Short abstract
It would be of great clinical value to find an indicator that can accurately evaluate the early-stage renal injury in primary hypertension. Previous findings have shown renalase not only plays an important role in hypertension but also closely correlates with kidney function. The purpose of this study is to investigate whether urinary renalase could be used as a predictive index of early-stage renal damage in patients with primary hypertension. Urinary albumin to creatinine ratio (UACR) was used to divide subjects with primary hypertension into two groups: a no renal damage (NRD) group (UACR <30 mg/g) and an early-stage renal damage (RD) group (UACR >30 mg/g). Subjects with normal examination results were randomly included in a healthy control (HC) group. Urinary renalase was determined through an enzyme-linked immunosorbent assay (ELISA). Urinary renalase continued to reduce among the HC (n = 81), NRD (n = 84) and RD group (n = 80), while systolic blood pressure (SBP) increased. Urinary renalase was negatively correlated with SBP in all the groups. Among the subjects with stage 1 primary hypertension, urinary renalase in the RD group was lower than the NRD group, while the UACR was higher, and urinary renalase was negatively correlated with the UACR. A multiple linear stepwise regression analysis showed that there was a linear regression relationship between the increase of the UACR and urinary renalase, heart rate (HR), SBP and serum creatinine. In addition, the standardized partial regression coefficient of urinary renalase was the highest. The performance of urinary renalase as a marker for the diagnosis of early-stage renal damage in patients with primary hypertension was 0.968 with a cut off value of 2.01 µg/ml. Taken together, urinary renalase was further decreased in patients with early-stage renal damage and primary hypertension, and consequently, it could be used as a predictive index.
Impact statement
In patients with early-stage kidney damage of primary hypertension, there are no obvious structural or functional changes, which leads to a high level of diagnostic omissions. Therefore, it would be of great clinical value to find an indicator that can accurately evaluate the early-stage renal injury in primary hypertension. Urinary albumin to creatinine ratio (UACR) is a classic indicator used in early-stage renal damage, but it is affected by many factors. Renalase, a protein discovered by Xu in 2005, not only plays an important role in hypertension but also closely correlates with kidney function. In our study, we found that urinary renalase was further decreased in patients with early-stage renal damage in primary hypertension, and it could be used as a predictive index. This finding could help to diagnose the early-stage renal damage in primary hypertension much earlier and improve the prognosis of these patients.
Keywords: Urinary renalase, primary hypertension, early-stage renal damage, urinary albumin to creatinine ratio, biomarker, predictive index
Introduction
Primary hypertension, a global public health problem and one of the most common clinical cardiovascular diseases, often causes damages to the heart, brain, kidney and other important target organs.1 In the early-stage kidney injury of primary hypertension, patients often do not have any obvious clinical manifestations. Once they develop symptoms, the kidney injury has usually entered into the middle or late stages. Research has demonstrated that the number of end-stage renal disease (ESRD) patients caused by primary hypertension has been increasing year by year.2,3 American data indicates that of all the ESRD causes, primary hypertension accounts for 24%,4 and dialysis registration data from Beijing suggests that it is the third biggest cause of ESRD. Therefore, early-stage detection and the accurate assessment of kidney injury in patients with primary hypertension are of great significance. Urinary albumin to creatinine ratio (UACR) is an important observation index in early-stage renal damage, but it can be easily affected by many factors, such as position, motion, diet, blood pressure (BP) and so on.5,6 Therefore, we need to explore a more accurate indicator.
Renalase, a protein discovered by Xu in 2005, is expressed within the kidney, myocardium, skeletal muscle, liver and so on.7,8 The majority of renalase is synthesized by renal tubular epithelial cells.9,10 Renalase is considered as an amine oxidase dependent on flavin adenine dinucleotide (FAD), and it can reduce BP through degrading catecholamines.7,11–14 Our team has proven that renalase gene polymorphism plays an important role in patients with hypertension and concomitant coronary heart diseases,15 and renalase protects the cardiomyocytes of Sprague-Dawley rats against ischemia and reperfusion injury by reducing myocardial cell necrosis and apoptosis.16 Other studies have shown that whether in acute kidney injury models (induced by 5/6 kidney resection, cisplatin or contrast agent intervention) or in acute kidney ischemia reperfusion models, plasma renalase levels are markedly lower than the control groups.17–21 Clinical studies have suggested that plasma renalase levels in end-stage renal disease (ESRD) patients are much lower than those with normal renal function.22–24
Therefore, renalase levels are not only related to BP but are also affected by renal function. Plasma renalase levels are lower in patients with hypertension than those who are normotensive, but the levels of urinary renalase in early-stage renal damage of primary hypertension remain unknown. Therefore, the target of this study is to observe the urinary renalase of these patients and to explore whether it could be used as a predictive index for early-stage renal damage in patients with primary hypertension.
Materials and methods
Study cohort
Subjects who were diagnosed as primary hypertension in both the outpatient and inpatient departments of cardiology in the Third Xiangya Hospital of Central South University from September 2017 to January 2018 were included at random. The inclusion criteria were as follows: (1) primary hypertension that met the diagnostic criteria of the 2010 guidelines of Hypertension Prevention and Treatment in China; (2) normal plasma creatinine and urea nitrogen and a negative urine protein test in the urine routine. The exclusion criteria were as follows: (1) acute or chronic kidney diseases; (2) acute or chronic infectious diseases; (3) fever; (4) secondary hypertension; (5) connective tissue or other autoimmune diseases; (6) malignant diseases, such as tumors.
There were 164 subjects with primary hypertension who were divided into two groups: a no renal damage (NRD) group (UACR <30 mg/g) and an early-stage renal damage (RD) group (UACR >30 mg/g). There were 84 subjects in the NRD group (44 males and 40 females) and 80 subjects in the RD group (42 males and 38 females). In addition, 81 healthy control (HC) subjects (43 males and 38 females) with normal physical and laboratory examination results were randomly included from the health management center of the Third Xiangya Hospital of Central South University.
Well-trained staff interviewed the participants and collected their data on demographics (age, gender, education, occupation, and physical activity), smoking history (if a patient had a history of smoking but had quit for more than one year this was recorded as a non-smoking history), family history of hypertension, hypertension course and so on. Standard measurement methods were used to measure height, weight and body mass index (BMI = weight (kg)/[height (m)]2). Physical examinations were also conducted.
This study was approved by the Institutional Review Board of the Third Xiangya Hospital of Central South University (No.: 2019-S544) and all the selected subjects signed the informed consent.
BP measurement
BP was measured by trained staff to obtain three readings using a standard mercury sphygmomanometer and defined as the mean value. The method was as follows: the subjects were instructed to rest for more than 5 min, and their right brachial pressure was measured while they were in a sitting position. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were determined as the first and fifth Korotkoff sounds, respectively. Pulse pressure was considered as the difference between the SBP and DBP. Subjects manifesting a SBP≧140 mmHg and/or DBP≧90 mmHg or using anti-hypertensive medications were defined as hypertensive.25
Biochemical analyses
Venous blood samples from all the subjects were collected to detect their biochemical indexes, such as blood glucose, creatinine and urea nitrogen with automatic biochemical analyzers (model 7600 and 7100, Hitachi, Ltd, Tokyo, Japan). At the same time, urine samples (midstream urine) were randomly collected to test urinary microalbumin, urinary creatinine and urinary renalase levels. The UACR was then calculated. Urinary microalbumin was determined by rate scattering turbidimetry using a kit (Yikang Biotechnology Co., Ltd, Hangzhou, China). Urinary creatinine and urinary sugar levels were detected by enzyme endpoint colorimetry with a kit (Yikang Biotechnology Co., Ltd, Hangzhou, China). Urinary renalase was assessed using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (SEC845Hu, USCN Business Co., Ltd, Wuhan, China), and all of the operations were in accordance with the manufacturer’s instructions.
Statistical analyses
Data were expressed as means ± standard deviation (SD) for normally distributed values, and as median (25th and 75th percentile) for non-normally distributed values. Student’s t tests for unpaired two samples were performed for normally distributed variables, while analysis of variance was conducted for more than two samples of data. Mann–Whitney U tests were conducted for non-normally distributed parameters. Chi-square tests were carried out to compare categorical variables. When residuals were normally distributed, Pearson’s correlation coefficients were used to determine correlations between the parameters. Otherwise, Spearman’s correlation coefficients were chosen. Multiple linear stepwise regression analysis was also conducted to determine the influence factors for the rise of UACR. A receiver operating characteristic (ROC) curve was performed by plotting the sensitivity and specificity of the urinary renalase values according to Youden’s index. Statistical analyses were performed with SPSS version 19.0 for Windows (SPSS Inc., Chicago, IL, USA). A two-tailed p < 0.05 was considered significant.
Results
Basic data of the subjects in three groups
Table 1 demonstrated that there were no statistically significant differences in the number of subjects, gender, age, hypertension course, smoking, family history of hypertension, creatinine, urea nitrogen and blood glucose among the three groups (p > 0.05). However, the BMIs of the NRD group and the RD group were higher than the HC group (p < 0.05).
Table 1.
Demographic and clinical characteristics for all the groups’ subjects: the healthy control (HC) group, the primary hypertension without renal damage (NRD) group and the primary hypertension with renal damage (RD) group.
| Parameters | HC (n = 81) | NRD (n = 84) | RD (n = 80) |
|---|---|---|---|
| Sex (M/F) | 43/38 | 44/40 | 42/38 |
| Age (year) | 56.29 ± 11.10 | 57.01 ± 10.85 | 57.89 ± 13.16 |
| Hypertension course (year) | – | 11.38 ± 8.75 | 12.61 ± 9.55 |
| Smoking (%) | 30.9% | 32.1% | 31.5% |
| Family history of hypertension (%) | 34.6% | 35.7% | 35.2% |
| BMI (kg/m2) | 23.35 ± 2.65 | 24.59 ± 2.94* | 25.54 ± 4.45* |
| Creatinine (μmol/l) | 67.85 ± 12.81 | 67.87 ± 11.81 | 70.10 ± 13.38 |
| Urea nitrogen (mmol/l) | 4.41 ± 1.05 | 4.38 ± 1.01 | 4.71 ± 1.14 |
| Blood glucose (mmol/l) | 5.07 ± 0.85 | 4.98 ± 0.87 | 5.12 ± 0.78 |
Non-normally distributed variables are expressed as the median (interquartile range). All of the values are expressed as means ± standard deviation (SD) or n, %.
HC: healthy control; NRD: primary hypertension without renal damage; RD: primary hypertension with renal damage; BMI: body mass index, BMI = weight (kg)/[height (m)]2.
*p < 0.05, vs. the HC group.
Comparisons of urinary renalase, SBP and UACR among the three groups
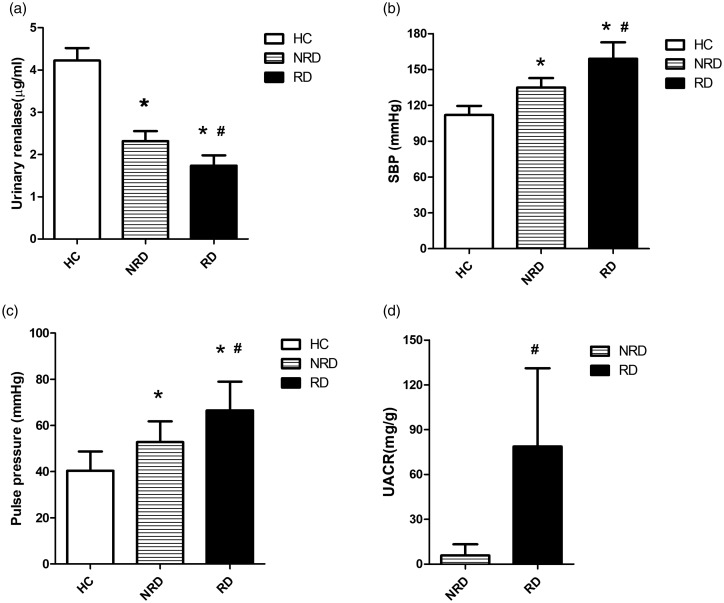
From the HC group to the NRD group to the RD group, urinary renalase continuously decreased (4.22 ± 0.29 vs. 2.32 ± 0.24 vs. 1.74 ± 0.25) µg/ml (p < 0.05, Figure 1(a)), and SBP continuously increased (111.90 ± 7.58 vs. 134.83 ± 7.94 vs. 159.03 ± 13.71) mmHg (p < 0.05, Figure 1(b)), as did pulse pressure (40.40 ± 8.34 vs. 52.81 ± 9.00 vs. 66.50 ±12.48) mmHg (p < 0.05, Figure 1(c)). The UACR was higher in the RD group than in the NRD group (p < 0.05, Figure 1(d)). In addition, urinary renalase was negatively correlated with SBP in the HC group (r = −0.589, p < 0.001), the NRD group (r = −0.523, p < 0.001) and the RD group (r = −0.337, p = 0.002), but there was no correlation between urinary renalase and pulse pressure (p > 0.05).
Figure 1.
Comparisons of urinary renalase, systolic blood pressure (SBP), pulse pressure and the urinary albumin to creatinine ratio (UACR) among the three groups. From the HC group to the NRD group to the RD group, urinary renalase continuously decreased (a), while SBP and pulse pressure continuously increased (b, c). The UACR also increased from the NRD group to the RD group (d). HC: healthy control; NRD: primary hypertension without renal damage; RD: primary hypertension with renal damage. *p < 0.05, vs. the HC group; #p < 0.05, vs. the NRD group.
The results of the subjects with stage 1 primary hypertension
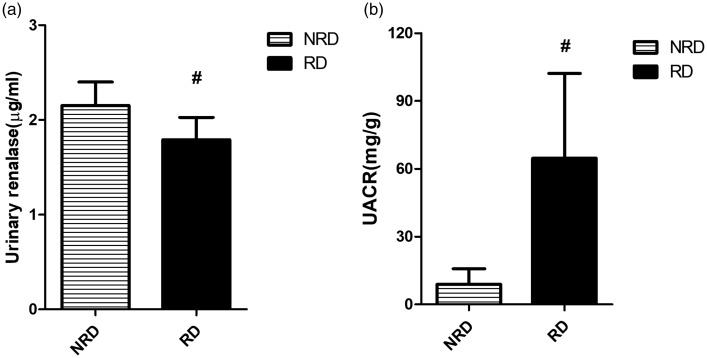
As shown in Table 2, the differences in gender, age, SBP and pulse pressure among the subjects with stage 1 primary hypertension were not significant (p > 0.05). However, urinary renalase in the RD group was still lower than the NRD group (1.79 ± 0.24 vs. 2.17 ± 0.25) µg/ml (p < 0.05, Figure 2(a)), while UACR was higher (64.68 ± 37.48 vs. 8.17 ± 7.14) mg/g (p < 0.05, Figure 2(b)). Urinary renalase was negatively associated with the UACR in both the NRD group (r = −0.365, p < 0.001) and the RD group (r = −0.778, p < 0.001).
Table 2.
Clinical characteristics of subjects with stage 1 primary hypertension from the primary hypertension without renal damage (NRD) group and the primary hypertension with renal damage (RD) group.
| Parameters |
Subjects with stage 1 primary hypertension |
|
|---|---|---|
| NRD (n=35) | RD (n=39) | |
| Urinary renalase (μg/ml) | 2.17 ± 0.25 | 1.79 ± 0.24a |
| UACR (mg/g) | 8.17 ± 7.14 | 64.68 ± 37.48a |
| SBP (mmHg) | 147.71 ± 4.32 | 151.54 ± 5.24 |
| Pulse pressure (mmHg) | 59.51 ± 9.56 | 62.56 ± 9.53 |
| Sex (M/F) | 19/16 | 22/17 |
| Age (year) | 58.14 ± 11.32 | 56.67 ± 12.95 |
Non-normally distributed variables are expressed as the median (interquartile range). All of the values are expressed as mean ± standard deviation (SD) or n, %.
NRD: primary hypertension without renal damage; RD: primary hypertension with renal damage; UACR: urinary albumin to creatinine ratio; SBP: systolic blood pressure.
ap < 0.05, vs. the NRD group.
Figure 2.
Comparisons of urinary renalase and the urinary albumin to creatinine ratio (UACR) between the NRD group and the RD group. Urinary renalase decreased from the NRD group to the RD group (a), while the UACR increased (b). NRD: primary hypertension without renal damage; RD: primary hypertension with renal damage. #p < 0.05, vs. the NRD group.
Multiple linear stepwise regression analysis
In the RD group, the UACR was taken as the dependent variable and urinary renalase, age, SBP, DBP, creatinine, BMI, pulse pressure and so on were used as independent variables to conduct a multiple linear stepwise regression analysis (α in = 0.05, α out = 0.10): Ŷ = 34.715–109.794X1+0.466X2 + 1.251X3+0.794X4 [X1 was urinary renalase, X2 was SBP, X3 was heart rate (HR), X4 was creatinine; p < 0.001]. The determination coefficient (R2) of the regression equation was 0.794 and Table 3 showed that the standardized partial regression coefficient of urinary renalase was the greatest.
Table 3.
Multiple linear stepwise regression analysis.
| Model | Non-standardized coefficients β | Standardized coefficients β | Standard error | T-value | p-value |
|---|---|---|---|---|---|
| Constant | 34.715 | – | 63.770 | 0.544 | 0.588 |
| Urinary renalase (μg/ml) | −109.794 | −0.515 | 16.537 | −6.639 | 0.000* |
| SBP (mmHg) | 0.466 | 0.122 | 0.220 | 2.113 | 0.038* |
| HR (bmp) | 1.251 | 0.236 | 0.357 | 3.508 | 0.001* |
| Creatinine (μmol/l) | 0.794 | 0.202 | 0.293 | 2.705 | 0.008* |
SBP: systolic blood pressure; HR: heart rate.
*p < 0.05.
ROC curve for urinary renalase to diagnose the early-stage renal damage in primary hypertension
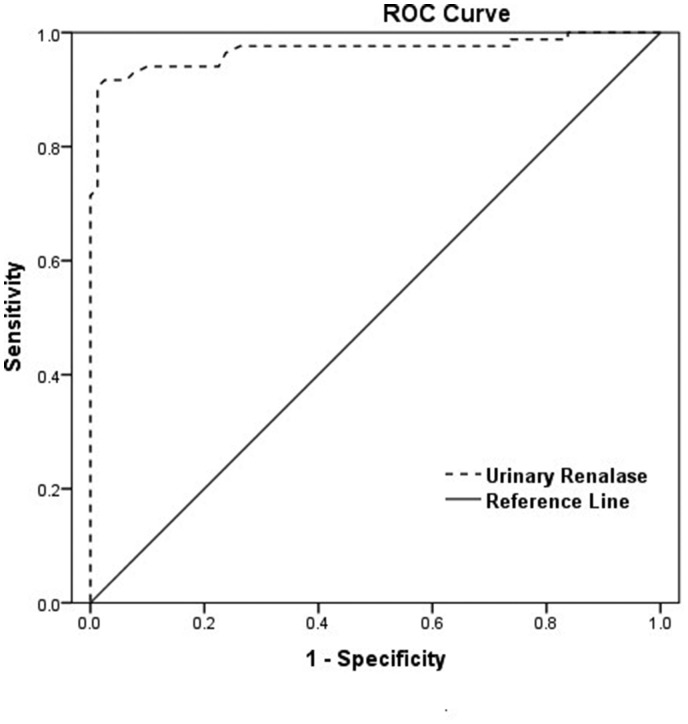
A ROC curve was plotted to determine the diagnostic performance of urinary renalase as a bio-marker for early-stage renal damage in subjects with primary hypertension (Figure 3). The area under the curve (AUC) of urinary renalase was 0.968 (95% CI: 0.940–0.997; p < 0.001). When the cut off value was set at 2.01 µg/ml, the sensitivity for early-stage renal damage in primary hypertension was 90.50% and the specificity was 98.80%.
Figure 3.
A receiver operator characteristic (ROC) curve was performed to assess urinary renalase as a predictive marker for early-stage renal damage in subjects with primary hypertension. The area under the curve (AUC) was 0.968 (95% CI: 0.940–0.997; p < 0.001), while sensitivity and specificity were 90.50% and 98.80%, respectively, using a cut off of 2.01 μg/ml.
Discussion
In the early-stage kidney damage of patients with primary hypertension, there are no obvious structural or functional changes, which leads to a high rate of diagnostic omissions. After the occurrence of renal injury in patients with primary hypertension, the BP will further rise. This continual increase in BP aggravates the renal damage and thus forms a vicious cycle.2 Therefore, it would be of great clinical value to look for an indicator that can accurately evaluate the early-stage renal injury in patients with primary hypertension. The UACR is a classic indicator used for early-stage renal damage, but it is affected by many factors and the result is unreliable.5,6,26,27 Therefore, researchers have been attempting to seek a more effective indicator. In this cohort of Chinese adults, we found that urinary renalase was further decreased in patients with early-stage renal damage and primary hypertension, and it could be used as a predictive index.
Previous studies have shown that after injecting intravenously recombinant renalase into rats, the rats’ BP, HR and myocardial contractility display a dose-dependent decrease.14 In addition, studies about renalase gene knockout mice and wild mice have revealed that the former would develop hypertension, with tachycardia and high catecholamine levels.28 This study verifies that urinary renalase is negatively correlated with SBP, which is consistent with the results of previous research.29,30 Furthermore, there is a lower correlation between urinary renalase and BP in the RD group than the NRD group. We speculate that when the kidney function is normal, the main effects of renalase are on the metabolism of catecholamine to reduce BP.7 Once kidney damage occurs, the urinary renalase level is largely affected and its correlation with BP decreases.
Due to the negative correlation between urinary renalase and BP, it is necessary to exclude any interference from BP to investigate whether the early-stage renal injury in primary hypertension is related to urinary renalase. The results from patients with stage 1 primary hypertension demonstrated that urinary renalase in the RD group was still lower than the NRD group, while the UACR was higher. Further analysis revealed that the increase of UACR was negatively correlated with urinary renalase, and their correlation in the RD group was greater than in the NRD group. It is worth noting that the UACR is a classic indicator for early-stage renal damage. Therefore, the result indicates that urinary renalase will further decrease in the early-stage renal damage in patients with primary hypertension. Other researchers have discovered that plasma renalase is closely related to renal function in patients with kidney or heart transplantations: the worse the degree of renal function, the lower the levels of renalase,17,18 and renalase in patients with ESRD is significantly lower than healthy people.23,31–33 At the same time, the inflammation, necrosis and apoptosis of the renal tubules in renalase gene knockout mice are more serious than wild mice, and their creatinine and urea nitrogen levels are much higher. After injecting the recombinant renalase protein into these mice, their renal functions are largely improved.34 All of these imply that renalase is associated with renal function, and renalase has a renal protective function. Our study further confirms the correlation between renalase and renal function.
Among patients with primary hypertension, there are many factors that influence the rise of the UACR. In order to determine which factor was the most important, a multivariate linear stepwise regression analysis was performed. The data from the RD group revealed that the increase of the UACR had a linear regression relationship with urinary renalase, HR, SBP and creatinine, and all of these independent variables explained 79.4% of the variations in the rise of the UACR. Furthermore, the effect of urinary renalase was the greatest. The ROC curve also supports that urinary renalase could be a good predictive indicator for early-stage renal damage in primary hypertension.
In conclusion, there was a linear regression relationship between the increase of the UACR and urinary renalase levels. The performance of urinary renalase as a marker for the diagnosis in early-stage renal damage for subjects with primary hypertension was 0.968. These results indicate that urinary renalase levels fall with hypertension and are further diminished when hypertension is complicated by renal disease. Therefore, we suggest that urinary renalase could be used in the prediction of renal disease in hypertension.
Authors’ contribution
N-NY and W-HJ conceived and designed the experiments; N-NY and M-YL performed the experiments; X-GL, Y-YW, J-YL, X-YZ, Z-WD, J-WW, X-XZ, H-LC and H-TT analyzed the data; M-YL contributed to the analysis tools and N-NY wrote the paper. All of the authors have read and approved the final manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (NSFC) Projects (grant number 81670335 and 81800271) and the New Xiangya Talent Projects of the Third XiangYa Hospital of Central South University (grant number 20170304).
ORCID iD
Wei-Hong Jiang https://orcid.org/0000-0001-8369-9694
References
- 1.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J 2018; 39:3021–104 [DOI] [PubMed] [Google Scholar]
- 2.Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet 2017; 389:1238–52 [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, Chen M, He Q, Liao Y, Yu X, Chen N, Zhang J-e, Hu Z, Liu F, Hong D, Ma L, Liu H, Zhou X, Chen J, Pan L, Chen W, Wang W, Li X, Wang H. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 2012; 379:815–22 [DOI] [PubMed] [Google Scholar]
- 4.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, Kasiske B, Kutner N, Liu J, St Peter W, Guo H, Gustafson S, Heubner B, Lamb K, Li S, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Thompson B, Wang C, Weinhandl E, Zaun D, Arko C, Chen S-C, Daniels F, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L. United States renal data system 2011 annual data report: Atlas of chronic kidney disease & end-stage renal disease in the United States. American Journal of Kidney Diseases: The Official Journal of the National Kidney Foundation 2012; 59:e1–420 [DOI] [PubMed] [Google Scholar]
- 5.Johnson DW, Jones GRD, Mathew TH, Ludlow MJ, Chadban SJ, Usherwood T, Polkinghorne K, Colagiuri S, Jerums G, MacIsaac R, Martin H. Australasian protelnuria C. Chronic kidney disease and measurement of albuminuria or proteinuria: a position statement. Med J Aust 2012; 197:224–5 [DOI] [PubMed] [Google Scholar]
- 6.Sehestedt T, Jeppesen J, Hansen TW, Wachtell K, Ibsens H, Torp-Petersen C, Hildebrandt P, Olsen MH. Risk prediction is improved by adding markers of subclinical organ damage to SCORE. Eur Heart J 2010; 31:883–91 [DOI] [PubMed] [Google Scholar]
- 7.Xu JC, Li GY, Wang PL, Velazquez H, Yao XQ, Li YY, Wu YL, Peixoto A, Crowley S, Desir GV. Renalase is a novel, soluble monoamine oxidase that regulates cardiac function and blood pressure. J Clin Invest 2005; 115:1275–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hennebry SC, Eikelis N, Socratous F, Desir G, Lambert G, Schlaich M. Renalase, a novel soluble FAD-dependent protein, is synthesized in the brain and peripheral nerves. Mol Psychiatry 2010; 15:234–6 [DOI] [PubMed] [Google Scholar]
- 9.Wang F, Xing T, Li J, Bai M, Hu R, Zhao Z, Tian S, Zhang Z, Wang N. Renalase’s expression and distribution in renal tissue and cells. PloS One 2012; 7:e46442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desir G. Novel insights into the physiology of renalase and its role in hypertension and heart disease. Pediatr Nephrol 2012; 27:719–25 [DOI] [PubMed] [Google Scholar]
- 11.Desir GV, Peixoto AJ. Renalase in hypertension and kidney disease. Nephrol Dial Transplant 2014; 29:22–8 [DOI] [PubMed] [Google Scholar]
- 12.Li G, Xu J, Wang P, Velazquez H, Li Y, Wu Y, Desir GV. Catecholamines regulate the activity, secretion, and synthesis of renalase. Circulation 2008; 117:1277–82 [DOI] [PubMed] [Google Scholar]
- 13.Desir GV, Tang L, Wang P, Li G, Sampaio-Maia B, Quelhas-Santos J, Pestana M, Velazquez H. Renalase lowers ambulatory blood pressure by metabolizing circulating adrenaline. J Am Heart Assoc 2012; 1:e002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desir GV. Regulation of blood pressure and cardiovascular function by renalase. Kidney Int 2009; 76:366–70 [DOI] [PubMed] [Google Scholar]
- 15.Li X, Jiang W, Li L, Huang R, Yang Q, Yang Y, Hong Y, Tang X. Renalase gene polymorphism in patients with hypertension and concomitant coronary heart disease. Kidney Blood Press Res 2014; 39:9–16 [DOI] [PubMed] [Google Scholar]
- 16.Li X, Xie Z, Lin M, Huang R, Liang Z, Huang W, Jiang W. Renalase protects the cardiomyocytes of Sprague-Dawley rats against ischemia and reperfusion injury by reducing myocardial cell necrosis and apoptosis. Kidney Blood Press Res 2015; 40:215–22 [DOI] [PubMed] [Google Scholar]
- 17.Przybylowski P, Malyszko J, Kozlowska S, Malyszko J, Koc-Zorawska E, Mysliwiec M. Serum renalase depends on kidney function but not on blood pressure in heart transplant recipients. Transplant Proc 2011; 43:3888–91 [DOI] [PubMed] [Google Scholar]
- 18.Malyszko J, Zbroch E, Malyszko JS, Koc-Zorawska E, Mysliwiec M. Renalase, a novel regulator of blood pressure, is predicted by kidney function in renal transplant recipients. Transplant Proc 2011; 43:3004–7 [DOI] [PubMed] [Google Scholar]
- 19.Zbroch E, Koc-Zorawska E, Malyszko J, Malyszko J, Mysliwiec M. Circulating levels of renalase, norepinephrine, and dopamine in dialysis patients. Ren Fail 2013; 35:673–9 [DOI] [PubMed] [Google Scholar]
- 20.Baraka A, El Ghotny S. Cardioprotective effect of renalase in 5/6 nephrectomized rats. J Cardiovasc Pharmacol Ther 2012; 17:412–6 [DOI] [PubMed] [Google Scholar]
- 21.Zhao B, Zhao Q, Li J, Xing T, Wang F, Wang N. Renalase protects against contrast-induced nephropathy in Sprague-Dawley rats. PloS One 2015; 10:e0116583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stec A, Semczuk A, Furmaga J, Ksiazek A, Buraczynska M. Polymorphism of the renalase gene in end-stage renal disease patients affected by hypertension. Nephrol Dial Transplant 2012; 27:4162–6 [DOI] [PubMed] [Google Scholar]
- 23.Huang Y-S, Lai J-B, Li S-F, Wang T, Liu Y-N, Zhang Q-X, Zhang S-y, Sun C-h, Hu N, Zhang X-Z. Relationship between renalase expression and kidney disease: an observational study in 72 patients undergoing renal biopsy. Curr Med Sci 2018; 38:268–76 [DOI] [PubMed] [Google Scholar]
- 24.Musialowska D, Malyszko J. Renalase – a new marker or just a bystander in cardiovascular disease: clinical and experimental data. Kardiologia Polska 2016; 74:937–42 [DOI] [PubMed] [Google Scholar]
- 25.Johnson KC, Whelton PK, Cushman WC, Cutler JA, Evans GW, Snyder JK, Ambrosius WT, Beddhu S, Cheung AK, Fine LJ, Lewis CE, Rahman M, Reboussin DM, Rocco MV, Oparil S, Wright JT. Blood pressure measurement in SPRINT (systolic blood pressure intervention trial). Hypertension (Hypertension ) 2018; 71:848–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang TJ, Evans JC, Meigs JB, Rifai N, Fox CS, D’Agostino RB, Levy D, Vasan RS. Low-grade albuminuria and the risks of hypertension and blood pressure progression. Circulation 2005; 111:1370–6 [DOI] [PubMed] [Google Scholar]
- 27.Gupta J, Mitra N, Kanetsky PA, Devaney J, Wing MR, Reilly M, Shah VO, Balakrishnan VS, Guzman NJ, Girndt M, Periera BG, Feldman HI, Kusek JW, Joffe MM, Raj DS, Investigators CS. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol 2012; 7:1938–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Y, Xu J, Velazquez H, Wang P, Li G, Liu D, Sampaio-Maia B, Quelhas-Santos J, Russell K, Russell R, Flavell RA, Pestana M, Giordano F, Desir GV. Renalase deficiency aggravates ischemic myocardial damage. Kidney Int 2011; 79:853–60 [DOI] [PubMed] [Google Scholar]
- 29.Lemiesz M, Tenderenda-Banasiuk E, Sosnowska D, Taranta-Janusz K, Wasilewska A. Serum renalase levels in adolescents with primary hypertension. Pediatr Cardiol 2018; 39:1258–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wybraniec MT, Mizia-Stec K, Trojnarska O, Chudek J, Czerwieńska B, Wikarek M, Więcek A. Low plasma renalase concentration in hypertensive patients after surgical repair of coarctation of aorta. J Am Soc Hypertens 2014; 8:464–74 [DOI] [PubMed] [Google Scholar]
- 31.Wybraniec MT, Mizia-Stec K. Renalase and biomarkers of contrast-induced acute kidney injury. Cardiorenal Med 2016; 6:25–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taranta-Janusz K, Wasilewska A, Roszkowska R. Is renalase a marker of decline in renal function in children with solitary kidney? Pediat Nephrol 2015; 30:1586–7 [DOI] [PubMed] [Google Scholar]
- 33.Wybraniec MT, Bozentowicz-Wikarek M, Chudek J, Mizia-Stec K. Urinary renalase concentration in patients with preserved kidney function undergoing coronary angiography. Nephrology (Carlton) 2018; 23:133–8 [DOI] [PubMed] [Google Scholar]
- 34.Lee HT, Kim JY, Kim M, Wang P, Tang L, Baroni S, D’Agati VD, Desir GV. Renalase protects against ischemic AKI. J Am Soc Nephrol 2013; 24:445–55 [DOI] [PMC free article] [PubMed] [Google Scholar]