Abstract
Background
DEPOSEIN (NCT01645839) was a randomized open-label phase III study to explore the role of intrathecal chemotherapy in patients with newly diagnosed leptomeningeal metastasis (LM), a common manifestation of breast cancer.
Methods
Patients with newly diagnosed LM defined by tumor cells in the cerebrospinal fluid or combination of clinical and neuroimaging signs of LM were randomized to receive systemic therapy alone (control group) or systemic therapy plus intrathecal liposomal cytarabine (experimental group). Progression-free survival related to LM (LM-PFS) was the primary endpoint.
Results
Thirty-seven and 36 patients were assigned to the control and the experimental groups. Median number of liposomal cytarabine injections in the experimental group was 5 (range 1–20). Focal radiotherapy was performed in 6 (16%) and 3 (8%) patients in the control and experimental groups. In the intent-to-treat population, median LM-PFS was 2.2 months (95% CI: 1.3–3.1) in the control versus 3.8 months (95% CI: 2.3–6.8) in the experimental group (hazard ratio 0.61, 95% CI: 0.38–0.98) (P = 0.04). Seventy-one patients have died. Median overall survival was 4.0 months (95% CI: 2.2–6.3) in the control versus 7.3 months (95% CI: 3.9–9.6) in the experimental group (hazard ratio 0.85, 95% CI: 0.53–1.36) (P = 0.51). Serious adverse events were reported in 22 and 30 patients, respectively. Quality of life until progression did not differ between groups.
Conclusion
The addition of intrathecal liposomal cytarabine to systemic treatment improves LM-related PFS. Confirmatory trials with optimized patient selection criteria and more active drugs may be required to demonstrate a survival benefit from intrathecal pharmacotherapy.
Keywords: brain, cerebrospinal, depocyte, neoplastic, meningitis
Key Points.
Leptomeningeal metastasis (LM) is an advanced stage manifestation of breast cancer.
First randomized trial showing a clinical benefit of intrathecal chemotherapy in LM.
Importance of the Study.
Patients with LM are treated in a highly variable fashion. DEPOSEIN is a randomized open-label phase III study to explore the addition of intrathecal liposomal cytarabine to systemic therapy for the treatment of newly diagnosed LM from breast cancer. The DEPOSEIN study shows that, compared with systemic chemotherapy alone, the combination of intrathecal chemotherapy with systemic chemotherapy prolongs leptomeningeal metastasis–related PFS. Quality of life until progression did not differ between groups, and, surprisingly, this local treatment prolongs overall survival by trend in a preplanned analysis in the adjusted intent-to-treat population. No differences in quality of life were observed between groups, although patients in the experimental group had more intensive treatment. DEPOSEIN thus confirms a role for intrathecal pharmacotherapy at least in subsets of LM patients. A larger confirmatory trial with optimized patient selection criteria may be required to demonstrate a survival benefit from intrathecal pharmacotherapy.
Leptomeningeal metastasis (LM) commonly represents a manifestation of advanced cancer. It affected 8% of patients with metastatic cancer already prior to 19781 and is seen most often with breast cancer, lung cancer, and melanoma. Prognosis remains poor, with an overall survival (OS) of around 4 months in breast cancer patients with LM (Supplementary Table 1).2–6 The main goals of LM treatment are to maintain neurological function and quality of life (QoL) and to prolong OS.7 Only 5 randomized trials have been performed in LM from solid tumors,8–12 all published more than 10 years ago and criticized for methodological limitations.13 Four trials included patients with LM from different primary tumors, compared different intrathecal chemotherapy regimens, and observed no major differences except for longer time to neurological progression with liposomal cytarabine compared with methotrexate in one trial.10 The only trial comparing intrathecal therapy with no intrathecal therapy11 revealed no benefit from intrathecal methotrexate. However, response evaluation was based on clinical examination only, a high rate of ventricular device complications with 18% reservoir revisions was observed, likely negatively affecting outcome in the experimental arm, and the trial was stopped prematurely for poor accrual. Thus, a role of intrathecal chemotherapy in LM from solid cancers remained unproven. Yet, a recent survey indicated widespread use of this treatment modality across Europe.14 Methotrexate, cytarabine, and thiotepa are available for intrathecal chemotherapy in most European countries and commonly given twice weekly. Liposomal cytarabine has a prolonged half-life, allowing for fewer administrations, every 14 days, and was therefore chosen as the experimental intervention in the DEPOSEIN trial. Its purpose was to determine whether intrathecal chemotherapy is of value in the treatment of LM from a chemosensitive tumor entity.
Patients and Methods
Study Design and Participants
DEPOSEIN (NCT01645839) was a phase III randomized open-label study which compared systemic treatment alone (control group) with systemic treatment combined with intrathecal liposomal cytarabine (experimental group) in patients with newly diagnosed LM from breast cancer. The primary aim was to demonstrate superior LM-related progression-free survival (LM-PFS) in the experimental group. Secondary endpoints included overall PFS, OS, response to treatment, safety, and benefit-risk ratio using the quality-adjusted time without symptoms of disease or toxicity (Q-TWiST) approach.15 Exploratory objectives included QoL and determination of prognostic factors.
The study was performed at 5 centers across France. Eligible patients were 18 years of age or older, female, had histologically confirmed breast cancer and newly diagnosed LM based on the presence of malignant cells in the cerebrospinal fluid (CSF), or the combination of typical clinical symptoms and signs and MRI findings. Meningeal nodules had to be less than 5 mm in diameter if no focal radiotherapy was planned, but could be larger if focal radiotherapy was planned. Irradiated nodules were not used as target lesions to define response. Patients had to have an Eastern Cooperative Oncology Group (ECOG) performance status score between 0 and 2, be candidates for systemic therapy, and have a life expectancy of at least 2 months. Brain metastases were allowed if asymptomatic and not considered to require whole brain radiotherapy. Prior whole brain radiotherapy for brain metastases or focal radiotherapy to spinal lesions was allowed, but prior craniospinal radiotherapy was not. No prior intrathecal chemotherapy or prior systemic treatment with high-dose cytarabine or methotrexate was allowed. Patients with ventriculoperitoneal shunts were excluded. Signed informed consent was obtained from all study participants. The protocol was approved by the ethics committees and competent authorities of all participating centers. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice Guidelines.
Randomization and Masking
Patients were randomly assigned to systemic treatment alone or systemic treatment plus intrathecal liposomal cytarabine chemotherapy. Randomization (1:1) was performed centrally by Oscar Lambret Cancer Center Research Unit staff using blocks of 6 patients, without stratification criteria. Neither investigators nor patients were masked to treatment allocation.
Interventions
Baseline assessment included clinical evaluation including weight, ECOG performance status, Medical Research Council scale, general examination, neurological evaluation (Supplementary Table 2), medical history, current medications, blood analysis with hematology, electrolytes, albumin, renal and hepatic functions, CSF cytological and biochemical analysis, standardized cerebrospinal MRI (Supplementary Table 3), extra-central nervous system evaluation (chest-abdominal-pelvic CT or PET scans), cognitive testing using the Montreal cognitive assessment,16 and health-related QoL questionnaires: generic European Organisation for Research and Treatment of Cancer QLQ-C30 in combination with the QLQ-BN20 brain-cancer specific module and the QLQ-C15-PAL module for palliative care. Supplementary Table 4 summarizes the schedule of assessments. Clinical evaluations were foreseen every 14 ± 2 days for 2 months and then every 28 ± 5 days. Cerebrospinal MRI was scheduled every 2 months.
All patients received systemic treatment selected by the treating physician prior to randomization according to molecular tumor characteristics, prior lines of treatments, and general status. Patients assigned to the experimental group received in addition intrathecal liposomal cytarabine 50 mg every 14 days for 2 months (5 injections) followed by monthly injections of 50 mg until progression, unacceptable toxicity, or for up to 1 year. Oral steroids (5–6 mg equivalent dexamethasone) were recommended for 5 consecutive days from the day of liposomal cytarabine injection to prevent chemical meningitis. In case of severe toxicity, the dose of cytarabine could be reduced to 25 mg. Intrathecal chemotherapy was initiated via the lumbar route, but the intraventricular route was encouraged.
Outcome Measurements
The primary endpoint was LM-PFS, defined as the time between randomization and first evidence of LM progression, based on clinical or imaging findings assessed by the treating center, or death from any cause. Secondary efficacy endpoints included LM-related response rate defined by neurological clinical evaluation, MRI or CSF analysis (Supplementary Tables 5‒8), overall PFS, and OS. In brief, absence or presence of diffuse or focal contrast enhancement, nodules, cortical infiltration, parenchymal extension, and hydrocephalus were captured. Nodules were rated as 0–5 mm, 5–10 mm, or more than 10 mm diameter. Complete response required the disappearance of all contrast-enhancing lesions and no evidence of new lesions. Partial response required a reduction of at least 30% of the sum of the 2 largest diameters for measurable lesions, no progression of non-measurable lesions, and no evidence of new lesions. Increase of at least 20% of the largest diameter for measurable targets, appearance of new leptomeningeal lesions, or progression of non-measurable lesions constituted progression.
All survival endpoints were computed from randomization. All adverse events (AEs) were categorized by MedRA preferred term name and system organ class and graded according to National Cancer Institute‒Common Terminology Criteria for Adverse Events (CTCAE) v4.0. For each type of AE, we considered the worst grade observed over the whole study period. A grade equal to or higher than 3 was classified as severe. Death occurring in the setting of an infection was considered as study treatment related when the patient was on treatment.
Sample Size
A previous phase II trial in LM from various solid cancers had revealed a median time to neurological progression of 60 days.10 When the protocol was designed, a doubling of LM-related PFS in the experimental arm was considered clinically meaningful. To show a difference of 30 days in LM-PFS with a hazard ratio (HR) of 0.50, bilateral alpha = 5%, beta = 20%, accrual of 7 years, and follow-up of at least 6 months, 66 events had to be observed. Considering the hypothesis of an exponential distribution of survival, the LM-PFS rates at 3 months were estimated at 12.5% and 35% in the 2 groups. To take into account a drop-out rate of 20%, 80 patients had to be enrolled. Inclusion was to be stopped when the requested number of 66 events was reached.
Statistical Analysis
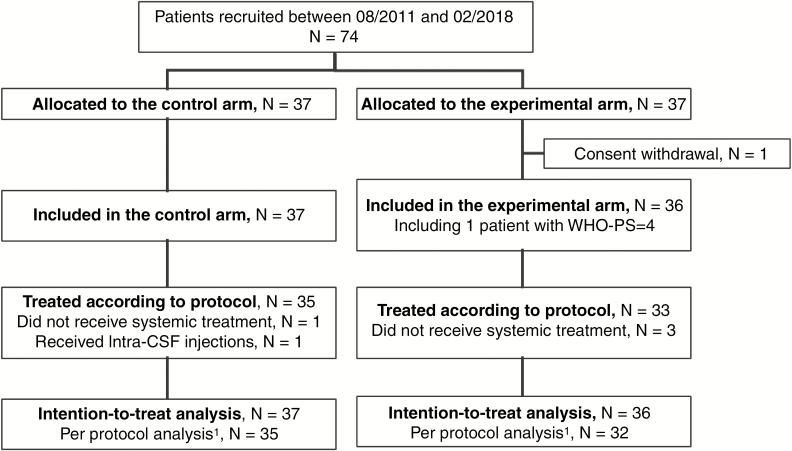
All randomized patients were included in the intent-to-treat (ITT) analysis. The per-protocol (PP) analysis excluded patients who did not fulfill the inclusion criteria and patients who did not start systemic treatment for reasons other than progression, patients in the experimental group who did not start intrathecal treatment for reasons other than progression, and control group patients treated with intrathecal liposomal cytarabine prior to further progression (Fig. 1). LM-PFS (main endpoint), overall PFS, and OS were estimated by the Kaplan–Meier method from the date of randomization.
Fig. 1.
CONSORT chart for the DEPOSEIN trial. For the per protocol analysis, we excluded 6 patients: 2 patients in the control group who did not receive treatment as planned in the protocol; 4 patients in the experimental group, 1 because she was included although not matching the eligibility criteria and 3 because they did not receive treatment as planned per protocol.1
Cox models were used to estimate the HR associated with the treatment effect (experimental vs control) after testing the proportional hazards assumption with the method of the scaled Schoenfeld residuals. Prespecified sensitivity analyses were performed adjusting for ECOG performance status (0 vs 1 vs 2 or more) at LM diagnosis, number of prior lines of systemic treatment (0 or 1 versus two or more), human epidermal growth factor receptor 2 (HER-2) status (negative versus positive), positivity of CSF cytology at LM diagnosis (negative or equivocal vs positive). A sensitivity analysis was also performed by excluding patients with a major protocol violation. We used a competing risk approach in order to estimate the treatment effect on the different components of the PFS where systemic progression without LM progression and death without progression were considered as competing events of LM progression. Cumulative incidence of the different events was obtained by the Kalbfleish and Prentice17 method, and the comparison between groups (control, experimental) was done using the Fine and Gray18 models, leading to the estimate of subdistribution HR.
Safety analyses were performed on the as-treated population, excluding patients who did not receive any treatment. For each category of AE, the proportion of patients having experienced an AE of any grade as well as the proportion of patients with severe AE were estimated by treatment group and displayed in a butterfly plot. The relative risk of having experienced a severe AE in the experimental group versus control was estimated. Details of grades per preferred term were tabulated by treatment group.
Q-TWiST was estimated from randomization until death with a restriction time set at 18 months. For this analysis, each patient’s OS duration was partitioned into 3 mutually exclusive health states: time spent with severe adverse event (grade 3 or 4) before progression (TOX), time spent after LM progression (LM-PROG), and time spent without severe adverse event before progression (TWiST). The time spent in each state was weighted by a health-state utility associated with each state, UTOX = 0.5, UTWiST = 1, and ULM-PROG = 0.5 and summed to calculate the Q-TWiST. Q-TWiST was compared between treatment groups using Bootstrap samples.
All randomized patients, for whom baseline measures of QoL were available, were included in the QoL analysis. For each dimension of the considered QoL questionnaire, the time until definitive deterioration of QoL was compared between treatment groups by log-rank test, and the effect of treatment was estimated by HR of QoL deterioration using Cox models. Statistical analyses were done with Stata/SE (version 15.0) statistical software.
Results
Accrual Period and Trial Participants
A total of 74 patients were enrolled from August 2011 to January 2018; 37 patients to each group. One patient from the experimental group withdrew consent (Fig. 1). Thus 73 patients represent the ITT population, 67 patients were treated per protocol. Baseline patient characteristics were similar between groups, except for mildly heavier pretreatment with systemic treatment and radiotherapy in the control group, which, however, had overall better performance status and a higher rate of HER-2 positive tumors (Table 1).
Table 1.
Patient characteristics at baseline
| Characteristics | Control Group(N = 37) | Experimental Group(N = 36) | All Patients(N = 73) | |||
|---|---|---|---|---|---|---|
| Breast cancer history | ||||||
| Age at breast cancer diagnosis | ||||||
| Years, median (range) | 47.5 | (29.7; 84.1) | 50.9 | (30.1; 75) | 50.2 | (29.7; 84.1) |
| Histology, n (%) | ||||||
| Invasive ductal carcinoma | 23 | 62% | 23 | 62% | 46 | 63% |
| Invasive lobular carcinoma | 9 | 24% | 9 | 25% | 18 | 25% |
| Other subtypes1 | 5 | 14% | 4 | 11% | 9 | 12% |
| Differentiation, n (%) | ||||||
| Moderate | 9 | 24% | 11 | 31% | 20 | 27% |
| Poor | 20 | 54% | 12 | 33% | 32 | 44% |
| Other | 1 | 3% | 1 | 3% | 2 | 3% |
| Unknown | 7 | 19% | 12 | 33% | 19 | 26% |
| T status per TNM at diagnosis of breast cancer | ||||||
| 1 | 5 | 14% | 9 | 25% | 14 | 19% |
| 2 | 12 | 32% | 9 | 25% | 21 | 29% |
| 3 | 5 | 14% | 4 | 11% | 9 | 12% |
| 4 | 5 | 14% | 2 | 6% | 7 | 10% |
| X (unknown) | 10 | 27% | 12 | 33% | 22 | 30% |
| N status per TNM at diagnosis of breast cancer | ||||||
| 0 | 6 | 16% | 8 | 22% | 14 | 19% |
| 1 | 11 | 30% | 10 | 28% | 21 | 29% |
| 2 | 5 | 14% | 2 | 6% | 7 | 10% |
| 3 | 2 | 5% | 2 | 6% | 4 | 5% |
| X (unknown) | 13 | 35% | 14 | 39% | 27 | 37% |
| M status per TNM at diagnosis of breast cancer | ||||||
| 0 | 22 | 59% | 21 | 58% | 43 | 59% |
| 1 | 5 | 14% | 8 | 22% | 13 | 18% |
| X (unknown) | 10 | 27% | 7 | 19% | 17 | 23% |
| Estrogen receptor status, n (%) | ||||||
| Negative | 11 | 30% | 9 | 25% | 20 | 27% |
| Positive | 24 | 65% | 27 | 75% | 51 | 70% |
| Unknown | 2 | 5% | 0 | 0% | 2 | 3% |
| Progesterone receptor status, n (%) | ||||||
| Negative | 17 | 46% | 11 | 31% | 28 | 38% |
| Positive | 18 | 49% | 24 | 67% | 42 | 58% |
| Unknown | 2 | 5% | 1 | 3% | 3 | 4% |
| HER-2 status, n (%) | ||||||
| Negative | 25 | 68% | 30 | 83% | 55 | 75% |
| Positive | 9 | 24% | 2 | 6% | 11 | 15% |
| Unknown | 3 | 8% | 4 | 11% | 7 | 10% |
| Triple-negative tumor, n (%) | ||||||
| No | 28 | 76% | 29 | 81% | 57 | 78% |
| Yes | 7 | 19% | 5 | 14% | 12 | 16% |
| Unknown | 2 | 5% | 2 | 6% | 4 | 5% |
| LM presentation | ||||||
| Age at LM diagnosis | ||||||
| Years, median (range) | 59 | (31; 87) | 57 | (41; 76) | 57 | (31; 87) |
| Body mass index classification at LM diagnosis | ||||||
| Underweight | 2 | 5% | 2 | 6% | 4 | 5% |
| Normal range | 16 | 43% | 18 | 50% | 34 | 47% |
| Overweight | 6 | 16% | 11 | 31% | 17 | 23% |
| Obese | 11 | 30% | 5 | 14% | 16 | 22% |
| Unknown | 2 | 5% | 0 | 0% | 2 | 3% |
| Systemic therapy before LM diagnosis, n (%) | 37 | 100% | 34 | 94% | 71 | 97% |
| Number of lines, median (range) | 3 | (1; 11) | 2 | (0; 8) | 3 | (0; 11) |
| Prior surgery for brain metastases, n (%) | 3 | 8% | 2 | 6% | 5 | 7% |
| Prior central nervous system radiotherapy, n (%) | 10 | 27% | 5 | 14% | 15 | 21% |
| Focal radiotherapy alone | 6 | 16% | 3 | 8% | 9 | 12% |
| Whole brain radiotherapy (± focal) | 4 | 11% | 2 | 6% | 6 | 8% |
| Neurological symptoms or signs, n (%) | 33 | 89% | 34 | 94% | 67 | 92% |
| MRI findings suggestive of LM diagnosis, n (%) | 37 | 100% | 34 | 94% | 71 | 97% |
| CSF cytology at LM diagnosis within 14 days prior to randomization, n (%) | ||||||
| Positive | 29 | 78% | 26 | 72% | 55 | 75% |
| Negative | 6 | 16% | 8 | 22% | 14 | 19% |
| Equivocal | 2 | 5% | 2 | 6% | 4 | 5% |
| CSF protein (7 missing data) | ||||||
| g/L, median (range) | 0.8 | (0.2; 7.1) | 0.7 | (0.3; 21.8) | 0.8 | (0.2; 21.8) |
| Patient characteristics at LM diagnosis | ||||||
| Time interval (years) between breast cancer diagnosis and LM diagnosis | ||||||
| Years, median (range) | 4.5 | (0.4; 25.4) | 7.3 | (0.1; 24) | 4.7 | (0.1; 25.4) |
| Breast cancer manifestations at LM diagnosis | ||||||
| Brain metastases, n (%) | 15 | 41% | 8 | 22% | 23 | 32% |
| Non-CNS metastatic sites, n (%) | 35 | 95% | 34 | 94% | 69 | 95% |
| Liver | 19 | 51% | 19 | 53% | 38 | 52% |
| Lung/pleura | 16 | 43% | 17 | 47% | 33 | 45% |
| Bone/bone marrow | 26 | 70% | 30 | 83% | 56 | 77% |
| Cutaneous | 6 | 16% | 4 | 11% | 10 | 14% |
| Lymph nodes | 14 | 38% | 5 | 14% | 19 | 26% |
| Other | 12 | 32% | 8 | 22% | 20 | 27% |
| LM as one of the initial sites of metastases (n, %) | 5 | 14% | 4 | 11% | 9 | 12% |
| ECOG performance status at LM diagnosis, n (%) | ||||||
| 0 | 7 | 19% | 3 | 8% | 10 | 14% |
| 1 | 16 | 43% | 15 | 42% | 31 | 42% |
| 2 | 14 | 38% | 17 | 47% | 31 | 42% |
| 4 | 0 | 0% | 1 | 3% | 1 | 1% |
| Montreal Cognitive Assessment (MoCA) | ||||||
| Score, median (range) | 25 | (9; 30) | 26 | (5; 30) | 26 | (5; 30) |
| Unknown | 7 | 1 | 8 | |||
| Medical Research Council scale at LM diagnosis | ||||||
| No neurological deficit | 11 | 30% | 5 | 14% | 16 | 22% |
| Light functional neurological disorder, allowing useful work | 15 | 41% | 14 | 39% | 29 | 40% |
| Moderate functional neurological disorder | 10 | 27% | 11 | 31% | 21 | 29% |
| Major functional neurological disorder | 1 | 3% | 4 | 11% | 5 | 7% |
| Unknown | 0 | 0% | 2 | 6% | 2 | 3% |
1 Other subtypes histology: 4 mixed ductal and lobular, 1 atypical medullary carcinoma, 1 ductal and micro papillary, 3 breast cancers not otherwise specified.
All patients in the experimental group received at least one intrathecal injection (Table 2). The median number of injections was 5 (range, 1–20); 3 patients had a dose reduction. Cytarabine was administered via lumbar route only in 24 patients, 12 patients received a ventricular reservoir. Thirty-six (97%) and 33 (92%) patients in the control and experimental groups received at least one dose of systemic treatment (Table 2, Supplementary Table 9). A second line of systemic treatment was administered in 11 patients (5 and 6 in the control and experimental groups), for non-LM progression prior to reaching the primary endpoint of LM-related progressive disease. Focal radiotherapy was administered in 6 (16%) and 3 (8%) patients in the control and experimental groups (Table 2). After end of study treatment, 30 patients (81%) in the control group and 23 patients (64%) in the experimental group received further treatment, including intrathecal chemotherapy in 15 patients in the control group and 2 patients in the experimental group.
Table 2.
Study treatment until further progression or end of study, and treatment at first LM progression in the ITT population
| Control Group (N = 37) | Experimental Group (N = 36) | |
|---|---|---|
| Intrathecal liposomal cytarabine until LM progression or end of study | ||
| Administration of liposomal cytarabine, n (%) | 1 (3%)1 | 36 (100%) |
| Duration of treatment, months, median (range) | 11.3 | 3.1 (0.4; 31.4) |
| Number of injections: median (range) | 14 | 5 (1–20) |
| Lumbar route only | 0 | 24 (67%) |
| Ventricular route | 1 | 12 (33%) |
| Time from randomization to first injection, days (range) | 0 | 0 (0; 13) |
| Systemic treatment, n (%)1until LM progression or end of study | 36 (97%) | 33 (92%) |
| Duration of treatment, months, median (range) | 2.4 (0.6; 44.8) | 3.6 (0.7; 18.2) |
| Type of systemic treatment (sometimes in combination) | ||
| Chemotherapy2 | 33 | 33 |
| Hormonal therapy | 1 | 1 |
| HER-2 targeted treatment | 7 | 2 |
| Other targeted treatment | 3 | 1 |
| Focal CNS radiotherapy, n (%) until LM progression or end of study(sometimes in combination)3 | 6 (16%) | 3 (8%) |
| Brain | 6 | 2 |
| Spine | 1 | 1 |
| Treatment at further LM progression, n (%)(possibly associated) | 30 (81%) | 23 (64%) |
| Intrathecal therapy | 15 | 2 |
| Focal radiotherapy | 1 | 2 |
| Systemic therapy | 19 | 10 |
1 Two patients in the control group and 4 patients in the experimental group had a major protocol violation. They are excluded from the per protocol population (see Fig. 1).
2 Systemic pharmacotherapy at study entry is detailed in Supplementary Table 9.
3 Six patients in the control group received focal radiotherapy: posterior fossa (n = 3), meningeal nodule at brain level (n = 1), cauda equina (n = 1), meningeal nodule at brain level and cauda equina (n = 1); 3 patients in the experimental group received focal radiotherapy: posterior fossa (n = 1), meningeal nodule at brain level (n = 1), cauda equina (n = 1).
Efficacy
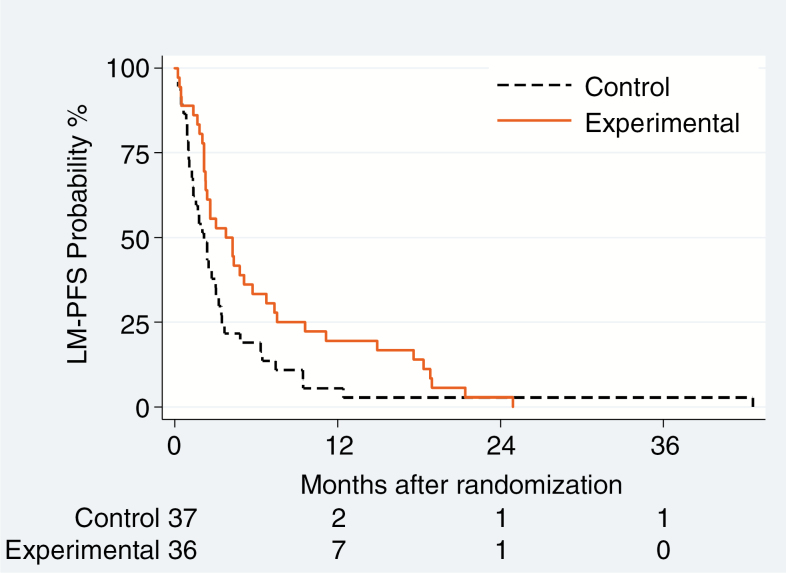
LM-related clinical improvement was reported in 1 (3%) and 6 (17%) patients in the control and experimental groups. MRI responses were observed in 3 (8%) in the control and 7 (19%) patients in the experimental groups. CSF cytology complete responses were observed in 5 (14%) versus 10 (28%) patients. All patients experienced an event, including LM progression in 51 patients, 30 and 21 in the control and experimental groups. There was a significant reduction of risk of LM progression or death in the experimental arm (HR = 0.61; 95% CI: 0.38–0.98; P = 0.04) in the ITT analysis. Median LM-PFS was 2.2 versus 3.8 months in the control and experimental groups, and the 6-month LM-PFS rate was 19% versus 33%. The estimated magnitude of treatment effect was larger in the adjusted analysis (HR = 0.47; 95% CI: 0.27–0.83; P = 0.01) (Table 3, Fig. 2A).
Table 3.
Treatment effect on efficacy outcomes ITT/PP populations
| ITT Population | Per Protocol Population1 | |||
|---|---|---|---|---|
| Outcome | Control Group | Experimental Group | Control Group | Experimental Group |
| N = 37 | N = 36 | N = 35 | N = 32 | |
| Best LM response 2 | ||||
| Clinical | ||||
| Improvement | 1 (3%) | 6 (17%) | 1 (3%) | 6 (19%) |
| Stabilization | 30 (81%) | 26 (72%) | 28 (80%) | 24 (75%) |
| Deterioration | 4 (11%) | 4 (11%) | 4 (11%) | 2 (6%) |
| Missing3 | 2 (5%) | 0 (0%) | 2 (6%) | 0 (0%) |
| Radiological | ||||
| PR | 3 (8%) | 7 (19%) | 3 (9%) | 7 (22%) |
| SD | 12 (32%) | 20 (56%) | 11 (31%) | 18 (56%) |
| PD | 11 (30%) | 5 (14%) | 11 (31%) | 5 (16%) |
| Missing or unevaluable4 | 11 (30%) | 4 (11%) | 10 (29%) | 2 (6%) |
| CSF cytology | ||||
| CR | 5 (14%) | 10 (28%) | 4 (11%) | 10 (31%) |
| SD | 22 (59%) | 23 (64%) | 21 (60%) | 21 (66%) |
| PD | 2 (5%) | 0 (0%) | 2 (7%) | 0 (0%) |
| Missing or unevaluable5 | 8 (22%) | 3 (8%) | 8 (23%) | 1 (3%) |
| LM-related PFS (LM-PFS) | ||||
| Number and type of events | 37 | 36 | 35 | 32 |
| LM-related progression | 30 | 21 | 29 | 19 |
| Death without LM-related progression | 7 | 15 | 6 | 13 |
| Median LM-PFS (95% CI) | 2.2 (1.3–3.1) | 3.8 (2.3–5.7) | 2.2 (1.3–3.0) | 4.3 (2.4–7.4) |
| LM-PFS rate (95% CI) | ||||
| at 2 months | 54.1 (36.9–68.4) | 80.6 (63.5–90.2) | 54.3 (36.6–69.0) | 90.6 (73.7–96.9) |
| at 4 months | 21.6 (10.2–35.8) | 50.0 (32.9–64.9) | 20.0 (8.8–34.4) | 56.3 (37.6–71.3) |
| at 6 months | 18.9 (8.3–32.8) | 33.3 (18.8–48.6) | 17.1 (7.0–31.1) | 37.5 (21.3–53.7) |
| Unadjusted HR (95% CI) | 1 (ref) | 0.61 (0.38–0.98) | 1 (ref) | 0.52 (0.31–0.85) |
| P-value | P = 0 · 04 | P = 0.009 | ||
| Adjusted HR (95% CI)6 | 1 (ref) | 0.47 (0.27–0.83) | 1 (ref) | 0.41 (0.23–0.74) |
| P-value | P = 0.01 | P = 0.003 | ||
| Overall PFS | ||||
| Number and type of first event | 37 | 36 | 35 | 32 |
| LM progression as first event | 27 | 16 | 26 | 13 |
| Systemic progression without LM prog. as first event | 7 | 15 | 6 | 14 |
| Death without reported progression | 3 | 5 | 3 | 5 |
| Median PFS (95% CI) | 2.0 (1.3–2.9) | 2.4 (2.0–4.4) | 2.0 (1.1–2.5) | 3.1 (2.2–5.7) |
| PFS rate (95% CI) | ||||
| at 2 months | 51.4 (34.4–66.0) | 69.4 (51.7–81.8) | 51.4 (34.0–66.4) | 78.1 (59.5–88.9) |
| at 4 months | 18.9 (8.3–32.8) | 41.7 (25.6–57.0) | 17.1 (7.0–31.1) | 46.9 (29.2–62.8) |
| at 6 months | 13.5 (4.9–26.4) | 25.0 (12.4–39.8) | 11.4 (3.6–24.2) | 28.1 (14.0–44.1) |
| Unadjusted HR (95% CI) | 1 (ref) | 0.66 (0.41–1.06) | 1 (ref) | 0.58 (0.35–0.94) |
| P-value | P = 0.09 | P = 0.03 | ||
| Adjusted HR (95% CI) 6 | 1 (ref) | 0.54 (0.31–0.95) | 1 (ref) | 0.47 (0.26–0.83) |
| P-value | P = 0.03 | P = 0.01 | ||
| Overall survival (OS) | ||||
| Number and type of deaths | 35 | 36 | 33 | 32 |
| Death related to neurological progression | 16 | 9 | 15 | 7 |
| Death related to extra-CNS progression | 4 | 6 | 3 | 6 |
| Death related to combined progression | 7 | 8 | 7 | 8 |
| Study treatment (systemic treatment) related death7 | 2 | 4 | 2 | 4 |
| Other7 | 2 | 4 | 2 | 4 |
| Unknown | 4 | 5 | 4 | 3 |
| Median OS (95% CI) | 4.0 (2.2–6.3) | 7.3 (3.9–9.6) | 4.0 (2.2–6.3) | 7.3 (3.9–12.6) |
| OS rate (95% CI) | ||||
| at 2 months | 70.3 (52.8–82.3) | 86.1 (69.8–94.0) | 68.6 (50.5–81.2) | 93.8 (77.3–98.4) |
| at 4 months | 51.4 (34.4–66.0) | 63.9 (46.1–77.2) | 51.4 (34.0–66.4) | 68.8 (49.7–81.8) |
| at 6 months | 40.5 (24.9–55.7) | 55.6 (38.1–69.9) | 40.0 (24.0–55.5) | 59.4 (40.5–74.0) |
| Unadjusted HR (95% CI) | 1 (ref) | 0.85 (0.53 -1.36) | 1 (ref) | 0.81 (0.50–1.32) |
| P-value | P = 0.51 | P = 0.40 | ||
| Adjusted HR (95% CI) 6 | 1 (ref) | 0.60 (0.35–1.02) | 1 (ref) | 0.55 (0.31–0.96) |
| P-value | P = 0.057 | P = 0.04 |
CR: complete response, PD: progressive disease, PR: partial response, ref: reference, SD: stable disease.
1 Per protocol population, excluding 2 and 4 patients in the control arm and the experimental arm, respectively, who had a major protocol violation (see Fig. 1).
2 Clinical data were collected every 14 ± 2 days for 2 months then every 28 days ± 5 days, CSF cytology data were collected every 14 ± 2 days in the experimental group and every 28 ± 5 days in the control group for 2 months and then every 28 days ± 5 days for the 2 groups; cerebrospinal MRI was performed every 2 months (see Supplementary Table 4).
3 Information about best clinical response is missing for 3 patients who went off study early (<2 mo).
4 Information about best radiological response is missing for 15 patients who had an early treatment termination, mainly due to clinical progression.
5 Information about best cytological response is missing for 11 patients who went out of study early (<2 mo), mainly due to clinical progression or death.
6 Model adjusted for ECOG performance status at LM diagnosis (0 vs 1 vs 2+), number of prior lines of systemic treatment (0–1 vs 2+), HER-2 status at diagnosis (positive vs negative, excluding 7 cases with missing data), and positivity of CSF cytology at LM diagnosis (positive vs negative or equivocal)
7 The deaths reported as first event without prior progression, as well as all other deaths not classified as related to disease progression are detailed in Supplementary Table 11.
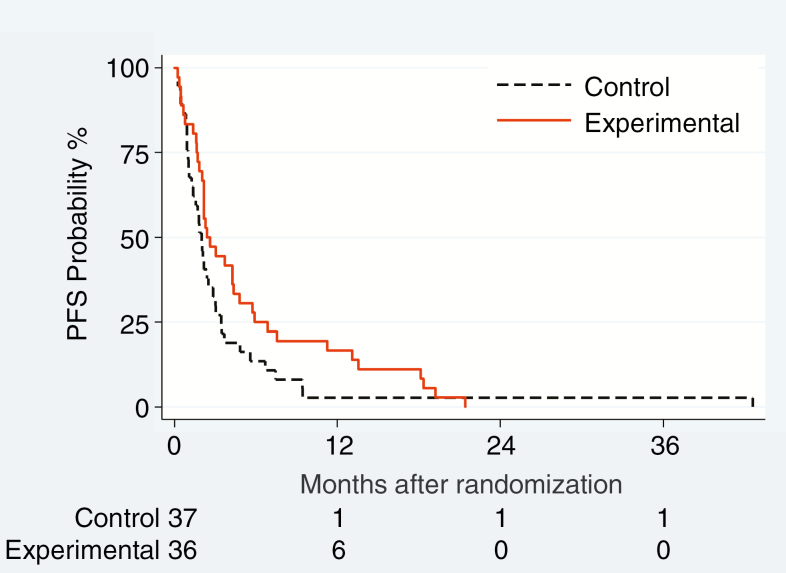
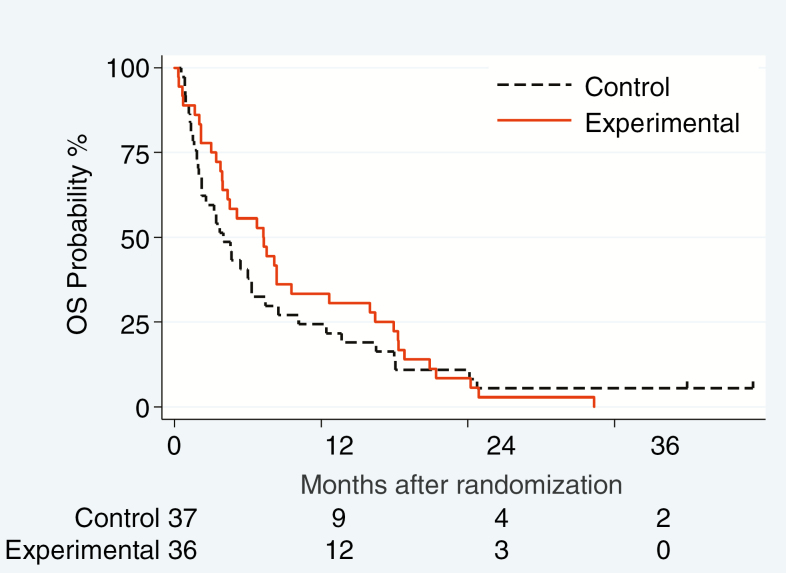
Fig. 2.
Progression-free survival related to leptomeningeal disease (LM-PFS) (A), overall PFS (B) and OS (C) in the ITT population
All patients had a PFS event. No patient had isolated progression of brain metastases without LM progression. In the unadjusted analysis performed on the ITT population, we observed a non-significant 34% reduction of risk of overall progression or death (HR = 0.66; 95% CI: 0.41–1.06; P = 0.09; Fig. 2B), with a median overall PFS of 2.0 versus 2.4 months in the control and experimental groups. The treatment effect was significant in the prespecified multivariate analysis (HR = 0.54; 95% CI: 0.31–0.95; P = 0.03). In the competing risk approach (Supplementary Table 10, Supplementary Fig. 1), the distribution of events differed between the treatment groups: there was a significant reduction of LM progression (P = 0.01), contrasting with a trend for an excess risk of systemic progression without LM progression (P = 0.053), in the experimental group.
Overall, 71 patients died and 2 patients are alive 41.8 and 47.2 months after study entry, despite having experienced LM progression. Death was attributed to LM-related neurological progression in 25 patients (16 and 9 patients), to a combination of neurological and extra-neurological disease in 15 patients (7 and 8 patients) (Table 3), and considered not directly related to disease progression in 21 patients (8 and 13 patients) (Supplementary Table 11). Median OS was 4.0 months (95% CI: 2.2–6.3) in the control and 7.3 months (95% CI: 3.9–9.6) in the experimental group. Considering the whole survival curve of the ITT population, the unadjusted estimate of HR was 0.85 (95% CI: 0.53–1.36, P = 0.51; Fig. 2C), and the adjusted HR was 0.60 (95% CI: 0.35–1.02, P = 0.057) (Table 3).
In the PP population, median LM-PFS was 2.2 months (95% CI: 1.3–3.0) in the control versus 4.3 months (95% CI: 2.4–7.4) in the experimental group (unadjusted HR = 0.52, 95% CI: 0.31–0.85, P = 0.009) (Supplementary Fig. 2A). Overall PFS was different, too, in the PP population, with 2.0 months (95% CI: 1.1–2.5) in the control versus 3.1 months (95% CI: 2.2–5.7) in the experimental group (Supplementary Fig. 2B) (unadjusted HR = 0.58, 95% CI: 0.35–0.94, P = 0.03). Median OS was 4.0 months (95% CI: 2.2–6.3) versus 7.3 months (unadjusted HR = 0.81, 95% CI: 3.9–12.6, P = 0.40). Of note, this difference became significant on adjusted analysis (adjusted HR = 0.55, 95% CI: 0.31–0.96, P = 0.04) (Supplementary Fig. 2C). Prognostic factors for OS are summarized in Supplementary Table 12.
Safety
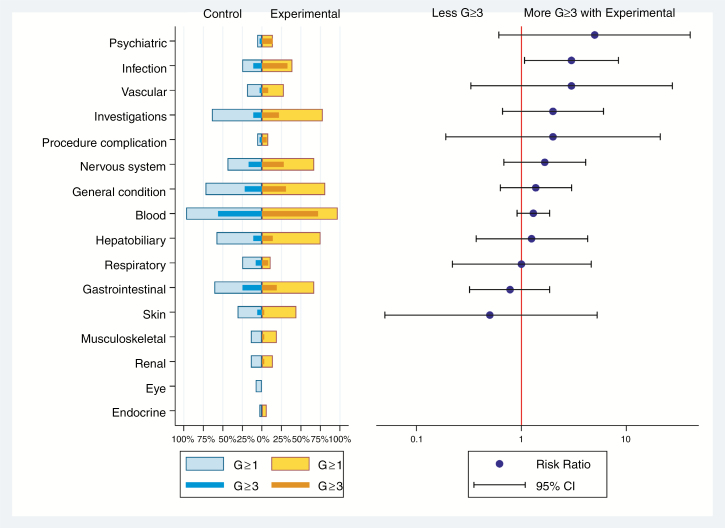
One patient received no study treatment and was not evaluated for safety. Five patients died from AE considered related to systemic treatment toxicity before LM progression. One control group patient died from gastrointestinal bleeding with severe thrombocytopenia before progression, 4 experimental group patients died from infection. Another control group patient died from systemic treatment toxicity (infection) administered after progression. There was no significant difference in the incidence of severe AE, except for more infections in the experimental group; further, chemical meningitis was seen in 3 patients in the experimental group (Supplementary Table 13, Fig. 3).
Fig. 3.
Butterfly plot of adverse events (related or not) in the 2 groups by system organ class. The panel on the left is a butterfly plot showing the proportion of patients experiencing an adverse event coded as related or not to the study treatment, whatever the grade (light blue for control group and yellow for experimental group), and a severe adverse event, grade ≥3 (dark blue for control group and orange for experimental group) according to group of randomization. The panel on the right displays the relative risk of a severe adverse event in patients in the experimental group relative to patients in the control group, with 95% CI. The toxicity items were pooled by system organ class. Details of adverse events are given in Supplementary Table 13.
The estimated mean duration of time spent without severe AE and before LM progression (TWiST) was 1.62 months (95% CI: 0.86–2.39) and 1.39 months (95% CI: 0.90–1.88) in the control and experimental groups. The estimated duration of time spent with severe AE (grade 3 or 4) before progression (TOX) was 1.82 months (95% CI: 0.73–29.1) and 4.93 (95% CI: 2.93–6.93), respectively, leading to an estimated Q-TWiST of 4.22 months (95% CI: 3.08–5.36) and 5.04 months (95% CI: 3.94–6.14), respectively. The difference was not significant (P = 0.33) with an absolute Q-TWiST gain of 0.82 months (95% CI: −0.82–2.46) in the experimental group compared with the control group (Supplementary Fig. 3).
QoL and Performance Status
We observed a trend favoring the experimental group regarding time until definitive deterioration for most dimensions of QLQ C-30, QLQ-BN20, and QLQ-C15PAL, with significant differences for 3 dimensions (Supplementary Tables 14‒17, Supplementary Fig. 4). The ECOG performance status decreased over time in both groups (Supplementary Table 18). No analysis of steroid use was performed because of the recommendation of primary steroid prophylaxis after liposomal cytarabine in the experimental group.
Discussion
DEPOSEIN is the first randomized trial to show a clinical benefit from intrathecal chemotherapy in LM from a defined type of solid cancer. We report a clinically meaningful gain in LM-related PFS when breast cancer patients with newly diagnosed LM received intrathecal liposomal cytarabine chemotherapy together with systemic treatment compared with systemic treatment alone (Fig. 2A). No differences in QoL were observed between groups, although patients in the experimental group had more intensive treatment, reflected by more CTCAE grade 3 or more infections. Quality of life may even have been maintained longer in the experimental group because of prolonged PFS.
Patient selection remains a challenge in LM trials, since the presentation of LM is highly variable. A classification into distinct subtypes as developed by the European Association for Neuro-Oncology/European Society for Medical Oncology task force7 was not available when DEPOSEIN was initiated. We based inclusion on positive CSF cytology in the majority of patients (Table 1), but a combination of typical clinical symptoms or signs and typical MRI findings in the absence of positive CSF cytology allowed inclusion, too.
LM-PFS was chosen as the primary endpoint, since intrathecal chemotherapy is a local treatment aimed at improving local control in the LM compartment. There was nevertheless improved overall PFS in the experimental arm in the adjusted ITT population (Table 3, Fig. 2B), suggesting that adequate coverage of the subarachnoid space sanctuary may affect global disease control. Accordingly, in the adjusted ITT population, risk of death was nearly significantly reduced in the experimental arm.
DEPOSEIN has some limitations. It was a small, open-label trial, without stratifications, leading to imbalances between groups. Control group patients had heavier pretreatment, but also more often HER-2-positive tumors, both of which were identified as prognostic factors (Table 1). There was major cross-over toward intrathecal therapy at LM progression. The type of systemic treatment was not standardized, but left at the discretion of the investigator as LM usually occurs in late stage disease when several lines of treatment have already been exhausted. There were no validated tools for the determination of response and these still do not exist.19 However, we used standardized scorecards for clinical and imaging evaluation during follow-up and had a priori defined criteria for determination of response. CSF cytology was not considered for the determination of LM-PFS for its low sensitivity and risk of misinterpretation, a concern that is still valid today.7 Finally, liposomal cytarabine is currently not available, indicating that the encouraging results from DEPOSEIN may need to be confirmed with other intrathecal agents with superior intrinsic antitumor activity. Standard cytarabine for intrathecal use is still available for intrathecal use, but would have to be administered twice weekly.
DEPOSEIN provides a major advance over previous clinical trials conducted in this therapeutic area, mostly because these earlier, nevertheless important trials were designed and conducted 10–20 years ago, with little statistical power, without challenging the concept of intrathecal chemotherapy as such, with one notable exception of a trial that was, however, not completed11 and most importantly including patients with LM from different primary tumors which would not be considered state of the art today. Furthermore, future clinical trials on intrathecal therapy should consider superior patient selection (eg, focus on patients with molecularly defined types of cancer), with positive CSF cytology and diffuse linear disease rather than bulky disease, which may not be reached by intrathecally administered agents. In conclusion, DEPOSEIN confirms a role for intrathecal pharmacotherapy in subsets of LM patients. New approaches using targeted agents such as trastuzumab for LM from breast cancer with HER-2 overexpression are currently explored (NCT01373710, NCT01325207).20
Funding
This investigator-initiated study was supported by Mundipharma. Oscar Lambret Center, Lille, France was the study sponsor. The funder had no role in the design of the study, data collection, monitoring, data analysis, interpretation, writing the report, and decision to submit the paper for publication.
Conflict of interest statement. ELR reports grants from Mundipharma, during the conduct of the study; grants from Amgen, personal fees from Abbvie, Daiichi Sankyo, Mundipharma, and Novartis, outside the submitted work. MW reports grants from Adastra, Abbvie, Dracen, OGD2, Piqur, Roche, grants and personal fees from MSD, Merck (EMD), Novocure, personal fees from Celgene, Orbus, BMS, Tocagen, outside the submitted work.
Authorship statement. All authors have significantly contributed to the manuscript. The author contributions are as follows: Experimental design: ELR Implementation: ELR, IR, TB, VL, JB, YMR, JB Data analysis and interpretation: ELR, JW, MCLD, MW, JB Manuscript writing: All authors. Final approval of manuscript: All authors.
Supplementary Material
Acknowledgments
We thank
• all patients and their relatives for their willingness to participate in this clinical trial.
• the following institutions (in alphabetical order) and investigators or study personnel participating in the trial (in alphabetical order): Dijon: M. Chaix, S. Ladoire and P. Lapierre; Lille: G. Aberi-Moska, S. Beldi, J. Courtial, S. Cousin, E. Decoupigny, P. Desmet, H. Jeazet, S. Jebert, G. Lauridant, L. Vanlemmens and M. Vanseymortier; Montpellier: N. Firmin and L. Meignant; Nice: D. Chauvière; Paris: M. Bourgoin and S. Taillibert.
•S. Taillibert for strategic discussions in the early phase of the project.
References
- 1. Posner JB, Chernik NL. Intracranial metastases from systemic cancer. Adv Neurol. 1978;19:579–592. [PubMed] [Google Scholar]
- 2. Yust-Katz S, Garciarena P, Liu D, et al. Breast cancer and leptomeningeal disease (LMD): hormone receptor status influences time to development of LMD and survival from LMD diagnosis. J Neurooncol. 2013;114(2):229–235. [DOI] [PubMed] [Google Scholar]
- 3. Le Rhun E, Taillibert S, Zairi F, et al. A retrospective case series of 103 consecutive patients with leptomeningeal metastasis and breast cancer. J Neurooncol. 2013;113(1):83–92. [DOI] [PubMed] [Google Scholar]
- 4. Abouharb S, Ensor J, Loghin ME, et al. Leptomeningeal disease and breast cancer: the importance of tumor subtype. Breast Cancer Res Treat. 2014;146(3):477–486. [DOI] [PubMed] [Google Scholar]
- 5. Morikawa A, Jordan L, Rozner R, et al. Characteristics and outcomes of patients with breast cancer with leptomeningeal metastasis. Clin Breast Cancer. 2017;17(1):23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Niwińska A, Pogoda K, Michalski W, Kunkiel M, Jagiełło-Gruszfeld A. Determinants of prolonged survival for breast cancer patient groups with leptomeningeal metastasis (LM). J Neurooncol. 2018;138(1):191–198. [DOI] [PubMed] [Google Scholar]
- 7. Le Rhun E, Weller M, Brandsma D, et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann Oncol. 2017;28(suppl_4):iv84-iv99. [DOI] [PubMed] [Google Scholar]
- 8. Grossman SA, Finkelstein DM, Ruckdeschel JC, Trump DL, Moynihan T, Ettinger DS. Randomized prospective comparison of intraventricular methotrexate and thiotepa in patients with previously untreated neoplastic meningitis. Eastern Cooperative Oncology Group. J Clin Oncol. 1993;11(3):561–569. [DOI] [PubMed] [Google Scholar]
- 9. Hitchins RN, Bell DR, Woods RL, Levi JA. A prospective randomized trial of single-agent versus combination chemotherapy in meningeal carcinomatosis. J Clin Oncol. 1987;5(10):1655–1662. [DOI] [PubMed] [Google Scholar]
- 10. Glantz MJ, Jaeckle KA, Chamberlain MC, et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res. 1999;5(11):3394–3402. [PubMed] [Google Scholar]
- 11. Boogerd W, van den Bent MJ, Koehler PJ, et al. The relevance of intraventricular chemotherapy for leptomeningeal metastasis in breast cancer: a randomised study. Eur J Cancer. 2004;40(18):2726–2733. [DOI] [PubMed] [Google Scholar]
- 12. Shapiro WR, Schmid M, Glantz M, Miller JJ. A randomized phase III/IV study to determine benefit and safety of cytarabine liposome injection for treatment of neoplastic meningitis. JCO. 2006;24(18_suppl): 1528–1528. [Google Scholar]
- 13. Chamberlain M, Soffietti R, Raizer J, et al. Leptomeningeal metastasis: a Response Assessment in Neuro-Oncology critical review of endpoints and response criteria of published randomized clinical trials. Neuro Oncol. 2014;16(9):1176–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Le Rhun E, Rudà R, Devos P, et al. Diagnosis and treatment patterns for patients with leptomeningeal metastasis from solid tumors across Europe. J Neurooncol. 2017;133(2):419–427. [DOI] [PubMed] [Google Scholar]
- 15. Gelber RD, Goldhirsch A, Cole BF. Evaluation of effectiveness: Q-TWiST. The International Breast Cancer Study Group. Cancer Treat Rev. 1993;19 Suppl A:73–84. [DOI] [PubMed] [Google Scholar]
- 16. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. [DOI] [PubMed] [Google Scholar]
- 17. Kalbfleisch JD, Prentice RL.. The Statistical Analysis of Failure Time Data. 2nd ed. Hoboken, NJ: Wiley-Blackwell; 2002. [Google Scholar]
- 18. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 19. Le Rhun E, Devos P, Boulanger T, et al. ; European Organisation for Research and Treatment of Cancer (EORTC) Brain Tumor Group (BTG) Central Nervous System (CNS) Metastases Committee and the EORTC BTG Imaging Committee The RANO Leptomeningeal Metastasis Group proposal to assess response to treatment: lack of feasibility and clinical utility and a revised proposal. Neuro Oncol. 2019;21(5):648–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bonneau C, Paintaud G, Trédan O, et al. Phase I feasibility study for intrathecal administration of trastuzumab in patients with HER2 positive breast carcinomatous meningitis. Eur J Cancer. 2018;95:75–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.