Abstract
Noninvasive tools for the prognosis of α-fetoprotein negative hepatocellular carcinoma (HCC) are urgently needed. The present study proposed a prognostic system based on preoperative plasma prothrombin time and fibrinogen (PT/Fbg system). With respect to α-fetoprotein (AFP)-negative HCC, we compared the prognostic value in PT/Fbg system, Glasgow Prognostic Score, and aminotransferase/aspartate aminotransferase ratio. The present study retrospectively analyzed patient characteristics, clinicopathological factors, and the level of pretreatment biomarkers in 628 patients with HCC. Patients with increased PT and Fbg levels were allocated a score of 2, patients with only one of these abnormalities were assigned score 1, and patients with neither of these abnormalities were allocated a score of 0. The following distributions of the PT/Fbg system scores were observed: 187 (29.78%) patients had a score of 0, 305 (30.65%) had a score of 1, and 134 (22.69%) patients had a preoperative score of 2. The prognostic significance of the PT/Fbg system was determined using univariate and multivariate Cox hazard analyses in AFP-negative HCC. Multivariate analysis revealed that patients with a higher PT/Fbg system exhibited worse overall survival (OS) than patients with a lower PT/Fbg system. Our study proposes preoperative evaluation of the plasma PT/Fbg system to predict the OS of patients with AFP-negative HCC.
Keywords: hepatocellular carcinoma, AFP-negative, prothrombin time, fibrinogen, prognosis
Introduction
Liver resection and transplantation are effective approaches for the treatment of hepatocellular carcinoma (HCC); however, the 5-year survival rate after curative resection remains low, at 54.1% to 61.5%, and ultimately results in poor overall survival (OS).1 Abdominal ultrasonography and serum α-fetoprotein (AFP) are widely used to detect HCC at an early stage. However, the diagnosis sensitivity of ultrasound is only 60%, and it is highly dependent on operator experience; ultrasound also has a poor ability to differentiate malignant nodules from benign nodules in the small cirrhotic liver.2 Alpha-fetoprotein has been used for the identification of HCC since the 1970s. However, the diagnostic power of AFP is continuously questioned and debated as follows: (1) Only 60% to 70% patients with HCC exhibit increased serum AFP3,4; (2) Approximately11% to 47% of patients with liver cirrhosis exhibit nonspecific elevation of serum AFP. Other traditional tumor markers, such as CEA and CA199, are used to screen and evaluate HCC, and these tumor markers also exhibit a low sensitivity in the detection of HCC. Therefore, the search for reliable and efficient serum biomarkers for the prognostic evaluation of HCC, especially in AFP-negative HCC (AFP levels <20 ng/mL), remains an urgent open task.
Several factors, including pathological stage, Glasgow Prognostic Score (GPS), and serum biomarkers, are used as independent predictors of survival in patients with a variety of common solid tumor. However, the prognostic value of these factors is not as good as expected. First, pathological stage (TNM stage), without consideration of the biological variability of the tumor itself, results in different clinical outcomes even within the same stage using similar treatment strategies. Second, GPS is based on C-reactive protein (CRP) and albumin levels, and large variations in clinical benefit were found because of the damaged liver function and nutritional deficiencies.5 Third, serum biomarkers, such as liver function tests, partially consist of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), and the ALT/AST ratio (LSR) exhibits a low sensitivity for HCC prognosis and related to liver functional impairment which might be caused by various diseases such as liver fibrosis and cirrhosis. Glasgow Prognostic Score and LSR also do not play a role in the progression of AFP-negative HCC.5,6
An abnormal coagulation system, including prothrombin time (PT), activated partial thromboplastin time, thrombin time, and fibrinogen (Fbg), is implicated in several pathological conditions, including cancer, and may be associated with aggressive tumor growth, progression, and poor survival, which was observed in lung cancer,7 penile cancer,8 and melanoma.9 Our previous study found that plasma coagulation biomarkers were predictive of survival in HCC and esophageal squamous cell carcinoma (ESCC).10,11 Our findings suggested that the combination of plasma PT and Fbg levels (PT/Fbg system) was a valuable predictor of survival in patients with HCC that the impaired coagulation parameters are associated with pathological stage of HCC: patients exhibited longer PT levels in advanced HCC than that in early-stage disease. Furthermore, positive relationship was found between higher Fbg and tumor number, tumor size, node metastasis, and pathological stage in HCC. Based on the previous study, we hypothesized that this coagulation biomarker would be a valuable biomarker in the prognosis of patients with AFP-negative HCC.
We performed a retrospective study to evaluate the prognostic value of the PT/Fbg system, GPS, and LSR and PT/Fbg system is the optimal method of assessing the prognosis of patients with AFP-negative HCC.
Materials and Methods
Ethics Approval and Consent to Participate
This study was approved by the Institute Research Ethics Committee of the Sun Yat-Sen University Cancer Center, Guangzhou, China (approval no. 2017-FXY-129). All patients provided written informed consent prior to enrollment in the study. The raw data underlying this article are available upon request to the corresponding author or the Research Data Deposit public platform (http://www.researchdata.org.cn, with the approval RDD Number as RDDA2018000385).
Patients
A total of 628 patients with AFP-negative HCC from Sun Yat-Sen University Cancer Center were recruited in our retrospective study between April 2008 and January 2015. Patient characteristics, clinicopathological factors, and survival times were extracted from the electronic medical record system, and coagulation biomarkers and HCC-related serum markers were extracted from the laboratory information system. Table 1 displays the retrieved data. Only the first records of hospitalizations were retained, and the levels of all the laboratory markers were tested prior to treatment. All of the patients met the diagnostic criteria for HCC. Patients with other conditions that may alter plasma coagulation levels, such as other tumors, pulmonary embolism, VTE, or disseminated intravascular coagulation, were excluded. Tumor staging was evaluated using the American Joint Committee on Cancer Staging system (AJCC, 2002; Greene) modified TNM staging classification.
Table 1.
Basic Characteristics of Patients With AFP-Negative HCC.
| Characteristics | No. (%) | 5-Year OS (Months) Mean (95% CI) | P Value |
|---|---|---|---|
| Gender (n) | |||
| Male | 561 (89.33%) | 30.3 (28.91-31.69) | .838 |
| Female | 67 (10.66%) | 29.4 (25.20-33.61) | |
| Age (years) | |||
| ≥57 | 333 (53.03%) | 30.43 (28.66-32.19) | .674 |
| <57 | 295 (46.97%) | 29.95 (27.97-31.93) | |
| TNM stage (n) | |||
| I and II | 442 (70.38%) | 33.43 (32.03-34.83) | <.001 |
| III and IV | 186 (29.62%) | 22.53 (19.89-25.18) | |
| T stage | |||
| T1-2 | 458 (72.93%) | 33.30 (31.90-34.70) | <.001 |
| T3-4 | 170 (27.07%) | 21.86 (19.14-24.58) | |
| Node stage | |||
| N0 | 587 (93.47%) | 30.81 (29.47-32.14) | .001 |
| N1-2 | 41 (6.53%) | 21.56 (15.41-27.72) | |
| Distant metastases | |||
| Yes | 29 (4.62%) | 23.31 (15.79-30.83) | .024 |
| No | 599 (95.38%) | 30.54 (29.21-31.87) | |
| Treatment | |||
| Resection | 305 (48.57%) | 33.51 (31.76-35.26) | <.001 |
| Local ablation | 83 (13.21%) | 31.92 (28.78-35.05) | |
| Interventional therapy | 203 (32.32%) | 25.89 (23.47-28.30) | |
| Other | 37 (5.89%) | 22.78 (15.94-29.63) | |
| ECOG | |||
| 0-1 | 613 (97.61%) | 30.27 (29.93-31.62) | .452 |
| 2 | 15 (2.39%) | 27.27 (21.46-33.07) | |
| Alcohol behavior | |||
| Previous/current | 222 (35.35%) | 29.99 (27.82-32.16) | .970 |
| Never | 391 (62.26%) | 30.18 (28.52-31.85) | |
| Family history of cancer | |||
| Yes | 127 (20.22%) | 31.43 (28.44-34.41) | .338 |
| No | 487 (77.55%) | 29.20 (28.42-31.38) | |
| HBs Ag | |||
| Negative | 90 (14.33%) | 22.74 (19.34-26.14) | <.001 |
| Positive | 380 (60.51%) | 31.87 (30.17-33.57) | |
| HBe Ag | |||
| Negative | 413 (65.76%) | 30.01 (28.38-31.64) | .752 |
| Positive | 57 (9.08%) | 30.93 (25.87-35.99) | |
| HBc Ab | |||
| Negative | 42 (6.69%) | 21.33 (16.34-26.33) | .001 |
| Positive | 428 (68.15%) | 30.98 (29.37-32.60) | |
| LSR | |||
| ≥1.065 | 240 (38.22%) | <.001 | |
| <1.065 | 388 (61.78%) | ||
| GPS | |||
| 0 | 438 (69.75%) | <.001 | |
| 1 | 154 (24.52%) | ||
| 2 | 36 (5.73%) | ||
| PT/Fbg system score | |||
| 0 | 187 (29.78) | 34.87 (32.71-37.04) | <.001 |
| 1 | 305 (48.57%) | 30.65 (28.75-32.55) | |
| 2 | 134 (21.34%) | 22.69 (19.87-25.52) |
Abbreviations: AFP, alpha-fetoprotein; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; GPS, Glasgow Prognostic Score; HBs Ag, hepatitis B surface antigen; HBe Ag, hepatitis B e-antigen; HBc Ab, hepatitis B core antibody; HCC, hepatocellular carcinoma; LSR, ALT/AST ratio; OS, overall survival; PT/Fbg, prothrombin time and fibrinogen.
Laboratory Measurements
All blood samples were collected between 7 am and 8 am. Plasma samples were collected into anticoagulation tubes, and serum samples were clotted at room temperature. Both samples were centrifuged at 3500 rpm/min for 10 minutes at room temperature. The Sysmex CA-7000 automatic coagulation analyzer (Sysmex Corporation, Kobe, Japan) was used to measure the levels of coagulation biomarkers. All reagents used in this study were provided by a kinetic nephelometric detection system using a Diagon Dia-Timer 4 (Diagon Ltd, Budapest, Hungary). A Hitachi 7600 automatic biochemical analyzer (Hitachi High Technologies, Tokyo, Japan) was used to measure the levels of CRP, albumin, ALT, and AST, and the reagents were provided by Wako Pure Chemical Industries (Japan). Informed consent was obtained from each patient prior to use of serum and plasma. All patients provided written informed consent. The Institute Research Ethics Committee of the Sun Yat-Sen University Cancer Center, Guangzhou, China, approved this study.
Follow-Up
All patients with HCC were advised to receive regular follow-ups after completion of the primary therapy according to clinical guidelines. Patients were generally followed up every 3 months in the first 2 years and annually thereafter for patients without evidence of recurrence in the following 3 to 5 years. Patients who did not visit our hospital as scheduled were telephoned for follow-ups to obtain the treatment information and living status (performed by The Medical Information Unit in our Cancer Center). The last follow-up occurred in June 2016. The outcome of our study was OS. Overall survival was defined as the time from the diagnosis of HCC to the date of the last follow-up or death. The LSR was calculated as the serum ALT level divided by the serum AST level.
Statistical Analysis
Data sets were analyzed using IBM SPSS software 16.0 (IBM, Chicago, Illinois). A receiver operating characteristic (ROC) curve was used to estimate the optimal cutoff values of laboratory biomarkers. ALT/AST ratio was calculated as the serum ALT level divided by the serum AST level. Glasgow Prognostic Score was estimated using CRP and albumin as follows: GPS 0, patients with a CRP ≤10 mg/L and albumin ≥35 g/L; GPS 1, patients with only higher CRP or lower albumin; GPS 2, patients in whom CRP was >10 mg/L and albumin concentration <35 g/L. Patients with elevated PT and Fbg levels were assigned a score of 2 in the PT/Fbg system, patients with only one of these biochemical abnormalities were assigned a score of 1, and patients without elevated PT and Fbg levels were assigned a score of 2. Univariate and multivariate analyses of clinical variables were performed using Cox proportional hazards regression models. The results of this survey were analyzed using Kaplan-Meier survival curves with the log-rank test and proportional hazard model. Correlation between the PT/Fbg system and clinical characteristics was assessed using χ2 tests. P values <.05 indicated statistically significant differences. All reported P values are 2 sided.
Results
Basic Characteristics of the Study Populations
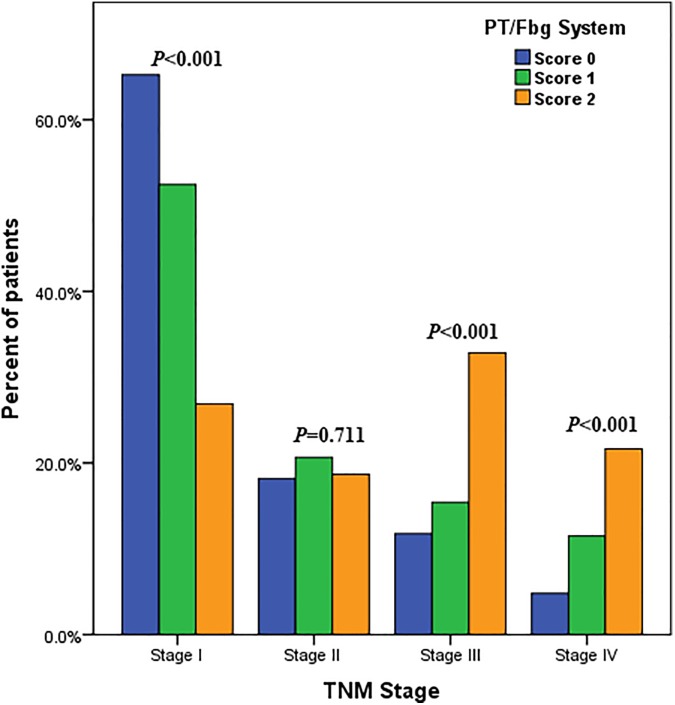
Table 1 shows the basic characteristics of the 628 consecutive AFP-negative HCC cases who were finally included to quantify the potential associations between the PT/Fbg system and AFP-negative HCC. A total of 89.33% patients were males, and 139 patients died of cancer. There were 380 (60.51%), 57 (9.08%), and 428 (68.15%) patients who were hepatitis B surface antigen (HBs Ag) positive, hepatitis B e-antigen (HBe Ag) positive, and hepatitis B core antibody (HBc Ab) positive, respectively. A total of 222 (35.35%) cases reported a history of drinking, and 127 (20.22%) cases had a family history of cancer. Of this, 305 (48.57%) patients received surgical resection, 83 (13.62%) patients received local ablation, 201 (32.01%) patients received interventional therapy, and 37 (5.89%) patients undergone other therapies (including chemoradiotherapy, targeted therapy). The median follow-up period was 31.0 months. TNM classifications of early and advanced stages were observed in 442 (70.38%) and 186 (29.62%) patients, respectively. Greater than 60% patients in TNM stage I had a PT/Fbg system score of 1, and approximately 10% of TNM stage III patients had a PT/Fbg system score of 0 (Figure 1).
Figure 1.
Relationship between preoperative PT/Fbg system score and TNM stage. Patients with early TNM stage had a lower PT/Fbg system score than patients with advanced TNM stage in AFP-negative HCC. Greater than 60% of patients in TNM stage I had a PT/Fbg system score of 0, approximately 20% of TNM stage II patients had a score of 0, approximately 10% of TNM stage III patients had a score of 0, and 5% of TNM stage II patients had a score of 0. AFP indicates alpha-fetoprotein; HCC, hepatocellular carcinoma; PT/Fbg, prothrombin time and fibrinogen.
Distribution of LSR, GPS, and PT/Fbg System Scores
The optimal cutoff point of LST was 34.8, which evaluated using ROC analysis. There were 387 (61.62%) lower LSR patients and 239 (38.06%) higher LSR patients, and the mean OS rates were 32.43 and 26.62 months, respectively. A total of 437 (69.59%) patients were assign a GPS score of 0, 153 (24.36%) patients were assigned score 1, and 46 (7.32%) patients were assigned score 2, and the mean OS rates were 34.19, 22.66, and 13.94 months, respectively. The optimal cutoff points for PT and Fbg were also defined using ROC. Patients without increased PT levels (<11.95 seconds) and Fbg levels (<2.88 g/L) were assigned a PT/Fbg system score of 0, patients with only one of these biochemical abnormalities were assigned a score of 1, and patients with elevated PT levels (≥11.95 seconds) and hypoalbuminemia (≥2.83 g/L) were assigned a score of 2. A total of 187 (29.78%) of these patients had an FA score of 0, 305 (48.57%) patients had an FA score of 1, and 134 (21.34%) patients had a preoperative FA score of 2, and the mean OS rates were 34.87, 30.65, and 22.69 months, respectively (Table 1).
Prognostic Values of LSR, GPS, and PT/Fbg System
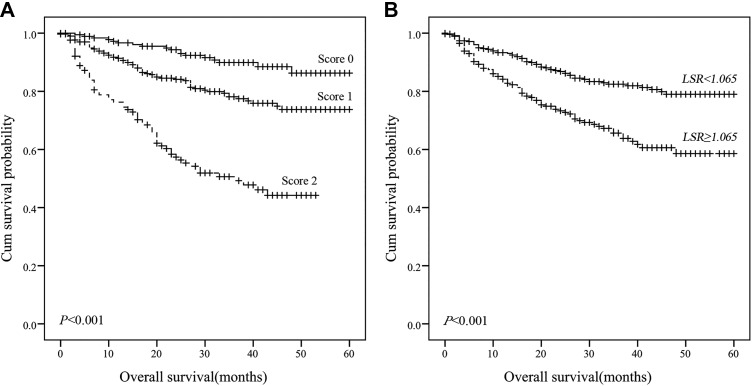
Univariate and multivariate analyses identified specific prognostic indexes associated with AFP-negative HCC. Table 2 shows that TNM stage (P < .001), T stage (P < .001), node stage (P < .001), distant metastases (P < .001), treatment (P < .001), HBs Ag (P < .001), HBc Ab (P = .001), LSR (P < .001), GPS (P < .001), and PT/Fbg system score (P < .001) were significantly associated with OS. Multivariate analysis demonstrated that patients with a higher PT/Fbg system score had worse OS than patients with lower PT/Fbg system scores (HR = 1.899; 95% confidence interval [CI]: 1.334-2.705; P < .001), and patients with LSR ≥34.8 had worse OS than patients with an LSR <34.8 (HR: 1.677; 95% CI: 1.141-2.465; P = .008). The survival curves of patients with AFP-negative HCC were constructed using the Kaplan-Meier method and compared using the log-rank test. ALT/AST ratio and PT/Fbg system scores were closely associated with OS that high LSR level and higher PT/Fbg system associated with shorter OS (P < .001, P < .01, respectively; Figure 2).
Table 2.
Univariate and Multivariate Analyses: Clinicopathological Factors, PT/Fbg System Score, and Overall Survival.
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Gender | ||||||
| Male vs female | 1.117 | 0.664-1.881 | .677 | |||
| Age (years) | ||||||
| <57 vs ≥57 | 1.320 | 0.940-1.855 | .109 | |||
| TNM stage | ||||||
| I-II vs III-IV | 5.044 | 3.592-7.083 | <.001 | 2.754 | 1.836-4.131 | <.001 |
| T stage | ||||||
| T1-2 vs T3-4 | 4.611 | 3.298-6.445 | <.001 | |||
| Node stage | ||||||
| N0 vs N1-2 | 4.102 | 2.618-6.424 | <.001 | |||
| Distant metastases | ||||||
| Yes vs No | 3.300 | 1.930-5.641 | <.001 | |||
| Treatment | ||||||
| Resection vs local ablation vs Interventional therapy vs other | 1.670 | 1.422-1.961 | <.001 | 1.299 | 1.068-1.579 | .009 |
| ECOG | ||||||
| 0-1 vs 2 | 0.300 | 0.042-2.149 | .231 | |||
| Alcohol behavior | ||||||
| Yes vs no | 0.948 | 0.668-1.347 | 0.767 | |||
| Family history of cancer | ||||||
| Yes vs no | 0.668 | 0.420-1.063 | .089 | |||
| HBs Ag | ||||||
| Negative vs positive | 0.444 | 0.294-0.670 | <.001 | 0.563 | 0.329-0.963 | .036 |
| HBe Ag | ||||||
| Negative vs positive | 0.886 | 0.486-1.612 | .691 | |||
| HBc Ab | ||||||
| Negative vs positive | 0.399 | 0.238-0.670 | .001 | 0.707 | 0.361-1.387 | .313 |
| LSR | ||||||
| ≥1.065 vs <1.065 | 2.222 | 1.591-3.103 | <.001 | 1.677 | 1.141-2.465 | .008 |
| GPS | ||||||
| Score 0 vs 1 vs 2 | 2.991 | 2.377-3.764 | <.001 | 1.390 | 0.991-1.950 | .057 |
| PT/Fbg system | ||||||
| Score 0 vs 1 vs 2 | 2.671 | 2.083-3.426 | <.001 | 1.899 | 1.334-2.705 | <.001 |
Abbreviations: CI, confidence interval; GPS, Glasgow Prognostic Score; HBs Ag, hepatitis B surface antigen; HBe Ag, hepatitis B e-antigen; HBc Ab, hepatitis B core antibody; LSR, ALT/AST ratio; PT/Fbg, prothrombin time and fibrinogen.
Figure 2.
Kaplan-Meier survival curves of 5-year overall survival in patients with AFP-negative HCC. A, Patients with lower PT/Fbg system scores exhibited better OS (P < .001); (B) Patients with lower LSR exhibited better OS (P < .001). AFP indicates alpha-fetoprotein; HCC, hepatocellular carcinoma; LSR, ALT/AST ratio; OS, overall survival; PT/Fbg, prothrombin time and fibrinogen.
Relationship Between PT/Fbg System Score and Clinicopathological Characteristics
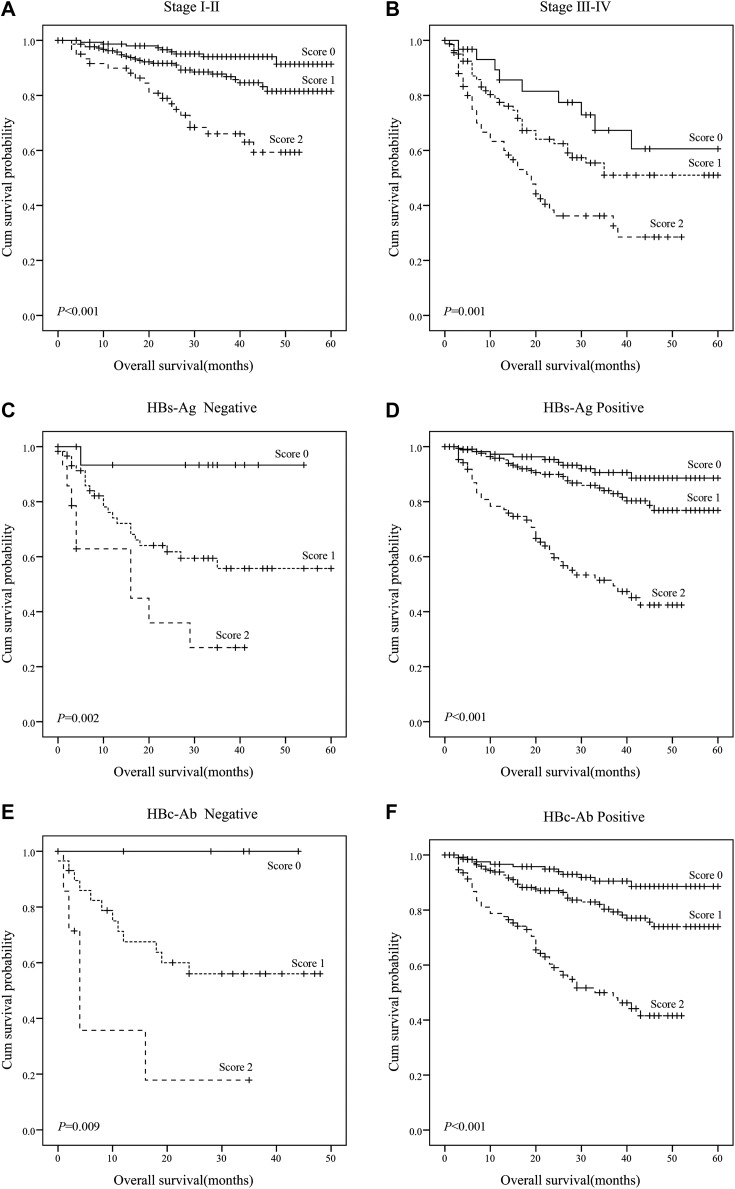
Tables 3 and 4 present the associations between PT/Fbg system score and clinicopathological variables in patients with AFP-negative HCC. The PT/Fbg system score was associated with TNM stage (P < .001), T stage (P < .001), node stage (P < .001), HBs Ag (P < .001), HBc Ab (P < .001), and treatment (P < .001). We analyzed the prognostic effect of the PT/Fbg system score in subgroups based on TNM stage, HBs Ag, and HBc Ab to further examine the relationship between PT/Fbg system and survival. Patients with a higher PT/Fbg system score exhibited significantly shorter OS than patients with a lower PT/Fbg system score in stage I-II (P < .001) and stage III-IV (P = .001) subgroups, HBs Ag negative (P = .002) and HBs Ag positive (P < .001) subgroups, and HBc Ab negative (P = .009) and HBc Ab positive (P < .001) subgroups (Figure 3).
Table 3.
Relationship Between Clinicopathological Factors and PT/Fbg System Score.
| Variables | PT/Fbg System Score, No. (%) | |||
|---|---|---|---|---|
| Score 0, 187 (29.78) | Score 1, 305 (48.57) | Score 2, 134 (21.44) | P Value | |
| Gender | ||||
| Male | 171 (91.44) | 265 (86.89) | 123 (91.79) | .163 |
| Female | 16 (8.56) | 40 (13.11) | 11 (8.21) | |
| Age (years) | ||||
| <53 | 90 (48.13) | 135 (44.26) | 69 (51.49) | .350 |
| ≥53 | 97 (51.87) | 170 (55.74) | 65 (48.51) | |
| TNM stage | ||||
| I | 122 (65.24) | 159 (52.13) | 36 (26.86) | <.001 |
| II | 34 (18.18) | 64 (20.98) | 25 (18.66) | |
| III | 22 (11.76) | 47 (15.41) | 44 (32.84) | |
| IV | 9 (14.82) | 35 (11.48) | 29 (21.64) | |
| T stage | ||||
| T1-2 | 159 (85.03) | 232 (76.07) | 65 (48.51) | <.001 |
| T3-4 | 28 (14.97) | 73 (23.93) | 69 (51.49) | |
| Node stage | ||||
| N0 | 182 (97.33) | 289 (94.75) | 114 (85.07) | <.001 |
| N1-2 | 5 (2.67) | 16 (5.25) | 20 (14.93) | |
| Distant metastases | ||||
| No | 181 (96.79) | 291 (95.41) | 125 (93.28) | .337 |
| Yes | 6 (3.21) | 14 (4.59) | 9 (6.72) | |
| Treatment | ||||
| Resection | 111 (59.36) | 148 (48.52) | 46 (34.33) | <.001 |
| Local ablation | 26 (13.90) | 49 (16.07) | 8 (5.97) | |
| Interventional therapy | 40 (21.39) | 97 (31.80) | 64 (47.76) | |
| Other | 10 (5.35) | 11 (3.61) | 16 (11.94) | |
| ECOG | ||||
| 0-1 | 182 (97.33) | 296 (97.05) | 133 (99.25) | .364 |
| 2 | 5 (2.67) | 9 (2.95) | 1 (0.75) | |
| Alcohol behavior | ||||
| Never | 112 (59.89) | 196 (64.26) | 81 (60.45) | .570 |
| Previous/current | 69 (36.90) | 102 (33.44) | 51 (38.06) | |
| Family history of cancer | ||||
| No | 139 (74.33) | 243 (79.67) | 103 (76.87) | .569 |
| Yes | 41 (21.93) | 57 (18.69) | 29 (21.64) | |
| HBs Ag | ||||
| Negative | 17 (9.09) | 59 (19.34) | 14 (10.45) | .001 |
| Positive | 113 (60.43) | 173 (56.72) | 93 (69.40) | |
| HBe Ag | ||||
| Negative | 118 (63.10) | 203 (66.55) | 91 (67.91) | .181 |
| Positive | 12 (6.42) | 29 (9.51) | 16 (11.94) | |
| HBc Ab | ||||
| Negative | 124 (66.31) | 203 (66.56) | 100 (74.63) | .025 |
| Positive | 6 (3.21) | 29 (9.51) | 7 (5.22) | |
| LSR | ||||
| ≥1.065 | 51 (27.27) | 123 (40.33) | 65 (48.51) | <.001 |
| <1.065 | 136 (72.73) | 182 (59.67) | 69 (51.49) | |
| GPS | ||||
| 0 | 178 (95.19) | 224 (73.44) | 35 (26.12) | <.001 |
| 1 | 8 (4.28) | 75 (24.59) | 70 (52.24) | |
| 2 | 1 (0.53) | 6 (1.97) | 29 (21.64) | |
Abbreviations: GPS, Glasgow Prognostic Score; HBs Ag, hepatitis B surface antigen; HBe Ag, hepatitis B e-antigen; HBc Ab, hepatitis B core antibody; LSR, ALT/AST ratio; PT/Fbg, prothrombin time and fibrinogen.
Table 4.
Relationship Between Child-Pugh Factors and PT/Fbg System Score.
| Variables | PT/Fbg System Score, No. (%) | |||
|---|---|---|---|---|
| Score 0, 187 (29.78) | Score 1, 305 (48.57) | Score 2, 134 (21.44) | P Value | |
| TBIL (mmol/L) | ||||
| <34.2 | 185 (98.93) | 295 (96.72) | 126 (94.03) | .061 |
| 34.2-51.3 | 2 (1.07) | 3 (0.98) | 3 (2.24) | |
| >51.3 | 0 (0) | 4 (1.31) | 5 (3.73) | |
| ALB (g/L) | ||||
| <28 | 0 (0) | 1 (0.33) | 6 (4.48) | <.001 |
| 28-34 | 2 (1.07) | 15 (4.92) | 28 (20.90) | |
| ≥35 | 185 (98.93) | 286 (93.77) | 100 (74.62) | |
| PT (s) | ||||
| ≤14 | 187 (100) | 284 (93.11) | 116 (86.57) | <.001 |
| 15-17 | 0 (0) | 17 (5.57) | 18 (13.43) | |
| ≥18 | 0 (0) | 1 (0.33) | 0 (0) | |
Abbreviation: PT/Fbg, prothrombin time and fibrinogen; TBIL, total bilirubin; ALB, albumin.
Figure 3.
The prognostic significance of PT/Fbg system scores in AFP-negative HCC. OS was significantly different in all subgroups. A, TNM stage I-II (P < .001); (B) TNM stage III-IV (P = .001); (C) HBs-Ag negative (P = .002); (D) HBs-Ag positive (P < .001); (E) HBC-Ab negative (P = .009); (F) HBC-Ab positive (P < .001). AFP indicates alpha-fetoprotein; HCC, hepatocellular carcinoma; OS, overall survival; PT/Fbg, prothrombin time and fibrinogen.
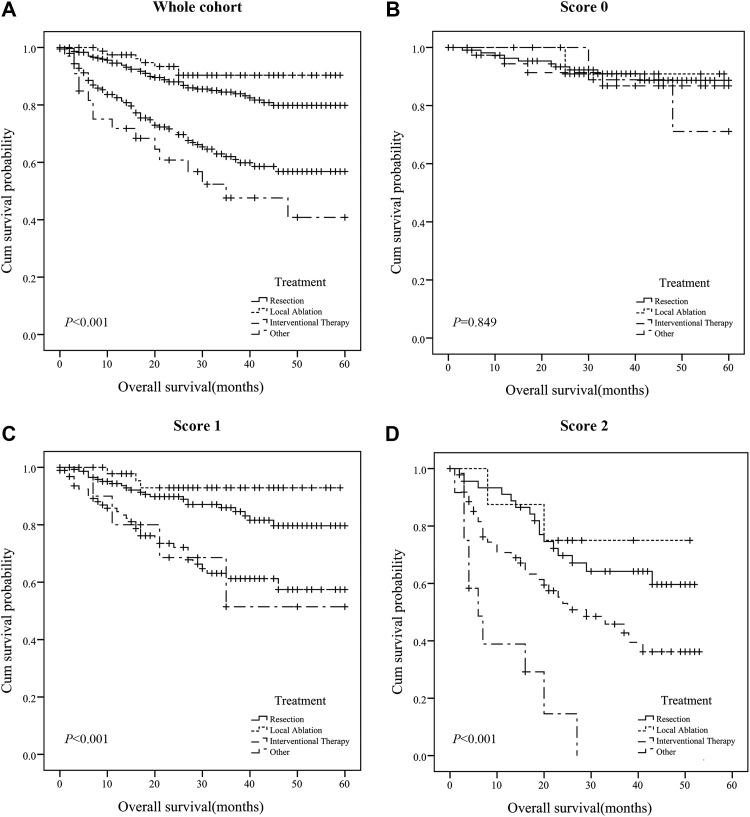
Moreover, we analyzed the prognostic effect of the PT/Fbg system score in subgroups based on treatment. Patients with a lower PT/Fbg system score exhibited similar OS among all the treatment strategies (P = .849). In PT/Fbg system score 1, most patients (48.52%) undergone resection, patients with interventional therapy and other therapies(including chemoradiotherapy, targeted therapy) exhibited similar OS, which were shorter than OS with resection and local ablation (P < .001). In PT/Fbg system score 2, most patients (47.76%) undergone interventional therapy, and the OS was shorter than resection and local ablation (P < .001; Figure 4).
Figure 4.
The association of PT/Fbg system and treatment in AFP-negative HCC. A, Prognostic significance of treatment in whole cohort; (B) Prognostic significance of treatment in PT/Fbg system score 0; (C) Prognostic significance of treatment in PT/Fbg system score 1; (D) Prognostic significance of treatment in PT/Fbg system score 2. AFP indicates alpha-fetoprotein; HCC, hepatocellular carcinoma; PT/Fbg, prothrombin time and fibrinogen.
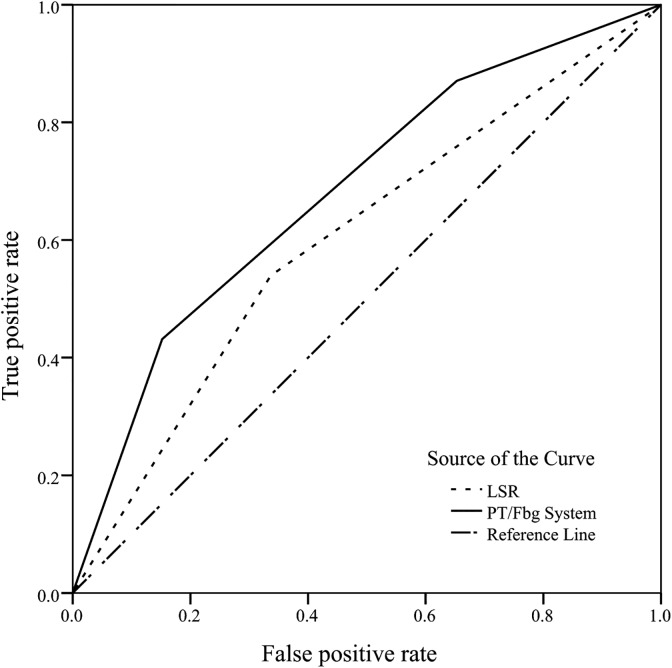
The AUC of PT/Fbg System Score in AFP-Negative HCC
The ROC curve was plotted to assess the discrimination ability of LSR and PT/Fbg system in patients with AFP-negative HCC, as shown in Figure 5. Assessed by AUC, the prognostic values of PT/Fbg system were 0.684 (95% CI: 0.633-0.734, P < .001), which was higher than LSR (AUC: 0.601, 95% CI: 0.547-0.655, P < .001).
Figure 5.
Discriminatory ability of PT/Fbg system score and LSR. LSR indicates ALT/AST ratio; PT/Fbg, prothrombin time and fibrinogen.
Discussion
Hepatocellular carcinoma is an extremely poor prognostic cancer that remains one of the most common and aggressive human malignancies worldwide.12 Alpha-fetoprotein is the best tumor marker of HCC, and it is used for the clinical diagnosis of liver cancer screening, prognostic judgment, and recurrence monitoring.13 However, recent studies reported that the sensitivity of AFP for the diagnosis of HCC is only 40% to 65%, and the specificity is 76% to 96%. Notably, AFP expression in many cases of liver cancer is not elevated or even expressed.14 Therefore, AFP-negative HCC is not as easily diagnosed, and it was the focus of our study.15 Numerous recent studies were performed to identify a diagnostic biomarker for AFP-negative HCC, but all of these potential candidates exhibit poor specificity and sensitivity.
Liver function tests are routine laboratory tests, and serum ALT and AST are the circulating transaminases in the body that are used as specific markers of liver dysfunction; ALT and AST catalyze the transfer of amino groups to generate products in gluconeogenesis and amino acid metabolism,16,17 and many earlier investigations noted the relationship between LSR and the risk of malignancies, including hepatocellular cancer,18 gastric cancer,19 and esophageal cancer.20 The probable mechanisms underlying these associations were that the subclinical inflammation may be associated with the change in LSR levels, which may continue to damage the tissue and cause some noninfectious diseases. The GPS is a scoring system based on inflammation (CRP and ALB), and it was validated as a useful tool for predicting the prognosis for various cancers, including gastric cancer,21 lung cancer,22 pancreatic cancer,23 and hepatocellular cancer.24 Glasgow Prognostic Score also measures inflammation factors, and a systemic inflammatory response is part of the tumor. The release of pro-inflammatory cytokines may stimulate liver production of CRP and increase the demand for certain amino acids. Cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor (TNF), may modulate the production of ALB by hepatocytes via an increase in the permeability of the microvasculature to increase the transcapillary passage of ALB.25 However, the clinical utility of LSR and GPS in patients with AFP-negative HCC has not been reported. Inflammation and LSR levels are not always elevated, and many other factors influence these states in patients with AFP-negative HCC.
Our previous study demonstrated that the combination of plasma PT and Fbg levels could be evaluated as a valuable predictor of survival in patients with HCC. However, the present study is the first study to review the prognostic value of coagulation system tests in AFP-negative HCC, and we defined the optimal cutoff values of PT and Fbg using ROC. Patients were divided into 3 groups using our PT/Fbg system: score 0, score 1, and score 2. We demonstrated that PT/Fbg system scores were associated with 5-year OS in patients with ESCC. Furthermore, we observed that patients with a higher PT/Fbg score exhibited significantly poor 5-year OS compared to patients with a lower PT/Fbg score both in the entire cohort (HR = 1.899; 95% CI: 1.334-2.705; P < .001) and in subgroups stratified by TNM stage (stage I-II and stage III-IV) and treatment (resection, local ablation, interventional therapy, and other). The present data shows that the preoperative PT/Fbg score is significantly associated with TNM stage and OS, indicating that patients with higher PT/Fbg score show more progressed disease and poorer prognosis. Therefore, based on the preoperative FA score which is independent of clinical stage, we can identify patients who have high risk of poor prognosis preoperatively (among all the treatment).
We also used the AUC values to compare the discriminatory ability of PT/Fbg system scores with LSR in patients with AFP-negative HCC. Our results demonstrated that the AUC value of the PT/Fbg system (AUC: 0.684, 95% CI: 0.633-0.734, P < .001) was higher than the other values. We further investigated whether the PT/Fbg system score was related to the clinical–pathological parameters of the tumor to determine the factors that may affect the plasma PT/Fbg system. PT/Fbg system scores correlated with TNM stage, tumor stage, node stage, HBs Ag, and HBc Ab. Notably, these factors were also relevant predictive factors of tumor progression. These findings demonstrated that the PT/Fbg system score predicted AFP-negative HCC prognosis and preoperatively identified patients who exhibited a high risk of recurrence and in whom additional treatments may be suggested. However, large-scale clinical trials are required to confirm the true value of this system.
Cancer is a pro-inflammatory state in which inflammatory cells actively participate in the occurrence of tumor development, such as tumor cell proliferation, survival, and migration. Systemic inflammation may not be severe in patients with AFP-negative HCC, and patients with abnormal GPS scores or LSR levels are uncommon. The liver plays an important role in the metabolism and synthesis of clotting factors. The ability of the synthesis of clotting factors and anticoagulation proteins is damaged in various liver diseases, such as hepatitis, liver cirrhosis, and HCC.26 Multivariate analysis demonstrated that the PT/Fbg system scores were significant prognostic factors of postoperative survival and related to TNM stage. Therefore, the PT/Fbg system is superior to GPS and LSR as a prognostic indicator in patients with AFP-negative HCC. The presumed mechanism was described previously: first, the clotting factors, tissue coagulation enzymes and fibrinolytic factors decline, which damages liver cells in AFP-negative HCC27; second, tumor cells directly produce various procoagulant activities and pro-inflammatory cytokines, including tissue factor, cancer procoagulant, TNF-α, IL-1b, and vascular endothelial growth factor.28-31 Therefore, the imbalance of tumor, coagulation, and inflammation in blood coagulation disorders promotes tumor growth, invasion, and metastasis.32
In conclusion, our results indicate that PT/Fbg system scoring is a promising novel biomarker that is complementary to AFP for the diagnosis of AFP-negative HCC. This system may help clinicians identify high-risk patients with AFP-negative HCC. There were some limitations to our study. Our study was a retrospective analysis in our hospital, and the result must be validated in large prospective multicenter trials. We expect that the PT/Fbg system scoring will facilitate personalized multidisciplinary treatments to improve outcomes for patients with AFP-negative HCC.
Acknowledgments
The authors thank the staff of the biochemical laboratory of Sun Yat-sen University Cancer Center who provided various biochemical markers, and all of the staff who supported our study.
Authors’ Note: M.M., X.W., and Y.S. contributed equally to this work. Due to ethical restrictions, the raw data underlying this paper are available upon request to the corresponding author or the Research Data Deposit public platform (www.researchdata.org.cn, with the approval RDD Number as RDDA2018000385).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Guangzhou Science and Technology planning project (201604016070).
ORCID iD: Minjie Mao, MD  https://orcid.org/0000-0002-5250-8186
https://orcid.org/0000-0002-5250-8186
Xueping Wang, MD  https://orcid.org/0000-0002-3519-3100
https://orcid.org/0000-0002-3519-3100
Shuqin Dai, MD  https://orcid.org/0000-0002-6776-8466
https://orcid.org/0000-0002-6776-8466
References
- 1. Xu Y, Liu AJ, Gao YX, et al. Expression of Ku86 and presence of Ku86 antibody as biomarkers of hepatitis B virus related hepatocellular carcinoma. Dig Dis Sci. 2014;59(3):614–622. [DOI] [PubMed] [Google Scholar]
- 2. Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30(1):37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen DS, Sung JL, Sheu JC, et al. Serum alpha-fetoprotein in the early stage of human hepatocellular carcinoma. Gastroenterology. 1984;86(6):1404–1409. [PubMed] [Google Scholar]
- 4. Paul SB, Gulati MS, Sreenivas V, et al. Evaluating patients with cirrhosis for hepatocellular carcinoma: value of clinical symptomatology, imaging and alpha-fetoprotein. Oncology. 2007;72(suppl 1):117–123. [DOI] [PubMed] [Google Scholar]
- 5. Matsuda S, Takeuchi H, Kawakubo H, et al. Cumulative prognostic scores based on plasma fibrinogen and serum albumin levels in esophageal cancer patients treated with transthoracic esophagectomy: comparison with the Glasgow Prognostic Score. Ann Surg Oncol. 2015;22(1):302–310. [DOI] [PubMed] [Google Scholar]
- 6. Giannini E, Botta F, Fasoli A, et al. Progressive liver functional impairment is associated with an increase in AST/ALT ratio. Dig Dis Sci. 1999;44(6):1249–1253. [DOI] [PubMed] [Google Scholar]
- 7. Tas F, Kilic L, Serilmez M, Keskin S, Sen F, Duranyildiz D. Clinical and prognostic significance of coagulation assays in lung cancer. Respir Med. 2013;107(3):451–457. [DOI] [PubMed] [Google Scholar]
- 8. Ma C, Zhou Y, Zhou S, Zhao K, Lu B, Sun E. Preoperative peripheral plasma fibrinogen level is an independent prognostic marker in penile cancer. Oncotarget. 2017;8(7):12355–12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bottasso B, Mari D, Coppola R, Santoro N, Vaglini M, Mannucci PM. Hypercoagulability and hyperfibrinolysis in patients with melanoma. Thromb Res. 1996;81(3):345–352. [DOI] [PubMed] [Google Scholar]
- 10. Wang XP, Mao MJ, He ZL, et al. A retrospective discussion of the prognostic value of combining prothrombin time (PT) and fibrinogen (Fbg) in patients with Hepatocellular carcinoma. J Cancer. 2017;8(11):2079–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li XH, Wang XP, Gu WS, et al. Clinical significance of preoperative thrombin time in patients with esophageal squamous cell carcinoma following surgical resection. PLoS One. 2015;10(10):e140323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340(10):745–750. [DOI] [PubMed] [Google Scholar]
- 13. Song P, Tobe RG, Inagaki Y, et al. The management of hepatocellular carcinoma around the world: a comparison of guidelines from 2001 to 2011. Liver Int. 2012;32(7):1053–1063. [DOI] [PubMed] [Google Scholar]
- 14. Asrih M, Lenglet S, Mach F, Montecucco F. Alpha-fetoprotein: a controversial prognostic biomarker for small hepatocellular carcinoma. World J Gastroenterol. 2013;19(3):328–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. She S, Xiang Y, Yang M, et al. C-reactive protein is a biomarker of AFP-negative HBV-related hepatocellular carcinoma. Int J Oncol. 2015;47(2):543–554. [DOI] [PubMed] [Google Scholar]
- 16. Wroblewski F, Ladue JS. Serum glutamic pyruvic transaminase in cardiac with hepatic disease. Proc Soc Exp Biol Med. 1956;91(4):569–571. [DOI] [PubMed] [Google Scholar]
- 17. Kim HC, Nam CM, Jee SH, Han KH, Oh DK, Suh I. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ. 2004;328(7446):983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang ZX, Jiang CP, Cao Y, Zhang G, Chen WB, Ding YT. Preoperative serum liver enzyme markers for predicting early recurrence after curative resection of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2015;14(2):178–185. [DOI] [PubMed] [Google Scholar]
- 19. Chen SL, Li JP, Li LF, Zeng T, He X. Elevated preoperative serum alanine aminotransferase/aspartate aminotransferase (ALT/AST) ratio is associated with better prognosis in patients undergoing curative treatment for gastric adenocarcinoma. Int J Mol Sci. 2016;17(6):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang H, Wang XP, Li XH, et al. Prognostic value of pretreatment serum alanine aminotransferase/aspartate aminotransferase (ALT/AST) ratio and gamma glutamyltransferase (GGT) in patients with esophageal squamous cell carcinoma. BMC Cancer. 2017;17(1):544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Crumley AB, McMillan DC, McKernan M, McDonald AC, Stuart RC. Evaluation of an inflammation-based prognostic score in patients with in operable gastro-oesophageal cancer. Br J Cancer. 2006;94(5):637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leung EY, Scott HR, McMillan DC. Clinical utility of the pretreatment Glasgow Prognostic Score in patients with advanced inoperable non-small cell lung cancer. J Thorac Oncol. 2012;7(4):655–662. [DOI] [PubMed] [Google Scholar]
- 23. La Torre M, Nigri G, Cavallini M, Mercantini P, Ziparo V, Ramacciato G. The Glasgow Prognostic Score as a predictor of survival in patients with potentially resectable pancreatic adenocarcinoma. Ann Surg Oncol. 2012;19(9):2917–2923. [DOI] [PubMed] [Google Scholar]
- 24. Horino K, Beppu T, Kuroki H, et al. Glasgow Prognostic Score as a useful prognostic factor after hepatectomy for hepatocellular carcinoma. Int J Clin Oncol. 2013;18(5):829–838. [DOI] [PubMed] [Google Scholar]
- 25. Barber MD, Ross JA, Fearon KC. Changes in nutritional, functional, and inflammatory markers in advanced pancreatic cancer. Nutr Cancer. 1999;35(2):106–110. [DOI] [PubMed] [Google Scholar]
- 26. Vlodavsky I, Ilan N, Nadir Y, et al. Heparanase, heparin and the coagulation system in cancer progression. Thromb Res. 2007;120(suppl 2):S112–S120. [DOI] [PubMed] [Google Scholar]
- 27. Aytac S, Turkay C, Bavbek N, Kosar A. Hemostasis and global fibrinolytic capacity in chronic liver disease. Blood Coagul Fibrinolysis. 2007;18(7):623–626. [DOI] [PubMed] [Google Scholar]
- 28. Rak J, Milsom C, May L, Klement P, Yu J. Tissue factor in cancer and angiogenesis: the molecular link between genetic tumor progression, tumor neovascularization, and cancer coagulopathy. Semin Thromb Hemost. 2006;32(1):54–70. [DOI] [PubMed] [Google Scholar]
- 29. Francis JL, Biggerstaff J, Amirkhosravi A. Hemostasis and malignancy. Semin Thromb Hemost. 1998;24(2):93–109. [DOI] [PubMed] [Google Scholar]
- 30. Donati MB, Gambacorti-Passerini C, Casali B, et al. Cancer procoagulant in human tumor cells: evidence from melanoma patients. Cancer Res. 1986;46(12 pt 1):6471–6474. [PubMed] [Google Scholar]
- 31. Dittman WA, Majerus PW. Structure and function of thrombomodulin: a natural anticoagulant. Blood. 1990;75(2):329–336. [PubMed] [Google Scholar]
- 32. Zwicker JI, Furie BC, Furie B. Cancer-associated thrombosis. Crit Rev Oncol Hematol. 2007;62(2):126–316. [DOI] [PubMed] [Google Scholar]