Abstract
Background
In epidemics of highly infectious diseases, such as Ebola, severe acute respiratory syndrome (SARS), or coronavirus (COVID‐19), healthcare workers (HCW) are at much greater risk of infection than the general population, due to their contact with patients' contaminated body fluids. Personal protective equipment (PPE) can reduce the risk by covering exposed body parts. It is unclear which type of PPE protects best, what is the best way to put PPE on (i.e. donning) or to remove PPE (i.e. doffing), and how to train HCWs to use PPE as instructed.
Objectives
To evaluate which type of full‐body PPE and which method of donning or doffing PPE have the least risk of contamination or infection for HCW, and which training methods increase compliance with PPE protocols.
Search methods
We searched CENTRAL, MEDLINE, Embase and CINAHL to 20 March 2020.
Selection criteria
We included all controlled studies that evaluated the effect of full‐body PPE used by HCW exposed to highly infectious diseases, on the risk of infection, contamination, or noncompliance with protocols. We also included studies that compared the effect of various ways of donning or doffing PPE, and the effects of training on the same outcomes.
Data collection and analysis
Two review authors independently selected studies, extracted data and assessed the risk of bias in included trials. We conducted random‐effects meta‐analyses were appropriate.
Main results
Earlier versions of this review were published in 2016 and 2019. In this update, we included 24 studies with 2278 participants, of which 14 were randomised controlled trials (RCT), one was a quasi‐RCT and nine had a non‐randomised design.
Eight studies compared types of PPE. Six studies evaluated adapted PPE. Eight studies compared donning and doffing processes and three studies evaluated types of training. Eighteen studies used simulated exposure with fluorescent markers or harmless microbes. In simulation studies, median contamination rates were 25% for the intervention and 67% for the control groups.
Evidence for all outcomes is of very low certainty unless otherwise stated because it is based on one or two studies, the indirectness of the evidence in simulation studies and because of risk of bias.
Types of PPE
The use of a powered, air‐purifying respirator with coverall may protect against the risk of contamination better than a N95 mask and gown (risk ratio (RR) 0.27, 95% confidence interval (CI) 0.17 to 0.43) but was more difficult to don (non‐compliance: RR 7.5, 95% CI 1.81 to 31.1). In one RCT (59 participants), people with a long gown had less contamination than those with a coverall, and coveralls were more difficult to doff (low‐certainty evidence). Gowns may protect better against contamination than aprons (small patches: mean difference (MD) −10.28, 95% CI −14.77 to −5.79). PPE made of more breathable material may lead to a similar number of spots on the trunk (MD 1.60, 95% CI −0.15 to 3.35) compared to more water‐repellent material but may have greater user satisfaction (MD −0.46, 95% CI −0.84 to −0.08, scale of 1 to 5).
Modified PPE versus standard PPE
The following modifications to PPE design may lead to less contamination compared to standard PPE: sealed gown and glove combination (RR 0.27, 95% CI 0.09 to 0.78), a better fitting gown around the neck, wrists and hands (RR 0.08, 95% CI 0.01 to 0.55), a better cover of the gown‐wrist interface (RR 0.45, 95% CI 0.26 to 0.78, low‐certainty evidence), added tabs to grab to facilitate doffing of masks (RR 0.33, 95% CI 0.14 to 0.80) or gloves (RR 0.22, 95% CI 0.15 to 0.31).
Donning and doffing
Using Centers for Disease Control and Prevention (CDC) recommendations for doffing may lead to less contamination compared to no guidance (small patches: MD −5.44, 95% CI −7.43 to −3.45). One‐step removal of gloves and gown may lead to less bacterial contamination (RR 0.20, 95% CI 0.05 to 0.77) but not to less fluorescent contamination (RR 0.98, 95% CI 0.75 to 1.28) than separate removal. Double‐gloving may lead to less viral or bacterial contamination compared to single gloving (RR 0.34, 95% CI 0.17 to 0.66) but not to less fluorescent contamination (RR 0.98, 95% CI 0.75 to 1.28). Additional spoken instruction may lead to fewer errors in doffing (MD −0.9, 95% CI −1.4 to −0.4) and to fewer contamination spots (MD −5, 95% CI −8.08 to −1.92). Extra sanitation of gloves before doffing with quaternary ammonium or bleach may decrease contamination, but not alcohol‐based hand rub.
Training
The use of additional computer simulation may lead to fewer errors in doffing (MD −1.2, 95% CI −1.6 to −0.7). A video lecture on donning PPE may lead to better skills scores (MD 30.70, 95% CI 20.14 to 41.26) than a traditional lecture. Face‐to‐face instruction may reduce noncompliance with doffing guidance more (odds ratio 0.45, 95% CI 0.21 to 0.98) than providing folders or videos only.
Authors' conclusions
We found low‐ to very low‐certainty evidence that covering more parts of the body leads to better protection but usually comes at the cost of more difficult donning or doffing and less user comfort, and may therefore even lead to more contamination. More breathable types of PPE may lead to similar contamination but may have greater user satisfaction. Modifications to PPE design, such as tabs to grab, may decrease the risk of contamination. For donning and doffing procedures, following CDC doffing guidance, a one‐step glove and gown removal, double‐gloving, spoken instructions during doffing, and using glove disinfection may reduce contamination and increase compliance. Face‐to‐face training in PPE use may reduce errors more than folder‐based training.
We still need RCTs of training with long‐term follow‐up. We need simulation studies with more participants to find out which combinations of PPE and which doffing procedure protects best. Consensus on simulation of exposure and assessment of outcome is urgently needed. We also need more real‐life evidence. Therefore, the use of PPE of HCW exposed to highly infectious diseases should be registered and the HCW should be prospectively followed for their risk of infection.
Plain language summary
Protective clothes and equipment for healthcare workers to prevent them catching coronavirus and other highly infectious diseases
Background
Healthcare workers treating patients with infections such as coronavirus (COVID‐19) are at risk of infection themselves. Healthcare workers use personal protective equipment (PPE) to shield themselves from droplets from coughs, sneezes or other body fluids from infected patients and contaminated surfaces that might infect them. PPE may include aprons, gowns or coveralls (a one‐piece suit), gloves, masks and breathing equipment (respirators), and goggles. PPE must be put on correctly; it may be uncomfortable to wear, and healthcare workers may contaminate themselves when they remove it. Some PPE has been adapted, for example, by adding tabs to grab to make it easier to remove. Guidance on the correct procedure for putting on and removing PPE is available from organisations such as the Centers for Disease Control and Prevention (CDC) in the USA.
This is the 2020 update of a review first published in 2016 and previously updated in 2019.
What did we want to find out?
We wanted to know:
what type of PPE or combination of PPE gives healthcare workers the best protection;
whether modifying PPE for easier removal is effective;
whether following guidance on removing PPE reduced contamination;
whether training reduced contamination.
What did we find?
We found 24 relevant studies with 2278 participants that evaluated types of PPE, modified PPE, procedures for putting on and removing PPE, and types of training. Eighteen of the studies did not assess healthcare workers who were treating infected patients but simulated the effect of exposure to infection using fluorescent markers or harmless viruses or bacteria. Most of the studies were small, and only one or two studies addressed each of our questions.
Types of PPE
Covering more of the body leads to better protection. However, as this is usually associated with increased difficulty in putting on and removing PPE, and the PPE is less comfortable, it may lead to more contamination. Coveralls are the most difficult PPE to remove but may offer the best protection, followed by long gowns, gowns and aprons. Respirators worn with coveralls may protect better than a mask worn with a gown, but are more difficult to put on. More breathable types of PPE may lead to similar levels of contamination but be more comfortable. Contamination was common in half the studies despite improved PPE.
Modified PPE
Gowns that have gloves attached at the cuff, so that gloves and gown are removed together and cover the wrist area, and gowns that are modified to fit tightly at the neck may reduce contamination. Also, adding tabs to gloves and face masks may lead to less contamination. However, one study did not find fewer errors in putting on or removing modified gowns.
Guidance on PPE use
Following CDC guidance for apron or gown removal, or any instructions for removing PPE compared to an individual’s own preferences may reduce self‐contamination. Removing gown and gloves in one step, using two pairs of gloves, and cleaning gloves with bleach or disinfectant (but not alcohol) may also reduce contamination.
User training
Face‐to‐face training, computer simulation and video training led to fewer errors in PPE removal than training delivered as written material only or a traditional lecture.
Certainty of the evidence
Our certainty (confidence) in the evidence is limited because the studies simulated infection (i.e. it was not real), and they had a small number of participants.
What do we still need to find out?
There were no studies that investigated goggles or face shields. We are unclear about the best way to remove PPE after use and the best type of training in the long term.
Hospitals need to organise more studies, and researchers need to agree on the best way to simulate exposure to a virus.
In future, simulation studies need to have at least 60 participants each, and use exposure to a harmless virus to assess which type and combination of PPE is most protective.
It would be helpful if hospitals could register and record the type of PPE used by their workers to provide urgently needed, real‐life information.
Search date
This review includes evidence published up to 20 March 2020.
Summary of findings
Background
Description of the condition
Over 59 million people are employed in the healthcare sector worldwide (WHO 2006). Some of these healthcare workers (HCW) are at risk of developing life‐threatening infectious diseases due to contact with patients’ blood or body fluids such as mucus, vomit or exhaled droplets. The risk of infection and its consequences vary, but it is well recognised as an occupational risk (Heptonstall 2010; Sepkowitz 2005). Especially during epidemics, these risks become more visible as the infection rate among HCW is higher than in the general population. Another risk of HCW infection is that infected HCWs will infect patients or that they will act as a vector for the transfer of the disease between patients. In addition, during epidemics, infected HCW will further diminish the capacity of an already overburdened healthcare system.
The 2013 to 2015 Ebola Virus Disease (EVD) epidemic put HCW at high risk of a disease with a very high fatality rate in the epidemic areas (Ebola 2014). According to the World Health Organization (WHO), healthcare workers were between 21 and 32 times more likely to be infected with Ebola than people in the general adult population (Forrester 2014; WHO 2015a). According to the statistics from the 2013‐2015 West Africa EVD epidemic, there were 1049 registered cases of infected HCW with 535 deaths (Kilmarx 2014; WHO 2015b).
Just a decade earlier during the 2002 to 2003 Severe Acute Respiratory Syndrome (SARS) epidemic, 20% of all patients were healthcare workers of whom about 10% lost their lives (WHO 2003).
During the COVID‐19 pandemic, HCW are at higher risk of infection than the general population, just as during other epidemics. Experts strongly urge the use of proper personal protective equipment (PPE) for the HCWs' and patients' safety (Adams 2020; Chang 2020). In a Chinese case‐series of 138 consecutive patients that were hospitalised in Wuhan, China during the month of January 2020, 30% were HCW, which is considerably higher than expected (Wang 2020). Remuzzi 2020 reports that in Lombardy, Italy as of 12 March 2020, 20% of HCW at intensive care units became infected, while Giwa 2020 estimates that at least 10% of HCW in Italy will become infected in spite of using PPE.
HCW may become infected through various routes of transmission, depending on the pathogen. Infection can occur through splashes and droplets of contaminated body fluids on non‐intact skin, or via needle‐stick injuries through intact skin. Infection can also occur when splashes or droplets of contaminated body fluids land on the mucous membranes in the eyes, mouth or nose, or when the same mucous membranes come into contact with contaminated skin, such as when rubbing the eyes with a hand carrying pathogens after touching a patient or contaminated surface (Siegel 2019). For EVD, contact transmission is the main route of transmission. For SARS, the highest risk of infection was due to inhalation of aerosols, but the disease was also transmitted through droplet and contact infection. For COVID‐19 the main route of exposure is through droplet transmission and contact transmission but other transmission routes are also possible (Chang 2020; Otter 2016; Peng 2020).
Here, we focus on highly infectious diseases, which means that contamination with infectious material can readily lead to clinical disease. We also focus on those infections that have serious consequences, such as a high case fatality rate, because the motivation of HCW to protect themselves will be different in situations where the risk is low and the consequences are not serious. The term 'high consequence pathogen' is also used but the list of what constitutes a high consequence pathogen varies from country to country. The European Network for Infectious Diseases defines highly infectious disease as an infectious disease easily transmitted from person to person, causing life‐threatening disease, presenting a serious hazard in healthcare settings and in the community, and requiring specific control measures (Brouqui 2009).
Description of the intervention
In the occupational health field, the 'hierarchy of controls' is best practice. This means that measures with a general effect such as control of exposure should have priority over more individual control measures such as PPE. Exposure of HCW can be best controlled by organisational measures that minimise the exposure to contaminated body fluids or infected patients. The most important preventive measure is the proper organisation of the hospital or healthcare unit to avoid unnecessary contact. Once this has been implemented, the main strategy for reducing physical exposure to highly infectious diseases is through PPE. Both in the European Union (EU) and in the USA, it is mandatory for employers to protect their workers against blood‐borne pathogens and other infections at work (OSHA 2012; EU 2010).
Coveralls, gowns, hoods, masks, goggles and face shields, among others, are used to prevent skin and mucous membranes from becoming contaminated and respirators are used to prevent inhalation. Depending on the transmission route and the specifics of the infection, different types of PPE are recommended. PPE in health care are usually considered as part of what is called transmission‐based precautions. Standard precautions or universal precautions are based on the principle that all blood, body fluids, secretions, excretions except sweat, non‐intact skin, and mucous membranes may contain transmissible infectious agents. Depending on anticipated exposure, hand hygiene and the use of PPE such as gloves, gowns, masks, eye protection (i.e. goggles or face shields) should be implemented. When the route(s) of transmission is (are) not completely interrupted using standard precautions alone, there are three categories that elaborate the precautions to be taken: contact precautions, droplet precautions, and airborne precautions (Siegel 2019).These precautions contain a number of measures including appropriate PPE to prevent the specific modes of transmission.
PPE will only be effective if the equipment can form a barrier between the HCW and the contaminated body fluids. Therefore, standards have been developed that, when complied with, ensure that PPE is of sufficient quality to protect against biohazards (Mäkelä 2014; NIOSH 2014). Even though the biohazard symbol (Figure 1), is widely used to indicate the presence of biohazards, it is not a label for protective clothing. For biohazards, these standards are based on laboratory tests that evaluate to what extent the fabric and the seams of protective clothing are leak‐tight, that is, are they impermeable for liquids, viruses, or both at certain pressure levels. The standards in the EU and the USA are different. PPE should contain a label that specifically indicates the standards against which it has been tested.
1.

International symbol indicating biohazards
Technical standards for PPE
Technical standards for PPE are complicated and the categorisation is confusing. In the EU, there is standard EN 14126 for clothing, specifically coveralls that protect workers against biological hazards from micro‐organisms. Clothing compliant with the standard EN 14126 is further classified according to routes of contamination and the circumstances in which contamination may occur (pressurized contaminated liquid, mechanical contact with substances containing contaminated liquid, contaminated liquid aerosols, contaminated solid particles) based on ISO 2004a and ISO 2004b test methods. There is a separate standard for surgical gowns, EN 13795, but this standard is specifically designed to protect the patient.
In the USA, ANSI/AAMI PB70 2012 standard classifies surgical and isolation gowns according to their liquid barrier performance with four levels of protection, with level 4 offering the most protection against viral and liquid penetration but level 1 offering only minimal water resistance. There are several differences between ANSI/AAMI PB70 2012 and EN 13795 surgical gown classifications. Because the test methods and performance requirements cannot be compared directly, it is difficult to assign equivalency between surgical gowns classified according to ANSI/AAMI PB70 2012 and EN 13795. There is also US standard NFPA 1999 which was specifically developed to address a range of different protective clothing items worn by emergency medical service first responders, and also applies to medical first receivers. NFPA 1999 lists many performance requirements for protective clothing used by emergency medical personnel, including (but not limited to) viral penetration resistance, tensile strength, liquid integrity, and seam strength.
To summarise, the qualities of protective clothing certified by different standards are not fully comparable and complex. Nonetheless, they all aim to ensure that protective clothing is of a quality that prohibits water and blood‐like fluids with virus particles, applied under a specified amount of pressure, from passing through. In addition, some standards have requirements that the whole piece of clothing, including the seams, must be non‐permeable to liquids (NFPA 1999).
Clothing that is manufactured according to the standards mentioned above, at the appropriate level of protection, is impermeable to body fluids and viruses and will technically prevent skin contamination. However, this review does not deal with the technical physical standards of equipment, but rather whether and how its use in practice will prevent contamination and infection.
Guidelines for choosing proper PPE
In 2014, the WHO developed a guideline for infection prevention and control of epidemic‐ and pandemic‐prone acute respiratory infections in health care. The guideline strongly recommends using appropriate PPE as determined by risk assessment (according to the procedure and suspected pathogen). Appropriate PPE when providing care to patients presenting with acute respiratory infection (ARI) syndromes may include a combination of: medical mask (surgical or procedure mask); gloves; long‐sleeved gowns; and eye protection (goggles or face shields). For aerosol‐generating procedures (AGPs) this combination including a surgical or a procedural mask or a particulate respirator is conditionally recommended. If splashing with blood or other body fluids is anticipated and gowns are not fluid‐resistant, a waterproof apron should be worn over the gown (WHO 2014).
For COVID‐19, recommendations for PPE are gloves, masks, goggles or face shields, and long‐sleeved gowns (WHO 2020a; WHO 2020b) with N95 respirators recommended over masks for AGPs, consistent with the WHO 2014 guideline. Masks are further described as medical mask (flat, pleated or cup‐shaped, affixed to head with a strap). Otherwise there are no quality criteria provided for the PPE parts. This is especially worrying because isolation gowns can have very different qualities, of which the end users are usually not aware (Kilinc‐Balci 2016). Most isolation gown models also leave the neck unprotected, which could be a source of contamination (Zamora 2006). Centers for Disease Control and Prevention (CDC) recommends that non‐sterile, disposable patient isolation gowns, which are used for routine patient care in healthcare settings, are appropriate for use by HCW when caring for patients with suspected or confirmed COVID‐19. Current US guidelines do not require use of gowns that conform to any standards (CDC 2020a). If there is a medium to high risk of contamination, CDC recommends isolation gowns that claim moderate to high barrier protection (ANSI/AAMI PB70 2012 level 3 or 4; CDC 2020b). For a proper overview of requirements for and use of isolation gowns see Kilinc‐Balci 2015 and Kilinc‐Balci 2016.
During the EVD epidemic, several guidelines became available for choosing proper PPE (Australian NHMRC 2010; CDC 2014; ECDC 2014; WHO 2016). Even though all guidelines propose using similar protective clothing, there are differences. For example, ECDC 2014 proposes taping gloves, boot covers and goggles onto the coveralls to prevent leaving any openings but the other guidelines do not recommend this. Most guidelines have recently been updated. There are also recommendations for the technical quality of the PPE to be used with Ebola. For gowns, WHO 2016 currently recommends EN 13795 high‐performance surgical gowns or ANSI/AAMI PB70 2012 level 3 (option 1), or level 4 (option 2), or equivalent. As the first option for coveralls, WHO currently recommends protection equivalent to EN 14126, level 3 protection against blood level 2 against viruses.
Overprotection can be a problem. Some propose using three layers of gloves, because according to their experience, this is best practice (Lowe 2014). However, it may make work more difficult, and eventually lead to an increased rather than a decreased risk of infection, especially during doffing (i.e. removing the PPE). For example, the combined use of several respirators probably does not lead to more protection, but considerably increases the burden on the worker (Roberge 2008a; Roberge 2008b).
Donning and doffing of PPE
Despite using proper PPE, probably the biggest risk of infection is associated with self‐contamination by HCW inappropriately removing the PPE (Fischer 2014). Some types of PPE make donning and doffing more difficult, thereby increasing the risk of contamination (Zamora 2006). There is evidence that when doffing PPE, the use of a double pair of gloves decreases the risk of contamination (Casanova 2012). How contamination of PPE occurs has also been clearly illustrated with a simulation study about cleaning up vomit (Makison 2014). The results of such simulation studies should increase HCW's confidence in executing the donning and doffing procedures correctly, and thus can also be an incentive for their uptake and compliance with the guidelines. Therefore, specific guidance has been developed for donning and doffing PPE (CDC 2014; WHO 2016).
Compliance with guidance on correct PPE use in health care is historically poor. HCW sometimes distrust infection control, and using PPE is stressful (Zelnick 2013). For respiratory protection such as masks and respirators, compliance has been reported to be around 50% on many occasions (Nichol 2008). Due to lack of proper fitting and incorrect use, real field conditions almost never match laboratory standards (Coia 2013; Howie 2005). Also, reports of hand hygiene show that there is still much room for improvement, and guidelines recommend education and training in combination with other implementation measures (WHO 2009). From reports of HCW, it is clear that most appropriate PPE is not user‐friendly in tropical conditions. It prevents heat loss through sweating because it is not made of breathable material. A common reason for a breach in the barrier of the PPE is the worker sweating and then instinctively wiping their face (Cherrie 2006).
In this review, we only concentrated on PPE for highly infectious diseases that have serious consequences for health, such as EVD and COVID‐19. We excluded other highly infectious, but less serious viral infections, such as norovirus, as we expected the effect of PPE to be different. We included SARS as it was highly infectious to HCW, sometimes fatal, and had similar recommendations on PPE use and training to COVID‐19.
We did not specifically study the effects of hand hygiene or of respiratory protection to prevent transmission through inhalation. Hand hygiene is also crucial in preventing skin contamination, but this has already been covered in another review (Gould 2010). The protective effect of different types of respiratory protection, and effects of interventions to increase their uptake are covered in two other reviews (Jefferson 2011; Luong Thanh 2016).
How the intervention might work
First, HCW, their supervisors, or occupational health professionals should choose the proper type of PPE, as indicated in the guidance described above. Then, the HCW needs to know how to don and doff PPE according to the guidelines provided. Next, the HCW needs to comply with established procedures for correctly using, donning and doffing PPE. Education and training are used to increase compliance. The emphasis in teaching the correct use of PPE is on doing everything slowly and carefully to minimise the risk of making a mistake. Often an assistant or buddy, sometimes coupled with a mirror, is used while donning PPE, while a hygienist supervises doffing.
Compliance can be increased by personal supervision and instruction, checklists, audits of performance, by providing feedback, and by allowing sufficient time for donning and doffing. Education and training on uptake and compliance with PPE should have an effect in both the short term and the long term (Northington 2007; Ward 2011). Education and training can be seen as one method to increase compliance (Gershon 2009; Hon 2008). Compliance with PPE can also be improved by providing sufficient, comfortable, well‐fitting, and more user‐ and patient‐friendly PPE. Compliance with guidelines has been studied for hand hygiene. There is some evidence that multifaceted interventions and staff involvement are important, but altogether, there is little evidence that allows firm conclusions (Gould 2010).
Why it is important to do this review
From studies conducted during the SARS epidemic and the EVD epidemic it has become clear that the use of gloves, gowns and masks each help to reduce the infection rate in HCW (Appendix 1, Verbeek 2016a). More consistent use of gloves, gowns, masks and goggles was each related to fewer infections among HCW. Also, theoretically, protecting the skin and the mucous membranes of the mouth nose and eyes will prevent transmission. We have therefore little doubt that in a technical sense PPE will help and that the minimum amount of PPE needed is gloves, gown, and mouth, nose and eye protection, as recommended by WHO and CDC. The guidance does not, however, indicate which type or quality‐level of PPE is most protective. In this review, we concentrate on finding out which PPE protects best by only including studies that compare one type of PPE against an alternative type of PPE, such as gowns against coveralls or goggles against face shields only when used as part of full PPE. We do not include studies that compare the use of PPE against no PPE, or studies comparing one type of PPE to another when not used as part of a set of full‐body PPE.
There is still uncertainty about the optimal type, composition, amount, and ways of using full‐body PPE to prevent skin and mucous membrane contamination of HCW while treating patients infected with highly infectious diseases. This is also reflected in the different ways guidelines for PPE are implemented in Europe (De Iaco 2012), and acknowledged in current WHO guidelines regarding EVD (WHO 2016). WHO realises that a safer, more comfortable and culturally appropriate protective system commensurate with the risk is needed and has provided guidance for industry, health workers, engineers, innovators, medical and scientific researchers, and others to re‐think, energise, and innovate for a better PPE system for the HCW responding to Ebola virus outbreaks in tropical climates (WHO 2018).
Since full‐body protection has mainly evolved as a direct result of experiences gained from the recent outbreaks of deadly viruses, there are still many types of PPE available with varying types of components. The comparative effectiveness of one type against another is still unknown. Regarding the equipment, there is uncertainty whether face shields protect as well as goggles, especially when goggles are combined with a hood. There is uncertainty whether and when double or triple gloves would be more protective than single gloves. Regarding suits, it is unclear if gowns are as protective as coveralls, and how breathable and impermeable for liquids or viruses they should be. Some argue that using more breathable material would decrease the risk of contamination (Kuklane 2015).
When it comes to donning and doffing procedures for EVD, there is uncertainty about the effect of integrity checks of gloves and other equipment, and whether gloves should be changed when highly contaminated. With doffing especially, it is unclear if this should be done in pairs with a helper buddy removing part of the PPE, or if this can be done alone. Another element of the doffing procedure that is uncertain is if spraying with a disinfectant such as chlorine spray is more protective than not using spray. It is not clear which disinfectant is the best antiviral: chlorine solution or alcohol gel, and at which concentration.
Also, for COVID‐19, different procedures for donning and doffing PPE are recommended. Giwa 2020 proposes a specific procedure of doffing PPE, but the procedure is not consistent with the procedures proposed by CDC (CDC 2020c). Others, including the CDC, have proposed that gown and gloves should be doffed in a one‐step procedure (Osei‐Bonsu 2019), to minimise self‐contamination.
It is also unclear what are the best ways to train HCW and how to best maintain the skills needed for proper use of PPE.
This review is a timely update of the Verbeek 2019 review, the results of which indicated that more research is still needed to answer the review's questions.
Objectives
To evaluate which type of full‐body PPE and which method of donning or doffing PPE have the least risk of contamination or infection for HCW, and which training methods increase compliance with PPE protocols. In particular, we evaluated the effect of:
different types of PPE on contamination and infection rates or on compliance (one type or component of full‐body protection PPE versus another);
different donning or doffing procedures on contamination and infection rates or on compliance (one procedure for donning and doffing full‐body PPE versus another); and
different types of education and training aiming to improve compliance with guidelines for full‐body PPE on compliance, contamination and infection rates, (one type of training versus another).
Methods
Criteria for considering studies for this review
Types of studies
Since the circumstances for evaluation studies are difficult during epidemics, we anticipated including a broad range of study designs.
We included any prospective or retrospective controlled field study. Field study here refers to a study that tests interventions with healthcare staff in a real‐life exposure situation. This also includes case‐control studies that compare the use of interventions retrospectively between cases that have become infected and comparable controls that did not get infected.
We also included randomised as well as non‐randomised prospective controlled studies that simulated exposure to contaminated body fluids with the use of marker chemicals or harmless viruses or bacteria.
We excluded studies without a comparison group, but did not exclude studies on the basis of type of comparison group.
Types of participants
For simulation studies, we included any type of participants (volunteers or HCW) using PPE designed for EVD or comparable highly infectious diseases with serious consequences.
For field studies, we included studies only if they were conducted with HCW or ancillary staff exposed to body fluids from patients in the form of splashes, droplets, or aerosols contaminated with particles of highly infectious diseases that have serious consequences for health such as EVD, SARS, or COVID‐19.
We excluded studies conducted with laboratory staff because the preventive measures in labs are more detailed and easier to comply with.
Types of interventions
1. We included studies that evaluated the effectiveness of different types of full‐body protection (PPE), or comparing different types, compositions, or amounts of the following PPE components:
body protection such as gowns, coveralls, or hazardous materials (hazmat) suits;
eye and face protection such as glasses, goggles, face shields or visors, or masks or hoods that cover the entire head;
hand protection: gloves; and
foot protection: overshoes or boots.
We defined PPE as any of the equipment listed above that is designed or intended to protect healthcare staff from contamination with infected patients' body fluids.
2. We included studies that evaluated the effectiveness of different PPE parts or different procedures or protocols for donning and doffing of the PPE.
For example, extra assistance during donning and doffing, extra disinfection, or the use of extra gloves to prevent contamination in comparison to standard protocols.
3. We included studies that evaluated the effectiveness of training to increase compliance with existing guidance on the selection or use of PPE, including but not limited to:
education (courses);
practical training;
information only (such as posters, guideline leaflets, etc.);
audit and feedback; or
monetary or organisational incentives.
Types of outcome measures
Primary outcomes
We included all studies that had measured the effectiveness of interventions as:
contamination of skin or clothing, measured with any type of test material to visualise contamination (e.g. stains made visible with UV light) or harmless viruses or bacteria;
infection with EVD, another viral haemorrhagic fever, or comparable highly infectious disease with serious consequences such as SARS, or COVID‐19;
compliance with guidance on selection of type and use of PPE measured, for example, with an observation checklist.
Secondary outcomes
User‐reported assessment of comfort and convenience
Costs or resource use
Time to don and doff the PPE
The secondary outcomes were not a criterion for including studies in this review.
Search methods for identification of studies
Electronic searches
We conducted a systematic literature search to identify all published and unpublished trials that could be considered eligible for inclusion in this review. We adapted the search strategy we developed for Medline through PubMed (see Appendix 2) for use in the other electronic databases. The literature search identified potential studies in all languages.
We searched the following electronic databases from inception to the dates presented underneath for identifying potential studies (search dates provided below). We searched with different interfaces for the various updates. The searches are listed in the appendices for all interfaces. For the 2020 update we did not search OSH‐Update because the earlier search yielded so little.
Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 3) via Wiley Online Library (Appendix 3);
MEDLINE (Ovid) (Appendix 2; Appendix 4) until 20 March 2020;
Embase (OVID) (Appendix 5; Appendix 6; Appendix 7) to 20 March 2020;
CINAHL (EBSCOhost) (Appendix 8; Appendix 9) to 20 March 2020;
NIOSHTIC (OSH‐UPDATE) (Appendix 10) to 31 December 2018;
NIOSHTIC‐2 (OSH‐UPDATE) to 31 December 2018;
HSELINE (OSH‐UPDATE) to 31 December 2018;
CISDOC (OSH‐UPDATE) to 31 December 2018;
We also conducted a search of ClinicalTrials.gov (www.ClinicalTrials.gov), and the WHO trials portal (www.who.int/ictrp/en/), which includes the Pan African Registry for potential studies on EVD for the 2016 and 2019 updates. For the 2020 update we searched the WHO trials portal for COVID 19/SARS‐CoV‐2. We searched all databases from their inception to the present for the first versions of the review. We searched from the earliest date of search to the present for updates of the review. We did not impose a restriction on language of publication.
Searching other resources
We checked reference lists of all primary studies and reviewed articles for additional references. For the 2016 version of the review, we contacted non‐governmental organisations involved in medical relief operations in the high‐risk EVD areas to identify additional unpublished materials on protection against EVD (Médécins Sans Frontières (MSF) and Save the Children). We also used Twitter to ask for unpublished reports from people in the field. Evidence Aid helped in locating relevant organisations and in asking them for unpublished reports. We also contacted DuPont, and 3M, PPE manufacturers, to request unpublished studies.
In addition, we used Google to find any unpublished or grey literature on our question that may not be available from the sources listed above by using the following terms: 'personal protective equipment ebola'. For the March 2020 update we conducted a search of Google Scholar using the search phrase ('SARS CoV 2' OR 'COVID' AND 'protective equipment' AND 'healthcare worker').
Data collection and analysis
Selection of studies
Pairs of review authors (JV, RS, BR, ET, BB, CT, SI, JR) independently screened titles and abstracts of all systematic search results to identify studies for inclusion. The same review authors coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text study reports/publication and pairs of review authors (JV, ET, BR, RS, BB, CT, SI, JR) independently screened the full text, identified studies for inclusion, and identified and recorded reasons for exclusion of the ineligible studies. We used the computer programme Covidence for the selection of references and full‐text studies. We resolved any disagreement through discussion, except in two cases where a third‐person assessment (SI or CT) was needed. We identified and excluded duplicates and collated multiple reports of the same study so that each study rather than each report is the unit of interest in the review. We recorded the selection process and completed a PRISMA flow diagram (Moher 2009), for the search for our original review (Figure 2), our updated review (Figure 3) and this update (Figure 4). We also completed a 'Characteristics of excluded studies' table.
2.
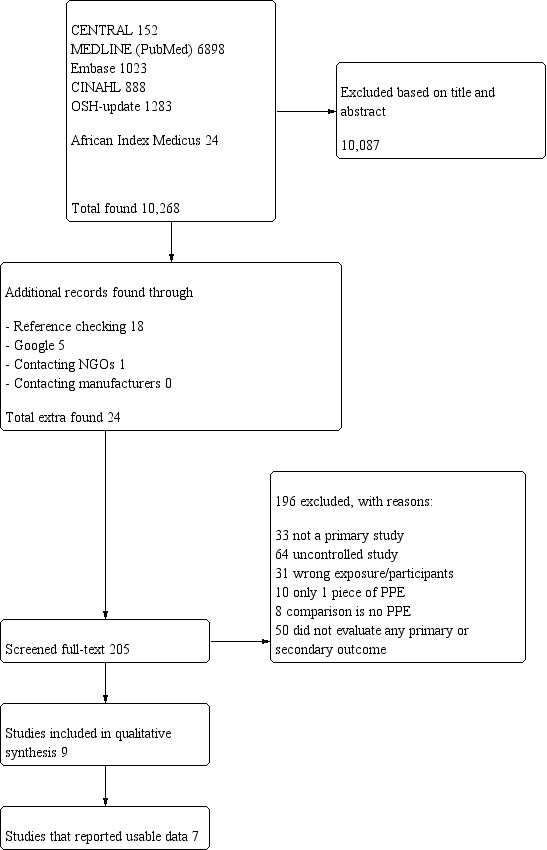
PRISMA study flow diagram for search up to January 2016
3.
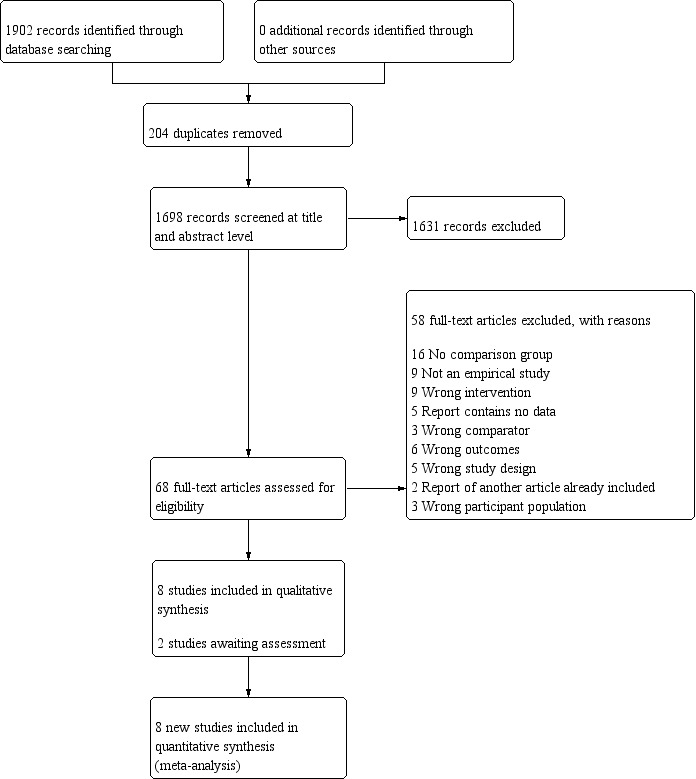
PRISMA study flow diagram for search between 2016 and 2018
4.
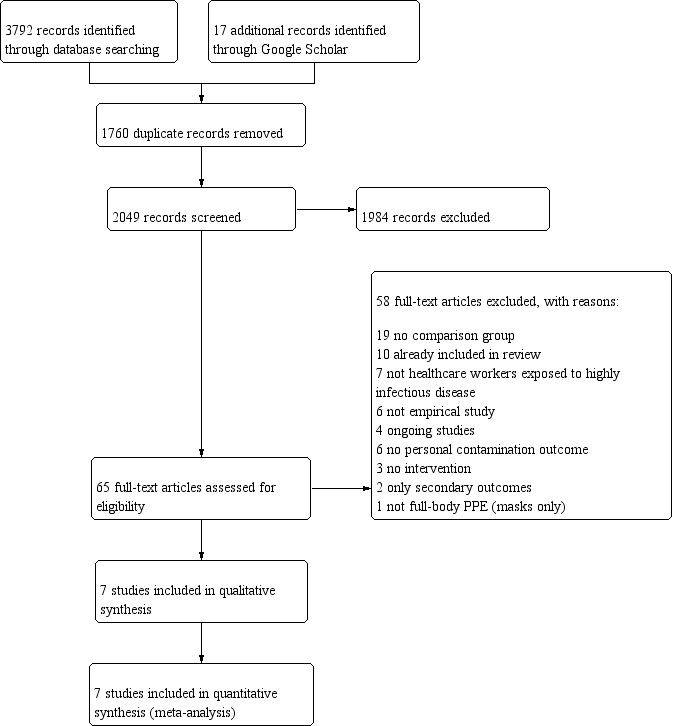
Study flow diagram for 2020 April update
Data extraction and management
We used Covidence for extracting study characteristics and outcome data. Two review authors (JV, BR, BB, ET, CT, RS, SI, JR) independently extracted the following study characteristics from included studies.
Methods: study design, total duration of study, study location, study setting, withdrawals, and date of study
Participants: number, mean age or age range, sex, severity of condition, diagnostic criteria if applicable, inclusion criteria, and exclusion criteria
Interventions: description of intervention, comparison, duration, intensity, content of both intervention and control condition, and co‐interventions
Outcomes: description of primary and secondary outcomes specified and collected, and at which time points reported
Notes: funding for trial, and notable conflicts of interest of trial authors, country where trial was conducted
Pairs of review authors (JV, BR, CT, SI, JR, ME, RS) independently extracted outcome data from included studies. We noted in the 'Characteristics of included studies' table if outcome data were not reported in a usable way. We resolved disagreements by consensus so there was no need to involve a third review author. One review author (JV or BR) transferred the data into Review Manager 5 (Review Manager 2014). We double‐checked that data had been entered correctly by comparing the data presented in the systematic review with the study reports. A second review author (CT or JV) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Pairs of two review authors (JV, BR, CT, SI, JR, ME, RS) independently assessed risk of bias for each randomised study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We resolved any disagreements by discussion so there was no need to involve another review author. We assessed the risk of bias according to the following domains in all RCTs.
Random sequence generation
Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data
Selective outcome reporting, and
Other bias
We rated each potential source of bias as high, low, or unclear and provided a quote from the study report or study author together with a justification for our judgment in the 'Risk of bias' table. We summarised the 'Risk of bias' judgements across different studies for each of the domains listed. For compliance, we considered blinding to PPE type significant for the outcome assessor only. Where information on risk of bias relates to unpublished data or correspondence with a study author, we noted this in the 'Risk of bias' table.
We considered randomised studies to have a low overall risk of bias when we judged random sequence generation and blinded outcome assessment to have a low risk of bias and none of the other domains to have a high risk of bias.
We used the domains blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other bias for all non‐randomised studies. Instead of the domains random sequence generation and allocation concealment, we used the following items as suggested in the ROBINS‐I tool (Sterne 2016), for the assessment of risk of bias in non‐randomised intervention studies.
-
Bias due to confounding. We made an overall assessment of risk of bias based on the following questions if the signalling question, 'Is confounding of the effect of intervention unlikely in this study?' was answered with no.
Did the authors use an appropriate analysis method that adjusted for all the critically important confounding domains?
Were confounding domains that were adjusted for measured validly and reliably by the variables available in this study? For this review question, we considered baseline differences between compared groups in the following factors significant: prior experience with PPE, healthcare qualification, or education of HCW, age and sex, ambient temperatures, and stressful activities.
-
Bias due to selection of participants into the study. We made an overall assessment of this risk of bias based on the following questions if the signalling questions, 'Was selection into the study unrelated to intervention or unrelated to outcome?, and 'Do start of follow‐up and start of intervention coincide for most participants?' were answered with no.
Were adjustment techniques used that are likely to correct for the presence of selection biases?
For case‐control studies: were the controls sampled from the population that gave rise to the cases, or using another method that avoids selection bias?
We considered the domains of confounding and selection of participants to yield high, low, or unclear risk of bias. For a non‐randomised study as a whole, we considered the study to have a low risk of bias if all domains received a judgment of low risk of bias comparable to an RCT. This means receiving a low 'Risk of bias' judgment on the two domains listed above as well as domains three to seven in the previous section.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
We judged studies to have a low overall risk of bias if we judged them to have a low risk of bias in the following domains: both random allocation and allocation concealment, or both confounding and selection bias, and incomplete outcome data and selective reporting. We considered the blinding of participants and outcome assessors less important because the outcomes were objective or we could not imagine that participants would have an interest in a certain type of attire and outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol (Verbeek 2015), and where there were deviations from it, we reported these in the 'Differences between protocol and review' section of the systematic review.
Measures of treatment effect
We entered the outcome data for each study into the data tables in Review Manager 2014 to calculate the treatment effects. We used risk ratios (RRs) for dichotomous outcomes, and mean differences (MDs) or standardised mean differences (SMDs) for continuous outcomes. When studies reported only effect estimates and their 95% confidence intervals or standard errors, we entered these data into Review Manager 2014 using the generic inverse variance method. When study authors used multivariate analyses, we used the most adjusted OR (odds ratios) or RRs. We ensured that higher scores for continuous outcomes had the same meaning for the particular outcome, explained the direction and reported where the directions were reversed, if this was necessary. If, in future updates of this review, we come across studies reporting results that we cannot enter in either way, we will describe them in the 'Characteristics of included studies' table, or we will enter the data into additional tables. For cohort studies that compare an exposed to a non‐exposed population we intended to report both the RR for the intervention versus the control at baseline and at follow‐up for dichotomous outcomes to indicate the change brought about by the intervention but we did not find any such studies.
Unit of analysis issues
If in future updates of this review we come across studies that employ a cluster‐randomised design and that report sufficient data to be included in the meta‐analysis but do not make an allowance for the design effect, we will calculate the design effect based on a fairly large assumed intra‐cluster correlation of 0.10. We based this assumption of 0.10 being a realistic estimate by analogy on studies about implementation research (Campbell 2001). We will follow the methods stated in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) for the calculations.
We intended to take the paired nature of the cross‐over design in the included studies into account in our data analysis. However, the included studies did not present sufficient data to do so and the results presented here are based on the unpaired test that is implemented in Review Manager 2014 which resulted in wider confidence intervals than with the use of a paired t‐test.
Dealing with missing data
We contacted investigators to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when a study was identified as abstract only). If in future updates of this review we come across studies where this is not possible, and the missing data are thought to introduce serious bias, we will explore the impact of including such studies in the overall assessment of results by a sensitivity analysis.
Similarly, if in future updates of this review we come across studies where numerical outcome data are missing, such as SDs or correlation coefficients and they cannot be obtained from the authors, we will calculate them from other available statistics such as P values, according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017).
Assessment of heterogeneity
We assessed the clinical homogeneity of the results of included studies based on similarity of population, intervention, outcome and follow‐up. We considered populations as similar when they were HCWs directly engaged in patient treatment (nurses, doctors, paramedics) versus those who were not involved in patient therapy directly (cleaning and transport staff).
We considered interventions as similar when they fell into one of the intervention categories as stated in Types of interventions.
We considered any assessment of contamination of the skin or mucous membranes as similar enough to combine.
We considered the following follow‐up times as similar: from immediately following a procedure up until the end of the work shift (short‐term), and any time after the incubation time (long‐term).
If in future updates of this review we come across studies with results that we can pool with meta‐analysis, we will use the I² statistic (Higgins 2003), to measure heterogeneity among the trials in each analysis. Where we identify substantial heterogeneity, we will report it and explore possible causes by prespecified subgroup analysis. We will regard an I² value above 50% as substantial heterogeneity (Deeks 2017).
Assessment of reporting biases
For a future update, if we are able to pool more than five trials in any single meta‐analysis, we will create and examine a funnel plot to explore possible small study biases.
Data synthesis
In future updates of this review we will pool data from studies we judge to be clinically homogeneous using Review Manager software (RevMan Web 2019). If more than one study provides usable data in any single comparison, we will perform meta‐analysis. We will use a random‐effects model when I² is above 40%; otherwise we will use a fixed‐effect model. When I² is higher than 75% we will not pool results of studies in meta‐analysis. We will include a 95% confidence interval (CI) for all estimates (Deeks 2017).
We will describe the results in the case of skewed data reported as medians and interquartile ranges.
Where multiple trial arms are reported in a single trial, we will include only the relevant arms. If two comparisons are combined in the same meta‐analysis, we will halve the control group to avoid double‐counting.
Subgroup analysis and investigation of heterogeneity
If future updates of this review find a sufficient number of studies, we will carry out the following subgroup analyses:
high income versus low and middle‐income countries; and
PPE that is certified for biological hazards versus PPE that does not have such a certification.
We will also use our primary outcomes in subgroup analyses, and we will use the Chi² test, as implemented in RevMan Web 2019, to test for subgroup interactions. At this time, we have not identified enough studies to allow for such a subgroup analysis.
Sensitivity analysis
If future updates of this review find a sufficient number of studies, we will perform sensitivity analyses defined a priori to assess the robustness of our conclusions. This involves including only studies we judge to have a low risk of bias. At this time we have not identified enough studies to allow such a sensitivity analysis.
Reaching conclusions
We based our conclusions only on findings from the quantitative or narrative synthesis of included studies that we judged to have the lowest risk of bias. Consequently, we used findings from non‐randomised studies when we did not find evidence from randomised studies. We avoided making recommendations for practice based on more than just the evidence, such as values and available resources. Our implications for research suggest priorities for future research and outline what the remaining uncertainties are in the area.
Summary of findings and assessment of the certainty of the evidence
Studies used numerous comparisons to measure the effect of PPE and we limited the 'Summary of findings' tables to the findings of the comparisons we judged most useful. We created a series of 'Summary of findings' tables to present the primary outcomes for different types of PPE (one type versus another) and donning or doffing procedures (one procedure versus another). We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of a body of evidence as it related to the studies that contributed results data for the prespecified outcomes. We used methods and recommendations described in Section 8.5 (Higgins 2017), and Chapter 12 (Schünemann 2017), of the Cochrane Handbook for Systematic Reviews of Interventions, using GRADEpro GDT software. We justified all decisions to down‐ or upgrade the certainty of evidence using footnotes and we made comments to aid reader's understanding of the review where necessary. With non‐randomised studies, we started at low‐certainty evidence and with randomised studies at high‐certainty evidence. In future updates of this review, if the outcomes are measured in many different ways, we will prioritise the reporting of outcomes as follows: infection rates, contamination rates and compliance rates.
Results
Description of studies
Results of the search
The search to January 2016 resulted in 10,268 references for screening (see Figure 2). From these references we selected 205 articles for full‐text assessment. Through checking the references of included articles we found 18 additional articles. We found another five articles by using Google, and we found one more through contacting NGOs (Tomas 2015). Our contacts with the manufacturers did not yield any responses or data. Most of the studies that we located outside our electronic searches were studies of PPE use during the SARS epidemic that did not make reference to any type of PPE in the title or abstract. For the same reason we did not locate Nyenswah 2015 because there was no reference to PPE. By using Google search, we found one additional article (Bell 2015), that was not indexed in any of the databases that we searched. Based on a request of one of the peer referees we also searched the African Index Medicus, which yielded 24 references but no new studies to include. Contacting PPE manufacturers did not lead to any responses. This added up to 205 papers that we checked full‐text for inclusion. Of these, we excluded 196. This resulted in nine included studies.
We updated the searches in Embase up to 22 May 2018, in Medline through PubMed up to 15 July 2018, in CINAHL up to 31 July 2018, in OSH‐update on 31 December 2018, and in CENTRAL up to 18 June 2019. We did not have access to Embase after May 2018 and used Scopus to update the Embase search up to 18 June 2019. This yielded 1698 new references after de‐duplication. We assessed 68 articles in full‐text and subsequently we excluded 58 articles. This resulted in 10 new studies that fulfilled our inclusion criteria (see Figure 3) of which we could include eight in the review and two were awaiting assessment.
For the 2020 update we reran the searches including the search word 'decontamination' and PPE as a MeSH term in Medline. We did not update the OSHupdate search because this yielded so little for the previous version. We also searched African Index Medicus but it did not add any new articles. Altogether we retrieved 3792 references through database searching and 17 additional records through searching Google Scholar. We removed 1760 duplicates (see Figure 4). Thus, we screened 2049 records, which led to 65 full‐text assessments. Of these, we excluded 58 records, mainly because the studies did not have a comparison or were already included in the review. The selection process finally resulted in seven new studies included in the review which includes the two studies awaiting assessment in the previous version of this review (Andonian 2019; Chughtai 2018; Drews 2019; Hajar 2019; Kpadeh Rogers 2019; Osei‐Bonsu 2019; Suen 2018).
Included studies
We contacted Bell 2015; Casalino 2015; Casanova 2016; Curtis 2018; Drews 2019; Hall 2018; Suen 2018 and we got additional information from all but Casanova 2016. We entered this information in the 'Characteristics of included studies' table.
Study types
We included 24 studies in total. Twenty‐two were simulation studies, of which 18 simulated exposure to contaminated body fluids and measured contamination outcomes, and four studies provided alternative PPE or procedures and measured compliance with donning and doffing procedures.
Of these simulation studies 14 were randomised trials (seven with parallel groups (Andonian 2019; Bell 2015; Curtis 2018; Hung 2015; Osei‐Bonsu 2019; Tomas 2016; Wong 2004), seven had a cross‐over design (Chughtai 2018; Hajar 2019; Guo 2014; Mana 2018; Strauch 2016; Suen 2018; Zamora 2006)), and one was a quasi‐RCT (Gleser 2018).
There were seven non‐randomised controlled studies (five with a cross‐over design ((Buianov 2004; Casanova 2012; Drews 2019; Kpadeh Rogers 2019; Hall 2018) and two with parallel groups (Casalino 2015; Casanova 2016)).
In addition, we found two retrospective cohort studies. One study evaluated the effect of PPE training on SARS infection rates and noncompliance with the doffing protocol (Shigayeva 2007). In this study, the authors located all HCW that had been exposed to SARS patients and assessed, by questionnaire, compliance with PPE guidelines and PPE doffing guidelines. Houlihan 2017 evaluated the risk of EVD infection according to donning and doffing practices and the use of disinfectant in HCW that had been deployed in West Africa during the EVD epidemic.
Participants
In the simulation studies, researchers included 816 intervention and 367 control participants, when we take into account that studies used a cross‐over design and thus all participants were intervention participants. In the cohort studies, there were 863 intervention and 232 control participants. Altogether there were 2278 participants.
The participants in all studies were healthcare workers with a mixture of occupations, but mainly physicians, nurses and respiratory technicians. One study included medical students during their internships (Casalino 2015). No studies included other healthcare staff such as people working in emergency services or cleaning staff.
In the two retrospective cohort studies, exposure of participants was to the SARS epidemic in one study (Shigayeva 2007), and to the EVD epidemic in another study (Houlihan 2017).
In the simulation studies, 12 studies simulated exposure using a fluorescent agent, three studies exposed participants to a harmless virus or microbes, and another three studies used both ways of exposure simulation. Studies used a wide range of different fluorescent agents and a range of exposure methods that varied from rubbing 0.5 mL of fluorescent agent over the gloved hands to throwing 100 mL of fluorescent agent onto the torso of the gown (see Table 17). The situation was similar in the studies that used viruses or bacteria to simulate exposure. Four studies simulated donning and doffing to assess compliance with guidance (Casalino 2015; Curtis 2018; Drews 2019; Hung 2015).
1. Exposure and outcome in simulation studies.
| Study ID | Exposure | Outcome | |||||||
| Agent | Name | Solution | Amount | Additions | Exposure method | Detection | Photographs | Measure | |
| Andonian 2019 | Fluorescent fluid/microbeads | Powder (Glitter Bug)/fluorescent 2‐μm polystyrene latex bead (PLSs) | Grape‐seed oil and water (1:6 oil‐to‐water ratio)/in aerosol | 75 mg/mL | ‐ | Pesticide hand sprayer: 5 sweeping passes of sprayer from head to feet on the front and back of the HCW/4 min of continuous aerosol generation while the HCW turned 90° every 60 s | UV‐light/PLS detection was performed by counting via epifluorescent microscopy | No | Number of body sites with fluorescent marker/with PLS |
| Bell 2015 | Fluorescent | Glogerm, Tide, Bright Dyes Orange Dye | Water | 100 mL | Oatmeal, chocolate powder, crushed cereal | 100 mL splashed on the front torso of their garment | UV LED black light, Chauvet | Yes | Contaminated yes/no |
| Buianov 2004 | Microbes | ? | 10^8 CFU/m3 | ? | ? | ? | ? | ? | ? |
| Casanova 2012 | Virus | MS2 | 10^5 PFU/5 muL | 25 muL | ? | Shoulder, respirator, eye protection, hand 5 drops of 5 muL | Swabs of face and hands; extraction gloves, scrubs | n/a | Any contamination yes/no; mean Log10 PFU recovered |
| Casanova 2016 | Virus | MS2, Phi6 | 10^8 MS2, 10^7 Phi6/5 muL | 25 muL | ? | Hand, shoulder, face‐shield, boot | Swabs of face and hands; extraction gloves, scrubs | n/a | Any contamination yes/no |
| Chughtai 2018 | Fluorescent | Fluorescent spray: Glitter Bug | ? | 0.5 mL | ? | Rubbed over hands; sprayed on front and sides from 1 m distance | UV light | No | People with contaminated patches |
| Gleser 2018 | Fluorescent | Schulke | ? | 5 mL | No | Distributed equally on the gloves | UV box | No | Hand contamination (yes/no) |
| Guo 2014 | Fluorescent | Glogerm | Oil and water | ? | No | Sprayed 3.8 g of the lotion onto the upper body of the subject at a distance of 60 cm from the participant | UV scan | No | Number of stains |
| Hajar 2019 | Fluorescent | Fluorescent solution (Super Blue Invisible Ink, Black Light World) | ? | 0.5 mL | ? | Rubbed over gloved hands appr 15 s | Black light | no | Sites per person/people with contamination |
| Hall 2018 | Fluorescent | VIOLET‐tool | Water, glycerol | 800 mL (blue UV) | Flour, salt | Manikin vomited, produced diarrhoea, sweat and cough | UV‐A strip lights | Yes | Yes/no and location (n = 12) |
| Kpadeh Rogers 2019 | Bacteria | Bacterial suspension of MSSA/GloGerm Mist liquid | ? | 50 muL each | ? | Rubbed the bacteria/fluorescent marker on their hands | Plated on tryptic soy agar for quantitative culturing. Plates were incubated overnight. | No | Number of CFUs of K pneumoniae and MSSA were calculated |
| Mana 2018 | Fluorescent | ? | ? | 0.5 mL | No | Rubbed over gloved hands; then contaminated front of the gown | Ultra light UV1 | No | Any contamination yes/no |
| Virus | Phi X174 | 10^8 PFU/0.5 mL | 0.5 mL | ? | Rubbed over de‐gloved hands for 10 sec | Swabs of hands and wrist; swabs of neck and chest | n/a | Any contamination yes/no; mean Log10 PFU recovered | |
| Osei‐Bonsu 2019 | Fluorescent/ bacteria | Glo Germ fluorescent powder/Staphylococcus epidermidis | ? | 1 mL of S epidermidis in a 0.5 McFarland suspension (1.5 10 8 CFU/mL) | ? | Wedge foam paint brush to coat participants with Glo Germfluorescent powder on both arms, hands, and the abdomen/dripping droplets over the PPE with a 1000 uL pipette | Black light/areas of apparent powder transfer were documented and cultured using cotton swabs, inoculated onto blood agar plates, and incubated for 48 h. | No | Number of people with contamination |
| Suen 2018 | Fluorescent | Fluorescent solution (UV GERM Hygiene Spray, Glow TecLtd | ? | 12 times 1.99 g | ? | Solution was sprayed onto the face shield, 2 upper limb/gloves and anterior surfaces of the gown | UV lamp (CheckPoint, 220– 240 V/50 Hz; Glow Tec Ltd., London, England) under dim light | No | Overall average of contaminated body sites |
| Strauch 2016 | Fluorescent | Glogerm | Oil | 25 mL | No | 1. brushed on masks 2. 1 mL on the hands | UV‐A light | Yes | Contaminated yes/no; intensity of UV light reflection |
| Tomas 2016 | Fluorescent | ? | ? | 0.5 mL | No | Rubbed over gloved hands | Ultra light UV1 | No | Contaminated hands/wrist yes/no |
| Virus | MS2 | 10^10 PFU /0.5 mL | 0.5 mL | ? | Gloved hands were inoculated | Swabs of hands and wrist | n/a | Contaminated hands/wrist yes/no; mean log10 PFU recovered | |
| Wong 2004 | Fluorescent | ? | Water | 100 mL | No | Sprayed the exposed part with an atomiser (participants were blindfolded during this process) | UV scan | Yes | Number of stains |
| Zamora 2006 | Fluorescent | Detection paste | ? | 100 mL | No | Paste on forearms and palms of the hands | UV lamp | No | Areas measured |
| CFU: colony forming units; HCW: healthcare worker; K pneumonia: Klebsiella pneumoniae;LED: light‐emitting diode; MS2: harmless virus; MSSA: methicillin‐sensitive Staphylococcus aureus;mL: millilitre; muL: microlitre; n/a: not applicable; PFU: plaque forming units; Phi6: harmless virus; PLS: polystyrene latex bead; UV: ultraviolet | |||||||||
Countries
Twelve studies were performed in the USA, four in China and Hong Kong, two in Canada, two in the UK, one each in Australia, Germany and Russia, and one was performed in three countries at the same time: France, Mexico and Peru (Casalino 2015). One study in Canada was performed during the SARS epidemic and one study in the UK was among HCW that had returned from the West‐African EVD epidemic.
Time period
All studies were conducted after the year 2000, with six before, and 18 after 2015.
Interventions and comparisons
Of the 24 included studies, 17 studies evaluated an intervention and a control condition. Four studies (Buianov 2004; Guo 2014; Houlihan 2017; Shigayeva 2007), evaluated two interventions. One study compared three types of PPE (Suen 2018), one study five types (Hall 2018), and one study 10 types (Chughtai 2018).
Fourteen studies compared one type of PPE to one or more other types. Eight studies compared two or more different ways of donning and doffing. One of these studies named the intervention 'enforced training' but we categorised it under different ways of doffing because it entailed giving instructions during the donning and doffing process versus not giving instructions (Casalino 2015). Three studies evaluated the effect of training.
Comparison of different types or parts of full‐body PPE
Fourteen simulation studies compared different types or parts of full‐body PPE outfits or compared an adapted design versus a standard design PPE, but all in a different way. Only a couple of studies were similar enough to allow us to combine their results. None of the included studies used a standardised classification of the properties of the PPE that protect against viral penetration such as the EN 14126.
Two simulation studies compared different types of masks or respirators as part of full‐body PPE. Buianov 2004 compared two different types of powered, air‐purifying respirator (PAPR) that were especially developed for this project in Russia to protect healthcare personnel against Ebola and similar viruses. Buianov 2004 also compared the effect of different airflow rates that varied from 50 L to 300 L per minute. The intervention participants were required to carry out a step test that lasted for four hours. The study authors did not describe the equipment they tested in sufficient detail for us to be able to judge their technical qualities. Zamora 2006 compared PPE combined with a PAPR in use at the study hospital with PPE without a PAPR according to CDC recommendations to prevent respiratory infection at the time of the study, the so‐called Enhanced Respiratory and Contact Precautions (E‐RCP).
Six simulation studies compared different types of gowns and protective clothing. Wong 2004 compared four types of PPE according to their material properties. First, they tested the material according to the American Association of Textile Chemists and Colorists' standards 22 and 127. We excluded the surgical‐gowns‐only category since it had no water repellency and insufficient viral barrier properties. Type A had good water repellency and water penetration resistance, but at the cost of poor air permeability. Type B had good water repellency and good air permeability, but poor water penetration resistance. Type C was the surgical gown with both poor water repellency and water penetration resistance. Type D, Barrierman, was made of Tyvek and had good water repellency, poor air permeability and fair water resistance. Bell 2015 compared commercially available PPE, compliant with CDC recommendations, with locally available clothing, such as rain coats that were thought to be as protective as the commercially available ones. Guo 2014 compared three types of PPE: a disposable water‐resistant, non‐woven gown, a reusable, woven, cotton gown, and a disposable non‐woven plastic apron. The second one was a cotton, water permeable gown, like a surgical gown. We left this arm out of the analysis because surgical gowns alone are not used for EVD. The study authors tested the fabrics for water repellency and liquid penetration according to the American Association of Textile Chemists and Colorists' standard 22. The gown and the apron received ratings of 4 and 5 respectively on a scale of 0 to 5 for water repellency. One simulation study compared different full‐body PPE ensembles. Hall 2018 compared five different PPE ensembles used in EVD surge units in hospitals, which all met the guidance of the Advisory Committee on Dangerous Pathogens endorsed by Public Health England (PHE). Three ensembles used gowns while two ensembles used coveralls. Some PPE ensembles were comprised of gowns with surgical caps and other ensembles of coveralls with hoods. Some PPE comprised boots only and others boot covers. Some taped the second pair of gloves whereas others did not. Suen 2018 compared three types of PPE, which differed with respect to the use of a waterproof gown, isolation gown, or coverall. Chughtai 2018 compared 10 different outfits that complied with guidance given by WHO or in specific countries, including the guidance for donning and doffing.
Modifications to existing PPE
Strauch 2016 compared a N95 filtering face piece respirator (FFR) mask to a modified FFR mask with tabs placed on the elastic band as a doffing aid. The study authors reported having evaluated contamination of the hands and head in two different trials but they reported their results in the same article.
Tomas 2016 compared a standard gown to a prototype seamless PPE that consisted of a polyethylene gown with nitrile gloves attached by a contact bond adhesive to enable the removal of the gown and gloves at the same time. Mana 2018 compared a standard polyethylene gown to a modified gown with a double elastic neck closure for easier removal, more gown coverage on the palm of the hand and smaller thumb holes and elastic wrist bands to create a snugger fit. Hajar 2019 also evaluated a gown with improved glove gown interface.
One simulation study compared different types of gloves. Gleser 2018 compared a modified glove with a small tab near the thumb to aid in glove removal without contamination to standard medical examination gloves. Both types of gloves were made of the same material from the same company. The study authors did not provide any more information.
Studies comparing different types of eye protection or footwear are missing.
Contamination rates are not only determined by the type of PPE but also by the donning and doffing procedures. All studies had a priori determined donning and doffing procedures. It should be noted that these studies evaluated the totality of the type of PPE inclusive of the donning and doffing procedure. We have described the procedures in the 'Characteristics of included studies' table.
Donning or doffing procedures (one procedure for donning or doffing versus another)
Eight studies compared different donning or doffing procedures.
Extra gloves
Casanova 2012 compared the effect of wearing two pairs of gloves with wearing one pair of gloves on contamination rates. We classified the study under methods of doffing because the intention of the double‐gloving was to decrease contamination during doffing. Doffing was done as per CDC recommendation, which describes how to do both single‐gloving and double‐gloving. Osei‐Bonsu 2019 also compared the CDC procedure for doffing with doffing with double gloves.
Structured procedures versus individual ways of donning and doffing
One simulation study compared individual's own versus recommended procedures. Guo 2014 compared the effect of doffing a gown or an apron according to an individual's own views versus the procedure recommended by CDC in the USA in 2007. Participants were given the following instructions: "Gown front and sleeves are contaminated! Unfasten neck, then waist ties. Remove gown using a peeling motion; pull gown from each shoulder toward the same hand. Gown will turn inside out. Hold removed gown away from body, roll into a bundle and discard into waste or linen receptacle".
Alternative procedures versus CDC procedure
One study (Osei‐Bonsu 2019) compared the CDC procedure for doffing with a one‐step procedure in which gloves are doffed at the same time as the gown.
Extra instruction
Two simulation studies compared the effect of extra assistance during donning or doffing versus no instructions. Casalino 2015 compared standard (unassisted) donning or doffing procedure to reinforced (extra assistance) procedures. The reinforcement consisted of an instructor saying out loud the next step of donning or doffing. The study authors used the reinforcement with both basic PPE (impermeable apron without a hood) and enhanced PPE (full‐body suit and hood). Andonian 2019 compared training in teamwork to conventional donning and doffing.
Disinfection procedures
Four simulation studies, and one field study, compared donning or doffing procedures with extra disinfection during the process. Casanova 2016 compared the self‐contamination of skin with two surrogate viruses when either an alcohol‐based hand rub or hypochlorite solution was used for the glove hygiene step of a PPE doffing protocol. Houlihan 2017 intended to compare the PPE removal with and without chlorine spray and also with and without assistance but there was collinearity between these variables and being in clinical work or in laboratory work. All those that were in clinical work reported having used chlorine spray and assistance whereas those in laboratory work did not. Therefore we could not analyse these data. Kpadeh Rogers 2019 compared the effect of alcohol‐based hand rub, quaternary ammonium or bleach to no glove disinfection. Osei‐Bonsu 2019 compared the recommended CDC procedure to the same procedure plus extra hand hygiene with alcohol‐based hand rub.
Type of training or education (one type of training or education versus another)
Three studies evaluated different training methods for donning and doffing procedures.
Hung 2015, a simulation study, compared a conventional training session for donning and doffing procedures to a procedure in which the conventional session was complemented with a computer simulation later.
Shigayeva 2007, a field study, evaluated the effect of active and passive training versus no training on compliance rates. We defined active training as training that involved any group or face‐to‐face interaction. We defined passive training as watching a video or receiving written instructions. This allowed us to make an indirect comparison between the effect of active and passive training. We calculated the effect of active training compared to passive training by subtracting the OR for passive training from the OR for active training, as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We calculated the variance of this indirect comparison by summing the variances of both direct comparisons. Then we calculated the standard error by taking the square root of the combined variance. We used this as input for the generic inverse variance method in Review Manager 2014.
Curtis 2018, a simulation study, compared a video‐based learning session on instructions for PPE use for patient decontamination as part of a disaster medicine training to a traditional lecture before participating in a practical exercise.
Outcomes
Infection rates
One study (Houlihan 2017), evaluated the effect of interventions on infection rates. The study authors measured the level of immunoglobulin G (IgG) specific for EVD in an oral fluid sample to assess if there had been undetected infections in HCW exposed to EVD.
Contamination outcomes
Simulation studies measured contamination either as the proportion of people contaminated, as the number of contaminated spots, or as the area of the body contaminated in studies using a fluorescent marker (see Table 17). Study authors measured contamination with the help of a UV lamp (when using fluorescent marker), or by directly measuring viral or microbe presence or viral or microbial load (when using a non‐pathogenic virus or microbes). However, across studies, different body locations were contaminated and also different body locations were measured for the contamination outcome. In the control groups there was a median of 67% of participants contaminated and across intervention groups this was 25%. There were two studies in which there were participants that had zero contamination with a specific PPE outfit (Chughtai 2018; Hall 2018).
Compliance with guidance: noncompliance rates with donning and doffing procedures
Ten studies evaluated the effect of interventions on noncompliance (Casalino 2015; Casanova 2012; Curtis 2018; Drews 2019; Hajar 2019; Hung 2015; Shigayeva 2007; Suen 2018; Zamora 2006)
Four contamination simulation studies (Casanova 2012; Drews 2019; Hajar 2019; Zamora 2006), measured non‐compliance as the number of participants that did not follow the correct order of the protocol, omitted elements, or did not use the correct equipment.
Shigayeva 2007 measured noncompliance in their training study as the number of violations against protocol as recorded from interviews. There were two different compliance outcomes. One was called consistent adherence and was calculated as the proportion of exposure episodes with full compliance with PPE. The other one was called unsafe doffing, measured if one or more of the elements of the doffing procedure were violated. We recalculated outcomes in such a way that they represented the frequency of noncompliance.
Hung 2015 measured compliance as a total score on a 16‐item checklist for donning and 20‐item checklist for doffing. To get results comparable to the other studies we subtracted the mean compliance values from the maximum score and used these as noncompliance values.
Casalino 2015 measured noncompliance as the number of errors per person for donning and for doffing and the number of people with one or more errors as measured by the specialist trainer or instructor, who also gave the spoken instructions in case of reinforcement. The study authors also measured critical errors, which were those where there was contact between skin and potentially contaminated PPE, but we did not consider this a valid measure of contamination and disregarded it. We took measurement of the errors at the last training session as the effect of the intervention. We disregarded the error measurements at earlier training sessions.
Suen 2018 measured non‐compliance as the average of the percentage errors of all items of a checklist.
Curtis 2018 measured compliance as the percentage of the maximum attainable score that an external evaluator gave on a practical skills test for both donning and doffing PPE.
Secondary and other relevant outcomes
No studies reported on costs or other economic outcomes such as resource use.
Wong 2004 and Lai 2011a measured time, and Wong 2004 and Drews 2019 measured satisfaction. Buianov 2004 measured heart rate and body temperature. We chose to report the results of this outcome as well, as we identified it as an additional outcome that appeared relevant to the questions being addressed.
Excluded studies
Description of case series or outbreak
One reason for excluding important studies was that the researchers only described a case‐series of HCW cases' use of PPE for EVD (Muyembe‐Tamfum 1999), Marburg Haemorraghic Fever infection (MHF) (Borchert 2007; Colebunders 2004; Jeffs 2007; Kerstiens 1999), Congo Crimean Haemorraghic Fever (CCHF) (Gozel 2013), or for SARS (Christian 2004; Ho 2003; Ofner 2003; Ofner‐Agostini 2006). None of these studies described the use of PPE by the cases in such detail that they could be replicated. In combination with the lack of a control condition, it is difficult to conclude how much PPE, or the lack thereof, contributed to the infection. The only different study of a series of cases during an outbreak was the study by Dunn 2015 that contained proper descriptions of PPE.
Description of PPE use only
We excluded studies if they only described how and what PPE was used without relation to an outcome (Beam 2016a; Beam 2016b; Franklin 2016; Lee 2017; Lowe 2014; Marklund 2002; Minnich 2003).
One type of PPE only, no comparison
Alraddadi 2016, Delaney 2016, Drew 2016, Elcin 2016, Luo 2011, Kwon 2017 and Tomas 2015 evaluated only one type of PPE without a comparison in a simulation study. Also for the 2020 update we excluded many studies because of the lack of a control group (Abualenain 2018; Casanova 2018; Kogutt 2019; Mumma 2018; Parveen 2018; Williams 2019; Weber 2018).
No infection rates contamination or compliance outcomes
Some studies measured only performance with PPE compared to no PPE use and not infection rates, contamination or compliance (Castle 2009; Coates 2000; Garibaldi 2019; Hendler 2000). Other studies did not measure personal but only environmental contamination (Jaffe 2019; Lai 2011; Porteous 2018; Visnovsky 2019).
Comparison with no PPE only
We excluded studies that only compared PPE use with no PPE and not with alternative PPE use (Lu 2006; Schumacher 2010; Teleman 2004).
Studies that evaluated only one type of PPE and not part of full‐body PPE
Ogendo 2008 measured eye protection only. Bearman 2007 measured universal glove use only. Chughtai 2013, Lindsley 2012 and Lindsley 2014 measured masks or face shields only. Even though these studies yield valuable information, it is unclear how well the results also cover the use of these items as part of full‐body protection and therefore we excluded these studies.
Participants not exposed to highly infectious diseases with serious consequences
Many studies evaluated PPE use for diseases other than EVD and related haemorraghic fevers, such as HIV or other nosocomial infections that were not considered highly infectious or having serious consequences, or both, and we excluded these studies (Anderson 2017; Bischoff 2019; Malik 2006; Makovicka 2018; Ransjo 1979; Sorensen 2008). In another study participants were not HCW (Kahveci 2019).
Training or simulation studies without a control group
There were a number of studies that evaluated training but that did not use a control group. This makes it difficult to draw inferences about the effect of one type of training compared to another (Abrahamson 2006; Beam 2014; Hon 2008; Northington 2007; Tomas 2015).
Inconsistent use of PPE during the SARS epidemic
After intensive discussion, we excluded 11 studies that measured the use of PPE (mask, gloves, gowns, goggles) during the SARS outbreak and related that to the risk of SARS infection. One line of thinking was that these studies did not fulfil the inclusion criteria because the comparison here was not clearly one type of PPE versus another type of PPE. Another line of thinking was that the studies compared different types of PPE composition and thus would fulfil the inclusion criteria. We finally decided to deal with these studies in the discussion section only (Ho 2004; Lau 2004; Le 2004; Liu 2009; Loeb 2004; Nishiura 2005; Park 2004; Pei 2006; Scales 2003; Seto 2003; Teleman 2004).
Risk of bias in included studies
See Figure 5 for an overview of our judgment of the risk of bias per study. Figure 6 gives an overview of risk of bias per domain. Since the figures contain the 'Risk of bias' assessments for both randomised and non‐randomised studies, not all cells are applicable to both study types and those that are not applicable remain empty.
5.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
6.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies
Allocation
Allocation was random in 14 studies but only five of them stated adequately what method they had used for generating the random sequence and where thus rated as at low risk of bias for random sequence generation. Five studies reported an appropriate method (Osei‐Bonsu 2019; Suen 2018; Wong 2004; Zamora 2006), and for one we received additional information from the study authors (Mana 2018). One used alternation and we rated it as having a high risk of bias (Gleser 2018). The other studies were rated at unclear risk of bias
Allocation concealment was unclear in all but two of the randomised studies (Mana 2018; Osei‐Bonsu 2019). We judged only these two studies to have a low risk of selection bias.
Blinding
In the simulation studies, the participants could not be blinded for the type of attire they were wearing or the type of donning or doffing procedure they were following. It is unclear if they could have contaminated themselves more with attire that they thought was not good, or they did not like, but for the majority of the studies we considered this unlikely and assessed the risk of performance bias to be low. For one study, Casalino 2015, we rated the risk of performance bias as high because the instructors who provided the intervention were very much aware if instruction was given or not and they were the also the assessors. We also rated the risk of performance bias as high for Drews 2019 and Hajar 2019 because the outcomes were subjective and the participants unblinded. We judged the risk of performance bias as low in 15 studies.
For the non‐randomised SARS study (Shigayeva 2007), we considered the risk of performance bias low because the study was retrospective and the participants did not know they were part of a study.
The risk of detection bias was unclear in most studies, as they did not report whether outcome assessors were blinded. We considered the risk to be high in one study (Casalino 2015), as providers of the intervention were also the assessors of compliance, and in a second study (Shigayeva 2007), because the intervention and the outcome were assessed with the same questionnaire at the same time. We judged the risk to be low in four studies because the study authors stated that assessors were blind to group status (Curtis 2018; Hung 2015; Mana 2018; Zamora 2006). We judged the risk of detection bias to be low for Houlihan 2017 because they used antibodies against Ebola, an objective outcome, which would not be affected by assessors' knowledge of treatment. All in all, we judged the risk of detection bias as low in eight studies.
Incomplete outcome data
We judged the risk of attrition bias to be low in 14 studies and unclear in 10 studies. All but two studies were short‐term experiments and therefore most had a complete follow‐up of all participants.
Selective reporting
It was difficult for us to judge selective reporting because none of the included studies had published a protocol. We judged seven studies (Andonian 2019; Casalino 2015; Casanova 2016; Chughtai 2018; Guo 2014; Kpadeh Rogers 2019; Suen 2018), to have a low risk of reporting bias as the study authors appeared to have reported all relevant data as specified in their articles' methods. We judged Bell 2015 to be at high risk of reporting bias because they did not report outcomes separately for the intervention and the control. We also judged Hung 2015 to have a high risk of reporting bias as the study authors did not fully report the results of the computer usability questionnaire. In addition, Gleser 2018 and Osei‐Bonsu 2019 did not fully report all results. In total we judged four studies to be at high risk of reporting bias.
Other potential sources of bias
We did not consider that any of the included studies were at risk of other sources of bias except for Gleser 2018, where we considered that there was a substantial financial conflict of interest because the first author was also the director of the company that produced the gloves that were part of the intervention.
Bias due to confounding (non‐randomised studies)
We judged there to be a low risk of bias due to confounding in six non‐randomised studies (Casanova 2012; Casanova 2016; Drews 2019; Hall 2018; Houlihan 2017; Shigayeva 2007), unclear risk in two non‐randomised studies (Casalino 2015; Kpadeh Rogers 2019), and a high risk in one non‐randomised study (Buianov 2004).
Bias due to selection of participants into the study (non‐randomised studies)
We judged there to be a low risk of bias due to selection of participants into the study for five non‐randomised studies (Buianov 2004; Casalino 2015; Casanova 2012; Hall 2018; Shigayeva 2007), and unclear for one study (Casanova 2016). We considered the risk of selection bias to be high in two studies. Houlihan 2017, because they recruited participants based on snowball sampling, and Kpadeh Rogers 2019, where different HCW performed tests with different bacteria.
Overall risk of bias per study
We judged none of the included studies to be at low risk of bias overall. According to our judgment they were all at either unclear (N = 15) or at high risk of bias (N = 9).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16
Summary of findings 1. Personal protective equipment (PPE) types: powered, air‐purifying respirator (PAPR) plus coverall versus N95 mask plus gown.
| PAPR versus enhanced respiratory and contact precautions (E‐RCP) attire for preventing contact with contaminated body fluids in healthcare staff | ||||||
|
Patient or population: healthcare staff
volunteers
Settings: simulation study
Intervention:
PPE with PAPR Control: E‐RCP attire according to 2005 CDC recommendation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| E‐RCP attire | PAPR attire | |||||
|
Any contamination: fluorescent marker Follow‐up: post intervention |
960 per 1000 | 259 per 1000 (163 to 413) | RR 0.27 (0.17 to 0.43) | 50 (1 cross‐over RCT) | ⊕⊝⊝⊝ Very low1,2,3 | Analyses presented in this table are unadjusted for the paired nature of the cross‐over design but similar to the results that the study authors presented while taking the cross‐over into account |
|
Compliance: noncompliance with donning guidance Follow‐up: post intervention |
40 per 1000 | 300 per 1000 (72 to 1000) | RR 7.5 (1.81 to 31.1) | 50 (1 cross‐over RCT) | ⊕⊝⊝⊝ Very low1,2,3 | |
|
Compliance: noncompliance with doffing guidance Follow‐up: post intervention |
240 per 1000 | 120 per 1000 (48 to 295) | RR 0.5 (0.2 to 1.23) | 50 (1 cross‐over RCT) | ⊕⊝⊝⊝ Very low1,2,3 | |
| *The basis for the assumed risk is the control group risk. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CDC: Centers for Disease Control and Prevention; CI: confidence interval; E‐RCP: enhanced respiratory and contact precautions; PAPR: powered, air‐purifying respirator; PPE: personal protective equipment; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Simulation study, downgraded one level for indirectness. 2One cross‐over study with 50 participants, downgraded one level for imprecision. 3HIgh risk of bias, downgraded one level for study limitations.
Summary of findings 2. Personal protective equipment (PPE) types: more protective versus less protective.
| Three types of PPE attire compared by number of contaminated spots | |||||
| Patient or population: healthcare worker volunteers Settings: simulation study Intervention: more protective attire, not permeable not breathable (A) Comparison: less protective attire: permeable but breathable (B); fairly permeable, not breathable (D) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Less protective type of PPE (B or D) | Most protective type of PPE attire (A) | ||||
|
Number of contaminated spots: trunk Fluorescent marker Follow‐up: post‐intervention |
The mean number of contaminated spots in control group B was 1.62 spots | The mean number of contaminated spots in the intervention group A was 1.60lower (3.35 lower to 0.15 higher) | 50 (1 study) | ⊕⊝⊝⊝ Very low1,2,3 | |
|
Number of contaminated spots: neck Fluorescent marker Follow‐up: post‐intervention |
The mean number of contaminated spots in control group B was 0.12 spots | The mean number of contaminated spots in the intervention group A was 0.7 higher (0.26 lower to 1.66 higher) | 50 (1 study) | ⊕⊝⊝⊝ Very low1,2,3 | |
|
Number of contaminated spots: foot Fluorescent marker Follow‐up: post‐intervention |
The mean number of contaminated spots in the control group B was 2.86 spots | The mean number of contaminated spots in the intervention group A was 0.96 lower (2.35 lower to 0.43 higher) | 50 (1 study) | ⊕⊝⊝⊝ Very low1,2,3 | |
|
Number of contaminated spots: palm Fluorescent marker Follow‐up: post‐intervention |
The mean number of contaminated spots in the control group B was 17.83 | The mean number of contaminated spots in the intervention group A was 7.72 lower (15.65 lower to 0.21 higher) | 50 (1 study) | ⊕⊝⊝⊝ Very low1,2,3 | |
|
Number of contaminated spots: trunk or neck Fluorescent marker Follow‐up: post‐intervention |
The mean number of contaminated spots in the control group D was 0 | Because no standard deviations were provided no analysis was possible | |||
|
Number of contaminated spots: foot Fluorescent marker Follow‐up: post‐intervention |
The mean number of contaminated spots in the control group D was 4.96 | The mean number of contaminated spots in the intervention group A was 4.1 lower (6.94 to 1.26 lower) | 50 (1 study) | ⊕⊝⊝⊝ Very low1,2,3 | |
|
Number of contaminated spots: palm Fluorescent marker Follow‐up: post‐intervention |
The mean number of contaminated spots in the control group D was 20.49 | The mean number of contaminated spots in the intervention group A was 12.76 lower (21.62 to 3.9 lower) | 50 (1 study) | ⊕⊝⊝⊝ Very low1,2,3 | |
|
Usability score Range 1 to 5, higher indicating better |
Mean score for group B was 4.02 | The mean score of intervention group A was 0.46 lower (0.84 to 0.08 lower) | 50 (1 study) |
⊕⊝⊝⊝ Very low1,2,3 | |
| Compliance with guidance | See comment | See comment | 0 (0 studies) |
See comment | No studies evaluated the effect of the interventions on compliance with guidance. |
| *The basis for the assumed risk is the control group risk. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group. CI: confidence interval; PPE: personal protective equipment | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
1Simulation study, downgraded one level for indirectness. 2One study with 100 participants, 25 participants per arm, downgraded one level for imprecision. 3Unclear risk of bias in the study, downgraded one level.
Summary of findings 3. Personal protective equipment (PPE) types: gowns versus aprons.
| Gowns versus aprons for preventing highly infectious diseases due to contact with contaminated body fluids in healthcare workers | |||||
| Patient or population: healthcare worker volunteers Settings: simulation study Intervention: gowns versus aprons | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Aprons | Gowns | ||||
|
Contamination with marker: individual type of doffing Follow‐up: post‐intervention |
The mean contamination with marker in the control groups was 16.98 small spots | The mean contamination with marker in the intervention groups was 10.28 lower (14.77 to 5.79 lower) | 50 (1 study) | ⊕⊝⊝⊝ Very low1,2,3 | Cross‐over study; the analyses were unadjusted for the paired nature of the data but similar to the analysis of the study authors, who took this into account |
|
Contamination with marker: CDC‐recommended doffing Follow‐up: post‐intervention |
The mean contamination with marker in the control groups was 1.88 small spots | The mean contamination with marker in the intervention groups was 0.62 lower (1.75 lower to 0.51 higher) | 50 (1 study) | ⊕⊝⊝⊝ Very low1,2,3 | |
| Compliance with guidance | See comment | See comment | 0 (0 studies) |
See comment | No studies evaluated the effect of the interventions on compliance with guidance. |
| *The basis for the assumed risk is the control group risk. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group. CDC: Centers for Disease Control and Prevention; CI: confidence interval | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
1Randomisation method unclear, downgraded one level. 2Simulation study, downgraded one level for indirectness. 3Single cross‐over study with 50 participants, downgraded one level for imprecision.
Summary of findings 4. Personal protective equipment (PPE) types: different types of PPE attire.
| One type of full‐body PPE compared to another type for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare workers | ||||
| Patient or population: healthcare workers Setting: simulation study Intervention: one type of full‐body PPE Comparison: another type | ||||
| Outcomes | Impact | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Any contamination | In 1 RCT (59 participants) people with a long gown had less contamination than
those with a coverall and those with a coverall less than those with an
isolation gown. In 1 observational study (11 participants) there were too few events to enable comparison of contamination rates between 5 types of PPE In 1 observational study (10 participants), out of 10 different ensembles there were contaminations in only 4, of these 3 used coveralls |
59 participants (1 RCT) 21 participants (2 observational studies) |
⊕⊕⊝⊝
Low1,2 ⊕⊝⊝⊝ Very low3 |
|
| Compliance | Isolation gown was easiest to don and doff, coverall was more difficult to doff | 59 participants (1 RCT) | ⊕⊝⊝⊝ Very low1,2 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). PPE: personal protective equipment; RCT: randomised controlled trial | ||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||
1 One study with 59 participants, downgraded by one level because of imprecision. 2Risk of bias in the study was unclear and so we downgraded by one level. 3The simulated exposure was very low. This resulted in a lack of power to detect differences, We downgraded by one level.
Summary of findings 5. Modified personal protective equipment (PPE): sealed gown‐glove interface versus standard gown.
| Sealed gown‐glove interface compared to standard gown for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare workers | ||||||
| Patient or population: healthcare workers Setting: simulation study Intervention: sealed gown‐glove interface Comparison: standard gown | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with traditional suit | Risk with sealed suit | |||||
|
Contamination: fluorescent lotion |
733 per 1000 | 198 per 1000 (66 to 572) | RR 0.27 (0.09 to 0.78) | 30 (1 RCT) | ⊕⊝⊝⊝ Very low1,2,3 | |
| Contamination: MS2 | 1000 per 1000 | 680 per 1000 (470 to 980) | RR 0.68 (0.47 to 0.98) | 30 (1 RCT) | ⊕⊝⊝⊝ Very low1,2,3 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Unclear risk of bias, downgraded by one level. 2This is a simulation study so we downgraded by one level because of indirectness. 3One study with 30 participants so we downgraded by one level because of imprecision.
Summary of findings 6. Modified personal protective equipment (PPE): gown ‐ easy to doff compared to standard gown.
| Easy‐to‐doff gown compared to standard gown for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare workers | ||||||
| Patient or population: healthcare workers Setting: simulation study Intervention: gown: easy to doff Comparison: standard gown | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with standard gown | Risk with easy‐to‐doff gown | |||||
|
Contamination: fluorescent marker |
419 per 1000 | 34 per 1000 (4 to 231) | RR 0.08 (0.01 to 0.55) | 62 (1 RCT) | ⊕⊕⊝⊝ Low1,2 | |
|
Contamination: bacteriophage |
613 per 1000 | 325 per 1000 (178 to 576) | RR 0.53 (0.29 to 0.94) | 62 (1 RCT) | ⊕⊕⊝⊝ Low1,2 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Simulation study, downgraded by one level. 2One small study, downgraded by one level.
Summary of findings 7. Modified personal protective equipment (PPE): gown with gown‐glove improvement compared to standard gown and gloves.
| Gown with gown‐glove improvement compared to standard gown and gloves for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare workers | ||||||
| Patient or population: healthcare workers Setting: simulation study Intervention: gown with gown‐glove improvement Comparison: standard gown and gloves | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with standard gown and gloves | Risk with gown with gown‐glove improvement | |||||
| People with contamination | 410 per 1000 | 185 per 1000 (107 to 320) | RR 0.45 (0.26 to 0.78) | 50 (2 RCTs) | ⊕⊕⊝⊝ Low1,2 | Cross‐over study analysed as parallel study |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Study at high risk of performance bias, otherwise unclear risk of bias, downgraded one level. 2Simulation study, downgraded by one level.
Summary of findings 8. Modified personal protective equipment (PPE): gloves with tab versus standard gloves.
| Gloves with tab compared to standard gloves for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare workers | ||||||
| Patient or population: healthcare workers Setting: simulation study Intervention: gloves with tab Comparison: standard gloves | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with standard gloves | Risk with gloves with tab | |||||
| Any contamination of hands | 733 per 1000 | 161 per 1000 (110 to 227) | RR 0.22 (0.15 to 0.31) | 317 (1 RCT) | ⊕⊝⊝⊝ Very low1,2 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Clusters of healthcare workers who were present at work were allocated to intervention or control on alternating days and so we downgraded by two levels because of study limitations. 2This is a simulation study so we downgraded by one level because of indirectness.
Summary of findings 9. Modified personal protective equipment (PPE): mask plus tabs versus standard masks.
| Mask tabs compared to no mask tabs for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare workers | ||||||
| Patient or population: healthcare workers Setting: simulation study Intervention: mask tabs Comparison: no mask tabs | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with No mask tabs | Risk with Mask tabs | |||||
| Contamination of mask from hands | 1000 per 1000 | 330 per 1000 (140 to 800) | RR 0.33 (0.14 to 0.80) | 20 (1 RCT) | ⊕⊝⊝⊝ Very low1,2,3 | Analyses presented in this table are unadjusted for the paired nature of the cross‐over design but similar to the results that the study authors presented while taking the cross‐over into account. |
| Contamination of head from hands | 867 per 1000 | 832 per 1000 (719 to 971) | RR 0.96 (0.83 to 1.12) | 20 (1 RCT) | ⊕⊝⊝⊝ Very low1,2,3 | Analyses presented in this table are unadjusted for the paired nature of the cross‐over design but similar to the results that the study authors presented while taking the cross‐over into account. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1The randomisation procedure was unclear and the cross‐over procedure was unclear so we downgraded by one level because of study limitations. 2This is a simulation study so we downgraded by one level because of indirectness. 3One study only with 20 participants and so we downgraded by one level because of imprecision.
Summary of findings 10. Procedures: doffing according to Centers for Disease Control and Prevention method versus individual doffing.
| Centers for Disease Control and Prevention (CDC) method versus individual doffing for preventing contact with contaminated body fluids in healthcare workers | ||||||
|
Patient or population: healthcare worker
volunteers
Settings: simulation study
Intervention:
CDC method of doffing Control: individual method of doffing | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Individual doffing method | CDC‐recommended doffing method | |||||
|
Contamination with fluor marker when using gowns Follow‐up: post‐intervention |
The mean contamination with fluor marker in the control group was 6.7 small spots | The mean contamination with fluor marker in the intervention group was 5.44 lower (7.43 to 3.45 lower) | 50 (1 study) | ⊕⊝⊝⊝ Very low1,2,3 | Cross‐over study; the analyses were unadjusted for the paired nature of the data but similar to the analysis of the study authors, who took this into account. | |
|
Contamination with fluor marker when using aprons Follow‐up: post‐intervention |
The mean contamination with fluor marker in the control group was 16.98 small spots | The mean contamination with fluor marker in the intervention group was 15.1 lower (19.28 to 10.92 lower) | 50 (1 study) | ⊕⊝⊝⊝ Very low1,2,3 | ||
| *The basis for the assumed risk is the control group risk. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group. CDC: Centers for Disease Control and Prevention; CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1The randomisation procedure was unclear and so we downgraded by one level due to study limitations. 2This is a simulation study so we downgraded by one level because of indirectness. 3One cross‐over study with 50 participants and so we downgraded by one level due to imprecision.
Summary of findings 11. Procedures: single‐step doffing compared to Centers for Disease Control and Prevention standard.
| Single‐step doffing compared to Centers for Disease Control and Prevention (CDC) standard for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare workers | ||||||
| Patient or population: preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare workers Setting: simulation study Intervention: single‐step doffing Comparison: CDC standard | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with CDC standard | Risk with single‐step doffing | |||||
| Fluorescent contamination | 917 per 1000 | 898 per 1000 (688 to 1000) | RR 0.98 (0.75 to 1.28) | 22 (1 RCT) | ⊕⊝⊝⊝ Very low1,2,3 | |
| Bacterial contamination | 667 per 1000 | 133 per 1000 (33 to 513) | RR 0.20 (0.05 to 0.77) | 27 (1 RCT) | ⊕⊝⊝⊝ Very low1,2,4 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CDC: Centers for Disease Control and Prevention; CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Simulation study, downgraded by one level. 2Large difference in effects between fluorescent contamination and bacterial contamination. 3Confidence Interval contains harms and benefits. 4Confidence interval contains both very large effects and very small effects.
Summary of findings 12. Procedures: doffing with double gloves compared to doffing with single gloves.
| Doffing with double gloves compared to doffing with single gloves for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare workers | ||||||
| Patient or population: healthcare workers Setting: simulation study Intervention: doffing with double gloves Comparison: doffing with single gloves | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with doffing with single gloves | Risk with doffing with double gloves | |||||
| Contamination: virus detected ‐ all body parts | 733 per 1000 | 249 per 1000 (125 to 484) | RR 0.34 (0.17 to 0.66) | 58 (1 RCT, 1 observational study) | ⊕⊝⊝⊝ Very low1,2,3 | |
| Contamination: virus detected ‐ face | 17 per 1000 | 73 per 1000 (9 to 606) | RR 4.39 (0.53 to 36.37) | 58 (1 RCT, 1 observational study) | ⊕⊝⊝⊝ Very low1,2,3 | |
| Contamination: virus detected ‐ shirt | 567 per 1000 | 572 per 1000 (448 to 731) | RR 1.01 (0.79 to 1.29) | 58 (1 RCT, 1 observational study) | ⊕⊝⊝⊝ Very low1,2,4 | |
| Contamination: virus detected ‐ pants | 611 per 1000 | 556 per 1000 (318 to 966) | RR 0.91 (0.52 to 1.58) | 36 (1 observational study) | ⊕⊝⊝⊝ Very low2,4 | |
| Non‐compliance: any error | 667 per 1000 | 720 per 1000 (467 to 1000) | RR 1.08 (0.70 to 1.67) | 36 (1 observational study) | ⊕⊝⊝⊝ Very low2,4 | |
| Contamination with fluorescent | 917 per 1000 | 898 per 1000 (688 to 1000) | RR 0.98 (0.75 to 1.28) | 22 (1 RCT) | ⊕⊝⊝⊝ Very low1,2,4 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Difference in viral/bacterial outcomes and fluorescent marker outcomes, downgraded one level. 2Simulation studies, downgraded one level. 3Confidence interval contains both very large and moderate effects. 4Confidence interval contains both harms and benefits.
Summary of findings 13. Procedures: donning and doffing with instructions compared to without instruction.
| Donning and doffing with instructions compared to without instructions for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare workers | ||||||
| Patient or population: healthcare workers Setting: simulation study Intervention: donning and doffing with instructions Comparison: without instructions | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with without instructions | Risk with donning and doffing with instructions | |||||
| People with one or more errors | 467 per 1000 | 145 per 1000 (51 to 434) | RR 0.31 (0.11 to 0.93) | 120 (1 observational study) | ⊕⊝⊝⊝ Very low1,2,3 | |
| Mean errors | The mean errors was 1.15 | MD 0.89 lower (1.36 lower to 0.41 lower) | ‐ | 120 (1 observational study) | ⊕⊝⊝⊝ Very low1,2 | |
| Fluorescence contamination | The mean fluorescence contamination was 11 | MD 5 lower (8.08 lower to 1.92 lower) | ‐ | 24 (1 RCT) | ⊕⊕⊝⊝ Low4,5 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Unblinded outcome assessors with subjective outcome, downgraded one level. 2Simulated donning/doffing, downgraded one level. 3Confidence Interval includes very large effect size and small effect size. 4Simulation study, downgraded one level. 5One small study only, downgraded one level.
Summary of findings 14. Procedures: doffing with extra sanitation of gloves compared to standard no sanitation.
| Doffing with extra sanitation of gloves compared to standard no sanitation for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare workers | ||||||
| Patient or population: healthcare workers Setting: simulation study Intervention: doffing with extra sanitation of gloves Comparison: standard no sanitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with standard no sanitation | Risk with doffing with extra sanitation of gloves | |||||
| Bacterial contamination: alcohol‐based hand rub | 667 per 1000 | 500 per 1000 (260 to 967) | RR 0.75 (0.39 to 1.45) | 46 (1 RCT, 1 observational study) | ⊕⊕⊝⊝ Low1,2 | In the observational study, bacterial contamination (2.2 CFUs) did not significantly reduce compared to no sanitation (2.4 CFUs) |
| Bacterial contamination: quaternary ammonium | 20 (1 observational study) | ⊕⊝⊝⊝ Very low1,3,4 | Bacterial contamination significantly reduced from 2.4 CFUs to 0 CFUs and compared to 2.2 CFUs without sanitation | |||
| Bacterial contamination: bleach | 20 (2 observational studies) | ⊕⊝⊝⊝ Very low1,3,4 | In one study, bacterial contamination significantly reduced from 2.4 CFUs to 0 CFUs and compared to 2.2 CFUs without sanitation. In another study there was collinearity between PPE use and other variables, which precluded analysis. | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFU: colony‐forming unit; CI: confidence interval; PPE: personal protective equipment; RCT: randomised controlled trial; RR: risk ratio | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Simulation study, downgraded by one level. 2Confidence interval contains both harms and benefits. 3Study at high risk of selection bias, downgraded one level. 4One small study with 20 participants, downgraded one level.
Summary of findings 15. Procedures: doffing with hypochlorite versus doffing with alcohol‐based glove sanitiser.
| Doffing with hypochlorite compared to doffing with alcohol‐based glove sanitiser for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare workers | ||||||
| Patient or population: healthcare workers Setting: simulation study Intervention: doffing with hypochlorite Comparison: doffing with alcohol‐based glove sanitiser | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with doffing with alcohol‐based glove sanitiser | Risk with doffing with hypochlorite | |||||
| Contamination: MS2 | Study population | RR 4.00 (0.47 to 34.24) | 15 (1 observational study) | ⊕⊝⊝⊝ Very low1,2,3 | ||
| 100 per 1000 | 400 per 1000 (47 to 1000) | |||||
| Contamination: Ph6 | Study population | Not estimable | 15 (1 observational study) | ‐⊕⊝⊝⊝ Very low1,2,3 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: Risk ratio | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Allocation to intervention was based on belonging to last five participants, which is an unclear selection procedure and so we downgraded by one level because of study limitations. 2This is a simulation study so we downgraded by one level because of indirectness. 3The study had a small number of participants and so we downgraded by one level because of imprecision.
Summary of findings 16. Teaching: video‐based learning versus traditional lecture.
| Video‐based learning compared to traditional lecture for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare workers | ||||||
| Patient or population: healthcare workers Setting: simulation studies Intervention: video‐based learning Comparison: traditional lecture | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with traditional lecture | Risk with video‐based learning | |||||
| Skills in PPE donning Assessed with assessment scale. Scale from: 0% to 100%; higher is better | The mean skills in PPE donning was 47.4% | MD 30.7% higher (20.14 higher to 41.26 higher) | ‐ | 26 (1 RCT) | ⊕⊝⊝⊝ Very low1,2,3 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; PPE: personal protective equipment; RCT: randomised controlled trial | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1The randomisation and allocation procedures were unclear and so we downgraded by one level because of study limitations. 2This is a simulation study so we downgraded by one level because of indirectness. 3One study with only 26 participants and so we downgraded by one level because of imprecision.
1. Different types of PPE compared
1a Different types of mouth and nose protection
1.1 Powered air‐purifying respirator (PAPR) versus PPE for enhanced respiratory and contact precautions (E‐RCP)
Outcome: contamination with fluorescent marker
Zamora 2006 found that the PAPR system in use in their hospital led to less contamination than using the E‐RCP system (RR 0.27, 95% CI 0.17 to 0.43; Analysis 1.1). Other ways of measuring contamination also led to less contamination with the PAPR system: contamination more than 1 cm (RR 0.21, 95% CI 0.12 to 0.36). The total contaminated area was also less with a mean difference of −81.10 cm² (95% CI −96.07 to −66.13). This was mainly due to a lack of protection of the neck in the E‐RCP system.
1.1. Analysis.

Comparison 1: PAPR versus E‐RCP Attire, Outcome 1: Any contamination
Outcomes: compliance with guidance ‐ donning and doffing noncompliance
Noncompliance with donning guidelines occurred more with the PAPR system as this consists of more elements (RR 7.50, 95% CI 1.81 to 31.10; Analysis 1.4; Zamora 2006). Noncompliance with doffing guidelines was more frequent with the E‐RCP system, but this was not statistically significant (RR 0.50; 95% CI 0.20 to 1.23; Analysis 1.5).
1.4. Analysis.

Comparison 1: PAPR versus E‐RCP Attire, Outcome 4: Donning noncompliance
1.5. Analysis.

Comparison 1: PAPR versus E‐RCP Attire, Outcome 5: Doffing noncompliance
Outcomes: donning and doffing time
The donning (MD = 259 seconds) and doffing time (MD = 337 seconds) were considerably longer with the PAPR system (Analysis 1.6; Analysis 1.7; Zamora 2006).
1.6. Analysis.

Comparison 1: PAPR versus E‐RCP Attire, Outcome 6: Donning time
1.7. Analysis.

Comparison 1: PAPR versus E‐RCP Attire, Outcome 7: Doffing time
1.2 One type of PAPR versus another and different airflow rates
Outcome: contamination with microbial aerosol
Buianov 2004 found that the suit that had the hood attached to the suit (СКБ‐I) had a lower 'contamination penetration rate' than the suits that had separate hoods and coveralls with a percentage of 8.10‐8 for the suit and 2.10‐1 for the coveralls. However, we could not understand the meaning of the penetration rate and we decided that we would not use these results for our conclusions (their results are not shown in data tables).
Outcomes: heart rate and body temperature
Buianov 2004 also found that contamination stopped beyond the 250 L/minute airflow rates. Body temperature and heart rates were also lower at these airflow rates.
1b Different types of body protection
1.3 Four types of PPE versus another
Wong 2004 compared four types of PPE according to their material properties. Type A had good water repellency and water penetration resistance but at the cost of poor air permeability. Type B had good water repellency and good air permeability but poor water penetration resistance. Type C was the surgical gown with both poor water repellency and water penetration resistance. Type D, Barrierman, was made of Tyvek and had good water repellency, poor air permeability, and fair water resistance.
Outcomes: contamination, user‐reported assessment of comfort and convenience ‐ usability, donning and doffing times
There were no considerable differences in contamination (Analysis 2.1) between Type A and Type B for face, neck, trunk, foot, or hand, but Type B scored about 10% higher on usability (MD −0.46, 95% CI −0.84 to −0.08; Analysis 2.2); this was due especially to better breathability of the fabric. There were no considerable differences in donning and doffing times (Analysis 2.3; Analysis 2.4).
2.1. Analysis.

Comparison 2: Four types of PPE attire compared, Outcome 1: A vs B Contamination, mean number of spots
2.2. Analysis.

Comparison 2: Four types of PPE attire compared, Outcome 2: A vs B Usability score (1‐5)
2.3. Analysis.

Comparison 2: Four types of PPE attire compared, Outcome 3: A vs B Donning time
2.4. Analysis.

Comparison 2: Four types of PPE attire compared, Outcome 4: A vs B Doffing time
There were considerable differences in contamination of the foot (MD −4.1 spots, 95% CI −6.94 to −1.26) and the hand (MD −12.76 spots, 95% CI −21.62 to −3.9) between Type A and Type D (Analysis 2.5). Donning (MD 33 seconds, Analysis 2.7) and doffing (MD 17 seconds, Analysis 2.8) times were also much worse for Type D. Usability was rated as not considerably differently (MD 0.25, 95% CI −0.12 to 0.62; Analysis 2.6).
2.5. Analysis.

Comparison 2: Four types of PPE attire compared, Outcome 5: A vs D Contamination, mean number of spots
2.7. Analysis.

Comparison 2: Four types of PPE attire compared, Outcome 7: A vs D Donning time
2.8. Analysis.

Comparison 2: Four types of PPE attire compared, Outcome 8: A vs D Doffing time
2.6. Analysis.

Comparison 2: Four types of PPE attire compared, Outcome 6: A vs D Usability score (1‐5)
It was unclear how many participants had no contamination. On average, all types of PPE had some contamination.
1.4 Formal PPE versus locally available PPE
Outcome: contamination with fluorescent marker
Bell 2015 compared contamination in four participants with formal PPE with four participants with locally available protective gear, such as raincoats. They found contamination in one participant in both study arms. The study was so small that it is difficult to draw conclusions (Analysis 3.1).
3.1. Analysis.

Comparison 3: Formal versus local available attire, Outcome 1: Contamination
1.5 Gown versus apron
Outcome: contamination with fluorescent marker
Guo 2014 compared a gown with an apron and found that the gown left less contamination than an apron, regardless of the way of doffing (Analysis 4.1; Analysis 4.2).
4.1. Analysis.

Comparison 4: Gown versus apron, Outcome 1: Contamination with marker; individual doffing
4.2. Analysis.

Comparison 4: Gown versus apron, Outcome 2: Contamination with marker; CDC doffing
1.6 Five types of PPE attire compared
Outcome: contamination with fluorescent marker
Hall 2018 compared post‐doffing contamination of five types of PPE ensembles used in different hospital wards across the UK. No analysis of contamination rates of the different suits was available since the authors reported the data on contamination sites only and not according to type of attire. They argued that the contamination rates were too low to provide a valid comparison.
1.7 Three different types of PPE attire compared
Outcome: contamination with fluorescent marker
Suen 2018 measured small and large patches of contamination in three different ensembles with PPE 1, a surgical gown used with EVD with a hood covering the neck, PPE 2, a coverall also used for EVD, and PPE 3, an isolation gown. They reported the median number of patches across 10 body sites and four environmental contamination sites. The median number of contaminations for small patches was respectively 5, 7 and 7 and for large patches it was 39, 43 and 47. These differences were reported as being statistically significantly different but there were insufficient data to check this. This would mean that a long gown protects better than a coverall and that the commonly used isolation gown protects least. According to the study authors, the reduced protection for the isolation gown is especially due to the lack of coverage of the neck, "which resulted in many small or extra‐large patches in the anterior and posterior neck region after spraying of the fluorescent solution onto the face shield and anterior surfaces of the gown".
Outcomes: compliance with guidance ‐ donning and doffing noncompliance
Suen 2018 also measured compliance and reported the average percentage of errors across the items measured. For PPE 1, PPE 2 and PPE 3, the averages for donning were 6.1, 6.0 and 3.7 and for doffing 3.0, 9.5 and 3.5. This seems to give an indication that coveralls are more difficult to doff.
Outcomes: time for donning and doffing
Suen 2018 also measured the time needed to don and doff the PPE (Analysis 5.1; Analysis 5.2). PPE 3, the isolation gown, was quickest to don and doff, while the coverall doffing took significantly longer, with on average more than 10 minutes for doffing. The attire with the long surgical gown took twice as long as the isolation gown to put on and was also slower to doff because more PPE items were used. We were not able to conduct a proper paired analysis because of the lack of detail in the study report. We analysed the trial as if it were a two‐group parallel trial, which leads to too wide confidence intervals.
5.1. Analysis.

Comparison 5: Three types of PPE compared, Outcome 1: Time for donning
5.2. Analysis.

Comparison 5: Three types of PPE compared, Outcome 2: Time for doffing
1.8 Ten different types of PPE ensembles compared
Outcome: contamination with fluorescent marker
Chughtai 2018 evaluated 10 different PPE ensembles recommended for use with EVD by global and national authorities. Six of these used coveralls and four used gowns. There were also differences in the use of a PAPR or a respiratory mask. Each ensemble was tested in total three times by part of 10 volunteers. There were only four ensembles that led to contamination: the ensemble recommended by WHO, North Carolina authorities, CDC and Health Canada. The first three consist of coveralls and the last one is a gown.
Outcome: user satisfaction
Chughtai 2018 also asked users to rate the ease of donning and doffing. The ECDC coverall and protocol was rated highest for ease of donning and doffing. Since there were only three ratings per ensemble, this has only a limited meaning.
2. Modifications versus standard gear
2.1 Sealed gown‐glove interface versus traditional gown‐glove interface
Outcome: contamination
Tomas 2016 found that participants doffing a gown that had continuous coverage of skin from arm to hand (sealed suit) were less likely to contaminate themselves with fluorescent lotion than those doffing traditional PPE of gown and glove (RR 0.27; 95% CI 0.09 to 0.78; Analysis 6.1). The study authors obtained similar results when they used MS2 bacteriophage as the contaminate (RR 0.68; 95% CI 0.47 to 0.98; Analysis 6.2).
6.1. Analysis.

Comparison 6: Gown sealed gloves versus standard gown, Outcome 1: Contamination fluorescent lotion
6.2. Analysis.

Comparison 6: Gown sealed gloves versus standard gown, Outcome 2: Contamination MS2
2.2 Easy‐doffing gown versus traditional gown
Outcome: contamination
Mana 2018 compared a gown with modified neck and wrist design to facilitate doffing with a traditional gown and found fewer people with contamination with both fluorescent marker (RR 0.08, 95% CI 0.01 to 0.55; Analysis 7.1) and with harmless virus (RR 0.53, 95% CI 0.29 to 0.94; Analysis 7.2). Even though we received additional information from the study authors we were unable to conduct a proper paired analysis.
7.1. Analysis.

Comparison 7: Gown easy to doff versus standard gown, Outcome 1: Contamination with fluorescent marker
7.2. Analysis.

Comparison 7: Gown easy to doff versus standard gown, Outcome 2: Contamination with bacteriophage
2.3. Modified gown‐glove interface versus standard gown‐glove interface
Outcome: contamination
Hajar 2019 modified the gown‐glove interface with more overlap between gown and glove. They evaluated this in two different groups. In one they compared the modified gown to a standard gown and in the other they added extra education to both intervention and control group. This led to considerably less contamination (RR 0.45, 95% CI 0.26 to 0.78, Analysis 8.1) in the meta‐analysis of the two trials. We could not take into account that the trials had a cross‐over design but analysed these as if they were parallel trials with twice the number of participants. This may have led to a slight overestimation of the precision.
8.1. Analysis.

Comparison 8: Gown with gown‐glove improvement vs standard gown‐gloves, Outcome 1: People with contamination
2.4. Modified‐inside gown versus standard gown
Outcome non‐compliance: errors during donning, doffing, performance
Drews 2019 redesigned the gown based on observed errors during doffing, donning and performing tasks. They found a similar number of people with errors while donning (RR 0.93, 95% CI 0.50 to 1.72; Analysis 9.1), while performing tasks (MD −0.30, 95% CI −0.67 to 0.07; Analysis 9.2) and while doffing (RR 0.81, 95% CI 0.33 to 2.00; Analysis 9.3).
9.1. Analysis.

Comparison 9: Gown with marked inside versus standard gown, Outcome 1: Noncompliance donning: people with errors
9.2. Analysis.

Comparison 9: Gown with marked inside versus standard gown, Outcome 2: Noncompliance: errors during performance
9.3. Analysis.

Comparison 9: Gown with marked inside versus standard gown, Outcome 3: Noncompliance doffing: people with errors
2.5 Gloves with tabs versus gloves without tabs
Outcome: contamination
Gleser 2018 found a decrease in people with contamination when doffing gloves with tab near thumb and wrist compared to standard gloves (RR 0.22, 95% CI 0.15 to 0.31; Analysis 10.1).
10.1. Analysis.

Comparison 10: Gloves with tab versus standard gloves, Outcome 1: Any contamination of hands
2.6 Masks with tabs versus masks without tabs
Outcome: contamination
Strauch 2016 found that contamination from hands to the head was less when the participant doffed a mask with tabs on the strap engineered as a doffing aid compared to a mask without tabs (RR 0.33, 95% CI 0.14 to 0.80; Analysis 11.1). There was no difference in contamination rates when participants doffed a contaminated mask that either had or did not have tabs (RR 0.96; 95% CI 0.83 to 1.12; Analysis 11.2).
11.1. Analysis.

Comparison 11: Mask with tabs versus no mask tabs, Outcome 1: Contamination of head from hands
11.2. Analysis.

Comparison 11: Mask with tabs versus no mask tabs, Outcome 2: Contamination of hands from mask
3. Changes in donning or doffing procedures
3.1 Double‐gloving versus single‐gloving
Outcome: contamination with MS2 virus
Both Casanova 2012 and Osei‐Bonsu 2019 found that contamination with the use of double gloves was less than with single gloves. We felt that the studies were comparable even though the first used harmless virus and the second harmless bacteria as the simulated exposure. When all contaminated sites were taken together the RR was 0.34 (95% CI 0.17 to 0.66; Analysis 12.1). For the specific body parts the reduction was less clear (Analysis 12.1). Also when measured with fluorescent marker, there was no difference between double‐ and single‐gloving (RR 0.98, 95% CI 0.75 to 1.28; Analysis 12.4).
12.1. Analysis.

Comparison 12: Doffing with double gloves versus doffing with single gloves, Outcome 1: Contamination: virus detected
12.4. Analysis.

Comparison 12: Doffing with double gloves versus doffing with single gloves, Outcome 4: Contamination with fluorescent
All participants had some level of contamination. Measured as the quantity of virus found, the hands were less contaminated after degloving when participants used double gloves but due to missing data we could not test this.
Outcome: compliance with guidance ‐ compliance errors
No more errors in compliance occurred with the donning or doffing protocol for double‐gloving compared to single gloving (RR 1.08, 95% CI 0.70 to 1.67; Analysis 12.3).
12.3. Analysis.

Comparison 12: Doffing with double gloves versus doffing with single gloves, Outcome 3: Non‐compliance: any error
3.2 CDC‐recommended procedure versus individual doffing
Outcome: contamination
Guo 2014 found that the CDC's recommended way of doffing a gown or an apron led to a different decrease in contamination compared to individually chosen doffing. When doffing the gown, there were 5.4 fewer smaller contamination patches (95% CI −7.4 to −3.4) and 5.2 fewer stains in the environment (95% CI −7.3 to −3.3), but no difference in small contamination patches on the hands, shoes or underwear. With doffing the apron, there were fewer smaller stains, stains on the hands, shoes, and environment, but more large stains and a similar number of stains on the underwear (Analysis 13.1; Analysis 13.2).
13.1. Analysis.

Comparison 13: CDC versus individual doffing, Outcome 1: Gown: contamination with fluor marker
13.2. Analysis.

Comparison 13: CDC versus individual doffing, Outcome 2: Apron: contamination with fluor marker
3.3 CDC‐recommended procedure versus single step
Outcome: contamination
Osei‐Bonsu 2019 evaluated doffing gown and gloves in a single step versus the standard gloves first procedure and found no difference in contamination with fluorescent marker (RR 0.98, 95% CI 0.75 to 1.28; Analysis 14.1) but with bacterial contamination there was a considerable difference (RR 0.20, 95% CI 0.05 to 0.77; Analysis 14.2). It is unclear what would cause this difference in effect between the two outcome measures. We would be inclined to assume that the bacterial simulation is more realistic than the fluorescent powder.
14.1. Analysis.

Comparison 14: Single‐step doffing vs CDC standard, Outcome 1: Fluorescent contamination
14.2. Analysis.

Comparison 14: Single‐step doffing vs CDC standard, Outcome 2: Bacterial contamination
3.4 Doffing with extra disinfection of gloves
a. Alcohol‐based sanitation of gloves versus no extra glove sanitation
Outcome: bacterial contamination
Osei‐Bonsu 2019 compared alcohol‐based glove sanitation versus no glove sanitation and found no considerable reduction in the number of people contaminated (RR 0.75, 95% CI 0.39 to 1.45; Analysis 15.1). Kpadeh Rogers 2019 found a non‐significant reduction in bacterial contamination from a median 2.4 colony‐forming units (CFUs) to 2.2 CFUs for both bacteria used when alcohol‐based hand rub was used versus no extra sanitation of gloves.
15.1. Analysis.

Comparison 15: Doffing with extra sanitation of gloves versus standard no sanitation, Outcome 1: Bacterial contamination
b. Quaternary ammonium versus no extra glove sanitation
Outcome: bacterial contamination
Kpadeh Rogers 2019 found a significant reduction in bacterial contamination from a median 2.4 CFUs to 0 CFUs for both bacteria used for simulating exposure when quaternary ammonium‐based hand rub was used versus no extra sanitation of gloves.
c. Bleach versus no extra glove sanitation
Outcome: bacterial contamination
Kpadeh Rogers 2019 found a significant reduction in bacterial contamination from a median 2.4 CFUs to 0 CFUs for both bacteria used for simulating exposure when bleach‐based hand rub was used versus no extra sanitation of gloves.
d. Hypochlorite sanitation versus alcohol‐based sanitation
Outcome: viral contamination
Casanova 2016 found non‐significantly greater self‐contamination of bacteriophage MS2 to the hands, face or scrubs when hypochlorite solution was used for the glove sanitising step of the doffing protocol compared to the use of an alcohol‐based hand rub (RR 4.00, 95% CI 0.47 to 34.24; Analysis 18.1). The study authors did not detect contamination of bacteriophage Ph6 when using either alcohol‐based hand rub or the hypochlorite solution (Analysis 18.2).
18.1. Analysis.

Comparison 18: Doffing with hypochlorite versus doffing with alcohol‐based glove sanitiser, Outcome 1: Contamination MS2
18.2. Analysis.

Comparison 18: Doffing with hypochlorite versus doffing with alcohol‐based glove sanitiser, Outcome 2: Contamination Ph6
e. Chlorine spray versus no spray
Houlihan 2017 compared the risk of HCW contracting Ebola when either using or not using a chlorine spray during the doffing of PPE. However, there was no variation in the use of chlorine spray among clinical workers. The use only varied between clinical and laboratory workers. Since it is not possible to disentangle risk of exposure and the use of hypochlorite solution, no conclusions can be drawn from this study with regard to PPE.
3.5 Additional spoken personal instructions versus no such instructions
3.5.1. Outcome: compliance with guidance ‐ noncompliance
Casalino 2015 found that there were substantially less noncompliance (people with one or more errors) after additional spoken instruction compared to no instructions with (RR 0.31, 95% CI 0.11 to 0.93) and also that the mean number of errors fell by on average almost one (MD −0.89, 95% CI −1.36 to −0.41) in the group with spoken instructions (Analysis 16.1; Analysis 16.2).
16.1. Analysis.

Comparison 16: Donning and doffing with instructions versus without instructions, Outcome 1: People with one or more errors
16.2. Analysis.

Comparison 16: Donning and doffing with instructions versus without instructions, Outcome 2: Non‐compliance: mean errors
Andonian 2019 organised team work between the person with PPE and doffing assistants who guided the donning and doffing process and found a decrease in the number of sites contaminated with either fluorescent marker or particles (MD −5.00, 95% CI −8.08 to −1.92). We assumed that the median reported by the study authors would be roughly equal to the mean and the interquartile range equalled, 1.35 SD.
3.5.2. Outcome: infection rate
One study compared infection rates between people who had instructions while donning and doffing versus rates in those without instructions. Due to the fact that the exposure was also different between these two groups, we were unable to draw conclusions about the protective effect of instructions (Houlihan 2017).
4. Training and instructions
4a. Training and instruction for proper and complete PPE use
4a.1 Active training versus passive training
4a.1.1 Outcome: compliance with guidance ‐ noncompliance with PPE guidance
Shigayeva 2007 defined consistent adherence as always wearing gloves, gown, mask, and eye protection. We transformed this to inconsistent use as being noncompliant with the guidance. The study found that active training led to less noncompliance than no training (OR 0.37, 95% CI 0.2 to 0.58). For passive training, they found a lower risk of noncompliance compared to no training (OR 0.58, 95% CI 0.33 to 1.00). For the indirect comparison, active versus passive training, the OR was 0.63 (95% CI 0.31 to 1.30; Analysis 20.1).
20.1. Analysis.

Comparison 20: Computer simulation versus no simulation, Outcome 1: Number of errors while donning
4b. Training and instruction for PPE donning and doffing
4b.1. Active versus passive instruction
4b.1.2. Outcome: compliance with guidance ‐ noncompliance with doffing procedures
Shigayeva 2007 found no considerable effect of active (OR 0.70, 95% CI 0.45 to 1.11) or passive training (OR 1.56, 95% CI 0.83 to 2.94) compared to no training on the number of errors in compliance with the doffing protocol. For the indirect comparison, active versus passive training, the OR was 0.45 (95% CI 0.21 to 0.98; Analysis 19.1).
19.1. Analysis.

Comparison 19: Active training in PPE doffing versus passive training, Outcome 1: Noncompliance doffing protocol
4b.2. Additional computer simulation versus no additional computer simulation
4b.2.1. Outcome: compliance with guidance ‐ noncompliance
Even though the number of errors was already low, Hung 2015 found that adding computer simulation reduced the number of errors with on average half an error for donning (MD, −0.52, 95% CI −0.90 to −0.14; Analysis 20.1) and with more than one error for doffing (MD −1.16, 95% CI −1.63 to −0.69; Analysis 20.2).
20.2. Analysis.

Comparison 20: Computer simulation versus no simulation, Outcome 2: Number of errors while doffing
4b.3 Video‐based learning versus traditional learning
Curtis 2018 compared skills in donning PPE when taught with a video‐based learning method versus a traditional lecture. Those that participated in the video learning had a higher mean score on the post‐exam than those who attended a traditional lecture. (MD 30.7, 95% CI 20.14 to 41.26; Analysis 21.1).
21.1. Analysis.

Comparison 21: Video‐based learning versus traditional lecture, Outcome 1: Skills in PPE donning
5. Subgroup and sensitivity analysis
We planned a subgroup analysis of studies conducted in high‐ versus low‐ and middle‐income countries. However, there were not enough studies for such a subgroup analysis to be meaningful.
We also planned a sensitivity analysis including only studies we judged to have a low risk of bias. As none of the included studies fulfilled this criterion, we could not perform this analysis.
6. Certainty of the evidence
We judged if there was a reason to downgrade the certainty of the evidence for each domain of GRADE. Since we judged all studies to have a high or unclear risk of bias, we downgraded the evidence for all comparisons by one level. We considered simulation studies to be indirect evidence, and downgraded the evidence yielded by these studies by one level as well. In addition, when there was only one small study, we downgraded because of imprecision. All in all, the certainty of the evidence is low to very low for all comparisons. For the non‐randomised studies, there were no reason to upgrade the certainty of the evidence.
Discussion
Summary of main results
Almost all findings are based on one or at most two small simulation studies. Therefore, we judged the certainty of the evidence as very low or low.
One type of PPE compared to another
One study found less contamination when a PAPR with hood and coverall was used compared to a gown and a N95 mask but there were more errors in donning with the PAPR (Table 1).
Three studies compared different types of body protection. One study found that more protective gear protected slightly better but was more uncomfortable because of lack of breathability (Table 2). Another study found gowns to be better than aprons (Table 3). The third study did not provide data.
Three studies compared more recently proposed PPE ensembles according to different guidelines. One study found too few contamination events to draw conclusions. Another study found that long gowns protected better than a coverall or isolation gown and the coverall was difficult to doff (Table 4).
Modifications versus standard attire
Three studies compared changes to gowns especially related to improved doffing and changed glove‐gown interface and found considerably less contamination (Table 5; Table 6; Table 7). One study modified the inside of the gown and the closure system but found no difference in errors with donning or doffing or during performance.
Two studies evaluated the effect of tabs to improve ease of donning and found less contamination with tabs on masks or gloves (Table 8; Table 9).
One type of donning or doffing procedure compared to another
There are eight studies that compared donning and doffing procedures.
Following CDC recommendations for doffing gowns and aprons compared to individually chosen ways may decrease the risk of contamination (Table 10). Doffing of gloves and gown in one step may also decrease the risk of contamination (Table 11).
For doffing, there is very low‐certainty evidence that double‐gloving as part of full‐body PPE may reduce the risk of contamination and reduce the viral load on the hands without increasing the frequency of noncompliance with the doffing protocol (Table 12). Instructions during doffing may increase compliance (Table 13). Adding extra steps to the process in the form of glove disinfection may not be effective for alcohol‐based rub but may decrease viral and bacterial contamination when quaternary ammonium or bleach is used (Table 14). There is no difference in contamination between using alcohol‐based hand rub during doffing and using chlorine based disinfection (Table 15).
One type of training versus another
Three studies compared training models. There is very low‐certainty evidence from one SARS‐related study and two simulation studies that more active training in PPE use decreases noncompliance with donning and doffing guidance more than passive training. The active training used in the studies was video or computer simulation or face‐to‐face training compared to lectures (passive) only (Table 16).
We found no audit reports or other unpublished reports or data from our contact efforts to manufacturers and other organisations.
Overall completeness and applicability of evidence
Most studies provided sparse descriptions of the level of chemical protection (ISO 2013), or viral protection (EN 14126; ISO 2004a), of the PPE they used, or the outfits used varied so much in their components that it was impossible to make uniform comparisons.
For some PPE parts such as face shields and goggles, we found no studies that compared the two. There is, however, evidence from studies with viruses that do not have serious consequences and from simulation studies with manikins that each protects compared to no intervention (Agah 1987; Lindsley 2014). In a thorough overview of face shields for infection prevention, Roberge 2016 concludes that even though face shields can considerably reduce droplet contamination of the face, more research is needed into their efficacy. Other technical laboratory studies without involvement of humans also support the findings of this review. Kahveci 2019 found that double gloving can reduce contamination by reducing the fluid leakages through the glove‐gown interface.
Doffing procedures are fairly easy to evaluate in simulation studies. We found several studies that confirmed that it is important to follow procedures. However, all studies were small and only the comparisons about double‐gloving disinfection procedures and spoken instructions had more than one study. It seems that it would not be difficult to perform more and better simulation studies to find out how important these procedures are.
Because studies seem feasible and because we searched exhaustively, there must be other reasons why there is so little evidence available with infection rates as an outcome. One of these is probably the highly politicised context in which such a study has to be performed during an epidemic. However, retrospective cohort and case‐control studies are possible as has been shown during the SARS epidemic. The studies conducted after the SARS epidemic show that the consistent use of PPE rather than type of PPE was most important (see Appendix 1). At the start of the epidemic, SARS patients were not appropriately diagnosed, and the importance of PPE was not immediately clear. PPE compliance was higher in the later stages, and infections occurred less frequently (Nishiura 2005). SARS also affected comparatively higher‐income countries such as China, Hong Kong and Canada. The experiences from retrospective studies during Ebola epidemics are similar. During the 1995 Ebola epidemic in Kikwit in the Democratic Republic of the Congo, a study also reported that once PPE and other control measures were used, there were very few HCW infections (Kerstiens 1999). Dunn 2015 is a case study from the Ebola epidemic that also provided systematic information on the use of PPE and infection rates. We reanalysed the excluded study by Dunn 2015 as a cohort study of exposed HCW (Verbeek 2016a). The risk ratio of contracting Ebola infection for HCW using gloves only versus those not using PPE was 0.16 (95% CI 0.04 to 0.71) indicating that using gloves already provides a lot of protection. For using gloves or a gown or more compared to no PPE, the RR was 0.03 (95% CI 0.00 to 0.57; Verbeek 2016a). This is very similar to the findings of the SARS studies mentioned above. It is also, to a certain extent, reassuring for those situations during an epidemic or in low‐ and middle‐ income countries, when sufficient PPE is not available (see Levy 2015), that some PPE decreases the risk of infection considerably. In this version of the review we were able to include one retrospective cohort study from the 2015 West Africa Ebola epidemic. Unfortunately, the information on PPE was not detailed enough to be able to draw conclusions.
While the included studies show that more active training prevented errors, it is not clear how long the effects of training last. Northington 2007 showed that at six months after training, only 14% of participants were able to correctly don and doff PPE. It is unclear from the included studies, if fit‐testing of masks is part of training. This is a commonly accepted prerequisite for proper functioning of respiratory protection.
We included only one study conducted in a low‐ and middle‐income country. Since most serious haemorrhagic fever epidemics occur in some parts of Africa, this is a serious disadvantage of the current evidence. However, in such resource‐poor settings, appropriate research is the lowest priority for the local decision makers. Consequently, the initiative has to come from WHO and international organisations that work in these epidemics.
Quality of the evidence
We rated the certainty of the evidence as very low or low for all comparisons, mainly because our conclusions are based on single studies or two small studies and all the included studies had a high or unclear risk of bias. The retrospective cohort studies have a high risk of recall bias because participants had to recall their use of PPE after the epidemic occurred. The simulation studies had small sample sizes or very few events across compared groups.
One of the major problems is that most of the studies did not indicate if the PPE that they used complied with one or more of the international standards for protective clothing and whether they used whether they used protective clothing that is constructed with viral resistant fabrics and seams. The lack of attention to the designation of PPE as being protective for viruses is also problematic in practice.Also the lack of description of the PPE significantly reduces the ability make clear conclusions.
The many different labels and standards that are in use to designate protection make it almost impossible for a HCW in practice to make the right choice. For EVD, it was especially problematic because HCW needed the highest standard of protection. The confusing language of infection control has also been reported for isolation practices in general. This is why Landers 2010 called for the adoption of internationally accepted and standardised category terms for isolation precautions. Others have tried to improve the standardisation by providing HCW with a summary card of the various types of precautions that have to be taken and indicated that this increased the implementation of precautionary measures (Russell 2015).
In simulation studies, it is not clear how well the exposure represents real life exposure. Some studies used 'high volume exposure to simulate splash' (Bell 2015), whereas other studies only used a powdered fluorescent marker spread in the room (Beam 2011). It is also not clear how well the fluorescent marker can indicate that there is no viral contamination. Casanova 2008 showed that in spite of no fluorescent marker being detected, there could still be viral contamination with bacteriophage MS2. On the other hand, Osei‐Bonsu 2019 did not find a difference in effect with fluorescent marker as the outcome but did find a difference with bacterial exposure.
Only one of the case studies that we collected (Dunn 2015), properly described the use of PPE. Better description would enable better analysis.
Potential biases in the review process
We excluded all studies that evaluated only one piece of PPE, such as goggles or masks. However, none of these excluded studies would have answered the questions that in our current review remained unanswered. From Casanova 2012, it became clear that using double gloves as part of full‐body PPE is important, because it facilitates the removal of the other pieces of PPE without contaminating the hands. This shows that it is important to consider the effect of one piece of PPE as part of full‐body PPE. In addition, seldom is there only one clear transmission route. Even with SARS, which, as a respiratory infection, was spread by droplets and aerosols, consistent use of other pieces of PPE besides respiratory protection was still important to prevent contact transmission. Therefore, we think that our strict inclusion criteria did not bias the results of our review.
We assumed that adherence to PPE use and training would work in a similar way between SARS, EVD, and simulation studies. However, there is an important difference. At the start of the SARS epidemic, the causal virus and its transmission were unclear and workers were probably not instructed well enough to protect themselves. On the other hand, it has been known for years that EVD is a highly contagious disease with a very high fatality rate. Thus, compliance and effectiveness of training concerning EVD might be higher than we concluded from the SARS study. In the SARS studies that we excluded, there was high heterogeneity in the effects of consistently wearing PPE that we could not explain. The heterogeneity in effect is also underpinned by studies that did not find any SARS infections in spite of imperfect protection with PPE. This means that at best the effectiveness of PPE is not fully understood.
Twelve of the included simulation studies are cross‐over studies. But the authors of only four studies analysed the data with tests that took into account the paired nature of the data: Zamora 2006 used the Mailand‐Gart test; Guo 2014 used repeated measures; and Casanova 2012 and Strauch 2016 the paired t‐test but the methods used in Mana 2018 were unclear. We could not use the results of these tests in our analyses in Review Manager 2014, which resulted in wider confidence intervals than using a paired analysis. There were insufficient data in the studies to properly adjust for the cross‐over effect in our analyses. However, all results that were reported as being statistically significant were also statistically significant in our analyses. Therefore, we think that this has not biased our results.
With the simulation studies the way exposure was simulated is an important element to consider. This varied highly between the studies. However, most studies used a worst case scenario, spraying fluorescent marker over large parts of the body but some studies applied only small amounts. Hall 2018 used a sophisticated manikin with an internal mechanism simulating exposure as described by Poller 2018. Future studies urgently need consensus from experts in the field on how exposure can be best simulated. This is best possible under the auspices of WHO or other internationally recognised bodies.
With the included non‐randomised studies, we assessed risk of bias with a hybrid version of the Cochrane 'Risk of bias tool' (Higgins 2017) and the recently developed ROBINS‐I tool (Sterne 2016). This might not have been the optimal way to assess risk of bias. However, we believe that the limitations of the available studies are profound and a more rigorous 'Risk of bias' assessment could not have lowered (or improved) our confidence in the evidence any further.
Agreements and disagreements with other studies or reviews
We found two other reviews that have evaluated the effect of PPE for highly infectious diseases with serious consequences in HCW: Hersi 2015 and Fischer 2015. Hersi 2015 was commissioned by WHO to underpin the PPE guidelines issued for HCW exposed to EVD. The authors originally included only controlled studies of interventions to protect HCW against EVD and similar haemorrhagic fever infections with infection rates as outcomes. During the review process the authors decided to also include case studies and case series but they were not able to draw conclusions from these studies because the PPE use was not well described. Fischer 2015 took a more pragmatic but unsystematic approach and included all articles pertaining to filovirus transmission and PPE and in addition articles that evaluated donning and doffing strategies. They conclude that there is a lack of evidence but that simulation studies could provide evidence for guidelines.
Heat stress and breathability is an important issue in PPE especially for Ebola. Kuklane 2015 argued that using other materials would substantially reduce the heat stress but these come at a tenfold higher price. Other researchers that have looked into this problem have found inconsistent results. Coca 2015 found that PPE on manikins led to a critical body core temperature of 38.4ºC in one hour. On the other hand, Grélot 2015 found that HCW caring for Ebola patients had only a 0.46ºC rise in core body temperature after being at work for one hour. Of the 25 workers studied, only four reached a core body temperature over 38.5ºC.
An independent panel of experts that evaluated the Ebola response concluded, among many other things, that a coordinated research effort is needed to build a better global system for infectious disease outbreak and response (Moon 2015). Their recommendation is that research funders should establish a worldwide research and development financing facility for outbreak‐relevant drugs, vaccines, diagnostics, and non‐pharmaceutical supplies (such as PPE). This is very much in line with what we experienced and found in this review.
Missair 2014 reviewed implications of EVD patient management for anaesthetists based on a literature review of all types of studies on EVD. This is why their inclusion criteria were very broad and non‐specific. Finally the authors relied on PPE guidelines as provided by WHO and MSF to make recommendations with no evidence of their comparability. This makes their results difficult to compare to ours.
Moore 2005 reviewed all measures to prevent healthcare workers from SARS and other respiratory pathogens in a narrative format, from 168 publications. They concluded that a positive safety climate is the most important factor for adherence to universal precautions. They recommend using adequate PPE, but they do not define 'adequate'. Their inclusion criteria were much broader than ours and their results are difficult to compare with ours. The same research group formulated valuable advice about research gaps based on this review but focused only on respiratory protection (Yassi 2005). They corroborate the findings of Jefferson 2011, that N95 respirators may not be superior, citing the early containment of the SARS epidemic without these in Hanoi.
The Cochrane Review by Jefferson 2008, updated in Jefferson 2011, evaluated the effect of physical interventions to interrupt the spread of respiratory viruses for all patient and staff populations. Even though they only included studies on respiratory infections and any type of protection for any person at risk, 10 studies in their review are about SARS and protecting HCW. The authors did not conduct a subgroup or additional analysis of these HCW studies because the infection risk for HCW is substantially different from the populations they protect. The Jefferson 2011 results are not applicable to HCW.
Authors' conclusions
Implications for practice.
In addition to other infection control measures, consistent use of full‐body personal protective equipment (PPE) can diminish the risk of infection for healthcare workers (HCW). EN (European) and ISO (international) standards for protective clothing and fabric permeability for viruses are helpful to determine which PPE should technically protect sufficiently against highly infectious diseases. However, the risk of contamination depends on more than just these technical factors. In simulation studies, contamination happened in almost all intervention and control arms.
For choosing between PPE types, there is very low‐certainty evidence, based on single‐exposure simulation studies. Covering more parts of the body leads to better protection but usually comes at the cost of more difficult donning (putting on) or doffing (taking off) and user comfort, and may therefore even lead to more contamination. A powered, air‐purifying respirator (PAPR) with a hood may protect better than an N95 mask with a gown but is more difficult to don. A long gown may be the best compromise between protection and ease of doffing. Coveralls may be more difficult to doff. A more breathable fabric may still lead to similar levels of contamination protection to less breathable fabric, and may be preferred by users.
For changes to PPE, there is low‐ to very low‐certainty evidence that adding tabs to gloves or masks or closer fit of gowns at the neck or the wrist may decrease contamination, even though one study could not show a decrease in donning or doffing errors.
For different procedures of donning and doffing, there is very low‐certainty evidence that double gloves, as part of PPE and following Centers for Disease Control and Prevention (CDC) guidelines, and providing users with help or spoken instructions during donning and doffing may reduce the risk of contamination. Extra disinfection of gloves with bleach or quaternary ammonium may decrease hand contamination but not alcohol‐based hand rub.
For various training procedures there is very low‐certainty evidence that more active training (including video or computer simulation or spoken instructions) may increase compliance with instructions compared to passive training (lectures or no added instructions). No studies compared methods to retain PPE skills needed for proper donning and doffing in the long term.
The certainty of the evidence is low to very low for all comparisons because conclusions are based on one or two small studies and a high or unclear risk of bias in studies, indirectness of evidence, and small numbers of participants. This means that we are uncertain about the estimates of effects and it is therefore possible that the true effects may be substantially different from the ones reported in this review.
Implications for research.
We concur with the World Health Organization (WHO) that there is a need to carry out a re‐evaluation of how PPE is standardised, designed, and tested (WHO 2018). What is missing is a harmonised set of PPE standards and a unified design for PPE to be used when taking care of patients with highly infectious diseases. This holds for PPE as used for preventing contact transmission as well as other ways of transmission. There is, for example, no unified technical standard for isolation gowns. There is also a need for a more transparent and uniform labelling of infection control measures, such as droplet precautions, and the protection level of PPE for HCW. We believe that this is an important prerequisite for the universal implementation of infection control measures for HCW.
Simulation studies are a feasible and relatively simple way to compare different types of PPE and to find out which protects best against contamination. It is a prerequisite for a reliable answer that methods of simulation studies are standardised in terms of exposure and outcome measurement. We recommend developing a core outcome set (COS) in this field that would provide critical outcomes measures to enable better comparisons and synthesis across trials. Viral marker bacteriophage MS2 seems to be the most sensitive marker and we would advocate using this. Studies should have sufficient power. A sample size of 62 would be needed to be able to detect a relatively large risk ratio of 0.5 with a large control group rate of contamination of 0.7, assuming α = 0.05 and β = 0.80. In addition, it would help evidence synthesis if study authors would better adhere to the appropriate reporting guidelines (Cheng 2016).
To find out how PPE behaves under real exposure, we need prospective follow‐up of HCW involved in the treatment of patients with highly infectious diseases, with careful registration of PPE, donning and doffing and risk of infection. Here, the effect sizes would be smaller and thus the sample size should be bigger than 60.
In addition, case‐control studies comparing PPE use among infected HCW and matched healthy controls, using rigorous collection of exposure data, can provide information about the effects of PPE on the risk of infection. The sample sizes should be much bigger than the current case studies because we would like to detect small but important differences in effect between various combinations of PPE such as gowns versus coveralls. There is a need for collaboration between organisations serving epidemic areas to carry out this important research in circumstances with limited resources, and during the throes of an outbreak.
We also need more randomised controlled studies of the effects of one type of training versus another, to find out which training works best, especially at long‐term follow‐up of one year or more. Here also, the effect size seems to be quite large and thus a sample size of around 60 seems to provide adequate power.
Feedback
Unified design for PPE, September 2019
Summary
We noted the timely and welcome update of the above review by Dr Verbeek and his team. As stated in the introduction, in epidemics of highly infectious diseases such as Ebola Virus Disease (EVD), healthcare workers (HCW) are at much greater risk of infection than the general population. Sadly the review comes at a time when this once again is being proved, with recent (20th July, 2019) data from the Democratic Republic of Congo (DRC) recording that since the beginning of the epidemic, the cumulative number of cases has been 2564 (2470 confirmed and 94 probable) with 1728 deaths (1634 confirmed and 94 probable cases). Of those, the cumulative number of confirmed/ probable cases among health workers is 138 (5% of all confirmed/probable cases) including 41 deaths (1). This comes shortly after the World Health Organization declared EVD in DRC a Public Health Emergency of International Concern (2). With the United Nations also recognising the seriousness of the emergency, by activating the Humanitarian System‐wide Scale‐Up to support the EVD response, this increases the possibility of HCW from around the globe being called upon to provide practical support in country, or travellers to affected countries returning with infection, and with it the need for personal protection from exposure to patients’ contaminated body fluids.
We were pleased that data from our recent research (3) was included in the review. In our study, we compared five PPE ensembles used in different high consequence infectious disease (HCID) units around the UK for examination of a ‘suspected case’, using a medical training manikin to expose HCW wearing the PPE to four different body fluid simulants, each tagged with different colour fluorochromes, and UV light to visualise any cross‐contamination during dry doffing. We note and accept the conclusions of the Review that “what is missing is a harmonised set of PPE standards and a unified design for PPE to be used when taking care of patients with highly infectious diseases”, also that the quality of the evidence was low because conclusions were based on single studies or on small numbers of participants.
While resources did not allow us to address the ‘small numbers’ issue, we have addressed the ‘unified design for PPE’ in a paper which was published after the Review literature search cut‐off date. In this follow‐up work, we presented the outcome of the initial research to the HCID units and reached a consensus on a unified PPE ensemble for examination of a suspected HCID case. Again, using HCW volunteers, we tested the unified PPE ensemble with fluorochromes as before, the result being no cross contamination events from 20 volunteers (4). In subsequent HCW training for one HCID unit, a further 40 challenges using 35 volunteers tested the PPE ensemble with only one cross contamination event through a known deviation from the doffing protocol (unpublished data).Therefore, there were 60 challenges with 54 volunteers with one breach. Public Health England plan in the near future to make written and video guidance available to demonstrate safe use of this unified PPE ensemble, and similar guidance is already available through Health Protection Scotland (5).
While more is needed, we believe this adds to the body of evidence required to ensure HCW can conduct the important business of patient care with confidence that they will be protected from potential infection.
Brian Crook(a), Anne Tunbridge(b), Bozena Poller(b), Samantha Hall(a), Cariad Evans(c) on behalf of the High Consequence Infectious Diseases Project Working Group UK
(a) Health and Safety Executive, Harpur Hill, Buxton SK17 9JN UK, (b) Sheffield Teaching Hospitals NHS Foundation Trust, Department of Infectious Diseases, Royal Hallamshire Hospital, Glossop Road, Sheffield S10 2JF, UK, (c) Sheffield Teaching Hospitals NHS Foundation Trust, Department of Virology, Northern General Hospital, Herries Road, Sheffield, S5 7AU, UK
References
1. DRC Ministry of Health. Epidemiological situation report, Fri 20 Jul 2019. Available at: https://mailchi.mp/sante.gouv.cd/ebola_kivu_20juil19?e=77c16511ad
2. WHO news release 17th July, 2019. Available at: https://www.who.int/news-room/detail/17-07-2019-ebola-outbreak-in-the-democratic-republic-of-the-congo-declared-a-public-health-emergency-of-international-concern
3. Hall S, Poller B, Bailey C, Gregory S, Clark R, Roberts P, Tunbridge A, Poran V, Evans C, Crook B. Use of ultraviolet‐fluorescence‐based simulation in evaluation of personal protective equipment worn for first assessment and care of a patient with suspected high‐consequence infectious disease. J Hosp Infect 2018;99: 218‐228
4. Poller B, Tunbridge A, Hall S, Beadsworth M, Jacobs M, Peters E, Schmid ML, Sykes A, Poran V, Gent N, Evans C, Crook B on behalf of the High Consequence Infectious Diseases Project Working Group. A unified personal protective equipment ensemble for clinical response to possible high consequence infectious diseases: A consensus document on behalf of Public Health England and the Health and Safety Executive. Journal of Infection 2018;77:496–502
5. NHS Education for Scotland. Viral Haemorrhagic Fever‐ The correct order for donning and the safe order for removal and disposal of Personal Protection Equipment. Available at:https://www.nes.scot.nhs.uk/education-and-training/by-theme-initiative/public-health/health-protection/travel-and-international-health/viral-haemorrhagic-fever.aspx
Reply
Thank you for the comments and for supporting the conclusions of our review. It is great to see that the use of PPE for highly infectious diseases is becoming standardised in the UK. Compared to the current diversity in outfits this is certainly an improvement.
We believe that controlled studies form the best evidence in showing the protective capabilities of PPE against highly infectious diseases. We have no doubts that PPE helps in preventing infection. The question remains what the best possible PPE is. Given that infections still occur among health care workers and that users are not very satisfied with the PPE ensembles currently in use, improvement is still possible. Therefore, we included only controlled studies that compared newly designed PPE with existing PPE. The 20 test of one type of PPE by 17 volunteers in the Poller 2018 study were an uncontrolled experiment. Unfortunately, the paper did not provide data on the test of volunteers but only reported that there were no contaminations. Without knowing further details of this study, for example how many times the volunteers tested the new PPE ensemble, it is difficult to judge the significance of this result.
We also noticed that the agreed PPE ensemble currently does not include tags on gloves and masks or a sealed gown‐glove combination. These are both aspects that are supported by some evidence in our updated Cochrane review, meaning that these may prevent contamination more than conventional PPE. Therefore, we think that the agreed PPE ensemble could still be improved. We also hope that the newly agreed ensemble, and any further improvements upon it, will be tested against the currently used ones in a sufficiently large randomised experiment of simulated exposure.
Contributors
Jos H Verbeek, Blair Rajamaki, Sharea Ijaz, Christina Tikka, Jani H Ruotsalainen, Riitta Sauni
Certainty of the evidence, November 2019
Summary
This is a large scale and important review. On the next update, the review may benefit from up‐to‐date application of GRADE. Authors should use the term 'certainty' rather than 'quality' of evidence. At present, the term 'certainty' features in GRADE tables, but 'quality' throughout the text. Although the authors GRADE all comparisons as very low certainty, in the abstract the authors present findings using the term 'may', for example: "may protect better". The accepted plain language for very low certainty evidence is 'we do not know', and the review may therefore over‐represent the certainty of evidence. The authors should consider how best to ensure the very low certainty of evidence is adequately reflected for each result presented.
Paul Hine, Honorary research fellow, Cochrane Infectious Diseases Group
Reply
Thank you very much for your comments on our review and pointing out the inconsistency in using quality and certainty of the evidence. We will repair this throughout the review with the next update.
We don't think that the phrase 'may improve' instead of ‘we don't know’ over‐represents the certainty of the evidence. At the beginning of the abstract we state: 'Evidence for all outcomes is based on single studies and is very low quality'. Recent GRADE guidance says that very low certainty evidence can be reported as 'may improve but the evidence is very uncertain'.1 This is also the guidance in the latest version of the Cochrane Handbook (Table 15.6.b). We will add the additional "but the evidence is very uncertain" to the phrase 'may improve' in the review update in line with the most recent GRADE guidance.
1Santesso N, Glenton C, Dahm P, Garner P, Akl E, Alper B, Brignardello‐Petersen R, Carrasco‐Labra A, De Beer H, Hultcrantz M, Kuijpers T, Meerpohl J, Morgan R, Mustafa R, Skoetz N, Sultan S, Wiysonge C, Guyatt G, Schünemann HJ, for the GRADE Working Group, GRADE guidelines 26: Informative statements to communicate the findings of systematic reviews of interventions, Journal of Clinical Epidemiology (2019)
Contributors
Jos H Verbeek, Blair Rajamaki, Sharea Ijaz, Christina Tikka, Jani H Ruotsalainen, Riitta Sauni
Comparisons, April 2020
Summary
The comparison of PAPR and gown, vs N95 and gown is a poor comparison. And should not be used as a fair comparison and will likely give HCW the wrong impression. PAPR/CAPR and gown vs N95 and face shield with gown should be the equal comparison arms. Please consider this as this will give people the wrong messaging as we know face shields are an important component to PPE and we should do a real comparison as to determine whether one protection is better than another.
Michael Bolaris
Reply
Thank you for comments. We were already quite strict with including only studies that intended to evaluate full‐body protection. We don't have control over the interventions and controls that have been evaluated in studies. This specific combination of intervention and control was evaluated in one trial as a result of the problems encountered with aerosol generating procedures during the SARS epidemic. We think that the results are still very useful for the current COVID‐19 pandemic. The difference in contamination with the two types of PPE was especially caused by contamination of the neck in the PPE that consisted of mask and gown but where the gown did not cover the neck. We doubt that a face‐shield would have made much difference here because it does not protect the neck. The current WHO COVIID‐19 guidelines suggest the use of a gown with face mask and goggles as minimum PPE. This PPE ensemble leaves a part of the body uncovered and the trial mentioned above shows that this can easily lead to contamination
Contributors
Jos H Verbeek, Blair Rajamaki, Sharea Ijaz, Riitta Sauni, Elaine Toomey, Bronagh Blackwood, Christina Tikka, Jani H Ruotsalainen, F Selcen Kilinc Balci
What's new
| Date | Event | Description |
|---|---|---|
| 24 April 2020 | Feedback has been incorporated | Feedback about comparisons and author response incorporated |
History
Protocol first published: Issue 4, 2015 Review first published: Issue 4, 2016
| Date | Event | Description |
|---|---|---|
| 30 March 2020 | New citation required and conclusions have changed | Seven new studies incorporated, revised to incorporate COVID‐19 developments, conclusions changed. |
| 30 March 2020 | New search has been performed | All searches updated, new studies incorporated, categorisation of interventions slightly changed, background revised to incorporate COVID‐19 developments, conclusions changed |
| 5 December 2019 | Feedback has been incorporated | Feedback about the certainty of the evidence and authors' response incorporated |
| 13 September 2019 | Amended | Feedback updated with more information from latest tests |
| 9 September 2019 | Feedback has been incorporated | Feedback and authors' response added |
| 22 July 2019 | Amended | In summary of findings tables we corrected the number of plusses for the quality of the evidence to match the very low quality evidence |
| 20 June 2019 | New citation required and conclusions have changed | We included eight new studies of which one is a field study and seven are simulation studies. This extended the evidence to other types of PPE. |
| 18 June 2019 | New search has been performed | Updated the databases:PubMed up to 15 July 2018, CENTRAL up to 18 June 2019, Scopus 18 June 2019, CINAHL 31 July 2018 and OSH‐Update up to 31 December 2018 |
Notes
Disclaimer. The findings and conclusions in this Cochrane systematic review are those of the authors and do not necessarily represent the official position of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention. Mention of product names does not imply endorsement
Acknowledgements
We thank the Cochrane Editorial Unit for providing financial support to undertake this review. For this 2020 update, we thank Ruth Foxlee for updating the searches, Bozena Poller and Nick Gent for peer referee comments, Euan S Shearer for consumer comments, Toby Lasserson, Liz Bickerdike, Anne‐Marie Sephani, Robin Featherstone, and Helen Wakeford for editorial input, and Denise Mitchell for copy‐editing the review.
We thank Kaisa Neuvonen, Erja Mäkelä and Raluca Mihalache and Michael Edmond for their contributions as authors to the previous version of this review (Verbeek 2016b). We thank Toby Lasserson, Hannah Ryan, Darrel Singh, Fiona Smaill, Mauriccio Ferri, Annalee Yassi, Nuala Livingstone and Julian Higgins for their comments on the text for the first publication of this review (Verbeek 2016b). We extend a special thankyou to Consol Serra for her editorial work. We thank Claire Allen from Evidence Aid for her help in trying to locate unpublished reports. We thank Alexey Pristupa for assessing the studies written in Russian.
Sharea Ijaz's time for this update was supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care West (CLAHRC West) at University Hospitals Bristol NHS Foundation Trust.
Appendices
Appendix 1. Effects of wearing personal protective equipment (PPE) consistently on the risk of SARS infection
Wearing PPE consistently versus wearing PPE inconsistently
During and just after the SARS epidemic a number of studies evaluated the impact of the use of PPE on SARS infection rates. Six of these studies were case‐control studies and five were retrospective cohort studies. Since information in these studies was collected in the same retrospective way by questionnaires and/or interviews we combined the results of these studies.
There were two studies (Le 2004; Park 2004), one in a single hospital in Vietnam and the other in multiple hospitals in the USA, that reported no cases in spite of sufficient exposure to SARS patients. The Vietnamese study claimed that this was because of the almost universal use of N95 masks later during the epidemic. The US study could not find an explanation because the use of PPE was not optimal in many cases. We could find no reasons to explain this result because these studies were similar to the other studies included. Also, in another hospital near the one in the Vietnamese study, SARS cases did occur among healthcare workers but this was more at the beginning of the epidemic and it was unclear how well PPE had been used (Reynolds 2006).
1 Consistent mask use versus inconsistent use
We were able to combine six studies (Liu 2009; Loeb 2004; Nishiura 2005; Scales 2003; Seto 2003; Teleman 2004), in a meta‐analysis that showed a beneficial effect of consistent mask use as part of PPE both in a fixed‐effect (OR 0.28, 95% CI 0.17 to 0.46, I² = 42%) and in a random‐effects meta‐analysis model (OR 0.27, 95% CI 0.13 to 0.53).
2 Consistent gown/suit use versus inconsistent use
Four studies (Loeb 2004; Nishiura 2005; Pei 2006; Teleman 2004), could be combined and showed that consistent gown use had a preventive effect on SARS infection both in a fixed‐ and random‐effects analysis (OR 0.22, 95% 0.10 to 0.50, I² = 53%). The data in Teleman 2004 were reported as OR 0.5, 95% CI 0.4 to 6.9 P = 0.6). However, this is an apparent mistake as the confidence interval does not fit with the OR nor with the P value. We corrected this to OR 0.5, 95% CI 0.04 to 6.9 which makes the results consistent.
3 Consistent glove use versus inconsistent use
Also consistent glove use in six studies (Loeb 2004; Nishiura 2005; Pei 2006; Scales 2003; Seto 2003; Teleman 2004), led to a decrease in the risk of SARS infection both in fixed‐effect meta‐analysis (OR 0.54 95% CI 0.33 to 0.89, I² = 0%) and in a random‐effects analysis (OR 0.53, 95% CI 0.28 to 1.01) but this was not statistically significant.
4 Consistent use of more than one PPE part versus inconsistent use
Ho 2004, Lau 2004, and Scales 2003 measured consistent use of more than one PPE part compared to no use at all. The combination of more than one PPE had a similar effect on SARS infection risk but this was not statistically significant, neither in the fixed‐effect analysis (OR 0.36, 95% CI 0.09 to 1.39, I² = 35%) nor in the random‐effects analysis (OR 0.37, 95% CI 0.07 to 1.98).
Appendix 2. MEDLINE search strategy 15 July 2019
#1
"Protective Clothing"[Mesh] OR gown*[tw] OR coverall*[tw] OR "protective layer"[tw] OR "protective layers"[tw] OR "surgical toga"[tw] OR apron*[tw] OR "smock"[tw] OR "smocks"[tw] OR "hazmat suit"[tw] OR (hazmat[tw] AND suit[tw]) OR "Gloves, Protective"[Mesh] OR "glove"[tw] OR "gloves"[tw] OR "Respiratory Protective Devices"[Mesh] OR "Masks"[Mesh] OR "mask"[tw] OR "masks"[tw] OR "air‐purifying respirator"[tw] OR "PAPR"[tw] OR "enhanced respiratory and contact precautions" OR "E‐RCP"[tw] OR "respiratory protection"[tw] OR "transparent panel"[tw] OR "surgical mask"[tw] OR "surgical masks"[tw] OR "filtering face piece"[tw] OR "filtering facepiece"[tw] OR "Eye Protective Devices"[Mesh] OR goggle*[tw] OR "visor"[tw] OR "facial protection equipment"[tw] OR "safety glass"[tw] OR "safety glasses"[tw] OR "safety spectacles"[tw] OR "personal protective equipment"[tw] OR "PPE"[tw] OR "protective equipment"[tw] OR overshoe*[tw] OR "shoe cover"[tw] OR "shoe covers"[tw] OR "rubber boot"[tw] OR "rubber boots"[tw] OR "head cover"[tw] OR "head covering"[tw] OR "face shield"[tw] OR "face shields"[tw] OR "surgical hood"[tw] OR "hood"[tw] OR "Equipment Contamination/prevention and control"[Mesh] OR "Infection Control"[Mesh] OR "infection control"[tiab] OR "gloving"[tw] OR "donning"[tw] OR "doffing"[tw]
#2
"Communicable Diseases"[Mesh] OR "infectious disease"[tiab] OR "infectious diseases"[tiab] OR "Disease Transmission, Infectious"[Mesh] OR "disease transmission"[tw] OR "Infectious Disease Transmission, Patient‐to‐Professional"[Mesh] OR "infection control precautions"[tw] OR "human‐to‐human transmission"[tw] OR "parenteral transmission"[tw] OR "Virus Diseases/prevention and control"[Mesh] OR "viral disease"[tw] OR "viral diseases"[tw] OR "Bacterial Infections/prevention and control"[Mesh] OR "bacterial infection"[tw] OR "filovirus"[tw] OR "Ebolavirus"[Mesh] OR "Hemorrhagic Fever, Ebola"[Mesh] OR "Ebola"[tw] OR "Marburg virus"[tw] OR "Lassa virus"[tw] OR "haemorrhagic fever"[tw] OR "HIV Infections/prevention and control"[Mesh] OR "HIV"[ti] OR "hiv infection"[tiab] OR "hiv transmission"[tw] OR "Influenza, Human/prevention and control"[Mesh] OR "SARS Virus"[Mesh] OR "Severe Acute Respiratory Syndrome Virus"[tw] OR "SARS"[tw] OR "MERS"[tw] OR "respiratory infection"[tw] OR "Influenza, Human/prevention and control"[Mesh] OR "influenza"[tiab] OR "Tuberculosis/prevention and control"[Mesh] OR "tuberculosis"[tiab] OR "Hepatitis A"[Mesh] OR "hepatitis a"[ti] OR "Hepatitis B/prevention and control"[Mesh] OR "hepatitis b"[ti] OR "Hepatitis C/transmission"[Mesh] OR "hepatitis c"[ti] OR "bioterrorism"[tw] OR "aerosol‐generating procedure"[tw] OR "Cross Infection"[Mesh] OR "bacterial contamination"[tw] OR "microbial contamination"[tw] OR "self‐contamination"[tw] OR "decontamination"[tw] OR "surface decontamination"[tw] OR "skin decontamination"[tw]
#3
"Health Personnel"[Mesh] OR "Personnel, Hospital"[Mesh] OR "health care worker"[tw] OR "health care workers"[tw] OR "health care personnel"[tw] OR "health personnel"[tw] OR "health‐personnel"[tw] OR "health provider"[tw] OR "health providers"[tw] OR "health care provider"[tw] OR "health care providers"[tw] OR "medical staff"[tw] OR "medical personnel"[tw]OR "medical professional"[tw] OR "medical worker"[tw] OR "medical workers"[tw] OR "dental personnel"[tw] OR "dental staff"[tw] OR "Dentists"[Mesh] OR "dentist"[tw] OR "dentists"[tw] OR "dental assistant"[tw] OR "dental assistants"[tw] OR "Dental Assistants"[Mesh] OR "nursing staff"[tw] OR "Nurses"[Mesh] OR "nurse"[tw] OR "nurses"[tw] OR "nursing assistant"[tw] OR "nursing assistants"[tw] OR "Nurses' Aides"[Mesh] OR "Nurse Midwives"[Mesh] OR "midwife"[tw] OR "midwives"[tw] OR "military‐medical personnel"[tw] OR "Physicians"[Mesh] OR "physician"[tw] OR "physicians"[tw] OR "emergency medical services"[tw] OR "Emergency Medical Services"[MeSH] OR "transporting patients"[tw] OR "patient transport"[tw] OR "Ambulances"[Mesh] OR "Allied Health Personnel"[Mesh] OR paramedic[tw] OR paramedics[tw] OR paramedical personnel[tw] OR "Burial"[Mesh] OR burial staff OR cleaning workers[tw] OR cleaner work OR cleaner[tw] OR cleaners[tw]
#4
(#1 AND #2 AND #3)
Appendix 3. CENTRAL search strategy 20 March 2020
#1 MeSH descriptor: [Personal Protective Equipment] explode all trees
#2 MeSH descriptor: [Protective Clothing] explode all trees
#3 MeSH descriptor: [Respiratory Protective Devices] explode all trees
#4 MeSH descriptor: [Masks] explode all trees
#5 MeSH descriptor: [Eye Protective Devices] explode all trees
#6 MeSH descriptor: [Equipment Contamination] explode all trees
#7 MeSH descriptor: [Infection Control] explode all trees and with qualifier(s): [methods ‐ MT]
#8 (glove* or gloving):ti,ab,kw
#9 (gown* or coverall* or (protective NEXT layer*) or (surgical NEXT toga*) or apron* or smock* or (hazmat NEXT suit*)):ti,ab,kw
#10 (mask* or (air NEXT purifying NEXT respirator*) or PAPR or "enhanced respiratory and contact precautions" or ERCP or "respiratory protection" or (transparent NEXT panel*) or (filtering NEXT face NEXT piece*) or (filtering NEXT facepiece*)):ti,ab,kw
#11 (goggle* or visor* or (safety NEXT glass*) or "safety spectacles" or overshoe* or (shoe NEXT cover*) or (rubber NEXT boot*) or (head NEXT cover*) or (face NEXT shield*) or hood* or "protective equipment" or PPE or donning or doffing):ti,ab,kw
#12 "infection control":ti,ab,kw
#13 {or #1‐#12}
#14 MeSH descriptor: [Health Personnel] explode all trees
#15 MeSH descriptor: [Personnel, Hospital] explode all trees
#16 ((health NEXT care NEXT worker*) or (healthcare NEXT worker*) or "health care personnel" or "healthcare personnel" or "health personnel" or (health NEXT provider*) or (health NEXT care NEXT provider*) or "medical staff" or "medical personnel" or (medical NEXT professional*) or (medical NEXT worker*) or "military‐medical personnel" or "military medical personnel"):ti,ab,kw
#17 MeSH descriptor: [Dentists] explode all trees
#18 MeSH descriptor: [Dental Assistants] explode all trees
#19 ("dental personnel" or "dental staff" or dentist* or (dental NEXT assistant*)):ti,ab,kw
#20 MeSH descriptor: [Nurses] explode all trees
#21 MeSH descriptor: [Nursing Assistants] explode all trees
#22 MeSH descriptor: [Nurse Midwives] explode all trees
#23 MeSH descriptor: [Nursing Staff] explode all trees
#24 (nurse or nurses or nursing or midwife OR midwives):ti,ab,kw
#25 MeSH descriptor: [Physicians] explode all trees
#26 physician*:ti,ab,kw
#27 MeSH descriptor: [Emergency Medical Services] explode all trees
#28 MeSH descriptor: [Ambulances] explode all trees
#29 ("emergency medical services" or "transporting patients" or "patient transport" or paramedic* or (ambulance NEXT worker*)):ti,ab,kw
#30 MeSH descriptor: [Allied Health Personnel] explode all trees
#31 MeSH descriptor: [Burial] explode all trees
#32 "burial staff":ti,ab,kw
#33 ("cleaning workers" or cleaner* or janitor*):ti,ab,kw
#34 {or #14‐#33}
#35 MeSH descriptor: [Communicable Diseases] explode all trees
#36 MeSH descriptor: [Disease Transmission, Infectious] explode all trees
#37 MeSH descriptor: [Virus Diseases] explode all trees and with qualifier(s): [prevention & control ‐ PC, transmission ‐ TM]
#38 MeSH descriptor: [Bacterial Infections] explode all trees and with qualifier(s): [prevention & control ‐ PC, transmission ‐ TM]
#39 MeSH descriptor: [Ebolavirus] explode all trees
#40 MeSH descriptor: [Hemorrhagic Fever, Ebola] explode all trees
#41 MeSH descriptor: [Marburg Virus Disease] explode all trees
#42 MeSH descriptor: [Lassa virus] explode all trees
#43 MeSH descriptor: [Influenza, Human] explode all trees and with qualifier(s): [prevention & control ‐ PC, transmission ‐ TM]
#44 MeSH descriptor: [SARS Virus] explode all trees
#45 MeSH descriptor: [Severe Acute Respiratory Syndrome] explode all trees
#46 MeSH descriptor: [Middle East Respiratory Syndrome Coronavirus] explode all trees
#47 MeSH descriptor: [HIV Infections] explode all trees and with qualifier(s): [prevention & control ‐ PC, transmission ‐ TM]
#48 MeSH descriptor: [Tuberculosis] explode all trees and with qualifier(s): [prevention & control ‐ PC]
#49 MeSH descriptor: [Hepatitis A] explode all trees and with qualifier(s): [prevention & control ‐ PC, transmission ‐ TM]
#50 MeSH descriptor: [Hepatitis B] explode all trees and with qualifier(s): [prevention & control ‐ PC, transmission ‐ TM]
#51 MeSH descriptor: [Cross Infection] explode all trees
#52 ((infectious NEXT disease*) or "disease transmission" or "infection control precautions" or "human‐to‐human transmission" or "human transmission" or "parenteral transmission"):ti,ab,kw
#53 ((viral NEXT disease*) or (bacterial NEXT infection*) or filovirus or ebola or "Marburg virus" or "Lassa virus" or "haemorrhagic fever" or "hemorrhagic fever" or (HIV NEAR/3 infection*) or "Severe Acute Respiratory Syndrome Virus" or SARS or "Middle East Respiratory Syndrome" or MERS or coronavirus* or (corona NEXT virus*) or COVID or "COVID 19" or “severe acute respiratory syndrome coronavirus 2” or "SARS CoV 2" or (SARS NEXT CoV*)):ti,ab,kw
#54 ("surface decontamination" or "skin decontamination" or "self contamination" or self‐contamination):ti,ab,kw
#55 {or #35‐#54}
Appendix 4. Medline OVID search strategy 20 March 2020
1 exp Personal Protective Equipment/
2 exp Protective Clothing/
3 exp Respiratory Protective Devices/
4 exp Masks/
5 exp Eye Protective Devices/
6 exp Equipment Contamination/
7 exp Infection Control/mt [Methods]
8 (glove* or gloving).ti,ab.
9 (gown* or coverall* or protective layer* or surgical toga* or apron* or smock* or hazmat suit*).ti,ab.
10 (mask or masks or air purifying respirator* or PAPR or "enhanced respiratory and contact precautions" or ERCP or "respiratory protection" or transparent panel* or filtering face piece* or filtering facepiece*).ti,ab.
11 (goggle* or visor* or facial protection equipment or safety glass* or safety spectacles or overshoe* or shoe cover* or rubber boot* or head cover* or face shield* or hood* or protective equipment or PPE or donning or doffing).ti,ab.
12 infection control.ti.
13 or/1‐12
14 exp Health Personnel/
15 exp Personnel, Hospital/
16 (health care worker* or healthcare worker* or health care personnel or health personnel or health care provider* or health provider* or medical staff or medical personnel or medical professional* or medical worker* or military medical personnel).ti,ab.
17 exp Dentists/
18 exp Dental Assistants/
19 (dental personnel or dental staff or dentist* or dental assistant*).ti,ab.
20 exp Nurses/
21 exp Nursing Assistants/
22 exp Nurse Midwives/
23 exp Nursing Staff/
24 (nurse or nurses or nursing or midwife or midwives).ti,ab.
25 exp Physicians/
26 physician*.ti,ab.
27 exp Emergency Medical Services/
28 exp Ambulances/
29 (emergency medical services or transporting patients or patient transport or paramedic* or ambulance worker*).ti,ab.
30 exp Allied Health Personnel/
31 exp Burial/
32 burial staff.ti,ab.
33 (cleaning worker* or cleaner* or janitor*).ti,ab.
34 or/14‐33
35 exp Communicable Diseases/
36 exp Disease Transmission, Infectious/
37 exp Virus Diseases/
38 exp Bacterial Infections/
39 exp Ebolavirus/
40 exp Hemorrhagic Fever, Ebola/
41 exp Marburg Virus Disease/
42 exp Lassa virus/
43 exp Influenza, Human/
44 exp SARS Virus/
45 exp Severe Acute Respiratory Syndrome/
46 exp Middle East Respiratory Syndrome Coronavirus/
47 exp HIV Infections/pc, tm [Prevention & Control, Transmission]
48 exp Tuberculosis/pc, tm [Prevention & Control, Transmission]
49 exp Hepatitis A/pc, tm [Prevention & Control, Transmission]
50 exp Hepatitis B/pc, tm [Prevention & Control, Transmission]
51 exp Cross Infection/
52 (infectious disease* or disease transmission or infection control precautions or (human* adj3 transmission) or parenteral transmission).ti,ab.
53 (viral disease* or viral infection* or bacterial infection* or filovirus or ebola* or Marburg virus or Lassa virus or h?emorrhagic fever or (HIV adj3 infection*) or Severe Acute Respiratory Syndrome Virus or SARS or Middle East Respiratory Syndrome or MERS or coronavirus* or corona virus* or COVID or severe acute respiratory syndrome coronavirus or SARS CoV 2 or SARS‐CoV‐2).ti,ab.
54 (skin decontamination or surface decontamination or self contamination).ti,ab.
55 or/35‐54
56 13 and 34 and 55
57 exp animals/ not humans.sh.
Appendix 5. Embase OVID search strategy 20 March 2020
1 exp protective equipment/
2 exp protective clothing/
3 exp mask/
4 exp eye protective device/
5 exp medical device contamination/
6 infection control/pc [Prevention]
7 (glove* or gloving).ti,ab.
8 (gown* or coverall* or protective layer* or surgical toga* or apron* or smock* or hazmat suit*).ti,ab.
9 (mask or masks or air purifying respirator* or PAPR or "enhanced respiratory and contact precautions" or ERCP or "respiratory protection" or transparent panel* or filtering face piece* or filtering facepiece*).ti,ab.
10 (goggle* or visor* or facial protection equipment or safety glass* or safety spectacles or overshoe* or shoe cover* or rubber boot* or head cover* or face shield* or hood* or protective equipment or PPE or donning or doffing).ti,ab.
11 infection control.ti.
12 or/1‐11
13 exp health care personnel/
14 exp hospital personnel/
15 (health care worker* or healthcare worker* or health care personnel or health personnel or health care provider* or health provider* or medical staff or medical personnel or medical professional* or medical worker* or military medical personnel).ti,ab.
16 exp dentist/
17 exp dental assistant/
18 (dental personnel or dental staff or dentist* or dental assistant*).ti,ab.
19 exp nurse/
20 exp nursing assistant/
21 exp nurse midwife/
22 exp nursing staff/
23 (nurse or nurses or nursing or midwife or midwives).ti,ab.
24 exp physician/
25 physician*.ti,ab.
26 exp emergency health service/
27 exp ambulance/
28 (emergency medical services or transporting patients or patient transport or paramedic* or ambulance worker*).ti,ab.
29 exp paramedical personnel/
30 exp burial/
31 burial staff.ti,ab.
32 (cleaning worker* or cleaner* or janitor*).ti,ab.
33 or/13‐32
34 exp communicable disease/
35 exp disease transmission/
36 exp virus infection/
37 exp bacterial infection/
38 exp ebolavirus/
39 exp Ebola hemorrhagic fever/
40 exp Marburg hemorrhagic fever/
41 exp Lassa virus/
42 exp filovirus infection/
43 exp influenza/
44 exp SARS coronavirus/
45 exp severe acute respiratory syndrome/
46 exp Middle East respiratory syndrome coronavirus/
47 exp Human immunodeficiency virus infection/pc [Prevention]
48 exp tuberculosis/pc [Prevention]
49 exp hepatitis/pc [Prevention]
50 exp cross infection/
51 (infectious disease* or disease transmission or infection control precautions or (human* adj3 transmission) or parenteral transmission).ti,ab.
52 (viral disease* or viral infection* or bacterial infection* or filovirus or ebola* or Marburg virus or Lassa virus or h?emorrhagic fever or (HIV adj3 infection*) or Severe Acute Respiratory Syndrome Virus or SARS or Middle East Respiratory Syndrome or MERS or coronavirus* or corona virus* or COVID or severe acute respiratory syndrome coronavirus or SARS CoV 2 or SARS‐CoV‐2).ti,ab.
53 (skin decontamination or surface decontamination or self contamination).ti,ab.
54 or/34‐53
55 12 and 33 and 54
56 exp experimental organism/
57 animal tissue/
58 exp animal disease/
59 exp carnivore disease/
60 exp bird/
61 exp experimental animal welfare/
62 exp animal husbandry/
63 animal behavior/
64 exp animal cell culture/
65 exp mammalian disease/
66 exp mammal/
67 exp marine species/
68 nonhuman/
69 animal.hw.
70 or/56‐69
71 70 not human/
72 55 not 71
Appendix 6. Scopus search strategy 18 June 2019
#1
"protective clothing" OR gown* OR coverall* OR "protective layer" OR "protective layers" OR "surgical toga" OR apron* OR smock OR smocks OR "hazmat suit" OR glove OR gloves OR "respiratory protective devices" OR mask OR "air‐purifying respirator" OR "PAPR" OR "enhanced respiratory and contact precautions" OR "E‐RCP" OR "respiratory protection" OR "transparent panel" OR "surgical mask" OR "surgical masks" OR "filtering face piece" OR "filtering facepiece" OR "eye protective device" OR goggle* OR visor OR "facial protection equipment" OR "safety glass" OR "safety glasses" OR "safety spectacles" OR "personal protective equipment" OR "PPE" OR "protective equipment" OR overshoe* OR "shoe cover" OR "shoe covers" OR "rubber boot" OR "rubber boots" OR "head cover" OR "head covering" OR "face shield" OR "face shields" OR "surgical hood" OR hood OR gloving OR donning OR doffing)
#2
"health care personnel" OR "hospital personnel" OR "health care worker" OR "health care workers" OR "health care personnel" OR "health personnel" OR "health‐personnel" OR "health provider" OR "health providers" OR "health care provider" OR "health care providers" OR "medical staff" OR "medical personnel" OR "medical professional" OR "medical worker" OR "medical workers" OR "dental personnel" OR "dental staff" OR "dentist" OR "dentists" OR "dental assistant" OR "dental assistants" OR "nursing staff" OR "nurse" OR "nurses" OR "nursing assistant" OR "nursing assistants" OR "midwife" OR "midwives" OR "military‐medical personnel" OR "physician" OR "physicians" OR "emergency medical services" OR "transporting patients" OR "patient transport" OR "ambulance" OR "paramedical personnel" OR paramedic OR paramedics OR "burial staff" OR "cleaning workers" OR cleaner OR cleaners
#3
"virus infection" OR "viral disease" OR "filovirus" OR "ebola" OR "marburg virus" OR "lassa virus" OR "haemorrhagic fever" OR "Severe Acute Respiratory Syndrome Virus" OR "SARS" OR "MERS" OR "bioterrorism" OR "bacterial contamination" OR "microbial contamination" OR "self‐contamination" OR "decontamination" OR "surface decontamination" OR "skin decontamination"
#4
LIMIT‐TO ( PUBYEAR, 2018 )
#5
#1 AND #2 AND #3 AND #4
Appendix 7. Embase search strategy embase.com 15 July 2016
#7
#6 NOT [medline]/lim) (646)
#6
#5 AND [embase]/lim (2,227)
#5
#4 AND [humans]/lim (5,270)
#4
#1 AND #2 AND #3 (5,675)
#3
'communicable disease'/de OR "infectious disease":ab,ti OR 'disease transmission'/de OR "disease transmission" OR "infection control precautions" OR "human‐to‐human transmission" OR "parenteral transmission" OR 'virus infection'/de OR "viral disease":ab,ti OR 'bacterial infection'/de OR "bacterial infection":ab,ti OR "filovirus" OR 'ebola virus'/de OR 'hemorrhagic fever ebola'/de OR "ebola" OR "marburg virus" OR "lassa virus" OR "haemorrhagic fever" OR 'sars coronavirus'/de OR "Severe Acute Respiratory Syndrome Virus" OR "SARS" OR "MERS" OR "bioterrorism" OR 'cross infection'/de OR "bacterial contamination" OR "microbial contamination" OR "self‐contamination" OR "decontamination" OR "surface decontamination" OR "skin decontamination" (323,524)
#2
'health care personnel'/de OR 'hospital personnel'/de OR "health care worker" OR "health care workers" OR "health care personnel" OR "health personnel" OR "health‐personnel" OR "health provider" OR "health providers" OR "health care provider" OR "health care providers" OR "medical staff" OR "medical personnel" OR "medical professional" OR "medical worker" OR "medical workers" OR “dental personnel” OR “dental staff” OR "dentist" OR "dentists" OR "dental assistant" OR "dental assistants" OR "nursing staff" OR 'nurses'/de OR "nurse" OR "nurses" OR "nursing assistant" OR "nursing assistants" OR 'nursing assistant'/de OR 'nurse midwife'/de OR "midwife" OR "midwives" OR "military‐medical personnel" OR 'physician'/de OR "physician" OR "physicians" OR "emergency medical services" OR “transporting patients” OR “patient transport” OR 'ambulance'/de OR 'paramedical personnel'/de OR "paramedical personnel" OR paramedic OR paramedics OR 'posthumous care'/de OR "burial staff" OR "cleaning workers" OR "cleaner work" OR cleaner OR cleaners (1,287,399)
#1
'protective clothing'/de OR gown* OR coverall* OR "protective layer" OR "protective layers" OR "surgical toga" OR apron* OR smock OR smocks OR "hazmat suit" OR (hazmat AND suit) OR glove OR gloves OR 'respiratory protective devices'/de OR 'mask'/de OR mask OR "air‐purifying respirator" OR "PAPR" OR "enhanced respiratory and contact precautions" OR "E‐RCP" OR “respiratory protection” OR "transparent panel" OR "surgical mask" OR "surgical masks" OR "filtering face piece" OR "filtering facepiece" OR 'eye protective device'/de OR goggle* OR visor OR "facial protection equipment" OR "safety glass" OR "safety glasses" OR "safety spectacles" OR "personal protective equipment" OR "PPE" OR "protective equipment" OR overshoe* OR "shoe cover" OR "shoe covers" OR "rubber boot" OR "rubber boots" OR "head cover" OR "head covering" OR "face shield" OR "face shields" OR "surgical hood" OR hood OR 'medical device contamination'/de OR 'infection control'/de OR 'infection control':ab,ti OR gloving OR donning OR doffing (160,118)
Appendix 8. CINAHL EBSCO search strategy 20 March 2020
S51 S10 AND S32 AND S50
S50 S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49
S49 TI ("surface decontamination" OR "skin decontamination") OR AB ("surface decontamination" OR "skin decontamination")
S48 AB (“viral disease” OR “viral diseases” OR “viral infection” OR “viral infections” OR “bacterial infection” OR “bacterial infections” OR filovirus OR ebola* OR “Marburg virus” OR “Lassa virus” OR “haemorrhagic fever” OR “hemorrhagic fever” OR “HIV infection” OR “HIV infections” OR “Severe Acute Respiratory Syndrome Virus” OR SARS OR “Middle East Respiratory Syndrome” OR MERS OR coronavirus* OR “corona virus” OR “corona viruses” OR COVID OR “severe acute respiratory syndrome coronavirus” OR “SARS CoV 2” OR “SARS‐CoV‐2”)
S47 TI (“viral disease” OR “viral diseases” OR “viral infection” OR “viral infections” OR “bacterial infection” OR “bacterial infections” OR filovirus OR ebola* OR “Marburg virus” OR “Lassa virus” OR “haemorrhagic fever” OR “hemorrhagic fever” OR “HIV infection” OR “HIV infections” OR “Severe Acute Respiratory Syndrome Virus” OR SARS OR “Middle East Respiratory Syndrome” OR MERS OR coronavirus* OR “corona virus” OR “corona viruses” OR COVID OR “severe acute respiratory syndrome coronavirus” OR “SARS CoV 2” OR “SARS‐CoV‐2”)
S46 TI (“infectious disease” OR “infectious diseases” OR “disease transmission” OR “infection control precautions” OR “human‐to‐human transmission” OR “parenteral transmission”) OR AB (“infectious disease” OR “infectious diseases” OR “disease transmission” OR “infection control precautions” OR “human‐to‐human transmission” OR “parenteral transmission”)
S45 (MH "Cross Infection+")
S44 (MH "Hepatitis B+/PC/TM")
S43 (MH "Hepatitis A/PC/TM")
S42 (MH "Tuberculosis+/PC/TM")
S41 (MH "HIV Infections+/TM/PC")
S40 (MH "Middle East Respiratory Syndrome") OR (MH "Middle East Respiratory Syndrome Coronavirus")
S39 (MH "SARS Virus") OR (MH "Severe Acute Respiratory Syndrome")
S38 (MH "Influenza, Human+")
S37 (MH "Hemorrhagic Fever, Ebola") OR (MH "Ebola Virus")
S36 (MH "Bacterial Infections+")
S35 (MH "Virus Diseases+")
S34 (MH "Disease Transmission, Patient‐to‐Professional") OR (MH "Disease Transmission, Professional‐to‐Patient")
S33 (MH "Communicable Diseases+")
S32 S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31
S31 TI ("cleaning worker" OR "cleaning workers" OR cleaner* or janitor*) OR AB ("cleaning worker" OR "cleaning workers" OR cleaner* or janitor*)
S30 (TI burial) or (AB burial)
S29 (MH "Allied Health Personnel+")
S28 TI (“emergency medical services” OR “transporting patients” OR “patient transport” OR paramedic* OR “ambulance worker” OR “ambulance workers”) OR AB (“emergency medical services” OR “transporting patients” OR “patient transport” OR paramedic* OR “ambulance worker” OR “ambulance workers”)
S27 (MH "Ambulances")
S26 (MH "Emergency Medical Services+")
S25 (TI physician*) OR (AB physician*)
S24 (MH "Physicians+")
S23 AB (nurse OR nurses OR nursing OR midwife OR midwives)
S22 TI (nurse OR nurses OR nursing OR midwife OR midwives)
S21 (MH "Nurse Midwives")
S20 (MH "Nursing Assistants")
S19 (MH "Nurses+")
S18 AB ("dental personnel" OR "dental staff" OR dentist* OR "dental assistant" OR "dental assistants")
S17 TI ("dental personnel" OR "dental staff" OR dentist* OR "dental assistant" OR "dental assistants")
S16 (MH "Dental Assistants")
S15 (MH "Dentists+")
S14 AB (“health care worker” OR “health care workers” OR “healthcare worker” OR “healthcare workers” OR “health care personnel” OR “health personnel” OR “health care provider” OR “health care providers” OR “health provider” OR “health providers” OR “medical staff” OR “medical personnel” OR “medical professional” OR “medical professionals” OR “medical worker” OR “medical workers” OR "military medical personnel")
S13 TI (“health care worker” OR “health care workers” OR “healthcare worker” OR “healthcare workers” OR “health care personnel” OR “health personnel” OR “health care provider” OR “health care providers” OR “health provider” OR “health providers” OR “medical staff” OR “medical personnel” OR “medical professional” OR “medical professionals” OR “medical worker” OR “medical workers” OR "military medical personnel")
S12 (MH "Personnel, Health Facility+")
S11 (MH "Health Personnel+")
S10 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9
S9 AB (glove* OR gloving OR gown* OR coverall* OR "protective layer" OR "protective layers" OR "surgical toga" OR apron* OR smock* OR "hazmat suit" OR “hazmat suits” OR mask* OR "air‐purifying respirator" OR PAPR OR "enhanced respiratory and contact precautions" OR E‐RCP OR ECPR OR "respiratory protection" OR "transparent panel" OR "surgical mask" OR "surgical masks" OR "filtering face piece" OR "filtering facepiece" OR goggle* OR visor* OR "facial protection equipment" OR "safety glass" OR "safety glasses" OR "safety spectacles" OR "personal protective equipment" OR PPE OR "protective equipment" OR overshoe* OR "shoe cover" OR "shoe covers" OR "rubber boot" OR "rubber boots" OR "head cover" OR "head covering" OR "face shield" OR "face shields" OR "surgical hood" OR "hood" OR "infection control OR donning OR doffing)
S8 TI (glove* OR gloving OR gown* OR coverall* OR "protective layer" OR "protective layers" OR "surgical toga" OR apron* OR smock* OR "hazmat suit" OR “hazmat suits” OR mask* OR "air‐purifying respirator" OR PAPR OR "enhanced respiratory and contact precautions" OR E‐RCP OR ECPR OR "respiratory protection" OR "transparent panel" OR "surgical mask" OR "surgical masks" OR "filtering face piece" OR "filtering facepiece" OR goggle* OR visor* OR "facial protection equipment" OR "safety glass" OR "safety glasses" OR "safety spectacles" OR "personal protective equipment" OR PPE OR "protective equipment" OR overshoe* OR "shoe cover" OR "shoe covers" OR "rubber boot" OR "rubber boots" OR "head cover" OR "head covering" OR "face shield" OR "face shields" OR "surgical hood" OR "hood" OR "infection control OR donning OR doffing)
S7 (MH "Infection Control+/PC")
S6 (MH "Equipment Contamination/PC")
S5 (MH "Respiratory Protective Devices")
S4 (MH "Gloves")
S3 (MH "Eye Protective Devices")
S2 (MH "Masks")
S1 (MH "Protective Clothing+")
Appendix 9. CINAHL search strategy 31 July 2018
S5 S4 MEDLINE records excluded (878)
S4 (S1 AND S2 AND S3) (2,584)
S3
(MH "Communicable Diseases") OR (TI "infectious disease") OR (AB "infectious disease") OR (MH "Disease Transmission) OR TX "disease transmission" OR (MH "Disease Transmission, Patient‐to‐Professional") OR TX "infection control precautions" OR TX "human‐to‐human transmission" OR TX "parenteral transmission" OR (MH "Virus Diseases/PC") OR TX "viral disease" OR TX "viral diseases" OR TX "bacterial infection" OR (MH "Bacterial infection/PC") OR TX "filovirus" OR TX "ebolavirus" OR (MH "Hemorrhagic Fever, Ebola") OR TX "ebola" OR TX "marburg virus" OR TX "lassa virus" OR TX "haemorrhagic fever" OR (MH "SARS Virus") OR TX "severe acute respiratory syndrome virus" OR TX "SARS" OR TX "MERS" OR TX "respiratory infection" OR TX "bioterrorism" OR TX "aerosol‐generating procedure" OR (MH "Cross Infection") OR TX "bacterial contamination" OR TX "microbial contamination" OR TX "self‐contamination" OR TX "decontamination" OR TX "surface decontamination" OR TX "skin decontamination" (37,937)
S2
(MH Protective Clothing) OR TX gown* OR TX coverall* OR TX "protective layer" OR TX "protective layers" OR TX "surgical toga" OR TX apron* OR TX "smock" OR TX "smocks" OR TX "hazmat suit" OR TX (hazmat AND suit) OR (MH "gloves protective") OR TX glove OR TX gloves OR (MH "Respiratory Protective Devices") OR (MH "Masks") OR TX mask OR TX masks OR TX "air‐purifying respirator" OR TX "PAPR" OR TX "enhanced respiratory and contact precautions" OR TX "E‐RCP" OR TX "respiratory protection" OR TX "transparent panel" OR TX "surgical mask" OR TX "surgical masks" OR TX "filtering face piece" OR TX "filtering facepiece" OR (MH "Eye Protective Devices") OR TX goggle* OR TX "visor" OR TX "facial protection equipment" OR TX "safety glass" OR TX "safety glasses" OR TX "safety spectacles" OR TX "personal protective equipment" OR TX "PPE" OR TX "protective equipment" OR TX overshoe* OR TX "shoe cover" OR TX "shoe covers" OR TX "rubber boot" OR TX "rubber boots" OR TX "head cover" OR TX "head covering" OR TX "face shield" OR TX "face shields" OR TX "surgical hood" OR TX "hood" OR (MH "Equipment Contamination/PC") OR (MH "Infection Control") OR (TI "infection control") OR (AB "infection control") OR TX "gloving" OR TX "donning" OR TX “doffing” (28,554)
S1
(MH "Health Personnel") OR TX health care workers OR TX health care personnel OR TX health personnel OR TX health‐personnel OR TX health providers OR TX health care providers OR TX medical staff OR TX medical personnel OR TX medical professional OR TX medical workers OR TX dental personnel OR TX dental staff OR (MH "Dentists") OR TX dentist OR TX dental assistant OR TX nursing staff OR (MH "Nurses") OR TX nurse OR TX nursing assistant OR (MH "Allied Health Personnel" OR (MH "Midwives") OR TX nurse midwife OR TX nurse midwives OR TX military‐medical personnel OR (MH “Physicians") OR TX physician OR TX emergency medical services OR (MH “Emergency Medical Services”) OR TX transporting patients OR TX patient transport OR (MH "Ambulance") OR (MH "Allied Health Personnel") OR TX paramedic OR TX paramedical personnel OR (MH "Burial") OR TX burial staff OR TX cleaning worker OR TX cleaner work OR TX cleaner OR TX cleaners (498,394)
Appendix 10. OSH‐update search strategy
| Step: | Hits: | Strategy: | ||
| #1 | 32657 | GW{protective clothing OR gown* OR coverall* OR protective layer* OR surgical toga* OR apron* OR smock* OR hazmat suit* OR (hazmat AND suit) OR glove* OR respiratory protective device* OR mask OR masks OR air purifying respirator* OR 'PAPR' OR 'enhanced respiratory and contact precautions' OR 'E‐RCP' OR respiratory protection OR transparent panel* OR surgical mask* OR filtering face piece OR eye protective device* OR goggle* OR visor OR facial protective equipment OR safety glass* OR safety spectacles OR personal protective equipment OR 'PPE' OR protective equipment OR overshoe* OR shoe cover* OR rubber boot* OR head cover* OR face shield* OR surgical hood OR hood OR equipment contamination OR infection control OR gloving OR donning OR doffing} | ||
| #2 | 11286 | GW{communicable disease* OR infectious disease* OR disease transmission OR infection control precautions OR human‐to‐human transmission OR parenteral transmission OR viral disease* OR bacterial infection* OR filovirus OR Ebolavirus OR hemorrhagic fever OR Ebola OR Marburg virus OR Lassa virus OR SARS virus OR severe acute respiratory syndrome virus OR 'SARS' OR 'MERS' OR respiratory infection* OR bioterrorism OR aerosol‐generating procedure OR cross infection* OR bacterial contamination OR microbial contamination OR self‐contamination OR decontamination OR surface decontamination OR skin decontamination} | ||
| #3 | 32599 | GW{health personnel OR health care worker* OR health care personnel OR health personnel OR health‐personnel OR health provider* OR health care provider* OR medical staff OR medical personnel OR medical professional OR medical worker* OR dental personnel OR dental staff OR dentist* OR dental assistant* OR nursing staff OR nurse* OR nursing assistant* OR nurses' aides OR nurse midwife OR nurse midwives OR midwife OR midwives OR military‐medical personnel OR physician* OR emergency medical services OR transporting patients OR patient transport OR ambulance* OR allied health personnel OR paramedic* OR paramedical personnel OR burial OR burial staff OR cleaning worker* OR cleaner work OR cleaner*} | ||
| #4 | 1250 | #1 AND #2 AND #3 | ||
| #5 | 742476 | DC{OUBIB OR OUCISD OR OUHSEL OR OUISST OR OUNIOC OR OUNIOS OR OURILO} | ||
| #6 | 1103 | #4 AND #5 |
Data and analyses
Comparison 1. PAPR versus E‐RCP Attire.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Any contamination | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.2 Contamination > 1 cm | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.3 Contamination area | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.4 Donning noncompliance | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.5 Doffing noncompliance | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.6 Donning time | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 1.7 Doffing time | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
1.2. Analysis.

Comparison 1: PAPR versus E‐RCP Attire, Outcome 2: Contamination > 1 cm
1.3. Analysis.

Comparison 1: PAPR versus E‐RCP Attire, Outcome 3: Contamination area
Comparison 2. Four types of PPE attire compared.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 A vs B Contamination, mean number of spots | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1.1 Face type A vs type B | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1.2 Trunk type A vs type B | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1.3 Neck type A vs type B | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1.4 Foot type A vs type B | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1.5 Palm type A vs type B | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2 A vs B Usability score (1‐5) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.3 A vs B Donning time | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.4 A vs B Doffing time | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.5 A vs D Contamination, mean number of spots | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.5.1 Face type A vs type D | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.5.2 Trunk type A vs type D | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.5.3 Neck type A vs type D | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.5.4 Foot type A vs type D | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.5.5 Palm type A vs type D | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.6 A vs D Usability score (1‐5) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.7 A vs D Donning time | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.8 A vs D Doffing time | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. Formal versus local available attire.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Contamination | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 4. Gown versus apron.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Contamination with marker; individual doffing | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1.1 small patches | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1.2 large patches | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1.3 hand | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1.4 shoe | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1.5 underwear | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1.6 environment | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.2 Contamination with marker; CDC doffing | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.2.1 small patches | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.2.2 large patches | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.2.3 hand | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.2.4 shoe | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.2.5 underwear | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.2.6 environment | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 5. Three types of PPE compared.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Time for donning | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1.1 PPE 1 vs PPE 3 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1.2 PPE 2 vs PPE 3 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.2 Time for doffing | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.2.1 PPE 2 vs PPE 3 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.2.2 PPE 2 vs PPE 3 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 6. Gown sealed gloves versus standard gown.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Contamination fluorescent lotion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.2 Contamination MS2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 7. Gown easy to doff versus standard gown.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Contamination with fluorescent marker | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.2 Contamination with bacteriophage | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 8. Gown with gown‐glove improvement vs standard gown‐gloves.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 People with contamination | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.26, 0.78] |
| 8.1.1 Improved vs standard | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.31, 0.81] |
| 8.1.2 Improved plus education vs standard plus education | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 0.96] |
Comparison 9. Gown with marked inside versus standard gown.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Noncompliance donning: people with errors | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.2 Noncompliance: errors during performance | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.3 Noncompliance doffing: people with errors | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 10. Gloves with tab versus standard gloves.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Any contamination of hands | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 11. Mask with tabs versus no mask tabs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Contamination of head from hands | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.2 Contamination of hands from mask | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 12. Doffing with double gloves versus doffing with single gloves.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Contamination: virus detected | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1.1 All body parts | 2 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.17, 0.66] |
| 12.1.2 Face | 2 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.39 [0.53, 36.37] |
| 12.1.3 Shirt | 2 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.79, 1.29] |
| 12.1.4 Pants | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.52, 1.58] |
| 12.2 Contamination: virus quantity | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.2.1 Dominant hand | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.2.2 Non‐dominant hand | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.2.3 Face | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.2.4 Shirt | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.2.5 Pants | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.3 Non‐compliance: any error | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.4 Contamination with fluorescent | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
12.2. Analysis.

Comparison 12: Doffing with double gloves versus doffing with single gloves, Outcome 2: Contamination: virus quantity
Comparison 13. CDC versus individual doffing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Gown: contamination with fluor marker | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1.1 small patch | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1.2 large patch | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1.3 hand | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1.4 shoe | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1.5 underwear | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1.6 environment | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.2 Apron: contamination with fluor marker | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.2.1 small patch | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.2.2 large patch | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.2.3 hand | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.2.4 shoe | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.2.5 underwear | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.2.6 environment | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 14. Single‐step doffing vs CDC standard.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 14.1 Fluorescent contamination | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 14.2 Bacterial contamination | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 15. Doffing with extra sanitation of gloves versus standard no sanitation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 15.1 Bacterial contamination | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 15.1.1 Alcohol‐based hand rub | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 16. Donning and doffing with instructions versus without instructions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 16.1 People with one or more errors | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.11, 0.93] |
| 16.1.1 Basic PPE | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.04, 0.62] |
| 16.1.2 Enhanced PPE | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.22, 0.98] |
| 16.2 Non‐compliance: mean errors | 1 | 120 | Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.36, ‐0.41] |
| 16.2.1 Basic PPE | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐1.15, ‐0.25] |
| 16.2.2 Enhanced PPE | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐1.87, ‐0.53] |
| 16.3 Fluorescence contamination | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
16.3. Analysis.

Comparison 16: Donning and doffing with instructions versus without instructions, Outcome 3: Fluorescence contamination
Comparison 17. Active training in PPE use versus passive training.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 17.1 Noncompliance with PPE | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected |
17.1. Analysis.

Comparison 17: Active training in PPE use versus passive training, Outcome 1: Noncompliance with PPE
Comparison 18. Doffing with hypochlorite versus doffing with alcohol‐based glove sanitiser.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 18.1 Contamination MS2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 18.2 Contamination Ph6 | 1 | 15 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
Comparison 19. Active training in PPE doffing versus passive training.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 19.1 Noncompliance doffing protocol | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected |
Comparison 20. Computer simulation versus no simulation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 20.1 Number of errors while donning | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 20.2 Number of errors while doffing | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 21. Video‐based learning versus traditional lecture.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 21.1 Skills in PPE donning | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andonian 2019.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel group For simulation study, what was used for the exposure (virus, fluorescent fluid etc): 1. The optimised fluorescent slurry consisted of fluorescent powder (Glitter Bug, Brevis Corporation, Salt Lake City, UT; 75 mg/mL) in a viscous suspension of grape‐seed oil and water (1:6 oil‐to‐water ratio) 2. Fluorescent 2‐μm polystyrene latex bead (PLSs) (G0200, Thermo Fisher Scientific, Waltham, MA) diluted in water. PLSs are commonly utilised in aerosol research and were used to simulate pathogens Exposure simulation: 1. Fluorescent tracer mixture was applied to PPE using 1000 mL in a pesticide hand sprayer (RL Flo‐Master, Lowell, MI; 2000 mL capacity) and 5 sweeping passes of sprayer from head to feet on the front and back of the HCW. 2. A PLS suspension (25 mL) was aerosolised using a 3‐jet Collison nebuliser (Mesa Laboratories, Inc, Butler, NJ) for 4 min of continuous aerosol generation while the HCW turned 90° every 60 s |
|
| Participants |
Baseline characteristics 48 participants were included in the study Enhanced doffing protocol
CDC doffing protocol
Overall
Inclusion criteria: not reported, but study authors included: adults (male/female) with no prior experience doffing enhanced PPE Excluded criteria: not reported |
|
| Interventions |
Intervention characteristics Enhanced doffing protocol: doffing with extra instructions
CDC doffing protocol
|
|
| Outcomes |
How the outcome was measured: from the fluorescent tracer slurry ‐
detection was by direct visualisation in a dark room using ultraviolet
light. (1) The number of body sites contaminated and (2) the extent of
contamination at each site were recorded. PLS detection was performed by (3)
counting via epifluorescent microscopy and (4) quantifying the number PLSs
per cm² of skin or per m3 of sampled air. (5) Teamwork dynamics
were assessed via video and coded using a task analysis of the process sets
and subsets (checklist). (6) The National Aeronautics and Space
Administration (NASA) Task Load Index (NASA‐TLX) questionnaire assessed
perceptions of workload during doffing (7) The Team Strategies and Tools to
Enhance Performance and Patient Safety Teamwork Attitudes Questionnaire
(T‐TAQ) assessed attitudes toward teamwork Body sites with fluorescent marker
Body sites with PLS
|
|
| Notes | Outcomes Median and IQR of 22 possible contaminated sites reported. For Fluor Marker: intervention 1 (1‐2) control 5 (2‐5) For PLS out of 12 possible contaminated sites: intervention 4 (2‐5) control 5 (5‐8). These were transformed to means and SDs for use in the data tables. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned to the control or intervention condition and then
to the role of HCW or DA." Judgement comment: method of random assignment not reported |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: method of allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "role as either HCW or DA." Quote: "After training, all participants were given the opportunity to ask questions and were informed about their randomly assigned" Quote: "Study participants were blind to their group assignment." Judgement comment: group assignment (intervention and control) was blinded; role was blinded to participants until after training and before the doffing intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The contamination forms were deidentified and assigned randomized
numbers for scoring purposes. Two IPs, blinded to experimental assignment,
independently scored each form" Judgement comment: infection preventionists were the outcome assessors and were blinded to intervention group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Forty‐eight study participants (35 females, 13 males) were randomly
assigned to the control (n = 22) or intervention group (n = 26)." Quote: "Participants in each study arm were randomly assigned to the role of control HCW (n = 11), control DA (n = 11), intervention HCW (n = 13), or intervention DA (n = 13). For the fluorescent tracer, 11 HCWs (84.6%) in the intervention group and all 13 control HCWs (100%) contaminated at least 1 body area." Quote: "Coding and scoring of teamwork behaviors exhibited in the videotaped doffing sessions were completed for 10 intervention and 11 control teams. Technical difficulties resulted in missing videotapes for 3 intervention teams." Judgement comment: main outcomes were listed within the methods (but scattered and hard to find). All recruited participants completed the interventions and outcomes were collected. 1 typographical error (I assume) in reporting fluorescent tracer contamination (they reported 13 control HCWs but there were only 11). 3 sets of teamwork behaviour outcomes recorded in videos from the intervention group were lost. However, despite the missing data, there was a plausible difference in median (IQR) between groups that may not have impacted the observed effect size. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: the availability of the study protocol is not reported in the paper, but it is clear that the published report includes all expected outcomes for this type of study. |
| Other bias | Low risk | Judgement comment: no other bias detected |
Bell 2015.
| Study characteristics | ||
| Methods | Randomised, 2 parallel groups; simulation study | |
| Participants | N = 8, nurses (6), physicians 2; women 7/8 Intervention: 4, control: 4 Volunteer healthcare providers, no further details provided Location: USA |
|
| Interventions |
Intervention: different types of PPE compared: commercially available
PPE: neck‐to‐ankle coverall (type not reported), water impermeable
surgical gown, knee‐length impermeable leggings, Stryker hood, double gloves
with outer arm‐length surgical gloves, N95 masks; meeting CDC
recommendations; each participant was assisted in PPE donning by an
experienced trainer. Control: local, readily available attire: 2 plastic gowns worn over the front and the back of the torso, rain‐suit pants and hood, spark‐shield as face‐cover, ankle length shoe covers, double gloves with outer arm‐length surgical gloves, N95 masks; meeting CDC recommendations; each participant was assisted in PPE donning by an experienced trainer. |
|
| Outcomes | Contamination: measured in mL of fluorescent agent with LED black light
after doffing. Random order of 2 types of exposure: high volume or standard. High volume meant 100 mL of fluorescent agent splashed on the torso. Standard meant working on a manikin contaminated with fluorescent agent. Fluorescent liquid mimicked body fluids and consisted of fluorescent powder, clothes detergent, fluorescent tablets |
|
| Notes | No funding or conflict of interest reported Apparently tape was used to put attire together; this resulted in more difficult doffing but no figures reported; costs of locally available equipment was USD 36 US, that of commercial material not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized to one of two PPE ensembles" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | High risk | Contamination outcomes reported but no separate outcomes for high or normal exposure, however small sample and no statistical analysis by study authors |
| Other bias | Low risk | No indication |
Buianov 2004.
| Study characteristics | ||
| Methods | Controlled simulation study, not randomised; probably cross‐over study | |
| Participants | N = 9 volunteers that carried out a 4‐h step test of average workload at a temperature of 20º C and 60% relative humidity, no further details provided | |
| Interventions |
Intervention: different types of PPE compared: different types of
respirators Positive pressure suit (special biological suit, СКБ‐I) consisting of a rubber hood connected to a PAPR and a 'dust‐proof' coverall in 1 piece with different rates of air supply: initially 250 L/min, then 50, 100, 150, 200, 250, 300 L/min. No information about the filtering piece. PPE was especially developed for highly infectious diseases such as Ebola, Marburg and Lassa fever intended for use by HCW, such as doctors, nurses and orderlies Comparison: 2 different types of positive pressure hoods (ЛИЗ‐4 and ПШБ‐3) together with a coverall type Biotekhnolog ‐1 Procedure: tests are carried out in a so‐called Meltserovsky room (individual room with quarantine). The pressure suit or hood and coverall is put on before entering and checked whether it functions by attaching the connecting pipe to the air supply system. Then the worker enters the buffer zone (gateway with entrance and exit) and proceeds to the individual measurement room. After the step test in the individual room the HCW goes to the buffer zone in order to treat the outside surface of the pressure suit. The worker attaches the suit to the connecting pipe of the air supply system and treats the suit with the help of aerosol disinfectant, usually 3%‐6% hydrogen peroxide (2‐3 aerosol generators are situated at different heights). After the aerosol rests are pumped out of the buffer zone the HCW leaves through the gateway, takes off the pressure suit and places it in the special container for final disinfection. |
|
| Outcomes | Contamination exposure: Participants were exposed to a microbial aerosol
with a concentration of 108 CFU/m3. No further details
on the spray aerosol provided. Contamination outcome measured aerosol particles on different parts of the body (neck, shoulder, forearm, chest, loin, thigh, shin) and the suit with 'washouts' and triple agar prints. Only data from triple agar prints are presented since the 'washouts' resulted in unreliable data (because the textile materials used in the pressure suit were impregnated with hydrophobic materials). Triple agar prints were taken from the outside surface of the pressure suit, inside surface of the pressure suit, clothes and skin areas at different parts of the body (neck, shoulder and forearm, chest, loin, thigh and shin). The outcome was both expressed as CFU/m³ and as penetration rate as a percentage of the outside that has leaked inside the PPE. It was unclear if these outcomes were expressed as an average across the participants and what the variation was. The study authors conclude that "despite the significant concentration of microbial aerosol in the experimental room (107‐105 cfu/m3) no microbial aerosol was measured on skin areas with air supply speeds of 250 L/min and higher". Additionally, the study authors assessed skin temperature, heart rate, breath rate, and moisture loss |
|
| Notes | Article in Russian, data retrieved with help of a native speaker (AP) Article difficult to judge due to cultural differences in style and translation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Confounding NRS | High risk | No confounders reported |
| Selection Bias NRS | Low risk | Selection of volunteers unrelated to intervention or to outcome. Start follow‐up and intervention coincide for all participants. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if data reported for all nine participants |
| Selective reporting (reporting bias) | Unclear risk | All data announced in methods reported in results |
| Other bias | Low risk | No other biases assessed |
Casalino 2015.
| Study characteristics | ||
| Methods | Controlled before‐after study of 2 training variants | |
| Participants | N = 120, 63% nursing students, 37% medical students Age 21.2 +/‐ 3.5 years, 35% male The study authors did not present demographic data per group Location: Paris (France), Lima (Peru), and Guadalajara (Mexico), in December 2014 and January 2015 with no previous training in PPE use, with no special intention to be involved in Ebola care |
|
| Interventions |
Intervention: doffing with extra instructions There were 2 intervention groups that only differed in type of PPE used 1. Basic PPE + reinforced training (N = 30); basic PPE consisted of boots, goggles, surgical mask, surgical cap, impermeable apron (11 pieces of equipment) with 6 steps for donning and 13 steps for doffing. 2. Enhanced PPE + reinforced training (N = 30); enhanced PPE consisted of boots, full‐body impermeable suit, hood with surgical cap and mask, double gloves, impermeable apron (9 pieces of equipment) with 6 steps for donning and 12 steps for doffing. Training for all participants consisted of 60 min of theoretical course including 10 min of donning instruction and 20 min of doffing instruction. In addition, there were 3 practical training sessions per 2 students who mutually assisted each other observed by a specialist trainer who intervened in case of non‐compliance. The sessions were held with 3‐day intervals. Compared to the control group the additional intervention was that the specialist trainer "repeated aloud each of the steps and technical skills or processes necessary" to comply with the standard during the practical training sessions. The sessions were also reviewed comprehensively. Control group: There were 2 control groups that differed in type of PPE used just as in the intervention groups 1. Basic PPE + conventional training (N = 30) 2. Enhanced PPE + conventional training (N = 30) These groups received the same training as the intervention group but the specialist‐trainer did not repeat aloud the necessary steps. |
|
| Outcomes |
Primary outcome: number of errors per person for donning and for
doffing and the number of people with ≥ 1 errors measured by the specialist
trainer. The study authors also measured critical errors, which were those
where there was contact between skin and potentially contaminated PPE, but
we did not consider this a valid measure of contamination and disregarded
this. We took measurement of the errors at the last training session as the
effect of the intervention. We disregarded the error measurements at earlier
training sessions. Secondary outcomes: errors for doffing of the gown, full‐body suit and boots; duration of donning and doffing in min at the last training session |
|
| Notes | Country: France, Peru Mexico; no funding reported; no conflict of interest
reported The first study author, Enrique Casalino, answered some of our questions regarding the study, but we were unable to retrieve more information on the group allocation and therefore classified the study as non‐randomised. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Confounding NRS | Unclear risk | None of the confounders mentioned |
| Selection Bias NRS | Low risk | Students were randomly chosen and did not have any experience or intention to use the knowledge and skills. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible but students could be motivated to perform better because of knowing that they were in the intervention group and not as a result of the oral instructions. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Providers were also the assessors of compliance. We asked study authors for more information but did not get any information that increased our confidence in the outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported if all data were available |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods section reported; no protocol available |
| Other bias | Low risk | No other biases assessed |
Casanova 2012.
| Study characteristics | ||
| Methods | Controlled simulation study, non‐randomised, first intervention then control condition for all participants | |
| Participants | N = 18 volunteer healthcare providers > 18 years of age; exclusion
criteria: pregnant, latex allergy, skin disorder, previous fit‐testing for
N95 respirator; 17/18 right handed, 18/18 previous experience with PPE Location: USA |
|
| Interventions |
Intervention: doffing with double gloves 2 pairs of latex gloves; inner glove under the cuff of the gown sleeve, the outer glove, 1 size larger worn over the gown cuff; in addition, full PPE consisted of contact isolation gown, N95 respirator and eye protection Control: 1 pair of latex gloves in addition to similar full PPE as in intervention group Doffing was performed according to CDC instructions: gloves, goggles, gown, mask or respirator in case of single gloves; in case of double gloves, outer pair of gloves first and inner pair last |
|
| Outcomes | 1. Contamination of the hands, face, gloves and scrubs with bacteriophage
MS2 virus; hands sampled with "glove juice method", face with a swab at the
edge of the N95 respirator, shirt, pants and gloves were immersed in beef
extract. All eluants were assayed by 'most probable number enrichment
infectivity assay' (MPN). Detection level 0.15 log 10 MPN; Used paired t‐test for the analysis of continuous data to take the cross‐over into account 2. Noncompliance with doffing guidelines Contamination with bacteriophage MS2 was put on front shoulder of the gown, right side of respirator, right front of eye protection and palm of dominant hand by simulated droplet contamination; before doffing participants had to perform neck and wrist pulses on manikin |
|
| Notes | No funding or conflict of interest reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Confounding NRS | Low risk | No apparent confounders for this type of study and outcome |
| Selection Bias NRS | Low risk | No apparent selection of participants into the study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding, but performance bias not likely because participants would not have an interest with either intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Some data only in figures and not in tables |
| Other bias | Low risk | No other biases anticipated |
Casanova 2016.
| Study characteristics | ||
| Methods | Non‐randomised simulation study How was the simulation performed? Each participant was verbally guided through the donning process of EVD PPE using the CDC protocol. After the exposure contamination was applied to the PPE worn, all participants performed a gown change on a manikin. Participants were then verbally guided through the doffing process using the CDC checklist either using a hypochlorite spray or an alcohol‐based hand rub for all six hand or glove cleaning steps during doffing. How was the exposure simulated? Exposure to a mixture of MS2 and Φ6 suspended in phosphate‐buffered saline was applied to 4 sites: (1) the palm of the dominant hand, (2) the shoulder of the gown opposite the dominant hand, (3) the top side of the face shield on the same side as the dominant hand, and (4) the toe of the rubber boot opposite the dominant hand. A total of 25 µL was applied to each site in 5 drops of 5 µL each to simulate droplet exposure, particularly small droplet exposure of which the HCW may not be aware. The mean virus titre applied to each site in 25 µL was 1 × 108 for MS2 and 5 × 107 for Φ6, based on reports of viral load in body fluids during acute phases of EVD. |
|
| Participants | N= 15 (11 RNs and 4 MDs) no further details given Intervention: 5, control: 10 Study participants were all members of the Ebola care team at a large tertiary care academic medical centre. Members of the Ebola team were > 18 years of age and had undergone extensive training in a simulation laboratory in the use of EVD‐specific PPE, including donning and doffing |
|
| Interventions |
Intervention: doffing with extra glove sanitation Hypochlorite glove sanitiser: liquid hypochlorite at a concentration of 1850 ppm was applied by spraying it on the gloves for each hand or glove sanitising step of the 16‐step doffing protocol that was used. This was the only alternation of the usual doffing protocol. Control: alcohol‐based hand rub: 70% ethanol gel was used for each hand or glove sanitising step of the 16‐step doffing protocol that was used |
|
| Outcomes | Contamination: 1. MS2 bacteriophage (non‐enveloped surrogate virus) 2. Φ6 bacteriophage (enveloped surrogate virus, such as Ebola) We took from the study authors' report contamination found on scrubs, or on the bare hands or on the face of the participant |
|
| Notes | Country: USA; no conflict in interests reported; funded with CDC grant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Confounding NRS | Low risk | Differences related to: 1. prior experience with PPE ‐ no 2. healthcare qualification or education of HCW ‐ no 3. age‐no information, unlikely 4. sex‐no information, unlikely 5. ambient temperatures ‐ no, assumed similar 6. stressful activities ‐ no |
| Selection Bias NRS | Unclear risk | Allocation to group was based on belonging to the last 5 participants |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were asked to close their eyes when simulated exposure was
applied to them. However, it is unlikely that they did not notice where
simulation exposure was applied. Participants were not blinded to the intervention, however, it is unlikely that they behaved differently with hypochlorite or alcohol sanitiser |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | No protocol, but selective reporting unlikely |
| Other bias | Low risk | No other bias observed |
Chughtai 2018.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: cross‐over Simulation study? If so, describe exposure simulation: after donning PPE, inert fluorescent lotion was applied on external surfaces of the PPE to simulate contamination. Participants were given 0.5 mL of lotion and were instructed to rub the lotion on their hand and apply to the PPE. Fluorescent lotion was also sprayed on the front and sides, from approximately 1 m, to mimic droplet infection. For simulation study: what was used for the exposure (virus, fluorescent fluid etc): fluorescent spray: GlitterBug. Glitterbug kits. Available from: glitterbug.net.au/products/. Accessed 2 January 2018 |
|
| Participants |
Baseline characteristics Overall
Included criteria: not reported other than "Staff and students of the University of New South Wales". Assuming adult, both genders Excluded criteria: excluded participants with any pre‐existing respiratory condition, heart disease, or pregnancy |
|
| Interventions |
Intervention characteristics: different types of PPE compared with
various donning and doffing protocols
|
|
| Outcomes |
Small patches of contamination
Large patches of contamination
Ease of use and comfort
|
|
| Notes | Outcomes For the WHO attire there were 4 large patches of contamination, for the North Caroline PPE 2, for the CDC PPE 1 small patch and for the Health Canada PPE there was 1 small patch of contamination | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned to use 3 different PPE protocols." Judgement comment: insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Participants did not have any training in PPE. They were provided
with the relevant protocol for donning/doffing, and procedures were examined
by a study investigator using a checklist. The study investigator read out
the donning and doffing steps, and participants followed the instructions.
Videos were shown if available for each protocol that was tested." Judgement comment: no blinding possible but participants and personnel were not aware which protocol would be better, we felt that it is unclear if performance bias is likely |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: procedures were examined by the study investigator who was aware of the PPE protocol. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: data for all participants provided |
| Selective reporting (reporting bias) | Low risk | Judgement comment: there was no protocol reported in the paper for this study. However, the outcomes of interest were listed in the methods and appear reasonable for the study. |
| Other bias | Low risk | Judgement comment: no other bias detected |
Curtis 2018.
| Study characteristics | ||
| Methods | RCT with parallel groups How was the simulation performed? Participants had to demonstrate skills in donning PPE, working with PPE and doffing PPE in a simulated practice setting where they were observed. At Station 1, participants were asked to don Level C PPE. At Station 2, the participants were asked to demonstrate the proper technique for administration of the Duodote auto‐injector to a simulated victim of nerve agent poisoning. Participants were then asked to use the Simple Triage and Rapid Treatment triage system for 6 different disaster scenarios that were described on cards attached to inflatable training manikins. At Station 3, participants were asked to decontaminate inflatable training manikins simulating contaminated victims of a hazardous materials incident. Following completion of the 3rd station, the participants doffed their Level C PPE and were asked to complete the post‐exercise comfort survey. |
|
| Participants | N = 30 volunteers. Emergency Medicine residents were randomised, results of
26 are reported. The study was conducted at an urban, academic, tertiary referral centre that provides training to Emergency Medicine residents in a 4‐year programme. All Emergency Medicine residents who attended the weekly educational conference were recruited for this study. As there were not any more residents available to participate at this single‐site study, the number needed to study for significance was not determined. Intervention: n = 13 (53% female), Control: n = 13 (46% female) |
|
| Interventions | Intervention: training: video‐based learning (VBL) A training video about specific content for the training modules was watched prior to completing a knowledge quiz and the practical exercises. An Emergency Medicine resident in the residency programme’s disaster medicine specialty track wrote, directed, and edited the video. The VBL modality was setup and viewed without faculty interaction. Both educational modalities contained identical educational content Control: traditional lecture (TL) A PowerPoint presentation that covered the same information as the video was presented prior to completing a knowledge quiz and the practical exercises. |
|
| Outcomes | Primary outcome: performance scores on proper donning of PPE on the practical exercises evaluated by a blinded trained evaluator | |
| Notes | Location: USA; no funding or conflict of interest reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "department research division consultant conducted a stratified randomisation of residents by post‐graduate year class level and assigned them to either the experimental (VBL) group or the control (TL)" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Study participants identified themselves on all study tests and surveys using employee identification numbers rather than their names" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study could not be blinded but unlikely that participants could have influenced the outcome because they knew to which group they belonged |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All evaluators were blinded as to which study participants had participated in the TL modality and which participated in the VBL modality." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data available for 13 out of 15 participants in both groups. Missing data were not related to the intervention. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol provided. Probably all outcomes reported |
| Other bias | Unclear risk | No other bias detected |
Drews 2019.
| Study characteristics | ||
| Methods | Design: a 2 (task) x 2 (gown) nested, repeated‐measurements design Group: cross‐over For simulation study: what was used for the exposure (virus, fluorescent fluid etc): no exposure was used only simulated tasks. Exposure simulation: all participants reviewed a brief presentation and were given an opportunity to ask questions on the design, attributes, and use of the redesigned gown. They were then introduced to the simulator and given patient information along with a brief description of the task they were to perform. The simulated patients were in isolation precautions with signage posted outside the patient room indicating the required PPE. Participants performed 2 scenarios, with a different gown (standard or re‐designed) made available prior to the start of each scenario. |
|
| Participants | Male %: not reported Age (m ± SD): not reported Occupations: nurses (50%) and nurses' aides Employment duration: not reported |
|
| Interventions |
Intervention: modified PPE: redesigned gown: gown redesign
considerations focused on improving the closure mechanism, providing visual
cues to demarcate the contaminated outer from the clean inner surfaces,
weighing down the gown material for better coverage, and making gown removal
easier by adding perforations to the tie. A closure mechanism using an
asymmetrical closure approach was favoured, with the gown secured by pulling
a single strap from the back to front. An adhesive strip covered by red tape
was placed at the end of the strap. Pulling the tape off the adhesive strip
allowed for strap securement to the front of the gown Control: standard gown |
|
| Outcomes | 1. Non‐adherence to proper use of PPE during donning, measured as: if and
how gown was closed 2. Non‐adherence of proper use of PPE during doffing, measured as: pulling gown from waist, balling up gown) 3. Non‐adherence of proper use of PPE during performance: measured as: exposure while squatting, tie or gown touches floor |
|
| Notes | Sponsorship source: This work was supported by the CDC (grant number P50
CA098252). The article appears as part of the supplement “Personal
Protective Equipment for Preventing Contact Transmission of Pathogens:
Innovations from CDC’s Prevention Epicenters Program,” sponsored by the
CDC’s Prevention Epicenters Program. Country: USA Setting Simulation learning center Authors name: Frank A. Drews Institution: Department of Psychology, and Division of Epidemiology, Department of Internal Medicine, University of Utah, Salt Lake City, US Email drews@psych.utah.edu Address: University of Utah, Department of Psychology, 380S 1530E BEH, Rm 502, Salt Lake City, UT 84112, US |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Confounding NRS | Low risk | Confounders:
|
| Selection Bias NRS | Unclear risk | No details of participants mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Personnel and participants could not be blinded and likely that the redesigned gown can have influenced behaviour |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessors mentioned. Adherance is a rather subjective evaluation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported |
| Selective reporting (reporting bias) | Unclear risk | No protocol mentioned and unclear if all outcomes reported |
| Other bias | Unclear risk | There was no washout period and it is unclear if the order of the experiments was random. It is only reported as counter balanced |
Gleser 2018.
| Study characteristics | ||
| Methods | Simulation study, quasi‐randomised study based on alternation How was the simulation performed? A volunteer HCW donned appropriate sized glove and then wetted each hand with fluorescent solution and distributed this solution equally on the glove's surfaces to simulate an external glove contamination. Immediately thereafter, the volunteer removed their gloves, and their hands were then examined using a UV Box (Hand Hygeine Teaching Box "Sharing Expertise; B. Braun, Melsungen, Germany) How was the exposure simulated? 5 mL of a fluorescent solution (Schülke Optics Training fluorescent lotion; Schülke & Mayr GmbH, Vienna, Austria) on each hand |
|
| Participants | N = 317 (~70% female) volunteer HCWs on 35 hospital wards in a tertiary
care university hospital Intervention: N = 146 (104 nurses, 53 physicians) Control: N = 171 (118 nurses, 53 physicians) |
|
| Interventions | Intervention: modified PPE: tabs on gloves Doffy Glove, modified nitrile gloves with a textured small flap (doffing aid) above the thumb area positioned laterally on the wrist when worn that can be gripped during glove removal Control: standard nitrile medical examination gloves made according to the same material formulation and manufacturing process by the same company on behalf of IP Gloves GmbH |
|
| Outcomes | Contamination: any visible fluorescence on the volunteer's skin | |
| Notes | Location: Germany; no funding or conflict of interest reported, however first author is also CEO of the start‐up that developed and market the new types of gloves. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Participants were randomised for the use of either standard gloves
or Doffy Gloves on an alternate daily basis" Judgement comment: quasi‐randomisation; big difference in number in intervention or control group |
| Allocation concealment (selection bias) | Unclear risk | No description provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study could not be blinded but unlikely that participants could have influenced the outcome, which was assessed by observers |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors of contamination were aware of which glove was used and subjective assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data reported |
| Selective reporting (reporting bias) | High risk | No study protocol provided |
| Other bias | High risk | Study authors have a big financial interest in a positive evaluation of their new product |
Guo 2014.
| Study characteristics | ||
| Methods | Randomised, multiple arm, cross‐over, simulation study | |
| Participants | N = 50; voluntary HCW who gave informed consent; excluded were those who
were allergic to the fluorescent marker; 34/50 female, 20/50 nurses, 10/50
doctors, 15/50 support staff, 5/50 allied health workers; age 32.9 ± 5.7
years average; working experience 10.9 ± 5.1 years Location: Hong Kong, China |
|
| Interventions |
Intervention: different types of PPE compared Intervention 1: N = 50 participants. 3 types of protective clothing: 1. Disposable, water‐resistant, non‐woven gown, 2. Reusable, woven cotton gown, 3. Disposable, non‐woven plastic apron; and 2 different removal methods: individually determined or CDC‐recommended. Each of the 50 participants was required to test the 3 different types of PPE followed by 1 of 2 different removal methods. Intervention 2: first the participant should doff according to their own views (individual method), then a CDC instruction video was shown and participants were asked to perform the donning or doffing method for gowns that was recommended by CDC in 2007: gown front and sleeves are contaminated! Unfasten neck, then waist ties. Remove gown using a peeling motion; pull gown from each shoulder toward the same hand. Gown will turn inside out. Hold removed gown away from body, roll into a bundle and discard into waste or linen receptacle. Control: cross‐over N = 50 participants. 3 types of protective clothing were compared against each other. |
|
| Outcomes |
A fluorescent powder (GloGermCo,Moab,UT) especially developed for determining hand hygiene compliance was used in this study. The Glo Germ powder was mixed with light olive oil and water to resemble human aerosol as closely as possible. The study authors used repeated measures analysis to take into account the cross‐over design of the study |
|
| Notes | Funding Hong Kong Polytechnic University; no conflict of interest declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Interventions were offered "in random order"; study authors asked for clarification |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding possible, but no performance bias expected as participants would not have an interest with any intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All data reported |
| Other bias | Low risk | Not detected |
Hajar 2019.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: cross‐over Exposure (virus, fluorescent fluid etc): fluorescent solution (Super Blue Invisible Ink, Black Light World) Exposure simulation: participants donned the gowns and nitrile gloves (DenvilleScientific, Holliston, MA) in their usual manner. The gloved hands were inoculated with 0.5 mL of fluorescent solution (Super Blue Invisible Ink, Black Light World) that was rubbed over the gloved hands until dry (~15 s). The participants removed their PPE in their usual manner; no education was provided. |
|
| Participants |
Baseline characteristics Overall
Included criteria: not reported (HCW) Excluded criteria: not reported |
|
| Interventions |
Intervention characteristics: modified PPE Increased‐coverage gown
Standard gown
Increased coverage gown plus education
Standard gown plus education
|
|
| Outcomes |
Contamination outcome assessment: contamination of the hands and
wrists was assessed using a black light, and the sites of contamination were
recorded. After a washout period of at least 5 min, an additional simulation
was conducted with cross‐over to the alternate gown. People with contamination
People with protocol deviation
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "personnel were randomized" Judgement comment: no reporting of random number generation |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: no reporting of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "We conducted 2 non‐blinded cross‐over trials to compare
contamination of personnel during simulations of contaminated PPE removal
with the standard versus the alternative design cover gown." Judgement comment: study authors stated they were non‐blinded. 1 gown was routinely used in the facility and participants may have had a biased preference for it. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: no mentioning of blinding of outcome assessors. Probably not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "total, 6 participants were excluded from the analysis because they
did not complete the second assessment because they were unavailable or
unable to be located after the initial assessment." Judgement comment: attrition was even in both trials, with 4 dropping out (2 each group) in trial 1 and 2 dropping out (1 each group) in trial 2. All data were reported for those analysed |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no compliance data provided for second trial |
| Other bias | Unclear risk | Judgement comment: washout period very short and unclear if all contaminant was cleared away |
Hall 2018.
| Study characteristics | ||
| Methods | Simulation study, non‐randomised cross‐over study How was the simulation performed? Prior to donning PPE volunteers were screened using Fluorescence Interactive Video Exposure System (FIVES) to ensure that there was no pre‐existing contamination on their skin or scrubs from the environment, previous tests or background fluorescence. Over disposable scrubs volunteers then donned the PPE ensembles under supervision by a buddy, and they were screened again prior to beginning the simulation exercise. After completing the exercise, volunteers were screened front and back using the FIVES system to qualitatively record contamination resulting from the simulation. PPE was then removed according to protocol under the supervision of a buddy, and screening was repeated to detect any post‐doffing contamination. How was the exposure simulated? 'Violet' (Visualising Infection with Optimised Light for Education and Training) was a medical training manikin adapted to deliver simulants of 4 fluorochrome‐tagged body fluids during a scenario based on a doctor and nurse undertaking clinical procedures with a suspected‐case patient. |
|
| Participants | N = 11 (7 nurses, 4 doctors) Volunteer healthcare providers were recruited via calling notices at the participating Infectious Disease (ID) units, gave informed consent and were free to withdraw at any time. 11 volunteers completed the simulation exercise up to 10 times depending on their availability. 5 volunteers (including 1 further doctor and nurse) acted as 'buddies' to assist with doffing. All volunteers were experienced in using the PPE ensembles adopted by their respective ID units, but if they used an ensemble from another unit, they had to undergo training to practice donning and doffing 10 times or until deemed competent by a staff trainer. Limiting the number of volunteers reduced user attributable variation. | |
| Interventions | Intervention: different types of PPE compared 5 'suspected case' PPE ensembles used in different infectious disease units around the UK. All models met the guidance of the Advisory Committee on Dangerous Pathogens endorsed by Public Health England. PPE components met their relevant material standards. All were donned and dry‐doffed according to the specific protocol relevant to the ensemble. The PPE ensembles varied but could broadly be grouped as a 'gown model' or a 'coverall model' but each had slight differences (e.g. use of hood vs surgical cap, boots vs boot covers, and different glove lengths and number of pairs). Control: basic‐level PPE (surgical mask, standard length apron, 1 pair short gloves, no standard footwear, scrubs and no buddy used for doffing) |
|
| Outcomes | Contamination: fluorescent areas seen on skin or scrubs of the volunteer post‐doffing | |
| Notes | Location: UK; no conflict of interested reported; funding was provided by Health and Safety Executive (HSE); Bozena Poller was funded by the Healthcare Infection Society’s Graham Ayliffe Training Fellowship | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Confounding NRS | Low risk | Differences related to:
|
| Selection Bias NRS | Low risk | Cross‐over trial; 11 participants did the simulation up to 10 times |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants knew which PPE they had on but it is unlikely that they could have influenced the outcome, which was an objective assessment by an observer. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The judgement of the contamination is subjective and the assessors were aware of the type of equipment but it is unclear if this could have influenced the outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "This resulted in a non‐trained volunteer participating in the role of the nurse for 1 simulation; their data were excluded from the final analysis, but their participation allowed data to be captured for their doctor partner. In total, 19, suspected case simulations captured 37 volunteers." |
| Selective reporting (reporting bias) | Unclear risk | No protocol provided |
| Other bias | Low risk | No other biases detected |
Houlihan 2017.
| Study characteristics | ||
| Methods | Retrospective cohort study Invitations to participate were sent to individuals known to the study authors, and through organisations supporting EMT deployment involving UK‐based staff, including non‐governmental organisations (NGOs), UK government‐affiliated institutions, and the London School of Hygiene and Tropical Medicine (LSHTM). The participants filled in a questionnaire with information about PPE use. They then underwent a blood test to assess their antibody status. The researchers assessed the participants' risk of being exposed to EVD based on an independent algorithm. |
|
| Participants | N = 300 individuals who returned to the UK or Ireland after responding to the West African EVD epidemic completed the survey. Of these, N = 268 returned material for IgG assessment (median age 36 years range 30‐45; 57% female; 35% lab staff, 26% physicians, 20% nurses, 19% other) In addition, there were N = 53 non‐exposed control participants included who had not left the UK (median age 35 years range 31‐40; 66% female) | |
| Interventions | Intervention: doffing with extra sanitation; doffing with extra
instructions There were 2 interventions that were of interest. (1) PPE removal with or without chlorine spray, (2) PPE removal with and without assistance. However, almost all clinical staff had used both interventions as compared to laboratory staff who had not used them. Because there was also a big difference in the likelihood of exposure between these 2 occupational groups, the effect of protection of these measures could therefore not be analysed. |
|
| Outcomes | Level of IgG antibody against Ebola Virus as an indicator of infection | |
| Notes | Country: UK; funding by Wellcome Trust: Enhancing Research Activity in Epidemic Situations. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript; 1 study author received funding from the Wellcome Trust via the University of Liverpool and also received non‐financial support from NHSBT, as part of the Convalescent Plasma Study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Confounding NRS | Low risk | Differences related to:
|
| Selection Bias NRS | High risk | Sample based on snowball sampling |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were not aware of exposure status when they reported their exposures. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researchers knew who was rated as 'high risk' but objective outcome measure. Therefore unlikely that it was influenced |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Convenience sample; from sample 10.7% did not react |
| Selective reporting (reporting bias) | Unclear risk | No protocol provided |
| Other bias | Low risk | No other sources of bias detected |
Hung 2015.
| Study characteristics | ||
| Methods | RCT, 2 parallel groups, 2 training variants | |
| Participants | Intervention group: N = 25, age 44% < 31 years, healthcare assistant
56%, nurse 44%, work experience < 6 years 44%, no gender reported Control group: N = 25, age 28% < 31 years, healthcare assistant 56%, nurse 44%, work experience < 6 years 48%, no gender reported All HCW of an outpatient department of a private hospital handling infectious patients before admission; able to read English, basic computer skills |
|
| Interventions |
Intervention: training: extra computer simulation All participants were asked to don and doff N95 respirator, face shield, cap, gown, gloves for "precautions against airborne danger". External observers rated the procedures for errors. All participants then attended a PPE‐training consisting of a 15‐min demonstration of donning and doffing by an "infection control link nurse". After 1 week the intervention group got the interactive computer simulation programme and again after 1 week was assessed for compliance with the donning and doffing procedures. Control: the control group was assessed for compliance with donning and doffing procedures 1 week after PPE training. The group did not get the computer simulation training. |
|
| Outcomes |
Primary outcome: score on 16‐item checklist for donning and 20‐item
checklist for doffing. Secondary outcome: IBM computer system usability questionnaire (CSUQ) consisting of 19 items with a 7‐point Likert response scale |
|
| Notes | Hong Kong China; funding: Hong Komg Research Grant Council; no conflict of interest reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The subjects were randomly assigned to the control and experimental group of the same size", page 53 |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind participants or providers but outcome objectively assessed by observers, unlikely that this was influenced |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Nurse assessing PPE compliance "was blinded about the research", page 53 |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported if all participants contributed data |
| Selective reporting (reporting bias) | High risk | Results of computer usability questionnaire not fully reported |
| Other bias | Low risk | No other biases assessed |
Kpadeh Rogers 2019.
| Study characteristics | ||
| Methods |
Study design: non‐RCT Study grouping: n/a Simulation of the exposure (virus, fluorescent fluid etc): for each experiment, the top gloves on both hands were directly inoculated with 50 μL of bacterial suspension and 50 μL of GloGerm Mist liquid fluorescent marker (GloGerm, Moab, UT) to give a final concentration of 108 CFU of bacteria. A high inoculum was used based on our pilot observations that organism recovery from gloves was reduced by 1–2 logs from the original inoculum. Fluorescent marker was added to visually trace bacterial transfer throughout all experiments. Exposure simulation: participants were asked to rub the bacteria/fluorescent marker on their hands in a standardised way. A research team member provided verbal instructions to ensure that doffing steps were performed per CDC protocols. Alcohol‐based hand rub, 63% alcohol (Steris Corp, Mentor, OH) and 2 US Environmental Protection Agency–registered hospital disinfectants, dispatch bleach disinfecting wipes (Clorox Healthcare, Oakland, CA) and Sani‐Cloth AF3 quaternary ammonium (“quat”) disinfecting wipes (PDI Healthcare, Montvale, NJ), were used for decontamination. Volunteers were asked to decontaminate in a manner that ensured they covered all parts of the glove surface including between all fingers. Using a single pump of the alcohol‐based hand rub, volunteers rubbed both gloved hands together, similar to routine hand hygiene in the hospital, until the gloves were completely dry. For wipe‐based decontamination, the volunteer used a single wipe to decontaminate both gloves with continuous wiping for at least 1 min. We ensured a total manufacturer‐recommended dwell or contact time, that is, time for which the glove surface remained visibly wet, of 3 min for quat and 1 min for bleach. |
|
| Participants |
Baseline characteristics 10 participants were enrolled, 10 per organism Overall
Included criteria: volunteers were asked to don 2 pairs of gloves and a gown, with the under gloves representing HCW hands and the top gloves representing the actual gloves worn for patient care. In total, 20 HCW (10 per organism) were enrolled. Excluded criteria: not reported Pretreatment: cross‐over trial. All participants used all 3 disinfectants and no disinfectant |
|
| Interventions |
Intervention characteristics: doffing with extra sanitation Alcohol‐based glove decontamination
Quat‐based glove decontamination
Bleach‐based glove decontamination
No glove decontamination
|
|
| Outcomes |
Outcome assessment for simulation study: at the end of the
experiment, gloves were sampled using a 3M sponge‐stick with 10 mL
neutralising buffer (St. Paul, MN) in a standardised manner to ensure
sampling of all surfaces. Sponge‐sticks were processed using previously
described methods. From the eluent, successive 1/10 dilutions were made and
plated on tryptic soy agar (Becton Dickinson, Sparks, MD) in triplicate for
quantitative culturing. Plates were incubated overnight, and the number of
CFUs of Klebsiella pneumoniae and Methicillin‐sensitive
Staphylococcus aureus (MSSA) were calculated. The eluent was also
enriched in gram‐negative broth (Becton Dickinson) for K. pneumoniae
and tryptic soy broth with salt (Remel, Lenexa, KS) for MSSA, incubated
overnight, and plated onto MacConkey agar and blood agar, respectively. Bacterial contamination (combined Staphylococcus and Klebsiella)
Bacterial contamination
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Confounding NRS | Unclear risk | Judgement comment: differences related to:
|
| Selection Bias NRS | High risk | Judgement comment: 10 HCW performed the trial with 1 type of bacteria and another 10 HCW performed the trial with the second type of bacteria. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement comment: participants knew which disinfectant they used, but it is unlikely that they could have influenced the outcome, which was an objective assessment by an observer. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement comment: outcome assessors unblinded but outcome fairly objective. Unlikely that they influenced the outcome measurement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: apparently data from all experiments reported |
| Selective reporting (reporting bias) | Low risk | No protocol but apparently all outcomes reported |
| Other bias | Low risk | Judgement comment: no other sources of bias detected |
Mana 2018.
| Study characteristics | ||
| Methods | Simulation study, randomised cross‐over study How was the simulation performed? Participants were instructed to don intervention or control gown and gloves in their usual manner. A lotion containing both exposures was rubbed onto the gloves and then the participants rubbed gloved hands on the front area of the gown to simulate contamination. Participants doffed the PPE again in their usual manner. How was the exposure simulated? Exposure to contamination was simulated by a lotion containing 0.5 mL of phosphate‐buffered saline containing 108 PFU of the enveloped virus bacteriophage Phi X174 (American Type Culture Collection (ATCC) 13706‐B1), and 0.5 mL of fluorescent lotion |
|
| Participants | N = 31 11 physicians (36%), 6 nurses (19%), 14 allied health personnel (45%) 31 paired simulations |
|
| Interventions |
Intervention: modified PPE: gown easy doffing Assure Wear Gown with Flexneck technology (AMD Ritmed, Tonawanda, NY) designed to allow easy removal at the neck and with increased skin coverage and snugness of fit at the wrist. The gown has a double elastic neck closure system to aid in removal, thumb loops with smaller holes and provides more palm coverage and elastic band around wrist to improve snugness of gown Control: Standard Safety Plus polyethylene gown (TIDI Products, Neenah, WI). Problems can occur with hand and wrist contamination due to skin exposure at the gown–glove interface despite the presence of a thumb loop intended to keep the gown in proximity to the gloves. A loose fit at the wrist and minimal coverage of the upper palm contributes to the potential for contamination. Contamination of the neck region often occurs when gowns do not easily come apart at the posterior neck, resulting in tearing of gown material. |
|
| Outcomes | UV contamination: a black light (Ultra LightUV1 by Grizzly Gear, SCS
Direct, Trumball, CT) was used to look for the fluorescent tracer on the
hands, wrist, neck and chest. Bacteriophage contamination: the participants' hands and wrist were swabbed with gauze to collect potential bacteriophage. Alcohol based hand sanitiser was used for hand hygiene and sterile gloves were donned prior to the participant swabbing their neck and chest, including their clothing to collect other potential contamination. |
|
| Notes | Location: USA; financial support: this work was supported by a Merit Review grant (no. 1 I01 BX002944‐01A1) from the Department of Veterans Affairs to C.J.D. AMD Ritmed provided the Assure Wear VersaGowns with Flexneck technology for testing, but they had no role in study design, analysis or interpretation of the data, or writing of the manuscript. Potential conflicts of interest: C.J.D. received research grants from Clorox, Merck, AvidBiotics, and GOJO, and has served on scientific advisory boards for 3M and Seres Health. All other study authors reported no conflicts of interest relevant to this article. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Healthcare personnel were randomised to perform simulations of
contaminated glove and gown removal using either the standard or alternative
design gown." Additional info from study authors: the random sequence was generated have used a List Randomizer from the web‐site: www.random.org/lists/, which provided a random listing of which gown will be used first for each participant. |
| Allocation concealment (selection bias) | Low risk | Additional info received from study authors: the allocation was irrevocable |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants could not be blinded but this is unlikely to have an effect on the outcome because this was assessed by observers |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Additional information from authors: it was not possible to blind outcome evaluators for the fluorescence evaluation because the gowns are visibly different. However, the outcome evaluators for the assessment of bacteriophage Phi X174 contamination were blinded to the identity of the study groups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Additional information from study authors: there were no missing data |
| Selective reporting (reporting bias) | Unclear risk | Additional information from study authors: we did not register the study protocol |
| Other bias | Low risk | No indication of other biases |
Osei‐Bonsu 2019.
| Study characteristics | ||
| Methods | Simulation study, randomised How was the simulation performed? 1. Glo Germ fluorescent powder (Glo Germ Company, Moab, UT) 2. 1 mL of Staphylococcus epidermidis in a 0.5 McFarland suspension (1.5 10 8 CFU/mL). The S epidermidis was genetically engineered to stably express a green fluorescent protein that is visible under a black light in bacterial cultures How was the exposure simulated? In order to simulate PPE contamination, after donning PPE, study assistants (KO, NM, and MD) used a wedge foam paint brush to liberally coat participants with Glo Germfluorescent powder (Glo Germ Company, Moab, UT) on both arms, hands, and the abdomen. The brush was dipped back into the powder after coating each arm or abdomen. These areas were thought most likely to be contaminated in the course of patient care activities at the bedside. Participants were then also coated with S Epidermidis in the same distribution on the body. The solution was applied by dripping droplets over the PPE with a 1000 uL pipette by the study staff. After the opportunity to review the assigned procedure and ask questions, participants were then asked to doff PPE under guided observation by the study investigators. There was no training or practice of the doffing techniques prior to the simulation. Prompts were given as needed to ensure the participants followed the assigned procedure. |
|
| Participants |
Inclusion criteria: clinical providers and microbiology laboratory
personnel as well as life safety administrators. Laboratory personnel and
life safety administrators do not use PPE in the context of patient care,
but do use it as occupational PPE (i.e. gowns, gloves, masks, and goggles)
in the laboratory or to train other staff on proper PPEusage. Exclusion criteria: individuals < 18 years of age or > 65 years of age; pregnancy or breastfeeding; history of joint replacements or other prosthetic medical devices; and active inflammatory skin conditions or open wounds. Differences between intervention groups in HCW profession type and duration of work experience Occupation: 18% MD, 67% RN, 16% non‐clinician Work experience average 5.2 years |
|
| Interventions | Interventions: different doffing procedures; Doffing with extra sanitation;
Doffing with double gloves; Doffing 1 step 1. CDC standard doffing procedure (Control intervention): prescribed procedure for doffing in the following order: gloves, goggles/face shield, gown, mask/respirator, hand hygiene 2. CDC 1 step: similar to CDC procedure but gloves and gown are doffed in 1 go. 3.CDC plus extra hand hygiene: CDC plus extra disinfection of gloves with alcohol‐based hand rub 4. CDC plus double gloves: similar to CDC procedure but 2 pairs of gloves used and the first pair is doffed first and the second pair last |
|
| Outcomes |
|
|
| Notes | Location: USA Corresponce: Michelle Doll: Michelle.Doll@vcuhealth.org. Address: Michelle Doll, MD, MPH, Virginia Commonwealth University Health System, 1300 E Marshall St, North Hospital, 2nd Fl, Rm 2‐100, PO Box 980019, Richmond, VA 23298 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participants were assigned a procedure by having them pick a
doffing procedure at random from a closed envelope." Judgement: likely to be random |
| Allocation concealment (selection bias) | Low risk | Quote: "Participants were assigned a procedure by having them pick a
doffing procedure at random from a closed envelope." Judgement: unlikely that participants or researchers could change assigned group |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement: personnel and participants could not be blinded, however unlikely that they could have an influence on the outcome which is fairly objective |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement: no information. However both outcomes fairly objective and unlikely that this changed the outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement: no information, apparently all data available |
| Selective reporting (reporting bias) | High risk | Not all outcomes fully reported: main outcome usability only reported as not significant |
| Other bias | Low risk | No other biases detected |
Shigayeva 2007.
| Study characteristics | ||
| Methods | Retrospective cohort study | |
| Participants | HCW who provided care or entered the room of a Toronto SARS patient who
required intubation, during the 24 h before and 4 h after intubation Eligible N = 879, analysed N = 795; age (median) = 41 years (range 21‐67 years); employment in current occupation (median) = 12 years (range 0‐43 years); 46% nurses, 14% physicians, 14% respiratory therapists, 10% imaging staff and 16% other; 1055 exposure episodes or shifts Active training intervention: N = 511 episodes (= 385 people), Passive training intervention: N = 236 episodes (= 178 people), Comparison no active training: N = 308 episodes (= 323 people) Location: Canada |
|
| Interventions |
Intervention: training Intervention 1: active training: participants answered that they had received any individual or group face‐to‐face training sessions Intervention 2: passive training: participants watched a video or got written information. Comparison: no training reported Other predictors of PPE studied in a multivariate generalised estimating equation logistic regression analysis in addition to training for both outcomes: phase of epidemic, occupation, work experience, hospital type, location of care, number of times patient's room entered, SARS diagnosis recognised, Apache II score of patient. |
|
| Outcomes | 1. Consistent adherences as proportion of exposure episodes. Participants
were interviewed based on a questionnaire 0.2‐10 months after the exposure.
Interviewers asked about consistent use of PPE: masks, gowns, gloves and eye
protection and possible predictors of their use, including training.
Consistent adherence was defined as always wearing gloves, a gown, a mask,
and eye protection. Consistent adherence was reported in 817/1055 (77%)
exposure episodes. Eye protection was least with 13.5% consistent and no PPE
in 23 episodes (2.2%). PPE use increased during epidemic from 34.6% at start
to 97.4% in the end. 2. Doffing as proportion of exposure episodes (safe, at some risk, or at risk). Participants were asked about their sequence of doffing PPE. Safe was defined as the sequence of removing gown and gloves, hand hygiene, mask, goggles, or safety glasses, hand hygiene. At some risk was considered if hand hygiene was performed only once. At risk if no hand hygiene was performed or hands touched potentially contaminated face. Doffing description was available for 810/1055 (77%) of exposure episodes; 15.4% qualified as safe, 63% as at some risk, and 22% as at risk. |
|
| Notes | Units of analysis used in studies: exposure episodes not people exposed,
based on work schedules, patient assignments and health records. There were
65 intubations of SARS patients of which 7 were not recognised as such at
the time of intubation. Funding Ontario Ministery of Health and Long term Care; no conflict of Interest reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Confounding NRS | Low risk | Adjustment in multiple regression analysis for education, work experience, and presumably for age and sex |
| Selection Bias NRS | Low risk | Whole cohort assessed that was working during the epidemic. Exposure to SARS patients clearly defined |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both the intervention and the outcome were assessed at the same time |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Both the intervention and the outcome were assessed with the same questionnaire at the same time |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 90% HCW participated for adherence and for 77% of shifts more or less reliable info about doffing available |
| Selective reporting (reporting bias) | Unclear risk | Not clear which predictors of adherence or safe doffing were tested and negative |
| Other bias | Low risk | No indication of other bias |
Strauch 2016.
| Study characteristics | ||
| Methods | Simulation study, cross‐over RCT How was the simulation performed? 2 different simulations of contamination of the Filtering Facepiece Respirator (FFR) were performed: 1 in which the FFR was contaminated but not the hands and another one in which the hands were contaminated but not the FFR 1. Contamination of the FFR and clean hands: 20 participants performed 3 trials of FFR with removal tabs (tab+) and tab‐ masks each in random order 2. Clean FFR and contamination of hands: 20 participants performed 1 tab+ trial and 1 tab‐ trial How was the exposure simulated? To contaminate the FFR, 7 mL of fluorescent tracer was brushed onto the entire outer surface of the test FFRs. As only the outer surface of the FFR was contaminated with the fluorescent tracer, transfer from the FFR to the hands would only occur if the FFR was doffed improperly by grasping the contaminated surface. 2. For the hand contamination test, 1 mL of fluorescent tracer was applied and rubbed into the hands of the test participant before removal of a clean FFR with or without tabs. The fluorescent tracer was prepared by suspending 1 g of GloGerm (GloGerm Company; Moab,UT) powder suspended in 25 mL of mineral oil. |
|
| Participants | N = 20 aged 18‐60 HCW
Volunteers employed as HCW, that were enrolled
in a respiratory protection programme and experienced in wearing FFRs were
preferred, but a potential participant was not excluded if all of the
qualities were not met. Volunteers were excluded if they had a history of skin cancer, sensitivity to UV light, or burns from a black light Country: USA |
|
| Interventions | Intervention: modified PPE: masks with tabs Mask with tabs; N‐95 mask with 4 red foam tabs attached to straps to assist in mask removal Control: mask with out tabs |
|
| Outcomes | Contamination of the hands resulting from exposure to a contaminated
mask Contamination of the head resulting from exposure to contaminated hands: the participant’s head, face and hair were photographed under UVA light for contamination with fluorescent tracer. |
|
| Notes | Location: USA; funding source and conflict of interest were not published; reported on Lumens as a measure of contaminate but the written results did not match those presented in figure. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "each subject doffed one randomly assigned FFR" Unclear how randomisation was performed |
| Allocation concealment (selection bias) | Unclear risk | Unclear if allocation was irrevocable |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not reported but unlikely to have influenced the outcome that was assessed by observers |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if there were missing data |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other biases detected |
Suen 2018.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: Simulation exposure (virus, fluorescent fluid etc): fluorescent solution (UV GERM Hygiene Spray, Glow Tec Ltd., London, England) that mimics contaminated bodily fluids or secretions spread via contact route. Exposure simulation: fluorescent solution was sprayed onto the face shield, 2 upper limb/gloves and anterior surfaces of the gown at a distance of 60 cm from the participants, which represents the length of a stethoscope, simulating the usual working distance between a patient and an HCW, with an average of 1.99 g fluorescent solution/per stroke. This value was determined using an electronic analytical balance with a precision of 0.1 g (NJW‐3000, Xiangxin, Taipei, Taiwan) via obtaining the average of 20 trial cases. A standard of 3 strokes was sprayed on each body part with a total of 12 strokes made for each case. The weight of the splash in 1 stroke was 1.99 g in this study when the density of the solution was assumed as 1. How was the simulation performed?: on the testing day, the participants watched a video about donning and doffing of the PPE ensembles to familiarise themselves with the procedures. The experiment was sequentially conducted in 3 areas. Area A was the ‘clean zone’, where the participants donned the working clothes and clean PPE ensemble in front of a mirror. Area B was the ‘preparation zone’, where the PPE of the participants was contaminated with a fluorescent solution. Area C was the ‘de‐gown and test‐zone’, wherein the participants were required to doff the PPE. |
|
| Participants |
Baseline characteristics Overall
Included criteria: HCWs that were willing to participate Excluded criteria: pregnant women and participants suffering from upper respiratory tract infection and respiratory diseases requiring treatment were excluded. Pretreatment: all participants tested the 3 PPE types |
|
| Interventions |
Intervention characteristics: different types of PPE compared PPE1
PPE2
PPE3
|
|
| Outcomes |
How was the outcome measured: 1. Areas in contamination were counted, measured, and categorised as small‐ (medium‐ (1 cm² to < 3 cm²), large‐ (≥ 3 cm² to 5 cm²) or extra‐large patch (≥ 5 cm²)The presence of fluorescent solution using UV lamp (CheckPoint, 220‐240 V / 50 Hz; Glow Tec Ltd., London, England) under a dim light. The participants’ hair and head, face, anterior/posterior neck, left/right arms, hands or wrists, upper/lower working clothes and shoes, along with the surrounding environment (rubbish bin cover, chair, faucet (tap), and sink). 2. Deviation rate is mean of 11 issues 10 issues and 9 issues resp for donning and same for doffing Overall small contamination sites
Overall extra‐large contamination sites
Overall deviation rate of donning PPE
Overall deviation rate of doffing PPE
Time of donning PPE
Time of doffing PPE
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random order as decided by a computer‐generated randomised table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement comment: not possible to blind but outcome objective and difficult to influence by providers and participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: data for all participants reported |
| Selective reporting (reporting bias) | Low risk | Judgement comment: no protocol provided but apparently all outcomes reported |
| Other bias | Low risk | Judgement comment: no other biases detected |
Tomas 2016.
| Study characteristics | ||
| Methods | Simulation study, RCT, parallel groups How was the simulation performed? Participants removed improved gowns and gloves in their usual manner. How was the exposure simulated? Gloved hands were inoculated with 0.5 mL phosphate‐buffered saline (PBS) containing 10^10 PFUs of MS2 and 0.5 mL fluorescent lotion and the solutions were rubbed over the gloved hands until dry. Bacteriophage MS2 was of the type 15597‐B1 (American Type Culture Collection,VA). |
|
| Participants | N = 30 HCW; no other information provided; asked study authors for more information | |
| Interventions |
Intervention: modified PPE: seamless gown‐glove interface A seamless PPE prototype in which adhesive material on the outer sleeve of the gown at the wrist attaches to the inner cuff of the gloves, providing continuous coverage of the wrist and hand. This design prevents exposure of skin and requires that gloves be peeled off as the gown is removed. The prototype seamless PPE consisted of polyethylene contact isolation gowns (SafetyPlus Polyethylene Gown, TIDI Products, Neenah, WI) and nitrile gloves (Denville Scientific, South Plainfield, NJ). Permanent contact bond adhesive (DAP Weldwood Contact Cement, DAP Products, Baltimore, MD) was applied circumferentially to the outer gown at the level of the wrist. Gloves were pressed to the gowns for 15 min and allowed to air dry for 24 h. Control: only described as standard PPE and assumed as gloves and gown |
|
| Outcomes | 1. Outcome assessment fluorescent: hand and wrist skin contamination with
the fluorescent lotion was assessed using a black light (Ultra Light UV1 by
Grizzly Gear, SCS Direct,Trumball, CT). 2. Outcome assessment bacteriophage: participants then wiped both hands and wrists with a sterile, pre‐moistened 4 x 4 gauze pad that was placed into a sterile container containing 10 mL PBS and mixed in a vortex mixer for 1 min to elute the bacteriophage. Aliquots of each elutant were serially diluted and cultured to quantify virus particles. |
|
| Notes | Location: USA; funding was provided by the Department of Veteran Affairs; 1 author, C.J.D. had previously received research grants from Clorox, Merck, AvidBiotics and GOJO and the same author also served on scientific advisory boards for 3M and Seres Health. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Healthcare personnel were randomized to perform simulations of
contaminated glove removal" We asked study authors for method of generation |
| Allocation concealment (selection bias) | Unclear risk | Unclear if irrevocable |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded but objectively measured outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on missing data provided |
| Selective reporting (reporting bias) | Unclear risk | No protocol published |
| Other bias | Low risk | No other biases detected |
Wong 2004.
| Study characteristics | ||
| Methods | Randomised, multiple‐arm, parallel‐group, simulation study | |
| Participants | Nursing students volunteering; N = 100 nursing students who had given written consent, 82% female, age 21 ± 1.2 years, 60% completed > 1 study year, all had been taught PPE use, none had been involved with SARS patients | |
| Interventions |
Intervention: different types of PPE compared 10 different brands and types of PPE at the time of the study in use in Hong Kong hospitals; 1 type was a surgical gown and 1 the brand Barrierman, probably Tyvek by DuPont, the others were denoted as White A, White, Green, Y‐HR‐9, Yellow, Blue, Blue‐9, B‐NHK‐9, B‐HR‐9. These were categorised into 4 categories: A: good water repellency and penetration resistance but poor air permeability; B good water repellency and air permeability but poor water penetration resistance; C: surgical gown with poor water repellency and penetration resistance and fair air permeability; D Barrierman, with good water repellency, poor air permeability and fair water penetration resistance. Types A,B, C, and D were compared against each other |
|
| Outcomes | 1. Usability rated by the users as the mean of 5‐point scales for:
instructions, comfort, ease of donning and doffing, and satisfaction 2. Donning and doffing time/durations in min 3. Contamination after spraying fluorescent marker on the trunk and doffing of PPE, measured as mean number of contaminated spots that light up in UV‐light |
|
| Notes | Hong Kong, China; funded by Hong Kong Infection Control Nurses’ Association, Hong Kong Polytechnic University; no conflict of interest is reported in the article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participantss were allocated a PPE using a random table page 91 |
| Allocation concealment (selection bias) | Unclear risk | Not reported and information asked from study authors did not lead to a higher confidence in allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not blinded; page 91 and discussion page 95 indicates that they knew what they were wearing, obviously, as PPE Type D was a 1‐piece construct, and they were asked to read manual for wearing. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported if any data were missing |
| Selective reporting (reporting bias) | Unclear risk | Apparently all data reported |
| Other bias | Low risk | No indication of other bias |
Zamora 2006.
| Study characteristics | ||
| Methods | Randomised, 2‐arm, cross‐over, simulation study | |
| Participants | Clincians from Queen's Hospital, Kingston, ON, Canada volunteering to
participate. N = 50; PAPR‐first N = 27, age 34.3 ± 8.7 years, height 171.8 ± 8.1, weight 76.3 ± 16.7, male 16/27, anaesthetists 19/27, prior PAPR training 15/27 Enhanced respiratory and contact precautions (E‐RCP) first N = 23, age 36.8 ± 9.8, height 172.3 ± 7.6, male 11/23, anaesthetist 10/23, prior PAPR training 18/23 Location: Canada |
|
| Interventions |
Intervention: different types of PPE compared: PAPR versus mask PPE with PAPR, consisting of Tyvek hood (3M), Bouffant hair cover, Spartan economy impact goggle, 3M air‐mate breathing tube, 3M HEPA filter unit, N95 mask, 3 pairs of gloves, Tyvek coverall with hood, 2 Tyvek boot covers, Astound impervious surgical gown. Doffing order: first gloves, turbo unit hose, hood, gown, second gloves, belt and battery, shoe covers, third gloves, wash hands, new gloves, coverall, second shoe covers, gloves, new gloves, goggles, hair cover, gloves, wash hands, new mask. Comparison: E‐RCP consisting of Bouffant hair cover, Spartan economy impact goggle, face shield (Splash shield), N95 mask, 2 pairs of gloves, Astound impervious gown. Doffing order: outer gloves, gown, inner gloves, wash hands, new gloves, face shield, hair cover, goggles, mask, gloves, wash hands. |
|
| Outcomes | 1. Number of participants with presence of contamination on base layer of
clothes or skin. Contamination measured with fluorescein solution (5 mL in
front of face shield and torso) plus invisible detection paste on forearms
and palms of the hands; assessment after removing of outer layer by
unblinded assessor with UV lamp; blinded evaluator then inspected all skin
and clothes and measured area of contamination. Secondary outcomes were:
contamination of inner layers of PAPR system, area size of contamination,
number of donning or doffing violations; time required for donning and
doffing. 2. Number of participants with donning or removal violation was defined as out of sequence removal, touching or tearing item of clothing, touching body part before hand washing. Used the Mainland‐Gart test for the analysis of cross‐over studies |
|
| Notes | Funding: Physicians' Services Incorporated Foundation and Clinical Teachers' Association of Queen's University; no Conflict of Interest declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomised by coin tossing |
| Allocation concealment (selection bias) | Unclear risk | Once started, order was known, but unclear if participants could still change groups and if there would be an interest to do so. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants knew attire |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Evaluators blind for attire |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Apparently all data collected and usable |
| Selective reporting (reporting bias) | Unclear risk | Apparently all outcomes reported |
| Other bias | Low risk | No indication of other bias |
CDC: Center for Disease Control and Prevention; CFU: colony‐forming unit; ECDC: European Centre for Disease Prevention and Control; EMT: emergency medical technician; EVD: Ebola virus disease; HCW: healthcare worker; IgG: immunoglobulin G; IQR: interquartile range; LED: light‐emitting diode; MD: Doctor of Medicine; MPN: most probable number; MSF: Médecins Sans Frontières; n/a: not applicable; PAPR: powered, air‐purifying respirator; PFU: plaque‐forming unit; PLS: polystyrene latex beads; PPE: personal protection equipment; RCT: randomised controlled trial; RN: Registered Nurse; SARS: severe acute respiratory syndrome; SD: standard deviation; UV: ultraviolet WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abrahamson 2006 | Uncontrolled study; 1 type of training only |
| Abualenain 2018 | No comparison group |
| Alraddadi 2016 | No comparison group |
| Anderson 2017 | No highly infectious disease exposure |
| Beam 2011 | No control group with an active intervention |
| Beam 2014 | Uncontrolled study; only 1 type of training in donning and doffing studied with video recordings |
| Beam 2016a | Not an empirical study |
| Beam 2016b | Not an empirical study |
| Bearman 2007 | Trial of universal gloving, not as part of full‐body PPE |
| Bischoff 2019 | No highly infectious disease exposure |
| Borchert 2007 | Description of use of PPE in MHF outbreak, not a case‐control or cohort study |
| Bosc 2016 | Wrong comparator |
| Buianov 1991 | Study compares 2 types of PPE for highly infectious diseases but does not measure contamination or infection as outcome, only physiological parameters (native speaker assessment AP) |
| Butt 2016 | Wrong comparator |
| Casanova 2008 | Not a comparative study; only studied 1 method of doffing |
| Casanova 2018 | No comparison group |
| Castle 2009 | Outcome only performance with PPE and not infection rate or adherence |
| Chandramohan 2018 | No comparison group |
| Christian 2004 | Investigation of cluster of SARS infected HCW; not a case‐control or cohort study |
| Chughtai 2013 | Overview focusing on mask use only, not part of full‐body PPE |
| Clay 2015 | Simulation study; military HCWs; no control group |
| Coates 2000 | Outcome performance only not infection rates or adherence |
| Coca 2015 | Wrong type of participants, thermal manikin study |
| Coca 2017 | Secondary outcomes only |
| Colebunders 2004 | Description of MHF outbreak; not a case‐control or cohort study |
| Cooper 2005 | Simulation study, but of facial protection only, no full‐body PPE involved |
| Delaney 2016 | No comparison group |
| Doll 2017a | No comparison group |
| Doll 2017b | No comparison group |
| Doshi 2016 | No comparison group |
| Drew 2016 | No comparison group |
| DuBose 2018 | Wrong study design |
| Dunn 2015 | Case study of spread of infection in 1 hospital; used in discussion section |
| Elcin 2016 | No comparison group |
| Fischer 2015 | Not a primary study, literature review |
| Fogel 2017 | Wrong study design |
| Foote 2017 | Wrong intervention |
| Franklin 2016 | Not an empirical study |
| Garibaldi 2019 | Secondary outcomes only |
| Gozel 2013 | Description of use of PPE among HCW exposed to CCHF; not case‐control or cohort study |
| Grélot 2015 | Measurement of thermal strain, no infection or contamination or compliance measured |
| Grélot 2016 | Measurement of thermal strain, no infection or contamination or compliance measured |
| Hendler 2000 | PPE versus no PPE; outcome performance only |
| Herlihey 2016 | No comparison group |
| Herlihey 2017 | No comparison group |
| Hersi 2015 | Not a primary study, rapid review |
| Ho 2003 | Descriptive study of SARS outbreak and HCWs use of PPE; not a case‐control or cohort study |
| Ho 2004 | Compares consistent versus inconsistent use of PPE, not 2 different types |
| Hon 2008 | Evaluation of on‐line PPE training; uncontrolled study, no comparison training |
| Hormbrey 1996 | Description of introduction of new clothing; no infection or adherence outcome |
| Huh 2020 | No comparison group |
| Jacob 2018 | Not empirical study |
| Jaffe 2019 | No personal contamination outcome |
| Jaques 2016 | Report contains no data |
| Jeffs 2007 | Description of control of MHF outbreak; not a case‐control or cohort study |
| Jinadatha 2015 | Wrong type of participants, investigation of disinfection on different PPE fabrics and components |
| Jones 2020 | Not empirical study |
| Kahveci 2019 | Participants not HCWs |
| Kang 2017 | Wrong study design |
| Kang 2017a | No comparison group |
| Kappes Ramirez 2018 | Wrong outcomes |
| Keane 1977 | Description of risk of HCW only; no evaluation of PPE safety |
| Kerstiens 1999 | Desription of Ebola outbreak; not case‐control or cohort study |
| Kilinc‐Balci 2016 | Not an empirical study |
| Kilinc‐Balci 2015 | Report contains no data |
| Kim 2015 | No control group, HCWs infected with MERS CoV |
| Ko 2004 | Description of risk of EMT staff; no evaluation of PPE safety |
| Kogutt 2019 | No comparison group |
| Kratz 2017 | Report contains no data |
| Kwon 2016 | No comparison group |
| Kwon 2017 | No comparison group |
| Lai 2005 | Study of SARS IgG prevalence in HCWs who did not become sick, no PPE use measured |
| Lai 2011 | No personal contamination measured only environmental contamination |
| Lange 2005 | Letter to the editor; not primary study |
| Lau 2004 | Compares consistent versus inconsistent use of PPE, not 2 different types |
| Le 2004 | Compares consistent versus inconsistent use of PPE, not 2 different types |
| Lee 2017 | Report contains no data |
| Lindsley 2012 | Test respiratory protection only; not part of full‐body PPE |
| Lindsley 2014 | Tests respiratory protection only; not part of full‐body PPE |
| Liu 2009 | Compares consistent versus inconsistent use of PPE, not 2 different types |
| Loeb 2004 | Compares consistent versus inconsistent use of PPE, not 2 different types |
| Low 2005 | A review of SARS and HCW; not a primary study |
| Lowe 2014 | Description of PPE use only; no adherence or infection outcomes |
| Lu 2006 | Comparison of viral load in patients infected outside and inside hospital; comparison is with no PPE |
| Lu 2020 | No Intervention |
| Luo 2011 | Simulation study of 1 Tyvek® (duPont) suit only, no comparison suit or no comparison doffing method |
| Ma 2004 | Retrospective case‐control study about PPE for SARS, compares consistent versus inconsistent use not 2 types |
| Makovicka 2018 | No highly infectious disease exposure |
| Malik 2006 | Participants not exposed to highly infectious diseases |
| Marklund 2002 | Description of Ebola patient transportation; not an intervention study |
| Matanock 2014 | Description of risk of infection of HCW compared to general population; no evaluation of PPE |
| McLaws 2016 | Not an empirical study |
| Mehtar 2015 | No control group, 2 infection prevention and control training courses |
| Minnich 2003 | Description of ambulance adaptation for transport of highly infected patients; not evaluation or intervention study |
| Mollura 2015 | Review; EVD within radiology wards and on imaging equipment |
| Moore 2005 | Review not intervention study |
| Morgan 2009 | Review of adverse effects of contact precautions |
| Mumma 2018 | No comparison group |
| Mumma 2019 | No Intervention |
| Muyembe‐Tamfum 1999 | Description of Ebola outbreak; not case‐control or cohort study |
| Nikiforuk 2017 | Wrong patient population |
| Nishiura 2005 | Compares consistent versus inconsistent use of PPE, not 2 different types |
| Northington 2007 | No comparison group; only 1 type of education with follow‐up |
| Novosad 2016 | No comparison group |
| Nyenswah 2015 | Case study of EVD cluster including HCWs, but insufficient information on PPE to draw any conclusions |
| Ofner 2003 | SARS case series only; no healthy controls; not case control or cohort study |
| Ofner‐Agostini 2006 | SARS case series only; no healthy controls; not case control or cohort study |
| Ogendo 2008 | Eye protection only; not part of full‐body PPE |
| Ong 2013 | No exposure to highly infectious diseases |
| Park 2004 | Compares consistent versus inconsistent use of PPE, not 2 different types |
| Parveen 2018 | No comparison group |
| Pei 2006 | Compares consistent versus inconsistent use of PPE, not 2 different types |
| Phan 2018 | Wrong intervention |
| Phrampus 2016 | No comparison group |
| Porteous 2018 | No personal contamination outcome |
| Quinn 2018 | Wrong outcomes |
| Ragazzoni 2015 | No control group, virtual reality simulation training study |
| Ransjo 1979 | No exposure to highly infectious diseases |
| Reynolds 2006 | Case‐control study evaluating SARS risk in HCWs in Vietnam but no inclusion of PPE use |
| Rosenberg 2016 | Report of publication of Tomas 2015 |
| Russell 2015 | No control group, no outcome, before/after summary card |
| Scales 2003 | Compares consistent versus inconsistent use of PPE, not 2 different types |
| Schumacher 2010 | Comparison is no PPE; outcome is performance time only |
| Scott Taylor 2017 | Wrong outcomes |
| Seto 2003 | Compares consistent versus inconsistent use of PPE, not 2 different types |
| Shao 2015 | Not a primary study, Chinese review |
| Sorensen 2008 | No exposure to highly infectious diseases |
| Su 2017 | No comparison group |
| Suen 2017 | No highly infectious disease exposure |
| Tartari 2015 | No control group, infection control readiness checklist (from 45 countries), no outcome |
| Teleman 2004 | Compares consistent versus inconsistent use of PPE, not 2 different types |
| Tomas 2015 | No comparison used only description of contamination in a simulation study |
| Tomas 2016a | Wrong intervention |
| Torres 2015 | Not a primary study, literature review |
| Visnovsky 2019 | No personal contamination outcome |
| Weber 2018 | No comparison group |
| Weber 2019 | No Intervention |
| West 2014 | Not a primary study but a commentary |
| Williams 2019 | No comparison group |
| Xi 2016 | No comparison group |
| Yin 2004 | Case‐control study of use of PPE for SARS, not comparing 2 different types of PPE |
| Yuan 2018 | Not empirical study |
| Zellmer 2015 | No control group, checklist for removing PPE |
| Zhou 2003 | Follow‐up of HCWs exposed to SARS and their PPE and protection measures, not comparative study |
CCHF: Crimean‐Congo haemorrhagic fever; EMT: emergency medical technician; EVD: Ebola virsu disease; HCW: healthcare worker; IgG: immunoglobulin G; MERS CoV; Middle East respiratory syndrome coronavirus; MHF: Marburg haemorrhagic fever; PPE: personal protective equipment; SARS: severe acute respiratory syndrome
Characteristics of ongoing studies [ordered by study ID]
ChiCTR2000029900.
| Study name | Renmin Hospital of Wuhan University |
| Methods | Unclear |
| Participants | HCWs exposed to COVID‐19 |
| Interventions | Infection and prevention strategies |
| Outcomes | Infection |
| Starting date | Not reported |
| Contact information | Chinese trial register |
| Notes |
ChiCTR2000030317.
| Study name | West China Hospital of Sichuan University |
| Methods | RCT |
| Participants | HCWs exposed to COVID‐19 |
| Interventions | Self‐made mask |
| Outcomes | Infection rate |
| Starting date | Not reported |
| Contact information | Chinese trials register |
| Notes |
ChiCTR2000030834.
| Study name | Tongji Hospital Tongji Medical College Huazhong University Wuhan China ‐a |
| Methods | Unclear |
| Participants | HCWs exposed to COVID‐19 |
| Interventions | Infection prevention and control |
| Outcomes | Infection |
| Starting date | Not reported |
| Contact information | Chinese trials register |
| Notes |
ChiCTR2000030895.
| Study name | Tongji Hospital Tongji Medical College Huazhong University Wuhan China ‐b |
| Methods | Not reported |
| Participants | HCWs |
| Interventions | Infection prevention and control |
| Outcomes | Infection |
| Starting date | Not reported |
| Contact information | Chinese trials register |
| Notes |
HCW: healthcare worker
Differences between protocol and review
We changed the title from 'Personal protective equipment for preventing highly infectious diseases due to contact with contaminated body fluids in health care staff' to 'Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff' to avoid confusion with the term 'contact precautions'.
We replaced the statement in the methods section: "We will also include audit reports or case reports of PPE failure in which there are no comparisons. We will not use these for drawing conclusions but only to compare with findings produced by the above study types. For audit reports, we will examine any reports of failed PPE or audits of health care staff being infected or contaminated" with "We intended to also include uncontrolled audit reports or case reports of PPE failure for descriptive purposes, but we did not find any. If we find any such reports in future updates of this review, we will not use them for drawing conclusions, but only to compare with findings produced by the above study types".
We added the following definition of PPE in the methods section because it was lacking: "We defined PPE as any of the above equipment designed or intended to protect healthcare staff from contamination with body fluids".
We added an extra outcome "Time to don and doff the PPE" because we stated in our protocol that we would add outcomes that we had not defined in advance and that we considered important.
We added a more detailed description of the specific resources that we searched in addition to the electronic databases, that is, the specific non‐governmental organisations (Médicins Sans Frontierès and Save the Children), and specific manufacturers (DuPont, 3M, and Alpha Pro Tech). We could not foresee in advance which parties we would be contacting.
When using the GRADE considerations to assess the certainty of the evidence, for non‐randomised studies, we started at the 'low‐certainty' level, rather than the 'moderate‐certainty' level outlined in the protocol, as per the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2017).
Contributions of authors
Conceiving the protocol: JV, SI, CT, JR, KN
Designing the protocol: JV, CT, JR, KN, ME, EM, RS
Coordinating the protocol and the review: JV, SI
Designing search strategies: KN
Data extraction: JV, BR, SI, CT, RS, BB, ET
Data analysis: JV
Writing the protocol and the review: JV, BR, FSKB, BB, ET
Providing general advice on the protocol and review: RS, FSKB
Sources of support
Internal sources
-
Cochrane Collaboration, UK
Bursary to Sharea Ijaz
-
Finnish Institute of Occupational Health, Finland
Salary for Jos Verbeek, Christina Mischke, Jani Ruotsalainen, Erja Mäkelä and Kaisa Neuvonen
-
National Institute for Occupational Safety and Health, USA
Salary for F Selcen Kilinc Balci
External sources
No sources of support supplied
Declarations of interest
Jos Verbeek: none known
Blair Rajamaki: none known
Sharea Ijaz: none known
Christina Mischke: none known
Jani Ruotsalainen: none known
F Selcen Kilinc Balci: none known
Riitta Sauni: none known
Bronagh Blackwood: none known
Elaine Toomley: none known
Edited (no change to conclusions)
References
References to studies included in this review
Andonian 2019 {published data only}
- Andonian J, Kazi S, Therkorn J, Benishek L, Billman C, Schiffhauer M, et al. Effect of an intervention package and teamwork training to prevent healthcare personnel self-contamination during personal protective equipment doffing. Clinical Infectious Diseases 2019; 69 Suppl 3:S248-S255. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bell 2015 {published data only}
- Bell T, Smoot J, Patterson JE, Smalligan R, Jordan R. Ebola virus disease: the use of fluorescents as markers of contamination for personal protective equipment. ID Cases 2015; 2:27-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buianov 2004 {published data only}
- Buianov VV, Kolesnikov NV, Malyshev NA, Suprun IP. Use of new individual protection substances in Mel'tser boxes. Vestnik Rossiiskoi Akademii Meditsinskikh Nauk / Rossiiskaia Akademiia Meditsinskikh Nauk 2004; 1:30-5. [PubMed] [Google Scholar]
Casalino 2015 {published data only}
- Casalino E, Astocondor E, Sanchez JC, Diaz-Santana DE, Del Aquila C, Carrillo JP. Personal protective equipment for the Ebola virus disease: a comparison of 2 training programs. American Journal of Infection Control 2015; 43(12):1281-7. [DOI] [PubMed] [Google Scholar]
Casanova 2012 {published data only}
- Casanova LM, Rutala WA, Weber DJ, Sobsey MD. Effect of single- versus double-gloving on virus transfer to health care workers' skin and clothing during removal of personal protective equipment. American Journal of Infection Control 2012; 40(4):369-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Casanova 2016 {published data only}
- Casanova LM, Teal LJ, Sickbert-Bennett EE, Anderson DJ, Sexton DJ, Rutala WA, et al. Assessment of self-contamination during removal of personal protective equipment for Ebola patient care. Infection Control and Hospital Epidemiology Oct 2016;37(10):1156-61. [DOI] [PubMed] [Google Scholar]
Chughtai 2018 {published data only}
- Chughtai AA, Chen X, Macintyre CR. Risk of self-contamination during doffing of personal protective equipment. American Journal of Infection Control 2018; 46(12):1329-34. [DOI: 10.1016/j.ajic.2018.06.003] [DOI] [PubMed] [Google Scholar]
Curtis 2018 {published data only}
- Curtis HA, Trang K, Chason KW, Biddinger PD. Video-based learning vs traditional lecture for instructing emergency medicine residents in disaster medicine principles of mass triage, decontamination, and personal protective equipment. Prehospital and Disaster Medicine 2018; 33(1):7-12. [DOI] [PubMed] [Google Scholar]
Drews 2019 {published data only}
- Drews FA, Mulvey D, Stratford K, Samore MH, Mayer J. Evaluation of a redesigned personal protective equipment gown. Clinical Infectious Diseases 2019; 69 Suppl 3:S199-205. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gleser 2018 {published data only}
- Gleser M, Schwab F, Solbach P, Vonberg RP. Modified gloves: a chance for the prevention of nosocomial infections. American Journal of Infection Control 2018; 46(3):266-9. [DOI] [PubMed] [Google Scholar]
Guo 2014 {published data only}
- Guo YP, Li Y, Wong PLH. Environment and body contamination: a comparison of two different removal methods in three types of personal protective clothing. American Journal of Infection Control 2014; 42(4):e39-e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hajar 2019 {published data only}
- Hajar Z, Mana TS, Tomas ME, Alhmidi H, Wilson BM, Donskey CJ. A crossover trial comparing contamination of healthcare personnel during removal of a standard gown versus a modified gown with increased skin coverage at the hands and wrists. Infection Control and Hospital Epidemiology 2019; 40(11):1278-80. [DOI: 10.1017/ice.2019.211] [DOI] [PubMed] [Google Scholar]
Hall 2018 {published data only}
- Hall S, Poller B, Bailey C, Gregory S, Clark R, Roberts P, et al. Use of ultraviolet-fluorescence-based simulation in evaluation of personal protective equipment worn for first assessment and care of a patient with suspected high-consequence infectious disease. Journal of Hospital Infection 2018; 99(2):218-28. [DOI] [PubMed] [Google Scholar]
Houlihan 2017 {published data only}
- Houlihan CF, McGowan CR, Dicks S, Baguelin M, Moore DA, Mabey D, et al. Ebola exposure, illness experience and Ebola antibody prevelence in international responders to the West African Ebola epidemic 2014-2016: a cross sectional study. PLoS Medicine 2017; 14(4):e1002300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hung 2015 {published data only}
- Hung PP, Choi KS, Chiang VC. Using interactive computer simulation for teaching the proper use of personal protective equipment. CIN: Computers, Informatics, Nursing 2015; 33(2):49-57. [DOI] [PubMed] [Google Scholar]
Kpadeh Rogers 2019 {published data only}
- Kpadeh-Rogers Z, Robinson GL, Alserehi H, Morgan DJ, Harris AD, Herrera NB, et al. Effect of glove decontamination on bacterial contamination of healthcare personnel hands. Clinical Infectious Diseases 2019; 69:S224-S227. [DOI: 10.1093/cid/ciz615] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson G, Kpadeh ZZ, Alserehi H, Morgan D, Harris AD, Johnson JK, et al. Effect of glove disinfection on bacterial contamination of healthcare worker hands. Open Forum Infectious Diseases 2018; 5 Suppl 1:S56. [Google Scholar]
Mana 2018 {published data only}
- Mana TS, Tomas ME, Cadnum JL, Jencson AL, Piedrahita CT, Donskey CJ. A randomized trial of two cover gowns comparing contamination of healthcare personnel during removal of personal protective equipment. Infection Control and Hospital Epidemiology 2018; 39(1):97-100. [DOI] [PubMed] [Google Scholar]
Osei‐Bonsu 2019 {published data only}
- NCT03192553. Alternative doffing strategies to prevent healthcare worker self-contamination when using personal protective equipment (PPE). NCT03192553 2017.
- Osei-Bonsu K, Masroor N, Cooper K, Doern C, Jefferson KK, Major Y, et al. Alternative doffing strategies of personal protective equipment to prevent self-contamination in the health care setting. American Journal of Infection Control 2019; 47(5):534-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Shigayeva 2007 {published data only}
- Shigayeva A, Green K, Raboud JM, Henry B, Simor AE, Vearncombe M, et al. Factors associated with critical-care healthcare workers' adherence to recommended barrier precautions during the Toronto severe acute respiratory syndrome outbreak. Infection Control 2007; 28(11):1275-83. [DOI] [PubMed] [Google Scholar]
Strauch 2016 {published data only}
- Strauch AL, Brady TM, Niezgoda G, Almaguer CM, Shaffer RE, Fisher EM. Assessing the efficacy of tabs on filtering facepiece respirator straps to increase proper doffing techniques while reducing contact transmission of pathogens. Journal of Occupational and Environmental Hygiene 2016; 13(10):794-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Suen 2018 {published data only}
- Suen LK, Guo YP, Tong DW, Leung PH, Lung D, Ng MS, et al. Self-contamination during doffing of personal protective equipment by healthcare workers to prevent Ebola transmission. Antimicrobial Resistance and Infection Control 2018; 7:157. [DOI: 10.1186/s13756-018-0433-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tomas 2016 {published data only}
- Tomas ME, Cadnum JL, Mana TS, Jencson AL, Donskey CJ. Seamless suits: reducing personnel contamination through improved personal protective equipment design. Infection Control and Hospital Epidemiology 2016; 37(6):742-4. [DOI] [PubMed] [Google Scholar]
Wong 2004 {published data only}
- Wong TK, Chung JW, Li Y, Chan WF, Ching PT, Lam CH, et al. Effective personal protective clothing for health care workers attending patients with severe acute respiratory syndrome. American Journal of Infection Control 2004; 32(2):90-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zamora 2006 {published data only}
- Zamora JE, Murdoch J, Simchison B, Day AG. Contamination: a comparison of 2 personal protective systems. Canadian Medical Association Journal 2006; 175(3):249-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abrahamson 2006 {published data only}
- Abrahamson SD, Canzian S, Brunet F. Using simulation for training and to change protocol during the outbreak of severe acute respiratory syndrome. Critical Care 2006; 10(1):1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Abualenain 2018 {published data only}
- Abualenain JT, Al-Alawi MM. Simulation-based training in Ebola personal protective equipment for healthcare workers: experience from King Abdulaziz University Hospital in Saudi Arabia. Journal of Infection and Public Health 2018; 11(6):796-800. [DOI: ] [DOI] [PubMed] [Google Scholar]
Alraddadi 2016 {published data only}
- Alraddadi BM, Al-Salmi HS, Jacobs-Slifka K, Slayton RB, Estivariz CF, Geller AI, et al. Risk factors for Middle East respiratory syndrome coronavirus infection among healthcare personnel. Emerging Infectious Diseases 2016; 22(11):1915-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2017 {published data only}
- Anderson DJ, Addison R, Lokhnygina Y, Warren B, Sharma-Kuinkel B, Rojas LJ, et al, CDC Prevention Epicenters Program. The Antimicrobial Scrub Contamination and Transmission (ASCOT) Trial: a three-arm, blinded, randomized controlled trial with crossover design to determine the efficacy of antimicrobial-impregnated scrubs in preventing healthcare provider contamination. Infection Control and Hospital Epidemiology 2017; 38(10):1147-54. [DOI: ] [DOI] [PubMed] [Google Scholar]
Beam 2011 {published data only}
- Beam EL, Gibbs SG, Boulter KC, Beckerdite ME, Smith PW. A method for evaluating health care workers' personal protective equipment technique. American Journal of Infection Control 2011; 39(5):415-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beam 2014 {published data only}
- Beam EL, Gibbs SG, Hewlett AL, Iwen PC, Nuss SL, Smith PW. Method for investigating nursing behaviors related to isolation care. American Journal of Infection Control 2014; 42(11):1152-6. [DOI] [PubMed] [Google Scholar]
Beam 2016a {published data only}
- Beam EL, Schwedhelm S, Boulter K, Kratochvil C, Lowe J, Hewlett A, et al. Personal protective equipment processes and rationale for the Nebraska Biocontainment Unit during the 2014 activations for Ebola virus disease. American Journal of Infection Control 2016; 44(3):340-2. [DOI] [PubMed] [Google Scholar]
Beam 2016b {published data only}
- Beam EL. Call for improvement in personal protective equipment guidance and research. American Journal of Infection Control 2016; 44(11):1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bearman 2007 {published data only}
- Bearman GM, Marra AR, Sessler CN, Smith WR, Rosato A, Laplante JK, et al. A controlled trial of universal gloving versus contact precautions for preventing the transmission of multidrug-resistant organisms. American Journal of Infection Control 2007; 35(10):650-5. [DOI] [PubMed] [Google Scholar]
Bischoff 2019 {published data only}
- Bischoff WE, Turner J, Russell G, Blevins M, Missaiel E, Stehle J. How well do N95 respirators protect healthcare providers against aerosolized influenza virus? Infection Control and Hospital Epidemiology 2019; 40(2):232-4. [DOI: 10.1017/ice.2018.326] [DOI] [PubMed] [Google Scholar]
Borchert 2007 {published data only}
- Borchert M, Mulangu S, Lefevre P, Tshomba A, Libande ML, Kulidri A, et al. Use of protective gear and the occurrence of occupational Marburg hemorrhagic fever in health workers from Watsa health zone, Democratic Republic of the Congo. Journal of Infectious Diseases 2007; 196 Suppl 2:S168-75. [DOI] [PubMed] [Google Scholar]
Bosc 2016 {published data only}
- Bosc J, Sanchez O, Carrie C, Revel P, Tentillier E, Biais M, et al. Impact of the personal protective equipment for Ebola virus disease on vascular access skills and the performance of airway: a manikin study. Annales Francaises de Medecine d'Urgence 2016; 6(3):172-8. [Google Scholar]
Buianov 1991 {published data only}
- Buianov VV, Elkin EN, Kolmykov VG, Kaplunov IuV, Malyshev NA, Kelli EI, et al. Use of personal protective equipment by pathologists and legal physicians working with particularly dangerous infectious diseases. Arkhiv Patologii 1991; 53(3):59-61. [PubMed] [Google Scholar]
Butt 2016 {published data only}
- Butt TS, Koutlakis-Barron I, AlJumaah S, Al Thawadi S, Al Mofada S. Infection control and prevention practices implemented to reduce transmission risk of Middle East respiratory syndrome-coronavirus in a tertiary care institution in Saudi Arabia. American Journal of Infection Control 2016; 44(5):605-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Casanova 2008 {published data only}
- Casanova L, Alfano-Sobsey E, Rutala WA, Weber DJ, Sobsey M. Virus transfer from personal protective equipment to healthcare employees' skin and clothing. Emerging Infectious Diseases 2008 2008; 14(8):1291-3. [DOI: 10.3201/eid1408.080085] [DOI] [PMC free article] [PubMed] [Google Scholar]
Casanova 2018 {published data only}
- Casanova LM, Erukunuakpor K, Kraft CS, Mumma JM, Durso FT, Ferguson AN, et al, Centers for Disease Control and Prevention Epicenters Program, Division of Healthcare Quality Promotion. Assessing viral transfer during doffing of Ebola-level personal protective equipment in a biocontainment unit. Clinical Infectious Diseases 2018; 66(6):945-9. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Castle 2009 {published data only}
- Castle N, Owen R, Hann M, Clark S, Reeves D, Gurney I. Impact of chemical, biological, radiation, and nuclear personal protective equipment on the performance of low- and high-dexterity airway and vascular access skills. Resuscitation 2009; 80(11):1290-5. [DOI] [PubMed] [Google Scholar]
Chandramohan 2018 {published data only}
- Chandramohan S, Krishna A, Navalkele B, Virdi P, Gill A, Javed I, et al. "All eyes on you": a covert observational study on contact precaution compliance in six hospitals at the Detroit Medical Center. Open Forum Infectious Diseases 2018; 5 Suppl 1:S173-S174. [DOI: ] [Google Scholar]
Christian 2004 {published data only}
- Christian MD, Loutfy M, McDonald LC, Martinez KF, Ofner M, Wong T, et al. Possible SARS coronavirus transmission during cardiopulmonary resuscitation. Emerging Infectious Diseases 2004; 10(2):287-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chughtai 2013 {published data only}
- Chughtai AA, Seale H, MacIntyre CR. Availability, consistency and evidence-base of policies and guidelines on the use of mask and respirator to protect hospital health care workers: a global analysis. BMC Research Notes 2013; 6(216):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clay 2015 {published data only}
- Clay KA, O'Shea MK, Fletcher T, Moore AJ, Burns DS, Craig D, et al. Use of an ultraviolet tracer in simulation training for the clinical management of Ebola virus disease. Journal of Hospital Infection 2015; 91(3):275-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Coates 2000 {published data only}
- Coates MJ, Jundi AS, James MR. Chemical protective clothing; a study into the ability of staff to perform lifesaving procedures. Journal of Accident & Emergency Medicine 2000; 17(2):115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coca 2015 {published data only}
- Coca A, DiLeo T, Kim JH, Roberge R, Shaffer R. Baseline evaluation with a sweating thermal manikin of personal protective ensembles recommended for use in West Africa. Disaster Medicine and Public Health Preparedness 2015; 9(5):536-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Coca 2017 {published data only}
- Coca A, Quinn T, Kim J-H, Wu T, Powell J, Roberge R, et al. Physiological evaluation of personal protective ensembles recommended for use in West Africa. Disaster Medicine and Public Health Preparedness 2017; 11(5):580-6. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Colebunders 2004 {published data only}
- Colebunders R, Sleurs H, Pirard P, Borchert M, Libande M, Mustin JP, et al. Organisation of health care during an outbreak of Marburg haemorrhagic fever in the Democratic Republic of Congo, 1999. Journal of Infection 2004; 48(4):347-53. [DOI] [PubMed] [Google Scholar]
Cooper 2005 {published data only}
- Cooper DM, Charles D, Durnell AJ, Anderson JM, Kern T, Self T. Assessment of personal protective equipment used for facial mucocutaneous exposure protection in nonhuman primate areas. Lab Animal 2005; 34(5):49-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
Delaney 2016 {published data only}
- Delaney HM, Lucero PF, Maves RC, Lawler JV, Maddry JK, Biever KA, et al. Ebola virus disease simulation case series: Patient with Ebola virus disease in the prodromal phase of illness (scenario 1), the "wet" gastrointestinal phase of illness (scenario 2), and the late, critically ill phase of disease (scenario 3). Simulation in Healthcare 2016; 11(2):106-16. [DOI] [PubMed] [Google Scholar]
Doll 2017a {published data only}
- Doll M, Feldman M, Hartigan S, Sanogo K, Stevens M, McReynolds M, et al. Acceptability and necessity of training for optimal personal protective equipment use. Infection Control and Hospital Epidemiology 2017; 38(2):226-9. [DOI] [PubMed] [Google Scholar]
Doll 2017b {published data only}
- Doll M, Feldman M, Hartigan S, Sanogo K, Stevens M, McReynolds M, et al. Acceptability and necessity of training for optimal personal protective equipment use. Infection Control and Hospital Epidemiology 2017; 38(2):226-9. [DOI: ] [DOI] [PubMed] [Google Scholar]
Doshi 2016 {published data only}
- Doshi RH, Hoff NA, Mukadi P, Mwanza A, Mukadi D, Wasiswa J, et al. Seroprevalence of Ebola virus among health care workers in the Tshuapa district, Democratic Republic of the Congo. American Journal of Tropical Medicine and Hygiene 2016; 95(5):50. [Google Scholar]
Drew 2016 {published data only}
- Drew JL, Turner J, Mugele J, Hasty G, Duncan T, Zaiser R, et al. Beating the spread: developing a simulation analog for contagious body fluids. Simulation in Healthcare 2016; 11(2):100-5. [DOI] [PubMed] [Google Scholar]
DuBose 2018 {published data only}
- DuBose JR, Matic Z, Sala MF, Mumma JM, Kraft CS, Casanova LM, et al, CDC Prevention Epicenters Program. Design strategies to improve healthcare worker safety in biocontainment units: learning from Ebola preparedness. Infection Control and Hospital Epidemiology 2018; 39(8):961-7. [DOI: ] [DOI] [PubMed] [Google Scholar]
Dunn 2015 {published data only}
- Dunn AC, Walker TA, Redd J, Sugerman D, McFadden J, Singh T, et al. Nosocomial transmission of Ebola virus disease on pediatric and maternity wards: Bombali and Tonkolili, Sierra Leone, 2014. American Journal of Infection Control 2015; 44(3):269-72. [DOI: 10.1016/j.ajic.2015.09.016] [PMID: ] [DOI] [PubMed] [Google Scholar]
Elcin 2016 {published data only}
- Elcin M, Onan A, Odabasi O, Saylam M, Ilhan H, Daylan Kockaya P, et al. Developing a simulation-based training program for the prehospital professionals and students on the management of Middle East respiratory syndrome. Simulation in Healthcare 2016; 11(6):394-403. [DOI] [PubMed] [Google Scholar]
Fischer 2015 {published data only}
- Fischer WA 2nd, Weber DJ, Wohl DA. Personal protective equipment: protecting health care providers in an Ebola outbreak. Clinical Therapeutics 2015; 37(11):2402-10. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fogel 2017 {published data only}
- Fogel I, David O, Balik CH, Eisenkraft A, Poles L, Shental O, et al. The association between self-perceived proficiency of personal protective equipment and objective performance: An observational study during a bioterrorism simulation drill. American Journal of Infection Control 2017; 45(11):1238-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Foote 2017 {published data only}
- Foote MM, Styles TS, Quinn CL. Assessment of hospital emergency department response to potentially infectious diseases using unannounced mystery patient drills - New York city, 2016. MMWR. Morbidity and Mortality Weekly Report 2017; 66(36):945-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Franklin 2016 {published data only}
- Franklin SM. A comparison of personal protective standards: caring for patients with Ebola virus. Clinical Nurse Specialist CNS 2016; 30(2):E1-8. [DOI] [PubMed] [Google Scholar]
Garibaldi 2019 {published data only}
- Garibaldi BT, Ruparelia C, Shaw-Saliba K, Sauer LM, Maragakis LL, Glancey M, et al. A novel personal protective equipment coverall was rated higher than standard Ebola virus personal protective equipment in terms of comfort, mobility and perception of safety when tested by health care workers in Liberia and in a United States biocontainment unit. American Journal of Infection Control 2019; 47(3):298-304. [DOI: ] [DOI] [PubMed] [Google Scholar]
Gozel 2013 {published data only}
- Gozel MG, Dokmetas I, Oztop AY, Engin A, Elaldi N, Bakir M. Recommended precaution procedures protect healthcare workers from Crimean-Congo hemorrhagic fever virus. International Journal of Infectious Diseases 2013; 17(11):e1046-50. [DOI] [PubMed] [Google Scholar]
Grélot 2015 {published data only}
- Grélot L, Koulibaly F, Maugey N, Janvier F, Foissaud V, Aletti M, et al. Moderate thermal strain in healthcare workers wearing personal protective equipment during treatment and care activities in the context of the 2014 Ebola virus disease outbreak. Journal of Infectious Diseases 2015 Dec 9;212:1462-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Grélot 2016 {published data only}
- Grélot L, Koulibaly F, Maugey N, Janvier F, Foissaud V, Aletti M, et al. Moderate thermal strain in healthcare workers wearing personal protective equipment during treatment and care activities in the context of the 2014 Ebola virus disease outbreak. Journal of Infectious Diseases 2016; 213(9):1462-5. [DOI] [PubMed] [Google Scholar]
Hendler 2000 {published data only}
- Hendler I, Nahtomi O, Segal E, Perel A, Wiener M, Meyerovitch J. The effect of full protective gear on intubation performance by hospital medical personnel. Association of Military Surgeons of the United States 2000; 165(4):272-4. [PubMed] [Google Scholar]
Herlihey 2016 {published data only}
- Herlihey TA, Gelmi S, Flewwelling CJ, Hall TN, Bañez C, Morita PP, et al. Personal protective equipment for infectious disease preparedness: a human factors evaluation. Infection Control and Hospital Epidemiology 2016; 37(9):1022-8. [DOI] [PubMed] [Google Scholar]
Herlihey 2017 {published data only}
- Herlihey TA, Gelmi S, Cafazzo JA, Hall TN. The impact of environmental design on doffing personal protective equipment in a healthcare environment: a formative human factors trial. Infection Control and Hospital Epidemiology 2017; 38(6):712-7. [DOI] [PubMed] [Google Scholar]
Hersi 2015 {published data only}
- Hersi M, Stevens A, Quach P, Hamel C, Thavorn K, Garritty C, et al. Effectiveness of personal protective equipment for healthcare workers caring for patients with filovirus disease: a rapid review. PloS One 2015; 10(10):e0140290. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ho 2003 {published data only}
- Ho AS, Sung JJ, Chan-Yeung M. An outbreak of severe acute respiratory syndrome among hospital workers in a community hospital in Hong Kong. Annals of Internal Medicine 2003; 139(7):564-7. [DOI] [PubMed] [Google Scholar]
Ho 2004 {published data only}
- Ho KY, Singh KS, Habib AG, Ong BK, Lim TK, Ooi EE, et al. Mild illness associated with severe acute respiratory syndrome coronavirus infection: lessons from a prospective seroepidemiologic study of health-care workers in a teaching hospital in Singapore. Journal of Infectious Diseases 2004; 189(4):642-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hon 2008 {published data only}
- Hon CY, Gamage B, Bryce EA, LoChang J, Yassi A, Maultsaid D, et al. Personal protective equipment in health care: Can online infection control courses transfer knowledge and improve proper selection and use? American Journal of Infection Control 2008; 36(10):e33-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hormbrey 1996 {published data only}
- Hormbrey PJ, Moore F, Skinner DV. Protective clothing in accident and emergency departments: cost versus risk benefit. Journal of Accident and Emergency Medicine 1996; 13(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huh 2020 {published data only}
- Huh S. How to train the health personnel for protecting themselves from novel coronavirus (COVID-19) infection during their patient or suspected case care. Journal of Educational Evaluation for Health Professions 2020; 17(101490061):10. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacob 2018 {published data only}
- Jacob JT, Herwaldt LA, Durso FT, CDC Prevention Epicenters Program. Preventing healthcare-associated infections through human factors engineering. Current Opinion in Infectious Diseases 2018; 31(4):353-8. [DOI: ] [DOI] [PubMed] [Google Scholar]
Jaffe 2019 {published data only}
- Jaffe G, Moriber N. Use of a double gloving technique to decrease cross-contamination by anesthesia providers. AANA Journal 2019; 87(4):307-12. [PubMed] [Google Scholar]
Jaques 2016 {published data only}
- Jaques PA, Gao P, Kilinc-Balci S, Portnoff L, Weible R, Horvatin M, et al. Evaluation of gowns and coveralls used by medical personnel working with Ebola patients against simulated bodily fluids using an Elbow Lean Test. Journal of Occupational and Environmental Hygiene 2016; 13(11):881-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jeffs 2007 {published data only}
- Jeffs B, Roddy P, Weatherill D, la Rosa O, Dorion C, Iscla M, et al. The Medecins Sans Frontieres intervention in the Marburg hemorrhagic fever epidemic, Uige, Angola, 2005. I. Lessons learned in the hospital. Journal of Infectious Diseases 2007; 196 Suppl 2:S154-61. [DOI] [PubMed] [Google Scholar]
Jinadatha 2015 {published data only}
- Jinadatha C, Simmons S, Dale C, Ganachari-Mallappa N, Villamaria FC, Goulding N, et al. Disinfecting personal protective equipment with pulsed xenon ultraviolet as a risk mitigation strategy for health care workers. American Journal of Infection Control 2015; 43(4):412-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jones 2020 {published data only}
- Jones RM, Bleasdale SC, Maita D, Brosseau LM, CDC Prevention Epicenters Program. A systematic risk-based strategy to select personal protective equipment for infectious diseases. American Journal of Infection Control 2020; 48(1):46-51. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kahveci 2019 {published data only}
- Kahveci Z, Selcen Kilinc-Balci F, Yorio PL. Critical investigation of glove–gown interface barrier performance in simulated surgical settings. Journal of Occupational and Environmental Hygiene 2019; 16(7):498-506. [DOI: 10.1080/15459624.2019.1600702] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kang 2017 {published data only}
- Kang J, Kim EJ, Choi JH, Hong HK, Han SH, Choi IS, et al. Difficulties in using personal protective equipment: training experiences with the 2015 outbreak of Middle East respiratory syndrome in Korea. Antimicrobial Resistance and Infection Control 2017; 6:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kang 2017a {published data only}
- Kang J, O'Donnell J, Colaianne B, Bircher N, Ren D, Smith KJ. Use of personal protective equipment among health care personnel: results of clinical observations and simulations. American Journal of Infection Control 2017; 45(1):17-23. [DOI] [PubMed] [Google Scholar]
Kappes Ramirez 2018 {published data only}
- Kappes Ramirez MS. Influence of undergraduate nursing student teaching methods on learning standard precautions and transmission-based precautions: Experimental research.. Nurse Education Today 2018; 61(ned, 8511379):101-5. [DOI: ] [DOI] [PubMed] [Google Scholar]
Keane 1977 {published data only}
- Keane E, Gilles HM. Lassa fever in Panguma Hospital, Sierra Leone, 1973-6. British Medical Journal 1977; 1(6073):1399-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kerstiens 1999 {published data only}
- Kerstiens B, Matthys F. Interventions to control virus transmission during an outbreak of Ebola hemorrhagic fever: experience from Kikwit, Democratic Republic of the Congo, 1995. Journal of Infectious Diseases 1999; 179 suppl 1:s263-7. [DOI] [PubMed] [Google Scholar]
Kilinc‐Balci 2015 {published data only}
- Kilinc-Balci FS, Nwoko J, Hillam T. Evaluation of the performance of isolation gowns. American Journal of Infection Control 2015; 43(6):S44. [Google Scholar]
Kilinc‐Balci 2016 {published data only}
- Kilinc Balci FS. Isolation gowns in health care settings: laboratory studies, regulations and standards, and potential barriers of gown selection and use. American Journal of Infection Control 2016; 44(1):104-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2015 {published data only}
- Kim SG. Healthcare workers infected with Middle East respiratory syndrome coronavirus and infection control. Journal of the Korean Medical Association 2015; 58(7):647-54. [Google Scholar]
Ko 2004 {published data only}
- Ko PC, Chen WJ, Ma MH, Chiang WC, Su CP, Huang CH, et al. Emergency medical services utilization during an outbreak of severe acute respiratory syndrome (SARS) and the incidence of SARS-associated coronavirus infection among emergency medical technicians. Academic Emergency Medicine 2004; 11(9):903-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kogutt 2019 {published data only}
- Kogutt BK, Sheffield JS, Garibaldi BT. 680: Assessing effectiveness of PPE in a simulated SVD of a highly infectious disease patient. American Journal of Obstetrics and Gynecology 2019; 220(1 Suppl):S451. [DOI: ] [Google Scholar]
Kratz 2017 {published data only}
- Kratz T, Verbeek L. Discussion of two infection prevention and control training approaches to enhance biosafety in primary healthcare facilities during an outbreak of Ebola virus disease. Tropical Medicine and International Health 2017; 22:167. [Google Scholar]
Kwon 2016 {published data only}
- Kwon JH, Burnham C-AD, Reske K, Liang S, Hink T, Wallace M, et al. Healthcare worker self-contamination during standard and Ebola virus disease personal protective equipment doffing. Open Forum Infectious Diseases 2016; 3:Supplement 1. [DOI: ] [Google Scholar]
Kwon 2017 {published data only}
- Kwon JH, Burnham CD, Reske KA, Liang SY, Hink T, Wallace MA, et al. Assessment of healthcare worker protocol deviations and self-contamination during personal protective equipment donning and doffing. Infection Control and Hospital Epidemiology 2017; 38(9):1077-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lai 2005 {published data only}
- Lai TS, Keung Ng T, Seto WH, Yam L, Law KI, Chan J. Low prevalence of subclinical severe acute respiratory syndrome-associated coronavirus infection among hospital healthcare workers in Hong Kong. Scandinavian Journal of Infectious Diseases 2005; 37(6/7):500/3. [DOI] [PubMed] [Google Scholar]
Lai 2011 {published data only}
- Lai JY, Guo YP, Or PP, Li Y. Comparison of hand contamination rates and environmental contamination levels between two different glove removal methods and distances. American Journal of Infection Control 2011; 39(2):104-11. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lange 2005 {published data only}
- Lange JH. SARS, emerging diseases, healthcare workers and respirators. Journal of Hospital Infection 2005; 60(3):293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lau 2004 {published data only}
- Lau JT, Fung KS, Wong TW, Kim JH, Wong E, Chung S, et al. SARS transmission among hospital workers in Hong Kong. Emerging Infectious Diseases 2004; 10(2):280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Le 2004 {published data only}
- Le DH, Bloom SA, Nguyen QH, Maloney SA, Le QM, Leitmeyer KC, et al. Lack of SARS transmission among public hospital workers, Vietnam. Emerging Infectious Diseases 2004; 10(2):265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2017 {published data only}
- Lee MH, Meerbach A, Straub J, Neidhardt I, Gresser N, Gies S, et al. Which personal protective equipment to provide? Challenges during the Ebola outbreak and lessons learned. Tropical Medicine and International Health 2017; 22:29. [Google Scholar]
Lindsley 2012 {published data only}
- Lindsley WG, King WP, Thewlis RE, Reynolds JS, Panday K, Cao G, et al. Dispersion and exposure to a cough-generated aerosol in a simulated medical examination room. Journal of Occupational and Environmental Hygiene 2012; 9(12):681-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lindsley 2014 {published data only}
- Lindsley WG, Noti JD, Blachere FM, Szalajda JV, Beezhold DH. Efficacy of face shields against cough aerosol droplets from a cough simulator. Journal of Occupational and Environmental Hygiene 2014; 11(8):509-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2009 {published data only}
- Liu W, Tang F, Fang LQ, Vlas SJ, Ma HJ, Zhou JP, et al. Risk factors for SARS infection among hospital healthcare workers in Beijing: a case control study. Tropical Medicine and International Health 2009; 14 Suppl 1:52-9.
Loeb 2004 {published data only}
- Loeb M, McGeer A, Henry B, Ofner M, Rose D, Hlywka T, et al. SARS among critical care nurses, Toronto. Tropical Medicine and International Health 2004; 10(2):251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Low 2005 {published data only}
- Low JG, Wilder-Smith A. Infectious respiratory illnesses and their impact on healthcare workers: a review. Annals of Academic Medicine Singapore 2005; 34(1):105-10. [PubMed] [Google Scholar]
Lowe 2014 {published data only}
- Lowe JJ, Jelden KC, Schenarts PJ, Rupp LE, Hawes KJ, Tysor BM, et al. Considerations for safe EMS transport of patients infected with Ebola virus. Prehospital Emergency Care 2015; 19(2):179-83. [DOI] [PubMed] [Google Scholar]
Lu 2006 {published data only}
- Lu YT, Chen PJ, Sheu CY, Liu CL. Viral load and outcome in SARS infection: the role of personal protective equipment in the emergency department. Journal of Emergency Medicine 2006; 30(1):7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 2020 {published data only}
- Lu D, Wang H, Yu R, Yang H, Zhao Y. Integrated infection control strategy to minimize nosocomial infection of corona virus disease 2019 among ENT healthcare workers. Journal of Hospital Infection 2020; 104:PII: S0195-6701(20)30092-X. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Luo 2011 {published data only}
- Luo CH, Yang S, Wen CY, Syu MY, Lin KH, Chiu SH, et al. Fluorescent aerosol leakage quantification for protective clothing with an entropy-based image processor for industrial and medical workers. Journal of Aerosol Science 2011; 42(7):491-6. [Google Scholar]
Ma 2004 {published data only}
- Ma HJ, Wang HW, Fang LQ, Jiang JF, Wei MT, Liu W, et al. A case-control study on the risk factors of severe acute respiratory syndromes among health care workers. Zhonghua Liu Xing Bing Xue Za Zhi 2004; 25(9):741-4. [PubMed] [Google Scholar]
Makovicka 2018 {published data only}
- Makovicka JL, Bingham JS, Patel KA, Young SW, Beauchamp CP, Spangehl MJ. Surgeon personal protection: an underappreciated benefit of positive-pressure exhaust suits. Clinical Orthopaedics and Related Research 2018; 476(6):1341-8. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Malik 2006 {published data only}
- Malik MH, Handford E, Staniford E, Gambhir AK, Kay PR. Comfort assessment of personal protection systems during total joint arthroplasty using a novel multi-dimensional evaluation tool. Annals of the Royal College of Surgeons England 2006; 88(5):465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marklund 2002 {published data only}
- Marklund LA. Transporting patients with lethal contagious infections. International Journal of Trauma Nursing 2002; 8(2):51-3. [DOI] [PubMed] [Google Scholar]
Matanock 2014 {published data only}
- Matanock A, Arwady MA, Ayscue P, Forrester JD, Gaddis B, Hunter JC, et al. Ebola virus disease cases among health care workers not working in Ebola treatment units - Liberia, June-August, 2014. Morbidity and Mortality Weekly Report 2014; 63(46):1077-81. [PMC free article] [PubMed] [Google Scholar]
McLaws 2016 {published data only}
- McLaws ML, Chughtai AA, Salmon S, MacIntyre CR. A highly precautionary doffing sequence for health care workers after caring for wet Ebola patients to further reduce occupational acquisition of Ebola. American Journal of Infection Control 2016; 44(7):740-4. [DOI] [PubMed] [Google Scholar]
Mehtar 2015 {published data only}
- Mehtar S, Hakizimana B, Infection Control Africa Network Education and Training Working Group. IPC training in Sierra Leone- ICAN's role in fighting Ebola. Antimicrobial Resistance and Infection Control 2015; 4 Suppl 1:12. [Google Scholar]
Minnich 2003 {published data only}
- Minnich G. A clean ride. Building EMS vehicles for easier cleaning & decontamination. JEMS : a Journal of Emergency Medical Services 2003; 28(5):104-15. [PubMed] [Google Scholar]
Mollura 2015 {published data only}
- Mollura DJ, Palmore TN, Folio LR, Bluemke DA. Radiology preparedness in Ebola virus disease: guidelines and challenges for disinfection of medical imaging equipment for the protection of staff and patients. Radiology 2015; 275(2):538-44. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2005 {published data only}
- Moore D, Gamage B, Bryce E, Copes R, Yassi A. Protecting health care workers from SARS and other respiratory pathogens: organizational and individual factors that affect adherence to infection control guidelines. American Journal of Infection Control 2005; 33(2):114-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morgan 2009 {published data only}
- Morgan DJ, Diekema DJ, Sepkowitz K, Perencevich EN. Adverse outcomes associated with contact precautions: a review of the literature. American Journal of Infection Control 2009; 37(2):85-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mumma 2018 {published data only}
- Mumma JM, Durso FT, Ferguson AN, Gipson CL, Casanova L, Erukunuakpor K, et al. Human factors risk analyses of a doffing protocol for Ebola-level personal protective equipment: mapping errors to contamination. Clinical Infectious Diseases 2018; 66(6):950-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mumma 2019 {published data only}
- Mumma JM, Durso FT, Casanova LM, Erukunuakpor K, Kraft CS, Ray SM, et al. Common behaviors and faults when doffing personal protective equipment for patients with serious communicable diseases. Clinical Infectious Diseases 2019; 69 Suppl 3:S214-S220. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Muyembe‐Tamfum 1999 {published data only}
- Muyembe-Tamfum JJ, Kipasa M, Kiyungu C, Colebunders R. Ebola outbreak in Kikwit, Democratic Republic of the Congo: discovery and control measures. Journal of Infectious Diseases 1999; 179 Suppl 1:S259-62. [DOI] [PubMed] [Google Scholar]
Nikiforuk 2017 {published data only}
- Nikiforuk AM, Cutts TA, Theriault SS, Cook BW. Challenge of liquid stressed protective materials and environmental persistence of Ebola virus. Scientific Reports 2017; 7(1):4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nishiura 2005 {published data only}
- Nishiura H, Kuratsuji T, Quy T, Phi NC, Van Ban V, Ha LE, et al. Rapid awareness and transmission of severe acute respiratory syndrome in Hanoi French Hospital, Vietnam. American Journal of Tropical Medicine and Hygiene 2005; 73(1):17-25. [PubMed] [Google Scholar]
Northington 2007 {published data only}
- Northington WE, Mahoney GM, Hahn ME, Suyama J, Hostler D. Training retention of level C personal protective equipment use by emergency medical services personnel. Academic Emergency Medicine 2007; 14(10):846-9. [DOI] [PubMed] [Google Scholar]
Novosad 2016 {published data only}
- Novosad S, Christensen BE, Staples EJ, Nakashima A, Christensen K, Atkinson A, et al. Evaluating healthcare workers for acquisition of Zika virus while providing critical care. Open Forum Infectious Diseases 2016; 3:Supplement 1. [DOI: ] [Google Scholar]
Nyenswah 2015 {published data only}
- Nyenswah T, Fallah M, Sieh S, Kollie K, Badio M, Gray A, et al. Controlling the last known cluster of Ebola virus disease - Liberia, January-February 2015. MMWR Morbidity and Mortality Weekly Report 2015; 64(18):500-4. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Ofner 2003 {published data only}
- Ofner M, Lem M, Sarwal S, Vearncombe M, Simor A. Cluster of severe acute respiratory syndrome cases among protected health care workers -Toronto, April 2003. Canada Communicable Disease Report 2003; 29(11):93-7. [PubMed] [Google Scholar]
Ofner‐Agostini 2006 {published data only}
- Ofner-Agostini M, Gravel D, McDonald LC, Lem M, Sarwal S, McGeer A, et al. Cluster of cases of severe acute respiratory syndrome among Toronto healthcare workers after implementation of infection control precautions: a case series. Infection Control 2006; 27(5):473-8. [DOI] [PubMed] [Google Scholar]
Ogendo 2008 {published data only}
- Ogendo SW, Awori MN, Omondi MA, Mulatya EM, Mugo PW. Risk of conjunctival contamination from blood splashes during surgery at the Kenyatta National Hospital, Nairobi. East African Medical Journal 2008; 85(9):432-7. [DOI] [PubMed] [Google Scholar]
Ong 2013 {published data only}
- Ong MS, Magrabi F, Post J, Morris S, Westbrook J, Wobcke W, et al. Communication interventions to improve adherence to infection control precautions: a randomised crossover trial. BMC Infectious Diseases 2013; 13:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2004 {published data only}
- Park BJ, Peck AJ, Kuehnert MJ, Newbern C, Smelser C, Comer JA, et al. Lack of SARS transmission among healthcare workers, United States. Emerging Infectious Diseases 2004; 10(2):244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parveen 2018 {published data only}
- Parveen SN, Gurley ES, Sazzad HM, Sultana R, Saiful Islam M, Knust B, et al. Identifying acceptable and feasible infection control interventions for Nipah encephalitis outbreaks in Bangladesh. American Journal of Infection Control 2018; 46(6):S24. [Google Scholar]
Pei 2006 {published data only}
- Pei LY, Gao ZC, Yang Z, Wei DG, Wang SX, Ji JM, et al. Investigation of the influencing factors on severe acute respiratory syndrome among health care workers. Journal of Peking University (Health Sciences) 2006; 38(3):271-5. [PubMed] [Google Scholar]
Phan 2018 {published data only}
- Phan L, Su YM, Weber R, Fritzen-Pedicini C, Edomwande O, Jones RM, et al. Environmental and body contamination from cleaning vomitus in a health care setting: a simulation study. American Journal of Infection Control 2018; 46(4):397-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Phrampus 2016 {published data only}
- Phrampus PE, O'Donnell JM, Farkas D, Abernethy D, Brownlee K, Dongilli T, et al. Rapid development and deployment of Ebola readiness training across an academic health system: the critical role of simulation education, consulting, and systems integration. Simulation in Healthcare 2016; 11(2):82-8. [DOI] [PubMed] [Google Scholar]
Porteous 2018 {published data only}
- Porteous GH, Bean HA, Woodward CM, Beecher RP, Bernstein JR, Wilkerson S, et al. A simulation study to evaluate improvements in anesthesia work environment contamination after implementation of an infection prevention bundle. Anesthesia and Analgesia 2018; 127(3):662-70. [DOI: ] [DOI] [PubMed] [Google Scholar]
Quinn 2018 {published data only}
- Quinn T, Kim JH, Seo Y, Coca A. Comparison of thermal manikin modeling and human subjects' response during use of cooling devices under personal protective ensembles in the heat. Prehospital and Disaster Medicine 2018; 33(4):1-9. [DOI] [PubMed] [Google Scholar]
Ragazzoni 2015 {published data only}
- Ragazzoni L, Ingrassia PL, Echeverri L, Maccapani F, Berryman L, Burkle FM, et al. Virtual reality simulation training for Ebola deployment. Disaster Medicine and Public Health Preparedness 2015; 9(5):543-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ransjo 1979 {published data only}
- Ransjo U. Attempts to control clothes-borne infection in a burn unit, 3. An open-roofed plastic isolator or plastic aprons to prevent contact transfer of bacteria. Journal of Hygiene 1979; 82(3):385-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reynolds 2006 {published data only}
- Reynolds MG, Anh BH, Thu VH, Montgomery JM, Bausch DG, Shah JJ, et al. Factors associated with nosocomial SARS-CoV transmission among healthcare workers in Hanoi, Vietnam, 2003. BMC Public Health 2006; 6:207. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosenberg 2016 {published data only}
- Rosenberg K, Zolot JP. A simple intervention reduces contamination risk in health care personnel. American Journal of Nursing 2016; 116(1):64-5. [Google Scholar]
Russell 2015 {published data only}
- Russell CD, Young I, Leung V, Morris K. Healthcare workers' decision-making about transmission-based infection control precautions is improved by a guidance summary card. Journal of Hospital Infection 2015; 90(3):235-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Scales 2003 {published data only}
- Scales DC, Green K, Chan AK, Poutanen SM, Foster D, Nowak K, et al. Illness in intensive care staff after brief exposure to severe acute respiratory syndrome. Emerging Infectious Diseases 2003; 9(10):1205-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schumacher 2010 {published data only}
- Schumacher J, Gray SA, Michel S, Alcock R, Brinker A. Respiratory protection during paediatric cardiopulmonary resuscitation. European Journal of Anaesthesiology 2011; 28:150. [Google Scholar]
Scott Taylor 2017 {published data only}
- Scott Taylor R, Pitzer M, Goldman G, Czysz A, Simunich T, Ashurst J. Comparison of intubation devices in level C personal protective equipment: a cadaveric study. American Journal of Emergency Medicine 2017; 36:922-5. [DOI] [PubMed] [Google Scholar]
Seto 2003 {published data only}
- Seto WH, Tsang D, Yung RW, Ching TY, Ng TK, Ho M, et al. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS). Lancet 2003; 361(9368):1519-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shao 2015 {published data only}
- Shao X-P, Zhou Q, Ju J-T, Xin H-G, Chen J, Wan C-l, et al. Evaluation of anti-Ebola training system in the PLA Medical Team to Liberia and some suggestion. Academic Journal Of Second Military Medical University 2015; 36(8):822-7. [Google Scholar]
Sorensen 2008 {published data only}
- Sorensen P, Ejlertsen T, Aaen D, Poulsen K. Bacterial contamination of surgeons gloves during shunt insertion: a pilot study. British Journal of Neurosurgery 2008; 22(5):675-7. [DOI] [PubMed] [Google Scholar]
Su 2017 {published data only}
- Su YM, Phan L, Edomwande O, Weber R, Bleasdale SC, Brosseau LM, CDC Prevention Epicenters Program. Contact patterns during cleaning of vomitus: a simulation study. American Journal of Infection Control 2017; 45(12):1312-17. [DOI] [PubMed] [Google Scholar]
Suen 2017 {published data only}
- Suen LK, Yang L, Ho SS, Fung KH, Boost MV, Wu CS, et al. Reliability of N95 respirators for respiratory protection before, during, and after nursing procedures. American Journal of Infection Control 2017; 45(9):974-8. [DOI: ] [DOI] [PubMed] [Google Scholar]
Tartari 2015 {published data only}
- Tartari E, Allegranzi B, Ang B, Calleja N, Collignon P, Hopman J, et al. Preparedness of institutions around the world for managing patients with Ebola virus disease: an infection control readiness checklist. Antimicrobial Resistance and Infection Control 2015; 4:22. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartari E, Falzon Parascandalo AR, Borg MA. Ensuring healthcare workers' safety in the management of Ebola virus disease: a novel competency assessment checklist for proper PPE use. Antimicrobial Resistance and Infection Control 2015; 4:Suppl 1. [Google Scholar]
Teleman 2004 {published data only}
- Teleman MD, Boudville IC, Heng BH, Zhu D, Leo YS. Factors associated with transmission of severe acute respiratory syndrome among health-care workers in Singapore. Epidemiology and Infection 2004; 132(5):797-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tomas 2015 {published data only}
- Tomas ME, Kundrapu S, Thota P, Sunkesula VC, Cadnum JL, Mana TS, et al. Contamination of health care personnel during removal of personal protective equipment. JAMA Internal Medicine 2015; 175(12):1904-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tomas 2016a {published data only}
- Tomas ME, Cadnum JL, Mana TSC, Jencson AL, Koganti S, Alhmidi H. Utility of a novel reflective marker visualized by flash photography for assessment of personnel contamination during removal of personal protective equipment. Infection Control and Hospital Epidemiology 2016; 37(6):711-3. [DOI] [PubMed] [Google Scholar]
Torres 2015 {published data only}
- Torres M, Hansen KN, Jerrard D. Ebola: a review for emergency providers. Emergency Medicine Clinics of North America 2015; 33(2):e1-18. [PMID: ] [DOI] [PubMed] [Google Scholar]
Visnovsky 2019 {published data only}
- Visnovsky LD, Zhang Y, Leecaster MK, Haroldsen C, Mulvey DL, Nevers M, et al. Effectiveness of a multisite personal protective equipment (PPE)–free zone intervention in acute care. Infection Control and Hospital Epidemiology 2019; 40(7):761-6. [DOI: 10.1017/ice.2019.111] [DOI] [PubMed] [Google Scholar]
Weber 2018 {published data only}
- Weber RT, Phan LT, Edomwande O, Fritzen-Pedicini CM, Bleasdale SC, Jones R. Environmental and personal protective equipment contamination during simulated healthcare activities. American Journal of Infection Control 2018; 46(6):S10-S11. [Google Scholar]
Weber 2019 {published data only}
- Weber RT, Phan LT, Fritzen-Pedicini C, Jones RM. Environmental and personal protective equipment contamination during simulated healthcare activities. Annals of Work Exposures and Health 2019; 63(7):784-96. [DOI: ] [DOI] [PubMed] [Google Scholar]
West 2014 {published data only}
- West K. Ebola outbreak 2014. Why we don't need moon suits. JEMS : a Journal of Emergency Medical Services 2014; 39(11):28-30. [PubMed] [Google Scholar]
Williams 2019 {published data only}
- Williams VR, Leis JA, Trbovich P, Agnihotri T, Lee W, Joseph B, et al. Improving healthcare worker adherence to the use of transmission-based precautions through application of human factors design: a prospective multi-centre study. Journal of Hospital Infection 2019; 103(1):101-5. [DOI: ] [DOI] [PubMed] [Google Scholar]
Xi 2016 {published data only}
- Xi H, Cao J, Liu J, Li Z, Kong X, Wang Y, et al. Improving health care workers' protection against infection of Ebola hemorrhagic fever through video surveillance. American Journal of Infection Control 2016; 44(8):922-4. [DOI] [PubMed] [Google Scholar]
Yin 2004 {published data only}
- Yin WW, Gao LD, Lin WS, Gao LD, Lin WS, Du L, et al. Effectiveness of personal protective measures in prevention of nosocomial transmission of severe acute respiratory syndrome. Zhonghua Liu Xing Bing Xue Za Zhi 2004; 25(1):18-22. [PubMed] [Google Scholar]
Yuan 2018 {published data only}
- Yuan S, Zhang Z-W, Li Z-L. Bacteriophage M13 may be used for the assessment of viral transfer during doffing of Ebola-level personal protective equipment. Infection Control and Hospital Epidemiology 2018; 39(6):762-3. [DOI: ] [DOI] [PubMed] [Google Scholar]
Zellmer 2015 {published data only}
- Zellmer C, Van Hoof S, Safdar N. Variation in health care worker removal of personal protective equipment. American Journal of Infection Control 2015; 43(7):750-1. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2003 {published data only}
- Zhou G, Qi Y, Li L. Investigation report on the SARS infection rate of the second medical team of Peking University First Hospital. Beijing Da Xue Xue Bao 2003; 35 Suppl:59-61. [PubMed] [Google Scholar]
References to ongoing studies
ChiCTR2000029900 {published data only}
- Renmin Hospital of Wuhan University. Research for risks associated with novel coronavirus pneumonia (COVID-19) in the hospital workers and nosocomial prevention and control strategy. ChiCTR2000029900 2020.
ChiCTR2000030317 {published data only}
- West China Hospital of Sichuan University. A prospective randomized controlled trial for a self-made gastroscope isolation mask for preventing and controlling the novel coronavirus pneumonia (COVID-19) epidemic period. ChiCTR2000030317 2020.
ChiCTR2000030834 {published data only}
- Tongji Hospital Tongji Medical College Huazhong University of Science and Technology Wuhan China. Epidemiological characteristics and antibody levels of COVID-19 infection of pediatric medical staff working in quarantine area. ChiCTR2000030834 2020.
ChiCTR2000030895 {published data only}
- Tongji Hospital Tongji Medical College Huazhong University of Science and Technology Wuhan China. Retrospective and prospective study on nosocomial infection in stomatology department under the background of COVID-19. ChiCTR2000030895 2020.
Additional references
Adams 2020
- Adams JG, Walls RM. Supporting the health care workforce during the COVID-19 global epidemic. JAMA 2020; 323:doi: 10.1001/jama.2020.3972. [PMID: ] [DOI] [PubMed] [Google Scholar]
Agah 1987
- Agah R, Cherry JD, Garakian AJ, Chapin M. Respiratory Syncytial Virus (RSV) infection rate in personnel caring for children with RSV infections. Routine isolation procedure vs routine procedure supplemented by use of masks and goggles. American Journal of Diseases of Children (1960) 1987; 141(6):695-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
ANSI/AAMI PB70 2012
- ANSI/AAMI. PB70: Liquid barrier performance and classification of protective apparel and drapes in health care facilities. Association for the Advancement of Medical Instrumentation 2012.
Australian NHMRC 2010
- Australian National Health and Medical Research Council. Australian guidelines for the prevention and control of infection in healthcare. www.nhmrc.gov.au/book/html-australian-guidelines-prevention-and-control-infection-healthcare-2010 (accessed 8 December 2014).
Brouqui 2009
- Brouqui P, Puro V, Fusco FM, Bannister B, Schilling S, Follin P, et al. Infection control in the management of highly pathogenic infectious diseases: consensus of the European Network of Infectious Disease. Lancet Infectious Diseases 2009; 9(5):301-11. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 2001
- Campbell MK, Mollison J, Grimshaw JM. Cluster trials in implementation research: estimation of intracluster correlation coefficients and sample size. Statistics in Medicine 2001; 20(3):391-9. [DOI] [PubMed] [Google Scholar]
CDC 2014
- Centers for Disease Control and Prevention. Guidance on personal protective equipment to be used by healthcare workers during management of patients with Ebola virus disease in U.S. Hospitals, including procedures for putting on (donning) and removing (doffing). www.cdc.gov/vhf/ebola/hcp/procedures-for-ppe.html (accessed 8 December 2014).
CDC 2020a
- Centers for Disease Control and Prevention. Conventional capacity strategies: use isolation gown alternatives that offer equivalent or higher protection. www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/isolation-gowns.html#conventional-capacity (accessed 31 March 2020).
CDC 2020b
- Centers for Disease Control and Prevention. Frequently asked questions about personal protective equipment. www.cdc.gov/coronavirus/2019-ncov/hcp/respirator-use-faq.html (accessed 2 April 2020).
CDC 2020c
- Centers for Disease Control and Prevention. Sequence for putting on personal protective equipment (PPE). www.cdc.gov/niosh/npptl/pdfs/PPE-Sequence-508.pdf (accessed 2 April 2020).
Chang 2020
- Chang, Xu H, Rebaza A, Sharma L, Dela Cruz CS. Protecting health-care workers from subclinical coronavirus infection. Lancet Respiratory Medicine 2020; 8(3):e13. [PMID: ] [DOI] [PMC free article] [PubMed]
Cheng 2016
- Cheng A, Kessler D, Mackinnon R, Chang TP, Nadkarni VM, Hunt EA, et al. Reporting guidelines for health care simulation research: extensions to the CONSORT and STROBE statements. Simulation in Healthcare 2016; 11(4):238-48. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cherrie 2006
- Cherrie JW, Semple S, Christopher Y, Saleem A, Hughson GW, Philips A. How important is inadvertent ingestion of hazardous substances at work? Annals of Occupational Hygiene 2006; 50(7):693-704. [PMID: ] [DOI] [PubMed] [Google Scholar]
Coia 2013
- Coia JE, Ritchie L, Adisesh A, Makison Booth C, Bradley C, Bunyan D, et al. Guidance on the use of respiratory and facial protection equipment. Journal of Hospital Infection 2013; 85(3):170-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation Covidence Systematic Review Software. Melbourne, Australia: Veritas Health Innovation, 2016.Available at www.covidence.org.
De Iaco 2012
- De Iaco G, Puro V, Fusco FM, Schilling S, Maltezou HC, Brouqui P, et al. European network for highly infectious diseases working group. Personal protective equipment management and policies: European network for highly infectious diseases data from 48 isolation facilities in 16 European countries. Infection Control and Hospital Epidemiology 2012; 33(10):1008-16. [DOI] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG (editors) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Ebola 2014
ECDC 2014
- European Center for Disease Prevention and Control. Safe use of personal protective equipment in the treatment of infectious diseases of high consequence. ECDC . [DOI: 10.2900/339505] [ISBN 978-92-9193-612-0] [DOI]
EN 13795
- CEN (European Committee for Standardization). EN 13795-2 Surgical drapes, gowns, clean air suits used as medical devices for patients, clinical staff and equipment. CEN (European Committee for Standardization) 2005.
EN 14126
- CEN (European Committee for Standardization). CSN EN 14126 Protective clothing - Performance requirements and tests methods for protective clothing against infective agents. CEN (European Committee for Standardization) 2003.
EU 2010
- European Commission. Council Directive 2010/32/EU of 10 May 2010 implementing the Framework Agreement on prevention from sharp injuries in the hospital and healthcare sector concluded by HOSPEEM and EPSU. eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32010L0032 (accessed 30 March 2015).
Fischer 2014
- Fischer WA 2nd, Hynes NA, Perl TM. Protecting health care workers from ebola: personal protective equipment is critical but is not enough. Annals of Internal Medicine 2014; 161(10):753-4. [DOI] [PubMed] [Google Scholar]
Forrester 2014
- Forrester JD, Hunter JC, Pillai SK, Arwady MA, Ayscue P, Matanock A, et al, Centers for Disease Control and Prevention (CDC). Cluster of Ebola cases among Liberian and U.S. health care workers in an Ebola treatment unit and adjacent hospital - Liberia, 2014. Morbidity and Mortality Weekly Report 2014; 63(41):925-9. [PMC free article] [PubMed]
Gershon 2009
- Gershon RR, Vandelinde N, Magda LA, Pearson JM, Werner A, Prezant D. Evaluation of a pandemic preparedness training intervention of emergency medical services personnel. Prehospital and Disaster Medicine 2009; 24(6):508-11. [DOI] [PubMed] [Google Scholar]
Giwa 2020
- Giwa AL, Desai A, Duca A. Novel 2019 coronavirus SARS-CoV-2 (COVID-19): an updated overview for emergency clinicians. Emergency Medicine Practice 2020; 22(5):1-28. [PMID: ] [PubMed] [Google Scholar]
Gould 2010
- Gould DJ, Moralejo D, Drey N, Chudleigh JH. Interventions to improve hand hygiene compliance in patient care. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD005186.pub3] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 20 March 2020. Hamilton (ON): McMaster University (developed by Evidence Prime).Available at gradepro.org.
Heptonstall 2010
- Heptonstall J, Cockcroft A. Occupational infections. In: Hunter's diseases of occupations. 10 edition. London UK: Hodder & Stoughton Ltd, 2010:729-744. [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.training.cochrane.org/handbook.
Higgins 2017
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Howie 2005
- Howie RM. Respiratory protection use. Occupational Environmental Medicine 2005; 62(6):423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
ISO 2004a
- International Organization for Standardization (ISO). Clothing for protection against contact with blood and body fluids - Determination of resistance of protective clothing materials to penetration by blood-borne pathogens - Test method using Phi-X 174 bacteriophage ISO 166604:2004. International Organization for Standardization 2004.
ISO 2004b
- International Organization for Standardization (ISO). Clothing for protection against contact with blood and body fluids - Determination of the resistance of protective clothing materials to penetration by blood and body fluids - Test method using synthetic blood, ISO 16603:2004. International Organization for Standardization (ISO) 2004.
ISO 2013
- International Organization for Standardization (ISO). Protective clothing - Protection against chemicals - Determination of resistance of protective clothing materials to permeation by liquids and gases ISO 6529:2013. International Organization for Standardization (ISO) 2013.
Jefferson 2008
- Jefferson T, Foxlee R, Del Mar C, Dooley L, Ferroni E, Hewak B, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ 2008; 12(7635):77-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jefferson 2011
- Jefferson T, Del Mar CB, Dooley L, Ferroni E, Al-Ansary LA, Bawazeer GA, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD006207.pub4] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kahveci 2019
- Kahveci Z, Kilinc-Balci FS, Yorio PL. Critical investigation of glove–gown interface barrier performance in simulated surgical settings. Journal of occupational and environmental hygiene 2019; 16:498/506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kilmarx 2014
- Kilmarx PH, Clarke KR, Dietz PM, Hamel MJ, Husain F, McFadden JD, et al, Centers for Disease Control and Prevention (CDC). Ebola virus disease in health care workers - Sierra Leone, 2014. Morbidity and Mortality Weekly Report 2014; 63(49):1168-71. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Kuklane 2015
- Kuklane K, Lundgren K, Gao C, Löndahl J, Hornyanszky ED, Östergren PO, et al. Ebola: improving the design of protective clothing for emergency workers allows them to better cope with heat stress and help to contain the epidemic. Annals of Occupational Hygiene 2015; 59(2):258-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Landers 2010
- Landers T, McWalters J, Behta M, Bufe G, Ross B, Vawdrey DK, et al. Terms used for isolation practices by nurses at an academic medical center. Journal of Advanced Nursing 2010; 66(10):2309-19. [DOI: 10.1111/j.1365-2648.2010.05398.x] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Levy 2015
- Levy B, Rao CY, Miller L, Kennedy N, Adams M, Davis R, et al. Ebola infection control in Sierra Leonean health clinics: a large cross-agency cooperative project. American Journal of Infection Control 2015; 43(7):752-5. [DOI: 10.1016/j.ajic.2015.03.011] [PMID: ] [DOI] [PubMed] [Google Scholar]
Luong Thanh 2016
- Luong Thanh BY, Laopaiboon M, Koh D, Sakunkoo P, Moe H. Behavioural interventions to promote workers' use of respiratory protective equipment. Cochrane Database of Systematic Reviews 2016, Issue 12. [DOI: 10.1002/14651858.CD010157.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Makison 2014
- Makison Booth C. Vomiting Larry: a simulated vomiting system for assessing environmental contamination from projectile vomiting related to norovirus infection. Journal of Infection Prevention 2014; 15(5):176-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Missair 2014
- Missair A, Marino MJ, Vu CN, Gutierrez J, Missair A, Osman B, et al. Anesthetic implications of Ebola patient management: a review of the literature and policies. Anesthesia and Analgesia 2014; 121(3):810-21. [DOI: 10.1213/ANE.0000000000000573] [PMID: ] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Medicine 6;7:e1000097. [DOI: 10.1371/journal.pmed1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moon 2015
- Moon S, Sridhar D, Pate MA, Jha AK, Clinton C, Delaunay S, et al. Will Ebola change the game? Ten essential reforms before the next pandemic. The report of the Harvard-LSHTM independent panel on the global response to Ebola. Lancet 2015; 386:2204-21. [DOI: 10.1016/S0140-6736(15)00946-0] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mäkelä 2014
- Mäkelä E, Mäkinen H. Protective clothing against chemical and biological hazards. oshwiki.eu/wiki/Protective_clothing_against_chemical_and_biological_hazards#cite_note-EN14126-11 (accessed 12 November 2014).
NFPA 1999
- National Fire Protection Association. Standard on protective clothing for emergency medical operations. National Fire Protection Association 2013.
Nichol 2008
- Nichol K, Bigelow P, O'Brien-Pallas L, McGeer A, Manno M, Holness DL. The individual, environmental, and organizational factors that influence nurses' use of facial protection to prevent occupational transmission of communicable respiratory illness in acute care hospitals. American Journal of Infection Control 2008; 36(7):481-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
NIOSH 2014
- The National Institute for Occupational Safety and Health (NIOSH). Considerations for selecting protective clothing used in health care for protection against microorganisms in blood and body fluids. www.cdc.gov/niosh/npptl/topics/ProtectiveClothing/default.html (accessed 2 January 2015).
OSHA 2012
- Occupational Safety & Health Administration (OSHA). Bloodborne pathogens. 1910.1030(d)(3) Personal Protective Equipment; www.osha.gov/law-regs.html (accessed 10 December 2014).
Otter 2016
- Otter JA, Donskey C, Yezli S, Douthwaite S, Goldenberg SD, Weber DJ. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. Journal of hospital infection 2016; 92(3):235-50. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peng 2020
- Peng X, Xu X, Li Y, Cheng L, Zhou X, Ren B. Transmission routes of 2019-nCoV and controls in dental practice. International Journal of Oral Science 2020; 12(1):9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Poller 2018
- Poller B, Hall S, Bailey C, Gregory S, Clark R, Roberts P, et al. ‘VIOLET’: a fluorescence-based simulation exercise for training healthcare workers in the use of personal protective equipment. Journal of Hospital Infection 2018; 99(2):229-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Remuzzi 2020
- Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet 2020; 395:pii: S0140-6736(20)30627-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
RevMan Web 2019 [Computer program]
- The Cochrane Collaboration Review Manager Web (RevMan Web). The Cochrane Collaboration, 2019.Available at: revman.cochrane.org.
Roberge 2008a
- Roberge RJ. Evaluation of the rationale for concurrent use of N95 filtering face piece respirators with loose-fitting powered air-purifying respirators during aerosol-generating medical procedures. American Journal of Infection Control 2008; 36(2):135-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roberge 2008b
- Roberge RJ. Effect of surgical masks worn concurrently over N95 filtering face piece respirators: extended service life versus increased user burden. Journal of Public Health Management and Practice 2008; 14(2):E19-26. [DOI] [PubMed] [Google Scholar]
Roberge 2016
- Roberge RJ. Face shields for infection control: a review. Journal of Occupational and Environmental Hygiene 2016; 13(4):235-42. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2017
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al, on behalf of the Cochrane Applicability and Recommendations Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Sepkowitz 2005
- Sepkowitz KA, Eisenberg L. Occupational deaths among healthcare workers. Emerging Infectious Diseases 2005; 11(7):1003-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Siegel 2019
- Siegel JD, Rhinehart E, Jackson M, Chiarello L, Healthcare Infection Control Practices Advisory Committee. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. www.cdc.gov/infectioncontrol/guidelines/isolation/index.ht accessed 28 March 2020;(updated July 2019).
Sterne 2016
- Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355:e4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verbeek 2016a
- Verbeek JH, Mihalache RC. More PPE protects better against Ebola. American Journal of Infection Control 2016; Jun 1;44(6):731. [DOI: 10.1016/j.ajic.2015.12.024] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2020
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323:doi: 10.1001/jama.2020.1585. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ward 2011
- Ward DJ. The role of education in the prevention and control of infection: a review of the literature. Nurse Education Today 2011; 31(1):9-17. [DOI] [PubMed] [Google Scholar]
WHO 2003
- World Health Organization (WHO). Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. www.who.int/csr/sars/country/table2004_04_21/en/ accessed 31 March 2020.
WHO 2006
- World Health Organization (WHO). Health workers: a global profile. World Health Organization (WHO), Geneva 2006.
WHO 2009
- World Health Organization (WHO). WHO guidelines on hand hygiene in health care: a summary. www.who.int/gpsc/5may/tools/who_guidelines-handhygiene_summary.pdf (accessed 8 December 2014).
WHO 2014
- World Health Organization (WHO). Infection prevention and control of epidemic- and pandemic-prone acute respiratory infections in health care; WHO guideline. World Health Organisation, Geneva, Switzerland 2014. [PubMed]
WHO 2015a
- World Health Organization. Health worker Ebola infections in Guinea, Liberia and Sierra Leone. file:///C:/Users/jver/Desktop/Ebola%20update%202017/WHO_EVD_SDS_REPORT_2015.1_eng.pdf 2015:1-15.
WHO 2015b
- WHO regional office for Africa. Overview of Ebola virus disease epidemic in West Africa. WHO regional office for Africa Outbreak Bulletin 2015; 5(5):2-3.
WHO 2016
- World Health Organization. Personal protective equipment for use in a filovirus disease outbreak. Rapid advice guideline. WHO 2016:1-53. [ISBN 978 92 4 154972 1 ] [PubMed]
WHO 2018
- WHO. Preferred product characteristics for personal protective equipment for the health worker on the frontline responding to viral hemorrhagic fevers in tropical climates. apps.who.int/medicinedocs/documents/s23448en/s23448en.pdf 2018:1-31. [ISBN 978-92-4-151415-6]
WHO 2020a
- World Health Organization (WHO). Rational use of personal protective equipment (PPE) for coronavirus disease (COVID-19). Interim Guidance issued 19 March 2020. apps.who.int/iris/bitstream/handle/10665/331498/WHO-2019-nCoV-IPCPPE_use-2020.2-eng.pdf (accessed 30 March 2020).
WHO 2020b
- World Health Organization (WHO). Advice on the use of masks in the community, during home care, and in health care settings in the context of COVID-19. Interim Guidance 19 March 2020. World Health Organization, Geneva, Switzerland 2020.
Yassi 2005
- Yassi A, Moore D, Fitzgerald JM, Bigelow P, Hon CY, Bryce E, BC Interdisciplinary Respiratory Protection Study Group. Research gaps in protecting healthcare workers from SARS and other respiratory pathogens: an interdisciplinary, multi-stakeholder, evidence-based approach. Journal of Occupational and Environmental Medicine 2005; 47(1):41-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zelnick 2013
- Zelnick JR, Gibbs A, Loveday M, Padayatchi N, O'Donnell MR. Health-care workers' perspectives on workplace safety, infection control, and drug-resistant tuberculosis in a high-burden HIV setting. Journal of Public Health Policy 2013; 34(3):388-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Verbeek 2016b
- Verbeek JH, Ijaz S, Mischke C, Ruotsalainen JH, Mäkelä E, Neuvonen K, et al. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011621.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Verbeek 2015
- Verbeek JH, Ijaz S, Mischke C, Ruotsalainen JH, Mäkelä E, Neuvonen K, et al. Personal protective equipment for preventing highly infectious diseases due to contact with contaminated body fluids in health care staff. Cochrane Database of Systematic Reviews 2015, Issue 4. [DOI: 10.1002/14651858.CD011621] [DOI] [PMC free article] [PubMed] [Google Scholar]
Verbeek 2019
- Verbeek JH, Rajamaki B, Ijaz S, Mischke C, Ruotsalainen JH, Mäkelä E, et al. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Cochrane Database of Systematic Reviews 2019, Issue 7. [DOI: 10.1002/14651858.CD011621.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


