Abstract
The birth tissue is predominantly comprised of amniotic membrane (AM) and umbilical cord (UC), which share the same cell origin as the fetus. These versatile biological tissues have been used to treat a wide range of conjunctival and corneal conditions since 1940. The therapeutic benefits of the birth tissue stem from its anti-inflammatory and anti-scarring properties that orchestrate regenerative healing. Although the birth tissue also contains many cytokines, growth factors, and proteins, the heavy chain 1–hyaluronic acid/pentraxin 3 (HC–HA/PTX3) matrix has been identified to be a major active tissue component responsible for AM/UC's multifactorial therapeutic actions. HC–HA/PTX3 complex is abundantly present in fresh and cryopreserved AM/UC, but not in dehydrated tissue. In this review, we discuss the tissue anatomy, the molecular mechanism of action based on HC–HA/ PTX3 to explain their therapeutic potentials, and the various forms available in ophthalmology.
Keywords: Amniotic membrane, ophthalmology, umbilical cord
Introduction
Wound healing process
Wound healing can be broken down into three phases: inflammation, proliferation, and maturation. The inflammatory phase during adult wound healing involves early infiltration of neutrophils and macrophages, followed by lymphocytes derived from the innate and adaptive immune responses. Ideally, neutrophils have a short life span and are eventually removed by M2 (anti-inflammatory) macrophages via phagocytosis. This process helps to resolve inflammation and set the stage for proliferation.[1] However, under pathological states, an extended lifespan of neutrophils prolongs inflammation. This activates M1 (pro-inflammatory) macrophages, which are ineffective in clearing apoptotic neutrophils and express pro-inflammatory and profibrogenic cytokines.[2] Accordingly, this often results in inflammation-mediated tissue injury and/or scarring.[3,4]
Contrary to the adult wound healing process, wound healing in the mammalian fetus is characterized as “scarless”, as it can regenerate tissue rather than form a scar.[5,6] Following injury to the embryo, the inflammatory response is less pronounced with decreased inflammatory cells that enter the wound,[7] as well as, the diminished production of pro-inflammatory cytokines.[8,9] In addition, scarring responses in fetal wound healing are downregulated.[10,11] While this regenerative wound healing mechanism has yet to be fully elucidated, the birth tissue might contribute to such a fascinating process.
Birth Tissue Anatomy and Histology
The birth tissue includes the umbilical cord (UC), amniotic fluid, and placenta, the latter of which is further comprised of the amniotic membrane (AM) (inner layer) and chorion (outer layer). The AM is a thin, tensile, semi-transparent membrane that forms a sac around the fetus and is in direct contact with the amniotic fluid. The chorion is a membrane that is attached to the outer surface of the AM and separates the amnion from the uterus, maternal blood, and maternal side of the decidua. The UC is a rope-like structure (roughly 40–60 cm long at term) that connects the placenta to the fetus. These birth tissues nourish the fetus and protect it from unwanted maternal immunological insults during development.
Developmentally, these tissues are derived from different cell types. The fertilized egg undergoes cell division to form the blastocyst, which possesses both an inner cell mass and an outer cell mass. The inner cell mass subsequently develops into the fetus, the AM, and the UC, whereas the outer cell mass forms the chorion and decidua. Hence, both the AM and UC share the same cellular origin with the fetus, whereas the outer cell mass tissues come in direct contact with maternal blood.[12]
Histologically, the AM has a relative thickness of 20–150 μm and consists of a simple epithelium, a thick basement membrane, and an avascular stroma rich in hyaluronic acid (HA).[13] The epithelium consists of a single layer of metabolically active cuboidal epithelial cells with microvilli that protrude into the amniotic fluid from the free surface. The epithelium is in close contact with the basement membrane, which is comprised of collagen (Types IV, VII, XV, XVI, XVII, and XVIII), fibulin, fibrillin, perlecan, agrin, fibronectin, and laminin (α3, β1, β2, β3, γ1, and γ2 chains).[14,15] The stroma is divided into three layers: the 5–20 μm compact layer (which is in contact with the basement membrane), the fibroblast layer (thickest layer of the amnion), and the spongy layer (which is adjacent to the chorion membrane). The acellular compact layer is thought to be the strongest layer of the amnion that contains collagen Types I, III, V, and VI and fibronectin. The fibroblast layer is composed of fibroblast cells and collagen (Types I, III, and VI), fibronectin, laminin, and nidogen. The spongy layer is predominantly formed of collagen Type III, as well as a few fibroblasts, Hofbauer cells (placenta macrophages), proteoglycans, and collagen Types I and IV. AM also contains growth factors including epidermal growth factor (EGF), basic fibroblast growth factor, transforming growth factor (TGF), hepatocyte growth factor, keratinocyte growth factor, and nerve growth factor (NGF).[16,17]
The UC contains the AM as its outer layer, which is exposed to the amniotic fluid. Inside are two umbilical arteries and one umbilical vein, which are embedded within a loose, proteoglycan-rich Wharton's jelly. The Wharton's jelly provides vascular support and prevents kinking of the vessels that exchange oxygen and nutrients between the fetus and mother, as there are no capillaries to support UC itself. The Wharton's jelly is comprised of collagen (Types I, II, VI, XII, XIX), fibronectin-I, lumican I, glycosaminoglycans, chondroitin/dermatan sulfate proteoglycans, and mesenchymal stem cells (SCs).[18,19] Aside from the fibrillar content (collagen and other proteins), many growth factors such as basic FGF, EGF, IGF-I, PDGF, and TGF-beta are found within the Wharton's jelly.[20]
Biological Properties of Birth Tissue
In general, AM and UC are known to provide anti-inflammatory and anti-scarring properties to support epithelial adhesion and differentiation.[21] AM and UC can be used as a permanent graft or a temporary biological bandage/patch, which may provide additional therapeutic actions depending on the indication. For instance, when AM is used as a biological bandage, it can act as a mechanical barrier to protect the ocular surface, prevent evaporation/dryness, and maintain a stable tear film. On the other hand, when used as a permanent graft, these tissues may serve as a scaffold for donor–recipient cell migration and integration.
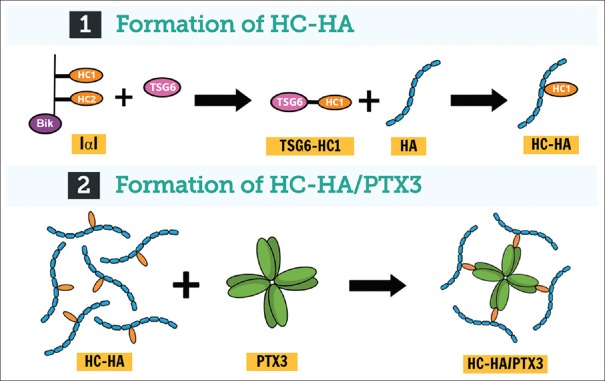
Although the AM and UC are known to contain multiple extracellular matrix components and growth factors, research efforts supported by the National Institutes of Health over the last decade have led us to discover and characterize heavy chain 1 [derived from inter-α-trypsin inhibitor]–HA/pentraxin 3 (HC–HA/ PTX3) as the major active tissue component uniquely present in both AM and UC that is responsible for their anti-inflammatory and anti-scarring properties.[22,23,24,25] The HC–HA/PTX3 complex is constitutively synthesized by human amniotic epithelial and stromal cells during pregnancy.[26,27] The biosynthetic pathway of HC–HA/ PTX3 is shown in Figure 1. High-molecular-weight HA is covalently linked with HC1 from inter-α-trypsin inhibitor through the catalytic action of tumor necrosis factor (TNF)-stimulating factor 6 (TSG-6), which is further complexed with PTX3 to form HC–HA/PTX3. Herein, we discuss the predominant molecular mechanisms of action of HC–HA/PTX3.
Figure 1.
Mechanism of heavy chain 1–hyaluronic acid/pentraxin 3 formation: (1) In the first step, tumor necrosis factor-stimulated gene 6 protein covalently binds heavy chain 1 of inter-α-trypsin inhibitor and transfers it to high-molecular-weight hyaluronan, at which time heavy chain 1 becomes conjugated and tumor stimulated gene 6 gets released. (2) In the second step, pentraxin 3 is tightly associated with the heavy chain 1–hyaluronic acid complex via binding with heavy chains
Anti-inflammatory
While the anti-inflammatory effects of AM can be observed clinically by a decrease in redness, various experiments have been performed to elucidate these exact mechanisms. Initial experiments were performed using preclinical animal models. In 2000, AM was shown to trap and prevent polymorphonuclear cell infiltration in a rabbit model of photorefractive keratectomy.[28,29,30,31] In 2001, the application of AM was shown to further promote rapid reduction of lymphocyte and macrophage infiltration in the corneal stroma of a murine model of HSV-1 necrotizing keratitis.[32] These initial experiments used the AM as a dressing, which raised the possibility that AM could act as a physical covering and barrier to inflammatory cell infiltration. In 2006, it was further reported that AM stromal matrix suppressed pro-inflammatory responses in vitro by promoting the apoptosis of interferon-α-activated, but not nonactivated macrophages, via downregulation of nuclear factor-κB and Akt–FKHR signaling pathways.[33] Further experiments using liquid AM extract supported the notion that the anti-inflammatory effect is more biological rather than mechanical/structural. It was demonstrated in 2008 that soluble AM extract reduced macrophage expression of pro-inflammatory cytokines such as TNF-α and interleukin (IL)-6 but upregulated the expression of anti-inflammatory cytokine IL-10.[34] One year later, the biochemical components of AM extract were purified in order to isolate the key components responsible for the anti-inflammatory effects. This research led to the identification of HC–HA/PTX3, which was shown to promote apoptosis of activated neutrophils and macrophages but not resting cells.[23,26] HC–HA/PTX3 was also shown to promote polarization of M2 macrophages and active phagocytosis of apoptotic neutrophils, which is a rapid process to prevent further pro-inflammatory response.[23] This indicates a seemingly selective effect that would modulate inflammation only in a pro-inflammatory environment.
Further experiments were also performed to demonstrate that the aforementioned anti-inflammatory actions extend beyond the innate response to the adaptive immune response. The adaptive immune system relies on B and T cells. CD4 + T cells become activated by contacting antigen-presenting cells, which then differentiate into Th1, Th2, Th17, or Treg. Th1 cells are known to promote pro-inflammatory responses[35,36] but can be downregulated by Tregs.[37] In-vitro studies evaluating AM derivatives showed that HC–HA/PTX3 suppressed Th1 CD4+ cells and promoted the expansion of Tregs to downregulate alloreactive responses.[38] This notion was supported in vivo by suppression of corneal allograft rejection following subconjunctival injection of HC–HA/PTX3.[38] In that study, the control group exhibited 0% corneal allograft survival by postoperative day 21, whereas 60% of allografts in the HC–HA/PTX3 group survived at postoperative day 31. This was also demonstrated in a murine model of chronic ocular graft versus host disease, wherein subconjunctival and subcutaneous injections of HC–HA/PTX3 significantly reduced the extent of infiltration of CD45+ CD4+ IL-17+ cells in the lacrimal glands.[39] This broad anti-inflammatory action of AM supports its potential in treating different types of ocular surface inflammation caused by different insults such as infection,[40,41] allergy,[42,43] and dryness.[44,45]
Anti-scarring
Fibroblasts are the major cells involved in the proliferation phase of wound healing. Their primary function is to produce collagen that provides structural integrity and minimizes the wound size. However, the collagen and glycosaminoglycans produced in the early phase of repair demonstrate an irregular fibril arrangement and composition, likely contributing to opacification and scarring. Inflammatory cells play an intricate role with fibroblasts by expressing mediators that stimulate fibroblast migration, proliferation, extracellular matrix production, and differentiation into myofibroblasts, which are the principal cells responsible for wound contraction. Fibroblasts, neutrophils, and macrophages subsequently secrete proteases that are responsible for collagen remodeling, ultimately replacing granulation tissue with a fibrotic scar. Thus, in order to prevent scarring, it is important to prevent excessive inflammation, collagen formation from fibroblasts, and differentiation of fibroblasts into myofibroblasts.
The anti-scarring phenomenon of birth tissue has been demonstrated in many clinical and preclinical studies. In rabbits, AM has been shown to reduce keratocyte (fibroblast) necrosis and reduce their differentiation into myofibroblasts (pro-scarring cells), which subsequently reduces further inflammation and prevents corneal haze after photorefractive keratectomy.[28,30,31,46] Further in-vitro experiments have shown molecular understandings of this anti-scarring mechanism, wherein human corneal, limbal, and conjunctival fibroblasts were shown to have decreased expression of TGF-β1-3 and TGF-β receptor within 8 h when cultured on the stromal side of AM.[47,48] Such effect was retained even if human[49] and mouse[50] keratocytes were exposed to serum or TGF-β1. This is consistent with other studies in which TGF-β1 is known to play a key role in scar formation, and inhibition of TGF-β can lead to reduced scarring.
It was subsequently reported that liquid-type AM extract prevents differentiation of fibroblasts into myofibroblasts.[51] Moreover, the myofibroblasts reversed to the fibroblast phenotype, which further supports the notion of a more biological action rather than structural action.[51] Further characterization was performed to show that this effect can be attributed to HC–HA/PTX3, which suppresses the TGF-β1 promoter activity of human corneal fibroblasts. HC–HA/PTX3's anti-scarring action has also been exhibited in vivo by mitigating excessive inflammation and fibrosis of chronic murine graft versus-host disease in the lacrimal gland and conjunctiva as evidenced by lack of infiltration of CD45+CD34 + collagen I + CXCR4 + fibrocytes and weak Mallory staining. These results collectively demonstrate the ability of AM and active component HC–HA/PTX3 to suppress TGF-β signaling and inhibit profibrotic and scarring actions.
Promotion of Epithelialization
The collective anti-inflammatory and anti-scarring effects of the AM help create a healthy environment for adhesion, growth, and differentiation of ocular surface cells. Furthermore, like AM, the ocular surface contains a basement membrane, which is responsible for anchoring the epithelial layer. AM's basement membrane contains Type IV collagen, laminin 1, laminin 5, and collagen VII similar to the cornea basement membrane, and these components are important for epithelial adhesion and growth.[14,52] For instance, laminin 5 has been shown to facilitate corneal epithelial adhesion,[53] and collagen VII forms anchoring fibrils that help stabilize the adhesion.[14] The overall aforementioned effects explain why AM can be used as a substrate to cultivate cells of the conjunctiva,[54,55,56,57] cornea,[58,59] limbus,[58,60,61,62,63,64] oral mucosa,[61,65,66] and endothelium[67] in vitro. These effects have been particularly advantageous for cases of limbal SC deficiency (LSCD) and explain why AM transplantation is effective in restoring vision in corneas with partial (i.e., <360° involvement) LSCD[68,69,70] and in augmenting the success of transplanting autologous[71,72] and allogeneic[73] SCs for total LSCD. It also helps explain why AM acts as a scaffold substrate (carrier) for ex-vivo expansion as alluded[74] and aids in-vivo expansion of limbal SCs in “simple limbal epithelial transplantation,” where the total percentage of limbal SCs removed from the donor eye has been reduced to <10%.[75,76]
More recently, studies have provided further insight into the regulatory mechanism of action of the limbal niche and how AM can act as a surrogate niche to support limbal epithelial SCs. These studies have demonstrated how limbal niche cells are necessary to promote limbal epithelial stem cell proliferation while maintaining stemness, and maintenance of this relationship on denuded AM results in consistent, robust epithelial outgrowth.[77] This was further verified in vitro, where SC quiescence and self-renewal were shown to be regulated through BMP and Wnt signaling pathways, respectively. Using this model, HC–HA/PTX3 was shown to suppress corneal fate decision of limbal epithelial SCs and upregulate their expression of quiescence markers.[22,78,79] Furthermore, HC–HA/PTX3 was shown to suppress canonical Wnt but activate noncanonical Wnt (planar cell polarity) and BMP canonical signaling in both limbal niche and epithelial cells.[22,78,79] Such effect helps further explain how AM is clinically useful for both in-vivo and ex-vivo expansion of limbal epithelial SCs.
Neuroregenerative Action
Early neurophysiologic changes after tissue inflammation and injury may trigger the generation of peripheral and central neuronal sensitization, leading to chronic pain. In this regard, a well-tolerated regenerative therapy may be invaluable in preventing the development of chronic pain conditions. Recently, AM has been shown to induce a neurotrophic and anti-inflammatory environment conducive of promoting corneal nerve regeneration,[44,80,81] and the restoration of the normal nerve function is postulated to be one mechanism in pain alleviation.[80] More specifically, John et al.[44] reported that single use of cryopreserved AM for a period of 3–5 days reduced pain for at least 3 months by promoting corneal nerve regeneration in patients suffering from dry eye disease (DED). In this prospective trial, twenty patients with chronic, moderate-to-severe DED were equally randomized to receive a self-retained device containing cryopreserved AM ((PROKERA® Slim [PKS], Bio-Tissue, Miami, FL), treatment group) or conventional maximum treatment (control group). The results showed that symptoms and signs such as corneal staining, pain, discomfort, and visual disturbances significantly improved in the study group receiving PROKERA but remained unchanged in the control group. In-vivo confocal microscopy was used to image the sub-basal corneal nerves, which showed a significant increase in the central corneal nerve density from 12,241 ± 5083 μm/mm2 at baseline to 16,364 ± 3734 μm/mm2 at 1 month and 18,827 ± 5453 μm/mm2 at 3 months (P = 0.015) in the treatment group, which was also associated with a significant increase in corneal sensitivity (P < 0.001).
The aforementioned results from the study by John et al.[44] conclude that the lasting effect (at least 3 months) of a single placement of AM for 3–5 days may be attributed to corneal nerve regeneration. Such hypothesis was further supported by McDonald et al.[45] and Morkin and Hamrah.[80] Morkin and Hamrah showed that the placement of a self-retained, cryopreserved AM for 6.4 ± 1.1 days led to 36.6% ±17.6% increase in total nerve density during a follow-up of 9.3 ± 0.8 months.[82] Such effect was also associated with a 72.5% ±8.4% improvement in pain severity (from 6.3 ± 0.8 to 1.9 ± 0.6, scale 1–10, P = 0.0003).[80] Studies have shown a beneficial effect in animal models as well.[44,80] The positive effect AM exerts on corneal nerve regeneration may be attributed to AM's anti-inflammatory and anti-scarring actions that indirectly lead to a pro-regenerative environment (similar to fetal scarless healing) or directly from the compositional matter of AM such as HC–HA/PTX3, neurotransmitters, and neurotrophins (NTs), which play significant roles in neuronal development and survival. Potential neurotrophic factors present in AM that may play a role include NGF, brain-derived neurotrophic factor, NT-3, and ephrin-A2.[16,17]
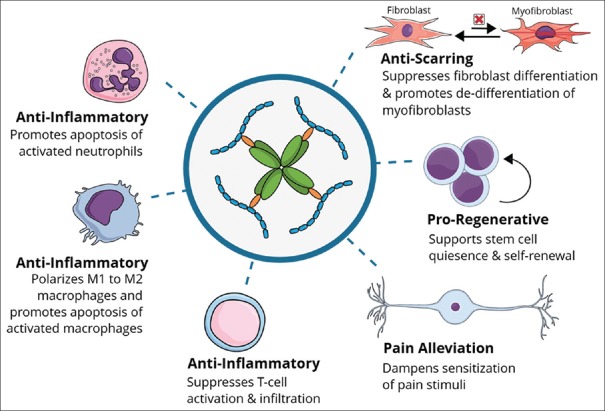
Overall, the cumulative research has demonstrated birth tissue to exert an antipain action indirectly through anti-inflammatory, anti-scarring, and growth-promoting effects[22,23,24,26,27,38,78] that promote regenerative healing including nerve regeneration [Figure 2 and Table 1]. Further investigation is underway including its clinical application in neurotrophic keratitis and dry eye disease.[84]
Figure 2.
Actions of heavy chain 1–hyaluronic acid/pentraxin 3 can indirectly reduce pain by exerting anti-inflammatory and anti-scarring effects. In addition, heavy chain 1– hyaluronic acid/pentraxin 3 directly reduces pain through its ability to dampen the sensitization of pain stimuli
Table 1.
Amniotic membrane and umbilical cord action by cell type
| Cell type | Action | Clinical implication | Supporting publication |
|---|---|---|---|
| Neutrophil | Promotes apoptosis of fMLP- or LPS-activated neutrophils | Reduces inflammation | [23] |
| No effect on resting (nonactivated) neutrophils | Reduces injury to host tissue | ||
| Macrophage | Promotes apoptosis of LPS-, IFN-γ, or LPS/ IFN-γ-activated macrophages | Reduces inflammation | [23,26,34,38] |
| No effect on resting macrophages | Reduces injury to host tissue | ||
| Promotes polarization of M1 (pro-inflammatory) macrophages to M2 (anti-inflammatory) macrophages | Removes inflammatory mediators and cell debris | ||
| Promotes macrophage phagocytosis of apoptotic neutrophils | |||
| Suppresses macrophage infiltration | |||
| T-Cell | Suppresses activation of CD4+T-cells | Reduces tissue inflammation in immunity and autoimmunity | |
| Promotes significant expansion of CD25+/FOXP3+T-cells Suppresses infiltration of CD45+CD4+IL-17+inflammatory immune cells |
Prolongs co-transplanted allograft survival | [38,83] | |
| Fibroblast | Downregulates TGF-β1, 2, and 3 and TGF-βR2 transcripts | Prevents scar formation | [26,39,46,47,48,49,50] |
| Suppresses TGF-β1 promoter activity | Prevents adhesion | ||
| Prevents pSMAD2/3 nuclear translocation | Promotes normal histologic structure and biomechanical strength | ||
| Reduces myofibroblast differentiation | Promotes range of motion and function | ||
| Suppresses CD45+CD34+collagen I+CXCR4+fibrocytes and HSP47+activated fibroblasts | Prevents structural degradation or failure | ||
| Myofibroblast | Reverses differentiated myofibroblasts back to fibroblasts without proliferation | Prevents scar formation and adhesion | [31,51] |
| Prevents expression of α-SMA | Reduces scarring | ||
| Promotes normal histologic structure | |||
| Retinal pigment epithelium | Suppresses proliferation of EGF/FGF-2-stimulated RPE |
Prevents proliferative vitreoretinopathy | [83] |
| Suppresses migration of TGF-β-stimulated human RPE |
Prevents scar formation | ||
| Prevents expression of α-SMA | |||
| Suppresses EMT of TGF-β-stimulated human RPE |
LPS=Lipopolysaccharide, IFN=Interferon, IL=Interleukin, TGF=Transforming growth factor, pSMAD=Phosphorylated Smad, EGF=Epidermal growth factor, FGF=Fibroblast growth factor, RPE=Retinal pigment epithelium, SMA=Smooth muscle actin, EMT=Epithelial–mesenchymal transition, fMLP=N-Formyl-methionyl-leucyl- phenylalanine, α-SMA=alpha-smooth muscle actin
Processing Methods and Products used in Ophthalmology
The biological properties of fresh birth tissue make it an ideal tissue graft for ophthalmic indications. However, as with any allograft tissue, the fresh tissue must be processed before clinical transplantation to a human recipient to prevent the introduction, transmission, and spread of communicable disease. Therefore, effective tissue preservation and storage procedures are essential to maintain the structural and biological properties of the fresh material.
To date, varying processing methods have been developed. In general, birth tissue is processed from donated human placental tissues following healthy, live, cesarean section, full-term births after evaluating donor eligibility, placenta suitability, and donor informed consent. Birth tissue obtained from vaginal delivery is normally avoided due to possible contamination from normal vaginal flora. The birth tissue is gently cleansed with agents such as saline to remove blood and potential contamination. Outside of those parameters, the processing methods of human birth tissue can drastically vary depending on the method of preparation and preservation. For example, two of the most commonly used methods are cryopreservation and dehydration. Cryopreservation refers to storage and transportation of tissue at low temperatures, which devitalizes the living cells but retains the natural structural and biological characteristics relevant to this tissue.[85] Contrarily, dehydration commonly refers to subjecting the tissue to heat to remove the moisture therein. Both methods aid in reducing chemical reactions and inhibiting microorganism growth, however, a head-to-head laboratory study found that only cryopreservation retains the HC–HA/PTX3 complex.[85] Further comparative studies are needed to demonstrate the clinical superiority of one preparation method over another, however, prior studies have demonstrated similar outcomes using fresh and cryopreserved AM.[86]
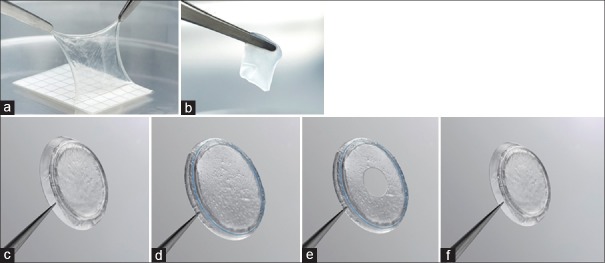
Cryopreserved AM was the first birth tissue product commercially available. Cryopreserved AM (AmnioGraft; Biotissue, Miami, FL, USA) was launched in 1997 and designated as a 361-human cell/tissue product in 2001 by the Food and Drug Administration (FDA) when used for ocular surface reconstruction. The FDA recognized that “AM that has not been dehydrated or decellularized may be used for wound repair and wound healing.” The AM sheet comes on a nitrocellulose carrier sheet that is removed prior to transplantation. The carrier sheet maintains the configuration of the AM graft, as the sticky stroma side of AM readily adheres to the sheet. To avoid suture-induced inflammation and medical costs associated with surgery, a self-retained cryopreserved AM product (PROKERA; Biotissue, Miami, FL, USA) was developed and cleared by the FDA in 2004 as a Class II medical device under 510(k) #K032104. In 2011, a cryopreserved UC (under the brand name AmnioGuard; Biotissue, Miami, FL, USA) was marketed and commonly used as a barrier graft over glaucoma shunt tubes. The thickness of the UC product is approximately 500–900 μm compared to 75–150 μm for the AM product.[13] These cryopreserved products [Figure 3] can be stored for 2 years (from the manufacturing date) at temperatures ranging from − 80°C to 4°C. Both cryopreserved AM and UC have been used clinically in ophthalmology to treat indications such as keratitis, band keratopathy, burns, keratoconjunctivitis, ulcers, basement membrane dystrophy, LSCD, epithelial defects, corneal erosion, Stevens–Johnson syndrome, and toxic epidermal necrolysis. Over the last year, publications have further demonstrated the utility of self-retained AM for Sjogren syndrome, filamentary keratitis, and HSV keratitis;[87,88,89] UC grafts for repair of the ocular surface (after excision of melanoma, carcinoma, and pterygium), socket contracture, orbital implant exposure, forniceal contracture, and entropion repair;[90] and AM grafts for macular hole repair, Stevens–Johnson syndrome, and toxic epidermal necrolysis.[91,92,93,94]
Figure 3.
Cryopreserved birth tissue products in ophthalmology: (a) Cryopreserved amniotic membrane was the first birth tissue product on the market. (b) The cryopreserved umbilical cord product (AmnioGuard) was available in 2010. The PROKERA family of devices comprises four models (c-f) of a biologic corneal bandage. (c) The PROKERA is designed such that an amniotic membrane is clipped to a dual polycarbonate ring system, allowing the membrane to act as a biological bandage when in contact with the cornea. (f) PROKERA PLUS model has two amniotic membrane layers, providing extra therapeutic benefit. (d) PROKERA SLIM and (e) PROKERA CLEAR both have a thinner, smaller ring (less plastic), while (e) PROKERA CLEAR also provides central aperture clearance
Dehydrated AM grafts (AmbioDry; OKTOS Surgical Corporation, Costa Mesa, CA, USA) have been used since 2002. The FDA recognized in 2005 that the dehydration and decellularization of the AM graft alters the characteristics of the original amniotic tissue. As such, the product should be only intended for wound covering. Aside from the dehydrated AM grafts that are intended to be used in surgical applications (e.g., Ambio2® single layer and Ambio5 multi-layered AM), an overlay AM disc (AmbioDisk; Katena, Denville, NJ, USA) can be placed under bandage contact lens for office-based applications and has been used since 2011–2012. Other dehydrated AM grafts including BioDOptix (Integra, Plainsboro Township, NJ, USA), Aril (Seed Biotech, Dallas, TX, USA), OculoMatrix (Skye Biologics, El Segundo, CA, USA), VisiDisc (Skye® Biologics, El Segundo, CA, USA), and AmioTek (ISP Surgical LLC, Boston, MA, USA) have also been released on the market. The dehydrated AM grafts can be stored at ambient room temperature for 3–5 years.
Overall, there are a variety of amniotic-derived products used in ophthalmology. Effective tissue preservation and storage procedures are essential for maintaining the structural and biological properties of the fresh material. As such, only cryopreserved products have been shown to retain the HC–HA/PTX3 complex, which has been identified as an active matrix component responsible for the observed anti-inflammatory and anti-scarring properties of the birth tissue.[85]
Conclusion
Of the birth tissue components, the AM and UC have been predominantly used in ophthalmology due to their known anti-inflammatory and anti-scarring effects, which create a healthy environment for the adhesion, growth, and differentiation of ocular surface cells. Although these tissues are known to contain multiple extracellular matrix components and growth factors, research efforts have demonstrated HC–HA/PTX3 to be a key matrix within these tissues that is responsible for the aforementioned therapeutic benefits and has been further shown to support limbal SC quiescence and stemness. Preservation of the HC–HA/PTX3 matrix has been shown when processing birth tissue via cryopreservation but not dehydration. Collectively, these data help explain why cryopreserved AM may promote regenerative wound healing, especially in severe forms of ocular surface damage and inflammation.
Financial support and sponsorship
The work was supported by a RO1 EY06819 grant (to SCGT) from the National Eye Institute, National Institutes of Health, Bethesda MD, USA.
Conflicts of interest
ST, OM, AL, and ST are employees of TissueTech, Inc. Dr. Tseng has obtained a patent for the method of preparation and clinical uses of amniotic membrane and has licensed the rights to Tissue tech, Inc., which procures and processes, and to Bio-Tissue, Inc, which is a subsidiary of TissueTech, Inc. to distribute cryopreserved amniotic membrane for clinical and research uses.
References
- 1.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–8. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N, et al. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12:231–8. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- 3.Koh TJ, DiPietro LA. Inflammation and wound healing: The role of the macrophage. Expert Rev Mol Med. 2011;13:e23. doi: 10.1017/S1462399411001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121:985–97. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rolfe KJ, Grobbelaar AO. A review of fetal scarless healing. ISRN Dermatol. 2012;2012:698034. doi: 10.5402/2012/698034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larson BJ, Longaker MT, Lorenz HP. Scarless fetal wound healing: A basic science review. Plast Reconstr Surg. 2010;126:1172–80. doi: 10.1097/PRS.0b013e3181eae781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowin AJ, Holmes TM, Brosnan P, Ferguson MW. Expression of TGF-beta and its receptors in murine fetal and adult dermal wounds. Eur J Dermatol. 2001;11:424–31. [PubMed] [Google Scholar]
- 8.Liechty KW, Adzick NS, Crombleholme TM. Diminished interleukin 6 (IL-6) production during scarless human fetal wound repair. Cytokine. 2000;12:671–6. doi: 10.1006/cyto.1999.0598. [DOI] [PubMed] [Google Scholar]
- 9.Liechty KW, Crombleholme TM, Cass DL, Martin B, Adzick NS. Diminished interleukin-8 (IL-8) production in the fetal wound healing response. J Surg Res. 1998;77:80–4. doi: 10.1006/jsre.1998.5345. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan KM, Lorenz HP, Meuli M, Lin RY, Adzick NS. A model of scarless human fetal wound repair is deficient in transforming growth factor beta. J Pediatr Surg. 1995;30:198–202. doi: 10.1016/0022-3468(95)90560-x. [DOI] [PubMed] [Google Scholar]
- 11.Olutoye OO, Yager DR, Cohen IK, Diegelmann RF. Lower cytokine release by fetal porcine platelets: A possible explanation for reduced inflammation after fetal wounding. J Pediatr Surg. 1996;31:91–5. doi: 10.1016/s0022-3468(96)90326-7. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg LE, Rosenberg DD. Human Genes and Genomes. 4. San Diego: Academic Press; 2012. Growth, Development, and Reproduction; pp. 27–50. [Google Scholar]
- 13.Tan EK, Cooke M, Mandrycky C, Mahabole M, He H, O’Connell J, et al. Structural and biological comparison of cryopreserved and fresh amniotic membrane tissues. J Biomater Tissue Eng. 2014;4:379–88. [Google Scholar]
- 14.Fukuda K, Chikama T, Nakamura M, Nishida T. Differential distribution of subchains of the basement membrane components type IV collagen and laminin among the amniotic membrane, cornea, and conjunctiva. Cornea. 1999;18:73–9. [PubMed] [Google Scholar]
- 15.Dietrich-Ntoukas T, Hofmann-Rummelt C, Kruse FE, Schlötzer-Schrehardt U. Comparative analysis of the basement membrane composition of the human limbus epithelium and amniotic membrane epithelium. Cornea. 2012;31:564–9. doi: 10.1097/ICO.0b013e3182254b78. [DOI] [PubMed] [Google Scholar]
- 16.Koizumi NJ, Inatomi TJ, Sotozono CJ, Fullwood NJ, Quantock AJ, Kinoshita S. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000;20:173–7. [PubMed] [Google Scholar]
- 17.López-Valladares MJ, Teresa Rodríguez-Ares M, Touriño R, Gude F, Teresa Silva M, Couceiro J. Donor age and gestational age influence on growth factor levels in human amniotic membrane. Acta Ophthalmol. 2010;88:e211–6. doi: 10.1111/j.1755-3768.2010.01908.x. [DOI] [PubMed] [Google Scholar]
- 18.Bańkowski E. Collagen of the umbilical cord and its alteration in EPH-gestosis (preeclampsia) Proc Indian Acad Sci (Chem Sci) 1999;111:207–13. [Google Scholar]
- 19.Jadalannagari S, Converse G, McFall C, Buse E, Filla M, Villar MT, et al. Decellularized Wharton's Jelly from human umbilical cord as a novel 3D scaffolding material for tissue engineering applications. PLoS One. 2017;12:e0172098. doi: 10.1371/journal.pone.0172098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sobolewski K, Małkowski A, Bańkowski E, Jaworski S. Wharton's jelly as a reservoir of peptide growth factors. Placenta. 2005;26:747–52. doi: 10.1016/j.placenta.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 21.FDA Decision Letter. Available from: http://www.fda.gov/downloads/CombinationProducts/JurisdictionalInformation/RFDJurisdictionalDecisions/RedactedDecisionLetters/UCM113701pdf2001 .
- 22.Tseng SC. HC-HA/PTX3 purified from amniotic membrane as novel regenerative matrix: Insight into relationship between inflammation and regeneration. Invest Ophthalmol Vis Sci. 2016;57:ORSFh1–8. doi: 10.1167/iovs.15-17637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He H, Zhang S, Tighe S, Son J, Tseng SC. Immobilized heavy chain-hyaluronic acid polarizes lipopolysaccharide-activated macrophages toward M2 phenotype. J Biol Chem. 2013;288:25792–803. doi: 10.1074/jbc.M113.479584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang S, Zhu YT, Chen SY, He H, Tseng SC. Constitutive expression of pentraxin 3 (PTX3) protein by human amniotic membrane cells leads to formation of the heavy chain (HC)-hyaluronan (HA)-PTX3 complex. J Biol Chem. 2014;289:13531–42. doi: 10.1074/jbc.M113.525287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shay E, He H, Sakurai S, Tseng SC. Inhibition of angiogenesis by HC·HA, a complex of hyaluronan and the heavy chain of inter-α-inhibitor, purified from human amniotic membrane. Invest Ophthalmol Vis Sci. 2011;52:2669–78. doi: 10.1167/iovs.10-5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He H, Li W, Tseng DY, Zhang S, Chen SY, Day AJ, et al. Biochemical characterization and function of complexes formed by hyaluronan and the heavy chains of inter-alpha-inhibitor (HC*HA) purified from extracts of human amniotic membrane. J Biol Chem. 2009;284:20136–46. doi: 10.1074/jbc.M109.021881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang S, He H, Day AJ, Tseng SC. Constitutive expression of inter-α-inhibitor (IαI) family proteins and tumor necrosis factor-stimulated gene-6 (TSG-6) by human amniotic membrane epithelial and stromal cells supporting formation of the heavy chain-hyaluronan (HC-HA) complex. J Biol Chem. 2012;287:12433–44. doi: 10.1074/jbc.M112.342873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park WC, Tseng SC. Modulation of acute inflammation and keratocyte death by suturing, blood, and amniotic membrane in PRK. Invest Ophthalmol Vis Sci. 2000;41:2906–14. [PubMed] [Google Scholar]
- 29.Wang MX, Gray TB, Park WC, Prabhasawat P, Culbertson W, Forster R, et al. Reduction in corneal haze and apoptosis by amniotic membrane matrix in excimer laser photoablation in rabbits. J Cataract Refract Surg. 2001;27:310–9. doi: 10.1016/s0886-3350(00)00467-3. [DOI] [PubMed] [Google Scholar]
- 30.Choi YS, Kim JY, Wee WR, Lee JH. Effect of the application of human amniotic membrane on rabbit corneal wound healing after excimer laser photorefractive keratectomy. Cornea. 1998;17:389–95. doi: 10.1097/00003226-199807000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Woo HM, Kim MS, Kweon OK, Kim DY, Nam TC, Kim JH. Effects of amniotic membrane on epithelial wound healing and stromal remodelling after excimer laser keratectomy in rabbit cornea. Br J Ophthalmol. 2001;85:345–9. doi: 10.1136/bjo.85.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heiligenhaus A, Bauer D, Meller D, Steuhl KP, Tseng SC. Improvement of HSV-1 necrotizing keratitis with amniotic membrane transplantation. Invest Ophthalmol Vis Sci. 2001;42:1969–74. [PubMed] [Google Scholar]
- 33.Li W, He H, Kawakita T, Espana EM, Tseng SC. Amniotic membrane induces apoptosis of interferon-gamma activated macrophages in vitro. Exp Eye Res. 2006;82:282–92. doi: 10.1016/j.exer.2005.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He H, Li W, Chen SY, Zhang S, Chen YT, Hayashida Y, et al. Suppression of activation and induction of apoptosis in RAW264.7 cells by amniotic membrane extract. Invest Ophthalmol Vis Sci. 2008;49:4468–75. doi: 10.1167/iovs.08-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76:241–51. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 36.Dong C, Flavell RA. Cell fate decision: T-helper 1 and 2 subsets in immune responses. Arthritis Res. 2000;2:179–88. doi: 10.1186/ar85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, et al. Intestinal tolerance requires gut homing and expansion of FoxP3+regulatory T cells in the lamina propria. Immunity. 2011;34:237–46. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 38.He H, Tan Y, Duffort S, Perez VL, Tseng SC. In vivo downregulation of innate and adaptive immune responses in corneal allograft rejection by HC-HA/PTX3 complex purified from amniotic membrane. Invest Ophthalmol Vis Sci. 2014;55:1647–56. doi: 10.1167/iovs.13-13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogawa Y, He H, Mukai S, Imada T, Nakamura S, Su CW, et al. Heavy Chain-Hyaluronan/Pentraxin 3 from Amniotic Membrane Suppresses Inflammation and Scarring in Murine Lacrimal Gland and Conjunctiva of Chronic Graft-versus-Host Disease. Sci Rep. 2017;7:42195. doi: 10.1038/srep42195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheha H, Liang L, Li J, Tseng SC. Sutureless amniotic membrane transplantation for severe bacterial keratitis. Cornea. 2009;28:1118–23. doi: 10.1097/ICO.0b013e3181a2abad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheha H, Tighe S, Cheng AM, Tseng SC. A stepping stone in treating dendritic keratitis. Am J Ophthalmol Case Rep. 2017;7:55–8. doi: 10.1016/j.ajoc.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shammas MC, Lai EC, Sarkar JS, Yang J, Starr CE, Sippel KC. Management of acute Stevens-Johnson syndrome and toxic epidermal necrolysis utilizing amniotic membrane and topical corticosteroids. Am J Ophthalmol. 2010;149:203–1300. doi: 10.1016/j.ajo.2009.08.040. [DOI] [PubMed] [Google Scholar]
- 43.Shay E, Khadem JJ, Tseng SC. Efficacy and limitation of sutureless amniotic membrane transplantation for acute toxic epidermal necrolysis. Cornea. 2010;29:359–61. doi: 10.1097/ICO.0b013e3181acf816. [DOI] [PubMed] [Google Scholar]
- 44.John T, Tighe S, Sheha H, Hamrah P, Salem ZM, Cheng AM, et al. Corneal nerve regeneration after self-retained cryopreserved amniotic membrane in dry eye disease. J Ophthalmol. 2017;2017:6404918. doi: 10.1155/2017/6404918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McDonald MB, Sheha H, Tighe S, Janik SB, Bowden FW, Chokshi AR, et al. Treatment outcomes in the DRy Eye Amniotic Membrane (DREAM) study. Clin Ophthalmol. 2018;12:677–81. doi: 10.2147/OPTH.S162203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi TH, Tseng SC. In vivo and in vitro demonstration of epithelial cell-induced myofibroblast differentiation of keratocytes and an inhibitory effect by amniotic membrane. Cornea. 2001;20:197–204. doi: 10.1097/00003226-200103000-00019. [DOI] [PubMed] [Google Scholar]
- 47.Lee SB, Li DQ, Tan DT, Meller DC, Tseng SC. Suppression of TGF-beta signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Curr Eye Res. 2000;20:325–34. [PubMed] [Google Scholar]
- 48.Tseng SC, Li DQ, Ma X. Suppression of transforming growth factor-beta isoforms, TGF-beta receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J Cell Physiol. 1999;179:325–35. doi: 10.1002/(SICI)1097-4652(199906)179:3<325::AID-JCP10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 49.Espana EM, He H, Kawakita T, Di Pascuale MA, Raju VK, Liu CY, et al. Human keratocytes cultured on amniotic membrane stroma preserve morphology and express keratocan. Invest Ophthalmol Vis Sci. 2003;44:5136–41. doi: 10.1167/iovs.03-0484. [DOI] [PubMed] [Google Scholar]
- 50.Kawakita T, Espana EM, He H, Hornia A, Yeh LK, Ouyang J, et al. Keratocan expression of murine keratocytes is maintained on amniotic membrane by down-regulating transforming growth factor-beta signaling. J Biol Chem. 2005;280:27085–92. doi: 10.1074/jbc.M409567200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li W, He H, Chen YT, Hayashida Y, Tseng SC. Reversal of myofibroblasts by amniotic membrane stromal extract. J Cell Physiol. 2008;215:657–64. doi: 10.1002/jcp.21345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Endo K, Nakamura T, Kawasaki S, Kinoshita S. Human amniotic membrane, like corneal epithelial basement membrane, manifests the alpha5 chain of type IV collagen. Invest Ophthalmol Vis Sci. 2004;45:1771–4. doi: 10.1167/iovs.03-0952. [DOI] [PubMed] [Google Scholar]
- 53.Kurpakus-Wheater M. Laminin-5 is a component of preserved amniotic membrane. Curr Eye Res. 2001;22:353–7. doi: 10.1076/ceyr.22.5.353.5494. [DOI] [PubMed] [Google Scholar]
- 54.Cho BJ, Djalilian AR, Obritsch WF, Matteson DM, Chan CC, Holland EJ. Conjunctival epithelial cells cultured on human amniotic membrane fail to transdifferentiate into corneal epithelial-type cells. Cornea. 1999;18:216–24. doi: 10.1097/00003226-199903000-00013. [DOI] [PubMed] [Google Scholar]
- 55.Meller D, Tseng SC. Conjunctival epithelial cell differentiation on amniotic membrane. Invest Ophthalmol Vis Sci. 1999;40:878–86. [PubMed] [Google Scholar]
- 56.Meller D, Dabul V, Tseng SC. Expansion of conjunctival epithelial progenitor cells on amniotic membrane. Exp Eye Res. 2002;74:537–45. doi: 10.1006/exer.2001.1163. [DOI] [PubMed] [Google Scholar]
- 57.Sangwan VS, Vemuganti GK, Singh S, Balasubramanian D. Successful reconstruction of damaged ocular outer surface in humans using limbal and conjunctival stem cell culture methods. Biosci Rep. 2003;23:169–74. doi: 10.1023/b:bire.0000007690.43273.73. [DOI] [PubMed] [Google Scholar]
- 58.Koizumi N, Fullwood NJ, Bairaktaris G, Inatomi T, Kinoshita S, Quantock AJ. Cultivation of corneal epithelial cells on intact and denuded human amniotic membrane. Invest Ophthalmol Vis Sci. 2000;41:2506–13. [PubMed] [Google Scholar]
- 59.Kinoshita S, Nakamura T. Development of cultivated mucosal epithelial sheet transplantation for ocular surface reconstruction. Artif Organs. 2004;28:22–7. doi: 10.1111/j.1525-1594.2004.07319.x. [DOI] [PubMed] [Google Scholar]
- 60.Schwab IR, Reyes M, Isseroff RR. Successful transplantation of bioengineered tissue replacements in patients with ocular surface disease. Cornea. 2000;19:421–6. doi: 10.1097/00003226-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 61.Koizumi N, Cooper LJ, Fullwood NJ, Nakamura T, Inoki K, Tsuzuki M, et al. An evaluation of cultivated corneal limbal epithelial cells, using cell-suspension culture. Invest Ophthalmol Vis Sci. 2002;43:2114–21. [PubMed] [Google Scholar]
- 62.Meller D, Pires RT, Tseng SC. Ex vivo preservation and expansion of human limbal epithelial stem cells on amniotic membrane cultures. Br J Ophthalmol. 2002;86:463–71. doi: 10.1136/bjo.86.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grueterich M, Tseng SC. Human limbal progenitor cells expanded on intact amniotic membrane ex vivo. Arch Ophthalmol. 2002;120:783–90. doi: 10.1001/archopht.120.6.783. [DOI] [PubMed] [Google Scholar]
- 64.Ban Y, Cooper LJ, Fullwood NJ, Nakamura T, Tsuzuki M, Koizumi N, et al. Comparison of ultrastructure, tight junction-related protein expression and barrier function of human corneal epithelial cells cultivated on amniotic membrane with and without air-lifting. Exp Eye Res. 2003;76:735–43. doi: 10.1016/s0014-4835(03)00033-2. [DOI] [PubMed] [Google Scholar]
- 65.Nakamura T, Endo K, Cooper LJ, Fullwood NJ, Tanifuji N, Tsuzuki M, et al. The successful culture and autologous transplantation of rabbit oral mucosal epithelial cells on amniotic membrane. Invest Ophthalmol Vis Sci. 2003;44:106–16. doi: 10.1167/iovs.02-0195. [DOI] [PubMed] [Google Scholar]
- 66.Nakamura T, Inatomi T, Sotozono C, Amemiya T, Kanamura N, Kinoshita S. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br J Ophthalmol. 2004;88:1280–4. doi: 10.1136/bjo.2003.038497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ishino Y, Sano Y, Nakamura T, Connon CJ, Rigby H, Fullwood NJ, et al. Amniotic membrane as a carrier for cultivated human corneal endothelial cell transplantation. Invest Ophthalmol Vis Sci. 2004;45:800–6. doi: 10.1167/iovs.03-0016. [DOI] [PubMed] [Google Scholar]
- 68.Hayashi R, Yamato M, Sugiyama H, Sumide T, Yang J, Okano T, et al. N-Cadherin is expressed by putative stem/progenitor cells and melanocytes in the human limbal epithelial stem cell niche. Stem Cells. 2007;25:289–96. doi: 10.1634/stemcells.2006-0167. [DOI] [PubMed] [Google Scholar]
- 69.Lavker RM, Sun TT. Epidermal stem cells: Properties, markers, and location. Proc Natl Acad Sci U S A. 2000;97:13473–5. doi: 10.1073/pnas.250380097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schlötzer-Schrehardt U, Dietrich T, Saito K, Sorokin L, Sasaki T, Paulsson M, et al. Characterization of extracellular matrix components in the limbal epithelial stem cell compartment. Exp Eye Res. 2007;85:845–60. doi: 10.1016/j.exer.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 71.Meallet MA, Espana EM, Grueterich M, Ti SE, Goto E, Tseng SC. Amniotic membrane transplantation with conjunctival limbal autograft for total limbal stem cell deficiency. Ophthalmology. 2003;110:1585–92. doi: 10.1016/S0161-6420(03)00503-7. [DOI] [PubMed] [Google Scholar]
- 72.Kheirkhah A, Casas V, Raju VK, Tseng SC. Sutureless amniotic membrane transplantation for partial limbal stem cell deficiency. Am J Ophthalmol. 2008;145:787–94. doi: 10.1016/j.ajo.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tseng SC, Prabhasawat P, Barton K, Gray T, Meller D. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol. 1998;116:431–41. doi: 10.1001/archopht.116.4.431. [DOI] [PubMed] [Google Scholar]
- 74.Tsai RJ, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000;343:86–93. doi: 10.1056/NEJM200007133430202. [DOI] [PubMed] [Google Scholar]
- 75.Sangwan VS, Basu S, MacNeil S, Balasubramanian D. Simple limbal epithelial transplantation (SLET): A novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br J Ophthalmol. 2012;96:931–4. doi: 10.1136/bjophthalmol-2011-301164. [DOI] [PubMed] [Google Scholar]
- 76.Amescua G, Atallah M, Nikpoor N, Galor A, Perez VL. Modified simple limbal epithelial transplantation using cryopreserved amniotic membrane for unilateral limbal stem cell deficiency. Am J Ophthalmol. 2014;158:469–7500. doi: 10.1016/j.ajo.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 77.Chen SY, Hayashida Y, Chen MY, Xie HT, Tseng SC. A new isolation method of human limbal progenitor cells by maintaining close association with their niche cells. Tissue Eng Part C Methods. 2011;17:537–48. doi: 10.1089/ten.tec.2010.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tseng SC, He H, Zhang S, Chen SY. Niche regulation of limbal epithelial stem cells: Relationship between inflammation and regeneration. Ocul Surf. 2016;14:100–12. doi: 10.1016/j.jtos.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen SY, Han B, Zhu YT, Mahabole M, Huang J, Beebe DC, et al. HC-HA/PTX3 purified from amniotic membrane promotes bmp signaling in limbal niche cells to maintain quiescence of limbal epithelial progenitor/stem cells. Stem Cells. 2015;33:3341–55. doi: 10.1002/stem.2091. [DOI] [PubMed] [Google Scholar]
- 80.Morkin MI, Hamrah P. Efficacy of self-retained cryopreserved amniotic membrane for treatment of neuropathic corneal pain. Ocul Surf. 2018;16:132–8. doi: 10.1016/j.jtos.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tighe S, Moein HR, Chua L, Cheng A, Hamrah P, Tseng SC. Topical cryopreserved amniotic membrane and umbilical cord eye drops promote re-epithelialization in a murine corneal abrasion model. Invest Ophthalmol Vis Sci. 2017;58:1586–93. doi: 10.1167/iovs.16-20834. [DOI] [PubMed] [Google Scholar]
- 82.Xiao X, Luo P, Zhao H, Chen J, He H, Xu Y, et al. Amniotic membrane extract ameliorates benzalkonium chloride-induced dry eye in a murine model. Exp Eye Res. 2013;115:31–40. doi: 10.1016/j.exer.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 83.He H, Kuriyan AE, Su CW, Mahabole M, Zhang Y, Zhu YT, et al. Inhibition of proliferation and epithelial mesenchymal transition in retinal pigment epithelial cells by heavy chain-hyaluronan/pentraxin 3. Sci Rep. 2017;7:43736. doi: 10.1038/srep43736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mead OG, Tighe S, Tseng SC. Amniotic membrane transplantation for managing dry eye and neurotrophic keratitis. Taiwan J Ophthalmol. 2020;10 doi: 10.4103/tjo.tjo_5_20. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cooke M, Tan EK, Mandrycky C, He H, O’Connell J, Tseng SC. Comparison of cryopreserved amniotic membrane and umbilical cord tissue with dehydrated amniotic membrane/chorion tissue. J Wound Care. 2014;23:465–74, 476. doi: 10.12968/jowc.2014.23.10.465. [DOI] [PubMed] [Google Scholar]
- 86.Adds PJ, Hunt CJ, Dart JK. Amniotic membrane grafts, “fresh” or frozen#x003F; A clinical and in vitro comparison. Br J Ophthalmol. 2001;85:905–7. doi: 10.1136/bjo.85.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shafer B, Fuerst NM, Massaro-Giordano M, Palladino V, Givnish T, Macchi I, et al. The use of self-retained, cryopreserved amniotic membrane for the treatment of Sjögren syndrome: A case series. Digit J Ophthalmol. 2019;25:21–5. doi: 10.5693/djo.01.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jennifer H, Sicks L. Sutureless amniotic membranes (PROKERA) for filamentary keratitis: A case series. J Dry Eye Occ Surf Dis. 2019;2:E10–6. [Google Scholar]
- 89.Maharaj R. Cryopreserved amniotic membrane (ProKera) treatment of anterior stromal haze secondary to HSV keratitis. J Dry Eye Occ Surf Dis. 2019;2:E2–6. [Google Scholar]
- 90.Slentz DH, Nelson CC. Novel use of cryopreserved ultra-thick human amniotic membrane for management of anophthalmic socket contracture. Ophthalmic Plast Reconstr Surg. 2019;35:193–6. doi: 10.1097/IOP.0000000000001264. [DOI] [PubMed] [Google Scholar]
- 91.Chen TH, Yang PT, Kikkawa DO, Ediriwickrema LS, Afshari NA, Lee JE, et al. A modified technique for bedside amniotic membrane application to the eyelid margins for Stevens-Johnson syndrome. Can J Ophthalmol. 2019;54:e118–e120. doi: 10.1016/j.jcjo.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 92.Shanbhag SS, Chodosh J, Saeed HN. Sutureless amniotic membrane transplantation with cyanoacrylate glue for acute Stevens-Johnson syndrome/toxic epidermal necrolysis. Ocul Surf. 2019;17:560–4. doi: 10.1016/j.jtos.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rizzo S, Caporossi T, Tartaro R, Finocchio L, Franco F, Barca F, et al. A human amniotic membrane plug to promote retinal breaks repair and recurrent macular hole closure. Retina. 2019;39(Suppl 1):S95–103. doi: 10.1097/IAE.0000000000002320. [DOI] [PubMed] [Google Scholar]
- 94.Reed D, Mehta A, Giles G, Phillips H, Santamaria J, DeMartelaere S, et al. 371 retrospective analysis of ocular involvement and outcomes following a novel approach to application of amniotic membrane graft in the management of Stevens-Johnson syndrome and toxic epidermal necrolysis at a level one burn center. J Burn Care Res. 2019;40:S162. [Google Scholar]