Abstract
Regardless of subtype, diffuse gliomas of adulthood are characterized by inexorable progression through treatment. Cancer recurrence in the context of therapy is by no means unique to gliomas. For many tumors residing outside the central nervous system (CNS), tissue-based analyses are routinely employed to document the molecular and cellular features of disease recurrence. Such interventions are inconsistently applied for gliomas, however, and lack rigorous standardization when they are. While many of the reasons underlying these discrepancies reflect pragmatic realities inherent to CNS disease, the suboptimal employment of histological and molecular assessment at recurrence nevertheless represents a missed opportunity to proactively guide patient management and increase knowledge. Herein, we address this quandary by pairing a succinct description of the histological, biological, and molecular characteristics of recurrent glioma with recommendations for how to better standardize and implement quality pathological assessment into patient management. We hope this review will prompt thoughtful revision of standard operating procedures to maximize the utility of glioma re-biopsy.
Keywords: glioma, adult, recurrence, pathology, pseudoprogression
The clinical, molecular, and histopathological heterogeneity of diffuse gliomas has been extensively characterized in recent years, revealing a broad spectrum of genomic alterations for therapeutic targeting, while also refining disease classification based on highly recurrent biomarkers.1 In particular, diffuse gliomas affecting adults are now robustly segregated into 3 subtypes based on mutations in isocitrate dehydrogenase genes (IDH1 and IDH2) and whole-arm chromosomal codeletion of 1p and 19q (1p/19q codeletion).2,3 These advances have transformed the practice of diagnostic neuropathology, which now incorporates integral biomarkers into the designation of specific disease entities, including glioblastoma (GBM), IDH wild-type (World Health Organization [WHO] grade IV), diffuse astrocytoma, IDH-mutant (WHO grade II), and oligodendroglioma, IDH-mutant and 1p/19q codeleted (WHO grade II).4 This schema delineates distinct glioma subclasses, each characterized by its own optimal management workflows, whose refinement in affected patient populations will continue moving forward.
Despite these significant developments in the diagnosis and management of primary adult glioma, much less is known about the unique biological features of glioma in the recurrent setting. The clinical relevance of this knowledge gap is considerable, given that adult diffuse gliomas almost invariably recur and progress over time. Recurrence is often a prerequisite for clinical trial enrollment, and almost all adult glioma patients eventually succumb to recurrent disease.5,6 One assumes that pathological assessment would play a similarly essential role in the management of recurrent glioma as it does for primary tumors. However, repeat biopsy of glioma at recurrence is by no means uniform practice, and the extent of histological examination and molecular testing performed on such specimens has not been rigorously standardized.7,8 Although practice patterns vary widely in these cases, neuropathologists in some cases may resort to descriptive diagnoses, such as “recurrent/residual high-grade glioma with treatment effect,” with minimal molecular workup. This reality has likely hindered progress on a deeper understanding of tumor progression, and with it the development of prognostic, predictive, and diagnostically relevant metrics to improve patient management. That being said, the full context of glioma recurrence and the constraints inherent to tumor re-resection often hamper subsequent tissue-based analyses. This review will address several topics relevant to recurrent glioma and the role of pathological assessment in its clinical management, including the underlying biology of treated glioma; the related issues of pseudoprogression, treatment effects, and adequate tumor sampling during re-resection; and the extent to which novel therapies may influence the morphological and clinical features of recurrence. We will also make recommendations to improve and better systematize the application of histological and molecular analysis to diffuse glioma in the recurrent setting.
Recurrent Glioma: Histopathological Considerations
The clinical course of diffuse gliomas in adults is characterized by almost invariable recurrence and progression, reflecting multiple distinct aspects of disease biology. Regardless of subtype, all diffuse gliomas by definition widely infiltrate surrounding brain tissue, precluding curative surgical resection. Ionizing radiation and alkylating chemotherapy are the mainstays of nonsurgical therapy for glioma, and both have repeatedly been shown to prolong median survival in affected patients.9,10 However, clinical responses to these standard-of-care interventions are not durable, and populations of resistant tumor cells almost invariably arise, often in a distribution within 2 cm of the initial lesion margin.11,12 Recurrence prompts additional therapeutic interventions that, while not uniformly applied across institutions, may involve re-irradiation, alternative chemotherapeutic approaches, and/or clinical trials for experimental treatments. Re-resection for further surgical debulking may also take place in this setting, although, as indicated above, such practice is not standardized.
Material obtained from glioma re-resection typically contains varying amounts of viable tumor, necrotic debris, and nonneoplastic brain elements with reactive changes; these latter two components represent the effects of cytotoxic therapy. Such treatment-related features can often obscure findings associated with histopathological progression in tumors initially classified as low or intermediate grade (WHO grades II–III), further complicating informative morphological analysis. Nevertheless, while delineating residual tumor from tumor regrowth and/or disease progression in this context can be somewhat subjective (see below), such distinctions may ultimately be less clinically relevant than the identification of bona fide viable tumor. To this point, some studies have shown that the presence of histologically confirmed viable tumor, in and of itself, is significantly associated with unfavorable prognosis.13,14 Such findings point to potential side benefits for recurrent glioma surgery, which has itself already been found to improve patient outcome in a large retrospective analysis.15 However, other studies have failed to demonstrate correlations between viable glioma at re-biopsy and clinical outcome,16 emphasizing the need for systematic validation grounded in consistently applied histopathological criteria. Spatial heterogeneity inherent to posttreatment GBM likely contributed to the disparate results detailed above, as none of the referenced studies systematically tracked the radiographic localization of specific biopsies, even at the level of enhancing versus nonenhancing disease.
Of course, effective histological analysis is predicated on adequate lesion sampling, by no means a certainty in cases of recurrent glioma. Even for primary glioma, no consensus exists regarding the procurement of adequate specimen for downstream analysis, with practice largely dependent on the philosophy and experience of individual neurosurgeons. As might be expected, higher numbers of tissue samples do correlate with improved diagnostic yield and accuracy.17 Radiographic guidance, especially using advanced brain tumor imaging (ABTI), can further facilitate this process,18,19 and intraoperative application of 5-aminolevulinic acid has been shown in a number of studies to improve resection precision while also decreasing the number of samples needed to obtain a definitive diagnosis.20–23 The extent to which these supporting technologies can be leveraged into operational standards for optimal glioma sampling in the recurrent setting remains to be seen. The notable molecular heterogeneity of recurrent glioma (see below) further emphasizes the importance of obtaining adequate resection material to inform patient management. Even in primary glioma, major genetic divergence has been shown to exist between tumor core and periphery, regions differentiated by hypoxia, and topographically distinct areas within the same tumor.24–29
Distinguishing true recurrence from pseudoprogression represents perhaps the most important quandary that effective pathological assessment can help to resolve in glioma patient management. Pseudoprogression is a clinical diagnosis whose biological substrate is robust cytotoxic response to radiochemotherapy or immunotherapy. It manifests as apparent radiographic disease progression (typically, though not always, within 12 weeks following completion of radiotherapy) that eventually resolves spontaneously without further treatment.30–32 As such, pseudoprogression mimics tumor recurrence and may initiate an unwarranted switch to a second-line chemotherapeutic or therapeutic nihilism. Some studies have associated molecular factors, such as IDH mutation and methylation of the O6-methylguanine-DNA methyltransferase (MGMT) promoter, with increased incidence of pseudoprogression in glioma.33–36 ABTI modalities also hold promise for identifying pseudoprogression cases, although robust methodology is still lacking at present.37,38 Nevertheless, histological confirmation of treatment effect without viable tumor, when available, remains a key finding supporting clinical diagnosis of pseudoprogression; this despite the inconsistent sampling issues characteristic of neurosurgical practice (see above). It is important to note, however, that no explicit standards currently exist for the histological diagnosis of pseudoprogression, regardless of whether it arises within 3 months of treatment initiation or as a later stage complication. Strategies to address this significant deficiency in patient management are described below.
Recurrent Glioma: Molecular Considerations
While the precise biological mechanisms driving therapeutic resistance and progression in glioma remain unclear, molecular heterogeneity almost certainly plays an important role. Even within established glioma subtypes, molecular heterogeneity exists, particularly for GBM.2,39,40 Large profiling studies have delineated a broad spectrum of recurrent genomic alterations characterizing GBM, along with 3 distinct molecular subclasses, each designated by transcriptional and DNA methylation signatures.39,41,42 Even within individual GBMs, a striking degree of molecular heterogeneity is often evident. Tumors composed of distinct yet coexisting cellular subclones, each harboring its own amplified receptor tyrosine kinase locus, have been documented by multiple groups.27,29 Moreover, regional sampling of GBM specimens has revealed significant genomic variability as a function of 3-dimensional space.43 Finally, single cell analysis has shown marked heterogeneity in transcriptional profiles across the neoplastic components of individual GBMs.44 One would expect highly divergent tumor subclones to behave differently in the context of treatment, setting the stage for recurrence.
The effects of frontline glioma therapy on global molecular profiles also speak to potential recurrence mechanisms. Temozolomide, the chemotherapeutic most frequently employed in the upfront treatment of glioma, has been repeatedly shown to induce a hypermutant state in a small subset of tumors.45–47 This phenotype is thought to result from mutational inactivation of one of several mismatch repair genes (MSH2, MSH6, MLH1, and others), after which affected tumors are prone to acquire numerous additional genomic alterations derived from alkylating agent–associated DNA damage.45,46 Therapy-induced hypermutation has now been identified in IDH-mutant lower-grade gliomas as well as GBMs and appears to affect genes encoding classical oncogenic pathway constituents—within the phosphatidylinositol-3 kinase network, for instance—in at least some cases.47 Hypermutation in IDH-mutant gliomas also appears to be associated with high-grade transformation to GBM, although a relatively small number of such cases have been analyzed thus far, largely restricted to MGMT methylated cases.47
Molecular evolution in the context of treatment is not limited to hypermutated gliomas alone. Multiple studies have now more generally addressed genomic alterations acquired during frontline therapy in small- to medium-sized sample cohorts. While no consistent patterns of pathogenic abnormalities have emerged, several compelling insights have been gleaned from these investigations. Perhaps not surprisingly, recurrence appears to be a complex phenomenon, featuring a multitude of clonal trajectories exhibiting varying degrees of similarities and discordances relative to the primary lesion in question.44,48 Not infrequently, genomic alterations acquired or lost at recurrence impact potentially targetable molecular networks.49–51 While recurrent clones may originate from any point during the molecular evolution of a glioma, it remains unclear which specific tumor cells act as fundamental drivers of the process.50,51 Recurrence-associated subclones may even arise years before diagnosis, consistent with the notion that relevant molecular features need not be treatment induced.49 Further supporting this conjecture, the extent of genomic alteration does not appear to directly correlate with treatment resistance, at least for GBM.49 In general, studies of glioma recurrence using patient-matched pre- and posttreatment sample sets have suffered from insufficient sample numbers, a deficiency that multi-institutional consortia are attempting to mitigate.52 Sufficiently powered investigations should further clarify the unique molecular mechanisms driving recurrence in glioma, ideally demonstrating specific molecular networks whose engagement directly promotes cellular resistance phenotypes.
Novel Therapeutic Agents: Bevacizumab as a Case Study
As alluded to above, surgical resection, ionizing radiation, and alkylating agent chemotherapy constitute the mainstays of glioma treatment, regardless of subtype. However, efforts to apply targeted therapies are ever present, and more widespread implementation of these agents in the near future will likely impact disease recurrence patterns from both histological and molecular perspectives. Bevacizumab, a monoclonal antibody targeting vascular endothelial growth factor A, serves as a particularly illustrative example of such considerations. Bevacizumab has been approved for the treatment of recurrent GBM since 2009 and has also been used in the upfront setting on a trial basis in combination with cytotoxic chemotherapeutics.53,54 As an angiogenesis inhibitor, its effects on peritumoral edema and radiographic markers of malignant glioma, most notably contrast enhancement by MRI, are well described. However, recent analyses have failed to demonstrate that bevacizumab prolongs survival in GBM patients, whether in the upfront or the recurrent setting.55–57 A distinctive recurrence pattern has been described for a subset of GBM patients treated with bevacizumab. Specifically, these cases are characterized by infiltrative tumor regrowth that is non–contrast enhancing by MRI and features so-called “normalization” of vasculature on histological analysis.58,59 Studies in xenograft and mouse models have begun to delineate the molecular foundations of bevacizumab recurrence in glioma.60 However, clinical translation of such findings lags behind, as there are still no established histopathological criteria for designating bevacizumab recurrence in routine specimens from the operating room. This quandary in part reflects the dearth of primary tissue from which such standards could be derived, given that gliomas treated with bevacizumab are rarely subjected to re-biopsy, due in part to legitimate concerns over increased bleeding risk and questions of clinical relevance.
Unique recurrence patterns and histopathological features may soon emerge in association with other targeted therapies. In particular, immune checkpoint inhibition might be expected to have specific effects on the cellular and molecular features of the tumor microenvironment that would be both observable and identifiable at biopsy. Accurately ascertaining such phenotypes, of course, will depend on the availability of adequate posttreatment tissue from immunotherapy trials.
Recommendations
The recommendations detailed below are made with the goal of further developing and improving standards of recurrent glioma management. It is hoped that their effective execution will address the significant knowledge gaps within this topic area.
Rigorously define “recurrent” versus “residual” glioma in diagnostic pathology
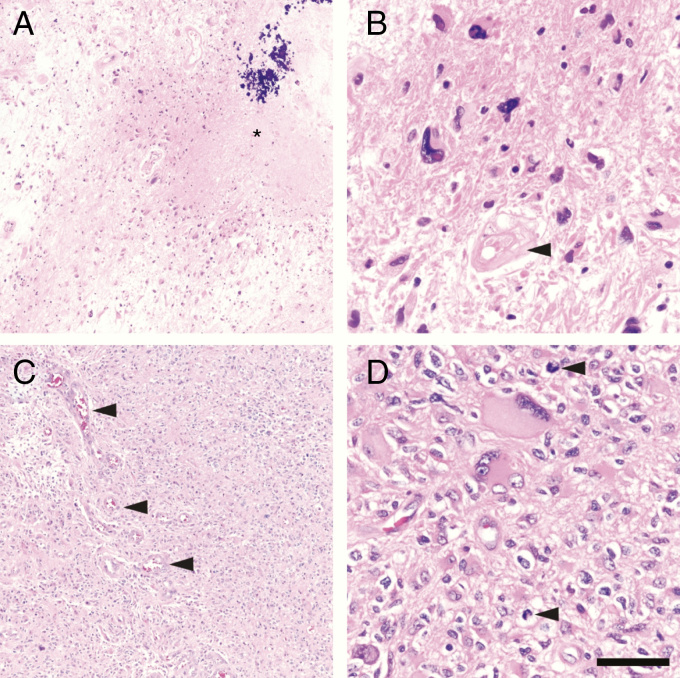
The reviewing pathologist should make every attempt to distinguish between recurrent and residual glioma, and avoid the mostly non-informative diagnosis of “recurrent/residual glioma.” For instance, the term “residual glioma” could be employed to specify glioma cells showing unequivocal treatment-related effects, such as severe nuclear atypia and ragged cell architecture, without signs of proliferation, such as elevated MIB-1/Ki67 immunolabeling (Fig. 1A, B). The term “recurrent glioma” could then be reserved for the designation of robust tumor regrowth, characterized by healthy-looking tumor cells exhibiting solid growth, dense cellularity, and proliferative activity (Fig. 1C, D). Findings of classic pseudopalisading necrosis and/or microvascular proliferation might be indicative of robust recurrence and even increased tumor grade (in the appropriate clinical setting), although care in such interpretations is required as necrosis and endothelial hyperplasia are also routinely seen as components of necrotizing treatment effect. Specific quantitative criteria for proliferative activity, based on MIB-1/Ki67 and/or phosphohistone H3 immunostaining, could also be applied, although their precise establishment would likely require multi-institutional consensus (see below). Finally, reviewing the relevant primary resection material could provide further insights into the expected cellular, architectural, and proliferative features of the lesion in question.
Fig. 1.
“Residual” versus “recurrent” GBM. (A) Patterns more suggestive of residual, posttherapy GBM include low-to-moderate cellularity tumor with large swaths of necrosis that may contain mineralization (*). (B) At higher power, the tumor cells still may be alive, but do not appear healthy. Such cells usually show marked nuclear atypia with very few mitoses, if any. Blood vessels often appear devitalized, hyalinized, and distorted (arrowhead). (C) Genuinely recurrent GBM, on the other hand, is more densely cellular, often with robust microvascular proliferation (arrowheads). (D) While recurrent GBM will still contain highly atypical cells, most cells will appear quite viable, with scattered mitoses (arrowheads). Scale bar = 250 µm in (A) and (C), and 50 µm in (B) and (D).
Better defining the distinction between the terms “residual” and “recurrent” would provide more precise signals to clinicians with regard to histopathological evidence of bona fide tumor regrowth. When both residual and recurrent tumor are identified, it may be preferable to only state the presence of recurrent tumor in the main diagnostic line, as this finding carries the greatest clinical relevance, and reserve acknowledgment of residual tumor for the “microscopic description” or “comment” sections of the report. Reporting the overall percentage of frankly recurrent tumor may also be of value, but as discussed above, the composition of any given resection may vary considerably based on the degree to which the lesion in question is effectively sampled.
Implementing these or similar morphological guidelines is, of course, easier said than done and has yet to be meaningfully standardized within the neuropathological community. Recent work has shown that considerable variability exists between neuropathologists in the evaluation of recurrent glioma specimens, even when specific histopathological features are assessed independently.61 Any effort to better standardize this process will require multi-institutional collaboration to establish robust, objective histopathological criteria that are consistently applied, ideally in a quantitative or semi-quantitative manner. Systematizing such analyses represents an excellent short-term goal upon which further refinements could be made to the recurrent/residual glioma distinction in the context of prospective studies. Finally, progress in this area will almost certainly require improved specimen sampling, perhaps in concert with radiographic guidance and frozen section analysis.
Study pseudoprogression in prospective cohorts with standardized pathological and radiographic correlates
The diagnosis of pseudoprogression currently lacks a radiographic and/or histological gold standard. Establishing these criteria will require prospective studies that combine adequate posttreatment tissue sampling with radiographic analysis (likely including ABTI) and clinical follow-up in GBM patients receiving standard upfront therapy. While no simple standards (eg, paraffin block number) exist for determining specimen adequacy in these contexts, analyzed tissue should be oriented to and representative of specific radiographic features, possibly with distinct areas of interest sampled independently. Such precision would enable robust correlations between key radiographic and histological findings.
Perform detailed molecular analysis on recurrent glioma biopsy material
As indicated above, diffuse gliomas of all subtypes undergo molecular evolution in the context of upfront therapy, frequently involving oncogenic pathways for which targeted compounds exist.47 As these treatment strategies continue to be incorporated into therapeutic trials, molecular profiling in the clinical setting should ideally be conducted not just on a patient’s primary specimen, but also at the time of recurrence, when most glioma patients are first considered for study enrollment. Purity and ploidy analysis of such genomic data could also inform the assessment of recurrent versus residual disease, through its designation of densely cellular neoplastic growth. Obtaining adequate recurrent glioma tissue by way of re-biopsy represents an obvious prerequisite for such testing, at least currently. Indeed, sampling multiple sites within a recurrent specimen might even be warranted in certain settings, in light of the significant cellular and molecular heterogeneity inherent to diffuse gliomas. Liquid biopsy via cerebrospinal fluid and/or plasma-based profiling may obviate the need for additional tissue at some point, although the sensitivity of these approaches may ultimately be lacking, particularly in the setting of extensive intratumoral heterogeneity. Regardless, studies required to ascertain the effectiveness of such alternatives will themselves require tumor sampling to determine extent of correlation between methodologies.
Substantially increasing the extent to which recurrent gliomas are subjected to extensive molecular profiling has obvious implications on cost of care, and arguments against the relevance of such testing, given the current lack of proven targeted treatment options for glioma, are not without merit. That clinical trials include molecular profiling performed on recurrent glioma specimens, therefore, takes on even greater importance, as the need for such testing can only be established in the context of formalized studies.
Systematically include posttreatment tissue assessment in clinical trials
Determining the impact that novel therapies have on the histological and molecular profiles of treated gliomas requires sufficient sampling at the time of progression, with specific considerations regarding specimen adequacy analogous, though likely not identical, to those described for pseudoprogression (see the second Recommendation above). As noted above for bevacizumab, even when distinct patterns of recurrence are reported in association with specific therapies, establishing robust criteria for their designation in the clinical setting requires a higher standard of evidence. Such challenges point to the necessity of systematic posttreatment tissue analysis as an integral component of trial design. Of course, balancing these concerns appropriately with legitimate clinical indications for re-resection represents a crucial ethical constraint that must be effectively addressed. It should be noted that multiple existing clinical trials routinely pursue collection of recurrent tumor specimens if and when they become available. Relevant strategies also include collection of postmortem tissues, especially if repeat neurosurgery is not clinically advisable. The logistics of patient transport and prolonged ischemic time are nontrivial obstacles to overcome in the postmortem setting, as are funding for such infrastructure and strained autopsy resources. Nevertheless, several institutions have developed highly successful programs through prioritized financial and personnel commitments, sometimes with the aid of philanthropic support. In this respect, pioneering work by pediatric groups aimed at advancing the molecular characterization of diffuse midline glioma (formerly diffuse intrinsic pontine glioma) has provided organizational templates for advancement.62,63 Autopsy programs have the potential to yield many tangible benefits, including (i) much larger tumor and nontumor samples than are possible to obtain through surgery, (ii) detailed mapping of tumor evolution in relation to 3D spread, (iii) studies of tumor and treatment effects on nonneoplastic brain tissue, (iv) procurement of cell lines from gliomas that have proven resistant to first- and second-line therapies, and (v) a sense of closure for many families and loved ones, especially if the postmortem director routinely discusses primary findings with the bereaved.
Conclusion
While much work remains to be done, it is imperative that the neuro-oncology community make better use of the opportunity recurrent gliomas provide to evaluate their biological and molecular evolution and incorporate findings into improved clinical management. This will likely require increasing the frequency, size, and sampling scope of glioma biopsies in the recurrent setting, shifting practice patterns. Indeed, establishing and executing operational criteria for the histological and molecular analysis of recurrent glioma will obviously require recurrent glioma tissue. Obtaining this material with clinical and radiographic correlation will likely improve its utility, and may even serve to minimize the extent of necessary tissue sampling. However, on a fundamental level, the importance of adequate specimen cannot be overstated.
Funding
No funding directly supported this study. The authors declare no conflicts of interest.
References
- 1. van den Bent MJ, Weller M, Wen PY, Kros JM, Aldape K, Chang S. A clinical perspective on the 2016 WHO brain tumor classification and routine molecular diagnostics. Neuro Oncol. 2017;19(5):614–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brat DJ, Verhaak RG, Aldape KD, et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372(26):2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huse JT. Establishing a robust molecular taxonomy for diffuse gliomas of adulthood. Surg Pathol Clin. 2016;9(3):379–390. [DOI] [PubMed] [Google Scholar]
- 4. Louis DN, Ohgaki H, Wiestler OD, et al. WHO Classification of Tumors of the Central Nervous System. Lyon, France: International Agency for Research on Cancer; 2016. [Google Scholar]
- 5. Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310(17):1842–1850. [DOI] [PubMed] [Google Scholar]
- 6. Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. [DOI] [PubMed] [Google Scholar]
- 7. Dardis C, Ashby L, Shapior W, Sanai N. Biopsy vs. extensive resection for first recurrence of glioblastoma: is a prospective clinical trail warranted? BMC Res Notes. 2015;8(414):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tully PA, Gogos AJ, Love C, Liew D, Drummond KJ, Morokoff AP. Reoperation for recurrent glioblastoma and its association with survival benefit. Neurosurgery. 2016;79(5):678–689. [DOI] [PubMed] [Google Scholar]
- 9. Garcia CR, Slone SA, Pittman T, St Clair WH, Lightner DD, Villano JL. Comprehensive evaluation of treatment and outcomes of low-grade diffuse gliomas. PLoS One. 2018;13(9):e0203639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cloughesy TF, Cavenee WK, Mischel PS. Glioblastoma: from molecular pathology to targeted treatment. Annu Rev Pathol. 2014;9:1–25. [DOI] [PubMed] [Google Scholar]
- 11. Burger PC, Dubois PJ, Schold SC Jr, et al. Computerized tomographic and pathologic studies of the untreated, quiescent, and recurrent glioblastoma multiforme. J Neurosurg. 1983;58(2):159–169. [DOI] [PubMed] [Google Scholar]
- 12. De Bonis P, Anile C, Pompucci A, et al. The influence of surgery on recurrence pattern of glioblastoma. Clin Neurol Neurosurg. 2013;115(1):37–43. [DOI] [PubMed] [Google Scholar]
- 13. Kim JH, Bae Kim Y, Han JH, et al. Pathologic diagnosis of recurrent glioblastoma: morphologic, immunohistochemical, and molecular analysis of 20 paired cases. Am J Surg Pathol. 2012;36(4):620–628. [DOI] [PubMed] [Google Scholar]
- 14. Woodworth GF, Garzon-Muvdi T, Ye X, Blakeley JO, Weingart JD, Burger PC. Histopathological correlates with survival in reoperated glioblastomas. J Neurooncol. 2013;113(3):485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ringel F, Pape H, Sabel M, et al. ; SN1 study group Clinical benefit from resection of recurrent glioblastomas: results of a multicenter study including 503 patients with recurrent glioblastomas undergoing surgical resection. Neuro Oncol. 2016;18(1):96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tihan T, Barletta J, Parney I, Lamborn K, Sneed PK, Chang S. Prognostic value of detecting recurrent glioblastoma multiforme in surgical specimens from patients after radiotherapy: should pathology evaluation alter treatment decisions? Hum Pathol. 2006;37(3):272–282. [DOI] [PubMed] [Google Scholar]
- 17. Jain D, Sharma MC, Sarkar C, Deb P, Gupta D, Mahapatra AK. Correlation of diagnostic yield of stereotactic brain biopsy with number of biopsy bits and site of the lesion. Brain Tumor Pathol. 2006;23(2):71–75. [DOI] [PubMed] [Google Scholar]
- 18. Iv M, Yoon BC, Heit JJ, Fischbein N, Wintermark M. Current clinical state of advanced magnetic resonance imaging for brain tumor diagnosis and follow up. Semin Roentgenol. 2018;53(1):45–61. [DOI] [PubMed] [Google Scholar]
- 19. Villanueva-Meyer JE, Mabray MC, Cha S. Current clinical brain tumor imaging. Neurosurgery. 2017;81(3):397–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg. 2000;93(6):1003–1013. [DOI] [PubMed] [Google Scholar]
- 21. Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ; ALA-Glioma Study Group Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392–401. [DOI] [PubMed] [Google Scholar]
- 22. Stummer W, Reulen HJ, Meinel T, et al. ; ALA-Glioma Study Group Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008;62(3):564–76; discussion 564. [DOI] [PubMed] [Google Scholar]
- 23. von Campe G, Moschopulos M, Hefti M. 5-Aminolevulinic acid-induced protoporphyrin IX fluorescence as immediate intraoperative indicator to improve the safety of malignant or high-grade brain tumor diagnosis in frameless stereotactic biopsies. Acta Neurochirurgica. 2012;154(4):585–588; discussion 588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Glas M, Rath BH, Simon M, et al. Residual tumor cells are unique cellular targets in glioblastoma. Ann Neurol. 2010;68(2):264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Inda MM, Bonavia R, Mukasa A, et al. Tumor heterogeneity is an active process maintained by a mutant EGFR-induced cytokine circuit in glioblastoma. Genes Dev. 2010;24(16):1731–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pistollato F, Abbadi S, Rampazzo E, et al. Intratumoral hypoxic gradient drives stem cells distribution and MGMT expression in glioblastoma. Stem Cells. 2010;28(5):851–862. [DOI] [PubMed] [Google Scholar]
- 27. Snuderl M, Fazlollahi L, Le LP, et al. Mosaic amplification of multiple receptor tyrosine kinase genes in glioblastoma. Cancer Cell. 2011;20(6):810–817. [DOI] [PubMed] [Google Scholar]
- 28. Sottoriva A, Spiteri I, Piccirillo SG, et al. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci U S A. 2013;110(10):4009–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Szerlip NJ, Pedraza A, Chakravarty D, et al. Intratumoral heterogeneity of receptor tyrosine kinases EGFR and PDGFRA amplification in glioblastoma defines subpopulations with distinct growth factor response. Proc Natl Acad Sci U S A. 2012;109(8):3041–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9(5):453–461. [DOI] [PubMed] [Google Scholar]
- 31. Clarke JL, Chang S. Pseudoprogression and pseudoresponse: challenges in brain tumor imaging. Curr Neurol Neurosci Rep. 2009;9(3):241–246. [DOI] [PubMed] [Google Scholar]
- 32. de Wit MC, de Bruin HG, Eijkenboom W, Sillevis Smitt PA, van den Bent MJ. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology. 2004;63(3):535–537. [DOI] [PubMed] [Google Scholar]
- 33. Brandes AA, Franceschi E, Tosoni A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26(13):2192–2197. [DOI] [PubMed] [Google Scholar]
- 34. Hygino da Cruz LC Jr., Rodriguez I, Domingues RC, Gasparetto EL, Sorensen AG. Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. AJNR Am J Neuroradiol. 2011;32(11):1978–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pouleau HB, Sadeghi N, Balériaux D, Mélot C, De Witte O, Lefranc F. High levels of cellular proliferation predict pseudoprogression in glioblastoma patients. Int J Oncol. 2012;40(4):923–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wick W, Chinot OL, Bendszus M, et al. Evaluation of pseudoprogression rates and tumor progression patterns in a phase III trial of bevacizumab plus radiotherapy/temozolomide for newly diagnosed glioblastoma. Neuro Oncol. 2016;18(10):1434–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brandes AA, Tosoni A, Spagnolli F, et al. Disease progression or pseudoprogression after concomitant radiochemotherapy treatment: pitfalls in neurooncology. Neuro Oncol. 2008;10(3):361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Verma N, Cowperthwaite MC, Burnett MG, Markey MK. Differentiating tumor recurrence from treatment necrosis: a review of neuro-oncologic imaging strategies. Neuro Oncol. 2013;15(5):515–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brennan CW, Verhaak RG, McKenna A, et al. ; TCGA Research Network The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372(26):2499–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sturm D, Witt H, Hovestadt V, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22(4):425–437. [DOI] [PubMed] [Google Scholar]
- 42. Wang Q, Hu B, Hu X, et al. Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell. 2017;32(1):42–56.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Suzuki H, Aoki K, Chiba K, et al. Mutational landscape and clonal architecture in grade II and III gliomas. Nat Genet. 2015;47(5):458–468. [DOI] [PubMed] [Google Scholar]
- 44. Patel AP, Tirosh I, Trombetta JJ, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344(6190):1396–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cahill DP, Levine KK, Betensky RA, et al. Loss of the mismatch repair protein MSH6 in human glioblastomas is associated with tumor progression during temozolomide treatment. Clin Cancer Res. 2007;13(7):2038–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hunter C, Smith R, Cahill DP, et al. A hypermutation phenotype and somatic MSH6 mutations in recurrent human malignant gliomas after alkylator chemotherapy. Cancer Res. 2006;66(8):3987–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Johnson BE, Mazor T, Hong C, et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343(6167):189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Verhaak RG, Hoadley KA, Purdom E, et al. ; Cancer Genome Atlas Research Network Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang J, Cazzato E, Ladewig E, et al. Clonal evolution of glioblastoma under therapy. Nat Genet. 2016;48(7):768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim H, Zheng S, Amini SS, et al. Whole-genome and multisector exome sequencing of primary and post-treatment glioblastoma reveals patterns of tumor evolution. Genome Res. 2015;25(3):316–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kim J, Lee IH, Cho HJ, et al. Spatiotemporal evolution of the primary glioblastoma genome. Cancer Cell. 2015;28(3):318–328. [DOI] [PubMed] [Google Scholar]
- 52. Consortium TG. Glioma through the looking GLASS: molecular evolution of diffuse gliomas and the Glioma Longitudinal Analysis Consortium. Neuro Oncol. 2018;20(7):873–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 54. Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5): 740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 56. Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wick W, Gorlia T, Bendszus M, et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377(20):1954–1963. [DOI] [PubMed] [Google Scholar]
- 58. de Groot JF, Fuller G, Kumar AJ, et al. Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro Oncol. 2010;12(3):233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Iwamoto FM, Abrey LE, Beal K, et al. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology. 2009;73(15):1200–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lu KV, Bergers G. Mechanisms of evasive resistance to anti-VEGF therapy in glioblastoma. CNS Oncol. 2013;2(1):49–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Holdhoff M, Ye X, Piotrowski AF, et al. The consistency of neuropathological diagnoses in patients undergoing surgery for suspected recurrence of glioblastoma. J Neurooncol. 2019;141(2): 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kambhampati M, Perez JP, Yadavilli S, et al. A standardized autopsy procurement allows for the comprehensive study of DIPG biology. Oncotarget. 2015;6(14):12740–12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tsoli M, Shen H, Mayoh C, et al. International experience in the development of patient-derived xenograft models of diffuse intrinsic pontine glioma. J Neurooncol. 2019;141(2):253–263. [DOI] [PubMed] [Google Scholar]