Abstract
PURPOSE:
To report corneal biomechanical parameters in young myopic Indian individuals.
METHODS:
It is a retrospective study where young myopic individuals aged between 19 and 36 years who have undergone corneal biomechanics assessment using Corvis ST between January 2017 and December 2017 were enrolled. Individuals with central corneal thickness (CCT) <500 microns, intraocular pressure (IOP) >21 mmHg, history of any systemic and ocular disease, any previous ocular surgery, high astigmatism, corneal disease such as keratoconus, poor scans quality, and individuals with any missing data were also excluded. Corneal biomechanical parameters were noted in mild to moderate and high myopia.
RESULTS:
We analyzed the 266 eyes of 266 myopic individuals, of which 167 and 99 eyes had mild to moderate and high myopia, respectively. All the individuals were matched for age, IOP, and CCT (P > 0.05). Twenty-three of 32 parameters were similar in different degrees of myopia whereas 9 parameters were significantly different in high myopes as compared to low to moderate myopes. First applanation (A1) parameters and Vinciguerra screening parameters were similar in both the groups (P > 0.05). Second applanation (A2) parameters were similar in both the groups (P > 0.05) except A2 time, A2 deformation, amplitude (DA) (P < 0.05). Highest concavity (HC) parameters were significantly different in both the groups (P < 0.05) except HCDA, HC deflection length, and HC delta arc length (P > 0.05).
CONCLUSIONS:
High myopic eyes showed a significantly higher maximum deflection amplitude, lesser A2 time and HC time, less A2DA, smaller HC radius than mild to moderate myopia indicating softer, more deformable corneas. However, better predictor of corneal biomechanics such as Stiffness parameters at A1 (SPA1), DA ratio max, integrated radius, and Corvis Biomechanical Index were similar among both the groups of myopia.
Keywords: Corneal biomechanical parameters, Corvis ST, myopia
Introduction
Myopia is one of the leading public health problems with increasing its prevalence globally. It has been estimated that globally by 2050, nearly 5 billion and 1 billion people would be affected by myopia and high myopia, respectively, with majority of myopia in the age group between 20 and 40 years.[1] The higher amount of myopia is associated with ocular comorbidities and thereby causing visual impairment leading to poor quality of life. All these facts are alarming and warrant the promising management plan for myopia. Perhaps, glasses and contact lenses are most common option to manage the myopia; refractive surgeries such as laser-assisted in situ keratomileusis, implantable contact lenses have positive impact on individual's quality of life.[2,3] However, poor screening strategies for refractive surgeries could result in postsurgery complications.
Corneal biomechanics has gained lot of attention in the field of refractive surgeries. Assessment of biomechanics parameters are important and cannot be neglected in patients who are undergoing refractive surgeries as it helps in predicting postsurgical complications such as ectasia. It also helps separate normal healthy eyes from ocular disease.[4] In clinical settings the assessment of Corneal Biomechanical properties have made possible with instruments such as ocular response analyzer (ORA) and Corvis ST. ORA is based on dynamic bidirectional applanation process, and biomechanical properties are characterized by corneal hysteresis and the corneal resistance factor,[5,6] whereas Corvis ST measures corneal dynamic deformation response to a puff of air to characterized corneal biomechanical properties.
Cornea in highly myopic eyes is weaker and more deformable.[7] A study by Shen et al. reported that corneal biomechanical properties measured using ORA are compromised in eyes with high myopia.[8] Another study has reported that corneal biomechanical properties measured using ORA are associated with refractive errors and could serve as an useful additional assessment for understanding the progression of myopia.[9]
Knowledge about the association between myopic refractive error and corneal biomechanics could provide more insights toward the management of myopia with refractive surgeries. The inference of this association would be helpful in laser ablative surgeries. Association between myopia and corneal biomechanics using Corvis ST has been studied in children;[10] however, limited literature is available in young myopic individuals. Ethnic variations of corneal biomechanics using ORA has been reported.[11] Secondly previous study has reported corneal biomechanics among high myopes and ignored eyes with low or moderate myopia.[7] To the best of our knowledge, no report has been published on myopic refractive error and corneal biomechanical properties measured using Corvis ST in young Indian individuals. Hence, the aim is to study the association between corneal biomechanical parameters and myopic refractive errors in young Indian individuals.
Methods
Study population
It is a retrospective study conducted in accordance with tenets of declaration of Helsinki, 2008 and was reviewed and approved by institutional ethics committee of Kenia Medical and Research Foundation, Mumbai (EC reg. details: ECR/1088/Inst/MH/2018; EC approval ref. no. 2018/02). Since this was retrospective study, informed consent was not obtained. Young myopic individuals aged between 19 and 36 years who have undergone corneal biomechanics assessment using Corvis ST between January 2017 and December 2017 were enrolled. Individuals with central corneal thickness (CCT) <500 microns, intraocular pressure (IOP) >21 mmHg, history of any systemic and ocular disease, history of any previous ocular surgery, high astigmatism, and corneal disease such as keratoconus were excluded. Corvis ST scans with poor quality and individuals with any missing data were also excluded. Two-hundred and sixty-six eyes of 266 patients were considered for final analysis. Following data were abstracted from each individual: demographic data such as age, gender, eye, date of examination, scan quality, ocular data such as refractive error, best corrected visual acuity, IOP, CCT, and corneal biomechanical parameters were noted.
Corneal biomechanics assessment
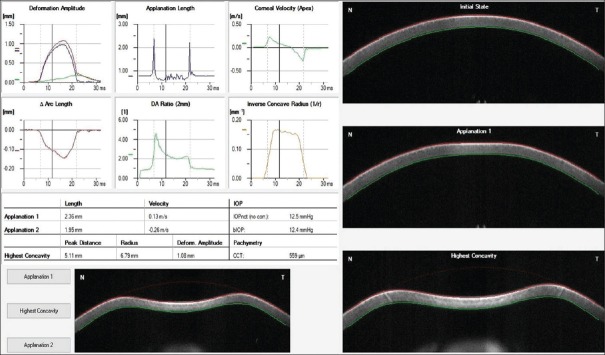
Corneal biomechanical properties were assessed using Corvis ST (Oculus Optikgeräte GmbH, Wetzlar, Germany) which records the deformation of the cornea to a defined air pulse using high-speed Scheimpflug camera. The camera takes over 4300 images per second and 576 points per image. IOP and corneal thickness can be measured with great precision on the basis of the Scheimpflug images. The process of corneal deformation and various corneal biomechanical parameters is shown in the Figure 1. All the parameters that were recorded using Corvis ST are enlisted in Table 1. Individual's images with quality scan as “OK” were considered for analysis. Corneal biomechanical parameters such as variables such as time, velocity, deformation and deflection amplitude, deflection lengths and area, delta arc lengths at first applanation (A1), second applanation (A2), highest concavity (HC), and maximum value (max), were noted. Also, SPA1, integrated radius (IR), Corvis Biomechanical Index (CBI) as provided by Vinciguerra screening reports was also recorded. Table 1 describes the each parameter in detail.
Figure 1.
Describes the process of corneal deformation and various corneal biomechanical parameters
Table 1.
Describes the corneal biomechanical parameters, its abbreviation and brief description
| Parameters | Abbreviation | Description |
|---|---|---|
| Biomechanically corrected intraocular pressure | bIOP | Derived by finite element simulations that take into account the influence of central corneal thickness, age, and DCR parameters |
| First applanation A1 | A1 | Moment at the first applanation of the cornea during the air puff |
| A1 time (ms) | A1T | Time from start to A1 |
| A1 velocity (m/s) | A1V | Velocity of corneal apex at A1 |
| A1 deformation amplitude | A1DA | Moving distance of the corneal apex from the initial position to that at the A1 Time |
| A1 deflection length | A1DL | Length of the flattened cornea at A1 |
| A1 deflection amplitude | A1DeflA | Similar to A1DA without whole eye movement |
| A1 delta arc length | A1dArclength | Change in Arclength from initial state to A1, in a defined 7-mm zone |
| Second applanation A2 | A2 | Moment at the first applanation of the cornea during the air puff |
| A2 time (ms) | A2T | Time from start to A2 |
| A2 velocity (m/s) | A2V | Velocity of corneal apex at A2 |
| A2 deformation amplitude | A2DA | Moving distance of the corneal apex from the initial position to that at A2 time |
| A2 deflection length | A2DL | Length of the flattened cornea at A2 |
| A2 deflection amplitude | A2DeflA | Similar to A2DA without whole eye movement |
| A2 delta arc length | A2dArclength | Change in arclength from initial state to A2, in a defined 7-mm zone |
| Highest concavity | HC | Moment that the cornea assumes its maximum concavity during the air puff |
| HC time | HCT | Time to reach the maximum deformation |
| Radius (mm) | Rad | Central curvature radius at the highest concavity |
| HC deformation amplitude | HCDA | Distance of the corneal apex movement from the initiation of the deformation to the highest concavity |
| HC deflection length | HCDL | Length of the flattened cornea at highest concavity |
| HC deflection amplitude | HCDeflA | Similar to HCDA without whole eye movement |
| Peak distance | PD | Distance between the two surrounding peaks of the cornea at the highest concavity |
| HC delta Arc length | HC dArclength | Change in arclength during the highest concavity moment from the initial state, in a defined 7-mm zone |
| Maximum | Max | Similar as HC |
| Max deformation amplitude | Max DA | Distance of the corneal apex movement from the initiation of the deformation to the highest concavity |
| Max deflection amplitude | Max DeflA | Similar to HCDeflA |
| Max delta arc length | Max dArclength | Change in arclength during the highest concavity moment from the initial state, in a defined 7-mm zone |
| Vinciguerra screening parameters | ||
| Deformation amplitude ratio max (2 mm) | DA ratio max | Ratio between the deformation amplitude at the apex and the average deformation amplitude measured at 2 mm from the center |
| Ambrósio’s relational thickness to the horizontal profile | ARTh | Describes thickness profile in the temporal-nasal direction and defined as corneal thickness thinnest to pachymetric progression |
| Integrated radius | INR | Area under the inverse concave radius versus time curve |
| Stiffness parameter at A1 | SP A1 | Describes corneal stiffness as defined by resultant pressure (Pr) divided by deflection amplitude at A1 |
| Corvis biomechanical index | CBI | Overall biomechanical index for keratoconus detection |
HC: Highest concavity, DCR: Dynamic corneal response
Statistical analysis
Data were entered in Microsoft Excel spreadsheet (Microsoft Corporation) and analyzed using Minitab 17 statistical software (Minitab LLC, State University, PA, USA). We calculated the means and standard deviation (SD) for continuous variables and proportions for the categorical variables. For statistical analysis, eyes were divided into two different groups (low to moderate myopia and high myopia) depending on the degree of spherical equivalent (SE) myopia. According to consensus WHO, high myopia is defined as the SE objective refractive error ≤–5.00 D, and the same criteria were used for the analysis. Two sample t-test was performed to compare biomechanical properties between two groups. Pearson's correlation coefficient (r) was used to assess the relationship between SE myopia and the Vinciguerra screening parameters. Univariate and stepwise multivariable linear regression models were constructed with corneal biomechanical parameters as the dependent variable and the relevant predictive factors such as age, SE refractive error, IOP, and CCT as independent variables.
Results
Demographics
We analyzed the 266 eyes of 266 myopic individuals, out of which 167 eyes had mild to moderate myopia, and 99 had high myopia. The SE refraction (SER) among all individuals examined ranged from −0.75 to −18.75D (SE). Significant differences in refraction were found between the three groups (P < 0.01). Two sample t-test revealed no statistical significant difference in age (P = 0.98), IOP (P = 0.10), and CCT (P = 0.48). There was no significant difference in the gender distribution among three groups (χ2 = 0.197, P = 0.66). Table 2 describes the mean ± SD age, refractive error, IOP and CCT.
Table 2.
Describes the demographic and ocular parameters
| Variable | Low myopia (167) | High myopia (99) | P |
|---|---|---|---|
| Age | 25.60±3.96 | 25.61±4.25 | 0.98 |
| Gender (male:female) | 67:100 | 37:62 | 0.66 |
| Spherical equivalent | −3.04±1.16 | −7.43±2.45 | <0.01 |
| CCT | 541.3±25.0 | 539.1±24.7 | 0.48 |
| IOP | 16.22±2.13 | 16.66±2.09 | 0.10 |
P<0.05 indicates statistical significance. CCT: Central corneal thickness, IOP: Intraocular pressure
Corneal biomechanical properties in different myopic groups
First applanation parameters were similar in both the groups (P > 0.05). Second applanation parameters were similar in both the groups (P > 0.05) except A2 time (P = 0.02) and A2 deformation amplitude (DA) (P < 0.001) which were found to be significantly different in both the groups. HC parameters were also significantly different except HCDA, HC deflection length, and HC dArc length (P > 0.05). Maximum DA, maximum inverse radius, and maximum delta arc length were similar whereas maximum deflection amplitude was significantly different in both the groups. Vinciguerra parameters were similar in both the groups (P > 0.05). Table 3 describes the mean ± SD of various corneal biomechanical parameters.
Table 3.
Describes the mean±standard deviation of corneal biomechanical properties
| Variable | Mild- moderate myopia (167) | High myopia (99) | P |
|---|---|---|---|
| First applanation A1 | |||
| A1 time (ms) | 7.395±0.23 | 7.434±0.22 | 0.16 |
| A1 velocity (m/s) | 0.149±0.02 | 0.149±0.02 | 0.88 |
| A1 deformation amplitude | 0.126±0.01 | 0.125±0.01 | 0.52 |
| A1 deflection length | 2.30±0.27 | 2.29±0.34 | 0.82 |
| A1 deflection amplitude | 0.094±0.01 | 0.095±0.01 | 0.46 |
| A1 deflection area | 0.167±0.02 | 0.169±0.02 | 0.64 |
| A1 delta arc length | −0.015±0.003 | −0.016±0.003 | 0.235 |
| Second applanation A2 | |||
| A2 time (ms) | 21.81±0.36 | 21.70±0.35 | 0.02 |
| A2 velocity (m/s) | −0.267±0.03 | −0.273±0.03 | 0.06 |
| A2 deformation amplitude | 0.36±0.06 | 0.33±0.05 | <0.001 |
| A2 deflection length | 3.08±1.09 | 3.10±1.21 | 0.89 |
| A2 deflection amplitude | 0.108±0.01 | 0.107±0.01 | 0.38 |
| A2 deflection area | 0.24±0.05 | 0.23±0.05 | 0.1 |
| A2 delta arc length | −0.022±0.001 | −0.021±0.001 | 0.107 |
| Highest concavity | |||
| HC time | 16.67±0.41 | 16.54±0.41 | 0.01 |
| Radius (mm) | 6.72±0.57 | 6.50±0.68 | 0.009 |
| HC deformation amplitude | 1.056±0.09 | 1.070±0.1 | 0.25 |
| HC deflection length | 5.77±1.23 | 5.52±1.33 | 0.14 |
| HC deflection amplitude | 0.89±0.08 | 0.93±0.09 | 0.005 |
| Peak distance | 4.95±0.23 | 5.02±0.21 | 0.015 |
| HC deflection area | 3.22±0.43 | 3.35±0.46 | 0.024 |
| HC delta arc length | −0.133±0.02 | −0.131±0.02 | 0.56 |
| Maximum | |||
| Max deformation amplitude | 1.056±0.09 | 1.070±0.1 | 0.25 |
| Max deflection amplitude | 0.91±0.08 | 0.94±0.09 | 0.01 |
| Max delta arc length | -0.148±0.02 | 0.147±0.02 | 0.66 |
| Max inverse radius | 0.183±0.02 | 0.186±0.02 | 0.15 |
| Vinciguerra screening parameter | |||
| Deformation amplitude ratio max (2 mm) | 4.48±0.38 | 4.51±0.42 | 0.54 |
| Ambrósio’s relational thickness to the horizontal profile | 423.9±68 | 436.8±82.6 | 0.19 |
| bIOP | 16.19±1.86 | 16.64±1.86 | 0.06 |
| Integrated radius | 8.83±0.93 | 8.94±1.0 | 0.38 |
| Stiffness parameter at A1 | 106.3±13.6 | 106.8±14.0 | 0.75 |
| Corvis biomechanical index | 0.086±0.16 | 0.057±0.12 | 0.09 |
P<0.05 indicates statistical significance
Association between refractive error and corneal biomechanical properties
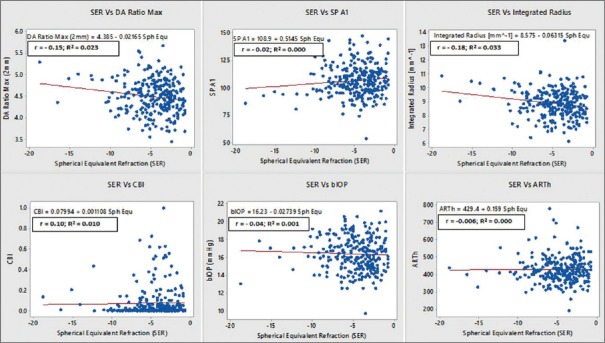
Pearson correlation revealed statistically significant (P < 0.05) but weak association (r = −0.3–−0.15, 0.3–0.1) of SE refractive error with A2 velocity, HCDA, A2DA, DA max, peak distance (PD), radius, deflection amplitude max, HC deflection amplitude, HC deflection area, A2 dArc length, and max inverse radius. We also studied the association between (SER) with Vinciguerra parameters. We found statistically significant (P < 0.05) but weak association of SER with DA ratio max (r = −0.15, P = 0.013), IR (r = −0.18, P = 0.003); however, it was not associated with biomechanically corrected IOP (bIOP) (r = −0.040, P = 0.51), Ambrósio rational thickness horizontal (ARTh) (r = 0.006, P = 0.92), SPA1 (r = 0.10, P = 0.09), and CBI (r = 0.021, P = 0.74) [Figure 2].
Figure 2.
Association between spherical equivalent refraction and vinciguerra screening parameters and biomechanically corrected intraocular pressure
Univariate regression
Univariate linear regression was carried out to study the relation of age, SER, IOP, and CCT with corneal biomechanical properties. We found that 4 of 32 biomechanical parameters were significantly associated with age. HC delta arclength, max inverse radius, DA ratio max, and IR were positively correlated with age. Thirteen of 32 parameters were significantly associated with SER. We did not find any relationship between first applanation parameters and SER. A2V, A2DA, and Radius were positively correlated whereas A2 delta arc length, HCDA, HC def amplitude, PD, HCDAr, maximum deformation and deflection amplitude, DA ratio max, max inverse radius, and IR were negatively correlated. Twenty-nine and 28 of 32 parameters were significantly associated with IOP and pachymetry, respectively. A2 deflection length was the only parameter which did not find association with any of the independent variable. Table 4 describes the coefficient (β) and P value for univariate linear regression analysis.
Table 4.
Describes the results of univariate linear regression model
| Variable | Age | Spherical equivalent | IOP | Pachymetry | ||||
|---|---|---|---|---|---|---|---|---|
| Coefficient (β) | P | Coefficient (β) | P | Coefficient (β) | P | Coefficient (β) | P | |
| First applanation A1 | ||||||||
| A1 time (ms) | −0.0013 | 0.69 | 0.0004 | 0.935 | 0.1046 | <0.001 | 0.0026 | <0.001 |
| A1 velocity (m/s) | 0.0002 | 0.53 | 0.0007 | 0.058 | −0.0056 | <0.001 | −0.0002 | <0.001 |
| A1 deformation amplitude | −0.0001 | 0.466 | 0.0001 | 0.751 | 0.0016 | <0.001 | 0.0001 | 0.003 |
| A1 deflection length | −0.0056 | 0.225 | −0.0003 | 0.959 | 0.0229 | 0.010 | 0.0007 | 0.377 |
| A1 deflection amplitude | 0.0000 | 0.954 | −0.0002 | 0.173 | 0.0008 | <0.001 | 0.0000 | 0.010 |
| A1 deflection area | −0.0004 | 0.254 | −0.0008 | 0.118 | 0.0028 | <0.001 | 0.0001 | 0.007 |
| A1 delta arc length | 0.0000 | 0.876 | 0.0001 | 0.365 | −0.0004 | <0.001 | −0.0000 | 0.002 |
| A2 time (ms) | −0.0062 | 0.255 | 0.006 | 0.458 | −0.1402 | <0.001 | −0.0012 | 0.180 |
| A2 velocity (m/s) | −0.0002 | 0.566 | 0.0023 | <0.001 | 0.0076 | <0.001 | 0.0002 | 0.012 |
| A2 deformation amplitude | −0.0006 | 0.480 | 0.0047 | <0.001 | −0.0047 | 0.005 | 0.0002 | 0.093 |
| A2 deflection length | −0.0273 | 0.12 | −0.044 | 0.084 | −0.0040 | 0.906 | −0.0019 | 0.522 |
| A2 deflection amplitude | 0.0000 | 0.863 | 0.0003 | 0.283 | 0.0008 | 0.016 | 0.0002 | <0.001 |
| A2 deflection area | 0.0010 | 0.172 | 0.0017 | 0.121 | 0.0019 | 0.171 | 0.0004 | <0.001 |
| A2 delta arc length | 0.0000 | 0.90 | −0.0003 | 0.010 | −0.0003 | 0.046 | −0.0001 | <0.001 |
| HC time | −0.0035 | 0.58 | 0.0116 | 0.213 | −0.0307 | 0.011 | 0.0026 | 0.011 |
| Radius (mm) | −0.0166 | 0.076 | 0.057 | <0.001 | 0.0851 | <0.001 | 0.0084 | <0.001 |
| HC deformation amplitude | 0.001 | 0.489 | −0.0074 | <0.001 | −0.0345 | <0.001 | −0.0008 | <0.001 |
| HC deflection length | 0.0137 | 0.481 | 0.031 | 0.285 | −0.0172 | 0.647 | −0.0040 | 0.221 |
| HC deflection amplitude | 0.0008 | 0.542 | −0.010 | <0.001 | −0.0328 | <0.001 | −0.0010 | <0.001 |
| Peak distance | 0.0012 | 0.72 | −0.0019 | <0.001 | −0.0773 | <0.001 | −0.0020 | <0.001 |
| HC deflection area | 0.0023 | 0.733 | −0.043 | <0.001 | −0.1545 | <0.001 | −0.0038 | 0.001 |
| HC delta arc length | 0.0009 | 0.003 | 0.0002 | 0.653 | 0.0023 | <0.001 | −0.0002 | <0.001 |
| Max deformation amplitude | 0.0009 | 0.489 | −0.0074 | <0.001 | −0.0345 | <0.001 | −0.0008 | <0.001 |
| Max deflection amplitude | 0.0007 | 0.620 | −0.0097 | <0.001 | −0.0334 | <0.001 | −0.0009 | <0.001 |
| Max delta arc length | 0.0008 | 0.031 | 0.0004 | 0.503 | 0.0040 | <0.001 | −0.0002 | <0.001 |
| Max inverse radius | 0.0008 | 0.002 | −0.0010 | 0.010 | −0.0014 | 0.007 | −0.0003 | <0.001 |
| Deformation amplitude ratio max (2 mm) | 0.0124 | 0.036 | −0.0217 | 0.013 | −0.1229 | <0.001 | −0.0085 | <0.001 |
| Ambrósio’s relational thickness to the horizontal profile | −0.180 | 0.872 | 0.159 | 0.923 | 6.213 | 0.004 | 0.9052 | <0.001 |
| bIOP | −0.0316 | 0.264 | −0.027 | 0.511 | 0.8227 | <0.001 | 0.0041 | 0.376 |
| Integrated radius | 0.0338 | 0.02 | −0.0631 | 0.003 | −0.2726 | <0.001 | −0.0176 | <0.001 |
| Stiffness parameter at A1 | 0.0130 | 0.95 | 0.0514 | 0.093 | 4.187 | <0.001 | 0.3430 | <0.001 |
| Corvis biomechanical index | 0.0021 | 0.33 | 0.0011 | 0.738 | −0.0267 | <0.001 | −0.0022 | <0.001 |
β indicates regression coefficient and P<0.05 indicates statistical significance. IOP: Intraocular pressure
Stepwise multivariate regression
The stepwise multivariable regression analyses demonstrated that age was positively associated with HC delta arc length, max delta arc length, max inverse radius, DA, and IR whereas negatively associated with A2T and bIOP. SE refractive error was positively associated with A2V, A2DA, radius, whereas negatively associated with 11 parameters. IOP was positively associated with 11 parameters whereas negatively associated with 14 parameters. CCT was positively associated with 8 parameters and negatively associated with 8 parameters. Table 5 describes the regression coefficients and P value for multivariate regression analysis.
Table 5.
Describes the results of Stepwise multivariate regression model
| Variable | Age | Spherical equivalent | IOP | Pachymetry | ||||
|---|---|---|---|---|---|---|---|---|
| Coefficient (β) | P | Coefficient (β) | P | Coefficient (β) | P | Coefficient (β) | P | |
| First applanation A1 | ||||||||
| A1 time (ms) | 0.1046 | <0.001 | ||||||
| A1 velocity (m/s) | −0.0007 | 0.006 | −0.0056 | <0.001 | ||||
| A1 deformation amplitude | 0.0015 | <0.001 | ||||||
| A1 deflection length | 0.0229 | <0.001 | ||||||
| A1 deflection amplitude | 0.0008 | <0.001 | ||||||
| A1 deflection area | 0.0028 | <0.001 | ||||||
| A1 delta arc length | −0.0004 | <0.001 | ||||||
| Second applanation A2 | ||||||||
| A2 time (ms) | −0.0079 | 0.007 | −0.1492 | <0.001 | 0.0025 | <0.001 | ||
| A2 velocity (m/s) | 0.0023 | <0.001 | 0.0076 | <0.001 | ||||
| A2 deformation amplitude | 0.0043 | 0.001 | −0.0059 | 0.001 | 0.0003 | 0.021 | ||
| A2 deflection length | ||||||||
| A2 deflection amplitude | 0.0002 | <0.001 | ||||||
| A2 deflection area | 0.0004 | <0.001 | ||||||
| A2 delta arc length | −0.0003 | 0.034 | −0.0001 | <0.001 | ||||
| Highest concavity | ||||||||
| HC time | −0.0436 | <0.001 | 0.0037 | 0.001 | ||||
| Radius (mm) | 0.0513 | <0.001 | 0.0634 | <0.001 | 0.0060 | <0.001 | ||
| HC deformation amplitude | −0.0074 | <0.001 | −0.0345 | <0.001 | ||||
| HC deflection length | ||||||||
| HC deflection amplitude | −0.0102 | <0.001 | −0.0328 | <0.001 | ||||
| Peak distance | −0.0195 | <0.001 | −0.0774 | <0.001 | ||||
| HC deflection area | −0.0428 | <0.001 | −0.1546 | <0.001 | ||||
| HC delta arc length | 0.0009 | 0.002 | 0.0033 | <0.001 | −0.0003 | <0.001 | ||
| Maximum | ||||||||
| Max deformation amplitude | −0.0074 | <0.001 | −0.0345 | <0.001 | ||||
| Max deflection amplitude | −0.0097 | <0.001 | −0.0334 | <0.001 | ||||
| Max delta arc length | 0.0008 | 0.014 | 0.0053 | <0.001 | −0.0004 | <0.001 | ||
| Max inverse radius | 0.0008 | 0.002 | −0.000 | 0.032 | −0.0002 | <0.001 | ||
| Vinciguerra screening parameters | ||||||||
| Deformation Amplitude ratio max (2 mm) | 0.0093 | 0.015 | −0.0161 | 0.005 | −0.1028 | <0.001 | −0.0057 | <0.001 |
| Ambrósio’s relational thickness to the horizontal profile | 0.905 | <0.001 | ||||||
| bIOP | −0.0243 | <0.001 | 0.9054 | <0.001 | −0.0272 | <0.001 | ||
| Integrated radius | 0.0275 | 0.009 | −0.0528 | 0.001 | −0.2336 | <0.001 | −0.0108 | <0.001 |
| Stiffness parameter at A1 | 3.285 | <0.001 | 0.2606 | <0.001 | ||||
| Corvis biomechanical index | −0.0208 | <0.001 | −0.0017 | <0.001 | ||||
β indicates regression coefficient and P<0.05 indicates statistical significance
Discussion
Corneal biomechanical properties are affected in various ocular diseases such as Keratoconus,[12,13] dry eyes,[4] glaucoma,[14] Fuchs’ dystrophy,[15] and ocular surgeries.[16,17,18] In the present study, we evaluated the corneal biomechanical properties among young Indian myopes in more details than previous studies.[10,19,20]
Instrument consideration
The newly updated Corvis ST software allowed us to evaluate as many as 32 corneal biomechanical parameters unlike previous studies.[4,7,13,17,18,19,20,21,22,23,24,25,26] Since we used a newer version, we had an opportunity to assess “HC deformation/deflection amplitude” which is identical to the “maximum deformation/deflection amplitude” from older version. Limited assessment of corneal biomechanical parameters with previous studies could be attributed to older softwares. To the best of our knowledge, this is the first report of quantitative assessment of the corneal biomechanics with larger sample size in young Indian myopes using Corvis ST. Our chief observation was 23 of 32 parameters were similar in different degrees of myopia whereas 9 parameters were significantly different in high myopes as compared to low to moderate myopes.
ORA describes corneal biomechanical properties in terms of corneal hysteresis and corneal resistance factor which were poor parameters for discriminating between mild keratoconus and normal corneas.[27] On the other hand, Corvis ST uses ultra-high speed Scheimpflug camera that records throughout the deformation process and thereby provide much more information on biomechanical properties as compared to ORA.[28] Corvis ST showed a good repeatability and reproducibility for IOP and dynamic corneal parameters measurement.[29] Furthermore, recently introduced parameters by Corvis ST have shown excellent specificity and sensitivity in discriminating between form fruste keratoconus and normal corneas.[12,30]
Corneal deformation parameters
In the present study, both the groups were age and gender matched. Although gender does not have much effect on corneal biomechanics,[20,22] previous studies have reported controversial report about the association between age and biomechanical properties. There are studies which have reported no association between age and corneal biomechanical properties.[8,10] On the other hand, age has found to be associated with various Corvis ST parameters such as HC time, A1T, A1DA, A1T, A2DA, and HCDA.[19,20] In the present study, age was associated with, HC delta arc length, max delta arc length, max inverse radius, DA ratio max 2 mm, and IR. This could be explained by an age dependent increase in crosslinking fibers in corneal stroma suggesting a stiffer cornea in older people.
The values of corneal deformations parameters obtained at first applanation, second applanation, and at HC are in agreement previous studies conducted in similar age group and low to moderate myopes.[20] A previous study in myopes conducted using ORA demonstrated that corneal biomechanical properties are affected in high myopes.[8] In our study, biomechanic parameters at first applanation were similar among both the groups. The A1T seems a valuable parameter in the diagnosis of keratoconic eyes;[13] however, in our study, we did not find any significant changes in A1T across both the groups. At second applanation, all the parameters were similar in both the groups except A2 time and A2 DA. A2 time and A2 DA were significantly lower in higher myopes as compared to low-moderate myopes. At highest concavity, HC time, radius was significantly lower, whereas deflection amplitude, PD, and deflection area were significantly higher in high myopes as compared to other groups. Maximum deflection amplitude was higher in high myopes. Maximum DA is one of the most repeatable and reproducible parameters. It serves as an indicator of corneal biomechanical properties where a thinner cornea is associated with a higher corneal deformation.[23] Maximum DA was similar in both the groups; however, the value was higher high myopes which is in agreement with previous study.[7] Furthermore, in the present study, the mean ± SD maximum DA in high myopes was 1.07 ± 0.10 which matches with previous report value of 1.07 ± 1.01.[7]
Impact of IOP and CCT on corneal biomechanics has been noted previously. Our results showed that both these parameters are associated with almost all corneal biomechanical parameters and confirmed the previous findings.[10,20] Interestingly, we found positive association of myopic refractive error with A2V, A2DA, and radius. Since myopic refractive error is negative, the reduction in myopic refractive error was associated with increase in value of corneal biomechanical properties. For example, univariate analysis showed that 1 diopter reduction in SE myopic refractive resulted in 0.0023, 0.0047, and 0.057 unit increase in A2V, A2DA, and radius, respectively. This showed that less myopic eyes have high A2V, A2DA, and radius suggesting less myopic eyes have better biomechanics which deceases with increase in myopic error.
Vinciguerra screening parameters
Newly developed Vinciguerra screening parameters are based on optimal combination of various biomechanical and ocular parameters and constructed using logistic regression. They provide much more information in predicting ectasia or separating healthy eyes from ectatic eyes.[30] To the best of our knowledge, this is the first report which describes the Vinciguerra parameters in different degrees of myopia. This could be helpful to understand measures of corneal deformation and biomechanical properties more comprehensively.
A lower value of ARTh indicates a thinner cornea and/or a faster thickness increase toward the periphery. Higher value of SPA1 suggest more stiffer cornea. In our study, we found nonsignifcant difference in ARTh and SPA1 among different degrees of myopia. The bIOP value was derived by finite element simulations that take into account the influence of CCT, age, and dynamic corneal response parameters and has been validated both experimentally and clinically.[29] We noted higher bIOP in high myopes however it was not statistically different from low to moderate myopes. Changes in biomechanical properties of the cornea may have an impact on IOP measurement, increasing the risk of glaucoma, especially in myopes due to its higher prevalence rate. BIOP can help in early screening of glaucoma.
The greater the DA ratio, the less resistant is the cornea to deformation. We noted higher DA ratio in high myopes suggesting softer corneas in high myopes. In the present study DA ratio in mild to moderate and high myopes was 4.48 ± 0.38, and 4.51 ± 0.42, respectively, which is comparable to normal eyes 4.30 ± 0.50.[12]
The CBI is much more sensitive in predicting the ectasia or early keratoconus.[30] CBI value ranges between 0 and 1 and values closer to 1 is associated with biomechanically weaker corneas.[12] A previous study of corneal biomechanics in normal and keratoconus eyes reported that CBI value in normal eyes ranges from 0.0 to 0.88 and having mean ± SD of 0.06 ± 0.14 in normal eyes.[12] In the present study of mean ± SD CBI in mild to moderate and high myopes was 0.086 ± 0.16, and 0.057 ± 0.12, respectively, and did not differ significantly among different groups of myopia. Based on CBI scale, value up to 0.1 is considered as normal, 0.25 is borderline, and values above 0.25 are considered as abnormal.
The strength of this study is larger sample size and detailed assessment of parameters which was possible due to latest software. The study has few limitations too. Since this was retrospective study, axial length which is primary reason for the high myopia was unavailable and therefore could not assess the impact of axial length on corneal biomechanics.
Conclusions
High myopic eyes showed a significantly higher maximum deflection amplitude, lesser A2 time and HC time, less A2DA, smaller HC radius than mild to moderate myopia indicating softer, more deformable corneas. However, better predictor of corneal biomechanics such as SPA1, DA ratio max, IR, and CBI were similar among both the groups of myopia.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors declare that there are no conflicts of interests of this paper.
References
- 1.Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123:1036–42. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Pesudovs K, Garamendi E, Elliott DB. A quality of life comparison of people wearing spectacles or contact lenses or having undergone refractive surgery. J Refract Surg. 2006;22:19–27. doi: 10.3928/1081-597X-20060101-07. [DOI] [PubMed] [Google Scholar]
- 3.Shams N, Mobaraki H, Kamali M, Jafarzadehpour E. Comparison of quality of life between myopic patients with spectacles and contact lenses, and patients who have undergone refractive surgery. J Curr Ophthalmol. 2015;27:32–6. doi: 10.1016/j.joco.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Long Q, Wang JY, Xu D, Li Y. Comparison of corneal biomechanics in Sjögren's syndrome and non-Sjögren's syndrome dry eyes by scheimpflug based device. Int J Ophthalmol. 2017;10:711–6. doi: 10.18240/ijo.2017.05.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luce DA. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J Cataract Refract Surg. 2005;31:156–62. doi: 10.1016/j.jcrs.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Zheng L, Zhao X, Xu Y, Chen S. Corneal biomechanics after small-incision lenticule extraction versus Q-value-guided femtosecond laser-assisted in situ keratomileusis. J Curr Ophthalmol. 2016;28:181–7. doi: 10.1016/j.joco.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He M, Wang W, Ding H, Zhong X. Corneal biomechanical properties in high myopia measured by dynamic scheimpflug imaging technology. Optom Vis Sci. 2017;94:1074–80. doi: 10.1097/OPX.0000000000001152. [DOI] [PubMed] [Google Scholar]
- 8.Shen M, Fan F, Xue A, Wang J, Zhou X, Lu F. Biomechanical properties of the cornea in high myopia. Vision Res. 2008;48:2167–71. doi: 10.1016/j.visres.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Jiang Z, Shen M, Mao G, Chen D, Wang J, Qu J, et al. Association between corneal biomechanical properties and myopia in Chinese subjects. Eye (Lond) 2011;25:1083–9. doi: 10.1038/eye.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He M, Ding H, He H, Zhang C, Liu L, Zhong X. Corneal biomechanical properties in healthy children measured by corneal visualization scheimpflug technology. BMC Ophthalmol. 2017;17:70. doi: 10.1186/s12886-017-0463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lazreg S, Mesplié N, Praud D, Delcourt C, Kamoun H, Chahbi M, et al. Comparison of corneal thickness and biomechanical properties between North African and French patients. J Cataract Refract Surg. 2013;39:425–30. doi: 10.1016/j.jcrs.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Ambrósio R, Jr, Lopes BT, Faria-Correia F, Salomão MQ, Bühren J, Roberts CJ, et al. Integration of scheimpflug-based corneal tomography and biomechanical assessments for enhancing ectasia detection. J Refract Surg. 2017;33:434–43. doi: 10.3928/1081597X-20170426-02. [DOI] [PubMed] [Google Scholar]
- 13.Elham R, Jafarzadehpur E, Hashemi H, Amanzadeh K, Shokrollahzadeh F, Yekta A, et al. Keratoconus diagnosis using Corvis ST measured biomechanical parameters. J Curr Ophthalmol. 2017;29:175–81. doi: 10.1016/j.joco.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W, Du S, Zhang X. Corneal deformation response in patients with primary open-angle glaucoma and in healthy subjects analyzed by Corvis ST. Invest Ophthalmol Vis Sci. 2015;56:5557–65. doi: 10.1167/iovs.15-16926. [DOI] [PubMed] [Google Scholar]
- 15.Clemmensen K, Hjortdal J. Intraocular pressure and corneal biomechanics in Fuchs’ endothelial dystrophy and after posterior lamellar keratoplasty. Acta Ophthalmol. 2014;92:350–4. doi: 10.1111/aos.12137. [DOI] [PubMed] [Google Scholar]
- 16.Ortiz D, Piñero D, Shabayek MH, Arnalich-Montiel F, Alió JL. Corneal biomechanical properties in normal, post-laser in situ keratomileusis, and keratoconic eyes. J Cataract Refract Surg. 2007;33:1371–5. doi: 10.1016/j.jcrs.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 17.Mastropasqua L, Calienno R, Lanzini M, Colasante M, Mastropasqua A, Mattei PA, et al. Evaluation of corneal biomechanical properties modification after small incision lenticule extraction using scheimpflug-based noncontact tonometer. Biomed Res Int. 2014;2014:290619. doi: 10.1155/2014/290619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirasawa K, Nakakura S, Nakao Y, Fujino Y, Matsuura M, Murata H, et al. Changes in corneal biomechanics and intraocular pressure following cataract surgery. Am J Ophthalmol. 2018;195:26–35. doi: 10.1016/j.ajo.2018.07.025. [DOI] [PubMed] [Google Scholar]
- 19.Valbon BF, Ambrósio R, Jr, Fontes BM, Alves MR. Effects of age on corneal deformation by non-contact tonometry integrated with an ultra-high-speed (UHS) scheimpflug camera. Arq Bras Oftalmol. 2013;76:229–32. doi: 10.1590/s0004-27492013000400008. [DOI] [PubMed] [Google Scholar]
- 20.Wang W, He M, He H, Zhang C, Jin H, Zhong X. Corneal biomechanical metrics of healthy Chinese adults using Corvis ST. Cont Lens Anterior Eye. 2017;40:97–103. doi: 10.1016/j.clae.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Kato Y, Nakakura S, Asaoka R, Matsuya K, Fujio Y, Kiuchi Y. Cataract surgery causes biomechanical alterations to the eye detectable by Corvis ST tonometry. PLoS One. 2017;12:e0171941. doi: 10.1371/journal.pone.0171941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long Q, Wang J, Yang X, Jin Y, Ai F, Li Y. Assessment of corneal biomechanical properties by corVis ST in patients with dry eye and in healthy subjects. J Ophthalmol. 2015;2015:380624. doi: 10.1155/2015/380624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hon Y, Lam AK. Corneal deformation measurement using scheimpflug noncontact tonometry. Optom Vis Sci. 2013;90:e1–8. doi: 10.1097/OPX.0b013e318279eb87. [DOI] [PubMed] [Google Scholar]
- 24.Tian L, Huang YF, Wang LQ, Bai H, Wang Q, Jiang JJ, et al. Corneal biomechanical assessment using corneal visualization scheimpflug technology in keratoconic and normal eyes. J Ophthalmol. 2014;2014:147516. doi: 10.1155/2014/147516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ambrósio R, Ramos I, Luz A, Faria FC, Steinmueller A, Krug M, et al. Dynamic ultra high speed Scheimpflug imaging for assessing corneal biomechanical properties. Rev Bras Oftalmol. 2013;72:99–102. [Google Scholar]
- 26.Sharifipour F, Panahi-Bazaz M, Bidar R, Idani A, Cheraghian B. Age-related variations in corneal biomechanical properties. J Curr Ophthalmol. 2016;28:117–22. doi: 10.1016/j.joco.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fontes BM, Ambrósio R, Jr, Jardim D, Velarde GC, Nosé W. Corneal biomechanical metrics and anterior segment parameters in mild keratoconus. Ophthalmology. 2010;117:673–9. doi: 10.1016/j.ophtha.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 28.Koprowski R, Lyssek-Boron A, Nowinska A, Wylegala E, Kasprzak H, Wrobel Z. Selected parameters of the corneal deformation in the Corvis tonometer. Biomed Eng Online. 2014;13:55. doi: 10.1186/1475-925X-13-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopes BT, Roberts CJ, Elsheikh A, Vinciguerra R, Vinciguerra P, Reisdorf S, et al. Repeatability and reproducibility of intraocular pressure and dynamic corneal response parameters assessed by the Corvis ST. J Ophthalmol. 2017;2017:8515742. doi: 10.1155/2017/8515742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vinciguerra R, Ambrósio R, Jr, Elsheikh A, Roberts CJ, Lopes B, Morenghi E, et al. Detection of keratoconus with a new biomechanical index. J Refract Surg. 2016;32:803–10. doi: 10.3928/1081597X-20160629-01. [DOI] [PubMed] [Google Scholar]