Abstract
Background and Purpose
Although exogenous hormone therapy (HT) use has been associated with increased risk of ischemic stroke in postmenopausal women, it remains unknown whether sex hormone levels contribute to ischemic stroke risk. We aimed to estimate associations between plasma sex hormone levels and ischemic stroke risk, by HT status, in a nested case-control study of postmenopausal women from the Nurses’ Health Study.
Methods
Women with confirmed incident ischemic stroke (n=419) were matched with controls (n=419) by age, HT use, and other factors. Plasma estradiol and testosterone levels were measured using liquid chromatography tandem mass spectrometry; sex hormone-binding globulin (SHBG) was assayed by electrochemiluminescence immunoassay. Associations of total and free estradiol and testosterone, the estradiol/testosterone ratio, and SHBG with ischemic stroke were estimated using conditional logistic regressions stratified by HT status with adjustment for matching and cardiovascular risk factors.
Results
Current HT users had different hormone profiles from never/past users. No clear linear trends were observed between estradiol (total or free) levels or the estradiol/testosterone ratio and ischemic stroke risk among either current users (Ptrend>0.1) or never/past users (Ptrend>0.6). For both current and never/past users, the associations between some of the sex hormones and ischemic stroke differed by body mass index (BMI) categories (Pinteraction≤0.04). For women with a BMI<25kg/m2, a higher estradiol/testosterone ratio was associated with significantly elevated ischemic stroke risk among current users (Ptrend=0.01), and higher levels of total and free estradiol were significantly associated with higher ischemic stroke risk among never/past users (Ptrend≤0.04). Testosterone and SHBG were not associated with ischemic stroke in either current or never/past users.
Conclusions
Our findings do not support a role of sex hormone levels in mediating ischemic stroke risk among postmenopausal women. Replications in additional larger studies are required.
Keywords: Estradiol, Testosterone, Sex hormone-binding globulin, Ischemic stroke, Hormone therapy, Postmenopausal women
Subject Terms: Cerebrovascular Disease, Ischemic Stroke, Epidemiology, Risk Factors, Women
Women have more stroke events than men.1 In the Women’s Health Initiative and other clinical trials, hormone therapy (HT) was associated with a higher risk of ischemic stroke among postmenopausal women.2-4 However, it remains unknown whether circulating sex hormone levels contribute to ischemic stroke risk in postmenopausal women, particularly in HT users. Only a few studies have investigated the relationship between sex hormone levels and ischemic stroke among postmenopausal women, and the conclusions remained inconsistent.5-9
In this nested case-control study, we prospectively evaluated associations between sex hormone levels and ischemic stroke risk in postmenopausal women. Because of the large differences in sex hormone levels by HT use, all analyses were performed separately in current and never/past users of HT.
Methods
Because of the sensitive nature of the data collected for this study, requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to the Nurses’ Health Study (NHS) at nhsaccess@channing.harvard.edu.
Study Population
Postmenopausal women from the NHS with confirmed incident ischemic stroke between blood collection (1989-1990)10 and June 2006 were included (n=419), and were matched to controls by age at blood collection, HT status, smoking, race, history of coronary heart disease, self-reported cancer, month of blood collection, and fasting. Participants who used estrogen-alone, estrogen-plus-progestogen, or other HT at blood collection were considered current HT users. Women consented to participate (Supplementary Methods). The study protocol was approved by the Institutional Review Boards of Brigham and Women’s Hospital.
Laboratory assessments
Plasma estradiol and testosterone were measured using liquid chromatography tandem mass spectrometry (LC-MS/MS). Sex hormone-binding globulin (SHBG) was measured by electrochemiluminescence immunoassay (Supplementary Methods). Free estradiol and free testosterone were calculated by the law of mass action.11
Statistical analysis
Conditional logistic regression was used to estimate ischemic stroke risk in relation to increasing quartiles of estradiol, testosterone, the estradiol/testosterone ratio, and SHBG. Multivariable models were adjusted for HT type (current users only), bilateral oophorectomy, body mass index (BMI), physical activity, alcohol intake, current aspirin use, and history of elevated cholesterol, hypertension, and diabetes. Stratified analyses by BMI, age at blood collection, and years since menopause were performed using unconditional logistic regressions with adjustment for matching factors and above covariates. Secondary and sensitivity analyses were also performed (Supplementary Methods).
Results
Women who developed ischemic stroke were more likely to have a history of hypertension and diabetes compared with controls (Table 1). Estradiol and SHBG levels were higher in current HT users than never/past users; no differences were seen by case-control status (Table I).
Table 1.
Baseline characteristics of study participants.
| Current HT users |
Never/past users of HT |
|||||
|---|---|---|---|---|---|---|
| Mean (SD) or No. (%) | Cases (n=198) |
Controls (n=198) |
P | Cases (n=221) |
Controls (n=221) |
P |
| Age, year† | 61.8 (5.1) | 61.8 (5.1) | 0.86 | 63.1 (4.4) | 63.1 (4.4) | 0.51 |
| BMI, kg/m2† | 25.2 (4.0) | 24.7 (4.1) | 0.18 | 26.2 (5.2) | 26.1 (5.3) | 0.85 |
| Bilateral oophorectomy | 64 (32.3%) | 70 (35.4%) | 0.52 | 37 (16.7%) | 31 (14.0%) | 0.43 |
| History of hypertension | 87 (43.9%) | 65 (32.8%) | 0.02 | 120 (54.3%) | 81 (36.7%) | 0.0002 |
| History of diabetes | 21 (10.6%) | 6 (3.0%) | 0.003 | 30 (13.6%) | 19 (8.6%) | 0.10 |
| History of elevated cholesterol | 93 (47.0%) | 90 (45.5%) | 0.76 | 112 (50.7%) | 110 (49.8%) | 0.85 |
| History of coronary heart disease† | 12 (6.1%) | 14 (7.1%) | 0.68 | 14 (6.3%) | 19 (8.6%) | 0.37 |
| Family history of MI prior to age 60 | 89 (45.0%) | 80 (40.4%) | 0.36 | 93 (42.1%) | 88 (39.8%) | 0.63 |
| Aspirin use >1 tabl/wk | 97 (49.0%) | 90 (45.5%) | 0.48 | 99 (44.8%) | 122 (55.2%) | 0.03 |
| Physically activity, MET-hour/wk | 15.6 (21.3) | 17.5 (20.7) | 0.36 | 15.1 (17.7) | 15.3 (16.3) | 0.92 |
| Alcohol consumption, g/m2 | 6.6 (12.1) | 5.9 (10.0) | 0.51 | 5.1 (9.4) | 4.8 (10.5) | 0.75 |
| Smoking status† | 0.94 | 0.93 | ||||
| Never smokers | 80 (40.4%) | 79 (39.9%) | 93 (42.1%) | 93 (42.1%) | ||
| Past smokers | 86 (43.4%) | 89 (45.0%) | 82 (37.1%) | 85 (38.5%) | ||
| Current smokers | 32 (16.2%) | 30 (15.2%) | 46 (20.8%) | 43 (19.5%) | ||
| HT type | 0.59 | |||||
| Estrogen alone | 108 (54.6%) | 114 (57.6%) | ||||
| Estrogen plus progestin | 62 (31.3%) | 55 (27.8%) | ||||
| Other hormones | 23 (11.6%) | 20 (10.1%) | ||||
| Unknown | 5 (2.5%) | 9 (4.6%) | ||||
Matching factors.
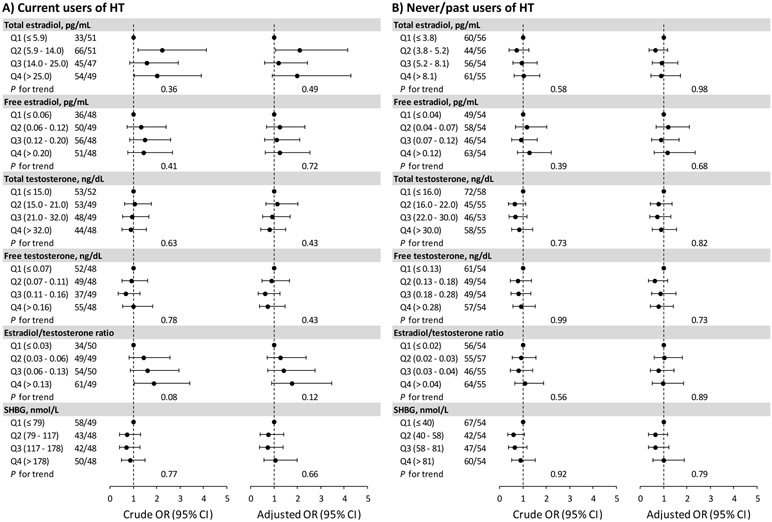
Estradiol, testosterone, the estradiol/testosterone ratio, and SHBG were not associated with ischemic stroke in current or never/past users of HT (multivariable Ptrend≥0.4; Figure 1). The results remained unchanged after additionally adjusting for other biomarkers (Table II), excluding women whose HT status changed after blood collection (Table III), or restricting the analysis to current HT users who took the most common estrogen preparation and dose.
Figure 1. Associations of sex hormone and SHBG levels with ischemic stroke.
Numbers of case/control were shown by quartiles of each marker.
We observed significant interactions of BMI and total and free estradiol with ischemic stroke in both current and never/past users of HT (Pinteraction≤0.04), and the interaction for the estradiol/testosterone ratio was also significant in current HT users (Pinteraction=0.02) (Figure 2). Among current HT users, associations between the estradiol/testosterone ratio and stroke differed significantly by years since menopause (Pinteraction=0.01; Table IV). Associations between sex hormone biomarkers and ischemic stroke did not vary by age (Pinteraction≥0.13; Table V). All interaction P-values were not significant after Bonferroni correction.
Figure 2. Adjusted associations of sex hormones and SHBG with ischemic stroke by BMI.
Numbers of case/control were shown by quartiles of each marker.
Discussion
In this nested case-control study with a large number of ischemic stroke cases, we did not observe significant associations between estradiol levels and ischemic stroke in current or never/past users of HT. Associations of estradiol levels (both current and never/past users) and the estradiol/testosterone ratio (current users only) with ischemic stroke may vary across BMI categories. No association for testosterone or SHBG with ischemic stroke was observed.
Our findings are consistent with previous studies that did not observed significant associations, but prior studies only focused on postmenopausal women who did not use HT.6-8 Only one study found that detectable total estradiol (vs. non-detectable by immunoassay) were associated with higher stroke risk among women who had established carotid atherosclerosis.5
We utilized the highly sensitive LC-MS/MS technique – the gold standard for assessing testosterone and estradiol levels in postmenopausal women,12 which ensured the accuracy and reliability of the assessment. Other strengths include the prospective study design and detailed covariate information in the NHS. Additionally, this study had the largest number of ischemic stroke cases in women thus far, but the statistical power was still limited for HT groups. Another limitation is that sex hormones and SHBG were only measured at one timepoint. We did not have information on atrial fibrillation, a known risk factor for ischemic stroke that has been associated with HT, at or prior to the blood collection.13 We cannot rule out residual confounding by unmeasured confounders.
Conclusions
Our data do not confirm an association of plasma sex hormone and SHBG levels with ischemic stroke risk in postmenopausal women who are either current or never/past HT users. Future studies with a larger sample size are needed.
Supplementary Material
Acknowledgements
We thank the NHS participants for their invaluable contribution.
Funding
The NHS is funded by the National Institutes of Health (UM1CA186107, R01CA49449, R01HL034594, and R01HL088521). Dr. Jie Hu is supported by a Research Fellowship Program from the Women’s Brain Initiative, Brigham and Women’s Hospital.
Footnotes
Disclosures
None.
Contributor Information
Jie Hu, Division of Women’s Health, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Jennifer H. Lin, Division of Preventive Medicine, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Monik C. Jiménez, Division of Women’s Health, Division of Preventive Medicine, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
JoAnn E. Manson, Division of Preventive Medicine, Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital, Boston, MA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA.
Susan E. Hankinson, Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital, Boston, MA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA; Department of Biostatistics and Epidemiology, School of Public Health and Health Science, University of Massachusetts Amherst, Amherst, MA.
Kathryn M. Rexrode, Division of Women’s Health, Division of Preventive Medicine, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
References
- 1.Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, et al. Sex differences in stroke: Epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: The women’s health initiative: A randomized trial. JAMA. 2003;289:2673–2684 [DOI] [PubMed] [Google Scholar]
- 3.Bath PM, Gray LJ. Association between hormone replacement therapy and subsequent stroke: A meta-analysis. BMJ. 2005;330:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hendrix SL, Wassertheil-Smoller S, Johnson KC, Howard BV, Kooperberg C, Rossouw JE, et al. Effects of conjugated equine estrogen on stroke in the women’s health initiative. Circulation. 2006;113:2425–2434 [DOI] [PubMed] [Google Scholar]
- 5.Glisic M, Mujaj B, Rueda-Ochoa OL, Asllanaj E, Laven JSE, Kavousi M, et al. Associations of endogenous estradiol and testosterone levels with plaque composition and risk of stroke in subjects with carotid atherosclerosis. Circ Res. 2018;122:97–105 [DOI] [PubMed] [Google Scholar]
- 6.Holmegard HN, Nordestgaard BG, Jensen GB, Tybjaerg-Hansen A, Benn M. Sex hormones and ischemic stroke: A prospective cohort study and meta-analyses. J Clin Endocrinol Metab. 2016;101:69–78 [DOI] [PubMed] [Google Scholar]
- 7.Lee JS, Yaffe K, Lui LY, Cauley J, Taylor B, Browner W, et al. Prospective study of endogenous circulating estradiol and risk of stroke in older women. Arch Neurol. 2010;67:195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scarabin-Carre V, Canonico M, Brailly-Tabard S, Trabado S, Ducimetiere P, Giroud M, et al. High level of plasma estradiol as a new predictor of ischemic arterial disease in older postmenopausal women: The three-city cohort study. J Am Heart Assoc. 2012;1:e001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao D, Guallar E, Ouyang P, Subramanya V, Vaidya D, Ndumele CE, et al. Endogenous sex hormones and incident cardiovascular disease in post-menopausal women. J Am Coll Cardiol. 2018;71:2555–2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hankinson SE, Willett WC, Manson JE, Hunter DJ, Colditz GA, Stampfer MJ, et al. Alcohol, height, and adiposity in relation to estrogen and prolactin levels in postmenopausal women. J Natl Cancer Inst. 1995;87:1297–1302 [DOI] [PubMed] [Google Scholar]
- 11.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–810 [DOI] [PubMed] [Google Scholar]
- 12.Stanczyk FZ, Lee JS, Santen RJ. Standardization of steroid hormone assays: Why, how, and when? Cancer Epidemiol Biomarkers Prev. 2007;16:1713–1719 [DOI] [PubMed] [Google Scholar]
- 13.Wong JA, Rexrode KM, Sandhu RK, Moorthy MV, Conen D, Albert CM. Menopausal age, postmenopausal hormone therapy and incident atrial fibrillation. Heart. 2017;103:1954–1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.