![]() An expanded version of this chapter is available online at ExpertConsult.
An expanded version of this chapter is available online at ExpertConsult.
Introduction
Pulmonary function tests permit accurate, reproducible assessment of the functional state of the respiratory system. It is worth emphasizing that pulmonary function tests do not diagnose specific diseases. Different diseases cause different patterns of abnormalities in a battery of pulmonary function tests. These patterns allow us to quantify the severity of respiratory disease, which enables us to detect disease early and characterize the natural history and response to treatment. It is important to remember, however, that these conclusions are based on inferences, not specific proofs. The accuracy of our inferences depends on a complete knowledge of the physiologic basis of the functions tested, properly validated equipment, and appropriate protocols. The purpose of this chapter is to describe these pulmonary function tests, reviewing briefly their physiologic basis, their equipment and protocol requirements, and their clinical results.
This chapter has an extended online version. A wealth of details of procedures, normal and predicted values, equations, and descriptions of techniques can be found in the online chapter.
Mechanical Properties of the Respiratory System
Measurements of Ventilatory Function
The physiologic determinants of airflow during quiet breathing, maximal airflow, lung volumes, and elastic recoil are reviewed in detail in Chapter 5. Figure 25-1 reviews the mechanisms involved in determining maximal airflow.
Figure 25-1.
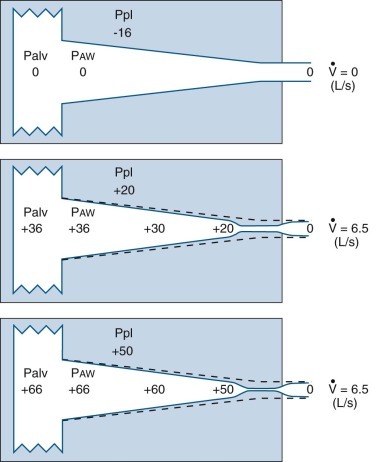
Model of expiratory flow limitation.
Top, The static relationships of pleural pressure (Ppl), alveolar pressure (Palv), and intraluminal airway pressure (Paw), and airway dimensions at a fixed lung volume. Middle and bottom, Conditions at the onset of maximal flow and with increased expiratory effort, respectively. Dotted lines show static airway dimensions for comparison with the dynamic state. All three panels show pressures (cm H2O) at the same lung volume: 60% of total lung capacity where lung elastic recoil pressure is +16 cm H2O and equals the transpulmonary pressure (Pl) (Pl = Palv − Ppl). Top, When conditions are static, Palv is zero (i.e., atmospheric) and flow (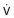 ) at the mouth is zero. Middle, The subject makes a forced expiratory effort at the same lung volume. Now
) at the mouth is zero. Middle, The subject makes a forced expiratory effort at the same lung volume. Now 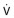 is 6.5 L/sec driven by Palv of +36 cm H2O. Because of the resistances down the airways from alveolus to mouth, the Paw decreases to the point where Paw = Ppl (+20 cm H2O, which is called the equal pressure point [EPP] because Ppl = Paw). Between the alveolus and the EPP, the airways are not compressed, but distal to the EPP there is compression and airway narrowing, because Ppl exceeds the pressure within the airways. For this lung volume, 6.5 L/sec is the maximal flow possible (see discussion of bottom panel, next). Bottom, The subject makes a forced expiratory effort starting at the same volume as in the top and middle panels (Pl = Palv − Ppl = +16). In this instance, the expiratory effort is markedly increased, reflected by the increased Ppl (+50 cm H2O) and Palv (+66 cm H2O). However, the flow generated is still only 6.5 L/sec because the increased effort succeeds only in compressing the airways more, dissipating the increased driving pressure across the increased resistance offered by the more narrowed airways; thus flow is maximum for this particular lung volume.
is 6.5 L/sec driven by Palv of +36 cm H2O. Because of the resistances down the airways from alveolus to mouth, the Paw decreases to the point where Paw = Ppl (+20 cm H2O, which is called the equal pressure point [EPP] because Ppl = Paw). Between the alveolus and the EPP, the airways are not compressed, but distal to the EPP there is compression and airway narrowing, because Ppl exceeds the pressure within the airways. For this lung volume, 6.5 L/sec is the maximal flow possible (see discussion of bottom panel, next). Bottom, The subject makes a forced expiratory effort starting at the same volume as in the top and middle panels (Pl = Palv − Ppl = +16). In this instance, the expiratory effort is markedly increased, reflected by the increased Ppl (+50 cm H2O) and Palv (+66 cm H2O). However, the flow generated is still only 6.5 L/sec because the increased effort succeeds only in compressing the airways more, dissipating the increased driving pressure across the increased resistance offered by the more narrowed airways; thus flow is maximum for this particular lung volume.
(Modified from Rodarte JR: Respiratory mechanics. In Basics of RD, New York, 1976, American Thoracic Society.)
Flow
Forced Spirometry
Indications.
There are several reasons for performing spirometry:
-
1.
In any occupation that is potentially hazardous to the lungs, individual workers should be monitored periodically by spirometry to detect and quantify evidence of pulmonary problems.
-
2.
Spirometry appears to be the best method to identify smokers at risk for developing severe chronic airflow obstruction.1
-
3.
Spirometry can indicate the statistical risk of specific surgical procedures for a group of patients but is probably not useful for the individual patient. Arterial oxygen desaturation is a much better indicator of the probability of a high risk associated with a surgical procedure (e.g., the need for prolonged postoperative mechanical ventilation) than is spirometry.2
-
4.
Many government agencies (e.g., the Social Security Administration) require results of spirometry to quantify impairment in patients who claim disability caused by chronic bronchitis or emphysema, as well as pneumoconioses, pulmonary fibrosis, and other pulmonary disorders.
-
5.
Spirometric results, including peak flow rates, are extremely useful in assessing the effectiveness of treatment in asthmatic patients. These simple tests are equally valuable for quantifying the effects of treatment in patients with other forms of chronic airflow obstruction, as well as many forms of restrictive disorders.
-
6.
Spirometry can be very sensitive for evaluating progression of disease, especially if baseline values, or results obtained early in the course of the illness, are available for comparison. Variation in the range of normal is so large that changes in serial test results are much more sensitive than a single value for detecting abnormal function. For example, changes in forced vital capacity were found to be predictive of survival time in idiopathic pulmonary fibrosis.3
-
7.
Spirometry is an excellent screening test for detection of chronic airflow obstruction, localizing and grading a critical orifice in the central airways, but may also be useful in detecting restrictive disorders.
Spirometry requires recording the volume of air inhaled and exhaled, plotted against time, during a series of ventilatory maneuvers. The resulting curves permit the determination as to whether the subject has a normal ventilatory reserve or an abnormal pattern characteristic of obstructive, restrictive, or mixed ventilatory abnormalities. None of these patterns is specific, although most diseases cause a predictable type of ventilatory defect. Spirometry alone cannot establish a diagnosis of a specific disease, but it is sufficiently reproducible to be useful in following the course of many different diseases. In addition, the results of spirometry make it possible to estimate the degree of exercise limitation due to a ventilatory defect (e.g., maximal voluntary ventilation [MVV] can be predicted from the forced expiratory volume in 1 second [FEV1])4 and to identify the type of patient likely to develop ventilatory failure after pneumonectomy.5, 6
The volumes of air inhaled and exhaled with relaxed and maximal effort can be measured easily with standard equipment. Lung volumes and capacities are defined in Figure 25-2 . The results are obtained and displayed in a standardized manner as a spirogram (Fig. 25-3 ). Tests can be performed with a simple recording spirometer, which is inexpensive enough to be standard equipment in a physician's office or the diagnostic laboratory of a small clinic or hospital. Recommended criteria for acceptable performance standards for equipment have been published.7 Although normal values have been established in a spectrum of subjects of different sex, age, size, and ethnic background, few have been reported using the standards of the American Thoracic Society (ATS).8, 9, 10, 11 Many samples are deficient in older subjects. Almost no data exist concerning the proper prediction equations to use in individuals of foreign extraction after the family has lived in the United States for several generations. Some regression equations that include “weight” as a determinant yield absurd values in very obese subjects.12 All these measurements depend heavily on patient understanding and cooperation and must be conducted by a well-trained technician able to communicate instructions clearly.
Figure 25-2.
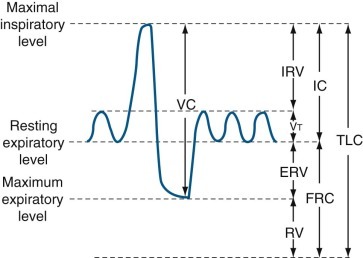
Lung volume and capacity.
Volumes: There are four volumes, which do not overlap: (1) tidal volume (Vt) is the volume of gas inhaled or exhaled during each respiratory cycle; (2) inspiratory reserve volume (IRV) is the maximal volume of gas inspired from end-inspiration; (3) expiratory reserve volume (ERV) is the maximal volume of gas exhaled from end-expiration; and (4) residual volume (RV) is the volume of gas remaining in the lungs following a maximal exhalation. Capacities: There are four capacities, each of which contains two or more primary volumes: (1) total lung capacity (TLC) is the amount of gas contained in the lung at maximal inspiration; (2) vital capacity (VC) is the maximal volume of gas that can be expelled from the lungs by a forceful effort following maximal inspiration, without regard for the time involved; (3) inspiratory capacity (IC) is the maximal volume of gas that can be inspired from the resting expiratory level; and (4) functional residual capacity (FRC) is the volume of gas in the lungs at resting end-expiration.
Figure 25-3.
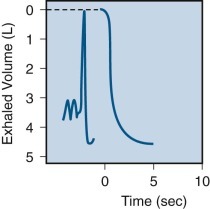
Spirogram obtained in a normal subject showing maneuvers to determine vital capacity and forced vital capacity.
On the tracing shown on the left, the subject breathes quietly (slow recording speed), then takes a maximal inspiration followed by a maximal expiration without concern for time (vital capacity). On the tracing shown to the right, after a maximal inspiration (not shown), with a rapid recording speed, the subject then exhales completely, forcefully, and as rapidly as possible (forced vital capacity).
Maximal-Effort Expiratory Vital Capacity.
To obtain a maximal-effort expiratory vital capacity (VC), the subject inhales maximally to total lung capacity (TLC) and then exhales as rapidly and forcefully as possible. When volume is recorded on the y-axis and time on the x-axis, the resulting curve is called the forced vital capacity (FVC) curve. Analysis of this curve permits computation of the volume exhaled during the time following the start of the maneuver (forced expiratory volume over time, or FEVt), the ratio of FEVt to total FVC, and average flow rates during different portions of the curve. The terms used in clinical spirometry, including these different components, are summarized in Table 25-1 .13
Table 25-1.
Terms Used for Spirometric Measurements
| Term | Previously Used Terms | Description |
|---|---|---|
| Vital capacity (VC) | Largest volume measured on complete exhalation after full inspiration | |
| Forced VC (FVC) | Timed VC, fast VC | VC performed with forced expiration |
| Forced expiratory volume with subscript indicating interval in seconds (FEVt) (e.g., FEV1) | Timed VC | Volume of gas exhaled in a given time during performance of FVC |
| Percentage expired in t seconds (FEVt%) (e.g., FEV1%) | Timed VC | FEVt expressed as percentage of FVC |
| Forced midexpiratory flow (FEF25%–75%) | Average flow rate during middle 50% of the FVC | Maximal midexpiratory flow |
| Forced expiratory flow with subscript indicating volume segment (FEFV1–V2) (e.g., FEF200–1200) | Maximal expiratory flow rate | Average rate of flow for a specified segment of FVC, most commonly 200–1200 mL in adults |
| Maximal voluntary ventilation (MVV) | Maximum breathing capacity (MBC) | Volume of air a subject can breathe with voluntary maximal effort for a given time |
Modified from Kory RC: Clinical spirometry: recommendation of the Section on Pulmonary Function Testing, Committee on Pulmonary Physiology, American College of Chest Physicians. Dis Chest 43:214, 1963.
Several useful variables may be derived from the maximal-effort FVC.
Forced Expiratory Volume Over Time.
The FEV1 is the measurement of dynamic volume most often used in conjunction with the FVC in analysis of spirometry (Fig. 25-4 ). The measurement incorporates the early, effort-dependent portion of the curve and enough of the midportion to make it reproducible and sensitive for clinical purposes. Forced expiratory volume (FEV) measurements taken at 0.5, 0.75, 2.0, and 3.0 seconds add little information to the FEV1 measurement. The forced expiratory volume exhaled in 6 seconds (FEV6) is useful, however, because it closely approximates FVC, has been shown to be a valid alternative to the conventional FEV1/FVC, and is easier for patients with severe airflow obstruction to attain.14 In addition, the end of the test is more clearly defined, permitting more reliable correspondence between measured and referenced values.15 Furthermore, as demonstrated by Swanney and associates,15 the degree of airflow obstruction, reflected in the FEV1/ FEV6 obtained from spirometry, can serve as an independent predictor of subsequent decline in lung function; it may therefore be used to detect smokers at higher risk for developing chronic obstructive pulmonary disease (COPD).15
Figure 25-4.
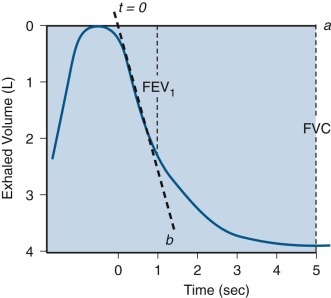
Measurement of forced expiratory volume in 1 second.
This diagram illustrates measurement of forced expiratory volume in 1 second (FEV1) using the back-extrapolation method to define time zero (i.e., the point during the forced vital capacity [FVC] maneuver when the subject began to blow as hard and as fast as possible). A solid horizontal line (a) indicates the level of maximal inhalation. A heavy dashed line (b) passes through the steepest portion of the volume-time tracing. The intersection point of these two lines becomes time zero, from which timing is initiated, as indicated; 1 second after time zero, the vertical dashed line is drawn, indicating FEV1, and 5 seconds later, another vertical dashed line is drawn, indicating FVC.
Forced Expiratory Volume Over Time as a Percentage of Forced Vital Capacity.
The ratio of FEVt to total FVC has been defined precisely in healthy subjects.16 It declines with age, but abnormally decreased ratios indicate airway obstruction; normal or increased ratios do not reliably exclude airway obstruction, particularly in the presence of a decreased FVC. When the FVC is decreased by an interstitial process or by chest wall restriction, and the airways are normal, the FEVt/FVC ratio is increased. (The FEVt/FVC ratio may also be increased in subjects who fail to make a maximal effort throughout the expiratory maneuver.) The absence of an increased ratio in patients in whom one would expect the ratio to be increased suggests the presence of concomitant airway obstruction. Absolute flow may be increased initially, probably because of outward traction of increased elastic forces on airway walls. However, because flow is volume dependent, it eventually decreases in restrictive disorders without airway obstruction, although precise quantification for the various types of pure restrictive disorders is not available. Examining exhaled volumes and flows as a percentage of predicted values may facilitate interpretation of the spirogram in patients with mixed ventilatory defects.
Average Forced Expiratory Flow.
The FEF25%–75%, or forced expiratory flow between 25% and 75% of FVC, was introduced as the maximal midexpiratory flow rate (Fig. 25-5 ). This measurement was intended to reflect the most effort-independent portion of the curve and the portion most sensitive to airflow in peripheral airways, where diseases of chronic airflow obstruction are thought to originate.17 These properties have gained support from clinical experience and theoretical analysis,18 and the FEF25%–75% is widely used currently. However, the FEF25%–75% shows marked variability in studies of large samples of healthy subjects, and the 95% confidence limits for normal values are so large that they limit its sensitivity in detecting disease in an individual subject.6, 19
Figure 25-5.
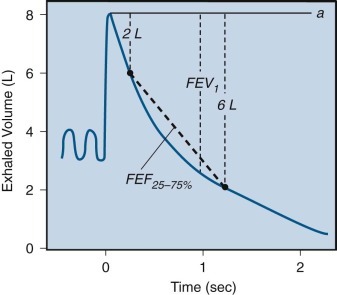
Determination of forced expiratory flow between 25% and 75% of total lung capacity (FEF25%–75%).
A heavy dashed line connects two points on the volume-time curve of the forced vital capacity (FVC) maneuver. One point is marked when 25% of the FVC has been exhaled (2 L); the other point is marked when 75% of the FVC (6 L) has been exhaled from the level of maximal inhalation indicated by the solid line (a). In this example, the elapsed time between these two points is 1 second; thus the calculated FEF25%–75% is 4 L/sec. FEV1, forced expiratory volume in 1 second.
Peak Expiratory Flow Rate.
Expiratory flow reaches a transient peak early in the forced expiratory maneuver. Peak flow manifests during the most effort-dependent portion of the expiratory maneuver, so decreased values can result from even slightly submaximal effort rather than from airway obstruction. Nevertheless, the ease of measuring peak flow with an inexpensive, small, portable device20 has made it a popular means of following the pattern of airflow obstruction on an ambulatory basis. For example, the test is used to monitor patients suspected of having occupational asthma and those who seem insensitive to the severity of bronchospasm. When a maximal effort is made, peak flow is largely a function of the caliber of large airways; it is also influenced by the transient flow caused by expulsion of air from compressed central airways. For these reasons, peak flow is abnormally decreased only in moderate to severe airway obstruction.
The national program to improve the management of patients with asthma based on the National Heart, Lung, and Blood Institute expert panel report21, 22 depends heavily on spirometry as well as the informed use of peak flowmeters for proper patient care. These devices are sufficiently accurate that peak flow measurements made in the morning and evening (before and after bronchodilator treatments) enable patients to participate effectively in their own care. The test provides a quantitative estimate of airway lability (change in peak flow > 20%) that correlates well with more sophisticated measures of airway hyperresponsiveness obtained by provocation testing. It also provides correlation of the clinical course with pulmonary function on a daily basis, provides an early warning that pulmonary function is deteriorating, and may be used as the basis of an action plan of treatment carried out by the patient.
Maximal Voluntary Ventilation.
The maximal voluntary ventilation (MVV) measurement is defined as the maximal volume of air that can be moved by voluntary effort in 1 minute. Subjects are instructed to breathe rapidly and deeply for 15 to 30 seconds, ventilatory volumes are recorded, and the maximal volume achieved over 15 consecutive seconds is expressed in liters per minute. Lung volumes are reported at the largest size possible within the chest and at body temperature (37° C) and standard pressure fully saturated with water vapor (760 mm Hg).
The observer should demonstrate the test; then the subject should choose his or her own respiratory rate and perform several practice runs. The respiratory frequency used in the MVV should be noted and recorded as a subscript (e.g., MVV90 or MVV110). Maximal levels are usually achieved between 70 and 120 breaths/min, but the choice of frequency does not greatly affect the test.23
This test is heavily dependent on subject cooperation and effort. Loss of coordination of respiratory muscles, musculoskeletal disease of the chest wall, neurologic disease, and deconditioning from any chronic illness, as well as ventilatory defects, decrease MVV, so the test is nonspecific. The MVV is decreased in patients with airway obstruction, but less so with mild or moderate restrictive defects because rapid, shallow breathing can compensate effectively for the decreased lung volume.
Despite these caveats, MVV can be useful in special circumstances. It correlates well with subjective dyspnea and is useful in evaluating exercise tolerance. It appears to have prognostic value in preoperative evaluation, possibly because the extrapulmonary factors to which it is sensitive are also important for recovery from a surgical procedure.24 It also provides a measure of respiratory muscle endurance that may be important in the evaluation of respiratory muscle fatigue, whether from obstructive or restrictive ventilatory defects or from specific neuromuscular diseases.25 In myasthenia gravis, for example, the patient can often produce maximal efforts for a short time, so that FVC and maximal inspiratory and expiratory pressures are normal. However, the effort cannot be sustained, so the MVV or repeated FVC values decrease, even within 12 to 15 seconds. The respiratory crisis of myasthenia gravis may happen rapidly and lead to respiratory failure. As a result, some investigators have suggested that MVV should never be measured in patients with myasthenia gravis, except under carefully controlled circumstances when it may be useful in evaluating treatment.6
Flow-Volume Relationships
General Principles.
The widespread availability of computer-based electronic pulmonary function test apparatus permits flow-volume curves to be as readily available in the physician's office as spirometry. All of the indications for spirometry probably apply equally to the flow-volume curve. This maneuver requires the subject to inspire and expire fully with maximal effort into an instrument that measures flow and volume simultaneously. These values are plotted on the two axes of an x-y recorder or computer monitor (Fig. 25-6 ). As summarized in Figure 25-1, analysis of these curves has contributed to the basic understanding of the mechanical events that limit maximal exhalation. Maximal flow clearly depends on lung volume: for every point on the lower two thirds of VC, a maximal flow exists that cannot be exceeded regardless of the effort exerted by the subject. Thus maximal flow must depend on mechanical characteristics of the lungs. Flow-volume curves also provide a useful way to display ventilatory data for diagnostic purposes.
Figure 25-6.
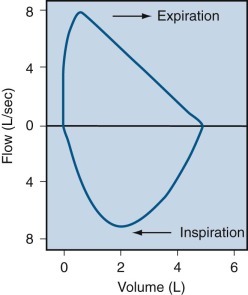
The flow-volume curve.
The tracing of the flow-volume curve is recorded during maximal inspiration and expiration in a normal subject.
By superimposition of repeated curves using graphic means or a computer, a maximal flow-volume envelope can be constructed for any subject. This envelope represents the maximal flow values of which the respiratory system is capable, and it may exceed the airflow rates achieved in any single maneuver. As illustrated in Figure 25-7 , the maximal flow-volume envelope can be approximated by having the subject make repeated trials of increasing effort or by having the subject cough repeatedly while flow-volume relationships are recorded. The flow-volume curve and FEV-time curve are mathematically interchangeable; either one can be derived graphically, or by computer analysis, from the other. This relationship can provide an internal check on the accuracy of the tests. Spirometric values can be computed from flow-volume curves. Thus values for both forced expiratory tests can be obtained with fewer efforts while still defining the maximal capacity of the respiratory system accurately. From a practical point of view, this means that the subject can generate the needed data with fewer maximal efforts and in a shorter time.
Figure 25-7.
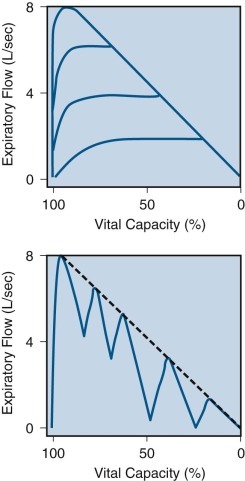
Flow-volume curves created by increasing effort and by coughing.
Top, Expiratory flow-volume curve recorded during a series of expirations with increasing efforts, finally producing a maximal flow-volume envelope. Bottom, Expiratory flow-volume curve recorded during coughing (solid line), approximating the maximal flow-volume envelope (dashed line).
On forced exhalation, the flow-volume curve has a characteristic appearance. The curve shows a rapid ascent to peak flow and subsequently a slow linear descent proportional to volume. The initial portion of the curve (the first 25% to 33% of the VC exhaled) depends on effort. As a subject exerts increasing effort during exhalation, associated with increasing intrathoracic pressure, increasing flow is generated. This portion of the curve has limited diagnostic use because its appearance depends primarily on the subject's muscular effort and cooperation rather than on the mechanical characteristics of the lung.
Shortly after development of peak flow, the curve follows a remarkably reproducible, effort-independent envelope as flow diminishes in proportion to volume until residual volume (RV) is reached. For each point on the volume axis, a maximal flow exists that cannot be exceeded regardless of the pressure generated by the respiratory muscles. Although this portion of the curve is very reproducible in a given subject from time to time, it is altered in a characteristic manner by the effect of diseases on the mechanical properties of the lungs. In most subjects older than age 30 and in patients with pulmonary disease, RV is determined by airway closure, so the flow-volume curve shows a progressive decrease in flow until RV is reached. In some young individuals, however, and perhaps in some patients with chest wall disease, RV is determined by chest wall rigidity, which limits maximal exhalation. In such cases, expiratory flow abruptly decreases to zero at low lung volumes.
On forced inhalation, the flow-volume curves are normally entirely effort dependent. The shape of the inspiratory portion is symmetrical with flow, increasing to a maximum midway through inspiration and then decreasing as inhalation proceeds to TLC. It is less influenced by diffuse airway or parenchymal disease. When central airway obstruction is suspected, the inspiratory limb of the flow-volume curve has great diagnostic usefulness, whereas ordinary spirometry reveals a nonspecific pattern.
Obstructive Ventilatory Defects.
Some studies suggest that early asymptomatic airway obstructive disorders may be associated with decreased maximal flow at low lung volumes,26 but sufficient numbers of anatomic studies that correlate findings in patients with emphysema and with central and peripheral airway lesions are not available.17, 27 The variability of the flow-volume curve at low lung volumes has made it difficult to interpret individual curves even when compared with studies of large populations.28
In patients with obstructive ventilatory patterns, peak flow is diminished. However, it is probable that abrupt emptying of large central airways associated with vigorous exhalation causes these central airways to be compressed, generating a brief period of relatively high flow, which preserves peak flow relative to flow at lower lung volumes. Furthermore, the usual linear descent of the flow-volume curve is disrupted by an exaggerated concavity of the descending limb of the curve. This curvilinear portion of the lower half of the flow-volume curve is characteristic of obstructive ventilatory patterns and suggests the presence of airflow obstruction even when the FVC, FEV1, and FEV1/FVC ratio are well preserved.29, 30, 30a
This loss of linearity relates to the severity of the obstruction as well as the type of disease. A decrease in volume is seen in conjunction with both obstructive and restrictive ventilatory defects, reflecting decreased VC. The decrease is relatively less in airway obstruction than in restrictive ventilatory defects, so the characteristic flow-volume curve in obstructive ventilatory defects tends to have its major axis oriented along the horizontal (volume) axis; in restrictive defects, the major axis appears to be along the vertical (flow) axis (see “Pathophysiologic Patterns” section).
When the tidal volume loop is superimposed on the flow-volume curve, comparison of the two may be useful in clinical evaluation. The difference between flow during tidal breathing and flow during maximal effort is a measure of pulmonary reserve. As the severity of airflow obstruction increases, the expiratory flow during the two maneuvers becomes superimposed, at first low in the lung volume, and then, as the disease becomes more severe, at higher lung volumes.
“Negative effort dependence” is present when expiratory airflow rates during quiet breathing exceed those during maximal effort. When present, this phenomenon suggests that the airways are less stable than normal, as may be seen in emphysema and in some forms of chronic bronchitis. (See later discussion of obstructive ventilatory defect in “Pathophysiologic Patterns” section for further details about this phenomenon.)
Finally, the relative position of the two curves on the volume axis is a graphic measure of the amount of expiratory volume in reserve. As this reserve decreases due to obesity, pregnancy, or ascites, the tidal volume loop moves closer to the RV.
Two other factors that affect flow-volume curves are upper airway obstruction and gas density.
Upper Airway Obstruction: Stenosis and Malacia.
Flow-volume curves may be especially helpful in identifying tracheal or other upper airway lesions as a cause of obstruction.31 Central airway obstruction (i.e., proximal to the tracheal carina) that is located within the thorax produces a plateau during forced exhalation instead of the usual rise to and descent from peak flow (Fig. 25-8 ). When more than 50% of the VC has been exhaled, the curve then follows the usual flow-volume envelope to RV. In patients with stridor, particular attention should be paid to the configuration of the inspiratory portion as well as the expiratory portion of the flow-volume curve. Lesions located in the trachea within the thorax cause decreased airflow particularly during exhalation; during inhalation, the posterior tracheal membrane is pulled out by negative intrathoracic pressure, so increased effort increases airflow rates and the inspiratory limb of the flow-volume curve can appear normal. Conversely, tracheal lesions located outside of the thorax cause decreased airflow during inhalation; during inhalation, the tracheal membrane is sucked in and is usually associated with stridor. It is possible to estimate the diameter of a stenotic lesion by analysis of the flow-volume curve with an accuracy of ±1 mm (eFig. 25-1), but the length of the flow-limiting segment must be confirmed by computed tomography (CT) scan to plan surgical correction, if required. Because a critical orifice located at the thoracic outlet is not affected by pressure above or below the lesion, airflow is limited equally during both inhalation and exhalation.32 Similarly, if a lesion is fixed and not altered by surrounding pressures, whether intra- or extrathoracic, airflow should be limited equally during inhalation and exhalation.
Figure 25-8.
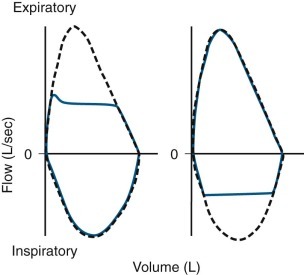
Flow-volume curves obtained from patients with upper airway obstruction.
Dashed line represents a curve obtained from a normal subject with the same vital capacity as that observed in the patients. Solid line indicates a curve obtained from a patient with intrathoracic obstruction (left) and from another patient with extrathoracic obstruction (right).
eFigure 25-1.
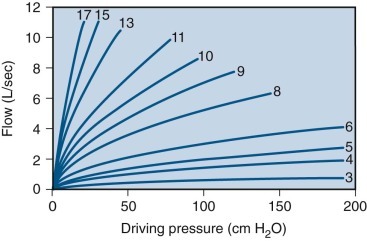
Relationship between driving pressure (x-axis) and airflow (y-axis) through a series of critical orifices of varying diameters (in mm).
Using this family of curves, it is possible to determine the diameter of a critical orifice in a patient with upper airway obstruction. Assuming a driving pressure of 100 cm H2O, the diameter is given by the curve closest to the maximum flow observed in the flow plateau obtained from the flow-volume loop obtained from the patient. Curves were constructed by using graded external resistances in a normal subject.
Gas Density.
Comparison of flow-volume curves obtained when the subject is breathing air and breathing low-density gas mixtures such as “heliox” (80% helium, 20% oxygen) has been advocated to detect early airway obstruction33 or localize the site of obstruction.14 During a forced exhalation, when flow limitation develops in large central airways where flow is turbulent, a low-density gas such as heliox increases maximal flow (defined by the increased maximal flow at 50% VC, or  ). As lung volume decreases, the flow-limiting segment moves into small peripheral airways, where flow is laminar and density independent. At this lung volume, air and heliox flow-volume curves can be superimposed; the lung volume at which flow becomes density independent is called the volume of isoflow. Clinical application of heliox in detecting airflow obstruction is generally not performed because a number of controversial issues have not been resolved.34, 35, 36
). As lung volume decreases, the flow-limiting segment moves into small peripheral airways, where flow is laminar and density independent. At this lung volume, air and heliox flow-volume curves can be superimposed; the lung volume at which flow becomes density independent is called the volume of isoflow. Clinical application of heliox in detecting airflow obstruction is generally not performed because a number of controversial issues have not been resolved.34, 35, 36
Restrictive Ventilatory Defects.
The increase in lung elastic recoil that accounts for the decrease in VC seen with restrictive defects also increases the force driving expiratory flow and pulling outward on airway walls; thus, the usual flow-volume curve in restrictive ventilatory defects is tall and narrow. Peak expiratory flow is relatively preserved, and the descending portion of the expiratory limb is linear, decreasing rapidly from peak flow to RV. The loop often maintains a nearly normal shape but appears miniaturized in all dimensions.
Lung Volumes
Vital Capacity and Other Static Lung Volumes.
The measurement of VC requires the subject to inhale as deeply as possible and then to exhale fully, taking as much time as required. Figure 25-2 illustrates the subdivisions of lung volume.37 The measurement can also be obtained by adding two of its components: the expiratory reserve volume, obtained by having the subject exhale maximally from the resting end-tidal level; and the inspiratory capacity, obtained by having the subject inspire fully from the resting end-tidal level. The sum of these two measurements yields the “combined VC”; as long as the resting end-tidal lung volume is the same for the two component maneuvers, the combined VC and the VC are equal. In patients with severe airflow obstruction the combined VC appears to be larger than the VC, suggesting the presence of poorly ventilated regions of lungs, or so-called trapped gas. This result probably reflects increased transmural pressure, which tends to cause airway closure during a large portion of the single maneuver—but only in the portion near RV during the combined VC maneuver.
A similar inference can be made by comparing the “slow VC” (performed without regard to time) and FVC, or by comparing inspired VC (maximal volume inhaled from RV to TLC) with the expired VC maneuver just described. Except for those subdivisions involving RV, each of the defined volumes can be recorded and measured by simple spirometry. The RV can be measured only by indirect methods (e.g., nitrogen washout, helium dilution, or body plethysmography). Figure 25-2 illustrates the fact that VC can be decreased in two different ways: by a decrease in TLC or by an increase in RV. Only measuring RV and TLC can differentiate these two causes.
The cause of a reduction in VC can often be inferred by analysis of maximal expiratory flow. Abnormally decreased flows support the diagnosis of an obstructive ventilatory defect, suggesting that the decreased VC is due to an increased RV (as in asthma, chronic bronchitis, and emphysema). Normal values for airflow make an obstructive ventilatory defect unlikely and suggest that a decrease in VC may be due to a decreased TLC. Restrictive ventilatory defects (e.g., pulmonary fibrosis, resection of lung tissue) decrease VC by decreasing TLC. Thus the finding of decreased VC alone is inadequate and nonspecific to assess decreased ventilatory reserve. Performance of complete spirometry (i.e., FVC and its subdivisions as well as VC) adds clarification of the mechanism and the severity of a ventilatory defect. Measurement of RV provides convincing proof of the presence or absence of overinflation or underinflation of the lungs.
Gas Dilution Methods.
The two most commonly used gas dilution methods for measuring lung volume are the open-circuit nitrogen (N2) method and the closed-circuit helium (He) method. Both methods use a physiologically inert gas that is poorly soluble in alveolar blood and lung tissues, and both are most often used to measure functional residual capacity (FRC), the volume of gas remaining in the lung at the end of a normal expiration. In the open-circuit method, all exhaled gas is collected while the subject inhales pure oxygen. By assuming values for the initial concentration of nitrogen in the lungs (alveolar nitrogen fraction varies slightly with the respiratory quotient but is assumed to be approximately 0.81) and, for the rate of nitrogen elimination from blood and tissues (about 30 mL/min), measurement of the total amount of nitrogen washed out from the lungs permits the calculation of the volume of nitrogen-containing gas present at the beginning of the maneuver (Fig. 25-9 ). In the closed-circuit helium dilution method (Fig. 25-10 ), the theory is similar. The subject rebreathes a gas mixture containing helium, a physiologically inert tracer gas, in a closed system until equilibration is achieved. If the volume and concentration of helium in the gas mixture rebreathed are known, measurement of the final equilibrium concentration of helium permits calculation of the volume of gas in the lungs at the start of the maneuver.
Figure 25-9.
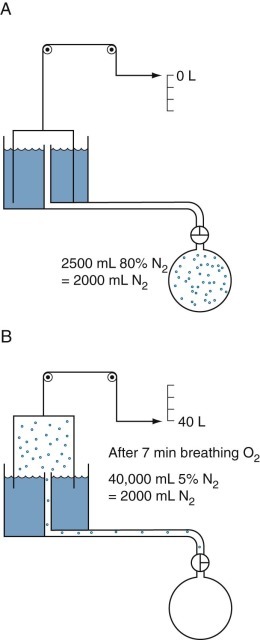
Open-circuit nitrogen method to measure functional residual capacity.
Dots represent nitrogen (N2) molecules. A, Initially all the N2 molecules are in the lungs (as 80% N2). B, When N2-free oxygen (“pure O2”) is breathed, the N2 molecules are washed out of the lungs and collected with the O2 as expired gas in the spirometer. The spirometer contains 40,000 mL of mixed expired gas with a N2 concentration of 5%. Thus the spirometer contains 0.05 × 40,000 = 2000 mL of N2; the remaining 38,000 mL of gas is mainly O2 used to wash the nitrogen out of the lungs, plus some carbon dioxide. The 2000 mL of N2 was distributed within the lungs at a concentration of 80% N2 when the washout began; therefore the alveolar volume in which the N2 was distributed was 2000/0.8 mL = 2500 mL. Corrections must be made for the small amount of N2 washed out of the blood and tissue when O2 is breathed and for the small amounts of N2 in “pure O2.”
Figure 25-10.
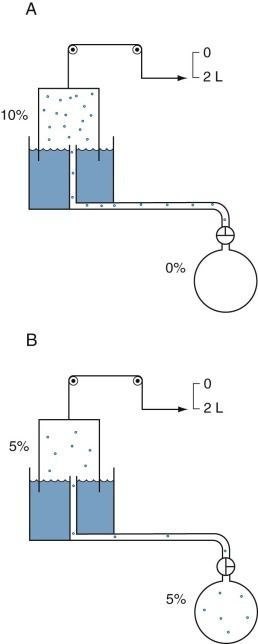
Closed-circuit helium method to measure functional residual capacity.
Dots represent molecules of helium (He). A, Initially, all He molecules are in the spirometer (as 10% He), and no molecules are in the lungs. If the spirometer contains 2000 mL of gas, of which 10% is He, then 2000 mL × 0.1, or 200 mL, of He is present in the spirometer before rebreathing. B, Rebreathing results in redistribution of the He molecules until equilibrium develops, at which time lung volume can be calculated. At the end of the test, the same amount of He (200 mL) must be redistributed in the lungs, tubing, and spirometer, assuming that He is inert and not soluble in blood or tissues.
An advantage of the open-circuit method is that it also permits an assessment of the uniformity of ventilation of the lungs by analyzing the slope of the change in nitrogen concentration over consecutive exhalations, by measuring the end-expiratory concentration of nitrogen after 7 minutes of washout,38 or by measuring the total ventilation required to reduce end-expiratory nitrogen to less than 2%.39 The open-circuit method is sensitive to leaks anywhere in the system (especially at the mouthpiece) and to errors in measurement of nitrogen concentration and exhaled volume. If a pneumotachygraph is used to measure volume, attention must be paid to the effects of viscosity changes in the exhaled gas, because it contains a progressively decreasing concentration of nitrogen. The open-circuit method shares several disadvantages with the closed-circuit method: it does not measure the volume of gas in poor communication with the airways (e.g., lung bullae); it assumes that the volume at which the measurement was made corresponds to the end-expiratory point on the spirometry tracing used to calculate expiratory reserve volume and inspiratory capacity (needed for the computation of RV and TLC from the measured FRC); and it requires a long period of reequilibration with room air before the test can be repeated. Measuring spirometric volumes immediately before measuring FRC as a combined, continuous sequence can eliminate the assumption of a constant or reproducible end-expiratory volume. This can be achieved with appropriate valves connected to the mouthpiece, which are available in many commercial systems.
Closed Circuit Methods.
The closed-circuit helium dilution method (see Fig. 25-10) is similar in its basic theory. It involves having the subject rebreathe a gas mixture containing helium, a physiologically inert tracer gas, in a closed system until equilibration is achieved. If the volume and concentration of helium in the gas mixture rebreathed are known, measurement of the final equilibrium concentration of helium permits calculation of the volume of gas in the lungs at the start of the maneuver.
In a closed-circuit method, a thermal-conductivity meter measures the helium concentration continuously, permitting return of the sampled gas to the system. Because the meter is sensitive to carbon dioxide, and because carbon dioxide must in any case be removed from a closed system, a carbon dioxide absorber is added. The removal of carbon dioxide results in a constant fall in the volume of gas in the closed circuit, as oxygen is consumed and the subject produces carbon dioxide. An equivalent amount of oxygen is therefore introduced as an initial bolus or as a continuous flow. In either case, it is important that the subject be “switched into” the system at the end-tidal point. It is possible to calculate the correction for an error in this point, but only if the subject is able to relax and exhale reproducibly to the actual end-tidal point while breathing from the circuit. In a cooperative subject the closed-circuit method also permits the measurement of inspiratory capacity, expiratory reserve volume, and VC from maneuvers recorded on the spirometer while the subject is switched into the system. This eliminates dependency on the identity of the value of end-tidal lung volume (FRC) at the time that the closed-circuit measurement is made and at the time that the subdivisions of spirometric volumes are measured.
Like the open-circuit method, the closed-circuit method is sensitive to errors caused by gas leaks and alinearity of the gas analyzer. It also fails to measure the volume of gas in lung bullae, and it cannot be repeated at short intervals. The test nevertheless gives reproducible results (the standard deviation [SD] of repeated measurements is 90 to 160 mL),40 and normal values are available from several studies of healthy subjects.6, 41
Two other measurements of lung volume can be obtained from the dilution of gases used in standard tests of lung function. One involves measurement of the mean concentration of nitrogen in the air exhaled after the VC inspiration of pure oxygen in the single-breath nitrogen washout test of the distribution of ventilation.42 The other involves measuring the change in concentration of the neon, helium, or methane used as the inert tracer gas in the single-breath measurement of the diffusing capacity for carbon monoxide (Dl CO).43 Indeed, the alveolar volume achieved during performance of the standard diffusing capacity maneuver is approximately TLC and must be calculated in order to measure Dl CO. Although the lung volume calculated from the single-breath nitrogen washout test of distribution is reported rarely, the TLC calculated from measurement of Dl CO is used commonly in many pulmonary function laboratories. Because the time for dilution of the tracer gas is short (10 seconds), true TLC is underestimated in patients with severe airway obstruction or uneven distribution of ventilation. FEV1 /FVC must be less than 0.40 for TLC measured by single-breath dilution to be underestimated significantly. In healthy subjects and in patients with mild airflow obstruction, the values obtained correspond well with those obtained by body plethysmography.6, 44
Radiographic Methods.
TLC and FRC can be estimated from chest radiographs, although what is measured is the combined air and tissue volume of the lungs; this is in contrast to the communicating gas volume that is measured by gas dilution methods and the compressible gas volume that is measured by body plethysmography.45
Body Plethysmography
Types of Plethysmographs.
There are three types of plethysmographs: pressure, volume, and pressure-volume.
Pressure (Closed-Type) Plethysmograph.
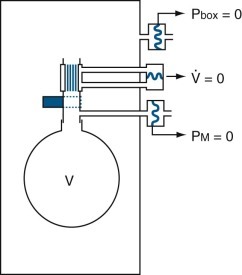
This type of plethysmograph has a closed chamber with a fixed volume in which the subject breathes the gas in the plethysmograph (or body box) (Fig. 25-11 ). Volume changes associated with compression or expansion of gas within the thorax are measured as pressure changes in gas surrounding the subject within the box. Volume exchange between lung and box does not directly cause pressure changes, although thermal, humidity, and carbon dioxide–oxygen exchange differences between inspired and expired gas do cause pressure changes. Thoracic gas volume and airway resistance are measured during rapid maneuvers, so small leaks are tolerated or are introduced to vent slow thermal-pressure drift. This device is best suited for measuring small volume changes because of its high sensitivity and excellent frequency response. It need not be leak-free, absolutely rigid, or refrigerated because the measurements are usually brief and are used to study rapid events.
Figure 25-11.
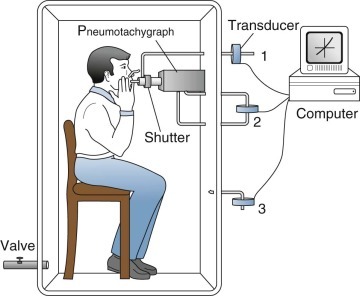
Pressure (closed-type) plethysmograph.
The subject breathes through a shutter/pneumotachygraph. The shutter is open during tidal breathing and for measurements of airway resistance, and closed for measurements of thoracic gas volume. When the shutter is closed, mouth pressure (equal to alveolar pressure at no flow) is measured by a pressure transducer (1). The pneumotachygraph measures airflow with another transducer (2), and the flow signal is integrated to volume electronically. The plethysmograph pressure is measured by a third transducer (3). The signals from the three transducers are processed by a computer. Excess box pressure caused by temperature changes when the subject sits in the closed box is vented through a valve.
Volume (Open-Type) Plethysmograph.
This type of plethysmograph (eFig. 25-2) has constant pressure and variable volume. When thoracic volume changes, gas is displaced through a hole in the box wall and is measured either with a spirometer or by integrating the flow through a pneumotachygraph (or flowmeter). This device is suitable for measuring small or large volume changes. To attain good frequency response, the impedance to gas displacement must be very small. This requires a low-resistance pneumotachygraph, a sensitive transducer, and a fast, drift-free integrator, or meticulous use of special spirometers; consequently this form of plethysmography is challenging and is used in the research setting only.
eFigure 25-2.
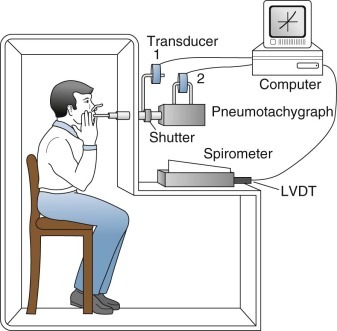
Volume (open-type) plethysmograph.
In this constant-pressure, variable-volume type of plethysmograph, the subject also breathes through a shutter/pneumotachygraph apparatus, which usually is located outside the plethysmograph itself. The shutter is open for tidal breathing, measurement of airway resistance, and spirometry. It is closed for measurement of thoracic gas volume. In the closed-shutter mode, mouth pressure is measured by a transducer (1) and approximates alveolar pressure with no flow and small volume changes. The pneumotachygraph measures flow via another transducer (2, above the pneumotachygraph). Flow is integrated electronically to obtain volume. Changes in volume of the plethysmograph, reflecting movement of the chest wall, are measured with a spirometer and a linear volume-displacement transducer (LVDT). The spirometer illustrated is a Krogh water-sealed spirometer with good frequency response and very small impedance to gas displacement. A low-resistance pneumotachygraph (flowmeter) with a fast, drift-free integrator may be used instead. Processing is usually performed by computer and permits slow and forced vital capacity maneuvers as well. However, neither approach is routine.
Pressure-Volume Plethysmograph.
This device (eFig. 25-3) combines features of both the closed and open types. As the subject breathes from the room, changes in thoracic gas volume compress or expand the air around the subject in the box and also displace it through a hole in the box wall. The compression or decompression of gas is measured as a pressure change; the displacement of gas is measured either by a spirometer connected to the box or by integrating airflow through a pneumotachygraph in the opening. At every instant, all of the change in thoracic gas volume is accounted for by adding the two components (pressure change and volume displacement). This combined approach has a wide range of sensitivities, permitting all types of measurements to be made with the same instrument (i.e., thoracic gas volume and airway resistance, spirometry, and flow-volume curves). The box has excellent frequency response and relatively modest requirements for the spirometer. The integrated flow version dispenses with water-filled spirometers and is tolerant of leaks.
eFigure 25-3.
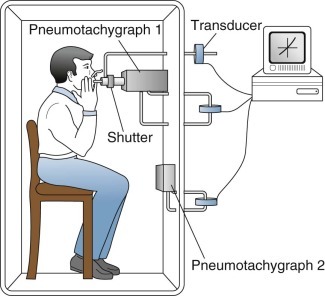
Pressure-volume (or flow) plethysmograph.
This type of plethysmograph combines features of the closed and open types. The subject breathes through a shutter/pneumotachygraph apparatus. The shutter is open for tidal breathing, measurement of airway resistance, and spirometry. It is closed for measurement of thoracic gas volume. In the closed position, mouth pressure (alveolar pressure) is measured by a transducer (top). The pneumotachygraph at the mouth (pneumotachygraph 1) measures airflow with another transducer (middle). This airflow at the mouth is integrated to obtain volume inhaled and exhaled at the mouth. Changes in plethysmograph or box volume resulting from movements of the chest wall are measured by a pneumotachygraph in the wall of the plethysmograph (pneumotachygraph 2) with a third transducer (bottom), and this signal is integrated to obtain volume change of the thorax. The signals from all three transducers usually are processed by computer to obtain slow and forced vital capacities as well as resistance and thoracic gas volumes.
In this type of plethysmograph, changes in lung volumes are computed from measurements of both box pressure (Pbox) and volume displacement to determine accurately the true volume change regardless of amplitude or frequency. Pbox is multiplied by a constant (Kbox) proportional to the gas volume in the box (i.e., by total box volume minus patient volume). Pbox is also divided by the box flowmeter resistance (Rbox) and multiplied by the integral of box flow to obtain the box volume (Vbox). These two signals are added together to yield the change in lung volume (ΔV):
| (1) |
The physical principles underlying this type of plethysmograph are illustrated in eFigure 25-4. The displacement volume, 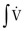 , is added to the plethysmograph compression volume, PboxKbox, to produce the “true” volume. If the volume change were instantaneous, the “true” volume event would be as illustrated in eFigure 25-4A. During this rapid inspiration, pressure in the plethysmograph increases abruptly and then decays exponentially (see eFig. 25-4B). If the plethysmograph flowmeter has a linear response, the plethysmograph flow signal (see eFig. 25-4C) will have a shape similar to that of the pressure signal (see eFig. 25-4B). The plethysmograph flow signal is integrated to determine volume (see eFig. 25-4D). The integrated flow signal attains the same level as that of the “true” volume event, but the shape of the integrated flow signal does not conform to that of the “true” volume event. The difference between the two waveforms is due to compression of the large volume of gas in the plethysmograph and is directly proportional to plethysmograph pressure. Thus, by adding a portion of the plethysmograph pressure to the integrated plethysmograph flow, the “true” volume event may be reconstructed accurately (see eFig. 25-4E) using Equation 1. The relative contributions of these two variables vary with frequency, but when added together, they always yield the total ΔV.
, is added to the plethysmograph compression volume, PboxKbox, to produce the “true” volume. If the volume change were instantaneous, the “true” volume event would be as illustrated in eFigure 25-4A. During this rapid inspiration, pressure in the plethysmograph increases abruptly and then decays exponentially (see eFig. 25-4B). If the plethysmograph flowmeter has a linear response, the plethysmograph flow signal (see eFig. 25-4C) will have a shape similar to that of the pressure signal (see eFig. 25-4B). The plethysmograph flow signal is integrated to determine volume (see eFig. 25-4D). The integrated flow signal attains the same level as that of the “true” volume event, but the shape of the integrated flow signal does not conform to that of the “true” volume event. The difference between the two waveforms is due to compression of the large volume of gas in the plethysmograph and is directly proportional to plethysmograph pressure. Thus, by adding a portion of the plethysmograph pressure to the integrated plethysmograph flow, the “true” volume event may be reconstructed accurately (see eFig. 25-4E) using Equation 1. The relative contributions of these two variables vary with frequency, but when added together, they always yield the total ΔV.
eFigure 25-4.
Physical principles underlying pressure-volume plethysmography.
A, The theoretical “true” instantaneous volume event. During this event, plethysmographic pressure increases rapidly and then decays exponentially (B). If the plethysmographic pneumotachygraph is linear, the flow signal has a shape similar to that of the pressure transducer (C). This flow signal is integrated to obtain volume (D), which reaches the same level as the true volume event, but the shape does not conform to the “true” event. The difference is a result of the compression of a large volume of gas in the plethysmograph and is directly proportional to the plethysmograph pressure. Therefore, by adding a portion of the plethysmograph pressure to the integrated plethysmograph flow (E), the true volume event is reconstructed accurately: ΔV = Pbox +  . Thus the true volume is obtained by adding the plethysmographic compression volume (Pbox) and the displacement volume (
. Thus the true volume is obtained by adding the plethysmographic compression volume (Pbox) and the displacement volume ( ). More precisely, (1) box pressure (Pbox) is multiplied by a constant (Kbox), a factor to correct pressure to volume that is proportional to the gas volume in the box (total box volume − patient volume); and (2) Pbox is also divided by the box flowmeter resistance (Rbox) to yield box flow (
). More precisely, (1) box pressure (Pbox) is multiplied by a constant (Kbox), a factor to correct pressure to volume that is proportional to the gas volume in the box (total box volume − patient volume); and (2) Pbox is also divided by the box flowmeter resistance (Rbox) to yield box flow ( ), and integrated to obtain volume (Vbox). These two signals are added together to yield the change in lung volume: ΔV = PboxKbox + Pbox/Rbox
), and integrated to obtain volume (Vbox). These two signals are added together to yield the change in lung volume: ΔV = PboxKbox + Pbox/Rbox  .
.
Thoracic Gas Volume.
The thoracic gas volume is the compressible gas in the thorax, whether or not it is in free communication with airways. By Boyle's law, pressure times the volume of the gas in the thorax is constant if its temperature remains constant (PV = P′V′). At end-expiration, alveolar pressure (Palv) equals atmospheric pressure (P) because there is no airflow; V (thoracic gas volume) is unknown (eFig. 25-5). Then, the airway is occluded and the subject makes small inspiratory and expiratory efforts against the occluded airway. During inspiratory efforts, the thorax enlarges (ΔV) and decompresses intrathoracic gas, creating a new thoracic gas volume (V′ = V + ΔV) and a new pressure (P′ = P + ΔP). A pressure transducer between the subject's mouth and the occluded airway measures the new pressure (P′). It is assumed that the mouth pressure (Pmouth) equals Palv during compressional changes while there is no airflow at the mouth, because pressure changes are equal throughout a static fluid system (Pascal's principle). Accordingly,
| (2) |
| (3) |
| (4) |
| (5) |
where P equals atmospheric pressure minus water vapor pressure (in mm Hg), assuming that alveolar gas is saturated with water vapor at body temperature; ΔV equals change in thoracic gas volume; and ΔPmouth equals change in Pmouth, which is equal to the change in alveolar pressure (ΔPalv). Then the thoracic gas volume is calculated as follows:
| (6) |
If a closed plethysmograph is used, ΔV is detected measuring increased plethysmographic pressure with a sensitive pressure transducer. If plethysmographic pressure is displayed on the x-axis and Pmouth Palv is displayed on the y-axis of an oscilloscope (Fig. 25-12 ), the slope of the line (α) can be measured during panting efforts against the closed airway:
| (7) |
| (8) |
eFigure 25-5.
The rectangle represents a closed, constant-volume, variable-pressure whole-body plethysmograph.
As described in eFigure 25-4, at end-expiration airflow is zero, thoracic gas volume (V) = functional residual capacity, and alveolar pressure (Palv) = mouth pressure (Pm) = barometric pressure (Pbar). When the subject inhales against an occluded shutter in the airway, airflow remains zero, but V increases by ΔV to V′ and Pm (= Palv) increases by ΔP (P + ΔP) to equal P′. When Pm is plotted against Pbox, the slope of the line (α) yields ΔV/ΔPalv, and V = ΔV/ΔPalv × Pbar, as indicated in the text. 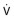 , airflow.
, airflow.
(Modified from Comroe JH Jr, Forster RE II, DuBois AB, et al: The lung: clinical physiology and pulmonary function tests, ed 2, Chicago, 1962, Year Book.)
Figure 25-12.
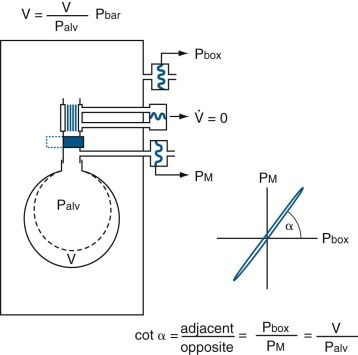
A closed, constant-volume, variable-pressure whole-body plethysmograph.
As described in eFigure 25-5, at end-expiration airflow is zero, thoracic gas volume (V) = functional residual capacity, and alveolar pressure (Palv) = mouth pressure (Pm) = barometric pressure (Pbar). The rectangle represents the plethysmograph. When the subject inhales against an occluded shutter in the airway, airflow remains zero, but V increases by ΔV to V′ and Pm (= Palv) increases by ΔP (P + ΔP) to equal P′. When Pm is plotted against box pressure (Pbox), the slope of the line (α) yields ΔV/ΔPalv, and V=ΔV/ΔPalv × Pbar, as indicated in the text.  , airflow.
, airflow.
(Modified from Comroe JH Jr, Forster RE II, DuBois AB, et al: The lung: clinical physiology and pulmonary function tests, ed 2, Chicago, 1962, Year Book.)
The thoracic gas volume usually measured is slightly larger than FRC unless the shutter is closed precisely after a normal tidal volume is exhaled. Connecting the mouthpiece assembly to a valve and spirometer (or pneumotachygraph and integrator), or using a pressure-volume plethysmograph, makes it possible to measure TLC and all its subdivisions in conjunction with the measurement of thoracic gas volume.
Technical Problems.
As might be expected, several problems may complicate these measurements. The most important are the following.
Effects of Heat, Humidity, and Respiratory Gas Exchange Ratio.
Effects of heat, humidity, and respiratory gas exchange ratio cause difficulties in obtaining stable baselines.
Changes in Outside Pressure.
Outside pressure changes can make it difficult to detect the “signal” relative to “noise.”46
Cooling.
Refrigeration is required for many of these boxes, but it can cause a variety of problems related to vibration and localized cooling (e.g., a cool body and a warm head may result because of poor circulation currents).
Underestimation of Mouth Pressure.
Stanescu and colleagues47 have reported that, in patients with asthma, lung volume measured by plethysmograph may be overestimated owing to an underestimation of Palv by measurements of Pmouth.
Compression Volume.
Commercial plethysmographs are now available that correct for these problems; some of these devices also take into account the compression of thoracic gas during a forced expiration.
Airway Resistance
General Principles.
Airway resistance (Raw) is easy to measure and is always related to the lung volume at which it is measured. It is useful to detect diseases such as asthma that are associated with increased airway smooth muscle tone. This can be accomplished by demonstrating that Raw is abnormally increased relative to lung volume, or by inducing significant relaxation of bronchomotor tone by administration of bronchodilator drugs. The test is very sensitive in detecting increased airway smooth muscle tone induced by provocative stimuli. This approach is useful in the assessment of nonspecific hyperirritability in response to pharmacologic agents, exercise, or cold air, or in response to specific agents such as allergens or chemicals (e.g., isocyanates) that are associated with occupational asthma (see “Bronchial Provocation” section). Measurements of Raw may also be useful in differential diagnosis of the type of airflow obstruction or localization of the major site of obstruction.
Raw is measured during airflow and represents the ratio of the driving pressure (between the alveoli [Palv] and mouth [Pmouth]) and instantaneous airflow ( ). In a closed plethysmograph, inspiration of 500 mL of gas from the box into the lungs increases plethysmographic pressure. At the start of inspiration, thoracic gas volume enlarges, and Palv (previously at atmospheric pressure) becomes subatmospheric throughout inspiration; thus alveolar gas occupies a larger volume. This decompression of thoracic gas is equivalent to adding a small volume of gas to the plethysmograph, so its pressure increases (as measured by a sensitive pressure transducer). The reverse results during exhalation, when alveolar gas is compressed. Thus
). In a closed plethysmograph, inspiration of 500 mL of gas from the box into the lungs increases plethysmographic pressure. At the start of inspiration, thoracic gas volume enlarges, and Palv (previously at atmospheric pressure) becomes subatmospheric throughout inspiration; thus alveolar gas occupies a larger volume. This decompression of thoracic gas is equivalent to adding a small volume of gas to the plethysmograph, so its pressure increases (as measured by a sensitive pressure transducer). The reverse results during exhalation, when alveolar gas is compressed. Thus  is measured continuously with a pneumotachygraph, Pmouth is measured with a pressure transducer connected to a side tap in the mouthpiece, and Palv is estimated continuously with the body plethysmograph (Fig. 25-13
).
is measured continuously with a pneumotachygraph, Pmouth is measured with a pressure transducer connected to a side tap in the mouthpiece, and Palv is estimated continuously with the body plethysmograph (Fig. 25-13
).
Figure 25-13.
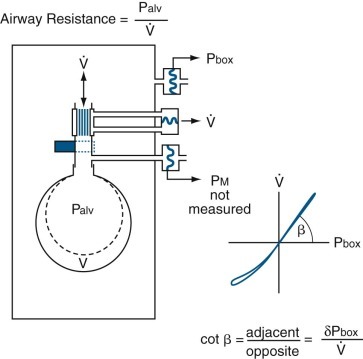
Measurement of airway resistance by plethysmography.
The rectangle represents a closed, constant-volume, variable-pressure, whole-body plethysmograph, as in eFig. 25-5. The subject is represented by a single alveolus and its conducting airway. The top pressure transducer measures pressure within the plethysmograph, or box pressure (Pbox). The middle pressure transducer measures the pressure drop across the pneumotachygraph connected in series with the open shutter to the airway, which yields airflow ( ). The bottom pressure transducer measures airway pressure (alveolar pressure during no flow, or Palv). During inspiration, the alveolus enlarges by ΔV from the original volume (broken line) to a new volume (solid line); during expiration, the alveolus returns to its original volume. Throughout inspiration, alveolar gas (previously at atmospheric pressure) is subatmospheric and therefore occupies more volume. This is the same as adding this increment of gas volume resulting from decompression of the alveolar gas to the plethysmograph, so Pbox increases and is recorded by the sensitive Pbox transducer. The reverse happens during expiration when alveolar gas is compressed. Thus alveolar pressure can be monitored throughout the respiratory cycle. When
). The bottom pressure transducer measures airway pressure (alveolar pressure during no flow, or Palv). During inspiration, the alveolus enlarges by ΔV from the original volume (broken line) to a new volume (solid line); during expiration, the alveolus returns to its original volume. Throughout inspiration, alveolar gas (previously at atmospheric pressure) is subatmospheric and therefore occupies more volume. This is the same as adding this increment of gas volume resulting from decompression of the alveolar gas to the plethysmograph, so Pbox increases and is recorded by the sensitive Pbox transducer. The reverse happens during expiration when alveolar gas is compressed. Thus alveolar pressure can be monitored throughout the respiratory cycle. When  is plotted against Pbox, the slope of the line (β) yields the ratio of ΔPbox/
is plotted against Pbox, the slope of the line (β) yields the ratio of ΔPbox/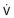 as indicated in the text.
as indicated in the text.
(Modified from Comroe JH Jr, Forster RE II, DuBois AB, et al: The lung: clinical physiology and pulmonary function tests, ed 2, Chicago, 1962, Year Book.)
In practice, Raw is determined by measuring the slope (β) of a curve of plethysmograph pressure (x-axis) displayed against airflow (y-axis) on a computer monitor during rapid, shallow breathing through a pneumotachygraph within the plethysmograph. Then, a shutter is closed across the mouthpiece, and the slope (α) of plethysmographic pressure (x-axis) displayed against Pmouth (y-axis) is measured during panting under static conditions. Because Pmouth equals Palv in a static system, the second step serves two purposes. First, it relates changes in plethysmographic pressure to changes in Palv in each subject. Palv is effectively measured during flow, provided that the ratio of lung to plethysmographic gas volume is constant, because Palv for a given plethysmographic pressure is the same whether or not flow is interrupted. Second, it relates Raw to a particular thoracic gas volume:

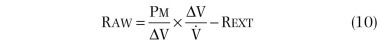
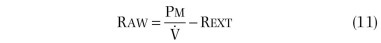
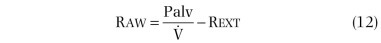
where Pm calibration is Pmouth calibration (cm H2O per cm), 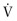 calibration is pneumotachygraph calibration (L⋅sec per cm), and Rext is resistance of breathing through mouthpiece and pneumotachygraph (cm H2O per L/sec).
calibration is pneumotachygraph calibration (L⋅sec per cm), and Rext is resistance of breathing through mouthpiece and pneumotachygraph (cm H2O per L/sec).
Physiologic Factors.
Several physiologic factors affect the values obtained during plethysmographic measurement of Raw.
Airflow.
Raw relates to a particular flow rate during continuous pressure-flow curves, so the slope may be read at any desired airflow rate. In general, Raw is measured at low flows, at which transmural compressive pressures across the airways are small and the relation to Palv is linear. Raw will be increased transiently with forced respiratory maneuvers in which airflow rates become limited by large transmural compressive pressures across the airways, by maximal dynamic airway compression, and by possible alterations in airway smooth muscle tone. Thus, to avoid artifacts, the standard approach is to measure Raw at low flows.
Volume.
Near TLC, resistance is small, but, near RV, resistance is large. Lung volume may be changed voluntarily to evaluate Raw at larger or smaller volumes in health and disease.
Transpulmonary Pressure.
Raw is related directly to lung elastic recoil pressure at any lung volume. Subjects with increased lung elastic recoil have a lower Raw at a given lung volume than normal subjects because of increased tissue tension pulling outward on airway walls. In contrast, loss of elastic recoil results in loss of tissue tension and decreased traction on airway walls, so Raw is increased. This relationship may be used to analyze the mechanism of airflow limitation in various obstructive ventilatory defects (e.g., bullous lung disease).48, 49
Airway Smooth Muscle Tone.
The airways are affected markedly by smooth muscle tone, depending on the state of inflation and the subject's previous pattern of breathing (referred to as “volume history”).50 These relationships are relevant to diseases in which smooth muscle tone is increased (e.g., asthma) or low lung volumes are encountered (e.g., during cough). Thus, bronchoconstriction is not demonstrable temporarily after a deep breath or at TLC in healthy subjects. Similarly, Raw in healthy subjects may be greater when a given lung volume is reached from RV than from TLC.
Panting.
Panting minimizes changes in the plethysmograph caused by thermal, water saturation, and carbon dioxide–oxygen exchange differences during inspiration and expiration; hence these factors may be neglected if measurements are made during panting. Panting also improves the signal-to-drift ratio, because each respiratory cycle is completed in a fraction of a second; gradual thermal changes and small leaks in the box become insignificant compared with volume changes attributable to compression and decompression of alveolar gas. The glottis stays open, rather than varying its position as it does during tidal breathing. Abdominal pressure changes are also minimized.
Quiet Breathing.
Increasingly laboratories are using commercial plethysmographs that estimate Raw during so-called quiet breathing, relying on computer software rather than panting to compensate for the effects of humidity, temperature, and gas exchange. In fact, the subject must breathe at higher than normal frequencies and tidal volumes to estimate RAW using this software. The limitation to this approach is that the average resistance values tend to be slightly higher than those observed during panting because the glottis is often partially closed during the measurement. Nonetheless, more and more laboratories are switching to this approach.
Stanescu and Rodenstein51 have demonstrated that, to avoid overestimation of thoracic gas volume, as described previously, panting must be done at 1 Hz; however, to measure Raw and avoid the temperature artifact, panting must be done at approximately 2 Hz, as advocated originally by DuBois and colleagues.52 This difference in the panting rates necessary for accurate measurements may prove impractical for clinical use. Alternatively, both artifacts may be avoided if the subjects breathe quietly at body temperature (37° C) and standard pressure fully saturated with water vapor (760 mm Hg) (BTPS) or may be compensated for electronically.53
Raw measured plethysmographically is not the average of unequal resistances throughout the lungs; rather, it is the average Palv per unit volume divided by average airflow rate at the mouth. It corresponds to average airway conductance (Gaw). Gaw = G1 + G2 + … +Gn, which is equivalent to adding resistances in parallel according to reciprocals: 1/Raw = (1/R1) + (1/R2) + … + (1/Rn). The control of these physiologic influences is often critical in determining specific factors that influence Gaw (or Raw) in a particular subject (e.g., loss of lung elastic recoil, airway smooth muscle spasm).
Impulse Oscillometry and Forced Oscillation Methods to Measure Respiratory Resistance (Rather Than Airway Resistance).54, 55
DuBois and colleagues52 described an oscillatory method to measure the mechanical properties of the lung and thorax. In contrast to the methods already described, the oscillation techniques use an external loudspeaker or similar device to generate and impose flow oscillations on spontaneous breathing, rather than using the respiratory muscles. Impulse oscillometry measures Raw and lung compliance independently of respiratory muscle strength and patient cooperation. Sound waves at various frequencies (3 to 20 Hz) are applied to the entire respiratory system (airways, lung tissue, and chest wall); a piston pump can be used to apply such pressure waves around the body in a whole-body respirator. With modern computer methods, the slow frequency changes in pressure, flow, and volume generated by the respiratory muscles during normal breathing are subtracted from the raw data, permitting analysis of the pressure-flow-volume relationships imposed by the oscillation device (eFig. 25-6).
eFigure 25-6.
Measurement of respiratory resistance by forced oscillation.
A loudspeaker may be driven to produce a sinusoidal oscillation at a single frequency, a sequence of sinusoidal oscillations at diverse single frequencies, or a random noise signal. The flow signal is integrated to yield tidal volume or, at the end of the study, inspiratory capacity. The recorded signals for mouth pressure (Pmo) and flow ( ) are directed to a Fourier analyzer, and the component of each signal caused by the applied oscillation is differentiated from changes caused by tidal breathing. Impedance (Zrs) is calculated over a wide range of frequencies. The impedance is subdivided into the in-phase and out-of-phase components of the primary signals. The in-phase signal is the resistance of the total respiratory system (Rrs), and the out-of-phase signal is the reactance (Xrs), sometimes called the imaginary part of the impedance. The reactance is related to the compliance and inertance of the respiratory system (see text).
) are directed to a Fourier analyzer, and the component of each signal caused by the applied oscillation is differentiated from changes caused by tidal breathing. Impedance (Zrs) is calculated over a wide range of frequencies. The impedance is subdivided into the in-phase and out-of-phase components of the primary signals. The in-phase signal is the resistance of the total respiratory system (Rrs), and the out-of-phase signal is the reactance (Xrs), sometimes called the imaginary part of the impedance. The reactance is related to the compliance and inertance of the respiratory system (see text).
(Modified from Hughes JMB, Pride NB: Lung function tests, London, 1999, WB Saunders, p 35.)
The elastic forces (pressures) of the lungs and chest wall oppose the volume changes induced by the applied pressure, which decrease as the frequency of oscillation increases. The total force or pressure that opposes the driving pressure applied by the loudspeaker, which can be measured as peak-to-peak pressure difference divided by peak-to-peak flow, is a combination of the resistance and reactance, which itself has elastic and inertial components. The reactance reaches a minimum at loudspeaker frequencies of approximately 3 to 8 Hz, where the resistance produces the only opposing force. This resistance is proportional to the Raw in healthy subjects and patients, although it does include a small component of lung tissue and chest wall resistance, as well as the resistance of the airways.
Values for the pulmonary resistance and total respiratory resistance primarily reflect Raw. The portion due to lung tissue resistance is about one fifth of the pulmonary resistance in healthy subjects. It is increased in patients with pulmonary fibrosis or kyphoscoliosis,56 but rarely to a level of clinical importance where it becomes the limiting resistance. The total resistance of the respiratory system (airway + lung + chest wall, or Rt = Raw + Rl + Rcw) usually is about 25% greater than the resistance of the airways in healthy subjects, or not much greater than pulmonary resistance. Again, although the chest wall resistance may be elevated in conditions such as kyphoscoliosis or parkinsonism, it rarely attains a level of clinical significance.
If the airways, lungs, and chest wall behaved as if they were a single bellows with frictional resistance, elasticity, and inertia, then the oscillations in airflow into and out of the lungs caused by the driving pressure produced by the loudspeaker across the respiratory system could be described as a function of the applied frequency by the following equations. At any frequency, the magnitude and phase shift of the reflected waves give a measure of impedance (Z) and reactance (X). The impedance is described by
| (13) |
where Z is mechanical impedance (cm H2O per L/sec) and is analogous to electrical impedance, R is resistance (cm H2O per L/sec), L is electrical inertance (cm H2O per L/sec2) and is analogous to electrical inductance, C is compliance (L/cm H2O) and is analogous to electrical capacitance, and f is the frequency of the driving pressure applied by the loudspeaker (Hz, or cycles per second).
The second equation describes the phase angle or lag (Θ) of the flow with respect to the applied pressure wave:
| (14) |
The inertial reactance (2πfL) corresponds to the electrical inductive reactance, which increases with frequency. The elastic reactance (2πfC) corresponds to the electrical capacitance, which decreases with increasing frequency.
The frequency at which the absolute values of these reactances are equal is called the resonant frequency. According to the Equation 14, the impedance (Z) becomes equal to the resistance of the respiratory system at the resonant frequency, which can be calculated from the following equation:
| (15) |
Landser and coworkers57 developed a device based on multiple frequencies (pseudorandom noise) in contrast to Dubois and colleagues52 and Michaelson and coworkers,58 who used a random noise signal. The technique requires little of the subject but to simply breathe quietly on a mouthpiece for 30 seconds; the computer does the complete analysis, yielding values for respiratory resistance at different frequencies. (The same device simultaneously estimates respiratory inertance and dynamic compliance.) This approach has been used extensively in Europe because it is fast, noninvasive, and reproducible; moreover, it seems to yield clinically meaningful results in healthy subjects before and after use of a bronchodilator, as well as in the evaluation of airflow obstruction in COPD, asthma, and congestive heart failure.59, 60, 61
It should be noted that the lungs and chest wall rarely respond to a driving pressure with diverse frequencies in the same way as a simple model assumed to be composed of single values of resistance, compliance, and inertance, as described earlier. The resonant frequency of the ribs is 7 to 10 Hz, whereas the resonant frequency of the abdomen and diaphragm is about 3 Hz. The air in the trachea, bronchi, and bronchioles has an inertial reactance that is significant at relatively high frequencies (≈6 Hz or greater). Consequently, when the whole system appears to be at resonant frequency, the impedance is probably not a pure resistance at all, but rather an admixture of other forces of the lungs and chest wall, as described earlier.62, 63, 64, 65
Bijaoui and colleagues66 have taken advantage of the impact of cardiogenic oscillations on the adjacent lungs to estimate mechanical output impedance of the lung. They observed that the beating heart creates small oscillations in flow that can be measured at the mouth when the glottis is open. Using the Fourier-domain ratio of these oscillations in pressure and flow, Bijaoui and coworkers calculated the respiratory impedance to be between 1.5 and 10 Hz. The real portion was similar to or smaller than the resistance measured simultaneously by the forced oscillation method. They suggested that they are measuring the flow resistance of the central and upper airway. This approach may prove to be useful to obtain information about the mechanical properties of the lungs without the need for an external source of applied flow.
Lung Elastic Recoil
General Principles.
Lung elastic recoil is an important physiologic characteristic of the lungs, which may change in qualitatively different ways in various diseases. In general, elastic recoil is increased in a restrictive ventilatory defect associated with decreased lung volumes. Conversely, in almost all forms of airflow obstruction, elastic recoil is decreased. Testing for elastic recoil is time-consuming, difficult to perform, expensive, and invasive. Thus the test may not be practical for the routine evaluation of patients with restrictive ventilatory defects but may be of great value in the assessment of various obstructive ventilatory defects, including those with isolated bullae or advanced emphysema, to determine whether patients will benefit from resection of nonfunctioning or very poorly functioning lung tissue. In other patients, it may be useful to differentiate emphysema from asthma or bronchitis. In evaluating patients with mixed ventilatory defects (e.g., emphysema plus fibrosis), the test may confirm the presence of both disorders.
Lung elastic recoil pressure, or transpulmonary pressure (Pl), is the difference between the pressure inside the lungs (the alveolar pressure) and the pressure outside the lungs (the pleural pressure [Ppl): Pl = Palv − Ppl. To maintain a sustained inspiration at a volume of three fourths of TLC with the mouth and glottis open, the muscles of inspiration must maintain a pleural pressure of approximately 12 cm H2O below atmospheric pressure (Ppl = −12 cm H2O). Under conditions of no flow and pressures at the mouth, alveoli, and atmosphere are equal: Pl = 0 − (−12 cm H2O). If the muscles of inspiration relax, allowing the chest wall to recoil inward, Ppl rises from −12 to 0 cm H2O and Palv from 0 to +12 cm H2O at the instant before flow begins. This example illustrates two of the principles that underlie measurement of lung recoil: (1) the pressure required to expand a lung to any volume is equal to the recoil pressure at that volume, and (2) under conditions of no flow, with the glottis open, Palv and Pmouth are identical. It is easy to measure Pmouth; absolute lung volume can be measured by any of a variety of methods already discussed; and the change in volume can be measured easily with a spirometer. All that is needed to measure lung elastic recoil pressure and lung compliance is a measurement of Ppl in relation to lung volume.
Because the esophagus passes through the pleural space, it seems reasonable to assume that pressure within the esophagus approximates Ppl. This assumption works as long as the sphincters of the upper and lower esophagus are competent and there is no force compressing the esophageal lumen, such as active contraction of the esophageal muscles or passive compression by surrounding mediastinal structures. Most of these conditions are met in subjects without esophageal disease who are sitting or standing upright.
Protocol for Measurement of Lung Elastic Recoil.
To preserve the patency of a tube placed in the esophagus to measure esophageal pressure, it is necessary to cover the end of the tube with a balloon. This complicates the situation, for now intraballoon pressure is assumed to reflect intraesophageal pressure, which in turn is assumed to reflect the surrounding Ppl. The artifacts caused by the balloon generally cause the measured pressure to be too positive, owing to the compression of the balloon by the walls of the esophagus (eFig. 25-7). A long (10 cm), narrow (2.5-cm perimeter), thin-walled (0.04 cm), highly compliant latex balloon containing a small amount (0.2 to 0.4 mL) of air can reduce these artifacts. The volume of air that minimizes this artifact varies slightly for different balloons. The volume can be determined for each balloon by suspending it vertically in water, with the top (proximal end) of the balloon at the surface, allowing it to empty before the tube is closed with a stopcock.67
eFigure 25-7.
Schematic drawing illustrating the position of an esophageal balloon in relation to adjacent structures.
The balloon is made of latex (wall thickness, 0.06 mm; length, 10 cm; circumference, 3.5 cm). The tubing is polyethylene (inner diameter, 0.14 cm; outer diameter, 0.19 cm) with holes placed in a spiral arrangement in the portion inside the balloon. The balloon is filled with 0.2 to 0.4 mL of air and positioned in the lower third of the esophagus. Intraesophageal pressure recorded from the catheter within the balloon is affected by the following factors in addition to static transpulmonary pressure: retractile pressure of balloon wall (1), pressure caused by resting esophageal tension (2), and pressure caused by mediastinal structures (3), including pulsations of the heart (4).
Ppl changes along a vertical gradient, with pressures being most negative at the base of the thoracic space. It is customary to measure pressure in the lower third of the esophagus, to estimate the pressure necessary to expand the greater proportion of the lungs. The balloon is advanced to the gastroesophageal junction (identified easily by the positive pressure caused by an inspiratory sniff) and then pulled back 10 cm.
Analysis
Compliance.
When the balloon is in place, the relationship between changes in lung volume and changes in Ppl can be measured.
Dynamic lung compliance refers to the ratio of the change in volume to the change in pressure over a tidal breath, with the pressure measured at moments of zero flow during breathing. Measurement of dynamic lung compliance at increasing respiratory frequencies allows estimation of the frequency dependence of compliance. A fall in dynamic lung compliance as frequency increases implies narrowing of some of the airways subtending alveoli. Thus, in the absence of abnormalities in total Raw or FEV1 (which, as described previously, are largely determined by resistance in large airways), decreased dynamic lung compliance suggests possible narrowing of small, peripheral airways.68
Static lung compliance is the slope of the pressure-volume curve of the lung obtained during deflation from TLC.
Having the subject inhale to TLC three times standardizes the volume history and ensures minimization of the changes due to the dynamics of entry of surface-active material into the air-liquid interface. On the third inhalation the subject pauses at TLC for 3 to 5 seconds and then exhales slowly, while flow is interrupted by closing the mouth shutter for 2 to 3 seconds at each of several volumes. Repeating this maneuver four or five times provides enough data to characterize the relationship between the change in lung volume and the change in Pl over the entire VC (see Fig. 25-14 ). To fix the resultant curve on the volume axis requires knowing absolute lung volume at some Pl. This is easily measured directly if the curve is obtained with the subject in a body plethysmograph. Alternatively, but less accurately, lung volume (TLC, FRC, or RV) measured at another time by a gas dilution technique, for example, may be assumed to be the same at the time of measurement of lung compliance.
Figure 25-14.
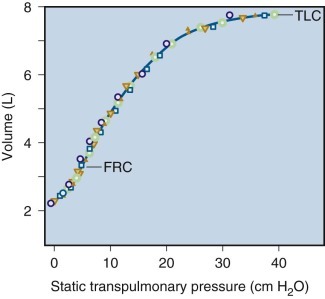
Static pressure-volume curve of the lungs during deflation in a normal subject.
Measurements were obtained during five different maneuvers. FRC, functional residual capacity; TLC, total lung capacity.
The data obtained are conveniently expressed in terms of lung compliance, the ratio of the change in lung volume to the change in Pl. However, it is clear that lung compliance changes with lung volume, with the highest values observed at volumes around FRC and lower values prevailing as the lungs are expanded more nearly to TLC (Fig. 25-14). Compliance therefore is usually reported as the slope of the curve at the point 0.5 L above FRC. However, when this convention is used, the value expressed for lung compliance is influenced by the determinants of FRC, rather than simply by the relationship between lung volume and distending pressure. Another value commonly calculated is the coefficient of retraction (lung elastic recoil pressure at TLC divided by TLC). Normal values are available for both compliance and the coefficient of retraction, although the great variability of these measurements limits their utility in individual patients. Because lung compliance is so dependent on lung volume (compliance can fall by 50% with resection of one lung, for example, even though the elastic properties of the remaining lung are unaltered), its variability can be somewhat reduced by correcting it for height, predicted TLC, or measured FRC.69
Maximum information about lung elastic recoil can be derived by analyzing the whole curve, when, for example, static lung elastic recoil pressure is plotted against lung volume expressed as a percentage of predicted TLC.70 Such a plot often makes it obvious whether a reduction in TLC is a function of the inability to generate an adequate lung elastic recoil pressure due to neural, muscular, or chest wall disease or is caused by a true loss of lung compliance. If lung compliance is reduced, it can still be difficult to determine whether the abnormality is due to a true increase in elastic forces or to a decrease in the number of alveoli communicating with the airways (see later discussion).
Exponential Analysis.
Gibson and Pride70 suggested that exponential analysis of the lung pressure-volume curve is superior to other approaches because it is less affected by patient effort and lung size, uses a greater range of the pressure-volume data, and mathematically describes the whole lung70a:
| (16) |
where V is the lung volume and Vmax is the maximal or extrapolated lung volume at infinite distending pressure. K is a constant describing the shape of the pressure-volume curve.
Exponential Analysis of Pressure-Volume Curve.
K is related to the incremental compliance (dV/dP) such that
| (17) |
where P is the lung elastic recoil pressure. When P is measured in centimeters of water, K has the dimensions of 1/cm H2O. To describe the curve fully requires the two parameters Vmax and A, which both have the dimensions of volume. A = Vmax − V0, where V0 equals the volume extrapolated to P = 0. A number of investigators have now used the approach in the evaluation of both restrictive and obstructive ventilatory defects (see later discussion).6, 70b, 70c
Fibrosis.
Exponential analysis of the pressure-volume curve appears to differentiate restriction due to loss of volume from that due to increased elastic properties.70b Gibson and colleagues70b reported that the elastic properties of the lungs in patients with diffuse interstitial fibrosis can be accounted for almost entirely by a loss of alveoli. This implies that the lungs of such patients consist of a population of completely obliterated, unventilated alveoli and a population of surrounding normal alveoli. Thompson and Colebatch70d confirmed these findings.
Emphysema.
Colebatch and associates309 reported that the constant K (describing the shape of the curve) falls outside the normal range in patients with pulmonary diseases (increased K in emphysema; decreased K in fibrosis). These results were confirmed by others.70b
Other researchers have examined this issue, and the results are more controversial. Gugger and coworkers70e found a significant correlation between elastic recoil pressure and both the FEV1 and the Dl CO. Lung density (measured by CT scans, which, in turn, correlate with the amount of emphysema measured by panel grading) correlated with both the natural logarithm of K and elastic recoil pressure of the lungs at 90% of TLC. Because elastic recoil pressure correlated with emphysema and with FEV1, their results suggest that loss of elastic recoil is one determinant of airflow limitation in patients with COPD.
Macklem and Eidelman70f and others165 have reexamined the effect of the elastic properties of emphysematous lungs on airflow obstruction. From published data in normal lungs and in patients with emphysema, they calculated specific lung elastance (change in lung elastic recoil pressure to produce a given fractional change in lung volume) for normal and emphysematous lungs. They found that specific lung elastance and the change in specific elastance with lung elastic recoil were increased in patients with emphysema compared with normal subjects. They speculated that this finding probably represents two distinct abnormalities in the elastic properties of emphysematous lungs: (1) an increase in resting length of alveolar walls, accounting for hyperinflation (TLC); and (2) a decrease in extensibility of alveolar walls once they become stressed (specific lung elastance). Surprisingly, these studies found no correlation between either of these factors and FEV1. They concluded that the change in elastic properties of the lungs in emphysema does not appear to account for flow limitation in this disease. Furthermore, because of the decreased extensibility of emphysematous lungs, they also suggested that these emphysematous regions are not only poorly perfused but also poorly ventilated; therefore they speculated that emphysema per se may not seriously disturb ventilation-perfusion relationships.70f
An equally surprising study of patients with severe expiratory airflow obstruction was reported by Gelb and colleagues.70g They documented that marked loss of lung elastic recoil, causing hyperinflation with increased TLC, associated with decreased Dl CO, can be present despite the absence of or only trivial emphysema on lung CT scans and in morphologic studies. These authors attributed the decreased Dl CO to errors related to inhomogeneity of ventilation and increased physiologic dead space. They attributed the severe, fixed expiratory airflow limitation to intrinsic disease of the bronchioles. They speculated that bronchiolar obstruction caused dynamic hyperinflation and gas trapping, leading to chronic loss of lung elastic recoil through unknown mechanisms, despite the absence of macroscopic emphysema. Thus the combination of increased TLC plus spuriously reduced Dl CO may be mistaken for emphysema; in such cases high-resolution lung CT scanning may help to clarify the source of lung hyperinflation as resulting from bronchiolar disease.
Clinical Applications of Flow-Volume Relationships
Normal Values
Spirometric values vary with height, gender, age, and ethnicity. Publications describing reference populations should include not only the prediction equations but also a means to define their lower limits. A lower limit can be estimated from a regression model: for spirometry, values below the 5th percentile are taken as below the “lower limit of normal.”28 There is no statistical basis for the common practice of using 80% of the predicted normal values for FEV1 and FVC as the lower limit of normal in adults. In fact, Miller et al71 studied 11,413 patients and found that using fixed cut points to determine whether lung function is abnormal could misdiagnose more than 20% of patients, which they found could be avoided by using the lower limit of normal based on the 5th percentile values.
Sources of Variability.
The ATS has published a formal recommendation on the selection of reference values and interpretative strategies for lung function tests, including FVC, FEV1, FEV1/FVC, FEV1/VC, and criteria defining a significant response to a bronchodilator for adult white and black men and women.6 This statement recommends reference values derived from the National Health and Nutrition Examination Survey (NHANES) III, which included whites, blacks, and Mexican Americans.72, 73, 74 In 2010 the Multi-Ethnic Study of Atherosclerosis lung study assessed the performance of the NHANES III reference equations in 3893 participants, of which approximately one third were Asian Americans. This study found the equations to be valid for the previously mentioned ethnic groups and, in addition, the investigators suggested that a correction factor of 0.88 be applied to the predicted and lower limits of normal values when comparing whites and Asian Americans.75
The ATS statement also emphasizes the importance of laboratory control of technical sources of variation, including strict adherence to ATS guidelines for equipment performance and calibration, minimizing temperature-related errors, careful validation of computer calculations when purchasing or changing equipment or software, and proper performance of the tests. Although certain within-individual sources of variation fall within the control of each laboratory, between-individual sources of variation are critical to the choice of appropriate reference values. Furthermore, environmental sources of variation pertinent to a given patient (in addition to other relevant clinical data) are likely to be known by the referring clinician. This information should be provided to the laboratory director, who should use it to evaluate the clinical relevance in a given lung function report. When short-term variation caused by disease, drugs, environment, smoking, laboratory instruments, or submaximal efforts is excluded, body position, head position, effort dependency of maximal flows, and circadian rhythms cause the primary residual sources of variation.6 Host factors (e.g., sex, size, aging, race, and past and present health), environmental factors, geographic factors, pollution, and socioeconomic factors cause variability among subjects.
Statistical Considerations.
Distributions of FEV1 and FVC in population studies are close to gaussian in the middle age range but not at the extremes. Furthermore, distributions of flow rates and ratios (FEV1/FVC) are not symmetrical.28 Therefore publications describing reference populations should include not only the prediction equations but also a means to define their lower limits. A lower limit can be estimated from a regression model. For spirometry, values below the 5th percentile are taken as below the “lower limit of normal.”28 If there are sufficient measurements within each category, percentiles can be estimated directly from the data. If the distribution of individual observations is close to gaussian, as it sometimes is in children, the value of the 5th percentile can be approximated.
However, comparisons of spirometric prediction equations indicate that there is good agreement using the 5th percentile. Furthermore, as stated previously, there is no statistical basis for the common practice of using 80% of the predicted normal values for FEV1 and FVC as the lower limit of normal in adults. For FEF25%–75% and for instantaneous airflow rates, this practice causes significant errors because the lower limits of normal for these values are close to 50% of predicted normal values. Using a fixed FEV1/FVC ratio as a lower limit of normal in adults also causes significant errors because this ratio is inversely related to age and height.28 However, using a fixed percentage of the predicted value as a lower limit of normal may be acceptable in children when the SD is proportional to the predicted mean value. In general, the lowest 5% of the reference populations may be considered as being below the lower limit of normal for any spirometric value.
The ATS suggests that individual laboratories use published reference equations that most closely describe the populations tested in their laboratories. It is useful to compare the results observed in 20 to 40 local subjects with those provided by the intended reference equations. These local subjects should be lifetime nonsmokers selected by age, ethnic group, and sex to match the population usually studied in the laboratory.75, 76, 77, 78, 79
Changes in Function over Time.
Changes in spirometric measurements can represent a true change or merely variability. A real change is more likely when a series of tests shows a consistent trend. A change varies in significance depending on the variable measured, the time period, and the type of patient. When the FVC and VC are followed in healthy, normal subjects, within-day changes of 5% or more, between-weeks changes of 11% to 12% or more, and yearly changes of 15% or more are probably clinically significant. In a classic epidemiologic study of the changes of FEV1 over time, normal men were found to have approximately a 40- to 50-mL decrease in FEV1 per year; the rate of loss increased in smokers, who were susceptible to the damaging effect of cigarette smoke and could return to a normal rate of loss with smoking cessation.79aMore recent studies using data from the Framingham Offspring cohort have expanded this analysis to include women, to enlarge the age range, and to standardize the spirometric measurements. In this study, both men and women nonsmokers were found to have an equivalent gradual loss of FEV1 with age (approximately 20 mL/year in men, 18 mL/year in women).80 There was an increase in loss with smoking (38 mL/year in men, 24 mL/year in women) and a benefit from quitting.
Flow-Volume Curves.
The range of normal for measurements derived from flow-volume curves has been even more difficult to define than that for spirometry. Correlations with sex, age, and height are poor and do not appear to decrease variability. Most published studies provide prediction equations for mean values only; a few report standard deviation or some other estimate of population variance, but this is of little use in predicting the lower limit of normal. Several investigators have analyzed this problem and have provided predicted mean values and estimates of the lower limit of normal values.6, 28
This wide range of normal values limits the interpretation of spirometric and flow-volume curves.81 If a subject has values in the very low normal range at a given time, the results may be normal for that person or may be significantly abnormal in a person whose VC or flow rates were much higher than average before the onset of the disease. In such cases a discrepancy between static and dynamic measurements, expressed as a percentage of predicted value, may yield a clue to this situation. For example, it would be unusual for a normal person to have a VC that is 115% of the predicted value and a FEF25%–75% that is 85% of the predicted normal value. These findings suggest the possibility of some form of airway obstruction. As with all laboratory tests, evaluation of the results in the clinical context may be helpful in interpretation.
Although the range of predicted values is large, the same pulmonary function tests tend to have reproducibility in the same subject. If variability is limited by careful standardization of pulmonary function testing, spirometry should be reproducible within 5% of the initial values obtained. In addition, if the patients are highly cooperative, the variability can be as small as 2% to 3%.82 Thus, repeated measurements of spirometry over time provide a sensitive way of monitoring disease. This reproducibility also accounts for the utility of performing spirometry initially in workers entering a job that will expose them to risks of obstructive or restrictive ventilatory defects.83
Pathophysiologic Patterns
The diagnosis and quantitation of airway obstruction are among the most common uses of pulmonary function tests. Raw, however, is not measured directly by spirometry. Variables derived from spirometry and flow-volume curves may be used to infer increased Raw from measurements of expiratory airflow achieved with a maximum effort by the subject. Because this maximum effort is not quantitated, the observer can only presume that the decreased flow is due to increased resistance, rather than a decreased effort to produce the flow. If necessary, the degree of effort can be determined using an intraesophageal balloon to estimate pleural pressure or, noninvasively, by estimating compression volume in a pressure-volume plethysmograph (see earlier discussion).
Obstructive Ventilatory Defect.
Despite the dependence on effort, reproducible patterns are obtained in normal subjects and in patients with obstructive ventilatory defects (Fig. 25-15 ). An inference of increased Raw can be made with reasonable assurance, and correlation with measurements made by body plethysmography is good.
Figure 25-15.
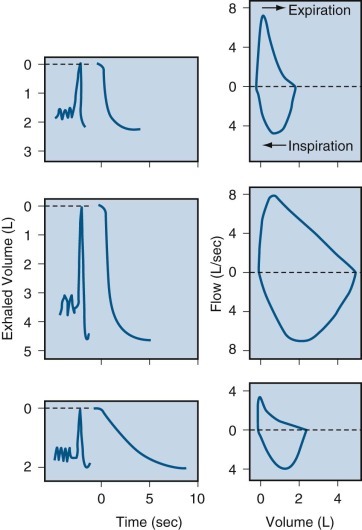
The three major patterns of flow.
Spirograms and flow-volume curves are shown which were obtained in a patient with a restrictive ventilatory defect (top), a normal subject (middle), and a patient with an obstructive ventilatory defect (bottom).
In patients with emphysema, most authors suggest that decreased maximal expiratory flow is thought to be due to the effect of loss of lung elastic recoil on airway dimensions, which results in an increased resistance to flow owing to increased compliance and collapse of airway walls.
In emphysema and other diffuse obstructive disorders, the decrease in expiratory flow is usually associated with decreased VC. The decreased VC results from “gas trapping” associated with increased RV. Actual measurement of RV may be necessary to document this phenomenon and to rule out a mixed restrictive and obstructive ventilatory defect. Expressed as percentages of predicted values, the decrease in VC in patients with obstructive ventilatory defects is relatively less severe than the decrease in airflow.
The pulmonary ventilation is limited ultimately by the highest flows that can be generated by the subject. Even during high-intensity exercise, most healthy subjects do not experience expiratory flow limitation.84 However, patients with COPD may experience expiratory flow limitation at low work rates during exercise or even at rest, as first suggested by Potter and colleagues.84 Potter's group reported that patients with advanced COPD often breathe on their maximal expiratory flow-volume curves during tidal breathing. They suggested that this phenomenon develops because of expiratory flow limitation (i.e., inability to increase flow beyond a limit at a given lung volume). The phenomenon of expiratory flow limitation has been studied extensively in COPD patients both at rest and during exercise.85, 86 Flows observed during tidal breathing in COPD patients often exceed the maximal expiratory flow-volume envelope.84, 85 This pattern has been termed “negative-effort dependence” and is attributed to abnormal compressibility or collapse of the airway walls; in this situation, tidal breathing involves less expiratory force, less collapse of highly collapsible airways, and slightly greater flow than seen with a maximal forced expiratory maneuver.
Expiratory Flow Limitation Assessed by Comparison of Tidal and Maximal Flow-Volume Curves
-
1.
Tidal and maximal flow curves are usually aligned on the assumption that TLC does not change during exercise and hence that changes in inspiratory capacity reflect changes in end-expiratory lung volume. Most reports indicate that TLC does not change with exercise,86, 87 but others have found that TLC does increase.88 In addition, this approach assumes that the patients can make a truly maximal inspiratory effort during exercise. In fact, some COPD patients are not able to perform these maneuvers during exercise.
-
2.
Maximal expiratory airflow depends on the volume and time history of the preceding inspiration.89, 90 However, the previous volume and time history always differ between tidal breathing and maximal inspiration. Therefore assessment of flow limitation by comparison of tidal and maximal flow-volume curves may lead to erroneous assumption of abnormal airway collapsibility, even if the measurements are made plethysmographically.
-
3.
In nearly all reports, the tidal and forced flow-volume loops were obtained from measurements of expired gas volume at the mouth. The assumption is made that both loops develop at the same lung volume when, in fact, the forced loop may be at a smaller volume due to gas compression during the forced maneuver. Such gas compression artifacts can be avoided by measuring volume with a body plethysmograph, as suggested by Ingram and Schilder.91
-
4.
Exercise may result in bronchodilation and other changes in the mechanical properties of the lungs, which may affect both the tidal and maximal flow-volume curves.86
-
5.
Evaluation of expiratory flow limitation has also been studied by comparison of tidal flow-volume curves with partial flow-volume curves, thereby keeping the previous volume history constant. Although theoretically appealing, this approach often neglects the effect of the previous time history (which affects both partial and maximal forced flow-volume curves)89, 92 and is not practical in most patients with COPD at rest, let alone during exercise.
Thus it appears that study of expiratory flow limitation on the basis of comparison of tidal with maximal flow-volume curves can be problematic. An alternative approach, called the negative expiratory pressure method, has been developed by Koulouris and associates93 (eFig. 25-8). This method does not require flow-volume maneuvers by the subject, nor must it be performed in a body plethysmograph. A negative pressure is applied at the mouth during a tidal expiration, and the ensuing expiratory flow-volume curve is compared with that of the previous control tidal expiration. With this method the volume and time history of the control and test breath are the same. The negative expiratory pressure method has been validated in mechanically ventilated patients by direct comparison with isovolume pressure-flow curves.94 It has also been used to study stable COPD patients at rest and during exercise.95, 96
eFigure 25-8.
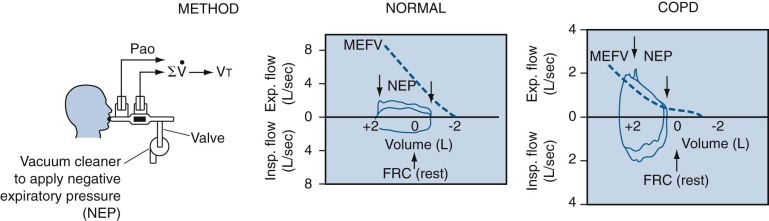
Measurement of expiratory flow limitation by negative expiratory pressure (NEP) method.
Left, The experimental setup. The subject breathes tidally through a pneumotachygraph that records flow ( ), which is integrated to yield tidal volume (Vt). After recording baseline Vt, a negative expiratory pressure of −5 cm H2O is applied to the subsequent Vt, in which the pressure at the airway opening (Pao) is reduced by 5 cm H2O. Right and center, Examples of tidal flow-volume curves. Both results were obtained during exercise. In the normal subject (center), expiratory flow increases, but except for a transient spike of flow, there is no change in flow in a patient with COPD (right). Note that the flow and volume scales are different in the two panels, as are the shapes of the curve in the normal individual (rectangular) and in the COPD patient (right). The change in volume during exercise is referred to as the functional residual capacity (FRC) at rest. Note the decrease in volume in the normal lung and the increase in volume in the COPD lung during exercise. The dashed lines indicate the full maximal flow-volume curves in both subjects. There is a large reserve of expiratory flow in the normal subject, whereas tidal expiratory flow exceeds the full flow-volume envelope at the same volume in the COPD patient. MEFV, maximal expiratory flow-volume.
), which is integrated to yield tidal volume (Vt). After recording baseline Vt, a negative expiratory pressure of −5 cm H2O is applied to the subsequent Vt, in which the pressure at the airway opening (Pao) is reduced by 5 cm H2O. Right and center, Examples of tidal flow-volume curves. Both results were obtained during exercise. In the normal subject (center), expiratory flow increases, but except for a transient spike of flow, there is no change in flow in a patient with COPD (right). Note that the flow and volume scales are different in the two panels, as are the shapes of the curve in the normal individual (rectangular) and in the COPD patient (right). The change in volume during exercise is referred to as the functional residual capacity (FRC) at rest. Note the decrease in volume in the normal lung and the increase in volume in the COPD lung during exercise. The dashed lines indicate the full maximal flow-volume curves in both subjects. There is a large reserve of expiratory flow in the normal subject, whereas tidal expiratory flow exceeds the full flow-volume envelope at the same volume in the COPD patient. MEFV, maximal expiratory flow-volume.
(Modified from Koulouris NG, Dimopoulou I, Volta P, et al: Detection of expiratory flow limitation during exercise in COPD patients. J Appl Physiol 82:723–731, 1997.)
Ninane and associates97 have offered another approach to the technical issues associated with comparing tidal breathing with maximal flow curves. They described a method to detect expiratory flow limitation by manual compression of the abdominal wall. In healthy subjects, abdominal compression causes decreased abdominal diameter, increased gastric and pleural pressures, and increased expiratory flow. This method has been used successfully to study expiratory flow limitation in neonates, but in adults with COPD abdominal compression fails to increase expiratory flow despite increased gastric and pleural pressures.97
Restrictive Ventilatory Defects.
A restrictive ventilatory defect is suggested by a decreased VC, reflecting limitation in chest excursion (which, according to the ATS expert panel, requires confirmation by a decreased TLC). Typical results consist of a decreased VC, little or no reduction in expiratory airflow, and relative preservation of MVV. Early in the development of an interstitial lung disease, before development of decreased lung volumes, volume-corrected flow (i.e., flow divided by the total lung capacity, to account for lung size) and FEV1/FVC ratios are increased. These increased airflow rates result from the increased force causing outward traction on airway walls. Thus airway diameters become larger than normal relative to lung volume, so airflow rates are increased. Because of the increased flows relative to lung volume, the usual flow-volume curve in restrictive defects is tall and narrow (see Fig. 25-15). With time, as the disease becomes more severe, lung volumes decrease, as reflected by a decreased VC. If the disease can be reversed, volumes return to normal first, then volume-corrected flows, and then the FEV1/FVC ratio.98, 99, 100
Distribution of Ventilation
For discussion of ventilation, blood flow, and gas exchange, see Chapter 4
Tests that measure distribution of ventilation are very sensitive to abnormalities in lung structure and function but are nonspecific. Thus they are useful for detecting the presence of abnormal function early, when other test results are normal, or to confirm the presence of airflow obstruction when other test results are only mildly abnormal. They are particularly important in the evaluation of patients with suspected upper airway obstruction to determine if there is associated disease of the airways distal to the trachea. They may be very useful in epidemiologic studies, such as evaluation of the effects of smoking or air pollution in large populations.
Measurements of Distribution of Ventilation
The physiologic determinants of distribution of ventilation are reviewed in Chapter 4. eFigure 25-9 illustrates the concept of uneven distribution of inspired gas.
eFigure 25-9.
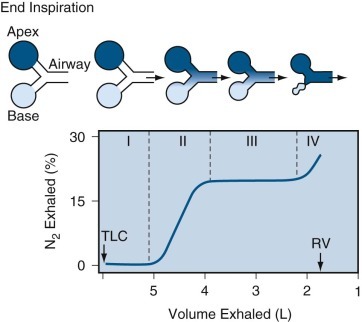
Relationship between nitrogen (N2) concentrations in different regions of lung (top) and the single-breath N2 washout test of distribution of ventilation (bottom).
Top, Schematic illustration of a ventilatory unit near the lung apex (dark blue) and a ventilatory unit near the base (light blue) subtended by a common airway. The intensity of color reflects the end-inspiratory concentration of the resident gas (N2) at the end of a single maximal inspiration of pure oxygen (O2) (at total lung capacity [TLC]). The differences in N2 concentration in each unit result from the effect of differences in regional residual volume (RV) and the distribution of inspired gas (see text). Bottom, At the start of exhalation, the gas (pure oxygen) in the conducting airway empties first, and 0% N2 is recorded (phase I). As exhalation continues, gas from both ventilatory units mixes in the airway, and the N2 concentration increases rapidly (phase II). With continued exhalation, mixed alveolar gas is recorded by the N2 analyzer (phase III). Finally, dependent airways at the base of the lungs close near RV (closing volume), and exhalation continues from the apical ventilatory unit of the lung only, which contains a higher N2 concentration than the basal unit (phase IV).
Resident Gas, Single-Breath Test
The single-breath nitrogen washout test (sometimes called the single-breath oxygen test) is designed to assess the uniformity of gas distribution in the lungs and the behavior of the dependent airways.101 At present, the most clinically useful aspect of the test is the measurement of the slope of phase III (alveolar gas plateau) to determine the uniformity of gas distribution.
Protocol.
The single-breath nitrogen washout test (sometimes called the single-breath oxygen test) is designed to assess the uniformity of gas distribution in the lungs and the behavior of the dependent airways.101 At present the most clinically useful aspect of the test is the measurement of the slope of phase III (alveolar gas plateau) to determine the uniformity of gas distribution. The subject inspires a single breath of pure oxygen from RV to TLC (inspiratory VC maneuver); nitrogen concentration at the mouth during exhalation is measured with a nitrogen analyzer or mass spectrometer. At end-inspiration, the dead space is filled with oxygen that has just been inspired (see eFig. 25-9). At the beginning of the subsequent expiratory VC maneuver, the nitrogen meter continues to record 0% nitrogen, because the first gas to leave the lungs is from conducting airways—the so-called anatomic dead space (phase I) (see eFig. 25-9). Subsequently, the nitrogen concentration increases in a sigmoid curve upward and reflects mixing of gas from dead space and alveoli (phase II). The slightly sloping plateau in phase III reflects the almost constant nitrogen concentration in alveolar gas. If inspired oxygen is distributed evenly to all alveoli so each has the same nitrogen concentration, then phase III of the nitrogen tracing is almost horizontal (alveolar plateau). However, if inspired oxygen is distributed unevenly (as happens to a small extent even in healthy subjects), then the end-inspiratory nitrogen concentrations are not equal throughout the lung. The concentrations of exhaled nitrogen from different alveoli are not recorded as a horizontal line; the first portion of phase III usually contains a lower nitrogen concentration than the last portion.
The analysis of these curves is not entirely objective. When the same observer reads such curves twice under “blind” conditions, agreement between the two measurements is poor. This variability appears to be due to differences between individual lungs; when a subject generates such a curve, usually all curves produced by that subject are difficult to analyze. On the other hand, if a subject generates a curve that is easy to analyze, most curves produced by that subject are reproducible. Obviously, analysis of these curves requires good judgment; some curves, although conforming to the criteria of acceptability, are unreadable and therefore should be ignored. It appears difficult, if not impossible, at present to establish a uniform set of criteria for this analysis.102
Normal Values.
The slope of phase III (percentage of nitrogen per liter) is determined as the line of best fit (by least-squares linear regression) between 70% of VC and the onset of phase IV. In most cases, the range about the mean of three measurements of the slope of phase III should not be greater than ±0.5% N2/L.103 The variations appear to be independent of the time of day at which the test is performed, but the slope of phase III does depend on inspiratory flow.104
The TLC measured by the single-breath nitrogen test correlates well with that measured by helium dilution in a population of men and women free from abnormalities of gas distribution, for both smokers and nonsmokers with and without symptoms.103 As expected, the measurement of TLC underestimates lung volume in patients with airway obstruction.6, 103
Other Tests
The methods just described use the resident-gas technique. Similar measurements made by bolus techniques and resident-gas techniques have been compared, and they show either close similarities in results or a systematic tendency for measurements of phase IV determined by the resident-gas technique to be slightly lower than those determined by the bolus technique.105
Other methods used to assess uniformity of distribution of ventilation include measurement of residual nitrogen following multiple-breath, open-circuit nitrogen washout106 and determination of helium mixing time during closed-circuit equilibration. In the multiple-breath nitrogen washout, for example, continuous breath-by-breath measurement of nitrogen concentration at the mouth during tidal breathing of pure oxygen is performed until end-tidal nitrogen concentration falls to less than 1%. The fall in end-tidal nitrogen concentration on a breath-by-breath basis is related to the cumulative volume of ventilation or breath number. By extending the nitrogen washout time to 30 minutes or more in subjects with severe chronic airway obstruction, estimates of lung volume may be obtained that compare favorably with those calculated by plethysmographic or radiographic methods.107
Exponential analysis of the end-tidal nitrogen concentration with time, cumulative ventilation, or breath number reveals that in normal subjects nitrogen concentration decreases in a single exponential curve. In the presence of uneven distribution of ventilation, the curve can be described by two or more exponentials. This analysis can be extended to estimate the size of poorly ventilated regions of the lung, but these multiple-breath tests are cumbersome and time-consuming, may have no anatomic correlates, and cannot be repeated rapidly (i.e., until all the added oxygen is washed out).
With the advent of rapidly acting infrared analyzers in commercial pulmonary function equipment, in which various filters are used in conjunction with an infrared analyzer to analyze methane, carbon monoxide, and acetylene to measure diffusing capacity and pulmonary blood flow, the opportunity has developed to assess distribution of ventilation using added inert gases, such as methane. The principle is the same as that for resident gases, but the modeling and mathematics are slightly altered. Reference equations have been published for values expected in healthy normal subjects.108, 109
A lung clearance index can be measured not only by resident N2 but also by exogenous tracer gas using a mass spectrometer or, more recently, a novel gas analyzer (Innocor), thus providing a potentially useful clinical tool as an early marker for disease in children and adults.110 With the use of exogenous tracer gases not found in normal air, patients can perform the washout tests while breathing air and not pure oxygen, as is needed for nitrogen washout. By breathing air, the patient avoids the unwanted changes in ventilation related to oxygen breathing. Sulfur hexafluoride, an inert gas, is a tracer used for this purpose.111
Clinical Applications
Tests of distribution of ventilation have been used widely in epidemiologic studies. Studies of cigarette smokers and studies of patients with mild airway obstruction have suggested that the single-breath nitrogen washout test (phase III) often has the most abnormal test results of lung function, and sometimes the only abnormal test results. The sensitivity of these tests of distribution may also prove useful in the field of occupational health for early detection of the effects of occupational hazards, but the practical value of these tests for occupational screening remains to be established.
The usefulness of tests of distribution of ventilation in clinical evaluation is well established. The single-breath nitrogen washout test has abnormal results in both restrictive and obstructive ventilatory defects. Presumably, this reflects its sensitivity to abnormalities in the mechanical properties of the lungs. Even though interstitial pulmonary fibrosis or emphysema may affect the lung diffusely, the process is never distributed homogeneously. Thus some regions of lung fill and empty more slowly than others, resulting in abnormal single-breath nitrogen test results. Why, then, is a test of distribution indicated in clinical evaluations? First, in mild disease, spirometry and clinical evidence may be equivocal, but tests of distribution may provide a more sensitive indicator of the presence of disease and the response to treatment. Second, not only is the test sensitive, but the degree of abnormality of the single-breath nitrogen washout test results is generally proportional to the amount of underlying lung disease. Third, the degree of abnormality of the test results may give an indication of the difficulties to be expected in gas exchange. When the closing volume (phase IV) is elevated above FRC, it is likely to be associated with atelectasis and hypoxia, particularly when narcotics or hypnotic drugs depress the drive to ventilation. Finally, in patients with suspected upper airway obstruction, a test of distribution of ventilation (e.g., single-breath nitrogen washout) may be the only way to assess whether there is associated disease of the airways distal to the carina.
Diffusion
Physiologists have devised a variety of methods to study the diffusion of gases across the alveolar-capillary membranes; many of these methods are useful clinically, and their physiologic foundations are discussed in Chapter 4. The advantages of physiologic tests for measuring diffusing capacity are that they permit diagnosis of an impaired surface area for the transfer of gases from the alveoli to the pulmonary capillaries, sometimes even during early stages of disease. Many pulmonary diseases are manifested by a diffusion defect when there is no abnormality apparent in other routine pulmonary function tests. These diseases include all interstitial lung diseases, asbestosis, scleroderma, lupus erythematosus, emphysema, pulmonary thromboembolism, diffuse metastatic cancer of the lungs,112 Pneumocystis jirovecii pneumonia, and rejection of a transplanted lung. There is now considerable evidence correlating the diffusing capacity and its subdivisions (membrane diffusing capacity [Dm] and pulmonary capillary blood volume [Vc]) with the morphometric study of normal lungs.113 Similar correlative studies of the lungs of patients with emphysema48 document the structural basis for the abnormal alveolar-capillary interface as a result of decreased numbers of patent pulmonary capillary segments.114 Finally, the tests are relatively simple (as far as the patient is concerned) and easy to repeat, making it practical to study the diffusing capacity frequently and to evaluate the effects of therapy or the natural history of the disease.
Measurements of Pulmonary Diffusing Capacity (Transfer Factor)
General Principles
The measurement of pulmonary diffusing capacity (also known as transfer factor) requires the use of a gas that is more soluble in blood than in lung tissues. Oxygen and carbon monoxide are the only two such gases known, and their chemical reaction with hemoglobin is responsible for this unusual pattern of “solubility.” Both molecules measure the same process, and estimates of Dl O2 can be made by multiplying the Dl CO by 1.23. However, the more difficult and time-consuming method of measurement by oxygen has been largely displaced by the carbon monoxide method.
For the standard Dl CO method, a low concentration of carbon monoxide is delivered to the lungs by adding about 0.3% carbon monoxide to inspired air. The mixed venous carbon monoxide concentration is assumed to be zero for all practical purposes (unless the test is repeated frequently over a short time). Molecules of carbon monoxide diffuse across the membrane, dissolve in the plasma, and then combine with hemoglobin. Carbon monoxide has a high affinity for hemoglobin, 210 times that of oxygen; thus carbon monoxide in the vicinity of a hemoglobin molecule binds avidly to it, and the partial pressure of dissolved carbon monoxide remains very low. Except in a patient with severe anemia, the available binding sites for carbon monoxide are so numerous that they cannot be saturated by the number of carbon monoxide molecules that diffuse from the air spaces to the capillary blood at the low concentrations of carbon monoxide used in the test. Therefore carbon monoxide transfer is not limited by pulmonary blood flow; instead, it is limited primarily by the alveolar-capillary diffusion rate and, to a lesser extent, by the red blood cell membrane diffusion rate and the chemical reaction rate between hemoglobin and carbon monoxide. The carbon monoxide transfer therefore can be considered a measure of the capillary surface area available for gas exchange.
In contrast, gases such as Freon, nitrous oxide, and acetylene are equally soluble in lung tissues and blood because they do not combine chemically with blood components. These gases diffuse across the alveolar-capillary membranes and quickly saturate the plasma; further diffusion is prevented until fresh blood enters the pulmonary capillaries. Thus these gases can be used to estimate pulmonary capillary blood flow to ventilated lung units.
There are some important differences in the transfer of carbon monoxide and oxygen. Both plasma and hemoglobin contain oxygen (but not carbon monoxide) when mixed venous blood enters the pulmonary capillaries. The rate of oxygen diffusion into blood depends on the alveolar-capillary Po 2 difference. As oxygen crosses the alveolar-capillary membranes, capillary Po 2 increases, narrows the alveolar-capillary Po 2 difference, and slows diffusion. Thus, before calculating a diffusing capacity for oxygen, blood Po 2 must be known at every point along the capillary and can be obtained by a combination of certain measurements and mathematical computations.115
Carbon Monoxide Methods for Clinical Measurement of Pulmonary Diffusing Capacity.
The Dl CO is calculated as follows:

To determine the amount of carbon monoxide transferred from alveolar gas to blood per minute, it is necessary to measure the mean alveolar carbon monoxide pressure and the mean pulmonary capillary carbon monoxide pressure. There are several tests available.
The standard single-breath Dl CO test is probably the most widely used and the best standardized of the various methods described. It has been used in the largest number of normal subjects and has been corrected for the effects of age, body size, sex, ethnic background, cigarette smoking, and physiologic factors.
The intrabreath method requires a special, very rapid infrared analyzer, but this is also commercially available. Because this method does not require a breath-hold and expiratory flow can be controlled by a critical orifice, the intrabreath method is probably the easiest of the four methods for sick patients to perform. With proper filters the same analyzer can be used to measure methane, acetylene, and carbon monoxide simultaneously. Diffusing capacity can be measured during exercise to define distensibility of the capillary bed using the intrabreath or three-gas iteration method, but this also requires extensive validation and establishment of predicted normal values.116, 117, 118
The three-gas iteration method may be more reproducible and is unaffected by a wide variety of factors that alter the single-breath or intrabreath methods, especially abnormalities in distribution of ventilation. More normal data are needed, as is validation in other laboratories, but the method is commercially available.
The steady-state, or rebreathing method, can also be measured during exercise, but is not widely used because its results are markedly affected by uneven distribution of ventilation or ventilation-perfusion abnormalities. The rebreathing method is more variable than the single-breath method and requires considerable patient cooperation to attain the rapid respiratory rate required.
Single-Breath Method.
In the single-breath method, the patient inhales a gas mixture containing 0.3% carbon monoxide and a low concentration of inert gas (0.3% neon, 0.3% methane, or 10% helium), then holds his or her breath for approximately 10 seconds. During the breath-hold, carbon monoxide leaves the air spaces and enters the blood. The larger the diffusing capacity, the greater the amount of carbon monoxide that enters the blood in 10 seconds.
The equation used in the single-breath method is as follows:
where Faco t is fractional alveolar carbon monoxide concentration at time (t), t is breath-hold time in seconds, Pbar is barometric pressure (in mm Hg), Va is alveolar volume (in mL) obtained from the ratio of inspired and expired inert gas concentrations and inspired volume, Faco 0 is the inspired carbon monoxide concentration corrected for dilution by the RV as estimated by the ratio of inspired and expired inert gas concentrations, and 60 is the conversion factor for seconds to minutes.
In the single-breath test, alveolar Pco is not maintained at a constant concentration, because carbon monoxide is absorbed during the period of breath-holding. Furthermore, the mean alveolar Pco is not the average of the Pco at the beginning and end of the breath-holding period. However, the mean alveolar Pco can be estimated and diffusing capacity measured. The single-breath test requires little time or cooperation from the patient except to inhale and to hold the breath for 10 seconds. Analyses are performed with an infrared analyzer or gas chromatograph, and no blood samples are needed. The test can be repeated a number of times rapidly, if desired. However, a measurement of the patient's RV is required, because a value for total alveolar volume during breath-holding must be calculated to measure carbon monoxide uptake. Furthermore, an inert gas such as helium, methane, or neon must be inhaled with carbon monoxide to correct for dilution of inspired carbon monoxide. This method has the disadvantages that carbon monoxide is a nonphysiologic gas, breath-holding is not a normal pattern of breathing, and breath-holding for 10 seconds may not be possible for patients with severe dyspnea or during exercise.
Factors such as inhalation time, breath-holding time, breath-holding lung volume, exhalation time, and the size and portion of alveolar gas sampled have all been shown to affect the single-breath Dl CO. Ogilvie and colleagues119 recognized that these discrepancies could exist either because diffusing capacity is not distributed homogeneously within the lung or because the single-breath equation ignores the fact that carbon monoxide uptake takes place during inhalation and exhalation as well as during breath-holding. They tried to circumvent these problems by standardizing the test.
Jones and Mead120 showed that, because the diffusion equation was valid only for breath-holding, there are errors in calculation of single-breath Dl CO owing to the nature of carbon monoxide uptake during inhalation and exhalation. Because delay in collection of the alveolar sample has been shown to cause an apparent increase in Dl CO, the ATS epidemiology standardization project23 developed a variation of the Jones and Mead method that took this problem into account and placed strong emphasis on an automated system, which standardized the procedure and is available in most modern commercial systems.
Diffusing Capacity
Steady-State Method.
In the steady-state method, the patient breathes a mixture of 0.1% carbon monoxide in air for several minutes through a one-way valve system. During the last 2 minutes, exhaled gas is collected in a plastic bag and analyzed for oxygen, carbon dioxide, and carbon monoxide concentrations (requiring rapidly responding gas analyzers). During collection, an arterial blood sample is drawn and analyzed for Pco
2.121 The amount of carbon monoxide transferred from the air spaces to capillary blood per minute (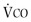 ) can be calculated from the inspired and expired gas concentrations and the volume of gas exhaled (
) can be calculated from the inspired and expired gas concentrations and the volume of gas exhaled (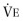 ) at standard conditions (i.e., STPD, where the “ideal gas” is corrected to standard pressure dry [760 − 47 mm Hg] and 0° C or 273° K, where 1 mole of ideal gas occupies 22.4 L.). The mean alveolar fractional concentration of carbon monoxide (Faco) is estimated from the Bohr equation for dead space volume divided by tidal volume (Vd/Vt). Assuming that Vd/Vt for carbon dioxide and carbon monoxide are the same, Faco can be calculated with the following equation, using the fractional concentrations of mixed expired carbon monoxide (Feco), mixed expired carbon dioxide (Feco
2), alveolar carbon dioxide (Faco
2), and inspired carbon monoxide (Fico):
) at standard conditions (i.e., STPD, where the “ideal gas” is corrected to standard pressure dry [760 − 47 mm Hg] and 0° C or 273° K, where 1 mole of ideal gas occupies 22.4 L.). The mean alveolar fractional concentration of carbon monoxide (Faco) is estimated from the Bohr equation for dead space volume divided by tidal volume (Vd/Vt). Assuming that Vd/Vt for carbon dioxide and carbon monoxide are the same, Faco can be calculated with the following equation, using the fractional concentrations of mixed expired carbon monoxide (Feco), mixed expired carbon dioxide (Feco
2), alveolar carbon dioxide (Faco
2), and inspired carbon monoxide (Fico):
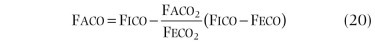
This value (alveolar carbon monoxide [Paco]) can now be used to calculate pulmonary diffusing capacity:Steady-state Dl
CO can be measured during tidal breathing, anesthesia, sleep, and exercise. Nevertheless, the method is not used widely because its results are more subject to error than those of the single-breath technique, especially in patients with uneven distribution of ventilation or with ventilation-perfusion abnormalities. In these conditions, a decreased Dl
CO (steady state) may reflect impairment of the ventilation-perfusion ratio 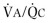 , where
, where 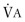 is alveolar ventilation and
is alveolar ventilation and 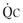 is pulmonary capillary blood flow, or an alteration of pulmonary gas transfer. A major problem is the estimation of alveolar carbon monoxide concentration. In addition, the method requires obtaining an arterial blood sample and is extremely sensitive to changes in breathing pattern. The “end-tidal” modification112 of the steady-state method does not require arterial blood samples but suffers from even more sources of error. Both approaches to the estimation of diffusing capacity really reflect ventilation-perfusion abnormalities in the lungs more than characteristics of the alveolar-capillary surface and functioning pulmonary capillaries. Marshall122 has reported another modification of this method, in which mixed venous Pco
2 was computed by an equilibration method that avoids arterial puncture but may be too imprecise for use in the Filley equations (Equations 21 and 22) at rest.
is pulmonary capillary blood flow, or an alteration of pulmonary gas transfer. A major problem is the estimation of alveolar carbon monoxide concentration. In addition, the method requires obtaining an arterial blood sample and is extremely sensitive to changes in breathing pattern. The “end-tidal” modification112 of the steady-state method does not require arterial blood samples but suffers from even more sources of error. Both approaches to the estimation of diffusing capacity really reflect ventilation-perfusion abnormalities in the lungs more than characteristics of the alveolar-capillary surface and functioning pulmonary capillaries. Marshall122 has reported another modification of this method, in which mixed venous Pco
2 was computed by an equilibration method that avoids arterial puncture but may be too imprecise for use in the Filley equations (Equations 21 and 22) at rest.
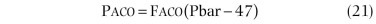
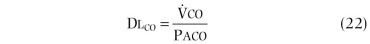
Rebreathing Method.
In the rebreathing method,123 the patient rebreathes the test gas (air plus a low concentration of carbon monoxide) from a reservoir, the volume of which equals the patient's FEV1. The patient exhales to RV before a valve is turned, then rebreathes from the reservoir, which should be emptied with each inspiration. Rebreathing continues for 30 to 45 seconds at a controlled rate of 30 breaths/min (to ensure mixing between lung and reservoir). The volume of RV plus reservoir, multiplied by the change in carbon monoxide concentration, equals the carbon monoxide volume transferred. Mean capillary Pco is neglected. Mean alveolar Pco is calculated from the same equation used in the single-breath method. The rebreathing method for measuring Dl CO is more variable and requires considerable patient cooperation, but it is less influenced by abnormalities in distribution of ventilation and is easier to use during exercise than the steady-state or single-breath technique. The rapid respiratory rate may be difficult for some patients to maintain and is not physiologic.
Three-Gas Iteration Method.
Graham and associates124 have described a method of calculating Dl CO that uses separate equations for the inhalation, breath-hold, and exhalation phases of the single-breath maneuver rather than trying to force them to fit the breath-hold equation. The method is now available commercially using a rapidly responding infrared analyzer. This method has been reported in a theoretical paper,125 analyzed in a lung model, and compared with the standard Ogilvie method,119 the Jones-Mead modification,120 and the ATS epidemiology standardization modification of the Ogilvie method.23 It has also been reported during exercise.116, 117, 118
Although Dl CO values calculated using the three conventional methods showed large changes with variations in inspiratory flow rates, inspiratory volumes, and collection times, the three-equation method yielded calculations of Dl CO that were minimally affected by these changes.124 These results agree with previous results obtained in the lung model, support the hypothesis that diffusing capacity is independent of lung volume, and indicate that the three-gas iteration method significantly improves the accuracy and precision of single-breath measurements.126
Intrabreath Method.
In the intrabreath (within-breath or exhaled) Dl CO method, Dl CO is measured at increments of the exhaled volume using a method devised by Newth and associates127and modified by Hallenborg and colleagues.128
Protocol.
As originally described, the subject performs two VC maneuvers, then exhales to RV and rapidly inhales a mixture containing 0.3% carbon monoxide, 15% helium, 21% oxygen, and the remainder nitrogen from a bag-in-box. After a 3-second breath-hold, the subject exhales while watching a flow signal to maintain a constant flow of 0.5 L/sec. Carbon monoxide concentrations are measured continuously with a rapidly responding infrared meter, and helium concentrations are measured with a mass spectrometer. Airflow is measured with a pneumotachygraph and integrated electrically to obtain volume. Data are converted from analog to digital form at 30 points per second and recorded by a digital computer to adjust carbon monoxide and helium recordings for time lags and to remove cardiac oscillations using a sliding, 30-point curve-averaging technique. The exhaled VC is divided into 2% decrements, and the corresponding exhaled carbon monoxide and helium values are used for calculations. The initial part of the VC, until the onset of phase III of the helium curve, is discarded as dead space. The lower portion of the VC, after the onset of phase IV of the helium curve, is also discarded because of uncertainties regarding contributions by dependent regions to expired gas concentrations. Approximately 40 data points are obtained over the lower 80% of the exhaled VC, and the rate of carbon monoxide uptake is calculated over 10% intervals of the exhaled VC (e.g., 20% to 30%, 22% to 32%). Alveolar volume is calculated at the midpoint of each 10% interval by subtracting the exhaled volume from TLC (measured by the single-breath helium dilution method). Intrabreath Dl CO is calculated in each interval by the Krogh equation129 and plotted against exhaled volume.
Although more complicated than the standard single-breath method, this technique makes no assumptions about the initiation of carbon monoxide uptake or the volume at which carbon monoxide uptake manifested, measures carbon monoxide uptake directly during the entire maneuver, and can be used during exercise as well as at rest. The method does require a rapidly responding carbon monoxide meter or special modification of a mass spectrometer (to measure C18O) to make the number of measurements of carbon monoxide concentration required during the single exhalation. The intrabreath method has been useful in detecting pulmonary hemorrhage128 and pulmonary vascular obstruction.130 The method is now available commercially using a rapidly responding infrared analyzer. With appropriate filters, the device can measure not only carbon monoxide but also methane (to measure tracer gas dilution) and acetylene (to measure pulmonary capillary blood flow to ventilated lung units), and the response to all three gases is linear.109, 131, 132, 133
Indications
The diffusing capacity is affected by those physiologic and pathophysiologic conditions that change the surface area of pulmonary capillaries available for gas exchange. For most purposes, one can think of Dl CO as a measure of the blood-filled capillaries in the lung.
It is interesting to consider the conditions that can increase the Dl CO. Indeed, many processes can increase the capillary blood surface area by increasing the blood volume in the lung, including recumbent posture, pregnancy, and obesity. Many pathologic processes that do not involve lung disease can also increase blood volume in the lung, such as congestive heart failure. Lung diseases that can increase blood volume are thought to include asthma, perhaps by increased intrathoracic negative pressures, and diffuse alveolar hemorrhage.
Most pathologic processes that involve the pulmonary capillaries decrease the Dl CO. Therefore the most common clinical indications for measuring Dl CO include evaluation of patients with diffuse interstitial lesions such as sarcoidosis and asbestosis,134 evaluation of patients suspected of having emphysema, for which several structure-function studies are now available,48 and assessment of patients with pulmonary vascular obstruction.135 It is important to recognize that the Dl CO depends on hemoglobin concentration; decreased Dl CO caused by severe anemia must not be misinterpreted as secondary to nonexistent lung disease. Thus Dl CO is routinely corrected for hemoglobin concentration, if known.
Standardization of the Single-Breath Methods
The ATS has recommended standardization of the test using criteria including rapid inspiration, inspired volume at least 90% of largest VC, breath-hold time between 9 and 11 seconds, and adequate washout and sample volumes. The mean of the acceptable tests is reported; if more than two tests are performed, the mean of all acceptable tests is reported. Calculations are standardized for breath-hold time and adjusted for dead space, gas collection conditions, and carbon dioxide concentration. Reproducibility of the two acceptable tests should be within 10% or 3 mL/min per mm Hg (at standard temperature, pressure, and dry [conditions] [STPD]), whichever is larger. When the ratio of Dl CO to alveolar volume (Dl CO/Va) is reported, Dl CO is at STPD and Va is at BTPS.
Interpretation
Interpretation of Diffusing Capacity
Hemoglobin.
Adjustment for hemoglobin is not mandatory but is desirable. Unadjusted values must always be reported even if the adjusted values are also reported. The adjustment should be made on the observed, not the predicted, value. Hemoglobin is reported in grams per deciliter, and the method of Cotes and associates136 should be used to make the adjustment:

where Dm is the membrane diffusing capacity and Vc is the pulmonary capillary blood volume.
Carboxyhemoglobin.
Heavy smokers may have as much as 10% to 12% carboxyhemoglobin in their blood, and therefore the back-pressure of carbon monoxide in mixed venous blood entering the pulmonary capillaries cannot be assumed to be zero in such individuals. The steady-state method is more sensitive to errors caused by this problem than the single-breath technique. Carbon monoxide back-pressure may be estimated, and Dl CO calculations may be corrected for back-pressure of carbon monoxide using the Haldane equation.136, 137 Alternatively, carboxyhemoglobin may be measured directly.138 In either case, the measured Dl CO is adjusted, and both the unadjusted and the adjusted values are reported. Dl CO measurements should not be performed on patients who have been breathing oxygen-enriched mixtures immediately before the test; at least 20 minutes of breathing room air should be allowed before measurement of Dl CO.
Graham and associates demonstrated that carbon monoxide back-pressure has a more complex effect than suggested in the ATS standardization statement. To adjust properly for the effect of carbon monoxide on the diffusing capacity, not only must the direct effect of carbon monoxide back-pressure build-up be corrected, but also the indirect anemia effect of increasing carboxyhemoglobin.139 The original ATS statement on this issue should be updated to account for both necessary adjustments.
Altitude.
As altitude increases and fractional concentration of inspired oxygen (Fio 2) remains constant, pressure of inspired oxygen (Pio 2) decreases and Dl CO increases approximately 0.35% for every 1 mm Hg decrease in alveolar Po 2:
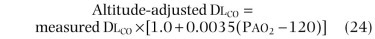
If alveolar Po 2 is not available, adjustments may be made for interpretative purposes, assuming a mean Pio 2 of 150 mm Hg at sea level:
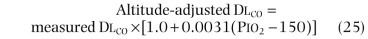
assuming Pio 2 = 0.21(Pbar − 47).
Normal Values for Pulmonary Diffusing Capacity
Body Size.
Body size is one of the factors that probably affect normal values6; diffusing capacity has been found to vary with body surface area (BSA, in square meters) according to the following equation, derived by Ogilvie and colleagues119:
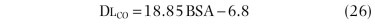
A better prediction140 is based on height (H, in centimeters) and age (A, in years):
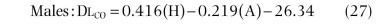
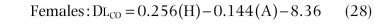
Values for Dl CO measured by analyzing the gases with a chromatograph are approximately 6% higher than values obtained with infrared meters.141
Age.
Maximal Dl O2 (i.e., Dl O2 during maximal exercise) has been found to decrease with increasing age according to the following equation:
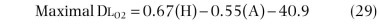
Age also affects Dl CO at rest (see previous discussion).
Lung Volume.
Diffusing capacity determined by the single-breath technique measured between FRC and TLC is relatively independent of lung volume in the same individual, although the diffusing capacity varies with lung volume among individuals, reflecting differences in alveolar-capillary surface with lung volume.48
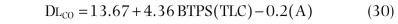
Exercise.
Exercise raises Dl CO and Dl O2 by enlarging the surface area of functioning alveoli in contact with pulmonary capillaries (eFig. 25-10). This is due primarily to recruitment of capillaries. Exercise causes an approximate doubling of pulmonary diffusing capacity and pulmonary capillary blood volume.142, 143 Huang and coworkers109 studied 105 healthy subjects with the intrabreath method and reported reference equations for diffusing capacity at rest and during exercise based on age, sex, and height. Hsia and associates143 found that Dl CO increased normally as cardiac output increased during exercise in patients who had undergone pneumonectomy compared with normal subjects. Although an upper limit to Dl CO with respect to oxygen uptake during exercise was observed by Stokes and colleagues,144 using the intrabreath carbon monoxide method, our group and Hsia et al found no upper limit to Dl CO in normal subjects or in pneumonectomy patients with respect to cardiac output during exercise.116, 145 Maximal values attained during exercise in patients who had undergone pneumonectomy were less than those attained by normal controls, as might be expected because the patients had been chronic smokers and probably had emphysema in the remaining lung.
eFigure 25-10.
Face-on views of freeze-dried, stained alveolar walls (200-µm-thick section) showing the distribution of the pulmonary capillary bed in anesthetized cats.
A, Zone I lobe. B, Zone II lobe. C, Zone III lobe. Morphometric analysis showed that 21% of the alveolar walls were occupied by red blood cells in zone I lobes, 43% in zone II lobes, and 61% in zone III lobes. Independent changes in pulmonary arterial or venous pressure were associated with changes in the pulmonary capillary blood volume over a threefold range.
(From Vriem CE, Staub NC: Pulmonary vascular pressures and capillary blood volume changes in anesthetized cats. J Appl Physiol 36:275–279, 1974.)
As reviewed by Ceretelli and DiPrampero,146 whether Dl
CO reaches a true plateau during exercise appears to depend on the method used and the level of exercise attained by the subjects. Kinker and associates147 used a modified steady-state method to determine breath-by-breath Dl
CO during exercise. They found that the rise of Dl
CO with increasing work was attenuated at high levels of exercise (maximal oxygen uptake, or  , approximately 4 L/min) in most subjects, suggesting that alveolar-capillary surface area tends to approach a maximum.147 These findings are consistent with studies of capillary perfusion patterns in single alveolar walls visualized through a transparent thoracic window implanted in anesthetized dogs.148 Results of this study agreed with a computer model of capillary flow developed by West and associates.149
, approximately 4 L/min) in most subjects, suggesting that alveolar-capillary surface area tends to approach a maximum.147 These findings are consistent with studies of capillary perfusion patterns in single alveolar walls visualized through a transparent thoracic window implanted in anesthetized dogs.148 Results of this study agreed with a computer model of capillary flow developed by West and associates.149
Body Position.
Dl CO is 15% to 20% greater in the supine than in the sitting position and 10% to 15% greater in the sitting than in the standing position, because of the effects of changes in posture on pulmonary capillary blood volume.
Alveolar Oxygen Pressure.
Alveolar Po 2 affects Dl CO because of the former's effect on the carbon monoxide reaction with hemoglobin. For example, diffusing capacity values measured at alveolar Po 2 values of 40 and 600 mm Hg are approximately 45 and 18 mL/min per mm Hg, respectively (see Fig. 25-11). Changes in Dl CO caused by variations in alveolar Po 2 in the physiologic range are much smaller. In patients with severe hypoxia (arterial Po 2 < 40 mm Hg), increased pulmonary blood flow and dilation of the pulmonary capillaries may increase the Dl CO. Hypoxia may also increase Dl CO as a result of its effect on the reaction rate between carbon monoxide and hemoglobin.
Subdivisions of the Total Diffusing Capacity.
It is possible to separate the pulmonary diffusing capacity into its two components: the membrane diffusing capacity (Dm) and the component related to the pulmonary capillaries (Vc). Nonetheless, it has been shown that almost all decreases in diffusing capacity are due to decreases in the capillary component (Vc).
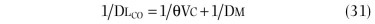
The method of separation depends on measurement of Dl CO at different alveolar oxygen pressures. When alveolar oxygen is increased by breathing enriched oxygen mixtures, oxygen molecules compete with carbon monoxide molecules for reactive sites on hemoglobin, thereby decreasing carbon monoxide uptake by the red blood cells. However, even at the higher oxygen concentration, transfer of carbon monoxide across the alveolar-capillary membranes is presumed to be unaffected. Thus measurements of Dl CO at 21% oxygen and at several higher concentrations of oxygen permit separation of the two components of the Dl CO (eFig. 25-11).150
eFigure 25-11.
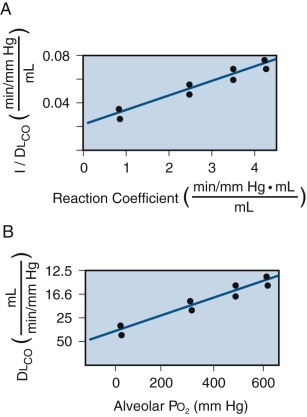
Subdivisions of the Dlco.
Experimental values of the diffusing capacity for carbon monoxide (DlCO) obtained at different alveolar Po2 values (x-axis in B) can be analyzed mathematically to obtain the subdivisions of total DlCO: the diffusing capacity of the membrane (Dm) and pulmonary capillary blood volume (Vc). As the alveolar Po2 was increased from 40 mm Hg to 600 mm Hg, the duplicate measurements of DlCO decreased from approximately 45 to 15 mL/min per mm Hg. Changing alveolar Po2 changes the reaction coefficient (θ), reflecting the change in hemoglobin affinity for carbon monoxide. The reaction coefficient is plotted against 1/DlCO in A. There is a linear relationship between 1/DlCO and 1/θ such that 1/DlCO = 1/θVc + 1/Dm. Under these conditions, Dm is derived from the value of the y-intercept and Vc from the slope of the line.
Diffusing Capacity for Nitric Oxide
The diffusing capacity of the lung for nitric oxide (Dl NO) is a relatively new pulmonary function test and similar in many ways to the more established Dl CO.151 It differs from the latter in being independent of Po2 and the hematocrit. It has been suggested that Dl NO can be used to describe pulmonary Dm directly.151a
Diffusion properties of nitric oxide are similar to those of carbon monoxide; however, its rate of reaction with red blood cells is much greater.152 Dl NO primarily reflects Dm, whereas Dl CO depends on both Dm and Vc.151, 152 In combination with Dl CO, Vc and Dm can be determined in a single maneuver, based on the equation for the serial connection of resistances.153 Using the combined Dl NO -Dl CO method, patients with COPD,154 pulmonary arterial hypertension,155 and parenchymal lung diseases156, 157 have been studied, as well as the effects of exercise.152, 153, 158 In addition, reference values have been published.159 The clinical utility of the Dl NO -Dl CO method remains to be determined by studies of healthy subjects under various experimental conditions and by studies of diverse patients by various laboratories.
Clinical Applications
The single-breath Dl CO can be used to differentiate airflow obstruction associated with intrinsic airway disorders from obstruction related to emphysema. A normal single-breath diffusing capacity in the setting of an obstructive pattern argues against the presence of emphysema.48 In fact, a normal or increased single-breath Dl CO associated with airflow obstruction is often associated with asthma.160 The single-breath Dl CO may be abnormal in patients with emphysema when there is no evidence of airflow obstruction, and it may become progressively more abnormal much more rapidly than tests of airway function, even when they do become abnormal.161 Several studies have demonstrated a correlation not only with the presence of emphysema but also with the amount of emphysema.134, 162, 163, 164, 165
Dl CO has also been used to study the earliest stages of emphysema. For example, some studies suggest that alveolar septal destruction may be seen in cigarette smokers before the development of either increased air space size or anatomic evidence of emphysema.166 In our laboratory, single-breath Dl CO was found to correlate with emphysema grade by panel grading167 from grade 1 to 100 (r = −0.73) in 50 patients whose lungs were studied at surgical resection, which was performed within 1 week of their pulmonary function tests (eFig. 25-12). However, for the milder, early emphysema at grade 30 or less, the intrabreath Dl CO appeared to be more sensitive and specific than the single-breath Dl CO.127
eFigure 25-12.
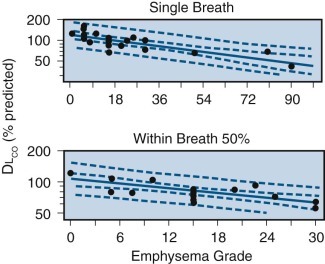
Correlation between diffusing capacity for carbon monoxide (DlCO) and emphysema grade.
Top, The single-breath DlCO, expressed as the log of the percentage of predicted normal, is displayed on the ordinate. Emphysema grade is displayed on the abscissa and was determined by the panel grading method on lung tissue resected from these patients within 1 week of pulmonary function testing. The solid line is the line of best fit (r = −0.73), the outer dashed lines show the 95% confidence limits for the points, and the inner dashed lines show the 95% confidence limits for the line. The single-breath DlCO correlates with the presence and amount of emphysema except when emphysema is mild (grades 0 to 30); when single-breath DlCO was plotted against emphysema for patients with minimal disease (grades 0 to 30), there was no significant correlation. Bottom, The intrabreath DlCO, expressed as the log of the percentage of predicted normal at 50% exhaled vital capacity, is displayed on the ordinate. Emphysema grade is displayed on the abscissa and was determined by the panel grading method on lung tissue resected from these patients within 1 week of pulmonary function testing. The solid line is the line of best fit (r = −0.77), the outer dashed lines show the 95% confidence limits for the points, and the inner dashed lines show the 95% confidence limits for the line. Thus the intrabreath DlCO correlates with the presence and amount of emphysema even when emphysema is mild (grades 0 to 30) and cannot be detected or quantified by the single-breath method.
Pulmonary Vascular Obstruction
The changes in Dl CO in the setting of pulmonary vascular obstruction can be complex, making it difficult to make a straightforward diagnosis. If pulmonary capillaries are occluded, single-breath Dl CO is decreased.114 In the presence of precapillary vascular obstruction, with the obstruction upstream of the capillaries, the single-breath Dl CO may be decreased,168 normal,169 or even increased, depending on the effect on pulmonary capillary blood volume. The capillary blood volume in turn depends on the relationship between pulmonary arterial pressure, pulmonary venous pressure (or left atrial pressure), and bronchial collateral blood flow. For example, bronchial arterial pressure may distend capillaries via collateral channels so that, even if pulmonary arteries are obstructed, a normal Dl CO may be maintained. In our laboratory, every patient with pulmonary vascular obstruction who had decreased single-breath Dl CO had a decreased pulmonary capillary blood volume.
Finally, capillary distention may vary in different parts of the lung (Fig. 25-16 ). According to the model of the zones of lung perfusion presented by West and colleagues,170 at the lower zone at the lung base, capillaries are distended by pulmonary arterial and pulmonary venous pressures. Even if the pulmonary arteries are obstructed, the capillaries are distended by pulmonary venous pressure and the Dl CO is maintained. Dl CO is decreased in this zone if the capillaries are occluded or if pulmonary venous pressure is decreased.
Figure 25-16.
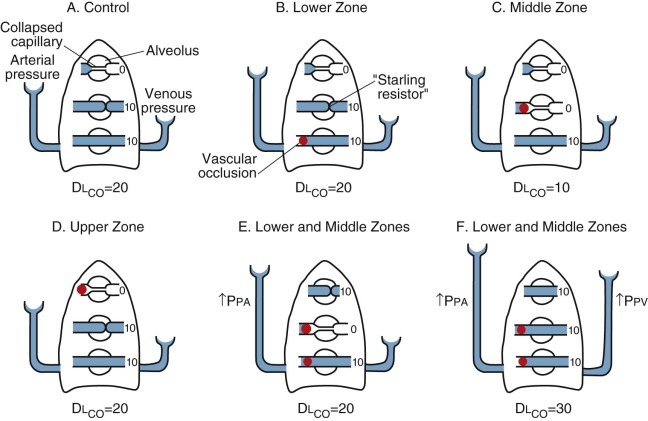
Theoretical model showing the effect of pulmonary arterial pressure (Ppa) and pulmonary venous pressure (Ppv) on pulmonary capillaries at different levels of the lungs.
The magnitude of Ppa or Ppv is indicated by the height of the fluid columns. For simplicity, the pressure in alveoli (Palv) is assumed to be equal to atmospheric pressure. Single-breath carbon monoxide diffusing capacity (DlCO) is given in arbitrary units indicating the relative contribution of various zones of the lung. A, In the control state, at the bottom of the lung, both Ppa and Ppv are greater than Palv, and both keep the capillaries open. In the middle zone, Ppa is greater than Palv and Ppv, so Ppa holds capillaries open. (The exact anatomy of capillaries in the zone in which Palv is greater than Ppv is unknown; in the diagram, the compressed segment at the end of the capillary is meant to suggest a “Starling resistor” effect.) In the upper zone, Palv is greater than Ppa and Ppv, and capillaries are “collapsed.” B, When arterial inflow is occluded to the lower zone (indicated by red solid sphere), Ppv is greater than Palv, so the capillaries in this zone remain distended and DlCO is unchanged. C, When arterial inflow to the middle zone is occluded, Palv is greater than Ppv and capillaries in this area are collapsed, so there is a decrease in DlCO. D, When arterial inflow to the upper zone is occluded, the capillaries are already collapsed, so there is no change in DlCO. E, When arterial inflows to the lower and middle zones are occluded simultaneously, capillaries in the middle zone may collapse. However, if Ppa increases, capillaries in the upper zone may become distended, and the net result may be no change in DlCO. Under these circumstances, if Ppv also increases (F), DlCO may actually increase.
(Modified from Nadel JA, Gold WM, Burgess JH: Early diagnosis of chronic pulmonary vascular obstruction: value of pulmonary function tests. Am J Med 44:16–25, 1968.)
In the middle zone of the lung, capillaries are distended by pulmonary arterial pressure only; they are not affected by pulmonary venous pressure. Dl CO would be decreased by pulmonary vascular obstruction alone; however, Dl CO would be normal if the obstruction led to an increase in pulmonary arterial pressure that then distended apical capillaries that were not perfused previously. In addition, the Dl CO would increase if the pulmonary venous pressure increased, as long as the pulmonary capillaries remained patent despite pulmonary artery occlusion in this zone.
In the upper zone at the lung apex, pulmonary capillaries may be nondistended because Palv exceeds both pulmonary arterial and venous pressures (assuming the lung is ever in this condition). In this situation, obstruction of pulmonary arteries would not affect Dl CO. Changes in Palv would affect the analysis of the test and thus might affect Dl CO.171 In conclusion, a decreased Dl CO may support the diagnosis of pulmonary vascular obstruction, but a normal Dl CO does not rule out this diagnosis.135
Restrictive Ventilatory Defects
Dl CO is reduced in interstitial pulmonary fibrosis and correlates with anatomic findings in resected lung tissue or on high-resolution CT scans. Although Dl CO is reduced in at least half of these patients, the test may be normal in at least one third more who have abnormal responses to exercise and have documented fibrosis by lung biopsy or CT scan.172 Dl CO is often decreased in patients with many other forms of pulmonary restriction. Dl CO (expressed as percentage of predicted normal) best reflects the extent of interstitial fibrosing alveolitis on chest CT scan associated with systemic sclerosis.173 Dl CO (expressed as percentage of predicted normal) also correlates closely with arterial oxygen desaturation during exercise in these patients. Dl CO is usually decreased in patients with asbestos-induced pleural fibrosis, who have a restrictive ventilatory defect without evidence of associated parenchymal abnormalities as documented by chest radiograph, bronchoalveolar lavage, and high-resolution CT scan.174 In interstitial pulmonary fibrosis, Dl CO may better define pulmonary gas exchange impairment than resting arterial Po 2, exercise alveolar-arterial Po 2 ((A-a)Po 2) differences, or 6-minute walk test arterial oxygen saturation.175, 176
When the diffusing capacity is reduced in patients with interstitial lung diseases, it is usually decreased out of proportion to the lung volumes; thus the 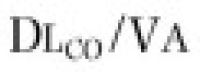 ratio is also decreased. However, this may not be the case in all patients with a restrictive defect. A patient with sarcoidosis, for example, may present with a TLC 50% of predicted normal, associated with a Dl
CO that is also 50% of predicted normal, in which case the
ratio is also decreased. However, this may not be the case in all patients with a restrictive defect. A patient with sarcoidosis, for example, may present with a TLC 50% of predicted normal, associated with a Dl
CO that is also 50% of predicted normal, in which case the 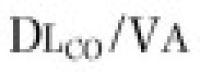 ratio is normal. Following treatment with systemic corticosteroids, the lung volume may return to normal, but diffusion may not, in which case the Dl
CO and
ratio is normal. Following treatment with systemic corticosteroids, the lung volume may return to normal, but diffusion may not, in which case the Dl
CO and 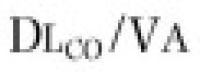 ratio may both be 50% of the predicted normal. In such cases, it is thought that the granulomas and fibrosis cause lasting damage to the alveolar membranes and capillaries, even though the lung volumes return to normal levels. Therefore clinical interpretation of the Dl
CO corrected for alveolar volume in patients with interstitial lung diseases is limited. It should not be assumed that a normal
ratio may both be 50% of the predicted normal. In such cases, it is thought that the granulomas and fibrosis cause lasting damage to the alveolar membranes and capillaries, even though the lung volumes return to normal levels. Therefore clinical interpretation of the Dl
CO corrected for alveolar volume in patients with interstitial lung diseases is limited. It should not be assumed that a normal 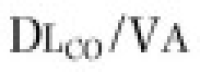 ratio indicates that the capillary beds are functioning normally.
ratio indicates that the capillary beds are functioning normally.
Rejection of Transplanted Lungs
Lung transplantation provides special challenges for physiologic evaluation. Dl CO is reported to be decreased abnormally in most patients with single-lung, double-lung, or heart and lung transplants. Great emphasis has been placed on the importance of detection of bronchiolitis obliterans in these patients as a potentially reversible manifestation of rejection that is lethal if treated inadequately or too late.177 Given the frequency of diffusion defects in patients with lung transplants, and given that rejection is mediated via the vascular bed, it is surprising that little emphasis has been placed on the potential value of serial evaluation of Dl CO to detect rejection early.178, 179
A major limitation to the use of simple lung function monitoring in single-lung transplant patients is the bias caused by the contribution of the native lung. Ikonen and associates used relative ventilation, perfusion, and ventilation-perfusion ratio of the transplanted lung, as determined with multidetector xenon-133 (133Xe) radiospirometry, to assess function of the graft specifically. Fractions of FEV1, FVC, and single-breath Dl CO were also determined using corresponding radiospirometric parameters to calculate their distribution between the lungs. This approach may have potential for distinguishing between acute rejection and infection.178
Regulation of Ventilation
Measurements of Regulation of Ventilation
Ventilatory regulation may be assessed by measuring the ventilator response to hypoxia or hypercapnia or by measuring the overall respiratory drive. The response to hypoxia and hypercapnia has been assessed using rebreathing methods, which are less time-consuming and tiring than classic steady-state methods. In one rebreathing method described by Severinghaus and associates,180 rapid step changes in the patient's Po 2 while Pco 2 is stabilized offer the advantage of a brief stable period of hypoxia. Respiratory drive can be assessed by measuring the inspiratory occlusion pressure at 100 msec (0.1 second), which is thought to reflect the entire neural output of the respiratory center. It is not influenced by conscious muscle effort and is less influenced by abnormal mechanical properties of the respiratory system than is measurement of ventilation. Other methods, including electromyographic measurements of the diaphragm, the measurement of isometric inspiratory loads, and the use of drugs that stimulate the carotid body, have not been used often enough to establish their clinical utility.181
The subject should be prepared for these tests according to the recommendations of the ATS Workshop on Assessment of Respiratory Control in Humans.182 To minimize distractions, the subject should be positioned so as to be screened from the meters, monitors, and manipulators; preferably, the subject's eyes should be closed during the test procedure.
The ventilatory responses to hypoxia and hypercapnia vary considerably even in normal individuals. To prevent extraneous influences from further increasing this variability, the following recommendations are made: (1) studies should be performed in the fasting state with the bladder empty, (2) the subject should be comfortable and should rest for at least 30 minutes before the test, (3) the room should be quiet, (4) body temperature should be determined, (5) tests may be performed in either the sitting or semisupine position, (6) consideration should be given to preliminary evidence suggesting that normal subjects may have greater hypoxic responses when using a nose clip and mouthpiece than when using a mask, and (7) tests should be performed in duplicate with at least 10 minutes of rest between tests.
Ventilatory responses to hypoxia and hypercapnia are potentially hazardous. The clinical condition of the patient should be considered when evaluating the potential hazardous effects of the test procedure, and the usual precautions for safety of the patient used in any stress test should be taken.
Breath-Holding Time
With nose clips in place, the subject exhales to RV, inhales to TLC, and holds his or her breath as long as comfortably possible. Analyses of expired gases can be made to estimate end-tidal carbon dioxide concentrations. Breath-holding time equals the average time in seconds from the end of inspiration to TLC until the first expiration. The test is repeated until the breath-holding times or end-tidal carbon dioxide concentrations are reproducible. The mean value for predicted breath-holding at TLC is 78 seconds.183 In six normal subjects studied by Davidson and colleagues,184 reproducibility of the test at TLC was 75 ± 3 seconds at sea level (mean ± standard error [SE]); subjects were trained until the expired end-tidal carbon dioxide was reproducible within 2 mm Hg.
Hypercapnic Response
As suggested by Read,185 a reservoir bag is filled with a volume equal to the subject's VC plus 1 L with a mixture containing 7% carbon dioxide and 93% oxygen. The subject breathes room air and exhales into the room. Expired flow and end-tidal carbon dioxide are recorded. After a period to establish a stable baseline, valves are turned so the subject breathes in and out of the reservoir bag. The test is continued until the subject stops because of dyspnea, until the end-tidal Pco 2 equals 9%, or until 4 minutes have elapsed.
The ventilation in liters per minute (BTPS), either breath-by-breath or averaged over 5 to 10 breaths, is plotted on the ordinate, and the mean end-tidal carbon dioxide (in mm Hg) is plotted on the abscissa for the same periods (eFig. 25-13). The slope change in  (
( in end-tidal Pco
2) is determined for all of the periods, preferably by linear least-squares regression analysis, eliminating the first 30 seconds of rebreathing.
in end-tidal Pco
2) is determined for all of the periods, preferably by linear least-squares regression analysis, eliminating the first 30 seconds of rebreathing.
eFigure 25-13.
Comparison of rebreathing and steady-state hypercapnic response curves.
Response lines defined by the steady-state method (solid symbols) and by the rebreathing method (open symbols) are shown for two experiments on the same subject. In one experiment, the steady-state points were defined by inhalation of each of four carbon dioxide mixtures for 20 minutes without intervening periods of air breathing. In the other experiment, the steady-state points were defined by inhalation of each carbon dioxide mixture for 30 minutes, with an intervening rest period of 30 minutes. The figure illustrates a difference in position of the response lines due to a smaller Pco2 gradient between arterial blood and chemoreceptor tissue during the rebreathing method. The close agreement in slope implies that the ratio of change in end-tidal Pco2 to change in chemoreceptor Pco2 is the same in the two methods.
(Modified from Read DJC: A clinical method for assessing the ventilatory response to carbon dioxide. Australas Ann Med 16:20–32, 1967.)
The variability among normal subjects is large.6, 185 The hypercapnic response has been shown to correlate with weight, height, and VC.186 In subjects studied on two occasions 15 minutes apart, the mean ± SE of the slope of the first test was 2.60 ± 0.11, and that of the second test was 2.46 ± 0.10. The mean ± SE of the intercept on the carbon dioxide axis was 32.42 ± 0.67 mm Hg for the first test and 31.17 ± 0.71 mm Hg for the second test. When 10 of the same subjects were retested as long as 2 years later, the differences in slopes from earlier values varied from 0.04 to 3.57 L/min per mm Hg, and the differences in intercepts on the abscissa varied from 0 to 7.6 mm Hg.
Hypoxic Response
Following the method of Rebuck and Campbell,187 a reservoir bag is filled with a volume equal to the VC of the subject plus 1 L with a mixture containing approximately 7% carbon dioxide, 70% nitrogen, and the balance oxygen. The subject breathes from and exhales into the room. Values for expired volume, end-tidal Po 2, end-tidal Pco 2, and oxygen saturation are recorded. When the end-tidal Pco 2 values become stable, appropriate valves are then turned so the subject rebreathes from the bag. The subject then takes three deep breaths to facilitate mixing; after these three breaths, the carbon dioxide value is recorded. Carbon dioxide is maintained at this level by manually adjusting flow through the carbon dioxide absorber. Rebreathing is continued until end-tidal Po 2 decreases to 45 mm Hg, oxygen saturation decreases to 75%, or the subject becomes distressed. If ventilation increases too rapidly, addition of oxygen at a rate of 125 to 200 mL/min will slow the rate of change.
Ventilation, in liters per minute BTPS (breath-by-breath or averaged over 5 to 10 breaths), is plotted on the ordinate, and the mean oxygen saturation (percentage) on the abscissa for the same periods (eFig. 25-14). The slope ( /1% desaturation) is calculated, preferably by linear least-squares regression analysis. These values are reported in terms of the mean end-tidal Pco
2 during the test.
/1% desaturation) is calculated, preferably by linear least-squares regression analysis. These values are reported in terms of the mean end-tidal Pco
2 during the test.
eFigure 25-14.
Hypoxic response curves, representing pooled results of four studies in two subjects.
A, Ventilation is plotted against oxygen saturation, producing linear responses. B, The more traditional hyperbolic relationship is obtained by plotting ventilation against alveolar Po2.
(Modified from Rebuck AS, Campbell EJM: A clinical method for assessing the ventilatory response to hypoxia. Am Rev Respir Dis 109:345–350, 1974.)
The variability of the hypoxic response during eucapnia in normal subjects is large. The difference in slopes indicates that the hypoxic response is very sensitive to the level of end-tidal Pco 2 selected. According to Rebuck and Campbell,187 repeated measurements in five subjects from day to day showed a variance within individuals of 0.76 and between individuals of 7.75.
Inspiratory Occlusion Pressure
When the patient is breathing room air or while the hypercapnic or hypoxic response is being tested, Pmouth at 100 msec (0.1 second), P0.1, or the maximum rate of inspiratory pressure change ([dP/dt]max), can be measured. Brief inspiratory occlusion should be performed randomly, always preceded by three or more tidal breaths.188 Out of view of the subject, the operator uses a syringe during expiration to close a Starling resistor arranged in series with the inspiratory channel so the channel is occluded. The syringe is decompressed as soon as possible after the inspiratory attempt is initiated. Recorder speed should be 50 mm/sec during the subject's inspiratory attempt. Alternatively, Pmouth and its differential (the change in pressure) can be measured in the 10 to 50 msec before the inspiratory valve opens. This approach takes advantage of the inherent resistance of the valve and can be measured at a slow recording speed for every breath without requiring use of a Starling resistor or other maneuvers by the operator.189 P0.1 should be measured every minute and at the same time after the inspiratory effort begins (e.g., 100 ± 10 msec).
The P0.1 measured during single partially occluded breaths, or the average (dP/dt)max of several breaths, is determined directly from the recording and plotted on the ordinate. Mean 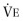 calculated from three or more breaths preceding inspiratory occlusion, end-tidal Pco
2, or arterial oxygen saturation is displayed on the abscissa (eFig. 25-15).
calculated from three or more breaths preceding inspiratory occlusion, end-tidal Pco
2, or arterial oxygen saturation is displayed on the abscissa (eFig. 25-15).
eFigure 25-15.
Inspiratory occlusion pressure at 100 msec (0.1 second) in response to hypercapnia.
Each curve is the mean regression of mouth pressure at 0.1 second against Pco2 for one subject. Inspiratory occlusion pressure may be used to measure the output of the respiratory center in response not only to hypercapnia, but also to hypoxia, exercise, and other factors.
(Modified from Whitelaw WA, Dernne J, Milic-Emili J: Occlusion pressure as a measure of respiratory center output in conscious man. Respir Physiol 23:181–199, 1975.)
Kryger and colleagues190 found a mean P0.1 of 2.6 cm H2O (range, 1.5 to 5.0 at an arterial Pco 2 of 39 to 42 mm Hg). Gelb and coworkers191 found a mean ± SD increase of 0.52 ± 0.19 cm H2O/mm Hg Pco 2 during increasing hypercapnia. Matthews and Howell189 found (dP/dt)max to vary during quiet breathing from 12.5 to 25 cm H2O/sec. During hypercapnia the increase in (dP/dt)max ranged from 0.6 to 4.6 cm H2O/sec per mm Hg carbon dioxide, and end-tidal Pco 2 increased from 50 to 60 mm Hg. Whitelaw and associates188 found a mean P0.1 ± SD of 13.2 ± 0.76 cm H2O during constant hypercapnia (end-tidal Pco 2 of 56 mm Hg). Matthews and Howell189 found that individual breath-to-breath (dP/dt)max varied up to 20%.
Clinical Applications
Carbon Dioxide Responses
In general there are three clinical conditions associated with abnormal carbon dioxide responses: decreased central chemoreceptor response to carbon dioxide, neuromuscular disease preventing a normal response to carbon dioxide, and abnormalities of the mechanical properties of the respiratory system.
Patients with decreased central chemoreceptor response to carbon dioxide may have varied defects. Decreased responsiveness may result from congenital abnormalities or may be acquired following trauma or inflammatory lesions in the central nervous system. Decreased responsiveness may also result from chronic carbon dioxide retention with associated increased bicarbonate levels and increased buffer capacity of blood and other tissue fluids.
Patients with neuromuscular disease have normal chemoreceptor responses from the ventilatory centers but an inadequate peripheral response. Thus patients with myasthenia gravis cannot respond because of the defective neuromuscular junction, and patients with poliomyelitis cannot respond because of damaged anterior horn cells. These patients have decreased inspiratory work and diminished maximal inspiratory force in response to inhaled carbon dioxide and can be diagnosed using tests of respiratory muscle strength.
Patients with chronic airflow obstruction, pulmonary restriction, or deformities of the chest wall have mechanical limitations to thoracic expansion in response to inhaled carbon dioxide. These patients have normal chemoreceptor responses from the ventilatory centers, a normal peripheral response, but a mechanical limitation that prevents the respiratory muscles from increasing ventilation normally. Thus the ventilatory response to inhaled carbon dioxide may be reduced, but the response reflected by diaphragmatic electromyography (EMG), the P0.1, is appropriate for the carbon dioxide.
Hypoxic Responses
There are few clinical indications for evaluation of hypoxic responses. The response to alveolar hypoxia has been used in patients with carotid body denervation to test the degree of depressed sensitivity to oxygen. Patients born at high altitude and patients with cyanotic congenital heart disease may have diminished response to hypoxia. The degree of abnormality can be assessed by administration of low-oxygen mixtures to breathe, but this is largely a research procedure.
In patients with chronic carbon dioxide retention, it may be worthwhile to test hypoxic responses because ventilation may be driven primarily by hypoxia. This possibility can be assessed by measuring the level of ventilation when the patient breathes room air and again with oxygen. Whereas normal subjects show a small decrease in ventilation, some patients with chronic carbon dioxide retention show a marked decrease in ventilation. Although this decreased response is unusual in patients with chronic airflow obstruction who are treated with oxygen, it is important to be aware that such a response can be seen in some patients, who may require assisted ventilation.
Ventilation-Perfusion Relationships
For discussion of ventilation, blood flow, and gas exchange, see Chapter 4.
Measurements of Ventilation-Perfusion Relationships
Inhaled air and pulmonary capillary blood flow are not distributed uniformly or in proportion to each other, even in the normal lung. Distributions of ventilation and blood flow are altered by posture, lung volume, and exercise not only in healthy subjects but even more so in patients with respiratory disease. The most common cause of arterial hypoxemia is increased mismatching of ventilation and perfusion, resulting in regional hypoventilation relative to perfusion (eTable 25-1). Whereas samples of alveolar gas and pulmonary capillary blood cannot be obtained to analyze gas exchange, inspired and expired gas (gas entering and leaving the alveoli) and mixed venous (blood entering the pulmonary capillaries) and arterial blood can be obtained and analyzed.
eTable 25-1.
Causes of Hypoxemia: The Effect on Alveolar-Arterial Po2 Differences and Arterial Pco2
| Cause | Effect on Alveolar Po2 | Effect on (A–a)Po2 | Effect on Arterial Pco2 |
|---|---|---|---|
| Normal lungs/inadequate oxygenation | |||
| Deficiency of oxygen in atmosphere | ↓ | ↔ | ↓ |
| Hypoventilation (neuromuscular disorder) | ↓ | ↔ | ↑ |
| Pulmonary disease | |||
| Hypoventilation (airway/parenchymal disorder) | ↓ | ↔ | ↑ |
| Diffusion abnormality | ↓*† | ↑*† | ↓ |
| Ventilation-perfusion imbalance | ↓† | ↑† | ↓ ↔, or ↑ |
| Right-to-left shunts | ↓ | ↑ | ↓, ↔, or ↑ |
| Inadequate transport/delivery of oxygen | |||
| Anemia | ↔ | ↔ | ↔ |
| General/localized circulatory insufficiency | ↔ | ↔ | |
| Inadequate tissue oxygenation | |||
| Abnormal tissue demand/poisoned enzymes/edema | ↔ | ↔ | ↔ |
↑, increased; ↔, no change; ↓, decreased.
Infrequently observed at rest but more likely during exercise.
Unless patient is hyperventilating.
Adapted from Comroe JH Jr, Forster RE II, DuBois AB, et al: Arterial blood oxygen, carbon dioxide and pH. In Comroe JH Jr, Forster RE II, DuBois AB, et al: The lung: clinical physiology and pulmonary function tests, ed 2, Chicago, 1962, Year Book, pp 140–161.
Resting Ventilation
Minute ventilation under resting conditions is defined as the amount of air exhaled per minute ( ). It can be measured readily using a recording spirometer equipped with a carbon dioxide absorber. (The measured expired volume must be corrected for the amount of absorbed carbon dioxide.) Many laboratories use a mouthpiece equipped with valves that separate inhaled and exhaled gases, permitting collection of exhaled air in a plastic bag or meteorologic balloon in preference to the use of a spirometer. Most commercial devices now direct expired gas through a pneumotachygraph and use a computer to integrate the flow signal to calculate expired volume. The volume of exhaled gas collected is then measured with a 120-L (Tissot) spirometer or with a dry-gas meter. For use at the bedside, a Wright respirometer192 is preferred; it is used commonly in surgical recovery rooms and critical care units. From
). It can be measured readily using a recording spirometer equipped with a carbon dioxide absorber. (The measured expired volume must be corrected for the amount of absorbed carbon dioxide.) Many laboratories use a mouthpiece equipped with valves that separate inhaled and exhaled gases, permitting collection of exhaled air in a plastic bag or meteorologic balloon in preference to the use of a spirometer. Most commercial devices now direct expired gas through a pneumotachygraph and use a computer to integrate the flow signal to calculate expired volume. The volume of exhaled gas collected is then measured with a 120-L (Tissot) spirometer or with a dry-gas meter. For use at the bedside, a Wright respirometer192 is preferred; it is used commonly in surgical recovery rooms and critical care units. From  , it is possible to estimate alveolar ventilation (see discussion later), using an assumed value for dead space (Vd):
, it is possible to estimate alveolar ventilation (see discussion later), using an assumed value for dead space (Vd):
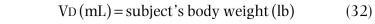
Measurement of resting  usually plays a minor role in routine assessment of pulmonary function, because patients with advanced disease of the lungs often breathe with a normal tidal volume and respiratory frequency. The attending physician may wish to obtain an accurate record of resting
usually plays a minor role in routine assessment of pulmonary function, because patients with advanced disease of the lungs often breathe with a normal tidal volume and respiratory frequency. The attending physician may wish to obtain an accurate record of resting  if hypoventilation or an abnormal respiratory pattern is suspected, such as that associated with central nervous system lesions or psychogenic disorders. Resting
if hypoventilation or an abnormal respiratory pattern is suspected, such as that associated with central nervous system lesions or psychogenic disorders. Resting  in normal subjects has been studied in detail: men breathe at an average rate of 16 breaths/min, women breathe at 19 breaths/min with much individual variation, and sighs happen at an average of 9 per hour in men and 10 per hour in women.193
in normal subjects has been studied in detail: men breathe at an average rate of 16 breaths/min, women breathe at 19 breaths/min with much individual variation, and sighs happen at an average of 9 per hour in men and 10 per hour in women.193
Because the attempts to measure  change an automatic, unconscious process to one of concern to the subject, it is difficult to obtain accurate measurements of the rate and pattern of resting ventilation. In addition to making measurements when the subject is unaware, investigators have used magnetometers attached to the chest wall and impedance plethysmography194 to measure ventilation and the pattern of breathing accurately.
change an automatic, unconscious process to one of concern to the subject, it is difficult to obtain accurate measurements of the rate and pattern of resting ventilation. In addition to making measurements when the subject is unaware, investigators have used magnetometers attached to the chest wall and impedance plethysmography194 to measure ventilation and the pattern of breathing accurately.
Measurement of resting  plays an important, but previously neglected, part in management of patients in danger of developing respiratory failure from hypoventilation (e.g., patients with obesity and sleep disorder syndromes). In such patients, and in patients in postoperative states, with drug intoxication, or with neuromuscular disease, measurement of
plays an important, but previously neglected, part in management of patients in danger of developing respiratory failure from hypoventilation (e.g., patients with obesity and sleep disorder syndromes). In such patients, and in patients in postoperative states, with drug intoxication, or with neuromuscular disease, measurement of  is as important as measurement of the usual vital signs (heart rate and blood pressure) and should be obtained at frequent intervals.
is as important as measurement of the usual vital signs (heart rate and blood pressure) and should be obtained at frequent intervals.
The Bohr Equation for Respiratory Dead Space
The Bohr equation, applied to a particular gas X, is as follows. Expired gas is the total volume of gas leaving the nose and mouth between the beginning and the end of a single exhalation (Ve). Va indicates the volume of alveolar gas contributed to the exhaled gas and does not refer to the total volume of gas in the alveoli. The amount of gas X in Ve, Va, or Vd is the product of its fractional concentration (Fx) and the volume in which gas X is contained. Therefore,
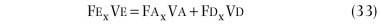
If the gas in question is carbon dioxide, this equation is simplified, because inspired air contains practically no carbon dioxide (Faco 2 = 0.0005), and the Bohr equation becomes
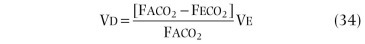
“Physiologic” Dead Space (also called Wasted Ventilation)
In the Bohr equation for respiratory dead space, Feco 2 and Ve can be measured easily, but Faco 2 is difficult to obtain, and Vd cannot be calculated unless the correct value for Faco 2 is known. Because there is almost always complete equilibrium between alveolar Pco 2 and end-pulmonary capillary Pco 2, arterial Pco 2 represents a mean alveolar Pco 2 over several respiratory cycles, provided that arterial blood is sampled over this same period and the patient does not have a significant venous-to-arterial shunt. Thus arterial Pco 2 can replace alveolar Pco 2, and the Bohr equation becomes
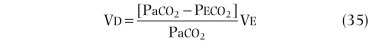
In the ideal case, anatomic and “physiologic” dead spaces are equal. However, in patients with uneven ventilation–blood flow ratios in the lung, the physiologic dead space is larger than the anatomic dead space, because regions with decreased blood flow in relation to ventilation act as regions of wasted ventilation or respiratory dead space.195
By substituting arterial Pco
2 for alveolar Pco
2 in the Bohr equation, it is possible to calculate physiologic dead space. This includes anatomic dead space and alveolar dead-space ventilation. The latter includes ventilation of alveoli without perfusion; alveoli with decreased perfusion and increased, normal, or slightly decreased ventilation; and alveoli with normal perfusion and marked overventilation. Because it is technically impossible to distinguish the various types of increased 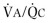 ratios, we assume that part of alveolar ventilation to regions with diminished perfusion goes to regions without any blood flow. That is, the physiologist assumes two compartments: one with and one without perfusion. Overventilation relative to perfusion wastes ventilation with respect to oxygen transfer because of the shape of the oxygen-hemoglobin dissociation curve. Little oxygen is added to blood by increasing alveolar Po
2 from 100 to 140 mm Hg. This excess ventilation is not wasted with respect to carbon dioxide elimination, because increased ventilation decreases arterial carbon dioxide. Regions with excess ventilation are usually accompanied by other regions with diminished ventilation and increased Pco
2. Ventilation is still “wasted” with respect to carbon dioxide, because it is not distributed proportionately to perfusion. Assessment of wasted ventilation is essential for the proper management of critically ill patients in the intensive care unit and for the diagnosis of patients with pulmonary vascular obstruction in the pulmonary exercise laboratory.
ratios, we assume that part of alveolar ventilation to regions with diminished perfusion goes to regions without any blood flow. That is, the physiologist assumes two compartments: one with and one without perfusion. Overventilation relative to perfusion wastes ventilation with respect to oxygen transfer because of the shape of the oxygen-hemoglobin dissociation curve. Little oxygen is added to blood by increasing alveolar Po
2 from 100 to 140 mm Hg. This excess ventilation is not wasted with respect to carbon dioxide elimination, because increased ventilation decreases arterial carbon dioxide. Regions with excess ventilation are usually accompanied by other regions with diminished ventilation and increased Pco
2. Ventilation is still “wasted” with respect to carbon dioxide, because it is not distributed proportionately to perfusion. Assessment of wasted ventilation is essential for the proper management of critically ill patients in the intensive care unit and for the diagnosis of patients with pulmonary vascular obstruction in the pulmonary exercise laboratory.
Alveolar Air Equation
The measurement of alveolar Po 2 and Pco 2 from analysis of a single sample of exhaled alveolar gas is subject to considerable error, but mean alveolar Po 2 can be calculated accurately. The underlying principle is based on the concept that at sea level the total pressure of gases (oxygen, carbon dioxide, nitrogen, and water) in the alveoli equals 760 mm Hg, and that if the partial pressures of any three of these four are known, the fourth can be obtained by subtraction. As derived in Chapter 4,
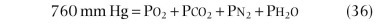
In general, water vapor pressure at 37° C is approximately 47 mm Hg, and this presents no problem. Arterial Pco 2 is used to represent mean alveolar Pco 2, because arterial blood coming from all the alveoli approaches an integrated value of alveolar Pco 2 with respect to different regions of the lung and to different times during the respiratory cycle. It is also assumed that Pn 2 = 563 mm Hg. This would be true if the respiratory gas exchange ratio (R) were 1 (i.e., the amount of carbon dioxide added to the alveoli equals the amount of oxygen removed from the alveoli per minute). Actually, the amount of oxygen removed per minute is greater than the amount of carbon dioxide added:
| (37) |
With an R of 0.8, the nitrogen molecules are slightly more concentrated, because the same number of nitrogen molecules is present in a smaller volume. If the alveolar nitrogen concentration increases to 81%, alveolar Pn 2 increases to 577 mm Hg and alveolar Po 2 falls to 96 mm Hg. It is therefore essential to measure R to calculate alveolar Pn 2 accurately. The precise formula (assuming inspired Pco 2 is zero) is
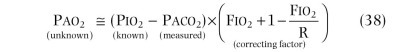
where Pio 2 (moist) at sea level is calculated as 20.93% of (760 − 47) = 149 mm Hg, and alveolar carbon dioxide pressure (Paco 2) is assumed to be equal to the arterial Pco 2, which can be measured accurately.
The alveolar air equation is often approximated to estimate alveolar-arterial oxygen differences for clinical purposes (assuming Paco 2 = Paco 2):
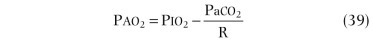
The alveolar-arterial Po2 difference ((A−a)Po 2) has been shown to be larger in older subjects than in younger ones.196 According to Mellemgaard,197 the regression with age is expressed as (A−a)Po 2 = 2.5 + 0.21 × age (in years). Mellemgaard's study was performed on 80 healthy, seated subjects whose ages ranged from 15 to 75 years.
Calculation of Alveolar Ventilation
Carbon dioxide in exhaled gas must all come from alveolar gas. As derived in Chapter 4, this equation is as follows:
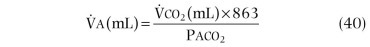
Relation of Alveolar Ventilation to Pulmonary Blood Flow
Equation 41, derived in Chapter 4, relates the factors that determine the adequacy of alveolar ventilation:where 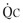 is pulmonary capillary blood flow,
is pulmonary capillary blood flow, 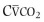 is the carbon dioxide concentration in mixed venous blood, Cc′co
2 is the carbon dioxide concentration in the end-pulmonary capillary blood,
is the carbon dioxide concentration in mixed venous blood, Cc′co
2 is the carbon dioxide concentration in the end-pulmonary capillary blood, 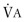 is alveolar ventilation, Paco
2 is alveolar carbon dioxide tension, and 863 is a constant to correct for changes from alveolar fraction to alveolar pressure of carbon dioxide. In any individual the mixed venous blood distributed to all pulmonary capillaries has the same carbon dioxide concentration, and end-pulmonary capillary blood has the same Pco
2 as alveolar gas; therefore alveolar Pco
2 is determined by the ratio
is alveolar ventilation, Paco
2 is alveolar carbon dioxide tension, and 863 is a constant to correct for changes from alveolar fraction to alveolar pressure of carbon dioxide. In any individual the mixed venous blood distributed to all pulmonary capillaries has the same carbon dioxide concentration, and end-pulmonary capillary blood has the same Pco
2 as alveolar gas; therefore alveolar Pco
2 is determined by the ratio 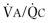 .
.
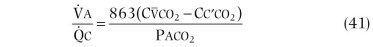
Calculation of Quantity of Venous-to-Arterial Shunt
For a more detailed discussion of pulmonary shunts, see Chapter 61 which discusses pulmonary arteriovenous malformations and other pulmonary vascular abnormalities.
When a patient has a venous-to-arterial shunt, arterial blood contains some mixed venous blood that has bypassed the lungs and some well-oxygenated blood that has passed through the pulmonary capillaries. The equation that expresses this relationship for blood is analogous to the Bohr equation for calculation of respiratory dead space:
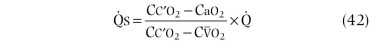
where 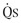 is shunt blood flow, Cc′o
2 is the oxygen content of end-capillary blood, Cao
2 is the oxygen content of arterial blood,
is shunt blood flow, Cc′o
2 is the oxygen content of end-capillary blood, Cao
2 is the oxygen content of arterial blood, 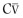 o
2 is the oxygen content of mixed venous blood, and
o
2 is the oxygen content of mixed venous blood, and 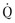 is total blood flow.
is total blood flow.
Arterial and mixed venous blood can be obtained, so the arterial concentration of oxygen and 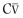 o
2 can be measured. The quantity of blood flowing through the shunt can be determined by having the patient breathe pure oxygen for a sufficient time to wash all of the nitrogen from the alveoli. Alveolar Po
2 is then equal to 760 − alveolar Ph
2
o − alveolar Pco
2, or approximately 673 mm Hg. Under these conditions, there is no alveolar-to-end-capillary difference, and end-capillary blood can be assumed to contain an amount equal to the oxygen capacity of hemoglobin plus 2.0 mL of dissolved oxygen per 100 mL.
o
2 can be measured. The quantity of blood flowing through the shunt can be determined by having the patient breathe pure oxygen for a sufficient time to wash all of the nitrogen from the alveoli. Alveolar Po
2 is then equal to 760 − alveolar Ph
2
o − alveolar Pco
2, or approximately 673 mm Hg. Under these conditions, there is no alveolar-to-end-capillary difference, and end-capillary blood can be assumed to contain an amount equal to the oxygen capacity of hemoglobin plus 2.0 mL of dissolved oxygen per 100 mL.
“Venous admixture” or “physiologic shunt” can be estimated by the method of Lilienthal and associates.198 “Shunt” means decreased 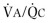 ratios and includes perfused alveoli without ventilation; very poorly ventilated alveoli with normal, increased, or slightly decreased perfusion; and ventilated alveoli with markedly increased perfusion. In this situation, the physiologist assumes two compartments: one with and one without a complete shunt.199
ratios and includes perfused alveoli without ventilation; very poorly ventilated alveoli with normal, increased, or slightly decreased perfusion; and ventilated alveoli with markedly increased perfusion. In this situation, the physiologist assumes two compartments: one with and one without a complete shunt.199
If a patient is given pure oxygen to breathe, it is possible to distinguish a right-to-left shunt from a ventilation-perfusion abnormality. Alveolar and arterial Po
2 values expected in an ideal lung, with 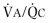 ratio imbalance, and with right-to-left shunt are given in eTable 25-1.
ratio imbalance, and with right-to-left shunt are given in eTable 25-1.
Pure oxygen replaces nitrogen with oxygen in all gas-exchange units that have patent airways, even in the presence of severe airway obstruction or pulmonary restriction; this leaves only oxygen, carbon dioxide, and water in the air spaces. Under these conditions,
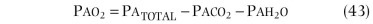
Total pressure (Pa
total) and water vapor pressure (Pah
2
o) are the same in all patent gas-exchange units; thus alveolar Po
2 differences between units exist only when there are differences in Pco
2. In ideal lungs or lungs with a  imbalance, the high alveolar Po
2 corrects the ventilation-perfusion imbalance; arterial Po
2 values are also high, provided all nitrogen is washed out of communicating units by oxygen.
imbalance, the high alveolar Po
2 corrects the ventilation-perfusion imbalance; arterial Po
2 values are also high, provided all nitrogen is washed out of communicating units by oxygen.
In most normal subjects, the right-to-left shunts are distal to the gas-exchange units (so-called postpulmonary shunts). These shunt vessels include bronchial veins, mediastinal-to-pulmonary veins, and thebesian vessels (left ventricular muscle to left ventricular cavity). In some patients, intracardiac shunts, pulmonary arteriovenous malformations, or perfusion of nonventilated alveoli produce pulmonary shunts. Most shunts in patients with pulmonary disorders involve perfusion of nonventilated alveoli. For clinical purposes the amount of right-to-left shunt may be estimated from the fall in arterial Po 2 below the expected value of 673 mm Hg, as long as the Po 2 is sufficient to saturate hemoglobin (i.e., more than 200 mm Hg). For every 2% shunt, Po 2 decreases 35 mm Hg.
Measurement of Ventilation-Perfusion Relationships Using Insoluble Gases
For a more detailed discussion of ventilation-perfusion relationships, see Chapter 4.
133Xe is a relatively insoluble gas with a blood-gas partition coefficient of approximately 0.13.200 When it is inhaled, 133Xe can be used to measure regional ventilation per unit lung volume, and when it is dissolved in saline and injected intravenously, it can be used to measure regional blood flow.201 When either of these procedures is followed by rebreathing in a closed circuit, a plateau is obtained that reflects the product of lung volume detected by the counter and the geometric factor for 133Xe; for this purpose, the subject is switched into a closed circuit at the end of the injection. Measurements that can be obtained are illustrated in eFigure 25-16. Following intravenous injection, peak activity reflects the appearance of the isotope distributed in proportion to pulmonary blood flow; because of its low blood-gas partition coefficient, about 85% of the isotope passes into the alveolar gas, where it remains as long as the subject holds his or her breath. On resumption of breathing, the distribution reflects ventilation of perfused tissue. A slow clearance implies units with a relatively low  ratio. Because of the overlap of many units (at least 107) with a single counting field, a functional definition of the
ratio. Because of the overlap of many units (at least 107) with a single counting field, a functional definition of the  ratio in this manner is more closely related to pulmonary gas exchange than a ratio obtained by dividing a measurement of regional ventilation by a separate measurement of regional perfusion.
ratio in this manner is more closely related to pulmonary gas exchange than a ratio obtained by dividing a measurement of regional ventilation by a separate measurement of regional perfusion.
eFigure 25-16.
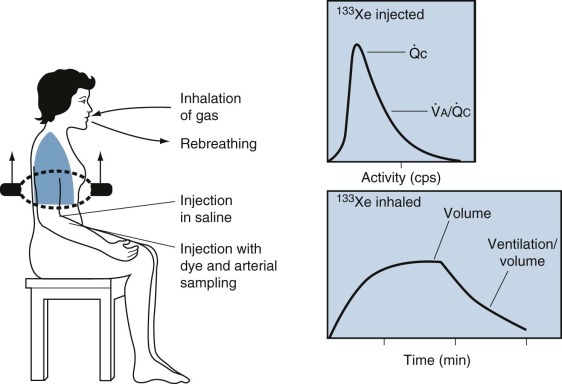
Assessment of regional lung function using xenon-133 (133Xe).
Top, After injection, the initial peak reflects the regional blood flow (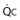 ); the isotope then passes into the gas phase, in which the clearance during normal breathing reflects the ventilation of lung tissue that is perfused. A slow washout indicates a low ventilation-perfusion ratio (
); the isotope then passes into the gas phase, in which the clearance during normal breathing reflects the ventilation of lung tissue that is perfused. A slow washout indicates a low ventilation-perfusion ratio (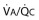 ). Bottom, During rebreathing, the plateau count rate when mixing is complete reflects the volume of lung gas in the field of counting. The slopes of the wash-in and washout curves indicate the ventilation per unit volume.
). Bottom, During rebreathing, the plateau count rate when mixing is complete reflects the volume of lung gas in the field of counting. The slopes of the wash-in and washout curves indicate the ventilation per unit volume.
(Modified from Cotes JE: Lung function, ed 4, Oxford, 1979, Blackwell Scientific.)
The lower graph of eFigure 25-16 shows a wash-in of 133Xe in a closed circuit followed by a washout. The equilibration plateau is evidence that the isotope concentration is the same in all alveoli. Local count rates then reflect the volume of alveolar gas in the counting fields. Perfusion per unit volume is obtained by dividing the peak counts for any region by the counts at equilibrium after intravenous injection. Both measurements should be made at the same lung volume, so geometric factors in the chest wall and differences in detector sensitivity do not influence the results.
If several VC breaths are taken at the beginning of the test, healthy subjects reach equilibration after rebreathing for 1 to 2 minutes or less. Patients with airway obstruction may not reach full equilibration in 20 minutes because isotope is accumulated in the blood and chest wall; rebreathing may then be terminated at 4 minutes. Ventilation per unit of lung volume may be obtained from the initial slope or half-time of the wash-in or washout of 133Xe (see eFig. 25-16). Beyond the half-time, the washout curve cannot be interpreted because of activity in the chest wall and in the recirculating blood. Ventilation per unit of lung volume may also be obtained from the activity during a breath held subsequent to taking in a tidal volume of 133Xe; activity is divided by the plateau level at the same lung volume.
Alternatively, a bolus of 133Xe may be injected close to the mouthpiece just before the start of inhalation, which is then continued until full inhalation. Under these circumstances the bolus is distributed in a pattern reflecting the early phase of inspiration starting at end-expiration. Because ventilation tends to be sequential, it is preferable to label the whole tidal breath. A bolus given at the beginning of inspiration after a maximal exhalation to RV is distributed preferentially to the lung apex. An inspiratory capacity breath of 133Xe reflects regional compliance, not regional ventilation, and measures the regional inspiratory capacity. Thus it is possible to use the gas dilution principle to calculate regional inspiratory capacity or regional VC using a variety of radioisotopes.202
The most widely used radioisotope study of the lung is the perfusion scan following intravenous injection of human serum albumin microspheres or microaggregates labeled with technetium-99m (99mTc).203 Particles are 20 to 50 µm in diameter and impact in small pulmonary vessels in proportion to local perfusion. Regional perfusion is measured, not perfusion per unit volume, so the volume of lung in the counting field influences the measurement. Calculations suggest that from 1 mg of protein, particles of 500, 100, and 30 µm in diameter obstruct, respectively, 0.12%, 0.31%, and 0.26% of the vascular bed.204 On this basis, injection is potentially hazardous in patients with severe pulmonary vascular disease, and deaths in this situation have been recorded. However, with reasonable precautions, the risk is minimal. Passage of particles into the systemic circulation through right-to-left intrapulmonary or intracardiac shunts does not appear to be accompanied by side effects. The radiation dose from most pulmonary isotopic procedures is low and confined primarily to the lungs. A typical 133Xe or 99mTc study yields 0.2 to 0.4 rad (the annual permitted dose is 5 rad).
Distribution of Ventilation-Perfusion Ratios
Distribution of perfusion in relation to ventilation of the lung may be analyzed on the basis of a region or lobe or for the lung as a whole and expressed in terms of physiologic shunt, physiologic dead space, and other compartments or in terms of ventilation-perfusion ratios. In an approach developed by Wagner and colleagues,205 the lung is assumed to consist of a large number of homogeneous compartments in parallel, each with its own ventilation, blood flow, and appropriate gas concentrations. Distribution of ventilation-perfusion ratios is evaluated with six inert gases of varying solubility dissolved in saline and infused intravenously and concurrently at a constant rate. Under these circumstances in the steady state, the amount of any gas exchanging between alveoli and pulmonary capillary blood is identical to that exchanging between alveoli and atmosphere. For each compartment, the quantity of gas is a function of the ventilation-perfusion ratio and the blood-gas partition coefficient for the gas in question, expressed as a fraction of that in the mixed venous blood. For the lung as a whole, the mixed arterial concentration is a blood flow–weighted mean of the values for several compartments, and the mean expired level is similarly a ventilation-weighted mean of the compartmental values. These parameters are measured directly, together with the cardiac output and the minute volume of ventilation. They are used to calculate the corresponding mixed venous and alveolar concentrations and then a distribution of ventilation-perfusion ratios that is compatible with the arterial and alveolar concentrations of all gases concurrently (eFigs. 25-17 and 25-18).
eFigure 25-17.
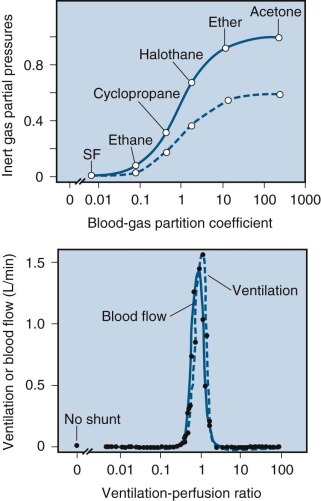
Distribution of ventilation-perfusion ratios in a normal person.
Top, The retention (arterial/venous, solid line) and excretion (expired/venous, dashed line) data points together with the curves for a homogeneous lung. Bottom, Continuous distribution of ventilation-perfusion ratios as found in a semirecumbent young (22-year-old) normal subject by means of the inert gas elimination method. Note the narrow dispersion and the absence of shunt. The dashed line indicates ventilation, and the solid line indicates blood flow. SF, sulfur hexafluoride.
(Modified from West JB: Ventilation/blood flow and gas exchange, ed 3, Oxford, 1977, Blackwell Scientific.)
eFigure 25-18.
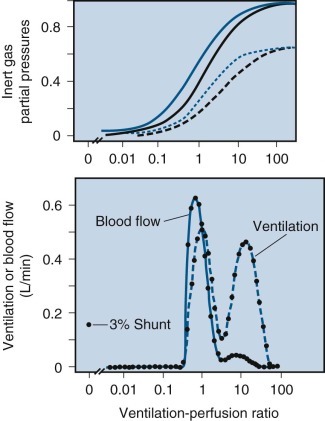
Distribution of ventilation-perfusion ratios in a COPD patient.
Top, Retention and excretion-solubility curves. Black lines indicate data from the patient; blue lines indicate data from the normal subject depicted in eFigure 25-17. Bottom, Continuous distribution of ventilation-perfusion ratios in a 60-year-old patient with chronic airway obstruction, predominantly emphysema. The dashed line indicates ventilation and the solid line indicates blood flow. Note the broad bimodal distribution with the large amount of ventilation going to lung units with very high ventilation-perfusion ratios.
(Modified from West JB: Ventilation/blood flow and gas exchange, ed 3, Oxford, 1977, Blackwell Scientific.)
The limitations of the method include the limited accuracy of current chromatographic techniques for gas analysis. In addition, it does not provide a unique solution, because the same arterial and alveolar gas concentrations could result from other distributions of ventilation and perfusion in the lung. Wagner and associates206 also reported a modification of the multiple inert gas method that permits estimation of the levels of inert gases in peripheral venous blood, rather than arterial blood, which may prove to be of considerable clinical interest.
Clinical Applications
The various methods of measuring ventilation-perfusion relationships have been used widely in the diagnosis and management of patients with various pulmonary disorders. This is not surprising because almost every pulmonary disease affects the delicate match between ventilation and perfusion early in the process, with the matching becoming worse as the disease progresses.
Understanding ventilation-perfusion mismatching may be essential to proper diagnosis and management. For example, measurement of physiologic dead space has provided insights into the gas exchange defects of the patient in the intensive care unit and of the patient with chronic pulmonary embolism who presents with the complaint of exertional dyspnea. Measurement of intrapulmonary shunts by having a patient breathe pure oxygen can be used to estimate the size of shunts and assess efficacy of therapeutic embolization of shunt vessels.
Although the measurement of distribution of ventilation-perfusion ratios has taught us a great deal about the pathophysiology of ventilation-perfusion matching in pulmonary disease, it has not been useful as a clinical tool. On the other hand, radioisotope lung scans are critically important in the management of many of our patients, not only those with pulmonary vascular problems, but also patients who have undergone a single-lung transplant, in whom we can understand the role played by the native lung as well as the graft.
Arterial Blood Gases
Measurements of Arterial Blood Gases
The physiologic determinants of arterial oxygen levels and acid-base balance are reviewed in detail in Chapter 4 and Chapter 7, respectively.
Invasive Measurements
pH.
The pH of blood is now measured almost entirely by the use of the pH electrode (eFig. 25-19). This device takes advantage of the discovery that an electrical potential difference exists across some types of glass membranes placed between solutions of different pH. By maintaining one side of the membrane at a known pH with a buffer solution (pH = 6.84), the pH of the solution placed on the other side of the membrane can be calculated from the potential difference generated, using the Nernst equation. The modern pH electrode is made up of two cells. The measurement half-cell consists of a fine capillary tube of pH-sensitive glass separating the introduced sample (as little as 25 µL) from the buffered solution, and a silver/silver chloride electrode to conduct the generated potential difference to the electronic circuitry. The reference half-cell usually contains a calomel (mercury/mercurous chloride) electrode in an electrolyte solution to provide a constant reference voltage and is connected to the measurement half-cell by a contact bridge to complete the circuit. These two cells are enclosed together in a sealed jacket and are maintained at a constant temperature (see eFig. 25-19).
eFigure 25-19.
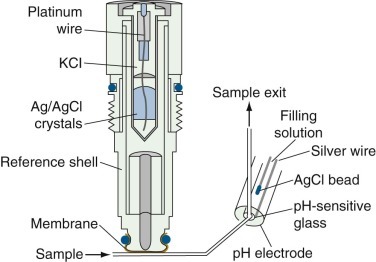
The structure of a pH electrode, which comprises two cells.
The measurement half-cell consists of a fine capillary tube of pH-sensitive glass separating the introduced sample of 25 µL from the buffered solution and a silver/silver chloride (Ag/AgCl) electrode to conduct the generated potential difference to the electronic circuitry. The reference half-cell usually contains a calomel (mercury/mercurous chloride) electrode in an electrolyte solution to provide a constant reference voltage and is connected to the measurement half-cell by a contact bridge to complete the circuit. Both cells are enclosed in a sealed jacket and maintained at a constant temperature. KCl, potassium chloride.
The potential difference generated across the glass membrane is a linear function of the pH. Thus it is usually adequate to calibrate the electrode with two buffered solutions of known pH that span a significant portion of the range expected in the samples to be measured. The normal range for arterial pH at sea level is 7.35 to 7.45 units. Even preliminary deviations from this range can be interpreted only by also examining the Pco 2, making use of the Henderson-Hasselbalch equation,
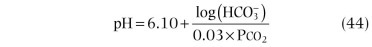
to infer whether the deviation in pH is due primarily to a metabolic or a respiratory cause and whether it is due to an acute or chronic disturbance. In clinical use the pH meter has proved to be a rugged, dependable device. Repeated measurements of a single sample by the same instrument fall within a narrow range of ±0.02 unit (±2 SD). Generally there is good agreement among the values obtained on unknown samples by the different instruments used by laboratories enrolled in quality-control programs (SD = 0.014 pH unit has been obtained in more than 800 laboratories).207 This remarkable accuracy depends on the integrity of the differential permeability of the glass membrane to hydrogen ions. The permeability may be altered by the deposition of protein or by the development of cracks on the membrane surface. Proper quality control requires that pH calibration be checked at one point before each series of pH determinations and at two major points every 4 hours. A number of standard phosphate buffer solutions are suitable for routine calibrations and are available commercially. Protein contamination of the membrane can be minimized by flushing the electrode with a cleaning solution at regular intervals (every 10 samples) and by taking care to follow injections of blood with injections of saline (not distilled water).
Carbon Dioxide.
Early chemical methods for measuring gas concentrations in blood were laborious and demanding. They involved liberating chemically bound oxygen and carbon dioxide in blood by adding chemical agents to a sample kept in a closed vessel. The quantity of gas released was then measured by a manometric208 or volumetric209 method. Carbon dioxide was then selectively absorbed, and the change in volume permitted calculation of the content of the blood sample of the two gases. These tedious and technically demanding methods gave accurate values for the content of the two gases in blood. Determination of pressure required measurement of the content of the gases in plasma alone, after separating plasma from red blood cells in a closed system. Alternatively, back-calculation of pressure could be made by measurement of blood hemoglobin content combined with measurements and calculations of the quantities of the gases transported in the cells and proteins of blood.
The breakthrough in the measurement of carbon dioxide came with the development of the membrane-covered carbon dioxide electrode (eFig. 25-20). This device exploits the principles of the pH electrode and the known relationship between Pco 2 and pH in a buffered solution. The sample to be analyzed is separated from a buffer solution by a membrane permeable to carbon dioxide. The carbon dioxide molecules that diffuse through the membrane alter the concentration of carbonic acid and therefore the concentration of hydrogen ion in the buffered solution:
| (45) |
eFigure 25-20.
The structure of a carbon dioxide electrode.
This electrode uses the combination of the relationship between Pco2 and pH in a buffered solution and the design of a pH electrode. The sample is separated from a buffer solution by a membrane permeable to carbon dioxide. The carbon dioxide molecules diffuse through the membrane, altering the concentration of carbonic acid, and therefore the hydrogen ion concentration in the buffered solution. The change in pH is read by a pH meter with output scaled in terms of Pco2.
A pH meter reads the resulting change in pH with the output scaled in terms of Pco 2. The time for response of the carbon dioxide electrode depends on the concentration and volume of the buffered solution, the diffusion properties of the artificial membrane, and the thickness of a second “stabilizing membrane” placed over the pH-sensitive glass. With silicone-rubber membranes, the 95% response time has been reduced to as little as 10 seconds (see eFig. 25-20).
Perhaps because it incorporates a pH electrode in its design, the Pco 2 electrode also has the advantages of precision and dependability if calibrated regularly. As with the pH electrode, a one-point calibration should be checked before each series of blood-gas measurements, and two-point calibrations every 4 to 8 hours or whenever the one-point calibration indicates the need for readjustment of more than 2 mm Hg Pco 2. The range of repeated measurements of samples of blood equilibrated under controlled conditions to Pco 2 of 20 to 60 mm Hg is ± 3.0 mm Hg, and tests with commercially available, sealed buffer solutions with different Pco 2 values show similar reproducibility with a variety of blood-gas measuring devices. The agreement among devices of laboratories enrolled in quality-control programs is also good.
The normal range of values for Pco 2 varies with altitude. At sea level, it ranges from 36 to 44 mm Hg.210 In Salt Lake City, Utah (elevation 1340 to 1520 m), the range is reported to be 30 to 40 mm Hg.211
Oxygen
Oxygen Pressure.
As with the measurement of Pco 2, the development of an accurate, stable electrode has almost entirely supplanted the use of older chemical methods for measuring total blood oxygen content and then back-calculating Po 2. The principle of the oxygen electrode differs from that of the pH and Pco 2 electrodes in that the oxygen electrode measures a current generated by the presence of the relevant molecule, rather than a potential difference. The device consists of platinum and silver electrodes placed in potassium chloride solutions, a polarizing voltage of 0.5 to 0.6 volt, and an electrolyte bridge to complete the circuit. Oxidation takes place at the silver electrode, where silver reacts with chloride ions to form silver chloride. This reaction produces electrons, which are consumed by the reduction of oxygen at the platinum electrode. The flow of electrons (current) is thus proportional to the concentration of oxygen at the platinum electrode (eFig. 25-21).
eFigure 25-21.
The structure of an oxygen electrode.
This electrode consists of platinum and silver electrodes placed in potassium chloride solutions, a polarizing voltage of 0.5 to 0.6 volts, and an electrolyte bridge to complete the circuit. Oxidation at the silver (Ag) electrode secondary to silver reacting with chloride ions to form silver chloride (AgCl) produces electrons that are consumed at the platinum electrode by reduction of oxygen. The flow of electrons (the current) is thus proportional to the concentration of oxygen at the platinum electrode.
Clark212 developed a useful electrode based on this principle. The important features of the Clark electrode are that it minimizes oxygen consumption by the use of a thin platinum electrode and ensures a constant diffusion distance between the surface of the electrode and the sample by covering the electrode with an oxygen-permeable membrane. The surface area of the platinum electrode and the permeability of the membrane to oxygen determine the sensitivity and response time of the electrode. However, the larger the electrode and the more permeable the membrane, the more rapidly oxygen is consumed, causing Po 2 to fall in small samples as the measurement is made. For most available devices, the compromises made result in a 95% response time of about 50 seconds.
A peculiarity of the oxygen electrode is that slightly different currents are generated when gases and liquids at the same Po 2 are introduced. The magnitude of the difference is usually 3% to 4%, depending on the electrode diameter, the nature and thickness of the membrane, and the flow of the sample around the electrode. A correction factor is sometimes introduced into the calculation of arterial Po 2 when gases are used for calibration. These factors, however, may not be related linearly to Po 2, resulting in errors when high oxygen pressures are measured, as in samples obtained from a patient to whom pure oxygen is given to estimate the magnitude of a right-to-left shunt. The simplest approach would seem to be calibration of the electrode with solutions, rather than gases. This is probably true, but large differences have been found for the same instrument using samples of different test solutions equilibrated to the same Po 2.213 In general, the more the oxygen-carrying capacity of the solution approximates that of blood, the smaller is the error. Thus the large interinstrument variability of oxygen pressure values reported for blind samples tested in a quality-control program may not reflect the variability that would be achieved if all tests were run with blood equilibrated to the appropriate oxygen tensions. For a single machine, the range of repeated measurements of Po 2 in blood is 3.0 mm Hg for Po 2 values from 20 to 150 mm Hg.210 In normal seated adult subjects, the predicted arterial Po 2 can be obtained from Mellemgaard's data197 with a SD around the regression line of approximately 6.0 mm Hg:
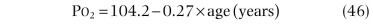
A final problem for some highly automated blood-gas machines appears only when samples of very high or very low Po 2 are tested. This is due to the error introduced by “contamination” of the sample chamber by the rinsing fluid.185 If the Po 2 of the rinsing fluid is similar to that of room air, then the persistence of a small amount of fluid does not much alter the Po 2 measured for blood samples with oxygen pressures between 60 and 100 mm Hg. The Po 2 of the rinsing fluid affects the values recorded for samples with oxygen pressures at either extreme. If the design of the machine permits, this source of error can be reduced by flushing the sample chamber with a fluid that has a Po 2 near the estimated value of the sample or by introducing consecutive specimens without flushing the chamber.
Oxygen Content.
Assessment of the adequacy of oxygen delivery requires not only measurement of the Po 2 in plasma but also measurement of the oxygen content in blood. Oxygen content, the sum of the oxygen bound to hemoglobin and that dissolved in plasma, can be measured directly by chemical or galvanic cell methods and can be estimated from the Po 2, the total hemoglobin concentration, and the percentage of oxyhemoglobin. The measurement or estimation of oxygen content of arterial and venous blood is required for calculating cardiac output by the Fick equation and for estimating the “shuntlike effect” in hypoxemic patients.
The chemical method for measuring total oxygen content involves liberating chemically bound oxygen and carbon dioxide from blood by adding ferricyanide, measuring the total quantity of gas displaced, and then absorbing the carbon dioxide with sodium hydroxide. This is the basis of the Van Slyke method, which served as the reference method for many years but is now used infrequently because of its demands on time and technical skill.208
Another method is the galvanic cell method, in which oxygen is chemically liberated from blood and transferred to a fuel cell, where a current is generated in proportion to the amount of oxygen delivered. This device yields values with an accuracy and precision similar to those obtained by the Van Slyke method.214
The method used most commonly for calculating oxygen content is measurement of the total hemoglobin concentration by the cyanmethemoglobin method,215 the percentage of oxyhemoglobin by a spectrophotometric method, and the dissolved oxygen as the product of arterial Po 2 and oxygen's solubility coefficient (0.003 mL per 100 mL blood).
Spectrophotometry is based on the discovery that substances differentially absorb various wavelengths of light. In the absence of other materials that absorb light at the same wavelength, the concentration of a substance in a solution is proportional to the amount of light absorbed. This method is especially applicable to hemoglobin analysis, because the various forms of hemoglobin (e.g., oxyhemoglobin, reduced hemoglobin, carboxyhemoglobin, sulfhemoglobin, methemoglobin) have characteristic spectra of light absorption. A simple two-wavelength spectrophotometer developed in 1900 could relate the amount of oxyhemoglobin to total hemoglobin but gave falsely high values when carboxyhemoglobin or methemoglobin was present. Three-wavelength instruments can simultaneously measure total hemoglobin, oxyhemoglobin, and carboxyhemoglobin; and a four-wavelength device is now marketed that enables measurement of methemoglobin as well.215
The importance of measuring carboxyhemoglobin lies not just in quantifying correctly the proportion of nonreduced hemoglobin that is actually available for carrying oxygen but also in identifying a cause of a shift in the position of the oxygen-hemoglobin dissociation curve. The presence of carboxyhemoglobin increases the affinity of adjacent hemoglobin molecules for oxygen, so the curve is shifted to the left (i.e., less oxygen is unloaded from oxyhemoglobin at normal tissue Po 2). Similar disorders may result from inherited abnormalities in hemoglobin structure, as with hemoglobin Chesapeake, for which 50% desaturation does not develop until the Po 2 is lowered to 19 mm Hg, as opposed to the normal 50% unloading point of 27 mm Hg.
Direct measurement of the Po 2 at which 50% of the binding sites on hemoglobin are saturated (P50) requires measurement of hemoglobin saturation after the blood sample is equilibrated at three oxygen pressures spanning the expected range (Po 2 values from 20 to 35 mm Hg are typical). Alternatively, a close estimate of Po 2 can be drawn from single measurements of Po 2 and hemoglobin saturation.215
Measurement of P50 is rarely needed in clinical practice. With the important exception of conditions in which carboxyhemoglobin is likely to be present in appreciable quantities (as in victims of fires or of exposure to closed-space combustion, or in heavy cigarette and cigar smokers), the estimation of blood oxygen content from measurements of arterial Po 2 and hemoglobin concentration usually provides sufficient information for decisions about clinical management.
Errors in the values used for such decisions arise most often from failures in the methods used for obtaining, transporting, and storing the sample of blood to be analyzed. For validity of the measurement, care must be taken to avoid contamination with room air or an excessive amount of anticoagulant when the sample is obtained, as well as leakage, diffusion, or consumption of gases while the sample is being transported and stored. For clinical utility, it is important that the sample be obtained with minimal discomfort and hazard. Syringes for arterial blood gas (ABG) collection are now designed to reduce the chance of a needle stick and to contain the optimal concentration of heparin.
Because of the adequacy of collateral flow in the event of occlusion of the sampled artery, the radial artery is the preferred site for obtaining the blood sample. However, in elderly patients and in patients with arteriosclerotic vascular disease, the adequacy of ulnar flow should be confirmed by the Allen test (appearance of palmar flush when the radial artery alone is decompressed). In infants, samples are most safely collected from the temporal or umbilical arteries. If radial and femoral arterial cannulation cannot be accomplished, then the brachial, axillary, and dorsalis pedis arteries should be considered. Sometimes physicians have been advised to avoid percutaneous cannulation of the brachial artery because of a lack of collateral vessels and the anatomic proximity to the median nerve. Aneurysm formation, thrombosis with loss of radial arterial pulse, and permanent median nerve neuropathy caused by hematoma have all been reported as complications of brachial cannulation. On the other hand, the literature associated with left heart catheterization states that percutaneous cannulation of the brachial artery is as “safe and effective” as surgical cutdown and arteriotomy.216
Venous blood gases (VBG) are increasingly used as alternative samples to estimate systemic carbon dioxide and pH that do not require ABG sampling. Using VBG in the intensive care unit is particularly attractive because most critically ill patients have a central venous catheter from which it is easy to obtain VBG quickly.
VBG analysis can be performed on a central venous sample (central venous catheter), mixed venous sample (pulmonary artery catheter), or peripheral venous sample (venipuncture).
Central venous samples have been well correlated with ABGs. Venous pH, serum  , and Pco
2 are used to assess ventilator and acid base status; arterial oxygen saturation to guide resuscitation during septic shock; but partial oxygen pressure in mixed venous blood has no practical value because the tissues have already extracted oxygen before reaching the venous system.
, and Pco
2 are used to assess ventilator and acid base status; arterial oxygen saturation to guide resuscitation during septic shock; but partial oxygen pressure in mixed venous blood has no practical value because the tissues have already extracted oxygen before reaching the venous system.
The difference between VBG and ABG depends on the site of venous sampling: central venous pH is usually 0.03 pH units lower than ABG, Pco
2 4 to 5 mm Hg higher than ABG, and little or no difference in serum  .217, 218 Mixed venous blood results are similar to central venous blood samples.219, 220 Similar comparisons have been published for peripheral venous pH and ABG.221, 222, 223, 224, 225
.217, 218 Mixed venous blood results are similar to central venous blood samples.219, 220 Similar comparisons have been published for peripheral venous pH and ABG.221, 222, 223, 224, 225
In clinical studies there is good agreement for pH and  between ABG and VBG in COPD patients, but not for Pco
2 or Po
2. To avoid misleading results, clinicians should avoid VBG in hemodynamically unstable patients and rely on ABGs instead.226, 227, 228 If serial monitoring of critically ill patients is done routinely, VBG and ABG should be compared periodically. Widespread clinical use of VBG is limited because of the lack of validation studies on clinical outcomes.229
between ABG and VBG in COPD patients, but not for Pco
2 or Po
2. To avoid misleading results, clinicians should avoid VBG in hemodynamically unstable patients and rely on ABGs instead.226, 227, 228 If serial monitoring of critically ill patients is done routinely, VBG and ABG should be compared periodically. Widespread clinical use of VBG is limited because of the lack of validation studies on clinical outcomes.229
An alternative to arterial puncture is to obtain a sample of “arterialized” capillary or venous blood. The assumption is that the vasodilation produced by heat or by application of vasodilator cream to a region with low metabolic activity will result in delivery of such an excess of arterial blood that local metabolism causes only small changes in Po 2, Pco 2, and pH. Under these circumstances, analysis of capillary or venous blood should provide close estimates of arterial values. The sites most commonly used for capillary sampling are the earlobe in adults and children and the lateral margin of the foot in infants; for sampling of arterialized venous blood, a dorsal hand vein is most commonly used. From any of these sites, the values obtained correlate well with arterial pH and Pco 2.230 The values for Po 2 are also accurate, except in patients with arterial hypotension or local reductions in flow to the sample site (as may develop with the vasoconstrictive response to severe hypoxemia), in newborns, and in patients with high arterial Po 2 (i.e., breathing oxygen-enriched gas mixtures).
Once the sample is obtained, it should be analyzed promptly or placed on ice to minimize the effects of continued cell metabolism on oxygen consumption. This is especially important for samples with very high white blood cell or platelet counts and in samples with Po 2 greater than 100 mm Hg.
However the sample is obtained, clinical interpretation of blood-gas values is possible only if the condition of the patient at the time of sampling is noted. Most important is a description of the oxygen pressure of the inspired gas mixture, but position, activity level, habitus, diet, and other factors can also influence arterial blood-gas values. Clearly, an excited or frightened patient may hyperventilate or breath-hold during arterial puncture. However, comparison with the values obtained from indwelling catheters shows that pain caused by arterial sampling does not routinely cause a change in alveolar ventilation.
Noninvasive Measurements
The appeal of an accurate, noninvasive means of assessing arterial blood-gas pressures is compelling, and several devices have been developed for transcutaneous measurement of oxygen saturation and pressure. Some devices already provide sufficiently accurate data on oxygen pressure to have been put into widespread clinical use. However, important physiologic and technologic barriers must be overcome for the development of a useful device for measuring carbon dioxide content and pressure.
Oxygen
Oximetry.
It was recognized more than 50 years ago that the principles of spectrophotometry could be applied to transcutaneous measurement of oxygen saturation in capillary blood by measuring the quantities of light at different wavelengths transmitted through or reflected from the earlobe. With dilation of local arterial vessels through application of heat or a vasodilating chemical (e.g., nicotine, alcohol), capillary oxygen saturation should approximate arterial oxygen saturation. Early ear oximeters were reported to give accurate data but were not widely accepted because of the practical difficulties in operating cumbersome instruments that were hard to calibrate, sensitive to changes in position, and likely to give unpredictable, unstable values.
The principle of oximetry depends on Beer's law, by which the amount of light absorbed by a solute in solution is related to the concentration of the unknown solute:
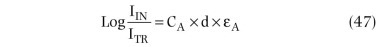
where IIN is the quantity of incident light, d is the distance through which light passes, CA is the concentration of the solute (e.g., hemoglobin), εA is the absorption coefficient, and ITR is the amount of light transmitted through A, the substance containing the solute.
When light is passed through tissue from the oximeter, the tissues absorb most of the light, and the amount of light absorbed does not vary with the cardiac cycle. During the cardiac cycle, however, there is a small increase in arterial blood, causing an increase in absorption of light. By comparing absorption at the peak and trough of the arterial pulse, the nonarterial sources of absorption become irrelevant (eFig. 25-22).
eFigure 25-22.
Factors influencing detection by pulse oximetry of light absorption through a pulsatile vascular bed.
Solid circles represent light absorption by hemoglobin in arterial blood and by the pulse of arterial blood; open circles represent light absorption by hemoglobin in venous blood; shaded zone represents absorption by tissue that absorbs incident light. d, distance through tissue that absorbs incident light.
The probe consists of two light-emitting diodes that emit light at specific wavelengths, usually 660 nm and 940 nm (eFig. 25-23). At these wavelengths the light absorption by oxyhemoglobin and by reduced hemoglobin is markedly different. A photodetector is placed across a vascular bed (finger, nose, or earlobe) from the light source. When the ratios (R) of pulsatile and baseline light absorption are compared at these two wavelengths, the ratio of oxyhemoglobin to reduced hemoglobin may be calculated:
| (48) |
eFigure 25-23.
Absorption spectrum of reduced hemoglobin (Hb) and oxyhemoglobin (HbO2).
Readings are made at 660 nm (red) and 940 nm (infrared) wavelengths.
The relationship between R and oxygen saturation was determined experimentally because there is no known function relating these two variables. A calibration curve was created by having healthy subjects with previously measured amounts of methemoglobin and carboxyhemoglobin breathe various hypoxic gas mixtures designed to produce oxygen saturations between 70% and 100%.231 A sample of arterial blood was obtained with each gas mixture, oxygen saturation was measured using a carbon monoxide oximeter (spectrophotometric hemoximeter), and the R value measured by the pulse oximeter was compared.
Because only two wavelengths are used, the pulse oximeter can measure only two substances, so it determines “functional saturation”:
| (49) |
Pulse oximeters are accurate when oxygen saturation is between 70% and 100%,232 but they may be inaccurate below that range. These devices may be misleading in the presence of abnormal hemoglobins (methemoglobin, carboxyhemoglobin, fetal hemoglobin), dyes (methylene blue, indocyanine green), increased bilirubin level, low perfusion states, anemia, increased venous pulsations, and external light sources.233
Continuous monitoring of oxygen saturation is considered the standard of care in operating rooms and recovery rooms.234 The limitation of oximetry is that it measures oxygen saturation, and the flattened shape of the upper portion of the oxygen-hemoglobin dissociation curve means that large changes in Po 2 result in small changes in arterial oxygen saturation. Oximetry is thus inherently insensitive to changes in Po 2 from the normal range that have diagnostic and clinical significance even if they do not result in important falls in oxygen delivery. The actual 95% confidence limits of ± 5% for arterial oxygen saturation reported for oximetry make this limitation all the more important.
Transcutaneous Oxygen Electrode.
The basic idea of the transcutaneous electrode is that a small polarographic electrode can measure the oxygen pressure in a bubble of gas trapped over the skin. Because the Po 2 at the surface of unwarmed skin is near zero, the success of the transcutaneous oxygen electrode depends on producing enough local vasodilation to compensate for the arterial-capillary gradient and also for the further loss of oxygen due to skin metabolism and imperfect diffusion of oxygen through the skin layer. This degree of vasodilation is achieved by warming the skin to 42° C. The increase in temperature causes local vasodilation and displaces the hemoglobin dissociation curve to the right. Thus the oxygen pressure is increased for any blood oxygen pressure, partially correcting for the losses of oxygen between the arterioles and the skin surface.
The skin surface electrode developed by Huch and associates235 in 1973 has proved accurate in continuous measurement of transcutaneous Po 2 in both healthy and sick newborns. Not surprisingly, transcutaneous Po 2 most severely underestimates arterial Po 2 when skin perfusion is decreased from hypotension. Arterial Po 2 was also underestimated in infants treated with tolazoline for pulmonary hypertension, possibly because the general peripheral vasodilation caused by the drug exceeded the effect of the preferential local vasodilation intended from local heating of the skin. These problems have not prevented the application of this noninvasive device for regulating oxygen therapy or ventilatory assistance to infants with neonatal respiratory distress syndrome, for monitoring apnea, for sleep studies, or for analyzing the impact of nursery procedures on oxygenation. The technique is not as well accepted for estimating Po 2 in adults, in whom the greater thickness of skin impairs oxygen diffusion. Carter and Banham236 reported that transcutaneous electrodes for measuring Po 2 and Pco 2 during exercise in adults with a variety of pulmonary disorders were reliable, provided the electrodes were kept at a slightly higher temperature (45° C) and the work rate intervals were gradual to allow for the slow response time. Unfortunately, most other workers have failed to confirm these findings,237 although the technique appears reliable for measuring the direction of change in arterial Po 2 in adult patients performing exercise238, 239 or during sleep studies.
Carbon Dioxide
Capnography.
The noninvasive measurement of Pco 2 is as important as the measurement of Po 2, especially in critical care units and operating rooms. The measurement of carbon dioxide during the respiratory cycle is called capnometry, and the display of the analog waveform is called a capnogram (eFig. 25-24). This measurement can be made using an infrared spectrometer, which is widely available. Care must be taken to calibrate the instrument regularly and to avoid interference by nitrous oxide, acetylene, and carbon monoxide.240 A mass spectrometer can be used to measure all the respiratory gases (carbon dioxide, oxygen, and nitrogen) as well as many anesthetic gases. This device is very rapid but very expensive. It is used most commonly in pulmonary and exercise laboratories and in operating rooms where sample gases from several patients may be tested in sequence. Capnography is valuable in detecting successful tracheal intubation versus esophageal intubation and in monitoring cardiopulmonary resuscitation. Capnography is also useful in detecting a variety of problems in ventilated patients, including an obstructed endotracheal tube, a disconnected airway, ventilator malfunction, severe pulmonary hypoperfusion, and pulmonary embolism.241, 242, 243
eFigure 25-24.
Normal capnogram.
During inspiration, Pco2 is zero. At the start of exhalation, Pco2 remains zero as gas from the anatomic dead space leaves the airway (comparable to phase I in the single-breath nitrogen washout test). Next, Pco2 rises rapidly as alveolar gas mixes with gas from the dead space (comparable to phase II in the single-breath nitrogen washout test), and then the Pco2 level stabilizes as gas from the dead space decreases and all the gas comes from alveoli containing carbon dioxide. The Pco2 at the end of the “alveolar plateau” is called the end-tidal Pco2 (comparable to phase III in the single-breath nitrogen washout test).
The end-tidal carbon dioxide may decrease suddenly in a life-threatening situation such as ventilator malfunction or a disconnected airway, as illustrated in eFigure 25-25. The end-tidal carbon dioxide data may be misleading when dead space is increased (increased anatomic dead space, dead space added in series to the airway of the patient, abnormally increased respiratory rate). In these situations there is an increased difference between arterial and end-tidal carbon dioxide, and the end-tidal level does not plateau. When wasted ventilation is increased because of regional increased ventilation relative to perfusion (e.g., restrictive or obstructive ventilatory defects, parallel dead space, or pulmonary vascular obstruction), differences between arterial and end-tidal carbon dioxide are also increased. In these situations the alveolar plateau is present but abnormally reduced. The shape of the waveform may be diagnostic of pulmonary vascular obstruction (eFig. 25-26).
eFigure 25-25.
Abnormal capnogram.
The sudden decrease in end-tidal Pco2 suggests a life-threatening situation in which the capnograph no longer detects carbon dioxide in the exhaled gas. This capnogram suggests the possibility of esophageal intubation, obstructed endotracheal tube, disconnected airway, or ventilator malfunction; these possibilities must be excluded before it can be assumed that the capnograph is malfunctioning.
eFigure 25-26.
Abnormal capnogram with an exponential decrease in end-tidal Pco2.
Note the progressively more sloping alveolar plateau, reflecting not only abnormal distribution of ventilation but also uneven perfusion relative to ventilation. This pattern suggests a potential life-threatening situation such as cardiac arrest, severe pulmonary hypoperfusion, or pulmonary embolism, as discussed in the text.
Colorimetric End-Tidal Carbon Dioxide.
In some centers, colorimetric measurement is used instead of capnography or other devices to monitor end-tidal carbon dioxide in critical care units, recovery rooms, and operating rooms. The method appears to be as accurate and sensitive as capnography. Both techniques appear useful in the management of critically ill patients.244
Transcutaneous Carbon Dioxide Electrode.
A device for transcutaneous measurement of Pco 2 has been developed. It also involves trapping gas above the skin layer, and measuring carbon dioxide pressure by photometric analysis with infrared light.245 The device has a long time-constant, and skin preparation requires stripping of the stratum corneum. Although the values for transcutaneous Pco 2 correspond closely to those for arterial Pco 2 in healthy subjects, erroneous values manifest with decreased skin perfusion, edema, and obesity.236 This electrode does appear useful in long-term monitoring,246 but its use in evaluating carbon dioxide transfer during exercise in adults is still controversial.237
New Technologies
Even at the bedside, in vitro blood-gas analysis requires limitation of the frequency of serial blood-gas measurements for two major reasons: blood loss and cost. Therefore a second major area of development involves in vivo or ex vivo blood-gas analyzers.247 These analyzers make it possible for the measurements to be made continuously, or as frequently as deemed desirable, without permanently removing blood or adding cost.248
So-called on-demand blood-gas monitors locate the sensors extravascularly but within the radial artery line, thereby avoiding the problems associated with intravascular measurements. Extravascular on-demand blood-gas analysis appears accurate, allows monitoring of trends of blood-gas changes, and decreases the risk for infection, the therapeutic decision time involved, and blood loss. However, until large patient studies are available, the clinical role of online blood-gas analysis cannot be clearly delineated.249
The successful relocation of blood-gas measurements from the standard clinical laboratory to a combination of blood-gas monitors and point-of-care analyzers at the bedside could change the practice of acute care medicine as did the introduction of laboratory-based blood-gas analysis more than 30 years ago. Therefore it is crucial for pulmonologists, intensivists, and their colleagues to be certain that these devices are accurate, reliable, and cost beneficial to avoid widespread application of yet another set of new technologies that provide more data, greater costs, and only questionable patient benefits.250, 251
Applications of Pulmonary Function Tests
Screening Studies
As suggested by Comroe and Nadel,252 screening pulmonary function tests should separate subjects who have normal lungs from those who have abnormal lungs in a few minutes of the patients' time, with little or no discomfort. The apparatus should be inexpensive and portable and should require little or no technical training to operate. Tests should be free from error and should pinpoint the specific functional abnormality and its location in a quantitative manner.
The single test that best fits this definition is spirometry. Spirometry is generally able to separate those with normal lungs from those with abnormal lungs; it is inexpensive, can be portable, and requires less training. Screening tests always include spirometry, with the understanding that the major limitation of spirometry is the inability to measure total lung volume. Additional tests may be added for screening pulmonary function as the situation demands.
Screening tests are useful for early detection of pulmonary or cardiopulmonary disease (e.g., emphysema, pulmonary fibrosis, pulmonary vascular disease); differential diagnosis of patients with dyspnea; detection of the presence, location, and extent of regional disease; evaluation of patients before surgical procedures; determination of the risk of certain diagnostic procedures; early detection of respiratory failure and monitoring of treatment in critical care units; quantitative evaluation of specific treatment in patients with known pulmonary disease; periodic examination of pulmonary function in workers whose occupations are associated with known pulmonary hazards; and epidemiologic studies of populations to provide clues regarding the pathogenesis of pulmonary disease.
In obstructive ventilatory defects, screening pulmonary function tests permit the diagnosis in asymptomatic patients on the basis of decreased FEF25%–75% from spirograms and decreased maximal flow at low lung volumes from flow-volume curves. In more advanced obstruction, FEV1, FEV1/FVC ratio, and maximal flows at all lung volumes may be abnormally decreased. The evidence of airway obstruction may be associated with uneven distribution of ventilation, as reflected by an abnormal single-breath nitrogen washout test, and associated hyperinflation, as reflected by increased RV and FRC. If the airway obstruction is severe (FEV1/FVC ratio < 0.4), the TLC measured by single-breath dilution may be underestimated significantly.43, 44
In restrictive ventilatory defects, if airway function is normal, screening pulmonary function tests permit an early diagnosis by the finding of an increased FEV1/FVC ratio associated with increased maximal expiratory airflow. With more advanced disease, TLC, VC, and associated lung volumes are decreased, with evidence of uneven distribution of ventilation. In mixed ventilatory defects, the interpretation of the spirogram may be aided by examining FEV1 as a percentage of predicted normal rather than as a percentage of FVC; however, mixed defects are more easily defined by measurement of TLC using a multiple-breath dilution technique or, preferably, body plethysmography.
In patients with neither a restrictive nor an obstructive ventilatory defect, the finding of an isolated decreased single-breath Dl CO may be the first clue to the presence of an interstitial process, emphysema, or pulmonary vascular obstruction. In general the degree of severity of a particular pulmonary pattern is indicated by the decrease in percentage of predicted values, illustrated in Table 25-2 .
Table 25-2.
Severity of Pulmonary Impairment229
| Impairment | FEV1* | TLC† | VC† | DlCO |
|---|---|---|---|---|
| Normal | ±95% CI | ±95% CI | ±95% CI | ±95% CI |
| Mild | <LLN and ≥70 | <LLN and ≥70 | <LLN and ≥70 | <LLN and ≥60 |
| Moderate | 60−69 | <70 and ≥60 | <70 and ≥60 | <60 and ≥40 |
| Moderately severe | 50–59 | <60 and ≥50 | ||
| Severe | 35–49 | <60 | <50 and ≥35 | <40 |
| Very severe | <35 | <35 |
CI, confidence interval; DlCO, carbon monoxide diffusion in the lung; FEV1, forced expiratory volume in 1 second; LLN, lower limit of the 95% confidence interval; TLC, total lung capacity; VC, vital capacity.
Airflow obstruction is based on a decreased FEV1/VC ratio. If the ratio is decreased below the lower 95% confidence interval (CI), the severity of airflow is graded on the percent predicted FEV1.
Pulmonary restriction is based on decreased TLC. If TLC is not available, a reduction in VC without a reduction in the FEV1/VC ratio is a “restriction of the volume excursion of the lung.”
If the screening test results are normal but the patient has symptoms, more complete pulmonary function studies are indicated. Such a diagnostic approach is essential if the ABG levels reveal evidence of chronic hyperventilation. Chronic hyperventilation (decreased arterial Pco 2 with evidence of renal compensation and a near-normal arterial pH) is observed in several major pulmonary disorders, probably reflecting an abnormal ventilatory drive.253, 254, 255 In patients with cough or with a history of wheezing with respiratory tract infections, bronchial provocation testing can determine whether the patient has abnormal airway responsiveness. If the patient complains of effort dyspnea or fatigue, particularly if the symptoms have caused a significant change in lifestyle and the screening tests do not explain the symptoms, an exercise test is indicated (see Chapter 26).
Patterns of Response
Obstructive Ventilatory Defects
Airway obstruction is characterized by a decrease in flow (Table 25-3 ). Supplementary data confirming obstruction include increased RV and Raw, uneven distribution of ventilation, and significant reversibility of airway obstruction, with or without decreased diffusing capacity. It is noteworthy to point out that some patients with normal lungs may have a decrease in the lower limits of normality for the FEV1/FVC ratio with a normal FEV1, and all other measured parameters may be normal. In this situation, this would be considered a normal variant.256Alternatively, if this pattern is observed in a subject with curvilinearity of the flow-volume curve, accompanied by other patterns suggesting airway obstruction, this could suggest early airway obstruction.
Table 25-3.
Obstructive Ventilatory Defect
| CHARACTERISTICS OF OBSTRUCTIVE VENTILATORY DEFECT |
|
| SUPPLEMENTAL DATA CONFIRMING OBSTRUCTION |
|
DlCO, diffusing capacity of the lung for carbon monoxide; MVV, maximal voluntary ventilation; RV, residual volume; VC, vital capacity.
Adapted from Welch MH: Ventilatory function of the lungs. In Guenter CA, Welch MH, editors: Pulmonary medicine, Philadelphia, 1977, JB Lippincott, pp 72–123.
Because of the increased incidence of obesity in the United States, it is important to be aware of the deleterious effect of obesity on patients with airflow obstruction. In addition, the increased mass of the chest wall decreases expiratory reserve volume, subsequently FRC, and then TLC. As the expiratory volume in reserve diminishes, tidal volume is shifted toward RV, graphically depicted when the tidal volume loop is superimposed on the flow-volume loop. As the patient breathes at lower lung volumes, Raw increases, (A–a)Po 2 differences increase, and respiratory symptoms increase. This situation is worsened in the supine posture. These effects of obesity are particularly limiting in patients with airflow obstruction. (see Chapter 98)
Reversibility.
Another approach to the differential diagnosis of the obstructive pattern is the assessment of reversibility of impaired expiratory airflow. Reversibility may appear acutely in response to administration of bronchodilator aerosols, chronically in response to a variety of airway treatments, or spontaneously during remission of bronchial asthma. Reversibility implies a better prognosis than fixed obstruction and may have considerable significance in planning a treatment program.
The ATS recommends that VC (slow or forced) and FEV1 be the primary spirometric indices used to determine bronchodilator response.6 Total expiratory time should be considered when using FVC to assess the dilator response because, in obstructed patients, FVC increases when expiratory time increases. A 12% increase above the prebronchodilator value and a 200-mL increase in either FVC or FEV1 indicate a positive bronchodilator response in adults. FEF25%–75% and instantaneous flow rates should be considered only secondarily in evaluating reversibility. They must be volume-adjusted, or the effect of changing FVC must be considered in the interpretation (Fig. 25-17 ). Ratios such as FEV1/VC should not be used to evaluate reversibility.
Figure 25-17.
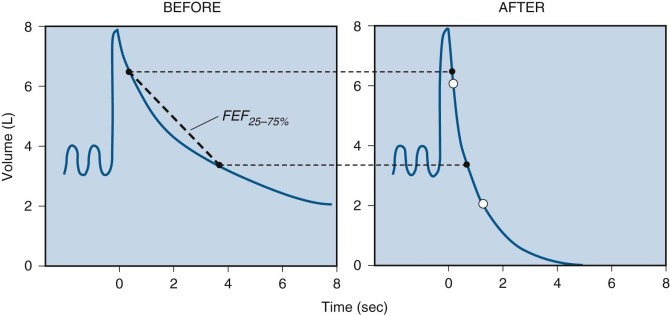
Schematic illustration of volume adjustment to calculate the isovolume FEF25%–75%, or forced expiratory flow between 25% and 75% of forced vital capacity.
Left, Before administration of bronchodilator, the FEF25%–75% is calculated from a line connecting two points on the volume-time curve of the forced vital capacity (FVC). One solid circle indicates when 25% of the FVC is exhaled (6.5 L), and the other solid circle indicates when 75% of the FVC is exhaled (3.5 L). This volume change (3.0 L) develops in 3.4 seconds, so the FEF25%–75% is 0.88 L/sec. Right, After administration of bronchodilator, one open circle indicates when 25% of the FVC is exhaled (6.0 L), and the other open circle indicates when 75% of the FVC is exhaled (2.0 L). This volume change develops in 1.3 seconds, so the FEF25%–75% is 3.0 L/sec. The values based on the “before” volumes from the pretreatment curve (solid circles) have been extended to the posttreatment (“after”) curve. The volume-adjusted or isovolume FEF25%–75% is determined from a line connecting the solid circles on the “after” graph. In this case the volume change is the same as that observed in the “before” graph, or 3.0 L, but it took place in only 0.6 second, so the isovolume FEF25%–75% is 5.0 L/sec, a marked improvement induced by the bronchodilator. This approach was developed because early reports indicated that some patients appeared to have significant improvement in forced expiratory volume in 1 second (FEV1) but not in FEF25%–75% when no volume adjustment was made in the calculation of FEF25%–75%. When a volume adjustment was made in the calculation of FEF25%–75%, there was improvement in both FEV1 and FEF25%–75%, as illustrated.
Eliasson and Degraff257 reported that these conventional criteria may still be misleading. In their study these criteria were not useful for distinguishing patients with asthma from those with other forms of chronic airway obstruction in a clinically defined population. Furthermore, when applied to a patient population, they resulted in selection of the most obstructed patients (a contradiction of the definition of reversibility). Instead, these authors suggested that the difference in FEV1 before and after bronchodilator administration (expressed either as an absolute value or as a percentage of predicted FEV1) appeared more appropriate as an expression of reversibility. Their study indicated that, when one compares results from two different bronchodilator studies, careful attention must be paid to the definitions of patient populations, the definitions of obstruction and reversibility, the degree of obstruction present, and the methods used to calculate bronchodilator response. Jain et al258 studied 321 physician-diagnosed asthmatic patients and found a significant proportion had increased RV and abnormal RV/TLC ratio in the presence of normal FEV1/FVC ratio and no significant bronchodilator response.
Failure to demonstrate significant responses to acute bronchodilator therapy does not rule out reversible airway obstruction. Many reports confirm that asthmatic patients with completely reversible airway obstruction may initially fail to respond to inhaled bronchodilators.259
Obstructive Ventilatory Defects
Reversibility.
Inhalation of albuterol (180 µg) 10 to 20 minutes before spirometry testing is used in many laboratories to test reversibility. Because many patients with asthma or other forms of airway disease do not respond initially to β-adrenergic agonists, particularly at low doses, it is worth considering evaluation of reversibility after administration of ipratropium bromide aerosol (36 µg). However, the maximal effect of ipratropium bromide may take 30 to 45 minutes. Furthermore, many patients with reversible airway obstruction do not respond to a standard clinical dose of either class of dilator, but airway obstruction still reverses completely if they are treated with larger doses. Thus, in some patients with airway obstruction who have never been treated before and who do not respond to a standard clinical dose of inhaled dilator, we often administer a cumulative dose-response protocol. Spirometry is measured before (baseline) and 15 minutes after administration of progressively increasing doses of albuterol (180 µg) or ipratropium bromide (36 µg) aerosol. The aerosol is administered every 15 minutes until a maximal increase in FEV1 or FVC is attained or limiting symptoms are reached. In this time interval, both agents produce at least 80% of their maximal response, and most patients respond maximally after receiving 8 to 10 puffs.
In fact, one of the pharmacologic benefits attributed to corticosteroids in this situation is that they enhance responsiveness to β-adrenergic agonists.260 We have observed that many patients with severe chronic airflow obstruction are undertreated with the usual treatment regimens. These patients show significant bronchodilation during exercise and in response to increased β-adrenergic treatment.
Bronchial Provocation.
Provocation tests may be extremely useful in the diagnosis and management of patients with asthma or occupational asthma and in the differential diagnosis of patients with chronic cough, wheezing, or intermittent dyspnea. Although many laboratories use spirometry to evaluate the airway response, measurement of Raw in a body plethysmograph is more sensitive, more specific for abnormalities in airway tone, and usually easier for the patient to perform than tests that depend on inspiration to TLC followed by a forced exhalation. In limited numbers of patients, tests with specific allergens may be helpful in the evaluation of allergic asthma. Similarly, in a small number of patients suspected of having occupational asthma, specific challenge with agents found in the workplace may be useful in the diagnosis. However, the referring physician should be aware that these challenge tests are dangerous and tedious, usually require hospitalization for observation, and may not be useful if the patient is exposed to multiple agents in the workplace. (When multiple agents are involved, provocation testing for each agent is usually not practical because it would require many weeks and repeated hospitalizations at great expense to assess each and every agent at multiple concentrations or doses [see Chapter 72].)
Tests of Nonspecific Airway Responsiveness.
Abnormal airway responsiveness is viewed by many as a characteristic feature of asthma. It may also be found in patients with chronic bronchitis and cystic fibrosis. Although a variety of stimuli have been used, including exercise and eucapnic ventilation, the most common stimuli are histamine and methacholine. Responses to these stimuli have good correlation and reproducibility.261 These agents are delivered in incremental concentrations until a desired effect on pulmonary function is achieved; usually, less than 0.1 mg/mL is the initial concentration to avoid inducing an inordinately severe reaction.
The nonspecific airway challenge begins with a diluent control aerosol, and responses are reported relative to the diluent value. The dose of agonist is expressed on the logarithmic abscissa as (1) cumulative inhalation breath units (the equivalent of one breath of a concentration containing 1 mg/mL); (2) cumulative amount of agonist (in micromoles) delivered from the nebulizer; and (3) the concentration inhaled (in milligrams per milliliter). The end point is the dose causing a decrease in FEV1 of 20% or a decrease in specific airway conductance of 40%. Pulmonary function measurements should be made 3 to 5 minutes after delivery of the aerosol and repeated in 5 minutes.
FEV1 is the most common test used to evaluate the outcome of this procedure, although specific Raw may be more sensitive. Medications, baseline airway function, respiratory infections, and exposure to specific allergens and chemical sensitizers influence responses. Bronchodilators, antihistamines, and other agents that decrease bronchial responsiveness should be withheld before the test.262
Compared to methacholine and histamine, which have direct effects on airway smooth muscle to cause airway narrowing, so-called indirect challenge tests (which cause airway narrowing indirectly by triggering mast cell degranulation by osmotic stimuli, or mediator release from inflammatory cells) may have an important place in the assessment of asthma. Such indirect challenge tests (including exercise-induced bronchoconstriction, eucapnic voluntary hyperpnea, hypertonic and hypotonic aerosols, and mannitol) are useful for monitoring treatment with inhaled corticosteroids.263 Indirect tests identify subjects with the potential for exercise-induced bronchoconstriction and therefore are useful for members of the armed services, firefighters, police, and elite athletes. A positive indirect test result suggests that inflammatory cells and their mediators are present in sufficient numbers and concentration to indicate that asthma is active at the time of the test. A negative test result in a known asthmatic patient means good control or mild disease. Healthy subjects do not experience bronchoconstriction during the indirect tests.263
Although histamine and methacholine are well-established agents for identifying airway hyperresponsiveness, the response to these agonists is not specific for the diagnosis of asthma. Both agents are better at excluding the diagnosis of asthma than making the diagnosis. Furthermore, neither agonist can establish or exclude the diagnosis of exercise-induced asthma, so they are not appropriate for assessment of persons at occupational risk or of athletes. Identification of airway hyperresponsiveness by pharmacologic agents does not indicate who will respond to inhaled corticosteroids, nor does it distinguish between the effects of different doses of steroids. Many asthmatic patients remain reactive to histamine and methacholine long after treatment, so airway hyperresponsiveness is not useful as a guide to withdrawal from steroid treatment.
According to Anderson and Brannan,263 dry powder mannitol can identify those patients with exercise-induced asthma who will respond to inhaled corticosteroids. A positive response to mannitol depends on activation of mast cells secondary to osmotic changes in the airways, release of leukotrienes and other mediators, and development of active inflammation in the airways.263 If the mannitol response is positive, sufficient numbers of inflammatory cells are present to release enough mediators to cause bronchoconstriction. The response to mannitol is reduced by corticosteroid therapy and may disappear within 6 to 8 weeks. Thus mannitol responsiveness may be able to predict the risk for a clinical flare during reduction of the corticosteroid dose. Mannitol alone may be able to identify those patients who will respond to inhaled corticosteroids and also (in patients already treated with corticosteroids) serve to guide the reduction of the steroid dose.264, 265, 266, 267, 268
Tests of Specific Airway Responsiveness.
Incremental allergen concentrations are given sequentially until the desired pulmonary function change develops. The response to inhaled allergen depends on both allergic sensitivity, as reflected by skin test, and nonspecific airway responsiveness, as reflected by histamine or methacholine responsiveness.
Most commercial allergens are obtained as lyophilized extracts or as concentrated solutions. These retain potency indefinitely when stored at −20° C. Thus the original guidelines of a starting concentration of ragweed pollen extract (AgE) that produces a 2+ reaction (larger than a 5-mm wheal) after intradermal injection is probably safe but may result in many doses having to be delivered in some patients. With a 2+ skin test at 0.0005 µg AgE/mL, an aerosol of 0.025 µg AgE/mL is used; with a 2+ skin test at 0.005 µg AgE/mL, an aerosol of 0.05 µg AgE/mL is used; and with a 2+ skin test at 0.05 µg AgE/mL, the same concentration of AgE is used in the aerosol challenge.
Aerosol delivery in North America is usually by (1) intermittent generation of aerosol during inspiration from a DeVilbiss 646 nebulizer connected to a dose-metering device that controls flow of compressed air at 20 psi for a fixed time, at a flow rate of 750 mL/min or less269; or (2) by a Wright nebulizer with aerosol delivered to a face mask with a nebulizer output of 0.13 to 0.16 mL/min.270 Respiratory rate, tidal volume, and inspiratory flow rate are kept constant for a fixed time interval, and the volume of aerosol solution administered is 3 mL.
Objective Evaluation of Lung Function in Management of Asthma.
Peak flowmeters play a very important role in National Institutes of Health–based guidelines for proper asthma management. Peak flowmeters offer advantages of convenience and portability; however, peak flowmeters are also less reproducible than standard spirometry. Thus it is critical that physicians recognize the importance of the use of spirometers in the initial assessment of the patient suspected of having asthma and in periodic monitoring of the management program.
According to the National Institutes of Health guideline for the diagnosis and management of asthma (Expert Panel Report No. 2, 1977), “spirometry measurements (FEV1, FVC, FEV1/FVC) before and after the patient inhales a short-acting bronchodilator should be undertaken for patients in whom the diagnosis of asthma is being considered.”271 Office-based physicians caring for asthma patients should have access to spirometry, which is useful both in diagnosis and in periodic monitoring of airway function. When office spirometry shows severe abnormalities or if questions arise regarding test accuracy or interpretation, the Expert Panel recommends further assessment in a specialized pulmonary function laboratory.
These objective measurements of pulmonary function (e.g., peak flow, spirometry) are necessary for the diagnosis of asthma because the medical history and physical examination do not reliably exclude other diagnoses or characterize the lung impairment. Physicians seem to be able to identify the presence of airflow obstruction,272 but they have a limited ability to assess the degree of obstruction273 or to predict whether it is reversible.272 Furthermore, large segments of our population, particularly older adults, appear to have undiagnosed airflow obstruction and also undiagnosed asthma. These patients are neither detected nor diagnosed properly without spirometric assessment.274, 275, 275a
Bullous Lung Disease.
In certain obstructive ventilatory defects, a variety of specific tests may prove useful. For example, in a patient with a localized bulla who is being considered for surgical resection of the lesion, it is important to show that the bulla, and not intrinsic airway disease or emphysema, is responsible for the pulmonary function abnormalities and disability. An exercise study can quantitate the disability caused by the bulla or associated disease. Physiologic studies relating Raw and 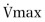 to static Pl can differentiate the effects of loss of lung elastic recoil from those of intrinsic airway disease. Radioisotope perfusion lung scans, pulmonary angiograms, and thin-section CT scans can determine whether the vascular defects are localized (i.e., bullae) or diffuse (i.e., emphysema). These studies may also indicate whether the bulla is compressing normal lung tissue. This possibility can be confirmed by a shunt study to determine whether compression of normal lung tissue by the bulla is having a shuntlike effect on arterial Po
2. Radioisotope ventilation lung scans also help determine whether the ventilatory defects are localized (i.e., bullae) or diffuse (i.e., emphysema). The single-breath Dl
CO is useful for detecting decreased numbers of pulmonary capillaries, reflecting the presence of pulmonary emphysema. Measurement of “trapped gas” by comparison of TLC measured by single-breath gas dilution and by body plethysmography should provide an estimate of the size of the bulla. This same multipronged approach may be useful for evaluating patients with advanced emphysema before consideration for possible surgical treatment (see later discussion).
to static Pl can differentiate the effects of loss of lung elastic recoil from those of intrinsic airway disease. Radioisotope perfusion lung scans, pulmonary angiograms, and thin-section CT scans can determine whether the vascular defects are localized (i.e., bullae) or diffuse (i.e., emphysema). These studies may also indicate whether the bulla is compressing normal lung tissue. This possibility can be confirmed by a shunt study to determine whether compression of normal lung tissue by the bulla is having a shuntlike effect on arterial Po
2. Radioisotope ventilation lung scans also help determine whether the ventilatory defects are localized (i.e., bullae) or diffuse (i.e., emphysema). The single-breath Dl
CO is useful for detecting decreased numbers of pulmonary capillaries, reflecting the presence of pulmonary emphysema. Measurement of “trapped gas” by comparison of TLC measured by single-breath gas dilution and by body plethysmography should provide an estimate of the size of the bulla. This same multipronged approach may be useful for evaluating patients with advanced emphysema before consideration for possible surgical treatment (see later discussion).
Emphysema: Lung Volume Reduction Surgery
Lung volume reduction surgery (LVRS) for emphysema, first introduced by Brantigan276 in 1954, is based on the theory that reduction in lung volume in patients with diffuse emphysema improves lung elastic recoil, increases radial traction on bronchi, and thus increases expiratory flow and relieves dyspnea. After initial disappointing results, this approach was revived as a therapy for COPD in the early 1990s, but it was not until Cooper and Patterson277 reported their first 20 operations using the sternotomy approach in 1995 that enthusiasm for LVRS increased dramatically.
The conventional explanations for the beneficial effects of LVRS are the increased elastic recoil at TLC278 and increased ability of inspiratory muscles to generate force.279 An important concept concerning the mechanism of LVRS was proposed by Fessler and Permutt.280 They developed a mathematical analysis and graphic model of the mechanism of improvement in both VC and expiratory airflow, based on their concept of the interaction between lung function and respiratory muscle function. They extended their analysis from LVRS to previously published data on mechanical properties of the lungs in patients with alpha1-antitrypsin deficiency, COPD, and asthma. In each of these diseases, a major determinant of airflow limitation is the ratio of residual volume to total lung capacity (RV/TLC). Their analysis suggested that RV/TLC determines the improvement in pulmonary function following surgical treatment of emphysema. Regardless of the underlying disease, impaired airflow appears to be due to the mismatch between the size of the lung and the size of the chest wall; surgical resection of lung tissue improves the matching. Fessler and Permutt also suggested that their analysis can be used to guide patient selection for LVRS.
Thus, when LVRS improves airflow limitation, it does so by improving the fit between lungs and chest wall by decreasing RV more than TLC. Although increased elastic recoil at TLC and increased ability of inspiratory muscles to generate force are the conventional explanations for the beneficial effects of LVRS, neither of these factors would necessarily increase VC as well as FEV1.
This analysis demonstrates that, regardless of the cause of increased RV (emphysema, increased airway closing pressure, or a normal lung contained in a chest wall that is too small), LVRS improves FEV1. The level of RV/TLC is of greater importance than the specific cause of the increased RV/TLC ratio. Furthermore, there is little difference in improvement in FEV1 whether the surgeon removes completely nonfunctional lung tissue or tissue with the same function as the lung left behind. The implications for selection criteria are straightforward: if increased FEV1 is the goal of LVRS, then the optimal candidates are those with the highest RV/TLC. Finally, the critical factor in comparing outcomes among patients, procedures, or centers is the amount of lung removed, which cannot be estimated accurately by weighing the resected specimens. Fessler and Permutt280 suggested that the best measurement of the fraction of lung resected may be derived from the ratio of residual volumes: 1 − RVA/RVB, where RVA is the residual volume before LVRS and RVB is the residual volume after LVRS. Several studies have examined the mechanisms responsible for improved function in these patients. Fessler and associates281 studied 78 patients and found that the results supported their model, as discussed previously; that is, RV/TLC is an important predictor of improvement in FVC because it reflects the mismatch in size between the hyperinflated lungs and the surrounding chest, and increased FVC is an important determinant of increased FEV1 after LVRS. Ingenito and associates282 performed an elegant study of 37 patients undergoing LVRS and found that increased FEV1 (increased by 28% ± 44%) correlated closely with increase in maximal flow of 78% ± 132%. The increased expiratory flow was largely due to increased lung recoil pressure, and FEV1 improved without changes in small airway conductance, airway closing pressure, or lung compliance. These results support the Fessler and Permutt concept that “resizing of the lung to the chest wall” is the primary mechanism by which LVRS improves function. In another study, Mineo and colleagues283 showed dramatic improvement in right heart function during exercise following LVRS; furthermore, the improvement in right ventricular ejection fraction during exercise correlated closely with the change in RV/TLC ratio, also supporting the Fessler and Permutt theory.
In a review of respiratory muscles, Laghi and Tobin284 strongly supported the Fessler and Permutt concept. They argued that an imbalance between hyperinflated lungs and a relatively small rib cage was primarily responsible for abnormal respiratory muscle function in patients with COPD; therefore reducing the volume of the lungs improves the match between the lungs and rib cage, and thereby the capacity of the respiratory muscles to generate pressure. They noted that most patients undergoing LVRS show improved expiratory flow and less hyperinflation and air trapping. These effects result from increased lung elastic recoil and better matching of lung and rib cage size, which also leads to decreased respiratory pressure required for tidal breathing and decreased cost of carbon dioxide removal. The mechanisms responsible for these benefits include improved alveolar ventilation, decreased operating lung volumes, decreased dynamic positive end-expiratory pressure, and decreased dynamic lung and chest wall stiffness. According to Laghi and Tobin, the surgery also improves the length-tension relationship of the respiratory muscles. They also noted improved coupling between inspiratory effort and output of the diaphragm, which correlated closely with improved 6-minute walk test results.
Long-term benefits are more difficult to identify. Improved FEV1 appears to peak at 3 to 6 months and then declines 100 to 150 mL or more over the subsequent year. Improvement in TLC and RV may be more stable in the first year. Gelb and associates285 reported that FEV1 decreased 141 ± 60 mL per year over 3.8 ± 1.2 years following surgery. Obviously, many more long-term data are needed before it is clear whether this procedure is useful in treatment of COPD.286, 287
Restrictive Ventilatory Defects
A parallel decrease in FEV1 and FVC with a normal or increased FEV1/FVC ratio suggests a restrictive defect, but this diagnosis requires a decreased TLC by plethysmograph or multiple-breath dilution method. Supplementary data confirming restriction include decreased single-breath Dl CO, uneven distribution of ventilation, chronic alveolar hyperventilation, and increased (A–a)Po 2 (Table 25-4 ). Because static lung elastic recoil pressure depends on lung volume, the diagnosis of a restrictive ventilatory defect does not usually require measurement of pressure-volume curves of the lung. In patients with mixed disease or in whom poor cooperation is suspected, measurement of pressure-volume curves may be helpful.
Table 25-4.
Restrictive Ventilatory Defect
| CHARACTERISTICS OF RESTRICTIVE VENTILATORY DEFECT |
|
| SUPPLEMENTAL DATA CONFIRMING RESTRICTIVE PATTERN |
|
(A–a)Po2, alveolar-arterial Po2 difference; MVV, maximal voluntary ventilation; TLC, total lung capacity; VC, vital capacity.
Adapted from Welch MH: Ventilatory function of the lungs. In Guenter CA, Welch MH, editors: Pulmonary medicine, Philadelphia, 1977, JB Lippincott, pp 72–123.
Pulmonary function tests have been widely accepted and used in the management of interstitial lung diseases. Although the tests performed have changed little over the past several decades, extensive literature has been published highlighting their clinical role in the diagnosis, staging, prognosis, and follow-up of patients with a wide variety of interstitial lung diseases. Pulmonary function testing aids in the evaluation and management of patients with interstitial lung disease. Such function tests can provide a baseline estimation of prognosis and can be used to monitor disease progression and response to therapy. The FVC and Dl CO are the most valuable serial measurements,288, 289 but further data are required to examine composite scoring and exercise gas exchange.290
Two groups have tried to develop a systemic approach to improving the initial evaluation of these patients. Wells and colleagues291 of Brompton Hospital developed a composite physiologic index using Dl CO, FVC, and FEV1, which is designed to reflect the morphologic extent of pulmonary fibrosis, with the goal of excluding confounding emphysema in patients with pulmonary fibrosis. Survival of 106 patients with pulmonary fibrosis was predicted more closely by the composite index than any single pulmonary function test. King and associates292 collated their extensive experience with interstitial lung disease at the National Jewish Medical and Research Center in a new scoring system and survival model for interstitial lung disease. They reviewed 238 patients with usual interstitial pneumonia confirmed by biopsy to develop a scoring system that would predict survival in newly diagnosed patients, based on clinical, radiologic, and physiologic data. In contrast to the Brompton index, the National Jewish Medical and Research Center system found that pulmonary function data contributed 45% of the score as follows: Dl CO/Va, 5%; (A–a)Po 2 at rest, 10%; and gas exchange during exercise, 30% (of which arterial Po 2 during exercise contributed 10.5%). Thus King and colleagues found that arterial hypoxemia is the most important single factor limiting exercise in these patients.
Three large referral centers have published qualitatively similar observations: a decrease in pulmonary function, especially FVC, with elapsed time after referral to the tertiary center predicts decreased survival in patients with idiopathic pulmonary fibrosis.290, 293, 294, 295 Apparently, the changes in pulmonary function as early as 6 months after referral, rather than baseline pulmonary function or histopathologic characteristics, are of critical importance with respect to ultimate outcome. Future studies are essential to determine the specific features of patients with idiopathic pulmonary fibrosis who experience varying rates of deterioration with time, hopefully to understand the mechanisms involved and to improve treatment. (For comparison with other patterns of abnormal function, see Table 25-5 .)
Table 25-5.
Patterns of Abnormal Function for Various Pulmonary Disorders
| Emphysema | Chronic Bronchitis | COPD | Asthma | RESTRICTION |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Test | Parenchymal | Chest Wall | Neuromuscular | PVO | CHF | ||||
| FVC (L) | (N)⇒ ↓ | (N)⇒ ↓ | (N)⇒ ↓ | (N)⇒ ↓ | ↓ | ↓ | N⇒ ↓ | N | ↓ |
| FEV1 (L) | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | N⇒ ↓ | N | ↓ |
| FEV1/FVC (%) | ↓ | ↓ | ↓ | N⇒ ↓ | N⇒↑ | N | N | N | N⇒ ↓ |
| FEF (L/sec) | ↓ | ↓ | ↓ | ↓ | N⇒ ↓ | ↓ | N⇒ ↓ | N | ↓ |
| PEF (L/sec) | ↓ | ↓ | ↓ | ↓ | N⇒ ↓ | ↓ | N⇒ ↓ | N | ↓ |
| MVV (L/min) | ↓ | ↓ | ↓ | ↓ | N⇒ ↓ | ↓ | N⇒ ↓ | N | ↓ |
| FEF50 (L/sec) | ↓ | ↓ | ↓ | ↓ | N⇒ ↓ | ↓ | N⇒ ↓ | N | ↓ |
| TLC (L) | ↑ | N⇒↑ | ↑ | N⇒↑ | ↓ | ↓ | N⇒ ↓ | N⇒↓* | ↓ |
| RV (L) | ↑ | ↑ | ↑ | ↑ | ↓ | ↓ | N⇒↑ | N | ↑⇒N⇒ ↓ |
| RV/TLC (%) | ↑ | ↑ | ↑ | ↑ | N | N⇒↑ | N⇒↑ | N | ↑⇒N⇒ ↓ |
| DlCO (mL/min/mm Hg) | ↓ | N⇒↓ | N⇒↓ | ↑⇒N | ↓ | N⇒↓ | N⇒↓ | ↓⇒N⇒↑ | ↓ |
| Pao2 (mm Hg) | N⇒↓ | ↓ | N⇒↓ | N⇒↓ | ↓ | N | N⇒↓ | N⇒↓ | N⇒↓ |
| Sao2 (%) | N⇒↓ | ↓ | N⇒↓ | N⇒↓ | ↓ | N | N⇒↓ | N⇒↓ | N⇒↓ |
| Paco2 (mm Hg) | N⇒↑ | ↑ | N⇒↑ | N⇒↓ | N⇒↓ | N | N⇒↑ | ↓ | N⇒↓ |
| pH (−log[H+]) | N⇒↓ | N⇒↓ | N⇒↓ | N⇒↑ | N⇒↑ | N | N⇒↓ | N | N⇒↑ |
| Raw (cm H2O/L/sec) | ↑ | ↑ | ↑ | ↑ | ↓⇒N⇒↑ | N⇒↑ | N⇒↑ | N | N⇒↑ |
| CstL (L/cm H2O) | ↑ | N | N⇒↑ | N⇒↑ | ↓ | N | N | N | N⇒↓ |
| CdynL (L/cm H2O) | ↓ | N⇒↓ | N⇒↓ | N⇒↓ | ↓ | N | N | N | N ⇒↓ |
| Pstmax (cm H2O) | ↓ | N | N⇒↓ | ↓ | N⇒↑ | N⇒↓ | N⇒↓ | N | N⇒↓ |
| Phase III (% N2/L) | ↑ | ↑ | ↑ | ↑ | N⇒↑ | N | N | N | N⇒↑ |
| Phase IV (% VC) | A | ↑⇒A | ↑⇒A | ↑⇒A | N⇒↑ | N | N | N | N⇒↑ |
| MEP (cm H2O) | N⇒↓ | ↑ | ↓⇒N⇒↑ | N | N⇒↓ | N⇒↓ | ↓↓ | N | N |
| MIP (cm H2O) | ↓ | N | N⇒↓ | N | N⇒↑ | N⇒↑ | ↓↓ | N | N |
A, often absent; N, normal; (N), occasionally normal; ⇒, to; ↑, increased; ↓, decreased. CdynL, dynamic compliance of the lung; CHF, congestive heart failure; CstL, static compliance of the lung; DlCO, diffusing capacity of the lung for carbon monoxide; Dl/Va, diffusing capacity of the lung/alveolar volume; FEF, forced expiratory flow; FEF50, forced expiratory flow after 50% of vital capacity exhaled; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; MEP, maximal expiratory pressure; MIP, maximal inspiratory pressure; MVV, maximal voluntary ventilation; Paco2, arterial Pco2; Pao2, arterial Po2; PEF, peak expiratory flow; Pstmax, maximal static pressure; PVO, pulmonary vascular obstruction; Raw, airway resistance; RV, residual volume; Sao2, arterial oxygen saturation; TLC, total lung capacity.
Volumes are decreased in the presence of primary pulmonary hypertension but not chronic thromboemboli.
Mixed Obstructive and Restrictive Ventilatory Defects
This pattern of pulmonary function defect is not common; however, the ATS guidelines do not provide suggestions for how to assess the components of a mixed ventilatory defect separately. It is logical to assume that using the FEV1 percentage predicted to grade the severity of obstruction in a mixed disorder will overestimate the severity due to the concomitant loss of volume contributed by restriction. A study by Gardner296 and coworkers has begun to address this issue by adjusting the FEV1 percentage predicted for the degree of restriction. They accomplished this by dividing the FEV1 percentage predicted by the TLC percentage predicted. In their cohort of 199 subjects with a mixed ventilatory defect, using the adjusted FEV1 percentage predicted, they classified 33% with severe or very severe obstruction, whereas, using the unadjusted FEV1 percentage predicted, they classified 76% in this category.296 They also showed that the correlation between the adjusted FEV1 percentage and RV/TLC was better than with the unadjusted FEV1percentage. Because this work has not been validated, there is no widespread consensus on its use in clinical practice; however, it is important for the clinician to be cognizant of the possibility of overestimating the severity of obstruction in a patient with a mixed physiologic defect.
Pulmonary Vascular Obstruction
Patients who have dyspnea during exertion, especially those with decreased Dl
CO without evidence of obstructive or restrictive ventilatory defects, deserve detailed pulmonary function studies of the pulmonary circulation. These studies should include exercise tests, especially when signs of pulmonary hypertension are absent and radiographic methods fail to demonstrate obstruction of large pulmonary arteries. In fact it is important to make the diagnosis of pulmonary vascular obstruction before the development of pulmonary hypertension if possible. Measurements of Vd/Vt may be normal at rest but increased during exercise, indicating ventilated but poorly perfused regions of lung. The diagnosis of pulmonary vascular obstruction may be made by Vd/Vt measurements during exercise provided that no  abnormalities exist as a result of restrictive or obstructive ventilatory defects. Pulmonary vascular obstruction may cause abnormalities in Vd/Vt
only during exercise, for several reasons. At rest, poorly perfused regions may be poorly ventilated; during exercise, ventilation may increase if deep breaths overcome smooth muscle constriction in peripheral airways.297 At rest, bronchial blood flow may maintain normal carbon dioxide output from a poorly perfused region; during exercise, the collateral blood flow may not be able to increase proportionately to ventilation. At rest, narrowed pulmonary arteries may perfuse poorly ventilated regions; with exercise, blood flow may fail to increase as much as the ventilation.
abnormalities exist as a result of restrictive or obstructive ventilatory defects. Pulmonary vascular obstruction may cause abnormalities in Vd/Vt
only during exercise, for several reasons. At rest, poorly perfused regions may be poorly ventilated; during exercise, ventilation may increase if deep breaths overcome smooth muscle constriction in peripheral airways.297 At rest, bronchial blood flow may maintain normal carbon dioxide output from a poorly perfused region; during exercise, the collateral blood flow may not be able to increase proportionately to ventilation. At rest, narrowed pulmonary arteries may perfuse poorly ventilated regions; with exercise, blood flow may fail to increase as much as the ventilation.
ABGs should be studied because most patients with pulmonary vascular obstruction appear to have an abnormal drive to ventilation, resulting in tachypnea and alveolar hyperventilation at rest and during exercise.297a This abnormal drive results in decreased arterial Pco 2 and partially compensated respiratory alkalosis. Arterial Po 2 should be measured; in many patients with pulmonary vascular obstruction, arterial Po 2 may be normal at rest but decreased during exercise (eTable 25-2). Breathing pure oxygen allows the determination of the presence of a right-to-left shunt, which is often dependent on posture or exercise. The shunt tends to increase under conditions that increase right-sided pressures relative to left-sided pressures, as during increased venous return during exercise, in the supine posture, or at high versus low lung volumes.
eTable 25-2.
Effects of Exercise, Respiratory Maneuvers, and Posture on Arterial Po2 (mm Hg) During Inhalation of Pure Oxygen
| SITTING ARTERIAL Po2 |
SUPINE ARTERIAL Po2 |
|||||
|---|---|---|---|---|---|---|
| Case no. | Rest | Slow Maximal Inspiration | Slow Maximal Expiration | Exercise | Rest | Slow Maximal Inspiration |
| 1* | 410 | 370 | 512 | 122 | ||
| 3† | 615 | 620 | 605 | 605 | 490 | 375 |
| (105) | (83) | |||||
| 5‡ | 410 | 240 | 480 | 440 | ||
| 7§ | 389 | 487 | 384 | |||
| (58) | ||||||
| 9‖ | 572 | 395 | 463 | 96 | 520 | |
| 10¶ | 426 | 130 | 330 | |||
| (72) | (62) | |||||
| 11‡ | 350 | 120 | 390 | 180 | ||
Numbers in parentheses indicate arterial Po2 during inhalation of room air.
Patent foramen ovale.
Abnormal pleural-pulmonary vessels.
No shunt at cardiac catheterization.
No abnormal vessels.
Po2 73 mm Hg during exercise while supine breathing pure oxygen.
Orthopnea relieved by breathing oxygen or sitting up.
“Poor Cooperation” Pattern
Pulmonary function tests in general depend heavily on the cooperation of the subject being tested. If a competent technician performs the procedures and recordings of the test tracings accompany the measurements, it is usually possible to determine the validity of the data. In some instances, particularly in cases involving financial compensation, the pulmonary function tests must be carried out as part of a complete clinical evaluation, and the evaluating physician involved must observe the test results generated. Nevertheless, “poor cooperation” can usually be identified on the basis of the features listed in Table 25-6 . The VC is decreased and does not show a smooth curve, reaching a maximum value. The decreased VC is often accompanied by relatively normal expiratory airflow, increased FEV1/FVC ratio, and decreased MVV. Supplemental data confirming invalid test results include uneven, slurred, or notched curves on inspection; poor reproducibility on repeated testing; and decreased maximum lung elastic recoil pressure. A valid restrictive pattern differs from a test with poor effort in that it is reproducible and shows smooth expiratory curves on direct examination, increased lung elastic recoil, and a normal or nearly normal MVV.
Table 25-6.
Poor Cooperation
| CHARACTERISTICS OF POOR COOPERATION |
|
| SUPPLEMENTARY RESULTS CONFIRMING PATTERN |
|
FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
Adapted from Welch MH: Ventilatory function of the lungs. In Guenter CA, Welch MH, editors: Pulmonary medicine, Philadelphia, 1977, JB Lippincott, pp 72–123.
“Nonspecific” Pattern
In a study of 80,929 pulmonary function test results, what has been termed the “nonspecific” pattern was described, which consists of a reduced FEV1 and/or FVC with a normal FEV1/FVC and normal TLC. This pattern was identified in 7702 subjects (or ≈10%).298 The ATS/European Respiratory Society consensus statement considers this pattern as representing incomplete inhalation and/or exhalation and ultimately classified this pattern as obstructive.299 The original study was followed up with a longitudinal study of 1284 subjects who had one or more pulmonary function tests performed 6 months or more after initial testing, with a median follow-up of 3 years. The authors used a multivariate, multinomial logistic regression model to study the association between different variables and the final pulmonary function pattern. Their findings revealed that the nonspecific pattern was reproduced in 64% of subjects. Roughly equal numbers of subjects (≈15%) went on to develop a restrictive or obstructive pattern, whereas 3% normalized and 2% showed a mixed pattern.300 These data highlight the importance of longitudinal follow-up in patients with conflicting pulmonary function patterns.
Role of Obesity
Obesity is common in the United States and contributes to increased risk for death from heart disease and diabetes. It also increases the risk from anesthesia and surgical procedures, especially for procedures involving the upper abdomen and thorax. Obesity also complicates life for individuals with pulmonary disease because it increases the work of breathing. Obesity also increases the symptoms and adverse physiologic consequences of airway obstruction. Because of the increased extrathoracic mass, these patients are forced to breathe at low lung volumes, where airway diameters are decreased. The increased work of breathing at low lung volumes and reduced airway diameters interacts with the increased work of breathing due to increased extrathoracic mass. These factors are particularly troublesome, especially if the obese patient develops even mild airflow obstruction.301Obesity is also often associated with sleep disorders and impaired regulation of ventilation. In general, obesity limits exercise tolerance, and it makes physical conditioning more difficult to attain and maintain. (For a summary of the effects of mild obesity on lung function, see eTable 25-3).
eTable 25-3.
Effects of Mild Obesity on Lung Function (Mean ± SD)
| Parameter | Grade 0 | Grade I | Grade II |
|---|---|---|---|
| BMI (kg/m2) | 20–24.9 | 25–29.9 | 30–40 |
| Smoking history (pack-years) | 29 (1–82)* | 26 (1–123)* | 27 (3–90)* |
| FRC (L) | 3.45 ± 0.71 | 3.17 ± 0.69 | 2.66 ± 0.74 |
| ERV (L) | 1.10 ± 0.50 | 0.77 ± 0.37 | 0.59 ± 0.34 |
| RV (L) | 2.32 ± 0.48 | 2.36 ± 0.52 | 2.13 ± 0.54 |
| TLC (L) | 6.74 ± 0.97 | 6.58 ± 1.02 | 6.33 ± 0.91 |
| FEV1 (L) | 3.15 ± 0.68 | 2.91 ± 0.56 | 3.14 ± 0.49 |
| FVC (L) | 4.12 ± 0.17 | 3.84 ± 0.71 | 3.94 ± 0.69 |
| PEF (L/min) | 456 ± 104 | 458 ± 98 | 470 ± 100 |
BMI, body mass index; ERV, expiratory reserve volume; FEV1, forced expiratory volume in 1 second; FRC, functional reserve capacity; FVC, forced vital capacity; PEF, peak expiratory flow; RV, residual volume; SD, standard deviation; TLC, total lung capacity.
Median (range).
Modified from Jenkins SC, Moxham J: The effects of mild obesity on lung function. Respir Med 1991:85:309–311.
The increased mass of the chest and abdominal walls and their contents results in decreased outward recoil of the chest wall and increased pressure within the abdomen. Expiratory reserve volume and FRC are decreased, especially when the obese subject is recumbent.302 The single-breath nitrogen washout test results are abnormally increased, and perfusion is increased to poorly ventilated, dependent lung zones at the bases.303 This results in airway closure, often at lung volumes greater than FRC, with associated arterial hypoxemia.304 Dl CO is often increased in mild to moderate obesity and is associated with an increased red blood cell mass, cardiac output, and central blood volume. On the other hand, in morbid obesity Dl CO is usually decreased secondary to airway closure and atelectasis.305 Ventilatory response to carbon dioxide is often reduced; in some subjects the ventilatory responses to both hypoxia and hypercapnia are abnormal.306 Fatty infiltration of respiratory muscles may decrease maximum respiratory pressures, aggravate the abnormal lung volumes, and inhibit the capacity to maintain the increased work of breathing. As mentioned, obese patients who also have asthma or other forms of obstructive ventilatory defects usually have increased symptoms relative to the severity of the airway obstruction because they are forced to breathe at low lung volumes, where airflow resistance is increased.306a
A large study of almost 1500 adults in the general population who were followed for 8 years revealed that the detrimental effect of weight gain might be reversible because lung function improved in all those who lost weight. Obese patients with ventilatory impairment should therefore be encouraged to lose weight.307, 308, 308a
Aging Lung
Aging is associated with a decrease in lung elastic recoil pressure at TLC and at all lower lung volumes. Colebatch and coworkers309 showed that the index of curvature in the exponential expression of lung elastic recoil (see “Lung Elastic Recoil” section) increases with age. They concluded that this change was related to increased alveolar size. These findings were confirmed by Knudson and Kaltenborn,310 and morphologic studies have confirmed the increased alveolar dimensions. The effect of age on airflow rates depends on whether the data are based on cross-sectional studies or longitudinal studies. Burrows and associates311 found that the progressive decline in FVC and FEV1 did not begin until the middle 30s, and that the subsequent decline in FEV1/FVC was linear with age, independent of FVC, similar in men and women, and much less severe than that described in cross-sectional studies. Gelb and Zamel312 reported decreased maximal flows but no change in lung elastic recoil pressure or Raw with age, suggesting that airway collapsibility increased with age. More recently, Babb and Rodarte313 confirmed work by Janssens and coworkers,314 indicating that decreases in maximal expiratory airflow and in the minimum pressure needed to generate maximal flow in older subjects were due to decreased static lung elastic recoil compared with that in younger subjects. VC decreases with age, whereas RV and closing volume increase with age,315 suggesting that lung emptying is limited with increasing age because of airway closure (see earlier discussion).316 MVV decreases approximately 30% between 30 and 70 years of age, probably as a consequence of decreased maximal respiratory pressures, decreased distensibility of the total respiratory system, decreased lung elastic recoil, and impaired coordination of the respiratory muscles.
The slope of the alveolar plateau measured in the single-breath nitrogen washout test increases with age. Although arterial Pco 2 does not change with age, arterial Po 2 declines316 and the (A–a)Pco 2 widens with age.317 These changes probably reflect the increase in closing volume relative to expiratory reserve volume. Georges and coworkers318 reported that Dl CO decreases because membrane diffusing capacity decreases after 40 years of age; pulmonary capillary blood volume is maintained until the seventh decade and then decreases rapidly. The accelerated decline in Dl CO over the age of 40 years was confirmed by Viegi and colleagues.319 These changes appear to be consistent with the results of morphologic studies of the aging lung, which show a decrease in the surface area of the alveoli and the capillary bed.
Abnormal Respiratory Muscle Function
Increasing attention has been focused on the evaluation of respiratory muscles to detect abnormal function as a cause of unexplained dyspnea or respiratory failure. Inspiratory muscles may become fatigued and fail to contract adequately despite effective neural stimulation. If not detected and treated adequately, respiratory failure may result. This problem may develop in patients with obstructive or restrictive ventilatory defects, neuromuscular disorders such as myasthenia gravis, cardiogenic shock, or sepsis.284
Inspiratory Muscle Function Tests.
Inspiratory muscle function may be tested clinically by measuring muscle strength or endurance. Common measurements include maximally negative airway pressure, maximum transdiaphragmatic pressure (Pdimax), MVV, diaphragmatic EMG, and fluoroscopy.
For more details about these measurements of muscle function, please see the online ![]() version of the chapter.
version of the chapter.
Maximally Negative Airway Pressure.
The maximally negative airway pressure measures the maximally negative airway pressure generated by an inspiratory effort against an occluded airway. In this test the subject inspires maximally from RV against an obstructed mouthpiece with a small leak (1 mm in diameter) to prevent closure of the glottis or development of pressure above the glottis by the muscles of the cheeks. A plateau pressure should be maintained for at least 1 second. Reproducible maximal values are difficult to obtain; the coefficient of variation is about 9% for duplicate tests for the maximal inspiratory pressure (MIP) and the maximal expiratory pressure (MEP).320
Maximum Transdiaphragmatic Pressure.
Pdimax is measured during the same maneuver by determining the difference between intragastric and esophageal pressures. These pressures should be measured at RV because maximally negative airway pressure is reduced at larger lung volumes. Conversely, MEPs are greatest at TLC. Because it is difficult to obtain cooperation from patients to exhale to RV, MIP, MEP, and Pdimax are often measured at FRC (which probably accounts in part for the reported variability). Healthy persons can sustain respiratory patterns requiring 40% of Pdimax for long periods without fatigue. When a patient must increase the Pdi/Pdimax ratio to overcome a resistive load, endurance decreases in proportion to the resistive load (eFig. 25-27). Normal subjects can inspire with varying degrees of diaphragm and rib cage contraction. Thus Pdimax may be decreased because of marked recruitment of the rib cage during the test. The range reported is accordingly large: 18 to 137 cm H2O. Training results in improved coordination and reproducible values with a coefficient of variation of 19%.321
eFigure 25-27.
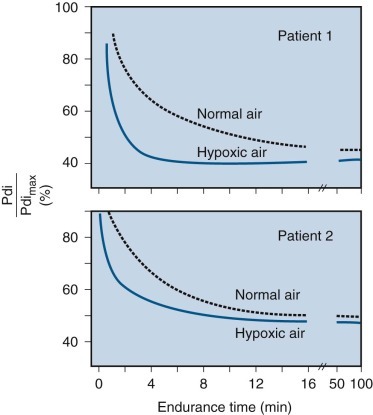
Diaphragmatic endurance in two patients in normal and hypoxic conditions.
The relationship between endurance and transdiaphragmatic pressure (Pdi) is expressed as a percentage of maximal transdiaphragmatic pressure (Pdimax). Data from two patients breathing normal and hypoxic air are shown. Added resistance leads to an increased Pdi/Pdimax ratio and increases the work of breathing. As the Pdi/Pdimax ratio increases, endurance rapidly decreases. This effect is more pronounced when the respiratory muscles are hypoxic.
(Modified from Roussos CS, Macklem PT: Diaphragmatic fatigue in man. J Appl Physiol 43:189–197, 1977.)
Similowski and colleagues322 have reported the use of cervical magnetic stimulation (CMS) as a method of phrenic nerve stimulation. They reported the results of comparisons of stimulated Pdi with the maximal Pdi obtained during the static combined expulsive-Mueller maneuver (Pdimax) and with the Pdi generated during a sniff test. Their results were comparable to those obtained in other studies using transcutaneous phrenic stimulation. They were highly reproducible in all the subjects. EMG data provided evidence of bilateral maximal stimulation. CMS is a nonspecific method and may stimulate various nervous structures. Co-contraction of neck muscles, including the sternomastoid, was present, but its influence in the CMS-induced Pdi appeared minimal. The method appears to avoid the pain of transcutaneous phrenic stimulation and the potential danger of needle stimulation of the phrenic nerves. However, subsequent studies showed that CMS stimulated many muscles of the upper thoracic cage as well as the diaphragm. Investigators have used chest wall electrodes to record the diaphragm compound action potential to assess the extent of stimulation of other muscles.323
Although initially promising, these studies have demonstrated difficulty in obtaining surface signals of acceptable quality.324 The variable shape and latency of the action potential induced by magnetic stimulation plus the shorter phrenic nerve conduction times with CMS compared with electrical stimulation indicate that diaphragm EMG after CMS is potentially unreliable, perhaps because chest wall electrodes also record electrical activity from other muscles. As demonstrated by Luo and associates,325 the method also appears unreliable for the measurement of phrenic nerve conduction time.
Maximum Voluntary Ventilation.
MVV measurements can also be used to assess endurance. Endurance decreases as Ve/MVV increases in a pattern similar to that observed with increasing Pdi/Pdimax ratio. In the absence of an external resistance, the largest ventilation that can be sustained more than 15 minutes is approximately 60% of the MVV.
Diaphragmatic Electromyography.
This is another technique used to evaluate respiratory muscle strength and endurance. Changes in respiratory muscle electrical activity appear to reflect closely other aspects of muscle contraction. Normally the phrenic nerve stimulates the diaphragm at a mixture of frequencies between 20 and 400 Hz. A graphic display of the EMG signal strength against frequency is called the power spectrum. When the EMG is analyzed over two limited frequency ranges (<50 Hz and >150 Hz), the power spectrum shifts to the low-frequency range as the diaphragm fatigues. This change precedes failure of contraction, and it therefore may be useful to predict decompensation of the respiratory muscles. Phrenic nerve conduction time may also be used to assess diaphragmatic paralysis or weakness. With recording electrodes placed over the rib cage above the diaphragm, the phrenic nerve is stimulated in the neck. Normal conduction time is 7.7 ± 0.8 msec.
Fluoroscopy.
Fluoroscopy may be used to evaluate diaphragmatic function. Decreased excursion should be evaluated with a lateral view using the “sniff test.” Decreased excursion alone is nonspecific, but decreased excursion associated with paradoxical motion during the sniff test is very specific. False-negative findings may result during spontaneous breathing because of contraction of abdominal muscles during exhalation, which produces downward motion of the diaphragm when the abdominal muscles relax at the beginning of inspiration. False-positive results may be produced by paradoxical motion limited to the anterior portion of the diaphragm.
Events Precipitating Respiratory Failure.
Any one of three events may precipitate respiratory failure: increased work of breathing, decreased energy supply, and decreased muscular efficiency.
Increased Work of Breathing.
Higher airflow resistance or greater elastic recoil of lung or chest wall, or both, may increase the work of breathing and the energy required of the respiratory muscles.
Decreased Energy Supply.
Reduction in the supply of vital metabolic substrates may limit the efficiency of respiratory muscles under certain circumstances. Reduction in cardiac output, arterial oxygen content, or extraction of oxygen from the blood (or a combination of these factors) may impair aerobic metabolism and compromise respiratory muscle function. Decreased oxygen delivery may be critically important under conditions that increase respiratory work. Oxygen consumption by respiratory muscles may rise 25-fold above baseline under conditions of high ventilatory requirement and increased Raw, and it may exceed supply. Forceful muscle contraction alone impedes blood flow to respiratory muscles in animals breathing against increased respiratory workloads. Other variables related to metabolism (e.g., hypercapnia, malnutrition, acidosis, electrolyte disorders) may also limit endurance.
Decreased Muscular Efficiency.
The number and distribution of fiber types determine inspiratory reserve. Disease processes and inactivity may alter the number and relative proportions of fibers in the diaphragm. Under certain conditions, changes in the distribution of fiber types or the loss of fibers may play an important role in the development or maintenance of respiratory failure. For example, atrophy of respiratory muscles is a potentially important problem in patients undergoing long-term mechanical ventilation.326
Muscular efficiency depends on mechanical factors as well as on structure.284 The position and configuration of the diaphragm at the beginning of inspiration determines the resting length of the muscle fibers. As FRC increases, the contour of the diaphragm flattens, and muscle fibers are not stretched to their optimal length. Acute hyperinflation (as in asthma) may cause a mechanical disadvantage of the diaphragm by shortening muscle fibers. In addition to changing muscle fiber length, hyperinflation also causes a mechanical disadvantage by changing the shape of the diaphragm. The Pdimax is determined by the radius of curvature of the diaphragm at any value of muscle tension (Laplace's law). Thus, increasing the radius of curvature of the diaphragm (i.e., flattening the diaphragm) greatly reduces its capacity to develop Pdi and to change lung volume.
Although abdominal muscles are usually regarded as expiratory, abdominal muscle tone may be needed to maintain the mechanical advantage of the diaphragm. Thus flaccidity of abdominal muscles contributes to the inefficient ventilation seen in paraplegic patients, especially when these patients are in the upright position.
VC is a useful test of respiratory muscle weakness because normally a small fraction of the muscle strength is required to inflate the lung. Furthermore, the curvilinear relationship between pressure and volume means that a greater loss of muscle strength (pressure) is required to produce a loss of volume; for example, a 50% decrease in MIP is associated with only a 15% decrease in VC. Although MIP may be reduced markedly before lung volume decreases, most patients with respiratory muscle weakness have decreased VC and decreased lung compliance. The latter finding is thought to result from atelectasis, which may be detectable in chest radiographs. FRC, inspiratory capacity, expiratory reserve volume, and TLC may also be decreased in association with decreased lung elastic recoil, suggestive of decreased outward recoil of the chest wall.
According to Rochester and Esau,327 substantial respiratory muscle strength can be lost without a change in spirometric values or ABG levels. There is a moderate decrease in MVV and increased RV, and MIP and MEP may be 50% of predicted normal. In advanced chronic disease, patients still may not have symptoms because they are not exercising. When patients reach the stage of poor cough, scoliosis, absent gag reflex, and MIP and MEP less than 50% of predicted normal, MVV is decreased more and RV enlarges further. Overt respiratory failure may develop abruptly, so it is important to follow these patients serially with VC and MIP/MEP studies.
Lung Transplantation
Lung transplantation can improve the quality of life and the capacity to exercise in patients with end-stage emphysema or interstitial lung disease, but it is not clear if it prolongs life.328, 328a In single-lung transplantation for emphysema, the radius of curvature of the dome of the diaphragm and the zone of apposition of the diaphragm with the chest wall on the side of the graft return to normal. The surface area of the dome also becomes smaller on the side of the graft compared with controls.329 This effect results from mediastinal displacement toward the graft, due to the lesser lung elastic recoil of the native (emphysematous) lung and greater lung elastic recoil of the graft. The disparity in elastic recoil may be increased if the graft is infected or undergoes rejection. It has also been suggested that mediastinal shift toward the graft may reflect dynamic hyperinflation of the native lung. Such dynamic hyperinflation seems unlikely because single-lung transplants do not show flow limitation during tidal breathing except during maximal exercise.330 Mediastinal displacement is usually counterbalanced by equal expansion of the rib cage on the side of the graft. Compensatory expansion of the rib cage on the graft side is not always sufficient to accommodate expansion of the contralateral hyperinflated lung. In rare cases the mediastinal shift can compromise function of the graft. This risk depends on the severity of obstruction and air trapping preoperatively.
When patients with a single-lung transplant inhale to TLC, they attain only 78% of the volume of matched controls. The smaller volume is due to mediastinal shift, mismatch in sizing the graft relative to the native lung and rib cage, and reduced capacity of inspiratory muscles to generate required pressures. Smaller inspiratory pressures may result from shorter operating length of the inspiratory muscles, steroid myopathy, or the respiratory myopathy caused by the vehicle used with cyclosporine.331
Transplantation of two lungs results in a normal TLC in patients with chronic hyperinflation (FRC about 1 L above predicted levels).332 Electrical stimulation and sniff-induced Pdi are not affected by single-lung transplantation. However, after bilateral lung transplantation, increased resting length of muscle fibers and normalization of the radius of curvature of the diaphragm lead to improved sniff pressure and normal MIP.333 It is still uncertain why MEP is only 70% of normal, even after double-lung transplantation. The fact that weakness of expiratory respiratory muscles and ankle dorsiflexors is equivalent suggests that these muscles may be vulnerable to a factor that does not affect the diaphragm, perhaps because the diaphragm is active continuously.334 Inspiratory muscle endurance does not change after single-lung or double-lung transplantation.
A major problem, well recognized by transplant surgeons, is the need to obtain a proper fit of the donor graft within the chest cavity of the recipient. As suggested by Fessler and Permutt (see earlier discussion of lung volume resection surgery), a mismatch in size of the single-lung (or double-lung) graft and the size of the surrounding chest, as reflected by RV/TLC, has a profound effect on pulmonary function. If the donor graft is too large for the chest cavity, regardless of how healthy the graft is, the respiratory muscles of the recipient are unable to generate sufficient pressures to improve expiratory airflow and reduce hyperinflation and air trapping.
In patients with a single-lung or double-lung transplant, maximal exercise capacity is about half normal. This is not the result of ventilatory limitation, as it is in COPD patients without transplantation. The ventilatory reserve during maximal exercise is similar in transplant patients and matched controls. Evidence suggests that exercise limitation after transplantation results from decreased strength and endurance of locomotor muscles. Compared with healthy controls, transplant patients have shorter time to exhaustion, greater acidosis in quadriceps during knee-extension exercise, decreased type I fibers, and markedly decreased mitochondrial oxidative capacity.335, 336
Airline Travel
The increase in air travel makes it important for family physicians and specialists to be able to advise patients concerning the medical risks involved. Cardiovascular and pulmonary problems are the most common reasons for excluding air travel. Hypoxia in the aircraft, despite pressurization to the equivalent of 6000 to 8000 feet altitude, may be dangerous for patients with unstable angina, severe congestive heart failure, or chronic airway obstruction.337 Patients with severe lung disease associated with arterial oxygen desaturation should be evaluated at sea level for the risk for hypoxia during air travel or during travel to high altitude. Measurement of arterial Po 2 at ground level (as close to departure as possible) is a good predictor of tolerance to altitude, because hypoxia is the most important stress for patients with pulmonary disease at high altitude. If arterial Po 2 at altitude is 55 mm Hg or greater with a saturation of 85% to 90% or greater, the patient should tolerate air travel reasonably well.
The arterial Po 2 at altitude in patients with chronic airflow obstruction may be estimated from the following equation338:
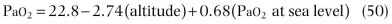
where altitude is given in thousands of feet. The use of FEV1 may enhance the accuracy of prediction of the arterial Po 2 at 8000 feet in patients with chronic airflow obstruction339:
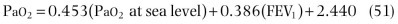
where FEV1 is given as percentage of predicted normal.
As suggested by Gong and associates,338 while the patient breathes hypoxic gas mixtures equivalent to the atmospheric oxygen at 8000 feet (15.1% oxygen, representing “worst case” cabin pressurization excluding accidental depressurization), measurements can be made of pulse oximetry or ABG levels combined with electrocardiographic monitoring at rest and during exercise. Although measurements of ABGs may be used to predict the likely oxygenation of pulmonary patients at altitude, more objective information may be obtained from a hypoxia-altitude simulation test. Berg and associates340 approached altitude simulation directly and studied patients with COPD [FEV1 0.97 L (±31.3%)] in a hypobaric chamber to simulate a commercial jet aircraft cabin at the equivalent of 8000 feet altitude. When breathing supplemental oxygen by nasal cannula at 4 L/min, the mean arterial Po 2 increased from 47.4 ± 6.3 mm Hg to 82.3 ± 14 mm Hg (n = 18). Supplementation of oxygen by 24% Venturi mask caused arterial Po 2 at 8000 feet to increase by 12.7 ± 3.8 mm Hg; a 28% Venturi mask caused arterial Po 2 at 8000 feet to increase by 19.7 ± 8.2 mm Hg. Compared with ground level, oxygen at 4 L/min (by nasal cannula) increased mean arterial Po 2 by 9.9 ± 12.6 mm Hg; 24% and 28% Venturi masks did not cause mean arterial Po 2 to increase above ground-level values. These changes could be evaluated accurately using a transmittance ear oximeter and less accurately using a reusable digital pulse oximeter.341
These results suggest that altitude simulation studies may be more accurate in assessing hypoxemia and the effect of treatment with supplemental oxygen than predictions of altitude arterial Po 2 based on studies at ground level.
Using altitude simulation (using low-oxygen mixtures or hypobaric chambers), it is possible to assess symptoms, tolerance to exercise, amount of supplemental oxygen required, and effects of controlled hypoxia on associated hematologic, cardiac, and neurologic disorders. Such information cannot be obtained from the prediction of altitude arterial Po 2 alone. Furthermore, the equations for estimation of hypoxia at altitude are relatively population-specific (i.e., limited to COPD patients) and only deal with the effects of altitude on arterial oxygenation.
Thus, although predictions may be useful in patients with COPD, they may be misleading in patients with other conditions. For example, in children with cystic fibrosis, simple spirometry and baseline arterial Po 2 may underestimate the individual response to air travel or altitude.342 In a study of 17 patients with restrictive ventilatory defects, Christensen and colleagues343 reported the effect of simulated air travel in a hypobaric chamber on ABGs, blood pressure, and cardiac frequency during rest and mild exercise, and the response to supplementary oxygen. They found that resting arterial Po 2 was much lower than predicted by an equation, and that arterial Po 2 fell further with light exercise. Thus, for patients with diseases other than COPD, it is probably prudent to use some form of altitude simulation to estimate the possible physiologic and clinical effects of altitude or air travel on patients with pulmonary disease.344, 345, 346
Infection Control and Safety
When patients with communicable infectious diseases are referred for pulmonary function tests, they always present a potential risk for transmission of infectious diseases to the technical and administrative staff of the laboratory, as well as to other patients who may be in the laboratory for studies at the same time. Pulmonary laboratory directors are familiar with the risk for spreading tuberculosis by aerosols produced by sputum-positive patients. There has also been the more theoretical possibility of transmitting tuberculosis from one patient to another via infected secretions, which may contaminate pulmonary function equipment. The increasing number of immunosuppressed patients in cancer treatment and transplantation programs has raised the possibility of increased risk for transmission of infection to such patients.
With the human immunodeficiency virus (HIV) epidemic and the recent severe acute respiratory syndrome epidemic and the possible risk for transmission of a lethal virus, this issue has been rigorously reassessed. It is not possible to screen everyone studied in a laboratory for HIV infection or the acquired immunodeficiency syndrome (AIDS) before testing. Furthermore, in hospitals in which large numbers of patients with chronic liver disease are evaluated for possible liver transplantation, there is a high prevalence of diverse hepatitis viruses, which are also difficult if not impossible to screen out before pulmonary function testing.
For these reasons, on the advice of the Infectious Disease Control Committee and the AIDS Advisory Committee at University of California, San Francisco, the laboratory with which I am affiliated has adopted a uniform protocol designed to protect all patients and technical staff from possible infectious diseases. When a patient is sent to the laboratory, the administrative assistant responsible for the schedule makes certain the requisition is completed. The requisition is designed to screen for possible HIV, tuberculosis, and other infectious diseases. The administrative assistant also checks for these possibilities with the referring physician when the study is scheduled. When the patient arrives at the laboratory for the study, the technicians check for possible communicable infectious diseases on the requisition, the medical record, and the questionnaire completed by the patient. If this review is negative, studies are performed. All patients are studied on the same equipment. All studies are performed with the patient breathing through a filter that traps particles as small as 0.2 µm in diameter and does not affect the results of the physiologic tests performed. It is assumed that infectious particles, whether bacterial, fungal, parasitic, or viral, will be carried in respiratory secretions of such a large size that all of them will be trapped by these filters. No infectious diseases have been transmitted from the equipment in this laboratory, a fact that reinforces these assumptions. The fact that the Centers for Disease Control and Prevention has not reported transmission of HIV or hepatitis virus via pulmonary function equipment also reinforces the assumptions.
Because of the expense of effective filters, an alternative and conservative policy might include the following: careful screening of patients before testing; use of disposable mouthpieces that are discarded after single-patient use or rubber mouthpieces that are changed between each patient and cleaned with high-level disinfection procedures; replacing external spirometer tubing between patients, with high-level disinfection and drying of the tubing between each use; replacing nose clips between each patient and discarding used nose clips or cleaning them with high-level disinfection procedures; changing the water in water-sealed spirometers at least monthly; and washing hands thoroughly before and after pulmonary function testing, with appropriate use of gloves when blood samples are to be collected.
Key Points.
-
▪
There are two main ventilatory patterns measured by pulmonary function tests: obstruction and restriction. Categorization of these patterns is dictated by the lung volumes, both static and forced, as well as the interpretation of the flow-volume loop.
-
▪
Because pulmonary function testing is effort dependent, technicians must be trained sufficiently and laboratories must adhere to high standards to obtain the most accurate and reproducible results possible.
-
▪
Measurements from spirometry alone can be useful in patients whose main physiologic impairment is airway obstruction. The findings from spirometry can provide clues that concomitant restriction is present; however, for assessment of restriction, lung volumes must be measured.
-
▪
In patients with reversible mild airway disease, the results of pulmonary function tests may be normal because the disease is dynamic and patients with mild airflow obstruction may spontaneously return to normal.
-
▪
The diffusing capacity measurement is used to determine defects of the alveolar-pulmonary capillary unit. A defect in the diffusing capacity alerts the physician that oxygen transfer, the alveolar-capillary membranes, and/or pulmonary capillaries may not be normal, and further evaluation of the underlying cause of this defect should be pursued.
-
▪
Methacholine provocation testing is used to identify whether abnormal airway responsiveness is present. Usually this indicates a diagnosis of asthma, but the clinician must understand that other conditions that involve airway inflammation, such as sarcoidosis, may also show abnormal airway responsiveness.
eFigure Image Gallery
Key Readings
- Arjomandi M, Haight T, Sadeghi N. Reduced exercise tolerance and pulmonary capillary recruitment with remote secondhand smoke exposure. PLoS ONE. 2012;7:e34393. doi: 10.1371/journal.pone.0034393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger AJ, Mitchell RA, Severinghaus JW. Regulation of respiration. N Engl J Med. 1977;297:92–97. doi: 10.1056/NEJM197707142970206. [DOI] [PubMed] [Google Scholar]
- Buist AS, Ross BB. Quantitative analysis of the alveolar plateau in the diagnosis of early airway obstruction. Am Rev Respir Dis. 1973;108:1078–1087. doi: 10.1164/arrd.1973.108.5.1078. [DOI] [PubMed] [Google Scholar]
- Collard HR, King TE, Jr, Bartelson BB. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168:538–542. doi: 10.1164/rccm.200211-1311OC. [DOI] [PubMed] [Google Scholar]
- Comroe JH, Jr, Nadel JA. Current concepts: screening tests of pulmonary function. N Engl J Med. 1970;282:1249–1253. doi: 10.1056/NEJM197005282822207. [DOI] [PubMed] [Google Scholar]
- Crapo RO, Casaburi R, Coates AL. Guidelines for methacholine and exercise challenge testing—1999. Am J Respir Crit Care Med. 2000;161:309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- Expert Panel Report 3 (EPR-3) Guidelines for the diagnosis and management of asthma—summary report 2007. National asthma education and prevention program. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- Gelb AF, Gold WM, Wright RR. Physiologic diagnosis of subclinical emphysema. Am Rev Respir Dis. 1973;107:50–63. doi: 10.1164/arrd.1973.107.1.50. [DOI] [PubMed] [Google Scholar]
- Gelb AF, McKenna RJ, Jr, Brenner M. Lung function 5 yr after lung volume reduction surgery for emphysema. Am J Respir Crit Care Med. 2001;163:1562–1566. doi: 10.1164/ajrccm.163.7.2009048. [DOI] [PubMed] [Google Scholar]
- Gingo MR, George MP, Kessinger CJ. Pulmonary function abnormalities in HIV-infected patients during the current antiretroviral therapy era. Am J Respir Crit Care Med. 2010;182:790–796. doi: 10.1164/rccm.200912-1858OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustini D, Giuntini C. Relationship between extent of pulmonary emphysema by high-resolution computed tomography and lung elastic recoil in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:585–589. doi: 10.1164/ajrccm.164.4.2010066. [DOI] [PubMed] [Google Scholar]
- Hankinson JL, Kawut SM, Shahar E. Performance of American Thoracic Society-recommended spirometry reference values in a multiethnic sample of adults: the multi-ethnic study of atherosclerosis (MESA) lung study. Chest. 2010;137:138–145. doi: 10.1378/chest.09-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie GE, Janson S, Boushey HA. Ethnic differences: word descriptors used by African American and white asthma patients during induced bronchoconstriction. Chest. 2000;117:935–943. doi: 10.1378/chest.117.4.935. [DOI] [PubMed] [Google Scholar]
- Janssens JP, Pache JC, Nicod LP. Physiological changes in respiratory function associated with ageing. Eur Respir J. 1999;13:197–205. doi: 10.1034/j.1399-3003.1999.13a36.x. [DOI] [PubMed] [Google Scholar]
- Mead J, Turner JM, Macklem PT. Significance of the relationship between lung recoil and maximum expiratory flow. J Appl Physiol. 1967;22:95–108. doi: 10.1152/jappl.1967.22.1.95. [DOI] [PubMed] [Google Scholar]
- Meyers DA, Goldberg AP, Bleecker ML. Relationship of obesity and physical fitness to cardiopulmonary and metabolic function in healthy older men. J Gerontol. 1991;46:M57–M65. doi: 10.1093/geronj/46.2.m57. [DOI] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Mohler JG, Collier CR, Brandt W. Blood gases. In: Clausen JL, editor. Pulmonary function testing guidelines and controversies: equipment, methods, and normal values. Grune & Stratton; Orlando, FL: 1984. pp. 223–258. [Google Scholar]
- Nadel JA, Gold WM, Burgess JH. Early diagnosis of chronic pulmonary vascular obstruction. Am J Med. 1968;44:16–25. doi: 10.1016/0002-9343(68)90233-7. [DOI] [PubMed] [Google Scholar]
- Rochester DF, Esau SA. Assessment of ventilatory function in patients with neuromuscular disease. Clin Chest Med. 1994;15:751–763. [PubMed] [Google Scholar]
- Wagner PD, Smith CM, Davies NJH. Estimation of ventilation-perfusion inequality by inert gas elimination without arterial sampling. J Appl Physiol. 1985;59:376–383. doi: 10.1152/jappl.1985.59.2.376. [DOI] [PubMed] [Google Scholar]
- West JB, Dollery CT. Distribution of blood flow and the pressure-flow relations of the whole lung. J Appl Physiol. 1965;20:175–183. [Google Scholar]
References
- 1.Pride NB. Assessment of long-term changes in airway function. Agents Actions. 1990;30:21–34. doi: 10.1007/978-3-0348-7488-5_2. [DOI] [PubMed] [Google Scholar]
- 2.Jayr C, Matthay MA, Goldstone J. Preoperative and intraoperative factors associated with prolonged mechanical ventilation. A study in patients following major abdominal vascular surgery. Chest. 1993;103:1231–1236. doi: 10.1378/chest.103.4.1231. [DOI] [PubMed] [Google Scholar]
- 3.Collard HR. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168(5):538–542. doi: 10.1164/rccm.200211-1311OC. [DOI] [PubMed] [Google Scholar]
- 4.Jones NL, Jones J, Edwards RHT. Exercise tolerance in chronic airway obstruction. Am Rev Respir Dis. 1971;103:477–491. doi: 10.1164/arrd.1971.103.4.477. [DOI] [PubMed] [Google Scholar]
- 5.Pontoppidan H, Geffin B, Lowenstein E. Acute respiratory failure in the adult. N Engl J Med. 1972;287:690–698. doi: 10.1056/NEJM197210052871404. [DOI] [PubMed] [Google Scholar]
- 6.American Thoracic Society Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis. 1991;144:1202–1216. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 7.Miller MR. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 8.Gardner RM, Baker CD, Broennle AM., Jr ATS statement: snowbird workshop on standardization of spirometry. Am Rev Respir Dis. 1979;119:831–838. doi: 10.1164/arrd.1979.119.5.831. [DOI] [PubMed] [Google Scholar]
- 9.Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis. 1981;123:859–864. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- 10.Morris JF, Koski A, Johnson LC. Spirometric standards for healthy nonsmoking adults. Am Rev Respir Dis. 1971;103:57–67. doi: 10.1164/arrd.1971.103.1.57. [DOI] [PubMed] [Google Scholar]
- 11.DaCosta JL. Pulmonary function studies in healthy Chinese adults in Singapore. Am Rev Respir Dis. 1971;104:128–131. doi: 10.1164/arrd.1971.104.1.128. [DOI] [PubMed] [Google Scholar]
- 12.Schoenberg JB, Beck GJ, Bouhuys A. Growth and decay of pulmonary function in healthy blacks and whites. Respir Physiol. 1978;33:367–393. doi: 10.1016/0034-5687(78)90063-4. [DOI] [PubMed] [Google Scholar]
- 13.Kory RC. Clinical spirometry: recommendation of the section on pulmonary function testing, committee on pulmonary physiology, American college of chest physicians. Dis Chest. 1963;43:214–219. [Google Scholar]
- 14.Jing JY. Should FEV1/FEV6 replace FEV1/FVC ratio to detect airway obstruction? A metaanalysis. Chest. 2009;135(4):991–998. doi: 10.1378/chest.08-0723. [DOI] [PubMed] [Google Scholar]
- 15.Swanney MP, Jensen RL, Crichton DA. FEV6 is an acceptable surrogate for FVC in the spirometric diagnosis of airway obstruction and restriction. Am J Respir Crit Care Med. 2000;162:917–919. doi: 10.1164/ajrccm.162.3.9907115. [DOI] [PubMed] [Google Scholar]
- 16.Morris JF, Temple WP, Koski A. Normal values for the ratio of one-second forced expiratory volume to forced vital capacity. Am Rev Respir Dis. 1973;108:1000–1003. doi: 10.1164/arrd.1973.108.4.1000. [DOI] [PubMed] [Google Scholar]
- 17.Cosio M, Ghezzo H, Hogg JC. The relations between structural changes in small airways and pulmonary-function tests. N Engl J Med. 1978;298:1277–1281. doi: 10.1056/NEJM197806082982303. [DOI] [PubMed] [Google Scholar]
- 18.Mead J, Turner JM, Macklem PT. Significance of the relationship between lung recoil and maximum expiratory flow. J Appl Physiol. 1967;22:95–108. doi: 10.1152/jappl.1967.22.1.95. [DOI] [PubMed] [Google Scholar]
- 19.Cochrane GM, Prieto F, Clark TJ. Intrasubject variability of maximal expiratory flow volume curve. Thorax. 1977;32:171–176. doi: 10.1136/thx.32.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright BM, McKerrow CB. Maximum forced expiratory flow rate as a measure of ventilatory capacity: with a description of a new portable instrument for measuring it. BMJ. 1959;5159:1041–1046. doi: 10.1136/bmj.2.5159.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Expert Panel Report 3 (EPR-3) Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 22.Sheffer AL, Chair EPoA Expert panel report, national heart, lung, and blood institute national asthma education program: guidelines for the diagnosis and management of asthma. J Allergy Clin Immunol. 1991;88:425–534. [PubMed] [Google Scholar]
- 23.Ferris BG. Epidemiology: standardization project. Am Rev Respir Dis. 1978;118:1–120. [PubMed] [Google Scholar]
- 24.Gaensler EA, Wright GW. Evaluation of respiratory impairment. Arch Environ Health. 1966;12:146–189. doi: 10.1080/00039896.1966.10664355. [DOI] [PubMed] [Google Scholar]
- 25.Rochester DF, Arora NS, Braun NMT. The respiratory muscles in chronic obstructive pulmonary disease (COPD) Bull Eur Physiopathol Respir. 1979;15:951–975. [PubMed] [Google Scholar]
- 26.Gelb AF, Hogg JC, Muller NL. Contribution of emphysema and small airways in COPD. Chest. 1996;109:353–359. doi: 10.1378/chest.109.2.353. [DOI] [PubMed] [Google Scholar]
- 27.Berend N, Thurlbeck WM. Correlations of maximum expiratory flow with small airway dimensions and pathology. J Appl Physiol. 1982;52:346–351. doi: 10.1152/jappl.1982.52.2.346. [DOI] [PubMed] [Google Scholar]
- 28.Knudson RJ, Lebowitz MD, Holberg CJ. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127:725–734. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- 29.Jayamanne DS, Epstein H, Goldring RM. Flow-volume curve contour in COPD: correlation with pulmonary mechanics. Chest. 1980;77(6):749–757. doi: 10.1378/chest.77.6.749. [DOI] [PubMed] [Google Scholar]
- 30.Ohwada A, Takahashi K. Concave pattern of a maximal expiratory flow-volume curve: a sign of airflow limitation in adult bronchial asthma. Pulm Med. 2012;2012:797495. doi: 10.1155/2012/797495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberline M, Schmidt GS, Browe RG. Chest wall strapping: an old physiology experiment with new relevance to small airways disease. Ann Am Thorac Soc. 2014 doi: 10.1513/AnnalsATS.201312-465OI. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyazawa T. Stenting at the flow-limiting segment in tracheobronchial stenosis due to lung cancer. Am J Respir Crit Care Med. 2004;169(10):1096–1102. doi: 10.1164/rccm.200312-1784OC. [DOI] [PubMed] [Google Scholar]
- 32.Lunn WW, Sheller JR. Flow volume loops in the evaluation of upper airway obstruction. Otolaryngol Clin North Am. 1995;28:721–729. [PubMed] [Google Scholar]
- 33.Despas PJ, Leroux M, Macklem PT. Site of airway obstruction in asthma as determined by measuring maximal expiratory flow breathing air and a helium-oxygen mixture. J Clin Invest. 1972;51:3235–3243. doi: 10.1172/JCI107150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balkissoon R, Kenn K. Asthma: vocal cord dysfunction (VCD) and other dysfunctional breathing disorders. Semin Respir Crit Care Med. 2012;33(6):595–605. doi: 10.1055/s-0032-1326959. [DOI] [PubMed] [Google Scholar]
- 35.Robertson DR. Effects of an external resistance on maximum flow in chronic obstructive lung disease: implications for recognition of coincident upper airway obstruction. Thorax. 1989;44(6):461–468. doi: 10.1136/thx.44.6.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li KYR, Tan LTK, Chong P. Between-technician variation in the measurement of spirometry with air and helium. Am Rev Respir Dis. 1981;124:196–198. doi: 10.1164/arrd.1981.124.2.196. [DOI] [PubMed] [Google Scholar]
- 37.Hutchinson J. On the capacity of the lungs and on the respiratory functions, with a view of establishing a precise and easy method of detecting diseases by the spirometer. Trans Med Soc Lond. 1846;29:137–252. doi: 10.1177/095952874602900113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fleming GM, Chester EH, Saniie J. Ventilation inhomogeneity using multibreath nitrogen washout: comparison of moment ratios and other indexes. Am Rev Respir Dis. 1980;121:789–794. doi: 10.1164/arrd.1980.121.5.789. [DOI] [PubMed] [Google Scholar]
- 39.Bouhuys A. Pulmonary nitrogen clearance in relation to age in healthy males. J Appl Physiol. 1963;18:297–300. doi: 10.1152/jappl.1963.18.2.297. [DOI] [PubMed] [Google Scholar]
- 40.Schaanning CG, Gulsvik A. Accuracy and precision of helium dilution technique and body plethysmography in measuring lung volumes. Scand J Clin Lab Invest. 1973;32:271–277. doi: 10.3109/00365517309082471. [DOI] [PubMed] [Google Scholar]
- 41.Boren HG, Kory RC, Syner JC. The Veteran's Administration-Army cooperative study of pulmonary function. II. The lung volume and its subdivisions in normal man. Am J Med. 1966;41:96–114. doi: 10.1016/0002-9343(61)90096-1. [DOI] [PubMed] [Google Scholar]
- 42.Martin R, Macklem PT. Division of Lung Disease, National Heart and Lung Institute, NIH; Bethesda: 1973. Suggested standardized procedures for closed volume determinations (nitrogen method) [Google Scholar]
- 43.Mitchell MM, Renzetti AD., Jr Evaluation of a single-breath method of measuring total lung capacity. Am Rev Respir Dis. 1968;97:571–580. doi: 10.1164/arrd.1968.97.4.571. [DOI] [PubMed] [Google Scholar]
- 44.Burns CB, Scheinhorn DJ. Evaluation of single-breath helium dilution total lung capacity in obstructive lung disease. Am Rev Respir Dis. 1984;97:580–583. doi: 10.1164/arrd.1984.130.4.580. [DOI] [PubMed] [Google Scholar]
- 45.Harris TR, Pratt PC, Kilburn KH. Total lung capacity measured by roentgenograms. Am J Med. 1971;50:756–763. doi: 10.1016/0002-9343(71)90183-5. [DOI] [PubMed] [Google Scholar]
- 46.Bryant GH, Hansen JE. An improvement in whole body plethysmography. Am Rev Respir Dis. 1975;112:464–465. doi: 10.1164/arrd.1975.112.3.464. [DOI] [PubMed] [Google Scholar]
- 47.Stanescu DC, Rodenstein P, Cauberghs M. Failure of body plethysmography in bronchial asthma. J Appl Physiol. 1982;52:939–948. doi: 10.1152/jappl.1982.52.4.939. [DOI] [PubMed] [Google Scholar]
- 48.Gelb AF, Gold WM, Wright RR. Physiologic diagnosis of subclinical emphysema. Am Rev Respir Dis. 1973;107:50–63. doi: 10.1164/arrd.1973.107.1.50. [DOI] [PubMed] [Google Scholar]
- 49.Gelb AF, Gold WM, Nadel JA. Mechanisms limiting airflow in bullous lung disease. Am Rev Respir Dis. 1973;107:571–578. doi: 10.1164/arrd.1973.107.4.571. [DOI] [PubMed] [Google Scholar]
- 50.Hahn HL, Graf PD, Nadel JA. Effect of vagal tone on airway diameters and on lung volume in anesthetized dogs. J Appl Physiol. 1976;41:581–589. doi: 10.1152/jappl.1976.41.4.581. [DOI] [PubMed] [Google Scholar]
- 51.Stanescu DC, Rodenstein DO. Is simpler better? New approaches for computing airway resistance. Bull Eur Physiopathol Respir. 1986;22:323–328. [PubMed] [Google Scholar]
- 52.DuBois AB, Brody AW, Lewis DH, Burgess BF. Oscillation mechanics of lung and chest in man. J Appl Physiol. 1956;8:587–594. doi: 10.1152/jappl.1956.8.6.587. [DOI] [PubMed] [Google Scholar]
- 53.Peslin R, Divivier C, Malvetio P. Frequency dependence of specific airway resistance in a commercialized plethysmograph. Eur Respir J. 1996;9:1747–1750. doi: 10.1183/09031936.96.09081747. [DOI] [PubMed] [Google Scholar]
- 54.Komarow HD. Impulse oscillometry in the evaluation of diseases of the airways in children. Ann Allergy Asthma Immunol. 2011;106(3):191–199. doi: 10.1016/j.anai.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Galant SP, Nickerson B. Lung function measurement in the assessment of childhood asthma: recent important developments. Curr Opin Allergy Clin Immunol. 2010;10(2):149–154. doi: 10.1097/ACI.0b013e328335ce48. [DOI] [PubMed] [Google Scholar]
- 56.Van Noord JA, Cauberghs M, Van de Woestijne KP. Total respiratory resistance and reactance in ankylosing spondylitis and kyphoscoliosis. Eur Respir J. 1991;4:9445–9951. [PubMed] [Google Scholar]
- 57.Landser FJ, Nagels J, Clement J. Errors in the measurement of total respiratory resistance and reactance by forced oscillations. Respir Physiol. 1976;28:289–301. doi: 10.1016/0034-5687(76)90024-4. [DOI] [PubMed] [Google Scholar]
- 58.Michaelson ED, Grassman ED, Peters WR. Pulmonary mechanics by spectral analysis of forced random noise. J Clin Invest. 1975;56:1210–1230. doi: 10.1172/JCI108198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goldman MD, Carter R, Klein R. Within- and between-day variability of respiratory impedance, using impulse oscillometry in adolescent asthmatics. Pediatr Pulmonol. 2002;34:312–319. doi: 10.1002/ppul.10168. [DOI] [PubMed] [Google Scholar]
- 60.Vink GR, Arets HG, van der Laag J. Impulse oscillometry: a measure for airway obstruction. Pediatr Pulmonol. 2003;35:214–219. doi: 10.1002/ppul.10235. [DOI] [PubMed] [Google Scholar]
- 61.Witte KK, Morice A, Clark AL. Airway resistance in chronic heart failure measured by impulse oscillometry. J Card Fail. 2002;8:225–231. doi: 10.1054/jcaf.2002.126916. [DOI] [PubMed] [Google Scholar]
- 62.Hamakawa H. Forced oscillation technique as a non-invasive assessment for lung transplant recipients. Adv Exp Med Biol. 2010;662:293–298. doi: 10.1007/978-1-4419-1241-1_42. [DOI] [PubMed] [Google Scholar]
- 63.Faria AC. Contrasting diagnosis performance of forced oscillation and spirometry in patients with rheumatoid arthritis and respiratory symptoms. Clinics (Sao Paulo) 2012;67(9):987–994. doi: 10.6061/clinics/2012(09)01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aarli BB. Reference values for within-breath pulmonary impedance parameters in asymptomatic elderly. Clin Respir J. 2012;7:245–252. doi: 10.111/j.1752-699. [DOI] [PubMed] [Google Scholar]
- 65.Mahut B. Relationships between respiratory and airway resistances and activity-related dyspnea in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2012;7:165–171. doi: 10.2147/COPD.S29745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bijaoui E, Baconnier PF, Bates JH. Mechanical output impedance of the lung determined from cardiogenic oscillations. J Appl Physiol. 2001;91:859–865. doi: 10.1152/jappl.2001.91.2.859. [DOI] [PubMed] [Google Scholar]
- 67.Leman R, Benson M, Jones JG. Absolute pressure measurements with hand-dipped and manufactured esophageal balloons. J Appl Physiol. 1974;37:600–603. doi: 10.1152/jappl.1974.37.4.600. [DOI] [PubMed] [Google Scholar]
- 68.Woolcock AJ, Vincent JN, Macklem PT. Frequency dependence of compliance as a test for obstruction in the small airways. J Clin Invest. 1969;48:1097–1105. doi: 10.1172/JCI106066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dawson A. Elastic recoil and compliance. In: Clausen JL, editor. Pulmonary function testing: guidelines and controversies. Academic; San Diego: 1982. pp. 193–204. [Google Scholar]
- 70.Gibson GJ, Pride NB. Lung distensibility: the static pressure-volume curve of the lungs and its use in clinical assessment. Br J Dis Chest. 1976;70:143–184. doi: 10.1016/0007-0971(76)90027-9. [DOI] [PubMed] [Google Scholar]
- Bogaard JM, Overbeck SE, Verbraak AF. Pressure-volume analysis of the lung with an exponential and linear-exponential model in asthma and COPD. Dutch CNSLD Study Group. Eur Respir J. 1995;8(9):1525–1531. [PubMed] [Google Scholar]
- Gibson GJ, Pride NB, Davis T. Exponential description of the static pressure-volume curve of normal and diseased lungs. Am Rev Respir Dis. 1979;120:799–811. doi: 10.1164/arrd.1979.120.4.799. [DOI] [PubMed] [Google Scholar]
- Sansores RH, Ramirez-Venegas A, Perez-Padilla R. Correlation between pulmonary fibrosis and the lung pressure-volume curve. Lung. 1996;174:315–323. doi: 10.1007/BF00176190. [DOI] [PubMed] [Google Scholar]
- Thompson MJ, Colebatch HJH. Decreased pulmonary distensibility in fibrosing alveolitis and its relation to decreased lung volume. Thorax. 1989;44:725–731. doi: 10.1136/thx.44.9.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gugger M, Gould G, Sudlow MF. Extent of pulmonary emphysema in man and its relation to the loss of elastic recoil. Clin Sci. 1991;80:353–358. doi: 10.1042/cs0800353. [DOI] [PubMed] [Google Scholar]
- Macklem PT, Eidelman D. Reexamination of the elastic properties of emphysematous lungs. Respiration. 1990;57:187–192. doi: 10.1159/000195842. [DOI] [PubMed] [Google Scholar]
- Gelb AF, Zamel N, Hogg JC. Pseudophysiologic emphysema resulting from severe small-airways disease. Am J Respir Crit Care Med. 1998;158:815–819. doi: 10.1164/ajrccm.158.3.9801045. [DOI] [PubMed] [Google Scholar]
- 71.Miller MR. Interpreting lung function data using 80% predicted and fixed thresholds misclassifies more than 20% of patients. Chest. 2011;139(1):52–59. doi: 10.1378/chest.10-0189. [DOI] [PubMed] [Google Scholar]
- 72.Pellegrino R. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 73.Control, C.f.D., Third National Health and Nutrition Examination Survey (NHANES III), 1988–94. National Center for Health Statistics. 1994;32 (Vital Health Stat Series No. 1) [Google Scholar]
- 74.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 75.Hankinson JL. Performance of American Thoracic Society-recommended spirometry reference values in a multiethnic sample of adults: the multi-ethnic study of atherosclerosis (MESA) lung study. Chest. 2010;137(1):138–145. doi: 10.1378/chest.09-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kumar R. Genetic ancestry in lung-function predictions. N Engl J Med. 2010;363(4):321–330. doi: 10.1056/NEJMoa0907897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ward H, Cooper B, Miller MR. Validation of lung function prediction equations from patient survival data. Eur Respir J. 2012;39(5):1181–1187. doi: 10.1183/09031936.00104911. [DOI] [PubMed] [Google Scholar]
- 78.Pesola GR, Huggins G, Sherpa TY. Abnormal predicted diffusion capacities in healthy Asians: an inequality with a solution. Respiration. 2006;73(6):799–807. doi: 10.1159/000094391. [DOI] [PubMed] [Google Scholar]
- 79.Pesola GR. Measured diffusion capacity versus prediction equation estimates in blacks without lung disease. Respiration. 2004;71(5):484–492. doi: 10.1159/000080633. [DOI] [PubMed] [Google Scholar]
- Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977;1(6077):1645–1648. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kohansal R. The natural history of chronic airflow obstruction revisited: an analysis of the Framingham offspring cohort. Am J Respir Crit Care Med. 2009;180(1):3–10. doi: 10.1164/rccm.200901-0047OC. [DOI] [PubMed] [Google Scholar]
- 81.McCarthy DS, Craig DB, Cherniak RM. Intraindividual variability in maximal expiratory flow-volume and closing volume in asymptomatic subjects. Am Rev Respir Dis. 1975;112:407–411. doi: 10.1164/arrd.1975.112.3.407. [DOI] [PubMed] [Google Scholar]
- 82.Wise RA, Connett J, Kurnow K. Selection of spirometric measurements in a clinical trial, the Lung Health Study. Am J Respir Crit Care Med. 1995;151:675–681. doi: 10.1164/ajrccm.151.3.7881655. [DOI] [PubMed] [Google Scholar]
- 83.Wang ML, McCabe L, Petsonk EL. Weight gain and longitudinal changes in lung function in steel workers. Chest. 1997;111:1526–1532. doi: 10.1378/chest.111.6.1526. [DOI] [PubMed] [Google Scholar]
- 84.Potter WA, Olafsson S, Hyatt RE. Ventilatory mechanics and expiratory flow limitation during exercise in patients with obstructive lung disease. J Clin Invest. 1971;50:910–919. doi: 10.1172/JCI106563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Babb TG, Viggiano R, Hurley B. Effect of mild-to-moderate airflow limitation on exercise capacity. J Appl Physiol. 1991;70:223–230. doi: 10.1152/jappl.1991.70.1.223. [DOI] [PubMed] [Google Scholar]
- 86.Stubbing DG, Pengelly LD, Morse JL. Pulmonary mechanics during exercise in subjects with chronic airflow obstruction. J Appl Physiol. 1980;49:511–515. doi: 10.1152/jappl.1980.49.3.511. [DOI] [PubMed] [Google Scholar]
- 87.O'Donnell DE, Webb KA. Exertional breathlessness in patients with chronic airflow limitation: the role of lung hyperinflation. Am Rev Respir Dis. 1993;148:1351–1357. doi: 10.1164/ajrccm/148.5.1351. [DOI] [PubMed] [Google Scholar]
- 88.Hanson JS, Tabakin BS, Caldwell EJ. Response of lung volumes and ventilation to posture change and upright exercise. J Appl Physiol. 1962;17:783–786. [Google Scholar]
- 89.D’Angelo E, Prandi E, Marazzini L. Dependence of maximal flow-volume curves on time course of preceding inspiration in patients with chronic obstruction pulmonary disease. Am J Respir Crit Care Med. 1994;150:1581–1586. doi: 10.1164/ajrccm.150.6.7952618. [DOI] [PubMed] [Google Scholar]
- 90.D’Angelo E, Prandi E, Milic-Emili J. Dependence of maximal flow-volume curves on time course of preceding inspiration. J Appl Physiol. 1993;70:2602–2610. doi: 10.1152/jappl.1993.75.3.1155. [DOI] [PubMed] [Google Scholar]
- 91.Ingram RH, Jr, Schilder DP. Effect of gas compression on pulmonary pressure, flow, and volume relationship. J Appl Physiol. 1966;21:1821–1826. doi: 10.1152/jappl.1966.21.6.1821. [DOI] [PubMed] [Google Scholar]
- 92.Wellman JJ, Brown R, Ingram RH., Jr Effect of volume history on successive partial expiratory flow-volume maneuvers. J Appl Physiol. 1976;41:153–158. doi: 10.1152/jappl.1976.41.2.153. [DOI] [PubMed] [Google Scholar]
- 93.Koulouris NG, Valta P, Lavoie A. A simple method to detect expiratory flow limitation during spontaneous breathing. Eur Respir J. 1995;8:306–313. doi: 10.1183/09031936.95.08020306. [DOI] [PubMed] [Google Scholar]
- 94.Valta P, Corbeil C, Lavoie A. Detection of expiratory flow limitation during mechanical ventilation. Am J Respir Crit Care Med. 1994;150:1311–1317. doi: 10.1164/ajrccm.150.5.7952558. [DOI] [PubMed] [Google Scholar]
- 95.Koulouris NG, Dimopoulou I, Valta P. Detection of expiratory flow limitation during exercise in COPD patients. J Appl Physiol. 1997;82:723–731. doi: 10.1152/jappl.1997.82.3.723. [DOI] [PubMed] [Google Scholar]
- 96.Koulouris NG. Methods for assessing expiratory flow limitation during tidal breathing in COPD patients. Pulm Med. 2012;2012:234145. doi: 10.1155/2012/234145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ninane V, Leduc D, Kafi SA. Detection of expiratory flow limitation by manual compression of the abdominal wall. Am J Respir Crit Care Med. 2001;163:1326–1330. doi: 10.1164/ajrccm.163.6.2004150. [DOI] [PubMed] [Google Scholar]
- 98.Flaherty KR, Martinez FJ. The role of pulmonary function testing in pulmonary fibrosis. Curr Opin Pulm Med. 2000;6:404–410. doi: 10.1097/00063198-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 99.Flaherty KR, Martinez FJ. Diagnosing interstitial lung disease: a practical approach to a difficult problem. Cleve Clin J Med. 2001;68:33–34. doi: 10.3949/ccjm.68.1.33. 37–38, 40–41, 45–49. [DOI] [PubMed] [Google Scholar]
- 100.Reich J. Supranormal expiratory flow rates in patients with interstitial lung disease. Chest. 1836;118:2000. doi: 10.1378/chest.118.6.1836. [DOI] [PubMed] [Google Scholar]
- 101.Rodenstein DO, Stanescu DC, Francis C. Demonstration of failure of body plethysmography in airway obstruction. J Appl Physiol. 1982;52:949–954. doi: 10.1152/jappl.1982.52.4.949. [DOI] [PubMed] [Google Scholar]
- 102.Craven N, Sidwall G, West P. Computer analysis of the single-breath nitrogen washout curve. Am Rev Respir Dis. 1976;113:445–449. doi: 10.1164/arrd.1976.113.4.445. [DOI] [PubMed] [Google Scholar]
- 103.Becklake MR, Leclerc M, Strobach H. The nitrogen closing volume test in population studies: sources of variation and reproducibility. Am Rev Respir Dis. 1975;111:141–147. doi: 10.1164/arrd.1975.111.2.141. [DOI] [PubMed] [Google Scholar]
- 104.Make B, Lapp NL. Factors influencing the measurement of closing volume. Am Rev Respir Dis. 1975;111:749–754. doi: 10.1164/arrd.1975.111.6.749. [DOI] [PubMed] [Google Scholar]
- 105.Buist AS, Ross BB. Quantitative analysis of the alveolar plateau in the diagnosis of early airway obstruction. Am Rev Respir Dis. 1973;108:1078–1087. doi: 10.1164/arrd.1973.108.5.1078. [DOI] [PubMed] [Google Scholar]
- 106.Cournand A, Baldwin EDF, Darling RC. Studies on intrapulmonary mixture of gases. IV. The significance of the pulmonary emptying rate and a simplified open circuit measurement of residual air. J Clin Invest. 1941;20:681–689. doi: 10.1172/JCI101261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fowler WS, Cornish ER, Jr, Kety SS. Lung function studies. VIII. Analysis of alveolar ventilation by pulmonary nitrogen clearance curves. J Clin Invest. 1952;31:40–50. doi: 10.1172/JCI102575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang YC, Helms MJ, MacIntyre NR. Normal values for single exhalation diffusing capacity and pulmonary capillary blood flow in sitting, supine positions, and during mild exercise. Chest. 1994;105:501–508. doi: 10.1378/chest.105.2.501. [DOI] [PubMed] [Google Scholar]
- 109.Huang YC, O’Brien SR, MacIntyre NR. Intrabreath diffusing capacity of the lung in healthy individuals at rest and during exercise. Chest. 2002;122:177–185. doi: 10.1378/chest.122.1.177. [DOI] [PubMed] [Google Scholar]
- 110.Horsley AR. Lung clearance index is a sensitive, repeatable and practical measure of airways disease in adults with cystic fibrosis. Thorax. 2008;63(2):135–140. doi: 10.1136/thx.2007.082628. [DOI] [PubMed] [Google Scholar]
- 111.Robinson PD, Goldman MD, Gustafsson PM. Inert gas washout: theoretical background and clinical utility in respiratory disease. Respiration. 2009;78(3):339–355. doi: 10.1159/000225373. [DOI] [PubMed] [Google Scholar]
- 112.Bates DV, Macklem PT, Christie RV. Respiratory function in disease. ed 2. WB Saunders; Philadelphia: 1971. The normal lung: physiology and methods of study; pp. 10–95. [Google Scholar]
- 113.Weibel ER. A simplified morphometric method for estimating diffusing capacity in normal and emphasematous human lungs. Am Rev Respir Dis. 1973;107:579–588. doi: 10.1164/arrd.1973.107.4.579. [DOI] [PubMed] [Google Scholar]
- 114.Gold WM, Youker J, Anderson S. Pulmonary-function abnormalities after lymphangiography. N Engl J Med. 1965;273:519–524. doi: 10.1056/NEJM196509022731003. [DOI] [PubMed] [Google Scholar]
- 115.Comroe JH, Jr, Forster RE, II, DuBois AB. The lung: clinical physiology and pulmonary function tests. ed 2. Year Book; Chicago: 1962. Useful data, equations and calculations; pp. 323–364. [Google Scholar]
- 116.Arjomandi M, Haight T, Sadeghi N. Reduced exercise tolerance and pulmonary capillary recruitment with remote secondhand smoke exposure. PLoS ONE. 2012;7:e34393. doi: 10.1371/journal.pone.0034393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang JS. Role of CO diffusing capacity during exercise in the preoperative evaluation for lung resection. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1435–1444. doi: 10.1164/ajrccm.162.4.2001117. [DOI] [PubMed] [Google Scholar]
- 118.Wang JS, Abboud RT, Wang LM. Effect of lung resection on exercise capacity and on carbon monoxide diffusing capacity during exercise. Chest. 2006;129(4):863–872. doi: 10.1378/chest.129.4.863. [DOI] [PubMed] [Google Scholar]
- 119.Ogilvie CM, Forster RE, Blakemore WS. A standardized breath holding technique for the clinical measurement of the diffusing capacity of the lung for carbon monoxide. J Clin Invest. 1957;36:1–17. doi: 10.1172/JCI103402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jones FS, Mead F. A theoretical and experimental analysis of anomalies in the estimation of pulmonary diffusing capacity by the single breath method. Q J Exp Physiol. 1961;46:131–143. doi: 10.1113/expphysiol.1961.sp001525. [DOI] [PubMed] [Google Scholar]
- 121.Filley GF, MacIntosh DJ, Wright GW. Carbon monoxide uptake and pulmonary diffusing capacity in normal subjects at rest and during exercise. J Clin Invest. 1954;33:530–539. doi: 10.1172/JCI102923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Marshall R. Methods of measuring pulmonary diffusing capacity and their significance. Proc R Soc Med Lond. 1958;51:101–104. doi: 10.1177/003591575805100206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lewis BM, Lin TH, Noe FE. The measurement of pulmonary diffusing capacity for carbon monoxide by a rebreathing method. J Clin Invest. 1958;38:2073–2086. doi: 10.1172/JCI103985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Graham BL, Mink JT, Cotton DJ. Improved accuracy and precision of single-breath CO diffusing capacity measurements. J Appl Physiol. 1981;51:1306–1313. doi: 10.1152/jappl.1981.51.5.1306. [DOI] [PubMed] [Google Scholar]
- 125.Graham BL, Dosman JA, Cotton DJ. A theoretical analysis of the single breath diffusing capacity for carbon monoxide. Trans Biomed Eng. 1980;27:221–227. doi: 10.1109/tbme.1980.326726. [DOI] [PubMed] [Google Scholar]
- 126.Cotton DJ, Graham BL, Mink JT. Pulmonary diffusing capacity in adult cystic fibrosis: reduced positional changes are partially reversed by hyperoxia. Clin Invest Med. 1990;13:82–91. [PubMed] [Google Scholar]
- 127.Newth CJL, Cotton DJ, Nadel JA. Pulmonary diffusing capacity measured at multiple intervals during a single exhalation in man. J Appl Physiol. 1977;43:617–625. doi: 10.1152/jappl.1977.43.4.617. [DOI] [PubMed] [Google Scholar]
- 128.Hallenborg C, Holden W, Menzel T. The clinical usefulness of a screening test to detect static pulmonary blood using a multiple breath analysis of diffusing capacity. Am Rev Respir Dis. 1979;119:349–353. doi: 10.1164/arrd.1979.119.3.349. [DOI] [PubMed] [Google Scholar]
- 129.Krogh M. The diffusion of gases through the lungs of man. J Physiol (Lond) 1915;49:271–296. doi: 10.1113/jphysiol.1915.sp001710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Holden WE, Hallenborg CP, Menzel TE. Effect of static or slowly flowing blood on carbon monoxide diffusion in dog lungs. J Appl Physiol. 1979;46:992–997. doi: 10.1152/jappl.1979.46.5.992. [DOI] [PubMed] [Google Scholar]
- 131.Zenger MR, Brenner M, Haruno M. Measurement of cardiac output by automated single breath technique and comparison with thermodilution and Fick methods in patients with cardiac disease. Am J Cardiol. 1993;71:105–109. doi: 10.1016/0002-9149(93)90719-s. [DOI] [PubMed] [Google Scholar]
- 132.Huang YC, Helms MJ, MacIntyre NC. Normal values for single exhalation diffusing capacity and pulmonary blood flow in sitting, supine positions, and during mild exercise. Chest. 1994;104:501–508. doi: 10.1378/chest.105.2.501. [DOI] [PubMed] [Google Scholar]
- 133.Huang YC, MacIntyre NR. Real-time gas analysis improves the measurement of single breath diffusing capacity. Am Rev Respir Dis. 1992;146:946–950. doi: 10.1164/ajrccm/146.4.946. [DOI] [PubMed] [Google Scholar]
- 134.Morrison NJ, Abboud RT, Muller NL. Pulmonary capillary blood volume in emphysema. Am Rev Respir Dis. 1990;141:53–61. doi: 10.1164/ajrccm/141.1.53. [DOI] [PubMed] [Google Scholar]
- 135.Nadel JA, Gold WM, Burgess JH. Early diagnosis of chronic pulmonary vascular obstruction. Am J Med. 1968;44:16–25. doi: 10.1016/0002-9343(68)90233-7. [DOI] [PubMed] [Google Scholar]
- 136.Cotes JE, Dabbs JM, Elwood PC. Iron-deficiency anaemia: its effect on transfer factor for the lung (diffusing capacity) and ventilation and cardiac frequency during sub-maximal exercise. Clin Sci. 1972;42:325–335. doi: 10.1042/cs0420325. [DOI] [PubMed] [Google Scholar]
- 137.Forster RE. Diffusion of gases. In: Fenn WO, Rahn H, editors. vol 1. American Physiological Society; Washington, DC: 1964. pp. 839–872. (Handbook of physiology. Section III: respiration). [Google Scholar]
- 138.American Thoracic Society Single-breath carbon monoxide diffusing capacity (transfer factor): recommendations for a standard technique. Am Rev Respir Dis. 1987;136:1299–1307. doi: 10.1164/ajrccm/136.5.1299. [DOI] [PubMed] [Google Scholar]
- 139.Graham BL, Mink JT, Cotton DJ. Effects of increasing carboxyhemoglobin on the single breath carbon monoxide diffusing capacity. Am J Respir Crit Care Med. 2002;165:1504–1510. doi: 10.1164/rccm.2108071. [DOI] [PubMed] [Google Scholar]
- 140.Crapo RO, Morris AH. Standardized single breath normal values for carbon monoxide diffusing capacity. Am Rev Respir Dis. 1981;123:185–189. doi: 10.1164/arrd.1981.123.2.185. [DOI] [PubMed] [Google Scholar]
- 141.Rankin J, McNeill RS, Forster RE. Influence of increased alveolar carbon dioxide tension on pulmonary diffusing capacity for CO in man. J Appl Physiol. 1960;15:543–549. doi: 10.1152/jappl.1960.15.4.543. [DOI] [PubMed] [Google Scholar]
- 142.Karp RB, Graf PD, Nadel JA. Regulation of pulmonary capillary blood volume by pulmonary arterial and left atrial pressures. Circ Res. 1968;22:1–10. doi: 10.1161/01.res.22.1.1. [DOI] [PubMed] [Google Scholar]
- 143.Hsia CC, Carlin JI, Wagner PD. Gas exchange abnormalities after pneumonectomy in conditioned foxhounds. J Appl Physiol. 1990;68:94–104. doi: 10.1152/jappl.1990.68.1.94. [DOI] [PubMed] [Google Scholar]
- 144.Stokes DL, Macintyre NR, Nadel JA. Nonlinear increases in diffusing capacity during exercise by seated and supine subjects. J Appl Physiol. 1981;51:858–863. doi: 10.1152/jappl.1981.51.4.858. [DOI] [PubMed] [Google Scholar]
- 145.Arjomandi M. Pulmonary function abnormalities in never-smoking flight attendants exposed to secondhand tobacco smoke in the aircraft cabin. J Occup Environ Med. 2009;51(6):639–646. doi: 10.1097/JOM.0b013e3181a7f048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ceretelli P, DiPrampero PE. Gas exchange during exercise. In: Farhi LE, Tenney SM, editors. vol IV. American Physiological Society; Bethesda, MD: 1987. pp. 297–339. (Handbook of physiology. Section 3: the respiratory system). Gas Exchange. [Google Scholar]
- 147.Kinker JR, Haffor AS, Stephan M. Kinetics of CO uptake and diffusing capacity in transition from rest to steady-state exercise. J Appl Physiol. 1992;72:1764–1772. doi: 10.1152/jappl.1992.72.5.1764. [DOI] [PubMed] [Google Scholar]
- 148.Okada O, Presson RG, Jr, Kirk KR. Capillary perfusion patterns in single alveolar walls. J Appl Physiol. 1992;72:1838–1844. doi: 10.1152/jappl.1992.72.5.1838. [DOI] [PubMed] [Google Scholar]
- 149.West JB, Schneider AM, Mitchell MM. Recruitment in networks of pulmonary capillaries. J Appl Physiol. 1975;39:976–984. doi: 10.1152/jappl.1975.39.6.976. [DOI] [PubMed] [Google Scholar]
- 150.Sankary RM, Turner J, Lipavsky AJA. Alveolar-capillary block in patients with AIDS and Pneumocystis carinii pneumonia. Am Rev Respir Dis. 1988;137:443–449. doi: 10.1164/ajrccm/137.2.443. [DOI] [PubMed] [Google Scholar]
- 151.Hughes JM, van der Lee I. The TL,NO/TL,CO ratio in pulmonary function test interpretation. Eur Respir J. 2013;41(2):453–461. doi: 10.1183/09031936.00082112. [DOI] [PubMed] [Google Scholar]
- Barisione G, Bacigalupo A, Scanarotti C. Mechanisms for reduced diffusing capacity in haematopoietic stem-cell transplantation recipients. Respir Physiol Neurobiol. 2014;194:54–61. doi: 10.1016/j.resp.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 152.Borland C. Steady-state measurement of NO and CO lung diffusing capacity on moderate exercise in men. J Appl Physiol. 2001;90(2):538–544. doi: 10.1152/jappl.2001.90.2.538. [DOI] [PubMed] [Google Scholar]
- 153.Tamhane RM, Johnson RL, Jr, Hsia CC. Pulmonary membrane diffusing capacity and capillary blood volume measured during exercise from nitric oxide uptake. Chest. 2001;120(6):1850–1856. doi: 10.1378/chest.120.6.1850. [DOI] [PubMed] [Google Scholar]
- 154.Moinard J, Guenard H. Determination of lung capillary blood volume and membrane diffusing capacity in patients with COLD using the NO-CO method. Eur Respir J. 1990;3(3):318–322. [PubMed] [Google Scholar]
- 155.Borland C, Cox Y, Higenbottam T. Reduction of pulmonary capillary blood volume in patients with severe unexplained pulmonary hypertension. Thorax. 1996;51(8):855–856. doi: 10.1136/thx.51.8.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.van der Lee I. Diffusing capacity for nitric oxide and carbon monoxide in patients with diffuse parenchymal lung disease and pulmonary arterial hypertension. Chest. 2006;129(2):378–383. doi: 10.1378/chest.129.2.378. [DOI] [PubMed] [Google Scholar]
- 157.Phansalkar AR. Nitric oxide diffusing capacity and alveolar microvascular recruitment in sarcoidosis. Am J Respir Crit Care Med. 2004;169(9):1034–1040. doi: 10.1164/rccm.200309-1287OC. [DOI] [PubMed] [Google Scholar]
- 158.Zavorsky GS. The relationship between single-breath diffusion capacity of the lung for nitric oxide and carbon monoxide during various exercise intensities. Chest. 2004;125(3):1019–1027. doi: 10.1378/chest.125.3.1019. [DOI] [PubMed] [Google Scholar]
- 159.van der Lee I. Diffusing capacity for nitric oxide: reference values and dependence on alveolar volume. Respir Med. 2007;101(7):1579–1584. doi: 10.1016/j.rmed.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 160.Van Noord JA, Clement J, Van de Woestijne KP. Total respiratory resistance and reactance in patients with asthma, chronic bronchitis, and emphysema. Am Rev Respir Dis. 1991;143:922–927. doi: 10.1164/ajrccm/143.5_Pt_1.922. [DOI] [PubMed] [Google Scholar]
- 161.Klein JS, Gamsu G, Webb WR. High-resolution CT diagnosis of emphysema in symptomatic patients with normal chest radiographs and isolated low diffusing capacity. Radiology. 1992;182:817–821. doi: 10.1148/radiology.182.3.1535900. [DOI] [PubMed] [Google Scholar]
- 162.Berend NC, Woolcock AJ, Marlin GE. Correlation between the function and structure of the lung in smokers. Am Rev Respir Dis. 1979;119:695–705. doi: 10.1164/arrd.1979.119.5.695. [DOI] [PubMed] [Google Scholar]
- 163.Gould GA, Redpath AT, Ryan M. Lung CT density correlates with measurements of airflow limitation and the diffusing capacity. Eur Respir J. 1991;4:141–146. [PubMed] [Google Scholar]
- 164.Wall M, Moe E, Eisenberg J. Pulmonary capillary blood volume in emphysema. Am Rev Respir Dis. 1990;141:53–61. doi: 10.1164/ajrccm/141.1.53. [DOI] [PubMed] [Google Scholar]
- 165.Baldi S. Relationship between extent of pulmonary emphysema by high-resolution computed tomography and lung elastic recoil in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(4):585–589. doi: 10.1164/ajrccm.164.4.2010066. [DOI] [PubMed] [Google Scholar]
- 166.Eidelman DH, Ghezzo H, Kim WD. The destructive index and early lung destruction in smokers. Am Rev Respir Dis. 1991;144:156–159. doi: 10.1164/ajrccm/144.1.156. [DOI] [PubMed] [Google Scholar]
- 167.Thurlbeck WM, Dunnill MS, Hartung W. A comparison of three methods of measuring emphysema. Hum Pathol. 1970;1:215–226. doi: 10.1016/s0046-8177(70)80035-1. [DOI] [PubMed] [Google Scholar]
- 168.Jones NL, Goodwin JF. Respiratory function in pulmonary thromboembolic disorders. Br Med J. 1965;5442:1089–1093. doi: 10.1136/bmj.1.5442.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wessel HU, Kezdi P, Cugell DW. Respiratory and cardiovascular function in patients with severe pulmonary hypertension. Circulation. 1964;29:825–832. doi: 10.1161/01.cir.29.6.825. [DOI] [PubMed] [Google Scholar]
- 170.West JB, Dollery CT, Naimaule A. Distribution of blood flow in isolated lung: relation to vascular and alveolar pressures. J Appl Physiol. 1964;19:713–724. doi: 10.1152/jappl.1964.19.4.713. [DOI] [PubMed] [Google Scholar]
- 171.West JB, Dollery CT. Distribution of blood flow and the pressure-flow relations of the whole lung. J Appl Physiol. 1965;20:175–183. [Google Scholar]
- 172.Rienmuller RK, Behr J, Kalender WA. Standardized quantitative high resolution CT in lung diseases. J Comp Assist Tomogr. 1991;15:742–749. doi: 10.1097/00004728-199109000-00003. [DOI] [PubMed] [Google Scholar]
- 173.Wells AU, Hansell DM, Rubens MB. Fibrosing alveolitis in systemic sclerosis: indices of lung function in relation to extent of disease on computed tomography. Arthritis Rheum. 1997;40:1229–1236. doi: 10.1002/1529-0131(199707)40:7<1229::AID-ART6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 174.Schwartz DA, Galvin JR, Dayton CS. Determinants of restrictive lung function in asbestos-induced pleural fibrosis. J Appl Physiol. 1990;68:1932–1937. doi: 10.1152/jappl.1990.68.5.1932. [DOI] [PubMed] [Google Scholar]
- 175.Ley B. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156(10):684–691. doi: 10.7326/0003-4819-156-10-201205150-00004. [DOI] [PubMed] [Google Scholar]
- 176.Wallaert B. Do we need exercise tests to detect gas exchange impairment in fibrotic idiopathic interstitial pneumonias? Pulm Med. 2012;2012:657180. doi: 10.1155/2012/657180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Glanville AR, Baldwin JC, Hunt SA. Long-term cardiopulmonary function after human heart-lung transplantation. Austral N Z J Med. 1990;20:208–214. doi: 10.1111/j.1445-5994.1990.tb01020.x. [DOI] [PubMed] [Google Scholar]
- 178.Ikonen T, Harjula AL, Kinnula V. Selective assessment of single-lung graft function with 133Xe radiospirometry in acute rejection and infection. Chest. 1996;109:879–884. doi: 10.1378/chest.109.4.879. [DOI] [PubMed] [Google Scholar]
- 179.Scott JP, Peters SG, McDougall JC. Posttransplantation physiologic features of the lung and obliterative bronchiolitis. Mayo Clin Proc. 1997;72:170–174. doi: 10.4065/72.2.170. [DOI] [PubMed] [Google Scholar]
- 180.Severinghaus J, Ozanne G, Massuda Y. Measurement of the ventilatory response to hypoxia. Chest. 1976;70:121–124. [PubMed] [Google Scholar]
- 181.Lourenco RV. Clinical methods for the study of regulation of breathing. Chest. 1976;70:109–112. doi: 10.1378/chest.70.1_supplement.109-a. [DOI] [PubMed] [Google Scholar]
- 182.Cherniack NS, Dempsey J, Fencl V. Workshop on assessment of respiratory control in humans. I. Methods of measurement of ventilatory responses to hypoxia and hypercapnia: conference report. Am Rev Respir Dis. 1977;115:177–181. doi: 10.1164/arrd.1977.115.1.177. [DOI] [PubMed] [Google Scholar]
- 183.Mithoefer JC. Breathholding. In: Fenn WO, Rahn H II, editors. Handbook of physiology. Section 3: respiration. American Physiological Society; Washington, DC: 1965. pp. 1011–1025. [Google Scholar]
- 184.Davidson JT, Whipp BJ, Wasserman K. Role of the carotid bodies in breath-holding. N Engl J Med. 1974;290:819–822. doi: 10.1056/NEJM197404112901502. [DOI] [PubMed] [Google Scholar]
- 185.Read DJC. A clinical method for assessing the ventilatory response to carbon dioxide. Australas Ann Med. 1967;16:20–32. doi: 10.1111/imj.1967.16.1.20. [DOI] [PubMed] [Google Scholar]
- 186.Patrick JM, Cotes JE. Hypoxic and hypercapnic ventilatory drives in man (correspondence) J Appl Physiol. 1976;40:1012. doi: 10.1152/jappl.1976.40.6.1012. [DOI] [PubMed] [Google Scholar]
- 187.Rebuck AS, Campbell EJM. A clinical method for assessing the ventilatory response to hypoxia. Am Rev Respir Dis. 1974;109:345–350. doi: 10.1164/arrd.1974.109.3.345. [DOI] [PubMed] [Google Scholar]
- 188.Whitelaw WA, Derenne J, Milic-Emili J. Occlusion pressure as a measure of respiratory center output in conscious man. Respir Physiol. 1975;23:181–199. doi: 10.1016/0034-5687(75)90059-6. [DOI] [PubMed] [Google Scholar]
- 189.Matthews AW, Howell JBL. The rate of isometric inspiratory pressure development as a measure of responsiveness to carbon dioxide in man. Clin Sci Mol Med. 1975;49:57–68. doi: 10.1042/cs0490057. [DOI] [PubMed] [Google Scholar]
- 190.Kryger MH, Yacoub O, Dosman J. Effect of meperidine on occlusion pressure responses to hypercapnia and hypoxia with and without external inspiratory resistance. Am Rev Respir Dis. 1976;114:333–340. doi: 10.1164/arrd.1976.114.2.333. [DOI] [PubMed] [Google Scholar]
- 191.Gelb AF, Klein E, Schiffman P. Ventilatory response and drive in acute and chronic obstructive pulmonary disease. Am Rev Respir Dis. 1977;116:9–16. doi: 10.1164/arrd.1977.116.1.9. [DOI] [PubMed] [Google Scholar]
- 192.Wright BM. Discussion on measuring pulmonary ventilation. In: Harbord RP, Woolmer R, editors. Symposium on pulmonary ventilation. John Sherratt; Altrincham, UK: 1959. p. 87. [Google Scholar]
- 193.Bendixen HH, Smith GM, Mead J. Pattern of ventilation in young adults. J Appl Physiol. 1964;19:195–198. doi: 10.1152/jappl.1964.19.2.195. [DOI] [PubMed] [Google Scholar]
- 194.Mead J, Loring SH. Analysis of volume displacement and length changes of the diaphragm during breathing. J Appl Physiol. 1982;53:750–755. doi: 10.1152/jappl.1982.53.3.750. [DOI] [PubMed] [Google Scholar]
- 195.Severinghaus JW, Stupfel M. Alveolar dead space as an index of distribution of blood flow in pulmonary capillaries. J Appl Physiol. 1957;10:335–348. doi: 10.1152/jappl.1957.10.3.335. [DOI] [PubMed] [Google Scholar]
- 196.Terman JW, Newton JL. Changes in alveolar and arterial gas tensions as related to altitude and age. J Appl Physiol. 1964;19:21–24. doi: 10.1152/jappl.1964.19.1.21. [DOI] [PubMed] [Google Scholar]
- 197.Mellemgaard K. The alveolar-arterial oxygen difference: size and components in normal man. Acta Physiol Scand. 1966;67:10–20. doi: 10.1111/j.1748-1716.1966.tb03281.x. [DOI] [PubMed] [Google Scholar]
- 198.Lilienthal JL, Jr, Riley RL, Premmel DD. An experimental analysis in man of the oxygen pressure gradient from alveolar air to arterial blood during rest and exercise at sea level and at altitude. Am J Physiol. 1946;147:199–216. doi: 10.1152/ajplegacy.1946.147.1.199. [DOI] [PubMed] [Google Scholar]
- 199.Riley RL, Cournand A. Analysis of factors affecting partial pressures of oxygen and carbon dioxide in gas and blood of lungs: theory. J Appl Physiol. 1951;4:77–101. doi: 10.1152/jappl.1951.4.2.77. [DOI] [PubMed] [Google Scholar]
- 200.Andersen AM, Ladefoged J. Partition coefficient of 133xenon between various tissues and blood in vivo. Scand J Clin Lab Invest. 1967;19:72–78. doi: 10.3109/00365516709093483. [DOI] [PubMed] [Google Scholar]
- 201.Bryan AC, Bentivoglio LG, Beerel F. Factors affecting regional distribution of ventilation and perfusion in the lung. J Appl Physiol. 1964;19:395–402. doi: 10.1152/jappl.1964.19.3.395. [DOI] [PubMed] [Google Scholar]
- 202.Milic-Emili J, Henderson JA, Dolovich MB. Regional distribution of inspired gas in the lung. J Appl Physiol. 1966;21:749–759. doi: 10.1152/jappl.1966.21.3.749. [DOI] [PubMed] [Google Scholar]
- 203.Wagner HN, Jr, Sabiston DC, Jr, Iio M. Regional pulmonary blood flow in man by radioisotope scanning. JAMA. 1964;187:601–603. doi: 10.1001/jama.1964.03060210051012. [DOI] [PubMed] [Google Scholar]
- 204.Harding LK, Horsfield K, Singhal SS. The proportion of lung vessels blocked by albumin microspheres. J Nucl Med. 1973;14:579–581. [PubMed] [Google Scholar]
- 205.Wagner PD, Saltzman HA, West JB. Measurement of continuous distributions of ventilation-perfusion ratios: theory. J Appl Physiol. 1974;36:588–599. doi: 10.1152/jappl.1974.36.5.588. [DOI] [PubMed] [Google Scholar]
- 206.Wagner PD, Smith CM, Davies NJH. Estimation of ventilation-perfusion inequality by inert gas elimination without arterial sampling. J Appl Physiol. 1985;59:376–383. doi: 10.1152/jappl.1985.59.2.376. [DOI] [PubMed] [Google Scholar]
- 207.Hansen JE, Clausen JL, Levy SE. Proficiency testing materials for pH and blood gases: The California Thoracic Society experience. Chest. 1986;89:214–217. doi: 10.1378/chest.89.2.214. [DOI] [PubMed] [Google Scholar]
- 208.Van Slyke DD, Neill JM. Determination of gases in blood and other solutions by vacuum extraction and manometric measurement. J Biol Chem. 1924;61:523–573. [PubMed] [Google Scholar]
- 209.Haldane JS, Smith JL. The oxygen tension of arterial blood. J Physiol (Lond) 1896;20:497. doi: 10.1113/jphysiol.1896.sp000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mohler JG, Collier CR, Brandt W. Blood gases. In: Clausen JL, editor. Pulmonary function testing guidelines and controversies: equipment, methods, and normal values. Grune & Stratton; Orlando, FL: 1984. pp. 223–258. [Google Scholar]
- 211.Morris AH, Kanner RE, Crapo RO. ed 2. Intermountain Thoracic Society; Salt Lake City: 1984. Blood gas analysis. [Google Scholar]
- 212.Clark LC., Jr Monitor and control of blood and tissue oxygen tensions. Trans Am Soc Artif Intern Organs. 1956;2:41–48. [Google Scholar]
- 213.Hansen JE, Stone ME, Ong ST. Evaluation of blood gas quality control and proficiency testing materials by tonometry. Am Rev Respir Dis. 1982;125:480–483. doi: 10.1164/arrd.1982.125.4.480. [DOI] [PubMed] [Google Scholar]
- 214.Kusumi F, Butts WC, Ruff WL. Superior analytic performance by electrolytic cell analysis of oxygen content. J Appl Physiol. 1973;35:299–300. doi: 10.1152/jappl.1973.35.2.299. [DOI] [PubMed] [Google Scholar]
- 215.Van Kampen EJ, Zijlstra WG. Standardization of hemoglobinometry. II. The hemoglobin cyanide method. Clin Chim Acta. 1961;6:538–544. doi: 10.1016/0009-8981(61)90145-0. [DOI] [PubMed] [Google Scholar]
- 216.Campagna AC, Matthay MA. Complications of invasive monitoring in the intensive care unit. Pulm Crit Care Update. 1991;6:1–6. [Google Scholar]
- 217.Malinoski DJ, Todd SR, Slone S. Correlation of central venous and arterial blood gas measurements in mechanically ventilated trauma patients. Arch Surg. 2005;140:1122–1125. doi: 10.1001/archsurg.140.11.1122. [DOI] [PubMed] [Google Scholar]
- 218.Walkey AJ, Farber HW, O'Donnell C. The accuracy of the central venous blood gas for acid-base monitoring. J Intensive Care Med. 2010;25:104–110. doi: 10.1177/0885066609356164. [DOI] [PubMed] [Google Scholar]
- 219.Ramakrishna MN, Hedge VD, Kumarswamy GN. Impact of preoperative mild renal dysfunction on short term outcome in isolated Coronary Artery Bypass (CABG) patients. Indian J Crit Care Med. 2008;12:158–162. doi: 10.4103/0972-5229.45075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ladakis C, Myrianthefs P, Karabinis A. Central venous and mixed venous oxygen saturation in critically ill patients. Respiration. 2001;68:279–285. doi: 10.1159/000050511. [DOI] [PubMed] [Google Scholar]
- 221.Gokel Y, Paydas S, Koseoglu Z. Comparison of blood gas and acid-base measurements in arterial and venous blood samples in patients with uremic acidosis and diabetic ketoacidosis in the emergency room. Am J Nephrol. 2000;20:319–323. doi: 10.1159/000013607. [DOI] [PubMed] [Google Scholar]
- 222.Brandenburg MA, Dire DJ. Comparison of arterial and venous blood gas values in the initial emergency department evaluation of patients with diabetic ketoacidosis. Ann Emerg Med. 1998;31:459–465. doi: 10.1016/s0196-0644(98)70254-9. [DOI] [PubMed] [Google Scholar]
- 223.Malatesha G, Singh NK, Bharija A. Comparison of arterial and venous pH, bicarbonate, PCO2 and PO2 in initial emergency department assessment. Emerg Med J. 2007;24:569–571. doi: 10.1136/emj.2007.046979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kelly AM, Kyle E, McAlpine R. Venous pCO(2) and pH can be used to screen for significant hypercarbia in emergency patients with acute respiratory disease. J Emerg Med. 2002;22:15–19. doi: 10.1016/s0736-4679(01)00431-0. [DOI] [PubMed] [Google Scholar]
- 225.Kelly AM, McAlpine R, Kyle E. Venous pH can safely replace arterial pH in the initial evaluation of patients in the emergency department. Emerg Med J. 2001;18:340–342. doi: 10.1136/emj.18.5.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Yildizdaş D, Yapicioğlu H, Yilmaz HL. Correlation of simultaneously obtained capillary, venous, and arterial blood gases of patients in a paediatric intensive care unit. Arch Dis Child. 2004;89:176–180. doi: 10.1136/adc.2002.016261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Adrogué HJ, Rashad M, Gorin AB. Assessing acid-base status in circulatory failure. Differences between arterial and central venous blood. N Engl J Med. 1989;320:1312–1316. doi: 10.1056/NEJM198905183202004. [DOI] [PubMed] [Google Scholar]
- 228.Weil MH, Rackow E, Trevino R. Difference in acid-base state between venous and arterial blood during cardiopulmonary resuscitation. N Engl J Med. 1986;315:153–156. doi: 10.1056/NEJM198607173150303. [DOI] [PubMed] [Google Scholar]
- 229.Lim BL, Kelly AM. A meta-analysis on the utility of peripheral venous blood gas analyses in exacerbations of chronic obstructive pulmonary disease in the emergency department. Eur J Emerg Med. 2010;17(5):246–248. doi: 10.1097/MEJ.0b013e328335622a. [DOI] [PubMed] [Google Scholar]
- 230.McQuitty JC, Lewiston NJ. Pulmonary function testing of children. In: Clausen JL, editor. Pulmonary function testing guidelines and controversies: equipment, methods and normal values. Grune & Stratton; Orlando, FL: 1982. pp. 321–330. [Google Scholar]
- 231.Dawson A. How should we report the oxygen saturation measured on the CO-oximeter? Calif Thorac Soc ABG Newslett. 1989;June:6–7. [Google Scholar]
- 232.Mendelson Y, Kent J, Shaharian A. Evaluation of the Datascope Accusat pulse oximeter in healthy adults. J Clin Monit. 1988;4:59–63. doi: 10.1007/BF01618108. [DOI] [PubMed] [Google Scholar]
- 233.Chapman KR, D’Urzo A, Rebuck AS. The accuracy and response characteristics of a simplified ear oximeter. Chest. 1983;83:860–864. doi: 10.1378/chest.83.6.860. [DOI] [PubMed] [Google Scholar]
- 234.Eichorn J, Cooper J, Cullen D. Standards for patient monitoring during anaesthesia at Harvard Medical School. JAMA. 1986;256:1017–1020. [PubMed] [Google Scholar]
- 235.Huch R, Huch A, Lumbers DW. Transcutaneous measurement of blood Po2 (tcPo2): method and application in perinatal medicine. J Perinat Med. 1973;1:183–191. doi: 10.1515/jpme.1973.1.3.183. [DOI] [PubMed] [Google Scholar]
- 236.Carter R, Banham SW. Use of transcutaneous oxygen and carbon dioxide tensions for assessing indices of gas exchange during exercise testing. Respir Med. 2000;94:350–355. doi: 10.1053/rmed.1999.0714. [DOI] [PubMed] [Google Scholar]
- 237.Planes C, Leroy M, Foray E. Arterial blood gases during exercise: validity of transcutaneous measurements. Arch Phys Med Rehabil. 2001;82:1686–1691. doi: 10.1053/apmr.2001.26248. [DOI] [PubMed] [Google Scholar]
- 238.Kesten S, Chapman KR, Rebuck AS. Response characteristics of a dual transcutaneous oxygen/carbon dioxide monitoring system. Chest. 1991;99:1211–1215. doi: 10.1378/chest.99.5.1211. [DOI] [PubMed] [Google Scholar]
- 239.Martin RJ, Beoglos A, Miller MJ. Increasing arterial carbon dioxide tension: influence on transcutaneous carbon dioxide tension measurements. Pediatrics. 1988;81:684–687. [PubMed] [Google Scholar]
- 240.Morley TF. Capnography in the intensive care unit. J Intern Med. 1990;5:209–223. [Google Scholar]
- 241.Goldberg JS, Rawle PR, Zehnder JL. Colorimetric end-tidal carbon dioxide monitoring for tracheal intubation. Anesth Anal. 1990;70:191–194. doi: 10.1213/00000539-199002000-00011. [DOI] [PubMed] [Google Scholar]
- 242.Bhende MS, Thompson AE, Howland DF. Validity of a disposable end-tidal carbon dioxide detector in verifying endotracheal tube position in piglets. Crit Care Med. 1991;19:566–568. doi: 10.1097/00003246-199104000-00020. [DOI] [PubMed] [Google Scholar]
- 243.Varon AJ, Morrina J, Civetta JM. Clinical utility of a colorimetric end-tidal CO2 detector in cardiopulmonary resuscitation and emergency intubation. J Clin Monit. 1991;7:289–293. doi: 10.1007/BF01619347. [DOI] [PubMed] [Google Scholar]
- 244.Edwards L. Randomised controlled crossover trial of the effect on PtCO2 of oxygen-driven versus air-driven nebulisers in severe chronic obstructive pulmonary disease. Emerg Med J. 2012;29(11):894–898. doi: 10.1136/emermed-2011-200443. [DOI] [PubMed] [Google Scholar]
- 245.Thiele FA, van Kempen LH. A micro method for measuring the carbon dioxide release by small skin areas. Br J Dermatol. 1972;86:463–471. doi: 10.1111/j.1365-2133.1972.tb16098.x. [DOI] [PubMed] [Google Scholar]
- 246.Janssens JP, Perrin E, Bennani I. Is continuous transcutaneous monitoring of Pco2 (TcPco2) over 8h reliable in adults? Respir Med. 2001;95:331–335. doi: 10.1053/rmed.2001.1045. [DOI] [PubMed] [Google Scholar]
- 247.Rais-Bahrami K, Rivera O, Mikesell GT. Continuous blood gas monitoring using an in-dwelling optode method: comparison to intermittent arterial blood gas sampling in ECMO patients. J Perinatol. 2002;22:472–474. doi: 10.1038/sj.jp.7210776. [DOI] [PubMed] [Google Scholar]
- 248.Weiss IK, Fink S, Harrison R. Clinical use of continuous arterial blood gas monitoring in the pediatric intensive care unit. Pediatrics. 1999;103:440–445. doi: 10.1542/peds.103.2.440. [DOI] [PubMed] [Google Scholar]
- 249.Mahutte CK. On-line arterial blood gas analysis with optodes: current status. Clin Biochem. 1998;31:119–130. doi: 10.1016/s0009-9120(98)00009-5. [DOI] [PubMed] [Google Scholar]
- 250.Peruzzi WT, Shapiro BA. Blood gas monitors. Respir Care Clin N Am. 1995;1:143–156. [PubMed] [Google Scholar]
- 251.Ross EM. Measuring arterial oxygenation in a high altitude field environment: comparing portable pulse oximetry with blood gas analysis. Wilderness Environ Med. 2013;24(2):112–117. doi: 10.1016/j.wem.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 252.Comroe JH, Jr, Nadel JA. Current concepts: screening tests of pulmonary function. N Engl J Med. 1970;282:1249–1253. doi: 10.1056/NEJM197005282822207. [DOI] [PubMed] [Google Scholar]
- 253.Bleecker ER, Cotton DJ, Fischer SP. The mechanism of rapid, shallow breathing after inhaling histamine aerosol in exercising dogs. Am Rev Respir Dis. 1976;114:909–916. doi: 10.1164/arrd.1976.114.5.909. [DOI] [PubMed] [Google Scholar]
- 254.Cotton DJ, Bleecker ER, Fischer SP. Rapid, shallow breathing after Ascaris suum antigen inhalation: role of vagus nerves. J Appl Physiol. 1977;42:101–106. doi: 10.1152/jappl.1977.42.1.101. [DOI] [PubMed] [Google Scholar]
- 255.Phillipson EA, Murphy E, Kozar LF. Role of vagal stimuli in exercise ventilation in dogs with experimental pneumonitis. J Appl Physiol. 1975;39:76–85. doi: 10.1152/jappl.1975.39.1.76. [DOI] [PubMed] [Google Scholar]
- 256.Barisione G. How to interpret reduced forced expiratory volume in 1 s (FEV1)/vital capacity ratio with normal FEV1. Eur Respir J. 2009;33(6):1396–1402. doi: 10.1183/09031936.00183708. [DOI] [PubMed] [Google Scholar]
- 257.Eliasson O, Degraff AC., Jr The use of criteria for reversibility and obstruction to define patient groups for bronchodilator trials: influence of clinical diagnosis, spirometric, and anthropometric variables. Am Rev Respir Dis. 1985;132:858–864. doi: 10.1164/arrd.1985.132.4.858. [DOI] [PubMed] [Google Scholar]
- 258.Jain VV. Lung volume abnormalities and its correlation to spirometric and demographic variables in adult asthma. J Asthma. 2013;50(6):600–605. doi: 10.3109/02770903.2013.789058. [DOI] [PubMed] [Google Scholar]
- 259.Shenfield GM, Hodson ME, Clarke SW. Interaction of corticosteroids and catecholamines in the treatment of asthma. Thorax. 1975;30:430–435. doi: 10.1136/thx.30.4.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Davies AO, Lefkowitz RJ. Corticosteroid-induced differential regulation of beta-adrenergic receptors in circulating human polymorphonuclear leukocytes and mononuclear leukocytes. J Clin Endocrinol Metab. 1980;51:599–605. doi: 10.1210/jcem-51-3-599. [DOI] [PubMed] [Google Scholar]
- 261.Hargreave FE, Ryan G, Thomson NC. Bronchial responsiveness to histamine or methacholine in asthma: measurement and clinical significance. J Allergy Clin Immunol. 1981;68:347–355. doi: 10.1016/0091-6749(81)90132-9. [DOI] [PubMed] [Google Scholar]
- 262.Crapo RO, Casaburi R, Coates AL. Guidelines for methacholine and exercise challenge testing—1999. Am J Respir Crit Care Med. 2000;161:309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 263.Anderson SD, Brannan JD. Methods for “indirect” challenge tests including exercise, eucapnic voluntary hyperpnea, and hypertonic aerosols. Clin Rev Allergy Immunol. 2003;24:27–54. doi: 10.1385/CRIAI:24:1:27. [DOI] [PubMed] [Google Scholar]
- 264.Currie GP, Haggart K, Lee DK. Effects of mediator antagonism on mannitol and adenosine monophosphate challenges. Clin Exp Allergy. 2003;33:783–788. doi: 10.1046/j.1365-2222.2003.01688.x. [DOI] [PubMed] [Google Scholar]
- 265.Daviskas E, Anderson SD, Eberl S. Inhalation of dry powder mannitol improves clearance of mucus in patients with bronchiectasis. Am J Respir Crit Care Med. 1999;159:1843–1848. doi: 10.1164/ajrccm.159.6.9809074. [DOI] [PubMed] [Google Scholar]
- 266.Leuppi JD, Brannan JD, Anderson SD. Bronchial provocation tests: the rationale for using inhaled mannitol as a test for airway hyperresponsiveness. Swiss Med Wkly. 2002;132:151–158. doi: 10.4414/smw.2002.09850. [DOI] [PubMed] [Google Scholar]
- 267.Leuppi JD, Salome CM, Jenkins CR. Markers of airway inflammation and airway hyperresponsiveness in patients with well-controlled asthma. Eur Respir J. 2001;18:444–450. doi: 10.1183/09031936.01.00058601. [DOI] [PubMed] [Google Scholar]
- 268.Nensa F. Assessment of airway hyperresponsiveness: comparison of spirometry and body plethysmography. Adv Exp Med Biol. 2013;755:1–9. doi: 10.1007/978-94-007-4546-9_1. [DOI] [PubMed] [Google Scholar]
- 269.Townley RG. Guidelines for bronchial inhalation challenge with pharmacologic and antigenic agents. Am Thorac Soc News. 1980;6:11–19. [Google Scholar]
- 270.Cockcroft DW, Killian DN, Melton JJA. Bronchial reactivity to inhaled histamine: a method and clinical survey. Clin Allergy. 1977;7:235–243. doi: 10.1111/j.1365-2222.1977.tb01448.x. [DOI] [PubMed] [Google Scholar]
- 271.Bye MR, Kerstein D, Barsh E. The importance of spirometry in the assessment of childhood asthma. Am J Dis Child. 1992;146:977–978. doi: 10.1001/archpedi.1992.02160200099037. [DOI] [PubMed] [Google Scholar]
- 272.Russell NJ, Crichton NJ, Emerson PA. Quantitative assessment of the value of spirometry. Thorax. 1986;41:360–363. doi: 10.1136/thx.41.5.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Shim CS, Williams MH., Jr Evaluation of the severity of asthma: patients versus physicians. Am J Med. 1980;68:11–13. doi: 10.1016/0002-9343(80)90155-2. [DOI] [PubMed] [Google Scholar]
- 274.Waterer GW, Wan JY, Kritchevsky SB. Airflow limitation is underrecognized in well-functioning older people. J Am Geriatr Soc. 2001;49:1032–1038. doi: 10.1046/j.1532-5415.2001.49205.x. [DOI] [PubMed] [Google Scholar]
- 275.Enright PL, McClelland RL, Newman AB. Underdiagnosis and undertreatment of asthma in the elderly: Health Study Research Group. Chest. 1999;116:603–613. doi: 10.1378/chest.116.3.603. [DOI] [PubMed] [Google Scholar]
- Inoue H, Niimi A, Takeda T. Pathophysiologial characteristics of asthma in the elderly: a comprehensive study. Ann Allergy Asthma Immunol. 2014 doi: 10.1016/j.anai.2014.08.002. pii:S1081-1206(14)00555-9 [Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 276.Brantigan O. The surgical treatment of pulmonary emphysema. W V Med J. 1954;50:283–285. [PubMed] [Google Scholar]
- 277.Cooper JD, Patterson GA. Lung-volume reduction surgery for severe emphysema. Chest Surg Clin N Am. 1995;5:815–831. [PubMed] [Google Scholar]
- 278.Sciurba FC, Rogers RM, Keenan RJ. Improvement in pulmonary function and elastic recoil after lung-reduction surgery for diffuse emphysema. N Engl J Med. 1996;334:1095–1099. doi: 10.1056/NEJM199604253341704. [DOI] [PubMed] [Google Scholar]
- 279.Teschler H, Stamatis G, El-Raouf Farhat AA. Effect of surgical lung volume reduction on respiratory muscle function in pulmonary emphysema. Eur Respir J. 1996;9:1779–1784. doi: 10.1183/09031936.96.09091779. [DOI] [PubMed] [Google Scholar]
- 280.Fessler HE, Permutt S. Lung volume reduction surgery and airflow limitation. Am J Respir Crit Care Med. 1998;157:715–722. doi: 10.1164/ajrccm.157.3.9608004. [DOI] [PubMed] [Google Scholar]
- 281.Fessler HE, Scharf SM, Permutt S. Improvement in spirometry following lung volume reduction surgery: application of a physiologic model. Am J Respir Crit Care Med. 2002;165:34–40. doi: 10.1164/ajrccm.165.1.2101149. [DOI] [PubMed] [Google Scholar]
- 282.Ingenito EP, Loring SH, Moy ML. Interpreting improvement in expiratory flows after lung volume reduction surgery in terms of flow limitation theory. Am J Respir Crit Care Med. 2001;163:1074–1080. doi: 10.1164/ajrccm.163.5.2001121. [DOI] [PubMed] [Google Scholar]
- 283.Mineo TC, Pompeo E, Rogliani P. Effect of lung volume reduction surgery for severe emphysema on right ventricular function. Am J Respir Crit Care Med. 2002;165:489–494. doi: 10.1164/ajrccm.165.4.2108129. [DOI] [PubMed] [Google Scholar]
- 284.Laghi F, Tobin MJ. Disorders of the respiratory muscles. Am J Respir Crit Care Med. 2003;168:10–48. doi: 10.1164/rccm.2206020. [DOI] [PubMed] [Google Scholar]
- 285.Gelb AF, McKenna RJ, Jr, Brenner M. Lung function 5yr after lung volume reduction surgery for emphysema. Am J Respir Crit Care Med. 2001;163:1562–1566. doi: 10.1164/ajrccm.163.7.2009048. [DOI] [PubMed] [Google Scholar]
- 286.Drazen JM, Epstein AM. Guidance concerning surgery for emphysema. N Engl J Med. 2003;348:2134–2136. doi: 10.1056/NEJMe030058. [DOI] [PubMed] [Google Scholar]
- 287.Ware JH. The National Emphysema Treatment Trial: how strong is the evidence? N Engl J Med. 2003;348:2055–2056. doi: 10.1056/NEJMp030068. [DOI] [PubMed] [Google Scholar]
- 288.Barros WG, Neder JA, Pereira CA, Nery LE. Clinical, radiographic and functional predictors of pulmonary gas exchange impairment at moderate exercise in patients with sarcoidosis. Respiration. 2004;71:367–373. doi: 10.1159/000079641. [DOI] [PubMed] [Google Scholar]
- 289.Alhamad EH, Lynch JP, III, Martinez FJ. Pulmonary function tests in interstitial lung disease: what role do they have? Clin Chest Med. 2001;22:715–750. doi: 10.1016/s0272-5231(05)70062-9. ix. [DOI] [PubMed] [Google Scholar]
- 290.Flaherty KR, Mumford JA, Murray S. Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2003;168:543–548. doi: 10.1164/rccm.200209-1112OC. [DOI] [PubMed] [Google Scholar]
- 291.Wells AU, Desai SR, Rubens MB. Idiopathic pulmonary fibrosis: a composite physiologic index derived from disease extent observed by computed tomography. Am J Respir Crit Care Med. 2003;167:962–969. doi: 10.1164/rccm.2111053. [DOI] [PubMed] [Google Scholar]
- 292.King TE, Jr, Tooze JA, Schwarz MI. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med. 2001;164:1171–1181. doi: 10.1164/ajrccm.164.7.2003140. [DOI] [PubMed] [Google Scholar]
- 293.Latsi PI, Du Bois RM, Nicholson AG. Fibrotic idiopathic interstitial pneumonia: the prognostic value of longitudinal functional trends. Am J Respir Crit Care Med. 2003;168:531–537. doi: 10.1164/rccm.200210-1245OC. [DOI] [PubMed] [Google Scholar]
- 294.Collard HR, King TE, Jr, Bartelson BB. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168:538–542. doi: 10.1164/rccm.200211-1311OC. [DOI] [PubMed] [Google Scholar]
- 295.Noble PW, Morris DG. Time will tell: predicting survival in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2003;168:510–511. doi: 10.1164/rccm.2306021. [DOI] [PubMed] [Google Scholar]
- 296.Gardner ZS, Ruppel GL, Kaminsky DA. Grading the severity of obstruction in mixed obstructive-restrictive lung disease. Chest. 2011;140(3):598–603. doi: 10.1378/chest.10-2860. [DOI] [PubMed] [Google Scholar]
- 297.Nadel JA, Colebatch HJH, Olsen CR. Location and mechanism of airway constriction after barium sulfate microembolism. J Appl Physiol. 1964;19:387–394. doi: 10.1152/jappl.1964.19.3.387. [DOI] [PubMed] [Google Scholar]
- Tsang JY, Hogg JC. Gas exchange and pulmonary hypertension following acute pulmonary thromboembolism: has the emperor got new clothes yet? Pulm Circ. 2014;4:220–236. doi: 10.1086/675985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Hyatt RE. Conditions associated with an abnormal nonspecific pattern of pulmonary function tests. Chest. 2009;135(2):419–424. doi: 10.1378/chest.08-1235. [DOI] [PubMed] [Google Scholar]
- 299.Pellegrino R. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 300.Iyer VN. The nonspecific pulmonary function test: longitudinal follow-up and outcomes. Chest. 2011;139(4):878–886. doi: 10.1378/chest.10-0804. [DOI] [PubMed] [Google Scholar]
- 301.Lin CK, Lin CC. Work of breathing and respiratory drive in obesity. Respirology. 2012;17:402–411. doi: 10.1111/j.1440-1843.2011.02124.x. [DOI] [PubMed] [Google Scholar]
- 302.Rochester D, Enson Y. Current concepts in the pathogenesis of the obesity-hypoventilation syndrome. Am J Med. 1974;57:402–420. doi: 10.1016/0002-9343(74)90135-1. [DOI] [PubMed] [Google Scholar]
- 303.Partridge MR, Ciofetta G, Hughes JMB. Topography of ventilation-perfusion ratios in obesity. Bull Eur Physiopathol Respir. 1979;14:765–773. [PubMed] [Google Scholar]
- 304.Sixt R, Bake B, Kral J. Closing volume and gas exchange before and after intestinal bypass operation. Scand J Respir Dis. 1976;95:65–67. [PubMed] [Google Scholar]
- 305.Meyers DA, Goldberg AP, Bleecker ML. Relationship of obesity and physical fitness to cardiopulmonary and metabolic function in healthy older men. J Gerontol. 1991;46:M57–M65. doi: 10.1093/geronj/46.2.m57. [DOI] [PubMed] [Google Scholar]
- 306.Zwillich CW, Sutton FD, Peirson DJ. Decreased hypoxic drive in the obesity-hypoventilation syndrome. Am J Med. 1975;59:343–348. doi: 10.1016/0002-9343(75)90392-7. [DOI] [PubMed] [Google Scholar]
- O'Donnell DE, Ciavaglia CE, Neder JA. When obesity and chronic obstructive pulmonary disease collide. Physiological and clinical consequences. Ann Am Thorac Soc. 2014;11:635–644. doi: 10.1513/AnnalsATS.201312-438FR. [DOI] [PubMed] [Google Scholar]
- 307.Bottai M, Pistelli F, Di Pede F. Longitudinal changes of body mass index, spirometry and diffusion in a general population. Eur Respir J. 2002;20:665–673. doi: 10.1183/09031936.02.01282001. [DOI] [PubMed] [Google Scholar]
- 308.Zerah-Lancner F. Airway responsiveness measured by forced oscillation technique in severely obese patients, before and after bariatric surgery. J Asthma. 2011;48(8):818–823. doi: 10.3109/02770903.2011.613508. [DOI] [PubMed] [Google Scholar]
- Hanson C, Rutten EP, Wouters EF. Influence of diet and obesity on COPD development and outcomes. Int J Chron Obstruct Pulmon Dis. 2014;9:723–733. doi: 10.2147/COPD.S50111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Colebatch HJH, Greaves IA, Ng CKY. Exponential analysis of elastic recoil and aging in healthy males and females. J Appl Physiol. 1979;47:683–691. doi: 10.1152/jappl.1979.47.4.683. [DOI] [PubMed] [Google Scholar]
- 310.Knudson RJ, Kaltenborn WT. Evaluation of lung elastic recoil by exponential curve analysis. Respir Physiol. 1981;46:29–42. doi: 10.1016/0034-5687(81)90066-9. [DOI] [PubMed] [Google Scholar]
- 311.Burrows B, Lebowitz MD, Camilli AE. Longitudinal changes in forced expiratory volume in one second in adults. Am Rev Respir Dis. 1986;133:974–980. doi: 10.1164/arrd.1986.133.6.974. [DOI] [PubMed] [Google Scholar]
- 312.Gelb AF, Zamel N. Effect of aging on lung mechanics in healthy nonsmokers. Chest. 1975;68:538–541. doi: 10.1378/chest.68.4.538. [DOI] [PubMed] [Google Scholar]
- 313.Babb TG, Rodarte JR. Mechanism of reduced maximal expiratory flow with aging. J Appl Physiol. 2000;89:505–511. doi: 10.1152/jappl.2000.89.2.505. [DOI] [PubMed] [Google Scholar]
- 314.Janssens JP, Pache JC, Nicod LP. Physiological changes in respiratory function associated with ageing. Eur Respir J. 1999;13:197–205. doi: 10.1034/j.1399-3003.1999.13a36.x. [DOI] [PubMed] [Google Scholar]
- 315.Crapo RO, Morris AH, Clayton PD. Lung volumes in healthy nonsmoking adults. Bull Eur Physiopathol Respir. 1982;18:419–425. [PubMed] [Google Scholar]
- 316.Davis C, Cambell EJM, Openshaw P. Importance of airway closure in limiting maximal expiration in normal man. J Appl Physiol. 1980;48:695–701. doi: 10.1152/jappl.1980.48.4.695. [DOI] [PubMed] [Google Scholar]
- 317.Hertle F, Georg E, Lange H-J. Die arteriellen Blutgaspartialdrucke und ihr Beziehungen zu alter und anthropometrischen Gröössen. Respiration. 1971;28:1–30. doi: 10.1159/000192800. [DOI] [PubMed] [Google Scholar]
- 318.Georges R, Saumon G, Loiseau A. The relationship of age to pulmonary membrane conductance and capillary blood volume. Am Rev Respir Dis. 1978;117:1069–1078. doi: 10.1164/arrd.1978.117.6.1069. [DOI] [PubMed] [Google Scholar]
- 319.Viegi G, Sherrill DL, Carrozzi L. An 8-year follow-up of carbon monoxide diffusing capacity in a general population sample of northern Italy. Chest. 2001;120:74–80. doi: 10.1378/chest.120.1.74. [DOI] [PubMed] [Google Scholar]
- 320.Black LF, Hyatt RE. Maximal respiratory pressures: normal values and relationship to age and sex. Am Rev Respir Dis. 1969;99:696–702. doi: 10.1164/arrd.1969.99.5.696. [DOI] [PubMed] [Google Scholar]
- 321.Davis JN. Phrenic nerve conduction in man. J Neurol Neurosurg Psychiatry. 1967;30:420–426. doi: 10.1136/jnnp.30.5.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Similowski T, Fleury B, Launois S. Cervical magnetic stimulation: a new painless method for bilateral phrenic nerve stimulation in conscious humans. J Appl Physiol. 1989;67:1311–1318. doi: 10.1152/jappl.1989.67.4.1311. [DOI] [PubMed] [Google Scholar]
- 323.Wragg S, Aquilina R, Morgan J. Comparison of cervical magnetic stimulation and bilateral percutaneous electrical stimulation of the phrenic nerve in normal subjects. Eur Respir J. 1994;7:1788–1792. doi: 10.1183/09031936.94.07101788. [DOI] [PubMed] [Google Scholar]
- 324.Mador MJ, Rodis A, Magaland UJ. Comparison of cervical magnetic and transcutaneous phrenic nerve stimulation before and after threshold loading. Am J Respir Crit Care Med. 1996;154:448–453. doi: 10.1164/ajrccm.154.2.8756821. [DOI] [PubMed] [Google Scholar]
- 325.Luo YM, Polkey MI, Johnson LC. Diaphragm EMG measured by cervical magnetic and electrical phrenic nerve stimulation. J Appl Physiol. 1998;85:2089–2099. doi: 10.1152/jappl.1998.85.6.2089. [DOI] [PubMed] [Google Scholar]
- 326.Wheeler AP, Marini JJ. Look to the physical examination for valuable diagnostic clues: avoiding the consequences of respiratory muscle fatigue. J Respir Dis. 1985;6:107–125. [Google Scholar]
- 327.Rochester DF, Esau SA. Assessment of ventilatory function in patients with neuromuscular disease. Clin Chest Med. 1994;15:751–763. [PubMed] [Google Scholar]
- 328.De Meester J, Smits JM, Persijn GG. Listing for lung transplantation: life expectancy and transplant effect, stratified by type of end-stage lung disease. The Eurotransplant experience. J Heart Lung Transplant. 2001;20:518–524. doi: 10.1016/s1053-2498(01)00241-8. [DOI] [PubMed] [Google Scholar]
- Backhus L, Sargent J, Cheng A. Outcome in lung transplantation after previous lung volume reduction surgery in a contemporary cohort. J Thorac Cardiovasc Surg. 2014;147:1678–1683. doi: 10.1016/j.jtcvs.2014.01.045. [DOI] [PubMed] [Google Scholar]
- 329.Cassart M, Verbandt Y, de Francquen P. Diaphragm dimensions after single-lung transplantation for emphysema. Am J Respir Crit Care Med. 1999;159:1992–1997. doi: 10.1164/ajrccm.159.6.9812052. [DOI] [PubMed] [Google Scholar]
- 330.Murciano D, Ferretti A, Boczkowski J. Flow limitation and dynamic hyperinflation during exercise in COPD patients after single lung transplantation. Chest. 2000;118:1248–1254. doi: 10.1378/chest.118.5.1248. [DOI] [PubMed] [Google Scholar]
- 331.Sanchez H, Zoll J, Bigard X. Effect of cyclosporin A and its vehicle on cardiac and skeletal muscle mitochondria: relationship to efficacy of the respiratory chain. Br J Pharmacol. 2001;133:781–788. doi: 10.1038/sj.bjp.0704129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Pinet C, Estenne M. Effect of preoperative hyperinflation on static lung volumes after lung transplantation. Eur Respir J. 2000;16:482–485. doi: 10.1034/j.1399-3003.2000.016003482.x. [DOI] [PubMed] [Google Scholar]
- 333.Wanke T, Merkle M, Formanek D. Effect of lung transplantation on diaphragmatic function in patients with chronic obstructive pulmonary disease. Thorax. 1994;49:459–464. doi: 10.1136/thx.49.5.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Pantoja JG, Andrade FH, Stoki DS. Respiratory and limb muscle function in lung allograft recipients. Am J Respir Crit Care Med. 1999;160:1205–1211. doi: 10.1164/ajrccm.160.4.9808097. [DOI] [PubMed] [Google Scholar]
- 335.Wang XN, Williams TJ, McKenna MJ. Skeletal muscle oxidative capacity, fiber type, and metabolites after lung transplantation. Am J Respir Crit Care Med. 1999;160:57–63. doi: 10.1164/ajrccm.160.1.9805092. [DOI] [PubMed] [Google Scholar]
- 336.Evans AB, Al-Himyary AJ, Hrovat MI. Abnormal skeletal muscle oxidative capacity after lung transplantation by 31P-MRS. Am J Respir Crit Care Med. 1997;155:615–621. doi: 10.1164/ajrccm.155.2.9032203. [DOI] [PubMed] [Google Scholar]
- 337.Rodenberg H. Prevention of medical emergencies during air travel. Am Fam Physician. 1988;37:263–271. [PubMed] [Google Scholar]
- 338.Gong H, Jr, Tashkin DP, Lee EY. Hypoxia-altitude simulation test: evaluation of patients with chronic airway obstruction. Am Rev Respir Dis. 1984;130:980–986. doi: 10.1164/arrd.1984.130.6.980. [DOI] [PubMed] [Google Scholar]
- 339.Dillard TA, Berg BW, Rajagopal KR. Hypoxemia during air travel in patients with chronic obstructive pulmonary disease. Ann Intern Med. 1989;111:362–367. doi: 10.7326/0003-4819-111-5-362. [DOI] [PubMed] [Google Scholar]
- 340.Berg BW, Dillard T, Rajagopal KR. Oxygen supplementation during air travel in patients with chronic obstructive lung disease. Chest. 1992;101:638–641. doi: 10.1378/chest.101.3.638. [DOI] [PubMed] [Google Scholar]
- 341.Mehm WJ, Dillard TA, Berg BW. Accuracy of oxyhemoglobin saturation monitors during simulated altitude exposure of men with chronic obstructive pulmonary disease. Aviat Space Environ Med. 1991;62:418–421. [PubMed] [Google Scholar]
- 342.Oades PJ, Buchdahl RM, Bush A. Prediction of hypoxaemia at high altitude in children with cystic fibrosis. BMJ. 1994;308:15–18. doi: 10.1136/bmj.308.6920.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Christensen CC, Ryg MS, Refvem OK. Effect of hypobaric hypoxia on blood gases in patients with restrictive lung disease. Eur Respir J. 2002;20:300–305. doi: 10.1183/09031936.02.00222302. [DOI] [PubMed] [Google Scholar]
- 344.Seccombe LM, Kelly PT, Wong CK. Effect of simulated commercial flight on oxygenation in patients with interstitial lung disease and chronic obstructive pulmonary disease. Thorax. 2004;59:966–970. doi: 10.1136/thx.2004.022210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Dillard TA, Ewald FW., Jr The use of pulmonary function testing in piloting, air travel, mountain climbing, and diving. Clin Chest Med. 2001;22(4):795–816. doi: 10.1016/s0272-5231(05)70067-8. x. [DOI] [PubMed] [Google Scholar]
- 346.Edvardsen A. Air travel and chronic obstructive pulmonary disease: a new algorithm for pre-flight evaluation. Thorax. 2012;67(11):964–969. doi: 10.1136/thoraxjnl-2012-201855. [DOI] [PubMed] [Google Scholar]


