Abstract
Background: Chemexfoliation is widely used to reverse signs of photodamage. Although photodamage can eventually lead to skin cancer, it remains unclear whether chemical peels also affect photocarcinogenesis. Moreover, concerns about the systemic and/or cutaneous toxicity of peeling agents have already arisen. Objective: This review sought primarily to summarize the data available on the effects of chemical peels on ultraviolet-induced skin carcinogenesis, focusing particular attention on actinic keratoses and cutaneous field cancerization. In addition, considerations about the systemic and/or cutaneous toxicity of peeling agents, particularly trichloracetic acid, are briefly discussed. Methods: The PubMed, MEDLINE, and SCOPUS databases were searched using the keywords “chemical peeling,” “actinic keratosis,” “cutaneous field cancerization,” “skin cancer,” “skin cancer prevention,” and “cutaneous and systemic carcinogenicity,” both alone and in combination with one another. Additional relevant references were also isolated from citations in the reviewed literature. Results: A total of 42 articles involving both in-vitro and in-vivo human and animal models were included for analysis. The data were mainly confined to laboratory animals. Conclusion: Apart from efficacy in clearing visible actinic keratoses, the findings point towards the possible clinical use of chemical peeling for the prevention of skin cancer. To date, no evidence on systemic toxicity following dermal exposure of humans to chemical peels has been identified.
Keywords: Actinic keratosis, cutaneous field cancerization, photocarcinogenesis, field therapy, photoaging
Chemical peels, available in a number of formulations, have long been used to treat a variety of aesthetic and medical skin conditions. Topical exfoliative agents, alone or in combination, are used to induce controlled cutaneous injury, ultimately resulting in epidermal turnover and collagen regeneration. Chemical peeling is not restricted to cosmetic interventions. Indications for chemical resurfacing include pigmentary disorders (e.g., ephelides, melasma, lentigines), inflammatory disorders (e.g., acne, rosacea), scarring (e.g., acne, traumatic, surgical), chronoaging and photoaging (e.g., superficial and medium-depth wrinkles), and precancerous lesions (e.g., actinic keratoses).1–3
Chemexfoliation has demonstrated efficacy in reversing signs of photoaging. However, as a result of chemical removal of the photoprotective stratum corneum, an enhanced but reversible degree of ultraviolet (UV) sensitivity can occur. Repeated skin resurfacing is also coupled with chronic tissue damage and inflammatory changes that might contribute to a long-term risk of skin cancer.4,5 Moreover, concerns about systemic toxicity have arisen when studies began linking specific peel agents, especially trichloracetic acid (TCA), to cancers in animals.6,7 The popularity of TCA peels as expressed in commercial formulas available to consumers at pharmacy chains and do-it-yourself videos on the internet prompted the New York Times to publish a cautioning article debating possible carcinogenicity in animal studies.8 On the other hand, several lines of evidence suggest a curative and/or protective effect on photocarcinogenesis, regardless of the kind of peeling formula.4 As the ultimate progression of photoaging is skin cancer, it is important to clarify whether the repetitive procedure of chemical peeling on photodamaged skin is safe and whether these chemicals can reduce or stimulate the formation of UV-induced skin cancers.
Here, we present a structured review on the safety profile and effect of chemical peeling on skin photocarcinogenesis, with special emphasis on actinic keratoses (AKs) and cutaneous field cancerization.
METHODS
The PubMed, MEDLINE, and SCOPUS databases were searched using the keywords “chemical peeling,” “actinic keratosis,” “cutaneous field cancerization,” “skin cancer,” “skin cancer prevention,” and “cutaneous and systemic carcinogenicity,” both alone and in combination with one another. Additional relevant references were also isolated from citations in the reviewed literature. A total of 42 articles involving both in-vitro and in-vivo human and animal models were included for analysis. The data were mainly confined to laboratory animals.
TUMORIGENIC ROLE OF CHEMICAL PEELING
Implications about the systemic and/or cutaneous toxicity of chemical peeling have arisen when studies began linking such to cancers in animals, suggesting a tumorigenic potential.6,7 TCA, a persistent metabolite of trichloroethylene (TCE), is formed in drinking water as a byproduct of the chlorination process and as a metabolite of industrial medications like chloral hydrate. Major human exposure to TCA directly occurs through the consumption of water disinfected with chlorinated solvents.9 However, although TCA has frequently been detected in the blood of humans occupationally exposed to TCE, no epidemiological evidence exists on the carcinogenicity of TCA in humans.9 The available experimental data, mainly confined to laboratory animals, appear to be inconsistent. A number of studies have reported TCA-promoted carcinogenesis, while others failed to replicate the same results.
Many studies found liver and/or renal toxicity in mouse models of TCA when such was administered in drinking water or by gavage.6,7,10–15 There is no evidence of carcinogenicity in rats exposed to TCA, though.9,16,17 In addition, TCA-induced toxicity was not observed in Chinese hamster ovary and mouse lymphoma cells.18–20 Although the bulk of TCA studies involve animals, very few in-vitro human studies hint at harm.9,21 Recent data have indicated a positive mutagenic effect of TCA (25, 50, and 100μg/mL) on human blood lymphocytes, suggesting a carcinogenic activity in human subjects exposed to TCA-contaminated water.9 On the contrary, prior studies support that this chemical requires very high doses (2,000 and 3,500μg/mL) to induce DNA mutation, considering it as a weak genotoxin.22,23
As previously reported, TCA has the capability for reversible binding with plasma proteins that play an important role in tissue distribution and elimination of the chemical.24–26 Lumpkin et al. reported 4- to 5-fold higher plasma levels of free TCA in mice than in rats.25 Moreover, the rate of binding of TCA to plasma protein was found to be consistently greater in humans than in rats or mice. The degree of binding capability with plasma protein can influence the noted species difference in the susceptibility of TCA toxicity.26 As a species-specific carcinogen, TCA might not be key in inducing human tumors at the low doses encountered in the environment.12
CUTANEOUS FIELD CANCERIZATION
Actinic keratoses (AKs) are thought to represent a spectrum along the continuum to invasive skin cancer. Chronic UV exposure is the major contributing factor for these precancerous epidermal lesions. The term cutaneous field cancerization was proposed to describe a common UV-induced precancerous skin state characterized by multiple clinical and subclinical AKs lesions occurring in areas of photodamaged skin. These multiple patches of premalignant disease can potentially progress into squamous cell carcinoma (SCC), a type of nonmelanoma skin cancer (NMSC).27,28
Given the high prevalence of AKs, several modalities have been used to prevent potential evolution to SCC. The concept of field cancerization has initiated a paradigm shift in AK therapy from lesion-directed (e.g., cryotherapy, curettage) to field-directed therapies. The latter address the entire field damage and thus target both clinically apparent and subclinical lesions expanding in perilesional sun damaged skin.27,28
In this context, chemical resurfacing might theoretically decrease the risk of SCC through ablating premalignant cells. Whereas chemical peeling is widely performed in cosmetics to combat signs of photodamage, its role in treating and/or preventing photocarcinogenesis has yet to be determined. As a consequence, chemical peels are only briefly mentioned or even omitted from current treatment recommendations for AKs and field cancerization.27–29
Given the evidence of the carcinogenicity of TCA, the safety of such products after repetitive and long-term performance needs to be evaluated. However, no information on systemic toxicity following dermal exposure of humans to chemical peels has been identified.9 Regarding cutaneous insults, chemical disruption of the photoprotective barrier of the stratum corneum increases the penetration of UV radiation into the skin. This enhanced UV sensitivity, however, is reversible and recovers to normal after one week. Chronic inflammation secondary to repeated skin damage during peeling might also stimulate carcinogenesis in the long run.4,5 However, no evidence of inflammatory infiltrates was seen with 30% salicylic acid (SA) in macrogol at 70 days in animal sun-damaged skin.30
STUDIES SUGGESTING A POSSIBLE LINK BETWEEN PEELS AND SKIN CARCINOGENESIS
A number of studies have suggested a possible link between chemical peeling and skin carcinogenesis (Table 1). Although the medical use of chemical peels has been complicated by cutaneous and ocular adverse events,2,31 the link is not proven in any human studies.
TABLE 1.
Studies suggesting a link with skin carcinogenesis
| PEELING FORMULATION | MATERIALS/SUBJECTS | RESULTS | STUDIES |
|---|---|---|---|
| Animal models | |||
| GA (1–7mg/cm2, pH 3.0) | Guinea pigs | Enhanced redness and swelling without increased PGE2 production or COX-2 protein expression | Park et al32 |
| 35% TCA | UV-irradiated hairless mice | Tumor formation mostly in TCA-treated sites; reduced total tumor count | Dainichi et al33 |
| Human models | |||
| 40% + 60% TCA | Normal human skin | Reduced epidermal LCs in treated vs. control area on Day 7 | Sakai et al34 |
| Jessner’s + 35% TCA, 70% GA + 40% TCA | Humans | Eruptive keratoacanthomas | Mohr et al35; Cox et al36 |
GA: glycolic acid; PGE2: prostaglandin E2; COX: cyclooxygenase; TCA: trichloracetic acid; LCs: Langerhans cells
Animal studies. Although the application of glycolic acid (GA) to the skin of guinea pigs was reported to enhance UVB-induced skin sensitivity (i.e., redness and swelling), it was not accompanied by increased prostaglandin E2 (PGE2) production or cyclooxygenase (COX)-2 protein expression.32
As reported by Dainichi et al,33 TCA-treated mice exhibited UVB-induced skin tumors in the form of SCC mainly located in the experimental area, suggesting a TCA-induced susceptibility of the peeled skin to UV-promoted tumorigenesis. However, the number and size of tumors were comparable in the treated versus the control mice, while the formation of tumors outside of the experimental area was significantly decreased in TCA-treated mice. This latter fact was attributed to the activation of systemic immunosurveillance by inflammatory cells recruited from the TCA-treated area. Although TCA did not exert a direct preventive effect, it was not photocarcinogenic itself.
Human studies. Sakai et al34 supported that 40% and 60% TCA peeling applications on normal human skin can significantly reduce the number of epidermal Langerhans cells (LCs) in the treated area relative to liquid nitrogen-treated skin. This change was considered indicative of the temporary impairment of skin immunity that might be involved in skin carcinogenesis.
Two cases of eruptive keratoacanthomas following peeling application (Jessner’s solution plus 35% TCA, 70% GA plus 40% TCA) to nonfacial skin on a background of extensive solar damage have also been published. All lesions completely resolved spontaneously or after topical diclofenac application without further complications. The authors concluded that keratoacanthomas represent an abnormal reaction to different types of skin injury, pointing toward a benign clinical course.35,36
STUDIES SUGGESTING A PROTECTIVE LINK BETWEEN PEELS AND CARCINOGENESIS
Animal studies. Photodamage can eventually lead to skin tumorigenesis. Since chemical peels have been used to reverse photodamage, several animal studies were designed to determine the effect of peels on UV-induced skin cancers. Mouse models provide convincing evidence for a positive association between chemical peels and skin cancer prophylaxis.5,37–42
In the mouse study by Hong et al,37 GA reduced UVA+B-induced skin tumor incidence as well as the number of both large tumors (>2mm) and tumors per mouse by 20 percent, 47 percent, and 55 percent, respectively. GA also delayed the timing of tumor development.37 Bair et al38 conducted an in-vivo experiment to determine the efficacy of topical aspirin and sodium salicylate (NAS) in preventing UVB-induced NMCS. In the SKH-1 mouse model, both NAS (10 or 40μmol) and aspirin (40μmol) significantly inhibited the rate of tumor development compared to the vehicle control (p<0.05). Contrary to aspirin, NAS was found to prevent UVB-induced thymine dimer formation in the mouse epidermis, most probably via a sun-screening mechanism of action.38
A preparation of 30% SA dissolved in polyethylene glycol (PEG) resulted in both delayed and reduced skin tumor formation in the treated versus the control mice after repetitive UVB irradiation. This SA-PEG formulation also restored phototoxic histological changes by suppressing p53 expression and normalizing keratinocyte differentiation in UVB-irradiated mice.39,40 The United States National Toxicology Program evaluated the effects of synthetic solar light on the skin of hairless mice treated with creams containing GA (4% or 10%) or SA (2% or 4%). Although GA had no effect on photocarcinogenesis, SA was observed to be photoprotective, reducing the incidence of skin tumors.41
Abdel-Daim et al5,42 conducted mouse studies to investigate the chemopreventive effect of three types of chemical peels (35% GA, 30% SA, and 10% and 35% TCA) after UV irradiation (UVB or UVA+B). All modalities achieved significantly decreased rates of cancer incidence mostly in the treated but also in the nontreated area compared to nonpeeled mice. Moreover, peeling reduced the p53-positive keratinocytes and suppressed the messenger RNA expression of COX-2 in the treated skin. Serum PGE2 level was also decreased in the treated versus the control mice.
Human studies. Despite the scarcity of data, a number of human studies have also noted that chemical resurfacing offers benefits in terms of skin cancer prophylaxis.40,43–49 Ahn et al43 demonstrated the ability of GA to inhibit UVB-induced cell cytotoxicity, apoptosis, and the expression of apoptosis-regulatory genes (p53, p21) in cultured immortalized human keratinocyte HaCaT cells. In human facial skin, which is primarily photoexposed, the SA-PEG formulation eliminated immature cornified cells and induced normal cornification of keratinocytes. Restoring this UV-induced structural atypia allows the stratum corneum to recover its barrier function.40
In line with animal observations, positive results were described in high-risk patients with precancerous skin lesions and extensive solar damage. In a split-face study comparing a combined formulation of Jessner’s solution and 35% TCA with topical 5% fluorouracil (FU) in patients with widespread facial AKs, both modalities reduced the number of visible AKs by 75 percent through 12 months. TCA was observed to be as effective as fluorouracil in preventing lesion recurrence for at least one year, but was superior in terms of morbidity. However, the prophylactic effect of peeling, although pronounced, was not sustained at 32 months.44 Another study with the same design but conducting pretreatment with 0.025% tretinoin also noted a reduction in AKs at the 12-month follow-up.45
In a randomized, prospective five-year trial, Hantash et al46 compared three facial resurfacing techniques—carbon dioxide laser, 30% TCA peel, topical 5% FU—in 34 patients with severe actinic damage or a history of NMSC. TCA peeling produced equivalent reductions in mean AK count at three months posttreatment compared with fluorouracil and laser ablation (89% versus 83% and 92% for FU and laser, respectively). Significantly decreased rates of cancer incidence and a trend towards a longer timespan until the occurrence of new NMSC was recorded with all three modalities relative to the control group. Of note, a remarkably lower incidence of NMSC in the TCA-treated group was observed compared to in the FU- and laser-treated groups (0.04 vs. 0.15 and 0.21 in laser- and FU-treated groups, respectively; p< 0.001). No adverse events were experienced.46
A prospective study evaluated the efficacy, safety, and recurrence outcomes of phenol peels in precancerous lesions of AKs and Bowen’s disease (BD). Of the 46 cases (32 AKs and 14 BD), 39 (84.8%; composed of 29 AKs and 10 BD) experienced a complete response after 1 to 8 treatment sessions over a median follow-up period of 2.8 to 3.5 years. Concerning recurrence, only two cases (4.3%; one AKs and one BD) recurred over a period of one year. No systemic side effects, such as serious arrhythmia, were observed.47
Recent half-side intrapatient studies comparing TCA peeling with 5-aminolevulinic acid photodynamic therapy (PDT) for the treatment of patients with multiple facial or scalp AKs also pointed toward beneficial effects.48,49 Although methylaminolevulinate PDT consistently performed better than 50% TCA peel in terms of clinical efficacy, the mean clearance rate of preexisting AKs by TCA was 66.1 percent at three months and remained stable at the six-month follow-up. Considering prevention, the recurrence rate after 12 months was 17.6 percent in the TCA group.48 In the series by Holzer et al,49 35% TCA peel was compared with aminolevulinic acid (ALA) PDT. Here, the mean clearance rate by TCA at the three- and 12-month follow-up points was 78.6 percent and 48.8 percent, respectively (vs. 88.9% and 73.7% for PDT). At the 12-month follow-up, the reduction in total lesion count by TCA was 31.9 percent (vs. 58.0% for PDT). However, no significant differences were observed between TCA and ALA PDT with regard to AK prevention. In both studies, treatment-related pain, evaluated by visual analog scale, was significantly higher for PDT than for TCA peels.
A summary of possible protective effects of chemical peeling on skin photocarcinogenesis is provided in Table 2.
TABLE 2.
Select studies suggesting a protective effect on skin carcinogenesis
| PEELING FORMULATION | MATERIALS/SUBJECTS | RESULTS | STUDIES |
|---|---|---|---|
| Animal models | |||
| GA (8mg/cm2) | UV-irradiated hairless mice | Reduced and delayed tumor formation | Hong et al37 |
| aspirin (10, 40pmol), NAS (10, 40pmol) | UV-irradiated hairless mice | 40pmol aspirin and both NAS doses inhibited tumor formation rate | Bair et al38 |
| 30% SA-PEG | UV-irradiated hairless mice | Reduced and delayed tumor formation; histological atypia restoration | Dainichi et al39; Dainichi et al40 |
| GA (4, 10%), SA (2, 4%) | Irradiated mice | SA was photoprotective | National Toxicology Program41 |
| 35% GA, 30% SA, 10% + 35% TCA | UV-irradiated hairless mice | Reduced tumor count, skin p53 and COX-2 expression, and serum PGE2 level | Abdel-Daim et al;5 Abdel-Daim et al42 |
| Human models | |||
| GA (0.01–10 mM) | Human keratinocyte cell line, HaCaT | Inhibition of UV-induced cytotoxicity and apoptosis | Ahn et al43 |
| Jessner’s + 35% TCA | Humans | 75% AKs clearance at 12 months; no recurrence for at least one year | Lawrence et al44 |
| 30% TCA | Humans | 89% AKs clearance at 3 months; reduced and delayed NMSC onset | Hantash et al46 |
| 100% pure phenol | Humans | 84.8% complete response (29 AKs, 10 BD) after 1–8 sessions; two recurrences over one year | Kaminaka et al47 |
| 50% TCA | Humans | 66.1% AKs clearance at 3 and 6 months; 17.6% recurrence at 12 months | Di Nuzzo et al48 |
| 35% TCA | Humans | 78.6% and 48.8% AKs clearance at 3 and 12 months | Holzer et al49 |
GA: glycolic acid; UV: ultraviolet; NAS: sodium salicylate; SA: salicylic acid; PEG: polyethylene glycol; TCA: trichloracetic acid; PGE2: prostaglandin E2; COX: cyclooxygenase; AKs: actinic keratoses; mo: months; NMSC: nonmelanoma skin cancer; BD: Bowen’s disease
CONCLUSION
Guidelines from the British Association of Dermatologists suggest the inclusion of chemical peels among valid field-directed treatment options for AKs.29 However, no level of evidence or strength of recommendation is provided, probably due to the current lack of randomized controlled trials. As the burden of NMSC is high, alternative and preventive modalities are clearly necessary. In this setting, chemical peels might have the potential to fill a therapeutic gap.
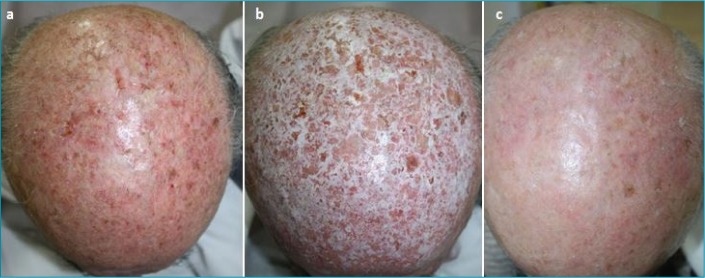
As presented, chemexfoliation has not only demonstrated efficacy in clearing visible AKs but also offers the benefit of treating the background of subclinical lesions (Figure 1). Various peeling formulas have shown promising results in terms of skin cancer prophylaxis in both human and animal studies. Although the precise mechanisms remain elusive, the findings suggest the possible clinical use of chemical peeling for the prevention of skin tumors, thus rendering it an alternative strategy for patients with extensive field damage.
FIGURE 1.
From left to right: multiple actinic keratoses of the scalp—Before (a), during (b), and two months after (c) medium-depth peel (Jessner’s solution and 35% TCA)
In addition, chemexfoliation is a versatile and safe therapy that can be applied repeatedly at frequent intervals. It demonstrates superiority in procedural ease, tolerance, healing time, patient adherence, and cost-effectiveness. Peels can also be combined with other resurfacing techniques, such as 5% FU, in an attempt to optimize outcomes and allow clinicians to tailor the treatment based on individual patient needs.
The need for further prospective studies with longer follow-up periods when utilizing chemical peels as preventive agents for carcinogenesis might sound trivial. However, as chemical peeling becomes increasingly sophisticated, it is realistic to expect that more optimized formulas will further improve the success in tumor prevention in photoaged skin.
REFERENCES
- Soleymani T, Lanoue J, Rahman Z. A practical approach to chemical peels: a review offundamentals and step-by-step algorithmic protocol for treatment. J Clin Aesthet Dermatol. 2018;11(8):21–28. [PMC free article] [PubMed] [Google Scholar]
- Lee KC, Wambier CG, Soon SL et al. Basic chemical peeling-superficial and medium-depth peels. J Am Acad Dermatol. 2019;81(2):313–324. doi: 10.1016/j.jaad.2018.10.079. [DOI] [PubMed] [Google Scholar]
- Kontochristopoulos G, Platsidaki E. Chemical peels in active acne and acne scars. Clin Dermatol. 2017;35(2):179–182. doi: 10.1016/j.clindermatol.2016.10.011. [DOI] [PubMed] [Google Scholar]
- Funasaka Y, Abdel-Daim M, Kawana S, Nishigori C. Effect of chemical peeling on the skin in relation to UV irradiation. Exp Dermatol. 2012;21(Suppl 1):31–35. doi: 10.1111/j.1600-0625.2012.01500.x. [DOI] [PubMed] [Google Scholar]
- Abdel-Daim M, Funasaka Y, Kamo T et al. Effect of chemical peeling on photocarcinogenesis. J Dermatol. 2010;37(10):864–872. doi: 10.1111/j.1346-8138.2010.00859.x. [DOI] [PubMed] [Google Scholar]
- DeAngelo AB, Daniel FB, Wong DM, George MH. The induction of hepatocellular neoplasia by trichloroacetic acid administered in the drinking water of the male B6C3F1 mouse. J Toxicol Environ Health A. 2008;71(16):1056–1068. doi: 10.1080/15287390802111952. [DOI] [PubMed] [Google Scholar]
- Pereira MA, Kramer PM, Conran PB, Tao L. Effect of chloroform on dichloroacetic acid and trichloroacetic acid-induced hypomethylation and expression of the c-myc gene and on their promotion of liver and kidney tumors in mice. Carcinogenesis. 2001;22(9):1511–1519. doi: 10.1093/carcin/22.9.1511. [DOI] [PubMed] [Google Scholar]
- Blum D. A Warning on chemical peels. New York Times. https://well.blogs.nytimes.com/2013/10/25/a-warning-on-chemical-peels. site. Oct 25, 2013. Accessed February 12, 2020.
- Varshney M, Chandra A, Chauhan LK, Goel SK. In vitro cytogenetic assessment of trichloroacetic acid in human peripheral blood lymphocytes. Environ SciPollutRes Int. 2014;21(2):843–850. doi: 10.1007/s11356-013-1949-6. [DOI] [PubMed] [Google Scholar]
- Tao I, Wang W, Li L et al. DNA hypomethylation induced by drinking water disinfection by-products in mouse and rat kidney. ToxicolSci. 2005;87(2):344–352. doi: 10.1093/toxsci/kfi257. [DOI] [PubMed] [Google Scholar]
- Bull RJ, Sasser LB, Lei XC. Interactions in the tumor-promoting activity of carbon tetrachloride, trichloroacetate, and dichloroacetate in the liver of male B6C3F1 mice. Toxicology. 2004;199(2–3):169–183. doi: 10.1016/j.tox.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Bull RJ, Orner GA, Cheng RS et al. Contribution of dichloroacetate and trichloroacetate to liver tumor induction in mice by trichloroethylene. Toxicol Appl Pharmacol. 2002;182(1):55–65. doi: 10.1006/taap.2002.9427. [DOI] [PubMed] [Google Scholar]
- Tao I, Yang S, Xie M et al. Hypomethylation and overexpression of c-jun and c-myc protooncogenes and increased DNA methyltransferase activity in dichloroacetic and trichloroacetic acid-promoted mouse liver tumors. Cancer Lett. 2000;158(2):185–193. doi: 10.1016/s0304-3835(00)00518-8. [DOI] [PubMed] [Google Scholar]
- Tao I, Kramer PM, Ge R, Pereira MA. Effect of dichloroacetic acid and trichloroacetic acid on DNA methylation in liver and tumors of female B6C3F1 mice. ToxicolSci. 1998;43(2):139–144. doi: 10.1006/toxs.1998.2449. [DOI] [PubMed] [Google Scholar]
- Pereira MA, Li K, Kramer PM. Promotion by mixtures of dichloroacetic acid and trichloroacetic acid of N-methyl-N-nitrosourea-initiated cancer in the liver offemale B6C3F1 mice. Cancer Lett. 1997;115(1):15–23. doi: 10.1016/s0304-3835(97)04699-5. [DOI] [PubMed] [Google Scholar]
- Bull RJ. Mode of action of liver tumor induction by trichloroethylene and its metabolites, trichloroacetate and dichloroacetate. Environ Health Perspect. 2000;108(Suppl 2):241–259. doi: 10.1289/ehp.00108s2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeAngelo AB, Daniel FB, Most BM, Olson GR. Failure of monochloroacetic acid and trichloroacetic acid administered in the drinking water to produce liver cancer in male F344/N rats. J Toxicol Environ Health. 1997;52(5):425–445. doi: 10.1080/00984109708984074. [DOI] [PubMed] [Google Scholar]
- Plewa MJ, Simmons JE, Richardson SD, Wagner ED. Mammalian cell cytotoxicity and genotoxicity of the haloacetic acids, a major class of drinking water disinfection by-products. Environ Mol Mutagen. 2010;51(8–9):871–878. doi: 10.1002/em.20585. [DOI] [PubMed] [Google Scholar]
- Plewa MJ, Kargalioglu Y, Vankerk D et al. Mammalian cell cytotoxicity and genotoxicity analysis of drinking water disinfection by-products. Environ Mol Mutagen. 2002;40(2):134–142. doi: 10.1002/em.10092. [DOI] [PubMed] [Google Scholar]
- Harrington-Brock K, Doerr CL, Moore M. Mutagenicity ofthree disinfection by-products: di- and trichloroacetic acid and chloral hydrate in L5178Y/TK +/- (-)3.7.2C mouse lymphoma cells. MutatRes. 1998;413(3):265–276. doi: 10.1016/s1383-5718(98)00026-6. [DOI] [PubMed] [Google Scholar]
- Zhang I, Xu L, Zeng Q et al. Comparison of DNA damage in human-derived hepatoma line (HepG2) exposed to the fifteen drinking water disinfection byproducts using the single cell gel electrophoresis assay. MutatRes. 2012;741(1–2):89–94. doi: 10.1016/j.mrgentox.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Moore MM, Harrington-Brock K. Mutagenicity of trichloroethylene and its metabolites: implications for the risk assessment of trichloroethylene. Environ Health Perspect. 2000;108(Suppl 2):215–223. doi: 10.1289/ehp.00108s2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay JM, Fox V, Griffiths K et al. Trichloroacetic acid: investigation into the mechanism of chromosomal damage in the in vitro human lymphocyte cytogenetic assay and the mouse bone marrow micronucleus test. Carcinogenesis. 1995;16(5):1127–1133. doi: 10.1093/carcin/16.5.1127. [DOI] [PubMed] [Google Scholar]
- Toxopeus C, Frazier JM. Kinetics of trichloroacetic acid and dichloroacetic acid in the isolated perfused rat liver. Toxicol Appl Pharmacol. 1998;152(1):90–98. doi: 10.1006/taap.1998.8505. [DOI] [PubMed] [Google Scholar]
- Lumpkin MH, Bruckner JV, Campbell JL et al. Plasma binding of trichloroacetic acid in mice, rats, and humans under cancer bioassay and environmental exposure conditions. Drug Metab Dispos. 2003;31(10):1203–1207. doi: 10.1124/dmd.31.10.1203. [DOI] [PubMed] [Google Scholar]
- Templin MV, Stevens DK, Stenner RD et al. Factors affecting species differences in the kinetics of metabolites of trichloroethylene. J Toxicol Environ Health. 1995;44(4):435–447. doi: 10.1080/15287399509531972. [DOI] [PubMed] [Google Scholar]
- Arenberger P, Arenbergerova M. New and current preventive treatment options in actinic keratosis. J Eur Acad Dermatol Venereol. 2017;31(Suppl 5):13–17. doi: 10.1111/jdv.14375. [DOI] [PubMed] [Google Scholar]
- Ceilley RI, Jorizzo JL. Current issues in the management of actinic keratosis. J Am Acad Dermatol. 2013;68(1 Suppl 1):S28–S38. doi: 10.1016/j.jaad.2012.09.051. [DOI] [PubMed] [Google Scholar]
- de Berker D, McGregor JM, Mohd Mustapa MF et al. British Association of Dermatologists’guidelines for the care of patients with actinic keratosis 2017. Br J Dermatol. 2017;176(1):20–43. doi: 10.1111/bjd.15107. [DOI] [PubMed] [Google Scholar]
- Isoda M, Ueda S, Imayama S, Tsukahara K. New formulation of chemical peeling agent: histological evaluation in sun-damaged skin model in hairless mice. J Dermatol Sci. 2001;27(Suppl 1):S60–S67. doi: 10.1016/s0923-1811(01)00111-6. [DOI] [PubMed] [Google Scholar]
- Costa IMC, Damasceno PS1, Costa MC1, Gomes KGP. Review in peeling complications. J Cosmet Dermatol. 2017;16(3):319–326. doi: 10.1111/jocd.12329. [DOI] [PubMed] [Google Scholar]
- Park KS, Kim HJ, Kim EJ et al. Effect of glycolic acid on UVB-induced skin damage and inflammation in guinea pigs. Skin Pharmacol Appl Skin Physiol. 2002;15(4):236–245. doi: 10.1159/000065970. [DOI] [PubMed] [Google Scholar]
- Dainichi T, Koga T, Furue M et al. Paradoxical effect of trichloroacetic acid (TCA) on ultraviolet B-induced skin tumor formation. J DermatolSci. 2003;31(3):229–231. doi: 10.1016/s0923-1811(03)00041-0. [DOI] [PubMed] [Google Scholar]
- Sakai A, Yamamoto Y, Uede K, Furukawa F. Changes of epidermal Langerhans cells in skin treated with trichloroacetic acid. Eur J Dermatol. 2005;15(4):239–242. [PubMed] [Google Scholar]
- Mohr B, 3rd, Fernandez MP, Krejci-Manwaring J. Eruptive keratoacanthomas after Jessners and trichloroacetic acid peel for actinic keratosis. DermatolSurg. 2013;39(2):331–333. doi: 10.1111/dsu.12017. [DOI] [PubMed] [Google Scholar]
- Cox S. Rapid development of keratoacanthomas after a body peel. DermatolSurg. 2003;29(2):201–203. doi: 10.1046/j.1524-4725.2003.29030.x. [DOI] [PubMed] [Google Scholar]
- Hong JT, Kim EJ, Ahn KS et al. Inhibitory effect of glycolic acid on ultraviolet-induced skin tumorigenesis in SKH-1 hairless mice and its mechanism of action. Mol Carcinog. 2001;31(3):152–160. doi: 10.1002/mc.1050. [DOI] [PubMed] [Google Scholar]
- Bair WB, 3rd, Hart N, Einspahr J et al. Inhibitory effects of sodium salicylate and acetylsalicylic acid on UVB-induced mouse skin carcinogenesis. Cancer Epidemiol Biomarkers Prev. 2002;11(12):1645–1652. [PubMed] [Google Scholar]
- Dainichi T, Ueda S, Isoda M et al. Chemical peeling with salicylic acid in polyethylene glycol vehicle suppresses skin tumour development in hairless mice. Br J Dermatol. 2003;148(5):906–912. doi: 10.1046/j.1365-2133.2003.05282.x. [DOI] [PubMed] [Google Scholar]
- Dainichi T, Amano S, Matsunaga Y et al. Chemical peeling by SA-PEG remodels photo-damaged skin: suppressing p53 expression and normalizing keratinocyte differentiation. J Invest Dermatol. 2006;126(2):416–421. doi: 10.1038/sj.jid.5700066. [DOI] [PubMed] [Google Scholar]
- National Toxicology and Program Photocarcinogenesis study of glycolic acid and salicylic acid (CAS Nos. 79–14-1 and 69–72-7) in SKH-1 mice (simulated solar light and topical application study) Natl Toxicol Program Tech Rep Ser. 2007;524:1–242. [PubMed] [Google Scholar]
- Abdel-Daim M, Funasaka Y, Kamo T et al. Preventive effect of chemical peeling on ultraviolet induced skin tumor formation. J DermatolSci. 2010;60(1):21–28. doi: 10.1016/j.jdermsci.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Ahn KS, Park KS, Jung KM et al. Inhibitory effect of glycolic acid on ultraviolet B-induced c-fos expression, AP-1 activation and p53-p21 response in a human keratinocyte cell line. Cancer Lett. 2002;186(2):125–135. doi: 10.1016/s0304-3835(02)00283-5. [DOI] [PubMed] [Google Scholar]
- Lawrence N, Cox SE, Cockerell CJ et al. A comparison of the efficacy and safety of Jessners solution and 35% trichloroacetic acid vs 5% fluorouracil in the treatment of widespread facial actinic keratoses. Arch Dermatol. 1995;131(2):176–181. [PubMed] [Google Scholar]
- Witheiler DD, Lawrence N, Cox SE et al. Long-term efficacy and safety of Jessner’s solution and 35% trichloroacetic acid vs 5% fluorouracil in the treatment of widespread facial actinic keratoses. DermatolSurg. 1997;23(3):191–196. doi: 10.1111/j.1524-4725.1997.tb00020.x. [DOI] [PubMed] [Google Scholar]
- Hantash BM, Stewart DB, Cooper ZA et al. Facial resurfacing for nonmelanoma skin cancer prophylaxis. Arch Dermatol. 2006;142(8):976–982. doi: 10.1001/archderm.142.8.976. [DOI] [PubMed] [Google Scholar]
- Kaminaka C, Yamamoto Y, Yonei N et al. Phenol peels as a novel therapeutic approach for actinic keratosis and Bowen disease: prospective pilot trial with assessment of clinical, histologic; and immunohistochemical correlations. J Am Acad Dermatol. 2009;60(4):615–625. doi: 10.1016/j.jaad.2008.11.907. [DOI] [PubMed] [Google Scholar]
- Di Nuzzo S, Cortelazzi C, Boccaletti V et al. Comparative study oftrichloroacetic acid vs. photodynamic therapy with topical 5-aminolevulinic acid for actinic keratosis of the scalp. Photodermatol Photoimmunol Photomed. 2015;31(5):233–238. doi: 10.1111/phpp.12164. [DOI] [PubMed] [Google Scholar]
- Holzer G, Pinkowicz A, Radakovic S et al. Randomized controlled trial comparing 35% trichloroacetic acid peel and 5-aminolaevulinic acid photodynamic therapy for treating multiple actinic keratosis. Br J Dermatol. 2017;176(5):1155–1161. doi: 10.1111/bjd.15272. [DOI] [PubMed] [Google Scholar]
- de Berker D, McGregor JM, Mohd Mustapa MF et al. British Association of Dermatologists’ guidelines for the care of patients with actinic keratosis 2017. Br J Dermatol. 2017;176(1):20–43. doi: 10.1111/bjd.15107. [DOI] [PubMed] [Google Scholar]