Abstract
Lectins are proteins of non-immune origin that bind specific carbohydrates without chemical modification. Coupled with the emerging biological and pathological significance of carbohydrates, lectins have become extensively used as research tools in glycobiology. However, lectin-based drug development has been impeded by high manufacturing costs, low chemical stability, and the potential risk of initiating an unfavorable immune response. As alternatives to lectins, non-protein small molecules having carbohydrate-binding properties (lectin mimics) are currently attracting a great deal of attention because of their ease of preparation and chemical modification. Lectin mimics of synthetic origin are divided roughly into two groups, boronic acid-dependent and boronic acid-independent lectin mimics. This article outlines their representative architectures and carbohydrate-binding properties, and discusses their therapeutic potential by reviewing recent attempts to develop antiviral and antimicrobial agents using their architectures. We also focus on the naturally occurring lectin mimics, pradimicins and benanomicins. They are the only class of non-protein natural products having a C-type lectin-like ability to recognize d-mannopyranosides in the presence of Ca2 + ions. Their molecular basis of carbohydrate recognition and therapeutic potential are also discussed.
Keywords: Antibiotic, Antiviral drug, Carbohydrate recognition, Lectin mimic, Pradimicin
Abbreviations
- 2D-DARR
two-dimensional dipolar-assisted rotational resonance
- AIDS
acquired immunodeficiency syndrome
- BAMP
2,5-bis-(aminomethyl)pyrrole
- CA
Cymbidium sp. agglutinin
- CP/MAS
cross-polarization/magic angle spinning
- CV-N
cyanovirin-N
- EGTA
ethylene glycol-bis(2-aminoethyl ether)-N,N,N′,N′-tetraacetic acid
- ELISA
enzyme-linked immunosorbent assay
- Gal
d-galactose
- Glc
d-glucose
- GlcNAc
N-acetyl-d-glucosamine
- GNA
Galanthus nivalis agglutinin
- HCV
hepatitis C virus
- HEA
Epipactis helleborine agglutinin
- HHA
Hippeastrum hybrid agglutinin
- HIV
human immunodeficiency virus
- ICD
induced circular dichroism
- LOA
Listera ovata agglutinin
- MALDI-MS
matrix-assisted laser desorption/ionization mass spectroscopy
- Man
d-mannose
- Me-α-Glc
methyl α-d-glucopyranoside
- Me-β-Glc
methyl β-d-glucopyranoside
- Me-α-GlcNAc
methyl 2-acetamido-2-deoxy-α-d-glucopyranoside
- Me-β-GlcNAc
methyl 2-acetamido-2-deoxy β-d-glucopyranoside
- Me-α-Man
methyl α-d-mannopyranoside
- Me2SO
dimethyl sulfoxide
- MIC
minimum inhibitory concentration
- NMR
nuclear magnetic resonance
- NPA
Narcissus pseudonarcissus agglutinin
- Oct-β-Gal
n-octyl β-d-galactopyranoside
- Oct-α-Gal
n-octyl α-d-galactopyranoside
- Oct-α-Glc
n-octyl α-d-glucopyranoside
- Oct-β-Glc
n-octyl β-d-glucopyranoside
- Oct-β-GlcNAc
n-octyl 2-acetamido-2-deoxy-β-d-glucopyranoside
- Oct-β-Man
n-octyl β-d-mannopyranoside
- Oct-α-Man
n-octyl α-d-mannopyranoside
- PET
photoinduced electron transfer
- PRM
pradimicin
- ROS
reactive oxygen species
- sLex
sialyl Lewis X
- SPR
surface plasmon resonance
- STD
saturation-transfer difference
- TF
Thomsen–Friedenreich
- TIMS
targeted imaging mass spectroscopy
- UDA
Urtica dioica agglutinin
- WGA
wheat-germ agglutinin
I. Introduction
Carbohydrates regulate a variety of biochemical pathways and disease processes, including protein folding, fertilization, embryogenesis, neuronal development, cell proliferation, microbial and viral infections, and cancer metastasis.1, 2, 3, 4, 5 Coupled with advances in understanding of the functions of carbohydrates in biological and pathological processes, the importance of carbohydrate-binding molecules has been rapidly growing as chemical tools in glycobiology. The most extensively used carbohydrate-binding molecules are lectins, which are defined as proteins of non-immune origin that bind specific carbohydrates without modifying them.6, 7 They have proved to be extremely useful tools not only for the isolation and characterization of glycoproteins but also for detection of dynamic spatiotemporal changes that are associated with pathological processes.8, 9, 10 In addition, several lectins derived from prokaryotic, plant, invertebrate, or vertebrate species having binding specificity for mannose, galactose, or N-acetylglucosamine (GlcNAc) have been found to inhibit the infection of enveloped viruses, such as human immunodeficiency virus (HIV) and hepatitis C virus (HCV).11, 12, 13 Representative lectins having antiviral activity are the mannose-specific agglutinins from Galanthus nivalis (GNA), Hippeastrum hybrid (HHA), Cymbidium sp. (CA), Narcissus pseudonarcissus (NPA), Epipactis helleborine (HEA), and Listera ovata (LOA), the GlcNAc-specific agglutinin from Urtica dioica (UDA), and the cyanobacterium-derived lectin (CV-N). These lectins show the dual mode of antiviral action, blockage of virus entry, and triggering the action of immune system by exposing cryptic immunogenic epitopes of the virus surface. The discovery of these unique anti-HIV properties of lectins that have never been observed in any of the existing chemotherapeutics has spurred intensive study of lectins as therapeutic agents.
While lectins are of great value as possible drug candidates as well as chemical tools in glycobiology research, they have several disadvantages due to their nature as proteins. First of all, large-scale production of proteins is generally both costly and time-consuming, and their relative low chemical stability may be problematic for long storage. From a therapeutic viewpoint, the bioavailability of proteins is likely to be poor, and concerns are also raised about the unfavorable biological properties of lectins, such as immunogenicity, inflammatory activity, cellular toxicity, mitogenic stimulation of human peripheral lymphocyte cells, and hemagglutination of human red blood cells. Under these situations, non-protein small-size molecules having carbohydrate-binding properties have been recently attracting a great deal of attention as alternatives to lectins of protein structure. Since small molecules can be prepared in reliable quantities and are easily “tuned” for structural optimizations, these “lectin mimics” may have potential benefit as research tools and drug candidates.
Lectin mimics of synthetic origin have been developed progressively during recent years.14, 15, 16, 17 On the basis of molecular-design principles, the synthetic lectin mimics may be divided roughly into two groups, boronic acid-dependent and boronic acid-independent lectin mimics. The former group possesses boronic acid motifs, which form tight and reversible covalent bonds with 1, 2- or 1, 3-diol groups of carbohydrates. While boronic acid itself has a low affinity for most common carbohydrates at physiological pH, the affinity can be enhanced by introduction of neighboring functional groups and/or use of two boronic acid motifs of proper alignment. In contrast, boronic acid-independent lectin mimics rely solely on non-covalent interactions for binding to carbohydrates. These types of lectin mimics have been intensively developed in the field of supramolecular chemistry. Representative molecular architectures include tripod- and cage-types, both of which incorporate, respectively, hydrophilic and hydrophobic surfaces for hydrogen bonding and CH/π interaction (a weak hydrogen bond occurring between CH groups and aromatic rings)18 with carbohydrates. A combination of these non-covalent interactions collectively realizes three-dimensional recognition of carbohydrates.
This article outlines the design concept, molecular architecture, and carbohydrate-binding properties of synthetic lectin mimics by using representative examples, and discusses their therapeutic potential by reviewing recent attempts to develop antiviral and antimicrobial agents using their architectures. We also focus on naturally occurring lectin mimics, pradimicins (PRMs) and benanomicins.19 They are the only class of non-protein natural products having a C-type lectin-like property of being able to recognize d-mannopyranosides (Man) in the presence of Ca2 + ions. Recent investigations have revealed an interesting similarity in molecular architecture between these natural products and tripod-type lectin mimics. Moreover, accumulated evidence suggests that they have unique antimicrobial profiles that differ from those of the major classes of antibiotics, and exhibit anti-HIV activity in a manner similar to lectins. These emerging concepts of the molecular basis of carbohydrate recognition and therapeutic potential of naturally occurring lectin mimics are also described.
II. Synthetic Lectin Mimics
1. Molecular Architecture of Boronic Acid-Dependent Lectin Mimics
Boronic acid-dependent lectin mimics contain more than one boronic acid moiety, which is responsible for their carbohydrate binding. Boronic acids act as Lewis acids because of the empty p-orbital on boron, and in the presence of Lewis bases such as hydroxyl groups, interconversion from sp2 to sp3 hybridization readily occurs.20, 21, 22 In aqueous media, a water molecule reversibly adds to a neutral trigonal form (I) of boronic acids to produce an anionic tetrahedral form (II) with a release of one proton (Scheme 1 ). In the alkaline pH range (pH > 10), diols can react with the tetrahedral form (II) to give stable cyclic boronate esters (IV). The general affinity of boronic acids for diols in carbohydrates is as follows: cis-1, 2-diol > 1, 3-diol ≫ trans-1, 2-diol. On the other hand, boronic acids exist predominately as the trigonal form (I) in the neutral pH range. As a result, reaction with diols produces chemically unstable adducts (III), which are readily hydrolyzed. Therefore, a high pH is generally required in order to favor the equilibrium toward cyclic boronate esters (IV).
Scheme 1.
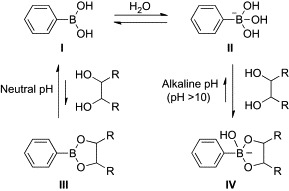
Binding of phenylboronic acid with a diol.
To realize arylboronic acid/diol association at neutral pH, several attempts have been made to stabilize their adducts. One of the most promising strategies is the introduction of dialkylaminomethyl groups to the ortho position of arylboronic acids (Scheme 2 ).23, 24 The covalently appended amino groups facilitate formation of a boronic ester and accelerate arylboronic acid/diol association by direct B–N coordination or “solvent-insertion” mechanism. Recent investigations by X-ray crystallography, 11B-NMR, and computational analysis support that the latter mechanism is predominant in protic solvents.25, 26, 27 By using this molecular architecture as a key structure, a number of boronic acid-dependent lectin mimics have been reported.14, 28 The majority of these works have been directed toward the development of molecular sensors for monitoring blood d-glucose as a key component of insulin-releasing implants for diabetes patients. In early landmark studies, Shinkai and coworkers developed carbohydrate-based photoinduced electron-transfer sensors (1, 2, Fig. 1 ).29, 30, 31 These compounds bound carbohydrates at neutral pH (pH 7.77) and showed increased fluorescence through suppression of the photoinduced electron transfer (PET) from the tertiary amino group to the anthracenyl group upon binding of the carbohydrate. While the binding selectivity of 1 for d-glucose was poor (the order of selectivity for 1 is d-fructose > d-allose ≈ d-galactose > d-glucose > ethylene glycol), compound 2 exhibited binding preference for d-glucose (the order of selectivity for 2 is d-glucose > d-allose > d-fructose ≈ d-galactose > ethylene glycol). This selectivity of 2 for d-glucose was explained by the spatial disposition of the two boronic acid moieties. The two inwardly facing boronic acid moieties are perfectly spaced and aligned for two pairs of diol groups (1,2- and 4,6-hydroxyl groups) of d-glucose.
Scheme 2.
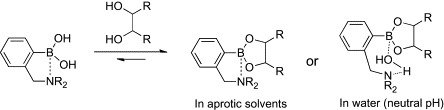
Binding of o-(N,N-dialkylaminomethyl)phenylboronic acid with a diol.
Fig. 1.
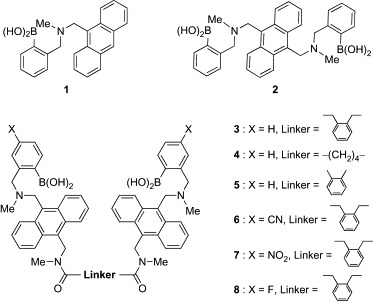
Boronic acid-dependent lectin mimics (1–8).
Using the same fluorescent reporter system, Wang and coworkers developed a series of dimer analogues of 1 (3–8, Fig. 1).32, 33 Of three dimers having different linkers (3–5), compound 3 showed the highest selectivity for d-glucose over d-fructose and d-galactose (Table I ), suggesting that the linker of 3 offers the appropriate orientation and distance of two boronic acid moieties for binding with d-glucose. Although the affinity of 3 for d-glucose was about three times lower than that of 2, compound 3 represents about 3-fold enhancement in selectivity for d-glucose over d-fructose. Based on these results, an additional three derivatives with electron-withdrawing groups, which generally enhance the carbohydrate affinity of arylboronic acids, were tested for their affinity and selectivity for carbohydrates. Although the introduction of cyano and nitro groups enhanced the affinity for d-glucose, the effects were more significant for binding to d-fructose and d-galactose. As a result, the selectivity of 6 and 7 for d-glucose was lower than that of the parent compound 3. The fluoro-substituted derivative (8) was inferior to 3 in both affinity and selectivity. Although the substituent effect on carbohydrate selectivity of arylboronic acids is hard to predict, it is interesting that a simple modification of the aryl group changes the binding preference for carbohydrates.
Table I.
Binding Constants of 2–8 for Carbohydrates in Phosphate Buffer (pH 7.4)
Introduction of chirality into the PET sensor was successfully performed by Shinkai and coworkers.34 The sensor 9 having the (R)-1,1′-binaphthyl moiety as the chiral and fluorophore building block discriminated d-glucose from l-glucose (Fig. 2 ). A maximum of a 4-fold increase in fluorescence intensity was observed upon binding of 9 to d-glucose, whereas l-glucose induced only a 2-fold increase. Competition experiments showed that 9 had the ability to recognize d-glucose selectively in the presence of l-glucose. Very recent examples of PET sensors using chiral units are the R and S enantiomers of 10, which contain (1R)- and (1S)-1-(1-naphthyl)ethanamines, respectively.35 The binding of d-glucose to R-10 caused a significant increase in fluorescence intensity, whereas smaller changes in fluorescence were observed in the case of S-10. These studies clearly demonstrate that selectivity of boronic acid-dependent lectin mimics can be finely tuned toward one optical isomer of carbohydrates.
Fig. 2.
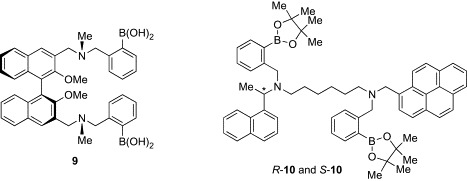
Boronic acid-dependent lectin mimics (9, 10).
In addition to these glucose sensors, chemical probes for carbohydrate chains have been also reported. One example is a fluorescent probe (11) for sialyl Lewis X (sLex) developed by Wang and coworkers (Fig. 3 ).36 The tetrasaccharide sLex is a well-known cell-surface carbohydrate antigen associated with the development and progression of many types of cancer.37, 38, 39, 40 Probes for sLex are therefore promising as the diagnostic agents for cancer. Compound 11 showed strong fluorescence enhancement upon binding to sLex in a 1:1 mixture of methanol and phosphate buffer (pH 7.4). Moreover, 11 was found to fluorescently label HEPG2 cells expressing high levels of sLex, while 11 stained neither HEP3B cells expressing Lewis Y nor COS7 cells lacking fucosylated antigens. Using this unique fluorescent sLex probe, matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS)-based imaging of cancer tissue has been performed.41 Targeted imaging mass spectrometry (TIMS) is a powerful method of imaging for histological analysis, which allows spatial visualization of a molecule of interest directly from tissue sections by the use of laser-reactive photo-cleavable molecular tags attached to affinity molecules.42, 43 Wang and coworkers synthesized a conjugate molecule (12) of 11 and a trityl-based tag, and TIMS analysis of cancer tissues having a high level of sLex was performed. The tumor region expressing sLex was successfully detected by the imaging, suggesting that MS-based imaging of cancer tissues using lectin mimic-based probes is feasible.
Fig. 3.
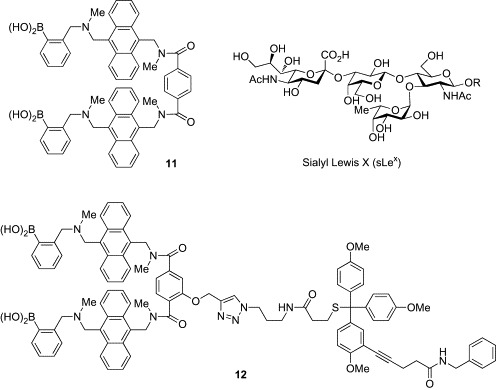
Probes for sialyl Lewis X (11, 12).
Although o-dialkylaminomethyl arylboronic acids have long stood as the established standard for the design of boronic acid-dependent lectin mimics, Hall and coworkers have subsequently introduced o-hydroxymethyl phenylboronic acid (benzoboroxole) as a promising alternative.44, 45 Benzoboroxole (13) is considered to exist in its cyclic dehydrated boronophthalide form (Scheme 3 ). The unusually small C–B–O dihedral angle of 13 facilitates production of an anionic boronate ester with a diol even at neutral pH by opening up the cone angle in the resulting tetrahedral structure. The boronate ester formation of 13 is also entropically more favorable than that of the simple phenylboronic acid, which requires the external hydroxyl ligand to form the anionic tetrahedral complex (Scheme 1). The most important and interesting feature of 13 is its capability to bind the pyranose form of carbohydrates in neutral water. Several lines of evidence suggest that carbohydrates bind to boronic acids, including o-dialkylaminomethyl arylboronic acids in their weakly populated furanose forms, and not in their pyranose form.46, 47 In contrast, 13 was shown to bind hexopyranosides of d-glucose, d-galactose, d-mannose, and d-fucose with weak but encouraging affinities (Table II ). Although the exact mode of complexation of 13 with them is not fully understood, the relatively high Lewis acidity and strained cyclic structure of 13 are thought to be possible contributing factors for its exceptional pyranoside-binding behavior.
Scheme 3.
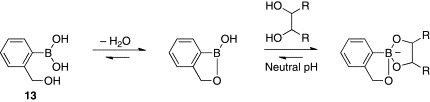
Binding of benzoboroxole (13) with a diol.
Table II.
Binding Constants of 13 for Hexopyranosides in Phosphate Buffer (pH 7.4)
| Hexopyranoside | Ka (M− 1)a |
|---|---|
| d-Glucose | 31 |
| Methyl α-d-glucopyranoside | 22 |
| Methyl β-d-glucopyranoside | 9 |
| Methyl α-d-galactopyranoside | 29 |
| Methyl β-d-galactopyranoside | 23 |
| Methyl α-d-mannopyranoside | 24 |
| Methyl α-d-fucopyranoside | 25 |
Data cited from Ref. 45.
By taking advantage of the pyranoside-binding capability, Hall and coworkers have developed a water-soluble receptor (14–16, Fig. 4 )48 for the Thomsen–Friedenreich (TF) disaccharide, an important tumor-associated carbohydrate antigen that is present in over 90% of cancers.49 The design of the receptor was based on the assumption that two units of benzoboroxoles would bind two diol units of the disaccharide, and the peptide backbone could be involved in hydrogen bonding and CH/π interaction with the disaccharide. The tri(ethylene glycol) component was introduced to increase the water solubility. A library of 400 examples having different peptide backbones was prepared by solid-phase peptide synthesis and was screened for binding to the TF antigen disaccharide by use of a competitive enzyme-linked immunosorbent assay (ELISA). The most potent receptor, showing an IC50 of 20 μM, was compound 14, having an electron-rich p-methoxyphenylalanine component and a furan, both of which are favorable for CH/π interaction with hydrogen atoms on the carbohydrate rings. The importance of the benzoboroxole moieties was confirmed by the weak affinities of control compounds (15: IC50 = 54 μM, 16: IC50 = 100 μM) for the TF disaccharide. These studies demonstrate that benzoboroxole (13) holds a great promise for the design of lectin mimics for carbohydrate biomarkers of biological importance.
Fig. 4.
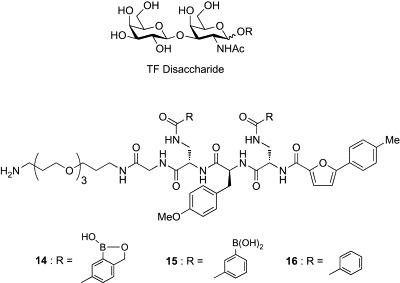
Receptors for TF disaccharide (14–16).
2. Antiviral Potential of Boronic Acid-Dependent Lectin Mimics
The use of lectins has been recently proposed as a novel and promising approach for antiviral chemotherapy.11, 12, 13 Lectins exhibit antiviral activity against a range of viruses such as HIV,50, 51 HCV,52, 53 coronavirus,54, 55 and influenza virus.56, 57 The antiviral effects of these lectins are ascribed to their specific binding to carbohydrate chains (glycans) on the viral envelope. It is well known that several proteins of the viral envelope are densely glycosylated. For example, gp120, the HIV-1 envelope glycoprotein, possesses 18–30 (an average of 24) N-linked glycans, which constitute about half of the molecular weight of the gp120 molecule.58 These glycans on the HIV-1 envelope play crucial roles in enabling an efficient entry of HIV into its susceptible target cells.59, 60 Binding of lectins to these glycans thus inhibits the efficient functioning of the envelope molecules during viral entry.61, 62, 63 Another important role of the envelope glycans is to hide the highly immunogenic epitopes from the immune system of the host.64, 65 Because of this glycan shield, the neutralizing antibody response is not elicited, and as a result, the immune system is not able to suppress HIV. However, these glycans are progressively deleted by long-term exposure of HIV to lectins through mutation of HIV.66, 67, 68, 69 Weakening of the glycan shield triggers the production of neutralizing antibodies against the immunogenic epitopes of gp120 previously hidden.
Despite the promising dual mode of anti-HIV action of lectins, concerns regarding their therapeutic use have been raised on account of the unacceptably high cost of large-scale production, low chemical stability, and unfavorable immunogenic response. Investigations are therefore ongoing to identify lectin mimics having high synthetic accessibility and high chemical stability. Boronic acid-dependent lectin mimics are undoubtedly potential candidates that exhibit lectin-like anti-HIV activities. The first attempt to evaluate antiviral activities of small-size boronic acid derivatives has been made by McGuigan and coworkers.70 In an initial screening, a diverse range of ortho-, meta-, and para-substituted monophenylboronic acids were tested for their antiviral activities against a broad range of viruses, including HIV-1, HIV-2, varicella zoster virus, and influenza virus (Fig. 5 ). Unfortunately, none of the compounds showed antiviral activity at subtoxic concentrations or bound to gp120 of HIV-1. The same research group therefore performed the second screening using a library of bisphenylboronic acids having a variety of linkers (Fig. 6 ).71 Although these compounds have a potential to bind two sets of diol groups in such glycans as 2–8, surface plasmon resonance (SPR) experiments revealed that none of the bisphenylboronic acids bound to gp120 of HIV-1, even at 50 μM. Obviously, all of the compounds lacked the ability to inhibit virus replication. Although the results were disappointing, these data are valuable for future research aimed at designing small-size phenylboronic acid derivatives having antiviral activity.
Fig. 5.
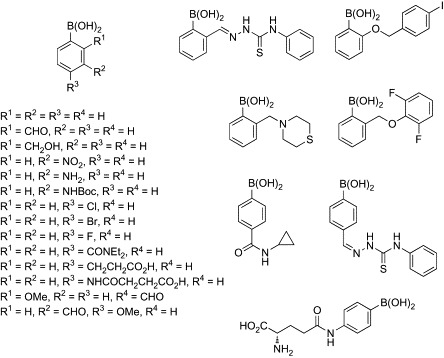
Library of monophenyl boronic acid compounds.
Fig. 6.

Library of bisphenylboronic acid compounds.
On the other hand, Kiser and coworkers have successfully developed water-soluble polymers of phenylboronic acids as promising anti-HIV agents (Fig. 7 ).72 The advantage of using the polymer structure is that multivalent interaction can be realized between the multiply linked boronic acid groups in the polymer and the abundant glycans on the viral envelope. Multivalent interactions can compensate for weak binding affinity of ligands because of entropic advantage and steric stabilization, and are often found in natural systems, including the carbohydrate–lectin interaction.73, 74 Moreover, they utilized benzoboroxole (13) for the boronic acid components, which can bind to such non-reducing carbohydrates as methyl α-d-mannopyranoside and methyl α-d-galactopyranoside at physiological pH, as already described.44, 45 The use of benzoboroxole is quite rational, because 30–50% of glycans in HIV-1 gp120 are high mannose-type oligosaccharides containing three terminal non-reducing mannose residues, and 50–63% are complex-type oligosaccharides terminating to some extent with non-reducing galactose groups (Fig. 8 ).58 Based on these molecular design concepts, the research group of Kisher synthesized benzoboroxole-functionalized oligomers (18) and polymers (20–22). In these compounds, 5-methacrylamido-2-hydroxymethylphenyl boronic acid-derived monomers were incorporated at different feed ratios into linear backbones with 2-hydroxypropylmethacrylamide. The SPR experiments revealed that the 25 mol% functionalized oligomer (18) showed an about 8-fold higher affinity for gp120 of HIV-1 than the simple benzoboroxole (13). On the other hand, no binding was detected for an oligomer lacking benzoboroxole components (17) at any concentrations, indicating that multivalent interaction of the benzoboroxole groups with gp120 glycans is responsible for the significant affinity of compound 18. Antiviral activity against HIV-1 was evaluated only for the polymer compounds (19–22) because polymers of larger molecular weight decrease cytotoxicity by preventing cellular uptake. While the 25 mol% functionalized polymer 20 showed only slightly higher activity (EC50 = 15 μM) than the control polymer without benzoboroxole groups (19, EC50 ≥ 50 μM), increasing the degree of functionalization markedly decreased the EC50 values. The 75 mol% functionalized polymer (22) exhibited a strong activity (EC50 = 0.015 μM) comparable to that of the natural lectin CV-N.
Fig. 7.
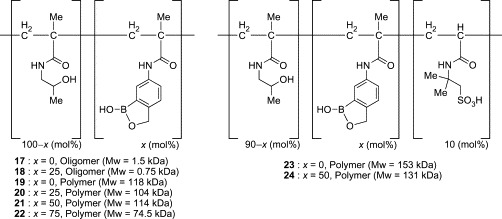
Benzoboroxole-functionalized polymers (17–24).
Fig. 8.
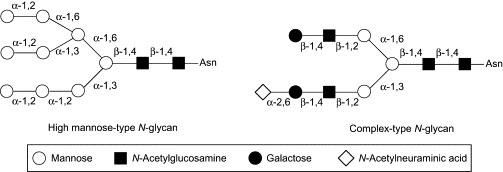
Structures of high mannose-type and complex-type N-glycans.
Kisher's group has also developed a second generation of the benzoboroxole-functionalized polymer, with 10 mol% of 2-acrylamido-2-methylpropanesulfonic acid (24).75 Incorporation of the negatively charged sulfonate groups was expected not only to increase the aqueous solubility of the polymer at neutral pH but also to increase the binding affinity to gp120 through electrostatic interaction with the positively charged peptide fragments in the V3 loop of gp120. In fact, an almost 100-fold increase in aqueous solubility was observed with the incorporation of the methylpropanesulfonic acid moieties, and compound 24 showed enhanced anti-HIV-1 activity (EC50 = 4 nM). A physical mixture of 21 and 23 did not demonstrate any significant increase in activity, suggesting that simultaneous ionic and covalent interactions possibly contribute to the observed synergistic improvement in antiviral activity of 24. Moreover, biocompatibility evaluations on VEC-100-reconstructed human vaginal tissue suggest that 24 is likely to be nontoxic. These studies provide the first example of boronic acid-dependent lectin mimics having anti-HIV activity and demonstrate that these polymers are promising candidates for further development as microbicides.
3. Molecular Architecture of Boronic Acid-Independent Lectin Mimics
In contrast to the boronic acid-dependent lectin mimics that utilize non-natural covalent interactions for carbohydrate binding, boronic acid-independent lectin mimics bind carbohydrates through natural non-covalent interactions. These receptors generally realize the carbohydrate recognition by a combination of hydrogen bonding, CH/π interaction, and metal coordination, in a manner similar to that of natural lectins.15, 16, 17 From this characteristic, boronic acid-independent lectin mimics are often referred to as “biomimetic carbohydrate receptors.” By imitating the binding motifs observed in the crystal structures of lectin–carbohydrate complexes, a number of boronic acid-independent lectin mimics have been designed. Among them, this section focuses on tripod-type and cage-type architectures and reviews the design concept and carbohydrate-binding properties of representative compounds.
The tripod-type receptors, in principle, incorporate three hydrophobic units interconnected by an arene spacer (Fig. 9 ). Whereas the apolar bottom face of this architecture interacts with CH groups of carbohydrates through CH/π interaction, the polar lateral face forms hydrogen bonds with the hydroxyl groups of carbohydrates. These non-covalent interactions collectively realize three-dimensional recognition of carbohydrates by the tripod-type receptors. Early examples are compounds 25 and 26 developed by Mazik and coworkers.76, 77, 78, 79 These receptors contain a benzene platform as the π-donor for CH/π interaction and three amidopyridine moieties as hydrophilic units (Fig. 10 ). 1H-NMR spectroscopic titrations in chloroform (Table III ) revealed that both receptors formed very strong 2:1 receptor–carbohydrate complexes with n-octyl β-d-glucopyranoside (Oct-β-Glc), n-octyl α-d-glucopyranoside (Oct-α-Glc), and n-octyl β-d-galactopyranoside (Oct-β-Gal). Replacement of the amidopyridine moieties by aminopyridine groups and incorporation of methyl or ethyl groups into the central phenyl ring (27, 28, Fig. 10) significantly increased the binding selectivity for Oct-β-Glc. In addition, curve fitting of the titration data for receptors 27 and 28 with Oct-β-Glc suggested the existence of both 1:1 and 1:2 receptor–carbohydrate complexes in chloroform, with strong association constants for 1:1 complexes. The results of these initial studies demonstrated that the tripod-type architecture is effective for the carbohydrate recognition, and subtle structural variation can lead to remarkable changes of the receptor properties.
Fig. 9.
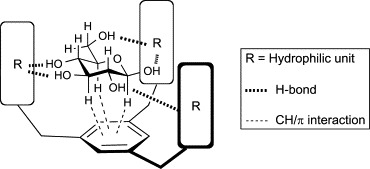
Typical architecture of tripod-type boronic acid-independent lectin mimics.
Fig. 10.

Tripod-type boronic acid-independent lectin mimics (25–28).
Table III.
Binding Constants of 25–40 for n-Octyl d-Glycosides in Chloroform
| β-Glc |
α-Glc |
β-Gal |
||||
|---|---|---|---|---|---|---|
| Receptor | K11a | K21b or K12c | K11 | K21 or K12 | K11 | K21 or K12 |
| 25d | 660 | 24,200 | 3640 | 82,450 | 420 | 50,770 |
| 26d | 440 | 22,600 | 2100 | 47,600 | 300 | 29,350 |
| 27d | 20,950 | 790 | 800 | – | 1360 | 211 |
| 28d | 48,630 | 1320 | 1310 | – | 3070 | 470 |
| 29e | 144,520 | 4330 | 24,880 | 1750 | NTf | NT |
| 30e | 39,800 | 1610 | 2280 | – | NT | NT |
| 31e | 18,900 | 2850 | 1840 | – | NT | NT |
| 32g | 191,730 | 8560 | 3160 | 1540 | 3320 | 300 |
| 33g | 156,100 | 10,360 | 2820 | 350 | 7470 | 1100 |
| 34h | > 100,000 | 10,000 | > 100,000 | NDi | 9550 | 1030 |
| 35j | > 100,000 | ND | > 100,000 | ND | 13,360 | 800 |
| 36k | > 100,000 | ND | 7450 | 1150 | > 100,000 | ND |
| 37k | 45,900 | 730 | 1280 | 250 | 38,000 | 1100 |
| 38l | 28,800 | 530 | 4360 | 210 | 44,540 | 1680 |
| 39l | 12,600 | 450 | 1660 | 280 | 19,400 | 940 |
| 40m | 69,500 | 1060 | 6810 | 100 | 148,700 | 1580 |
1:1 receptor–carbohydrate association constant
2:1 receptor–carbohydrate association constant;
1:2 receptor–carbohydrate association constant
Data cited from Ref. 79
Data cited from Ref. 80;
Not tested
Data cited from Ref. 81
Data cited from Ref. 82
Not detected
Data cited from Ref. 83;
Data cited from Ref. 84
Data cited from Ref. 85
Data cited from Ref. 86.
Since these discoveries, Mazik's group has performed systematic studies using the tripodal architecture and found that a variety of functional groups, such as heteroaromatic, guanidinium, carboxylate, crown ether, hydroxy, amide, and oxime-based groups, were suitable for the hydrophilic units.87 The tripod-type receptors reported by Mazik's group are also shown in Fig. 11 (29–40). Receptors 29, 30, and 31 contain, respectively, the primary amide, amino, and hydroxyl groups instead of one aminopyridine unit of 28.80 These functional groups were incorporated as analogues of the side chains of Asn, Gln, Lys, and Ser, which are often observed in the carbohydrate-binding motifs of natural lectins. While the binding profiles of 30 and 31 with the amino and hydroxyl groups, respectively, were almost similar to that of the parent compound 28, significant enhancement in affinity for Oct-β-Glc was observed in 29 with two primary amide groups (Table III). Receptors 32 and 33 containing imidazole and indole rings, which mimic the side chains of His and Trp, respectively, were also tested for their binding to Oct-β-Glc, Oct-α-Glc, and Oct-β-Gal.81 Marked enhancements in binding to all glycosides were observed in both compounds. 1H-NMR analysis suggested that CH/π interaction might contribute significantly to the complexation of 32 and 33 with Oct-β-Glc. On the other hand, introduction of an unnatural phenanthroline ring to the hydrophilic unit (34) was shown to be particularly effective in the binding to Oct-β- and Oct-α-Glc.82 Molecular-modeling calculations suggested that the binding pocket of 34 has the correct shape and size for encapsulation of these glucopyranosides.
Fig. 11.
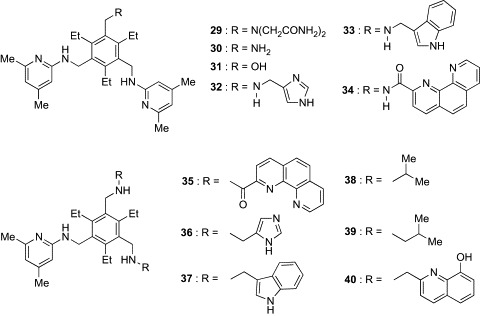
Tripod-type boronic acid-independent lectin mimics (29–40).
Based on the observation that the phenanthroline, imidazole, and indole rings were valuable building blocks for hydrophilic units, a new series of receptors (35–37) containing two units of these building blocks were designed.83, 84 Although the affinities of both 34 and 35, having, respectively, one and two phenanthroline units, for Oct-β-Glc and Oct-α-Glc were too large to be calculated in chloroform, titration experiments in 5% Me2SO–chloroform revealed that 35 was a superior β-Glc binder to 34 (K 11 for 34 = 36,530 M− 1, K 11 for 35 = 78,400 M− 1). These results indicate that the incorporation of the second phenanthroline unit causes further enhancement of the binding affinity of the receptor. On the other hand, significant changes in binding preference were observed in 36 and 37, containing, respectively, two imidazole and indole units. Whereas the affinities of these receptors for Oct-β-Glc were markedly decreased as compared to those of 32 and 33 with, respectively, one imidazole and indole unit, they displayed a high level of affinity toward Oct-β-Gal. The binding selectivity for Oct-β-Gal was further increased in 38 and 39, which have the alkyl side chains of Val and Leu in the hydrophilic units, respectively.85 Their binding affinities for Oct-β-Gal were slightly higher than those for Oct-β-Glc. Van der Waals contacts between the branched alkyl groups and glycosides probably contribute to the binding capacity of 38 and 39. The most effective receptor for Oct-β-Gal reported to date is 40, a compound with two 8-hydroxyquinoline units.86 Receptor 40 displayed more than 2-fold selectivity for Oct-β-Gal over Oct-β-Glc and Oct-α-Glc, and its affinity for Oct-β-Gal was extremely potent. In addition to these tripod-type receptors for monosaccharides, Mazik's group has developed the tetrapod-type receptors 41 and 42 (Fig. 12 ).88 Combination of the dimesitylmethane scaffold and four aminopyridine units was expected to provide a cavity suitable for disaccharides. Indeed, these receptors exhibited a strong binding preference for n-dodecyl β-maltoside (K 21 ≥ 100,000 M− 1 for 41 and 42) over Oct-β-Glc (K 12 = 630 M− 1 for 41, K 12 = 560 M− 1 for 42) in chloroform, indicating that these relatively large architectures fit well to the disaccharide structures.
Fig. 12.
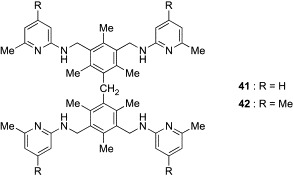
Tetrapod-type boronic acid-independent lectin mimics (41, 42).
Roelens and coworkers have developed a series of pyrrolic tripodal receptors using the triethylbenzene scaffold, representatives of which are shown in Fig. 13 (43–47). These receptors were evaluated against a set of n-octyl glycosides of biologically relevant monosaccharides, including Glc, Gal, Man, and GlcNAc. Roelens's group has assessed the binding affinities of the receptors using the original parameter, (intrinsic median binding concentration) descriptor, which is defined as the total concentration of receptor necessary for binding 50% of the ligand when the fraction of bound receptor is zero.89 The value can be viewed as a global dissociation constant in chemical systems involving any number of complex species. Table IV summarizes values of 43–47 for the set of n-octyl glycosides in chloroform or acetonitrile. The progenitors 43 and 44, having imino and amino groups, respectively, displayed modest to high affinity for all n-octyl glycosides in chloroform.89 Both receptors had a preference for Oct-β-Glc and Oct-β-GlcNAc, indicating that the pyrrolic tripodal architecture is well suited for binding to carbohydrates having all-equatorial arrays of polar functionality. However, the binding selectivity was completely different in 45, which has an acetal substituent in the α position of the pyrrole.90 Receptor 45 showed a striking affinity for Oct-β-Man, which was too strong to be evaluated in chloroform. Titration experiments in acetonitrile revealed that the affinity of 45 for Oct-β-Man was almost one order of magnitude greater than those of the other glycosides. NMR experimental data combined with molecular-modeling calculations suggested that this notable selectivity of 45 for Oct-β-Man was derived from a conformational restriction induced by the cyclic acetal substituents on the pyrrole unit.92 The resulting narrower binding-cleft of 45 fits preferentially to Oct-β-Man. Based on these promising results, Roelens's group developed a new generation of Man-selective receptors incorporating chiral trans-1,2-diaminocyclohexane units (46, 47).91, 93, 94 Receptors 46 and 47 displayed substantial affinity and selectivity for Oct-α- and Oct-β-Man, respectively. Replacement of the diamines of 46 and 47 with the corresponding enantiomers decreased both affinity and selectivity, indicating that the chirality of the receptor plays a key role in determining the recognition properties of the receptors. In regard to affinity and selectivity, 46 and 47 are the most effective Man receptors reported to date.
Fig. 13.
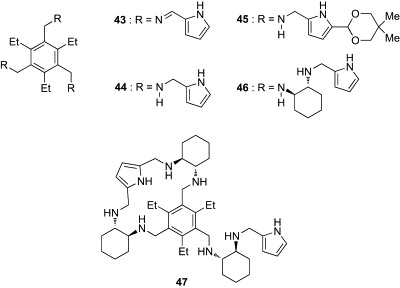
Tripod-type boronic acid-independent lectin mimics (43–47).
Table IV.
Intrinsic Median Binding Concentrations of 43–47 for n-Octyl d-Glycosides in Chloroform or Acetonitrile
|
(μM) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Receptor | α-Glc | β-Glc | α-Gal | β-Gal | α-Man | β-Man | α-GlcNAc | β-GlcNAc |
| 43a, b | 268 | 4.8 | 368 | 120 | 262 | 660 | 1179 | 30 |
| 44a, b | 570 | 24 | 790 | 70 | 43 | 37 | 72 | 18 |
| 45a, c | 570 | 39 | 2250 | 185 | 2.8 | < 1 | 6.4 | 6.9 |
| 45c, d | 25,600 | 7940 | 28,300 | 10,290 | 5850 | 680 | 5880 | 6990 |
| 46d, e | 1251 | 929 | 1245 | 2450 | 127 | 873 | 1070 | 905 |
| 47d, e | 2493 | 1248 | 3183 | 1532 | 286 | 83 | 1573 | 1298 |
Miller and coworkers have generated a novel class of tripod-type receptors having a cis-1,3,5-trisubstituted cyclohexane scaffold (Fig. 14 ).95 To achieve the conformational restriction of the receptors, three pyridine (48) or quinoline (49) rings were directly attached to the cyclohexane ring. 1H NMR, UV, and fluorescence titration experiments demonstrated that both compounds strongly bound to n-octyl glycosides in chloroform and even in a polar solvent, methanol (Table V ). It is noteworthy that 48 displayed the highest binding affinity reported to date for Oct-α-Glc in chloroform and retained its micromolar range of affinities in methanol. These results indicate that cyclohexane ring can also serve as an effective scaffold for designing tripod-type receptors.
Fig. 14.

Tripod-type boronic acid-independent lectin mimics (48, 49).
Table V.
Binding Constants of 48 and 49 for n-Octyl d-Glycosides in Chloroform or Methanol
Another representative architecture of boronic acid-independent lectin mimics is the cage type, which consists of two parallel aromatic surfaces held apart by hydrophilic groups (Fig. 15 ).16, 96 Carbohydrates can be fully enclosed by this architecture, in which the roof and floor of the aromatic rings provide apolar surfaces for CH/π interaction, and polar pillars of hydrophilic units are capable of hydrogen bonding with carbohydrates. Because of this structural feature, the cage-type receptors specifically recognize carbohydrates such as β-Glc and β-GlcNAc that bear all-equatorial arrays of polar functionality and all-axial arrays of hydrophobic CH groups.
Fig. 15.

Typical architecture of cage-type boronic acid-independent lectin mimics.
In a pioneering study, Davis and coworkers constructed a prototype 50 having two biphenyl and eight isophthalamide groups (Fig. 16 ).97 Molecular modeling suggested that the cavity created by this architecture was sufficiently large to accept a β-Glc molecule, making up to six intermolecular hydrogen bonds and several CH/π interactions. This prediction was confirmed by binding studies employing fluorescence-titration experiments in chloroform. Receptor 50 bound Oct-β-Glc with substantial affinity (K a = 300,000 M− 1) and reasonable selectivity over Oct-α-Glc (K a = 13,000 M− 1) and Oct-β-Gal (K a = 100,000 M− 1). Moreover, NMR experiments revealed that 50 was capable of binding Oct-β-Glc (K a = 980 M− 1), even in the presence of methanol (CDCl3:CD3OH = 92:8). Davis's group has also developed an extended variant of 50 (compound 51) as a selective receptor for disaccharides having all-equatorial arrays of polar functionality.98 While no binding was detected with Oct-β-Glc, n-octyl β-lactoside [β-Gal-(1 → 4)-Glc], n-octyl β-maltoside [α-Glc-(1 → 4)-Glc], and n-dodecyl α-maltoside in CDCl3:CD3OH (92:8), compound 51 significantly bound n-octyl β-cellobiose [β-Glc-(1 → 4)-Glc] with K a of 7000 M− 1.
Fig. 16.
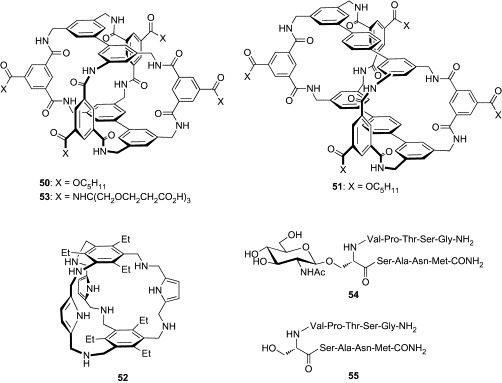
Cage-type boronic acid-independent lectin mimics (50–53) and (glyco)peptides (54, 55).
Roelens and coworkers also reported a cage-type receptor incorporating aminopyrrolic groups (52, Fig. 16), which was active in an organic solvent.99 The roof and floor of 52 were constructed from 1,3,5-triethylbenzene, which has been extensively employed for the core scaffold in tripod-type receptors, as already discussed. Although the binding cavity of 52 is smaller relative to that of Davis's receptor (50), compound 52 was capable of enclosing Oct-β-Glc with a K a of 48,300 M− 1 in chloroform. Moreover, compound 52 was found not to bind either Oct-α-Glc or both isomers of Oct-Gal and Oct-Man, indicating that the architecture of 52 is also effective for the recognition of carbohydrates having an all-equatorial array of polar functionality.
Based on the promising results of this early work, the Davis group investigated the effectiveness of the cage architecture in aqueous media. After the successful development of a water-soluble variant of compound 50 (53, Fig. 16),100 in which a tricarboxylate group is installed in each hydrophilic component, its binding property against a panel of 22 carbohydrates was thoroughly examined (Table VI ).101 In accordance with the binding preference of 50 in organic solvents, compound 53 displayed substantial affinity for methyl β-d-glucopyranoside (Me-β-Glc) in water (K a = 28 M− 1). However, it was found that methyl 2-acetamido-2-deoxy β-d-glucopyranoside (Me-β-GlcNAc) was a better target for 53 (K a = 630 M− 1). The affinity of compound 53 for Me-β-GlcNAc was comparable to that of a natural lectin, wheat-germ agglutinin (WGA, K a = 730 M− 1), which has classically been used to bind GlcNAc residues. It is also noteworthy that 53 was far more discriminatory than WGA. Whereas WGA exhibits significant binding to several 2-acetamido sugars, including GlcNAc, Me-α-GlcNAc, N-acetylneuraminic acid, and N,N′-diacetyl-d-chitobiose, the affinities of 53 for other 21 carbohydrates were more than one order of magnitude weaker than that for Me-β-GlcNAc. Since β-GlcNAc attached to the hydroxyl group of serine or threonine is a common posttranslational modification of proteins, binding experiments of 53 with a glycopeptide containing a GlcNAc residue were also conducted using 54 (Fig. 16). This was based on the sequence from GlcNAc-linked casein kinase II.102 1H NMR titration experiments in D2O demonstrated that 53 bound to 54 with significant affinity (K a = 1040 M− 1). On the other hand, binding to the aglycosyl peptide 55 was negligible, supporting the conclusion that 53 binds 54 through the GlcNAc residue.
Table VI.
Binding Constants of Compound 53 and the Natural Lectin, Wheat-Germ Agglutinin (WGA), for Carbohydrates in Aqueous Solution
|
Ka (M− 1)a |
||
|---|---|---|
| Carbohydrate | 53 | WGA |
| Me-β-GlcNAc | 630b | 730c |
| N-Acetyl-d-glucosamine (GlcNAc) | 56b | 410d |
| Methyl β-d-glucopyranoside | 28b | |
| Me-α-GlcNAc | 24e | 480c |
| Cellobiose | 17b | |
| d-Glucose | 9b | |
| 2-Deoxy-d-arabino-hexose | 7b | |
| Methyl α-d-glucopyranoside | 7b | |
| d-Xylose | 5b | |
| d-Ribose | 3b | |
| d-Galactose | 2b | |
| l-Fucose | 2b | |
| N-Acetyl-d-galactosamine (GalNAc) | 2b | 60c |
| N-Acetyl-d-mannosamine (ManNAc) | 2b | 60c |
| d-Arabinose | 2b | |
| d-Lyxose | ≤ 2b | |
| d-Mannose | ≤ 2b | |
| l-Rhamnose | ≤ 2b | |
| Maltose | ≤ 2b | |
| Lactose | ≤ 2b | |
| N-Acetylmuramic acid (MurAc) | 0b | |
| N-Acetylneuraminic acid (Neu5Ac) | 0b | 560b |
| N,N′-Diacetylchitobiose | 0b | 5,300d |
Data cited from Ref. 101
Measured by 1H NMR titration in D2O
Estimated from inhibitory activity of WGA precipitation induced by N-acetyl-d-glucosamine
Measured by isothermal titration calorimetry in H2O
Measured by induced circular dichroism in H2O.
Elegant work by Davis and coworkers has also developed a water-soluble cage-type receptor for disaccharides.103 Although the extended receptor 51 was successful in discriminating n-octyl β-cellobiose from monosaccharides and other disaccharides in chloroform, the cavity of this relatively flexible architecture had a tendency to collapse in aqueous solution by the hydrophobic contacts of the aromatic rings of the roof and floor. To avoid this phenomenon, Davis's group employed a meta-terphenyl structure in place of the para-terphenyl group of 51 and incorporated the fifth isophthalamide spacer. The resulting “rigid” variant of 51 (56, Fig. 17 ) bearing carboxylate groups was shown to bind cellobiose (K a = 560–600 M− 1) and methyl β-cellobioside (K a = 850–910 M− 1) with good affinities in aqueous solutions (Table VII ). The binding affinities of compound 56 for carbohydrates were evaluated by 1H NMR, induced circular dichroism (ICD), and fluorescence titrations. To obtain reliable K a values, at least two methods were used for each carbohydrate. While 56 showed moderate affinities for xylobiose (K a = 250–270 M− 1) and N,N′-diacetylchitobiose (K a = 120 M− 1), both of which are also all-equatorial disaccharides, only weak binding was detected for monosaccharides and other disaccharides (K a ≤ 25 M− 1). Interestingly, compound 56 can distinguish between cellobiose and lactose, the structures of which differ from each other only by one asymmetric center.
Fig. 17.
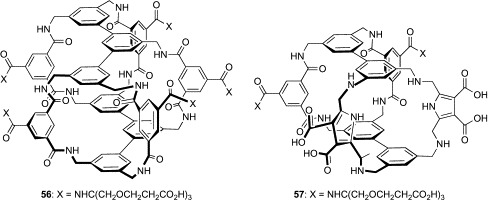
Cage-type boronic acid-independent lectin mimics (56, 57).
Table VII.
Binding Constants of Compound 56 for Carbohydrates in Aqueous Solution, as Measured by 1H NMR, ICD, and Fluorescence Titrations
|
Ka (M− 1)a |
|||
|---|---|---|---|
| Carbohydrate | 1H NMR | ICD | Fluorescence |
| Cellobiose | 600 | 580 | 560 |
| Methyl β-cellobioside | 910 | 850 | |
| Xylobiose | 250 | 270 | |
| N, N′-Diacetylchitobiose | 120 | 120 | |
| Lactose | 11 | 14 | |
| Mannobiose | 13 | 9 | |
| Maltose | 15 | 11 | |
| Gentiobiose | 12 | 5 | |
| Trehalose | NDb | ND | |
| Sucrose | ND | ND | |
| d-Glucose | 11 | 12 | ND |
| d-Ribose | ND | ND | |
| N-Acetyl-d-glucosamine | 24 | 19 | |
Data cited from Ref. 103
Not detected.
In subsequent work, an interesting hybrid molecule of 52 and 53 has been generated.104 In the new water-soluble receptor 57 (Fig. 17), two isophthalamide spacers of 53 are replaced by two 2,5-bis(aminomethyl)pyrrole (BAMP) spacers of 52. The isophthalamide and BAMP spacers are similar to each other in length, but very different in hydrogen-bonding characteristics. In the isophthalamide spacer, two sets of the amide hydrogen and amide carbonyl groups can act as hydrogen-bond donors and acceptors, respectively. On the other hand, the BAMP spacer contains one pyrrole ring as a hydrogen-bond donor and two amino groups, which can act either as donors or acceptors, depending on the pH of the solution. 1H NMR titration experiments under basic conditions (pD = 13) demonstrated that the affinity of 57 for d-glucose (K a = 18 M− 1) was twice as high as that of 53 (K a = 9 M− 1). However, 57 bound weakly to Me-β-Glc (K a = 4 M− 1) and GlcNAc (K a = 7 M− 1), in contrast to 53 (K a = 28 M− 1 for Me-β-Glc, K a = 56 M− 1 for GlcNAc). Although the binding test under neutral conditions has not been performed because of solubility problems with 57, this study suggests that selectivity among all-equatorial carbohydrates can be tuned though modification of the hydrogen-bonding property of the cage-type receptors.
As already discussed, the affinity and selectivity of some cage-type receptors for carbohydrates are almost comparable with those of natural lectins. In this regard, the cage-type receptors are often called as “synthetic lectins.” Since synthetic lectins are superior to natural lectins in terms of chemical stability, synthetic accessibility, and ease of structural modification, they have great potential for applications in glycobiology research.
4. Antiviral and Antimicrobial Potential of Boronic Acid-Independent Lectin Mimics
Coupled with the successful development of tripod-type and cage-type lectin mimics, their biomedical applications have begun to be explored. When considering their application to anti-HIV and antimicrobial therapies, it would be desirable to design receptors capable of binding to Man, which is a main component of high mannose-type oligosaccharides on the HIV envelope, and cell-wall mannans of pathogenic yeasts and bacteria.105, 106 In this respect, the cage-type receptors are unlikely to be useful in applications as anti-HIV and antimicrobial agents because of their weak preference for Man. On the other hand, several tripod-type receptors have been shown to bind Man in organic solvents. Although none of tripod-type receptors thus far reported can bind carbohydrates in aqueous media, the tripod-type architecture has an advantage with regard to its simplicity in structure, which allows for easy preparation of libraries of compounds for systematic studies. By exploiting this advantage, attempts to develop tripod-type receptors with anti-HIV and antimicrobial activities have been recently made.
Pérez-Pérez and coworkers have synthesized a library of tripod-type compounds and have evaluated their anti-HIV activities.107 The library is classified into three groups, the monomers (58–66, Fig. 18 ), dimers (67–75, Fig. 19 ), and trimers (76–81, Fig. 20 ) of the 1,3,5-triazine motif, incorporating two aromatic amino acid components (Phe, Tyr, or Trp). Each triazine motif is considered to have the tripod-type architecture, in which the 1,3,5-triazine ring is a core scaffold and the amino acid and amine groups can act as hydrophilic components. The carboxylate groups of the amino acid residues are also crucial for increasing the water solubility of the compounds. The dimers and trimers were designed based on the assumption that multivalent interaction can be realized between the two or three triazine motifs and glycans on the viral envelope. The anti-HIV activity of these compounds was evaluated as the inhibitory activity against HIV-1(IIIB) and HIV-2(ROD) replication in the CEM T-cell culture (Table VIII ). While the monomers (58–66) were devoid of anti-HIV activity even at 250 μM, significant activity was observed in several dimers and trimers. Among the dimers, the Trp-containing 69 and 72 showed moderate anti-HIV activity, with EC50 values of 65 and 56 μM for HIV-1(IIIB) and 63 and 81 μM for HIV-2(ROD), respectively. Enhanced activity was observed in the trimers, especially in those having triethylbenzene as the central unit. Thus, 79–81 afforded EC50 values of 16–33 μM for both HIV-1(IIIB) and HIV-2(ROD). The same order in anti-HIV activity (trimer > dimer ≫ monomer) was also observed in binding studies to HIV-1 gp120. SPR analysis revealed that the trimer 79 bound strongly to gp120, with K dvalue of 1.61 μM. On the other hand, the corresponding dimer 72, which exhibited low antiviral activity, showed a much lower amplitude of binding, and binding of the corresponding inactive monomer 63 was barely detected. The strong correlation for the mono-, di-, and trimer between antiviral activity and binding affinity to HIV-1 gp120 possibly indicates that the anti-HIV activity of these tripod-type compounds is ascribed to the binding to HIV-1 gp120, and that a certain level of multivalent interactions is required to show both antiviral and binding activities. One remaining question is whether the observed affinity to HIV-1 gp120 is due to binding to the glycans or do the polyanionic compounds interact with protein areas of gp120, such as the positively charged V3 loop. Additionally, the anti-HIV activities of these compounds seem still modest compared to the activities of natural lectins. For example, the reported EC50 values of CV-N for HIV-1(IIIB) and HIV-2(ROD) are 0.003 and 0.002 μM, respectively,68, 108 and thus both of these are more than three orders of magnitude lower than those of 79. Nevertheless, this study provides strong evidence that anti-HIV drug discovery based on the tripod-type architecture is a viable approach.
Fig. 18.

Monomers (58–66) of 1,3,5-triazine compounds.
Fig. 19.

Dimers (67–75) of 1,3,5-triazine compounds.
Fig. 20.

Trimers (76–81) of 1,3,5-triazine compounds.
Table VIII.
Anti-HIV Activities of 67–81 in Human T-Lymphocyte Cell Cultures
| EC50 (μM)a |
||
|---|---|---|
| Compound | HIV-1(IIIB) | HIV-2(ROD) |
| 67 | 106 | 121 |
| 68 | ≥ 250 | ≥ 250 |
| 69 | 65 | 63 |
| 70 | > 10b | > 10 |
| 71 | 148 | 80 |
| 72 | 56 | 81 |
| 73 | ≥ 250 | ≥ 250 |
| 74 | > 250 | > 250 |
| 75 | 112 | 120 |
| 76 | 65 | 216 |
| 77 | ≥ 250 | ≥ 250 |
| 78 | 20 | 65 |
| 79 | 16 | 22 |
| 80 | 26 | 24 |
| 81 | 18 | 33 |
Data cited from Ref. 107
Precipitation was detected at higher concentration.
In other work, Roelens and coworkers have explored the potential of tripod-type receptors as the antimicrobial agents.109 As already described, Roelens's group has developed a family of aminopyrrolic tripod-type compounds, some of which proved to be effective receptors for Man in polar organic solvents. The pradimicins and benanomicins (see the later section) are small-size Man receptors that occur in nature. They are reported to show potent antimicrobial activity against a wide variety of fungi and yeasts, including clinically important pathogens, by binding to mannans on the cell wall. Focusing on the similarity of Man-binding properties of the aminopyrrolic tripod-type compounds and pradimicin-related natural products, Roelens's group performed the first investigation on the antimicrobial activity of aminopyrrolic tripod-type receptors. In preliminary screening, the progenitor of the family, compound 44, and the most effective Man receptors, 46 and 47, were tested for their antimicrobial activities against Candida tropicalis, Pichia norvegensis, Prototheca wickerhamii, and Prototheca zopfii (Table IX ). All compounds were shown to inhibit markedly the growth of the four microorganisms. In particular, the minimum inhibitory concentration (MIC) values of 44 were comparable for those of the well-known antibiotics, amphotericin B and ketoconazole. Structural segments (82–87, Fig. 21 ) of compound 44 were less active or inactive, indicating that all structural components are essential for antimicrobial activity. Analogues of 44 were also prepared, and representative examples (88–92) are shown in Fig. 22 . Antimicrobial assay using a variety of yeast and yeast-like microorganisms revealed that none of the modifications, including introduction of functional groups into the pyrrole rings (88–90) and variation of substitution pattern on the benzene platform (91, 92), enhanced the activity of the parent compound 44 (Table X ). The binding affinity of these compounds for Oct-α-Man was also evaluated in acetonitrile. Although this affinity may not reflect the binding for mannans in water, a broad correlation was detected between the antimicrobial activity and Man-binding affinity; compounds without antimicrobial activity lacked Man-binding ability, whereas compounds having antimicrobial activity showed detectable binding for Man. However, perfect correlation was not obtained. On the basis of these results, Roelens and coworkers proposed the interesting hypothesis that Man binding of the tripod-type compounds would facilitate the approach to the microbial cells. This would trigger a process that would subsequently involve penetration across the membrane into the cytoplasm, where the compounds might exert antimicrobial activity by interacting with a specific target. Although further research is necessary to validate this hypothesis, this pioneering study emphasizes the promising potential of tripod-type carbohydrate receptors as antimicrobial agents having a novel mode of action.
Table IX.
Antibiotic Activities of Compounds 44, 46, 47, and 82–87 Against Yeast and Yeast-Like Microorganisms
| MIC (μg/mL)a |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Species | 44 | 46 | 47 | 82 | 83 | 84 | 85 | 86 | 87 |
| Candida tropicalis | 4 | 8 | 16 | 512 | 128 | 64 | 256 | NDb | ND |
| Pichia norvegensis | 2 | 4 | 16 | 512 | 64 | 16 | 64 | ND | ND |
| Prototheca wickerhamii | 2 | 4 | 16 | ND | 16 | 64 | 256 | ND | ND |
| Prototheca zopfii | 8 | 4 | 16 | ND | 32 | 128 | 256 | ND | ND |
Data cited from Ref. 109
Not determined (> 1024 μg/mL).
Fig. 21.
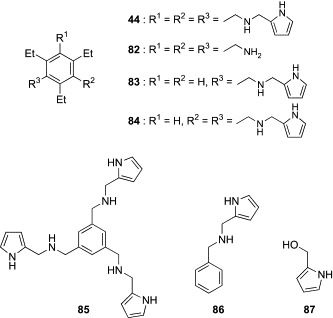
Aminopyrrolic tripod-type receptor (44) and its structural segments (82–87).
Fig. 22.
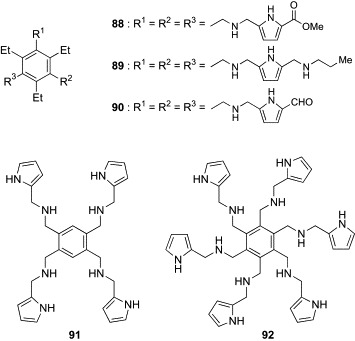
Analogues (88–92) of the aminopyrrolic tripod-type receptor (44).
Table X.
Antibiotic Activities of 44 and 88–92 Against Yeast and Yeast-Like Microorganisms
| MIC (μg/mL)a |
||||||
|---|---|---|---|---|---|---|
| Species | 44 | 88 | 89 | 90 | 91 | 92 |
| Candida albicans | 16 | 256 | 64 | 512 | 1024 | 256 |
| Candida glabrata | 8 | 64 | 128 | ND | 1024 | 256 |
| Meyerozyma guilliermondii | 8 | 64 | 64 | 512 | 1024 | 64 |
| Candida parapsilosis | 8 | 64 | NDb | ND | ND | 512 |
| Candida tropicalis | 4 | 32 | 8 | 128 | 512 | 32 |
| Pichia norvegensis | 2 | 8 | 8 | 32 | 64 | 8 |
| Clavispora lusitaniae | 32 | 128 | 128 | 512 | ND | 256 |
| Yarrowia lipolytica | 8 | 128 | 32 | 256 | 512 | 64 |
| Oichia kudriavzevii | 16 | 64 | 128 | 128 | 512 | 64 |
| Kluyveromyces marxianus | 8 | 8 | 8 | 128 | 256 | 32 |
| Prototheca wickerhamii | 2 | 128 | 256 | 256 | 256 | 32 |
| Prototheca zopfii | 8 | 128 | 256 | 1024 | 256 | 64 |
Data cited from Ref. 109
Not determined (> 1024 μg/mL).
III. Naturally Occurring Lectin Mimics
1. Antimicrobial and Carbohydrate-Binding Profiles of Pradimicins and Benanoimicins
While there are a number of publications describing synthetic lectin mimics, only a few natural products possessing carbohydrate-binding ability have been reported. Pradimicins (PRMs) and benanomicins are the only family of naturally occurring lectin mimics with non-peptidic skeletons. In 1988, three PRMs (PRM-A, B, C) and two benanomicins (benanomicin A, B) were first isolated from actinomycetes as potential antibiotics, by Oki's and Kondo's groups, respectively.110, 111 Interestingly, these compounds were found to share a common aglycon, namely benzo[a]naphthacenequinone, and differ only in the glycon components (Fig. 23 ). Since then, benanomicin B was shown to be the same as PRM-C,112, 113 and 12 additional members of the PRM class demonstrating antimicrobial activity have been identified.114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124 These structurally unique natural products show potent in vitro antimicrobial activity against a wide variety of yeasts and fungi, and high in vivo therapeutic efficacy in murine models against Candida albicans, Cryptococcus neoformans, and Aspergillus fumigatus. Table XI, Table XII list the in vitro and in vivo antimicrobial profiles of PRM-A. Since there is no cross-resistance to other antimicrobial agents, such as amphotericin B, 5-fluorocytosine, and ketoconazole, this family of compounds is chemically and functionally different from the other major classes of antimicrobial agents. The initial biochemical study using C. albicans found that PRM-A causes K+ leakage from the cells and binds to the outer surface of C. albicans cells in a Ca2 +-dependent manner.126 The absorption of PRM-A on C. albicans cells was found to originate from binding to mannan in the yeast cell wall. These results collectively suggest that the antimicrobial action of PRM-A is associated with alteration of the membrane permeability by binding to cell-wall mannan. This putative mechanism explains well the fact that PRM-A does not induce K+ leakage from human erythrocytes or other mammalian cells in the presence of Ca2 + ion.
Fig. 23.
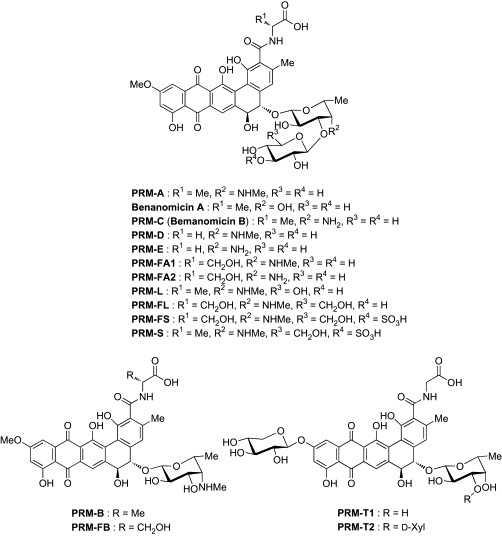
Pradimicins (PRMs) and benanomicins.
Table XI.
In Vitro Antibiotic Activities of PRM-A and BMY-28864 (93)
| MIC (μg/mL)a |
||
|---|---|---|
| Species | PRM-A | BMY-28864 (93) |
| Saccharomyces cerevisiae | ||
| ATCC 9763 | 12.5 | 3.1 |
| Candida albicans | ||
| IAM 4888 | 50 | 6.3 |
| A9540 | 50 | 6.3 |
| ATCC 38247 | 3.1 | 0.8 |
| ATCC 32354 | 12.5 | 6.3 |
| Candida tropicalis | ||
| IFO 10241 | >100 | 50 |
| CS-07 | 25 | 6.3 |
| Candida parapsilosis | ||
| CS-08 | >100 | 3.1 |
| Candida krusei | ||
| A15052 | 25 | 3.1 |
| Cryptococcus neoformans | ||
| D49 | 1.6 | 1.6 |
| IAM 4514 | 0.8 | 1.6 |
| CS-01 | 1.6 | 1.6 |
| Aspergillus fumigatus | ||
| IAM 2034 | 3.1 | 3.1 |
| IAM 2530 | 1.6 | 3.1 |
| Aspergillus flavus | ||
| FA 21436 | 6.3 | 6.3 |
| CS-18 | 25 | 100 |
| Sporothrix schenckii | ||
| IFO 8158 | 1.6 | 3.1 |
| Trichophyton mentagrophytes | ||
| No. 4329 | 6.3 | 12.5 |
| D155 | 3.1 | 12.5 |
Data cited from Ref. 125.
Table XII.
In Vivo Activities of PRM-A and BMY-28864 (93) Against Candida, Cryptococcus, and Aspergillus Systematic Infection in Mice (n = 5)
| PD50 (mg/kg)a, b |
||
|---|---|---|
| Species | PRM-A | BMY-28864 (93) |
| Candida albicans A9540 | 8.9 | 9 |
| Cryptococcus neoformans IAM 4514 | 11 | 11 |
| Aspergillus fumigatus IAM 2034 | 16 | 36 |
50% protective dose
Data cited from Ref. 125.
The structure–microbial activity relationship of PRM-A has been also thoroughly examined by using naturally occurring congeners and artificial derivatives (Fig. 24 ). Replacement of the d-Ala component with l-amino acids, including l-Ala, abolished antimicrobial activity.127, 128 On the other hand, d-α-aminobutanoic acid, d-Phe, d-Asp, d-2,3-diaminopropanoic acid, d-Lys, Gly, and d-Ser derivatives retained the activity, indicating that stereochemistry at position 17 is crucial for the antimicrobial action of PRM-A, while the side chain of d-Ala plays only a marginal role. The amide hydrogen at position 16 seems to be important, because a complete loss of activity was observed in sarcosine (N-methylglycine) and d-Pro derivatives. The role of the carboxyl group at position 18 was also examined, using the ester and amide derivatives.129 Whereas the esters, butyl amide, and dipeptidyl derivatives showed much diminished activity, the amide, N-methylamide, and N,N-dimethylamide derivatives were as effective as PRM-A. These observations suggest that the carboxyl group at position 18 is not essential, but there are some electronic and steric requirements around this group. On the other hand, the antimicrobial activity of PRM-A is quite sensitive to chemical modifications of the benzo[a]naphthacenequinone group.130 Methylation of the 1- and 9-hydroxyl groups, substitution at positions 4 and 10, and reduction of 13-ketone group significantly decreased or abolished the activity. The position 11 is the sole site to be modified without losing activity. 11-Demethoxy-, 11-O-demethyl-, and 11-O-ethyl derivatives were as active as PRM-A. These results indicate that the benzo[a]naphthacenequinone group of PRM-A, except for position 11, plays a pivotal role in the antimicrobial action. Regarding the disaccharide group of PRM-A, the d-xylose component is apparently not essential, because PRM-B, which lacks the d-xylose group, is indistinguishable from PRM-A in its antimicrobial activity.115 Similarly, comparison of PRM-A with benanomicins A and B (PRM-C) suggests that the methylamino group at position 4′ of the 4,6-dideoxy-4-methylamino-d-galactose substituent is of little importance,110 which is further supported by the observations that the activity is retained after introduction of various alkyl and acyl groups onto the methylamino group.131 In contrast, antimicrobial evaluations of synthetic derivatives having d-fucose, l-arabinose, and d-galactose in place of the 4,6-dideoxy-4-methylamino-d-galactose group showed that the stereochemistry at position 1′ and the existence of the methyl group at position 6′ are fairly important for the biological activity of PRM-A.132
Fig. 24.
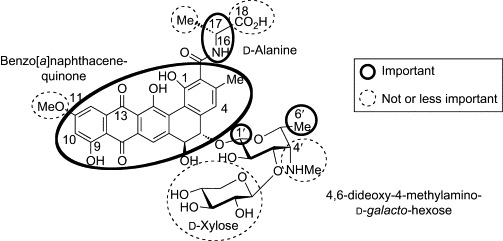
Structural requirements for antifungal activity of PRM-A.
During the foregoing studies on structure–activity relationships of PRM-A, Oki's group has developed a semisynthetic derivative showing augmented biological and physicochemical properties. BMY-28864 (93, Fig. 25 ), which was prepared by reductive N-methylation of PRM-FA2, displays an in vitro and in vivo antimicrobial profile (Table XI, Table XII) similar to that of PRM-A, but has greater water solubility than PRM-A (0.02 mg/mL for PRM-A, > 20.0 mg/mL for 93 in phosphate-buffered saline containing 0.9 mM CaCl2 and 0.5 mM MgCl2, pH 7.2, 25 °C).125, 133, 134 The availability of sample and handling convenience enabled further studies on the mode of antimicrobial action of PRMs by using compound 93. As the first and foremost step, the carbohydrate binding of 93 was thoroughly examined. In the early studies, PRM-A was found to produce an insoluble precipitate with yeast mannan in the presence of Ca2 + ion.126 Thus, quantitative analysis of the precipitate of 93 with Ca2 + ion and methyl α-d-mannopyranoside (Me-α-Man) was initially performed.135 The molar quantity of 93, Ca2 + ion, and Me-α-Man in the precipitate was determined by UV/visible spectrophotometry, atomic absorption spectrometry, and the phenol–sulfuric acid methods, respectively. The molar component ratio of 93:Ca2 +:Me-α-Man was found to be 2:1:4. The same ratio was obtained when the concentration of 93 was varied, or d-mannose was used instead of Me-α-Man for forming the precipitate, indicating that the precipitate of 93 with Ca2 + ion and Me-α-Man is a true chemical complex and not a simple mixture. By taking advantage of this complex-forming property, the carbohydrate specificity of 93 was subsequently evaluated.136 As shown in Table XIII , compound 93 produced precipitates with Me-β-Man, the α and β anomers of p-nitrophenyl d-mannopyranoside, p-aminophenyl d-mannopyranoside, d-fructose, d-arabinose, and d-lyxose, as well as d-mannose and Me-α-Man, while other carbohydrates did not form precipitates at all. The complex formation with these carbohydrates was also confirmed by UV–visible spectrophotometric analysis. It is noteworthy that the carbohydrate specificity of 93 is quite high; the compound has the ability to discriminate d-mannose from l-mannose, and the 2-, 3-, and 4-epimers of d-mannose (d-glucose, d-altrose, and d-talose, respectively), d-mannosamine, and N-acetyl-d-mannosamine. These results indicate that 93 recognizes the 2-, 3-, and 4-hydroxyl groups of d-mannose, and the configuration and substituent at position 1 are not essential. However, it remains unclear as to whether the 6-hydroxyl group of d-mannose is necessary for binding to 93. In addition, there is no plausible explanation available concerning the reason why d-fructose, d-arabinose, and d-lyxose formed precipitates with 93. Oki's group proposed that these carbohydrates have an arrangement similar to 2-, 3-, and 4-hydroxyl groups of d-mannose (Fig. 26 ), and thus bind to 93 in the same manner as d-mannose. However, l-galactose and l-fucose, both of which do not form precipitates with 93, can also mimic the array of 2-, 3-, and 4-hydroxyl groups of d-mannose. Further studies are necessary to clarify these issues. In addition, however, the metal specificity of 93 was examined by UV–visible spectrophotometric analysis and two biological assays, yeast-cell absorption, and potassium leakage induction tests.136 The results collectively suggest that Sr2 + and Cd2 + ions, which have ionic radii similar to that of the Ca2 + ion (1.05 Å for Ca2 +, 1.18 Å for Sr2 +, 0.99 Å for Cd2 +), can also form the ternary complexes with 93 and mannan.
Fig. 25.
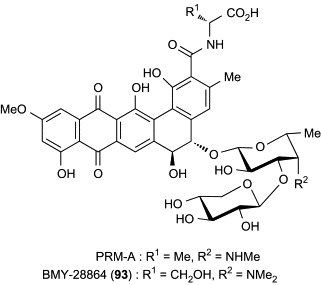
PRM-A and BMY-28864 (93).
Table XIII.
Precipitation of Compound 93 with Carbohydrates in the Presence of Ca2 + Ion
| Carbohydrate | Precipitation (%) in Water (pH 7.0) |
|---|---|
| Hexoses | |
| d-Allosea | 0 |
| d-Altrosea | 0 |
| d-Galactosea | 0 |
| l-Galactosea | 0 |
| d-Glucosea | 0 |
| Methyl α-d-glucopyranosidea | 0 |
| d-Gulosea | 0 |
| d-Idosea | 0 |
| d-Talosea | 0 |
| d-Mannosea | 100 |
| l-Mannoseb | 0 |
| d-Mannose 6-phosphatea | 0 |
| Methyl α-d-mannopyranosidea | 100 |
| Methyl β-d-mannopyranosideb | 100 |
| p-Nitrophenyl α-d-mannopyranosideb | 100 |
| p-Nitrophenyl β-d-mannopyranosideb | 100 |
| p-Aminophenyl α-d-mannopyranosideb | 100 |
| d-Fructosea | 100 |
| d-Fucoseb | 0 |
| d-Rhamnoseb | 0 |
| d-Sorbosea | 0 |
| N-Acetyl-d-glucosaminea | 0 |
| N-Acetyl-d-mannosaminea | 0 |
| d-Glucosaminea | 0 |
| d-Mannosaminea | 0 |
| Pentoses | |
| d-Arabinoseb | 100 |
| l-Arabinoseb | 0 |
| d-Riboseb | 0 |
| d-Lyxoseb | 100 |
| d-Xyloseb | 0 |
| Nonose and disaccharides | |
| N-Acetylneuraminic acida | 0 |
| Lactoseb | 0 |
| Maltoseb | 0 |
| Sucroseb | 0 |
Fig. 26.
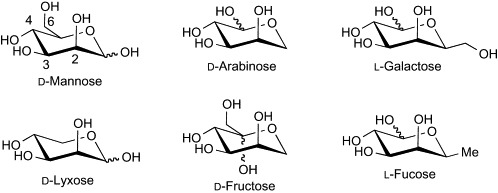
Comparison of d-mannose with d-arabinose, d-lyxose, d-fructose, l-galactose, and l-fucose.
2. Molecular Basis of Carbohydrate Recognition by PRMs
Since the discovery that pradimicins have a C-type lectin-like property, interaction analysis of compound 93 with Ca2 + ion and d-mannose has been attempted in an effort to understand the molecular basis of carbohydrate recognition by pradimicins. Oki's group examined the Ca2 + salt-forming ability of PRM-A, compound 93, and their derivatives (94–100, Fig. 27 ) and found that the ester derivatives (94, 95, 98) formed no Ca2 + salts, whereas PRM-A, 93, and the other derivatives (96, 97, 99, and 100), having the carboxyl group free, produced the Ca2 + salts with a PRM:Ca2 + ratio of 2:1.137 Although the hydroxyl groups on the benzo[a]naphthacenequinone group also have the potential to bind Ca2 + ion, these observations suggest that the carboxyl group of PRMs is a possible binding site for Ca2 + ion. This hypothesis was partially supported by solution NMR analysis of the formation of the Ca2 + salt of 93.138, 139, 140 Significant change in chemical shifts upon addition of CaCl2 was observed for the proton signals at positions 3-Me, 4, and 7 of 93. On the other hand, the proton signals at positions 10 and 12 showed little change, indicating that the Ca2 +-binding site is near the A ring but not around the E ring of PRMs. The Man-binding process of PRMs was investigated using 93 by Lee and coworkers.141 UV–visible spectrophotometric analysis demonstrated that two molecules of PRM bind four molecules of Man in two separate steps. As shown in Scheme 4 , the [PRM2/Ca2 +] salt initially binds two molecules of Man to form the ternary [PRM2/Ca2 +/Man2] complex, which then incorporates another two molecules of Man to form the ultimate ternary [PRM2/Ca2 +/Man4] complex. This complicated three-component equilibrium, along with the aggregation-forming property of PRMs, has collectively made it difficult to analyze the molecular interaction of PRMs and Man by conventional methods of solution NMR.
Fig. 27.
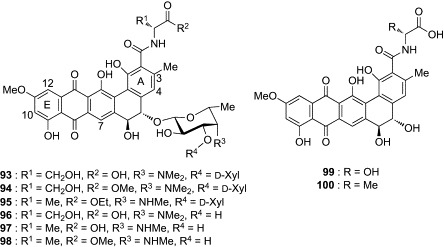
Derivatives (94–100) of BMY-28864 (93).
Scheme 4.
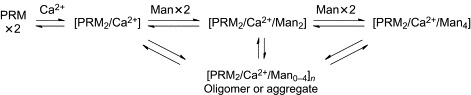
Complex-forming equilibrium of PRM with Ca2 + ion and Man.
Our group has performed solid-state NMR experiments using solid aggregates of PRM-A complexed with Me-α-Man. The first stage of the analysis explored the role of Ca2 + ion on the binding of Man with PRM-A.142 As already described, Cd2 + ion can act as a surrogate for Ca2 + ion in the binding of Man to PRMs. Accordingly, we conducted cross-polarization/magic angle spinning (CP/MAS) 113Cd-NMR spectroscopic experiments with solid samples of the PRM-A/113Cd2 + salt and the ternary PRM-A/113Cd2 +/Me-α-Man complex (Fig. 28 ). The 113Cd NMR spectrum of the PRM-A/113Cd2 + salt exhibited a broad signal around δ = –50 ppm, which is similar to those reported for solid cadmium compounds containing two carboxyl groups, such as Cd(OAc)2·H2O (δ = –46 ppm) and Cd(O2CCH2CH2CO2)·2H2O (δ = –52 ppm).143, 144 This result indicates that the 113Cd2 + ion binds to the carboxyl group of PRM-A and also supports the hypothesis that Ca2 + ion bridges the carboxylate groups of two PRM molecules to produce the Ca2 + salts having a PRM:Ca2 + ratio of 2:1. In contrast, the ternary PRM-A/113Cd2 +/Me-α-Man complex exhibited a sharp signal at δ = –135 ppm, which was markedly shifted upfield (> 80 ppm) in comparison with that of the PRM-A/113Cd2 + salt (δ = –50 ppm), suggesting the occurrence of a change in 113Cd2 + coordination upon binding of Me-α-Man to the PRM-A/113Cd2 + salt. Since 113Cd signals upfield of δ = –100 ppm are observed only for 113Cd2 + coordinated with more than six oxygen ligands,143 it is reasonable to assume that the hydroxyl group(s) of Me-α-Man coordinates to 113Cd2 + ion in the PRM-A/113Cd2 + salt. The realistic implication of these results is that PRM-A binds at least one molecule of Man in a Ca2 +-mediated manner through its carboxylate group.
Fig. 28.

Solid-state CP/MAS 113Cd-NMR spectra of (A) PRM-A/113Cd2 + and (B) PRM-A/113Cd2 +/Me-α-Man complexes. The signals with an asterisk are the spinning side bands of the 113Cd signal at δ = –135 ppm.
The solid-state 113Cd-NMR spectroscopic analysis suggested the possibility that one of the binding sites for Man is located in the proximity of the carboxyl group of PRM-A. On the basis of this assumption, our subsequent analysis was directed at identifying the Man-binding site in PRM-A.145 Several lines of evidence have suggested that PRM-A possesses two binding sites exhibiting different affinities for Man.135, 141 Although it is undoubtedly difficult to search simultaneously two Man-binding sites of PRM-A, we successfully prepared an aggregate composed solely of the [PRM-A2/Ca2 +/Me-α-Man2] complex, in which only the Man-binding site of stronger affinity was occupied by Me-α-Man. Me-α-Man was found to be released from the weaker binding site during the process of washing the aggregate composed of the [PRM-A2/Ca2 +/Me-α-Man4] complex. The simple 1:1 complexes of biosynthetically 13C-enriched PRM-As and Me-α-[13C6]Man facilitated the analysis, by two-dimensional dipolar-assisted rotational resonance (2D-DARR), of the stronger binding site for Man.146, 147, 148 In 2D-DARR spectra, dipolar interactions between 13C nuclei that are located within a 6 Å distance can be detected as cross peaks, permitting evaluation of intermolecular close contacts between PRM-A and Me-α-Man. The 2D-DARR analyses detected close interactions between the d-alanine residue (C-17, 17-Me, C-18) and the 3-Me, C-4, C-5, and C-14 of PRM-A with Me-α-Man (Fig. 29 ). These close contacts are simultaneously compatible when Man is located on the same face of the A ring as the d-alanine group and the C-14 carbon atom, suggesting that the cavity consisting of the d-alanine group and the ABC rings is the stronger binding site for Man (Fig. 30 ). Similar CP/MAS 113Cd-NMR and DARR experiments using compound 93 confirmed that the mode of binding of Ca2 + ion and Man is nearly identical between PRM-A and 93.149
Fig. 29.
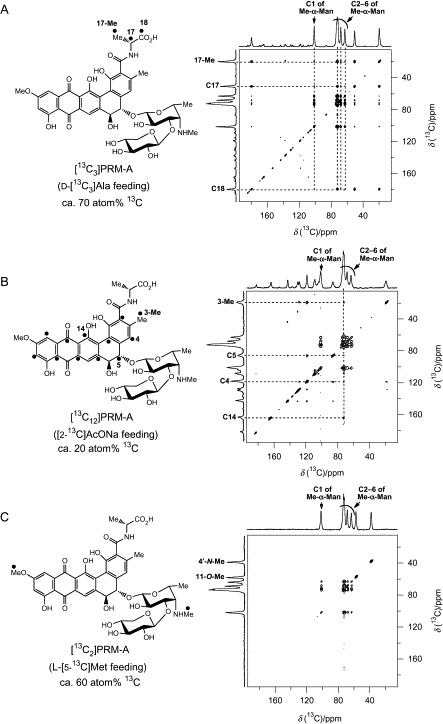
2D-DARR spectra of [13C-labeled PRM-A2/Ca2 +/Me-α-[13C6]Man2] complexes.
Fig. 30.
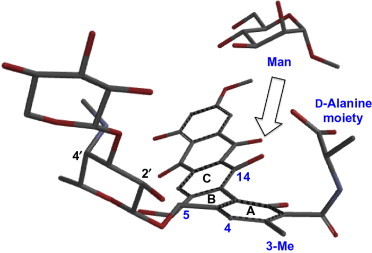
Possible Man-binding conformation of PRM-A.
Based on the results of the solid-state NMR analyses and the previous structure–activity relationship studies, we proposed a model for the Man binding of PRM-A (Fig. 31 ).145 The coordination of Ca2 +, hydrogen bonding, and CH/π interaction might all be involved in the interaction of PRM-A with Man. It is particularly interesting that the architecture of PRM-A, the naturally occurring lectin mimic, seems conceptually similar to that of tripod-type lectin mimics (Fig. 9); the A ring provides the hydrophobic surface for CH/π interaction, and the d-alanine, anthraquinone, and disaccharide components serve as hydrophilic units. Although more investigations are necessary to validate this model, these studies provide an important step toward the full elucidation of the molecular basis of the recognition of Man by PRM-A.
Fig. 31.
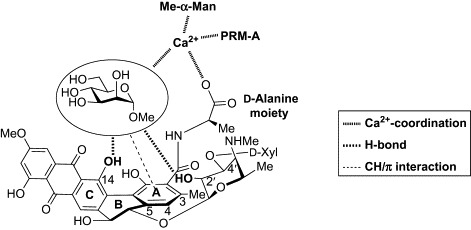
Man-binding model of PRM-A.
3. Antiviral Profile and Mode of Action of Pradimicins
In the late 1980s, PRM-A and benanomicins A and B were discovered to block effectively HIV infection in cell-free as well as in cell-to-cell infection systems in vitro.150, 151 The subsequent study by Oki's group provided evidence that PRM-A inhibits an early step in HIV infection.152 Preincubation of the cells with HIV at 0 °C, followed by incubation with PRM-A at 37 °C, resulted in complete inhibition of HIV infection, whereas PRM-A did not inhibit the infection after preincubation at 37 °C. No inhibitory effect was observed when the viruses treated with PRM-A were washed prior to infection. Moreover, addition of mannan or ethylene glycol-bis(2-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA) prevented the inhibitory effect of PRM-A. Thus, it has been assumed that PRM-A interferes with the viral fusion process through binding to the glycan on the HIV envelope in the presence of Ca2 + ion.
The antiviral properties of PRM-A were later studied in more detail by Balzarini and coworkers.153 PRM-A was shown to inhibit the cytopathic effects induced by HIV-1 and HIV-2, although its EC50 values are in the lower micromolar range, and thus quite higher than those of natural lectins (Table XIV ). Time-of-drug-addition studies demonstrated that PRM-A as well as the mannose-specific plant lectin HHA needed to be present from the beginning of the virus infection to fully suppress the infection. This observation supports the assumption that an early event in the infection cycle of HIV is the target of therapeutic intervention by PRM-A. The inhibition of the HIV entry process by PRM-A through interaction with gp120 was further confirmed by the complete loss of the inhibitory effect of PRM-A against VSV-G-pseudotyped HIV-1, which lacks gp120 in the envelope. The SPR analysis revealed that PRM-A significantly bound to gp120 in a Ca2 +-dependent manner, with a K d value of ∼ 2.7 μM, suggesting the possibility that PRM-A inhibits entry of the virus by binding to high mannose-type glycans of gp120 in a manner similar to those of natural lectins. In accordance with this hypothesis, HIV-1 strains selected under increasing pressure of PRM-A were found to contain up to eight different glycan deletions in gp120, most of which were high mannose-type glycans. Such deletion at similar glycosylation sites was also observed for such mannose-specific plant lectins as HHA, GNA, and CN-V. Thus, PRM-A can act as a lectin in terms of glycan recognition, antiviral activity, and pattern of drug resistance.
Table XIV.
Anti-HIV Activities of Natural Lectins, PRM-A, and PRM-S in MT-4 Cell Cultures
Balzarini's group has also examined the antiviral action of PRM-S, a water-soluble derivative of PRM-A having a d-glucose 3-sulfate group instead of the terminal d-xylose group (Fig. 23).154 PRM-S also inhibited the cytopathic effect of HIV-1 and HIV-2, showing EC50 values similar to those of PRM-A (Table XIV), and the binding capacity of PRM-S for gp120 was comparable to that of PRM-A. Drug resistance selection experiments using PRM-S demonstrated that PRM-S, like PRM-A, selected for drug-resistant mutant virus strains contained deletions at various N-glycosylation sites of HIV-1 gp120, showing that PRM-S as well as PRM-A has a strong preference for binding to high mannose-type glycans. Subsequently, Bewley and coworkers have made a direct observation of the binding of PRM-S with the trisaccharide, α-Man-(1 → 3)-[α-Man-(1 → 6)]-Man, using saturation-transfer difference (STD) NMR.155 Moreover, PRM-S and PRM-A were found to inhibit, efficiently and dose dependently, the binding of HIV-1 to the DC-SIGN-expressing cells, indicating that these compounds prevent virus capture by DC-SIGN-expressing cells and subsequent transmission to T lymphocytes. PRM-S and PRM-A were also investigated for their potential to induce chemokines and cytokines. In contrast to the prokaryotic mannose-specific lectin CV-N, which dramatically stimulates the production of a wide range of cytokines and chemokines,156 neither compound had any stimulatory effect on the chemo/cytokine production levels in mononuclear cells of peripheral blood.
On the basis of the unique antiviral properties of PRM-A and PRM-S, as well as lectins, Balzarini has proposed a novel therapeutic concept for HIV treatment that is entirely different from all currently existing therapeutic methods.11, 12, 13 Exposure of HIV to carbohydrate receptors puts the virus in the dilemma of either becoming eliminated from its host by being kept suppressed by the carbohydrate receptors or escaping the pressure from the carbohydrate receptors by mutating glycosylation sites in gp120, and thereby becoming prone to neutralization and elimination by the immune system. In contrast to the conventional strategies of designing drugs that have as high a genetic barrier as possible to delay the development of drug resistance, the proposed approach makes use of viral variability and the inherent viral replication to generate mutant virus strains having deletions of glycosylation sites on gp120. Moreover, this therapeutic concept is unique in regard to its use of concerned action of drug chemotherapy—triggering the immune system in the host (“self-vaccination”) by the administration of one single drug. It is also noteworthy that carbohydrate receptors can interact with a target gp120 molecule in a non-stoichiometric manner. All existing anti-HIV drugs, such as reverse-transcriptase inhibitors and protease inhibitors, bind to only one target molecule. As a result, one or a few mutations that appear in a target usually elicit a marked degree of drug resistance. On the other hand, several carbohydrate receptor molecules can bind to one gp120 molecule bearing 20–29 glycans at the same time, resulting in a high genetic barrier for HIV to overcome. Obviously, this effect becomes more significant for the smaller carbohydrate receptors, PRM-A and PRM-S, because a larger number of molecules can simultaneously bind to gp120 without steric hindrance. They also have additional advantages over protein lectins in regard to chemical stability and productivity. Although their antiviral activity is less pronounced than that of lectins, these lectin mimics possessing beneficial characteristics for drug development may be very suitable candidates as conceptually novel anti-HIV drugs.
In addition to the anti-HIV activity, PRM-A has been shown to possess antiviral activity against HCV,52 coronaviruses,55 and simian immunodeficiency virus.157 PRM-A also inhibits the entry process of these viruses by binding to the viral envelope-associated glycans. Therefore, PRM-A and its derivatives may qualify as potential antiviral drug candidates for infectious diseases derived from these viruses.
IV. Conclusion and Future Prospects
The emerging biological and pathological significance of carbohydrates has increased the importance of carbohydrate receptors as diagnostic and therapeutic agents as well as research tools in glycobiology. A number of efforts have been made to utilize lectins for glycobiology research and have yielded fruitful results. However, their application in medicinal research has been hampered by the potential disadvantages presented by their protein nature. Consequently, there is a rapidly growing interest in small molecules having carbohydrate-binding properties (lectin mimics). Progress in the study of synthetic lectin mimics has laid an excellent foundation for the future development of diagnostic and therapeutic agents based on carbohydrate recognition. The boronic acid-dependent lectin mimics are designed to form covalent bonds with carbohydrates in aqueous media, and several examples have been successfully shown to act as possible sensors for such biologically important biomarkers as sialyl Lewis X and the TF antigen. An especially encouraging result was obtained for boronic acid-dependent lectin mimics having peptide backbones, in which such non-covalent interactions as hydrogen bonding and CH/π interaction also contribute to carbohydrate recognition. The combination of covalent interactions with boronic acid components and non-covalent interactions with other functional groups may be a promising strategy for designing a new generation of boronic acid-dependent lectin mimics that can act as diagnostic and therapeutic agents.
In parallel development, intensive work in the area of supramolecular chemistry has generated the boronic acid-independent lectin mimics, which rely solely on non-covalent interactions with carbohydrates. A number of the tripod-type lectin mimics have been shown to bind effectively carbohydrates in organic media. However, exact prediction of the binding preference is still difficult, and carbohydrate recognition in aqueous media by means of the tripod-type architecture remains an important goal for future studies. Meanwhile, the cage-type lectin mimics have successfully demonstrated recognition in aqueous solution of those carbohydrates having the all-equatorial substitution mode. However, at present, no strategy exists for designing cage-type lectin mimics that recognize other carbohydrates. Rapidly evolving computational methods may overcome these problems.
While only initial attempts have been made up to the present to develop synthetic lectin mimics having antiviral and antimicrobial activities, the naturally occurring lectin mimics, pradimicins and benanomicins, have become established as promising candidates as therapeutic agents having a novel mode of action. They express antiviral and antimicrobial activities by binding to Man residues of glycans on the viral envelope and the microbial cell wall. It is particularly interesting that these natural products appear to constitute highly sophisticated tripod-type lectin mimics, and they possibly provide a unique guide for designing a new therapeutic leads having the tripod-type architecture. With further advancements in these fields of research, novel therapeutic drugs based on lectin mimics are likely to emerge in the near future.
Acknowledgments
The research described in this article was conducted in collaboration with Professor Yasuhiro Igarashi of Toyama Prefectural University, and Professor Kiyonori Takegoshi, Dr. Yuichi Masuda, Mr. Takashi Doi, and Mr. Keita Yamada of Kyoto University. The work was partly supported by a Fund for Seeds of Collaborative Research and an Incentive Research Grant from RIKEN, and a MEXT Grant-in-Aid for Young Scientists (B) and Scientific Research on Innovative Areas “Chemical Biology of Natural Products.”
References
- 1.Larkin A., Imperiali B. The expanding horizons of asparagine-linked glycosylation. Biochemistry. 2011;50:4411–4426. doi: 10.1021/bi200346n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee Y.C. Warfare between pathogens and hosts: The trickery of sugars. Trends Glycosci. Glycotechnol. 2010;22:95–106. [Google Scholar]
- 3.Myrrey H.E., Hsieh-Wilson L.C. The chemical neurobiology of carbohydrates. Chem. Rev. 2008;108:1708–1731. doi: 10.1021/cr078215f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lau K.S., Dennis J.W. N-Glycans in cancer progression. Glycobiology. 2008;18:750–760. doi: 10.1093/glycob/cwn071. [DOI] [PubMed] [Google Scholar]
- 5.Dwek R.A. Glycobiology: Toward understanding the function of sugars. Chem. Rev. 1996;96:683–720. doi: 10.1021/cr940283b. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein I.J., Hughes R.C., Monsigny M., Osawa T., Sharon N. What should be called a lectin? Nature. 1980;285:66. [Google Scholar]
- 7.Sharon N., Lis H. History of lectins: From hemagglutinins to biological recognition molecules. Glycobiology. 2004;14:53R–62R. doi: 10.1093/glycob/cwh122. [DOI] [PubMed] [Google Scholar]
- 8.Hirabayashi J. Concept, strategy and realization of lectin-based glycan profiling. J. Biochem. 2008;144:139–147. doi: 10.1093/jb/mvn043. [DOI] [PubMed] [Google Scholar]
- 9.Dai Z., Zhou J., Qui S., Liu Y., Fan J. Lectin-based glycoproteomics to explore and analyze hepatocellular carcinoma-related glycoprotein markers. Electrophoresis. 2009;30:2957–2966. doi: 10.1002/elps.200900064. [DOI] [PubMed] [Google Scholar]
- 10.Gupta G., Surolia A., Sampathkumar S. Lectin microarrays for glycomic analysis. OMICS. 2010;14:419–436. doi: 10.1089/omi.2009.0150. [DOI] [PubMed] [Google Scholar]
- 11.Balzarini J. Targeting the glycans of glycoproteins: A novel paradigm for antiviral therapy. Nat. Rev. Microbiol. 2007;5:583–597. doi: 10.1038/nrmicro1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balzarini J. Carbohydrate-binding agents: A potential future cornerstone for the chemotherapy of enveloped viruses? Antivir. Chem. Chemother. 2007;18:1–11. doi: 10.1177/095632020701800101. [DOI] [PubMed] [Google Scholar]
- 13.François K.O., Balzarini J. Potential of carbohydrate-binding agents as therapeutics against enveloped viruses. Med. Res. Rev. 2012;32:349–387. doi: 10.1002/med.20216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin S., Cheng Y., Reid S., Li M., Wang B. Carbohydrate recognition by boronolectins, small molecules, and lectins. Med. Res. Rev. 2010;30:171–257. doi: 10.1002/med.20155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazik M. Recent developments in the molecular recognition of carbohydrates by artificial receptors. RSC Adv. 2012;2:2630–2642. [Google Scholar]
- 16.Walker D.B., Joshi G., Davis A.P. Progress in biomimetic carbohydrate recognition. Cell. Mol. Life Sci. 2009;66:3177–3191. doi: 10.1007/s00018-009-0081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakagawa Y., Ito Y. Carbohydrate-binding molecules with non-peptidic skeletons. Trends GlycoSci. Glycotechnol. 2012;24:1–12. [Google Scholar]
- 18.Nishio M. The CH/π hydrogen bond in chemistry. Conformation, supramolecules, optical resolution and interactions involving carbohydrates. Phys. Chem. Chem. Phys. 2011;13:13873–13900. doi: 10.1039/c1cp20404a. [DOI] [PubMed] [Google Scholar]
- 19.Fukagawa Y., Ueki T., Numata K., Oki T. Pradimicins and benanomicins, sugar-recognizing antibiotics: Their novel mode of antifungal action and conceptual significance. Actinomycetologia. 1993;7:1–22. [Google Scholar]
- 20.Springsteen G., Wang B. A detailed examination of boronic acid–diol complexation. Tetrahedron. 2002;58:5291–5300. [Google Scholar]
- 21.Lorand J.P., Edwards J.O. Polyol complexes and structure of the benzeneboronate ion. J. Org. Chem. 1959;24:769–774. [Google Scholar]
- 22.Yan J., Springsteen G., Deeter S., Wang B. The relationship among pKa, pH, and binding constants in the interactions between boronic acids and diols—It is not as simple as it appears. Tetrahedron. 2004;60:11205–11209. [Google Scholar]
- 23.Wulff G. Selective binding to polymers via covalent bonds: The construction of chiral cavities as specific receptor sites. Pure Appl. Chem. 1982;54:2093–2102. [Google Scholar]
- 24.Wulff G., Lauer M., Böhnke H. Rapid proton transfer as cause of an unusually large neighboring group effect. Angew. Chem. Int. Ed. 1984;23:741–742. [Google Scholar]
- 25.Franzen S., Ni W., Wang B. Study of the mechanism of electron-transfer quenching by boron–nitrogen adducts in fluorescent sensors. J. Phys. Chem. B. 2003;107:12942–12948. [Google Scholar]
- 26.Ni W., Kaur G., Springsteen G., Wang B., Franzen S. Regulating the fluorescence intensity of an anthracene boronic acid system: A B–N bond or a hydrolysis mechanism? Bioorg. Chem. 2004;32:571–581. doi: 10.1016/j.bioorg.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Zhu L., Shabbir S.H., Gray M., Lynch V.M., Sorey S., Anslyn E.V. A structural investigation of the N–B interaction in an o-(N, N-dialkylaminomethyl)arylboronate system. J. Am. Chem. Soc. 2006;128:1222–1232. doi: 10.1021/ja055817c. [DOI] [PubMed] [Google Scholar]
- 28.Yan J., Fang H., Wang B. Boronolectins and fluorescent boronolectins: An examination of the detailed chemistry issues important for the drug design. Med. Res. Rev. 2005;25:490–520. doi: 10.1002/med.20038. [DOI] [PubMed] [Google Scholar]
- 29.James T.D., Sandanayake K.R.A.S., Shinkai S. Novel photoinduced electron-transfer sensor for saccharides based on the interaction of boronic acid and amine. J. Chem. Soc. Chem. Commun. 1994:477–478. [Google Scholar]
- 30.James T.D., Sandanayake K.R.A.S., Shinkai S. A glucose-selective molecular fluorescence sensor. Angew. Chem. Int. Ed. 1994;33:2207–2209. [Google Scholar]
- 31.James T.D., Sandanayake K.R.A.S., Iguchi R., Shinkai S. Novel saccharide-photoinduced electron transfer sensors based on the interaction of boronic acid and amine. J. Am. Chem. Soc. 1995;117:8982–8987. [Google Scholar]
- 32.Karnati V.V., Gao X., Gao S., Yang W., Ni W., Sankar S., Wang B. A glucose-selective fluorescence sensor based on boronic acid-diol recognition. Bioorg. Med. Chem. Lett. 2002;12:3373–3377. doi: 10.1016/s0960-894x(02)00767-9. [DOI] [PubMed] [Google Scholar]
- 33.Kaur G., Fang H., Gao X., Li H., Wang B. Substituent effect on anthracene-based bisboronic acid glucose sensors. Tetrahedron. 2006;62:2583–2589. [Google Scholar]
- 34.James T.D., Sandanayake K.R.A.S., Shinkai S. Chiral discrimination of monosaccharides using a fluorescent molecular sensor. Nature. 1995;374:345–347. [Google Scholar]
- 35.Xing Z., Wang H., Cheng Y., Zhu C., James T.D., Zhao J. Selective saccharide recognition using modular diboronic acid fluorescent sensors. Eur. J. Org. Chem. 2012:1223–1229. [Google Scholar]
- 36.Yang W., Gao S., Gao X., Karnati V.V.R., Ni W., Wang B., Hooks W.B., Carson J., Weston B. Diboronic acids as fluorescent probes for cells expressing sialyl Lewis X. Bioorg. Med. Chem. Lett. 2002;12:2175–2177. doi: 10.1016/s0960-894x(02)00339-6. [DOI] [PubMed] [Google Scholar]
- 37.Takada A., Ohmori K., Yoneda T., Tsuyuoka K., Hasegawa A., Kiso M., Kannagi R. Contribution of carbohydrate antigens sialyl Lewis A and sialyl Lewis X to adhesion of human cancer cells to vascular endothelium. Cancer Res. 1993;53:354–361. [PubMed] [Google Scholar]
- 38.Kannagi R. Molecular mechanism for cancer-associated induction of sialyl Lewis X and sialyl Lewis A expression—The Warburg effect revisited. Glycoconj. J. 2004;20:353–354. doi: 10.1023/B:GLYC.0000033631.35357.41. [DOI] [PubMed] [Google Scholar]
- 39.Jorgensen T., Berner A., Kaalhus O., Tveter K.J., Danielsen H.E., Bryne M. Up-regulation of the oligosaccharide sialyl Lewis X: A new prognostic parameter in metastatic prostate cancer. Cancer Res. 1995;55:1817–1819. [PubMed] [Google Scholar]
- 40.Idikio H.A. Sialyl Lewis X, gleason grade and stage in nonmetastatic human prostate cancer. Glycoconj. J. 1997;14:875–877. doi: 10.1023/a:1018502424487. [DOI] [PubMed] [Google Scholar]
- 41.Dai C., Cazares L.H., Wang L., Chu Y., Wang S.L., Troyer D.A., Semmes O.J., Drake R.R., Wang B. Using boronolectin in MALDI-MS imaging for the histological analysis of cancer tissue expressing the sialyl Lewis X antigen. Chem. Commun. 2011;47:10338–10340. doi: 10.1039/c1cc11814e. [DOI] [PubMed] [Google Scholar]
- 42.Thiery G., Anselmi E., Audebourg A., Darii E., Abarbri M., Terris B., Tabet J.C., Gut I.G. Improvements of targeted multiplex mass spectroscopy imaging. Proteomics. 2008;8:3725–3734. doi: 10.1002/pmic.200701150. [DOI] [PubMed] [Google Scholar]
- 43.Lemaire R., Stauber J., Wisztorski M., Van Camp C., Desmons A., Deschamps M., Proess G., Rudlof I., Woods A.S., Day R., Salzet M., Fournier I. Tag-mass: Specific molecular imaging of transcriptome and proteome by mass spectrometry based on photocleavable tag. J. Proteome Res. 2007;6:2057–2067. doi: 10.1021/pr0700044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dowlut M., Hall D.G. An improved class of sugar-binding boronic acids, soluble and capable of complexing glycosides in neutral water. J. Am. Chem. Soc. 2006;128:4226–4227. doi: 10.1021/ja057798c. [DOI] [PubMed] [Google Scholar]
- 45.Bérubé M., Dowlut M., Hall D.G. Benzoboroxoles as efficient glycopyranoside-binding agents in physiological conditions: Structure and selectivity of complex formation. J. Org. Chem. 2008;73:6471–6479. doi: 10.1021/jo800788s. [DOI] [PubMed] [Google Scholar]
- 46.Norrild J.C., Eggert H. Evidence for mono- and bisdentate boronate complexes of glucose in the furanose form. application of 1JC–C coupling constants as a structural probe. J. Am. Chem. Soc. 1995;117:1479–1484. [Google Scholar]
- 47.Bielecki M., Eggert H., Norrild J.C. A fluorescent glucose sensor binding covalently to all five hydroxyl groups of α-D-glucofuranose. A reinvestigation. J. Chem. Soc. Perkin Trans. 1999;2:449–455. [Google Scholar]
- 48.Pal A., Bérubé M., Hall D.G. Design, synthesis, and screening of a library of peptidyl bis(boroxoles) as oligosaccharide receptors in water: Identification of a receptor for the tumor marker TF-antigen disaccharide. Angew. Chem. Int. Ed. 2010;49:1492–1495. doi: 10.1002/anie.200906620. [DOI] [PubMed] [Google Scholar]
- 49.Yu L. The oncofetal Thomsen-Friedenreich carbohydrate antigen in cancer progression. Glycoconj. J. 2007;24:411–420. doi: 10.1007/s10719-007-9034-3. [DOI] [PubMed] [Google Scholar]
- 50.Balzarini J. Inhibition of HIV entry by carbohydrate-binding proteins. Antiviral Res. 2006;71:237–247. doi: 10.1016/j.antiviral.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 51.Botos I., Wlodawer A. Proteins that bind high-mannose sugars of the HIV envelope. Prog. Biophys. Mol. Biol. 2005;88:233–282. doi: 10.1016/j.pbiomolbio.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 52.Bertaux C., Daelemans D., Meertens L., Cormier E.G., Reinus J.F., Peumans W.J., Van Damme E.J., Igarashi Y., Oki T., Schols D., Dragic T., Balzarini J. Entry of hepatitis C virus and human immunodeficiency virus is selectively inhibited by carbohydrate-binding agents but not by polyanions. Virology. 2007;366:40–50. doi: 10.1016/j.virol.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 53.Helle F., Wychowski C., Vu-Dac N., Gustafson K.R., Voisset C., Dubuisson J. Cyanovirin-N inhibits hepatitis C virus entry by binding to envelope protein glycans. J. Biol. Chem. 2006;281:25177–25183. doi: 10.1074/jbc.M602431200. [DOI] [PubMed] [Google Scholar]
- 54.Kumaki Y., Wandersee M.K., Smith A.J., Zhou Y., Simmons G., Nelson N.M., Bailey K.W., Vest Z.G., Li J.K.-K., Chan P.K., Smee D.F., Barnard D.L. Inhibition of severe acute respiratory syndrome coronavirus replication in a lethal SARS-CoV BALB/c mouse model by stinging nettle lectin, Urtica dioica agglutinin. Antiviral Res. 2011;90:22–32. doi: 10.1016/j.antiviral.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van der Meer F.J.U.M., de Haan C.A.M., Schuurman N.M.P., Haijema B.J., Verheije M.H., Bosch B.J., Balzarini J., Egberink H.F. The carbohydrate-binding plant lectins and the non-peptidic antibiotic pradimicin A target the glycans of the coronavirus envelope glycoproteins. J. Antimicrob. Chemother. 2007;60:741–749. doi: 10.1093/jac/dkm301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O'Keefe B.R., Smee D.F., Turpin J.A., Saucedo C.J., Gustafson K.R., Mori T., Blakeslee D., Buckheit R., Boyd M.R. Potent anti-influenza activity of cyanovirin-N and interactions with viral hemagglutinin. Antimicrob. Agents Chemother. 2003;47:2518–2525. doi: 10.1128/AAC.47.8.2518-2525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sato Y., Hirayama M., Morimoto K., Yamamoto N., Okuyama S., Hori K. High mannose-binding lectin with preference for the cluster of α1-2-mannose from the green alga Boodlea coacta is a potent entry inhibitor of HIV-1 and influenza viruses. J. Biol. Chem. 2011;286:19446–19458. doi: 10.1074/jbc.M110.216655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leonard C.K., Spellman M.W., Riddle L., Harris R.J., Thomas J.N., Gregory T.J. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J. Biol. Chem. 1990;265:10373–10382. [PubMed] [Google Scholar]
- 59.Rudd P.M., Elliott T., Cresswell P., Wilson I.A., Dwek R.A. Glycosylation and the immune system. Science. 2001;291:2370–2376. doi: 10.1126/science.291.5512.2370. [DOI] [PubMed] [Google Scholar]
- 60.Smith A.E., Helenius A. How viruses enter animal cells. Science. 2004;304:237–242. doi: 10.1126/science.1094823. [DOI] [PubMed] [Google Scholar]
- 61.Balzarini J., Schols D., Neyts J., Van Damme E., Peumans W., De Clercq E. Alpha-(1-3)- and alpha-(1-6)-D-mannose-specific plant lectins are markedly inhibitory to human immunodeficiency virus and cytomegalovirus infections in vitro. Antimicrob. Agents Chemother. 1991;35:410–416. doi: 10.1128/aac.35.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Balzarini J. Targeting the glycans of gp120: A novel approach aimed at the Achilles heel of HIV. Lancet Infect. Dis. 2005;5:726–731. doi: 10.1016/S1473-3099(05)70271-1. [DOI] [PubMed] [Google Scholar]
- 63.Balzarini J., Van Laethem K., Hatse S., Vermeire K., De Clercq E., Peumans W., Van Damme E., Vandamme A.M., Bölmstedt A., Schols D. Profile of resistance of human immunodeficiency virus to mannose-specific plant lectins. J. Virol. 2004;78:10617–10627. doi: 10.1128/JVI.78.19.10617-10627.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Losman B., Bolmstedt A., Schønning K., Björndal A., Westin C., Fenyö E.M., Oloffson S. Protection of neutralization epitopes in the V3 loop of oligomeric human immunodeficiency virus type 1 glycoprotein 120 by N-linked oligosaccharides in the V1 region. AIDS Res. Hum. Retroviruses. 2001;17:1067–1076. doi: 10.1089/088922201300343753. [DOI] [PubMed] [Google Scholar]
- 65.Wei X., Decker J.M., Wang S., Hui H., Kappes J.C., Wu X., Salazar-Gonzalez J.F., Salazar M.G., Kilby J.M., Saag M.S., Komarova N.L., Nowak M.A., Hahn B.H., Kwong P.D., Shaw G.M. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 66.Balzarini J., Van Laethem K., Hatse S., Froeyen M., Peumans W., Van Damme E., Schols D. Carbohydrate-binding agents cause deletions of highly conserved glycosylation sites in HIV GP120: A new therapeutic concept to hit the achilles heel of HIV. J. Biol. Chem. 2005;280:41005–41014. doi: 10.1074/jbc.M508801200. [DOI] [PubMed] [Google Scholar]
- 67.Balzarini J., Van Laethem K., Hatse S., Froeyen M., Van Damme E., Bolmstedt A., Peumans W., De Clercq E., Schols D. Marked depletion of glycosylation sites in HIV-1 gp120 under selection pressure by the mannose-specific plant lectins of Hippeastrum hybrid and Galanthus nivalis. Mol. Pharmacol. 2005;67:1556–1565. doi: 10.1124/mol.104.005082. [DOI] [PubMed] [Google Scholar]
- 68.Balzarini J., Van Laethem K., Peumans W., Van Damme E., Bolmstedt A., Gago F., Schols D. Mutational pathways, resistance profile, and side effects of cyanovirin relative to human immunodeficiency virus type 1 strains with N-glycan deletions in their gp120 envelopes. J. Virol. 2006;80:8411–8421. doi: 10.1128/JVI.00369-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Witvrouw M., Fikkert V., Hantson A., Pannecouque C., O'keefe B.R., McMahon J., Stamatatos L., de Clercq E., Bolmstedt A. Resistance of human immunodeficiency virus type 1 to the high-mannose binding agents cyanovirin N and concanavalin A. J. Virol. 2005;79:7777–7784. doi: 10.1128/JVI.79.12.7777-7784.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trippier P.C., McGuigan C., Balzarini J. Phenylboronic-acid-based carbohydrate binders as antiviral therapeutics: Monophenylboronic acids. Antivir. Chem. Chemother. 2010;20:249–257. doi: 10.3851/IMP1632. [DOI] [PubMed] [Google Scholar]
- 71.Trippier P.C., Balzarini J., McGuigan C. Phenylboronic-acid-based carbohydrate binders as antiviral therapeutics: Bisphenylboronic acids. Antivir. Chem. Chemother. 2011;21:129–142. doi: 10.3851/IMP1707. [DOI] [PubMed] [Google Scholar]
- 72.Jay J.I., Lai B.E., Myszka D.G., Mahalingam A., Langheinrich K., Katz D.F., Kiser P.F. Multivalent benzoboroxole functionalized polymers as gp120 glycan targeted microbicide entry inhibitors. Mol. Pharm. 2010;7:116–129. doi: 10.1021/mp900159n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mammem M., Choi S.K., Whitesides G.M. Polyvalent interactions in biological systems: Implication for design and use of multivalent ligands and inhibitors. Angew. Chem. Int. Ed. 1998;37:2754–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 74.Brewer C.F., Miceli M.C., Baum L.G. Clusters, bundles, arrays and lattices: Novel mechanisms for lectin-saccharide-mediated cellular interactions. Curr. Opin. Struct. Biol. 2002;12:616–623. doi: 10.1016/s0959-440x(02)00364-0. [DOI] [PubMed] [Google Scholar]
- 75.Mahalingam A., Geonnotti A.R., Balzarini J., Kiser P.F. Activity and safety of synthetic lectins based on benzoboroxole-functionalized polymers for inhibition of HIV entry. Mol. Pharm. 2011;8:2465–2475. doi: 10.1021/mp2002957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mazik M., Bandmann H., Sicking W. Molecular recognition of carbohydrates by artificial polypyridine and polypyrimidine receptors. Angew. Chem. Int. Ed. 2000;39:551–554. doi: 10.1002/(sici)1521-3773(20000204)39:3<551::aid-anie551>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 77.Mazik M., Sicking W. Molecular recognition of carbohydrates by artificial receptors: Systematic studies towards recognition motifs for carbohydrates. Chem. Eur. J. 2001;7:664–670. doi: 10.1002/1521-3765(20010202)7:3<664::aid-chem664>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 78.Mazik M., Radunz W., Sicking W. High α/β-anomer selectivity in molecular recognition of carbohydrates by artificial receptors. Org. Lett. 2002;4:4579–4582. doi: 10.1021/ol0201759. [DOI] [PubMed] [Google Scholar]
- 79.Mazik M., Radunz W., Boese R. Molecular recognition of carbohydrates with acyclic pyridine-based receptors. J. Org. Chem. 2004;69:7448–7462. doi: 10.1021/jo048979k. [DOI] [PubMed] [Google Scholar]
- 80.Mazik M., Kuschel M. Amide, amino, hydroxyl and aminopyridine groups as building blocks for carbohydrate receptors. Eur. J. Org. Chem. 2008:1517–1526. [Google Scholar]
- 81.Mazik M., Kuschel M. Highly effective acyclic carbohydrate receptors consisting of aminopyridine, imidazole, and indole recognition units. Chem. Eur. J. 2008;14:2405–2419. doi: 10.1002/chem.200701269. [DOI] [PubMed] [Google Scholar]
- 82.Mazik M., Hartmann A. Phenanthroline unit as a building block for carbohydrate receptors. J. Org. Chem. 2008;73:7444–7450. doi: 10.1021/jo8005842. [DOI] [PubMed] [Google Scholar]
- 83.Mazik M., Hartmann A., Jones P.G. Highly effective recognition of carbohydrates by phenanthroline-based receptors: α- versus β-anomer binding preference. Chem. Eur. J. 2009;15:9147–9159. doi: 10.1002/chem.200900664. [DOI] [PubMed] [Google Scholar]
- 84.Mazik M., Hartmann A. Recognition properties of receptors consisting of imidazole and indole recognition units towards carbohydrates. Beilstein J. Org. Chem. 2010;6:9. doi: 10.3762/bjoc.6.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mazik M., Sonnenberg C. Isopropylamino and isobutylamino groups as recognition sites for carbohydrates: Acyclic receptors with enhanced binding affinity toward β-galactosides. J. Org. Chem. 2010;75:6416–6423. doi: 10.1021/jo100982x. [DOI] [PubMed] [Google Scholar]
- 86.Mazik M., Geffert C. 8-Hydroxylquinoline as a building block for artificial receptors: Binding preferences in the recognition of glycopyranosides. Org. Biomol. Chem. 2011;9:2319–2326. doi: 10.1039/c0ob00960a. [DOI] [PubMed] [Google Scholar]
- 87.Mazik M. Molecular recognition of carbohydrates by acyclic receptors employing noncovalent interactions. Chem. Soc. Rev. 2009;38:935–956. doi: 10.1039/b710910p. [DOI] [PubMed] [Google Scholar]
- 88.Mazik M., Buthe A.C. Recognition properties of receptors based on dimesitylmethane-derived core: Di- vs. monosaccharide preference. Org. Biomol. Chem. 2009;7:2063–2071. doi: 10.1039/b901173k. [DOI] [PubMed] [Google Scholar]
- 89.Nativi C., Cacciarini M., Francesconi O., Vacca A., Moneti G., Ienco A., Roelens S. Pyrrolic tripodal receptors efficiently recognizing monosaccharides. Affinity assessment through a generalized binding descriptor. J. Am. Chem. Soc. 2007;129:4377–4385. doi: 10.1021/ja068754m. [DOI] [PubMed] [Google Scholar]
- 90.Nativi C., Cacciarini M., Francesconi O., Moneti G., Roelens S. A β-mannoside-selective pyrrolic tripodal receptor. Org. Lett. 2007;9:4685–4688. doi: 10.1021/ol701959r. [DOI] [PubMed] [Google Scholar]
- 91.Nativi C., Francesconi O., Gabrielli G., Vacca A., Roelens S. Chiral diaminopyrrolic receptors for selective recognition of mannosides, part 1: Design, synthesis, and affinities of second-generation tripodal receptors. Chem. Eur. J. 2011;17:4814–4820. doi: 10.1002/chem.201002871. [DOI] [PubMed] [Google Scholar]
- 92.Ardá A., Venturi C., Nativi C., Francesconi O., Cañada F.J., Jiménez-Barbero J., Roelens S. Selective recognition of β-mannosides by synthetic tripodal receptors: A 3D view of the recognition mode by NMR. Eur. J. Org. Chem. 2010:64–71. [Google Scholar]
- 93.Ardá A., Venturi C., Nativi C., Francesconi O., Gabrielli G., Cañada F.J., Jiménez-Barbero J., Roelens S. A chiral pyrrolic tripodal receptor enantioselectively recognizes β-mannose and β-mannosides. Chem. Eur. J. 2010;16:414–418. doi: 10.1002/chem.200902514. [DOI] [PubMed] [Google Scholar]
- 94.Ardá A., Cañada F.J., Nativi C., Francesconi O., Gabrielli G., Ienco A., Jiménez-Barbero J., Roelens S. Chiral diaminopyrrolic receptors for selective recognition of mannosides, part 2: A 3D view of the recognition modes by X-ray, NMR spectroscopy, and molecular modeling. Chem. Eur. J. 2011;17:4821–4829. doi: 10.1002/chem.201002872. [DOI] [PubMed] [Google Scholar]
- 95.Palde P.B., Gareiss P.C., Miller B.L. Selective recognition of alkyl pyranosides in protic and aprotic solvents. J. Am. Chem. Soc. 2008;130:9566–9573. doi: 10.1021/ja802229f. [DOI] [PubMed] [Google Scholar]
- 96.Davis A.P. Synthetic lectin. Org. Biomol. Chem. 2009;7:3629–3638. doi: 10.1039/b909856a. [DOI] [PubMed] [Google Scholar]
- 97.Davis A.P., Wareham R.S. A tricyclic polyamide receptor for carbohydrates in organic media. Angew. Chem. Int. Ed. 1998;37:2270–2273. doi: 10.1002/(SICI)1521-3773(19980904)37:16<2270::AID-ANIE2270>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 98.Lecollinet G., Dominey A.P., Velasco T., Davis A.P. Highly selective disaccharide recognition by a tricyclic octaamide cage. Angew. Chem. Int. Ed. 2002;41:4093–4096. doi: 10.1002/1521-3773(20021104)41:21<4093::AID-ANIE4093>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 99.Francesconi O., Ienco A., Moneti G., Nativi C., Roelens S. A self-assembled pyrrolic cage receptor specifically recognizes β-glucopyranosides. Angew. Chem. Int. Ed. 2006;45:6693–6696. doi: 10.1002/anie.200602412. [DOI] [PubMed] [Google Scholar]
- 100.Klein E., Crump M.P., Davis A.P. Carbohydrate recognition in water by a tricyclic polyamide receptor. Angew. Chem. Int. Ed. 2005;44:298–302. doi: 10.1002/anie.200461409. [DOI] [PubMed] [Google Scholar]
- 101.Ferrand Y., Klein E., Barwell N.P., Crump M.P., Jiménez-Barbero J., Vicent C., Boons G.J., Ingale S., Davis A.P. A synthetic lectin for O-linked β-N-acetylglucosamine. Angew. Chem. Int. Ed. 2009;48:1775–1779. doi: 10.1002/anie.200804905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kreppel L.K., Hart G.W. Regulation of a cytosolic and nuclear O-GlcNAc transferase. J. Biol. Chem. 1999;274:32015–32022. doi: 10.1074/jbc.274.45.32015. [DOI] [PubMed] [Google Scholar]
- 103.Ferrand Y., Crump M.P., Davis A.P. A synthetic lectin analog for biomimetic disaccharide recognition. Science. 2007;318:619–622. doi: 10.1126/science.1148735. [DOI] [PubMed] [Google Scholar]
- 104.Joshi G., Davis A.P. New H-bonding patterns in biphenyl-based synthetic lectins; pyrrolediamine bridges enhance glucose-selectivity. Org. Biomol. Chem. 2012;10:5760–5763. doi: 10.1039/c2ob25900a. [DOI] [PubMed] [Google Scholar]
- 105.Gozalbo D., Roig P., Villamon E., Gil M.L. Candida and candidiasis: The cell wall as a potential molecular target for antifungal therapy. Curr. Drug Targets Infect. Disord. 2004;4:117–135. doi: 10.2174/1568005043341046. [DOI] [PubMed] [Google Scholar]
- 106.Mora-Montes H.M., Ponce-Noyola P., Villagómez-Castro J.C., Gow N.A.R., Flores-Carreón A., López-Romero E. Protein glycosylation in Candida. Future Microbiol. 2009;4:1167–1183. doi: 10.2217/fmb.09.88. [DOI] [PubMed] [Google Scholar]
- 107.Lozano V., Aguado L., Hoorelbeke B., Renders M., Camarasa M.J., Schols D., Balzarini J., San-Félix A., Pérez-Pérez M.J. Targeting HIV entry through interaction with envelope glycoprotein 120 (gp120): Synthesis and antiviral evaluation of 1,3,5-triazines with aromatic amino acids. J. Med. Chem. 2011;54:5335–5348. doi: 10.1021/jm200560r. [DOI] [PubMed] [Google Scholar]
- 108.Boyd M.R., Gustafson K.R., McMahon J.B., Shoemaker R.H., O'Keefe B.R., Mori T., Gulakowski R.J., Wu L., Rivera M.I., Laurencot C.M., Currens M.J., Cardellina J.H., II, Buckheit R.W., Jr., Nara P.L., Pannell L.K., Sowder R.C., II, Henderson L.E. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: Potential applications to microbicide development. Antimicrob. Agents Chemother. 1997;41:1521–1530. doi: 10.1128/aac.41.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nativi C., Francesconi O., Gabrielli G., Simone I.D., Turchetti B., Mello T., Mannelli L.D.C., Ghelardini C., Buzzini P., Roelens S. Aminopyrrolic synthetic receptors for monosaccharides: A class of carbohydrate-binding agents endowed with antibiotic activity versus pathogenic yeasts. Chem. Eur. J. 2012;18:5064–5072. doi: 10.1002/chem.201103318. [DOI] [PubMed] [Google Scholar]
- 110.Takeuchi T., Hara H., Naganawa H., Okada M., Hamada M., Umezawa H., Gomi S., Sezaki M., Kondo S. New antifungal antibiotics, benanomicins A and B from an actinomycete. J. Antibiot. 1988;41:807–811. doi: 10.7164/antibiotics.41.807. [DOI] [PubMed] [Google Scholar]
- 111.Oki T., Konishi M., Tomatsu K., Tomita K., Saitoh K., Tsunakawa M., Nishio M., Miyaki T., Kawaguchi H. Pradimicin, a novel class of potent antifungal antibiotics. J. Antibiot. 1988;41:1701–1704. doi: 10.7164/antibiotics.41.1701. [DOI] [PubMed] [Google Scholar]
- 112.Gomi S., Sezaki M., Kondo S. The structures of new antifungal antibiotics, benanomicins A and B. J. Antibiot. 1988;41:1019–1028. doi: 10.7164/antibiotics.41.1019. [DOI] [PubMed] [Google Scholar]
- 113.Tsunakawa M., Nishio M., Ohkuma H., Tsuno T., Konishi M., Naito T., Oki T., Kawaguchi H. The structures of pradimicins A, B, and C: A novel family of antifungal antibiotics. J. Org. Chem. 1989;54:2532–2536. [Google Scholar]
- 114.Tomita K., Nishio M., Saitoh K., Yamamoto H., Hoshino Y., Ohkuma H., Konishi M., Miyaki T., Oki T. Pradimicins A, B, and C: New antifungal antibiotics. I. Taxonomy, production, isolation and physico-chemical properties. J. Antibiot. 1990;43:755–762. doi: 10.7164/antibiotics.43.755. [DOI] [PubMed] [Google Scholar]
- 115.Oki T., Tenmyo O., Hirano M., Tomatsu K., Kamei H. Pradimicins A, B, and C: New antifungal antibiotics. II. In vitro and in vivo biological activities. J. Antibiot. 1990;43:763–770. doi: 10.7164/antibiotics.43.763. [DOI] [PubMed] [Google Scholar]
- 116.Kondo S., Gomi S., Ikeda D., Hamada M., Takeuchi T. Antifungal and antiviral activities of benanomicins and their analogues. J. Antibiot. 1991;44:1228–1236. doi: 10.7164/antibiotics.44.1228. [DOI] [PubMed] [Google Scholar]
- 117.Sawada Y., Nishio M., Yamamoto H., Hatori M., Miyaki T., Konishi M., Oki T. New antifungal antibiotics, pradimicins D and E glycine analogues of pradimicins A and C. J. Antibiot. 1990;43:771–777. doi: 10.7164/antibiotics.43.771. [DOI] [PubMed] [Google Scholar]
- 118.Sawada Y., Hatori M., Yamamoto H., Nishio M., Miyaki T., Oki T. New antifungal antibiotics pradimicins FA-1 and FA-2: D-serine analogs of pradimicins A and C. J. Antibiot. 1990;43:1223–1229. doi: 10.7164/antibiotics.43.1223. [DOI] [PubMed] [Google Scholar]
- 119.Saitoh K., Suzuki K., Hirano M., Furumai T., Oki T. Pradimicins FS and FB, new pradimicin analogs: Directed production, structures and biological activities. J. Antibiot. 1993;46:398–405. doi: 10.7164/antibiotics.46.398. [DOI] [PubMed] [Google Scholar]
- 120.Saitoh K., Sawada Y., Tomita K., Tsuno T., Hatori M., Oki T. Pradimicins L and FL: New pradimicin congeners from Actinomadura verrucosospora subsp. neohibisca. J. Antibiot. 1993;46:387–397. doi: 10.7164/antibiotics.46.387. [DOI] [PubMed] [Google Scholar]
- 121.Furumai T., Hasegawa T., Kakushima M., Suzuki K., Yamamoto H., Yamamoto S., Hirano M., Oki T. Pradimicins T1 and T2, new antifungal antibiotics produced by an actinomycete. I. Taxonomy, production, isolation, physico-chemical and biological properties. J. Antibiot. 1993;46:589–597. doi: 10.7164/antibiotics.46.589. [DOI] [PubMed] [Google Scholar]
- 122.Hasegawa T., Kakushima M., Hatori M., Aburaki S., Kakinuma S., Furumai T., Oki T. Pradimicins T1 and T2, new antifungal antibiotics produced by an actinomycete. II. Structures and biosynthesis. J. Antibiot. 1993;46:598–605. doi: 10.7164/antibiotics.46.598. [DOI] [PubMed] [Google Scholar]
- 123.Saitoh K., Tenmyo O., Yamamoto S., Furumai T., Oki T. Pradimicin S, a new pradimicin analog. I. Taxonomy, fermentation and biological activities. J. Antibiot. 1993;46:580–588. doi: 10.7164/antibiotics.46.580. [DOI] [PubMed] [Google Scholar]
- 124.Saitoh K., Tsuno T., Kakushima M., Hatori M., Furumai T., Oki T. Pradimicin S, a new pradimicin analog. II. Isolation and structure elucidation. J. Antibiot. 1993;46:406–411. doi: 10.7164/antibiotics.46.406. [DOI] [PubMed] [Google Scholar]
- 125.Oki T., Kakushima M., Nishio M., Kamei H., Hirano M., Sawada Y., Konishi M. Water-soluble pradimicin derivatives, synthesis and antifungal evaluation of N, N-dimethyl pradimicins. J. Antibiot. 1990;43:1230–1235. doi: 10.7164/antibiotics.43.1230. [DOI] [PubMed] [Google Scholar]
- 126.Sawada Y., Numata K., Murakami T., Tanimichi H., Yamamoto S., Oki T. Calcium-dependent anticandidal action of pradimicin A. J. Antibiot. 1990;43:715–721. doi: 10.7164/antibiotics.43.715. [DOI] [PubMed] [Google Scholar]
- 127.Kakushima M., Nishio M., Numata K., Konishi M., Oki T. Effect of stereochemistry at the C-17 position on the antifungal activity of pradimicin A. J. Antibiot. 1990;43:1028–1030. doi: 10.7164/antibiotics.43.1028. [DOI] [PubMed] [Google Scholar]
- 128.Okuyama S., Kakushima M., Kamachi H., Konishi M., Oki T. Synthesis and antifungal activities of alanine-exchanged analogs of pradimicin A. J. Antibiot. 1993;46:500–506. doi: 10.7164/antibiotics.46.500. [DOI] [PubMed] [Google Scholar]
- 129.Nishio M., Ohkuma H., Kakushima M., Ohta S., Iimura S., Hirano M., Konishi M., Oki T. Synthesis and antifungal activities of pradimicin A derivatives modification of the alanine moiety. J. Antibiot. 1993;46:494–499. doi: 10.7164/antibiotics.46.494. [DOI] [PubMed] [Google Scholar]
- 130.Aburaki S., Okuyama S., Hoshi H., Kamachi H., Nishio M., Hasegawa T., Masuyoshi S., Iimura S., Konishi M., Oki T. Synthesis and antifungal activity of pradimicin derivatives modifications on the aglycon part. J. Antibiot. 1993;46:1447–1457. doi: 10.7164/antibiotics.46.1447. [DOI] [PubMed] [Google Scholar]
- 131.Kamachi H., Iimura S., Okuyama S., Hoshi H., Tamura S., Shinoda M., Saitoh K., Konishi M., Oki T. Synthesis and antifungal activities of pradimicin derivatives, modification at C4’-position. J. Antibiot. 1992;45:1518–1525. doi: 10.7164/antibiotics.45.1518. [DOI] [PubMed] [Google Scholar]
- 132.Aburaki S., Yamashita H., Ohnuma T., Kamachi H., Moriyama T., Masuyoshi S., Kamei H., Konishi M., Oki T. Synthesis and antifungal activity of pradimicin derivatives modifications of the sugar part. J. Antibiot. 1993;46:631–640. doi: 10.7164/antibiotics.46.631. [DOI] [PubMed] [Google Scholar]
- 133.Sawada Y., Murakami T., Ueki T., Fukagawa Y., Oki T., Nozawa Y. Mannan-mediated anticandidal activity of BMY-28864, a new water-soluble pradimicin derivative. J. Antibiot. 1991;44:119–121. doi: 10.7164/antibiotics.44.119. [DOI] [PubMed] [Google Scholar]
- 134.Kakushima M., Masuyoshi S., Hirano M., Shinoda M., Ohta A., Kamei H., Oki T. In vitro and in vivo antifungal activities of BMY-28864, a water-soluble pradimicin derivative. Antimicrob. Agents Chemother. 1991;35:2185–2190. doi: 10.1128/aac.35.11.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ueki T., Numata K., Sawada Y., Nakajima T., Fukagawa Y., Oki T. Studies on the mode of antifungal action of pradimicin antibiotics I. Lectin-mimic binding of BMY-28864 to yeast mannan in the presence of calcium. J. Antibiot. 1993;46:149–161. doi: 10.7164/antibiotics.46.149. [DOI] [PubMed] [Google Scholar]
- 136.Ueki T., Oka M., Fukagawa Y., Oki T. Studies on the mode of antifungal action of pradimicin antibiotics III. Spectrophotometric sequence analysis of the ternary complex formation of BMY-28864 with D-mannopyranoside and calcium. J. Antibiot. 1993;46:465–477. doi: 10.7164/antibiotics.46.465. [DOI] [PubMed] [Google Scholar]
- 137.Ueki T., Numata K., Sawada Y., Nishio M., Ohkuma H., Toda S., Kamachi H., Fukagawa Y., Oki T. Studies on the mode of antifungal action of pradimicin antibiotics II. D-Mannopyranoside-binding site and calcium-binding site. J. Antibiot. 1993;46:455–464. doi: 10.7164/antibiotics.46.455. [DOI] [PubMed] [Google Scholar]
- 138.Hu M., Ishizuka Y., Igarashi Y., Oki T., Nakanishi H. NMR study of pradimicin derivative BMY-28864 and its interaction with calcium ions in D2O. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1999;55:2547–2558. doi: 10.1016/s1386-1425(99)00106-7. [DOI] [PubMed] [Google Scholar]
- 139.Hu M., Ishizuka Y., Igarashi Y., Oki T., Nakanishi H. NMR, UV–Vis and CD study on the interaction of pradimicin BMY-28864 with divalent cations of alkaline earth metal. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1999;56:181–191. doi: 10.1016/s1386-1425(99)00196-1. [DOI] [PubMed] [Google Scholar]
- 140.Hu M., Ishizuka Y., Igarashi Y., Oki T., Nakanishi H. Interaction of three pradimicin derivatives with divalent cations in aqueous solution. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2000;56:1233–1243. doi: 10.1016/s1386-1425(00)00229-8. [DOI] [PubMed] [Google Scholar]
- 141.Fujikawa K., Tsukamoto Y., Oki T., Lee Y.C. Spectroscopic studies on the interaction of pradimicin BMY-28864 with mannose derivatives. Glycobiology. 1998;8:407–414. doi: 10.1093/glycob/8.4.407. [DOI] [PubMed] [Google Scholar]
- 142.Nakagawa Y., Masuda Y., Yamada K., Doi T., Takegoshi K., Igarashi Y., Ito Y. Solid-state NMR spectroscopic analysis of the Ca2 +-dependent mannose binding of pradimicin A. Angew. Chem. Int. Ed. 2011;50:6084–6088. doi: 10.1002/anie.201007775. [DOI] [PubMed] [Google Scholar]
- 143.Summers M.F. 113Cd NMR spectroscopy of coordination compounds and proteins. Coord. Chem. Rev. 1988;86:43–134. [Google Scholar]
- 144.Xia H., Rayson G.D. 113Cd-NMR spectrometry of Cd2 + binding sites on algae and higher plant tissues. Adv. Environ. Res. 2002;7:157–167. [Google Scholar]
- 145.Nakagawa Y., Doi T., Masuda Y., Takegoshi K., Igarashi Y., Ito Y. Mapping of the primary mannose binding site of PRM-A. J. Am. Chem. Soc. 2011;133:17485–17493. doi: 10.1021/ja207816h. [DOI] [PubMed] [Google Scholar]
- 146.Takegoshi K., Nakamura S., Terao T. 13C–1H dipolar-assisted rotational resonance in magic-angle spinning NMR. Chem. Phys. Lett. 2001;344:631–637. [Google Scholar]
- 147.Takegoshi K., Nakamura S., Terao T. 13C–1H dipolar-driven 13C–13C recoupling without 13C rf irradiation in nuclear magnetic resonance of rotating solids. J. Chem. Phys. 2003;118:2325–2341. [Google Scholar]
- 148.Morcombe C.R., Gaponenko V., Byrd R.A., Zilm K.W. Diluting abundant spins by isotope edited radio frequency field assisted diffusion. J. Am. Chem. Soc. 2004;126:7196–7197. doi: 10.1021/ja047919t. [DOI] [PubMed] [Google Scholar]
- 149.Nakagawa Y., Doi T., Takegoshi K., Igarashi Y., Ito Y. Solid-state NMR analysis of calcium and D-mannose binding of BMY-28864, a water-soluble analogue of pradimicin A. Bioorg. Med. Chem. Lett. 2012;22:1040–1043. doi: 10.1016/j.bmcl.2011.11.106. [DOI] [PubMed] [Google Scholar]
- 150.Tanabe A., Nakashima H., Yoshida O., Yamamoto N., Tenmyo O., Oki T. Inhibitory effect of new antibiotic, pradimicin A on infectivity, cytopathic effect and replication of human immunodeficiency virus in vitro. J. Antibiot. (Tokyo) 1988;41:1708–1710. doi: 10.7164/antibiotics.41.1708. [DOI] [PubMed] [Google Scholar]
- 151.Hoshino H., Seki J., Takeuchi T. New antifungal antibiotics, benanomicins A and B inhibit infection of T-cell with human immunodeficiency virus (HIV) and syncytium formation by HIV. J. Antibiot. 1989;42:344–346. doi: 10.7164/antibiotics.42.344. [DOI] [PubMed] [Google Scholar]
- 152.Tanabe-Tochikura A., Tochikura T.S., Yoshida O., Oki T., Yamamoto N. Pradimicin A inhibition of human immunodeficiency virus: Attenuation by mannan. Virology. 1990;176:467–473. doi: 10.1016/0042-6822(90)90016-k. [DOI] [PubMed] [Google Scholar]
- 153.Balzarini J., Van Laethem K., Daelemans D., Hatse S., Bugatti A., Rusnati M., Igarashi Y., Oki T., Schols D. Pradimicin A, a carbohydrate-binding nonpeptidic lead compound for treatment of infections with viruses with highly glycosylated envelopes, such as human immunodeficiency virus. J. Virol. 2007;81:362–373. doi: 10.1128/JVI.01404-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Balzarini J., François K.O., Van Laethem K., Hoorelbeke B., Renders M., Auwerx J., Liekens S., Oki T., Igarashi Y., Schols D. Pradimicin S, a highly soluble nonpeptidic small-size carbohydrate-binding antibiotic, is an anti-HIV drug lead for both microbicidal and systematic use. Antimicrob. Agents Chemother. 2010;54:1425–1435. doi: 10.1128/AAC.01347-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shahzad-ul-Hussan S., Ghirlando R., Dogo-Isonagie C.I., Igarashi Y., Balzarini J., Bewley C.A. Characterization and carbohydrate specificity of pradimicin S. J. Am. Chem. Soc. 2012;134:12346–12349. doi: 10.1021/ja303860m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Huskens D., Vermeire K., Vandemeulebroucke E., Balzarini J., Schols D. Safety concerns for the potential use of cyanovirin-N as a microbicidal anti-HIV agent. Int. J. Biochem. Cell Biol. 2008;40:2802–2814. doi: 10.1016/j.biocel.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 157.François K.O., Auwerx J., Schols D., Balzarini J. Simian immunodeficiency virus is susceptible to inhibition by carbohydrate-binding agents in a manner similar to that of HIV: Implications for further preclinical drug development. Mol. Pharmacol. 2008;74:330–337. doi: 10.1124/mol.108.047621. [DOI] [PubMed] [Google Scholar]


