Abstract
AIM:
The aim of the study is to report the results of conjunctival-limbal autograft (CLAU) transplantation and penetrating keratoplasty (PK) in eyes with limbal stem cell deficiency (LSCD) due to chemical or thermal injury.
METHODS:
Thirty-one eyes of the 31 patients, who had unilateral LSCD due to chemical or thermal injury, were included in the study. Bilaterally affected cases and LSCD due to Steven-Johnson syndrome and mucous membrane pemphigoid were excluded from the study. All patients underwent a complete ophthalmologic examination. The surgical procedures, postoperative complications, ocular surface status, and visual outcomes were noted.
RESULTS:
In the CLAU group, regular corneal epithelium and ambulatory vision (≤1.0 logarithm of the minimum angle of resolution [20/200]) were achieved in 81% of eyes, including 22 eyes (71%) that were assessed after a mean follow-up period of 58 months, respectively. The 5-year survival rate of corneal allograft was 33%, 4 ± 13.9 in the CLAU applied eyes. In addition, the corneal graft clarity maintenance rate was found to be higher in patients having ≥12 months duration between CLAU and PK, which is statistically significant (62% vs. 23%, P = 0.046).
CONCLUSION:
Waiting at least 1 year after CLAU transplantation to perform PK increases corneal clarity. Eyelid problems, even if the eyelids were reconstructed properly, remain a major risk factor for the development of the epithelial disorder in the early and late postoperative period in CLAU applied eyes.
Keywords: Chemical injury, conjunctival-limbal autograft transplantation, eyelid reconstruction, limbal stem cell deficiency, penetrating keratoplasty
Introduction
To maintain corneal clarity, the integrity of corneal epithelium is crucial. The corneal epithelium originates from the limbal stem cells located in the limbus.[1] A decrease in the number of limbal stem cells due to ocular surface injury leads to corneal opacity and scar development.[2] In such cases, if this limbal stem cell deficiency (LSCD) is not adjusted properly before corneal transplantation, it is not possible to maintain the clarity of the transplanted corneal graft. LSCD treatment cannot be managed by corneal transplantation alone. It is possible to treat such cases by limbal stem cell transplantation or cultivated limbal epithelial transplantation to the donor side.[3,4,5,6,7]
After the suggestion of the XYZ hypothesis related to the regeneration of corneal epithelium by Thoft and Friend, various new ocular surface transplantation techniques have emerged and improved the management and prognosis of eyes with LSCD.[8] Limbal autograft and allograft transplantation can restore depleted limbal stem cell populations, and normal corneal phenotype can be reestablished.[6]
In this study, we aimed to report our experience with the treatment modalities, follow-up challenges, and long-term outcomes of a group of patients with LSCD due to chemical or thermal ocular surface injury. In addition, the long-term results of sequentially applied penetrating keratoplasty (PK) in conjunctival-limbal autograft (CLAU)-transplanted eyes were evaluated in this study, but first in the literature.
Methods
Retrospective medical records of patients who underwent conjunctival autograft limbal stem cell transplantation between November 1995 and January 2014 at the Istanbul University, Faculty of Medicine's Ophthalmology Department, were reviewed. The study was approved by the Istanbul University, the Istanbul Faculty of Medicine, and the Surgical and Pharmaceutical Research Ethics Board (IRB approval date is 23/10/2015 and the number is 1824). Informed consent is not required due to the retrospective nature of the study.
Patients who underwent limbal stem cell transplantation due to LSCD and received follow-up assessment at least 1-year postoperatively were involved in the study. The existence of conjunctivalization, which was defined as conjunctival epithelium with a vascular component encroaching over corneal surface, along with the existence of irregular corneal epithelium as determined by fluorescein staining under biomicroscopic examination were the diagnostic criteria of LSCD. In addition, the absence of limbal palisades of Vogt, the presence of superficial neovascularization on the cornea and the presence of goblets cells on the corneal surface in the impression cytology were additional criteria in defining LSCD [Figure 1].[9] The cases which were followed up with <1 year postoperatively, bilaterally affected cases and LSCD due to Steven-Johnson syndrome and mucous membrane pemphigoid were excluded from the study.
Figure 1.
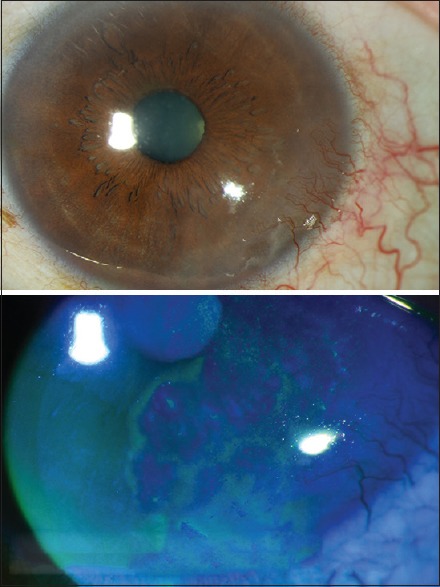
Appearance of partial limbal stem cell deficiency. Note the conjunctivalization in the inferior temporal quadrant and irregular fluorescein staining of abnormal corneal epithelium
The best-corrected visual acuities (BCVAs), biomicroscopic findings, intraocular pressures (IOPs), and fundoscopic findings of all participants were evaluated and recorded. The cases, in which fundoscopic examination was not possible, were evaluated using B-scan ultrasonography. The patients who applied to the clinic 4 months after the injury incident were classified as the chronic group; those who applied within 4 months after injury were classified as the acute group. In cases of patients who were in the acute phase during their first clinic visit, it was decided that treatment with topical medications and/or amniotic membrane transplantation (AMT) should be carried out until inflammation subsided. In cases of patients who were in the chronic phase during their initial clinic visit, existing lid problems, tear film instability, and chronic inflammation were treated before the limbal transplantation (LT) was applied.
Patients were grouped according to corneal involvement and limbal ischemia degree using the Roper–Hall classification system.[10] According to this classification, Stage 1 disease is defined as corneal epithelial defect along with the absence of limbal ischemia; Stage 2 disease is defined as clear iris detail, despite the existence of corneal haze with limbal ischemia occupying <1/3 of the limbus; Stage 3 disease is defined as blurry iris detail with limbal ischemia occupying >1/3 but <1/2 of the limbus; and Stage 4 disease is defined as corneal opacification with no iris detail, and limbal ischemia occupying >1/2 of the limbus. The same surgeon operated in all cases (NA).
The recipient's eye (s) is prepared by performing a conjunctival peritomy, followed by a superficial keratectomy to remove abnormal epithelium and fibrovascular pannus in all LT candidates. In CLAU, two limbal grafts measuring approximately 6 mm at the limbus (3-h quadrants) and extending 5–8 mm posterior to the limbus are demarcated at the 12 and 6 o’clock positions. Dissection toward the cornea is extended through the limbal palisades of Vogt to ensure the isolation of stem cells. The graft is then transferred to the recipient's eye, taking care to maintain the epithelial and limbal orientation of the graft. The CLAUs are secured with 10-0 nylon interrupted sutures to the recipient limbus, ensuring the grafts overlap the cornea 1 mm peripherally. During the follow-up period, any comorbidities, such as glaucoma and cataracts, are managed with proper surgical intervention. Complications related to the proper epithelization of the ocular surface (persistent epithelial defect) which has occurred within the first 2 months after CLAU transplantation were classified as the early postoperative period complications [Figure 2].
Figure 2.
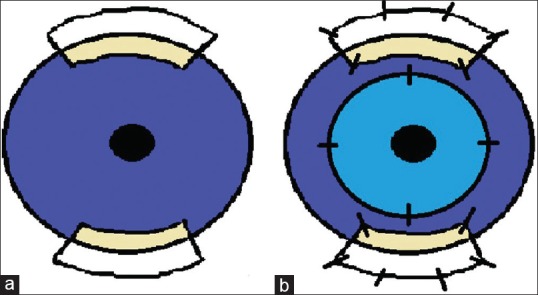
(a) Diagram illustrating the harvestment of autolimbal explants at the 6 and 12 o’clock positions of the recipient eye. Two limbal autografts measuring approximately 6 mm at the limbus (3-h quadrants) and extending 5–8 mm posterior to the limbus are demarcated at the 12 and 6 o’clock positions. (b) Conjunctival-limbal autograft transplantation with corneal allograft. The conjunctival-limbal autograft are secured with 10-0 nylon interrupted sutures to the recipient limbus, ensuring the grafts overlap the cornea 1 mm peripherally
Concerning the severity of inflammation during the postoperative period, topical steroids, nonpreserved teardrops, and antibiotics were ordered for each patient. Persistent corneal epithelial defects (PED) in the postoperative period were managed with AMT, temporary/ permanent tarsorrhaphy, and autologous serum.
After normal corneal epithelium was achieved, corneal transplantation was suggested to remove persistent central corneal opacity that restricted visual improvement. PK was applied at least 6 months after the CLAU procedure. Corneal edema, endothelial rejection, glaucoma, limbal deficiency recurrence (defined as the failure to maintain normal corneal epithelium and conjunctivalization recurrence), and visual acuity were noted in PK-applied eyes during the follow-up. The eyelid defects were reconstructed before the LT. If there was any suspicion regarding abnormal lid function, lid reconstruction was reapplied. Despite proper treatment, some of the cases showed chronic cicatricial eyelid disorder during follow-up. The primary success criterion was defined as the existence of healthy corneal epithelium, which was defined as the absence of fluorescein staining of the ocular surface, and the secondary success criterion was the improvement in visual acuity compared to preoperative rates.
Statistical analyses were performed using the SPSS software, Version 22 (SPSS Inc., Chicago, IL, USA). The variables were investigated using the Kolmogorov–Smirnov/Shapiro–Wilk test to determine whether or not they were typically distributed. The acquired data were reported in average, standard deviation, and percentage. In categorical variability comparison, Chi-square and Fischer exact tests were used. Comparisons between groups were performed using the Student's t-test and Mann–Whitney U-test. Cumulative survival rates of healthy epithelium and corneal graft clarity were analyzed using the Kaplan–Meier survival test. Statistically, the significance rate was determined to be P < 0.05.
Results
Patient demographics and conjunctival-limbal autograft transplantation results
There were 27 men and 4 women who underwent CLAU transplantation. The mean age at presentation was 31 ± 13 (4–53 years). The right eye involvement was seen in 74% (n = 23) of cases. There were 5 (16%) thermal, 3 (10%) acidic, and 23 (74%) alkaline injuries.
Fifteen cases were in the acute phase (48%), and 16 cases were in the chronic phase (52%) at presentation. Of the cases where patients were in the acute phase during the first visit, two cases had a second-degree injury, five cases had a third-degree injury, and eight cases had a fourth-degree injury, according to the Roper–Hall classification. Of the cases where patients were in the acute phase, it was decided that treatment with topical anti-inflammatory medications should be administered in 12 cases, and AMT should be done in three cases until the inflammation is subsided. CLAU transplantation was performed in the cases presenting in the acute phase after a mean time of 6 ± 8 months (1.5–30 months) from the initial visit. Of the 16 cases who were in the chronic phase, the right eye was affected in 11 cases (69%). Eleven cases were male (69%), and the mean age at presentation was 43 ± 16 (4–53 years).
We performed CLAU transplantation in 22 eyes, CLAU combined with AMT in seven eyes and CLAU combined with PK in two eyes. Secondary CLAU surgery was performed due to irregular corneal surface persistence in one case. In this study, 13 patients had severe eyelid problems at presentation. Reconstruction surgery was performed before PK in some but not all cases. Some of the patients also showed recurrence in cicatricial eyelid problems during follow-ups. Eyelid reconstruction surgery was performed in eight cases before and in two cases immediately after CLAU surgery (n = 10). CLAU with AMT was performed for symblepharon reconstruction in five cases.
Corneal epithelization delay was seen in ten cases after CLAU transplantation. Three of them needed AMT for PED treatment. Tectonic PK was required in one case due to spontaneous corneal perforation. In other cases, epithelial defects were treated by a therapeutic contact lens, autologous serum eye drops, and tarsorrhaphy. Sixty percent of eyes that had epithelization problems in the early postoperative period after the CLAU procedure had a history of eyelid surgery. Normal corneal epithelization was completed in all cases (100%) after an average of 25 ± 21 days (2–120 days).
Results of long-term survival of subsequently applied corneal grafts
Eighteen PK surgeries were performed in 16 eyes, in which normal corneal epithelium was achieved after CLAU. PK was performed at a mean time of 23 ± 17 months (4–65 months) after the CLAU procedure in 14 eyes. Simultaneous PK with CLAU was performed in two eyes. PK was repeated in two eyes due to one case in the endothelial rejection of the previous corneal graft and due to descemetocele formation in the other.
In the early postoperative period, PED was observed in two cases after PK because of recurrent cicatricial eyelid problems. One had graft failure due to PED. Therapeutic PK was required in the other case due to descemetocele development.
During follow-ups, corneal graft rejection attacks were observed in six cases. The epithelium rejection attack was observed in one case after 9 months. Endothelial rejection attack was observed in five eyes after a mean time of 5 ± 3 months (1–10 months), and we observed graft failure in two of six cases (33%) despite intense topical and systemic steroid treatment. Corneal graft failure was eventually observed in 11 cases (55%) after 21 ± 15 months of follow-up (5–67 months). The reason for the failure was PED due to depletion of limbal cells during the follow-up in three cases, chronic corneal endothelial insufficiency in five cases, endothelial rejection in two cases, and herpetic keratitis in one case.
As mentioned above, in this study, the eyelid problems were resolved in some cases before the application of CLAU, but not in all cases. We did not observe any statistically significant difference between the eyes that underwent lid reconstruction before the CLAU procedure and those that did not, in terms of corneal graft survival rates (P = 0.43). Furthermore, the corneal graft clarity maintenance rate was found to be higher in patients having ≥12 months duration between CLAU and PK, which was statistically significant (62% vs. 23%, P = 0.046) [Graph 1]. However, normal corneal epithelium was preserved in the cases of eyelid reconstruction applied prior to CLAU (six eyes, 19%) compared to the other cases; in the other cases, the irregular corneal epithelium (conjunctivalization) encroaching on the corneal graft was observed after a mean follow-up of 58 ± 44 months (12–219 months) (P = 0.04) [Graph 2]. The cumulative corneal graft survival rate after PK was found to be 33% ± 14% in the 5th year of the follow-up period [Graph 3 and Figure 3]. Secondary glaucoma was seen in three cases after PK, and cyclodestructive surgery was performed in two cases. Optic atrophy was seen in two eyes that had glaucoma before PK.
Graph 1.
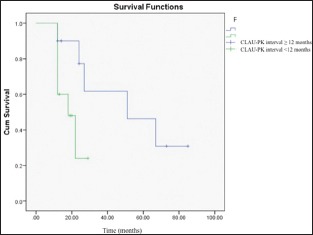
Corneal allograft survival rate comparison in which the conjunctival-limbal autograft-penetrating keratoplasty intervals were ≥12 months and <12 months
Graph 2.
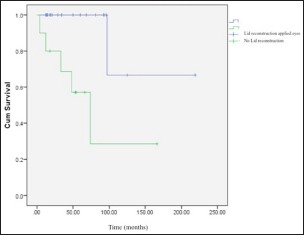
The survival rate of proper corneal epithelium comparison between eyes, in which lid reconstruction was applied before the conjunctival-limbal autograft, and lid reconstruction was not applied before the conjunctival-limbal autograft (P = 0.04)
Graph 3.
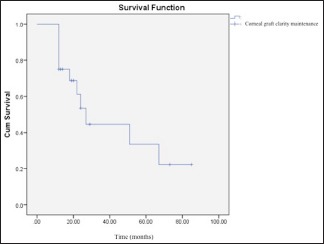
Cumulative corneal allograft survival rate in penetrating keratoplasty-applied eyes (33%, 4 ± 13.9) in the 5th year after the procedure
Figure 3.
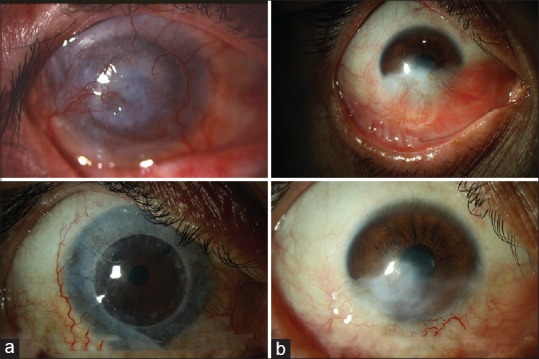
Preoperative and last follow-up appearance of eyes having limbal stem cell deficiency. Penetrating keratoplasty along with conjunctival-limbal autograft has been applied in the first case (a). In the second case, conjunctival-limbal autograft has been applied in the inferior quadrant. Note the conjunctivalization was significantly decreased, and corneal stroma was clearer than the preoperative state (b)
Best-corrected visual acuity results at the final visit
Twenty-one eyes had >1.0 logarithm of the minimum angle of resolution (logMAR) (20/200) visual acuity preoperatively. Visual acuity was improved in 81% of cases (n = 25), at the end of the follow-up. The mean BCVA was significantly improved in patients in whom visual acuity was ≤1.0 logMAR (20/200) at presentation (P <0.01). Visual acuity achieved ambulatory visual acuity level (≤1,0 logMar [20/200]) in 22 eyes (71%) after CLAU in 58 ± 44 months (median: 48 months, range: 12–219 months). Visual acuity was detected ≤0,3 logMar (20/40) in 10 eyes (32%) after an average of 34 ± 23 months (13–94 months) of the follow-up period.
Discussion
In this study, the existence of normal corneal epithelium and improvement in visual acuity were accepted as the surgical success criteria.
In general, the success rate of CLAU transplantation is perfectly fine.[11] Kenyon and Tseng have applied CLAU transplantation within 2–4 weeks of the incidence of chemical injuries and stated that after an average 18 ± 18.5 months (2–45 months) of follow-up, visual acuity improved in 88% of cases, and failure of epithelialization was observed in 11.5% of cases.[11] Similarly, Yao et al. succeeded in the restoration of the ocular surface in 32 out of 34 eyes, in which CLAU transplantation combined with deep anterior lamellar keratoplasty was applied after a mean follow-up time of 27 ± 15.4 months, with a success rate of 94.1%.[12] In their study, which was related to the timing of the LT and involved 16 patients, they observed that the epithelialization rate after CLAU transplantation was two times faster in eyes in the late period (>4 months) after injury than eyes in the early period (<4 months) (8.3 ± 6.7 days vs. 15 ± 6.1 days, respectively).[13] In other similar studies,[14,15] a waiting time of at least 3 months has been proposed to observe the avascular limbal area before CLAU transplantation. In our study, CLAU transplantation was applied to the patients at least 3 months after injury, and during this period, adnexal pathologies (cicatricial entropion, symblepharon, fornix stenosis, and tear deficiency) were seen to have been overcome. Normal epithelialization was achieved within the first 2 weeks postoperatively in 21 out of 31 of eyes (68%), and the mean epithelialization time was 25 ± 21 days (2–120). Epithelialization failed in only four eyes (13%) during the postoperative period, in which three of them had severe recurrent cicatricial eyelid disorders despite reconstruction. Our results are compatible with the literature.
The normal corneal epithelium was preserved in 81% of eyes, in which a simultaneous or staged PK procedure was applied during a mean follow-up of 58 ± 44 months (12–219 months). Visual acuity increased significantly compared to that at presentation, and it exceeded the ambulatory visual acuity level in 71% of eyes (P = 0.01). Vision improvement was also found to be highly significant in eyes that had an ambulatory vision (≤1.0 logMAR [20/200]) at presentation (P < 0.01).
In the literature, there are no studies about the long-term results of simultaneous or staged corneal transplant combined with CLAU. Kenyon and Tseng suggested that it would be appropriate to apply keratoplasty 1 year after the LT.[11] In our study, corneal clarity maintenance rate was found to be significantly higher in patients having PK after a mean duration of ≥12 months rather than <12 months (P = 0.046). Thus, waiting to perform PK until at least 1 year after CLAU transplantation increases corneal clarity maintenance in CLAU patients. Frucht-Pery et al. have published a small series of cases involving three patients who underwent PK three to 6 months after CLAU transplantation. Graft epithelization was completed within 7–12 days, and none of the patients had a recurrent epithelial defect or graft rejection.[16] Yao et al. reported that ocular surface restoration was achieved at the end of 27 ± 15.4 months’ follow-up in 32 of 34 eyes (94.1%) that had undergone deep anterior lamellar keratoplasty combined with CLAU transplantation.[12] In Rao et al.'s cohort, PK was applied to seven CLAU-transplanted eyes, and visual acuity was improved in 81.3% of the cases during the follow-up period.[13]
In our study, the corneal clarity maintenance rate at 5 years postoperatively was 33.4% ± 13.9%, and the most common cause of graft failure was identified as the chronic loss of corneal allograft endothelium (45%) during the follow-up. The epithelialization problem after PK was observed in two eyes that had prior eyelid problems. Signs of rejection attack were observed in the corneal tissue of 6–20 eyes (30%), all of which emerged in the 1st postoperative year. Treatment of corneal rejection attacks decreased the rate of permanent rejection by 67%.
Another question currently awaiting response is related to the capacity of stem cells in the transplanted limbus to maintain their long-term function. In almost all series published so far, the follow-up period ranges from 12 to 27 months. It has been reported in the literature that a decrease in the function of limbal tissue has been observed in some of the recipients’ eyes. Williams et al. reported the development of limbal graft failure in two of nine eyes, in which CLAU was previously applied.[17] In another study, Jenkins et al. observed recurrent epithelial defect problems in two eyes, in which CLAU was applied and reported that this may have been related to the reduction of limbal stem cell functions over time.[18] Some factors that may cause the long-term failure of limbal autograft tissues have been described in the literature. These include inflammation and subclinical limbal insufficiency in the donor tissue.[19] In our study, it was found that the survival rate of the normal corneal epithelium was indirectly associated with eyelid problems (P = 0.04). The possibility of development of corneal epithelial irregularities is still higher in eyes having serious eyelid problems even if reconstructions have been performed. As well, eyes that failed in epithelialization or eyes that had delayed epithelialization, contrary to expectations, had a history of eyelid reconstruction in 60% of cases. This indicates that eyelid problems are one of the primary reasons for the complications observed in the early postoperative period. In our study, corneal graft failure was eventually observed in 11 cases (55%) after 21 ± 15 months of follow-up (5–67 months). The reason for the failure was PED in three cases (27%) due to depletion of limbal cells during the follow-up. This finding has not yet been well described in the literature.
In the literature, the rate of postoperative glaucoma after LT with PK ranged from 26% to 32%.[20,21] In our study, glaucoma is developed in 2 of 31 eyes (6%) after CLAU transplantation and in 3 of 16 eyes (19%) after PK. The reason for glaucoma development in CLAU applied eyes is interpreted as the consequence of alkali injury-induced trabecular meshwork damage. Surgical intervention was required in two cases, in which the IOP was unable to be controlled with topical medications. Increase in visual acuity could not be achieved due to glaucomatous optic atrophy in two eyes. It has been reported in the literature that postoperative bacterial keratitis rates range from 8% to 14%.[20,22] In our series, herpetic keratitis was seen in one eye.
Conclusion
As a result, LT is still the most important treatment option in severe ocular surface diseases. Suppression of inflammation in the preoperative period and awareness of the risk of secondary glaucoma, performing eyelid reconstruction in crucial situations and waiting for PK at least 1 year after LT are essential factors for surgical success. Eyelid problems, even if the eyelids were appropriately reconstructed, remain a significant risk factor for the development of the epithelial disorder in the early and late postoperative periods in CLAU-transplanted eyes. The success rate of surgery would decrease with increased duration of follow-up in the autograft LT procedure even if the risk factors were removed. The staged procedure is much more convenient than the simultaneous procedure in terms of corneal allograft clarity maintenance in limbal autograft employed eyes. One should be careful in terms of infectious keratitis and secondary glaucoma in the follow-up period.
Consent to publish
Written informed consent for publication was obtained from all of the participants.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors declare that there are no conflicts of interests of this paper.
Acknowledgments
Special thanks to Fedai Abi, who is the former secretary of the cornea section, for supporting us during the collection of data.
References
- 1.Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: Implications on epithelial stem cells. Cell. 1989;57:201–9. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- 2.Dua HS, Azuara-Blanco A. Limbal stem cells of the corneal epithelium. Surv Ophthalmol. 2000;44:415–25. doi: 10.1016/s0039-6257(00)00109-0. [DOI] [PubMed] [Google Scholar]
- 3.Dua HS, Azuara-Blanco A. Allo-limbal transplantation in patients with limbal stem cell deficiency. Br J Ophthalmol. 1999;83:414–9. doi: 10.1136/bjo.83.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holland EJ. Epithelial transplantation for the management of severe ocular surface disease. Trans Am Ophthalmol Soc. 1996;94:677–743. [PMC free article] [PubMed] [Google Scholar]
- 5.Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, De Luca M, et al. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990–3. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- 6.Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G, et al. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147–55. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 7.Tan DT, Ficker LA, Buckley RJ. Limbal transplantation. Ophthalmology. 1996;103:29–36. doi: 10.1016/s0161-6420(96)30737-9. [DOI] [PubMed] [Google Scholar]
- 8.Thoft RA, Friend J. Biochemical transformation of regenerating ocular surface epithelium. Invest Ophthalmol Vis Sci. 1977;16:14–20. [PubMed] [Google Scholar]
- 9.Dua HS, Miri A, Alomar T, Yeung AM, Said DG. The role of limbal stem cells in corneal epithelial maintenance: Testing the dogma. Ophthalmology. 2009;116:856–63. doi: 10.1016/j.ophtha.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 10.Roper-Hall MJ. Thermal and chemical burns. Trans Ophthalmol Soc U K. 1965;85:631–53. [PubMed] [Google Scholar]
- 11.Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96:709–22. doi: 10.1016/s0161-6420(89)32833-8. [DOI] [PubMed] [Google Scholar]
- 12.Yao YF, Zhang B, Zhou P, Jiang JK. Autologous limbal grafting combined with deep lamellar keratoplasty in unilateral eye with severe chemical or thermal burn at late stage. Ophthalmology. 2002;109:2011–7. doi: 10.1016/s0161-6420(02)01258-7. [DOI] [PubMed] [Google Scholar]
- 13.Rao SK, Rajagopal R, Sitalakshmi G, Padmanabhan P. Limbal autografting: Comparison of results in the acute and chronic phases of ocular surface burns. Cornea. 1999;18:164–71. doi: 10.1097/00003226-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Morgan S, Murray A. Limbal autotransplantation in the acute and chronic phases of severe chemical injuries. Eye (Lond) 1996;10(Pt 3):349–54. doi: 10.1038/eye.1996.72. [DOI] [PubMed] [Google Scholar]
- 15.Ronk JF, Ruiz-Esmenjaud S, Osorio M, Bacigalupi M, Goosey JD. Limbal conjunctival autograft in a subacute alkaline corneal burn. Cornea. 1994;13:465–8. doi: 10.1097/00003226-199409000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Frucht-Pery J, Siganos CS, Solomon A, Scheman L, Brautbar C, Zauberman H, et al. Limbal cell autograft transplantation for severe ocular surface disorders. Graefes Arch Clin Exp Ophthalmol. 1998;236:582–7. doi: 10.1007/s004170050125. [DOI] [PubMed] [Google Scholar]
- 17.Williams KA, Brereton HM, Aggarwal R, Sykes PJ, Turner DR, Russ GR, et al. Use of DNA polymorphisms and the polymerase chain reaction to examine the survival of a human limbal stem cell allograft. Am J Ophthalmol. 1995;120:342–50. doi: 10.1016/s0002-9394(14)72164-6. [DOI] [PubMed] [Google Scholar]
- 18.Jenkins C, Tuft S, Liu C, Buckley R. Limbal transplantation in the management of chronic contact-lens-associated epitheliopathy. Eye (Lond) 1993;7(Pt 5):629–33. doi: 10.1038/eye.1993.145. [DOI] [PubMed] [Google Scholar]
- 19.Basti S, Mathur U. Unusual intermediate-term outcome in three cases of limbal autograft transplantation. Ophthalmology. 1999;106:958–63. doi: 10.1016/S0161-6420(99)00516-3. [DOI] [PubMed] [Google Scholar]
- 20.Solomon A, Ellies P, Anderson DF, Touhami A, Grueterich M, Espana EM, et al. Long-term outcome of keratolimbal allograft with or without penetrating keratoplasty for total limbal stem cell deficiency. Ophthalmology. 2002;109:1159–66. doi: 10.1016/s0161-6420(02)00960-0. [DOI] [PubMed] [Google Scholar]
- 21.Shimazaki J, Shimmura S, Tsubota K. Donor source affects the outcome of ocular surface reconstruction in chemical or thermal burns of the cornea. Ophthalmology. 2004;111:38–44. doi: 10.1016/j.ophtha.2003.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Tsubota K, Satake Y, Kaido M, Shinozaki N, Shimmura S, Bissen-Miyajima H, et al. Treatment of severe ocular-surface disorders with corneal epithelial stem-cell transplantation. N Engl J Med. 1999;340:1697–703. doi: 10.1056/NEJM199906033402201. [DOI] [PubMed] [Google Scholar]


