Short abstract
Mesenchymal stem cells (MSCs) can act as a carrier in tumor therapy, and tumor suppressor gene-modified MSCs can reach and suppress the tumor. However, in the colon cancer microenvironment, MSCs could promote tumor growth and create the environment that is conducive to the survival of cancer stem cells (CSCs). This study discovered MSCs from three sources (bone marrow, adipose, placenta) could induce the stemness and epithelial–mesenchymal transition (EMT) of HCT116 in vitro, meanwhile adipose- and placenta-derived MSCs increase the proportion of CD133+/CD44+ HCT116. Then, we explored the interaction mechanism between CD133+/CD44+ HCT116 and MSCs by the bioinformatics and in vitro assays. After CD133+/CD44+ HCT116 were co-cultured with MSCs, many cytokines in MSCs were stimulated, including interleukin-8 (IL-8). The binding of IL-8/CXCR2 activates the downstream mitogen-activated protein kinase (MAPK) signaling pathway in colon CSCs, thereby promoting the stemness and EMT. However, inhibition of IL-8/CXCR2/Erk1/2 could reverse the effect of MSCs on CSC stemness. In addition, MSCs co-cultured with CD133+/CD44+ HCT116 produce a carcinoma-associated fibroblast phenotype via intracellular FGF10–PKA–Akt–β-catenin signaling, which can be attenuated by IL-8 peptide inhibitor. To conclude, IL-8 promotes the interaction between colon CSCs and MSCs, and activates the MAPK signaling pathway in colon CSCs, which provides a theoretical basis for the application of MSCs in clinical practice.
Impact statement
MSCs have the property of chemotaxis and they can migrate to the tumor site by paracrine pathway in the tumor environment, and then interact with tumor cells. Although a mass of studies have been conducted about the impact of MSCs on tumors, it is still controversial whether the exogenous MSCs promote or inhibit tumor growth. In this work, we evaluated the effects of MSCs from three sources (bone marrow, adipose, placenta) on the proliferation, stemness, and metastasis of the colon cancer cells both in vitro and in vivo. Then, we proved the IL-8/CXCR2/MAPK and FGF10–PKA–Akt–β-catenin signaling pathway which mediate the interplay between MSC and CD133+/CD44+ colon cancer cell. This research aims to provide a theoretical basis for the safe application of MSCs in the clinical treatment of colon cancer.
Keywords: Mesenchymal stem cell, colon cancer stem cell, stemness, interleukin-8, mitogen-activated protein kinase
Introduction
Colon cancer is the second most common cancer killer worldwide and the fourth major cause of cancer-related mortality.1 According to the results of the American Cancer Association, the five-year survival rate of stage I colorectal cancer patients was 93.2%, while that of stage IV was only 8.1%. It can be seen that the prognosis of advanced colon cancer is not optimistic, and the cause of death is mostly the metastasis of tumor to liver, lung, abdominal lymph nodes, and other organs.2 Many studies have confirmed that cancer progression and distant metastasis are mainly related to the occurrence of epithelial–mesenchymal transition (EMT), while mesenchymal stem cells (MSCs) can promote EMT of cancer cells. MSCs have the property of chemotaxis and they can migrate to the tumor site by paracrine pathway in the tumor environment, and then interact with tumor cells.3 Therefore, MSCs can act as a carrier in tumor therapy, and tumor suppressor gene-modified MSCs can reach and suppress the tumor. For example, MSCs genetically modified to mediate anti-high mobility group box 1 activity can target colon cancer cells;4 placenta-derived MSCs expressing endostatin can suppress colorectal peritoneal tumorigenesis.5
Although plentiful studies have been conducted about the effects of MSCs on tumors, it is still controversial whether the exogenous MSCs promote or inhibit tumor growth. We must assess whether the in vivo transfusion of MSCs could lead to tumor progression before their clinical application. So, it is necessary to clarify the interaction mechanism between MSC and colon cancer cell. After being recruited to the tumor site by various chemokines, MSCs can change the phenotype of cancer cells and induce malignant behavior of tumors by secreting various cytokines, matrix hydrolases, and immunoregulators.6 Three phenotypes of cancer cells are worthy of our attention: (a) the ability to regulate cancer stem cells (CSCs) or to initiate tumors,7,8 (b) the ability to resist chemotherapy, and (c) the ability to metastasize. Above all, CSCs have received much attention in the field of cancer research, which plays a critical role in tumor initiation, metastasis, invasion, and drug resistance. For example, labeled human bone marrow-derived MSCs are concentrated in the tumor site of the orthotopic breast cancer xenografts, and the proportion of CSCs is increased by the paracrine secretion of cytokines IL-6 and CXCL7.8 Thus, the potential link between MSCs and CSCs is worth exploring in the colorectal cancer. In addition, the sources of MSCs have different effects on tumors. This may be due to the different immunomodulatory capacity, migration ability, and secreted cytokines of MSCs from different sources.9 In this research, we compared the effects of human MSCs from three different sources (bone marrow, adipose, placenta) on the stemness, EMT, and proliferation phenotypes of colon cancer cell line HCT116. Animal experiments explored the effects of three types of MSCs on the growth and metastasis of colon cancer xenograft. Microarray analysis and in vitro experiments explored the molecular mechanisms that mediate the interaction between human MSC and colon CSC.
Materials and methods
Cell culture, reagents, and transfection
Human placenta-derived mesenchymal stem cells (PMSCs), human bone marrow-derived mesenchymal stem cells (BMSCs), and human adipose-derived mesenchymal stem cells (AMSCs) were bought from Sciencell Research Laboratories, Inc. (CA, USA). MSCs were cultivated in MSC medium (ScienCell, Cat. No. 7501) in a humidified atmosphere at 37°C. Parental HCT116 and CD133+/CD44+ HCT116 were cultured in DMEM/F12 serum-free medium (Gibco, CA, USA) supplemented with 10% FBS and stem cell growth factors (20 ng/mL EGF, 10 ng/mL bFGF (PeproTech, NJ, USA), 2% B27 and 5 µg/mL insulin (Gibco, CA, USA)), respectively. In the co-culture system, HCT116 was cultured in the upper chamber of 0.4 µm transwell plate (TS) or cultured with conditioned medium of MSCs (CM). MSCs of 80% cell density were refreshed with DMEM/F12 basic medium and cultured for 24 h; the supernatant was then filtrated and stored at −20°C. SB225002 (CXCR2 inhibitor) and U0126 (Erk1/2 inhibitor) were purchased from MedChemExpress LLC. IL-8 peptide inhibitor (Cat. No. 62401) was purchased from ANASPEC, Inc., California.
Construction of IL-8 (NM_NM_000584.4) knockdown vector
Primers were designed according to the shRNA sequence given by the Sigma-Aldrich website (http://rnaidesigner.thermofisher.com/rnaiexpress/) and the pSilencer2.1_U6 vector instructions; the sequences were as follows: sh#1[TCTCTTGGCAGCCTTCCTGATTTCTGCAGTTCAAGAGACTGCAGAAATCAGGAAGGCTGCCAAGAGATTTTTT]; sh#2[GAACTTAGATGTCAGTGCATTCAAGAGATGCACTGA CATCTAAGTTCTTTTTT].
Animal experiments
Six-week-old male balb/c nude mice were purchased from JSJ Lab (Shanghai, China) and maintained in a sterile environment with modest temperature and humidity. The animal protocol in this study conformed to guidelines for the care and use of laboratory animals authorized by Ministry of Science and Technology of the People’s Republic of China. The animal study was approved by Shanghai Longhua Hospital. Mice were divided into eight groups at random (four mice per group). First of four groups were subcutaneously inoculated with 5 × 105 HCT116 and 1 × 106 MSCs (AMSCs, BMSCs, or PMSCs) or 5 × 105 HCT116 alone; another four groups were first injected subcutaneously with 5 × 105 HCT116. When the subcutaneous tumors were grown at 50 mm3, 1 × 106 MSCs (AMSCs, BMSCs, or PMSCs) or 150 µL saline were injected through the tail vein every four days for a total of four times. The CT-26 peritoneal metastasis model of six-week-old male balb/c mice (5 × 105 CT26 cells blended with Matrigel at a ratio of 1:1) was divided into four groups (five mice per group). On the day of modeling, 1 × 106 MSCs (AMSCs, BMSCs, or PMSCs) or 150 µL saline were injected into the tail vein every four days for a total of four times. Mice were sacrificed with 160 mg/kg sodium pentobarbital.
Real-time RT-PCR qPCR
Total RNA was extracted using the Trizol reagent (Invitrogen). The cDNAs were synthesized by a reverse transcription kit (Transgene Biotech, Beijing, China). qPCR assay followed the instruction of TransStart Top Green qPCR SuperMix (Transgene Biotech). GAPDH was used as the internal control. The sequences of specific primers are listed in Table 1.
Table 1.
Oligonucleotide primers used in the real-time PCR.
| Gene | Sequence (5′→3′) |
|---|---|
| Vimentin | F:GAAGAGAACTTTGCCGTTG |
| R:GAAGGTGACGAGCCATTT | |
| Twist1 | F:AGCCTGAGCAACAGCGA |
| R:ACAGCCCGCAGACTTCTT | |
| E-cadherin | F:GGTTGATCCTGGCTTTGTT |
| R:GCCCTGTTGTCCTTCTTTT | |
| α-SMA(alpha-smooth muscle actin) | F:CGTGGCTATTCCTTCGTT |
| R:ACGTTCATTTCCGATGGT | |
| FAPA(fibroblaat activation protein alpha) | F:ATAATGACCCCAGTGCTTTT |
| R:TATTCCCTTTCCCAGACCT | |
| Tenascin-c | F:AAACTGCCCTCCTTACCTG |
| R:GGACCCACAATGACTTCCT | |
| FGF10(fibroblast growth factor 10) | F:TGGTGTCTTCCGTCCCT |
| R:TGATTGTAGCTCCGCACAT | |
| FGFR1(fibroblast growth factor receptor 1) | F:CCTGGGCAACAGAGAAAAC |
| R:GAAGCACTGGCAAAACCA | |
| FGFR2(fibroblast growth factor receptor 2) | F:CATTGCGCGTAGTCCAT |
| R:CCGGTCCTCTTCCATATCT | |
| Akt(protein kinase B) | F:AAGCCCCAGGTCACGTC |
| R:TCGCTGTCCACACACTCC | |
| PKAc (protein kinase A) | F:TTAACGTCCAGCCTTCCC |
| R:TTGGCCTCTTCTGTTCCC | |
| β-catenin | F:TGGGACCTTGCATAACCT |
| R:TTCACCAGGGCAGGAAT | |
| CXCL1(CXC chemokine ligand 1) | F:GGCAAATCCAACTGACCA |
| R:ACATTAGGCGCAATCCAG | |
| CXCL2(CXC chemokine ligand 2) | F:AACCGAAGTCATAGCCACA |
| R:CAGGAACAGCCACCAATAA | |
| CXCL3(CXC chemokine ligand 3) | F:TTTCCAGTCTCAACCATGC |
| R:GTGTGGCTCCCCAAGAT | |
| CXCL6(CXC chemokine ligand 6) | F:CCCTTCTTTCCACACTGC |
| R:GGTTTCCTCGTGCCTTC | |
| CXCL8(CXC chemokine ligand 8) | F:GTGCTGTGTTGAATTACGGA |
| R:TTGACTGTGGAGTTTTGGC | |
| IL-32 (interleukin-32) | F:GGGTGAAGGAGAAGGTGGT |
| R:GGGCTCCGTAGGACTGG | |
| CXCR1(C-X-C chemokine receptor 1) | F:CCTGCCCTTCTTCCTTTT |
| R:ACACCATCCGCCATTTT | |
| CXCR2(C-X-C chemokine receptor 2) | F:CGCAATGTGACTTAATGCC |
| R:CTTTTGGGGCTCAGGTTT | |
| EGFR(epidermal growth factor receptor) | F:GCCTGAAAACAGGACGGA |
| R:GAGGGAGCGTAATCCCAAG | |
| ADAM10(a disintegrin andmetalloproteinase domain 10) | F:TATTATGTGCCCCGTGTTC |
| R:TAGCCTTGATTGGCAGTTG | |
| CD133(cluster differentiation 133) | F:GCATCCATCAAGTGAAACC |
| R:ACCAGGCCATCCAAATC | |
| CD44(cluster differentiation 44) | F:AGTCCCTGGATCACCGA |
| R:CCTCTTGGTTGCTGTCTCA | |
| KLF4(kruppel like factor 4) | F:AGGAGCCCAGCCAGAAA |
| R:TCCAGTCACAGACCCCATC |
Colony formation assay
HCT116 was cultured in a six-well plate (1 × 104 cells per well). Photos were taken under the inverted microscope on the 14th day.
Western blot
Total proteins were extracted and separated on a 10% SDS-PAGE gel, then transferred onto PVDF membranes. After blocked with 4% BSA-TBST for 1.5 h, membranes were incubated with different primary antibodies overnight such as β-catenin, p-β-catenin(ser552), p-β-catenin(ser675), p-β-catenin(ser33/37/41), N-cadherin, Vimentin, KLF4, EpCAM and GAPDH (Santa Cruz), CD133 (Proteintech), Slug, α-SMA, E-cadherin, IL-8, Erk1/2, p-Erk1/2, JNK, p-JNK, p38, p-p38, β-actin and PCNA (Abways), CXCR2 (Affinity), PKA and Akt (CST), FGF10 (Abclonal). β-actin or GAPDH was used as the loading control.
Immunohistochemistry
Immunohistochemical staining of paraffin-embedded clinical tissue slides was conducted. IL-8 anti-human antibody (Abways) was diluted with PBS at a ratio of 1:100. Eight fields of view were randomly photoed under microscope. The mean optical density value of the positive protein in each field was analyzed by Image-Pro Plus 6.0 software.
In vitro limiting dilution assay
Cells were diluted into the final concentration of 1000, 100, 10, 1 cells/well in the low attach 96-well plate. Two weeks later, we examined which well contained cell spheres and counted. We then used Extreme Limiting Dilution Analysis online software to analyze the cancer cell initiating frequency (http://bioinf.wehi.edu.au/software/elda/).
Stem cell sphere formation assay
We suspended the dissociated spheres in 2 mL SFM, took the required number of live cells (counting with a hemocytometer in presence of trypan blue solution to exclude dead cells), and adjusted to a final concentration of 100 cells/100 µL in SFM. We pipetted 400 µL/well of SFM in each 24-well plate for a total of six wells for each treatment, seeded 100 µL of cells in SFM (100 cells/well), and placed the plate in an incubator at 37°C with 5% CO2. Spheres can be counted under an optical microscope with 20× objective after seven days.
Flow cytometry analysis
The digested cells were centrifuged at 300×g and blocked with 2.5% BSA at 37°C for 1 h; cells were incubated with CD44-FITC and CD133-PE (eBioscience) antibodies at 4°C for 45 min, then washed and resuspended in 500 µL PBS, followed by flow cytometry (BD) detection.
Statistical analysis
Statistical analysis of the data was performed by GraphPad Prism 5.0 software. All the data were expressed as mean ± SD (or SEM) of biological replicates. Student’s t-test was applied to evaluate the difference between each test group and control group. P <0.05 was considered statistically significant.
Results
Three different sources of MSCs increase the stemness and EMT phenotype of HCT116
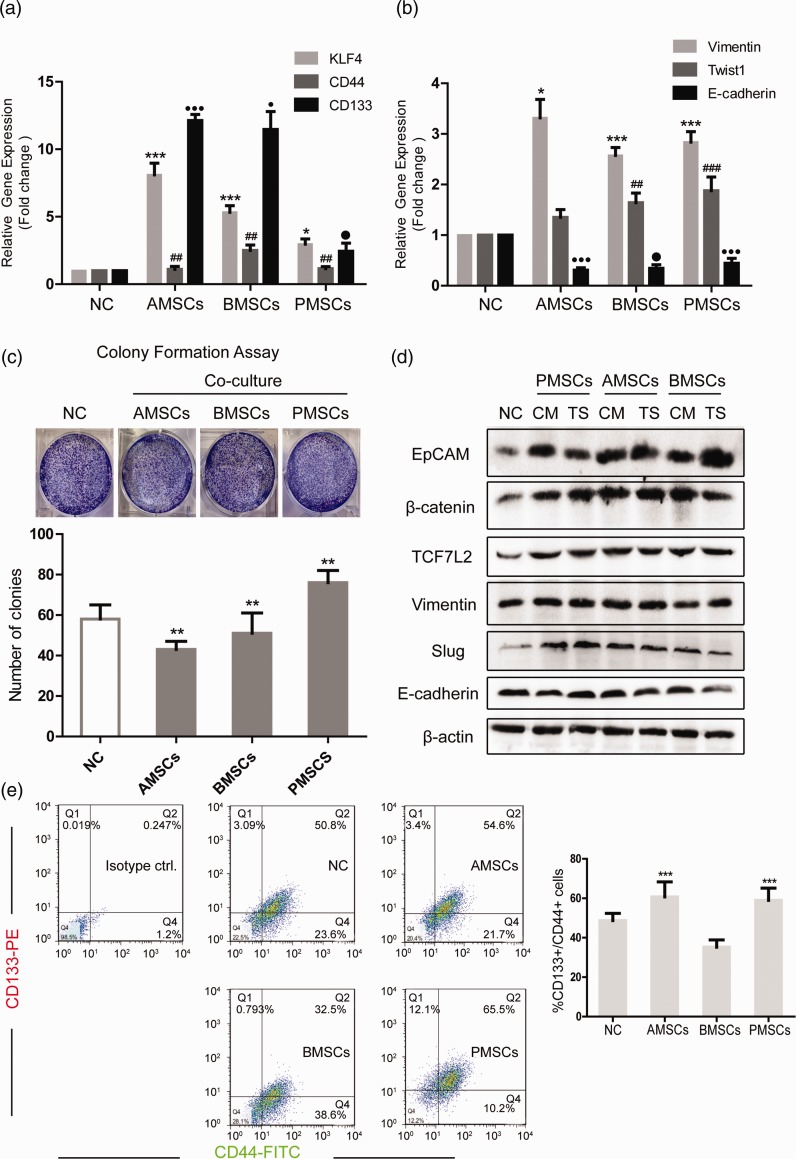
MSCs play a double role in the tumor microenvironment, that is promoting or inhibiting tumor progression. The discrepancy may result from tissue sources, individual variability, and the methods of MSC injection.4 In this study, colon cancer cell line HCT116 was co-cultured with human MSCs from three different tissue sources (bone marrow, adipose, placenta) by CM or TS (0.4 µm Transwell) co-culture method. qRT-PCR detected the levels of stemness-related molecules (CD133, CD44, KLF4) of HCT116 after co-culture. The results suggested that the levels of three genes were all augmented after co-culture. Among them, the expression of KLF4 and CD133 was significantly increased after co-culture with AMSCs and BMSCs (Figure 1(a)). We then detected the expression of EMT-related molecules (Vimentin, Twist1, E-cadherin) after co-culture. The results showed that the expression of Vimentin and Twist1 in HCT116 after co-culture was significantly increased, and the level of E-cadherin was greatly decreased (Figure 1(b)). Besides, the colony formation ability of HCT116 after co-culture with AMSCs or BMSCs was slightly decreased and was increased after co-culture with PMSCs (Figure 1(c)). Western blot examined the levels of stemness-related and EMT-related proteins of HCT116 after co-culture and the levels of EpCAM, β-catenin, TCF7L2, and Slug were increased evidently after co-culture with three kinds of MSCs under the condition of CM or TS. Vimentin was increased in the PMSCs co-culture group. E-cadherin was reduced in the PMSCs (CM) group, AMSCs (TS) group, and BMSCs (TS) group (Figure 1(d)). CD133 and CD44 are well-known cell surface markers widely applied in colon CSC sorting. The ratio of CD133+/CD44+ HCT116 was increased after co-culture with AMSCs or PMSCs, indicating an increase in the proportion of colon cancer stem-like cells (Figure 1(e)).
Figure 1.
Three different sources of MSCs increase the stemness and EMT phenotype of HCT116. HCT116 was cultured in CM from three different sources of MSCs for 48 h. (a, b) qRT-PCR detected the levels of stemness-related molecules KLF4, CD44, CD133 in HCT116 and EMT marker molecules Vimentin, Twist1, E-cadherin. (c) Colony formation assays detected the sphere formation ability of HCT116. (d) Western blot detected the levels of stemness-related proteins EpCAM, β-catenin, TCF7L2 and EMT marker proteins Vimentin, Slug, E-cadherin in HCT116 under two co-culture conditions (CM and TS). (e) Flow cytometry detected the proportion of CD133+/CD44+ double positive cells in HCT116. Data shown are the results (mean ± SD) from three independent experiments. The asterisks indicate a significant difference (***P < 0.001, **P < 0.01, *P < 0.05), the # indicates a significant difference (###P < 0.001, ##P < 0.01) and the ● indicates a significant difference (●●●P < 0.001, ●P < 0.05) between MSC co-culture group and NC group. Images are representative of experiments with similar results. (A color version of this figure is available in the online journal.)
AMSCs: human adipose-derived mesenchymal stem cells; BMSCs: human bone marrow-derived mesenchymal stem cells; CM: conditioned medium from MSCs; EpCAM: epithelial cell adhesion molecule; KLF4: kruppel like factor 4; NC: negative control; PMSCs: human placenta-derived mesenchymal stem cells; TS: 0.4 µm Transwell co-culture in which HCT116 was placed in the upper chambers.
Therefore, MSCs from three sources could induce the stemness and EMT phenotype of colon cancer cells in vitro, meanwhile AMSCs and PMSCs increase the proportion of colon CSCs.
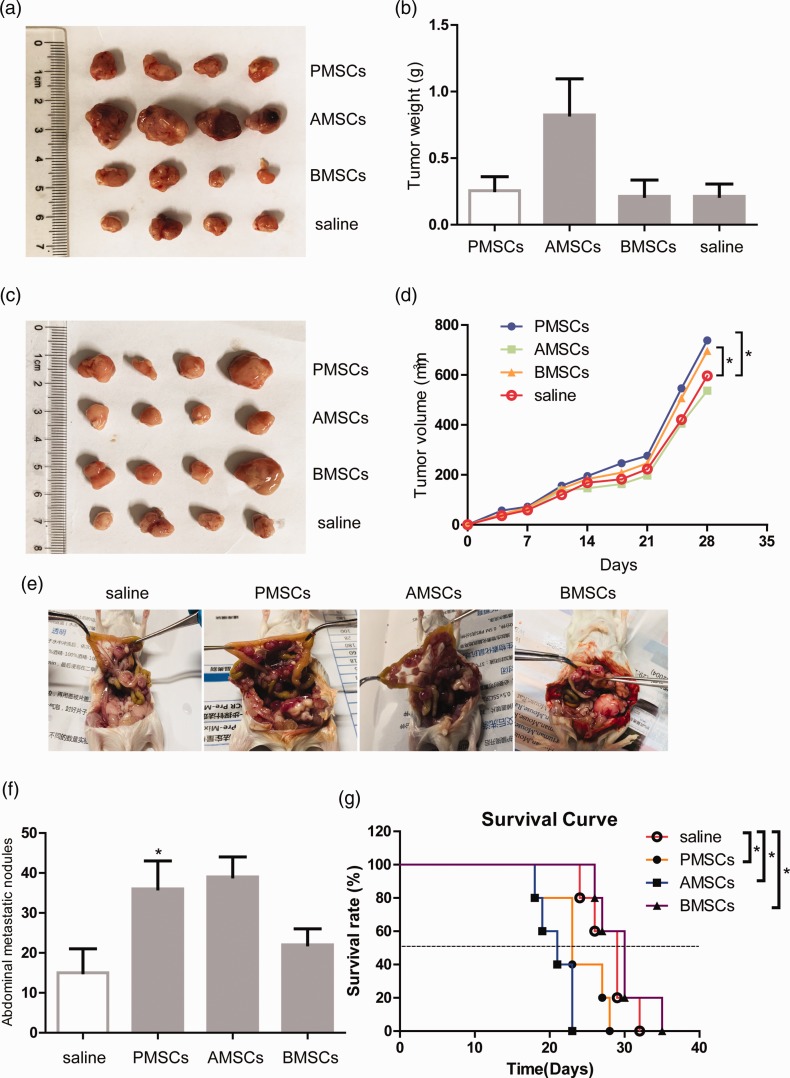
The in vivo effect of MSCs on colon cancer xenografts
The dual effect of MSCs on cancer cells is related to the tissue source, co-culture methods, in vivo injection methods, and the tumor microenvironment. Therefore, we constructed a subcutaneous tumor model and a peritoneal metastasis model to investigate the impact of three sources of MSCs on the growth and metastasis of xenograft tumors in mice. Four groups of balb/c nude mice were subcutaneously injected with the mixture of 5 × 105 HCT116 and 1 × 106 MSCs (AMSCs, BMSCs, or PMSCs) or 5 × 105 HCT116 alone (four mice per group). After one month, the mice in the AMSC group have the largest tumor (Figure 2(a) and (b)). Meanwhile, another four groups of nude mice were first subcutaneously inoculated with 5 × 105 HCT116 (four mice per group). When the tumors were grown at 50 mm3 (on day 7), 1 × 106 MSCs were injected through the tail vein every four days for a total of four times. After one month, the tumor volume of the PMSC group and the BMSC group is larger than the control group, which is statistically significant (Figure 2(c) and (d)). Next, we constructed a CT-26 peritoneal metastasis model of balb/c mice (5 × 105 CT26 with Matrigel). On the day of modeling, 1 × 106 MSCs (AMSCs, BMSCs, or PMSCs) or 150 µL saline (five mice per group) were injected into the tail vein every four days for a total of four times. In contrast to the control group, liver metastasis was obvious in the PMSC group (data not shown). Three kinds of MSCs all promoted abdominal metastasis. The survival rate of mice in the PMSC group and AMSC group decreased (Figure 2(e) to (g)).
Figure 2.
The in vivo effect of MSCs on colon cancer xenografts. (a) Balb/c nude mice were subcutaneously inoculated with 5 × 105 HCT116 and 1 × 106 MSCs (four mice per group). (b) One month later, the tumor weight of the mice was examined. (c) Balb/c nude mice were inoculated subcutaneously with 5 × 105 HCT116. When the subcutaneous tumors were grown at 50 mm3, 1 × 106 MSCs or 150 µl saline (four mice per group) were injected through the tail vein every four days for a total of four times. (d) One month later, the tumor volume of the mice was examined. The CT-26 peritoneal metastasis model of balb/c mice (5 × 105 CT26 cells blended with Matrigel) was constructed. On the day of modeling, 1 × 106 MSCs or 150 µl saline (five mice per group) was injected into the tail vein every four days for a total of four times. The liver metastasis (e), abdominal metastatic nodules (f), and survival rate (g) of the mice were observed. The data for each histogram are presented as mean±SEM. The asterisks indicate a significant difference (*P<0.05) between MSC treatment group and saline group. (A color version of this figure is available in the online journal.)
AMSCs: human adipose-derived mesenchymal stem cells; BMSCs: human bone marrow-derived mesenchymal stem cells; CM: conditioned medium from MSCs; NC: negative control; PMSCs: human placenta-derived mesenchymal stem cells.
In conclusion, MSCs may have different impact on colon cancer in vitro and in vivo. Also, different injection methods may affect the growth of colon cancer cells in vivo.
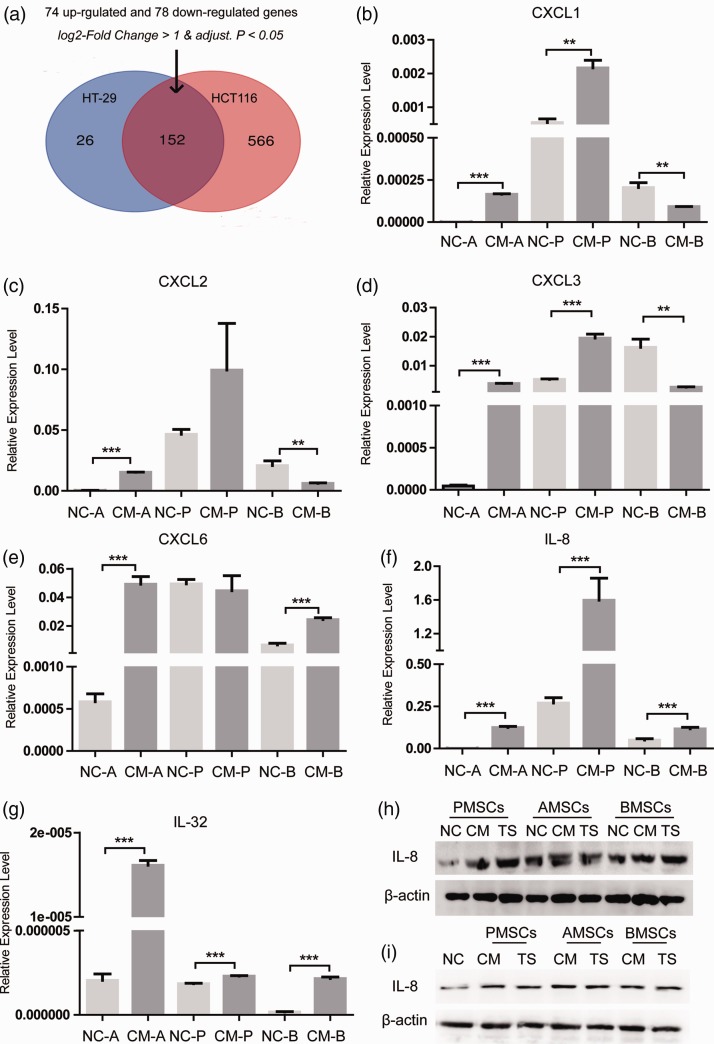
Cytokines regulate the interaction between MSC and colon CSC
MSCs migrate to the tumor site and interact with tumor cells via paracrine signals. Certain cytokines secreted by MSCs can regulate tumor growth and metastasis. MSCs secrete IL-6 through STAT3 to increase the number of colorectal CSCs and promote tumor initiation.10 To explore the interaction between colon CSC and MSC, we first sorted CD133+/CD44+ HCT116 (HCT116++) by fluorescence-activated cell sorting which we have performed in the previous research.11 Then, we extracted the raw data of GSE106325 from GEO DataSets, in which BMSCs cultured alone was the control group and BMSCs co-cultured with HT29 or HCT116 was the experimental group. The differential genes of HT-29 group and HCT116 group in the GSE106325 chip were crossed. After co-culture, there were 74 genes with elevated expression and 78 genes with decreased expression. (Figure 3(a), Supplement 1A). Among 152 genes, we found six cytokines (CXCL1, 2, 3, 6, IL-8 and IL-32). qRT-PCR detected their expression in three kinds of MSCs after co-culture with HCT116+/+ and the levels of all cytokines in the AMSC group were increased. In the PMSC group, except for CXCL6, the expression of other five cytokines was increased. In the BMSC group, the expression of CXCL1, 2, 3 was decreased, and CXCL6, IL-8, and IL-32 were increased (Figure 3(b) to (g)). IL-8, also known as CXCL8, plays a vital role in the self-renewal, chemoresistance, and tumorigenesis in tumor-initiating cells of lung, breast, and colon cancers.12 TCGA database analysis and immunohistochemistry results showed that the levels of IL-8 mRNA were significantly augmented in tumor tissues compared with adjacent normal mucosa (Supplement 1C, 1D). The available information on in situ tumor samples is shown in Table 2. It is reported that advanced TNM stage, poor differentiation, and distant metastasis were related with IL-8.13 But, we found no significant correlation of IL-8 with relapse-free survival, overall survival, and distant metastasis-free survival in the Kaplan–Meier plotter database (Supplement 1B). Then, Western blot demonstrated that the expression of IL-8 in PMSCs and HCT116+/+ was both enhanced after co-culture, indicating that IL-8 could be secreted in the co-culture environment by both MSC and CSC (Figure 3(h) and (i)).
Figure 3.
Cytokines regulate the interaction between MSCs and colon CSCs. (a) The differential genes of HT-29 group and HCT116 group in the GSE106325 chip were crossed. (b) to (g) qRT-PCR detected the expression of cytokines CXCL1, 2, 3, 6, IL-8, and IL-32 in MSCs from three different sources after co-culture with HCT116+/+. (h, i) Western blot detected the expression of IL-8 in PMSCs and HCT116+/+ under the condition of co-culture. Data shown are the results (mean ± SD) from three independent experiments. Statistical differences between two groups were expressed as ***P<0.001, **P<0.01. (A color version of this figure is available in the online journal.)
AMSCs: human adipose-derived mesenchymal stem cells; BMSCs: human bone marrow-derived mesenchymal stem cells; PMSCs: human placenta-derived mesenchymal stem cells; TS: 0.4 µm Transwell co-culture in which HCT116 was placed in the upper chambers.
Table 2.
Available information on in situ tumor samples of clinical patients.
| No. of patients | Age | Sex | Colonic adenocarcinoma pathological grading | Is the tumor metastasized? |
|---|---|---|---|---|
| #17-21978 | 78 | Male | III | Yes |
| #17-27583 | 86 | Male | II | No |
| #17-30660 | 62 | Female | II | No |
| #17-27586 | 70 | Female | II–III | Yes |
| #17-26788 | 58 | Male | II–III | Yes |
| #17-27636 | 77 | Female | II–III | Yes |
| #17-27636 | 44 | Male | III | Yes |
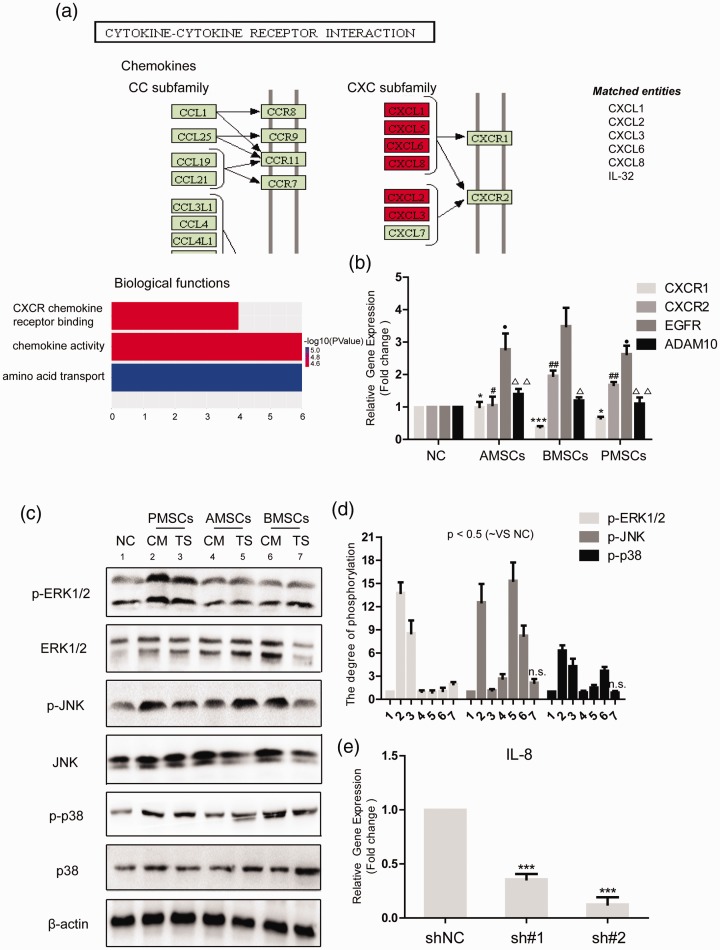
IL-8/CXCR2/mitogen-activated protein kinase (MAPK) signaling pathway is activated in HCT116++ after co-culture with MSCs
CXC chemokines bind to G-protein-coupled receptors, mainly CXCR1 and CXCR2, that play an important role in tumor formation and progression.14 The interaction between cytokine and cytokine receptor is highlighted in the co-culture of BMSCs with colon cancer cells through GO analysis (Figure 4(a)). First, we detected the mRNA levels of CXCR1 and CXCR2 in HCT116+/+ after co-culture with MSCs and the expression of CXCR2 was greatly augmented in the BMSC group and PMSC group while the level of CXCR1 did not change in the AMSC group and was decreased in the BMSC group and PMSC group. Therefore, we hypothesized that IL-8 secreted under the co-culture condition may activate the downstream signaling of CSCs by binding to the CXCR2 receptor (Figure 4(b)). IL-8 activates MAPK signaling pathway characterized by enhanced phosphorylated Erk1/2 levels.12 MAPKs, mainly Erk, JNK, and p38, regulate the inflammatory response, inducing inflammatory cytokines and chemokines.15 To further investigate the impact of secreted IL-8 on the CSCs, the activation of MAPK signaling pathway, including Erk1/2, JNK, and p38, was detected. The results demonstrated that MSCs significantly promoted the phosphorylation of Erk1/2, JNK, and p-38 in CSCs (Figure 4(c) and (d)). Meanwhile, qRT-PCR detected the levels of EGFR and ADAM10 in HCT116+/+. The results showed that the mRNA levels of EGFR and ADAM10 were enhanced in CSCs after co-culture with MSCs (Figure 4(b)). We suggested that MSCs could activate MAPK signaling pathway in CSCs through IL-8/CXCR2. Next, we constructed two IL-8 knockdown plasmids to explore the impact of IL-8 on the stemness of CSCs. The knockdown effect was examined by qPCR assay (Figure 4(e)).
Figure 4.
IL-8/CXCR2/MAPK signaling pathway is activated in HCT116+/+ after co-culture with MSCs. (a) After the GO analysis of the differential genes from GSE106325, we found that cytokines shall interact with cytokine receptors under the co-culture condition. We hypothesized that MSCs regulate the stemness of colon CSCs through a cytokine network. (b) qRT-PCR detected the mRNA levels of CXCR1, CXCR2, EGFR, and ADAM10 after HCT116+/+ was co-cultured with three different sources of MSCs. (c) Western blot detected the activation of MAPK signaling after HCT116+/+ was co-cultured with three different sources of MSCs. (d) The degree of phosphorylation was normalized to GAPDH as an internal control. (e) qRT-PCR verified the knockdown efficiency of two IL-8 knockdown plasmids sh#1 and sh#2 in HCT116+/+. Data shown are the results (mean±SD) from three independent experiments. Statistical differences between two groups were expressed as ***P < 0.001, *P < 0.05; ##P < 0.01, #P < 0.05; ●P < 0.05; △△p < 0.01, △p < 0.05. Images are representative of experiments with similar results. (A color version of this figure is available in the online journal.)
ADAM10: a disintegrin and metalloproteinase domain 10; AMSCs: human adipose-derived mesenchymal stem cells; BMSCs: human bone marrow-derived mesenchymal stem cells; CM: conditioned medium from MSCs; CXCR: C-X-C chemokine receptor; EGFR: epidermal growth factor receptor; p-ERK1/2: phospho-extracellular regulated protein kinases1/2; p-JNK: phospho-c-Jun N-terminal kinase; NC: negative control; PMSCs: human placenta-derived mesenchymal stem cells; TS: 0.4 µm Transwell co-culture in which HCT116 was placed in the upper chambers.
IL-8/CXCR2/MAPK signaling pathway regulates CSC phenotype of HCT116+/+
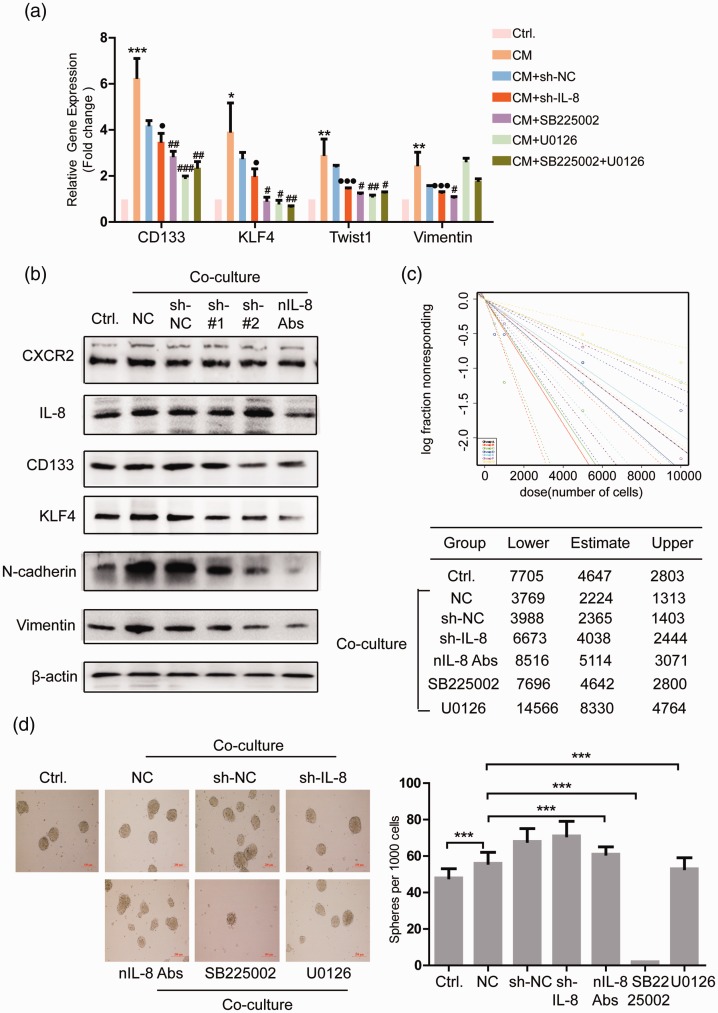
First, we detected the impact of knockdown of IL-8 in PMSCs (sh-IL-8), inhibition of CXCR2 receptor and Erk1/2 in HCT116+/+ on the expression of CD133, KLF4, Twist1, Vimentin of HCT16+/+. The results showed that inhibition of IL-8 and CXCR2 expression could attenuate the effect of co-culture on the expression of these four genes. Inhibition of the Erk1/2 in CSCs attenuates the effect of co-culture on the enhanced expression of CD133, KLF4, and Twist1 (Figure 5(a)). Western blot detected the effects of knockdown of IL-8 in PMSCs or inhibition of IL-8 expression in co-culture system with IL-8 peptide inhibitor (nIL-8 Abs) on HCT116+/+ stemness- and EMT-related proteins. The results showed that CXCR2 expression was slightly decreased by nIL-8 Abs, and the levels of CD133, KLF4, N-cadherin, and Vimentin were all decreased by shIL-8 and nIL-8 Abs (Figure 5(b)).
Figure 5.
IL-8/CXCR2/MAPK signaling pathway regulates CSC phenotype of HCT116+/+. (a) qRT-PCR detected the impact of knockdown of IL-8 in PMSCs, inhibition of CXCR2 receptor, Erk1/2 signaling in HCT116+/+, and IL-8 expression in co-culture system on the expression of CD133, KLF4, Twist1, Vimentin in CSCs. (b) Western blot detected the effect of knockdown of IL-8 in PMSCs or inhibition of IL-8 expression in co-culture system on HCT116+/+ stemness- and EMT-related proteins. In vitro limiting dilution assay (c) and sphere formation assay (d) detected the effect of knockdown of IL-8 in PMSCs, inhibition of CXCR2 receptor, Erk1/2 signaling in HCT116+/+, and IL-8 expression in co-culture system on HCT116++ self-renewal ability. Data shown are the results (mean±SD) from three independent experiments. Statistical differences between two groups were expressed as ***P < 0.001, **P < 0.01, *P < 0.05; ###P < 0.001, ##P < 0.01, #P < 0.05; ●●●P < 0.001, ●P < 0.05. Images are representative of experiments with similar results. (A color version of this figure is available in the online journal.)
CM: conditioned medium from MSCs; CXCR2: C-X-C chemokine receptor 2; KLF4: kruppel like factor 4; NC: negative control.
Then, in vitro limited dilution assay and sphere formation assay examined the effect of knockdown of IL-8 in PMSCs, inhibition of CXCR2 receptor, Erk1/2 in HCT116+/+ and IL-8 expression in co-culture system on HCT116+/+ self-renewal ability. The results showed that inhibition of IL-8/CXCR2/Erk1/2 attenuated the effect of co-culture on HCT116+/+ self-renewal ability (Figure 5(c)). However, in the sphere formation assay, only SB225002 (CXCR2 inhibitor) can significantly inhibit the proliferation of CSCs (Figure 5(d)).
In conclusion, inhibition of IL-8/CXCR2/Erk1/2 could reverse the impact of PMSC on the stemness of colon CSC.
Colon CSCs promote cancer-associated fibroblast (CAF) phenotype of PMSCs via FGF10–PKA–Akt–β-catenin signaling
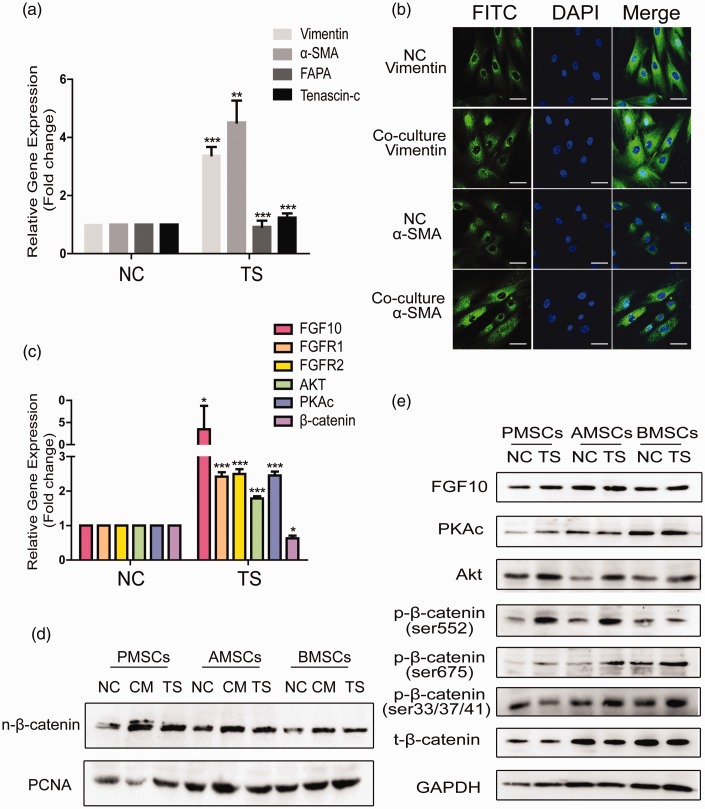
Studies16,17 proved that MSCs can differentiate into CAF-like cells after its consecutive exposure to cancer cell CM. We first examined whether HCT116+/+ can promote the differentiation of PMSCs into CAFs by detecting the levels of CAF markers Vimentin, α-SMA, FAPA, and Tenascin-c in PMSCs. The results showed that the expression of Vimentin and α-SMA was significantly increased after co-culture (Figure 6(a)). We then examined the fluorescence intensity and distribution of Vimentin and α-SMA by the confocal microscope and found the strengthened intensity of Vimentin and α-SMA and nuclear translocation of Vimentin in the co-culture group (Figure 6(b)). It is reported that some EMT-related genes are stimulated in AMSCs via the canonical Wnt signaling when co-cultured with HCT116.18 Next, we detected the levels of ZEB1/2, MMP2, MMP-9 (data not shown), FGF-10, and β-catenin in MSCs after co-culture with CSCs. The results showed that the levels of FGF-10 and its receptors FGFR1 and FGFR2 were upregulated in MSCs after co-culture, but total β-catenin was not changed at all (Figure 6(c)). Intriguingly, we found the levels of nuclei β-catenin were increased in the MSCs after co-culture with CSCs (Figure 6(d)). β-catenin can also be activated non-canonically via receptor tyrosine kinase activation featured by Akt-dependent and protein Kinase A (PKA) mediated phosphorylation of β-catenin at Serine-552.19 Then, we detected the activation of FGF10–PKA–Akt–β-catenin signaling pathway in PMSCs after co-culture with CSCs. The results showed that the expression of FGF10, PKA, Akt, p-β-catenin(ser552), and p-β-catenin(ser675) was obviously increased in the PMSCs after co-culture (Figure 6(e)).
Figure 6.
After co-culture with colon CSCs, FGF10–PKAc–Akt–β-catenin signaling is activated in PMSCs. (a) qRT-PCR detected the levels of Vimentin, α-SMA, FAPA, and Tenascin-c in PMSCs when co-cultured with HCT116+/+ by TS. (b) Immunofluorescence detected the fluorescence intensity and distribution of Vimentin and α-SMA in PMSCs after co-culture with HCT116+/+. Scale bar: 20 µm. qRT-PCR (c) and Western blot (e) detected the activation of FGF10–PKA–Akt–β-catenin signaling in PMSCs under the co-culture condition of TS. (d) Western blot detected the levels of β-catenin in the nucleus of three different sources of MSCs after co-culture with HCT116+/+ under the condition of CM or TS, respectively. (A color version of this figure is available in the online journal.)
α-SMA: alpha-smooth muscle actin; AMSCs: human adipose-derived mesenchymal stem cells; BMSCs: human bone marrow-derived mesenchymal stem cells; CM: conditioned medium from MSCs; DAPI: 4',6-diamidino-2-phenylindole; FAPA: fibroblaat activation protein alpha; FGF10: fibroblast growth factor 10; FITC: fluorescein isothiocyanate; GADPH: glyceraldehyde-3-phosphate dehydrogenase; NC: negative control; PCNA: proliferating cell nuclear antigen; PKAc: protein kinase A; PMSCs: human placenta-derived mesenchymal stem cells; TS: 0.4 µm Transwell co-culture in which MSCs were placed in the upper chambers.
In conclusion, PMSCs co-cultured with HCT116+/+ could produce a CAF phenotype and the intracellular FGF10–PKA–Akt–β-catenin signaling pathway was activated.
nIL-8 abs could attenuate the activated PKA-Akt-β-catenin signaling pathway in PMSCs
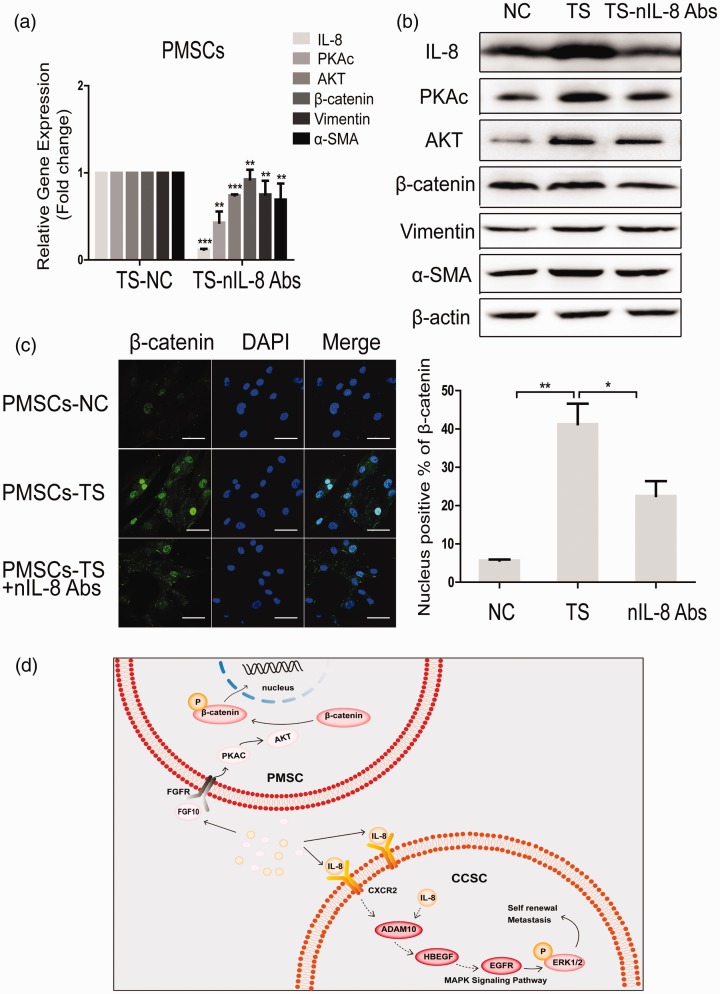
First, we examined the impact of IL-8 peptide inhibitor (nIL-8 Abs) on the CAF phenotype and non-canonical β-catenin signaling in PMSCs. The results showed that nIL-8 Abs attenuated the levels of IL-8, PKA, Akt, β-catenin, and α-SMA in PMSCs co-cultured with CSCs under the condition of TS co-culture (Figure 7(a) and (b)). Immunofluorescence showed that the intranuclear fluorescence intensity of β-catenin was increased in PMSCs after co-culture with HCT116+/+ and was significantly attenuated after the addition of IL-8 peptide inhibitor into the co-culture system (Figure 7(c)).
Figure 7.
nIL-8 Abs could attenuate the activated PKA-Akt-β-catenin signaling pathway in PMSCs. qRT-PCR (a) and Western blot (b) detected the effect of IL-8 peptide inhibitors on the expression of PKA, Akt, β-catenin, Vimentin, and α-SMA in PMSCs under TS co-culture condition. (c) Immunofluorescence assay detected intranuclear fluorescence intensity of β-catenin in PMSCs after co-culture with HCT116+/+. Quantification is shown at bottom. Scale bar: 20 µm. (d) A diagram of the mechanism by which PMSC interacts with HCT116+/+. Data shown are the results (mean±SD) from three independent experiments. Statistical differences between two groups were expressed as ***P < 0.001, **P < 0.01, *P < 0.05. Images are representative of experiments with similar results. (A color version of this figure is available in the online journal.)
ADAM10: a disintegrin and metalloproteinase domain 10; AKT: protein kinase B; α-SMA: alpha-smooth muscle actin; CCSC: colon cancer stem cell; CXCR2: C-X-C chemokine receptor 2; DAPI: 4',6-diamidino-2-phenylindole; EGFR: epidermal growth factor receptor; ERK1/2: phospho-extracellular regulated protein kinases1/2; FGFR: fibroblast growth factor receptor; FGF10: fibroblast growth factor 10; HBEGF: heparin-binding epidermal growth factor; MAPK: mitogen-activated protein kinase; NC: negative control; PKAc: protein kinase A; PMSCs: human placenta-derived mesenchymal stem cells; TS: 0.4 µm Transwell co-culture in which PMSCs were placed in the upper chambers.
Therefore, the conclusion of this study can be summarized as follows: After co-culture of CCSC and MSC, IL-8 secreted by MSC binds to CCSC cell membrane receptor CXCR2, then activates downstream MAPK signaling pathway, thus enhancing the stemness and EMT of CCSC. At the same time, CCSC activates FGF10–PKA–Akt–β-catenin signaling in MSC, promoting the nuclear translocation of β-catenin and a CAF phenotype (Figure 7(d)).
Discussion
MSCs are a category of adult stem cells that develop from the mesoderm with the general features of stem cells, namely the self-renewal ability and multidirectional differentiation capability.20 With the deepening research on the biological characteristics of MSCs, its clinical application is also widespread. MSCs are low in immunogenicity, making them susceptible to exogenous gene expression.21 At the same time, MSC has excellent tumor tropism. These advantages make MSCs useful in animal models of a variety of malignant solid tumors. For example, MSCs could be genetically modified to secrete different anticancer molecules, such as tissue necrosis factor (TNF), TNF-related apoptosis-inducing ligand, or IFN-β. MSCs modified with IFN-β were inoculated into a human melanoma xenograft model and the tumor growth was decreased and the survival rate of mouse was increased.22 In addition, TRAIL-modified MSCs can effectively inhibit the growth of intracranial glioma.23 There are many sources of MSCs. It is worth mentioning that placenta-derived MSCs have a longer life span and higher proliferation than MSCs derived from other tissues.24 Thus, many researchers believe the placenta may serve as a favorable source of MSCs for clinical use.
Preclinical data have demonstrated that MSCs are stromal cells involved in promoting the initiation and development of colon cancer.25 MSCs promote CRC through the interplay between MSCs and colon cancer cells.26 Therefore, we were meant to assess the safety of MSCs for clinical treatment in colon cancer. To explore the effects of different sources of MSCs on colon cancer cells, HCT116 was cultured in CM from three different sources of MSCs (bone marrow, adipose, placenta). The results suggested that the levels of KLF4, CD44, and CD133 in the three co-cultured groups were evidently higher than that in the control group, meanwhile the expression of EMT-related genes Vimentin and Twist1 was increased, and E-cadherin was decreased. The proliferation ability of HCT116 after co-culture with PMSCs was increased. After co-culture of HCT116 with AMSCs and PMSCs, the proportion of CD133+/CD44+ subpopulations was increased. The balb/c nude mice were inoculated subcutaneously, and the tumor mass of AMSC group increased significantly. When the subcutaneous tumor grew to 50 mm3, the MSCs were injected through the tail vein, and the tumor volume of the PMSC group and AMSC group increased. The CT-26 peritoneal metastasis model of balb/c mice was constructed and MSCs were injected into the tail vein. Liver metastasis was evident in the PMSC group, and all three MSCs promoted abdominal metastasis. The survival rate of mice in AMSCs and PMSCs decreased. In summary, MSCs from three sources can induce the stemness and EMT of colon cancer cells in vitro, and AMSCs and PMSCs increase the proportion of colon CSCs.
Cytokines play a key role in intercellular communication and mediate stem cell phenotype. Tumor microenvironment can produce VEGF, TGF-β, matrix metalloproteinase, SDF-1, IGF, PDGF, Wnt, and Hedgehog ligands, which stimulate CSC self-renewal and angiogenesis.27 When MSCs migrate toward the tumor, they can interact with tumor cells through paracrine signals. Different sources of MSCs secrete different cytokines in the tumor microenvironment. After being affected by cytokines secreted by MSCs, the tumor microenvironment can self-evolve and facilitate metastasis, which has a great impact on the risk assessment of metastatic cancer patients.28 In this work, the effects of colon CSCs on cytokines in MSCs were analyzed by bioinformatics and in vitro assays. These cytokines function through two high-affinity receptors: CXCR1 and CXCR2. The GEO enrichment of differentially expressed genes in GSE106325 indicates that the interaction between cytokine and cytokine receptor is enhanced in the MSC–CSC co-culture system. The cytokine IL-8 was significantly enhanced in the MSCs and CSCs of the three co-culture groups. Many researchers have discovered that MSC helps to construct the CSC niche, which provides a powerful tumor microenvironment for the maintenance of CSC stemness, thereby promoting cancer cell metastasis, including breast, ovarian, lung, and colon cancer.7 Tumor-infiltrating MSCs can support CSCs through a variety of autocrine and/or paracrine pathways, that is MSCs can secrete some cytokines, IL-6, IL-8, and CCL-5 to promote stemness of the cancer cell.29 For instance, breast cancer can produce IL-6, which attracts MSCs, thereby producing a CSC-supporting cytokine CXCL7.8 MSCs in colon cancer maintain the stemness of CSCs by secreting the Notch ligand Jagged-1.30 In the pancreatic ductal gland mouse xenograft model, MSCs positively regulated CSCs through the STAT3–CXCL10–CXCR3 paracrine signal axis.31 IL-8 can initiate an intracellular downstream MAPK signaling by binding to the CXCR1/2 receptor.32 Our studies indicate that IL-8 secreted by MSCs activates the CXCR2/MAPK signaling pathway of CSCs. Inhibition of IL-8 in co-culture systems can inhibit the stemness and EMT of CSCs. Inhibition of CXCR2 receptor and Erk1/2 also inhibits the self-renewal ability of CSCs.
In addition, MSCs can be differentiated into cancer associated fibroblasts (CAFs). The role of MSCs in the tumor microenvironment is based on the assumption that MSCs are precursors of CAFs.33 CAFs are important cell groups in the tumor microenvironment, which are involved in vascular construction and promote tumor cell infiltration and metastasis. In this study, co-culture of CSCs with MSCs increased the expression of CAF markers α-SMA and Vimentin in MSCs and also elevated MSC migration-related FGF10. This work demonstrated for the first time that Wnt non-canonical pathway is activated in MSCs after co-culture with CSCs.
To conclude, we are the first to co-culture MSCs from three different sources with colon CSCs in vitro. We found inhibition of IL-8/CXCR2/MAPK signaling pathway could reverse the effect of MSCs on CSC stemness, which provides a theoretical basis for the application of MSCs in clinical practice.
Supplemental Material
Supplemental material, EBM910690 Supplemental Material for Mesenchymal stem cells maintain the stemness of colon cancer stem cells via interleukin-8/mitogen-activated protein kinase signaling pathway by Xiaoying Ma, Jiajun Liu, Xiaotong Yang, Kai Fang, Peiyong Zheng, Xin Liang and Jianwen Liu in Experimental Biology and Medicine
Authors’ contributions
PYZ, XL, and JWL participated in the design, interpretation of the studies, and review of the manuscript; XYM, JJL, XTY, and KF conducted the experiments; XYM analyzed the data and wrote the manuscript; JWL applied for the funds.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval and consent to participate
Seven clinical CRC samples were collected with ethical approval from the research ethics committees of Shanghai Tenth People’s Hospital.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by the National Natural Science Foundation of China (No. 81873050).
ORCID iD
Jianwen Liu https://orcid.org/0000-0003-4149-4257
SUPPLEMENTAL MATERIAL
Supplemental material for this article is available online.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68:7–30 [DOI] [PubMed] [Google Scholar]
- 2.Iplik ES, Ertugrul B, Kozanoglu I, Baran Y, Cakmakoglu B. An answer to colon cancer treatment by mesenchymal stem cell originated from adipose tissue. Iran J Basic Med Sci 2018; 21:465–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramdasi S, Sarang S, Viswanathan C. Potential of mesenchymal stem cell based application in cancer. Int J Hematol Oncol Stem Cell Res 2015; 9:95–103 [PMC free article] [PubMed] [Google Scholar]
- 4.Kikuchi H, Yagi H, Hasegawa H, Ishii Y, Okabayashi K, Tsuruta M, Hoshino G, Takayanagi A, Kitagawa Y. Therapeutic potential of transgenic mesenchymal stem cells engineered to mediate anti-high mobility group box 1 activity: targeting of colon cancer. J Surg Res 2014; 190:134–43 [DOI] [PubMed] [Google Scholar]
- 5.Zhang D, Zheng L, Shi H, Chen X, Wan Y, Zhang H, Li M, Lu L, Luo S, Yin T, Lin H, He S, Luo Y, Yang L. Suppression of peritoneal tumorigenesis by placenta-derived mesenchymal stem cells expressing endostatin on colorectal cancer. Int J Med Sci 2014; 11:870–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Malley G, Heijltjes M, Houston AM, Rani S, Ritter T, Egan LJ, Egan LJ, Ryan AE. Mesenchymal stromal cells (MSCs) and colorectal cancer: a troublesome twosome for the anti-tumour immune response. Oncotarget 2016; 7:60752–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan TS, Shaked Y, Tsai KK. Targeting the interplay between cancer fibroblasts, mesenchymal stem cells, and cancer stem cells in desmoplastic cancers. Front Oncol 2019; 9:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalwitz G, Endres M, Neumann K, Skriner K, Ringe J, Sezer O, Sittinger M, Haupl T, Kaps C. Gene expression profile of adult human bone marrow-derived mesenchymal stem cells stimulated by the chemokine CXCL7. Int J Biochem Cell Biol 2009; 41:649–58 [DOI] [PubMed] [Google Scholar]
- 9.Rivera-Cruz CM, Shearer JJ, Figueiredo Neto M, Figueiredo ML. The immunomodulatory effects of mesenchymal stem cell polarization within the tumor microenvironment niche. Stem Cells Int 2017; 2017:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hossain A, Gumin J, Gao F, Figueroa J, Shinojima N, Takezaki T, Priebe W, Villarreal D, Kang SG, Joyce C, Sulman E, Wang Q, Marini FC, Andreeff M, Colman H, Lang FF. Mesenchymal stem cells isolated from human gliomas increase proliferation and maintain stemness of glioma stem cells through the IL-6/gp130/STAT3 pathway. Stem Cells 2015; 33:2400–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma X, Liu J, Li J, Li Y, Le VM, Li S, Liang X, Liu L, Liu J. miR-139-5p reverses stemness maintenance and metastasis of colon cancer stem-like cells by targeting E2-2. J Cell Physiol 2019; 234:22703–18 [DOI] [PubMed] [Google Scholar]
- 12.Tang KH, Ma S, Lee TK, Chan YP, Kwan PS, Tong CM, Ng IO, Man K, To KF, Lai PB, Lo CM, Guan XY, Chan KW. CD133(+) liver tumor-initiating cells promote tumor angiogenesis, growth, and self-renewal through neurotensin/interleukin-8/CXCL1 signaling. Hepatology 2012; 55:807–20 [DOI] [PubMed] [Google Scholar]
- 13.Mohammadi M, Kaghazian M, Rahmani O, Ahmadi K, Hatami E, Ziari K, Talebreza A. Overexpression of interleukins IL-17 and IL-8 with poor prognosis in colorectal cancer induces metastasis. Tumour Biol 2016; 37:7501–5 [DOI] [PubMed] [Google Scholar]
- 14.Ha H, Debnath B, Neamati N. Role of the CXCL8-CXCR1/2 axis in cancer and inflammatory diseases. Theranostics 2017; 7:1543–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan P, Zhu H, Yin L, Wang L, Xie P, Ye J, Jiang X, He X. Integrin alphavbeta6 promotes lung cancer proliferation and metastasis through upregulation of IL-8-Mediated MAPK/ERK signaling. Transl Oncol 2018; 11:619–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shinagawa K, Kitadai Y, Tanaka M, Sumida T, Kodama M, Higashi Y, Tanaka S, Yasui W, Chayama K. Mesenchymal stem cells enhance growth and metastasis of colon cancer. Int J Cancer 2010; 127:2323–33 [DOI] [PubMed] [Google Scholar]
- 17.Wen S, Niu Y, Yeh S, Chang C. BM-MSCs promote prostate cancer progression via the conversion of normal fibroblasts to cancer-associated fibroblasts. Int J Oncol 2015; 47:719–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen D, Liu S, Ma H, Liang X, Ma H, Yan X, Yang B, Wei J, Liu X. Paracrine factors from adipose-mesenchymal stem cells enhance metastatic capacity through Wnt signaling pathway in a colon cancer cell co-culture model. Cancer Cell Int 2015; 15:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Utley S, James D, Mavila N, Nguyen MV, Vendryes C, Salisbury SM, Phan J, Wang KS. Fibroblast growth factor signaling regulates the expansion of A6-expressing hepatocytes in association with AKT-dependent beta-catenin activation. J Hepatol 2014; 60:1002–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin E, Jiang Y, Deng Y, Lapsiwala R, Lin T, Blau C. Cancer stem cells, endothelial progenitors, and mesenchymal stem cells: “seed and soil” theory revisited. Gastrointest Cancer Res 2018; 2:169–74 [PMC free article] [PubMed] [Google Scholar]
- 21.Francois S, Usunier B, Forgue-Lafitte ME, L’Homme B, Benderitter M, Douay L, Gorin NC, Larsen AK, Chapel A. Mesenchymal stem cell administration attenuates colon cancer progression by modulating the immune component within the colorectal tumor microenvironment. Stem Cells Transl Med 2019; 8:285–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han S, Park C, Ahn J, Park S, Jung W, Seo K, Ra J, Kang S, Lee H, Youn H. Pro-apoptotic and growth-inhibitory effect of IFN-β-overexpressing canine adipose tissue-derived mesenchymal stem cells against melanoma cells. Anticancer Res 2015; 35:4749–56 [PubMed] [Google Scholar]
- 23.Loebinger MR, Sage EK, Davies D, Janes SM. TRAIL-expressing mesenchymal stem cells kill the putative cancer stem cell population. Br J Cancer 2010; 103:1692–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu Y, Liu H, Lou G, Zhang Q, Wu C. Human placenta mesenchymal stem cells expressing exogenous kringle1-5 protein by fiber-modified adenovirus suppress angiogenesis. Cancer Gene Ther 2014; 21:200–8 [DOI] [PubMed] [Google Scholar]
- 25.Chen K, Liu Q, Tsang LL, Ye Q, Chan HC, Sun Y, Jiang X. Human MSCs promotes colorectal cancer epithelial-mesenchymal transition and progression via CCL5/beta-catenin/slug pathway. Cell Death Dis 2017; 8:e2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu XB, Liu Y, Wang GH, Xu X, Cai Y, Wang HY, Li YQ, Meng HF, Dai F, Jin JD. Mesenchymal stem cells promote colorectal cancer progression through AMPK/mTOR-mediated NF-kappaB activation. Sci Rep 2016; 6:21420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye J, Wu D, Wu P, Chen Z, Huang J. The cancer stem cell niche: cross talk between cancer stem cells and their microenvironment. Tumour Biol 2014; 35:3945–51 [DOI] [PubMed] [Google Scholar]
- 28.Takigawa H, Kitadai Y, Shinagawa K, Yuge R, Higashi Y, Tanaka S, Yasui W, Chayama K. Mesenchymal stem cells induce epithelial to mesenchymal transition in colon cancer cells through direct cell-to-Cell contact. Neoplasia 2017; 19:429–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhong W, Tong Y, Li Y, Yuan J, Hu S, Hu T, Song G. Mesenchymal stem cells in inflammatory microenvironment potently promote metastatic growth of cholangiocarcinoma via activating Akt/NF-κB signaling by paracrine CCL5. Oncotarget 2017; 8:73693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiu Y, Guo J, Mao R, Chao K, Chen BL, He Y, Zeng ZR, Zhang SH, Chen MH. TLR3 preconditioning enhances the therapeutic efficacy of umbilical cord mesenchymal stem cells in TNBS-induced colitis via the TLR3-Jagged-1-Notch-1 pathway. Mucosal Immunol 2017; 10:727–42 [DOI] [PubMed] [Google Scholar]
- 31.Yue C, Shen S, Deng J, Priceman SJ, Li W, Huang A, Yu H. STAT3 in CD8+ T cells inhibits their tumor accumulation by downregulating CXCR3/CXCL10 axis. Cancer Immunol Res 2015; 3:864–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Q, Li A, Tian Y, Wu JD, Liu Y, Li T, Chen Y, Han X, Wu K. The CXCL8-CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev 2016; 31:61–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang X, Hao J, Mao Y, Jin ZQ, Cao R, Zhu CH, Liu XH, Liu C, Ding XL, Wang XD, Chen D, Wu XZ. bFGF promotes migration and induces cancer-associated fibroblast differentiation of mouse bone mesenchymal stem cells to promote tumor growth. Stem Cells Dev 2016; 25:1629–39 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, EBM910690 Supplemental Material for Mesenchymal stem cells maintain the stemness of colon cancer stem cells via interleukin-8/mitogen-activated protein kinase signaling pathway by Xiaoying Ma, Jiajun Liu, Xiaotong Yang, Kai Fang, Peiyong Zheng, Xin Liang and Jianwen Liu in Experimental Biology and Medicine