Short abstract
Studies have shown that metabolic diseases, such as obesity, are significantly associated with intestinal flora imbalance. The amplification of opportunistic pathogens induced by the glyoxylic acid cycle contributes to intestinal flora imbalance. Promising, though, is that saturated hydrogen can effectively improve the occurrence and development of metabolic diseases, such as obesity. However, the specific mechanism of how saturated hydrogen operates is still not very clear. In this study, after a high-fat diet, the level of total cholesterol, total glyceride, and low-density lipoprotein in the peripheral blood of mice increased, and that of high-density lipoprotein decreased. Intestinal fatty acid metabolism-related gene Apolipoprotein E (ApoE), fatty acid synthase (FAS), intestinal fatty acid-binding protein (I-FAPB), acetyl-CoA carboxylase 1 (ACC1), peroxisome proliferator-activated receptor γ (PPARγ), and stearoyl-CoA desaturase 1 (SCD1) increased significantly. Bacteroides, Bifidobacteria, and Lactobacillus counts in feces decreased considerably, while Enterobacter cloacae increased. The activity of isocitrate lyase in feces increased markedly. Treatment of mice with saturated hydrogen led to decreased total cholesterol, total glyceride, and low-density lipoprotein and increased high-density lipoprotein in the peripheral blood. FAS and I-FAPB gene expression in the small intestine decreased. Bacteroides, Bifidobacteria, and Lactobacillus in feces increased significantly, whereas Enterobacter cloacae decreased. The activity of isocitrate lyase also diminished remarkably. These results suggest that saturated hydrogen could improve intestinal structural integrity and lipid metabolism disorders by inhibiting the glyoxylic acid cycle of the intestinal flora.
Impact statement
Past studies have shown that hydrogen can improve metabolic disorders, but its mechanism of action remains unclear. It is well known that metabolic diseases, such as obesity, are significantly associated with changes in the intestinal flora. The glyoxylic acid cycle is an essential metabolic pathway in prokaryotes, lower eukaryotes, and plants and could be the portal for mechanisms related to metabolic disorders. Many opportunistic pathogenic bacteria can recycle fatty acids to synthesize sugars and other pathogenic substances using the glyoxylic acid cycle. So, the glyoxylic acid cycle may be involved in intestinal dysbacteriosis under high-fat diet. This study, therefore, seeks to provide the mechanism of how hydrogen improves metabolic diseases and a new basis for the use of hydrogen in the treatment of metabolic disorders.
Keywords: Saturated hydrogen, obesity, glyoxylic acid cycle, lipid metabolism, intestinal flora, isocitrate lyase
Introduction
Obesity is a chronic metabolic disease, as well as a chronic inflammatory response.1 The pathogenesis of obesity largely remains unclear. However, factors, such as intestinal flora imbalance, are currently being studied for their relationship with the pathogenesis of this disorder.2 Based on the “second genome” regulating human health, the fundamental components of the intestinal flora have been identified as Bacteroides, Eubacterium, Bif idobacterium, Peptostreptococcus, Fusobacterium, Ruminococcus, Clostridium, and Lactobacillus. Among them, Bacteroides, Bif idobacterium, Lactobacillus, and Ruminococcus are the main factors significantly related to obesity.3–5 Duca et al.6 transplanted a high ratio of Firmicutes/Bacteroides from the intestines of obese rats into sterile mice and successfully duplicated the obesity phenotype, a finding consistent with the hypothesis that intestinal flora may play an important role in obesity.
The glyoxylic acid cycle is a metabolic pathway in prokaryotes, lower eukaryotes, and plants, and it is essential for the energy metabolism of these organisms. Studies have shown that the glyoxylate cycle also exists in many opportunistic pathogens. These opportunistic pathogens use the host's fatty acid degradation products to synthesize substances needed for their growth and reproduction, split macrophages from the inside, and then expand in large quantities in the host.7 Fatty acids are decomposed into acetyl-CoA by beta-oxidation, which combines with oxaloacetate produced from two acetates through the glyoxylic cycle to form citric acid. Under the action of aconitase, citric acid produces isocitrate, which is broken by isocitrate lyase (ICL) to produce glyoxylic acid and succinic acid. Next, glyoxylic acid and acetyl-CoA produce malic acid under the catalysis of malic acid synthetase. The malic acid is dehydrogenated to oxaloacetic acid under the catalysis of malic acid dehydrogenase. The succinic acid produced in this cycle can be used to synthesize sugars and other cellular components, which means ICL is the critical enzyme in the glyoxylic acid cycle that may be involved in intestinal dysbacteriosis under high-fat diet.8
Hydrogen (H2) has antioxidant, anti-inflammatory, and anti-apoptotic properties and is the most abundant chemical element in the world.9 In 2007, Ohsawa et al.10 confirmed that inhaling 2% hydrogen could lead to selective scavenging of hydroxyl radicals (OH) and peroxynitrite anions (ONOO−), which significantly enhance cerebral ischemia-reperfusion injury in rats. Studies have confirmed that oxidative stress plays a vital role in the progression of metabolic syndrome.11 After long-term drinking of hydrogen-rich water by obese mice with type 2 diabetes, liver malondialdehyde level, fat content, blood sugar, and blood TG levels all decreased,12,13 suggesting that hydrogen could improve metabolic disorders of lipids and glucose, as well as the metabolic syndrome.
At present, the mechanism of saturated hydrogen action in metabolic diseases is not clear. This study investigated whether saturated hydrogen plays a therapeutic role in lipid metabolism disorders and dysbacteriosis.
Materials and methods
Animal grouping
Five-week-old C57BL6/J male mice weighing 16–20 g (purchased from Hunan Tianqin Biological Technology Co., Ltd) were acclimatized for a week during 12 h of a light/dark cycle at a constant temperature of 25°C and supplied with sterile water (once a day). Six healthy mice were randomly selected, executed, and their blood, small intestines, and feces collected. The remaining mice were then randomized into normal, saturated hydrogen (H2), high-fat (HF), and saturated hydrogen + high-fat (HF + H2) groups (n = 20 each). Normal and H2 group mice were fed with sterile water and low-fat control feed, while HF and H2 + HF group animals were given sterile water and 60% high-fat model feed (purchased from Nantong Trophy Feed Technology Co., Ltd).
Additionally, mice in the normal and HF groups were given 0.5 mL of saline intragastrically once a day, and those in the H2 and HF + H2 groups were given 0.5 mL of saturated hydrogen saline once a day. Saturated hydrogen saline was prepared at the Center of Modern Analysis and Detection of Central South University by dissolving molecular hydrogen in normal saline at high pressure (13.5 Mpa). The formulated saturated hydrogen solution was prepared once every week and stored in aluminum packaging to ensure the hydrogen concentration remained >0.6 mmol/L.
The weights of the mice were measured once a week and recorded. Six mice from each group were sacrificed in the 2nd, 4th, and 6th weeks after the start of the experiment. Blood, feces, and small intestines were collected for subsequent analyses. This study was conducted per the Declaration of Helsinki and approved by the Medical Ethical Committee of the Xiangya School of Medicine.
Monitoring body weight and TC, TG, LDL, and HDL concentrations in peripheral blood
Mice were weighed on days 0, 7, 14, 21, 28, 35, and 42, and the results were recorded appropriately. Blood samples were collected from veins using the eyeball extraction method; 35 µL of blood was collected from each mouse for blood lipid tests according to the specific operation steps of blood lipid tester and blood lipid test card (Aikang Biotechnology Co., Ltd, Hangzhou, China).
Determining the expression of ApoE, FAS, I-FAPB, ACC1, PPARγ, SCD1, ZO-1, and occludin in the small intestine using real-time quantitative PCR
Primers were designed using the Primer Premier 5.0 program (Table 1) and synthesized by Dingguochangsheng Biotechnology Co., Ltd, Beijing, China. Small intestine tissues were collected from each group of mice, and total RNA was extracted with TRizol. RNA purity was analyzed using a nucleic acid analyzer. cDNA was synthesized by reverse transcription and amplified using a qPCR kit (TansGen Biotech Technology Co., Ltd, Beijing, China) according to the manufacturer’s instructions. The reaction mixture consisted of 1.5 µL cDNA, 12.5 µL SYBR Green qPCR Master Mix, 1.5 µL each of the forward and reverse primers, and diethylpyrocarbonate (DEPC) water, totaling 25 µL. qPCR was performed at 94°C for 3 min, and 35 cycles of 94°C for 45 s, 51°C for 45 s, and 72°C for 45 s, and the relative mRNA expression was calculated using the 2-△△Ct method.14
Table 1.
Primers for quantitative polymerase chain reaction
| Gene | Sequence | Length(bp) |
|---|---|---|
| β-actin | Forward: GTGACGTTGACATCCGTAAAGA | 22 |
| Reverse: GTAACAGTCCGCCTAGAAGCAC | 22 | |
| ApoE | Forward: CAGACCTTTGGGAAGAGCGG | 20 |
| Reverse: CCAGTCTGCGGAGGGAAAAA | 20 | |
| FAS | Forward: GGCCCCTCTGTTAATTGGCT | 20 |
| Reverse: GGATCTCAGGGTTGGGGTTG | 20 | |
| I-FAPB | Forward: GGACTGGACCTCTGCTTTCC | 20 |
| Reverse: CACCGAGCTCAAACACAACA | 20 | |
| ACC1 | Forward: ATGTGGCCTGGGTAGATCCT | 20 |
| Reverse: CGGACAAGGTAAGCCCCAAT | 20 | |
| PPARγ | Forward: GACGTTTGTGGCTGGTCAAG | 20 |
| Reverse: CAGTGGGGAGAGAGGACAGA | 20 | |
| SCD1 | Forward: TGGAGACGGGAGTCACAAGA | 20 |
| Reverse: ACACCCCGATAGCAATATCCAG | 20 | |
| ZO-1 | Forward: CTGGTGAAGTCTCGGAAAAATG | 22 |
| Reverse: CATCTCTTGCTGCCAAACTATC | 22 | |
| occludin | Forward: TGCTTCATCGCTTCCTTAGTAA | 22 |
| Reverse: GGGTTCACTCCCATTATGTACA | 22 | |
| Bacteroides | Forward: TGACAGTGAGAGATTTGCTGCGTA | 24 |
| Reverse: TCAGCCGACATTTCCTCTTCCGA | 23 | |
| Bifidobacteria | Forward: GATTCTGGCTCAGGATGAACGC | 22 |
| Reverse: CTGATAGGACGCGACCCCAT | 20 | |
| Lactobacillus | Forward: AGCAGTAGGGAATCTTCCA | 19 |
| Reverse: CACCGCTACACATGGAG | 17 | |
| Enterobacter cloacaeRuminococcus | Forward: TATCCGTCGCCCATACAGA | 19 |
| Reverse: TTGATCCGATCCACCACCForward: GCACAAGCAGTGGAGTReverse: CTTCCTCCGTTTTGTCAA | 181618 | |
| 16S rRNA | Forward: CCTACGGGAGGCAGCAG | 17 |
| Reverse: ATTACCGCGGCTGCTGG | 17 |
Hematoxylin-eosin staining
After anesthetizing mice with ether, 2–3 cm of small intestine tissues were excised and rinsed with a 0.9% sodium chloride solution. The resected small intestine tissues were fixed with a 10% formaldehyde solution for 48 h, embedded in paraffin, and cut into 4 µm-thick sections. The tissue sections were then heated to 60°C for 2 h, dewaxed with xylene (twice, 15 min each), and dehydrated using an increasing alcohol gradient (75%, 95%, and 100%) for 5 min each. HE staining was performed, and the specimens were observed for pathological morphology under an optical microscope.
Immunofluorescence
Excised small intestine tissue sections were processed as described above and boiled with 0.01 M citrate buffer for antigen retrieval. The tissues were then washed twice with PBS (3 min each), fixed with 4% paraformaldehyde for 5 min, washed with PBS again (same as the first time), blocked with normal goat serum for 20 min, and incubated overnight with 5 µL of ZO-1 (bs-1329R-50, BEIJING Bioss Biological Technology Co., Ltd) or occludin (bs-10011R-50, BEIJING Bioss Biological Technology Co., Ltd) rabbit anti-mouse primary antibody in a wet box at 4°C.
On the following day, the tissue sections were washed thrice with PBS, incubated with a FITC-conjugated goat anti-rabbit secondary antibody (bs-0295-FITC, BEIJING Bioss Biological Technology Co., Ltd) at room temperature for 1 h, washed three times with PBS, and counterstained with DAPI (100 ng/mL) for 10 min. After removing moisture from the tissues, the slides were sealed with an anti-fluorescence quenching agent.
Determining the expression of bacteroides, bifidobacteria, lactobacillus, Enterobacter cloacae, and ruminococcus in feces using qPCR
Primers were designed using the Primer Premier 5.0 program (Table 1) and synthesized by Beijing Dingguochangsheng Biotechnology Co., Ltd. Feces were collected from the intestinal tract and the total DNA extracted according to the operation steps of the centrifugal, columnar, fecal genomic DNA extraction kit (purchased from Beijing Tiangen Biotechnology Co., Ltd). The extracted DNA was purified using a nucleic acid analyzer, and qPCR was performed as described above.
Determining the activity of ICL in feces
Fecal samples (0.1 g) were taken from each mouse for the determination of the activity of ICL according to the instruction of the ICL kit (JH01017, COMIN Biotechnology Co., Ltd, Suzhou, China).
Statistical analysis
The SPSS21.0 software was used for statistical analysis, and the data were expressed as mean ± standard deviation. The t-test was used for comparisons between two groups, variance analysis was used for comparisons among multiple groups, and the least significant difference (LSD) was used for comparisons between intra-group differences. P-value <0.05 was considered statistically significant.
Results
Saturated hydrogen inhibits the increase in body weights and improves dyslipidemia induced by a high-fat diet
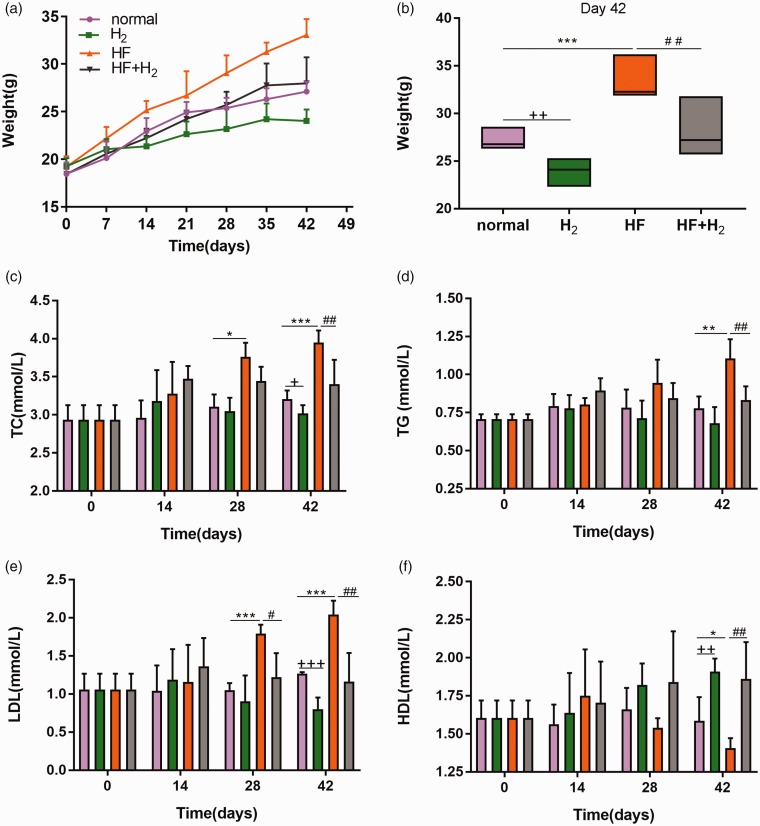
As shown in Figure 1(a), the average weight of mice in the HF + H2 group on the 42nd day was 34.05 ± 2.15 g, 20% higher than that of mice in the normal group, which was 27.45 ± 1.15 g, meaning that the average weight of mice in the HF + H2 group met the weight standard of obese mice. The average weights of mice in the H2 and HF + H2 groups decreased after treatment with saturated hydrogen when compared with those in the corresponding normal and HF groups, as shown in Figure 1(b), indicating that saturated hydrogen inhibited the increase in body weights of mice.
Figure 1.
Saturated hydrogen inhibits the increase in body weights and improves dyslipidemia induced by a high-fat diet (n = 6). (a) Changes of weight with time. (b) Weight of mice on day 42. (c–f) represented the changes of TC, TG, LDL and HDL in peripheral blood of mice with time (*P < 0.05, **P < 0.01 and ***P < 0.001 vs. normal; +P < 0.05, ++P < 0.01 and +++P < 0.001 vs. normal; #P < 0.05 and ##P < 0.01 vs. HF). (A color version of this figure is available in the online journal.)
On the 28th day, TC and LDL in the HF group increased significantly compared with those in the normal group, and LDL in the HF + H2 group decreased considerably compared with that in the HF group (Figure 1(c) and (e)). On the 42nd day, TC, TG, and LDL in the HF group increased markedly compared with those in the normal group, which decreased substantially after saturated hydrogen treatment (Figure 1(c) to (e)). However, HDL in the HF group decreased remarkably compared with that in the normal group, and it increased dramatically in the HF + H2 group compared with the HF group (Figure 1(f)), suggesting that saturated hydrogen reduces TC, TG, and LDL levels and increases the content of HDL in the peripheral blood of obese mice.
Saturated hydrogen improves abnormal small intestinal fatty acid metabolism induced by a high-fat diet
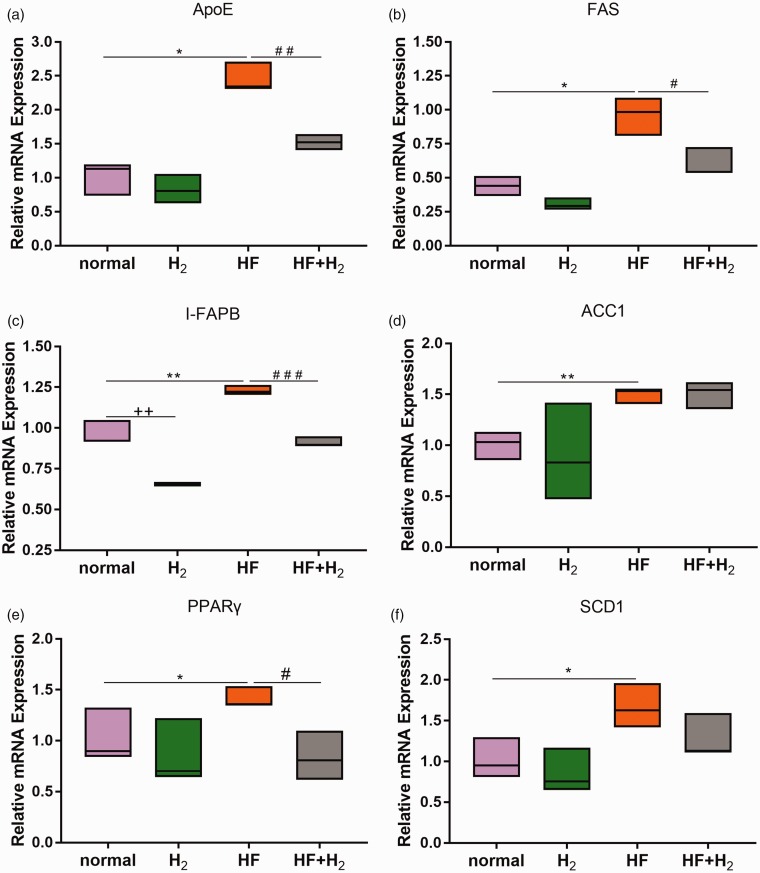
qPCR was used to determine the expression of fatty acid metabolism-related genes in the small intestinal tissues of mice on day 42. The results (Figure 2(a) to (e)) showed that the expression levels of ApoE, FAS, I-FAPB, ACC1, PPARγ, and SCD1 in the HF group were significantly higher than those in the normal group, while ApoE, FAS, I-FAPB, and PPARγ levels in the HF + H2 group were substantially lower than those in the HF group. We did not, however, find significant changes in ACC1 and SCD1 in the HF + H2 group compared with the HF group. The findings with considerable changes indicate that obese mice had abnormal fatty acid metabolism, which can be rectified using saturated hydrogen.
Figure 2.
Saturated hydrogen improves abnormal small intestinal fatty acid metabolism induced by a high-fat diet (n = 6). (a–f) showed the expression of ApoE, FAS, I-FAPB, ACC1, PPARγ, and SCD1 genes in small intestine tissues on day 42 using qPCR (*P < 0.05 and **P < 0.01 vs. normal; ++P < 0.01 vs. normal; #P < 0.05 and ##P < 0.01 vs. HF). (A color version of this figure is available in the online journal.)
Saturated hydrogen helps maintain the integrity of the small intestinal mucosa during a high-fat diet
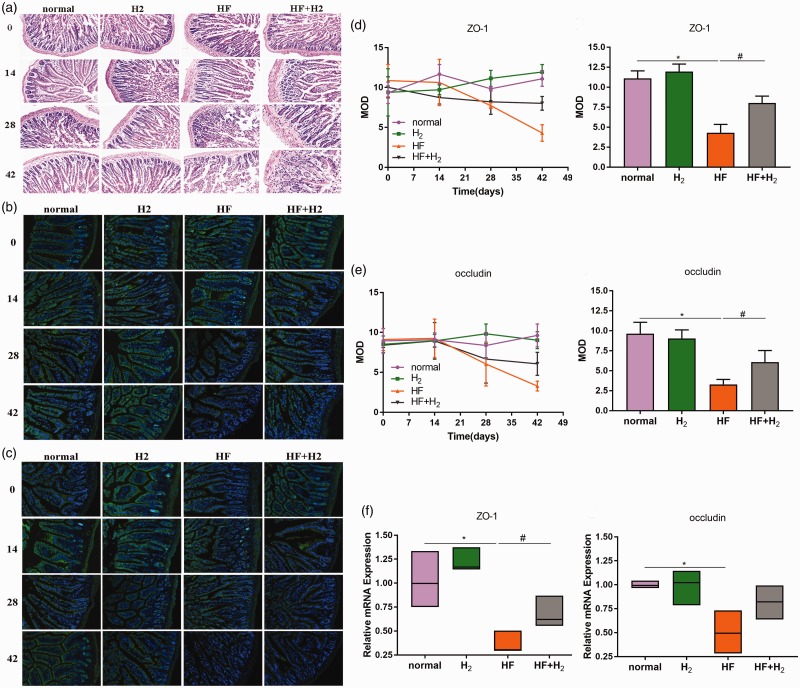
As shown in Figure 3(a), the small intestinal microvilli had slight exfoliation on day 28, which became more severe on day 42. Compared with the HF group, the degree of exfoliation of the HF + H2 group was much less severe.
Figure 3.
Saturated hydrogen helps maintain the integrity of the small intestinal mucosa during a high-fat diet (n = 6). (a) HE staining of small intestinal tissues. (b) Immunofluorescence of ZO-1 protein in small intestine tissues. (c) Immunofluorescence of occludin protein in small intestine tissues. (d) was the mean optical density (MOD) of ZO-1 protein and its statistical analysis on day 42. (e) was the MOD of occludin protein and its statistical analysis on the day 42. (f) showed the expression of ZO-1 and occludin mRNA in small intestine tissues on day 42 using qPCR (*P < 0.05 vs. normal; #P < 0.05 vs. HF). (A color version of this figure is available in the online journal.)
In the HF group, the expression of the intestinal tight junction protein ZO-1 (Figure 3(b) and (d)) and occludin (Figure 3(c) and (e)) decreased from day 28 to day 42 but increased in the HF + H2 group compared with the HF group. At the same time, the expression of ZO-1 and occludin genes in the small intestinal tract, as detected by qPCR, was consistent with the results from the immunofluorescence test (Figure 3(f)). These results suggest that saturated hydrogen is beneficial because it maintains or recovers the expression of ZO-1 and occludin in the small intestine, thus maintaining intestinal integrity.
Saturated hydrogen improves dysbacteriosis induced by a high-fat diet
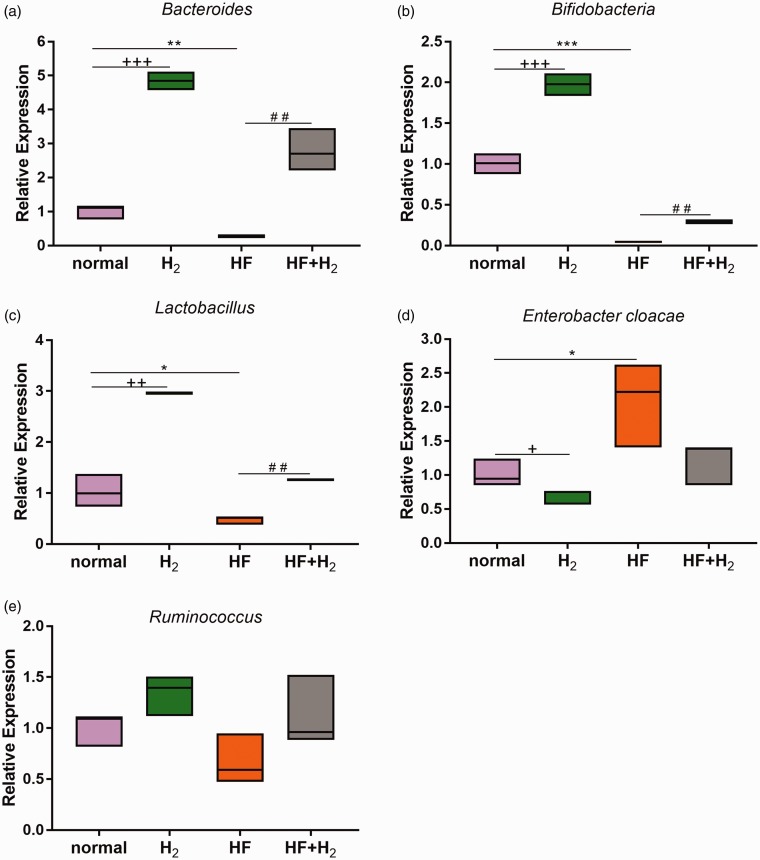
The qPCR findings (Figure 4(a) to (d)), Bacteroides, Bif idobacteria, and Lactobacillus decreased significantly in the HF group, while Enterobacter cloacae increased when compared with the normal group. Compared with the HF group, Bacteroides, Bif idobacteria, and Lactobacillus increased substantially, while Enterobacter cloacae decreased in the HF + H2 group. There was no significant difference in Ruminococcus levels between all four groups (Figure 4(e)). These results indicate that saturated hydrogen promoted the growth of Bacteroides, Bif idobacteria, Lactobacillus and inhibited Enterobacter cloacae in the intestines of both normal and obese mice.
Figure 4.
Saturated hydrogen improves dysbacteriosis induced by a high-fat diet (n = 6). (a–e) were the changes of Bacteroides, Bifidobacteria, Lactobacillus, Enterobacter cloacae, and Ruminococcus gene expression in feces of mice on day 42 using qPCR (*P < 0.05, **P < 0.01 and ***P < 0.001 vs. normal; +P < 0.05, ++P < 0.01 and +++P < 0.001 vs. normal; ##P < 0.01 vs. HF). (A color version of this figure is available in the online journal.)
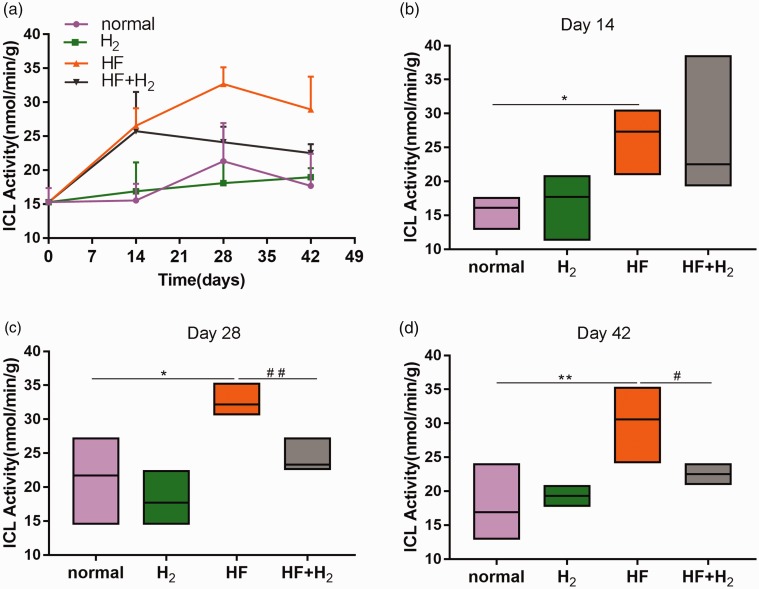
The changes in intestinal flora were significantly related to the activity of ICL
The activity of ICL in the HF and HF + H2 groups was considerably higher than that in the normal and H2 groups from the 14th day (Figure 5(a) and (b)). The activity of ICL in the HF group continued to increase on the 28th day and was higher than that in the HF + H2 group, becoming more apparent on the 42nd day (Figure 5(a) to (d)). These results indicate that saturated hydrogen could inhibit the activity of ICL induced by a high-fat diet. Also, the change in ICL activity in feces was consistent with the number of Bacteroides, Bif idobacteria, and Lactobacillus, and correlated negatively with the number of Enterobacter cloacae.
Figure 5.
The changes in intestinal flora were significantly related to the activity of ICL (n = 6). (a) The change of ICL activities in feces with time. (b–d) represented the ICL activities on days 14, 28, and 42 (*P < 0.05 and **P < 0.01 vs. normal; #P < 0.05 and ##P < 0.01 vs. HF). (A color version of this figure is available in the online journal.)
Discussion
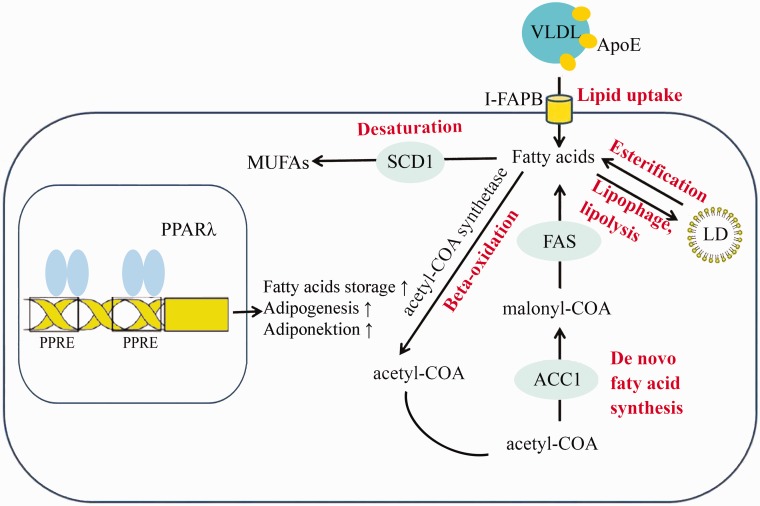
This study revealed that the levels of TC, TG, and LDL in the peripheral blood of mice fed with a high-fat diet were significantly higher than those in normal mice, while the levels of HDL were substantially lower than those in normal mice. The levels of lipid metabolism-related genes ApoE, FAS, I-FAPB2, ACC1, PPARγ, and SCD1 in the small intestinal tissues of mice fed with a high-fat diet, whose functions are shown in Figure 6, were considerably higher than those in mice fed with a normal diet. Moreover, the expression of the intestinal tight junction protein ZO-1 and occludin decreased from day 28 to day 42 after a high-fat diet. We also found that the dysbacteriosis was induced by a high-fat diet and the change in ICL activity in feces was consistent with the number of Bacteroides, Bif idobacteria, and Lactobacillus, and correlated negatively with the number of Enterobacter cloacae.
Figure 6.
A schematic diagram of lipid metabolism-related genes detected in the small intestine tissues. HF promotes the expression of ApoE, FAS, I-FAPB, ACC1, PPARγ, and SCD1. Saturated hydrogen inhibits the expression of ApoE, I-FAPB, FAS, and PPARγ induced by a high-fat diet. (A color version of this figure is available in the online journal.)
Apolipoprotein (Apo) is a lipoprotein that transports blood lipids to various tissues for metabolism and utilization. Among them, Apo E is the ligand of the LDL-c receptor and is mainly involved in the transport and uptake of LDL-c.15 I-FAPB is a member of the cytoplasmic fatty acid-binding protein family; it helps in the process of fatty acid desorption from the plasma membrane to the cytoplasm and exists solely in the small intestine where it is involved in the absorption of fatty acids from diets. In addition to regulating the intake of fatty acids and intracellular transport, I-FAPB can also control the level of free fatty acids in cells.16–18 FAS, as a key enzyme for fatty acid synthesis, is a rate-limiting enzyme for fatty acid regeneration. The expression of FAS gene in the human adipose tissue is related to obesity and type 2 diabetes mellitus and can lead to fat deposition in the body when overexpressed.19,20ACC is a biotin enzyme that catalyzes the reaction of Acetyl CoA + ATP + HCO3−→Malonyl CoA + ADP + Pi. During the synthesis of fatty acids, Malonyl-CoA adds 2-carbon units to the extended chain of fatty acids, which plays a vital role in the extension stage of fatty acid synthesis.21 Peroxisome proliferator-activated receptors (PPAR)γ is a nuclear transcription factor that can be activated by fatty acids and exogenous peroxisome proliferators. Upon activation, PPARγ induces the expression of apolipoprotein and lipoprotein lipase, thus regulating fat metabolism, inflammation, immunity, and cell differentiation. PPARγ and its ligands are closely related to the inflammatory responses induced by obesity.22–25 SCD is a rate-limiting enzyme for the synthesis of monounsaturated fatty acids and a key control point for lipid formation and lipid oxidation. A change in SCD activity leads to abnormal lipid metabolism and various diseases related to lipid metabolism.26
Our research showed that saturated hydrogen inhibited the increase in ApoE, FAS, I-FAPB, and PPARγ induced by a high-fat diet but had no significant influence on ACC1 and SCD1, indicating that lipid metabolism disorder appeared in mice fed with a high-fat diet, and hydrogen could alleviate the disease by inhibiting lipid transport, lipid uptake, lipid oxidation, fatty acid synthesis, and more.
We also found in this investigation that the number of Lactobacillus, Bif idobacteria, and Bacteroides in the intestinal tract of the HF group mice decreased significantly, while the number of Enterobacter cloacae increased. Lactobacillus and Bif idobacteria are probiotics in the intestinal tract, maintaining the microecological balance and protecting the normal physiological function of the intestinal tract. Enterobacter cloacae is an opportunistic pathogen in the intestinal tract, which produces endotoxins. Studies have shown that the colonization of Enterobacter cloacae into sterile mice causes severe obesity and insulin resistance.27 Changes in intestinal flora may result in the decreased expression of intestinal tight-linking proteins ZO-1 and occludin, leading to increased intestinal permeability and decreased intestinal mucosal integrity. Moreover, intestinal bacterial metabolites, such as endotoxin, can enter the blood circulation, trigger inflammation, and lead to obesity.5,28 After saturated hydrogen treatment, the number of Lactobacillus, Bif idobacteria, and Bacteroides increased, the number of Enterobacter cloacae decreased, and the expression of ZO-1 and occludin proteins increased, suggesting that saturated hydrogen plays a protective role in the intestinal tract by promoting benign intestinal bacteria and reducing the growth of opportunistic pathogenic bacteria.
The activity of ICL in the intestinal tract of the HF group mice increased gradually with the increase in body weights but was inhibited significantly by saturated hydrogen. The expression and activity of ICL directly affect the formation of related products through the glyoxylic acid cycle, thus inducing the growth and reproduction of intestinal opportunistic pathogenic bacteria.29 Therefore, we speculate that saturated hydrogen influences the glyoxylic acid circulation of intestinal microorganisms through its antioxidant properties, thus playing a role in regulating intestinal flora. However, further in vitro and in vivo studies are needed to validate our findings.
Authors’ contributions
QX, YQ, SM and WL carried out the study. QX performed the statistical analysis. TY and WG participated in the design of the study. All authors read and approved the submission.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Grants 31670121 and 31771277 from National Natural Science Foundation of China.
ORCID iD
Yurong Tan https://orcid.org/0000-0002-0283-2105
References
- 1.Wang N, Zhao TT, Li SM, Sun X, Li ZC, Li YH, Li DS, Wang WF. Fibroblast growth factor 21 exerts its anti-inflammatory effects on multiple cell types of adipose tissue in obesity. Obesity 2019; 27:399–408 [DOI] [PubMed] [Google Scholar]
- 2.Moore WE, Holdeman LV. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol 1974; 27:961–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xing YW, Lei GT, Wu QH, Jiang Y, Huang MX. Procyanidin B2 protects against diet-induced obesity and non-alcoholic fatty liver disease via the modulation of the gut microbiota in rabbits. World J Gastroenterol 2019; 25:955–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo Y, Huang ZP, Liu CQ, Qi L, Sheng Y, Zou DJ. Modulation of the gut microbiome: a systematic review of the effect of bariatric surgery. Eur J Endocrinol 2018; 178:43–56 [DOI] [PubMed] [Google Scholar]
- 5.Shen J, Obin MS, Zhao L. The gut microbiota, obesity and insulin resistance. Mol Aspects Med 2013; 34:39–58 [DOI] [PubMed] [Google Scholar]
- 6.Duca FA, Sakar Y, Lepage P, Devime F, Langelier B, Doré JF, Covasa M. Replication of obesity and associated signaling pathways through transfer of microbiota from obese-prone rats. Diabetes 2014; 63:1624–36 [DOI] [PubMed] [Google Scholar]
- 7.Timm J, Post FA, Bekker LG, Walther GB, Wainwright HC, Manganelli R, Chan WT, Tsenova L, Gold B, Smith I, Kaplan G, McKinney JD. Differential expression of iron-, carbon-, and oxygen-responsive mycobacterial genes in the lungs of chronically infected mice and tuberculosis patients. Proc Natl Acad Sci U S A 2003; 100:14321–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKinney JD, Höner zu Bentrup K, Muñoz-Elías EJ, Miczak A, Chen B, Chan WT, Swenson D, Sacchettini JC, Jacobs WR, Jr, Russell DG. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 2000; 406:735–8 [DOI] [PubMed] [Google Scholar]
- 9.Shi QH, Wei W, Ran JH, Wang SY, Liu ZX, Ge D, Chen P, Fu JF. Hydrogen therapy reduces oxidative stress-associated risks following acute and chronic exposure to high-altitude environment. Biomed Environ Sci 2015; 28:239–41 [DOI] [PubMed] [Google Scholar]
- 10.Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med 2007; 13:688–94 [DOI] [PubMed] [Google Scholar]
- 11.Yara S, Lavoie JC, Levy E. Oxidative stress and DNA methylation regulation in the metabolic syndrome. Epigenomics 2015; 7:283–300 [DOI] [PubMed] [Google Scholar]
- 12.Kamimura N, Nishimaki K, Ohsawa I, Ohta S. Molecular hydrogen improves obesity and diabetes by inducing hepatic FGF21 and stimulating energy metabolism in db/db mice. Obesity 2011; 19:1396–403 [DOI] [PubMed] [Google Scholar]
- 13.Song G, Lin Q, Zhao H, Liu M, Ye F, Sun Y, Yu Y, Guo S, Jiao P, Wu Y, Ding G, Xiao Q, Qin S. Hydrogen activates ATP-binding cassette transporter A1-dependent efflux ex vivo and improves high-density lipoprotein function in patients with hypercholesterolemia: a double-blinded, randomized, and placebo-controlled trial. J Clin Endocrinol Metab 2015; 100:2724–33 [DOI] [PubMed] [Google Scholar]
- 14.Qin L, Qiu K, Hu C, Wang L, Wu G, Tan Y. Respiratory syncytial virus promoted the differentiation of Th17 cells in airway microenvironment through activation of notch-1/Delta3. J Med Microbiol 2019; 68:649–56 [DOI] [PubMed] [Google Scholar]
- 15.Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjærg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med 2014; 371:32–41 [DOI] [PubMed] [Google Scholar]
- 16.Storch J, Thumser AE. The fatty acid transport function of fatty acid-binding proteins. Biochim Biophys Acta 2000; 1486:28–44 [DOI] [PubMed] [Google Scholar]
- 17.Hsu KT, Storch J. Fatty acid transfer from liver and intestinal fatty acid-binding proteins to membranes occurs by different mechanisms. J Biol Chem 1996; 271:13317–23 [DOI] [PubMed] [Google Scholar]
- 18.Alpers DH, Bass NM, Engle MJ, DeSchryver-Kecskemeti K. Intestinal fatty acid binding protein may favor differential apical fatty acid binding in the intestine. Biochim Biophys Acta 2000; 1483:352–62 [DOI] [PubMed] [Google Scholar]
- 19.Roy R, Gautier M, Hayes H, Laurent P, Osta R, Zaragoza P, Eggen A, Rodellar C. Assignment of the fatty acid synthase (FASN) gene to bovine chromosome 19 (19q22) by in situ hybridization and confirmation by somatic cell hybrid mapping. Cytogenet Cell Genet 2001; 93:141–2 [DOI] [PubMed] [Google Scholar]
- 20.Berndt J, Kovacs P, Ruschke K, Klöting N, Fasshauer M, Schön MR, Körner A, Stumvoll M, Blüher M. Fatty acid synthase gene expression in human adipose tissue: association with obesity and type 2 diabetes. Diabetologia 2007; 50:1472–80 [DOI] [PubMed] [Google Scholar]
- 21.Tong L. Acetyl-coenzyme a carboxylase: crucial metabolic enzyme and attractive target for drug discovery. Cell Mol Life Sci 2005; 62:1784–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM. PPARγ signaling and metabolism: the good, the bad and the future. Nat Med 2013; 19:557–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang B, Yuan HD, Kim DY, Quan HY, Chung SH. Cinnamaldehyde prevents adipocyte differentiation and adipogenesis via regulation of peroxisome proliferator-activated receptor-γ (PPARγ) and AMP-activated protein kinase (AMPK) pathways. J Agric Food Chem 2011; 59:3666–73 [DOI] [PubMed] [Google Scholar]
- 24.Gross B, Pawlak M, Lefebvre P, Staels B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat Rev Endocrinol 2017; 13:36–49 [DOI] [PubMed] [Google Scholar]
- 25.Rosen ED, Spiegelman BM. PPARgamma: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem 2001; 276:37731–4 [DOI] [PubMed] [Google Scholar]
- 26.Wong BX, Kyle RA, Myhill PC, Croft KD, Quinn CM, Jessup W, Yeap BB. Dyslipidemic diabetic serum increases lipid accumulation and expression of stearoyl-CoA desaturase in human macrophages. Lipids 2011; 46:931–41 [DOI] [PubMed] [Google Scholar]
- 27.Fei N, Zhao L. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J 2013; 7:880–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des 2009; 15:1546–58 [DOI] [PubMed] [Google Scholar]
- 29.Serrano JA, Bonete MJ. Bonete, sequencing, phylogenetic and transcriptional analysis of the glyoxylate bypass operon (ace) in the halophilic archaeon Haloferax volcanii. Biochim Biophys Acta 2001; 1520:154–62 [DOI] [PubMed] [Google Scholar]