Short abstract
Peritoneum is one of the most common metastatic sites of colorectal cancer (CRC). It has been reported that cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (HIPEC) prolongs the lifespan of patients with peritoneal carcinomatosis of colorectal origin (CRC-PC), while the drugs used for HIPEC are limited. We investigated the application of recombinant mutant human tumor necrosis factor-α (rmhTNF) combined with raltitrexed in the HIPEC treatment in a mice model with CRC-PC. In vitro, we detected the cytotoxicity and apoptosis of human colorectal cancer cells by 3–(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay, Western blot, and TdT-mediated dUTP Nick End Labeling (TUNEL) assay. In vivo, we established xenograft models of CRC-PC and assessed the antitumor effect by in vivo imaging, peritoneal cancer index scoring, and TUNEL assay. The results showed that the combination of rmhTNF and raltitrexed under hyperthermia with a temperature of 42°C inhibited the growth of colorectal cancer cells significantly in vitro, and after HIPEC treatments with rmhTNF and raltitrexed, peritoneal tumor growth was prohibited in vivo. Our findings about the efficacy of rmhTNF and raltitrexed used for HIPEC to treat CRC-PC will provide experimental data and basis for their potential clinical application.
Impact statement
Colorectal peritoneal carcinomatosis exhibits poor prognosis and presents a treatment challenge. At present, the main treatment is surgery, supplemented by hyperthermic intraperitoneal chemotherapy (HIPEC), but the drugs used for HIPEC are limited. Our study found that the combination of recombinant mutant human TNF-α (rmhTNF) and raltitrexed (RTX) under hyperthermia with a temperature of 42°C had antitumor effect both in vitro and vivo. The findings will provide experimental data and basis for the potential clinical application of rmhTNF and RTX, which might offer patients a new choice of therapeutic drugs.
Keywords: Recombinant mutant human TNF-α, hyperthermic intraperitoneal chemotherapy, hyperthermia, raltitrexed, colorectal cancer, peritoneal carcinomatosis
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer mortality and the fourth in diagnosis among all types of human malignant neoplasm.1 The obvious clinical symptoms usually occur in the advanced stage of cancer, and tumor cells are likely to spread and seed into the peritoneal cavity.2,3 This process is called peritoneal metastasis (PM), and finally forms peritoneal carcinomatosis (PC). PM originated from CRC is associated with poor prognosis, with an average survival of only 5–24 months.4,5 Maximum cytoreductive surgery (CRS) to remove macroscopic tumors from the abdominal cavity, and then intraperitoneal chemotherapy to eradicate all residual microscopic diseases are currently the standard treatment for advanced CRC.6–8
Hyperthermic intraperitoneal chemotherapy (HIPEC) followed by CRS is the most proactive way of dealing with PC,9 showing significant survival rate increase.10 HIPEC is a process of intraperitoneal infusion of chemotherapeutic drugs at precise temperature and perfusion rates.11 HIPEC mainly includes intraperitoneal chemotherapy and hyperthermia (HT). HT refers to heating tumor tissue to 40–43°C to further increase the lethality of selected drugs by enhancing cytotoxicity.12 Besides, free tumor cells, tiny tumor nodules and subclinical lesions can be cleared away by HIPEC, because HIPEC has the ability to directly target and mechanically flush cancer cells.11,13 At present, the most commonly used drugs for HIPEC are all synergistic with heat, including mitomycin C and platinum-based cytotoxic drugs (i.e. cisplatin, oxaliplatin, and carboplatin).14,15 Although these drugs are effective in the treatment of PC, the absolute advantage of any specific drug cannot be derived.16
Tumor necrosis factor-α (TNF-α) is a natural cytokine product activated by macrophages. TNF-α is a potential anticancer drug which is effective for various malignancies.17 TNF-α can inhibit the proliferation of tumor cells and increase the infiltration of chemotherapeutic agents into tumor tissues.18 However, clinical trial studies have shown that TNF-α cannot be used for systemic chemotherapy because of its dose-limiting toxicity.19 Thus, recombinant mutant human TNF-α (rmhTNF) was prepared by replacing Pro 8, Ser 9, Asp 10, and Leu 157 with Arg 8, Lys 9, Arg 10, and Gln 157 and deleting the first seven amino acids at the N-terminal.20 rmhTNF has been used to treat malignant pleural effusions, non-Hodgkin’s lymphoma, and non-small cell lung cancer, with much lower toxicity and better efficacy than TNF-α.20,21 It has also been reported that rmhTNF combined with traditional chemotherapeutic drugs can enhance the antitumor effect.22,23
Raltitrexed (RTX) is a folate analogue belonging to the family of antimetabolites with antitumor activity related to the inhibition of thymidine synthase enzyme. RTX has been reported to be effective in many types of cancer,24–26 especially advanced CRC.27,28 Our previous studies confirmed that HIPEC with RTX was a therapeutic strategy to treat CRC-PC in mouse models without any severe untoward effects accompanied.11,29 Besides, compared to the systemic chemotherapy with the intravenous drip of RTX, HIPEC could promote the intracellular absorption of RTX according to pharmacokinetic studies.11
Here, we assessed the effects of rmhTNF, rmhTNF + HT, and rmhTNF + RTX + HT on human CRC cells in vitro. Then, we established the CRC-PC xenograft mice model to evaluate the efficacy of rmhTNF and rmhTNF + RTX under intraperitoneal perfusion chemotherapy (IPEC) or HIPEC. The findings would provide experimental data and basis for the potential clinical application of rmhTNF and rmhTNF + RTX in the treatment of CRC-PC.
Materials and methods
Reagents
Reagents were obtained from the following sources: rmhTNF from Weike Biopharmaceutical, Shanghai, China; and RTX from Chia Tai Tianqing Pharmaceutical, Nanjing, China. The reagents were dissolved in normal saline and stored under −20°C.
Cell culture
Human CRC cell lines (HCT116, HCT116-luc, SW480, HCT8 and LOVO) were purchased from the Cell Bank of Chinese Academy of Science and cultured in RPMI1640 medium (Gibco, USA) which was supplemented with 10% fetal bovine serum (FBS, Gibco), 100 units/mL penicillin and 100 μg/mL streptomycin (Gibco). Cell was cultured in a humidified incubator with 5% CO2 at 37°C.
Cytotoxicity assay in vitro
Cells seeded in 96-well plates at a density of 1000 cells per well for overnight, and then treated with different concentrations of drugs in the test culture medium containing 3% FBS and 1 μg/mL actinomycin D (MCE, USA) for 24, 48, or 72 h.30,31 For HT treatment, cells were cotreated with drugs and HT at 42°C for 0, 4, 6 or 8 h, and then returned to 37°C until the incubation time reached 24 or 48 h. The cell viability was determined by using MTT (Sigma, USA) assay. The half inhibitory concentrations (IC50) were calculated by the GraphPad Prism 5 software.
TdT-mediated dUTP nick end labeling assay
The TUNEL assay was used to detect DNA breaks, which was determined by One Step TUNEL Apoptosis Assay Kit (Beyotime Biotechnology, China) according to the manufacturer’s instructions. The stained cells were measured by flow cytometry (BD, USA). The tumor tissues were counterstained with 4ʹ,6-diamidino-2-phenylindole (DAPI, Sigma, USA). Images were acquired with the fluorescence microscope (Nikon, Japan). The quantification of positive cells was obtained by Image-J.
Western blot analysis
Protein samples were extracted from cells and electrophoresed on sodium dodecyl sulfate polyacrylamide gel electrophoresis gels. The procedures were accomplished as previously reported.32 The primary antibodies against cleaved caspase3 and β-actin were purchased from Proteintech, China, and cleaved caspase9 were purchased from Wanleibio, China. The reactive protein was probed with horseradish peroxidase-conjugated secondary antibodies (Proteintech, China) and evaluated using an enhanced chemiluminescent system (Tanon, China).
Animals and tumor xenotransplantation
Athymic male BALB/c nude mice, four to five weeks old, were purchased from the Shanghai Laboratory Animal Resource Center. The animals were maintained in a pathogen-free environment and provided with autoclaved food, water, and cages. All experimental procedures were carried out according to the Institutional Animal Care Guidelines.
Luciferase-expressing HCT116-luc cells were suspended in PBS containing 500 μg/mL Matrigel (Corning BD Matrigel Basement Membrane Matrix,) and injected into the abdomen of each mouse in a total volume of 100 μL (2.5 × 106 cells).
IPEC and HIPEC treatments
After inoculation, animals were randomly assigned to six mice each group for different treatments for 30 min according to our previous study.11,29 The difference between IPEC and HIPEC was the temperature of perfusate. The temperature of IPEC was 37°C, and that of HIPEC was 42°C. rmhTNF (2500 IU/g per body weight) and RTX (0.335 μg/g per body weight) were administered every time. The dose of rmhTNF was referred to previous literatures,20 and that of RTX was chosen from our previous study.11,29 The remaining identical perfusion process was performed as previously reported.11,29
In vivo imaging
Firstly, D-luciferin potassium salt (150 μg/g per body weight, PerkinElmer, USA) was injected into the abdomen of mice. Then after 5 min, 1% phenobarbital sodium was used to anesthetize the mice before in vivo imaging. The fluorescence signals were captured and detected by in vivo imaging system (Kodak, USA).
Peritoneal cancer index scoring
According to previous reports by Aarts et al.,33 we opened the abdominal cavity, divided it into four quadrants, and analyzed the intraperitoneal tumor growth semi-quantitatively. The scoring rules were as follows: 0 (none); 1 (little, diameters of 1–2 mm, 1–2 sites); 2 (moderate, diameters of 2–5 mm, 1–2 sites); 3 (abundant, diameters of >5 mm, >2 sites). The e PCI score was the sum of the four quadrants (0–12).
Histopathological assay
The tumor tissues were harvested after animals were sacrificed. The procedure of histopathological assay was accomplished by conventional hematoxylin-eosin (H&E) staining in accordance with standard techniques.34
Statistical analysis
Experimental data were expressed by the mean ± standard deviation (SD) of three independent experiments and analyzed by using Student’s t test and Mann–Whitney U test to evaluate the significance of differences between the untreated and treated group. The significant differences were labeled as *P < 0.05, **P < 0.01, ***P < 0.001, and P values were derived from two-tailed tests.
Results
rmhTNF suppressed the survival of human CRC cells
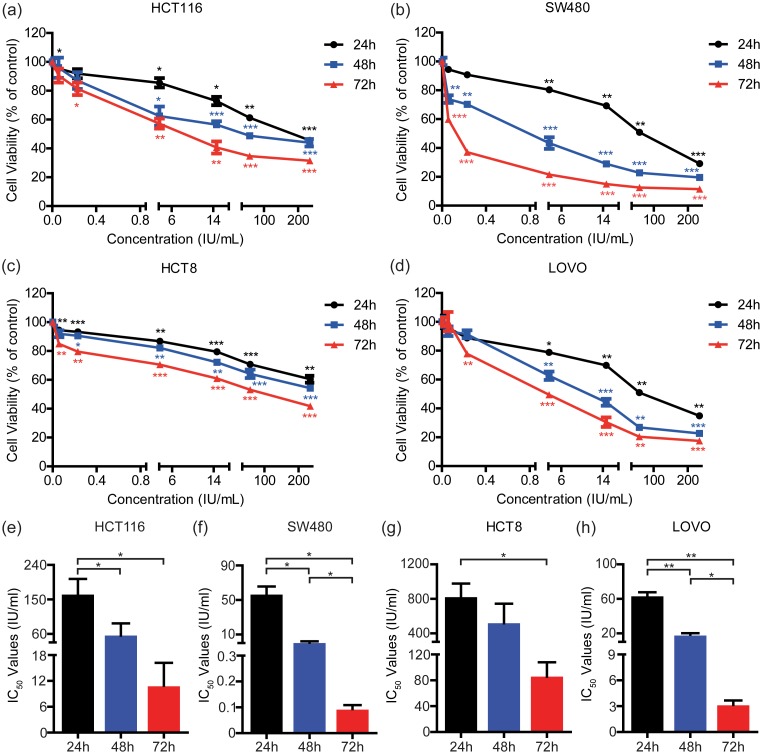
To determine the potential of rmhTNF to inhibit the growth of human CRC cells in vitro, HCT116, SW480, HCT8, and LOVO were incubated with different concentrations of rmhTNF for 24, 48, or 72 h. The cell viability was detected by the MTT assay (Figure 1(a) to (d)), and the IC50 values were calculated by the GraphPad Prism 5 software (Figure 1(e) to (h)). We found that rmhTNF could induce death in CRC cells in a dose-dependent and time-dependent manner. Remarkably, the incubation of rmhTNF for 72 h significantly suppressed the survival of human CRC cells compared with that for 24 h. HCT116 and HCT8 were less susceptive to rmhTNF than SW480 and LOVO.
Figure 1.
rmhTNF suppressed the survival of human CRC cells. HCT116 (a), SW480 (b), HCT8 (c) and LOVO (d) cells were incubated with different concentrations of rmhTNF for 24, 48, or 72 h, respectively. The cell viability was detected by the MTT assay. *P < 0.05, **P < 0.01, ***P < 0.001 vs. untreated groups (from 24, 48, and 72 h, respectively) (Student’s t test). The IC50 values of HCT116 (e), SW480 (f), HCT8 (g), and LOVO (h) cells were calculated by the GraphPad Prism 5 software. *P < 0.05, **P < 0.01, ***P < 0.001 (Student’s t test). Results represented mean ± SD of three replicates. IC50: half inhibitory concentrations. (A color version of this figure is available in the online journal.)
HT enhanced rmhTNF-mediated cell death in human CRC cells
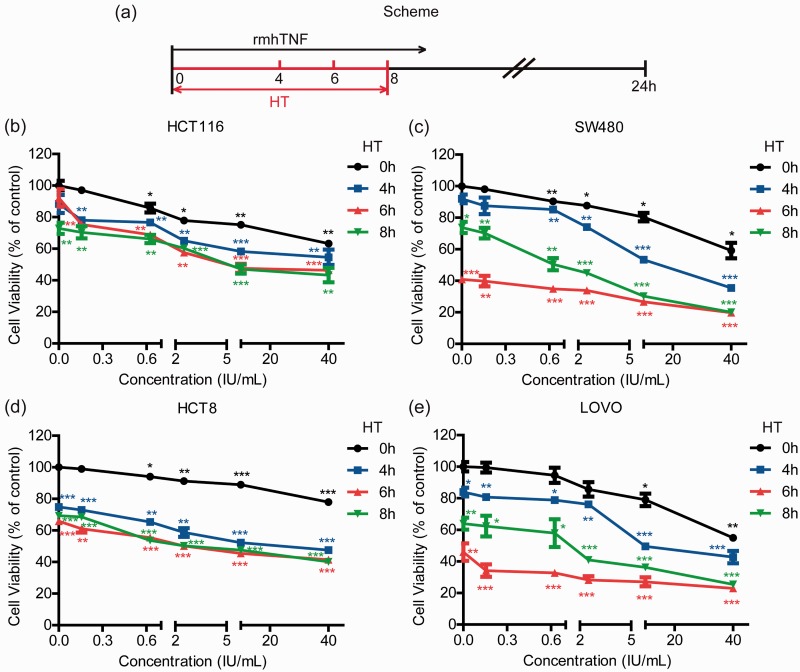
To investigate the effects of HT in vitro, we used the MTT assay to examine the cytotoxicity of rmhTNF on human CRC cells under HT. The incubation time of HT (42°C) was 0, 4, 6, or 8 h respectively, and then cells were allowed to recover at 37°C with a total of 24-h treatment (Figure 2(a)). Figure 2(b) to (e) shows that HT could decrease the cell viability in all four cell lines. HT for 4 or 6 h significantly increased rmhTNF-induced cell death compared to the control group, while HT for 8 h did not go on expediting death. The optimal time of HT for rmhTNF was 6 h. In addition, HT further enhanced rmhTNF-mediated cell death in HCT116 and HCT8, which were not so sensitive to rmhTNF.
Figure 2.
HT enhanced rmhTNF-mediated death in human CRC cells. (a) Scheme of treatments. HCT116 (b), SW480 (c), HCT8 (d), and LOVO (e) cells were cotreated with rmhTNF and HT (42°C) for 0, 4, 6, or 8 h, and then returned to 37°C until the incubation time reached 24 h. The cell viability was detected by the MTT assay. Results represented mean ± SD of three replicates. *P < 0.05, **P < 0.01, ***P < 0.001 vs. untreated group (no drug and HT for 0 h) (Student’s t test). HT: hyperthermia; rmhTNF: recombinant mutant human TNF-α. (A color version of this figure is available in the online journal.)
rmhTNF + RTX + HT combination treatment effectively inhibited the viability rate in human CRC cells
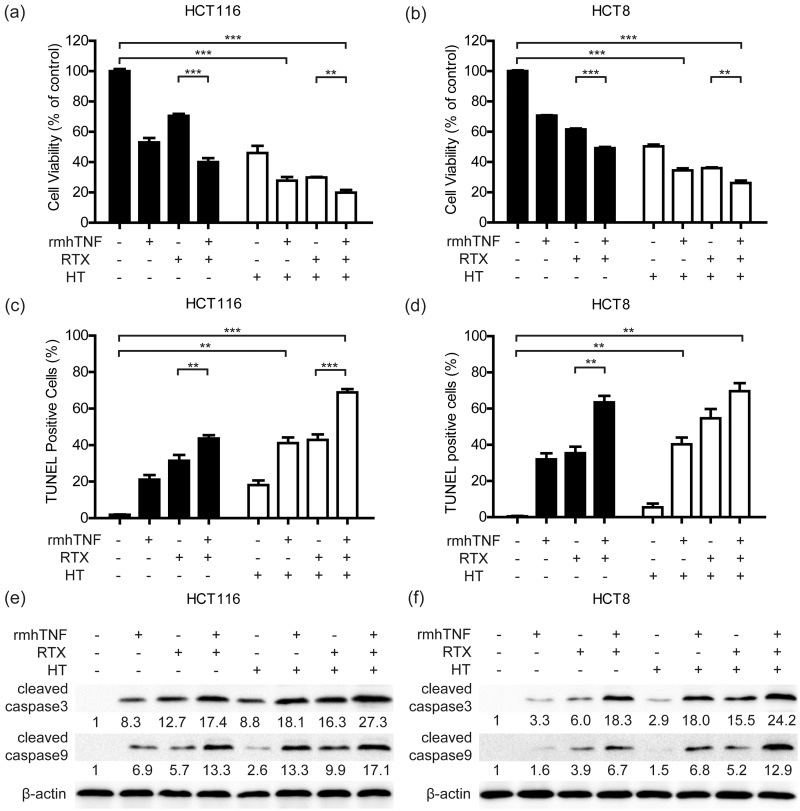
RTX has been frequently applied to treat with advanced CRC cases with PM in clinic.27,28 Our previous study identified that RTX + HT had therapeutic effects both in vitro and in vivo.11 To verity the efficacy of rmhTNF + RTX + HT combination treatment in vitro, HCT116 and HCT8 were incubated with different conditions for 48 h (rmhTNF: 40 IU/mL; RTX: 10 µg/mL; HT: 6 h). The cell viability was evaluated with the MTT assay (Figure 3(a) and (b)). We found that rmhTNF + RTX depressed the activity of cancer cells, and rmhTNF + RTX + HT was the most effective treatment.
Figure 3.
rmhTNF+RTX+HT combination treatment effectively inhibited the viability rate in human CRC cells. HCT116 (a) and HCT8 (b) cells were cotreated with rmhTNF (40 IU/mL), RTX (10 μg/mL), and HT (42°C) for 6 h, and then returned to 37°C until the incubation time reached 48 h. The cell viability was detected by the MTT assay. HCT116 (c) and HCT8 (d) cells were collected for TUNEL staining. The TUNEL-positive cells were detected by flow cytometry. *P < 0.05, **P < 0.01, ***P < 0.001 vs. untreated groups (Student’s t test). The protein expression of cleaved caspase3 and cleaved caspase9 in HCT116 (e) and HCT8 (f) samples harvested 6 h after different treatments were detected by Western blot. β-actin was the normalization for the loading. Results represented mean ± SD of three replicates. rmhTNF: recombinant mutant human TNF-α; RTX: raltitrexed; HT: hyperthermia; TUNEL: TdT-mediated dUTP nick end labeling.
To detect the proportion of apoptotic cells, the cells were collected for TUNEL staining. The results showed that the higher proportion of apoptotic cells was detected in rmhTNF + RTX and rmhTNF + RTX + HT groups (Figure 3(c) and (d)). The proapoptotic proteins, including cleaved caspase3 and cleaved caspase9 were further investigated in HCT116 and HCT8 using Western blot (Figure 3(e) and 3(f)). The results showed that rmhTNF + RTX + HT combination treatment greatly increased levels of cleaved caspase3 and cleaved caspase9. In short, rmhTNF + RTX + HT combination treatment effectively inhibited the viability in human CRC cells.
HIPEC with rmhTNF + RTX could significantly inhibit the tumor growth in the CRC-PC xenograft model
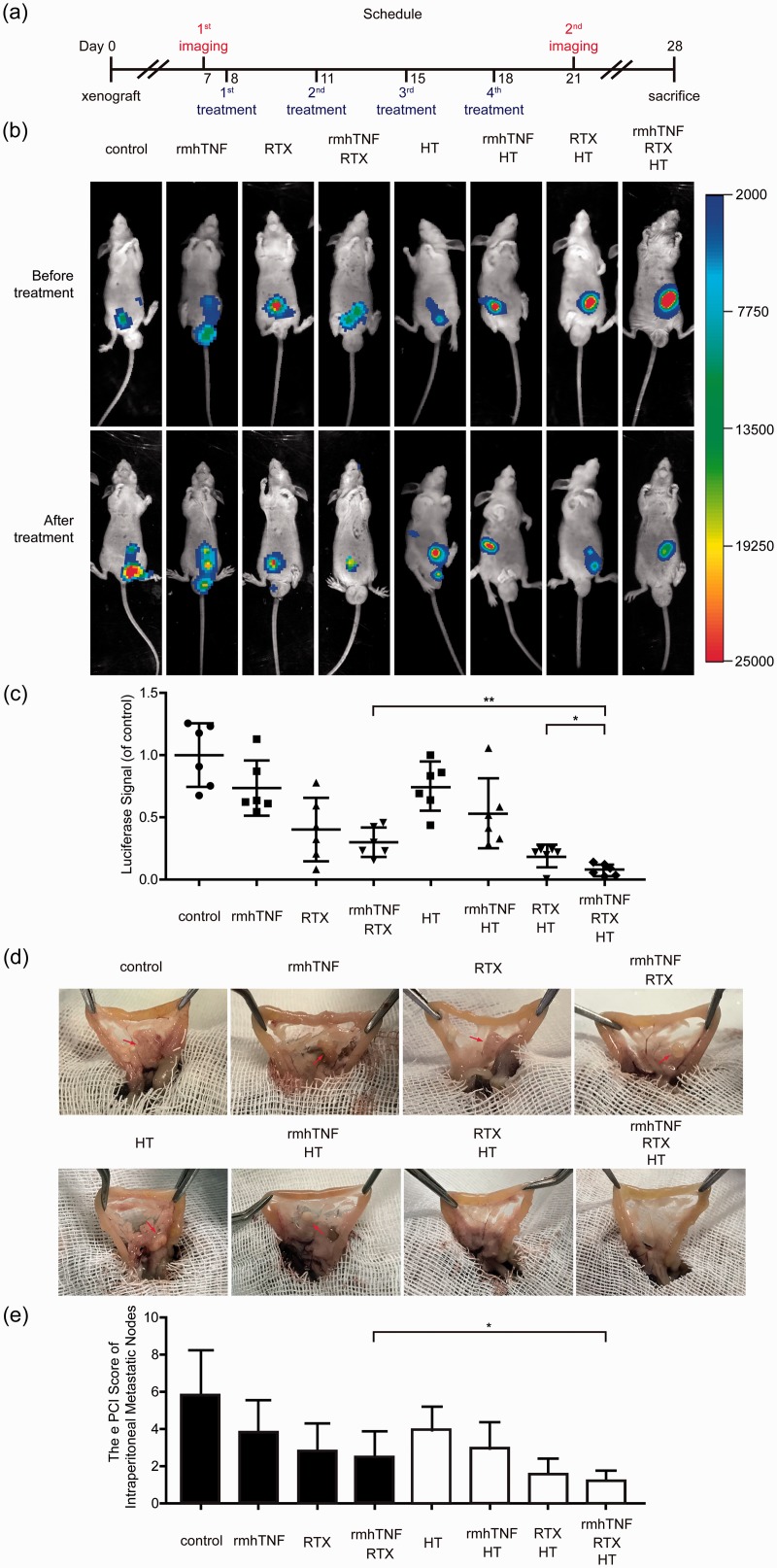
To identify the effect of rmhTNF + RTX in vivo, the CRC-PC xenograft model was established by using luciferase-expressing HCT116 (HCT116-luc). The tumor growth could be observed by in vivo fluorescence imaging system. The scheme and schedule of treatments are shown in Figure 4(a). Each mouse was given four times of chemotherapy as a course, and treated in eight different ways: control (IPEC with saline), rmhTNF (IPEC with rmhTNF), RTX (IPEC with RTX), rmhTNF + RTX (IPEC with rmhTNF and RTX), HT (HIPEC with saline), rmhTNF + HT (HIPEC with rmhTNF), RTX + HT (HIPEC with RTX), and rmhTNF + RTX + HT (HIPEC with rmhTNF and RTX). Pictures were captured by in vivo fluorescence imaging system before and after treatments (Figure 4(b)), and the analysis of fluorescence intensities is shown in Figure 4(c). The results showed that RTX and rmhTNF + RTX without HT could mildly suppress the tumor growth, while RTX and rmhTNF + RTX under HIPEC could significantly inhibit the tumor growth.
Figure 4.
HIPEC with rmhTNF+RTX could significantly inhibit the tumor growth and had good therapeutic effect on the CRC-PC xenograft model. (a) Scheme and schedule of imaging and treatments. (b) The tumors in mice were visualized by in vivo imaging system before or after a course of chemotherapy. Representative images of each group were shown above. (c) The fluorescence intensities were quantitatively analyzed by Kodak in vivo imaging system. Each point stood for an independent mouse. (d) The representative macroscopic views of tumor nodules in each group were shown. (e) The pathology system scores of tumor nodules were evaluated by e PCI scoring after mice were sacrificed and dissected. Each group had six mice. Results represented mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 (Mann–Whitney U test). rmhTNF: recombinant mutant human TNF-α; RTX: raltitrexed; HT: hyperthermia; HIPEC: hyperthermic intraperitoneal chemotherapy; e PCI: peritoneal cancer index. (A color version of this figure is available in the online journal.)
Twenty-eight days after inoculation, the mice were sacrificed and dissected. Diffuse tumor nodules were found in the peritoneal cavity of mice. Figure 4(d) shows the macroscopic views of these nodules, and e PCI scoring system was used to evaluate the extent of tumor diffusion (Figure 4(e)). The views and scores showed that there were fewer nodules existed in peritoneal cavities after HIPEC treatments especially in the rmhTNF + RTX + HT group. HIPEC with rmhTNF and RTX had the best therapeutic effect on the CRC-PC xenograft model among other groups.
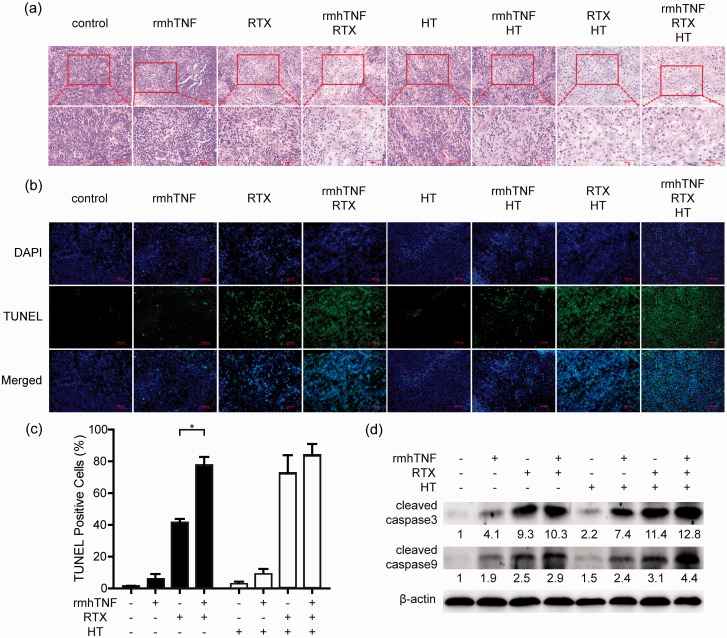
HIPEC with rmhTNF + RTX increased CRC cell damage in vivo
H&E staining confirmed the morphology of tumor tissues that were harvested after mice were sacrificed (Figure 5(a)). Some morphologic features of apoptosis could be especially observed at high magnification in the rmhTNF + RTX + HT group, including cell irregular shrinkage and nuclear condensation. Besides, TUNEL assay was used to detect the proportion of apoptotic tissues (Figure 5(b)), and the quantification of TUNEL-positive cells is shown in Figure 5(c). Compared with the control group, higher level of apoptosis was observed in the rmhTNF + RTX, RTX + HT, and rmhTNF + RTX + HT groups, indicating that the combination drug therapy and HT from HIPEC could increase CRC cell damage in vivo. The expression of proapoptotic proteins including cleaved caspase3 and cleaved caspase9 in the tumor tissues was detected by Western blot (Figure 5(d)). The expression of cleaved caspase3 and cleaved caspase9 was up-regulated after rmhTNF + RTX + HT combination treatments in mice. The results indicated that rmhTNF + RTX + HT increased the apoptosis of tumor tissues. Taken together, rmhTNF + RTX + HT could significantly suppress the tumor growth by increasing CRC cell damage in the CRC-PC xenograft mice.
Figure 5.
HIPEC with rmhTNF+RTX increased CRC cell damage in vivo. (a) Representative images of H&E staining of tumor tissues from different treatment groups. Scale bars of upper and lower images were 100 µm and 50 µm, respectively. (b–c) Apoptotic tumor tissues were assessed by TUNEL staining (positive stain = green, nuclei = blue (DAPI)). Representative staining images were shown in (b) and quantification of TUNEL-positive cells was presented in (c). The scale bars were 100 µm. (d) The protein expression of cleaved caspase3 and cleaved caspase9 in tumor tissues from different treatment groups was detected by Western blot. β-actin was the normalization for the loading. Results represented mean ± SD of three replicates. *P < 0.05, **P < 0.01, ***P < 0.001 (Student’s t test). rmhTNF: recombinant mutant human TNF-α; RTX: raltitrexed; HT: hyperthermia; HIPEC: hyperthermic intraperitoneal chemotherapy; H&E: hematoxylin-eosin; DAPI: 4ʹ,6-diamidino-2-phenylindole; TUNEL: TdT-mediated dUTP nick end labeling. (A color version of this figure is available in the online journal.)
Discussion
PM is a well-known indicator of poor prognosis in patients with CRC, and has long been considered as an incurable disease.35 The median progression-free survival time is 6–12 months with palliative treatment.36 Despite the implementation of CRS and HIPEC improving the prognosis of PM, the clinical outcome is affected by the selection of chemotherapeutic drugs.8
TNF-α was limited in the clinical application, because it was implicated in septic shock, cachexia, and fever37,38 and the maximum tolerable dose of TNF-α was much lower than the effective antitumor dose.39 For this reason, only topical administration such as “isolated limb perfusion (ILP)” and “isolated hepatic perfusion (IHP),”40,41 could reduce systemic toxicity through isolation and extracorporeal circulation. Several methods are currently being investigated to improve the efficacy and reduce the systemic toxicity of TNF-α. For example, through NGR peptide tumor vascular-targeted delivery, “NGR-hTNF” was able to decrease toxicity and increase antitumor activities.42 Furthermore, rmhTNF was created by gene engineering technology with high activity and low toxicity.20 However, adverse reactions still occurred in clinical systemic administration of rmhTNF.21 Considering various advantages of HIPEC, we thought of HIPEC with rmhTNF to further develop the clinical application. Firstly, because of the existence of peritoneum-plasma barrier, rmhTNF was limited to transmit from peritoneum to the whole body. Secondly, the abdominal cavities were flushed with saline to remove the residual drugs at the end of HIPEC. What’s more, HT had a direct cytotoxic effect on malignant tumor cells in hypoxic, nutrient-deprived, and low-PH environment by affecting the distribution of the plasma membrane and the modulation of the transmembrane efflux pumps.43,44 Thereby, HIPEC helped rmhTNF to restrict its toxicity and improve its efficacy.
Combined therapy of rmhTNF and conventional chemotherapy has co-activating and sensitivity improving effects on the treatment of various tumors in previous pre-clinical and clinical trials. Xia et al.31 found that the inhibitory effect of rmhTNF combined with cisplatin was significantly greater than cisplatin alone on human lung adenocarcinoma cell line A549 in vitro. The clinical results from Liu et al.45 showed that rmhTNF in combination with general chemotherapeutic agents (cyclophosphamide, doxorubicin, cisplatin, mitomycin C, leucovorin and 5-Fluorouracil (5-Fu)) is an effective and secure means in treating advanced malignant tumor. Moreover, Shang et al.46 proved that rmhTNF was able to enhance the apoptotic effect of 5-Fu on gastric cancer cell lines. RTX is used as an alternative for 5-Fu in clinic.11 Compared with 5-Fu, RTX was interrelated with a significantly lower incidence of cardiac and hematologic toxicity for patients.47 Here, we demonstrated that rmhTNF could further enhance the effect of RTX on CRC.
In this study, the combination of rmhTNF, RTX, and HT was confirmed to be effective on human CRC cells in vitro and in vivo, respectively. In vitro, rmhTNF alone could inhibit cell growth to some extent. And for better results, the cells were exposed to 42°C for a specified time after the administration of drugs, so as to simulate HIPEC in clinic. Besides, the addition of RTX to rmhTNF and HT could further suppress the survival of human CRC cells as we expected. In vivo, we identified whether rmhTNF or rmhTNF + RTX could affect the tumor growth under IPEC (without HT) or HIPEC treatments. The results showed that: (1) because of HT, HIPEC could increase the therapeutic effect of chemotherapeutic drugs. (2) Compared with rmhTNF or RTX, the combination of rmhTNF and RTX got the best efficacy, possibly due to the synergistic effect of these two drugs.48 The specific mechanism would need to be further studied in the next stage of the experiment.
The HIPEC system used on mice in this study was developed in our previous work, and it simulated the clinical HIPEC process pretty well.11 There was no significant decrease in body weight of the mice in each group throughout the administration (data not shown), which meant our HIPEC system and the chemotherapeutic drugs were of good tolerance.
However, certain limitations still existed in the present study. The penetration of chemotherapeutic drugs played an important role in HIPEC. According to the pharmacokinetic research of Li et al.,49 the concentration of rmhTNF was detected by radio chromatography and enzyme-linked immunosorbent assay which needed to prepare specific antibodies. Both methods were not readily available. Hopefully, the difficulty in the detection of rmhTNF could be overcome in the future.
We found that rmhTNF combined with RTX under HIPEC treatments had an antitumor effect on CRC-PC both in vitro and in vivo. Our findings will provide the basis for further investigation on the efficacy of rmhTNF in clinical practice.
Authors’ contributions
JL, XL and GC designed and supervised the study; RW and GC contributed experimental materials and methods; QG, CS and XW performed the experiments; QG and RW analyzed the data; QG wrote the manuscript; XL reviewed the manuscript. All authors read and approved the final manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (grant 81873050) and the Laboratory Animal Program of Science and Technology Commission of Shanghai Municipality (grant 19140902100 and 19140902101).
ORCID iD
Jianwen Liu https://orcid.org/0000-0003-4149-4257
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68:394–424 [DOI] [PubMed] [Google Scholar]
- 2.Tiwari AK, Laird-Fick HS, Wali RK, Roy HK. Surveillance for gastrointestinal malignancies. World J Gastroenterol 2012; 18:4507–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coccolini F, Gheza F, Lotti M, Virzi S, Iusco D, Ghermandi C, Melotti R, Baiocchi G, Giulini SM, Ansaloni L, Catena F. Peritoneal carcinomatosis. World J Gastroenterol 2013; 19:6979–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pelz JO, Chua TC, Esquivel J, Stojadinovic A, Doerfer J, Morris DL, Maeder U, Germer CT, Kerscher AG. Evaluation of best supportive care and systemic chemotherapy as treatment stratified according to the retrospective peritoneal surface disease severity score (PSDSS) for peritoneal carcinomatosis of colorectal origin. BMC Cancer 2010; 10:689–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Y, Alzahrani NA, Chua TC, Liauw W, Morris DL. Impacts of low peritoneal cancer index on the survival outcomes of patient with peritoneal carcinomatosis of colorectal origin. Int J Surg 2015; 23:181–5 [DOI] [PubMed] [Google Scholar]
- 6.Bhandare M, Patil P, Pai V, Bhamre R, Engineer R, Ostwal V, Saklani A. Peritoneal carcinomatosis in colorectal cancers – management perspective needs a change. Clin Colorectal Cancer 2017; 16:e1–e6 [DOI] [PubMed] [Google Scholar]
- 7.Vallicelli C, Cavaliere D, Catena F, Coccolini F, Ansaloni L, Poiasina E, Abongwa HK, Simone BD, Alberici L, Framarini M, Verdecchia GM. Management of peritoneal carcinomatosis from colorectal cancer: review of the literature. Int J Colorectal Dis 2014; 29:895–8 [DOI] [PubMed] [Google Scholar]
- 8.Helderman R, Loke DR, Kok HP, Oei AL, Tanis PJ, Franken N, Crezee J. Variation in clinical application of hyperthermic intraperitoneal chemotherapy: a review. Cancers 2019; 11:78–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verwaal VJ, Ruth S, Witkamp A, Boot H, Slooten G, Zoetmulder F. Long-term survival of peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol 2005; 12:65–71 [DOI] [PubMed] [Google Scholar]
- 10.Verwaal VJ, Bruin S, Boot H, Slooten G. Tinteren Hv. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol 2008; 15:2426–32 [DOI] [PubMed] [Google Scholar]
- 11.Qiu C, Li Y, Liang X, Qi Y, Chen Y, Meng X, Zheng H, Xu Y, Cai S, Cai G, Liu J. A study of peritoneal metastatic xenograft model of colorectal cancer in the treatment of hyperthermic intraperitoneal chemotherapy with raltitrexed. Biomed Pharmacother 2017; 92:149–56 [DOI] [PubMed] [Google Scholar]
- 12.Jung H. Interaction of thermotolerance and thermosensitization induced in CHO cells by combined hyperthermic treatments at 40 and 43°C. Radiat Res 1982; 91:433–46 [PubMed] [Google Scholar]
- 13.Sugarbaker P, Ihemelandu C, Bijelic L. Cytoreductive surgery and HIPEC as a treatment option for laparoscopic resection of uterine leiomyosarcoma with morcellation: early results. Ann Surg Oncol 2015; 23:1501–7 [DOI] [PubMed] [Google Scholar]
- 14.Neuwirth MG, Alexander HR, Karakousis GC. Then and now: cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC), a historical perspective. J Gastrointest Oncol 2016; 7:18–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quenet F, Elias D, Roca L, Goere D, Ghouti L, Pocard M, Facy O. A UNICANCER phase III trial of hyperthermic intraperitoneal chemotherapy (HIPEC) for colorectal peritoneal carcinomatosis. Eur J Surg Oncol 2019; 45:e17 [Google Scholar]
- 16.Shan L, Bai B, Lv Y, Xie B, Huang X, Zhu H. Lobaplatin suppresses proliferation and peritoneal metastasis of colorectal cancer in a preclinical model. Biomed Pharmacother 2018; 108:486–91 [DOI] [PubMed] [Google Scholar]
- 17.Haranaka K, Satomi N, Sakurai A. Antitumor activity of murine tumor necrosis factor (TNF) against transplanted murine tumors and heterotransplanted human tumors in nude mice. Int J Cancer 1984; 34:263–7 [DOI] [PubMed] [Google Scholar]
- 18.Roberts NJ, Zhou S, Jr., LAD, Holdhoff M. Systemic use of tumor necrosis factor alpha as an anticancer agent (review. Oncotarget 2011; 2:739–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lejeune FJ, Ruegg C, Lienard D. Clinical applications of TNF-α in cancer. Curr Opin Immunol 1998; 10:573–80 [DOI] [PubMed] [Google Scholar]
- 20.Yan Z, Zhao N, Wang Z, Li B, Bao C, Shi J, Han W, Zhang Y. A mutated human tumor necrosis factor-alpha improves the therapeutic index in vitro and in vivo. Cytotherapy 2006; 8:415–23 [DOI] [PubMed] [Google Scholar]
- 21.Li M, Xu T, Zhang Z, Xue X, Zhang C, Qin X, Li W, Hao Q, Zhang W, Zhang Y. Phase II multicenter, randomized, double-blind study of recombinant mutated human tumor necrosis factor-alpha in combination with chemotherapies in cancer patients. Cancer Sci 2012; 103:288–95 [DOI] [PubMed] [Google Scholar]
- 22.Jiang C, Niu J, Li M, Teng Y, Wang H, Zhang Y. Tumor vasculature-targeted recombinant mutated human TNF-α enhanced the antitumor activity of doxorubicin by increasing tumor vessel permeability in mouse xenograft models. PLoS One 2014; 9:e87036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji W, Yuan M, Zhang L, Zhang H, Jiao J, Gao Z. Effect of p53beta on human gastric cancer cells treated with recombinant mutated human TNF and cisplatin. Mol Med Rep 2017; 15:3865–70 [DOI] [PubMed] [Google Scholar]
- 24.Xue S, Chen Y, Qin S, Yang A, Wang L, Xu H, Geng H. Raltitrexed induces mitochondrialmediated apoptosis in SGC7901 human gastric cancer cells. Mol Med Rep 2014; 10:1927–34 [DOI] [PubMed] [Google Scholar]
- 25.Zhao H, Zhang Y, Sun J, Zhan C, Zhao L. Raltitrexed inhibits HepG2 cell proliferation via G0/G1 cell cycle arrest. Oncol Res 2016; 23:237–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding W, Liu S, Ma J, Pu J, Wang H, Zhang S, Sun X. Raltitrexed increases radiation sensitivity of esophageal squamous carcinoma cells. Cancer Cell Int 2019; 19:36–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ransom D, Wilson K, Fournier M, Simes RJ, Gebski V, Yip D, Tebbutt N, Karapetis CS, Ferry D, Gordon S, Price TJ. Final results of Australasian gastrointestinal trials group ARCTIC study: an audit of raltitrexed for patients with cardiac toxicity induced by fluoropyrimidines. Ann Oncol 2014; 25:117–21 [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Wu J, Cheng K, Li Z, Luo D, Qiu M, Gou H, Yi C, Li Q, Wang X. S-1 plus raltitrexed for refractory metastatic colorectal cancer: a phase II trial. Oncologist 2019; 24:591–e165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Chen Y, Qiu C, Ma X, Lei K, Cai G, Liang X, Liu J. 17-allylamino-17-demethoxygeldanamycin impeded chemotherapy through antioxidant activation via reducing reactive oxygen species-induced cell death. J Cell Biochem 2018; 120:1560–76 [DOI] [PubMed] [Google Scholar]
- 30.Wang H, Yan Z, Shi J, Han W, Zhang Y. Expression, purification, and characterization of a neovasculature targeted rmhTNF-alpha in Escherichia coli. Protein Expr Purif 2006; 45:60–5 [DOI] [PubMed] [Google Scholar]
- 31.Xia L, Zhou Y. rmhTNF-α combined with cisplatin inhibits proliferation of A549 cell line in vitro. Chin Med Sci J 2014; 29:185–7 [DOI] [PubMed] [Google Scholar]
- 32.Zhang A, Zheng Y, Que Z, Zhang L, Lin S, Le V, Liu J, Tian J. Astragaloside IV inhibits progression of lung cancer by mediating immune function of tregs and CTLs by interfering with IDO. J Cancer Res Clin Oncol 2014; 140:1883–90 [DOI] [PubMed] [Google Scholar]
- 33.Aarts F, Hendriks T, Boerman OC, Koppe MJ, Oyen WJG, Bleichrodt RP. A comparison between radioimmunotherapy and hyperthermic intraperitoneal chemotherapy for the treatment of peritoneal carcinomatosis of colonic origin in rats. Ann Surg Oncol 2007; 14:3274–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mao R, Zou F, Yang L, Lin S, Li Y, Ma M, Yin P, Liang X, Liu J. The loss of MiR-139-5p promotes colitis-associated tumorigenesis by mediating PI3K/AKT/wnt signaling. Int J Biochem Cell Biol 2015; 69:153–61 [DOI] [PubMed] [Google Scholar]
- 35.Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg 2002; 89:1545–50 [DOI] [PubMed] [Google Scholar]
- 36.Kuramochi H, Ando M, Itabashi M, Nakajima G, Kawakami K, Hamano M, Hirai E, Yokomizo H, Okuyama R, Araida T, Yoshimatsu K, Kameoka S, Hayashi K. Phase II study of bevacizumab and irinotecan as second-line therapy for patients with metastatic colorectal cancer previously treated with fluoropyrimidines, oxaliplatin, and bevacizumab. Cancer Chemother Pharmacol 2017; 79:579–85 [DOI] [PubMed] [Google Scholar]
- 37.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 2001; 104:487–501 [DOI] [PubMed] [Google Scholar]
- 38.Kriegler M, Perez C, DeFay K, Albert I, Lu SD. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell 1988; 53:45–53 [DOI] [PubMed] [Google Scholar]
- 39.Schiller JH, Storer BE, Witt PL, Alberti D, Tombes MB, Arzoomanian R. Biological and clinical effects of intravenous tumor-necrosis-factor-alpha administered 3 times weekly. Cancer Res 1991; 51:1651–8 [PubMed] [Google Scholar]
- 40.Spacek M, Mitas P, Vocka M, Lacina L, Hodkova G, Spunda R. Isolated perfusion of the upper extremity with TNF-alpha – double venous cannulation. Klin Onkol 2017; 30:213–9 [DOI] [PubMed] [Google Scholar]
- 41.Ye H, Lu C, Zheng S. Drug selection in isolated hepatic perfusion for nonresectable liver tumors: recent trends and perspectives. World Chinese J Digestol 2008; 23:2621–5 [Google Scholar]
- 42.Santoro A, Rimassa L, Sobrero AF, Citterio G, Sclafani F, Carnaghi C, Pessino A, Caprioni F. Phase II study of NGR-hTNF, a selective vascular targeting agent, in patients with metastatic colorectal cancer after failure of standard therapy. Eur J Cancer 2010; 46:2746–52 [DOI] [PubMed] [Google Scholar]
- 43.Zee J, Gonzalez DG, Rhoon GCv, Dijk J, Putten W, Hart A. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Lancet 2000; 355:1119–25 [DOI] [PubMed] [Google Scholar]
- 44.Roti J. Cellular responses to hyperthermia (40-46 degrees C): cell killing and molecular events. Int J Hyperthermia 2008; 24:3–15 [DOI] [PubMed] [Google Scholar]
- 45.Liu X, Zhang X, Zheng Z, Lu H, Wu X, Huang C, Wang C, Guang G. The effect of chemotherapy combined with recombination mutant human tumor necrosis factor on advanced cancer. J Transl Med 2004; 2:33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shang ZM, Tang JD, Jiang QQ, Guo A, Zhang N, Gao ZX, Ji WS. Role of Δ133p53 in tumor necrosis factor-induced survival of p53 functions in MKN45 gastric cancer cell line. Eur Rev Med Pharmacol Sci 2015; 19:2416–22 [PubMed] [Google Scholar]
- 47.Guo J, Zhang H, Gao S, Zhang P, Li X, Chen H, Wang X, Zhu X. Hepatic artery infusion with raltitrexed or 5-fluorouracil for colorectal cancer liver metastasis. World J Gastroenterol 2017; 23:1406–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li G, Dong S, Qu J, Sun Z, Huang Z, Ye L, Liang H, Ai X, Zhang W, Chen X. Synergism of hydroxyapatite nanoparticles and recombinant mutant human tumour necrosis factor-alpha in chemotherapy of multidrug-resistant hepatocellular carcinoma. Liver Int 2010; 30:585–92 [DOI] [PubMed] [Google Scholar]
- 49.Li M, Qin X, Xue X, Zhang C, Yan Z, Han W, Komarck C, Wang TD, Zhang Y. Safety evaluation and pharmacokinetics of a novel human tumor necrosis factor-alpha exhibited a higher antitumor activity and a lower systemic toxicity. Anticancer Drugs 2010; 21:243–51 [DOI] [PMC free article] [PubMed] [Google Scholar]