Short abstract
Schwann cell and nerve conduit are crucial for nerve regeneration and re-myelination after peripheral nerves injury. To investigate the effects of Salidroside on autogenous epimysium conduit mixed with BD matrigel and RSC96 Schwann cells on an animal model with 5 mm sciatic nerve defect injury in rat, motor function, muscle reinnervation, immunohistochemical staining, retrograded tracing and Western blot were used in this study. The results showed that Salidroside enhanced the compound effects of epimysium conduit mixed with BD matrigel and RSC96 Schwann cells to improve the sciatic functional index and the gastrocnemius muscle weight ratio, which were better than EPM group at 8 weeks and 12 weeks post operation. Immunofluorescence and Western blot results of P75NTR showed that Salidroside improved the sciatic nerve regeneration, and retrograded tracing of CTB-Alexa 488 also supported that Salidroside was better to promote CTB tracer transporting from the distal nerve defect to the ipsilateral dorsal root ganglion and ventral horn of L3-L5 spinal cord on post-operation 8 weeks and 12 weeks. Our results demonstrated that Salidroside improved the effect of autogenous epimysium conduit mixed with BD matrigel and RSC96 Schwann cells on sciatic nerve regeneration in our study.
Impact statement
Peripheral nerve injury and regeneration remain a major challenge. Although nerve conduit and Schwann cells have been used to study the nerve regeneration, our results demonstrated that Salidroside improved the regenerative effect in a rat model with sciatic nerve injury model, following a combined application of autogenous epimysium conduit mixed with Schwann cells. Different concentrations of Salidroside combining autogenous epimysium conduit and Schwann cells were applied to compare the epimysium conduit group and the epimysium conduit combining Schwann cells group. Based on the results of motor function and muscle reinnervation evaluation, as well as neuronal tracing and expression of P75NTR, our study for the first time suggests that Salidroside may improve the regeneration effect on the sciatic nerve following a combined application of epimysium conduit and RSC96 Schwann cells in rats.
Keywords: Salidroside, RSC96 Schwann cells, autologous epimysium conduit, combination application, nerve regeneration, sciatic nerve injury
Introduction
Peripheral nerve injury has a high incidence, and is often caused by trauma, resulting in widespread disability and substantial costs to society.1 Peripheral nerve defect may lead to injury gap and disruption of myelin sheaths and axons. Although nerve autografts are often used to repair peripheral nerve, there also exist a low functional recovery rate and permanent denervation at the donor site, peripheral nerve tissue engineering is therefore seen as a perspective strategy for creating a better environment for the regeneration of the damaged nerve.2
Peripheral nerve tissue engineering involves nerve guide conduit, Schwann-like cells or Schwann cells (SCs) as seeding cells, and a suitable three-dimensional microenvironment produced by bioactive molecules to promote nerve regeneration to bridge the nerve gap.3–5 The nerve conduit provides a good regeneration chamber for the peripheral nerve injury to repair, and essential neurotrophic factors and regeneration factors in the nerve conduit also promote nerve regeneration.6 SCs or Schwann-like cells are the primary seeding cells similar to epithelial cells, are dedifferentiated and proliferated to promote peripheral nerve regeneration.3 BD matrigel is a kind of solubilized basement membrane, effective in attaching and differentiating normal and transformed anchorage-dependent epithelioid and other cell types, supporting dimensional culture of Schwann cells in vivo for peripheral nerve regeneration.7,8
Salidroside is a glucoside of tyrosol, derived from the plant Rhodiola rosea. Several reports demonstrated that salidroside exhibits neurotrophic and neuroprotective activities by regulating the response of oxidative stress, inflammation, apoptosis, and neural regeneration.9 Previous studies reported that Salidroside significantly promote SCs survival and proliferation,10 and it also can attenuate colistin-induced neurotoxicity in RSC96 Schwann cells.11
However, there is no previous study focused on the effects of combinations of Salidroside, epimysium conduit, and Schwann cells in nerve regeneration. In this study, the effect of Salidroside combined with autogenous epimysium conduit and RSC96 Schwann cells was investigated on the regeneration of sciatic nerve defect model of rat.
Materials and methods
Experimental animals and groups
Male SD rats (150 ± 20 g) were housed in a temperature- and humidity-controlled room at the Fujian Medical University Laboratory Animal Center on a 12 h–12-h light/dark cycle, and given ad libitum food and water. Room temperature was between 22 and 25°C.
All the animals were randomly assigned into five groups. (1) EPM group, in which rats with 5 mm sciatic nerve defect only received autogenous epimysium conduit bridging both stumps of the nerve defect; (2) EPM + RSC96 group, in which rats with 5 mm sciatic nerve defect were treated with autogenous epimysium conduit filled with matrigel and RSC96 Schwann cells to bridge both stumps of the nerve defect; (3) 0.1 mM Salidroside mixture group, in which rats with 5 mm sciatic nerve defect were treated with autogenous epimysium conduit filled with matrigel, RSC96 Schwann cells, and 0.1 mM Salidroside mixture to bridge both stumps of the nerve defect. (4) 0.2 mM Salidroside mixture group, in which rats with 5 mm sciatic nerve defect were treated with autogenous epimysium conduit filled with matrigel, RSC96 Schwann cells, and 0.2 mM Salidroside mixture to bridge both stumps of the nerve defect. (5) 0.4 mM Salidroside mixture group, in which rats with 5 mm sciatic nerve defect were treated with autogenous epimysium conduit filled with matrigel, RSC96 Schwann cells, and 0.4 mM Salidroside mixture to bridge both stumps of the nerve defect. Salidroside (Aladdin, Shanghai, China) was dissolved by the culture medium ranging from 0.1 mM to 0.4 mM before use in this study.
In the current study, the Salidroside mixture with RSC96 Schwann cells and autogenous epimysium conduit was used to demonstrate the regenerative effect after 5 mm sciatic nerve defect injury in rats. In order to eliminate the complicated physiological function of gonadal hormone in this study, we only used male rats as experimental animal like most of the other published articles. All the experimental procedures were approved by the research ethics committee of Fujian Medical University and abided by the guidelines of Fujian Medical University Institutional Animal Care and Use Committee. The number and suffering of animals used have been minimized.
Autologous epimysium conduit preparation
The method of epimysium conduit preparation was as described previously.12 All the operations were performed by using a surgical microscope (WPI, USA). Rats were anesthetized intraperitoneally with pentobarbital (50 mg/kg), and a U-shaped surgical incision was made on the inguinal region beside the midline to expose the right external oblique to obtain a 10 mm × 6 mm epimysium slice. Then four corners of the epimysium slice were fixed with 6–0 nylon sutures and temporarily stored in the low temperature normal saline solution for subsequent epimysium conduit preparation (Figure 1). The incision was sutured by 5–0 surgical sutures.
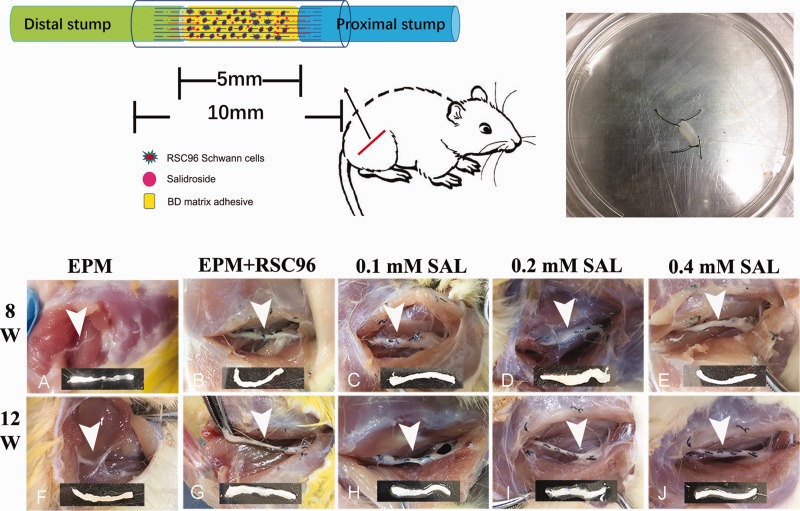
Figure 1.
Diagram of animal surgery for sciatic nerve injury model and gross observation of regenerative sciatic nerve. An autologous epimysium conduit was performed to bridge both stumps of the nerve defect. BD matrigel, Schwann cells, and Salidroside (SAL) mixture were injected inside the epimysium conduit during operation (top left). The epimysium slice were obtain from the right external oblique and fixed with 6–0 nylon sutures for subsequent epimysium conduit preparation (top right). In post-operation 8 weeks, poor bridge of the stumps was observed in the sham group (a), while the sciatic nerve injury in other groups was completely connected together by regenerative tissue (b–e); in post-operation 12 weeks (f–j), the regenerative nerves of all the groups were relatively coarser than before, and the 0.2 mM Salidroside mixture group (i) and 0.4 mM Salidroside mixture group (j) were better regenerative. Arrowhead showed the epimysium conduit bridge the sciatic nerve injury, and below was the representative segment of regeneration sciatic nerve tissue. (A color version of this figure is available in the online journal.)
Animal surgery of sciatic nerve injury animal model
After the autologous epimysium slice preparation and the rat was still under anesthesia, then another surgical incision in the right hind limb dorsal skin was made; subsequently, the sciatic nerve was exposed by bluntly separating the skin and hip muscle (Figure 1). The prepared autologous epimysium slice was took out from the normal saline solution to wrap the sciatic nerve longitudinally, and then two terminals of the epimysium slice were fixed to the proximal and distal perineural tissue of the sciatic nerve, respectively. The sciatic nerve with 5-mm long defect between the two terminals of the epimysium was excised, then the bilateral edges of epimysium was carefully sutured together to form an autologous epimysium conduit to bridge both stumps of the nerve defect.
According to different treatments in five groups, BD matrigel (BD Biosciences, MA, USA), 1 × 108 cells/mL RSC96 Schwann cells (Cell Bank of Typical Culture Preservation Committee of Chinese Academy of Science, Shanghai, China) or Salidroside (Aladdin, shanghai, China) with different concentrations (0.1 mM, 0.2 mM, or 0.4 mM) mixtures were injected into the epimysium conduit by 26-gauge syringe, respectively. The epimysium conduit was closed by 6–0 sutures carefully without leakage; 5–0 sutures were used to close surgical incisions at last.
Motor function testing by sciatic functional index
The rat walking track was recorded to evaluate the motor function of hind limbs by sciatic functional index (SFI) at 8 weeks and 12 weeks post-operation, as previously described.13 Briefly, the print length from the heel to the 3rd toe of experimental and the normal sides (EPL, NPL), the toe spread between the 1st toe and 5th toe of experimental and normal sides (ETS, NTS), while the intermediate toe spread between the 2nd toe and 4th toe of experimental and the normal sides (EIT, NIT) were measured in different groups during walking. The SFI value can be calculated by the following formula
SFI = 109.5(ETS-NTS)/NTS-38.3(EPL-NPL)/NPL + 13.3(EIT-NIT)/NIT-8.8
The SFI value of −100 indicates total impairment, while the value of 0 indicates normal function.
Ratios of the gastrocnemius muscle weight
The gastrocnemius muscles were resected in hind limbs soon after the rats were sacrificed at 8 weeks and 12 weeks post-operation, then weighed by an electronic balance (Sartorius, Germany). The ratio of gastrocnemius muscle weight was calculated from the experimental limb to the control limb.
Retrograde tracing of the sciatic nerve with CTB-Alexa 488
At 8 weeks and 12 weeks post-operation, the sciatic nerve segment with epimysium conduit of different groups (n = 4) was exposed after intraperitoneally pentobarbital (50 mg/kg) anesthesia, and 1 μL 0.5% Alexa Fluor 488-conjugated cholera toxin subunit B solution (CTB-Alexa 488, Invitrogen, Thermo Fisher, USA) was injected into the distal stump outside of the epimysium conduit with a microsyringe. The rats were sacrificed after 72 h, and transcardially perfused by 4% paraformaldehyde in 0.2 M PBS. The segments of sciatic nerve containing epimysium conduit, dorsal root ganglion, and L3–L5 segment of spinal cord were quickly removed, then cryoprotected in 30% (W/V) sucrose solutions for 48 h at 4°C.
Segments were sectioned at 10 μm thickness with a cryostat microtome (Leica CM1950, Germany), then washed with 0.01 M PBS and DAPI nuclear staining. As the sections combined with CTB-Alexa 488, they were cover slipped with Fluoromount-G and observed under fluorescence microscope.
Immunohistochemical staining
Each group of rats (n = 5), at either 8 weeks or 12 weeks post-operation, were anaesthetized with pentobarbital (200 mg/kg) and transcardially perfused with 0.01 M phosphate-buffered saline, followed by 4% paraformaldehyde phosphate buffer (pH 7.4). The segment of sciatic nerve including the epimysium conduit was obtained and then cryoprotected in 0.1 M phosphate buffer containing 30% sucrose (W/V) at 4°C overnight. Longitudinal sections of the sciatic nerve were serially cut at 10 μm thickness using cryostat microtome (Leica CM1950, Germany).
Sections were preincubated in 3% bovine serum albumin for 30 min, followed by incubating primary antibodies: mouse monoclonal antibody against p75 (1:200, Abcam, MA, USA), rabbit anti-IBA1 (1:200, WAKO, Tokyo, Japan), rabbit anti-Ki67 monoclonal antibody (1:200, Abcam, MA, USA), mouse anti-neurofilament monoclonal antibody (1:50, Abcam, MA, USA), rabbit anti-MBP monoclonal antibody (1:1000, CST, MA, USA) at room temperature overnight, and then continuously incubated at 4°C for 48 h. The sections were further incubated with the following secondary antibodies at 4°C overnight: goat anti-rabbit 488 (1:500, Invitrogen, CA, USA) and biotinylated donkey anti-mouse IgG (1:200; Vector, CA, USA). Finally, the sections were incubated with Cy3-avidin (1:1500, Jackson ImmunoResearch, PA, USA) at room temperature for 4 h, and DAPI (1:1000, Invitrogen, CA, USA) stained nuclei, respectively.
Western blot
Total proteins were extracted from the segment of sciatic nerve tissues within the epimysium conduit at 8 weeks and 12 weeks post-operation, then the proteins were transfer onto a polyvinylidene fluoride (PVDF) membrane (Millipore, MA, USA), and followed by blocking with 5% (w/v) nonfat milk. After that, the PVDF membranes were incubated overnight at 4°C with the following antibodies: Rabbit anti-P75NTR (1:1000, Millipore, MA, USA), mouse anti-β-actin (1:1000, TransGen, Beijing, China). Following washing, the membranes were incubated with secondary antibodies using goat anti-rabbit IgG (H + L)-HRP (1:5000, Bioworld, OH, USA) or goat anti-mouse IgG-HRP (1:100,000, EarthOX, CA, USA) at room temperature for 2 h. Finally, Immobilon Western Chemiluminescent reagent (Merck Millipore P90720) was used to detect the bands.
Statistical analysis
All data were presented as the mean ± SEM. The quantification of CTB-488-positive neurons and Iba-1-positive macrophage/microglial cells in five sections was performed under the microscopic fields. The bands intensity signal of Western blot was quantified with Image J software (version 1.8.0, National Institutes of Health). The results were analyzed by two-way ANOVA followed by the Sidak’s multiple comparisons test, time point and groups were the variables used as factors in the two-way ANOVA. P < 0.05 was considered statistically significant.
Results
Gross observation of regenerative sciatic nerve
After the regenerative sciatic nerves were harvested at 8 weeks and 12 weeks post-operation, the sciatic nerves were observed in macroscopic observations. Poor bridging of the sciatic nerve defect was observed in the EPM group at 8 weeks post-operation (Figure 1(a)), while other groups were completely connected together by regenerative tissue (Figure 1(b) to (e)). At 12 weeks post-operation, the regenerative sciatic nerves of all the groups were relatively coarser than before (Figure 1(f) to (j)), especially the 0.2 mM and 0.4 mM Salidroside mixture groups, which recovered better, being observed to be more engorged and in relatively good condition.
Walking track assessment by SFI
The SFI values are shown in Table 1. Except for the EPM group, the SFI in all the other groups progressively improved with different degrees from 8 weeks to 12 weeks post-operation. At 8 weeks post-operation, the 0.4 mM Salidroside mixture group showed a relatively better recovery compared to other groups (P < 0.001). At 12 weeks post-operation, no significant recovery in the EPM group was observed, while the other groups recovered better than before, especially the 0.4 mM Salidroside mixture group, which showed the best effect of promoted motor function recovery (P < 0.001).
Table 1.
Evaluations for sciatic nerve regeneration by sciatic functional index.
| Groups | SFI | SFI |
|---|---|---|
| Post-operation 8 weeks M ± SD (n=5) | Post-operation 12 weeks M ± SD (n=5) | |
| EPM | −98.709 ± 6.444 | −101.201 ± 10.878 |
| EPM + RSC96 | −63.155 ± 5.904a | −55.410 ± 3.468a |
| 0.1 mM Salidroside | −75.805 ± 4.594 | −54.625 ± 4.557a |
| 0.2 mM Salidroside | −65.814 ± 5.809a | −61.723 ± 4.321a |
| 0.4 mM Salidroside | −57.562 ± 6.662a | −32.806 ± 2.834a,d |
M: mean; SD: standard deviation; SFI: sciatic functional index.
aStatistically significant vs. EPM group, P < 0.05.
bStatistically significant vs. EPM + RSC96 group, P < 0.05.
cStatistically significant vs. 0.1 mM salidroside group, P < 0.05.
dStatistically significant vs. 0.2 mM salidroside group, P < 0.05.
Wet weight analysis of gastrocnemius muscle
The gastrocnemius muscles from both hind limbs were harvested and immediately weighed at 8 weeks and 12 weeks post-operation to calculate the ratios of gastrocnemius muscle for trophism analysis is shown in Table 2. Compared to the EPM group and EPM+RSC96 group, the ratios of gastrocnemius muscle in the Salidroside mixture groups obviously improved, especially the 0.4 mM Salidroside mixture group at 8 weeks post-operation and the 0.1 mM Salidroside mixture group at 12 weeks post-operation (P < 0.001).
Table 2.
Ratios of the gastrocnemius muscle weight.
| Groups | Ratios | Ratios |
|---|---|---|
| Post-operation 8 weeks M ± SD (n=5) | Post-operation 12 weeks M ± SD (n=5) | |
| EPM | 0.226 ± 0.036 | 0.319 ± 0.076 |
| EPM + RSC96 | 0.433 ± 0.054a | 0.533 ± 0.014a |
| 0.1 mM Salidroside | 0.501 ± 0.027a,b | 0.631 ± 0.017a,b |
| 0.2 mM Salidroside | 0.485 ± 0.034a | 0.603 ± 0.008a |
| 0.4 mM Salidroside | 0.569 ± 0.016a,b | 0.606 ± 0.034a |
M: mean; SD: standard deviation.
aStatistically significant vs. EPM group, P < 0.05.
bStatistically significant vs. EPM + RSC96 group, P < 0.05.
cStatistically significant vs. 0.1 mM Salidroside group, P < 0.05.
dStatistically significant vs. 0.2 mM Salidroside group, P < 0.05.
Retrograde tracer results of sciatic nerve
CTB-Alexa 488 (green) injection into the right sciatic nerve in the distal epimysium conduit retrograde transported to produce ipsilateral labeling of dorsal root ganglion (Figure 2) and ventral horn of L3-L5 spinal cord (Figure 3) at 8 weeks and 12 weeks post-operation.
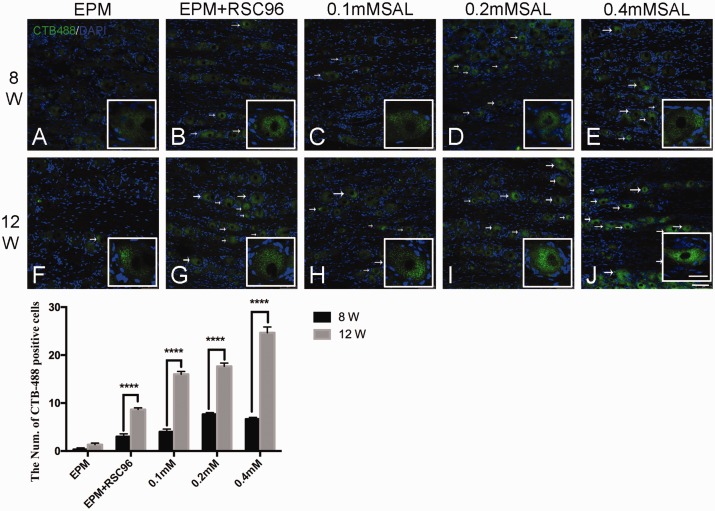
Figure 2.
Retrograde labeling of CTB-Alexa 488 (green) in the dorsal root ganglion. In post-operation 8 weeks, no obvious CTB-Alexa 488-positive granules in the sham group were observed (a), while other groups (b–e) were only relatively obvious than the sham group; In post-operation 12 weeks, the CTB-Alexa 488-positive granules in the different groups increased than post-operation 8 weeks, and the CTB-Alexa 488-positive granules in the Salidroside (SAL) mixture groups (h–j) were relatively more than the sham group and BDSC group (f–g), especially the 0.4 mM Salidroside mixture group (j). White arrow showed the CTB-Alexa 488-labeled neuron. Histogram showed the count of CTB-Alexa 488-labeled neurons in the dorsal root ganglion. n = 4; Bar = 75 μm. (Enlarged view bar = 30 μm) ****P < 0.0001. (A color version of this figure is available in the online journal.)
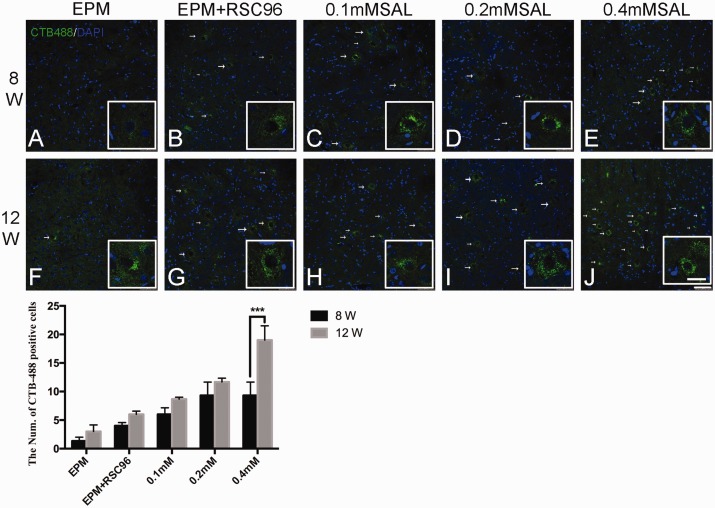
Figure 3.
Retrograde labeling of CTB-Alexa 488 (green) in the anterior horn of L3–L5 spinal cord. In post-operation 8 weeks, CTB-labeled neurons were absent in the right ventral horn in sham group and BDSC group (a–b), but only a few CTB-labeled neurons were observed in the Salidroside (SAL) mixture groups (c–e). In post-operation 12 weeks, CTB-labeled neurons presented in all the groups (f–j), and the 0.4 mM Salidroside mixture group (j) was relatively more than other groups. White arrow showed the CTB-Alexa 488-labeled neuron. Histogram showed the count of CTB-Alexa 488-labeled neurons in the anterior horn of L3–L5. n = 4; Bar = 75 μm. (Enlarged view bar = 30 μm) ***P < 0.001. (A color version of this figure is available in the online journal.)
In the ipsilateral dorsal root ganglion of L3-L5, there was no obvious CTB-Alexa 488-labeled neuron in different groups at 8 weeks post-operation (Figure 2(a) to (e)), while some of them were observed at 12 weeks post-operation (Figure 2(f) to (j)). The CTB-Alexa 488-labeled neurons in the Salidroside mixture groups (Figure 2(h) to (j)) were relatively more than the EPM group and EPM+RSC96 group (Figure 2(f) to (g)), especially in the 0.4 mM Salidroside mixture group (Figure 2(j)).
In the ventral horn of L3-L5 spinal cord at 8 weeks post-operation (Figure 3), there was no obvious CTB-Alexa 488-labeled neurons in the EPM group and EPM+RSC96 group (Figure 3(a) and (b)), while there were a few CTB-Alexa 488 labeled-neurons in the Salidroside mixture groups (Figure 3(c) and (d)). At 12 weeks post-operation, the CTB-Alexa 488-labeled neurons were observed in all groups (Figure 3(f) to (j)), and the 0.2 mM and 0.4 mM Salidroside mixture groups showed more than other groups (Figure 3(i) to (j)).
Immunofluorescence staining in the sciatic nerve
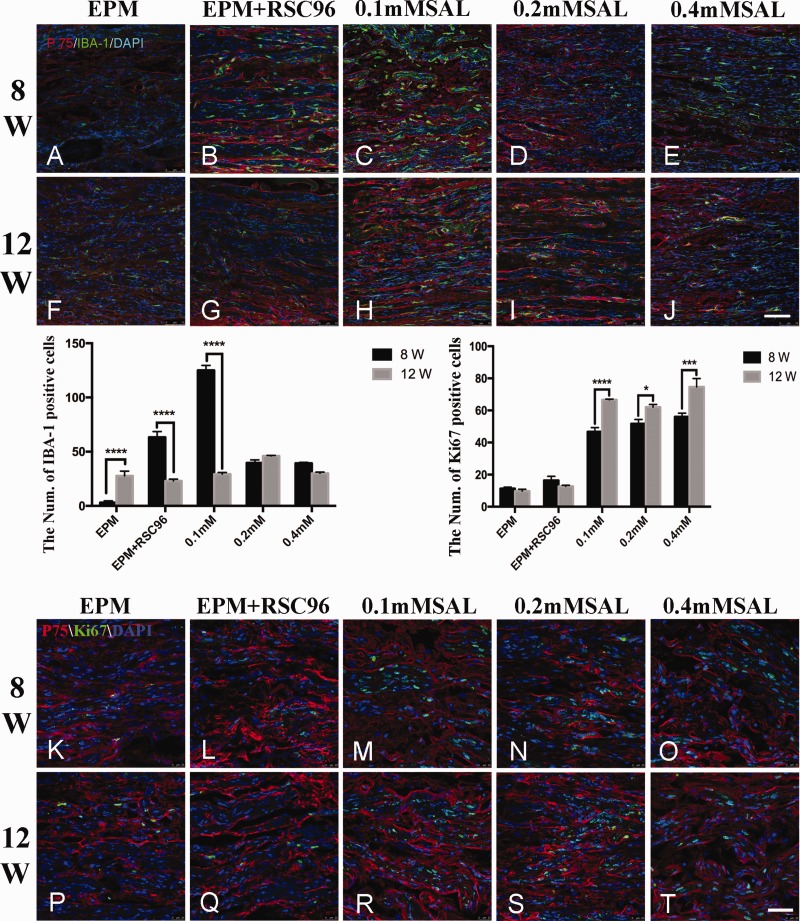
At 8 weeks post-operation, as shown in Figure 4, the regenerative nerve tissues were arranged in a disordered way in the EPM group (Figure 4(a)), and the expression of P75NTR was relatively weak, but no Iba-1 immunoreactivity was observed; although the regenerative nerve tissues were still randomly arranged in the EPM+RSC96 group (Figure 4(b)), the expressions of P75NTR and Iba-1 slightly increased over the EPM group. In the Salidroside mixture groups (Figure 4(c) to (e)), the expressions of P75NTR were relatively higher than in the EPM group, and the expressions of P75NTR and Iba-1 in the 0.1 mM Salidroside mixture group (Figure 4(c)) were more obvious than others, but the arrangement of the regenerative nerve tissues in the 0.4 mM Salidroside mixture group (Figure 4(e)) seemed to be more orderly than in any other group.
Figure 4.
Immunofluorescence staining of P75NTR, IBA-1, and Ki67 in regenerated sciatic nerve. In post-operation 8 weeks, the regenerative nerve fibers were disordered arrangement and only a few P75NTR was observed in the sham group (a). Compared with the sham group, the regenerative nerve fibers in the other groups arranged relatively regular (b–e), P75NTR and Iba-1 immunoreactivities were also observed. In post-operation 12 weeks, the regenerative nerve fibers in all the groups (f–j) obviously improved than before, P75NTR immunoreactivities in different Salidroside (SAL) mixture groups were better than sham group (f) and BDSC group (g), and the expression of Iba-1 in 0.2 mM Salidroside mixture group (i) was also lower than other group. Histograms showed the count of IBA-1-positive cells and Ki67-positive cells in regenerative sciatic nerve. Ki67-positive cells were observed in all groups at post-operation 8 weeks and 12 weeks, but there were more obvious in the Salidroside mixture groups with different concentrations than EPM group and EPM + RSC96 group (k–t). n = 5; Bar = 100 μm. (A color version of this figure is available in the online journal.)
At 12 weeks post-operation, the expressions of P75NTR and Iba-1 in the EPM group and EPM+RSC96 group were still relatively low (Figure 4(f) to (g)), but the regenerative nerve tissues were improved, although they were still randomly arranged. The expressions of P75NTR in the Salidroside mixture groups (Figure 4(h) to (j)) were higher than those at 8 weeks post-operation, and there was only a small expression of Iba-1. Most of the regenerative nerve tissues were relatively neatly arranged, especially in the 0.4 mM Salidroside mixture group (Figure 4(j)).
According to the results of proliferation marker Ki67 staining in the regenerative sciatic nerve tissues, Ki67-positive cells were observed in all groups at post-operation 8 weeks and 12 weeks, but there were more obvious in the Salidroside mixture groups with different concentrations than EPM group and EPM+RSC96 group (Figure 4(k) to (t)).
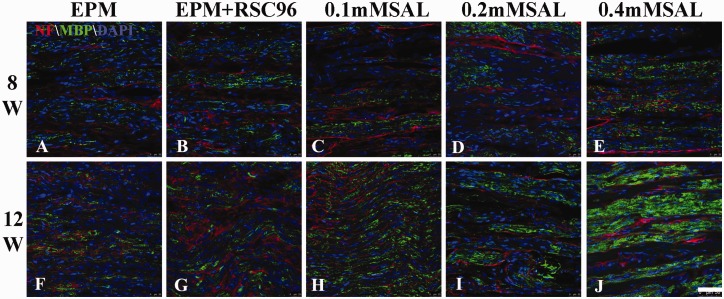
To better describe the arrangement of regenerative nerve tissue, the immunofluorescence staining of neurofilament and MBP was also performed in the study. At 8 weeks post-operation, the neurofilament and MBP-positive regenerative tissues longitudinally distributed along the sciatic nerve, and their expression in 0.4 mM Salidroside mixture group was more obvious than other groups, while the EPM group showed fainter expression (Figure 5(a) to (e)). At 12 weeks post-operation, the regenerative nerve tissues in all groups (Figure 5(f) to (j)) seemed to be arranged relatively regular and more intensive than those at 8 weeks post-operation, especially the neurofilament in 0.4 mM Salidroside mixture group was observed more expression than others and better alignment (Figure 5).
Figure 5.
Immunofluorescence staining of Neurofilament and MBP in regenerated sciatic nerve. At 8 weeks post-operation, the neurofilament (red) and MBP- (green) positive regenerative tissues longitudinally distributed along the sciatic nerve, and their expression in 0.4 mM Salidroside mixture group was more obvious than other groups, while the EPM group showed fainter expression. At 12 weeks post-operation, the regenerative nerve tissues in all groups seemed to be arranged relatively regular and more intensive than those at 8 weeks post-operation, especially the neurofilament in 0.4 mM Salidroside mixture group was observed more expression than others and better alignment. n = 5; Bar = 50 μm. (A color version of this figure is available in the online journal.)
Figure 6.
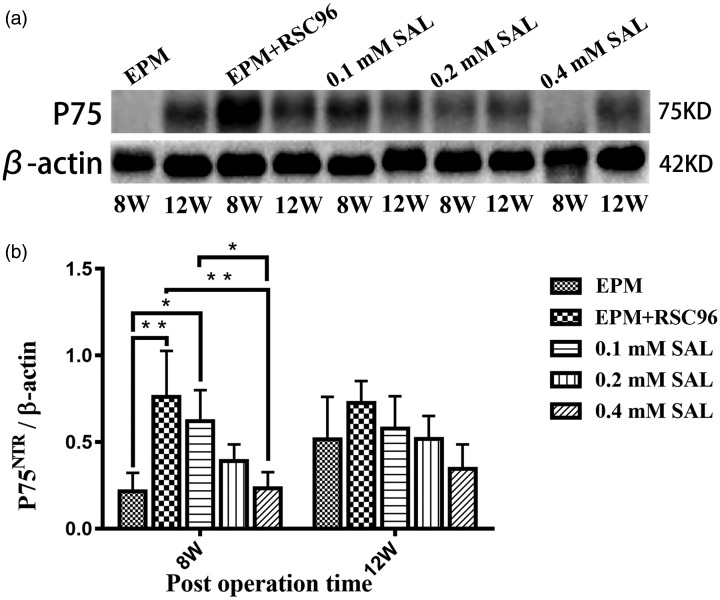
Western blot of P75NTR in the regenerative sciatic nerve. The expression of P75NTR in the sham group was not obvious in post-operation 8 weeks; all the other Salidroside (SAL) mixture groups were higher than sham group. In post-operation 12 weeks, there were no significant difference in the expression of P75NTR in all the groups (n = 5). *P < 0.05, **P < 0.01.
The Western blot results of P75NTR
As shown in Figure 1, the regenerative nerve tissues were not fully bridged with the nerve defect at 8 weeks post-operation in the EPM group, so the total protein extracted from the nerve tissues was relatively less, and as a result the expression of P75NTR in the EPM group was not obvious, while all the other groups were higher than the EPM group (Figure 6(a)). The P75NTR expressed the most in the EPM+RSC96 group (P < 0.01), and it gradually decreased in the 0.1 mM, 0.2 mM, and 0.4 mM Salidroside mixture groups. There were no significant differences in the expression of P75NTR in all the groups at 12 weeks post-operation (P > 0.05) (Figure 6 (b)).
Discussion
Previous studies have shown that after nerve injury, the proliferation of Schwann cells surrounding the axon forms a myelin sheath to promote axon regeneration.14,15 After peripheral nerve injury, the resting Schwann cells are activated to produce chemokine to attract phagocytes to clear the myelin sheath and axon fragments, and extend along the basal layer in the Bungner zone, providing a scaffold for the growth of new axons.16 Schwann cells also released various neurotrophic factors and extracellular matrix components provide nutrients for nerve cell survival and axon growth and material support. BD Matrigel provided a mimetic structure and physical properties of basement membrane to benefit the culture and differentiation of Schwann cells in the epimysium conduit in vitro, which may improve the regeneration of nerve injury by Schwann cells in the nerve conduit.12,17 Nerve conduit and BD Matrigel may control cell behavior, cell attachment, and proliferation, which also contribute to axons sprouting, cells migration, and bridge the nerve gap.
In this study, Salidroside administration to the autogenous epimysium conduit with RSC96 Schwann cells generally improved the regenerative nerve tissue and functional recovery in the rat sciatic nerve. The motor function of hind limb evaluated by SFI was significantly recovered in the 0.4 mM Salidroside mixture group, while the ratio of gastrocnemius muscle in the 0.1 mM Salidroside mixture group was better than other groups. A small number of CTB-Alexa 488-labeled neurons were observed in the ipsilateral dorsal root ganglion and ventral horn of the spinal cord in the groups with Schwann cell and Salidroside administration, which also supported the functional connections of the sciatic nerve defect.
Salidroside, as one of the active components of Rhodiola rosea L, has been reported to have neuroprotective effects.18–20 A previous study showed that Salidroside may induce Schwann cell proliferation by significantly up-regulating the expression of neurotrophic factors BDNF and GDNF to contribute to functional recovery in the rat model of sciatic nerve injury.18 As a low affinity receptor of BDNF, P75NTR binds to BDNF to form a neurotrophic factor receptor complex and participates in the activation of several signal transduction pathways.21 It was found that P75NTR and BDNF were up-regulated after peripheral nerve injury, and BDNF stimulated myelination by activating P75NTR in Schwann cells and axons.21–24 In our study, the expression of P75NTR in the Salidroside administration groups was relatively lower than the EPM+RSC96 group at post-operation 8 weeks, but there was no significant differences among all the groups at post-operation 12 weeks. It was suggested that Salidroside may regulate neurotrophic factors to affect the proliferation of Schwann cells to promote nerve regeneration of peripheral nerve injury in the early phase. Besides, studies also showed that Salidroside can protect Schwann cells from oxygen-free radical damage, promote the proliferation ability of Schwann cells, and enhance cell vitality.25
Compared with the previous literatures using a PLGA conduit and salidroside on peripheral nerve regeneration,26 the effect of Salidroside combined with epimysium conduit showed that the arrangement of regenerative tissue seemed to be more regular and tight in our study, and the regenerative effect was superior to the synthetic polyurethane conduit.
In our study, Iba-1-positive macrophage/microglia was observed in the early phase of sciatic nerve injury. We considered that the small amount of macrophage/microglia in the EPM group might be activated in response to mild chemical or inflammatory stimulation at 8 weeks post-operation, while other exogenous RSC96 Schwann cells may stimulating many activated macrophage/microglia; meanwhile, 0.1 mM Salidroside treatment could not easily eliminate the activation, so the amount of Iba-1-positive macrophage/microglia in EPM+RSC96 group and 0.1 mM Salidroside mixture group was significantly higher than other groups. Only high concentration Salidroside treatment in 0.2 mM and 0.4 mM Salidroside mixture groups alleviated the expression of macrophage/microglia. At 12 weeks post-operation, most of the Iba-1-positive macrophage/microglia in different groups were in relatively low expression level, which could indicate that nerve regeneration was in the relatively stable period. Therefore, these results could be closely related to the synergistic effects of epimysium conduit, BD matrigel, RSC96 Schwann cells, and Salidroside on nerve regeneration, that epimysium conduit provides a good regeneration chamber, BD matrigel creates the environment for RSC96 Schwann cells survival and proliferation to guide the newly axons sprouting direction and migration of cells, Salidroside regulates oxidative stress response and inflammation to exhibit neurotrophic and neuroprotective activities, and ultimately promotes the regeneration of sciatic nerve injury.
In conclusion, this study demonstrated that Salidroside combined with epimysium conduit and Schwann cell intervention may play a better role in promoting sciatic nerve regeneration and improved the motor function recovery repair in the sciatic nerve injury animal model of rats.
Authors’ contributions
Jiaqi Li and Yongguang Zhang designed and performed the experiments, Zhimin Yang and Jingxian Zhang help to perform the experiments, Jiaqi Li, Ren Lin and Daoshu Luo analyzed the data including statistical analysis, and Daoshu Luo prepared and revised the manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by National Natural Science Foundation of China (81671100; 81501896) and Natural Science Foundation of Fujian province of China (2015J05166).
ORCID iDs
Jiaqi Li https://orcid.org/0000-0002-8767-0909
Daoshu Luo https://orcid.org/0000-0001-9013-1052
References
- 1.Ciaramitaro P, Mondelli M, Logullo F, Grimaldi S, Battiston B, Sard A, Scarinzi C, Migliaretti G, Faccani G, Cocito D; Italian network for traumatic N. Traumatic peripheral nerve injuries: epidemiological findings, neuropathic pain and quality of life in 158 patients. J Peripher Nerv Syst 2010; 15:120–7 [DOI] [PubMed] [Google Scholar]
- 2.Chrzaszcz P, Derbisz K, Suszynski K, Miodonski J, Trybulski R, Lewin-Kowalik J, Marcol W. Application of peripheral nerve conduits in clinical practice: a literature review. Neurol Neurochir Pol 2018; 52:427–35 [DOI] [PubMed] [Google Scholar]
- 3.Han GH, Peng J, Liu P, Ding X, Wei S, Lu S, Wang Y. Therapeutic strategies for peripheral nerve injury: decellularized nerve conduits and Schwann cell transplantation. Neural Regen Res 2019; 14:1343–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Luca AC, Lacour SP, Raffoul W, di Summa PG. Extracellular matrix components in peripheral nerve repair: how to affect neural cellular response and nerve regeneration? Neural Regen Res 2014; 9:1943–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang Z, Song Y, Qiao J, Yang Y, Zhang W, Liu W, Han B. Rat sciatic nerve regeneration across a 10-mm defect bridged by a chitin/CM-chitosan artificial nerve graft. Int J Biol Macromol 2019; 129:997–1005 [DOI] [PubMed] [Google Scholar]
- 6.Panagopoulos GN, Megaloikonomos PD, Mavrogenis AF. The present and future for peripheral nerve regeneration. Orthopedics 2017; 40:e141–e56 [DOI] [PubMed] [Google Scholar]
- 7.Kleinman HK, McGarvey ML, Liotta LA, Robey PG, Tryggvason K, Martin GR. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry 1982; 21:6188–93 [DOI] [PubMed] [Google Scholar]
- 8.de Guzman RC, Loeb JA, VandeVord PJ. Electrospinning of matrigel to deposit a basal lamina-like nanofiber surface. J Biomater Sci Polym 2010; 21:1081–101 [DOI] [PubMed] [Google Scholar]
- 9.Zhong Z, Han J, Zhang J, Xiao Q, Hu J, Chen L. Pharmacological activities, mechanisms of action, and safety of salidroside in the central nervous system. Drug Des Devel Ther 2018; 12:1479–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu H, Lv P, Wu H, Zhang K, Xu F, Zheng L, Zhao J. The proliferation enhancing effects of salidroside on Schwann cells in vitro. Evid Based Complement Alternat Med 2017; 2017:4673289. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Lu Z, Jiang G, Chen Y, Wang J, Muhammad I, Zhang L, Wang R, Liu F, Li R, Qian F, Li J. Salidroside attenuates colistin-induced neurotoxicity in RSC96 Schwann cells through PI3K/Akt pathway. Chem Biol Interact 2017; 271:67–78 [DOI] [PubMed] [Google Scholar]
- 12.Yang XN, Jin YQ, Bi H, Wei W, Cheng J, Liu ZY, Shen Z, Qi ZL, Cao Y. Peripheral nerve repair with epimysium conduit. Biomaterials 2013; 34:5606–16 [DOI] [PubMed] [Google Scholar]
- 13.Wang T, Ito A, Aoyama T, Nakahara R, Nakahata A, Ji X, Zhang J, Kawai H, Kuroki H. Functional evaluation outcomes correlate with histomorphometric changes in the rat sciatic nerve crush injury model: a comparison between sciatic functional index and kinematic analysis. PLoS One 2018; 13:e0208985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olson HE, Rooney GE, Gross L, Nesbitt JJ, Galvin KE, Knight A, Chen B, Yaszemski MJ, Windebank AJ. Neural stem cell- and Schwann cell-loaded biodegradable polymer scaffolds support axonal regeneration in the transected spinal cord. Tissue Eng Part A 2009; 15:1797–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jessen KR, Mirsky R. The repair Schwann cell and its function in regenerating nerves. J Physiol 2016; 594:3521–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein D, Martini R. Myelin and macrophages in the PNS: an intimate relationship in trauma and disease. Brain Res 2016; 1641:130–8 [DOI] [PubMed] [Google Scholar]
- 17.de Ruiter GC, Spinner RJ, Yaszemski MJ, Windebank AJ, Malessy MJ. Nerve tubes for peripheral nerve repair. Neurosurg Clin N Am 2009; 20:91–105, vii [DOI] [PubMed] [Google Scholar]
- 18.Sheng QS, Wang ZJ, Zhang J, Zhang YG. Salidroside promotes peripheral nerve regeneration following crush injury to the sciatic nerve in rats. Neuroreport 2013; 24:217–23 [DOI] [PubMed] [Google Scholar]
- 19.Zhang B, Wang Y, Li H, Xiong R, Zhao Z, Chu X, Li Q, Sun S, Chen S. Neuroprotective effects of salidroside through PI3K/Akt pathway activation in Alzheimer’s disease models. Drug Des Devel Ther 2016; 10:1335–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Yu H, Zhao X, Lin X, Tan C, Cao G, Wang Z. Neuroprotective effects of salidroside against beta-amyloid-induced oxidative stress in SH-SY5Y human neuroblastoma cells. Neurochem Int 2010; 57:547–55 [DOI] [PubMed] [Google Scholar]
- 21.Saxena S, Howe CL, Cosgaya JM, Hu M, Weis J, Kruttgen A. Differences in the surface binding and endocytosis of neurotrophins by p75NTR. Mol Cell Neurosci 2004; 26:292–307 [DOI] [PubMed] [Google Scholar]
- 22.Lykissas MG, Batistatou AK, Charalabopoulos KA, Beris AE. The role of neurotrophins in axonal growth, guidance, and regeneration. Curr Neurovasc Res 2007; 4:143–51 [DOI] [PubMed] [Google Scholar]
- 23.Meeker R, Williams K. Dynamic nature of the p75 neurotrophin receptor in response to injury and disease. J Neuroimmune Pharmacol 2014; 9:615–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo D, Lin R, Luo L, Li Q, Chen T, Qiu R, Li Y. Glial plasticity in the trigeminal root entry zone of a rat trigeminal neuralgia animal model. Neurochem Res 2019; 44:1893–902 [DOI] [PubMed] [Google Scholar]
- 25.Cornejo M, Nambi D, Walheim C, Somerville M, Walker J, Kim L, Ollison L, Diamante G, Vyawahare S, de Bellard ME. Effect of NRG1, GDNF, EGF and NGF in the migration of a Schwann cell precursor line. Neurochem Res 2010; 35:1643–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu H, Lv P, Zhu Y, Wu H, Zhang K, Xu F, Zheng L, Zhao J. Salidroside promotes peripheral nerve regeneration based on tissue engineering strategy using Schwann cells and PLGA: in vitro and in vivo. Sci Rep 2017; 7:39869. [DOI] [PMC free article] [PubMed] [Google Scholar]