The common cold, also known as upper respiratory tract infection (URI), is an acute, self-limited viral infection of the upper airway that may involve the lower respiratory tract as well. The characteristic symptom complex consisting of rhinorrhea, nasal congestion, and sore or scratchy throat is familiar to all adults. Colds are the most common cause of human illness and are responsible for significant absenteeism from school and work. Children are especially susceptible because: (1) they have not yet acquired immunity to many of the viruses; (2) they have poor personal hygiene practices; and (3) they have frequent close contact with other children who are excreting virus.
Etiology
Colds are common because some of the causative viruses do not produce lasting immunity after infection and some viruses have numerous serotypes (Table 26-1 ). Cold viruses that do not produce lasting immunity include respiratory syncytial virus (RSV), parainfluenza viruses (PIVs), and coronaviruses (HCoVs). Cold viruses that have numerous serotypes but produce lasting serotype-specific immunity after infection include rhinoviruses, adenoviruses, influenza viruses, and enteroviruses.1
TABLE 26-1.
Immunity to Common Cold Viruses
| Virus | No. of Serotypes |
|---|---|
| Long-lasting immunity not produced by infection (repeated infection with same serotype usual) | |
| Respiratory syncytial virus (RSV) | 1 |
| Parainfluenza virus | 4 |
| Human coronavirus | 2 |
| Immunity produced by infection (reinfection with same serotype uncommon) | |
| Rhinovirus | >100 |
| Adenovirus | ≥33 |
| Influenza | 3a |
| Echovirus | 31 |
| Coxsackievirus group A | 3 |
| Coxsackievirus group B | 6 |
Type A subtypes change.
Modified from Hendley JO. Immunology of viral colds. In: Veldman JE, McCabe BF, Huizing EH, et al. (eds) Immunobiology, Autoimmunity, Transplantation in Otorhinolaryngology. Amsterdam, Kugler Publications, 1985, pp 257–260.
Rhinoviruses, with at least 100 serotypes, are the most common cause of URIs in children and adults. At least 50% of colds in adults are caused by rhinoviruses. Other viruses that cause URIs are HCoVs, RSV, human metapneumovirus, influenza virus, parainfluenza viruses, adenoviruses, echoviruses, and coxsackieviruses A and B. Human bocavirus (HBoV), discovered in 2005, has been reported in children with symptomatic upper respiratory tract infection (>10%) and may also be present in asymptomatic children, making its role in causing illness unclear.2, 3, 4 Some of these viruses cause characteristic syndromes; for example, RSV causes bronchiolitis in children 2 years or younger, influenza viruses cause febrile respiratory illness with severe lower respiratory tract involvement, adenoviruses cause pharyngoconjunctival fever, parainfluenza viruses cause croup in young children, HBoV is associated with wheezing, and enteroviruses cause a variety of illnesses, including aseptic meningitis and herpangina.
Epidemiology
In temperate climates in the northern hemisphere, the predictable yearly epidemic of colds begins in September and continues unabated until spring. This sustained epidemic curve is a result of successive waves of different respiratory viruses moving through the community (Figure 26-1 ). The epidemic begins with a sharp rise in the frequency of rhinovirus infections in September (after children return to school), which is followed by PIVs in October and November. RSV and HCoVs circulate during the winter months, whereas infection due to influenza virus peaks in the late winter. The epidemic finally ends with a small resurgence of rhinovirus infections in the spring. Adenovirus infection occurs at a constant rate throughout the cold season.5
Figure 26-1.
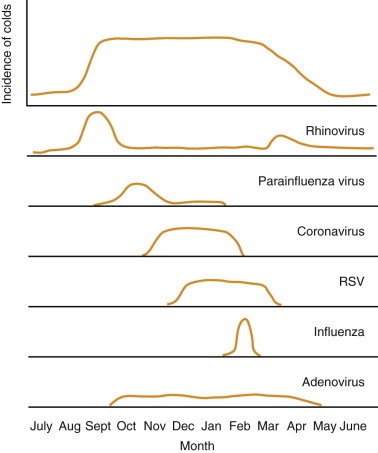
Schematic diagram of the incidence of colds and frequency of causative viruses. RSV, respiratory syncytial virus.
(Redrawn from Hendley JO. The common cold. In: Goldman L, Bennett JC (eds) Cecil Textbook of Medicine, 21st ed. Philadelphia, WB Saunders, 2000, pp 1790–1793.)
The frequency of colds varies with age. A 10-year study of families with children who did not attend a childcare facility showed that the peak incidence of colds occurs in preschool children 1 to 5 years old, with a frequency of 7.4 to 8.3 colds per year. Infants younger than 1 year averaged 6.7 colds per year, and teenagers averaged about 4.5 colds per year. Mothers and fathers experienced about 4 colds per year.6 With the greater exposure of children to other preschool children in childcare facilities, the frequency of colds in children younger than 6 years has increased. Thus, the typical preschool child experiences at least one URI per month throughout the cold season.
Viral transmission occurs primarily in the home setting, although the exact mechanism of spread has not been clearly established. Colds can be spread by: (1) small-particle (<5 µm in diameter) aerosol, which infects when inhaled; or (2) large-particle (>10 µm in diameter) droplets, which infect by landing on nasal or conjunctival mucosa; or (3) direct transfer via hand-to-hand contact.7 Small-particle aerosol is an effective method of transfer for influenza virus8 and coronavirus9 but not for RSV.10 Rhinoviruses are most likely spread by large-particle droplets or direct transfer. Rhinoviruses can survive as long as 2 hours on human hands and up to several days on other surfaces. Studies in young adults have shown that infected individuals commonly have rhinovirus on their hands, which can be efficiently transferred to the hands of uninfected individuals during brief contact; infection then results when the uninfected person transfers the virus from the hands on to his or her nasal or conjunctival mucosa. Sneezing and coughing are ineffective modes of rhinovirus transmission,11 although there is some evidence that virus could also be transmitted by aerosols generated by coughing, talking, and breathing.12 Inoculation of the oral mucosa with rhinovirus13 or RSV14 does not result in infection.
Pathogenesis
Symptoms of the common cold do not appear to result from destruction of nasal mucosa, because nasal biopsy specimens from young adults with both natural and experimentally induced colds show intact nasal epithelium during symptomatic illness.15, 16 Study by in situ hybridization of nasal biopsy specimens obtained during rhinovirus infection indicates that replication occurs in only a small number of epithelial cells.17, 18 Furthermore, in vitro studies have shown that rhinovirus and coronavirus produce no detectable cytopathic effect when replicating in a cultured monolayer of nasal epithelial cells, whereas influenza virus A and adenovirus produce obvious damage.19
The symptoms of the common cold appear to result from release of cytokines and other mediators from infected nasal epithelial cells as well as from an influx of polymorphonuclear cells (PMNs). Nasal washings of volunteers experimentally infected with rhinovirus showed a 100-fold increase in PMN concentration 1 to 2 days after inoculation.20 This influx of PMNs coincides with onset of symptoms and correlates with the presence of a colored nasal discharge.21 A yellow or white nasal discharge may result from the higher number of PMNs, whereas the enzymatic activity of PMNs (due to myeloperoxidase and other enzymes) may cause a green nasal discharge. A potent chemoattractant for PMNs is produced by cells in culture infected with rhinovirus.22 This chemoattractant has been identified as interleukin-8 (IL-8).23 Elevated levels of IL-8 and other cytokines (IL-1β, IL-6) also have been demonstrated in the nasal secretions of infected individuals.24, 25 Furthermore, elevated levels of albumin and kinins (predominantly bradykinin) in nasal secretions have been shown to coincide with the onset of symptoms in experimental rhinovirus infection.20 The elevated concentration of albumin and kinins likely results from exudation of plasma proteins due to greater vascular permeability in the nasal submucosa. The method by which viral infection initiates this vascular leak has not yet been determined. The release of kinins resulting from plasma exudation may augment the symptoms of the cold; bradykinin alone can cause rhinitis and sore throat when sprayed into the noses of uninfected individuals.26
The paranasal sinuses usually are involved during an uncomplicated cold. In one study, computed tomographic (CT) scans obtained during the acute phase of illness revealed abnormalities of one or more sinuses in 27 (87%) of 31 young adults.27 Without antibiotic therapy, there was complete resolution or marked improvement of the sinus abnormalities in 11 (79%) of the 14 subjects in whom second CT scans were obtained 2 weeks later. In another study, MRI revealed that 60% of children with upper respiratory tract infections had major abnormalities in their paranasal sinuses; these tended to resolve without antibiotic therapy.28 It is not known whether these sinus abnormalities result from viral infection of the sinus mucosa or from impaired sinus drainage secondary to viral rhinitis. Nose-blowing can generate enough pressure to force fluid from the nasopharynx into the paranasal sinuses, suggesting that nose-blowing may force mucus containing viruses, bacteria, and inflammatory mediators into the paranasal sinuses during a cold.29
The middle ear can also be involved during uncomplicated colds. Studies in school-aged children have shown that two-thirds will develop abnormal middle-ear pressures within 2 weeks after onset of a cold.30 Otitis media was not diagnosed during the study, as ears were not examined and none of the children sought medical care. It is not known whether the abnormal middle-ear pressures result from viral infection of the mucosa of the middle ear and eustachian tube or from viral nasopharyngitis with secondary eustachian tube dysfunction.
Clinical Manifestations
Symptoms of the common cold do not vary by specific causative virus. In older children and adults, rhinorrhea, nasal obstruction, and sore or scratchy throat are typical. The rhinorrhea is initially clear but may become colored as the illness proceeds. Cough or sneeze may be present. Fever (>38°C) is uncommon in adults. Other symptoms are malaise, sinus fullness, and hoarseness. Objective findings are minimal except for mild erythema of the nasal mucosa or pharynx. Symptoms resolve in 5 to 7 days.
Compared with adults, infants and preschool children with colds are more likely to have fever (>38°C) and moderate enlargement of the anterior cervical lymph nodes (Table 26-2 ).1 Rhinorrhea may not be noticed until the nasal discharge becomes colored. Nasal congestion can disrupt sleep and can lead to fatigue and irritability. The illness often persists in infants and preschool children for 10 to 14 days.31
TABLE 26-2.
Characteristics of Viral Colds in Adults and Young Children
| Characteristic | Adults | Children <6 years |
|---|---|---|
| Frequency | 2–4 per year | One per month, September–April |
| Fever | Rare | Common during first 3 days |
| Nasal manifestations | Congestion | Colored nasal discharge |
| Duration of illness | 5–7 days | 14 days |
Modified from Hendley JO. Epidemiology, pathogenesis, and treatment of the common cold. Semin Pediatr Infect Dis 1998;9:50–55.
Differential Diagnosis
The differential diagnosis of a cold includes allergic rhinitis, vasomotor rhinitis, intranasal foreign body, and sinusitis. A diagnosis of allergic rhinitis is suggested by a seasonal pattern of clear rhinorrhea, absence of associated fever, and family history of allergy. Possible associated conditions are asthma and eczema. Physical findings consistent with allergic rhinitis include allergic “shiners” and “nasal salute.” The detection of numerous eosinophils upon microscopic examination of the nasal mucus using Hansel stain confirms the diagnosis of allergic rhinitis. A diagnosis of vasomotor rhinitis is suggested by a chronic course without fever or sore throat. The diagnosis of bacterial sinusitis is suggested by persistent rhinorrhea or cough or both for greater than 10 days.32
Clinical Approach
The diagnosis of a cold is based on history and physical examination; generally, laboratory tests are not useful. The rapid test for detecting RSV, influenza, parainfluenza, and adenovirus antigens in nasal secretions can be used to confirm the diagnosis. RSV, rhinovirus, influenza viruses, parainfluenza viruses, and adenoviruses also can be isolated in cell culture. HCoV cannot be detected reliably in cell culture, so serologic titer rise can be used for diagnosis, if necessary. Polymerase chain reaction assays for diagnosis of all the respiratory viruses are available in research laboratories and increasingly in clinical laboratories; there is lack of standardization and validation for many tests offered. Other methods of detection can be used but are rarely needed.
Management
At present, no antiviral agents are available that are effective for treatment of the common cold. Although an array of medications may be used to relieve symptoms, there is little scientific evidence to support the use of symptomatic treatments in children. Because the common cold is a self-limited illness with symptomatology that is largely subjective, a substantial placebo effect can suggest that various treatments have some efficacy. Inadequate blinding of placebo recipients in a study can make an ineffective treatment appear effective.
In adults with colds, first-generation antihistamines (i.e., chlorpheniramine) have been shown to provide modest symptomatic relief, with decreases in nasal discharge, sneezing, nose-blowing, and duration of symptoms.33 This effect is presumably due to the anticholinergic effects of these medications. A randomized, double-blind, placebo-controlled study in preschool children with URIs showed that treatment with an antihistamine–decongestant combination (brompheniramine maleate and phenylpropanolamine hydrochloride) produced no improvement in cough, rhinorrhea, or nasal congestion, although a larger proportion of the treated children (47% versus 26%) were asleep 2 hours after treatment.34
Numerous decongestants, antitussives, and expectorants are available over the counter, but there is no evidence to support their use in children. A study of phenylephrine, a topical decongestant, in children 6 to 18 months old showed no decrease in nasal obstruction with its use during a URI.35 In a study comparing placebo, dextromethorphan, and codeine for cough suppression in children 18 months to 12 years old, cough decreased in all patients within 3 days, but there was no difference in cough reduction among the three treatment groups.36 Guaifenesin, an expectorant, has not been shown to change the volume or quality of sputum or the frequency of cough in young adults with colds.37 Echinacea preparations, commonly believed to be effective in the treatment of the common cold, have been shown to have no effect on the prevention or treatment of rhinovirus infection38 as has intranasal zinc gluconate for treatment of colds39 or prevention of experimental rhinovirus colds.40 In January, 2008, the U.S. Food and Drug Administration issued an advisory strongly recommending that over-the-counter cold and cough medications not be given to infants because of the risk of life-threatening side effects.
Antibiotics have no role in the treatment of uncomplicated URIs in children. Antibiotic therapy does not hasten resolution of the viral infection or reduce the likelihood of occurrence of secondary bacterial infection.41 Antibiotics are only indicated in cases of secondary bacterial infection, such as sinusitis and acute otitis media.
Thus, supportive measures remain the mainstay of treatment of the common cold in children. Bulb suction with saline drops (about 1 teaspoon salt in 2 cups of water) may help relieve nasal congestion and remove secretions. A recent study suggests that honey given at bedtime may help reduce cough in children with upper respiratory tract infections, although honey is not recommended for children under the age of 12 months because of the risk of exposure to C. botulinum spores.42
Complications
The common cold usually resolves in about 10 to 14 days in infants and children. New-onset fever and earache during this period may herald the development of bacterial otitis media, which occurs in about 5% of colds in preschool children. Persistence of nasal symptoms for longer than 10 days has been thought to signify the development of a secondary bacterial sinusitis. However, a recent study found that 20 children hospitalized for preseptal or orbital cellulitis, indicative of bacterial sinusitis, had symptoms of acute respiratory tract infection for 7 days or less prior to hospitalization, suggesting that the complications of rhinosinusitis can occur during the first few days of a cold.43 Bacterial pneumonia is an uncommon secondary infection. For children with underlying reactive airways disease, wheezing is common during the course of a viral URI; at least 50% of asthma exacerbations in children are associated with viral infection. Children who experience more than one lower respiratory tract infection (such as croup or bronchiolitis) during their first year of life have an increased risk of asthma thereafter.44 Other complications are epistaxis, eustachian tube dysfunction, conjunctivitis, and pharyngitis.
Recent Advances
The symptoms of the common cold appear to result from effects of inflammatory mediators released in response to the viral infection of the respiratory tract. As the determinants of this process are further elucidated, treatments may be developed that can interrupt or ameliorate release of inflammatory mediators and thus prevent or reduce the symptoms of the common cold. Vaccines are unlikely to be useful for prevention, given the large number of serotypes of some cold viruses as well as the lack of lasting immunity to others. The use of alcohol-based hand gels has been suggested as a means of reducing secondary transmission of respiratory illnesses in the home,45 but this was not shown to be effective in one field trial.46 Also, virucidal tissues have been shown to be effective in preventing viral passage and transmission, and may reduce secondary transmission by about 30%.47, 48 Until new methods are developed, prevention of the common cold is limited to avoiding self-inoculation (transfer of virus from contaminated fingers to nasal or conjunctival mucosa) by removing virus through handwashing or by killing virus with application of a virucide to the hands.
References
- 1.Hendley JO. Epidemiology, pathogenesis, and treatment of the common cold. Semin Pediatr Infect Dis. 1998;9:50–55. doi: 10.1016/S1045-1870(98)80051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Longtin J, Bastien M, Gilca R. Human bocavirus infections in hospitalized children and adults. Emerg Infect Dis. 2008;14:217–220. doi: 10.3201/eid1402.070851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruohola A, Waris M, Allander T. Viral etiology of common cold in children, Finland. Emerg Infect Dis. 2009;15:344–345. doi: 10.3201/eid1502.081468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arden KE, Chung AB, Lambert SB. Newly identified respiratory viruses in children with asthma exacerbation not requiring admission to hospital. Med Virol. 2010;82:1458–1461. doi: 10.1002/jmv.21819. [DOI] [PubMed] [Google Scholar]
- 5.Hendley JO. The common cold. In: Cecil RL, Bennett JC, Goldman L, editors. Cecil Textbook of Medicine. 21st ed. WB Saunders; Philadelphia: 2000. pp. 1790–1793. [Google Scholar]
- 6.Dingle JH, Badger GF, Jordan WS. Press of Case Western Reserve University; Cleveland: 1964. Illness in the Home. [Google Scholar]
- 7.Hendley JO, Gwaltney JM., Jr Mechanisms of transmission of rhinovirus infections. Epidemiol Rev. 1988;10:242–258. [PubMed] [Google Scholar]
- 8.Moser MR, Bender TR, Margolis HS. An outbreak of influenza aboard a commercial airliner. Am J Epidemiol. 1979;110:1–6. doi: 10.1093/oxfordjournals.aje.a112781. [DOI] [PubMed] [Google Scholar]
- 9.Turner RB, Meschievitz CK, Streisand AC. Mechanism of transmission of coronavirus 229E in human volunteers. Clin Res. 1987;35:145a. [Google Scholar]
- 10.Hall CB, Douglas RG. Modes of transmission of respiratory syncytial virus. J Pediatr. 1981;99:100–103. doi: 10.1016/s0022-3476(81)80969-9. [DOI] [PubMed] [Google Scholar]
- 11.Gwaltney JM, Moskalski PB, Hendley JO. Hand-to-hand transmission of rhinovirus colds. Ann Intern Med. 1978;88:463–467. doi: 10.7326/0003-4819-88-4-463. [DOI] [PubMed] [Google Scholar]
- 12.Stelzer-Brais S, Oliver BG, Blazey AJ. Exhalation of respiratory viruses by breathing, coughing, talking. J Med Virol. 2009;81:1674–1679. doi: 10.1002/jmv.21556. [DOI] [PubMed] [Google Scholar]
- 13.Hendley JO, Wenzel RP, Gwaltney JM., Jr Transmission of rhinovirus colds by self-inoculation. N Engl J Med. 1973;288:1361–1364. doi: 10.1056/NEJM197306282882601. [DOI] [PubMed] [Google Scholar]
- 14.Hall CB, Douglas RG, Schnabel KC. Infectivity of respiratory syncytial virus by various routes of inoculation. Infect Immun. 1981;33:779–783. doi: 10.1128/iai.33.3.779-783.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winther B, Brofeldt S, Christensen B. Light and scanning electron microscopy of nasal biopsy material from patients with naturally acquired common colds. Acta Otolaryngol (Stockh) 1984;97:309–318. doi: 10.3109/00016488409130994. [DOI] [PubMed] [Google Scholar]
- 16.Winther B, Farr B, Turner RB. Histopathologic examination and enumeration of polymorphonuclear leukocytes in the nasal mucosa during experimental rhinovirus colds. Acta Otolaryngol (Stockh) 1984;413(suppl):19–24. doi: 10.3109/00016488409128537. [DOI] [PubMed] [Google Scholar]
- 17.Bardin PG, Johnston SL, Sanderson G. Detection of rhinovirus infection of the nasal mucosa by oligonucleotide in situ hybridization. Am J Respir Cell Mol Biol. 1994;10:207–213. doi: 10.1165/ajrcmb.10.2.8110476. [DOI] [PubMed] [Google Scholar]
- 18.Arruda E, Boyle TR, Winther B. Localization of human rhinovirus replication in the upper respiratory tract by in situ hybridization. J Infect Dis. 1995;171:1329–1333. doi: 10.1093/infdis/171.5.1329. [DOI] [PubMed] [Google Scholar]
- 19.Winther B, Gwaltney JM, Jr, Hendley JO. Respiratory virus infection of monolayer cultures of human nasal epithelial cells. Am Rev Respir Dis. 1990;141:839–845. doi: 10.1164/ajrccm/141.4_Pt_1.839. [DOI] [PubMed] [Google Scholar]
- 20.Naclerio RM, Proud D, Lichtenstein LM. Kinins are generated during experimental rhinovirus colds. J Infect Dis. 1988;157:133–142. doi: 10.1093/infdis/157.1.133. [DOI] [PubMed] [Google Scholar]
- 21.Winther B, Brofeldt S, Gronborg H. Study of bacteria in the nasal cavity and nasopharynx during naturally acquired common colds. Acta Otolaryngol (Stockh) 1984;98:315–320. doi: 10.3109/00016488409107569. [DOI] [PubMed] [Google Scholar]
- 22.Turner RB. Rhinovirus infection of human embryonic lung fibroblasts induces the production of a chemoattractant for polymorphonuclear leukocytes. J Infect Dis. 1988;157:346–350. doi: 10.1093/infdis/157.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turner RB. American Society for Microbiology; Orlando, FL: 1994. Elaboration of interleukin 8 (IL-8) from fibroblast (MRC-5) cells and human nasal epithelium in response to rhinovirus (RV) challenge (abstract B43). Abstracts of the 34th Interscience Conference on Antimicrobial Agents and Chemotherapy. [Google Scholar]
- 24.Noah TL, Becker S. Respiratory syncytial virus-induced cytokine production by a human bronchial epithelial cell line. Am J Physiol. 1993;265:L472–L478. doi: 10.1152/ajplung.1993.265.5.L472. [DOI] [PubMed] [Google Scholar]
- 25.Noah TL, Henderson FW, Wortman IA. Nasal cytokine production in viral acute upper respiratory infection of childhood. J Infect Dis. 1995;171:584–592. doi: 10.1093/infdis/171.3.584. [DOI] [PubMed] [Google Scholar]
- 26.Proud D, Reynolds CJ, Lacapra S. Nasal provocation with bradykinin induces symptoms of rhinitis and a sore throat. Am Rev Respir Dis. 1988;137:613–616. doi: 10.1164/ajrccm/137.3.613. [DOI] [PubMed] [Google Scholar]
- 27.Gwaltney JM, Jr, Phillips CD, Miller RD. Computed tomographic study of the common cold. N Engl J Med. 1994;330:25–30. doi: 10.1056/NEJM199401063300105. [DOI] [PubMed] [Google Scholar]
- 28.Kristo A, Uhari M, Luotonen J. Paranasal sinus findings in children during respiratory infections evaluated with magnetic resonance imaging. Pediatrics. 2003;111:e586–e589. doi: 10.1542/peds.111.5.e586. [DOI] [PubMed] [Google Scholar]
- 29.Gwaltney JM, Jr, Hendley JO, Phillips CD. Nose blowing propels nasal fluid into the paranasal sinuses. Clin Infect Dis. 2000;30:387–391. doi: 10.1086/313661. [DOI] [PubMed] [Google Scholar]
- 30.Winther B, Hayden FG, Arruda E. Viral respiratory infection in schoolchildren: effects on middle ear pressure. Pediatrics. 2002;109:826–832. doi: 10.1542/peds.109.5.826. [DOI] [PubMed] [Google Scholar]
- 31.Butler CC, Kinnersley P, Hood K. Clinical course of acute infection of the upper respiratory tract in children: a cohort study. Br Med J. 2003;327:1088–1089. doi: 10.1136/bmj.327.7423.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wald ER. Sinusitis in children. N Engl J Med. 1992;326:319–323. doi: 10.1056/NEJM199201303260507. [DOI] [PubMed] [Google Scholar]
- 33.Howard JC, Kantner TR, Lilienfield LS. Effectiveness of antihistamines in the symptomatic management of the common cold. JAMA. 1979;242:2414–2417. [PubMed] [Google Scholar]
- 34.Clemens CJ, Taylor JA, Almquist JR. Is an antihistamine-decongestant combination effective in temporarily relieving symptoms of the common cold in preschool children? J Pediatr. 1997;130:463–466. doi: 10.1016/s0022-3476(97)70211-7. [DOI] [PubMed] [Google Scholar]
- 35.Turner RB, Darden PM. Effect of topical adrenergic decongestants on middle ear pressure in infants with common colds. Pediatr Infect Dis J. 1996;15:621–624. doi: 10.1097/00006454-199607000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Taylor JA, Novack AH, Almquist JR. Efficacy of cough suppressants in children. J Pediatr. 1993;122:799–802. doi: 10.1016/s0022-3476(06)80031-4. [DOI] [PubMed] [Google Scholar]
- 37.Kuhn JJ, Hendley JO, Adams KF. Antitussive effect of guaifenesin in young adults with natural colds: objective and subjective assessment. Chest. 1982;82:713–718. doi: 10.1378/chest.82.6.713. [DOI] [PubMed] [Google Scholar]
- 38.Turner RB, Bauer R, Woelkart K. An evaluation of Echinacea angustifolia in experimental rhinovirus infections. N Engl J Med. 2005;353:341–348. doi: 10.1056/NEJMoa044441. [DOI] [PubMed] [Google Scholar]
- 39.Caruso TJ, Prober CG, Gwaltney JM., Jr Treatment of naturally acquired common colds with zinc: a structured review. Clin Infect Dis. 2007;45:569–574. doi: 10.1086/520031. [DOI] [PubMed] [Google Scholar]
- 40.Turner RB. Ineffectiveness of intranasal zinc gluconate for prevention of experimental rhinovirus colds. Clin Infect Dis. 2001;33:1865–1870. doi: 10.1086/324347. [DOI] [PubMed] [Google Scholar]
- 41.Glasziou P, Del Mar C. Upper respiratory tract infection. Clin Evid. 2000;3:737–742. [Google Scholar]
- 42.Paul IM, Beiler J, McMonagle A. Effect of honey, dextromethorphan, and no treatment on nocturnal cough and sleep quality for coughing children and their parents. Arch Pediatr Adolesc Med. 2007;161:1140–1146. doi: 10.1001/archpedi.161.12.1140. [DOI] [PubMed] [Google Scholar]
- 43.Kristo A, Uhari M. Timing of rhinosinusitis complications in children. Pediatr Infect Dis J. 2009;28:769–770. doi: 10.1097/INF.0b013e3181a3aa7f. [DOI] [PubMed] [Google Scholar]
- 44.Nafstad P, Brrunekreef B, Skrondal A. Early respiratory infections, asthma, and allergy: 10-year follow-up of the Oslo birth cohort. Pediatrics. 2005;116e:e255–e262. doi: 10.1542/peds.2004-2785. [DOI] [PubMed] [Google Scholar]
- 45.Lee GM, Salomon JA, Friedman JF. Illness transmission in the home: a possible role for alcohol-based hand gels. Pediatrics. 2005;115:852–860. doi: 10.1542/peds.2004-0856. [DOI] [PubMed] [Google Scholar]
- 46.Sandora TJ, Taveras EM, Shih EM. A randomized, controlled trial of a multifaceted intervention including alcohol-based hand sanitizer and hand-hygiene education to reduce illness transmission in the home. Pediatrics. 2005;116:587–594. doi: 10.1542/peds.2005-0199. [DOI] [PubMed] [Google Scholar]
- 47.Farr BM, Hendley JO, Kaiser DL. Two randomized, controlled trials of virucidal nasal tissues in the prevention of natural upper respiratory infections. Am J Epidemiol. 1988;128:1162. doi: 10.1093/oxfordjournals.aje.a115059. [DOI] [PubMed] [Google Scholar]
- 48.Longin IM, Monto AS. Efficacy of virucidal nasal tissues in interrupting familial transmission of respiratory agents. Am J Epidemiol. 1988;128:639. doi: 10.1093/oxfordjournals.aje.a115011. [DOI] [PubMed] [Google Scholar]


