Severe fever with thrombocytopenia syndrome virus (SFTSV) is a tick-borne pathogen that causes severe hemorrhagic fever. Although SFTSV poses a serious threat to public health and was recently isolated, its pathogenesis remains unclear. In particular, the relationship between SFTSV infection and the host cell cycle has not been described. Here, we show for the first time that both asynchronized and synchronized SFTSV-susceptible cells arrest at the G2/M checkpoint following SFTSV infection and that the accumulation of cells at this checkpoint facilitates viral replication. We also identify a key mechanism underlying SFTSV-induced G2/M arrest, in which SFTSV NSs interacts with CDK1 to inhibit formation and nuclear import of the cyclin B1-CDK1 complex, thus preventing it from regulating cell cycle progression. Our study highlights the key role that NSs plays in SFTSV-induced G2/M arrest.
KEYWORDS: cell cycle, cyclin B1-CDK1 complex, G2/M arrest, SFTSV
ABSTRACT
Severe fever with thrombocytopenia syndrome virus (SFTSV) is a newly identified phlebovirus associated with severe hemorrhagic fever in humans. While many viruses subvert the host cell cycle to promote viral growth, it is unknown whether this is a strategy employed by SFTSV. In this study, we investigated how SFTSV manipulates the cell cycle and the effect of the host cell cycle on SFTSV replication. Our results suggest that cells arrest at the G2/M transition following infection with SFTSV. The accumulation of cells at the G2/M transition did not affect virus adsorption and entry but did facilitate viral replication. In addition, we found that SFTSV NSs, a nonstructural protein that forms viroplasm-like structures in the cytoplasm of infected cells and promotes virulence by modulating the interferon response, induces a large number of cells to arrest at the G2/M transition by interacting with CDK1. The interaction between NSs and CDK1, which is inclusion body dependent, inhibits formation and nuclear import of the cyclin B1-CDK1 complex, thereby leading to cell cycle arrest. Expression of a CDK1 loss-of-function mutant reversed the inhibitive effect of NSs on the cell cycle, suggesting that this protein is a potential antiviral target. Our study provides new insight into the role of a specific viral protein in SFTSV replication, indicating that NSs induces G2/M arrest of SFTSV-infected cells, which promotes viral replication.
IMPORTANCE Severe fever with thrombocytopenia syndrome virus (SFTSV) is a tick-borne pathogen that causes severe hemorrhagic fever. Although SFTSV poses a serious threat to public health and was recently isolated, its pathogenesis remains unclear. In particular, the relationship between SFTSV infection and the host cell cycle has not been described. Here, we show for the first time that both asynchronized and synchronized SFTSV-susceptible cells arrest at the G2/M checkpoint following SFTSV infection and that the accumulation of cells at this checkpoint facilitates viral replication. We also identify a key mechanism underlying SFTSV-induced G2/M arrest, in which SFTSV NSs interacts with CDK1 to inhibit formation and nuclear import of the cyclin B1-CDK1 complex, thus preventing it from regulating cell cycle progression. Our study highlights the key role that NSs plays in SFTSV-induced G2/M arrest.
INTRODUCTION
Severe fever with thrombocytopenia syndrome virus (SFTSV) is a newly discovered tick-borne pathogen that causes severe hemorrhagic fever. Patients with SFTSV infection often have acute fever, accompanied by thrombocytopenia, leukocyte reduction, gastrointestinal symptoms, regional lymph node swelling and other symptoms. Some patients will go on to develop multiple-system failure or even die (1–4). SFTSV was first discovered in China and has subsequently been reported in Korea and Japan, with mortality rates ranging from 12 to 50% (5–8). Although several vaccines and drugs have been shown to be effective in cell or animal models, to date none have been used for clinical prevention or treatment in humans (9–12), as little is known about how the virus interacts with host cells.
SFTSV is an enveloped, single-stranded, negative-sense RNA virus belonging to the genus Banyangvirus in family Phenuiviridae (13). SFTSV consists of three RNA segments: the large (L) segment encodes the RNA-dependent RNA polymerase (RdRp), which mediates viral RNA replication and synthesis; the medium (M) segment encodes the viral envelope glycoproteins, glycoprotein N (Gn) and glycoprotein C (Gc), which mediate fusion between viruses and host cell membranes (14); and the small (S) segment encodes the viral nucleocapsid protein (NP) and the nonstructural protein (NSs) (15–17). NP is the most abundant protein in SFTSV particles and infected cells and plays a protective role in viral replication and assembly (17). NSs, which is potentially an important virulence factor for SFTSV, inhibits the innate antiviral response of host cells (18–20). Ning et al. found that SFTSV NSs sequesters STAT2 in inclusion bodies (IBs), thereby inhibiting STAT2 phosphorylation and blocking activation of the type I interferon (IFN) signal pathway (21). Another study found that NSs reduces STAT1 phosphorylation at residue S727, thereby inhibiting type I and III IFN signal transduction (22). However, little else is known about the virus’s pathogenesis and especially the interaction between the virus and the host.
Viral infections can cause a variety of different diseases, and their pathogenesis is often complex. Viral subversion of the host cell cycle has become a topic of intensive research in recent years (23–25). The cell cycle of eukaryotic cells is strictly controlled and progresses from the G0 phase through the G1, S, G2, and M phases (26, 27). The cell cycle always progresses in the same order, and this progress is regulated by various cyclic regulatory factors such as cyclins, cyclin-dependent kinases (CDKs), and CDK inhibitors (CKIs) (28). Cell cycle regulation plays an important role in cell division, cell differentiation, and carcinogenesis (29). However, when a cell becomes infected with a virus, the virus uses the metabolic system of the host cell to synthesize its own nucleic acids, proteins, and other components to promote virus assembly and replication. Viruses can disrupt the cell cycle process through several regulatory pathways and multiple regulatory points, and expression of viral proteins facilitates viral replication. Many viruses, including DNA viruses, retroviruses, and RNA viruses, interact with host factors that regulate cell cycle progression to facilitate their own replication (29). For example, hepatitis B virus (HBV), an enveloped DNA virus, induces G1-phase arrest in hepatocytes to promote viral replication (30). The type 1 human immunodeficiency virus (HIV-1) inhibits cell proliferation by blocking infected cells at the G2/M checkpoint (31). RNA viruses, whose primary site of replication is normally in the cytoplasm, can also interfere with the host cell cycle; for example, infectious bronchitis virus (IBV) induces S and G2 arrest (32). In addition, Rift Valley fever virus (RVFV) arrests the host cell cycle at the S phase in order to promote its own replication (23). However, it is unknown whether SFTSV, an important pathogen, manipulates the host cell cycle.
The aim of this study was to investigate the relationship between SFTSV infection and the host cell cycle. In this study, we showed that infection with SFTSV alters cell cycle progression in asynchronous and synchronous cells and that there is a relationship between cell cycle arrest and viral replication. We further showed that expression of the viral protein NSs alone induces cell cycle arrest. Finally, we further explored the molecular mechanism of NSs-induced cell cycle arrest. The findings from this study increase our understanding of the pathogenic mechanisms of SFTSV and provide a potential target for the treatment and prevention of SFTSV infection.
RESULTS
SFTSV manipulates the host cell cycle to arrest cells at the G2/M transition.
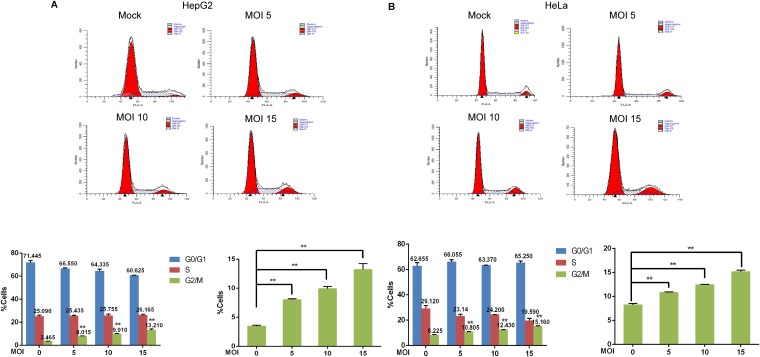
To determine whether SFTSV manipulates the cell cycle, HepG2, HeLa, THP-1, and 293T cells were infected with SFTSV at a multiplicity of infection (MOI) of 10 PFU/cell (10 MOI) or mock infected. At 48 h postinfection, the cells were collected for cell cycle distribution analysis. We observed a 1-fold increase in the percentage of cells arrested at the G2/M transition following infection with SFTSV compared with mock-infected cells. The cell cycle distribution of HeLa cells was assessed at 6, 12, 24, and 48 h after infection with SFTSV or UV-inactivated SFTSV. SFTSV-induced arrest at the G2/M phase increased with the duration of infection, while inactivated virus could not induce G2/M-phase cell cycle arrest (data not shown). However, the degree of cell cycle arrest induced in the early phase of SFTSV infection was indistinguishable from that observed in the control group, presumably due to low viral infection efficiency and slow viral proliferation. To determine whether SFTSV induces cell cycle arrest in a dose-dependent manner, HepG2 cells and HeLa cells were infected with SFTSV at different MOIs (0 MOI, 5 MOI, 10 MOI, and 15 MOI), or were mock infected. After 48 h, the cells were collected, and flow cytometry was used to detect changes in the cell cycle distribution. In total, 3.465% of mock-infected HepG2 cells were at the G2/M transition, but 8.015%, 9.91%, and 13.21% of HepG2 cells were in the G2/M phase after infection with SFTSV at 5 MOI, 10 MOI, or 15 MOI, respectively (Fig. 1A). Similarly, 8.225% of mock-infected HeLa cells arrested at the G2/M transition, while 10.805%, 12.43%, and 15.16% of HeLa cells were arrested at the G2/M transition after infection with SFTSV at 5 MOI, 10 MOI, or 15 MOI, respectively (Fig. 1B). Collectively, these data indicated that both HepG2 cells and HeLa cells were arrested at the G2/M transition after infection with SFTSV, and the number of cells at the G2/M transition increased as viral infection titer increased, indicating that cell cycle arrest induced by SFTSV infection was related to the severity of SFTSV infection.
FIG 1.
Infection with SFTSV modulates the host cell cycle by arresting cells at the G2/M phase. HepG2 cells (A) and HeLa cells (B) were infected with SFTSV at different MOIs (0, 5, 10, or 15) for 48 h, and the cell cycle distribution was then analyzed by flow cytometry (**, P < 0.01).
Effect of SFTSV infection on synchronized cells.
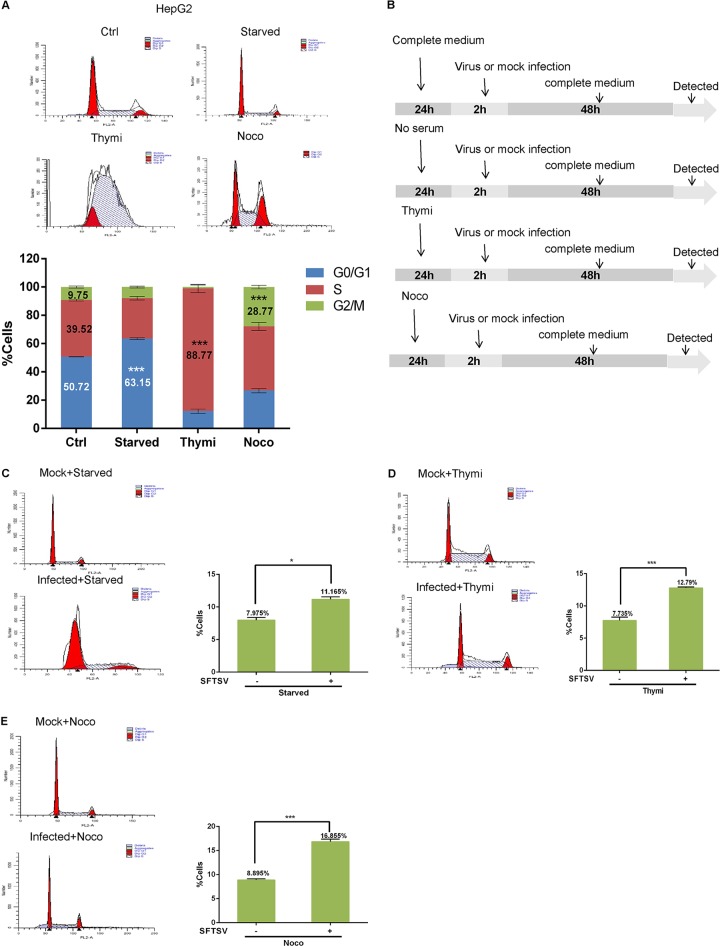
When studying the effect of viruses on the host cell cycle, errors often occur due to the cells being in different phases of the cell cycle. However, synchronizing cell populations so that most of the cell are in a relatively similar phase of the cell cycle makes it possible to effectively verify the effect of viral infection on the cell cycle. To further confirm that SFTSV infection leads to cell cycle arrest at the G2/M transition, we synchronized cells in different phases, infected them with SFTSV, and then assessed the cell cycle distribution. The treatment scheme is shown in Fig. 2B. Consistent with previous reports, serum deprivation resulted in 63.15% of HepG2 cells arresting at the G0/G1 transition, culturing with 0.85 mM thymidine resulted in 88.77% of HepG2 cells arresting in the S phase, and 28.77% of HepG2 cells arrested at the G2/M transition when treated with 50 ng/ml nocodazole (Fig. 2A). Subsequently, the synchronized cells were infected with SFTSV (10 MOI) or mock infected for 2 h, fresh medium was added, and the cells were allowed to grow for another 48 h. In total, 7.975% of HepG2 cells that had been synchronized at the G0/G1 transition and then mock infected reached that G2/M transition, while 11.165% of HepG2 cells that had been synchronized at the G0/G1 transition and then infected with SFTSV reached this transition (Fig. 2C). Similarly, 7.735% of HepG2 cells that had been synchronized in the S phase and then mock infected reached the G2/M transition, while 12.79% of HepG2 cells that had been synchronized in the S phase and then infected with SFTSV reached this transition (Fig. 2D). Finally, 8.895% of HepG2 cells that had been synchronized at the G2/M transition remained at this transition in the uninfected group, while 16.855% of HepG2 cells that had been synchronized at the G2/M transition remained at this transition in the infected group (Fig. 2E). Similar results were obtained in HeLa cells (data not shown). These data confirm that SFTSV infection induces G2/M checkpoint arrest.
FIG 2.
Effect of SFTSV infection on synchronized cells. (A) HepG2 cells were blocked at the G0/G1, S, or G2/M phase by growth in serum-free culture, culturing with 0.85 mM thymidine (Thymi), or culturing with 50 ng/ml nocodazole (Noco), respectively, for 24 h. (B) Cell synchronization experimental workflow. (C, D, and E) HepG2 cells were synchronized at different phases, infected with SFTSV (10 MOI), and then cultured in fresh medium for another 48 h. The cell cycle distribution was analyzed by flow cytometry (*, P < 0.05; ***, P < 0.001).
Synchronization at the G2/M transition promotes SFTSV replication.
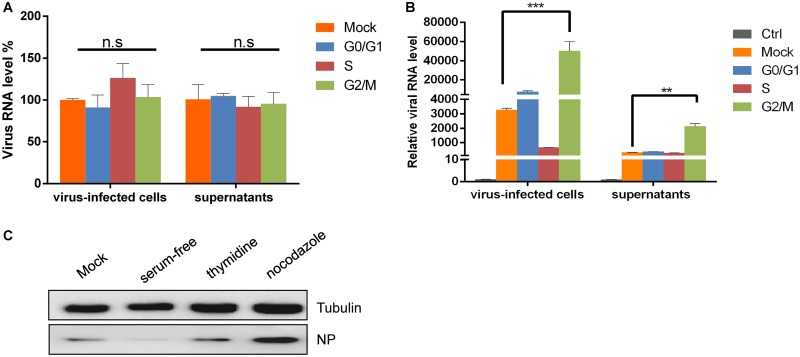
The cell cycle status of host cells is often crucial for viral replication (33). To explore the possible benefits of different cell cycle phases for SFTSV replication, we synchronized cells at different phases and then assessed viral replication. First, we examined the relationship between cell cycle arrest and virus adsorption. HepG2 cells (Fig. 3A) and HeLa cells (data not shown) were cultured in serum-containing medium (as a control), serum-free medium (G0/G1 transition synchronization), medium containing 0.85 mM thymidine (S-phase synchronization), or 50 ng/ml nocodazole (G2/M transition synchronization) for 24 h, according to previously described protocols. The cells then were infected with SFTSV (0.1 MOI) or were mock infected for 30 min, after which the cells and supernatants were immediately collected to detect the viral RNA level by quantitative real-time PCR (qRT-PCR). We found that, compared with the control group (in which untreated cells were infected with SFTSV at the same MOI), cell cycle arrest at the G0/G1 transition, in S phase, or at the G2/M transition did not affect virus adsorption.
FIG 3.
G2/M phase synchronization promotes SFTSV proliferation. (A) HepG2 cells were cultured in complete medium, serum-free medium, medium containing 0.85 mM thymidine, or medium containing 50 ng/ml nocodazole for 24 h and then infected with SFTSV (0.1 MOI) for 30 min. The cells and supernatants were collected immediately and analyzed by qRT-PCR. (B) HepG2 cells were treated as described above. After infection with SFTSV (0.1 MOI), fresh complete medium, serum-free medium, medium containing 0.85 mM thymidine, or medium containing 50 ng/ml nocodazole was added to the culture and left for another 48 h. The cells and supernatants were collected for qRT-PCR analysis (n.s, P > 0.05; **, P < 0.01; ***, P < 0.001). (C) HepG2 cells were treated as described above but infected with SFTSV at an MOI of 1. After 48 h, Western blotting was performed to detect NP expression.
To further assess the effects of synchronization at different cell cycle stages on SFTSV replication, SFTSV was allowed to adsorb to HepG2 cells for 2 h, and then fresh culture medium, serum-free medium, medium containing 0.85 mM thymidine, or medium containing 50 ng/ml nocodazole was added, and the cells were incubated for another 48 h. The cells and supernatants were then collected for qRT-PCR analysis. We found that arrest at the G2/M transition promoted SFTSV replication in both HepG2 and HeLa cells. Compared with the control group (described above), viral replication in HepG2 cells increased 16-fold, and the amount of virus in the HepG2 supernatants increased 6-fold (Fig. 3B), while viral replication in HeLa cells increased 13-fold, and the amount of virus in the HeLa supernatants increased 4-fold (data not shown). Western blot analysis showed that G2/M-phase arrest promoted NP expression but that a G0/G1-phase block inhibited NP expression (Fig. 3C). We hypothesized that serum-free medium does not provide sufficient amino acids for cell/viral protein translation. Therefore, our data suggest that SFTSV infection can arrest cells at the G2/M transition, which promotes SFTSV replication.
SFTSV NSs mediates cell cycle alterations.
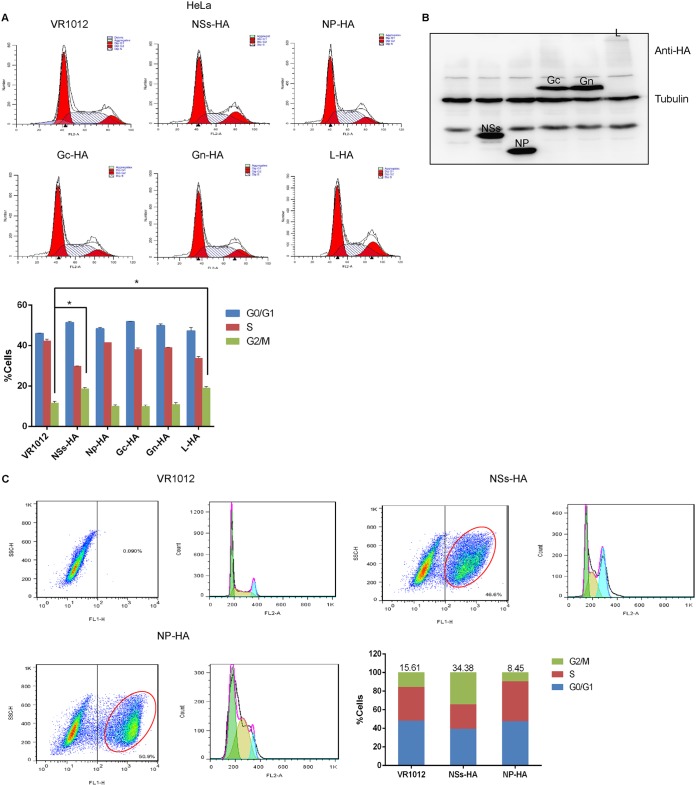
In order to further explore the relationship between viral proteins and the cell cycle, we transfected cells with recombinant plasmids encoding nonstructural protein (NSs)-hemagglutinin (HA), NP-HA, Gc-HA, Gn-HA, or L-HA (or empty vector, VR1012). For all the subsequent experiments, VR1012 was used as the empty control vector, unless otherwise indicated. After 48 h, the cells were collected, stained with propidium iodide (PI) and analyzed by flow cytometry. We found that both HeLa cells (Fig. 4A) and 293T cells (data not shown) expressing NSs-HA and L-HA arrested at the G2/M transition. The viral proteins were detected by Western blotting (Fig. 4B). To further verify the effect of NSs on the cell cycle, HeLa cells were transfected with empty vector or plasmids encoding NSs-HA or NP-HA. Flow cytometry analysis showed that 34.38% of HeLa cells expressing NSs were at the G2/M transition, which represents an increase of more than 20% compared with the mock-infected group. However, there was no significant difference in the proportion of cells at the G2/M transition between cells expressing NP and the control group (Fig. 4C). These data suggest that NSs induces cell cycle arrest.
FIG 4.
The SFTSV nonstructural protein (NSs) mediates cell cycle modulation. (A) HeLa cells were transfected with plasmids encoding VR1012, NSs-HA, NP-HA, Gc-HA, Gn-HA, or L-HA. The cells were collected at 48 h, and the cell cycle distribution was analyzed by flow cytometry. (B) Detection of protein expression by Western blotting. (C) HeLa cells were transfected with plasmids encoding VR1012, NSs-HA, or NP-HA for 48 h, after which the cells were collected, fixed, permeabilized, blocked, and incubated with anti-HA antibodies. Flow cytometry was used to detect cells expressing the exogenous proteins and analyze their cell cycle distribution (*, P < 0.05).
NSs interacts with CDK1, preventing the cyclin B1-CDK1 complex from entering the nucleus.
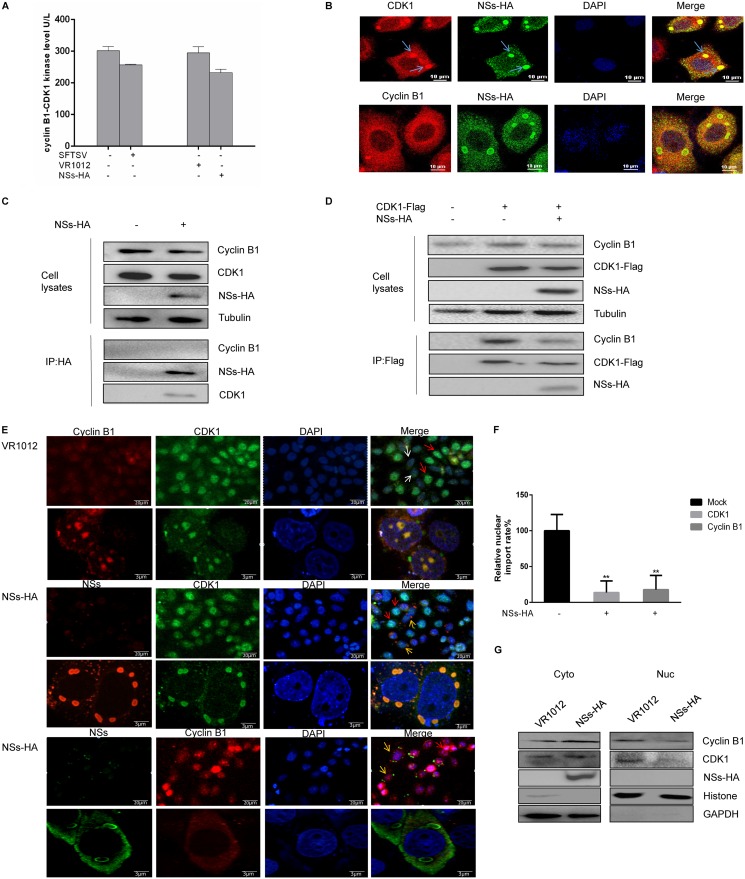
The cyclin-dependent kinase CDK1 is the catalytic subunit of the highly conserved protein kinase complex known as M-phase-promoting factor (MPF), which plays vital roles in the G1/S and G2/M transitions of the eukaryotic cell cycle. There is increasing evidence that CDK1 is also a common target of viral proteins that regulate the host cell cycle machinery to facilitate viral survival or replication (24, 34). We assessed cyclin B1-CDK1 kinase levels in HeLa cells infected with SFTSV or transfected with NSs-HA according to the manufacturer’s instructions. We found that the levels of cyclin B1-CDK1 kinase expressed by both SFTSV-infected and NSs-expressing cells were lower than that expressed by the control cells (Fig. 5A). To determine whether NSs induces G2/M arrest through CDK1, HeLa cells were transfected with a recombinant plasmid encoding NSs-HA or with empty vector as a control. Next, at 48 h after transfection, the cells were fixed, permeabilized, blocked, and incubated with antibodies. The immunofluorescence assay results showed that CDK1 colocalized with NSs IBs and that cyclin B1 did not colocalize with these structures (Fig. 5B). TBK1 and NSs are known to interact in an IB-dependent manner (35, 36), and we also observed colocalization of CDK1 and TBK1 in IBs in NSs-expressing cells (data not shown). We then transfected 293T cells with a plasmid encoding NSs-HA and found that NSs interacted with CDK1 but did not interact with cyclin B1, as determined by coimmunoprecipitation (Fig. 5C). Next, we focused on the cyclin B1-CDK1 complex, which is the key mitotic regulator of the G2/M transition. Equal amounts of recombinant plasmids encoding NSs-HA or CDK1-Flag were transfected into 293T cells (control cells were transfected with the plasmid encoding CDK1-Flag and an empty plasmid). Coimmunoprecipitation analysis showed that NSs expression resulted in decreased binding of cyclin B1 to CDK1 (Fig. 5D), indicating that NSs inhibited the formation of the cyclin B1-CDK1 complex.
FIG 5.
NSs interacts with CDK1 and inhibits the cyclin B1-CDK1 complex from entering the nucleus. (A) HeLa cells were infected with SFTSV (10 MOI) or transfected with a plasmid encoding NSs-HA for 48 h, after which the cyclin B1-CDK1 level was measured using a human CDK1/cyclin B kinase expression level detection kit. (B) HeLa cells were transfected with a plasmid encoding NSs-HA. After 48 h, the cells were fixed, permeabilized, blocked, and incubated with antibodies. A laser confocal microscope was used to observe colocalization of cyclin B1 with NSs, CDK1, and NSs. (C) 293T cells were transfected with plasmids encoding VR1012 or NSs-HA and collected at 48 h. Coimmunoprecipitation was performed to detect interactions between cyclin B1, CDK1, and NSs. (D) 293T cells were transfected with plasmids encoding VR1012 alone, VR1012 and CDK1-Flag, or NSs-HA and CDK1-Flag. The cells were collected at 48 h, and the effect of NSs on formation of the cyclin B1-CDK1 complex was analyzed by immunoprecipitation. (E) HeLa cells were transfected with plasmids encoding VR1012 or NSs-HA. After 48 h, the cells were fixed, permeabilized, blocked, and incubated with antibodies. Localization of cyclin B1 and CDK1 to the nucleus was observed by confocal laser microscopy. The blue arrows indicate colocalization of NSs and CDK1, the red arrows indicate the nuclear cyclin B1-CDK1 complex, the white arrows indicate the cytoplasmic cyclin B1-CDK1 complex, and the yellow arrows indicate cells expressing NSs in which the cyclin B1-CDK1 complex localized to the cytoplasm. (F) Gen5 software was used to perform a statistical analysis of localization of the cyclin B1-CDK1 complex to the nucleus (**, P < 0.01). (G) 293T cells were transfected with plasmids encoding VR1012 or NSs-HA and collected at 48 h. Localization of the cyclin B1-CDK1 complex was determined by nuclear separation and Western blotting.
In the G2 phase, cyclin B1 accumulates and forms a complex with CDK1 in the cytoplasm. For a cell to enter mitosis, the cyclin B1-CDK1 complex must be imported into the nucleus (31). Therefore, we next asked whether NSs expression induced arrest at the G2/M transition by preventing this complex from entering the nucleus. Immunofluorescence analysis showed that the amounts of CDK1 and cyclin B1 in the nuclei of cells expressing NSs were decreased compared with those in cells that did not express NSs (Fig. 5E). The nuclear and cytoplasmic signals were separated using a Lionhear FX automated microscope and Gen5 software, and the fluorescence intensity of the nucleoplasm was calculated. The fluorescence intensity of the nucleus was three times greater than that of the cytoplasm, suggesting that the cyclin B1-CDK1 complex had entered the nucleus. Statistical analysis showed that if the rate of entry of the cyclin B1-CDK1 complex into the nucleus in the control group was considered 100%, that in the group expressing NSs was ∼30% (Fig. 5F). The nuclear and cytoplasmic fractions were then analyzed according to previously published protocols. Western blotting demonstrated that the proportions of CDK1 and cyclin B1 decreased in the nucleus and increased in the cytoplasm in cells expressing NSs-HA (Fig. 5G), indicating that NSs regulates the host cell cycle by inhibiting cyclin B1-CDK1 complex nuclear entry. These data suggest that NSs can arrest cells at the G2/M transition by sequestering CDK1 in IBs, which inhibits the formation of the cyclin B1-CDK1 complex and thereby prevents it from entering the nucleus.
The interaction between CDK1 and NSs is IB dependent.
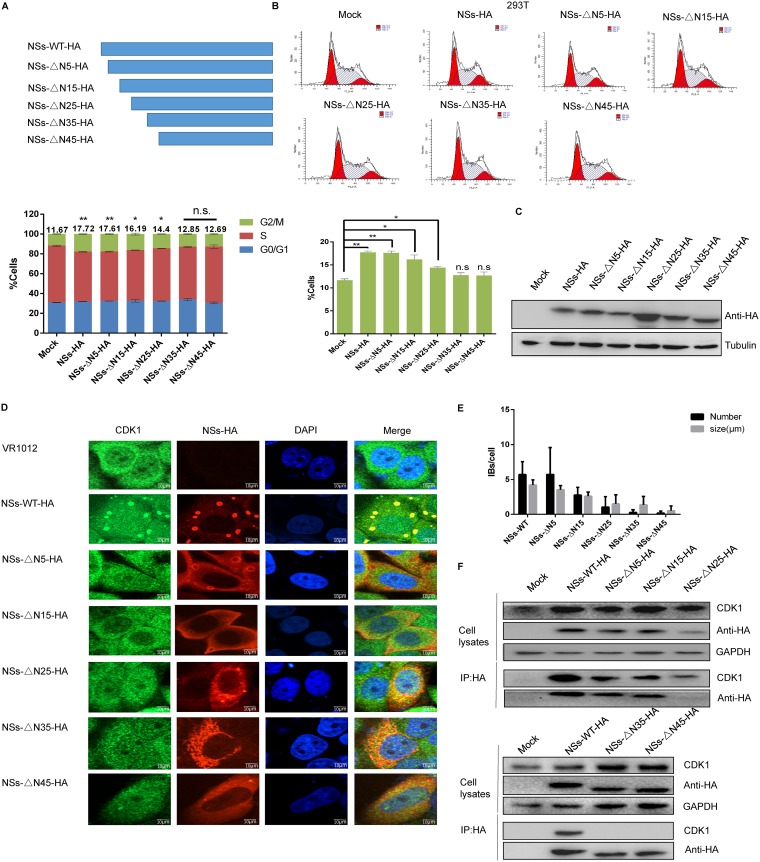
Previous studies have shown that NSs and CDK1 colocalize in IBs, which led us to hypothesize that CDK1 is also sequestered in IBs, thereby inhibiting its function. To verify this hypothesis, we constructed recombinant plasmids encoding a panel of N-terminally truncated NSs variants (Fig. 6A) and then used flow cytometry analysis to determine the effect of expression of this protein on the 293T cell cycle distribution at 48 h after transfection. We found that, while wild-type NSs induced cell arrest at the G2/M transition, progressively larger truncations of the N terminus correlated with a gradual weakening of G2/M transition arrest. 293T cells expressing NSs-△N35-HA or NSs-△N45-HA showed no arrest at the G2/M transition, similar to the case for the control group (Fig. 6B), and the same results were observed in HeLa cells (data not shown). Western blot analysis showed that N-terminal truncation did not affect protein expression (Fig. 6C). Laser confocal microscopy showed that the number and diameter of IBs decreased gradually with the truncation of the NSs N terminus, and localization of CDK1 to the IBs also decreased gradually (Fig. 6D). Using the Gen5 “spot” function, we quantified IBs, defined by their fluorescence intensity and a size range of 0.5 to 6 μm. Statistical analysis showed that the number and size of IBs decreased as the proportion of the N terminus that was truncated increased and that NSs-△N45-HA no longer formed IBs (Fig. 6E). To further assess whether the interaction between CDK1 and NSs was IB dependent, 293T cells were transfected with the N-terminally truncated NSs plasmids. Coimmunoprecipitation showed that CDK1 interacted with wild-type NSs but not with NSs-△N45-HA, indicating that the interaction between CDK1 and NSs is IB dependent (Fig. 6F).
FIG 6.
The interaction between CDK1 and NSs is IB dependent. (A) Schematic of mutant NSs with N-terminal truncations. (B) 293T cells were transfected with plasmids encoding VR1012, NSs-WT-HA, NSs-△N5-HA, NSs-△N15-HA, NSs-△N25-HA, NSs-△N35-HA, or NSs-△N45-HA. The cells were collected at 48 h and analyzed by flow cytometry (*, P < 0.05; **, P < 0.01). (C) Western blotting was performed to detect expression of the recombinant proteins. (D) HeLa cells were transfected with plasmids encoding VR1012, NSs-WT-HA, NSs-△N5-HA, NSs-△N15-HA, NSs-△N25-HA, NSs-△N35-HA, or NSs-△N45-HA. After 48 h, the cells were fixed, permeabilized, blocked, and incubated with antibodies. A laser confocal microscope was used to observe colocalization of CDK1 and NSs. (E) Gen5 software was used to perform a statistical analysis of the number and size of IBs formed by NSs. (F) 293T cells were transfected with plasmids encoding VR1012, NSs-WT-HA, NSs-△N5-HA, NSs-△N15-HA, NSs-△N25-HA, NSs-△N35-HA, or NSs-△N45-HA and collected at 48 h. Interactions between CDK1 and NSs were detected by immunoprecipitation.
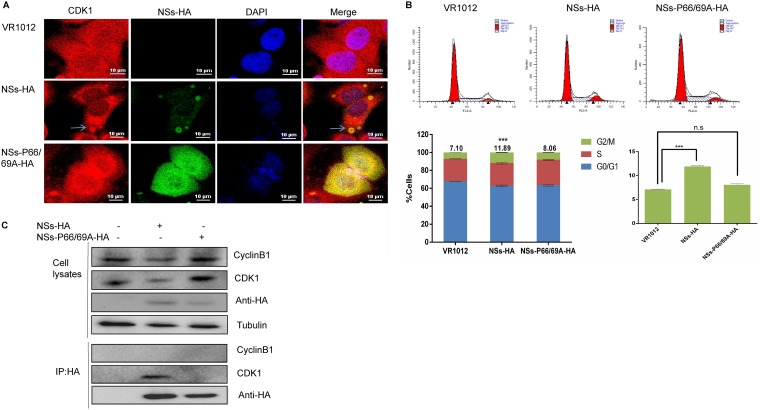
Moriyama et al. found that changing the proline residues at positions 66 and 69 of NSs to alanine resulted in the absence of IB formation in the cytoplasm (36). To further explore the relationship between the ability of NSs to form IBs and cell cycle arrest, we constructed a recombinant plasmid expressing mutant NSs with alanine residues at positions 66 and 69 and transfected it into HeLa cells. Laser confocal microscopy 48 h later showed that NSs-P66/69A-HA did not form IBs and did not colocalize with CDK1 (Fig. 7A). Next, 293T cells were transfected with an empty plasmid or with plasmids expressing NSs-HA or NSs-P66/69A-HA. After 48 h, the cell cycle distribution was analyzed by flow cytometry. We found that 11.89% of the cells expressing wild-type NSs were at the G2/M transition, while only 8.06% of the cells expressing the NSs mutant were at this transition, and there was no significant difference between the cells expressing the NSs mutant and the control group (Fig. 7B). Coimmunoprecipitation showed that wild-type NSs interacted with CDK1 but that NSs-P66/69A did not (Fig. 7C). Taken together, these results suggest that the ability of truncated or mutant NSs proteins to interact with CDK1 was weakened when their ability to form IBs decreased, thus inhibiting their ability to induce cycle arrest. Therefore, the capacity of NSs to arrest the cell cycle is related to its ability to form IBs.
FIG 7.
The NSs-P66/69A-HA mutant does not induce cell cycle arrest. (A) HeLa cells were transfected with plasmids encoding VR1012, NSs-HA, or NSs-P66/69A-HA. After 48 h, the cells were fixed, permeabilized, blocked, and incubated with antibodies. Laser confocal microscopy was used to observe formation of IBs by wild-type or mutant NSs and colocalization of these proteins with CDK1. (B) 293T cells were transfected with plasmids encoding VR1012, NSs-HA, or NSs-P66/69A-HA. The cells were collected 48 h later, and the cell cycle distribution was analyzed by flow cytometry (n.s, P > 0.05; ***, P < 0.001). (C) 293T cells were transfected with plasmids encoding VR1012, NSs-HA, or NSs-P66/69A-HA. The cells were collected, and interactions between NSs and CDK1 were detected by immunoprecipitation.
Expression of an exogenous loss-of-function CDK1 deletion mutant reverses the inhibitory effect of NSs on cell cycle progression.
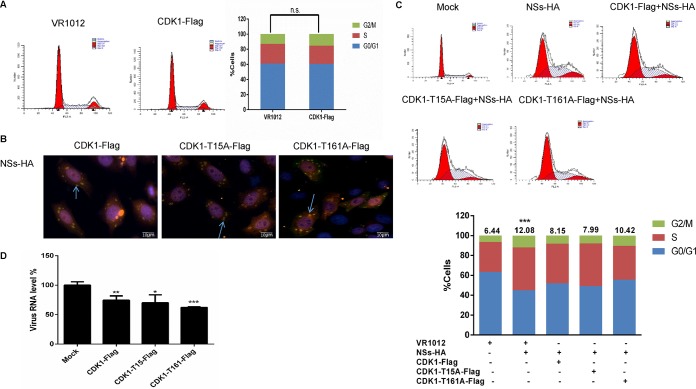
CDK1 plays an important role in the G2/M transition (37, 38). 293T cells were transfected with a recombinant plasmid encoding CDK1-Flag. Flow cytometry analysis showed that there was no significant change in cell cycle distribution in these cells compared with the control group, indicating that exogenous CDK1 protein did not lead to G2/M arrest (Fig. 8A). To further study the effect of exogenous CDK1 on the cell cycle, we constructed plasmids encoding CDK1 with T15A and T161A mutations (these two amino acids are associated with CDK1 activity and cyclin B1 binding). Immunofluorescence analysis showed that the mutant CDK1 protein still colocalized with the IBs formed by NSs in HeLa cells (Fig. 8B). We speculated that the exogenous CDK1 bound to NSs, thus interfering with the interaction between NSs and the endogenous CDK1. 293T cells were transfected with an empty plasmid or plasmids encoding NSs-HA, CDK1-Flag and NSs-HA, CDK1-T15A-Flag and NSs-HA, or CDK1-T161A-Flag and NSS-HA, and cell cycle changes were analyzed after 48 h. Compared with the control group, the proportion of cells at the G2/M transition increased 1-fold in cells expressing NSs, but this proportion was lower in cells expressing mutant CDK1 variants (Fig. 8C). Similar results were observed in HeLa cells (data not shown). Next, 293T cells were transfected with empty plasmid or plasmids encoding CDK1-Flag, CDK1-T15A-Flag, or CDK1-T161A-Flag. After 24 h, SFTSV was allowed to adsorb to the 293T cells, and the cells were incubated for another 48 h. The cells were then collected for qRT-PCR analysis. We found that the exogenously expressed CDK1 protein variants all inhibited SFTSV replication (Fig. 8D). These findings suggest that exogenous CDK1 can bind to NSs, thus inhibiting NSs binding to endogenous CDK1 and decreasing host cell cycle arrest.
FIG 8.
A nonfunctional CDK1 deletion mutant counteracts NSs-mediated inhibition of cell cycle progression. (A) 293T cells were transfected with plasmids encoding VR1012 or CDK1-Flag. The cells were collected 48 h later and analyzed by flow cytometry. (B) HeLa cells were transfected with plasmids encoding CDK1-Flag and NSs-HA, CDK1-T15A-Flag and NSs-HA, or CDK1-T161A-Flag and NSs-HA. After 48 h, the cells were fixed, permeabilized, blocked, and incubated with antibodies. Laser confocal microscopy was used to observe the colocalization of NSs with wild-type or mutant CDK1. (C) 293T cells were transfected with plasmids encoding VR1012 alone, VR1012 and NSs-HA, CDK1-Flag and NSs-HA, CDK1-T15A-Flag and NSs-HA, or CDK1-T161A-Flag and NSs-HA. After 48 h, the cells were collected and analyzed by flow cytometry. (D) 293T cells were transfected with empty plasmid or plasmids encoding CDK1-Flag, CDK1-T15A-Flag, or CDK1-T161A-Flag. After 24 h, SFTSV (0.1 MOI) was allowed to adsorb to the 293T cells, and the cells were incubated for another 48 h. The cells were then collected for qRT-PCR analysis (***, P < 0.001).
DISCUSSION
Halting the proliferation of virus-infected cells is an important way to regulate viral replication. Viruses bypass cell cycle checkpoints and modulate cell proliferation pathways in a variety of different ways. Many DNA and RNA viruses or the proteins that they encode can target key regulators of the cell cycle, leading to changes in cell cycle progression that facilitate viral replication (29). Many DNA viruses primarily infect quiescent or differentiated cells that contain low levels of deoxynucleotides, but a limited deoxynucleotide supply is detrimental to viral replication (39). Therefore, viruses often induce quiescent cells to enter the cell cycle (for example, by progressing to the S phase) to produce factors required for viral proliferation. Retroviruses and other RNA viruses also interfere with the host cell cycle to promote viral replication, translation, and assembly (31, 32). SFTSV is a newly discovered phlebovirus that causes a severe hemorrhagic fever disease with a high mortality rate and has recently spread to East Asian countries. However, even though this is an important pathogen that causes SFTS, there have been few studies on the pathogenesis of SFTSV, and a relationship between viral infection and host cell cycle regulation has not been reported. In this study, we found that the number of both asynchronized and synchronized cells susceptible to SFTSV infection (HepG2 cells and HeLa cells) that arrested at the G2/M transition increased 1-fold after virus infection. qRT-PCR analysis showed that arrest at the G2/M transition did not affect viral adsorption but did promote viral replication (Fig. 3). Our data show that the cell cycle arrest at the G2/M transition induced by SFTSV infection provides favorable conditions for viral replication.
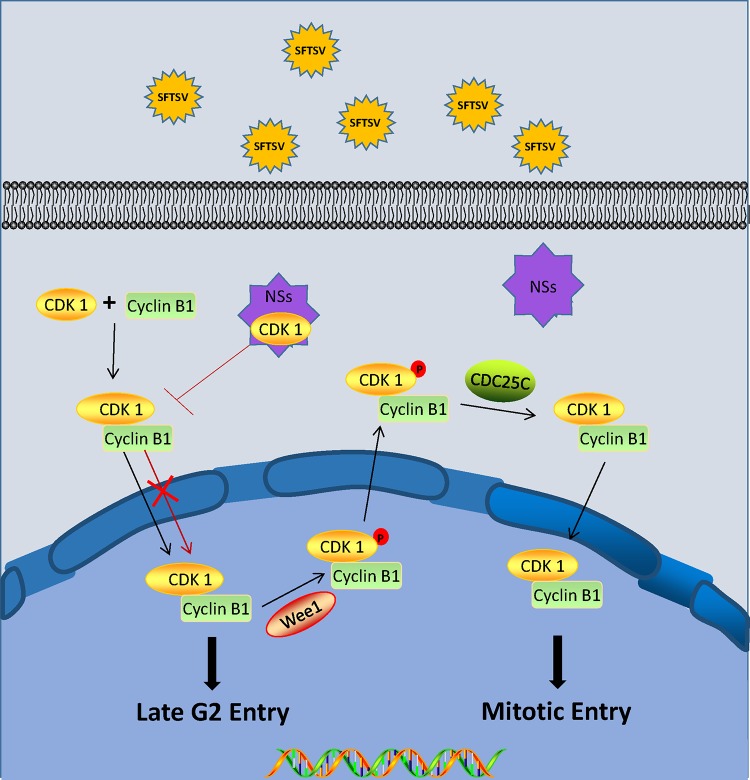
The G2/M transition is the last checkpoint in the cell cycle, and passing this checkpoint allows the cell to divide into two daughter cells. Studies have shown that viruses induce arrest at the G2/M transition via a variety of different methods (40–43). The HIV Vpr protein induces host cell arrest at the G2/M transition by activating ATR to interact with Cdc25C, thereby inhibiting its phosphatase activity (44, 45). The polyomavirus agnoprotein JC inhibits CDK activity by promoting P21 expression (46), and the human papillomavirus (HPV) type 1E4 protein maintains CDK1 phosphorylation (thereby inactivating its activity) (47). In addition, viral infections can also inhibit the nuclear accumulation of cyclin B1-CDK1 complexes. For example, the HPV16 E4 protein retains the cyclin B1-CDK1 kinase complex in the cytoplasm, resulting in cell cycle arrest (48). Chiu et al. found that the reovirus P17 protein induces G2 arrest by interacting with CDK1 (49). The mechanisms by which these viruses induce cell cycle arrest seem to vary widely and are not always fully understood. In this study, we found that infection with SFTSV or expression of NSs induced arrest at the G2/M transition (Fig. 4). NSs sequesters the TBK1/iKKɛ/IRF3 complex, RIG-I, the E3 ligase TRIM25, and the signaling molecules IRF3 and STAT2 in IBs, thus blocking activation of downstream signaling pathways and evading the innate immune response (50, 51). Immunofluorescence results showed that CDK1 colocalized with NSs in IBs, while cyclin B1 did not colocalize with the IBs (Fig. 5B). We hypothesized that NSs can induce cell cycle progression changes by interacting with CDK1, in a manner similar to that for reovirus P17. Immunoprecipitation analysis confirmed our hypothesis. Figure 9 displays our current working model of the signaling events that occur following SFTSV infection, and specifically NSs expression, resulting in subsequent arrest at the G2/M transition and increased viral replication. Although we have demonstrated that the interaction between NSs and CDK1 induces arrest at the G2/M transition, many viruses can induce cell cycle changes through various pathways, and viral proteins may also have multiple roles in cell cycle regulation. Therefore, further research is needed to determine whether SFTSV induces cell cycle arrest through other pathways.
FIG 9.
Proposed model for SFTSV NSs-induced G2-phase arrest. NSs sequesters CDK1 in IBs, inhibiting cyclin B1-CDK1 complex formation and entry into the nucleus and thereby leading to cell cycle arrest.
Our results indicate that SFTSV NSs-induced cell cycle arrest at the G2/M transition promotes its replication. In addition, as shown in Fig. 4, the SFTSV L protein also induced an increase in the proportion of cells arrested at the G2/M transition. These findings suggest that NSs and L may function in the context of SFTSV infection to enhance the percentage of cells at the G2/M transition. Interestingly, another phlebovirus, RVFV, differs from SFTSV in that G2/M synchronization inhibits, rather than promotes, its replication. It is possible that this is due to differences in the location, form, and function of the two NSs proteins. RVFV NSs forms a large filamentous fibril bundle in the nucleus, regulates protein expression by inhibiting transcription, and activates the ATM arm of the DNA damage response (DDR) pathway, resulting in a cell cycle arrest (23, 52, 53). In contrast, SFTSV NSs is expressed in the cytoplasm, where it forms IBs that sequester various regulatory proteins, which may change the distribution of (and therefore interfere with the function of) cell cycle regulatory proteins (21, 54, 55). Although SFTSV and RVFV belong to the family Phenuiviridae (13, 56, 57), our data show that G2/M arrest is conducive to SFTSV replication, while previous data show that S-phase blockade promotes RVFV proliferation. Although both SFTSV and RVFV cause changes in the host cell cycle, the processes and outcomes are different, which may explain why SFTSV and RVFV have different prevalence and pathogenic characteristics.
In summary, our research has shown a novel function for SFTSV NSs in inducing cell cycle arrest and directly promoting viral replication. Unlike RVFV NSs, SFTSV NSs induces cell cycle arrest at the G2/M transition by sequestering CDK1, which is a key regulatory protein that controls cell cycle progression, in NSs-induced cytoplasmic structures, thus inhibiting the formation and nuclear import of the cyclin B1-CDK1 complex. These findings increase our understanding of SFTSV infection and may help identify new antiviral targets to inhibit viral replication and limit the acute and rapid pathogenesis of SFTSV.
MATERIALS AND METHODS
Reagents and chemicals.
Thymidine (catalog no. T1895), nocodazole (catalog no. M1404), and Anti-Flag M2 Affinity Gel (catalog no. A2220) were purchased from Sigma. Anti-HA affinity matrix (catalog no. 11815016001) was purchased from Roche. Primary antibodies to CDK1 (catalog no. 9611s) and cyclin B1 (catalog no. 9915s) were purchased from Cell Signaling Technology. The primary antibody to P-CDK1 (catalog no. AP0016) was purchased from ABclonal Technology. DAPI (4′,6-diamidino-2-phenylindole) (catalog no. H-1200) and the DNA content quantitation assay (cell cycle; catalog no. CA1510) were acquired from Solarbio Life Science. The enhanced chemiluminescence (ECL) reagent was obtained from Thermo Fisher Scientific, Inc. (USA).
Viruses and cell lines.
SFTSV strain HB29 (GenBank accession no. NC_018136, NC_018138, and NC_018137) was obtained from Dexin Li (Chinese Center for Disease Control and Prevention). The experiments were performed with HeLa cervical carcinoma cells (HeLa cells), HepG2 hepatic carcinoma cells (HepG2 cells), and HEK293T human embryonic kidney 293 cells (293T cells) that were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (100 IU/ml) and incubated at 37°C with 5% CO2. All cell lines were purchased from the American Type Culture Collection (ATCC).
Cell synchronization.
HepG2 cells and HeLa cells were synchronized in G0/G1 phase by depriving them of serum for 24 h. For S-phase synchronization, a final concentration of 0.85 mM thymidine was added to the growth medium and left for 24 h. For G2/M synchronization, 50 ng/ml of nocodazole was added to the growth medium and left for 24 h (58). The cells were then infected with SFTSV and maintained in complete medium for 48 h.
Cell cycle analysis by flow cytometry.
Cells were stained with propidium iodide (PI) to measure the nuclear DNA content, as described previously (23, 58). The cell cycle distribution was determined using the cell cycle analysis kit according to the manufacturer’s protocol. A BD FACSCalibur fully automatic multicolor analysis flow cytometry system (FACScan; BD) was used to analyze the PI-stained cells, and at least 15,000 cells were counted for each sample. ModFit LT, version 2.0 (Verity Software House), was used for the data analysis.
qRT-PCR of viral RNA.
For quantitative real-time PCR (qRT-PCR), cells synchronized in the G0/G1, S, or G2/M phase were infected with SFTSV (0.1 MOI) for 30 min, after which the medium was aspirated, and the cells were washed three times with DMEM to remove any unadsorbed SFTSV. The infected cells or supernatants were then collected to determine viral RNA levels. Alternatively, the SFTSV (0.1 MOI) was allowed to adsorb to the cells for 2 h, after which the cells were incubated in fresh serum-free medium, medium containing 0.85 mM thymidine, or medium containing 50 ng/ml nocodazole for another 48 h. Total RNA was extracted using QIAzol lysis reagent. RNA was reverse transcribed into cDNA using TransScript First-Strand cDNA Synthesis SuperMix (catalog no. AT301; Transgen Biotech). S-segment-specific primers were used to determine the quantities of viral mRNAs (59), which were normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA.
Coimmunoprecipitation and immunoblotting.
293T cells were transfected with various combinations of plasmids. After 48 h, the cells were lysed in lysis buffer, and the lysates were incubated with anti-HA affinity matrix or anti-Flag M2 affinity gel at 4°C for 6 h. After seven washes, the immunoprecipitates were boiled in SDS loading buffer and analyzed by Western blotting.
Immunofluorescence.
HeLa cells were transfected with NSs-HA plasmids, CDK1-Flag plasmids, or NSs deletion mutant plasmids. After 48 h, the cells were fixed with paraformaldehyde (4%) for 15 min, permeabilized with 0.5% Triton X-100 for 10 min, blocked with 1% bovine serum albumin (BSA) for 20 min, and then probed with specific antibodies. DAPI was used to counterstain with nuclei, and the cells were observed using a laser confocal scanning microscope system. Data were acquired and statistical analyses were performed using a Lionhear FX automated microscope and Gen5 software from BioTek Instruments, Inc. (Winooski, VT, USA).
Cytoplasmic and nuclear separation experiments.
293T cells were transfected with a plasmid encoding NSs-HA or with empty vector. After 48 h, the cells were collected and suspended in 100 μl cytoplasmic lysate (containing 1:1,000 protease inhibitor cocktail and 1:2,000 dithiothreitol [DTT]). The cells were allowed to lyse on ice for 20 min and then subjected to shearing by aspirating and expelling the solution 200 times with a 200-μl pipette to lyse any remaining unbroken cells. Next, the mixture was centrifuged at 4°C and 8,000 × g for 20 min, and the supernatant (the cytoplasmic lysate) was removed for later investigation. The precipitate was washed five times with phosphate-buffered saline (PBS), and then 50 μl nuclear lysis buffer and 4× SDS loading buffer were added. The samples were detected and analyzed by Western blotting.
Assessment of cyclin B1-CDK1 kinase expression levels.
HeLa cells (5 × 105) were infected with SFTSV (10 MOI) or transfected with a plasmid encoding NSs-HA (1 μg) and collected at 48 h. The cells were then collected by centrifugation, freeze-thawed three times, resuspended in 80 μl PBS, sonicated for 2 min, and centrifuged for 10 min at 12,000 rpm. The supernatants were analyzed using the human CDK1/cyclin B kinase expression level detection kit (Meike, catalog no. MK4058A) according to the manufacturer’s instructions.
Statistical analysis.
All results were analyzed with GraphPad Prism and are presented as means ± standard deviations (SD). Statistical significance was determined using the two-tailed Student unpaired t test (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Data availability.
All data are fully available without restriction.
ACKNOWLEDGMENTS
We thank Dexin Li for providing critical reagents. We thank LetPub (Waltham, MA) for linguistic assistance during the preparation of the manuscript.
This work was supported by the National Key Research and Development Program of China (2017YFA0205102), the National Science Foundation of China (31270201), the National Science Foundation of Tianjin (16JCQNJC09800) and the Natural Science Foundation of Tianjin City (18JCZDJC34500).
Conceptualization, Z.W. and T.W.; Data Curation, L.X. and K.Z.; Formal Analysis: S.L., J.K., and X.L.; Funding Acquisition, J.K., W.H., and T.W.; Investigation, S.L. and H.L.; Methodology, S.L., H.L., and L.X.; Project Administration, Z.W. and T.W.; Resources, X. L. and T.W.; Supervision, Z.W. and T.W.; Visualization, S.L., H.L., and K.Z.; Writing – Original Draft, S.L.; Writing – Review & Editing: Z.W. and T.W.
All authors declare that no competing interests exist.
REFERENCES
- 1.Yun Y, Heo ST, Kim G, Hewson R, Kim H, Park D, Cho NH, Oh WS, Ryu SY, Kwon KT, Medlock JM, Lee KH. 2015. Phylogenetic analysis of severe fever with thrombocytopenia syndrome virus in South Korea, and migratory bird routes between China, South Korea, and Japan. Am J Trop Med Hyg 93:468–474. doi: 10.4269/ajtmh.15-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gai ZT, Zhang Y, Liang MF, Jin C, Zhang S, Zhu CB, Li C, Li XY, Zhang QF, Bian PF, Zhang LH, Wang B, Zhou N, Liu JX, Song XG, Xu A, Bi ZQ, Chen SJ, Li DX. 2012. Clinical progress and risk factors for death in severe fever with thrombocytopenia syndrome patients. J Infect Dis 206:1095–1102. doi: 10.1093/infdis/jis472. [DOI] [PubMed] [Google Scholar]
- 3.Zhan J, Wang Q, Cheng J, Hu B, Li J, Zhan F, Song Y, Guo D. 2017. Current status of severe fever with thrombocytopenia syndrome in China. Virol Sin 32:51–62. doi: 10.1007/s12250-016-3931-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qi R, Qin XR, Wang L, Han HJ, Cui F, Yu H, Liu JW, Yu XJ. 2019. Severe fever with thrombocytopenia syndrome can masquerade as hemorrhagic fever with renal syndrome. PLoS Negl Trop Dis 13:e0007308. doi: 10.1371/journal.pntd.0007308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu B, Cai K, Liu M, Li W, Xu J, Qiu F, Zhan J. 2018. Laboratory detection and molecular phylogenetic analysis of severe fever with thrombocytopenia syndrome virus in Hubei Province, central China. Arch Virol 163:3243–3254. doi: 10.1007/s00705-018-3993-5. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi T, Maeda K, Suzuki T, Ishido A, Shigeoka T, Tominaga T, Kamei T, Honda M, Ninomiya D, Sakai T, Senba T, Kaneyuki S, Sakaguchi S, Satoh A, Hosokawa T, Kawabe Y, Kurihara S, Izumikawa K, Kohno S, Azuma T, Suemori K, Yasukawa M, Mizutani T, Omatsu T, Katayama Y, Miyahara M, Ijuin M, Doi K, Okuda M, Umeki K, Saito T, Fukushima K, Nakajima K, Yoshikawa T, Tani H, Fukushi S, Fukuma A, Ogata M, Shimojima M, Nakajima N, Nagata N, Katano H, Fukumoto H, Sato Y, Hasegawa H, Yamagishi T, Oishi K, Kurane I, Morikawa S, Saijo M. 2014. The first identification and retrospective study of severe fever with thrombocytopenia syndrome in Japan. J Infect Dis 209:816–827. doi: 10.1093/infdis/jit603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H, Zhang LK, Li SF, Zhang SF, Wan WW, Zhang YL, Xin QL, Dai K, Hu YY, Wang ZB, Zhu XT, Fang YJ, Cui N, Zhang PH, Yuan C, Lu QB, Bai JY, Deng F, Xiao GF, Liu W, Peng K. 2019. Calcium channel blockers reduce severe fever with thrombocytopenia syndrome virus (SFTSV) related fatality. Cell Res 29:739–753. doi: 10.1038/s41422-019-0214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim KH, Yi J, Kim G, Choi SJ, Jun KI, Kim NH, Choe PG, Kim NJ, Lee JK, Oh MD. 2013. Severe fever with thrombocytopenia syndrome, South Korea, 2012. Emerg Infect Dis 19:1892–1894. doi: 10.3201/eid1911.130792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwak JE, Kim YI, Park SJ, Yu MA, Kwon HI, Eo S, Kim TS, Seok J, Choi WS, Jeong JH, Lee H, Cho Y, Kwon JA, Jeong M, Maslow JN, Kim YE, Jeon H, Kim KK, Shin EC, Song MS, Jung JU, Choi YK, Park SH. 2019. Development of a SFTSV DNA vaccine that confers complete protection against lethal infection in ferrets. Nat Commun 10:3836. doi: 10.1038/s41467-019-11815-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tani H, Fukuma A, Fukushi S, Taniguchi S, Yoshikawa T, Iwata-Yoshikawa N, Sato Y, Suzuki T, Nagata N, Hasegawa H, Kawai Y, Uda A, Morikawa S, Shimojima M, Watanabe H, Saijo M, Tani H, Fukuma A, Fukushi S, Taniguchi S, Yoshikawa T, Iwata-Yoshikawa N, Sato Y, Suzuki T, Nagata N, Hasegawa H, Kawai Y, Uda A, Morikawa S, Shimojima M, Watanabe H, Saijo M. 2016. Efficacy of T-705 (Favipiravir) in the treatment of infections with lethal severe fever with thrombocytopenia syndrome virus. mSphere 1:e00061-15. doi: 10.1128/mSphere.00061-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong F, Li D, Wen D, Li S, Zhao C, Qi Y, Jangra RK, Wu C, Xia D, Zhang X, Deng F, Chandran K, Zou Z, Yuan F, Zheng A. 2019. Single dose of a rVSV-based vaccine elicits complete protection against severe fever with thrombocytopenia syndrome virus. NPJ Vaccines 4:5. doi: 10.1038/s41541-018-0096-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu S, Liu H, Zhang K, Li X, Duan Y, Wang Z, Wang T. 2019. Proteasome inhibitor PS-341 effectively blocks infection by the severe fever with thrombocytopenia syndrome virus. Virol Sin 34:572–582. doi: 10.1007/s12250-019-00162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abudurexiti A, Adkins S, Alioto D, Alkhovsky SV, Avsic-Zupanc T, Ballinger MJ, Bente DA, Beer M, Bergeron E, Blair CD, Briese T, Buchmeier MJ, Burt FJ, Calisher CH, Chang C, Charrel RN, Choi IR, Clegg JCS, de la Torre JC, de Lamballerie X, Deng F, Di Serio F, Digiaro M, Drebot MA, Duan X, Ebihara H, Elbeaino T, Ergunay K, Fulhorst CF, Garrison AR, Gao GF, Gonzalez JJ, Groschup MH, Gunther S, Haenni AL, Hall RA, Hepojoki J, Hewson R, Hu Z, Hughes HR, Jonson MG, Junglen S, Klempa B, Klingstrom J, Kou C, Laenen L, Lambert AJ, Langevin SA, Liu D, Lukashevich IS. 2019. Taxonomy of the order Bunyavirales: update 2019. Arch Virol 164:1949–1965. doi: 10.1007/s00705-019-04253-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He CQ, Ding NZ. 2012. Discovery of severe fever with thrombocytopenia syndrome bunyavirus strains originating from intragenic recombination. J Virol 86:12426–12430. doi: 10.1128/JVI.01317-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, Zhang L, Zhang QF, Popov VL, Li C, Qu J, Li Q, Zhang YP, Hai R, Wu W, Wang Q, Zhan FX, Wang XJ, Kan B, Wang SW, Wan KL, Jing HQ, Lu JX, Yin WW, Zhou H, Guan XH, Liu JF, Bi ZQ, Liu GH, Ren J, Wang H, Zhao Z, Song JD, He JR, Wan T, Zhang JS, Fu XP, Sun LN, Dong XP, Feng ZJ, Yang WZ, Hong T, Zhang Y, Walker DH, Wang Y, Li DX. 2011. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med 364:1523–1532. doi: 10.1056/NEJMoa1010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Y, Jin C, Zhan F, Wang X, Liang M, Zhang Q, Ding S, Guan X, Huo X, Li C, Qu J, Wang Q, Zhang S, Zhang Y, Wang S, Xu A, Bi Z, Li D. 2012. Host cytokine storm is associated with disease severity of severe fever with thrombocytopenia syndrome. J Infect Dis 206:1085–1094. doi: 10.1093/infdis/jis452. [DOI] [PubMed] [Google Scholar]
- 17.Lei XY, Liu MM, Yu XJ. 2015. Severe fever with thrombocytopenia syndrome and its pathogen SFTSV. Microbes Infect 17:149–154. doi: 10.1016/j.micinf.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Santiago FW, Covaleda LM, Sanchez-Aparicio MT, Silvas JA, Diaz-Vizarreta AC, Patel JR, Popov V, Yu X-J, García-Sastre A, Aguilar PV. 2014. Hijacking of RIG-I signaling proteins into virus-induced cytoplasmic structures correlates with the inhibition of type I interferon responses. J Virol 88:4572–4585. doi: 10.1128/JVI.03021-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rezelj VV, Li P, Chaudhary V, Elliott RM, Jin D-Y, Brennan B, Rezelj VV, Li P, Chaudhary V, Elliott RM, Jin D-Y, Brennan B. 2017. Differential antagonism of human innate immune responses by tick-borne phlebovirus nonstructural proteins. mSphere 2:e00234-17. doi: 10.1128/mSphere.00234-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brennan B, Rezelj VV, Elliott RM. 2017. Mapping of transcription termination within the S segment of SFTS phlebovirus facilitated generation of NSs deletant viruses. J Virol 91:e00743-17. doi: 10.1128/JVI.00743-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ning YJ, Feng K, Min YQ, Cao WC, Wang M, Deng F, Hu Z, Wang H. 2015. Disruption of type I interferon signaling by the nonstructural protein of severe fever with thrombocytopenia syndrome virus via the hijacking of STAT2 and STAT1 into inclusion bodies. J Virol 89:4227–4236. doi: 10.1128/JVI.00154-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaudhary V, Zhang S, Yuen KS, Li C, Lui PY, Fung SY, Wang PH, Chan CP, Li D, Kok KH, Liang M, Jin DY. 2015. Suppression of type I and type III IFN signalling by NSs protein of severe fever with thrombocytopenia syndrome virus through inhibition of STAT1 phosphorylation and activation. J Gen Virol 96:3204–3211. doi: 10.1099/jgv.0.000280. [DOI] [PubMed] [Google Scholar]
- 23.Baer A, Austin D, Narayanan A, Popova T, Kainulainen M, Bailey C, Kashanchi F, Weber F, Kehn-Hall K. 2012. Induction of DNA damage signaling upon Rift Valley fever virus infection results in cell cycle arrest and increased viral replication. J Biol Chem 287:7399–7410. doi: 10.1074/jbc.M111.296608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu P, Zhou Z, Xiong M, Zou W, Deng X, Ganaie SS, Kleiboeker S, Peng J, Liu K, Wang S, Ye SQ, Qiu J. 2017. Parvovirus B19 NS1 protein induces cell cycle arrest at G2-phase by activating the ATR-CDC25C-CDK1 pathway. PLoS Pathog 13:e1006266. doi: 10.1371/journal.ppat.1006266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin H, Qu J, Peng Q, Gan R. 2019. Molecular mechanisms of EBV-driven cell cycle progression and oncogenesis. Med Microbiol Immunol 208:573–583. doi: 10.1007/s00430-018-0570-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vermeulen K, Van Bockstaele DR, Berneman ZN. 2003. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif 36:131–149. doi: 10.1046/j.1365-2184.2003.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harper JV, Brooks G. 2005. The mammalian cell cycle: an overview. Methods Mol Biol 296:113–153. doi: 10.1385/1-59259-857-9:113. [DOI] [PubMed] [Google Scholar]
- 28.Lim S, Kaldis P. 2013. Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development 140:3079–3093. doi: 10.1242/dev.091744. [DOI] [PubMed] [Google Scholar]
- 29.Bagga S, Bouchard MJ. 2014. Cell cycle regulation during viral infection. Methods Mol Biol 1170:165–227. doi: 10.1007/978-1-4939-0888-2_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gearhart TL, Bouchard MJ. 2010. Replication of the hepatitis B virus requires a calcium-dependent HBx-induced G1 phase arrest of hepatocytes. Virology 407:14–25. doi: 10.1016/j.virol.2010.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davy C, Doorbar J. 2007. G2/M cell cycle arrest in the life cycle of viruses. Virology 368:219–226. doi: 10.1016/j.virol.2007.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dove B, Brooks G, Bicknell K, Wurm T, Hiscox JA. 2006. Cell cycle perturbations induced by infection with the coronavirus infectious bronchitis virus and their effect on virus replication. J Virol 80:4147–4156. doi: 10.1128/JVI.80.8.4147-4156.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feuer R, Mena I, Pagarigan R, Slifka MK, Whitton JL. 2002. Cell cycle status affects coxsackievirus replication, persistence, and reactivation in vitro. J Virol 76:4430–4440. doi: 10.1128/jvi.76.9.4430-4440.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bassini A, Pierpaoli S, Falcieri E, Vitale M, Guidotti L, Capitani S, Zauli G. 1999. Selective modulation of the cyclin B/CDK1 and cyclin D/CDK4 complexes during in vitro human megakaryocyte development. Br J Haematol 104:820–828. doi: 10.1046/j.1365-2141.1999.01264.x. [DOI] [PubMed] [Google Scholar]
- 35.Zhang S, Zheng B, Wang T, Li A, Wan J, Qu J, Li CH, Li D, Liang M. 2017. NSs protein of severe fever with thrombocytopenia syndrome virus suppresses interferon production through different mechanism than Rift Valley fever virus. Acta Virol 61:289–298. doi: 10.4149/av_2017_307. [DOI] [PubMed] [Google Scholar]
- 36.Moriyama M, Igarashi M, Koshiba T, Irie T, Takada A, Ichinohe T, Moriyama M, Igarashi M, Koshiba T, Irie T, Takada A, Ichinohe T. 2018. Two conserved amino acids within the NSs of severe fever with thrombocytopenia syndrome phlebovirus are essential for anti-interferon activity. J Virol 92:e00706-18. doi: 10.1128/JVI.00706-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stewart M. 2007. Molecular mechanism of the nuclear protein import cycle. Nat Rev Mol Cell Biol 8:195–208. doi: 10.1038/nrm2114. [DOI] [PubMed] [Google Scholar]
- 38.Shi Y, Guo S, Wang Y, Liu X, Li Q, Li T. 2018. Lamprey Prohibitin2 arrest G2/M phase transition of HeLa Cells through down-regulating expression and phosphorylation level of cell cycle proteins. Sci Rep 8:3932. doi: 10.1038/s41598-018-22212-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swanton C, Jones N. 2001. Strategies in subversion: de-regulation of the mammalian cell cycle by viral gene products. Int J Exp Pathol 82:3–13. doi: 10.1046/j.1365-2613.2001.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang Z, Liu R, Lin Y, Liang C, Tan J, Qiao W. 2015. HIV-1 Vpr protein activates the NF-kappaB pathway to promote G2/M cell cycle arrest. Virol Sin 30:441–448. doi: 10.1007/s12250-015-3654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berger G, Lawrence M, Hue S, Neil SJ. 2015. G2/M cell cycle arrest correlates with primate lentiviral Vpr interaction with the SLX4 complex. J Virol 89:230–240. doi: 10.1128/JVI.02307-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bressy C, Droby GN, Maldonado BD, Steuerwald N, Grdzelishvili VZ. 2019. Cell cycle arrest in G2/M phase enhances replication of interferon-sensitive cytoplasmic RNA viruses via inhibition of antiviral gene expression. J Virol 93:e01885-18. doi: 10.1128/JVI.01885-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song L, Han X, Jia C, Zhang X, Jiao Y, Du T, Xiao S, Hiscox JA, Zhou EM, Mu Y. 2018. Porcine reproductive and respiratory syndrome virus inhibits MARC-145 proliferation via inducing apoptosis and G2/M arrest by activation of Chk/Cdc25C and p53/p21 pathway. Virol J 15:169. doi: 10.1186/s12985-018-1081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lai M, Zimmerman ES, Planelles V, Chen J. 2005. Activation of the ATR pathway by human immunodeficiency virus type 1 Vpr involves its direct binding to chromatin in vivo. J Virol 79:15443–15451. doi: 10.1128/JVI.79.24.15443-15451.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terada Y, Yasuda Y. 2006. Human immunodeficiency virus type 1 Vpr induces G checkpoint activation by interacting with the splicing factor SAP145. Mol Cell Biol 26:8149–8158. doi: 10.1128/MCB.01170-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Darbinyan A, Darbinian N, Safak M, Radhakrishnan S, Giordano A, Khalili K. 2002. Evidence for dysregulation of cell cycle by human polyomavirus, JCV, late auxiliary protein. Oncogene 21:5574–5581. doi: 10.1038/sj.onc.1205744. [DOI] [PubMed] [Google Scholar]
- 47.Knight GL, Turnell AS, Roberts S. 2006. Role for Wee1 in inhibition of G2-to-M transition through the cooperation of distinct human papillomavirus type 1 E4 proteins. J Virol 80:7416–7426. doi: 10.1128/JVI.00196-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davy CE, Jackson DJ, Raj K, Peh WL, Southern SA, Das P, Sorathia R, Laskey P, Middleton K, Nakahara T, Wang Q, Masterson PJ, Lambert PF, Cuthill S, Millar JB, Doorbar J. 2005. Human papillomavirus type 16 E1 E4-induced G2 arrest is associated with cytoplasmic retention of active Cdk1/cyclin B1 complexes. J Virol 79:3998–4011. doi: 10.1128/JVI.79.7.3998-4011.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chiu HC, Huang WR, Liao TL, Wu HY, Munir M, Shih WL, Liu HJ. 2016. Suppression of vimentin phosphorylation by the avian reovirus p17 through inhibition of CDK1 and Plk1 impacting the G2/M phase of the cell cycle. PLoS One 11:e0162356. doi: 10.1371/journal.pone.0162356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu X, Qi X, Qu B, Zhang Z, Liang M, Li C, Cardona CJ, Li D, Xing Z. 2014. Evasion of antiviral immunity through sequestering of TBK1/IKKepsilon/IRF3 into viral inclusion bodies. J Virol 88:3067–3076. doi: 10.1128/JVI.03510-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qu B, Qi X, Wu X, Liang M, Li C, Cardona CJ, Xu W, Tang F, Li Z, Wu B, Powell K, Wegner M, Li D, Xing Z. 2012. Suppression of the interferon and NF-kappaB responses by severe fever with thrombocytopenia syndrome virus. J Virol 86:8388–8401. doi: 10.1128/JVI.00612-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ly HJ, Ikegami T. 2016. Rift Valley fever virus NSs protein functions and the similarity to other bunyavirus NSs proteins. Virol J 13:118. doi: 10.1186/s12985-016-0573-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Le May N, Dubaele S, Proietti De Santis L, Billecocq A, Bouloy M, Egly JM. 2004. TFIIH transcription factor, a target for the Rift Valley hemorrhagic fever virus. Cell 116:541–550. doi: 10.1016/s0092-8674(04)00132-1. [DOI] [PubMed] [Google Scholar]
- 54.Sun Q, Qi X, Zhang Y, Wu X, Liang M, Li C, Li D, Cardona CJ, Xing Z. 2016. Synaptogyrin-2 promotes replication of a novel tick-borne bunyavirus through interacting with viral nonstructural protein NSs. J Biol Chem 291:16138–16149. doi: 10.1074/jbc.M116.715599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ning YJ, Wang M, Deng M, Shen S, Liu W, Cao WC, Deng F, Wang YY, Hu Z, Wang H. 2014. Viral suppression of innate immunity via spatial isolation of TBK1/IKKepsilon from mitochondrial antiviral platform. J Mol Cell Biol 6:324–337. doi: 10.1093/jmcb/mju015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park ES, Shimojima M, Nagata N, Ami Y, Yoshikawa T, Iwata-Yoshikawa N, Fukushi S, Watanabe S, Kurosu T, Kataoka M, Okutani A, Kimura M, Imaoka K, Hanaki K, Suzuki T, Hasegawa H, Saijo M, Maeda K, Morikawa S. 2019. Severe fever with thrombocytopenia syndrome phlebovirus causes lethal viral hemorrhagic fever in cats. Sci Rep 9:11990. doi: 10.1038/s41598-019-48317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tercero B, Terasaki K, Nakagawa K, Narayanan K, Makino S. 2019. A strand-specific real-time quantitative RT-PCR assay for distinguishing the genomic and antigenomic RNAs of Rift Valley fever phlebovirus. J Virol Methods 272:113701. doi: 10.1016/j.jviromet.2019.113701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang ZY, Zhong T, Wang Y, Song FM, Yu XF, Xing LP, Zhang WY, Yu JH, Hua SC, Yu XF. 2017. Human enterovirus 68 interferes with the host cell cycle to facilitate viral production. Front Cell Infect Microbiol 7:29. doi: 10.3389/fcimb.2017.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun Y, Liang M, Qu J, Jin C, Zhang Q, Li J, Jiang X, Wang Q, Lu J, Gu W, Zhang S, Li C, Wang X, Zhan F, Yao W, Bi Z, Wang S, Li D. 2012. Early diagnosis of novel SFTS bunyavirus infection by quantitative real-time RT-PCR assay. J Clin Virol 53:48–53. doi: 10.1016/j.jcv.2011.09.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are fully available without restriction.