Abstract
Nowadays the advent of innovative high-throughput sequencing allows obtaining high-quality microbiome profiling. However, PCR-based tests are still considered the “golden standard” for many clinical applications. Here, we designed a qPCR-based platform with fluorescent-labeled oligonucleotide probes for assessing human gut microbiome composition. The system allows conducting qualitative and semiquantitative analysis for 12 prokaryotic taxa that are prevalent in the human gut and associated with diseases, diet, age and other factors. The platform was validated by comparing microbiome profile data obtained with two different methods - the platform and high-throughput 16S rRNA sequencing - across 42 stool samples. The test can form the basis for precise and cost-efficient microbiome assay for large-scale surveys including clinical trials with interventions related to diet and disease risks.
Keywords: Quantitative real-time PCR, Gut microbiome, Taxonomic profiling, Microbial biomarkers, Diet, Nutrition assessment technique, Responders
Highlights
-
•
The qPCR platform provides semiquantitative analysis for 12 microbial taxa prevalent in the human gut.
-
•
Comparison with high-throughput 16S rRNA sequencing of stool samples showed a high correlation between the methods.
-
•
The platform is a promising method for the assessing links of microbiome composition to diet, diseases and other factors.
1. Introduction
The gut microbiome is virtually an organ of the human body. The gut is colonized with 1013 of microbes belonging to thousands of species. Alterations of microbiome composition are linked to lifestyle changes, short- and long-term diet [1], probiotics intake [1,2], medications including antibiotics [3] and diseases including obesity, type 2 diabetes [4], colorectal cancer [5], Crohn's disease [6] and others. Based on the discovered associations, a number of microbial taxa have been proposed as potential biomarkers. For example, the abundance of Akkermansia muciniphila, a mucin-degrading bacterium is inversely associated with body fat mass and glucose intolerance [7]. Disrupted gut community structure is also one of the risk factors for cardiovascular diseases [8]. Many of the bacteria belonging to Clostridiales order are efficient producers of butyrate an other short-chain fatty acids known to possess anti-inflammatory, immunomodulatory and other important activities including gut epithelium integrity maintainence [9,10].
The task of typing and enumerating gut microbial taxa is highly important. There are a plethora of methods for addressing it, including cultivation-based methods, PCR approaches and high-thoughput sequencing. Despite the recent progress with culturomics [11], routine stool cultivation allows to enumerate a narrow range of gut microbes with low accuracy. The methods based on microbiome sequencing are becoming more popular - the “shotgun” metagenomics as well as amplicon-based surveys (using 16S rRNA gene for prokaryotes and ITS region for yeast, etc.). Unlike the culture-dependent approaches, the sequencing gives a total semiquantitative portrait of the microbiome - capturing the uncultivable diversity as well.
An alternative method for taxonomic and functional profiling of microbial communities are hybridization microarrays that allow high-throughput detection of thousands of targets at a time including prokaryotes, viruses, yeast and protozoa [[12], [13], [14]]. Although microarrays capture low-abundant microbes and can be cost-efficient on a large scale, they only detect a fixed set of microbial targets and require a specialized microarray analysis instrument.
Another category for microbiome profiling methods is based on PCR using oligonucleotide sequences specific to selected microbial clades. The real-time PCR (qPCR) is superior to the above-mentioned methods in terms of generally available equipment, cheaper sample preparation, flexibility in application and robustness for diagnostic applications. Human gut-specific PCR panels have been described before. One of them describes a qPCR test for detecting 4 taxa using SYBR Green: Bacteroidetes, Firmicutes, Bacteroides thetaiotaomicron and Enterococcus spp. [15]. In a more extensive manner, a real-time quantitative PCR-based analysis platform termed 'GUt Low-Density Array' (GULDA) with SYBR Green for 27 bacterial taxa was introduced [16]. Detailed clade-focused applications are available - for example, for detecting all known species of human gut-dwelling bifidobacteria [17] using genus- and species-specific primers and probes for qPCR and further proceeding with PCR product restriction for species identification.
In this article we describe a qPCR-based platform for taxonomic profiling of gut microbiome targeting taxa that are prevalent in the gut and/or associated with important diseases, diet, populations, age and other factors. The platform provides a flexible tool for analyzing an individual microbiome composition (e.g., from a stool sample) to assess its general deviation from the reference population distributions, as well as quantify potential microbial biomarkers of disbyosis - universal as well as disease-specific.
2. Materials and methods
2.1. Selection of targets
The target taxa were selected based on the principles of combining the taxa differentially abundant in subjects with major diseases (obesity, type 2 diabetes, inflammatory bowel diseases [IBD] and atherosclerosis), the taxa enriched in probiotic representatives and the “driver” taxa of the human gut microbiome. We collected a statistical summary of the targets’ abundance in the 16S rRNA datasets of stool samples collected from the Russian general population during the OhMyGut crowdfunded project [1] and other studies (see Supplementary Table 2).
2.2. Primers and probes design
The HITdb v1.00 database was used as a non-redundant curated set of 16S rRNA genes specific for human gut microbiota [18]. In order to identify primers and probes targeting specific groups of gut microbes, the gene sequences were analyzed to identify the regions that were both highly conservative within a given group and sufficiently variable to separate the group from other members of the gut microbiome.
On the first step, nucleotide sequences belonging to a certain taxon were extracted from the database and aligned using the Aligner tool [19]. The obtained alignment was then used to define regions conservative within a given taxon. For this, we split the alignment into overlapping 20-mer blocks (set of columns) and only highly conservative blocks (showing 90% agreement in the consensus sequence with <3 degenerate positions) were selected.
On the next step, we checked the taxon-specificity of each conservative block. For this, a BLAST database was constructed using all the sequences from HITdb except those for the analyzed taxon. A conservative block was considered taxon-specific if, during a search against the BLAST database, it had aligned to <1% of non-specific targets with 100% identity and coverage. In case when no such taxon-specific conservative blocks were identified, all the blocks were combined into pairs of blocks separated from each other by 50–300 bases (typical qPCR fragment size). A pair of blocks was considered taxon-specific if the blocks had not aligned to the same non-specific target simultaneously.
2.3. DNA extraction
Stool DNA was extracted as described previously [1]. For facilitating PCR amplification, additional purification from PCR inhibitors was performed using AmpliPrime DNK-Sorb-B (Federal State Institution Central Research Institute of Epidemiology of Rospotrebnadzor, Russia) according to the manufacturer's instructions.
2.4. Cloning and production of recombinant plasmids containing DNA fragments for amplifying target loci
A 1200-1400 bp fragment of 16S rDNA was amplified as follows. The master mix in a reaction volume of 50 μL included: 1x Taq polymerase buffer (65 mM Tris-НCl [pH 8.9]); 16 mM (NH4)2SO4; 0.05% Tween 20; 3.5 mM MgCl2, 0.2 mM dNTPs, 50 ng genomic bacterial DNA or DNA isolated from human feces, 0.3 μM solutions of oligonucleotide primers, 1U Taq-polymerase (Biosan), 0.5U Pfu-polymerase (Biosan). Amplification was performed in Tercyc thermal cycler (DNK-Tehnologiya) under the following program: 15 min at 95 °C for initial denaturation, 30 cycles of 10 s at 95 °C for denaturation, 10 s at 60 °C for annealing, 60 s at 72 °C for elongation. The amplification products were hydrolyzed using the respective restriction endonucleases (SibEnzyme) and ligated using pBluscriptII SK(+) vector hydrolyzed with the same endonucleases within 3 h with 100U T4 DNA ligases (Biosan). Competent cells of E. coli strain XL1-Blue (Stratagene) were transformed with a ligase mixture. In the plasmid clones selected according to the results of specific PCR, the nucleotide sequence of the insert was determined by Sanger sequencing for confirmation. Plasmid DNA was extracted from 100 mL of overnight culture on LB medium using QIAGEN Plasmid Midi Kit (QIAGEN) according to the manufacturer's instructions.
2.5. Measurement of exact plasmid concentrations, preparation of reference samples for qPCR
Concentrations of the obtained reference plasmid DNA were measured by spectrophotometry and fluorometry (Qubit BR kit, Invitrogen). Two μg DNA was hydrolyzed by restriction endonuclease SalGI for linearization. The obtained linear standards were diluted to a concentration of 107 to 100 copies of plasmid DNA per μL in a sterile buffer containing 10 mM TrisHCl pH 7.6 and poly-A RNA (5 ng per μL). DNA concentration in the obtained standards was measured using digital PCR on a QX100 Droplet Digital PCR System (Bio-Rad) platform according to the manufacturer's instructions. For this purpose, 20 μL PCR mixture containing the analyzed DNA (<66 ng per 20 μL), 1X PCR mixture (Bio-Rad), 300 nM oligonucleotide primers and 100 nM TaqMan probe were prepared. To obtain microdroplets, 20 μl of the prepared PCR mixture and 70 μl of oil to generate drops were placed in open DG8 cartridges. 40 μL of the obtained microdroplets was transferred to a 96-well PCR plate, sealed with foil and placed in a thermal cycler. Amplification program: 96 °C–10 min then 50 cycles were performed as follows: 96 °C–30 s, 57 °C–60 s with final warming for 10 min at 98 °C. Then the microdroplets were read and analyzed using a Droplet Reader instrument; the obtained data were processed in the QuantaSoft program (Bio-Rad).
2.6. qPCR
Quantitative qPCR was carried out using a CFX96 thermal cycler (BioRad) in 96-well PCR plate (PCR-96M2-HS-C; Axygen). Sequences of primers and TaqMan probes are provided in Supplementary Tables 2 and 3. Amplification for each sample was carried out in two replicates in a total volume of 20 μL containing: 65 mM Tris-HCl (pH 8.9), 16 mM (NH4)2SO4, 3.0 mM MgCl2, 0.05% Tween 20, 0.2 mM dNTPs (BIOSAN LLC), 0.3 μM oligonucleotide primers with 0.1 μM TaqMan probe, 1 U hot start Taq polymerase (ICBFM SB RAS), 2 μL target DNA or plasmid standard. Amplification was carried out according to the following protocol:
-
1.
95 °C - 15 min
-
2.
95 °C - 10 s
60 °C - 30 s FAM/Green, R6G/JOE/HEX/VIC/Yellow and ROX/Orange; Cy5/Red
72 °C - 30 s
Repeat step #2 45 times
To quantify the DNA of the target microbial taxa in a sample, the method of calibration curves was used: successive 10-fold dilutions of plasmid standards were obtained and then amplified. The concentration range of calibration standards is shown in Supplementary Table 4.
Gene amplification levels obtained by PCR were recorded as Ct values that are inversely related to the initial DNA concentration. Quantity Mean was calculated automatically by the CFX96 (BioRad) software control program based on the set calibration curve values. After automatic data processing, the inaccuracies of automatic reading for some of the curves were corrected by manual alignment. The quantity values were exported to a spreadsheet for further analysis and comparison of data with the results of 16S rRNA gene sequencing.
2.7. Comparison of qPCR and 16S rRNA sequencing microbiome profiles
The library preparation for stool DNA samples and Illumina MiSeq high-throughput sequencing was performed as described previously [2] targeting V4 region of the gene with the following modification of 515F–806R primers: GTGBCAGCMGCCGCGGTAA and GACTACNVGGGTMTCTAATCC. Data analysis has been done using Knomics-Biota system [20]. Reads were denoised using DADA2 algorithm [21] with the variable trimming length (from 251 to 253 bp). The taxonomic classification of the denoised reads has been done using QIIME2 naive-bayes classifier [22] and GreenGenes database [18] with pre-trimmed and clustered with 97% identity sequences using TaxMan [23] and CD-HIT software (version 4.8.1) [24]. The relative abundance of each taxon was calculated by rarefying to 3000 reads per sample and normalizing to 100%. Proportions of genera and families were obtained by summing the levels of their member amplicon-sequencing variants (ASV). The qPCR and 16S rRNA values for target taxa abundance were compared using Spearman's correlation. Multiple comparison adjustment has been done using the Benjamini-Hochberg method. During PERMANOVA analysis, abundance values of PCR targets were used as predictors and Bray-Curtis dissimilarity matrix calculated from 16S rRNA composition - as output.
3. Results
The developed qPCR platform allows semiquantitative detection of major gut microbial taxa using qPCR with oligonucleotide probes. It provides an efficient way to characterize the major components of the human gut microbiome in relation to diseases, dietary interventions as well as for comparison with other factors. The platform includes specific oligonucleotide probes for detection and quantification of the microbes listed in Table 1 .
Table 1.
List of the 12 target taxa in the platform.
| Taxon | Associations | References |
|---|---|---|
| Akkermansia | Inversely associated with metabolic signatures of obesity including insulin resistance and fasting glucose level. Suggested marker for calorie restriction diet efficiency prediction. | [7,[25], [26], [27], [28]]. |
| Bacteroides | One of the dominant genera in adult gut microbiome; linked to “Western diet”. Can change in abundance after high-fiber dietary interventions. | [1,29,30] |
| Bifidobacterium | Dominates in the gut community of healthy infants. Inversely associated with obesity and Crohn's disease. Abundant in certain Eurasian populations. Contains many probiotic strains. Can increase in abundance after intervention with probiotics. | [31] [2,[32], [33], [34]] |
| Blautia | One of the dominant genera in adult gut microbiome. Linked to type 2 diabetes. | [4,33] |
| Christensenellaceae | Inversely associated with obesity and IBD and directly - to Parkinson's disease; members of the family can promote reduction of excessive weight. | [32,35,36] |
| Enterobacteriaceae | Positively associated with metabolic disorders and IBD. | [6,32,37,38] |
| Enterococcus | Linked to inflammation, can carry a wide range of virulence factors and antibiotic resistance genes. Its intestinal domination increases the risk of bacteremia in immunocompromised patients. Prevalent in infant microbiome. | [39,40]; [41]. |
| Lactobacillus | Inversely associated with type 2 diabetes, ulcerative colitis. Contains many probiotic strains. | [25,42] |
| Methanobrevibacter | Linked to eating behavior, anorexia nervosa, body mass index, obesity, IBD. | [[43], [44], [45]] |
| Prevotella | One of the dominant genera in adult gut microbiome. Increased in rural populations of the world. Linked to diet high in fiber and low levels in animal protein and fat. Maternal carriage during pregnancy is reversely linked to food allergy in the offspring. |
[[46], [47], [48]] |
| [Ruminococcus] (from Lachnospiraceae family) | High abundance is linked to IBD. | [49,50] |
| Streptococcus | High abundance is associated with liver cirrhosis, as well as to the increased risk of bacteremia in immunocompromised patients. | [51,52]. [53] |
3.1. Selection of targets
The final list included 12 taxa shown in Table 1. The number of taxa was selected to balance the scope of detection with the economical expenses due to the probe design and analysis cost.
3.2. Results of primers and probes design
Following the in silico analysis described in the Materials and Methods, the consensus sequences were derived from taxon-specific conservative blocks (or pairs of blocks). Primers and probes were designed based on the consensus sequences using Vector NTI software [54]. The following requirements had to be fulfilled: melting temperature (Tm) — 58–62 °C for a primer and 65–75 °C for a probe, GC content — 40–65%, no hairpins with the ΔG <-3 kcal/mol and >2 kcal/mol, no primer-dimers with the ΔG < −5 kcal/mol.
The 12 pairs of primers and probes selected in this way are specific to the investigated taxa. The qPCR-based platform contains a pair of primers and an oligonucleotide probe for each taxon.
3.3. Analytical characteristics of the qPCR platform
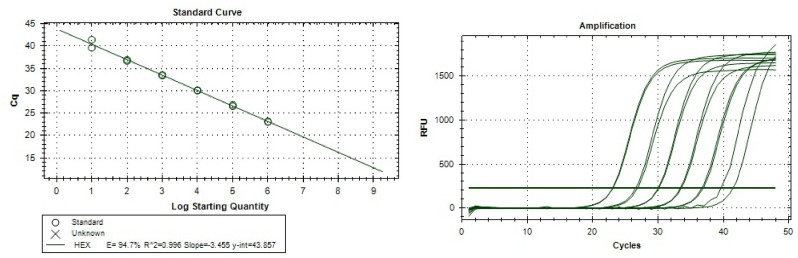
The platform characteristics were calculated based on the amplification of consecutive 10-fold dilutions of plasmid standards. An example of amplification for one of the primer sets is shown in Fig. 1 . All primer sets worked in the efficiency range of 81–128.9%, the coefficient of determination was high (R2 > 0.979) confirming high reproducibility of the results. Detailed analytical characteristics are listed in Supplementary Table 5. Due to multitude of targeted taxa, the specificity of the qPCR platform was evaluated via comparison with the results of high-throughput 16S rRNA sequencing.
Fig. 1.
Example of amplification results (for Blautia target).
3.4. Evaluating the qPCR platform on stool samples
For validation of the qPCR platform, we selected 42 stool samples from generally healthy Russian population that, according to previously performed 16S rRNA sequencing, were representative of global gut microbiome diversity and contained widely varying proportions of the target taxa of the qPCR platform. Each of the stool DNA samples were amplified using each of the primer pairs and probes (listed in Supplementary Tables 2 and 3). In each qPCR run, the analysis was performed for each taxon on all samples accompanied with negative control and a series of positive controls. The positive controls were plasmids in 4 dilutions each used to assess PCR efficacy (concentrations are shown in Supplementary Table 4).
Two repeats were performed for each qPCR experiment to obtain more precise results. Standard curve was drawn by plotting the logarithmic input DNA concentration vs. mean cycle threshold (mCt). Overall, the differences between the replicates for stool samples were significantly lower than between samples (delta Ct 0.54 ± 0.29 vs. 5.31 ± 4.63, p = 0; see Supplementary Fig. S1). In case if the discrepancy between the two replicates is more than 1 cycle it is recommended that the experiment should be repeated and the outlying replicate be removed.
3.5. Comparison with 16S rRNA sequencing microbiome profiles
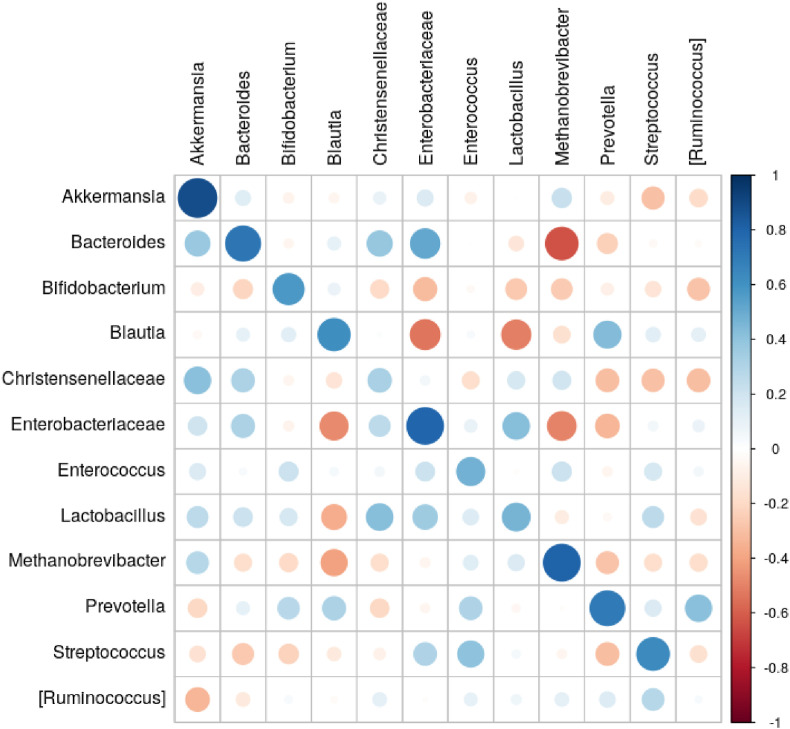
The same stool DNA samples were also subject to 16S rRNA library preparation (V4 region) and sequencing. The taxonomic classification of each read was used to obtain the total composition of each sample and the relative abundance of each identified taxon. Overall, when the abundance values across all taxa and samples were pooled, the correlation between the qPCR and 16S rRNA sequencing was significant (Spearman's r = 0.66, p = 0). For 11 of the 12 target taxa, their levels were significantly correlated between the two methods (FDR-adjusted p < 0.05) (see Fig. 2 and Supplementary Tables 6 and 7; per-sample comparison is shown in Supplementary Fig. S2). The remaining taxon [Ruminococcus] produced insignificant correlation (r = 0.03, FDR-adjusted p = 0.8414).
Fig. 2.
Correlation between levels of target taxa evaluated using qPCR values and 16S rRNA gene sequencing. Rows - 16S rRNA, columns - PCR data; color shows Spearman's correlation coefficient.
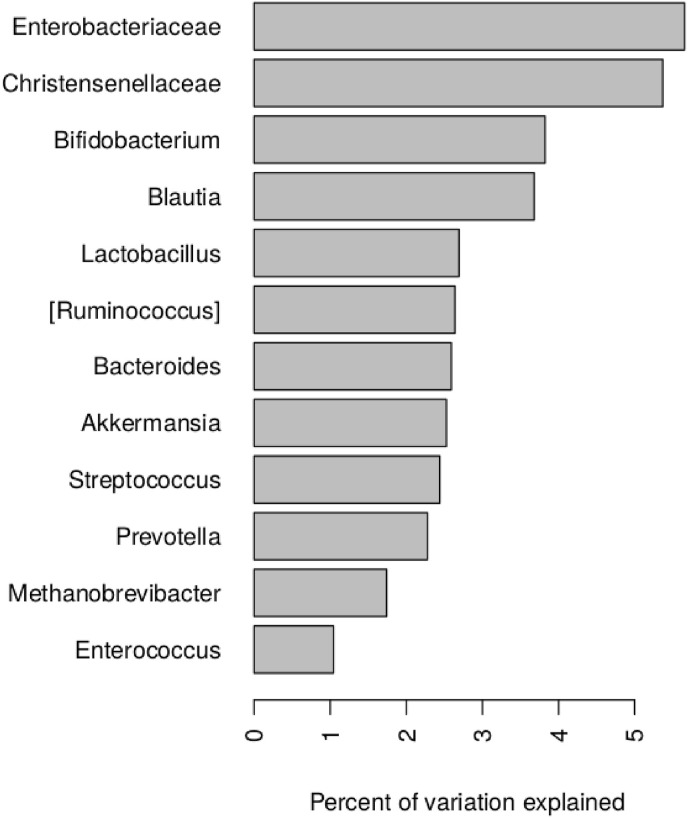
Fig. 3 shows the percentage of variance in community structure according to NGS explained by the level of each PCR target; totally all targets explain 36.5% of it.
Fig. 3.
Percentage of variance of NGS-derived community structure explained by target qPCR taxa (assessed using PERMANOVA).
4. Discussion
Considerable conformance of our qPCR platform with the results of high-throughput sequencing suggests that the panel is a prototype applicable for profiling major components of the human gut microbiome. The analyzed sample types can range from stool samples - as the non-invasive method of choice in such studies - to swabs and biopsies as well as contents of human intestinal tract simulators. Large-scale datasets obtained via the standard described protocol and using the same DNA extraction protocol can be subsequently subject to statistical analysis like the ones used for NGS microbiome data to conduct exploratory analysis and reveal associations with factors like diseases, diet and lifestyle [20].
The platform was designed in a way to reach equilibrium between the coverage of the human gut microbiome diversity and cost per analysis. Subsequently, as the gut microbiome is highly complex, many prevalent taxa are not currently included among the targets. According to our comparison with NGS results, only a third of the total composition variance is explained by the platform. Our results show that the other families and genera that are abundant and variable across the test samples according to 16S rRNA sequencing but not targeted by the PCR test include Lachnospiraceae spp. (9.2 ± 4.3%), Faecalibacterium (5.9 ± 5.0%) and Ruminococcus (3.4 ± 3.0%). These along with their members are potential candidates to be included in the prospective extended panel - along with various members of the Firmicutes phylum including Dorea, Roseburia and others. However, the taxonomy within the phylum especially its Clostridiales order is rather ambiguous hence further elaboration of the primers and probes will be required.
One of the methods for microbiome profiling that can be viewed as an alternative to the proposed panel is Nanopore sequencing. Its advantages includes the possibility to obtain the total microbial composition in an untargeted way. However, although the analysis time (~12 h) takes shorter than for 16S rRNA on Illumina platform, compared to qPCR it takes longer (qPCR: 2–3 h), is less cost-efficient and scalable and requires more expensive equipment [55].
For some of the taxa, we observed a reduced correlation of qPCR levels with the 16S rRNA sequencing results. At least for some of the taxa they could be due to a lower dynamic range provided by NGS (at given coverage) - so that the values below 1 read could be detected only by qPCR. A low correlation for [Ruminococcus] could be due to above-described complexity of Clostridiales phylogeny and need to improve the alignment of taxonomic annotations with the 16S rRNA database.
While our method is cultivation-independent and allows to capture a variety of taxa undetected during a classic stool test, its taxonomic detection accuracy is limited by the level of genus. A single genus can include species and strains with a highly variable gene content resulting in dramatically different phenotypes - from probiotic to human pathogen like in the case of Escherichia coli [6] - or correlating to opposing dietary patterns as shown for Prevotella copri [56]. Despite this limitation, a general-level profile of the gut microbiome composition provided by the proposed platform can be useful for assessing the general degree of dysbiosis. It can help in more advanced tasks like predicting response to drug therapy (as suggested by the discovered link between cancer immunotherapy response and Bacteroides levels [57]) or diet (for example, high-fiber diet [1]), measuring contribution of gut microbes to microbiome of built environments [58] as well as rapidly assessing the gut microbiome dysbiosis in patients with viral infections like COVID-19 (sometimes characterized by low presence of Lactobacillus and Bifidobacterium [59]).
One of the problems of the PCR approach is related to normalization. To address this issue, the set could be extended by adding a universal pair of primers (and a probe) targeting total prokaryotic content that can be used for normalization purposes (for example, by dividing signals from other taxa by it). Although this would reflect the microbial concentration in the analyzed DNA sample, this concentration could not directly correspond to the concentration in the subject's stool, as it can change considerably during the extraction. One of the suggested ways to approach absolute normalization is to collect an exactly equal volume of stool each time. Still, our platform allows us to assess the pairwise ratios between the targeted taxa. Consideration of ratios in the context of microbiome data is especially relevant as for many techniques - including metagenomics - they are often inherently compositional implying use of statistical approaches based on ratios, for instance, the balances [60]. The ratios and their derivatives can further be used as microbial community features to explore the associations with clinical status, diet and other factors. Finally, the proposed platform can be used for prioritization purposes: basing on its results for a specific subject, one can opt for performing additional in-depth analysis using other qPCR platforms targeting microbial genes (like virulence factors and antibiotic resistance determinants) or pathogenic taxa.
5. Conclusions
The developed qPCR platform is a perspective technique for rapid evaluation of human gut microbiome composition. Positive correlation with the data obtained using a different method demonstrates that the qPCR method can be applied for larger cohorts during the clinical and observational studies related to diet and diseases. Further larger clinical trials are needed to identify the method specificity and accuracy when it comes to individual microbiome analysis.
Funding
This work was financially supported by the Skolkovo Foundation for Development of the Center for Elaboration and Commercialization of New Technologies (#G94/16 to Knomics LLC).
Authors’ contributions
AD conceived the project. AD, TA and PA managed the project. KI, FM and KS performed the experiments. KN, CL, FM, KI, and KS performed the data analysis. KI, KN, TA, PA and CL wrote the manuscript. All authors read and approved the final manuscript.
CRediT authorship contribution statement
Irina Kurina: Writing - original draft, Visualization, Writing - review & editing, Formal analysis, Investigation, Validation. Anna Popenko: Methodology, Resources, Writing - original draft, Writing - review & editing, Supervision, Project administration. Natalia Klimenko: Software, Validation, Formal analysis, Data curation, Writing - original draft, Writing - review & editing, Visualization. Stanislav Koshechkin: Validation, Formal analysis, Investigation, Resources, Data curation, Project administration, Writing - review & editing. Liubov Chuprikova: Formal analysis, Investigation, Writing - original draft, Writing - review & editing, Data curation, Visualization. Maxim Filipenko: Validation, Resources, Data curation, Visualization, Formal analysis, Investigation. Alexander Tyakht: Software, Validation, Data curation, Visualization, Methodology, Writing - original draft, Writing - review & editing, Project administration, Supervision. Dmitry Alexeev: Project administration, Funding acquisition, Methodology, Conceptualization.
Decalartion of competing interest
The authors declare that they have no competing interests.
Acknowledgements
The authors would like to thank Maria Kardakova for performing language editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mcp.2020.101570.
Contributor Information
Irina Kurina, Email: kurina@atlas.ru.
Alexander Tyakht, Email: a.tyakht@gmail.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Klimenko Natalia S., Tyakht Alexander V., Popenko Anna S., Vasiliev Anatoly S., Altukhov Ilya A., Ischenko Dmitry S., Shashkova Tatiana I. Microbiome responses to an uncontrolled short-term diet intervention in the frame of the citizen science project. Nutrients. 2018;10(5) doi: 10.3390/nu10050576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volokh Olesya, Klimenko Natalia, Berezhnaya Yulia, Alexander Tyakht, Nesterova Polina, Anna Popenko, Alexeev Dmitry. Human gut microbiome response induced by fermented dairy product intake in healthy volunteers. Nutrients. 2019;11(3) doi: 10.3390/nu11030547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olekhnovich Evgenii I., Manolov Alexander I., Samoilov Andrey E., Prianichnikov Nikita A., Malakhova Maja V., Tyakht Alexander V., Pavlenko Alexander V. Shifts in the human gut microbiota structure caused by quadruple eradication therapy. Front. Microbiol. 2019;10(August):1902. doi: 10.3389/fmicb.2019.01902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egshatyan Lilit, Kashtanova Daria, Anna Popenko, Tkacheva Olga, Alexander Tyakht, Alexeev Dmitry, Karamnova Natalia. Gut microbiota and diet in patients with different glucose tolerance. Endocrine Connect. 2016;5(1):1–9. doi: 10.1530/EC-15-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wirbel Jakob, Pyl Paul Theodor, Kartal Ece, Zych Konrad, Kashani Alireza, Milanese Alessio, Fleck Jonas S. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat. Med. 2019;25(4):679–689. doi: 10.1038/s41591-019-0406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tyakht Alexander V., Manolov Alexander I., Kanygina Alexandra V., Ischenko Dmitry S., Kovarsky Boris A., Popenko Anna S., Pavlenko Alexander V. “Genetic diversity of Escherichia coli in gut microbiota of patients with Crohn's disease discovered using metagenomic and genomic analyses. BMC Genom. 2018;19(1):968. doi: 10.1186/s12864-018-5306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dao Maria Carlota, Everard Amandine, Aron-Wisnewsky Judith, Sokolovska Nataliya, Prifti Edi, Verger Eric O., Kayser Brandon D. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65(3):426–436. doi: 10.1136/gutjnl-2014-308778. [DOI] [PubMed] [Google Scholar]
- 8.Karlsson Fredrik H., Frida Fåk, Nookaew Intawat, Tremaroli Valentina, Fagerberg Björn, Petranovic Dina, Bäckhed Fredrik, Nielsen Jens. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 2012;3:1245. doi: 10.1038/ncomms2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machiels Kathleen, Joossens Marie, Sabino João, De Preter Vicky, Arijs Ingrid, Eeckhaut Venessa, Ballet Vera. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63(8):1275–1283. doi: 10.1136/gutjnl-2013-304833. [DOI] [PubMed] [Google Scholar]
- 10.Sanna Serena, van Zuydam Natalie R., Mahajan Anubha, Alexander Kurilshikov, Vich Vila Arnau, Võsa Urmo, Mujagic Zlatan. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 2019;51(4):600–605. doi: 10.1038/s41588-019-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lagier Jean-Christophe, Grégory Dubourg, Million Matthieu, Cadoret Frédéric, Bilen Melhem, Fenollar Florence, Anthony Levasseur, Rolain Jean-Marc, Fournier Pierre-Edouard, Raoult Didier. Culturing the human microbiota and culturomics. Nat. Rev. Microbiol. 2018;16(May):540–550. doi: 10.1038/s41579-018-0041-0. [DOI] [PubMed] [Google Scholar]
- 12.Thissen James B., Be Nicholas A., McLoughlin Kevin, Gardner Shea, Rack Paul G., Shapero Michael H., Rowland Raymond R.R., Tom Slezak, Jaing Crystal J. Axiom microbiome Array, the next generation microarray for high-throughput pathogen and microbiome analysis. PloS One. 2019;14(2) doi: 10.1371/journal.pone.0212045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Kaoutari, Abdessamad, Armougom Fabrice, Leroy Quentin, Vialettes Bernard, Million Matthieu, Raoult Didier, Bernard Henrissat. Development and validation of a microarray for the investigation of the CAZymes encoded by the human gut microbiome. PloS One. 2013;8(12) doi: 10.1371/journal.pone.0084033. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Rajilić-Stojanović, Mirjana Hans G.H., Molenaar Douwe, Kajander Kajsa, Anu Surakka, Smidt Hauke, Willem M., de Vos Development and application of the human intestinal tract chip, a phylogenetic microarray: analysis of universally conserved phylotypes in the abundant microbiota of young and elderly adults. Environ. Microbiol. 2009 doi: 10.1111/j.1462-2920.2009.01900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bahl Martin Iain, Anders Bergström, Licht Tine Rask. Freezing fecal samples prior to DNA extraction affects the Firmicutes to Bacteroidetes ratio determined by downstream quantitative PCR analysis. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Lett. 2012;329(2):193–197. doi: 10.1111/j.1574-6968.2012.02523.x. [DOI] [PubMed] [Google Scholar]
- 16.Bergström Anders, Licht Tine R., Wilcks Andrea, Andersen Jens B., Schmidt Line R., Grønlund Hugo A., Vigsnaes Louise K., Michaelsen Kim F., Bahl Martin I. Introducing GUt low-density Array (GULDA) - a validated approach for qPCR-based intestinal microbial community analysis. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Lett. 2012 doi: 10.1111/1574-6968.12004. [DOI] [PubMed] [Google Scholar]
- 17.Venema Koen, Maathuis Annet J.H. A PCR-based method for identification of bifidobacteria from the human alimentary tract at the species level. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Lett. 2003;224(1):143–149. doi: 10.1016/S0378-1097(03)00436-1. [DOI] [PubMed] [Google Scholar]
- 18.Ritari Jarmo, Salojärvi Jarkko, Lahti Leo, Willem M., de Vos Improved taxonomic assignment of human intestinal 16S rRNA sequences by a dedicated reference database. BMC Genom. 2015;16(December):1056. doi: 10.1186/s12864-015-2265-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cole James R., Wang Qiong, Fish Jordan A., Chai Benli, McGarrell Donna M., Sun Yanni, Brown C. Titus, Porras-Alfaro Andrea, Kuske Cheryl R., Tiedje James M. Ribosomal database project: data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2014;42(Database issue):D633–D642. doi: 10.1093/nar/gkt1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Efimova Daria, Alexander Tyakht, Anna Popenko, Vasilyev Anatoly, Altukhov Ilya, Dovidchenko Nikita, Vera Odintsova. Knomics-biota - a system for exploratory analysis of human gut microbiota data. BioData Min. 2018;11(November):25. doi: 10.1186/s13040-018-0187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Callahan Benjamin J., McMurdie Paul J., Rosen Michael J., Han Andrew W., Johnson Amy Jo A., Holmes Susan P. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016;13(7):581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolyen Evan, Rideout Jai Ram, Dillon Matthew R., Bokulich Nicholas A., Abnet Christian C., Al-Ghalith Gabriel A., Alexander Harriet. Author correction: reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019;37(9):1091. doi: 10.1038/s41587-019-0252-6. [DOI] [PubMed] [Google Scholar]
- 23.Brandt Bernd W., Bonder Marc J., Huse Susan M., Zaura Egija. TaxMan: a server to trim rRNA reference databases and inspect taxonomic coverage. Nucleic Acids Res. 2012;40 doi: 10.1093/nar/gks418. Web Server issue): W82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu Limin, Niu Beifang, Zhu Zhengwei, Wu Sitao, Li Weizhong. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics. 2012;28(23):3150–3152. doi: 10.1093/bioinformatics/bts565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forslund Kristoffer, Hildebrand Falk, Nielsen Trine, Falony Gwen, Le Chatelier Emmanuelle, Sunagawa Shinichi, Prifti Edi. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262–266. doi: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Everard Amandine, Belzer Clara, Geurts Lucie, Ouwerkerk Janneke P., Druart Céline, Bindels Laure B., Guiot Yves. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. U.S.A. 2013;110(22):9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macchione I.G., Lopetuso L.R., Ianiro G., Napoli M., Gibiino G., Rizzatti G., Petito V., Gasbarrini A., Scaldaferri F. Akkermansia muciniphila: key player in metabolic and gastrointestinal disorders. Eur. Rev. Med. Pharmacol. Sci. 2019;23(18):8075–8083. doi: 10.26355/eurrev_201909_19024. [DOI] [PubMed] [Google Scholar]
- 28.Brahe L.K., Le Chatelier E., Prifti E., Pons N., Kennedy S., Hansen T., Pedersen O., Astrup A., Ehrlich S.D., Larsen L.H. Specific gut microbiota features and metabolic markers in postmenopausal women with obesity. Nutr. Diabetes. 2015;5(June):e159. doi: 10.1038/nutd.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.David Lawrence A., Maurice Corinne F., Carmody Rachel N., Gootenberg David B., Button Julie E., Wolfe Benjamin E., Ling Alisha V. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Gary D., Chen Jun, Hoffmann Christian, Bittinger Kyle, Chen Ying-Yu, Keilbaugh Sue A., Bewtra Meenakshi. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bäckhed Fredrik, Roswall Josefine, Peng Yangqing, Feng Qiang, Jia Huijue, Kovatcheva-Datchary Petia, Yin Li. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17(6):852. doi: 10.1016/j.chom.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Imhann Floris, Vila Arnau Vich, Bonder Marc Jan, Fu Jingyuan, Gevers Dirk, Visschedijk Marijn C., Spekhorst Lieke M. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut. 2018;67(1):108–119. doi: 10.1136/gutjnl-2016-312135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tyakht Alexander V., Kostryukova Elena S., Popenko Anna S., Belenikin Maxim S., Pavlenko Alexander V., Larin Andrey K., Karpova Irina Y. Human gut microbiota community structures in urban and rural populations in Russia. Nat. Commun. 2013;4:2469. doi: 10.1038/ncomms3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Russell D.A., Ross R.P., Fitzgerald G.F., Stanton C. Metabolic activities and probiotic potential of bifidobacteria. Int. J. Food Microbiol. 2011;149(1):88–105. doi: 10.1016/j.ijfoodmicro.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Goodrich Julia K., Waters Jillian L., Poole Angela C., Sutter Jessica L., Koren Omry, Blekhman Ran, Beaumont Michelle. Human genetics shape the gut microbiome. Cell. 2014;159(4):789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrov V.A., Saltykova I.V., Zhukova I.A., Alifirova V.M., Zhukova N.G., Dorofeeva Yu B., Tyakht A.V. “Analysis of gut microbiota in patients with Parkinson's disease. Bull. Exp. Biol. Med. 2017;162(6):734–737. doi: 10.1007/s10517-017-3700-7. [DOI] [PubMed] [Google Scholar]
- 37.Fei Na, Zhao Liping. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J. 2013;7(4):880–884. doi: 10.1038/ismej.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lupp Claudia, Robertson Marilyn L., Wickham Mark E., Sekirov Inna, Champion Olivia L., Gaynor Erin C., Brett Finlay B. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of enterobacteriaceae. Cell Host Microbe. 2007;2(3):204. doi: 10.1016/j.chom.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Palmer Kelli L., Godfrey Paul, Griggs Allison, Kos Veronica N., Zucker Jeremy, Desjardins Christopher, Cerqueira Gustavo. Comparative genomics of enterococci: variation in Enterococcus faecalis, clade structure in E. Faecium, and defining characteristics of E. Gallinarum and E. Casseliflavus. mBio. 2012 doi: 10.1128/mbio.00318-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dubin Krista A., Mathur Deepti, McKenney Peter T., Taylor Bradford P., Littmann Eric R., Peled Jonathan U., van den Brink MarcelR.M., Ying Taur, Pamer Eric G., Xavier Joao B. Diversification and evolution of vancomycin-resistant Enterococcus faecium during intestinal domination. Infect. Immun. 2019;87(7) doi: 10.1128/IAI.00102-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fanaro S., Chierici R., Guerrini P., Vigi V. Intestinal microflora in early infancy: composition and development. Acta Paediatr. 2007 doi: 10.1111/j.1651-2227.2003.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 42.Jones R.M. The use of Lactobacillus casei and Lactobacillus paracasei in clinical trials for the improvement of human health. Microbiota Gastrointest. Pathophysiol. 2017 doi: 10.1016/b978-0-12-804024-9.00009-4. [DOI] [Google Scholar]
- 43.Million M., Angelakis E., Maraninchi M., Henry M., Giorgi R., Valero R., Vialettes B., Raoult D. Correlation between body mass index and gut concentrations of Lactobacillus reuteri, Bifidobacterium animalis, methanobrevibacter smithii and Escherichia coli. Int. J. Obes. 2013;37(11):1460–1466. doi: 10.1038/ijo.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Schwensen, Hanna Ferløv, Kan Carol, Treasure Janet, Høiby Niels, Sjögren Magnus. A systematic review of studies on the faecal microbiota in anorexia nervosa: future Research may need to include microbiota from the small intestine. Eat. Weight Disord.: EWD. 2018;23(4):399–418. doi: 10.1007/s40519-018-0499-9. [DOI] [PubMed] [Google Scholar]
- 45.Mbakwa Catherine A., Penders John, Savelkoul Paul H., Thijs Carel, Dagnelie Pieter C., Mommers Monique, Arts Ilja C.W. Gut colonization with methanobrevibacter smithii is associated with childhood weight development. Obesity. 2015;23(12):2508–2516. doi: 10.1002/oby.21266. [DOI] [PubMed] [Google Scholar]
- 46.De Filippo, Carlotta, Cavalieri Duccio, Di Paola Monica, Ramazzotti Matteo, Poullet Jean Baptiste, Massart Sebastien, Collini Silvia, Pieraccini Giuseppe, Lionetti Paolo. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural africa. Proc. Natl. Acad. Sci. U.S.A. 2010;107(33):14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schnorr Stephanie L., Candela Marco, Rampelli Simone, Centanni Manuela, Consolandi Clarissa, Basaglia Giulia, Turroni Silvia. Gut microbiome of the hadza hunter-gatherers. Nat. Commun. 2014;5(April):3654. doi: 10.1038/ncomms4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vuillermin Peter J., Martin O'Hely, Collier Fiona, Allen Katrina J., Tang Mimi L.K., Harrison Leonard C., Carlin John B. Maternal carriage of Prevotella during pregnancy associates with protection against food allergy in the offspring. Nat. Commun. 2020;11(1):1452. doi: 10.1038/s41467-020-14552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tailford Louise E., David Owen C., Walshaw John, Crost Emmanuelle H., Hardy-Goddard Jemma, Le Gall Gwenaelle, Willem M., de Vos, Taylor Garry L., Juge Nathalie. Discovery of intramolecular trans-sialidases in human gut microbiota suggests novel mechanisms of mucosal adaptation. Nat. Commun. 2015;6(July):7624. doi: 10.1038/ncomms8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Png Chin Wen, Lindén Sara K., Gilshenan Kristen S., Zoetendal Erwin G., McSweeney Chris S., Sly Lindsay I., McGuckin Michael A., Florin Timothy H.J. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am. J. Gastroenterol. 2010;105(11):2420–2428. doi: 10.1038/ajg.2010.281. [DOI] [PubMed] [Google Scholar]
- 51.Qin Nan, Yang Fengling, Li Ang, Prifti Edi, Chen Yanfei, Shao Li, Guo Jing. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513(7516):59–64. doi: 10.1038/nature13568. [DOI] [PubMed] [Google Scholar]
- 52.Taur Ying, Xavier Joao B., Lipuma Lauren, Ubeda Carles, Goldberg Jenna, Gobourne Asia, Lee Yeon Joo. “Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation.” clinical infectious diseases. Off. Publ. Infect. Dis. Soc. Am. 2012;55(7):905–914. doi: 10.1093/cid/cis580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dubinkina Veronika B., Tyakht Alexander V., Odintsova Vera Y., Yarygin Konstantin S., Kovarsky Boris A., Pavlenko Alexander V., Ischenko Dmitry S. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome. 2017;5(1):141. doi: 10.1186/s40168-017-0359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu G. Vector NTI, a balanced all-in-one sequence analysis suite. Briefings Bioinf. 2004 doi: 10.1093/bib/5.4.378. [DOI] [PubMed] [Google Scholar]
- 55.Wongsurawat Thidathip, Nakagawa Mayumi, Omar Atiq, Coleman Hannah N., Jenjaroenpun Piroon, Allred James I., Angela Trammel, Puengrang Pantakan, Ussery David W., Nookaew Intawat. An assessment of oxford Nanopore sequencing for human gut metagenome profiling: a pilot study of head and neck cancer patients. J. Microbiol. Methods. 2019;166(November):105739. doi: 10.1016/j.mimet.2019.105739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Filippis, Francesca, Pasolli Edoardo, Tett Adrian, Tarallo Sonia, Naccarati Alessio, De Angelis Maria, Neviani Erasmo. Distinct genetic and functional traits of human intestinal Prevotella copri strains are associated with different habitual diets. Cell Host Microbe. 2019;25(3):444–453. doi: 10.1016/j.chom.2019.01.004. e3. [DOI] [PubMed] [Google Scholar]
- 57.Gopalakrishnan V., Spencer C.N., Nezi L., Reuben A., Andrews M.C., Karpinets T.V., Prieto P.A. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klimenko Natalia S., Tyakht Alexander V., Toshchakov Stepan V., Shevchenko Margarita A., Korzhenkov Aleksei A., Afshinnekoo Ebrahim, Mason Christopher E., Alexeev Dmitry G. Co-occurrence patterns of bacteria within microbiome of moscow subway. Comput. Struct. Biotechnol. J. 2020;18(February):314–322. doi: 10.1016/j.csbj.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu Kaijin, Cai Hongliu, Shen Yihong, Ni Qin, Chen Yu, Hu Shaohua, Li Jianping. Management of corona virus disease-19 (COVID-19): the Zhejiang experience. J. Zhejiang Univ. Med. Sci. 2020;49(1) doi: 10.3785/j.issn.1008-9292.2020.02.02. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rivera-Pinto J., Egozcue J.J., Pawlowsky-Glahn V., Paredes R., Noguera-Julian M., Calle M.L. Balances: a New perspective for microbiome analysis. mSystems. 2018;3(4) doi: 10.1128/mSystems.00053-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.