Abstract
Following a stressful event, the hypothalamus-pituitary-adrenal axis mediates the release of the stress hormone cortisol (corticosterone in rodents; CORT). Elevated CORT binds to glucocorticoid receptors to mediate physiological responses including facilitating memory formation. Previous work from our laboratory demonstrated that male rats exposed to chronic stress demonstrate enhanced contextual fear memories and sensitized CORT responses to subsequent stress exposure; however, this is unknown in female rats. The experiments here tested whether chronic stress enhances fear memory formation in female rats and whether the sensitized CORT response in chronic stress rats contributes to their enhanced fear memory. Studies first examined CORT responses to contextual fear conditioning in male and female rats and examined whether chronic stress enhanced the formation of contextual fear memories 24hrs later. Studies then used metyrapone, a CORT synthesis inhibitor, to investigate whether blockade of plasma CORT would eliminate the chronic stress-induced enhancement in contextual fear memory. Results show that female rats have greater CORT responses than males, and chronic stress sensitizes the CORT response to fear conditioning in both sexes. However, female rats do not show enhanced contextual fear memory following chronic stress. Chronically stressed male rats show greater memory acquisition and show greater contextual fear memory 24hrs later following fear conditioning. Metyrapone dampens contextual fear memory in all rats but does not eliminate the enhancement in freezing behavior in chronic stress rats. Collectively, these studies indicate sensitized CORT responses in chronically stressed rats is likely not the mechanism by which chronic stress facilitates memory formation.
Keywords: Corticosterone, Memory, Stress, Sex
Chronic stress impacts an individual’s behavior such as restlessness and avoidance of nonharmful stimuli, which are more broadly classified as anxiety [1]. Clinically, diagnoses of anxiety disorders range from generalized anxiety disorder, panic disorder, social anxiety disorder, and post-traumatic stress disorder (PTSD) [2]. These diagnoses are all characterized by an enhanced fear response and more specifically an underlying enhanced fear memory. Notably, women are more likely than men to develop anxiety [3,4]; however, research conducted on anxiety in rodents is mostly completed in males. Thus, an investigation into whether female rats are susceptible to enhanced fear memory formation following chronic stress is needed.
Corticosterone (CORT), a stress hormone important for liberating energy in response to a stressor [5] has been demonstrated to have a role in modulating memory formation [6,7]. McReynolds et al. found CORT was sufficient to enhance memory consolidation by administering a single injection of CORT immediately after inhibitory avoidance conditioning [7]. Additionally, Roozendaal et al. discovered that glucocorticoids enhance memory consolidation via α1-adrenergic receptor coupling to glucocorticoid receptors (GR); however, their laboratory only utilized acute stressors [8,9].
Chronic stress often primes the CORT response to subsequent acute stress exposure [10] and has also been demonstrated to enhance fear memory formation in rodents [11,12]. It is currently unknown whether the chronic stress enhancement of fear memory is caused by the sensitization of the CORT response. The present work investigated whether CORT is necessary for the chronic stress enhancement of fear memory.
Studies first characterized whether CORT production is enhanced in chronically stressed male and female rats following fear conditioning. Second, we tested whether female rats develop enhanced fear memories. Third, we tested whether CORT is necessary systemically for chronic stress enhanced fear memories.
We used both male and female Fischer rats (Harlan, Indianapolis, Indiana) since this strain is highly stress responsive and more susceptible to stress-induced pathology compared to many other rat strains [13–15]. Adult rats (250–350g) were single-housed in standard rat cages, and given access to food and water ad libitum, except when undergoing food restriction stress (see below). Rats were kept on a 12:12hr light–dark cycle (lights on at 08:00). All animals were handled according to the Animal Welfare Act and The Guide for the Care and Use of Laboratory Animals. The Kent State University Institutional Animal and Care Committee approved all procedures.
The rats were exposed to a series of stressors following an established protocol [13,16] or were left undisturbed as home cage control animals. This four day repeated stress paradigm was chosen since we previously demonstrated that it results in increased norepinephrine turnover in the amygdala, sensitized β-adrenergic receptor mediated responses, and enhanced fear conditioning to contextual cues [11,13,17]. On the morning (08:00–10:00) of day 1, chronic stress rats were placed in DecapiCone rodent restrainers (Braintree Scientific, Inc., Braintree, MA) for 60min, before being returned to their home cage. At 15:00hr, food was removed from chronic stress rat cages for 18hr. On day 2, chronic stress rats were placed in novel habitats, containing 35μL trimethylthiazoline (a component of fox feces) to simulate predator odor. Rats were then placed back in their home cages but were housed in constant light conditions overnight. On the morning of day 3, animals were exposed to restraint stress again for 60min, then at 15:00hr their bedding material was dampened with approximately 1500mL distilled water. On day 4, rats were exposed to forced swim for 5min in glass cylinders measuring 49 × 18.7cm (inner height and diameter, respectively) and were filled approximately to the 37.5cm line with water at a temperature of 21°C. Following this task, rats were placed in cages containing dry bedding. Control rats were in their home cage for the entire duration of the chronic stress paradigm.
Twenty-four hrs following the termination of the last stressor, rats were placed in a 21.59 × 21.59 × 27.94cm conditioning chamber (Lafayette Instrument Company, Lafayette, IN) with a floor consisting of a series of electrically conductive steel bars for a total of 5min. At min 2 and min 4, rats received a foot shock (1.5mA for 2s). Following fear conditioning, rats were placed back into their home cages.
For study 1, rats (n=5–6/group) were decapitated immediately following fear conditioning to collect blood and measure plasma CORT. The CORT was measured using the Corticosterone ELISA Kit (Enzo, NY; CAT: ADI-901-097). Plasma was diluted 1:50 and heated for 1hr at 70°C to release CORT from corticosterone-binding globulin. Manufacturer instructions were followed after the dissociation step. In study 2, a novel group of female rats were separated into either chronic stress or no chronic stress groups (n=8/group). All rats went through the contextual fear memory paradigm and freezing behavior was recorded 24hr following fear conditioning. In study 3, two hrs prior to fear conditioning, 100mg/kg of metyrapone (Sigma-Aldrich; CAT:1443001) or saline by intraperitoneal injection into the rats (n=12/group). We previously demonstrated this dose and time as sufficient to block CORT synthesis [16]. Rats were then returned to their home cage for the 2hr duration.
For study 2 and study 3, 24hr following fear conditioning, rats were placed back into the conditioning chamber and freezing behavior was recorded via a C615 HD Webcam (Logitech, Silicon Valley, CA) for 15min. Freezing behavior was evaluated to measure contextual fear memory (ability to remember the fearful context) and was defined as complete immobility, except for movements necessary for respiration. Scoring was performed by a trained researcher blind to group assignment. Scores were obtained by checking the video every 10sec for 15min where one point was assigned for each instance of freezing behavior, with a maximum possible score of 90pts. In study 3, fear acquisition (ability to acquire the memory) was also recorded during the time of fear conditioning. Acquisition time points were separated into “0–2 min”; “2–4 min”; and “4–5 min” for analysis of freezing behavior.
A repeated measures analysis (Figure 1A) and a two-way ANOVA (Figure 1B) were utilized to determine statistical significance (alpha=0.05) in study 1. A t-test was utilized to analyze statistical significance (alpha=0.05) on the effect of chronic stress on female contextual fear memory in study 2 (Figure 2). A repeated measures analysis (Figure 3A) and a two-way ANOVA test (Figure 3B) were utilized to analyze statistical significance (alpha=0.05) in contextual fear acquisition and fear memory in study 3.
Fig 1. Prior chronic stress exposure sensitizes the CORT response.
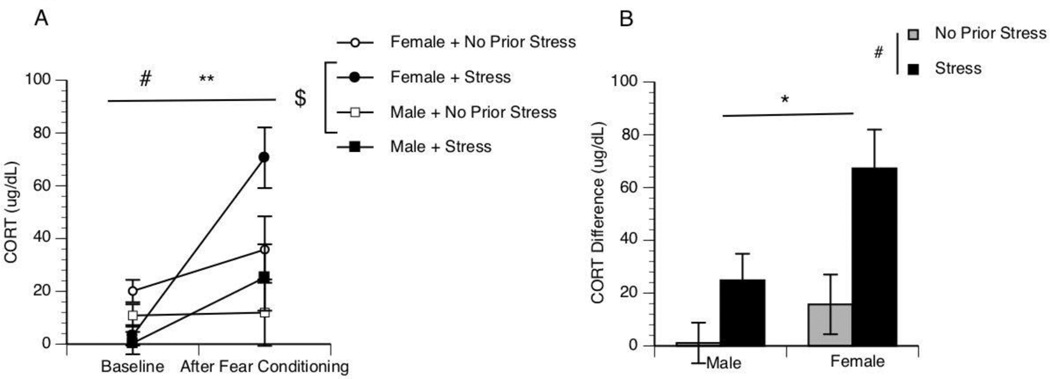
Baseline CORT was collected from male and female rats. Rats were then either chronically stressed or not chronically stressed. All rats then received fear conditioning. Immediately following fear conditioning, trunk blood was collected. Baseline CORT and After Fear Conditioning CORT are reported. ** Effect of Time x Stress, # Effect of Time x Sex, $ Effect of Sex. The mean CORT differences (CORT After Fear Conditioning - CORT Baseline) for each group are reported (3B). * Effect of Stress p<0.05; # Effect of Sex p<0.05.
Fig 2. Female rats do not show enhanced contextual fear memory.
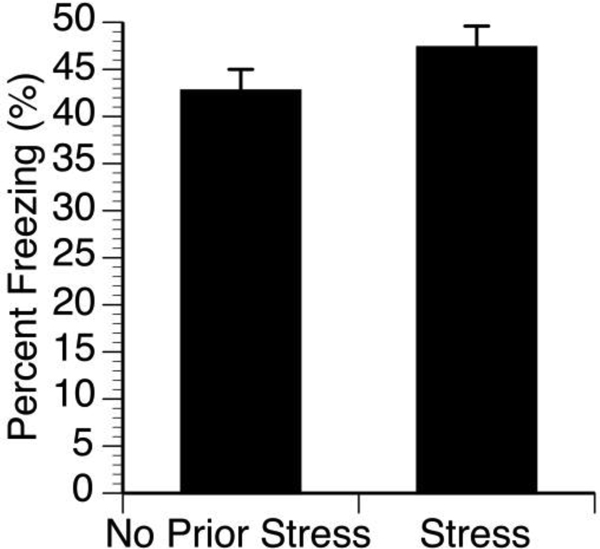
Female rats were separated into either chronic stress or no chronic stress groups and all rats went through contextual fear memory paradigm. Following 24hr, freezing behavior was recorded.
Fig 3. Metyrapone failed to protect animals from chronic stress induced enhancement in freezing behavior.
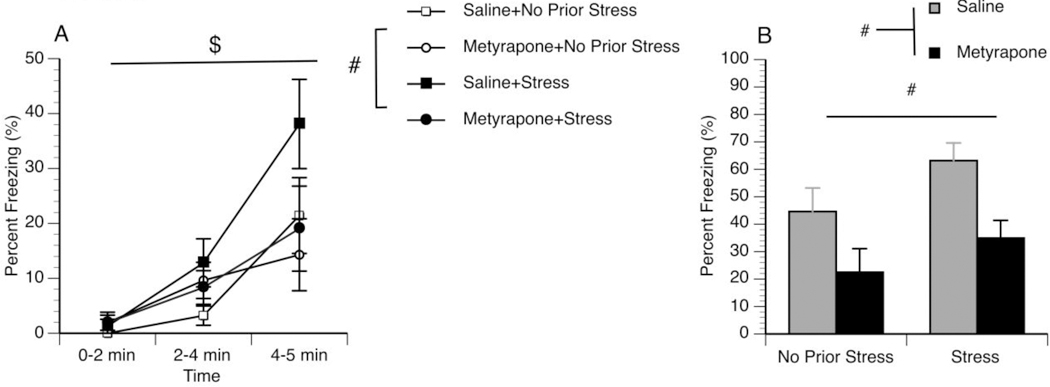
Rats were either not chronically stressed or chronically stressed. In addition, rats were administered saline or metyrapone 2hrs prior to fear conditioning. All rats underwent the fear conditioning paradigm. Throughout the fear conditioning paradigm (acquisition), rats were scored for percent freezing (3A). Freezing behavior is presented across time: 0–2 min (prior to foot shock); 2–4 min (after one foot shock); and 4–5 min (after two foot shocks). $ Effect of time p<0.05; # Effect of Stress p<0.05. Fear memory was assessed 24hrs following conditioning when rats were returned to the same context and percent freezing was measured (3B). The first five minutes of contextual fear memory is reported. # Main effect of stress and drug treatments p=<0.05.
To understand the sensitization of CORT following chronic stress, baseline CORT was first measured in both male and female (prior to chronic stress). Chronically stressed and not chronically stressed control animals underwent fear conditioning and were immediately decapitated to determine the change in plasma CORT. A repeated measures analysis revealed a significant effect of Time x Stress [F(1,17)=10.26, p=0.005] and effect of Time x Sex [F(1,17)=5.885, p=0.027] (Figure 1A). A two-way ANOVA revealed a significant main effect of CORT in chronically stressed rats compared to not chronically stressed rats [F(1,17)=10.26, p=0.005]. In addition, the two-way ANOVA revealed a significant main effect of sex [F(1,17)=5.88, p=0.027] in which females had a significantly greater CORT response compared to males. There was no interaction present [F(1,17)=1.395, p=0.254] (Figure 1B).
To determine whether female rats are susceptible to chronic stress enhancement in fear memory, female rats were separated into either chronic stress or no chronic stress groups and all rats went through contextual fear memory paradigm. There was no significant difference in freezing behavior between chronic stress and no chronic stress groups [t(14)= −0.356, p=0.727] (Figure 2).
To examine the role of CORT on fear acquisition and memory, metyrapone (CORT synthesis inhibitor) or saline was administered systemically to animals exposed to 4-days of chronic stress or non-stressed control male rats 2hrs prior to fear conditioning. A repeated measures analysis revealed that during the acquisition of the fear memory, chronically stressed rats had a significant increase in freezing [F(1,44)=2.942, p=0.047, one-tailed] (Figure 3A). In addition, there was a significant effect of time during the acquisition phase of fear conditioning [F(1,44)=36.11, p=0.0001] (Figure 3A). However, the data do not reveal a significant effect of metyrapone during acquisition [F(1,44)=1.438, p=0.237]. Moreover, there was no interaction between drug treatment and stress condition [F(1,44)=1.771, p=0.190] (Figure 3A). We also examined the effects of metyrapone on fear memory formation 24hrs later. A two-way ANOVA analysis revealed a significant main effect of chronic stress in which chronically stressed rats had a significantly greater contextual fear memory compared to no chronic stress rats [F(1,44)=4.13, p=0.048]. The two-way ANOVA analysis also revealed a significant main effect of metyrapone administration in which metyrapone dampened fear memory [F(1,44)=10.98, p=0.002]. There was no interaction between metyrapone treatment and stress condition [F(1,44)=0.183, p=0.671] (Figure 3B).
Studies presented here investigated the role of CORT on contextual fear memory. It was discovered that chronic stress increases the CORT response in both males and females with females having greater CORT responses compared to males. However, female rats do not show enhanced contextual fear memory following chronic stress. Male rats that undergo chronic stress have sensitized acquisition during fear conditioning and sensitized contextual fear memory 24hrs after fear conditioning. Metyrapone has no effect on acquisition but dampens contextual fear memory 24hrs later; however, chronic stress sensitizes contextual fear memory in males. The molecular mechanism of this phenomenon was unclear. Previous research has shown that CORT was a candidate molecule since chronically stressed rats have greater basal production of CORT and sensitized CORT production to a subsequent stressor [10], and CORT is sufficient to enhance memory consolidation [7]. For this reason, this work investigated CORT’s role in fear memory. Camp and Johnson [11] demonstrated that chronic stress enhances contextual fear memory in male rats. One of the limitations of their work is that females were not utilized, thus it was unknown whether female rats are susceptible to chronic stress enhanced contextual fear memory like males. We first characterized the CORT response to fear conditioning in females compared to males. Both male and female rats with a prior history of stress showed greater CORT levels following fear conditioning. Moreover, females had an even greater CORT response compared to males (Figure 1). This is significant because it would initially suggest that the sensitized CORT is a prime candidate for the mechanism behind chronic stress induced contextual fear memory. For this reason, we repeated Camp and Johnson [11] chronic stress protocol to examine fear memory in female rats. Surprisingly, even though female rats demonstrate greater CORT responses to fear conditioning compared to males, females failed to show enhanced contextual fear memories following conditioning (Figure 2). This is the first indication that sensitized CORT responses following chronic stress is not sufficient to enhance fear memories. Gruene et al.’s work suggest that female rodents express a more active fear response (darting) compared to males, which may explain the sexual dimorphic phenomenon [18]. Since female rats were not susceptible to chronic stress-induced enhancement in contextual fear memory, the remaining studies only utilized male rats.
To further evaluate the role of CORT in facilitating fear memory formation, chronically stressed male rats and not chronically stressed controls were administered metyrapone prior to fear conditioning. The data demonstrate that chronic stress sensitizes memory acquisition (Figure 3A) and enhances contextual fear memory when measured 24 hours after fear conditioning (Figure 3B). Metyrapone fails to dampen memory acquisition (Figure 3A) but does disrupt the formation of fear memories (Figure 3B). This suggests that CORT is not contributing toward acquisition of the memory but is important for the consolidation of the memory. Interestingly, the two-way ANOVA failed to reveal an interaction between metyrapone and chronic stress. This indicates that the greater fear response in chronic stress animals is not dependent on a sensitized CORT response during fear conditioning.
There are limitations to these studies. First, we characterized systemic CORT; however, it is still unclear the role CORT has in the brain. Future studies could investigate availability of CORT within specific brain regions. One area of interest is the basolateral amygdala, a brain region important for connecting the stressful stimuli with the environment [19,20]. Second, the clinical data suggest women actually have less CORT compared to men [21,22] and are more susceptible to fearful memories [3,4], which is opposite for the data we presented here in rats (Figure 1 and Figure 2). Kessler et al. found that clinically, women are more likely to develop anxiety and twice as likely to develop PTSD [4,23], but the data here suggest female rats are not susceptible to the enhanced fear memories. CORT can suppress norepinephrine release [16,24], thus low CORT in women (and here in male rats) may result in more prolonged increase norepinephrine, which has been heavily implicated in facilitating memory formation [16,25]. Future studies will investigate whether greater CORT responses actually provide protective mechanisms against enhanced contextual memory in female rats.
Acknowledgments
Funding
This work was supported by the National Institutes of Health: MH11049-01A1.
Footnotes
Declaration of Competing Interest
The authors state no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Eiland L, McEwen BS, Early life stress followed by subsequent adult chronic stress potentiates anxiety and blunts hippocampal structural remodeling, Hippocampus 22 (2012) 82–91. 10.1002/hipo.20862. [DOI] [PubMed] [Google Scholar]
- [2].Crocq MA, The history of generalized anxiety disorder as a diagnostic category, Dialogues Clin. Neurosci 19 (2017) 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kessler RC, Wai TC, Demler O, Walters EE, Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication, Arch. Gen. Psychiatry 62 (2005) 617–627. 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Foa EB, Street GP, Women and traumatic events., J. Clin. Psychiatry 62 Suppl 17 (2001) 29–34. http://www.ncbi.nlm.nih.gov/pubmed/11495093 (accessed August 4, 2019). [PubMed] [Google Scholar]
- [5].Heck AL, Handa RJ, Sex differences in the hypothalamic-pituitary-adrenal axis’ response to stress: an important role for gonadal hormones, Neuropsychopharmacology 44 (2019) 45–58. 10.1038/s41386-018-0167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pugh CR, Tremblay D, Fleshner M, Rudy JW, A Selective Role for Corticosterone in Contextual-Fear Conditioning, Behav. Neurosci Ill (1997) 503–511. http://psycnet.apa.org/fulltext/1997-04750-004.pdf (accessed February 12, 2018). [PubMed] [Google Scholar]
- [7].McReynolds JR, Holloway-Erickson CM, Parmar TU, McIntyre CK, Corticosterone-induced enhancement of memory and synaptic Arc protein in the medial prefrontal cortex., Neurobiol. Learn. Mem 112 (2014) 148–57. 10.1016/j.nlm.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Roozendaal B, Quirarte GL, McGaugh JL, Glucocorticoids interact with the basolateral amygdala beta-adrenoceptor--cAMP/cAMP/PKA system in influencing memory consolidation., Eur. J. Neurosci 15 (2002) 553–60. http://www.ncbi.nlm.nih.gov/pubmed/11876783 (accessed August 4, 2019). [DOI] [PubMed] [Google Scholar]
- [9].Roozendaal B, Brunson KL, Holloway BL, McGaugh JL, Baram TZ, Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation, Proc. Natl. Acad. Sci 99 (2002) 13908–13913. 10.1073/pnas.212504599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lowrance SA, Ionadi A, McKay E, Douglas X, Johnson JD, Sympathetic nervous system contributes to enhanced corticosterone levels following chronic stress., Psychoneuroendocrinology 68 (2016) 163–70. 10.1016/j.psyneuen.2016.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Camp RM, Johnson JD, Repeated stressor exposure enhances contextual fear memory in a beta-adrenergic receptor-dependent process and increases impulsivity in a non-beta receptor-dependent fashion., Physiol. Behav 150 (2015) 64–8. 10.1016/j.physbeh.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jones ME, Lebonville CL, Barrus D, Lysle DT, The Role of Brain Interleukin-1 in Stress-Enhanced Fear Learning, Neuropsychopharmacology 40 (2015) 1289–1296. 10.1038/npp.2014.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Camp RM, Remus JL, Kalburgi SN, Porterfield VM, Johnson JD, Fear conditioning can contribute to behavioral changes observed in a repeated stress model, Behav. Brain Res 233 (2012) 536–544. 10.1016/j.bbr.2012.05.040. [DOI] [PubMed] [Google Scholar]
- [14].Porterfield VM, Zimomra ZR, Caldwell EA, Camp RM, Gabella KM, Johnson JD, Rat strain differences in restraint stress-induced brain cytokines., Neuroscience. 188 (2011) 48–54. 10.1016/j.neuroscience.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Uchida S, Nishida A, Hara K, Kamemoto T, Suetsugi M, Fujimoto M, Watanuki T, Wakabayashi Y, Otsuki K, McEwen BS, Watanabe Y, Characterization of the vulnerability to repeated stress in Fischer 344 rats: possible involvement of microRNA-mediated downregulation of the glucocorticoid receptor, Eur. J. Neurosci 27 (2008) 2250–2261. 10.1111/j.1460-9568.2008.06218.x. [DOI] [PubMed] [Google Scholar]
- [16].Barnard DF, Gabella KM, Kulp AC, Parker AD, Dugan PB, Johnson JD, Sex differences in the regulation of brain IL-1β in response to chronic stress, Psychoneuroendocrinology. 103 (2019) 203–211. 10.1016/j.psyneuen.2019.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Porterfield VM, Gabella KM, Simmons MA, Johnson JD, Repeated stressor exposure regionally enhances beta-adrenergic receptor-mediated brain IL-1β production, Brain. Behav. Immun 26 (2012) 1249–1255. 10.1016/j.bbi.2012.08.001. [DOI] [PubMed] [Google Scholar]
- [18].Gruene TM, Flick K, Stefano A, Shea SD, Shansky RM, Sexually divergent expression of active and passive conditioned fear responses in rats, Elife. 4 (2015). 10.7554/eLife.11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Maren S, Neurotoxic basolateral amygdala lesions impair learning and memory but not the performance of conditional fear in rats., J. Neurosci 19 (1999) 8696–703. 10.1523/JNEUROSCI.19-19-08696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kishi T, Tsumori T, Yokota S, Yasui Y, Topographical projection from the hippocampal formation to the amygdala: A combined anterograde and retrograde tracing study in the rat, J. Comp. Neurol 496 (2006) 349–368. 10.1002/cne.20919. [DOI] [PubMed] [Google Scholar]
- [21].Zimmer C, Basler H-D, Vedder H, Lautenbacher S, Sex differences in cortisol response to noxious stress., Clin. J. Pain 19 (n.d.) 233–9. http://www.ncbi.nlm.nih.gov/pubmed/12840617 (accessed August 4, 2019). [DOI] [PubMed] [Google Scholar]
- [22].Van Cauter E, Leproult R, Kupfer DJ, Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol., J. Clin. Endocrinol. Metab 81 (1996) 2468–2473. 10.1210/jcem.81.7.8675562. [DOI] [PubMed] [Google Scholar]
- [23].Kessler RC, Chiu WT, Demler O, Walters EE, Walters EE, Prevalence, Severity, and Comorbidity of 12-Month DSM-IV Disorders in the National Comorbidity Survey Replication, Arch. Gen. Psychiatry 62 (2005) 617 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pacák K, Kvetnanský R, Palkovits M, Fukuhara K, Yadid G, Kopin IJ, Goldstein DS, Adrenalectomy augments in vivo release of norepinephrine in the paraventricular nucleus during immobilization stress., Endocrinology 133 (1993) 1404–1410. 10.1210/endo.133.3.8396018. [DOI] [PubMed] [Google Scholar]
- [25].LaLumiere RT, Buen T-V, McGaugh JL, Post-training intra-basolateral amygdala infusions of norepinephrine enhance consolidation of memory for contextual fear conditioning., J. Neurosci 23 (2003) 6754–8. 10.1523/JNEUROSCI.23-1706754.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


