RBCs are phagocytized and digested by sinusoidal macrophages. 2, Spherocytes. Spherocytes form when the membrane of immunoglobulin- or C3b-bound RBCs are phagocytized by macrophages, without removing the entire RBC from circulation. Compared to normal erythrocytes, spherocytes appear smaller, more eosinophilic, and lack central pallor. 3, RBC aggregation (agglutination). RBC aggregation occurs when antierythrocyte immunoglobulins (immunoglobulin M [IgM] or high concentrations of IgG) bind multiple erythrocytes simultaneously. 4, Ghost cells. Antierythrocyte antibodies bind RBC surface antigens, resulting in complement activation and formation of the membrane attack complex (MAC). MACs form membrane pores, resulting in rupture of RBCs, and the release of hemoglobin into the circulation. Ghost cells are RBC membrane remnants that lack cytoplasm (hemoglobin).
(Courtesy Dr. K.M. Boes, College of Veterinary Medicine, Virginia Polytechnic Institute and State University; and Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
Figure 13-13.

Immune-Mediated Hemolytic Anemia, Canine Blood, Dog.
A, Spherocytosis. Numerous spherocytes, several ghost cells, and one polychromatophil. Wright-Giemsa stain. B, Macroscopic autoagglutination. Note the grossly visible agglutination. C, Microscopic agglutination. Note the grapelike cluster of erythrocytes. Wright-Giemsa stain. (Courtesy Dr. K.M. Boes, College of Veterinary Medicine, Virginia Polytechnic Institute and State University.)
Ghost cells are ruptured red blood cell membranes devoid of cytoplasmic contents (see Figs. 13-12 and 13-13, A). They indicate intravascular hemolysis and may be seen with a variety of hemolytic disorders, including those with immune-mediated, infectious, oxidative, or fragmentation causes. In the case of immune-mediated hemolytic anemia, antibody or complement binds to red blood cell membranes and activates the complement membrane attack complex (see Fig. 13-12). This causes pore formation in the red blood cell membrane and release of cytoplasmic contents into the plasma. Ghost cells are eventually cleared from circulation by phagocytic macrophages, mainly within the spleen.
Oxidative damage to erythrocytes occurs when normal antioxidative pathways that generate reducing agents (such as reduced nicotinamide adenine dinucleotide [NADH], reduced nicotinamide adenine dinucleotide phosphate [NADPH], and reduced glutathione [GSH]) are compromised or overwhelmed, resulting in hemolytic anemia, abnormal hemoglobin function, or both. Hemolysis caused by oxidative damage may be extravascular or intravascular, or a combination. Evidence of oxidative damage to erythrocytes may be apparent on blood smear examination as Heinz bodies or eccentrocytes or on gross examination as methemoglobinemia.
Heinz bodies are foci of denatured globin that interact with the erythrocyte membrane. They are usually subtly evident on routine Wright-stained blood smears as pale circular inclusions or blunt, rounded protrusions of the cell margin but are readily discernible on smears stained with new methylene blue (Fig. 13-14 ). Cats are particularly susceptible to Heinz body formation and may have low numbers of Heinz bodies normally. There is no unanimity of opinion, but some clinical pathologists believe that the presence of Heinz bodies in up to 10% of all erythrocytes in cats is within normal limits. This predisposition is believed to reflect unique features of the feline erythrocyte, whose hemoglobin has more sulfhydryl groups (preferential sites for oxidative damage) than do erythrocytes of other species and may also have lower intrinsic reducing capacity. It is also possible that the feline spleen does not have as efficient a “pitting” function (splenic structure and function are discussed in more detail later in this chapter).
Figure 13-14.
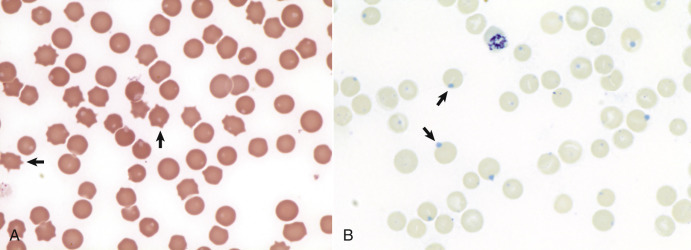
Heinz Bodies, Blood Smears.
A, Feline blood smear. With routine staining, Heinz bodies appear as pale circular intraerythrocytic inclusions that may protrude (arrows) from the margin of the cell. Wright's stain. B, Canine blood smear. Using a supravital stain, Heinz bodies are blue inclusions (arrows) and easier to see. New methylene blue stain.
(Courtesy Dr. M.M. Fry, College of Veterinary Medicine, University of Tennessee.)
Eccentrocytes, evident as erythrocytes in which one side of the cell has increased pallor (Fig. 13-15, A ), are another manifestation of oxidative damage. They form because of cross-linking of membrane proteins, with adhesion of opposing areas of the cell's inner membrane leaflet, and displacement of most of the hemoglobin toward the other side. The fused membranes may fragment off of the eccentrocyte, leaving a slightly ruffled border; this cellular morphologic abnormality is called a pyknocyte (see Fig. 13-15, B).
Figure 13-15.
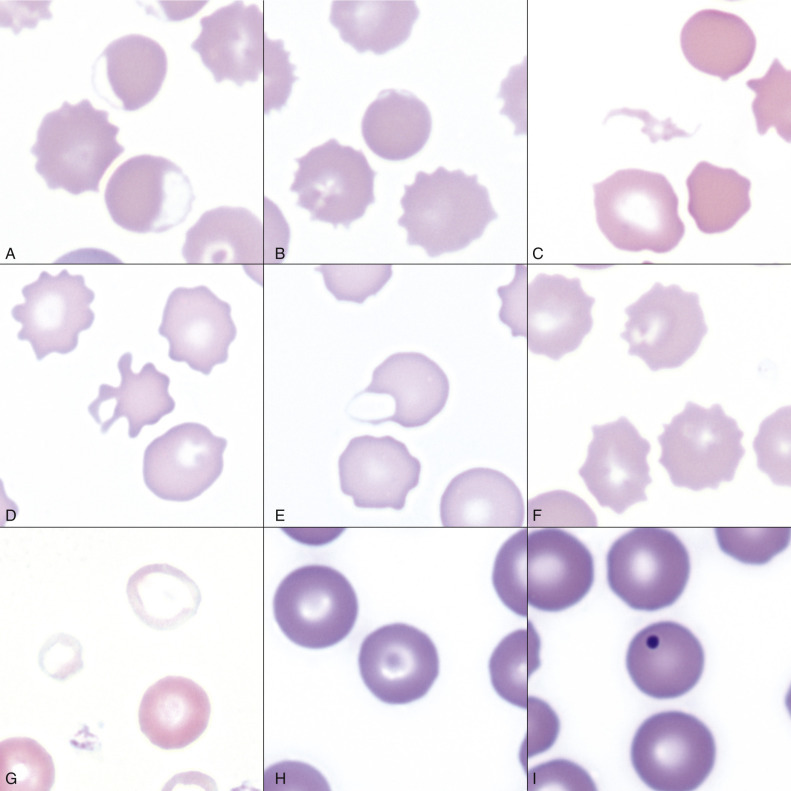
Common Erythrocyte Morphologic Abnormalities.
A, Blood from a dog that was administered a continuous rate infusion of propofol. The dog developed oxidant-induced hemolytic anemia with eccentrocytes. Wright-Giemsa stain. B, Blood from the same dog as in A, showing a pyknocyte. Note the spherocyte-like appearance of the pyknocyte, except for a small portion of the red blood cell membrane that is ruffled. Wright-Giemsa stain. C, A schistocyte in the blood of a dog with mechanical fragmentation hemolysis from disseminated intravascular coagulation. Wright-Giemsa stain. D, Blood from a dog with hemangiosarcoma, showing an acanthocyte. Wright-Giemsa stain. E, A keratocyte, exhibiting what appears to be a ruptured “vesicle” in blood from a dog. Wright-Giemsa stain. F, Blood from a dog with crenation artifact showing echinocytes. Wright-Giemsa stain. G, Blood from a dog with iron deficiency anemia. Note the patient's microcytic and hypochromic cell (left) and the normocytic hypochromic cell (top), as well as the normocytic normochromic erythrocyte (bottom right) from a recent blood transfusion. Wright-Giemsa stain. H, Blood from a dog. The center erythrocyte is a target cell, or codocyte. Wright-Giemsa stain. I, Blood from a dog shows a Howell-Jolly body, which is round, deeply basophilic remnant of the erythrocyte's nucleus. Wright-Giemsa stain.
(Courtesy Dr. K.M. Boes, College of Veterinary Medicine, Virginia Polytechnic Institute and State University.)
Oxidative insult may also result in conversion of hemoglobin (iron in the Fe2+ state) to methemoglobin (iron in the Fe3+ state), which is incapable of binding oxygen. Methemoglobin is produced normally in small amounts but reduced back to oxyhemoglobin by the enzyme cytochrome-b 5 reductase (also known as methemoglobin reductase). Methemoglobinemia results when methemoglobin is produced in excessive amounts (because of oxidative insult) or when the normal pathways for maintaining hemoglobin in the Fe2+ state are impaired (as in cytochrome-b 5 reductase deficiency). When present in sufficiently high concentration (approximately 10% of total hemoglobin), methemoglobin imparts a grossly discernible chocolate color to the blood.
By itself, mechanical fragmentation hemolysis tends to cause mild or no anemia. Mechanical fragmentation results from trauma or shearing of erythrocytes within blood vessels. Normal erythrocytes may be flowing through abnormal vasculature, such as with heart valve defects, intravascular fibrin deposition (e.g., disseminated intravascular coagulation), vasculitis, or hemangiosarcoma. Alternatively, the red blood cells may be particularly fragile within normal blood vasculature, as occurs with iron deficiency. In either instance, microscopic evidence of mechanical fragmentation includes the presence of erythrocyte fragments (schistocytes [see Fig. 13-15, C]), erythrocytes with irregular cytoplasmic projections (acanthocytes), erythrocytes with blister-like projections (keratocytes), or ghost cells (see Figs. 13-13, A, 13-15, D, and 13-15, E). Schistocytes are the only red blood cell morphologic abnormality specific for mechanical fragmentation because all other morphologic abnormalities can be seen with other disease processes. For example, ghost cells may be observed with other types of hemolysis.
Nonregenerative anemia is characterized by a lack of reticulocytosis on the CBC; however, reticulocytosis does not occur in horses even in the context of regeneration. Most often this is a result of decreased production in the marrow (i.e., erythroid hypoplasia). Erythrocytes circulate for a long time, so anemias caused by decreased production tend to develop slowly.
The most common form of nonregenerative anemia is known as anemia of inflammation or anemia of chronic disease. In this form of anemia, erythrocytes are decreased in number but are typically normal in size and hemoglobin concentration (so-called normocytic, normochromic anemia). It has long been known that patients with inflammatory or other chronic disease often become anemic, and that this condition results in increased iron stores in the bone marrow. Sequestration of iron may be a bacteriostatic evolutionary adaptation because many bacteria require iron as a cofactor for growth. In recent years, investigators have begun to elucidate the molecular mechanisms underlying anemia of inflammation. Hepcidin, an acute phase protein and antimicrobial peptide synthesized in the liver, is a key mediator that limits iron availability. Hepcidin expression increases with inflammation, infection, or iron overload and decreases with anemia or hypoxia. Hepcidin exerts its effects by causing functional iron deficiency. It binds to and causes the degradation of the cell surface iron efflux molecule, ferroportin, thus inhibiting both absorption of dietary iron from the intestinal epithelium and export of iron from macrophages and hepatocytes into the plasma (Fig. 13-16 ).
Figure 13-16.
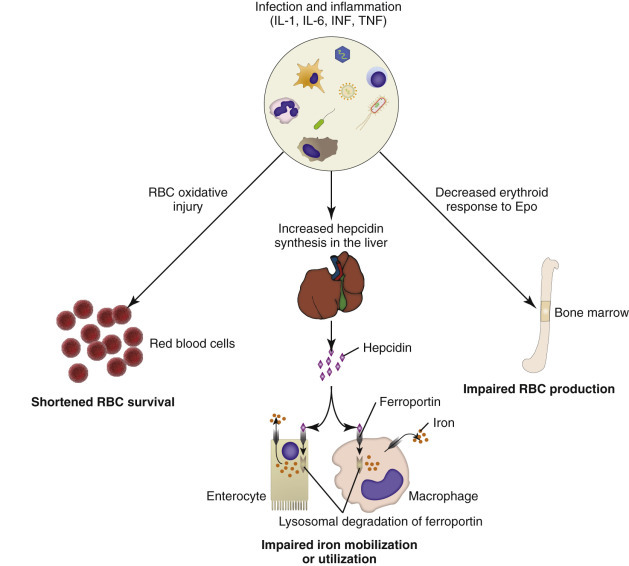
Mechanisms of Anemia in Inflammatory Diseases.
Inflammatory mediators, including interleukin-1 (IL-1), interleukin-6 (IL-6), interferon (INF), and tumor necrosis factor (TNF), cause anemia of inflammatory disease due to oxidative hemolysis, iron sequestration within enterocytes and macrophages, and impaired erythroid responsiveness to erythropoietin (Epo). During homeostasis the membrane transport molecule, ferroportin, transports iron from the cytosol to the extracellular space. The iron is then used for various physiologic processes, including hemoglobin production within bone marrow erythroid precursors. During times of inflammation the liver increases production of hepcidin, which binds ferroportin and causes its internalization and lysosomal degradation. With fewer membrane ferroportin molecules, less iron is absorbed from the diet and mobilized from macrophages. RBC, Red blood cell.
(Courtesy Dr. K.M. Boes, College of Veterinary Medicine, Virginia Polytechnic Institute and State University; and Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
Anemia of inflammation involves factors besides decreased iron availability. Inflammatory cytokines are likely to inhibit erythropoiesis by oxidative damage to and triggering apoptosis of developing erythroid cells, by decreasing expression of Epo and stem cell factor, and by decreasing expression of Epo receptors. In addition, experimentally induced sterile inflammation in cats resulted in shortened erythrocyte survival, indicating that anemia of inflammation is likely also a function of increased erythrocyte destruction.
Other causes of decreased erythropoiesis are listed in Table 13-2. Specific examples of diseases causing nonregenerative anemia by these mechanisms are discussed later in this chapter.
Neutropenia.
Neutropenia refers to a decrease in the concentration of neutrophils in circulating blood. Neutropenia may be caused by decreased production, increased destruction, altered distribution, or a demand for neutrophils in tissues that exceeds the rate of granulopoiesis.
Decreased production is evident on bone marrow examination as granulocytic hypoplasia. This usually results from an insult that affects multiple hematopoietic lineages, such as chemical insult, radiation, neoplasia, infection, or fibrosis, but may also be caused by a process that preferentially targets granulopoiesis. In marked contrast to erythrocytes, neutrophils have a very short life span in circulation. Once released from the bone marrow, a neutrophil is in the bloodstream only for hours before migrating into the tissues. When neutrophil production ceases, a reserve of mature neutrophils in the bone marrow storage pool may be adequate to maintain normal numbers of circulating neutrophils for a few days; however, after the bone marrow storage pool is depleted, neutropenia rapidly ensues.
Immune-mediated neutropenia is a rare but recognized condition in domestic animals. Bone marrow findings range from granulocytic hypoplasia to hyperplasia, depending on where the cells under immune attack are in their differentiation programs. Neutropenia with no evidence of decreased production and in which other causes of neutropenia have been excluded may be a result of destruction of neutrophils before they leave the bone marrow, a condition known as ineffective granulopoiesis. Like other forms of ineffective hematopoiesis, this condition is often presumed to be immune mediated; in cats this condition may occur as a result of infection of hematopoietic cells with FeLV.
As presented in the earlier section on Granulopoiesis and Monocytopoiesis (Myelopoiesis), neutrophils within the blood vasculature are in two compartments: a circulating pool, consisting of those cells flowing freely in the blood, and a marginating pool, consisting of those cells transiently interacting with the endothelial surface. (In reality, neutrophils are constantly shifting between these two pools, but the proportion of cells in either pool normally remains fairly constant in any given species.) Circulating neutrophils are part of the blood sample collected during routine venipuncture and are thus counted in the CBC, whereas marginating neutrophils are not. Pseudoneutropenia refers to the situation in which there is an increased proportion of neutrophils in the marginating pool. This may occur because of decreased blood flow or in response to stimuli, such as endotoxemia, that increase expression of molecules promoting interaction between neutrophils and endothelial cells. This mechanism of neutropenia is rarely observed in clinical practice.
Neutropenia may also result from increased demand for neutrophils in the tissue. How rapidly such a situation develops depends not only on the magnitude of the inflammatory stimulus but also on the reserve of postmitotic neutrophils in the bone marrow. The size of this reserve, or storage pool, is species dependent. In dogs this pool contains the equivalent of 5 days' normal production of neutrophils. Cattle represent the other extreme in that they have a small storage pool and thus are predisposed to becoming neutropenic during times of acute inflammation. Horses and cats are somewhere between the two extremes, closer to cattle and dogs, respectively. It stands to reason that the clinical significance of neutropenia because of a supply and demand imbalance is also species dependent. In dogs, neutropenia as a result of inflammation is an alarming finding because it is evidence of a massive tissue demand for neutrophils that has exhausted the patient's storage pool and is exceeding the rate of granulopoiesis in the bone marrow. However in cows, neutropenia is commonly noted in a wide range of conditions involving acute inflammation and does not necessarily indicate an overwhelming demand.
Eosinopenia/Basopenia.
Eosinopenia and basopenia are decreased concentrations of blood eosinophils and basophils, respectively. In many laboratories, CBC reference values for eosinophils and basophils are as low as zero cells per microliter, precluding detection of eosinopenia or basopenia. When detectable, eosinopenia is often a result of stress (i.e., glucocorticoid mediated).
Monocytopenia.
Monocytopenia denotes a decreased concentration of monocytes in blood; it is of little to no pathologic significance by itself.
Thrombocytopenia.
Thrombocytopenia refers to a decrease in the concentration of circulating platelets. Mechanisms of thrombocytopenia include decreased production, increased destruction, increased consumption, and altered distribution.
Decreased production may occur because of a condition affecting cells of multiple hematopoietic lineages, including megakaryocytes, or because of one specifically depressing thrombopoiesis. In either case, decreased thrombopoiesis is evident as megakaryocytic hypoplasia upon bone marrow examination. General causes of decreased hematopoiesis outlined earlier in the sections on anemia and neutropenia also apply to thrombocytopenia.
Increased platelet destruction due to immune-mediated thrombocytopenia (IMTP) is a fairly common disease in dogs and may also occur in other species. Thrombocytopenia with immune-mediated thrombocytopenia is often severe (e.g., <10,000 platelets/µL), resulting in spontaneous multisystemic hemorrhage.
Increased use of platelets occurs with hemorrhage and disseminated intravascular coagulation. Thrombocytopenia secondary to hemorrhage is often mild to moderate, whereas disseminated intravascular coagulation may cause mild to severe thrombocytopenia, often with evidence of mechanical fragmentation hemolysis (e.g., schistocytes). Disseminated intravascular coagulation is a syndrome in which hypercoagulability leads to increased consumption of both platelets and coagulation factors in the plasma, with subsequent hypocoagulability and susceptibility to bleeding. Risk factors for developing disseminated intravascular coagulation include severe inflammation, such as sepsis or pancreatitis, neoplasia, and organ failure.
The spleen normally contains a significant proportion of total platelet mass (up to one-third in some species), and abnormalities involving the spleen may result in changes in the number of circulating platelets. For example, splenic congestion may result in platelet sequestration and thrombocytopenia, and splenic contraction may cause thrombocytosis.
Lymphopenia.
Lymphopenia refers to a decreased concentration of lymphocytes in blood. It is a common hematologic finding in sick animals. Usually the precise mechanism of lymphopenia is not clear but is often presumed secondary to endogenous glucocorticoid excess that occurs with stress. Excess glucocorticoids, either endogenous or exogenous, cause an altered distribution of lymphocytes; there is increased trafficking of lymphocytes from blood to lymphoid tissue, and decreased egress of lymphocytes from lymphoid tissue to blood. At higher concentrations of glucocorticoids, lymphocytes are destroyed. Other causes of lymphotoxicity include chemotherapeutic agents, radiation therapy, and some infectious agents. Lymphopenia may occur with various mechanisms, including loss of lymphocyte-rich lymphatic fluid (e.g., gastrointestinal disease, repeated drainage of chylous effusions), and disruption of the normal lymphoid tissue architecture because of generalized lymphadenopathy (e.g., lymphoma, blastomycosis). Some hereditary immunodeficiencies, such as severe combined immunodeficiency or thymic aplasia, can cause lymphopenia due to lymphoid aplasia.
Erythrocytosis.
An increase in the measured red cell mass above the normal range is known as erythrocytosis. The term polycythemia is often used interchangeably with erythrocytosis, but technically and for the purposes of this chapter, polycythemia refers to a specific type of leukemia called primary erythrocytosis or polycythemia vera.
Causes of erythrocytosis are either relative or absolute. Relative erythrocytosis results from a fluid deficit or an altered distribution of erythrocytes within the body (i.e., the body's total erythrocyte mass is not increased). It occurs most frequently with dehydration, when the decreased proportion of water in the blood results in hemoconcentration. It is observed less frequently with epinephrine-mediated splenic contraction, wherein erythrocytes move from the spleen into peripheral circulation. Erythrocytosis from splenic contraction occurs to the most pronounced degree in horses and cats, especially in young, healthy animals.
Absolute erythrocytosis is a true increase in red blood cell mass due to erythroid neoplasia or hyperplasia and includes causes of primary and secondary erythrocytosis. Primary erythrocytosis, or polycythemia vera, is a neoplastic proliferation of erythroid cells with a predominance of mature erythrocytes. Diagnosis is based on a marked increase in red cell mass (hematocrit in normally hydrated dogs ranges from 65% to >80%), an absence of hypoxemia, an absence of other tumors, and a normal or decreased plasma Epo concentration.
Secondary erythrocytosis refers to Epo-mediated erythroid hyperplasia causing an increased red blood cell mass. The erythroid hyperplasia may be an appropriate response to chronic hypoxia, such as occurs with right-to-left cardiac shunts or chronic pulmonary disease. Rarely, an Epo-secreting tumor may cause inappropriately elevated levels of Epo in the absence of hypoxia.
Absolute erythrocytosis, whether primary or secondary, causes increased viscosity of the blood, resulting in impaired blood flow and microvasculature distention. Affected individuals are at increased risk for tissue hypoxia, thrombosis, and hemorrhage. Clinical signs of hyperviscosity syndrome may include erythematous mucous membranes (Fig. 13-17 ), prolonged capillary refill time, prominent scleral vessels, evidence of thrombosis or hemorrhage, and secondary signs related to specific organ systems affected (e.g., neurologic and cardiovascular signs).
Figure 13-17.
Absolute Erythrocytosis, Hyperviscosity Syndrome, Erythematous Mucous Membranes, Cat.
Erythema of mucous membranes is one of the signs associated with hyperviscosity syndrome. In this case the oral mucous membranes are deeper red (arrows) than normal because of an abnormally high concentration of erythrocytes and associated sludging of blood. Hyperviscosity syndrome may also occur as the result of increased plasma immunoglobulin concentration.
(Courtesy Dr. C. Patrick Ryan, Veterinary Public Health, Los Angeles Department of Health Services; and Noah's Arkive, College of Veterinary Medicine, The University of Georgia.)
Neutrophilia.
Neutrophilia, an increased blood concentration of neutrophils, occurs in response to a number of different stimuli, which are not mutually exclusive. Major mechanisms of neutrophilia are shown in Fig. 13-18 . Understanding the CBC findings characteristic of these responses is an important part of clinical veterinary medicine. Inflammation can result in neutropenia, as discussed earlier, or neutrophilia, as discussed next. However, before moving on to a discussion of inflammatory neutrophilia and the so-called left shift, it is important to mention two other common causes of neutrophilia: glucocorticoid excess and epinephrine excess. Less common causes of neutrophilia, such as leukocyte adhesion deficiency and neoplasia, are discussed later in the chapter.
Figure 13-18.
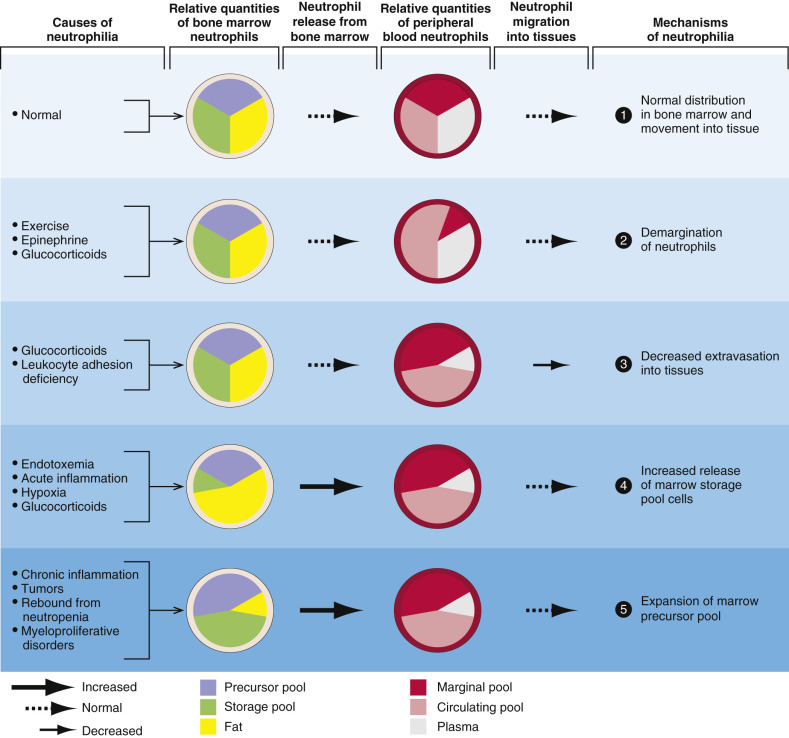
Mechanisms of Neutrophilic Leukocytosis.
1, Neutrophils and their precursors are distributed in five pools: a bone marrow precursor pool, which includes mitotically active and inactive immature cells; a bone marrow storage pool, consisting of mitotically inactive mature neutrophils; a peripheral blood marginating pool; a peripheral blood circulating pool; and a tissue pool. The relative size of each pool is represented by the size of its corresponding wedge. The peripheral blood neutrophil count measures only neutrophils within the circulating peripheral blood pool, which can be enlarged by (2) increased demargination, (3) diminished extravasation into tissue, (4) increased release of cells from the marrow storage pool, and (5) expansion of the marrow precursor pool.
(Courtesy Dr. K.M. Boes, College of Veterinary Medicine, Virginia Polytechnic Institute and State University; and Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
Glucocorticoid excess, either because of endogenous production or exogenous administration, results in a CBC pattern known as the stress leukogram, characterized by mature neutrophilia (i.e., increased concentration of segmented neutrophils without immature neutrophils) and lymphopenia, with or without monocytosis and eosinopenia. Mechanisms contributing to glucocorticoid-mediated neutrophilia include the following:
-
•
Increased release of mature neutrophils from the bone marrow storage pool
-
•
Decreased margination of neutrophils within the vasculature, with a resulting increase in the circulating pool
-
•
Decreased migration of neutrophils from the bloodstream into tissues
The magnitude of neutrophilia tends to be species dependent, with dogs having the most pronounced response (up to 35,000 cells/µL) and in decreasing order of responsiveness, cats (30,000 cells/µL), horses (20,000 cells/µL), and cattle (15,000 cells/µL) having less marked responses. With long-term glucocorticoid excess, neutrophil numbers tend to normalize, whereas lymphopenia persists.
Epinephrine release results in a different pattern, known as physiologic leukocytosis or excitement leukocytosis, characterized by mature neutrophilia (like the glucocorticoid response) and lymphocytosis (unlike the glucocorticoid response). This phenomenon is short lived (i.e., <1 hour). Neutrophilia occurs primarily because of a shift of cells from the marginating to the circulating pool. Physiologic leukocytosis is common in cats (especially when they are highly stressed during blood collection) and horses, less common in cattle, and uncommon in dogs.
Of course, neutrophilia may also indicate inflammation, and inflammatory stimuli of varying magnitude and duration produce different patterns of neutrophilia. A classic hematologic finding in patients with increased demand for neutrophils is the presence of immature forms in the blood, known as a left shift. Not all inflammatory responses have a left shift, but the presence of a left shift almost always signifies active demand for neutrophils in the tissue. The magnitude of a left shift is assessed by the number of immature cells and their degree of immaturity. The mildest form is characterized by increased numbers of band neutrophils, the immediate predecessor to the segmented neutrophil normally found in circulation. Progressively immature predecessors are seen with increasingly severe inflammation. A left shift is considered orderly if the number of immature neutrophils in circulation decreases as they become progressively immature. The term degenerative left shift is sometimes used to describe cases in which the number of immature forms exceeds the number of segmented neutrophils. As with glucocorticoid-mediated neutrophilia, the typical magnitude of neutrophilia caused by inflammation varies by species, with dogs having the most pronounced response.
It might be useful to think of neutrophil kinetics in terms of a producer-consumer model in which the bone marrow is the factory, and the tissues (where the neutrophils eventually go) are the customers. The bone marrow storage pool is the factory inventory, and the neutrophils in the bloodstream are in delivery to the customer. Within the blood vessels, circulating neutrophils are on the highway, and marginating neutrophils are temporarily pulled off to the side of the road. During health, there is an even flow of neutrophils from the factory to the customer. Thus the system is in steady state, and neutrophil numbers remain relatively constant and within the normal range. However, disease states may perturb this system at multiple levels. Decreased granulopoiesis is analogous to a factory working below normal production level. Ineffective granulopoiesis is analogous to goods that are produced at a normal to increased rate but are damaged during manufacturing and never leave the factory. A left shift is analogous to the factory meeting increased customer demand by shipping out unfinished goods. Cases of persistent, established inflammation are characterized by bone marrow granulocytic hyperplasia and mature neutrophilia, analogous to a factory that has had time to adjust to increased demand and is meeting it more efficiently by increasing its output.
Eosinophilia/Basophilia.
Eosinophilia and basophilia are increased concentrations of blood eosinophils and basophils, respectively. They may occur with parasitism, hypersensitivity reactions, paraneoplastic responses (e.g., lymphoma, mast cell neoplasia, or leukemia), and nonparasitic infectious disease. Eosinophilia has also been documented with hypoadrenocorticism and rare idiopathic conditions (e.g., hypereosinophilic syndrome). Most cases of eosinophilia and basophilia are due to eosinophilic and basophilic hyperplasia within the bone marrow in response to inflammatory growth factors. However, cortisol deficiency is thought to cause eosinophilia in dogs with hypoadrenocorticism.
Monocytosis.
Monocytosis is an increased concentration of monocytes in blood. It most commonly occurs with excessive glucocorticoids or inflammation and uncommonly to rarely with monocytic leukemia, immune-mediated neutropenia, and cyclic hematopoiesis. With excessive endogenous or exogenous glucocorticoids, monocytes shift from the marginating pool to the circulating pool. This stress monocytosis is most common in dogs, less frequent in cats, and rare in horses and cattle. Inflammatory diseases cause monocytosis by cytokine-mediated monocytic hyperplasia in the bone marrow.
Thrombocytosis.
Thrombocytosis, or an increased concentration of platelets in the blood, is a relatively common, nonspecific finding in veterinary patients. In the vast majority of cases, thrombocytosis is reactive—a response to another, often apparently unrelated, disease process. Examples of conditions having reactive thrombocytosis include inflammatory and infectious diseases, iron deficiency, hemorrhage, endocrinopathies, and neoplasia. Factors that may contribute to reactive thrombocytosis include increased plasma concentration of thrombopoietin, inflammatory cytokines (e.g., IL-6), or catecholamines. Thrombocytosis may also occur as part of a regenerative response in patients recovering from thrombocytopenia, as a result of redistribution after splenic contraction, or within the several weeks after splenectomy. In these cases, thrombocytosis is transient. In the case of splenectomy, thrombocytosis may be marked but normalizes after several weeks. Because the body's total platelet mass regulates thrombopoiesis, and a significant portion of the platelet mass is normally in the spleen, it makes sense that splenectomized animals develop thrombocytosis. However, the reason that the number of circulating platelets normalizes in these individuals in the weeks after splenectomy is not clear. There is also a rare form of megakaryocytic leukemia known as essential thrombocythemia, which is characterized by marked thrombocytosis.
Lymphocytosis.
Lymphocytosis refers to an increase in the concentration of lymphocytes in blood circulation. There are several causes of lymphocytosis, including age, excessive epinephrine, chronic inflammation, hypoadrenocorticism, and lymphoid neoplasia; lymphoid neoplasms are presented later in the chapter. Young animals normally have higher concentrations of lymphocytes than older animals, and normal healthy young animals may have counts that exceed adult reference values. Because this is not pathologic lymphocytosis, but normal physiologic variation, it is often termed pseudolymphocytosis of young animals. As discussed earlier in the section on neutrophilia, lymphocytosis is also a feature of epinephrine-mediated physiologic leukocytosis, resulting from redistribution of lymphocytes from the blood marginating pool into the blood circulating pool. Epinephrine-mediated lymphocytosis may be more marked than neutrophilia, particularly in cats (lymphocyte counts of > 20,000/µL are not uncommon). Antigenic stimulation may result in lymphocytosis, which may be marked in rare cases (up to approximately 30,000/µL in dogs and 40,000/µL in cats); however, this is not usually the case, even when there is clear evidence of increased immunologic activity in lymphoid tissues. In cases of antigenic stimulation, it is common for a minority of lymphocytes to have “reactive” morphologic features—larger lymphocytes with more abundant, deeply basophilic cytoplasm and more open chromatin (Fig. 13-19 ). Just as glucocorticoid excess can cause lymphopenia, glucocorticoid deficiency (hypoadrenocorticism) can cause lymphocytosis, or lack of lymphopenia during conditions of stress that typically result in glucocorticoid-mediated lymphopenia.
Figure 13-19.
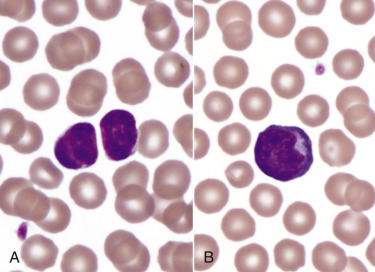
Lymphocytosis (B), Lymphocytes, Canine Blood Smear.
A, Small lymphocytes, the predominant type of lymphocyte in the blood under normal conditions. B, A reactive lymphocyte, characterized by mildly increased size and an increased amount of basophilic cytoplasm, from a recently vaccinated 16-week-old dog. Wright's stain.
(Courtesy Dr. M.M. Fry, College of Veterinary Medicine, University of Tennessee.)
A condition known as persistent lymphocytosis (PL) occurs in approximately 30% of cattle infected with the bovine leukemia virus (BLV). The condition is defined as an increase in the blood concentration of lymphocytes above the reference interval for at least 3 months. This form of lymphocytosis is a nonneoplastic proliferation (i.e., hyperplasia) of B lymphocytes. In the absence of other disease, cattle with persistent lymphocytosis are asymptomatic. However, cattle infected with BLV, especially those animals with persistent lymphocytosis, are at increased risk for developing B lymphocyte lymphoma.
Secondary Abnormal Structure or Function of Blood Cells.
The preceding section focused on abnormalities in the number of blood cells. There are also various acquired and congenital conditions involving abnormal structure or function of blood cells. This section briefly discusses abnormal blood cell structure or function occurring secondary to other underlying disease. Primary disorders of blood cells are discussed later in the chapter in the section on specific diseases.
Morphologic abnormalities detected on routine microscopic examination of blood smears may provide important clues about underlying disease processes. Poikilocytosis is a broad term referring to the presence of abnormally shaped erythrocytes in circulation. E-Table 13-1 lists conditions with and mechanisms involved in the formation of a number of specific types of erythrocyte morphologic abnormalities, and Fig. 13-15 shows some examples.
E-Table 13-1.
Common Erythrocyte Morphologic Abnormalities
| Term | Description | Common Causes or Conditions |
|---|---|---|
| Macrocyte | Abnormally large | Regenerative anemia Feline leukemia virus infection (cats) Congenital in some poodle dogs |
| Microcyte | Abnormally small | Iron deficiency Portosystemic shunts Normal in Akita and Shiba dogs |
| Polychromasia | Bluish color (see Figs. 13-5 and 13-8) | Reticulocytosis (erythroid hyperplasia) |
| Basophilic stippling | Fine punctate inclusions (see Fig. 13-8) | Lead toxicity Regenerative anemia (especially ruminants) |
| Howell-Jolly body | Small, round, blue-black inclusion, usually off center (see Fig. 13-15) | Regenerative anemia Splenic dysfunction Congenital in some poodle dogs |
| Hypochromasia | Increased central pallor (see Fig. 13-15) | Iron deficiency |
| Heinz body | Pale round inclusion, may bulge from cell margin; stains blue with new methylene blue (see Fig. 13-14) | Oxidative damage |
| Poikilocyte | Nonspecific term for shape abnormality—specific types of poikilocytosis listed below | See below |
| Spherocyte | Appears abnormally small with uniform staining (see Fig. 13-13) | Extravascular hemolysis |
| Schistocyte | Small, irregular RBC fragment, often crescent shaped (see Fig. 13-15) | Microangiopathies (e.g., disseminated intravascular coagulation, hemangiosarcoma, vasculitis) Increased fragility (e.g., iron deficiency) |
| Acanthocyte | Few irregular projections (see Fig. 13-15) | Abnormal lipid metabolism (e.g., liver disease) Microangiopathies (as per schistocytes) Increased fragility (as per schistocytes) Common normal finding in young goats |
| Echinocyte | Many relatively uniform projections (see Fig. 13-15) | Usually in vitro artifact Normal in pigs Some envenomations |
| Eccentrocyte | Eccentric staining (see Fig. 13-15) | Oxidative damage |
| Leptocyte | Thin, often hypochromic and/or folded | Reticulocytosis Iron deficiency |
| Codocyte (“target cell”) | Type of leptocyte with area of dense staining within central pallor (see Fig. 13-15) | Reticulocytosis Liver disease |
| Keratocyte | Intact or ruptured “blister” (see Fig. 13-15) | Conditions causing schistocytosis and/or acanthocytosis |
| Stomatocyte | Type of leptocyte with linear central pallor | Reticulocytosis |
RBC, Red blood cell.
The acquired neutrophil morphologic abnormality known as toxic change (Fig. 13-20 ) reflects accelerated production of neutrophils as part of the inflammatory response. Features of toxic change include increased cytoplasmic basophilia, the presence of small blue-gray cytoplasmic inclusions known as Döhle bodies (often noted incidentally in cats), and in more severe cases, cytoplasmic vacuolation. Although not causing impaired neutrophil function, toxic change occurs during granulopoiesis and thus is technically a form of dysplasia (e.g., Döhle bodies are foci of aggregated endoplasmic reticulum). Toxic change may accompany any inflammatory response, but in general the more marked the toxic change, the higher the index of suspicion for infection or endotoxemia. Other secondary changes to neutrophils may not be evident morphologically. For example, studies in human beings and dogs have shown that individuals with cancer have abnormal neutrophil function (including phagocytic activity, killing capacity, and oxidative burst activity) before initiation of therapy. The clinical significance of this finding is not clear.
Figure 13-20.
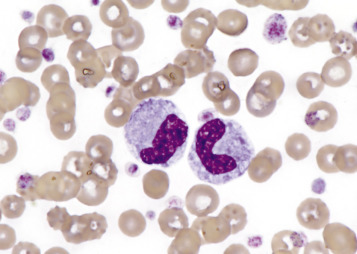
Toxic Change, Neutrophils, Canine Blood Smear.
Two band neutrophils with basophilic, foamy cytoplasm indicate toxic change. This dog also has reactive thrombocytosis. Wright's stain.
(Courtesy Dr. M.M. Fry, College of Veterinary Medicine, University of Tennessee.)
Platelet function disorders, also known as thrombopathies or thrombopathias, may be primary or secondary. Many conditions are known or suspected to cause secondary platelet dysfunction (hypofunction or hyperfunction) by altering platelet adhesion or aggregation or by mechanisms that are not fully understood. Box 13-2 shows underlying conditions having secondary platelet dysfunction.
Box 13-2. Conditions Known or Suspected to Cause Secondary Platelet Dysfunction in Animals.
Secondary Platelet Hypofunction
Underlying Disease
Uremia
Antiplatelet antibodies (also cause immune-mediated thrombocytopenia)
Infection (BVDV, FeLV)
Hyperglobulinemia
Increased fibrinolytic products
Hypoammonemia
Snake envenomation
Drugs or Other Exogenous Agents
Platelet inhibitors
NSAIDs—irreversible (aspirin) or reversible inhibition of cyclooxygenase
Colloidal plasma expanders (e.g., hydroxyethyl starch)
Other drugs and exogenous agents (many)
Secondary Platelet Hyperfunction
Underlying Disease
Infection (heartworm and RMSF in dogs, FIP, pasteurellosis in cattle)
Inflammation
Neoplasia
Taurine deficiency in cats
Nephrotic syndrome
BVDV, Bovine viral diarrhea virus; FeLV, feline leukemia virus; FIP, feline infectious peritonitis, NSAIDs, nonsteroidal antiinflammatory drugs; RMSF, Rocky Mountain spotted fever.
Portals of Entry/Pathways of Spread
Invading cells or microorganism gain access to the bone marrow or blood circulation either hematogenously or by trauma. Trauma may be as obvious as a gaping wound or as subtle as the bite of an insect. Portals of entry for the bone marrow are summarized in Box 13-3 . Diseases that arise from the bone marrow, such as leukemia, typically spread to other tissues hematogenously.
Box 13-3. Portals of Entry into Bone Marrow.
Bone Marrow
Hematogenously
Direct penetration (trauma)
Defense Mechanisms/Barrier Systems
The bone marrow is encased by a protective shell of cortical bone, and blood supply to the marrow provides access to systemic humoral and cellular defenses. Of course, leukocytes themselves function as an essential part of inflammation and immune function, as discussed briefly in the section on Granulopoiesis and Monocytopoiesis (Myelopoiesis) and in greater detail in Chapters 3 and 5.
Biochemical steps in the glycolytic pathway or linked to it generate antioxidant molecules that enable erythrocytes to withstand oxidative insults throughout their many days in circulation. In addition to producing energy in the form of adenosine triphosphate (ATP), glycolysis generates NADH, which helps convert the oxidized, nonfunctional form of hemoglobin, known as methemoglobin, back to its active, reduced state. Another antioxidant erythrocyte metabolic pathway, the pentose shunt or hexose monophosphate shunt, generates NADPH to help maintain glutathione in the reduced state.
Disorders of Domestic Animals
Aplastic Anemia (Aplastic Pancytopenia)
Aplastic anemia, or more accurately aplastic pancytopenia, is a rare condition characterized by aplasia or severe hypoplasia of all hematopoietic lineages in the bone marrow with resulting cytopenias. The term aplastic anemia is a misnomer because affected cells are not limited to the erythroid lineage.
Many of the conditions reported to cause aplastic anemia do so only rarely or idiosyncratically; more frequently, they cause other hematologic or nonhematologic abnormalities. A partial list of reported causes of aplastic anemia in domestic animals includes the following:
-
•Chemical agents
-
•Antimicrobial agents (dogs, cats)
-
•Chemotherapeutic agents (dogs, cats)
-
•Phenylbutazone (horses, dogs)
-
•Bracken fern (cattle, sheep)
-
•Estrogen (dogs)
-
•Trichloroethylene (cattle, sheep)
-
•Aflatoxin B1 (horses, cattle, dogs, pigs)
-
•
-
•Infectious agents
-
•Ehrlichia (Ehrlichiosis [dogs, cats])
-
•Parvovirus (dogs, cats)
-
•FeLV (cats)
-
•Feline immunodeficiency virus (cats)
-
•Lentivirus (Equine infectious anemia [horses])
-
•Idiopathic (horses, cattle, dogs, cats)
-
•
Most of these causes, especially the chemical agents, are directly cytotoxic to HSCs or progenitor cells, resulting in their destruction. However, another proposed mechanism is disruption of normal stem cell function because of mutation or perturbation of hematopoietic cells and/or their microenvironment. This pathogenesis is mostly recognized in retroviral infections.
Aplastic anemia occurs in both acute and chronic forms. Most of the chemical causes result in acute disease. Grossly, affected animals may show signs of multisystemic infection and hemorrhage due to severe neutropenia and thrombocytopenia, respectively. Severe neutropenia typically develops within 1 week of an acute insult to the bone marrow, and severe thrombocytopenia occurs in the second week. This sequence is a result of the circulating life spans of each cell type; in health, neutrophils have a blood half-life of 5 to 10 hours, whereas platelets circulate for 5 to 10 days. The development of signs of anemia, such as pale mucous membranes, is more variable. The presence and severity of anemia depends on how rapidly the marrow recovers from the insult and the erythrocyte life span of the particular species.
Microscopically, bone marrow is hypocellular with markedly reduced hematopoietic cells. Hematopoietic cells are replaced with adipose tissue, and there is a variable inflammatory infiltrate of lymphocytes, plasma cells, and macrophages. In addition, there may be necrosis, hematopoietic cell apoptosis, and an increase in phagocytic macrophages. Fig. 13-21 shows bone marrow aspirates from a dog with pancytopenia from acute 5-fluorouracil toxicosis, before and during recovery.
Figure 13-21.
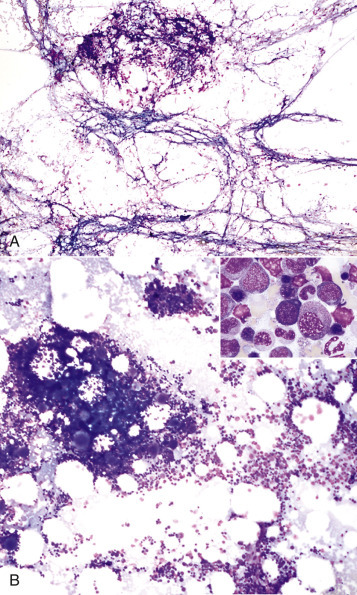
Aplastic Anemia, Canine Bone Marrow Aspirate.
A, Bone marrow aspirate from a dog 8 days after ingestion of a toxic dose of 5-fluorouracil shows stromal cells but a lack of developing blood cells. B, Bone marrow aspirate from the same dog 1 week later, after resumption of hematopoiesis. Inset, Higher magnification of Figure 13-21, B, shows early- and late-stage erythroid and granulocytic precursors. Wright's stain.
(Courtesy Dr. M.M. Fry, College of Veterinary Medicine, University of Tennessee.)
Congenital Disorders
Many inherited or presumably inherited disorders of blood cells have been recognized in domestic animals, including rare or sporadic cases and conditions that are of questionable clinical relevance. This section and the later sections covering species-specific disorders are not comprehensive but instead focus on the more common, well-characterized, or recently reported conditions.
Erythropoietic Porphyrias.
Porphyrias are a group of hereditary disorders in which porphyrins accumulate in the body because of defective heme synthesis. Inherited enzyme defects in hemoglobin synthesis have been identified in Holstein cattle, Siamese cats, and other cattle and cat breeds, resulting in bovine congenital erythropoietic porphyria and feline erythropoietic porphyria, respectively. Accumulation of toxic porphyrins in erythrocytes causes hemolytic anemia, whereas accumulation of porphyrins in tissues and fluids produces discoloration, including red-brown teeth, bones, and urine (see Fig. 1-59). Because of the circulation of the photodynamic porphyrins in blood, these animals have lesions of photosensitization of the nonpigmented skin. All affected tissues, including erythrocytes, exhibit fluorescence with ultraviolet light. Histologically, animals may exhibit perivascular dermatitis, as well as multisystemic porphyrin deposition, hemosiderosis, EMH, and marrow erythroid hyperplasia. Cats may show evidence of renal disease, including hypercellular glomeruli, thickened glomerular and tubular basement membranes, and tubular epithelial lipidosis, degeneration, and necrosis. Other porphyrias have been diagnosed in cattle, pigs, and cats but are not known to cause hemolytic anemia.
Pyruvate Kinase Deficiency.
Pyruvate kinase (PK) deficiency is an inherited autosomal recessive condition due to a defective R-type PK isoenzyme that is normally present in high concentrations in mature erythrocytes. To compensate for this deficiency, there is persistence of the M2-type PK isoenzyme, which is less stable than the R-type isoenzyme. The disease is reported in many dog breeds and fewer cat breeds (e.g., Abyssinian, Somali, and domestic shorthair). Erythrocyte PK deficiency results in decreased ATP production and shortened erythrocyte life spans. In dogs the hemolytic anemia is typically chronic, moderate to severe, extravascular, and strongly regenerative. With chronicity, hemolytic anemia causes enhanced intestinal absorption of iron and subsequent hemosiderosis, especially of the liver and bone marrow. Dogs typically die at 1 to 5 years of age of hemochromatosis-induced liver and bone marrow failure. However, cats with PK deficiency typically show no clinical signs, have milder anemia, and do not develop organ failure. Grossly, affected animals have lesions attributed to hemolytic anemia, including splenomegaly, pale mucous membranes, and rarely icterus. Dogs with end-stage disease have cirrhosis, myelofibrosis, and osteosclerosis. Dogs with PK deficiency do not necessarily have the same genetic defect, so mutation-specific DNA-based assays are required. In contrast, a single DNA-based test is available to detect the common mutation affecting Abyssinian, Somali, and domestic shorthair cats.
Cytochrome-b5 Reductase Deficiency.
Deficiency of cytochrome-b 5 reductase (Cb5R, also known as methemoglobin reductase), the enzyme that catalyzes the reduction of methemoglobin (Fe3+) to hemoglobin (Fe2+), has been recognized in many dog breeds and in domestic shorthair cats. It is probably an autosomal recessive trait. Affected animals may have cyanotic mucous membranes or exercise intolerance but usually lack anemia and clinical signs of disease. Life expectancies are normal.
Glucose-6-Phosphate Dehydrogenase Deficiency.
Deficiency of glucose-6-phosphate dehydrogenase (G6PD), the rate-controlling enzyme of the pentose phosphate pathway (PPP), has been reported in an American saddlebred colt, its dam, and one male dog. The PPP is an antioxidative pathway that generates NADPH, which maintains glutathione in its reduced form (GSH). Therefore in animals with G6PD deficiency, oxidants are not scavenged, and erythrocyte oxidative injury occurs. The colt with G6PD deficiency had severe oxidative hemolytic anemia with eccentrocytes on blood smear evaluation. However, the colt's dam only had eccentrocytes, and showed no hematologic signs of disease.
Leukocyte Adhesion Deficiency.
Leukocyte adhesion deficiency (LAD) is a fatal autosomal recessive defect of leukocyte integrins, in particular the β2 chain (also known as cluster of differentiation [CD] 18 [CD18]). Disease has been recognized in Holstein cattle (known as bovine leukocyte adhesion deficiency [BLAD]) and Irish setter dogs (known as canine leukocyte adhesion deficiency [CLAD]) (see Chapter 3). Without normal expression of this adhesion molecule, leukocytes have severely impaired abilities to migrate from the blood into tissues. As a result, animals with leukocyte adhesion deficiency have marked neutrophilia with nonsuppurative multisystemic infections. Blood neutrophils often have nuclei with greater than five nuclear segments, termed hypersegmented neutrophils, due to neutrophil aging within blood vessels (Fig. 13-22 ). These animals are highly susceptible to infections and usually die at a young age.
Figure 13-22.
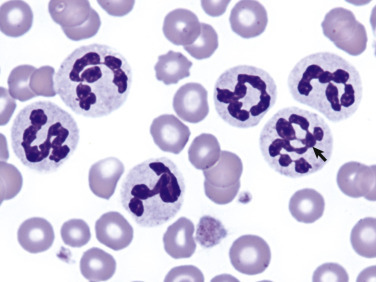
Leukocyte Adhesion Deficiency, Canine Blood Smear.
Neutrophils in animals with leukocyte adhesion deficiency cannot migrate into the tissues, resulting in marked neutrophilia and morphologic signs of aging, such as nuclear hypersegmentation (arrow). Wright-Giemsa stain.
(Courtesy Dr. K.M. Boes and Dr. K. Zimmerman, College of Veterinary Medicine, Virginia Polytechnic Institute and State University.)
Pelger-Huët Anomaly.
Pelger-Huët anomaly (PHA) is a condition of hyposegmented granulocytes due to a lamin B receptor mutation. It has been described in dogs, cats, horses, and rabbits, especially in certain breeds. In Australian shepherd dogs the mode of inheritance is autosomal dominant with incomplete penetrance. Most cases of Pelger-Huët anomaly are the heterozygous form and of no clinical significance. However, skeletal abnormalities, stillbirths, and/or early mortality may accompany Pelger-Huët anomaly in rabbits and cats, especially homozygotes. In Pelger-Huët anomaly the nuclei of neutrophils, eosinophils, and basophils fail to segment, resulting in band-shaped, bean-shaped, or round nuclei. Although the nuclear shape is similar to that of an inflammatory left shift, healthy animals with Pelger-Huët anomaly do not have clinical signs or other laboratory findings indicating inflammation. For example, neutrophils in healthy animals with Pelger-Huët anomaly have mature (clumped) chromatin and do not show signs of toxicity (Fig. 13-23 ). An acquired, reversible condition mimicking Pelger-Huët anomaly, known as pseudo–Pelger-Huët anomaly, is occasionally noted in animals with infectious disease, neoplasia, or drug administration.
Figure 13-23.
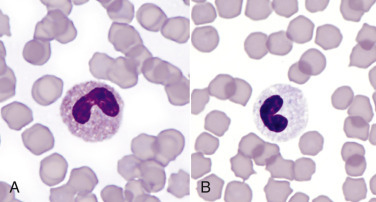
Pelger-Huët Anomaly, Feline Blood Smear.
Eosinophil (A) and neutrophil (B) have hyposegmented nuclei with mature, condensed chromatin. Wright's stain.
(Courtesy Dr. M.M. Fry, College of Veterinary Medicine, University of Tennessee.)
Chédiak-Higashi Syndrome.
Chédiak-Higashi syndrome (CHS) is a rare autosomal recessive defect in the lysosomal trafficking regulator (LYST) protein. The syndrome has been identified in Hereford, Brangus, and Japanese black cattle, Persian cats, and several nondomestic species. The defective LYST protein results in granule fusion in multiple cell types, including granulocytes, platelets, and melanocytes, as well as abnormal cell function. Individuals with Chédiak-Higashi syndrome have severely impaired cellular innate immunity because of neutropenia, impaired leukocyte chemotaxis, and impaired killing by granulocytes and cytotoxic lymphocytes. Platelets lack the dense granules that normally contain key bioactive molecules involved in hemostasis, including platelet agonists, such as ADP and serotonin. In vitro platelet aggregation is severely impaired. As a result, animals with Chédiak-Higashi syndrome exhibit oculocutaneous albinism (due to altered distribution of melanin granules) and are prone to infection and bleeding. Blood smear evaluation reveals granulocytes with large cytoplasmic granules.
Glanzmann Thrombasthenia.
Glanzmann thrombasthenia (GT) is an inherited platelet function defect caused by a mutated αIIb subunit of the integrin αIIbβ3 (also known as glycoprotein IIb-IIIa [GPIIb-IIIa]). The disorder has been recognized in Great Pyrenees and otterhound dogs and several horse breeds, including a quarter horse, a standardbred, a thoroughbred-cross, a Peruvian Paso mare, and an Oldenburg filly. The αIIbβ3 molecule has multiple functions but is best known as a fibrinogen receptor that is essential for normal platelet aggregation. Bleeding tendencies vary widely between affected individuals but mainly occur on mucosal surfaces. The condition is characterized by an in vitro lack of response to all platelet agonists and severely impaired clot retraction (i.e., whole blood samples without anticoagulant often fail to clot). Molecular testing is available to detect diseased or carrier states in dogs and horses.
CalDAG-GEFI Thrombopathia.
Calcium diacylglycerol guanine nucleotide exchange factor I (CalDAG-GEFI) is a molecule within the signaling pathway that results in platelet activation in response to platelet agonists. Mutated CalDAG-GEFI has been documented in basset hound, Eskimo spitz, and Landseer dogs, and Simmental cattle. All reported mutations have a bleeding tendency. In vitro platelet aggregation responses to platelet agonists, such as ADP, collagen, and thrombin, are absent or impaired.
von Willebrand Disease
von Willebrand disease (vWD) is the most common canine hereditary bleeding disorder and has also been described in many other domestic species. The disease actually refers to a group of inherited conditions characterized by a quantitative or qualitative deficiency of vWF. This factor is a multimeric glycoprotein that is stored in platelet α-granules and endothelial cells and circulates as a complex with coagulation factor VIII. Its primary functions are to stabilize factor VIII and mediate platelet binding to other platelets and subendothelial collagen. Although not technically a platelet disorder, von Willebrand disease is often classified as such because it results in a loss of normal platelet function. Different types of von Willebrand disease vary in terms of mode of inheritance and severity of clinical disease. Type I von Willebrand disease is characterized by low plasma vWF concentration but normal multimeric proportions and a mild to moderate clinical bleeding tendency; it has been reported in many dog breeds. Type II von Willebrand disease is characterized by low vWF concentration, absence of large multimers, and a moderate to severe bleeding tendency; it has been reported in German short-haired pointer and German wirehaired pointer dogs. Type III von Willebrand disease is characterized by absence of vWF and a severe bleeding tendency; familial and sporadic cases have been reported in numerous dog breeds. The buccal mucosal bleeding time is prolonged with von Willebrand disease, often with adequate concentrations of platelets and normal prothrombin time and partial thromboplastin time (PTT). However, PTT may be mildly prolonged because vWF stabilizes factor VIII, and deficiency of vWF results in enhanced factor VIII degradation. Grossly, affected animals exhibit bleeding tendencies, especially in the form of gingival bleeding, epistaxis, and hematuria or at sites of injections, venipuncture, or surgery.
Hereditary Coagulation Factor Deficiencies
Inherited coagulation factor deficiencies have been documented in most domestic species, including deficiencies of prekallikrein and factors I, II, VII, VIII, IX, X, XI, and XII. Of these disorders, hereditary coagulation factor VIII (hemophilia A) and factor IX (hemophilia B) deficiencies are most common. Hemophilia A has been recognized in horses, cattle, dogs, and cats, and hemophilia B occurs in dogs and cats. Both disorders have an X-linked recessive mode of inheritance, meaning that clinical disease is more common in males. Affected males have variable tendencies to bleed, depending on the severity of the deficiency, exposure to trauma, and size and activity level of the affected individual. Carrier females are usually asymptomatic. Laboratory tests often reveal adequate platelets, normal prothrombin times, and prolonged partial thromboplastin times.
Hereditary γ-Glutamyl Carboxylase Defect
Hereditary defects in γ-glutamyl carboxylase, the enzyme required for normal carboxylation of vitamin K–dependent coagulation factors, have been recognized in a flock of Rambouillet sheep and two Devon rex cats from the same litter. The genetic defect is not known in cats, but in sheep it is an autosomal recessive trait that results in a premature stop codon and truncated γ-glutamyl carboxylase. In sheep there is increased lamb mortality with excessive bleeding during parturition, especially through the umbilicus or into subcutaneous tissues.
Toxicoses
Oxidative Agents.
A variety of oxidative toxins cause hemolytic anemia and/or methemoglobinemia in domestic species. More common or well-characterized oxidants are listed here:
-
•
Horses—Acer rubrum (red maple)
-
•
Ruminants—Brassica spp. (cabbage, kale, and rape), copper
-
•
Dogs—Acetaminophen, propofol, zinc
-
•
Cats—Acetaminophen, propofol, propylene glycol
-
•
All species—Allium spp. (chives, garlic, and onions)
In horses, red maple leaves and bark are toxic, especially wilted or dried leaves. The toxic principle is believed to be gallic acid. Plants that contain high concentrations of nitrates, such as cabbage, kale, and rape, may cause oxidative injury to erythrocytes; cattle are more susceptible than sheep and goats. However, sheep are more prone to copper toxicosis relative to other ruminants. The condition occurs in animals that have chronically accumulated large amounts of copper in the liver through the diet. The copper is then acutely released during conditions of stress, such as shipping or starvation. Continuous rate infusions of the anesthetic propofol may cause oxidative hemolytic anemia in dogs and cats, but single or multiple single doses are not expected to cause clinical hemolysis. Zinc toxicosis has been identified in a wide range of animals; however, it is most common in dogs due to their indiscriminate eating habits. Common sources include pennies, batteries, paints, creams, automotive parts, screws, nuts, and coating on galvanized metals. Propylene glycol is an odorless, slightly sweet solvent and moistening agent in many foods, drugs, and tobacco products. Although it is “generally recognized as safe” for animal foods other than for cats by the Food and Drug Administration, it has been banned from cat food since 1996.
Grossly and microscopically, animals show varying signs of oxidative hemolysis and/or methemoglobinemia, as previously presented in the section discussing anemias (see Bone Marrow and Blood Cells, Dysfunction/Responses to Injury, Blood Cells, Abnormal Concentrations of Blood Cells, Anemia). In sheep with copper toxicosis, hemoglobinuric nephrosis, frequently described as gunmetal-colored kidneys with port wine–colored urine, is a classic postmortem lesion.
Snake Envenomation.
Hemolytic anemia from snake envenomation has been reported in horses, dogs, and cats. It is most commonly reported with viper and pit viper envenomations, including those from rattlesnakes. Hemolysins within viper venom directly injure erythrocytes, causing intravascular hemolysis. Other mechanisms of hemolysis include the action of phospholipase A2 on erythrocyte membranes and erythrocyte mechanical fragmentation due to intravascular coagulation and vasculitis. Nonhemolytic lesions depend on the venom's additional components and may include hemorrhage, paralysis, and/or tissue edema, inflammation, and necrosis. On blood smear evaluation, animals with snake envenomation may have ghost cells, spherocytes, and/or echinocytes (see Figs. 13-13 and 13-15).
Avitaminosis K
Antagonism of vitamin K leads to production of a nonfunctional form of some coagulation factors and resulting coagulopathy; a similar condition results from vitamin K deficiency. Conditions with avitaminosis K include poisoning with coumarin-related molecules, fat malabsorption (vitamin K is a fat-soluble vitamin) caused by primary intestinal disease or impaired biliary outflow (uncommon), dietary deficiency (rare), and antibiotics that interfere with vitamin K absorption or usage.
A number of coagulation factors—factors II, VII, IX, and X (collectively known as the vitamin K–dependent factors), as well as the regulatory molecules protein C and protein S—must undergo carboxylation to be functional. This posttranslational modification is catalyzed by the enzyme γ-glutamyl carboxylase, and requires vitamin K as a cofactor. Vitamin K is oxidized during the carboxylation reaction and is converted back into its active reduced form by the enzyme vitamin K epoxide reductase. Coumarin-related rodenticides, such as warfarin, act by inhibiting vitamin K epoxide reductase, resulting in an absence of vitamin K in its active reduced form (E-Fig. 13-2). This inhibition lasts until the rodenticide is metabolized and cleared. How long this takes depends on the half-life of the rodenticide and dose, but it may take many weeks. Second-generation rodenticides, such as bromadoline and brodifacoum, are more potent than warfarin, with longer half-lives. Spoiled sweet clover contains dicumarol, which causes coagulopathy by the same mechanism.
E-Figure 13-2.
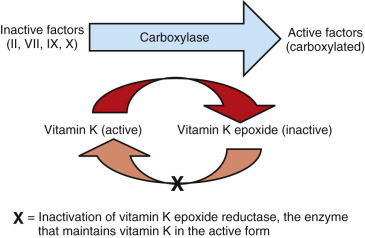
Mechanism of Anticoagulant Rodenticide Toxicity.
Anticoagulant rodenticides inhibit the enzyme that converts vitamin K back to its active reduced form.
Laboratory findings include prolonged coagulation times (prothrombin time [PT], PTT, and activated clotting time [ACT]). Early in the course of rodenticide and related toxicoses, PT may be the only one of these tests that is prolonged because factor VII has the shortest half-life of the vitamin K–dependent factors. However, the other tests become prolonged as nonfunctional forms of the other factors accumulate. In uncomplicated cases, patients are not thrombocytopenic.
A wide range of hemorrhagic lesions may occur in affected individuals, including ecchymoses, epistaxis, gingival bleeding, hematomas, hemoptysis, melena or hematochezia, hematuria, and other forms of hemorrhage. There are also lesions with regenerative anemia, such as pale mucous membranes and splenomegaly. Histologically, there is hemorrhage, EMH, and marrow erythroid hyperplasia.
The treatment of cases of rodenticide and related toxicoses is regular administration of exogenous vitamin K1 until the toxin is cleared (determined by repeat coagulation testing after withholding treatment).
Nutritional and Metabolic Disorders
Severe malnutrition is probably a cause of nonregenerative anemia in all species attributable to combined deficiencies of molecular building blocks, energy, and essential cofactors. By far the most commonly recognized specific deficiency that results in anemia is iron deficiency. Other specific nutritional deficiencies causing anemia in animals are uncommon or rare. Acquired cobalamin (vitamin B12) and folate deficiencies are recognized as causes of anemia in human beings but are rare in animals.
Iron Deficiency Anemia.
Iron deficiency is usually not a primary nutritional deficiency but rather occurs secondary to depletion of iron stores via chronic blood loss. The most common route of loss is through the gastrointestinal tract (e.g., neoplasia in older animals or hookworm infection in puppies). Chronic blood loss may also be caused by marked ectoparasitism (e.g., pediculosis in cattle or massive flea burden in kittens and puppies), neoplasia in locations other than the gastrointestinal tract (e.g., cutaneous hemangiosarcoma), coagulation disorders, and repeated phlebotomy of blood donor animals. Rapidly growing nursing animals may be iron deficient when compared with adults because milk is an iron-poor diet. In most cases this has little clinical significance (and in fact is normal). An important exception is piglets with no access to iron, which may cause anemia, failure to thrive, and increased mortality. Neonatal piglets are routinely given parenteral iron for this reason. Copper deficiency can cause iron deficiency in ruminants and may occur because of copper-deficient forage or impaired usage of copper by high dietary molybdenum or sulfate. It is believed that copper deficiency impairs production of ceruloplasmin, a copper-containing enzyme involved in gastrointestinal iron absorption.
Iron deficiency causes anemia by impaired hemoglobin synthesis. Iron is an essential component of hemoglobin, and when it is absent, hemoglobin synthesis is depressed. Because erythrocyte maturation is dependent upon obtaining a critical hemoglobin concentration, maturing erythroid precursors undergo additional cell divisions during iron-deficient states. These additional cell divisions result in small erythrocytes, termed microcytes (see Fig. 13-15, G). However, erythrocytes with low hemoglobin concentrations are produced when microcyte formation can no longer compensate for iron deficiency. The classic hematologic picture with iron deficiency anemia is microcytic (i.e., decreased MCV), hypochromic (i.e., decreased MCHC) anemia. Microcytes and hypochromasia (see Fig. 13-15, G) may also be discernible on blood smear examination as erythrocytes that are abnormally small and paler-staining, respectively. Early iron deficiency anemia is poorly regenerative, whereas continued hemorrhage and iron loss cause nonregenerative anemia. Additional hematologic changes may include evidence of erythrocyte mechanical fragmentation (e.g., schistocytes) and reactive thrombocytosis.
Hypophosphatemic Hemolytic Anemia.
Marked hypophosphatemia is recognized as a cause of intravascular hemolytic anemia in postparturient dairy cows and diabetic animals receiving insulin therapy. In postparturient cows, hypophosphatemia results from increased loss of phosphorus in their milk. Insulin therapy may cause hypophosphatemia by shifting phosphorus from the extracellular space to the intracellular space. In either case, marked hypophosphatemia (e.g., 1 mg/dL in cows, or ≤ 1.5 mg/dL in cats) is thought to decrease erythrocyte production of ATP, leading to inadequate energy required for maintenance of membrane and cytoskeletal integrity. An accompanying decrease in reducing capacity and increase in methemoglobin concentration have also been noted in experimental studies of hypophosphatemic hemolytic anemia in dairy cattle, suggesting that oxidative mechanisms may also contribute to anemia. Affected animals are anemic and hemoglobinuric. Gross postmortem findings include pallor, decreased viscosity of the blood, and lesions arising from the underlying metabolic derangement (e.g., discolored pale yellow and swollen liver due to hepatic lipidosis). Renal tubular necrosis and hemoglobin pigment within the tubules is evident microscopically.
Infectious Diseases
This section covers infectious agents within the same genus that are recognized to cause disease in multiple species. Other infectious agents with more limited host specificity (e.g., cytauxzoonosis in cats, feline and equine retroviruses) are covered in later sections on species-specific diseases. Throughout both sections, diseases are organized by taxonomy (protozoal, bacterial and rickettsial, and viral).
Babesiosis (Piroplasmosis).
Babesia spp. and Theileria spp., presented in the next section, are members of the order Piroplasmida, and are generally referenced as piroplasms. These organisms are morphologically similar but have different life cycles; Babesia spp. are primarily erythrocytic parasites, whereas Theileria spp. sequentially parasitize leukocytes and then erythrocytes. Both are protozoan parasites spread by ticks, but other modes of transmission are possible (e.g., biting flies, transplacental, and blood transfusions). Evidence is accumulating that dog fighting also transmits Babesia gibsoni infection.
Babesia organisms are typically classified as large (2 to 4 µm) or small (<2 µm) with routine light microscopy (Fig. 13-24 ). Over 100 Babesia species have been identified, some of which are listed here, along with their relative microscopic size in parentheses:
-
•
Horses—Babesia caballi (large)
-
•
Cattle—Babesia bigemina (large), Babesia bovis (small)
-
•
Sheep and goats—Babesia motasi (large), Babesia ovis (small)
-
•
Dogs—Babesia canis (large), Babesia conradae, B. gibsoni (small)
-
•
Cats—Babesia cati, Babesia felis, Babesia herpailuri (small)
Figure 13-24.
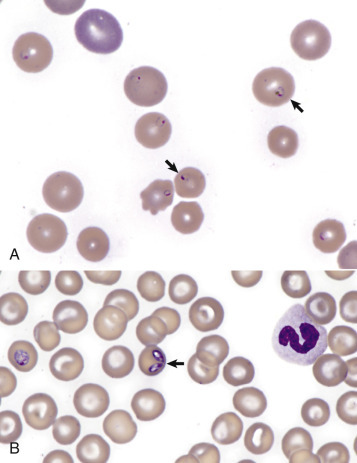
Babesiosis, Canine Blood Smear.
A, Small form (arrows) of Babesia (consistent with Babesia gibsonii). B,Babesia canis (arrow) organisms infecting erythrocytes. Wright's stain.
(Courtesy Dr. M.M. Fry, College of Veterinary Medicine, University of Tennessee.)
Geographic distributions vary with the species, but most have higher prevalences in tropical and subtropical regions. For example, equine and bovine babesiosis are endemic in parts of Africa, the Middle East, Asia, Central and South America, the Caribbean, and Europe. Both were eradicated from the United States and are now considered exotic diseases in that country. Of the previously mentioned species, only agents of canine babesiosis are thought to be endemic in the United States.
Babesiosis may cause intravascular and extravascular hemolytic anemia via direct red blood cell injury, the innocent bystander effect, and secondary immune-mediated hemolytic anemia. Infection with highly virulent strains may cause severe multisystemic disease. In these cases, massive immunostimulation and cytokine release cause circulatory disturbances, which may result in shock, induction of the systemic inflammatory response, and multiple organ dysfunction syndromes.
Babesia organisms can usually be detected on a routine blood smear in animals with acute disease. Infected erythrocytes may be more prevalent in capillary blood, so blood smears made from samples taken from the pinna of the ear or the nail bed may increase the likelihood of detecting organisms microscopically. Buffy coat smears also have an enriched population of infected erythrocytes. PCR-based tests are the most sensitive assay for detecting infection in animals with very low levels of parasitemia.
At necropsy, gross lesions are mainly related to hemolysis and include pale mucous membranes, icterus, splenomegaly, dark red or black kidneys, and reddish-brown urine. The cut surface of the congested spleen oozes blood. The gallbladder is usually distended with thick bile. Less common lesions include pulmonary edema, ascites, and congestion, petechiae, and ecchymoses of organs, including the heart and brain. Parasitized erythrocytes are best visualized on impression smears of the kidney, brain, and skeletal muscle.
Microscopic findings in the liver and kidney are typical of a hemolytic crisis and include anemia-induced degeneration, necrosis of periacinar hepatocytes and cholestasis, and hemoglobinuric nephrosis with degeneration of tubular epithelium. Erythroid hyperplasia is present in the bone marrow. In animals that survive the acute disease, there is hemosiderin accumulation in the liver, kidney, spleen, and bone marrow. In chronic cases there is hyperplasia of macrophages in the red pulp of the spleen.
Theileriosis (Piroplasmosis).
Theileria spp. are tick-borne protozoal organisms that infect many domestic and wild animals worldwide. Numerous Theileria spp. have been documented, but only the more economically or regionally important species are mentioned here. Diseases with the greatest economic impact in ruminants are East Coast fever (Theileria parva infection) and tropical theileriosis (Theileria annulata infection).
-
•
Horses—Theileria equi (formerly Babesia equi)
-
•
Cattle—Theileria annulata, Theileria buffeli, T. parva
-
•
Sheep and goats—Theileria lestoquardi (formerly Theileria hirci)
Like babesiosis, theileriosis is generally restricted to tropical and subtropical regions, including parts of Africa, Asia, the Middle East, and Europe. Except for T. buffeli, all previously listed species are exotic to the United States.
Infection is characterized by schizonts within lymphocytes or monocytes, and pleomorphic intraerythrocytic piroplasms (merozoites and trophozoites). Within host leukocytes the parasite induces leukocyte cellular division, which expands the parasitized cell population. Infected cells disseminate throughout the lymphoid system via the lymphatic and blood vessels. The infected leukocyte may block capillaries, causing tissue ischemia. Later in infection some schizonts cause leukocyte lysis and release of merozoites. Merozoites then invade and parasitize erythrocytes, causing hemolytic anemia. Possible mechanisms of anemia in theileriosis include invasion of erythroid precursors by merozoite stages and associated erythroid hypoplasia (as occurs with T. parva infection), immune-mediated hemolysis, mechanical fragmentation because of vasculitis or microthrombi, enzymatic destruction by proteases, and oxidative damage.
Gross and microscopic lesions are similar to those of babesiosis, except that cattle with East Coast fever tend not to develop hemolytic anemia. In acute East Coast fever, lymph nodes are enlarged, edematous, and hemorrhagic. But with chronic cases they may be shrunken. There is often splenomegaly, hepatomegaly, and hemorrhagic enteritis with white foci of lymphoid infiltrates (pseudoinfarcts) in the liver and kidney. Microscopically, infected leukocytes may block capillaries.
African Trypanosomiasis.
Trypanosomes are flagellated protozoa that can infect all domesticated animals. The most important species that cause disease are Trypanosoma congolense, Trypanosoma vivax, and Trypanosoma brucei ssp. brucei. Disease is most common in parts of Africa where the biologic vector, the tsetse fly, exists. However, T. vivax has spread to Central and South America and the Caribbean, where other biting flies transmit the parasite mechanically. In Africa, cattle are mainly affected due to the feeding preferences of the tsetse fly. African trypanosomiasis must be distinguished from nonpathogenic trypanosomiasis, such as Trypanosoma theileri infection in cattle.
Animals become infected when feeding tsetse flies inoculate metacyclic trypanosomes into the skin of animals. The trypanosomes grow for a few days, causing a localized chancre sore, and then sequentially enter the lymph nodes and bloodstream. Trypanosomal organisms do not infect erythrocytes but rather exist as free trypomastigotes (i.e., flagellated protozoa with a characteristic undulating membrane) in the blood (Fig. 13-25, A ) or as amastigotes in tissue. The mechanism of anemia is believed to be immune mediated. Cattle with acute trypanosomiasis have significant anemia, which initially is regenerative, but less so with time. The extent of parasitemia is readily apparent with T. vivax and T. theileri infections because the organisms are present in large numbers in the blood. This is in contrast to T. congolense, which localizes within the vasculature of the brain and skeletal muscle. Chronically infected animals often die secondary to poor body condition, immunosuppression, and concurrent infections.
Figure 13-25.
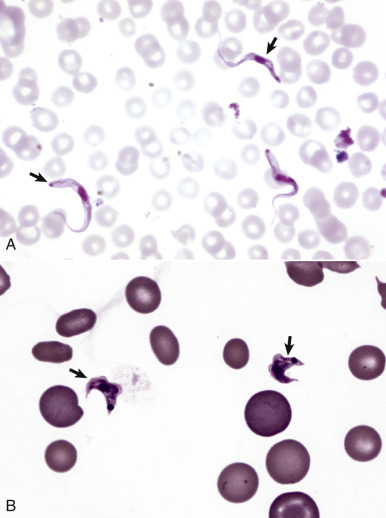
Trypanosomiasis, Bovine (A) and Canine (B) Blood Smears.
A, The trypomastigote life stage of trypanosomes is a flagellated protozoan (arrows) with an undulating membrane, kinetoplast, and nucleus. They may be identified in a wet mount made from the buffy coat portion of the packed cells. B,Trypanosoma cruzi trypomastigotes from a dog with Chagas's disease. Wright-Giemsa stain.
(A courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee. B courtesy Dr. K.M. Boes, College of Veterinary Medicine, Virginia Polytechnic Institute and State University.)
Gross examination of animals with acute disease often reveals generalized lymphadenomegaly, splenomegaly, and petechiae on serosal membranes. An acute hemorrhagic syndrome may occur in cattle, resulting in lesions of severe anemia (e.g., pale mucous membranes) and widespread mucosal and visceral hemorrhages. Main lesions of chronic infections include signs of anemia, lymphadenopathy (e.g., enlarged or atrophied lymph nodes), emaciation, subcutaneous edema, pulmonary edema, increased fluid in body cavities, and serous atrophy of fat.
American Trypanosomiasis (Chagas's Disease).
Trypanosoma cruzi is the flagellated protozoal agent of American trypanosomiasis. Infections have been reported in more than 100 mammal species in South America, Central America, and the southern United States, but dogs and cats are among the more common domestic hosts.
Infected triatomine insects, or “kissing bugs,” defecate as they feed on their mammalian host, releasing infective T. cruzi organisms. The parasite then enters the body through mucous membranes or breaks in the skin. Like the other trypanosomes described previously, T. cruzi lives in the blood as extracellular trypomastigotes (see Fig. 13-25, B) and in the tissues as intracellular amastigotes.
Trypanosoma cruzi primarily causes heart disease. Lesions of acute disease include a pale myocardium, subendocardial and subepicardial hemorrhages, and yellowish-white spots and streaks. There may also be secondary lesions, such as pulmonary edema, ascites, and congestion of the liver, spleen, and kidneys. In chronic disease the heart may be enlarged and flaccid with thin walls. Microscopically, there is often myocarditis and amastigotes within cardiomyocytes.
Anaplasmosis, Ehrlichiosis, Heartwater, and Tick-Borne Fever.
Anaplasmosis, ehrlichiosis, heartwater, and tick-borne fever are tick-borne diseases caused by small, pleomorphic, Gram-negative, obligate intracellular bacteria within the order Rickettsiaceae, also colloquially known as rickettsias. As a group, rickettsias primarily infect hematopoietic cells and endothelial cells. Rickettsias that predominantly infect endothelial cells (e.g., Rickettsia rickettsii [Rocky Mountain spotted fever]), or cause gastrointestinal disease (e.g., Neorickettsia helminthoeca [salmon poisoning disease] and Neorickettsia risticii [Potomac horse fever]) are discussed elsewhere (see Chapters 4 and 7). Less commonly, transmission may occur via blood transfusions or blood-contaminated medical supplies.
Rickettsias that infect erythrocytes include the following species (the disease name follows in parentheses):
-
•
Cattle—Anaplasma marginale, Anaplasma centrale (bovine anaplasmosis)
-
•
Sheep and goats—Anaplasma ovis (ovine and caprine anaplasmosis, respectively)
Anaplasma marginale and A. ovis have worldwide distributions, but A. centrale is mostly restricted to South America, Africa, and the Middle East.
Bovine anaplasmosis causes anemia mainly by immune-mediated extravascular hemolysis. The severity of disease in infected animals varies with age. Infected calves under 1 year of age rarely develop clinical disease, whereas cattle 3 years of age or older are more likely to develop severe, potentially fatal, illness. The reason for this discrepancy is not clear. Indian cattle (Bos indicus) are more resistant to disease than European cattle (Bos taurus). Surviving cattle become chronic carriers (and thus reservoirs for infection of other animals) and develop cyclic bacteremia, which is typically not detectable on blood smears. Splenectomy of carrier animals results in marked bacteremia and acute hemolysis. PCR testing is the most sensitive means of identifying animals with low levels of bacteremia.
Grossly, acute disease causes lesions of acute hemolytic anemia, including pale mucous membranes, low blood viscosity, icterus, splenomegaly, hepatomegaly, and a distended gallbladder. In animals with acute disease it is usually easy to detect A. marginale organisms on routine blood smear evaluation (Fig. 13-26 ) or impression smears from cut sections of the spleen. However, in recovering animals, the organisms may be difficult to find.
Figure 13-26.
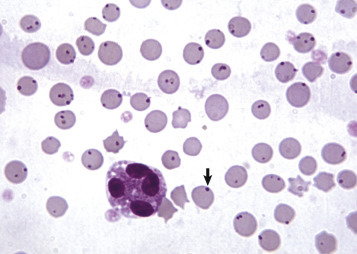
Anaplasmosis, Bovine Blood Smear.
Note the darkly stained Anaplasma marginale organisms (arrow), most of which are located on the edges of the erythrocytes. Anaplasmosis causes anemia mainly by immune-mediated extravascular hemolysis.
(Courtesy Dr. J. Simon, College of Veterinary Medicine, University of Illinois.)
Rickettsias that infect leukocytes are broadly divided into those that preferentially infect granulocytes (Anaplasma phagocytophilum [previously Ehrlichia equi, the agent of human granulocytic ehrlichiosis, and Ehrlichia phagocytophila] and Ehrlichia ewingii), mononuclear cells (E. canis and Ehrlichia chaffeensis), or both (Ehrlichia ruminantium [previously Cowdria ruminantium]). Anaplasma platys (previously Ehrlichia platys) infects platelets. Some of these agents, such as A. phagocytophilum and E. ruminantium, are proven to also infect endothelial cells. The rickettsias have variable host ranges, including the domestic hosts listed here (the disease name follows in parentheses):
-
•
Horses—A. phagocytophilum (equine granulocytic ehrlichiosis)
-
•
Cattle—A. phagocytophilum (tick-borne fever), E. ruminantium (heartwater)
-
•
Sheep and goats—A. phagocytophilum (tick-borne fever), E. ruminantium (heartwater)
-
•
Dogs—A. phagocytophilum, E. ewingii (canine granulocytic ehrlichiosis [E-Fig. 13-3]); A. platys (canine cyclic thrombocytopenia); E. canis, E. chaffeensis (canine monocytic ehrlichiosis)
-
•
Cats—A. phagocytophilum, possibly E. canis (feline ehrlichiosis)
E-Figure 13-3.
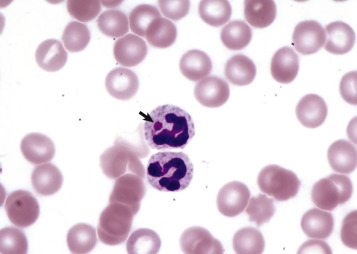
Granulocytic Ehrlichiosis, Anaplasma phagocytophila, Canine Blood Smear.
The top neutrophil contains an inclusion (arrow) consistent with an A. phagocytophila morula. Wright's stain.
(Courtesy Dr. M.M. Fry, College of Veterinary Medicine, University of Tennessee.)
Most of these rickettsias have worldwide distributions. However, E. ewingii has been reported only in the United States, and E. ruminantium is endemic only in parts of Africa and the Caribbean. Although A. phagocytophilum has a wide geographic distribution, strain variants are regionally restricted. For example, A. phagocytophilum causes disease in ruminants in Europe, but it has not been documented in ruminants in the United States.
Reservoirs of disease vary, depending upon the rickettsial species. Cattle are the reservoir host for E. ruminantium, canids are the reservoir host for A. platys and E. canis, and the other rickettsias have wildlife reservoirs.
Pathogenesis of disease involves endothelial cell, platelet, and leukocyte dysfunction. Those agents that infect endothelial cells cause vasculitis and increased vascular permeability of small blood vessels. If only plasma is lost, then there is hypotension and tissue edema. However, more severe vasculitis causes microvascular hemorrhage with the potential for platelet consumption thrombocytopenia, disseminated intravascular coagulation, and hypotension. Infection of platelets may cause thrombocytopenia by direct platelet lysis, immune-mediated mechanisms, or platelet sequestration within the spleen. Pathogenesis of leukocyte dysfunction is unclear, but may involve sepsis, inhibited leukocyte function, endothelial cell activation, and platelet consumption. Chronic E. canis infection may cause aplastic anemia with pancytopenia by an unknown mechanism. Some studies indicate that German shepherd dogs with ehrlichiosis are predisposed to have particularly severe clinical disease. Some breeds of cattle (Bos taurus), sheep (merino), and goats (Angora and Saanen) are more susceptible to heartwater.
Upon blood smear evaluation, thrombocytopenia is the most common hematologic abnormality; anemia and neutropenia occur less frequently. In early stages of infection, blood cells may contain morulae, which are clusters of rickettsial organisms within cytoplasmic, membrane-bound vacuoles (Fig. 13-27 ). Examination of buffy coat smears increases the probability of detecting the organism. Chronic infection may cause lymphocytosis, particularly of granular lymphocytes. Anaplasma platys causes recurrent marked thrombocytopenia.
Figure 13-27.
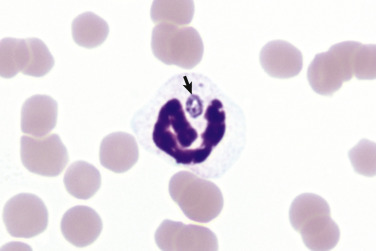
Granulocytic Ehrlichiosis, Equine Blood Smear.
The neutrophil contains an inclusion (arrow) consistent with an Anaplasma phagocytophilum morula. Wright-Giemsa stain.
(Courtesy Dr. K.M. Boes, College of Veterinary Medicine, Virginia Polytechnic Institute and State University.)
In general, more common gross lesions are splenomegaly, lymphadenomegaly, and pulmonary edema and hemorrhage. More severe cases may also exhibit multisystemic petechiae, ecchymoses, and edema, cavitary effusions, and effusive polyarthropathy. Hydropericardium gives heartwater its name but is more consistently found in small ruminants than in cattle. Chronically infected dogs are emaciated. The bone marrow is hyperplastic and red in the acute disease but becomes hypoplastic and pale in dogs with chronic E. canis infection. Equine anaplasmosis is often mild but may cause edema and hemorrhages. Disease in cats is rare and poorly documented.
Histologic findings include generalized perivascular plasma cell infiltration, which is most pronounced in animals with chronic disease. Multifocal, nonsuppurative meningoencephalitis, interstitial pneumonia, and glomerulonephritis are present in most dogs with the disease. Rickettsial organisms are difficult to detect histologically; examination of Wright-Giemsa–stained impression smears of lung, liver, lymph nodes, and spleen is a more effective method for detecting the morulae within leukocytes. Heartwater is often diagnosed by observing morulae in endothelial cells of Giemsa-stained squash preparations of brain. Rickettsial diseases are often diagnosed on the basis of serologic testing, but PCR testing is more sensitive.
Clostridial Diseases.
Certain Clostridium spp. may cause potentially fatal hemolytic anemias in animals; nonhemolytic lesions are presented elsewhere (see Chapters 4, 7, 8, and 19Chapter 4Chapter 7Chapter 8Chapter 19). Clostridium haemolyticum and Clostridium novyi type D cause the disease in cattle known as bacillary hemoglobinuria. (The phrase “red water” has also been used for this disease and for hemolytic anemias in cattle caused by Babesia spp.) Similar naturally occurring disease has been reported in sheep. In cattle the disease is caused by liver fluke (Fasciola hepatica) migration in susceptible animals. Ingested clostridial spores may live in Kupffer cells for a long time without causing disease. However, when migrating flukes cause hepatic necrosis, the resulting anaerobic environment stimulates the clostridial organisms to proliferate and elaborate their hemolytic toxins, causing additional hepatic necrosis. The mechanism of hemolysis involves a bacterial β-toxin (phospholipase C or lecithinase), which enzymatically degrades cell membranes, causing acute intravascular hemolysis. Bacillary hemoglobinuria also occurs with liver biopsies in calves.
Clostridium perfringens type A causes intravascular hemolytic anemia in lambs and calves—a condition known as yellow lamb disease, yellows, or enterotoxemic jaundice because of the characteristic icterus. The organism is a normal inhabitant of the gastrointestinal tract in these animals but may proliferate abnormally in response to some diets. C. perfringens causes intravascular hemolytic anemia in horses with clostridial abscesses, and clostridial mastitis in ewes. C. perfringens type A produces hemolytic α-toxin, which also has phospholipase C activity.
Leptospirosis.
Leptospirosis is recognized as a cause of hemolytic anemia in calves, lambs, and pigs. Specific leptospiral organisms that cause hemolytic disease include Leptospira interrogans serovars pomona and ictohaemorrhagiae.
Leptospira organisms are ubiquitous in the environment. Infection occurs percutaneously and via mucosal surfaces and is followed by leptospiremia; organisms then localize preferentially in certain tissues (e.g., kidney, liver, and pregnant uterus). Proposed mechanisms of hemolytic disease include immune-mediated (immunoglobulin M [IgM] cold agglutinin) extravascular hemolysis and enzymatic (phospholipase produced by the organism) intravascular hemolysis. Leptospirosis can also cause many disease manifestations besides hemolysis (e.g., renal failure, liver failure, abortion, and other conditions) that are not discussed here.
In addition to anemia, common findings in animals with leptospirosis-induced hemolysis include hemoglobinuria and icterus. On necropsy, renal tubular necrosis, which occurs in part because of hemoglobinuria (hemoglobinuric nephrosis), may also be present.
Hemotropic Mycoplasmosis (Hemoplasmosis).
The term hemotropic mycoplasmas, or hemoplasmas, encompasses a group of bacteria, formerly known as Haemobartonella or Eperythrozoon spp., that infect erythrocytes of many domestic, laboratory, and wild animals. Hemotropic mycoplasmas affecting common domestic species are as follows:
-
•
Cattle—Mycoplasma wenyonii
-
•
Camelids—“Candidatus Mycoplasma haemolamae”
-
•
Sheep and goats—Mycoplasma ovis
-
•
Pigs—Mycoplasma suis (E-Fig. 13-4)
-
•
Dogs—Mycoplasma haemocanis, “Candidatus Mycoplasma haematoparvum”
-
•
Cats—Mycoplasma haemofelis, “Candidatus Mycoplasma haemominutum,” “Candidatus Mycoplasma turicensus”
E-Figure 13-4.
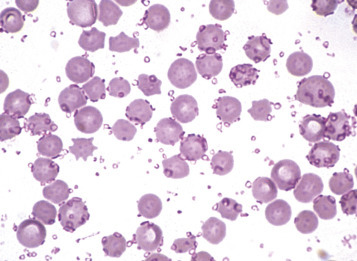
Hemotropic Mycoplasmosis, Porcine Blood Smear.
Blood smear from a splenectomized pig infected with Mycoplasma suis (formerly Eperythrozoon suis). Note the small oval to ring-shaped organisms attached to the surface of the erythrocytes and free in the protein of the blood smear. Wright's stain.
(Courtesy Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
Like other mycoplasmas, hemoplasmas are small (0.3 to 3 µm in diameter) and lack a cell wall. They are epicellular parasites, residing in indentations and invaginations of red blood cell surfaces. The mode of transmission is poorly understood, but blood-sucking arthropods are believed to play a role; transmission in utero, through biting or fighting, and transfusion of infected blood products are also suspected.
Effects of infection vary from subclinical to fatal anemia, depending on the specific organism, dose, and host susceptibility. Most hemoplasmas are more likely to cause acute illness in individuals that are immunocompromised or have concurrent disease. However, M. haemofelis is an exception and tends to cause acute hemolytic anemia in immunocompetent cats. Anemia occurs mainly because of extravascular hemolysis, but intravascular hemolysis also occurs. Although the pathogenic mechanisms are not completely understood, an immune-mediated component is highly probable, as well as direct red blood cell injury by the bacteria and the innocent bystander effect. Hemotropic mycoplasmas induce cold agglutinins in infected individuals, although it is not clear whether these particular antibodies are important in the development of hemolytic anemia.
When detected on routine blood smear evaluation, the organisms are variably shaped (cocci, small rods, or ring forms) and sometimes arranged in short, branching chains (Fig. 13-28 ). The organisms may also be noted extracellularly, in the background of the blood smear, especially if the smear is made after prolonged storage of the blood in an anticoagulant tube.
Figure 13-28.
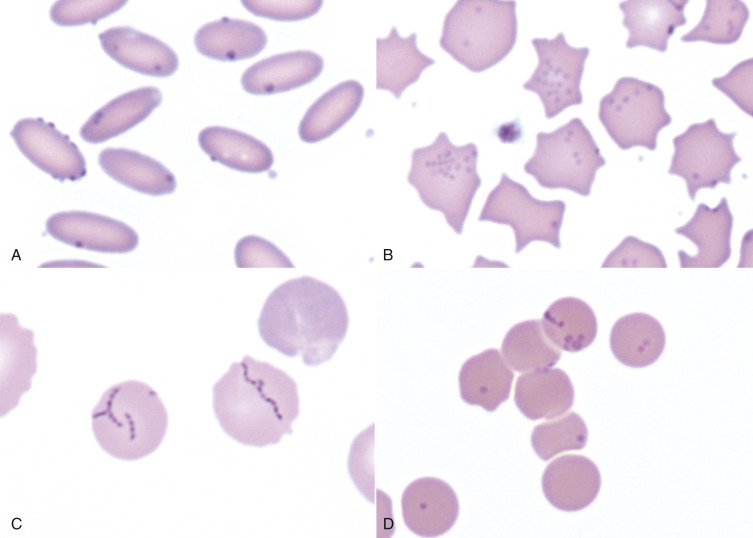
Hemotropic Mycoplasmosis, Alpaca (A), Porcine (B), Canine (C), and Feline (D) Blood Smears.
Blood smears from an alpaca with Mycoplasma haemolamae(A), a pig with Mycoplasma suis(B), a dog with Mycoplasma haemocanis(C), and a cat with Mycoplasma haemominutum(D) infections. Note the small oval to ring-shaped organisms attached to the surface of the erythrocytes and free in the background of the blood smear. Wright-Giemsa stain.
(Courtesy Dr. K.M. Boes, College of Veterinary Medicine, Virginia Polytechnic Institute and State University.)
In animals dying of acute hemoplasma infection, the gross findings are typical of extravascular hemolysis, with pallor, icterus, splenomegaly, and distended gallbladder (Fig. 13-29 ). Additional lesions documented in cattle include scrotal and hind limb edema and swelling of the teats. Microscopic lesions in the red pulp of the spleen include congestion, erythrophagocytosis, macrophage hyperplasia, EMH, and increased numbers of plasma cells. Bone marrow has varying degrees of erythroid hyperplasia, depending on the duration of hemolysis.
Figure 13-29.
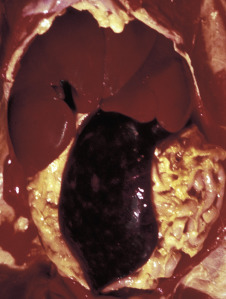
Mycoplasma haemofelis, Cat.
Note the splenomegaly, hepatomegaly, and icterus caused by infection of erythrocytes with this hemotropic parasite. Splenomegaly and icterus are the result of increased destruction (extravascular hemolysis) of infected erythrocytes.
(Courtesy College of Veterinary Medicine, University of Illinois.)
Immune-Mediated Disorders
Immune-Mediated Hemolytic Anemia.
Immune-mediated hemolytic anemia is a condition characterized by increased destruction of erythrocytes because of binding of immunoglobulin to red blood cell surface antigens. It is a common, life-threatening condition in dogs but also has been described in horses, cattle, and cats. Immune-mediated hemolytic anemia may be idiopathic (also called primary immune-mediated hemolytic anemia or autoimmune hemolytic anemia) or secondary to a known initiator, termed secondary immune-mediated hemolytic anemia. Although the cause of idiopathic immune-mediated hemolytic anemia is unknown, certain dog breeds (e.g., cocker spaniels) are predisposed to developing disease, suggesting the possibility of a genetic component. Causes of secondary immune-mediated hemolytic anemia include certain infections (e.g., hemoplasmosis, babesiosis, and theileriosis), drugs (e.g., cephalosporins, penicillin, and sulfonamides), vaccines, and envenomations (e.g., bee stings). Immune-mediated hemolysis directed at nonself antigens, such as in neonatal isoerythrolysis, is presented later.
In most cases of idiopathic immune-mediated hemolytic anemia, the reactive antibody is IgG, and the hemolysis is extravascular (i.e., erythrocytes with surface-bound antibody are phagocytized by macrophages, mainly in the spleen). IgM and/or complement proteins may also contribute to idiopathic immune-mediated hemolytic anemia. Complement factor C3b usually acts as an opsonin that promotes phagocytosis and extravascular hemolysis. However, formation of the complement membrane attack complex on red blood cell surfaces causes intravascular hemolysis; this mechanism more commonly occurs with IgM autoantibodies. Most immunoglobulins implicated in immune-mediated hemolytic anemia are reactive at body temperature (warm hemagglutinins). A smaller portion, usually IgM, are more reactive at lower temperatures, causing a condition known as cold hemagglutinin disease. This results in ischemic necrosis at anatomic extremities (e.g., tips of the ears), where cooling of the circulation causes autoagglutination of erythrocytes and occlusion of the microvasculature. Typically immune-mediated hemolytic anemia targets mature erythrocytes, causing a marked regenerative response. However, as discussed earlier in the chapter, immune-mediated destruction of immature erythroid cells in the bone marrow may also occur, resulting in nonregenerative anemia.
Pathogenesis of secondary immune-mediated hemolytic anemia is dependent upon the cause. Erythrocytic parasites may cause immune-mediated hemolysis by altering the red blood cell surface and exposing “hidden antigens” that are not recognized as self-antigens by the host's immune system. Alternatively, the immune attack may be directed at the infectious agent, but erythrocytes are nonspecifically destroyed because of their close proximity—this is called the innocent bystander mechanism. Certain drugs, such as penicillin, may cause immune-mediated hemolytic anemia by binding to erythrocyte membranes and forming drug-autoantigen complexes that induce antibody formation, termed hapten-dependent antibodies. Other proposed mechanisms include binding of drug-antibody immune complexes to the erythrocyte membrane, or induction of a true autoantibody directed against an erythrocyte antigen.
Hematologic, gross, and histopathologic abnormalities are typical of those of hemolytic anemia, as presented in the earlier section on Bone Marrow and Blood Cells, Dysfunction/Responses to Injury, Blood Cells, Abnormal Concentrations of Blood Cells, Anemia). In brief, there may be spherocytes and autoagglutination on blood smear evaluation, icterus and splenomegaly on gross examination, and EMH, erythrophagocytic macrophages, and hypoxia-induced or thromboemboli-induced tissue necrosis on histopathologic examination. Dogs with immune-mediated hemolytic anemia also frequently develop an inflammatory leukocytosis and coagulation abnormalities (prolonged coagulation times, decreased plasma antithrombin concentration, increased plasma concentration of fibrin degradation products, thrombocytopenia, and disseminated intravascular coagulation). Intravascular hemolysis plays a relatively insignificant role in most cases of immune-mediated hemolytic anemia, but evidence of intravascular hemolysis (e.g., ghost cells, red plasma and urine, dark red kidneys) is noted occasionally, presumably in those cases in which IgM and complement are major mediators of hemolysis.
Neonatal Isoerythrolysis.
Neonatal isoerythrolysis (NI) is a form of immune-mediated hemolytic anemia in which colostrum-derived maternal antibodies react against the newborn's erythrocytes. It is common in horses (Fig. 13-30 ) and has been reported in cattle, cats, and some other domestic and wildlife species. In horses, neonatal isoerythrolysis occurs as a result of immunosensitization of the dam from exposure to an incompatible blood type inherited from the stallion (e.g., transplacental exposure to fetal blood during pregnancy or mixing of maternal and fetal blood during parturition). A previously mismatched blood transfusion produces the same results. Some equine blood groups are more antigenic than others; in particular, types Aa and Qa are very immunogenic in mares. In cattle, neonatal isoerythrolysis has been caused by vaccination with whole blood products or products containing erythrocyte membrane fragments. Neonatal isoerythrolysis has been produced experimentally in dogs, but there are no reports of naturally occurring disease. In cats the recognized form of neonatal isoerythrolysis does not depend on prior maternal immunosensitization but on naturally occurring anti-A antibodies in queens with type B blood. Affected animals are young (hours to days old) with typical gross and microscopic changes of immune-mediated hemolytic anemia.
Figure 13-30.
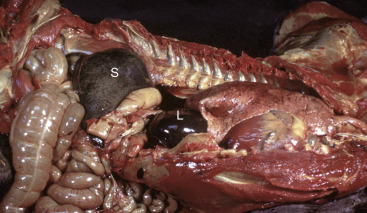
Neonatal Isoerythrolysis, Foal.
Note the enlarged spleen (S) (also liver [L]) and icterus. The newborn foal had colostrum-derived maternal antibodies, which reacted against its own erythrocytes. Macrophages in the splenic red pulp remove erythrocytes whose membranes have bound antibody.
(Courtesy College of Veterinary Medicine, University of Illinois.)
Pure Red Cell Aplasia.
Pure red cell aplasia (PRCA) is a rare bone marrow disorder characterized by absence of erythropoiesis and severe nonregenerative anemia. Primary and secondary forms of pure red cell aplasia have been described in dogs and cats. Primary pure red cell aplasia is apparently caused by immune-mediated destruction of early erythroid progenitor cells, a presumption supported by the response of some patients to immunosuppressive therapy and by the detection of antibodies inhibiting erythroid colony formation in vitro in some dogs. Administration of recombinant human erythropoietin (rhEpo) has been identified as a cause of secondary pure red cell aplasia in dogs, cats, and horses, presumably caused by induction of antibodies against rhEpo that cross-react with endogenous Epo. Experimentation with the use of species-specific recombinant Epo has produced mixed results. Dogs treated with recombinant canine Epo have not developed pure red cell aplasia. However, in experiments reported thus far involving cats treated with recombinant feline Epo, at least some animals have developed pure red cell aplasia. Parvoviral infection has been suggested as a possible cause of secondary pure red cell aplasia in dogs. Infection with FeLV subgroup C causes secondary erythroid aplasia in cats, probably because of infection of early-stage erythroid precursors. Grossly, animals with pure red cell aplasia have pale mucous membranes without indicators of hemolysis (e.g., icterus). Microscopic examination of the bone marrow shows an absence or near absence of erythroid precursors with or without lymphocytosis, plasmacytosis, and myelofibrosis; production of other cell lines (e.g., neutrophils and platelets) is normal or hyperplastic.
Immune-Mediated Neutropenia.
Immune-mediated neutropenia is a rare condition that has been reported in horses, dogs, and cats. This disease is characterized by severe neutropenia from immune-mediated destruction of neutrophils or their precursors. The range of causes is presumably similar to that of other immune-mediated cytopenias (e.g., immune-mediated hemolytic anemia, pure red cell aplasia, and immune-mediated thrombocytopenia). Affected animals may have infections, such as dermatitis, conjunctivitis, or vaginitis, which are secondary to marked neutropenia and a compromised innate immune system. Microscopically, there may be neutrophil hyperplasia, maturation arrest, or aplasia in the bone marrow, depending on which neutrophil maturation stage is targeted for destruction. Marrow lymphocytosis and plasmacytosis may be marked (e.g., >60% of nucleated cells). The diagnosis may be supported by flow cytometric detection of immunoglobulin bound to neutrophils but is most often made on the basis of exclusion of other causes of neutropenia and response to immunosuppressive therapy.
Immune-Mediated Thrombocytopenia.
Immune-mediated thrombocytopenia (IMTP) is a condition characterized by immune-mediated destruction of platelets. It is a fairly common condition in dogs and is less frequent in horses and cats. The disease is usually idiopathic but may be secondary to infection (e.g., equine infectious anemia and ehrlichiosis), drug administration (e.g., cephalosporins and sulfonamides), neoplasia, and other immune-mediated diseases. When immune-mediated thrombocytopenia occurs together with immune-mediated hemolytic anemia, the condition is called Evans's syndrome. The thrombocytopenia is often severe (e.g., < 20,000 platelets/µL), resulting in varying degrees of bleeding tendencies, mainly in skin and mucous membranes. Microscopically, there are multifocal perivascular hemorrhages in multiple tissues, and the bone marrow exhibits megakaryocytic and erythroid hyperplasia. Rarely, immune-mediated destruction of megakaryocytes may cause megakaryocytic hypoplasia, termed amegakaryocytic thrombocytopenia.
Neonatal Alloimmune Thrombocytopenia.
A form of immune-mediated thrombocytopenia, known as neonatal alloimmune thrombocytopenia, is recognized in neonatal pigs and foals. The pathogenesis of this disease is virtually identical to that of neonatal isoerythrolysis as a cause of anemia: a neonate inheriting paternal platelet antigens absorbs maternal antibodies against these antigens through the colostrum. In principle, a similar situation may occur after platelet-incompatible transfusion of blood or blood products containing platelets. Gross and microscopic changes are similar to those of immune-mediated thrombocytopenia except that the animal is young (e.g., 1 to 3 days).
Inflammatory Disorders
Hemophagocytic Syndrome.
Hemophagocytic syndrome is a term used to describe the proliferation of nonneoplastic (i.e., polyclonal), well-differentiated but highly erythrophagic macrophages. The condition is rare but has been recognized in dogs and cats. Unlike hemophagocytic histiocytic sarcoma, which is a neoplastic proliferation of phagocytic macrophages, hemophagocytic syndrome is secondary to an underlying disease, such as neoplasia, infection, or an immune-mediated disorder. The primary disease process causes increased production of stimulatory cytokines, which results in macrophage proliferation and hyperactivation. These activated macrophages phagocytize mature hematopoietic cells and hematopoietic precursors at an enhanced rate, resulting in one or more cytopenias. Affected animals usually have lesions of the primary disease, as well as signs of the anemia (e.g., pale mucous membranes), neutropenia (e.g., bacterial infections), and thrombocytopenia (e.g., petechiae and ecchymoses). Microscopically, phagocytic macrophages are found in high numbers in the bone marrow and commonly in other tissues, including lymph nodes, spleen, and liver. Additional bone marrow findings reported in animals with hemophagocytic syndrome vary widely, ranging from hypoplasia to hyperplasia of cell lines with peripheral cytopenias.
Disseminated Intravascular Coagulation.
Disseminated intravascular coagulation is a syndrome characterized by continuous activation of both coagulation and fibrinolytic pathways and is also known as consumptive coagulopathy. It is not a primary disease, but rather a secondary complication of many types of underlying disease, including severe inflammation, organ failure, and neoplasia. It is included in the section on Inflammatory Disorders because the coagulation cascade is closely linked to inflammatory pathways.
Disseminated intravascular coagulation is a consumptive coagulopathy resulting from activation of both coagulation and fibrinolytic pathways. It is a secondary complication of many types of underlying disease, including many infectious diseases, trauma, burns, heat stroke, immune-mediated disease, hemolysis, shock, neoplasia, organ failure, obstetric complications, and noninfectious inflammatory disease, such as pancreatitis. It is common in critically ill domestic animals. Disseminated intravascular coagulation involves an initial hypercoagulable phase, resulting in thrombosis and ischemic tissue damage, and a subsequent hypocoagulable phase as a result of consumption of coagulation factors and platelets, resulting in hemorrhage (E-Fig. 13-5). The pathogenesis of disseminated intravascular coagulation typically involves the release of tissue factor (thromboplastin) and subsequent activation of coagulation pathways and platelets but may also involve defective normal inhibition of coagulation or defective fibrinolysis. Classically diagnosis of disseminated intravascular coagulation is based on clinical evidence of hemorrhage and/or thromboembolic disease and a triad of laboratory findings: thrombocytopenia, usually moderate (below the lower reference value but above 50,000/µL); prolonged coagulation times (prothrombin time and/or partial thromboplastin time); and decreased fibrinogen or increased concentration of plasma fibrin degradation products or D-dimer. Milder forms of disseminated intravascular coagulation that do not meet all of the diagnostic criteria also occur. Decreased plasma antithrombin (antithrombin III) concentration and schistocytosis are other laboratory abnormalities often found in patients with disseminated intravascular coagulation.
E-Figure 13-5.
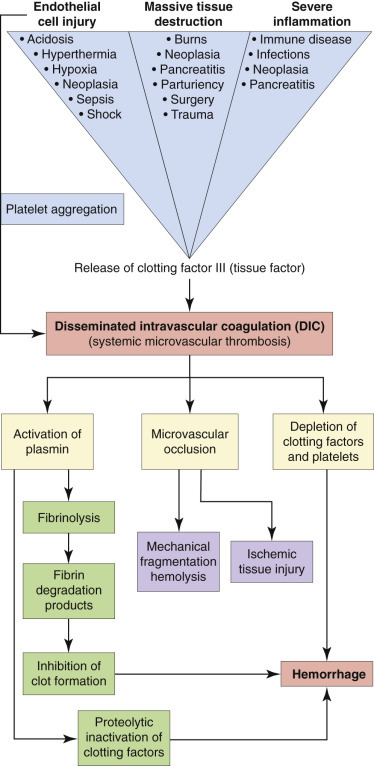
Pathophysiology of Disseminated Intravascular Coagulation.
(Courtesy Dr. K.M. Boes, College of Veterinary Medicine, Virginia Polytechnic Institute and State University; and Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
Hematopoietic Neoplasia
The term hematopoietic neoplasia encompasses a large and diverse group of clonal proliferative disorders of hematopoietic cells. Historically, numerous systems have been used to classify hematopoietic neoplasms in human medicine, some of which have been applied inconsistently to veterinary species (examples include the Kiel classification and National Cancer Institute Working Formulation). The World Health Organization (WHO) classification of hematopoietic neoplasia was first published in 2001 (updated in 2008) and is based on the principles defined in the Revised European-American Classification of Lymphoid Neoplasms (REAL) from the International Lymphoma Study Group. The WHO classification system is considered the first true worldwide consensus on the classification of hematopoietic malignancies and integrates information on tumor topography, cell morphology, immunophenotype, genetic features, and clinical presentation and course. A veterinary reference of the WHO classification system, published in 2002, was later validated in 2011 using the canine model of lymphoma. This project, modeled after the study to validate the system in human beings, yielded an overall accuracy (i.e., agreement on a diagnosis) among pathologists of 83%. Currently this classification system is accepted as the method of choice in both human and veterinary medicine.
The WHO classification broadly categorizes neoplasms primarily according to cell lineage: myeloid, lymphoid, and histiocytic. This distinction is based on the fact that the earliest commitment of a pluripotent HSC is to either a lymphoid or nonlymphoid lineage. Many pathologists and clinicians distinguish leukemias from other hematopoietic neoplasms. Leukemia refers to a group of hematopoietic neoplasms that arise from the bone marrow and are present within the blood. Leukemia may be difficult to differentiate from other forms of hematopoietic neoplasms that originate outside of the bone marrow but infiltrate the bone marrow and blood. For simplicity, cases of secondary bone marrow or blood involvement may not be considered leukemia but rather the “leukemic phase” of another primary neoplasm. It is now recognized that certain lymphomas and leukemias are different manifestations of the same disease (e.g., chronic lymphocytic leukemia and small lymphocytic lymphoma), and the designation of lymphoma or leukemia is placed on the tissue with the largest tumor burden.
Based on their degree of differentiation, leukemias are classified as acute or chronic. Acute leukemias are poorly differentiated or undifferentiated, meaning that there are high percentages of early progenitor and precursor cells, including lymphoblasts, myeloblasts, monoblasts, erythroblasts, and/or megakaryoblasts. In contrast, well-differentiated cells predominate in chronic leukemias. Because well-differentiated cells also predominate with nonneoplastic proliferations, chronic leukemias must be differentiated from reactive processes, such as those cells that occur in chronic and/or granulomatous inflammation. Diagnosis of chronic leukemia is often made by excluding all other causes for the proliferating cell type. For example, causes of relative and secondary erythrocytosis are excluded to be able to diagnose polycythemia vera. Furthermore, the designation of acute or chronic also refers to the disease's clinical course. Acute leukemias tend to have an acute onset of severe and rapidly progressive clinical signs, whereas animals with chronic leukemia typically have indolent, slowly progressive disease. This classification scheme is summarized in Table 13-4 . Subcategories exist within each of these groups, as discussed further later.
Table 13-4.
Basic Classification of Leukemias
| Leukemia | Basic Diagnostic Criteria |
|---|---|
| Acute undifferentiated leukemia |
|
| Lymphoid leukemia |
|
| Acute lymphoblastic leukemia |
|
| Chronic lymphocytic leukemia |
|
| Multiple myeloma |
|
| Myeloid leukemia |
|
| Myelodysplastic syndrome |
|
| Acute myeloid leukemia |
|
| Chronic myeloid leukemia |
|
Diagnostic Techniques Used to Classify Hematopoietic Neoplasms
Before the discussion of specific diseases, it is worthwhile to describe the diagnostic techniques required to classify hematopoietic neoplasms that are becoming increasingly available for routine use in veterinary medicine. Immunophenotyping refers to the use of antibodies recognizing specific molecules expressed on different cell types to determine the identity of a cell population of interest. Immunophenotyping on the basis of these lineage-specific or lineage-associated markers can be performed on histologic sections (immunohistochemistry [see Fig. 13-86]), air-dried cytologic examination smears (immunocytochemistry), or by laser analysis of cells in suspension in blood or buffer solutions (flow cytometry). In cases of lymphoid neoplasia, immunophenotyping most routinely refers to determination of B or T lymphocyte origin. Clonality assays, PCR for antigen receptor rearrangement (PARR), can help identify neoplastic lymphoid proliferations on the basis of clonal rearrangements of genes encoding lymphocyte antigen receptors. In terms of practical application the PARR assay is most useful in helping to distinguish lymphoid neoplasms from those nonneoplastic lymphoid proliferations mimicking neoplasia. Cytogenetic testing has not been routinely used in veterinary medicine, though several genetic mutations have been identified in dogs. For example, breakpoint cluster region-Abelson (BCR-ABL) translocations have been identified in some canine leukemias, including acute myeloblastic leukemia and chronic monocytic leukemia. Dogs with Burkitt-like lymphoma have a translocation leading to constitutive c-Myc expression.
Figure 13-86.

T Cell–Rich Large B Cell Lymphoma, Skin, Horse.
A, The majority of the cells are small T lymphocytes and are mixed with fewer neoplastic large pleomorphic B lymphocytes. H&E stain. B, The small reactive T lymphocytes are strongly CD3 positive. Immunohistochemistry with anti-CD3, hematoxylin counterstain.
(Courtesy Dr. A.C. Durham, School of Veterinary Medicine, University of Pennsylvania.)
Types of Hematopoietic Neoplasia
This section discusses examples of myeloid neoplasms, including myelodysplastic syndrome, myeloid leukemias, and mast cell neoplasms (technically a form of myeloid neoplasia), and lymphoid neoplasms, including lymphoid leukemias and multiple myeloma. Other lymphoid neoplasms, such as the numerous subtypes of lymphoma and extramedullary plasmacytomas (EMPs), as well as histiocytic disorders are described in the section on Lymphoid/Lymphatic System, Disorders of Domestic Animals, Neoplasia. Additional discussion of hematopoietic neoplasia occurs in the species-specific sections at the end of this chapter.
Myeloid Neoplasia
Myelodysplastic Syndrome.
Myelodysplastic syndrome (MDS) most commonly occurs in dogs and cats and may be caused by FeLV infection in cats. The disease refers to a group of clonal myeloid proliferative disorders with ineffective hematopoiesis in the bone marrow, resulting in cytopenias of more than one cell line. Hematopoietic proliferation in bone marrow with concurrent peripheral blood cytopenias is likely a result of increased apoptosis of neoplastic cells within the bone marrow, before their release into circulation. Clinical illness and death often result from secondary manifestations, such as secondary infections or cachexia, attributable to the effects of cytopenias and/or transformation of the neoplasm into acute myeloid leukemia. Gross lesions are dependent upon the type and severity of the cytopenias. However, essential microscopic findings within the bone marrow are normal or increased cellularity, dysplasia of myeloid cells, and fewer than 20% myeloblasts and “blast equivalents.”3
Acute Myeloid Leukemia.
Acute myeloid leukemia (AML) is uncommon in domestic animals but most frequently occurs in dogs and cats. In veterinary species, acute myeloid leukemia is most commonly of neutrophil, monocyte, and/or erythroid origin, with rare reports of eosinophil, basophil, or megakaryocytic lineages. It is caused by FeLV infection in cats. Evaluations of blood smears show many early myeloid precursors, including myeloblasts and blast equivalents (Fig. 13-31, A ). In dogs the total leukocyte concentration averages approximately 70,000/µL; anemia, neutropenia, and thrombocytopenia commonly occur. Grossly, animals show lesions attributed to anemia, neutropenia, and thrombocytopenia, such as pale mucous membranes, secondary infections, and multisystemic bleeding, respectively. Neoplastic cells often infiltrate tissues, resulting in splenomegaly, hepatomegaly, and lymphadenomegaly. Microscopically, myeloid cells efface (replace) the bone marrow and infiltrate extramedullary tissues, especially lymphoid tissue.
Figure 13-31.

Leukemia, Canine Blood Smears.
A, Acute myeloid leukemia. A dog with acute myelomonocytic leukemia has a marked leukocytosis (52,200 white blood cells/µL) with myeloid blasts (arrows) differentiating into dysplastic neutrophils (arrowheads) and monocytes (m). Modified Wright's stain. B, Chronic myeloid leukemia. A dog with chronic myelomonocytic leukemia (arrows) has a marked leukocytosis (138,300 white blood cells/µL) with a predominance of mature neutrophils (105,108/µL) and monocytes (26,277/µL). Wright-Giemsa stain. C, Acute lymphoblastic leukemia. Note the large lymphoid cells with immature (fine) chromatin and nucleoli (arrows). The dog also had pancytopenia due to neoplastic myelophthisis. Modified Wright's stain. D, Chronic lymphocytic leukemia (CLL). Small lymphocytes predominate in CLL. The neoplastic lymphocytes have clumped chromatin and no to rare nucleoli (arrows). Most canine CLLs are of T lymphocyte origin with a granular phenotype. Modified Wright's stain.
(Courtesy Dr. K.M. Boes, College of Veterinary Medicine, Virginia Polytechnic Institute and State University.)
Chronic Myeloid Leukemia.
Chronic myeloid leukemia (CML), also called chronic myelogenous leukemia or myeloproliferative neoplasia, is rare in animals. Most reported cases occur in dogs and cats. There are various subclassifications of chronic myeloid leukemia, including excessive production of erythrocytes (polycythemia vera), platelets (essential thrombocythemia), neutrophils (chronic neutrophilic leukemia), monocytes (chronic monocytic leukemia), neutrophils and monocytes (chronic myelomonocytic leukemia), eosinophils (chronic eosinophilic leukemia), or basophils (chronic basophilic leukemia). Complete peripheral blood count analysis often reveals very high concentrations of the neoplastic cells, such as greater than 50,000 to 100,000 leukocytes/µL (see Fig. 13-31, B) or 2,000,000 platelets/µL. Cellular morphologic features are often normal, but slight dysplasia may be observed. Later in the disease there may be cytopenias of nonneoplastic cell types.
Animals with polycythemia vera often have red mucous membranes and lesions of hyperviscosity syndrome, such as bleeding and dilated, tortuous retinal vessels. Essential thrombocythemia results in multisystemic bleeding due to dysfunctional platelets, or multisystemic infarcts from hyperaggregability and excessive platelets. Chronic myeloid leukemias of leukocytes often result in splenomegaly, hepatomegaly, and lymphadenomegaly because of infiltration by the neoplastic cells. Histologically, the bone marrow shows proliferation of the neoplastic cell type characterized by dysplasia and low numbers (e.g., <20%) of myeloblasts and blast equivalents.
Mast Cell Neoplasia.
Mast cell tumors (MCTs) of the skin and other sites are common in animals (see Chapters 6, 7, and 17Chapter 6Chapter 7Chapter 17), but mast cell leukemia is rare. In cats, MCTs are the most common neoplasm in the spleen (E-Fig. 13-6). Mast cells normally are not present in the blood vascular system, but the finding of mast cells in the blood (mastocytemia) is highly suggestive of disseminated mast cell neoplasia (systemic mastocytosis) in cats. However, mastocytemia does not necessarily indicate myeloid neoplasia in dogs. In fact, one study found that the severity of mastocytemia in dogs was frequently higher in animals without MCTs than those with MCTs and that random detection of mast cells in blood smears usually is not the result of underlying MCT.
E-Figure 13-6.
Feline Splenic Mast Cell Tumor
A, Sheets of neoplastic mast cells fill the splenic parenchyma. H&E stain. B, Astral blue stain highlights the positively staining (turquoise) metachromatic granules.
(Courtesy Dr. A.C. Durham, School of Veterinary Medicine, University of Pennsylvania.)
Granulocytic Sarcoma.
Granulocytic sarcoma is a poorly characterized extramedullary proliferation of myeloid precursors, most often of eosinophilic or neutrophilic cell lines. Although rare, there are reports of granulocytic sarcoma in dogs, cats, cattle, and pigs, and it may arise in a number of sites, such as lung, intestine, lymph nodes, liver, kidney, skin, and muscle.
Lymphoid Neoplasia
Lymphoid Leukemia
Acute Lymphoblastic Leukemia.
Acute lymphoblastic leukemia (ALL) is uncommon in dogs and cats, and rare in horses and cattle. In a recent immunophenotype study of 51 cases of acute lymphoblastic leukemia in dogs, 47 arose from B lymphocytes and 4 arose from double-negative T lymphocytes that were immunonegative for CD4 and CD8 markers. In the blood of animals with acute lymphoblastic leukemia, there are typically many medium to large lymphoid cells with deeply basophilic cytoplasm, reticular to coarse chromatin, and prominent, multiple nucleoli (see Fig. 13-31, C). In affected dogs the mean blood lymphoid concentration is approximately 70,000/µL, but cats with acute lymphoblastic leukemia often have low numbers of neoplastic cells in the circulation. As with animals with acute myeloid leukemia, anemia, neutropenia, and thrombocytopenia commonly occur. Gross and microscopic lesions also are similar to those that occur in cases of acute myeloid leukemia, except that neoplastic cells may differentiate into morphologically identifiable lymphoid cells.
Chronic Lymphocytic Leukemia.
Chronic lymphocytic leukemia (CLL) is uncommon in veterinary medicine. It is predominantly a disease of middle-aged to older dogs but is also documented in horses, cattle, and cats. Most canine chronic lymphocytic leukemia cases are of T lymphocyte origin, typically cytotoxic T lymphocytes expressing CD8. In cats the majority of chronic lymphocytic leukemia cases have a T helper lymphocyte immunophenotype. A CBC often shows very high numbers of small lymphocytes with clumped chromatin and scant cytoplasm. Proliferating cytotoxic T lymphocytes frequently contain a few pink cytoplasmic granules when stained with most methanol-based Romanowsky stains (e.g., Wright-Giemsa). However, these granules may not be appreciated with some aqueous-based Romanowsky stains (e.g., Diff-Quik). Although the number of total blood lymphocytes is often greater than 100,000/µL, relatively mild lymphocytosis (e.g., 15,000/µL) has been reported. Seventy-five percent of affected dogs also have anemia, and 15% have thrombocytopenia. Autopsy findings depend on the stage of disease. In advanced cases with marked infiltration of organs with neoplastic cells, there is often uniform splenomegaly, hepatomegaly, and lymphadenomegaly, and the bone marrow is highly cellular (E-Fig. 13-7; see Fig. 13-31, D). Other lesions depend on whether there are concurrent cytopenias, such as anemia, neutropenia, and thrombocytopenia, and if the neoplastic cells produce excessive immunoglobulin. Lesions caused by excessive immunoglobulin are further discussed in the section on multiple myeloma. Histologically, the bone marrow is densely cellular with well-differentiated lymphocytes. Small lymphocytes infiltrate and often efface in the architecture of the lymph nodes and spleen. The liver may have dense accumulations of neoplastic cells in the connective tissue around the portal triad.
E-Figure 13-7.
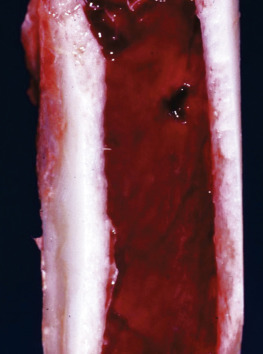
Chronic Leukemia, Hypercellularity, Bone Marrow, Dog.
Grossly, the marrow consists entirely of hematopoietic tissue (red) and no fat (yellow). In this case, hypercellularity of the marrow is attributable to neoplastic proliferation, but hyperplasia may cause similar gross findings.
(Courtesy Dr. B.C. Ward, College of Veterinary Medicine, University of Mississippi; and Noah's Arkive, College of Veterinary Medicine, The University of Georgia.)
Plasma Cell Neoplasia.
Plasma cell neoplasms are most easily categorized as myeloma or multiple myeloma, which arises in the bone marrow, and extramedullary plasmacytoma, which as the name implies involves sites other than bone; the latter is discussed in the section on Lymphoid/Lymphatic System, Disorders of Domestic Animals: Lymph Nodes, Neoplasia, Plasma Cell Neoplasia.
Multiple Myeloma.
Multiple myeloma (MM) is a rare, malignant tumor of plasma cells that arises in the bone marrow and usually secretes large amounts of immunoglobulin. The finding of neoplastic plasma cells in blood samples or smears is rare. Dogs are affected more frequently than other species, but multiple myeloma has also been reported in horses, cattle, cats, and pigs. Diagnosis of multiple myeloma is based on finding a minimum of two or three (opinions vary) of the following abnormalities:
-
•
Markedly increased numbers of plasma cells in the bone marrow (Fig. 13-32, A )
-
•
Monoclonal gammopathy
-
•
Radiographic evidence of osteolysis
-
•
Light chain proteinuria
Figure 13-32.
Multiple Myeloma and Monoclonal Gammopathy.
A, Canine bone marrow aspirate. Many of the neoplastic plasma cells in the bone marrow aspirate have pink-tinged cytoplasm (arrow), the result of a high concentration of immunoglobulin. Wright's stain. B, Multiple myeloma, cat. Agarose gels and densitometry tracings showing results of serum electrophoresis. The serum has a high concentration of a monoclonal immunoglobulin (the dark band [arrow] on the right of the gel, corresponding to the tall peak on the right of the tracing). C, Normal cat. Agarose gels and densitometry tracings showing results of serum electrophoresis. The serum has a normal distribution of protein fractions, the most abundant being albumin (the dark band [arrow] on the left of the gel, corresponding to the tall peak on the left of the tracing).
(A courtesy Dr. M.M. Fry, College of Veterinary Medicine, University of Tennessee. B and C courtesy Dr. S.A. Kania, College of Veterinary Medicine, University of Tennessee.)
The classic laboratory finding in patients with multiple myeloma is hyperglobulinemia, which results from the excessive production of immunoglobulin or an immunoglobulin subunit by the neoplastic cells. This homogeneous protein fraction is often called paraprotein or M protein. Paraproteins produced from the same clone of plasma cells have the same molecular weight and electric charge. Therefore they have the same migration pattern using serum protein electrophoresis, which results in a tall, narrow spike in the globulins region, termed monoclonal gammopathy (see Fig. 13-32, B). The term gammopathy is used because most immunoglobulins migrate in the γ-region of an electrophoresis gel. However, some immunoglobulins, especially immunoglobulin A (IgA) and IgM, migrate to the β-region. Occasionally, biclonal or other atypical electrophoretic patterns may be seen with multiple myeloma as a result of protein degradation, protein complex formation, binding to other proteins, or when the tumor includes more than one clonal population. It is important to note that monoclonal gammopathy is not specific to multiple myeloma but has also been reported with lymphoma, chronic lymphocytic leukemia, canine ehrlichiosis, and canine leishmaniasis. Definitively distinguishing monoclonal from polyclonal gammopathy requires immunoelectrophoresis or immunofixation using species-specific antibodies recognizing different immunoglobulin subclasses and subunits.
Occasionally, multiple myeloma cells produce only the immunoglobulin light chain. An immunoglobulin monomer consists of two heavy chains and two light chains connected by disulfide bonds. These light chains may deposit in tissues and cause organ dysfunction, especially renal failure. When the light chains form amyloid deposits, the disease is called amyloid light chain amyloidosis. But if the light chains deposit as nonamyloid granules, it is termed light chain deposition disease. Light chains are low-molecular-weight proteins that pass through the glomerular filter into the urine, wherein they are also known as Bence Jones proteins. They tend to not react with urine dipstick protein indicators and are most specifically detected by electrophoresis and immunoprecipitation.
In addition to aiding in the diagnosis of multiple myeloma, paraproteins have an important role in pathogenesis of disease. These proteins may inhibit platelet function, increase blood viscosity, deposit in glomerular basement membranes (see Chapter 11; see Figs. 11-27 and 11-28, or precipitate at cool temperatures, which results in bleeding tendencies, hyperviscosity syndrome, glomerulopathies, and cryoglobulinemia, respectively. Hyperviscosity syndrome refers to the clinical sequelae of pathologically increased blood viscosity, which are slowed blood flow and loss of laminar flow. Clinical signs include mucosal hemorrhages, visual impairment due to retinopathy, and neurologic signs, such as tremors and abnormal aggressive behavior. Cryoglobulinemia is the condition in which proteins, typically IgM, precipitate at temperatures below normal body temperature (cold agglutinins). Precipitation often occurs in blood vessels of the skin and extremities, such as the ears and digits, and results in ischemic necrosis.
In multiple myeloma the neoplastic proliferation of plasma cells results in osteolysis. Work with human cell cultures has shown that osteoclasts support the growth of myeloma cells, and that direct contact between the two cell types increases the myeloma cell proliferation and promotes osteoclast survival. Increased osteoclast activity causes osteolysis, but the exact mechanism is not known. Osteolysis often results in bone pain, lytic bone lesions on radiographs, hypercalcemia, and increased serum alkaline phosphatase activity. Later in disease, osteolysis may cause pathologic fractures.
Morphologically, myeloma cells tend to grow in sheets that displace normal hematopoietic cells in the bone marrow. A proposed diagnostic criterion of multiple myeloma is that plasma cells constitute 30% or more of the nucleated cells in the marrow. Well-differentiated plasma cells are round with abundant basophilic cytoplasm (due to increased rough endoplasmic reticulum) and a perinuclear pale zone (enlarged Golgi apparatus for the production of immunoglobulin); anisocytosis and anisokaryosis are often mild but may be marked. Some plasma cell neoplasms have a bright eosinophilic fringe due to accumulated IgA (see Fig. 13-32, A). Nuclei are round with clumped chromatin and often peripherally placed with the cytoplasm; binucleation and multinucleation are common. Poorly differentiated myeloma cells may lack and/or display less characteristic features. Osteolysis of bone may be present microscopically. Common sites of metastasis include the spleen, liver, lymph nodes, and kidneys.
Disorders of Horses
Congenital Disorders
Flavin Adenine Dinucleotide Deficiency.
Flavin adenine dinucleotide (FAD) is a cofactor for cytochrome-b 5 reductase, the enzyme that maintains hemoglobin in its functional reduced state, and for glutathione reductase, an enzyme that also protects erythrocytes from oxidative damage. Reported in a Spanish mustang mare and a Kentucky mountain saddle horse gelding, erythrocyte FAD deficiency is a result of an abnormal riboflavin kinase reaction, which is the first reaction in converting riboflavin to FAD. Clinicopathologic changes include persistent methemoglobinemia of 26% to 46%, eccentrocytosis, a slightly decreased or normal hematocrit, and erythroid hyperplasia in the bone marrow.
Infectious Diseases
Equine Infectious Anemia Virus.
Equine infectious anemia virus (EIAV), the agent of equine infectious anemia, is a lentivirus that infects cells of the monocyte-macrophage system in horses (also ponies, donkeys, and mules). The virus is mechanically transmitted by biting flies, such as horseflies and deer flies. Less common routes of transmission include blood transfusions, contaminated medical equipment, and transplacentally. Disease may present in acute, subacute, and chronic forms and is potentially fatal. After an acute period of fever, depression, and thrombocytopenia that lasts 1 to 3 days, there is a prolonged period of recurrent fever, thrombocytopenia, and anemia. In most cases, clinical disease subsides within a year, and horses become lifelong carriers and reservoirs of EIAV.
EIAV causes anemia by both immune-mediated hemolysis and decreased erythropoiesis. Hemolysis is typically extravascular but may have an intravascular component during the acute phase. Decreased erythropoiesis may result from direct suppression of early-stage erythroid cells by the virus, as well as anemia of inflammation. Thrombocytopenia likely results from immune-mediated platelet destruction and suppressed platelet production.
Animals dying during hemolytic crises are pale with mucosal hemorrhages and dependent edema. The spleen and liver are enlarged, dark, and turgid, and they and other organs have superficial subcapsular hemorrhages. Petechiae are evident beneath the renal capsule and throughout the cortex and medulla. The bone marrow is dark red as a result of replacement of fat by hematopoietic tissue; the extent of replacement is an indication of the duration of the anemia.
The severity of microscopic lesions is dependent on the chronicity of the disease, and they are most significant in the spleen, liver, and bone marrow. As would be anticipated, microscopic findings of the spleen are predominantly influenced by the number and activity of macrophages, which is a reflection of the duration of the disease and the frequency of hemolytic episodes. Hemosiderin-laden macrophages persist for months to years; therefore large numbers are consistent with chronicity. Kupffer cell hyperplasia with hemosiderin stores and periportal infiltrates of lymphocytes are the most significant changes in the liver. Bone marrow histologic findings vary depending on the duration of the disease. In most animals the marrow is cellular because of the replacement of fat by intense, orderly erythropoiesis. Granulocytes are relatively less numerous, and plasma cells are increased. As in the spleen, hemosiderin-laden macrophages are present in large numbers in chronic cases. Emaciated animals with chronic disease have serous atrophy of fat (see E-Fig. 13-1).
Clinical findings with viremic episodes include fever, depression, icterus, petechial hemorrhages, lymph node enlargement, and dependent edema. Equine infectious anemia infection is diagnosed on the basis of the Coggins test, an agarose gel immunodiffusion test for the presence of the antibody against the virus.
Disorders of Ruminants (Cattle, Sheep, and Goats)
Congenital Disorders
Congenital Dyserythropoiesis in Polled Herefords.
A syndrome of congenital dyserythropoiesis and alopecia occurs in polled Hereford calves. The cause and pathogenesis of this often fatal disease are unknown. Early in disease there is hyperkeratosis and alopecia of the muzzle and ears, which progresses to generalized alopecia and hyperkeratotic dermatitis. Histologically, there is orthokeratotic hyperkeratosis with dyskeratosis, as well as erythroid hyperplasia, dysplasia, and maturation arrest in the bone marrow. Ineffective erythropoiesis results in nonregenerative to poorly regenerative anemia.
Erythrocyte Band 3 Deficiency in Japanese Black Cattle.
Erythrocyte band 3 is integral membrane protein that connects to the cytoskeleton and aids in erythrocyte stability. A hereditary deficiency of this protein has been identified in Japanese black cattle, resulting in increased erythrocyte fragility, spherocytosis, intravascular hemolytic anemia, and retarded growth. Affected calves show lesions consistent with hemolytic anemia, including pale mucous membranes, icterus, and splenomegaly. Histologically, there are bilirubin accumulations in the liver, and hemosiderin in renal tubules.
Infectious Diseases
Bovine Leukemia Virus.
Bovine leukemia virus is discussed in the later section on lymphoma (see Lymphoid/Lymphatic System, Disorders of Domestic Animals: Lymph Nodes, Neoplasia, Lymphoma).
Bovine Viral Diarrhea Virus.
BVDV infection may cause thrombocytopenia in cattle, and a thrombocytopenic hemorrhagic syndrome has been specifically caused by type II BVDV infection. Investigations of the mechanism of BVDV-induced thrombocytopenia have resulted in varying, sometimes conflicting, conclusions. More than one study has shown viral antigen within bone marrow megakaryocytes and circulating platelets. Evidence of impaired thrombopoiesis (megakaryocyte necrosis, megakaryocyte pyknosis, and degeneration) and increased thrombopoiesis (megakaryocytic hyperplasia, increased numbers of immature megakaryocytes) in the bone marrow has been reported in type II BVDV–infected animals, including concurrent megakaryocyte necrosis and hyperplasia in some experimental subjects. Calves infected with type II BVDV also have impaired platelet function.
Cattle with the hemorrhagic syndrome are severely thrombocytopenic and neutropenic with multisystemic hemorrhages, particularly of the digestive tract, spleen, gallbladder, urinary bladder, and lymph nodes. Histologic lesions include hemorrhage, epithelial necrosis of enterocytes, intestinal erosions, crypt proliferation with microabscesses, and lymphoid depletion of the gut-associated lymphoid tissue, Peyer's patches, and spleen. Lesions of the bone marrow are variable, as previously described.
Immune-Mediated Disorders
Bovine Neonatal Pancytopenia.
Bovine neonatal pancytopenia (BNP) is caused by alloantibodies absorbed from colostrum, resulting in a hemorrhagic syndrome in calves. The syndrome was first recognized in Europe in the early 2000s and has since been experimentally correlated with prior vaccination of affected calves' dams with a commercial BVDV vaccine (Pregsure BVD; Pfizer Animal Health). The vaccine has since been voluntarily recalled from the market. It is thought that vaccination induces alloantibody formation by the dam. The alloantibodies are ingested by the calf and bind to the calf's hematopoietic progenitor cells, resulting in functional compromise of those cells. Acutely affected calves are less than a year of age and have peripheral thrombocytopenia and neutropenia. Death results from thrombocytopenia-induced hemorrhages or neutropenia-induced secondary infections, including pneumonia, enteritis, and septicemia. Within the bone marrow there is erythroid, myeloid, and megakaryocytic hypoplasia.
Disorders of Dogs
Congenital Disorders
Cyclic Hematopoiesis.
Cyclic hematopoiesis (also known as lethal gray collie disease) is an autosomal recessive disorder of pluripotent HSCs in gray collie dogs. A defect in the adaptor protein complex (AP3) results in defective intracellular signaling and predictable fluctuations in concentrations of blood cells that occur in 14-day cycles. The pattern is cyclic marked neutropenia, and in a different phase, cyclic reticulocytosis, monocytosis, and thrombocytosis. Production of key cytokines involved in regulation of hematopoiesis is also cyclic. Neutropenia predisposes affected animals to infection, and many die of infectious causes. Affected animals have dilute hair coats and lesions with acute or chronic infectious disease, especially of the lungs, gastrointestinal tract, and kidneys. Dogs older than 30 weeks of age have systemic amyloidosis, which occurs because of cyclic increases in concentration of acute phase proteins during phases of monocytosis.
Phosphofructokinase Deficiency.
Inherited autosomal recessive deficiency of the erythrocyte glycolytic enzyme, phosphofructokinase (PFK), is described in English springer spaniel, American cocker spaniel, and mixed-breed dogs. There are three genes encoding PFK enzymes, designated M-PFK in muscle and erythrocytes, L-PFK in liver, and P-PFK in platelets. A point mutation in the gene coding for M-PFK results in an unstable, truncated molecule. Erythrocytes in PFK-deficient dogs have decreased ATP and 2,3-diphosphoglycerate (2,3-DPG) production and increased fragility under alkaline conditions. The disease is characterized by chronic hemolysis with marked reticulocytosis. The marked regenerative response may compensate for the ongoing hemolysis; therefore affected animals are not necessarily anemic. However, acute intravascular hemolytic episodes may occur with hyperventilation-induced alkalemia. Lesions are typical of hemolytic anemia and include pale mucous membranes, icterus, hepatosplenomegaly, and dark red urine with microscopic EMH and marrow erythroid hyperplasia. A single DNA-based test is available to detect the common mutation.
Erythrocyte Structural Abnormalities.
Congenital erythrocyte structural abnormalities may occur with abnormal membrane composition or defective proteins within the membrane or cytoskeleton. Some of these morphologic changes occur concurrently with clinical disease, but others do not.
Hereditary stomatocytosis is recognized in Alaskan malamutes, Drentse patrijshonds, and schnauzers. The specific defects are not known, but they are likely different in the various dog breeds. However, all affected dogs have stomatocytes on blood smear evaluation, as identified by their slit-shaped area of central pallor. Erythrocytes also have increased osmotic fragility and decreased survival. Schnauzers are clinically healthy and not anemic but do have reticulocytosis, suggesting that the hemolytic anemia is compensated by erythroid hyperplasia. Mild to marked hemolytic anemia is documented in Alaskan malamutes and Drentse patrijshonds. Alaskan malamutes have concurrent short-limb dwarfism, and Drentse patrijshonds have hypertrophic gastritis and polycystic kidney disease.
Other (presumably heritable) erythrocyte abnormalities in dogs that do not have clinical signs include elliptocytosis caused by band 4.1 deficiency or β-spectrin mutation, and familial macrocytosis and dyshematopoiesis in poodles.
Scott's Syndrome.
An inherited thrombopathy resembling Scott's syndrome in human beings, in which platelets lack normal procoagulant activity, has been recognized in a family of German shepherd dogs. The specific defect in these dogs has not been identified on the molecular level but involves impaired expression of phosphatidylserine on the platelet surface. Affected dogs have a mild to moderate clinical bleeding tendency characterized by epistaxis, hyphema, intramuscular hematoma formation, and increased hemorrhage with surgery.
Macrothrombocytopenia.
Macrothrombocytopenia is an inherited condition in Cavalier King Charles spaniels in which there are lower than normal concentrations of platelets with enlarged and giant platelets. The condition is caused by defective β1-tubulin, which results in impaired microtubule assembly. Affected dogs are asymptomatic but may have abnormal platelet aggregation in vitro.
Infectious Diseases
Canine Distemper.
Canine distemper virus preferentially infects lymphoid, epithelial, and nervous cells and is presented in greater detail in the lymphoid section. Canine distemper virus may also infect other hematopoietic cells, including erythrocytes, nonlymphoid leukocytes, and platelets (Fig. 13-33 ), and can cause decreased peripheral blood concentrations of neutrophils, lymphocytes, monocytes, and platelets during viremia. The thrombocytopenia is a result of virus-antibody immune complexes on platelet membranes and direct viral infection of megakaryocytes.
Figure 13-33.
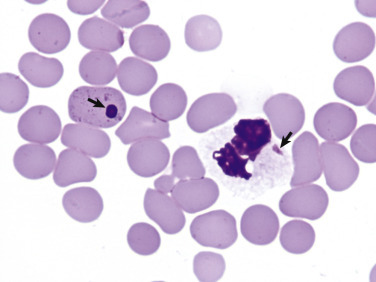
Canine Distemper Viral Inclusions, Canine Blood Smear.
Note the viral inclusions within the erythrocyte and neutrophil (arrows). Inclusions may also be present within other leukocyte types, or rarely, platelets. Diff-Quik stain.
(Courtesy Dr. K.M. Boes, College of Veterinary Medicine, Virginia Polytechnic Institute and State University.)
Disorders of Cats
Congenital Disorders
Increased Erythrocyte Osmotic Fragility.
A condition characterized by increased erythrocyte osmotic fragility has been described in Abyssinian and Somali cats. The specific defect has not been identified, but PK deficiency (which has been reported in these breeds) was excluded as the cause. Affected cats have chronic intermittent severe hemolytic anemia and often have other lesions secondary to hemolytic anemia (e.g., splenomegaly and hyperbilirubinemia).
Infectious Diseases
Cytauxzoonosis.
Cytauxzoonosis is a severe, often fatal disease of domestic cats caused by the protozoal organism, Cytauxzoon felis. Disease is relatively common in the south central United States, particularly during summer months. Bobcats (Lynx rufus) and other wild felids are thought to be wildlife reservoirs of disease. C. felis is transmitted by a tick vector, Dermacentor variabilis, which is probably essential for infectivity of the organism.
Cytauxzoonosis has a schizogenous phase within macrophages throughout the body (especially liver, spleen, lung, lymph nodes, and bone marrow) that causes systemic illness. These schizont-containing macrophages enlarge and accumulate within the walls of veins, eventually causing vessel occlusion, circulatory impairment, and tissue hypoxia. Later in disease, merozoites released from schizonts enter erythrocytes, resulting in an erythrocytic phase of infection. Infected domestic cats often have nonregenerative anemia, but the pathogenesis for the anemia is unclear. However, it likely represents preregenerative hemolytic anemia because erythrocyte phagocytosis is a prominent finding in many organs. Infected cats often also develop neutropenia and thrombocytopenia, which likely result from inflammation and disseminated intravascular coagulation, respectively.
On blood smear evaluation, signet ring–shaped erythrocytic inclusions (piroplasms) may be observed during the erythrocytic phase of disease (Fig. 13-34 ). These inclusions closely resemble small-form Babesia (see Fig. 13-24, A) and some Theileria organisms. Postmortem examination typically shows pallor, icterus, splenomegaly, enlarged and red lymph nodes, diffuse pulmonary congestion and edema, and multisystemic petechiae and ecchymoses. Vascular obstruction may cause marked distention of abdominal veins. Cavitary effusions are present in some cats. Microscopically, large, schizont-laden macrophages accumulate within venous and sinusoidal lumens and often completely occlude the lumens (Fig. 13-35 ). Erythrophagocytosis, thrombosis, and histologic changes of ischemia are common, especially within the spleen, liver, and lungs.
Figure 13-34.

Cytauxzoonosis, Feline Blood Smear.
Erythrocytes parasitized by Cytauxzoon felis contain signet ring–shaped inclusions (arrows). (Courtesy Dr. K.M. Boes, College of Veterinary Medicine, Virginia Polytechnic Institute and State University.)
Figure 13-35.

Cytauxzoonosis, Tissue Aspirate (A) and Biopsy (B), Cat.
A, Lymph node aspirate. A large macrophage (center of figure) is laden with schizonts of Cytauxzoon felis. Wright's stain. B, Splenic macrophages are filled with Cytauxzoon organisms. H&E stain.
(A courtesy Dr. D.F. Edwards, College of Veterinary Medicine, University of Tennessee. B courtesy Dr. A.R. Doster, University of Nebraska; and Noah's Arkive, College of Veterinary Medicine, The University of Georgia.)
Affected cats typically become acutely ill with fever, pallor, and icterus and usually die within 2 to 3 days. For many years, cytauxzoonosis was considered to be almost always fatal. However, a recent report, in which numerous cats from a subregion of the endemic area in the United States survived infection with an organism with greater than 99% homology to Cytauxzoon felis, suggests the emergence of a less virulent strain.
Feline Leukemia Virus.
FeLV is an oncogenic, immunosuppressive lentivirus that causes hematologic abnormalities of widely varying types and severity. Manifestations of disease caused by FeLV infection vary depending on dose, viral genetics, and host factors, but normal hematopoiesis is probably suppressed to some degree in all cases.
FeLV infects hematopoietic precursor cells soon after the animal is exposed and continues to replicate in hematopoietic and lymphatic tissue of animals that remain persistently viremic. The virus disrupts normal hematopoiesis by inducing genetic mutations, by other direct effects of the virus on infected hematopoietic cells, or by an altered host immune system. Hematologic changes include dysmyelopoiesis with resultant cytopenias or abnormal cell morphologic features, and neoplastic transformation of hematopoietic cells (leukemia). A notable form of dysplasia is the presence of macrocytic erythrocytes (macrocytes) and metarubricytosis in the absence of erythrocyte regeneration (inappropriate metarubricytosis). The relatively uncommon subgroup C viruses cause erythroid hypoplasia, probably because of infection of early-stage erythroid precursors. FeLV may be detected in megakaryocytes and platelets in infected cats and may result in platelet abnormalities, including thrombocytopenia, thrombocytosis, increased platelet size, and decreased function. Proposed mechanisms of FeLV-induced thrombocytopenia include direct cytopathic effects, myelophthisis, and immune-mediated destruction. Platelet life span and function have been shown to be decreased in FeLV-positive cats. Persistently viremic cats are immunosuppressed and are prone to developing other diseases, including infectious diseases, bone marrow disorders, and lymphoma.
CBC abnormalities attributed to FeLV infection include various cytopenias, especially nonregenerative anemia, which may be persistent or cyclical. Regenerative anemia may also occur with FeLV infection, often because of coinfection with M. haemofelis. Hematopoietic cell dysplasia or neoplasia may also be evident. Grossly, infected cats are often pale, but other lesions are dependent upon the presence of other cytopenias or concurrent disease. Microscopically, the bone marrow is hypocellular, normocellular, or hypercellular. There may be erythroid hypoplasia, erythroid hyperplasia with maturation arrest, or acute leukemia.
Feline Immunodeficiency Virus.
Feline immunodeficiency virus (FIV), another feline lentivirus, causes anemia in a minority of infected cats. Immunosuppressive effects of FIV from thymic depletion are discussed elsewhere. It is generally accepted that anemia does not result directly from FIV infection but instead develops because of concurrent disease such as coinfection with FeLV or hemotropic mycoplasma, other infection, or malignancy. The severity and type of anemia in FIV-infected cats depends on the other specific disease processes involved.
Lymphoid/Lymphatic System
The thymus, spleen, lymph nodes, and lymph nodules, including MALT, are classified as part of both the lymphoid and immune systems. The lymphoid system (also known as lymphatic system in some texts) is broadly categorized into primary and secondary lymphoid organs. The main primary lymphoid organs include thymus, bone marrow, and bursa of Fabricius in birds and are the sites at which the B and T lymphocytes proliferate, differentiate, and mature. In mammals, lymphocytes arise from HSCs in the bone marrow, and B lymphocytes continue to develop at this site. Ruminants also have B lymphocyte proliferation and maturation within their Peyer's patches. Progenitor T lymphocytes migrate from bone marrow to mature and undergo selection in the thymus. The spleen, lymph nodes, and lymph nodules are secondary lymphoid organs and are responsible for the immune responses to antigens, such as the production of antibody and cell-mediated immune reactions. At these sites, lymphocytes are activated by antigens and undergo clonal selection, proliferation, and differentiation (see also Chapter 5). In addition, the spleen and lymph nodes contain cells of the monocyte-macrophage system and thus also participate in the phagocytosis of cells and materials.
The bone marrow is described in the first section of this chapter. The remaining primary lymphoid organ, the thymus, is described first in this section, followed by the secondary lymphoid organs: spleen, lymph nodes, and diffuse and nodular lymphatic tissues.
Dissection and Fixation of Lymphoid/Lymphatic Tissues
Errors from selection of inappropriate sampling sites and artifacts from compression and incorrect fixation for histopathologic and immunohistochemical examinations are common in routine veterinary pathologic analysis. The identification and remedies for these problems are discussed in E-Appendix 13-2.
Thymus
Structure and Function
The thymus is essential for the development and function of the immune system, specifically for the differentiation, selection, and maturation of T lymphocytes generated in the bone marrow (see also Chapter 5). The basic arrangement of the thymus in domestic animals consists of paired cervical lobes (left and right), an intermediate lobe at the thoracic inlet, and a thoracic lobe, which may be bilobed. The cervical lobes are positioned ventrolateral to the trachea, adjacent to the carotid arteries, and extend from the intermediate lobe at the thoracic inlet as far cranially as the larynx. The intermediate lobe bridges between the cervical and the thoracic lobe. The right thoracic lobe is usually small or completely absent. The left lobe lies in the ventral aspect of the cranial mediastinum (except in the ruminant, where it is dorsal) and extends caudally as far as the pericardium.
Horse—The cervical lobes in foals are small, and the thoracic lobe constitutes the bulk of the thymus.
Ruminant—The cervical lobes are large. The left and right thoracic lobes are fused and unlike other domestic animals, lie in the dorsal aspect of the cranial mediastinum.
Pig—The cervical lobes are large.
Dog—The cervical lobes regress very early and thus appear absent. The thoracic lobe extends caudally to the pericardium.
Cat—The cervical lobes are small, and the thoracic lobe, which forms the majority of the thymus, extends caudally to the pericardium and molds to its surface.
The thymus is referred to as a lymphoepithelial organ and hence is composed of epithelial and lymphoid tissue. Formed from the endoderm of the third pharyngeal pouch in the fetus, the thymic epithelium is infiltrated by blood vessels from the surrounding mesoderm, resulting in the development of the thymic epithelial reticulum. The lymphocyte population consists of bone marrow–derived progenitor cells, which fill spaces within the epithelial network. A connective tissue capsule surrounds the thymus, and attached thin septa subdivide the tissue into partially separated lobules. Each lobule is composed of a central medulla and surrounding cortex (Fig. 13-36 ).
Figure 13-36.

Lobular Organization of the Thymus.
The thymus consists of several incomplete lobules. Each lobule contains an independent outer cortical region, and the central medullary region is shared by adjacent lobules. Trabeculae, extensions of the capsule down to the corticomedullary region, form the boundary of each lobule. The cortex consists of stromal cells, cortical epithelial cells, macrophages, and developing T lymphocytes (thymocytes). Major histocompatibility complex class I and II molecules are present on the surface of the cortical epithelial cells. The characteristic deep blue staining of the cortex in histologic preparation reflects the predominant dense population of T lymphocytes as compared with the less basophilic medulla, which contains a lower number of thymocytes.
(Courtesy Dr. A.C. Durham, School of Veterinary Medicine, University of Pennsylvania; and Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
The thymic cortex consists mainly of an epithelial reticulum and lymphocytes (Fig. 13-37 ). The stellate cells of the epithelial reticulum have elongate branching cytoplasmic processes that connect to adjacent epithelial cells through desmosomes, thus forming a supportive network (cytoreticulum). The lymphoid component is composed of differentiating lymphocytes derived from progenitor (also known as precursor) T lymphocytes in the bone marrow. The medulla is composed of similar epithelial reticular cells, many of which are much larger than those in the cortex and have a more obvious epithelial structure. Some of the epithelial reticular cells form thymic corpuscles, also called Hassall's corpuscles, which are distinctive keratinized epithelial structures (see Fig. 13-37). Interdigitating dendritic cells (DCs) are also present within the medulla, but there are far fewer lymphocytes than in the cortex.
Figure 13-37.
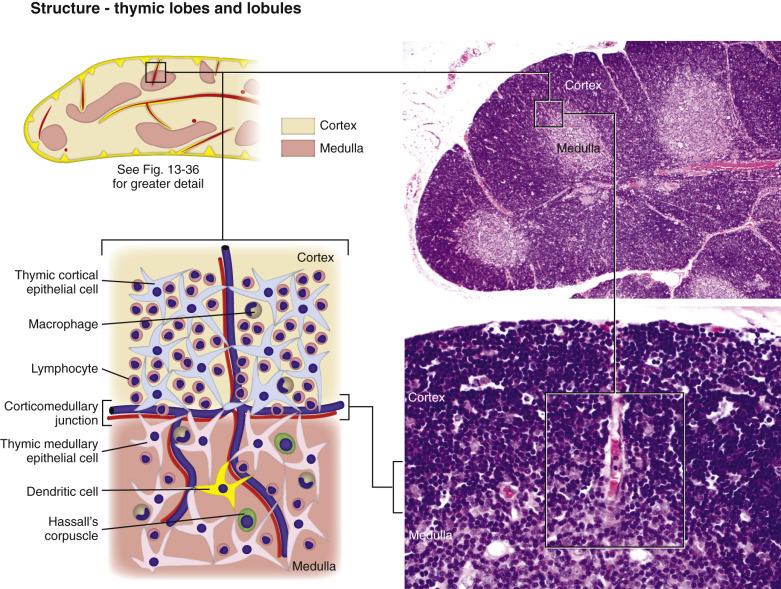
Cell Populations in Lobules of the Thymus.
The functional thymus consists of two cell populations: stromal cells and thymocytes. The stroma consists mainly of epithelial cells present beneath the capsule, lining trabeculae and blood vessels, and forming the supportive network (cytoreticulum) within the cortex and medulla; the medulla also contains Hassall's corpuscles. Macrophages within the cortex and medulla are involved in the removal of apoptotic thymocytes eliminated during clonal selection.
(Courtesy Dr. A.C. Durham, School of Veterinary Medicine, University of Pennsylvania and Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
The progenitor T lymphocytes released from the bone marrow into the blood enter the thymus in the subcapsular zone of the cortex and begin the differentiation and selection processes, developing into mature naïve T lymphocytes as they traverse the thymic cortex to the medulla. In the cortex, T lymphocytes that recognize self-molecules (major histocompatibility complex [MHC] molecules) but not self-antigens are permitted to mature by a process called positive selection. Cells that do not recognize MHC molecules are removed by apoptosis. Those T lymphocytes that recognize both MHC molecules and self-antigens are removed by macrophages at the corticomedullary junction, a process called negative selection. Because of the rigid differentiation requirements attributable to MHC restriction and tolerance (positive and negative selection, respectively), only a small fraction (<5%) of the developing T lymphocytes that arrive at the thymus from the bone marrow survive. Mature naïve T lymphocytes exit the thymus through postcapillary venules in the corticomedullary region, enter the circulation, and recirculate through secondary lymphoid tissues, primarily located in the paracortex of lymph nodes and the periarteriolar sheaths of the spleen. In these specialized sites, the mature naïve T lymphocytes are activated upon exposure to their specific antigens and undergo additional phases of development to differentiate into effector and memory cells.
The thymus attains its maximal mass relative to body weight at birth and involutes after sexual maturity; the rate of involution may vary among domestic species. The lymphoid and epithelial components are gradually replaced by loose connective tissue and fat, although remnants remain histologically, even in aged animals.
Dysfunction/Responses to Injury
The responses of the thymus to injury and causes are listed in Boxes 13-4 and 13-5 . The most common change is lymphoid atrophy caused by physical and physiologic stresses, toxins, drugs, and viral infections.
Box 13-4. Responses of the Thymus to Injury.
Lymphoid atrophy (see Box 13-5)
- Inflammation—rare
- Infectious agents (e.g., porcine circovirus type 2)
Hemorrhage and hematomas
- Neoplasia
- Thymoma
- Lymphoma
Box 13-5. General Causes of Lymphoid Atrophy in Lymphoid Organs.
Lack of antigenic stimulus
- Toxins
- For example, halogenated aromatic hydrocarbons, metals (lead, mercury), mycotoxins
- Chemotherapeutic agents
- For example, azathioprine, cyclophosphamide, cyclosporin A, corticosteroids
- Ionizing radiation (+/−)
- For example, when lymphoid tissue is present within therapeutic field
- Viruses
- For example, CDV, canine and feline parvovirus, FIV, BVDV, classic swine fever virus, EHV-1
Malnutrition and Cachexia
Aging
BVDV, Bovine viral diarrhea virus; CDV, canine distemper virus; EHV-1, equine herpesvirus 1; FIV, feline immunodeficiency virus.
Atrophy.
Because the thymus does not contain any lymphopoietic tissue, it depends on the bone marrow for the supply of progenitor T lymphocytes. Thus thymic lymphoid atrophy can be the result of either an inadequate supply of lymphocytes from the bone marrow or lysis of lymphocytes (lymphocytolysis) in the thymus. Thymic atrophy must be differentiated from involution, which normally begins at sexual maturity. This distinction is difficult to make, unless the change is extreme or age-matched control animals are available for comparison.
Inflammation.
Inflammation of the thymus is rare. Neutrophils and macrophages are often present within keratinized Hassall's corpuscles during involution and should not be mistaken for a true thymitis. Thymitis has been reported in salmon poisoning disease of dogs (see Chapter 7), epizootic bovine abortion (see Chapter 18), and in pigs infected with porcine circovirus type 2 (PCV2). Necrosis and secondary infiltrates of neutrophils and macrophages may be seen in other infectious diseases (e.g., equine herpesvirus 1 [EHV-1]).
Hemorrhage and Hematomas.
Thymic enlargement is often the result of hemorrhage, hematomas, or neoplasia and is discussed further in the section on Lymphoid/Lymphatic System, Disorders of Domestic Animals: Thymus, Disorders of Dogs.
Neoplasia.
Primary tumors of the thymus are thymomas, arising from the epithelial component, and lymphomas and are discussed further in the section on Disorders of Domestic Animals: Thymus.
Portals of Entry/Pathways of Spread
The main portal of entry to the thymus is hematogenous. Portals of entry used by microorganisms and other agents and substances to access the lymphatic system are summarized in Box 13-6 . These portals include the blood vessels (hematogenous spread by microorganisms free in the plasma or within circulating leukocytes or erythrocytes), afferent lymphatic vessels (lymphatic spread), direct penetration, or through M (for “microfold”) cells and DCs in MALT.
Box 13-6. Portals of Entry into Lymphoid Organs.
- Thymus
- Hematogenous
- Spleen
- Hematogenous
- Direct penetration
- Lymph node
- Hematogenous
- Afferent lymphatic vessels
- MALT
- Hematogenous
- Migrating macrophages
- Dendritic cells
- M cells (Peyer's patches)
MALT, Mucosa-associated lymphoid tissue.
Defense Mechanisms/Barrier Systems
Defense mechanisms used by the thymus to protect itself against microorganisms and other agents are the innate and adaptive immune responses, discussed in Chapters 3, 4, and 5Chapter 3Chapter 4Chapter 5. Viruses, bacteria, and particles arriving in the lymph and blood interact with cells of the monocyte-macrophage system through phagocytosis and antigen processing and presentation. Hyperplasia of the macrophages often occurs concurrently. Antigen processing and presentation are followed by an immune response resulting in proliferation of B lymphocytes, plasma cells, and the subsequent production of antibody; proliferation of T lymphocytes may also occur.
Spleen
The relationships between anatomic structures and the different functions of the spleen are complicated. There are also anatomic differences among domestic animal species and confusion about the correct and up-to-date terminology. The following brief discussion aims to define the terms used in this chapter. The term splenic sinusoid is used to describe a vascular structure present in the sinusal spleen (also known as sinusoidal spleen); dogs are the only domestic animal with true splenic sinusoids. The term red pulp vascular spaces is used (as opposed to “sinus”) to describe the vascular spaces in the red pulp of both the nonsinusal and nonsinusoidal spleens of all domestic animals.4 The other terms used here include marginal sinus, marginal zone, periarteriolar lymphoid sheath (PALS), periarteriolar macrophage sheath (PAMS), and splenic lymphoid follicles.
Structure
The spleen is located in the left cranial hypogastric region of the abdomen, where it is typically suspended in the gastrosplenic ligament between the diaphragm, stomach, and the body wall. The exception is in domestic ruminants, where it is closely adhered to the left dorsolateral aspect of the rumen. The gross shape and size of the spleen vary markedly among domestic animals, but generally it is a flattened, elongated organ. Some species, notably birds, demonstrate seasonal variation in splenic shape and size.
The spleen is covered by a thick capsule composed of smooth muscle and elastic fibers, from which numerous intertwining fibromuscular trabeculae extend into the parenchyma. These trabeculae and reticular cells form a spongelike supportive matrix for the parenchyma of the mammalian spleen in all domestic species. In cattle and horses the three muscular layers of the capsule lie perpendicular to each other, forming a capsule thicker than that of carnivores. Carnivores, small ruminants, and pigs have interwoven smooth muscle within the splenic capsule, and pigs also have abundant elastic fibers within the capsule.
The spleen differs from many other organs in the organization of its parenchyma. Instead of a cortex and medulla, the spleen is divided into two distinct structural and functional components: the red pulp and white pulp (Fig. 13-38 ). With hematoxylin and eosin (H&E) staining, red pulp appears red-pink because of the abundance of red blood cells, whereas white pulp appears blue-purple because of the heavy concentration of lymphocytes. The white pulp consists of splenic follicles, populated by B lymphocytes; the PALS, inhabited by T lymphocytes; and the marginal zone at the periphery of follicles. Macrophages, antigen-presenting cells, and trafficking B and T lymphocytes populate the marginal zone. The radial arteries, branches of the central artery (also known as central arteriole), and capillaries from both red and white pulp drain into the marginal sinus of the marginal zone, although the latter has not been shown to be the case in all species to the same degree (e.g., the cat has a small marginal sinus but a well-developed PAMS) (Figs. 13-39 and 13-40 ). The red pulp consists of cells of the monocyte-macrophage system, PAMS, sinusoids (dogs, rats, and human beings only), red pulp vascular spaces, and associated stromal elements such as reticular cells, fibroblasts, and trabecular myocytes. The labyrinth of the splenic red pulp vascular spaces serves as both a functional and physical filter for circulating blood cells.
Figure 13-38.
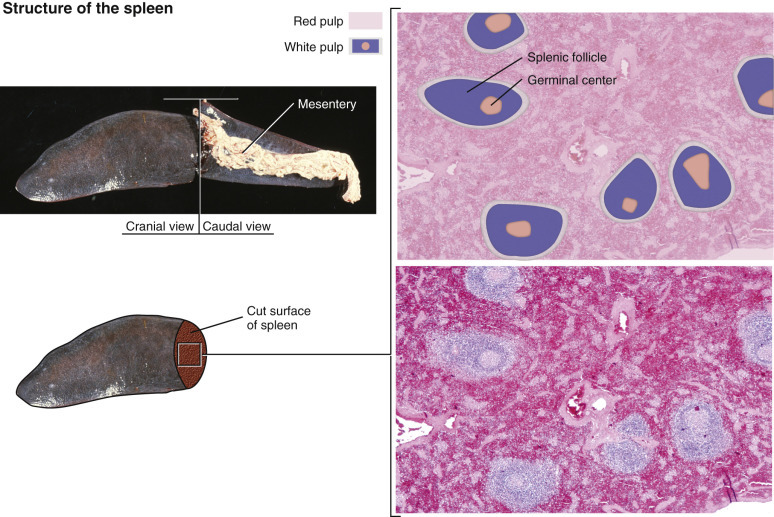
Structure of the Spleen—Red and White Pulp.
The spleen is organized into two distinct components. The red pulp consists of cells of the monocyte-macrophage system, periarteriolar macrophage sheaths, sinusoids (dogs, rats and human beings only), red pulp vascular spaces, and associated stromal elements such as reticular cells, fibroblasts and trabecular myocytes. The white pulp is composed of splenic follicles (B lymphocytes), periarteriolar sheaths (T lymphocytes), and the marginal zone.
(Courtesy Dr. A.C. Durham, School of Veterinary Medicine, University of Pennsylvania; Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee; and Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
Figure 13-39.
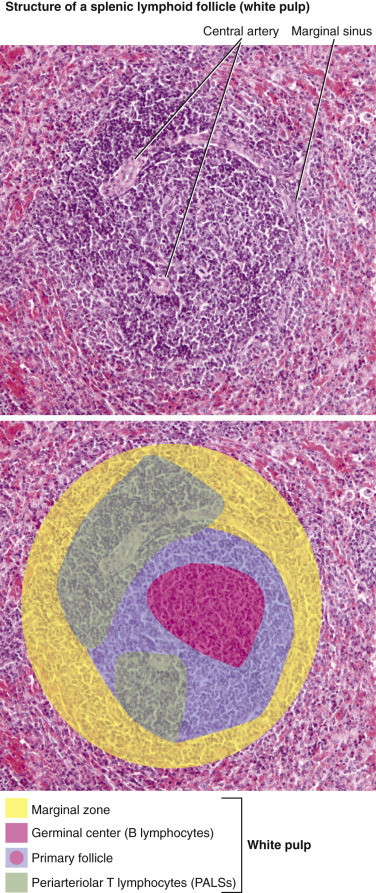
Structure of a Splenic Lymphoid Follicle (White Pulp).
The splenic white pulp is organized into periarteriolar sheaths (PALSs) around central arteries composed mainly of T lymphocytes, splenic follicles primarily composed of B lymphocytes, and the marginal zone, which forms the outer rim of the white pulp nodule. When exposed to antigen, the splenic lymphoid follicles develop germinal centers.
(Courtesy Dr. A.C. Durham, School of Veterinary Medicine, University of Pennsylvania; Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee; and Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
Figure 13-40.
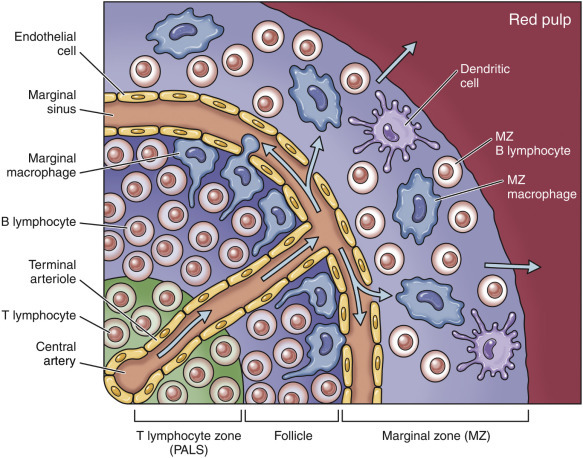
Structure of the Marginal Zone in the Splenic Follicle.
Antigens, bacteria, particles, and other material enter the follicle via the central arteries and reach the marginal sinus, where they are phagocytized by macrophages of the marginal zone. Once captured by these macrophages, blood-borne antigens are processed and presented to the lymphocytes within the white pulp.
The blood circulation of the spleen is particularly suited to enable its functions, namely, (1) filtering and clearing the blood of particulate matter and senescent cells; (2) transporting recirculating lymphocytes and naïve B and T lymphocytes to the follicle and PALS, respectively, to fulfill their specific immune functions; and (3) storage of blood in some domestic animal species (dog, cat, and horse) (Fig. 13-41 ). Phagocytosis is particularly effective in the spleen because blood flows through areas within the red pulp that are populated with increased concentrations of macrophages, namely, within the marginal sinuses, in cuffs around the penicillar arteries (PAMS), diffusely on the reticular walls of the red pulp vascular spaces, and along the sinusoids in dogs. Trafficking of naïve and recirculating lymphocytes is facilitated by the proximity of the marginal sinus to the follicular germinal centers and PALS.
Figure 13-41.
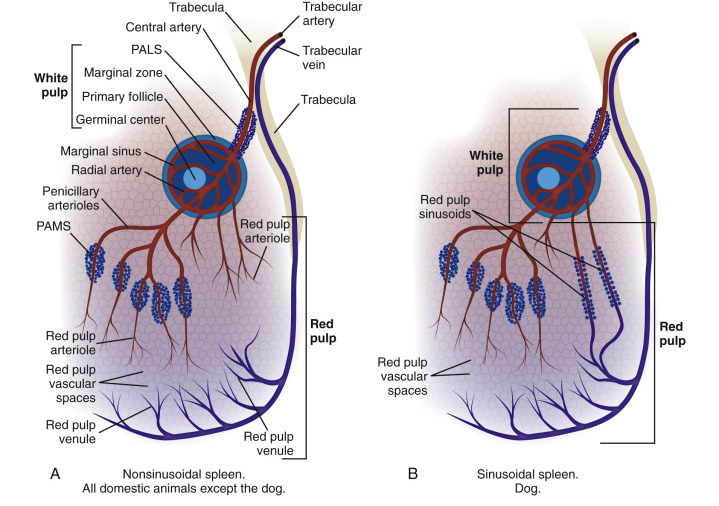
Major Pathways of Blood Flow in Nonsinusoidal and Sinusoidal Spleens.
A, Nonsinusoidal spleen, all domestic animals except the dog. The splenic artery enters at the hilus and divides into arteries, which enter the trabeculae. When a trabecular artery emerges from a trabecula it becomes the central artery and is encased in a periarteriolar lymphoid sheath (PALS), which is composed of T lymphocytes. It then enters the splenic follicle and gives off branches—the radial arteries, which supply the marginal sinus and marginal zone. The central artery emerges from the splenic follicle to enter the red pulp and branches into the penicillary arterioles, which are enclosed in a cuff of macrophages—the periarteriolar macrophage sheath (PAMS). The emerging penicillar arteries branch into arterioles and capillaries that supply the red pulp vascular spaces (see Fig. 13-43). The red pulp vascular spaces also receive blood from capillaries draining from the marginal sinus and drain into the splenic venules and then into the trabecular veins and splenic vein. B, Sinusoidal spleen, dog. The blood flow is essentially the same but with the additional feature that arterioles from the marginal sinus drain into the sinusoids and some blood from the red pulp vascular space passes through slits in the sinusoidal wall to enter the sinusoid (see Fig. 13-42). This is the site of pitting and erythrophagocytosis. Note that the major flow in A is sequentially past concentrations of macrophages in the marginal sinus, PAMS, and red pulp vascular spaces. In B there is the additional route from the marginal zone into the sinusoids. The figure does not illustrate variations in anatomy in the different domestic species such as a small marginal sinus in the cat and large PAMS in the dog, pig, and cattle, or variations in the blood supply, such as the sheathed arteries emptying into the marginal sinus.
(Courtesy Dr. A.C. Durham, School of Veterinary Medicine, University of Pennsylvania; Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee; and Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
Maps of the vascular blood flow in sinusoidal and nonsinusoidal spleens are illustrated in Figure 13-41, Figure 13-42, Figure 13-43 . The celiac artery is the major branch of the abdominal aorta from which the splenic artery arises. The splenic artery enters the splenic capsule at the hilus, where it branches and enters the fibromuscular trabeculae as trabecular arteries to supply the splenic parenchyma. Trabecular arteries become the central arteries of the white pulp and are surrounded by cuffs of T lymphocytes forming the PALS. The splenic follicles, populated by B lymphocytes, are eccentrically embedded within or just adjacent to the PALS. The central arteries send branches—the radial arteries—to supply the marginal sinus surrounding the splenic follicles. Thus the cells at the circumferences of the follicles are brought into intimate contact with blood-borne antigens and trafficking B and T lymphocytes in the marginal sinus. As a result of this pattern of blood flow, macrophages in the marginal sinus have the first opportunity to phagocytize antigens, bacteria, particles, and other material before macrophages in the sinusoids (in the dog) or in the PAMS and red pulp vascular spaces (all other domestic animals). In the dog the marginal sinus drains into the sinusoids, but in other domestic animals it drains into the red pulp vascular spaces.
Figure 13-42.
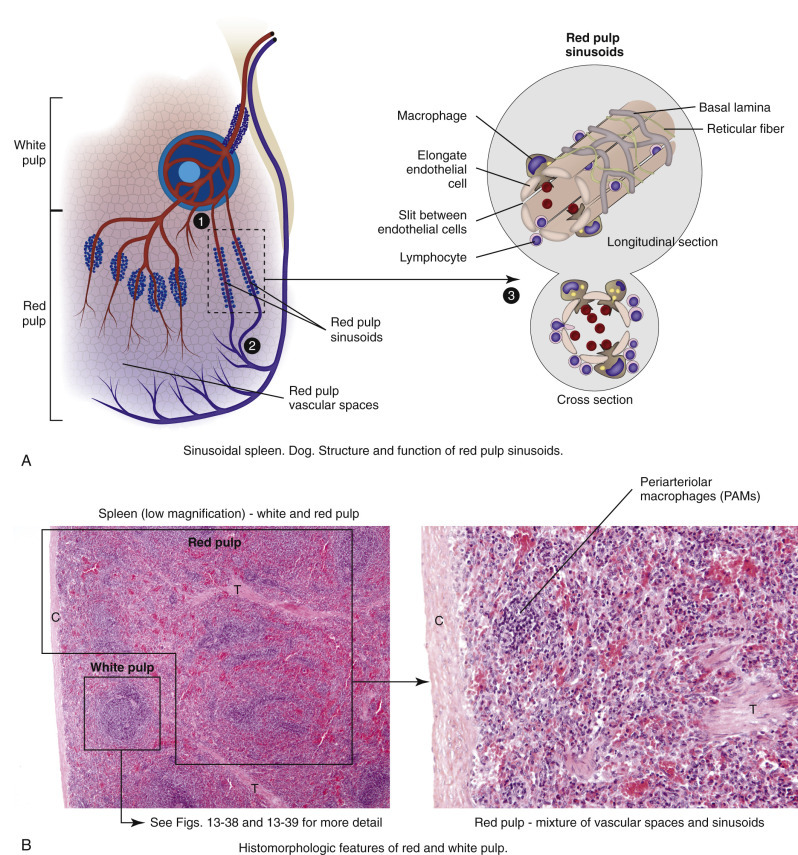
Vascular Flow in the Red Pulp of Dogs—Sinusoids.
A, Sinusoidal spleen, dog. Structure and function of red pulp sinusoids. 1, Branches of the central arteries of the white pulp and vessels from the marginal sinus enter into the sinusoids. 2, Sinusoids are lined by a discontinuous endothelium, and these empty into splenic venules, creating a closed system of circulation. 3, The red pulp of the dog spleen consists of both sinusoids and red pulp vascular spaces. B, Histomorphologic features of red and white pulp. C, capsule; T, trabeculae.
(Courtesy Dr. A.C. Durham, School of Veterinary Medicine, University of Pennsylvania; Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee; and Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
Figure 13-43.
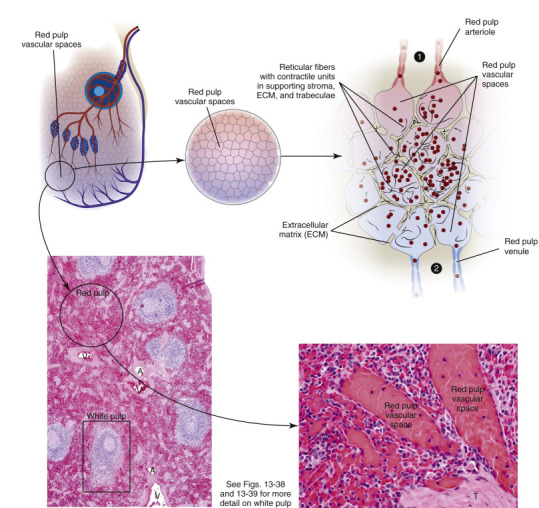
Vascular Flow in the Red Pulp of Domestic Animals—Red Pulp Vascular Spaces.
Red pulp vascular spaces occur in all domestic animals. 1, Blood travels into red pulp arterioles to enter red pulp vascular spaces in both types of spleens. 2, Blood leaves red pulp vascular spaces via red pulp venules and the trabecular veins. Red pulp vascular spaces are lined by reticular cells, and macrophages attached to these reticular walls provide constant surveillance of the blood. A, Artery; V, vein; T, trabecula.
(Courtesy Dr. A.C. Durham, School of Veterinary Medicine, University of Pennsylvania; Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee; and Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
The central arteries leave the white pulp, enter the red pulp, and branch into smaller penicillar arterioles. Each arteriole is surrounded by a sheath of macrophages known as periarteriolar macrophage sheaths (PAMS, previously known as ellipsoids), which are notably prominent in pigs, dogs, and cats. In horses, cattle, pigs, and cats the terminal branches of the penicillar arterioles empty into the red pulp vascular spaces lined by reticular cells. Because the red pulp vascular spaces are not lined by endothelium, this type of circulation is known as an open system. This system is in contrast to the sinusoidal spleen of the dog (also of the rat and human beings), where the branches of the central artery of the white pulp and vessels from the marginal sinus enter into the sinusoids, which are lined by a discontinuous endothelium, and these empty into splenic venules. This type of circulation is known as a closed system because the blood flow is through blood vessels (arterioles, capillaries, sinusoids, and venules), all of which are lined by endothelium. Although circulation in the red pulp is anatomically open in nonsinusoidal spleens, under certain conditions (e.g., during splenic contraction) the circulation is functionally closed, and the blood in the red pulp is diverted into “channels” lined by reticular cells. Because the dog has both sinusoids and red pulp vascular spaces, it has both open and closed splenic circulations, which may allow for both fast and slow flows of blood depending on the physiologic need of the animal. Blood flowing through the sinusoids or red pulp vascular spaces is under the surveillance of macrophages. In dogs the pseudopodia of these perisinusoidal macrophages project into the sinusoidal lumen through the spaces in the discontinuous endothelium. In all domestic animals, blood in the red pulp vascular spaces is under surveillance of macrophages attached to the reticular walls. Blood from the red pulp vascular spaces and sinusoids then drains into the splenic venules, splenic veins, and ultimately into the portal vein, which empties into the liver.
Function
The spleen filters blood and removes foreign particles, bacteria, and erythrocytes that are senescent, have structural membrane abnormalities, or are infected with hemotropic parasites. As a secondary lymphoid organ, its immunologic functions include the activation of macrophages to process and present antigen, the proliferation of B lymphocytes and production of antibody and biologic molecules, and the interaction of T lymphocytes and antigens. In some species the spleen stores significant quantities of blood (Box 13-7 ). The functions of the spleen are best considered on the basis of the two main components of the spleen: the red and white pulp and the anatomic systems contained within them (monocyte-macrophage system, red pulp vascular spaces, and hematopoiesis in the red pulp, and the B and T lymphocyte systems within the white pulp).
Box 13-7. Functions of the Spleen.
Red Pulp
Filtration (Monocyte-Macrophage System)
Removal (phagocytosis) of foreign material and bacteria
- Removal of erythrocytes
- Senescent erythrocytes
- Damaged erythrocytes (e.g., immune-mediated anemias)
- Parasitized erythrocytes (e.g., hemotropic parasites)
Storage (Red Pulp Vascular Spaces)
Storage of blood (in storage spleens)
Hematopoiesis
- Extramedullary hematopoiesis
- Severe demand (e.g., anemias)
- Degenerative/inflammatory conditions without concomitant hematologic disease
- Incidental (e.g., within nodules of hyperplasia)
- Monocytes within splenic cords
- Reserve for generating tissue macrophages in response to inflammation
White Pulp
Immunologic Functions
- PALS, splenic lymphoid follicle
- Lymphocyte transformation and proliferation
- Production of antibodies
- Marginal zone
- Homing of circulating lymphocytes in the blood
- Phagocytosis and processing of antigen
- Macrophage activation
PALS, Periarteriolar lymphoid sheath.
Red Pulp
Monocyte-Macrophage System.
Within the red pulp, macrophages are located in the marginal sinus, PAMS, and attached to the reticular walls of the red pulp vascular spaces. In the dog, macrophages are also located perisinusoidally. The supportive reticular network of the red pulp vascular spaces is composed of a fine meshwork of reticular fibers made of type III collagen, on which macrophages are dispersed. Exactly in which of these concentrations of macrophages phagocytosis of blood-borne particles takes place depends upon (1) the sequence in which they are exposed to the incoming blood, (2) the concentration of macrophages in these areas (e.g., the cat marginal sinus is small and thus not a major site of clearance; there is a compensatory increase in PAMS for phagocytosis), and (3) the functions of the macrophages. Some of the macrophages in the marginal sinus and marginal zone are responsible for phagocytosis of particulate matter and others for the trapping and ingestion of antigens and antigen-antibody complexes. Macrophages responsible for phagocytosis of blood-borne foreign material (Fig. 13-44 ), bacteria, and senescent and/or damaged erythrocytes (e.g., as seen in immune-mediated anemias and infections with hemotropic parasites) are also found in the red pulp. In the dog, sinusoidal macrophages remove entire erythrocytes (erythrophagocytosis), as well as portions of an erythrocyte's membrane and cytoplasmic inclusions, such as nuclear remnants like Heinz bodies, by a process called pitting. As such, the presence of large numbers of nuclear remnants in erythrocytes in canine blood smears may indicate malfunction of the sinusoidal system. The normal rate of removal of senescent erythrocytes from the circulating blood does not cause an increase in size of the spleen; however, splenomegaly can be observed when large numbers of defective erythrocytes must be removed, as in cases of severe acute hemolytic anemia. Nonsinusoidal spleens lack the fenestrated endothelium and perisinusoidal macrophages of canine sinusoids that allow for slow processing of red blood cells to determine which are to be returned to the circulation, pitted, or phagocytized. Instead, the macrophages of the red pulp perform these functions, and phagocytized cells remain in the red pulp vascular spaces. The location of the primary sites of pitting in nonsinusoidal spleens is unclear, but it is likely that most erythrophagocytosis takes place in the red pulp vascular spaces. The cat's spleen is deficient in pitting, and removal of Heinz bodies is slow; however, some erythrophagocytosis does occur in the marginal sinus.
Figure 13-44.
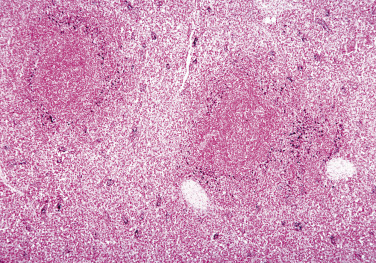
Phagocytosis of Foreign Material by Macrophages of the Splenic Marginal Zone (Calf Injected Intravenously with Micronized Carbon Particles).
Carbon particles (black pigment) are present in macrophages of the marginal zone. Macrophages phagocytize blood-borne foreign material, bacteria, viruses, and senescent and/or damaged erythrocytes (as in immune-mediated anemias and infections with hemotropic parasites). Eosin counterstain.
(Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
The macrophages of the sinusoids, marginal sinus, and red pulp vascular spaces are of bone marrow origin. From the bone marrow these cells circulate in the blood as monocytes and migrate into the spleen. Some macrophages are replenished by local proliferation. For example, after phagocytizing large amounts of material from the blood, the macrophages of the PAMS migrate through the wall of the cuff into the adjacent red pulp, denuding the PAMS of macrophages. After 24 hours, local residual macrophages have proliferated to repopulate the PAMS. The fixed macrophages elsewhere in the body, namely, those in connective tissue, lymph nodes (sinus histiocytes), liver (Kupffer cells), lung (pulmonary intravascular macrophages and pulmonary alveolar macrophages), and brain (resident and perivascular microglial cells), are also derived from bone marrow (see Chapters 5, 8, 9, and 14Chapter 5Chapter 8Chapter 9Chapter 14).
Red Pulp Vascular Spaces
Storage or Defense Spleens.
Spleens are also classified as either storage or defense spleens, based on whether or not they can store significant volumes of blood. The ability to store blood in the spleen depends on the fibromuscular composition of the splenic capsule and trabeculae. Splenic capsules and trabeculae with a low percentage of smooth muscle and elastic fibers cannot expand and contract and are designated as defense spleens. These are found in rabbits and human beings. The spleens of other domestic animal species have storage and defense functions but are classified as storage spleens because the extensive smooth muscle of the capsule and trabeculae allows the spleen to expand and contract. The spleens of ruminants and pigs are intermediate in their amount of smooth muscle and thus have limited storage capacity. Equine, canine, and feline spleens all have considerable storage and contractile capacity because of their muscular capsule, increased numbers of trabeculae, and the relatively small amount of splenic parenchyma devoted to white pulp. The storage capacity in dogs and horses is remarkable: It has been claimed that the canine spleen can store one-third of the dog's erythrocytes while the animal sleeps and the equine spleen holds one-half of the animal's circulating red cell mass (which is considered advantageous because it reduces the viscosity of the circulating blood). Storage spleens expand and contract quickly under the influence of the autonomic nervous system, via sympathetic and vagal fibers in the trabeculae and reticular walls of the red pulp vascular spaces and other circulatory disruptions, such as hypovolemic and/or cardiogenic shock. Thus storage spleens may be either grossly enlarged and congested or small with a wrinkled surface and a dry parenchyma depending on whether the spleen is congested from stored blood or shrunken from contraction (see Uniform Splenomegaly and Small Spleens).
Hematopoietic Tissue.
In the developing fetus the liver is the primary site of hematopoiesis, with the spleen making a minor contribution. Shortly before or after birth, hematopoiesis ceases in the liver and spleen, and the bone marrow becomes the primary hematopoietic organ. Under certain conditions, such as severe demand due to prolonged anemia, splenic hematopoiesis can be reactivated; this outcome is called extramedullary hematopoiesis (EMH). Studies have indicated that splenic EMH in dogs and cats most commonly occurs with degenerative or inflammatory conditions (e.g., hematomas, thrombosis) and may occur without concomitant hematologic disease (see Uniform Splenomegaly with a Firm Consistency). It is also found in splenic nodular hyperplasia (see Splenic Nodules with a Firm Consistency). In some species, such as the mouse, EMH is a normal function of the adult spleen and not necessarily a response to disease or hypoxic challenge. The splenic red pulp also contains large numbers of monocytes, which function as a reserve for generating tissue macrophages in response to ongoing tissue inflammation in the body.
White Pulp.
White pulp consists of PALS, each with a splenic lymphoid follicle surrounded by a marginal zone. Normally these foci of white pulp are so small that they may not be visible on gross examination of a cross section of the spleen. However, if nodules are enlarged either by lymphoid hyperplasia, amyloid deposits, or a neoplastic process (e.g., lymphoma), they can become grossly visible on the cut surface, initially as 0.5- to 1.0-mm white circular foci scattered through the red pulp. In animals with storage spleens, the distention of the red pulp from stored blood separates the foci of white pulp (PALS and lymphoid follicles), making white pulp appear sparser. Splenic white pulp is organized around central arteries in the form of PALS, which are populated primarily by T lymphocytes (see Figure 13-38, Figure 13-39, Figure 13-40). Primary splenic follicles are located eccentrically in PALS and are primarily composed of B lymphocytes. When exposed to antigen, the splenic lymphoid follicles develop germinal centers (see Lymphoid/Lymphatic System, Lymph Nodes, Function). Macrophages in the white pulp follicles remove apoptotic B lymphocytes not selected for expansion because of low binding affinity for antigen. Failure of these macrophages to phagocytize has been experimentally correlated with decreased production of growth factors like TGF-β and increased production of inflammatory cytokines that predispose the animal to autoimmune conditions.
The marginal zone surrounds the marginal sinus at the interface of the white and red pulp and consists of macrophages, DCs, and T and B lymphocytes. The blood supply of the marginal sinus is from the radial branches of the central artery, and it serves as the portal of entry into the spleen for recirculating B and T lymphocytes. From here, T lymphocytes migrate to the PALS and B lymphocytes to the germinal centers. Macrophages in the marginal zone capture blood-borne antigens, process them, and present them to the lymphocytes. B lymphocytes that recognize antigens corresponding to their receptors are activated, enter the follicle, and proliferate.
Macrophages in the marginal zone are phenotypically distinct from those in the red pulp. The red pulp macrophages function primarily to filter the blood by phagocytizing particles and by removing senescent or infected erythrocytes and pathogenic bacteria and fungi. Marginal zone macrophages are divided into two types based on their location and the type of cell surface receptors they possess. The first group is positioned toward the periphery of the marginal zone, whereas the second group, the marginal metallophilic macrophages (so called for their silver staining positivity), is at the inner margin of the marginal zone closer to the splenic follicle and PALS. It has been difficult to generate mammalian models that eliminate one of the two classes of marginal zone macrophages, so the degree to which one group specializes in a particular function is not clear. Some marginal zone macrophages actively phagocytize particulate matter or bacteria (e.g., septicemias caused by Streptococcus pneumoniae, Listeria monocytogenes, Campylobacter jejuni, or Bacillus anthracis) in the blood (see Fig. 13-40). They also play a similar role in limiting the spread of viral infections. Other marginal zone macrophages phagocytize and process antigens. Thus macrophages of the marginal zone serve to bridge the innate and adaptive immune responses by secreting inflammatory cytokines to activate other immune cells and providing receptor-based activation of marginal zone lymphocytes. Studies have shown that a loss of marginal zone macrophages coincides with decreased antigen trapping by resident B lymphocytes of the marginal zone and consequently a decrease in the early IgM response to antigens.
Dysfunction/Responses to Injury
The responses of the spleen to injury (Box 13-8 ) include acute inflammation, hyperplasia of the monocyte-macrophage system, hyperplasia of lymphoid tissues, atrophy of lymphoid tissues, storage of blood or contraction to expel reserve blood, and neoplasia. These responses are also best considered on the basis of the two main components of the spleen, the red and white pulp, and the anatomic systems associated with each.
Box 13-8. Responses of the Spleen to Injury.
Red Pulp
Inflammation
Acute inflammation with fibrin and necrosis
Abscesses, microabscesses
Granulomatous inflammation (diffuse, multifocal, focal)
Monocyte-Macrophage System
Hyperplasia
- Infectious agents
- Bacteremia/septicemia
- Facultative intracellular pathogens (e.g., Mycobacterium bovis)
- Fungi
Chronic hemolytic disease
Chronic splenic congestion
Red Pulp Vascular Spaces
Congestion
Blood storage or expulsion
White Pulp
Lymphoid hyperplasia (PALS, splenic lymphoid follicle)
Macrophage hyperplasia (marginal zone)
Atrophy (PALS, splenic lymphoid follicle; see Box 13-5)
Neoplasia
- Primary
- Lymphoma
- Sarcoma (e.g., hemangiosarcoma, histiocytic sarcoma, leiomyosarcoma)
Metastatic
PALS, Periarteriolar lymphoid sheath.
Red Pulp
Monocyte-Macrophage System.
The distribution and function of macrophages in the spleen is described earlier in the section on Structure and Function. These interactions are complex, and their relationships to both innate and adaptive immunity are areas of intense study (see also Chapter 5). To facilitate filtering, all of the blood in the body passes through the spleen at least once a day, and 5% of the cardiac output goes to the spleen. In dogs, blood flow and transit time depend on whether the spleen is contracted or distended; blood flow is slower in the distended spleen. The extent to which macrophages of the monocyte-macrophage system phagocytize particles depends to a large degree on the sequence in which they receive blood. In most species, macrophages of the marginal sinus are the first to receive blood, and consequently phagocytized particles and bacteria tend to be more concentrated here initially. However, there are differences among domestic animal species; the cat, for instance, has a comparatively small marginal sinus, and thus the PAMS play a larger role in phagocytosis.
The spleen is able to mount a strong response to blood-borne pathogens, which has been demonstrated in several studies. The blood of immunized rabbits injected intravenously with pneumococci cleared 98% of those bacteria within 15 minutes and 100% within an hour. The blood of dogs injected with 1 billion pneumococci per pound of body weight into the splenic artery was cleared of all bacteria in 65 minutes. After splenectomy, blood-borne organisms multiply rapidly and may disseminate widely in the body to cause an overwhelming postsplenectomy infection. Studies have also shown that the phagocytic function of the spleen is critical in the control of plasmodium (causative agent of malaria) in human beings and babesiosis in cattle. If the number of pathogenic bacteria in the circulation exceeds the capacity of the splenic macrophages, as in cases of severe septicemia, it may result in acute splenic congestion (see Uniform Splenomegaly with a Bloody Consistency). This may be followed by inflammation with areas of necrosis, fibrin deposition, and infiltration by neutrophils in bacteremias of pyogenic bacteria. The marginal zone can be the initial site of response to blood-borne antigens and bacteria delivered by the radial branches of the central arteries to the marginal sinus. Similar to the response of the red pulp vascular spaces, the marginal zone can become congested and with time (only hours with highly pathogenic organisms) may contain aggregates of neutrophils and macrophages. Histologically, the congestion and inflammation form a complete or partial concentric ring around the circumference of the splenic nodule (see Anthrax).
Hyperplasia of the red pulp macrophages is also seen in chronic hemolytic diseases, because there is a prolonged need for phagocytosis of erythrocytes. Similarly, chronic splenic congestion, usually the result of portal or splenic vein hypertension, can lead to proliferation of the macrophages present on the walls of the red pulp vascular spaces and results in thickening of the reticular walls between the red pulp vascular spaces. Macrophages in the red pulp also proliferate in response to fungi and facultative intracellular pathogens (e.g., Mycobacterium bovis) arriving hematogenously to the spleen. The number of red pulp macrophages may be augmented by monocytes recruited from the blood to form granulomatous inflammation, which may be diffuse or multifocal/focal (e.g., blastomycosis and tuberculosis, respectively).
Red Pulp Vascular Spaces.
The main response to injury of the red pulp vascular spaces is congestion (see Uniform Splenomegaly with a Bloody Consistency), as well as the storage of blood or contraction to expel reserve blood.
White Pulp.
The responses to injury within the white pulp are most pronounced in the splenic lymphoid follicles. Lymphoid follicular hyperplasia is a response to antigenic stimuli and results in the formation of secondary follicles; marked hyperplasia may be grossly evident. Hyperplasia of splenic lymphoid follicles follows a similar sequence of events and morphologic changes as seen in other secondary lymphoid organs and is discussed in more detail in Lymphoid/Lymphatic System, Lymph Nodes, Dysfunction/Responses to Injury. Similarly, atrophy of splenic lymphoid follicles has similar causes as lymphoid atrophy in other lymphoid organs (see Box 13-5). Briefly, atrophy occurs in response to lack of antigenic stimulation (e.g., from regression after antigenic stimulation has ceased), from the effects of toxins, antineoplastic chemotherapeutic agents, microorganisms, radiation, malnutrition, wasting/cachectic diseases, or aging, or when the bone marrow and thymus fail to supply adequate numbers of B and T lymphocytes, respectively. The follicles are depleted of lymphocytes, and with time, germinal centers and follicles disappear. The amount of the total lymphoid tissue is reduced, and the spleen may be smaller.
The response to injury of the monocyte-macrophage system in the marginal sinus and marginal zone is also phagocytosis and proliferation.
Capsule and Trabeculae.
Lesions in the capsule and trabeculae are uncommon and include splenic capsulitis secondary to peritonitis, and complete or partial rupture of the splenic capsule, usually due to trauma.
Portals of Entry/Pathways of Spread
The two main portals of entry to the spleen for infectious agents are hematogenous spread and direct penetration. The splenic capsule is thick, and thus direct penetration is less common. Inflammation from an adjacent peritonitis is unlikely to penetrate the capsule into the splenic parenchyma. Cattle with traumatic reticulitis may have foreign objects migrate into the ventral extremity of the spleen, causing a splenic abscess. Splenic abscesses also develop secondary to perforation of the gastric wall in horses, due to foreign body penetration, gastric ulcers, or gastric inflammation. Portals of entry used by microorganisms and other agents and substances to access the lymphoid/lymphatic system are summarized in Box 13-6.
Defense Mechanisms/Barrier Systems
Defense mechanisms used by the spleen to protect itself against microorganisms and other agents are the innate and adaptive immune responses, discussed in Chapters 3, 4, and 5Chapter 3Chapter 4Chapter 5. Other defense mechanisms are structural in nature to protect against external trauma and include the thick fibrous capsule of the spleen.
Lymph Nodes
Structure
Lymph nodes are soft, pale tan, round, oval or reniform organs with a complex three-dimensional structure. On gross examination of a cross section of lymph nodes, two main areas are visible: an outer rim of cortex and an inner medulla (Fig. 13-45 ). To understand the pathologic response of the lymph node, it is important to consider its anatomic components and their relationship with antigen processing (Fig. 13-46 ):
-
•
Stroma—Capsule, trabeculae, and reticulum
-
•
Cortex—“Superficial” or “outer” cortex (lymphoid follicles, B lymphocytes)
-
•
Paracortex—“Deep” or “inner” cortex (T lymphocytes)
-
•
Medulla—Medullary sinuses and medullary cords
-
•
Blood vessels—Arteries, arterioles, high endothelial venules (HEVs), efferent veins
-
•
Lymphatic vessels—Lymphatic afferent and efferent vessels; lymphatic sinuses (subcapsular, trabecular, and medullary)
-
•
Monocyte-macrophage system—Sinus histiocytes
Figure 13-45.
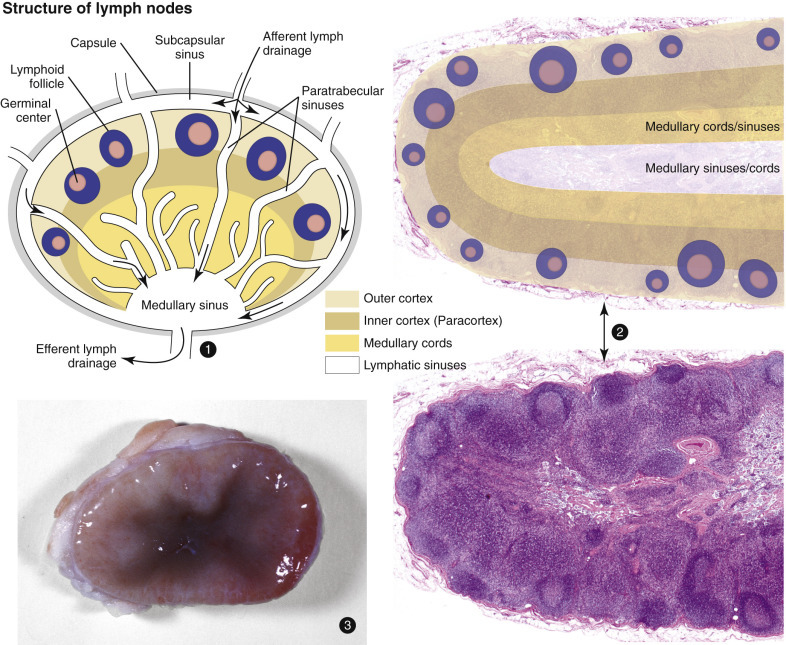
Structure of a Lymph Node.
1 and 2, Lymph node architecture consists of an outer cortex composed of lymphoid follicles (B lymphocytes), inner/deep paracortex (T lymphocytes), and medulla (medullary cords and sinus). Lower right, Histologic section. H&E stain. 3, Gross photograph of a lymph node: The cortex (both outer and inner) is pale tan-pink, and the medulla is dark red.
(Courtesy Dr. A.C. Durham, School of Veterinary Medicine, University of Pennsylvania; Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee; and Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
Figure 13-46.
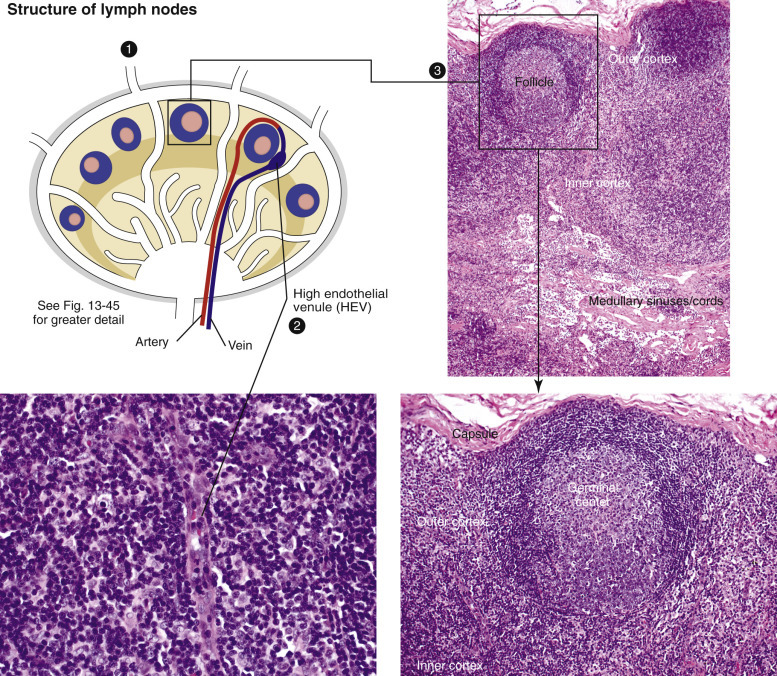
Cellular Zones of a Lymph Node.
1, Antigen arrives in the afferent lymphatic vessels, empties into the subcapsular sinus, and drains into the trabecular and medullary sinuses. 2, As antigens travel through the sinuses, they are captured and processed by macrophages and dendritic cells (DCs), or antigen-bearing DCs in blood can enter through high endothelial venules (HEVs). B lymphocytes encounter DCs charged with antigen, are activated, and migrate to a primary follicle to initiate germinal center formation, creating secondary follicles. 3 and lower right image, lymphoid follicles. Germinal centers have a distinct polarity (superficial or light zone and a deep dark zone), and the mantle cell rim partially encircles the germinal center and is wider over the light pole of the follicle.
(Courtesy Dr. A.C. Durham, School of Veterinary Medicine, University of Pennsylvania; Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee; and Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
Stroma.
The lymph node is enclosed by a fibrous capsule penetrated by multiple afferent lymphatic vessels, which empty into the subcapsular sinus (see also Figs. 13-45 and 13-46). At the hilus, efferent lymphatic vessels and veins exit, and arteries enter the node. Fibrous trabeculae extend from the capsule into the parenchyma to provide support to the node and to house vessels and nerves. The lymph node is also supported by a meshwork of fibroblastic reticular cells and fibers. Besides providing structural support, this reticulum helps form a substratum for the migration of lymphocytes and antigen-presenting cells to the follicles and facilitates the interaction with B and T lymphocytes.
Cortex.
The outer/superficial cortex contains the lymphoid follicles (also referred to as lymphoid nodules) (see Figs. 13-45 and 13-46). The follicles are designated as primary if they consist mainly of small lymphocytes: Mature naïve B lymphocytes expressing receptors for specific antigens exit the bone marrow and circulate through the bloodstream, lymphatic vessels, and secondary lymphoid tissues. On their arrival at lymph nodes, B lymphocytes exit through HEVs in the paracortex and home to a primary follicle (which also contains follicular DCs in addition to the resting B lymphocytes). Lymphoid follicles with germinal centers are designated as secondary follicles: B lymphocytes that recognize the antigen for which they are expressing receptors are activated and proliferate to form the secondary lymphoid follicles characterized by prominent germinal centers. Germinal centers are areas with a specialized microenvironment that support the proliferation and further development of B lymphocytes to increase their antigen and functional capacity (see Lymphoid/Lymphatic System, Lymph Nodes, Function). The mantle cell zone surrounds the germinal center and consists of small inactive mature naïve B lymphocytes and a smaller population of T lymphocytes (approximately 10%).
Paracortex.
The diffuse lymphoid tissue of the paracortex (also referred to as the deep or inner cortex) consists mainly of T lymphocytes, as well as macrophages and DCs (see Figs. 13-45 and 13-46). This region contains the HEVs through which B and T lymphocytes migrate from the blood into the lymphoid follicles and paracortex, respectively. T and B lymphocytes may also enter the lymph node via the lymphatic vessels.
Medulla.
The medulla is composed of medullary cords and medullary sinuses (see Figs. 13-45 and 13-46). The medullary cords contain macrophages, lymphocytes, and plasma cells. In a stimulated node the cords become filled with antibody-secreting plasma cells. The medullary sinuses are lined by fibroblastic reticular cells and contain macrophages (“sinus histiocytes”), which cling to reticular fibers crossing the lumen of the sinus. These macrophages phagocytize foreign material, cellular debris, and bacteria from the incoming lymph.
Vasculature: Blood Vessels, Lymphatic Vessels, and Lymphatic Sinuses.
The blood vessels of the lymph node include arteries, arterioles, veins, and postcapillary venules (HEVs) lined by specialized cuboidal endothelium (see Figs. 13-45 and 13-46). Approximately 90% to 95% of lymphocytes enter lymph nodes through the HEVs, which also play an important role in lymph fluid balance. The lymphatic vasculature consists of afferent lymphatic vessels, which pierce the capsule and drain into the subcapsular sinus. Lymph continues to drain through the trabecular sinuses to the medullary sinuses and finally exits at the hilus via efferent lymphatic vessels.
All lymph nodes receive afferent lymphatic vessels from specific areas of the body. The term lymphocenter is often used in veterinary anatomy to describe a lymph node or a group of lymph nodes that is consistently present at the same location and drains from the same region in all species. For example, the popliteal lymph node, caudal to the stifle, drains the distal hind limb. The tracheobronchial nodes (bronchial lymphocenter), located at the tracheal bifurcation, collect lymph from the lungs and send it to the mediastinal nodes or directly to the thoracic duct. Because lymph from a single afferent lymphatic vessel drains into a discrete region of a lymph node, only these regions of the node may be affected by the contents of a single draining lymph vessel (e.g., antigen, infectious organisms, or metastatic neoplasms [Fig. 13-47 ]).
Figure 13-47.
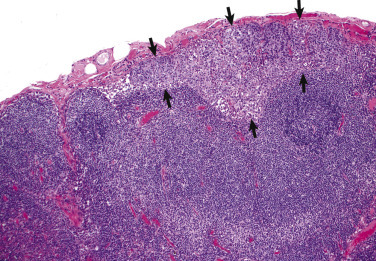
Subcapsular Sinuses with Metastatic Carcinoma, Lymph Node, Dog.
Subcapsular sinuses are sites for embolization, lodgment, invasion, and growth of neoplastic emboli (arrows), most commonly carcinomas. Emboli initially lodge in that portion of the lymph node drained by the branch of the afferent lymphatic vessel draining the site of the primary carcinoma. H&E stain.
(Courtesy Dr. A.C. Durham, School of Veterinary Medicine, University of Pennsylvania.)
The lymph node of the pig has a different structure. The afferent lymphatic vessels enter at the hilus instead of around the periphery of the node and empty lymph into the center of the node. The lymph drains to the “subcapsular” sinus (the equivalent of the medullary sinuses of other domestic animals) and then into several efferent lymphatic vessels, which pierce the outer capsule. This reversal of flow is the result of an inverted nodal architecture, with the cortex in the middle of the node surrounded by the medulla at the periphery. Thus a pig lymph node that is draining an area of hemorrhage will have blood accumulate in the periphery (subcapsular) instead of in the center of the node (which may be grossly visible).
Function
The functions of the lymph node are (1) to filter lymph of particulate matter and microorganisms, (2) to facilitate the surveillance and processing of incoming antigens via interactions with B and T lymphocytes, and (3) to produce B lymphocytes and plasma cells. Material arriving in the lymph can be subdivided into free particles and larger molecules, small molecules and free antigens, and antigen within DCs. It is helpful to consider the paths taken by particles, molecules, antigens, and cells arriving at a lymph node. The following account describes the journey of an antigen as it enters a lymph node to trigger an immune response.
Antigen in the lymph arriving in the afferent lymphatic vessels empties into the subcapsular sinus. Hydrostatic pressure here is low, and reticular fibers crossing the sinus impede flow, and thus particles tend to settle, which facilitates phagocytosis by the sinus macrophages. Lymph then flows down the trabecular sinuses that line the outer surface of fibrous trabeculae, to the medullary sinus, and eventually exits via efferent vessels. As antigens within the lymph travel through the sinuses, they are captured and processed by macrophages and DCs. Alternatively, DCs charged with antigen can migrate within blood vessels to the node and enter the paracortex via the HEVs. Circulating B lymphocytes also enter across the HEVs, and if they encounter antigen-bearing DCs, there is a local reaction involving the appropriate T helper lymphocytes, B lymphocytes, and DCs. This results in the migration of the activated B lymphocytes to a primary follicle, where they initiate formation of a germinal center.
Germinal centers, upon migration of antigen-activated B lymphocytes, develop a characteristic architecture. Distinct polarity composed of a superficial or light zone and a deep dark zone is present in cases of antigenic stimulation. The light zone, orientated at the source of antigen, consists mainly of small lymphocytes, called centrocytes, which have moderate amounts of pale eosinophilic cytoplasm. The cells of the dark zone, called centroblasts, are large, densely packed lymphocytes with scant cytoplasm, giving this area a darker appearance on H&E staining. The centroblasts undergo somatic mutations of the variable regions of the immunoglobulin gene, followed by isotype class switching (from IgM to IgG or IgA). During this process most centroblasts undergo apoptosis, and cell fragments are phagocytized by macrophages, which are then termed tingible (stainable) body macrophages. The cells that have survived the affinity maturation process are now called centrocytes and along with T lymphocytes and follicular DCs, populate the germinal center light zone. These post–germinal center B lymphocytes leave the follicle as plasma cell precursors (immunoblasts or plasmablasts) and migrate from the cortex to the medullary cords, where they mature and excrete antibody into the efferent lymph. Some of these cells may colonize the region surrounding the mantle cell zone to form a marginal zone. Marginal zones are apparent only in situations of prolonged and intense immune stimulation and serve as a reservoir of memory cells. The elliptical mantle cell cuff is wider over the light pole of the follicle, though in instances of strong antigenic stimulation, the cuffs can completely encircle the germinal center.
Dysfunction/Responses to Injury
Responses to injury are listed in Box 13-9 , and the responses are discussed on the basis of the following systems: sinus histiocytes of the monocyte-macrophage system, cortex, paracortex, and medulla (medullary sinuses and medullary cords).
Box 13-9. Responses of the Lymph Node to Injury.
Hyperplasia
Sinus histiocytosis (monocyte-macrophage system)
Follicular hyperplasia (B lymphocytes)
Paracortical hyperplasia (T lymphocytes)
Atrophy
Lymphoid atrophy (see Box 13-5)
Inflammation
Acute or chronic lymphadenitis
Neoplasia
Primary (lymphoma)
Metastatic
Generally, enlarged lymph nodes can be distributed in several different patterns in the body. First, all lymph nodes throughout the body (systemic or generalized) may be enlarged (lymphadenopathy or lymphadenomegaly). This pattern is usually attributed to systemic infectious, inflammatory, or neoplastic processes. If a single lymph node or regional chain of nodes is enlarged, then the area drained by that node should be checked for lesions (e.g., evaluate the oral cavity if the mandibular lymph nodes are enlarged). Thus it is important to know the area drained by specific lymph nodes. Mesenteric lymph nodes are normally larger because of follicular hyperplasia and sinus histiocytosis, because these nodes continuously receive and respond to barrages of antigens and bacteria from the intestinal tract.
Sinus Histiocytes (Monocyte-Macrophage System).
Sinus histiocytes (macrophages) are part of the monocyte-macrophage system and the first line of defense against infectious and noninfectious agents in the incoming lymph. In response to these draining agents, there is hyperplasia of the macrophages (“sinus histiocytosis”), most notable in the medullary sinuses (Fig. 13-48 ). Leukocytes, often monocytes, may harbor intracellular pathogens (e.g., Mycobacterium spp., cell-associated viruses such as parvovirus), arrive in the blood or lymph, infect the lymph node, and then are disseminated throughout the lymphoid tissues of the body via the efferent lymph and circulating blood.
Figure 13-48.
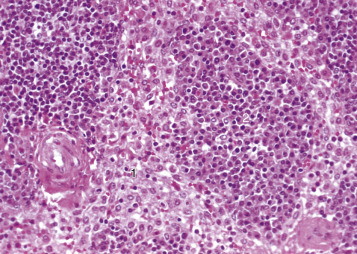
Medullary Sinus Histiocytosis and Medullary Cord Plasmacytosis, Lymph Node, Dog.
1, The medullary sinuses are filled with histiocytes (macrophages) in response to drainage of infectious and noninfectious agents in the incoming lymph. 2, The medullary cords are filled with plasma cells and fewer lymphocytes. Plasma cell precursors are formed in the germinal centers, mature into plasma cells, and migrate to the medullary cords. The presence of large numbers of plasma cells in the medullary cords indicates ongoing production of antibody due to an antigenic stimulus.
(Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Cortex (Lymphoid Follicles).
Follicular hyperplasia of the cortex is discussed in the section Lymphoid/Lymphatic System, Lymph Node, Function. An antigenically stimulated lymph node that is undergoing follicular hyperplasia is enlarged and has a taut capsule, and the cut surface may bulge. Histologically, the follicles contain active germinal centers with antigenic polarity (light and dark zones) (Figs. 13-49 and 13-50 ; also see Fig. 13-46). Depending on the duration and continued exposure to the antigen, there may also be concomitant paracortical hyperplasia and medullary cord plasmacytosis. Less florid follicular reactions will have smaller separated germinal centers, whereas nodes receiving persistent high levels of antigen stimulation may have coalescing germinal centers (termed “atypical benign follicular hyperplasia”). In such cases of chronic strong antigenemia, the highly reactive nodes may also exhibit colonization of lymphocytes into perinodal fat, and germinal centers may contain irregular lakes of eosinophilic material, known as follicular hyalinosis. As the immune response declines, there is follicular lymphoid depletion and the concentration of lymphocytes in the germinal centers is reduced, allowing the underlying follicular stroma (including DCs and macrophages) to become visible. With ongoing lymphocyte depletion, the mantle cell zones are thinned, less populated, and discontinuous. Eventually, residual mantle cells collapse into the follicular stroma, forming clusters of small dark cells within the bed of DCs and macrophages, referred to as fading follicles.
Figure 13-49.
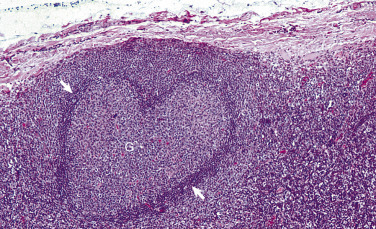
Benign Follicular Hyperplasia, Lymph Node, Dog.
Antigenic stimulation results in a secondary follicle with germinal center formation (G). The centroblasts of the germinal center undergo somatic mutations and isotype class switching, a process during which most centroblasts undergo apoptosis and cell fragments are phagocytized by tingible body macrophages. The cells that have survived the affinity maturation process (centrocytes) leave the germinal center as plasma cell precursors. Some of these cells migrate to the medullary cords, where they mature and excrete antibody into the efferent lymph, whereas others colonize the region surrounding the mantle cell zone to form a marginal zone. In instances of strong antigenic stimulation, the mantle cell cuffs can completely encircle the germinal center (arrows). H&E stain.
(Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Figure 13-50.

Benign Follicular Hyperplasia, Chronic Demodicosis, Prescapular Lymph Node, Dog.
There is diffuse hyperplasia of the lymphoid follicles (F) with prominent and often coalescing germinal centers. H&E stain.
(Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Paracortex.
Paracortical atrophy may result from a variety of causes, including deficiency in lymphocyte production in the bone marrow, reduced differential selection of lymphocytes in the thymus, or destruction of lymphocytes in the lymph node by viruses, radiation, and toxins directly on the lymphocytes in the lymph node (see Box 13-5). Examination of H&E-stained sections allows evaluation of follicular activity in the cortex and the concentration of plasma cells in the medullary cords, which serve as a reasonable estimate of B lymphocyte activity for comparison.
Paracortical hyperplasia may have a nodular or diffuse appearance depending on which and how many afferent lymphatic vessels are draining antigen. This reaction may precede or be concurrent with the germinal center reaction of follicular hyperplasia. Proliferation of T lymphocytes has been reported in the paracortex (and PALS of the spleen) in malignant catarrhal fever (MCF) in cattle and in pigs with porcine reproductive and respiratory syndrome. PCV2 can cause a diffuse proliferation of macrophages within the paracortex.
Medulla (Medullary Sinuses and Cords).
Responses to injury by the medullary sinuses are dilation of the sinuses and proliferation of histiocytes (“sinus histiocytosis”). Sinus macrophages proliferate in response to a wide variety of particulate matter in the lymph, including bacteria and erythrocytes (erythrophagocytosis) draining from a hemorrhagic area (see Lymphoid/Lymphatic System, Disorders of Domestic Animals: Lymph Nodes, Pigmentation of Lymph Nodes). Dilation of the sinuses due to edema occurs with many underlying conditions, including chronic cardiac failure or drainage from an acutely inflamed area. As the inflammation progresses, the sinuses become filled with neutrophils, macrophages, and occasionally fibrin, in addition to the hyperplastic resident sinus histiocytes (see Fig. 13-48). Depending on the intensity of the inflammation, the adjacent parenchyma may become affected (see Lymphoid/Lymphatic System, Disorders of Domestic Animals: Lymph Nodes, Enlarged Lymph Nodes [Lymphadenomegaly], Acute Lymphadenitis).
As pointed out in the section on Lymph Nodes, Function, after activation and proliferation of B lymphocytes in the follicle, the immunoblasts formed there move to and mature in the medullary cords, which as a result are distended with plasma cells that secrete antibody into the efferent lymphatic vessels (“medullary plasmacytosis”). The concentration of medullary plasma cells correlates with the activity of the germinal centers. As the immune response subsides, the number of plasma cells decreases and the medullary cords return to their resting state populated by few lymphocytes and scattered plasma cells.
Portals of Entry/Pathways of Spread
The two main portals of entry to the lymph node for infectious agents and antigens are afferent lymphatic vessels (lymphatic spread) and blood vessels (hematogenous spread). Portals of entry used by microorganisms and other agents and substances to access the lymphoid/lymphatic system are summarized in Box 13-6. Infectious microorganisms, either free within the lymph or within lymphocytes or monocytes, are transported to regional lymph nodes through lymphatic vessels. Agents may escape removal by phagocytosis in one lymph node and be transported via efferent lymphatic vessels to the next lymph node in the chain and cause an inflammatory or immunologic response there. This process can continue serially down a lymph node chain, and if the agent is not removed, it may eventually be transported via the lymphatic vessels to either the cervical or thoracic ducts and then disseminated throughout the body.
Although most pathogens are transported to lymph nodes via afferent lymphatic vessels, bacteria can be transported to lymph nodes hematogenously (free or within leukocytes such as monocytes) in septicemias and bacteremias. Direct penetration of a lymph node is uncommon, because it is protected by a thick fibrous capsule. Occasionally, inflammatory cells or neoplasms can extend directly into nodal parenchyma from adjacent tissues.
Defense Mechanisms/Barrier Systems
Defense mechanisms used by the lymphatic system to protect itself against microorganisms and other agents are the innate and adaptive immune responses, discussed in Chapters 3, 4, and 5Chapter 3Chapter 4Chapter 5. Other defense mechanisms are structural in nature to protect against external trauma and include the thick fibrous capsules of lymph nodes.
Hemal Nodes
Structure and Function
Hemal nodes are small, dark red to brown nodules found most commonly in ruminants, mainly sheep, and have also been reported in horses, primates, and some canids. Their architecture resembles that of a lymph node with lymph follicles and sinuses, except that in the hemal node, sinuses are filled with blood (E-Fig. 13-8). Because erythrophagocytosis can be present, it is presumed that hemal nodes can filter blood and remove senescent erythrocytes, but as their blood supply is small, their functional importance is not clear.
E-Figure 13-8.
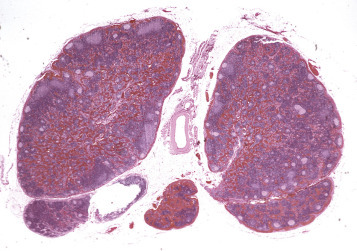
Hemal Node, Ruminant.
Hemal nodes resemble lymph nodes except that the sinuses are filled with blood. H&E stain.
(Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Mucosa-Associated Lymphoid Tissue
Structure and Function
MALT is the initial site for mucosal immunity and is crucial in the protection of mucosal barriers. MALT is composed of both diffuse lymphoid tissues and aggregated lymphoid (also known as lymphatic) nodules, which can be subcategorized based on their anatomic location: (1) bronchus-associated lymphoid tissue (BALT), which is often at the bifurcation of the bronchi and bronchioles; (2) tonsils (pharyngeal and palatine) form a ring of lymphoid tissue at the oropharynx; (3) nasal-, larynx-, and auditory tube–associated lymphoid tissues (NALT, LALT, and ATALT, respectively) within the nasopharyngeal area; (4) gut-associated lymphoid tissue (GALT), which includes Peyer's patches and diffuse lymphoid tissue in the gut wall; (5) conjunctiva-associated lymphoid tissue (CALT); (6) other lymphoid nodules (e.g., genitourinary tract) (Fig. 13-51 ).
Figure 13-51.
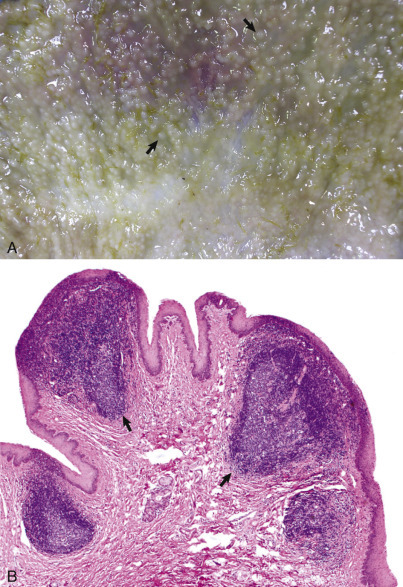
Lymphoid Follicular Hyperplasia, Mucosa-Associated Lymphoid Tissue.
A, The mucosa contains multifocal, slightly raised, soft white nodules (arrows). B, Prominent lymphoid follicles (arrows) have formed within the submucosa. H& E stain.
(Courtesy Dr. A.C. Durham, School of Veterinary Medicine, University of Pennsylvania.)
Diffuse lymphoid tissue consists of lymphocytes and DCs within the lamina propria of the mucosa of the alimentary, respiratory, and genitourinary tracts. These cells intercept and process antigens, which then travel to regional lymph nodes to initiate the immune response, leading ultimately to the secretion of IgA, IgG, and IgM.
Solitary lymphoid nodules are localized concentrations of lymphocytes (mainly B lymphocytes) in the mucosa and consist of defined but unencapsulated clusters of small lymphocytes (primary lymphoid nodule). They are usually not grossly visible in the resting or antigenically unstimulated state, but upon antigenic stimulation, they proliferate and form germinal centers and surrounding mantle cell zones (secondary lymphoid nodules).
Aggregated lymphoid nodules consist of groups of lymph nodules, the most notable of which are the tonsils and Peyer's patches. The aggregated lymphoid follicles of the Peyer's patches are most obvious in the ileum. The latter are covered by a specialized epithelium, the follicle-associated epithelium (FAE). The FAE is the interface between the Peyer's patches and the luminal microenvironment and consists of enterocytes and interdigitated M cells. M cells transport (via endocytosis, phagocytosis, pinocytosis, and micropinocytosis) antigens, particles, bacteria, and viruses from the intestinal lumen to the underlying area rich in DCs , which deliver the material to the lymphoid tissue of the Peyer's patches. M cells also express IgA receptors, which allows for the capture and transport of bacteria entrapped by IgA. The proportion of enterocytes and M cells within the FAE is modulated by the luminal bacterial composition. For instance, M cells increase in animals transferred from pathogen-free housing to the normal environment. M cells may also be exploited as a portal for entry by some microbes (see Lymphoid/Lymphatic System, Portals of Entry/Pathways of Spread). Table 13-5 lists the interactions of the MALT with different microorganisms.
Table 13-5.
Function of Mucosa-Associated Lymphoid Tissue (MALT) in Viral and Bacterial Diseases in Livestock
| Function | Species | Microorganism |
|---|---|---|
| TONSILS | ||
| Portal of entry | Sheep and goats | Chlamydia psittaci |
| Initial site of infection | Cattle | BVDV |
| Early site of infection | Cattle | BHV-1 |
| Site of replication | Pig | Porcine circovirus type 2 PMWS infection |
| Carriers (reservoirs) | Horses | Streptococcus equi subsp. zooepidemicus |
| Cattle | Mannheimia haemolytica | |
| Sheep |
Salmonella spp. Pasteurella haemolytica Scrapie agent (PrPSc) |
|
| Pigs |
Mycoplasma spp. Streptococcus suis Salmonella spp. Yersinia pseudotuberculosis |
|
| GALT (PEYER'S PATCHES) | ||
| Portal of entry | Cattle |
Brucella abortus Mycobacterium avium ssp. paratuberculosis |
| Sheep and goats | Yersinia tuberculosis | |
BVDV, Bovine viral diarrhea virus; BHV-1, bovine herpesvirus 1; PMWS, postweaning multisystemic wasting syndrome.
Data from Liebler-Tenorio EM, Pabst R: Vet Res 37:257-280, 2006.
Dysfunction/Responses to Injury
The responses of MALT to injury are similar to those of other lymphoid tissues: hyperplasia, atrophy, and inflammation (Box 13-10 ).
Box 13-10. Responses of Mucosa-Associated Lymphoid Tissue to Injury.
- Hyperplasia
- Lymphoid hyperplasia with germinal center formation due to antigenic stimulation
- Atrophy
- Lymphoid atrophy (see Box 13-5)
- Inflammation
- Granulomatous (Johne's disease; see Diseases of Ruminants)
Hyperplasia.
Hyperplasia of lymphoid nodules is a response to antigenic stimulation and consists of activation of germinal centers with subsequent production of plasma cells (see Fig. 13-51, B). Lymphoid nodule hyperplasia is often present in chronic disease conditions, such as BALT hyperplasia in chronic Dictyocaulus spp. (horses, cattle, sheep, and goats) or Metastrongylus spp. (pigs) associated bronchitis or bronchiolitis. Mycoplasma spp. pneumonias of sheep and pigs display marked BALT hyperplasia that can encircle bronchioles and bronchi (“cuffing pneumonia”).
Hyperplastic lymphoid nodules can be so enlarged that they become grossly visible as discrete white plaques or nodules (see Fig. 13-51, A). They can be seen in the conjunctiva of the eyelids and the third eyelid in chronic conjunctivitis, the pharyngeal mucosa in chronic pharyngitis, the gastric mucosa in chronic gastritis, and the urinary bladder in chronic cystitis (follicular cystitis). The normal fetus has no detectable BALT, though it may be present in fetuses aborted due to infectious disease.
Atrophy.
Atrophy of the diffuse lymphoid tissue and lymphoid nodules has the same causes as atrophy affecting other lymphoid tissues (see Box 13-5) and includes lack of antigenic stimulation, cachexia, malnutrition, aging, viral infections, or failure to be repopulated by B lymphocytes from the bone marrow or T lymphocytes from the thymus. Lymphocytolysis of germinal center lymphocytes of Peyer's patches is a characteristic lesion in BVDV infection in ruminants and canine and feline parvovirus infections (“punched-out Peyer's patches) (see Chapters 4 and 7).
Portals of Entry/Pathways of Spread
The main portals of entry to MALT for infectious agents are hematogenous spread and through migrating macrophages, DCs , and M cells. Pathogenic bacteria such as Escherichia coli, Yersinia pestis, Mycobacterium avium ssp. paratuberculosis (MAP), L. monocytogenes, Salmonella spp., and Shigella flexneri can invade the host from the lumen of the intestine through dendritic or M cells. Some viruses (e.g., reovirus) may be transported by M cells. The scrapie prion protein (PrPSc) may also accumulate in Peyer's patches. Many viruses, such as bovine coronavirus, BVDV, rinderpest virus, malignant catarrhal fever virus, feline panleukopenia virus, and canine parvovirus, cause lymphocyte depletion within the MALT. Portals of entry used by microorganisms and other agents and substances to access the lymphoid system are summarized in Box 13-6.
Defense Mechanisms/Barrier Systems
Defense mechanisms used by MALT to protect itself against microorganisms and other agents are the innate and adaptive immune responses, discussed in Chapters 3, 4, and 5Chapter 3Chapter 4Chapter 5.
Disorders of Domestic Animals: Thymus
Congenital Disorders
Congenital disorders of the thymus are discussed in detail in Chapter 5. Summaries of the gross and microscopic morphologic changes are described in the sections on Disorders of Horses and Disorders of Dogs.
Thymic cysts can be found within the developing and mature thymus and in thymic remnants in the cranial mediastinum. Thymic cysts are often lined by ciliated epithelium and represent developmental remnants of branchial arch epithelium and are usually of no significance.
Inflammatory and Degenerative Disorders
Thymitis is an uncommon lesion and may be seen in PCV2 infection (see Disorders of Pigs and also Chapter 4), enzootic bovine abortion (see Chapter 18), and salmon poisoning disease of dogs (see Chapter 7). Infectious agents more commonly cause thymic atrophy. Variable degrees of acquired immunodeficiency can be also be caused by toxins, chemotherapeutic agents and radiation, malnutrition, aging, and neoplasia. Of infectious agents, viruses most commonly infect and injure lymphoid tissues and include the following: EHV-1 in aborted foals (Fig. 13-52 ), classic swine fever virus, BVDV, canine distemper virus, canine and feline parvovirus, and FIV; severe thymic lymphoid depletion is an early lesion in FIV-infected kittens.
Figure 13-52.

Equine Herpesvirus 1, Spleen, Aborted Foal.
Most of the splenic follicle is occupied by nuclear debris, the result of lymphocytolysis. note: lymphocytolysis may be caused by other infectious and noninfectious agents (e.g., chemotherapeutic drugs). H&E stain.
(Courtesy College of Veterinary Medicine, University of Illinois.)
Environmental toxins, such as halogenated aromatic hydrocarbons (e.g., polychlorinated biphenyls and dibenzodioxins), lead, and mercury have a suppressive effect on the immune system. Halogenated aromatic hydrocarbons cause dysfunction of DCs through several mechanisms that lead to atrophy of the primary and secondary lymphoid organs. Heavy metals, such as lead, mercury and nickel, are immunosuppressive and generally affect the levels of B and T lymphocytes, NK cells, and inflammatory cytokines. Other metals, such as selenium, zinc, and vanadium, may be immunostimulatory at low doses. The immunotoxic mechanisms may differ and include chelation of molecules and effects on protein synthesis, cell membrane integrity, and nucleic acid replication. The toxic effects of mycotoxins such as fumonisins B1 and B2 (secondary fungal metabolites produced by members of the genus Fusarium) and aflatoxin (produced by Aspergillus flavus) include lymphocytolysis in the thymic cortex.
Chemotherapeutic drugs inhibit the cell cycle through various mechanisms, and thus all dividing cells, including lymphocytes, bone marrow cells, and enterocytes, are sensitive to their effects. As such, bone marrow suppression, immunosuppression, and gastrointestinal disturbances are common side effects of anticancer drugs. Purine analogues (e.g., azathioprine) compete with purines in the synthesis of nucleic acids, whereas alkylating agents like cyclophosphamide cross-link DNA and inhibit the replication and activation of lymphocytes. Cyclosporin A specifically inhibits the T lymphocyte signaling pathway by interfering with the transcription of the IL-2 gene. Methotrexate, a folic acid antagonist, blocks the synthesis of thymidine and purine nucleotides. The immunosuppressive effects of some of these agents is desirable for the treatment of immune-mediated disease (e.g., immune-mediated hemolytic anemia) or to prevent allograft rejection after transplantation. Corticosteroids may be given at an immunosuppressive dose, though the degree of suppression is highly variable among species. Local or palliative treatment of cancer may include radiotherapy (ionizing radiation) to target and damage the DNA of the neoplastic cells. Although some immunosuppression may be noted, particularly if bone marrow or lymphoid tissue is within the therapeutically irradiated field, mounting evidence suggests that radiotherapy can induce a cascade of proimmunogenic effects that engage the innate and adaptive immune systems to contribute to the destruction of tumor cells.
Malnutrition and cachexia, which may occur with cancer, lead to secondary immunosuppression through several complex metabolic and neurohormonal aberrations. Thymic function may be impaired in young malnourished animals, resulting in a decrease in circulating T lymphocytes and subsequent depletion of T lymphocyte regions of secondary lymphoid organs. Lymphoid atrophy may result from physiologic and emotional stress, which can cause the release of catecholamines and glucocorticoids.
Aging
As part of the general effects of aging in cells (see Chapter 1), all lymphoid organs decrease in size (atrophy) with advancing age. In the case of the thymus this reduction in size occurs normally after sexual maturity and is more appropriately termed thymic involution. The term involution should be reserved for normal physiologic processes in which an organ either returns to normal size after a period of enlargement (e.g., postpartum uterus) or regresses to a more primitive state (e.g., thymic involution).
Neoplasia
Because the thymus has both lymphoid and epithelial components, neoplasms may arise from either component. Thymic lymphoma arises from the T lymphocytes in the thymus (and very rarely B lymphocytes). It is most often seen in young cats and cattle and less frequently in dogs (Fig. 13-53 ) (see Hematopoietic Neoplasia). Thymomas arise from the epithelial component and are usually benign neoplasms that occupy the cranial mediastinum of older animals. Histologically, these neoplasms consist of clustered or individualized neoplastic epithelial cells, often outnumbered by nonneoplastic small lymphocytes (“lymphocyte-rich thymoma”). Thymomas are common in goats and often contain large cystic structures. Immune-mediated diseases, including myasthenia gravis and immune-mediated polymyositis, occur with thymomas in dogs, and also rarely in cats. Myasthenia gravis is caused by autoantibodies directed toward the acetylcholine receptors, which lead to destruction of postsynaptic membranes and reduction of acetylcholine receptors at neuromuscular junction. Megaesophagus and aspiration pneumonia are common sequelae to this condition.
Figure 13-53.

Thymic Lymphoma, Cat.
The large pale tan mass (M) fills the cranial mediastinum and caudally displaces the lungs. H, Heart. (Courtesy Dr. A.C. Durham, School of Veterinary Medicine, University of Pennsylvania.)
Miscellaneous Disorders
Thymic Hyperplasia.
Asymptomatic hyperplasia may occur in juvenile animals in association with immunizations and results in symmetrical increase in the size of the thymus. Autoimmune lymphoid hyperplasia of the thymus has germinal center formation and occurs with myasthenia gravis.
Thymic Hematomas.
See Disorders of Dogs.
Disorders of Domestic Animals: Spleen
Congenital Disorders
Asplenia or the failure of a spleen to develop in utero occurs rarely in animals, and the effect on the animal's immune status is uncertain. (Splenic aplasia is present in certain strains of mice, but because these are usually maintained under either germ-free or specific pathogen–free [SPF] conditions, the effect of asplenia cannot be evaluated.) Congenital immunodeficiency diseases are described in detail in Chapter 5, and in the sections on Disorders of Horses and Disorders of Dogs.
Splenomegaly
Gross examination of the spleen involves deciding whether the spleen is enlarged (splenomegaly), normal, or small (see E-Appendix 13-2). Diffuse enlargement of the spleen may be due to congestion (termed bloody spleen) or other infiltrative disease (termed meaty spleen). The cut surface of congested spleens will exude blood, whereas meaty spleens are more firm and do not readily ooze blood. The diseases and disorders having splenomegaly are discussed using the following categories, which list the common causes of uniform splenomegaly (Table 13-6 ):
-
•
Uniform splenomegaly with a bloody consistency (bloody spleen) (Fig. 13-54, A )
-
•
Uniform splenomegaly with a firm consistency (meaty spleen) (see Fig. 13-54, B)
-
•
Splenic nodules with a bloody consistency
-
•
Splenic nodules with a firm consistency
Table 13-6.
Common Causes of Uniform Splenomegaly in Domestic Animals
| Species | Congested (Bloody) Spleen | Firm (Meaty) Spleen |
|---|---|---|
| Horse |
|
|
| Cattle, sheep, and goat |
|
|
| Pig |
|
|
| Dog and cat |
|
|
EIA, Equine infectious anemia; GDV, gastric dilatation and volvulus; IMHA, immune-mediated hemolytic anemia.
Figure 13-54.
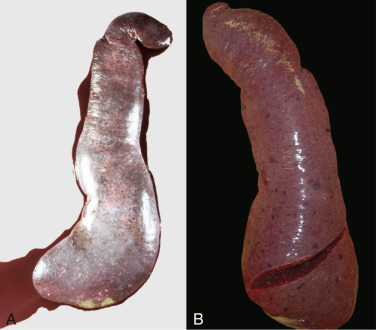
Uniform Splenomegaly.
A, Congested bloody spleen. This condition occurs secondary to compromises in vascular flow into and out of the spleen (e.g., torsion), from intravenous barbiturates (e.g., euthanasia or anesthesia), and from acute hyperemia due to septicemia. B, Meaty spleen. This condition may be due to proliferation of macrophages in cases of chronic septicemias, hemolytic diseases, diffuse granulomatous disease, or neoplasia (e.g., lymphoma).
(A courtesy College of Veterinary Medicine, University of Illinois. B courtesy Dr. A.C. Durham, School of Veterinary Medicine, University of Pennsylvania.)
Uniform Splenomegaly with a Bloody Consistency—Bloody Spleen.
The common causes of a bloody spleen are (1) congestion (due to gastric volvulus with splenic entrapment, splenic volvulus [all of which compress the splenic vein], and barbiturate euthanasia, anesthesia, or sedation), (2) acute hyperemia (due to septicemia), and (3) acute hemolytic anemia (due to an autoimmune disorder or an infection with a hemotropic parasite).
Congestion
Splenic Torsion.
Torsion of the spleen occurs most commonly in pigs and dogs; in dogs this usually involves both spleen and stomach and is seen more often in deep-chested breeds (see Chapter 7). In contrast to ruminants, in which the spleen is firmly attached to the rumen, the spleens of dogs and pigs are attached loosely to the stomach by the gastrosplenic ligament. It is the twisting of the spleen around this ligament that results initially in occlusion of the veins, causing splenic congestion, and later in occlusion of the artery, causing splenic infarction. In dogs the spleen is uniformly and markedly enlarged and may be blue-black from cyanosis. It is often folded back on itself (visceral surface to visceral surface) in the shape of the letter “C.” Treatment for this condition is most often splenectomy.
Barbiturate Euthanasia, Anesthesia, or Sedation.
Intravenous injection of barbiturates induces acute passive congestion in the spleen due to relaxation of smooth muscle in the capsule and trabeculae. This phenomenon is seen most dramatically at autopsy (syn: necropsy) in horses and dogs that have been euthanized or anesthetized with barbiturates. Grossly, the spleen is extremely enlarged (Fig. 13-55 ), and the cut surface bulges and oozes copious blood. Because of the splenic distention, the splenic capsule can be fragile and easily ruptured. Histologically, the red pulp is distended by erythrocytes, and the lymphoid tissues of the white pulp are small and widely separated (Fig. 13-56 ). Electric stunning of pigs at slaughter may result in a large congested spleen; the mechanism is unknown, but it should not be confused with a pathologically congested spleen. Splenic congestion in acute cardiac failure is rarely seen in animals.
Figure 13-55.
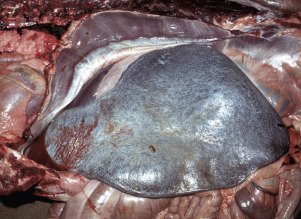
Splenic Congestion From Barbiturate Euthanasia, Horse.
The spleen is enlarged and congested from storage of blood.
(Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Figure 13-56.
Splenic Congestion From Barbiturate Euthanasia, Dog.
The red pulp vascular spaces are markedly distended by blood. One white pulp splenic follicle is present in the lower right. H&E stain.
(Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Acute Congestion/Hyperemia.
Acute septicemias may cause acute hyperemia and concurrent acute congestion of marginal zones and splenic red pulp. Microbes are transported hematogenously to these sites, where they are rapidly phagocytized by macrophages. Enormous numbers of intravenous bacteria can be cleared by the spleen from the blood in 20 to 30 minutes, but when this defensive mechanism is overwhelmed, the outcome is usually fatal. The response of the spleen depends on the duration of the disease. In acutely fatal cases, such as anthrax and fulminating salmonellosis, distention by blood may be the only gross finding. If the animal survives longer, as in swine erysipelas and the less virulent forms of salmonellosis, there may be sufficient time for neutrophils and macrophages to accumulate in the marginal sinuses, marginal zones, and splenic red pulp vascular spaces.
Anthrax.
B. anthracis, the causative agent of anthrax, is a Gram-positive, large, endospore-forming bacillus, which grows in aerobic to facultative anaerobic environments. Anthrax is primarily a disease of ruminants, especially cattle and sheep (see Chapters 4, 7, 9, and 10Chapter 4Chapter 7Chapter 9Chapter 10). Once the spores are ingested, they replicate locally in the intestinal tract, spread to regional lymph nodes, and then disseminate systemically through the bloodstream, resulting in septicemia. B. anthracis produces exotoxins, which degrade endothelial cell membranes and enzyme systems.
Grossly, the spleen is uniformly enlarged and dark red to bluish-black and contains abundant unclotted blood. In peracute cases the only histologic lesion may be marked congestion of the marginal sinuses and the splenic red pulp vascular spaces. At low magnification, congestion of the marginal sinus may appear as a circumferential red ring around the splenic follicle, and there is marked lymphocytolysis of follicles and PALS. Intravascular free bacilli are noted and may be seen in impression smears of peripheral blood, presumably because death is so rapid from the anthrax toxin that there is insufficient time for phagocytosis to take place. If the animal lives longer, scattered neutrophils are present in the marginal sinuses and red pulp vascular spaces (Fig. 13-57 ). Anthrax cases are not normally autopsied because exposure to air causes the bacteria to sporulate—anthrax spores are extremely resistant and readily contaminate the environment.
Figure 13-57.
Anthrax, Spleen, Monkey.
(See Fig. 13-40 for schematic illustration of the marginal zone.)
A, Acute septicemias may cause acute congestion of the marginal zone (double-headed line) and then of the red pulp vascular spaces (not shown). B, Higher magnification of A with marginal zone (double-headed line) and central artery (C) of the follicle. C, Higher magnification of B with small aggregates of neutrophils within the marginal zone (arrows).D, Higher magnification of B with accumulation anthrax bacilli within the marginal zone (arrows). This form produces anthrax toxins, which cause severe tissue injury, resulting in inflammation and cell death. All H&E stain.
(Courtesy Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois. Photographed from slides provided by Toxicology Battelle Columbus to the Wednesday Slide Conference [2003-2004, Conference 13, Case 1], Armed Forces Institute of Pathology, Department of Veterinary Pathology.)
Acute Hemolytic Anemias.
Hemolytic diseases, including acute babesiosis, hemolytic crises in equine infectious anemia, and immune-mediated hemolytic anemia, can cause marked splenic congestion. The splenic congestion is due to the process of removal (phagocytosis) and storage of large numbers of sequestered parasitized and/or altered erythrocytes from the circulation. Histologically, there is dilation of the red pulp vascular spaces with erythrocytes and erythrophagocytes. With chronicity there is hyperplasia of the red pulp macrophages, hemosiderosis, and reduced congestion because the number of sequestered diseased erythrocytes is diminished.
Uniform Splenomegaly with a Firm Consistency—Meaty Spleen.
The three general categories of conditions leading to uniform splenomegaly with a firm meaty consistency are (1) marked phagocytosis of cells, debris, or foreign agents/material; (2) proliferation or infiltration of cells as occurs in diffuse lymphoid and histiocytic hyperplasia, diffuse granulomatous disease (E-Table 13-2), EMH, and neoplasia; (3) storage of materials in storage diseases or amyloidosis. It is important to recognize that more than one of these processes can occur in the same patient (e.g., dogs with immune-mediated hemolytic anemia may have both marked erythrophagocytosis and EMH). The appearance of the cut surface of a meaty spleen depends on the underlying cause. In diffuse marked lymphoid hyperplasia, large, disseminated, discrete, white, bulging nodules are visible. Spleens with diffuse infiltrative neoplasms, such as lymphoma, are pink–light purple on cut surface.
E-TABLE 13-2.
Granulomatous Diseases of the Spleen
| Disorders | Agents |
|---|---|
| Noninfectious |
|
| Infectious |
|
| |
|
Modified from Nieman RS, Attilo O: Disorders of the spleen in major problem in pathology, Philadelphia, 1999, WB Saunders.
Phagocytosis and Proliferation of Cells
Diffuse Lymphoid Hyperplasia.
Lymphoid hyperplasia has been described in detail in the section on Dysfunction/Responses to Injury. In cases of prolonged antigenic stimulation the lymphoid follicles throughout the splenic parenchyma can become enlarged and visible on gross examination (Fig. 13-58 ), leading to diffuse splenomegaly. In contrast to B lymphocyte hyperplasia of the lymphoid follicles, certain diseases (e.g., malignant catarrhal fever in cattle) may lead to T lymphocyte hyperplasia of the PALS.
Figure 13-58.

Lymphoid Hyperplasia, Cross Section of Spleen, Dog.
The hyperplastic white pulp follicles are grossly evident as 1 to 3 mm in diameter pale gray-white foci. These structures are not visible in the normal spleen but become enlarged and visible from marked lymphoid hyperplasia.
(Courtesy Dr. S. Wolpert, USDA/FSIS; and Noah's Arkive, College of Veterinary Medicine, The University of Georgia.)
Diffuse Histiocytic Hyperplasia and Phagocytosis.
Splenomegaly from hyperplasia and increased phagocytosis of splenic macrophages is a response to the need to engulf organisms in prolonged bacteremia or parasitemia from hemotropic organisms. Whereas acute hemolytic anemias cause splenomegaly with congestion (bloody spleen), with chronicity there is decreased sequestration of diseased erythrocytes and hence less congestion. Therefore in cases of chronic hemolytic disease, splenomegaly is attributed to diffuse proliferation of macrophages, phagocytosis, and concurrent hyperplasia of the white pulp due to ongoing antigenic stimulation. For example, equine infectious anemia has cyclical periods of viremia, with immune-mediated damage to erythrocytes and platelets, and phagocytosis to remove altered erythrocytes and platelets. These cycles result in proliferation of red pulp macrophages, hyperplasia of hematopoietic cells (EMH) to replace those lost, and hyperplasia of lymphocytes in the white pulp.
Diffuse Granulomatous Disease.
Chronic infectious diseases may cause a uniformly firm and enlarged spleen, mostly due to macrophage hyperplasia and phagocytosis, diffuse lymphoid hyperplasia, or diffuse granulomatous disease. Diffuse granulomatous diseases (see E-Table 13-2) occur in (1) intracellular facultative bacteria that infect macrophages (e.g., Mycobacterium spp., Brucella spp., and Francisella tularensis); (2) systemic mycoses (e.g., Blastomyces dermatitidis, Histoplasma capsulatum) (see Lymphoid/Lymphatic System, Disorders of Domestic Animals: Lymph Nodes, Enlarged Lymph Nodes [Lymphadenomegaly]) (Fig. 13-59, A and B ), and (3) protozoal infections that infect macrophages (e.g., Leishmania spp.). Some of these organisms may also produce nodular spleens with the formation of discrete to coalescing granulomas (e.g., M. bovis) (see Splenic Nodules with a Firm Consistency).
Figure 13-59.
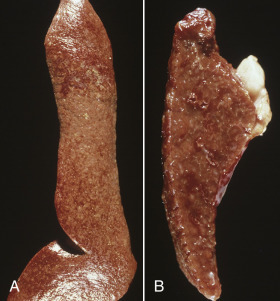
Histoplasmosis, Spleen, Dog.
A, There is uniform splenomegaly with a firm consistency (meaty spleen). B, Cross section of spleen. The red pulp has been almost completely replaced by diffuse granulomatous inflammation.
(Courtesy Department of Veterinary Biosciences, The Ohio State University; and Noah's Arkive, College of Veterinary Medicine, The University of Georgia.)
Extramedullary Hematopoiesis.
EMH is the development of blood cells in tissues outside the medullary cavity of the bone (E-Fig. 13-9). The formation of single or multiple lineages of hematopoietic cells is often observed in many tissues and commonly in the spleen. The ability of blood cell precursors to home, proliferate, and mature in extramedullary sites relies on the presence of HSCs and pathophysiologic changes in the microenvironment (i.e., extracellular matrix, stroma, and chemokines). In the spleen, HSCs have been found within vessels and adjacent to endothelial cells to form a vascular niche; thus splenic EMH occurs in the red pulp, both within the red pulp vascular spaces and sinusoids (of the dog). The predilection for EMH to occur varies among species (for instance, splenic EMH persists throughout adulthood in mice), and the underlying mechanisms are not completely understood, but four major theories to explain the causes of EMH are (1) severe bone marrow failure; (2) myelostimulation; (3) tissue inflammation, injury, and repair; and (4) abnormal chemokine production.
E-Figure 13-9.
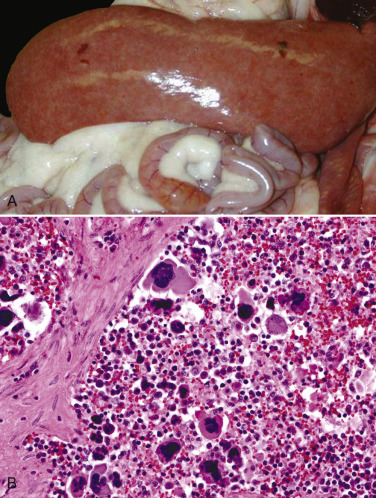
Extramedullary Hematopoiesis.
A, Marked diffuse splenomegaly (meaty spleen) from a ferret. B, The splenic parenchyma contains numerous erythroid and myeloid precursors and megakaryocytes. H&E stain.
(Courtesy Dr. A.C. Durham, School of Veterinary Medicine, University of Pennsylvania.)
Because splenic EMH is often observed in animals without obvious hematologic abnormalities, tissue inflammation, injury, and repair is the most likely mechanism of EMH in this organ. In dogs and cats EMH occurs most frequently with degenerative and inflammatory disorders, such as lymphoid nodular hyperplasia, hematomas, thrombi, histiocytic hyperplasia, inflammation (e.g., fungal splenitis), and neoplasia. EMH in multiple tissues may be observed in chronic cardiovascular or respiratory conditions, chronic anemia, or chronic suppurative diseases in which there is an excessive tissue demand for neutrophils that exceeds the supply available from the marrow (e.g., canine pyometra).
Primary Neoplasms.
Primary neoplastic diseases of the spleen arise from cell populations that normally exist in the spleen and include hematopoietic components, such as lymphocytes, mast cells, and macrophages, and stromal cells, such as fibroblasts, smooth muscle, and endothelium. The primary neoplasms that result in diffuse splenomegaly are the round cell tumors, including lymphoma (Fig. 13-60 ), leukemia, visceral mast cell tumor, and histiocytic sarcoma. It is important to note that all of these types of neoplasms can produce nodular lesions instead of—or along with—a diffusely enlarged spleen. The different types of lymphoma in domestic animals are discussed in the section on Hematopoietic Neoplasia. Secondary neoplasms of the spleen are due to metastatic spread and most often form nodules in the spleen, not a uniform splenomegaly.
Figure 13-60.
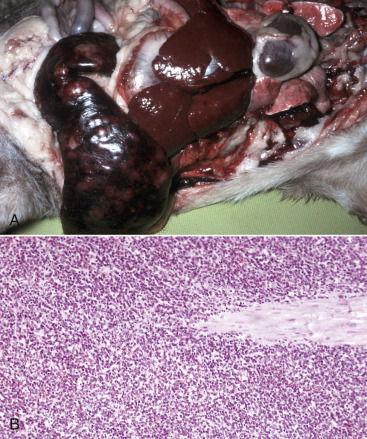
Lymphoma, Spleen and Liver.
A, Dog. There is diffuse splenomegaly and multiple tan splenic nodules. Mild hepatomegaly with an irregular surface corresponds to the neoplastic infiltration into the portal areas. B, Cow. The spleen is diffusely infiltrated by neoplastic lymphocytes, which have completely obliterated all normal architecture (absence of the red and white pulp). H&E stain.
(A courtesy College of Veterinary Medicine, University of Illinois. B courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Storage of Material
Amyloid.
The accumulation of amyloid in the spleen may occur with primary (AL) or secondary (AA) amyloidosis (see Chapters 1 and 5). Rarely, severe amyloid accumulation may cause uniform splenomegaly (Fig. 13-61 ), in which the spleen is firm, rubbery to waxy, and light brown to orange. Microscopically, amyloid is usually in the splenic follicles, which if large enough, are grossly visible as approximately 2-mm-diameter gray nodules. Amyloid deposition can also be seen within the walls of splenic veins and arterioles. Plasma cells tumors within the spleen may also be associated with amyloid (AL) deposits.
Figure 13-61.
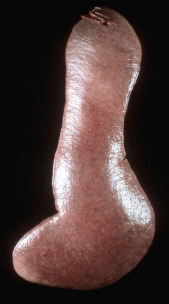
Splenic Amyloid, Dog.
The spleen is enlarged, pale tan, firm, and waxy in this advanced case of amyloidosis.
(Courtesy College of Veterinary Medicine, University of Illinois.)
Lysosomal Storage Diseases.
Storage diseases are a heterogeneous group of inherited defects in metabolism characterized by accumulation of storage material within the cell (lysosomes). Genetic defects, which result in the absence of an enzyme, the synthesis of a catalytically inactive enzyme, the lack of activator proteins, or a defect in posttranslational processing, can lead to a storage disease. Acquired storage diseases are caused by exogenous toxins, most often plants that inhibit a particular lysosomal enzyme (e.g., swainsonine toxicity due to indolizidine alkaloid found in Astragalus and Oxytropis plant spp.). Storage diseases typically occur in animals less than 1 year of age. In general, these substrates are lipids and/or carbohydrates that accumulate in the cells, the result of the lack of normal processing within lysosomes. Major categories of stored materials include mucopolysaccharides, sphingolipids, glycolipids, glycoproteins, glycogen, and oligosaccharides. Macrophages are commonly affected by storage diseases, and thus accumulations of macrophages within several organs, including splenic macrophages, Kupffer cells of the liver, and macrophages in the brain are often observed.
Splenic Nodules with a Bloody Consistency.
The most common disorders of the spleen with bloody nodules are (1) hematomas, including those induced by nodular hyperplasia or occurring with hemangiosarcoma, (2) incompletely contracted areas of the spleen, (3) acute splenic infarcts, and (4) hemangiosarcomas. The term nodule has been applied rather loosely here. In some of these conditions, such as incompletely or irregularly contracted areas of the spleen, the elevated area of the spleen is not as well defined as the term nodule would imply.
Hematomas.
Bleeding into the red pulp to form a hematoma is confined by the splenic capsule, and produces a red to dark red, soft, bulging, usually solitary mass of varying size (2 to 15 cm in diameter) (Fig. 13-62 ). Resolution of a splenic hematoma progresses over days to weeks, through the stages of coagulation and breakdown of the blood into a dark red-brown soft mass (Fig. 13-63, A ), infiltration by macrophages that phagocytize erythrocytes and break down hemoglobin to form hematoidin and hemosiderin (see Fig. 13-63, B), and repair leading to fibrosis. On occasion the capsule (splenic capsule and visceral peritoneum) over the hematoma can rupture, resulting in hemoperitoneum, hypovolemic shock, and death.
Figure 13-62.
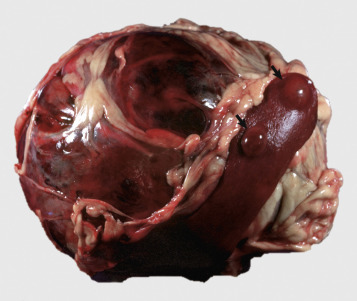
Hematoma, Spleen, Dog.
The ventral extremity of the spleen has a large hematoma on its visceral surface. Note the two nodules on the dorsal extremity (arrows) of splenic nodular hyperplasia, a common site for hematomas to arise.
(Courtesy College of Veterinary Medicine, University of Illinois.)
Figure 13-63.
Subcapsular Hematoma, Spleen, Dog.
A, Note the separation of the splenic capsule from the underlying parenchyma by a mass of blood. B, The hematoma is subjacent to the capsule. Hematoidin (yellow) or hemosiderin (brown) pigments may be seen in or around these lesions as a result of the breakdown of erythrocytes. H&E stain.
(Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
The origin or cause of many hematomas is unknown. Some are due to trauma, and others may also be induced by splenic nodular hyperplasia. It is postulated that as the splenic follicles become hyperplastic they distort the adjacent marginal zone and marginal sinus, which compromises their drainage into sinusoids and red pulp vascular spaces. The result is an accumulation of pooled blood surrounding the hyperplastic nodule, which leads to hematoma formation. Splenic hematomas can also occur secondary to the rupture of hemangiosarcomas within the spleen.
Incompletely Contracted Areas of the Spleen.
Incompletely or irregularly contracted areas of the spleen are caused by failure of the smooth muscle to contract in response to circulatory shock (hypovolemic, cardiogenic, or septic) or sympathetic “fight-or-flight” response, resulting in a lack of splenic evacuation of stored blood. Grossly, incompletely contracted areas are characterized by multiple, variably sized and irregularly shaped, dark red to black, raised, soft, blood-filled “nodules.” These areas are usually at the margins of the spleen, and the intervening tissues are depressed and pink-red, corresponding to the contracted portions of red pulp devoid of blood. Incompletely contracted areas may be confused with acute splenic infarcts or hematomas on gross examination.
Acute Splenic Infarcts.
Splenic infarcts are wedge-shaped or triangular hemorrhagic lesions that occur primarily at the margins of the spleen. In dogs, splenic infarcts most often occur with hypercoagulable states (e.g., liver disease, renal disease, Cushing's disease), neoplasia, and cardiovascular disease. Splenic vein thrombi may occur in association with traumatic reticulitis, splenic abscesses, portal vein thrombosis, and arterial thrombosis in bovine theileriosis in cattle. Valvular endocarditis may also lead to multiorgan infarcts, including the spleen. Splenic infarcts are common in pigs with classical swine fever.
Acute splenic infarcts may not always be grossly visible in the early stages but develop into discrete, dark red and blood-filled, bulging, wedge-shaped foci with the base toward the splenic capsule (Fig. 13-64, A ). With chronicity the lesion becomes gray-white and contracted due to fibrosis (see Fig. 13-64, B).
Figure 13-64.
Acute and Chronic Splenic Infarcts, Spleen, Dogs.
A, Acute splenic infarct (asterisk). Acute infarcts are red-black wedge-shaped foci filled with blood. B, Chronic splenic infarct. Chronic infarcts are wedge-shaped (asterisk), pale gray-white, firm, and often contracted due to fibrosis.
(A courtesy Dr. A.C. Durham, School of Veterinary Medicine, University of Pennsylvania. B courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Hemangiosarcoma.
Hemangiosarcoma is a malignant neoplasm of endothelial cells and is a common primary tumor of the spleen, especially in dogs. Benign splenic hemangiomas are extraordinarily rare. Grossly, hemangiosarcomas may appear as single, multifocal, or coalescing dark red-purple masses and cannot be easily differentiated from a hematoma (Fig. 13-65 ). On cut surface they are bloody with varying amounts of soft red neoplastic tissue; in more solid areas the neoplasm can be slightly more firm and white-tan. Metastatic spread occurs early in the disease process. Seeding of the peritoneum results in numerous discrete red-black masses throughout the omentum and serosa of abdominal organs, and hematogenous spread to liver and lung are common. Hemangiosarcomas in dogs also occur in the right atrium of the heart, retroperitoneal fat, and skin (dermal and/or subcutaneous) and multiorgan hemangiosarcomas are described in horses, cats, and cattle. Because hemangiosarcomas have often metastasized at the time of initial diagnosis, it may be difficult (and futile) to determine the primary site. Histologically, hemangiosarcomas are composed of plump neoplastic endothelial cells, which wrap around stroma to form haphazardly arranged and poorly defined blood-filled vascular spaces (Fig. 13-66 ).
Figure 13-65.
Hemangiosarcoma, Spleen, Dog.
A, There are multiple neoplastic nodules on the dorsal extremity and a large nodule on the ventral extremity of the spleen. B, The ventral mass has been incised to reveal the cut surface of the hemangiosarcoma.
(Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Figure 13-66.

Hemangiosarcoma, Spleen, Dog.
Neoplastic endothelial cells form haphazardly organized blood-filled vascular channels. Mitotic figure (arrow). H&E stain.
(Courtesy Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
Splenic Nodules with a Firm Consistency.
The most common disorders of the spleen with firm nodules are (1) lymphoid nodular hyperplasia, (2) complex nodular hyperplasia, (3) primary neoplasms, (4) secondary metastatic neoplasms, (5) granulomas, and (6) abscesses.
Lymphoid and Complex Nodular Hyperplasia.
Primary Neoplasms.
The primary neoplastic diseases of the spleen that result in firm nodules include lymphoma (multiple subtypes), histiocytic sarcoma, leiomyoma, leiomyosarcoma, fibrosarcoma, myelolipomas, liposarcomas, myxosarcomas, undifferentiated pleomorphic sarcomas, solid hemangiosarcomas, and rare reports of primary chondrosarcomas. These locally extensive neoplasms may be solitary or multiple, raised above the capsular surface, but usually confined by the capsular surface. The consistency and cut surface appearance varies depending on the type of neoplasm; spindle cell tumors like leiomyosarcomas and fibrosarcomas will be white and firm, liposarcomas and myelolipomas are soft and bulging, and myxomatous neoplasms are gelatinous. It is important to remember that many round cell neoplasms, such as lymphoma, mast cell tumors, plasma cell tumors, myeloid neoplasms, and histiocytic sarcomas, can form nodules or diffuse splenic enlargement (or both).
Metastatic Neoplasms.
Neoplasms that metastasize to the spleen usually result in enlarged nodular spleens (Fig. 13-67 ) and include any number of sarcomas, carcinomas, or malignant round cell tumors. Metastatic sarcomas can include fibrosarcomas, leiomyosarcomas, chondrosarcomas, and osteosarcomas. Mammary, prostatic, pulmonary, anal sac gland and neuroendocrine carcinomas may metastasize widely to abdominal viscera, including the spleen.
Figure 13-67.

Metastatic Carcinoma, Spleen, Cow.
The firm, lobulated, white mass is an undifferentiated carcinoma, which has metastasized to the spleen.
(Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Granulomas and Abscesses.
Microorganisms that cause diffuse granulomatous splenitis and uniform splenomegaly may also cause focal to multifocal nodular lesions (e.g., Mycobacterium spp., fungal organisms) (see Diffuse Granulomatous Diseases of the Spleen and also Enlarged Lymph Nodes). Although there are a large number of diseases and conditions commonly caused by bacteremia (e.g., navel ill, joint ill, chronic respiratory infections, bacterial endocarditis, chronic skin diseases, castration, tail docking, and ear trimming and/or notching), these rarely result in visible splenic abscesses. Pyogranulomas and abscesses in the spleen (multifocal chronic suppurative splenitis) that do develop after septicemia and/or bacteremia are usually caused by pyogenic bacteria such as Streptococcus spp., Rhodococcus equi (Fig. 13-68 ), Trueperella pyogenes (Fig. 13-69 ), and Corynebacterium pseudotuberculosis. Cats with the wet or dry form of feline infectious peritonitis virus may have nodular pyogranulomatous and lymphoplasmacytic inflammatory foci throughout the spleen. Splenic abscesses due to direct penetration by a migrating foreign body are reported in cattle (from the reticulum) and less commonly in the horse (from the stomach). Perforating gastric ulcers in horses due to Gasterophilus and Habronema spp. have also reportedly led to adjacent splenic abscesses. Granulomas and abscesses bulge from the capsule and cut surfaces, and the exudate can vary in amount, texture, and color depending on the inciting organism and the age of the lesion.
Figure 13-68.
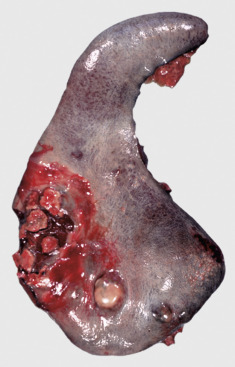
Multiple Subcapsular Splenic Abscesses, Rhodococcus equi, Spleen, Horse.
(Courtesy Dr. P. Carbonell, School of Veterinary Science, University of Melbourne.)
Figure 13-69.
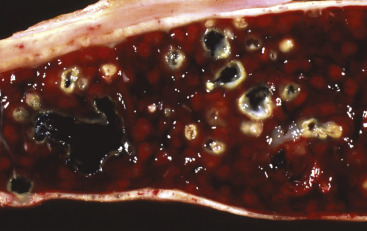
Chronic Multifocal Suppurative Splenitis (Splenic Abscesses), Trueperella pyogenes, Spleen, Cow.
Multiple encapsulated yellow-white abscesses are present throughout the parenchyma of the spleen as a result of a previous bacteremia.
(Courtesy Department of Veterinary Biosciences, The Ohio State University; and Noah's Arkive, College of Veterinary Medicine, The University of Georgia.)
Small Spleens (Splenic Hypoplasia and Atrophy)
The most common diseases or conditions that have small spleens are (1) developmental anomalies, (2) aging changes, (3) wasting and/or cachectic diseases, and (4) splenic contraction.
Developmental Anomalies
Splenic Hypoplasia.
Primary immunodeficiency diseases can result in splenic hypoplasia, as well as small thymuses and lymph nodes (which may be so small as to be grossly undetectable in some diseases). These diseases affect young animals and involve defects in T and/or B lymphocytes (Fig. 13-70 ). Spleens are exceptionally small, firm, and pale red and lack lymphoid follicles and PALS. These diseases and their pathologic findings are discussed in Chapter 5 and in the sections on Disorders of Horses and Disorders of Dogs.
Figure 13-70.

Severe Combined Immunodeficiency Disease, Spleen, Arabian Foal.
There is notable absence of the white pulp (the large pale pink areas are splenic trabeculae). H&E stain.
(Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Congenital Accessory Spleens.
Accessory spleens can be either congenital or acquired (see Splenic Rupture). Congenital accessory spleens are termed splenic choristomas, which are nodules of normal splenic parenchyma in abnormal locations. These are usually small and may be located in the gastrosplenic ligament, liver, or pancreas (see Fig. 13-74, B).
Figure 13-74.
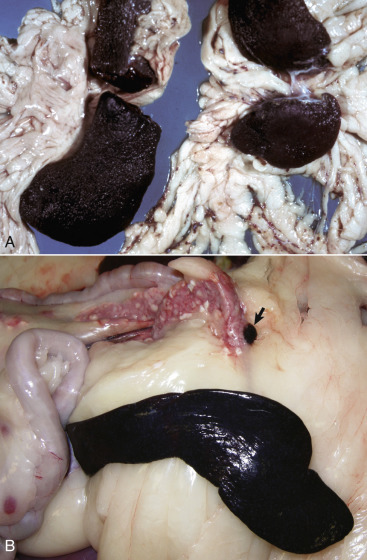
Accessory Spleens and Splenic Choristoma, Dogs.
A, Accessory spleens. The spleen had been broken into several parts, and the rupture sites have healed by fibrosis. These small pieces of spleen (also referred to as daughter or progeny spleens) are found on the gastrosplenic ligament. B, Splenic choristomas. These nodules of normal splenic parenchyma are usually small and may be located in the gastrosplenic ligament, liver, or pancreas. Splenic choristoma (arrow).
(A courtesy Dr. H.B. Gelberg, College of Veterinary Medicine, Oregon State University. B courtesy Dr. A.C. Durham, School of Veterinary Medicine, University of Pennsylvania.)
Splenic Fissures.
Fissures in the splenic capsule are elongated grooves whose axes run parallel to the borders of the spleen. This developmental defect is seen most commonly in horses but also occurs in other domestic animals and has no pathologic significance. The surface of the fissure is smooth and covered by the normal splenic capsule.
Aging Changes.
As part of the general aging change of cells as the body ages, there is reduction in the number of B lymphocytes produced by the bone marrow and decline of naïve T lymphocytes due to age-related thymic involution. Consequently, there is lymphoid atrophy in secondary lymphoid organs. The spleen is small, and its capsule may be wrinkled. Microscopically, the white pulp is atrophied, and splenic follicles, if present, lack germinal centers. Sinuses may also collapse from a reduced amount of blood, possibly because of anemia, which makes the red pulp appear fibrous.
Wasting/Cachectic Diseases.
Any chronic disease, such as starvation, systemic neoplasia, and malabsorption syndrome, may produce cachexia. Starvation has a marked effect on the thymus, which results in atrophy of the T lymphocyte areas in the spleen and lymph nodes, which is in part mediated by leptin. B lymphocyte development is also diminished, because B lymphocytes require accessory signals from helper T lymphocytes to undergo somatic hypermutation and immunoglobulin isotype switching.
Splenic Contraction.
Contraction of the spleen is a result of contraction of the smooth muscle in the capsule and trabeculae of storage spleens. It can be induced by the activation of the sympathetic “fight-or-flight” response and is seen in patients with heart failure or shock (cardiogenic, hypovolemic, and septic shock) and also occurs in acute splenic rupture that has resulted in massive hemorrhage (hemoabdomen/hemoperitoneum). The contracted spleen is small, its surface is wrinkled, and the cut surface is dry.
Miscellaneous Disorders of the Spleen
Hemosiderosis.
Hemosiderin is a form of storage iron derived chiefly from the breakdown of erythrocytes, which normally takes place in the splenic red pulp. Thus some splenic hemosiderosis is to be expected, and the amount varies with the species (it is most extensive in the horse). Excessive amounts of splenic hemosiderin are seen when erythropoiesis is reduced (less demand for iron) or from the rapid destruction of erythrocytes in hemolytic anemias (increased stores of iron), such as those caused by immune-mediated hemolytic anemias or hemotropic parasites. Excess splenic hemosiderin may also occur in conditions such as chronic heart failure or injections of iron dextran or as focal accumulations at the sites of old hematomas, infarcts, or trauma-induced hemorrhages. Hemosiderin is also present in siderofibrotic plaques.
Siderofibrotic Plaques.
Siderofibrotic plaques are also known as siderocalcific plaques and Gamna-Gandy bodies. Grossly, they are gray-white to yellowish, firm, dry encrustations on the splenic capsule. Usually they are most extensive along the margins of the spleen but can be elsewhere on the capsule (Fig. 13-71 ) and sometimes in the parenchyma. With H&E staining these plaques are a multicolored mixture of yellow (hematoidin), golden brown (hemosiderin), purple-blue (hematoxylinophilic calcium mineral), and pink (eosinophilic fibrous tissue) (Fig. 13-72 ; E-Figs. 13-10 and 13-11). Siderofibrotic plaques are extremely common in aged dogs and may represent sequelae to previous hemorrhages from trauma to the spleen.
Figure 13-71.
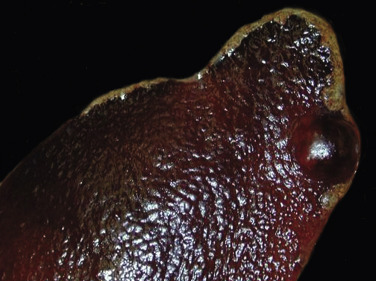
Siderofibrotic Plaques, Spleen, Macroscopic View, Dog.
Siderofibrotic plaque along the margins (golden-brown area) of the spleen; a focal nodule of hyperplasia is also present. Both are common lesions in older dogs.
(Courtesy Dr. A.C. Durham, School of Veterinary Medicine, University of Pennsylvania.)
Figure 13-72.

Siderofibrotic Plaques, Spleen, Microscopic View, Dog.
A, The thick splenic capsule contains fibrosis connective tissue (pink), linear bands of mineral (dark purple), small lakes of hematoidin pigment (yellow), and hemosiderin-laden macrophages (brown). H&E stain. B, The plaque is composed of fibrous connective tissue, hemosiderin (blue) and hematoidin (orange) pigments, and mineral. Prussian blue reaction.
(A courtesy Dr. A.C. Durham, School of Veterinary Medicine, University of Pennsylvania. B courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
E-Figure 13-10.

Siderofibrotic Plaques and Nodular Hyperplasia/Hematoma, Spleen, Dog.
A, Multiple confluent raised yellow-white plaques are present on the capsular surface of the body of the spleen. Note the nodule of hyperplasia/hematoma (incised). B, The plaque consists of fibrous connective tissue of the capsule, mineral/hemosiderin (purple-blue), hemosiderophages (brown), and large areas of hematoidin (orange-yellow) pigment. H&E stain.
(A courtesy College of Veterinary Medicine, University of Illinois. B courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
E-Figure 13-11.
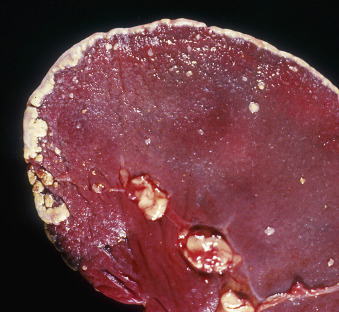
Siderofibrotic Plaques, Spleen, Macroscopic View, Dog.
Note the yellow-white plaques on the capsular surface and along the border of the spleen. These plaques may be the result of healing of sites of previous trauma and hemorrhage.
(Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Splenic Rupture.
Splenic rupture is most commonly caused by trauma, such as from an automobile accident or being kicked by other animals. Thinning of the capsule from splenomegaly can render the spleen more susceptible to rupture, and this may occur at sites of infarcts, hematomas, hemangiosarcomas, and lymphoma. In acute cases of splenic capsular rupture, the spleen is contracted and dry and the surface wrinkled from the marked blood loss (Fig. 13-73 ). In more severe cases the spleen may be broken into two or more pieces, and small pieces of splenic parenchyma may be scattered throughout the omentum and peritoneum (sometimes called splenosis) (Fig. 13-74, A ). Clotted blood, fibrin, and omentum may adhere to the surface at the rupture site. If the rupture is not fatal, the spleen heals by fibrosis, and there may be a capsular scar. Occasionally there are two or more separate pieces of spleen adjacent to each other and sometimes joined by scar tissue in the gastrosplenic ligament. The functional capabilities of the small accessory spleens are questionable, although erythrophagocytosis, hemosiderosis, hyperplastic nodules, EMH, and neoplasia can be present in these nodules.
Figure 13-73.
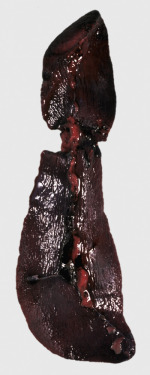
Acute Splenic Rupture, Spleen, Dog.
The spleen has been almost transected by recent trauma. Because of the loss of blood, the spleen is contracted, the surface is wrinkled, and the exposed parenchymal surface is dry.
(Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Accessory spleens due to traumatic rupture should be distinguished from peritoneal seeding of hemangiosarcoma and the developmental anomaly splenic choristomas (see Fig. 13-74, B), which are nodules of normal splenic parenchyma in abnormal locations (such as liver and pancreas).
Chronic Splenic Infarcts.
In the early stage, splenic infarcts are hemorrhagic and may elevate the capsule (see Splenic Nodules with a Bloody Consistency). However, as the lesions age and fibrous connective tissue is laid down, they shrink and become contracted and often depressed below the surface of the adjacent capsule.
Parasitic Cysts.
Occasionally, parasitic cystic nodules are present within the spleen. These cysts are intermediate stages of Echinococcus granulosus and Cysticercus tenuicollis and are seen most commonly in wild animal species.
Disorders of Domestic Animals: Lymph Nodes
Small Lymph Nodes
The diseases or conditions with small lymph nodes are (1) congenital disorders, (2) lack of antigenic stimulation, (3) viral infections, (4) cachexia and malnutrition, (5) aging, and (6) radiation.
Congenital Disorders.
Primary immunodeficiency diseases are described in detail in Chapter 5 and in the sections on Disorders of Horses and Disorders of Dogs. Neonatal animals with primary immunodeficiency diseases often have extremely small to undetectable lymph nodes. In dogs and horses with severe combined immunodeficiency disease (SCID), lymphoid tissues, including lymph nodes from affected animals, are often grossly difficult to identify and characterized by an absence of lymphoid follicles. Congenital hereditary lymphedema has been reported in certain breeds of cattle and dogs. Grossly, the most severely affected animals have generalized subcutaneous edema (see Fig. 2-10) and effusions. In severe cases the peripheral and mesenteric lymph nodes are hypoplastic and characterized by an absence of follicles. Nodes draining an edematous area may be grossly enlarged from marked sinus edema.
Lack of Antigenic Stimulation.
The size of the lymph node depends on the level of phagocytosis and antigenic stimulation; lymph nodes that are not receiving antigenic stimuli (e.g., SPF animals) will be small with low numbers of primary lymphoid follicles and few, if any, secondary follicles or plasma cells in the medullary cords. Conversely, nodes receiving constant antigenic material (such as those draining the oral cavity or intestines) are large with active secondary lymphoid follicles. The number of follicles increases or decreases with changes in the intensity of the antigenic stimuli, and the germinal centers go through a cycle of activation, depletion, and rest, as described previously (see Lymph Nodes, Function). As the antigenic response wanes, germinal centers become depleted of lymphocytes, and lymphoid follicles become smaller.
Viral Infections.
Many viral infections of animals target lymphocytes and cause the destruction of lymphoid tissue. Of infectious agents, viruses most commonly infect and injure lymphoid tissues and include the following: EHV-1 in aborted foals, classic swine fever virus, BVDV, canine distemper virus, and canine and feline parvovirus.
Although some viruses destroy lymphoid tissue, others can lead to lymph node hyperplasia (e.g., follicular B lymphocyte hyperplasia in FIV and paracortical T lymphocyte hyperplasia in malignant catarrhal fever virus) or cause neoplasia (e.g., FeLV, BLV, and Marek's disease).
Cachexia and Malnutrition.
Malnutrition and cachexia, which occur with cancer, lead to secondary immunosuppression through several complex metabolic and neurohormonal aberrations. Starvation has marked effect on the thymus with resultant atrophy of the T lymphocyte areas in the spleen and lymph nodes and may also affect B lymphocyte development. Lymphoid atrophy may result from physiologic and emotional stress and the concurrent release of catecholamines and glucocorticoids. Glucocorticoids reduce B and T lymphocytes via redistribution of these cells and glucocorticoid-induced apoptosis. T lymphocytes are more sensitive to glucocorticoid-induced apoptosis than are B lymphocytes.
Aging.
As part of the general aging change of cells as the body ages, there is reduction in the number of lymphocytes produced by the bone marrow and regressed thymus, and consequently a reduction in the B and T lymphocytes in secondary lymphoid organs, resulting in lymphoid atrophy. Consequently, lymph nodes are small, with loss of B and T lymphocytes and plasma cells in the cortical follicles, paracortex, and medullary cords, respectively.
Radiation.
Local or palliative treatment of cancer may include radiotherapy (ionizing radiation) to target and damage the DNA of the neoplastic cells. Although some immunosuppression may be noted, particularly if bone marrow or lymphoid tissues are within the irradiated field, mounting evidence suggests that radiotherapy can induce a cascade of proimmunogenic effects that engage the innate and adaptive immune systems to contribute to the destruction of tumor cells. Fibrosis of tissues within the irradiated field also occurs as mainly a late effect of chronic radiation.
Enlarged Lymph Nodes (Lymphadenomegaly)
Conditions causing lymphadenomegaly include (1) lymphoid hyperplasia (follicular or paracortical), (2) hyperplasia of the sinus histiocytes (monocyte-macrophage system), (3) acute or chronic lymphadenitis, (4) lymphoma, and (5) metastatic neoplasia.
Lymphoid Hyperplasia and Hyperplasia of the Monocyte-Macrophage System.
Detailed descriptions of lymphoid follicular hyperplasia, paracortical hyperplasia, and hyperplasia of sinus histiocytes are in the sections on Lymph Nodes, Function, and Lymph Node, Dysfunction/Responses to Injury. Follicular lymphoid hyperplasia can involve large numbers of lymph nodes, as in a systemic disease, or can be localized to a regional lymph node draining an inflamed or antigenically stimulated (e.g., vaccine injection) area.
Acute Lymphadenitis.
Lymph nodes draining sites of infection and inflammation may develop acute lymphadenitis (e.g., retropharyngeal lymph nodes draining the nasal cavity with acute rhinitis, tracheobronchial lymph nodes in animals with pneumonia (Fig. 13-75 ), and mammary [supramammary] lymph nodes in animals with mastitis). Grossly, affected lymph nodes in acute lymphadenitis are red and edematous, have taut capsules, and may have necrotic areas (Fig. 13-76 ). In some instances the afferent lymphatic vessels may also be inflamed (lymphangitis). The material draining to the regional lymph node may be microorganisms (bacteria, parasites, protozoa, and fungi), inflammatory mediators, or a sterile irritant. In septicemic diseases, such as bovine anthrax, the lymph nodes are markedly congested and the sinuses filled with blood. Examination of these lymph nodes should include culturing for bacteria and the examination of smears and histologic sections for bacteria and fungi. Pyogenic bacteria, such as Streptococcus equi ssp. equi in horses (Fig. 13-77 ), Streptococcus porcinus in pig, and Trueperella pyogenes in cattle and sheep, cause acute suppurative lymphadenitis (see Disorders of Horses and Disorders of Pigs).
Figure 13-75.
Acute Lymphadenitis, Tracheobronchial Lymph Nodes, Pig.
The tracheobronchial lymph nodes are draining the cranial lung lobes, which are consolidated due to severe pneumonia. The nodes are enlarged and reddened. This appearance is due to the “reversed” anatomic arrangement in the pig lymph node; the blood-filled sinuses are obvious at the surface.
(Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Figure 13-76.

Acute Lymphadenitis, Lymph Node, Dog.
Acute lymphadenitis usually occurs when a regional lymph node drains a site of inflammation caused by microorganisms and subsequently becomes infected. The lymph node is firm and enlarged with a tense capsule. The cut surface bulges and is wet with blood, edema, and an inflammatory cell infiltrate.
(Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Figure 13-77.

Acute Suppurative Lymphadenitis, Equine Strangles (Streptococcus equi ssp. equi), Dorsal View of Larynx, Left and Right Retropharyngeal Lymph Nodes, Horse.
The lymph nodes are grossly distended with suppurative inflammation (pus).
(Courtesy College of Veterinary Medicine, University of Illinois.)
Histologically, the subcapsular, trabecular, and medullary sinuses and the parenchyma of the cortex and medulla have focal to coalescing foci of neutrophilic inflammation, necrosis, and fibrin deposition (Fig. 13-78 ). If inflammation in the lymph node continues for several days or longer, the lymph node is further enlarged by follicular hyperplasia and plasmacytosis of the medullary cords from the expected immune response.
Figure 13-78.
Acute Lymphadenitis (Early), Lymph Node, Dog.
A, The sinuses and the parenchyma of the cortex and medulla have coalescing foci of neutrophilic inflammation, necrosis, hemorrhage, and fibrin deposition. H&E stain. B, The medullary sinus contains numerous macrophages (sinus histiocytosis) and fewer neutrophils. This is the type of early response seen when a lymph node drains an inflamed area. Medullary cords are filled with lymphocytes and plasma cells. H&E stain.
(A courtesy Dr. A.C. Durham, School of Veterinary Medicine, University of Pennsylvania. B courtesy Dr. H.B. Gelberg, College of Veterinary Medicine, Oregon State University.)
Chronic Lymphadenitis.
The types of chronic lymphadenitis include chronic suppurative lymphadenitis, diffuse granulomatous inflammation, and discrete granulomas. In chronic suppurative inflammation, abscesses range in size from small microabscesses to large abscesses that occupy and obliterate the whole node. Recurrent bouts of chronic lymphadenitis (e.g., regional lymph node draining chronic mastitis in cows) lead to fibrosis and lymphoid hyperplasia, in addition to chronic abscesses. The classic example of chronic suppurative lymphadenitis with encapsulated abscesses is caseous lymphadenitis, a disease of sheep and goats caused by C. pseudotuberculosis (Figs. 13-79 and 13-80 ) (also see Disorders of Ruminants). It is also the cause of ulcerative lymphangitis in cattle and horses and pectoral abscesses in horses.
Figure 13-79.

Caseous Lymphadenitis, Corynebacterium pseudotuberculosis, Lymph Node, Sheep.
The entire lymph node is replaced by an abscess. This is an early stage of caseous lymphadenitis, before the pus has become inspissated and lamellated.
(Courtesy Dr. K. Read, College of Veterinary Medicine, Texas A&M University; and Noah's Arkive, College of Veterinary Medicine, The University of Georgia.)
Figure 13-80.
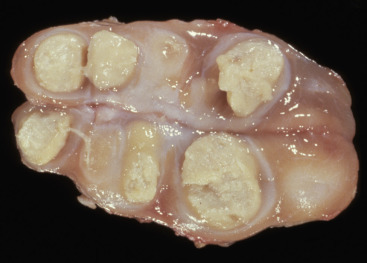
Chronic Caseous Lymphadenitis, Corynebacterium pseudotuberculosis, Lymph Node, Sheep.
Three encapsulated chronic abscesses contain yellow-white caseous pus.
(Courtesy Dr. W. Crowell, College of Veterinary Medicine, The University of Georgia; and Noah's Arkive, College of Veterinary Medicine, The University of Georgia.)
Focal Granulomatous Lymphadenitis.
Classic examples of focal to multifocal granulomatous lymphadenitis are Mycobacterium tuberculosis complex, which includes M. bovis among others. Members of M. avium complex cause similar lesions and have been described in a number of species, including dogs, cats, primates, pigs, cattle, sheep, horses, and human beings. Infection may begin by inhalation of aerosol droplets containing the bacilli, which may spread via the lymphatic vessels to regional lymph nodes, resulting in granulomatous lymphangitis and lymphadenitis (Fig. 13-81 ). Initially lesions in the lymphatic system are confined to the lymphatic vessels (granulomatous lymphangitis) and regional lymph nodes (e.g., the tracheobronchial lymph nodes in the case of pulmonary tuberculosis), but once disseminated in the lymph or blood, lymph nodes throughout the body will have lesions. Well-organized granulomas consist of a central mass of macrophages with phagocytized mycobacteria, surrounded by epithelioid and foamy macrophages and occasional multinucleated giant cells (Langhans type). These inflammatory nodules are surrounded by a layer of lymphocytes enclosed in a fibrous capsule. Over time the center of the granuloma may undergo caseous necrosis due to the high lipid and protein content of the dead macrophages (see Chapter 3). In bovine Johne's disease the mesenteric lymph nodes draining the infected intestine can have noncaseous granulomas (Fig. 13-82 ).
Figure 13-81.
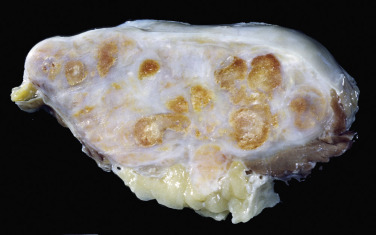
Tuberculosis (Mycobacterium bovis), Lymph Node, Ox.
The normal architecture of the lymph node has been completely obliterated by multiple yellow-brown caseating granulomas, typical of M. bovis lesions.
(Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Figure 13-82.
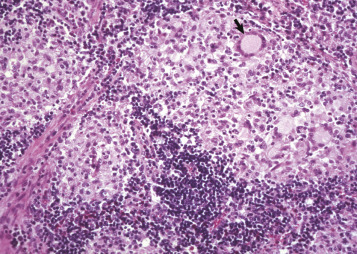
Johne's Disease (Mycobacterium avium ssp. paratuberculosis), Lymph Node, Ox.
Several noncaseating granulomas (areas of pallor) have replaced the normal lymphoid tissue (blue). Note the Langhans giant cell (arrow). H&E stain.
(Courtesy College of Veterinary Medicine, University of Illinois.)
Diffuse Granulomatous Lymphadenitis.
Coalescing to diffuse granulomatous lymphadenitis is seen in disseminated fungal infections such as blastomycosis, cryptococcosis (E-Fig. 13-12), and histoplasmosis (see Disorders in Dogs). In feline cryptococcosis (most often Cryptococcus neoformans), the inflammatory response may be mild due to the thick polysaccharide capsule, which has strong immunomodulatory properties and promotes immune evasion and survival within the host. Therefore the nodal enlargement is due mainly to a large mass of organisms (see E-Fig. 13-12). Pigs with PCV2 infection may have a multifocal to diffuse infiltrate of macrophages and multinucleated giant cells of varying severity (see Disorders of Pigs).
E-Figure 13-12.
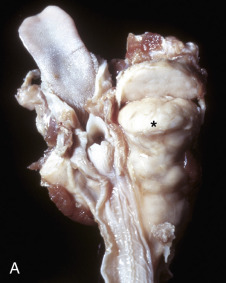

Cryptococcosis (Cryptococcus neoformans), Cats.
A, Right mandibular lymph node. The lymph node (asterisk) is grossly enlarged with complete loss of architecture. B, The fungal organisms (Cryptococcus neoformans) have thick nonstaining capsules (arrows) and a lightly basophilic nuclear structure. Variable amounts of granulomatous inflammation may be seen in cases of Cryptococcus. H&E stain.
(A courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee; B courtesy Dr. A.C. Durham, School of Veterinary Medicine, University of Pennsylvania.)
Secondary (Metastatic) Neoplasms.
Carcinomas typically metastasize via lymphatic vessels to the regional lymph node. Other common metastatic neoplasms include mast cell tumor and malignant melanoma. Although sarcomas most often metastasize hematogenously, some more aggressive sarcomas (e.g., osteosarcoma) may spread to regional lymph nodes. Histologically, single cells or clusters of neoplastic cells travel via the afferent lymphatic vessels and are deposited in a sinus, usually the subcapsular sinus (see Fig. 13-47). Here the cells proliferate and can ultimately occupy the whole lymph node, as well as drain to the next lymph node in the chain.
Pigmentation of Lymph Nodes
Red discoloration is caused by (1) draining erythrocytes from hemorrhagic or acutely inflamed areas, (2) acute lymphadenitis with hyperemia and/or hemorrhage, (3) acute septicemias with endotoxin-induced vasculitis or disseminated intravascular coagulation, and (4) dependent areas in postmortem hypostatic congestion. Blood in pig lymph nodes is especially obvious due to the inverse anatomy (the equivalent of the medullary sinuses are subcapsular and thus readily visible in the unsectioned node). Initially erythrocytes fill trabecular and medullary sinuses and then rapidly undergo erythrophagocytosis by proliferating sinus macrophages. Hemosiderin deposition occurs within 7 to 10 days in these macrophages, imparting a brown discoloration of the node.
Black discoloration is often present in the tracheobronchial lymph nodes due to draining of carbon pigment (pulmonary anthracosis, see Chapter 9). Black ink from skin tattoos will drain to the regional lymph node. These pigments are usually noted within the medullary sinus macrophages.
Brown discoloration may be due to melanin, parasitic hematin, or hemosiderin. Melanin pigment is seen in animals with chronic dermatitis when melanocytes are damaged and their pigment is released into the dermis and phagocytized by melanomacrophages (pigmentary incontinence) and drained to the regional lymph node. The mandibular lymph nodes often contain numerous melanomacrophages in animals with heavily pigmented oral mucosa, presumably due to chronic low levels of inflammation. This must be distinguished from metastatic malignant melanomas. Lymph nodes draining areas of congenital melanosis may have melanin deposits.
Parasitic hematin pigment is produced by Fascioloides magna (cattle) and Fasciola hepatica (sheep) in the liver and then transported via the lymphatic vessels to the hepatic lymph nodes.
Hemosiderin, an erythrocyte breakdown product, may form in a hemorrhagic node or arrive in hemosiderophages draining from congested, hemorrhagic, or inflamed areas. Drainage of iron dextran from an intramuscular injection may also cause hemosiderin pigment accumulation within the draining lymph node.
Green discoloration is rare and may be caused by green tattoo ink (often used in black animals); ingestion of blue-green algae, which drain to mesenteric lymph nodes; massive eosinophilic inflammation; and in mutant Corriedale sheep, which have a genetic defect that results in a deficiency in the excretion of bilirubin and phylloerythrin by the liver. The phylloerythrin or a metabolite stains all the tissues of the body a dark green, except for the brain and spinal cord, which are protected by the blood-brain barrier.
Miscellaneous discolorations of lymph nodes may be seen with intravenously injected dyes (e.g., methylene blue or trypan blue) or subcutaneous drug injections. Lymph nodes may be yellow in severely icteric patients. The pigmented strain of MAP (Johne's disease) may impart an orange discoloration in the mesenteric lymph nodes of sheep.
Miscellaneous Lymph Node Disorders
Inclusion Bodies.
Many viruses produce inclusion bodies, and some of these occur in lymph nodes. These viruses include EHV-1 in horses, bovine adenovirus, cytomegalic virus in inclusion body rhinitis and PCV2, herpesvirus of pseudorabies in pigs, and rarely parvovirus in dogs and cats.
Emphysema.
Emphysema in lymph nodes is a consequence of emphysema in their drainage fields and is seen most frequently in tracheobronchial lymph nodes in bovine interstitial emphysema and in porcine mesenteric lymph nodes in intestinal emphysema (see Chapter 7). The appearance of the lymph node varies with the extent of the emphysema. In severe cases the lymph node is light, puffy, and filled with discrete gas bubbles, and the cut surface may be spongy. Histologically, the sinuses are distended with gas and lined by macrophages and giant cells. This change has been considered a foreign body reaction to the gas bubbles. Macrophages and giant cells are also seen in afferent lymphatic vessels (granulomatous lymphangitis).
Vascular Transformation of Lymph Node Sinus (Nodal Angiomatosis).
Vascular transformation of the sinuses is a nonneoplastic reaction to blocked efferent lymphatic vessels or veins. This pressure-induced lesion results in the formation of anastomosing vascular channels and may be confused with a nodal vascular neoplasm. These proliferative but noninvasive masses usually begin in the subcapsular sinuses and may be followed by lymphoid atrophy, erythrophagocytosis/hemosiderosis, and fibrosis. The blockage may be caused by malignant neoplasms of the tissues that the lymph node drains (e.g., thyroid carcinoma with nodal angiomatosis of the mandibular lymph node).
Neoplasia
See section on Bone Marrow, Disorders of Domestic Animals, Hematopoietic Neoplasia for a discussion of the WHO classification of hematopoietic neoplasia that predominantly arise and proliferate within bone marrow. This section will cover neoplasms of lymphoid tissue(s) arising outside of bone marrow.
Lymphoma.
The term lymphoma (also known as lymphosarcoma) encompasses a diverse group of malignancies arising in lymphoid tissue(s) outside of bone marrow. Grossly, there may be diffuse to nodular enlargement of one or more lymph nodes (Fig. 13-83 ), and the cut surface is soft, white, and bulging with loss of normal corticomedullary architecture. There is great variation in the clinical manifestations and cytopathologic features of lymphoma, which underlie the importance of classification to better predict the clinical behavior and outcome. An understanding of lymphocyte maturation is crucial, because the WHO classification of lymphoma postulates a normal cell counterpart for each type of lymphoma (when possible). In other words, lymphoma can arise at any stage in the development/maturation of a lymphocyte—from precursor lymphocytes (B or T lymphoblasts) to mature lymphoid B and T, lymphocytes and NK cells (Table 13-7 and Box 13-11 ).
Figure 13-83.
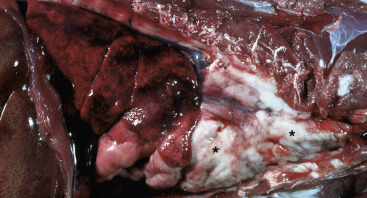
Lymphoma, Cranial Mediastinal Lymph Nodes, Cat.
The cranial mediastinal lymph nodes are grossly enlarged (asterisks), fill the cranial thoracic cavity, and have caudally displaced the lungs and heart.
(Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Table 13-7.
Common Lymphoma Diagnoses in Domestic Animals Using the WHO Classification System
| Lymphoma Subtype | Pattern | Cell Size | Grade | Postulated Normal Cell Counterpart | Defining Histopathologic Features |
|---|---|---|---|---|---|
| DLBCL | Diffuse | Large | Mid to high | Germinal center or post–germinal center (activated) B lymphocyte | Multiple nucleoli (centroblastic variant) or single central nucleoli (immunoblastic variant) |
| PTCL | Diffuse | Large | Mid to high | Activated mature T lymphocyte | Nuclei of variable size and shape; eosinophils may be present |
| Burkitt-like lymphoma | Diffuse | Intermediate | High | Either germinal center or post–germinal center (activated) B lymphocyte | Uniform nuclear size with multiple distinct small nucleoli; numerous tingible body macrophages |
| T-LBL | Diffuse | Intermediate | High | Naïve T lymphocyte | Dispersed chromatin that obscures nuclear detail |
| TZL | Nodular | Small to intermediate | Indolent | Activated mature T lymphocyte; paracortical | Abundant clear cytoplasm, sharp shallow nuclear indentations; expansion of paracortex with peripheralization of “fading” follicles |
| MZL | Nodular | Intermediate | Indolent | Post–germinal center marginal zone B lymphocyte | Prominent single central nucleoli; expansion of marginal zone with encircling of “fading” follicles |
| TCRLBCL | Nodular/diffuse | Large | Indolent to low | Germinal center B lymphocyte | Large irregularly-shaped nuclei with 1-2 prominent nucleoli; numerous small reactive T lymphocytes |
| EATCL | Diffuse | Small to intermediate | Low | Intestinal intraepithelial T lymphocytes | Hyperchromatic nuclei with dispersed chromatin |
DLBCL, Diffuse large B cell lymphoma; EATCL, enteropathy-associated T cell lymphoma; MZL, marginal zone lymphoma; PTCL, peripheral T cell lymphoma; TCRLBCL, T cell–rich large B cell lymphoma; T-LBL, T cell lymphoblastic lymphoma; TZL, T zone lymphoma, WHO, World Health Organization.
Box 13-11. Histologic Classification of Hematopoietic Tumors of the Lymphoid System in Domestic Animals.
B Lymphocyte Lymphoid Neoplasms
Precursor B Lymphocyte
B cell lymphoblastic leukemia/lymphoma
Mature B Lymphocyte
B cell chronic lymphocytic leukemia/lymphoma
B cell lymphocytic lymphoma intermediate type
Lymphoplasmacytic
- Follicular lymphomas
- Mantle cell lymphoma
- Follicular center cell lymphoma (I, II, III)
- Nodal marginal zone lymphoma
- Splenic marginal zone lymphoma
Extranodal marginal zone lymphoma of MALT
Hairy cell leukemia
- Plasmacytic tumors
- Indolent plasmacytoma
- Anaplastic plasmacytoma
- Plasma cell myeloma
- Large B cell lymphomas
- T cell–rich large B cell lymphoma
- Diffuse large B cell lymphoma
- Thymic B cell lymphoma
- Intravascular large B cell lymphoma
Burkitt-like lymphoma
T Lymphocyte and NK Cell Lymphoid Neoplasms
Precursor T Lymphocyte
T cell lymphoblastic leukemia/lymphoma
Mature T Cell/NK Lymphocyte
- Large granular lymphoproliferative disorders
- T cell chronic lymphocytic leukemia
- T cell LGL lymphoma/leukemia
- NK cell chronic lymphocytic leukemia
- Cutaneous T cell lymphomas
- Cutaneous epitheliotropic lymphoma
- Cutaneous nonepitheliotropic lymphoma
Extranodal/Peripheral T cell lymphoma
Adult T cell–like lymphoma/leukemia
Angioimmunoblastic lymphoma
Angiotropic lymphoma
Intestinal T cell lymphoma
Anaplastic large cell lymphoma
MALT, Mucosa-associated lymphoid tissue; LGL, large granular lymphocyte; NK, natural killer.
Modified from Valli VE, Jacobs RM, Parodi AL, et al: Histologic classification of hematopoietic tumors of domestic animals. In World Health Organization international histological classification of tumors in domestic animals, second series (vol 8), Washington, DC, 2002, Armed Forces Institute of Pathology.
Pathologists use gross features, histomorphologic features, immunophenotype (B or T lymphocyte), and clinical characteristics to classify lymphomas. The morphologic features used in histopathologic classification are the following:
-
•
Histologic pattern—Nodular or diffuse.
-
•
Cell size—The nuclei of the neoplastic lymphocytes are compared to the diameter of a red blood cell (RBC ≅ 5 µm). Small is less than 1.5 times the diameter of an RBC; intermediate is 1.5 to 2.0 times the diameter of an RBC; large is more than 2.0 times the diameter of an RBC.
-
•
Grade—Mitotic figures are counted in a single high-power (400×) field. Indolent is 0 to 1; low is 2 to 5; mid is 6 to 10; high is more than 10.
Although there are numerous subtypes of lymphoma recognized under the WHO system, a detailed discussion of each subtype is outside the scope of this textbook. However, a select number of subtypes are more commonly seen in domestic animals (see Table 13-7) and currently best described in the dog (see Disorders of Dogs, Neoplasms, Lymphomas). The most common types in dogs are large cell lymphomas and include diffuse large B cell lymphoma and peripheral T cell lymphoma. T cell–rich large B cell lymphoma is thought to be a variant of diffuse large B cell lymphoma with a distinctive reactive T lymphocyte infiltrate. Intermediate cell lymphomas include B or T lymphocyte lymphoblastic lymphomas and Burkitt-like lymphoma (both high grade), marginal zone lymphoma, and the intermediate cell variant of T zone lymphomas (both indolent and nodular). Small cell lymphomas most commonly diagnosed in domestic animal species include enteropathy-associated T cell lymphoma, commonly seen in the cat, T zone lymphoma (small cell variant), and small cell lymphoma. Cutaneous lymphomas are most often of T lymphocyte origin and may be epitheliotropic or nonepitheliotropic, and a distinct entity of inflamed T cell lymphoma has been recently described in dogs (see Chapter 17).
Plasma Cell Neoplasia.
Plasma cell neoplasms are most easily categorized as myeloma or multiple myeloma, which arises in the bone marrow, and extramedullary plasmacytoma, which as the name implies involves sites other than bone.
Multiple Myeloma.
See Bone Marrow and Blood Cells, Disorders of Domestic Animals, Types of Hematopoietic Neoplasia, Plasma Cell Neoplasia.
Extramedullary Plasmacytomas.
Extramedullary plasmacytomas are most commonly diagnosed in the skin of dogs (also cats and horses), where they constitute 1.5% of all canine cutaneous tumors (see Chapter 17). The pinnae, lips, digits, and chin are the most commonly affected locations, and most lesions are solitary, though multiple plasmacytomas are infrequently diagnosed. Other tissues affected include the oral cavity, intestine (colorectal in particular), liver, spleen, kidney, lung, and brain; of these, the oral cavity and intestine (colorectal) are involved most often. In one study, extramedullary plasmacytomas represented 5% of all canine oral tumors and 28% of all extramedullary plasmacytomas diagnosed. Most cutaneous extramedullary plasmacytomas are benign, and complete excision is usually curative; oral cavity and colorectal extramedullary plasmacytomas are likely to behave in a similar manner. More aggressive forms may occur at any site.
As with multiple myeloma, the neoplastic cells composing the tumor may vary from well differentiated to pleomorphic, often within the same tumor. The cells often have a characteristic perinuclear Golgi clearing or “halo,” and the more pleomorphic cells exhibit karyomegaly and binucleation (Fig. 13-84 ). Extramedullary plasmacytomas may produce monoclonal immunoglobulins with resulting monoclonal gammopathy. Amyloid deposition (which may mineralize) is also observed in a proportion of cases. Differentiation from other round cell tumors may be aided by immunohistochemistry (MUM1/IRF4 is particularly sensitive and specific for plasma cell neoplasms).
Figure 13-84.
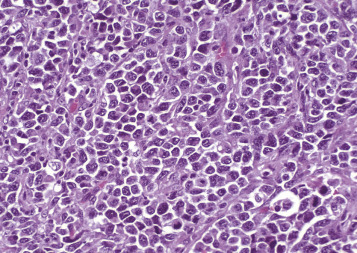
Plasmacytoma (Extramedullary), Oral Cavity, Dog.
Note the moderately well-differentiated plasma cells arranged in small clusters separated by a fibrovascular stroma. H&E stain.
(Courtesy College of Veterinary Medicine, University of Illinois.)
Histiocytic Disorders.
Histiocytic disorders are frequently diagnosed in dogs and occur less often in cats. Briefly, histiocytes are categorized as macrophages and DCs, the latter of which are subdivided into Langerhans cells (LCs), found in skin, gastrointestinal, respiratory, and reproductive epithelia (mucosae), and interstitial DCs (iDC), located in perivascular spaces of most organs. The term interdigitating DCs describes DCs (either resident or migrating) found in T lymphocyte regions of lymph nodes (paracortex) and spleen (PALS); interdigitating DCs consist of both LCs and iDCs. These lineages can be differentiated using immunohistochemical stains. Histiocytic disorders that are diagnosed in veterinary medicine at this time include the following: canine cutaneous histiocytoma, canine LC histiocytosis, canine cutaneous and systemic histiocytosis, feline pulmonary LC histiocytosis, feline progressive histiocytosis, dendritic cell leukemia in the dog, and histiocytic sarcoma and hemophagocytic histiocytic sarcoma in both dogs and cats.
Lymph node involvement is seen in many of these conditions. Rare reports of regional lymph node metastasis in cases of solitary canine cutaneous histiocytoma have been published. Lymphatic invasion with subsequent regional nodal involvement may be seen in dogs with LC histiocytosis, which is a poor prognostic indicator and likely reflects systemic infiltration. The normal architecture of tracheobronchial lymph nodes is often effaced in cats with pulmonary LC histiocytosis.
Canine reactive histiocytoses are not clonal neoplastic proliferations but likely reflect an immune dysregulation consisting of activated dermal iDCs (and T lymphocytes). They are categorized as cutaneous histiocytosis (CH), involving skin and draining lymph nodes, and a more generalized systemic histiocytosis (SH), affecting skin and other sites (e.g., lung, liver, bone marrow, spleen, lymph nodes, kidneys, and orbital and nasal tissues).
Histiocytic Sarcoma Complex.
Histiocytic sarcomas (HSs) are neoplasms of iDCs and therefore can arise in almost any tissue, frequently the spleen, lung, skin, meninges, lymph nodes, bone marrow, and synovium. Secondary involvement of the liver is common as the disease progresses. This neoplasm is most commonly diagnosed in dogs, and a lower incidence is seen in cats. Localized histiocytic sarcoma may be a focal solitary lesion or multiple nodules within a single organ. Disseminated histiocytic sarcoma describes lesions that involve distant sites and has replaced the term malignant histiocytosis. Breed predispositions to histiocytic sarcoma complex are seen in Bernese mountain dogs, Rottweilers, golden retrievers, and flat-coated retrievers, though the disease can occur in any breed. Histiocytic sarcoma complex is considered to have a rapid and highly aggressive course, and the clinical signs depend on the particular organ(s) involved.
Grossly, affected organs may be uniformly enlarged and/or contain multiple coalescing white-tan nodules. Tissue architecture is effaced by sheets of pleomorphic round to spindle-shaped cells. There is marked cellular atypia with numerous karyomegalic and multinucleated neoplastic cells (Fig. 13-85 ).
Figure 13-85.
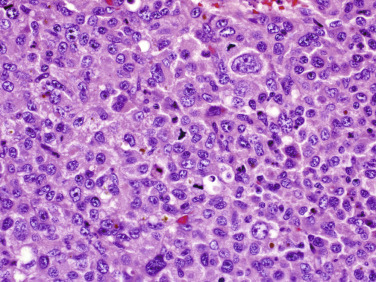
Histiocytic Sarcoma, Spleen, Dog.
The neoplastic cells are markedly pleomorphic with karyomegalic cells, binucleation, and numerous mitotic figures. H&E stain.
(Courtesy Dr. A.C. Durham, School of Veterinary Medicine, University of Pennsylvania.)
Hemophagocytic Histiocytic Sarcoma.
Hemophagocytic histiocytic sarcoma is seen in dogs and cats and is a neoplasm of macrophages of the spleen and bone marrow. Clinically, dogs present with hemolytic regenerative anemia and thrombocytopenia, thus mimicking Evans's syndrome, though they are Coombs negative. This form of histiocytic sarcoma carries the worst prognosis of the histiocytic sarcomas, which is likely in part related to the severe anemia and coagulopathy. It is characterized by a non–mass forming infiltrate of histiocytes within the bone marrow and splenic red pulp, causing diffuse splenomegaly. The neoplastic cells exhibit marked erythrophagocytosis, but the severe cellular pleomorphism seen in the histiocytic sarcoma complex may be lacking. The neoplastic cells are often intermixed with EMH and plasma cells. Metastasis is frequently to the liver, where the cells concentrate within the sinuses. Tumor emboli within the lung are often present.
Disorders of Domestic Animals: Mucosa-Associated Lymphoid Tissue
MALT is involved in a variety of ways with bacteria and viruses, and these are summarized for large animals in Table 13-5. These interactions include being a portal of entry for pathogens (e.g., Salmonella spp., Yersinia pestis, MAP, and L. monocytogenes); a site of replication for viruses (e.g., BVDV); a site for hematogenous infection (e.g., panleukopenia virus and parvovirus); and a site of gross or microscopic lesions in some viral diseases. Bovine coronavirus, BVDV, rinderpest virus, malignant catarrhal fever virus, feline panleukopenia virus, and canine parvovirus cause lymphocyte depletion within the MALT.
Disorders of Horses
Severe Combined Immunodeficiency
Severe combined immunodeficiency disease of Arabian foals is an autosomal recessive primary immunodeficiency disorder characterized by the lack of functional T and B lymphocytes caused by a genetic mutation in the gene encoding for DNA-dependent protein kinase catalytic subunit (DNA-PKcs). This enzyme is required for receptor gene rearrangements involved in the maturation of lymphocytes, and the resulting loss of functional T and B lymphocytes leads to a profound susceptibility to infectious diseases. Though normal at birth, these foals develop diarrhea and pneumonia by approximately 10 days of age, often due to adenovirus, Cryptosporidium parvum, and Pneumocystis carinii infections. Affected foals often die before 5 months of age. Lymph nodes and thymus are small and often grossly undetectable, and the spleen is small and firm due to the absence of white pulp (see Fig. 13-70). The development of genetic tests to identify carriers of the disorder has led to a decrease in the prevalence of severe combined immunodeficiency disease. Recently, severe combined immunodeficiency disease was diagnosed in a single Caspian filly, though the exact genetic defect was not determined. Congenital immunodeficiency diseases are also discussed in detail in Chapter 5.
Strangles
Streptococcus equi ssp. equi, the etiologic agent of equine strangles, is inhaled or ingested after direct contact with the discharge from infected horses or from a contaminated environment. The bacteria attach to the tonsils, penetrate into deeper tissues, enter the lymphatic vessels, drain to regional lymph nodes (mandibular, retropharyngeal, and occasionally parotid and cervical lymph nodes), and cause large abscesses (see Fig. 13-77). Retropharyngeal enlargement from abscesses may lead to compression of the pharynx and subsequent respiratory stridor and dysphagia. Abscesses may rupture and discharge pus through a sinus to the skin surface or spread medially into guttural pouches, where residual pus dries and hardens to form chondroids (which serve as a nidus for live bacteria to persist in carrier animals). In up to 20% of these cases, ruptured abscess material may spread via blood or lymph to other organs (metastatic abscess formation, bastard strangles), including lung, liver, kidney, synovia, mesenteric and mediastinal lymph nodes, spleen, and occasionally brain. Purpura hemorrhagica, a type III hypersensitivity reaction, may result in necrotizing vasculitis in some horses with repeated natural exposure to S. equi ssp. equi or after vaccination in horses that have had strangles.
Rhodococcus Equi Infection
The typical manifestation of R. equi infection is chronic suppurative bronchopneumonia with abscesses (see Chapter 9). Approximately 50% of foals also develop intestinal lesions characterized by pyogranulomatous ulcerative enterotyphlocolitis, often over Peyer's patches, and pyogranulomatous lymphadenitis of mesenteric and colonic lymph nodes (see Chapter 7; see Fig. 13-68). Large abdominal abscesses may be the only lesion in the abdomen and presumably originate from an infected mesenteric lymph node. The diffuse lymphatic tissue in the lamina propria may contain granulomatous inflammation with the phagocytized bacteria. Mediastinal pyogranulomatous lymphadenitis may compress the trachea, causing respiratory distress. R. equi lesions also can develop in the liver, kidney, spleen, or nervous tissue.
Lymphoma
Lymphoma is the most common malignant neoplasm in horses and mostly affects adult animals (mean age 10 to 11 years) with no apparent breed or sex predisposition. The most frequent anatomic locations of equine lymphoma are multicentric, cutaneous, and gastrointestinal tract.
Multicentric lymphoma, defined as involving at least two organs (excluding the regional lymph nodes), is the most common manifestation, followed by skin and gastrointestinal tract types. Solitary locations have been reported in the mediastinum, lymph nodes, ocular/orbital region, brain, spinal cord, oral cavity, and spleen. Of the multicentric lymphomas, the most frequently observed type is T cell–rich large B cell lymphoma (TCRLBCL), reportedly in one study affecting 34% of the cases. Peripheral T cell lymphoma (PTCL) was the second most common, followed by diffuse large B cell lymphoma (DLBCL).
The most common lymphoma type in the gastrointestinal tract is also T cell–rich large B cell lymphoma, followed by enteropathy-associated T cell lymphoma. Cutaneous lymphomas in horses account for up to 3% of all equine skin tumors. T cell–rich large B cell lymphoma is again the most common lymphoma subtype in the skin, representing up to 84% of all cutaneous lymphomas, and most frequently presents clinically as multiple skin masses. Cutaneous T cell lymphoma (CTCL) is the second most common form and arises as smaller solitary nodules. Thoroughbreds may have a higher incidence of cutaneous T cell lymphoma compared to other breeds. Overall, horses with cutaneous T cell–rich large B cell lymphoma appear to have a longer survival time than horses with other types of lymphoma of the skin. Progesterone receptor–positive lymphomas have also been identified in horses, and there is one report of subcutaneous tumor regression following removal of an ovarian granulosa-theca cell tumor. There may be an increased frequency of lymphoma in horses diagnosed with equine herpesvirus 5 (EHV-5, gammaherpesvirus), when compared to healthy horses, although the exact cause-effect role of this observation in lymphomagenesis5 is not yet known.
Histologically, the hallmark features of T cell–rich large B cell lymphoma include a majority of small (nuclei approximately the size of an RBC), reactive, mature T lymphocytes admixed with a neoplastic population of large B lymphocytes whose nuclei are two to three times the diameter of an equine RBC. These large atypical cells are often binucleated and have prominent eosinophilic nucleoli (Fig. 13-86 ). The large cells may be observed in mitosis or in necrosis as single cells with retracted cytoplasm and pyknotic nuclei. T cell–rich large B cell lymphoma is often accompanied by the presence of a dense fibrovascular network.
Disorders of Ruminants (Cattle, Sheep, and Goats)
Johne's Disease
Johne's disease primarily affects domestic and wild ruminants (and rarely pigs and horses) and is due to infection by MAP. The characteristic lesions include granulomatous enteritis usually confined to the ileum, cecum, and proximal colon; lymphangitis; and lymphadenitis of regional lymph nodes (see Fig. 13-82). The bacteria are ingested, engulfed by the M cells overlying Peyer's patches, and then transported to macrophages in the lamina propria and submucosa. Among cattle, sheep, goats, and wild ruminants, there is wide variation in the severity, distribution of lesions, primary inflammatory cell type (lymphocytes, epithelioid macrophages, multinucleated giant cells), and numbers of bacteria within lesions (multibacillary or paucibacillary). Histologically, the architecture of the ileocecal lymph nodes may be partially replaced by aggregates of epithelioid macrophages and multinucleated giant cells, and the remaining nodal tissue contains large secondary follicles with reactive germinal centers. Cattle tend to have noncaseating granulomas, whereas sheep and goats may have granulomas with necrotic caseous centers and mineralization. Variable numbers of acid-fast bacilli are detected within epithelioid macrophages. The intestinal lesions of Johne's disease are described in detail in Chapter 7.
Anthrax
Anthrax is caused by B. anthracis, a Gram-positive bacillus found in spore form in soil. Cattle, sheep, and goats become infected when grazing on infected soil, and infection causes fulminant septicemia. The spleen in infected animals is markedly enlarged and congested (see Uniform Splenomegaly with a Bloody Consistency; see also Chapter 4).
Bovine Viral Diarrhea
Bovine viral diarrhea is caused by BVDV, a pestivirus. Cattle are the natural host, but other animals such as alpacas, deer, sheep, and goats are also affected. BVDV preferentially infects cells of the immune system, including macrophages, DCs, and lymphocytes. The associated lesions in lymphoid tissues are severe lymphoid depletion in mesenteric lymph nodes and Peyer's patches, whose intestinal surface may be covered by a fibrinonecrotic membrane. Histologically, there is marked lymphocytolysis and necrosis of germinal centers in Peyer's patches and cortices of lymph nodes. There is thymic atrophy because the thymus is markedly depleted of lymphocytes and may consist of only collapsed stroma and few scattered lymphocytes. BVD is discussed in detail in Chapters 4 and 7.
Splenic Abscesses
Splenic abscesses can be the result of bacteremia (see Fig. 13-69) or direct penetration by a foreign body from the reticulum (see Spleen and also Portals of Entry/Pathways of Spread).
Caseous Lymphadenitis
C. pseudotuberculosis is a Gram-positive intracellular bacterium that causes caseous lymphadenitis, a chronic suppurative disease of sheep and goats. The bacterium may enter through skin wounds (e.g., shearing cuts in sheep, tagging, tail docking, or castration), drain to the regional lymph node, and then be disseminated in lymph and circulating blood to external and internal lymph nodes, as well as other internal organs, including lung. External abscesses are most often detected in the “jaw and neck” region, specifically in the mandibular and parotid lymph nodes. On gross examination the abscesses are encapsulated and filled with greenish semifluid pus due to an infiltrate of eosinophils (see Fig. 13-79). Over time the abscesses lose the greenish hue, and contents become inspissated to form the characteristic concentric laminations (see Fig. 13-80); old abscesses may reach a diameter of 4 to 5 cm.
Bovine Lymphoma
Bovine lymphoma is broadly classified into enzootic and sporadic forms. The enzootic form, called enzootic bovine leukosis (EBL), is caused by BLV, a retrovirus common in cattle. There is a higher prevalence in dairy cattle compared to beef breeds. BLV is transmitted horizontally (e.g., blood, milk/colostrum, saliva) or iatrogenically (e.g., rectal sleeves, instruments/equipment). Following infection, BLV invades and integrates into the genome of infected B lymphocytes, resulting in a polyclonal B lymphocyte lymphocytosis in approximately 30% of cattle. In approximately 1% to 5% of BLV-infected cattle, a single clone will emerge, leading to the development of B lymphocyte leukemia/lymphoma. The average incubation period between infection and development of lymphoma is 7 to 8 years, and this low conversion rate suggests that the latency period may be longer than the life span of most animals (dairy cattle seldom live to the 7- to 8-year peak incidence of lymphoma occurrence). Other contributing variables, such as genetic background, coinfections, and environmental factors, may also play a role in lymphomagenesis. The exact mechanism of BLV-induced tumorigenesis is poorly understood. Recently BLV microRNAs (miRNAs) were identified in preleukemic and malignant B lymphocytes, which showed repression of structural and regulatory gene expression. These findings suggested that miRNAs may play a key role in tumor onset and progression.
Grossly, multiple tissues may be affected in cattle that develop lymphoma, including peripheral lymph nodes (cephalic, cervical, sublumbar) (Fig. 13-87 ), abdominal lymph nodes, retrobulbar region, abomasum, liver, spleen, heart, urogenital tract, bone marrow, vertebral canal (Fig. 13-88 ), and spinal cord. One study indicates most of these high-grade lymphomas are diffuse large cell lymphomas (66%), and approximately 20% are intermediate cell lymphomas (Burkitt-like and lymphoblastic lymphomas).
Figure 13-87.
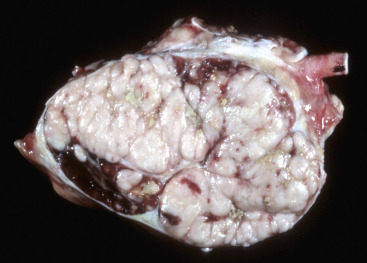
Lymphoma, Bovine Lymph Node.
The normal architecture of lymph node has been replaced by white lobules of neoplastic lymphocytes.
(Courtesy College of Veterinary Medicine, University of Illinois.)
Figure 13-88.

Lymphoma (Asterisks), Vertebral Canal, Epidural Space, Cow.
S, spinal cord.
(Courtesy Dr. J.M. King, College of Veterinary Medicine, Cornell University.)
The sporadic form of bovine lymphoma is most often of T lymphocyte immunophenotype and has three subcategories: cutaneous, calf, and thymic. There is no known viral cause for the sporadic form, and each subcategory has a much smaller prevalence compared to the enzootic form. Of the three sporadic forms, the cutaneous form seems to be the most common and manifests itself as multiple skin nodules in 1- to 3-year-old cattle. The calf form presents as generalized lymphadenopathy with weight loss, lethargy, and weakness in calves less than 6 months old. The thymic form is reportedly more common in beef cattle, 6 to 24 months of age.
Disorders of Pigs
Postweaning Multisystem Wasting Syndrome
PCV2, a small single-stranded DNA virus, is highly prevalent in the domestic pig population. Several clinical syndromes are attributed to PCV2 infection and collectively termed PCV-associated diseases (PCVADs). These include postweaning multisystemic wasting syndrome (PMWS), porcine respiratory disease complex (PRDC), porcine dermatitis and nephropathy syndrome, and enteric disease (see Chapters 4 and 9).
The major postmortem findings of postweaning multisystemic wasting syndrome are poor body condition, enlarged lymph nodes, and interstitial pneumonia. The lesions of the lymphoid system are commonly observed in the tonsil, spleen, Peyer's patches, and lymph nodes. Some pigs have all lymphoid tissues affected, whereas others may have only one or two affected lymph nodes. The characteristic microscopic lesions are lymphoid depletion of both follicles and paracortex with replacement by histiocytes, mild to severe granulomatous inflammation with multinucleated giant cells, and intrahistiocytic sharply demarcated, spherical, basophilic cytoplasmic inclusion bodies. Necrosis of prominent lymphoid follicles (necrotizing lymphadenitis) is occasionally observed, and PCV2 can be detected within the necrotic regions. The loss of lymphocytes may be due to reduced production in the bone marrow, decreased proliferation in the secondary lymphoid organs, or necrosis of lymphocytes.
Porcine Reproductive and Respiratory Syndrome
Porcine reproductive and respiratory syndrome (PRRS) is caused by an arterivirus and causes two overlapping clinical syndromes: reproductive failure and respiratory disease. The virus is transmitted by contact with body fluids (saliva, mucus, serum, urine, and mammary secretions and from contact with semen during coitus), but often it first colonizes tonsils or upper respiratory tract. The virus has a predilection for lymphoid tissues (spleen, thymus, tonsils, lymph nodes, Peyer's patches). Viral replication takes place in macrophages of the lymphoid tissues and lungs, though porcine reproductive and respiratory syndrome virus antigen is found in resident macrophages in many tissues and may persist in tonsil and lung macrophages. The result of this infection is a reduction in the phagocytic and functional capacity of macrophages of the monocyte-macrophage system. As a consequence, there is reduction in resistance to common bacterial and viral pathogens. Most porcine reproductive and respiratory syndrome–infected pigs are coinfected with one or more pathogens, including Streptococcus suis and Salmonella choleraesuis. Infection with Bordetella bronchiseptica and Mycoplasma hyopneumoniae appear to increase the duration and severity of the interstitial pneumonia.
The major lesions are interstitial pneumonia and generalized lymphadenopathy, and tracheobronchial and mediastinal lymph nodes are most commonly affected. Coinfections often complicate the gross and histopathologic changes. Lymph nodes are enlarged, pale tan, occasionally cystic, and firm; some strains of virus also cause nodal hemorrhage. Microscopically, the lesions in the lymph nodes, tonsils, and spleens consist of varying degrees of follicular and paracortical hyperplasia and lymphocyte depletion in follicular germinal centers.
Porcine Jowl Abscess
Streptococcus porcinus causes jowl abscesses in pigs. The bacteria colonize the oral cavity and spread to infect tonsils and regional lymph nodes. The mandibular lymph nodes are the most often affected and have multiple, 1- to 10-cm abscesses; the retropharyngeal and parotid lymph nodes may also be involved (Fig. 13-89 ). This once-prevalent disease is now rare, presumably due to improvements in husbandry, feeder design, and hygiene. It is occasionally isolated in pigs with bacteremia.
Figure 13-89.
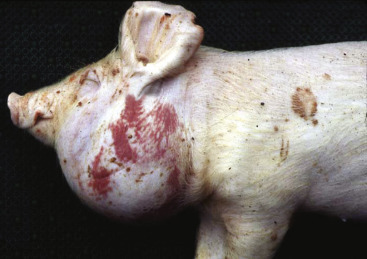
Jowl Abscess, Pig.
The mandibular lymph nodes are markedly enlarged from a suppurative lymphadenitis caused by Streptococcus porcinus.
(Courtesy Dr. J.M. King, College of Veterinary Medicine, Cornell University.)
Lymphoma
Lymphoma is the most frequently reported cancer of pigs based on abattoir surveys. Affected pigs are typically less than 1 year of age, and there is no reported breed predisposition, although a hereditary basis is suspected in cases arising in inbred herds. The two main forms of porcine lymphoma are thymic/mediastinal and multicentric; the latter is more common. Spleen, liver, kidney, bone marrow, and lymph nodes are affected in the multicentric form, with visceral lymph nodes reportedly more commonly involved than peripheral nodes. A recent study of lymphoma in 17 pigs found the majority to be multicentric, and subtypes included the following: B lymphoblastic leukemia/lymphoma, follicular lymphoma, diffuse and intestinal large B cell lymphoma, and peripheral T cell lymphoma. One case each of thymic B cell and T cell lymphomas were also described.
Disorders of Dogs
Severe Combined Immunodeficiency Disease
Several types of severe combined immunodeficiency diseases have been described in dogs. A mutation in DNA-PKcs (similar to Arabian horses) with an autosomal recessive mode of inheritance is seen in Jack Russell terriers. An X-linked form of severe combined immunodeficiency disease is well described in basset hounds and is caused by mutations in the common γ-chain (γc) subunit of the receptors for IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21. A similar disease is seen in Cardigan Welsh corgi puppies, though it is an autosomal mode of inheritance in this breed. The mutation inhibits the signal transduction pathways initiated by any of these cytokines, which are critical for the proliferation, differentiation, survival, and function of B and T lymphocytes. Affected dogs have normal numbers of circulating B lymphocytes that are unable to class switch to IgG or IgA and reduced numbers of T lymphocytes, which are nonfunctional due to the inability to express IL receptors. Affected puppies are remarkably susceptible to bacterial and viral infections and rarely survive past 3 to 4 months of age. The thymus of these dogs is small and consists of only small dysplastic lobules with a few of Hassall's corpuscles. Tonsils, lymph nodes, and Peyer's patches are often grossly unidentifiable due to the severe lymphocyte hypoplasia. Congenital immunodeficiency diseases are also discussed in detail in Chapter 5.
Thymic Hematomas
Thymic hemorrhage and hematomas have been reported in dogs and are most often seen in young animals. A variety of causes are described, including ingestion of anticoagulant rodenticides (warfarin, dicumarol, diphacinone, and brodifacoum), dissecting aortic aneurysms, trauma (e.g., automobile accident), and idiopathic/spontaneous. Histologically, hemorrhage variably expands the thymic lobules and septa, and in severe cases the lobular architecture is obscured by hemorrhage. In cases of anticoagulant rodenticide toxicosis, the medulla appears to be the main site of hemorrhage.
Gastrosplenic Volvulus
See Uniform Splenomegaly with a Bloody Consistency (also see Figs. 7-72 and 7-73).
Splenic Hematomas, Incomplete Splenic Contraction, Acute Splenic Infarcts, and Hemangiosarcomas
See the section on Splenic Nodules with a Bloody Consistency for discussion on splenic hematomas (including those induced by nodular hyperplasia or occurring with hemangiosarcoma), incomplete splenic contraction, acute splenic infarcts, and hemangiosarcomas.
Siderofibrotic Plaques, Splenic Rupture, and Accessory Spleens
See the section on Miscellaneous Disorders of the Spleen for discussions on siderofibrotic plaques, splenic rupture, and accessory spleens.
Lymphoid and Complex Splenic Nodular Hyperplasia
Splenic nodular hyperplasia is common in dogs and categorized based on their cellular components as lymphoid nodular hyperplasia or complex nodular hyperplasia. Hematomas may arise within nodules of hyperplasia (see Splenic Nodules with a Bloody Consistency).
Lymphoid (or simple) nodular hyperplasia consists of a focal well-demarcated mass composed of discrete to coalescing aggregates of lymphocytes. The lymphocytes may form follicular structures with germinal centers and/or consist of a mixture of lymphocytes with mantle and marginal zone cell morphologic features. The intervening tissue is often congested and may contain plasma cells, but stroma is not observed (Fig. 13-90 ; E-Fig. 13-13).
Figure 13-90.
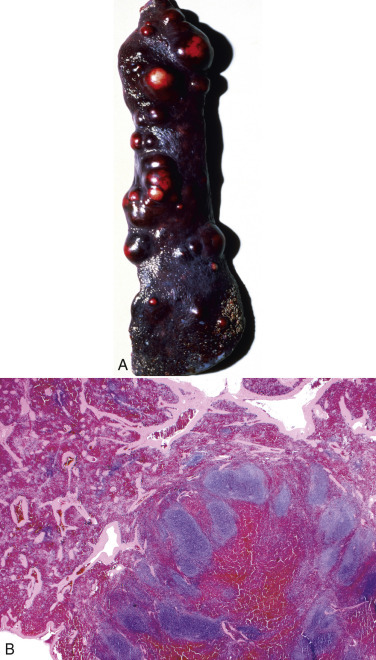
Lymphoid (Simple) Splenic Nodular Hyperplasia
A, Nodular hyperplasia, spleen, dog. B, The well-demarcated nodule (lower right of image) is composed of hyperplastic lymphoid follicles, and the intervening tissue is congested. H&E stain.
(A courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee. B courtesy Dr. A.C. Durham, School of Veterinary Medicine, University of Pennsylvania.)
E-Figure 13-13.

Nodular Hyperplasia, Spleen, Dog.
A, A hemispheric 4-cm diameter nodule protrudes from the capsular surface. B, Cross section of the nodular mass with mottled red and white areas composed of red blood cells and hyperplastic follicles.
(A courtesy College of Veterinary Medicine, University of Illinois. B courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Complex nodular hyperplasia is a focal mass that contains two proliferative components: lymphoid and stroma (E-Fig. 13-14). The lymphoid component resembles lymphoid nodular hyperplasia described above. There is proliferation of the intervening stromal tissues with fibroplasia, smooth muscle hyperplasia, and histiocytic hyperplasia; EMH and plasma cells may also be present.
E-Figure 13-14.

Complex Splenic Nodular Hyperplasia.
The splenic nodule contains a lymphoid component of discrete to merging nodular aggregates of lymphocytes, characteristic of simple lymphoid nodular hyperplasia. In addition, there is proliferation of splenic stromal elements with fibroplasia, smooth muscle hyperplasia, and histiocytic hyperplasia. H&E stain.
(Courtesy Dr. A.C. Durham, School of Veterinary Medicine, University of Pennsylvania.)
Splenic Fibrohistiocytic Nodules
It has recently come to light that the entity splenic fibrohistiocytic nodule (SFHN), first described in 1998, is not a single condition, but in fact a complex group of diseases. Our better understanding of the spectrum of diseases once described under the term splenic fibrohistiocytic nodule is due to increasing knowledge of histiocytic disorders and immunochemistry. The original definition of splenic fibrohistiocytic nodule is a nodule characterized by a stromal population of histiocytoid and spindle cells intermixed with lymphocytes. Grading was based on the lymphocyte percentage of the population (e.g., > 70% lymphocytes = grade 1; < 40% lymphocytes = grade 3); dogs with grade 1 splenic fibrohistiocytic nodule had a much better 1-year survival rate, and dogs with grade 3 nodules may develop sarcomas (often malignant fibrous histiocytoma, a now outdated term).
With our increasing knowledge of histiocytic disorders and additional immunohistochemical stains, diseases that likely were encompassed by the term splenic fibrohistiocytic nodule include the following: complex and lymphoid nodular hyperplasia (see earlier), stromal sarcoma, histiocytic sarcoma, marginal zone hyperplasia, marginal zone lymphoma, and diffuse large B cell lymphoma (see Lymphoid/Lymphatic System, Disorders of Domestic Animals: Lymph Nodes, Neoplasia, Lymphoma).
Histoplasmosis
Histoplasma capsulatum can cause a disseminated fungal disease that is widely endemic, particularly in areas with major river valleys and temperate or tropical climates (e.g., midwestern and southern United States). Free-living organisms in the mycelial phase produce macroconidia and microconidia that are inhaled and converted to the yeast phase in the lung. Yeasts are phagocytized and harbored by macrophages of the monocyte-macrophage system. In some dogs the disease is limited to the respiratory tract and causes dyspnea and coughing. However, in most dogs, the disease is disseminated throughout the body, predominantly affecting the liver, spleen, gastrointestinal tract, bone marrow, skin, and eyes; primary gastrointestinal disease is also reported. The clinical signs in cases of disseminated histoplasmosis include wasting, emaciation, fever, respiratory distress, diarrhea with hematochezia or melena, and lameness.
The clinicopathologic changes of disseminated histoplasmosis may include neutrophilia, monocytosis, nonregenerative anemia in chronic infections, changes in total serum protein level, and liver enzyme level elevations with hepatic involvement. The anemia is likely a result of chronic inflammation, Histoplasma infection of the bone marrow, and/or intestinal blood loss in dogs with GI disease. Cytologic examination is useful for the diagnosis of histoplasmosis (tracheal wash preparations, aspirates of bone marrow and lymph nodes), where organisms are often visible in macrophages (E-Fig. 13-15).
E-Figure 13-15.
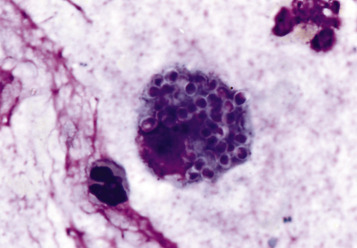
Histoplasmosis, Feline Transtracheal Wash.
A macrophage is laden with small, oval, encapsulated yeast forms. Wright's stain.
(Courtesy Dr. M.M. Fry, College of Veterinary Medicine, University of Tennessee.)
Grossly, there is hepatosplenomegaly, the intestines are thickened and corrugated, and the lymph nodes are uniformly enlarged (Fig. 13-91 ) with loss of normal architecture (somewhat similar to lymphoma, though the nodes tend to be more firm in histoplasmosis). Histologically within the node, there is a multifocal to coalescing infiltrate of epithelioid macrophages with intracytoplasmic, small (2 to 4 µm in diameter) yeast organisms with spherical basophilic central bodies surrounded by a clear halo (Fig. 13-92 ).
Figure 13-91.
Mesenteric Lymph Node, Diffuse Granulomatous Lymphadenitis, Histoplasmosis, Dog.
The lymph node is enlarged, the cut surface shows loss of architecture, and the tissue bulges because of the diffuse granulomatous inflammation (see Fig. 13-92).
(Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Figure 13-92.
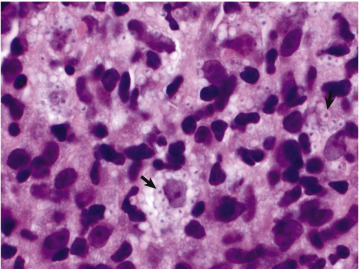
Histoplasmosis, Lymph Node, Dog.
Diffuse granulomatous lymphadenitis. Macrophages contain the phagocytized Histoplasma capsulatum organisms (arrows). H&E stain.
(Courtesy Dr. A.C. Durham, School of Veterinary Medicine, University of Pennsylvania.)
Leishmaniasis
Leishmaniasis is a disease of the monocyte-macrophage system caused by protozoa of the genus Leishmania. It occurs in dogs and other animals and is endemic in parts of the United States, Europe, Mediterranean, Middle East, Africa, and Central and South America. The protozoa proliferate by binary fission in the gut of the sand fly and become flagellated organisms, which are introduced into mammals by insect bites, where they are phagocytized by macrophages and assume a nonflagellated form. Cutaneous and/or visceral forms of the disease are observed. In the visceral form, dogs are emaciated and have general enlargement of abdominal lymph nodes and hepatosplenomegaly (E-Fig. 13-16). Histologically, the lymph node sinuses and splenic red pulp are filled with macrophages that contain intracytoplasmic, round, 2-µm-diameter organisms with a small kinetoplast. Though there is an initial stage of lymphoid hyperplasia in the spleen and lymph node, subsequent lymphoid atrophy occurs with chronicity. The atrophy is due to impairment of follicular DCs, B lymphocyte migration, and germinal center formation. There may be lymphoid atrophy of the spleen and lymph nodes in severe chronic infections.
E-Figure 13-16.
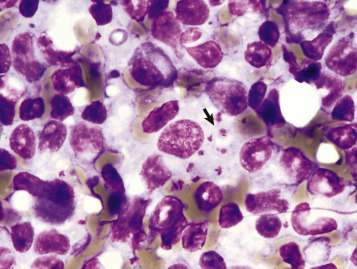
Leishmaniasis, Leishmania spp., Canine Popliteal Lymph Node Aspirate.
A macrophage contains multiple amastigotes with oval nuclei and smaller bar-shaped kinetoplasts (arrow). Wright's stain.
(Courtesy Dr. M.M. Fry, College of Veterinary Medicine, University of Tennessee.)
Canine Distemper
Canine distemper virus preferentially infects lymphoid, epithelial, and nervous cells (see Chapter 14). Dogs are exposed through contact with oronasal secretions, and the virus infects macrophages within the lymphoid tissue of the tonsil and respiratory tract (including tracheobronchial lymph nodes) and later disseminates to the spleen, lymph nodes, bone marrow, MALT, and hepatic Kupffer cells. The virus causes necrosis of lymphocytes (especially CD4 T lymphocytes) and depression of lymphopoiesis in the bone marrow, leading to severe immunosuppression. Dogs are therefore susceptible to secondary infections, including Bordetella bronchiseptica, Toxoplasma gondii, Nocardia, Salmonella spp., and generalized demodicosis.
Canine Parvovirus
Canine parvovirus type 2 (CPV-2) is a highly contagious disease of dogs spread through the fecal-oral route or oronasal exposure to contaminated fomites. The virus has tropism for rapidly dividing cells, and replication begins in the lymphoid tissues of the oropharynx, thymus, and mesenteric lymph nodes and then is disseminated to the small intestinal crypt epithelium. By infecting lymphoid tissues, canine parvovirus type 2 causes immunosuppression directly through lymphocytolysis and indirectly though bone marrow depletion of lymphocyte precursors. There is marked lymphoid atrophy of thymus and follicles of the spleen, lymph nodes, and MALT—particularly of Peyer's patches to produce the classic gross lesion of depressed oval regions of the mucosa (so-called punched-out Peyer's patches).
Neoplasms
Thymomas.
See Lymphoid/Lymphatic System, Disorders of Domestic Animals: Thymus, Neoplasia.
Lymphomas.
Lymphoma is the most common hematologic malignancy in the dog. Using the WHO classification scheme, several lymphoma subtypes are identified in dogs and clinically range from slow-growing indolent tumors to highly aggressive tumors. Of all domestic animal species, lymphoma is the most extensively studied in dogs. The most common clinical presentation in dogs is generalized lymphadenopathy, with or without clinical signs such as lethargy and inappetence.
The majority of lymphomas in dogs are large cell mid- to high-grade lymphomas, and up to half of all lymphoma cases are subtyped as diffuse large B cell lymphoma. Diffuse large B cell lymphomas are further subdivided into centroblastic or immunoblastic based on nucleolar morphologic features (see Table 13-7 and Box 13-11), although it is unclear if this difference has any prognostic significance. Histologically, lymph node architecture is most often completely effaced by sheets of large neoplastic cells, which may invade through the capsule and colonize the perinodal tissue. These dogs are often treated with chemotherapy and achieve remission. The overall median survival time for dogs with diffuse large B lymphocyte lymphoma is approximately 7 months, although this number varies based on the study and the grade of the tumor (as determined by mitotic figures). Peripheral T cell lymphomas–not otherwise specified are the second most common subtype in dogs. This category includes all T cell lymphomas that do not fit into the other categories (e.g., T zone lymphoma, enteropathy-associated T cell lymphoma, and hepatosplenic T cell lymphoma). Peripheral T cell lymphoma also effaces nodal architecture, and when compared to diffuse large B cell lymphoma, there is more variation in nuclear size and morphologic features. Dogs with this subtype tend to have shorter survival times.
Intermediate cell size, high-grade lymphomas are less common in dogs, and the two most frequently encountered subtypes are lymphoblastic lymphoma (LBL) and Burkitt-like lymphoma (BLL). Lymphoblastic lymphoma may be of B or T lymphocyte origin, though T cell lymphoblastic lymphoma is more common of the two. It is important to recognize a common misuse of the term “lymphoblast” in lymphoblastic lymphoma—by definition in lymphoblastic lymphoma, lymphoblasts are intermediate-sized cells with a distinct dispersed chromatin pattern, and not the large lymphocytes seen in cases of diffuse large B cell lymphoma or peripheral T cell lymphoma. T lymphocyte lymphoblastic lymphoma is an aggressive disease that is often resistant to treatment. Burkitt-like lymphoma is a high-grade lymphoma of B lymphocytes.
Numerous other subtypes of lymphoma have been reported in dogs, including several forms of cutaneous lymphomas, most often of T lymphocyte origin and epitheliotropic (see Chapter 17). Hepatosplenic T cell lymphoma, thought to be of γ/δ T lymphocyte origin, affects the liver and spleen without significant nodal involvement. Hepatocytotropic T cell lymphoma is a distinct form of lymphoma with tropism for the hepatic cords; clusters or individual neoplastic lymphocytes invade the hepatic cords, without hepatocyte degeneration. Intravascular lymphoma is a proliferation of large neoplastic lymphocytes within blood vessels of many tissues, leading to progressive occlusion and subsequent thromboses and infarcts. This neoplasm does not form an extravascular mass, and neoplastic cells are not found in peripheral blood smears or bone marrow.
Indolent lymphomas constitute up to 29% of all canine lymphomas. Indolent lymphomas in dogs, in descending order of frequency, include T zone lymphoma (TZL), marginal zone lymphoma (MZL), mantle cell lymphoma (MCL), and follicular lymphoma (FL). Mantle cell lymphoma and follicular lymphoma are less commonly diagnosed than T zone lymphoma and marginal zone lymphoma; therefore the reader is referred to the Suggested Readings to learn more on mantle cell lymphoma and follicular lymphoma.
T Zone Lymphoma.
T zone lymphoma is the most common indolent lymphoma in dogs (Fig. 13-93 ). It presents as a solitary or multiple peripheral lymphadenomegaly (often mandibular lymph nodes) in otherwise healthy-appearing dogs. The characteristic histopathologic architecture is a nodular expansion of the paracortex by neoplastic cells, which push atrophied “fading” cortical follicles against the thinned capsule and trabeculae. This unique architectural feature is best highlighted with immunohistochemical stains (often CD3 for T lymphocytes and CD79a, pax5, or CD20 for B lymphocytes). The neoplastic cells are small to intermediate in size with pale eosinophilic cytoplasm and oval nuclei with sharp shallow indentations. Mitotic figures are rare. Dogs with this lymphoma subtype tend to be diagnosed with an advanced stage of the disease, likely because they present clinically healthy, without loss of appetite or activity level. Even so, dogs with T zone lymphoma have a relatively long survival time compared to other lymphomas: reports on median survival time range from 13 to 33 months, and data suggest that dogs who do not receive chemotherapy actually have longer median survival times.
Figure 13-93.
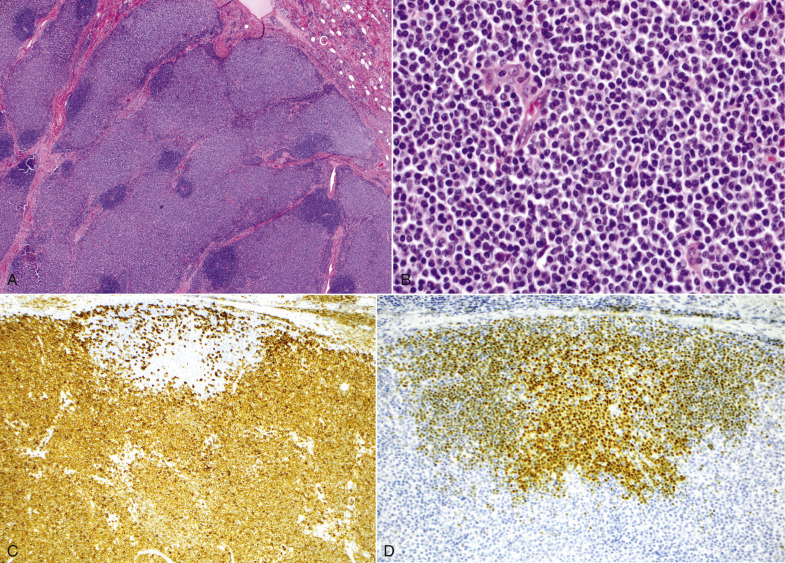
T Zone Lymphoma, Lymph Node, Dog.
A, The characteristic histopathologic architecture is a nodular expansion of the paracortex by neoplastic cells, which push atrophied “fading” cortical follicles against the capsule (C) and trabeculae (interconnected pink bands). H&E stain. B, The neoplastic cells are small to intermediate in size, and mitotic figures are rare. H&E stain. C, The neoplastic cells are T lymphocytes. Immunohistochemistry anti-CD3, hematoxylin counterstain. D, The remnants of the “fading” cortical follicles are composed of B lymphocytes. Immunohistochemistry anti-pax5, hematoxylin counterstain.
(Courtesy Dr. A.C. Durham, School of Veterinary Medicine, University of Pennsylvania.)
Marginal Zone Lymphoma.
Marginal zone lymphoma is an indolent B lymphocyte neoplasm derived from the cells of the marginal zone of lymphoid follicles. Most marginal zone lymphomas (and mantle cell lymphomas) are assumed to originate in the spleen with slow spread to lymph nodes and often present as a mottled white-red smooth spherical splenic mass. Histopathologic assessment of tissue architecture is needed for a diagnosis of marginal zone lymphoma and is characterized by a distinct nodular pattern in which the lighter-staining neoplastic marginal zone cells form a dense cuff around small foci of darkly stained mantle cells (fading follicles). The neoplastic marginal zone lymphocytes are intermediate in size and have a single prominent central nucleolus. Mitotic figures are often rare or absent early on and increase with disease progression.
Differentiating between marginal zone lymphoma and marginal zone hyperplasia (which refers to a proliferation of marginal zone cells and contains a mixture of small and intermediate lymphocytes) is challenging because marginal zone lymphoma arises on the background of marginal zone hyperplasia. Additionally, lymphoid and complex nodular hyperplasia are common in the dog spleen (see Disorders of Dogs), and it is possible that many cases of nodular hyperplasia contain areas of marginal zone lymphoma. Therefore immunophenotyping and molecular clonality are ultimately required for a definitive diagnosis of marginal zone lymphoma. The overall median survival time in dogs with splenic marginal zone lymphoma after splenectomy is approximately 13 months (even longer if it is diagnosed as an incidental finding).
Plasmacytomas.
See Disorders of Domestic Animals: Lymph Nodes, Neoplasia, Plasma Cell Neoplasia, Extramedullary Plasmacytomas (see Fig. 13-84).
Disorders of Cats
Feline Panleukopenia (Parvovirus)
Feline panleukopenia, caused by the single-stranded DNA virus feline parvovirus (FPV), is a highly contagious and often lethal disease of cats and other Felidae, as well as other species (including raccoons, ring-tailed cats, foxes, and minks). FPV is transmitted by the fecal-oral route through contact with infected body fluids, feces, or fomites. Following intranasal or oral infection, the virus initially replicates in the macrophages in the lamina propria of the oropharynx and regional lymph nodes, followed by viremia, which distributes the virus throughout the body. Because FPV requires rapidly multiplying cells in the S phase of division for its replication, replication occurs in mitotically active tissues (lymphoid tissue, bone marrow, and intestinal mucosa). By infecting lymphoid tissues, FPV causes immunosuppression directly through lymphocytolysis and indirectly through depletion of lymphocyte precursors in the bone marrow. Consequently there is marked lymphoid atrophy of thymus, spleen, lymph node, and MALT (particularly Peyer's patches).
Mast Cell Tumors
See Bone Marrow and Blood Cells, Disorders of Domestic Animals, Types of Hematopoietic Neoplasia, Myeloid Neoplasia, Mast Cell Neoplasia and see E-Fig. 13-6.
Lymphoma
Lymphoma is the most commonly diagnosed neoplasm in cats, and the incidence is reportedly the highest for any species. Mediastinal or multicentric lymphomas are seen in young, FeLV-infected cats (see Fig. 13-53). With the advent of FeLV vaccine and routine testing, the prevalence of FeLV-associated lymphoma is decreased. Currently the alimentary tract is the most commonly affected site, and typically occurs in cats greater than 10 years of age (Figs. 13-94 and 13-95 ). Other miscellaneous sites commonly affected are brain, spinal cord, eye, kidney, and nasopharynx.
Figure 13-94.
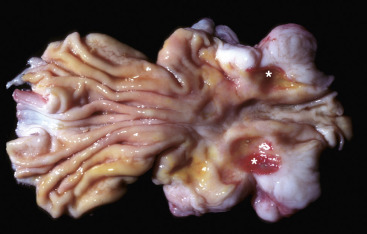
Alimentary Lymphoma, Stomach, Cat.
The stomach mucosa is markedly thickened by the neoplastic cells (gray-white areas in the right half of the image); focal ulcers are also noted (asterisks).
(Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Figure 13-95.
Intestinal Small Cell Lymphoma, Jejunum, Cat.
Enteropathy-associated T cell lymphoma. A, The neoplastic cells expand the lamina propria of intestinal villi and submucosa, lifting the crypts from the muscularis mucosa. B, Higher magnification of a villus. The neoplastic lymphocytes in the lamina propria are small and often colonize in clusters within the epithelium. H&E stain.
(Courtesy Dr. A.C. Durham, School of Veterinary Medicine, University of Pennsylvania.)
The retrovirus FeLV has long been recognized as a cause of lymphoma in cats—the risk for lymphoma is increased sixtyfold in infected cats. Before the advent of a vaccine in 1985, approximately 70% of cats (mainly young animals) with lymphoma were FeLV positive. FeLV infects T lymphocytes and can cause myelodysplastic syndrome, acute myeloid leukemias (see Myeloid Neoplasia), and T lymphocyte leukemia/lymphoma. In the latter the mediastinum (thymus, mediastinal, and sternal lymph nodes) is the site most commonly involved, although a multicentric distribution also occurs. Routine FeLV vaccination has led to a significant decrease in the prevalence of FeLV infection, which has resulted in a decrease in the proportion of mediastinal lymphomas.
The risk for developing lymphoma in FIV-infected cats is fivefold to sixfold higher than in uninfected cats. Cats that underwent kidney transplantation and thus received immunosuppressive drug therapy had a similar risk for developing lymphoma. Both FIV-infected and posttransplantation cats predominantly developed extranodal, high-grade, diffuse large B lymphocyte lymphomas. This form is also the most common subtype in human immunodeficiency virus and posttransplantation patients caused by the Epstein-Barr virus (EBV). Therefore it is reasonable to question whether these two groups of immunosuppressed cats may be more prone to infection by a gammaherpesvirus similar to EBV, leading to lymphoma. Recently a novel feline gammaherpesvirus (FcaGHV1) was discovered in domestic cats with a 16% prevalence in North America, and further studies to investigate its role in lymphomagenesis are needed.
The overall incidence of feline lymphomas has increased, mainly due to an increase in gastrointestinal lymphomas. Mucosal T cell lymphoma, also known as enteropathy-associated T cell lymphoma (EATCL type II), is the most common and arises from diffuse MALT of the small intestine. The neoplastic cells are small (nuclei are equal to the diameter of a feline RBC), mitotic figures are infrequent (low grade), and mucosal and crypt epitheliotropism is common (see Fig. 13-95). A diagnosis of this subtype of lymphoma may be difficult (particularly in endoscopic biopsy samples), because this disease often is multifocal and concurrent with or arises within lymphoplasmacytic inflammatory bowel disease (IBD). The neoplastic lymphocytes are morphologically similar to the inflammatory lymphocytes. Early small cell mucosal T cell lymphomas often require additional diagnostic testing, namely, immunohistochemistry and molecular clonality testing (PCR for antigen receptor rearrangement [PARR]) to confirm a clonal neoplasm.
Transmural T cell lymphomas also occur focally or multifocally in the small intestine of cats (best classified as enteropathy-associated T cell lymphoma type I) and by definition must extend into the submucosa and muscularis. Some tumors invade the serosa and adjacent mesentery. T cell large granular lymphocyte (LGL) lymphoma is often diagnosed, and the intestinal segments orad and aborad to the transmural mass may also have mucosal lymphoma. Gastrointestinal B cell lymphomas are less prevalent in cats but occur in the stomach, jejunum, and ileocecocolic region as transmural lesions. Most are diagnosed as diffuse large B cell lymphomas.
Lymphomas in other sites also occur less frequently in cats. The upper respiratory tract (nasal and/or nasopharyngeal region) is a relatively rare site for lymphoma. However, lymphoma is the most common primary nasal tumor, and diffuse large B cell lymphomas (of immunoblastic type) are the predominant subtype. Both cutaneous (cutaneous T cell lymphoma) and subcutaneous lymphomas (usually large cell lymphomas) are rare. Presumed solitary ocular lymphomas have also been reported.
T cell–rich large B cell lymphoma, also referred to as feline Hodgkin-like lymphoma in some studies, is composed of a mixture of reactive small lymphocytes and large neoplastic B lymphocytes, many of which may be binucleated and/or have prominent nucleoli (thus resembling the Reed-Sternberg cells of human Hodgkin's lymphoma). This disease is typically characterized by a distinctive clinical presentation of an indolent unilateral neoplasm of the cervical lymph nodes, which spreads slowly to adjacent nodes within the chain. However, a proportion of cases may go on to develop into a more aggressive multicentric large to anaplastic B lymphocyte lymphoma that can affect peripheral and central nodes and multiple organs.
Suggested Readings
Suggested Readings are available at www.expertconsult.com.
Footnotes
For a glossary of abbreviations and terms used in this chapter see E-Glossary 13-1.
For tests to evaluate platelet function or immune-mediated thrombocytopenia see E-Appendix 13-1.
“Blast equivalents” include other stages of immature myeloid cells, such as abnormal promyelocytes, monoblasts, promonocytes, erythroblasts, and megakaryoblasts.
There are numerous synonyms and misuse of terms within the literature, which have contributed to the confusion over terminology for red pulp vascular spaces. These terms include reticular space, red pulp, splenic cords, sinuses, red pulp sinuses, sinus spaces, pulp spaces, mesh space of the spleen, reticular cell-lined meshwork, interstices of the reticulum network, blood-filled reticular meshwork of the red pulp, chordal spaces, splenic cords, and cords of Billroth. The latter two terms are defined as the red pulp between the sinusoids, which most domestic animals do not have (except the dog). Therefore the term red pulp vascular spaces is more appropriate.
The growth and development of a lymphoma.
Suggested Readings
- Alexandre-Pires G. Intermediary spleen microvasculature in Canis familiaris—morphological evidence of a closed and open type. Anat Histol Embryol. 2003;32:263–270. doi: 10.1046/j.1439-0264.2003.00469.x. [DOI] [PubMed] [Google Scholar]
- Avery PR, Avery AC. Molecular methods to distinguish reactive and neoplastic lymphocyte expansions and their importance in transitional neoplastic states. Vet Clin Pathol. 2004;33:196–207. doi: 10.1111/j.1939-165x.2004.tb00374.x. [DOI] [PubMed] [Google Scholar]
- Beutler E, Lichtman MA, Coller BS, editors. Williams hematology. ed 6. McGraw-Hill; New York: 2001. [Google Scholar]
- Boudreaux MK. Characteristics, diagnosis, and treatment of inherited platelet disorders in mammals. J Am Vet Med Assoc. 2008;233:1251–1259. doi: 10.2460/javma.233.8.1251. [DOI] [PubMed] [Google Scholar]
- Burkhard MJ, Bienzle D. Making sense of lymphoma diagnostics in small animal patients. Vet Clin Small Anim. 2013;43:1331–1347. doi: 10.1016/j.cvsm.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Carrade DD. Canine granulocytic anaplasmosis: a review. J Vet Intern Med. 2009;23:1129–1141. doi: 10.1111/j.1939-1676.2009.0384.x. [DOI] [PubMed] [Google Scholar]
- Chaplin DD. Lymphoid tissues and organs. In: Paul WE, editor. Fundamental immunology. ed 5. Lippincott Williams & Wilkins; Philadelphia: 2003. [Google Scholar]
- Charles JA. Lymph nodes and thymus. In: Sims LD, Glastonbury JRW, editors. Pathology of the pig: a diagnostic guide. Barton, A.C.T.: Pig Research and Development Corp; Victoria, Australia: 1996. [Google Scholar]
- Constantinescu G, Schaller O, editors. Illustrated veterinary anatomical nomenclature. ed 3 rev. Thieme; Stuttgart, Germany: 2011. [Google Scholar]
- Cowell RL. ed 3. Mosby; St. Louis: 2008. Diagnostic cytology and hematology of the dog and cat. [Google Scholar]
- Durham AC, Pillitteri CA, Myint MS. Two hundred three cases of equine lymphoma classified according to the World Health Organization (WHO) classification criteria. Vet Pathol. 2013;50:86–93. doi: 10.1177/0300985812451603. [DOI] [PubMed] [Google Scholar]
- Ganz T. Hepcidin: a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102:783–788. doi: 10.1182/blood-2003-03-0672. [DOI] [PubMed] [Google Scholar]
- George JW. Changes to bovine hematology reference intervals from 1957 to 2006. Vet Clin Pathol. 2010;39:138–148. doi: 10.1111/j.1939-165X.2009.00208.x. [DOI] [PubMed] [Google Scholar]
- Harvey JW. Saunders; Philadelphia: 2001. Atlas of veterinary hematology: blood and bone marrow of domestic animals. [Google Scholar]
- Harvey JW. Pathogenesis, laboratory diagnosis, and clinical implications of erythrocyte enzyme deficiencies in dogs, cats, and horses. Vet Clin Pathol. 2006;35:144–156. doi: 10.1111/j.1939-165x.2006.tb00108.x. [DOI] [PubMed] [Google Scholar]
- Hoover EA, Mullins JI. Feline leukemia virus infection and diseases. J Am Vet Med Assoc. 1991;199:1287–1297. [PubMed] [Google Scholar]
- Jacobs RM, Messick JB, Valli VE. Tumors of the hemolymphatic system. In: Meuten DJ, editor. Tumors in domestic animals. ed 4. Iowa State Press; Ames: 2002. [Google Scholar]
- Jain NC, Blue JT, Grindem CB. Proposed criteria for classification of acute myeloid leukemia in dogs and cats. Vet Clin Pathol. 1991;20:63–82. doi: 10.1111/j.1939-165x.1991.tb00571.x. [DOI] [PubMed] [Google Scholar]
- Janeway CA, Travers P, Walport M. ed 5. Garland Publishing; New York: 2001. Immunobiology: the immune system in health and disease. [Google Scholar]
- Johns JL, Christopher MM. Extramedullary hematopoiesis: a new look at the underlying stem cell niche, theories of development, and occurrence in animals. Vet Pathol. 2012;49:508–523. doi: 10.1177/0300985811432344. [DOI] [PubMed] [Google Scholar]
- Kaushansky K. Lineage-specific hematopoietic growth factors. N Engl J Med. 2006;354:2034–2045. doi: 10.1056/NEJMra052706. [DOI] [PubMed] [Google Scholar]
- Keller SM, Vernau W, Hodges J. Hepatosplenic and hepatocytotropic T-cell lymphoma: two distinct types of T-cell lymphoma in dogs. Vet Pathol. 2013;50:281–290. doi: 10.1177/0300985812451625. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum AL. ed 2. Mosby; St. Louis: 2007. Histology and cell biology: an introduction to pathology. [Google Scholar]
- Kikumi O, Takemi O, Hikaru T. Lymphoid neoplasms in swine. J Vet Med Sci. 2012;74:149–154. doi: 10.1292/jvms.11-0277. [DOI] [PubMed] [Google Scholar]
- Kumar V, Abbas AK, Fausto N, Aster JC. ed 9. Saunders; Philadelphia: 2014. Robbins & Cotran pathologic basis of disease. [Google Scholar]
- McOrist S, Sims LD. The spleen. In: Sims LD, Glastonbury JRW, editors. Pathology of the pig: a diagnostic guide. Barton, A.C.T.: Pig Research and Development Corp; Victoria, Australia: 1996. [Google Scholar]
- McQuiston JH, McCall CL, Nicholson WL. Ehrlichiosis and related infections. J Am Vet Med Assoc. 2003;223:1750–1756. doi: 10.2460/javma.2003.223.1750. [DOI] [PubMed] [Google Scholar]
- Messick JB. Hemotropic mycoplasmas (hemoplasmas): a review and new insights into pathogenic potential. Vet Clin Pathol. 2004;33:2–13. doi: 10.1111/j.1939-165x.2004.tb00342.x. [DOI] [PubMed] [Google Scholar]
- Meyer J. Clinical, laboratory, and histopathologic features of equine lymphoma. Vet Pathol. 2006;43:914–924. doi: 10.1354/vp.43-6-914. [DOI] [PubMed] [Google Scholar]
- Miller CA, Durham AC, Schaffer PA. Classification and clinical features in 88 cases of equine cutaneous lymphoma. J Vet Diagn Invest. 2015;27:86–91. doi: 10.1177/1040638714561653. [DOI] [PubMed] [Google Scholar]
- Moore AS, Frimberger AE, Sullivan N. Histologic and immunohistochemical review of splenic fibrohistiocytic nodules in dogs. J Vet Intern Med. 2012;26:1164–1168. doi: 10.1111/j.1939-1676.2012.00986.x. [DOI] [PubMed] [Google Scholar]
- Moore PF, Rodriguez-Bertos A, Kass PH. Feline gastrointestinal lymphoma: mucosal architecture, immunophenotype, and molecular clonality. Vet Pathol. 2012;49:658–668. doi: 10.1177/0300985811404712. [DOI] [PubMed] [Google Scholar]
- Opriessnig T, Langohr I. Current state of knowledge on porcine circovirus type 2-associated lesions. Vet Pathol. 2012;50:23–38. doi: 10.1177/0300985812450726. [DOI] [PubMed] [Google Scholar]
- Perryman LE. Molecular pathology of severe combined immunodeficiency in mice, horses, and dogs. Vet Pathol. 2004;41:95–100. doi: 10.1354/vp.41-2-95. [DOI] [PubMed] [Google Scholar]
- Sellon DC, Fuller FJ, McGuire TC. The immunopathogenesis of equine infectious anemia virus. Virus Res. 1994;32:111–138. doi: 10.1016/0168-1702(94)90038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton GH, Linenberger ML. Hematologic abnormalities associated with retroviral infections in the cat. Semin Vet Med Surg (Small Anim) 1995;10:220–233. [PubMed] [Google Scholar]
- Sims LD, Glastonbury JRW, editors. Pathology of the pig: a diagnostic guide. Barton, A.C.T.: Pig Research and Development Corp; Victoria, Australia: 1996. [Google Scholar]
- Spangler WL, Kass PH. Pathologic and prognostic characteristics of splenomegaly in dogs due to fibrohistiocytic nodules: 98 cases. Vet Pathol. 1998;35:488–498. doi: 10.1177/030098589803500603. [DOI] [PubMed] [Google Scholar]
- Spangler WL, Kass PH. Splenic myeloid metaplasia, histiocytosis, and hypersplenism in the dog (65 cases) Vet Pathol. 1999;36:583–593. doi: 10.1354/vp.36-6-583. [DOI] [PubMed] [Google Scholar]
- Stockham SL, Scott MA. ed 2. Blackwell Publishing; Ames: 2008. Fundamentals of veterinary clinical pathology. [Google Scholar]
- Stuetzer B, Hartmann K. Feline parvovirus infection and associated diseases. Vet J. 2014;201:150–215. doi: 10.1016/j.tvjl.2014.05.027. [DOI] [PubMed] [Google Scholar]
- Troyer RM, Beatty JA, Stutzman-Rodriguez KR. Novel gammaherpesviruses in North American domestic cats, bobcats, and pumas: identification, prevalence, and risk factors. J Virol. 2014;88:3914–3924. doi: 10.1128/JVI.03405-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valli VE, Kiupel M, Bienzle D. Hematopoietic system. In: Maxie MG, editor. ed 6. vol 2. Elsevier; St Louis: 2016. (Jubb, Kennedy, and Palmer's pathology of domestic animals). [Google Scholar]
- Valli VE. Blackwell Publishing; Ames: 2007. Veterinary comparative hematopathology. [Google Scholar]
- Valli VE, Jacobs RM, Parodi AL. World Health Organization international histological classification of tumors in domestic animals. second series. Armed Forces Institute of Pathology; Washington, DC: 2002. Histologic classification of hematopoietic tumors of domestic animals. [Google Scholar]
- Valli VE, Kass PH, Myint MS. Canine lymphomas: association of classification type, disease stage, tumor subtype, mitotic rate, and treatment with survival. Vet Pathol. 2013;50:738–748. doi: 10.1177/0300985813478210. [DOI] [PubMed] [Google Scholar]
- Valli VE, Myint MS, Barthel A. Classification of canine malignant lymphomas according to the World Health Organization criteria. Vet Pathol. 2011;48:198–211. doi: 10.1177/0300985810379428. [DOI] [PubMed] [Google Scholar]
- Valli VE, Vernau W, De Lorimier LP. Canine indolent nodular lymphoma. Vet Pathol. 2006;43:241–256. doi: 10.1354/vp.43-3-241. [DOI] [PubMed] [Google Scholar]
- Vardiman JW, Thiele J, Arber DA. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- Wardrop KJ. The Coombs' test in veterinary medicine: past, present, future. Vet Clin Pathol. 2005;34:325–334. doi: 10.1111/j.1939-165x.2005.tb00057.x. [DOI] [PubMed] [Google Scholar]
- Weiss DJ. A retrospective study of the incidence and the classification of bone marrow disorders in the dog at a veterinary teaching hospital (1996-2004) J Vet Intern Med. 2006;20:955–961. doi: 10.1892/0891-6640(2006)20[955:arsoti]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Weiss DJ, Wardrop KJ, editors. Schalm's veterinary hematology. ed 6. Wiley-Blackwell; Ames: 2010. [Google Scholar]
- Workman HC, Vernau W. Chronic lymphocytic leukemia in dogs and cats: the veterinary perspective. Vet Clin North Am Small Anim Pract. 2003;33:1379–1399. doi: 10.1016/s0195-5616(03)00120-7. [DOI] [PubMed] [Google Scholar]
E-Appendix 13-1 Tests to Evaluate Platelet Function or Immune-Mediated Thrombocytopenia
-
•
Bleeding time (template bleeding time, buccal mucosal bleeding time). This assay assesses primary hemostasis (platelet plug formation) by measuring the time interval between inflicting of standardized wound and cessation of bleeding. Sedation may be required. In small animals the test is usually performed on the buccal mucosa; in large animals it may be performed on the distal limb. Prolonged bleeding time may be because of a platelet function defect, von Willebrand disease, or a vascular defect. The sensitivity of this test is low; reference intervals are species and site dependent (can perform test on a normal animal as a control). This test is contraindicated in cases of thrombocytopenia because significant thrombocytopenia can cause a prolonged bleeding time (invalidates interpretation of test results).
-
•
Clot retraction test. This assay assesses retraction of a clot, in which platelets play an essential role. This is a crude test that is rarely performed. Different protocols are described. Significant thrombocytopenia invalidates interpretation of test results.
-
•Tests to characterize platelet function abnormalities more specifically are available through specialized laboratories.
-
•Aggregometry—to assess platelet aggregation in response to different physiologic agonists.
-
•Adhesion assays—to assess the ability of platelets to adhere to a substrate (e.g., collagen).
-
•Flow cytometry—to assay for expression of surface molecules.
-
•PFA-100—an instrument that simulates a damaged blood vessel, by measuring time for a platelet plug to occlude an aperture; to date, this instrument has mainly been used in research applications.
-
•Thromboelastography (TEG)—global assessment of hemostasis (platelets, coagulation, and fibrinolysis) based on viscoelastic analysis of whole blood.
-
•
-
•Tests for immune-mediated thrombocytopenia (IMT).
-
•Flow cytometry—to detect immunoglobulin bound to the platelet surface, using a fluorescent-labeled antibody.
-
•Bone marrow immunofluorescent antibody (IFA) test—to detect bound immunoglobulin. Sometimes referred to as the “antimegakaryocyte antibody test,” this assay actually detects the presence of immunoglobulin nonspecifically: a smear of a bone marrow aspirate is incubated with a fluorescent-labeled antibody to species-specific immunoglobulin.
-
•
Tests for Evaluating the Coagulation System
-
•Activated partial thromboplastin time (aPTT or PTT)
-
•Required sample: citrated plasma.
-
•Measures time for fibrin clot formation after addition of a contact activator, calcium, and a substitute for platelet phospholipid.
-
•Deficiencies/dysfunction in intrinsic and/or common coagulation pathway (all factors except for VII and XIII) causes prolongation of PTT.
-
•Insensitive test—prolongation requires 70% deficiency.
-
•Other causes of prolongation include polycythemia (less plasma per unit volume, so excess amount of citrate is available to chelate calcium) and heparin therapy.
-
•
-
•Activated clotting time (ACT)
-
•Required sample: nonanticoagulated whole blood in special ACT tube (diatomaceous earth as contact activator).
-
•Used in practice setting—performed by warming sample to body temperature, monitoring for clot formation; normal clotting times are within 60 to 90 seconds in dogs, 165 seconds in cats.
-
•Less sensitive version of PTT—prolongation requires 95% deficiency.
-
•Severe thrombocytopenia may cause prolongation.
-
•
-
•One-stage prothrombin time (OSPT or PT)
-
•Required sample: citrated plasma.
-
•Measures time for fibrin clot formation after addition of tissue factor (TF; thromboplastin), calcium, and a substitute for platelet phospholipid.
-
•Deficiencies/dysfunction in extrinsic (factor VII) and/or common coagulation pathway cause prolongation of PT.
-
•Insensitive test—prolongation requires 70% deficiency.
-
•
-
•Proteins induced by vitamin K antagonism or absence (PIVKA) test
-
•Required sample: citrated plasma.
-
•Essentially a version of the PT using an especially sensitive thromboplastin reagent.
-
•PIVKA are inactive (uncarboxylated) vitamin K–dependent factors; an increase in PIVKA is not specific for vitamin-K antagonism but may be an earlier and more sensitive detector than PT or PTT.
-
•
-
•Thrombin time (TT)
-
•Required sample: citrated plasma.
-
•Measures time for fibrin clot formation after thrombin (factor IIa) is added.
-
•Defects directly involving formation and/or polymerization of fibrin prolong this test (i.e., if the lesion is upstream of the conversion of fibrinogen to fibrin, the TT will be normal). Hypofibrinogenemia or dysfibrinogenemia causes prolongation of the TT.
-
•
-
•Fibrinogen
-
•Required sample: citrated plasma.
-
•Fibrinogen concentration measured based on time to clot formation after addition of thrombin; this is essentially the same as the TT mentioned earlier and is a more accurate method than the heat precipitation method.
-
•Decreased fibrinogen may be because of increased consumption (disseminated intravascular coagulation) or decreased production (liver disease).
-
•Increased fibrinogen is associated with inflammation, renal disease, and dehydration.
-
•
-
•Fibrin degradation products (FDPs)
-
•Required sample: special FDP tube.
-
•Used in the practice setting.
-
•Performed by adding blood to a special tube containing thrombin and a trypsin inhibitor (sample clots almost instantly in normal dogs and cats) and incubating two dilutions of serum (1 : 5 and 1 : 20) with polystyrene latex particles coated with sheep anti-FDP antibodies (should be negative in normal dogs and cats, but positive results have been reported in normal cats).
-
•
-
•D-dimer
-
•Required sample: citrated plasma.
-
•Latex agglutination test.
-
•To date, only validated in dogs and horses.
-
•Assay detects a specific type of FDP resulting from breakdown of cross-linked fibrin; concentration of plasma D-dimer indicates the degree of fibrinolysis; often used as part of a disseminated intravascular coagulation panel; can be used as a negative predictor to rule out pathologic thrombosis (e.g., pulmonary thromboembolism); also increased when there is appropriate clotting.
-
•
-
•Antithrombin (antithrombin III [ATIII])
-
•Required sample: citrated plasma.
-
•Decreased because of decreased production (liver disease), loss (protein-losing nephropathy or enteropathy), consumption (disseminated intravascular coagulation), chronic heparin therapy.
-
•
-
•Specific factor assays
-
•Required sample: citrated plasma.
-
•Performed at specialized laboratories.
-
•
E-Appendix 13-2 Methods of Gross and Microscopic Examination
Thymus
Because the thymus involutes after sexual maturity, evaluation of whether it is smaller than normal is difficult to discern unless the change is extreme or age-matched control animals are available. Before sexual maturity the thymus is easily identified as a lobular white to gray organ with a thin capsule. After sexual maturity the gland is often grossly indistinguishable from adipose connective tissue within the cranial mediastinum, although microscopic remnants may remain. An extremely small thymus in a neonatal animal should be considered abnormal and may indicate a primary immunodeficiency or secondary lymphoid depletion caused by extreme stress, often due to infectious diseases. Enlargement of the thymus is most often due to neoplasia. Serial sectioning of the thymus allows for gross identification of neoplasms, cysts, or hematomas.
Spleen
Spleens vary in size within the same species and among the different species of domestic animals. The spleen can be enlarged (splenomegaly), normal in size, or small (atrophy), and the surface can be smooth, wrinkled, or nodular. The appearance of the cut surface of the spleen in normal animals depends on the amount of stroma (e.g., trabeculae are prominent in ruminants); the size and visibility of the white pulp, which reflects the amount of lymphoid tissue; and whether the red pulp is congested with blood. During an autopsy (syn: necropsy), the spleen is dissected free and checked for torsion of the gastrosplenic ligament (in nonruminants). The spleen is then sliced transversely at approximately 5-mm intervals (serial sectioning), and the cut surfaces are checked for lesions. Specimens are taken for tests that require fresh tissue (e.g., bacteriologic and virologic examinations), and the remaining cross-sections are placed in fixative (10% buffered neutral formalin).
A diffusely enlarged spleen should be serially sectioned to determine if the splenomegaly is due to congestion. The cut surface of severely congested spleens is red to bluish-black and exudes blood (bloody spleens), whereas cut surfaces of noncongested enlarged spleens ooze little blood (meaty spleens) and the color depends on how much of the normal parenchyma is replaced by inflammatory cells, stored materials, or neoplastic cells (see Splenomegaly and Table 13-5). The spleen may be measured and weighed, but because of the wide variation in the dimensions and weight of normal spleens and the amount of blood stored, this information is difficult to interpret. It is essential that spleens with one or more nodules also be serially sectioned and the nodules evaluated for size, shape, and consistency. Nodules may be dark red and ooze blood on cut surface, white-tan with a more firm texture, or a mixture of both. Multiple wedge sections that include the interface between a nodule and the adjacent nonmass spleen should be collected, because the center of the nodules often consists only of hemorrhage and necrosis, and neoplasms may be missed.
The color of the capsular surface of the spleen also varies among species of domestic animals and depends on the opacity or translucence of the splenic capsule. The degree of opacity of the capsule is a function of its thickness and the amount of collagen. The splenic capsules of horses and ruminants are thick and usually appear gray because the color of the red pulp is not visible through the capsule. In the pig, dog, and cat, the splenic capsule is thin, and thus the surface of the spleen is red. The tenseness of the capsule depends on how much the splenic parenchyma is distended; storage spleens devoid of blood usually have a wrinkled surface. Irregular contraction of storage spleens is common, especially in dogs, and consists of nonuniform areas of congestion with intermingled contracted and wrinkled regions.
Lymph Nodes
Lymph nodes should be dissected free of fat and connective tissue, and any firm attachment to adjacent tissues should be noted because these attachments may indicate neoplastic infiltration through the capsule. Gross examination includes evaluating size (measurement or weight), shape, and whether the capsule is intact. The cut surface is examined for the presence of bulging tissue, edema, congestion, exudate, discoloration (see Pigmentation), obscuration of the normal architecture, and masses such as abscesses, granulomas, and discrete neoplasms.
Microscopic Examination
Cytologic evaluation of superficial lymph nodes through fine-needle aspirates provides excellent cellular detail and often yields a diagnosis. However, diagnosis of certain diseases (including lymphomas for complete World Health Organization [WHO] classification) requires architectural assessments, and therefore cytologic or small histologic samples are not sufficient. Tru-Cut biopsies are not ideal, but a 2-mm Tru-Cut needle may provide adequate tissue.
The surgeon or pathologist must handle lymph nodes carefully to minimize artifacts. Compression (e.g., squeezing with forceps) may cause crush artifacts, usually resulting in nuclear “streaming.” Immediately after removal, imprints/impression smears should be prepared and then kept away from formalin fumes. Formalin fixation (in this case by formaldehyde fumes) destroys the differential staining seen with Romanowsky stains such as Wright's and Giemsa and results in diffuse blue staining. Prompt transfer of biopsy or postmortem specimens into fixative is crucial because delayed fixation can lead to numerous artifacts, including an artificial decrease in mitotic index (up to 40% reduction with a more than 12-hour delay in fixation); this reduction can alter tumor classification and grade. The current recommendation for the duration of formalin fixation is 16 to 32 hours; complete fixation of 2- to 4-mm thick tissues is likely to be achieved after 24 to 48 hours. Both underfixation and overfixation may lead to difficulties with antigen retrieval for immunohistochemistry, though underfixation is considered the more common and serious problem.
Thinly slicing some nodes may be difficult, and allowing the node to fix for 1 hour before slicing may help. Some pathologists prefer not to incise very small lymph nodes to avoid compression artifacts, but instead nick the capsule to allow formalin penetration. However, fixation of unincised lymph nodes can also cause compression artifacts because the fibrous capsule contracts in the fixative. Once fixed, nodes should be cut in uniformly thick cross section to include both the cortex and medulla. Transverse sections are usually sufficiently small to allow the entire cross section of most lymph nodes to fit on one microscopic slide, which facilitates histologic interpretation. The longitudinal plane is preferred for porcine lymph nodes because the location and amount of cortex and medulla vary at different sites in transverse sections.


