Abstract
Background
Progress in extending the survival of glioblastoma (GBM) patients has been slow. A better understanding of why patient survival remains poor is critical to developing new strategies. Postmortem studies on GBM can shed light on why patients are dying.
Methods
The brains of 33 GBM patients were autopsied and examined for gross and microscopic abnormalities. Clinical-pathologic correlations were accomplished through detailed chart reviews. Data were compared with older published autopsy GBM studies that predated newer treatment strategies, such as more extensive surgical resection and adjuvant temozolomide.
Results
In older GBM autopsy series, mass effect was observed in 72% of brains, with herniation in 50% of all cases. Infiltration of tumor into the brainstem was noted in only 21% of those older cases. In the current series, only 10 of 33 (30%) GBMs showed mass effect (P = 0.0003), and only 1 (3%) showed herniation (P < 0.0001). However, extensive GBM infiltration of the brainstem was present in 22 cases (67%, P < 0.0001), with accompanying destruction of the pons and white matter tracts. There was a direct correlation between longer median patient survival and the presence of brainstem infiltration (16.1 mo in brainstem-invaded cases vs 9.0 mo in cases lacking extensive brainstem involvement; P = 0.0003).
Conclusions
With improving care, severe mass effect appears to be less common in GBM patients today, whereas dissemination, including life-threatening brainstem invasion, is now more pronounced. This has major implications regarding preclinical GBM models, as well as the design of clinical trials aimed at further improving patient survival.
Keywords: glioblastoma, midbrain, pons, medulla, brainstem, postmortem, autopsy
Key Points.
Widespread tumor infiltration, especially involving the brainstem, now appears to be a greater contributor to GBM patient death than mass effect.
This could improve the preclinical modeling of GBM and the design and implementation of clinical trials.
Importance of the Study.
Despite advances in neurosurgery and adjuvant therapies, as well as numerous clinical trials, GBM survival has not substantially improved in recent years. The reasons for this are not fully understood, in part because the precise causes of death in GBM patients are often unclear. The current study demonstrates that the pathologic features of end-stage GBM have changed compared with autopsy cohorts from several decades ago, wherein extensive dissemination of tumor into vital structures like the brainstem is now more common, and herniation due to mass effect is less common. This has multiple implications for the field, including why overall progress in improving GBM patient outcomes has been so slow, why many therapies that seemed promising in preclinical animal models have been disappointing in humans, and how to better conceptualize GBM as a disease of the entire nervous system.
Glioblastoma (GBM) remains not only an incurable disease, but a disease in which patients’ median overall survival is only a few months better than it was 20 years ago. Numerous high-impact papers have advanced our knowledge of the underlying molecular biology of GBM, and clinical trials have tested a wide variety of therapies, including small-molecule inhibitors, virotherapy, and immunotherapy. Despite all this, there have been very few improvements on the standard of care since 2005, which includes surgical resection followed by radiation and temozolomide (RT/TMZ).1,2 One recent improvement has been the addition of tumor-treating fields (TTF) to RT/TMZ, but even with this therapy, GBM is still lethal.3,4 Revisiting our approach to these tumors, with respect to both preclinical modeling and clinical trial design, could therefore be beneficial.
An effective way to reevaluate the nature of GBM is to study the pattern of tumor spread in the postmortem setting and relate it to end-stage patient symptoms. However, the largest autopsy GBM studies were all published decades ago, before our modern treatment strategies. Based on a series of 50 glioma patients published in 1983, Giangaspero and Burger reported that, at the end of life, around half had notable mass effect from the tumor, and half of those had transtentorial herniation.5 In 1991, Silbergeld et al reported 117 autopsy gliomas spanning 1961–1989, and found that 61% showed mass effect from the tumor and radionecrosis, sufficient to cause herniation.6 Since then, aside from a couple of studies demonstrating that drop metastases do occur in GBM,7,8 no systematic effort has been made to evaluate the pattern of tumor spread in the brain at the end of life, including clinicopathologic correlation with the conditions under which GBM patients are dying.
Based on those older studies, there is a prevailing assumption that most GBM patients die of mass effect from the tumor. For example, in one end-of-life paper published in 2010, seventy-three percent of GBM patients were thought to have brain herniation caused by the tumor, but no pathologic confirmation was done.9 The current study reports results from a rapid autopsy program, initiated by the Northwestern Nervous System Tumor Bank in 2015, to begin addressing this gap in our understanding of end-stage GBMs in the current era of therapy.
Materials and Methods
Postmortem Cohort Collection
Neuro-oncologists discussed the option of tissue donation with GBM patients and their designated caregivers when patients were approaching the end of life. Those who provided informed consent, according to a protocol approved by the Northwestern institutional review board, were then tracked. At the time of passing, the body was transported to Northwestern Memorial Hospital, where the brain and spinal cord were removed. Samples of unfixed tumor were stored in the vapor phase of liquid nitrogen, and the remaining brain and spinal cord were suspended in 10% neutral buffered formalin for approximately 14 days. At the time of brain sectioning, portions of key brain and spinal cord regions, as well as extra sampling of tumor, were collected for histologic processing as paraffin-embedded tissue blocks. Mass effect was assessed at time of brain sectioning, and was categorized as mild, moderate, or severe. Mild mass effect was defined as some displacement of adjacent structures; moderate mass effect was defined as midline shift; and severe mass effect was defined as uncal or tonsillar herniation of the brain into the posterior fossa or foramen magnum, respectively. Detailed electronic chart reviews were performed on each patient, including a review of all hospice notes when available.
To obtain data from old GBM autopsy cohorts, a PubMed search was done using combinations of the words “postmortem,” “autopsy,” “glioblastoma,” and “glioma.” Each result was screened, and papers that were both relevant and available for full review (ie, not just abstracts) were selected for comparison to the current postmortem cohort.
Tissue Analyses
Standard immunohistochemistry analysis was performed using Ki67 antibody (clone MIB-1, Agilent M7240, diluted at 1:500). Hematoxylin and eosin staining was performed, and Luxol fast blue (LFB) was used in order to study white matter. Tumor infiltration was categorized as either extensive or microscopic tumor infiltration. Extensive tumor infiltration was defined as the presence of numerous tumor cells with accompanying tissue damage, whereas microscopic tumor infiltration was the presence of only scattered single tumor cells and no tissue damage. Leptomeningeal dissemination was defined as the spread of tumor, via the leptomeningeal compartment, to locations distant from the original surgical site, such as other parts of the cerebrum, posterior fossa, and spinal cord.
Molecular profiling was performed using a targeted next-generation sequencing panel, GlioSeq, to assess for single nucleotide variants and indels, copy number variations, and structural alterations of key genes.10 Promoter methylation of O6-methylguanine-DNA methyltransferase (MGMT) was determined by pyrosequencing.
Statistical Analyses
Differences between mean values of 2 groups were compared using two-sample t-test or Mann–Whitney, as appropriate. Fisher’s exact tests were done when comparing categorical variables (eg, brainstem invasion); Spearman correlations were done between overall survival and brainstem invasion or bevacizumab and brainstem invasion, and differences in survival between groups was calculated by log-rank tests. P-values less than 0.05 were considered significant. Graph generation and statistical analyses were performed with GraphPad Prism 5 software.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Northwestern University institutional review board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Results
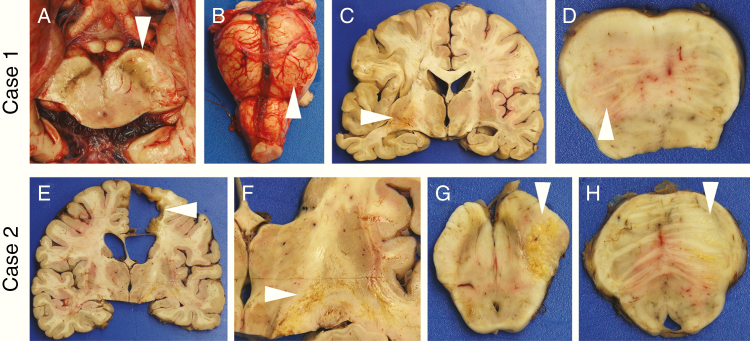
Between 2015 and 2019, the brains and spinal cords of 33 isocitrate dehydrogenase 1 (IDH1) wild-type and IDH1 mutant GBM patients underwent detailed postmortem gross and microscopic examination (Supplementary Table 1). Seven (21%) showed mild mass effect at the original tumor site, defined as some displacement of adjacent structures. Only 2 cases showed moderate mass effect in the form of midline shift, and only 1 had severe mass effect from the tumor, with subfalcine, uncal, and tonsillar herniation (Supplementary Fig. 1). Instead, most of the brains showed only a resection cavity and/or focal necrosis in the original tumor site (Fig. 1c and Fig. 1e). In several cases, gross abnormalities were readily apparent in the midbrain and pons, indicative of direct tumor extension through the brain parenchyma (Fig. 1, Supplementary Fig. 2).
Fig. 1.
Gross findings in postmortem GBMs. At the time of brain removal in Case 1, separation of the posterior fossa contents via midbrain transection revealed a swollen, discolored left cerebral peduncle (a, arrowhead) and left pons (b, arrowhead). After formalin fixation, coronal sections showed a relatively small area of hemorrhage and necrosis in the left temporal lobe (c, arrowhead), but no notable mass effect or midline shift. Axial sections of the brainstem again found the left pons to be swollen and discolored (d, arrowhead). In Case 2, the original tumor site in the right frontal lobe showed a resection cavity surrounded by glial scar (e, arrowhead), as well as streaks of yellow necrosis through the internal capsule headed toward the right cerebral peduncle (f, arrowhead). No mass effect was noted. Sections of the midbrain (g) and pons (h) revealed swelling and necrosis in the right cerebral peduncle and basis pontis, respectively (arrowheads). In (d), (g), and (h), anterior is up.
In all cases, microscopic analysis of the original tumor sites revealed a consistent pattern of recurrent/residual GBM interspersed with varying amounts of therapy-related necrosis (Figures 2–3, Supplementary Figures 3–4). The latter was distinguishable from spontaneous tumor necrosis by its near-complete destruction of all cells within a relatively large area, as well as extensive vascular hyalinization. In 31/33 cases (94%), infiltrating tumor cells were present all the way down through white matter tracts into the midbrain and pons, and in most cases were also readily identified in the medulla (Supplementary Table 1, Figures 2–3, Supplementary Figures 3–5). Only Cases 6 and 18 showed a few scattered tumor cells in the white matter of the upper cervical spinal cord (not shown). Some infiltrating GBM cells within the brainstem had highly atypical nuclei similar to cells around the matching original tumor sites (Figures 2–3). Pseudopalisading tumor necrosis within the brainstem was also observed in many cases (Figures 2–3, Supplementary Figures 3 and 5), without any therapy-related necrosis. This matched the data on radiotherapy, which indicated that none of the patients received significant doses of radiation to the posterior fossa (Supplementary Table 3).
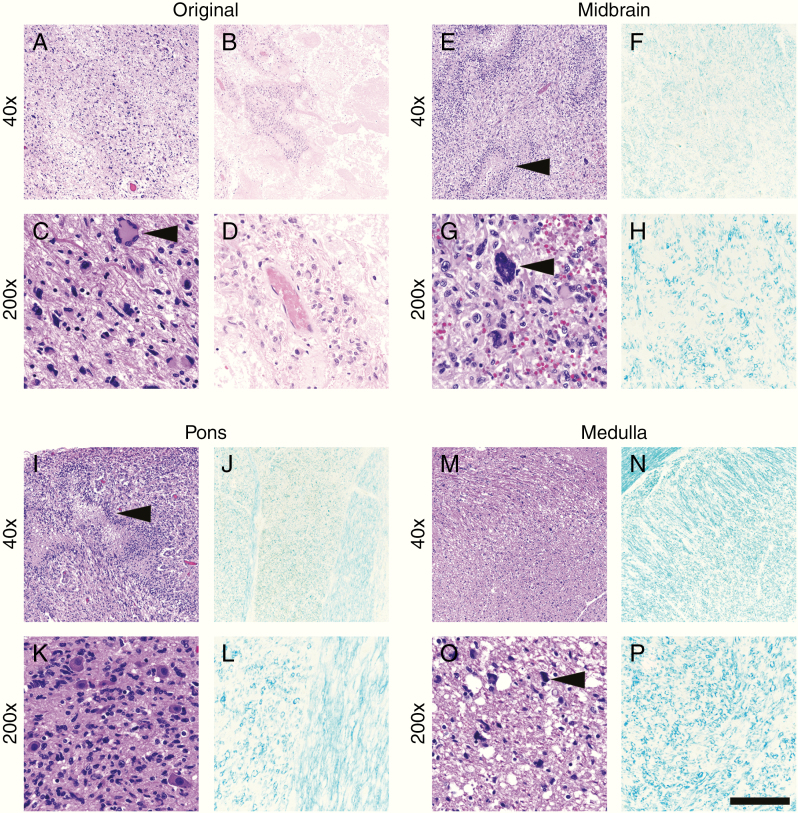
Fig. 2.
Case 1 histology. The original tumor site in the left temporal lobe showed large areas of viable tumor (a) and therapy-induced cellular atypia (c, arrowhead), as well as abundant therapy-related necrosis (b, d). In contrast, sections of the midbrain (e, g) and pons (i, k) showed viable tumor with pseudopalisading necrosis (e and i, arrowheads). At higher magnification, scattered tumor nuclei with therapy-associated atypia were observed (g, arrowhead). Tumor infiltration was heavy around pontine neurons (k). In the medulla, overall tumor burden was lighter (m, o), but still readily apparent (o, arrowhead). LFB highlighted massive loss of myelination in the midbrain (f, h), pons (j, l), and medulla (n, p). Compare with the undamaged white matter in Fig. 4. Scale bar in (p) = 400 µm at 40x and 80 µm at 200x.
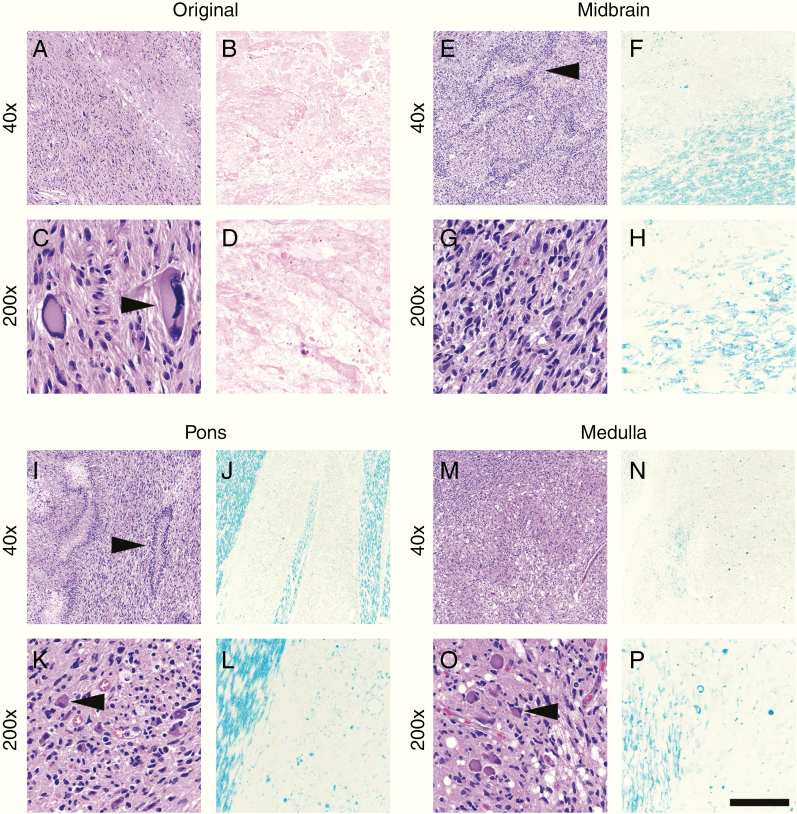
Fig. 3.
Case 2 histology. As in Case 1, the GBM remaining in the original right frontal lobe site had a mixture of viable cells (a, c) and therapy-related necrosis (b, d). Tumor cells with therapy-induced atypia were frequent (c, arrowhead). The midbrain (e, g), pons (i, k), and medulla (m, o) all showed extensive tumor infiltration with pseudopalisading necrosis (e and i, arrowheads). Neurons comprising cranial nerve nuclei in the pons and medulla were surrounded by tumor (k and o, arrowheads). Extensive damage to the right cerebral peduncle in the midbrain (f, h) and white matter tracts in the right pons (j, l) and right medulla (n, p) was readily apparent via LFB. There was preferential loss of vertically oriented white matter tracts (right area in l) alongside mostly intact horizontal tracts of the middle cerebellar peduncles (left area in l). Compare with the undamaged white matter in Fig. 4. Scale bar in (p) = 400 µm at 40x and 80 µm at 200x.
In 22/33 cases (67%), there was extensive tumor infiltration in the brainstem, associated with tissue damage observable by LFB staining of white matter myelin (Figures 2–3, Supplementary Figures 3–6). This damage was extensive and consistently followed the neuroanatomic path of the white matter tracts in relation to the original tumor. For example, in Case 1, which had a left temporal lobe tumor, the infiltrating GBM cells and loss of myelinated white matter were observed most prominently in the left cerebral peduncle of the midbrain (Fig. 2e–h, Supplementary Fig. 5), as well as the left anterior pons (Fig. 2i–l) and the left medullary pyramid (Fig. 2m–p). Case 2, with a right frontal GBM, showed a pattern of tumor infiltration and damage favoring the right side of the brainstem (Fig. 3, Supplementary Fig. 5), although extension to the contralateral side was common. The ascending/descending white matter tracts in the pons and medulla sustained the worst damage, whereas the horizontally oriented tracts, such as the middle cerebellar peduncles traversing the anterior pons, were mostly spared (Fig. 2j, l) (Fig. 3j, l) (Supplementary Figures 3 and 5). Although direct intraparenchymal tumor extension was not observed below the upper cervical spinal cord in any case, anterograde (Wallerian) degeneration was frequently apparent all the way down the spinal cord (Supplementary Fig. 6m–r).
Of the remaining 11/33 cases (33%), 9 showed only microscopic infiltration of scattered single cells into the midbrain and pons, with intact, normal-appearing white matter (Fig. 4, Supplementary Fig. 7). A study of the cases in which little to no brainstem spread was present suggested a direct relationship with shorter overall survival; in other words, the more rapid the death after initial presentation and diagnosis, the less likely there was extensive infiltration of the brainstem (Supplementary Table 1). Indeed, cases with extensive brainstem infiltration had an aggregate median overall survival of 17.4 months, compared with 9.0 months without such infiltration (P = 0.002) (Supplementary Fig. 8). For example, the right parietal GBM in Case 3, which was not resected, spread to the bilateral hippocampi (Fig. 4a–d); this matched the patient’s rapidly progressing dementia and overall survival of only 5.4 months (Supplementary Table 1).
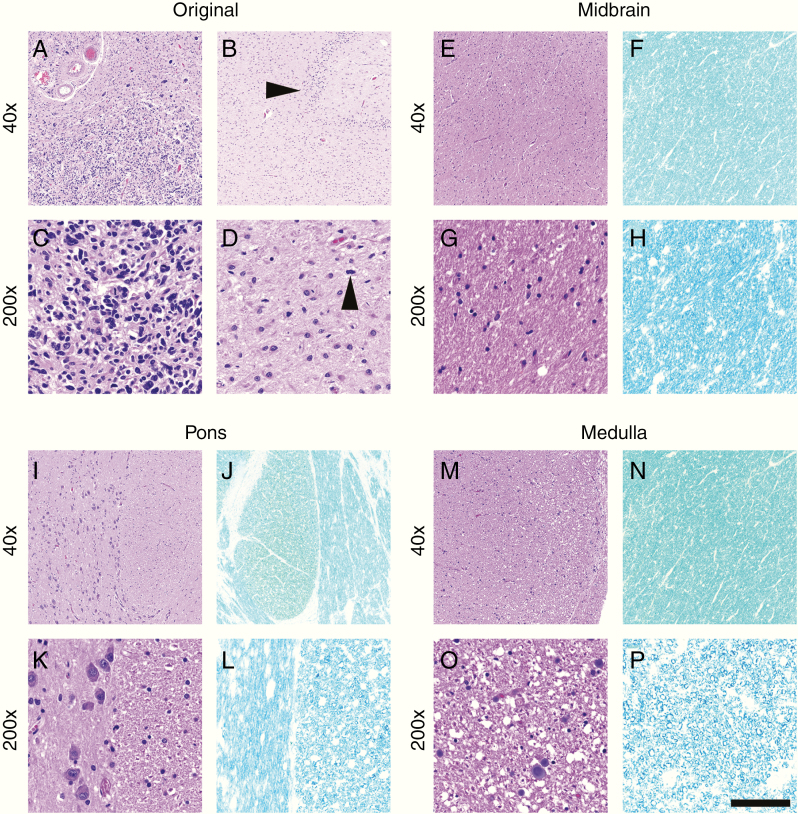
Fig. 4.
Case 3 histology. In this case, the right parietal GBM, which was only biopsied, not resected, showed virtually no therapy-related changes (a, c) and spread to both hippocampal formations. Arrowhead in (b) denotes residual dentate gyrus in Ammon’s horn of the right hippocampus. Tumor mitoses were abundant in all sections, including within the hippocampi (d, arrowhead). Unlike Cases 1 and 2 (Figures 2 and 3), very little tumor infiltration or white matter damage (via LFB stain) was present in the midbrain (e–h), pons (i–l), or medulla (m–p). However, scattered infiltrating tumor cells and mitoses were found on detailed microscopic examination (see Supplementary Fig. 7). Scale bar in (p) = 400 µm at 40x and 80 µm at 200x.
Twenty-eight cases were IDH1 wild-type glioblastoma, and 4 were IDH1 mutant glioblastoma (IDH1 status was unknown in Case 14). All 4 of the IDH1mut GBMs showed extensive brainstem invasion, compared with 18/28 (64%) IDH1wt GBMs (Supplementary Table 1), but this difference was not statistically significant (P = 0.28 by Fisher’s exact test). IDH1wt GBMs with extensive brainstem infiltration had a median survival of 14.6 months, while IDH1wt GBMs without extensive infiltration had a median survival of 9.0 months (P = 0.04). Six of 7 (86%) tumors with MGMT promoter methylation showed brainstem infiltration, whereas only 15/25 (60%) of MGMT-unmethylated tumors showed such infiltration. This difference, however, was not statistically significant (P = 0.37).
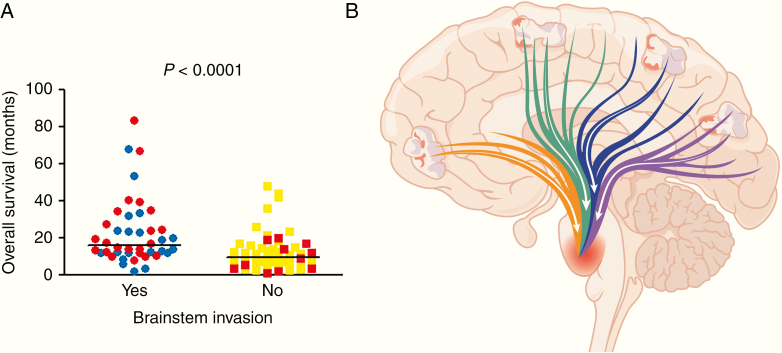
A literature search identified 3 other studies with detailed characterization of glioma infiltration in postmortem cases: Giangaspero and Burger,5 Onda et al,11 and Silbergeld et al.6 Those 3 studies totaled 181 cases from the 1960s through the 1980s. Compared with those older cohorts, patients in the current cohort were of similar age (54.2 vs 55.4 years) and male:female ratio (2.3 vs 2.2) (Table 1, Supplementary Table 4). Median overall survival in the current cohort was 2.3 months longer than in the older cohorts (14.3 vs 12.0 months), but this did not reach significance (P = 0.16). The most striking differences were in the autopsy findings, as cases in the current cohort were much less likely to show signs of mass effect (30.3% vs 72.0%, P = 0.0003) or herniation (3.0% vs 49.7%, P < 0.0001), but were more likely to have extensive tumor infiltration into the brainstem (66.7% vs 21.0%, P < 0.0001). Of note, histologic comparisons between the current and older cohorts necessarily rely on interpretation of images and textual descriptions in the older cohorts; from these, it is apparent that what was described as brainstem involvement in the older cohorts equates to “extensive invasion” in the current cohort, not just rare scattered microscopic tumor cells. The positive correlation between prolonged survival and brainstem invasion held when combining the older and current cohorts (16.1 mo vs 9.0 mo, P = 0.0003) (Fig. 5a).
Table 1.
Cohort comparisons
| Cohort | Current | Previously Published | P |
|---|---|---|---|
| Years | 2015–2019 | 1960s–1980s | |
| N | 33 | 181 | |
| mean patient age, y | 54.2 | 55.4 | 0.5 |
| male:female ratio | 2.3 | 2.2 | 1.0 |
| median overall survival, mo* | 14.3 | 12.0 | 0.16 |
| # with mass effect (%) | 10/33 (30.3) | 36/50 (72.0) | 0.0003 |
| # with herniation (%) | 1/33 (3.0) | 83/167 (49.7) | <0.0001 |
| # with extensive brainstem invasion (%) | 22/33 (66.7) | 38/181 (21.0) | <0.0001 |
Data from the current cohort of postmortem GBM cases were compared with similar studies from several decades ago.5,6,10P-value for survival was calculated via log-rank test; all others were calculated via Fisher’s exact test. *Only two of the prior studies had extractable survival data (N = 64).5,10 See Supplementary Table 4 for additional details on the cohorts from those prior studies. Bonferroni-adjusted P = 0.009.
Fig. 5.
Brainstem invasion and overall survival in postmortem GBMs. (A) The overall survival of patients in the current cohort was integrated with published data from several decades ago,5,6,11 and sorted by the presence or absence of brainstem invasion. Red circles or squares = cases from current cohort; blue circles or yellow squares = cases from older published cohorts. P-value was calculated via log-rank test. Bars = median survival. (B) Multiple large tracts of myelinated axons extend from the cortex through the midbrain into the pons, including fronto-pontine (orange), corticospinal (green), parieto-pontine (blue), and occipito-pontine (purple). (Temporo-pontine tracts are above and below the plane of the diagram.) Such tracts allow a GBM, arising anywhere in the cerebrum, direct access to vital brainstem structures.
Among the 22 cases that showed significant brainstem infiltration of GBM on postmortem histologic examination, 16 (73%) also had at least some signal abnormalities on MRI obtained a median of 47 days before death (Supplementary Table 1, Supplementary Fig. 9). None of the 11 cases with minimal brainstem infiltration by histology showed MRI anomalies in the brainstem a median of 40 days before death. In 6 cases, there was a prominent gliomatosis-like pattern of brainstem infiltration, which was not apparent on the patient’s last MRI. Thus, the overall radiology/pathology concordance was 27/33, or 82%.
The monoclonal antibody inhibitor of vascular endothelial growth factor (VEGF), bevacizumab, was administered to 23/33 (70%) patients in the cohort, with a median duration of 3.0 months (mean 3.8 mo). Both the duration of bevacizumab (ρ = 0.40, P = 0.02) and length of overall survival (ρ = 0.49, P = 0.004) correlated with the presence of brainstem invasion on Spearman’s rank correlation. Twenty-six of 33 cases had detailed molecular profiling done by next-generation sequencing (Supplementary Table 2), but there were no clear correlations between specific molecular alterations and brainstem invasion.
A review of medical records and hospice notes showed that 17/22 (77%) cases with extensive brainstem infiltration had documented evidence of premortem brainstem symptoms that consistently worsened as the patient approached death. Such symptoms included varying combinations of dysphagia/dysarthria, aphasia, diplopia, ataxia, decreased alertness, and hypersomnolence. In contrast, of the 11 cases without extensive brainstem invasion, only Case 3 had documented dysphagia late in the course of disease, and Case 26 had decreased alertness, hypersomnolence, and ataxia.
Regarding leptomeningeal spread of disease, most of our cases showed increased leptomeningeal cellularity in the form of infiltrating mononuclear inflammatory cells, especially in postsurgical and post-RT areas. Yet only 6/33 (18%) cases showed leptomeningeal dissemination to sites distal from the main tumor (Supplementary Table 1). In 2/6 cases, only minute amounts of microscopic disease were present. In 4/6 cases, much heavier spread into the leptomeningeal space was observed, extending all the way down into the nerve roots of the lumbar spinal cord (Supplementary Figures 10 and 11).
Discussion
Although postmortem examinations were far more common in decades past than they are today,12,13 they can still add a great deal of valuable information, especially when comparing disease patterns with older published work. Today, moderate-to-severe mass effect and herniation appear to be far less common in end-stage GBM patients than they were just a few decades ago. Conversely, extensive tumor infiltration into the pons by way of large white matter tracts, which used to be relatively infrequent, is now a common feature of advanced GBM (Table 1).5,6,11 Several decades ago, patients were often treated with just radiation, yet even without adjuvant chemotherapy, postmortem examination of many of the original resection sites demonstrated relatively little viable tumor. In 1979, Dr Peter Burger wrote, “the scant quantity of residual tumor in the majority of cases fosters optimism” that markedly prolonged survival of GBM patients might soon be a reality.14 Unfortunately, that has not proven to be the case, as median survival for GBM patients treated with surgical resection and RT/TMZ is still less than 2 years, even when the tumors have MGMT promoter methylation.1,2 This, plus the myriad disappointments of high-profile clinical trials in recent years, suggests that the ultimate driver of death for many GBM patients may not be sufficiently addressed in our current approaches, and that a fundamental rethinking of these tumors is needed.
The pons (Latin for “bridge”) connects the cerebrum with the medulla and spinal cord, and along with the other structures of the rhombencephalon (cerebellum and medulla) is the earliest to mature in the mammalian brain. This is because a functional pons is essential for independent life, containing cranial nerve nuclei that facilitate breathing, chewing, swallowing, equilibrium, conjugate eye movements, and sleep-wake cycles. The pons also acts as a gateway for all ascending and descending sensorimotor pathways connecting the brain with the body, as well as the main lateral fibers connecting the cerebellar hemispheres. Therefore, any disruption of the pons can have catastrophic consequences for a patient, in a way that damage to a comparable amount of cerebral tissue may not. The pons is connected to the cerebrum by just a few centimeters of readily traversed white matter tracts through the midbrain. Such tracts include the descending corticospinal motor tracts arising in the primary motor cortex, as well as tracts from all lobes in the cerebral cortex that synapse with pontine nuclei.15 Thus, a GBM arising virtually anywhere in the brain has at least one route of direct access to the pons (Fig. 5B). And, because the migration of GBM along white matter tracts takes time, it follows that the longer a patient survives with a GBM, the more opportunity a tumor has to spread to the pons (Fig. 5A, Supplementary Fig. 8). Even in cases where the patient survived less than 6 months, scattered individual tumor cells were still seen in the pons (Supplementary Table 1, Supplementary Fig. 7).
Our data clearly show a positive correlation between heavy brainstem invasion and survival, and we observe much higher rates of such invasion than what was reported in older cohorts. Yet the overall median survival of our cohort is not significantly longer than that of those older cohorts (Table 1). Although this study is not designed to experimentally elucidate the reason(s) for this apparent paradox, it is plausible that better neurosurgical resection (and re-resection) of primary tumor, plus adjuvant therapies like TMZ and bevacizumab, have caused a shift in GBM recurrence patterns from bulky local recurrence with massive radiation necrosis to a more disseminated disease. Some radiology-focused papers, published in this current era, have suggested as much.16,17 And instead of a solid large mass of tumor and necrosis at the original site, surgical cavities were noted in 22/33 (67%) cases, as demonstrated by Case 2 (Fig. 1). Such cavities might act as buffers against mass effect.
While brainstem dysfunction is characteristic of the final stages for the majority of GBM patients today, rather than mass effect and herniation, there are still other factors that contribute to death. Case 3 had severe dementia-like symptoms, directly attributable to heavy bilateral infiltration of the hippocampi (Supplementary Table 1, Fig. 4). Cases 10, 11, 15, 18, and 20 had pulmonary emboli, a common debilitating paraneoplastic complication of GBM.18 Cases 8, 10, and 30 had intracerebral hemorrhage. Case 11 had complications related to the surgical resection. Cases 15, 16, and 29 had sepsis, possibly related to chemotherapy as well as the systemic immunosuppressive effect that GBM can exert.19,20 However, for many GBM patients, extensive brainstem infiltration may represent a barrier to further improvement in survival—one that is not being sufficiently addressed by current preclinical and clinical approaches.
Our results showing heavy leptomeningeal dissemination via pathology in only 12% of our cases comports with a prior study, in which 24/595 (4%) of patients on clinical trials showed leptomeningeal dissemination via radiology and CSF cytology.21 In that study, leptomeningeal dissemination was a late event, occurring a median of 11.9 months following diagnosis of GBM, with death occurring a median of 3.5 months after dissemination.
The biomedical field as a whole has made substantial progress in advancing the treatment of many cancers, but not GBM.22 The current study demonstrates the unique challenge of improving survival in brain cancer patients, since brainstem destruction is obviously not a feature of cancers arising elsewhere in the body. These data also further highlight the limitations of prevailing preclinical animal models of brain cancer, including patient-derived xenografts and transgenic mice, from which new clinical trials are created. Very few of those models recapitulate the cerebrum-to-brainstem spread of GBM, via single-cell infiltration, which is so frequent in humans. Instead, death in those animal models is nearly always due to severe mass effect from the tumor—a mechanism that does not appear to be as relevant in most GBM patients anymore. For example, bevacizumab did not extend overall survival in GBM patients, despite highly encouraging effects in preclinical models.23–25 But the GBMs in those models grow as solid masses, and do not infiltrate on a single-cell level like they do in humans. Although blocking angiogenesis could inhibit a tumor with a solid growth pattern, infiltration of GBM cells through the white matter does not require new blood vessel formation. Thus, VEGF inhibition could not be expected to have any suppressive effect on invasion into critical sites like the brainstem. In fact, experimental data have suggested that bevacizumab may actually enhance glioma infiltration.26,27 In addition to newer surgical techniques, TMZ, and slightly longer median survival, the use of bevacizumab in GBM is another major difference between the current and older cohorts.
Regarding clinical trials, those that only enroll patients with recurrent tumor after frontline RT/TMZ has failed, as determined by radiologic evaluation of the original tumor site, may be too late in testing novel therapeutics that otherwise might have been effective with earlier administration. Furthermore, experimental treatments that are directed only toward the original tumor site probably do not affect distal spread. Based on the current data, by the time the tumor shows radiologic recurrence in the supratentorium, at least some of it is probably already in the pons. Strategies that are designed to target infiltrating tumor cells throughout the entire neuraxis, including immunotherapies, might therefore have the best chance of success if they are employed as early in the course of disease as possible.
These findings also call into question the prevailing dogma of RT field design, which employs a volumetric expansion of several centimeters around the tumor/cavity/edema into brain tissue. This strategy was based on pathologic studies correlating antemortem imaging with scattered tumor cells,28,29 but even this large volume RT might not reach tumor cells en route to the brainstem (Supplementary Table 3). Perhaps even larger volumes could reach such cells and enhance other therapies, or if this is not feasible, smaller volumes would at least minimize the negative effects of RT on the patient’s quality of life. Regarding the latter, some radiation oncologists have begun targeting smaller volumes, without an obvious diminution of efficacy.30
This cohort includes only patients who volunteered to donate their brain and/or spinal cord. Thus, this cohort does not consist of consecutive patients, so there is a potential for selection bias in this study. However, it is unclear what sort of bias would have prompted patients disproportionately suffering from brainstem invasion to volunteer for postmortem studies. The older autopsy cohorts were collected and reported with varying methods, which make comparisons to our modern cohort somewhat imprecise. However, their detailed descriptions of gross and microscopic patterns allowed us to extrapolate to our current cohort. Median survival in the current cohort was 14.3 months, similar to the reported median survival of GBM patients treated with RT/TMZ.2 Four of 33 patients (12%) had IDH1mut tumors (Supplementary Table 1), which is within the 5–15% range reported among GBMs.31 However, the rate of brainstem invasion between IDH1mut and IDH1wt GBM was not statistically different (see the “Results” section). The frequency of MGMT promoter methylation in the current cohort was only 21% (Supplementary Table 1), which is lower than the expected rate of ~40%,1 but because of the link between prolonged survival and brainstem invasion, this might have been expected to reduce the frequency of brainstem extension, not the other way around. Furthermore, it is not yet clear whether GBM has a unique tropism toward the brainstem, or whether tumor migration along axons is random, and tumor cells are simply being funneled by multiple large white matter tracts into a relatively small area.
In conclusion, this postmortem data has multiple implications for the study and management of GBM in the current era. First, it may be advisable to focus more attention on the brainstem, especially in patients who show apparently stable tumor at the original supratentorial site, yet are still clinically declining. As an example, while gliomas are currently only graded, not staged, it may be possible to further refine prognosis through a radiologic or clinical staging system that assesses brainstem involvement. Second, new preclinical GBM models that feature more diffuse brainstem infiltration might be better at predicting the clinical efficacy of new treatments. Finally, experimental therapies that kill GBM cells may have a better chance at extending patient survival, especially if they are used earlier, without waiting for tumor to radiologically recur near the original supratentorial site.
Funding
This study was funded by NIH grant R01NS102669 (to C.H.), by the Northwestern SPORE in Brain Cancer P50CA221747, and by the Lou Malnati Cancer Research Foundation. This work was additionally supported by NIH grant R01NS107833 (to M.G.C.), Grant 2015215 from the Doris Duke Charitable Foundation (to M.G.C.), and by Kathy and Mark Frederickson (to M.G.C.).
Supplementary Material
Acknowledgments
The authors thank Sarah Gentile, Jennifer Matthews, and Brooke Robertson for autopsy assistance; Kimberly Liggett for acquiring hospice notes; Andrea Charest for Figure 5B; and the Lou and Jean Malnati Brain Tumor Institute for supporting the Northwestern Nervous System Tumor Bank. Most of all, the authors thank the courageous donors, and their families, for supporting this endeavor through their great gift to humanity.
Conflict of interest statement. The authors declare that they have no conflict of interest.
Authorship statement. M.R.D. generated data tables and drafted the manuscript. K.S.D., S.G., P.K., R.V.L., J.J.R., and R.S. provided cohort patients and manuscript edits. K.L.K., M.M., A.S., R.J., and K.M. assisted with postmortem collections. M.G.C., S.S., and T.K. provided manuscript edits. C.H. conceived of the project, performed analyses, prepared figures, and edited the manuscript.
References
- 1. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 3. Stupp R, Wong ET, Kanner AA, et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012;48(14):2192–2202. [DOI] [PubMed] [Google Scholar]
- 4. Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Giangaspero F, Burger PC. Correlations between cytologic composition and biologic behavior in the glioblastoma multiforme. A postmortem study of 50 cases. Cancer. 1983;52(12):2320–2333. [DOI] [PubMed] [Google Scholar]
- 6. Silbergeld DL, Rostomily RC, Alvord EC Jr. The cause of death in patients with glioblastoma is multifactorial: clinical factors and autopsy findings in 117 cases of supratentorial glioblastoma in adults. J Neurooncol. 1991;10(2):179–185. [DOI] [PubMed] [Google Scholar]
- 7. Vertosick FT Jr., Selker RG. Brain stem and spinal metastases of supratentorial glioblastoma multiforme: a clinical series. Neurosurgery. 1990;27(4):516–521; discussion 521–522. [DOI] [PubMed] [Google Scholar]
- 8. Willard N, Kleinschmidt-DeMasters BK. Massive dissemination of adult glioblastomas. Clin Neuropathol. 2015;34(6):330–342. [DOI] [PubMed] [Google Scholar]
- 9. Sizoo EM, Braam L, Postma TJ, et al. Symptoms and problems in the end-of-life phase of high-grade glioma patients. Neuro Oncol. 2010;12(11):1162–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nikiforova MN, Wald AI, Melan MA, et al. Targeted next-generation sequencing panel (GlioSeq) provides comprehensive genetic profiling of central nervous system tumors. Neuro Oncol. 2016;18(3):379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Onda K, Tanaka R, Takahashi H, Takeda N, Ikuta F. Cerebral glioblastoma with cerebrospinal fluid dissemination: a clinicopathological study of 14 cases examined by complete autopsy. Neurosurgery. 1989;25(4):533–540. [PubMed] [Google Scholar]
- 12. Hamza A. Declining rate of autopsies: implications for anatomic pathology residents. Autops Case Rep. 2017;7(4):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nemetz PN, Tanglos E, Sands LP, Fisher WP Jr, Newman WP 3rd, Burton EC. Attitudes toward the autopsy—an 8-state survey. MedGenMed. 2006;8(3):80. [PMC free article] [PubMed] [Google Scholar]
- 14. Burger PC, Mahley MS Jr, Dudka L, Vogel FS. The morphologic effects of radiation administered therapeutically for intracranial gliomas: a postmortem study of 25 cases. Cancer. 1979;44(4):1256–1272. [DOI] [PubMed] [Google Scholar]
- 15. Kamali A, Kramer LA, Frye RE, Butler IJ, Hasan KM. Diffusion tensor tractography of the human brain cortico-ponto-cerebellar pathways: a quantitative preliminary study. J Magn Reson Imaging. 2010;32(4):809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ogura K, Mizowaki T, Arakawa Y, et al. Initial and cumulative recurrence patterns of glioblastoma after temozolomide-based chemoradiotherapy and salvage treatment: a retrospective cohort study in a single institution. Radiat Oncol. 2013;8:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tejada S, Aldave G, Marigil M, Gallego Perez-Larraya J, Dominguez PD, Diez-Valle R. Factors associated with a higher rate of distant failure after primary treatment for glioblastoma. J Neurooncol. 2014;116(1):169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Unruh D, Schwarze SR, Khoury L, et al. Mutant IDH1 and thrombosis in gliomas. Acta Neuropathol. 2016;132(6):917–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Woroniecka K, Chongsathidkiet P, Rhodin K, et al. T-cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clin Cancer Res. 2018;24(17):4175–4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chongsathidkiet P, Jackson C, Koyama S, et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med. 2018;24(9):1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mandel JJ, Yust-Katz S, Cachia D, et al. Leptomeningeal dissemination in glioblastoma: an inspection of risk factors, treatment, and outcomes at a single institution. J Neurooncol. 2014;120(3):597–605. [DOI] [PubMed] [Google Scholar]
- 22. Noone AM, Howlader N, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2015. 2018. https://seer.cancer.gov/csr/1975_2015/.
- 23. Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 25. Wick W, Gorlia T, Bendszus M, et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377(20):1954–1963. [DOI] [PubMed] [Google Scholar]
- 26. de Groot JF, Fuller G, Kumar AJ, et al. Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro Oncol. 2010;12(3):233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lu KV, Chang JP, Parachoniak CA, et al. VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell. 2012;22(1):21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Halperin EC, Bentel G, Heinz ER, Burger PC. Radiation therapy treatment planning in supratentorial glioblastoma multiforme: an analysis based on post mortem topographic anatomy with CT correlations. Int J Radiat Oncol Biol Phys. 1989;17(6):1347–1350. [DOI] [PubMed] [Google Scholar]
- 29. Kelly PJ, Daumas-Duport C, Kispert DB, Kall BA, Scheithauer BW, Illig JJ. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg. 1987;66(6):865–874. [DOI] [PubMed] [Google Scholar]
- 30. Chang EL, Akyurek S, Avalos T, et al. Evaluation of peritumoral edema in the delineation of radiotherapy clinical target volumes for glioblastoma. Int J Radiat Oncol Biol Phys. 2007;68(1):144–150. [DOI] [PubMed] [Google Scholar]
- 31. Horbinski C. What do we know about IDH1/2 mutations so far, and how do we use it? Acta Neuropathol. 2013;125(5):621–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.