Abstract
Background
The novel coronavirus disease 2019 (COVID-19) has become a global health emergency. The cumulative number of new confirmed cases and deaths are still increasing out of China. Independent predicted factors associated with fatal outcomes remain uncertain.
Research Question
The goal of the current study was to investigate the potential risk factors associated with fatal outcomes from COVID-19 through a multivariate Cox regression analysis and a nomogram model.
Study Design and Methods
A retrospective cohort of 1,590 hospitalized patients with COVID-19 throughout China was established. The prognostic effects of variables, including clinical features and laboratory findings, were analyzed by using Kaplan-Meier methods and a Cox proportional hazards model. A prognostic nomogram was formulated to predict the survival of patients with COVID-19.
Results
In this nationwide cohort, nonsurvivors included a higher incidence of elderly people and subjects with coexisting chronic illness, dyspnea, and laboratory abnormalities on admission compared with survivors. Multivariate Cox regression analysis showed that age ≥ 75 years (hazard ratio [HR], 7.86; 95% CI, 2.44-25.35), age between 65 and 74 years (HR, 3.43; 95% CI, 1.24-9.5), coronary heart disease (HR, 4.28; 95% CI, 1.14-16.13), cerebrovascular disease (HR, 3.1; 95% CI, 1.07-8.94), dyspnea (HR, 3.96; 95% CI, 1.42-11), procalcitonin level > 0.5 ng/mL (HR, 8.72; 95% CI, 3.42-22.28), and aspartate aminotransferase level > 40 U/L (HR, 2.2; 95% CI, 1.1-6.73) were independent risk factors associated with fatal outcome. A nomogram was established based on the results of multivariate analysis. The internal bootstrap resampling approach suggested the nomogram has sufficient discriminatory power with a C-index of 0.91 (95% CI, 0.85-0.97). The calibration plots also showed good consistency between the prediction and the observation.
Interpretation
The proposed nomogram accurately predicted clinical outcomes of patients with COVID-19 based on individual characteristics. Earlier identification, more intensive surveillance, and appropriate therapy should be considered in patients at high risk.
Key Words: COVID-19, fatal outcome, nomogram, risk factors
Abbreviations: ACE2, angiotensin-converting enzyme 2; AST, aspartate aminotransferase; CHD, coronary heart disease; COVID-19, coronavirus disease 2019; CVD, cerebrovascular disease; HFNC, high-flow nasal cannula; HR, hazard ratio; IMV, invasive mechanical ventilation; NIV, noninvasive ventilation; PCT, procalcitonin; RAS, renin-angiotensin system; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
Since December 2019, China has been experiencing an outbreak of the novel coronavirus disease 2019 (COVID-19). It has become the newest global health threat, and > 800,000 people have been infected. Europe and the United State have become the epicenters of COVID-19, with a total of 464,212 and 163,199 confirmed cases as of April 1, 2020, respectively.1 Severe cases can develop adverse outcomes, and the numbers of death are persistently increasing outside of China. The case fatal ratio in Italy was up to 11.7%.
As the pandemic evolves, it is urgent that the risk factors associated with fatal outcomes be identified. Earlier intensive surveillance or treatment could then be performed to save lives. Previous reports have described the clinical characteristics of patients with COVID-19, and differences between subjects with mild and severe disease were compared.2, 3, 4 Nonetheless, these previous studies have limitations, including relatively small sample sizes, single-center observations, and use of the univariate analysis alone. Independent predicted factors of fatal outcome remain uncertain. It has also been suggested that the use of a single risk factor could not thoroughly estimate the comprehensive clinical outcome of individual patients.5 Therefore, based on a national cohort, the current study attempted to investigate the potential risk factors associated with fatality by using a multivariate Cox regression analysis and a nomogram model.
Patients and Methods
Study Patients
Led by the National Health Commission of the People’s Republic of China, a retrospective cohort to study COVID-19 admitted cases from 575 hospitals throughout China was established.6 The diagnosis of COVID-19 was made based on the World Health Organization interim guidance.7 The diagnosis was confirmed by a positive result of real-time reverse transcriptase-polymerase chain reaction assay or high-throughput sequencing findings from nasal or pharyngeal swab specimens. By the cutoff time of January 31, 2020, a total of 2,007 cases diagnosed with laboratory-confirmed COVID-19 were collected, and 417 cases were excluded because of incomplete medical records.
The study was approved by the ethics commission of the First Affiliated Hospital of Guangzhou Medical University (Institutional Review Board: 202092). Due to the urgent need to collect data on this emerging infectious disease, the requirement for written informed consent was waived.
Design and Data Extraction
After careful medical chart review, clinical data (including epidemiologic history, demographic data, clinical symptoms and signs, comorbidities, radiologic assessments, laboratory findings upon admission, treatments, and clinical outcome) were extracted from electronic medical records. Laboratory assessments consisted of CBC, blood chemistry, coagulation test, liver and renal function, electrolytes, C-reactive protein, procalcitonin (PCT), lactate dehydrogenase, and creatine kinase. All medical records were copied and sent to the data-processing center in Guangzhou Institute of Respiratory Health, under the coordination of the National Health Commission. A team of experienced respiratory clinicians reviewed and abstracted the data, and data were entered into a computerized database and cross-checked. The primary end point of outcome was death. Independent predicted factors associated with the fatal outcome were investigated.
Statistical Analysis
Continuous variables are described by using mean ± SD or median (interquartile range). Categorical variables are described as number (percentage). Statistical comparisons between continuous variables were performed with an independent Student t test for normally distributed data; otherwise, the Mann-Whitney U test was performed. The χ2 test and Fisher exact test were applied to categorical variables as appropriate. Survival curves were plotted by using the Kaplan-Meier method and compared by using the log-rank test.
Cox regression analysis was used for univariate and multivariate analyses. To estimate risk factors associated with fatal outcome, variables (including baseline characteristics and laboratory findings) were assessed by using univariate Cox regression analyses. A final model selection was performed via a backward stepdown selection process with the Akaike information criterion. A nomogram was built based on the results of multivariate analysis and through the rms package in R version 3.3.1 (R Foundation for Statistical Computing; http://www.r-project.org/). The maximum score of each variable was set as 100. The performance of the nomogram was measured based on the Harrell concordance index (C-index). The nomogram was also evaluated by comparing between nomogram-predicted and observed Kaplan-Meier estimates of survival probability. Bootstraps of 1,000 resamples were set, and calibration curves were calculated by using regression analysis. All statistical analysis was performed by using R version 3.3.1. P values < .05 were considered statistically significant.
Results
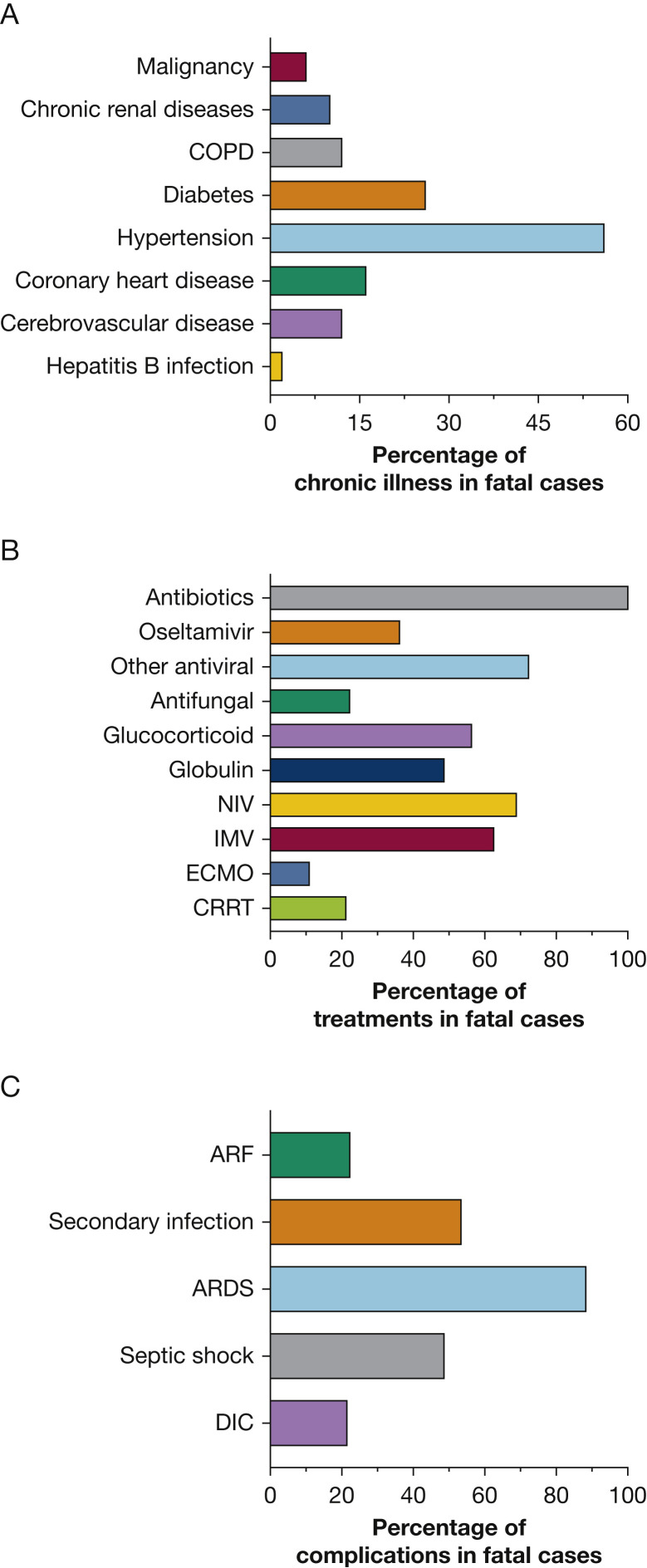
Of the 1,590 cases included in this cohort, 50 deaths were reported by January 31, 2020. The median age of fatal cases was 69 years (range, 51-86 years); 30 of these subjects were male. The median duration from initial treatment to death was 11 days (interquartile range, 7-16.5 days). Thirty-five (70%) cases reported one or more co-existing illness: 28 (56.0%) with hypertension, 13 (26.0%) with diabetes, eight (16.0%) with coronary heart disease (CHD), six (12.0%) with cerebrovascular disease (CVD), six (12.0%) with COPD, and five (10.0%) with renal disease (Fig 1 A). The most common symptoms since disease onset were fever (87.5%), cough (77.1%), and dyspnea (74.0%). The incidence of dyspnea in fatal cases was higher than that in nonfatal cases (19.1%; P < .001). Other symptoms included fatigue, headache, myalgia, nausea or vomiting, diarrhea, and chills (e-Table 1).
Figure 1.
A-C, Clinical characteristics of 50 fatal cases with coronavirus disease 2019. The percentages of coexisting chronic illness in fatal cases (A), treatments in fatal cases (B), and complications in fatal cases (C). ARF = acute renal failure; CRRT = continuous renal replacement therapy; DIC = disseminated intravascular coagulation; ECMO = extracorporeal membrane oxygenation; IMV = invasive mechanical ventilation; NIV = noninvasive ventilation.
On admission, the following abnormalities of laboratory findings were observed in the fatal cases: lymphopenia (97.6%), elevated levels of C-reactive protein (100%), lactose dehydrogenase (91.4%), D-dimer (87.2%), aspartate aminotransferase (AST) (68.6%), alanine aminotransferase (54.3%), leukocytes (42.5%), PCT (41.2%), and total bilirubin (39.0%). Compared with nonfatal cases, more prominent laboratory abnormalities and abnormal chest radiographs were observed in fatal cases (ie, leukopenia, lymphopenia, elevated C-reactive protein, PCT, lactose dehydrogenase, AST, alanine aminotransferase, total bilirubin, creatine kinase, creatinine, D-dimer level) according to univariate analysis (e-Table 1).
All patients with fatal outcomes had received antibiotic therapy. Antifungal therapy was administered to 22%; 36.1% of patients received oseltamivir; and 56.3% received systemic glucocorticoids. Noninvasive ventilation (NIV) and invasive mechanical ventilation (IMV) were performed in 68.8% and 62.5% of cases, respectively. The median age of fatal cases who received NIV and IMV were 69 years (range, 53-84 years) and 66 years (range, 51-82 years). Overall, 10.8% of cases were given extracorporeal membrane oxygenation, 21.1% with continuous renal replacement therapy. ARDS, secondary infection, and septic shock were the most commonly reported complications (Fig 1B, 1C). e-Figure 1 shows the progression of a 63-year-old patient with a fatal outcome.
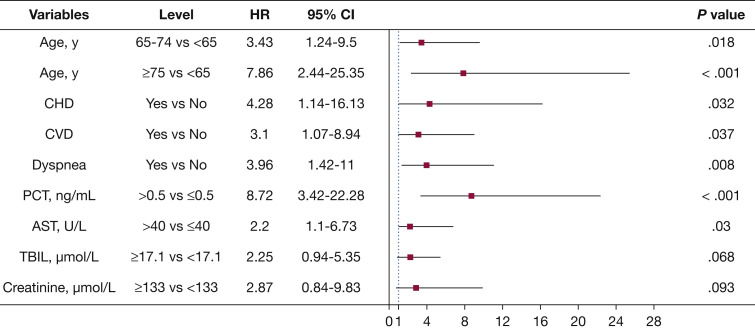
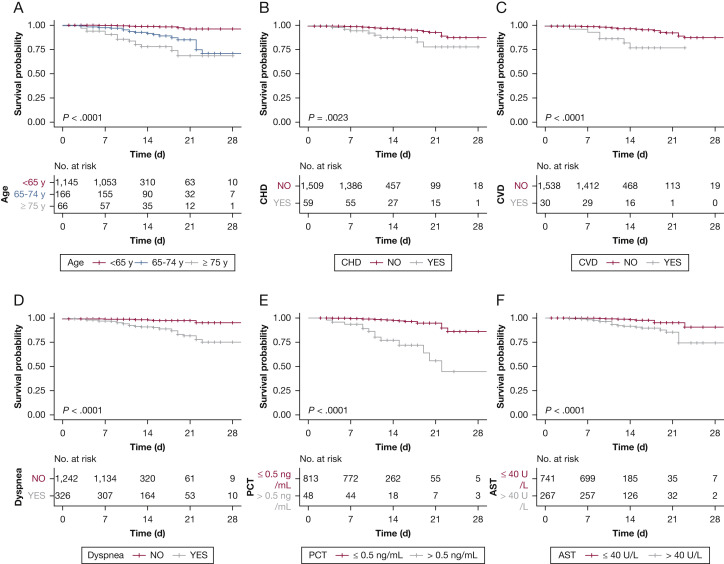
The multivariate Cox regression model displays some independent predicted factors for the fatal outcome. Age ≥ 75 years (hazard ratio [HR], 7.86; 95% CI, 2.44-25.35), age between 65 and 74 years (HR, 3.43; 95% CI, 1.24-9.5), CHD (HR, 4.28; 95% CI,1.14-16.13), CVD (HR, 3.1; 95% CI, 1.07-8.94), dyspnea (HR, 3.96; 95% CI,1.42-11), PCT > 0.5 ng/mL (HR, 8.72; 95% CI, 3.42-22.28), AST > 40 U/L (HR, 2.2; 95% CI, 1.1-6.73) were independent risk factors associated with fatal outcomes (Fig 2 ). The univariate Cox analysis of risk factors related to the fatal outcome is shown in e-Table 2. Kaplan-Meier survival plots for these prognostic factors are shown in Figure 3 .
Figure 2.
Risk factor of the fatal outcome in the multivariate Cox proportional hazards regression model. The figure presents the HRs and the 95% CIs associated with the end point. AST = aspartate aminotransferase; CHD = coronary heart disease; CVD = cerebrovascular disease; HR = hazard ratio; PCT = procalcitonin; TBIL = total bilirubin.
Figure 3.
Kaplan-Meier survival plots for different prognostic factors. The figure displays the Kaplan-Meier survival plots according to (A) age, (B) CHD, (C) CVD, (D) dyspnea, (E) PCT, and (F) AST. See Figure 2 legend for expansion of abbreviations.
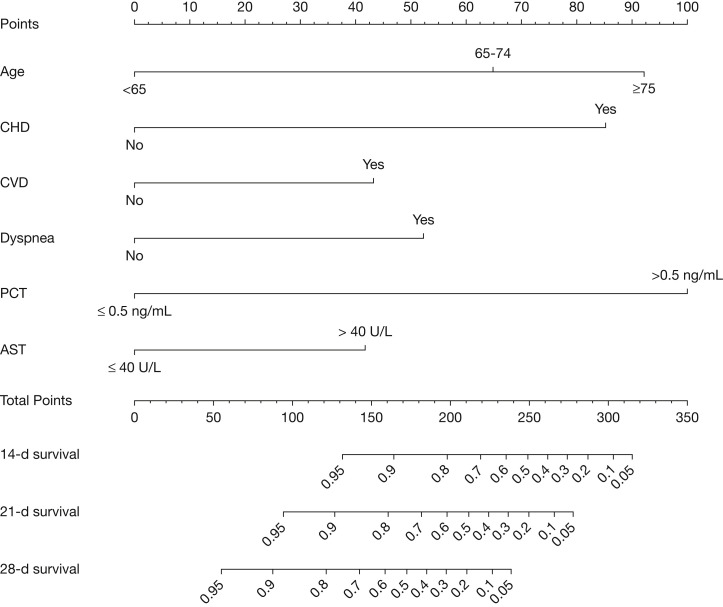
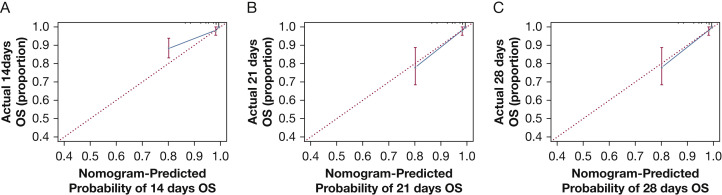
The nomogram was constructed based on the final multivariate model. To calculate 14-, 21-, and 28-day overall survival probability, we first identified each factor based on the points scale at the top of the nomogram and then summed the points of each factor. Finally, the 14-, 21-, and 28-day overall survival probability was obtained based on the bottom point scale of the nomogram (Fig 4 ). The calibration plots on bootstrap resampling validation are shown in Figure 5 . The C-index for prediction of overall survival was 0.91 (95% CI, 0.85-0.97), indicating that the nomogram is consistent with the actual observation for patients with COVID-19.
Figure 4.
Prognostic nomogram for predicting the overall survival probability of patients with coronavirus disease 2019. Prognostic patient’s value is located on each variable axis, and a line is drawn upward to determine the number of point nomogram for predicting overall survival probability of patients with coronavirus disease 2019. The sum of these numbers is located on the Total Points axis, and a line is drawn downward to the survival axes to determine the likelihood of 14-day, 21-day, and 28-day survival. See Figure 2 legend for expansion of abbreviations.
Figure 5.
Calibration curves of the nomogram predicting OS in patients with coronavirus disease 2019. Calibration curves of the nomogram predict 14-day (A), 21-day (B), and 28-day (C) OS in patients with coronavirus disease 2019. Nomogram-predicted probability of OS is plotted on the x-axis; actual OS is plotted on the y-axis. OS = overall survival.
Discussion
Our study unraveled the clinic features and risk factors for fatal outcome in subjects with laboratory-confirmed COVID-19 based on a national cohort. By January 31, 2020, the National Health Commission had documented 11,791 patients with laboratory-confirmed COVID-19 in China. The current cohort represents 13.5% of infected patients and is able to provide a more panoramic picture regarding the fatal cases with COVID-19.
In our study, some independent risk factors for fatal outcome were found by using a multivariate Cox regression analysis. The study first developed a nomogram model to accurately predict clinical outcomes of patients with COVID-19 based on individual characteristic risk factors. The prognostic nomogram performed well in predicting survival, supported by the C-index (0.91; 95% CI, 0.85-0.97) and the calibration curve, which is helpful for further understanding and improving clinical strategies against the disease.
The overall confirmed case fatality ratio was 3.14% in the current cohort, which resembles that in the March 8, 2020, World Health Organization report (ie, 3.39%).1 This is lower than the earlier reports from the single site in Wuhan, China.3 , 8 The difference might be due to the difficulties in performing diagnosis tests for all suspected subjects and insufficient admission for mild patients at the early stage. Although the case fatal ratio is obviously lower than that of severe acute respiratory syndrome (SARS) (10%)9 and Middle Eastern respiratory syndrome (36%),10 the transmissibility of COVID seems to be much higher. A recent study has found the the novel 2019 coronavirus (SARS coronavirus 2 [SARS-CoV-2]) spike protein has an approximately 10- to 20-fold higher affinity than SARS-CoV binding to functional host-cell receptor angiotensin-converting enzyme 2 (ACE2), which may be a potential mechanism for the large number of infected people around the world.11
Consistent with previous reports,12 , 13 the current study indicated that all age groups are susceptible to SARS-CoV-2. However, elderly patients have more severe disease and generally have a higher probability of a fatal outcome. Previous reports indicated that elderly people were more commonly critically ill with COVID-19.2-4 Our study showed that advanced age is the strongest risk factor for a fatal outcome. It also found that risk of death was increased 7.86-fold in patients aged ≥ 75 years and increased 3.43-fold in patients aged between 65 and 74 years, compared with patients aged < 65 years. In the nomogram model, age was the most important predictor for the fatal outcome. Based on a report from the China Center for Disease Control and Prevention that included 44,672 confirmed cases (updated through February 11, 2020), the case-fatality rate reached 14.8% in patients aged ≥ 80 years (208 of 1,408) and 8.0% in patients aged 70 to 79 years (312 of 3,918).12 More intensive surveillance is needed in elderly patients. Earlier reports showed that male individuals were more commonly infected, which may be due to a higher probability of occupational exposure.3 , 8 However, subsequent reports showed an equivalence of sex distribution in infected cases.14 The current study indicates that sex is not the core factor accounting for death based on the multivariate Cox regression analysis.
Nonsurvivors present with a higher proportion of various coexisting chronic illness in univariate analysis. Previous studies reported similar findings.2 , 4 , 15 CHD and CVD are confirmed to be independent risk factors for death through the multivariate Cox regression analysis and were entered into the nomogram model. As the functional receptor of SARS-CoV-2, ACE2 is mainly expressed not only in lung alveolar type II epithelial cells but also in blood vessels.16 , 17ACE2 also serves as a negative regulatory factor for the renin-angiotensin system (RAS) via the ACE2/angiotensin-(1-7) axis.18 Exhaustion of ACE2 could activate RAS and enhance susceptibility to heart failure or pulmonary edema.19 SARS-CoV-2 may act similarly as SARS-CoV and result in an overactivation of RAS in patients with preexisting chronic cardiovascular diseases, which leads to a fatal outcome.20 Although hypertension is common in nonsurvivors, whether RAS inhibitors (ACE inhibitors or angiotensin II receptor blockers) in patients with hypertension is a protector requires further investigation.
Dyspnea at disease onset was reported in 74% of fatal cases, and it was proved to be an independent risk factor for death. With the progression of disease in some cases, hypoxemia would worsen, and ARDS may develop. It is imperative to identify these patients with hypoxemia and provide appropriate intervention. ARDS is the most common complication associated with death, following by secondary infection and septic shock. Pathologic evidence of ARDS in cases of COVID-19 showed obvious desquamation of pneumocytes, hyaline membrane formation, and pulmonary edema,21 which is similar to that seen in SARS and Middle East respiratory syndrome coronavirus infections. In our cohort, NIV and IMV were performed in 68.8% and 62.5% of fatal cases, respectively. Eleven percent of cases were managed with extracorporeal membrane oxygenation. There is still controversy regarding the use of the high-flow nasal cannula (HFNC) or NIV in patients with ARDS. However, two studies indicated that early application of HFNC or HFNC with prone positioning could be considered as first-line therapy in acute respiratory failure and may help avoid intubation in patients with ARDS.22 , 23
A series of laboratory abnormalities on admission were more common in fatal cases compared with the survivors. Some independent laboratory predictors of the fatal outcome were found via multivariate Cox regression analysis. PCT is a calcitonin propeptide synthesized by C cells of the thyroid gland and released from leukocytes, and it is significantly increased in bacterial infections and sepsis. It has been proved to have a high specificity for bacterial infection and a good correlation with severity of illness.24 PCT-guided antibiotic stewardship has been shown to reduce antibiotic use, with fewer side effects and an improvement in clinical outcomes.25 In the nomogram model of the current study, an elevated level of PCT (> 0.5 ng/mL) was a strong reliable predictor for case fatality. The results indicate that secondary bacterial infections at an earlier stage may play an important role in progression of the disease.
Elevated AST (> 40 U/L) levels were also associated with a greater risk for death in the nomogram model. Increased creatinine (≥133 μmol/L) and total bilirubin (> 17.1 μmol/L) levels might be associated with the fatal outcome. Because ACE2 is widely expressed in multiple organs besides the lung, it is possible that SARS-CoV-2 could invade the local tissues and lead to both direct and indirect damage. The ensuing activation of immune response and cytokine storm will also play an important role in organ dysfunction. Overactivation of peripheral T cells, present as increases of CC4+CCR6+Th17 and high cytotoxicity of CD8 T cells, may contribute to immune injury in severe cases of COVID-19.21 In addition, the use of nonsteroidal antiinflammatory drugs, antivirus agents, antibiotics, and traditional Chinese medicine may be associated with liver and renal injury. These findings call for multidisciplinary cooperation and monitoring of the organ dysfunction.
The current study had some limitations. First, in some cases, there was incomplete documentation of the history, symptoms, or laboratory findings in the electronic database, even after making efforts regarding feedback and recollection. Some diagnoses of co-existing illness were from patients’ self-reports at admission, which might lead to recall bias. Although the cohort had a broad coverage of all patients and regions, the nonresponse bias cannot be fully excluded. Second, as a retrospective and observational study, we currently could not set up a validation cohort to assess the predictive accuracy due to the urgent timeline of this special situation. However, we used the bootstrap resampling cohort and showed that the C-index is ideal, suggesting the nomogram is a good model for predicting outcomes. Likewise, the calibration curves for survival probability also showed a good consistency between the prediction and the observation.
Conclusions
Collectively, the current study provides evidence that advanced age, dyspnea, CHD, CVD, and elevated PCT and AST levels are independent risk factors associated with fatal outcome. The nomogram proposed in this study objectively predicted the prognosis of patients with COVID-19. Earlier identification, more intensive surveillance, and appropriate therapy should be considered in these patients at high risk.
Acknowledgments
Author contributions: N. Zhong and S. L.. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. R. C., W. L., M. J., W. G., C. Z., N. Zhong, and S. L. participated in study conception. Z. N., Y. H., L. L., H. S., H. L., Y. P., L. W., Y. Liu, Y. H., P. P., J. W., Jiaxing Liu, Z. C., G. L., Z. Z., S. Q., J. Luo, C. Y., S. Z., X. L., L. C., F. Y., J. Z., N. Zhang, Y. L., and J. H. recruited patients. R. C., W. L., M. J., W. G., C. Z., T. W., C. T., L. S., and Jiaxing Liu. performed data analysis. R. C., W. L., W. G., M. J., C. Z., S. L., and N. Zhong drafted and revised the paper. All authors approved the final draft of the manuscript for publication.
Financial/nonfinancial disclosures: None declared.
Other contributions: The authors sincerely thank all the health-care providers fighting against this public crisis and all the patients involved in the study. They thank the hospital staff (e-Appendix 1 provides a full list of the staff) for their efforts in collecting the information. The authors are indebted to the coordination of Drs Zong-jiu Zhang, Ya-hui Jiao, Bin Du, Xin-qiang Gao, and Tao Wei (National Health Commission), Yu-fei Duan and Zhi-ling Zhao (Health Commission of Guangdong Province), and Zi-jing Liang, Qing-hui Huang, Wen-xi Huang, and Ming Li (Guangzhou Institute of Respiratory Health), who greatly facilitated the collection of patient data. They also thank Drs Li-qiang Wang, Wei-peng Cai, and Zi-sheng Chen (the Sixth Affiliated Hospital of Guangzhou Medical University), Chang-xing Ou, Xiao-min Peng, Si-ni Cui, Yuan Wang, Mou Zeng, Xin Hao, Qi-hua He, Jing-pei Li, Xu-kai Li, Wei Wang, Li-min Ou, Ya-lei Zhang, Jing-wei Liu, Xin-guo Xiong, Wei-juna Shi, San-mei Yu, Run-dong Qin, Si-yang Yao, Bo-meng Zhang, Xiao-hong Xie, Zhan-hong Xie, Wan-di Wang, Xiao-xian Zhang, Hui-yin Xu, Zi-qing Zhou, Ying Jiang, Ni Liu, Jing-jing Yuan, Zheng Zhu, Jie-xia Zhang, Hong-hao Li, Wei-hua Huang, Lu-lin Wang, Jie-ying Li, Li-fen Gao, Jia-bo Gao, Cai-chen Li, Xue-wei Chen, Jia-bo Gao, Ming-shan Xue, Shou-xie Huang, Jia-man Tang, Wei-li Gu, and Jin-lin Wang (Guangzhou Institute of Respiratory Health) for their dedication to data entry and verification. The authors express sincere sympathies and deep condolences to the victims and bereaved families.
Additional information: The e-Appendix, e-Figure, and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: The authors have reported to CHEST that no funding was received for this study.
Drs R. Chen, Liang, Jiang, Guan, and Zhan contributed equally as co-first authors.
Supplementary Data
References
- 1.World Health Organization Coronavirus disease 2019 (COVID-19) situation report-72. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200401-sitrep-72-covid-19.pdf?sfvrsn=3dd8971b_2
- 2.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. [DOI] [PMC free article] [PubMed]
- 4.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;368(2):m641–m643. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kattan M.W. Judging new markers by their ability to improve predictive accuracy. J Natl Cancer Inst. 2003;95(9):634–635. doi: 10.1093/jnci/95.9.634. [DOI] [PubMed] [Google Scholar]
- 6.Liang W., Guan W., Chen R. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. Jan 28, 2020. https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf?sfvrsn=bc7da517_6&download=true
- 8.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lew T.W.K., Kwek T.K., Tai D. Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290(3):374–380. doi: 10.1001/jama.290.3.374. [DOI] [PubMed] [Google Scholar]
- 10.Hui D.S., Azhar E.I., Kim Y.J., Memish Z.A., Oh M.D., Zumla A. Middle East respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission. Lancet Infect Dis. 2018;18(8):e217–e227. doi: 10.1016/S1473-3099(18)30127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wrapp D., Wang N., Corbett K.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention [published online ahead of print February 24, 2020]. JAMA. 10.1001/jama.2020.2648. [DOI] [PubMed]
- 13.Wei M., Yuan J., Liu Y., Fu T., Yu X., Zhang Z.J. Novel coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020;323(13):1313–1314. doi: 10.1001/jama.2020.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145–151. [in Chinese] [Google Scholar]
- 15.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study [published online ahead of print, February 24, 2020] [published correction appears in Lancet Respir Med. 2020;8(4):e26]. Lancet Respir Med. 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed]
- 16.Lu R., Zhao X., Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel V.B., Zhong J.C., Grant M.B., Oudit G.Y. Role of the ACE2/angiotensin 1-7 axis of the renin-angiotensin system in heart failure. Circ Res. 2016;118(8):1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wösten-van Asperen R.M., Lutter R., Specht P.A. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-(1-7) or an angiotensin II receptor antagonist. J Pathol. 2011;225(4):618–627. doi: 10.1002/path.2987. [DOI] [PubMed] [Google Scholar]
- 20.Kuba K., Imai Y., Rao S. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [published correction appears in Lancet Respir Med. 2020;8(4):e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding L., Wang L., Ma W., He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24(1) doi: 10.1186/s13054-020-2738-5. 28-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Messika J., Ben Ahmed K., Gaudry S. Use of high-flow nasal cannula oxygen therapy in subjects with ARDS: a 1-year observational study. Respir Care. 2015;60(2):162–169. doi: 10.4187/respcare.03423. [DOI] [PubMed] [Google Scholar]
- 24.de Jong E., van Oers J.A., Beishuizen A. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis. 2016;16(7):819–827. doi: 10.1016/S1473-3099(16)00053-0. [DOI] [PubMed] [Google Scholar]
- 25.Schuetz P., Beishuizen A., Broyles M. Procalcitonin (PCT)-guided antibiotic stewardship: an international experts consensus on optimized clinical use. Clin Chem Lab Med. 2019;57(9):1308–1318. doi: 10.1515/cclm-2018-1181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.