Abstract
WHO has listed several priority diseases with epidemic potential for which there are no, or insufficient, medical countermeasures. In response, the Bill & Melinda Gates Foundation (with support from PricewaterhouseCoopers) coordinated subject matter experts to create a preparedness plan for Disease X. Disease X is caused by Pathogen X, an infectious agent that is not currently known to cause human disease, but an aetiologic agent of a future outbreak with epidemic or pandemic potential. We have identified crucial areas for acceleration in medical countermeasure product development and international coordination. We have also reviewed novel platforms and process improvements related to manufacturing, which could revolutionise the response to the next pandemic. Finally, we created several coordination and engagement guides. These guides range from the rational design of an intervention target product profile, to the key facets of vaccine and therapeutic development, to accelerated manufacturing and regulatory mechanisms. In this Personal View, we provide a high-level summary of the outcomes of the medical countermeasure development workstream, intended for a broad audience including academia, medical countermeasure developers, and multilateral coordinating bodies. We hope that they might find this piece useful in prioritising strategic investments and efforts to accelerate medical countermeasure development. We observed that in-depth analyses of clinical trial design, chemistry, manufacturing and control activities, and accelerated regulatory pathways are necessary for shortening the timelines for the product development of medical countermeasures. We intend to cover these topics in future publications.
Introduction
The 2014–16 Ebola epidemic had mostly waned before successful medical countermeasures were deployed.1 Arguably, many lives could have been saved if these countermeasures had arrived sooner. In 2016, WHO's Research and Development Blueprint was launched to decrease the time for development, assessment, and authorisation of medical countermeasures for the world's most dangerous pathogens. Although this effort has provided unprecedented coordination, past epidemics have revealed many scientific and technical issues that remain unresolved. The rapid development of diagnostics, vaccines, and therapeutics in the wake of an epidemic involves a complex and interdependent stakeholder ecosystem: these stakeholders might have different priorities, interests, and activities resulting in misaligned goals and delays. Additionally, uncertainty remains as to who will pay the costs, offset the risk, and accelerate research, clinical trials, and product development for medical countermeasures. The global community has an opportunity to align and coordinate these efforts across stakeholder groups.
In this Personal View, we discuss the rapid development of medical countermeasures for Pathogen X, an infectious agent currently unknown to cause human disease, but with epidemic or pandemic potential. Although this Personal View is based on our 2018 convening, we believe that the recommendations continue to be valid. The goal of the convening was to focus the few resources in pandemic preparedness on the crucial and persistent barriers that remain across research, clinical trials, and manufacturing before and during an epidemic. We attempt to define Pathogen X and present the challenges, opportunities, and priorities in the acceleration of diagnostic, vaccine, and small molecule development in preparation for an epidemic. Also, we present coordination guides that have been shown to be successful in planning and prioritising development activities. We hope that this content will enable funders, academia, and product developers (ie, biotechnology and pharmaceutical companies) to better navigate the epidemic medical countermeasure space. The content should also support stakeholders in coordinating and engaging with developers of medical countermeasures, regulators, and government officials. Additionally, we recognise that accelerated manufacturing platforms and regulatory procedures are key drivers of epidemic medical countermeasure development, and they will be covered in future publications elsewhere.
Key messages.
-
•
Emerging pathogens continue to pose a serious threat to global health. More lives can be saved if medical countermeasures are deployed in time. We convened subject matter experts in preclinical development, clinical development, manufacturing, and regulatory assessment to discuss how the development and approval of medical countermeasures could be accelerated both before and during an epidemic.
-
•
Disease X will result from Pathogen X: a pathogen that is previously unknown to cause human disease but possesses epidemic or pandemic potential.
-
•
There are key challenges that span across preclinical, clinical, and the manufacturing phases of medical countermeasure product development, including low sample and reagent availability, challenges in manufacturing at scale, and efficient operation of harmonised clinical trials across borders.
-
•
Investments can be made now to accelerate the availability of medical countermeasures during a pandemic.
-
•
Because end-to-end product development is a complex process with many interdependent decisions, the intervention target product profile can be used to set standards so that medical countermeasure developers have a clear understanding of the hurdles that need to be met for successful development and use of their products.
The epidemiology of Pathogen X
Pathogen X could be any pathogen including but not limited to viruses, bacteria, fungi, parasites, or prions. Of the 400 emerging infectious disease events recorded since 1940, bacteria (including rickettsia) account for 54%, whereas viral or prion pathogens (25%), protozoa (11%), fungi (6%), and helminths (3%) are less common.2 Although viral pathogens represent a small proportion of the pathogens that account for emerging infectious disease events, the most devastating recent emergence events—namely, HIV, influenza H1N1 and H5H1, severe acute respiratory syndrome coronavirus, Lassa virus, Ebola virus, and Middle East respiratory syndrome coronavirus—have involved RNA viruses.3, 4 They can replicate in numerous host species, and their error-prone reverse transcriptase enables high mutability resulting in the evasion of host responses. Further, 94% of zoonotic viruses affecting humans are RNA viruses.5, 6
This zoonotic transmission often occurs where human activities take place in a landscape of wildlife, insect, and microbial diversity.7 Their emergence is largely driven by anthropogenic changes; as humans alter their patterns of land use for agriculture, trade, livestock rearing, and travel, these pathogens have an opportunity to cross species and establish localised emergence. A synergy of enhanced virulence and population dynamics act to drive transmission from these self-limiting emergence events into sustained person-to-person transmission. Thus, pandemic emergence is assumed to be likely to occur because of the following risk factors: human activities near wildlife, creation of animal source foods with little monitoring of employees and a poorly understood supply chain, insect and tick vectors, extreme population density, and constrained surveillance and laboratory capacity.8, 9, 10
Consequently, we can hypothesise that the advent of a catastrophic outbreak involving Disease X is likely to result from the zoonotic transmission of a highly virulent RNA virus11 from an area where a convergence of risk factors and population dynamics will result in sustained person-to-person transmission. This premise does not negate the need for measures against other types of pathogens of pandemic importance, but the work on Disease X that we have done is modelled on the development of medical countermeasures against this particular pathogen archetype. However, the development of medical countermeasures does not demand that the epidemiology of Disease X is known, and many conserved elements of product development remain that do not vary on the basis of established pathophysiology or epidemiology. In the first instance, development of medical countermeasures for Disease X will inevitably include plans to detect cases and mobilise the movement of samples and data to developers.
Sample and data sharing
One of the most important barriers to the development of diagnostics is the paucity of serum and blood samples available to validate clinical assays. Challenges to the availability of such samples include logistical difficulties in storing and transporting biohazardous samples from remote areas, obtaining sufficient informed consent from patients, and political complications from countries that refuse to transit or accept hazardous specimens.12 To overcome these operational and political obstacles, an opportunity exists to establish regional biorepositories and formalise multilateral agreements concerning the collection and transport of specimens. The Nagoya Protocol13—a treaty designed to ensure the fair and equitable sharing of benefits arising out of the use of genetic resources—could not only be expanded to include other biological material, but also enhanced to include exceptions or fast track options for emergency research on pathogens.14
Issues of intellectual property and benefit sharing continue to hamper the development of medical countermeasures during epidemics. Stakeholders need to negotiate intellectual property contracts and agreements to facilitate the sharing of pathogen sequences,15 clinical samples, and diagnostic platforms before outbreaks occur.16 For example, the Pandemic Influenza Preparedness Framework17 was negotiated over 4 years and resulted in a cooperative approach to benefit sharing, as well as substantial cost sharing of the laboratory-based surveillance system. Since its institution, there has been more open sharing of influenza samples. Although the Pandemic Influenza Preparedness Framework is limited by its inability to address sharing of pathogen genetic sequence data, it represents a model on which sample and sequence sharing can be based.
Opportunities for accelerating the development of medical countermeasures against Disease X
The product development cycle remains lengthy for de-novo medical countermeasures: diagnostic tests typically take 2–5 years to develop and 5–10 years to complete development, before procurement can be initiated. The timeline for vaccines and small molecules is even longer, largely because of the requirement for human safety and efficacy data. Here, we list technological and process opportunities for shortening the development times of diagnostics, vaccines, clinical trials, and small molecules for Disease X (table 1 ). Technological opportunities speed up discovery and translation of new products. Process opportunities facilitate early alignment and more effective collaborations. These opportunities cover both near-term (ie, immediately) and medium-term (ie, over 5–10 years), and are intended to be unaffected by the knowledge of the specific identity of the pathogen.
Table 1.
Overview of challenges and opportunities related to the development of diagnostics, vaccines, and therapeutics during an epidemic
| Opportunities | |
|---|---|
| Diagnostic development | |
| Lengthy regulatory process to analytically validate a new diagnostic assay | Expand the use of prequalified platforms: prequalification of diagnostic platforms (including the instruments and their associated reagents) before outbreaks means that only pathogen-specific components will need to be qualified during an epidemic. This prequalification is an opportunity for regulators to fast track the approval of pathogen-specific assays for emergency use. |
| Insufficient global manufacturing capacity | Accelerate opportunities for platform diagnostic companies to expand their footprint within low-income and middle-income countries by establishing infrastructure (instruments, consumables, human resources) for routine clinical use so that it is ready in case of an epidemic, with trained staff and accessible support channels. |
| Vaccine development | |
| Unknown biology of Pathogen X hinders identification of an appropriate immunisation target | Expand databases of conserved pathogen sequences and targets to more quickly triage and identify homologies among pathogens. The database can be expanded by proactively researching new infectious agents in animals and humans. Develop broad-spectrum and narrow-spectrum antivirals and homologous targets, as identified in multiple pathogens. |
| Insufficient available and standardised animal models | Standardise and validate animal models of disease transmission and pathogenesis that are sufficient for research and able to satisfy requirements for licensure (either through traditional, emergency use, or Animal Rule pathways). This process requires considerable investment in basic research and would probably run in parallel to clinical development if an emergency demands it. Expand curated databases of existing animal models and data to inform members of scientific advisory boards who are empowered to make decisions pertaining to standards for animal-use protocols and interpretation of study results. |
| Insufficient standardised viral stock strains hinder preclinical research and manufacturing processes | Empower a centralised research unit to propagate protocols of the International Organization for Standardization for isolating and growing pathogens. Use innovative technologies such as synthetic biology to rapidly synthesise nucleotide sequences or even to predictively stockpile vaccine-ready viral seeds. |
| Clinical trials | |
| Few standardised clinical protocol designs | Standardise and pre-approve clinical trial designs and protocols before outbreaks to reduce their harried development during an epidemic. Establish pathogen-independent and adaptive master protocols of clinical trials that can expand and enrol patients as necessary during an epidemic. Increase accessibility of surveillance and epidemiological data to support clinical development planning and study design (eg, incidence and clinical characterisation of disease in symptomatic populations). Ensure that master clinical trial protocols that are prepositioned at the country level undergo conditional regulatory approval, and thus only require modification for modality and indication. Protocol development is a separate activity under the Epidemic Preparedness Research and Development Blueprint.22, 23 |
| Loosely coordinated clinical trials, particularly when capability of good clinical practice is insufficient | Pre-select clinical sites and expand infrastructure and expertise within high-risk areas by running trials in a non-epidemic context to establish a prepared infrastructure and capabilities.24, 25 Expand emergency operations centres. |
| Small molecules | |
| Toxicology studies remain lengthy for small molecules, effectively hindering the de novo development of drugs to control epidemics | Continue to repurpose small molecule agents that have already undergone the required safety and toxicity studies. This process requires establishing intellectual property sharing agreements and compounding libraries of drugs that have already completed safety studies but were not commercially pursued. Pre-screen these compounds during an epidemic to identify signals of efficacy and then fast track them into clinical development. |
Technological opportunities for research and development acceleration
Access to convalescent serum samples is important to establish whether neutralising antibodies are present and to develop potential antigens for vaccine development. The first broad category of enabling technologies include rapid antibody development platforms for therapeutic or prophylaxis candidates and assay reagents.18 Presently, the time taken to translate manufactured antibodies from discovery through to the clinic remains a hurdle to the broad applicability of this therapeutic. Improving speed or decreasing cost of goods can enable these platforms to be routinely used in the context of epidemic response.
Expanding prequalified and validated rapid response vaccine delivery platform technologies that are approved for human use could revolutionise development times for vaccines.19, 20 A nearer-term objective would be to license a platform for a known pathogen with commercial potential. Such licensing could probably increase the regulators' confidence that the platform can be successfully applied to prevent disease from a new pathogen. Such platforms include plug-and-play recombinant vectors3 and nucleic acid vaccines,21 which require swapping gene cassettes for an antigen of choice (table 1). These platforms promise to accelerate progression into clinical development by using existing (even if partial) toxicity data, bridging studies, and optimised manufacturing plans. Establishing the efficacy of each candidate within the platforms remains the most serious challenge, which can currently only be addressed through empirical testing. The Coalition for Epidemic Preparedness Innovation is funding development of several RNA-based and DNA-based platforms to address this need.26, 27, 28
Another strategic opportunity to accelerate development of effective therapeutics across modalities is through development of broad and narrow spectrum therapeutics against priority pathogen families (table 1). Broad spectrum drugs (eg, immune modulating compounds) are inherently more useful, but subject to considerable technical risk as they advance in development. Narrow spectrum drugs are more achievable in the near term.
Similar technological opportunities are also available for diagnostic products, including expanding open plug-and-play platforms that can be multiplexed.29 Ideally, these systems would be designed with prequalified reagents that are readily available for assay development. These systems require minimal customisation and can be quickly validated to develop and manufacture point-of-care diagnostics that are easy to use. Prevalidated components can also enable stockpiling of common reagents, which reduces the burden to coordinate the supply chain and distribution of multiple assay components during an emergency.
Although diagnostic assays during an epidemic are most likely to be based on nucleic acids, it will be important to co-develop antibody tests. Lateral flow assays are often inexpensive and easy to distribute and administer, which can be important for point-of-care use in remote areas.29 Additionally, the evolution of more complex assays that can discriminate between the immune response to active infections or past exposure will also be important. These antibody-dependent tests can benefit from antibody discovery platforms that identify candidate reagents necessary for diagnostic platforms.
Manufacturing of medical countermeasures remains a highly specialised pursuit, for which biotechnology companies and large pharmaceutical corporations have core competencies. Cell-based manufacturing requires the use of complex optimisation combined with time-consuming iterative regulatory approval. Expanding an open access library of approved substrates could reduce the time required to develop stable cell lines and achieve better yield, capacity, and costs. Furthermore, developing universal cell substrates through genetic engineering that can serve as hosts for all viruses could simplify and accelerate this process. It is still probable that most manufacturing substrates will require time-consuming optimisation; the complexity of these processes can be addressed with modular manufacturing approaches30 and manufacturing optimisation platforms such as microreactors.
Process opportunities
The development of small molecule therapeutics poses specific issues related to off-target toxicities and meeting the existing safety specifications. A way to overcome this challenge is to establish libraries of compounds that can be drawn on in emergency situations: these libraries might contain all known and approved compounds (including those out of production), or candidates with some toxicology data that might have not been efficacious for their original indication.31, 32 These compounds can progress faster through the development pipeline than a de novo lead. Several pharmaceutical and biotechnology companies have large compound libraries that could be indexed and used for these efforts.
Vaccine adjuvants are crucial for improving vaccine efficacy. Few types of adjuvants are currently approved for use in licensed vaccines. Establishing a library and stockpiles of prequalified adjuvants with reciprocal access agreements would allow for the rapid screening and identification of enhanced vaccine formulations. These libraries could also be expanded to include adjuvants that are used in animal vaccines, which have the added benefit of already having de facto animal safety data.
To facilitate efficient clinical trial design during an epidemic, it will be important to establish effective and empowered governance to oversee and guide decisions of clinical development plans. This governance would facilitate rapid decision making for clinical development (including protocol design, endpoint selection, and site selection). It could take the form of a designated pathogen-specific governance body, like a scientific advisory board, and optimise numerous funding and coordination workstreams created by WHO.33, 34, 35
Clinical development could further be aided through instituting adaptive clinical trials using simplified and standardised protocols, which are overseen by a data safety monitoring board to centralise safety and efficacy review (table 1). Adaptive trial approaches allow for the collapsing of study arms following interim analyses. Study acceleration and improved statistical power for the resulting study arms can result. Some of these approaches can also allow for meaningful data generation during fast-burning or sporadic outbreaks that otherwise would be too difficult to incorporate into standard trial designs.
Improving standards for assays across different laboratories and countries for parameters such as sensitivity and specificity as well as for data reporting and management systems can also facilitate the development of diagnostic products. This improvement will address the variability of standards and test methods across different diagnostic laboratories; additionally, it will facilitate and synchronise information sharing and conformance of data between countries, developers, and national and global cloud-based registries. Finally, expanding regional emergency reference laboratory consortia (especially in high-risk regions) and a base level of laboratory expertise (in all countries) can accelerate the solution of logistical and quality issues, and the overall mobilisation and delivery of diagnostics.
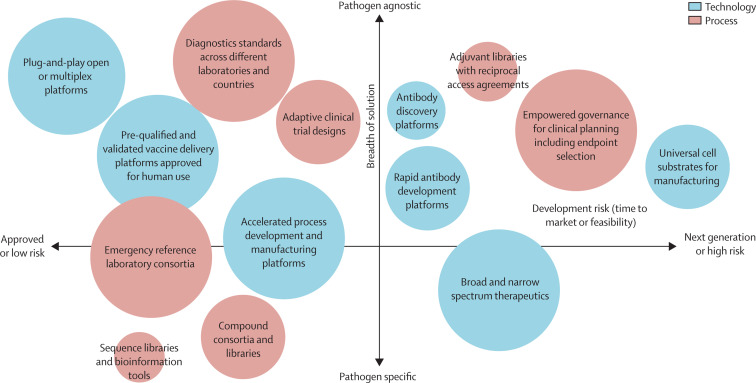
As a myriad of process and technological opportunities exists, we asked subject matter experts to prioritise investment in various opportunities on the basis of the breadth of applicability and market readiness. For the global health community to prioritise investment, we propose that the high-impact, pathogen-agnostic, and low-risk opportunities should be considered as priorities for near-term initiatives, and that pathogen-agnostic and high-risk opportunities should be considered as priorities for the medium term (figure ).
Figure.
Opportunities for accelerating the development of diagnostics, biologics, and small molecule therapeutics for Pathogen X—evaluation of risk and breadth
Large circles indicate a high effect, medium-sized circles a medium effect, and small circles a low effect.
End-to-end product development
Effective decisions drive product development. Although these decisions are arrayed along product development timelines, they should be evaluated and decided holistically, not sequentially. We suggest that the first step after the identification of Disease X is the development of a global intervention target product profile (iTPP). The iTPP should be created by funders or oversight bodies, or both, with input from disease and product development experts, to provide clear guidance to medical countermeasure manufacturers with respect to what product attributes are needed to address a novel pathogen. Each key variable (eg, efficacy, stability, safety, storage conditions) will have minimum and optimistic targets. The minimum target sets the absolute lower bound of acceptable product attributes, whereas optimistic targets can be aspirational or provide guidance about specific attributes that might be problematic (eg, cost of goods or efficacy). Product developers can then create integrated product development plans that outline the complete scope of manufacturing as well as clinical and regulatory activities required to develop their product. It should be noted that a single iTPP could lead to multiple product candidates under development. Ideally, each of the products would be differentiated with respect to meeting various iTPP parameters. Use of these documents at the start of an epidemic can increase transparency between stakeholder organisations, improve communication, align interests, and focus resources on a common set of priorities. We provide a sample iTPP template for a vaccine targeting a novel pathogen in table 2 .
Table 2.
Sample intervention target product profile for vaccines that target Pathogen X
| Minimum product attributes: the minimum attributes are used as a potential go or no-go decision point for continuing development. | Optimistic product attributes: the optimistic attributes should reflect what will achieve an ideal global health impact. | |
|---|---|---|
| Indication | Describe specific details related to the putative indication that would be listed on the product label (eg, prevention of disease vs reduction in disease severity or duration). | Consider how the product could be modified to increase impact. For example, is cure or prevention possible, as opposed to reduction of disease severity? |
| Target population | Which age groups are the target population? Could it include infants, adolescents, and adults? Explore the potential of expanding the target population into subpopulations, such as first responders or medical professionals. | Special populations (including pregnant or older individuals) are often listed as post-licensure commitments. |
| Efficacy or immunogenicity | Use numerical values whenever possible, ideally capturing seroconversion or seroprotection endpoints. For example, achieve protective antibody titres in more than 80% of recipients. | Specify the degree of improvement relative to the minimum requirements. Push for breakthroughs in efficacy. |
| Duration of protection | Duration should be sufficient to protect the population at risk for the period of the initial wave of the pandemic. | Consider whether it is possible to ensure extended protection or longer intervals between boosting (for vaccines). |
| Onset of Immunity (specific to vaccines) | Typically use 2–4 weeks for most vaccines. | Can onset of immunity be shortened by using novel adjuvants? |
| Safety | Acceptable risk or benefit profile will depend on the case fatality rate and long-term health effect of the new pathogen. | Consider what can be done to improve the toxicity profile of the compound when the risk-benefit profile is appropriate. |
| Presentation | Single-dose versus multi-dose vials is the typical listing. | Identify other presentations of interest. Examples are prefilled syringes or special delivery systems (such as electroporation devices). |
| Dosing schedule and route of administration | Will one dose or two doses be required to achieve efficacy? If more than one dose is required, specify the intervals between the doses. Consider whether administration will be parenteral, subcutaneous, intramuscular, or intravenous; other potential routes are oral sublingual, intranasal, mucosal, and skin using microneedles. | Consider how to make the candidate more suitable for use in the target population at the required scale. For example, a one-dose regimen is preferable to a two-dose regimen. |
| Stability or shelf life | Specify the storage temperature that is needed (eg, refrigerated vs frozen). If frozen, indicate the specific temperature needed for stability (eg, −20°C ranging to −70°C). | Consider how to remove cold-chain requirements or make the product suitable for a wider range of temperatures. For example, refrigerated products might be preferable to frozen products. Point-of-care diagnostics that have been validated for use for up to 40°C might be preferable for tropical climates. |
| Product registration path | Identify scientific assessment or registration plan and targets. For example, find a traditional biologics license application via the US Food and Drug Administration. | Given a satisfactory risk-benefit profile, consider accelerated pathways, such as the Animal Rule, the emergency use assessment and listing procedure, or conditional market authorisation. |
| Other | Potentially include cost of goods sold, target countries and delivery channels, and other attributes specific to the pathogen. | Consider how to drive innovation by setting aggressive targets, potentially using novel or easy-to-scale technologies. |
We additionally provide a catalogue of these decisions (table 3 ) along with key considerations for each of the decisions. Together with an iTPP, these decisions can guide medical countermeasure product development. During a pandemic, rapid and effective decision making is of upmost importance. However, decisions made early in the medical countermeasure development process can result in downstream ramifications that can slow down development or even require restarting at an earlier development stage. By laying out the decisions and choices up front, unintended consequences can become evident earlier and medical countermeasure developers can potentially avoid non-productive pathways. Although this example is not exhaustive, it represents a sample of some of the essential decisions that are related to an instance of medical countermeasure development.
Table 3.
Overview of key decision points in medical countermeasure development
| Description | Key considerations | |
|---|---|---|
| Response tools | Select a combination of diagnostics, vaccines, and therapeutic products on the basis of the epidemic response and control strategy | Epidemic or pandemic potential of the pathogen (eg, virulence, latency, pathophysiology); technical feasibility (eg, similarity to known pathogen, antigenic diversity, biomarkers); availability of other public health or epidemic control measures (eg, vector control, social distancing) |
| Product strategy | Generate a product-specific target product profile to define essential product performance specifications | Indication for use (eg, pre-exposure or post-exposure prophylaxis, suppressive therapy); vaccination strategy and target population (eg, herd vs ring vaccination, use in paediatric populations or pregnant women, geographical considerations); durability of protection, product formulation stability and storage, route of administration, production, co-administration or combination therapy |
| Target selection | Identify and prioritise potential targets for vaccine and drug candidate development that meet the target product profile | Understanding of the natural history, biology, pathogenesis, and genetics of the pathogen (eg, viral life cycle, entry mechanisms, hosts, genomic sequence); mechanism of action or immunological response, and product development biomarkers |
| Animal model | Select and develop well-defined and standardised animal models that recapitulate the pathogenesis of human disease to do efficacy studies | Correlation of animal and human response, ease of use, animal rule requirements; availability, standardisation, and validation of reagents and related assays; toxicology studies or data required for drug repurposing |
| Platform selection | Vaccine type (eg, attenuated and recombinant protein with or without adjuvants), therapeutics (eg, small molecules, antibodies), diagnostics (eg, lateral flow, nucleic acid technology) | Safety profile, speed of development, complexity, ability to culture pathogen, immunological response (eg, humoral vs cellular, duration); availability of verification specimens |
| Manufacturing | Select suitable platform for manufacture of quality product at required scale | Biocontainment requirements, scalability, available capacity, formulation, dose selection, regulatory requirements for release assay validation, and qualification or validation of good manufacturing practices |
| Clinical development plan | Define clinical trial design (geography, sites, sample size, control groups) including clinical endpoints (survival vs disease prevention) | Expertise of clinical trial sites, capacity, disease incidence or epidemiology studies; established infrastructure (including patient recruitment and enrolment, data collection and management); coordination between operational (ie, outbreak response) and research groups |
| Manufacturing partner | Identify qualified partners with the required manufacturing capabilities of good manufacturing practices and the capacity to meet product specifications | Technology transfer plan, requisite infrastructure (capital equipment, talent, vendor support), access to raw materials, fill-finish capability |
| Delivery | Define product access and delivery methods across the supply chain | Supply chain requirements (cold chain or thermostability); in-country operations, transfer and import or export agreements; means of dissemination (eg, fixed posts vs house-to-house campaigns) |
Additional considerations
Most medical products nowadays are global commodities with complex development and manufacturing pathways that often involve multiple jurisdictions. Depending on the epidemic situation, the benefit-to-risk ratio, and the regulatory pathway pursued, innovators might need to manufacture at risk without any assurances of return on their investment. These realities present challenges that can act as deterrents for innovators to enter the product development space in epidemic response. These challenges are particularly acute when the target population is located in an area with an unfamiliar regulatory landscape, or when the target population does not have the expertise to routinely handle new technologies and approaches, and most importantly, when the economic return on investment—if existing—is expected to be minimal. To resolve this issue, product developers should engage early with regulators in areas where medical countermeasures are manufactured and with regulators in target countries, as well as liaise with WHO programme and prequalification units. Together, they should align on appropriate non-clinical models and study designs, and deploy assistance in coordinating late-stage trials. This approach can clarify clinical trial endpoints that would be supportive for accelerated regulatory approval. Alternatively, several accelerated pathways exist that derive human efficacy data from animal surrogates or intermediate clinical endpoints, and provide considerable scientific and agency support.36, 37, 38, 39 Although chemistry, manufacturing, and control (CMC) activities and regulatory considerations are not within the scope of this Personal View, the working group recognises the need for alignment in these areas and intends to disseminate the output of their conversations in these areas imminently. Subsequent publications will aim to provide a detailed analysis of the key CMC activities and investments that will assist in reducing the time to manufacture licensed products for infectious diseases, and aim to improve coordination and understanding of the frameworks that would facilitate the use of accelerated regulatory pathways, including the use of animal surrogates for human efficacy and intermediate endpoints or biomarkers.
Conclusion
Our intent for this Personal View is to provide a high-level overview of opportunities that can shorten the time to development of medical countermeasures, discussing technological, process, planning, and coordination activities that could be beneficial. We have taken a somewhat unorthodox approach: although we believe that Pathogen X is likely to be a highly virulent RNA virus, we discuss only the aspects of product development that are largely conserved across pathogens. We believe that these high-impact, pathogen-agnostic, and low-risk opportunities enable the best near-term preparedness for Disease X. We also advocate for the use of iTPPs that outline the full characteristics required for developing de novo medical countermeasures. We described an array of variables that could be considered up front: early consideration might avoid unintended delays and unproductive avenues. This content can be used by global academic and product development partners to accelerate the provision of medical countermeasures during an outbreak.
This online publication has been corrected. The corrected version first appeared at thelancet.com/infection on October 16, 2020
Acknowledgments
Acknowledgments
We thank the subject matter experts and members of the Disease X Working Group who gave their time and extensive experience towards this endeavour (listed in appendix).
Contributors
All authors contributed equally to the conceptualisation and execution of the Personal View.
Declaration of interests
SS and AC are employees of the Gates Foundation at the time of writing; however, this Personal View does not necessarily represent the views of the Gates Foundation. MCK and VVG are employees of PricewaterhouseCoopers at the time of writing; however, this Personal View does not necessarily represent the views of PricewaterhouseCoopers. We declare no competing interests.
Supplementary Material
References
- 1.Kaner J, Schaack S. Understanding Ebola: the 2014 epidemic. Global Health. 2016;12:53. doi: 10.1186/s12992-016-0194-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones KE, Patel NG, Levy MA. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rauch S, Jasny E, Schmidt KE, Petsch B. New vaccine technologies to combat outbreak situations. Front Immunol. 2018;9:1963. doi: 10.3389/fimmu.2018.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johns Hopkins Bloomberg School of Public Health The characteristics of pandemic pathogens. http://www.centerforhealthsecurity.org/our-work/pubs_archive/pubs-pdfs/2018/180510-pandemic-pathogens-report.pdf
- 5.Woolhouse ME. Adair K, Brierley L. RNA viruses: a case study of the biology of emerging infectious diseases. Microbiol Spectr. 2013;1 doi: 10.1128/microbiolspec.OH-0001-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kreuder Johnson C, Hitchens PL, Smiley Evans T. Spillover and pandemic properties of zoonotic viruses with high host plasticity. Sci Rep. 2015;5:14830. doi: 10.1038/srep14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morse SS, Mazet JA, Woolhouse M. Prediction and prevention of the next pandemic zoonosis. Lancet. 2012;380:1956–1965. doi: 10.1016/S0140-6736(12)61684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salyer SJ, Silver R, Simone K, Barton Behravesh C. Prioritizing zoonoses for global health capacity building—themes from One Health Zoonotic Disease Workshops in 7 countries, 2014–2016. Emerg Infect Dis. 2017;23 doi: 10.3201/eid2313.170418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly TR, Karesh WB, Johnson CK. One Health proof of concept: bringing a transdisciplinary approach to surveillance for zoonotic viruses at the human-wild animal interface. Prev Vet Med. 2017;137(Pt B):112–118. doi: 10.1016/j.prevetmed.2016.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwind JS, Goldstein T, Thomas K, Mazet JA, Smith WA. Capacity building efforts and perceptions for wildlife surveillance to detect zoonotic pathogens: comparing stakeholder perspectives. BMC Public Health. 2014;14:684. doi: 10.1186/1471-2458-14-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woolhouse ME, Howey R, Gaunt E, Reilly L, Chase-Topping M, Savill N. Temporal trends in the discovery of human viruses. Proc Biol Sci. 2008;275:2111–2115. doi: 10.1098/rspb.2008.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fidler DP. Negotiating equitable access to influenza vaccines: global health diplomacy and the controversies surrounding avian influenza H5N1 and pandemic influenza H1N1. PLoS Med. 2010;7:e1000247. doi: 10.1371/journal.pmed.1000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Secretariat of the Convention on Biological Diversity Montreal Nagoya protocol on access to genetic resources and the fair and equitable sharing of benefits arising from their utilization to the convention on biological diversity. 2014. https://www.cbd.int/abs/doc/protocol/nagoya-protocol-en.pdf
- 14.Knauf S, Abel L, Hallmaier-Wacker LK. The Nagoya protocol and research on emerging infectious diseases. Bull World Health Organ. 2019;97:379. doi: 10.2471/BLT.19.232173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO WHO R&D Blueprint meeting on pathogen genetic sequence data (GSD) sharing in the context of public health emergencies. https://www.who.int/blueprint/meetings-events/meeting-report-pathogen-genetic-sequence-data-sharing.pdf
- 16.WHO Policy statement on data sharing by the World Health Organization in the context of public health emergencies. 2016. https://www.who.int/ihr/procedures/SPG_data_sharing.pdf
- 17.Eurosurveillance editorial team Agreement on a pandemic influenza preparedness framework for the sharing of viruses and benefit sharing. Euro Surveill. 2011;16 [PubMed] [Google Scholar]
- 18.Graham BS, Mascola JR, Fauci AS. Novel vaccine technologies: essential components of an adequate response to emerging viral diseases. JAMA. 2018;319:1431–1432. doi: 10.1001/jama.2018.0345. [DOI] [PubMed] [Google Scholar]
- 19.Johns Hopkins Center for Health Security Vaccine platforms: state of the field and looming challenges. http://www.centerforhealthsecurity.org/our-work/pubs_archive/pubs-pdfs/2019/190423-OPP-platform-report.pdf
- 20.WHO Evaluation of ideas for potential platforms to support development and production of health technologies for priority infectious diseases with epidemic potential. 2016. https://www.who.int/medicines/ebola-treatment/R-D-Blueprint_Evaluation-of-platform-technologies-for-priority-patho.pdf?ua=1
- 21.Maruggi G, Zhang C, Li J, Ulmer JB, Yu D. mRNA as a transformative technology for vaccine development to control infectious diseases. Mol Ther. 2019;27:757–772. doi: 10.1016/j.ymthe.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO InterVax Tool: decision support for vaccine efficacy trial design during an emerging outbreak. http://vaxeval.com/
- 23.Genetic Engineering & Biotechnology News A small-footprint, integrated, and automated platform for viral production. https://www.genengnews.com/resources/tutorial/a-small-footprint-integrated-and-automated-platform-for-viral-production/
- 24.WHO Good participatory practice guidelines for trials of emerging (and re-emerging) pathogens that are likely to cause severe outbreaks in the near future and for which few or no medical countermeasures exist (GPP-EP) https://www.who.int/blueprint/what/norms-standards/GPP-EPP-December2016.pdf?ua=1
- 25.WHO Key actions for good participatory practices in trials of emerging (and re-emerging) pathogens—GPP-EP. https://www.who.int/docs/default-source/blue-print/key-actions-gpp-ep-20161207.pdf?sfvrsn=a34efbe7_2
- 26.Thess A, Grund S, Mui BL. Sequence-engineered mRNA without chemical nucleoside modifications enables an effective protein therapy in large animals. Mol Ther. 2015;23:1456–1464. doi: 10.1038/mt.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coalition for Epidemic Preparedness Innovations CEPI launches new call for innovative platform technologies to rapidly respond to Disease X. 2019. https://cepi.net/news_cepi/cepi-launches-new-call-for-innovative-platform-technologies-to-rapidly-respond-to-disease-x/
- 28.Coalition for Epidemic Preparedness Innovations Our platform technology. https://cepi.staging.tegu.hexdigital.com/research_dev/technology/
- 29.Nayak S, Blumenfeld NR, Laksanasopin T, Sia SK. Point-of-care diagnostics: recent developments in a connected age. Anal Chem. 2017;89:102–123. doi: 10.1021/acs.analchem.6b04630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WHO Design of vaccine efficacy trials to be used during public health emergencies—points of considerations and key principles. http://www10.who.int/blueprint/what/norms-standards/AP1_guidelines_Online_Consultation.pdf
- 31.Kim YJ, Cubitt B, Chen E. The ReFRAME library as a comprehensive drug repurposing library to identify mammarenavirus inhibitors. Antiviral Res. 2019;169:104558. doi: 10.1016/j.antiviral.2019.104558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su Lab Reframedb. https://reframedb.org/
- 33.WHO Global Coordination Mechanism. 2017. http://www.who.int/blueprint/what/improving-coordination/global_coordination_mechanism/en/
- 34.WHO An R&D blueprint for action to prevent epidemics. 2016. https://www.who.int/blueprint/what/improving-coordination/workstream_5_document_on_financing.pdf
- 35.WHO Financing of R&D preparedness and response to epidemic emergencies 2015. https://apps.who.int/medicinedocs/documents/s22474en/s22474en.pdf
- 36.Allio T. Product development under FDA's animal rule: understanding FDA's expectations and potential implications for traditional development programs. Ther Innov Regul Sci. 2016;50:660–670. doi: 10.1177/2168479016641717. [DOI] [PubMed] [Google Scholar]
- 37.US Food and Drug Administration Emergency use authorization of medical products and related authorities—guidance for industry and other stakeholders. 2017. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/emergency-use-authorization-medical-products-and-related-authorities
- 38.WHO Emergency Use Assessment and Listing Procedure (EUAL) for candidate vaccines for use in the context of a public health emergency. https://www.who.int/medicines/news/EUAL-vaccines_7July2015_MS.pdf
- 39.The European Medicines Agency Support for early access. https://www.ema.europa.eu/en/human-regulatory/overview/support-early-access
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.