CEREBROSPINAL FLUID
Cerebrospinal fluid (CSF) evaluation is a mainstay in the diagnosis of central nervous system (CNS) disease because it is relatively simple to collect and has the potential to provide valuable information. Lesions of the CNS do not consistently cause CSF abnormalities related to the location and extent of the lesion. Although CSF evaluation infrequently provides a definitive diagnosis, it may be of benefit in documenting normal or abnormal features and, in combination with other tests, determining a diagnosis or differential diagnoses (Bohn et al., 2006; Bush et al., 2002; Chrisman, 1992; Cook and DeNicola, 1988; Fenner, 2000; Rand, 1995). CSF collection is recommended as a part of virtually any diagnostic investigation of CNS disease of unknown cause when contraindications to its collection are not present.
The submission of a properly collected specimen is necessary to obtain reliable and accurate information. Proper interpretation of the sample requires knowledge of the clinical presentation, collection site, and specimen-handling considerations. The presence of artifacts or contaminants may interfere with an appropriate interpretation unless the conditions surrounding the collection are known. Experience in interpretation of cytologic specimens from the species of interest and knowledge of the limitations of cytology or types of pathologic processes likely to be reflected in CSF are also important and can only be gained by diligent study of the literature and specimens over time.
Cerebrospinal fluid is formed primarily by the ultrafiltration and secretion through the choroid plexuses of the lateral, third, and fourth ventricles. Other sites that secrete CSF include the ependymal linings of the ventricles and blood vessels of the subarachnoid spaces and pia mater. Fluid then escapes from the fourth ventricle into the subarachnoid spaces and central canal of the spinal cord. It is then absorbed predominantly from the subarachnoid spaces via veins in the arachnoid and subarachnoid villi that project into subdural venous sinuses.
Collection of Cerebrospinal Fluid
Contraindications to CSF Collection
Cerebrospinal fluid collection is not indicated in cases in which a cause is obvious, such as known trauma or intoxication (Parent and Rand, 1994). Because anesthesia is required for collection of CSF in small animals, CSF collection is contraindicated in cases in which anesthesia is contraindicated (Carmichael, 1998; Cook and DeNicola, 1988).
Cerebrospinal fluid collection is contraindicated in cases with increased intracranial pressure. Increased intracranial pressure should be suspected with acute head trauma, active or decompensated hydrocephalus, anisocoria, papilledema, or cerebral edema. Expansile mass lesions and unstable CNS or systemic conditions may result in increased intracranial pressure or decreased pressure in the spinal compartment relative to the intracranial compartment. In these situations herniation of the brain may result in severe compromise of brain function, tetraplegia, stupor/coma, and/or death (Parent and Rand, 1994). Physical and neurologic examination, history, presentation, and results of imaging studies are of benefit in determining if these conditions are likely before the decision to collect CSF.
Even in cases with potential for brain herniation, the risks associated with CSF collection may be acceptable if the cause of patient deterioration is not apparent. Risk of herniation may be reduced by administration of dexamethasone (0.25 mg/kg IV) just before induction of anesthesia and by hyperventilation of the patient with oxygen during the procedure (Fenner, 2000). Except in cases in which dexamethasone is administered prophylactically because of suspected increased intracranial pressure, CSF collection should predate corticosteroid administration because of potential alteration of CSF composition (Rand, 1995).
Complications of CSF Collection
As with any medical procedure, the risks and benefits of CSF collection should be considered for individual cases. The potential exists for iatrogenic trauma to the spinal cord and/or brainstem from the collection needle, but is minimized by attention to anatomic landmarks and careful collection procedures (Carmichael, 1998; Parent and Rand, 1994). Risk of introduction of infectious agents into the CNS is minimized by adherence to the basic principles of aseptic technique and correct preparation of the site of collection (Cook and DeNicola, 1988).
Slight to moderate blood contamination is a common complication of collection associated with penetration of the dorsal vertebral sinuses or small vessels within the meninges; this may complicate interpretation of the fluid analyses and cytology, but has not been found to be harmful to the patient (Carmichael, 1998; Fenner, 2000).
Ketamine should not be used to anesthetize cats for CSF collection because it increases intracranial pressure and may induce seizures; gas anesthesia should be used (Parent and Rand, 1994).
If three unsuccessful attempts at CSF collection occur, abandonment of the procedure is recommended to decrease the probability of repeated penetration of the spinal cord, which may result in serious complications or death. Practice on cadavers before performing collection in live clinical cases has been recommended.
Equipment for CSF Collection
Clippers, scrub, and alcohol to surgically prepare the site of collection are needed. Sterile gloves should be worn during the procedure. A sterile disposable or resterilizable spinal needle with stylet is used. A 20- to 22-gauge, 1.5-inch needle with a polypropylene hub is recommended for most cases, although smaller needles may be needed in very small dogs and cats and longer needles may be needed in large dogs (Carmichael, 1998; Cook and DeNicola, 1988; Parent and Rand, 1994; Rand, 1995). Several needles should be available since replacement may be needed if the needle is inserted off the midline and enters a venous sinus (Cook and DeNicola, 1988).
Sterile plain tubes for collection of CSF are recommended. Some authors indicate that EDTA is not used because clotting is rare and EDTA may falsely elevate the protein concentration of CSF (Parent and Rand, 1994). However, others recommend addition of samples to EDTA if blood contamination is present or if elevated nucleated cell count, bacteria, elevated protein concentration, and/or the presence of fibrinogen are suspected because these may lead to clotting (Carmichael, 1998). If glucose determination is desired, it is recommended to collect CSF into fluoride/oxalate; this may not be necessary if CSF contains few erythrocytes and is analyzed rapidly.
Plastic containers are recommended because leukocytes may adhere to glass. Use of only a few plain, sterile containers makes collection easier and simpler and increases the probability of maximum yield of CSF.
Collection Volume
Carmichael (1998) indicates that approximately 1 mL of CSF per 5 kg of body weight can be collected safely. It may be dangerous to remove more than 1 mL of CSF per 30 seconds, more than 4 to 5 mL of CSF from the dog, more than 0.5 to 1 mL of CSF from the adult cat or more than 10 to 20 drops of CSF from the kitten. Rand (1990) indicates that 1.0 to 1.5 mL of CSF can usually be collected from the cat. The cat may be susceptible to meningeal hemorrhage if too much fluid is withdrawn.
Cerebellomedullary Cistern Collection
Collection at this site is indicated to classify lesions affecting the meninges of the head and neck when the clinical signs involve seizures, generalized incoordination, head tilt, or circling.
Preparation of the site should include clipping of the hair from the head and neck, from the anterior margin of the pinna to the level of the third cervical vertebra and laterally to the level of the lateral margins of the pinnae. This area should be scrubbed for a sterile procedure (Cellio, 2001).
The animal is positioned in lateral recumbency with the head and vertebral column positioned at an angle of approximately 90 degrees. Excessive flexion of the neck may result in elevation of intracranial pressure and increase the potential for brain herniation (Fenner, 2000) or may result in occlusion of the endotracheal tube (Carmichael, 1998). The nose should be held or propped so that its long axis is parallel to the table and it should not be allowed to rotate in either direction. The point of insertion is located on the midline approximately half way between the external occipital protuberance and the craniodorsal tip of the dorsal spine of C2 (axis) and just rostral to the anterior margins of the wings of C1 (atlas). The needle is inserted at the intersection of a line connecting the anterior borders of the wings of the atlas and a line drawn from the occipital crest to the dorsal border of the axis along the midline. Puncture of the skin first with an 18-gauge needle or a scalpel blade is helpful in overcoming skin resistance in thick-skinned animals. Alternatively, the skin can be pinched and lifted so that the needle can be safely pushed through the skin with a twisting motion.
The needle should be inserted with the bevel oriented cranially. It should be held perpendicular to the skin surface and gradually advanced with the stylet in place. Periodically the needle should be stabilized and the stylet withdrawn to determine if CSF is present. Occasionally, a sudden loss of resistance may be felt as the subarachnoid space is entered, but this may not be recognized in all cases. If the collector suspects that the needle has been inserted too deeply, the stylet may be removed and the needle slowly withdrawn a few millimeters at a time, watching for the appearance of fluid in the hub. If the needle hits bone during insertion, slight redirection of the needle cranially or caudally should be attempted to enter the atlanto-occipital space.
If opening pressure readings are taken, CSF fluid sample is taken by directing the flow of CSF through the manometer by way of a three-way stopcock. If pressure readings are not obtained, CSF may be collected directly from the spinal needle hub by dripping into a test tube or gentle aspiration of drops as they collect at the hub using a syringe. Attachment of a syringe to the needle with aspiration of CSF is not recommended because suction may result in contamination with blood or meningeal cells or obstruction of CSF flow by aspirated meningeal trabeculae. Careful aspiration is acknowledged to be necessary for collections in some cases. Passage of the needle through the spinal cord to underlying bone should be avoided at the cerebellomedullary cistern because it may cause damage to the cord and/or cause blood contamination of the CSF sample. On completion of collection of CSF, the needle is smoothly withdrawn. Replacement of the stylet is not necessary.
If the fluid appears bloody at the onset of collection, replacement of the stylet for 30 to 60 seconds may result in clearing of the blood. If the first few drops of CSF are still slightly bloody, they can be collected separately from the following drops that are often clear. If rate of flow of CSF is slow, the needle should be rotated slightly to make sure that it is clear at the luminal tip. If this not effective, rate of flow may be increased by compression of the jugular veins, resulting in expansion of the venous sinuses and increased CSF pressure.
Appearance of abundant fresh blood from the collection needle indicates that the point of the needle is most likely off the midline and in a lateral venous sinus. A new approach with a fresh, clean needle is recommended if the first attempt results in frank blood consistent with puncture of the venous sinus.
Lumbar Cistern Collection
Both cerebellomedullary and lumbar cistern specimens may be collected. Collection of a cerebellomedullary specimen is recommended prior to thoracolumbar myelography to ensure that a diagnostic CSF sample will be obtained because lumbar puncture alone may not be sufficient. The collection of CSF from the lumbar cistern is more difficult and more likely to be contaminated with blood than that from the cerebellomedullary cistern (Chrisman, 1992). Sometimes no fluid or only a very small amount of fluid can be obtained owing to the small size of the lumbar subarachnoid space. Lumbar puncture may be preferred in cases with localized spinal disease because it may be more likely to confirm abnormality than cerebellomedullary cistern collections (Thomson et al., 1990).
The dorsal midline is clipped and prepared between the midsacrum and L3, extending laterally to the wings of the ilium. The animal is placed in lateral recumbency and the back is flexed slightly to open the spaces between the dorsal laminae of the vertebrae. The L5-6 or L6-7 spaces are most commonly used in dogs because the subarachnoid space rarely extends to the lumbosacral junction. In cats collection can frequently be made from the lumbosacral space.
The dorsal spinous process of L7 lies between the wings of the ilia and is usually smaller than that of L6. To collect from the L5-6 intervertebral space, the needle is inserted just off the midline at the caudal aspect of the L6 dorsal spinous process and advanced at an angle cranioventrally and slightly medially to enter the spinal canal between the dorsal laminae of L5 and L6. Misdirection laterally into the paralumbar muscles or underestimation of the length of needle required might result in advancement of the needle to the hub without encountering bone.
Cerebrospinal fluid may be collected from the dorsal subarachnoid space, or the needle may be passed through the nervous structures to the floor of the spinal canal and CSF collected from the ventral subarachnoid space. The stylet is removed and the needle may be carefully withdrawn a few millimeters to allow for fluid flow. The rate of flow is usually slower than from the cerebellomedullary cistern. Rate of flow may be increased by jugular compression.
Cerebrospinal Fluid Opening Pressure
CSF pressure is measured with a standard spinal fluid manometer as the fluid is collected (Simpson and Reed, 1987). CSF opening pressure should be measured to confirm a supposed increase in intracranial pressure due to a space-occupying mass or cerebral edema. Its normal range is less than 170 mmH2O (Lipsitz et al., 1999) and 100 mmH2O, for the dog and cat, respectively (Chrisman, 1991).
Handling of Cerebrospinal Fluid Specimens
Cells lyse rapidly in the low-protein milieu of CSF, so cell counts and cytologic preparations of unfixed fluid should be done within 30 to 60 minutes of collection (Fry et al., 2006). The likelihood of misinterpretation due to sample deterioration depends on the initial protein concentration of the sample and how long analysis is delayed. For example, if the CSF protein concentration is >50 mg/dl, a delay in analysis of <12 hours is unlikely to alter final interpretation. Addition of an equal volume of 4% to 10% neutral buffered formalin or 50% to 90% alcohol is recommended for fixation of specimens that cannot be immediately delivered to a laboratory and processed immediately (Carmichael, 1998). Alternatively, the addition of one drop of 10% formalin to 1 to 2 mL of CSF may be used to preserve cells for cell counts and morphologic examination when submitted to a referral laboratory, keeping in mind that cell counts will be affected but may not be clinically significant. Refrigeration will help retard cellular degeneration. Cellular stability can be increased by addition of fresh, frozen, or thawed serum or plasma (Bienzle et al., 2000) or by addition of 20% albumin (Fenner, 2000). If a CSF sample is not analyzed within 1 hour from collection, Fry and co-workers (2006) recommend to divide the fluid into 2 aliquots, an unadulterated aliquot for total nucleated cell count and protein measurement and an aliquot added with 20% of fetal calf serum (or 10% autologous serum) for differential cell count and morphologic evaluation. For those samples of insufficient volume (<0.5 mL total), hetastarch (1:1) can be added to CSF for all routine assays. In the last situation, the dilutional effect of adding a stabilizing agent must be taken into account when calculating results. Protein and enzyme concentrations in CSF are relatively stable, and submission to the laboratory by routine delivery, postal delivery, or courier is usually sufficient for accurate determinations (Carmichael, 1998).
Laboratory Analysis of CSF
Usually at least 1 to 2 mL of CSF is available from dogs or cats. The analysis for cell counts requires approximately 0.5 mL (500 μl total or 250 μl for duplicate erythrocyte count and nucleated cell count, respectively). The volume required for chemical protein determination will vary, depending on the equipment and method used, but can be expected to be on the order of 200 to 250 μl for large, automated pieces of equipment. Taking these figures into account, approximately 0.25 to 1.25 mL of CSF should be available for cytologic evaluation and/or other tests.
Routine analysis of CSF is recommended in all cases in which it is collected; specialized analyses may be needed in selected cases. Routine analyses of CSF includes the following: macroscopic evaluation, quantitative analysis (erythrocyte count, nucleated cell count, and total protein), and microscopic evaluation as summarized in Table 14-1 . If the volume of CSF is small and all tests are not likely to be obtained, the clinician should rank the tests in order of preference when the specimen is submitted to the laboratory. Rand (1995) indicates that the most useful diagnostic tests, in decreasing order, are nucleated and erythrocyte counts, sedimentation cytology, protein concentration, and cytocentrifuge cytology.
TABLE 14-1.
Routine Evaluation of CSF
| Component of CSF Evaluation | Normal CSF | Abnormal CSF | Comments/Notes | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Macroscopic Evaluation | |||||||||||||||||
| Color | Colorless | Pink, red xanthochromic (yellow to yellow-orange). Occasional gray to green color may be seen. |
|
||||||||||||||
| Turbidity | Clear, turbidity absent | Turbid or cloudy—slight, moderate, or marked |
|
||||||||||||||
| Erythrocyte (RBC) count | Zero RBC considered normal, but frequently present in small numbers | Variable | Standard hemocytometer | ||||||||||||||
| Nucleated cell count |
|
Variable | Standard hemocytometer | ||||||||||||||
| Specific gravity | 1.004-1.006 | Most within reference interval for normal CSF | Of questionable value because only relatively marked increases in total protein result in changes that are detectable by specific gravity measurement | ||||||||||||||
| Total Protein (Microprotein) | |||||||||||||||||
| Quantitation | Most commonly cited reference intervals indicate usually <30 mg/dl (cerebellomedullary) or <45 mg/dl (lumbar cistern) | Increased total protein seen in a variety of conditions | Microprotein method and reference values may vary with laboratory; use laboratory-established reference values. | ||||||||||||||
| Estimation |
|
Most sensitive to albumin; detects ranges of protein that are useful for evaluation of most canine and feline CSF specimens; good correlation with standard dye-binding microprotein determinations. | |||||||||||||||
| Ames Multistix* (urine dipstick) | Trace to 1 + protein on urine dipstick is within normal limits | ||||||||||||||||
| Microscopic Evaluation | |||||||||||||||||
| Cell population | Lymphocytes and monocytoid cells predominate; very few mature, nondegenerated neutrophils may be present. A few erythrocytes may be seen. | Variable |
|
||||||||||||||
N-Multistix SG, Bayer, Miles, Diagnostic Division, Elkhart, IN.
Effect of Blood Contamination
Various formulas have been used to predict the effect of blood contamination on protein concentration and nucleated cell count in CSF (Parent and Rand, 1994; Rand et al, 1990). Rand (1995) indicates that red blood cell (RBC) counts greater than 30 cells/μl in CSF will have a profound effect on the total and differential cell counts. However, in a study (Hurtt and Smith, 1997) of iatrogenic blood contamination effects of total protein and nucleated cell counts in CSF, the RBC count was not significantly correlated with nucleated cell count or protein concentration in CSF from clinically normal dogs or those with neurologic disease. The study concluded that high CSF nucleated cell counts and protein concentrations are indicative of neurologic disease, even if samples contain up to 13,200 RBC/μl. Although blood contamination may make interpretation of CSF more difficult, red or pink CSF or CSF with a high RBC count should not be discarded as a useless specimen because cytologic evaluation may detect abnormalities (Chrisman, 1992).
Macroscopic Evaluation
Normal CSF is clear, colorless, and transparent and does not coagulate Deviations from normal should be recorded as part of the macroscopic evaluation and are often graded 1 to 4 + or as slight, moderate, or marked. Turbidity (Fig. 14-1A ) is reported to be detectable if greater than 500 cells/μl are present (Fenner, 2000) or if at least 200 leukocytes/μl or 700 erythrocytes/μl are present (Parent and Rand, 1994).
FIGURE 14-1.

A, Turbid CSF. Dog. There is marked turbidity or cloudiness as apparent against the background of newsprint. This animal had a steroid-responsive meningitis with a count of 760 nucleated cells/μl. Turbidity is reported to be detectable if greater than 200 leukocytes/μl are present. B, Xanthochromic CSF. Dog. The fluid has a yellow-orange discoloration and moderate turbidity from a dog with subarachnoidal hemorrhage of inflammatory origin.
Red to pink discoloration may be associated with iatrogenic contamination with blood or pathologic hemorrhage. Erythrophages or siderophages in a rapidly processed CSF specimen with fixative added immediately following collection support pathologic hemorrhage as an underlying cause. Xanthochromia is the yellow to yellow-orange discoloration (Fig. 14-1B) associated with pathologic hemorrhage due to trauma, vasculitis, severe inflammation, disc extrusion, or necrotic or erosive neoplasia. Occasionally xanthochromia will be seen with leptospirosis, cryptococcosis, toxoplasmosis, ischemic myelopathy, coagulopathy, or hyperbilirubinemia.
Quantitative Analysis
Cell Counts
Erythrocyte and nucleated cell counts are often done using standard hemocytometer techniques. In general, collections from normal animals from the cerebellomedullary cistern have slightly higher numbers of cells and slightly lower protein levels than those from the lumbar cistern.
To count nucleated cells, charge both chambers of the hemocytometer with undiluted CSF and place the unit in a humidified Petri dish for 15 minutes to allow cells to adhere to the glass. All nucleated cells are counted in the 10 large squares (four corner squares and one center square on each side) for a total nucleated cell count per microliter. Cell counts for erythrocytes are performed similarly. A study conducted to evaluate the usefulness of an automated cell counter in counting and differentiating cell types from canine CSF (Ruotsala et al., 2008) determined moderate correlation between this method and a hemocytometer for leukocyte values and excellent correlation for erythrocytes; however, cell differentials were much more variable. Results from this study also suggested that lymphocytes may be underestimated by manual microscopy in favor of large mononuclears.
Reference intervals for feline CSF erythrocyte counts are reported to range from 0 to 30 red blood cells per microliter (Parent and Rand, 1994). Reference intervals for feline CSF nucleated cell counts are reported to be less than 5 cells/μl, 0 to 2 cells/μl (Parent and Rand, 1994), less than 3 cells/μl (Chrisman, 1992), and less than 8 cells/μl (Cook and DeNicola, 1988).
Reference intervals for canine CSF erythrocyte counts are reported to be zero (Chrisman, 1992). Reference intervals for canine CSF nucleated cell counts are reported to be less than 5 cells/μl (Cook and DeNicola, 1988), and less than 6 cells/μl for cerebellomedullary cistern collections or less than 5 cells/μl for lumbar cistern collections (Chrisman, 1992).
Absence of elevation of nucleated cell counts in CSF does not preclude the need for cytomorphologic evaluation with a differential cell count or estimate because abnormalities in cell type or morphology may be present even when CSF nucleated cell counts are within normal limits.
Protein
Reference intervals for CSF total protein values may vary slightly with the laboratory and testing method used, but cerebellomedullary CSF protein is usually less than 25 to 30 mg/dl and lumbar cistern collections less than 45 mg/dl in dogs and cats (Chrisman, 1992; Fenner, 2000). Refractometer total protein evaluation is not accurate for assessment of CSF since the concentration of protein is quite low compared to serum or plasma and clinically significant changes may not be easily detectable on the refractometer scale. Special analytic techniques most often available at commercial or reference laboratories and not available in practice are needed owing to the minute protein concentration in CSF. Due to the minute amounts present, CSF protein analysis may be referred to as “microprotein.” An estimate of CSF protein content can be obtained using urine dipsticks. A membrane microconcentrator technique followed by agarose gel electrophoresis was recently described for measurement of cerebrospinal fluid proteins in dogs (Gama et al., 2007).
Increased CSF protein concentration may be caused by an alteration in the blood-brain barrier and leakage from plasma or increased local synthesis. Quantitative tests for detection of the components of CSF protein are covered under the heading of Other Tests. Differential diagnoses and examples of processes causing elevated CSF protein are covered under the heading of Protein Abnormalities in CSF.
Albumin accounts for 80% to 95% of the total protein in normal CSF. Qualitative tests to detect increased globulins in CSF are the Pandy and Nonne-Apelt tests. Use of these tests is limited because of the qualitative nature and absence of specificity regarding underlying cause. Normal CSF contains little if any globulin that can be detected by these methods.
Other Tests
Other tests that have been recommended by various authors or used in specific situations include electrophoretic determination of albumin and determination of total immunoglobulin levels. In combination with the serum albumin level and serum immunoglobulin, these can be used to calculate the albumin quotient (AQ) and immunoglobulin G (IgG) index. The AQ is equal to the CSF albumin divided by serum albumin times 100. AQ greater than 2.35 suggests an altered blood-brain barrier with increased protein in CSF associated with leakage from plasma. The IgG index is equal to the (CSF IgG/serum IgG) divided by (CSF albumin/serum albumin). An IgG index greater than 0.272 with a normal AQ suggests intrathecal production of IgG. An increased IgG index and increased AQ are suggestive of an altered blood-brain barrier as the source of IgG (Chrisman, 1992).
Alterations in electrophoretic protein fractions have been reported to be useful in identifying inflammatory, degenerative, and neoplastic disease in combination with clinical signs. In general, dogs with canine distemper often have elevated CSF gamma globulins, most likely related to intrathecal production, while dogs with granulomatous meningoencephalitis (GME) may have elevated CSF beta and gamma globulins (Chrisman, 1992).
In a more recent work, Behr and co-workers (2006) found, using high-resolution protein electrophoresis, a strong linear correlation between CSF total protein concentration and AQ suggesting that an increased CSF total protein concentration is an indicator of blood-brain barrier dysfunction; moreover, although unexpected, the same authors found that electrophoretic profiles in a series of 94 dogs with different neurologic diseases were not characteristic of any particular disease concluding that high-resolution electrophoresis of paired CSF and serum samples cannot be considered a valuable ancillary diagnostic tool for canine neurologic diseases.
Detection of specific antibodies within the CSF and comparison with serum levels may be useful in diagnosis of infectious meningoencephalitides, including infectious canine hepatitis, canine herpesvirus, canine parvovirus, canine parainfluenza virus, canine distemper virus, ehrlichiosis, Rocky Mountain spotted fever, Lyme disease (borreliosis), Toxoplasma gondii, Neospora caninum, Encephalitozoon cuniculi infection, Babesia spp. infection, cryptococcosis (Berthelin et al., 1994), and blastomycosis. On the contrary, measurement of anticoronavirus IgG in CSF of cats with confirmed feline infectious peritonitis involving the central nervous system is considered of equivocal clinical use (Boettcher et al., 2007). Serial titers for serum IgG to show rising titer are helpful to demonstrate active disease. The presence of IgM in serum or CSF is considered more specific than IgG or total immunoglobulin levels for detection of active disease (Chrisman, 1992).
Glucose measurement in CSF and comparison with serum or plasma glucose levels are frequently cited. Normal CSF glucose is approximately 60% to 80% of the serum or plasma concentration (Fenner, 2000). However, changes in CSF glucose concentration in serum or plasma are not immediately reflected in CSF and may take 1 to 3 hours before they are apparent in CSF (Cook and DeNicola, 1988). The ratio between blood glucose and CSF glucose is frequently reduced in bacterial infections of the CNS in humans and has been reported to occur in some cases of pyogenic infections of the CNS, CSF hemorrhage, or blood contamination that may result in increased utilization of glucose by cells. However, the relationship between bacterial encephalitis and decreased CSF glucose compared with serum or plasma glucose may depend on multiple factors, including the blood glucose level, degree of permeability of the blood-brain barrier, and presence or absence of glycolytic cells or microorganisms. Fenner (2000) states that the reduction in glucose does not occur in dogs. Significant reductions in CSF glucose concentrations have been reported in human malignant disorders involving the leptomeninges and it is considered a relatively specific finding for this condition (Chamberlain, 1995).
Measurement of various electrolytes and enzymes in CSF has been reported. Their interpretation may be limited because of increases associated with altered blood-brain barrier permeability, concurrent evaluation of serum values, low benefit for cost, and poor correlation or specificity for particular pathologic processes or conditions (Chrisman, 1992; Cook and DeNicola, 1988; Parent and Rand, 1994).
Aerobic and anaerobic bacterial cultures are recommended for all CSF samples with degenerated neutrophils or when bacteria are identified cytologically.
Nevertheless, the CSF culture rarely assist in the identification of microorganisms: in a series of 8 confirmed cases of canine bacterial meningoencephalomyelitis, the CSF culture was positive in only 1 sample and the cultured bacteria was Corynebacterium spp. (Radaelli and Platt, 2002). Several factors likely contribute to poor culture performance in veterinary medicine —e.g., small volumes of CSF are collected for culture, the organisms are mostly confined to the brain parenchyma, some organisms are slow growing or require nonstandard culture techniques, and animal has received antibiotic therapy before sampling (Fenner, 1998).
Staphylococcus, Streptococcus, Klebsiella, Escherichia coli and Pasteurella are aerobic bacteria that may cause CNS infection; Fusobacterium, Bacteroides, Peptostreptococcus, Clostridium, and Eubacterium are anaerobic species that have been reported.
The immunophenotype of cerebrospinal fluid mononuclear cells in the dog has been examined (Duque et al., 2002). According to authors, the main advantage of standardized immunophenotyping is having a more objective classification of mononuclear cells compared with the relative inconsistency of this classification when based solely on microscopic appearance.
Cytologic Evaluation of CSF
Cytologic evaluation of CSF is recommended for all collections as a valuable part of CSF evaluation. When necessary, clinicians are asked to rank tests in order of preference if less than 1-mL total volume is submitted. Usually, cell counts and total protein concentration are requested, followed by cytology. If additional CSF is available, other tests may be requested, depending on the differential diagnoses suggested by clinical signs, presentation, history, imaging studies, and results of cell counts, protein, and cytologic evaluation. Rand (1995) indicates that the most useful diagnostic tests in decreasing order are nucleated cell and erythrocyte counts, sedimentation cytology, protein concentration, and cytocentrifuge cytology.
Methods of Cytologic Preparation
Standardization of the volume used for cytologic evaluation may be of benefit in minimizing analytic variation and aid in interpretation, although evaluation of multiple preparations or preparations from larger volumes of CSF may increase the likelihood of detection of minor abnormalities. Because CSF is normally of low cellularity and increases in cellularity may not result in large numbers of cells, concentration of the cells is required. Cytocentrifugation, sedimentation, or membrane filtration techniques may be used for concentration of cells.
Cytocentrifugation is most commonly available in reference or commercial laboratories in which the volume of submissions justifies purchase of specialized equipment. The membrane filtration technique requires special staining that is not commonly available in practice, but which may be available at some reference or commercial laboratories. Several sedimentation techniques have been described and are suitable for use in practice or commercial or reference laboratories to which rapid submission of CSF specimens is possible (Cook and DeNicola, 1988; Parent and Rand, 1994). Readers are referred to these sources for more detail on construction of a sedimentation apparatus and preparation of sedimentation specimens. A sample device is demonstrated in Fig. 14-2A&B .
FIGURE 14-2.

A-D, In-house CSF sedimentation device. A, Unassembled sedimentation device with materials needed including 1 mL modified insulin syringe barrel, filter paper with hole punched, glass slide, two binder clips, and Eppendorf tube for CSF collection. B, Partially assembled sedimentation device. C, Assembled sedimentation device demonstrating the attachment of the binder clips to the barrel flanged portions. D, The tube made from the syringe barrel is filled with as little as 100 μl CSF by transfer pipette or as the figure demonstrates using a Butterfly needle. The added fluid is allowed to sit undisturbed for 1 hour. Cells concentrate and settle on to the exposed area of the glass slide.
Sedimentation preparations should be made if a specimen cannot be delivered immediately to the laboratory for cytologic processing. Prepared slides can then be sent to a commercial or reference laboratory for interpretation or stained and evaluated by clinicians at the practice.
Cytocentrifuge or sedimentation preparations are most commonly air-dried and stained with Romanowsky stains that are commonly available in commercial or reference laboratories and clinical practice laboratories. Membrane-filtration specimens require wet-fixation and stains appropriate for this method, commonly Papanicolaou, Trichrome, or H & E. Wet-fixation and these staining methods may also be used on cytocentrifuge or sedimentation preparations and are appropriate for formalin- or alcohol-fixed specimens. Cytocentrifuge or membrane-filtration preparatory and staining techniques may vary with laboratory, technical training, and pathologist preference. Summaries of cytopreparatory and staining techniques for cytospin and membrane filtration specimens and specimens fixed in formalin or alcohol are available from a variety of sources. Interested readers are referred to Keebler and Facik (2008) as a recent comprehensive review.
Special stains may be indicated in some cases. Gram stain may be useful for confirmation and identification of categories of bacteria. India ink or new methylene blue preparations have been reported to be helpful in identification of fungal infections, especially cryptococcosis. Periodic acid-Schiff stain may be used to demonstrate positive intracellular material in dogs with globoid cell leukodystrophy. Luxol fast blue can be used to demonstrate myelin in CSF specimens (Mesher et al., 1996).
Cytologic Features of CSF
Several reviews of differential diagnoses and features of normal and abnormal cytology of canine and feline CSF with photomicrographs are available (Baker and Lumsden, 2000; Desnoyers et al., 2008). Cytologic features that may be found in canine and feline CSF are summarized in Table 14-2 . Differential diagnoses associated with abnormal CSF findings are summarized in Table 14-3 .
TABLE 14-2.
Cytologic Features of Cerebrospinal Fluid in Dogs and Cats
| Cell or Feature | Description | Significance |
|---|---|---|
| Lymphocytes | Morphologically similar to those in peripheral blood; 9-15 μm in diameter, scant to moderate, pale basophilic cytoplasm with round to ovoid, slightly indented nucleus | Predominant cell type in normal CSF from healthy dogs; present in normal CSF from healthy cats |
| Reactive lymphocytes | Morphologically similar to those in peripheral blood; greater amount of cytoplasm and more deeply basophilic cytoplasm than normal lymphocytes; may see prominent perinuclear clear zones and coarse chromatin patterns | Not present in normal CSF from healthy animals, but not specific for underlying condition |
| Monocytoid cells | Large mononuclear cell; 12-15 μm diameter; moderate amount, pale basophilic, often finely foamy cytoplasm; nuclear shape variable to amoeboid; chromatin pattern open to lacy | Present in CSF from healthy animals in low numbers |
| Activated monocytoid cells | Morphologically resemble macrophages in many sites; larger than “normal” monocytoid cells (>12-15 μm diameter); increased amount of cytoplasm that is often paler than normal and possibly vacuolated; nuclei become round to oval and eccentric; chromatin with increased coarseness | Activation associated with irritation, inflammation, or degenerative processes; often phagocytic; reported in cats to be commonly associated with extensive necrosis |
| Neutrophils | Morphologically similar to those in peripheral blood; polymorphonuclear leukocytes | May be present in low numbers (up to 25% of total nucleated cells) in normal CSF from healthy animals |
| Ependymal lining cells | Uniform, round to cuboidal mononuclear cells; individual cells or in cohesive clusters; eccentric, round nuclei; uniformly granular to coarse chromatin; moderate amount of finely granular cytoplasm | May be present in normal CSF from healthy animals in low numbers; not consistently present in normal or abnormal conditions |
| Choroid plexus cells | Indistinguishable from ependymal lining cells (see above description) | May be present in normal CSF from healthy animals in low numbers; not consistently present in normal or abnormal conditions |
| Subarachnoid lining cells/leptomeningeal cells | Mononuclear cells with moderate to abundant pale basophilic cytoplasm; round to oval eccentric nuclei; uniform, delicate chromatin pattern; indistinct cytoplasm margins; single or in small clusters | May be present in normal CSF from healthy animals in low numbers; not consistently present in normal or abnormal conditions |
| Hematopoietic cells | Morphologically similar to those in bone marrow or other locations | Myeloid and erythroid precursors and erythroblastic island reported as contaminants of canine CSF with lumbar collections |
| Eosinophils | Morphologically similar to those in peripheral blood; polymorphonuclear leukocytes with eosinophilic granules with shape characteristic for species | Occasionally cells seen in normal CSF from healthy dogs or cats; may be seen as a nonspecific part of an active inflammatory response; also consider parasitic, hypersensitivity, or neoplastic processes (primary or metastatic) |
| Plasma cells | Morphologically similar to those in other locations; eccentric nuclei with prominent chromatin (“clockface” pattern); moderately abundant cytoplasm, moderately to deeply basophilic with perinuclear clear zone (Golgi apparatus) | Not present in normal CSF from healthy dogs or cats; may be part of nonspecific reactive or inflammatory process with response to antigenic stimulation |
| Bacteria | Morphology varies with type, may include cocci, rods of various sizes, coccobacilli, or filamentous forms | Not present in normal CSF from healthy dogs or cats; may be contaminants if collection process or tube are not sterile or if CSF collected close to death; pathologic role likely if suppurative meningitis is present and supported by intracellular location |
| Neural tissue | Nerve cells morphologically similar to those in nervous tissue; very large cell with prominent nucleolus, abundant cytoplasm, and three to four tentacle-like cytoplasmic processes; neuropil/myelin represented by amorphous, acellular background material | Reported as contaminant in canine CSF associated with accidental puncture of spinal cord; myelin fragments may be associated with demyelination |
| Paracellular coiled “ribbons” | Coiled, homogeneous, basophilic material within phagocytic vacuoles | Reported in CSF obtained at postmortem; hypothesized to represent denatured myelin, myelin figures, or myelin fragments |
| Neoplastic cells | Abnormal cell type or number for location (benign tumors) or atypical features fulfilling criteria for malignancy (malignant tumors); morphology may vary with cell type of origin and degree of differentiation | May be primary or metastatic; presence requires communication with subarachnoid space or ventricles; absence of tumor does not rule out its presence without contribution of cells to the CSF |
| Fungi/Yeast/Protozoa | Appearance varies with type; may be primary or opportunistic infections | Characteristic morphology associated with various common pathologic organisms; demonstration of organisms in conjunction with clinical signs and results of other testing increases confidence in diagnosis of fungal or protozoal disease |
| Mitotic Figures | Recognized by characteristic nuclear configurations of cells undergoing mitosis; cell type of origin not identifiable during the mitotic cycle | Rare mitotic figures reported in normal CSF from healthy animals; presence indicates proliferative process, often neoplasia |
TABLE 14-3.
Differential Diagnoses Associated with Cytologic Features of Inflammation in the CSF
| Cytologic Features | Special Considerations or Differential Diagnoses | Comments |
|---|---|---|
|
|
|
| Marked neutrophilic inflammation (suppurative meningitis) | Bacterial infection | May be focal (abscess) or diffuse (meningoencephalomyelitis); intracellular bacterial confirms diagnosis |
| Predominance of neutrophils (>50%), often with increased CSF protein |
|
|
| Mixed cell inflammation with a variety of cell types (no single cell type predominant) | Often interpreted to represent granulomatous inflammation—consider fungal, protozoal, parasitic, or rickettsial infection | Presence of fungal or protozoal organisms is confirmatory |
| Mixture of macrophages, lymphocytes, neutrophils, and sometimes plasma cells, with or without elevated CSF protein, with or without pleocytosis | Some idiopathic inflammatory or degenerative diseases Inadequately treated chronic bacterial infections or early response to antibacterial treatment |
|
| Nonsuppurative inflammation (mononuclear pleocytosis) | Viral, bacterial, fungal, protozoal, parasitic, or rickettsial infection | Especially non-FIP viral meningoencephalomyelitis in cats and canine distemper infection in dogs |
| Pleocytosis with predominance of mononuclear cells, especially lymphocytes |
|
|
| Eosinophilic inflammation | Parasitic, protozoal, bacterial, viral, or rickettsial infections | Uncommon manifestation reported with a variety of types of disease |
| Pleocytosis with predominance of eosinophils |
|
|
Cytologic Features of Normal CSF
Normal CSF from healthy dogs and cats contains primarily mononuclear cells (Fig. 14-3 ) and is indicated to be a mixture of lymphocytes and large mononuclear (monocytoid) cells. The percentages of lymphocytes and monocytoid cells may vary with the method used for cytologic preparations, but lymphocytes are reported to be the predominant nucleated cell type in normal canine and feline CSF. However, Parent and Rand (1994) report monocytoid cells as the predominant type in normal CSF from healthy cats. They indicate monocytoid cells compose 69% to 100% of the nucleated cells, lymphocytes 0% to 27%, neutrophils 0% to 9% macrophages 0% to 3%, and eosinophils 0% to less than 1% of nucleated cells. Occasional neutrophils or eosinophils as within normal limits may be present as long as these cell types do not represent more than 10% or 1% of the nucleated cells, respectively. Low numbers of mature, nondegenerate neutrophils are occasionally seen in normal CSF from healthy dogs and cats and that rare eosinophils may be present. Occasional choroid plexus cells, ependymal cells, meningeal lining cells, or mitotic figures may be seen in normal CSF from dogs or cats (Chrisman, 1992; Rand, 1995).
FIGURE 14-3.

Cell types found in CSF. Dog. Two small mononuclear cells (lymphocytes), one large mononuclear (monocytoid) at (arrow), one nondegenerate neutrophil, and one erythrocyte are present. (Wright-Giemsa; HP oil.)
Accidental Puncture Contaminants
Christopher (1992) described bone marrow elements as contaminants in canine CSF associated with bone marrow aspiration during lumbar cistern collections of CSF. These may not have been from bone marrow aspiration but possibly from a site of extramedullary hematopoiesis as was discovered in five dogs with hematopoietic elements within the interstitium of the choroid plexus at the level of the 4th ventricle (Bienzle et al., 1995). Myelin-like material, neurons (Fig. 14-4A ), and neuropil (Fig. 14-4B) have been reported as contaminants of canine CSF associated with accidental puncture of the spinal cord during cerebellomedullary cistern collection (Fallin et al., 1996) because of the absence of significant neurologic deficits in the patient.
FIGURE 14-4.

A, Neuron. CSF. Dog. Accidental puncture of nervous tissue during collection at the cerebellomedullary cistern demonstrating the large size of the neuron compared with a neutrophil and erythrocytes. Basophilic granular material within the neuronal cell cytoplasm is presumed to be Nissl bodies. (Wright-Giemsa; HP oil.) B, Nervous tissue with microglial cells. Dog. CSF from the same case of a dog with cervical pain as in A. (Wright-Giemsa; HP oil.)
(A, From Fallin CW, Raskin RE, Harvey JW: Cytologic identification of neural tissue in the cerebrospinal fluid of two dogs, Vet Clin Pathol 25:127-29, 1996.)
© 2010
CSF Presentation and Interpretation
Normal CSF Findings in the Presence of Disease
No abnormalities may be detected in CSF in many cases of neurologic disease, although some animals with the same conditions may have abnormalities detected in CSF. CSF abnormality is not detected in the majority of cases of idiopathic epilepsy, congenital hydrocephalus, intoxication, metabolic or functional disorders, vertebral disease, or myelomalacia. A significant proportion of cases with neurologic disease due to feline infectious peritonitis, distemper encephalitis, neoplasia, or GME may have CSF that is within normal limits. In a series of 17 dogs with neurologic symptoms due to spinal arachnoid cysts, CSF analysis was unremarkable (Skeen et al., 2003). Absence of cytologic abnormality does not rule out the possibility of neurologic disease not reflected in the CSF.
Protein Abnormalities in CSF
Elevated total protein may occur in the absence of cytologic abnormalities and may be referred to as “albuminocytologic dissociation.” Elevated total protein as the sole abnormality or in combination with increases in nucleated cell count and/or cytologic abnormality in CSF may occur with inflammatory, degenerative, compressive, or neoplastic disease (Carmichael, 1998). Elevated protein may occur in association with increased permeability of the blood-brain barrier, local necrosis, interruption of normal CSF flow and absorption, or intrathecal globulin production (Chrisman, 1992). Elevated CSF protein without increases in CSF nucleated cell count has been reported with viral nonsuppurative encephalomyelitis, or with neoplasia, acute spinal cord injury, and compressive spinal cord lesions. In the cat, CSF protein concentration may provide some help in categorizing disease groups as markedly elevated protein concentrations should increase the index of suspicion only for feline infectious peritonitis (Singh et al., 2005). In a series of 56 cases of canine intracranial meningiomas, increased total protein concentration, in the presence of a normal total nucleated cell count, was detected in 16 (30%) dogs (Dickinson et al., 2006). Elevated total protein without pleocytosis may occur with neoplasia, ischemic myelopathy, postseizure activity, fever, disc extrusion, degenerative myelopathy (Clemmons, 1991), myelomalacia, or GME.
Increased Cell Type Percentages without Increased Total Nucleated Cell Counts
Increased percentages of either neutrophils or eosinophils may occur without an increase in the total white cell count in a variety of neurologic disorders. If blood contamination is ruled out, increased neutrophil percentages greater than 10% to 20% and eosinophil percentages greater than 1% should be considered unusual. Increased neutrophils may indicate mild or early inflammation, a lesion that does not contact the meninges or ependymal cells, or previous use of drugs such as glucocorticoids and antibiotics, which reduce the inflammatory response. Conditions to consider include degenerative intervertebral disc disease, spinal fractures, or cerebrovascular disorders such as infarcts. Increased eosinophils without increased total white blood cell (WBC) count may occur with parasite migration or protozoal disease (Desnoyers et al., 2008).
Pleocytosis
Increases in the total nucleated cell count of the CSF is termed pleocytosis, which is further defined by the predominant cell type, that is, neutrophilic, eosinophilic, mononuclear, or mixed cell pleocytosis. Pleocytosis is graded as mild (6 to 50 cells/μl in dogs and cats), moderate (51 to 200 cells/μl and 51 to 1000 cells/μl, in dogs and cats, respectively), or marked (>200 cells/μl and >1000 cells/μl, in dogs and cats, respectively) (Chrisman, 1992; Singh, 2005).
Neutrophilic Pleocytosis
Neutrophilic pleocytosis has been associated with a wide variety of active inflammatory disorders, including trauma, postmyelographic aseptic meningitis, fibrocartilagineus embolic myelopathy, myelomalacia, hemorrhage, neoplasia, and mycotic and bacterial meningitis (Mariani et al., 2002; Mikszewski et al., 2006). It may be seen with abscesses communicating with the ventricles or subarachnoid space, early viral infections, feline infectious peritonitis, Rocky Mountain spotted fever, discospondylitis, acquired hydrocephalus, necrosis, or GME. Marked neutrophilic pleocytosis is most often found with bacterial or fungal meningoencephalitis, neoplasia (Fig. 14-5 ), steroid-responsive meningitis, or necrotizing vasculitis (Chrisman, 1992). Demonstration of bacteria, fungi, yeast, or protozoa in CSF can confirm the presence of these infections. A variety of bacterial types—Cryptococcus, Blastomyces, Histoplasma, Neospora caninum, and ehrlichial organisms—have been demonstrated in CSF (Gaitero et al., 2006; Singh et al., 2005). Parasites such as Toxocara canis, Dirofilaria immitis, Cuterebra larva, or Cysticercus that may cause neurologic disease have not been reported to be seen in CSF cytology preparations. The presence of marked neutrophilic pleocytosis or increasing numbers of neutrophils in sequential CSF collections has been reported to be an unfavorable prognostic finding. Neoplasia should be considered as the most likely diagnosis in a cat more than 7 years of age with progressive clinical neurologic signs of greater than 4 weeks' duration (Rand et al., 1994).
FIGURE 14-5.

Neutrophilic pleocytosis. CSF. Dog. Nucleated cell count was 1018/μl and 240 mg/dl protein with a history of head tilt and hemiplegia related to a cranial meningioma. Nondegenerate neutrophils composed 83% of the cell population. (Wright-Giemsa; HP oil.)
Feline infectious peritonitis (FIP), a coronavirus infection, is a common cause of neutrophilic pleocytosis in the cat (Fig. 14-6A&B ). The main neurologic signs are depression, tetraparesis, head tilt, nystagmus, and intention tremor (Baroni and Heinold, 1995). It accounted for 44% of 61 feline cases of inflammatory CNS disease (Rand et al., 1994). Parent and Rand (1994) indicate that marked neutrophilic pleocytosis with a nucleated count of more than 100 cells/μl and neutrophils greater than 50% is commonly seen with FIP, along with increased CSF protein (usually greater than 200 mg/dl). They indicate a high probability of FIP if a cat is less than 4 years of age and shows multifocal neurologic signs referable to the cerebellum and/or brainstem, protracted course of illness, and CSF protein greater than 200 mg/dl. Later in the course of the disease, a mixed cellular population may be found with large mononuclear cells and lymphocytes present to a significant degree (Fig. 14-7A&B ). Similar results were found by Singh and others (2005) in a series of 11 cats with FIP. CSF analysis in that study was characterized as suppurative in seven cats, mixed in one, and mononuclear in three. Five cats had marked elevations in the CSF white cells count (>1000 cells/μl), three cats had moderate elevations (51 to 1000 calls/μl), two cats had mild elevations (6 to 50 cells/μl), and one cat had insufficient sample for a white cells count.
FIGURE 14-6.

Neutrophilic pleocytosis. CSF. Cat. Same case A-B. A, This direct smear is made from fluid from a kitten with 5-day duration of ataxia. A high nucleated cell count supported use of a direct smear to evaluate leukocytes. The case was diagnosed as FIP by positive titer and histologic examination. Numerous erythrocytes and several nondegenerate neutrophils characterize the cells present. Acute hemorrhage was evident but is not demonstrated in this field. (Wright, HP oil) B, Section of midbrain and third ventricle demonstrating multifocal perivascular infiltrates in a cat with FIP. The proximity of the infiltrates to the ventricle contributed to the neutrophilic pleocytosis. (H & E; LP.)
FIGURE 14-7.

Mixed cell pleocytosis with neutrophilic predominance. CSF. Cat. A, Increased numbers of large mononuclear cells consistent with macrophages were present in a cat with fever, high titers for FIP, and histopathologic support of FIP at necropsy. Duration of disease was several months, accounting for the more mononuclear response than the case in Fig. 14-6. (Wright-Giemsa; HP oil.) B, Chronicity of infection with FIP is suggested by the presence of plasma cells indicated by the Mott cell (center). Nondegenerate cells and erythrocytes are also seen. Plasma cells are not seen in the CSF of healthy animals but rather in viral infections and tumors. (MGG; HP oil.)
(A, Courtesy of Rick Alleman, University of Florida.)
Only 11 of 19 cats in one study (Baroni and Heinold, 1995) demonstrated high serum antibody titers, indicating that CSF analysis was essential for a correct diagnosis. Non-FIP viral meningoencephalitis that involved 37% of the inflammatory cases reported by Rand et al. (1994) was considered most likely with cats less than 3 years of age having progressive neurologic disease and focal neurologic signs referable to the thalamocortex. In these cases, the nucleated cell count was less than 50 cells/μl and CSF protein was less than 100 mg/dl. Non-FIP viral meningoencephalitis usually carries a favorable prognosis for recovery.
Steroid-responsive suppurative meningitis-arteritis (Fig. 14-8 ) has been recognized in young to middle-aged dogs that present with signs of fever, cervical pain, hyperesthesia, and paresis. CSF pleocytosis is often greater than 500 cells/μl with greater than 75% nondegenerate neutrophils if glucocorticoids have not been recently administered (Chrisman, 1992). Bacteria are not observed or cultured and improvement is often seen within 72 hours following glucocorticoid administration; long-term prognosis is good. One report investigated the immunologic response in these cases finding IgG and IgA synthesis intrathecally and suggested the humoral response is primary, rather than the result of a generalized immune complex disease (Tipold et al., 1995). In another more recent paper Behr and Cauzinille (2006) evaluated clinical findings and prognosis in a series of 12 cases of aseptic suppurative meningitis in juvenile Boxers of which 10 of the dogs exhibited the acute form of the disease and had more than 100 nucleated cells/μl with neutrophils ranging from 72% to 100%. The two other dogs presented with a more chronic form that produced a mixed pleocytosis, with neutrophils about 60% of total nucleated cells. An abnormal cell count of mixed cell population or mononuclear cells in the CSF are seen in the protracted form, and monitoring of CSF cell count in dogs with this condition seems to be a sensitive indicator of success of treatment (Cizinauskas et al., 2000).
FIGURE 14-8.

Neutrophilic pleocytosis. CSF. Dog. Generalized nonseptic inflammatory response in a 1-year-old dog exhibiting fever and cervical, thoracic, and lumbar pain. Nucleated cell count was 106/μl with 41 mg/dl protein and 3700 RBC/μl. Three nondegenerate neutrophils, one large mononuclear cell, and one lymphocyte are present. Multiple joints were similarly affected in this case. An immune-mediated corticosteroid-responsive meningitis was suspected. (Wright-Giemsa; HP oil.)
Necrotizing vasculitis, a syndrome of aseptic suppurative meningitis in young Bernese Mountain Dogs involving the leptomeningeal arteries, has been described. Animals presented with severe cervical pain and neurologic deficits. Total WBC counts are generally greater than 1000 cells/μl, with nondegenerate neutrophils predominating. A similar condition has been reported in Beagles and sporadic cases have been described in a variety of other breeds (Caswell and Nykamp, 2003). Clinical improvement occurred with corticosteroid administration.
Bacterial meningoencephalitis (Fig. 14-9A&B ) is suspected if greater than 75% neutrophils are present in the CSF regardless of the total cell count. Bacteremia is usually the cause with septic emboli to the brain as a result. Untreated cases often produce marked pleocytosis with greater than 1000 cells/μl. Intracellular location of bacteria and accompanying inflammation are particularly important in eliminating the possibility of bacterial contamination associated with nonsterile collection technique or nonsterile collection tubes. Neutrophils affected may display mild to severe karyolysis.
FIGURE 14-9.

A, Neutrophilic pleocytosis. CSF. Cat. Direct smear of cloudy CSF indicated increased cellularity with many degenerate neutrophils present. Associated with the karyolytic neutrophils shown are intracellular, small rod-shaped bacteria that were cultured as Enterobacter sp. (Wright; HP oil.) B, Septic meningoencephalitis. CSF. Bacterial infection. Cat. Several rod bacteria are present with the cytoplasm of the neutrophil. Several erythrocytes surround the inflammatory cell. (MGG; HP oil.)
Identification of bacteria on CSF cytology is even rarer than positive bacterial culture. Bacteria were identified by using CSF cytology in 62 of 109 (57%) adult humans, 0 of 14 (0%) dogs, and 2 of 5 (40%) cats with confirmed bacterial CNS infection (Messer et al., 2006). The difficulty of identifying bacteria in CSF often forces the clinician to rely on inflammatory changes in the CSF to make a presumptive diagnosis of bacterial CSF infection.
Eosinophilic Pleocytosis
Cerebrospinal fluid pleocytosis that is predominantly eosinophilic is rare. Increased eosinophils in CSF may be present in association with a nonspecific acute inflammatory response, but also can be seen with parasitic, hypersensitivity, neoplastic processes, protozoal infection including toxoplasmosis (Fig. 14-10A&B ) and neosporosis, or Cryptococcus infection. Steroidresponsive meningoencephalitis with a predominance of eosinophils (Fig. 14-10C) has been described in dogs and cats (Chrisman, 1992). Sometimes migrating internal parasites, Prototheca infection, canine distemper virus infection, or rabies may cause eosinophilic pleocytosis (Chrisman, 1992).
FIGURE 14-10.

Eosinophilic pleocytosis. A, Cisternal CSF. Dog. This acutely paraparetic animal with upper motor neuron dysfunction to the rear legs was diagnosed as having toxoplasmosis by serum titer. Total WBC count was 124/μl with high normal protein. Eosinophils accounted for 98% of the cell population. Peripheral eosinophilia was not evident. (Wright-Giemsa; HP oil.) B, Cisternal CSF. Cat. Note the predominance of typical bi-lobate eosinophils in the cerebrospinal fluid from this case of toxoplasmosis confirmed by PCR of CSF. (MGG; HP oil.) C, CSF. Dog. The nucleated cell count was 125/μl and eosinophils represented 85% of the nucleated cells. Several nondegenerate neutrophils and a large, foamy macrophage are also present. The final diagnosis was eosinophilic steroid-responsive meningoencephalitis. (MGG; HP oil.)
Steroid-responsive eosinophilic meningitis has been reported in dogs and cats. Finding greater than 80% eosinophils with mild to marked pleocytosis present and finding no evidence of protozoal, parasitic, or fungal infection usually supports the diagnosis. In the canine study, Golden Retrievers were overrepresented, which may suggest a breed predisposition to this condition (Fig. 14-11 ). Animals usually respond to glucocorticoid therapy with dramatic decreases in cell numbers and changes in differential percentages. An allergic or type I hypersensitivity reaction is suspected in some cases.
FIGURE 14-11.

Eosinophilic pleocytosis. CSF. Dog. This sample is from a Golden Retriever whose CSF cell count was 43/μl and whose protein was 77 mg/dl. The cell differential indicated 43% eosinophils, 50% lymphocytes, and 7% large mononuclear phagocytes. Three eosinophils and two small lymphocytes are shown. An idiopathic eosinophilic meningoencephalitis associated with this breed was suspected. (Wright-Giemsa; HP oil.)
Mononuclear Pleocytosis
Mononuclear pleocytosis of CSF usually presents with increased lymphocytes in viral, protozoal, or fungal infection, uremia, intoxication, vaccine reaction, GME, and discospondylitis. It may be seen with necrotizing encephalitis, steroid-responsive meningoencephalomyelitis, ehrlichiosis, or treated bacterial meningoencephalitis. However, monocytoid/macrophage cells may also predominate in these conditions and most commonly with cryptococcosis (Figs. 14-12 and 14-13 ). Mononuclear pleocytosis was noted in two cats with cuterebriasis (Glass et al., 1998) and in another cat with cerebral cholesterol granuloma (Fluhemann et al., 2006). The appearance of CSF macrophages containing vacuoles and pink-purple amorphous granular material in a young cat with mononuclear pleocytosis, elevated protein content, seizures, incoordination, and tremors indicated the presence of a lysosomal storage disease (GM2-gangliosidosis) (Johnsrude et al., 1996). A recent report of necrotizing meningoencephalitis in a young miniature Poodle demonstrated the predominance and pleocytosis of large granular lymphocytes (Garma-Aviña and Tyler, 1999). The most frequent noninflammatory neurologic diseases of the CNS in the cat are neoplasia and ischemic encephalopathy, which usually present with an elevated CSF protein and slight lymphocytic pleocytosis or normal nucleated cell counts (Rand et al., 1994). Hemorrhagic conditions may be accompanied by a mononuclear pleocytosis composed of foamy macrophages (Fig. 14-14A&B ). Mononuclear cells may take on changes that are nonspecific
FIGURE 14-12.

Cryptococcosis with mononuclear pleocytosis. CSF. Dog. Clusters of basophilic-staining extracellular yeast forms measuring approximately 10 to 20 μm in diameter are present. Three yeast forms are indicated by arrows. The fluid contained a total nucleated cell count of 60/μl of which 85% were mononuclear phagocytes. Several mononuclear cells are pictured that have abundant foamy to vacuolated pale cytoplasm indicating reactivity. (Wright-Giemsa; HP oil.)
FIGURE 14-13.

Cryptococcosis. CSF. Dog. These spherical organisms display frequent budding. (New methylene blue; HP oil.)
(Courtesy of Rick Alleman, University of Florida.)
FIGURE 14-14.

Acute hemorrhage with mononuclear pleocytosis. CSF. Dog. A, This animal had a history of seizures and dementia. Nucleated cell count was 190/μl and protein 72 mg/dl. Mononuclear phagocytes accounted for 91% of the cell population. (Wright-Giemsa, HP oil) B, Same case as A. Several vacuolated, phagocytic macrophages with engulfed erythrocytes (arrows) are shown. (Wright-Giemsa; HP oil.)
Necrotizing encephalitis in small breed dogs (Figs. 14-17 and 14-18A-D )—Pugs, Maltese, Shih Tzus, French Bulldogs, and Yorkshire Terriers—is reported to demonstrate multifocal to massive necrosis and nonsuppurative inflammation of the cerebrum and meninges that is fatal or leads to euthanasia (Uchida et al., 1999; Stalis et al., 1995; Timmann et al., 2007; Tipold et al., 1993). These dogs are usually less than 4 years of age and present frequently with seizures, depression, and ataxia; they do not respond significantly to glucocorticoids. The CSF presents with mild to moderate pleocytosis, generally greater than 200 cells/μl, and these are predominantly lymphocytes, generally greater than 70%. CSF protein concentration is often greater than 50 mg/dl. The cause is considered unknown but some Pugs appear to exhibit an autoantibody against astrocytes that has been detected in the CSF by indirect immunofluorescence assay, confirming an immune-mediated syndrome. A similar population of cells may be found in the CSF in GME necessitating a histologic examination of the brain to detect the necrotizing lesions.
FIGURE 14-15.
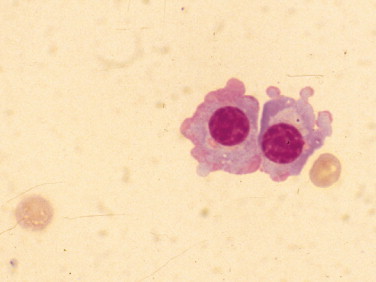
Flaming plasma cells. CSF. Dog. High-normal nucleated cell count and increased protein (361 mg/dl) were present in a suspected case of granulomatous meningoencephalomyelitis. The term “flaming” is used to describe the red-pink periphery of the cytoplasm. (Wright-Giemsa; HP oil.)
FIGURE 14-16.

Granular large mononuclear phagocytes. CSF. Dog. Highly granulated and phagocytic-appearing cells in a case of suspected granulomatous meningoencephalomyelitis. (Wright-Giemsa; HP oil.)
(Courtesy of Rick Alleman, University of Florida.)
FIGURE 14-17.

Lymphocytic pleocytosis. CSF. Dog. This example of Pug encephalitis is characterized by pleocytosis (265 cells/μl) with lymphocytic predominance (87%). Lymphocytes shown are small to medium size with normal morphology. (Wright-Giemsa; HP oil.)
FIGURE 14-18.

Lymphocytic pleocytosis. CSF. Dog. Same case A-D. A, This 6-year-old Maltese presented with acute seizures that were unresponsive to glucocorticoids and anticonvulsants. Fluid indicated total nucleated cell count of 430/μl and 3+ protein on chemistry dipstick. Lymphocytes accounted for 82%, large mononuclear cells 11%, and nondegenerate neutrophils 7% of the cell population. Shown are many lymphocytes, one of which is a granular lymphocyte (arrow) and three large mononuclear cells demonstrating various nuclear shapes and cytoplasmic features. (Wright-Giemsa; HP oil.) B, Mononuclear pleocytosis is evident in this field with two large mononuclear cells, one of which displays marked cytoplasmic vacuolization consistent with demyelination. One granular lymphocyte and one erythrocyte are also present. (Wright-Giemsa; HP oil.) C, Maltese with nonsuppurative necrotizing meningoencephalitis. Dense accumulations of mononuclear cells along the meninges extend into the parenchyma. There is gliosis and neuronal necrosis evident in the parenchyma. (H&E; LP.) D, Severe, focally extensive, perivascular meningoencephalitis. Cells present consist mostly of lymphocytes and plasma cells, with smaller numbers of large mononuclear phagocytes. (H&E; HP oil.)
Granulomatous meningoencephalomyelitis (GME) (Fig. 14-19 ) is an idiopathic inflammatory disease of the CNS in primarily young to middle-aged female dogs (Sorjonen, 1990). A study of 42 dogs found a high percentage of affected animals were toy or terrier breeds (Munana and Luttgen, 1998). Clinical signs of fever, ataxia, tetraparesis, cervical hyperesthesia, and seizures have been reported. Designation of the clinical signs into focal or multifocal was helpful in determining prognosis, with dogs having focal clinical signs surviving longer. Lesions are histologically found in both white and gray matter of the brain and predominantly the white matter of the caudal brainstem and spinal cord. The CSF may have a mild to moderate lymphocytic, mixed cell pleocytosis, or occasionally neutrophilic predominance (Chrisman, 1992). Nucleated cell counts had a median of 250 cells/μl (range 0 to 11,840) with the majority having counts greater than 100 cells/μl (Munana and Luttgen, 1998). In this same study, dogs with multifocal signs all had pleocytosis, whereas some of the dogs with focal signs had normal cell counts. The predominant cell type was lymphocytic (52%), monocytic (21%), neutrophilic (10%), and mixed cell (17%). CSF protein is variably elevated, with a mean value of 256 mg/dl (range 13 to 1119) as reported by Bailey and Higgins (1986). Differentiation must be made from infectious diseases and idiopathic necrotizing encephalitis, all of which may appear similar cytologically. Electrophoretic separation of CSF proteins in GME has shown increases in the alpha and beta globulin fractions (Sorjonen, 1990); however, these fractions are generally decreased in canine distemper (Chrisman, 1992). Both GME and canine distemper may have increased gamma globulins. GME lesions involve widely disseminated perivascular, lymphocytic-granulomatous meningeal, and parenchymal infiltrates. Necrosis and demyelination are major features in necrotizing encephalitis and may be present to a minor extent in GME. GME cases with lesions that involve the caudal brainstem or spinal cord progress slowly, permitting longer survival. Radiation has been recommended as an adjunct to treatment, especially in dogs with focal clinical signs. The disease is poorly responsive to glucocorticoids, although an immune-mediated etiology has been suggested (Kipar et al., 1998). In this study, it was determined that the GME inflammatory lesions are composed of predominantly CD3 antigen–positive T-lymphocytes and a heterogeneous population of activated macrophages with MHC class II expression suggesting a T-cell–mediated delayed-type hypersensitivity of an organ-specific autoimmune disease.
FIGURE 14-19.

Mixed cell pleocytosis. CSF. Dog. This young dog presented with neck pain. Shown are numerous small and medium-sized lymphocytes (70%), several nondegenerate neutrophils (18%), and fewer numbers of large mononuclear cells (12%), one of which demonstrates large cytoplasmic vacuoles. Total WBC count was 208/μl and protein increased to 256 mg/dl. The dog died 5 days later and histopathology indicated an idiopathic condition with moderate to marked, multifocal, nonsuppurative meningoencephalitis and mild, multifocal vacuolization and neuronal necrosis. (Wright-Giemsa; HP oil.)
Canine viral infections such as canine distemper infection (Fig. 14-20A&B ) and rabies infection (Fig. 14-21 ) each present with CSF that exhibits a lymphocytic pleocytosis. Cell counts may be variable, ranging from normal to greater than 50 cells/μl, and lymphocytes represent the predominant cell population, accounting for greater than 60% of the cells present. One report (Abate et al, 1998) indicated that the CSF in distemper cases had an increase in macrophages, an increase in total protein concentration, an increase of the gamma-globulin fraction by electrophoretic separation, and the presence of cellular inclusions. Another report from Amude et al (2006) describes a marked CSF pleocytosis (554 cells/μl) with protein content within normal limits in a 7-month-old dog with PCR-confirmed viral distemper. In this case, the nucleated cell differential count was 70% lymphocytes, 25% neutrophils, and 5% monocytes. Diagnosis of canine distemper often involves suggestive history, clinical signs, and evidence of serum or CSF IgM in response to active infection by canine distemper virus. In addition, RT-PCR on CSF is considered a useful, fast, and specific method to diagnose canine distemper virus infection (Amude et al., 2006).
FIGURE 14-20.

CSF. Dog. A, Lymphocytic pleocytosis. Pleocytosis (292 cells/μl), elevated CSF protein concentration (126 mg/dl), and lymphocyte predominance (72%) were detected in a cerebellomedullary cistern sample from a dog with acute ataxia and head tilt. Canine distemper titer levels were present in the CSF suggesting a viral-induced encephalopathy, which responded completely by 6 months with glucocorticoid therapy. Shown are numerous small lymphocytes, one neutrophil, and one large mononuclear cell. (Wright-Giemsa; HP oil.) B, Distemper inclusion. Eosinophilic inclusion, the homogenous oval structure above the nucleus within a large mononuclear cell, represents viral proteins from a dog diagnosed with canine distemper. (Wright-Giemsa; HP oil.)
(B, From Alleman AR, Christopher MM, Steiner DA, et al: Identification of intracytoplasmic inclusion bodies in mononuclear cells from the cerebrospinal fluid of a dog with canine distemper, Vet Pathol 29:84-85, 1992.)
FIGURE 14-21.
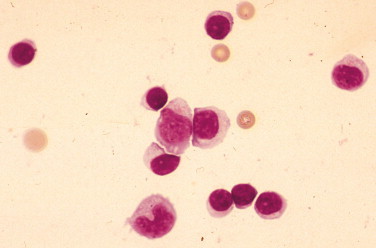
Lymphocytic pleocytosis. Rabies infection. CSF. Dog. Six-month-old stray presented with weakness on one hind leg that progressed over the course of a week to bilateral forelimb paresis and later seizures. The clinical presentation of a leg bite wound with cytologic appearance of CSF warranted euthanasia and subsequent diagnosis of rabies. If infectious agents are suspected, gloves and facial mask must be worn when handling diagnostic specimens and cytocentrifugation must be covered to prevent aerosolization. Note the predominance of small lymphocytes in addition to two large mononuclear cells. The nucleated cell count was 1140 cells/μl and protein 366 mg/dl in this fluid. (Wright-Giemsa; HP oil.)
(Courtesy of Rose Raskin, University of Florida.)
Mixed Cell Pleocytosis
Pleocytosis with a mixture of cell types may be seen with a variety of underlying causes including GME, FIP, canine distemper, steroid-responsive meningoencephalomyelitis (Fig. 14-22 ), toxoplasmosis, neosporosis, Sarcocystosis (Fig. 14-23A-C ), encephalitozoonosis, cryptococcosis, blastomycosis, aspergillosis, histoplasmosis, degenerative disc disease, ischemia, and neoplasia (Chrisman, 1992).
FIGURE 14-22.
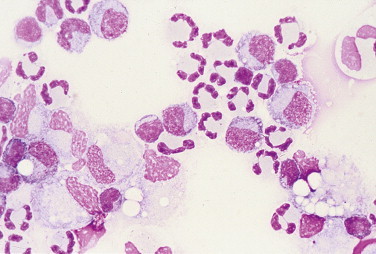
Mixed cell pleocytosis. CSF. Dog. This sample is from an adult female Cairn Terrier with 4-month history of neck pain and muscle spasms that were responsive to glucocorticoids. Mononuclear phagocytes (52%) were mostly reactive as indicated by a foamy or vacuolated cytoplasm and evidence of phagocytized debris. Neutrophils composed 35% and lymphocytes 13% of the total cell population. (Wright-Giemsa; HP oil.)
FIGURE 14-23.

Mixed cell pleocytosis. Cisternal CSF. Cat. Same case A-C. A, This shows several Sarcocystis sp. merozoites within the cytoplasm of three large mononuclear cells. This 5-month-old cat presented with paraparesis and pain upon palpation of the spine. PCR and gene sequencing established the specific diagnosis. (Wright; HP oil.) B, This shows rare containment of the Sarcocystis merozoite within a neutrophil. Neutrophils appear mostly nondegenerate and account for 80% of the nucleated cell population along with 11% lymphocytes and 9% large mononuclear cells. The cytocentrifuge preparation was highly cellular but insufficient fluid did not allow an accurate cell count. (Wright; HP oil.) C, This shows an extracellular, pear-shaped merozoite that measures approximately 2 to 3 × 5 μm (Wright; HP oil.)
(A-C, Courtesy of Rose Raskin, Purdue University.)
Neural Tissue Injury Findings
In addition to blood contamination encountered during collection, the presence of erythrocytes in a cytologic preparation may result from cranial or spinal hemorrhage. Macrophages with engulfed erythrocytes (Fig. 14-24A&B ) may be seen in cases of acute spinal cord injury such as intervertebral disc herniation, neoplasia, inflammation, or degenerative conditions. Chronic hemorrhage will be indicated by the presence of hemosiderin-laden macrophages.
FIGURE 14-24.
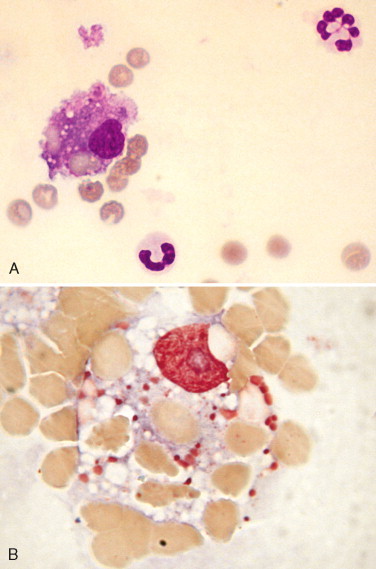
Erythrophagocytosis. CSF. Dog. A, This lumbar site collection was bloody with nucleated cells 84/μl, RBC 7000/μl, and protein 104 mg/dl. A car-related injury caused a thoracic spinal fracture that contributed to the acute hemorrhage exhibited in this example. A macrophage with engulfed red cells is present along with a hypersegmented neutrophil. (Wright-Giemsa; HP oil.) B, A macrophage with engulfed red cells is present and surrounded by erythrocytes. (MGG; HP oil.)
(A, Courtesy of Rick Alleman, University of Florida.)
Homogeneous “ribbons” of basophilic material hypothesized to represent degenerated myelin, as myelin figures or myelin fragments (Figs. 14-25A&B and 14-26 ), have been reported in a postmortem collection of CSF from a dog (Fallin et al., 1996). Spinal cord infarction with diffuse myelomalacia in a dog resulted in the presence of foamy macrophages in the CSF (Mesher et al., 1996). Luxol fast blue staining of the amorphous eosinophilic material found within the macrophages was positive in this case, suggestive of myelin. Similar myelin-like extracellular material was found in a dog with spinal subdural hemorrhage secondary to an intervertebral disk protrusion (Bauer et al., 2006). Other demyelinating conditions such as degenerative myelopathy may present with free myelin (Fig. 14-27A&B ).
FIGURE 14-25.

Same case A-B. A, Myelomalacia. CSF. Dog. Patient presented with acute paraplegic and absent deep pain related to a disc protrusion at LI-2. A myelogram confirmed dorsal spinal compression from T11 to L1. A cerebellomedullary cistern sample was taken 4 days postsurgery at the time of euthanasia. Pictured are two macrophages with large lipid-filled cytoplasmic vacuoles and basophilic ribbon material extracellularly. Necropsy confirmed a necrotic spinal cord in the areas shown compressed on the presurgery myelogram. (Wright-Giemsa; HP oil.) B, Myelin figures. CSF. Dog. Pictured are basophilic ribbon structures that likely represent phospholipids, derived from damaged cytomembranes. (Wright-Giemsa; HP oil.)
FIGURE 14-26.

Myelin ribbon. Lumbar CSF. Dog. An eosinophilic ribbon structure is present along with two mononuclear cells. It is likely this represents phospholipids, derived from damaged membranes. (MGG; HP oil.)
FIGURE 14-27.

Myelin. CSF. Dog. Same case A-B. A, Mixed-breed dog with a history of degenerative myelopathy with normal nucleated cell count and increased protein (62 mg/dl). Foamy, phagocytic macrophages were present (not shown). Collections of eosinophilic foamy material are shown extracellularly. (Wright-Giemsa; HP oil.) B, Extracellular material stained positive for myelin. Demyelination was suspected in this dog. (Luxol fast blue; HP oil.)
Mikszewski et al. (2006) described diagnostic evaluations of five cats with myelomalacia due to fibrocartilagineus embolism. In those animals results of CSF were abnormal in four cats with neutrophilic pleocytosis detected in three cases.
Neural Cystic and Neoplastic Lesion Findings in CSF
Rare developmental defects have been demonstrated within the brain and in one case the CSF contained numerous mature squamous epithelium, consistent with an epidermoid cyst (Fig. 14-28A&B ). Spinal arachnoid cysts, also referred to as meningeal cysts and leptomeningeal cysts, have been reported as an uncommon cause of neurologic deficits in the dog and cat generally related to a normal CSF analysis (Galloway, 1999).
FIGURE 14-28.

Epidermoid cyst. CSF. Dog. Same case A-B. A, Direct smear of creamy, opaque fluid with a nucleated cell count of 80,000/μl taken from the cerebellomedullary cistern of a dog with a 3-month duration of seizures. Numerous large, blue-green cells are evident at low magnification. (Romanowsky-type stain; IP.) B, Squamous epithelium is present as keratinized (upper left) and intermediate (lower right) squamous epithelium along with numerous nondegenerate neutrophils. (Romanowsky-type stain; HP oil.)
(A-B, From a glass slide submitted by Joseph Spano to the 1988 ASVCP case review.)
© 2010
With neoplasia, more often the protein concentration is elevated, with only occasional neoplastic cells present in the CSF. This will depend on the location of the mass with its proximity to the ventricle, its involvement with the meninges, or its communication with the subarachnoid space in order to have access to the CSF. CSF was collected in 51 dogs with primary intracranial neoplasia in which the fluid was normal in 10% of cases, characterized by an elevated cell count in 58% of cases, and characterized as albuminocytologic dissociation in 30% of cases. A mixed cell pleocytosis was the most common cytologic abnormality in the 51 samples while cellular atypia or neoplastic cells were detected in only two dogs with CNS lymphoma (Snyder et al., 2006). The authors concluded that in the case of CNS lymphoma, CSF analysis may be helpful in achieving a diagnosis. However, because of the inflammatory nature of CSF seen with primary intracranial neoplasia, this test may not be helpful in differentiating between neoplastic and other inflammatory diseases.
Similar results were found in a series of CSF analyses from 28 cats with intracranial neoplasia (Troxel et al., 2003) in that albuminocytologic dissociation was noted in 8 (28.6%) cats. The remaining 20 (71.4%) cats had varied increases in nucleated cell counts while a definitive diagnosis of lymphoma was made in only one cat in which lymphoid blast cells were detected in the CSF.
Dickinson et al. (2006) tested the hypothesis that predominantly neutrophilic pleocytosis is a typical finding in dogs with intracranial meningioma by evaluating the characteristic of cisternal CSF in a series of 56 dogs with confirmed disease. Results were significantly different from those routinely reported in veterinary literature. Pleocytosis was detected in only 27% of dogs, and pleocytosis with a predominance of neutrophils was detected only in 19% of dogs. They concluded in particular that neutrophilic pleocytosis may not be detected in CSF samples from dogs with meningiomas located within the middle or rostral portion of the cranial fossae.
The presence of mitotic cells in the CSF is unusual and often indicates a proliferative population such as a neoplasm. The presence of immature lymphocytes is highly diagnostic for the presence of CNS lymphoma (FIGURE 14-29, FIGURE 14-30, FIGURE 14-31 ). Well-differentiated lymphoid malignancies may not be readily distinguished from a lymphocytic pleocytosis involving granular lymphocytes (Fig. 14-32A&B ). Other round cell tumors (Sheppard et al., 1997; Greenberg et al., 2004) are less common and include encephalic and spinal plasma cell tumors (Fig. 14-33A-C ), histiocyticappearing neoplasms (Fig. 14-33D) that are difficult to distinguish from GME (Zimmerman et al., 2006). Medulloblastoma should be considered as another differential diagnosis for atypical round cells in the CSF of young dogs (Thompson et al., 2003). Rarely, individualized cells from choroid plexus papillomas may be found in the CSF as large round cells (see discussion later under “Neoplasms of Neuroepithelial Cells”).
FIGURE 14-29.
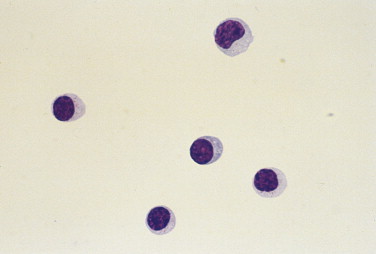
Lymphocytic pleocytosis. CSF. Cat. Cerebellomedullary collection contained 60 nucleated cells/μl, protein 140 mg/dl, and 80% lymphocytes in a cat with hind-limb paresis, urinary and fecal incontinence, and flaccid anal tone and tail. Intermediate-sized lymphocytes predominate in the field shown. Myelogram revealed a lumbar spinal cord mass that was cytologically diagnosed as large cell lymphoma. (Wright-Giemsa; HP oil.)
(Courtesy of Rick Alleman, University of Florida.)
FIGURE 14-30.

Lymphoma. CSF. Dog. Clinical signs involved a head tilt with ataxia of 3 months' duration. Increased protein (170 mg/dl) and pleocytosis (1417 cells/μl) were present in the clear fluid from the cerebellomedullary site. A mixed population of small, well-differentiated lymphocytes and large lymphoid blast cells (greater than 50%) together accounted for 99% of the cell population. Blast cells often contain a single prominent nucleolus. (Wright-Giemsa; HP oil.)
FIGURE 14-31.
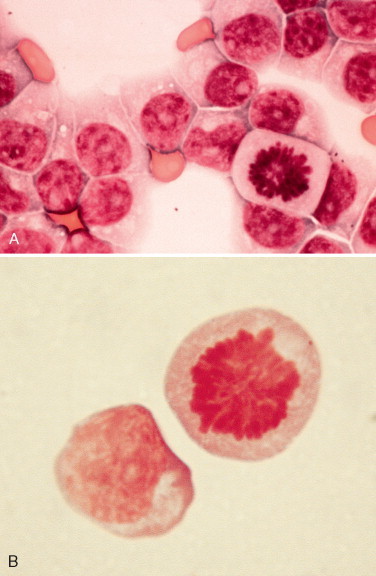
Lymphoma. Dog. A, CSF. Cream-colored CSF from the cerebellomedullary cistern of a dog with vestibular deficits. The fluid had marked pleocytosis of 109,400 nucleated cells/μl and increased protein of 220 mg/dl. A monomorphic population composing 92% of the cells involved large lymphoid blast cells with a prominent single nucleolus. Pictured are the blast cells along with a normal-appearing mitotic figure. (Wright; HP oil.) B, Cisternal CSF. This dog presented with dementia and circling. Of the two cells shown, the left cell is a blast cell with a single prominent nucleolus. The cell on the right is a normal-appearing mitotic figure, common in neoplastic CSF. (MGG; HP oil.)
FIGURE 14-32.

Granular cell lymphoma. CSF. Dog. A, Shown are three granular cell lymphocytes found in fluid collected from the cerebellomedullary cistern from a dog with granular cell lymphocyte leukemia that originated within the spleen. Two months later the dog presented with dementia and cerebellar signs. The fluid had moderate pleocytosis (32 cells/μl), increased protein (69 mg/dl), few erythrocytes (520/μl) with 91% lymphocytes. The granules are very fine and lightly eosinophilic (most prominent in the uropod or cytoplasmic projection of the lower center cell). (Wright-Giemsa; HP oil.) B, Shown are three granular cell lymphocytes with prominent paranuclear eosinophilic granules from a dog with intestinal and splenic granular cell lymphoma. Note the red cell for size comparison. Some artifact is apparent from cytocentrifugation as indicated by the surface blebbing and nuclear incontinence. (MGG; HP oil.)
FIGURE 14-33.

A, Plasma cell tumor. CSF. Dog. Two large mononuclear cells and two plasmacytoid cells are shown from the spinal fluid with marked mononuclear pleocytosis (27,600 nucleated cells/μl) and increased protein (greater than 2000 mg/dl). A primary encephalic plasma cell tumor involving the brainstem was diagnosed at necropsy with diagnostic support by electron microscopy and immunocytochemistry. (Wright-Giemsa; HP oil.) B, Plasma cell. CSF. Dog. Two lymphoid cells are shown, one of which displays a plasmacytoid appearance with an irregular nuclear outline. (MGG; HP oil.) C, Spinal plasmacytoma. Lumbar CSF. Dog. A large atypical plasma cell with pink and blue cytoplasm is shown against a proteinaceous background with numerous erythrocytes. Compare the size of this neoplastic cell with the small lymphocyte on the lower left. (MGG; HP oil.) D, Multinucleated cells. CSF. Dog. A tumor of unknown origin in the area of the thalamus produced clinical signs of pain initially and later tetraparesis. The fluid had mild pleocytosis (21 nucleated cells/μl) and elevated protein (70 mg/dl). Large mononuclear cells (59%) predominated followed by lymphocytes (37%). The pleomorphism of the large mononuclear cells along with many giant, multinucleated forms as shown supported a neoplastic process rather than an inflammatory disease. (Wright; HP oil.)
Finding metastatic carcinoma cells in the CSF is confirmed to be the gold standard for the diagnosis of leptomeningeal carcinomatosis in humans. Patients with extensive meningeal involvement are more likely to have a positive CSF cytology (66%) than those with only focal involvement of the leptomeninges (38%). In two cases of canine leptomeningeal carcinomatosis CSF, cytology demonstrated atypical cells with high or normal cell counts (Pumarola and Balash, 1996; Stampley et al., 1986).
CYTOLOGY OF NERVOUS SYSTEM TISSUE
Collection and Cytologic Preparation of Nervous System Tissues
When an intracranial or spinal mass is suspected, the veterinary neurologist often relies on sophisticated imaging such as computed tomography (CT) and magnetic resonance to identify the lesion. Even if imaging can give some information about the location, the size, and the relationship to other surrounding structures, very often the differential diagnosis for the mass found on imaging includes inflammatory (sterile or septic) lesion or benign and malignant tumor.
Since each disease has a different prognosis and requires different therapies, a definitive diagnosis needs to be sought but this can be achieved only by histologic examination. Intraoperative cytology has been applied worldwide in human neuropathology as a method for obtaining a rapid and accurate diagnosis even on very small specimens collected from stereotactic needle biopsy or intracranial and spinal surgery (Di Stefano et al., 1998, Tilgner et al., 2005). In human neurosurgery, where stereotactic biopsies are a routine way of obtaining samples of brain tissue in the least invasive manner possible with the fewest complications, cytology has emerged as the preferred diagnostic tool over the traditional frozen section technique (Shah et al., 1998).
Elective employment of smear cytology in neuropathology is based on several considerations: it is very simple and quick to perform, it requires few materials and equipment, and the specimens can be prepared directly in the operating or adjacent room. Small pieces of tissue can be examined and smears can be repeated several times with different portions of the same specimens. Various fast-staining techniques can be used. A recent study confirmed the adequacy of smear preparation for intracranial lesions in dogs and cats. Smear preparations appeared to be of greater diagnostic value, with fewer nondiagnostic specimens, when compared with touch preparations (Long et al., 2002). Moissonnier et al. (2002) demonstrated that stereotactic CT-guided brain biopsy can be considered as a valued technique in the neurologic workup of patients with brain diseases and an early cytologic assessment is considered important even during conventional intracranial and spinal surgery.
The interpretation of smear samples from nervous system lesions requires considerable experience, not only in general cytology, but also in normal and abnormal cytology of the specific features of nervous system tissue.
Cytology of Normal Nervous System Tissues
Very little information is available on normal nervous tissue cytology in dogs and cats. Cytology of normal cerebral, cerebellar, and spinal cord tissue of these animals shows a close resemblance to their human counterparts (Herzberg, 1999).
In one study (De Lorenzi et al., 2004) authors collected several samples by core needle biopsy from different areas of brain and cerebellum of dogs and cats without neurologic disorders in five dogs and five cats. From the two edges of the biopsy sample, a small fragment was removed with a scalpel blade and smeared on a glass slide a “squash” or “crush” technique (Fig. 14-34 ). The small portion (approx. 1 mm3) of the biopsy material was placed between two slides and squashed firmly until it becomes a thin film. Then, with a firm, even motion the slides were quickly pulled apart horizontally. All the cytologic samples were air dried, then placed in an automatic slide stainer with May-Grünwald Giemsa (MGG) stain. The rest of the biopsy was fixed in 10% neutral-buffered formalin, and processed routinely as 5-μm sections stained with H&E, then examined by a neuropathologist to confirm the absence of pathologic changes.
FIGURE 14-34.

Squash prep technique. Brain. A, Cut a tiny portion of the brain tissue biopsy for the smear. B, Place the small fragment near the frosted end of a standard glass slide. C, Apply pressure using a second slide, maintain compression, then slide the top slide over the held bottom slide. D, The tissue is smeared out, resulting in an oval cytologic preparation.
Central Nervous System Cells
Central nervous system cells have two main origins, the neuroectoderm and mesenchyme. Neurons and glial cells (astrocytes, oligodendrocytes, Schwann cells, ependymal cells, and choroid plexus cells) are considered of neuroectodermal origin; meningeal cells and microglia are of mesenchymal origin.
In normal CNS tissue smears it is usually possible to recognize many of cellular and noncellular elements, while it is often difficult to identify the exact nature of every cell. A short description of the normal cytologic features found in this study is provided in the following paragraphs.
Normal cerebral tissue is an easy-to-smear tissue of low cellular density. In general, the grey matter contains neurons and a few nonmyelinated fibers, while the white matter displays myelinated axonal fibers.
Neuropil
Neuropil is the term used to define the dense network of fine glial processes, neuronal processes (axons and dendrites), and fibrils in the gray matter of the CNS (Fig. 14-35A&B ). The neuropil is particularly prominent with MGG staining, in which it appears blue-purple. The characteristic and almost distinctive blue staining and foaminess shown by normal neuropil is particularly important to recognize since it is rarely, if ever, present in tumors and in most pathologic lesions.
FIGURE 14-35.

Neuropil. A, Brain cortex aspirate. Dog. Accidental puncture of cerebral cortex during aspiration of sinus cavity. Demonstrates the vacuolated foamy and amorphous basophilic appearance of neuronal and glial processes of the gray matter. (Wright; HP oil.) B, Normal brain cortex. Squash preparation. Cat. Present is a neuron (large nucleus with prominent nucleolus) and several hyperchromatic glial cell nuclei within a meshwork of fibrillary processes known as neuropil. Blood vessels are present within the neuropil. (MGG; HP oil.)
(A, Courtesy of Rose Raskin, Purdue University.)
Neurons
Neurons are the principal components of the CNS. More than any other cell, neurons vary in size from location to location. Most neurons are very large cells (Figs. 14-4A and 14-36 ), measuring up to 40 μm in diameter, but their size can vary from 5 μm (granular layer of the cerebellum) to 100 μm (motor cortex). Despite this variation in size, neurons share a common morphologic feature in both dogs and cats, which is an angulated shape with multiple and branching cytoplasmic processes composed of dendrites and a single axon. The real number of extensions of these specialized structures cannot be evaluated by MGG stain, as special stains are needed for this purpose. All neurons have a very large, centrally placed nucleus and frequently a single, prominent nucleolus. The cytoplasm is usually abundant and granular, due to the presence of Nissl substance, the rough endoplasmic reticulum often so abundant as to obscure the nucleus. In some areas of the brain, the cytoplasm may contain melanin pigments (neuromelanin) and microvacuoles (neuromediators). Smears from the cerebellar cortex have a highly characteristic appearance as the cellularity is usually higher than in the brain cortex with sheets of small, hyperchromatic granular cells (Fig. 14-37A ) from the inner, granular layer that are occasionally intermixed with large Purkinje cells (Fig. 14-37B).
FIGURE 14-36.

Neuron. Normal brain cortex. Squash preparation. Cat. A neuron with a prominent nucleolus is shown. Neurons vary in size and shape depending on location. Common morphologic features shared by most neurons include single, centrally placed nucleus with prominent nucleolus, and angulated cytoplasm. Note the basophilic, granular cytoplasm that is rough endoplasmic reticulum (Nissl substance). Small hyperchromatic glial cell nuclei are noted in the background. (MGG; HP oil.)
FIGURE 14-37.

Cerebellar cortex. Squash preparation. Dog. A, Granular cells. Several small hyperchromatic neuron nuclei are shown from the inner granular layer of the cerebellar cortex. Note the hypercellularity and linear arrangement of the nuclei within the neuropil. Due to their small size and near absence of cytoplasm, these cells can be confused with lymphoid cells. (MGG; HP oil.) B, Purkinje cell. Present is a large, distinctive, flask-shaped neuron with single, central nucleus and characteristic single large, extended axon. The numerous highly branched dendrites for this cell are usually not evident with MGG stain. The cytoplasm surrounding the nucleus contains basophilic granular material known as Nissl bodies or substance. Several hyperchromatic granular cells appear in the background. (MGG; HP oil.)
Astrocytes
Astrocytes have a supportive function to neurons and are distributed throughout the nervous system. The characteristic star-shaped appearance cannot be evaluated with MGG stain and the cells appear as small, oval, naked nuclei, which measure between 7 and 10 μm, surrounded by neuropil (Fig. 14-38A ). In response to neural tissue injury in the brain and spinal cord, there is a proliferation and hypertrophy of the resident neuroglial cells, which include the astrocyte, a supporting cell with branched cellular projections (Fig. 14-38B&C).
FIGURE 14-38.
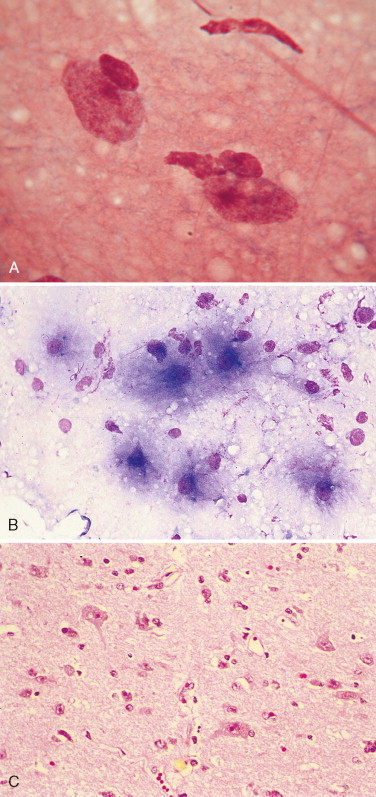
A, Normal astrocytes. Squash preparation. Dog. In MGG-stained samples, astrocytes appear as small, oval, naked nuclei that measure between 7 to 10 μm. Here two are surrounded by neuropil. (MGG; HP oil.) Same case B-C. Astrocytosis. Cat. B, Brain aspirate. Six large cells with a wispy basophilic cytoplasm are evident in this aspirate from a cat with a 14-day progression of dementia and head pressing. Nuclei are round to oval with a single small prominent nucleolus and the nuclear-to-cytoplasmic ratio is mildly increased. Cytologically, a neoplasm was suspected. (Wright-Giemsa; HP oil.) C, Brain histology. MRI revealed an intracranial mass. Tissue biopsy revealed normal gray matter with hypertrophied astrocytes, which is a nonspecific reaction. Although neoplastic cells were not found, adjacent neoplasia could not be ruled out. (H&E; HP oil.)
Oligodendrocytes
These are the myelin-forming cells of the central nervous system. In smears their nuclei are smaller (5 to 7 μm) and rounder than astrocytes and, like astrocytes, their cytoplasm is not well defined (Fig. 14-39 ). Due to their size and shape, oligodendrocytes can be mistaken for lymphocytes. Oligodendrocytes may surround neurons in a process called satellitosis.
FIGURE 14-39.

Oligodendrocyte. Normal brain cortex. Squash preparation. Dog. In MGG-stained smears, oligodendrocytes look like round, naked nuclei that often surround neurons in a process called satellitosis. (MGG; HP oil.)
Ependymal and Choroid Plexus Cells
Choroid plexus cells can be considered as specialized ependymal cells, lining the brain ventricles and the central canal of the spinal cord. These neuroepithelial cells show similar cytomorphologic features as they are usually organized in small, loose clusters and small sheets of cuboidal to columnar cells with a single, small, round, and centrally placed nucleus (Fig. 14-40 ).
FIGURE 14-40.
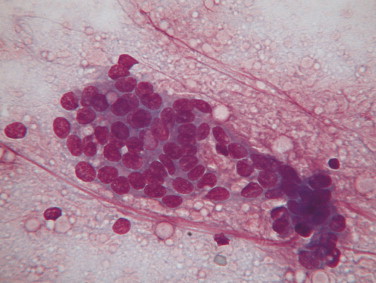
Normal choroid cells. Squash preparation. Dog. These lining cells arranged as small sheets of cuboidal to columnar cells have a uniform nuclear appearance and high nuclear-to-cytoplasmic ratio. The single small, round, and centrally placed nuclei are arranged occasionally in a palisade or linear arrangement. Choroid cells are cytologically indistinguishable from ependymal cells. (MGG; HP oil.)
Meningeal Cells
Meningeal cells are only rarely seen and recognized in brain smears. The cells are usually organized in sheets or in loose clusters showing a rather pleomorphic storiform pattern. Cell borders are poorly defined and the nuclear shape ranges from round to oval to elongate (Fig. 14-41 ). Occasionally, cells are organized in pseudoacinar structures mimicking a glandular origin, which is more common in meningiomas but can also be identified in normal meningeal tissue.
FIGURE 14-41.

Normal meningeal cells. Squash preparation. Dog. The dense collection of oval nuclei is shown with associated eosinophilic streaming and whirling cytoplasm. This loose aggregate of pleomorphic meningeal cells organized in a pseudoacinar arrangement is a feature more common in meningiomas but that can be seen in samples from normal brain. (MGG; HP oil.)
Microglia
These neuroglial cells derive from bone marrow elements, likely macrophages that have specific phagocytic functions. They have small and elongated nuclei, which explains their title as “rod cells.” In many smears from brain cortex, microglia are localized in perivascular areas. When reactive, they show lipophagocytosis, filling the cytoplasm with well-defined vacuoles producing a foamy appearance (Fig. 14-42A&B ).
FIGURE 14-42.

Microglial cells. Normal brain cortex. Squash preparation. A, Dog. Several perivascular microglial cells are shown in the center, which display an abundant foamy cytoplasm. The background contains other glial cells along with neural fibrils. (MGG; HP oil.) B, Cat. A single vacuolated, lipid-filled microglial cell is noted adjacent to a blood vessel. When reactive, these macrophage-derived cells undergo lipophagocytosis. (MGG; HP oil.)
Cytology of Pathologic Nervous System Tissues
Inflammatory lesions of the central nervous system can mimic neoplastic proliferation both clinically and radiographically. Cytology can be an extremely useful tool in distinguishing a true tumor from an inflammatory or reactive lesion. By using Romanowsky stain, many infectious agents can be easily identified and recognized. In a recent report (Falzone et al., 2008), a space-occupying lesion in the temporal lobe of an adult cat was diagnosed cytologically as brain toxoplasmosis. The squash prep (Fig. 14-43 ) showed intense reactive gliosis surrounding round, encapsulated tissue cysts ranging from 15 μm to more than 100 μm that contained a number of elongated nucleated bradyzoites identified as Toxoplasma sp.
FIGURE 14-43.

Toxoplasmosis tissue cyst. Brain mass. Squash preparation. Cat. A round, encapsulated structure or tissue cyst is filled with nucleated bradyzoites of Toxoplasma sp. (MGG; HP oil.)
While cytologic profiles of smears of various types of human CNS tumors have been extensively described, there are very few reports in the veterinary literature that describe cytologic findings of nervous system lesions and the diagnostic accuracy of this technique.
While most describe the cytologic features from single cases or small series, Vernau et al. (2001) described the cytologic features of smears obtained from 93 primary brain tumors in dogs and cats. More recently, De Lorenzi et al. (2006) sdetermined an accuracy value of 92.8% with a cytologic evaluation of smear samples of nervous system lesions from 42 cases in dogs and cats. In this study, once the smear considered abnormal, the specimen was designated as non-neoplastic or neoplastic. The non-neoplastic group consisted of tissues derived from inflammation, cyst, granuloma, or scar lesion, while the neoplastic group included lesions of neuroepithelial origin (neural, glial, and ependymal/choroidal proliferations) or of non-neuroepithelial origin, divided further into epithelial, mesenchymal, and round cell tumors.
The distinction between neuroepithelial and nonneuroepithelial depends to a large extent on pattern recognition and individual cell morphology. Therefore, misinterpretations may be due to different factors, such as the overlapping of morphologic features in neoplastic and non-neoplastic lesions and the primitive tumor heterogeneity.
Cytologic features suggesting a neuroepithelial origin include highly cellular smears related to the soft texture of the specimen, fine fibrillar background, perivascular arrangement with processes approaching the vascular lumen, round nuclei with finely stippled chromatin, and endothelial cell proliferation. Cytologic features of non-neuroepithelial lesions can vary considerably due to the extreme heterogeneous morphology of these groups. Nevertheless, the presence of cohesive cellular clusters or epithelial sheets; tightly packed spindle cells; whorls; large, round cells with prominent nucleoli and discernable cytoplasm; and numerous inflammatory cells suggests consideration of non-neuroepithelial tumors.
More specific cytologic features are described for different tumors.
Neoplasms of the Meninges and Nerve Sheaths
Non-neuroepithelial tumors that arise from the arachnoid meningeal layer are termed meningiomas. These are the most common intracranial tumor in dogs and cats. The tumors are derived from leptomeningocytes that associate with neural crest tissue and have both epithelial and fibroblastic ultrastructural characteristics. As a result, these tumors have several variant forms (Montoliu et al., 2006) that are found both in cervical and lumbar regions of the spinal cord as well as intracranially and within the retrobulbar region (Zimmerman et al., 2000). Spinal cord meningiomas are mostly extramedullary but few reports note the radiographic presentation of them as intramedullary (Hopkins et al., 1995). According to Bailey and Higgins (1986), meningiomas had the highest prevalence of pleocytosis, with a predominance of neutrophils often associated with necrosis. The meningioma was unique of all the tumors reviewed in this study, being the only tumor with CSF nucleated cell counts greater than 50/μl. Nevertheless in a more recent work (Dickinson et al., 2006) pleocytosis was detected in only 27% of 56 dogs with confirmed intracranial meningioma, and pleocytosis with a predominance of neutrophils was detected in only 19% of dogs with the conclusion that neutrophilic pleocytosis, especially with total nucleated cells count greater than 50 cells/μl, was not typical in CSF samples from dogs with intracranial meningiomas. Myelography, MRI, and CT are imaging tools used currently to identify tumors of the brain and spinal cord. Fine-needle aspirations, crush preparations (De Lorenzi et al., 2006; Moissonier et al., 2002), and incisional cutting needles (Platt et al., 2002) have been used to obtain cytologic and tissue samples for biopsy. The cytologic features of meningiomas have been discussed in several reports (Zimmerman et al., 2000; Hopkins et al., 1995).
Crush preparations from wet-fixed, rapid HE–stained meningiomas from 44 dogs and 7 cats were evaluated (Vernau et al., 2001). At low magnification, tumor cells were broken up into many clusters or cohesive cell aggregates, as well as separated into individual cells. Meningioma cells had round to slightly elongate, uniform-sized nuclei with a small, prominent nucleolus, diffusely coarse chromatin, and a well-defined nuclear border. Rarely there were intranuclear cytoplasmic evaginations, but these were plentiful in some individual tumor cells of the meningothelial subtype (Fig. 14-44A ). More elongated cells sometimes had a central bar or fold through the longitudinal axis of their nucleus. There were variable amounts of eosinophilic, granular, wispy to solid cytoplasm that appeared round to elongate, often with a polar location. Mitotic figures were extremely rare. Some tumors had marked cellular anaplasia or nuclear atypia. Neutrophils were usually found in those tumors that had histologic foci of necrosis and focal accumulations.
FIGURE 14-44.

A, Meningioma with nuclear inclusion. Squash preparation from surgical biopsy. Dog. A single meningothelial cell contains an intranuclear inclusion that is thought to represent cytoplasmic evagination. Cytoplasmic evagination is an uncommon feature in meningioma cells but may be more commonly encountered in meningiomas of meningothelial origin. (MGG; HP oil.) Same case B-C. Meningioma. CSF. Dog. B, Large clump of cells in a sample taken from the cerebellomedullary cistern of a dog having a spinal cord lesion in the C1-2 region that presented with weakness. (Wright-Giemsa; HP oil.) C, Higher magnification of the cell clusters showing plump cells with oval to round eccentric nuclei and occasional prominent nucleoli. The cytoplasm contains an eosinophilic secretory material. Necropsy confirmed the presence of a locally extensive meningioma. (Wright-Giemsa; HP oil.)
Tumors with a sarcomatous appearance may have a disseminated nature. Two examples of spinal cord meningiomas with a meningotheliomatous appearance are shown (Figs. 14-44B&C and 14-45A&B ). Meningiomas with a more common spindle cell cytologic appearance and a psammomatous histologic appearance are included (FIGURE 14-46, FIGURE 14-47, FIGURE 14-48 ).
FIGURE 14-45.

Meningioma. Tissue imprint. Dog. Same case A-B. A, A spinal cord mass from a dog with a 2-year history of neck pain and front leg paresis was obtained at surgery. Cytologic features demonstrate cohesive ball formation with epithelial-like appearance. (Wright; IP.) B, Individual meningeal cell with histiocytic appearance. The cytoplasm is abundant with eosinophilic secretory material that was positive for acid mucopolysaccharides. (Wright; HP oil.)
FIGURE 14-46.
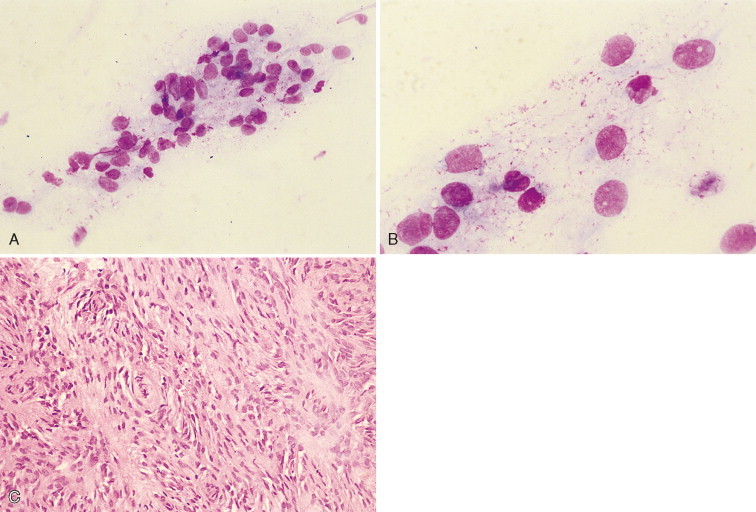
Meningioma. Dog. Same case A-C. A-B, Tissue imprint. A, Progressive quadriparesis was present with a circular lesion on MRI suggesting an intramedullary disease. Large cellular aggregates of mesenchymal-appearing cells are shown against a pink, finely granular background. (Wright-Giemsa; HP oil.) B, Higher magnification of a meningioma demonstrating the round to oval nucleus with finely granular chromatin, small nucleoli, and lightly basophilic cytoplasm that forms wispy tails. A finely granular eosinophilic material surrounds the cells and is seen within the cytoplasm as well. (Wright-Giemsa; HP oil.) C, Tissue section. Interweaving bundles of spindle cells are prominent with dense collagenous bands separating the cells. A small psammoma body with presumed calcified center is present (left center). (H&E; IP.)
FIGURE 14-47.
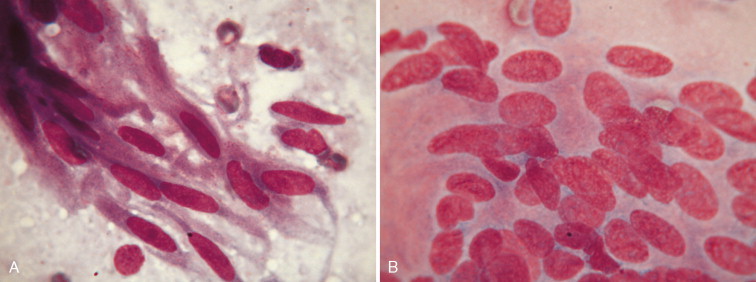
Meningioma. Spindle pattern. Squash preparation from surgical biopsy. Dog. A, Spindle-like tumor cells are arranged in a storiform pattern. These elongate cells with oval nuclei have a single small but prominent nucleolus. The cytoplasm is basophilic with wispy, pointed ends. (MGG; HP oil.) B, The tumor cells have oval or elongate uniform nuclei with moderately coarse chromatin. The cytoplasmic borders are not as defined as those in A and instead are almost nondetectable with cells appearing as an aggregate of multiple nuclei. The cytoplasm is eosinophilic. (MGG; HP oil.)
FIGURE 14-48.

A, Meningioma. Perivascular pattern. Squash preparation from surgical biopsy. Dog. The spindle-shaped cells are strongly associated with an eosinophilic linear capillary blood vessel. Meningiomas have several variant forms and cytology may be insufficient to determine these subtypes. This perivascular pattern may be observed in multiple tumor types. (MGG; HP oil.) Same case B-C. B, Meningioma whorl. Squash preparation from surgical biopsy. Cat. A cohesive aggregate of meningeal cells are present in a distinctive whorl formation characteristic of a psammomatous meningioma. Nuclei are round or slightly elongated and the cell border is poorly defined. (MGG; IP.) C, Transitional meningioma. Cerebrum. Tissue section. Cat. Same case as in (B). Island and whorl patterns of neoplastic meningothelial cells characterized by spindloid cells. (H&E; IP.)
Another tumor associated with the meninges is the granular cell tumor, an uncommon tumor of animals and humans that occurs both within or outside the nervous system. The cell of origin is unclear but the morphologic appearance is thought to be the result of the accumulation of lysosomes, as demonstrated by electron microscopy (Sharkey et al., 2004), that reflect metabolic derangements in the cell. The cytologic features include large, round cells with eccentric nuclei and cytoplasm distended by many variably stained eosinophilic granules (Fig. 14-49A-C ). Cells stain strongly positive for PAS as well as for the immunocytochemical stain for ubiquitin. Other staining reactions for these cells include a variably positive reaction for S-100, a-1-antichymotrypsin, a-1antitrypsin, and vimentin as well as a negative reaction for glial fibrillary acidic protein, pancytokeratins, and markers for subpopulations of leukocytes and macrophages (Higgins et al., 2001; Sharkey et al., 2004).
FIGURE 14-49.

Granular cell tumor. Dog. Same case A-C. A-B, Tissue imprint. A, This 10-year old Golden Retriever became aggressive and began having seizures 2 months before cerebral tumor detection by MRI. A group of pleomorphic round granular cells as defined morphologically are present with variable degrees of cytoplasmic granularity. One poorly granular cell (bottom center) has abundant cytoplasm and a small round nucleus. (Wright-Giemsa; HP oil.) B, One cell measuring approximately 50μm contains numerous pink-purple, coarse granules in the cytoplasm that are thought to be the result of the accumulation of lysosomes as demonstrated by electron microscopy. Note two poorly granular adjacent cells with small round nuclei and single small nucleoli. (Wright-Giemsa; HP oil.) C, Histologic section. The meningeal tumor consisted of a mixed cell population displaying a psammomatous meningioma with characteristic whorl pattern and a granular cell tumor that appears to arise from the meningeal layer. (H&E; IP.)
(A-C, Courtesy of Rose Raskin, University of Florida.)
Peripheral nerve sheath tumors (PNST) may be encountered in cytologic preparations. These are most often associated with peripheral nerve roots and include those of the neural crest–derived Schwann cell that assists in myelination as well as fibroblastic connective tissue cells that surround nerve bundles. A cytologic distinction among benign PNST (Schwannoma and neurofibroma) can be difficult or impossible (Fig. 14-50 ). Moreover, sometimes benign Schwannomas and neurofibromas can show cytologic features of atypia so that differentiation between benign and malignant PNST becomes even more difficult.
FIGURE 14-50.

Peripheral nerve sheath tumor. Squash preparation from surgical biopsy. Dog. A dense collection of pleomorphic, elongated cells with oval nuclei is organized in a storiform pattern with focal palisade arrangement. Distinction between Schwannoma and neurofibroma is not possible by cytology. The diagnosis of PNST can be more easily performed if a neoplasm with these cytomorphologic features is associated with a peripheral nerve root. (MGG; IP.)
A diagnosis of suspect PNST can be made in the presence of neoplasms associated with a peripheral nerve root, showing cytologic features of moderate to high cellularity. The cells are mainly grouped in thick fragments even if smaller clusters or single cells can be present and are characteristically spindle-shaped with elongated nuclei and inconspicuous nucleoli.
An unusual cytologic presentation of PNST from the forelimb of one cat was described (Tremblay et al., 2005). The cells revealed a pleomorphic population of individual round cells resembling histiocytes or plasma cells with round, central to eccentric nuclei, basophilic cytoplasm, numerous mitotic figures, and large, multinucleated cells.
A benign nerve sheath tumor is shown (Fig. 14-51A&B ). Malignant nerve sheath tumors may be locally extensive and recur more often. The histologic distinction between malignant fibroblasts and malignant Schwann cells is not readily discernible without immunohistochemistry and electron microscopy. A presumed neurofibrosarcoma is shown (Fig. 14-52A&B ).
FIGURE 14-51.

Benign nerve sheath tumor. Dog. Same case A-B. A, Tissue imprint. Two intact plump spindle cells demonstrate minimal anaplastic features. A compressive extradural lesion was found in the spinal canal at the nerve root region of C2-3. Clinical presentation included tetraparesis, ataxia, cervical pain, and Horner syndrome. (Wright-Giemsa; HP oil.) B, Tissue section. Neoplastic mesenchymal cells with eosinophilic fibrillary cell borders are arranged loosely within a fibroblastic stroma. (H&E; HP oil.)
FIGURE 14-52.

Malignant nerve sheath tumor. Tissue imprint. Dog. Same case A-B. A, Clinical presentation included paraparesis that progressed to tetraparesis. A mass within the spinal canal at the C2-3 nerve root was resected but recurred 2 months later. Spindle cells predominate with two populations present. Some cells have elongated fusiform nuclei and others have plump round to oval nuclei. The cytoplasm forms tails more distinct on the more elongated cells. (Wright-Giemsa; HP oil.) B, Aggregate of neoplastic cells with associated amorphous eosinophilic collagenous stroma. Cells have oval nuclei with coarse chromatin, small distinct nucleoli, and vacuolated scant pale blue cytoplasm. Histologic diagnosis was neurofibrosarcoma. (Wright-Giemsa; HP oil.)
Neoplasms of Neuroepithelial Cells
Gliomas refer to neoplasms of specific neuroglial cells that include oligodendrocytes and astrocytes. Tumors from these cells most often produce a normal CSF related to their deep parenchymal location. One report of oligodendrogliomas in cats described their cytologic features as they appeared in cytospin preparations of the CSF (Dickinson et al., 2000). Cells were large with nuclei four to six times the size of red cells. Nuclei were eccentric within a densely basophilic, moderately abundant cytoplasm. An aspirate from an oligodendrogliomas presenting as a brain mass is shown (Fig. 14-53A-C ). Normally these cells are responsible for myelination of neurons in the CNS and appear as small cells with condensed chromatin. Tumors often demonstrate a unique honeycomb appearance and increased proliferation of blood vessels.
FIGURE 14-53.
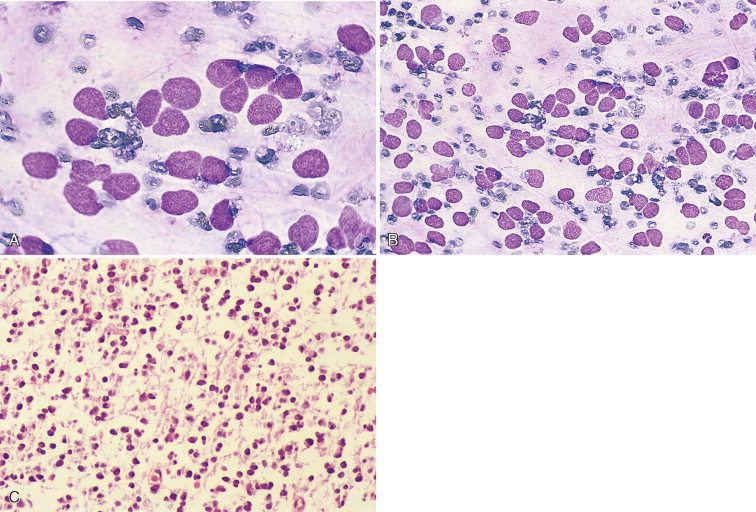
Oligodendroglioma. Dog. Same case A-C. A-B, Tissue aspirate. A, This brain specimen was taken from a dog with demented behavior. MRI identified a 5-cm mass in the cerebrum that extended into the lateral ventricle. Nuclei measured two to three times a red cell and appeared free with clear area present around the cell when viewed against the proteinaceous background. Nuclei are round to oval with fine chromatin and indistinct nucleoli. (Wright-Giemsa; HP oil.) B, A monomorphic population of large mononuclear cells arranged in loose sheets or small clusters. (Wright-Giemsa; HP oil.) C, Tissue section. Tissue section showing linear arrays of round hyperchromatic cells surrounded by clear spaces producing a honeycomb appearance. (H&E; HP oil.)
Astrocytes provide nutritional support to neurons, act as metabolic buffers or detoxifiers, and assist in repair and scar formation. They have been described as histiocytic in appearance (Fernandez et al., 1997). An example of reactive astrocytes was described previously (see Fig. 14-38A&B). Astrocytomas examined by crush preparation preserve the cytoplasmic features of an eccentrically placed nucleus within a moderately abundant basophilic cytoplasm (Fig. 14-54A-C ). Glial fibrillary acidic protein (GFAP) is a marker used to distinguish astrocytes from other neuroglial and meningeal cells but may sometimes yield a positive reaction in neuroepithelial tumors, such as ependymoma and choroid plexus tumor (Fernandez et al., 1997), and meningeal tumors (Montoliu, 2006).
FIGURE 14-54.

Astrocytoma. A, Brain. Squash preparation. Dog. Pleomorphic cells are individualized having an eccentrically placed nucleus with prominent nucleoli and moderately abundant, deeply basophilic cytoplasm. One cell appears to contain a vacuole. This appearance strongly resembles a plasma cell tumor. (MGG; HP oil.) B, Spinal mass. Squash preparation from surgical biopsy. Cat. This intradural spinal mass is composed of large cells with a histiocytic appearance having an eccentrically placed nucleus and abundant pink, granular cytoplasm. Also present is a normalappearing mitotic figure. A positive immunohistochemical stain for glial fibrillary acid protein was determined, which confirmed the astrocytic origin. (MGG; HP oil.) C, Spinal mass. Tissue section. Cat. Medium-grade astrocytoma (anaplastic astrocytoma) with astrocytic neoplastic population characterized by high cellularity and pleomorphism, marked nuclear atypia, and numerous mitotic figures. (H&E; IP.)
Neuroepithelial cells also include ependymal cells and choroid plexus cells. The ependymal cells line the ventricular system of the brain and central canal of the spinal cord. The ependymoma is a rare tumor (Fig. 14-55 ) that was reported in the cat with neoplastic cells found in the CSF with a moderately elevated protein content, mild pleocytosis, and macrophages as the predominant cell type along with evidence of chronic hemorrhage. The neoplastic cells were described as large cells having nuclear hyperchromatism, prominent nucleoli, and moderately abundant, highly basophilic agranular cytoplasm appearing singly and in clusters. An anaplastic ependymoma in a dog was diagnosed from partial positive staining with GFAP and vimentin but a negative reaction with S100, CD3, and cytokeratin (Fernandez et al., 1997). The choroid plexus cells represent a highly vascular portion of the pia mater that projects into the ventricles of the brain and is thought to secrete the CSF. Positive cytokeratin staining is expected in choroid plexus tumors. A cytologic example of a choroid plexus tumor is shown as an imprint of the tumor mass and within the CSF (Fig. 14-56A&B ). The choroid plexus tumors were associated with an increased protein content and increased nucleated cell count in a study (Bailey and Higgins, 1986). These cells appear similar to mesothelial cells in the CSF, having a large, round nucleus with abundant, well-defined, deeply basophilic cytoplasm having surface projections (Fig. 14-56C). A papillary appearance with epithelial features can be seen in cytologic preparations of choroid plexus papilloma (Fig. 14-56A). Two choroid plexus carcinomas were cytologically misdiagnosed as choroid plexus papillomas (De Lorenzi et al., 2006). Both of these tumors occurred within a ventricular site and consisted of large, pedunculated, friable masses that presented cytologically as well-formed papillary structures, usually showing a fine fibrovascular core covered by uniform, cuboidal or columnar epithelium having minimal cytologic atypia with moderate pleomorphism (Fig. 14-57A ). The final histologic diagnosis of carcinoma was made based of the presence of single-cell infiltration into the fibrovascular cores, suggesting that a definitive diagnosis of choroid plexus carcinoma can be made only after thorough histologic examination of the resected specimen (Fig. 14-57B).
FIGURE 14-55.

Ependymoma. Spinal mass. Squash preparation from surgical biopsy. Dog. These monomorphic cuboidal cells are arranged in a tight cluster with an acinar pattern. Cellular atypia is present as mild anisokaryosis and occasionally prominent nucleoli. Nuclei are round with uniform chromatin features. Cytoplasm is scant to moderately abundant and pink-blue with fine granularity. (MGG; HP oil.)
FIGURE 14-56.

Same case A-C. A-B, Choroid plexus papilloma. Tissue imprint. Dog. A, Seizure, dementia, ataxia, and tetraparesis were clinical signs present in this dog in which MRI diagnosed a ventricular mass. The sample was highly cellular with large, dense, cohesive clusters of cells having moderately abundant, deeply basophilic cytoplasm. (Wright-Giemsa; HP oil.) B, Higher magnification demonstrating the tight cohesion between cells. The nuclear-to-cytoplasmic ratio is high. The nucleus is round with finely granular chromatin and large prominent nucleoli. The cytoplasmic is basophilic and finely granular. (Wright-Giemsa; HP oil.) C, Choroid plexus papilloma with presumed myelin fragments. CSF. Dog. The fluid had increased protein (98 mg/dl) and a normal nucleated cell count. One neoplastic cell was present in two cytospin preparations. The nucleus is very large and round with dispersed chromatin and a single prominent nucleolus. The cytoplasm is dark blue with smooth surface projections. To the right of the cell is granular, gray-blue material consistent with myelin, which suggests a degenerative process. (Wright-Giemsa; HP oil.)
FIGURE 14-57.

Choroid plexus carcinoma. Dog. Same case A-B. A, Squash preparation. Cells are grouped in tight clusters; the upper left in an acinar arrangement. Cellular atypia include moderate anisokaryosis, prominent nucleoli, coarse chromatin, and high nuclear-to-cytoplasmic ratio. These cytomorphologic features resemble metastatic carcinomas from well-differentiated tumor cells. (MGG; HP oil.) B, Tissue section. Papillary figures characterized by cuboidal to columnar neoplastic neuroepithelial cells of one or more layers in thickness. The adjacent subependymal brain tissue appears to be infiltrated by the tumor. (H&E; LP.)
A potential diagnostic pitfall among tumors of different neuroectodermal and non-neuroectodermal origin can arise with ependymal or choroid plexus tumors and metastasis from well-differentiated adenocarcinomas. In fact, these two categories of neoplasms can share some analogous cytomorphologic findings such as epithelioid aspects, with cohesive glandlike fragments, round to polygonal cellular shape, and well-defined cytoplasm with distinct margins and roundish nuclei often with evident nucleoli. Additional information, such as the breed and the age of the patient, the precise location within the brain or the spinal cord, and a complete clinical history, especially the presence of a known primary tumor, may be critical in making the correct diagnosis.
Extramedullary Tumors
Two different papers describe the cytologic features of a canine thoracolumbar spinal tumor (nephroblastoma) in two young dogs (De Lorenzi et al., 2007; Neel and Dean, 2000). In the more recent case report, a triphasic pattern, denoting the coexistence in the same smear of three different tumor cell populations, was described in analogy with human renal nephroblastoma.
THE FUTURE OF NERVOUS SYSTEM CYTOLOGY
Cerebrospinal fluid cytology and fluid analysis are likely to continue to be an important part of the investigation of neurologic disease in dogs and cats. During the last decade, there has been an increased use of sophisticated imaging techniques with increased surgical investigation and surgical and medical treatment of neurologic disease. These trends indicate there may be opportunity to expand the use of nervous system cytology to include examination of fine-needle aspirates of lesions identified within the brain or spinal cord, ventricular fluids obtained by direct aspiration or by cannula, and squash preparations of small biopsy specimens or tissue fragments. All of these techniques are currently used in cytologic evaluations of human patients (Bigner, 1997) and to a limited extent in veterinary medicine (De Lorenzi et al., 2006; Moissoinier et al., 2002; Vernau et al., 2001). There may be increased demand and/or need for development of special preparative techniques such as cell blocks and immunocytochemistry for more precise identification of primary or metastatic tumors, inflammatory cell types, or immunophenotype of malignant or nonmalignant lymphoid proliferations. This has occurred in human medicine for the purpose of diagnosis, prognosis, and disease monitoring.
A recent study (Tilgner et al., 2005) retrospectively analyzed 5000 consecutive stereotactic brain biopsy smear preparations from 4589 patients. In this huge series of cases the intraoperative, cytologic diagnosis was correct in 90.3% of cases. The authors conclude that intraoperative diagnosis with stereotactic biopsy has high validity and that immediate treatment based on smear preparations can be justified. Although in a much smaller series of cases, similar results have been published in veterinary literature as well, with a satisfactory cytologic diagnosis obtained in more than 90% of examined cases (De Lorenzi et al., 2006).
The future of veterinary nervous system evaluation may include types of collection, preparation, and analysis that differ significantly from those currently used. Even if histopathologic and immunohistochemical evaluation of biopsy specimens remains the cornerstone of the final diagnosis, neurocytopathology can be considered, in experienced hands, an extremely useful tool in the workup of patients with nervous system diseases. New approaches using biochemical markers in the CSF (Turba et al., 2007) may provide future areas of diagnostic tools in veterinary medicine. To avoid inappropriate conclusions, diagnostic data provided by cytology should always be in accord and consistent with those derived from an accurate clinicoradiologic investigation. The combination of these approaches should significantly enhance the accuracy of smear cytology.
SUMMARY
Cerebrospinal fluid evaluation is an important part of the investigation of neurologic disease. An alteration in CSF protein content, cell counts, or cytologic findings that provides a definitive diagnosis is infrequent. However, when considered in combination with clinical signs, presentation, history, and results of other tests, a high degree of confidence can be achieved in establishing a clinical diagnosis. Knowledge of methods of collection, sites of collection, methods for analysis, and possible contaminants is an important part of establishing expertise in laboratory and cytologic evaluation of the nervous system. In the future, emerging techniques, new testing methods, and new applications of current test methods may provide information different from or complementary to existing techniques.
REFERENCES
- Abate O, Bollo E, Lotti D. Cytological, immunocytochemical and biochemical cerebrospinal fluid investigations in selected central nervous system disorder of dogs. Zentralbl Veterinarmed [B] 1998;45:73–85. doi: 10.1111/j.1439-0450.1998.tb00769.x. [DOI] [PubMed] [Google Scholar]
- Amude AM, Alfieri AA, Balarin MRS. Cerebrospinal fluid from a 7-month-old dog with seizure-like episodes. Vet Clin Pathol. 2006;35:119–122. doi: 10.1111/j.1939-165x.2006.tb00101.x. [DOI] [PubMed] [Google Scholar]
- Bailey CS, Higgins RJ. Characteristics of cerebrospinal fluid associated with canine granulomatous meningoencephalomyelitis: a retrospective study. J Am Vet Med Assoc. 1986;188:418–421. [PubMed] [Google Scholar]
- Baker R, Lumsden JH. Color atlas of cytology of the dog and cat. Mosby; St. Louis: 2000. pp. 95–115. [Google Scholar]
- Baroni M, Heinold Y. A review of the clinical diagnosis of feline infectious peritonitis viral meningoencephalomyelitis. Prog Vet Neurol. 1995;6:88–94. [Google Scholar]
- Bauer NB, Basset H, O'Neill EJ. Cerebrospinal fluid from a 6-year-old dog with severe neck pain. Vet Clin Pathol. 2006;35:123–125. doi: 10.1111/j.1939-165x.2006.tb00102.x. [DOI] [PubMed] [Google Scholar]
- Behr S, Cauzinille L. Aseptic suppurative meningitis in juvenile boxer dogs: retrospective study of 12 cases. J Am Anim Hosp Assoc. 2006;42:277–282. doi: 10.5326/0420277. [DOI] [PubMed] [Google Scholar]
- Behr S, Trumel C, Cauzinille L. High resolution protein electrophoresis of 100 paired canine cerebrospinal fluid and serum. J Vet Intern Med. 2006;20:657–662. doi: 10.1892/0891-6640(2006)20[657:hrpeop]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Berthelin CF, Legendre AM, Bailey CS. Cryptococcosis of the nervous system in dogs, Part 2: Diagnosis, treatment, monitoring, and prognosis. Prog Vet Neurol. 1994;5:136–145. [Google Scholar]
- Bienzle D, Kwiecien JM, Parent JM. Extramedullary hematopoiesis in the choroid plexus of five dogs. Vet Pathol. 1995;32:437–440. doi: 10.1177/030098589503200417. [DOI] [PubMed] [Google Scholar]
- Bienzle D, McDonnell JJ, Stanton JB. Analysis of cerebrospinal fluid from dogs and cats after 24 and 48 hours of storage. J Am Vet Med Assoc. 2000;216:1761–1764. doi: 10.2460/javma.2000.216.1761. [DOI] [PubMed] [Google Scholar]
- Bigner SH. Central nervous system. In: Bibbo M, editor. Comprehensive cytopathology. WB Saunders; Philadelphia: 1997. pp. 477–492. [Google Scholar]
- Boettcher IC, Steinberg T, Matiasek K. Use of anti-coronavirus antibody testing of cerebrospinal fluid for diagnosis of feline infectious peritonitis involving the central nervous system. J Am Vet Med Assoc. 2007;230:199–206. doi: 10.2460/javma.230.2.199. [DOI] [PubMed] [Google Scholar]
- Bohn AA, Willis TB, West CL. Cerebrospinal fluid analysis and magnetic resonance imaging in the diagnosis of neurologic disease in dogs: a retrospective study. Vet Clin Pathol. 2006;35:315–320. doi: 10.1111/j.1939-165x.2006.tb00138.x. [DOI] [PubMed] [Google Scholar]
- Bush WW, Barr C, Darrin EW. Results of cerebrospinal fluid analysis, neurological examination findings, and age at the onset of seizures as predictors for results of magnetic resonance imaging of the brain in dogs examined because of seizures: 115 cases (1992–2000) JAVMA. 2002;220:781–784. doi: 10.2460/javma.2002.220.781. [DOI] [PubMed] [Google Scholar]
- Carmichael N. Nervous system. In: Davidson M, Else R, Lumsden J, editors. Manual of small animal clinical pathology. British Small Animal Veterinary Association; Cheltenham, UK: 1998. pp. 235–240. [Google Scholar]
- Caswell JL, Nykamp SG. Intradural vasculitis and hemorrhage in full sibling Welsh springer spaniels. Can Vet J. 2003;44:137–139. [PMC free article] [PubMed] [Google Scholar]
- Cellio BC. Collecting, processing, and preparing cerebrospinal fluid in dogs and cats. Compend Contin Educ Pract Vet. 2001;23:786–794. [Google Scholar]
- Chamberlain MC. Comparative spine imaging in leptomeningeal metastases. J Neuro Oncol. 1995;23:233–238. doi: 10.1007/BF01059954. [DOI] [PubMed] [Google Scholar]
- Chrisman CL. Special ancillary investigations. In: Chrisman CL, editor. Problems in small animal neurology. ed 2. Lea & Febiger; Philadelphia: 1991. pp. 81–117. [Google Scholar]
- Chrisman CL. Cerebrospinal fluid analysis. Vet Clin North Am Small Anim Pract. 1992;22:781–810. doi: 10.1016/s0195-5616(92)50077-8. [DOI] [PubMed] [Google Scholar]
- Christopher MM. Bone marrow contamination of canine cerebrospinal fluid. Vet Clin Pathol. 1992;21:95–98. doi: 10.1111/j.1939-165x.1992.tb00592.x. [DOI] [PubMed] [Google Scholar]
- Cizinauskas S, Jaggy A, Tipold A. Long-term treatment of dogs with steroid-responsive meningitis-arteritis: clinical, laboratory and therapeutic results. J Small Anim Pract. 2000;41:295–301. doi: 10.1111/j.1748-5827.2000.tb03205.x. [DOI] [PubMed] [Google Scholar]
- Clemmons RM: Therapeutic considerations for degenerative myelopathy of German Shepherds, New Orleans, Proceedings of the 9th ACVIM Forum, 1991, pp 773-775.
- Cook JR, DeNicola DB. Cerebrospinal fluid. Vet Clin North Am Small Anim Pract. 1988;18:475–499. doi: 10.1016/s0195-5616(88)50051-7. [DOI] [PubMed] [Google Scholar]
- De Lorenzi D, Bernardini M, Mandara MT. Nuove applicazioni in citologia diagnostica veterinaria: il sisterna nervoso centrale. In Proceedings of the 48th SCIVAC National Congress, Rimini, Italy, 2004, pp 136-138.
- De Lorenzi D, Mandara MT, Tranquillo M. Squash-prep cytology in the diagnosis of canine and feline nervous system lesions: a study of 42 cases. Vet Clin Pathol. 2006;35:208–214. doi: 10.1111/j.1939-165x.2006.tb00116.x. [DOI] [PubMed] [Google Scholar]
- De Lorenzi D, Baroni M, Mandara MT. A true “triphasic” pattern: thoracolumbar spinal tumor in a young dog. Vet Clin Pathol. 2007;36:200–203. doi: 10.1111/j.1939-165x.2007.tb00210.x. [DOI] [PubMed] [Google Scholar]
- Desnoyers M, Bédard C, Meinkoth JH. Cerebrospinal fluid analysis. In: Cowell RL, Tyler RD, Meinkoth JH, DeNicola DB, editors. Diagnostic cytology and hematology of the dog and cat. ed 3. Mosby; St. Louis: 2008. pp. 215–234. [Google Scholar]
- Dickinson PJ, Keel MK, Higgins RJ. Clinical and pathologic features of oligodendrogliomas in two cats. Vet Pathol. 2000;37:160–167. doi: 10.1354/vp.37-2-160. [DOI] [PubMed] [Google Scholar]
- Dickinson PJ, Sturges BK, Kass PH. Characteristics of cisternal cerebrospinal fluid associated with intracranial meningiomas in dogs: 56 cases (1985–2004) J Am Vet Med Assoc. 2006;228:564–567. doi: 10.2460/javma.228.4.564. [DOI] [PubMed] [Google Scholar]
- Di Stefano D, Scucchi LF, Cosentino L. Intraoperative diagnosis of nervous system lesions. Acta Cytol. 1998;42:346–356. doi: 10.1159/000331614. [DOI] [PubMed] [Google Scholar]
- Duque C, Parent J, Bienzle D. The immunophenotype of blood and cerebrospinal fluid mononuclear cells in dogs. J Vet Intern Med. 2002;16:714–719. doi: 10.1892/0891-6640(2002)016<0714:tiobac>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Fallin CW, Raskin RE, Harvey JW. Cytologic identification of neural tissue in the cerebrospinal fluid of two dogs. Vet Clin Pathol. 1996;25:127–129. doi: 10.1111/j.1939-165x.1996.tb00982.x. [DOI] [PubMed] [Google Scholar]
- Falzone C, Baroni M, De Lorenzi D. Toxoplasma gondii brain granuloma in a cat: diagnosis using cytology from an intraoperative sample and sequential magnetic resonance imaging. J Small Anim Pract. 2008;49:95–99. doi: 10.1111/j.1748-5827.2007.00421.x. [DOI] [PubMed] [Google Scholar]
- Fenner WR. Diseases of the brain. In: Ettinger SJ, Feldman EC, editors. Textbook of veterinary internal medicine. ed 5. WB Saunders; Philadelphia: 2005. pp. 552–602. [Google Scholar]
- Fenner WR. Central nervous system infections. In: Greene CE, editor. Infectious diseases of the dog and cat. ed 2. WB Saunders; Philadelphia: 1998. pp. 647–657. [Google Scholar]
- Fernandez FR, Grindem CB, Brown TT. Cytologic and histologic features of a poorly differentiated glioma in a dog. Vet Clin Pathol. 1997;26:182–186. doi: 10.1111/j.1939-165x.1997.tb00733.x. [DOI] [PubMed] [Google Scholar]
- Fluhemann G, Konar M, Jaggy A. Cerebral cholesterol granuloma in a cat. J Vet Intern Med. 2006;20:1241–1244. doi: 10.1892/0891-6640(2006)20[1241:ccgiac]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Fry MM, Vernau W, Kass PH. Effects of time, initial composition, and stabilizing agents on the results of canine cerebrospinal fluid analysis. Vet Clin Pathol. 2006;35:72–77. doi: 10.1111/j.1939-165x.2006.tb00090.x. [DOI] [PubMed] [Google Scholar]
- Gaitero L, Anor S, Montoliu P. Detection of Neospora caninum tachyzoites in canine cerebrospinal fluid. J Vet Intern Med. 2006;20:410–414. doi: 10.1892/0891-6640(2006)20[410:doncti]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Galloway AM, Curtis NC, Sommerlad SF. Correlative imaging findings in seven dogs and one cat with spinal arachnoid cyst. Vet Radiol Ultrasound. 1999;40:445–452. doi: 10.1111/j.1740-8261.1999.tb00373.x. [DOI] [PubMed] [Google Scholar]
- Gama FGV, Santana AE, de Campos Filho E. Agarose gel electrophoresis of cerebrospinal fluid proteins of dogs after sample concentration using a membrane microconcentrator technique. Vet Clin Pathol. 2007;36:85–88. doi: 10.1111/j.1939-165x.2007.tb00187.x. [DOI] [PubMed] [Google Scholar]
- Garma-Aviña A, Tyler JW. Large granular lymphocyte pleocytosis in the cerebrospinal fluid of a dog with necrotizing meningoencephalitis. J Comp Pathol. 1999;121:83–87. doi: 10.1053/jcpa.1998.0297. [DOI] [PubMed] [Google Scholar]
- Glass EN, Cornetta AM, deLahunta A. Clinical and clinicopathologic features in 11 cats with Cuterebra larvae myiasis of the central nervous system. J Vet Intern Med. 1998;12:365–368. doi: 10.1111/j.1939-1676.1998.tb02136.x. [DOI] [PubMed] [Google Scholar]
- Greenberg MJ, Schatzberg SJ, deLahunta A. Intracerebral plasma cell tumor in a cat: a case report and literature review. J Vet Intern Med. 2004;18:581–585. doi: 10.1892/0891-6640(2004)18<581:ipctia>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Herzberg AJ. Neurocytology. In: Herzberg AJ, Raso DS, Silverman JF, editors. Color atlas of normal cytology. Churchill Livingstone; New York: 1999. pp. 415–443. [Google Scholar]
- Higgins RJ, LeCouteur RA, Vernau KM. Granular cell tumor of the canine central nervous system: two cases. Vet Pathol. 2001;38:620–627. doi: 10.1354/vp.38-6-620. [DOI] [PubMed] [Google Scholar]
- Hopkins AL, Garner M, Ackerman N. Spinal meningeal sarcoma in a Rottweiler puppy. J Small Anim Pract. 1995;36:183–186. doi: 10.1111/j.1748-5827.1995.tb02880.x. [DOI] [PubMed] [Google Scholar]
- Hurtt AE, Smith MO. Effects of iatrogenic blood contamination of results of cerebrospinal fluid analysis in clinically normal dogs and dogs with neurologic disease. J Am Vet Med Assoc. 1997;211:866–867. [PubMed] [Google Scholar]
- Johnsrude JD, Alleman AR, Schumacher J. Cytologic findings in cerebrospinal fluid from two animals with GM2-gangliosidosis. Vet Clin Pathol. 1996;25:80–83. doi: 10.1111/j.1939-165x.1996.tb00995.x. [DOI] [PubMed] [Google Scholar]
- Keebler CM, Facik M. Cytopreparatory techniques. In: Bibbo M, Wilbur D, editors. Comprehensive cytopathology. ed 3. Saunders; St. Louis: 2008. pp. 977–1003. [Google Scholar]
- Kipar A, Baumgartner W, Vogl C. Immunohistochemical characterization of inflammatory cells in brains of dogs with granulomatous meningoencephalitis. Vet Pathol. 1998;35:43–52. doi: 10.1177/030098589803500104. [DOI] [PubMed] [Google Scholar]
- Lipsitz D, Levitski RE, Chauvet AE. Magnetic resonance imaging of a choroid plexus carcinoma and meningeal carcinomatosis in a dog. Vet Radiol Ultrasound. 1999;40:246–250. doi: 10.1111/j.1740-8261.1999.tb00356.x. [DOI] [PubMed] [Google Scholar]
- Long SN, Anderson TJ, Long FHA. Evaluation of rapid staining techniques for cytologic diagnosis of intracranial lesions. AJVR. 2002;3:381–386. doi: 10.2460/ajvr.2002.63.381. [DOI] [PubMed] [Google Scholar]
- Mariani CL, Platt SR, Scase TJ. Cerebral phaeohyphomycosis caused by Cladosporum spp. in two domestic shorthair cats. J Am Anim Hosp Assoc. 2002;38:225–230. doi: 10.5326/0380225. [DOI] [PubMed] [Google Scholar]
- Mesher CI, Blue JT, Guffroy MRG. Intracellular myelin in cerebrospinal fluid from a dog with myelomalacia. Vet Clin Pathol. 1996;25:124–126. doi: 10.1111/j.1939-165x.1996.tb00981.x. [DOI] [PubMed] [Google Scholar]
- Messer JS, Kegge SJ, Cooper ES. Meningoencephalomyelitis caused by Pasteurella multocida in a cat. J Vet Intern Med. 2006;20:1033–1036. doi: 10.1892/0891-6640(2006)20[1033:mcbpmi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Mikszewski JS, Van Winkle TJ, Troxel MT. Fibrocartilagineous embolic myelopathy in five cats. J Am Anim Hosp Assoc. 2006;42:226–233. doi: 10.5326/0420226. [DOI] [PubMed] [Google Scholar]
- Moissonnier P, Blot S, Devauchelle P. Stereotactic CT-guided brain biopsy in the dog. J Small Anim Prac. 2002;43:115–123. doi: 10.1111/j.1748-5827.2002.tb00041.x. [DOI] [PubMed] [Google Scholar]
- Montoliu P, Añor S, Vidal E. Histological and immunohistochemical study of 30 cases of canine meningioma. J Comp Path. 2006;135:200–207. doi: 10.1016/j.jcpa.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Munana KR, Luttgen PJ. Prognostic factors for dogs with granulomatous meningoencephalomyelitis: 42 cases (1982–1996) J Am Vet Med Assoc. 1998;212:1902–1906. [PubMed] [Google Scholar]
- Neel J, Dean GA. A mass in the spinal column of a dog (nephroblastoma) Vet Clin Pathol. 2000;29:87–89. doi: 10.1111/j.1939-165x.2000.tb00409.x. [DOI] [PubMed] [Google Scholar]
- Parent JM, Rand JS. Cerebrospinal fluid collection and analysis. In: August JR, editor. Consultations in feline internal medicine. ed 2. Saunders; Philadelphia: 1994. pp. 385–392. [Google Scholar]
- Platt SR, Alleman AR, Lanz OI. Comparison of fine-needle aspiration and surgical-tissue biopsy in the diagnosis of canine brain tumors. Vet Surg. 2002;31:65–69. doi: 10.1053/jvet.2002.29456. [DOI] [PubMed] [Google Scholar]
- Pumarola M, Balash M. Meningeal carcinomatosis in a dog. Vet Rec. 1996;25:523–524. doi: 10.1136/vr.138.21.523. [DOI] [PubMed] [Google Scholar]
- Radaelli ST, Platt SR. Bacterial meningoencephalomyelitis in dogs a retrospective study of 23 cases (1990–1999) J Vet Intern Med. 2002;16:159–163. doi: 10.1892/0891-6640(2002)016<0159:bmidar>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Rand JS. The analysis of cerebrospinal fluid in cats. In: Bonagua JD, Kirk RW, editors. Kirk's current veterinary therapy XII: small animal practice. Saunders; Philadelphia: 1995. pp. 1121–1126. [Google Scholar]
- Rand JS, Parent J, Jacobs R. Reference intervals for feline cerebrospinal fluid: cell counts and cytological features. Am J Vet Res. 1990;51:1044–1048. [PubMed] [Google Scholar]
- Rand JS, Parent J, Percy D. Clinical, cerebrospinal fluid and histological data from thirty-four cats with primary noninflammatory disease of the central nervous system. Can Vet J. 1994;35:174–181. [PMC free article] [PubMed] [Google Scholar]
- Ruotsala K, Poma R, da Costa RC. Evaluation of the ADVIA 120 for analysis of canine cerebrospinal fluid. Vet Clin Pathol. 2008;37:242–248. doi: 10.1111/j.1939-165X.2008.00036.x. [DOI] [PubMed] [Google Scholar]
- Shah AB, Muzumdar GA, Chitale AR. Squash preparation and frozen section in intraoperative diagnosis of central nervous system tumors. Acta Cytol. 1998;41:1149–1154. doi: 10.1159/000332104. [DOI] [PubMed] [Google Scholar]
- Sharkey LC, McDonnell JJ, Alroy J. Cytology of a mass on the meningeal surface of the left brain in a dog. Vet Clin Pathol. 2004;33:111–114. doi: 10.1111/j.1939-165x.2004.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Sheppard BJ, Chrisman CL, Newell SM. Primary encephalic plasma cell tumor in a dog. Vet Pathol. 1997;34:621–627. doi: 10.1177/030098589703400612. [DOI] [PubMed] [Google Scholar]
- Simpson ST, Reed RB. Manometric values of normal cerebrospinal fluid pressure in dogs. J Am Anim Hosp Assoc. 1987;23:629. [Google Scholar]
- Singh M, Foster DJ, Child J. Inflammatory cerebrospinal fluid analysis in cats: clinical diagnosis ad outcome. J Feline Med Surg. 2005;7:77–93. doi: 10.1016/j.jfms.2004.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeen TM, Olby NJ, Munana KR. Spinal arachnoid cysts in 17 dogs. J Am Anim Hosp Assoc. 2003;39:271–282. doi: 10.5326/0390271. [DOI] [PubMed] [Google Scholar]
- Snyder JM, Shofer FS, Van Winkle TJ. Canine intracranial primary neoplasia: 173 cases (1986–2003) J Vet Intern Med. 2006;20:669–765. doi: 10.1892/0891-6640(2006)20[669:cipnc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Sorjonen DC. Clinical and histopathological features of granulomatous meningoencephalomyelitis in dogs. J Am Anim Hosp Assoc. 1990;26:141–147. [Google Scholar]
- Stalis IH, Chadwick B, Dayrell-Hart B. Necrotizing meningoencephalitis of Maltese dogs. Vet Pathol. 1995;32:230–235. doi: 10.1177/030098589503200303. [DOI] [PubMed] [Google Scholar]
- Stampley AR, Swaynev DE, Prasse KW. Meningeal carcinomatosis secondary to a colonic signet-ring cell carcinoma in a dog. J Am Anim Hosp Assoc. 1986;23:655–658. [Google Scholar]
- Thompson CA, Russell KE, Levine JM. Cerebrospinal fluid from a dog with neurologic collapse. Vet Clin Pathol. 2003;32:143–146. doi: 10.1111/j.1939-165x.2003.tb00328.x. [DOI] [PubMed] [Google Scholar]
- Thomson CE, Kornegay JN, Stevens JB. Analysis of cerebrospinal fluid from the cerebellomedullary and lumbar cisterns of dogs with focal neurologic disease: 145 cases (1985–1987) J Am Vet Med Assoc. 1990;196:1841–1844. [PubMed] [Google Scholar]
- Tilgner J, Herr M, Ostertag C. Validation of intraoperative diagnoses using smear preparations from stereotactic brain biopsies: intraoperative versus final diagnosis–influence of clinical factors. Neurosurgery. 2005;56:257–265. doi: 10.1227/01.neu.0000148899.39020.87. [DOI] [PubMed] [Google Scholar]
- Timmann D, Konar M, Howard J. Necrotizing encephalitis in a French bulldog. J Small Anim Pract. 2007;48:339–342. doi: 10.1111/j.1748-5827.2006.00239.x. [DOI] [PubMed] [Google Scholar]
- Tipold A, Fatzer R, Jaggy A. Necrotizing encephalitis in Yorkshire terriers. J Small Anim Pract. 1993;34:623–628. [Google Scholar]
- Tipold A, Vandevelde M, Zurbriggen A. Neuroimmunological studies in steroid-responsive meningitis-arteritis in dogs. Res Vet Sci. 1995;58:103–108. doi: 10.1016/0034-5288(95)90060-8. [DOI] [PubMed] [Google Scholar]
- Tremblay N, Lanevschi A, Doré M. Of all the nerve! A subcutaneous forelimb mass in a cat. Vet Clin Pathol. 2005;34:417–420. doi: 10.1111/j.1939-165x.2005.tb00073.x. [DOI] [PubMed] [Google Scholar]
- Troxel MT, Vite CH, Van Winkle TJ. Feline intracranial neoplasia: review of 160 cases (1985–2001) J Vet Intern Med. 2003;17:850–859. doi: 10.1111/j.1939-1676.2003.tb02525.x. [DOI] [PubMed] [Google Scholar]
- Turba ME, Forni M, Gandini G. Recruited leukocytes and local synthesis account for increased matrix metalloproteinase-9 in cerebrospinal fluid of dogs with central nervous system neoplasm. J Neurooncol. 2007;81:123–129. doi: 10.1007/s11060-006-9213-2. [DOI] [PubMed] [Google Scholar]
- Uchida K, Hasegawa T, Ikeda M. Detection of an autoantibody from pug dogs with necrotizing encephalitis (pug dog encephalitis) Vet Pathol. 1999;36:301–307. doi: 10.1354/vp.36-4-301. [DOI] [PubMed] [Google Scholar]
- Vernau KM, Higgins RJ, Bollen AW. Primary canine and feline nervous system tumours: intraoperative diagnosis using the smear technique. Vet Pathol. 2001;38:47–57. doi: 10.1354/vp.38-1-47. [DOI] [PubMed] [Google Scholar]
- Zimmerman KL, Bender HS, Boon GD. A comparison of the cytologic and histologic features of meningiomas in four dogs. Vet Clin Pathol. 2000;29:29–34. doi: 10.1111/j.1939-165x.2000.tb00394.x. [DOI] [PubMed] [Google Scholar]
- Zimmerman K, Almy F, Carter L. Cerebrospinal fluid from a 10-year-old dog with a single seizure episode. Vet Clin Pathol. 2006;35:127–131. doi: 10.1111/j.1939-165x.2006.tb00103.x. [DOI] [PubMed] [Google Scholar]


