Introduction
Non-domestic felids are susceptible to many of the same diseases as their domestic counterparts. Infectious diseases uncommon in domestic cats, such as canine distemper virus, however, can cause episodic mortalities in wild and captive felids. The solitary nature of some species may help limit transmission of infectious diseases; however, expansion of human populations and their domestic animals provides increased opportunities for contact making naïve wild felids especially vulnerable to cross-species infectious disease transmission. Human-wildlife conflict also results in other forms of morbidity and mortality (e.g. retribution poisoning) and fragmented habitats increase the chance of vehicular trauma. Over half of all non-domestic felids are listed as vulnerable or endangered and many have decreasing wild populations (see Supplemental Materials Table e1).
Table e1.
Taxonomy and Conservation Status of Felidae
| Genus | Species | Common Names | Red List Statusa, b | Year Assessed | Population Trends |
|---|---|---|---|---|---|
| Acinonyx | jubatus | Cheetah, Hunting Leopard | Vuc | 2015 | Decreasing |
| Caracal | aurata | African Golden Cat, Golden Cat | VU | 2015 | Decreasing |
| Caracal | caracal | Caracal, African Caracal, Asian Caracal, Desert Lynx | LC | 2016 | Unknown |
| Catopuma | badia | Borneo Bay Cat, Bay Cat, Bornean Bay Cat | EN | 2016 | Decreasing |
| Catopuma | temminckii | Asiatic Golden Cat, Golden Cat, Temminck’s Cat | NT | 2015 | Decreasing |
| Felis | bieti | Chinese Mountain Cat, Chinese Alpine Steppe Cat, Chinese Desert Cat, Chinese Steppe Cat, Grass Cat | VU | 2015 | Decreasing |
| Felis | chaus | Jungle Cat, Reed Cat, Swamp Cat | LC | 2016 | Decreasing |
| Felis | margarita | Sand Cat, Sand Dune Cat | LC | 2016 | Unknown |
| Felis | nigripes | Black-footed Cat, Small-spotted Cat | VU | 2016 | Decreasing |
| Felis | silvestris | Wild Cat, Wildcat | LC | 2015 | Decreasing |
| Herpailurus | yagouaroundi | Jaguarundi, Eyra Cat | LC | 2015 | Decreasing |
| Leopardus | colocolo | Pampas Cat, Chilean Pampa Cat | NT | 2016 | Decreasing |
| Leopardus | geoffroyi | Geoffroy’s Cat | LC | 2015 | Stable |
| Leopardus | guigna | Guiña, Chilean Cat, Kodkod | VU | 2015 | Decreasing |
| Leopardus | guttulus | Southern Tiger Cat, Southern Little Spotted Cat | VU | 2016 | Decreasing |
| Leopardus | jacobita | Andean Cat, Andean Mountain Cat, Mountain Cat | EN | 2016 | Decreasing |
| Leopardus | pardalis | Ocelot | LC | 2015 | Decreasing |
| Leopardus | tigrinus | Northern Tiger Cat | VU | 2016 | Decreasing |
| Leopardus | wiedii | Margay, Tree Ocelot | NT | 2015 | Decreasing |
| Leptailurus | serval | Serval | LC | 2015 | Stable |
| Lynx | canadensis | Canada Lynx, American Lynx | LC | 2016 | Stable |
| Lynx | lynx | Eurasian Lynx | LC | 2015 | Stable |
| Lynx | pardinus | Iberian Lynx, Pardel Lynx, Spanish Lynx | EN | 2015 | Increasing |
| Lynx | rufus | Bobcat, Bay Lynx | LC | 2016 | Stable |
| Neofelis | diardi | Sunda Clouded Leopard, Enkuli Clouded Leopard, Sunda Clouded Leopard, Sunda Islands Clouded Leopard, Sundaland Clouded Leopard | VU | 2015 | Decreasing |
| Neofelis | nebulosa | Clouded Leopard | VU | 2016 | Decreasing |
| Otocolobus | manul | Pallas’s Cat, Manul | NT | 2016 | Decreasing |
| Panthera | leo | Lion, African Lion | VU | 2016 | Decreasing |
| Panthera | onca | Jaguar | NT | 2008 | Decreasing |
| Panthera | pardus | Leopard | VU | 2016 | Decreasing |
| Panthera | tigris | Tiger | ENc | 2015 | Decreasing |
| Panthera | uncia | Snow Leopard, Ounce | EN | 2008 | Decreasing |
| Pardofelis | marmorata | Marbled Cat | NT | 2016 | Decreasing |
| Prionailurus | bengalensis | Leopard Cat, Iriomote Cat | LCc | 2015 | Stable |
| Prionailurus | planiceps | Flat-headed Cat | EN | 2015 | Decreasing |
| Prionailurus | rubiginosus | Rusty-spotted Cat | NT | 2016 | Decreasing |
| Prionailurus | viverrinus | Fishing Cat | VU | 2016 | Decreasing |
| Puma | concolor | Puma, Cougar, Deer Tiger, Mountain Lion, Red Tiger | LC | 2015 | Decreasing |
En, Endangered; LC, least concern; NT, near threatened; VU, vulnerable.
Status is periodically reviewed, see www.IUCNredlist.org for more detail and current status.
Some subspecies are currently listed as critically endangered (CR), such as the Asiatic cheetah, Malayan tiger, South China tiger, Sumatran tiger, and Iriomote cat; see www.IUCNredlist.org for more detail and current status.
Unique features
Many aspects of the anatomy and clinical pathology of non-domestic felids are similar to those in domestic cats. All felids have the same dental formula (I3/3, C1/1, PM2-3/2, M1/1 in each arcade). Placentation in felids is zonary with an endotheliochorial maternal-fetal interface.
Despite a wide range in body sizes and coloration, there are only a few external and internal anatomic differences among the species of Felidae. Clouded leopards have unusually long canine teeth in comparison to other extant felids. Claws in the cheetah are only semi-retractable and the metacarpal and metatarsal pads are ridged for improved traction during high speed runs. Cheetahs have relatively large hearts in proportion to their body size. Captive black footed cats appear to have enlarged adrenals glands. Because these cats have a normal corticomedullary ratio, the extent to which this enlargement is associated with captivity is uncertain.
Internally, the distal esophagus in cheetahs and some members of the Panthera lineage (lions, tigers, leopards) have prominent folds (Fig. 10.1 ). This can be confusing especially during clinical procedures to obtain gastric biopsies as the folds in the distal esophagus can be mistaken for gastric rugae. Histologically, these folds are composed of stratified squamous epithelium (as for the remainder of the distal esophagus) overlying connective tissue. The purpose of these folds is not certain. In some species, most notably lions, the meninges can contain foci of extramedullary hematopoiesis and/or hemosiderin-laden macrophages without history of previous trauma and hemorrhagei
Figure 10.1.
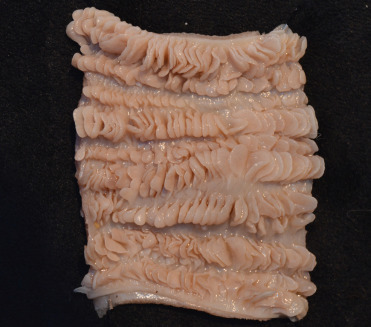
Normal distal esophagus of a cheetah (fixed specimen).
Note the prominent folds and papilla along the mucosal surface.
A common incidental finding in cheetahs is ectatic pancreatic ducts (Munson, 1993). This lesion occurs in approximately one third of captive cheetahs and has been noted in wild cheetahs. In many cases, these ectatic ducts are grossly evident and appear as variably sized, clear, fluid filled cystic structures within the pancreatic parenchyma. Epithelium lining these ducts is low cuboidal and not necessarily flattened suggesting that the ducts are enlarged and not cystic due to obstruction. Fibrosis may or may not be present. No functional significance appears to correlate with their presence or absence and there is no association with pancreatitis.
The mesonephric duct, also known as the Wolffian duct, is an embryonal paired structure in mammals that differentiates into the epididymis, vas deferens and seminal vesicles in males. In females, the ducts regress in the absence of anti-Müllerian hormone. However, cysts can develop in remnant ducts adjacent to the ovary (Fig. 10.2 ). Mesonephric duct cysts occur in all felids and are a common paraovarian cyst in female cheetahs. Cysts are adjacent to but not within the ovary, lined by low cuboidal to flattened, ciliated epithelium and have small amounts of smooth muscle within the cyst wall. There is no association between the presence of these cysts and fertility (Munson, 1993).
Figure 10.2.
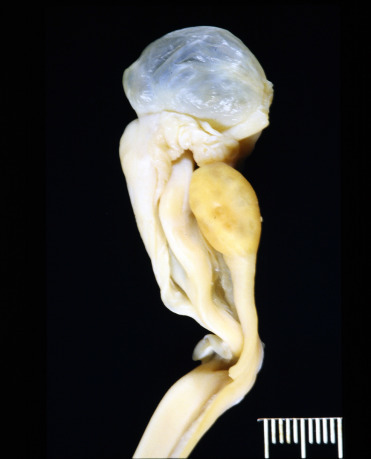
Cystic mesonephric duct remnant (Wolffian duct remnant) in a cheetah.
These cysts are adjacent and not within the ovary (paraovarian) and are incidental findings but can be large and clear fluid filled.
(Photo Courtesy of the Association of Zoos and Aquariums Cheetah Species Survival Plan Pathology Archive)
Hepatic telangiectasia is not an uncommon incidental finding in some of the larger felids including cheetahs, snow leopards, cougars, and lions (Munson, 1993, Bernard et al., 2015). In most cases, lesions develop because of sinusoidal dilation due to loss of integrity of the reticulin network rather than hepatocellular loss (as with the parenchymal form of peliosis hepatis). The pathogenesis of this lesion is unknown. Grossly, multiple dark red areas are noted throughout the liver. Histologically, these variably sized, blood-filled cyst-like spaces are lined by endothelium.
Non-infectious diseases
Nutritional
Cranial malformations and secondary neurologic disorders have been diagnosed in young (typically <2yrs) African lions worldwide. All affected lions have been captive at the time of diagnosis though some were wild born (Bartsch et al., 1975). Similar lesions have been reported in cheetahs (De Risio et al., 2010). Common presenting signs include incoordination, ataxia, head tilt, star-gazing, and or opisthotonus. Characteristic gross lesions include nodular thickening of the bones of the skull, osseous tentorium cerebelli, and dorsal arch of the atlas with narrowing of the foramen magnum, secondary cerebellar herniation, and dilation of the ventricles and central canal of the spinal cord (Figs. 10.3A and B). Affected lions may have compression of the anterior spinal cord. Histologically, bone is thickened, with large, irregular islands of fibrocartilage and woven bone with retained cartilage cores. Osteoclasts are only rarely noted. In the brain and spinal cord, there is histologic evidence of hemorrhage, rarefaction and thinning of the cerebellar folia with loss of all cell layers, and gliosis. Wallerian degeneration is most prominent in the cervical spinal cord. Leptomeninges are often thickened by fibrous connective tissue.
Figure 10.3.
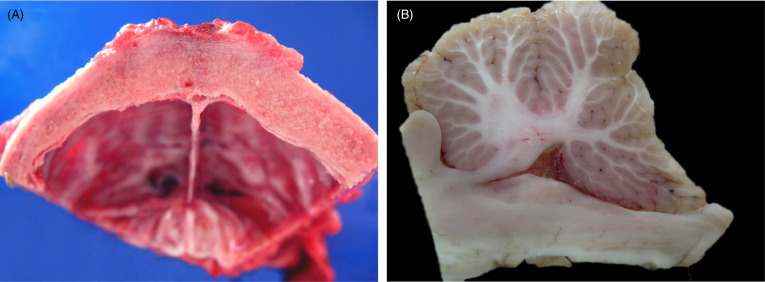
Cranial malformation in an African lion presumed secondary to hypovitaminosis A.
(A) Diffuse thickening of the parietal bones of the skull. (B) Herniation of the cerebellar vermis in a lion with osseous cranial malformations. There is caudal dorsoventral flattening of the cerebellar vermis resulting in a “wedge-shaped” appearance.
Low serum and hepatic vitamin A levels have been reported in many affected lions. Similar lesions have also been documented in other species with Vitamin A deficiency although lesions have not been reproduced in domestic cats (Gershoff et al., 1957) and the pathogenesis is not well understood. In hypovitaminosis A, bone production occurs at sites of normal resorption and the paucity of osteoclasts within lesions suggests a potential link though the role of vitamin A in bone development and maintenance is not completely understood. Treatment in many clinical cases has been unrewarding although improvement has been noted in mild cases given excessive Vitamin A supplementation and in other cases following surgical reduction of bone (Shamir et al., 2008, McCain et al., 2008). Whether the poor response to replacement therapy is due to the chronicity of lesions or alternative pathogenesis is uncertain. Many affected lions are fed diets similar to those of other captive felids that do not develop these lesions. Many reports include multiple cubs from the same litter suggesting genetics may contribute. A separate myelopathy associated with hypovitaminosis A with diffuse spinal cord white matter degeneration in the absence of bone lesions has been diagnosed in a captive lion (Maratea et al., 2006).
Toxic
Toxic levels of mercury, specifically monomethyl and inorganic mercury, have been found in tissues from free-ranging Florida panthers and in hair from museum specimens collected as early as the 1890’s (Roelke et al., 1991, Newman et al., 2005). Mercury levels are high within the Everglades ecosystem and exposure of panthers is likely through predation of piscivorous prey, particularly raccoons (Duvall and Barron, 2000, Barron et al., 2004). It is hypothesized that these contaminants contribute to some of the reproductive, endocrine and immune system problems noted in this species (Roelke et al., 1991, Facemire et al., 1995).
Congenital/Genetic
As a result of habitat loss, geographic isolation from other populations of pumas, and small population numbers, the Florida panther has become increasingly inbred (Roelke et al., 1993). This may be a primary contributing factor in several congenital defects noted in free-ranging panthers including atrial septal defects and cryptorchidism. Ostium secundum atrial septal defects have been diagnosed in up to 18% of panthers (Cunningham et al., 1999, Buergelt et al., 2002) and are thought to have been the cause of death in 9% of studied animals. Bilateral cryptorchidism varies from 49 - 54% depending on the study (Buergelt et al., 2002, Mansfield and Land, 2002). Texas cougars were introduced into the population in 1995 to increase genetic diversity. Since that time, none of the progeny resulting from this introduction have been cryptorchid (Mansfield and Land, 2002).
Multiple ocular colobomas are common in snow leopards in zoos in Europe and the United States (Schaffer et al. 1988; Martin et al. 1997). Ocular colobomas are defects in one or more structures of the eye. Those that occur in the eyelids, cornea, iris, ciliary body, choroid, retina and/or optic disc result from failure of the embryonic fissure to close correctly during embryogenesis (Gripenberg et al. 1985; Barnett & Lewis 2002). A similar suite of lesions has also been described in a captive Texas cougar (Cutler, 2002). To date, there are no reports of the condition in free-ranging snow leopards. In all cases, unilateral or bilateral eyelid colobomas with agenesis of a small to extensive region (typically the lateral and/or central areas) of the upper lid/s occurs (Fig. 10.4 ) and appears as an area in which there is direct transition from the periocular skin to the bulbar conjunctiva. Other lesions include microphthalmia or anophthalmia, and choroidal or optic nerve colobomas. Persistent pupillary membranes, slender, pigmented, fibrovascular strands of remnant fetal membranes that extend from the iris to the anterior lens capsule, posterior surface of the cornea, or other parts of the iris, are relatively common concurrent lesions. Histologic lesions include choroid hypoplasia, retinal cysts, retinal dysplasia (retinal folds), and cupping or cystic ectasia of the optic nerve. Multiple hypotheses have been proposed but to date none have been proven (Gripenberg et al. 1985; Esson 2001; Barnett & Lewis 2002).
Figure 10.4.
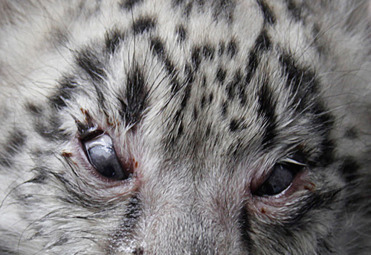
Ocular colobomas in a snow leopard.
There is an abrupt transition from haired skin to the bulbar conjunctiva due to agenesis of the eyelids. Lesions develop in the upper lids and often affect the central (seen here) and/or lateral aspects of the lids.
(Photo Courtesy of the Wildlife Conservation Society)
Pallas’ cats in North American zoos have been diagnosed with polycystic kidneys. Affected cats are generally adults at the time of diagnosis. Cysts are confined to the kidneys, and appear as multiple, large (up to 3 cm diameter), fluid filled tubular cysts lined by low cuboidal to attenuated epithelium in the renal cortex. Because several affected cats have been siblings or closely related, a genetic cause is suspected.
Age-related/Degenerative
Spondylosis (spondylosis deformans; ankylosing spondylosis) is a degenerative disease of the vertebral column that has been described in a variety of captive, non-domestic felids (Kolmstetter et al., 2000). Commonly considered a disease of large cats, spondylosis can also occur in medium and small cats. Rare cases have been identified in free-ranging felids. The spectrum of lesions varies from small bone spurs on the lateral or ventral edges of the articular surfaces of vertebral bodies to large, boney bridges or callus-like proliferations of bone between adjacent vertebral bodies and sclerosis of vertebral end plates (Fig. 10.5 ). Primary factors important in its development include intervertebral disc degeneration, disc herniation and intervertebral space collapse, and degeneration of the articular facets of the vertebral bodies. Affected animals may be asymptomatic or show decreased activity, weakness, ataxia, proprioceptive deficits or paresis. While lesions can occur anywhere along the vertebral column, those in the lumbar spine and lumbosacral junction are most common. Axonal damage and Wallerian degeneration in the spinal cord are associated with disc herniation and compression but can be due to osteophyte formation, and compressive lesions may occur in spinal nerves due to bone expansion.
Figure 10.5.
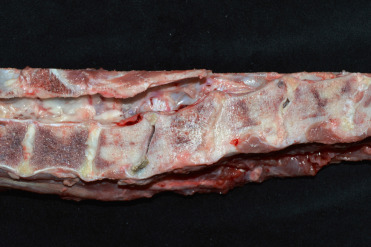
Intervertebral disc degeneration and spondylosis in an African lion.
Collapse of multiple disc spaces is associated with dorsal extrusion of degenerate disc material into the vertebral canal (spinal cord has been removed). Bridging spondylosis, seen as smooth, nodular proliferative bone between adjacent vertebra, is present along the ventral aspect of multiple vertebrae and there is increased bone (end plate sclerosis) in multiple affected vertebral bodies.
Inflammatory Non-infectious
Progressive membranous glomerulonephritis has been described in wild and captive Iberian lynx (Jiménez et al., 2008). Lesion progression occurs with age irrespective of gender. Histologically, there is diffuse membranous glomerulonephritis with rare sclerotic glomeruli. Glomerular basement membranes have increased amounts of laminin, fibronectin and type IV collagen, even in the early stages. Ultrastructurally, in addition to thickened basement membranes, foot processes are effaced in some cases and electron-dense deposits compatible with immune complexes are present in the intramembranous spaces. Positive immunogold staining for IgM and IgG but not IgA are present in these complexes. In addition to glomerular lesions, interstitial lymphoplasmacytic infiltrates are present and increase with severity of the glomerular lesions. Interstitial fibrosis is also noted in more severe cases. Proteinuria and low urine specific gravity, consistent with renal dysfunction, as well as azotemia and hyperphosphatemia are seen in some cases. Underlying viral infections are not associated and thus a genetic predisposition has been hypothesized.
Miscellaneous
Veno-occlusive disease (VOD) is described in cheetahs and snow leopards (Gosselin et al., 1988, Munson and Worley, 1991). Disease is characterized by progressive accumulation of collagen in perisinusoidal spaces (space of Disse) and subintimal spaces of central and sublobular veins. The result is progressive attenuation or obliteration of vascular lumens with collapse of regional cords, atrophy and loss of hepatocytes surrounding affected vessels and subsequent fibrosis (Fig. 10.6A and B). Subendothelial damage, thought to be the primary lesion, is evident ultrastructurally. Chronic cases can progress to cirrhosis. In severe acute cases, centrilobular to massive hepatic necrosis with hemorrhagecan be seen. Clinically, affected cats can be asymptomatic or have signs of hepatic failure including hypoproteinemia, serous or chylous abdominal effusion, elevated liver enzymes (aspartate aminotransferase, alanine aminotransferase), and icterus (Terrell et al., 2003). In rare cases, cheetahs have exhibited neurologic signs presumably due to hepatic encephalopathy. A dietary cause for VOD, including phytoestrogens (a cause of the disease in humans) or elevated dietary Vitamin A, has been proposed but not proven (Setchell et al., 1987, Gosselin et al., 1988). The diagnosis of VOD in a few wild cheetahs seems to refute the hypothesis that captive diets are causative (Munson et al., 2005).
Figure 10.6.
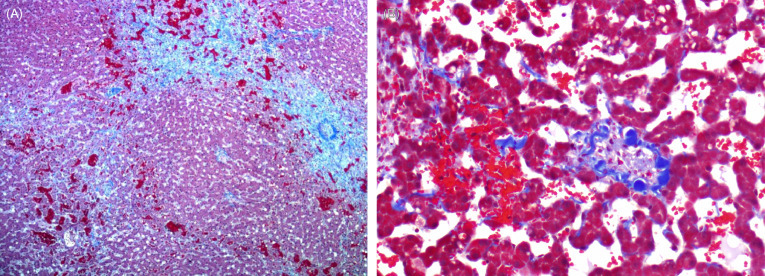
Veno-occlusive disease in the liver of a cheetah.
(A) Complete occlusion of a central vein with broad bands of fibrosis extending from central regions into the adjacent parenchyma (Masson’s Trichrome). (B) Higher magnification of a central vein with fibrous connective tissue occluding the lumen and extending into the adjacent sinusoids. Masson’s Trichrome.
Among felids, amyloidosis is a concern in cheetahs and black-footed cats (Papendick et al., 1997, Terio et al., 2008) although the pathogenesis differs.
Captive black-footed cats have a very high prevalence of systemic AA amyloidosis (Terio et al., 2008); a single, free-ranging juvenile black-footed cat with small amounts of amyloid has been described but the prevalence in and impact on the wild population is unknown. Amyloid deposition occurs most commonly in the renal medulla, glomeruli, spleen, and gastrointestinal tract (Fig. 10.7 ). Affected black-footed cats commonly develop amyloid associated renal failure at a young age (around 4y). Amyloidosis is not associated with chronic inflammation and heritability estimations suggest it may have a genetic component.
Figure 10.7.
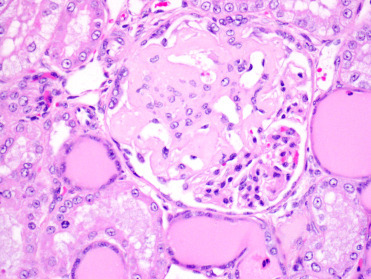
Glomerular amyloidosis in a black-footed cat.
Segmental amyloid deposition within the glomerulus obliterates capillary and urinary spaces with loss of podocytes. Adjacent tubules contain abundant brightly eosinophilic homogenous proteinaceous material consistent with protein leakage from the damaged glomerulus.
In cheetahs, amyloid fibrils are commonly deposited in the renal medulla and can be associated with papillary necrosis (Papendick et al., 1997); less common is deposition in the hepatic perisinusoidal space (space of Disse). These amyloid deposits contain full serum amyloid A (SAA) protein as well as fragmented AA protein (Bergström et al., 2006, Johnson et al., 1997). Unlike black-footed cats, concurrent inflammation, commonly gastritis, is common in cheetahs; however, not all not all cheetahs with inflammation develop amyloidosis. The amino acid sequence of the cheetah AA protein is similar to the sequence in domestic cat breeds with familial amyloidosis, suggesting a structural predisposition and genetic component to disease pathogenesis. Additionally, single nucleotide polymorphisms have been identified in genes regulating transcription of SAA1 suggesting a possible genetic role in increased production of SAA (Zhang et al., 2008b). Different alleles of this gene have been associated with differing SAA serum concentrations in cheetahs but no significant allelic difference has been identified in the presence or absence of amyloidosis in tissues at necropsy (Franklin et al, 2016). Furthermore, if amyloidosis was solely due to a genetic predisposition, we would expect to find amyloidosis in free-ranging cheetahs. To date, this has not been documented (Munson et al., 2005; Terio, Mitchell unpublished) suggesting that disease development is multi-factorial. In some species, AA amyloidosis is thought to be transmissible and AA fibrils have been identified in cheetah feces (Zhang et al., 2008a). Epidemiological studies of amyloidosis in captive cheetahs that control for concurrent inflammation have not found evidence of amyloid transmission in captive North American facilities (McLean et al., 2015).
In addition to systemic amyloidosis, beta amyloid deposits have been described in the brains of some cheetahs. A subset of these had neurofibrillary tangles suggestive of Alzheimer’s disease (Serizawa et al., 2012). While cognitive declines were suggested in 1 case, this is difficult to assess in wild animals and the relative advanced age of affected cats suggests that it is unlikely to be of significance to in situ conservation.
Glomerulosclerosis is a primary renal disease of captive cheetahs that is only rarely documented in free-ranging cheetahs (Munson 1993; Munson et al., 1999, Munson et al., 2005). Glomerular basement membranes are segmentally to globally thickened with individual sclerotic glomeruli (Bolton and Munson, 1999). Some Bowman’s capsule and tubular basement membranes are similarly thickened. Glomerular inflammation and synechia are lacking. Ultrastructurally, membranes contain homogeneous and fibrillar material that lacks electron-dense deposits (Fig. 10.8 ). In more severe lesions, there is fusion of podocyte foot processes. Thickened membranes are histochemically positive for collagen, glycoproteins and reticulin and immunopositive for the advanced glycosylation end products pyrraline and pentosidine. Associated lesions include cortical atrophy, nephrosclerosis, and interstitial fibrosis with lymphoplasmacytic inflammation.
Figure 10.8.
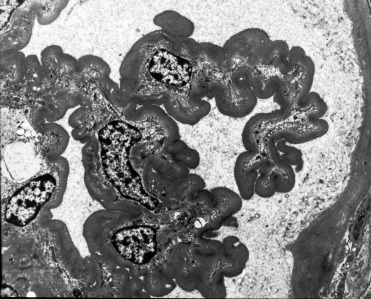
Glomerulosclerosis in a cheetah.
Basement membranes are diffusely thickened by homogeneous material. There is loss of podocytes and endothelial cells and the membranes have collapsed around the remaining mesangial cells. Transmission electron micrograph.
(Reprinted with permission L.A. Bolton and L. Munson, Veterinary Pathology 36: 14-22, 1999)
Several mechanisms have been suggested, but none have been confirmed. The presence of advanced glycosylation end products within glomerular basement membranes of cheetahs parallels lesions in diabetic nephropathy of humans; however, affected cheetahs are generally not diabetic. As the lesions are similar to old rat nephropathy which is linked in some rat strains to high protein diets, the large proportion of skeletal meat in the diet of captive cheetahs (in contrast to wild cheetahs that eat more of the whole carcass) may contribute to disease predisposition in captivity. A genetic predisposition is possible but unlikely since wild cheetahs, which share the species’ renowned homogeneity (O’Brien et al. 1985), generally lack these lesions. Stress-induced hypercorticoidism with subsequent hyperglycemia and or glomerular hypertension with capillary basement membrane damage has also been proposed (Bolton and Munson, 1999, Terio et al., 2004).
Since the mid 1970’s, sporadic cases of oxalate nephrosis have been reported in captive cougars, jaguars, leopards, and most commonly, cheetahs (Mitchell et al., 2017). Affected cats are found dead or present in acute renal failure with severe azotemia, and poly- to anuria. Crystals may be noted in the urine. Histologically, characteristic birefringent crystals forming rosettes are present in renal tubules and associated with epithelial necrosis, tubular dilation and cellular casts. In more chronic cases, there may be tubular regeneration. Crystals are positive for calcium and in some cases basement membranes are mineralized. Ethylene glycol toxicosis has been suspected in some cases but never proven and cases have occurred in areas of the world that do not use antifreeze. Additionally, approximately 10% of the North American and South African captive cheetah populations have histologically evident oxalate crystals at the time of death, making widespread toxicity unlikely. In cheetahs, there is no association between risk factors such as concurrent renal disease (amyloidosis, glomerulosclerosis, interstitial nephritis) or gastritis and the severity of oxalate nephrosis. Some of the most severe cases occur in young cheetahs suggesting an inherent predisposition, although the pathogenesis remains uncertain.
In 1996, leukoencephalopathy was diagnosed in cheetahs at multiple captive breeding facilities from all geographic regions of North America (Brower et al., 2014). This disease had not been diagnosed in captive cheetahs previously, but was similar to lesions in two captive Florida panthers. Affected cheetahs were older (median and mean age of 12 years) and presented with ataxia, suspected blindness, altered behavior, disorientation, proprioceptive deficits and, in some cases. seizures. At necropsy, lesions included bilateral cavitation within the white matter of the centrum semiovale and corona radiata as well as hydrocephalus. Histologically, there were numerous reactive astrocytes, myelin vacuolation and degeneration, and cavitation. The cause of leukoencephalopathy has not been determined. Given the lesions, lack of viral evidence, and epidemiology of the outbreak, exposure of cheetahs to some common neurotoxin through feed, pharmaceuticals or vaccines was considered the most likely cause. No cases have been identified since 2005, and as more times passes, the likelihood of identifying the cause is waning.
A myelopathy has been described in captive born cheetahs housed in Europe and the United Arab Emirates (Walzer and Kübber-Heiss, 1995; Walzer et al., 2003, Robert and Walzer, 2009). Age of onset varies from 2.5 months to 12 years with a rapid and fatal course. Onset is acute with ataxia, paresis and variable progression to dragging of the hind limbs. Histologically, there is bilaterally symmetric degeneration of the white matter tracts of the spinal cord. Myelin loss exceeds that of axonal loss suggesting a primary demyelinating disorder. Gliosis and degeneration of the Purkinje and granular cells of the cerebellum also occur. The pathogenesis is unknown, although copper deficiency has been suspected.
Endometrial hyperplasia (EH) occurs spontaneously in a number of species of zoo felids and is significantly more common in melengestrol acetate (MGA) contracepted animals (Munson et al., 1995, Munson et al., 2002). It is characterized grossly by mild to marked expansion of the endometrium with or without macroscopic cyst formation. Histologically, there is proliferation of endometrial glands with adenomatous projections (adenomatous hyperplasia), dilated cystic glands (cystic hyperplasia), or expansion of the endometrium by irregular folds and/or polypoid projections. Hyperplastic glands are lined by crowded, densely packed, well-differentiated cuboidal to columnar epithelial cells that are asynchronous with the estrous cycle stage. In cystic glands, epithelial cells may be attenuated. In more severe cases, there may also be endometrial fibrosis and endometrial or stromal mineralization. Hyperplasia develops earlier and is significantly more common in MGA-treated animals; risk is increased with increased treatment duration (>72 months). In addition to MGA treatment, age is an additional risk factor while parity is associated with decreased risk. The pathogenesis of MGA-associated EH is unclear; however, exposure of estrogen primed endometrium to prolonged progestins, the latter inducing secretory differentiation of epithelial cells, is suspected.
Neoplastic
Non-domestic felids are susceptible to many of the same neoplasms as domestic cats. Tumors have been described mainly in captive animals. A few tumors of particular note based on species predilections are noted here; additional information on the pathogenesis of these and other common neoplasms is available in the Supplemental Materials. Splenic myelolipomas occur in both captive and wild cheetahs. They commonly present as multiple, variable sized masses composed of mature adipose with varying amounts of hematopoietic cells (Fig. 10.9 ). These masses may be mistaken for more nefarious tumors on ultrasound but are incidental and benign. Benign and malignant biliary tumors are common in many of the large cats. Depending on the extent of parenchyma affected, animals may have associated clinical signs of hepatic dysfunction. Tumors that are generally uncommon in cats but have species predilections include transitional cell carcinomas in fishing cats, pheochromocytomas in clouded leopards, and ovarian carcinomas in jaguars (Fig. 10.10 A and B). Snow leopards can develop squamous cell carcinomas associated with papillomavirus infection (see below). Reproductive tract leiomyomas are common in older female felids and can predispose to other complications such as hydrometra and pyometra. Uterine and mammary carcinomas have been associated with the use of progestin-based contraceptives (e.g. MGA).
Figure 10.9.
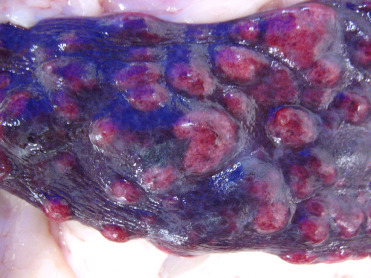
Splenic myelolipomas in a cheetah.
Myelolipomas in cheetahs can be single but are often multiple and appear as variably sized, unencapsulated, well-demarcated, expansile masses that are light to dark red/purple.
Figure 10.10.
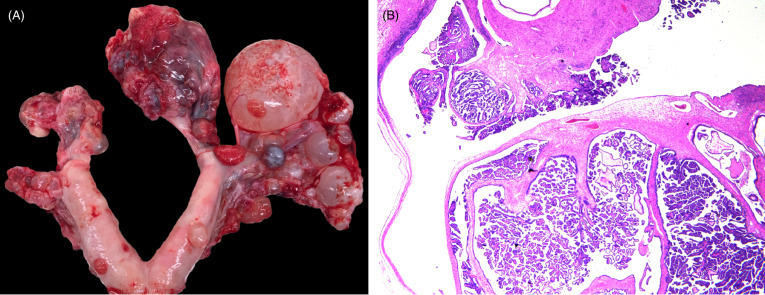
Ovarian cystadenocarcinoma in a jaguar.
(A) The ovaries are effaced by a numerous, variably sized, cystic, tan, red or purple nodular masses. Local metastasis is seen along the uterine serosa. (B) The cystadenocarcinoma is composed of multiple cystic spaces with irregular papillary projections lined by fronds of neoplastic epithelial cells.
(Photos Courtesy of S. Corner, Michigan State University)
Infectious diseases
Free-ranging felids are exposed to a variety of pathogens and are generally well-adapted to their presence due to a long history of co-evolution. However, many felid habitats have become highly fragmented, increasing the risk of disease due to perturbation of normal host-parasite relationships and exposure to novel pathogens (Munson et al., 2010). While the solitary and reclusive nature of felids decreases intraspecific contact rates, fragmentation of habitat results in increased contact with other species, particularly domestic dogs and cats, exposing them to pathogens for which there may not be individual or population level immunity. For example, Feline leukemia virus (FeLV) does not appear to be endemic in most free-ranging felid populations but has resulted in mortalities after introduction (see below). In captivity, despite vaccination and generally excellent medical care, increased animal density facilitates transmission.
DNA Viruses
All captive felids are commonly infected with feline herpesvirus and can develop mild disease with sneezing, nasal discharge, loss of appetite and ocular lesions. Many wild felid populations also have serologic evidence of exposure although disease has not been described (Munson et al., 2010). Vaccination with modified live vaccines may, in some circumstances, induce disease in non-domestic felids (Rivas et al., 2015). More severe upper respiratory tract disease with corneal ulcers and/or keratitis have been noted in both Pallas’ cats and cheetahs. Cheetahs have also been diagnosed with unusual skin lesions due to feline herpesvirus 1 (FHV-1) (Scherba et al., 1988, Junge et al., 1991, Munson et al., 2004b, Witte et al., 2013). Skin lesions have not been noted in wild cheetahs despite evidence of seropositivity (Munson et al., 2004a, Munson et al., 2005, Thalwitzer et al., 2010) but have been documented in wild-caught captive-held cats (Flacke et al., 2015). Lesions present as ulceration and often plaque like proliferative lesions on the face and/or forelimbs, or sites in contact with lacrimal (tear) and salivary gland secretions (Fig. 10.11 A). Biopsy is essential to rule out neoplasia. Histologically, there are dense dermal infiltrates of eosinophils, plasma cells and mast cells. The intact epithelium adjacent to the ulcer may be hyperplastic (Fig 10.11B). Eosinophilic nuclear inclusions can be found in the intact epithelium of the skin, adnexa and rarely in salivary gland ducts
Figure 10.11.
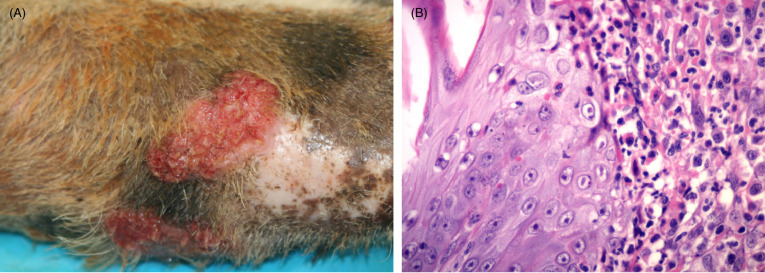
Feline herpesviral dermatitis in a cheetah.
(A) Lesions present as ulcerated, raised plaque like areas. The forelimb is a common site. Similar lesions can be noted on the face. (B) The epidermis is thickened and epithelial cells contain characteristic eosinophilic to amphophilic intranuclear inclusions. Dermal inflammation is typically mixed and, as is this case, can include numerous eosinophils and plasma cells. Mast cells as well as neutrophils in regions with ulceration may also be seen.
Immune responses to FHV-1 in cheetahs have been studied in vitro using peripheral blood mononuclear (lymphocyte and monocyte) cell (PBM) cultures (PBMC) (Miller-Edge and Worley, 1992). In these studies, cheetah PBM as a group had significantly lower proliferative responses than domestic cats to FHV-1, although individual responses varied widely. Interestingly, the cheetah PBM responses improved when they were first cultured with interleukin-2 (IL-2), a TH-1 cytokine critical to an effective cell-mediated immune response. Other studies have found low levels of IL-2 in captive cheetahs as opposed to wild counterparts suggesting that ineffective immune responses may play a role in disease susceptibility (Terio et al., 2014).
Parvoviruses, single stranded DNA viruses, naturally infect a wide range of carnivores including Felidae. The host range of the feline subgroup is poorly defined but felids are considered susceptible to both feline panleukopenia virus (FPL) and canine parvovirus 2 (CPV 2) variants. Little is known about the epidemiology of these viruses in non-domestic species, including their role in free-ranging hosts as natural reservoirs and the importance of bi-directional transmission between and among domestic and wild carnivores. Carnivore breeding or housing facilities may be particularly prone to recurrent disease outbreaks even if vaccination in late pregnancy using an inactivated vaccine is practiced (Lane et al., 2016). The virus is hardy, particularly in cool, moist, shaded environments, so even solitary, widely dispersed free-ranging carnivores may be exposed at marking sites, latrines or play trees shared with other carnivores.
Clinical signs and pathological lesions are rarely documented in wild felids (Steinel et al., 2000, Duarte et al., 2009; Lane et al., 2016). Differing outcomes depend on the timing of infection and may include: reduced litter size, severe lymphoid depletion with or without typical cerebellar or intestinal lesions, or classical lymphoid and intestinal disease in older cats characterized by intestinal hemorrhage, villous atrophy and collapse, necrosis and regeneration of crypt epithelium and opportunistic bacterial catarrhal to necrohemorrhagic enteritis (Fig. 10.12 A and B). Viral inclusions in the intestine and shedding are more common early in the course of disease, which facilitates diagnosis by fecal electron microscopy, immunohistochemistry or virus isolation. Disease caused by CPV in felids may be milder than that caused by FPL.
Figure 10.12.
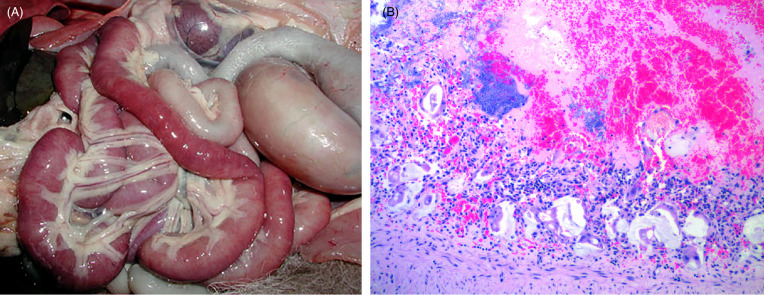
Feline panleukopenia virus (parvovirus) enteritis in a cheetah.
(A) The small intestines are segmentally congested and flaccid. (B) Characteristic lesions include severe, acute crypt epithelial cell necrosis with villous blunting and villous collapse. Intraluminal and intralesional hemorrhage and opportunistic bacterial colonization are common.
Papillomaviruses (PV) are non-enveloped, circular, DNA viruses in the family Papillomaviridae (Rector et al. 2007, Munday 2014). Most PVs are species specific and many have specific tissue tropism. In non-domestic felids, papillomaviruses have been detected and are thought to play a role in the development of oral and cutaneous papillomas with transformation in some cases causing squamous cell carcinomas and sarcoids (Munday, 2014). Basilar epithelial cells are the primary site of infection, and viral replication and assembly occurs in maturing epithelial cells.
Oral papillomas associated with PuPV-1 (also known as UuPV-1) are a relatively common tumor of snow leopards in many zoo collections (Ott Joslin et al., 2000, Sundberg et al., 2000); to our knowledge, they have not been detected in free ranging snow leopards. They arise as single to multiple, variably sized, non-pigmented plaques and or papillary exophytic masses commonly on the ventral surface of the tongue (Fig. 10.13 A). Characteristic histologic lesions include mild to marked mucosal hyperplasia and hyperkeratosis with expansion of the suprabasilar layers of the epithelium and evidence of orderly maturation. A sharp line of demarcation is typically present between lesional and non-lesional tissue. In papillary lesions, underlying fibrous stroma may contain mild inflammation. Viral cytopathologic changes occur in the granular cell layer and include formation of large epithelial cells containing abundant gray-blue, granular cytoplasm, prominent, large basophilic, cytoplasmic keratohyalin granules, and koilocytes (large cells with perinuclear clearing). Inclusions are typically rare and infection can be confirmed by Papillomavirus immunohistochemistry (IHC) (Fig. 10.13B), in situ hybridization, electron microscopy or by PCR.
Figure 10.13.
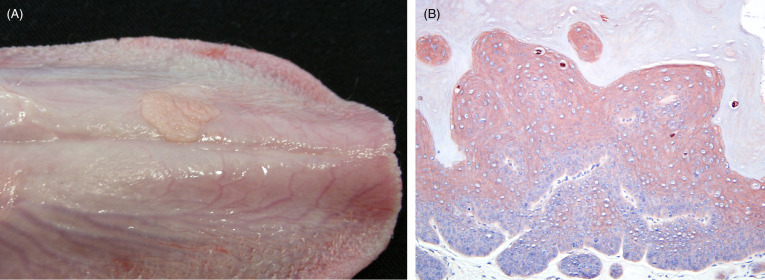
Oral papilloma on the tongue of a snow leopard.
(A) Two non-pigmented, raised, flat plaques are present just lateral to midline on the ventral surface of the tongue. (B) Mucosal epithelial hyperplasia and hyperkeratosis with intense cytoplasmic staining within individual koilocytes. Papillomavirus immunohistochemistry.
In contrast to other non-domestic felids, squamous cell carcinomas (SCC) are more common in snow leopards. Oral SCCs in snow leopards may be due to PV induced hyperplasia and transformation but SCC are less commonly PV IHC positive than are benign papillomas. However, there can be an inverse relationship between the degree of cellular dysplasia and positive immunolabelling and the absence of immunolabelling should not be taken to infer the lack of PV involvement (Munday, 2014). Tumors are commonly sublingual (similar to papilloma location) but can occur in other locations in the oral cavity. A relationship between those that appear to arise in tonsillar crypts or salivary gland and PV infection is unclear. The morphophology is typical of SCC.
Cutaneous plaques, papillomas or squamous cell carcinomas (SCC) have been uncommonly reported in captive snow leopards and associated with PuPV-2 (Ott Joslin et al., 2000, Sundberg et al., 2000) . They can occur in any location but are more often described on the face and forelimbs and less commonly on the feet. Some have been positive for PV by IHC. Research is needed to better understand the pathogenesis and predilection for transformation in snow leopards.
Sarcoids have been described in a group of captive African lions and a captive mountain lion (Schulman et al. 2003, Orbell et al. 2011). In the African lions, tumors develop as single or multiple perioral masses while in the mountain lion the lesions are between the lip and nasal planum. Tumors in both have gross and histologic features typical of sarcoids including densely packed spindle to stellate cells overlain by hyperkeratotic epidermis with long rete pegs. Tissue from affected felids has been PCR positive for PV with a 96-97% sequence similarity to FeSarPV. Surgical resection has not been associated with recurrence in the African lions but regrowth occurred a year after surgical resection in the mountain lion.
RNA Viruses
Canine distemper virus (CDV) has become a significant disease concern for captive and wild felids (Appel et al., 1994, Roelke-Parker et al., 1996, Daoust et al., 2009, Meli et al., 2010, Origgi et al., 2012, Nagao et al., 2012, Seimon et al., 2013). Interestingly, retrospective studies of archived tissues have identified CDV infection in captive lions and tigers prior to the first described outbreak suggesting that CDV infections in felids is not a new phenomenon (Myers et al., 1997). CDV lesions in felids are similar to those in other carnivores (see Chapter 9) including bronchointerstitial pneumonia (Fig. 10.14 A) and non-suppurative encephalitis (Fig. 10.14B) with slight variations in manifestation that may be due to viral strain, tropism and pathogenicity. Co-infections may also be important in the outcome of viral infection, although they are not required for fatal infection (Munson et al., 2008).
Figure 10.14.
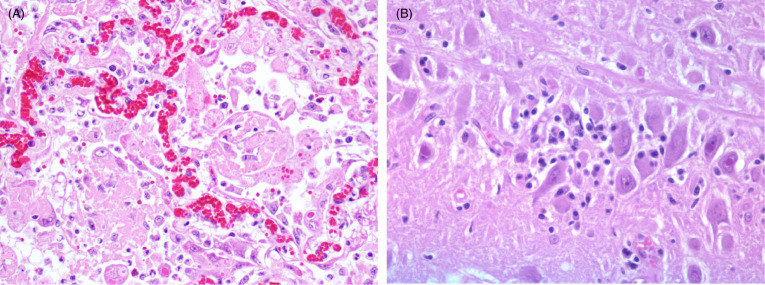
Canine distemper virus bronchointerstitial pneumonia and encephalitis in two tigers.
(A) Alveoli are lined by plump Type II pneumocytes and syncytial cells with characteristic intracytoplasmic and intranuclear inclusion bodies. Alveolar lumens contain numerous foamy macrophages and sloughed epithelial cells. (B) Multiple neurons and astrocytes contain eosinophilic, intranuclear inclusion bodies with mild non-suppurative, lymphoplasmacytic inflammation.
In wild felids, CDV infection has commonly been attributed to unvaccinated domestic dogs (Roelke-Parker et al., 1996). In the Serengeti ecosystem, serological data suggested spillover from dogs as the source of wildlife outbreaks. However, after implementation of a vaccination program, exposure in lions became asynchronous from those in dogs, which highlighted the importance of other sympatric carnivores as reservoirs or a source of infection (Viana et al., 2015). Once infected, whether there is cat to cat spread is uncertain, though models predict that lions may be able to sustain an epidemic due to their social nature (Craft et al., 2009).
Whether felids are naturally susceptible to CDV, similar to other carnivores, or accidental spillover hosts susceptible to only some viral strains is an area of active research. All wild felid populations tested to date have some degree of seropositivity, suggesting that exposure and infection is widespread (Munson et al., 2010). Analysis of multiple sites on the CDV-H gene which encodes the hemagglutinin protein important for binding to host receptors have found no consistent amino acid sequence to identify those strains that infect felids (Terio and Craft, 2013). Additional research is needed to better understand CDV infection dynamics in felids as well as the ecology of disease spread in multi-host ecosystems.
Feline enteric coronavirus (FCoV) infection can cause mild enteritis as well as fatal feline infectious peritonitis (FIP) in non-domestic felids. Lesions and clinical signs of FIP in non-domestic felids are similar to those described in domestic cats and include high protein effusions within the abdominal and or thoracic cavities and pyogranulomatous inflammation centered on vessels. In 1983 an epidemic of FIP in a captive cheetah population caused > 60% mortality (Evermann et al., 1988, Heeney et al., 1990). It was hypothesized that lack of heterogeneity at MHC loci encoding peptides that mediate immune responses to pathogens (O’Brien et al., 1985) was an important factor in the high mortality rate. However, subsequent studies and comparison with data from wild cheetah populations that also lack MHC heterogeneity suggest that extrinsic environmental factors and possibly stress may also be significant in disease pathogenesis (Munson et al., 2005, Terio et al., 2004).
Feline calicivirus is a common, usually self-limiting infection in free-ranging and captive felids. Characteristic lesions are vesicles and ulcers primarily on the tongue, oral and nasal mucosa; they are less common on the foot pads (Kadoi et al., 1997). Infection is highly contagious and can thus spread quickly. Clinical infection has occurred after vaccination with modified-live vaccines in non-domestic felids. Non-domestic felids are also susceptible to systemic calicivirus infections, which can lead to sloughing of glossal epithelium and death (Harrison et al., 2007). Dual-strain killed vaccines may be protective (Harrison et al., 2014).
Avian influenza caused by an influenza type A virus of the Orthomyxoviridae family is a known pathogen of domestic and non-domestic felids. Multiple experimental studies in domestic cats have shown that cats exposed to various influenza A viruses can become infected and develop disease, and that viral spread between cats can occur (Paniker and Nair, 1972, Hinshaw et al., 1981, Kuiken et al., 2004, Rimmelzwaan et al., 2006). Virus is shed through aerozolized respiratory secretions and feces. In 2003-2004, captive clouded leopards, leopard cats and tigers died of highly pathogenic avian influenza (HPAI) H5N1 after being fed infected chicken and quail (Keawcharoen et al., 2004). In one zoo, there was evidence of tiger to tiger viral spread (Thanawongnuwech et al., 2005). Additional cases of H5N1 in captive tigers have occurred in subsequent outbreaks (He et al., 2015, Hu et al., 2016). Clinical signs in affected felids are primarily high fever and respiratory distress. Lesions at gross necropsy have included pulmonary consolidation and hemorrhage with tracheal and bronchial exudates. Histologically, cats have pulmonary hemorrhage, edema and necrosis of alveolar walls as well as meningoencephalitis and necrotizing hepatitis. The impact of influenza viruses on wild populations of felids is unknown. In addition to H5N1 viruses, non-fatal H1N1 viruses have been recovered from cheetahs with clinical respiratory signs (Crossley et al., 2012).
Feline immunodeficiency virus (FIV) is prevalent worldwide and many species of felids, both free-ranging and captive, have serological evidence of exposure. Of the non-domestic felids, FIV infection has been most extensively studied in African lions. In contrast to domestic cats, there is controversy as to whether disease is associated with FIV infections in non-domestic felids. Only a single case of FIV-associated lymphoma has been described (Poli et al., 1995). Alterations in lymphocyte subsets with depletion of CD4+ T cells occur in both captive and free-ranging lions and pumas infected with FIV (Bull et al., 2003, Roelke et al., 2006, Roelke et al., 2009). In wild lions, different FIV(Ple) subtypes are associated with different mortality rates (Troyer et al., 2011) but there is no evidence of differential susceptibility to other infectious agents in FIV infected felids (Turnbull et al., 1992, Schroder et al., 1998, Munson et al., 1995). In addition to lions, mountain lions (pumas), bobcats, Pallas’ cats, leopards, and cheetahs can be infected with their own lentiviruses or those of other felids (Brown et al., 1994, Carpenter et al., 1996, Barr et al., 1997, Carpenter et al., 1998, Troyer et al., 2005, Lee et al., 2014).
As previously noted, Feline leukemia virus (FeLV) is not endemic in most wild felid populations (Munson et al., 2010); the exception is free-ranging wildcats (Duarte et al., 2012). This may be due to increased contact between wildcats and feral domestic cats. FeLV has been associated with clinical disease and mortality in free-ranging Iberian lynx, Florida panthers, and a mountain lion (Jessup et al, 1993; Cunningham et al., 2008, Meli et al., 2009) and has been associated with lymphoma in a single captive cheetah (Marker et al., 2003). Viremia has been linked to anemia, lymphopenia and lymphadenopathy, and death in some cats with associated sepsis. In the Iberian lynx and Florida panther viral sequence analysis indicates similarity to strains previously described in domestic cats, suggesting possible cross-species transmission (Brown et al., 2008, Meli et al., 2009).
Bluetongue (BT) virus serotype 8 has been associated with disease in captive Eurasian lynx fed stillborn or aborted fetuses from farms with confirmed BT infection (Jauniaux et al., 2008). Anemia, subcutaneous hematomas, petechial hemorrhages, pulmonary congestion, edema and death were noted macroscopically and histologic lesions included vasculitis, edema, and reactive endothelial cells. Free-ranging felids have evidence of infection with bluetongue virus but disease has not been reported (Alexander et al., 1994). Transmission is presumed from infected prey; transmission by biting midges, as occurs in ruminants, has not been ruled out.
Bacteria
Both captive and free-ranging felids are susceptible to tuberculosis due mycobacterial infections, mainly by Mycobacterium bovis (bTB). Disease has been described in free-ranging African lions, leopards, cheetah, Iberian lynx, and bobcat (Keet et al 1996; Briones et al., 2000, Bruning-Fann et al., 2001, Pérez et al., 2001, Aranaz et al., 2004, Cleaveland et al., 2005; Kirberger et al., 2006; Schmidbauer et al., 2007, Michel et al., 2009, Martinez et al., 2013, Miller et al., 2015). Wild African lions, leopard and cheetah are affected with the same spoligotype and variable number tandem repeat (VNTR) genotypes isolated from sympatric buffalo, while free-ranging lynx in Spain are infected with spoligotypes identical to those in cattle, wild boar and fallow deer. These findings suggest that cats are infected from prey. Free-ranging cheetahs, leopards and lynx are considered spillover hosts, but lions may be able to serve as maintenance hosts. The long-term effects of bTB on free-ranging lion populations is uncertain (Cleaveland et al., 2005, Ferreira and Funston, 2010, Viljoen et al., 2015). Seroconversion of necropsy staff and keepers has been reported, emphasizing the importance of appropriate personal protective equipment (Helman et al., 1998, Morris et al., 1996, Watering et al., 1972).
Clinical signs, ante-mortem tests and clinical pathology in felids have been reviewed (de Lisle et al., 2002, Maas et al., 2013, Miller et al., 2015, Viljoen et al., 2015). The pathogenesis of mycobacterial infections is determined by multiple host factors including host immunity, co-infections, genetics, population density, contact rates, longevity and behavior (including, gregariousness, denning, scavenging and grooming behavior); pathogen virulence and genetics; and ecological factors including feral cat, host and prey geographic distribution (Clarke et al., 2016). Concurrent infection with FIV appears to play no role in the pathogenesis of bTB in free-ranging lions or lynx (Maas et al., 2012, Peña et al., 2006, Spencer et al., 1992). Increased susceptibility to bTB or recrudescence of latent infections is related to in-breeding in free-ranging lions (Trinkel et al., 2011) and recent episodes of stress or concurrent disease in captive felids (Cho et al., 2006, Martinez et al., 2013, Morris et al., 1996).
Lesions among felid species are similar and disease is often advanced. External findings include emaciation, decubital ulcers, cutaneous alopecia, corneal opacity, poorly healed bite wounds, and elbow and hock hygromas (Figs. 10.15 A, B). Internally, there can be localized or systemic granulomatous inflammation within the lung (Fig. 10.16 A) and lymph nodes or other sites such as the spleen, kidney, eye or adrenal glands. Pulmonary cavitations (<4cm diameter) caused by bronchiectasis with luminal mucus are most common in the diaphragmatic lung lobes. These lesions can rupture and result in pneumothorax as well as hydrothorax and pleural adhesions. Arthritis and proliferative osteitis are other common findings. Histologically, granulomas, multinucleate giant cells, necrosis and mineralization are rare. Rather there are expansile, non-encapsulated aggregates of macrophages, epithelioid cells, lymphocytes, plasma cells and neutrophils along bronchioles and in alveolar walls (Fig. 10.16B). While acid-fast bacteria may be numerous in airways, lung and joint exudates, bacteria are rare in pulmonary macrophages (Fig. 10.16C). Lymphoid atrophy is common. Granulomatous lymphadenitis, panophthalmitis, choroiditis, uveitis, conjunctivitis (with or without retinal detachment), pleuritis, mural enteritis and renal medullary amyloidosis may be present, and osteomyelitis, myositis and periostitis may be accompanied by secondary hypertrophic osteoarthropathy. Granulomatous arthritis may be associated with joint capsule mineralization and ossified fragments in the joint space (Keet et al., 1996, Kirberger et al., 2006).
Figure 10.15.
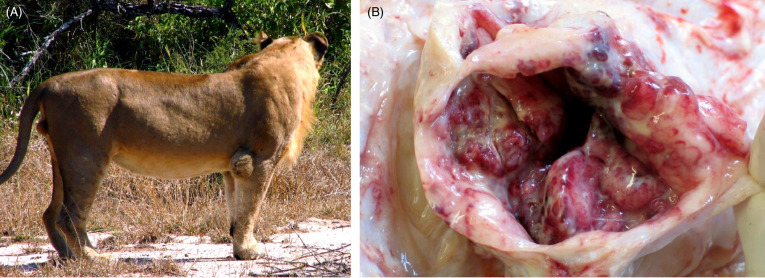
Mycobacterial hygroma in a lion.
(A) Infection with Mycobacterium bovis can lead to hygromas, distended, sac-like structures, over the elbow and hock joints. (B) The opened joint capsule is markedly thickened and there is a chronic, hemorrhagic synovitis within the elbow joint.
(Courtesy of the Roy Bengis, South African Department of Agriculture, Forestry and Fisheries)
Figure 10.16.
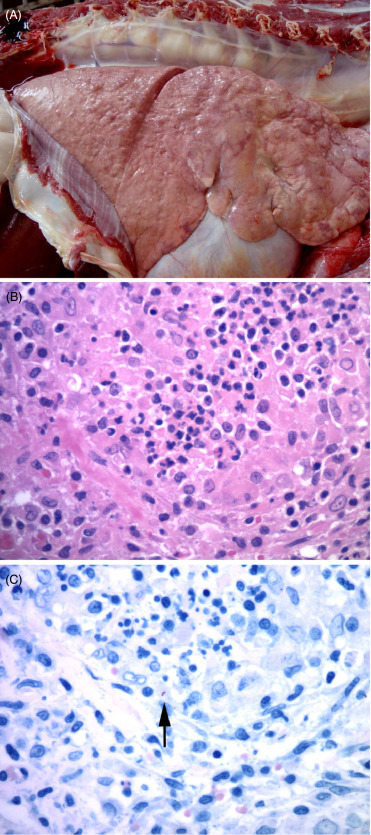
Mycobacterium bovis pneumonia in an African lion.
(A) The lungs contain multiple distinct granulomas and other more diffuse areas of granulomatous inflammation. (Photo Courtesy of Lin-Marie de Klerk-Lorist, South African Department of Agriculture, Forestry, and Fisheries) (B) Characteristic granulomatous inflammation with aggregates of epithelioid macrophages, fewer lymphocytes and plasma cells. (C) Rare, intracellular, acid fast bacilli (arrow) are present within lesions, typical of Mycobacterium tuberculosis complex infections. Ziehl-Neelsen.
Cases of bTB have been described in captive cheetahs, leopards, lions, lynx, puma, snow leopard and tigers (Eulenberger et al., 1992, Jones, 1953). Clinical diagnostic and pathological features are similar to those described in free-ranging felids. Disease due to M. avium has been described in a captive Bengal tiger (Cho et al., 2006) and M. bovis caprae has been isolated from a captive adult Siberian tiger (Lantos et al., 2003). Non-tuberculous mycobacteria (NTM) have also been isolated from the oropharynx of clinically healthy captive pumas in Argentina (Traversa et al., 2009).
Typical lesions and culture results should rule out other bacterial infections unless prolonged antibacterial therapy has been administered prior to death. Mycobacterial isolation from affected tissues, the gold standard diagnostic test, is slow and insensitive. Acid fast stains, immunohistochemistry and molecular tests may provide valuable interim results while waiting for culture. Speciation, typing (spoligotyping and VNTR), PCR and DNA sequencing on fresh or paraffin embedded tissues may be valuable for determining the source of infection, rule out NTM infection and assess the risk to personnel (Cousins and Florisson, 2005, O’Halloran et al., 2016).
Carnivores worldwide are susceptible to anthrax, caused by the Gram-positive endospore-forming multi-host bacterium Bacillus anthracis. Infection is typically through ingestion of infected prey. Confirmed and suspected cases have been described in captive and free-ranging Amur leopard, bobcat, caracal, cheetah, clouded leopard, golden cat, jungle cat, leopard, leopard cat, lion, lynx, ocelot, panther, puma, serval and tiger (Tubbesing, 1997, Yilmaz and Yumusak, 2015). Serological titers in free-ranging lions correlate with geographic areas of anthrax incidence and periods of higher rainfall. Regular exposure may be protective since high mortality occurs in lions after periods of low anthrax incidence whereas non-scavenging felids, such as cheetahs, are highly susceptible to fatal disease (Good et al., 2008, Hugh-Jones and Vos, 2002, Lembo et al., 2011, Turnbull et al., 1992).
Infection occurs through skin defects, ingestion or inhalation of spores that gain entry through oropharyngeal or intestinal tract epithelia, or pulmonary alveolar membranes. Spores in macrophages or mast cells are transported to the major draining lymph nodes of the neck or intestinal tract where they germinate, replicate and produce toxin and then spread systemically causing septicemia. The toxin complex consists of two separate protein components (edema and lethal toxins) and their shared cell receptor binding protein (protective antigen). Severe edema at the site of entry and draining lymph nodes is caused by the edema toxin and is modulated by macrophage production of cytokines including interleukin-1 (IL-1), tumor necrosis factor alpha (TNF-α), IL-6 and IL-8; while lethal toxin inactivates mitogen-activated protein kinases and inhibits IL-1β expression with resultant cell necrosis, reduced phagocytosis and altered blood clotting. Death is due to edema, shock, acute renal failure and central nervous system mediated anoxia (Ha et al., 2016, Hoover et al., 1994).
Disease in felids is usually mild, transient and localized with head and neck swelling (Fig. 10.17 ), lethargy and anorexia; acute death may occur. Marked lip, gingival, submandibular and lingual edema, serosanguinous ascites, marked edema of the gastric and/or intestinal wall, pancreatic congestion and edema, multifocal acute agonal subepicardial and endocardial hemorrhages and rapid putrefaction of the spleen, liver, kidney and parotid salivary glands are described in fatal cases. Histologically, abundant fibrin-rich edema fluid containing large numbers of neutrophils and variable numbers of spore-forming bacilli expand the affected subcutaneous adipose tissue and gastric wall. Fibrinoid vasculitis may be present in the skin and renal blood vessels; acute necrotizing and hemorrhagic lymphadenitis and necrotizing enteritis are also seen. Asphyxiation and aspiration of gastric contents may occur.
Figure 10.17.

Anthrax cellulitis and edema in an African lion.
Note the marked swelling of the head and neck due to edema and cellulitis.
(Courtesy Roy Bengis, South African Department of Agriculture, Forestry and Fisheries)
Differentiation from cytotoxic snake bites is necessary. To reduce environmental contamination, carcasses of suspected cases should not be opened to limit exposure, including mechanical transfer of spores by arthropods. Carcasses should be disposed of by incineration, rendering or burial. Cytological preparations of tissue smears, fluids aspirated from edematous lesions or tissue lesions stained with Giemsa stains show variable numbers of basophilic blunt -ended, bacterial rods with a well-developed capsule that may form short chains (see Chapter 1). Bacterial morphology may alter after death. Organisms in affected tissues may not stain with Gram stains if antibiotics have been administered before death, but silver stains usually remain positive. Bacteria may be cultured from unstained blood, tissue or exudate smears. Molecular tests for anthrax are largely used for human epidemiological studies and research. As anthrax is an OIE listed, reportable zoonotic disease, appropriate protective measures should be taken.
Helicobacter sp. are spiral bacteria that commonly colonize the stomachs of free-ranging and captive felids (Kinsel et al., 1998a, Kinsel et al., 1998b). Helicobacter sp. are generally an incidental finding with only rare individual cases of gastritis reported in most felid species (Schroder et al., 1998). The exception are cheetah in which the majority (>95%) of captive cheetah have associated lymphoplasmacytic gastritis (Eaton et al., 1993; Munson, 1993). Grossly, stomachs are thickened with a reddened cobblestone appearance; some cases have loss of rugae and atrophy (Fig. 10.18 A). Histologically, there are large numbers of lymphocytes and plasma cells within the lamina propria as well as intraepithelial (CD3+CD8α+) T cells (Fig. 10.18B). Severe cases are characterized by large numbers (>70%) of lamina proprial activated B (CD79a+ CD21-) and plasma cells (Terio et al., 2012). Some lymphoid aggregates along the base of the lamina propria have follicular differentiation and mucosal atrophy is present in some cases. Clinical signs in cheetahs range from weight loss to chronic vomiting and regurgitation; in rare cases, cheetahs are euthanized due to chronic gastritis. Cheetahs with gastritis can develop secondary amyloidosis (Papendick et al., 1997). Wild cheetahs are also infected with Helicobacter sp. and the types of Helicobacter are similar to those in captive cheetahs suggesting that bacteria alone do not cause disease and that host factors are important in disease development (Terio et al., 2005, 2014).
Figure 10.18.
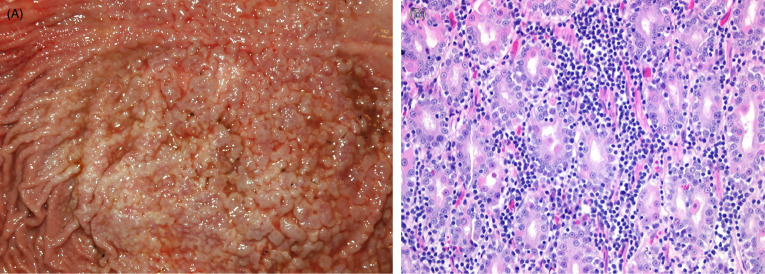
Helicobacter-associated gastritis in a captive cheetah.
(A) The gastric mucosa is markedly thickened and reddened with a cobblestone appearance. (B) Large numbers of plasma cells and lymphocytes are present within the lamina propria and within the gland epithelium. Individual epithelial cells are necrotic and sloughed into gland lumens.
Salmonella sp. can cause enteritis in felids but fecal culture of Salmonella sp. should be interpreted with caution as the bacteria can be shed from asymptomatic cats (Clyde et al., 1997, Venter et al., 2003). In one study, Salmonella, primarily S. typhimurium, was isolated from >90% of cats. The source of Salmonella has been presumed to be contaminated feed as Salmonella have been isolated from dietary meat.
Plague, due to infection with Yersinia pestis, occurs in felids primarily due to consumption of infected rodents. Disease is uncommon in captivity as food sources are managed, but can occur, especially with “opportunistic” predation on wild rodents. Lesions are typical and include necrotizing and suppurative pneumonia, splenitis, hepatitis and lymphadenitis with intralesional Gram negative coccobacilli. Disease has also occurred in free-ranging Canada lynx where lesions were predominately in the lungs (Wild et al., 2006).
Hemoplasmas (hemotropic Mycoplasmas ) have been reported in multiple wild felids. The pathogenicity of infection is uncertain. In some cases, association between infection and disease has not been found (Munson et al., 2008) and in other cases they are co-infectious agents so their contribution to disease outcome is unclear (Geret et al., 2011).
Fungi
Cryptococcosis, caused by the dimorphic fungus Cryptococcus gattii, has been described in captive and free-ranging cheetahs (Illnait-Zaragozi et al., 2011, Millward and Williams, 2005). Inhalation of infectious basidiospores may be common in cheetahs because of their habit of using play trees and aerosolizing the organism through slapping the ground with their forelimbs when threatened. The outcome of infection depends on host immune status, fungal virulence factors including capsule thickness, and the capacity to combat host oxidative processes (Lester et al., 2011). While the predisposition of the king cheetah coat variant to clinical disease suggests an inherited susceptibility in this subtype (Berry et al., 1997, Bolton et al., 1999, Picard et al., 1998), neither the limited genetic variation of the cheetah nor underlying viral infections are predisposing (Miller-Edge and Worley,1992; Millward and Williams, 2005). The role of chronic stress in captive cheetahs (Terio et al., 2004) in the susceptibility to this disease is uncertain.
Ulcerative skin nodules and discrete to coalescing white, light gray to tan, soft to gelatinous space occupying foci in the nose and underlying bone or oral mucosa, lungs, pleura, meninges, brain, spinal cord, kidney, optic nerve, and retrobulbar adipose tissue as well as draining lymph nodes may be present. Histologically, uninucleate thin-walled spherical to oval yeast (2-20 μm diameter) with single, narrow-based budding and a wide clear capsule are pathognomonic. Inflammation is variable and may include macrophages (with phagocytosed organisms), multinucleate giant cells, lymphocytes and plasma cells. Lesions are often present on the ventral brain surface and are associated with the meninges, ventricles and spinal cord central canal. Cytology of lesions or centrifuged cerebrospinal fluid using Romanowsky-type stains is diagnostic as is histology since Cryptococcus is distinctive and is the only fungus with a mucinous capsule that stains with Alcian blue and Mayer’s mucicarmine. Genotyping by various molecular methods has replaced serotyping (Lester et al., 2011).
Dermatophytosis due to Trichophyton mentagrophytes or Microsporum spp . can occur in any felid, captive or wild, and causes alopecia, pyoderma and hyperkeratosis (Rotstein et al., 1999). Topical treatment may be effective (Sykes and Ramsay, 2007). In cheetahs, topical anti-fungal treatments are recommended as griseofulvin has been associated with lethargy, diarrhea and bone marrow suppression (Wack et al., 1992).
Parasites
Felids can be infected with a wide variety of parasites, some which are associated with disease and others that are asymptomatic in immune competent hosts. Some parasites, such as Sarcoptes scabeii, have negatively impacted wild populations. The lesions and pathogenesis of mange in felids is similar to other species. Selected metazoan, protozoan and ectoparasites are described below and presented in the Supplemental Materials (Table e2).
Table e2.
Additional Selected Parasites of Nondomestic Felids
| Etiology | Location in Host | Associated Disease (If Any) | Species Affected | References |
|---|---|---|---|---|
| Metazoan | ||||
| Aelurostrongylus abstrusus | Lung | Unknown | African lion, serval, caracal, leopard cat, ocelot | a, b, h, n, g |
| Cylicospirura spp. | Stomach and proximal duodenum | Nodular granulomatous inflammation surrounding worms; rare cases of rupture | Mountain lions and bobcats | c, f |
| Echinococcus oligarthrus | Gastrointestinal tract | None | Central/South American Felids (Puma, jaguar, ocelot, wild cat, pampas cat, bobcat, lynx) | r |
| Taenia spp. | Gastrointestinal tract | None | All felids susceptible | r |
| Trichinella spp. | Striated muscle | None | All felids susceptible | r |
| Protozoan | ||||
| Babesia spp. | Intraerythrocytic | Variable anemia; can be important copathogen in CDV epidemics | Lion, serval, black-footed cat, leopard | o, k |
| Cytauxzoon spp. | Intrahistiocytic | C. felis associated with anemia, lethargy, high fever associated with numerous large intravascular macrophages containing numerous schizonts; pathogenicity of other Cytauxzoon species is unknown | North American felids (mountain lion, Florida panther, bobcats) infected with C. felis; Captive felids housed in the southern United States; C. manul in Pallas’ cats from Mongolia; Cytauxzoon sp. in lynx and wildcats from Europe | e, m, p, t |
| Ehrlichia spp. | None | Jaguar, Mountain lion, African lion, Iriomote cat, Tsushima leopard cat | d, s, u | |
| Giardia spp. | Small intestine | Loss of microvilli, malabsorption/maldigestion | All felids susceptible | r |
| Hepatozoon felis and H. spp. | White blood cells | None | All felids susceptible | r |
| Sarcocystis spp. | Skeletal muscle | None to minimal inflammation surrounding ruptured cysts | All felids susceptible | r |
| Ectoparasite | ||||
| Sarcoptes scabeii, Notoedres cattii | Hair loss, crusting dermatitis | Hyperkeratotic dermatitis | All felids susceptible | i, j, l, q, v, w, x |
Bjork, K.E., Averbeck, G.A., Stromberg, B.E., 2000. Parasites and parasite stages of free-ranging wild lions (Panthera leo) of northern Tanzania. J. Zoo Wildl. Med. 31, 56-61.
Di Cesare, A., Laiacona, F., Iorio, R., Marangi, M., Menegotto, A., 2016. Aelurostrongylus abstrusus in wild felids of South Africa. Parasitol. Res. 115, 3731-3735.
Ferguson, J.A., Woodberry, K., Gillin, C.M., Jackson, D.H., Sanders, J.L., Madigan, W., Bildfell, R.J., Kent, M.L., 2011. Cyclicospirura species (Nematoda: Spirocercidae) and stomach nodules in cougars (Puma concolor) and bobcats (Lynx rufus) in Oregon. J. Wildl. Dis. 471, 140-153.
Filoni, C., Catão-Dias, J.L., Bay, G., Durigon, E.L., Jorge, R.S., Lutz, H., Hofmann-Lehmann, R., 2006. First evidence of feline herpesvirus, calicivirus, parvovirus, and Ehrlichia exposure in Brazilian free-ranging felids. Journal of Wildlife Diseases 42. 470-477.
Gallusová, M., Jirsová, D., Mihalca, A.D., Gherman, C.M., D’Amico, G., Qablan, M.A., Modry, D., 2016. Cytauxzoon infections in wild felids from Carpathian-Danubian-Pontic Space: Further evidence for a different Cytauxzoon species in European felids. J. Parasitol. 102, 377-380.
Gillin, C.M., Jackson, D.H., Stroud, R.K., Woodberry, K.E., 2005. Spatial distribution and potential impact of nodular stomach worms (Cylicospirura spp.) to survival of free ranging mountain lions (Puma concolor) in Oregon. Proceedings AAZV, AAWV, AZA/NAG Joint Conference, 251.
Glenn, B.L., A.A. Kocan, and E.F. Blouin. 1983. Cytauxzoonosis in bobcats. J. Am. Vet. Med. Assoc. 183, 1155-1158.
González, P., Carbonell, E., Urios, V., Rozhnov, W., 2007. Coprology of Panthera tigris altaica and Felis bengalensis euptilurus from the Russian Far East. J. Parasitol. 93, 948-950.
Holt, G., Berg, C., 1990. Sarcoptic mange in red fox and other wild mammals in Norway. Norsk Veterinartidsskrift 102, 427-432.
Mörner, T., 1992. Sarcoptic mange in Swedish wildlife. Rev. Sci. Tech. 11,1115–1121.
Munson, L., Terio, K.A., Kock, R., Mlengeya, T., Roelke, M.E., Dubovi, E., Summers, B., Sinclair, A.R., Packer, C., 2008. Climate extremes promote fatal co-infections during canine distemper epidemics in African lions. PLoS One 3, e2545.
Mwanzia, J.M., Kock, R.A., Wambna, J.M., Kock, N.D., Jarrett, O., 1995. An outbreak of sarcoptic mange in free living cheetah (Acinonyx jubatus) in the Mara region of Kenya.
Proceedings AAZV/WDA/AAWV Joint Conference, 95-102.
Nietfeld, J.C., Pollock, C., 2002. Fatal cytauxzoonosis in a free-ranging bobcat (Lynx rufus). J. Wildl. Dis. 38, 607-610.
Patton, S., Rabinowitz, A., Randolph, S., Jojnson, S.S., 1986. A coprological survey of parasits of wild neotropical felidae. J. Parasitol. 72, 517-520.
Penzhorn, B.L., 2006. Babesiosis of wild carnivores and ungulates. Vet. Parasitol. 138, 11-21.
Rotstein, D.S., Taylor, S.K., Harvey, J.W., Bean, J., 1999. Hematologic effects of cytauxzonosis in Florida panthers and Texas cougars in Florida. J. Widl. Dis. 35, 613-617.
Ryser-Degiorgis, M.P., Ryser, A., Bacciarini, L.N., Angst, C., Gottstein, B., Janovsky, M., Breitenmoser, U., 2002. Notoedric and sarcoptic mange in free-ranging lynx from Switzerland. J. Wildl. Dis. 38, 228-232.
Samuel, W.M., Pybus, M.J., Kocan, A.A., 2008. Parasitic Diseases of Wild Mammals, second ed. Iowa State University Press, Ames, Iowa.
Tateno, M., Nishio, T., Sakuma, M., Nakanishi, N., Izawa, M., Asari, Y., Okamura, M., Maruyama, S., Miyama, T.S., Setoguchi, A., Endo, Y., 2013. Molecular epidemiologic survey of Bartonella, Ehrlichia, and Anaplasma infections in Japanese Iriomote and Tsushima leopard cats. J. Wildl. Dis. 49, 646-652.
Veronesi, F., Ravagnan, S., Cerquetella, M., Carli, E., Olivieri, E., Santoro, A., Pesaro, S., Berardi, S., Rossi , G., Ragni, B., Beraldo, P., Capelli, G., 2016. First Detection of Cytauxzoon spp. infection in European wildcats (Felis silvestris silvestris) of Italy. Ticks Tick Borne Dis. 7, 853-585.
Widmer, C.E., Azevedo, F.C., Almeida, A.P., Ferreira, F., Labruna, M.B., 2011. Tick-borne bacteria in free-living jaguars (Panthera onca) in Pantanal, Brazil. Vector Borne Zoonotic Dis. 11, 1001-1005.
Young, E., Zumpt, F., Whyte, I. J., 1972a. Notoedres cati infestation of the cheetah: preliminary report. J. South Afr. Vet. Assoc. 43, 205.
Young, E., Zumpt, F., Whyte, I. J., 1972b. Sarcoptic mange in free-living lions. J. South Afr. Vet. Med. Assoc. 43, 226.
Zumpt, F., Ledger, J. A., 1973. Present epidemiological problems of a sarcoptic mange in wild and domestic animals. J. Southern Afr. Wildl. Manag. Assoc. 3, 119-120.
Ollulanus tricuspis is a gastric trichostrongylid that occurs worldwide and is recorded in non-domestic felids including captive cheetahs, lions and tigers (Breuer et al., 1993, Chauvier and Chabaud, 1964, Collett et al., 2000, Hasslinger et al., 1982, Lensink et al., 1979) as well as in free-ranging wild cats, pumas and tigers (Brglez and Zeleznik, 1976, Mandal and Choudhury, 1985). Infected free-ranging pumas have been seropositive for FeLV, which may have predisposed to infection (Jessup et al., 1993, Rickard and Foreyt, 1992). Clinical signs include vomiting, inappetance, intermittent bloody or mucoid diarrhea and ill-thrift. Grossly, cats have an uneven gastric mucosal surface, small raised pale pink plaques, hemorrhage and or areas of ulceration. Histologically, abundant globule leukocytes and multifocal lymphoplasmacytic gastritis with fibrosis and glandular atrophy as well as moderately hyperplastic basilar mucosal-associated and submucosal lymphoid tissue are reported. Mucus cell hyperplasia is noted in some cases. Nematode sections may be rare but occur in the surface mucus and gastric pits (Fig. 10.19 ). Since the life cycle is completed in the stomach, infections may be overlooked due to the small size of the nematode (c. 1 mm long), virtual absence of adults, larvae or eggs in feces, and often small numbers of parasites in the mucosa. In cheetahs, histological differentiation from Helicobacter-associated gastritis is necessary.
Figure 10.19.
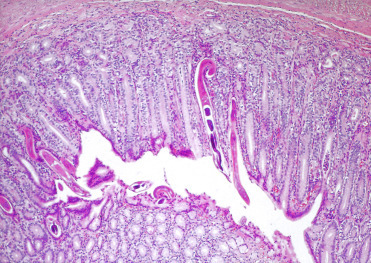
Gastritis associated with Ollulanus tricuspis in a tiger.
Lymphoplasmacytic gastritis is associated with embedded trichostrongyle nematodes.
(Reprinted with permission from Breuer et al, Berliner und Münchener Tierärztliche Wochenschrift 106(2): 47-49, 1993)
Toxoplasma gondii infects both captive and wild felids but is only rarely associated with disease. In contrast to most felids, captive Pallas’ cats are uniquely susceptible to infection with Toxoplasma, resulting in high neonatal mortality (Kenny et al., 2002). Common lesions in neonates include necrotizing encephalitis, pneumonia and/or hepatitis (Fig. 10.20 ). Necrotizing to granulomatous inflammation may also occur in other tissues such as the spleen, kidney and adipose. Disease also occurs in aged Pallas’ cats and manifests primarily as encephalomyelitis. In these aged cats, disease may be related to declining immune function and recrudescence of latent infection. A survey of wild Pallas’ cats in Mongolia found that <13% are seropositive and organisms could not be identified in the feces or in tissues (Brown et al., 2005). This suggests the possibility that Pallas’ cat may have evolved without exposure to this parasite and thereby did not develop a commensal relationship. Others have suggested that immune deficiencies may contribute to the susceptibility (Ketz-Riley et al., 2003).
Figure 10.20.
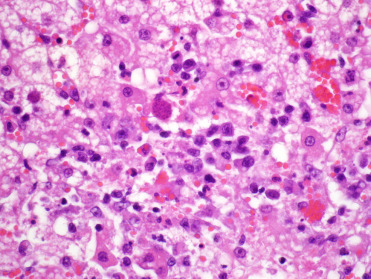
Toxoplasma gondii hepatitis in a Pallas’ cat. Multiple areas of hepatic necrosis and neutrophilic inflammation are associated with protozoal zoites.
Prions
Several captive cheetah in Europe developed spongiform encephalopathy with vacuolation in the neuropil and neurons of the midbrain, thalamus and hypothalamus. The obex, brainstem, and cerebellum were affected to a lesser degree. Lesions were associated with deposition of prion protein (PrPSc) within glial cells and neurons. Infection occurred presumably from eating contaminated meat during bovine spongiform encephalopathy (BSE) outbreaks (Peet and Curran 1992; Kirkwood et al., 1995, Baron et al., 1997, Lezmi et al., 2003, Eiden et al., 2010). Prion protein (PNRP) sequences are similar among cheetahs and other felids that lack evidence of disease, such as mountain lions who prey upon deer and elk affected by chronic wasting disease (CWD), suggesting that spongiform encephalopathy in cheetah is not due to a genetic susceptibility (Stewart et al., 2012). However, the role of dosage as well as differences between BSE and CWD are unknown in disease pathogenesis.
E-Slides
-
10.e1
Gastritis, Cheetah, Stomach. The gastric lamina propria contains large numbers of lymphocytes and plasma cells that separate glands. Few lymphocytes are present within gland epithelium and there is multifocal necrosis of gland epithelium. Glands contain approximately 10 micron long spiral bacteria morphologically consistent with Helicobacter sp. (See Fig. 10.18). eSlide: VM04945
-
10.e2
AA amyloidosis, Black-footed cat, Kidney and Spleen. Amyloid deposition in black-footed cats can be segmental to global within glomeruli and within the renal interstitium. Secondary interstitial fibrosis and inflammation are common as is protein within tubules. In the spleen, amyloid is present within lymphoid follicles and within the wall of arterioles. Amyloid in black-footed cats is AA type (See Fig. 10.7). eSlide: VM04947
-
10.e3
Veno-occlusive disease, cheetah, Liver, Masson’s Trichrome. Veno-occlusive disease which occurs in cheetahs and snow leopards is characterized by progressive accumulation of collagen within hepatic perisinusoidal spaces and subintimal spaces of central and sub-lobular veins. In this slide, some central veins are completed occluded. In other areas, extensive fibrosis disrupts hepatic cords. (See Fig. 10.6). eSlide: VM04948
-
10.e4
Transitional cell carcinoma, fishing cat, urinary bladder. Arising from the urothelium is an exophytic and invasive neoplasm. Neoplastic cells are arranged in arborizing papillary projections, variably sized nests, islands and few glands supported by a fibrovascular stroma. Neoplastic cells are crowded and disorganized, polygonal with moderate anisocytosis and anisokaryosis. Nucleoli are prominent and mitoses are common. Neoplastic cells are also present within lymphatics. eSlide: VM05038
-
10.e5
Feline herpesviral dermatitis, cheetah, skin. The epidermis is thickened and regionally ulcerated. Within remaining epidermis and in adnexal structures, some epithelial cells contain characteristic intranuclear inclusions. Inflammatory cell infiltrate contains large numbers of eosinophils and plasma cells. Virus isolation and sequencing has confirmed the virus as Feline herpesvirus 1. (See Fig. 10.11). Also on this slide are sections of uterus with few cystic glands. eSlide: VM05041
Common neoplasms in felids
Single, multiple, and multilocular biliary cysts are common postmortem findings in African and Asian lions as well as African leopard, clouded leopard, snow leopard, jaguar, tiger, mountain lion, and cheetah (Bernard et al., 2015, Gerhauser et al., 2009, Lucena et al., 2011 Pettan-Brewer et al., 1999; Yu et al., 2007). Cysts vary in size, some being quite large, and contain clear to dark green (bile) or bloody fluid. Histologically, cysts are lined by flattened to low cuboidal epithelium supported by a few layers of fibrous connective tissue. Although in a few individual cases, cysts have also been noted in the kidney, mutations in PKD1 (polycystic kidney disease 1) have not been identified and the vast majority of lions with hepatic cysts do not have concurrent renal cysts (Bernard et al., 2015; Gerhauser et al., 2009). Biliary adenomas and adenocarcinomas (Fig. e1 ) are less common but do occur in lions and leopards (Bernard et al., 2015, Lepri et al., 2013, Sakai et al., 2003). In most cases, these tumors are cystic rather than solid.
Figure e1.
Biliary carcinoma in an African lion.
Numerous variably sized, solid to cystic structures, some of which are filled with dark green fluid (bile) efface the normal hepatic parenchyma.
Lymphoma, while it can occur in any nondomestic felid, is most common in older (>14 years) African lions. In one study (Harrison et al., 2010), the majority of tumors were T cell (CD3+ CD79a − ) with primary splenic involvement. Liver and lymph nodes were also commonly affected. Tumors in these lions were not associated with FeLV or FIV infection, although there is a case report of an FIV associated lymphoma in a lion (Poli et al., 1995). In contrast, a single Namibian cheetah has been diagnosed with FeLV associated multicentric T cell lymphoma (Marker et al., 2003).
Multiple myeloma has been diagnosed in single captive African lion, jaguar, and snow leopard (Port et al., 1981; Kappeli et al., 2009; Tordiffe et al., 2013). Lesions in these species resemble the disease in domestic animals including intraosseous neoplasms with osteolysis and bony destruction, monoclonal gammopathy, excretion of light chains in the urine, and neoplasms composed of sheets of round cells with clear cytoplasmic margins and immunoreactivity to CD79a and other B cell markers. Spread to extramedullary sites appears to be common in nondomestic felids.
Leiomyomas and leiomyosarcomas are benign and malignant smooth muscle tumors, respectively. They can originate in smooth muscle anywhere in the body. In zoo felids, they appear to be relatively common in the female genital tract (Fig. e2 ) (Chassy et al., 2002). Leiomyomas have been diagnosed more common in large cats than small cats, which may reflect true incidence or collection bias. Tumors occur more commonly in the uterus than in the ovary or broad ligament; leiomyomas can also occur in the cervix. Tumors may be single or multiple and appear grossly as expansile or pedunculated, fleshy to firm, light pink/tan to tan/white masses. In the uterus, they appear to originate more commonly from the intramyometrial smooth muscle than from subserosal or subendometrial smooth muscle. Tumors are often well-demarcated but can blend with adjacent normal tissue or have edges that vary from being well to poorly defined but they are not invasive. Leiomyomas are composed of dense, interwoven bundles and fascicles of well-differentiated, elongate cells with moderate amounts of fibrillar, eosinophilic cytoplasm, and oval to elongate, normochromatic, vesicular nuclei with a single nucleolus. Mitoses are typically absent or rare (<1 per 10 high power field). Tumoral necrosis may be present but inflammation is uncommon and metastasis has not been described. Leiomyosarcomas are less common in zoo felids than leiomyomas. They are poorly demarcated, invasive, firm, multilobulated masses that often replace normal architecture. Cells have mild to moderate cellular pleomorphism with anisocytosis, anisokaryosis, occasional multinucleation, and one to a few nucleoli. Mitosis are much higher than in leiomyomas and are often common (several to over 20 per 10 high power fields). Tumor necrosis and inflammation are more common in leiomyosarcomas than in leiomyomas, and foci of tumoral mineralization, ossification, and local or distant metastasis may be present. Immunohistochemical labeling is useful in differentiating leiomyomas and leiomyosarcomas from other mesenchymal tumors, and will be positive for vimentin, smooth muscle actin, and/or desmin though labeling with the smooth muscle specific markers may be less common in leiomyosarcomas than leiomyomas. Possible complications of either tumor include obstruction along the reproductive tract and in leiomyosarcomas, adhesions between the tumor and adjacent structures due to invasive growth (Chassy et al., 2002, Siegal-Willott et al., 2005). Progestin contraception, in particular long-term treatment with melengestrol acetate (MGA) has been associated with mammary gland carcinogenesis in zoo felids (Harrenstien et al., 1996, McAloose et al., 2007). In a review of the reproductive histories of 52 zoo felids with uterine leiomyomas, there was no association between progestin contraception and tumor presence; in five zoo felids with leiomyosarcoma, four had been treated with MGA; however, an association between the two was not significant and further investigation was deemed necessary (Chassy et al., 2002).
Figure e2.
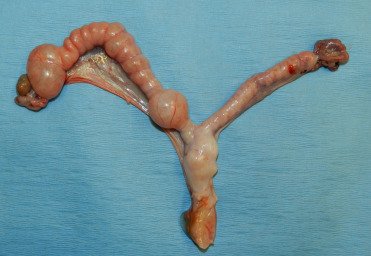
Leiomyoma and hydrometra in a serval.
This multinodular mass expands the wall of the uterine body. Obstruction to the outflow of uterine secretions has resulted in fluid retention and dilation of one uterine horn.
Captive fishing cats have a high incidence of transitional cell carcinomas (TCC) (Sutherland-Smith et al., 2004). Mean age in affected cats is 10.8 years and no sex predilection has been noted. Both captive bred and wild-caught captive held cats have developed these tumors. However, it is unknown if this disease occurs in wild fishing cats. Tumors commonly develop in the trigone region of the bladder. Histologically, lesions are primarily mural, although exophytic papillary proliferation does occur in about half of the cases (Landolfi and Terio, 2006). Carcinoma in situ occurs in, presumably, early lesions. Invasive portions of the neoplasms express cyclo-oxygenase 2 (COX-2) and therefore treatment with COX-2 inhibitors may slow disease progression (Landolfi and Terio, 2006). A dietary component to the pathogenesis has been suspected because in captivity cats had commonly been fed commercial carnivore diets consisting primarily of beef with little fish. Nutritional studies did find significantly increased levels of saturated fatty acids in cats with TCC compared to cats without tumors (Marshal et al., 2012). As some of these fatty acids are correlated with TCC in humans, recommendations have been made to decrease beef and increase fish content of captive diets.
Among nondomestic felids, only jaguars commonly develop ovarian papillary cystadenocarcinomas (Bossart and Hubbell, 1986, Munson, 1994). This tumor is a significant cause of mortality in captive female jaguars affecting breeding age as well as geriatric cats but its prevalence in wild jaguars is unknown (Hope and Deem, 2006). Diagnosis is common at necropsy. Affected jaguars will have multiple, variably sized cystic masses many of which are bilateral with spread along the uterine horns and abdominal carcinomatosis. Histologically, tumors are composed of papillary projections lined by neoplastic polygonal cells overlying a thin connective tissue stroma. Cysts formed by neoplastic cells contain proteinic fluid and or hemorrhage. An important differential is peritoneal mesothelioma and immunohistochemical studies are underway to provide markers to characterize ovarian tumors in jaguars (S. Corner, personal communication). In jaguars, the occurrence of this tumor has not been correlated with progestin (melengestrol acetate, MGA) contraception (Kazensky et al., 1998). Interestingly, jaguars also have a high prevalence of mammary cancer (Bryan et al., 2015, Munson, 1994), suggesting that abnormalities in genes, such as BRCA may be important in the pathogenesis.
Pheochromocytomas, an uncommon neoplasm in domestic felids, are common in clouded leopards (Corner et al., 2016). Many neoplasms are solitary expansile masses; however, a few have invaded the vena cava causing fatal hemoabdomen. By electron microscopy, neoplastic cells contain both epinephrine and norepinephrine granules. Retinal detachment, in some cases, suggests that tumors may be functional.
Splenic myelolipomas are a common benign neoplasm of cheetahs occurring in both captive and wild cats (Munson, 1993). Masses are commonly multiple throughout the parenchyma and can be greater than 2 cm in diameter. Masses are composed of well-differentiated adipose with variable amounts of admixed hematopoietic tissue.
Mammary gland (MG) carcinoma has been diagnosed in many species of large and small zoo felids. More common reports in large cats may reflect collection bias; however, the relatively high incidence of these cancers in jaguars may represent a true genetic predisposition (see previous). Tumors typically develop in older animals (Harrenstien et al., 1996). In general, and as occurs in domestic cats, MG cancers in zoo felids are typically aggressive, have multiple patterns, and many have local, distant, or occasionally widespread metastasis at the time of diagnosis (McAloose et al., 2007).
MG carcinomas typically present as variably sized, single or multiple, demarcated to invasive tumors in one or more of the mammary glands. On cut section, tumors are typically composed of firm, dense, light tan to light pink tissue. Ulceration of overlying skin may be seen (Fig. e3 A). Histologically, the majority contain multiple morphologic patterns (Harrenstien et al., 1996, McAloose et al., 2007). Most cancers have some areas of tubulopapillary growth with formation of tubular, solid, cribriform, comedone, and less commonly mucinous patterns (Fig. e3B). Regardless of morphologic pattern, tumors are typically multinodular to multilobular with scant to small amounts of fibrous or fibrovascular stroma although moderate intertubular stroma may be seen in tubular patterns. Focal to multifocal inflammation, typically lymphoplasmacytic, neutrophilic, or mixed, can be seen though is not a consistent finding; necrosis may also be present but is not as common as intratubular necrotic cellular debris.
Figure e3.
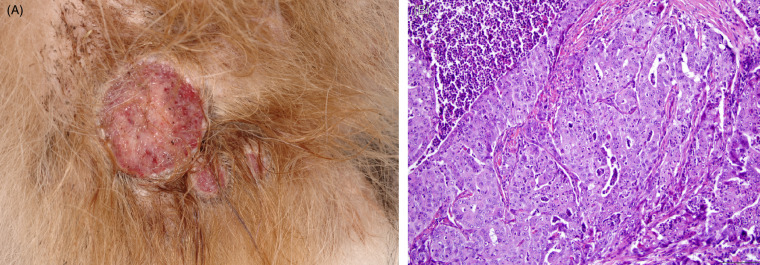
Mammary carcinoma in a melengestrol acetate (MGA) contracepted tiger.
S3a. Multiple raised pink to tan mammary masses with ulceration of the overlying skin. S3b. Multiple histologic patterns are common in mammary gland carcinomas. In this case, there are areas of comedone, solid and tubular growth separated by small amounts of fibrous stroma. Intratubular inflammation and necrotic debris is also present.
(Photos courtesy of the Wildlife Conservation Society)
Tumors vary from well to poorly differentiated based on anisocytosis, anisokaryosis and chromatin pattern, with poorly differentiated tumors having more marked degrees of anisocytosis and anisokaryosis, a vesicular rather than uniform chromatin pattern, and multiple rather than single nucleoli. In general, most MG carcinomas in zoo felids are moderately to poorly differentiated and of high grade based on the percentage of tubule formation, mitotic count and nuclear morphology [using criteria of Elston and Ellis (Elston and Ellis, 1991)]. Metastasis is common and most often to local and distant lymph nodes, lung, and liver. As occurs in domestic cats, MG carcinomas in zoo felids are more commonly positive for progesterone receptors than for estrogen receptors, though testing has only been performed on a handful of animals (McAloose et al., 2007).
While MG carcinomas occur spontaneously, a significant increase in incidence is seen in zoo felids on continuous, long-term (> 3 years) melengestrol acetate (MGA) contraception (Harrenstien et al., 1996). Use of this potent synthetic progestin in implant form (subcutaneous or intramuscular) began in the 1970s and continued until concerns about its association with mammary and uterine cancers and cystic endometrial hyperplasia arose in the 1990s (Harrenstien et al., 1996, Munson et al., 1995, Munson et al., 2002). Though not completely worked out, concurrent exposure to estrogen and this potent progestin is suspected as a predisposing factor in MGA-associated MG carcinogenesis. Felids are seasonally polyestrous and typically ovulate only if bred (induced ovulators). When not bred, they are exposed to recurrent peaks of estrogen as waves of follicles develop and then undergo atresia (Munson and Moresco, 2007). Because MGA does not suppress ovulation (Kazensky et al., 1998), treated cats are likely exposed to concurrent intermittent high levels of estrogen (during folliculogenesis) and this potent progestin. In women, dogs, and cats, cumulative endogenous and exogenous steroid hormone exposure are risk factors in MG carcinogenesis (Munson and Moresco, 2007). This combination is also suspected as a predisposing factor in carcinogenesis in zoo felids as estrogen and progesterone promote MG interlobular duct and lobuloalveolar growth, respectively (McAloose et al., 2007). Interestingly, there are no significant differences in spontaneous and MGA-associated MG cancers including the age of development, morphology, grade, or occurrence of metastasis. In either group, MG cancer may be associated with hypercalcemia or anemia.
Spontaneous or MGA-associatedendometrial hyperplasia (EH) and neoplasia are also described in a number of species of zoo felids; both appear to be significantly more common in MGA treated animals (Munson et al., 1995, Munson et al., 2002). Endometrial hyperplasia is described within the main text for this chapter. Endometrial carcinoma in zoo felids is similar to that in other species. On gross examination, tumors present as a firm, unencapsulated, light tan to white, demarcated to poorly demarcated thickened areas within the uterine wall. Histologically, there are proliferative neoplastic endometrial cells that form papillary intraluminal growths (Munson et al., 1995). Invasion of clusters of neoplastic endometrial cells into the adjacent stroma is seen.
References
- Bernard J.M., Newkirk K.M., McRee A.E., Whittemore J.C., Ramsay E.C. Hepatic lesions in 90 captive nondomestic felids presented for autopsy. Vet. Pathol. 2015;52:369–376. doi: 10.1177/0300985814532822. [DOI] [PubMed] [Google Scholar]
- Bossart G.D., Hubbell G. Ovarian papillary cystadenocarcinoma in a jaguar. J. Zoo Wildl. Med. 1986;14:73–76. [Google Scholar]
- Bryan L.K., Edwards J.F., Hoppes S.M. Pathology in practice. J. Am. Vet. Med. Assoc. 2015;247:1117–1119. doi: 10.2460/javma.247.10.1117. [DOI] [PubMed] [Google Scholar]
- Chassy L.M., Gardner I., Plotka E.D., Munson L. Genital tract smooth muscle tumors are common in zoo felids but are not associated with melengestrol acetate contraceptive treatment. Vet. Pathol. 2002;39:379–385. doi: 10.1354/vp.39-3-379. [DOI] [PubMed] [Google Scholar]
- Corner S., Walsh T., Padilla L., MacNeill A., Wallig M., Kiupel M., Terio K. Histologic and immunohistochemical characterization of pheochromocytomas in 20 clouded leopards (Neofelisnebulosa) Vet. Pathol. 2017;54:269–276. doi: 10.1177/0300985816664791. [DOI] [PubMed] [Google Scholar]
- Elston C., Ellis I. Pathological prognostic factors in breast cancer I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- Gerhauser I., Philipp U., Distl O., Beineke A. Multiple cyst formation in the liver and kidneys of a lion (Panthera leo): a case of polycystic kidney disease? Eur J. Wildl. Res. 2009;55:433–437. [Google Scholar]
- Harrenstien L.A., Munson L., Seal U.S. The American Zoo and Aquarium Association Mammary Cancer Study Group Mammary cancer in captive wild felids and risk factors for its development: a retrospective study of the clinical behavior of 31 cases. J. Zoo Wildl. Med. 1996;27:468–476. [Google Scholar]
- Harrison T.M., McKnight C.A., Sikarskie J.G., Kitchell B.E., Garner M.M., Raymond J.T., Fitzgerald S.D., Valli V.E., Agnew D., Kiupel M. Malignant lymphoma in African lions (Panthera leo) Vet. Pathol. 2010;47:952–957. doi: 10.1177/0300985810375054. [DOI] [PubMed] [Google Scholar]
- Hope K., Deem S.L. Retrospective study of morbidity and mortality of captive jaguars (Pantheraonca) in NorthAmerica. 1982-2002. Zoo Biol. 2006;25:501–512. [Google Scholar]
- Kappeli U., Eulenberger U., Nitzl D., Bley C.R., Steffen F., Sydler T. Clinical challenge: multiple myeloma. J. Zoo Wildl. Med. 2009;40:398–401. doi: 10.1638/2008-0120.1. [DOI] [PubMed] [Google Scholar]
- Kazensky C., Munson L., Seal U. The effects of melengestrol acetate on the ovaries of captive wild felids. J. Zoo Wildl. Med. 1998;29:1–5. [PubMed] [Google Scholar]
- Landolfi J., Terio K.A. Transitional cell carcinoma in fishing cats (Prionailurus viverrinus): pathology and expression of cyclooxygenase-1, -2, and p53. Vet. Pathol. 2006;43:674–681. doi: 10.1354/vp.43-5-674. [DOI] [PubMed] [Google Scholar]
- Lepri E., Sforna M., Brachelente C., Vitellozzi G. Cholangiocarcinoma of intrahepatic bile ducts with disseminated metastases in an African lion (Panthera leo) J. Zoo Wildl. Med. 2013;44:509–512. doi: 10.1638/2012-0221R1.1. [DOI] [PubMed] [Google Scholar]
- Lucena R.B., Fighera R.A., Barros C.S.L. Peribiliary cysts in an African lion. Pesquisa Veterinária Brasileira. 2011;31:165–168. [Google Scholar]
- Marker L., Munson L., Basson P.A., Quackenbush S. Multicentric T-cell lymphoma associated with feline leukemia virus infection in a captive Namibian cheetah (Acinonyx jubatus) J. Wildl. Dis. 2003;39:690–695. doi: 10.7589/0090-3558-39.3.690. [DOI] [PubMed] [Google Scholar]
- Marshall E., Swanson W., Kelley R., Kennedy J., Terio K., Garabed R., Buffington T. Investigation of epidemiologic and nutritional factors associated with a global epizootic of transitional cell carcinoma in fishing cats (Prionailurus viverrinus) Proceedings of the American Association of Zoo Veterinarians. 2012:244–245. [Google Scholar]
- McAloose D., Munson L., Naydan D. Histologic features of mammary carcinomas in zoo felids treated with melengestrol acetate (MGA) contraceptives. Vet. Pathol. 2007;44:320–326. doi: 10.1354/vp.44-3-320. [DOI] [PubMed] [Google Scholar]
- Munson L. Diseases of captive cheetahs (Acinonyx jubatus): Results of the cheetah research council pathology survey. 1989-1992. Zoo Biol. 1993;12:105–124. [Google Scholar]
- Munson L. A high prevalence of ovarian papillary cystadenocarcinomas in jaguars. Vet. Pathol. 1994;31:604. [Google Scholar]
- Munson L., Gardner I., Mason R.J., Chassy L., Seal U. Endometrial hyperplasia and mineralization in zoo felids treated with melengestrol acetate contraceptives. Vet. Pathol. 2002;39:419–427. doi: 10.1354/vp.39-4-419. [DOI] [PubMed] [Google Scholar]
- Munson L., Moresco A. Comparative pathology of mammary gland cancers in domestic and wild animals. Breast Dis. 2007;28:7–21. doi: 10.3233/bd-2007-28102. [DOI] [PubMed] [Google Scholar]
- Munson L., Stokes E., Harrenstien L. Uterine cancer in zoo felids on progestin contraceptives. Vet. Pathol. 1995;32:578. [Google Scholar]
- Pettan-Brewer K.C., Lowenstine L.J. Intrahepatic cysts and hepatic neoplasms in felids, ursids, and other zoo and wild animals. In: Fowler M.E., Miller R.E., editors. Zoo and Wild Animal Medicine Current Therapy 4. Elsevier; Philadelphia: 1999. pp. 423–428. [Google Scholar]
- Poli A., Abramo F., Cavicchio P., Bandecchi P., Ghelardi E., Pistello M. Lentivirus infection in an African lion: a clinical, pathological and virological study. J. Wildl. Dis. 1995;1:70–74. doi: 10.7589/0090-3558-31.1.70. [DOI] [PubMed] [Google Scholar]
- Port C.D., Maschgan E.R., Pond J., Scarpelli D.G. Multiple neoplasia in a jaguar (Pantheraonca) J. Comp. Pathol. 1981;91:115–122. doi: 10.1016/0021-9975(81)90051-7. [DOI] [PubMed] [Google Scholar]
- Sakai H., Yanai T., Yonemaru K., Hirata A., Masegi T. Gallbladder adenocarcinomas in two captive African lions (Pantheraleo) J. Zoo Wildl. Med. 2003;34:302–306. doi: 10.1638/1042-7260(2003)034[0302:GAITCA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Siegal-Willott J., Henrikson T., Carpenter J., Andrews G. Chronic obstipation in a leopard (Panthera pardus) caused by intrapelvic uterine leiomyoma compression of the distal colon. J. Zoo Wildl. Med. 2005;36:534–537. doi: 10.1638/03-123.1. [DOI] [PubMed] [Google Scholar]
- Sutherland-Smith M., Harvey C., Campbell M., McAloose D., Rideout B., Morris P. Transitional cell carcinoma in fishing cats (Prionailurisviverrinus) J. Zoo Wildl. Med. 2004;35:370–380. doi: 10.1638/03-106. [DOI] [PubMed] [Google Scholar]
- Tordiffe A.S.W., Cassel N., Lane E.P., Reyers F. Multiple myeloma in a captive lion (Pantheraleo) J. South Afr. Vet. Assoc. 2013;84:949. [Google Scholar]
- Yu C.H., Kim K., Hwang T.D., Yhee N., Moon J.Y., Hur C.T., Sur T.Y.J.H. Peribiliary cysts associated with severe liver disease: a previously unrecognized tumor in a lion (Pantheraleo) J. Vet. Diagn. Invest. 2007;19:709–712. doi: 10.1177/104063870701900617. [DOI] [PubMed] [Google Scholar]
References
- Alexander K.A., MacLachlan N.J., Kat P.W., House C., O’Brien S.J., Lerche N.W., Sawyer M., Frank L.G., Holekamp K., Smale L., McNutt J.W., Laurenson M.K., Mills M.G.L., Osburn B.I. Evidence of natural bluetongue virus among African carnivores. The American Journal of Tropical Medicine and Hygiene. 1994;51:568–576. [PubMed] [Google Scholar]
- Appel M.J.G., Yates R.A., Foley A.L., Bernstein J.J., Santinelli S., Spielman L.D., Miller L.D., Arp L.H., Anderson M., Barr M., Pearce-Kelling S., Summers B.A. Canine distemper virus epizootic in lions, tigers, and leopards in North America. Journal of Veterinary Diagnostic Investigation. 1994;6:277–288. doi: 10.1177/104063879400600301. [DOI] [PubMed] [Google Scholar]
- Aranaz A., De Juan L., Montero N., Sanchez C., Galka M., Delso C., Alvarez J., Romero B., Bezos J., Vela A.I., Briones V., Mateos A., Dominguez L. Bovine tuberculosis (Mycobacterium bovis) in wildlife in Spain. Journal of Clinical Microbiology. 2004;42 doi: 10.1128/JCM.42.6.2602-2608.2004. 2602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett K., Lewis J. Multiple ocular colobomas in the snow leopard (Uncia uncia) Veterinary Ophthalmology. 2002;5:197–199. doi: 10.1046/j.1463-5224.2002.00219.x. [DOI] [PubMed] [Google Scholar]
- Baron T., Belli P., Madec J.Y., Moutou F., Vitaud C., Savey M. Spongiform encephalopathy in an imported cheetah in France. Veterinary Record. 1997;141:270–271. doi: 10.1136/vr.141.11.270. [DOI] [PubMed] [Google Scholar]
- Barr M.C., Zou L., Hoose W.A., Avery R.J. Proviral organization and sequence analysis of feline immunodeficiency virus isolated from a Pallas’ cat. Virology. 1997;8:84–91. doi: 10.1006/viro.1996.8358. [DOI] [PubMed] [Google Scholar]
- Barron M.G., Duvall S.E., Barron K.J. Retrospective and current risks of mercury to panthers in the Florida Everglades. Ecotoxicology. 2004;13:223–229. doi: 10.1023/b:ectx.0000023567.42698.38. [DOI] [PubMed] [Google Scholar]
- Bartsch R.C., Imes G.D., Smit J.P. Vitamin A deficiency in captive African lion cub Panthera leo (Linneaus, 1758). Onderstepoort. Journal of Veterinary Research. 1975;42:43–54. [PubMed] [Google Scholar]
- Bengis R.G., Kriek N.P.J., Keet D.F., Raath J.P., deVos V., Huchzermeyer H.F.A.K. An outbreak of bovine tuberculosis in a free-living African buffalo (Syncerus caffer-Sparrman) population in the Kruger National Park: A preliminary report. Onderstepoort Journal of Veterinary Research. 1996;63:15–18. [PubMed] [Google Scholar]
- Bergström J., Ueda M., Une Y., Sun X., Misumi S., Shoji S., Ando Y. Analysis of amyloid fibrils in the cheetah (Acinonyx jubatus) Amyloid. 2006;13:93–98. doi: 10.1080/13506120600722621. [DOI] [PubMed] [Google Scholar]
- Bernard J.M., Newkirk K.M., McRee A.E., Whittemore J.C., Ramsay E.C. Hepatic lesions in 90 captive nondomestic felids presented for autopsy. Veterinary Pathology. 2015;52 doi: 10.1177/0300985814532822. 369-76. [DOI] [PubMed] [Google Scholar]
- Berry W.L., Jardine J.E., Espie I.W. Pulmonary cryptococcoma and cryptococcal meningoencephalitis in a king cheetah (Acinonyx jubatus) Journal of Zoo and Wildlife Medicine. 1997;28 485-90. [PubMed] [Google Scholar]
- Bolton L.A., Munson L. Glomerulosclerosis in captive cheetahs (Acinonyx jubatus) Veterinary Pathology. 1999;36:14–22. doi: 10.1354/vp.36-1-14. [DOI] [PubMed] [Google Scholar]
- Bolton L.A., Lobetti R.G., Evezard D.N., Picard J.A., Nesbit J.W., van Heerden J., Burroughs R.E. Cryptococcosis in captive cheetah (Acinonyx jubatus): two cases. Journal of the South African Veterinary Association. 1999;70:35–39. doi: 10.4102/jsava.v70i1.748. [DOI] [PubMed] [Google Scholar]
- Breuer W., Hasslinger M.A., Hermanns W. Chronic gastritis caused by Ollulanus tricuspis (Leuckart 1865) in a tiger. Berliner und Munchener Tierarztliche Wochenschrift. 1993;106:47–49. [PubMed] [Google Scholar]
- Brglez J., Zeleznik Z. The pathogenicity of ollulanosis in wild cat (Felis silvestris Schreber) Internationalen Symposiums uber die Erkrankungen der Zootiere, Innsbruck, Austria. 1976:233–234. [Google Scholar]
- Briones V., de Juan L., Sanchez C., Vela A.I., Galka M., Montero, Goyache J., Aranaz A., Dominguez L. Bovine tuberculosis and the endangered Iberian lynx. Emerging Infectious Diseases. 2000;6 doi: 10.3201/eid0602.000214. 189-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower A.I., Munson L., Radcliffe R.W., Citino S.B., Lackey L.B., Van Winkle T.J., Stalis I., Terio K.A., Summers B.A., de Lahunta A. Leukoencephalopathy of mature captive cheetahs and other large felids: a novel neurodegenerative disease that came and went? Veterinary Pathology. 2014;51:1013–1021. doi: 10.1177/0300985813506917. [DOI] [PubMed] [Google Scholar]
- Brown E.W., Yuhki N., Packer C., O’Brien S.J. A lion lentivirus related to feline immunodeficiency virus: epidemiologic and phylogenetic aspects. Journal of Virology. 1994;68:5953–5968. doi: 10.1128/jvi.68.9.5953-5968.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M., Lappin M.R., Brown J.L., Munkhtsog B., Swanson W.F. Exploring the ecologic basis for extreme susceptibility of Pallas’ cats (Otocolobus manul) to fatal toxoplasmosis. Journal of Wildlife Diseases. 2005;41:691–700. doi: 10.7589/0090-3558-41.4.691. [DOI] [PubMed] [Google Scholar]
- Brown M.A., Cunningham M.W., Roca A.L., Troyer J.L., Johnson W.E., O’Brien S.J. Genetic characterization of feline leukemia virus from Florida panthers. Emerging Infectious Diseases. 2008;14:252–259. doi: 10.3201/eid1402.070981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruning-Fann C.S., Schmitt S.M., Fitzgerald S.D., Fierke J.S., Friedrich P.D., Kaneene J.B., Clarke K.A., Butler K.L., Payeur J.B., Whipple D., Cooley T.M., Miller J.M., Muzo D.P. Bovine tuberculosis in free-ranging carnivores from Michigan. Journal of Wildlife Diseases. 2001;37:58–64. doi: 10.7589/0090-3558-37.1.58. [DOI] [PubMed] [Google Scholar]
- Buergelt C.D., Homer B.L., Spalding M.G. Causes of mortality in the Florida panther (Felis concolor coryi) Annals of the New York Academy of Sciences. 2002;969:350–353. doi: 10.1111/j.1749-6632.2002.tb04403.x. [DOI] [PubMed] [Google Scholar]
- Bull M.E., Kennedy-Stoskopf S., Levine J.F., Loomis M., Gebhard D.G., Tompkins W.A. Evaluation of T lymphocytes in captive African lions (Panthea leo) infected with feline immunodeficiency virus. American Journal of Veterinary Research. 2003;64:1293–1300. doi: 10.2460/ajvr.2003.64.1293. [DOI] [PubMed] [Google Scholar]
- Carpenter M.A., Brown E.W., Culver M., Johnson W.E., Pecon-Slattery J., Brousset D., O’Brien S.J. Genetic and phylogenetic divergence of feline immunodeficiency virus in the puma (Puma concolor) Journal of Virology. 1996;70:6682–6693. doi: 10.1128/jvi.70.10.6682-6693.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter M.A., Brown E.W., MacDonald D.W., O’Brien S.J. Phylogeographic patterns of feline immunodeficiency virus genetic diversity in the domestic cat. Virology. 1998;251:234–243. doi: 10.1006/viro.1998.9402. [DOI] [PubMed] [Google Scholar]
- Chauvier G., Chabaud A.G. Ollulanose du lion. Annales de Parasitologie Humaine et Comparee. 1964;39:791–793. [Google Scholar]
- Cho H.S., Kim Y.H., Park N.Y. Disseminated mycobacteriosis due to Mycobacterium avium in captive Bengal tiger (Panthera tigris) Journal of Veterinary Diagnostic Investigation. 2006;18:312–314. doi: 10.1177/104063870601800318. [DOI] [PubMed] [Google Scholar]
- Clarke C., van Helden P., Miller M., Parsons P. Animal-adapted members of the Mycobacterium tuberculosis complex endemic to the southern African subregion. Journal of the South African Veterinary Association. 2016;87 doi: 10.4102/jsava.v87i1.1322. a1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaveland S., Mlengeya T., Kazwala R.R., Michel A., Kaare M.T., Jones S.L., Eblate E., Shirima G.M., Packer C. Tuberculosis in Tanzanian wildlife. Journal of Wildlife Diseases. 2005;41:446–453. doi: 10.7589/0090-3558-41.2.446. [DOI] [PubMed] [Google Scholar]
- Clyde V.L., Ramsay E.C., Bemis D.A. Fecal shedding of Salmonella in exotic felids. Journal of Zoo Wildlife Medicine. 1997;28:148–152. [PubMed] [Google Scholar]
- Collett M.G., Pomroy W.E., Guilford W.G., Johnstone A.C., Blanchard B.J., Mirams S.G. Gastric Ollulanus tricuspis infection identified in captive cheetahs (Acinonyx jubatus) with chronic vomiting. Journal of the South African Veterinary Association. 2000;71:251–255. doi: 10.4102/jsava.v71i4.727. [DOI] [PubMed] [Google Scholar]
- Cousins D.V., Florisson N. A review of tests available for use in the diagnosis of tuberculosis in non-bovine species. Revue Scientifique et Technique. 2005;24:1039–1059. [PubMed] [Google Scholar]
- Craft M.E., Volz E., Packer C., Meyers L.A. Distinguishing epidemic waves from disease spillover in a wildlife population. In: Proceedings of the Royal Society B: Biological Sciences. 2009;276:1777–1785. doi: 10.1098/rspb.2008.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley B., Hietala S., Hunt T., Benjamin G., Martinez M., Darnell D., Rubrum A., Webby R. Pandemic (H1N1) 2009 in captive cheetah. Emerging Infectious Diseases. 2012;18:315–317. doi: 10.3201/eid1802.111245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M.W., Dunbar M.R., Buergelt C.D., Homer B.L., Roelke-Parker M.E., Taylor S.K., King R., Citino S.B., Glass C. Atrial septal defects in Florida panthers. Journal of Wildlife Diseases. 1999;35:519–530. doi: 10.7589/0090-3558-35.3.519. [DOI] [PubMed] [Google Scholar]
- Cunningham M.W., Brown M.A., Shindle D.B., Terrell S.P., Hayes K.A., Ferree B.C., McBride R.T., Blankenship E.L., Jansen D., Citino S.B., Roelke M.E., Kiltie R.A., Troyer J.L., O’Brien S.J. Epizootiology and Management of feline leukemia virus in the Florida puma. Journal of Wildlife Diseases. 2008;44:537–552. doi: 10.7589/0090-3558-44.3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler T. Bilateral eyelid agenesis repair in a captive Texas cougar. Veterinary Ophthalmology. 2002;5:143–148. doi: 10.1046/j.1463-5224.2002.00237.x. [DOI] [PubMed] [Google Scholar]
- Daoust P.Y., McBurney S.R., Godson D.L., van de Bildt M.W., Osterhaus A.D. Canine-distemper virus-associated encephalitis in free-living lynx (Lynx Canadensis) and bobcats (Lynx rufus) of eastern Canada. Journal of Wildlife Diseases. 2009;45:611–624. doi: 10.7589/0090-3558-45.3.611. [DOI] [PubMed] [Google Scholar]
- de Lisle G.W., Bengis R.G., Schmitt S.M., O’Brien D.J. Tuberculosis in free-ranging wildlife: detection, diagnosis and management. Revue Scientifique et Technique. 2002;21:317–334. doi: 10.20506/rst.21.2.1339. [DOI] [PubMed] [Google Scholar]
- De Risio L., Beltran E., de Stefani A., Holloway A., Matiasek K. Neurological dysfunction and caudal fossa overcrowding in a young cheetah with hypovitaminosis A. Veterinary Record. 2010;167:534–536. doi: 10.1136/vr.c4802. [DOI] [PubMed] [Google Scholar]
- Duarte M.D., Barros S.C., Henriques M., Fernandes T.L., Bernardino R., Monteiro M., Fevereiro M. Fatal infection with feline panleukopenia virus in two captive wild carnivores (Panthera tigris and Panthera leo) Journal of Zoo and Wildlife Medicine. 2009;40:354–359. doi: 10.1638/2008-0015.1. [DOI] [PubMed] [Google Scholar]
- Duarte A., Fernandes M., Santos N., Tavares L. Virological survey in free-ranging wildcats (Felis silvestris) and feral domestic cats in Portugal. Veterinary Microbiology. 2012;158:400–404. doi: 10.1016/j.vetmic.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvall S.E., Barron M.G. A screening level probabilistic risk assessment of mercury in Everglades food webs. Ecotoxicology and Environmental. 2000;47:298–305. doi: 10.1006/eesa.2000.1949. Safety. [DOI] [PubMed] [Google Scholar]
- Eaton K.A., Radin M.J., Kramer L.W., Wack R.F., Sherding R., Krakowka S., Fox J.G., Morgan D.R. Epizootic gastritis associated with gastric spiral bacilli in cheetahs (Acinonyx jubatus) Veterinary Pathology. 1993;30:55–63. doi: 10.1177/030098589303000107. [DOI] [PubMed] [Google Scholar]
- Eiden M., Hoffmann C., Balkema-Buschmann A., Müller M., Baumgartner K., Groschup M.H. Biochemical and immunohistochemical characterization of feline spongiform encephalopathy in a German captive cheetah. The Journal of General Virology. 2010;91:2874–2883. doi: 10.1099/vir.0.022103-0. [DOI] [PubMed] [Google Scholar]
- Esson D. A modification of the Mustarde technique for the surgical repair of a large feline eyelid coloboma. Veterinary Ophthalmology. 2001;4:159–160. doi: 10.1046/j.1463-5224.2001.00174.x. [DOI] [PubMed] [Google Scholar]
- Eulenberger K., Elze K., Schuppel K.-., Seifert S. Tuberkulose und ihre bekampfung bei primaten und feliden im Leipziger zoologische garten von 1951-1990. Erkrankungen der Zootiere. 1992:7–15. [Google Scholar]
- Evermann J.F., Heeney J.L., Roelke M.E., McKiernan A.J., O’Brien S.J. Biology and pathologic consequences of feline infectious peritonitis virus infection in the cheetah. Archives of Virology. 1988;120:155–171. doi: 10.1007/BF01310822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facemire C.F., Gross T.S., Guillette L.J., Jr. Reproductive impairment in the Florida panther: nature or nuture? Environmental Health Perspectives. 1995;4:79–86. doi: 10.1289/ehp.103-1519283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira S.M., Funston P.J. Estimating lion population variables: prey and disease effects in Kruger National Park, South Africa. Wildlife Research. 2010;37:194–206. [Google Scholar]
- Flacke G.L., Schmidt-Küntzel A., Marker L. Treatment of chronic herpesviral dermatitis in a captive cheetah (Acinonyx jubatus) in Namibia. Journal of Zoo and Wildlife Medicine. 2015;46:641–646. doi: 10.1638/2014-0206.1. [DOI] [PubMed] [Google Scholar]
- Franklin A.D., Schmidt-Küntzel A., Terio K.A., Marker L.L., Crosier A.E. Serum amyloid A protein concentration in blood is influenced by genetic differences in the cheetah (Acinonyx jubatus) Journal of Heredity. 2016;107:115–121. doi: 10.1093/jhered/esv089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geret C.P., Cattori V., Meli M.L., Riond B., Martínez F., López G., Vargas A., Simón M.A., López-Bao J.V., Hofmann-Lehmann R., Lutz H. Feline leukemia virus outbreak in the critically endangered Iberian lynx (Lynx pardinus): high-throughput sequencing of envelope variable region A and experimental transmission. Archives of Virology. 2011;156:839–854. doi: 10.1007/s00705-011-0925-z. [DOI] [PubMed] [Google Scholar]
- Gershoff S.N., Andrus S.B., Hegsted D.M., Lentini E.A. Vitamin A deficiency in cats. Laboratory Investigation. 1957;6:227–240. [PubMed] [Google Scholar]
- Good K.M., Houser A., Arntzen L., Turnbull P.C.B. Naturally acquired anthrax antibodies in a cheetah (Acinonyx jubatus) in Botswana. Journal of Wildlife Diseases. 2008;44:721–723. doi: 10.7589/0090-3558-44.3.721. [DOI] [PubMed] [Google Scholar]
- Gosselin S.J., Loudy D.L., Tarr M.J., Balistreri W.F., Setchell K.D.R., Johnston J.O., Kramer L.W., Dresser B.L. Veno-occlusive disease of the liver in captive cheetah. Veterinary Pathology. 1988;25:48–57. doi: 10.1177/030098588802500107. [DOI] [PubMed] [Google Scholar]
- Gripenberg U., Blomqvist L., Pamil P., Soderlund V., Tarkkanen A., Wahlberg C., Varvio-Aho S.-L., Virta-Ranta-Knowles K. Multiple ocular coloboma (MOC) in snow leopards. Hereditas. 1985;103:221–229. doi: 10.1111/j.1601-5223.1985.tb00505.x. [DOI] [PubMed] [Google Scholar]
- Ha S., Reid C., Meshkibaf S., Kim S.O. Inhibition of interleukin 1 (Il-1) expression by anthrax lethal toxin (letx) is reversed by histone deacetylase 8 (hdac8) inhibition in murine macrophages. Journal of Biological Chemistry. 2016;291:8745–8755. doi: 10.1074/jbc.M115.695809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison T.M., Sikarskie J., Kruger J., Wise A., Mullaney T.P., Kiupel M., Maes R.K. Systemic calicivirus epidemic in captive exotic felids. J Zoo Wildl Med. 2007;38(2) doi: 10.1638/1042-7260(2007)038[0292:SCEICE]2.0.CO;2. 292-9. [DOI] [PubMed] [Google Scholar]
- Harrison T.M., Harrison S.H., Sikarskie J.G., Armstrong D. Humoral response to calicivirus in captive tigers given a dual-strain vaccine. Journal of Zoo and Wildlife Medicine. 2014;45 doi: 10.1638/2012-0069R2.1. 23-8. [DOI] [PubMed] [Google Scholar]
- Hasslinger M.A., Wittmann F.X., Wiesner H., Rietschel W. On the incidence of Ollulanus tricuspis (Leuckart, 1865) in Felidae of a zoological garden. Veterinary Medical Review. 1982:220–228. [Google Scholar]
- He S., Shi J., Qi X., Huang G., Chen H., Lu C. Lethal infection by a novel reassortment H5N1 avian influenza A virus in a zoo-housed tiger. Microbes and Infection. 2015;17:54–61. doi: 10.1016/j.micinf.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Heeney J.L., Evermann J.F., McKerinan A.J., Marker-Kraus L., Roelke M.E., Bush M., Wildt D.E., Meltzer D.G., Colly L., Lukas J. Prevalence and implications of feline coronavirus infections of captive and free-ranging cheetahs (Acinonyx jubatus) Journal of Virology. 1990;64:1964–1972. doi: 10.1128/jvi.64.5.1964-1972.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helman R.G., Russell W.C., Jenny A., Miller J., Payeur J. Diagnosis of tuberculosis in two snow leopards using polymerase chain reaction. Journal of Veterinary Diagnostic Investigation. 1998;10:89–92. doi: 10.1177/104063879801000118. [DOI] [PubMed] [Google Scholar]
- Hinshaw V.S., Webster R.G., Easterday B.C., Bean W.J. Replication of avian influenza A viruses in mammals. Infection and Immunity. 1981;34 doi: 10.1128/iai.34.2.354-361.1981. 354-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover D.L., Friedlander A.M., Rogers L.C., Yoon I.K., Warren R.L., Cross A.S. Anthrax edema toxin differentially regulates lipopolysaccharide-induced monocyte production of tumor-necrosis-factor-alpha and interleukin-6 by increasing intracellular cyclic-amp. Infection and Immunity. 1994;62:4432–4439. doi: 10.1128/iai.62.10.4432-4439.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu T., Zhao H., Zhang Y., Zhang W., Kong Q., Zhang Z., Cui Q., Qiu W., Deng B., Fan Q., Zhang F. Fatal influenza A (H5N1) virus infection in zoo-housed tigers in Yunnan province. China. Scientific Reports. 2016;6:25845. doi: 10.1038/srep25845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugh-Jones M.E., Vos V. Anthrax and wildlife. Revue Scientifique et Technique - Office International des Epizooties. 2002;21:359–383. doi: 10.20506/rst.21.2.1336. de. [DOI] [PubMed] [Google Scholar]
- Illnait-Zaragozi M.T., Hagen F., Fernandez-Andreu C.M., Martinez-Machin G.F., Polo-Leal J.L., Boekhout T., Klaassen C.H., Meis J.F. Reactivation of a Cryptococcus gattii infection in a cheetah (Acinonyx jubatus) held in the National Zoo, Havana, Cuba. Mycoses. 2011;54:E889–E892. doi: 10.1111/j.1439-0507.2011.02046.x. [DOI] [PubMed] [Google Scholar]
- Jauniaux T.P., De Clercq K.E., Cassart D.E., Kennedy S., Vandenbussche F.E., Vandemeulebroucke E.L., Vanbinst T.M., Verheyden B.I., Goris N.E., Coignoul F.L. Bluetongue in Eurasian lynx. Emerging Infectious Diseases. 2008;14:1496–1498. doi: 10.3201/eid1409.080434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessup D.A., Pettan K.C., Lowenstine L.J., Pedersen N.C. Feline leukemia virus infection and renal spirochetosis in a free-ranging cougar (Felis concolor) Journal of Zoo and Wildlife Medicine. 1993;24:73–79. [Google Scholar]
- Jiménez M.A., Sánchez B., Pérez Alenza M.D., García P., López J.V., Rodriquez A., Muñoz A., Martínez F., Vargas A., Peña L. Membranous glomerulonephritis in the Iberian lynx (Lynx pardinus) Veterinary Immunology and Immunopathology. 2008;121:34–43. doi: 10.1016/j.vetimm.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K.H., Sletten K., Munson L., O’Brien T.D., Papendick R., Westermark P. Amino acid sequence analysis of amyloid protein A (AA) from cheetahs (Acinonyx jubatus) with a high prevalence of AA amyloidosis. Amyloid. 1997;4:171–177. [Google Scholar]
- Jones O.G. Tuberculosis in a cheetah: the use of antibiotics and modern chemotherapeutic agents. The Veterinary Record. 1953;65:453–454. [Google Scholar]
- Junge R.E., Miller E., Boever W.J., Scherba G., Sundberg J. Persistent cutaneous ulcers associated with feline herpesvirus type 1 infection in a cheetah. Journal of the American Veterinary Medical Association. 1991;198:1057–1058. [PubMed] [Google Scholar]
- Kadoi K., Kiryu M., Iwabuchi M., Kamata H., Yukawa M., Inaba Y. A strain of calcivirus isolated from lions with vesicular lesions on tongue and snout. New Microbiologica. 1997;20:141–148. [PubMed] [Google Scholar]
- Keawcharoen J., Oraveerakul K., Kuiken T., Fouchier R.A.M., Amonsin A., Payungporn S., Noppornpanth S., Wattanodoen S., Theamboonlers A., Tantilertcharoen R., Pattanarangsan R., Arya N., Ratanakorn P., Osterhaus A.D.M.E., Poovorawan Y. Avian influenza H5N1 in tigers and leopards. Emerging Infectious Diseases. 2004;10 doi: 10.3201/eid1012.040759. 2189-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keet D.F., Kriek N.P., Penrith M.L., Michel A., Huchzermeyer H. Tuberculosis in buffaloes (Syncerus caffer) in the Kruger National Park: spread of the disease to other species. Onderstepoort Journal of Veterinary Research. 1996;63:239–244. [PubMed] [Google Scholar]
- Kenny D.E., Lappin M.R., Knightly F., Baier J., Brewer M., Getzy D.M. Toxoplasmosis in Pallas’ cats (Otocolobus felis manul) Journal of Zoo and Wildlife Medicine. 2002;33 doi: 10.1638/1042-7260(2002)033[0131:TIPCOF]2.0.CO;2. 131-8. [DOI] [PubMed] [Google Scholar]
- Ketz-Riley C.J., Ritchey J.W., Hoover J.P., Johnson C.M., Barrie M.T. Immunodeficiency associated with multiple concurrent infections in captive Pallas’ cats (Otocolobus manul) Journal of Zoo and Wildlife Medicine. 2003;34 doi: 10.1638/01-112. 239-45. [DOI] [PubMed] [Google Scholar]
- Kinsel M.J., Briggs M.B., Venzke K., Forge O., Murnane R.D. Gastric spiral bacteria and intramuscular sarcocysts in African lions from Namibia. Journal of Wildlife Diseases. 1998;34:317–324. doi: 10.7589/0090-3558-34.2.317. [DOI] [PubMed] [Google Scholar]
- Kinsel M.J., Kovarik P., Murnane R.D. Gastric spiral bacteria in small felids. Journal of Zoo and Wildlife Medicine. 1998;29:214–220. [PubMed] [Google Scholar]
- Kirberger R.M., Keet D.F., Wagner W.M. Radiologic abnormalities of the appendicular skeleton of the lion (Panthera leo): Incidental findings and Mycobacterium bovis-induced changes. Veterinary Radiology and Ultrasound. 2006;47:145–152. doi: 10.1111/j.1740-8261.2006.00121.x. [DOI] [PubMed] [Google Scholar]
- Kirkwood J.K., Cunningham A.A., Flach E.J., Thornton S.M., Wells G.A.H. Spongiform encephalopathy in another captive cheetah (Acinonyx jubatus): Evidence for variation in susceptibility or incubation periods between species? Journal of Zoo and Wildlife Medicine. 1995;26:577–582. [Google Scholar]
- Kolmstetter C., Munson L., Ramsay E.C. Degenerative spinal disease in large felids. Journal of Zoo and Wildlife Medicine. 2000;31 doi: 10.1638/1042-7260(2000)031[0015:DSDILF]2.0.CO;2. 15-9. [DOI] [PubMed] [Google Scholar]
- Kuiken T., Rimmelzwaan G., van Riel D., van Amerongen G., Baars M., Fouchier R., Osterhaus A. Avian H5N1 influenza in cats. Science. 2004;306 doi: 10.1126/science.1102287. 241. [DOI] [PubMed] [Google Scholar]
- Lane E.P., Brettschneider H., Caldwell P., Oosthuizen A., Dalton D.L., du Plessis L., Steyl J., Kotze A. Feline panleukopaenia virus in captive non-domestic felids in South Africa. Ondersterpoort Journal of Veterinary Research 83. 2016:a1099. doi: 10.4102/ojvr.v83i1.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantos A., Niemann S., Mezosi L., Sos E., Erdelyi K., David S., Parsons L.M., Kubica T., Rusch-Gerdes S., Somoskovi A. Pulmonary tuberculosis due to Mycobacterium bovis subsp. caprae in captive Siberian tiger. Emerging Infectious Diseases. 2003;9:1462–1464. doi: 10.3201/eid0911.030297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.S., Bevins S.N., Serieys L.E., Vickers W., Logan K.A., Aldredge M., Boydston E.E., Lyren L.M., McBride R., Roelke-Parker M., Pecon-Slattery J., Troyer J.L., Riley S.P., Boyce W.M., Crooks K.R., VandeWoude S. Evolution of puma lentivirus in bobcats (Lynx rufus) and mountain lions (Puma concolor) in North America. Journal of Virology. 2014;88 doi: 10.1128/JVI.00473-14. 7727-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembo T., Hampson K., Auty H., Beesley C.A., Bessell P., Packer C., Halliday J., Fyumagwa R., Hoare R., Ernest E., Mentzel C., Mlengeya T., Stamey K., Wilkins P.P., Cleaveland S. Serologic Surveillance of Anthrax in the Serengeti Ecosystem, Tanzania, 1996-2009. Emerging Infectious Diseases. 2011;17:387–394. doi: 10.3201/eid1703.101290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lensink B.M., Rijpstra A.C., Erken A.H.M. Ollulanus infections in captive Bengal tigers. Zoologische Garten. 1979;49:121–126. [Google Scholar]
- Lester S.J., Malik R., Bartlett K.H., Duncan C.G. Cryptococcosis: update and emergence of Cryptococcus gattii. Veterinary Clinical Pathology. 2011;40:4–17. doi: 10.1111/j.1939-165X.2010.00281.x. [DOI] [PubMed] [Google Scholar]
- Lezmi S., Benscik A., Monks E., Petit T., Baron T. First case of feline spongiform encephalopathy in a captive cheetah born in France: PrP9sc) analysis in various tissues revealed unexpected targeting of kidney and adrenal gland. Histochemistry and Cell Biology. 2003;119:415–422. doi: 10.1007/s00418-003-0524-5. [DOI] [PubMed] [Google Scholar]
- Maas M., Keet D.F., Rutten V.P.M.G., Heesterbeek J.A.P., Nielen M. Assessing the impact of feline immunodeficiency virus and bovine tuberculosis co-infection in African lions. In: Proceedings of the Royal Society B-Biological Sciences. 2012;279:4206–4214. doi: 10.1098/rspb.2012.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas M., Michel A.L., Rutten V.P.M.G. Facts and dilemmas in diagnosis of tuberculosis in wildlife. Comparative Immunology Microbiology and Infectious Diseases. 2013;36:269–285. doi: 10.1016/j.cimid.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Mandal D., Choudhury A. Helminth parasites of wild tiger of Sundarbans Forest, West Bengal, India. Internationalen Symposiums uber die Erkrankungen der Zootiere, Torino, Italy. 1985:499–501. [Google Scholar]
- Mansfield K.G., Land E.D. Cryptorchidism in Florida panthers: prevalence, features, and influence of genetic restoration. Journal of Wildlife Diseases. 2002;38:693–698. doi: 10.7589/0090-3558-38.4.693. [DOI] [PubMed] [Google Scholar]
- Maratea K.A., Hooser S.B., Ramos-Vara J.A. Degenerative myelopathy and vitamin A deficiency in a young, black-maned lion (Panthera leo) Journal of Veterinary Diagnostic Investigation. 2006;18 doi: 10.1177/104063870601800617. 608-11. [DOI] [PubMed] [Google Scholar]
- Marker L., Munson L., Basson P.A., Quackenbush S. Multicentric T-cell lymphoma associated with feline leukemia virus infection in a captive Namibian cheetah (Acinonyx jubatus) Journal of Wildlife Diseases. 2003;39 doi: 10.7589/0090-3558-39.3.690. 690-5. [DOI] [PubMed] [Google Scholar]
- Martin C., Stiles J., Willis M. Feline colobomatous syndrome. Veterinary Comparative Ophthalmology. 1997;7:39–43. [Google Scholar]
- Martinez F., Manteca X., Pastor J. Retrospective study of morbidity and mortality of captive Iberian lynx (Lynx pardinus) in the ex situ conservation programme (2004-June 2010) Journal of Zoo and Wildlife Medicine. 2013;44:845–852. doi: 10.1638/2011-0165R4.1. [DOI] [PubMed] [Google Scholar]
- McCain S., Souza M., Ramsay E., Schumacher J., Hecht S., Thomas W. Diagnosis and surgical treatment of a Chiari I-like malformation in an African lion (Panthera leo) Journal of Zoo and Wildlife Medicine. 2008;39 doi: 10.1638/2007-0157.1. 421-7. [DOI] [PubMed] [Google Scholar]
- McLean K., Garabed R. Is amyloidosis in cheetahs (Acinonyx jubatus), transmissible? In: Proceedings Annual Meeting of the American Association of Zoo Veterinarians. 2015 [Google Scholar]
- Meli M.L., Cattori V., Martínez F., López G., Vargas A., Simón M.A., Zorrilla I., Muñoz A., Palomares F., López-Bao J.V., Pastor J., Tandon R., Willi B., Hofmann-Lehmann R., Lutz H. Feline leukemia virus and other pathogens as important threats to the survival of the critically endangered Iberian lynx (Lynx pardinus) PLoS One. 2009;4:e4744. doi: 10.1371/journal.pone.0004744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meli M.L., Simpler P., Cattori V., Martínez F., Vargas A., Palomares F., López-Bao J.V., Simón M.A., López G., León-Vizcaino L., Hofmann-Lehmann R., Lutz H. Importance of canine distemper virus (CDV) infection in free-ranging Iberian lynxes (Lynx pardinus) Veterinary Microbiology. 2010;146:132–137. doi: 10.1016/j.vetmic.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Michel A.L., Coetzee M.L., Keet D.F., Mare L., Warren R., Cooper D., Bengis R.G., Kremer K., van Helden P. Molecular epidemiology of Mycobacterium bovis isolates from free-ranging wildlife in South African game reserves. Veterinary Microbiology. 2009;133:335–343. doi: 10.1016/j.vetmic.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Miller M., Buss P., Hofmeyr J., Olea-Popelka F., Parsons S., van Helden P. Antemortem Diagnosis of Mycobacterium bovis infection in free-ranging African lions (Panthera leo) and implications for transmission. Journal of Wildlife Diseases. 2015;51:493–497. doi: 10.7589/2014-07-170. [DOI] [PubMed] [Google Scholar]
- Miller-Edge M.A., Worley M.B. In-vitro responses of cheetah mononuclear-cells to feline herpesvirus-1 and Cryptococcus neoformans. Veterinary Immunology and Immunopathology. 1992;30:261–274. doi: 10.1016/0165-2427(92)90143-E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward I.R., Williams M.C. Cryptococcus neoformans granuloma in the lung and spinal cord of a free-ranging cheetah (Acinonyx jubatus) A clinical report and literature review, Journal of the South African Veterinary Association. 2005;76:228–232. doi: 10.4102/jsava.v76i4.432. [DOI] [PubMed] [Google Scholar]
- Mitchell, E.P., Church, M.E., Neser, S.M,, Yakes, B.J., Evans, E.R., Reimschuessel, R., Lemberger K., Thompson, P.N., Terio, K.A. , 2017. Pathology and epidemiology of oxalate nephrosis in cheetahs. Veterinary Pathology, 54, 977-985. [DOI] [PubMed]
- Morris P.J., Thoen C.O., Legendre A.M. Pulmonary tuberculosis in an African lion (Panthera leo) Journal of Zoo and Wildlife Medicine. 1996;27:392–396. [Google Scholar]
- Munday J. Papillomaviruses in felids. Veterinary Journal. 2014;199:340–347. doi: 10.1016/j.tvjl.2013.11.025. [DOI] [PubMed] [Google Scholar]
- Munson L. Diseases of captive cheetahs (Acinonyx jubatus): Results of the cheetah research council pathology survey, 1989-1992. Zoo Biology. 1993;12:105–124. [Google Scholar]
- Munson L., Worley M.B. Veno-occlusive disease in snow leopards (Panthera uncia) from zoological parks. Veterinary Pathology. 1991;28:37–45. doi: 10.1177/030098589102800106. [DOI] [PubMed] [Google Scholar]
- Munson L., Stokes E., Harrenstien L. Uterine cancer in zoo felids on progestin contraceptives. Veterinary Pathology. 1995;32:578. [Google Scholar]
- Munson L., Nesbit J.W., Meltzer D.G.A., Colly L.P., Bolton L., Kriek N.P.J. Diseases of captive cheetahs (Acinonyx jubatus jubatus) in South Africa: a 20year retrospective survey. Journal of Zoo and Wildlife Medicine. 1999;30:342–347. [PubMed] [Google Scholar]
- Munson L., Gardner I., Mason R., Chassy L., Seal U. Endometrial hyperplasia and mineralization in zoo felids treated with melengestrol acetate contraceptives. Veterinary Pathology. 2002;39:419–427. doi: 10.1354/vp.39-4-419. [DOI] [PubMed] [Google Scholar]
- Munson L., Marker L., Dubovi E., Spencer J.A., Evermann J.F., O’Brien S.J. Serosurvey of viral infections in free-ranging Namibian cheetahs (Acinonyx jubatus) Journal of Wildlife Diseases. 2004;40:23–31. doi: 10.7589/0090-3558-40.1.23. [DOI] [PubMed] [Google Scholar]
- Munson L., Wack R., Duncan M., Montali R.J., Boon D., Stalis I., Crawshaw G.J., Cameron K.N., Mortenson J., Citino S., Zuba J., Junge J.E. Chronic eosinophilic dermatitis associated with persistent feline herpes virus infection in cheetahs (Acinonyx jubatus) Veterinary Pathology. 2004;41:170–176. doi: 10.1354/vp.41-2-170. [DOI] [PubMed] [Google Scholar]
- Munson L., Terio K.A., Worley M., Jago M., Bagot-Smith A., Marker L. Extrinsic factors significantly affect patterns of disease in free-ranging and captive cheetah (Acinonyx jubatus) populations. Journal of Wildlife Diseases. 2005;41:542–548. doi: 10.7589/0090-3558-41.3.542. [DOI] [PubMed] [Google Scholar]
- Munson L., Terio K.A., Kock R., Mlengeya T., Roelke M.E., Dubovi E., Summers B., Sinclair A.R.E., Packer C. Climate extremes promote fatal co-infections during canine distemper epidemics in African lions. PloS One. 2008;3:e2545. doi: 10.1371/journal.pone.0002545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson L., Terio K.A., Ryser-Degeorgis M., Lane E., Courchamp F. Wild Felid Diseases: Conservation Implications and Management Strategies. In: MacDonald D., Loveridge A., editors. Biology and Conservation of Wild Felids. Oxford University Press; Oxford: 2010. pp. 237–259. [Google Scholar]
- Myers D.L., Zurbriggen A., Lutz H., Pospischil A. Distemper: not a new disease in lions and tigers. Clinical and Diagnostic Laboratory Immunology. 1997;4 doi: 10.1128/cdli.4.2.180-184.1997. 180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao Y., Nishio Y., Shimoda H., Tamura S., Shimojima M., Goto M., Une Y., Sato A., Ikebe Y., Maeda K. An outbreak of canine distemper virus in tigers (Panthera tigris): possible transmission from wild animals to zoo animals. Journal of Veterinary Medical Science. 2012;74:699–705. doi: 10.1292/jvms.11-0509. [DOI] [PubMed] [Google Scholar]
- Newman J., Zillioux E., Rich E., Liang L., Newman C. Historical and other patterns of monomethyl and inorganic mercury in the Florida panther (Felis concolor coryi) Archives of Environmental Contaminants and Toxicology. 2005;48:75–80. doi: 10.1007/s00244-003-0130-5. [DOI] [PubMed] [Google Scholar]
- O’Brien S.J., Roelke M.E., Marker L., Newman A., Winkler C.A., Meltzer D., Colly L., Evermann J.F., Bush M., Wildt D.E. Genetic basis for species vulnerability in the cheetah. Science. 1985;227:1428–1434. doi: 10.1126/science.2983425. [DOI] [PubMed] [Google Scholar]
- O’Halloran C., Gunn-Moore D., Hope J. Diagnosis of feline mycobacteriosis. Veterinary Record. 2016;178:145–1145. doi: 10.1136/vr.i686. [DOI] [PubMed] [Google Scholar]
- Orbell G., Young S., Munday J. Cutaneous sarcoids in captive African lions associated with feline sarcoid-associated papillomavirus infection. Veterinary Pathology. 2011;48:1176–1179. doi: 10.1177/0300985810391111. [DOI] [PubMed] [Google Scholar]
- Origgi F.C., Plattet P., Sattler U., Robert N., Casaubon J., Mavrot F., Pewsner M., Wu N., Giovannini S., Oevermann A., Stoffel M.H., Gaschen V., Segner H., Ryser-Degiorgis M.P. Emergence of canine distemper virus strains with modified molecular signature and enhanced neuronal tropism leading to high mortality in wild carnivores. Veterinary Pathology. 2012;49 doi: 10.1177/0300985812436743. 913-29. [DOI] [PubMed] [Google Scholar]
- Ott Joslin J., Garner M., Collins D., Kamaka E., Sinabaldi K., Meleo K., Montali R., Sundberg J., Jenson A.B., Ghim S., Davidow B., Hargis A.M., West K., Clark T., Haines D. Viral Papilloma and squamous cell carcinomas in snow leopards (Uncia uncia) In: Proceedings AAZV and IAAAM Joint Conference. 2000:155–158. [Google Scholar]
- Paniker C.K.J., Nair C.M.G. Experimental infection of animals with influenza types A and B. WHO Bulletin. 1972;47 461-3. [PMC free article] [PubMed] [Google Scholar]
- Papendick R.E., Munson L., O’Brien T.D., Johnson K.H. Systemic amyloidosis in captive cheetahs (Acinonyx jubatus) Veterinary Pathology. 1997;34 doi: 10.1177/030098589703400602. 549-56. [DOI] [PubMed] [Google Scholar]
- Peet R.L., Curran J.M. Spongiform encephalopathy in an imported cheetah (Acinonyx jubatus) Australian Veterinary Journal. 1992;69:171. doi: 10.1111/j.1751-0813.1992.tb07506.x. [DOI] [PubMed] [Google Scholar]
- Peña L., Garcia P., Jiménez M., Benito A., Pérez-Alenza M., Sánchez B. Histopathological and immunohistochemical findings in lymphoid tissues of the endangered Iberian lynx (Lynx pardinus) Comparative Immunology Microbiology and Infectious Diseases. 2006;29:114–126. doi: 10.1016/j.cimid.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez J., Calzada J., León-Vizcaíno L., Cubero M.J., Velarde J., Mozos E. Tuberculosis in an Iberian lynx (Lynx pardina) Veterinary Record. 2001;148 doi: 10.1136/vr.148.13.414. 414-5. [DOI] [PubMed] [Google Scholar]
- Picard J.A., Lane E., Louw M. Cryptococcosis in cheetahs. In: Proceedings of a symposium on cheetahs as game ranch animals, Onderstepoort, South Africa. 1998:136–141. [Google Scholar]
- Poli A., Abramo F., Cavicchio P., Bandecchi P., Ghelardi E., Pistello M. Lentivirus infection in an African lion: a clinical, pathological and virological study. Journal of Wildlife Diseases. 1995;1 doi: 10.7589/0090-3558-31.1.70. 70-4. [DOI] [PubMed] [Google Scholar]
- Rector A., Lemey P., Tachezy R., Mostmans S., Ghim S.J., Van Doorslaer K., Roelke M., Bush M., Montali R.J., Joslin J., Burk R.D., Jenson A.B., Sundberg J.P., Shapiro B., Van Ranst M. Ancient papillomavirus-host co-speciation in Felidae. Genome Biology. 2007;8:R57. doi: 10.1186/gb-2007-8-4-r57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard L.G., Foreyt W.J. Gastrointestinal parasites of cougars (Felis concolor) in Washington and the first report of Ollulanus tricuspis in a sylvatic felid from North America. Journal of Wildlife Diseases. 1992;28:130–133. doi: 10.7589/0090-3558-28.1.130. [DOI] [PubMed] [Google Scholar]
- Rimmelzwaan G.F., van Riel D., Baars M., Bestebroer T.M., van Amerongen G., Fouchier R.A.M., Osterhaus A.D.M.E., Kuiken T. Influenza A virus (H5N1) infection in cats causes systemic disease with potential novel routes of virus spread within and between hosts. American Journal of Pathology. 2006;168:176–183. doi: 10.2353/ajpath.2006.050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas A.E., Langan J.N., Colegrove K.M., Terio K., Adkesson M.J. Herpesvirus and calicivirus infection in a black-footed cat (Felis nigripes) family group following vaccination. Journal of Zoo and Wildlife Medicine. 2015;46 doi: 10.1638/2013-0216R1.1. 141-5. [DOI] [PubMed] [Google Scholar]
- Robert, N., Walzer, C., 2009. Pathological disorders in captive cheetahs (Patologías de guepardos en cautividad). In: Iberian Lynx Ex Situ Conservation: An Interdisciplinary Approach (Conservación Ex Situ del Lince Ibérico: Un Enfoque Multidisciplinar). Vargas, A., Breitenmoser-Wu¨rsten, C., and Breitenmoser, U. Eds. Fundación Biodiversidad in collaboration with: IUCN Cat Specialist Group, Madrid. pp. 265-272.
- Roelke M.E., Schulz D.P., Facemire C.F., Sundlof S.F. Mercury contamination in the free-ranging endangered Florida panther (Felis concolor coryi) In: Proceedings American Association of Zoo Veterinarians. 1991 277-83. [Google Scholar]
- Roelke M.E., Martenson J.S., O’Brien S.J. The consequences of demographic reduction and genetic depletion in the endangered Florida panther. Current Biology. 1993;3:340–350. doi: 10.1016/0960-9822(93)90197-v. [DOI] [PubMed] [Google Scholar]
- Roelke M.E., Pecon-Slattery J., Taylor S., Citino S., Brown E., Packer C., VandeWoode S., O’Brien S.J. T-Lymphocyte profiles in FIV-infected wild lions and pumas reveal CD4 depletion. Journal of Wildlife Diseases. 2006;42:234–248. doi: 10.7589/0090-3558-42.2.234. [DOI] [PubMed] [Google Scholar]
- Roelke M.E., Brown M.A., Troyer J.L., Winterbach H., Winterbach C., Hemson G., Smith D., Johnson R.C., Pecon-Slattery J., Roca A.L., Alexander K.A., Klein L., Martelli P., Krishnasamy K., O’Brien S.J. Pathological manifestations of feline immunodeficiency virus (FIV) infection in wild African lions. Virology. 2009;390:1–12. doi: 10.1016/j.virol.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelke-Parker M.E., Munson L., Packer C., Kock R., Cleaveland S., Carpenter M., O’Brien S.J., Posposchil A., Hofmann-Lehmann R., Lutz H., Mwamengele G.L.M., Mgasa M.N., Machange G.A., Summers B.A., Appel M.J.G. A canine distemper virus epidemic in Serengeti lions (Panthera leo) Nature. 1996;379:441–445. doi: 10.1038/379441a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotstein D.S., Thomas R., Helmick K., Citino S.B., Taylor S.K., Dunbar M.R. Dermatophyte infections in free-ranging Florida panthers (Felis concolor coryi) Journal of Zoo and Wildlife Medicine. 1999;30:281–284. [PubMed] [Google Scholar]
- Schaffer E., Wiesner H., VonHegel G. Multiple ocular coloboma with persistent pupillary membrane in the snow leopard (Panthera uncia) Tierarztl Prax. 1988;16:87–91. [PubMed] [Google Scholar]
- Scherba G., Hajjar A.M., Pernikoff D.S., Sundberg J., Basgall E.J., Leon-Monzon M., Nerukar L., Reichmann M.E. Comparison of a cheetah herpesvirus isolate to feline herpesvirus type 1. Archives of Virology. 1988;100:89–97. doi: 10.1007/BF01310910. [DOI] [PubMed] [Google Scholar]
- Schmidbauer S., Wohlsein P., Kirpal G., Beineke A., Mueller G., Mueller H., Moser I., Baumgartner W. Outbreak of Mycobacterium bovis infection in a wild animal park. Veterinary Record. 2007;161:304–307. doi: 10.1136/vr.161.9.304. [DOI] [PubMed] [Google Scholar]
- Schroder H.-D., Ludwig C., Jakob W., Reischl U., Stolte M., Lehn N. Chronic gastritis in tigers associated with Helicobacter acinonyx. Journal of Comparative Pathology. 1998;119:67–73. doi: 10.1016/s0021-9975(98)80072-8. [DOI] [PubMed] [Google Scholar]
- Schulman F., Krafft A., Janczewski T., Mikaelian I., Irwin J. Cutaneous fibropapilloma in a mountain lion (Felis concolor) Journal of Zoo and Wildlife Medicine. 2003;34:179–183. doi: 10.1638/1042-7260(2003)034[0179:CFIAML]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Seimon T.A., Miquelle D.G., Chang T.Y., Newton A.L., Korotkova I., Ivanchuk G., Lyubchenko E., Tupikov A., Slabe E., McAloose D. Canine distemper virus: an emerging disease in wild endangered Amur tigers (Panthera tigris altaica) mBio. 2013;4 doi: 10.1128/mBio.00410-13. e00410-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serizawa S., Chambers J.K., Une Y. Beta amyloid deposition and neurofibrillary tangles spontaneously occur in the brains of captive cheetahs (Acinonyx jubatus) Veterinary Pathology. 2012;49:304–312. doi: 10.1177/0300985811410719. [DOI] [PubMed] [Google Scholar]
- Setchell K.D., Gosselin S.J., Welsh M.B., Johnston J.O., Balistreri W.F., Kramer L.W., Dresser B.L., Tarr M.J. Dietary estrogens – a probable cause of infertility and liver disease in captive cheetahs. Gastroenterology. 1987;93 doi: 10.1016/0016-5085(87)91006-7. 225-33. [DOI] [PubMed] [Google Scholar]
- Shamir M.H., Shilo Y., Fridman A., Chai O., Reifen R., Miara L. Sub-occipital craniectomy in a lion (Panthera leo) with occipital bone malformation and hypovitaminosis A. Journal of Zoo and Wildlife Medicine. 2008;39 doi: 10.1638/2006-0064.1. 455-9. [DOI] [PubMed] [Google Scholar]
- Spencer J.A., Vandijk A.A., Horzinek M.C., Egberink H.F., Bengis R.G., Keet D.F., Morikawa S., Bishop D.H.L. Incidence of feline immunodeficiency virus reactive antibodies in free-ranging lions of the Kruger national park and the Etosha national park in southern Africa detected by recombinant FIV p24 antigen. Onderstepoort Journal of Veterinary Research. 1992;59:315–322. [PubMed] [Google Scholar]
- Steinel A., Munson L., van Vuuren M., Truyen U. Genetic characterization of feline parvovirus sequences from various carnivores. Journal of General Virology. 2000;81:345–350. doi: 10.1099/0022-1317-81-2-345. [DOI] [PubMed] [Google Scholar]
- Stewart P., Campbell L., Skogtvedt S., Griffen K.A., Arnemo J.M., Tryland M., Girling S., Miller M.W., Tranulis M.A., Goldmann W. Genetic predictions of prion disease susceptibility in carnivore species based on variability of the prion gene coding region. PLoS One. 2012;7:e50623. doi: 10.1371/journal.pone.0050623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg J.P., Van Ranst M., Montali R., Homer B.L., Miller W.H., Rowland P.H., Scott D.W., England J.J., Dunstan R.W., Mikaelian I., Jenson A.B. Feline papillomas and papillomaviruses. Veterinary Pathology. 2000;37:1–10. doi: 10.1354/vp.37-1-1. [DOI] [PubMed] [Google Scholar]
- Sykes J.M., Ramsay E.C. Attempted treatment of tigers (Panthera tigris) infected with Microsporum canis. Journal of Zoo and Wildlife Medicine. 2007;38:252–257. doi: 10.1638/1042-7260(2007)038[0252:ATOTPT]2.0.CO;2. 4th. [DOI] [PubMed] [Google Scholar]
- Terio K.A., Craft M.E. Canine Distemper Virus (CDV) in another big cat: should CDV be renamed Carnivore Distemper Virus? mBio. 2013;4 doi: 10.1128/mBio.00702-13. e00702-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terio K.A., Marker L., Munson L. Evidence for chronic stress in captive but not free-ranging cheetahs (Acinonyx jubatus) based on adrenal morphology and function. Journal of Wildlife Diseases. 2004;40:259–266. doi: 10.7589/0090-3558-40.2.259. [DOI] [PubMed] [Google Scholar]
- Terio K.A., Munson L., Marker L., Aldridge B.M., Solnick J.V. Comparison of Helicobacter spp. in cheetahs (Acinonyx jubatus) with and without gastritis. Journal of Clinical Microbiology. 2005;43:229–234. doi: 10.1128/JCM.43.1.229-234.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terio K.A., O’Brien T.D., Lamberski N., Famula T.R., Munson L. Amyloidosis in black-footed cats (Felis nigripes) Veterinary Pathology. 2008;45:393–400. doi: 10.1354/vp.45-3-393. [DOI] [PubMed] [Google Scholar]
- Terio K.A., Munson L., Moore P.F. Characterization of the gastric immune response in cheetahs (Acinonyx jubatus) with Helicobacter-associated gastritis. Veterinary Pathology. 2012;49:824–833. doi: 10.1177/0300985811412620. [DOI] [PubMed] [Google Scholar]
- Terio K., Whitham J., Chosy J., Sanchez C., Marker L., Wielebnowski N. Associations between gastritis, temperament and management risk factors in captive cheetahs (Acinonyx jubatus) In: Proceedings of the American Association of Zoological Veterinarians. 2014 [Google Scholar]
- Terrell S.P., Fontenot D.K., Miller M.A., Weber M.A. Chylous ascites in a cheetah (Acinonyx jubatus) with venoocclusive liver disease. Journal of Zoo and Wildlife Medicine. 2003;34:380–384. doi: 10.1638/02-081. [DOI] [PubMed] [Google Scholar]
- Thalwitzer S., Wachter B., Robert N., Wibbelt G., Müller T., Lonzer J., Meli M.L., Bay G., Hofer H., Lutz H. Seroprevalences to viral pathogens in free-ranging and captive cheetahs (Acinonyx jubatus) on Namibian farmland. Clinical and Vaccine Immunology. 2010;17:232–238. doi: 10.1128/CVI.00345-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanawongnuwech R., Amonsin A., Tantilercharoen R., Damronwatanpokin S., Theamboonlers A., Payungporn S., Nanthapornphiphat K., Rantanamungklanon S., Lekdumrongsak T., Kesdangsakonwut S., Tunhikorn S., Poovorawant Y. Possible tiger-to-tiger transmission of avian influenza H5N1. Emerging Infectious Diseases. 2005;11:699–701. doi: 10.3201/eid1105.050007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traversa M.J., Etchechoury I., Jorge M.C., Schettino D.M., Bernadelli A., Zumarraga M., Paolicchi F., Cataldi A., Canal S. Mycobacterial isolation from Felis concolor in captivity. Brazilian Journal of Veterinary Research and Animal Science. 2009;46:25–31. [Google Scholar]
- Trinkel M., Cooper D., Packer C., Slotow R. Inbreeding depression increases susceptibility to bovine tuberculosis in lions: an experimental test using an inbred-outbred contrast through translocation. Journal of Wildlife Diseases. 2011;47:494–500. doi: 10.7589/0090-3558-47.3.494. [DOI] [PubMed] [Google Scholar]
- Troyer J.L., Pecon-Slattery J., Roelke M.E., Johnson W., Vandewoude S., Vazquez-Salat N., Brown M., Frank L., Woodroffe R., Winterbach C., Winerbach H., Hemson G., Bush M., Alexander K.A., Revilla E., O’Brien S.J. Seroprevalence and genomic divergence of circulating strains of feline immunodeficiency virus among Felidae and Hyaenidae species. Journal of Virology. 2005;79:8282–8294. doi: 10.1128/JVI.79.13.8282-8294.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer J.L., Roelke M.E., Jespersen J.M., Baggett N., Buckley-Beason V., MacNulty D., Craft M., Packer C., Pecon-Slattery J., O’Brien S.J. FIV diversity: FIV Ple subtype composition may influence disease outcome in African lions. Veterinary Immunology and Immunopathology. 2011;143 doi: 10.1016/j.vetimm.2011.06.013. 338-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubbesing, U.H., 1997. An outbreak of anthrax amongst wild carnivore species held in captivity. In: Proceedings of a symposium on lions and leopards as game ranch animals, Onderstepoort, South Africa, 163-7.
- Turnbull P.C.B., Doganay M., Lindeque P.M., Aygen B., Mclaughlin J. Serology and anthrax in humans, livestock and etosha-national-park wildlife. Epidemiology and Infection. 1992;108:299–313. doi: 10.1017/s0950268800049773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venter E.H., van Vuuren M., Carstens J., van der Walt M.L., Nieuwoudt B., Steyn H., Kriek N.P.J. A molecular epidemiologic investigation of Salmonella from a meat source to the feces of captive cheetah (Acinonyx jubatus) Journal of Zoo Wildlife Medicine. 2003;34:76–81. doi: 10.1638/1042-7260(2003)34[0076:AMEIOS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Viana M., Cleaveland S., Matthiopoulos J., Halliday J., Packer C., Craft M.E., Hampson K., Czupryna A., Dobson A.P., Dubovi E.J., Ernest E., Fyumagwa R., Hoare R., Hopcraft J.G., Horton D.L., Kaare M.T., Kanellos T., Lankester F., Mentzel C., Mlengeya T., Mzimbiri I., Takahashi E., Willett B., Haydon D.T., Lembo T. Dynamics of a morbillivirus at the domestic-wildlife interface: Canine distemper virus in domestic dogs and lions. In: Proceedings of the National Academy of Sciences. 2015;112 doi: 10.1073/pnas.1411623112. 1464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viljoen I.M., van Heiden P.D., Millar R.P. Mycobacterium bovis infection in the lion (Panthera leo): Current knowledge, conundrums and research challenges. Veterinary Microbiology. 2015;177:252–260. doi: 10.1016/j.vetmic.2015.03.028. [DOI] [PubMed] [Google Scholar]
- Wack R.F., Kramer L.W., Cupps W. Griseofulvin toxicity in four cheetahs (Acinonyx jubatus) Journal of Zoo and Wildlife Medicine. 1992;23:442–446. [Google Scholar]
- Walzer C., Kübber-Heiss A. Progressive hind limb paralysis in adult cheetahs (Acinonyx jubatus) Journal of Zoo and Wildlife Medicine. 1995;26:430–435. [Google Scholar]
- Walzer C., Url A., Robert N., Kübber-Heiss A., Nowotny N., Schmidt P. Idiopathic acute onset myelopathy in cheetah (Acinonyx jubatus) cubs. Journal of Zoo and Wildlife Medicine. 2003;34:36–46. doi: 10.1638/1042-7260(2003)34[0036:IAOMIC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Watering C.C.V.d., Zwart P., Bakker J. Cavernous tuberculosis of the lungs and secondary hypertrophic osteo-arthropathy in a Siberian tiger (Panthera tigris) Journal of Small Animal Practice. 1972;13:321–327. doi: 10.1111/j.1748-5827.1972.tb06852.x. [DOI] [PubMed] [Google Scholar]
- Wild M.A., Shenk T.M., Spraker T.R. Plague as a mortality factor in Canada lynx (Lynx canadensis) reintroduced to Colorado. Journal of Wildlife Diseases. 2006;42:646–650. doi: 10.7589/0090-3558-42.3.646. [DOI] [PubMed] [Google Scholar]
- Witte C.L., Lamberski N., Rideout B.A., Fields V., Teare C.S., Barrie M., Haefele H., Junge R., Murray S., Hungerford L.L. Development of a case definition for clinical feline herpesvirus infection in cheetahs (Acinonyx jubatus) housed in zoos. Journal of Zoo and Wildlife Medicine. 2013;44:634–644. doi: 10.1638/2012-0183R.1. [DOI] [PubMed] [Google Scholar]
- Yilmaz R., Yumusak N. An anthrax outbreak in wild felidae kept in a local zoo. Kafkas Universitesi Veteriner Fakultesi Dergisi. 2015;21:441–444. [Google Scholar]
- Zhang B., Une Y., Fu X., Yan J., Ge F., Yao J., Sawashita J., Mori M., Tomozawa H., Kametani F., Higuchi K. Fecal transmission of AA amyloidosis in the cheetah contributes to high incidence of disease. In: Proceedings of the National Academy of Sciences USA. 2008;105:7263–7268. doi: 10.1073/pnas.0800367105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Une Y., Ge F., Fu X., Qian J., Zhang P., Sawashita J., Higuchi K., Mori M. Characterization of the cheetah serum amyloid A1 gene: critical role and functional polymorphism of a cis-acting element. The Journal of Heredity. 2008;99 doi: 10.1093/jhered/esn015. 355-63. [DOI] [PubMed] [Google Scholar]


