Abstract
Summary: Nutritional status has been indicated as a contributing factor to age‐related dysregulation of the immune response. Vitamin E, a lipid‐soluble antioxidant vitamin, is important for normal function of the immune cells. The elderly are at a greater risk for vitamin E intake that is lower than recommended levels. Vitamin E supplementation above currently recommended levels has been shown to improve immune functions in the aged including delayed‐type hypersensitivity skin response and antibody production in response to vaccination, which was shown to be mediated through increased production of interleukin (IL)‐2, leading to enhanced proliferation of T cells, and through reduced production of prostaglandin E2, a T‐cell suppressive factor, as a result of a decreased peroxynitrite formation. Vitamin E increased both cell‐dividing and IL‐producing capacities of naïve T cells, but not memory T cells. The vitamin E‐induced enhancement of immune functions in the aged was associated with significant improvement in resistance to influenza infection in aged mice and a reduced risk of acquiring upper respiratory infections in nursing home residents. Further studies are needed to determine the signaling mechanisms involved in the upregulation of naïve T‐cell function by vitamin E as well as the specific mechanisms involved in reduction of risk for upper respiratory infections.
Introduction
Vitamin E is the most effective chain‐breaking, lipid‐soluble antioxidant in biologic membranes of all cells. Immune cells are particularly enriched in vitamin E because their high polyunsaturated fatty acid content puts them at especially high risk for oxidative damage (1, 2). Free‐radical damage to immune cell membrane lipids may ultimately impair their ability to respond normally to pathogenic challenge. Available evidence suggests beneficial effects of supplemental vitamin E on immune function and related diseases. Vitamin E is perhaps one of the most studied nutrients in relation to its immunoregulatory effect. Results from animal and human studies indicate that vitamin E deficiency impairs both humoral and cell‐mediated immune functions (3, 4), while supplementation with vitamin E above the recommended levels has been shown to enhance immune response and to be associated with increased resistance against several pathogens (5, 6).
In a series of animal and human studies, we as well as others have shown that supplementation with vitamin E above currently recommended levels improves T cell‐mediated function in the aged (5, 7, 8, 9). This chapter summarizes studies related to the role of vitamin E in modulating the immune response in the aged with a focus on underlying mechanisms and clinical implications.
Vitamin E: definition, sources, and intakes
Vitamin E is a generic description for all tocols and tocotrienols that exhibit the biological activity of α‐tocopherol. There are eight naturally occurring forms of vitamin E: α‐, β‐, γ‐, and δ‐tocopherols and α‐, β‐, γ‐, and δ‐tocotrienols ( Fig. 1 ). Chemically synthesized α‐tocopherol contains eight stereoisomers and is designated all‐rac‐α‐tocopherol (historically and incorrectly called dl‐α‐tocopherol), while naturally occurring stereoisomer of α‐tocopherol is RRR‐α‐tocopherol (formerly called d‐α‐tocopherol). It has been generally accepted that biological activities of different forms of vitamin E correlate with antioxidant activities in the order of α > β > γ > δ (10). However, recent studies (11, 12) suggest that biological effects of vitamin E may not necessarily correlate with antioxidant activities of different forms of vitamin E. Since 1980, one international unit (IU) of vitamin E has been defined as 1 mg of all‐rac‐α‐tocopheryl acetate, 0.67 mg of RRR‐α‐tocopherol, or 0.74 mg of RRR‐α‐tocopheryl acetate. These differences in definitions of units among different forms of α‐tocopherol are based on rat fetal absorption assay and adjustment for the differences in molecular weights of esters of α‐tocopherol (13).
Figure 1.
Structure of vitamin E isomers.
Signs of vitamin E deficiency in animals include fetal resorption, necrotizing muscle disease, central and peripheral nerve degeneration, red blood cell hemolysis, and impairment of immune functions (10). Vitamin E deficiency in human occurs rarely in normal condition. However, vitamin E deficiency can be caused by genetic defects in α‐tocopherol‐transfer protein, fat malabsorption syndrome, and genetic defects in lipoprotein synthesis, or can be observed in low‐birth weight infants. Pathology of vitamin E deficiency in humans includes peripheral neuropathy, skeletal myopathy, reduced RBC half‐life, and immunological impairments (4, 10).
Various forms of vitamin E occur in foods in different proportions. The content of vitamin E in food varies depending on storage, processing, and preparation procedures. Vegetable oils and nuts contain high amount of vitamin E. Major sources of vitamin E that contribute to dietary vitamin E in the US diet are fats and oils, vegetables, poultry and meat, and fishes (14). While wheat germ oil, sunflower oil, safflower oil, canola oil, and olive oil provide vitamin E mostly in the form of α‐tocopherol, corn oil, soybean oil, sesame oil, and peanut oil contain mainly γ‐tocopherol.
The recommended dietary allowance (RDA) for vitamin E is currently set at 15 mg/day of α‐tocopherol for adults (ages above 19) (13), increased from 10 mg recommended in the tenth edition of the RDA book (15). The average daily intake of vitamin E in US and other western countries is estimated to be around 10 mg. Certain groups of population, such as the elderly, are at greater risk for inadequate dietary intake of vitamin E (16, 17). Ryan et al. (16) reported that over 40% of elderly (65 – 98 years) had intakes of vitamin E that were below two‐thirds the 1989 RDA. In another study by Panemangalore and Lee (17), 37% of elderly subjects (average age of 73 years old) consumed below two‐thirds RDA, and 12% had low lipid‐adjusted plasma tocopherol status.
Vitamin E and immune function in the aged
The beneficial effect of dietary vitamin E supplementation above the recommended levels, especially in the aged, has been shown in animal studies and human clinical trials (5, 7, 18) ( Table 1 ).
Table 1.
Vitamin E supplementation and immune response in the aged animals and humans
| Subjects | Age | Amount and duration of supplementation* | Effects on immune function | Reference |
|---|---|---|---|---|
| Old mice (C57BL) | 22 months | 500 mg/kg diet for 6 weeks | ⇑Lymphocyte proliferation (ConA, LPS) ⇑DTH ⇑IL‐2 production ⇓PGE2 production | Meydani et al. (7) |
| Rats (Fisher) | 12 weeks | 585 mg/kg diet for 12 months dl‐α‐tocopheryl nicotinate | ⇑Lymphocyte proliferation (ConA, PHA) ⇑IL‐2 production | Sakai et al. (8) |
| Young and old mice (C57BL) | 6 weeks and 22 months | 500 IU for 9 weeks | ⇑Lymphocyte proliferation in young ⇔Lymphocyte proliferation in old ⇑IFN‐γ in young under restraint stress ⇔IFN‐γ in old under restraint stress | Wakikawa et al. (101) |
| Sedentary young and elderly | 22–29, 55–74 | 800 IU/day for 48 days | ⇓IL‐6 secretion ⇓Exercise‐enhanced IL‐1β secretion | Cannon et al. (102) |
| Institutionalized elderly | 63–93 | 200 mg/day for 4 months | ⇑Total serum protein; α‐2 and β‐2 globulin fractions | Ziemlanski et al. (103) |
| Institutionalized adults and elderly (n = 103) | 24–104 | 200, 400 mg/day for 6 months | ⇔Antibody development to influenza virus | Harman & Miller (104) |
| Elderly (n = 74) | ≥65 | 100 mg/day for 3 months | ⇔Lymphocyte proliferation ⇔IgG, IgA levels | De Waart et al. (20) |
| Elderly (n = 161) | 65–80 | 50, 100 mg/day for 6 months | ⇑Number of positive DTH response with 100 mg ⇑Diameter of induration of DTH response in a subgroup with 100 mg ⇔ IL‐2 production | Pallast et al. (21) |
| Elderly (n = 32) | ≥60 | 800 mg/day for 30 days | ⇑Lymphocyte proliferation ⇑DTH ⇑IL‐2 production ⇓PGE2 production | Meydani et al. (5) |
| Elderly (n = 88) | ≥65 | 60, 200, 800 mg/day for 235 days | ⇑DTH and antibody titer to hepatitis B and tetanus with 200 and 800 mg | Meydani et al. (18) |
Supplemented with dl‐α‐tocopheryl acetate unless indicated.
Meydani et al. (7) showed that increasing the level of dietary vitamin E from 30 to 500 ppm significantly increases plasma vitamin E levels, DTH, lymphocyte proliferation to Con A, and interleukin (IL)‐2 production in old mice; this effect of vitamin E was associated with a decrease in prostaglandin (PG) E2 production. Vitamin E‐supplemented animals from this study also had a lower incidence of kidney amyloidosis than control (fed 30 ppm vitamin E) animals (19). Another recent study (8) confirmed these findings. Sakai et al. (8) reported that vitamin E supplementation (585 mg/kg diet) for 12 months significantly improved T‐cell‐mediated function compared with rats fed a control diet containing 50 mg vitamin E per kilogram.
Supplementation with vitamin E (800 mg per day) in healthy elderly over 60 years old resulted in a significant increase in delayed‐type hypersensitivity (DTH) response, in vitro T‐cell proliferation, and IL‐2 production and a significant decrease in plasma lipid peroxide concentration and production of the T‐cell‐suppressive PGE2 (5) ( Fig. 2 ). In a subsequent study, Meydani et al. (18) investigated the effect of 4.5 months of vitamin E supplementation on in vivo indices of immune function in healthy elderly over 65 years old; 88 subjects were supplemented with placebo, 60, 200, and 800 mg of dl‐α‐tocopherol. All three vitamin E‐supplemented groups showed a significant increase in DTH response compared with baseline. Subjects in the 200 mg/day group showed a significantly greater increase in median percentage change of DTH compared with those in the placebo group (65 versus 17%, P = 0.04) and a significant increase in antibody titers to hepatitis B and tetanus vaccines.
Figure 2.
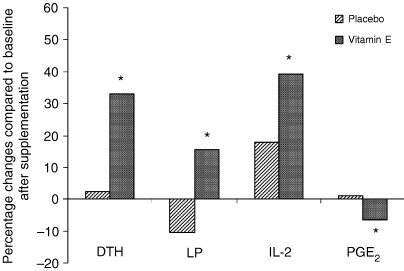
Effect of vitamin E supplementation (800 mg/day for 30 days) on immune response of healthy older adults.*Significant changes from baseline at P < 0.05. DTH, delayed‐type hypersensitivity response; LP, lymphocyte proliferation; IL‐2, interleukin‐2; PGE2, prostaglandin E2. Adapted from Meydani and Han (100).
Lee and Wan (9) reported a significant increase in the proliferative response to PHA or LPS and a significant decrease in plasma malondialdehyde and urinary DNA adduct 8‐hydroxy‐2′‐deoxyguanosine levels after short‐term supplementation with vitamin E (400 IU of dl‐α‐tocopherol/day for 28 days) in Chinese adults. De Waart et al. (20) observed no significant changes in mitogenic response to ConA and PHA or levels of IgG and IgA against Penicillium after 3‐month supplementation with vitamin E at 100 mg/day. The lower dose of vitamin E as well as the use of previously frozen lymphocytes for determination of mitogenic response and evaluation of antibody levels without previous specific vaccination may have contributed to the discrepancy observed between the results of De Waart et al. (20) and those of Meydani et al. (5, 18) and Lee and Wan (9). Pallast et al. (21) supplemented healthy elderly subjects (65 – 80 years old) with 50 or 100 mg/day of vitamin E for 6 months. Subjects in the vitamin E‐supplemented group showed a significant increase in DTH (induration diameter and number of positive responses) compared with their own baseline values. Only the change in the number of positive DTH responses tended to be larger in the 100 mg‐supplemented group than the placebo group (P = 0.06). A significantly greater improvement in cumulative DTH score and the number of positive DTH responses was observed in a subgroup of subjects who received 100 mg of vitamin E and had a low‐baseline DTH reactivity. There was no significant difference in PHA‐stimulated IL‐2 production between the vitamin E‐treated groups relative to the placebo group.
Differences in results among human studies may reflect the differences in vitamin E status at baseline and supplementation dose resulting in varied levels of changes in plasma vitamin E levels and methodology ( Fig. 3 ). Considering the results from the study by Meydani et al. (18) in which subjects in the upper tertile of serum vitamin E concentration (>48.4 µmol/l) after supplementation had higher antibody response to hepatitis B as well as higher DTH responses than those in the lower tertile of serum vitamin E (19.9 – 34.7 µmol/l), the amount of increase in vitamin E levels achieved in the studies by others (20, 21) might not have been adequate to observe a highly significant effect. It is also noteworthy that Lee and Wan (9) observed a significant increase in cell‐mediated immune response with a 13.4 µmol/l increase in plasma vitamin E level, a level of increase comparable to those observed by De Waart et al. (20) and Pallast et al. (21) with 100 mg supplementation. A difference in baseline vitamin E status may explain the varied results observed in these latter studies, as subjects in the study by Lee and Wan (9) had significantly lower plasma vitamin E levels at baseline (14 µmol/l) compared with those in the studies by De Waart et al. (20) (33 µmol/l) and Pallast et al. (21) (31 µmol/l). As shown in Fig. 3 , changes in plasma vitamin E levels up to 25 µmol/l is linearly associated with a change in DTH response. Further increase in plasma vitamin E level, however, does not seem to be associated with an additional improvement in DTH. A 25 µmol/l increase in plasma vitamin E can be achieved by consuming 200 mg/day of vitamin E.
Figure 3.
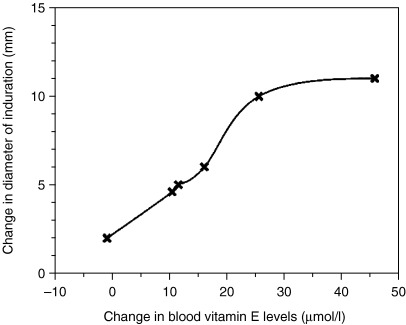
Relationship between changes in delayed‐type hypersensitivity response and changes in blood vitamin E levels following supplementation with different doses of vitamin E. Reproduced from Meydani and Han (100) with permission.
Mechanisms of immunostimulatory effect of vitamin E in the aged
There are several possible mechanisms for the immunostimulatory effect of vitamin E in the aged. Vitamin E can enhance T‐cell‐mediated function by directly influencing membrane integrity, and signal transduction in T cells, or indirectly by reducing the production of suppressive factors such as PGE2 by macrophages (Mφ).
As mentioned above, the immunostimulatory effect of vitamin E in both old mice and humans was associated with a reduction in PGE2 production (5, 7). Thus, we proposed that the enhancing effect of vitamin E on T‐cell‐mediated function might be attributed in part to its inhibition of PGE2 production. In a coculture study, Beharka et al. (22) isolated Mφ and T cells from young (6 months) and old (23 months) C57BL mice and preincubated the cells in the presence of 20 µg/ml of vitamin E or vehicle solution. Mφ and T cells from young or old mice, preincubated with or without vitamin E, were added together in different combinations and stimulated by ConA. The results showed that supplementation of both T cells and Mφ from old mice with vitamin E significantly increased T‐cell proliferation and IL‐2 production by old T cells compared to those of control culture. When either Mφ or T cell was supplemented with vitamin E, these changes were still present, but the magnitude of the increase was smaller. When vitamin E‐supplemented Mφ from old mice were added to cocultures containing T cells from young mice, proliferation, but not IL‐2 production, was improved. These results indicate that vitamin E improves T‐cell function in aged by two mechanisms: (i) by directly affecting T cells, and (ii) indirectly by reducing Mφ and PGE2 production.
Role of macrophages in the age‐associated T‐cell defects
Among the T‐cell suppressive factors produced by Mφ, PGE2 is the most prominent and thus most intensively studied. While PGE2 is necessary for T‐cell function, at higher concentrations, it has been shown to inhibit T‐cell proliferation, including both CD4+ and CD8+ T cells, with CD4+ T cells being most affected and thus the focus of majority of the studies. This effect of PGE2 on T‐cell function is the consequence of altered early signaling events in T‐cell activation (23) and results in altered cytokine profile of T cells. PGE2 has been consistently shown to inhibit Th1 cytokine IL‐2 and interferon (IFN)‐γ production as well as IL‐2 receptor expression (24, 25). On the other hand, depending on the stimulation condition, PGE2 upregulates or has no effect on the production of Th2 cytokines IL‐4, IL‐5, and IL‐10 (26). Thus PGE2, in general, promotes a shift in T‐cell cytokine production from Th1 type to Th2 type.
Studies have shown that Mφ and spleen cells from old mice and peripheral blood mononuclear cells from elderly human subjects synthesize significantly more PGE2 than their young counterparts (5, 7, 27, 28). In a study by Franklin et al. (29), addition of peritoneal Mφ from old rats to splenocytes from young rats significantly inhibited ConA‐stimulated splenocyte proliferation; this inhibitory effect was also observed when PGE2 was added. These findings were further confirmed and extended by Beharka et al. (22) who showed that cocultures of Mφ isolated from old mice with T cells from young mice exhibited lower T‐cell proliferation and IL‐2 production compared to those of young Mφ with young T cell. Exogenous addition of PGE2, at the concentrations produced by old Mφ, decreased T‐cell proliferation and IL‐2 production by young T cells. Addition of cyclooxygenase (COX) inhibitor indomethacin or antioxidant nutrient vitamin E inhibited PGE2 production and improved T‐cell proliferation and IL‐2 production. The association between PGE2 and age‐associated decrease in T‐cell‐mediated immune response was further supported by in vivo studies (5, 7), which showed that dietary vitamin E supplementation to old mice and human subjects improved their T‐cell proliferation, IL‐2 production, and DTH response. In these studies, improved T‐cell‐mediated immune response was accompanied by a reduced PGE2 production ( Fig. 2 ). Taken together, these results suggest a key role for PGE2 in T‐cell immunosenescence.
Mechanism of upregulated PGE2 production with aging
Since PGE2 synthesis is increased with aging and elevated levels of PGE2 contributes to the suppressed T‐cell immunity in old animals and humans and might also contribute to other age‐related pathologies, we investigated the underlying mechanism(s) for age‐associated upregulation in PGE2 synthesis.
Like other eicosanoids, biosynthesis of PGE2 is accomplished in a metabolic cascade starting from its precursor fatty acid, arachidonic acid (AA) ( Fig. 4 ). AA is present in membrane phospholipids and released by the hydrolytic action of phospholipase A2 (PLA2). Released AA is metabolized to unstable intermediate prostanoids by COX, also called PGH2 synthase. COX has bifunctional catalytic properties. It oxygenates and cyclizes AA to form PGG2 via its COX function, and this is followed by the reduction of PGG2 to PGH2 via its peroxidase function. PGH2 is then converted to PGE2 by the terminal synthase, PGE2 synthase (PGES). PGE2 synthesis is mainly determined by the availability of the substrate AA and the activity of enzyme COX. As AA in total cellular fatty acid or in phospholipids fraction has been shown either to remain unchanged or to decrease with age, we focused on the rate‐limiting enzyme COX.
Figure 4.
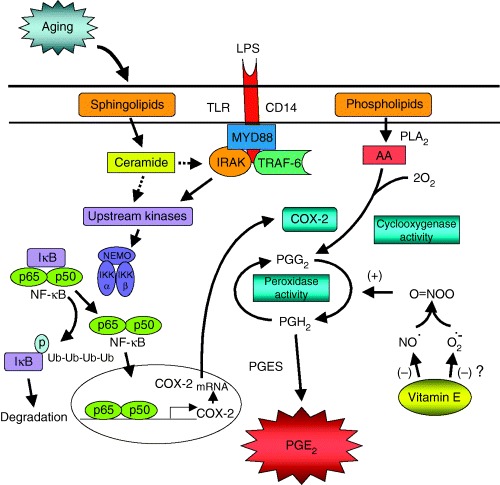
Mechanisms for age and vitamin E‐induced changes in macrophage PGE2. Binding of LPS to its receptor CD14 at Mφ surface triggers the activation of IκB kinase (IKK) complex, including IKKα, IKKβ, and IKKγ/NF‐κB essential modulator (NEMO), downstream of TRAF‐6. IKK is a major kinase responsible for the phosphorylation of IκB. Phosphorylated IκB is further ubiquitinated and eventually degraded, leaving the NF‐κB dimer free to translocate to nucleus. IκB degradation is increased in murine Mφ, which results in an increased translocation of dimer to nucleus. In the nucleus, NF‐κB binds to the promoter region of COX‐2 gene and initiates its transcription. The resulting mRNA of COX‐2 encodes synthesis of COX‐2 enzyme. COX‐2 catalyzes metabolism of AA to PGH2, which is further isomerized to PGE2. The age‐associated increase in activation leads to higher expression of COX‐2, COX‐2 activity, and PGE2 production. Although only the LPS‐induced activation pathway is illustrated here, age‐associated upregulation in COX‐2 expression and transcription process is not stimulus‐specific. The higher COX‐2 expression in old Mφ is shown to be due to higher level of sphingolipid ceramide. LPS‐stimulated Mφ produce more ceramide in old than in young mice. Ceramide upregulates COX‐2 expression through NF‐κB activation. Ceramide addition to young Mφ increases COX‐2‐specific NF‐κB activation, COX‐2 expression, and PGE2 production. Ceramide‐induced COX‐2 upregulation is diminished in the presence of NF‐κB decoy or IκB inhibitor. The mechanism by which ceramide activates NF‐κB degradation can only be speculated at this time (shown as dotted lines) and remains to be determined. Vitamin E inhibits COX activity but has no effect on expression levels of protein and mRNA of either COX‐1 or COX‐2.Vitamin E modulates COX activity post‐translationally, through its inhibitory effect on the production of peroxynitrite, a molecule shown to enhance COX activity without affecting COX enzyme expression. Mφ from old mice produce more NO than those from young mice. Vitamin E reduces NO production, and thus peroxynitrite synthesis.
There are different isoenzymes of COX, a constitutive form (COX‐1) and an inducible form (COX‐2). COX‐1 is constitutively expressed and is believed to be responsible for producing prostanoids to maintain physiological functions such as gastric protection and renal function. In contrast, COX‐2 is regulated by growth factors, tumor promoters, cytokines, mitogens, glucocorticoids, and bacterial endotoxin, and is implicated in inflammatory responses and pathological changes in numerous disorders. Recently, the presence of a third form of COX, referred to as COX‐3, has been proposed (30); however, little is known about its significance.
Hayek et al. (31) reported on the role of COX in the age‐related increase in PGE2 synthesis. They first showed that LPS‐stimulated peritoneal Mφ from old (24 months) C57BL/6NIA mice produced more PGE2 than those from young (6 months). PGE2 measured in this manner represents its accumulated synthesis utilizing endogenous substrate AA with all three enzymatic reactions, i.e. PLA2, COX, and PGE synthetase (PGES) involved. Thus, to test the hypothesis that age‐associated increase in PGE2 is due to an increase in COX activity, Hayek et al. (31) compared COX activity between young and old Mφ in the presence of adequate level of AA and showed that COX activity was significantly higher in old compared to young Mφ. PGES is not considered a rate‐limiting factor in PGE2 production, although recent publications suggested that it couples to COX and may participate in regulation of PGE2 synthesis.
Hayek et al. (31) further showed that higher COX activity in old Mφ is due to increased COX‐2 protein synthesis preceded by an elevated COX‐2 mRNA expression. This elevated COX‐2 mRNA expression was later shown to be due to a higher rate of transcription and not a change in the stability of COX‐2 mRNA (32). No age‐related difference in COX‐1 protein or mRNA expression was observed (31). Age‐associated increase in COX‐2 expression is not limited to Mφ or LPS stimulation. In addition to LPS, IL‐1β stimulates peritoneal Mφ from old mice to produce more PGE2 than those from young mice (33); calcium ionophore or T‐cell mitogens also stimulate more PGE2 production in the splenocytes of old mice or peripheral blood mononuclear cells of humans compared with their young counterparts (5, 7, 28). Age‐related increase in COX products has also been observed in mouse lungs (34), human platelets (35), and human urine (36).
Ceramide is a sphingolipid second messenger generated from the hydrolysis of membrane sphingomyelin under the action of sphingomyelinase (SMase), or by de novo synthesis. Ceramide is involved in the regulation of cell differentiation, proliferation, and apoptosis through multiple signaling pathways (37). LPS can induce intracellular ceramide generation, while ceramide and SMase mimic LPS action in murine Mφ (38). Age‐related increase in brain and liver ceramide and neutral SMase levels has been reported 39, 40, 41). In a senescence model of WI‐38 human diploid fibroblasts, Venable et al. (42) found that endogenous levels of ceramide and neutral SMase activity are significantly elevated as the cells entered the senescent phase. Thus, we hypothesized that ceramide might mediate the age‐associated increase in COX‐2 expression and thus PGE2 production. This was supported by the work of Claycombe et al. (32), which showed that Mφ from old mice generate significantly more intracellular ceramide than those from young mice within 30 – 60 min of LPS stimulation. Addition of exogenous ceramide to the Mφ from young mice dose‐dependently increased PGE2 production and COX activity. Ceramide also further enhanced LPS‐stimulated PGE2 production and COX‐2 protein expression. This effect of ceramide was shown to be due to its stimulatory effect on COX‐2 mRNA transcription (32).
As age‐associated increase in COX‐2 mRNA expression is due to its upregulated transcription in which ceramide plays a role, we further investigated the mechanism involved in COX‐2 transcriptional activation. The binding sites for several nuclear transcription factors, such as nuclear factor κB (NF‐κB), nuclear factor interleukin‐6, and cAMP‐responsive element (CRE), have been identified on the promoter region of the COX‐2 gene 43, 44, 45). Another redox‐sensitive transcription factor, activator protein‐1 (AP‐1), has also been shown to be involved in COX‐2‐transcriptional regulation by binding to the CRE binding site (46, 47). No significant difference in LPS‐stimulated AP‐1 or CREB activity was found between young and old Mφ. However, NF‐κB‐binding activity, both consensus and COX‐2 specific, was higher in old Mφ compared to that of young (33). Further studies indicated that the higher NF‐κB activation might be due to increased ΙκB degradation. The link between NF‐κB activity and COX‐2 activation was supported by the observation that COX‐2 mRNA and protein expression, COX activity, and PGE2 production were reduced when activation was blocked by using either a pharmacological inhibitor of IκB phosphorylation or a specific NF‐κB decoy (33). The authors further showed that ceramide stimulates by itself and also potentiates LPS‐induced PGE2 production and COX‐2 expression, and this effect of ceramide was mediated through inducing NF‐κB activation (33). A summary of different steps involved in age‐associated upregulation of COX‐2 and the role of ceramide and NF‐κB is presented in Fig. 4 .
Effect of vitamin E on PGE2 production and its underlying mechanism
To determine the mechanism of vitamin E‐induced decrease in PGE2 production, Wu et al. (48) conducted a study in which young (6 months) and old (24 months) C57BL/6NIA mice were fed semisynthetic diets containing 30 ppm (control) or 500 ppm (supplementation) vitamin E for 30 days. At the end of the feeding period, LPS‐stimulated Mφ from old mice fed the control diet had significantly higher production of PGE2 at 12 and 24 h compared to those from young mice fed the control diet. Furthermore, Wu et al (48). showed that vitamin E exerts its effect by decreasing COX‐2 activity. However, LPS‐induced COX‐2 protein expression was not altered by vitamin E supplementation, and similarly, COX‐2 mRNA expression was not affected. Thus, the vitamin E‐induced decrease in COX activity of Mφ from old mice is not due to its regulation of COX transcription or translation; rather, it appears that vitamin E exerts its effect post‐translationally ( Fig. 4 ). Similarly, other investigators (49, 50) reported no effect of vitamin E on COX expression.
COX activity requires the presence of oxidant hydroperoxides 51, 52, 53). The lag phase in attaining maximal COX activity was shortened or eliminated by endogenous or exogenous hydroperoxides while being delayed by antioxidants (53). Vitamin E is an effective biological antioxidant and a chain‐breaking free‐radical scavenger, and therefore may attenuate COX activity by scavenging the oxidant hydroperoxide necessary for COX activation.
Free‐radical nitric oxide (NO) has been shown to be involved in the regulation of COX activity and eicosanoids synthesis ( Fig. 4 ). It has been suggested that NO stimulates COX activity via direct stimulation of the enzyme (54). LPS‐stimulated peritoneal Mφ from old mice were shown to produce more NO than those from young mice (55, 56), while dietary supplementation with vitamin E was shown to reduce NO production in Mφ from old mice (55). NO can be further metabolized to peroxynitrite (ONOO) in the presence of superoxide, and ONOO has been shown to increase the activity of COX without affecting its expression (57). Therefore, we hypothesized that decreased NO and thus ONOO formation may mediate the inhibition of COX activity by vitamin E. This hypothesis was tested in a study conducted by Beharka et al. (55) in which young (6 months) and old (24 months) mice were fed 30 (control) or 500 ppm (supplementation) vitamin E for 30 days. Results from this study confirmed previous findings that Mφ from old mice produced more NO than those from young mice. Furthermore, the age‐associated increase of NO was reduced by vitamin E supplementation. Vitamin E supplementation did not affect LPS‐induced superoxide generation, but reduced the further potentiated superoxide generation in the presence of superoxide‐generating agents. Addition of NO donor to cell culture to increase NO levels did not change PGE2 production and COX activity in either young or old mice. However, when NO donor was added in the presence of superoxide to elevate ONOO levels in the culture, vitamin E‐induced inhibition in COX activity in the Mφ from old mice was diminished. On the other hand, when NO and superoxide inhibitors were added to Mφ from old mice fed control diet to block generation of ONOO, COX activity was significantly reduced. These results suggest that vitamin E reduces COX activity in old Mφ by decreasing NO production, which leads to lower production of ONOO in Mφ from old mice ( Fig. 4 ).
Direct effect of vitamin E on T cells
As mentioned above, our previous study indicated that vitamin E has a direct effect on T‐cell functions independent of its effect on Mφ PGE2 production (22). To evaluate if vitamin E has a direct, PGE2‐independent effect on T‐cell responses, T cells purified from the spleens of young and old mice were preincubated with vitamin E or vehicle control (58). Activation‐induced cell division of T cells from old mice was lower than that by young, and the production of IL‐2 following 48‐h activation was less by T cells from old mice. The defect in proliferation and IL‐2 production was specific to naïve T cells and was not observed in memory T cells. Furthermore, there was an age‐related decline in both the number of IL‐2+ T cells and the amount of IL‐2 produced per cell and an age‐related decline in cell division within naïve T‐cell subpopulation ( Fig. 5A,B ). Vitamin E increased both cell‐dividing capability, total IL‐2 production as well as the number of IL‐2+ T cells, and the amount of IL‐2 produced per naïve T cell ( Fig. 5A,B ). These data indicate that naïve T cells exhibit the greatest age‐related defect and show that supplemental vitamin E has a direct immunoenhancing effect on naïve T cells from old mice. The differential effect of vitamin E on naïve and memory T cells may be due to an underlying difference in the susceptibility of these cells to oxidative stress‐induced damage (59).
Figure 5.
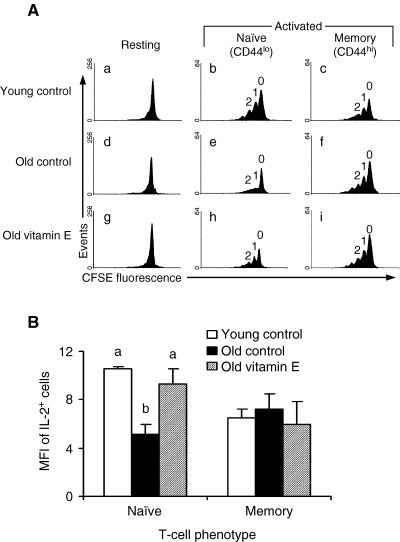
Effects of age and vitamin E. (A) Effects of age and vitamin E on the progression of T cells through cell‐cycle division. Purified T cells were preincubated with 46 µm vitamin E for 4 h, labeled with carboxyfluoroscein succinimidyl ester (CFSE), and activated with immobilized anti‐CD3 and soluble anti‐CD28 mAb for 48 h. Cells were harvested, stained for CD44 expression, and analyzed on a flow cytometer. One representative histogram for each of young control (a, b, and c), old control (d, e, and f), and old preincubated with vitamin E (g, h, and i) are shown. Cell‐cycle division patterns are shown for unactivated T cells (a, d, and g), activated naïve (CD44lo) T cells (b, e, and h), and activated memory (CD44hi) T cells (c, f, and i). Peaks representing cell division cycles 0, 1, and 2 are also indicated. (B) Effect of age and vitamin E on intracellular IL‐2 production by naïve and memory T‐cell subsets. Purified T cells (n = 5) were preincubated with 46 µm vitamin E for 4 h and activated with immobilized anti‐CD3 and soluble anti‐CD28 mAb for 48 h. Cells were treated with monensin, an inhibitor of IL‐2 secretion, for the last 10 h of activation. Harvested cells were stained with fluorochrome‐conjugated anti‐CD44 mAb, permeabilized, and stained with fluorochrome‐conjugated anti‐IL‐2. T cells were divided into naïve and memory phenotypes based on low or high expression of the CD44 antigen, respectively. Cell fluorescence was measured on a flow cytometer. Bars represent the linearized mean fluorescence intensity (MFI) of IL‐2+ T cells. Bars with different letters within each phenotype are significantly different (P < 0.05) by an ANOVA followed by Tukey's HSD posthoc procedure. Reproduced with permission from Adolfsson et al. J Immunol 2001;167:3809–3817. Copyright 2001, The American Association of Immunologists, Inc.
In summary, vitamin E enhances T‐cell function in old mice (i) by directly improving the cell dividing and IL‐2‐producing capacity of naïve T cells and (ii) by indirectly reducing the production of T‐cell suppressive factor PGE2 from Mφ ( Fig. 6 ). The mechanisms through which vitamin E improves cell‐cycle division and IL‐2 production are currently under investigation. Vitamin E may impact several different steps of the activation and proliferation process such as effective immune synapse formation, free‐radical‐sensitive signal‐transduction pathways or cell‐cycle‐related molecules. Our preliminary results indicate that vitamin E may improve effective immune synapse formation in naïve T cells from old mice (60). In addition, microarray analysis of gene‐expression profiles of purified T cells indicated that vitamin E supplementation in old mice increased the expression of cell‐cycle‐related proteins, including cyclin B, Cdc2 (Cdk1), and Cdc6 (61). Cyclin B and Cdc2 are important for entry of cells into M‐phase of the cell cycle, and Cdc6 is a key regulator in early step of DNA replication (62, 63). Additional ongoing studies would further determine the key steps in naïve T‐cell activation and cell‐cycle division, which are influenced by vitamin E.
Figure 6.
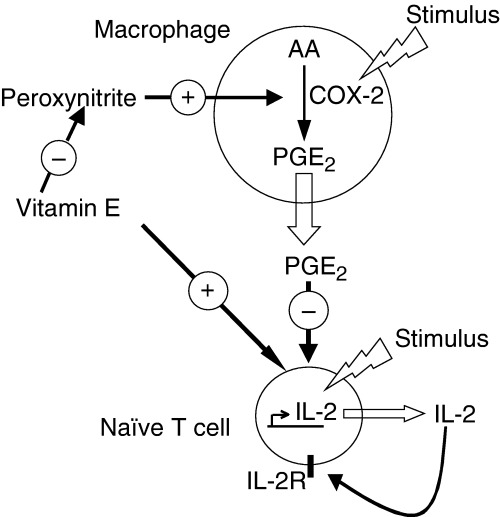
Supplemental vitamin E increases the function of T cells from the aged by at least two different mechanisms. Vitamin E enhances T‐cell function indirectly by reducing the age‐related increase in the production of T‐cell‐suppressive PGE2 by macrophages by reducing peroxynitrite formation. Additionally, a direct PGE2‐independent effect of vitamin E on the function of naïve T cells in the aged has also been shown. Reproduced with permission from Adolfsson et al. J Immunol 2001;167:3809–3817. Copyright 2001, The American Association of Immunologists.
Vitamin E and infections
The immunostimulatory effect of vitamin E has been shown to be associated with resistance to infections. Most of the animal studies that investigated the effect of vitamin E on infectious diseases reported a protective effect despite the variations in the dose and duration of the supplementation, infectious organisms involved, and route of administration. Vitamin E supplementation in old mice resulted in significantly lower viral titer on 2, 5, and 7 days postinfection and preserved antioxidant nutrient status following influenza virus infection. A significant inverse correlation was observed between hepatic vitamin E levels and lung viral titers (6, 64). Preservation of antioxidant status might protect tissues from oxidative stress caused by the virus itself or by the host's effector mechanisms. Han et al. (65) showed that vitamin E supplementation of old mice infected with influenza significantly increased production of IL‐2 and IFN‐γ. The change in IFN‐γ production significantly correlated with the decrease in viral titer. These results suggest that the enhancement of Th1 response is one of the mechanisms through which vitamin E provides protection against influenza infection in the old mice. Dysregulation of Th1 and Th2 functions are observed with aging. Decreased production of and responsiveness to IL‐2 as a result of aging have been demonstrated (66). In addition, aged mice were shown to have decreased ability to produce IFN‐γ following infection with Legionella pneumophilia or influenza virus (65, 67, 68). These changes in Th1/Th2 balance can contribute to delayed clearance and recovery from influenza infection. Th1 clones are cytolytic in vitro against influenza‐infected target cells, and adoptive transfer of a Th1 clone protects against lethal challenge with influenza virus in vivo (69, 70). Whereas, Th2 clones are noncytolytic and failed to promote recovery from lethal infection after adoptive transfer (70). Furthermore, in vivo treatment with IL‐4 suppressed CTL response and IFN‐γ production, and delayed viral clearance (71).
Only a limited number of studies have investigated the effect of vitamin E on resistance against infections in humans. The subjects in these studies were mainly elderly. Infections, particularly respiratory infections, are common in the elderly, resulting in decreased daily activity, prolonged recovery times, increased health care service utilization, and more frequent complications, including death 72, 73, 74).
It is predicted that 43% of all elderly persons will be admitted to a nursing home, with >85% of them admitted to the long‐term (>1 year) care facilities (75). Infections occur more frequently in nursing home residents than among independent‐living elderly 72, 73, 74, 76), and RI is a major cause of morbidity and mortality (73, 77, 78).
Contributing to the increased incidence of infection with age is the well‐described decline in immune response. For example, there is higher morbidity and mortality from cancer, pneumonia, and postoperative complications in those who have diminished delayed‐type hypersensitivity skin test responses (79, 80).
Nutritional status is an important determinant of immune function (81). Nutritional supplementation has been shown to enhance older subjects' immune response (82, 83). Meydani et al. (5, 18) showed that vitamin E supplementation improved immune response, including DTH and response to vaccines in healthy elderly. Furthermore, they reported a nonsignificant (P < 0.09), 30% lower incidence of self‐reported infections among the groups supplemented with vitamin E (60, 200, or 800 mg/day for 235 days) compared with the placebo group (18). As infection was not the primary outcome, the study did not have enough power to detect significant differences in the incidence of infections. To overcome these limitations, we conducted a large double‐blind, placebo‐controlled trial to determine the effect of 1‐year supplementation with vitamin E on objectively recorded respiratory infections in elderly nursing home residents (84).
In this randomized, double‐blind study, 617 people aged over 65 residing at 33 nursing homes in the Boston area who met the study's eligibility criteria received either a placebo or 200 IU of vitamin E (dl‐α‐tocopherol) daily for 1 year. All participants received a capsule containing half the recommended daily allowance of essential vitamins and minerals. The main outcomes of the study were incidence of respiratory tract infections, number of persons and number of days with respiratory infections (upper and lower), and number of new antibiotic prescriptions for respiratory infections among all randomized participants and those who completed the study.
Significantly fewer vitamin E‐supplemented subjects acquired one or more respiratory infections (65 versus 74%, RR = 0.87, CI = 0.73–0.99, P = 0.035), or upper respiratory infections (50 versus 62%, RR = 0.81, CI = 0.65–0.96, P = 0.015). However, supplementation with vitamin E had no significant effect on the incidence or duration of all respiratory infection taken together, or on upper or lower respiratory tract infections measured separately. Further analysis on the foremost respiratory infection, common cold, indicated that the vitamin E group had a lower incidence of common colds (0.66 versus 0.83 per subject–year, RR = 0.80, CI = 0.64–1.00, P = 0.046), and fewer subjects in the vitamin E group acquired one or more common colds (46 versus 57%, RR = 0.79, CI = 0.63–0.96, P = 0.016) (84). The vitamin E‐treated group also had fewer days with common cold per person–year compared to the placebo group, but the difference did not reach statistical significance (22% less, P = 0.11).
In conclusion, the results of this clinical trial show that vitamin E supplementation significantly reduces the risk for acquiring respiratory infections in elderly. In particular, vitamin E supplementation reduced the incidence rate of common colds and the number of subjects who acquire a cold among elderly nursing home residents. A nonsignificant reduction in the duration of colds was also observed. Because of the high rate and more severe morbidity associated with common colds in this age group, these findings have important implications for the well being of the elderly as well as for the economic burden associated with their care.
Colds are common afflictions for all age groups, and they account for 30% of absenteeism in the USA across all age groups (85). Rhinoviruses and coronaviruses represent the majority of the documented causes of colds 86, 87, 88). They exacerbate COPD (89) and are known to be associated with lower respiratory infections in the elderly (88, 90, 91). For example, a prospective cohort study of community‐based elderly found that rhinoviruses were associated with lower respiratory symptoms in nearly two‐thirds of episodes: about one‐fifth of patients were confined to bed, and 26% were unable to perform routine household activities (91). Constitutional and lower respiratory tract symptoms and signs have been reported to be more common in the elderly compared to younger adults infected with cold viruses (90). Nursing home populations may also be at risk for epidemic outbreaks of rhinovirus infections (92). The common cold is generally less severe than influenza. However, its much higher incidence and its recognized morbidity in the elderly (88, 90, 91, 92) make it an important public health problem in this age group (93). This is particularly relevant, as at present no clinically useful vaccine or antiviral therapy is available to combat colds.
The economic impact of noninfluenza‐related viral upper respiratory infections in general, and in the elderly in particular, has been overlooked. Fendrick et al. (93) showed that because of their high attack rate, these diseases are responsible for an economic burden, which approaches $40 billion annually. Thus, our finding that vitamin E supplementation reduces the common cold by 22% has significant implications for the elderly in reducing the burden of diseases and associated health care costs. Currently, there are 34 million elderly in the US. The observation that vitamin E reduced the risk for acquiring all respiratory infections by 20% will translate into about 7 million fewer elderly acquiring respiratory infection.
Previous studies on vitamin E and infection in the elderly have demonstrated mixed results. A retrospective study showed that subjects with plasma vitamin E levels above 16.7 mg/l had a significantly lower mean number of infections compared to those with plasma vitamin E levels below 12.2 mg/l (1.0 versus 2.3, 95% CI for difference = 0.12–2.48) (94). Hemila et al. (95) evaluated the effect of long‐term vitamin E and beta‐carotene supplementation on the incidence of common cold episodes from a cohort of 21 796 male smokers from the Alpha‐Tocopherol Beta‐Carotene Cancer Prevention Study. Common cold episodes were queried three times per year during a 4‐year follow‐up period. Fifty milligram of vitamin E supplementation resulted in slightly lower incidence of cold among subjects 65 years of age or older (RR = 0.95); this reduction was the greatest among older city dwellers who smoked fewer than 15 cigarettes per day (RR = 0.72).
A recent double‐blind trial of Dutch elderly (96) living in the community reported a rate ratio for all RI among those receiving vitamin E as 1.12 (95% CI = 0.88–1.25), compared to those not receiving vitamin E. The Dutch study differed from that of Meydani et al. (84) in terms of the population and the way that respiratory infections were diagnosed. While our subjects were institutionalized elderly, their incidence of respiratory infection was similar to the community‐dwelling Dutch elderly (96). Furthermore, we have previously shown that vitamin E is effective in improving the immune response in community‐dwelling elderly (5, 18), and although it did not have sufficient power to demonstrate statistical significance, one of these studies showed a 30% reduction of infection in independently living elderly (18).
In the Dutch study (96), subjects self‐reported their infections by telephone, and then the infections were confirmed by nurse visits. However, absence of infection in those not reporting was not confirmed, thus making the study results susceptible to reporting biases. In our study, the presence and type of RI, or absence, was documented by infectious disease specialists based on review of data gathered by trained research nurses during weekly subject interviews, review of medical records, and physical examination focused on respiratory infections using standardized case definitions (76, 90, 97, 98, 99). Furthermore, our results indicate that vitamin E reduces upper respiratory infections, particularly common colds, with no effect on lower respiratory infections or seasonal allergies. Graat et al. (96) did not differentiate between types of infections or between respiratory infection and allergies, and thus might have overlooked any vitamin E effect on upper respiratory infections. In addition, in our study adherence was checked by nursing home medication records and by periodic plasma vitamin E measurements, whereas the Graat et al. (96) study measured plasma vitamin E levels only at baseline. Table 2 summarizes the results from animal and human studies that investigated the effects of vitamin E supplementation on infectious diseases in aged animals and humans.
Table 2.
Effect of vitamin E supplementation on infectious diseases in animals and humans
| Subjects | Age | Dose and duration of supplementation | Infection organism and route of infection | Results: effects of vitamin E supplementation | Reference |
|---|---|---|---|---|---|
| Nursing home residents | >65 | 200 IU/day for 1 year | Natural incidence of respiratory infections | Fewer numbers of subjects with all and upper respiratory infections Lower incidence of common cold No effect on lower respiratory infection | Meydani et al. (84) |
| Male smokers | 50‐69 years | 50 mg/day for median of 6.1 years | Natural incidence of pneumonia | No overall effect on the incidence of pneumonia. Among the subjects who had initiated smoking at a later age (>21) | Hemila et al. (105) |
| Non‐institutionalized individuals | >60 years | 200 mg/day for median of 441 days | Natural incidence and severity of self‐reported acute respiratory tract infections | No effect on incidence and severity of acute respiratory tract infections | Graat et al. (96) |
| Male smokers | 50 mg/day during 4‐year follow‐up | Natural incidence of common cold episodes | Lower incidence of common cold. Reduction was greatest among older city dwellers who smoked fewer than 15 cigarettes per day | Hemila et al. (95) | |
| Mice (C57BL) | 22 months | 500 mg/kg diet for 6 weeks | Influenza by nasal inoculation | Lower viral titer | Heyek et al. (64) |
| Mice (C57BL) | 22 months | 500 mg/kg diet for 6 weeks | Influenza by nasal inoculation | Lower viral titer Higher IL‐2 and IFN‐γ production | Han et al. (65) |
Conclusion
Several investigations have demonstrated that vitamin E significantly enhances immune functions in the elderly. A dose–response relationship can be demonstrated between delayed‐type hypersensitivity skin response and plasma vitamin E levels up to 25 µmol/l of increase in plasma vitamin E levels following supplementation. Elderly with higher plasma vitamin E levels also had a better response to hepatitis B vaccine than those with lower plasma vitamin E levels. Results from cellular and molecular mechanistic studies have shown that immunoregulatory effects of vitamin E are mediated indirectly by reducing the production of suppressive factors such as PGE2 by Mφ and directly by increasing cell‐division capacity and IL‐2 production by naïve T cells. Further studies are needed to determine the mechanism of vitamin E‐induced enhancement of naïve T‐cell function. Preliminary studies indicate that vitamin E might affect membrane‐associated early events in activation of T cells.
Animal studies strongly suggest that vitamin E supplementation improves resistance against infections. Specifically in aged, improved resistance to influenza infection following vitamin E supplementation has been reported. Recent clinical trials also suggest beneficial effect of vitamin E supplementation in reducing the risk of upper respiratory infections, particularly common cold in the elderly. Further controlled clinical trials in humans are required to determine if the beneficial effect of vitamin E in elderly is specific to viral infections or can be extended to other pathogens. The observation that a single nutrient can reverse/reduce specific age‐related defects in immune response is encouraging, as nutrient supplementation presents a practical and inexpensive strategy to reduce the burden of immune‐related diseases in elderly.
References
- 1. Coquette A, Vray B, Vanderpas J. Role of vitamin E in the protection of the resident macrophage membrane against oxidative damage. Arch Int Physiol Biochim 1986;94: 529–534. [PubMed] [Google Scholar]
- 2. Hatman LJ, Kayden HJ. A high‐performance liquid chromatographic method for the determination of tocopherol in plasma and cellular elements of the blood. J Lipid Res 1979;20: 639–645. [PubMed] [Google Scholar]
- 3. Gebremichael A, Levy EM, Corwin LM. Adherent cell requirement for the effect of vitamin E on in vitro antibody synthesis. J Nutr 1984;114: 1297–1305. [DOI] [PubMed] [Google Scholar]
- 4. Kowdley KV, Mason JB, Meydani SN, Cornwall S, Grand RJ. Vitamin E deficiency and impaired cellular immunity related to intestinal fat malabsorption. Gastroenterology 1992;102: 2139–2142. [DOI] [PubMed] [Google Scholar]
- 5. Meydani SN, et al. Vitamin E supplementation enhances cell‐mediated immunity in healthy elderly subjects. Am J Clin Nutr 1990;52: 557–563. [DOI] [PubMed] [Google Scholar]
- 6. Han SN, Meydani SN. Vitamin E and infectious diseases in the aged. Proc Nutr Soc 1999;58: 697–705. [DOI] [PubMed] [Google Scholar]
- 7. Meydani SN, Meydani M, Verdon CP, Shapiro AC, Blumberg JB, Hayes KC. Vitamin E supplementation suppresses prostaglandin E2 synthesis and enhances the immune response of aged mice. Mech Ageing Dev 1986;34: 191–201.DOI: 10.1016/0047-6374(86)90034-5 [DOI] [PubMed] [Google Scholar]
- 8. Sakai S, Moriguchi S. Long‐term feeding of high vitamin E diet improves the decreased mitogen response of rat splenic lymphocytes with aging. J Nutr Sci Vitaminol 1997;43: 113–122. [DOI] [PubMed] [Google Scholar]
- 9. Lee C‐YJ, Wan JM‐F. Vitamin E supplementation improves cell‐mediated immunity and oxidative stress of Asian men and women. J Nutr 2000;130: 2932–2937. [DOI] [PubMed] [Google Scholar]
- 10. Traber MG. Vitamin E In: Shils ME, Olson JA, Shike M, Ross AC, eds. Modern Nutrition in Health and Disease. Baltimore: Williams & Wilkins, 1999: 347–362. [Google Scholar]
- 11. Wu D, Meydani M, Beharka AA, Serafini M, Martin KR, Meydani SN. In vitro supplementation with different tocopherol homologues can affect the function of immune cells in old mice. Free Radic Biol Med 2000;28: 643–651.DOI: 10.1016/S0891-5849(99)00276-2 [DOI] [PubMed] [Google Scholar]
- 12. Jiang Q, Christen S, Shigenaga MK, Ames BN. γ‐Tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr 2001;74: 714–722. [DOI] [PubMed] [Google Scholar]
- 13. Food and Nutrition Board. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, Carotenoids. Washington, DC: National Academy Press, 2000. [PubMed] [Google Scholar]
- 14. Murphy SP, Subar AF, Block G. Vitamin E intake and sources in the United States. Am J Clin Nutr 1990;52: 361–367. [DOI] [PubMed] [Google Scholar]
- 15. National Research Council. Fat‐Soluble Vitamins. Recommended Dietary Allowances. Washington, DC: National Academy Press, 1989:. 78–114. [Google Scholar]
- 16. Ryan A, Craig LD, Finn SC. Nutrient intakes and dietary patterns of older Americans: a national survey. J Geront 1992;47: M145–M150. [DOI] [PubMed] [Google Scholar]
- 17. Panemangalore M, Lee CJ. Evaluation of the indices of retinol and alpha‐tocopherol status in free‐living elderly. J Gerontol 1992;47: B98–B104. [DOI] [PubMed] [Google Scholar]
- 18. Meydani SN, et al. Vitamin E supplementation and in vivo immune response in healthy subjects. JAMA 1997;277: 1380–1386.DOI: 10.1001/jama.277.17.1380 [DOI] [PubMed] [Google Scholar]
- 19. Meydani SN, et al. Antioxidants in experimental amyloidosis of young and old mice In: Glenner GG, et al., eds. Fourth International Symposium on Amyloidosis. New York: Plenum Press, 1986:. 683–692. [Google Scholar]
- 20. De Waart F, Portengen L, Doekes G, Verwaal CJ, Kok FJ. Effect of 3 months vitamin E supplementation on indices of the cellular and humoral immune response in elderly subjects. Br J Nutr 1997;78: 761–774. [DOI] [PubMed] [Google Scholar]
- 21. Pallast EG, et al. Effect of 50‐ and 100‐mg vitamin E supplements on cellular immune function in noninstitutionalized elderly persons. Am J Clin Nutr 1999;69: 1273–1281. [DOI] [PubMed] [Google Scholar]
- 22. Beharka AA, Wu D, Han SN, Meydani SN. Macrophage prostaglandin production contributes to the age‐associated decrease in T cell function which is reversed by the dietary antioxidant vitamin E. Mech Ageing Dev 1997;93: 59–77. [DOI] [PubMed] [Google Scholar]
- 23. Choudhry MA, Ahmed Z, Sayeed MM. PGE (2)‐mediated inhibition of T cell p59 (fyn) is independent of cAMP. Am J Physiol 1999;277: C302–C309. [DOI] [PubMed] [Google Scholar]
- 24. Betz M, Fox BS. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J Immunol 1991;146: 108–113. [PubMed] [Google Scholar]
- 25. Anastassiou ED, Paliogianni F, Balow JP, Yamada H, Boumpas DT. Prostaglandin E2 and other cyclic AMP‐elevating agents modulate IL‐2 and IL‐2R alpha gene expression at multiple levels. J Immunol 1992;148: 2845–2852. [PubMed] [Google Scholar]
- 26. Hilkens CM, Snijders A, Snijdewint FG, Wierenga EA, Kapsenberg ML. Modulation of T‐cell cytokine secretion by accessory cell‐derived products. Eur Respir J Suppl 1996;22: 90s–94s. [PubMed] [Google Scholar]
- 27. Bartocci A, Maggi FM, Welker RD, Veronese F. Age‐related immunosuppression: putative role of prostaglandins In: Powels TJ, Backman RS, Hohn KV, Ramwell P, eds. Prostaglandins and Cancer. New York: Alan R. Riss, 1982:. 725–730. [Google Scholar]
- 28. Hayek MG, Meydani SN, Meydani M, Blumberg JB. Age differences in eicosanoid production of mouse splenocytes: effects on mitogen‐induced T‐cell proliferation. J Gerontol 1994;49: B197–B207. [DOI] [PubMed] [Google Scholar]
- 29. Franklin RA, Arkins S, Li YM, Kelley KW. Macrophages suppress lectin‐induced proliferation of lymphocytes from aged rats. Mech Ageing Dev 1993;67: 33–46.DOI: 10.1016/0047-6374(93)90110-D [DOI] [PubMed] [Google Scholar]
- 30. Chandrasekharan NV, et al. COX‐3, a cyclooxygenase‐1 variant inhibited by acetaminophen and other analgesic/ antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci USA 2002;99: 13926–13931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hayek MG, et al. Enhanced expression of inducible cyclooxygenase with age in murine macrophages. J Immunol 1997;159: 2445–2451. [PubMed] [Google Scholar]
- 32. Claycombe KJ, et al. Ceramide mediates age‐associated increase in macrophage cycooxygenase‐2 expression. J Biol Chem 2002;277: 30784–30791. [DOI] [PubMed] [Google Scholar]
- 33. Wu D, Marko M, Claycombe K, Paulson KE, Meydani SN. Ceramide‐induced and age‐associated increase in macrophage COX‐2 expression is mediated through upregulation of NF‐kappa B activity. J Biol Chem 2003;278: 10983–10992.DOI: 10.1074/jbc.M207470200 [DOI] [PubMed] [Google Scholar]
- 34. Meydani SN, Shapiro AC, Meydani M, Blumberg JB. Lung eicosanoid synthesis is affected by age, dietary fat and vitamin E. J Nutr 1992;122: 1627–1633. [DOI] [PubMed] [Google Scholar]
- 35. Vericel E, Croset M, Sedivy P, Coupron PH, Dechavanne M, Lagarde M. Platelets and aging. I. Aggregation, arachidonic acid metabolism and antioxidant status. Thromb Res 1988;49: 331–342.DOI: 10.1016/0049-3848(88)90313-1 [DOI] [PubMed] [Google Scholar]
- 36. Wilson TW, McCaulay J, Walsey TA. Effects of aging on responses to furosemide. Prostaglandins 1989;38: 675–687.DOI: 10.1016/0090-6980(89)90049-X [DOI] [PubMed] [Google Scholar]
- 37. Cutler RG, Mattson MP. Sphingomyelin and ceramide as regulators of development and lifespan. Mech Ageing Dev 2001;122: 895–908. [DOI] [PubMed] [Google Scholar]
- 38. Joseph CK, et al. Bacterial lipopolysaccharide has structural similarity to ceramide and stimulates ceramide‐activated protein kinase in myeloid cells. J Biol Chem 1994;269: 17606–17610. [PubMed] [Google Scholar]
- 39. Lightle SA, Oakley JI, Nikolova‐Karakashian MN. Activation of sphingolipid turnover and chronic generation of ceramide and sphingosine in liver during aging. Mech Ageing Dev 2000;120: 111–125.DOI: 10.1016/S0047-6374(00)00191-3 [DOI] [PubMed] [Google Scholar]
- 40. Palestini P, Masserini M, Fiorilli A, Calappi E, Tettamanti G. Age‐related changes in the ceramide composition of the major gangliosides present in rat brain subcellular fractions enriched in plasma membranes of neuronal and myelin origin. J Neurochem 1993;61: 955–960. [DOI] [PubMed] [Google Scholar]
- 41. Petkova DH, Momchilova‐Pankova AB, Markovska TT, Koumanov KS. Age‐related changes in rat liver plasma membrane sphingomyelinase activity. Exp Gerontol 1988;23: 19–24.DOI: 10.1016/0531-5565(88)90016-2 [DOI] [PubMed] [Google Scholar]
- 42. Venable ME, Lee JY, Smyth MJ, Bielawska A, Obeid LM. Role of ceramide in cellular senescence. J Biol Chem 1995;270: 30701–30708.DOI: 10.1074/jbc.270.51.30701 [DOI] [PubMed] [Google Scholar]
- 43. Inoue H, Nanayama T, Hara S, Yokoyama C, Tanabe T. The cyclic AMP response element plays an essential role in the expression of the human prostagladin‐endoperoxide synthase 2 gene in differentiated U937 monocytic cells. FEBS Lett 1994;350: 51–54.DOI: 10.1016/0014-5793(94)00731-4 [DOI] [PubMed] [Google Scholar]
- 44. Xie W, Fletcher BS, Andersen RD, Herschman HR. v‐src induction of the TIS10/PGS2 prostaglandin synthase gene is mediated by an ATF/CRE transcription response element. Mol Cell Biol 1994;14: 6531–6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yamamoto K, Arakawa T, Ueda N, Yamamoto S. Transcriptional roles of nuclear factor κB and nuclear factor‐interleukin‐6 in the tumor necrosis factor α‐dependent induction of cyclooxygenase‐2 in MC3T3–E1 cells. J Biol Chem 1995;270: 31315–31320.DOI: 10.1074/jbc.270.52.31315 [DOI] [PubMed] [Google Scholar]
- 46. Subbaramaiah K, Lin DT, Hart JC, Dannenberg AJ. Peroxisome proliferator‐activated receptor gamma ligands suppress the transcriptional activation of cyclooxygenase‐2. Evidence for involvement of activator protein‐1 and CREB‐binding protein/p300. J Biol Chem 2001;276: 12440–12448.DOI: 10.1074/jbc.M007237200 [DOI] [PubMed] [Google Scholar]
- 47. Von Knethen A, Brune B. Superinduction of cyclooxygenase‐2 by NO(*) and agonist challenge involves transcriptional regulation mediated by AP‐1 activation. Biochemistry 2000;39: 1532–1540. [DOI] [PubMed] [Google Scholar]
- 48. Wu D, et al. Age‐associated increase in PGE2 synthesis and COX activity in murine macrophages is reversed by vitamin E. Am J Physiol (Cell Physiol) 1998;275: C661–C668. [DOI] [PubMed] [Google Scholar]
- 49. O'Leary KA, De Pascual‐Tereasa S, Needs PW, Bao YPNMOB, Williamson G. Effect of flavonoids and vitamin E on cyclooxygenase‐2 (COX‐2) transcription. Mutat Res 2004;551: 245–254. [DOI] [PubMed] [Google Scholar]
- 50. Jiang Q, Elson‐Schwab I, Courtemanche C, Ames BN. Gamma‐tocopherol and its major metabolite, in contrast to alpha‐tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc Natl Acad Sci USA 2000;97: 11494–11499.DOI: 10.1073/pnas.200357097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Smith WL, Eling TE, Kulmacz RJ, Marnett LJ, Tsai A. Tyrosyl radicals and their role in hydroperoxide‐dependent activation and inactivation of prostaglandin endoperoxide synthase. Biochemistry 1992;31: 3–7. [DOI] [PubMed] [Google Scholar]
- 52. Kulmacz RJ, Wang L‐H. Comparison of hydroperoxide inhibitor requirements for the cyclooxygenase activities of prostaglandin H synthase‐1 and ‐2. J Biol Chem 1995;270: 24019–24023.DOI: 10.1074/jbc.270.41.24019 [DOI] [PubMed] [Google Scholar]
- 53. Hemler ME, Lands WEM. Evidence for a peroxide‐initiated free radical mechanism of prostaglandin biosynthesis. J Biol Chem 1980;255: 6253–6261. [PubMed] [Google Scholar]
- 54. Salvemini D, Settle SL, Masferrer JL, Seibert K, Currie MG, Needleman P. Regulation of prostaglandin production by nitric oxide; an in vivo analysis. Br J Pharmacol 1995;114: 1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Beharka AA, Wu D, Serafini M, Meydani SN. Mechanism of vitamin E inhibition of cyclooxygenase activity in macrophages from old mice: role of peroxynitrite. Free Radic Biol Med 2002;32: 503–511. [DOI] [PubMed] [Google Scholar]
- 56. Chen L‐C, Pace J, Russell S, Morrison D. Altered regulation of inducible nitric oxide synthase expression in macrophages from senescent mice. Infect Immun 1996;64: 4288–4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Landino LM, Crews BC, Timmons MD, Morrow JD, Marnett LJ. Peroxynitrite, the coupling product of nitric oxide and superoxide, activates prostaglandin biosynthesis. Proc Natl Acad Sci USA 1996;93: 15069–15074.DOI: 10.1073/pnas.93.26.15069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Adolfsson O, Huber BT, Meydani SN. Vitamin E‐enhanced IL‐2 production in old mice: naive but not memory T cells show increased cell division cycling and IL‐2‐producing capacity. J Immunol 2001;167: 3809–3817. [DOI] [PubMed] [Google Scholar]
- 59. Lohmiller JJ, Roellich KM, Toledano A, Rabinovitch PS, Wolf NS, Grossmann A. Aged murine T‐lymphocytes are more resistant to oxidative damage due to the predominance of the cells possessing the memory phenotype. J Gerontol A Biol Sci Med Sci 1996;51: B132–B140. [DOI] [PubMed] [Google Scholar]
- 60. Ahmed T, Marko M, Wu D, Chung HK, Huber BT, Meydani SN. Vitamin E supplementation reverses the age associated decrease in immune synapse formation in CD4+ cells. FASEB J 2004: 18: A8DOI: 10.1096/fj.02-1212rev [DOI] [PubMed] [Google Scholar]
- 61. Han SN, Adolfsson O, Lee C‐K, Prolla TA, Ordovas J, Meydani SN. Vitamin E and gene expression in immune cells. Ann NY Acad Sci 2004;1031: 96–101 DOI: 10.1196/annals.1331.010 [DOI] [PubMed] [Google Scholar]
- 62. Quadri RA, Arbogast A, Phelouzat MA, Boutet S, Plastre O, Proust JJ. Age‐associated decline in cdk1 activity delays cell cycle progression of human T lymphocytes. J Immunol 1998;161: 5203–5209. [PubMed] [Google Scholar]
- 63. Pelizon C. Down to the origin: Cdc6 protein and the competence to replicate. Trends Cell Biol 2003;13: 110–113.DOI: 10.1016/S0962-8924(03)00024-2 [DOI] [PubMed] [Google Scholar]
- 64. Hayek MG, et al. Vitamin E supplementation decreases lung virus titers in mice infected with influenza. J Infect Dis 1997;176: 273–276. [DOI] [PubMed] [Google Scholar]
- 65. Han SN, et al. Vitamin E supplementation increases T helper 1 cytokine production in old mice infected with influenza virus. Immunology 2000;100: 487–493.DOI: 10.1046/j.1365-2567.2000.00070.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Miller RA. The aging immune system: primer and prospectus. Science 1996;273: 70–74. [DOI] [PubMed] [Google Scholar]
- 67. Fujio H, Kawamura I, Miyamoto H, Mitsuyama M, Yoshida S‐I. Decreased capacity of aged mice to produce interferon‐gamma in Legionella pneumophila infection. Mech Ageing Dev 1995;81: 97–106.DOI: 10.1016/0047-6374(95)01588-Q [DOI] [PubMed] [Google Scholar]
- 68. Mbawuike IN, et al. Reversal of age‐related deficient influenza virus‐specific CTL responses and IFN‐γ production by monophosphoryl lipid A. Cell Immunol 1996;173: 64–78.DOI: 10.1006/cimm.1996.0252 [DOI] [PubMed] [Google Scholar]
- 69. Lukacher A, Morrison L, Braciale V, Braciale T. T Lymphocyte Function in Recovery from Experimental Viral Infection: the Influenza Model. New York: Rockefeller University Press, 1986. [Google Scholar]
- 70. Graham MB, Braciale VL, Braciale TJ. Influenza virus‐specific CD4+ T helper type 2 T lymphocytes do not promote recovery from experimental virus infection. J Exp Med 1994;180: 1273–1282.DOI: 10.1084/jem.180.4.1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Moran TM, Isobe H, Fernandez‐Sesma A, Schulman JL. Interleukin‐4 causes delayed virus clearance in influenza virus‐infected mice. J Virol 1996;70: 5230–5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Farber BF, Brennen C, Puntereri AJ, Brody JP. A prospective study of nosocomial infections in a chronic care facility. J Am Geriatr Soc 1984;32: 499–502. [DOI] [PubMed] [Google Scholar]
- 73. Crossley KB, Peterson PK. Infections in the elderly. Clin Infect Dis 1996;22: 209–215. [DOI] [PubMed] [Google Scholar]
- 74. Mehr DR, Foxman B, Colombo P. Risk factors for mortality from lower respiratory infections in nursing home patients. J Fam Prac 1992;34: 585–591. [PubMed] [Google Scholar]
- 75. Gabrel CS. Characteristics of elderly nursing home current residents and discharges: data from the 1997 National Nursing Home Survey. Adv Data 2000;312: 1–15. [PubMed] [Google Scholar]
- 76. Ruben FL, et al. Clinical infections in the non‐institutionalized geriatric age group: methods utilized and incidence of infections. Am J Epidemiol 1995;141: 145–157. [DOI] [PubMed] [Google Scholar]
- 77. Muder RR. Pneumonia in residents of long‐term care facilities. Epidemiology, etiology, management, and prevention. Am J Med 1998;105: 319–330.DOI: 10.1016/S0002-9343(98)00262-9 [DOI] [PubMed] [Google Scholar]
- 78. Marston BJ, et al. Incidence of community‐acquired pneumonia requiring hospitalization. Results of a population‐based active surveillance Study in Ohio. The Community‐Based Pneumonia Incidence Study Group. Arch Intern Med 1997;157: 1709–1718.DOI: 10.1001/archinte.157.15.1709 [DOI] [PubMed] [Google Scholar]
- 79. Wayne SJ, Rhyne RL, Garry PJ, Goodwin JS. Cell‐mediated immunity as a predictor of morbidity and mortality in the aged. J Gerontol Med Sci 1990;45: M45–M48. [DOI] [PubMed] [Google Scholar]
- 80. Cohn JR, Hohl CA, Buckley CE. The relationship between cutaneous cellular immune responsiveness and mortality in a nursing home population. J Am Geriatr Soc 1983;31: 808–809. [DOI] [PubMed] [Google Scholar]
- 81. Keusch GT, Wilson CS, Waksal SD. Nutrition, host defenses, and the lymphoid system. Adv Host Def Mech 1983;2: 275–306. [Google Scholar]
- 82. Chandra RK. Effect of vitamin and trace‐element supplementation on immune responses and infection in elderly subjects. Lancet 1992;340: 1124–1127. [DOI] [PubMed] [Google Scholar]
- 83. Meydani SN, Santos MS. Aging: nutrition and immunity In: Gershwin ME, German JB, Keen CL, eds. Nutrition and Immunology: Principles and Practice. Totowa, NJ: Humana Press Inc., 2000: 403–421. [Google Scholar]
- 84. Meydani SN, et al. Vitamin E and respiratory tract infections in elderly nursing home residents: a randomized controlled trial. J Am Med Assoc 2004;292: 828–836.DOI: 10.1001/jama.292.7.828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Monto AS, Ullman BM. Acute respiratory illness in an American community. The Tecumseh study. J Am Med Assoc 1974;227: 164–169.DOI: 10.1001/jama.227.2.164 [DOI] [PubMed] [Google Scholar]
- 86. Arruda E, Pitkaranta A, Witek TJ, Doyle CA, Hayden FG. Frequency and natural history of rhinovirus infections in adults during autumn. J Clin Microbiol 1997;35: 2864–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Makela MJ, et al. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol 1998;36: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nicholson KG, Kent J, Hammersley V, Cancio E. Acute viral infections of upper respiratory tract in elderly people living in the community: comparative, prospective, population based study of disease burden. BMJ 1997;315: 1060–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Seemungal T, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;164: 1618–1623. [DOI] [PubMed] [Google Scholar]
- 90. Falsey AR, et al. The ‘common cold’ in frail older persons: impact of rhinovirus and coronavirus in a senior daycare center. J Am Geriatr Soc 1997;45: 706–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nicholson KG, Kent J, Hammersley V, Cancio E. Risk factors for lower respiratory complications of rhinovirus infections in elderly people living in the community: prospective cohort study. BMJ 1996;313: 1119–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wald TG, Shult P, Krause P, Miller BA, Drinka P, Gravenstein S. A rhinovirus outbreak among residents of a long‐term care facility. Ann Intern Med 1995;123: 588–593. [DOI] [PubMed] [Google Scholar]
- 93. Fendrick AM, Monto AS, Nightengale B, Sarnes M. The economic burden of non‐influenza‐related viral respiratory tract infection in the United States. Arch Intern Med 2003;163: 487–494.DOI: 10.1001/archinte.163.4.487 [DOI] [PubMed] [Google Scholar]
- 94. Chavance M, Herbeth B, Fournier C, Janot C, Vernhes G. Vitamin status, immunity and infections in an elderly population. Eur J Clin Nutr 1989;43: 827–835. [PubMed] [Google Scholar]
- 95. Hemila H, Kaprio J, Albanes D, Heinonen OP, Virtamo J. Vitamin C, vitamin E, and beta‐carotene in relation to common cold incidence in male smokers. Epidemiology 2002;13: 32–37.DOI: 10.1097/00001648-200201000-00006 [DOI] [PubMed] [Google Scholar]
- 96. Graat JM, Schouten EG, Kok FJ. Effect of daily vitamin E and multivitamin‐mineral supplementation on acute respiratory tract infections in elderly persons. J Am Med Assoc 2002;288: 715–721.DOI: 10.1001/jama.288.6.715 [DOI] [PubMed] [Google Scholar]
- 97. Tyrrell DA, Cohen S, Schlarb JE. Signs and symptoms in common colds. Epidemiol Infect 1993;111: 143–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Dykewicz MS, et al. Diagnosis and management of rhinitis: complete guidelines of the Joint Task Force on Practice Parameters in Allergy, Asthma and Immunology. American Academy of Allergy, Asthma, and Immunology. Ann Allergy Asthma Immunol 1998;81: 478–518. [DOI] [PubMed] [Google Scholar]
- 99. McGeer A, et al. Definition of infection for surveillance in long‐term care facilities. Am J Infect Control 1991;19: 1–7. [DOI] [PubMed] [Google Scholar]
- 100. Meydani SN, Han SN, eds. Nutrient Regulation of the Immune Response: the Case of Vitamin E, 8th edn Washington, DC: ILSI Press, 2001. [Google Scholar]
- 101. Wakikawa A, Utsuyama M, Wakabayashi A, Kitagawa M, Hirokawa K. Vitamin E enhances the immune functions of young but not old mice under restraint stress. Exp Gerontol 1999;34: 853–862.DOI: 10.1016/S0531-5565(99)00055-8 [DOI] [PubMed] [Google Scholar]
- 102. Cannon JG, et al. Acute phase response in exercise. II. Associations between vitamin E, cytokines, and muscle proteolysis. Am J Physiol 1991;260: R1235–R1240. [DOI] [PubMed] [Google Scholar]
- 103. Ziemlanski S, Wartanowicz M, Klos A, Raczka A, Klos M. The effect of ascorbic acid and alpha‐tocopherol supplementation on serum proteins and immunoglobulin concentration in the elderly. Nutr Int 1986;2: 1–5. [Google Scholar]
- 104. Harman D, Miller RW. Effect of vitamin E on the immune response to influenza virus vaccine and the incidence of infectious disease in man. Age 1986;9: 21–23. [Google Scholar]
- 105. Hemila H, Virtamo J, Albanes D, Kaprio J. Vitamin E and beta‐carotene supplementation and hospital‐treated pneumonia incidence in male smokers. Chest 2004;125: 557–565. [DOI] [PubMed] [Google Scholar]


