Rotavirus is a highly infectious pathogen that causes severe diarrhea. Replication of RV in the small intestine is restricted to homologous host species, and host range restriction is substantially determined by the interferon response. In this study, we demonstrate that during infection, RV bystander cells resist exogenous IFN-mediated STAT1 signaling and transcription. In a suckling mouse model, ectopically stimulating different intestinal interferon receptors during RV infection eliminates several innate and inflammatory antiviral responses. Different intestinal inflammatory cytokines were also suppressed by homologous RV, as was intestinal damage in response to endotoxin. The ability of RV to suppress IFN-mediated receptors likely impacts intestinal cell homeostasis, as the cleavage of multiple intestinal caspases during RV infection is mediated by the IFN-STAT1 signaling pathway. Together, our results provide a mechanism underlying both the remarkable interferon resistance of homologous RV and its ability to prevent substantial inflammatory damage to the small bowel.
KEYWORDS: caspase cleavage, endotoxin, gut inflammation, interferon receptor, interferons, negative feedback, rotavirus, STAT1
ABSTRACT
Rotaviruses (RV) cause acute severe diarrhea in the absence of substantial intestinal inflammation. They are also highly infectious in their homologous host species. The replication capacity of RV in the small bowel is substantially due to its ability to inhibit different types of interferons (IFNs). Here, we found that during RV infection in vitro, both virus-infected and uninfected bystander cells resist STAT1 phosphorylation and interferon regulatory factor 7 (IRF7) induction in response to exogenous interferon (IFN). Functionally, cellular transcription in response to stimulation with IFN, but not intracellular double-stranded RNA (dsRNA), was inhibited by RV. Further, IFNAR1 stimulation during RV infection significantly repressed a set of virus-induced transcripts. Regulation of IFN signaling in vivo was studied in suckling mice using the highly infectious murine EW RV strain. Kinetic studies indicated that sustained EW RV replication and IFN induction in the small intestine are accompanied by significant decreases in IFN-stimulated transcripts. Lipopolysaccharide (LPS)-mediated intestinal damage, driven by STAT1-induced inflammation, was also prevented in EW RV-infected mice. Remarkably, by ectopically stimulating either IFNAR1 or IFNGR1 in EW RV-infected mice, we could eliminate several intestinal antiviral and inflammatory transcriptional responses to RV. In contrast to infection with homologous RV, infection with a STAT1-sensitive heterologous RV strain induced IFN-stimulated transcripts, inflammatory cytokines, and intestinal expression of STAT1-pY701. Finally, RV strain-specific STAT1 regulation also likely determines the intestinal activation of multiple caspases. The simian RRV strain, but not murine EW RV, uniquely triggers the cleavage of both extrinsic and intrinsic caspases (caspases 8, 9, and 3) in a STAT1-mediated manner. Collectively, our findings reveal efficient reprograming of multiple IFN receptors toward a negative-feedback mode of signaling, accompanied by suppression of IFN-mediated antiviral, apoptotic, and inflammatory functions, during natural RV intestinal infection.
IMPORTANCE Rotavirus is a highly infectious pathogen that causes severe diarrhea. Replication of RV in the small intestine is restricted to homologous host species, and host range restriction is substantially determined by the interferon response. In this study, we demonstrate that during infection, RV bystander cells resist exogenous IFN-mediated STAT1 signaling and transcription. In a suckling mouse model, ectopically stimulating different intestinal interferon receptors during RV infection eliminates several innate and inflammatory antiviral responses. Different intestinal inflammatory cytokines were also suppressed by homologous RV, as was intestinal damage in response to endotoxin. The ability of RV to suppress IFN-mediated receptors likely impacts intestinal cell homeostasis, as the cleavage of multiple intestinal caspases during RV infection is mediated by the IFN-STAT1 signaling pathway. Together, our results provide a mechanism underlying both the remarkable interferon resistance of homologous RV and its ability to prevent substantial inflammatory damage to the small bowel.
INTRODUCTION
Rotaviruses (RVs) are nonenveloped segmented double-stranded RNA (dsRNA) viruses causing severe dehydrating diarrhea in many mammals and an estimated 200,000 human infant deaths annually. Acute RV infection in a homologous host (i.e., the host species in which a specific RV strain replicates to high titer and spreads from individual to individual) is an extremely efficient process; severe diarrhea can be initiated by exposure to a very small infectious inoculum (1). The host innate interferon (IFN) response is a multipronged line of defense against many viral pathogens, as its induction can activate hundreds of different antiviral genes and trigger inflammatory signals, thus eliminating infected cells, limiting the spread of virus infection to uninfected bystander cells, and shaping both the magnitude and quality of subsequent adaptive immune responses (2).
All three major IFN types (IFN-α/β, -γ, and -λ) are induced during homologous RV infection of the small bowel (3, 4), but their antiviral effects on viral replication are modest at best. Although relatively poorly understood, the inflammatory effects of IFNs are also likely impaired during RV infection, which is associated with only mild intestinal inflammation compared to that with other acute diarrheal pathogens (5–7). Previously, in a suckling mouse model of RV disease, we demonstrated that the IFNAR1, IFNGR1, and IFNLR1 receptors, as well as their downstream convergent transcription factor STAT1, play an important role in restricting the replication of heterologous RV (RV not typically isolated from the symptomatically infected host species) (1, 3, 4, 8, 9). Depletion of IFN receptors (IFNRs) in suckling mice also results in an acute biliary inflammatory disease (often lethal) following infection with a heterologous (rhesus RRV) but not a homologous (murine EW RV) strain (8). How homologous RV efficiently restricts the ability of different IFNs to execute their antiviral and inflammatory programs is not well understood, but this knowledge is important both for effective strategies to combat RV-associated mortalities and to better understand the various pathogenic potentials of naturally circulating RV strains.
(This article was submitted to an online preprint archive [10].)
RESULTS
RV restricts IFN-directed STAT1 activation and IRF7 expression.
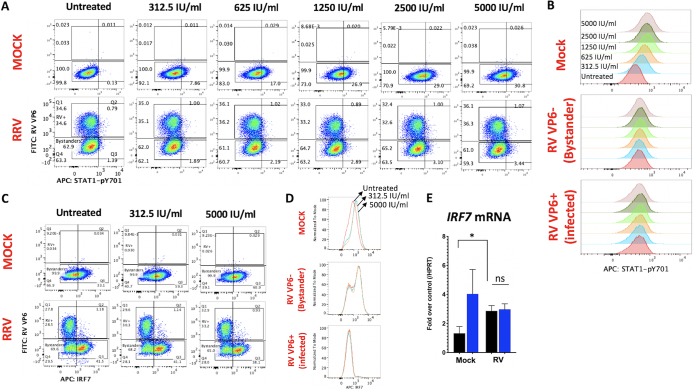
Prior studies using several RV strains identified two distinct RV strategies to regulate IFN induction, which are exemplified by either porcine (degrades β-TRCP) or simian (degrades interferon regulatory factors [IRFs]) RV strains (11). In contrast to this distinction, we reported that both porcine SB1-A and simian RRV strains mediated the lysosomal degradation of endogenous IFNAR1, IFNGR1, and IFNLR1 subunits in RV-infected (RV+) cells (12) and prevented IFN-directed STAT1 phosphorylation at the Y701 epitope (13), which is a critical convergent signal transduction event downstream of the different IFNRs. Surprisingly, SB1-A was also found to inhibit IFN-directed STAT1-Y701 phosphorylation in RV− bystander cells, which express normal levels of IFN receptors (13). Here, we extended our studies by examining whether IFN signaling is similarly inhibited in RV− bystander cells by the simian RV strain RRV. HT-29 cells were infected with RRV at a multiplicity of infection (MOI) resulting in approximately 35% RV-infected VP6+ cells (Fig. 1A, column 1) and at 12 h postinfection (hpi) the cells were stimulated for 2 h with increasing doses of purified universal type I IFN (IFN-A/D) to induce STAT1-Y701 phosphorylation (Fig. 1A, columns 2 to 6). Analysis of RV VP6 and STAT1-pY701 expression by fluorescence-activated cell sorting (FACS) demonstrated that mock-infected controls underwent a dose-dependent increase in STAT1-pY701 expression in response to ectopic IFN (Fig. 1A, row 1, and B). In contrast, both RRV VP6+ (RV+) and RRV VP6− (bystander) cell populations effectively resisted IFN-stimulated STAT1 phosphorylation, even at the highest concentration of IFN-A/D tested (Fig. 1A, row 2, and B). These findings demonstrate that the IFN signaling pathway is inhibited in uninfected bystander cells in vitro by the simian RV (RRV), similar to our earlier findings using a porcine RV (13). Thus, although uninfected RV bystander cells express normal levels of IFNRs (12) during infection, their ability to transduce IFN-directed signals is substantially impaired.
FIG 1.
Rotavirus inhibits IFN-stimulated cellular responses in vitro. (A and B) HT-29 cells were infected with RRV (or mock infected) at an MOI of 1. At 10 hpi, cells were stimulated with purified IFN-β at the indicated concentrations for 2 h and analyzed by flow cytometry. (C and D) cells were infected with RRV and analyzed by FACS as for panels A and B. (E) Cells were infected in triplicate as for panels C and D and at 12 hpi were stimulated with PBS (black bars) or 500 IU/ml of purified IFN-β (blue bars) for 6 h before analysis by qRT-PCR for IRF7 and hypoxanthine phosphoribosyltransferase (HPRT) transcript levels. *, P < 0.02; ns, not significant. Data shown are representative of two or more independent experiments.
To extend these observations, we examined the effect of RRV on levels of endogenous IRF7, an IFN-stimulated protein that is also targeted for degradation by the RRV-encoded NSP1 protein (14). Analysis of RV VP6 and IRF7 by FACS was performed in HT-29 cells as described above (Fig. 1C). In mock controls, IRF7 was constitutively expressed by ∼33% of the cells even in the absence of IFN stimulation. Following stimulation with either a low (312.5-IU/ml) or high (5,000-IU/ml) dose of IFN-A/D, a similar increase in IRF7 expression occurred (∼60% cells were IRF7+ after IFN stimulation at either dosage) (Fig. 1C, row 1, and D), indicating that relatively low IFN concentrations are sufficient to maximally induce IRF7 expression in HT-29 cells. Following RV infection, RRV VP6− cells exhibited elevated basal IRF7 expression in the absence of any exogenous IFN stimulation (i.e., ∼43% of all cells, almost entirely derived from the VP6− gate, were IRF7+), which could possibly be triggered by low levels of IFN induced earlier during RV infection (15, 16). However, IFN stimulation failed to induce any further increase in IRF7 expression in RRV VP6− cells (Fig. 1C, row 2, D). In contrast, RV+ HT-29 cells expressed substantially lower levels of IRF7 (only ∼4% of VP6+ cells were IRF7+). Notably, no detectable increase in IRF7 expression occurred in infected cells following IFN-A/D stimulation at either dose. Overall, in these cell monolayers, the percentage of HT-29 cells expressing IRF7 after stimulation with 5,000 IU/ml IFN was lower during RRV infection (∼39% of all cells) than during mock infection (∼61% of all cells). By quantitative reverse transcription-PCR (qRT-PCR) analysis, we confirmed that although RRV infection induced IRF7 transcription compared to that in mock controls, it prevented any further IFN-stimulated IRF7 transcription (Fig. 1E). These initial data clearly demonstrate that RV-infected cells are highly resistant to IFN-mediated induction of IRF7 or STAT1-pY701. In addition, the findings indicate that although RRV induces IRF7 in bystander cells during infection, it effectively prevents further IRF7 induction by saturating doses of IFN stimulus, which is in general agreement with the observed blockage of STAT1 phosphorylation in RV bystander cells (Fig. 1A). Since optimal IFN-directed STAT1-pY701 and IRF7 induction is important for IFNR positive feed-forward transcriptional amplification (17), it seemed likely that RV infection might perturb this process in both infected and bystander cells.
RV perturbs the IFN-stimulated feed-forward cellular transcriptional response.
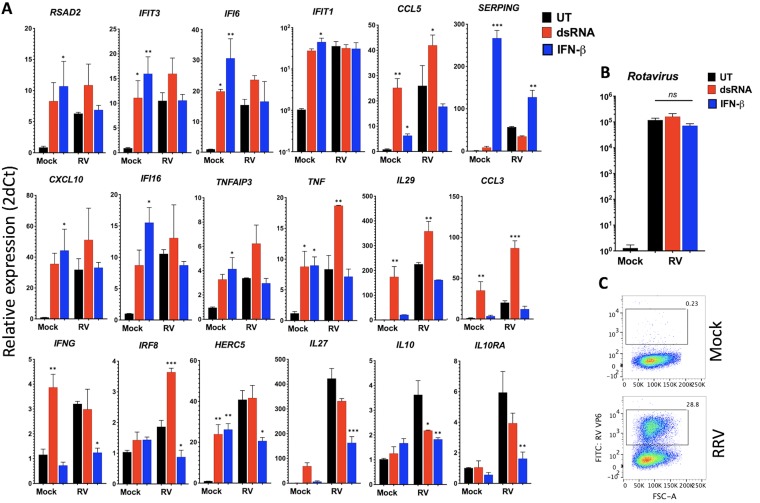
To gain further insight into the regulation of the IFN signaling response by RV, we next examined the ability of ectopic IFN to upregulate antiviral and inflammatory transcription following RRV infection in HT-29 cells. Since several interferon-stimulated genes (ISGs) and inflammatory transcripts upregulated during IFN signaling are also induced by intracellular dsRNA (18), primarily through NF-κB and IRF3 signaling pathways, we compared cellular transcriptional responses to either exogenously applied IFN-β or intracellular long dsRNA introduced by transfection. Following RV infection for 6 h, cells were stimulated with purified IFN-β or with liposomally complexed long dsRNA for an additional 6 h and analyzed by qRT-PCR. The results (Fig. 2A) demonstrate that mock-infected HT-29 cells underwent significant upregulation of several transcripts in response to IFN and/or dsRNA stimulation (IFIT3, IFI6, CCL5, TNF, and HERC5 were induced by both types of stimuli; SERPING, CXCL10, IFI16, and TNFAIP3 by IFN-A/D; and IL-29, CCL3, and IFNG by dsRNA only). In RV-infected samples, significant transcriptional upregulation of CCL5, TNF, IL-29, CCL3, and IRF8 occurred only in response to intracellular dsRNA. In addition, RSAD2, IFIT3, IFI6, CXCL10, IFI16, and TNFAIP3 transcripts were also weakly, but not significantly, elevated in response to dsRNA during RV infection. In contrast, we failed to detect any significant upregulation of the measured transcripts in response to IFN-β, with the sole exception of SERPING, whose induction was weaker in RV-infected samples than in mock-infected controls (Fig. 2A). The stimulus-specific RV effect on cellular transcription was unlikely due to RV replication sensitivity, which was unaltered by either IFN-β or dsRNA stimulation (Fig. 2B).
FIG 2.
Rotavirus infection inhibits IFN-stimulated feed-forward cellular transcription. (A and B) HT-29 cells grown in triplicate wells were infected with the RRV strain at an MOI of 1 (or mock infected) for 6 h and then stimulated with 2 μg of >200-bp-long dsRNA–liposome complexes or 500 IU/ml of purified IFN-β. At 12 hpi, cells were lysed for RNA purification and then analyzed by qRT-PCR for expression of the indicated host (A) and RV (B) transcripts. ns, not significant; *, P < 0.02; **, P < 0.002; ***, P < 0.0002 (significance is shown relative to results for untreated samples in the mock- and RV-infected groups). (C) Cells were infected as for panel A and B, and the percentage of cells expressing RV VP6 antigen before lysis was determined by FACS. The boxes indicate the gates used to estimate RV-infected cells in the monolayer. Data shown are representative of two experiments.
Interestingly, ectopic stimulation of IFNAR1 during RRV infection also significantly repressed the expression of a set of transcripts from their levels in the unstimulated RRV samples (IFNG, IRF8, HERC5, IL-27, IL-10, and IL10RA) (Fig. 2A, bottom row). Although RV infection completely prevented (or repressed) the IFN-directed upregulation of transcripts, by FACS analysis we found that only ∼30% of the cells in the monolayer were RRV VP6+ (Fig. 2C). Hence, these results suggested that RV inhibits IFN-directed cellular transcription both in RV-infected and in uninfected bystander cells.
RV also restricts intestinal IFN amplification responses in vivo.
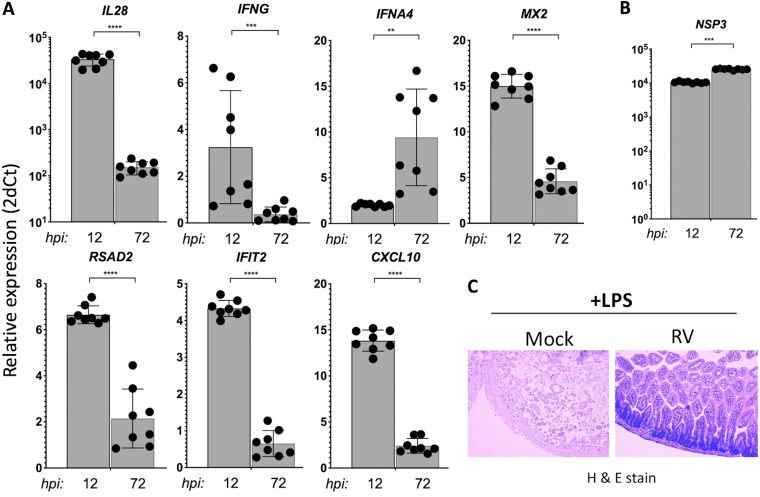
The transcriptional amplification of IFN genes (e.g., IL-28 and IFNG), antiviral genes (e.g., MX2), and inflammatory cytokine genes (e.g., CXCL10) occurs via STAT1 activation as part of the IFN feed-forward signaling circuit (19). We next examined the kinetics of selected IFN-stimulated transcripts in the small intestines of suckling mice infected with a highly infectious homologous murine EW RV strain (1) for either 12 h or 72 h (Fig. 3A and B). This analysis revealed that IFNA4 (a primarily induced IFN gene [17]) and RV NSP3 transcripts increase significantly between 12 and 72 hpi. In contrast, a significant decrease in RV-induced intestinal type II and III IFN gene (IL-28 and IFNG), RSAD2, IFIT2, and CXCL10 transcription occurred in EW RV-infected mice between 12 and 72 hpi (Fig. 3A). RV-induced transcription of MX2, a specific marker of the intestinal epithelial response to IFN stimulation (20), also significantly declined by 72 hpi. These results indicate that during murine EW RV infection in suckling mice, despite sustained viral replication and IFN induction, several intestinal IFN amplification responses are downregulated by 72 hpi. To further examine this finding, we directly tested whether intestinal damage caused by endotoxin, which mediates inflammation in a STAT1-dependent manner (21), is also downregulated by prior infection with EW RV. Suckling mice orally infected with EW RV (or mock infected) for 72 h were systemically administered endotoxin, and the resulting intestinal damage was assessed 6 h later. In mock-infected pups, parenteral administration of endotoxin led to severe intestinal necrosis and tissue destruction (Fig. 3C). In contrast, suckling mice that were infected with EW RV prior to endotoxin challenge exhibited minimal small intestinal damage (Fig. 3C). These results further indicate that RV infection in the small bowel suppresses the intestinal IFN amplification responses.
FIG 3.
Effects of homologous RV infection on intestinal IFN amplification responses. (A and B) Suckling mice were infected with murine EW RV or were mock infected (n = 3 to 8 mice per group) for the times indicated, and small intestines were collected to analyze the expression of the transcripts shown. Data shown are fold change in EW RV- versus mock-infected mice. (C) Suckling mice were mock or EW RV infected and 3 days later were parenterally administered purified endotoxin (15 μg/pup) for 6 h before harvesting small intestines for histological examination by hematoxylin-and-eosin staining. Data shown are representative of 2 pups per group.
RV reprograms intestinal IFN type I and II receptors toward negative feedback signaling.
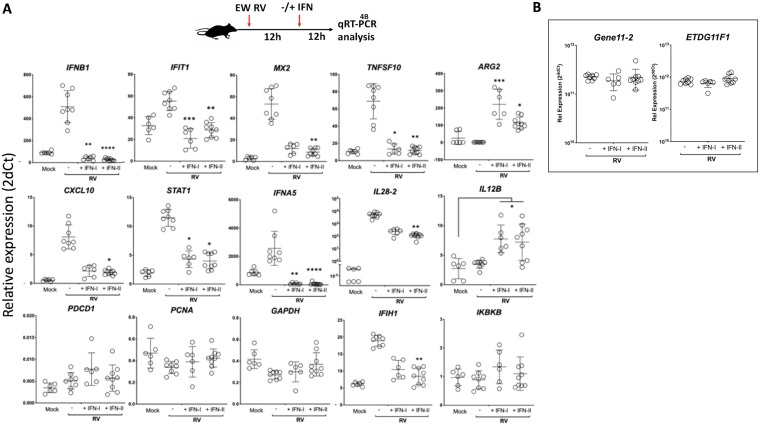
Since our data in vitro (Fig. 2A) suggested that ectopic IFN-stimulated transcription was suppressed during RV infection, we next examined intestinal transcriptional responses to ectopically administered type I and type II IFNs in suckling mice infected with murine RV. Following EW RV infection for 12 h, pups were parenterally administered either purified IFN-A/D or IFN-γ (or phosphate-buffered saline [PBS]), and small intestines were collected after an additional 12 h for transcriptional qRT-PCR analysis (Fig. 4A). In the absence of any ectopic IFN stimulation, RV infection transcriptionally induced several intestinal IFN genes, ISGs, and inflammatory genes, as expected from our prior findings (Fig. 3A). Remarkably, ectopic stimulation of IFNAR1 or IFNGR1 resulted in either a significant suppression (IL-28 and IFIH1) or the complete elimination (IFNB1, IFNA5, IFIT1, MX2, TNFSF10, CXCL10, and STAT1) of several of the innate transcriptional responses to RV. A similar IFN-dependent effect was not observed for PDCD1, PCNA, GAPDH, or IKBKB transcripts or for ARG2 and IL12B, whose expression during RV infection significantly increased following administration of either type of IFN (Fig. 4A). To ascertain whether the suppressive effect of IFN stimulation on transcription could be explained by a restriction of EW RV replication, we measured intestinal RV RNA by qRT-PCR using two different EW RV-specific assays (Fig. 4B). These results demonstrated that EW RV replication was not significantly altered following either IFN-A/D or IFN-γ stimulation under these conditions(Fig. 4B). The data from these experiments reveal that during RV infection, both type I and II IFNRs in the small intestine can efficiently suppress antiviral and inflammatory transcription when ectopically stimulated with their respective cognate ligands.
FIG 4.
Rotavirus reprograms intestinal IFNAR1 and IFNGR1 toward a negative-feedback mode of transcription. A schematic of the experimental approach used is shown at the top. Mice infected with EW RV (or mock infected) for 12 h were administered purified universal IFN-A/D (IFN-I), murine IFN-γ (IFN-II), or PBS by the intraperitoneal route. Twelve hours later, mice were sacrificed and small intestinal RNA analyzed by qRT-PCR for different host (A) or RV gene (B) transcripts. *, P < 0.02; **, P < 0.002; ***, P < 0.0002 (significance in each panel is relative to PBS-treated EW RV-infected mice, unless indicated otherwise). Data were measured from 3 to 5 pups per group.
Intestinal IFN signaling is regulated differently during infection with homologous versus heterologous RVs.
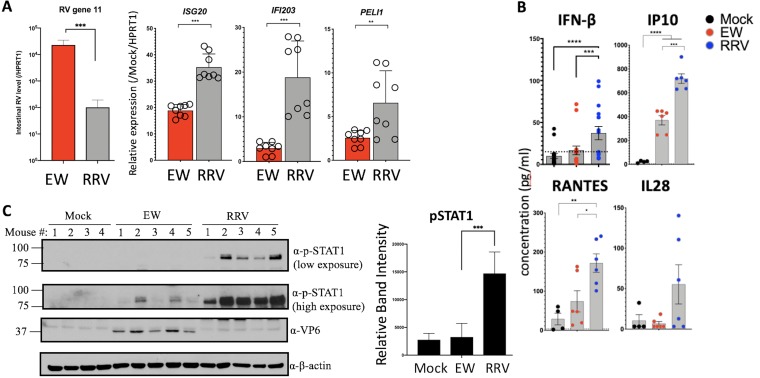
The finding that murine EW RV impairs IFN signaling in suckling mice is consistent with studies in mice (8) and human enteroids (22) that demonstrate that RV replication in the homologous host species is IFN resistant. In contrast, we previously observed that replication of the heterologous simian RRV strain in suckling mice is highly sensitive to different types of IFN and the presence of STAT1 (by 2 to 4 logs) (3, 4, 8, 9). We thus compared intestinal IFN-stimulated antiviral genes and cytokines in mice infected with the heterologous simian RRV or homologous murine EW or mock infected. As shown in Fig. 5A, at 12 hpi the intestinal replication of heterologous RRV was substantially and significantly lower than that of the homologous EW RV. However, RRV infection triggered relatively higher transcription levels of the ISG20, IFI203, and PELI1 genes than EW RV infection (Fig. 5A). In addition, heterologous RV infection also results in significantly elevated intestinal protein levels of IFN-β and the IFN-stimulated proinflammatory cytokines IP10 and RANTES compared to those after homologous RV infection (Fig. 5B). The expression of type III IFN (IL-28) was also elevated in RRV-infected pups compared to that in either the EW RV- or mock-infected groups.
FIG 5.
Differences between effects of homologous and heterologous RV strains on IFN-stimulated genes, inflammatory cytokine expression, and STAT1-Y701 activation in vivo. (A) Suckling mice (n = 3 to 5 pups per group) were infected with the homologous murine EW or heterologous simian RRV RV strain (or mock infected). At 12 hpi, mice were sacrificed for subsequent analysis of small intestinal transcripts by qRT-PCR. Data shown are fold relative to mock-infected mice. (B) Mice infected as for panel A were sacrificed, and small intestinal biopsy specimens were analyzed for the expression of the proteins shown by either ELISA (IFN-β) or Luminex-based cytokine assays. (C) Mice infected as for panel A were sacrificed and small intestines analyzed for immunoblotting analysis. Densitometric quantitation of pSTAT1 bands is shown in the bar diagram at the right. *, P < 0.02; **, P < 0.002; ***, P < 0.0002.
Analysis of intestines collected from EW- and RRV-infected mice at 1 day postinfection (dpi) by immunoblotting showed that compared to either mock or EW infection, RRV induces significant phosphorylation of STAT1 at Y701 (Fig. 5C), which is a convergent signaling response to different types of IFNs in the intestinal epithelium (12). Collectively, these findings are consistent with the conclusion that in contrast to homologous murine RV, heterologous simian RV strongly activates IFN-dependent STAT1 and proinflammatory responses in the small bowel.
STAT1 determines the cleavage of multiple intestinal caspases during RV infection in vivo.
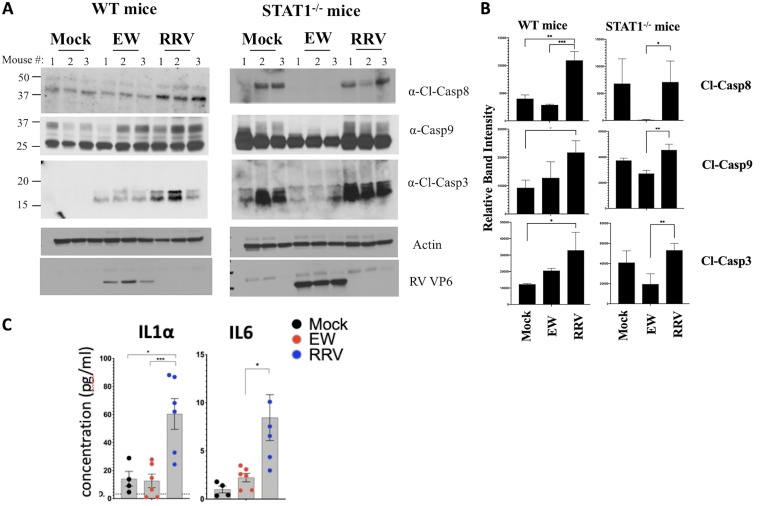
Different IFNs, as well as several IFN-stimulated cytokines (such as IP10 and RANTES), induce caspase-regulated cell death to potentiate inflammation (23, 24). Differences between EW and RRV strains in their ability to regulate pSTAT1 and cytokine expression prompted us to examine the status of intestinal caspases following infection of suckling mice with these two RV strains. Using immunoblotting analysis, the cleavage statuses of the initiator caspases 8 and 9 and the executioner caspase 3 were examined in the small intestines of wild-type (WT) and STAT1−/− mice infected with either EW or RRV RV (Fig. 6A). WT mice infected with RRV RV for 24 h undergo substantially higher levels of cleavage of caspases 8, 9, and 3 compared to those in their mock- or EW RV-infected counterparts (Fig. 6A and B), indicating that activation of both extrinsic and intrinsic intestinal apoptotic pathways is induced by heterologous RRV more efficiently than by the homologous murine EW strain.
FIG 6.
Homologous rotavirus inhibits the STAT1-mediated cleavage of multiple intestinal caspases. (A and B) Either wild-type (WT) or STAT1-deficient mice were infected with the EW or RRV RV strain (or mock infected). Twenty-four hours later, mice were sacrificed, and small intestinal lysates were analyzed by immunoblotting (A). Densitometric estimation of bands is shown in panel B. (C) Mice infected as for panels A and B were sacrificed and intestines analyzed for the expression of cytokines by Luminex-based assays. *, P < 0.02; **, P < 0.002; ***, P < 0.0002.
Of note, the enhanced cleavage of caspases during RRV infection was dependent on the intestinal IFN response. Specifically, we found that differences in caspase 8, 9, and 3 cleavage between mock- and RRV-infected WT pups were abrogated following infection in STAT1 knockout mice (Fig. 6A and B). The cleavage of caspases during EW RV infection was also STAT1 mediated. However, EW RV-infected STAT1−/− mice demonstrated cleaved caspases 8, 9, and 3 at levels much lower than those in mock-infected controls or RRV-infected mice (Fig. 6A and B), indicating that the basal cleavage efficiency of multiple caspases (i.e., occurring in the absence of infection) was impaired by homologous EW but not by heterologous RRV RV.
Impaired caspase 8 cleavage can spontaneously induce acute intestinal inflammation through necroptosis, resulting in the secretion of the proinflammatory factor IL-1α (25). Therefore, we examined IL-1α expression in the small intestine during EW and RRV infection (Fig. 6C). While the intestinal level of IL-1α was no different between mock- and EW RV-infected suckling mice at 1 dpi, it was significantly elevated after infection with RRV RV (26) (Fig. 6C). A similar difference was also observed for IL-6, another common marker of intestinal inflammation (27) (Fig. 6C). Thus, although murine EW RV inhibits caspase 8 cleavage compared to simian RRV, it avoids inducing the proinflammatory cytokine IL-1α or IL-6 by a yet-unknown mechanism. Collectively, these data identify an unrecognized role for STAT1 in mediating the cleavage of multiple intestinal caspases during RV infection. The findings also indicate that the ability of homologous RV to regulate IFN and STAT1 signaling in vivo (Fig. 4 and 5) likely dampens IFN-regulated cell death and inflammation.
DISCUSSION
Although they have been isolated from many different mammalian species, RVs are generally highly infectious only in their homologous host species. Robust RV replication occurs predominantly in the small intestinal mature villous tip epithelium and causes severe diarrhea but only mild inflammation (6, 7). We and others demonstrated that both RV intestinal replication and accompanying inflammation are likely regulated by host interferon (IFN) responses. Specifically, intestinal replication of a heterologous simian RRV strain can be substantially rescued in suckling mice lacking IFNAR1, IFNGR1, and/or IFNLR1 receptors or the convergent downstream transcription factor STAT1 (3). In the absence of STAT1, infection with the normally restricted RRV strain causes severe inflammation (mostly in the biliary tract), systemic spread, and mortality (8). In contrast, replication of a homologous murine EW RV strain is quite resistant to IFNs (8), and mice infected with EW RV are subsequently protected from lethal endotoxemia (12), which is STAT1 mediated (21). Paradoxically, EW RV robustly induces all three major IFN types (3, 4), raising the possibility that subsequent IFNR-directed antiviral and inflammatory signaling pathways are effectively blocked during infection.
How homologous RV successfully inhibits the ability of intestinal IFNs to mediate antiviral and inflammatory effects has not been well understood and was therefore examined in this study. We found that between 12 and 72 hpi, mice infected with murine EW RV exhibit sustained viral replication and increased transcription of the primary (i.e., induced prior to IFN amplification) murine type I IFN gene (IFNA4) (Fig. 3A and B) (17). However, transcriptional induction of the IFNL2/3, MX2, IFNG, RSAD2, IFIT2, and CXCL10 genes (Fig. 3A) in response to ongoing EW RV replication was considerably diminished by 72 hpi. Together, these data suggest that ongoing murine EW RV replication continues to trigger intestinal IFN induction relatively later during infection but restricts intestinal IFN amplification. This conclusion is further supported by our finding that suckling mice infected with EW RV for 72 h are protected from severe small intestinal injury that normally occurs following systemic LPS exposure (Fig. 3B). This injury was reported to be driven primarily by STAT1 signaling in the mouse model of endotoxemia (21). Using an alternate approach, we established that suckling mice infected with the STAT1-sensitive (3) simian RRV strain exhibit significantly higher levels of intestinal ISG transcriptional induction (Fig. 5A), IFN-β protein expression (Fig. 5B), and STAT1-pY701 expression (Fig. 5C) than those infected with the homologous murine EW RV strain. In addition, expression of the IFN-stimulated proinflammatory cytokines IP10 (28, 29) and RANTES (29–31) is also higher in the intestines of RRV-infected mice (Fig. 5B). Despite these higher levels of IFN responses to RRV at 12 to 24 hpi, previously we demonstrated that RRV-mediated transcriptional responses return to baseline levels by 48 hpi (3). In contrast, in murine EW RV-infected mice, significant transcriptional upregulation is sustained at 48 hpi, likely continually triggered by homologous viral replication occurring in the small bowel of these animals. A plausible conclusion is that heterologous RRV triggers a relatively efficient but short-lived IFN and inflammatory response which is sufficient to curtail viral replication but which is insufficient to restrict homologous RV replication despite a more prolonged replication pattern. Collectively, these data reinforce the notion that infection with homologous RV in vivo results in an inhibition of both IFN-mediated antiviral and inflammatory functions in the small intestine.
Caspase cleavage is a key parameter for determining both the magnitude and nature of inflammatory responses driven by IFNs and other cytokines (32, 33). Exposure to different IFNs can either induce caspase 8-mediated apoptosis (34) or, alternatively, trigger inflammatory cell death (necroptosis) under circumstances when caspase 8 cleavage is impaired (35). The data presented (Fig. 6) demonstrate that murine EW RV-infected mice express significantly reduced cleavage of intestinal caspases 8, 9, and 3 compared to RRV RV-infected mice, with cleavage levels being similar to those in the mock-infected control pups (Fig. 6A and B). In contrast, infection with RRV triggered the cleavage of all three caspases in the small intestine, indicating that both extrinsic and intrinsic apoptosis pathways are induced by the heterologous RRV despite its restricted replication capacity. Interestingly, activation of different caspases by RRV was contingent on the presence of STAT1, as differences in cleaved caspase 8, 9, and 3 expression between RRV- and mock-infected animals were eliminated in a STAT1-deficient background (Fig. 6A and B). The genetic ablation of STAT1 during EW RV infection resulted in cleaved caspase levels lower than those in uninfected mock controls. This was particularly evident for cleaved caspase 8 and 3 expression in the EW RV-infected STAT1−/− mice, which were at or below the limit of detection (Fig. 6A). A likely explanation for these findings is that during the course of infection, EW RV regulation of IFN feed-forward signaling (Fig. 4) and STAT1 phosphorylation (Fig. 5C) inhibits the efficiency of intestinal caspase 8 and 3 cleavage. The inhibition of caspase 8 cleavage has been reported to induce spontaneous intestinal inflammation, primarily via necroptosis (25). However, expression of IL-1α, a member of the IL-1 family that is released from necroptotic cells (36), and of the proinflammatory cytokine IL-6 was not detected in EW RV-infected (or mock-infected control) pup intestines but was indeed significantly induced by RRV RV (Fig. 6C). Future studies will be required to understand how homologous RV inhibits the cleavage of intestinal caspases yet avoids causing intestinal inflammation. These findings nevertheless reveal a STAT1-dependent intestinal caspase activation pathway that is likely regulated by homologous RV to control inflammation.
Findings in this study and earlier reports (3, 4, 8, 9, 12) indicate that mechanisms by which RV regulates STAT1 signaling are likely critical determinants of rotaviral pathogenicity in vivo. We found earlier that several RVs (porcine, bovine, and simian RV strains) mediate the lysosomal degradation of multiple IFN receptor subunits (i.e., IFNAR1, IFNGR1, and IFNLR1) in RV VP6+ (i.e., infected) cells, whereas RV VP6− (i.e., bystander) cells expressed normal levels of the IFN receptors (12). Remarkably, a porcine RV strain (SB1-A) was shown to efficiently inhibit IFN-directed STAT1-Y701 phosphorylation in uninfected (i.e., VP6−) bystander cells (13), indicating the existence of an alternative STAT1-inhibitory mechanism that operates beyond the virus-infected cell. In this study, we found that the simian RRV strain can also block IFN-β-mediated STAT1-pY701 induction in both VP6-positive and -negative HT-29 cell populations (Fig. 1A and B). In addition, RRV infection prevented IFN-β-directed induction of the IRF7 protein in both infected and bystander cells (Fig. 1C). Together with our previous observation that both simian and porcine RV strains can mediate IFNR degradation (12), these findings indicate that RV strategies to inhibit STAT1 signaling in infected and bystander cells are conserved across strains that differ in the manner in which they regulate the IFN induction phase (11).
Findings of this study also revealed a potential mechanism by which RV confers resistance to ectopic IFN stimulation to uninfected bystander cells (13). Specifically, under infection conditions in vitro where only ∼30% of HT-29 cells were RRV VP6+, ectopic stimulation with IFN-I failed to evoke significant cellular transcriptional responses (Fig. 2). This inhibitory effect of RRV specifically targeted IFN signaling, as several of these measured transcripts could be induced during RRV infection by intracellular dsRNA stimulation (Fig. 2A). In addition, ectopic IFNAR1 stimulation during RRV infection also resulted in a significant decline of certain innate immune transcripts induced in response to RV (Fig. 2A). These collective findings not only demonstrate that RV infection can block IFNR-directed feed-forward transcription but also suggest that RV represses transcription following ectopic IFN stimulation, which is characteristic of an IFNR-directed negative-feedback transcription mode (37).
This interesting possibility is supported by our studies in the suckling mouse model using purified IFN types I and II, which we earlier showed induces STAT1-Y701 in the small intestine with rapid kinetics (i.e., 30 min) when administered intraperitoneally (12). Remarkably, ectopically stimulating either IFN type receptor relatively early during EW RV infection (i.e., at 12 hpi) substantially repressed several measured intestinal antiviral transcriptional responses (Fig. 4A). In particular, intestinal induction of both type I (IFNB1 and IFNA5) and type III (IL-28) IFN transcripts by EW RV was significantly dampened when IFNAR1 or IFNGR1 was ectopically stimulated. In addition, RV-induced MX2 transcription, which is reported to be primarily IFNLR1 driven in the intestinal epithelial compartment (20), was also eliminated following stimulation with either type I or II IFN. Ectopic stimulation of IFNGR1 and IFNAR1 has been reported to elicit negative-feedback signaling leading to IFN resistance in the context of cancer and chronic viral infections (2, 37). Interestingly, norovirus (NoV) causes species-specific infections resulting in severe diarrhea in humans but not in mice. In contrast to our findings with RV, murine NoV promotes gut inflammation in synergy with commensal and pathogenic bacteria and under genetic contexts (38). In addition, homologous murine NoV (MNoV) exacerbates lethality and inflammation caused by systemic bacterial infections in a type I IFN-mediated manner (38). Thus, the ability of homologous RV to inhibit intestinal inflammation mediated by IFNs may be a unique property among diarrheagenic enteric viruses, although we do not currently have good diarrhea models in mice for enteric adenoviruses or astroviruses to test this comparison more fully. The ability of homologous RV to elicit IFNAR1- and IFNGR1-stimulated feedback signaling also suggests a potential mechanism underlying the ability of EW RV-infected mice to resist lethal endotoxemia (12) and the accompanying intestinal damage (Fig. 3C). It will be interesting to identify RV-mediated effectors involved in the broad suppression of multiple intestinal IFNR-dependent antiviral and inflammatory functions. As both type I and III IFNs were found to exert a profound antiviral activity against heterologous RV (3), one possibility is that the endogenous induction of these IFNs during homologous EW RV infection (3, 4, 16) serves to dampen intestinal antiviral, apoptotic, and inflammatory transcription and promote efficient viral replication.
MATERIALS AND METHODS
Viruses and reagents.
The tissue culture-adapted simian RRV and porcine SB1-A RV strains were propagated in African green monkey kidney cells (MA104) as previously described (15). Virus titers were determined by plaque assays in MA104 cells. HT-29 cells were plated in 6-well or 24-well cluster plates, and completely confluent monolayers were infected 2 to 3 days later as described previously (13).The non-tissue-culture-adapted murine EW RV strain was prepared as an intestinal homogenate from RV-infected Sv129 suckling mice. The 50% diarrheal dose (DD50) was determined as described previously (9). Human intestinal epithelial HT29 and embryonic kidney HEK293 cells were purchased from the American Type Culture Collection (ATCC) and maintained in Advanced Dulbecco modified Eagle medium (DMEM)-Ham’s F12 (Cellgro) or DMEM (Cellgro) containing 10% fetal calf serum (Invitrogen) supplemented with nonessential amino acids, glutamine, penicillin, and streptomycin. Purified human IFN-β (PBL) was used for stimulation of cells. Purified carrier-free universal type I IFN-A/D (PBL), murine IFN-γ (PBL), and LPS (Sigma) were used in animal studies. High-molecular-weight LyoVec poly(I·C) (InvivoGen) was used at 2.5 μg/ml.
Mouse infection and tissue harvest.
All mice were housed in the VA Palo Alto Health Care System (VAPAHCS) Veterinary Medicine Unit, and all experimentation followed VAPAHCS Institutional Animal Care and Use Committee-approved protocols. Three- to 5-day-old Sv129 pups were infected via oral gavage with 104 DD50 of murine EW RV or 4 × 106 PFU of the simian RRV or mock infected as described previously (9). At the indicated time points, the small intestines were removed. Sections were collected in TRIzol for RNA purification or in 2× Laemmli buffer for protein analysis by immunoblotting, as described previously (4), and stored at –80°C or fixed in 4% paraformaldehyde for processing and subsequent analyses as described previously (12).
Mouse stimulation and Luminex assays.
To ectopically stimulate IFNAR1 and IFNGR1, suckling mice infected with 104 DD50 of murine EW RV (or mock infected) for 12 h were intraperitoneally administered purified murine IFN-γ (1 μg IFN-γ per pup) or universal type I IFN-A/D (1 μg per pup) and sacrificed 12 h later for analysis of transcripts by qRT-PCR as described previously (4). For endotoxin stimulation, 3- to 5-day-old Sv129 mice were orally inoculated by gavage with 104 DD50 of murine RV-EW. Mice were intraperitoneally injected with PBS or purified LPS (Sigma; 10 mg/kg body weight) at 3 dpi and sacrificed 6 h later for analysis of paraformaldehyde-fixed and paraffin embedded small bowel tissue sections by hematoxylin-and-eosin staining. For measurement of intestinal cytokines, mouse small intestines were harvested and weighed, and pooled tissues (from 3 pups) were homogenized in ice-cold PBS containing a cocktail of protease and phosphatase inhibitors (Sigma) using a handheld micropestle (Thermo Fisher). Lysates were clarified by two sequential rounds of centrifugation at 10,000 × g for 10 min and analyzed for cytokine levels at the Human Immune Monitoring Center at Stanford University. Mouse 38-plex kits were purchased from eBioscience/Affymetrix and used according to the manufacturer’s recommendations. Plates were read using a Luminex 200 instrument with a lower bound of 50 beads per sample per cytokine. Custom assay control beads by Radix BioSolutions were added to all wells. Murine IFN-β was measured using a commercial enzyme-linked immunosorbent assay (ELISA) kit as per the manufacturer’s instructions (PBL).
Western blot analysis.
Preparation of cell lysates and immunoblotting in vitro were performed as described previously (15). For mouse samples, intestinal tissue pieces were lysed in radioimmunoprecipitation assay buffer (RIPA) supplemented with protease and phosphatase inhibitors (Roche). Equal volumes of 2× Laemmli buffer (Sigma-Aldrich) were added to each sample. Viscosity was reduced by passing repeatedly through a 22-gauge syringe, and samples were boiled for 5 min prior to analysis by SDS-PAGE and immunoblotting. Blots were probed with primary antibodies directed toward the following antigens: STAT1-pY701, cleaved caspase 8, caspase 9, and cleaved caspase 3 (Cell Signaling Technologies); RV VP6 (Santa Cruz Biotechnologies); and β-actin (Sigma). The blots were subsequently incubated with either anti-rabbit or anti-mouse secondary antibodies conjugated to horseradish peroxidase (HRP) (Amersham), incubated with ECL detection reagent, and exposed to film (GE Healthcare). All densitometry quantifications of protein blots were performed using ImageJ software analysis and normalized to actin protein bands.
Transcriptional analysis.
For in vivo analysis of transcripts, mice were sacrificed and sections of the small intestine were collected on ice in TRIzol (Life Technologies). For in vitro studies, cells were first washed in PBS and then lysed in TRIzol. Total RNA was extracted following the manufacturer’s instructions and subjected to DNase digestion before use in qRT-PCR. Synthesis of cDNA and subsequent microfluidics PCR on the Fluidigm platform was done as described earlier (4) using commercially available TaqMan assays (Thermo Fisher) and RV NSP5-specific assays described earlier (3, 4, 9). Serial 10-fold dilutions of mouse or human reference RNA (Agilent) and blank controls were run in duplicate for each PCR run.
Flow cytometry analysis.
Staining and flow cytometry analysis of HT-29 cells were performed as described previously (13). Cells were plated in 24-well cluster plates and infected with RV. Infected cells were harvested at 6 hpi or 16 hpi and transfected cells at 48 h after transfection for flow cytometry analysis. Cells were washed in PBS and fixed at room temperature for 10 min using 1.6% (vol/vol) methanol-free paraformaldehyde (Electron Microscopy Services). Cells were washed in FACS staining buffer (PBS containing 0.5% [wt/vol] bovine serum albumin) and permeabilized in cold methanol at 4°C for 10 min. Cells were washed in FACS staining buffer, stained using antibodies directed to STAT1-pY701 or IRF7 (from BD Biosciences) or to the RV VP6 antigen (13) at 4°C for 30 min, and washed prior to analysis by flow cytometry on an LSRII instrument (BD Biosciences). The flow data were analyzed using FlowJo software.
Statistical analysis.
Analysis of variance (ANOVA) was carried out by the Kruskal-Wallis method using the Dunn correction for multiple comparisons. Protein bands from SDS-PAGE blots were analyzed using the Holm-Sidak t test. P values of <0.05 were considered significant, as indicated in the figure legends. All error bars represent the standard error of the mean.
ACKNOWLEDGMENTS
This work was supported by grants 1RO1 AI021362 (H.B.G.) and 1RO1 AI1125249 (H.B.G.) from the National Institutes of Health and grant 1IO 1BX000158 from the Department of Veterans Affairs (H.B.G.). We acknowledge the Department of Biotechnology (DBT), Government of India, for Associateship support to N.D.N. (BT/20/NE/2011dtd 01/08/2017).
REFERENCES
- 1.Broome RL, Vo PT, Ward RL, Clark HF, Greenberg HB. 1993. Murine rotavirus genes encoding outer capsid proteins VP4, and VP7 are not major determinants of host range restriction and virulence. J Virol 67:2448–2455. doi: 10.1128/JVI.67.5.2448-2455.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ivashkiv LB, Donlin LT. 2014. Regulation of type I interferon responses. Nat Rev Immunol 14:36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin JD, Feng N, Sen A, Balan M, Tseng HC, McElrath C, Smirnov SV, Peng J, Yasukawa LL, Durbin RK, Durbin JE, Greenberg HB, Kotenko SV. 2016. Distinct roles of type I and type III interferons in intestinal immunity to homologous and heterologous rotavirus infections. PLoS Pathog 12:e1005600. doi: 10.1371/journal.ppat.1005600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sen A, Rothenberg ME, Mukherjee G, Feng N, Kalisky T, Nair N, Johnstone IM, Clarke MF, Greenberg HB. 2012. Innate immune response to homologous rotavirus infection in the small intestinal villous epithelium at single-cell resolution. Proc Natl Acad Sci U S A 109:20667–20672. doi: 10.1073/pnas.1212188109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenberg HB, Estes MK. 2009. Rotaviruses: from pathogenesis to vaccination. Gastroenterology 136:1939–1951. doi: 10.1053/j.gastro.2009.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lundgren O, Svensson L. 2001. Pathogenesis of rotavirus diarrhea. Microbes Infect 3:1145–1156. doi: 10.1016/s1286-4579(01)01475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris AP, Estes MK. 2001. Microbes and microbial toxins: paradigms for microbial-mucosal interactions. VIII. Pathological consequences of rotavirus infection and its enterotoxin. Am J Physiol Gastrointest Liver Physiol 281:G303–G310. doi: 10.1152/ajpgi.2001.281.2.G303. [DOI] [PubMed] [Google Scholar]
- 8.Feng N, Kim B, Fenaux M, Nguyen H, Vo P, Omary MB, Greenberg HB. 2008. Role of interferon in homologous and heterologous rotavirus infection in the intestines and extraintestinal organs of suckling mice. J Virol 82:7578–7590. doi: 10.1128/JVI.00391-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng N, Yasukawa LL, Sen A, Greenberg HB. 2013. Permissive replication of homologous murine rotavirus in the mouse intestine is primarily regulated by VP4 and NSP1. J Virol 87:8307–8316. doi: 10.1128/JVI.00619-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sen A, Namsa ND, Feng N, Greenberg HB. 2019. Rotavirus re-programs multiple IFN receptors and restricts their intestinal antiviral and inflammatory functions. bioRxiv https://www.biorxiv.org/content/10.1101/702837v2. [DOI] [PMC free article] [PubMed]
- 11.Arnold MM, Patton JT. 2011. Diversity of interferon antagonist activities mediated by NSP1 proteins of different rotavirus strains. J Virol 85:1970–1979. doi: 10.1128/JVI.01801-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sen A, Sharma A, Greenberg HB, Sen A, Sharma A, Greenberg HB. 2017. Rotavirus degrades multiple type interferon receptors to inhibit IFN signaling and protects against mortality from endotoxin in suckling mice. J Virol 92:e01394-17. doi: 10.1128/JVI.01394-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sen A, Rott L, Phan N, Mukherjee G, Greenberg HB. 2014. Rotavirus NSP1 protein inhibits interferon-mediated STAT1 activation. J Virol 88:41–53. doi: 10.1128/JVI.01501-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnold MM, Barro M, Patton JT. 2013. Rotavirus NSP1 mediates degradation of interferon regulatory factors through targeting of the dimerization domain. J Virol 87:9813–9821. doi: 10.1128/JVI.01146-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sen A, Feng N, Ettayebi K, Hardy ME, Greenberg HB. 2009. IRF3 inhibition by rotavirus NSP1 is host cell and virus strain dependent but independent of NSP1 proteasomal degradation. J Virol 83:10322–10335. doi: 10.1128/JVI.01186-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sen A, Pruijssers AJ, Dermody TS, Garcia-Sastre A, Greenberg HB. 2011. The early interferon response to rotavirus is regulated by PKR and depends on MAVS/IPS-1, RIG-I, MDA-5, and IRF3. J Virol 85:3717–3732. doi: 10.1128/JVI.02634-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marie I, Durbin JE, Levy DE. 1998. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J 17:6660–6669. doi: 10.1093/emboj/17.22.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalet A, Gatti E, Pierre P. 2015. Integration of PKR-dependent translation inhibition with innate immunity is required for a coordinated anti-viral response. FEBS Lett 589:1539–1545. doi: 10.1016/j.febslet.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Crotta S, Davidson S, Mahlakoiv T, Desmet CJ, Buckwalter MR, Albert ML, Staeheli P, Wack A. 2013. Type I and type III interferons drive redundant amplification loops to induce a transcriptional signature in influenza-infected airway epithelia. PLoS Pathog 9:e1003773. doi: 10.1371/journal.ppat.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pott J, Mahlakoiv T, Mordstein M, Duerr CU, Michiels T, Stockinger S, Staeheli P, Hornef MW. 2011. IFN-lambda determines the intestinal epithelial antiviral host defense. Proc Natl Acad Sci U S A 108:7944–7949. doi: 10.1073/pnas.1100552108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karaghiosoff M, Steinborn R, Kovarik P, Kriegshauser G, Baccarini M, Donabauer B, Reichart U, Kolbe T, Bogdan C, Leanderson T, Levy D, Decker T, Muller M. 2003. Central role for type I interferons and Tyk2 in lipopolysaccharide-induced endotoxin shock. Nat Immunol 4:471–477. doi: 10.1038/ni910. [DOI] [PubMed] [Google Scholar]
- 22.Saxena K, Simon LM, Zeng XL, Blutt SE, Crawford SE, Sastri NP, Karandikar UC, Ajami NJ, Zachos NC, Kovbasnjuk O, Donowitz M, Conner ME, Shaw CA, Estes MK. 2017. A paradox of transcriptional and functional innate interferon responses of human intestinal enteroids to enteric virus infection. Proc Natl Acad Sci U S A 114:E570–E579. doi: 10.1073/pnas.1615422114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Apelbaum A, Yarden G, Warszawski S, Harari D, Schreiber G. 2013. Type I interferons induce apoptosis by balancing cFLIP and caspase-8 independent of death ligands. Mol Cell Biol 33:800–814. doi: 10.1128/MCB.01430-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chawla-Sarkar M, Leaman DW, Borden EC. 2001. Preferential induction of apoptosis by interferon (IFN)-beta compared with IFN-alpha2: correlation with TRAIL/Apo2L induction in melanoma cell lines. Clin Cancer Res 7:1821–1831. [PubMed] [Google Scholar]
- 25.Gunther C, Martini E, Wittkopf N, Amann K, Weigmann B, Neumann H, Waldner MJ, Hedrick SM, Tenzer S, Neurath MF, Becker C. 2011. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature 477:335–339. doi: 10.1038/nature10400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gabay C. 2006. Interleukin-6 and chronic inflammation. Arthritis Res Ther 8(Suppl 2):S3. doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atreya R, Neurath MF. 2005. Involvement of IL-6 in the pathogenesis of inflammatory bowel disease and colon cancer. Clin Rev Allergy Immunol 28:187–196. doi: 10.1385/CRIAI:28:3:187. [DOI] [PubMed] [Google Scholar]
- 28.Majumder S, Zhou LZ, Chaturvedi P, Babcock G, Aras S, Ransohoff RM. 1998. p48/STAT-1alpha-containing complexes play a predominant role in induction of IFN-gamma-inducible protein, 10 kDa (IP-10) by IFN-gamma alone or in synergy with TNF-alpha. J Immunol 161:4736–4744. [PubMed] [Google Scholar]
- 29.Wen X, Kudo T, Payne L, Wang X, Rodgers L, Suzuki Y. 2010. Predominant interferon-gamma-mediated expression of CXCL9, CXCL10, and CCL5 proteins in the brain during chronic infection with Toxoplasma gondii in BALB/c mice resistant to development of toxoplasmic encephalitis. J Interferon Cytokine Res 30:653–660. doi: 10.1089/jir.2009.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim MO, Suh HS, Brosnan CF, Lee SC. 2004. Regulation of RANTES/CCL5 expression in human astrocytes by interleukin-1 and interferon-beta. J Neurochem 90:297–308. doi: 10.1111/j.1471-4159.2004.02487.x. [DOI] [PubMed] [Google Scholar]
- 31.Sung PS, Hong SH, Lee J, Park SH, Yoon SK, Chung WJ, Shin EC. 2017. CXCL10 is produced in hepatitis A virus-infected cells in an IRF3-dependent but IFN-independent manner. Sci Rep 7:6387. doi: 10.1038/s41598-017-06784-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feltham R, Vince JE, Lawlor KE. 2017. Caspase-8: not so silently deadly. Clin Transl Immunology 6:e124. doi: 10.1038/cti.2016.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wurstle ML, Laussmann MA, Rehm M. 2012. The central role of initiator caspase-9 in apoptosis signal transduction and the regulation of its activation and activity on the apoptosome. Exp Cell Res 318:1213–1220. doi: 10.1016/j.yexcr.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Balachandran S, Roberts PC, Kipperman T, Bhalla KN, Compans RW, Archer DR, Barber GN. 2000. Alpha/beta interferons potentiate virus-induced apoptosis through activation of the FADD/caspase-8 death signaling pathway. J Virol 74:1513–1523. doi: 10.1128/jvi.74.3.1513-1523.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thapa RJ, Nogusa S, Chen P, Maki JL, Lerro A, Andrake M, Rall GF, Degterev A, Balachandran S. 2013. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc Natl Acad Sci U S A 110:E3109–18. doi: 10.1073/pnas.1301218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.England H, Summersgill HR, Edye ME, Rothwell NJ, Brough D. 2014. Release of interleukin-1alpha or interleukin-1beta depends on mechanism of cell death. J Biol Chem 289:15942–15950. doi: 10.1074/jbc.M114.557561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim HS, Kim DC, Kim HM, Kwon HJ, Kwon SJ, Kang SJ, Kim SC, Choi GE. 2015. STAT1 deficiency redirects IFN signalling toward suppression of TLR response through a feedback activation of STAT3. Sci Rep 5:13414. doi: 10.1038/srep13414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baldridge MT, Turula H, Wobus CE. 2016. Norovirus regulation by host and microbe. Trends Mol Med 22:1047–1059. doi: 10.1016/j.molmed.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]