To the editor,
I appreciate the interest shown by Sora Yasri, and Viroj Wiwanitkit on my proposal that bioactive lipids can inactivate Coronavirus (COVID-19) and thus, may be of significant clinical benefit (1). The following are the likely actions of bioactive lipids with specific reference to Coronavirus (COVID-19).
COVID-19 and ACE2
COVID-19 uses angiotensin-converting enzyme 2 (ACE2) and the cellular protease transmembrane protease serine 2 (TMPRSS2) to enter the target cells. The spike glycoprotein (S protein) of COVID-19 is needed to mediate receptor recognition and facilitate membrane fusion. The S protein is cleaved during the COVID-19 infection into S1 and S2 subunits. S1 contains the receptor binding domain (RBD) that binds to the peptidase domain (PD) of ACE2, whereas S2 is needed for membrane fusion. The ectodomain of the SARS-CoV-2 S protein binds to the PD of ACE2 (2). ACE2 is a type 1 integral membrane glycoprotein that is expressed and active in most tissues and its highest expression is observed in the kidney, the endothelium, the lungs, and in the heart.
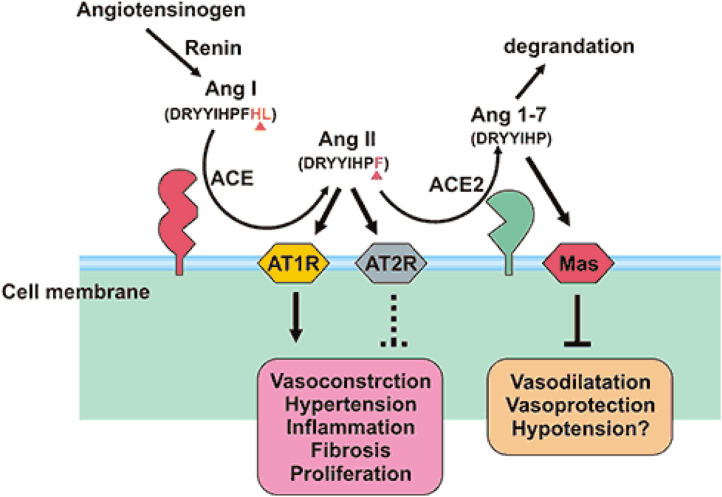
The renin-angiotensin system (RAS) is a modulator of vascular function and is needed for regulation of blood pressure, natriuresis, and blood volume control. RAS is needed for regulating regional renal blood flow. The RAS has a number of different regulatory components and effector peptides to facilitate the dynamic control of vascular function (Figure 1 ). Angiotensin I (Ang I) is metabolized by angiotensin-converting enzyme (ACE) to form angiotensin II (Ang II) that is metabolized by ACE2 to produce angiotensin (2, 3, 4, 5, 6, 7, 8) (Ang 1–7), a vasodilator. Though the focus has been on ACE and Ang II, recent studies showed that ACE2 is needed to maintain the balance of the RAS. Acquired or genetic deficiency of ACE2 leads to an increase in tissue and circulating levels of Ang II and reduced levels of Ang 1–7. Thus, ACE2 controls vasoconstriction and blood pressure. Alternatively, reducing ACE activity can lead to a reduction in the circulating levels of Ang II that suppresses the necessity of ACE2. SARS-CoV-2 (COVID-19) leads to downregulation of the ACE2 receptor, but not ACE, through binding of the spike protein with ACE2. It is noteworthy that bioactive lipids inhibit the activity of ACE (3) and thus, may indirectly suppress the expression of ACE2 leading to reduction in the availability of ACE receptors to SARS-CoV-2 (COVID-19) to bind to enter the target cell. The other possibility is that bioactive lipids can get incorporated into the cell membrane phospholipids and alter the membrane fluidity that, in turn, changes the expression of receptors both their number and affinity to the respective proteins (4, 5, 6). Thus, it is proposed that supplementation of bioactive lipids can possibly, suppress ACE2 expression and decrease the affinity of SARS-CoV-2 (COVID-19) to ACE2.
Figure 1.
ACE2 has a strong affinity for type 1 and type 2 receptors of Ang II and regulates blood pressure, humoral balance, inflammation, cell proliferation, hypertrophy, and fibrosis. ACE2 may play a role in regulating cardiovascular and renal functions and fertility. ACE2 is a suppressor of renin-angiotensin system (RAS). ACE catalyzes angiotensin I (AngI) to convert to the potent vasoconstrictor angiotensin II (AngII), whereas ACE2 cleaves Ang I to produce inactive angiotensin 1-9 peptide (Ang 1-9). Ang 1-9 is converted to angiotensin 1-7 (Ang 1-7) by ACE. ACE2 hydrolyzes AngII to Ang (1-7), and the efficiency of hydrolyzing AngII is 400 times than that of AngI. It suggests that ACE2 mainly generates Ang 1-7 by hydrolyzing AngII. Furthermore, Ang 1-7 acts on the Mas receptor to exert cardiovascular protection of diastolic blood vessels, anti-inflammatory, anti-proliferation, anti-fibrosis, anti-alveolar epithelial cell apoptosis, and anti-oxidative stress, thereby antagonizing the biological effects of Ang II. The ACE/AngⅡ/AT1R axis and the ACE2/Ang 1-7/MAS axis balance each other.
Bioactive Lipids and Imunocytes
Bioactive lipids can induce leakage and lysis of microbial cell membranes, disrupt viral protein envelopes, inhibit respiratory activity, interfere with transportation of amino acids, and uncouple oxidative phosphorylation (7, 8, 9, 10). Alveolar macrophages, leukocytes, T and B cells, NK cells and other immunocytes can release AA and other unsaturated fatty acids to inactivate SARS-CoV-2 (COVID-19), SARS and MERS, which are all enveloped viruses. It is possible that the ability of immunocytes to release bioactive lipids could be one of the fundamental innate immune response mechanisms needed to protect from various infections. Thus, failure of these immunocytes to secrete adequate amounts of bioactive lipids to inactivate various microbes including Coronary virus (COVID-19) may render a person more susceptible to infection. It has been shown that alveolar macrophages and other immunocytes in the lungs release bioactive lipids into the alveolar fluid and surrounding tissues to bring about their antimicrobial action (11,12). In this context, the work of Juers JA, et al. (12) in noteworthy. It was reported that alveolar macrophages do not kill in vitro unless the microorganisms have been incubated with alveolar lining material before phagocytosis. The factor that enhanced the microbicidal capacity of alveolar macrophages was found to be present in the lipid fraction of the alveolar lining material, which could be one of the bioactive lipids. These results imply that there is a close interaction/crosstalk between alveolar lining material and alveolar macrophages. This is further supported by the observation that activated macrophages release bioactive lipid to kill tumor cells (13).
The ability of various immunocytes (NK cells, cytotoxic tumor lymphocytes: CTL cells, lymphokine activated killer cells, dendritic cells, leukocytes, etc.,) to release cytotoxic molecules such as perforin and granzyme, cytokines IL-6, TNF-α, and IFN-γ is needed to kill tumor and infected cells. It is noteworthy that NK cells and CTLs induce apoptosis of tumor cells even when perforin, granzyme pathway is inactivated and is dependent on the expression of soluble phospholipase A2 (sPLA2) (14). This suggests that one additional mechanism by which NK cells and CTLs, even if do not secrete perforin and granzyme, induce apoptosis of tumor cells and those that harbor intracellular microbes is by activating sPLA2. It is likely that perforin and granzyme may have the ability to activate sPLA2 that needs to be established. But what is certain is the fact that PLD (phospholipase D) activation is necessary in the CD16-triggered signaling cascade that leads to NK cytotoxic granule exocytosis that, in turn, is associated with AA release (15, 16, 17). In this context, it is interesting to note that lipids are a constitutive component of cytolytic granules of CTL, NK and γδT cells (18) and cytokine activated macrophages release bioactive lipids to induce apoptosis of tumor cells (13,19, 20, 21).
To exhibit their functions adequately, macrophages need cooperation of tissue-resident memory CD8+ T cells both for pathogen sensing and rapid protection of barrier tissues, a function that is reminiscent of what is described by Juers JA, et al. (12) on the close interaction/crosstalk between alveolar lining material and alveolar macrophages for the latter (macrophages) to bring about their ability to eliminate invading microbes and tumor cells. Thus, there is close cooperation not only between epithelial cells and macrophages but also between macrophages and other T cells. This close association among various tissue epithelial and other cells, macrophages and tissue resident T cells (that are needed for local immune response) seem to depend on their (mainly tissue resident memory T cells) ability to uptake fatty acids for their survival from the local tissues/cells that is regulated by the type of fatty acid-binding protein (FABP) expressed by the T cells. It was reported that tissue resident memory T cells show varying patterns of FABP isoform usage that are determined by tissue-derived factors. It appears that tissue resident memory T cells (and possibly other immunocytes including macrophages) modify their FABP expression depending on the tissue in which they are located or relocated. Thus, tissue resident memory T cells when relocated to different organs modify their FABP expression in line with their new location implying that each tissue resident memory T cells have or need a specific pattern of fatty acids both for their survival and action (22). This interesting observation also argues in favor of the suggestion that each tissue and their resident memory T cells (and macrophages and other immunocytes) need a specific type of bioactive lipid to bring about their action tailored to their location and hence, the necessity of different types of bioactive lipids. This argument can be extended to suggest that, possibly, to inactivate a specific type of bacteria, fungi or virus a specific type or types of bioactive lipid(s) is needed depending on the tissue involved and microbe invading the tissues.
Macrophages are needed both for defense against infections and to clear debris and enhance tissue repair. Macrophages adopt initially an inflammatory phenotype (M1 type) to clear debris and bacteria and later change their phenotype to M2 type to produce anti-inflammatory cytokines and lipoxin A4 (LXA4) from AA, resolvins from eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) and protectins and maresins from DHA to dampen inflammation (7,23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34) restore homeostasis.
It is noteworthy that pro-inflammatory TNF-α and IL-6 inhibit the activities of desaturases that are essential for the generation of AA, EPA and DHA from their precursors LA and ALA respectively (35). Hence, in instances where there is substantial degree of inflammation due to high levels of IL-6 and TNF-α (as it may happen in COVID-19) it may lead to a deficiency of AA, EPA and DHA and decreased generation of LXA4, resolvins, protectins and maresins due to their precursor deficiency. AA, EPA, DHA, LXA4, resolvins, protectins and maresins are potent suppressors of IL-6 and TNF-α formation. Thus, there appears to be a delicate balance maintained between PUFAs and their metabolites and pro-inflammatory cytokines (7). This suggests that administration of PUFAs and their metabolites LXA4, resolvins, protectins and maresins suppress inappropriate production of IL-6 and TNF-α to resolve inflammation and enhance recovery and also prevent cytokine storm (17) that may occur in COVID-19.
Bioactive Lipids and Hematopoiesis
AA is the precursor of both prostaglandin E2 (PGE2) and LXA4. In general, it is believed that PGE2 is pro-inflammatory whereas LXA4 is an anti-inflammatory molecule. But, under some very specific conditions PGE2 shows anti-inflammatory actions and is highly beneficial (29,36,37). This anti-inflammatory action of PGE2 seems to depend on its ability to enhance LXA4 formation (29). This suggests that once the inflammation reaches a peak in which PGE2 plays a significant role, the metabolism of AA is redirected to form LXA4 instead of PGE2 indicating a crosstalk between PGE2 and LXA4. This switching of AA metabolism from PGE2 to LXA4 (and probably vice versa, if need be) appears to be a crucial event in the resolution of inflammation in which anti-inflammatory cytokines play a role (34). In this context, it is interesting to note that 15-PGDH–(15-prostaglandin dehydrogenase, a prostaglandin degrading enzyme) deficient mice showed a twofold increase in PGE2 levels in several tissues including bone marrow, colon, and liver, increased fitness of these tissues with enhanced hematopoietic capacity. These 15-PGDH deficient animals not only had rapid liver regeneration after partial hepatectomy but also showed accelerated recovery of neutrophils, platelets, and erythrocytes (38). These results are supported by other studies which showed that PGE2 regulates haematopoietic stem cell homeostasis, promotes wound healing and tissue regeneration and modulates stem cell trafficking, events that ultimately promote hematopoiesis (39, 40, 41, 42). These evidences suggest that administration of AA and other bioactive lipids to those with SARS-CoV-2 (COVID-19) infection, who may develop fulminant activation of coagulation, resulting in widespread microvascular thrombosis and consumption of coagulation factors as evidenced by thrombocytopenia, prolongation of the PT/INR, PTT, elevation of D-dimer, and decreased fibrinogen levels, may show early recovery due to increased formation of PGE2 (from AA).
Bioactive Lipids and COVID-19 Associated Mortality
It has been reported that mortality due to SARS-CoV-2 (COVID-19) infection is higher in those with diabetes mellitus, hypertension and cardiovascular diseases and the elderly. Previously, we showed that plasma levels of AA and other bioactive lipid shave been shown to be low in diabetes mellitus, hypertension and coronary heart disease (43) and plasma LXA4 levels, a potent anti-inflammatory bioactive lipid, fall with age (44) that may explain adverse outcome in them. Furthermore, it has been shown that it is safe to administer GLA and AA intravenously (45) and oral supplementation of AA enhances LXA4 formation with little or no change in PGE2 formation (that shifts the balance more towards anti-inflammatory events) without increasing the severity of underlying condition with no adverse events (46, 47, 48). Previously, we showed that bioactive lipids (especially AA) have cytoprotective actions against many chemicals, prevents both alloxan and streptozotocin-induced apoptosis of pancreatic β cells and ameliorate development of both type 1 and type 2 diabetes mellitus that is associated with suppression of inflammation and increase in the formation of LXA4 (49, 50, 51, 52, 53, 54, 55).
Conclusions
It is evident from the preceding discussion that bioactive lipids especially AA have many beneficial actions that may be of benefit in the prevention and management of SARS-CoV-2 (COVID-19) infection and other enveloped viruses. Bioactive lipids are useful in view of their ability not only to inactivate enveloped viruses but also several other harmful microbes, enhance macrophage phagocytic capacity, augment wound healing and tissue regeneration, promote hematopoiesis and protect normal cells from both endogenous and exogenous cytotoxic agents with relatively few adverse actions. Hence, efforts need to be made to test their efficacy in SARS-CoV-2 (COVID-19) infection. It is possible to deliver bioactive lipids orally, parenterally and as nasal drops.
(ARCMED_2020_435)
Supplementary data
References
- 1.Das U.N. Can bioactive lipids inactivate coronavirus (COVID-19)? Arch Med Res. 2020;51 doi: 10.1016/j.arcmed.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan R., Zhang Y., Li Y. Structural basis for the recognition of the SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar K.V., Das U.N. Effect of cis-unsaturated fatty acids, prostaglandins and free radicals on angiotensin converting enzyme activity in vitro. Proc Soc Exp Biol Med. 1997;214:374–379. doi: 10.3181/00379727-214-44106. [DOI] [PubMed] [Google Scholar]
- 4.Das U.N. Insulin resistance and hyperinsulinemia: Are they secondary to an alteration in the metabolism of essential fatty acids? Med Sci Res. 1994;22:243–245. [Google Scholar]
- 5.Das U.N. GLUT-4, tumor necrosis factor, essential fatty acids and daf-genes and their role in insulin resistance and non-insulin dependent diabetes mellitus. Prostaglandins Leukot Essent Fatty Acids. 1999;60:13–20. doi: 10.1054/plef.1998.0003. [DOI] [PubMed] [Google Scholar]
- 6.Candelario J., Chachisvilis M. Activity of bradykinin B2 receptor is regulated by long-chain polyunsaturated fatty acids. PLoS One. 2013;8:e68151. doi: 10.1371/journal.pone.0068151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Das U.N. Arachidonic acid and other unsaturated fatty acids and some of their metabolites function as endogenous antimicrobial molecules: a review. J Adv Res. 2018;11:57–66. doi: 10.1016/j.jare.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheu C.W., Konings W.N., Freese E. Effects of acetate and other short chain fatty acids on sugar and amino acid uptake of Bacillus subtilis. J Bacteriol. 1972;111:525–530. doi: 10.1128/jb.111.2.525-530.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ingram L.O., Eaton L.C., Erdos G.W., Tedder T.F., Vreeland N.L. Unsaturated fatty acid requirement in Escherichia coli. Mechanism of palmitate-induced inhibition of growth by strain WNl. J Membr Biol. 1982;1982:31–40. doi: 10.1007/BF01870466. [DOI] [PubMed] [Google Scholar]
- 10.Fay J.P., Farias R.N. Inhibitory action of a nonmetabolizable fatty acid on the growth of Escherichia coli. Role of metabolism and outer membrane integrity. J Bacteriol. 1977;1977:790–795. doi: 10.1128/jb.132.3.790-795.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Territo M.C., Golde D.W. The function of human alveolar macrophages. J Reticuloendothel Soc. 1979;25:111–120. [PubMed] [Google Scholar]
- 12.Juers J.A., Rogers R.M., McCurdy J.B., Cook W.W. Enhancement of bactericidal capacity of alveolar macrophages by human alveolar lining material. J Clin Invest. 1976;58:271–275. doi: 10.1172/JCI108468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlager S.I., Madden L.D., Meltzer M.S., Bara S., Mamula M.J. Role of macrophage lipids in regulating tumoricidal activity. Cell Immunol. 1983;77:52–68. doi: 10.1016/0008-8749(83)90006-0. [DOI] [PubMed] [Google Scholar]
- 14.Costa-Junior H.M., Hamaty F.C., da Silva Farias R. Apoptosis inducing factor of a cytotoxic T cell line: involvement of a secretory phospholipase A2. Cell Tissue Res. 2006;324:255–266. doi: 10.1007/s00441-005-0095-y. [DOI] [PubMed] [Google Scholar]
- 15.Milella M., Gismondi A., Roncaioli P. Beta 1 integrin crosslinking inhibits CD16-induced phospholipase D and secretory phospholipase A2 activity and granule exocytosis in human NK cells: role of phospholipase D in CD16-triggered degranulation. J Immunol. 1999;162:2064–2072. [PubMed] [Google Scholar]
- 16.Parmentier J.H., Muthalif M.M., Nishimoto A.T. 20-Hydroxyeicosatetraenoic acid mediates angiotensin ii-induced phospholipase D activation in vascular smooth muscle cells. Hypertension. 2001;37:623–629. doi: 10.1161/01.hyp.37.2.623. [DOI] [PubMed] [Google Scholar]
- 17.Das U.N. Can bioactive lipid(s) augment anti-cancer action of immunotherapy and prevent cytokine storm? Arch Med Res. 2019;50:342–349. doi: 10.1016/j.arcmed.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Baranov V., Nagaeva O., Hammarstrom S. Lipids are a constitutive component of cytolytic granules. Histochem Cell Biol. 2000;14:167–171. doi: 10.1007/s004180000174. [DOI] [PubMed] [Google Scholar]
- 19.Schlager S.I., Meltzer M.S., Madden L.D. Role of membrane lipids in the immunological killing of tumor cells, II. Effector cell lipids. Lipids. 1983;18:483–488. doi: 10.1007/BF02535789. [DOI] [PubMed] [Google Scholar]
- 20.Schlager S.I., Ohanian S.H. Role of membrane lipids in the immunological killing of tumor cells, I. Target cell lipids. Lipids. 1983;18:475–482. doi: 10.1007/BF02535788. [DOI] [PubMed] [Google Scholar]
- 21.Schlager S.I., Meltzer M.S. Role of macrophage lipids in regulating tumoricidal activity. II. Internal genetic and external physiologic regulatory factors controlling macrophage tumor cytotoxicity also control characteristic lipid changes associated with tumoricidal cells. Cell Immunol. 1983;80:10–19. doi: 10.1016/0008-8749(83)90089-8. [DOI] [PubMed] [Google Scholar]
- 22.Stolp B., Thelen F., Ficht X. Salivary gland macrophages and tissue-resident CD8+ T cells cooperate for homeostatic organ surveillance. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.aaz4371. [DOI] [PubMed] [Google Scholar]
- 23.Frizzell H., Fonseca R., Christo S.N. Organ-specific isoform selection of fatty acid–binding proteins in tissue-resident lymphocytes. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.aay9283. [DOI] [PubMed] [Google Scholar]
- 24.Bouchery T., Harris N.L. Specific repair by discerning macrophages. Science. 2017;356:1014. doi: 10.1126/science.aan6782. [DOI] [PubMed] [Google Scholar]
- 25.Salina A.C., Souza T.P., Serezani C.H., Medeiros A.I. Efferocytosis-induced prostaglandin E2 production impairs alveolar macrophage effector functions during Streptococcus pneumoniae infection. Innate Immun. 2017;23:219–227. doi: 10.1177/1753425916684934. [DOI] [PubMed] [Google Scholar]
- 26.Norris P.C., Arnardottir H., Sanger J.M., Fichtner D., Keyes G.S., Serhan C.N. Resolvin D3 multi-level proresolving actions are host protective during infection. Prostaglandins Leukot Essent Fatty Acids. 2018;138:81–89. doi: 10.1016/j.plefa.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramon S., Dalli J., Sanger J.M., Winkler J.W., Aursnes M., Tungen J.E. The protectin PCTR1 is produced by human M2 macrophages and enhances resolution of infectious inflammation. Am J Pathol. 2016;186:962–973. doi: 10.1016/j.ajpath.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalli J., Winkler J.W., Colas R.A., Arnardottir H., Cheng C.Y., Chiang N. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem Biol. 2013;20:188–201. doi: 10.1016/j.chembiol.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan M.M., Moore A.R. Resolution of inflammation in murine autoimmune arthritis is disrupted by cyclooxygenase-2 inhibition and restored by prostaglandin E2-mediated lipoxin A4 production. J Immunol. 2010;184:6418–6426. doi: 10.4049/jimmunol.0903816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu B., Walker J., Spur B., Rodriguez A., Yin K. Effects of Lipoxin A4 on antimicrobial actions of neutrophils in sepsis. Prostaglandins Leukot Essent Fatty Acids. 2015;94:55–64. doi: 10.1016/j.plefa.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Walker J., Dichter E., Lacorte G., Kerner D., Spur B., Rodriguez A. Lipoxin a4 increases survival by decreasing systemic inflammation and bacterial load in sepsis. Shock. 2011;36:410–416. doi: 10.1097/SHK.0b013e31822798c1. [DOI] [PubMed] [Google Scholar]
- 32.Wu B., Capilato J., Pham M.P., Walker J., Spur B., Rodriguez A. Lipoxin A4 augments host defense in sepsis and reduces Pseudomonas aeruginosa virulence through quorum sensing inhibition. FASEB J. 2016;30:2400–2410. doi: 10.1096/fj.201500029R. [DOI] [PubMed] [Google Scholar]
- 33.Das U.N. HLA-DR expression, cytokines and bioactive lipids in sepsis. Arch Med Sci. 2014;10:325–335. doi: 10.5114/aoms.2014.42586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Das U.N. Current and emerging strategies for the treatment and management of systemic lupus erythematosus based on molecular signatures of acute and chronic inflammation. J Inflamm Res. 2010;3:143–170. doi: 10.2147/JIR.S9425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Das U.N. Is sepsis a pro-resolution deficiency disorder? Med Hypotheses. 2013;80:297–299. doi: 10.1016/j.mehy.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Mayer K., Schmidt R., Muhly-Reinholz M., Bögeholz T., Gokorsch S., Grimminger F., Seeger W. In vitro mimicry of essential fatty acid deficiency in human endothelial cells by TNFα impact of ω-3 versus ω-6 fatty acids. J Lipid Res. 2002;43:944–951. [PubMed] [Google Scholar]
- 37.Duffin R., O’Connor R.A., Crittenden S. Prostaglandin E2 constrains systemic inflammation through an innate lymphoid cell–IL-22 axis. Science. 2016;351:1333–1338. doi: 10.1126/science.aad9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y., Desai A., Yang S.Y. Inhibition of the prostaglandin-degrading enzyme 15-PGDH potentiates tissue regeneration. Science. 2015;348:1223. doi: 10.1126/science.aaa2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.North T.E., Goessling W., Walkley C.R. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li F., Huang Q., Chen J. Apoptotic cells activate the “Phoenix Rising” pathway to promote wound healing and tissue regeneration. Sci Signal. 2010;3(110):ra13. doi: 10.1126/scisignal.2000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoggatt J., Mohammad K.S., Singh P. Differential stem- and progenitor-cell trafficking by prostaglandin E2. Nature. 2013;495:365–369. doi: 10.1038/nature11929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diaz M.F., Li N., Lee H.J. Biomechanical forces promote blood development through prostaglandin E2 and the cAMP–PKA signaling axis. J Exp Med. 2015;212:665–680. doi: 10.1084/jem.20142235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Das U.N. Essential fatty acid metabolism in patients with essential hypertension, diabetes mellitus and coronary heart disease. Prostaglandins Leukot Essent Fatty Acids. 1995;52:387–391. doi: 10.1016/0952-3278(95)90066-7. [DOI] [PubMed] [Google Scholar]
- 44.Gangemi S., Pescara L., D'Urbano E., Basile G., Nicita-Mauro V., Davì G., Romano M. Aging is characterized by a profound reduction in anti-inflammatory lipoxin A4 levels. Exp Gerontol. 2005;40:612–614. doi: 10.1016/j.exger.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Giamarellos-Bourboulis E.J., Mouktaroudi M., Adamis T. n-6 Polyunsaturated fatty acids enhance the activities of ceftazidime and amikacin in experimental sepsis caused by multidrug-resistant Pseudomonas aeruginosa. Antimicrobial Agents Chemother. 2004;48:4713–4717. doi: 10.1128/AAC.48.12.4713-4717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tateishi N., Kakutani S., Kawashima H. Dietary supplementation of arachidonic acid increases arachidonic acid and lipoxin A4 contents in colon but does not affect severity or prostaglandin E2 content in murine colitis model. Lipids Health Dis. 2014;13:30. doi: 10.1186/1476-511X-13-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tateishi N., Kaneda Y., Kakutani S. Dietary supplementation with arachidonic acid increases arachidonic acid content in paw but does not affect arthritis severity or prostaglandin E2 content in rat adjuvant-induced arthritis model. Lipids Health Dis. 2015;14:3. doi: 10.1186/1476-511X-14-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kakutani S., Ishikura Y., Tateishi N. Supplementation of arachidonic acid-enriched oil increases arachidonic acid contents in plasma phospholipids but does not increase their metabolites and clinical parameters in Japanese healthy elderly individuals: a randomized controlled study. Lipids Health Dis. 2011;10:241. doi: 10.1186/1476-511X-10-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suresh Y., Das U.N. Protective action of arachidonic acid against alloxan-induced cytotoxicity and diabetes mellitus. Prostaglandins Leukot Essent Fatty Acids. 2001;64:37–52. doi: 10.1054/plef.2000.0236. [DOI] [PubMed] [Google Scholar]
- 50.Suresh Y., Das U.N. Long-chain polyunsaturated fatty acids and chemically-induced diabetes mellitus: Effect of ω-6 fatty acids. Nutrition. 2003;19:93–114. doi: 10.1016/s0899-9007(02)00856-0. [DOI] [PubMed] [Google Scholar]
- 51.Suresh Y., Das U.N. Long-chain polyunsaturated fatty acids and chemically-induced diabetes mellitus: Effect of ω-3 fatty acids. Nutrition. 2003;19:213–228. doi: 10.1016/s0899-9007(02)00855-9. [DOI] [PubMed] [Google Scholar]
- 52.Naveen K.V.G., Naidu V.G.M., Das U.N. Arachidonic acid and lipoxin A4 attenuate alloxan-induced cytotoxicity to RIN5F cells in vitro and type 1 diabetes mellitus in vivo. Biofactors. 2017;43:251–271. doi: 10.1002/biof.1336. [DOI] [PubMed] [Google Scholar]
- 53.Naveen K.V.G., Naidu V.G.M., Das U.N. Arachidonic acid and lipoxin A4 attenuate streptozotocin-induced cytotoxicity to RIN5F cells in vitro and type 1 and type 2 diabetes mellitus in vivo. Nutrition. 2017;35:61–80. [Google Scholar]
- 54.Naveen K.V.G., Naidu G.V.M., Das U.N. Amelioration of streptozotocin-induced type 2 diabetes mellitus in Wistar rats by arachidonic acid. Biochem Biophys Res Commun. 2018;496:105–113. doi: 10.1016/j.bbrc.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 55.Siresha B., Das U.N. PUFAs, BDNF and lipoxin A4 inhibit chemical-induced cytotoxicity of RIN5F cells in vitro and streptozotocin-induced type 2 diabetes mellitus in vivo. Lipids Health Dis. 2019;18:214. doi: 10.1186/s12944-019-1164-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.