Abstract
BACKGROUND:
The U.S. experienced nearly 48,000 opioid overdose deaths in 2017. Treatment of opioid use disorder (OUD) with buprenorphine is a recommended part of primary care, yet little is known about current U.S. practices in this setting. This observational study reports the prevalence of documented OUD and OUD treatment with buprenorphine among primary care patients in six large health systems.
METHODS:
Adults with ≥2 primary care visits during a three-year period (10/1/2013–9/30/2016) in six health systems were included. Data were obtained from electronic health record and claims data, with measures, assessed over the three-year period, including indicators for documented OUD from ICD 9 and 10 codes and OUD treatment with buprenorphine. The prevalence of OUD treatment was adjusted for age, gender, race/ethnicity, and health system.
RESULTS:
Among 1,368,604 primary care patients, 13,942 (1.0%) had documented OUD, and among these, 21.0% had OUD treatment with buprenorphine. For those with documented OUD, the adjusted prevalence of OUD treatment with buprenorphine varied across demographic and clinical subgroups. OUD treatment was lower among patients who were older, women, Black/African American and Hispanic (compared to white), non-commercially insured, and those with non-cancer pain, mental health disorders, greater comorbidity, and more opioid prescriptions, emergency department visits or hospitalizations.
CONCLUSIONS:
Among primary care patients in six health systems, one in five with an OUD were treated with buprenorphine, with disparities across demographic and clinical characteristics. Less buprenorphine treatment among those with greater acute care utilization highlights an opportunity for systems-level changes to increase OUD treatment.
Keywords: opioid use disorder, primary care, treatment, buprenorphine, health services research
1.1. INTRODUCTION
The U.S. is in the midst of an opioid crisis, with nearly 48,000 opioid overdose deaths in 2017, and federal agencies have mobilized to prevent and treat opioid use disorders (OUDs).1–3 Evidence-based treatment of OUD includes medication treatment with methadone, buprenorphine or naltrexone.4,5 However, the large volume of individuals needing treatment for an OUD currently outstrips OUD treatment capacity, and improving access to OUD treatment, by addressing capacity and the multiple interrelated barriers (e.g., financial, regulatory, geographical, attitudinal), is a national priority.6,7 Methadone, an effective treatment for OUD, is available through federally-regulated opioid treatment programs (OTPs).6,8 Most OTPs operate near capacity, with varying regional availability, and serve a small fraction of patients who need treatment.6 Alternatively, buprenorphine, the first-line recommendation for most patients,5,9 or extended-release naltrexone10 can be provided in general medical settings, like primary care, as well as specialty addiction treatment settings. Buprenorphine can be prescribed by physicians, and as of 2018, by other prescribers, who complete required training and obtain a waiver through the Drug Enforcement Administration (DEA), but additional barriers to prescribing remain.11 Extended-release naltrexone, as effective as buprenorphine once initiated, does not require a waiver to prescribe.10 Due to the significant gap between the need for and availability of medication treatment for OUD, increasing OUD treatment in primary care is widely recommended.12–15
Despite these recommendations, the prevalence of recognized OUD and medication treatment of OUD among diverse primary care patients in the U.S. is unknown. Prior studies in primary care studied recruited samples using diagnostic interviews to assess OUD or were restricted to specific patient subgroups (e.g. chronic pain).16–18 Other studies described the prevalence of OUD and OUD treatment, in general and insured populations, including patients covered by Medicare, commercial insurance and the Veterans Administration (VA).9,19–26 Nonetheless, evident gaps in care remain and population-based prevalence estimates of OUD and OUD treatment among primary care patients are needed to bridge these gaps.
The present study is a secondary analysis of electronic health record (EHR) and claims data collected for Phase 1 of the Primary Care Opioid Use Disorders (PROUD) pragmatic implementation trial of collaborative care utilizing nurse care management of patients with OUD within the National Institutes on Drug Abuse Clinical Trials Network. The objective is to describe the prevalence and clinical characteristics of primary care patients with a documented OUD and the prevalence of buprenorphine treatment among primary care patients with an OUD, overall and across patient subgroups.
1.2. MATERIAL AND METHODS
1.2.1. Setting and Population
This cross-sectional, three-year, observational study of patients seen in primary care relied on EHR and claims data from health systems included in PROUD Phase 1, a pre-randomization pilot study to assess the feasibility of cohort identification, data collection, and health system participation required for inclusion in the trial (Phase 2). Six of eleven health systems invited into Phase 1 provided data necessary for the present study. These health systems, Kaiser Permanente (KP) Washington, KP Northwest, KP Northern California, KP Colorado, Health Partners, and Multicare, in six states (Minnesota, Wisconsin, Colorado, California, Oregon, and Washington), included five that were integrated health insurance and care delivery systems, with access to claims for care received outside the system, and another that was a fee-for-service community health care system serving a relatively rural population. All health systems use Epic® EHR and provided EHR data.
Each health system collected data on patients seen in primary care clinics selected by the site investigator, with four health systems including all primary care clinics, one providing information for five large clinics (e.g., ≥ 20,000 patients per clinic; range: 5–25 clinics per health system) and another for most clinics not in close proximity to specialty addiction treatment services. Data elements included patient demographics, diagnoses, procedures, utilization from all settings (e.g., outpatient, inpatient, urgent care, and emergency department), as well as pharmacy orders and/or dispensing and procedure codes (henceforth referred to as prescriptions). Diagnoses were based on International Classification of Disease-9th edition (ICD-9-CM; until September 30, 2015) or ICD-10-CM diagnostic codes (starting October 1, 2015).
Patients were included if they were ≥ 18 years old during the first study year, with at least 2 visits to any primary care clinic within a system during a 3-year period (Oct 1, 2013 – September 30, 2016). This study and waivers of consent and HIPAA authorization were approved by Kaiser Permanente Washington (KPW) Institutional Review Board (IRB), with each of five additional health system IRBs ceding to KPW IRB.
1.2.2. Measures
1.2.2.1. Documented OUD
Patients were classified as having a documented OUD diagnosis if they had one or more health care encounter anywhere within the system during the 3-year period with an ICD-9/10 code for OUD—active or in remission—based on EHR and/or claims data. Remission codes were included as providers could differ in OUD diagnosis coding (e.g., active vs. remission) when patients received opioid agonists for OUD treatment. A small proportion of patients with no documented ICD code for OUD but a prescription for buprenorphine used for OUD treatment (defined below) were also coded as having a documented OUD. An indicator for OUD in remission included patients with only remission ICD codes documented during the study period.
1.2.2.2. OUD Treatment
OUD treatment was defined as any documented prescription of FDA-approved buprenorphine for OUD, including oral or implanted, with or without naloxone, at any time during the 3-year period. Buprenorphine transdermal patches approved for pain were excluded. As a secondary treatment outcome, extended-release naltrexone based on procedure code, pharmacy order or dispensing was considered OUD treatment with naltrexone if the patient had a documented OUD. Oral naltrexone was not included in the definition of evidence-based OUD treatment due to low efficacy for OUD and more prevalent use for alcohol use disorders.27 Methadone treatment for OUD from OPTs is not included in analyses because data were not available for all health systems for the entire 3 year study period; the one year prevalence in 2016 was available for five systems and ranged 0%−11.4%.
1.2.2.3. Demographic and Clinical Characteristics
Gender, race/ethnicity, type of health insurance (e.g, Medicare, commercial/private, state subsidized, including Medicaid and uninsured) and Charlson Comorbidity index for burden of medical comorbidity28 were determined at the time of study entry (i.e., initial visit to a study clinic during the study). ICD 9/10 codes documented anytime during the 3-year period were used to create an indicator for any non-cancer pain, developed based on the National Pain Strategy29,30 as well as indicators for any mental health disorder diagnosis (e.g., depression, anxiety, serious mental illness, other disorders, including attention-deficit and eating disorders), any substance use disorder diagnosis, including indicators for tobacco, alcohol and other substance (e.g., cannabis, stimulants and other drugs) and opioid overdose. Counts for opioid prescriptions (e.g., orders or dispensing records, not including buprenorphine for OUD treatment) were categorized as 0, 1, 2–3, 4–6, 7–10 and > 10, while number of hospitalizations and emergency department visits (including urgent care) were categorized as 0, 1, 2 and ≥ 3 for encounters anytime during the 3-year study period.
1.2.3. Analyses
Analyses described the characteristics of the sample, stratified by documented OUD at any time during the 3-year study, with chi-square tests of independence used to test for differences between those with and without documented OUD. The unadjusted and adjusted prevalence of OUD treatment among patients with documented OUD was estimated overall and within patient demographic and clinical subgroups. Logistic regression, adjusted for age, gender, race/ethnicity and health system, was used to estimate the prevalence of OUD treatment with buprenorphine, with 95% confidence intervals, for each subgroup of patients with OUD (e.g., women vs. men). Post-hoc sensitivity analyses evaluated removal of patients with less than 90-days between OUD diagnosis and study end to allow for treatment capture, a requirement for ≥2 documented OUD diagnoses, rather than ≥1, used by other studies for OUD confirmation, and additional adjustment for insurance status to assess for potential confounding between OUD treatment prevalence and race/ethnicity. Results are presented as the average adjusted prevalence of OUD treatment for each subgroup, with significant differences in prevalence across subgroups estimated using post-estimation Wald tests.31
1.3. RESULTS
The study sample included 1,368,604 primary care patients aged 18 and older. Overall, the sample was primarily white and female, with commercial or employer-based health insurance (Table 1).
Table 1.
Characteristics of primary care patients with at least 2 visits to primary care during the 3-year period (Oct 1, 2013-Sept 30, 2016)
| Documented OUD | No Documented OUD | ||||
|---|---|---|---|---|---|
| (13,942) | (1,354,662) | ||||
| N | % | N | % | p-value | |
| Age | <0.001 | ||||
| 18–25 | 2,131 | (15.3) | 138,843 | (10.2) | |
| 26–35 | 2,891 | (20.7) | 214,663 | (15.8) | |
| 36–45 | 2,367 | (17.0) | 217,526 | (16.1) | |
| 46–55 | 2,758 | (19.8) | 252,535 | (18.6) | |
| 56–65 | 2,362 | (16.9) | 265,157 | (19.6) | |
| 66–75 | 932 | (6.7) | 163,385 | (12.1) | |
| >75 | 501 | (3.6) | 102,553 | (7.6) | |
| Gender | <0.001 | ||||
| Female | 7,404 | (53.1) | 770,231 | (56.9) | |
| Male | 6,537 | (46.9) | 584,373 | (43.1) | |
| Race/Ethnicity | <0.001 | ||||
| Hispanic | 859 | (6.2) | 128,123 | (9.5) | |
| White | 10,966 | (78.7) | 929,064 | (68.6) | |
| Black/African American | 926 | (6.6) | 80,064 | (5.9) | |
| Asian | 170 | (1.2) | 135,606 | (10.0) | |
| Native American/Alaska Native | 149 | (1.1) | 5,338 | (0.4) | |
| Hawaiian/Pacific Islander | 40 | (0.3) | 6,413 | (0.5) | |
| Multiracial | 456 | (3.3) | 24,696 | (1.8) | |
| Other | 100 | (0.7) | 10,232 | (0.8) | |
| Unknown | 276 | (2.0) | 35,126 | (2.6) | |
| Insurance type* | <0.001 | ||||
| Medicare | 2,406 | (22.9) | 237,094 | (25.1) | |
| Commercial or private | 6,128 | (58.3) | 642,976 | (68.0) | |
| State subsidized | 1,674 | (15.9) | 46,273 | (4.9) | |
| Uninsured | 308 | (2.9) | 18,836 | (2.0) | |
| Charlson co-morbidity index score | <0.001 | ||||
| 0 | 12,830 | (92.0) | 1,297,528 | (95.8) | |
| 1 | 213 | (1.5) | 13,785 | (1.0) | |
| 2+ | 899 | (6.4) | 43,349 | (3.2) | |
| Any non-cancer pain diagnosis | 12,420 | (89.1) | 1,019,994 | (75.3) | <0.001 |
| Any mental health disorder diagnosis | 11,225 | (80.5) | 462,151 | (34.1) | <0.001 |
| Tobacco use disorder | 8,395 | (60.2) | 214,561 | (15.8) | <0.001 |
| Alcohol use disorder | 3,965 | (28.4) | 47,711 | (3.5) | <0.001 |
| Other SUD disorder diagnosis | 7,346 | (52.7) | 27,103 | (2.0) | <0.001 |
| Cannabis use disorder | 2,307 | (16.5) | 13,314 | (1.0) | <0.001 |
| Stimulant disorder | 2,520 | (18.1) | 6,538 | (0.5) | <0.001 |
| Other drug use disorders | 6,157 | (44.2) | 12,939 | (1.0) | <0.001 |
| Opioid overdose | 511 | (3.7) | 622 | (0.0) | <0.001 |
| Opioid prescriptions (count) | <0.001 | ||||
| 0 | 3,886 | (27.9) | 809,527 | (59.8) | |
| 1 | 1,364 | (9.8) | 240,520 | (17.8) | |
| 2–3 | 1,423 | (10.2) | 148,952 | (11.0) | |
| 4–6 | 1,041 | (7.5) | 60,445 | (4.5) | |
| 7–10 | 827 | (5.9) | 28,405 | (2.1) | |
| >10 | 5,401 | (38.7) | 66,813 | (4.9) | |
| Emergency department visits* | <0.001 | ||||
| 0 | 3,645 | (34.7) | 637,823 | (67.5) | |
| 1 | 1,789 | (17.0) | 167,711 | (17.7) | |
| 2 | 1,210 | (11.5) | 64,724 | (6.8) | |
| 3+ | 3,872 | (36.8) | 74,921 | (7.9) | |
| Hospitalizations | <0.001 | ||||
| 0 | 8,848 | (63.5) | 1,165,973 | (86.1) | |
| 1 | 2,209 | (15.8) | 115,276 | (8.5) | |
| 2 | 1,083 | (7.8) | 39,766 | (2.9) | |
| 3 | 1,802 | (12.9) | 33,647 | (2.5) | |
One health system excluded from this variable for lack of data; OUD=opioid use disorder, Other SUD=other substance use disorder diagnoses, not including alcohol, opioid or tobacco use disorders; p-value obtained from chi-square tests of independence for differences between patients with and without documented OUD across patient characteristics
A total of 13,942 (1.0%) had a documented OUD during the 3-year study period (n=13,787 with an OUD diagnosis and 155 prescribed buprenorphine without an OUD diagnosis). Among the patients with an OUD diagnosis code, 92.7% had at least one active OUD diagnosis, and 26.2% had at least one remission diagnosis, with 7.3% having an OUD in remission diagnosis only.
Compared to patients without a documented OUD, those with documented OUD were more likely to be younger (p <0.001), white (p <0.001), male (p<0.001) and had a higher prevalence of state subsidized insurance (p <0.001; Table 1). Patients with documented OUD compared to patients without OUD had a higher prevalence of all other measured diagnoses (Table 1) (i.e., mental health and substance use disorder diagnoses, opioid-related overdose, non-cancer pain, and medical comorbidity), as well as a higher prevalence of opioid prescriptions, emergency department visits and hospitalizations.
Among patients with a documented OUD, the unadjusted prevalence of buprenorphine treatment was 21.0% (95% CI 20.3%−21.7%), and the unadjusted prevalence of naltrexone was 0.9% (0.8–1.1%). Post-hoc sensitivity analyses to remove patients with less than 90 days between diagnosis and study end did not meaningfully change OUD treatment prevalence. Additionally, among patients with ≥2 documented OUD diagnoses (n=9,461) rather than ≥1, OUD treatment prevalence was 28.3% (27.4%−29.3%) and 3.7% (3.3–4.1%) for buprenorphine and naltrexone, respectively (Supplements 1 and 2).
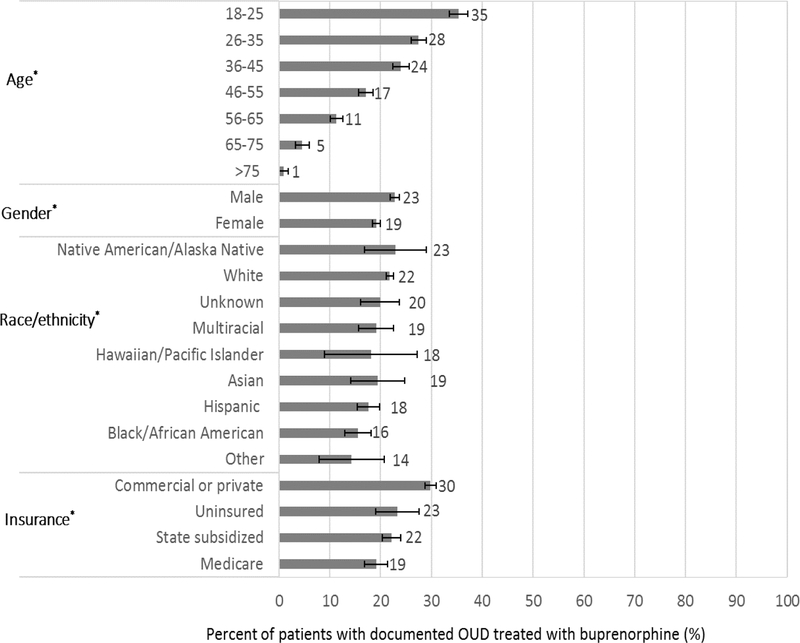
The adjusted prevalence of OUD treatment with buprenorphine among patients with documented OUD differed across all measured patient demographic characteristics (p-values <0.001; Figure 1). The adjusted prevalence of OUD treatment was highest among patients 18–25 years old, 35.3% (33.5–37.1%) and lowest among patients older than 75, 0.9% (0.0–1.8%). Men were more likely than women to have documented OUD treatment, while commercially insured patients were more likely than patients with other insurance to have documented OUD treatment (Figure 1). The estimated prevalence of OUD treatment varied across racial and ethnic groups and although confidence intervals overlapped for most, estimates for Black/African American (15.5% [95% CI 12.9–18.0%]) Hispanic, (17.6% [15.4–19.9%]), and other race/ethnicity patients were lower than for white patients, 21.8% (95% CI 21.1–22.5%; Table 2). In post-hoc sensitivity analyses that included insurance as an additional adjustment covariate for the five health systems that provided insurance data,32 these differences across race/ethnicity remained (Supplement 3).
FIGURE 1.
Prevalence of OUD treatment with buprenorphine across demographic subgroups of patients with documented OUD, adjusted for age, gender, race/ethnicity and health system; Error bars represent 95% confidence intervals; *p-value <0.001 for difference in OUD treatment within a subgroup; OUD=opioid use disorder
Table 2.
Unadjusted and adjusted prevalence of OUD treatment with buprenorphine across subgroups in patients with documented OUD (n=13,942) seen in primary care clinics during the 3-year period (Oct 1, 2013-Sept 30, 2016)
| N | Unadjusted % | adjustedǂ % | 95% CI (adjusted) | p-value | ||
|---|---|---|---|---|---|---|
| Age | ||||||
| 18–25 | 2,131 | 37.0 | 35.3 | (33.5- | 37.1) | <0.0001 |
| 26–35 | 2,891 | 27.7 | 27.5 | (26.0- | 29.0) | |
| 36–45 | 2,367 | 24.0 | 24.0 | (22.4- | 25.6) | |
| 46–55 | 2,758 | 16.7 | 17.1 | (15.7- | 18.4) | |
| 56–65 | 2,362 | 11.2 | 11.3 | (10.1- | 12.5) | |
| 66–75 | 932 | 4.3 | 4.6 | (3.2- | 5.9) | |
| >75 | 501 | 0.8 | 0.9 | (0.0- | 1.8) | |
| Gender | ||||||
| Female | 7,404 | 18.1 | 19.2 | (18.4- | 20.1) | <0.0001 |
| Male | 6,537 | 24.2 | 22.8 | (21.9- | 23.7) | |
| Race | ||||||
| Hispanic | 859 | 20.6 | 17.6 | (15.4- | 19.9) | <0.0001 |
| White | 10,966 | 22.0 | 21.8 | (21.1- | 22.5) | |
| Black/African American | 926 | 10.4 | 15.5 | (12.9- | 18.0) | |
| Asian | 170 | 20.6 | 19.4 | (14.1- | 24.6) | |
| Native American/Alaska Native | 149 | 21.5 | 22.9 | (16.8- | 29.0) | |
| Hawaiian/Pacific Islander | 40 | 27.5 | 18.1 | (9.1- | 27.2) | |
| Multiracial | 456 | 16.9 | 19.1 | (15.6- | 22.5) | |
| Other | 100 | 14.0 | 14.3 | (8.0- | 20.7) | |
| Unknown | 276 | 25.7 | 19.9 | (16.1- | 23.7) | |
| Insurance type | ||||||
| Medicare | 2,406 | 8.7 | 19.1 | (16.8- | 21.5) | <0.0001 |
| Commercial or private | 6,128 | 34.1 | 29.8 | (28.7- | 30.8) | |
| State subsidized | 1,674 | 26.3 | 22.1 | (20.2- | 23.9) | |
| Uninsured | 308 | 24.4 | 23.3 | (18.9- | 27.6) | |
| Charlson co-morbidity index | ||||||
| 0 | 12,830 | 22.3 | 21.5 | (20.9- | 22.2) | <0.0001 |
| 1 | 213 | 6.6 | 15.6 | (8.9- | 22.2) | |
| 2+ | 899 | 5.8 | 10.1 | (7.7- | 12.5) | |
| Any non-cancer pain diagnosis | ||||||
| Not Present | 1,522 | 40.9 | 28.8 | (26.9- | 30.7) | <0.0001 |
| Present | 12,420 | 18.5 | 19.7 | (19.0- | 20.4) | |
| Any mental health disorder diagnosis | ||||||
| Not Present | 2,717 | 26.1 | 23.2 | (21.8- | 24.6) | 0.0005 |
| Present | 11,225 | 19.8 | 20.4 | (19.7- | 21.1) | |
| Tobacco use disorder | ||||||
| Not Present | 5,547 | 18.2 | 21.1 | (20.0- | 22.1) | 0.8493 |
| Present | 8,395 | 22.8 | 20.9 | (20.2- | 21.7) | |
| Alcohol use disorder | ||||||
| Not Present | 9,977 | 20.6 | 21.2 | (20.5- | 21.9) | 0.3163 |
| Present | 3,965 | 22.0 | 20.5 | (19.4- | 21.6) | |
| Other SUD disorder diagnosis | ||||||
| Not Present | 6,596 | 17.8 | 19.8 | (18.9- | 20.7) | 0.0014 |
| Present | 7,346 | 23.9 | 21.9 | (21.0- | 22.7) | |
| Cannabis | ||||||
| Not Present | 11,635 | 19.7 | 21.1 | (20.4- | 21.8) | 0.462 |
| Present | 2,307 | 27.7 | 20.5 | (19.1- | 21.9) | |
| Stimulants | ||||||
| Not Present | 11,422 | 19.3 | 20.6 | (20.0- | 21.3) | 0.0531 |
| Present | 2,520 | 28.5 | 22.2 | (20.8- | 23.6) | |
| Other drug use disorders | ||||||
| Not Present | 7,785 | 19.0 | 19.6 | (18.8- | 20.5) | <0.0001 |
| Present | 6,157 | 23.4 | 22.6 | (21.6- | 23.5) | |
| Documented OUD diagnosis type | <0.0001 | |||||
| Active at least once during study | 12,773 | 21.0 | 21.1 | (20.5- | 10.0) | |
| Remission only | 1,014 | 9.3 | 8.5 | (6.9- | 21.8) | |
| Any opioid use | ||||||
| Not Present | 3,886 | 32.6 | 28.8 | (27.5- | 30.1) | <0.0001 |
| Present | 10,056 | 16.5 | 17.6 | (16.9- | 18.3) | |
| Opioid prescriptions (count) | ||||||
| 0 | 3,886 | 32.6 | 29.8 | (28.4- | 31.1) | <0.0001 |
| 1 | 1,364 | 28.4 | 25.8 | (23.7- | 27.8) | |
| 2–3 | 1,423 | 25.2 | 23.5 | (21.5- | 25.5) | |
| 4–6 | 1,041 | 24.0 | 23.4 | (21.1- | 25.7) | |
| 7–10 | 827 | 16.9 | 17.7 | (15.3- | 20.2) | |
| >10 | 5,401 | 9.7 | 11.5 | (10.6- | 12.4) | |
| Opioid overdose | ||||||
| Not Present | 13,431 | 20.8 | 20.9 | (20.3- | 21.6) | 0.5892 |
| Present | 511 | 24.9 | 21.8 | (18.7- | 25.0) | |
| Emergency department visits* | ||||||
| 0 | 3,645 | 32.5 | 32.1 | (30.6- | 33.5) | <0.0001 |
| 1 | 1,789 | 30.3 | 29.0 | (27.1- | 31.0) | |
| 2 | 1,210 | 26.6 | 25.7 | (23.4- | 28.0) | |
| 3+ | 3,872 | 19.8 | 20.7 | (19.4- | 21.9) | |
| Hospitalizations | ||||||
| 0 | 8,848 | 24.2 | 23.4 | (22.6- | 24.2) | <0.0001 |
| 1 | 2,209 | 19.2 | 18.3 | (16.9- | 19.7) | |
| 2 | 1,083 | 15.0 | 16.1 | (14.0- | 18.2) | |
| 3+ | 1,802 | 11.0 | 14.1 | (12.4- | 15.8) | |
Excludes one health system; OUD=opioid use disorder, Other SUD=other substance use disorder diagnoses, not including alcohol, opioid or tobacco use disorders;
Adjusted for age, gender, race/ethnicity and health system
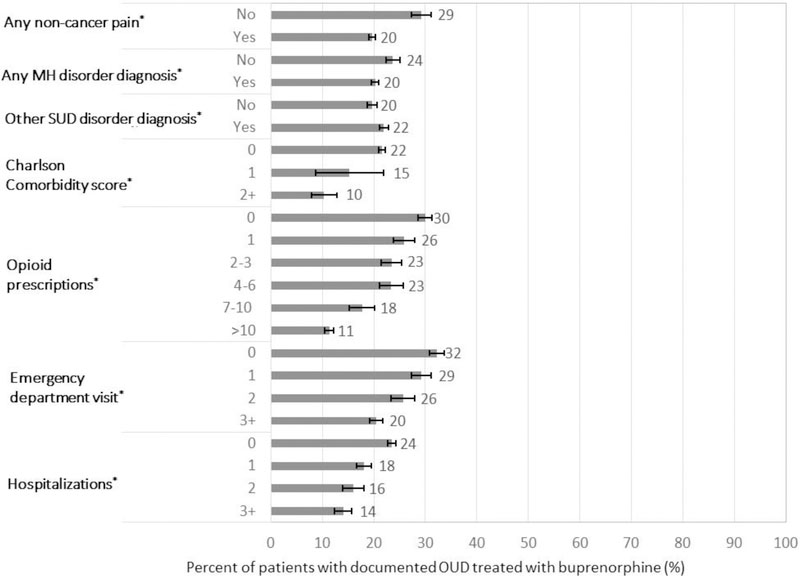
Among patients with documented OUD, the adjusted prevalence of OUD treatment with buprenorphine also varied based on mental health, substance use disorder and medical comorbidity (Figure 2). Although the prevalence of OUD treatment with buprenorphine was not associated with documented opioid overdose or tobacco, cannabis or alcohol use disorders (Table 2), the prevalence of documented OUD treatment was higher among patients with other SUDs compared to those without, but lower for patients with non-cancer pain and mental health diagnoses, compared to those without (p-values <0.001; Figure 2). The prevalence of treatment also decreased as Charlson comorbidity scores increased (p <0.001).
FIGURE 2.
Prevalence of OUD treatment with buprenorphine across clinical subgroups of patients with documented OUD, adjusted for age, gender, race/ethnicity and health system; Error bars represent 95% confidence intervals; *p-value <0.001 for difference in OUD treatment within a subgroup; MH=mental health, OUD=opioid use disorder, Other SUD=other substance use disorder diagnoses, not including alcohol, opioid or tobacco use disorders
Among patients with OUD, the adjusted prevalence of OUD treatment with buprenorphine was lower among patients with any opioid prescription (17.6% [16.9–18.3%]; p<0.001) compared to those without prescriptions (28.8% [27.5–30.1%]; p<0.001; Table 2). Moreover, the prevalence of OUD treatment decreased as the number of opioid prescriptions increased, from 25.8% (95% CI 23.7–27.8%) among those with 1 opioid prescription to 11.5% (10.6–12.4%) among those with more than 10 opioid prescriptions (p<0.001; Figure 2).
The adjusted prevalence of OUD treatment with buprenorphine was also lower among patients with documented OUD who visited emergency departments or were hospitalized. The prevalence of OUD treatment decreased as the numbers of visits to emergency departments and hospitalizations increased (p-values <0.001; Figure 2).
1.4. DISCUSSION
Despite widespread agreement that patients with OUD can be treated in primary care,12–15 this study reveals low prevalence rates of OUD treatment with buprenorphine and naltrexone in a large, geographically-diverse, real-world sample of primary care patients with OUD documented in their EHRs. This 3-year cross-sectional study of largely insured primary care patients from six health systems in Minnesota, Wisconsin, Colorado, Washington, Oregon, and California found that approximately 1% of patients had a documented OUD, yet one-in-five of those had been treated with buprenorphine during that time. Further, the prevalence of OUD treatment in primary care patients with OUD varied across demographic subgroups. Those who were older, Black/African American, Hispanic, and not commercially insured were less likely to have documented OUD treatment with buprenorphine. Moreover, the greater patients’ medical and mental health comorbidity—including non-cancer pain and opioid prescriptions for pain—the less likely they were to receive OUD treatment with buprenorphine. Primary care patients with OUD who had greater comorbidity scores and more visits to emergency departments or hospitalizations were least likely to have OUD treatment with buprenorphine documented.
The prevalence of documented OUD in this study is higher than that among VA patients (0.79% in 2010), Medicare members (0.30%−0.65%) based on 2008–2010 health insurance data, and commercially insured enrollees aged 12 and older (0.30% in 2015).20,25,26 The prevalence of documented OUD in this study is more than 2-fold greater than the 0.48% in a prior study by Morgan et al. of commercially-insured patients in 2014.9 This difference could reflect the 3-year prevalence estimates reported here, evaluation of data contemporaneous with increasing provider awareness and recognition of OUD, restriction to primary care patients, five of six sites being integrated health care delivery systems – many with internal addiction treatment programs, or that this study included primary care patients 18 years and older compared to insured individuals 12 years and older in Morgan et al. Yet, the population-based primary care OUD prevalence found in this study (1%) contrasts with a 2016 study of predominantly unemployed/disabled patients from five urban primary care clinics across four states that observed prevalence rates of OUD of 3.4% for heroin and 2.9% for prescription opioid use based on recruited-patient interviews.16 Prior studies restricted to primary care patients with non-cancer pain had a higher prevalence of OUD (4%−26%) than the prevalence of documented OUDs observed in the present study (1.2%), likely due to differences in samples and/or measures (e.g., self-report vs. diagnoses in EHR).17,18 Thus, the documented prevalence of OUD found here is likely an underestimate of the true prevalence.
In prior studies of patients with OUD, the prevalence of OUD treatment with buprenorphine, while low, varied widely depending on health setting or insurance coverage. The prevalence of OUD treatment with buprenorphine among VA patients with OUD was 14.1% in 2010, comparable to the 2016 prevalence of OUD medication treatment reported by Morgan et al.9,25 A 2013 study found 27% of Medicare9 and approximately 39% of privately insured patients with OUD received any medication treatment sometime between 2006–2011, although the latter prevalence is likely an overestimate due to inclusion of other medications that treat alcohol use disorders (i.e., disulfiram, acamprosate).24 The present study captured some but likely not all buprenorphine treatment for patients who paid out-of-pocket or received buprenorphine through OTPs or other addiction treatment programs outside the capture of the health system.6,33
The difference in OUD treatment across racial and ethnic groups among patients with documented OUD in this study is consistent with previous research.32,34 Black/African American, Hispanic and other nonwhite race/ethnicity patients with OUD were less likely to have documentation of OUD treatment compared to white patients, even after sensitivity analyses accounted for insurance status. Due to multiple factors, Black/African American and Hispanic patients have been more likely to receive OUD treatment in OTPs compared to white patients, 32,35 which may account for differences found here. These differences may also highlight the need for health systems to target improvements in OUD treatment access for nonwhite patients.
Perhaps most surprising was the lower prevalence of OUD treatment among patients who appear to have the poorest health status based on comorbidity scores, use of emergency departments, and hospitalizations. Most often, the greater a patient’s comorbidity, the more health care received, thus the more appropriate care received.36 Yet, that was not true for OUD treatment in this study. Patients with documented OUD who had evidence of greater comorbidity—other substance use, mental health, and medical comorbidity—and those who received more care in emergency departments and hospitals were least likely to receive OUD treatment. The finding that the sickest primary care patients with OUD, who had the greatest contact with the health system, were least likely to receive OUD treatment likely reflects the many, well-catalogued patient-, provider- and system-level barriers to OUD treatment, including provider workload and lack of DEA-waiver training, insufficient system supports for prescribing medications for OUD, limited OUD treatment focus in acute care settings, and lack of coordination across delivery settings.33,37–40 To address such barriers, innovative programs have extended OUD treatment-initiation to acute care settings.41,42 These findings could also reflect the benefits of sustained medication treatment for OUD, which could reduce comorbidity and emergency department and hospital use.43–46 Future longitudinal research will be needed to address those possibilities. Nonetheless, a simple fact likely underlies a portion of the gap in OUD treatment observed in this study. In contrast to other life-saving medical treatments for serious chronic medical conditions,47 primary care providers have not been expected to treat OUD. In recognition of this, the U.S. Surgeon General recently announced that primary care providers, along with all other eligible providers, should be expected to treat patients with OUD.48
These data, collected as part of a feasibility study, a critical first-step in the successful launch of the PROUD multi-site pragmatic implementation trial, have important limitations. Diagnosis of OUD in medical settings is subject to a variety of biases that could be reflected here. Some patients with OUD were likely unrecognized or undocumented by medical providers, with the documented OUD prevalence potentially representing less than half of patients with an OUD,49 while a likely small proportion of OUD diagnoses may have been erroneously documented when providers assigned ICD codes for patients physically dependent on opioids. Additionally, this study’s estimate of OUD treatment prevalence could be an overestimate if some patients were diagnosed with OUD only when seeking treatment for OUD. In addition, this study of OUD treatment underestimates treatment of OUD overall in primary care patients; as noted in the five health systems that provided 1-year data on methadone maintenance, 0–11.4% additional patients with OUD received methadone treatment in an OTP. Despite the higher OUD treatment prevalence among patients with ≥2 OUD diagnoses, the potential bias of documenting OUD during medication treatment suggests that use of a single OUD diagnosis likely results in a less biased sample for estimation of treatment prevalence. Further underestimates of OUD treatment could occur if patients received treatment with buprenorphine (and/or methadone) outside the health systems; however, the magnitude of this under-ascertainment is likely small given the limited capacity of OTPs among states in this study.6 Exclusion of transdermal buprenorphine and oral naltrexone, due to rare OUD treatment use and limits to determining indication, could have also led to modest underestimates of OUD treatment. Assessment of the duration and retention of patients in OUD in treatment, buprenorphine for short-term withdrawal from opioids rather than long-term OUD treatment, and the timing of acute care relative to OUD diagnosis and treatment were beyond the scope of the study. Due to large sample size, some differences between patients with and without documented OUD may not be clinically meaningful. This study did not exclude primary care patients with cancer diagnoses, which could have influenced the prevalence of opioid dispensing. Moreover, although assessment of opioid pill counts may be instructive, this study was limited to prescription counts. Finally, generalizability to primary care clinics in other health care settings and in other U.S. states, including Eastern states, is unknown.
1.5. CONCLUSIONS
Despite limitations, this study has important strengths and findings. This study included nearly 1.4 million primary care patients from urban and rural communities in six health systems serving patients in six states. It provides a stark picture of the gap in treatment for patients with OUD. Among nearly 14,000 patients with documented OUD, only one in five had evidence of OUD treatment with buprenorphine. Given the low capacity for OUD treatment outside these health care settings, these findings, which health system leaders can use to guide treatment initiatives, indicate a critical need for systems-level changes in the provision of OUD treatment. Among the many suggested primary care OUD treatment models, coordinated, multidisciplinary models that support providers in offering OUD treatment, like the one currently being tested in the PROUD trial, demonstrate the most promise.15,50–55 Yet, the most important change may be for health care leaders to set explicit expectations and provide necessary support for all medical and mental health care setting providers to treat patients with OUD with effective OUD treatment, just as would be expected for patients with other complex, life-threatening and/or chronic conditions.33
Supplementary Material
Highlights.
The OUD prevalence among primary care patients in 6 health systems was 1.0%
For patients with OUD, the prevalence of treatment with buprenorphine was 21.0%
Patients with poorest health and greatest acute care use had the lowest treatment
System changes are needed to increase treatment of OUD in primary care patients
Acknowledgments
Funding Resource:
This study was made possible by research support from the National Institute on Drug Abuse (NIDA) under Award Numbers UG1DA040314, UG1DA013034, UG1DA040316, UG1DA013720, 1U10DA015831, U10DA013714, 5UG1DA013035, 1UG1DA049436, UG1DA020024, and HHSN271201400028C. Dr. Thakral was supported by training grant T32-AG-0276-7709.
Role of Funding Source:
The content is solely the responsibility of the authors and does not necessarily represent the official views of NIDA. The NIDA Clinical Trials Network (CTN) Research Development Committee reviewed the study protocol and the NIDA CTN publications committee reviewed and approved the manuscript for publication. The funding organization had no role in the collection, management, analysis, and interpretation of the data or decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
Dr. Saxon is on the advisory board for Alkermes Pharmaceuticals, has received research support from MedicaSafe, Inc., and receives royalties from UpToDate. Dr. Campbell has received support through her institution from the Industry PMR Consortium, a consortium of companies working together to conduct post-marketing studies required by the Food and Drug Administration that assess known risks related to opioid analgesic use. Dr. Binswanger is employed by Colorado Permanente Medical Group, a for-profit medical group, and received royalties from UpToDate. Dr. Yarborough has received support through her institution from Syneos Health to conduct FDA-mandated post-marketing research on the risks of opioid analgesic use. Dr. Bradley is employed by Washington Permanente Medical Group, a for-profit medical group. Dr. Cunningham owns stock and stock options in Quest Diagnostics. Dr. Hechter has received support through her institution from the Industry PMR Consortium, a consortium of companies working together to conduct post-marketing studies required by the Food and Drug Administration that assess known risks related to extended-release, long-acting opioid analgesics. Dr. Murphy is a consultant for Indivior and Alkermes.
There are no other conflicts of interest.
REFERENCES
- 1.U. S. Department of Health & Human Services. National Opioid Crisis: Help, Resources and Information. 2018; https://www.hhs.gov/opioids/. Accessed June 11, 2018.
- 2.Center for Disease Control and Prevention. Opioid Overdose. 2018; https://www.cdc.gov/drugoverdose/. Accessed June 11, 2018.
- 3.Collins FS, Koroshetz WJ, Volkow ND. Helping to End Addiction Over the Long-term: The Research Plan for the NIH HEAL Initiative. JAMA. 2018;320(2):129–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuckit MA. Treatment of Opioid-Use Disorders. N Engl J Med. 2016;375(16):1596–1597. [DOI] [PubMed] [Google Scholar]
- 5.Dunlap B, Cifu AS. Clinical Management of Opioid Use Disorder. JAMA. 2016;316(3):338–339. [DOI] [PubMed] [Google Scholar]
- 6.Jones CM, Campopiano M, Baldwin G, McCance-Katz E. National and State Treatment Need and Capacity for Opioid Agonist Medication-Assisted Treatment. Am J Public Health. 2015;105(8):e55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma A, Kelly SM, Mitchell SG, Gryczynski J, O’Grady KE, Schwartz RP. Update on Barriers to Pharmacotherapy for Opioid Use Disorders. Curr Psychiatry Rep. 2017;19(6):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine Maintenance Versus Placebo or Methadone Maintenance for Opioid Dependence. Cochrane Database Syst Rev. 2014(2):CD002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan JR, Schackman BR, Leff JA, Linas BP, Walley AY. Injectable Naltrexone, Oral Naltrexone, and Buprenorphine Utilization and Discontinuation Among Individuals Treated for Opioid Use Disorder in a United States Commercially Insured Population. J Subst Abuse Treat. 2018;85:90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JD, Nunes EV Jr, Novo P, et al. Comparative Effectiveness of Extended-Release Naltrexone Versus Buprenorphine-Naloxone for Opioid Relapse Prevention (X:BOT): A Multicentre, Open-Label, Randomised Controlled Trial. Lancet. 2018;391(10118):309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutchinson E, Catlin M, Andrilla CH, Baldwin LM, Rosenblatt RA. Barriers to Primary Care Physicians Prescribing Buprenorphine. Ann Fam Med. 2014;12(2):128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Guidelines for the Psychosocially Assisted Pharmacological Treatment of Opioid Dependence. 2009; https://www.ncbi.nlm.nih.gov/books/NBK143185/ Accessed June 11, 2018. [PubMed]
- 13.Substance Abuse and Mental Health Services Administration. Medication and Counseling Treatment: Medications to Treat Opioid Addiction. 2018; https://www.samhsa.gov/medication-assisted-treatment/treatment/buprenorphine. Accessed June 11, 2018.
- 14.American Society of Addiction Medicine. Public Policy Statement on the Regulation of Office-Based Opioid Treatment. 2018; https://www.asam.org/docs/default-source/public-policy-statements/statement-on-regulation-of-obot.pdf?sfvrsn=df8540c2_2. Accessed June 11, 2018.
- 15.Korthuis PT, McCarty D, Weimer M, et al. Primary Care-Based Models for the Treatment of Opioid Use Disorder: A Scoping Review. Ann Intern Med. 2017;166(4):268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNeely J, Wu LT, Subramaniam G, et al. Performance of the Tobacco, Alcohol, Prescription Medication, and Other Substance Use (TAPS) Tool for Substance Use Screening in Primary Care Patients. Ann Intern Med. 2016;165(10):690–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleming MF, Balousek SL, Klessig CL, Mundt MP, Brown DD. Substance Use Disorders in a Primary Care Sample Receiving Daily Opioid Therapy. J Pain. 2007;8(7):573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boscarino JA, Rukstalis M, Hoffman SN, et al. Risk Factors for Drug Dependence Among Out-Patients on Opioid Therapy in a Large US Health-Care System. Addiction. 2010;105(10):1776–1782. [DOI] [PubMed] [Google Scholar]
- 19.Lembke A, Chen JH. Use of Opioid Agonist Therapy for Medicare Patients in 2013. JAMA Psychiatry. 2016;73(9):990–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dufour R, Joshi AV, Pasquale MK, et al. The Prevalence of Diagnosed Opioid Abuse in Commercial and Medicare Managed Care Populations. Pain Pract. 2014;14(3):E106–115. [DOI] [PubMed] [Google Scholar]
- 21.Oliva EM, Harris AH, Trafton JA, Gordon AJ. Receipt of Opioid Agonist Treatment in the Veterans Health Administration: Facility and Patient Factors. Drug Alcohol Depend. 2012;122(3):241–246. [DOI] [PubMed] [Google Scholar]
- 22.Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription Opioid Use, Misuse, and Use Disorders in U.S. Adults: 2015 National Survey on Drug Use and Health. Ann Intern Med. 2017;167(5):293–301. [DOI] [PubMed] [Google Scholar]
- 23.Wu LT, Zhu H, Swartz MS. Treatment Utilization Among Persons with Opioid Use Disorder in the United States. Drug Alcohol Depend. 2016;169:117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donohue JM, Barry CL, Stuart EA, et al. Effects of Global Payment and Accountable Care on Medication Treatment for Alcohol and Opioid Use Disorders. J Addict Med. 2018;12(1):11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliva EM, Trafton JA, Harris AH, Gordon AJ. Trends in Opioid Agonist Therapy in the Veterans Health Administration: Is Supply Keeping Up with Demand? Am J Drug Alcohol Abuse. 2013;39(2):103–107. [DOI] [PubMed] [Google Scholar]
- 26.Thomas CP, Ritter GA, Harris AHS, Garnick DW, Freedman KI, Herbert B. Applying American Society of Addiction Medicine Performance Measures in Commercial Health Insurance and Services Data. J Addict Med. 2018;12(4):287–294. [DOI] [PubMed] [Google Scholar]
- 27.Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral Naltrexone Maintenance Treatment for Opioid Dependence. Cochrane Database Syst Rev. 2011(4):CD001333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quan H, Sundararajan V, Halfon P, et al. Coding Algorithms for Defining Comorbidities in ICD-9-CM and ICD-10 Administrative Data. Med Care. 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 29.Interagency Pain Research Coordinating Committee. National Pain Strategy: A Comprehenisve Population Health-Level Strategy for Pain. Washington, D.C.: Department of Health and Human Services;2015. [Google Scholar]
- 30.Tian TY, Zlateva I, Anderson DR. Using Electronic Health Records Data to Identify Patients with Chronic Pain in a Primary Care Setting. J Am Med Inform Assoc. 2013;20(e2):e275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basu A, Rathouz PJ. Estimating Marginal and Incremental Effects on Health Outcomes Using Flexible Link and Variance Function Models. Biostatistics. 2005;6(1):93–109. [DOI] [PubMed] [Google Scholar]
- 32.Hansen H, Siegel C, Wanderling J, DiRocco D. Buprenorphine and methadone treatment for opioid dependence by income, ethnicity and race of neighborhoods in New York City. Drug Alcohol Depend. 2016;164:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wakeman SE, Barnett ML. Primary Care and the Opioid-Overdose Crisis - Buprenorphine Myths and Realities. N Engl J Med. 2018;379(1):1–4. [DOI] [PubMed] [Google Scholar]
- 34.Manhapra A, Quinones L, Rosenheck R. Characteristics of Veterans Receiving Buprenorphine vs. Methadone for Opioid Use Disorder Nationally in the Veterans Health Administration. Drug Alcohol Depend. 2016;160:82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krawczyk N, Feder KA, Fingerhood MI, Saloner B. Racial and ethnic differences in opioid agonist treatment for opioid use disorder in a U.S. national sample. Drug Alcohol Depend. 2017;178:512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Starfield B, Kinder K. Multimorbidity and Its Measurement. Health Policy. 2011;103(1):3–8. [DOI] [PubMed] [Google Scholar]
- 37.Uebelacker LA, Bailey G, Herman D, Anderson B, Stein M. Patients’ Beliefs About Medications are Associated with Stated Preference for Methadone, Buprenorphine, Naltrexone, or no Medication-Assisted Therapy Following Inpatient Opioid Detoxification. J Subst Abuse Treat. 2016;66:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hurstak EE, Kushel M, Chang J, et al. The Risks of Opioid Treatment: Perspectives of Primary Care Practitioners and Patients from Safety-Net Clinics. Subst Abus. 2017;38(2):213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schuman-Olivier Z, Connery H, Griffin ML, et al. Clinician Beliefs and Attitudes about Buprenorphine/Naloxone Diversion. Am J Addict. 2013;22(6):574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polydorou S, Ross S, Coleman P, et al. Integrating Buprenorphine Into an Opioid Treatment Program: Tailoring Care for Patients With Opioid Use Disorders. Psychiatr Serv. 2017;68(3):295–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trowbridge P, Weinstein ZM, Kerensky T, et al. Addiction consultation services - Linking hospitalized patients to outpatient addiction treatment. J Subst Abuse Treat. 2017;79:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA. 2015;313(16):1636–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lo-Ciganic WH, Gellad WF, Gordon AJ, et al. Association Between Trajectories of Buprenorphine Treatment and Emergency Department and In-Patient Utilization. Addiction. 2016;111(5):892–902. [DOI] [PubMed] [Google Scholar]
- 44.Baser O, Chalk M, Fiellin DA, Gastfriend DR. Cost and Utilization Outcomes of Opioid-Dependence Treatments. Am J Manag Care. 2011;17 Suppl 8:S235–248. [PubMed] [Google Scholar]
- 45.Tkacz J, Volpicelli J, Un H, Ruetsch C. Relationship Between Buprenorphine Adherence and Health Service Utilization and Costs Among Opioid Dependent Patients. J Subst Abuse Treat. 2014;46(4):456–462. [DOI] [PubMed] [Google Scholar]
- 46.Schwarz R, Zelenev A, Bruce RD, Altice FL. Retention on Buprenorphine Treatment Reduces Emergency Department Utilization, but Not Hospitalization, Among Treatment-Seeking Patients with Opioid Dependence. J Subst Abuse Treat. 2012;43(4):451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sordo L, Barrio G, Bravo MJ, et al. Mortality Risk During and After Opioid Substitution Treatment: Systematic Review and Meta-Analysis of Cohort Studies. BMJ. 2017;357:j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.U. S. Department of Health & Human Services Office of the Surgeon General. Facing Addiction in America: The Surgeon General’s Spotlight on Opioids. Washington, DC: 2018. [PubMed] [Google Scholar]
- 49.Barocas JA, White LF, Wang J, et al. Estimated Prevalence of Opioid Use Disorder in Massachusetts, 2011–2015: A Capture-Recapture Analysis. Am J Public Health. 2018;108(12):1675–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weinstein ZM, Kim HW, Cheng DM, et al. Long-Term Retention in Office Based Opioid Treatment with Buprenorphine. J Subst Abuse Treat. 2017;74:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.LaBelle CT, Han SC, Bergeron A, Samet JH. Office-Based Opioid Treatment with Buprenorphine (OBOT-B): Statewide Implementation of the Massachusetts Collaborative Care Model in Community Health Centers. J Subst Abuse Treat. 2016;60:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine Treatment for Hospitalized, Opioid-Dependent Patients: A Randomized Clinical Trial. JAMA Intern Med. 2014;174(8):1369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-Assisted Therapies--Tackling the Opioid-Overdose Epidemic. N Engl J Med. 2014;370(22):2063–2066. [DOI] [PubMed] [Google Scholar]
- 54.Alford DP, LaBelle CT, Kretsch N, et al. Collaborative care of opioid-addicted patients in primary care using buprenorphine: five-year experience. Arch Intern Med. 2011;171(5):425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lagisetty P, Klasa K, Bush C, Heisler M, Chopra V, Bohnert A. Primary care models for treating opioid use disorders: What actually works? A systematic review. PLoS One. 2017;12(10):e0186315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.