To the editor,
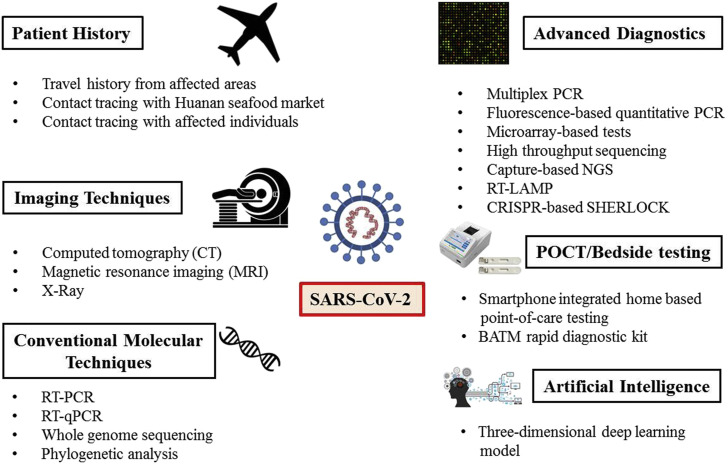
We read with great interest the article by Rahimi and Abadi about the Coronavirus Disease 2019 (COVID-19) (1), discussing concerning questions, and particularly the urgent, evidence-based, and practical measures needed. We would like to compliment, and add a synthesis of the current technological advances on emerging approaches to improve control and particularly diagnosis of this emerging pandemic (Figure 1 ).
Figure 1.
Key points related to the current technological advances on emerging approaches to improve control and particularly diagnosis of the SARS-CoV-2/COVID-19 pandemic.
Now with more than 2.1 million cases, the COVID-19 pandemic is leading to substantial economic losses, psychological and social impacts. Caused by the Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), this emerging disease has been extended to more than 200 countries facilitated by inappropriate and insufficient preventive measures along with unrestricted travel (2).
Various molecular tests targeting E gene, for screening, and RdRp gene, for confirmation, like reverse transcription-PCR (RT-PCR), real-time reverse transcription-PCR (RT-qPCR), whole-genome sequencing, phylogenetic analysis are widely used for the diagnosis of COVID-19 (3). Besides, RT-qPCR is currently considered as the most reliable diagnostic test for COVID-19 worldwide. However, high false-negative results of RT-PCR forced the clinicians in China to consider characteristic clinical manifestations also before giving a final diagnosis to avoid the unintentional spread of the disease. Advanced diagnostic strategies like multiplex PCR, fluorescence-based quantitative PCR, microarray-based tests, high throughput sequencing, and capture-based NGS were also explored for the rapid and simultaneous detection of multiple pathogens. A reverse transcriptional loop-mediated isothermal amplification (RT-LAMP) based method referred to as iLACO has been designed for the rapid and colorimetric detection of SARS-CoV-2 (4). Recently, a smartphone integrated home-based point-of-care testing (POCT) tool consisting of a paper-based POCT combined with LAMP assay was also described to avoid travel requirements in order to reach hospital for testing (5).
Moreover, home-based POCT allows the patients to screen themselves in-home and share the test results with healthcare workers without visiting the hospital. Furthermore, real-time monitoring of patients was also initiated by an interactive web-based dashboard. A CRISPR-based SHERLOCK (Specific High Sensitivity Enzymatic Reporter UnLOCKing) technique has also been developed for detection of SARS-CoV-2 in less than an hour without much instrumentation. In a study, a combination of CRISPR, RT-PCR, and metagenomic next-generation sequencing (mNGS) was employed for the diagnosis of COVID-19 and confirmed the clinical diagnosis effectively (Figure 1).
Various cell lines like Vero CCL81, Vero E6, human embryonic kidney cells (HEK-293T), and human liver cells (HUH7.0) were reported to be permissive for SARS-CoV-2. They may be used for propagation and isolation of the virus (3). Additionally, human airway epithelial cells were reported to be most appropriate for virus isolation and light microscopy along with transmission electron microscopy, found useful for visualization of the virus.
In a study, artificial intelligence (AI) using a three-dimensional deep learning model was reported highly sensitive and specific for the diagnosis of COVID-19 from CT images (Figure 1). A combination of disease history, clinical manifestations, molecular tests, and radiological findings is crucial and imperative for making an accurate and useful diagnosis. Moreover, there is always a need for rapid, accurate, and highly sensitive diagnostic assays to stop the menace created by the worldwide spread of this virus by early detection and follow up of subsequent timely implementation of appropriate prevention, control and mitigation strategies.
Funding
None.
Conflicts of Interest
None.
(ARCMED_2020_408)
Supplementary data
References
- 1.Rahimi F., Talebi Bezmin Abadi A. Practical Strategies Against the Novel Coronavirus and COVID-19—the Imminent Global Threat. Arch Med Res. 2020 doi: 10.1016/j.arcmed.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez-Morales A.J., MacGregor K., Kanagarajah S. Going global-travel and the 2019 novel coronavirus. Travel Med Infect Dis. 2020;33:101578. doi: 10.1016/j.tmaid.2020.101578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhama K, Sharun K, Tiwari R, et al. Coronavirus Disease 2019–COVID-19. Preprints 2020. 2020030001. doi: 10.20944/preprints202003.0001.v2.
- 4.Yu L., Wu S., Hao X. Rapid colorimetric detection of COVID-19 coronavirus using a reverse tran-scriptional loop-mediated isothermal amplification (RT-LAMP) diagnostic plat-form: iLACO. medRxiv. 2020 doi: 10.1101/2020.02.20.20025874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang T., Wang Y.C., Shen C.F. Point-of-care RNA-based diagnostic device for COVID-19. Diagnostics (Basel) 2020;10(3):165. doi: 10.3390/diagnostics10030165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.