Abstract
Neurotrophic keratitis (NK), a degenerative disease caused by damage to the trigeminal nerve, abolishes both tearing and blinking reflexes, thus causing the most severe forms of dry eye disease (DED). Conversely, the increasing severity of DED also leads to progressive loss of corneal nerve density, potentially resulting in NK. Both diseases manifest the same spectrum of corneal pathologies including inflammation and corneal epithelial keratitis, which can progress into vision-threatening epithelial defect and stromal ulceration. This review summarizes the current literature regarding outcomes following sutured and sutureless cryopreserved amniotic membrane (AM) in treating DED as well as epithelial defects and corneal ulcers due to underlying NK. These studies collectively support the safety and effectiveness of cryopreserved AM in restoring corneal epithelial health, improving visual acuity in eyes with NK and DED, and alleviating symptomatic DED. Future randomized controlled trials are warranted to validate the above findings and determine whether such clinical efficacy lies in promoting corneal nerve regeneration.
Keywords: Amniotic membrane, corneal ulcer, dry eye, epithelial defect, neurotrophic keratitis
Introduction
The ocular surface encompasses both the cornea and the conjunctiva that lies between the upper and lower eyelids. As a body surface exposed to the outside environment, the ocular surface must serve as a protective barrier against trauma, pathogens, and desiccation. While the skin accomplishes this through a dry and water-impermeable keratinized epithelium, the ocular surface is covered by a nonkeratinized epithelium that expresses membrane-associated mucin to help maintain a stable tear film. A preocular tear film nourishes the avascular cornea with nutrients, oxygen, and growth factors in the open-eye state, all the while providing a smooth refractive surface for vision. Disruption of the tear film is a hallmark of dry eye disease (DED), which can ultimately give rise to sight-threatening epithelial defects and corneal ulcers. Therefore, a stable preocular tear film is integral in maintaining a healthy ocular surface.
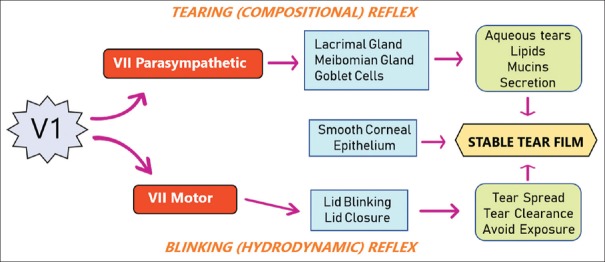
The key mechanism in maintaining a stable preocular tear film is through the neuroanatomic integration of the ocular surface epithelium and all external adnexae by the ophthalmic branch of the trigeminal nerve (V1) [Figure 1]. From the trigeminal ganglion, the ophthalmic branch enters the cornea at the limbus, where the nerve axon loses its myelin sheath, branches, and anastomose to form a subepithelial nerve plexus and sub-basal nerve plexus. This unique nerve distribution renders the corneal epithelium the most densely innervated tissue in the human body, more than 300–600 times that of the skin.[1] Corneal nerves provide trophic support to the ocular surface through the release of numerous trophic factors such as substance P, calcitonin gene-related peptide, and nerve growth factor (NGF), which have been shown to upregulate epithelial stem cell proliferation and promote corneal healing.[2,3,4,5] On the contrary, the corneal epithelium expresses NGF and glial cell-derived neurotrophic factor, which have been shown to play an important role in promoting corneal nerve regeneration following injury.[6] Thus, corneal nerves and epithelial cells mutually support each other through the release of neuropeptides and growth factors, collectively promoting corneal epithelial cell proliferation, migration, and differentiation as well as corneal nerve repair and survival.
Figure 1.
Neuroanatomic integration to maintain ocular surface health. Ocular surface health is maintained by a stable tear film, which comprises both compositional and hydrodynamic components. The former includes ocular surface epithelium producing mucins, meibomian glands producing meibum, and lacrimal glands producing aqueous tears. The latter includes eyelids, which blink at appropriate frequencies to enable tear distribution, tear clearance, and eye closure to prevent evaporation. The compositional factors are controlled by the tearing (compositional) reflex, whereas the hydrodynamic factors are controlled by the blinking (hydrodynamic) reflex. Both the tearing reflex and blinking reflex are stimulated by sensory input from the first (ophthalmic) branch of the trigeminal nerve, with output mediated by the parasympathetic and the motor branch of the facial nerve, respectively
Corneal sensitivity mediated by trigeminal sensory innervation triggers two reflex arcs: one through the facial motor branch to elicit the blinking (hydrodynamic) reflex and the other through the facial parasympathetic branch to elicit the tearing (compositional) reflex [Figure 1]. The blinking reflex governs intermittent eyelid closure at appropriate frequencies to prevent tear evaporation and facilitates effective tear distribution and clearance into the nasolacrimal drainage system. The tearing reflex permits the ocular surface epithelium to produce mucins, meibomian glands to produce meibum, and lacrimal glands to produce aqueous tears. These three key tear components are able to form a stable tear film through the assistance of the blinking reflex. A deficiency or dysfunction in any component of either reflex can destabilize the tear film and cause DED. Thus, we conclude that the neuroanatomic integration of these two reflexes is vital in maintaining a healthy ocular surface [Figure 1].
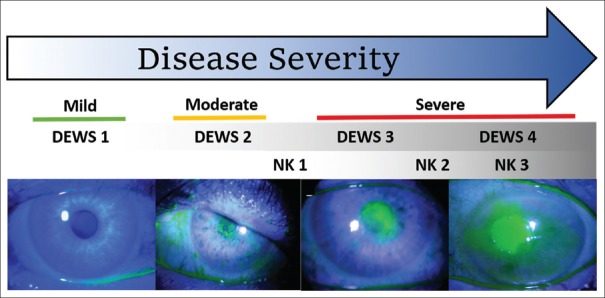
Corneal nerves react to injury of the ocular surface by triggering the blinking and tearing reflexes. However, damage to the corneal nerves dampens sensory input, thus diminishing these protective reflexes and compromising renewal and healing of the corneal epithelium. This pathological state can lead to a spectrum of sight-threatening complications that progressively affect the corneal epithelium (such as epithelial keratitis and epithelial defect) and underlying corneal stroma (such as neurotrophic keratitis [NK] and corneal ulcers/perforation). NK is a degenerative disease of the corneal epithelium and stroma caused by damage of trigeminal innervation that impairs corneal sensitivity. Because both tearing and blinking reflexes are compromised as a result of impaired corneal nerve function, NK results in the most severe form of DED.[7] Common causes of NK include herpetic keratitis, trauma, corneal surgery, diabetes, and neurosurgical procedures. NK is graded by the severity of corneal damage as proposed by Mackie:[8] Stage 1 (mild) is characterized by epithelial keratitis, Stage 2 (moderate) exhibits recurrent or persistent epithelial defect, and Stage 3 (severe) exhibits stromal ulceration, which may progress to stromal melting and perforation [Figure 2].
Figure 2.
The overlap between neurotrophic keratitis and dry eye disease pathologies. NK results in corneal epithelial keratitis (neurotrophic keratitis 1), epithelial defect (neurotrophic keratitis 2), and stromal ulcer (neurotrophic keratitis 3). These corneal pathologies are also observed in moderate-to-severe dry eye disease, graded as DEWS 2–4. The tear film breaks up into dry spots as evidenced by fluorescein staining in mild dry eye disease or DEWS 1
On the other hand, DED is defined as “a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.”[9] Ocular discomfort and visual fluctuations as a result of DED represent the most frequent complaints in ophthalmic practice.[10] Despite the existence of various underlying pathogenic processes, inflammation is a common denominator in DED. While inflammation is a fundamental process in corneal wound healing, prolonged or chronic inflammation can induce further damage to the corneal epithelium and underlying nerves.[11] DED severity can be classified by DEWS (International Dry Eye Workshop) Level 1–4[10] and includes corneal abnormalities such as epithelial keratitis (Level 2–3) and persistent epithelial defect and corneal ulcer (Level 3–4) [Figure 2]. Therefore, corneal pathologies of NK are intimately connected with those of DED, as NK Stages 1–3 overlap with DEWS Level 2–4 [Figure 2]. Furthermore, studies using in vivo confocal microscopy (IVCM) have found compromised corneal sub-basal nerves in patients with a variety of ocular conditions involving DED.[12,13,14,15,16,17,18,19] In a recent prospective controlled study, 10 eyes of 10 mice were exposed to dry eye stress (DES) for 4 weeks and compared to a control group with 10 eyes of 10 mice.[20] Corneal sensitivity in the DES group significantly decreased from baseline at 2 and 4 weeks and was significantly lower than the control group at both time points (P < 0.0001). Furthermore, there was a statistically significant decrease in the mean nerve fiber density in the DES group at 4 weeks (P = 0.0038). This nerve fiber density (1570 ± 506 pixels/frame) was also significantly lower compared to the control group (2538 ± 933 pixels/ frame) (P < 0.0001).
Therefore, we can conclude that DED can lead to progressive loss of corneal nerve density, resulting in NK. Conversely, NK can cause severe DED by abolishing the blinking and tearing reflexes. Collectively, these findings support the notion that NK and DED are interrelated with a causal relationship; as such, they manifest the same spectrum of inflammation and corneal pathologies including epithelial keratitis, epithelial defect, and stromal ulceration.
Standardized Care
Conventional care for NK and DED aims to restore the tear film and improve corneal epithelial integrity by halting the progression of corneal damage and promoting corneal epithelial healing. Preservative-free artificial tears are the mainstay of therapy for all forms of NK and DED as they lubricate and protect the ocular surface. The treatment of severe aqueous-deficient DED can be escalated to include temporary occlusion of the tear ducts with punctal plugs or cauterization. However, punctal occlusion or cauterization is not advised for cases with underlying inflammatory disorders, as delayed tear drainage of inflammatory factors on the ocular surface can exacerbate inflammation and further induce epithelial defect.[21] A short course of corticosteroids is sometimes recommended to control inflammation in moderate-to-severe DED; nevertheless, this should be used with caution in the presence of epithelial defect as steroids may inhibit healing and increase the risk of corneal melting. For severe ocular surface disorders in DED, therapeutic contact lenses are also available. Bandage contact lenses (BCLs) are useful in corneal wound healing by protecting advancing epithelial cells from being sloughed off, maintaining a stable fluid layer over the cornea, and alleviating discomfort when blinking. However, cases that utilize BCL should be monitored carefully as use of BCL can increase the risk of infection. Surgical treatment such as tarsorrhaphy and keratoplasty are often limited to severe cases with corneal ulcers and epithelial defects resistant to medical treatment. Tarsorrhaphy is commonly used to protect the cornea from rubbing against the eyelid and decrease tear evaporation rate; however, poor cosmetic outcome remains a major concern for patients. While the aforementioned treatments help to restore the damaged corneal surface, they do not aid in treating the underlying neurotrophic state through nerve regeneration.
Amniotic membrane (AM) has been extensively used in ocular surface surgery due to its ability to provide mechanical protection of the epithelial surface as well as its innate anti-inflammatory, antiscarring, and pro-regenerative properties.[22,23] Herein, we summarize how both sutured and sutureless cryopreserved AM have been successfully applied to restore homeostasis of the ocular surface, with focus placed on the preclinical and clinical evidence supporting the use of AM for NK and DED manifesting corneal epithelial keratitis, persistent epithelial defect, and corneal stromal ulcer. This review excludes those patients presenting with severe corneal melt (e.g., descemetocele) and corneal perforation.
Amniotic Membrane
Structure and composition
AM is the innermost layer of the placenta and shares the same cellular origin as the fetus, as they both arise from the inner cell mass during development.[24,25] The AM comprises three layers: the monolayer epithelium, thick basement membrane, and avascular stroma. AM contains many growth factors such as NGF, keratinocyte growth factor, and hepatocyte growth factor, all of which are implicated in promoting corneal epithelial wound healing.[26,27,28] The basement membrane resembles that of the corneal epithelium[29] and can serve as a scaffold for limbal epithelial stem cells.[30] The stroma can be subdivided into a compact, fibroblast, and spongy layer. The compact layer is composed of collagens (Type I and III) produced by mesenchymal cells in the fibroblast layer, which provide the AM with structural and mechanical integrity. The outermost spongy layer encompasses a loose, connective network predominant in Type III collagen and is a rich source of proteoglycans and glycoproteins.
Biological properties
It has long been established that AM exerts anti-inflammatory, antiscarring, and antiangiogenic properties. Heavy chain (HC)-hyaluronan (HA)/pentraxin 3 (PTX3) proteoglycan complex has recently been purified from AM and is characterized as a major biochemical tissue component responsible for its unique healing properties.[22,31] For example, HC-HA/PTX3, but not HA, facilitates rapid apoptosis and clearance of neutrophils,[32] which is essential in resolving chronic inflammation.[33,34] HC-HA/PTX3 also promotes the polarization of pro-inflammatory M1 macrophages toward M2 activation,[32,35] which express significantly more anti-inflammatory IL-10 and suppress T-cell proliferation and activation.[36] While these anti-inflammatory/immune effects can reduce scarring indirectly, evidence has also shown that the AM stroma directly inhibits scarring by inhibiting myofibroblast differentiation.[37,38] AM and HC-HA/PTX3 have also been shown to downregulate transforming growth factor-beta (TGF-β) signaling in human corneal fibroblasts.[38,39] In addition, AM has been shown to reduce corneal neovascularization following AM transplantation,[40,41,42] which is due in part to its ability to suppress the viability and tube formation of cultured human umbilical vein endothelial cells.[41,43] This is especially important since maintaining corneal avascularity is required for corneal transparency and optimal vision. The abundant presence of NGF in the AM stroma[27,44,45,46,47] further supports its mode of action in treating NK and DED.
Preclinical evidence of amniotic membrane in promoting corneal epithelial wound healing
Several preclinical studies have demonstrated the safety and efficacy of cryopreserved AM in treating NK and DED manifesting corneal ulcer, persistent corneal epithelial defect, and epithelial keratitis. In rabbits, sutured cryopreserved AM was shown to enhance corneal wound healing after photorefractive keratectomy[48,49] and phototherapeutic keratectomy[50,51] using excimer laser ablation.[48,49,50,51] Woo et al.[51] found that sutured AM resulted in significantly faster epithelial healing at 72 h; however, Choi et al.[49] and Wang et al.[50] found that corneal epithelial healing was completed within 72 h regardless of treatment. Following transepithelial keratectomy, transmission electron microscopy showed that the AM stromal matrix attracted PMNs, which led to a significant reduction of keratocyte apoptosis and inflammatory cell infiltration in the corneal stroma compared to eyes with no treatment.[48,49,50,51] Application of sutured AM after keratectomy significantly reduced corneal haze,[49,50,51] which was correlated with a more regular architecture of regenerated stromal lamella at 8 weeks[51] and a reduction in both stromal fibroblast cellularity and epithelial hyperplasia at 12 weeks.[50] In mice, a temporary AM secured by tarsorrhaphy has also been shown to promote the healing of HSV-1-induced necrotizing keratitis.[52] Rapid and marked regression of stromal keratitis was noted in all mice in the AMT group, and all but one ulcer (92%) achieved complete healing within 2 days. In contrast, tarsorrhaphy alone only mildly improved stromal inflammation, and only two (17%) ulcers healed.[52] Marked reduction of inflammatory cell infiltration by day 2 after AMT was associated with reduced expression and activity of matrix metalloproteinase (MMP)-8 and MMP-9 and increased localization of TIMP-19.[52]
In a prospective randomized controlled study, human AM extract has been shown to ameliorate benzalkonium chloride (BAC)-induced DED in a murine model.[53] Topical application of human AM extract resulted in significantly longer tear breakup time on days 3 and 6, lower fluorescein staining scores on day 3, and lower inflammation on day 6. Further analysis showed that human AM extract reduced inflammatory infiltration and levels of tumor necrosis factor-α, interleukin-1 beta (IL-1β), and IL-6, thus explaining the decreased TUNEL-positive cells in the cornea and increased goblet cells in the conjunctiva in BAC-treated mice.
Clinical evidence of cryopreserved amniotic membrane in treating dry eye disease
The use of AMT in ophthalmology was first documented more than 70 years ago and later resurfaced in 1995 when Kim and Tseng[54] reported its use in ocular surface reconstruction. Since then, AMT has been successfully utilized to treat a variety of ophthalmic conditions manifesting corneal ulcer, persistent epithelial defect, and epithelial keratitis.[55] The increasing popularity of AMT was made possible with the advent of AM cryopreservation,[56] which devitalizes all living cells while preserving extracellular matrices and growth factors/cytokines during long-term storage. Herein, we summarize the clinical evidence of cryopreserved AM in managing DED.
Self-retained cryopreserved AM (PROKERA, Bio-Tissue, Miami, FL, USA) has been shown to successfully manage the signs and symptoms of moderate-to-severe DED (DEWS Level 2–4) in several studies that included 121 eyes of 103 patients.[56,57,58] PROKERA was inserted for an average of 4.5 days (range: 2–11 days). Overall, there were no adverse events aside from mild discomfort, which lead to early PROKERA removal from 4 eyes (4%) after 2 days due to intolerance.[58] After treatment, the overall severity of DED was reduced from DEWS Level 3 to that of DEWS Level 1 at 1 and 3 months.[57,58] This was accompanied with a significant decrease in corneal staining from an overall average of 2.2 at baseline to 0.5 by 3 months.[56,57,58] Furthermore, tear film breakup time was significantly improved from 8 s at baseline to 15 s at 3 months.[57] These benefits were correlated with an increase in corneal nerve density and corneal sensitivity, which was measured using IVCM and the Bonnet-Crochet esthesiometer, respectively.[57] In contrast, individuals receiving conventional maximum treatment without PROKERA showed no significant changes in DEWS score, fluorescein staining, tear film breakup time, and corneal nerve density at 1 and 3 months despite conventional treatment use throughout the study duration.[57] All studies showed improvement in the quality of vision and visual symptoms following PROKERA use. Cheng et al.[56] reported visual acuity (VA) improvement from 20/25 at baseline to 20/23 at a median of 2.5 months, and John et al.[57] reported VA improvement from 20/46 to 20/40 at 3 months; however, these improvements were not statistically significant. Furthermore, PROKERA has been shown to provide short-term relief of autoimmune-related DED refractory to conventional and systemic immunotherapies.[59,60] Shafer et al.[59] found PROKERA relieved symptomatic DED secondary to Sjögren's syndrome for up to 1 month in 6 eyes. The device was removed in one patient after 48 h due to foreign body sensation and mucopurulent discharge. Cheng et al.[60] reported findings in one patient with rheumatoid arthritis-induced DED. Ten PROKERA placements over 4 years alleviated ocular symptoms for an average of 4 ± 2 months and improved VA from 20/400 to 20/70.
Furthermore, PROKERA has been shown to alleviate neuropathic corneal pain due to corneal nerve damage from DED.[57,61] In one retrospective case series, 6 eyes of 6 female patients (62 ± 8 years) displayed marked improvement in pain after PROKERA placement for an average of 7 ± 3 days.[61] Late-onset discomfort resulted in the removal of PROKERA in one patient before the complete dissolution of AM on day 6. Nevertheless, average pain severity decreased from 5.4 ± 2.7 out of 10 at baseline to 0.8 ± 0.9 at the end of follow-up (range: 8–14 months). This was accompanied by a significant increase in total sub-basal nerve density. One patient with severe pain (9/10) reported recurrence of symptomatic pain at 2.3 and 6.1 months, which was resolved completely (0/10) following additional placement of PROKERA at the time of each recurrence.
Clinical evidence of cryopreserved amniotic membrane in treating neurotrophic keratitis
Cryopreserved AM has also been successfully used to treat corneal epithelial defects and ulcers caused by NK and unresponsive to prior treatment as reported in 3 prospective studies[62,63,64] and 12 retrospective studies[65,66,67,68,69,70,71,72,73,74,75,76,77] [Table 1]. Cases that presented with active infection, descemetocele, or perforation were excluded. Underlying etiologies of NK included penetrating keratoplasty, herpes simplex keratitis, herpes zoster ophthalmicus, neuropathy, diabetes mellitus, acoustic neuroma removal, and radiation. The outcome parameters evaluated were healing rate, time to epithelialization, rate of VA improvement (as defined by >2 lines), duration of follow-up, number of recurrences, and complications or adverse events. In addition, changes in ocular surface health were noted. Single or multilayer AM was chosen depending on the ulcer depth, and AMT techniques included (i) “inlay or graft” technique, where one or more layers of AM are cut to the defect size and placed in the ulcer bed with the top layer fixed to the edge of the defect, (ii) “overlay or patch” technique, where an oversized AM covers the entire cornea and limbus area, or (iii) the “sandwich” technique that is a combination of both the inlay and overlay techniques. Two studies utilized self-retained sutureless AM through the PROKERA device. The overall average follow-up period was 12.3 months, which ranged from 3 to 20 months depending on the study [Table 1]. Collectively, AM has demonstrated a success rate of 88.9% (144/162) in achieving rapid and complete healing of defects and/or ulcers in an average of 18.4 days (range: 6-30 days) [Table 1]. VA improved more than 2 lines in 52.3% (57/109) of patients by the last follow-up [Table 1]. Furthermore, healing at the ulcer site was accompanied with a reduction in ocular inflammation[62,64,66,67,72,76] as evidenced by a decrease in corneal edema and conjunctival and limbal hyperemia.[68,70] A decrease in stromal vascularization was noted in three studies.[67,70,71]
Table 1.
Amniotic membrane transplantation for managing neurotrophic keratitis manifesting epithelial defect and corneal ulcera
| Study (years) | Design | n, eye/patient | Age | Gender (male/female) | AM method | Healing rate | Epithelial healing (days) | Rate of vision improvement | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|
| Lee and Tseng (1997)[65] | RS | 4/4 | 67.5±25.5 | 3/1 | Inlay; S/M | 100% (4/4) | 29.8±11.9 | NA | 5.8±5.5 |
| Kruse et al. (1999)[62] | PS | 10/10 | 56.9±16.6 | 7/3 | Inlay; M | 100% (10/10) | Range: 21-28 | 44.4% (4/9) | 12±0 |
| Chen et al. (2000)[66] | RS | 15/14 | 62.1±23.1 | 10/4 | Inlay or sandwich; S/M | 80% (12/15) | 16.6±9.0 | 46.7% (7/15) | 18.4±13.9 |
| Prabhasawat et al. (2001)[67] | RS | 9/9 | 49.7±27.1 | 6/3 | Inlay; S | 88.9% (8/9) | 24.8±18.4 | 22.2% (2/9) | 13.1±9.8 |
| Gris et al. (2002)[68]b | RS | 5/5 | 52.8±24.8 | 5/0 | Inlay; S | 100% (5/5) | 22.8±11.6 | NA | 17 (12-23) |
| Iveković et al. (2002)[69] | RS | 11/11 | 38.5±10.8 | 6/5 | Inlay; S/M | 100% (11/11) | 11.4±4.7 | 27.3% (3/11) | 19.7±6.0 |
| Dogru et al. (2003)[70] | RS | 4/4 | 59.0±13.4 | 4/0 | Inlay; S/M | 100% (4/4) | 22.0±5.5 | 100% (4/4) | 11.8±2.6 |
| Heiligenhaus et al. (2003)[71] | RS | 7/7 | 62.3±10.6 | 3/4 | Inlay or sandwich; S/M | 100% (7/7) | 17.0±7.0 | 71.4% (5/7) | 11±3.4 |
| Hick et al. (2005)[72] | RS | 16/16 | 62.6±19.6 | 9/7 | Inlay; S/M | 93.7% (15/16) | 20.7±13.0 | 37.5% (6/16) | 12.3±8.3 |
| Khokhar et al. (2005)[63] | RCT | 15/15 | 37.3±17.1 | 7/8 | Inlay, overlay, or sandwich; S/M | 73.3% (11/15) | 23.8±13.9 | 40% (6/15) | 3±0 |
| Seitz et al.(2009)[73] | RS | 8/8 | 58.9±20.6 | 5/3 | Overlay or sandwich; S/M | 87.5% (7/8) | 18.1±11.9 | NA | 17.9±12.4 |
| Nubile et al. (2011)[64] | PS | 9/9 | 60.0±12.8 | 5/4 | Sandwich; M | 88.9% (8/9) | 13.8±4.7 | NA | 12±0 |
| Suri et al. (2013)[74]c | RS | 11/11 | 69.5±10.4 | 8/3 | PROKERA | 63.6% (7/11) | 11.2±10.4 | NA | 3.1±1.3 |
| Turkoglu et al. (2014)[75] | RS | 19/19 | 59.3±7.9 | 15/4 | Overlay; S/M | 89.5% (17/19) | 20.0±4.6 | 89.5% (17/19) | 6.8±3.9 |
| Cheng and Tseng (2017)[76] | RS | 4/4 | 71.3±9.9 | 1/3 | PROKERA | 100% (4/4) | 5.75±2.9 | 75% (3/4) | 20.3±21.7 |
| Schuerch et al. (2019)[77]d | RS | 15/15 | 75±9 | 7/8 | Sandwich; M | 93.3% (14/15) | 35 (24-61)c | NA | Range: 6-NA |
| Total | 162/161 | 58.9±17.4 | 101/60 | 88.9% (144/162) | 18.4±10.2 | 52.3% (57/109) | 12.3±8.5 |
aValues are expressed as mean±SD unless otherwise indicated, bFollow-up reported as mean (range), cHealing rate includes two eyes with preexisting descemetocele, dTime to epithelial healing was estimated from a box and whisker plot and reported as median (IQR). AM=Amniotic membrane, NA=Not available, RS=Retrospective series, PS=Prospective series, RCT=Randomized clinical trial, S=Single layer, M=Multiple layers, IQR=Interquartile range, SD=Standard deviation
Two long-term (12 months) prospective studies have shown an overall success in 10 of 10 eyes (100%)[62] and 8 of 9 eyes (89%)[64] through multilayer AMT to achieve complete epithelialization within 4 weeks in corneas with persistent epithelial defect and stromal ulceration caused by severe NK. Following transplantation, AM gradually dissolved over a period of 12 months, but stromal thickness remained stable.[62] Anterior segment optical coherence tomography showed the mean residual stromal thickness at the ulcer bed before surgery to be 222 ± 70 μm, the mean thickness of AM layers at the same site was 394 ± 80 μm, whereas the mean total corneal thickness was 623 ± 51 μm at day 1 postsurgery.[64] A progressive reduction in thickness to 420 ± 61 μm at 6 months occurred, after which time the thickness stabilized. Confocal microscopy confirmed that the AM integrated entirely with the corneal stroma along with overlying epithelialization, which occurred 14 ± 5 days postsurgery in the successful cases.[64] Confocal microscopy also showed that the AM patch was degraded during the first few weeks following surgery, whereas the integrated amniotic tissues underwent progressive modifications characterized by early loss of amniotic epithelial cells, changes in fibrillar structure, and migration into the AM stroma by corneal stroma-derived cells.[64]
A histopathological correlation of two corneas with neurotrophic ulcers that received AMT and subsequent corneal transplantation revealed complete epithelialization over the basement membrane of the AM graft, which was slowly reabsorbed in one cornea without stromal vascularization and inflammatory reaction.[78] In the other cornea with stromal vascularization, the AM graft was rapidly reabsorbed due to the abundance of inflammatory cells and was replaced by new fibrotic stroma that was different from that in the rest of the cornea but helped to maintain corneal thickness.[78] In another retrospective study of 10 eyes, impression cytology showed a significant improvement in squamous metaplasia grading and the goblet cell density after AMT.[70] In addition, corneal wound healing promoted by sutured AM resulted in a significant improvement in tear film breakup time that was correlated with increased corneal sensitivity, suggesting that corneal nerve regeneration may have taken place.[70]
Overall, complications included two cases of corneal perforation: one following sutured AMT[63] and the other following sutureless PROKERA.[74] In addition, another case showed progressive corneal thinning to which conjunctival patching was conducted to prevent imminent perforation.[70] Progressive thinning and perforation have been recognized as a natural disease progression of severe NK. Furthermore, a total of 11 recurrences were noted in 5 studies, giving an overall recurrence rate of 6.8% (11/162).[62,64,72,73,74]
Conclusion and Discussion
Both preclinical and clinical evidence support the notion that sutured and sutureless cryopreserved AM transplantation can successfully be used to treat DED and NK. These studies collectively support the safety and effectiveness of cryopreserved AM in expediting restoration of corneal epithelial health and epithelialization, improving VA in eyes with NK and DED, and alleviating symptomatic DED. Future randomized controlled trials are warranted to validate the above findings and determine whether such clinical efficacy lies in promoting corneal nerve regeneration.
Financial support and sponsorship
The work was supported by a RO1 EY06819 grant (to SCGT) from the National Eye Institute, National Institutes of Health, Bethesda, MD, USA.
Conflicts of interest
OM, ST, and ST are employees of TissueTech. Dr Tseng has obtained a patent for the method of preparation and clinical uses of amniotic membrane and has licensed the rights to TissueTech, Inc, which procures and processes, and to Bio-Tissue, Inc, which is a subsidiary of TissueTech, Inc, to distribute cryopreserved amniotic membrane for clinical and research uses.
References
- 1.Zander E, Weddell G. Observations on the innervation of the cornea. J Anat. 1951;85:68–99. [PMC free article] [PubMed] [Google Scholar]
- 2.Okada Y, Sumioka T, Ichikawa K, Sano H, Nambu A, Kobayashi K, et al. Sensory nerve supports epithelial stem cell function in healing of corneal epithelium in mice: The role of trigeminal nerve transient receptor potential vanilloid 4. Lab Invest. 2019;99:210–30. doi: 10.1038/s41374-018-0118-4. [DOI] [PubMed] [Google Scholar]
- 3.Shi X, Wang L, Clark JD, Kingery WS. Keratinocytes express cytokines and nerve growth factor in response to neuropeptide activation of the ERK1/2 and JNK MAPK transcription pathways. Regul Pept. 2013;186:92–103. doi: 10.1016/j.regpep.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang L, Di G, Qi X, Qu M, Wang Y, Duan H, et al. SubstancePpromotes diabetic corneal epithelial wound healing through molecular mechanisms mediated via the neurokinin-1 receptor. Diabetes. 2014;63:4262–74. doi: 10.2337/db14-0163. [DOI] [PubMed] [Google Scholar]
- 5.Mikulec AA, Tanelian DL. CGRP increases the rate of corneal re-epithelialization in an in vitro whole mount preparation. J Ocul Pharmacol Ther. 1996;12:417–23. doi: 10.1089/jop.1996.12.417. [DOI] [PubMed] [Google Scholar]
- 6.Di G, Qi X, Zhao X, Zhang S, Danielson P, Zhou Q. Corneal epithelium-derived neurotrophic factors promote nerve regeneration. Invest Ophthalmol Vis Sci. 2017;58:4695–702. doi: 10.1167/iovs.16-21372. [DOI] [PubMed] [Google Scholar]
- 7.Tseng SC. A practical treatment algorithm for managing ocular surface and tear disorders. Cornea. 2011;30(Suppl 1):S8–S14. doi: 10.1097/ICO.0b013e318228218c. [DOI] [PubMed] [Google Scholar]
- 8.Mackie IA. St Louis: Mosby; 1973. Neuroparalytic (neurotrophic) keratitis Symposium on Contact Lenses: Transactions of the New Orleans Academy of Ophthalmology. [Google Scholar]
- 9.Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276–83. doi: 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 10.The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 11.Shaheen BS, Bakir M, Jain S. Corneal nerves in health and disease. Surv Ophthalmol. 2014;59:263–85. doi: 10.1016/j.survophthal.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Labbé A, Alalwani H, Van Went C, Brasnu E, Georgescu D, Baudouin C. The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease. Invest Ophthalmol Vis Sci. 2012;53:4926–31. doi: 10.1167/iovs.11-8708. [DOI] [PubMed] [Google Scholar]
- 13.Levy O, Labbé A, Borderie V, Hamiche T, Dupas B, Laroche L, et al. Increased corneal sub-basal nerve density in patients with Sjögren syndrome treated with topical cyclosporine A. Clin Exp Ophthalmol. 2017;45:455–63. doi: 10.1111/ceo.12898. [DOI] [PubMed] [Google Scholar]
- 14.Tepelus TC, Chiu GB, Huang J, Huang P, Sadda SR, Irvine J, et al. Correlation between corneal innervation and inflammation evaluated with confocal microscopy and symptomatology in patients with dry eye syndromes: A preliminary study. Graefes Arch Clin Exp Ophthalmol. 2017;255:1771–8. doi: 10.1007/s00417-017-3680-3. [DOI] [PubMed] [Google Scholar]
- 15.Tuisku IS, Konttinen YT, Konttinen LM, Tervo TM. Alterations in corneal sensitivity and nerve morphology in patients with primary Sjögren's syndrome. Exp Eye Res. 2008;86:879–85. doi: 10.1016/j.exer.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Tepelus TC, Chiu GB, Maram J, Huang J, Chopra V, Sadda SR, et al. Corneal features in ocular graft-versus-host disease by in vivo confocal microscopy. Graefes Arch Clin Exp Ophthalmol. 2017;255:2389–97. doi: 10.1007/s00417-017-3759-x. [DOI] [PubMed] [Google Scholar]
- 17.Kheirkhah A, Qazi Y, Arnoldner MA, Suri K, Dana R. In vivo confocal microscopy in dry eye disease associated with chronic graft-versus-host disease. Invest Ophthalmol Vis Sci. 2016;57:4686–91. doi: 10.1167/iovs.16-20013. [DOI] [PubMed] [Google Scholar]
- 18.Labbé A, Liang Q, Wang Z, Zhang Y, Xu L, Baudouin C, et al. Corneal nerve structure and function in patients with non-sjogren dry eye: Clinical correlations. Invest Ophthalmol Vis Sci. 2013;54:5144–50. doi: 10.1167/iovs.13-12370. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg ME, Tervo TM, Gallar J, Acosta MC, Müller LJ, Moilanen JA, et al. Corneal morphology and sensitivity in lattice dystrophy type II (familial amyloidosis, Finnish type) Invest Ophthalmol Vis Sci. 2001;42:634–41. [PubMed] [Google Scholar]
- 20.Simsek C, Kojima T, Nagata T, Dogru M, Tsubota K. Changes in murine subbasal corneal nerves after scopolamine-Induced dry eye stress exposure. Invest Ophthalmol Vis Sci. 2019;60:615–23. doi: 10.1167/iovs.18-26318. [DOI] [PubMed] [Google Scholar]
- 21.Prabhasawat P, Tseng SC. Frequent association of delayed tear clearance in ocular irritation. Br J Ophthalmol. 1998;82:666–75. doi: 10.1136/bjo.82.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tseng SC. HC-HA/PTX3 purified from amniotic membrane as novel regenerative matrix: Insight into relationship between inflammation and regeneration. Invest Ophthalmol Vis Sci. 2016;57:ORSFh1–8. doi: 10.1167/iovs.15-17637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tseng SC, Espana EM, Kawakita T, Di Pascuale MA, Li W, He H, et al. How does amniotic membrane work? Ocul Surf. 2004;2:177–87. doi: 10.1016/s1542-0124(12)70059-9. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg LE, Rosenberg DD, editors. Human Genes and Genomes. 4. San Diego: Academic Press; 2012. Growth, Development, and Reproduction; pp. 27–50. [Google Scholar]
- 25.Jones RE, Lopez KH, editors. Human Reproductive Biology. 4th ed. 10. San Diego: Academic Press; 2014. Pregnancy; pp. 175–204. [Google Scholar]
- 26.He M, Han T, Wang Y, Wu YH, Qin WS, Du LZ, et al. Effects of HGF and KGF gene silencing on vascular endothelial growth factor and its receptors in rat ultraviolet radiationinduced corneal neovascularization. Int J Mol Med. 2019;43:1888–99. doi: 10.3892/ijmm.2019.4114. [DOI] [PubMed] [Google Scholar]
- 27.Touhami A, Grueterich M, Tseng SC. The role of NGF signaling in human limbal epithelium expanded by amniotic membrane culture. Invest Ophthalmol Vis Sci. 2002;43:987–94. [PubMed] [Google Scholar]
- 28.Wang L, Wu X, Shi T, Lu L. Epidermal growth factor (EGF)-induced corneal epithelial wound healing through nuclear factor κB subtype-regulated CCCTC binding factor (CTCF) activation. J Biol Chem. 2013;288:24363–71. doi: 10.1074/jbc.M113.458141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Endo K, Nakamura T, Kawasaki S, Kinoshita S. Human amniotic membrane, like corneal epithelial basement membrane, manifests the alpha5 chain of type IV collagen. Invest Ophthalmol Vis Sci. 2004;45:1771–4. doi: 10.1167/iovs.03-0952. [DOI] [PubMed] [Google Scholar]
- 30.Yeh HJ, Yao CL, Chen HI, Cheng HC, Hwang SM. Cryopreservation of human limbal stem cells ex vivo expanded on amniotic membrane. Cornea. 2008;27:327–33. doi: 10.1097/ICO.0b013e31815dcfaf. [DOI] [PubMed] [Google Scholar]
- 31.Zhang S, Zhu YT, Chen SY, He H, Tseng SC. Constitutive expression of pentraxin 3 (PTX3) protein by human amniotic membrane cells leads to formation of the heavy chain (HC)-hyaluronan (HA)-PTX3 complex. J Biol Chem. 2014;289:13531–42. doi: 10.1074/jbc.M113.525287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He H, Zhang S, Tighe S, Son J, Tseng SC. Immobilized heavy chain-hyaluronic acid polarizes lipopolysaccharide-activated macrophages toward M2 phenotype. J Biol Chem. 2013;288:25792–803. doi: 10.1074/jbc.M113.479584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hampson P, Hazeldine J, Lord JM. Neutrophil apoptosis and its induction as a potential treatment for chronic inflammatory disease. Curr Opin Hematol. 2013;20:10–5. doi: 10.1097/MOH.0b013e32835b06be. [DOI] [PubMed] [Google Scholar]
- 34.El Kebir D, Filep JG. Role of neutrophil apoptosis in the resolution of inflammation. ScientificWorldJournal. 2010;10:1731–48. doi: 10.1100/tsw.2010.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He H, Tan Y, Duffort S, Perez VL, Tseng SC. In vivo downregulation of innate and adaptive immune responses in corneal allograft rejection by HC-HA/PTX3 complex purified from amniotic membrane. Invest Ophthalmol Vis Sci. 2014;55:1647–56. doi: 10.1167/iovs.13-13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oishi S, Takano R, Tamura S, Tani S, Iwaizumi M, Hamaya Y, et al. M2 polarization of murine peritoneal macrophages induces regulatory cytokine production and suppresses T-cell proliferation. Immunology. 2016;149:320–8. doi: 10.1111/imm.12647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi TH, Tseng SC. In vivo and in vitro demonstration of epithelial cell-induced myofibroblast differentiation of keratocytes and an inhibitory effect by amniotic membrane. Cornea. 2001;20:197–204. doi: 10.1097/00003226-200103000-00019. [DOI] [PubMed] [Google Scholar]
- 38.Tseng SC, Li DQ, Ma X. Suppression of transforming growth factor-beta isoforms, TGF-beta receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J Cell Physiol. 1999;179:325–35. doi: 10.1002/(SICI)1097-4652(199906)179:3<325::AID-JCP10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 39.He H, Li W, Tseng DY, Zhang S, Chen SY, Day AJ, et al. Biochemical characterization and function of complexes formed by hyaluronan and the heavy chains of inter-alpha-inhibitor (HC*HA) purified from extracts of human amniotic membrane. J Biol Chem. 2009;284:20136–46. doi: 10.1074/jbc.M109.021881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim JC, Tseng SC. The effects on inhibition of corneal neovascularization after human amniotic membrane transplantation in severely damaged rabbit corneas. Korean J Ophthalmol. 1995;9:32–46. doi: 10.3341/kjo.1995.9.1.32. [DOI] [PubMed] [Google Scholar]
- 41.Jiang A, Li C, Gao Y, Zhang M, Hu J, Kuang W, et al. In vivo and in vitro inhibitory effect of amniotic extraction on neovascularization. Cornea. 2006;25:S36–40. doi: 10.1097/01.ico.0000247211.78391.af. [DOI] [PubMed] [Google Scholar]
- 42.Shao C, Sima J, Zhang SX, Jin J, Reinach P, Wang Z, et al. Suppression of corneal neovascularization by PEDF release from human amniotic membranes. Invest Ophthalmol Vis Sci. 2004;45:1758–62. doi: 10.1167/iovs.03-0882. [DOI] [PubMed] [Google Scholar]
- 43.Shay E, He H, Sakurai S, Tseng SC. Inhibition of angiogenesis by HC·HA, a complex of hyaluronan and the heavy chain of inter-α-inhibitor, purified from human amniotic membrane. Invest Ophthalmol Vis Sci. 2011;52:2669–78. doi: 10.1167/iovs.10-5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uchida S, Inanaga Y, Kobayashi M, Hurukawa S, Araie M, Sakuragawa N. Neurotrophic function of conditioned medium from human amniotic epithelial cells. J Neurosci Res. 2000;62:585–90. doi: 10.1002/1097-4547(20001115)62:4<585::AID-JNR13>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 45.Koizumi NJ, Inatomi TJ, Sotozono CJ, Fullwood NJ, Quantock AJ, Kinoshita S. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000;20:173–7. [PubMed] [Google Scholar]
- 46.Davis GE, Engvall E, Varon S, Manthorpe M. Human amnion membrane as a substratum for cultured peripheral and central nervous system neurons. Brain Res. 1987;430:1–0. [PubMed] [Google Scholar]
- 47.Davis GE, Blaker SN, Engvall E, Varon S, Manthorpe M, Gage FH. Human amnion membrane serves as a substratum for growing axons in vitro and in vivo . Science. 1987;236:1106–9. doi: 10.1126/science.3576223. [DOI] [PubMed] [Google Scholar]
- 48.Park WC, Tseng SC. Modulation of acute inflammation and keratocyte death by suturing, blood, and amniotic membrane in PRK. Invest Ophthalmol Vis Sci. 2000;41:2906–14. [PubMed] [Google Scholar]
- 49.Choi YS, Kim JY, Wee WR, Lee JH. Effect of the application of human amniotic membrane on rabbit corneal wound healing after excimer laser photorefractive keratectomy. Cornea. 1998;17:389–95. doi: 10.1097/00003226-199807000-00009. [DOI] [PubMed] [Google Scholar]
- 50.Wang MX, Gray TB, Park WC, Prabhasawat P, Culbertson W, Forster R, et al. Reduction in corneal haze and apoptosis by amniotic membrane matrix in excimer laser photoablation in rabbits. J Cataract Refract Surg. 2001;27:310–9. doi: 10.1016/s0886-3350(00)00467-3. [DOI] [PubMed] [Google Scholar]
- 51.Woo HM, Kim MS, Kweon OK, Kim DY, Nam TC, Kim JH. Effects of amniotic membrane on epithelial wound healing and stromal remodelling after excimer laser keratectomy in rabbit cornea. Br J Ophthalmol. 2001;85:345–9. doi: 10.1136/bjo.85.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heiligenhaus A, Bauer D, Meller D, Steuhl KP, Tseng SC. Improvement of HSV-1 necrotizing keratitis with amniotic membrane transplantation. Invest Ophthalmol Vis Sci. 2001;42:1969–74. [PubMed] [Google Scholar]
- 53.Xiao X, Luo P, Zhao H, Chen J, He H, Xu Y, et al. Amniotic membrane extract ameliorates benzalkonium chloride-induced dry eye in a murine model. Exp Eye Res. 2013;115:31–40. doi: 10.1016/j.exer.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 54.Kim JC, Tseng SC. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995;14:473–84. [PubMed] [Google Scholar]
- 55.Liu J, Sheha H, Fu Y, Liang L, Tseng SC. Update on amniotic membrane transplantation. Expert Rev Ophthalmol. 2010;5:645–61. doi: 10.1586/eop.10.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng AM, Zhao D, Chen R, Yin HY, Tighe S, Sheha H, et al. Accelerated restoration of ocular surface health in dry eye disease by self-retained cryopreserved amniotic membrane. Ocul Surf. 2016;14:56–63. doi: 10.1016/j.jtos.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.John T, Tighe S, Sheha H, Hamrah P, Salem ZM, Cheng AMS, et al. Corneal nerve regeneration after self-retained cryopreserved amniotic membrane in dry eye disease. J Ophthalmol. 2017;2017:1–10. doi: 10.1155/2017/6404918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McDonald MB, Sheha H, Tighe S, Janik SB, Bowden FW, Chokshi AR, et al. Treatment outcomes in the DRy eye amniotic membrane (DREAM) study. Clin Ophthalmol. 2018;12:677–81. doi: 10.2147/OPTH.S162203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shafer B, Fuerst NM, Massaro-Giordano M, Palladino V, Givnish T, Macchi I, et al. The use of self-retained, cryopreserved amniotic membrane for the treatment of Sjögren syndrome: A case series. Digit J Ophthalmol. 2019;25:21–5. doi: 10.5693/djo.01.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheng AM, Tighe S, Sheha H, Tseng SC. Adjunctive role of self-retained cryopreserved amniotic membrane in treating immune-related dry eye disease. Int Ophthalmol. 2018;38:2219–22. doi: 10.1007/s10792-017-0708-y. [DOI] [PubMed] [Google Scholar]
- 61.Morkin MI, Hamrah P. Efficacy of self-retained cryopreserved amniotic membrane for treatment of neuropathic corneal pain. Ocul Surf. 2018;16:132–8. doi: 10.1016/j.jtos.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kruse FE, Rohrschneider K, Völcker HE. Multilayer amniotic membrane transplantation for reconstruction of deep corneal ulcers. Ophthalmology. 1999;106:1504–10. doi: 10.1016/S0161-6420(99)90444-X. [DOI] [PubMed] [Google Scholar]
- 63.Khokhar S, Natung T, Sony P, Sharma N, Agarwal N, Vajpayee RB. Amniotic membrane transplantation in refractory neurotrophic corneal ulcers: A randomized, controlled clinical trial. Cornea. 2005;24:654–60. doi: 10.1097/01.ico.0000153102.19776.80. [DOI] [PubMed] [Google Scholar]
- 64.Nubile M, Dua HS, Lanzini M, Ciancaglini M, Calienno R, Said DG, et al. In vivo analysis of stromal integration of multilayer amniotic membrane transplantation in corneal ulcers. Am J Ophthalmol. 2011;151:809–220. doi: 10.1016/j.ajo.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 65.Lee SH, Tseng SC. Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am J Ophthalmol. 1997;123:303–12. doi: 10.1016/s0002-9394(14)70125-4. [DOI] [PubMed] [Google Scholar]
- 66.Chen HJ, Pires RT, Tseng SC. Amniotic membrane transplantation for severe neurotrophic corneal ulcers. Br J Ophthalmol. 2000;84:826–33. doi: 10.1136/bjo.84.8.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prabhasawat P, Tesavibul N, Komolsuradej W. Single and multilayer amniotic membrane transplantation for persistent corneal epithelial defect with and without stromal thinning and perforation. Br J Ophthalmol. 2001;85:1455–63. doi: 10.1136/bjo.85.12.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gris O, del Campo Z, Wolley-Dod C, Güell JL, Bruix A, Calatayud M, et al. Amniotic membrane implantation as a therapeutic contact lens for the treatment of epithelial disorders. Cornea. 2002;21:22–7. doi: 10.1097/00003226-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 69.Iveković B, Tedeschi-Reiner E, Petric I, Novak-Laus K, Bradić-Hammoud M. Amniotic membrane transplantation for ocular surface reconstruction in neurotrophic corneal ulcera. Coll Antropol. 2002;26:47–54. [PubMed] [Google Scholar]
- 70.Dogru M, Yildiz M, Baykara M, Ozçetin H, Ertürk H. Corneal sensitivity and ocular surface changes following preserved amniotic membrane transplantation for nonhealing corneal ulcers. Eye (Lond) 2003;17:139–48. doi: 10.1038/sj.eye.6700346. [DOI] [PubMed] [Google Scholar]
- 71.Heiligenhaus A, Li H, Hernandez Galindo EE, Koch JM, Steuhl KP, Meller D. Management of acute ulcerative and necrotising herpes simplex and zoster keratitis with amniotic membrane transplantation. Br J Ophthalmol. 2003;87:1215–9. doi: 10.1136/bjo.87.10.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hick S, Demers PE, Brunette I, La C, Mabon M, Duchesne B. Amniotic membrane transplantation and fibrin glue in the management of corneal ulcers and perforations: A review of 33 cases. Cornea. 2005;24:369–77. doi: 10.1097/01.ico.0000151547.08113.d1. [DOI] [PubMed] [Google Scholar]
- 73.Seitz B, Das S, Sauer R, Mena D, Hofmann-Rummelt C. Amniotic membrane transplantation for persistent corneal epithelial defects in eyes after penetrating keratoplasty. Eye (Lond) 2009;23:840–8. doi: 10.1038/eye.2008.140. [DOI] [PubMed] [Google Scholar]
- 74.Suri K, Kosker M, Raber IM, Hammersmith KM, Nagra PK, Ayres BD, et al. Sutureless amniotic membrane ProKera for ocular surface disorders: Short-term results. Eye Contact Lens. 2013;39:341–7. doi: 10.1097/ICL.0b013e3182a2f8fa. [DOI] [PubMed] [Google Scholar]
- 75.Turkoglu E, Celik E, Alagoz G. A comparison of the efficacy of autologous serum eye drops with amniotic membrane transplantation in neurotrophic keratitis. Semin Ophthalmol. 2014;29:119–26. doi: 10.3109/08820538.2013.768678. [DOI] [PubMed] [Google Scholar]
- 76.Cheng AMS, Tseng SC. Self-retained amniotic membrane combined with antiviral therapy for herpetic epithelial keratitis. Cornea. 2017;36:1383–6. doi: 10.1097/ICO.0000000000001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schuerch K, Baeriswyl A, Frueh BE, Tappeiner C. Cornea 2019. 2019. Oct 16, Efficacy of amniotic membrane transplantation for the treatment of corneal ulcers. Published online ahead of print. [DOI] [PubMed] [Google Scholar]
- 78.Gris O, Wolley-Dod C, Güell JL, Tresserra F, Lerma E, Corcostegui B, et al. Histologic findings after amniotic membrane graft in the human cornea. Ophthalmology. 2002;109:508–12. doi: 10.1016/s0161-6420(01)00969-1. [DOI] [PubMed] [Google Scholar]